- 1Enzyme and Microbial Biochemistry Laboratory, Department of Chemistry, Indian Institute of Technology Delhi, New Delhi, India
- 2Department of Basic Medical Science, College of Applied Medical Science, King Khalid University (KKU), Khamis Mushait, Abha, Saudi Arabia
In the recent times, nanomaterials have emerged in the field of biology, medicine, electronics, and agriculture due to their immense applications. Owing to their nanoscale sizes, they present large surface/volume ratio, characteristic structures, and similar dimensions to biomolecules resulting in unique properties for biomedical applications. The chemical and physical methods to synthesize nanoparticles have their own limitations which can be overcome using biological methods for the synthesis. Moreover, through the biogenic synthesis route, the usage of microorganisms has offered a reliable, sustainable, safe, and environmental friendly technique for nanosynthesis. Bacterial, algal, fungal, and yeast cells are known to transport metals from their environment and convert them to elemental nanoparticle forms which are either accumulated or secreted. Additionally, robust nanocarriers have also been developed using viruses. In order to prevent aggregation and promote stabilization of the nanoparticles, capping agents are often secreted during biosynthesis. Microbial nanoparticles find biomedical applications in rapid diagnostics, imaging, biopharmaceuticals, drug delivery systems, antimicrobials, biomaterials for tissue regeneration as well as biosensors. The major challenges in therapeutic applications of microbial nanoparticles include biocompatibility, bioavailability, stability, degradation in the gastro-intestinal tract, and immune response. Thus, the current review article is focused on the microbe-mediated synthesis of various nanoparticles, the different microbial strains explored for such synthesis along with their current and future biomedical applications.
Introduction
Nanoparticles have found increasing industrial and biomedical applications in recent times. Particles within the size of 10–1,000 nm are considered as nanoparticles (Arshad, 2017). However, in general for most applications, <100 nm is deemed to be effective for applications due to easier penetration and similar sizes to biomolecules. The smaller size of nanomaterials provide myriad research opportunities for biologists. Owing to their dimensions matching the scale of biomolecules, nanomaterials have the ability to interact with complex biological systems in unique ways. This rapidly expanding field has allowed for the design and development of multifunctional nanoparticles to diagnose target and treat diseases such as cancer (Sardar et al., 2014; Pastorino et al., 2019). Nanoscale molecules, components, and devices are essentially of the same scale as biological entities and can easily cross the blood-tissue barriers. New approaches such as drug delivery through nanocarriers are being used for targeted and controlled delivery to the specific site. They help in improving drug efficacy and decrease the drug toxicity in disease therapy (Blanco et al., 2015; Pastorino et al., 2019; Ahmad et al., 2021). Further, nanocarriers interact with the biomolecules on the cell surface and within the cell in ways that do not alter these molecules' biochemical properties and behavior (Pastorino et al., 2019; Gao et al., 2020; Stillman et al., 2020). Such ready access to a living cell's interior allows remarkable advantages on the clinical and basic research frontiers. These days, with unique optical properties such as fluorescence and surface plasmon resonance (SPR), nanomaterials are achieving increasing attention in biomedical applications (Wang et al., 2007; Boisselier and Astruc, 2009; Aminabad et al., 2019; Elahi et al., 2019) especially in developing optics-based analytical techniques used for bioimaging (Xia, 2008; Chisanga et al., 2019) and biosensing (Kumar et al., 2019; Celiksoy et al., 2020; Noori et al., 2020). For such biomedical applications, a metal surface's biocompatibility is a key consideration and metal nanoparticles synthesized using biological systems, provide metals ions with high biocompatibility.
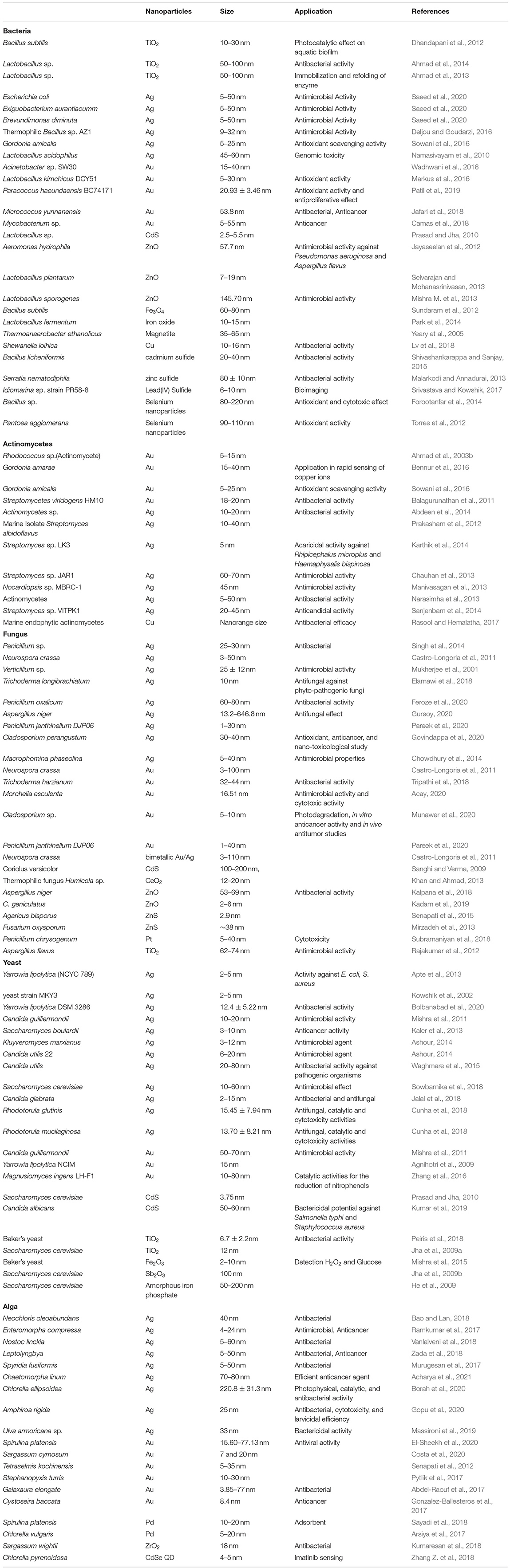
Various nanoparticle synthesis methods include physical, chemical, and biological routes (Chen and Mao, 2007; Ahmad et al., 2015; Khatoon et al., 2015; Mazumder et al., 2016; Abdulla et al., 2021). The different physical, chemical, and biological methods of nano-synthesis are depicted in Figure 1. Green synthesis approaches such as biological means provide a sustainable, economical and less harsh nanoparticle synthesis method compared to chemical or physical methods. Moreover, biological synthesis offers control over size and shape for required applications. This is now well-known that many organisms can produce inorganic materials either intra or extracellularly (Senapati et al., 2004). Organisms such as bacteria, actinomycetes, fungi, yeasts, viruses, and algae are being explored as reducing or stabilizing agents to synthesize metal nanoparticles such as gold, silver, copper, cadmium, platinum, palladium, titanium, and zinc, which find uses in numerous industrial and biomedical application. Hence, the current review article is focused on the microbial-mediated synthesis of various nanoparticles and their applications in multiple sectors, with a particular focus on the biomedical and pharmaceutical industry.
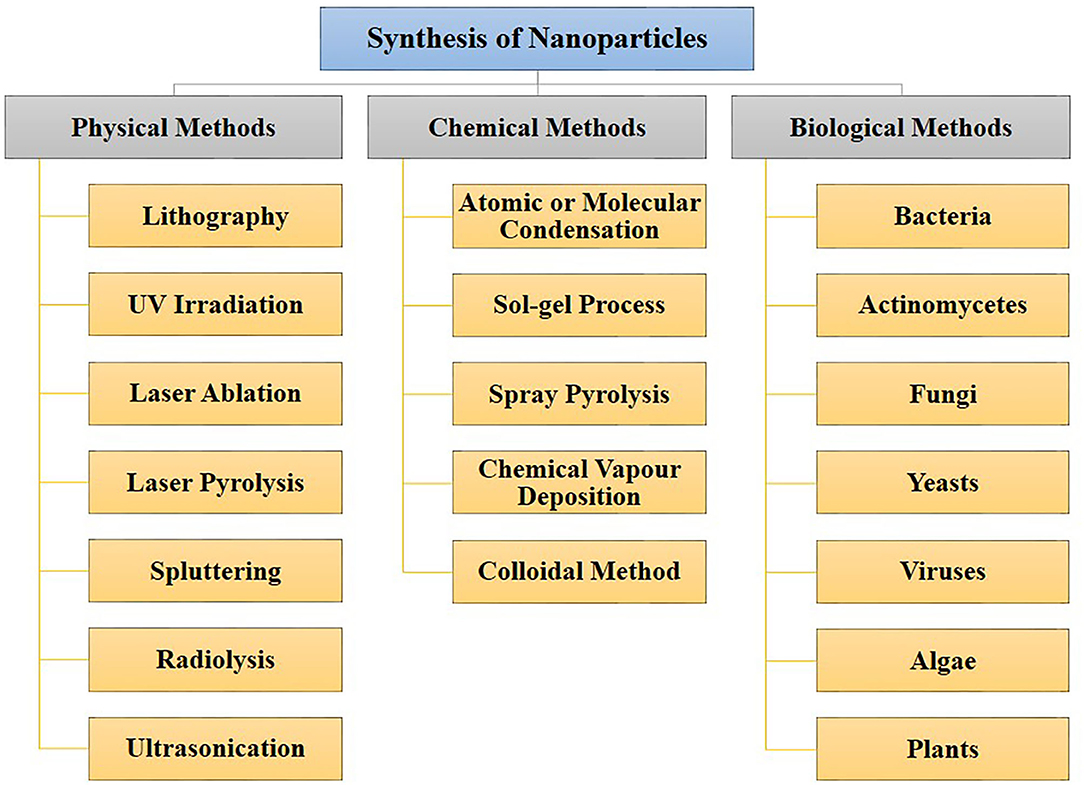
Figure 1. Different approaches for nanoparticles synthesis. Nanoparticles can be synthesized through physical, chemical, and biological routes.
Synthesis of Nanoparticles by Microbial Strains
There are three primary approaches to the synthesis of nanoparticles, namely physical, chemical, and biological. These three approaches of nanoparticles synthesis belong to either the top-down or bottom-up methods. The top-down approach involves the mechanical method of reducing size by gradually breaking down the bulk materials into the nanoscale structure. On the other hand, the bottom-up method is based on the assembly of atoms or molecules in the nanoscale range into the molecular structure. The bottom-up method depends on the nanoparticles' chemical and biological synthesis while top-down approaches generally refer to the physical or chemical route (Gan and Li, 2012; Lombardo et al., 2020). UV irradiation, sonochemistry, radiolysis, laser ablation, are physical methods to synthesize metallic nanoparticles (Kundu et al., 2008; Mohamed and Abu-Dief, 2018; Maric et al., 2019; Sadrolhosseini et al., 2019; Silva et al., 2019; Amulya et al., 2020). These methodologies have their limitations. While physical and chemical methods have successfully generated nanoparticles of high purity and desired size, these processes are typically costly and require toxic chemicals. The chemical synthesis process may lead to the existence of certain toxic chemical species becoming adsorbed on the surface of nanoparticles, which may lead to detrimental effects in medical applications; these nanoparticles may also have direct interaction with the human body, where the related toxicity becomes important. Thus, one of nanotechnology's primary objectives is to establish an eco-friendly production process that can provide low toxicity nanoparticles. Several investigators have focused their interest on biological methods of synthesizing metal nanoparticles to achieve this objective, as these are fast, cost-effective and eco-friendly. For this reason, the biological synthesis of nanoparticles includes a vast range of species in nature, such as viruses, bacteria, fungi, algae, plants (using their enzymes, proteins, DNA, lipids, and carbohydrates, etc.). Bacteria that reduce metals are found environmental-friendly catalysts for bioremediation as well as materials synthesis. In fact, microbes may help in the synthesis of diverse metal oxides through respiration processes (Kim et al., 2018). Electrons can be moved from reduced organic to oxidized inorganic compounds through microbial dissimilatory anaerobic respiration, thus promoting the formation of crystal/nanoparticles along with bioremediation processes. It is well-documented that the genus Shewanella are able to do the oxidation of organic acids as electron donors and reduction of inorganic metals as electron acceptors (Heidelberg et al., 2002; Harris et al., 2018). Further, the mechanism for detoxifying the immediate cell environment has been developed by microorganisms such as bacteria by reducing toxic metal species into metal nanoparticles (Deplanche and Macaskie, 2008; Murray et al., 2017). Also, biomolecules secreted by bacteria was used as capping as well as stabilizing agents of nanoparticles synthesis. The nanoparticle synthesis by the microbial process is depicted in Figure 2. The nanoparticles are usually formed following the way where metal ions are first trapped on the surface or inside of the microbial cells. The trapped metal ions are then reduced to nanoparticles in the presence of enzymes. In general, microorganisms impact the mineral formation in two distinct ways. They can modify the composition of the solution so that it becomes supersaturated or more supersaturated than it previously was with respect to a specific phase. A second means by which microorganisms can impact mineral formation is through the production of organic polymers, which can impact nucleation by favoring (or inhibiting) the stabilization of the very first mineral seeds. Microbes, which are regarded as potent eco-friendly green nanofactories, have the potential to control the size and shape of biological nanoparticles. Even though plant-extract based nanoparticle synthesis is a well-known biological nanosynthesis platform, nanoparticles synthesized this way may become polydisperse in nature due to the presence of phytochemicals as well as have difference in yield due to seasonal variations (Mishra A. et al., 2013; Mishra et al., 2016; Ovais et al., 2018; Sadaf et al., 2020; Ahmad et al., 2021). Thus, these are the distinct advantages pertaining to the synthesis of nanoparticles by microbes as compared to plants. Therefore, many microorganisms are considered to be potential candidates for synthesis of nanoparticles (Priyadarshini et al., 2013). The list of microorganisms used for the synthesis of nanoparticles is summarized in Table 1.
Figure 2. Mechanistic representation of the synthesis of nanoparticle by microbes. Formation of nanoparticles by microbes involves metal capture, enzymatic reduction, and capping. Metal ions are first trapped on the surface or inside of the microbial cells and then reduced to nanoparticles in the presence of enzymes. The enzyme serves as the nucleation site, providing electrons to the metal for its reduction. Microorganisms can impact mineral formation through the production of organic polymers, which can impact nucleation by favoring (or inhibiting) the stabilization of the very first mineral seeds.
Nanoparticle Synthesis by Bacteria
Production of reduced metal ions by microbes arises from their remarkable ability to adapt themselves to conditions of environmental stress (Kulkarni et al., 2015). Therefore, supernatants of various bacteria such as Pseudomonas proteolytic, Pseudomonas meridiana, Pseudomonas Antarctica, Arthrobacter gangotriensis, and Arthrobacter kerguelensis act as microbial cell factories finding applications as reducing agents in the synthesis of silver nanoparticles (Shaligram et al., 2009; Singh et al., 2015). Silver nanoparticles (AgNPs) synthesized by using Bacillus brevis have recently demonstrated remarkable antimicrobial properties against Staphylococcus aureus and Salmonella typhi multidrug-resistant strains (Saravanan et al., 2018). Pseudomonas stutzeri is another bacterial strain which has been found to accumulate AgNPs through an intracellular mechanism (Klaus et al., 1999). In Bacillus sp., silver nanoparticles have also been synthesized in intracellular periplasmic space (Pugazhenthiran et al., 2009). The organisms that reside in gold mines would be more able to resist soluble gold toxicity and efficiently produce gold nanoparticles (Srinath et al., 2018). When Acinetobacter sp. SW30 was incubated with different concentrations of gold chloride and different cell density, it showed enormous variation in the color of gold nanoparticles (AuNP) containing colloidal solution, suggesting variation in size and shape. Surprisingly, at the lowest cell density and HAuCl4 salt concentration, monodispersed spherical AuNP of size ~19 nm was observed, whereas cell number increase resulted in polyhedral AuNP (~39 nm) formation. Amino acids are implicated in the gold salt reduction, while amide groups assist in AuNP stabilization (Wadhwani et al., 2016). Also, inside the lactic acid bacteria cells, nanocrystals of silver, gold, and their alloys have been biosynthesized (Nair and Pradeep, 2002). In order to synthesize gold nanoparticles (AuNPs), two separate strains of Pseudomonas aeruginosa were used in one sample, producing AuNPs of different sizes (Husseiny et al., 2007). Rhodopseudomonas capsulate mediated extracellular synthesis of gold nanoparticles of various sizes and shapes was also reported. The strain was used to generate spherical (10–50 nm) and triangular plate (50–400 nm) AuNPs (He et al., 2007). ZnO nanoflowers were synthesized using Serratia ureilytica and further used on cotton fabrics to provide antimicrobial activities against E. coli and S. aureus (Dhandapani et al., 2014). Lactobacillus plantarum has also been reported to biosynthesize ZnO nanoparticles (Selvarajan and Mohanasrinivasan, 2013). The gram-negative bacterial strain Aeromonas hydrophila has been explored for the biosynthesis of ZnO nanoparticles with further antimicrobial applications (Jayaseelan et al., 2012). Triangular CuO nanoparticles have been developed using Halomonas elongate which displayed antimicrobial activity against E. coli and S. aureus (Rad et al., 2018). In another study, super paramagnetic iron oxide nanoparticles of about 29.3 nm dimensions were manufactured using Bacillus cereus strain. As an application, their anti-cancer effects were reported against the MCF-7 (breast cancer) and 3T3 (mouse fibroblast) cell lines in a dose-dependent manner (Fatemi et al., 2018). A rapid, convenient method for the synthesis of manganese and zinc nanoparticles by reducing manganese sulfate and zinc sulfate using Streptomyces sp. (intracellular route) has been reported. The scale of NPs for manganese and zinc was between 10 and 20 nm (Waghmare et al., 2011). Bacillus amyloliquifaciens strain KSU-109 produced surfactin, which helped in the synthesis of stable cadmium sulfide nanoparticles of average size of 3–4 nm (Singh et al., 2011). Escherichia coli E-30 and Klebsiella pneumoniae K-6 have been shown to synthesize cadmium sulfide nanoparticles with average size ranging from 3.2 to 44.9 nm and showed highest antimicrobial activity on A. fumigatus, G. candidum, B. subtilis, S. aureus, and E. coli strains (Abd Elsalam et al., 2018). Serratia marcescens mediated synthesized antimony sulfide nanoparticles were reported with size range <35 nm (Bahrami et al., 2012), while Pseudomonas aeruginosa ATCC 27853 mediated synthesis of selenium nanoparticles were reported with a size of 96 nm (Kora and Rastogi, 2016). Lead nanoparticles synthesized using Cocos nucifera were reported with 47 nm size and also showed good activity against S. aureus (Elango and Roopan, 2015). The bacterial strains isolated from Gabal El Sela in Eastern Dessert, Egypt have been used for the biosynthesis of uranium nanoparticles intracellularly with size ranging from 2.9 to 21.13 nm (Abostate et al., 2018).
Cyanobacteria are a phylum of photosynthetic bacteria widely explored for their capacity to synthesize nanoparticles due to the presence of bioactive components, which help in stabilizing and functionalizing the nanoparticles, resulting in fewer steps in synthesis. Their high-growth rate also facilitates higher biomass production to aid in nanosynthesis. In most cases, cell-free extracts of the cyanobacterial biomass are used for nanosynthesis. Aqueous extracts of the cyanobacterium Oscillatoria limnetica has been useful in synthesizing silver nanoparticles by reduction and further stabilizing them. The size of the nanoparticles was 3.30–17.97 nm and they showed anti-cancer and anti-microbial activity (Hamouda et al., 2019). A similar Ag-NPs synthesis by Microchaete sp. NCCU-342 was pursued using aqueous biomass extracts and spherical, polydispersed nanoparticles of 60–80 nm size were obtained (Husain et al., 2019). Silver nanoparticles synthesized from Desertifilum sp. (4.5–26 nm) showed antibacterial activity and cytotoxic effects against HepG2, MCF-7, and Caco-2 cancer cells (Hamida et al., 2020). Other cyanobacterial strains explored for nanoparticle synthesis include Scytonema sp., Nostoc sp., Phormidium sp. (Al Rashed et al., 2018). One interesting study used filamentous cyanobacterium, Plectonema boryanum (strain UTEX 485) biomass reacted with AgNO3. Silver nanoparticles were found to precipitate on the surface as well as inside of the cyanobacterium cell. Intracellular nanoparticles were found to be of the size (<10 nm), while that of extracellular ones exhibited size in the range of (1–200 nm) (Lengke et al., 2007a). P. boryanum is also reported to reduce gold (III)-chloride solutions to form Au nanoparticles intracellularly via formation of gold (I) sulfide (Lengke et al., 2006b); this species is also known to produce platinum and palladium NPs (Lengke et al., 2006a, 2007b). Thus, cyanobacteria present a promising platform for biogenic nanosynthesis with widespread applications.
Nanoparticle Synthesis by Actinomycetes
Actinomycetes have gained significant attention because they are the least studied, but important for metal nanoparticle synthesis (Golinska et al., 2014). Actinomycetes are considered superior groups among microbial species of commercial importance due to the development of various bioactive components and extracellular enzymes through their saprophytic behavior (Kumar et al., 2008). For the biosynthesis and characterization of gold nanoparticles, only a few of the genera such as Thermomonospora, Nocardia, Streptomyces, and Rhodococcus have been identified from actinomycetes (El-Batal et al., 2015). Streptomyces species are considered to be the dominant biosynthesis contender (Zonooz et al., 2012). In actinomycetes, intracellular reduction of metal ions takes place on the surface of mycelia along with cytoplasmic membranes, leading to the formation of nanoparticles (Ahmad et al., 2003b). Some researchers suggested that the possible mechanism of intracellular synthesis of metal nanoparticles occurs by trapping Ag+ ions on cell surface, likely through electrostatic interactions between Ag+ and negatively charged groups of carboxylate in mycelial cell wall enzymes. Enzymes present in the cell wall leading to the formation of silver nuclei decrease the silver ions, subsequently expanding by further decrease and accumulation of Ag+ ions on these nuclei (Abdeen et al., 2014). A different mechanism for the intracellular synthesis of silver nanoparticles by using lactic acid bacteria was suggested by Sintubin et al. (2009). Furthermore, several other researchers have also documented the intracellular synthesis of metal nanoparticles utilizing actinomycetes strains (Usha et al., 2010; Balagurunathan et al., 2011; Prakasham et al., 2012; Sukanya et al., 2013).
Nanoparticle Synthesis by Fungi
Another biogenic route of biosynthesis of various metal nanoparticles involves successful application of myco-nanotechnological approaches. Similar to bacteria/cyanobacteria, nanosynthesis may be extracellular or intracellular in nature. In the intracellular route, metal salts in the mycelia, which fungi can use, are converted into a less toxic form (Molnar et al., 2018; Rajeshkumar and Sivapriya, 2020). The use of fungal extracts involves extracellular biosynthesis (Zhao et al., 2018; Rajeshkumar and Sivapriya, 2020). In the biosynthesis of nanoparticles, fungi are comparatively more resourceful than bacteria due to many bioactive metabolites, high aggregation, and improved production (Castro-Longoria et al., 2011; Alghuthaymi et al., 2015). Several filamentous fungi have been reported to be capable in AuNP biosynthesis. In order to biosynthesize AuNPs, this study employed various methods. The authors suggested that fungal secreted compounds and media components could be used to stabilize the nanoparticles (Molnar et al., 2018; Guilger-Casagrande and de Lima, 2019). Three different fungal strains (namely Fusarium oxysporum, Fusarium sp., and Aureobasidium pullulans) were used by another group to biosynthesize the reported AuNPs. The authors suggested that biosynthesis happened inside fungal vacuoles, and that sugar reduction was involved in tailoring the shape of AuNPs. Additionally, fungus produced the secondary metabolite contain protein or biomolecules which act as capping as well as stabilizing agents (Zhang et al., 2011). Several Fusarium oxysporum strains have been used in another study to generate extracellular silver metal nanoparticles in the 20–50 nm range (Ahmad et al., 2003a). The metal ion reduction by nitrate-dependent reductase and extracellular shuttle quinone was confirmed by UV-Visible, fluorescence, and enzymatic activity analysis (Duran et al., 2005, 2007). Kumar and their groups formed in vitro silver nanoparticles (10–25 nm) stabilized in the presence of reduced cofactor nicotinamide adenine dinucleotide phosphate (NADPH) by a capping peptide using the nitrate reductase enzyme isolated from Fusarium oxysporum, along with phytochelatinin, and 4-hydroxyquinoline (Kumar et al., 2007). Another study indicated that the synthesis of monodispersed AgNPs of 9.4 nm size was mediated by Rhizopus stolonifera extracts, although condition optimization resulted in AgNPs of 2.86 nm (Abdelrahim et al., 2017). The extracellular synthesis of AgNPs utilizing Candida glabrata suggested strong antimicrobial activity (Jalal et al., 2018). ZnO nanoparticles mediated by Aspergillus niger indicated excellent antibacterial potential, while the Bismarck brown dye was also degraded by up to 90% (Kalpana et al., 2018). Cobalt oxide nanoparticles have recently been fabricated using Aspergillus nidulans (Vijayanandan and Balakrishnan, 2018). Biosynthesis of platinum nanoparticles of size range 100–180 nm from the Fusarium oxysporum fungus was documented (Riddin et al., 2006). The fungi Verticillium sp., Fusarium oxysporum sp., and Aspergillus flavus have shown the ability to produce nanoparticles either extracellularly or intracellularly (Mukherjee et al., 2002; Bhainsa and D'Souza, 2006). To create natural nanofactories, the change from bacteria to fungi has the added benefit that downstream biomass processing and handling can be much more straightforward.
Nanoparticle Synthesis by Yeasts
Yeast strains of several genera are known to employ different mechanisms for nanoparticle synthesis resulting in significant variations in size, particle position, monodispersity, and other properties. One study found that glutathione (GSH) and two classes of metal-binding ligands-metallothioneins and phytochelatins (PC) were generated by detoxification mechanisms in yeast cells. These molecules have a role to play in deciding the mechanism for nanoparticle synthesis and stabilize the resulting complexes in most of the yeast species studied (Hulkoti and Taranath, 2014). Often as a resistance mechanism, yeast cells in the vicinity of toxic metals can change the absorbed metal ions into complex polymer compounds that are not toxic to the cell. Typically, these nanoparticles synthesized in the yeast are referred to as “semiconductor crystals” or “quantum semiconductor crystals” (Dameron et al., 1989). Yeasts cells are particularly well-known for their ability to synthesize semiconductor nanoparticles, particularly that of cadmium sulfide (CdS). There are reports on the production of other metal nanoparticles, particularly AgNPs, by yeasts, including Pichia capsulata (Subramanian et al., 2010), Candida guilliermondii (Mishra et al., 2011), Saccharomyces boulardii (Kaler et al., 2013), Kluyveromyces marxianus (Ashour, 2014), Candida utilis (Waghmare et al., 2015), Candida lusitaniae (Eugenio et al., 2016), Saccharomyces cerevisiae (Sowbarnika et al., 2018), Candida glabrata (Jalal et al., 2018), Candida albicans (Ananthi et al., 2018), Rhodotorula glutinis, and Rhodotorula mucilaginosa (Cunha et al., 2018). The silver-tolerant yeast strain MKY3 was used for the production of silver nanoparticles (Kowshik et al., 2002).
Nanoparticle Synthesis by Algae
The use of algae for the biosynthesis of nanoparticles is also increasingly becoming common. In order to synthesize ZnO nanoparticles, Sargassum muticum was used and was reported to decrease angiogenesis in HepG2 cells in addition to apoptotic effects (Sanaeimehr et al., 2018). In the biosynthesis of AuNPs, Sargassum crassifolium, a macroalgae along with sea grass, has been utilized. Interestingly in this study, a blue shift in the UV absorption spectra was observed after increasing the concentration of S. crassifolium, which was attributed to a decreased size of the nanoparticles due to increased nucleation centers in the reductant (Maceda et al., 2018). CuO nanoparticles of around 7 nm dimensions have been synthesized biogenically using Cystoseira trinodis and reported to have improved antibacterial and antioxidant properties, along with methylene blue degradation potential (Gu et al., 2018). Using Sargassum ilicifolium, aluminum oxide nanoparticles with ~20 nm size were produced (Koopi and Buazar, 2018). Various algae strains, for example Turbinaria conoides, Laminaria japonica, Acanthophora spicifera, and Sargassum tenerrimum have been reported to synthesize gold nanoparticles (Ghodake and Lee, 2011; Swaminathan et al., 2011; Vijayaraghavan et al., 2011; Ramakrishna et al., 2016). Using Spirulina platensis, synthesis of novel core (Au)-shell (Ag) nanoparticles has also been investigated (Govindaraju et al., 2008).
Nanoparticle Synthesis by Viruses
Viruses have emerged as promising candidates as nanoparticles for biomedical applications, owing to their biocompatibility, biodegradability, capacity of mass production, programmable scaffolds, and ease of genetic manipulation for desired characteristics. Viral bodies, themselves are naturally occurring nanoparticles due to their 20–500 nanometer dimensions. Their robustness along with ability to detect changes in the environment to release their genetic material has been exploited in biomedical applications. The major applications of viral nanoparticles has been in gene delivery, drug delivery, as vaccines/immunotherapeutics and in imaging and theranostics. Mostly mammalian viruses are used in gene delivery while bacteriophages and plant viruses have been explored for drug delivery, vaccines, and immunotherapeutics. Viral nanoparticles (VNPs) can also be tagged with several ligands for targeting, therapeutics or imaging agents for myriad biomedical applications (Steinmetz, 2010). A similar class of materials are virus-like particles (VLPs) derived from the protein coating of the viruses (Chung et al., 2020). These nanoparticles can be of bacteriophage, plant or animal viral origin and are dynamic, self-assembling moieties with symmetrical, monodisperse structures. Production of viral nanoparticles involve generation in a host body (whether a bacteria, animal, or plant), further chemical conjugation and tuning, followed by evaluation in-vitro and in-vivo (Steinmetz, 2010). A major consideration for using VNPs is regarding their toxicity, especially for human pathogens. Thus, bacteriophages and plant viruses are preferred, compared to mammalian viruses such as adenoviruses. Additionally, immunogenicity of the viral particle affects their accumulation in the tissue as well as clearance. Attachment to molecules such as PEG, often helps in shielding of specific biointeractions (Bruckman et al., 2008). Various VNPs and VLPs have been exploited to deliver chemotherapeutic drugs. VLPs modified with targeting peptide with a load of doxorubicin, cisplatin, and 5-fluorouracil were found to be effective in human hepatocellular carcinoma cells (Ashley et al., 2011). Tobacco-Mosaic Virus derived VNPs used to carry cisplatin have been used in platinum-resistant ovarian cancer cells (Franke et al., 2017). Bacteriophage fd based nanoparticles with peptides specifically targeting pathogenic bacteria such as Staphylococcus aureus; and loaded with antibiotic such as chloramphenicol have found better antibacterial action than chloramphenicol alone (Yacoby et al., 2006). Viral nanoparticles also find application as MRI contrast agents, having large rotational correlation times due to their rigid structures, which results in high relaxivity. Additionally, owing to their polyvalent nature, a high number of contrast agents such as gadolinium can be chelated to their interior or exterior surfaces (Steinmetz, 2010). Such nanoparticles have also been explored to develop vaccines against pathogens such as hepatitis B, HIV, and Neospora caninum (Oh and Han, 2020).
In addition, one important precaution to be emphasized relates to the handling of bacterial or viral strains that might be harmful or pathogenic to humans. Thus, in order to implement microorganism-mediated nanosynthesis on a large scale for commercial exploitation, utmost importance is to be given to associated biological safety issues as well.
Biological Application of Microbial Synthesized Nanomaterials
Due to their controlled sizes, unique properties, biocompatible nature, non-toxicity, microbial nanoparticles find myriad biomedical applications. They have found major applications in the biomedical and pharmaceutical fields as antimicrobials, anti-biofilm agents, antioxidants, anti-cancer agents, and diagnostic or imaging agents, some of which are discussed here and shown in Figure 3.
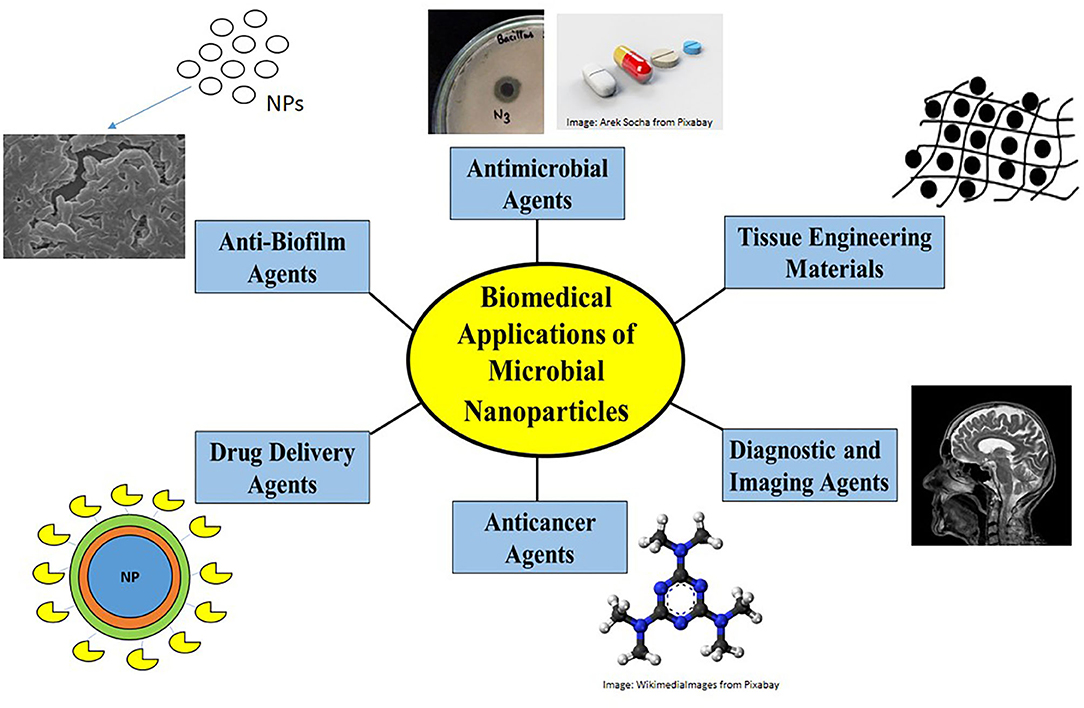
Figure 3. Biomedical applications of nanoparticles. Nanoparticles find biomedical applications as antimicrobial agents by disrupting membrane structure or by generating ROS, as anti-biofilm agents in preventing antimicrobial resistance, as drug-delivery agents to carry drug loads, as anti-cancer agents causing apoptosis, as diagnostic/imaging agents in MRI and biosensors and as anticoagulants/anthelmintics/tissue engineering materials.
Antimicrobial Agents
In general, several metallic nanoparticles such as that of silver, copper, zinc, magnesium, gold, and titanium are known for their antimicrobial properties. The mode of antimicrobial action attributed to the nanoparticles include disruption of membrane structure, pore formation on the microbial cell wall, inhibition of biofilm formation or production of reactive oxygen species (ROS) in case of metal oxide nanoparticles (Busi and Rajkumari, 2019). The antimicrobial property is heavily dependent on the nanoparticle size and shape, with smaller, monodispersed nanoparticles (with resulting larger surface to volume ratio) displaying greater antimicrobial tendencies (Duran et al., 2010). The search for novel antimicrobial nanoparticles has been fuelled by the rise of multidrug resistance (MDR) phenotype among pathogenic strains. An important advantage of biogenic synthesis is the inherent presence of natural stabilizing or capping agents such as polysaccharides or proteins on the nanoparticle surface upon synthesis, which reduces post-production steps to a large extent. AuNPs synthesized using the culture supernatant of Ochrobactrum rhizosphaerae were found to be coated with glycolipoprotein, with potent antibiotic activity against Vibrio cholerae. In case of fungally synthesized nanoparticles, the capping agents are generally proteinaceous in nature. Example of Ag-NPs synthesized intracellularly by the mushroom fungus, Schizophyllum commune and that of by Trichoderma viride showed capping by proteins and exhibited antibacterial activity against strains such as Bacillus subtilis, Pseudomonas sp., Trichophyton mentagrophytes, K. pneumonia, Trichophyton simii, Trichophyton rubrum, E. coli, B. subtilis, and Klebsiella planticola, respectively (Chitra and Annadurai, 2013; Arun et al., 2014). The Silver nanoparticles generally act due to the release of Ag+ ions which can disrupt bacterial membranes as well as interfere with DNA and protein synthesis. Similarly, gold nanoparticles, due to their photocatalytic activity, can be developed in conjugation with photosensitizers for antimicrobial photodynamic therapy. On exposure to near Infrared radiation (NIR), the heat produced destroys the bacterial cell wall (Busi and Rajkumari, 2019). Often conjugation of traditional antibiotic moieties to nanoparticles have been found to enhance their effect. AuNPs synthesized from the fungi, T. viride attached to vancomycin showed suppression of growth in vancomycin resistant S. aureus and E. coli, due to the proposed binding of vancomycin-AuNPs to the S. aureus transpeptidase, in place of terminal peptides of the glycopeptidyl precursors and easy transport across membrane in case of E. coli, leading to cell-wall lysis (Fayaz et al., 2011). Loading of multiple drugs such as ciprofloxacin, gentamycin, vancomycin and rifampicin on AuNPs, biogenically synthesized from B. subtilis exhibited growth suppression in S. haemolyticus and S. epidermidis due to enhanced surface area provided by the NPs for the drugs to bind (Roshmi et al., 2015). From the above examples, it is interesting to observe that the nanoparticles synthesized using the extracts of one microorganism are effective in the killing other microbial species and enhances the activity of existing antibiotics to overcome antimicrobial resistance phenotypes.
Anti-biofilm Agents
The increasing incidences of antibiotic resistance are a major challenge in the area of antibiotic/antimicrobial development. An important reason for bacterial infection and their multidrug-resistant phenotype arises from the ability of the organism to form biofilms which make them resistant to drugs. Microbes such as Staphylococcus aureus, Acinetobacter baumannii, Escherichia coli, Pseudomonas aeruginosa, are known to cause opportunistic infections due to biofilm formation and thus, inhibiting it is a significant aspect explored in case of biogenic nanoparticles. Additionally, biomedical and dental devices are at high-risk of transmitting infections due to biofilm formation and nanoparticle coating has been examined as an effective option to avoid this. In most studies, the biofilm formation is generally assessed by cell staining (by crystal violet) and absorbance measurements or by observation under electron microscopes. In one research, TiO2 nanoparticles were synthesized utilizing Bacillus subtilis biomass. Afterwards, microbe-rich pond water was used for the growth of biofilm in solution or on glass slides along with the nanoparticles followed by irradiation of polychromatic light; the TiO2 nanoparticles acted as a photocatalyst releasing H2O2 to inhibit the biofilm growth (Dhandapani et al., 2012). Another early investigation, synthesized microbial Se and Te nanoparticles from the intracellular extracts of Stenotrophomonas maltophilia SeITE02 and Ochrobactrum sp. MPV1, which displayed distinct antimicrobial and anti-biofilm capabilities against both planktonic cells and biofilm cells of E. coli JM109, S. aureus ATCC 25923, and P. aeruginosa PAO1 with production of ROS suggested as the possible mechanism (Zonaro et al., 2015). The disinfectant properties of silver nanoparticles are pretty well-known. Silver nanoparticles harvested intracellularly from B. licheniformis biomass exhibited 90% anti-biofilm activity against P. aeruginosa and S. epidermidis (Kalishwaralal et al., 2010). Additionally, gold-silver bimetallic nanoparticles biogenically synthesized using the γ-proteobacterium Shewanella oneidensis MR-1, showed antimicrobial properties and were able to inhibit biofilm growth of P. aeruginosa, S. aureus, E. coli, and Enterococcus faecalis cultures at a concentration of 250 μM (Ramasamy and Lee, 2016). Fungi such as Phanerochaete chrysosporium have also showed promising biofilm eradication capability. Silver nanoparticles (~45 nm diameter) obtained from the extracellular extracts of the fungus were able to act on E. coli and C. albicans, even though the cell wall of both the strains are different (Estevez et al., 2020). An interesting negative effect of biofilm formation is observable in membranes, mostly used for wastewater treatment, where biofouling caused by microbial consortia present in the wastewater slurry, reduces the efficacy of the bioreactor. Microbial silver nanoparticles (bio-Ag0) of around 11 nm size, synthesized by Lactobacillus fermentum LMG 8900 were embedded in polyethersulfone (PES) membranes, and were further tested on (E. coli and P. aeruginosa) and another mixed culture in an activated sludge bioreactor. The membranes showed remarkable antibacterial and anti-biofilm activity in both cases over a test period of 9 weeks (Zhang et al., 2011). All the above instances reveal an excellent potential of microbial nanoparticles in inhibition and eradication of biofilm formation.
Drug-Delivery Agents
Biogenic nanoparticles are important candidates over conventional ones as drug delivery agents due to their stability, biocompatibility, bioavailability, controlled drug release characteristics, targeted delivery and non-toxic nature. Such nano-agents can include nanospeheres, water soluble polymers, emulsions, micelles, and liposomes (Meng et al., 2010; Srivastava et al., 2021). As drug- carriers, what is needed is the ability to encapsulate a particular drug and release it conditionally at the disease site. Moreover, delivery agents should be able to cross the blood-tissue and cellular barriers for inter and intracellular transport in order to achieve targeted delivery of the drug-load at site (Fariq et al., 2017). However, it is pertinent to assess their safety to normal cells and efficacy in cancer cells at the very outset. Magnetotactic bacteria are known to convert magnetic greigite Fe3S4 and/or magnetite Fe3O4 into bilayer membrane bound structures known as magnetosomes, which can be used to encapsulate and carry drugs (Vargas et al., 2018; Ahmad et al., 2019). Bacterial magnetosomes loaded with doxorubicin were tested on H22 tumor-bearing mice and showed higher tumor suppression than doxorubicin alone (Sun et al., 2009). Magnetosomes from Magnetospirillum gryphiswaldense loaded with anti-4-1BB agonistic antibody have been used as immunotherapy against cancer in TC-1 mouse models (Tang et al., 2019). Taxol conjugated to gold nanoparticles obtained from the fungus Humicola sp. has been used for anti-tumor drug-delivery applications (Khan et al., 2014). Biogenic gold nanoparticles functionalized with moieties such as transferrin also hold potential to cross the blood-brain barrier to target drugs into the brain (Tripathi et al., 2015).
Anti-cancer Agents
As an extension to the above section, pristine biosynthesized nanoparticles, without drug load have also been extensively used to develop anti-cancer agents. Platinum nanoparticles Saccharomyces boulardii were found to be effective against A431 epidermoid carcinoma and MCF-7 breast cancer cell lines (Borse et al., 2015). Gold nanoparticles biosynthesized from Streptomyces cyaneus exhibited anticancer activity in vitro against HEPG-2 human liver cancer cells and MCF-7 breast cancer cells, respectively. The plausible mechanism of action of the nanoparticles is through mitochondrial apoptosis, DNA impairment and induced detention of cytokinesis (El-Batal et al., 2015). Silver nanoparticles synthesized from the water extract of the endophytic fungi, Cladosporium perangustum has been found to reduce the viability of the MCF-7 cells through enhancement in the levels of caspase-3, caspase-7, caspase-8, and caspase-9 expression (Govindappa et al., 2020). Biocompatible terbium oxide nanoparticles synthesized using the biomass of fungus Fusarium oxysporum were effective in dose-dependent cytotoxicity in MG-63 and Saos-2 cell-lines while being non-toxic to primary osteoblasts; ROS production was enhanced and apoptosis was confirmed with nanoparticle treatment (Iram et al., 2016). ZnO nanoparticles biosynthesized from Rhodococcus pyridinivorans, loaded with anthraquinone showed cell-death in HT-29 colon carcinoma cells as compared to normal cells, and can thus find application as an anti-cancer agent (Kundu et al., 2014). AuNPs obtained from the fungi Helminthosporium solani conjugated to doxorubicin had higher uptake and comparable cytotoxicity in HEK293 cells compared to doxorubicin alone (Kumar et al., 2008). Similar gold and gadolinium oxide nanoparticles Humicola sp. could be conjugated to taxol or doxorubicin for anti-cancer applications (Syed et al., 2013; Khan et al., 2014). One interesting study used biomineralised magnetic nanoparticles (from magnetotactic bacteria), guided by MRI to convert the energy of near-infrared light into heat thus resulting in ablation of tumor cells with no-known toxicity. This was termed as a photothermal effect and the bacterial nanoparticles acted as a theranostic (therapy + diagnostic) in this case (Chen et al., 2016). Several in-vivo studies have revealed the potential of bacterial magnetic nanoparticles. In another study, BALB/C mouse were immunized with bacterial magnetosomes to observe their immune response, and found to have not so significant response, proving their drug delivery potential (Meng et al., 2010).
Diagnostics and Imaging Agents
In general, nanoparticles find increasing applications in diagnostics and as biosensors often conjugated to diagnostic enzymes (Rossi et al., 2004; Ghosh et al., 2018a,b). In recent times, biogenic nanoparticles have also been explored as biosensors and in imaging modalities such as MRI. In MRI, contrast agents comprising of magnetites are found to be synthesized by several Gram negative magnetotactic bacteria (MTB) in the form of magnetosomes, which are intracellular organelles with a lipid bilayer enclosing crystals of magnetic iron oxides (Uebe and Schuler, 2016). Bacterial magnetosomes display higher r2 relaxivity than synthesized nanoparticles and have shown application in targeting human epidermal growth factor receptor-2 (HER2) expressing tumor cells. Relaxivity is a measure of how sensitive a contrast agent is. For similar compounds, a molecule with higher relaxivity would provide equivalent contrast at a lower dose compared to a low relaxivity compound. A lower dose may lower the risk of the nanoparticle toxicity (Jacques et al., 2010). In orthotopic breast cancer models, intravenous administration of HER2-targeting bacterial magnetosomes, showed augmented contrast in the MR signals (Zhang Y. et al., 2018). Another study created RGD-peptide expressing magnetosomes by generic engineering Magnetospirillum magneticum AMB-1 strain, which targeted αvβ3 integrins-overexpressing brain tumor cells in gliomas as evident in MRI (Boucher et al., 2007, 2017; Zhao, 2017). A theranostic photothermal therapy of cancer using magnetic nanoparticles of the same bacterial strain under the guidance of MRI was achieved in vitro and in vivo by another group (Chen et al., 2016). An interesting study employed magneto-endosymbionts as living contrast agent in the iPSC-derived cardiomyocytes, which could be tracked by MRI and cleared out within 1 week, thus enhancing biocompatibility (Mahmoudi et al., 2016). Bacteriogenic metal nanoparticles such as that of copper, palladium and gold have also been explored for their potential in biosensing (Rai et al., 2016; Ghosh, 2018). In an interesting study, AuNPs synthesized from Candida albicans were conjugated to liver cancer cell surface specific antibodies. Thus, when used to probe into liver cancer cells, they could uniquely bind to the liver cancer specific surface antigen, thus distinguishing them from normal cells. Such nanoparticles could thus find application as a diagnostic or as a carrier of anti-cancer drugs (Chauhan et al., 2011).
Other Medical Uses
As is evident, microbial synthesized nanoparticles find more than the above stated pharmaceutical applications. One early study employed the biomass of Brevibacterium casei to reduce AgNO3 and HAuCl4 to obtain silver and gold nanoparticles from the intracellular extracts, which were further explored as an anti-coagulant of human plasma (Kalishwaralal et al., 2010). From fungal species, gold nanoparticles derived from Nigrospora oryzae displayed anthelmintic activity (paralysis and death) against the cestode parasite Raillietina sp. (Kar et al., 2014). Antimicrobial carbon dots (CDs) were synthesized by hydrothermal method from cell free supernatant of Lactobacillus acidophilus and they showed antimicrobial activity against Escherichia coli (Gram-negative) and Listeria monocytogenes (Gram-positive) (Kousheh et al., 2020). Nanocellulose is another nanoscale material which is predominantly synthesized by bacteria. Scaffolds based on nanocellulose (NC) have pivotal applications in tissue engineering (TE) like to repair, improve or replace damaged tissues and organs, including skin, blood vessel, nerve, skeletal muscle, heart, liver, and ophthalmology, mainly due to the biocompatibility, water absorption, water retention, optical transparency, and chemo-mechanical properties (Luo et al., 2019). Some of these nanocelluloses has been clinically approved and available in the market in the form of patents for wound healing, burn treatment and cosmetic applications (Brown et al., 2015).
Conclusion and Future Prospects
Nanoparticles synthesized by microbes prove promising for several biomedical and therapeutic applications due to their controlled biocompatible dimensions and unique properties. Methods of biosynthesis are also beneficial since nanoparticles are often coated with a lipid layer/biomolecules that gives physiological solubility and stability, which is essential for biomedical applications and is the bottleneck of other synthetic methods. However, biogenic nanoparticles pose a few challenges which need to be addressed for large scale applications. Till now, the lack of monodispersity, time intensive production process, low production rates, and batch to batch variations has limited their use on commercial scale. There are some important aspects which might be considered in the process of synthesis of well-characterized nanoparticles. For the synthesis of highly stable and well-characterized NPs, biological protocols may be used when critical aspects such as organism types, inheritable and genetic properties of organisms, optimal conditions for cell growth and enzyme activity, optimal reaction conditions, and biocatalyst state selection have been considered. Additionally, most biomedical studies with microbial nanoparticles have been accomplished in-vitro and large scale clinical trials and safety tests are of utmost importance to realize their effects in-vivo. Thus, with further in-depth studies, it is hoped that microbial nanoparticles will hold immense potential in medicine and healthcare.
Author Contributions
SG and RA conceptualized and prepared the manuscript. SK has critically reviewed the manuscript. MZ helped in critically assessing the manuscript and addressing the review comments with inputs which were further included in the revised manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abd Elsalam, S. S., Taha, R. H., Tawfeik, A. M., El-Monem, A., Mohamed, O., and Mahmoud, H. A. (2018). Antimicrobial activity of bio and chemical synthesized cadmium sulfide nanoparticles. Egypt. J. Hosp. Med. 70, 1494–1507. doi: 10.12816/0044675
Abdeen, S., Geo, S., Praseetha, P. K., and Dhanya, R. P. (2014). Biosynthesis of silver nanoparticles from actinomycetes for therapeutic applications. Int. J. Nano Dimens. 5, 155–162. doi: 10.5101/nbe.v5i1.p39-45
Abdelrahim, K., Mahmoud, S. Y., Ali, A. M., Almaary, K. S., Mustafa, A. E. Z. M. A., and Husseiny, S. M. (2017). Extracellular biosynthesis of silver nanoparticles using Rhizopus stolonifer. Saudi J. Biol. Sci. 24, 208–216. doi: 10.1016/j.sjbs.2016.02.025
Abdel-Raouf, N., Al-Enazi, N. M., and Ibraheem, I. B. M. (2017). Green biosynthesis of gold nanoparticles using Galaxaura elongata and characterization of their antibacterial activity. Arab. J. Chem. 10, S3029–S3039. doi: 10.1016/j.arabjc.2013.11.044
Abdulla, N. K., Siddiqui, S. I., Fatima, B., Sultana, R., Tara, N., Hashmi, A. A., et al. (2021). Silver based hybrid nanocomposite: a novel antibacterial material for water cleansing. J. Clean. Prod. 284:124746. doi: 10.1016/j.jclepro.2020.124746
Abostate, M. A., Saleh, Y., Mira, H., Amin, M., Al Kazindar, M., and Ahmed, B. M. (2018). Characterization, kinetics and thermodynamics of biosynthesized uranium nanoparticles (UNPs). Artif. Cells Nanomed. Biotechnol. 46, 147–159. doi: 10.1080/21691401.2017.1301460
Acay, H. (2020). Utilization of Morchella esculenta-mediated green synthesis golden nanoparticles in biomedicine applications. Prep. Biochem. Biotechnol. 51, 127–136. doi: 10.1080/10826068.2020.1799390
Acharya, D., Satapathy, S., Somu, P., Parida, U. K., and Mishra, G. (2021). Apoptotic effect and anticancer activity of biosynthesized silver nanoparticles from marine algae chaetomorpha linum extract against human colon cancer cell HCT-116. Biol. Trace Elem. Res. 199, 1812–1822. doi: 10.1007/s12011-020-02304-7
Agnihotri, M., Joshi, S., Kumar, A. R., Zinjarde, S., and Kulkarni, S. (2009). Biosynthesis of gold nanoparticles by the tropical marine yeast Yarrowia lipolytica NCIM 3589. Mater. Lett. 63, 1231–1234. doi: 10.1016/j.matlet.2009.02.042
Ahmad, A., Mukherjee, P., Senapati, S., Mandal, D., Khan, M. I., Kumar, R., et al. (2003a). Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surfaces B Biointerfaces 28, 313–318. doi: 10.1016/S0927-7765(02)00174-1
Ahmad, A., Senapati, S., Khan, M. I., Kumar, R., Ramani, R., Srinivas, V., et al. (2003b). Intracellular synthesis of gold nanoparticles by a novel alkalotolerant actinomycete, Rhodococcus species. Nanotechnology 14:824. doi: 10.1088/0957-4484/14/7/323
Ahmad, F., Ashraf, N., Ashraf, T., Zhou, R.-B., and Yin, D.-C. (2019). Biological synthesis of metallic nanoparticles (MNPs) by plants and microbes: their cellular uptake, biocompatibility, and biomedical applications. Appl. Microbiol. Biotechnol. 103, 2913–2935. doi: 10.1007/s00253-019-09675-5
Ahmad, R., Khatoon, N., and Sardar, M. (2013). Biosynthesis, characterization and application of TiO2 nanoparticles in biocatalysis and protein folding. J. Proteins Proteomics 4, 115–121.
Ahmad, R., Khatoon, N., and Sardar, M. (2014). Antibacterial effect of green synthesized TiO2 nanoparticles. Adv. Sci. Lett. 20, 1616–1620. doi: 10.1166/asl.2014.5563
Ahmad, R., Mohsin, M., Ahmad, T., and Sardar, M. (2015). Alpha amylase assisted synthesis of TiO2 nanoparticles: structural characterization and application as antibacterial agents. J. Hazard. Mater. 283, 171–177. doi: 10.1016/j.jhazmat.2014.08.073
Ahmad, R., Srivastava, S., Ghosh, S., and Khare, S. K. (2021). Phytochemical delivery through nanocarriers: a review. Colloids Surfaces B Biointerfaces 197:111389. doi: 10.1016/j.colsurfb.2020.111389
Al Rashed, S., Al Shehri, S., and Moubayed, N. M. S. (2018). Extracellular biosynthesis of silver nanoparticles from Cyanobacteria. Biomed. Res. 29, 2859–2862. doi: 10.4066/biomedicalresearch.29-17-3209
Alghuthaymi, M. A., Almoammar, H., Rai, M., Said-Galiev, E., and Abd-Elsalam, K. A. (2015). Myconanoparticles: synthesis and their role in phytopathogens management. Biotechnol. Biotechnol. Equip. 29, 221–236. doi: 10.1080/13102818.2015.1008194
Aminabad, N. S., Farshbaf, M., and Akbarzadeh, A. (2019). Recent advances of gold nanoparticles in biomedical applications: State of the art. Cell Biochem. Biophys. 77, 123–137. doi: 10.1007/s12013-018-0863-4
Amulya, M. A. S., Nagaswarupa, H. P., Kumar, M. R. A., Ravikumar, C. R., and Kusuma, K. B. (2020). Sonochemical synthesis of MnFe2O4 nanoparticles and their electrochemical and photocatalytic properties. J. Phys. Chem. Solids 148:109661. doi: 10.1016/j.jpcs.2020.109661
Ananthi, V., Prakash, G. S., Rasu, K. M., Gangadevi, K., Boobalan, T., Raja, R., et al. (2018). Comparison of integrated sustainable biodiesel and antibacterial nano silver production by microalgal and yeast isolates. J. Photochem. Photobiol. B Biol. 186, 232–242. doi: 10.1016/j.jphotobiol.2018.07.021
Apte, M., Sambre, D., Gaikawad, S., Joshi, S., Bankar, A., Kumar, A. R., et al. (2013). Psychrotrophic yeast Yarrowia lipolytica NCYC 789 mediates the synthesis of antimicrobial silver nanoparticles via cell-associated melanin. AMB Express 3:32. doi: 10.1186/2191-0855-3-32
Arshad, A. (2017). Bacterial synthesis and applications of nanoparticles. Nano Sci Nano Technol. 11:119.
Arsiya, F., Sayadi, M. H., and Sobhani, S. (2017). Green synthesis of palladium nanoparticles using Chlorella vulgaris. Mater. Lett. 186, 113–115. doi: 10.1016/j.matlet.2016.09.101
Arun, G., Eyini, M., and Gunasekaran, P. (2014). Green synthesis of silver nanoparticles using the mushroom fungus Schizophyllum commune and its biomedical applications. Biotechnol. Bioproc. Eng. 19, 1083–1090. doi: 10.1007/s12257-014-0071-z
Ashley, C. E., Carnes, E. C., Phillips, G. K., Durfee, P. N., Buley, M. D., Lino, C. A., et al. (2011). Cell-specific delivery of diverse cargos by bacteriophage MS2 virus-like particles. ACS Nano 5, 5729–5745. doi: 10.1021/nn201397z
Ashour, S. M. (2014). Silver nanoparticles as antimicrobial agent from Kluyveromyces marxianus and Candida utilis. Int. J. Curr. Microbiol. Appl. Sci. 3, 384–396.
Bahrami, K., Nazari, P., Sepehrizadeh, Z., Zarea, B., and Shahverdi, A. R. (2012). Microbial synthesis of antimony sulfide nanoparticles and their characterization. Ann. Microbiol. 62, 1419–1425. doi: 10.1007/s13213-011-0392-5
Balagurunathan, R., Radhakrishnan, M., Rajendran, R. B., and Velmurugan, D. (2011). Biosynthesis of gold nanoparticles by actinomycete Streptomyces viridogens strain HM10. J. Biochem. Biophys. 48, 331–335.
Bao, Z., and Lan, C. Q. (2018). Mechanism of light-dependent biosynthesis of silver nanoparticles mediated by cell extract of Neochloris oleoabundans. Colloids Surfaces B Biointerfaces 170, 251–257. doi: 10.1016/j.colsurfb.2018.06.001
Bennur, T., Khan, Z., Kshirsagar, R., Javdekar, V., and Zinjarde, S. (2016). Biogenic gold nanoparticles from the Actinomycete Gordonia amarae: application in rapid sensing of copper ions. Sens. Actuators B Chem. 233, 684–690. doi: 10.1016/j.snb.2016.04.022
Bhainsa, K. C., and D'Souza, S. F. (2006). Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigatus. Colloids Surfaces B Biointerfaces 47, 160–164. doi: 10.1016/j.colsurfb.2005.11.026
Blanco, E., Shen, H., and Ferrari, M. (2015). Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 33:941. doi: 10.1038/nbt.3330
Boisselier, E., and Astruc, D. (2009). Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 38, 1759–1782. doi: 10.1039/b806051g
Bolbanabad, E. M., Ashengroph, M., and Darvishi, F. (2020). Development and evaluation of different strategies for the clean synthesis of silver nanoparticles using Yarrowia lipolytica and their antibacterial activity. Process Biochem. 94, 319–328. doi: 10.1016/j.procbio.2020.03.024
Borah, D., Das, N., Das, N., Bhattacharjee, A., Sarmah, P., Ghosh, K., et al. (2020). Alga-mediated facile green synthesis of silver nanoparticles: photophysical, catalytic and antibacterial activity. Appl. Organomet. Chem. 34:e5597. doi: 10.1002/aoc.5597
Borse, V., Kaler, A., and Banerjee, U. C. (2015). Microbial synthesis of platinum nanoparticles and evaluation of their anticancer activity. Int. J. Emerg. Trends Electr. Electron 11, 26–31.
Boucher, A. A., Hunt, G. E., Karl, T., Micheau, J., Mcgregor, I. S., and Arnold, J. C. (2007). Heterozygous neuregulin 1 mice display greater baseline and delta(9)-tetrahydrocannabinol-induced c-Fos expression. Neuroscience 149, 861–870. doi: 10.1016/j.neuroscience.2007.08.020
Boucher, M., Geffroy, F., Preveral, S., Bellanger, L., Selingue, E., Adryanczyk-Perrier, G., et al. (2017). Genetically tailored magnetosomes used as MRI probe for molecular imaging of brain tumor. Biomaterials 121, 167–178. doi: 10.1016/j.biomaterials.2016.12.013
Brown, R. M. Jr., Czaja, W., Jeschke, M., and Young, D. J. (2015). Multiribbon Nanocellulose as a Matrix for Wound Healing. Texas: Google Patents.
Bruckman, M. A., Kaur, G., Lee, L. A., Xie, F., Sepulveda, J., Breitenkamp, R., et al. (2008). Surface modification of tobacco mosaic virus with “click” chemistry. Chembiochem 9, 519–523. doi: 10.1002/cbic.200700559
Busi, S., and Rajkumari, J. (2019). “Microbially synthesized nanoparticles as next generation antimicrobials: scope and applications,” in Nanoparticles in Pharmacotherapy, ed A. M. Grumezescu (Elsevier), 485–524. doi: 10.1016/B978-0-12-816504-1.00008-9
Camas, M., Camas, A. S., and Kyeremeh, K. (2018). Extracellular synthesis and characterization of gold nanoparticles using Mycobacterium sp. BRS2A-AR2 isolated from the aerial roots of the Ghanaian mangrove plant, Rhizophora racemosa. Indian J. Microbiol. 58, 214–221. doi: 10.1007/s12088-018-0710-8
Castro-Longoria, E., Vilchis-Nestor, A. R., and Avalos-Borja, M. (2011). Biosynthesis of silver, gold and bimetallic nanoparticles using the filamentous fungus Neurospora crassa. Colloids Surfaces B Biointerfaces 83, 42–48. doi: 10.1016/j.colsurfb.2010.10.035
Celiksoy, S., Ye, W., Wandner, K., Schlapp, F., Kaefer, K., Ahijado-Guzman, R., et al. (2020). Plasmonic nanosensors for the label-free imaging of dynamic protein patterns. J. Phys. Chem. Lett. 11, 4554–4558. doi: 10.1021/acs.jpclett.0c01400
Chauhan, A., Zubair, S., Tufail, S., Sherwani, A., Sajid, M., Raman, S. C., et al. (2011). Fungus-mediated biological synthesis of gold nanoparticles: potential in detection of liver cancer. Int. J. Nanomedicine 6:2305. doi: 10.2147/IJN.S23195
Chauhan, R., Kumar, A., and Abraham, J. (2013). A biological approach to the synthesis of silver nanoparticles with Streptomyces sp JAR1 and its antimicrobial activity. Sci. Pharm. 81, 607–624. doi: 10.3797/scipharm.1302-02
Chen, C., Wang, S., Li, L., Wang, P., Chen, C., Sun, Z., et al. (2016). Bacterial magnetic nanoparticles for photothermal therapy of cancer under the guidance of MRI. Biomaterials 104, 352–360. doi: 10.1016/j.biomaterials.2016.07.030
Chen, X., and Mao, S. S. (2007). Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem. Rev. 107, 2891–2959. doi: 10.1021/cr0500535
Chisanga, M., Muhamadali, H., Ellis, D. I., and Goodacre, R. (2019). Enhancing disease diagnosis: biomedical applications of surface-enhanced Raman scattering. Appl. Sci. 9:1163. doi: 10.3390/app9061163
Chitra, K., and Annadurai, G. (2013). Antimicrobial activity of wet chemically engineered spherical shaped ZnO nanoparticles on food borne pathogen. Int. Food Res. J. 20, 59–64.
Chowdhury, S., Basu, A., and Kundu, S. (2014). Green synthesis of protein capped silver nanoparticles from phytopathogenic fungus Macrophomina phaseolina (Tassi) Goid with antimicrobial properties against multidrug-resistant bacteria. Nanoscale Res. Lett. 9:365. doi: 10.1186/1556-276X-9-365
Chung, Y. H., Cai, H., and Steinmetz, N. F. (2020). Viral nanoparticles for drug delivery, imaging, immunotherapy, and theranostic applications. Adv. Drug Deliv. Rev. 156, 214–235. doi: 10.1016/j.addr.2020.06.024
Costa, L. H., Hemmer, J. V., Wanderlind, E. H., Gerlach, O. M. S., Santos, A. L. H., Tamanaha, M. S., et al. (2020). Green synthesis of gold nanoparticles obtained from algae sargassum cymosum: optimization, characterization and stability. BioNanoScience 10, 1049–1062. doi: 10.1007/s12668-020-00776-4
Cunha, F. A., Da Cso Cunha, M., Da Frota, S. M., Mallmann, E. J. J., Freire, T. M., Costa, L. S., et al. (2018). Biogenic synthesis of multifunctional silver nanoparticles from Rhodotorula glutinis and Rhodotorula mucilaginosa: antifungal, catalytic and cytotoxicity activities. World J. Microbiol. Biotechnol. 34:127. doi: 10.1007/s11274-018-2514-8
Dameron, C. T., Reese, R. N., Mehra, R. K., Kortan, A. R., Carroll, P. J., Steigerwald, M. L., et al. (1989). Biosynthesis of cadmium sulphide quantum semiconductor crystallites. Nature 338, 596–597. doi: 10.1038/338596a0
Deljou, A., and Goudarzi, S. (2016). Green extracellular synthesis of the silver nanoparticles using thermophilic Bacillus sp. AZ1 and its antimicrobial activity against several human pathogenetic bacteria. Iranian J. Biotechnol. 14:25. doi: 10.15171/ijb.1259
Deplanche, K., and Macaskie, L. E. (2008). Biorecovery of gold by Escherichia coli and Desulfovibrio desulfuricans. Biotechnol. Bioeng. 99, 1055–1064. doi: 10.1002/bit.21688
Dhandapani, P., Maruthamuthu, S., and Rajagopal, G. (2012). Bio-mediated synthesis of TiO2 nanoparticles and its photocatalytic effect on aquatic biofilm. J. Photochem. Photobiol. B Biol. 110, 43–49. doi: 10.1016/j.jphotobiol.2012.03.003
Dhandapani, P., Siddarth, A. S., Kamalasekaran, S., Maruthamuthu, S., and Rajagopal, G. (2014). Bio-approach: ureolytic bacteria mediated synthesis of ZnO nanocrystals on cotton fabric and evaluation of their antibacterial properties. Carbohydr. Polym. 103, 448–455. doi: 10.1016/j.carbpol.2013.12.074
Duran, N., Marcato, P. D., Alves, O. L., De Souza, G. I. H., and Esposito, E. (2005). Mechanistic aspects of biosynthesis of silver nanoparticles by several Fusarium oxysporum strains. J. Nanobiotechnol. 3:8. doi: 10.1186/1477-3155-3-8
Duran, N., Marcato, P. D., Conti, R. D., Alves, O. L., Costa, F., and Brocchi, M. (2010). Potential use of silver nanoparticles on pathogenic bacteria, their toxicity and possible mechanisms of action. J. Braz. Chem. Soc. 21, 949–959. doi: 10.1590/S0103-50532010000600002
Duran, N., Marcato, P. D., De Souza, G. I. H., Alves, O. L., and Esposito, E. (2007). Antibacterial effect of silver nanoparticles produced by fungal process on textile fabrics and their effluent treatment. J. Biomed. Nanotechnol. 3, 203–208. doi: 10.1166/jbn.2007.022
Elahi, N., Kamali, M., and Baghersad, M. H. (2019). Recent biomedical applications of gold nanoparticles: a review. Talanta 184, 537–556. doi: 10.1016/j.talanta.2018.02.088
Elamawi, R. M., Al-Harbi, R. E., and Hendi, A. A. (2018). Biosynthesis and characterization of silver nanoparticles using Trichoderma longibrachiatum and their effect on phytopathogenic fungi. Egypt. J. Biol. Pest Control 28:28. doi: 10.1186/s41938-018-0028-1
Elango, G., and Roopan, S. M. (2015). Green synthesis, spectroscopic investigation and photocatalytic activity of lead nanoparticles. Spectrochimi. Acta Part A Mol. Biomol. Spectroscopy 139, 367–373. doi: 10.1016/j.saa.2014.12.066
El-Batal, A., Mona, S., and Al-Tamie, M. (2015). Biosynthesis of gold nanoparticles using marine Streptomyces cyaneus and their antimicrobial, antioxidant and antitumor (in vitro) activities. J. Chem. Pharm. Res. 7, 1020–1036.
El-Sheekh, M. M., Shabaan, M. T., Hassan, L., and Morsi, H. H. (2020). Antiviral activity of algae biosynthesized silver and gold nanoparticles against Herps Simplex (HSV-1) virus in vitro using cell-line culture technique. Int. J. Environ. Health Res. 6, 1–12. doi: 10.1080/09603123.2020.1789946
Estevez, M. B. N., Raffaelli, S., Mitchell, S. G., Faccio, R., and Alborés, S. S. (2020). Biofilm eradication using biogenic silver nanoparticles. Molecules 25:2023. doi: 10.3390/molecules25092023
Eugenio, M., Muller, N., Frases, S., Almeida-Paes, R., Lima, L. M. T. R., Lemgruber, L., et al. (2016). Yeast-derived biosynthesis of silver/silver chloride nanoparticles and their antiproliferative activity against bacteria. RSC Adv. 6, 9893–9904. doi: 10.1039/C5RA22727E
Fariq, A., Khan, T., and Yasmin, A. (2017). Microbial synthesis of nanoparticles and their potential applications in biomedicine. J. Appl. Biomed. 15, 241–248. doi: 10.1016/j.jab.2017.03.004
Fatemi, M., Mollania, N., Momeni-Moghaddam, M., and Sadeghifar, F. (2018). Extracellular biosynthesis of magnetic iron oxide nanoparticles by Bacillus cereus strain HMH1: characterization and in vitro cytotoxicity analysis on MCF-7 and 3T3 cell lines. J. Biotechnol. 270, 1–11. doi: 10.1016/j.jbiotec.2018.01.021
Fayaz, A. M., Girilal, M., Mahdy, S. A., Somsundar, S. S., Venkatesan, R., and Kalaichelvan, P. T. (2011). Vancomycin bound biogenic gold nanoparticles: a different perspective for development of anti VRSA agents. Process Biochem. 46, 636–641. doi: 10.1016/j.procbio.2010.11.001
Feroze, N., Arshad, B., Younas, M., Afridi, M. I., Saqib, S., and Ayaz, A. (2020). Fungal mediated synthesis of silver nanoparticles and evaluation of antibacterial activity. Microsc. Res. Tech. 83, 72–80. doi: 10.1002/jemt.23390
Forootanfar, H., Adeli-Sardou, M., Nikkhoo, M., Mehrabani, M., Amir-Heidari, B., Shahverdi, A. R., et al. (2014). Antioxidant and cytotoxic effect of biologically synthesized selenium nanoparticles in comparison to selenium dioxide. J. Trace Elem. Med. Biol. 28, 75–79. doi: 10.1016/j.jtemb.2013.07.005
Franke, C. E., Czapar, A. E., Patel, R. B., and Steinmetz, N. F. (2017). Tobacco mosaic virus-delivered cisplatin restores efficacy in platinum-resistant ovarian cancer cells. Mol. Pharm. 15, 2922–2931. doi: 10.1021/acs.molpharmaceut.7b00466
Gan, P. P., and Li, S. F. Y. (2012). Potential of plant as a biological factory to synthesize gold and silver nanoparticles and their applications. Rev. Environ. Sci. Bio. Technol. 11, 169–206. doi: 10.1007/s11157-012-9278-7
Gao, C., Wang, Y., Ye, Z., Lin, Z., Ma, X., and He, Q. (2020). Biomedical micro-/nanomotors: from overcoming biological barriers to in vivo imaging. Adv. Mater. 33:2000512. doi: 10.1002/adma.202000512
Ghodake, G., and Lee, D. S. (2011). Biological synthesis of gold nanoparticles using the aqueous extract of the brown algae Laminaria japonica. J. Nanoelectr. Optoelectr. 6, 268–271. doi: 10.1166/jno.2011.1166
Ghosh, S. (2018). Copper and palladium nanostructures: a bacteriogenic approach. Appl. Microbiol. Biotechnol. 102, 7693–7701. doi: 10.1007/s00253-018-9180-5
Ghosh, S., Ahmad, R., Gautam, V. K., and Khare, S. K. (2018b). Cholesterol-oxidase-magnetic nanobioconjugates for the production of 4-cholesten-3-one and 4-cholesten-3, 7-dione. Bioresour. Technol. 254, 91–96. doi: 10.1016/j.biortech.2018.01.030
Ghosh, S., Ahmad, R., and Khare, S. K. (2018a). Immobilization of cholesterol oxidase: an overview. Open Biotechnol. J. 12, 176–188. doi: 10.2174/1874070701812010176
Golinska, P., Wypij, M., Ingle, A. P., Gupta, I., Dahm, H., and Rai, M. (2014). Biogenic synthesis of metal nanoparticles from actinomycetes: biomedical applications and cytotoxicity. Appl. Microbiol. Biotechnol. 98, 8083–8097. doi: 10.1007/s00253-014-5953-7
Gonzalez-Ballesteros, N., Prado-Lopez, S., Rodraguez-Gonzalez, J. B., Lastra, M., and Rodraguez-Arguelles, M. C. (2017). Green synthesis of gold nanoparticles using brown algae Cystoseira baccata: its activity in colon cancer cells. Colloids Surfaces B Biointerfaces 153, 190–198. doi: 10.1016/j.colsurfb.2017.02.020
Gopu, M., Kumar, P., Selvankumar, T., Senthilkumar, B., Sudhakar, C., Govarthanan, M., et al. (2020). Green biomimetic silver nanoparticles utilizing the red algae Amphiroa rigida and its potent antibacterial, cytotoxicity and larvicidal efficiency. Bioproc. Biosyst. Eng. 44, 217–223. doi: 10.1007/s00449-020-02426-1
Govindappa, M., Lavanya, M., Aishwarya, P., Pai, K., Lunked, P., Hemashekhar, B., et al. (2020). Synthesis and characterization of endophytic fungi, Cladosporium perangustum mediated silver nanoparticles and their antioxidant, anticancer and nano-toxicological study. BioNanoScience 10, 1–14. doi: 10.1007/s12668-020-00719-z
Govindaraju, K., Basha, S. K., Kumar, V. G., and Singaravelu, G. (2008). Silver, gold and bimetallic nanoparticles production using single-cell protein (Spirulina platensis) Geitler. J. Mater. Sci. 43, 5115–5122. doi: 10.1007/s10853-008-2745-4
Gu, H., Chen, X., Chen, F., Zhou, X., and Parsaee, Z. (2018). Ultrasound-assisted biosynthesis of CuO-NPs using brown alga Cystoseira trinodis: characterization, photocatalytic AOP, DPPH scavenging and antibacterial investigations. Ultrason. Sonochem. 41, 109–119. doi: 10.1016/j.ultsonch.2017.09.006
Guilger-Casagrande, M., and de Lima, R. (2019). Synthesis of silver nanoparticles mediated by fungi: a review. Front. Bioeng. Biotechnol. 7:287. doi: 10.3389/fbioe.2019.00287
Gursoy, N. (2020). Fungus-mediated synthesis of silver nanoparticles (agnp) and inhibitory effect on Aspergillus spp. in combination with antifungal agent. Cumhuriyet Sci. J. 41, 311–318. doi: 10.17776/csj.653627
Hamida, R. S., Abdelmeguid, N. E., Ali, M. A., Bin-Meferij, M. M., and Khalil, M. I. (2020). Synthesis of silver nanoparticles using a novel cyanobacteria Desertifilum sp. extract: their antibacterial and cytotoxicity effects. Int. J. Nanomedicine 15:49. doi: 10.2147/IJN.S238575
Hamouda, R. A., Hussein, M. H., Abo-Elmagd, R. A., and Bawazir, S. S. (2019). Synthesis and biological characterization of silver nanoparticles derived from the cyanobacterium Oscillatoria limnetica. Sci. Rep. 9:13071. doi: 10.1038/s41598-019-49444-y
Harris, H. W., Sanchez-Andrea, I., Mclean, J. S., Salas, E. C., Tran, W., El-Naggar, M. Y., et al. (2018). Redox sensing within the genus Shewanella. Front. Microbiol. 8:2568. doi: 10.3389/fmicb.2017.02568
He, S., Guo, Z., Zhang, Y., Zhang, S., Wang, J., and Gu, N. (2007). Biosynthesis of gold nanoparticles using the bacteria Rhodopseudomonas capsulata. Mater. Lett. 61, 3984–3987. doi: 10.1016/j.matlet.2007.01.018
He, W., Zhou, W., Wang, Y., Zhang, X., Zhao, H., Li, Z., et al. (2009). Biomineralization of iron phosphate nanoparticles in yeast cells. Mater. Sci. Eng. C 29, 1348–1350. doi: 10.1016/j.msec.2008.10.030
Heidelberg, J. F., Paulsen, I. T., Nelson, K. E., Gaidos, E. J., Nelson, W. C., Read, T. D., et al. (2002). Genome sequence of the dissimilatory metal ion-reducing bacterium Shewanella oneidensis. Nat. Biotechnol. 20, 1118–1123. doi: 10.1038/nbt749
Hulkoti, N. I., and Taranath, T. C. (2014). Biosynthesis of nanoparticles using microbes-a review. Colloids Surfaces B Biointerfaces 121, 474–483. doi: 10.1016/j.colsurfb.2014.05.027
Husain, S., Afreen, S., Yasin, D., Afzal, B., and Fatma, T. (2019). Cyanobacteria as a bioreactor for synthesis of silver nanoparticles-an effect of different reaction conditions on the size of nanoparticles and their dye decolorization ability. J. Microbiol. Methods 162, 77–82. doi: 10.1016/j.mimet.2019.05.011
Husseiny, M. I., Abd El-Aziz, M., Badr, Y., and Mahmoud, M. A. (2007). Biosynthesis of gold nanoparticles using Pseudomonas aeruginosa. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 67, 1003–1006. doi: 10.1016/j.saa.2006.09.028
Iram, S., Khan, S., Ansary, A. A., Arshad, M., Siddiqui, S., Ahmad, E., et al. (2016). Biogenic terbium oxide nanoparticles as the vanguard against osteosarcoma. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 168, 123–131. doi: 10.1016/j.saa.2016.05.053
Jacques, V., Dumas, S., Sun, W.-C., Troughton, J. S., Greenfield, M. T., and Caravan, P. (2010). High relaxivity MRI contrast agents part 2: optimization of inner-and second-sphere relaxivity. Invest. Radiol. 45:613. doi: 10.1097/RLI.0b013e3181ee6a49
Jafari, M., Rokhbakhsh-Zamin, F., Shakibaie, M., Moshafi, M. H., Ameri, A., Rahimi, H. R., et al. (2018). Cytotoxic and antibacterial activities of biologically synthesized gold nanoparticles assisted by Micrococcus yunnanensis strain J2. Biocatal. Agric. Biotechnol. 15, 245–253. doi: 10.1016/j.bcab.2018.06.014
Jalal, M., Ansari, M. A., Alzohairy, M. A., Ali, S. G., Khan, H. M., Almatroudi, A., et al. (2018). Biosynthesis of silver nanoparticles from oropharyngeal candida glabrata isolates and their antimicrobial activity against clinical strains of bacteria and fungi. Nanomaterials 8:586. doi: 10.3390/nano8080586
Jayaseelan, C., Rahuman, A. A., Kirthi, A. V., Marimuthu, S., Santhoshkumar, T., Bagavan, A., et al. (2012). Novel microbial route to synthesize ZnO nanoparticles using Aeromonas hydrophila and their activity against pathogenic bacteria and fungi. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 90, 78–84. doi: 10.1016/j.saa.2012.01.006
Jha, A. K., Prasad, K., and Kulkarni, A. R. (2009a). Synthesis of TiO2 nanoparticles using microorganisms. Colloids Surfaces B Biointerfaces 71, 226–229. doi: 10.1016/j.colsurfb.2009.02.007
Jha, A. K., Prasad, K., and Prasad, K. (2009b). A green low-cost biosynthesis of Sb2O3 nanoparticles. Biochem. Eng. J. 43, 303–306. doi: 10.1016/j.bej.2008.10.016
Kadam, V. V., Ettiyappan, J. P., and Balakrishnan, R. M. (2019). Mechanistic insight into the endophytic fungus mediated synthesis of protein capped ZnO nanoparticles. Mater. Sci. Eng. B 243, 214–221. doi: 10.1016/j.mseb.2019.04.017
Kaler, A., Jain, S., and Banerjee, U. C. (2013). Green and rapid synthesis of anticancerous silver nanoparticles by Saccharomyces boulardii and insight into mechanism of nanoparticle synthesis. Biomed Res. Int. 2013:872940. doi: 10.1155/2013/872940
Kalishwaralal, K., Deepak, V., Pandian, S. R. K., Kottaisamy, M., Barathmanikanth, S., Kartikeyan, B., et al. (2010). Biosynthesis of silver and gold nanoparticles using Brevibacterium casei. Colloids Surfaces B Biointerfaces 77, 257–262. doi: 10.1016/j.colsurfb.2010.02.007
Kalpana, V. N., Kataru, B. A. S., Sravani, N., Vigneshwari, T., Panneerselvam, A., and Rajeswari, V. D. (2018). Biosynthesis of zinc oxide nanoparticles using culture filtrates of Aspergillus niger: antimicrobial textiles and dye degradation studies. OpenNano 3, 48–55. doi: 10.1016/j.onano.2018.06.001
Kar, P. K., Murmu, S., Saha, S., Tandon, V., and Acharya, K. (2014). Anthelmintic efficacy of gold nanoparticles derived from a phytopathogenic fungus, Nigrospora oryzae. PLoS ONE 9:e84693. doi: 10.1371/journal.pone.0084693
Karthik, L., Kumar, G., Kirthi, A. V., Rahuman, A. A., and Rao, K. V. B. (2014). Streptomyces sp. LK3 mediated synthesis of silver nanoparticles and its biomedical application. Bioproc. Biosyst. Eng. 37, 261–267. doi: 10.1007/s00449-013-0994-3
Khan, S. A., and Ahmad, A. (2013). Fungus mediated synthesis of biomedically important cerium oxide nanoparticles. Mater. Res. Bull. 48, 4134–4138. doi: 10.1016/j.materresbull.2013.06.038
Khan, S. A., Gambhir, S., and Ahmad, A. (2014). Extracellular biosynthesis of gadolinium oxide (Gd2O3) nanoparticles, their biodistribution and bioconjugation with the chemically modified anticancer drug taxol. Beilstein J. Nanotechnol. 5, 249–257. doi: 10.3762/bjnano.5.27
Khatoon, N., Ahmad, R., and Sardar, M. (2015). Robust and fluorescent silver nanoparticles using Artemisia annua: biosynthesis, characterization and antibacterial activity. Biochem. Eng. J. 102, 91–97. doi: 10.1016/j.bej.2015.02.019
Kim, T.-Y., Kim, M. G., Lee, J.-H., and Hur, H.-G. (2018). Biosynthesis of nanomaterials by Shewanella species for application in lithium ion batteries. Front. Microbiol. 9:2817. doi: 10.3389/fmicb.2018.02817
Klaus, T., Joerger, R., Olsson, E., and Granqvist, C.-G. R. (1999). Silver-based crystalline nanoparticles, microbially fabricated. Proc. Natl. Acad. Sci. U.S.A. 96, 13611–13614. doi: 10.1073/pnas.96.24.13611
Koopi, H., and Buazar, F. (2018). A novel one-pot biosynthesis of pure alpha aluminum oxide nanoparticles using the macroalgae Sargassum ilicifolium: a green marine approach. Ceram. Int. 44, 8940–8945. doi: 10.1016/j.ceramint.2018.02.091
Kora, A. J., and Rastogi, L. (2016). Biomimetic synthesis of selenium nanoparticles by Pseudomonas aeruginosa ATCC 27853: an approach for conversion of selenite. J. Environ. Manage. 181, 231–236. doi: 10.1016/j.jenvman.2016.06.029
Kousheh, S. A., Moradi, M., Tajik, H., and Molaei, R. (2020). Preparation of antimicrobial/ultraviolet protective bacterial nanocellulose film with carbon dots synthesized from lactic acid bacteria. Int. J. Biol. Macromol. 155, 216–225. doi: 10.1016/j.ijbiomac.2020.03.230
Kowshik, M., Ashtaputre, S., Kharrazi, S., Vogel, W., Urban, J., Kulkarni, S. K., et al. (2002). Extracellular synthesis of silver nanoparticles by a silver-tolerant yeast strain MKY3. Nanotechnology 14:95. doi: 10.1088/0957-4484/14/1/321
Kulkarni, R. R., Shaiwale, N. S., Deobagkar, D. N., and Deobagkar, D. D. (2015). Synthesis and extracellular accumulation of silver nanoparticles by employing radiation-resistant Deinococcus radiodurans, their characterization, and determination of bioactivity. Int. J. Nanomedicine 10:963. doi: 10.2147/IJN.S72888
Kumar, R. M. P., Venkatesh, A., and Moorthy, V. H. S. (2019). Nanopits based novel hybrid plasmonic nanosensor fabricated by a facile nanofabrication technique for biosensing. Mater. Res. Express 6:1150b6. doi: 10.1088/2053-1591/ab33b9
Kumar, S. A., Abyaneh, M. K., Gosavi, S. W., Kulkarni, S. K., Pasricha, R., Ahmad, A., et al. (2007). Nitrate reductase-mediated synthesis of silver nanoparticles from AgNO3. Biotechnol. Lett. 29, 439–445. doi: 10.1007/s10529-006-9256-7
Kumar, S. A., Peter, Y.-A., and Nadeau, J. L. (2008). Facile biosynthesis, separation and conjugation of gold nanoparticles to doxorubicin. Nanotechnology 19:495101. doi: 10.1088/0957-4484/19/49/495101
Kumaresan, M., Anand, K. V., Govindaraju, K., Tamilselvan, S., and Kumar, V. G. (2018). Seaweed Sargassum wightii mediated preparation of zirconia (ZrO2) nanoparticles and their antibacterial activity against gram positive and gram negative bacteria. Microb. Pathog. 124, 311–315. doi: 10.1016/j.micpath.2018.08.060
Kundu, D., Hazra, C., Chatterjee, A., Chaudhari, A., and Mishra, S. (2014). Extracellular biosynthesis of zinc oxide nanoparticles using Rhodococcus pyridinivorans NT2: multifunctional textile finishing, biosafety evaluation and in vitro drug delivery in colon carcinoma. J. Photochem. Photobiol. B Biol. 140, 194–204. doi: 10.1016/j.jphotobiol.2014.08.001
Kundu, S., Maheshwari, V., and Saraf, R. F. (2008). Photolytic metallization of Au nanoclusters and electrically conducting micrometer long nanostructures on a DNA scaffold. Langmuir 24, 551–555. doi: 10.1021/la702416z
Lengke, M. F., Fleet, M. E., and Southam, G. (2006a). Synthesis of platinum nanoparticles by reaction of filamentous cyanobacteria with platinum (IV) - chloride complex. Langmuir 22, 7318–7323. doi: 10.1021/la060873s
Lengke, M. F., Fleet, M. E., and Southam, G. (2007a). Biosynthesis of silver nanoparticles by filamentous cyanobacteria from a silver (I) nitrate complex. Langmuir 23, 2694–2699. doi: 10.1021/la0613124
Lengke, M. F., Fleet, M. E., and Southam, G. (2007b). Synthesis of palladium nanoparticles by reaction of filamentous cyanobacterial biomass with a palladium (II) chloride complex. Langmuir 23, 8982–8987. doi: 10.1021/la7012446
Lengke, M. F., Ravel, B., Fleet, M. E., Wanger, G., Gordon, R. A., and Southam, G. (2006b). Mechanisms of gold bioaccumulation by filamentous cyanobacteria from gold (III) - chloride complex. Environ. Sci. Technol. 40, 6304–6309. doi: 10.1021/es061040r
Lombardo, D., Calandra, P., Pasqua, L., and Magazu, S. (2020). Self-assembly of organic nanomaterials and biomaterials: the bottom-up approach for functional nanostructures formation and advanced applications. Materials 13:1048. doi: 10.3390/ma13051048
Luo, H., Cha, R., Li, J., Hao, W., Zhang, Y., and Zhou, F. (2019). Advances in tissue engineering of nanocellulose-based scaffolds: a review. Carbohydr. Polym. 224:115144. doi: 10.1016/j.carbpol.2019.115144
Lv, Q., Zhang, B., Xing, X., Zhao, Y., Cai, R., Wang, W., et al. (2018). Biosynthesis of copper nanoparticles using Shewanella loihica PV-4 with antibacterial activity: novel approach and mechanisms investigation. J. Hazard. Mater. 347, 141–149. doi: 10.1016/j.jhazmat.2017.12.070
Maceda, A. F., Ouano, J. J. S., Que, M. C. O., Basilia, B. A., Potestas, M. J., and Alguno, A. C. (2018). Controlling the absorption of gold nanoparticles via green synthesis using Sargassum crassifolium extract. Key Eng. Mater. 765, 44–48. doi: 10.4028/www.scientific.net/KEM.765.44
Mahmoudi, M., Tachibana, A., Goldstone, A. B., Woo, Y. J., Chakraborty, P., Lee, K. R., et al. (2016). Novel MRI contrast agent from magnetotactic bacteria enables in vivo tracking of iPSC-derived cardiomyocytes. Sci. Rep. 6:26960. doi: 10.1038/srep26960
Malarkodi, C., and Annadurai, G. (2013). A novel biological approach on extracellular synthesis and characterization of semiconductor zinc sulfide nanoparticles. Appl. Nanosci. 3, 389–395. doi: 10.1007/s13204-012-0138-0
Manivasagan, P., Venkatesan, J., Senthilkumar, K., Sivakumar, K., and Kim, S.-K. (2013). Biosynthesis, antimicrobial and cytotoxic effect of silver nanoparticles using a novel Nocardiopsis sp. MBRC-1. BioMed Res. Int. 2013:287638. doi: 10.1155/2013/287638
Maric, I., Stefanic, G., Gotic, M., and Jurkin, T. (2019). The impact of dextran sulfate on the radiolytic synthesis of magnetic iron oxide nanoparticles. J. Mol. Struct. 1183, 126–136. doi: 10.1016/j.molstruc.2019.01.075
Markus, J., Mathiyalagan, R., Kim, Y.-J., Abbai, R., Singh, P., Ahn, S., et al. (2016). Intracellular synthesis of gold nanoparticles with antioxidant activity by probiotic Lactobacillus kimchicus DCY51T isolated from Korean kimchi. Enzyme Microb. Technol. 95, 85–93. doi: 10.1016/j.enzmictec.2016.08.018
Massironi, A., Morelli, A., Grassi, L., Puppi, D., Braccini, S., Maisetta, G., et al. (2019). Ulvan as novel reducing and stabilizing agent from renewable algal biomass: application to green synthesis of silver nanoparticles. Carbohydr. Polym. 203, 310–321. doi: 10.1016/j.carbpol.2018.09.066
Mazumder, J. A., Ahmad, R., and Sardar, M. (2016). Reusable magnetic nanobiocatalyst for synthesis of silver and gold nanoparticles. Int. J. Biol. Macromol. 93, 66–74. doi: 10.1016/j.ijbiomac.2016.08.073
Meng, C., Tian, J., Li, Y., and Zheng, S. (2010). Influence of native bacterial magnetic particles on mouse immune response. Wei Sheng Wu Xue Bao 50, 817–821.
Mirzadeh, S., Darezereshki, E., Bakhtiari, F., Fazaelipoor, M. H., and Hosseini, M. R. (2013). Characterization of zinc sulfide (ZnS) nanoparticles biosynthesized by Fusarium oxysporum. Mater. Sci. Semiconduct. Proc. 16, 374–378. doi: 10.1016/j.mssp.2012.09.008
Mishra, A., Ahmad, R., Perwez, M., and Sardar, M. (2016). Reusable green synthesized biomimetic magnetic nanoparticles for glucose and H2O2 detection. Bionanoscience 6, 93–102. doi: 10.1007/s12668-016-0197-x
Mishra, A., Ahmad, R., and Sardar, M. (2015). Biosynthesized iron oxide nanoparticles mimicking peroxidase activity: application for biocatalysis and biosensing. J. Nanoeng. Nanomanufactur. 5, 37–42. doi: 10.1166/jnan.2015.1220
Mishra, A., Ahmad, R., Singh, V., Gupta, M. N., and Sardar, M. (2013). Preparation, characterization and biocatalytic activity of a nanoconjugate of alpha amylase and silver nanoparticles. J. Nanosci. Nanotechnol. 13, 5028–5033. doi: 10.1166/jnn.2013.7593
Mishra, A., Tripathy, S. K., and Yun, S.-I. (2011). Bio-synthesis of gold and silver nanoparticles from Candida guilliermondii and their antimicrobial effect against pathogenic bacteria. J. Nanosci. Nanotechnol. 11, 243–248. doi: 10.1166/jnn.2011.3265
Mishra, M., Paliwal, J. S., Singh, S. K., Selvarajan, E., Subathradevi, C., and Mohanasrinivasan, V. (2013). Studies on the inhibitory activity of biologically synthesized and characterized zinc oxide nanoparticles using lactobacillus sporogens against Staphylococcus aureus. J. Pure Appl. Microbiol 7, 1–6.
Mohamed, W. S., and Abu-Dief, A. M. (2018). Synthesis, characterization and photocatalysis enhancement of Eu2O3-ZnO mixed oxide nanoparticles. J. Phys. Chem. Solids 116, 375–385. doi: 10.1016/j.jpcs.2018.02.008
Molnar, Z., Bodai, V., Szakacs, G., Erdelyi, B., Fogarassy, Z., Safran, G., et al. (2018). Green synthesis of gold nanoparticles by thermophilic filamentous fungi. Sci. Rep. 8:3943. doi: 10.1038/s41598-018-22112-3
Mukherjee, P., Ahmad, A., Mandal, D., Senapati, S., Sainkar, S. R., Khan, M. I., et al. (2001). Fungus-mediated synthesis of silver nanoparticles and their immobilization in the mycelial matrix: a novel biological approach to nanoparticle synthesis. Nano Lett. 1, 515–519. doi: 10.1021/nl0155274
Mukherjee, P., Senapati, S., Mandal, D., Ahmad, A., Khan, M. I., Kumar, R., et al. (2002). Extracellular synthesis of gold nanoparticles by the fungus Fusarium oxysporum. ChemBioChem 3, 461–463. doi: 10.1002/1439-7633(20020503)3:5<461::AID-CBIC461>3.0.CO;2-X
Munawer, U., Raghavendra, V. B., Ningaraju, S., Krishna, K. L., Ghosh, A. R., Melappa, G., et al. (2020). Biofabrication of gold nanoparticles mediated by the endophytic Cladosporium species: photodegradation, in vitro anticancer activity and in vivo antitumor studies. Int. J. Pharm. 588:119729. doi: 10.1016/j.ijpharm.2020.119729
Murray, A. J., Zhu, J., Wood, J., and Macaskie, L. E. (2017). A novel biorefinery: biorecovery of precious metals from spent automotive catalyst leachates into new catalysts effective in metal reduction and in the hydrogenation of 2-pentyne. Miner. Eng. 113, 102–108. doi: 10.1016/j.mineng.2017.08.011
Murugesan, S., Bhuvaneswari, S., and Sivamurugan, V. (2017). Green synthesis, characterization of silver nanoparticles of a marine red alga Spyridia fusiformis and their antibacterial activity. Int. J. Pharm. Pharm. Sci. 9, 192–197. doi: 10.22159/ijpps.2017v9i5.17105
Nair, B., and Pradeep, T. (2002). Coalescence of nanoclusters and formation of submicron crystallites assisted by Lactobacillus strains. Crystal Growth Design 2, 293–298. doi: 10.1021/cg0255164
Namasivayam, S. K. R., Gnanendra, E. K., and Reepika, R. (2010). Synthesis of silver nanoparticles by Lactobaciluus acidophilus 01 strain and evaluation of its in vitro genomic DNA toxicity. Nano Micro Lett. 2, 160–163. doi: 10.1007/BF03353635
Narasimha, G., Alzohairy, M., Khadri, H., and Mallikarjuna, K. (2013). Extracellular synthesis, characterization and antibacterial activity of Silver nanoparticles by Actinomycetes isolative. Int J Nano Dimens 4, 77–83. doi: 10.7508/IJND.2013.01.010
Noori, R., Ahmad, R., and Sardar, M. (2020). “Nanobiosensor in health sector: the milestones achieved and future prospects,” in Nanobiosensors for Agricultural, Medical and Environmental Applications, eds M. Mohsin, R. Naz, and A. Ahmad (Singapore: Springer), 63–90. doi: 10.1007/978-981-15-8346-9_4
Oh, J.-W., and Han, D.-W. (2020). Virus-based nanomaterials and nanostructures. Nanomaterials 10:567. doi: 10.3390/nano10030567
Ovais, M., Khalil, A. T., Ayaz, M., Ahmad, I., Nethi, S. K., and Mukherjee, S. (2018). Biosynthesis of metal nanoparticles via microbial enzymes: a mechanistic approach. Int. J. Mol. Sci. 19:4100. doi: 10.3390/ijms19124100
Pareek, V., Bhargava, A., and Panwar, J. (2020). Biomimetic approach for multifarious synthesis of nanoparticles using metal tolerant fungi: a mechanistic perspective. Mater. Sci. Eng. B 262:114771. doi: 10.1016/j.mseb.2020.114771
Park, K.-Y., Jeong, J.-K., Lee, Y.-E., and Daily Iii, J. W. (2014). Health benefits of kimchi (Korean fermented vegetables) as a probiotic food. J. Med. Food 17, 6–20. doi: 10.1089/jmf.2013.3083
Pastorino, F., Brignole, C., Di Paolo, D., Perri, P., Curnis, F., Corti, A., et al. (2019). Overcoming biological barriers in neuroblastoma therapy: the vascular targeting approach with liposomal drug nanocarriers. Small 15:1804591. doi: 10.1002/smll.201804591
Patil, M. P., Kang, M.-J., Niyonizigiye, I., Singh, A., Kim, J.-O., Seo, Y. B., et al. (2019). Extracellular synthesis of gold nanoparticles using the marine bacterium Paracoccus haeundaensis BC74171T and evaluation of their antioxidant activity and antiproliferative effect on normal and cancer cell lines. Colloids Surfaces B Biointerfaces 183:110455. doi: 10.1016/j.colsurfb.2019.110455
Peiris, M. M. K., Guansekera, T., Jayaweera, P. M., and Fernando, S. S. N. (2018). TiO2 nanoparticles from Baker's yeast: a potent antimicrobial. J. Microbiol. Biotechnol. 28, 1664–1670. doi: 10.4014/jmb.1807.07005
Prakasham, R. S., Kumar, B. S., Kumar, Y. S., and Shankar, G. G. (2012). Characterization of silver nanoparticles synthesized by using marine isolate Streptomyces albidoflavus. J. Microbiol. Biotechnol. 22, 614–621. doi: 10.4014/jmb.1107.07013
Prasad, K., and Jha, A. K. (2010). Biosynthesis of CdS nanoparticles: an improved green and rapid procedure. J. Colloid Interface Sci. 342, 68–72. doi: 10.1016/j.jcis.2009.10.003
Priyadarshini, S., Gopinath, V., Priyadharsshini, N. M., Mubarakali, D., and Velusamy, P. (2013). Synthesis of anisotropic silver nanoparticles using novel strain, Bacillus flexus and its biomedical application. Colloids Surfaces B Biointerfaces 102, 232–237. doi: 10.1016/j.colsurfb.2012.08.018
Pugazhenthiran, N., Anandan, S., Kathiravan, G., Prakash, N. K. U., Crawford, S., and Ashokkumar, M. (2009). Microbial synthesis of silver nanoparticles by Bacillus sp. J. Nanopart. Res. 11:1811. doi: 10.1007/s11051-009-9621-2
Pytlik, N., Kaden, J., Finger, M., Naumann, J., Wanke, S., Machill, S., et al. (2017). Biological synthesis of gold nanoparticles by the diatom Stephanopyxis turris and in vivo SERS analyses. Algal Res. 28, 9–15. doi: 10.1016/j.algal.2017.10.004
Rad, M., Taran, M., and Alavi, M. (2018). Effect of incubation time, CuSO4 and glucose concentrations on biosynthesis of copper oxide (CuO) nanoparticles with rectangular shape and antibacterial activity: taguchi method approach. Nano Biomed. Eng. 10, 25–33. doi: 10.5101/nbe.v10i1.p25-33
Rai, M., Ingle, A. P., Birla, S., Yadav, A., and Santos, C. A. D. (2016). Strategic role of selected noble metal nanoparticles in medicine. Crit. Rev. Microbiol. 42, 696–719. doi: 10.3109/1040841X.2015.1018131
Rajakumar, G., Rahuman, A. A., Roopan, S. M., Khanna, V. G., Elango, G., Kamaraj, C., et al. (2012). Fungus-mediated biosynthesis and characterization of TiO2 nanoparticles and their activity against pathogenic bacteria. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 91, 23–29. doi: 10.1016/j.saa.2012.01.011
Rajeshkumar, S., and Sivapriya, D. (2020). “Fungus-mediated nanoparticles: characterization and biomedical advances,” in Nanoparticles in Medicine, ed A. Shukla (Singapore: Springer), 185–199. doi: 10.1007/978-981-13-8954-2_7
Ramakrishna, M., Babu, D. R., Gengan, R. M., Chandra, S., and Rao, G. N. (2016). Green synthesis of gold nanoparticles using marine algae and evaluation of their catalytic activity. J. Nanostruct. Chem. 6, 1–13. doi: 10.1007/s40097-015-0173-y
Ramasamy, M., and Lee, J. (2016). Recent nanotechnology approaches for prevention and treatment of biofilm-associated infections on medical devices. Biomed Res. Int. 2016:1851242. doi: 10.1155/2016/1851242
Ramkumar, V. S., Pugazhendhi, A., Gopalakrishnan, K., Sivagurunathan, P., Saratale, G. D., Dung, T. N. B., et al. (2017). Biofabrication and characterization of silver nanoparticles using aqueous extract of seaweed Enteromorpha compressa and its biomedical properties. Biotechnol. Rep. 14, 1–7. doi: 10.1016/j.btre.2017.02.001
Rasool, U., and Hemalatha, S. (2017). Marine endophytic actinomycetes assisted synthesis of copper nanoparticles (CuNPs): characterization and antibacterial efficacy against human pathogens. Mater. Lett. 194, 176–180. doi: 10.1016/j.matlet.2017.02.055
Riddin, T. L., Gericke, M., and Whiteley, C. G. (2006). Analysis of the inter-and extracellular formation of platinum nanoparticles by Fusarium oxysporum f. sp. lycopersici using response surface methodology. Nanotechnology 17:3482. doi: 10.1088/0957-4484/17/14/021
Roshmi, T., Soumya, K. R., Jyothis, M., and Radhakrishnan, E. K. (2015). Effect of biofabricated gold nanoparticle-based antibiotic conjugates on minimum inhibitory concentration of bacterial isolates of clinical origin. Gold Bull. 48, 63–71. doi: 10.1007/s13404-015-0162-4
Rossi, L. M., Quach, A. D., and Rosenzweig, Z. (2004). Glucose oxidase-magnetite nanoparticle bioconjugate for glucose sensing. Anal. Bioanal. Chem. 380, 606–613. doi: 10.1007/s00216-004-2770-3
Sadaf, A., Ahmad, R., Ghorbal, A., Elfalleh, W., and Khare, S. K. (2020). Synthesis of cost-effective magnetic nano-biocomposites mimicking peroxidase activity for remediation of dyes. Environ. Sci. Pollut. Res. 27, 27211–27220. doi: 10.1007/s11356-019-05270-3
Sadrolhosseini, A. R., Rashid, S. A., Shafie, S., and Soleimani, H. (2019). Laser ablation synthesis of Ag nanoparticles in graphene quantum dots aqueous solution and optical properties of nanocomposite. Appl. Phys. A 125:82. doi: 10.1007/s00339-018-2233-x
Saeed, S., Iqbal, A., and Ashraf, M. A. (2020). Bacterial-mediated synthesis of silver nanoparticles and their significant effect against pathogens. Environ. Sci. Pollut. Res. 27, 37347–37356. doi: 10.1007/s11356-020-07610-0
Sanaeimehr, Z., Javadi, I., and Namvar, F. (2018). “Antiangiogenic and antiapoptotic effects of green-synthesized zinc oxide nanoparticles using Sargassum muticum algae extraction,” in Cancer Nanotechnology, eds A. Tiwari, A. P. F. Turner (Scrivener Publishing) 9, 1–16. doi: 10.1186/s12645-018-0037-5
Sanghi, R., and Verma, P. (2009). A facile green extracellular biosynthesis of CdS nanoparticles by immobilized fungus. Chem. Eng. J. 155, 886–891. doi: 10.1016/j.cej.2009.08.006
Sanjenbam, P., Gopal, J. V., and Kannabiran, K. (2014). Anticandidal activity of silver nanoparticles synthesized using Streptomyces sp. VITPK1. J. Mycol. Med. 24, 211–219. doi: 10.1016/j.mycmed.2014.03.004
Saravanan, M., Barik, S. K., Mubarakali, D., Prakash, P., and Pugazhendhi, A. (2018). Synthesis of silver nanoparticles from Bacillus brevis (NCIM 2533) and their antibacterial activity against pathogenic bacteria. Microb. Pathog. 116, 221–226. doi: 10.1016/j.micpath.2018.01.038
Sardar, M., Mishra, A., and Ahmad, R. (2014). “Biosynthesis of metal nanoparticles and their applications,” in Biosensors and Nanotechnology, eds A. Tiwari and A. P. F. Turner (Beverly, MA: Scrivener Publishing), 239–266. doi: 10.1002/9781118773826.ch8
Sayadi, M. H., Salmani, N., Heidari, A., and Rezaei, M. R. (2018). Bio-synthesis of palladium nanoparticle using Spirulina platensis alga extract and its application as adsorbent. Surfaces Interfaces 10, 136–143. doi: 10.1016/j.surfin.2018.01.002
Selvarajan, E., and Mohanasrinivasan, V. (2013). Biosynthesis and characterization of ZnO nanoparticles using Lactobacillus plantarum VITES07. Mater. Lett. 112, 180–182. doi: 10.1016/j.matlet.2013.09.020
Senapati, S., Mandal, D., Ahmad, A., Khan, M. I., Sastry, M., and Kumar, R. (2004). Fungus mediated synthesis of silver nanoparticles: a novel biological approach. Indian J. Phys. 78, 101–105.
Senapati, S., Syed, A., Moeez, S., Kumar, A., and Ahmad, A. (2012). Intracellular synthesis of gold nanoparticles using alga Tetraselmis kochinensis. Mater. Lett. 79, 116–118. doi: 10.1016/j.matlet.2012.04.009
Senapati, U. S., Jha, D. K., and Sarkar, D. (2015). Structural, optical, thermal and electrical properties of fungus guided biosynthesized zinc sulphide nanoparticles. Res. J. Chem. Sci. 2231:606X.
Shaligram, N. S., Bule, M., Bhambure, R., Singhal, R. S., Singh, S. K., Szakacs, G., et al. (2009). Biosynthesis of silver nanoparticles using aqueous extract from the compactin producing fungal strain. Proc. Biochem. 44, 939–943. doi: 10.1016/j.procbio.2009.04.009
Shivashankarappa, A., and Sanjay, K. R. (2015). Study on biological synthesis of cadmium sulfide nanoparticles by Bacillus licheniformis and its antimicrobial properties against food borne pathogens. Nanosci. Nanotechnol. Res. 3, 6–15.
Silva, N., Ramirez, S., Diaz, I., Garcia, A., and Hassan, N. (2019). Easy, quick, and reproducible sonochemical synthesis of CuO nanoparticles. Materials 12:804. doi: 10.3390/ma12050804
Singh, B. R., Dwivedi, S., Al-Khedhairy, A. A., and Musarrat, J. (2011). Synthesis of stable cadmium sulfide nanoparticles using surfactin produced by Bacillus amyloliquifaciens strain KSU-109. Colloids Surfaces B Biointerfaces 85, 207–213. doi: 10.1016/j.colsurfb.2011.02.030
Singh, D., Rathod, V., Ninganagouda, S., Hiremath, J., Singh, A. K., and Mathew, J. (2014). Optimization and characterization of silver nanoparticle by endophytic fungi Penicillium sp. isolated from Curcuma longa (turmeric) and application studies against MDR E. coli and S. aureus. Bioinorg. Chem. Appl. 2014:408021. doi: 10.1155/2014/408021
Singh, R., Shedbalkar, U. U., Wadhwani, S. A., and Chopade, B. A. (2015). Bacteriagenic silver nanoparticles: synthesis, mechanism, and applications. Appl. Microbiol. Biotechnol. 99, 4579–4593. doi: 10.1007/s00253-015-6622-1
Sintubin, L., De Windt, W., Dick, J., Mast, J., Van Der Ha, D., Verstraete, W., et al. (2009). Lactic acid bacteria as reducing and capping agent for the fast and efficient production of silver nanoparticles. Appl. Microbiol. Biotechnol. 84, 741–749. doi: 10.1007/s00253-009-2032-6
Sowani, H., Mohite, P., Munot, H., Shouche, Y., Bapat, T., Kumar, A. R., et al. (2016). Green synthesis of gold and silver nanoparticles by an actinomycete Gordonia amicalis HS-11: mechanistic aspects and biological application. Proc. Biochem. 51, 374–383. doi: 10.1016/j.procbio.2015.12.013
Sowbarnika, R., Anhuradha, S., and Preetha, B. (2018). Enhanced antimicrobial effect of yeast mediated silver nanoparticles synthesized from baker's yeast. Int. J. Nanosci. Nanotechnol. 14, 33–42.
Srinath, B. S., Namratha, K., and Byrappa, K. (2018). Eco-friendly synthesis of gold nanoparticles by Bacillus subtilis and their environmental applications. Adv. Sci. Lett. 24, 5942–5946. doi: 10.1166/asl.2018.12224
Srivastava, P., and Kowshik, M. (2017). Fluorescent lead (IV) sulfide nanoparticles synthesized by Idiomarina sp. strain PR58-8 for bioimaging applications. Appl. Environ. Microbiol. 83, 1–15. doi: 10.1128/AEM.03091-16
Srivastava, S., Ahmad, R., and Khare, S. K. (2021). Alzheimer's disease and its treatment by different approaches: a review. Eur. J. Med. Chem. 216:113320. doi: 10.1016/j.ejmech.2021.113320
Steinmetz, N. F. (2010). Viral nanoparticles as platforms for next-generation therapeutics and imaging devices. Nanomed. Nanotechnol. Biol. Med. 6, 634–641. doi: 10.1016/j.nano.2010.04.005
Stillman, N. R., Kovacevic, M., Igor, B., and Sabine, H. (2020). In silico modelling of cancer nanomedicine, across scales and transport barriers. NPJ Comput. Mater. 6, 1–10. doi: 10.1038/s41524-020-00366-8
Subramanian, M., Alikunhi, N. M., and Kandasamy, K. (2010). In vitro synthesis of silver nanoparticles by marine yeasts from coastal mangrove sediment. Adv. Sci. Lett. 3, 428–433. doi: 10.1166/asl.2010.1168
Subramaniyan, S. A., Sheet, S., Vinothkannan, M., Yoo, D. J., Lee, Y. S., Belal, S. A., et al. (2018). One-pot facile synthesis of Pt nanoparticles using cultural filtrate of microgravity simulated grown P. chrysogenum and their activity on bacteria and cancer cells. J. Nanosci. Nanotechnol. 18, 3110–3125. doi: 10.1166/jnn.2018.14661
Sukanya, M. K., Saju, K. A., Praseetha, P. K., and Sakthivel, G. (2013). Therapeutic potential of biologically reduced silver nanoparticles from actinomycete cultures. J. Nanosci. 2013:940719. doi: 10.1155/2013/940719
Sun, J.-B., Wang, Z.-L., Duan, J.-H., Ren, J., Yang, X.-D., Dai, S.-L., et al. (2009). Targeted distribution of bacterial magnetosomes isolated from Magnetospirillum gryphiswaldense MSR-1 in healthy Sprague-Dawley rats. J. Nanosci. Nanotechnol. 9, 1881–1885. doi: 10.1166/jnn.2009.410
Sundaram, P. A., Augustine, R., and Kannan, M. (2012). Extracellular biosynthesis of iron oxide nanoparticles by Bacillus subtilis strains isolated from rhizosphere soil. Biotechnol. Bioproc. Eng. 17, 835–840. doi: 10.1007/s12257-011-0582-9
Swaminathan, S., Murugesan, S., Damodarkumar, S., Dhamotharan, R., and Bhuvaneshwari, S. (2011). Synthesis and characterization of gold nanoparticles from alga Acanthophora spicifera (VAHL) boergesen. Int. J. Nanosci. Nanotechnol. 2, 85–94.
Syed, A., Raja, R., Kundu, G. C., Gambhir, S., and Ahmad, A. (2013). Extracellular biosynthesis of monodispersed gold nanoparticles, their characterization, cytotoxicity assay, biodistribution and conjugation with the anticancer drug doxorubicin. J. Nanomed. Nanotechol. 4, 156–161.
Tang, Y.-S., Wang, D., Zhou, C., and Zhang, S. (2019). Preparation and anti-tumor efficiency evaluation of bacterial magnetosome-anti-4-1BB antibody complex: Bacterial magnetosome as antibody carriers isolated from Magnetospirillum gryphiswaldense. Biotechnol. Appl. Biochem. 66, 290–297. doi: 10.1002/bab.1724
Torres, S. K., Campos, V. L., Leon, C. G., Rodriguez-Llamazares, S. M., Rojas, S. M., Gonzalez, M., et al. (2012). Biosynthesis of selenium nanoparticles by Pantoea agglomerans and their antioxidant activity. J. Nanopart. Res. 14, 1–9. doi: 10.1007/s11051-012-1236-3
Tripathi, R. M., Shrivastav, A., and Shrivastav, B. R. (2015). Biogenic gold nanoparticles: as a potential candidate for brain tumor directed drug delivery. Artif. Cells Nanomed. Biotechnol. 43, 311–317. doi: 10.3109/21691401.2014.885445
Tripathi, R. M., Shrivastav, B. R., and Shrivastav, A. (2018). Antibacterial and catalytic activity of biogenic gold nanoparticles synthesised by Trichoderma harzianum. IET Nanobiotechnol. 12, 509–513. doi: 10.1049/iet-nbt.2017.0105
Uebe, R., and Schuler, D. (2016). Magnetosome biogenesis in magnetotactic bacteria. Nat. Rev. Microbiol. 14:621. doi: 10.1038/nrmicro.2016.99
Usha, R., Prabu, E., Palaniswamy, M., Venil, C. K., and Rajendran, R. (2010). Synthesis of metal oxide nano particles by Streptomyces sp. for development of antimicrobial textiles. Global J. Biotechnol. Biochem. 5, 153–160.
Vanlalveni, C., Rajkumari, K., Biswas, A., Adhikari, P. P., Lalfakzuala, R., and Rokhum, L. (2018). Green synthesis of silver nanoparticles using Nostoc linckia and its antimicrobial activity: a novel biological approach. Bionanoscience 8, 624–631. doi: 10.1007/s12668-018-0520-9
Vargas, G., Cypriano, J., Correa, T., Leao, P., Bazylinski, D. A., and Abreu, F. (2018). Applications of magnetotactic bacteria, magnetosomes and magnetosome crystals in biotechnology and nanotechnology: mini-review. Molecules 23:2438. doi: 10.3390/molecules23102438
Vijayanandan, A. S., and Balakrishnan, R. M. (2018). Biosynthesis of cobalt oxide nanoparticles using endophytic fungus Aspergillus nidulans. J. Environ. Manage. 218, 442–450. doi: 10.1016/j.jenvman.2018.04.032
Vijayaraghavan, K., Mahadevan, A., Sathishkumar, M., Pavagadhi, S., and Balasubramanian, R. (2011). Biosynthesis of Au (0) from Au (III) via biosorption and bioreduction using brown marine alga Turbinaria conoides. Chem. Eng. J. 167, 223–227. doi: 10.1016/j.cej.2010.12.027
Wadhwani, S. A., Shedbalkar, U. U., Singh, R., Vashisth, P., Pruthi, V., and Chopade, B. A. (2016). Kinetics of synthesis of gold nanoparticles by Acinetobacter sp. SW30 isolated from environment. Indian J. Microbiol. 56, 439–444. doi: 10.1007/s12088-016-0598-0
Waghmare, S. R., Mulla, M. N., Marathe, S. R., and Sonawane, K. D. (2015). Ecofriendly production of silver nanoparticles using Candida utilis and its mechanistic action against pathogenic microorganisms. 3 Biotech 5, 33–38. doi: 10.1007/s13205-014-0196-y
Waghmare, S. S., Deshmukh, A. M., Kulkarni, S. W., and Oswaldo, L. A. (2011). Biosynthesis and characterization of manganese and zinc nanoparticles. Univer. J. Environ. Res. Technol. 1, 64–69.
Wang, H. U. I., Brandl, D. W., Nordlander, P., and Halas, N. J. (2007). Plasmonic nanostructures: artificial molecules. Acc. Chem. Res. 40, 53–62. doi: 10.1021/ar0401045
Xia, Y. (2008). Nanomaterials at work in biomedical research. Nat. Mater. 7, 758–760. doi: 10.1038/nmat2277
Yacoby, I., Shamis, M., Bar, H., Shabat, D., and Benhar, I. (2006). Targeting antibacterial agents by using drug-carrying filamentous bacteriophages. Antimicrob. Agents Chemother. 50, 2087–2097. doi: 10.1128/AAC.00169-06
Yeary, L. W., Moon, J.-W., Love, L. J., Thompson, J. R., Rawn, C. J., and Phelps, T. J. (2005). Magnetic properties of biosynthesized magnetite nanoparticles. IEEE Trans. Magn. 41, 4384–4389. doi: 10.1109/TMAG.2005.857482
Zada, S., Ahmad, A., Khan, S., Yu, X., Chang, K., Iqbal, A., et al. (2018). Biogenic synthesis of silver nanoparticles using extracts of Leptolyngbya JSC-1 that induce apoptosis in HeLa cell line and exterminate pathogenic bacteria. Artif. Cells Nanomed. Biotechnol. 46, S471–S480. doi: 10.1080/21691401.2018.1499663
Zhang, X., He, X., Wang, K., and Yang, X. (2011). Different active biomolecules involved in biosynthesis of gold nanoparticles by three fungus species. J. Biomed. Nanotechnol. 7, 245–254. doi: 10.1166/jbn.2011.1285
Zhang, X., Qu, Y., Shen, W., Wang, J., Li, H., Zhang, Z., et al. (2016). Biogenic synthesis of gold nanoparticles by yeast Magnusiomyces ingens LH-F1 for catalytic reduction of nitrophenols. Colloids Surfaces A Physicochem. Eng. Aspects 497, 280–285. doi: 10.1016/j.colsurfa.2016.02.033
Zhang, Y., Ni, Q., Xu, C., Wan, B., Geng, Y., Zheng, G., et al. (2018). Smart bacterial magnetic nanoparticles for tumor-targeting magnetic resonance imaging of HER2-positive Breast cancers. ACS Appl. Mater. Interfaces 11, 3654–3665. doi: 10.1021/acsami.8b15838
Zhang, Z., Chen, J., Yang, Q., Lan, K., Yan, Z., and Chen, J. (2018). Eco-friendly intracellular microalgae synthesis of fluorescent CdSe QDs as a sensitive nanoprobe for determination of imatinib. Sens. Actuat. B Chem. 263, 625–633. doi: 10.1016/j.snb.2018.02.169
Zhao, D. (2017). Bacteriogenic magnetic nanoparticles as magnetic resonance imaging contrast agents. Transl. Cancer Res. 6:S512. doi: 10.21037/tcr.2017.03.81
Zhao, X., Zhou, L., Riaz Rajoka, M. S., Yan, L., Jiang, C., Shao, D., et al. (2018). Fungal silver nanoparticles: synthesis, application and challenges. Crit. Rev. Biotechnol. 38, 817–835. doi: 10.1080/07388551.2017.1414141
Zonaro, E., Lampis, S., Turner, R. J., Qazi, S. J. S., and Vallini, G. (2015). Biogenic selenium and tellurium nanoparticles synthesized by environmental microbial isolates efficaciously inhibit bacterial planktonic cultures and biofilms. Front. Microbiol. 6:584. doi: 10.3389/fmicb.2015.00584
Keywords: nanoparticles, microbial, synthesis, biogenic, metals, biocompatible, biomaterial, therapeutic
Citation: Ghosh S, Ahmad R, Zeyaullah M and Khare SK (2021) Microbial Nano-Factories: Synthesis and Biomedical Applications. Front. Chem. 9:626834. doi: 10.3389/fchem.2021.626834
Received: 06 November 2020; Accepted: 15 March 2021;
Published: 16 April 2021.
Edited by:
Karishma R. Pardesi, Savitribai Phule Pune University, IndiaReviewed by:
Rohini Kitture, Independent Researcher, Pune, IndiaKhushboo Jani, St. John's University, United States
Hina Singh, University of California, Riverside, United States
Copyright © 2021 Ghosh, Ahmad, Zeyaullah and Khare. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shubhrima Ghosh, c2h1YmhyaW1hLmdob3NoQGdtYWlsLmNvbQ==; Razi Ahmad, cmF6aS5qbWlAZ21haWwuY29t; Sunil Kumar Khare, c2traGFyZUBjaGVtaXN0cnkuaWl0ZC5hYy5pbg==
 Shubhrima Ghosh
Shubhrima Ghosh Razi Ahmad
Razi Ahmad Md. Zeyaullah2
Md. Zeyaullah2