- 1State Key Laboratory of Lithospheric Evolution, Institute of Geology and Geophysics, Chinese Academy of Sciences, Beijing, China
- 2Innovation Academy for Earth Science, Chinese Academy of Sciences, Beijing, China
The recent advances in analytical methods of Re-Os and PGE in geological materials including sample dissolution, chemical separation, mass spectrometric determinations, as well as the developments of matrix-matched reference materials for data quality control are thoroughly reviewed. Further, the in-situ measurement methods for Re-PGE mass fractions and 187Os/188Os ratios, as well as the measurement methods for stable isotope ratios of Re and PGE are also briefly reviewed. This review stands as a comprehensive reference for researchers to consider in the development of measurement methods for Re-PGE mass fractions and 187Os/188Os ratios in geological materials.
Introduction
Rhenium, Os, and other PGE (here PGE mainly referring to Ir, Ru, Pt, and Pd; Rh is a mono-isotopic element and not reported in most studies that measure PGE abundances using isotope-dilution methodologies, thus not is focused on this review) are classified as highly siderophile and strongly chalcophile/sulphophile elements (e.g., Shirey and Walker, 1998; Barnes and Ripley, 2016). In addition, Rhenium, Os, and other PGE also show significant organo-phile properties (e.g., Stein and Hannah, 2015). Among these elements, 187Re is decayed to 187Os through β− decay (λ = 1.666 × 10−11 a−1, t1/2 = 42.3 Ga; Smoliar et al., 1996) and 190Pt is decayed to 186Os through α decay (λ = 1.477 × 10−12 a−1, t1/2 = 469.3 Ga; Begemann et al., 2001).
The Re-Os and PGE systems have been widely applied in geosciences in the last decades due to their unique geochemical characteristics (e.g., Allègre and Luck, 1980; Morgan, 1986; Shirey and Walker, 1998; Stein and Hannah, 2015; Day, 2013; Day et al., 2016a; Barnes and Ripley, 2016; Harvey and Day, 2016). The application fields mainly include: 1) tracing planetary formation and evolution, particularly for tracing the late accretion history of Earth, Mar and Moon’s mantle (e.g., Day et al., 2007; Walker, 2009; Walker, 2015; Day et al., 2016a; Walker, 2016; Tait and Day, 2018); 2) tracing the evolution of Earth’s mantle, particularly for subcontinental lithospheric mantle (SCLM) dating (e.g., Walker et al., 1989; Shirey and Walker, 1998; Chu et al., 2009; Rudnick and Walker, 2009); 3) tracing the source of volcanic rock systems (e.g., Xu et al., 2007; Chu et al., 2013; Day, 2013; Chu et al., 2017); 4) dating metal sulfide ore deposits, particularly using molybdenite and low-level highly radiogenic (LLHR) sulfide Re-Os geochronology methods (e.g., Du et al., 1995; Stein et al., 2000; Stein, 2014; Stein and Hannah, 2015); 5) tracing the relative proportions of crust and mantle contributions to sedimentary (e.g., Stein and Hannah, 2015) or magmatic sulfide ore deposits (e.g., Shirey and Walker, 1998; Barnes and Ripley, 2016); 6) tracing the sulfide and/or PGE alloy segregation histories in magmatic systems (e.g., Barnes and Ripley, 2016); 7) age determinations of sedimentations such as in black shales (source rocks for oils) (Ravizza and Turekian, 1989; Creaser et al., 2002; Stein and Hannah, 2015) and even organic-rich carbonates (Rooney et al., 2014; Zhao et al., 2015); 8) investigation of global paleo-environments in earth’s history (e.g., Ravizza and Peucker-Ehrenbrink, 2003; Turgeon and Creaser, 2008; Sato et al., 2013; Stein and Hannah, 2015); 9) dating of petroleum system and fingerprinting hydrocarbon-source rock correlation (Selby and Creaser, 2005a; Selby et al., 2007; Finlay et al., 2011; Finlay et al., 2012; Lillis and Selby, 2013; Georgiev et al., 2016). In addition, although still controversial (e.g., Ireland et al., 2011; Day and O’Driscoll, 2019), the Pt-Os system has potential for tracing the core-mantle interaction (Walker et al., 1997).
Nevertheless, since the Re, Os, and other PGE are usually found in low abundance in geological samples (commonly in pg·g−1 to ng·g−1 levels) and are mainly hosted in trace phases such as sulfides, alloys, and other trace phases, rather than the major silicates (e.g., Lorand and Luguet, 2016), precise determination of Os isotope ratios and Re, PGE mass fractions in geological materials is difficult (Reisberg and Meisel, 2002; Meisel and Horan, 2016). The extremely low procedural blank for Re, Os, and other PGE is a prerequisite for the low uncertainty 187Os/188Os ratio and Re-PGE mass fraction measurement results. In recent decades, a lot of progress has been made in analytical methods for Re-Os and PGE in geological (and environmental) materials, including sample digestion, chemical separation, mass spectrometric determinations, as well as the development of matrix-matched reference materials for data quality control. Accordingly, recent advances in analytical methods (mainly for bulk analysis) for Re-Os and PGE in geological samples are briefly reviewed here.
Sample Dissolution
For bulk Re-Os and PGE analysis of geological materials, the first step is to dissolve the Re-Os and PGE-bearing phases in samples with low procedural blanks. Currently, the sample digestion methods for Re-Os and PGE analysis mainly include: 1) NiS fire assay; 2) Low-temperature acid attack; 3) Alkali fusion, or acid dissolution combined with alkali fusion techniques; 4) Carius tube acid digestion; and 5) HPA (High Pressure Asher) acid digestion.
NiS Fire Assay
The NiS fire assay method can digest large masses (could be >10 g) of samples for the Re-Os and PGE analysis, thus can reduce the “nugget effect” as Re-Os and PGE are usually hosted in heterogeneously distributed trace minerals such as sulfides and alloys (Ravizza and Pyle, 1997; Reisberg and Meisel, 2002). The drawback for the NiS fire assay method is the relatively high reagent blank. Carbonyl nickel or purified nickel (e.g., Sun and Sun, 2005) and sublimed S are preferred for fusion to achieve a lower blank level.
Ravizza and Pyle (1997) developed a NiS fire assay method for Os isotope and PGE analysis from the same sample digest with low procedural blanks. After filtration of the insoluble residue resulting from dissolution of the NiS bead, the filter paper containing the PGE-rich concentrate can be digested with an oxidant such as HNO3 to distill Os for isotopic analysis first, then the residue is processed for further analysis of other PGE (e.g., Hassler et al., 2000; Sun et al., 2009). Nevertheless, Re cannot be quantitatively recovered by the conventional NiS pre-concentration, thus Re is usually determined on a separate sample aliquot. Sun et al. (2009) developed an improved Fe-Ni sulfide fire assay method to allow for determinations of Re, PGE mass fractions and Os isotopic ratios from one sample digest.
Some researchers digested large masses (could be up to >50 g) of rock samples using the NiS fire assay method to obtain sufficient Os (30–200 ng) for ultra-high precision 186Os/188Os measurements by NTIMS (Negative Thermal Ionization Mass Spectrometry), and Os blanks are <1 pg per gram of sample fused (e.g., Brandon et al., 1998; Ireland et al., 2011; Day et al., 2017).
Low-Temperature Acid Attack
Low-temperature acid dissolution method means using HF + HCl + ethanol or HF + HBr in a PFA beaker for the sample digestion (Walker, 1988; Birck et al., 1997; Reisberg and Meisel, 2002). The advantage of the method is that an extremely low procedural blank can be achieved (Os blank <0.05 pg) (Birck et al., 1997; Gannoun et al., 2007). The disadvantage of the method is that complete dissolution of Os-bearing refractory phases, particularly for peridotites, often cannot be achieved (e.g., Meisel et al., 2003a; Qi et al., 2011).
Gannoun et al. (2007) have compared the HF-HBr PFA beaker dissolution with the Carius tube acid digestion for Re-Os analysis of MORB (mid-ocean ridge basalts). Their results indicate that both techniques yield undistinguishable Os mass fractions and 187Os/188Os ratios for MORB samples. Nevertheless, this is not true for all basalts, in particular for the very fresh clinopyroxene rich ones (e.g., Ishikawa et al., 2014).
Qi et al. (2011) developed a PFA bomb HF dissolution method for PGE analysis. Their results show that when using the PFA bomb method, even with a dissolution temperature of 190°C, the ultramafic rocks cannot be effectively digested by HF-HCl but can be effectively dissolved by HF-HCl-HNO3. As oxidized HNO3 is used, the method can only be used for PGE analysis other than Os.
Alkali Fusion, Acid Dissolution Combined with Alkali Fusion Techniques
Fusion with NaOH/Na2O2 has been used with success to dissolve refractory minerals such as spinel, chromite and PGE alloys for Re-Os-PGE analysis (Morgan and Walker, 1989; Sun et al., 2001; Reisberg and Meisel, 2002; Meisel et al., 2003a). This method is useful in particular for total digestion of meteorites (e.g., Morgan and Walker, 1989; Becker and Walker, 2003; Yokoyama et al., 2007; Yokoyama et al., 2010). The main drawbacks for the alkali fusion are that the reagent blanks are usually quite high, and that spike-sample equilibration sometimes cannot be completely achieved (e.g., Qi and Zhou, 2008). In addition, typically only 0.5 g sample can be digested.
Acid attack combined with Na2O2 fusion has been applied for effective sample digestion for Re-Os-PGE analysis. For example, Qi et al. (2004) reported a profound acid attack combined with a Na2O2 fusion method to lower the blank level for PGE analysis other than Os, including the following steps: first, HF, HNO3, and HCl are used to decompose 10 g of the sample; subsequently the fluoride residue from the acid digestion is minimized using H3BO3 for complexation; finally, minimal sodium peroxide is used for fusion of mini-residues.
Carius Tube Acid Digestion
Shirey and Walker (1995) first adopted the Carius tube method (Gordon, 1943; Wichers, et al., 1944; Gordon et al., 1944) for Re-Os isotopic analysis. The Carius tube method permits the dissolution of samples in oxidizing solutions (usually reverse aqua regia) at high temperatures without the loss of volatile OsO4. Typically, 2 g of silicate samples can be digested. For digestion of a sulfide such as pyrite, and a bitumen sample, addition of H2O2 is proven to be helpful (e.g., Qi et al., 2010). The Carius tube acid digestion method can achieve low procedural blanks for Re-Os and PGE. It is well-accepted that HNO3 is the main contributor of Os blank (Birck et al., 1997). It is widely reported that HNO3 can be pre-treated with the H2O2 purging (and N2 bubbling) to reduce its Os blank (e.g., Qi et al., 2010; Chu et al., 2013; Wang and Becker, 2014; Chu et al., 2015a; Yang et al., 2015; Day et al., 2016b; Day et al., 2017; Seo et al., 2018) (note: this technique originally from R. Creaser, University of Alberta, Canada). Usually, after two H2O2 purging purifications of HNO3, the procedural blanks of Os for the Carius tube method could be <0.3 pg.
KClO3/NaClO3-HCl has been used for digestion of Ir, Ru metal with the Carius tube method (e.g., Wichers, et al., 1944; Meisel et al., 2001; Savard et al., 2010; Chu et al., 2015a; Ren et al., 2016).
Meisel et al. (2003a) argued that for a serpentinized peridotite reference material (RM), UB-N, temperatures between 230 and 240°C, digested in Carius tubes were insufficient to completely digest its HSE-bearing spinels and HSE alloys; the highest yields of HSE were obtained at temperatures of ≥300°C in the HPA. Differently, Harvey et al. (2011) found little difference between HPA and Carius tube results for UB-N.
Becker et al. (2006) reported a modified method (c.f., Gordon et al., 1944) by putting the sealed Carius tube into a pressure steel jacket containing dry ice. The CO2 pressure that builds up inside the steel pressure vessel upon heating balances the internal pressure in the Carius tube, such that the digestion temperature could be as high as 345°C. For some mantle xenoliths, samples digested at 345°C give higher Os mass fractions and lower 187Os/188Os ratios compared to digestions at 220–230 °C. Similarly, Qi et al. (2007) put the Carius tubes in a steel pressure vessel containing water to prevent the explosion of the Carius tubes; 12 g silicate sample can be digested, and the digestion temperature can be set to 320°C thus allowing a more completed digestion of refractory minerals.
For silicate samples, some researchers used de-silicification with HF, before or after dissolution using aqua regia in a Carius tube (e.g., Meisel et al., 2003a; Qi and Zhou, 2008; Ishikawa et al., 2014; Li et al., 2015; Day et al., 2016b). Qi and Zhou (2008) reported that, for ultramafic reference material (RM) OKUM, about 4–15% of the PGE are in the silicate phase, which cannot be leached out by aqua regia even when digested at 300 °C with the modified Carius tube technique. Ishikawa et al. (2014) reported that: 1) improved results in RM TDB-1 for Re and Ru are obtained by additional treatment with hydrofluoric acid before or after Carius tube processing (c.f. Table 1); 2) for RM BIR-1, it is necessary to use HF desilicification to improve HSE recoveries, particularly Ru (c.f. Table 1). Li et al. (2015) reported that: 1) for RMs BHVO-2, TDB-1, and AGV-2 the HF desilicification increases the Re extraction efficiency (by 9–15%) and thus a small proportion of Re likely resides in silicate phases; 2) for RMs WGB-1 and WPR-1, Re extraction efficiencies obtained by the Carius tube digestion with and without HF desilicification are similar, indicating that Re in these rocks may dominantly reside in sulfides; 3) HF desilicification increases Os extraction efficiency in some RMs (e.g., BHVO-2 and AGV-2), suggesting that a portion of Os-rich trace phases may occur as inclusions in the silicate phases at ∼75 μm sizes. Differently, Day et al. (2016b) demonstrated that no systematic differences in HSE abundances are observed between data obtained by the Carius tube method with and without HF-desilicification for some intraplate basalts. Further, Day et al. (2016b) emphasized that: 1) if a Carius tube acid digestion to liberate Os, followed by HF-desilicification to obtain Re and Pt abundances, the measured Re/Os and Pt/Os may not correspond with measured 187Os/188Os and 186Os/188Os; 2) the sample solutions after the HF acid desilicification are much more complex with respect to potentially interfering elements, thus more complex column chemistry procedures are usually required.
Qi et al. (2010) developed an improved Carius tube for determination of low levels of Re and Os in pyrites. The pyrites are predigested in an open Carius tube, the produced Os is trapped by a chilled HCl solution. The trapped Os is then put back to the Carius tube for the further complete digestion. The technique allows increasing the sample mass of pyrites to about 3 g.
For Re-Os isotopic analysis of quartz-bearing molybdenite, Lawley and Selby (2012) used concentrated HF to pre-dissolve quartz to isolate molybdenite at room temperature in a PFA beaker, prior to Carius tube digestion.
Selby and Creaser (2003) reported that, in case of Re-Os isotope analysis for organic-rich sedimentary rocks such as black shales, CrO3-H2SO4 (20% CrO3 in 2 mol/L H2SO4) is preferred for sample digestion in the Carius tube. Different from aqua regia, CrO3-H2SO4 can selectively dissolve the hydrogenous Re and Os without dissolving or significantly leaching clastic or detrital minerals (Selby and Creaser, 2003; Kendall et al., 2004). This is critical for Re–Os geochronology and Os isotope stratigraphy of organic-rich sedimentary rocks. With the CrO3-H2SO4 digestion method, black shale Re–Os geochronology with an age measurement uncertainty <1% (even 0.5%) can be achieved (e.g., Kendall et al., 2004; Selby and Creaser, 2005b; Kendall et al., 2006; Zhu et al., 2013). Differently, Yin et al. (2017) used HNO3-H2O2 for digestion of organic-rich sedimentary rock to reduce the leaching Os from crustal detrital minerals (c.f. Table 1).
HPA Acid Digestion Method
The HPA (high pressure asher) digestion method was first used for Re-Os and PGE analysis of silicate samples by Meisel’s group from Montanuniversität Leoben, Austria (Meisel et al., 2001; Meisel et al., 2003a; Meisel et al., 2003b; Meisel and Moser, 2004a; Meisel and Moser, 2004b). The method uses a high pressure asher (HPA, Anton Paar, Graz) which employs reusable quartz glass containers to maintain a high temperature and high pressure under controlled conditions to allow a rapid and more complete sample digestion. Up to 3 g of silicate sample powder can be digested in reverse aqua regia in the HPA vessels at 300–320°C. The HPA digestion method has a lower PGE blank level compared to the Carius tube method, particularly Pt and Pd (e.g., Meisel et al., 2001; Meisel et al., 2003b). Thus, the HPA method is particularly useful for digestion of low PGE samples such as Apollo lunar basalts, which contain extremely low Re, Os and other PGE (at least twenty times lower than terrestrial basalts) (e.g., Day et al., 2007; Day and Walker, 2015). The main disadvantage of this technique is the high cost of the apparatus. Some researchers argued that even with the HPA method, for some silicate samples HF de-silicification is still necessary (e.g., Dale et al., 2012; Ishikawa et al., 2014).
Chen and Sharma (2009) and Seo et al. (2018) used HPA to allow for digestion of as much as 50 ml seawater or snow water, using 0.5 ml 8% CrO3-6 mol/L H2SO4 or 2.5 ml HNO3 + 1.5 ml H2O2 as oxidant, digested at 300°C to provide full Os oxidation.
Some researchers (e.g., Georgiev et al., 2016; Hurtig et al., 2020) used HPA for digestion of oil samples with HNO3 for Re-Os analysis, which essentially allows for larger samples to be processed compared to the Carius tube digestion.
Chemical Separation
Osmium Extraction
After sample digestion, two main classes of techniques can be used to separate Os from the dissolved sample matrix: distillation and liquid-liquid extraction (Reisberg and Meisel, 2002).
Distillation
After Carius or HPA digestion, the Os can be directly distilled (e.g., Shirey and Walker, 1995; Chen and Sharma, 2009). For alkali fusion or NiS fusion methods, the Os can be oxidized by the addition of a strong oxidizing agent, such as Ce(SO4)2 + H2SO4, CrO3 + H2SO4, HNO3, or H2O2 solution (e.g., Walker, 1988; Morgan and Walker, 1989; Ravizza and Pyle, 1997; Markey et al., 1998; Hassler et al., 2000; Sun et al., 2001; Sun et al., 2009). Traditionally, the distillations are performed in assembled Pyrex glassware and large quantities (∼100 ml) of distillation solution are used (e.g., Shirey and Walker, 1995; Markey et al., 1998; Sun et al., 2001; Sun et al., 2009). The distillation apparatus can be pre-distilled with the pure reagents to reduce the blank level. Nägler and Frei (1997) first used commercially available PFA (Savillex) vials and tubing for the miniaturized distillation. Some authors developed in situ Caius tube distillation methods without the use of distillation flask to further reduce the Os blank (e.g., Qi et al., 2010). Jin et al. (2013) developed a device for a batch of simultaneous Carius tube in situ distillations, which can offer significantly higher sample throughput. Woodhouse et al. (1999) developed a method for the direct distillation of Os from large volumes of seawater (1–1.5 L, addition of H2O2 in H2SO4 to oxidize the spiked Os) for subsequent Os isotopic analysis.
Liquid-Liquid Extractions
CCl4/CHCl3 extraction is the most frequently used method for extraction Os from the sample matrix (Cohen and Waters, 1996; Shen et al., 1996). After the sample is digested by the Carius tube, the Os can be extracted from the reverse aqua regia or CrO3-H2SO4 solution directly into the CCl4 or CHCl3. Subsequently, the Os is back-extracted by HBr.
Another frequently used method involves extraction of OsO4 into liquid Br2 developed by Birck et al. (1997). For the HF-HBr sample dissolution method, CrO3 dissolved in HNO3 and liquid Br2 are used for oxidation of Os to OsO4 and Br2 extraction (Birck et al., 1997). For the Carius tube or HPA dissolution method, as Os has already be oxidized to OsO4, it is plausible to perform the Os extraction without the addition of CrO3 (e.g., Paul et al., 2009; Seo et al., 2018). For example, Paul et al. (2009) used the HPA method for digestion of 50 ml surface or subsurface waters, at high temperature (≥250°C), using 0.5 ml H2O2 and 0.5 ml H2SO4 as an oxidant to oxidize Os species. Subsequently, the OsO4 is directly extracted by addition of 2 ml liquid Br2. In order to compensate for the small ratio of Br2 to water (about 2:50), they increased the extraction time and perform the procedure twice.
The Br2 extraction technique allows extremely low Os blanks to be achieved (Birck et al., 1997), permitting the analysis of extremely small quantities of Os. Levasseur et al. (1998) developed a technique by adding a mixture of Br2, CrO3, and H2SO4 to a spiked water sample (∼50 ml) in a 120 ml PFA pressure vessel, and heating at 90°C in an oven for at least 72 h to achieve spike-sample equilibration. The Os is oxidized to OsO4 and directly extracted from water into Br2 for subsequent mass spectrometric measurement. The total procedural blanks are <20 fg.
Micro-Distillation
For NTIMS Os isotope ratio measurements, the final Os purification is usually performed using a micro-distillation method developed by Birck et al. (1997). Birck et al. (1997) reported that the Os recovery during micro-distillation is about 70–90%. Recently, Nakanishi et al. (2019) further investigated the factors that affect the Os recovery during micro-distillation. They found that the most critical factor controlling the chemical yield of Os during micro-distillation is the extent of the dilution of the reductant (HBr) in the tip of the conical beaker by H2O evaporated from the oxidant. The H2O evaporation (and resultant HBr dilution) is suppressed by minimizing the volume of the oxidant solution and increasing the concentration of H2SO4 in the CrO3-H2SO4 oxidant solution as high as possible. In addition, the micro-distillation temperature should be <80°C, to reduce the evaporation of H2O into the HBr.
Day et al. (2016b, 2017) performed two or three micro-distillations to further improve the Os purity for NTIMS Os isotope ratio measurements.
Pearson et al. (1998); Harvey et al. (2006); Gannoun et al. (2007); Harvey et al. (2011) and Warren and Shirey (2012) used a method similar to the micro-distillation method for Os isotopic analysis of individual hand-picked sulfide grains. Rhenium and Os spikes and the sulfide grains are put onto the inverted cap of the conical 5 ml PFA vial and dried, CrO3-H2SO4 is then added to cover the sulfide grains to do the micro-digestion and micro-distillation. Pearson et al. (1998) have compared replicate micro-digestion dissolutions of single sulfide grains with a mini Carius-tube dissolution of a large, multi-crystal aggregate. The results indicate that the micro-digestion technique is effective at digesting the sulfides, and in achieving Os spike–sample equilibration.
Separation of Re and the PGE
Separation of Re
In the case of only Re-Os analysis, the most commonly used method for Re separation is the anion exchange method (AG1-X8) (e.g., Morgan and Walker, 1989; Pearson and Woodland, 2000; Chu et al., 2015a). Re can be further purified by a small secondary anion-exchange column (e.g., Morgan and Walker, 1989; Du et al., 2004) or single anion beads clean-up method (Georgiev et al., 2018). As Cr6+ may be retained by the anion exchange resin as CrO42− to some extent, when CrO3-H2SO4 digestion method is used, it is necessary to reduce Cr6+ to Cr3+ using C2H5OH, H2O2, or H2SO3 (e.g., Selby and Creaser, 2003) before sample loading onto the column.
Another frequently used method to separate Re from sample matrix is NaOH (>5 mol/L)-acetone extraction (Du et al., 1995; Du et al., 2004; Georgiev et al., 2018). The NaOH can be pre-purified by acetone extraction to remove Re. The Re extracted by acetone can be further purified by a small anion exchange column (Du et al., 2004) or a resin bead clean-up step (Georgiev et al., 2018) if NTIMS method is applied for the Re measurement.
Sun et al. (2010) used only HNO3 for digestion of molybdenite in a Carius tube; after Os distillation, the Re is directly measured by ICP-MS without additional purification.
Separation of Re and Ir-Ru-Pt-Pd
When isotope-dilution (ID) method is applied, the most frequently used methods are cation (e.g., Meisel et al., 2003b; Qi et al., 2004; Shinotsuka and Suzuki, 2007; Fischer-Gödde et al., 2011; Li et al., 2014) or anion exchange methods (e.g., Rehkämper and Halliday, 1997; Pearson and Woodland, 2000; Chu et al., 2015a).
Cation Exchange
PGE and Re exist as chloro-complex anions and ReO4− respectively in dilute HCl media, while major matrix elements exist as cations. Thus, PGE and Re can be separated collectively from sample matrix with high recovery using the cation exchange methods (e.g., Fischer-Gödde et al., 2011; Li et al., 2014). The main drawback with cation exchange separations is that relatively large amounts of resin are necessary to absorb the non-PGE ions to provide a good analyte/matrix separation and cleaning of the resin with large volumes of clean acid is required. In addition, some interfering elements such as Zr, Hf may not be efficiently separated from PGE (ZrO interferes with Pd; HfO interferes with Ir-Pt) (Qi et al., 2004; Shinotsuka and Suzuki, 2007; Li et al., 2014).
Meisel et al. (2003b) developed a low pressure (application of 2 Bar N2) cation exchange method with a long and thin column (1 m length, 4.2 mm inner diameter) that is coupled to a quadrupole ICP-MS for on-line matrix separation to have a full control over interference removal (e.g., Pd from Cd). Qi et al. (2004); Qi et al. (2007) used Te-coprecipitation to collect PGE from Na2O2 fusion or modified Carius tube digestion first, followed by a column packed with cation exchange (upper part) and HDEHP extraction (lower part) resins for further interfering elements removal. They concluded that the HDEHP resin is very efficient for removal of the Zr and Hf. Similarly, Ren et al. (2016) used a tandem assembly of mini-cation and Ln resin columns (both 5 mm inner diameter, 50 mm length) for the purification of Ru, Rh, Pd, Ir and Pt after a fire assay sample digestion. Shinotsuka and Suzuki (2007) further purified the PGE separated from cation exchange columns by solvent extraction using N-benzoyl-N-phenylhydroxylamine (BPHA) as an extractant to remove Zr, Hf, Mo. Differently, Li et al. (2014) coated the BPHA extractant onto an Amberchrom CG-71 m chromatographic grade material to make a column for a further removal of Zr, Hf and Mo from PGE. More recently, Zhou et al. (2019) reported that the abundance of interfering elements tends to increase in the eluent when conventional ion-exchange purification procedures are applied to de-silicified samples for Carius tube or HPA digestion method. They used an Ln column to further remove Zr, Hf after a cation exchange separation of PGE.
Anion Exchange
In contrast to cation exchange, with the anion exchange method, the PGE and Re are strongly retained on the resin (typically AG 1−X8) in dilute HCl media as chloro-complex anions and ReO4− respectively, while most major and trace elements pass through the column directly (e.g., Rehkämper and Halliday, 1997; Pearson and Woodland, 2000; Meisel et al., 2001; Chu et al., 2015a). Thus, a small amount of resin is sufficient for the separation. The anion exchange method has the advantage of being able to separate Re and PGE into different fractions in subgroups such as Re + Ru and Pt + Ir, and thus is suitable for ID measurements of Re, Ru, Ir-Pt and Pd individually by MC-ICP-MS with high precision (e.g., Day et al., 2003; Chu et al., 2015a). The main drawback for anion exchange method mainly includes: 1) it is required to use high molarity acids (HCl or HNO3) to elute Re-PGE, particularly Ir-Pt and Pd; 2) the recovery of some elements such as Ir, Ru, Pd is relatively low and unstable (e.g., Chu et al., 2015a). Meisel et al. (2001) digested the resin absorbed Re-PGE in an HPA and the solution was measured with ICP-MS directly to overcome these problems. Again, it is necessary to reduce Cr6+ to Cr3+ with H2O2 or C2H5OH prior to anion exchange chromatography for high Cr sample (e.g., Meisel et al., 2003a; Dale et al., 2012). In addition, Zr and Hf can also form stable anion complexes in HCl media, which can sometimes be absorbed by the anion resin, creating oxide interferences on Pd (ZrO+) and Ir-Pt (HfO+) (Pearson and Woodland, 2000; Meisel et al., 2001; Chu et al., 2015a; Day et al., 2016b). Some researchers used 1 mol/L HCl-1 mol/L HF to elute Zr-Hf prior to PGE elution (Pearson and Woodland, 2000; Day et al., 2016b). Chu et al. (2015a) used an Ln column for secondary purifications of the Ir-Pt and Pd to remove Zr, Hf.
It should be noted that, although the PGE belong to the least abundant group of elements in the Earth’s crust, they may be elevated through anthropogenic influence on the environment, for example the use of automobile catalytic converters (Meisel et al., 2003b; Meisel and Horan, 2016). Consequently, as an example, the road dust has relatively high PGE content, particularly Pt, Pd (e.g., Zhao et al., 2014). Thus, a dust-free ultraclean lab is important for the PGE chemistry, particularly for low PGE samples.
Mass Spectrometric Determination
Os Isotopic Measurement
Currently, there are mainly two kinds of mass spectrometric method for Os isotopic analysis: NTIMS and MC-ICP-MS (Multiple Collector-Inductively Coupled Plasma-Mass Spectrometry). For samples with extremely high 187Os/188Os, such as molybdenite, quadrupole or single collector magnetic sector ICP-MS can also be applied.
NTIMS
The most widely used technique for Os isotopic analysis is NTIMS (Creaser et al., 1991; Völkening et al., 1991). Osmium is measured as OsO3− on Pt-filament material using Ba(OH)2 as an electron emitter. The ion yield (ions detected by collector/atoms loaded) of OsO3− can be >10% (Birck et al., 1997; Birck, 2001). Therefore, the NTIMS technique can analyze extremely low amounts of Os (down to several pico-grams) with high precision. For example, the NTIMS technique has been used for Os isotopic analysis of samples with extremely low Os mass fractions, such as seawaters (e.g., Levasseur et al., 1998; Woodhouse et al., 1999; Chen and Sharma, 2009), river and surface/subsurface waters (e.g., Sharma and Wasserburg, 1997; Paul et al., 2009), snow or ice water (Seo et al., 2018), ferromanganese crusts (e.g., Klemm et al., 2008), oils (e.g., Selby and Creaser, 2005a), hydrothermal sulfides (e.g., Stein et al., 2000), MORB (e.g., Gannoun et al., 2007) and lunar basalts (e.g., Day and Walker, 2015). For such kinds of studies, in addition to instrumentation, the most important factor that affects the data quality is the total procedural Os blank (should be extremely low).
Liu and Pearson (2014) and Wang et al. (2017) have used multi-collector Faraday cups equipped with 1012 and 1013 Ω amplifiers for high-precision Os isotopic analysis of low Os samples (could be as low as ∼0.025 ng), respectively.
An important problem associated with the NTIMS method is the correction of the minor oxide species such as Os16O217O− and Os16O218O− interferences on the signals of the primary Os16O3− (e.g., Liu et al., 1998). Luguet et al. (2008); Chatterjee and Lassiter (2015) and Chu et al. (2015b) have reported in-run isobaric oxide correction methods for Os isotopic analysis with an improved precision.
For samples with low mass fractions of common Os and high mass fractions of radiogenic 187Os, such as molybdenite and LLHR sulfides, there is no isotopic ratios for internal mass fractionation correction. These problems can be resolved using a double Os spike (188Os–190Os, Markey et al., 2003; or 186Os-188Os, Qu et al., 2001) or using common Os as a double spike if the molybdenites contain no common Os (e.g., Suzuki et al., 1992; Selby and Creaser, 2001; Li et al., 2017). When utilizing NTIMS for Os measurements and a double spike or a common Os is used for mass fractionation correction, molybdenite Re-Os dating can provide ages with <0.1% level measurement uncertainties (Markey et al., 2003; Li et al., 2017).
MC-ICP-MS
Another technique used for Os isotopic analysis is ICP-MS (for isotopic analysis, mainly use MC-ICP-MS) (e.g., Schoenberg et al., 2000; Nowell et al., 2008a). The main advantage of ICP-MS measurements is the rapidity relative to NTIMS measurements. Nevertheless, currently, the Os ion yield, and thus, the precision of ICP-MS analysis is significantly lower than that of NTIMS [ion yield: traditional MC-ICP-MS, ∼0.08% for Os introduction in a reduced state (e.g., Nowell et al., 2008a); NTIMS >10% (e.g., Birck et al., 1997; Birck, 2001)]. Although the oxidized state of the Os (as OsO4 in H2O) for introduction into the ICP source has a much higher ion yield (at least 30 times higher than introduction of Os in a reduced state; e.g., Sun et al., 2001), the OsO4 has an extremely strong memory effect in the introduction system of ICP-MS (e.g., Sun et al., 2001; Sun et al., 2009). It has been reported that dilute NH2OH·HCl or H2NNH2·H2O can allow a more effective rinsing between Os measurements for the OsO4 introduction method (Meisel et al., 2001; Sun et al., 2001; Sun et al., 2009). In addition, more complex interfering isobaric elemental ions are usually generated by the plasma, compared to the NTIMS method.
One advantage for ICP-MS is a rapid sparging technique that can be used to extract OsO4 from the sample solution and transferred directly into the machine for isotope ratio measurements (Hassler et al., 2000; Norman et al., 2002; Nozaki et al., 2012; Sen and Peucker-Ehrenbrink, 2014). The elimination of the nebulizer and spray chamber assembly with the sparging method minimizes memory problems. A problem is that signal intensities usually decrease throughout a sparging analysis, which limits the analytical precision of the Os isotope ratio measurement results. Jin et al. (2011) developed a method to directly transfer OsO4 vapor from a Carius tube into an ICP-MS source for Os measurement, with a solution containing Ir being aspirated for mass bias correction.
Nozaki et al. (2012) reported that the sparging method combined with CrO3-H2SO4 digestion of organic-rich sedimentary rocks and multi-ion-counters (MIC) in the mass spectrometry is expected to be a powerful tool for reconstructing the secular change in marine Os isotope compositions with high sample throughput. Sen and Peucker-Ehrenbrink (2014) suggested to use the fast-sparging method to screen large numbers of oil and their source rock samples for finding a set of samples with spread in 187Re/188Os for further high-precision isochron analyses by N-TIMS.
Determination of Re and PGE Mass Fractions
Rhenium and the PGE mass fractions are usually measured by ICP-MS with ID method. Some labs use NTIMS for determination of Re (e.g., Markey et al., 2007; Liu and Selby, 2018). When NTIMS is used, it should be noted that trace amount of Re is probably present in the Pt filament (e.g., Day et al., 2003), and thus often Ni filament is applied with Ba(NO3)2 as an ion emitter. In addition, the thermal fractionation effect for Re isotopes with the NTIMS method is difficult to correct. Suzuki et al. (2004) developed a method for low uncertainty Re isotope ratio measurements by NTIMS using a total evaporation technique.
Measurements of isotope ratio of the Re and PGE for ID quantification is by ICP-MS, either single collector analysis using a magnetic sector field (ICP-SFMS, e.g., Element XR) (e.g., Fischer-Gödde et al., 2011; Wang and Becker, 2014) or quadrupole (ICP-QMS) mass spectrometer (e.g., Pearson and Woodland, 2000; Meisel et al., 2003b; Qi et al., 2004; Li et al., 2014; Day et al., 2016b), or by MC-ICP-MS (e.g., Day et al., 2003; Chu et al., 2015a). The mass bias effects are usually corrected by the sample-standard bracketing (SSB) method (e.g., Day et al., 2003; Chu et al., 2015a). In case of only Re analysis, the mass bias effect can also be corrected using doped Ir (e.g., Schoenberg et al., 2000; Day et al., 2003). The ICP-QMS and ICP-SFMS can measure all of the PGE and Re in a single aliquot and monitor the interfering elements in-run. Comparatively, the MC-ICP-MS technique has the highest sensitivity (or signal/noise ratio), thus is more suitable for analysis of extremely low amounts of Re and PGE (e.g., Day et al., 2003). However, it is difficult to monitor all interfering elements in run with the MC-ICP-MS technique. It is useful to use a desolvator to reduce isobaric oxide interferences for PGE determination using ICP-MS (e.g., Pearson and Woodland, 2000; Fischer-Gödde et al., 2011).
Stable Isotope Ratio Measurements for Re and PGE
Due to the unique chemical characteristics of Re and PGE, the stable isotopic ratios of Re and PGE are also useful for investigating the planetary formation, differentiation processes, as well as the Earth’s paleoredox history (Hoefs, 2018), and particularly nucleosynthetic isotope variations in the solar-system (e.g., Becker and Walker, 2003; Yokoyama et al., 2007, 2010; Chen et al., 2010; Fischer- Gödde et al., 2015; Yokoyama and Walker, 2016).
Nanne et al. (2017) described high-precision MC-ICP-MS and N-TIMS Os isotope ratio measurement methods using a 188Os-190Os double spike technique. More recently, Zhu et al. (2018) provided a new mass fractionation correction method for MC-ICP-MS to determine the absolute Os isotope ratios which does not rely on Nier’s values (192Os/188Os = 3.083, Nier, 1937). They determined Os isotope ratios using NRC (National Research Council Canada) IRIS-1 Ir for normalization. The Ir isotope ratio of IRIS-1 has been certified by NRC, determining by MC-ICP-MS using NIST SRM 997 Tl and NIST SRM 989 Re for normalization (Zhu et al., 2017). It should be noted that, the Os isotope ratios traceable to NRC IRIS-1 Ir probably are closer to the “true values” but with a larger uncertainty compared to those arbitrarily normalized to 192Os/188Os = 3.083. A precise MC-ICP-MS technique to measure Re isotope variations has been presented by Miller et al. (2009). Yokoyama et al. (2007); Yokoyama et al. (2010) determined Os isotope ratios in chondrites in order to resolve possible nucleosynthetic isotope anomalies.
Creech et al. (2013); Creech et al. (2014) described a precise MC-ICPMS technique measuring 198Pt/194Pt ratios (using a 196Pt–198Pt double spike) relative to the IRMM 010 Standard. 11 international geological standard reference materials were determined for 198Pt/194Pt variations. Creech et al. (2017) described a MC-ICP-MS technique by measuring 106Pd/105Pd ratios (using a 106Pd–110Pd double spike) in a variety of terrestrial and extraterrestrial materials. Hunt et al. (2018) developed a method for measuring Pt isotope ratios of ion meteorite samples. More recently, Creech et al. (2020) used a MC-ICP-MS equipped with 1013 Ω amplifiers for Pt isotope ratio measurements of samples with low Pt abundances.
Ruthenium isotope ratios can be measured using either NTIMS (e.g., Becker and Walker, 2003; Chen et al., 2010) or MC-ICP-MS (Becker et al., 2002; Fischer- Gödde et al., 2015). Chen et al. (2010) used a mixture of Ba(NO3)2 and Ba(OH)2 (in the approximate ratio of 1:10) as ion emitter to improve the ionization efficiencies of RuO3− for NTIMS analysis. The Ru can be separated from sample matrix by cation (Becker et al., 2002; Becker and Walker, 2003; Fischer- Gödde et al., 2015) or anion exchange (Chen et al., 2010) chromatography and then be further purified by distillation or micro-distillation methods (distillation as RuO4) similar to that for Os. The above techniques have been used to determine Ru isotope ratios of meteorites to resolve Ru isotope anomalies (e.g., Becker and Walker, 2003; Chen et al., 2010; Fischer- Gödde et al., 2015). Recently, Hopp et al. (2016) developed a MC-ICP-MS method to measure precise 102Ru/99Ru ratios (using a 98Ru–101Ru double spike) to resolve natural Ru stable isotope variations.
In-situ Techniques for Os Isotope Ratio and Re, PGE Mass Fraction Measurements
LA-(MC)-ICP-MS
LA (Laser ablation)-MC-ICP-MS is the most important in-situ technique for Os isotope ratio measurements. A problem associated with LA-MC-ICP-MS method is the isobaric interference of 187Re on 187Os (Nowell et al., 2008b). Thus, the method is only suitable for 187Os/188Os measurements of minerals with high Os but low Re mass fractions (at least 187Re/188Os ratios <0.5, Nowell et al., 2008b), such as primary mantle sulfides (e.g., Pearson et al., 2002; Alard et al., 2002; Alard et al., 2005) and Os-bearing alloys (e.g., Hirata et al., 1998; Walker et al., 2005; Nowell et al., 2008b). Another problem is the 186W interference on 186Os if 186Os/188Os is required to measure (Hirata et al., 1998; Walker et al., 2005; Nowell et al., 2008b). For Pt-bearing PGA (Platinum Group Alloy) analyses by LA-MC-ICP-MS, it is the better to use 189Os/188Os rather than 192Os/188Os for instrumental mass fractionation correction because of isobaric interferences on 192Os from 192Pt (Hirata et al., 1998; Nowell et al., 2008b).
Pearson et al. (2002) firstly established a LA-MC-ICP-MS technique for direct measurement of mantle sulfides containing Os at trace element (tens or hundreds of μg·g−1) levels. For sulfides enclosed in silicate minerals (e.g., olivine) larger than 50 μm in diameter and with Os contents >40 μg·g−1, the method can give 187Os/188Os ratios with a precision of 0.1% (2 RSE). The technique has been used to demonstrate distinct differences in Os isotopic composition between sulfide enclosed in silicates and intrastitial sulfides in peridotites (Pearson et al., 2002; Alard et al., 2002; Alard et al., 2005). The sulfide inclusions in silicates preserve significantly lower 187Os/188Os than interstitial sulfides and accordingly produce significantly older and more realistic Re-Os age information. Interstitial sulfides typically have lower Os (10–30 μg·g−1), higher Re and give analyses with higher uncertainty (∼1–2%) but still provide valuable information.
Hirata et al. (1998) firstly used LA-MC-ICP-MS for Os isotope ratio measurements of PGE alloys such as iridosmines. Walker et al. (2005) used LA-MC-ICP-MS for 186Os/188Os and 187Os/188Os measurements of Os–Ir–Ru alloy grains from southwestern Oregon, United States. Compared to the SIMS (Secondary Ionization Mass Spectrometry) technique (e.g., Meibom et al., 2002; Meibom and Frei, 2002), the LA-MC-ICP-MS can measure isotope ratios of PGE alloys with a lower measurement uncertainty at a lower analytical cost. Nowell et al. (2008b) presented a rapid (40 s acquisition time) LA-MC-ICP-MS methodology suitable for applying Pt–Os and Re–Os geochronology approaches to single PGA grains, for use in dating chromitite deposits and identifying and dating multiple sources in alluvial PGA deposits.
The LA-ICP-MS techniques has been widely used for determination of PGE mass fractions in mantle sulfide (e.g., Alard et al., 2000; Lorand et al., 2008; Lorand et al., 2010; Lorand and Luguet, 2016). For the measurement, a synthetic PGE doped NiS bead (PGE-A) is used as an external calibration standard. Their results show that sulfides enclosed in silicate phases have high osmium and iridium abundances but low Pd/Ir ratios, whereas pentlandite-dominated interstitial sulfides show low osmium and iridium abundances and high Pd/Ir ratios. They interpreted the silicate-enclosed sulfides as the residues of melting processes and interstitial sulfides as the crystallization products of sulfide-bearing (metasomatic) fluids. Lorand et al. (2008); Lorand et al. (2010) used time resolved analysis by LA-ICP-MS to find the PGE alloy micro-nugget in mantle sulfide grains.
LA-ICP-MS has also been extensively applied for the PGE analysis of iron meteorites, chondrites, as well as some achondrite meteorite groups (Campbell and Humayun, 1999; Day et al., 2016a).
Parent-daughter (187Re–187Os) decoupling within molybdenite crystals has ever been confirmed by LA-(MC)-ICP-MS studies (Stein et al., 2003; Selby and Creaser, 2004).
Scanning Electron Microscope, Synchrotron Radiation X-Ray Fluorescence and Atom Probe Microscopy In-Situ Techniques
The scanning electron microscope (SEM) technique has frequently been used to find and detect PGE micro-nuggets in rock samples (e.g., Luguet et al., 2007; Lorand et al., 2008; Lorand et al., 2010; Lorand and Luguet, 2016).
Kogiso et al. (2008) employed microbeam SR-XRF to detect micrometer-scale platinum-group minerals in an orogenic lherzolite sample. Daly et al. (2017a) used SR-XRF for in-situ analysis of PGE-rich refractory metal nuggets in carbonaceous chondrites.
Atom probe microscopy (APM) is a relatively new in situ tool for quantifying elemental and isotopic compositions at a sub-nanometer spatial resolution (<0.02 μm3 in volume) in geological materials (Reddy et al., 2020). Parman et al. (2015) applied the laser-assisted APM technique for measurements of Pt, Fe, Ir, Ni, Rh, Ru, and Cu mass fractions and Pt, Ru, Ir isotope ratios of Pt3Fe (isoferroplatinum) inclusions in an Os-Ir alloy (osmiridium) grain. Daly et al. (2017b) performed APM analyses of sub-micrometer PGE-rich refractory metal nuggets contained within a Sc-Zr–rich ultrarefractory inclusion from the ALH 77307 CO3.0 meteorite. More recently, Daly et al. (2018) employed the laser-assisted APM technique to measure Re and Os isotope ratios of pure Os, pure Re, and synthetic Re-Os-bearing alloys, demonstrating that the APM technique permits nanoscale Os isotope ratio measurements of Os-bearing alloys to yield similar, though less precise, ages to NTIMS analyses. At current, development and application of the analytically expensive and highly-specific APM technique in geosciences is still in its early stages (Reddy et al., 2020).
Reference Materials for Data Quality Control of Re-Os and PGE Analysis
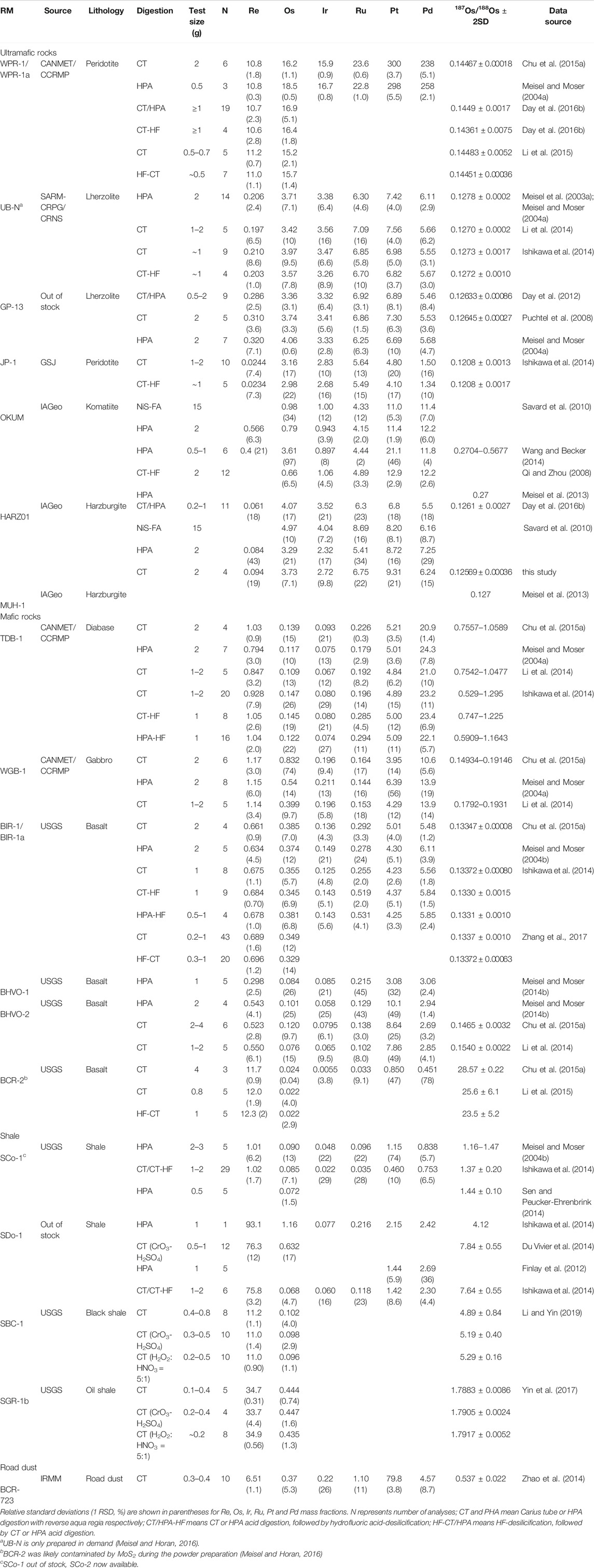
Reference materials (RMs) play a key role in method development and quality control in routine analyses (Meisel and Horan, 2016). Table 1 lists recently published 187Os/188Os and Re-PGE mass fraction data for the frequently used rock RMs (except for BCR-723, which is an environmental RM).
Ultramafic Rock Reference Materials
Among ultramafic RMs (Table 1), WPR-1/WPR-1a (altered peridotite), UB-N (serpentinized Lherzolite), and GP-13 (Lherzolite) are relatively easy to digest and proven to be relatively homogenous in Re-PGE mass fractions and 187Os/188Os ratios at 0.5–2 g sample size levels (Table 1) (e.g., Meisel et al., 2003a; Meisel and Moser, 2004a; Putchtel et al., 2008; Li et al., 2014; Day et al., 2012; Chu et al., 2015a; Day et al., 2016b), indicating that the Re-PGE are mainly hosted in base metal sulfides (e.g., Meisel et al., 2003b; Meisel et al., 2013; Day et al., 2016b; Meisel and Horan, 2016). Nevertheless, UB-N is only prepared on demand (Meisel and Horan, 2016); this means that the PGE composition can possibly change from batch to batch. GP-13 is now not available.
MUH-1 (harzbugite) and OKUM (komatiite) were prepared, bottled at the same time and tested for homogeneity. In addition their major and trace element mass fractions have been certified according to ISO Guides (Meisel and Horan, 2016). Qi and Zhou (2008) showed that OKUM is relatively homogeneous in PGE mass fractions at 2 g test portions (OPY-1 in Qi and Zhou (2008) is the same as OKUM; Meisel, personal communication) (Table 1). Nevertheless, Wang and Becker (2014) demonstrated that this RM is highly heterogeneous both in Re-PGE mass fractions and 187Os/188Os ratios, in particular between bottles. HARZ01 was also prepared by IAGeo limited as a harzbugite RM. It has been shown that harzbugite RMs (HARZ-01/MUH-1) are heterogeneous in Re-PGE mass fractions but relatively homogeneous in 187Os/188Os ratios (Table 1; Savard et al., 2010; Meisel et al., 2013; Day et al., 2016b; Chu, unpublished data). It is likely that the Re-PGE bearing phases in these harzburgite RMs (HARZ-01/MUH-1) are typically alloys and laurites (Meisel et al., 2013; Day et al., 2016b; Meisel and Horan, 2016), which are difficult to digest.
Mafic Rock Reference Materials
BIR-1a has been proven to be homogenous both in Re-PGE mass fractions and 187Os/188Os ratios at 0.5–2 g test portions (BIR-1 is out of stock, now BIR-1a available) (Table 1) (e.g., Meisel and Moser, 2004b; Li et al., 2014; Ishikawa et al., 2014; Chu et al., 2015a; Zhang et al., 2017) and thus is recommended as a basaltic RM for Re-Os and PGE. Basaltic RM USGS BHVO-2 has been widely reported for Re-PGE mass fractions and 187Os/188Os ratios but not definitely homogeneous at a 2 g sample size level (Table 1) (e.g., Meisel and Moser, 2004b; Ishikawa et al., 2014; Li et al., 2014; Li et al., 2015; Chu et al., 2015a). USGS BCR-2 was found to have high Re (∼11.7 ng·g−1), low Os mass fractions (∼24 pg·g−1) and a very high 187Os/188Os (∼28.57) and seems homogenous at a 2 g sample size level (Table 1) (e.g., Chu et al., 2015a; Li et al., 2015). Nevertheless, BCR-2 was likely contaminated by MoS2 during the powder preparation (Meisel and Horan, 2016), thus cannot be regarded as a matrix-matched basaltic RM for Re-Os.
TDB-1 (diabase) and WGB-1 (gabbro) have been certified for Pd, Pt, and Au mass fractions by CCRMP, and widely used as mafic rock RMs for Re-Os and PGE analysis (Savard et al., 2010; Meisel and Horan, 2016). However, these RMs are not homogeneous in term of Re-PGE mass fractions and 187Os/188Os ratios at a 2 g test portion level (Table 1). The replicate analyses of Re-Os for TDB-1 and WGB-1 even show strong 187Os/188Os-187Re/188Os correlations to construct errorchrons (e.g., Ishikawa et al., 2014; Li et al., 2014; Chu et al., 2015a).
Shale
USGS RMs SDo-1 and SCo-1 have been reported for Re-PGE mass fractions and 187Os/188Os ratios (e.g., Meisel and Moser, 2004b; Finlay et al., 2012; Du Vivier et al., 2014; Ishikawa et al., 2014; Sen and Peucker-Ehrenbrink, 2014; Table 1). Now SCo-1 is out of stock, but SCo-2 is available. SDo-1 is out of stock.
USGS RMs SBC-1 and SGR-1b are relatively homogeneous in Re, Os mass fractions and 187Os/188Os ratios in 0.2–0.5 test portions (Table 1) and thus recommended as Re-Os RMs for black shale (Li and Yin, 2019) and oil shale (Yin et al., 2017), respectively. Nevertheless, PGE mass fraction data have not reported for these RMs up to now.
Road Dust
BCR-723 has been certified for Pt, Pd and Rh as a road dust RM by IRMM (Zischka et al., 2002). This RM has been recently reported for Re, Os, Ir, Ru, Rh, Pt and Pd fractions and 187Os/188Os ratios (Table 1) (Meisel et al., 2003b; Zhao et al., 2014).
Molybdenite
JDC-1 and HLP-1 were launched as molybdenite Re, Os concentrations and Re-Os age RMs as described in detail by Du et al. (2004). Markey et al. (2007) developed a molybdenite Re-Os age RM, NIST RM 8599. They emphasized that, RM8599 is not a RM for Re and 187Os mass fractions, but only for the accuracy of 187Re–187Os ages. The Re and 187Os mass fractions vary but are perfectly coupled such that the age is highly reproducible. The reported Re-Os data for these molybdenite RMs are given in Table 2.
Oil
NIST RM 8505 whole oil and its homogenized powered asphaltene separates were recommended as Re-Os matrix-matched oil RMs by Liu and Selby (2018). Hurtig et al. (2020) also reported Re-Os data for NIST RM 8505 crude oil and another oil RM, NIST RM 1634c. Although the Re, Os mass fractions for NIST RM 8505 whole oil reported by Hurtig et al. (2020) are consistently higher than those reported by Liu and Selby (2018), the 187Os/188Os data between the two labs are consistent within stated precisions. The reported Re-Os data for these oil RMs are summarized in Table 3.
Os Solution Reference Materials
The Os solution RMs are mainly used for monitoring the instrument status and inter-laboratory comparison of Os isotope ratio measurement results. The mostly used Os solution RMs include: JMC Os standard from University of Maryland (UMd) and DROsS from Durham University (or IAGeo, International Association of Geoanalysts). The other Os solution RMs mainly include: Os standard from Dept. Terrestrial Magmatism (DTM) and LOsST from Montanuniversität Leoben. These Os solution RMs have quite different 187Os/188Os ratios (Table 4).
Osmium Gravimetric Calibration Standards
Selby and Creaser (2001) and Markey et al. (2007) reported that the actual Os content of (NH4)2OsCl6 can be precisely determined by ignition under an atmosphere of 2% H2/98% N2. Nevertheless, the question still remains if it is accurate enough to calculate the stoichiometry. With this method, they produced an Os gravimetric standard for calibration of Os spike (usually 190Os).
Concluding Remarks and Prospects
In the last two to three decades, a lot of progress has been made in the development of methods for Os isotope ratio and Re-PGE mass fraction measurements in geological samples. This progress mainly include the improvements of sample digestion, analyte/matrix separation, and mass spectrometric determination methods for bulk sample analyses, as well as the development of in-situ measurement techniques.
The developments of analytical methods allow for more accurate determinations of Os isotope ratios and Re-PGE mass fractions in various geological materials. In the current state-of-the-art Re-Os and PGE analysis, complete digestion of Re-PGE bearing minerals with low procedural blank, and selective dissolution of targeted Re-PGE bearing phases dependent on analytical purposes still remains challenging. In addition, the stoichiometry of the Os gravimetric calibration standard remains questionable. We also require further efforts in developing and providing well-characterized, matrix-matched RMs. In the future, in addition to instrumentation, the most important aspect for Os isotope ratio and Re-PGE mass fraction measurements is to further lower the laboratory blank levels of these elements or to increase sample amounts for digestion for low Os samples to improve the sample/blank ratios, such that the applications of the Re-Os and PGE systems in geosciences can be further extended. These may include more accurate LLHR sulfide dating of metal ore deposits, more accurate black shale Re-Os dating and Os isotope stratigraphy, and more accurate dating of petroleum system. In addition, complete or selective sample digestion dependent on analytical purposes, is also important for Re-Os geochronology and PGE geochemistry in the future.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Funding
This research was financially supported by the National Key Research and Development Project of China (Grant 2020YFA0714803) and the National Natural Science Foundations of China (Grant 42073050).
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Alard, O., Griffin, W. L., Lorand, J. P., Jackson, S. E., and O’Reilly, S. Y. (2000). Non-chondritic distribution of the highly siderophile elements in mantle sulphides. Nature 407, 891–894. doi:10.1038/35038049
Alard, O., Griffin, W. L., Pearson, N. J., Lorand, J. P., and O’Reilly, S. Y. (2002). New insights into the Re–Os systematics of sub-continental lithospheric mantle from in situ analysis of sulphides. Earth Planet. Sci. Lett. 203, 651–663. doi:10.1016/S0012-821X(02)00799-9
Alard, O., Luguet, A., Pearson, N. J., Griffin, W. L., Lorand, J. P., Bannoun, A., et al. (2005). In-situ Os isotopes in abyssal peridotites bridge the isotopic gap between MORB and their source mantle. Nature 436, 1005–1008. doi:10.1038/nature03902
Allègre, C.-J., and Luck, J.-M. (1980). Osmium isotopes as petrogenetic and geological tracers. Earth Planet. Sci. Lett. 48, 148–154. doi:10.1016/0012-821X(80)90177-6
Barnes, S.-J., and Ripley, E. M. (2016). Highly siderophile and strongly chalcophile elements in magmatic ore deposits. Rev. Mineral. Geochem. 81, 725–774. doi:10.2138/rmg.2016.81.12
Becker, H., Dalpe, C., and Walker, R. J. (2002). High-precision Ru isotopic measurements by multi-collector ICP-MS. Analyst 127, 775–780. doi:10.1039/b200596d
Becker, H., Horan, M. F., Walker, R. J., Gao, S., Lorand, J. P., and Rudnick, R. L. (2006). Highly siderophile element composition of the Earth’s primitive upper mantle: constraints from new data on peridotite massifs and xenoliths. Geochem. Cosmochim. Acta. 70, 4528–4550. doi:10.1016/j.gca.2006.06.004
Becker, H., and Walker, R. J. (2003). In search of extant Tc in the early solar system: 98Ru and 99Ru abundances in iron meteorites and chondrites. Chem. Geol. 196, 43–56. doi:10.1016/S0009-2541(02)00406-0
Begemann, F., Ludwig, K. R., Lugmair, G. W., Min, K., Nyquist, L. J., Patchett, P. J., et al. (2001). Call for an improved set of decay constants for geochronological use. Geochem. Cosmochim. Acta. 65, 111–121. doi:10.1016/S0016-7037(00)00512-3
Birck, J.-L., Roy-Barman, M., and Capmas, F. (1997). Re–Os isotopic measurements at the femtomole level in natural samples. Geostand. Newsl. 20, 19–27. doi:10.1111/j.1751-908X.1997.tb00528.x
Birck, J. L. (2001). The precision and sensitivity of thermal ionisation mass spectrometry (TIMS): an overview of the present status. Geostand. Geoanal. Res. 25, 253–259. doi:10.1111/j.1751-908X.2001.tb00600.x
Brandon, A. D., Walker, R. J., Morgan, J. W., Norman, M. D., and Prichard, H. M. (1998). Coupled 186Os and 187Os evidence for core-mantle interaction. Science 280, 1570–1573. doi:10.1126/science.280.5369.1570
Campbell, A. J., and Humayun, M. (1999). Trace element microanalysis in iron meteorites by laser ablation ICPMS. Anal. Chem. 71, 939–946. doi:10.1021/ac9808425
Chatterjee, R., and Lassiter, J. C. (2015). High precision Os isotopic measurement using N-TIMS: quantification of various sources of error in 186Os/188Os measurements. Chem. Geol. 396, 112–123. doi:10.1016/j.chemgeo.2014.12.014
Chen, C., and Sharma, M. (2009). High precision and high sensitivity measurements of osmium in seawater. Anal. Chem. 81, 5400–5406. doi:10.1021/ac900600e
Chen, J. H., Papanastassiou, D. A., and Wasserburg, G. J. (2010). Ruthenium endemic isotope effects in chondrites and differentiated meteorites. Geochem. Cosmochim. Acta. 74, 3851–3862. doi:10.1016/j.gca.2010.04.013
Chu, Z.-Y., Harvey, J., Liu, C.-Z., Guo, J.-H., Wu, F.-Y., Tian, W., et al. (2013). Source of highly potassic basalts in northeast China: evidence from Re–Os, Sr–Nd–Hf isotopes and PGE geochemistry. Chem. Geol. 357, 52–66. doi:10.1016/j.chemgeo.2013.08.007
Chu, Z.-Y., Li, C.-F., Chen, Z., Xu, J.-J., Di, Y.-K., and Guo, J.-H. (2015b). High-precision measurement of 186Os/188Os and 187Os/188Os: isobaric oxide corrections with in-run measured oxygen isotope ratios. Anal. Chem. 87, 8765–8771. doi:10.1021/acs.analchem.5b01689
Chu, Z., Yan, Y., Zeng, G., Tian, W., Li, C., Yang, Y., et al. (2017). Petrogenesis of Cenozoic basalts in central-eastern China: constraints from Re–Os and PGE geochemistry. Lithos. 278-281, 72–83. doi:10.1016/j.lithos.2017.01.022
Chu, Z. Y., Wu, F. Y., Walker, R. J., Rudnick, R. L., Pitcher, L., Puchtel, I. S., et al. (2009). Temporal evolution of the lithospheric mantle beneath the eastern North China Craton. J. Petrol. 50, 1857–1898. doi:10.1093/petrology/egp055
Chu, Z. Y., Yan, Y., Chen, Z., Guo, J., Yang, Y. Y., Li, C. F., et al. (2015a). A comprehensive method for precise determination of Re, Os, Ir, Ru, Pt, Pd concentrations and Os isotopic compositions in geological samples. Geostand. Geoanal. Res. 39, 151–169. doi:10.1111/j.1751-908X.2014.00283.x
Cohen, A. S., and Waters, F. G. (1996). Separation of osmium from geological materials solvent extraction for analysis by TIMS. Anal. Chim. Acta. 332, 269–275. doi:10.1016/0003-2670(96)00226-7
Creaser, R. A., Papanastassiou, D. A., and Wasserburg, G. J. (1991). Negative thermal ion mass spectrometry of osmium, rhenium and iridium. Geochem. Cosmochim. Acta. 55, 397–401. doi:10.1016/0016-7037(91)90427-7
Creaser, R. A., Sannigrahi, P., Chacko, T., and Selby, D. (2002). Further evaluation of the Re–Os geochronometer in organic-rich sedimentary rocks: a test of hydrocarbon maturation effects in the Exshaw formation, Western Canada Sedimentary Basin. Geochem. Cosmochim. Acta. 66, 3441–3452. doi:10.1016/S0016-7037(02)00939-0
Creech, J., Baker, J., Handler, M., and Bizzarro, M. (2014). Platinum stable isotope analysis of geological standard reference materials by double-spike MC-ICPMS. Chem. Geol. 363, 293–300. doi:10.1016/j.chemgeo.2013.11.009
Creech, J., Baker, J., Handler, M., Schiller, M., and Bizzarro, M. (2013). Platinum stable isotope ratio measurements by double-spike multiple collector ICPMS. J. Anal. At. Spectrom. 28, 853–865. doi:10.1039/c3ja50022e
Creech, J. B., Moynier, F., and Bizarro, M. (2017). Tracing metal silicate segregation and late veneer in the Earth and the ureilite parent body with palladium stable isotopes. Geochim. Cosmochim. Acta 216, 24–41. doi:10.1016/j.gca.2017.04.040
Creech, J. B., Schaefer, B. F., and Turner, S. P. (2020). Application of 1013 Ω Amplifiers in Low-Signal Plasma-Source Isotope Ratio Measurements by MC-ICP-MS: A Case Study with Pt Isotopes. Geostand. Geoanal. Res. 44, 223–229. doi:10.1111/ggr.12310
Dale, C. W., Macpherson, C. G., Pearson, D. G., Hammond, S. J., and Arculus, R. J. (2012). Inter-element fractionation of highly siderophile elements in the Tonga Arc due to flux melting of a depleted source. Geochem. Cosmochim. Acta. 89, 202–225. doi:10.1016/j.gca.2012.03.025
Daly, L., Bland, P. A., Dyl, K. A., Forman, L. V., Evans, K. A., Trimby, P. W., et al. (2017a). In situ analysis of refractory metal nuggets in carbonaceous chondrites. Geochem. Cosmochim. Acta. 216, 61–81. doi:10.1016/j.gca.2016.11.030
Daly, L., Bland, P. A., Saxey, D. W., Reddy, S. M., Fougerouse, D., Rickard, W. D. A., et al. (2018). Defining the potential of nanoscale Re-Os isotope systematics using atom probe microscopy. Geostand. Geoanal. Res. 42, 279–299. doi:10.1111/ggr.12216
Daly, L., Bland, P. A., Saxey, D. W., Reddy, S. M., Fougerouse, D., Rickard, W. D. A., et al. (2017b). Nebula sulfidation and evidence for migration of “freefloating” refractory metal nuggets revealed by atom probe microscopy. Geology 45, 847–850. doi:10.1130/G39075.1
Day, J. M. D., Walker, R. J., and Warren, J. M. (2017). 186Os–187Os and highly siderophile element abundance systematics of the mantle revealed by abyssal peridotites and Os-rich alloys. Geochem. Cosmochim. Acta. 200, 232–254. doi:10.1016/j.gca.2016.12.013
Day, J. M. D., Brandon, A. D., and Walker, R. J. (2016a). Highly siderophile elements in earth, mars, the moon, and asteroids. Rev. Mineral. Geochem. 81, 161–238. doi:10.2138/rmg.2016.81.04
Day, J. M. D. (2013). Hotspot volcanism and highly siderophile elements. Chem. Geol. 341, 50–74. doi:10.1016/j.chemgeo.2012.12.010
Day, J. M. D., and O’Driscoll, B. (2019). Ancient high Pt/Os crustal contaminants can explain radiogenic 186Os in some intraplate magmas. Earth Planet. Sci. Lett. 519, 101–108. doi:10.1016/j.epsl.2019.04.039
Day, J. M. D., Pearson, D. G., and Nowell, G. M. (2003). “High precision rhenium and platinum isotope dilution analyses by plasma ionisation multi-collector mass spectrometry,” In Plasma source mass spectrometry: applications and emerging technologies. Editors J. G. Holland, and S. D. Tanner (Cambridge: The Royal Society of Chemistry), 374–390. doi:10.1039/9781847551689-00374
Day, J. M. D., Pearson, D. G., and Taylor, L. A. (2007). Highly siderophile element constraints on accretion and differentiation of the earth–moon system. Science 315, 217–219. doi:10.1126/science.1133355
Day, J. M. D., and Walker, R. J. (2015). Highly siderophile element depletion in the Moon. Earth Planet. Sci. Lett. 423, 114–124. doi:10.1016/j.epsl.2015.05.001
Day, J. M. D., Walker, R. J., Qin, L., and Rumble, D. (2012). Late accretion as a natural consequence of planetary growth. Nat. Geosci. 5, 614–617. doi:10.1038/ngeo1527
Day, J. M. D., Waters, C. L., Schaefer, B. F., Walker, R. J., and Turner, S. (2016b). Use of hydrofluoric acid desilicification in the determination of highly siderophile element abundances and Re–Pt–Os isotope systematics in mafic–ultramafic rocks. Geostand. Geoanal. Res. 40, 49–65. doi:10.1111/j.1751-908X.2015.00367.x
Du, A., He, H., Yin, N., Zou, X., Sun, Y., Sun, D., et al. (1995). A study of the rhenium-osmium geochronometry of molybdenite. Acta Geol. Sin. 8, 171–181. doi:10.1111/j.1755-6724.1995.mp8002004.x
Du, A., Wu, S., Sun, D., Wang, S., Qu, W., Markey, R., et al. (2004). Preparation and certification of Re-Os dating reference materials: molybdenites HLP and JDC. Geostand. Geoanal. Res. 28, 41–52. doi:10.1111/j.1751-908X.2004.tb01042.x
Du Vivier, A. D. C., Selby, D., Sageman, B. B., Jarvis, I., Gröcke, D. R., and Voigt, S. (2014). Marine 187Os/188Os isotope stratigraphy reveals the interaction of volcanism and ocean circulation during oceanic anoxic event 2. Earth Planet. Sci. Lett. 389, 23–33. doi:10.1016/j.epsl.2013.12.024
Finlay, A. J., Selby, D., and Osborne, M. J. (2012). Petroleum source rock identification of United Kingdom atlantic margin oil fields and the Western Canadian oil sands using platinum, palladium, osmium and rhenium: implications for global petroleum systems. Earth Planet. Sci. Lett. 313–314, 95–104. doi:10.1016/j.epsl.2011.11.003
Finlay, A. J., Selby, D., and Osborne, M. J. (2011). Re-Os geochronology and fingerprinting of United Kingdom Atlantic margin oil: temporal implications for regional petroleum systems. Geology 39, 475–478. doi:10.1130/G31781.1
Fischer-Gödde, M., Becker, H., and Wombacher, F. (2011). Rhodium, gold and other highly siderophile elements in orogenic peridotites and peridotite xenoliths. Chem. Geol. 280, 365–383. doi:10.1016/j.chemgeo.2010.11.024
Fischer-Gödde, M., Burkhardt, C., Kruijer, T. S., and Kleine, T. (2015). Ru isotope heterogeneity in the solar protoplanetary disk. Geochem. Cosmochim. Acta. 168, 151–171. doi:10.1016/j.gca.2015.07.032
Gannoun, A., Burton, K. W., Parkinson, I. J., Alard, O., Schiano, P., and Thomas, L. E. (2007). The scale and origin of the osmium isotope variations in mid-ocean ridge basalts. Earth Planet. Sci. Lett. 259, 541–556. doi:10.1016/j.epsl.2007.05.014
Georgiev, S. V., Stein, H. J., Hannah, J. L., Galimberti, R., Nali, M., Yang, G., et al. (2016). Re-Os dating of maltenes and asphaltenes within single samples of crude oil. Geochem. Cosmochim. Acta. 179, 53–75. doi:10.1016/j.gca.2016.01.016
Georgiev, S. V., Zimmerman, A., Yang, G., Goswami, V., Hurtig, N. C., Hannah, J. L., et al. (2018). Comparison of chemical procedures for Re-isotopic measurements by NTIMS. Chem. Geol. 483, 151–161. doi:10.1016/j.chemgeo.2018.03.006
Gordon, C. L. (1943). Modification of the Carius combustion tube to minimize losses by explosion: pressures attained on heating nitric acid to 300°C. J. Res. Natl. Bur. Stand. 30, 107–111. doi:10.6028/jres.030.009
Gordon, C. L., Schlecht, W. G., and Wichers, E. (1944). Use of sealed tubes for the preparation of acid solutions of samples for analysis, or for small-scale refining: pressures of acids heated above 100°C. J. Res. Natl. Bur. Stand. 33, 457–470. doi:10.6028/jres.033.027
Harvey, J., Dale, C. W., Gannoun, A., and Burton, K. W. (2011). Osmium mass balance in peridotite and the effects of mantle derived sulphides on basalt petrogenesis. Geochem. Cosmochim. Acta. 75, 5574–5596. doi:10.1016/j.gca.2011.07.001
Harvey, J., and Day, J. M. D. (2016). Introduction to highly siderophile and strongly chalcophile elements in high temperature geochemistry and cosmochemistry. Rev. Mineral. Geochem. 81, 3–15. doi:10.1515/9781501502095
Harvey, J., Gannoun, A., Burton, K. W., Rogers, N. W., Alard, O., and Parkinson, I. J. (2006). Ancient melt extraction from the oceanic upper mantle revealed by Re–Os isotopes from the Mid-Atlantic Ridge. Earth Planet. Sci. Lett. 244, 606–621. doi:10.1016/j.epsl.2006.02.031
Hassler, D. R., Peucker-Ehrenbrink, B., and Ravizza, G. E. (2000). Rapid determination of Os isotopic composition by sparging OsO4 into a magnetic-sector ICP-MS. Chem. Geol. 166, 1–14. doi:10.1016/S0009-2541(99)00180-1
Hirata, T., Hattori, M., and Tanaka, T. (1998). In-situ osmium isotope ratio analyses of iridosmines by laser ablation-multiple collector inductively coupled plasma mass spectrometry. Chem. Geol. 144, 269–280. doi:10.1016/S0009-2541(97)00138-1
Hoefs, J. (2018). “Isotope fractionation processes of selected elements,” in Stable isotope geochemistry, springer textbooks in earth sciences, geography and environment. (Cham: Springer), 53–227. doi:10.1007/978-3-319-78527-1_2
Hopp, T., Fischer-Gödde, M., and Kleine, T. (2016). Ruthenium stable isotope measurements by double spike MC-ICP-MS. J. Anal. At. Spectrom. 31, 1515–1526. doi:10.1039/c6ja00041j
Hunt, A. C., Ek, M., and Schöonbäachler, M. (2018). Separation of platinum from palladium and iridium in iron meteorites and accurate high-precision determination of platinum isotopes by multi-collector ICP-MS. Geostand. Geoanal. Res. 41, 633–647. doi:10.1111/ggr.12176
Hurtig, N. C., Georgiev, S. V., Zimmerman, A., Yang, G., Goswami, V., Hannah, J. L., et al. (2020). Re-Os geochronology for the NIST RM 8505 crude oil: the importance of analytical protocol and uncertainty. Chem. Geol. 539, 1–17. doi:10.1016/j.chemgeo.2019.119381
Ireland, T. J., Walker, R. J., and Brandon, A. D. (2011). 186Os–187Os systematics of Hawai-ian picrites revisited: new insights into Os isotopic variations in ocean island basalts. Geochem. Cosmochim. Acta. 75, 4456–4475. doi:10.1016/j.gca.2011.05.015
Ishikawa, A., Senda, R., Suzuki, K., Dale, C. W., and Meisel, T. (2014). Re-evaluating digestion methods for highly siderophile element and 187Os isotope analysis: evidence from geological reference materials. Chem. Geol. 384, 27–46. doi:10.1016/j.chemgeo.2014.06.013
Jin, X. D., Li, W. J., Xiang, P., Sakyi, P. A., Zhu, M. T., and Zhang, L. C. (2013). A contribution to common carius tube distillation techniques. J. Anal. At. Spectrom. 28, 396–404. doi:10.1039/c2ja10374e
Jin, X., Du, A., Li, W., Xiang, P., Sakyi, P. A., and Zhang, L. (2011). A new modification of the sample introduction system for Os isotope ratio measurements. J. Anal. At. Spectrom. 26, 1245–1252. doi:10.1039/C1JA00004G
Kendall, B., Creaser, R. A., and Selby, D. (2006). Re–Os geochronology of postglacial black shales in Australia: constraints on the timing of “sturtian” glaciation. Geology 34, 729–732. doi:10.1130/G22775.1
Kendall, B. S., Creaser, R. A., Ross, G. M., and Selby, D. (2004). Constraints on the timing of marinoan “snowball Earth” glaciation by 187Re–187Os dating of a neoproterozoic, post-glacial black shale in Western Canada. Earth Planet. Sci. Lett. 222, 729–740. doi:10.1016/j.epsl.2004.04.004
Klemm, V., Frank, M., Levasseur, S., Halliday, A. N., and Hein, J. R. (2008). Seawater osmium isotope evidence for a middle miocene flood basalt event in ferromanganese crust records: earth Planet. Sci. Lett. 273, 175–183. doi:10.1016/j.epsl.2008.06.028
Kogiso, T., Suzuki, K., Suzuki, T., Shinotsuka, K., Uesugi, K., Takeuchi, A., et al. (2008). Detecting micrometer-scale platinum-group minerals in mantle peridotite with microbeam synchrotron radiation X-ray fluorescence analysis. Geochem. Geophys. Geosyst. 9, Q03018. doi:10.1029/2007GC001888
Lawley, C. J. M., and Selby, D. (2012). Re-Os geochronology of quartz-enclosed ultrafine molybdenite: implications for ore geochronology. Econ. Geol. 107, 1499–1505. doi:10.2113/econgeo.107.7.1499
Levasseur, S., Birck, J. L., and Allègre, C. J. (1998). Direct measurement of femtomoles of osmium and the 187Os/186Os ratio in seawater. Science 282, 272–274. doi:10.1126/science.282.5387.272
Li, J., Jiang, X.-Y., Xu, J.-F., Zhong, L.-F., Wang, X.-C., Wang, G.-Q., et al. (2014). Determination of platinum-group elements and Re–Os isotopes using ID-ICP-MS and N-TIMS from a single digestion after two-stage column separation. Geostand. Geoanal. Res. 383, 37–50. doi:10.1111/j.1751-908X.2013.00242.x
Li, J., and Yin, L. (2019). Rhenium–osmium isotope measurements in marine shale reference material SBC-1: implications for method validation and quality control. Geostand. Geoanal. Res. 43, 497–507. doi:10.1111/ggr.12267
Li, J., Zhao, P. P., Liu, J., Wang, X. C., Yang, A. Y., Wang, G. Q., et al. (2015). Reassessment of hydrofluoric acid desilicification in the Carius tube digestion technique for Re–Os isotopic determination in geological samples. Geostand. Geoanal. Res. 39, 17–30. doi:10.1111/j.1751-908X.2014.00299.x
Li, Y., Selby, D., Condon, D., and Tapster, S. (2017). Cyclic magmatic-hydrothermal evolution in porphyry systems: high-precision U-Pb and Re-Os geochronology constraints on the Tibetan qulong porphyry Cu-Mo deposit. Econ. Geol. 112, 1419–1440. doi:10.5382/econgeo.2017.4515
Lillis, P. G., and Selby, D. (2013). Evaluation of the rhenium-osmium geochronometer in the phosphoria petroleum system, bighorn basin of wyoming and montana, USA. Geochem. Cosmochim. Acta. 118, 312–330. doi:10.1016/j.gca.2013.04.021
Liu, J., and Pearson, D. G. (2014). Rapid, precise and accurate Os isotope ratio measurements of nanogram to sub-nanogram amounts using multiple Faraday collectors and amplifiers equipped with 1012 Ω resistors by N-TIMS. Chem. Geol. 363, 301–311. doi:10.1016/j.chemgeo.2013.11.008
Liu, J., and Selby, D. (2018). A matrix-matched reference material for validating petroleum Re-Os measurements. Geostand. Geoanal. Res. 42 (1), 1–17. doi:10.1111/ggr.12193
Liu, Y., Huang, M., Masuda, A., and Inoue, M. (1998). High-precision determination of osmium and rhenium isotope ratios by in-situ oxygen isotope correction using negative thermal ionisation mass spectrometry. Int. J. Mass Spectrom. Ion Process. 173, 163–175. doi:10.1016/S0168-1176(97)00270-X
Lorand, J.-P., and Luguet, A. (2016). Chalcophile and siderophile elements in mantle rocks: trace elements controlled by trace minerals. Rev. Mineral. Geochem. 81, 441–488. doi:10.2138/rmg.2016.81.08
Lorand, J.-P., Alard, O., and Luguet, A. (2010). Platinum-group element micronuggetts and refertilization process in the Lherz peridotite (northeastern Pyrenees, France). Earth Planet. Sci. Lett. 289, 298–310. doi:10.1016/j.epsl.2009.11.017
Lorand, J.-P., Luguet, A., Alard, O., Bezos, A., and Meisel, T. (2008). Distribution of platinum group elements in orogenic lherzolites: a case study in a Fontête Rouge lherzolite (French Pyrenees). Chem. Geol. 248, 174–194. doi:10.1016/j.chemgeo.2007.06.030
Luguet, A., Nowell, G. M., and Pearson, D. G. (2008). 184Os/188Os and 186Os/188Os measurements by negative thermal ionisation mass spectrometry (N-TIMS): effects of interfering element and mass fractionation corrections on data accuracy and precision. Chem. Geol. 248, 342–362. doi:10.1016/j.chemgeo.2007.10.013
Luguet, A., Shirey, S. B., Lorand, J.-P., Horan, M. F., and Carlson, R. W. (2007). Residual platinum-group minerals from highly depleted harzburgites of the Lherz (France) and their role in HSE fractionation of the mantle. Geochem. Cosmochim. Acta. 71, 3082–3097. doi:10.1016/j.gca.2007.04.011
Markey, R. J., Hannah, J. L., Morgan, J. W., and Stein, H. J. (2003). A double spike for osmium analysis of highly radiogenic samples. Chem. Geol. 200, 395–406. doi:10.1016/S0009-2541(03)00197-9
Markey, R. J., Stein, H. J., and Morgan, J. W. (1998). Highly precise Re–Os dating of molybdenite using alkaline fusion and NTIMS. Talanta 45, 935–946. doi:10.1016/S0039-9140(97)00198-7
Markey, R., Stein, H. J., Hannah, J. L., Zimmerman, A., Selby, D., and Creaser, R. A. (2007). Standardizing Re–Os geochronology: a new molybdenite reference material (Henderson, USA) and the stoichiometry of Os salts. Chem. Geol. 244, 74–87. doi:10.1016/j.chemgeo.2007.06.002
Meibom, A., and Frei, R. (2002). Evidence for an ancient osmium isotopic reservoir in earth. Science 296, 516–518. doi:10.1126/science.1069119
Meibom, A., Sleep, N. H., Chamberlain, C. P., Coleman, R. G., Frei, R., Hren, M. T., et al. (2002). Re–Os isotopic evidence for long-lived heterogeneity and equilibration processes in the Earth’s upper mantle. Nature 419, 705–708. doi:10.1038/nature01067
Meisel, T., Burnham, O. M., Kriete, C., Syed, N., Bokhari, H., and Schulz, T. (2013). Osmium isotope and PGE reference materials OKUM and MUH-1. Goldschmidt Conference 77, 1734. doi:10.1180/minmag.2013.077.5.13
Meisel, T., Fellner, N., and Moser, J. (2003b). A simple procedure for the determination of platinum group elements and rhenium (Ru, Rh, Pd, Re, Os, Ir and Pt) using ID-ICP-MS with an inexpensive on-line matrix separation in geological and environmental materials. J. Anal. At. Spectrom. 18, 720–726. doi:10.1039/b301754k
Meisel, T., and Horan, M. F. (2016). Analytical methods for the highly siderophile elements. Rev. Mineral. Geochem. 81, 89–106. doi:10.2138/rmg.2016.81.02
Meisel, T., Moser, J., Fellner, N., Wegscheider, W., and Schoenberg, R. (2001). Simplified method for the determination of Ru, Pd, Re, Os, Ir and Pt in chromitites and other geological materials by isotope dilution ICPMS and acid digestion. Analyst. 126, 322–328. doi:10.1039/b007575m
Meisel, T., and Moser, J. (2004b). Platinum-group element and rhenium concentrations in low abundance reference materials. Geostand. Geoanal. Res. 28, 233–250. doi:10.1111/j.1751-908X.2004.tb00740.x
Meisel, T., and Moser, J. (2004a). Reference materials for geochemical PGE analysis: new analytical data for Ru, Rh, Pd, Os, Ir, Pt and Re by isotope dilution ICP-MS in 11 geological reference materials. Chem. Geol. 208, 319–338. doi:10.1016/j.chemgeo.2004.04.019
Meisel, T., Reisberg, L., Moser, J., Carignan, J., Melcher, F., and Brügmann, G. (2003a). Re–Os systematics of UB-N, a serpentinized peridotite reference material. Chem. Geol. 201, 161–179. doi:10.1016/S0009-2541(03)00234-1
Miller, C. A., Peucker-Ehrenbrink, B., and Ball, L. (2009). Precise determination of rhenium isotope composition by multi-collector inductively-coupled plasma mass spectrometry. J. Anal. At. Spectrom. 24, 1069–1078. doi:10.1039/b818631f
Morgan, J. W. (1986). Ultramafic xenoliths: clues to Earth’s late accretionary history. J. Geophys. Res. 91, 12375–12387. doi:10.1029/JB091iB12p12375
Morgan, J. W., and Walker, J. M. (1989). Isotopic determinations of rhenium and osmium in meteorites by using fusion, distillation and ion-exchange separations. Anal. Chim. Acta. 222, 291–300. doi:10.1016/S0003-2670(00)81904-2
Nägler, T. F., and Frei, R. (1997). “Plug in” Os distillation. Schweiz. Mineral. Petrog. Mitt. 77, 123–127. doi:10.5169/seals-58474
Nakanishi, N., Yokoyama, T., and Ishikawa, A. (2019). Refinement of the micro-distillation technique for isotopic analysis of geological samples with pg-level Osmium Contents. Geostand. Geoanal. Res. 43, 231–243. doi:10.1111/ggr.12262
Nanne, J. A., Millet, M. A., Burton, K. W., Dale, C. W., Nowell, G. M., and Williams, H. M. (2017). High precision osmium stable isotope measurements by double spike MC-ICP-MS and N-TIMS. J. Anal. At. Spectrom. 32, 749–765. doi:10.1039/c6ja00406g
Nier, A. O. (1937). The isotopic composition of osmium. Phy. Rev. 52, 885–892. doi:10.1103/PhysRev.52.885
Norman, M., Bennett, V., McCulloch, M., and Kinsley, L. (2002). Osmium isotopic compositions by vapor phase sample introduction using a multi-collector ICP-MS. J. Anal. At. Spectrom. 17, 1394–1397. doi:10.1039/b204518d
Nowell, G. M., Luguet, A., Pearson, D. G., and Horstwood, M. A. (2008a). Precise and accurate 186Os/188Os and 187Os/188Os measurements by multi-collector plasma ionisation mass spectrometry (MC- ICP-MS) part I: solution analyses. Special issue on highly siderophile element geochemistry. Chem. Geol. 248, 363–393. doi:10.1016/j.chemgeo.2007.10.020
Nowell, G. M., Pearson, D. G., Parman, S. W., Luguet, A., and Hanski, E. (2008b). Precise, accurate 186Os/188Os and 187Os/188Os measurements by Multi-Collector Plasma Ionisation Mass Spectrometry, part II: laser ablation and its application to single-grain Pt-Os and Re-Os geochronology. Chem. Geol. 248, 394–425. doi:10.1016/j.chemgeo.2007.12.004
Nozaki, T., Suzuki, K., Ravizza, G., Kimura, J. I., and Chang, Q. (2012). A method for rapid determination of Re and Os isotope compositions using ID-MC-ICP-MS combined with the sparging method. Geostand. Geoanal. Res. 36, 131–148. doi:10.1111/j.1751-908X.2011.00125.x
Parman, S. W., Diercks, D. R., Gorman, B. P., and Cooper, R. F. (2015). Atom probe tomography of isoferroplatinum. Am. Mineral. 100, 852–860. doi:10.2138/am-2015-4998
Paul, M., Reisberg, L., and Vigier, N. (2009). A new method for analysis of osmium isotopes and concentrations in surface and subsurface water samples. Chem. Geol. 258, 136–144. doi:10.1016/j.chemgeo.2008.09.018
Pearson, D. G., Shirey, S. B., Harris, J. W., and Carlson, R. W. (1998). Sulphide inclusions in diamonds from the Koffiefontein kimberlite, South Africa: constraints on diamond ages and mantle Re–Os systematics. Earth Planet. Sci. Lett. 160, 311–326. doi:10.1016/S0012-821X(98)00092-2
Pearson, D. G., and Woodland, S. J. (2000). Solvent extraction/anion exchange separation and determination of PGE’s (Os, Ir, Pt, Pd, Ru) and Re-Os isotopes in samples by isotope dilution. Chem. Geol. 165, 87–107. doi:10.1016/S0009-2541(99)00161-8
Pearson, N. J., Alard, O., Griffin, W. L., Jackson, S. E., and O’Reilly, S. Y. (2002). In situ measurement of Re–Os isotopes in mantle sulfides by laser ablation multicollector-inductively coupled plasma mass spectrometry: analytical methods and preliminary results. Geochem. Cosmochim. Acta. 66, 1037–1050. doi:10.1016/S0016-7037(01)00823-7
Puchtel, I. S., Walker, R. J., James, O. B., and Kring, D. A. (2008). Osmium isotope and highly siderophile element systematics of lunar impact melt rocks: implications for the late accretion history of the Moon and Earth. Geochem. Cosmochim. Acta. 72, 3022–3042. doi:10.1016/j.gca.2008.04.006
Qi, L., Gao, J. F., Huang, X. W., Hu, J., Zhou, M. F., and Zhong, H. (2011). An improved digestion technique for determination of platinum-group elements in geological samples. J. Anal. At. Spectrom. 26, 1900–1904. doi:10.1039/c1ja10114e
Qi, L., and Zhou, M. F. (2008). Determination of platinum-group elements in OPY-1: comparison of results using different digestion techniques. Geostand. Geoanal. Res. 32, 377–387. doi:10.1111/j.1751-908X.2008.00893.x
Qi, L., Zhou, M. F., Gao, J. F., and Zhao, Z. (2010). An improved Carius tube technique for determination of low concentrations of Re and Os in pyrites. J. Anal. At. Spectrom. 25, 585–589. doi:10.1039/b919016c
Qi, L., Zhou, M. F., and Wang, C. Y. (2004). Determination of low concentrations of platinum group elements in geological samples by ID-ICP-MS. J. Anal. At. Spectrom. 19, 1335–1339. doi:10.1039/b400742e
Qi, L., Zhou, M. F., Wang, C. Y., and Sun, M. (2007). Evaluation of a technique for determining Re and PGEs in geological samples by ICP-MS coupled with a modified Carius tube digestion. Geochem. J. 41, 407–414. doi:10.2343/geochemj.41.407
Qu, W., Du, A., and Zhao, D. (2001). Determination of 187Os in molybdenite by ICP-MS with neutron-induced 186Os and 188Os spikes. Talanta 55, 815–820. doi:10.1016/S0039-9140(01)00506-9
Ravizza, G., and Peucker-Ehrenbrink, B. (2003). Chemostratigraphic evidence of Deccan volcanism from the marine osmium isotope record. Science 302, 1392–1395. doi:10.1126/science.1089209
Ravizza, G., and Pyle, D. (1997). PGE and Os isotope analyses of single sample aliquots with NiS fire assay preconcentration. Chem. Geol. 141, 251–268. doi:10.1016/S0009-2541(97)00091-0
Ravizza, G., and Turekian, K. K. (1989). Application of the 187Re-187Os system to black shale geochronometry. Geochem. Cosmochim. Acta. 53, 3257–3262. doi:10.1016/0016-7037(89)90105-1
Reddy, S. M., Saxey, D. W., Rickard, W. D. A., Fougerouse, D., Montalvo, S. D., Verberne, R., et al. (2020). Atom probe tomography: development and application to the geosciences. Geostand. Geoanal. Res. 44, 5–50. doi:10.1111/ggr.12313
Rehkämper, M., and Halliday, A. N. (1997). Development and application of new ion-exchange techniques for the separation of the platinum group and other siderophile elements from geological samples. Talanta 44, 663–672. doi:10.1016/S0039-9140(96)02100-5
Reisberg, L., and Meisel, T. (2002). The Re-Os isotopic system: a review of the analytical techniques. Geostand. Newsl. 26, 249–267. doi:10.1111/j.1751-908X.2002.tb00633.x
Ren, M. H., Sun, Y. L., Wang, C. Y., and Sun, S. L. (2016). Determination of platinum-group elements in geological samples by isotope dilution-inductively coupled plasma-mass spectrometry combined with sulfide fire assay preconcentration. Geostand. Geoanal. Res. 40, 67–83. doi:10.1111/j.1751-908X.2015.00349.x
Rooney, A. D., Macdonald, F. A., Strauss, J. A., Dudás, F. Ö., Hallmann, C., and Selby, D. (2014). Re‐Os geochronology and coupled Os-Sr isotope constraints on the Sturtian snowball Earth. Proc. Nat. Acad. Sci. 111, 51–56. doi:10.1073/pnas.1317266110
Rudnick, R. L., and Walker, R. J. (2009). Interpreting ages from Re–Os isotopes in peridotites. Lithos. 112, 1083–1095. doi:10.1016/j.lithos.2009.04.042
Sato, H., Onoue, T., Nozaki, T., and Suzuki, K. (2013). Osmium isotope evidence for a large late triassic impact event. Nat. Commun. 4, 2455. doi:10.1038/ncomms3455
Savard, D., Barnes, S.-J., and Meisel, T. (2010). Comparison between nickel–sulfur fire assay Te Co-precipitation and isotope dilution with high-pressure asher acid digestion for the determination of platinum-group elements, rhenium and gold. Geostand. Geoanal. Res. 34, 281–291. doi:10.1111/j.1751-908X.2010.00090.x
Schoenberg, R., Nägler, T. F., and Kramers, K. (2000). Precise and accurate Os and Re isotopic measurements by multicollector ICP-MS down to the picogram level. Int. J. Mass Spectrom. 197, 85–94. doi:10.1016/S1387-3806(99)00215-8
Selby, D., and Creaser, R. A. (2005a). Direct radiometric dating of hydrocarbon deposits using rhenium-osmium isotopes. Science 308, 1293–1295. doi:10.1126/science.1111081
Selby, D., and Creaser, R. A. (2005b). Direct radiometric dating of the Devonian-Mississippian time-scale boundary using the Re–Os black shale chronometer. Geology 33, 545–548. doi:10.1130/G21324.1
Selby, D., Creaser, R. A., and Fowler, M. G. (2007). Re–Os elemental and isotopic systematics in crude oils. Geochem. Cosmochim. Acta. 71, 378–386. doi:10.1016/j.gca.2006.09.005
Selby, D., and Creaser, R. A. (2004). Macroscale NTIMS and microscale LA-MC-ICP-MS Re–Os isotopic analysis of molybdenite: testing spatial restrictions for reliable Re–Os age determinations, and implications for the decoupling of Re and Os within molybdenite. Geochem. Cosmochim. Acta. 68, 3897–3908. doi:10.1016/j.gca.2004.03.022
Selby, D., and Creaser, R. A. (2001). Re–Os geochronology and systematics in molybdenite from the Endako porphyry molybdenum deposit, British Columbia, Canada. Econ. Geol. 96, 197–204. doi:10.2113/gsecongeo.96.1.197
Selby, D., and Creaser, R. A. (2003). Re–Os geochronology of organic rich sediments: an evaluation of organic matter analysis methods. Chem. Geol. 200, 225–240. doi:10.1016/S0009-2541(03)00199-2
Sen, I. S., and Peucker-Ehrenbrink, B. (2014). Determination of osmium concentrations and 187Os/188Os of crude oils and source rocks by coupling high-pressure, high-temperature digestion with sparging OsO4 into a multicollector inductively coupled plasma mass spectrometer. Anal. Chem. 86, 2982–2988. doi:10.1021/ac403413y
Seo, J. H., Sharma, M., Osterberg, E. C., and Jackson, B. P. (2018). Determination of osmium concentration and isotope composition at ultra-low level in polar ice and snow. Anal. Chem. 90, 5781–5787. doi:10.1021/acs.analchem.8b00150
Sharma, M., and Wasserburg, G. J. (1997). Osmium in the rivers. Geochem. Cosmochim. Acta. 61, 5411–5416. doi:10.1016/S0016-7037(97)00329-3
Shen, J. J., Papanastassiou, D. A., and Wasserburg, G. J. (1996). Precise Re-Os determinations and systematics of iron meteorites. Geochem. Cosmochim. Acta. 60, 2887–2900. doi:10.1016/0016-7037(96)00120-2
Shinotsuka, K., and Suzuki, K. (2007). Simultaneous determination of platinum group elements and rhenium in rock samples using isotope dilution inductively coupled plasma mass spectrometry after cation exchange separation followed by solvent extraction. Anal. Chim. Acta. 603, 129–139. doi:10.1016/j.aca.2007.09.042
Shirey, S. B., and Walker, R. J. (1995). Carius tube digestion for low blank rhenium-osmium analysis. Anal. Chem. 34, 2136–2141. doi:10.1021/ac00109a036
Shirey, S. B., and Walker, R. J. (1998). The Re–Os isotope system in cosmochemistry and high-temperature geochemistry. Annu. Rev. Earth Planet. Sci. 26, 423–500. doi:10.1146/annurev.earth.26.1.423
Smoliar, M. I., Walker, R. J., and Morgan, J. W. (1996). Re–Os ages of Group IIA, IIIA, IVA and IVB iron meteorites. Science 271, 1099–1102. doi:10.1126/science.271.5252.1099
Stein, H., and Hannah, J. (2015). “Rhenium–Osmium geochronology: sulfides, shales, oils, and mantle,” In Encyclopedia of scientific dating methods. Editors J. W. Rink, and J. W. Thompson (Dordrecht: Springer Press), 707–723. doi:10.1007/978-94-007-6304-3
Stein, H. J. (2014). “Dating and tracing the history of ore formation,” In Treatise on geochemistry. 2nd Edn. Editors H. D. Holland, and K. K. Turekian (Oxford: Elsevier Press), 87–118. doi:10.1016/B978-0-08-095975-7.01104-9
Stein, H. J., Morgan, J. W., and Scherstén, A. (2000). Re–Os dating of low-level highly-radiogenic (LLHR) sulfides: the Harnäs gold deposit, southwest Sweden records continental scale tectonic events. Econ. Geol. 95, 1657–1671. doi:10.2113/gsecongeo.95.8.1657
Stein, H., Scherstén, A., Hannah, J., and Markey, R. (2003). Sub-grain scale decoupling of Re and 187Os and assessment of laser ablation ICP-MS spot dating in molybdenite. Geochem. Cosmochim. Acta. 67, 3673–3686. doi:10.1016/S0016-7037(03)00269-2
Sun, Y. L., Chu, Z. Y., Sun, M., and Xia, X. P. (2009). An improved Fe–Ni sulfide fire assay method for determination of Re, platinum group elements, and Os isotopic ratios by inductively coupled plasma- and negative thermal ionization-mass spectrometry. Appl. Spectrosc. 63, 1232–1237. doi:10.1366/000370209789806966
Sun, Y. L., and Sun, M. (2005). Nickel sulfide fire assay improved for pre-concentration of platinum group elements in geological samples: a practical means of ultra-trace analysis combined with inductively coupled plasma-mass spectrometry. Analyst. 130, 664–669. doi:10.1039/b416844e
Sun, Y. L., Xu, P., Li, J., He, K., Chu, Z. Y., and Wang, Y. C. (2010). A practical method for determination of molybdenite Re–Os age by inductively coupled plasma-mass spectrometry combined with Carius tube-HNO3 digestion. Anal. Methods 2, 575–581. doi:10.1039/b9ay00258h
Sun, Y. L., Zhou, M. F., and Sun, M. (2001). Routine Os analysis by isotope dilution inductively coupled plasma mass spectrometry: OsO4 in water solution gives high sensitivity. J. Anal. At. Spectrom. 16, 345–349. doi:10.1039/b008533m
Suzuki, K., Miyata, Y., and Kanazawa, N. (2004). Precise Re Isotope ratio measurements by negative thermal Ionization mass Spectrometry (NTI-MS) using total evaporation technique. Int. J. Mass Spectrom. 235, 97–101. doi:10.1016/j.ijms.2004.04.006
Suzuki, K., Lu, Q., Shimizu, H., and Masuda, A. (1992). Determination of osmium abundance in molybdenite mineral by isotope dilution mass spectrometry with microwave digestion using potassium dichromate as oxidizing agent. Analyst. 117, 1151–1156. doi:10.1039/AN9921701151
Tait, K. T., and Day, J. M. D. (2018). Chondritic late accretion to Mars and the nature of shergottite reservoirs. Earth Planet. Sci. Lett. 494, 99–108. doi:10.1016/j.epsl.2018.04.040
Turgeon, S. C., and Creaser, R. A. (2008). Cretaceous oceanic anoxic event 2 triggered by a massive magmatic episode. Nature 454, 323–326. doi:10.1038/nature07076
Völkening, J., Walczyk, T., and Heumann, K. G. (1991). Osmium isotope ratio determinations by negative thermal ionization mass-spectrometry. Int. J. Mass Spectrom. Ion Process. 105, 147–159. doi:10.1016/0168-1176(91)80077-Z
Walker, R. J., Brandon, A. D., Bird, J. M., Piccoli, P. M., McDonough, W. F., and Ash, R. D. (2005). 187Os–186Os systematics of Os–Ir–Ru alloy grains from southwestern Oregon. Earth Planet. Sci. Lett. 230, 211–226. doi:10.1016/j.epsl.2004.11.009
Walker, R. J., Carlson, R. W., Shirey, S. B., and Boyd, F. R. (1989). Os, Sr, Nd, and Pb isotope systematics of southern African peridotite xenoliths: implications for the chemical evolution of the subcontinental mantle. Geochem. Cosmochim. Acta. 53, 1583–1595. doi:10.1016/0016-7037(89)90240-8
Walker, R. J. (2009). Highly siderophile elements in the Earth, Moon and Mars: update and implications for planetary accretion and differentiation. Chem. Erde. 69, 101–125. doi:10.1016/j.chemer.2008.10.001
Walker, R. J. (1988). Low-blank chemical separation of rhenium and osmium from gram quantities of silicate rock for measurement by resonance ionization mass spectrometry. Anal. Chem. 60, 1231–1234. doi:10.1021/ac00162a026
Walker, R. J., Morgan, J. W., Smoliar, M. I., Beary, E., Czamanske, G. K., and Horan, M. F. (1997). Applications of the 190Pt–186Os isotope system to geochemistry and cosmochemistry. Geochem. Cosmochim. Acta. 61, 4799–4808. doi:10.1016/S0016-7037(97)00270-6
Walker, R. J. (2015). “Rhenium–Osmium dating (meteorites),” In Encyclopedia of scientific dating methods. Editors J. W. Rink, and J. W. Thompson (Dordrecht: Springer Press), 703–707. doi:10.1007/978-94-007-6304-3
Walker, R. J. (2016). Siderophile elements in tracing planetary formation. Geochem. Perspect. 5, 1–145.
Wang, G., Sun, T., and Xu, J. (2017). A comparison using Faraday cups with 1013 Ω amplifiers and a secondary electron multiplier o measure Os isotopes by negative thermal ionization mass spectrometry. Rapid Commun. Mass Spectrom. 31, 1616–1622. doi:10.1002/rcm.7943
Wang, Z., and Becker, H. (2014). Abundances of sulfur, selenium, tellurium, rhenium and platinum-group elements in eighteen reference materials by isotope dilution sector-field ICP-MS and negative TIMS. Geostand. Geoanal. Res. 38, 189–209. doi:10.1111/j.1751-908X.2013.00258.x
Warren, J. M., and Shirey, S. B. (2012). Lead and osmium isotopic constraints on the oceanic mantle from single abyssal peridotite sulfides. Earth Planet. Sci. Lett. 359–360, 279–293. doi:10.1016/j.epsl.2012.09.055
Wichers, E., Schlecht, W. G., and Gordon, C. L. (1944). Attack of refractory platiniferous materials by acid mixtures at elevated temperatures. J. Res. Natl. Bur. Stand. 33, 363–381.
Woodhouse, O. B., Ravizza, G., Kenison Falkner, K., Statham, P. J., and Peucker-Ehrenbrink, B. (1999). Osmium in seawater: vertical profiles of concentration and isotopic composition in the eastern Pacific Ocean. Earth Planet. Sci. Lett. 173, 223–233.
Xu, J. F., Suzuki, K., Xu, Y. G., Mei, H. J., and Li, J. (2007). Os, Pb, and Nd isotope geochemistry of the Permian Emeishan continental flood basalts: insights into the source of a large igneous province. Geochem. Cosmochim. Acta. 71, 2104–2119. doi:10.1016/j.gca.2007.01.027
Yang, G., Zimmerman, A., Stein, H., and Hannah, J. (2015). Pretreatment of nitric acid with hydrogen peroxide reduces total procedural Os blank to femtogram levels. Anal. Chem. 87, 7017–7021. doi:10.1021/acs.analchem.5b01751
Yin, L., Li, J., Liu, J. G., Li, C., Sun, S. L., Liang, H. Y., et al. (2017). Precise and accurate Re–Os isotope dating of organic-rich sedimentary rocks by thermal ionization mass spectrometry with an improved H2O2-HNO3 digestion procedure. Int. J. Mass Spectrom. 421, 263–270. doi:10.1016/j.ijms.2017.07.013
Yokoyama, T., Alexander, C. M. O., and Walker, R. J. (2010). Osmium isotope anomalies in chondrites: results for acid residues and related leachates. Earth Planet. Sci. Lett. 291, 48–59. doi:10.1016/j.epsl.2009.12.048
Yokoyama, T., Rai, V. K., Alexander, M. O., Lewis, R. S., Carlson, R. W., Shirey, S. B., et al. (2007). Osmium isotope evidence for uniform distribution of s- and r process components in the early solar system. Earth Planet. Sci. Lett. 259, 567–580. doi:10.1016/j.epsl.2007.05.017
Yokoyama, T., and Walker, R. J. (2016). Nucleosynthetic isotope variations of siderophile and chalcophile elements in the Solar System. Rev. Mineral. Geochem. 81, 107–160. doi:10.2138/rmg.2016.81.03
Zhang, J., Li, J., Long, X., Sun, S., Yin, L., and Dai, M. (2017). Rhenium-osmium isotope measurements of geological reference material BIR-1a: evaluation of homogeneity and implications for method validation and quality control. Geostand. Geoanal. Res. 41, 649–658. doi:10.1111/ggr.12180
Zhao, H., Li, C., Jiang, X., Zhou, L., Li, X., Qu, W., et al. (2015). Direct radiometric dating of limestone from Changxing Permian-Triassic boundary using the Re-Os geochronometer. Chin. Sci. Bull. 60, 2209–2215. doi:10.1360/N972015-00409
Zhao, P. P., Li, J., Zhong, L. F., Sun, S. L., and Xu, J. F. (2014). A method for determination of PGE–Re concentrations and Os isotopic compositions in environmental materials. Anal. Methods 6, 5537–5545. doi:10.1039/c3ay42064g
Zhou, X. Y., Tanaka, R., Yamanaka, M., Sakaguchi, C., and Nakamura, E. (2019). A method to suppress isobaric and polyatomic interferences for measurements of highly siderophile elements in desilicified geological samples. Geostand. Geoanal. Res. 43, 611–633. doi:10.1111/ggr.12280
Zhu, B., Becker, H., Jiang, S.-Y., Pi, D.-H., Fischer-Gödde, M., and Yang, J.-H. (2013). Re–Os geochronology of black shales from the neoproterozoic doushantuo formation, yangtze platform, south China. Precambrian Res. 225, 67–76. doi:10.1016/j.precamres.2012.02.002
Zhu, Z., Meija, J., Tong, S., Zheng, A., Zhou, L., and Yang, L. (2018). Determination of the isotopic composition of osmium using MC-ICPMS. Anal. Chem. 90, 9281–9288. doi:10.1021/acs.analchem.8b01859
Zhu, Z., Meija, J., Zheng, A., Mester, Z., and Yang, L. (2017). Determination of the isotopic composition of iridium using Multicollector-ICPMS. Anal. Chem. 89, 9375–9382. doi:10.1021/acs.analchem.7b022061
Keywords: Re-Os, PGE, analytical method, NTIMS, ICP-MS, geoscience
Citation: Chu Z (2021) Analytical Methods for Os Isotope Ratios and Re-PGE Mass Fractions in Geological Samples. Front. Chem. 8:615839. doi: 10.3389/fchem.2020.615839
Received: 10 October 2020; Accepted: 21 December 2020;
Published: 17 February 2021.
Edited by:
Zhaochu Hu, China University of Geosciences Wuhan, ChinaReviewed by:
James Martin Dines Day, University of California, San Diego, United StatesThomas C. Meisel, University of Leoben, Austria
Copyright © 2021 Chu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhuyin Chu, emh5Y2h1QG1haWwuaWdnY2FzLmFjLmNu
 Zhuyin Chu
Zhuyin Chu