
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Chem., 08 January 2021
Sec. Medicinal and Pharmaceutical Chemistry
Volume 8 - 2020 | https://doi.org/10.3389/fchem.2020.616863
This article is part of the Research TopicPhosphonate Chemistry in Drug Design and DevelopmentView all 8 articles
The use of the phosphonate motif featuring a carbon-phosphorous bond as bioisosteric replacement of the labile P–O bond is widely recognized as an attractive structural concept in different areas of medicinal chemistry, since it addresses the very fundamental principles of enzymatic stability and minimized metabolic activation. This review discusses the most influential successes in drug design with special emphasis on nucleoside phosphonates and their prodrugs as antiviral and cancer treatment agents. A description of structurally related analogs able to interfere with the transmission of other infectious diseases caused by pathogens like bacteria and parasites will then follow. Finally, molecules acting as agonists/antagonists of P2X and P2Y receptors along with nucleotidase inhibitors will also be covered. This review aims to guide readers through the fundamentals of nucleoside phosphonate therapeutics in order to inspire the future design of molecules to target infections that are refractory to currently available therapeutic options.
Natural nucleosides are the building blocks of RNA and DNA and are composed of a nucleobase and a sugar moiety. Nucleoside analogs are structurally modified in either the sugar and/or base moiety. In order to become biologically active, nucleoside analogs need, very often, to be converted to their phosphorylated counterparts furnishing nucleotide analogs. Many nucleoside analogs are not phosphorylated effectively in cells, with the first phosphorylation often being the rate-limiting step. In principle, the administration of nucleoside monophosphate analog allows the first phosphorylation step in the nucleoside activation process to be skipped, therefore bypassing the rate-limiting step in the conversion. However, the phosphorus-oxygen bond of a nucleoside monophosphate is susceptible to enzymatic hydrolysis by phosphatases and phosphodiesterases and hence nucleoside-phosphate analogs cannot be pursued as drug candidates. It has encouraged medicinal chemists to synthesize nucleoside phosphonates as mimics of nucleoside phosphates. A major advantage of nucleoside phosphonates is their chemical and metabolic stability, as the phosphorus-carbon bond is not susceptible to enzymatic hydrolysis.
Various nucleoside phosphonates have been prepared as phosphate isosteres. Examples include deoxy nucleoside phosphonates in which the oxygen atom in α-position of the phosphorus is either removed or replaced by a methylene moiety. The phosphonomethoxy (P–C–O) functionality, in which the 5′-oxygen and 5′-carbon are swapped from place, emerged as the most promising isoster of the phosphonooxymethyl group (P–O–C) of the naturally occurring nucleoside-monophosphate. Its success is due to the fact that it is isopolar and isosteric with the phosphate group. Another important characteristic of nucleoside phosphonates is their ability to function as substrates for various kinases and hence to undergo enzymatic phosphorylation that converts them into diphospho phosphonate analogs. This is especially important for antiviral and antitumoral drugs, as in these cases, the pharmacologically active species is a nucleoside triphosphate analog.
Although metabolically stable, phosphonates are negatively charged at physiological pH, and hence, are not able to penetrate the lipid-rich cell membrane, which hampers their cellular activity (either antiviral, antitumoral, antibacterial, or antiparasitic). A variety of phosphonate prodrug strategies have been developed, which are mainly pursued in the antiviral area, but recently attracted attention in other therapeutic fields as the antibacterials and antiparasitics.
Previous reviews dealing with nucleoside phosphonates are mainly focused on their antiviral properties (Mackman and Cihlar, 2011; Pertusati et al., 2012; Baszczynski and Janeba, 2013; Macchi et al., 2015) or synthetic procedures (Pradere et al., 2014; Shen and Hong, 2019). Although nucleoside phosphonates have been historically mainly pursued because of their antiviral properties, they exhibit a wide range of other biological activities, such as antitumoral, antibacterial, and antiparasitic. In addition, nucleoside phosphonates are known to interfere in the purinergic signaling process. This review focuses on different biological properties associated with nucleoside phosphonates and is organized according to the therapeutic area or biological target. Only nucleoside phosphonates with demonstrated biological activity, either in vitro or in vivo, are highlighted in this review. For a full discussion of synthesis and structure-activity relationships (SAR), the reader is referred to the original research papers.
Herpesviruses (Herpesviridiae family) are ubiquitous, large double stranded DNA viruses responsible for a variety of latent and recurrent infections in specific tissues. Among the more than hundred recognized species, nine types of herpesviruses are known to routinely use humans as their primary host, including herpes simplex virus types 1 and 2 (HSV-1 and HSV-2), varicella-zoster virus (VZV), human cytomegalovirus (HCMV), human herpesviruses 6A, 6B, and 7 (HHV-6A, HHV-6B, and HHV7), as well as oncogenic Epstein-Barr virus (EBV) and human herpesvirus 8 (HHV8) or Kaposi's sarcoma-associated herpesvirus (KSHV). In addition, a herpesvirus carried by macaques known as B virus may also occasionally infect humans. Diseases caused by human herpesviruses pose a particularly significant risk of morbidity and mortality in severely immunocompromised individuals. An important breakthrough in herpesvirus therapy coincided with the development of nucleoside analogs bearing an acyclic pseudo-sugar moiety (e.g., acyclovir, ganciclovir, and penciclovir), which culminated in the late 1980's with the discovery of acyclic nucleoside phosphonates (ANPs) (de Clercq and Holy, 2005). ANPs are nucleotide analogs characterized by a non-hydrolysable P–C bond linking a phosphonate moiety to various aliphatic side chains of purine and pyrimidine acyclic nucleosides. Unlike “classical” anti-herpes nucleosides, the intracellular activation of ANPs is not dependent on a viral-encoded kinase to promote their initial phosphorylation. Generally, ANPs are divided in different subclasses depending on the chemical structure of their side chain residue, to whom corresponds a specific antiviral activity spectrum (de Clercq, 2007). In particular, the presence of a 2′-hydroxymethyl group as in the 1-(3-hydroxy-2-phosphonylmethoxypropyl) series (HPMPs, Figures 1A,B) led to broad-spectrum agents with potent and selective activity against a variety of herpesviruses as well as other double-stranded DNA viruses (pox-, polyoma-, papilloma-, and adenoviruses) (Declercq et al., 1986, 1987). The antiviral potency of HPMPs was found to be strongly influenced by the stereochemistry at the C2′ position with a preference for the (S)- over (R)-enantiomers. The cytosine analog (S)-HPMPC 1a (cidofovir, Vistide®) is the flagship member of HPMPs (Declercq et al., 1987), and the first ANP to have been clinically approved in 1996 for the treatment of HCMV retinitis, the most common intraocular opportunistic infection affecting AIDS patients. It has also found “off-label” use for the systemic or topical treatment of herpesvirus infections resistant to first line therapy and other diseases, such as papillomatosis and progressive multifocal leukoencephalopathy due to JC polyomavirus. Moreover, (S)-HPMPC administered by parenteral injection has been shown to protect against lethal smallpox infection. Within the cell, (S)-HPMPC is converted by cellular kinases to its diphosphate metabolite (S)-HPMPCpp, which acts as a competitive substrate for incorporation into the viral genome by a DNA polymerase. (S)-HPMPCpp was found to possess a 25- to 50-fold greater affinity for viral over host polymerases, thus allowing for selective inhibition of DNA virus replication.
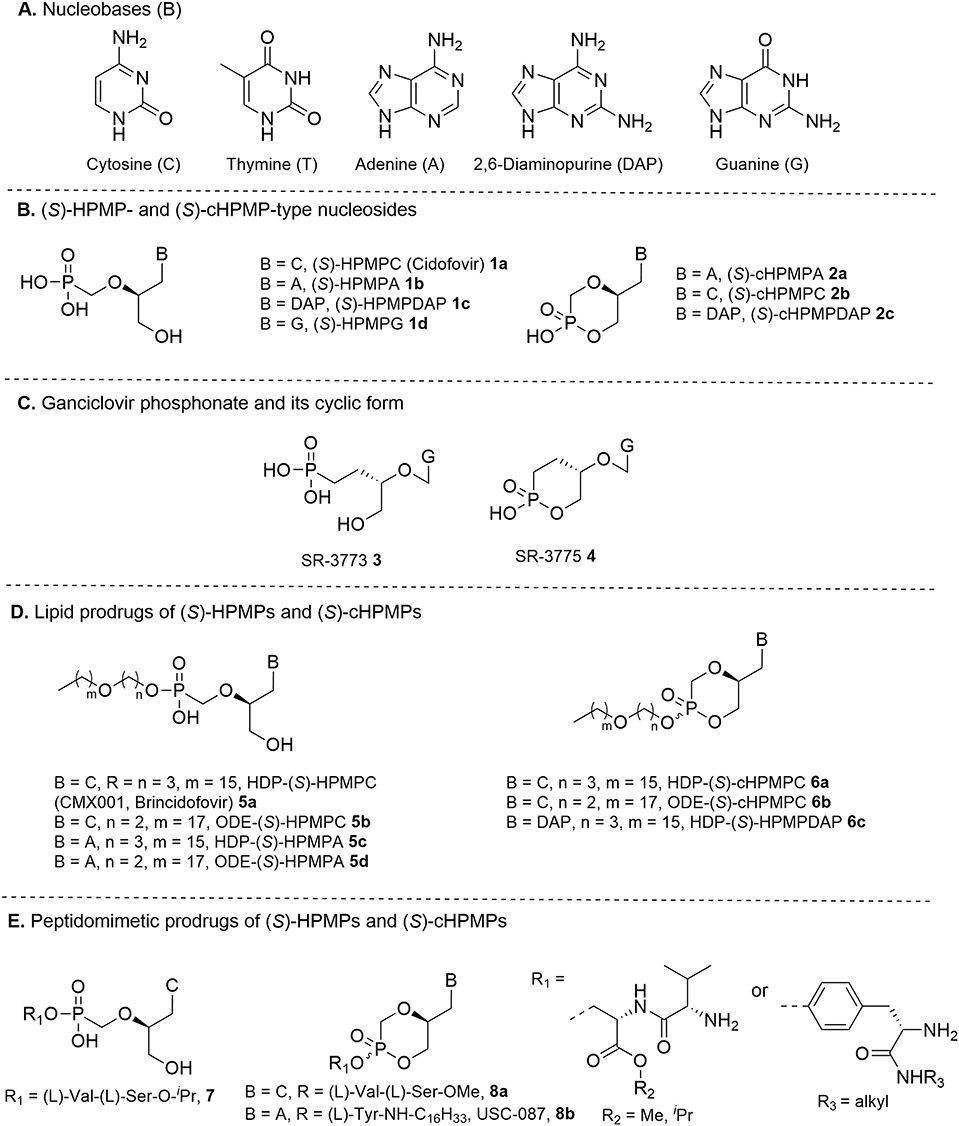
Figure 1. (A) Common pyrimidine and purine nucleobases found in nucleoside phosphonates; (B) (S)-HPMP and (S)-cHPMP-type nucleosides; (C) ganciclovir phosphonate and its cyclic form; (D) lipid prodrugs of (S)-HPMPs and (S)-cHPMPs; (E) peptidomimetic prodrugs of (S)-HPMPs and (S)-cHPMPs.
As compared to (S)-HPMPC, the adenine congener (S)-9-(3-hydroxy-2-phosphonylmethoxypropyl)adenine (S)-HPMPA 1a also displayed a similarly potent in vitro inhibitory activity against a broad panel of DNA viruses (Declercq et al., 1986), with EC50 values in the 0.08–13 μM range for different herpesviruses (HSV-1, HSV-2, VZV, HCMV, EBV, and HHV-6), 0.8–0.17 μM for adenoviruses (Ad5 and Ad8), 2.3 μM for vaccinia virus, and 15 μM for human polyoma virus. (S)-HPMPA was also reported to inhibit human hepatis B virus (HBV) with an EC50 = 1.5 μM (Yokota et al., 1991). However, a less favorable safety profile emerged from in vitro toxicity studies that halted its progressing into clinical development (Andrei et al., 1991). Other purine containing derivatives, i.e., 2,6-diaminopurine and guanine congeners (S)-HPMDAP 1c and (S)-HPMG 1d, were also found to exert broad anti-DNA virus activity in vitro.
Studies in multiple animal models as well as clinical testing in humans evidenced nephrotoxicity as a clear dose-limiting side effect of (S)-HPMPC (Bischofberger et al., 1994; Lalezari et al., 1995, 1997, 1998), and a major obstacle to the clinical application of ANPs. Upon intravenous administration, the drug accumulates in the kidney with a concentration 100-fold higher than other tissues, leading to local cellular toxicity that manifests as elevated levels of serum creatinine as well as proteins in the urine. This adverse effect was explained by the interaction of (S)-HPMPC with renal organic anion transporter 1 (OAT1) and consequential higher transport rates at the level of the basolateral and/or luminal membranes of the proximal convoluted tubule (PCT) cells compared to the efflux of the drug at the luminal side (Ho et al., 2000).
In this regard, it was demonstrated how a particularly beneficial effect could be derived from the generation of cyclic phosphonoesters, which were simply obtained by an intramolecular esterification reaction between the primary free 3′-OH group and neighboring phosphonic acid moiety (Rosenberg and Holy, 1987; Bischofberger et al., 1994). These cyclic congeners of HPMPs, as exemplified by (S)-cHPMPA 2a and (S)-cHPMPC 2b (Figure 1B), were found to retain the pronounced anti-herpesvirus potency of their parent compounds by acting as their intracellular prodrugs, while allowing for greater selectivity indexes. It was demonstrated that (S)-cHPMPC underwent an efficient cCMP phosphodiesterase-mediated in vivo conversion to the parent phosphonate (Mendel et al., 1997), leading to accumulation of similar levels of the active metabolite (S)-HPMPCpp. On the other hand, several studies documented that (S)-cHPMPC administered to diverse animal species (e.g., rats, rabbits, and monkeys) contributed to a substantially lower nephrotoxicity than (S)-HPMPC due to the presence of a modified charge that made it less susceptible to the action of organic anion transporters at the level of PCT cells.
Owing to the success of HPMPs, the introduction of a phosphonate group into the side chain of acyclic nucleosides licensed for clinical use as anti-herpesvirus agents [e.g., acyclovir and ganciclovir (GSV)] was also investigated. The (R)-enantiomer of ganciclovir phosphonate 3 (Figure 1C) exhibited potent activity against HMCV both in cell culture and murine models (Smee et al., 1994), while its cyclic phosphonate analog 4 was associated with a substantially reduced toxicity as a result of a similar effect as that observed for cyclic HPMPs (Smee and Reist, 1996).
While the antiviral activities of all these compounds are indeed remarkable, their utility, especially in clinical practice, is mitigated by their poor oral bioavailability. It is not surprising that the presence of ionizable groups at physiological pH restricts their ability to penetrate the lipid-rich cellular membrane, thus requiring parental administration in order to be effective. During the past years, various solutions have been sought to develop orally active prodrug forms with enhanced absorption and pharmacological parameters, few of which have progressed to in vivo or clinical studies. Prodrug derivatization was performed both at the level of acyclic and cyclic HPMP nucleoside phosphonates by covalently linking a suitable promoiety via an ester or amide bond. Below we provide selected illustrative examples for both categories.
In a systematic comparative study with a set of alkoxyalkyl monoesters of various ANPs analogs, Hostetler et al. defined the design principles for generating lipid-HPMP conjugates that could be sufficiently hydrophobic to enable improved cellular uptake and absorption by mimicking natural cellular membrane phospholipids (Figure 1D). Side chain moieties up to 20–21 atoms long appeared to be necessary for achieving optimal antiviral activity combined with enhanced oral bioavailability. Several in vitro studies demonstrated that the most active analogs hexadecyloxypropyl (HDP)- and octadecyloxyethyl (ODE)-(S)-HPMPC 5a and 5b showed a similar multiple log increases in antiviral activities over their parent molecule against herpesviruses (e.g., up to 1,000-fold for HCMV) (Beadle et al., 2002; Williams-Aziz et al., 2005). Low nanomolar EC50 values were obtained against wild-type as well as GCV-resistant HCMV strains (EC50 = 0.9 nM), VZV (EC50 = 0.1–0.4 nM), HSV-1 (EC50 = 0.02–0.06 μM), HSV-2 (EC50 = 0.008–0.01 μM), HHV-8 (0.02–0.03 μM), and EBV (0.03–0.10 μM). In addition, alkoxyalkyl esters HDP-(S)-HPMPC and ODE-(S)-HPMPC exhibited >100-fold enhanced in vitro activity vs. the parent nucleoside against cells infected with orthopoxviruses (poxviruses), such as vaccinia virus (VV) and cowpox (CV) (Kern et al., 2002), adenovirus (Hartline et al., 2005), BK polyomavirus (BKPyV) (Randhawa et al., 2006), and papillomavirus. Further evidence for the versatility of this prodrug approach was provided with the synthesis of related prodrugs of (S)-HPMPA (compounds 5c and 5d) (Beadle et al., 2006; Lebeau et al., 2006), (S)-cHPMPC (compounds 6c and 6d) (Beadle et al., 2002; Kern et al., 2002), and (S)-cHPMPDAP (6c) (Krecmerova et al., 2010), which likewise allowed for a significant enhancement of bioavailability as well as antiviral potency against herpesviruses and poxviruses.
HDP-(S)-HPMPC 5a, also known as CMX001 or brincidofovir, is the most well-investigated compound of this series, and the only orally available HPMP prodrug to have undergone clinical development so far (Chimerix Inc.). Originally intended as an oral formulation to treat smallpox in the event of a variola virus accidental outbreak or for biodefence purposes, it later underwent clinical trials for the treatment of HCMV and ADV infections. Brincidofovir remains intact in the plasma throughout the body and is absorbed in the small intestine by means of facilitated and passive diffusion processes. In vitro metabolic studies reported 10- to 20-fold increased membrane permeation rates and longer intracellular half-life (10 days) for brincidofovir when compared to (S)-HPMPC (2.7 days) (Aldern et al., 2003). Brincidofovir is converted intracellularly to (S)-HPMPCpp after cleavage of its lipid moiety and phosphorylation by intracellular kinases. Analysis of cellular metabolites showed that levels of (S)-HPMPCpp were 100-fold greater with brincidofovir than those observed with (S)-HPMPC.
Oral administration of brincidofovir proved as effective as parental (S)-HPMPC in the treatment of herpes- and poxvirus infection in several animal models, however, unlike (S)-HPMPC, brincidofovir was not a substrate of OAT1 and did not concentrate in renal proximal tubules, as shown by studies evaluating tissue distribution of radiolabeled species in mice (Ciesla et al., 2003).
Following promising Phase I safety and pharmacokinetic studies (Painter et al., 2012), brincidofovir was evaluated in a Phase II dose escalation trial in HCMV-seropositive recipients of hematopoietic-cell transplant (HCT), which identified 100 mg orally twice weekly as the maximum tolerated dosage to significantly reduce the incidence of HCMV (Marty et al., 2013). However, a subsequent phase III placebo-controlled trial in the same patient population showed that the incidence of clinically significant CMV infections and mortality was similar and higher, respectively, when comparing brincidofovir-treated patients and placebo recipients. Furthermore, serious adverse gastrointestinal events (digestive symptoms), such as acute graft-vs.-host disease (GVHD) and diarrhea were more frequently observed among treatment group participants (Marty et al., 2019).
Although clinical trials of brincidofovir for the prevention of HCMV are currently suspended, its evaluation for the treatment of disseminated adenovirus infections is ongoing and a phase II trial in pediatric and adult allogenic HSCT recipients was recently completed (Grimley et al., 2017) (ClinicalTrials.gov Identifier: NCT02087306; Recruitment Status: Completed). Oral administration of brincidofovir twice a week was reported to decrease mortality and led to an early and marked decline in HAdV viremia in subjects with HAdV levels of >1,000 genome copies/mL at baseline, although the results were not statistically significant.
An additional approach that was devised to counter the challenges associated with the oral delivery of HPMPs include the use of non-toxic biomoieties, such as single amino acids or peptides, with the aim of further facilitate gastrointestinal drug absorption by transporter-mediated uptake (Figure 1E). By linking a P-OH group of either (S)-HPMPC 1a or (S)-cHPMPC 2c to various dipeptides via the side chain hydroxy group of a (L)-serine alkyl ester residue, Mckenna et al. prepared a series of peptidomimetic conjugates that inhibited HCMV replication in the submicromolar range, while exhibiting enhanced permeability and bioavailability relative to the parent molecules (McKenna et al., 2005; Eriksson et al., 2008; Peterson et al., 2011). In a murine model, oral administration of (L)-Val-(L)-Ser prodrug conjugates 7 and 8a led to a 15- and 8-fold enhancement in plasma levels of total (S)-HPMPC species, respectively, over those obtained from dosing with the parent drug (Eriksson et al., 2008; Nadel et al., 2014). While the uptake mechanism underpinning the improved absorption of these prodrugs might rely on the function of specific transporters located in the gastrointestinal tract, further studies demonstrated that these compounds were not substrates of the peptide-specific intestinal transporter human hPEPT1 (hPEPT1) (Peterson et al., 2010).
Another proof of principle was achieved with a series of N-alkyl tyrosinamide ester prodrugs of (S)-cHPMPC and (S)-cHPMPA (Zalcharova et al., 2011). These derivatives underwent a simple activation pathway by generating the parent cyclic form as well as an acyclic form bearing the respective prodrug moiety, which was also endowed with in vitro antiviral activity. This prodrug design afforded analogs with a comparable in vitro inhibitory potency as the corresponding parent molecules against HCMV, HSV-1, VV, and CV, corresponding to IC50 values ranging from 0.1 to 50 μM, while enabling superior oral bioavailability in vivo.
The most promising example of this series described so far consists of an N-hexadecyl tyrosinamide congener of (S)-cHPMPA identified as USC-087 (8b), whose efficacy against various human adenovirus types was demonstrated in cell culture and in vivo. In the immunosuppressed Syrian hamster model, oral administration of USC-087 completely prevented or significantly decreased mortality against HAdV infection up to 4 days post-lethal intravenous challenge. Owing to the ability to decrease virus burden and liver damage, protection of the animal was achieved even when the administration of 8b started 4 days after challenge (Toth et al., 2018).
A more recent application of the prodrug strategy to HPMPs can be found in the synthesis of phosphonobisamidate (9) and aryloxyphosphonamidate analogs (10a–f) of (S)-HPMPA and (S)-cHPMPA, respectively (Figure 2) (Luo et al., 2018). Overall, these compounds displayed a dramatic enhancement of antiviral activity compared to the reference drug (S)-HPMPC when tested against several herpesviruses including HSV-1, HSV-2, VZV, and HCMV, which was explained by their increased lipophilicity (as expressed by their clogP values). Within the (S)-cHPMPA n-amyl ester amidate series, the EC50 values varied depending on the amino acid moiety but largely persisted in the low nanomolar range. Selectivity indexes up to 20,000 and 1,800 against VZV and HCMV, respectively, were obtained. Furthermore, these prodrugs were found to be stable in human plasma with t1/2 values exceeding 1 h, while undergoing fast metabolism in human liver microsomes.
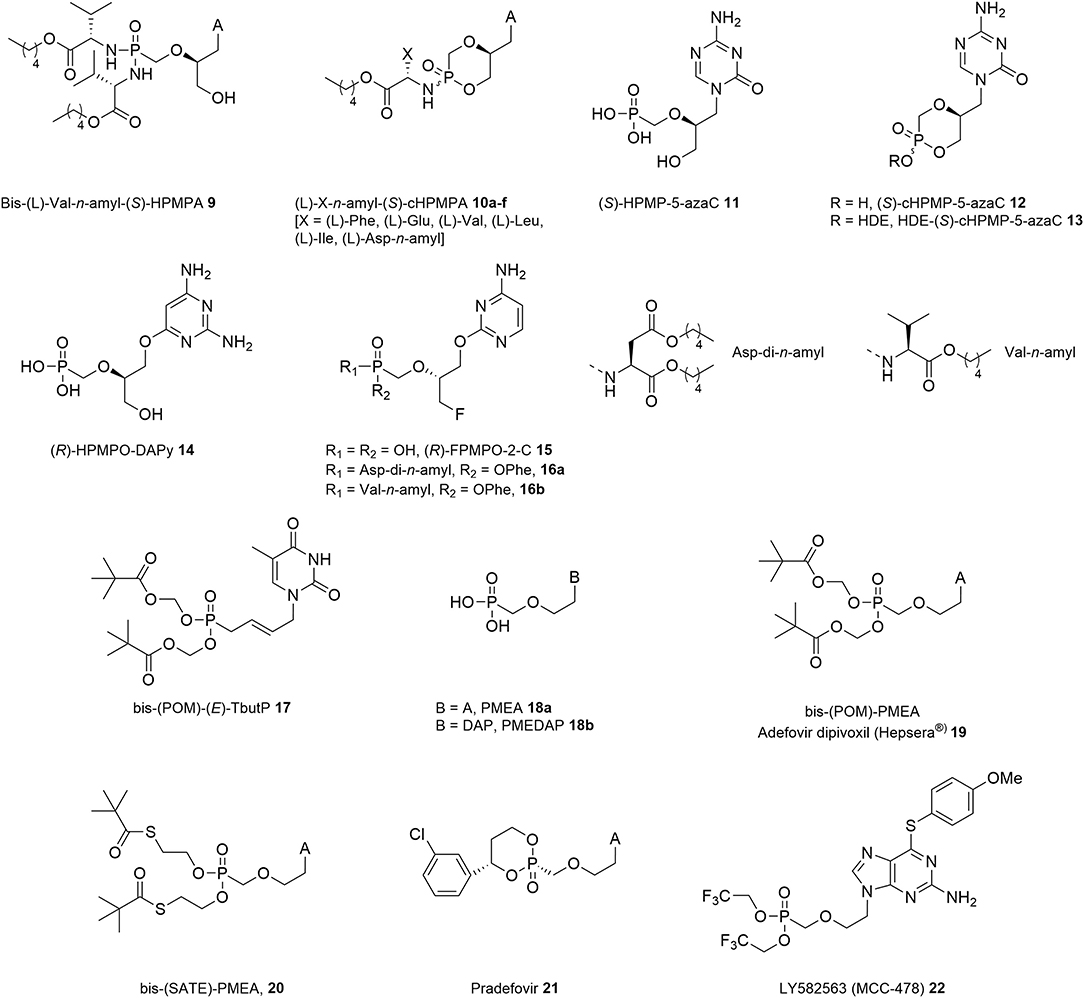
Figure 2. Phosphonamidate prodrugs of (S)-HPMPA and (S)-cHPMPA, (S)-HPMP-5-aza C and its prodrugs, O-6 and O-2-alkylated HPMP analogs, an alkenyl based bis-(POM) ANP prodrug, PME-type nucleosides and prodrugs.
The first generation HPMPs was promptly complemented by other ANPs combining a similar acyclic motif with non-natural pyrimidine nucleobases, which showed comparable antiviral activity in cell culture and animal models as the first generation HPMPs, but have not yet been assessed for their clinical potential. One such compound that deserves a special mentioning is the sym-triazine isomeric analog of (S)-HPMPC, namely (S)-HPMP-5-azaC (11, Figure 2), which displayed a broad anti-DNA virus spectrum closely resembling that of its parent drug with equipotent inhibitory activity against HSV-1 (EC50 = 0.07 μg/mL), HSV-2 (EC50 = 0.25 μg/mL), and vaccinia virus (EC50 = 2.56 μg/mL), while being 2- to 7-fold more active against VZV, HCMV, HHV-6, and Ad2 (EC50 = 0.02–0.71 μg/mL) (Krecmerova et al., 2007a). In contrast, both its (R)-enantiomer and isomeric 6-azacytosine analogs were significantly less active. In addition, (S)-HPMP-5-azaC also proved to be effective inhibiting Moloney murine sarcoma virus (MSV)-induced transformation of C3H/3T3 cell cultures (EC50 = 2.2 μg/mL).
In analogy to previous work on HPMPs, the corresponding cyclic form (S)-cHPMP-5-azaC 12 revealed strong in vitro activity against DNA viruses, with EC50 values in the range of 0.06–3.1 μg/mL, comparable with those of (S)-HPMP-5-azaC, (S)-HPMPC, and (S)-cHPMPC. Further derivatization of (S)-cHPMP-5-azaC with a lipid ester moiety led to a general enhancement of antiviral activity, with the most potent hexadecyloxyethyl (HDE) prodrug 13 exhibiting a 58- to 250-fold increased activity (Krecmerova et al., 2007b). As compared to (S)-HPMPC, (S)-HPMP-5-azaC displayed a 2- to 16-fold higher selectivity index against adeno-, pox, and herpesviruses in cell culture. However, despite their favorable toxicity profile, neither 5-aza-(S)-HPMPC nor its HDE prodrug were further pursued for their therapeutic potential, mainly due to the instability and complex metabolic pathways involving chemical decomposition and enzymatic deamination.
Another interesting compound was discovered by linking the HPMP pseudosugar synthon through an oxygen atom to the C6 of 2,4-diaminopyrimidine (DAPy) in place of the classical N1-pyrimidine linkage (Figure 2). The resulting (R)-HPMPO-DAPy 14 inhibited HSV-1, HSV-2, and VZV replication at a level comparable to (S)-HPMPA (1b) with EC50 values in the 0.51–51 μM range, while being a poor inhibitor of HCMV (EC50 = 139 μM vs. 0.63 μM of 1b) (Balzarini et al., 2004). (R)-HPMPO-DAPy proved also to be active in cell culture against other DNA viruses, including adenovirus type 2 (EC50 = 5.4–10 μM), albeit to a 3- to 10-fold lesser extent than the parent compounds (S)-HPMPA and (S)-HPMPC (Naesens et al., 2005), and especially poxviruses, e.g., camel pox, cowpox, vaccinia, and orf virus (Balzarini et al., 2004; Dal Pozzo et al., 2005; de Clercq et al., 2005; Duraffour et al., 2007). Promising results in animal models mimicking disseminated vaccinia further emphasized the antiviral potential of 14 in rivaling the utility of (S)-HPMPC (1a) for the treatment of human poxvirus infections and suppression of vaccine-related complications following immunization of immunocompromised hosts (de Clercq and Neyts, 2004; Stittelaar et al., 2006).
Recently, Herdewijn et al. described a new family of O-alkylated ANP amidate prodrugs with potent activity against different strains of HMCV and VZV (Figure 2) (Luo et al., 2020). The main difference with the previous series is that the aliphatic side chain is linked to the 2-O-position of a pyrimidine base rather than the 6-O position. Among the parent phosphonates, (R)-O-2-alkylated 3-fluoro-2-(phosphonomethoxy)propyl cytosine (FPMPC) 15 was found to be a moderately active inhibitor of HCMV and VZV with EC50 values in the 0.80–2.36 and 3.79–25.3 μM range, respectively. Interestingly, subsequent derivatization of (R)-O-2-alkylated FPMPC as its L-aspartic acid or L-valine containing phenoxyamidate prodrugs 16a and 16b led to a marked improvement of activity with EC50 values ranging between 0.094 and 0.54 μM along with low cellular toxicity. In addition, it should be noted that early attempts to tune the activity of HPMPs through replacement of the 3′-hydroxyl group by a fluorine atom resulted in loss of antiviral activity against herpesviruses (Balzarini et al., 1993; Luo et al., 2017). However, diamyl aspartate phosphonoamidate prodrugs of purine containing N-alkylated FPMP analogs exhibited submicromolar anti-VZV, while (S)-aspartate-FPMPC was endowed with an EC50 value of 0.76 μM against HCMV (Luo et al., 2017).
A series of 5-substituted pyrimidine ANPs bearing an unsaturated acyclic side chain was also synthesized along with their bis-pivaloyloxymethyl (POM) ester prodrugs (Topalis et al., 2011). Among them, bis-POM-(E)-thymidine-but-2-enyl phosphonate 17 was found to be the most potent inhibitor of HSV-1 and HSV-2 with IC50 in the 3 and 6 μM range, respectively, as well as VZV (IC50 = 0.19 μM), while the corresponding (Z)-configured congener was completely devoid of anti-DNA virus activity. It was argued that the observed in vitro antiviral activity correlated with the ability of 17 to act as a substrate for human thymidine monophosphate (TMP) kinase.
According to the latest WHO estimates, about 38 million people worldwide are living with HIV (2019 WHO report), while chronic HBV infection accounts for a global prevalence of 257–291 million affected individuals (2015 WHO Global hepatitis report and 2016 Polaris Observatory study). Due to the highly contagious capacity and common transmission routes shared by these bloodborne viruses, high rates (5–20%) of HIV-HBV co-infection are frequent, which may exacerbate clinical complications, such as the progression of the HBV infection to liver cirrhosis and hepatocellular carcinoma. Moreover, antiviral drug resistance may often develop after prolonged treatments usually resulting in progressive loss of clinical benefit.
The evolution of ANP inhibitors occurred in parallel with structural simplifications at the acyclic chain and developed into an important achievement with regard to their activity spectrum. By extending their seminal studies on ANPs, Holy, and De Clercq conducted similar investigations into the SAR profile of related 2-phosphonylmethoxyethyl (PME) derivatives (Figure 2). In striking contrast to the HPMP series, PME nucleosides lacking chirality due to the absence of the 2′-substituent exerted a distinctive 2-fold inhibitory effect against both DNA viruses of the herpes (Declercq et al., 1986, 1987) and hepadnavirus (Yokota et al., 1991; Heijtink et al., 1994) families as well as retroviruses of different species (Pauwels et al., 1988; Naesens et al., 1997). On the other hand, no appreciable activity was observed against adeno-, pox-, and papillomaviruses. Furthermore, only purine containing PMEs exhibited antiviral activity that comprise the most successful member of this class, i.e., PMEA or adefovir (18a, Figure 2) (Declercq et al., 1986). This adenine congener is arguably one of the most interesting ANPs due to its broad activity spectrum, and was selected as candidate for further clinical development especially in view of its potent reverse transcriptase inhibitory activity in various subtypes of HIV-1 clinical isolates and several animal models. While exhibiting a 5-fold increased antiretroviral (MSV) efficacy than 18a, the diaminopurine containing congener PMEDAP 18a (Figure 2), displayed an inferior toxicity profile (Naesens et al., 1989).
These initial findings motivated the generation of an entire family of phosphonodiester prodrugs of PMEA, as a means for oral delivery (Figure 2). Eventually, adefovir came to the market in 2002 as its acyloxy ester prodrug adefovir dipivoxil [bis-(POM)-PMEA, ADV, Hepsera®, 19] (Starrett et al., 1992; Cundy et al., 1994). Although initially evaluated for the treatment of HIV-infected individuals, the compound proved too nephrotoxic at the indicated efficacy dose (125 mg-dose once daily) to permit long term use (Kahn et al., 1999). It was nonetheless approved for oral chronic anti-HBV therapy as in this case administration at submaximally efficacious doses (10 mg-dose once daily) was sufficient to achieve an effective inhibitory activity (Hadziyannis et al., 2003; Marcellin et al., 2003; Peters et al., 2004).
Complementary approaches to the bis-(POM) prodrug strategy were considered in order to minimize systemic distribution and especially kidney exposure to PMEA and its metabolites, while enhancing liver targeting delivery.
Symmetrical phosphonodiester prodrugs of PMEA bearing carboxyesterase-labile S-acyl-thioethyl (SATE) masking moieties, as exemplified by bis(t-Bu-SATE)-PMEA 20 (Figure 2), exhibited antiviral potencies against HIV-1 similar to bis-(POM)-PMEA (EC50 = 0.03–0.65 μM for 20 vs. 0.04–0.08 μM for 19), while being characterized by a greater stability in human gastric juice and human serum (Benzaria et al., 1996). However, further clinical development of this class of prodrugs was precluded by concerns associated with the mutagenic potential of their degradation byproducts.
A remarkably pronounced enhancement of plasma stability compared to 19 was observed with the selectively liver-activated cyclic 1-aryl-1,3-propanyl ester prodrug of PMEA (21, Figure 2), also known as pradefovir (MB-06866) (Lin et al., 2006). The design of 21 was based on the HepDirect™ prodrug technology, which relied on the oxidative conversion of 21 to PMEA by the action of the hepatic cytochrome P450 isozyme CYP3A4, consequently allowing for decreased non-hepatic activation. Preclinical evaluation of pradefovir in rats showed a 12-fold improvement in the liver/kidney ratio for 21 over 19 combined with a good oral bioavailability (42%) as a mesylate salt formulation (Reddy et al., 2008). A 48-weeks Phase II trial involving chronically infected HBV patients further confirmed that pradefovir mesylate (dosed at 5–30 mg/day) was well-tolerated and significantly more active than 19 (dosed at 10 mg/day), without producing significant changes in kidney function markers (Lee et al., 2006). Although further clinical evaluation of 21 was discontinued in USA and Europe due to the potential carcinogenic effect observed in animal studies, a Phase III trial for the treatment of patients with chronic HBV infections is currently ongoing in China to determine (ClinicalTrials.gov Identifier: NCT04543565; Recruitment Status: Recruiting) (Zhang et al., 2020).
In contrast to the broad spectrum of activity characteristic of PMEs bearing natural nucleobases, bis(trifluoroethyl) esters of 2-amino-6-arylthio 9-[2-(phosphonomethoxy)ethyl]purines, and particularly 6-(methoxyphenyl)thio substituted derivative 22 (LY582563, Figure 2), were found to possess potent anti-HBV antiviral activity in vitro against wild-type and lamivudine resistant variants, while lacking inhibitory activity against HIV-1 (Ono-Nita et al., 2002; Sekiya et al., 2002). Compound 22 was absorbed in the gastrointestinal tract when administered orally to mice and high concentrations of its main active metabolite were observed in the liver (Sekiya et al., 2002). Its clinical efficacy for the treatment of HBV-infected patients was demonstrated in phase I studies (Wise et al., 2002).
The presence of a methyl group at the 2′-position of the side-chain of ANPs define an additional series of derivatives endowed by a yet diverse antiviral activity spectrum, with the most notorious example being the (R)-enantiomer of 9-(2-phosphonylmethoxypropyl)adenine [(R)-PMPA] (23a, Figure 3), also known as tenofovir (Balzarini et al., 1993). PMPs with a purine nucleobase displayed pronounced antiretroviral activity in cell culture, e.g., against HIV-1, HIV-2, and MSV, while completely lacking anti-herpesvirus activity. These analogs also resulted potent inhibitors of hepadnaviruses including human and duck HBV (Heijtink et al., 1994). Although in these early studies the 2,6-diaminopurine congener (R)-PMPDAP 23b was found to be up to 35- and 8-fold more potent than (R)-PMPA in inhibiting HIV and HBV replication, respectively, it was (R)-PMPA that later entered and underwent successful clinical development. In order to enable oral administration, (R)-PMPA is currently marketed as its bis(isopropyloxycarbonyloxymethyl) ester prodrug tenofovir disoproxil fumarate (24) (Naesens et al., 1998; Robbins et al., 1998) for the treatment of HIV and chronic HBV infections (Fung et al., 2002; Kuo et al., 2004; Heathcote et al., 2011), either as single drug (TDF, Viread®) or in a fixed-dose combination with other antiretroviral agents, such as emtricitabine (Truvada®) and efavirenz (Atripla®). TDF offered clear advantages in terms of bioavailability of the active drug (25 vs. 2% the dianionic parent compound 23a) upon oral administration to mice, as well as good tissue distribution and biological stability (Kearney et al., 2004). However, chronic treatment with TDF has been linked to lactic acidosis, Fanconi syndrome, acute renal failure, and bone loss mainly due to its cleavage in the systemic circulation (Duarte-Rojo and Heathcote, 2010). Therefore, alternative prodrugs strategies have been explored to decrease plasma exposure to (R)-PMPA.
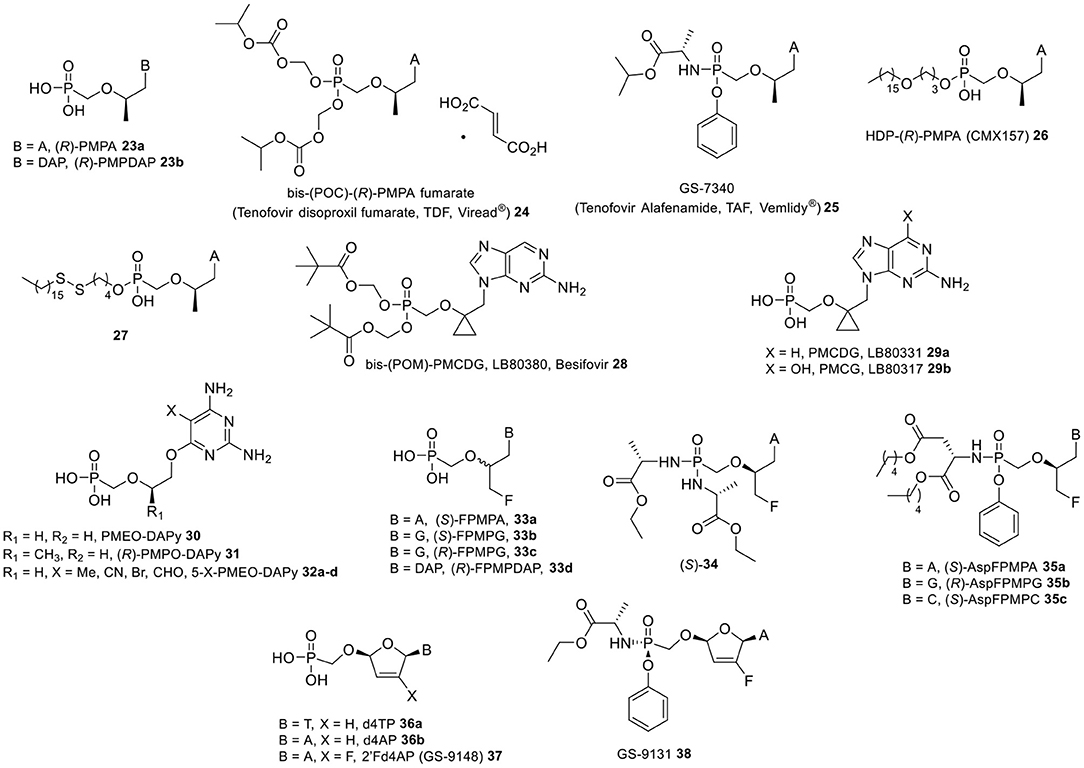
Figure 3. PMP-type nucleosides and prodrugs, Besifovir and its metabolites, PMEO- and PMPO-DAPy-type nucleosides, FPMP-type nucleosides and prodrugs, 2′, 3′-didehydro-2′,3′-dideoxyribose nucleoside phosphonates, and prodrugs.
For instance, the alanyl phenyl ester phophonoamidate prodrug of (R)-PMPA, namely tenofovir alafenamide 25 (TAF), was specifically designed to target lymphatic cells and tissue, where it was shown to undergo fast cleavage mediated by a lysosomal carboxypeptidase cathepsin A, while being endowed with higher plasma stability than 24 as well as little or no nephrotoxicity (Lee et al., 2005; Birkus et al., 2007). Notably, TAF was found to be 1,000-fold more active against HIV-1 (EC50 = 5 nM) when compared to the parent nucleoside 23a (EC50 = 5 μM), and was also licensed for clinical use in adults infected with chronic HBV (Ray et al., 2016).
Lipid conjugate ester prodrugs of (R)-PMPA have also been shown to provide a valuable mean for facilitating tissue targeted delivery, while minimizing systemic exposure to the parent drug. For instance, HDP-(R)-PMPA 26 (CMX-157, tenofovir exalidex TXL™) was shown to remain intact in the bloodstream, while undergoing cleavage by an intracellular hydrolase (phospholipase C and/or sphingomylenase), to liberate (R)-PMPA within the cytosol. This prodrug was reported to have 267- and 4.5-fold more pronounced in vitro activity than (R)-PMPA against HIV-1 and HBV, respectively (Painter et al., 2007), and could effectively suppress the replication of nucleos(t)de reverse transcriptase inhibitor (NRTI)-resistant HIV variant strains with low nanomolar EC50 values (Lanier et al., 2010). It showed good oral bioavailability in vivo, while being well-tolerated at doses up to 200 mg/kg in rats and monkeys with gastrointestinal toxicity being dose-limiting (Lanier et al., 2009). In early phase clinical trials, 26 showed a favorable tolerability and good pharmacokinetic properties, retaining significant antiviral activity for up to a week after a single administration. A multiple ascending dose proof of concept Phase II study involving HBV infected subjects treated orally with CMX157 at increasing dose levels up to 100 mg to evaluate the safety and tolerability was completed (ClinicalTrials.gov Identifier: NCT02710604; Recruitment Status: Completed) (Tanwandee et al., 2017).
Liotta et al. recently developed disulfide-based lipid conjugates of (R)-PMPA that exhibited a 600-fold improved potency over the parent nucleoside phosphonate against HIV-1 and significantly higher therapeutic index of >100,000 (vs. 9,500 of TDF) for the most potent conjugate bearing a C16 lipid chain along with a S–S moiety five atoms away from the phosphonate (compound 27). These conjugates were also potent inhibitors of HBV replication in hepatocytes with EC50 values comparable to TDF, and were stable in human plasma for >24 h (Giesler and Liotta, 2016; Giesler et al., 2016). Although it was demonstrated that these prodrugs had the potential to undergo intramolecular cleavage upon disulfide reduction, the principal mechanism accountable for the release of the parent drug and consequent in vitro antiviral activity seemed to be enzymatic hydrolysis, most likely mediated by the same enzymes involved in the decomposition of CMX-157 and other lipid conjugates.
In analogy to (R)-HPMPO-DAPy 14 (Figure 2), O-6-alkylated 2,4-diaminopyrimidine ANPs bearing either a PME or PMP aliphatic chain, i.e., PMEO-DAPy (30) and (R)-PMPO-DAPy (31) (Figure 3), were also synthesized and reported to be equipotent to their parent drugs [PMEA (18a) and (R)-PMPA (23a), respectively] in cells infected with HIV-1, HIV-2, and HBV (both wild-type and lamivudine-resistant strains), while being relatively non-toxic (Balzarini et al., 2002; de Clercq et al., 2005; Ying et al., 2005). Moreover, compounds 30 and 31 also inhibited MSV-induced cell transformation both in vitro (EC50 = 0.05–0.15 μM) and in a murine infection model (Balzarini et al., 2002). PMEO-DAPy derivatives featuring various substituents (e.g., Me, CN, Br, and CHO) at the 5-position of the DAPy base (compounds 32a-d, Figure 3) were also shown to be potent inhibitors of HIV and HBV replication (Hockova et al., 2003; Ying et al., 2005). Notably, the 5-methyl PMEO-DAPy derivative 32a was ~3- to 10-fold more active than PMEO-DAPy 30 against HIV and MSV including drug-resistant clinical isolates of HIV-1, while being more effective than PMEA in inhibiting retroviruses in vivo (Balzarini et al., 2007). The antiviral activity spectrum of PMEO-DAPy 30 was explained by the base pairing ability of the DAPy nucleobase, which was revealed to mimic the natural purine adenine counterpart allowing for hydrogen pairing to a pyrimidine nucleobase (Herman et al., 2010). In particular, PMEO-DAPy-pp was shown to be incorporate by HIV RT opposite to thymine (in DNA) or uracil (in RNA).
Since minor modifications at the side-chain of ANPs appeared to have a substantial impact on their antiviral activity spectrum, many different structural motifs have been explored for further antiviral profiling. Besifovir (LB80380, 28, Figure 3), an orally available ANP dipivoxyl maleate prodrug featuring a cyclopropyl moiety at the 2′-position, is a clinical development candidate for the treatment of HBV (Choi et al., 2004; Papatheodoridis and Manolakopoulos, 2006). Besifovir was shown to undergo rapid deacetylation in the liver and intestine to its parent 2′-9-(2′-phosphonomethoxycyclopropyl)deoxyguanine 29a (PMCDG, LB80331), which was further metabolized by enzyme oxidases (e.g., aldehyde and/or xanthine oxidase) to its corresponding guanine analog 29b (PMCG) (Choi et al., 2004; Yuen et al., 2009). The diphosphate derivative of 29b represents the active metabolite inhibiting viral DNA replication and eventually exerting the antiviral effect (Yuen et al., 2009). Besifovir was shown to exhibit high efficacy against both wild-type and HBV mutants resistant to lamivudine, adefovir, entecavir, and telbivudine studies as well as in animal models of HBV infection (Fung et al., 2008).
Results from various phase II trials indicated that 28 could significantly reduce the viral load in HBV-infected patients treated with escalating doses up to 240 mg/day for 4 weeks (Yuen et al., 2006), while also effectively suppressing HBV replication in treatment-naïve (Lai et al., 2014) and lamivudine-resistant chronic HBV patients (Yuen et al., 2010). Besifovir was also found to be clinically effective during a 2-years trial in. Although being generally well-tolerated and showing no significant nephrotoxicity, a high percentage of patients suffered from depletion of carnitine, suggesting that long-term use of besifovir needs to be accompanied by a carnitine supplement (Yuen et al., 2015). Recently, a phase III study demonstrated that 28 (150 mg) exerted antiviral efficacy comparable to TDF (300 mg) after 48 weeks of treatment with durable effects up to 96 weeks, together with comparably reduced bone and kidney toxicities (Ahn et al., 2019). These results led to the approval of 28 in South Korea (Besivo®).
As discussed in the previous section, 3-fluoro-2-(phosphonomethoxy)propyl (FPMP) nucleosides are side-chain fluorinated ANPs largely devoid of antiviral activity against a broad range of DNA viruses (Balzarini et al., 1993). As an exception, purine containing FPMP analogs (33a–d, Figure 3) were found to be effective inhibitors of HBV DNA synthesis (Heijtink et al., 1994; Luo et al., 2017), besides showing moderate antiretroviral activity against HIV-1 and HIV-2 in various cell lines (Balzarini et al., 1993; Luo et al., 2017). Notably, (S)-FPMPG 33b was at least as potent as its (R) counterpart 33c in inhibiting both HIV and HBV replication with the lowest EC50 values within this series (0.28–6.65 and 0.49–0.59 μM, respectively). On the other hand, the (S) enantiomer of FPMPA 33a was 5- to 7-fold more active than (R)-FPMPA with EC50 values in the 3–6 and 1 μM range for HIV and HBV, respectively. The diaminopurine containing congener (R)-FPMPDAP 33d was also endowed with antiretroviral activity (EC50 = 4.3 and 4.6 μM against HIV-1 and HIV-2, respectively) (Balzarini et al., 1993).
In an early report describing the application of the prodrug approach to FPMPs, a bis(amino acid) phosphonamidate of (S)-FPMPA (34) was synthesized that resulted in a 13-fold improved anti-HIV activity (EC50 = 0.54 μM) compared to the parent phosphonate (EC50 = 7.04 μM) (Jansa et al., 2011). Recently, a more systematic study revealed how the use of a diamyl aspartate phenoxyamidate motif to mask the phosphonate acid functionality of FPMPs resulted in a drastically enhanced antiretroviral potency by a factor up to 1,500, depending on the nucleobase moiety (Luo et al., 2017). Additionally, up to 160-fold improvements in anti-HBV activity were also observed, while some members of this family of prodrugs also displayed a broader spectrum of activity compared to their parent molecules by inhibiting herpesvirus replication. The (S)-FPMPA amidate prodrug 35a emerged as the most active compound of this series, exhibiting anti-HIV-1 activity at low nanomolar concentrations in different cell lines along with excellent potency against HBV (EC50 = 0.01 μM) and VZV (EC50 = 0.05 μM). This prodrug was found to be stable in acid and human plasma media, while being efficiently metabolized in human liver microsomes with a half-life of 2 min. The (R) isomeric guanine derivative (R)-AspFPMPG 35b also appeared to be a selective HIV, HBV, and VZV inhibitor, while being non-toxic to human hepatoblastoma cells. Notably, although a weak or no antiviral activity was generally associated with FPMP pyrimidine derivatives, the (S)-aspartate prodrug of FPMPC (35c) demonstrated anti-HCMV activity with an EC50 value up to 0.76 μM. Further studies are ongoing to determine the clinical potential of these compounds.
Numerous attempts have been made to identify cyclic nucleoside phosphonate (CNP) candidate antivirals; however, only a few of them have demonstrated to be effective HIV and HBV inhibitors with a promising therapeutic potential. Regardless of their closer structural resemblance to natural nucleoside monophosphates, CNPs mostly appear to be poor substrates for both viral and cellular kinases as well as being inefficiently incorporated by target polymerases. Extensive synthetic efforts on ribose-modified analogs helped to assemble important information about the SAR of these compounds (Mackman and Cihlar, 2011), revealing basic structural and electronic requirements for a favorable binding interaction with replicating target enzymes. For instance, the presence of a oxygen atom at the β-position to the phosphorus and a pKa value of the phosphonic acid functionality comparable to that of a phosphate moiety seemed to be fundamental prerequisites for potent antiretroviral activity. This is evident from thymine 2′,3′-didehydro-2′,3′-dideoxyribose nucleoside phosphonate 36a (d4TP) and especially its adenine congener 36b (d4AP) (Figure 1A and Figure 3), which were reported to exert a potent inhibitory effect on the replication of retroviruses including HIV-1 (Kim et al., 1991). Since d4AP was associated with mitochondrial toxicity, a fluorine atom was introduced at the 2′ position of the unsaturated ring moiety in order to increase the selectivity for HIV-1 reverse transcriptase (RT), while reducing the extent of the interaction with mitochondrial DNA polymerase γ. Although the resulting 2′Fd4AP (37, GS-9148) largely overcame this limitation showing more favorable pharmacological parameters than d4AP (Cihlar et al., 2008), further prodrug conjugation was needed for enhancing tissue specific delivery. Specifically, mono(ethyl-L-alanine) phosphonoamidate prodrug GS-9131 (38) did not lead to an improvement in the in vitro antiviral activity of 37; however, about 20-fold high intracellular levels of the diphosphate metabolite of 37 were detected in PBMCs after oral administration of 38 to dogs (Cihlar et al., 2008; Ray et al., 2008). Following these observations, GS-9131 progressed into clinical evaluation for the treatment of patients infected with NRTI-resistant HIV-1, but failed to meet the targeted antiviral response in a Phase II trial (ClinicalTrials.gov Identifier: NCT03472326; Recruitment Status: Terminated).
Another series of CNPs that constitute attractive clinical development candidates with a dual inhibitory activity against HIV and HBV are α-L-2′-deoxythreose nucleoside phosphonate analogs, in which a phosphonomethoxy group was bound at the 3′-position of a four-carbon sugar moiety (Figure 4) (Wu et al., 2005; Liu et al., 2016). This study further highlighted the decisive role played by the spatial proximity between the α-phosphorus atom and nucleobase, as comparatively greater bond distances, e.g., in modified five-carbon sugar CNPs bearing a phosphonate group at the primary hydroxyl group, appeared to be detrimental for antiviral activity. In vitro, phosphonomethoxydeoxythreosyl adenine (PMDTA) 39a displayed low-micromolar EC50 values against HIV-1 and HIV-2 (4.69 and 5.23 μM, respectively) without affecting normal cell proliferation. The corresponding thymidine containing congener PMDTT 39b was slightly less active with EC50 values in the 20 μM range. Likewise, PMDTA showed potent anti-HBV activity with an EC50 value of 0.5 μM, while PMDTT was 10-fold less potent (EC50 = 40.2 μM).
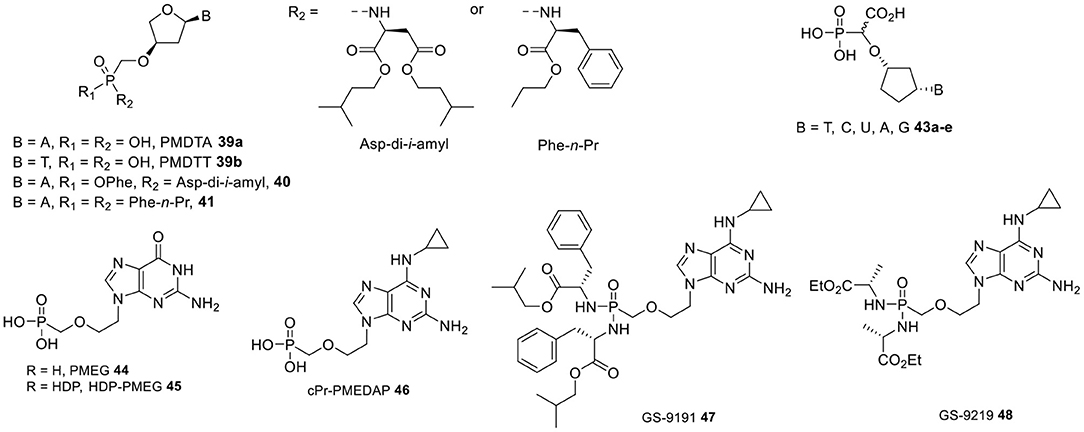
Figure 4. PMDT-type nucleosides and prodrugs, cyclopentyl α-carboxynucleoside phosphonates, PMEG and its prodrugs.
The use of the prodrug strategy was absolutely critical to boost the antiviral potency of these analogs. A variety of phosphonomonoamidate and phosphonodiamidate prodrugs were synthesized that showed a 100–1,000 increase of anti-HIV potency depending on the prodrug moiety, while 50- to 150-fold enhancements in activity against HBV were observed. The most potent congeners of this series were the L-aspartic acid diisoamyl ester phenoxy and L-phenylalanine propyl ester phosphonobisamidate prodrug of PMDTA 40 and 41 that exhibited anti-HIV and anti-HBV activities in the low nanomolar range (EC50 values up to 0.002 and 0.01 μM, respectively) and selectivity indexes of more than 300. These prodrugs were found to be reasonably stable at pH 8, while undergoing fast metabolic activation to the parent PMDTA in the presence of a carboxypeptidase Y type enzyme and human liver enzymes (Liu et al., 2016).
As mentioned above, the generally accepted intracellular metabolic cascade responsible for the antiviral effects of the majority of nucleoside phosphonates consists of two consecutive phosphorylation steps followed by interaction of the resulting diphosphate metabolite with a target replication enzyme and incorporation into viral DNA, which eventually induce absolute viral DNA chain termination. In contrast, alpha-carboxynucleoside phosphonates (α-CNPs) are a class of structural mimics of natural 2′-deoxynucleotide 5′-triphosphates that were conceived to enable direct inhibition of viral DNA polymerases without the need for prior metabolic activation. This concept is best illustrated by cyclopentyl α-CNPs containing either a pyrimidine or purine base (43a–e, Figure 4), whose ability to be efficiently recognized and strongly inhibit HIV-1 reverse transcriptase (IC50 = 0.19–4.3 μM) was demonstrated in a cell-free HIV-1-RT assay (Keane et al., 2015; Balzarini et al., 2017). Both the phosphonate and carboxyl groups were found to be required for activity, which resided in the L-enantiomer of the compounds.
Structural studies aimed to disclose the molecular interactions between α-CNPs and the target enzyme revealed that these compounds bound in the HIV reverse transcriptase binding site in a nucleobase-specific manner competing with an incoming dNTP, but did not undergo incorporation into a growing viral DNA chain (Balzarini et al., 2015) No antiviral activity was observed in cell culture for any of these compounds; this is most likely due to an inefficient uptake by virus-infected cells as a consequence of the presence of a strong negative charge (Keane et al., 2015). It is interesting to note that the substitution of the cyclopentane ring with a flexible acyclic butenyl moiety shifted the selectivity spectrum of α-CNPs from HIV- toward herpesvirus-encoded DNA polymerases (John et al., 2015).
The clinical indication of nucleoside phosphonates is not restricted to the treatment of viral infections, but also include, albeit to a comparably much less exploited extent, anticancer chemotherapy. Preclinical studies in a number of murine models demonstrated the additional potential of ANP antiviral therapeutics (S)-HPMPC (1a) and PMEA (18a) to induce widespread apoptosis or to inhibit the growth of choriocarcinoma (Hatse et al., 1998), nasopharyngeal (Neyts et al., 1998), and cervical (Andrei et al., 1998b) carcinoma, as well as vascular tumors (Liekens et al., 1998, 2001). However, these compounds were not further pursued for clinical development against cancer.
On the other hand, the guanine congener of PMEA, i.e., PMEG (44, Figure 4), exhibited a remarkably potent in vitro cytostatic and antiproliferative activity against leukemic and solid tumor cell lines (Paborsky et al., 1997; Franek et al., 1999) including certain types of HPV-transformed cancer cells (Andrei et al., 1998a), which translated in a unique therapeutic profile. Its antitumor activity was confirmed in vivo using transplantable murine tumor models of lymphocytic leukemia (P388) and melanoma (B16) (Rose et al., 1990). In addition, PMEG effectively suppressed the growth of proliferative lesions induced by cotton tail papillomavirus (CRPV) and human papillomavirus type 11 (HPV-11) infections of rabbits and human foreskin xenografts in athymic mice, respectively (Kreider et al., 1990). However, the narrow safety margins observed upon exposure to 44 (Kreider et al., 1990) and high prevalence of kidney and gastrointestinal tract toxicities combined with the typically poor cellular permeability of ANPs posed serious limitations to its practical utility as anticancer agent. Therefore, PMEG was replaced in preclinical studies by its modified 2,6-diaminopurine prodrug N6-cyclopropyl PMEDAP (46, cPrPMEDAP, Figure 4), which proved equally cytostatic in vitro against a variety of tumor cell lines (Hatse et al., 1999), while allowing for higher antitumor efficacy and selectivity in a rat choriocarcinoma tumor model (Naesens et al., 1999). By virtue of its susceptibility to enzymatic deamination, cPr-PMEDAP can in fact undergo intracellular conversion to 44, while minimizing toxicity due to diminished plasma exposure to PMEG. PMEG was shown to act via its diphosphate form PMEGpp formed upon cellular kinase phosphorylation, which was found to be a potent inhibitor of human polymerases α, δ, and ε involved in cellular DNA replication (Kramata et al., 1996). In vitro, PMEGpp was described to be efficiently recognized and incorporated into growing DNA chains by these polymerases (Cihlar and Chen, 1997; Pisarev et al., 1997; Kramata et al., 1998), resulting in termination of DNA synthesis with consequent arrest of cell division in S phase followed by induction of apoptosis. Resistance to both PMEG and cPr-PMEDAP was associated with a decreased capacity of the resistant cells to phosphorylate PMEG upon introduction of point mutations in the enzyme guanylate kinase (Mertlikova-Kaiserova et al., 2011).
The next step in prodrug design was carried out at Gilead Sciences with the synthesis of two bis-phosphonoamidate prodrugs, named GS-9191 (47) and GS-9219 (48, VDC-1101, Tanovea®, generic name: rabacfosadine), in an attempt to balance increased cellular penetration with tissue specific intracellular delivery and metabolic activation.
GS-9191 bearing a highly lipophilic bis(L-phenylalanine isobutylester)amidate moiety was purposefully prepared for topical skin administration with the aim of promoting selective accumulation of the active metabolite PMEGpp in the epithelial layer (Wolfgang et al., 2009). The mechanism of conversion of GS-9191 to PMEGpp was elucidated and found to be initiated in lysosomes by the carboxypeptidase cathepsin A (CatA)-mediated hydrolysis of a carboxylester bond of one of the amidate moieties (Birkus et al., 2011). The unmasked carboxyl group further displaces the L-phenylalanine isobutyl ester of the other amidate moiety, followed by release of cPrPMEDAP from cPrPMEDAP-Phe upon either enzymatic or pH dependent spontaneous cleavage. In subsequent steps, the cPr-PMEDAP metabolite undergoes translocation to the cytosol where further deamination most likely by N6-methyl-AMP aminohydrolase (Schinkmanova et al., 2006) and phosphorylation occur.
GS-9191 exerted a markedly more potent in vitro antiproliferative effect compared to its metabolic products cPrPMEDAP or PMEG against a broad spectrum of cervical carcinoma cell lines transformed by human papillomavirus (HPV) with an EC50 as low as 0.03 nM. Generally, non-HPV-infected and primary cells were less sensitive to the compound with EC50 values ranging between 1 and 15 nM (Wolfgang et al., 2009). In the cottontail rabbit papillomavirus (CRPV) infection model, topical administration of GS-9191 led to a dose-dependent decrease in the size of HPV-induced lesions, with cure achieved at the highest dose (0.1%) after 5 weeks. A topical ointment formulation of GS-9191 proved to be well-tolerated and clinically effective for the treatment of genital warts in a Phase II trial (ClinicalTrials.gov Identifier: NCT00499967, Recruitment status: completed). Graceway Pharmaceuticals, 6 November 2009 posting date. Graceway Pharmaceuticals acquires worldwide rights to GS 9191 from Gilead Sciences. Graceway Pharmaceuticals, Bristol, TN. http://www.gracewaypharma.com/news/0035-graceway-pharmaceuticals-acquires-worldwide-rights-gs-9191-gilead-~sciences).
The relatively more hydrophilic bis(L-alanine ethyl ester)amidate prodrug derivative GS-9219 was proposed to undergo a similar stepwise conversion pathway as GS-9191 leading to the intracellular release of PMEGpp. In this case, the adenosine deaminase like (ADAL) protein was shown to be primarily involved in the deamination step of cPr-PMEDAP to PMEG, since mutations affecting the enzyme activity promoted resistance to both GS-9219 and its cPr-PMEDAP metabolite (Frey et al., 2013).
GS-9219 displayed a preferential in vitro antiproliferative activity against activated lymphocytes and tumor cells of hematopoietic origin (EC50 = 27–1,043 nM) vs. quiescent lymphocytes (EC50 = 17.2 μM) and solid tumor cell lines (EC50 > 10 μM) (Reiser et al., 2008). A substantial in vivo efficacy combined with either no or low-grade adverse events was also shown in a series of preclinical and phase I/II studies in pet dogs with advanced-stage non-Hodgkin's lymphoma (NHL, 79% antitumor response rate) (Reiser et al., 2008; Vail et al., 2009), multiple myeloma (81% antitumor response rate) (Thamm et al., 2014), and cutaneous T-cell lymphoma (45% antitumor response rate) (Morges et al., 2014). Eventually, GS-9219 was licensed for clinical use in the systemic treatment of hematological malignancies in canines, and showed particularly useful in treating naïve multicentric (Thamm et al., 2017) and relapsing B-cell lymphoma (Saba et al., 2018). Based on these encouraging results, GS-9219 was also selected for evaluation in humans, but unfortunately failed to show an acceptable safety profile in a dose-escalating Phase I/II study involving patients with relapsed or refractory non-Hodgkin's lymphoma (NHL), chronic lymphocytic leukemia (CLL), and multiple myeloma (MM) (ClinicalTrials.gov Identifier: NCT00499239; Recruitment status: completed).
Subsequent work included the description of two additional prodrug analogs identified as GS-343074 and GS424044, whose structures have not been disclosed. In contrast to the activity spectrum of GS-9191, these compounds displayed considerable in vitro antiproliferative activity against multiple canine solid cancer cell lines, and seemed to favor a mechanism of action based on a cytotoxic rather than cytostatic effect upon induction of cancer cell apoptosis (Lawrence et al., 2015).
Finally, it should be mentioned that the alkoxyalkyl prodrug approach developed by Hostetler was also applied to PMEG, and ODE-PMEG 45 especially turned out to exert significant antiproliferative activity in vitro against different human cervical carcinoma cell lines (Valiaeva et al., 2010). In a murine model, intratumoral injection of 25 μg of 45 daily for 21 days resulted in near complete disappearance of measurable tumors [vs. 100 μg of ODE-(S)-HPMPC 5b], suggesting that ODE-PMEG may find use for local or topical treatment of cervical dysplasia.
The edema factor (EF) is an important virulence factor from Bacillus anthracis. Adefovir diphosphate is a potent inhibitor of the adenylyl cyclase activity of EF with high affinity (Ki = 27 nM). Another bacterium, Bordetella pertussis, secretes several virulence factors, and among them, CyaA is an adenylyl cyclase toxin (ACT) that is a tight binder of adefovir diphosphate. Based on the adefovir-diphosphate ACT crystal structure, a number of 2-substituted PMEA analogs were prepared and converted to their isopropyl ester bis(L-phenylalanine)bisamidate prodrugs. All the bisamidate prodrugs exhibited overall lower activity than the positive control adefovir dipivoxil when tested for their ability to inhibit ACT activity in macrophages. The 2-fluoro derivative 49 (Figure 5) emerged as the most potent in this series with an IC50 of 0.14 μM for the bacterial AC, whereas it has no effect on mammalian ACs. In addition, this compound lacked cytotoxicity against a panel of cancer cell lines and also did not display any toxicity toward normal cells (Cesnek et al., 2015).
A small series of racemic acyclic nucleoside diphosphophosphonates bearing a 5-chloro-anthraniloyl substituent attached through various linkers to the acyclic chain were prepared (Figure 5) and evaluated as potential inhibitors of the AC activity of the EF from Bacillus anthracis. In this case, compound 50 showed the best inhibitory activity (IC50 = 69 nM), followed by 51 (IC50 = 613 nM) and 52 (IC50 = 140 nM). As these three diphospho-phosphonate analogs are potent inhibitors of AC in a cell-free, biochemical assay, the corresponding lipophilic isopropyl ester bis(L-phenylalanine) prodrugs were synthesized. Only the prodrug 53, derived from the parent compound 51 showed modest inhibition of AC activity in murine macrophages, with an IC50 value of 12 μM, whereas other prodrugs lacked activity.
Hypoxanthine-guanine phosphoribosyltransferase (HGPRT) catalyzes the formation of the 6-oxopurine nucleoside monophosphates, IMP and GMP from 5-phospho-α-D-ribosyl-1-pyrophosphate and hypoxanthine or guanine. It is an important enzyme in the purine salvage pathway and it has been shown to be essential for the growth of Mycobacterium tuberculosis, the causative agent of tuberculosis. A series of various acyclic nucleoside phosphonates with a 6-oxo-purine nucleobase (either guanine or hypoxanthine) was synthesized. The most potent inhibitor was the bisphosphonate analog 54, displaying a Ki value of 0.69 μM (Figure 5). Unfortunately, this compound lacks selectivity, as it is equally active against the human HGPRT. For evaluation in cellular assays, the lipophilic ethyl L-phenylalanine phosphonoamidate prodrug 55 was prepared, that showed anti-tuberculosis activity against a virulent strain of M. tuberculosis (H37Rv), with an IC50 value of 9 μM (Eng et al., 2015).
Pyrrolidine bisphosphonates (Figure 5) with either a hypoxanthine or guanine nucleobase (compounds 56 and 57, respectively) were found to be very potent inhibitors of mycobacterial HGPRT (Ki values for both analogs was 60 nM). Unfortunately, both derivatives are 20- to 60-fold more potent against human HGPRT with a Ki value of 1 and 3 nM, respectively (Eng et al., 2018).
Bacteria, such as E. coli, possess two 6-oxopurine phosphoribosyltransferases (PRTases): a xanthine-guanine phosphoribosyltransferases (XGPRT) and a hypoxanthine PRTase (HPRT). The introduction of a second phosphonate group on a 2-(phosphonoethoxy)ethyl (PEE) side chain yielded compound 58 (Figure 6) with very high affinity for EcXGPRT (Ki = 10 nM). Replacing the guanine nucleobase with hypoxanthine affords the nucleoside phosphonate analog 59 which is a good inhibitor of HPRT (Ki = 0.8 μM). To confer rigidity to the acyclic, flexible linker moiety and possibly tighter binding, cyclic pyrrolidine derivatives of guanine were prepared, from which compound 60 emerged as the most potent congener. In order to mask the negative charges of the phosphonate groups and thus to increase cell permeability, prodrugs were synthesized. It was shown that the phosphonoamidate prodrug 61 arrested the growth of M. tuberculosis in cell culture, with IC50 values of 5 μM, and lacked cytotoxicity against a human lung carcinoma cell line (A549) (Keough et al., 2013a).
There are four Plasmodium species responsible for human malaria: falciparum, vivax, ovale, and malariae. P. falciparum (Pf) is the cause of the most lethal form of malaria. Hypoxanthine-guanine-xanthine phosphoribosyltransferase (HGXPRT) catalyzes the formation of the 6-oxopurine nucleoside monophosphates, inosine monophosphate (IMP), guanosine monophosphate (GMP) and xanthosine monophosphate (XMP), from 5-phospho-α-D-ribosyl-1-pyrophosphate and hypoxanthine, guanine, or xanthine. It is an important enzyme in the purine salvage pathway because parasites of the genus Plasmodium lack the de novo pathway for purine nucleotide biosynthesis.
The first, structurally simple, acyclic nucleoside phosphonates that were identified as inhibitors of PfHGXPRT were 9-[2-(2-phosphonoethoxy)ethyl]guanine 62 (PEEG) and 9-[2-(2-phosphonoethoxy)ethyl]hypoxanthine 63 (PEEHx), that displayed Ki values against PfHGXPRT of 0.1 and 0.3 μM, respectively (Figure 6). In addition, both compounds are selective for the malaria enzyme as the Ki values for the human HGPRT are 10-fold higher (Keough et al., 2009).
The effect of branching of the phosphonoethoxyethyl chain on the inhibition of PfHGXPRT and human HGPRT was studied by adding small alkyl chains either to the carbon attached directly to the phosphorus atom (α-branched derivatives) or to the adjacent carbon (β-branched derivatives). The α-branched derivatives completely lacked HG(X)PRT (human as well as malaria), whereas among the β-substituted derivatives, especially the guanosine congeners are active. The methyl and ethyl substituted analogs (compounds 64 and 65) are endowed with Ki values for PfHGXPRT of 1 and 5 μM, respectively. Remarkably, these β-branched derivatives bind 10 times more strongly to the human enzyme, when compared to the P. falciparum counterpart (Hocková et al., 2009).
The addition of a fluoromethylene group on the PEE linker created 3-fluoro-(2-phosphonoethoxy)propyl derivatives having a chiral center (Figure 6). Both enantiomers were equally active against PfHGXPRT (Ki = 1 μM), although their affinity for the PvHGXPRT and the human HGPRT was different. The (S)-enantiomer 66 showed modest activity for PvHGXPRT (Ki = 3.4 μM) and was quite potent as human HGXPRT inhibitor (Ki = 0.1 μM). The (R)-enantiomer 67 lacked activity for PvHGXPRT (Ki = 29 μM) and was moderately active as human HGPRT inhibitor (Ki = 4.7 μM) (Baszczynski et al., 2013).
(S)-3-Hydroxy-2-(phosphonoethoxy)propyl (HPEP) derivatives with a hypoxanthine and guanine nucleobase (compounds 68 and 69, respectively) were synthesized (Figure 7). Both analogs did inhibit PfHGXPRT (Ki = 2 μM for the hypoxanthine analog 68 and 0.1 μM for the guanine derivative 69), but also affected the human HGPRT (Ki = 0.5 and 0.6 μM, for the hypoxanthine and guanine analog, respectively) (Kaiser et al., 2015). Further SAR studies focused on structural variation of the acyclic linker. Shortening the PEE linker usually gave a decreased activity, although one particular analog, with a two carbon atom linker yielded compound 70, which is only slightly less active as PfHGXPRT inhibitor (Ki = 2.2 μM), but has a diminished selectivity profile (Ki value for the human enzyme = 3.4 μM). Changing the position of the oxygen in the acyclic part of PEEG afforded compound 71 having a Ki of 5 μM vs. PfHGXPRT (Cesnek et al., 2012). A number of derivatives with a 2-hydroxy-3-(phosphonomethoxy)propyl side chain also have been prepared. The hypoxanthine congener 72 inhibited both PfHGXPRT and PvHGPRT with Ki values of 2 and 5 μM, respectively, but did not inhibit the human enzymes at a concentration of 30 μM. In contrast, the guanosine analog 73 had a Ki value of 1.4 and 10 μM for PfHGXPRT and PvHGPRT, respectively, and was equally active against the human enzyme (Ki = 4 μM) (Krečmerová et al., 2012).
Very recently, a series of β-hydroxy-ANPs with different chain lengths was prepared (Figure 7). Among a set of 24 derivatives, a phosphonate with a guanine nucleobase, a n-butyl linker, and a β-hydroxy group with the R-configuration, was the most promising one (compound 74). Remarkably, the free phosphonate displayed potent activity in a cellular assay when tested against P. falciparum infected red blood cells (IC50 = 74 nM). This compound was equally active against multidrug resistant and chloroquine resistant P. falciparum strains. In addition, it is completely devoid of cytotoxicity when tested against a human cancer cell line (K562) (Cheviet et al., 2020).
Guided by available X-ray crystal structures, a new series of ANPs, containing a second phosphonate group, designed to occupy an additional pocket in the enzyme, were prepared (Figure 7). Compound 75 turned out to be a very potent inhibitor of PfHGXPRT (Ki = 0.07 μM) and PvHGPRT (Ki = 0.6 μM). This compound was also endowed with potent inhibition of human HGPRT with a Ki value of 0.03 μM. These bisphosphonates were highly polar and unable to cross the cell membranes. Therefore, a phosphoramidate-type prodrug was synthesized to increase its antimalarial activity. This prodrug 76 did show activity against different P. falciparum strains with IC50 values in the 10 μM range (Keough et al., 2013b). The insertion of an additional methylene linker afforded compound 77, which was a very potent inhibitor of the human enzyme (Ki = 0.006 μM), with still very good affinity for PfHGXPRT (0.07 μM). The corresponding Protide 78 was able to inhibit the growth of P. falciparum (IC50 between 5 and 7 μM) and was devoid of cytotoxicity.
In an effort to increase affinity and/or selectivity for PfHGXPRT and PvHGPRT vs. human HGPRT, the oxygen in the phosphonate side chain was replaced by a nitrogen atom, yielding an aza-ANP (Figure 8). When two phosphonate groups were connected via an ethyl linker to this central nitrogen, and having a guanosine nucleobase, a potent, but non-selective inhibitor was obtained (compound 79), with Ki values for human HGPRT, PfHGXPRT, and PvHGPRT of 0.2, 0.3, and 0.9 μM, respectively.
Replacing one of the phosphonate group by a carboxylic acid as an alternative ionizable moiety yielded compound 80 which was especially active against PvHGPRT, having a Ki value of 50 nM. A number of hypoxanthine based analogs, either having a terminal carboxylic acid (compound 81) or carboxylic acid methyl ester group (compound 82), were discovered that show promising selectivity for PfHGXPRT (Ki values of 0.4 and 0.1 μM, respectively), lacking activity for the human counterpart (Ki > 200 μM) (Hockova et al., 2012). A prodrug of 82 (compound 83) was able to arrest the growth of P. falciparum in cell culture and did not display cytotoxicity when evaluated against different human cancer cell lines (Hockova et al., 2015).
In a follow-up study, a new series of aza-ANPs with a central nitrogen atom substituted with two different phosphonate containing side chains, was prepared (Figure 8). The guanine based analog 84 was a potent compound with Ki values of 0.19 and 0.03 μM against PfHGXPRT and PvHGPRT, respectively. When 8-bromoguanine was installed as nucleobase, a more potent compound 85 was obtained with a Ki value of 0.08 μM (PfHGXPRT) and 0.01 μM (PvHGPRT). Unfortunately, both derivatives also displayed potent inhibition of the human HGXPRT with Ki values of 0.08 and 0.04 μM, for the guanine and 8-bromo-guanine congener, respectively. A phosphoramidate prodrug 86 was able to suppress P. falciparum replication in cultured erythrocytes (IC50 = 6 μM), and lacked cytotoxicity against human cells (Keough et al., 2015).
The conformational lock of the nitrogen-containing side chain was achieved by the synthesis of a series of pyrrolidine phosphonate analogs (Figure 8). The limited SAR demonstrated that the stereochemistry of the carbon atom bearing the phosphonomethoxy group and the nature of the nucleobase determines HGPRT inhibition. Compound 87 was the most potent with a Ki value of 0.6 μM (P. falciparum). Importantly, this compound did not have any affinity for the human enzyme (Pohl et al., 2015). A novel series of pyrrolidine nucleoside bisphosphonates (PNBPs) was recently published (exemplified by compound 88) with low nM activity for the various HGPRT enzymes (Ki values of 6, 2, and 1 nM for the PvHGPRT, PfHGXPRT, and human HGPRT, respectively) (Keough et al., 2018).
Structurally similar to the azaANPS are the acyclic immucillin phosphonates (AIPs). A typical structure feature of this compound family is the presence of 9-deaza-hypoxanthine as nucleobase and hence these compounds can be considered as acyclic C-nucleoside phosphonates. Compounds 89 and 90 are both competitive inhibitors of PfHGXPRT with Ki values of 10.6 and 0.65 nM, respectively. Both analogs displayed much lower affinities for the human enzyme with Ki values of 4,940 and 385 nM. Hence, these AIPs are more than 400-fold selective for the parasite enzyme. As these free phosphonates did not show activity against cultured parasites, a series of lysophospholipid prodrugs were synthesized from which analog 91 was the most potent one, as it inhibited the growth of various P. falciparum strains with an IC50 in the 2 μM range (Hazleton et al., 2012; Clinch et al., 2013).
The introduction of rigidity into the acyclic chain of AIPs was achieved by the insertion of heterocyclic structures between the 9-deazahypoxanthine group and the phosphonate moiety (Figure 9). Among a series of 18 compounds, triazole derivative 92 was the most potent with Ki values of 2 and 1 μM for PfHGXPRT and PvHGPRT, respectively, and no inhibition of the human enzyme was observed at 71 μM (Kaiser et al., 2017).
Recently, thia-ANPs were described containing a sulfur in various oxidation states (sulfide, sulfoxide, and sulfone) and at different positions of the acyclic chain (Figure 9). The most potent congeners from this series (compounds 93 and 94) are the direct counterparts from PEEG and PEEHx, respectively, with similar binding data to PfHGXPRT. The corresponding phosphoramidate prodrugs 95 and 96 do show antimalaria effects in cell culture with IC50 values in the 4–6 μM range.
Extracellular nucleotides function as signaling molecules through the activation of nucleotide receptors. These receptors are referred to as purinergic P2 receptors. In contrast to P1 receptors, which are activated by the nucleoside adenosine, P2 receptors are activated by nucleotides. On the basis of their signaling properties, P2 receptors can be further subdivided into metabotropic P2Y receptors that belong to the rhodopsin-like branch of family A G-protein coupled receptors, and ionotropic P2X receptors that are nucleotide-gated ion channels.
Two subfamilies are distinguished within the P2Y receptors. P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11 receptors are referred to as the P2Y1-like subfamily, whereas the P2Y12, P2Y13, and P2Y14Rs belong to the P2Y12-like subfamily. The P2Y receptor family is activated by either or both adenine and uracil nucleotides. Adenosine-5′-diphosphate (ADP) is the preferred natural agonist of P2Y1, P2Y12, and P2Y13 receptors, whereas uridine-5′-diphosphate (UDP) is the physiological agonist of P2Y6 and P2Y14. Other P2 receptors are preferentially activated by nucleoside-5′-triphosphates. Adenosine-5′-triphosphate (ATP) binds and activates P2Y2, P2Y4, and P2Y11, whereas uridine-5′-triphosphate (UTP) functions as the natural agonist of P2Y2 and P2Y4 receptors. P2X receptors are further subdivided into P2X1-7 receptors with the natural, endogenous agonist of these P2X receptors being ATP.
The natural ligands of the P2 receptors are highly charged nucleoside phosphates. Although these nucleotides are usually hydrolytically stable in the pH range of about 4–11, they are metabolically labile, as they are rapidly degraded in biological environment by enzymatic hydrolysis. Therefore, besides structural modification of the natural nucleoside-di-, and triphosphates, medicinal chemistry programs focused on the synthesis of nucleoside phosphonates as metabolically stable analogs of the natural agonists.
P2X receptors are ion channels that are permeable for various cations (sodium, potassium and calcium). Since these receptors are activated by ATP, most P2X receptor agonists are based on structural variation of ATP. Analogs in which methylene groups replaced oxygen atoms of the phosphate chain of ATP are known to act as P2X agonists (Figure 10). α,β-Methylene-adenosine-triphosphate (compound 97) acts preferentially on P2X1 and P2X3 receptors, with somewhat lower potency at the P2X4 receptor. β,γ-methylene-ATP (compound 98) was the most potent at the P2X1 receptor, lacking activity for other P2X receptors. Although agonists, as well as antagonists, based on different types of chemistry are known for various other P2X receptors, none of them is based on a nucleoside phosphonate scaffold.
A few acyclic nucleoside phosphonates were found to act as P2Y1 antagonists (Figure 10). MRS2496 (compound 99) acted as an inhibitor of ADP-induced activation of rat platelets with a Ki value of 0.68 μM. The longer chain homolog 100 was much less active (Ki = 25 μM), indicating a crucial role of the spacer length (Xu et al., 2002). Later on, it was demonstrated that MRS2496 had strong binding affinity for the P2Y1 receptor as measured by a radioligand-based binding assay, with a Ki value of 0.76 μM and that it also inhibited the ADP-induced aggregation of human platelets with an IC50 values of 62.8 nM (Cattaneo et al., 2004).
Several 2-thiomethyl ATP analogs were synthesized as P2Y1 agonists (Figure 10) (Eliahu et al., 2009). The most potent analog within this series carried a β,γ-methylene bridge (compound 101) and had an EC50 value of 80 nM. The corresponding difluoromethylene derivative (compound 102) was 10-fold less active as P2Y1 receptor agonist (EC50 = 0.76 μM), whereas the dichloromethylene analog (compound 103) was 100-fold less active (EC50 = 9.54 μM). Replacing the non-bridging oxygen on the phosphorus with BH3 created an additional chiral center yielding compound and the diastereomers were separated. In case of the difluoromethylene analogs (compound 104), one isomer lacked activity, whereas the corresponding diastereomer was modestly active as P2Y1 receptor agonist (EC50 = 2.13 μM). For the 2-thiomethyl-α-borano-β,γ-dichloromethylene ATP analog (compound 105), one diastereomer displayed quite potent P2Y1R agonism (EC50 = 0.57 μM), with the other diastereomer being 2-fold less active (EC50 = 1.2 μM). As 2-thiomethyl ADP was a selective and highly potent P2Y1 receptor agonist, suffering from low chemical and enzymatic stability, the α,β-bridging oxygen was replaced with chemical and metabolically stable dihalomethylene moieties. The difluoro analog (compound 106) was more potent than the dichloro counterpart (compound 107) with EC50 values of 0.98 and 3.10 μM, respectively (Eliahu et al., 2010).
Quite a number of P2Y2 agonists are known in literature, and most of them are derived from the natural agonists ATP and UTP. Most medicinal chemistry campaigns focused on UTP as lead structures due to the expected higher selectivity of pyrimidine nucleotides vs. the other P2 receptor subtypes that are activated by the adenine nucleotides.
β,γ-methylene UTP 108 showed weak activity as P2Y2 agonist (EC50 = 73 μM), although the corresponding β,γ-difluoromethylene analog 109 displayed an improved activity as P2Y2 agonist (EC50 = 4.9 μM), and especially the dichloromethylene congener showed promising P2Y2 agonism (EC50 = 0.612 μM). This dihalo modification was combined with 5-bromo-uracil as nucleobase, yielding the β,γ-dichloromethylene-5-bromo-UTP analog 110, that was endowed with an EC50 value of 0.354 μM (Figure 10) (El-Tayeb et al., 2006).
As it was well-known that substitution of the 2-oxo group by a 2-thio functionality enhanced the affinity of UTP for the P2Y2 receptor, this structural modification has been combined with the introduction of phosphonate groups. 2-Thio-β,γ-difluoromethylene-UTP (compound 111) and 2-thio-β,γ-dichloromethylene-UTP (compound 112) are both endowed with low μM agonistic activity when tested against the P2Y2 receptor (EC50 values of 1.63 and 2.51 μM, respectively) (Ko et al., 2008). As an expansion of the SAR study, the dichloromethylene and difluoromethylene modification was combined with a 4-thio-uridine nucleobase, affording compounds 113 and 114, that exhibited EC50 values of 1.81 and 0.134 μM, respectively (El-Tayeb et al., 2011).
Uridine-5′-(diphospho)-phosphonate 115 and uridine 5′-(diphospho)-vinylphosphonate 116 behaved as full agonists for the P2Y2 receptor, although the potencies were reduced by a factor 16–76, when compared to the natural ligand UTP (Figure 11). On the other hand, uridine 5′-phospho-phosphonate 117 and uridine 5′-methylene-phosphonate 118 both acted as partial agonists, with EC50 values of 3.4 and 1.6 μM, respectively (Cosyn et al., 2009). The 5′-methylene derivative 118 was selected as lead structure for further SAR exploration. It led to the discovery of a 5-(4-fluorophenyl) modified uridine 5′-phosphonate derivative 119, that was a P2Y2 receptor agonist (EC50 = 0.4 μM), although the maximal effect observed was only 43% of that observed with UTP. Additional pharmacological profiling suggested that this compound activated the P2Y2 receptor in a allosteric manner (van Poecke et al., 2012).
Phosphonate analogs were also prepared as analogs of dinucleoside tetraphosphate derivatives (Figure 11). The insertion of a β,γ-difluoro- or β,γ-dichloro-methylene linker afforded compounds that were less active at the P2Y2 receptor when compared with the tetraphosphate analog (EC50 = 0.21 μM). The difluoro analog 120 (EC50 = 2.27 μM) was more potent than the dichloro analog 121 (EC50 = 7.77 μM), suggesting that it is a better mimic of the phosphate ester bond (Ko et al., 2008).
A series of analogs in which the ribose moiety of the natural uracil nucleotides was replaced by various acyclic ribose-mimetic structures was also prepared (Figure 11). These acyclic nucleotide analogs were devoid of any agonistic activity at the P2Y2 receptor, but, in contrast, behaved as weak antagonist with an IC50 value of 92 μM for compound 122 (Sauer et al., 2009).
UTP analogs in which the phosphorus-β,γ-oxygen bridge was replaced by a dihalomethylene moiety were evaluated for P2Y6 agonism. β,γ-dichloromethylene-UTP showed an EC50 value of 6.2 μM at the P2Y6 receptor, but was more potent at the P2Y2 receptors (EC50 = 0.612 μM). On the other hand, this compound showed very high selectivity vs. the P2Y4 receptor (EC50 > 100 μM). The closely related β,γ-difluoromethylene-UTP analog (compound 109) is 2-fold more potent at the P2Y6 receptor (EC50 = 2.63 μM), than at the P2Y2 receptor (EC50 = 4.9 μM), whereas it completely lacked agonistic activity at the P2Y4 receptor (EC50 > 100 μM), similarly as the dichloromethylene analog.
Based on these data, these phosphonate modifications were combined with structural variations of the uracil nucleobase. 5-Bromo-β,γ-dichloromethylene-UTP 110 was discovered to be a full agonist at the P2Y6 receptor, with an EC50 value of 120 nM (El-Tayeb et al., 2006). This UTP analog shows some selectivity for the P2Y6 receptor, as it is 33- and 3-fold less active against the P2Y4 and P2Y2 receptors, respectively. 2-Thio-β,γ-dichloromethylene-UTP 112 showed moderate potency at the P2Y6 (EC50 = 7.7 μM) and at the P2Y4 receptor (EC50 = 3.85 μM), but is especially attractive as a P2Y2 agonist (EC50 = 0.794 μM) (El-Tayeb et al., 2011).
The introduction of an α,β-methylene substituent into the natural P2Y6 agonist UDP 123 (EC50 = 0.30 μM) yielded analog 124 that was embarked with only a slight decrease in P2Y6 activity (EC50 = 0.66 μM). The corresponding 5-iodo congener 125 has a similar agonistic activity on the P2Y6 receptor (EC50 = 0.13 μM). Strikingly, the α,β-difluoromethylene analog 126 was completely inactive (Ko et al., 2008; Maruoka et al., 2010). The potency-enhancing 4-methoxyimino modification was combined with an α,β-methylene substitution yielding analog 127 which promising activity as a selective P2Y6R agonist (EC50 = 0.678 μM), lacking activity for the P2Y4 and P2Y2 receptors (EC50 > 10 μM).
A number of nucleoside triphosphate mimics containing a dichloromethylene linker between the β- and γ-phosphate group and an n-propyl-thio substituent at position 2 of the adenine nucleobase were prepared (Figure 11). AR-C67085 (compound 128) lacked antagonistic activity against the P2Y11 receptor, but was a potent agonist of the P2Y11 receptor. The EC50 values of AR-C67085 for IP3 and cyclic AMP accumulation were respectively 8.9 and 1.5 μM, as compared to respectively 72 and 17.4 μM for the natural agonist ATP (Communi et al., 1999).
The presence of a thio-group at the α-phosphate position yielded a chiral center (compound 129). The Sp diastereomer displayed an EC50 of 0.6 μM, whereas an EC50 of 0.85 μM was found for the Rp isomer.
Substitution of the non-bridging oxygen atom of the α-phosphate group by a borano group yielded compound 130. The EC50 values of 0.03 μM for the Sp isomer and 0.1 μM for the Rp diastereomer indicated a diastereoselective preference of the P2Y11 receptor (Haas et al., 2013).
Among the P2 receptors, P2Y12 has been studied most extensively, because of its proven value as drug target for the development of antithrombotic drugs. AR-C66096 (compound 131) and AR-C67085 (compound 128) are both ATP mimics with a dihalomethylene bridged 5′-triphosphate moiety and a thiopropyl group at position 2 of the adenine ring. Both analogs inhibit the ADP induced aggregation of blood platelets, which is consistent with P2Y12 antagonism. A structurally related analog (compound 132, AR-C69931, Cangrelor) received marketing approval as an intravenous P2Y12 inhibitor for the treatment of thrombosis.
Replacement of the bridging oxygen of the diphosphate group of UDP (compound 123) with a methylene (compound 124) or difluoromethylene group (compound 126) was well-tolerated for agonistic activity at the P2Y14 receptor, resulting in high potency compounds endowed with EC50 values of 11 and 63 nM, respectively. Both compounds also show good activity at the P2Y6 receptor. Combining a carbon bridge with a known potency-enhancing uracil modification resulted in in 2-thio-α,β-methylene analog (compound 133) that displayed excellent potency at the P2Y14 receptor with an EC50 value of 0.92 nM (Figure 11). Combining the α,β-difluoromethylene and 5-iodo-uracil moiety afforded compound 134 with good agonistic activity at the P2Y14 receptor (EC50 = 142 nM) (Das et al., 2010).
Nucleotide signaling through P2X and P2Y receptors is predominantly modulated through the hydrolysis of the extracellular nucleotides by plasma membrane bound ecto-nucleotidases that have an extracellular active site. The ecto-nucleotidases are divided in four classes. The first family include the ectonucleoside triphosphate diphosphohydrolases (E-NTPDases). The E-NTPDases are nucleotide-specific and hydrolyze nucleoside triphosphates and diphosphates yielding the corresponding nucleoside monophosphates. Eight isoenzymes (NTPDase1-8) have been identified in mammals and they represent the major nucleotide-hydrolyzing enzymes involved in purinergic signaling. The second group is an ecto-5′-nucleotidase, also known as CD73, which hydrolyzes AMP to adenosine. Nucleotide pyrophosphatase/phosphodiesterase (NPP) enzymes constitute the third class. The seven isozymes of this family are capable of hydrolyzing phosphate esters of different substrates ranging from nucleotides to lipids. Among these isozymes, NPP1, NPP3, NPP4, and NPP5 are the only isozymes that act on nucleotide substrates. Finally, alkaline phosphatases finally hydrolyze nucleoside tri-, di-, and monophosphates, pyrophosphate and a large variety of additional monoesters of phosphoric acid. In this review, only ecto-nucleotidases for which inhibitors based on a nucleoside phosphonate scaffold are known are discussed.
6-N,N-diethyl-β,γ-dibromomethylene adenosine triphosphate (also known as ARL 67156, compound 135) is a weak inhibitor of NTPDase1 (CD39) and NTPDase3, with Ki values of 11 and 18 μM, respectively (Figure 12) (Levesque et al., 2007). In an effort to obtain novel, drug-like NTPDase 2 inhibitors, the triphosphate group of the natural nucleoside triphosphates was replaced by a phosphonic acid diester moiety linked via an amide bond and a linker to the nucleoside core. The most potent compounds that emerged from this series (compound 136) was endowed with a Ki value of 8.2 μM for NTPDase2, and lacked activity against NTPDase1 and 3.
Although several non-nucleotide NPP inhibitors are known in literature, the best studied inhibitors of NPP1 are substrate analogs (Figure 12) (Lee and Muller, 2017). Adenosine 5′-(α,β-methylene)diphosphate (compound 137) and adenosine 5′-(α,β-methylene)triphosphate (compound 138) are both weak inhibitors of NPP1 (Ki values of 16.5 and 3.32 μM, respectively, when evaluated against ATP as the natural substrate) (Lee et al., 2017). Because of the presence of zinc ions in the active site of NPP1, a number of thiophosphate was prepared as sulfur is known to have a strong affinity for this metal. The introduction of a sulfur atom instead of an oxygen at the terminal phosphate moiety afforded compound 139, which showed potent inhibition of the human membrane-bound NPP1 with a Ki value in the low nanomolar range (Ki = 0.02 μM), using p-nitrophenyl-5′-thymidine monophosphate (p-Nph-5′-TMP) as artificial substrate (Nadel et al., 2014). In addition to the insertion of metabolically stable methylene moieties, a borano group was introduced into the α-phosphate, yielding compound 140, which, was found to inhibit human membrane-associated NPP1 with a Ki of 0.5 μM, when tested vs. p-Nph-5′-TMP as a substrate (Lecka et al., 2013). In addition, this compound has a promising selectivity profile as it lacked activity against various other ectonucleotidases (NPP3, NTPDase1-3). The corresponding dichloro-β,γ-methylene derivative 141 showed a reduced inhibitory potency (Ki = 18 μM). The insertion of a dichloromethylene linker between the β- and γ-phosphate moiety combined with a thiophosphate group yielded compound 142, displaying a Ki value of 0.68 μM (Nadel et al., 2014).
Besides its inhibition toward NTPDase 1 and 3, 6-N,N-diethyl-D-β,γ-dibromomethylene ATP (ARL 67156, compound 135) is also a weak inhibitor of human NPP1 (Ki = 12 μM against the artificial substrate p-NO2-Ph-TMP) (Levesque et al., 2007).
In order to study the SAR of UTP analogs as NPP-1 inhibitors, both Pα non-bridging oxygen atoms of UTP were replaced by sulfur and a methylene linker was inserted yielded compound 143 that was endowed with a NPP1 Ki value of 1.11 μM (Figure 12) (Zelikman et al., 2018). In addition, a series of acyclic phosphonates, derived from UTP, was prepared (Nassir et al., 2019b). The acyclic UDP analogs with an α,β-bridging methylene or dichloromethylene group (compounds 144 and 145) displayed only moderate inhibitory effect on human NPP1 (54 and 51% inhibition at 100 μM, respectively). Dithio substitution on the Pα-phosphate turned these analogs into more potent NPP1 inhibitors with Ki values of 0.94 and 0.73 μM, for the methylene (compound 146) and dichloromethylene (compound 147) derivative, respectively.
Similarly, a series of acyclic nucleoside phosphonates carrying adenine as nucleobase was synthesized (Figure 12). Adenine-N9-(methoxy)-ethyl-β-bisphosphonate 148 was a dual NPP1/CD73 inhibitor (Ki values of 9.6 and 12.6 μM, respectively, when tested vs. the natural substrate ATP) and lacked activity against NPP3, NTPDase1, and tissue non-specific alkaline phosphatase (TNAP) (Nassir et al., 2019a). The analogs in which the α-phosphate groups was replaced by a dithiophosphate group only showed modest NPP1 inhibition, with IC50 values of 111 and 25.9 μM, for the methylene (compound 149) and dichloromethylene (compound 150) derivative, respectively, when tested vs. natural ATP as the substrate.
Ecto-5′-nucleotidase, also known as CD73, is an ecto-5′-nucleotidase which hydrolyzes AMP to adenosine and is considered as a promising drug target for immune-oncology. The most potent CD73 inhibitors are all nucleoside-5′-diphosphate analogs (Figure 13). The ADP analog 137 is endowed with a Ki value of 88 nM for human CD73. Structural modification of the nucleobase allowed to further increase the potency. A benzyladenosine analog (compound 151) showed a Ki value of 2.21 nM and has a promising selectivity profile as it had no activity on any other ectonucleotidases (Bhattarai et al., 2015). Structural modification at position 2 of the nucleobase was tolerated as the 2-iodo and 2-chloro-adenosine analogs (compounds 152 and 153) were potent CD73 inhibitors, displaying Ki values of 3 and 5 nM, respectively. In addition, it was demonstrated that the 2-iodo congener was metabolically stable in the presence of human plasma and rat liver microsomes (Bhattarai et al., 2020). Combination of a 2-chloro substituent with a N-Me-benzyl group furnished compound 154, a very potent CD73 inhibitor (Ki = 0.318 nM) (Bhattarai et al., 2019). Nucleotide analogs with a nucleobase that deviate more from the natural adenine base are also known. An example includes compound 155, that had a pyrazolo[3,4-b]pyridine nucleobase. The presence of a 2-chloro and a 6-benzylamino substituent made this compound a very potent CD73 inhibitor, displaying a Ki value of 0.005 nM (Bowman et al., 2019). Besides purine nucleoside phosphonates, pyrimidine nucleoside phosphonates, are also known to act as potent CD73 inhibitors. The synthesis of a series of N4-substituted-cytosine derivatives afforded potent CD73 inhibitors. Especially analogs carrying a bulky benzyloxy substituent (as exemplified by compound 156) were potent and selective CD73 inhibitors (Ki = 7.96 nM).
The heterogeneous key roles played by naturally occurring nucleoside phosphates in numerous biochemical processes has stimulated medicinal chemists to design nucleoside phosphonates as their isosteric and isopolar counterparts with increased resistance toward in vivo enzymatic degradation. Over the past 40 years, this concept has been the subject of extensive synthetic efforts that resulted in the marketing of a limited number of nucleoside phosphonate drugs. The most noteworthy examples are cidofovir, adefovir, and tenofovir that received approval for the clinical treatment of different viral infections. In addition, a variety of other nucleoside phosphonate analogs, both cyclic as well as acyclic, with excellent activity against retroviruses and/or DNA viruses have been described in the literature. However, an in-depth, side-by-side comparison with the existing agents is required to identify competitive advantages in terms of efficacy, toxicity, and/or resistance profile in order to justify their further development as antiviral drugs. Nucleoside phosphonate analogs with potent activity against RNA viruses remain elusive up to now. As current outbreaks of infectious diseases are mainly caused by RNA viruses, such as the Ebola virus (EBOV) and severe acute respiratory syndrome coronavirus 2 (SARS-CoV2), there is a huge interest in the discovery of broad-spectrum antiviral agents based on a nucleoside phosphonate scaffold that may be active against various RNA viruses.
The application of nucleoside phosphonate chemistry in other therapeutic areas has been lagging behind in comparison to the antiviral scene. Outside the antiviral area, only two nucleoside phosphonates were licensed for medical use. Specifically, Rabacfosadine received marketing approval by the FDA for the treatment of lymphoma in dogs, whereas Cangrelor was licensed for clinical use as an antiplatelet drug.
The main drawbacks associated with nucleoside phosphonates are their high polarity and consequentially limited cellular permeability as well as poor oral bioavailability. In the antiviral/antitumoral area, these hurdles were overcome with the synthesis of a variety of prodrugs. Similar promoieties were used to derivatize nucleoside phosphonate analogs that showed promising biochemical potency against isolated bacterial or parasitic targets. Penetration of small molecules through the bacterial membrane (especially for Gram-negative pathogens) remains in fact a major challenge in antibacterial drug discovery. However, it is likely that prodrugs that are optimized for antiviral and antitumoral drug discovery are not optimal for antibiotics, therefore highlighting the need for the development of new prodrug strategies specifically designed for bacterial drug delivery.
Several nucleoside phosphonates acting as agonists/antagonists of various purinergic receptors or ecto-nucleotidase inhibitors are known; however, only a few showed potent activity. In addition, selectivity between the different receptors and/or enzymes is still an issue for several of these compounds. Therefore, an expanded chemical space needs to be explored to gain access to analogs with potency in the nanomolar range and an acceptable selectivity window before these compounds can be used as pharmacological tools. Moreover, prior to use these compounds in preclinical animal models, they will have to be converted to a suitable prodrug.
Nonetheless, the continuously increasing awareness of the most influential factors that needs to be taken into consideration in this research field can be reasonably expected to sustain the future translation of fundamental research into new nucleoside phosphonate drugs.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ahn, S. H., Kim, W., Jung, Y. K., Yang, J. M., Jang, J. Y., Kweon, Y. O., et al. (2019). Efficacy and safety of besifovir dipivoxil maleate compared with tenofovir disoproxil fumarate in treatment of chronic hepatitis B virus infection. Clin. Gastroenterol. Hepatol. 17, 1850–1859. doi: 10.1016/j.cgh.2018.11.001
Aldern, K. A., Ciesla, S. L., Winegarden, K. L., and Hostetler, K. Y. (2003). Increased antiviral activity of 1-O-hexadecyloxypropyl-2-C-14 cidofovir in MRC-5 human lung fibroblasts is explained by unique cellular uptake and metabolism. Mol. Pharmacol. 63, 678–681. doi: 10.1124/mol.63.3.678
Andrei, G., Snoeck, R., Piette, J., Delvenne, P., and de Clercq, E. (1998a). Antiproliferative effects of acyclic nucleoside phosphonates on human papillomavirus (HPV)-harboring cell lines compared with HPV-negative cell lines. Oncol. Res. 10, 523–531.
Andrei, G., Snoeck, R., Piette, J., Delvenne, P., and de Clercq, E. (1998b). Inhibiting effects of cidofovir (HPMPC) on the growth of the human cervical carcinoma (SiHa) xenografts in athymic nude mice. Oncol. Res. 10, 533–539.
Andrei, G., Snoeck, R., Schols, D., Goubau, P., Desmyter, J., and Declercq, E. (1991). Comparative activity of selected antiviral compounds against clinical isolates of human cytomegalovirus. Eur. J. Clin. Microbiol. Infect. Dis. 10, 1026–1033. doi: 10.1007/BF01984924
Balzarini, J., Das, K., Bernatchez, J. A., Martinez, S. E., Ngure, M., Keane, S., et al. (2015). Alpha-carboxy nucleoside phosphonates as universal nucleoside triphosphate mimics. Proc. Natl. Acad. Sci. U. S. A. 112, 3475–3480. doi: 10.1073/pnas.1420233112
Balzarini, J., Holy, A., Jindrich, J., Naesens, L., Snoeck, R., Schols, D., et al. (1993). Differential antiherpesvirus and antiretrovirus effects of the (S) and (R) enantiomers of acyclic nucleoside phosphonates–potent and selective in vitro and in vivo antiretrovirus activities of (R)-9-(2-phosphonomethoxypropyl)-2,6-diaminopurine. Antimicrob. Agents Chemother. 37, 332–338. doi: 10.1128/AAC.37.2.332
Balzarini, J., Menni, M., Das, K., van Berckelaer, L., Ford, A., Maguire, N. M., et al. (2017). Guanine alpha-carboxy nucleoside phosphonate (G-alpha-CNP) shows a different inhibitory kinetic profile against the DNA polymerases of human immunodeficiency virus (HIV) and herpes viruses. Biochem. Pharmacol. 136, 51–61. doi: 10.1016/j.bcp.2017.04.001
Balzarini, J., Pannecouque, C., de Clercq, E., Aquaro, S., Perno, C. F., Egberink, H., et al. (2002). Antiretrovirus activity of a novel class of acyclic pyrimidine nucleoside phosphonates. Antimicrob. Agents Chemother. 46, 2185–2193. doi: 10.1128/AAC.46.7.2185-2193.2002
Balzarini, J., Pannecouque, C., Naesens, L., Andrei, G., Snoeck, R., de Clercq, E., et al. (2004). 6-2-Phosphonomethoxy)alkoxy-2,4-diaminopyrimidines: a new class of acyclic pyrimidine nucleoside phosphonates with antiviral activity. Nucleosides Nucleotides Nucleic Acids 23, 1321–1327. doi: 10.1081/NCN-200027573
Balzarini, J., Schols, D., van Laethem, K., de Clercq, E., Hockova, D., Masojidkova, M., et al. (2007). Pronounced in vitro and in vivo antiretroviral activity of 5-substituted 2,4-diamino-6-2-(phosphonomethoxy)ethoxy pyrimidines. J. Antimicrob. Chemother. 59, 80–86. doi: 10.1093/jac/dkl454
Baszczynski, O., Hocková, D., Janeba, Z., Holý, A., Jansa, P., Dračínský, M., et al. (2013). The effect of novel [3-fluoro-(2-phosphonoethoxy)propyl]purines on the inhibition of Plasmodium falciparum, Plasmodium vivax and human hypoxanthine–guanine–(xanthine) phosphoribosyltransferases. Eur. J. Med. Chem. 67, 81–89. doi: 10.1016/j.ejmech.2013.06.032
Baszczynski, O., and Janeba, Z. (2013). Medicinal chemistry of fluorinated cyclic and acyclic nucleoside phosphonates. Med. Res. Rev. 33, 1304–1344. doi: 10.1002/med.21296
Beadle, J. R., Hartline, C., Aldern, K. A., Rodriguez, N., Harden, E., Kern, E. R., et al. (2002). Alkoxyalkyl esters of cidofovir and cyclic cidofovir exhibit multiple-log enhancement of antiviral activity against cytomegalovirus and herpesvirus replication in vitro. Antimicrob. Agents Chemother. 46, 2381–2386. doi: 10.1128/AAC.46.8.2381-2386.2002
Beadle, J. R., Wan, W. B., Ciesla, S. L., Keith, K. A., Hartline, C., Kern, E. R., et al. (2006). Synthesis and antiviral evaluation of alkoxyalkyl derivatives of 9-(S)-(3-hydroxy-2-phosphonomethoxypropyl)adenine against cytomegalovirus and orthopoxviruses. J. Med. Chem. 49, 2010–2015. doi: 10.1021/jm050473m
Benzaria, S., Pelicano, H., Johnson, R., Maury, G., Imbach, J. L., Aubertin, A. M., et al. (1996). Synthesis, in vitro antiviral evaluation, and stability studies of bis(S-acyl-2-thioethyl) ester derivatives of 9-2-(phosphonomethoxy)ethyl adenine (PMEA) as potential PMEA prodrugs with improved oral bioavailability. J. Med. Chem. 39, 4958–4965. doi: 10.1021/jm960289o
Bhattarai, S., Freundlieb, M., Pippel, J., Meyer, A., Abdelrahman, A., Fiene, A., et al. (2015). alpha,beta-Methylene-ADP (AOPCP) derivatives and analogues: development of potent and selective ecto-5′-nucleotidase (CD73) inhibitors. J. Med. Chem. 58, 6248–6263. doi: 10.1021/acs.jmedchem.5b00802
Bhattarai, S., Pippel, J., Meyer, A., Freundlieb, M., Schmies, C., Abdelrahman, A., et al. (2019). X-Ray co-crystal structure guides the way to subnanomolar competitive ecto-5′-nucleotidase (CD73) inhibitors for cancer immunotherapy. Adv. Ther. 2:1900075. doi: 10.1002/adtp.201900075
Bhattarai, S., Pippel, J., Scaletti, E., Idris, R., Freundlieb, M., Rolshoven, G., et al. (2020). 2-Substituted alpha,beta-methylene-ADP derivatives: potent competitive ecto-5′-nucleotidase (CD73) inhibitors with variable binding modes. J. Med. Chem. 63, 2941–2957. doi: 10.1021/acs.jmedchem.9b01611
Birkus, G., Kutty, N., Frey, C. R., Shribata, R., Chou, T. F., Wagner, C., et al. (2011). Role of cathepsin A and lysosomes in the intracellular activation of novel antipapillomavirus agent GS-9191. Antimicrob. Agents Chemother. 55, 2166–2173. doi: 10.1128/AAC.01603-10
Birkus, G., Wang, R., Liu, X. H., Kutty, N., MacArthur, H., Cihlar, T., et al. (2007). Cathepsin A is the major hydrolase catalyzing the intracellular hydrolysis of the antiretroviral nucleotide phosphonoamidate prodrugs GS-7340 and GS-9131. Antimicrob. Agents Chemother. 51, 543–550. doi: 10.1128/AAC.00968-06
Bischofberger, N., Hitchcock, M. J., Chen, M. S., Barkhimer, D. B., Cundy, K. C., Kent, K. M., et al. (1994). 1-[((S)-2-Hydroxy-2-oxo-1,4,2-dioxaphosphorinan-5-yl)methyl] cytosine, an intracellular prodrug for (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine with improved therapeutic index in vivo. Antimicrob. Agents Chemother. 38, 2387–2391. doi: 10.1128/AAC.38.10.2387
Bowman, C. E., da Silva, R. G., Pham, A., and Young, S. W. (2019). An exceptionally potent inhibitor of human CD73. Biochemistry 58, 3331–3334. doi: 10.1021/acs.biochem.9b00448
Cattaneo, M., Lecchi, A., Ohno, M., Joshi, B. V., Besada, P., Tchilibon, S., et al. (2004). Antiaggregatory activity in human platelets of potent antagonists of the P2Y1 receptor. Biochem. Pharmacol. 68, 1995–2002. doi: 10.1016/j.bcp.2004.06.026
Cesnek, M., Hocková, D., Holý, A., Dračínský, M., Baszczynski, O., Jersey, J. D, et al. (2012). Synthesis of 9-phosphonoalkyl and 9-phosphonoalkoxyalkyl purines: Evaluation of their ability to act as inhibitors of Plasmodium falciparum, Plasmodium vivax and human hypoxanthine–guanine–(xanthine) phosphoribosyltransferases. Bioorg. Med. Chem. 20, 1076–1089. doi: 10.1016/j.bmc.2011.11.034
Cesnek, M., Jansa, P., Šmídková, M., Mertlíková-Kaiserová, H., Dračínský, M., Brust, T. F., et al. (2015). Bisamidate prodrugs of 2-substituted 9-[2-(phosphonomethoxy)ethyl]adenine (PMEA, adefovir) as selective inhibitors of adenylate cyclase toxin from Bordetella pertussis. ChemMedChem 10, 1351–1364. doi: 10.1002/cmdc.201500183
Cheviet, T., Wein, S., Bourchenin, G., Lagacherie, M., Périgaud, C., Cerdan, R., et al. (2020). β-Hydroxy- and β-aminophosphonate acyclonucleosides as potent inhibitors of Plasmodium falciparum growth. J. Med. Chem. 63, 8069–8087. doi: 10.1021/acs.jmedchem.0c00131
Choi, J. R., Cho, D. G., Roh, K. Y., Hwang, J. T., Ahn, S., Jang, H. S., et al. (2004). A novel class of phosphonate nucleosides. 9-(1-Phosphonomethoxycyclopropyl)methyl guanine as a potent and selective anti-HBV agent. J. Med. Chem. 47, 2864–2869. doi: 10.1021/jm0305265
Ciesla, S. L., Trahan, J., Wan, W. B., Beadle, J. R., Aldern, K. A., Painter, G. R., et al. (2003). Esterification of cidofovir with alkoxyalkanols increases oral bioavailability and diminishes drug accumulation in kidney. Antivir. Res. 59, 163–171. doi: 10.1016/S0166-3542(03)00110-4
Cihlar, T., and Chen, M. S. (1997). Incorporation of selected nucleoside phosphonates and anti-human immunodeficiency virus nucleotide analogues into DNA by human DNA polymerases alpha, beta and gamma. Antivir. Chem. Chemother. 8, 187–195. doi: 10.1177/095632029700800302
Cihlar, T., Ray, A. S., Boojamra, C. G., Zhang, L., Hui, H., Laflamme, G., et al. (2008). Design and profiling of GS-9148, a novel nucleotide analog active against nucleoside-resistant variants of human immunodeficiency virus type 1, and its orally bioavailable phosphonoamidate prodrug, GS-9131. Antimicrob. Agents Chemother. 52, 655–665. doi: 10.1128/AAC.01215-07
Clinch, K., Crump, D. R., Evans, G. B., Hazleton, K. Z., Mason, J. M., Schramm, V. L., et al. (2013). Acyclic phosph(on)ate inhibitors of Plasmodium falciparum hypoxanthine-guanine-xanthine phosphoribosyltransferase. Bioorg. Med. Chem. 21, 5629–5646. doi: 10.1016/j.bmc.2013.02.016
Communi, D., Robaye, B., and Boeynaems, J.-M. (1999). Pharmacological characterization of the human P2Y11 receptor. Br. J. Pharmacol. 128, 1199–1206. doi: 10.1038/sj.bjp.0702909
Cosyn, L., van Calenbergh, S., Joshi, B. V., Ko, H., Carter, R. L., Kendall Harden, T., et al. (2009). Synthesis and P2Y receptor activity of nucleoside 5′-phosphonate derivatives. Bioorg. Med. Chem. Lett. 19, 3002–3005. doi: 10.1016/j.bmcl.2009.04.027
Cundy, K. C., Fishback, J. A., Shaw, J. P., Lee, M. L., Soike, K. F., Visor, G. C., et al. (1994). Oral bioavailability of the antiretroviral agent 9-(2-phosphonylmethoxyethyl)adenine (PMEA) from 3-formulations of the prodrug bis(pivaloyloxymethyl)-PMEA in fasted male cynomolgus monkeys. Pharm. Res. 11, 839–843. doi: 10.1023/A:1018925723889
Dal Pozzo, F., Andrei, G., Holy, A., van Den Oord, J., Scagliarini, A., de Clercq, E., et al. (2005). Activities of acyclic nucleoside phosphonates against Orf virus in human and ovine cell monolayers and organotypic ovine raft cultures. Antimicrob. Agents Chemother. 49, 4843–4852. doi: 10.1128/AAC.49.12.4843-4852.2005
Das, A., Ko, H., Burianek, L. E., Barrett, M. O., Harden, T. K., and Jacobson, K. A. (2010). Human P2Y14 receptor agonists: truncation of the hexose moiety of uridine-5′-diphosphoglucose and its replacement with alkyl and aryl groups. J. Med. Chem. 53, 471–480. doi: 10.1021/jm901432g
de Clercq, E. (2007). Acyclic nucleoside phosphonates: past, present and future–bridging chemistry to HIV, HBV, HCV, HPV, adeno-, herpes-, and poxvirus infections: the phosphonate bridge. Biochem. Pharmacol. 73, 911–922. doi: 10.1016/j.bcp.2006.09.014
de Clercq, E., Andrei, G., Balzarini, J., Leyssen, P., Naesens, L., Neyts, J., et al. (2005). Antiviral potential of a new generation of acyclic nucleoside phosphonates, the 6-2-(phosphonomethoxy)alkoxy-2,4-diaminopyrimidines. Nucleosides Nucleotides Nucleic Acids 24, 331–341. doi: 10.1081/NCN-200059772
de Clercq, E., and Holy, A. (2005). Acyclic nucleoside phosphonates: a key class of antiviral drugs. Nat. Rev. Drug Discov. 4, 928–940. doi: 10.1038/nrd1877
de Clercq, E., and Neyts, J. (2004). Therapeutic potential of nucleoside/nucleotide analogues against poxvirus infections. Rev. Med. Virol. 14, 289–300. doi: 10.1002/rmv.439
Declercq, E., Holy, A., Rosenberg, I., Sakuma, T., Balzarini, J., and Maudgal, P. C. (1986). A novel selective broad-spectrum anti-DNA virus agent. Nature 323, 464–467. doi: 10.1038/323464a0
Declercq, E., Sakuma, T., Baba, M., Pauwels, R., Balzarini, J., Rosenberg, I., et al. (1987). Antiviral activity of phosphonylmethoxyalkyl derivatives of purine and pyrimidines. Antivir. Res. 8, 261–272. doi: 10.1016/S0166-3542(87)80004-9
Duarte-Rojo, A., and Heathcote, E. J. (2010). Efficacy and safety of tenofovir disoproxil fumarate in patients with chronic hepatitis B. Therap. Adv. Gastroenterol. 3, 107–119. doi: 10.1177/1756283X09354562
Duraffour, S., Snoeck, R., Krecmerova, M., van Den Oord, J., de Vos, R., Holy, A., et al. (2007). Activities of several classes of acyclic nucleoside phosphonates against camelpox virus replication in different cell culture models. Antimicrob. Agents Chemother. 51, 4410–4419. doi: 10.1128/AAC.00838-07
Eliahu, S., Martin-Gil, A., Perez de Lara, M. J., Pintor, J., Camden, J., Weisman, G. A., et al. (2010). 2-MeS-beta,gamma-CCl2-ATP is a potent agent for reducing intraocular pressure. J. Med. Chem. 53, 3305–3319. doi: 10.1021/jm100030u
Eliahu, S. E., Camden, J., Lecka, J., Weisman, G. A., Sévigny, J., Gélinas, S., et al. (2009). Identification of hydrolytically stable and selective P2Y1 receptor agonists. Eur. J. Med. Chem. 44, 1525–1536. doi: 10.1016/j.ejmech.2008.07.015
El-Tayeb, A., Qi, A., and Muller, C. E. (2006). Synthesis and structure-activity relationships of uracil nucleotide derivatives and analogues as agonists at human P2Y2, P2Y4, and P2Y6 receptors. J. Med. Chem. 49, 7076–7087. doi: 10.1021/jm060848j
El-Tayeb, A., Qi, A., Nicholas, R. A., and Muller, C. E. (2011). Structural modifications of UMP, UDP, and UTP leading to subtype-selective agonists for P2Y2, P2Y4, and P2Y6 receptors. J. Med. Chem. 54, 2878–2890. doi: 10.1021/jm1016297
Eng, W. S., Hocková, D., Špaček, P., Janeba, Z., West, N. P., Woods, K., et al. (2015). First crystal structures of Mycobacterium tuberculosis 6-oxopurine phosphoribosyltransferase: complexes with GMP and pyrophosphate and with acyclic nucleoside phosphonates whose prodrugs have antituberculosis activity. J. Med. Chem. 58, 4822–4838. doi: 10.1021/acs.jmedchem.5b00611
Eng, W. S., Rejman, D., Pohl, R., West, N. P., Woods, K., Naesens, L. M. J., et al. (2018). Pyrrolidine nucleoside bisphosphonates as antituberculosis agents targeting hypoxanthine-guanine phosphoribosyltransferase. Eur. J. Med. Chem. 159, 10–22. doi: 10.1016/j.ejmech.2018.09.039
Eriksson, U., Peterson, L. W., Kashemirov, B. A., Hilfinger, J. M., Drach, J. C., Borysko, K. Z., et al. (2008). Serine peptide phosphoester prodrugs of cyclic cidofovir: synthesis, transport, and antiviral activity. Mol. Pharm. 5, 598–609. doi: 10.1021/mp8000099
Franek, F., Holy, A., Votruba, I., and Eckschlager, T. (1999). Acyclic nucleotide analogues suppress growth and induce apoptosis in human leukemia cell lines. Int. J. Oncol. 14, 745–752. doi: 10.3892/ijo.14.4.745
Frey, C. R., Andrei, G., Votruba, I., Cannizzaro, C., Han, B., Fung, W. C., et al. (2013). Mutations in adenosine deaminase-like (ADAL) protein confer resistance to the antiproliferative agents N-6-cyclopropyl-PMEDAP and GS-9219. Anticancer Res. 33, 1899–1912.
Fung, H. B., Stone, E. A., and Piacenti, F. J. (2002). Tenofovir disoproxil fumarate: A nucleotide reverse transcriptase inhibitor for the treatment of HIV infection. Clin. Ther. 24, 1515–1548. doi: 10.1016/S0149-2918(02)80058-3
Fung, J., Lai, C. L., and Yuen, M. F. (2008). LB80380: a promising new drug for the treatment of chronic hepatitis B. Expert Opin. Invest. Drugs 17, 1581–1588. doi: 10.1517/13543784.17.10.1581
Giesler, K. E., and Liotta, D. C. (2016). Next-generation reduction sensitive lipid conjugates of tenofovir: antiviral activity and mechanism of release. J. Med. Chem. 59, 10244–10252. doi: 10.1021/acs.jmedchem.6b01292
Giesler, K. E., Marengo, J., and Liotta, D. C. (2016). Reduction sensitive lipid conjugates of tenofovir: synthesis, stability, and antiviral activity. J. Med. Chem. 59, 7097–7110. doi: 10.1021/acs.jmedchem.6b00428
Grimley, M. S., Chemaly, R. F., Englund, J. A., Kurtzberg, J., Chittick, G., Brundage, T. M., et al. (2017). Brincidofovir for asymptomatic adenovirus viremia in pediatric and adult allogeneic hematopoietic cell transplant recipients: a randomized placebo-controlled phase II trial. Biol. Blood Marrow Transplant. 23, 512–521. doi: 10.1016/j.bbmt.2016.12.621
Haas, M., Ben-Moshe, I., Fischer, B., and Reiser, G. (2013). Sp-2-propylthio-ATP-alpha-B and Sp-2-propylthio-ATP-alpha-B,beta-gamma-dichloromethylene are novel potent and specific agonists of the human P2Y(1)(1) receptor. Biochem. Pharmacol. 86, 645–655. doi: 10.1016/j.bcp.2013.06.013
Hadziyannis, S. J., Tassopoulos, N. C., Heathcote, E. J., Chang, T. T., Kitis, G., Rizzetto, M., et al. (2003). Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N. Engl. J. Med. 348, 800–807. doi: 10.1056/NEJMoa021812
Hartline, C. B., Gustin, K. M., Wan, W. B., Ciesla, S. L., Beadle, J. R., Hostetler, K. Y., et al. (2005). Ether lipid-ester prodrugs of acyclic nucleoside phosphonates: activity against adenovirus replication in vitro. J. Infect. Dis. 191, 396–399. doi: 10.1086/426831
Hatse, S., Naesens, L., de Clercq, E., and Balzarini, J. (1999). N(6)-cyclopropyl-PMEDAP: a novel derivative of 9-(2-phosphonylmethoxyethyl)2-6-diaminopurine (PMEDAP) with distinct metabolic, antiproliferative, and differentiation-inducing properties. Biochem. Pharmacol. 58, 311–323. doi: 10.1016/S0006-2952(99)00091-X
Hatse, S., Naesens, L., Degreve, B., Segers, C., vandeputte, M., Waer, M., et al. (1998). Potent antitumor activity of the acyclic nucleoside phosphonate 9-(2-phosphonylmethoxyethyl)adenine in choriocarcinoma-bearing rats. Int. J. Cancer 76, 595–600.
Hazleton, K. Z., Ho, M.-C., Cassera, M. B., Clinch, K., Crump, D. R., Rosario, I., et al. (2012). Acyclic immucillin phosphonates: second-generation inhibitors of Plasmodium falciparum hypoxanthine-guanine-xanthine phosphoribosyltransferase. Chem. Biol. 19, 721–730. doi: 10.1016/j.chembiol.2012.04.012
Heathcote, E. J., Marcellin, P., Buti, M., Gane, E., de Man, R. A., Krastev, Z., et al. (2011). Three-year efficacy and safety of tenofovir disoproxil fumarate treatment for chronic hepatitis B. Gastroenterology 140, 132–143. doi: 10.1053/j.gastro.2010.10.011
Heijtink, R. A., Kruining, J., Dewilde, G. A., Balzarini, J., Declercq, E., and Schalm, S. W. (1994). Inhibitory effects of acyclic nucleoside phosphonates on human hepatitis-B virus and duck hepatitis-B virus-infections in tissue-culture. Antimicrob. Agents Chemother. 38, 2180–2182. doi: 10.1128/AAC.38.9.2180
Herman, B. D., Votruba, I., Holy, A., Sluis-Cremer, N., and Balzarini, J. (2010). The acyclic 2,4-diaminopyrimidine nucleoside phosphonate acts as a purine mimetic in HIV-1 reverse transcriptase DNA polymerization. J. Biol. Chem. 285, 12101–12108. doi: 10.1074/jbc.M109.096529
Ho, E. S., Lin, D. C., Mendel, D. B., and Cihlar, T. (2000). Cytotoxicity of antiviral nucleotides adefovir and cidofovir is induced by the expression of human renal organic anion transporter 1. J. Am. Soc. Nephrol. 11, 383–393.
Hockova, D., Holy, A., Masojidkova, M., Andrei, G., Snoeck, R., de Clercq, E., et al. (2003). 5-Substituted-2,4-diamino-6-2-(phosphonomethoxy)ethoxylpyrimidines-acyc lic nucleoside phosphonate analogues with antiviral activity. J. Med. Chem. 46, 5064–5073. doi: 10.1021/jm030932o
Hocková, D., Holý, A., Masojídková, M., Keough, D. T., de Jersey, J., Guddat, L. W., et al. (2009). Synthesis of branched 9-[2-(2-phosphonoethoxy)ethyl]purines as a new class of acyclic nucleoside phosphonates which inhibit Plasmodium falciparum hypoxanthine–guanine–xanthine phosphoribosyltransferase. Bioorg. Med. Chem. 17, 6218–6232. doi: 10.1016/j.bmc.2009.07.044
Hockova, D., Janeba, Z., Naesens, L., Edstein, M. D., Chavchich, M., Keough, D. T., et al. (2015). Antimalarial activity of prodrugs of N-branched acyclic nucleoside phosphonate inhibitors of 6-oxopurine phosphoribosyltransferases. Bioorg. Med. Chem. 23, 5502–5510. doi: 10.1016/j.bmc.2015.07.038
Hockova, D., Keough, D. T., Janeba, Z., Wang, T. H., de Jersey, J., and Guddat, L. W. (2012). Synthesis of novel N-branched acyclic nucleoside phosphonates as potent and selective inhibitors of human, Plasmodium falciparum and Plasmodium vivax 6-oxopurine phosphoribosyltransferases. J. Med. Chem. 55, 6209–6223. doi: 10.1021/jm300662d
Jansa, P., Baszczynski, O., Dracinsky, M., Votruba, I., Zidek, Z., Bahador, G., et al. (2011). A novel and efficient one-pot synthesis of symmetrical diamide (bis-amidate) prodrugs of acyclic nucleoside phosphonates and evaluation of their biological activities. Eur. J. Med. Chem. 46, 3748–3754. doi: 10.1016/j.ejmech.2011.05.040
John, J., Kim, Y., Bennett, N., Das, K., Liekens, S., Naesens, L., et al. (2015). Pronounced inhibition shift from HIV reverse transcriptase to herpetic DNA polymerases by increasing the flexibility of alpha-carboxy nucleoside phosphonates. J. Med. Chem. 58, 8110–8127. doi: 10.1021/acs.jmedchem.5b01180
Kahn, J., Lagakos, S., Wulfsohn, M., Cherng, D., Miller, M., Cherrington, J., et al. (1999). Efficacy and safety of adefovir dipivoxil with antiretroviral therapy–a randomized controlled trial. JAMA J. Am. Med. Assoc. 282, 2305–2312. doi: 10.1001/jama.282.24.2305
Kaiser, M. M., Baszczynski, O., Hocková, D., Poštová-Slavětínská, L., Dračínský, M., Keough, D. T., et al. (2017). Acyclic nucleoside phosphonates containing 9-deazahypoxanthine and a five-membered heterocycle as selective inhibitors of plasmodial 6-oxopurine phosphoribosyltransferases. ChemMedChem 12, 1133–1141. doi: 10.1002/cmdc.201700293
Kaiser, M. M., Hocková, D., Wang, T.-H., Dračínský, M., Poštová-Slavětínská, L., Procházková, E., et al. (2015). Synthesis and evaluation of novel acyclic nucleoside phosphonates as inhibitors of Plasmodium falciparum and human 6-oxopurine phosphoribosyltransferases. ChemMedChem 10, 1707–1723. doi: 10.1002/cmdc.201500322
Keane, S. J., Ford, A., Mullins, N. D., Maguire, N. M., Legigan, T., Balzarini, J., et al. (2015). Design and synthesis of alpha-carboxy nucleoside phosphonate analogues and evaluation as HIV-1 reverse transcriptase-targeting agents. J. Org. Chem. 80, 2479–2493. doi: 10.1021/jo502549y
Kearney, B. P., Flaherty, J. F., and Shah, J. (2004). Tenofovir disoproxil fumarate–clinical pharmacology and pharmacokinetics. Clin. Pharmacokinet. 43, 595–612. doi: 10.2165/00003088-200443090-00003
Keough, D. T., Hocková, D., Holý, A., Naesens, L. M. J., Skinner-Adams, T. S., Jersey, J., et al. (2009). Inhibition of hypoxanthine-guanine phosphoribosyltransferase by acyclic nucleoside phosphonates: a new class of antimalarial therapeutics. J. Med. Chem. 52, 4391–4399. doi: 10.1021/jm900267n
Keough, D. T., Hockova, D., Janeba, Z., Wang, T. H., Naesens, L., Edstein, M. D., et al. (2015). Aza-acyclic nucleoside phosphonates containing a second phosphonate group as inhibitors of the human, Plasmodium falciparum and vivax 6-oxopurine phosphoribosyltransferases and their prodrugs as antimalarial agents. J. Med. Chem. 58, 827–846. doi: 10.1021/jm501416t
Keough, D. T., Hocková, D., Rejman, D., Špaček, P., Vrbková, S., Krečmerová, M., et al. (2013a). Inhibition of the Escherichia coli 6-oxopurine phosphoribosyltransferases by nucleoside phosphonates: potential for new antibacterial agents. J. Med. Chem. 56, 6967–6984. doi: 10.1021/jm400779n
Keough, D. T., Rejman, D., Pohl, R., Zbornikova, E., Hockova, D., Croll, T., et al. (2018). Design of Plasmodium vivax hypoxanthine-guanine phosphoribosyltransferase inhibitors as potential antimalarial therapeutics. ACS Chem. Biol. 13, 82–90. doi: 10.1021/acschembio.7b00916
Keough, D. T., Spacek, P., Hockova, D., Tichy, T., Vrbkova, S., Slavetinska, L., et al. (2013b). Acyclic nucleoside phosphonates containing a second phosphonate group are potent inhibitors of 6-oxopurine phosphoribosyltransferases and have antimalarial activity. J. Med. Chem. 56, 2513–2526. doi: 10.1021/jm301893b
Kern, E. R., Hartline, C., Harden, E., Keith, K., Rodriguez, N., Beadle, J. R., et al. (2002). Enhanced inhibition of orthopoxvirus replication in vitro by alkoxyalkyl esters of cidofovir and cyclic cidofovir. Antimicrob. Agents Chemother. 46, 991–995. doi: 10.1128/AAC.46.4.991-995.2002
Kim, C. U., Luh, B. Y., and Martin, J. C. (1991). Regiospecific and highly stereoselective electrophilic addition to furanoid glycals–synthesis of phosphonate nucleotide analogs with potent activity against HIV. J. Org. Chem. 56, 2642–2647. doi: 10.1021/jo00008a013
Ko, H., Carter, R. L., Cosyn, L., Petrelli, R., de Castro, S., Besada, P., et al. (2008). Synthesis and potency of novel uracil nucleotides and derivatives as P2Y2 and P2Y6 receptor agonists. Bioorg. Med. Chem. 16, 6319–6332. doi: 10.1016/j.bmc.2008.05.013
Kramata, P., Downey, K. M., and Paborsky, L. R. (1998). Incorporation and excision of 9-(2-phosphonylmethoxyethyl)guanine (PMEG) by DNA polymerase delta and epsilon in vitro. J. Biol. Chem. 273, 21966–21971. doi: 10.1074/jbc.273.34.21966
Kramata, P., Votruba, I., Otova, B., and Holy, A. (1996). Different inhibitory potencies of acyclic phosphonomethoxyalkyl nucleotide analogs toward DNA polymerases alpha, delta, and epsilon. Mol. Pharmacol. 49, 1005–1011.
Krečmerová, M., Dračínský, M., Hocková, D., Holý, A., Keough, D. T., and Guddat, L. W. (2012). Synthesis of purine N9-[2-hydroxy-3-O-(phosphonomethoxy)propyl] derivatives and their side-chain modified analogs as potential antimalarial agents. Bioorg. Med. Chem. 20, 1222–1230. doi: 10.1016/j.bmc.2011.12.034
Krecmerova, M., Holy, A., Andrei, G., Pomeisl, K., Tichy, T., Brehova, P., et al. (2010). Synthesis of ester prodrugs of 9-(s)-3-hydroxy-2-(phosphonomethoxy)propyl-2,6-diaminopurine (HPMPDAP) as anti-poxvirus agents. J. Med. Chem. 53, 6825–6837. doi: 10.1021/jm901828c
Krecmerova, M., Holy, A., Piskala, A., Masojidkova, M., Andrei, G., Naesens, L., et al. (2007a). Antiviral activity of triazine analogues of 1-(S)-3-hydroxy-2-(phosphonomethoxy)propyl cytosine (cidofovir) and related compounds. J. Med. Chem. 50, 1069–1077. doi: 10.1021/jm061281+
Krecmerova, M., Holy, A., Pohl, R., Masojidkova, M., Andrei, G., Naesens, L., et al. (2007b). Ester prodrugs of cyclic 1-(S)-3-hydroxy-2-(phosphonomethoxy)propyl-5-azacytosine: Synthesis and antiviral activity. J. Med. Chem. 50, 5765–5772. doi: 10.1021/jm0707166
Kreider, J. W., Balogh, K., Olson, R. O., and Martin, J. C. (1990). Treatment of latent rabbit and human papillomavirus infections with 9-(2-phosphonylmethoxy)ethylguanine (PMEG). Antivir. Res. 14, 51–58. doi: 10.1016/0166-3542(90)90065-F
Kuo, A., Dienstag, J. L., and Chung, R. T. (2004). Tenofovir disoproxil fumarate for the treatment of lamivudine-resistant hepatitis B. Clin. Gastroenterol. Hepatol. 2, 266–272. doi: 10.1016/S1542-3565(04)00017-5
Lai, C. L., Ahn, S. H., Lee, K. S., Um, S. H., Cho, M., Yoon, S. K., et al. (2014). Phase IIb multicentred randomised trial of besifovir (LB80380) versus entecavir in Asian patients with chronic hepatitis B. Gut 63, 996–1004. doi: 10.1136/gutjnl-2013-305138
Lalezari, J. P., Drew, W. L., Glutzer, E., James, C., Miner, D., Flaherty, J., et al. (1995). (S)-1-3-Hydroxy-2-(phosphonylmethoxy)propyl cytosine (cidofovir)–results of a phase I/II study of a novel antiviral nucleotide analog. J. Infect. Dis. 171, 788–796. doi: 10.1093/infdis/171.4.788
Lalezari, J. P., Holland, G. N., Kramer, F., McKinley, G. F., Kemper, C. A., Ives, D. V., et al. (1998). Randomized, controlled study of the safety and efficacy of intravenous cidofovir for the treatment of relapsing cytomegalovirus retinitis in patients with AIDS. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 17, 339–344. doi: 10.1097/00042560-199804010-00008
Lalezari, J. P., Stagg, R. J., Kuppermann, B. D., Holland, G. N., Kramer, F., Ives, D. V., et al. (1997). Intravenous cidofovir for peripheral cytomegalovirus retinitis in patients with AIDS–a randomized, controlled trial. Ann. Intern. Med. 126, 257–263. doi: 10.7326/0003-4819-126-4-199702150-00001
Lanier, E. R., Ptak, R. G., Lampert, B. M., Keilholz, L., Hartman, T., Buckheit, R. W., et al. (2010). Development of hexadecyloxypropyl tenofovir (CMX157) for treatment of infection caused by wild-type and nucleoside/nucleotide-resistant HIV. Antimicrob. Agents Chemother. 54, 2901–2909. doi: 10.1128/AAC.00068-10
Lanier, R., Lampert, B., Trost, L., Almond, M., and Painter, G. (2009). Development of hexadecyloxypropyl tenofovir (CMX157) for HIV: potential for use as a microbicide and therapeutic. Antiviral Res. 82:A43. doi: 10.1016/j.antiviral.2009.02.094
Lawrence, J. A., Huelsmeyer, M. K., Thamm, D. H., Tumas, D. B., Birkus, G., Kurzman, I., et al. (2015). Novel acyclic nucleotide analogues GS-343074 and GS-424044 demonstrate antiproliferative and pro-apoptotic activity in canine neoplastic cell lines. Vet. Comp. Oncol. 13, 246–254. doi: 10.1111/vco.12038
Lebeau, I., Andrei, G., Dal Pozzo, F., Beadle, J. R., Hostetler, K. Y., De Clercq, E., et al. (2006). Activities of alkoxyalkyl esters of cidofovir (CDV), cyclic CDV, and (S)-9-(3-hydroxy-2-phosphonylmethoxypropyl)adenine against orthopoxviruses in cell monolayers and in organotypic cultures. Antimicrob. Agents Chemother. 50, 2525–2529. doi: 10.1128/AAC.01489-05
Lecka, J., Ben-David, G., Simhaev, L., Eliahu, S., Oscar, J. Jr., Luyindula, P., et al. (2013). Nonhydrolyzable ATP analogues as selective inhibitors of human NPP1: a combined computational/experimental study. J. Med. Chem. 56, 8308–8320. doi: 10.1021/jm400918s
Lee, K. S., Lim, S. G., Chuang, W. L., Hwang, S. G., Cho, M., Lai, M. Y., et al. (2006). Safety, tolerability and antiviral activity of pradefovir mesylate in patients with chronic hepatitis B virus infection: 48-week analysis of a phase 2 study. J. Hepatol. 44, S274–S274. doi: 10.1016/S0168-8278(06)80742-7
Lee, S.-Y., Sarkar, S., Bhattarai, S., Namasivayam, V., de Jonghe, S., Stephan, H., et al. (2017). Substrate-dependence of competitive nucleotide pyrophosphatase/phosphodiesterase 1 (NPP1) inhibitors. Front. Pharmacol. 8:54. doi: 10.3389/fphar.2017.00054
Lee, S. Y., and Muller, C. E. (2017). Nucleotide pyrophosphatase/phosphodiesterase 1 (NPP1) and its inhibitors. Medchemcomm 8, 823–840. doi: 10.1039/C7MD00015D
Lee, W. A., He, G. X., Eisenberg, E., Cihlar, T., Swaminathan, S., Mulato, A., et al. (2005). Selective intracellular activation of a novel prodrug of the human immunodeficiency virus reverse transcriptase inhibitor tenofovir leads to preferential distribution and accumulation in lymphatic tissue. Antimicrob. Agents Chemother. 49, 1898–1906. doi: 10.1128/AAC.49.5.1898-1906.2005
Levesque, S. A., Lavoie, E. G., Lecka, J., Bigonnesse, F., and Sevigny, J. (2007). Specificity of the ecto-ATPase inhibitor ARL 67156 on human and mouse ectonucleotidases. Br. J. Pharmacol. 152, 141–150. doi: 10.1038/sj.bjp.0707361
Liekens, S., Andrei, G., Vandeputte, M., De Clercq, E., and Neyts, J. (1998). Potent inhibition of hemangioma formation in rats by the acyclic nucleoside phosphonate analogue cidofovir. Cancer Res. 58, 2562–2567.
Liekens, S., Verbeken, E., de Clercq, E., and Neyts, J. (2001). Potent inhibition of hemangiosarcoma development in mice by cidofovir. Int. J. Cancer 92, 161–167. doi: 10.1002/1097-0215(200102)9999:9999<::AID-IJC1183>3.0.CO
Lin, C. C., Fang, C., Benetton, S., Xu, G. F., and Yeh, U. T. (2006). Metabolic activation of pradefovir by CYP3A4 and its potential as an inhibitor or inducer. Antimicrob. Agents Chemother. 50, 2926–2931. doi: 10.1128/AAC.01566-05
Liu, C., Dumbre, S. G., Pannecouque, C., Huang, C. S., Ptak, R. G., Murray, M. G., et al. (2016). Amidate prodrugs of deoxythreosyl nucleoside phosphonates as dual inhibitors of HIV and HBV replication. J. Med. Chem. 59, 9513–9531. doi: 10.1021/acs.jmedchem.6b01260
Luo, M., Groaz, E., Andrei, G., Snoeck, R., Kalkeri, R., Ptak, R. G., et al. (2017). Expanding the antiviral spectrum of 3-fluoro-2-(phosphonomethoxy)propyl acyclic nucleoside phosphonates: diamyl aspartate amidate prodrugs. J. Med. Chem. 60, 6220–6238. doi: 10.1021/acs.jmedchem.7b00416
Luo, M., Groaz, E., de Jonghe, S., Snoeck, R., Andrei, G., and Herdewijn, P. (2018). Amidate prodrugs of cyclic 9-(s)-3-hydroxy-2(phosphonomethoxy)propyl adenine with potent anti-herpesvirus activity. ACS Med. Chem. Lett. 9, 381–385. doi: 10.1021/acsmedchemlett.8b00079
Luo, M., Groaz, E., Snoeck, R., Andrei, G., and Herdewijn, P. (2020). Amidate prodrugs of o-2-alkylated pyrimidine acyclic nucleosides display potent anti-herpesvirus activity. ACS Med. Chem. Lett. 11, 1410–1415. doi: 10.1021/acsmedchemlett.0c00090
Macchi, B., Romeo, G., Chiacchio, U., Frezza, C., Giofre, S. V., Marino-Merlo, F., et al. (2015). “Phosphonated nucleoside analogues as antiviral agents,” in Therapy of Viral Infections, eds Diederich, W. E., and Steuber, H., (Berlin; Heidelberg: Springer-Verlag), 53–91. doi: 10.1007/7355_2013_28
Mackman, R. L., and Cihlar, T. (2011). “Acyclic and cyclic nucleoside phosphonates,” in Antiviral Drug Strategies, ed E. DeClercq (Weinheim: Wiley-VCH Verlag & Co), 91–128. doi: 10.1002/9783527635955.ch5
Marcellin, P., Chang, T., Lim, S. G., Tong, M. J., Sievert, W., Shiffman, M. L., et al. (2003). Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N. Engl. J. Med. 348, 808–816. doi: 10.1056/NEJMoa020681
Marty, F. M., Winston, D. J., Chemaly, R. F., Mullane, K. M., Shore, T. B., Papanicolaou, G. A., et al. (2019). A randomized, double-blind, placebo-controlled phase 3 trial of oral brincidofovir for cytomegalovirus prophylaxis in allogeneic hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 25, 369–381. doi: 10.1016/j.bbmt.2018.09.038
Marty, F. M., Winston, D. J., Rowley, S. D., vance, E., Papanicolaou, G. A., Mullane, K. M., et al. (2013). CMX001 to prevent cytomegalovirus disease in hematopoietic-cell transplantation. N. Engl. J. Med. 369, 1227–1236. doi: 10.1056/NEJMoa1303688
Maruoka, H., Barrett, M. O., Ko, H., Tosh, D. K., Melman, A., Burianek, L. E., et al. (2010). Pyrimidine ribonucleotides with enhanced selectivity as P2Y(6) receptor agonists: novel 4-alkyloxyimino, (S)-methanocarba, and 5′-triphosphate gamma-ester modifications. J. Med. Chem. 53, 4488–4501. doi: 10.1021/jm100287t
McKenna, C. E., Kashemirov, B. A., Eriksson, U., Amidon, G. L., Kish, P. E., Mitchell, S., et al. (2005). Cidofovir peptide conjugates as prodrugs. J. Organomet. Chem. 690, 2673–2678. doi: 10.1016/j.jorganchem.2005.03.004
Mendel, D. B., Cihlar, T., Moon, K., and Chen, M. S. (1997). Conversion of 1-((S)-2-hydroxy-2-oxo-1,4,2-dioxaphosphorinan-5-yl)methyl cytosine to cidofovir by an intracellular cyclic CMP phosphodiesterase. Antimicrob. Agents Chemother. 41, 641–646. doi: 10.1128/AAC.41.3.641
Mertlikova-Kaiserova, H., Rumlova, M., Tloustova, E., Prochazkova, E., Holy, A., and Votruba, I. (2011). Point mutations in human guanylate kinase account for acquired resistance to anticancer nucleotide analogue PMEG. Biochem. Pharmacol. 82, 131–138. doi: 10.1016/j.bcp.2011.04.002
Morges, M. A., Burton, J. H., Saba, C. F., Vail, D. M., Burgess, K. E., and Thamm, D. H. (2014). Phase II evaluation of VDC-1101 in canine cutaneous T-cell lymphoma. J. Vet. Intern. Med. 28, 1569–1574. doi: 10.1111/jvim.12429
Nadel, Y., Lecka, J., Gilad, Y., Ben-David, G., Forster, D., Reiser, G., et al. (2014). Highly potent and selective ectonucleotide pyrophosphatase/phosphodiesterase I inhibitors based on an adenosine 5′-(alpha or gamma)-thio-(alpha,beta- or beta,gamma)-methylenetriphosphate scaffold. J. Med. Chem. 57, 4677–4691. doi: 10.1021/jm500196c
Naesens, L., Balzarini, J., Rosenberg, I., Holy, A., and Declercq, E. (1989). 9-(2-Phosphonylmethoxyethyl)-2,6-diaminopurine (PMEDAP)–a novel agent with anti-human immunodeficiency virus activity in vitro and potent anti-moloney murine sarcoma-virus activity in vivo. Eur. J. Clin. Microbiol. Infect. Dis. 8, 1043–1047. doi: 10.1007/BF01975167
Naesens, L., Bischofberger, N., Augustijns, P., Annaert, P., van den Mooter, G., Arimilli, M. N., et al. (1998). Antiretroviral efficacy and pharmacokinetics of oral bis(isopropyloxycarbonyloxymethyl) 9-(2-phosphonylmethoxypropyl)adenine in mice. Antimicrob. Agents Chemother. 42, 1568–1573. doi: 10.1128/AAC.42.7.1568
Naesens, L., Hatse, S., Segers, C., Verbeken, E., de Clercq, E., Waer, M., et al. (1999). 9-(2-Phosphonylmethoxyethyl)-N-6-cyclopropyl-2,6-diaminopurine: a novel prodrug of 9-(2-phosphonyl-methoxyethyl)guanine with improved antitumor efficacy and selectivity in choriocarcinoma-bearing rats. Oncol. Res. 11, 195–203.
Naesens, L., Lenaerts, L., Andrei, G., Snoeck, R., van Beers, D., Holy, A., et al. (2005). Antiadenovirus activities of several classes of nucleoside and nucleotide analogues. Antimicrob. Agents Chemother. 49, 1010–1016. doi: 10.1128/AAC.49.3.1010-1016.2005
Naesens, L., Snoeck, R., Andrei, G., Balzarini, J., Neyts, J., and DeClercq, E. (1997). HPMPC (cidofovir), PMEA (adefovir) and related acyclic nucleoside phosphonate analogues: a review of their pharmacology and clinical potential in the treatment of viral infections. Antivir. Chem. Chemother. 8, 1–23. doi: 10.1177/095632029700800101
Nassir, M., Mirza, S., Arad, U., Lee, S., Rafehi, M., Yaw Attah, I., et al. (2019a). Adenine-(methoxy)-ethoxy-Palpha,alpha-dithio-triphosphate inhibits pathologic calcium pyrophosphate deposition in osteoarthritic human chondrocytes. Org. Biomol. Chem. 17, 9913–9923. doi: 10.1039/C9OB02199J
Nassir, M., Pelletier, J., Arad, U., Arguin, G., Khazanov, N., Gendron, F. P., et al. (2019b). Structure-activity relationship study of NPP1 inhibitors based on uracil-N1-(methoxy)ethyl-beta-phosphate scaffold. Eur. J. Med. Chem. 184, 111754. doi: 10.1016/j.ejmech.2019.111754
Neyts, J., Sadler, R., de Clercq, E., Raab-Traub, N., and Pagano, J. S. (1998). The antiviral agent cidofovir (S)-1-(3-hydroxy-2-phosphonyl-methoxypropyl)cytosine has pronounced activity against nasopharyngeal carcinoma grown in nude mice. Cancer Res. 58, 384–388.
Ono-Nita, S. K., Kato, N., Shiratori, Y., Carrilho, F. J., and Omata, M. (2002). Novel nucleoside analogue MCC-478 (LY582563) is effective against wild-type or lamivudine-resistant hepatitis B virus. Antimicrob. Agents Chemother. 46, 2602–2605. doi: 10.1128/AAC.46.8.2602-2605.2002
Paborsky, L., Mao, C., Kramata, P., Compton, M., and Toole, J. (1997). “Potent anti-proliferative activity of a novel nucleotide analogue, PMEG, against human solid tumor cell lines,” in Proceedings of the American Associations Cancer Research (Orlando, FL), 100.
Painter, G. R., Almond, M. R., Trost, L. C., Lampert, B. M., Neyts, J., de Clercq, E., et al. (2007). Evaluation of hexadecyloxypropyl-9-R-2-(phosphonomethoxy)propyl adenine, CMX157, as a potential treatment for human immunodeficiency virus type 1 and hepatitis B virus infections. Antimicrob. Agents Chemother. 51, 3505–3509. doi: 10.1128/AAC.00460-07
Painter, W., Robertson, A., Trost, L. C., Godkin, S., Lampert, B., and Painter, G. (2012). First pharmacokinetic and safety study in humans of the novel lipid antiviral conjugate CMX001, a broad-spectrum oral drug active against double-stranded DNA viruses. Antimicrob. Agents Chemother. 56, 2726–2734. doi: 10.1128/AAC.05983-11
Papatheodoridis, G. V., and Manolakopoulos, S. (2006). Drug evaluation: LB-80380–a novel phosphonate nucleoside for the potential treatment of HBV infection. Curr. Opin. Mol. Ther. 8, 352–357.
Pauwels, R., Balzarini, J., Schols, D., Baba, M., Desmyter, J., Rosenberg, I., et al. (1988). Phosphonylmethoxyethyl purine derivatives, a new class of anti-human immunodeficiency virus agents. Antimicrob. Agents Chemother. 32, 1025–1030. doi: 10.1128/AAC.32.7.1025
Pertusati, F., Serpi, M., and McGuigan, C. (2012). Medicinal chemistry of nucleoside phosphonate prodrugs for antiviral therapy. Antivir. Chem. Chemother. 22, 181–203. doi: 10.3851/IMP2012
Peters, M. G., Hann, H. W., Martin, P., Heathcote, E. J., Buggisch, P., Rubin, R., et al. (2004). Adefovir dipivoxil alone or in combination with lamivudine in patients with lamivudine-resistant chronic hepatitis B. Gastroenterology 126, 91–101. doi: 10.1053/j.gastro.2003.10.051
Peterson, L. W., Kim, J. S., Kijek, P., Mitchell, S., Hilfinger, J., Breitenbach, J., et al. (2011). Synthesis, transport and antiviral activity of Ala-Ser and Val-Ser prodrugs of cidofovir. Bioorg. Med. Chem. Lett. 21, 4045–4049. doi: 10.1016/j.bmcl.2011.04.126
Peterson, L. W., Sala-Rabanal, M., Krylov, I. S., Serpi, M., Kashemirov, B. A., and McKenna, C. E. (2010). Serine side chain-linked peptidomimetic conjugates of cyclic HPMPC and HPMPA: synthesis and interaction with hPEPT1. Mol. Pharm. 7, 2349–2361. doi: 10.1021/mp100186b
Pisarev, V. M., Lee, S. H., Connelly, M. C., and Fridland, A. (1997). Intracellular metabolism and action of acyclic nucleoside phosphonates on DNA replication. Mol. Pharmacol. 52, 63–68. doi: 10.1124/mol.52.1.63
Pohl, R., Slavetinska, L. P., Eng, W. S., Keough, D. T., Guddat, L. W., and Rejman, D. (2015). Synthesis, conformational studies, and biological properties of phosphonomethoxyethyl derivatives of nucleobases with a locked conformation via a pyrrolidine ring. Org. Biomol. Chem. 13, 4693–4705. doi: 10.1039/C5OB00097A
Pradere, U., Garnier-Amblard, E. C., Coats, S. J., Amblard, F., and Schinazi, R. F. (2014). Synthesis of nucleoside phosphate and phosphonate prodrugs. Chem. Rev. 114, 9154–9218. doi: 10.1021/cr5002035
Randhawa, P., Farasati, N. A., Shapiro, R., and Hostetler, K. Y. (2006). Ether lipid ester derivatives of cidofovir inhibit polyomavirus BK replication in vitro. Antimicrob. Agents Chemother. 50, 1564–1566. doi: 10.1128/AAC.50.4.1564-1566.2006
Ray, A. S., Fordyce, M. W., and Hitchcock, M. J. M. (2016). Tenofovir alafenamide: a novel prodrug of tenofovir for the treatment of human immunodeficiency virus. Antivir. Res. 125, 63–70. doi: 10.1016/j.antiviral.2015.11.009
Ray, A. S., Vela, J. E., Boojamra, C. G., Zhang, L., Hui, H., Callebaut, C., et al. (2008). Intracellular metabolism of the nucleotide prodrug GS-9131, a potent anti-human immunodeficiency virus agent. Antimicrob. Agents Chemother. 52, 648–654. doi: 10.1128/AAC.01209-07
Reddy, K. R., Matelich, M. C., Ugarkar, B. G., Gomez-Galeno, J. E., Dare, J., Ollis, K., et al. (2008). Pradefovir: a prodrug that targets adefovir to the liver for the treatment of hepatitis B. J. Med. Chem. 51, 666–676. doi: 10.1021/jm7012216
Reiser, H., Wang, J. Y., Chong, L., Watkins, W. J., Ray, A. S., Shibata, R., et al. (2008). GS-9219–a novel acyclic nucleotide analogue with potent antineoplastic activity in dogs with spontaneous non-Hodgkin's lymphoma. Clin. Cancer Res. 14, 2824–2832. doi: 10.1158/1078-0432.CCR-07-2061
Robbins, B. L., Srinivas, R. V., Kim, C., Bischofberger, N., and Fridland, A. (1998). Anti-human immunodeficiency virus activity and cellular metabolism of a potential prodrug of the acyclic nucleoside phosphonate 9-R-(2-phosphonomethoxypropyl)adenine (PMPA), bis(isopropyloxymethylcarbonyl)PMPA. Antimicrob. Agents Chemother. 42, 612–617. doi: 10.1128/AAC.42.3.612
Rose, W. C., Crosswell, A. R., Bronson, J. J., and Martin, J. C. (1990). In vivo antitumor activity of 9-[(2-phosphonylmethoxy)ethyl]-guanine and related phosphonate nucleotide analogues. J. Natl. Cancer Inst. 82, 510–512. doi: 10.1093/jnci/82.6.510
Rosenberg, I., and Holy, A. (1987). Acyclic nucleotide analogs. 2. Synthesis of potential prodrugs and metabolites of 9-(s)-(3-hydroxy-2-phosphonylmethoxypropyl)adenine. Collect. Czechoslov. Chem. Commun. 52, 2792–2800. doi: 10.1135/cccc19872792
Saba, C. F., Vickery, K. R., Clifford, C. A., Burgess, K. E., Phillips, B., Vail, D. M., et al. (2018). Rabacfosadine for relapsed canine B-cell lymphoma: efficacy and adverse event profiles of 2 different doses. Vet. Comp. Oncol. 16, E76–E82. doi: 10.1111/vco.12337
Sauer, R., El-Tayeb, A., Kaulich, M., and Muller, C. E. (2009). Synthesis of uracil nucleotide analogs with a modified, acyclic ribose moiety as P2Y(2) receptor antagonists. Bioorg. Med. Chem. 17, 5071–5079. doi: 10.1016/j.bmc.2009.05.062
Schinkmanova, M., Votruba, I., and Holy, A. (2006). N-6-Methyl-AMP aminohydrolase activates N-6-substituted purine acyclic nucleoside phosphonates. Biochem. Pharmacol. 71, 1370–1376. doi: 10.1016/j.bcp.2006.01.013
Sekiya, K., Takashima, H., Ueda, N., Kamiya, N., Yuasa, S., Fujimura, Y., et al. (2002). 2-Amino-6-arylthio-9- 2-(phosphonomethoxy)ethyl purine bis(2,2,2-trifluoroethyl) esters as novel HBV-specific antiviral reagents. J. Med. Chem. 45, 3138–3142. doi: 10.1021/jm020036x
Shen, G. H., and Hong, J. H. (2019). Synthesis of nucleoside phosphonate analogs having phosphonodifluoromethylene moieties and their biological activities. J. Fluor. Chem. 224, 1–34. doi: 10.1016/j.jfluchem.2019.04.014
Smee, D. F., and Reist, E. J. (1996). Potent anti-murine cytomegalovirus activity and reduced nephrotoxicity of ganciclovir cyclic phosphonate. Antimicrob. Agents Chemother. 40, 1964–1966. doi: 10.1128/AAC.40.8.1964
Smee, D. F., Sugiyama, S. T., and Reist, E. J. (1994). Nucleotide analogs related to acyclovir and ganciclovir are effective against murine cytomegalovirus infections in balb/c and severe combined immunodeficient mice. Antimicrob. Agents Chemother. 38, 2165–2168. doi: 10.1128/AAC.38.9.2165
Starrett, J. E., Tortolani, D. R., Hitchcock, M. J. M., Martin, J. C., and Mansuri, M. M. (1992). Synthesis and in vitro evaluation of a phosphonate prodrug–bis(pivaloyloxymethyl)9-(2-phos-phonylmethoxyethyl)adenine. Antivir. Res. 19, 267–273. doi: 10.1016/0166-3542(92)90084-I
Stittelaar, K. J., Neyts, J., Naesens, L., van Amerongen, G., van Lavieren, R. F., Holy, A., et al. (2006). Antiviral treatment is more effective than smallpox vaccination upon lethal monkeypox virus infection. Nature 439, 745–748. doi: 10.1038/nature04295
Tanwandee, T., Chatsiricharoenkul, S., Thongsawat, S., Sukeepaisarnjaroen, W., Tangkijvanich, P., Komolmit, P., et al. (2017). Pharmacokinetics, safety and antiviral activity of CMX157, a novel prodrug of tenofovir, administered as ascending multiple doses to healthy volunteers and Hepatitis B virus-infected subjects. J. Hepatol. 66, S24–S25. doi: 10.1016/S0168-8278(17)30311-2
Thamm, D. H., Vail, D. M., Kurzman, I. D., Babusis, D., Ray, A. S., Sousa-Powers, N., et al. (2014). GS-9219/VDC-1101-a prodrug of the acyclic nucleotide PMEG has antitumor activity in spontaneous canine multiple myeloma. BMC Vet. Res. 10:30. doi: 10.1186/1746-6148-10-30
Thamm, D. H., Vail, D. M., Post, G. S., Fan, T. M., Phillips, B. S., Axiak-Bechtel, S., et al. (2017). Alternating rabacfosadine/doxorubicin: efficacy and tolerability in naive canine multicentric lymphoma. J. Vet. Intern. Med. 31, 872–878. doi: 10.1111/jvim.14700
Topalis, D., Pradere, U., Roy, V., Caillat, C., Azzouzi, A., Broggi, J., et al. (2011). Novel antiviral C5-substituted pyrimidine acyclic nucleoside phosphonates selected as human thymidylate kinase substrates. J. Med. Chem. 54, 222–232. doi: 10.1021/jm1011462
Toth, K., Spencer, J. F., Ying, B. L., Tollefson, A. E., Hartline, C. B., Richar, E. T., et al. (2018). USC-087 protects Syrian hamsters against lethal challenge with human species C adenoviruses. Antivir. Res. 153, 1–9. doi: 10.1016/j.antiviral.2018.03.001
Vail, D. M., Thamm, D. H., Reiser, H., Ray, A. S., Wolfgang, G. H. I., Watkins, W. J., et al. (2009). Assessment of GS-9219 in a pet dog model of non-Hodgkin's lymphoma. Clin. Cancer Res. 15, 3503–3510. doi: 10.1158/1078-0432.CCR-08-3113
Valiaeva, N., Trahan, J., Aldern, K. A., Beadle, J. R., and Hostetler, K. Y. (2010). Antiproliferative effects of octadecyloxyethyl 9-2-(phosphonomethoxy)ethyl guanine against Me-180 human cervical cancer cells in vitro and in vivo. Chemotherapy 56, 54–59. doi: 10.1159/000292582
van Poecke, S., Barrett, M. O., Santhosh Kumar, T., Sinnaeve, D., Martins, J. C., Jacobson, K. A., et al. (2012). Synthesis and P2Y(2) receptor agonist activities of uridine 5′-phosphonate analogues. Bioorg. Med. Chem. 20, 2304–2315. doi: 10.1016/j.bmc.2012.02.012
Williams-Aziz, S. L., Hartline, C. B., Harden, E. A., Daily, S. L., Prichard, M. N., Kushner, N. L., et al. (2005). Comparative activities of lipid esters of cidofovir and cyclic cidofovir against replication of herpesviruses in vitro. Antimicrob. Agents Chemother. 49, 3724–3733. doi: 10.1128/AAC.49.9.3724-3733.2005
Wise, S., Soon, D., Lowe, S., Teng, C. H., Yeo, K. P., Braun, D., et al. (2002). Potent antiviral activity of LY582563 (MCC-478) in chronic hepatitis B infected patients. J. Hepatol. 36, 137–137. doi: 10.1016/S0168-8278(02)80490-1
Wolfgang, G. H. I., Shibata, R., Wang, J., Ray, A. S., Wu, S., Doerrfler, E., et al. (2009). GS-9191 is a novel topical prodrug of the nucleotide analog 9-(2-phosphonylmethoxyethyl)guanine with antiproliferative activity and possible utility in the treatment of human papillomavirus lesions. Antimicrob. Agents Chemother. 53, 2777–2784. doi: 10.1128/AAC.00103-09
Wu, T. F., Froeyen, M., Kempeneers, V., Pannecouque, C., Wang, J., Busson, R., et al. (2005). Deoxythreosyl phosphonate nucleosides as selective anti-HIV agents. J. Am. Chem. Soc. 127, 5056–5065. doi: 10.1021/ja043045z
Xu, B., Stephens, A., Kirschenheuter, G., Greslin, A. F., Cheng, X., Sennelo, J., et al. (2002). Acyclic analogues of adenosine bisphosphates as P2Y receptor antagonists: phosphate substitution leads to multiple pathways of inhibition of platelet aggregation. J. Med. Chem. 45, 5694–5709. doi: 10.1021/jm020173u
Ying, C., Holy, A., Hockova, D., Havlas, Z., de Clercq, E., and Neyts, J. (2005). Novel acyclic nucleoside phosphonate analogues with potent anti-hepatitis B virus activities. Antimicrob. Agents Chemother. 49, 1177–1180. doi: 10.1128/AAC.49.3.1177-1180.2005
Yokota, T., Mochizuki, S., Konno, K., Mori, S., Shigeta, S., and Declercq, E. (1991). Inhibitory effects of selected antiviral compounds on human hepatitis-B virus-DNA synthesis. Antimicrob. Agents Chemother. 35, 394–397. doi: 10.1128/AAC.35.2.394
Yuen, M. F., Kim, J., Kim, C. R., Ngai, V., Yuen, J. C. H., Min, C., et al. (2006). A randomized placebo-controlled, dose-finding study of oral LB80380 in HBeAg-positive patients with chronic hepatitis B. Antivir. Ther. 11, 977–983.
Yuen, M. F., Ahn, S. H., Lee, K. S., Um, S. H., Cho, M., Yoon, S. K., et al. (2015). Two-year treatment outcome of chronic hepatitis B infection treated with besifovir vs. entecavir: results from a multicentre study. J. Hepatol. 62, 526–532. doi: 10.1016/j.jhep.2014.10.026
Yuen, M. F., Han, K. H., Um, S. H., Yoon, S. K., Kim, H. R., Kim, J., et al. (2010). Antiviral activity and safety of LB80380 in hepatitis B e antigen-positive chronic hepatitis B patients with lamivudine-resistant disease. Hepatology 51, 767–776. doi: 10.1002/hep.23462
Yuen, M. F., Lee, S. H., Kang, H. M., Kim, C. R., Kim, J., Ngai, V., et al. (2009). Pharmacokinetics of LB80331 and LB80317 following oral administration of LB80380, a new antiviral agent for chronic hepatitis B (CHB), in healthy adult subjects, CHB patients, and mice. Antimicrob. Agents Chemother. 53, 1779–1785. doi: 10.1128/AAC.01290-08
Zalcharova, V. M., Serpi, M., Krylov, I. S., Peterson, L. W., Breitenbach, J. M., Borysko, K. Z., et al. (2011). Tyrosine-based 1-(S) 3-hydroxy-2-(phosphonomethoxy)propyl cytosine and -adenine ((S)-HPMPC and (S)-HPMPA) prodrugs: synthesis, stability, antiviral activity, and in vivo transport studies. J. Med. Chem. 54, 5680–5693. doi: 10.1021/jm2001426
Zelikman, V., Pelletier, J., Simhaev, L., Sela, A., Gendron, F.-P., Arguin, G., et al. (2018). Highly selective and potent ectonucleotide pyrophosphatase-1 (NPP1) inhibitors based on uridine 5′-Pα,α-dithiophosphate analogues. J. Med. Chem. 61, 3939–3951. doi: 10.1021/acs.jmedchem.7b01906
Keywords: nucleoside phosphonate, antivirals, anticancer drugs, antibacterials, antiparasitic agents, purinergic signaling
Citation: Groaz E and De Jonghe S (2021) Overview of Biologically Active Nucleoside Phosphonates. Front. Chem. 8:616863. doi: 10.3389/fchem.2020.616863
Received: 13 October 2020; Accepted: 30 November 2020;
Published: 08 January 2021.
Edited by:
Petri Turhanen, University of Eastern Finland, FinlandReviewed by:
Samuele Maramai, University of Siena, ItalyCopyright © 2021 Groaz and De Jonghe. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elisabetta Groaz, ZWxpc2FiZXR0YS5ncm9hekBrdWxldXZlbi5iZQ==; Steven De Jonghe, c3RldmVuLmRlam9uZ2hlQGt1bGV1dmVuLmJl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.