
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Chem. , 19 November 2020
Sec. Supramolecular Chemistry
Volume 8 - 2020 | https://doi.org/10.3389/fchem.2020.610093
This article is part of the Research Topic Host-Guest Chemistry of Macrocycles View all 17 articles
Fluorescent supramolecular polymers (FSP) have attracted considerable attention in recent years. Particularly, the incorporation of aggregation-induced emission (AIE) property to the FSP will bring this material into practical applications. Herein, we designed and synthesized a cyanostilbene motif derived ditopic benzo-21-crown-7 (B21C7) as a host molecule (H). The cyanostilbene motif endows H with AIE property while the B21C7 motif renders it with the capability to complex with electron deficient guest molecules. Upon the addition of a ditopic dialkylammonium salt molecule (G), a novel FSP with blue luminescent property can be constructed. This B21C7-based host-guest FSP with blue fluorescence may have potential application in supramolecular luminescent materials.
Supramolecular polymers, in which ordered and highly directional polymeric arrays of monomeric building blocks are brought together by reversible non-covalent bonds, are outstanding materials that generally exhibit stimuli-responsive and self-healing properties (Aida et al., 2012; Yang et al., 2015; Wehner and Würthner, 2020). The driving force of supramolecular polymers usually includes hydrogen bonds (Xiao et al., 2019b,e; Datta et al., 2020; Qi et al., 2020), metal-ligand bonds (Zheng et al., 2016; Shi et al., 2019), host-guest complexation (Guo et al., 2010; Harada et al., 2014; Wang et al., 2018; Xiao and Wang, 2018; Xiao et al., 2018, 2019d; Chen et al., 2019), donor–acceptor interaction (Han et al., 2018), π-π stacking (Wagner et al., 2019; Xiao et al., 2019c), or a combination of them (Li et al., 2012; Wei et al., 2015; Xiao et al., 2020b). In recent years, supramolecular fluorescent materials have drawn much attention, such as fluorescent molecular switches (Cheng et al., 2017), fluorescent sensors (Kumawat et al., 2019), fluorescent metallaclip (Wang et al., 2019), and artificial light-harvesting systems (Xiao et al., 2019g, 2020a). Particularly, the development of fluorescent supramolecular polymers (FSPs) has attracted more and more interest because of their potential application in the area of dynamic luminescent materials (Ji et al., 2013; Zhang et al., 2017, 2018; Li et al., 2019). Moreover, the emergence of aggregation-induced emission (Hong et al., 2011) (AIE) fluorophores laid the foundation for the application of FSP in practice.
Macrocycle-based host-guest interaction is an important driving force in supramolecular chemistry. For example, we have reviewed a series of supramolecular materials based on pillararene (Xiao et al., 2019a,f,h). Benzo-21-crown-7 (B21C7) is one of the most important crown ethers (Zhang et al., 2007), and it shows interesting applications like adhesive materials (Dong et al., 2017; Zhang et al., 2019). B21C7 is the smallest crown ether that can complex with dialkylammonium salts, leading to a relatively strong host-guest interaction. Cyanostilbene is a well-known fluorophore and shows interesting AIE behavior, which is usually used for the construction of rotaxanes (Lee et al., 2013), nanoparticles with near-infrared emission (Shi et al., 2016), hydrogen-bonded supramolecular polymer (Lavrenova et al., 2017), and light-harvesting system (Kim et al., 2018; Sun et al., 2020). To the best of our knowledge, the integration of B21C7 unit and cyanostilbene unit to a host molecule to construct FSP has not yet been reported so far.
Previously, we have reported some orthogonal supramolecular polymers based on B21C7 (Xiao et al., 2013, 2020c). Herein, we designed and synthesized a new host molecule H, which bears both cyanostilbene motif and B21C7 units (Figure 1). The cyanostilbene motif endows H with the property of AIE, while B21C7 unit endows H with the capability to bind dialkylammonium salt. In the presence of guest molecule G (a ditopic dialkylammonium salt compound), a novel AA/BB-type FSP can be fabricated by crown ether-based host-guest complexation. Furthermore, the FSP could be assembled into macroscopic fibers with blue fluorescence from concentrated solution. This crown ether-based FSP with beautiful blue fluorescence both in solution and in the solid state may have potential application in supramolecular luminescent materials.
The synthesis of organic molecules H and G are straightforward. Compound H was synthesized from B21C7-based derivative A (Lu et al., 2018) and cyanostilbene-derived compound 5 (Scheme 1). As shown in Scheme 1, starting from p-anisaldehyde and 4-methoxybenzyl cyanide, compound 4 was prepared in ethanol solution by condensation reaction. Demethylation of compound 4 with boron tribromide in dichloromethane (DCM) yields compound 5 (Gu et al., 2012). Compound G was synthesized according to literature report (Li et al., 2018). The compounds that have not been reported previously are carefully characterized by 1H NMR, 13C NMR, and HR-MS (Supplementary Figures 1–4).
In order to examine whether the cyanostilbene motif bridged B21C7 is AIE active, the fluorescence spectra of H in mixed H2O/Tetrahydrofuran (THF) solutions were investigated. As shown in Figure 2, H shows an obvious AIE behavior. There is no fluorescence emission when H was in pure THF (a good solvent for H). When water (a poor solvent for H) content was increased gradually to 80%, a moderate emission was observed. The absorption spectrum of H is shown in Supplementary Figure 5. The emission wavelength was at 480 nm when exited at 365 nm. Upon increasing water content to 95%, the fluorescence intensity of H exhibits a dramatic enhancement with a bright blue color. The dried powder of H was obtained as a light yellow solid, which exhibited intense blue luminescence under irradiation at 365 nm at room temperature (Figure 2B). Interestingly, exposing the sample to DCM vapor resulted in a distinct change of color from blue to green within only 30 s (Figure 2B). Notably, the blue color can be recovered by heating the sample to remove the DCM (Figure 2B).
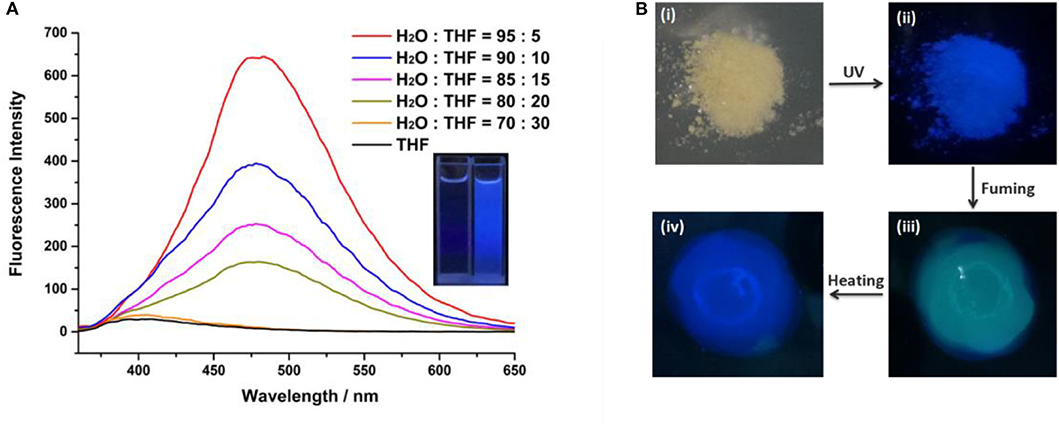
Figure 2. (A) Fluorescence emission spectra of H versus H2O fraction in THF/H2O mixtures (λex = 365 nm, [H] = 5 × 10−5 M). Inset: photographs of H in THF and H in THF/H2O = 5: 95 mixtures ([H] = 5 × 10−5 M); and (B) solid powder of H under natural light (i) and UV light (ii), fumigation with DCM (iii), and color recovered by heating (iv).
Supramolecular polymerization of H and G was first investigated by concentration-dependent 1H NMR. It was measured in mixed CDCl3/CD3CN (1:1, v/v) at concentrations in the range of 2–64 mM (Figure 3). The concentration-dependent 1H NMR spectra showed a complex picture owing to the slow-exchange complexation of the B21C7 motif with the dialkylammonium salt on the NMR timescale. It should be noted that the peak splitting is relatively sharp at low concentrations (2–8 mM), suggesting that the cyclic oligomers are predominant species at this stage. As the concentration increases, these peaks became broad, indicating the formation of high-molecular-weight assemblies, such as supramolecular polymers. By contrast, the concentration-dependent 1H NMR spectra of individual H shows no chemical shift change upon concentration increasing (Supplementary Figure 6).
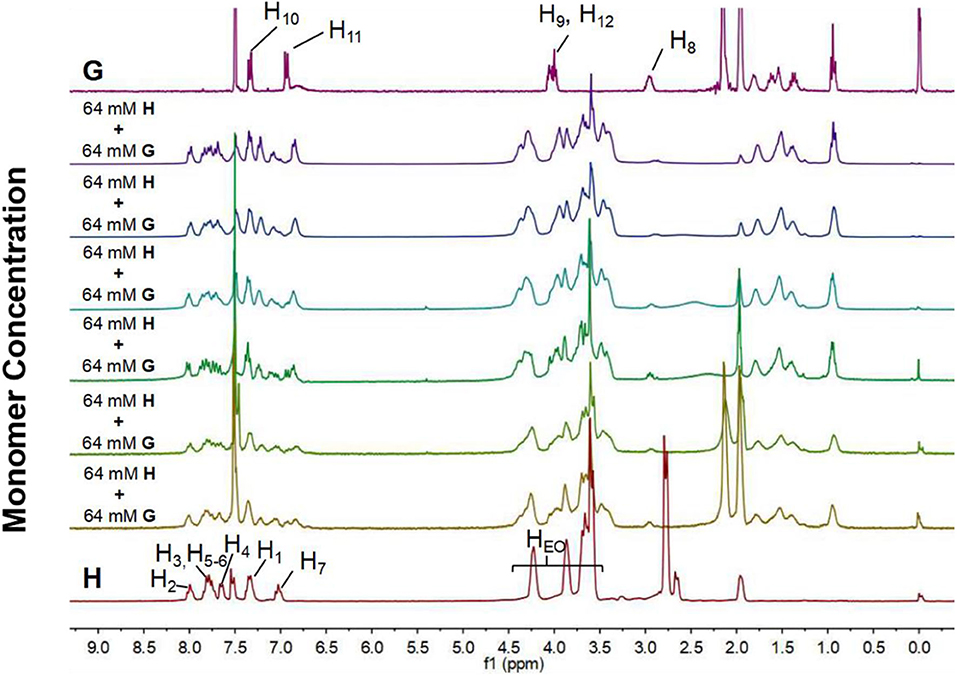
Figure 3. 1H NMR spectra (300 MHz, CDCl3/CD3CN = 1/1, v/v, 298 K) of individual H and G, and mixtures of them at different monomer concentrations ([H]/[G] = 1/1).
To further study the supramolecular polymerization driven by crown ether-based host-guest interaction, viscosity measurements were performed by using a micro-Ubbelohde viscometer. A double logarithmic curve of specific viscosity toward monomer concentration is depicted in Figure 4. During the low concentration range, the slope value was tested to be 1.04, which is the characteristic of cyclic oligomers with constant size. When the concentration increased to 9 mM, a steeper curve with a slope 1.84 was obtained, suggesting that the cyclic oligomers are gradually transformed into supramolecular polymers. This phenomenon was in line with concentration-dependent 1H NMR.
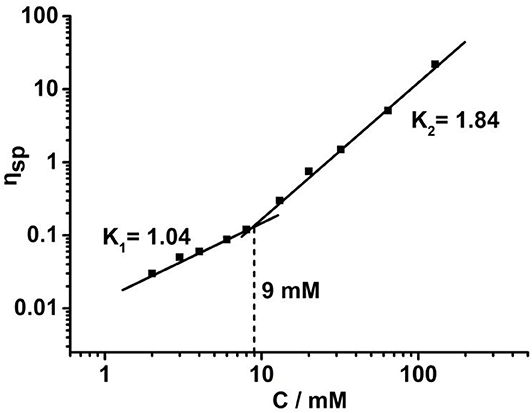
Figure 4. Specific viscosity of H/G complex vs. the monomer concentration in CDCl3/CH3CN (1:1, v/v) solutions (298 K).
The formation of supramolecular polymers was further evidenced by its processibility. Fibers can be drawn from a concentrated solution of the host and guest with a molar ratio of H/G = 1/1 (Figure 5A). Such fibers can be only made from entanglements of large aggregates. By contrast, no fiber can be pulled out from the concentrated solution of individual H or individual G. The fiber is colorless under visible light (Figure 5A) and generates blue fluorescence under UV light (Figure 5B). This fiber still has fluorescent luminescence after preparation for several days, indicating that the supramolecular polymer has potential applications in the area of supramolecular luminescent materials. The photophysical property of the supramolecular polymer was further investigated by solid-state fluorescence spectroscopy. As shown in Figure 5C, the emission wavelength of H shows a hypochromic shift from 480 nm in solution to 415 nm in the solid state. Compared with individual H, the supramolecular polymer in the solid state exhibited a stronger emission and bathochromic shift to 430 nm, indicating that the incorporation of the guest affected the packing of the fluorophores in the solid state, resulting in modified luminescent properties.
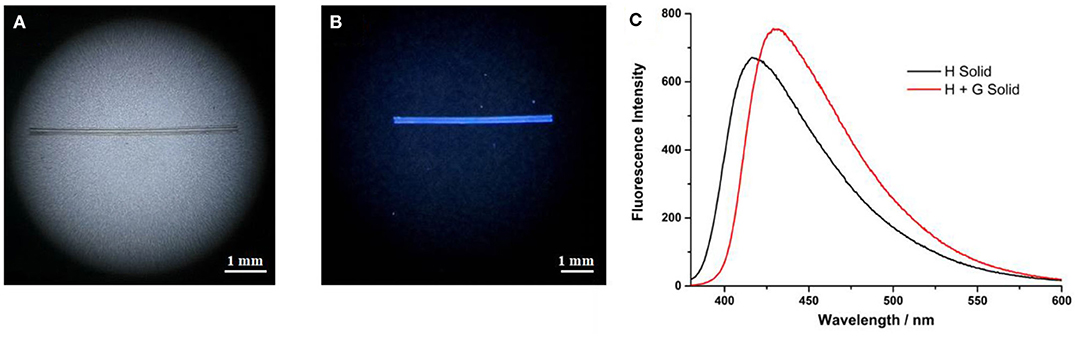
Figure 5. (A) A rod-like fiber formed from H and G under visible light; (B) the rod-like fiber under UV (365 nm) lamp irradiation; and (C) fluorescence spectra of H and H-G in the solid state, λex = 365 nm.
All chemicals, reagents, and solvents were purchased from commercial suppliers and used, unless otherwise stated, without further purification. If needed, solvents were dried by literature-known procedures. All yields were given as isolated yields. The 1H NMR and 13C NMR spectra were recorded with a Bruker AVANCE III (300 MHz) spectrometer and calibrated against the residual proton signal or natural abundance carbon resonance of the used deuterated solvent from tetramethylsilane as the internal standard. The chemical shifts δ are indicated in ppm and the coupling constants J in Hz. The multiplicities are given as s (singlet), d (doublet), dd (doublet of doublets), t (triplet), and m (multiplet). High-resolution electrospray ionization mass spectra (HR-ESI-MS) were recorded on an Agilent Technologies 6540 UHD Accurate-Mass. Fluorescence measurements were performed on an Agilent Cary Eclipse spectrofluorometer. Viscosity measurements were carried out with Ubbelohde microviscometers (Shanghai Liangjing Glass Instrument Factory, 0.40 mm inner diameter) at 298 K in chloroform and acetonitrile.
To a solution of compound 5 (237 mg, 1.0 mmol) in THF (15 mL) was added compound A (1.26 g, 3.0 mmol) and 4-dimethylaminopyridine (18 mg, 0.15 mmol) at room temperature under N2 atmosphere. Then the Et3N (303 mg, 3.0 mmol) was added with vigorous stirred over 15 min. The reaction mixture was heated at 70°C for 2 h and then poured into water (100 mL). The resulting mixture was extracted with DCM (50 mL × 3) and the combined extracts were washed with H2O (100 mL × 3), brine (50 mL × 3), dried over anhydrous Na2SO4 and concentrated under reduced pressure. The resulting residue was chromatographed over silica gel (DCM:MeOH = 60:1, v/v) to afford compound H as a light yellow solid (460 mg, 0.46 mmol), yield: 46%. 1H NMR (300 MHz, CDCl3): δ (ppm) = 7.98 (d, J = 9.0 Hz, 2H, ArH), 7.85 (dd, J = 8.4, 2.1 Hz, 2H, ArH), 7.74 (d, J = 8.7 Hz, 2H, ArH), 7.69 (d, J = 1.8 Hz, 2H, ArH), 7.54 (s, 1H, alkene-H), 7.32 (m, 4H, ArH), 6.96 (d, J = 8.7 Hz, 2H, ArH), 4.33–4.19 (m, 8H, -OCH2CH2O-), 4.03–3.93 (m, 8H, -OCH2CH2O-), 3.82 (m, 8H, -OCH2CH2O-), 3.76 (m, 8H, -OCH2CH2O-), 3.69 (s, 16H, -OCH2CH2O-). 13C NMR (75 MHz, CDCl3): δ (ppm) = 164.6, 164.5, 153.7, 153.7, 152.6, 151.8, 148.5, 141.2, 132.0, 131.2, 130.6, 127.2, 124.9, 124.8, 122.6, 122.5, 121.7, 121.6, 117.9, 114.9, 112.3, 110.8, 71.4, 71.3, 71.2, 71.1, 71.0, 71.0, 70.6, 69.6, 69.5, 69.4, 69.2. HR-ESI-MS: m/z calcd for [C53H64NO18]+ = 1002.4118, found = 1002.4120.
In summary, we have successfully synthesized a cyanostilbene motif bridged ditopic B21C7 compound, which exhibits good AIE property and vapochromic behavior. Supramolecular polymers can be fabricated by such a host compound with a ditopic dialkylammonium salt guest. The host-guest supramolecular polymerization was fully characterized by concentration-dependent 1H NMR, viscosity measurements, and fiber formation test. Fluorescent properties of the supramolecular polymers in the solid state are also studied, which suggests that the supramolecular polymers can enhance the AIE of the individual host. The FSP described in this work may have potential applications in the field of dynamic luminescent materials.
The original contributions generated for the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
TX conceived and designed the study and wrote and revised the manuscript. HW conducted the synthetic experiments. All authors analyzed and interpreted the data.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We gratefully thank the financial support from the National Natural Science Foundation of China (21702020).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2020.610093/full#supplementary-material
Aida, T., Meijer, E. W., and Stupp, S. I. (2012). Functional supramolecular polymers. Science 335, 813–817. doi: 10.1126/science.1205962
Chen, Y., Sun, S., Lu, D., Shi, Y., and Yao, Y. (2019). Water-soluble supramolecular polymers constructed by macrocycle-based host-guest interactions. Chin. Chem. Lett. 30, 37–43. doi: 10.1016/j.cclet.2018.10.022
Cheng, M., Zhang, J., Ren, X., Guo, S., Xiao, T., Hu, X. Y., et al. (2017). Acid/base-controllable fluorescent molecular switches based on cryptands and basic N-heteroaromatics. Chem. Commun. 53, 11838–11841. doi: 10.1039/C7CC07469G
Datta, S., Kato, Y., Higashiharaguchi, S., Aratsu, K., Isobe, A., Saito, T., et al. (2020). Self-assembled poly-catenanes from supramolecular toroidal building blocks. Nature 583, 400–405. doi: 10.1038/s41586-020-2445-z
Dong, S., Leng, J., Feng, Y., Liu, M., Stackhouse, C. J., Schonhals, A., et al. (2017). Structural water as an essential comonomer in supramolecular polymerization. Sci. Adv. 3:eaao0900. doi: 10.1126/sciadv.aao0900
Gu, X., Yao, J., Zhang, G., Yan, Y., Zhang, C., Peng, Q., et al. (2012). Polymorphism-dependent emission for di(p-methoxylphenyl)dibenzofulvene and analogues: optical waveguide/amplified spontaneous emission behaviors. Adv. Funct. Mater. 22, 4862–4872. doi: 10.1002/adfm.201201482
Guo, D.-S., Chen, S., Qian, H., Zhang, H.-Q., and Liu, Y. (2010). Electrochemical stimulus-responsive supramolecular polymer based on sulfonatocalixarene and viologen dimers. Chem. Commun. 46, 2620–2622. doi: 10.1039/b925157j
Han, Y., Tian, Y., Li, Z., and Wang, F. (2018). Donor-acceptor-type supramolecular polymers on the basis of preorganized molecular tweezers/guest complexation. Chem. Soc. Rev. 47, 5165–5176. doi: 10.1039/C7CS00802C
Harada, A., Takashima, Y., and Nakahata, M. (2014). Supramolecular polymeric materials via cyclodextrin-guest interactions. Acc. Chem. Res. 47, 2128–2140. doi: 10.1021/ar500109h
Hong, Y., Lam, J. W. Y., and Tang, B. Z. (2011). Aggregation-induced emission. Chem. Soc. Rev. 40, 5361–5388. doi: 10.1039/c1cs15113d
Ji, X., Yao, Y., Li, J., Yan, X., and Huang, F. (2013). A supramolecular cross-linked conjugated polymer network for multiple fluorescent sensing. J. Am. Chem. Soc. 135, 74–77. doi: 10.1021/ja3108559
Kim, H.-J., Nandajan, P. C., Gierschner, J., and Park, S. Y. (2018). Light-harvesting fluorescent supramolecular block copolymers based on cyanostilbene derivatives and cucurbit[8]urils in aqueous solution. Adv. Funct. Mater. 28:1705141. doi: 10.1002/adfm.201705141
Kumawat, L. K., Abogunrin, A. A., Kickham, M., Pardeshi, J., Fenelon, O., Schroeder, M., et al. (2019). Squaramide-naphthalimide conjugates as “turn-on” fluorescent sensors for bromide through an aggregation-disaggregation approach. Front. Chem. 7:354. doi: 10.3389/fchem.2019.00354
Lavrenova, A., Balkenende, D. W. R., Sagara, Y., Schrettl, S., Simon, Y. C., and Weder, C. (2017). Mechano- and thermoresponsive photoluminescent supramolecular polymer. J. Am. Chem. Soc. 139, 4302–4305. doi: 10.1021/jacs.7b00342
Lee, S., Chen, C.-H., and Flood, A. H. (2013). A pentagonal cyanostar macrocycle with cyanostilbene CH donors binds anions and forms dialkylphosphate [3]rotaxanes. Nat. Chem. 5, 704–710. doi: 10.1038/nchem.1668
Li, B., He, T., Shen, X., Tang, D., and Yin, S. (2019). Fluorescent supramolecular polymers with aggregation induced emission properties. Polym. Chem. 10, 796–818. doi: 10.1039/C8PY01396A
Li, S.-L., Xiao, T., Lin, C., and Wang, L. (2012). Advanced supramolecular polymers constructed by orthogonal self-assembly. Chem. Soc. Rev. 41, 5950–5968. doi: 10.1039/c2cs35099h
Li, X., Wang, L., Deng, Y., Luo, Z., Zhang, Q., Dong, S., et al. (2018). Preparation of cross-linked supramolecular polymers based on benzo-21-crown-7/secondary ammonium salt host-guest interactions. Chem. Commun. 54, 12459–12462. doi: 10.1039/C8CC07657J
Lu, C., Zhang, M., Tang, D., Yan, X., Zhang, Z., Zhou, Z., et al. (2018). Fluorescent metallacage-core supramolecular polymer gel formed by orthogonal metal coordination and host–guest interactions. J. Am. Chem. Soc. 140, 7674–7680. doi: 10.1021/jacs.8b03781
Qi, L., Ding, Y., Xiao, T., Wu, H., Diao, K., Bao, C., et al. (2020). Supramolecular polymerization of dioxyphenylene bridged UPy derivatives and their host-guest behaviors with the bipyridinium-based cyclophane. Chin. J. Org. Chem. doi: 10.6023/cjoc202006070
Shi, B., Jie, K., Zhou, Y., Zhou, J., Xia, D., and Huang, F. (2016). Nanoparticles with near-infrared emission enhanced by pillararene-based molecular recognition in water. J. Am. Chem. Soc. 138, 80–83. doi: 10.1021/jacs.5b11676
Shi, B., Zhou, Z., Vanderlinden, R. T., Tang, J.-H., Yu, G., Acharyya, K., et al. (2019). Spontaneous supramolecular polymerization driven by discrete platinum metallacycle-based host–guest complexation. J. Am. Chem. Soc. 141, 11837–11841. doi: 10.1021/jacs.9b06181
Sun, G., Qian, W., Jiao, J., Han, T., Shi, Y., Hu, X.-Y., et al. (2020). A highly efficient artificial light-harvesting system with two-step sequential energy transfer based on supramolecular self-assembly. J. Mater. Chem. A 8, 9590–9596. doi: 10.1039/D0TA03169K
Wagner, W., Wehner, M., Stepanenko, V., and Wurthner, F. (2019). Supramolecular block copolymers by seeded living polymerization of perylene bisimides. J. Am. Chem. Soc. 141, 12044–12054. doi: 10.1021/jacs.9b04935
Wang, S., Xu, Z., Wang, T., Xiao, T., Hu, X.-Y., Shen, Y.-Z., et al. (2018). Warm/cool-tone switchable thermochromic material for smart windows by orthogonally integrating properties of pillar[6]arene and ferrocene. Nat. Commun. 9:1737. doi: 10.1038/s41467-018-03827-3
Wang, Y., Cai, Y., Cao, L., Cen, M., Chen, Y., Zhang, R., et al. (2019). An amphiphilic metallaclip with enhanced fluorescence emission in water: synthesis and controllable self-assembly into multi-dimensional micro-structures. Chem. Commun. 55, 10132–10134. doi: 10.1039/C9CC04809J
Wehner, M., and Würthner, F. (2020). Supramolecular polymerization through kinetic pathway control and living chain growth. Nat. Rev. Chem. 4, 38–53. doi: 10.1038/s41570-019-0153-8
Wei, P., Yan, X., and Huang, F. (2015). Supramolecular polymers constructed by orthogonal self-assembly based on host-guest and metal-ligand interactions. Chem. Soc. Rev. 44, 815–832. doi: 10.1039/C4CS00327F
Xiao, T., Feng, X., Wang, Q., Lin, C., Wang, L., and Pan, Y. (2013). Switchable supramolecular polymers from the orthogonal self-assembly of quadruple hydrogen bonding and benzo-21-crown-7-secondary ammonium salt recognition. Chem. Commun. 49, 8329–8331. doi: 10.1039/c3cc44525a
Xiao, T., Qi, L., Zhong, W., Lin, C., Wang, R., and Wang, L. (2019a). Stimuli-responsive nanocarriers constructed from pillar[n]arene-based supra-amphiphiles. Mater. Chem. Front. 3, 1973–1993. doi: 10.1039/C9QM00428A
Xiao, T., and Wang, L. (2018). Recent advances of functional gels controlled by pillar[n]arene-based host–guest interactions. Tetrahedron Lett. 59, 1172–1182. doi: 10.1016/j.tetlet.2018.02.028
Xiao, T., Wu, H., Sun, G., Diao, K., Wei, X., Li, Z.-Y., et al. (2020a). An efficient artificial light-harvesting system with tunable emission in water constructed from H-bonded AIE supramolecular polymer and Nile Red. Chem. Commun. 56, 12021–12024. doi: 10.1039/D0CC05077F
Xiao, T., Xu, L., Götz, J., Cheng, M., Würthner, F., Gu, J., et al. (2019b). Supramolecular polymerization and cyclization of dioxynaphthalene motif bridged bifunctional UPys: minor variations in the molecular skeleton and drastic differences in self-assembly. Mater. Chem. Front. 3, 2738–2745. doi: 10.1039/C9QM00595A
Xiao, T., Xu, L., Wang, J., Li, Z.-Y., Sun, X.-Q., and Wang, L. (2019c). Biomimetic folding of small organic molecules driven by multiple non-covalent interactions. Org. Chem. Front. 6, 936–941. doi: 10.1039/C9QO00089E
Xiao, T., Xu, L., Zhong, W., Zhou, L., Sun, X.-Q., Hu, X.-Y., et al. (2018). Advanced functional materials constructed from pillar[n]arenes. Isr. J. Chem. 58, 1183–1193. doi: 10.1002/ijch.201800026
Xiao, T., Xu, L., Zhou, L., Sun, X.-Q., Lin, C., and Wang, L. (2019d). Dynamic hydrogels mediated by macrocyclic host–guest interactions. J. Mater. Chem. B 7, 1526–1540. doi: 10.1039/C8TB02339E
Xiao, T., Zhong, W., Qi, L., Gu, J., Feng, X., Yin, Y., et al. (2019e). Ring-opening supramolecular polymerization controlled by orthogonal non-covalent interactions. Polym. Chem. 10, 3342–3350. doi: 10.1039/C9PY00312F
Xiao, T., Zhong, W., Xu, L., Sun, X.-Q., Hu, X.-Y., and Wang, L. (2019f). Supramolecular vesicles based on pillar[n]arenes: design, construction, and applications. Org. Biomol. Chem. 17, 1336–1350. doi: 10.1039/C8OB03095B
Xiao, T., Zhong, W., Zhou, L., Xu, L., Sun, X.-Q., Elmes, R. B. P., et al. (2019g). Artificial light-harvesting systems fabricated by supramolecular host–guest interactions. Chin. Chem. Lett. 30, 31–36. doi: 10.1016/j.cclet.2018.05.034
Xiao, T., Zhou, L., Sun, X.-Q., Huang, F., Lin, C., and Wang, L. (2020b). Supramolecular polymers fabricated by orthogonal self-assembly based on multiple hydrogen bonding and macrocyclic host–guest interactions. Chin. Chem. Lett. 31, 1–9. doi: 10.1016/j.cclet.2019.05.011
Xiao, T., Zhou, L., Wei, X., Li, Z., and Sun, X. (2020c). Supramolecular copolymers driven by quadruple hydrogen bonding and host-guest interactions. Chin. J. Org. Chem. 40, 944–949. doi: 10.6023/cjoc201911014
Xiao, T., Zhou, L., Xu, L., Zhong, W., Zhao, W., Sun, X.-Q., et al. (2019h). Dynamic materials fabricated from water soluble pillar[n]arenes bearing triethylene oxide groups. Chin. Chem. Lett. 30, 271–276. doi: 10.1016/j.cclet.2018.05.039
Yang, L., Tan, X., Wang, Z., and Zhang, X. (2015). Supramolecular polymers: historical development, preparation, characterization, and functions. Chem. Rev. 115, 7196–7239. doi: 10.1021/cr500633b
Zhang, C., Li, S., Zhang, J., Zhu, K., Li, N., and Huang, F. (2007). Benzo-21-crown-7/secondary dialkylammonium salt [2]Pseudorotaxane- and [2]rotaxane-type threaded structures. Org. Lett. 9, 5553–5556. doi: 10.1021/ol702510c
Zhang, C.-W., Ou, B., Jiang, S.-T., Yin, G.-Q., Chen, L.-J., Xu, L., et al. (2018). Cross-linked AIE supramolecular polymer gels with multiple stimuli-responsive behaviours constructed by hierarchical self-assembly. Polym. Chem. 9, 2021–2030. doi: 10.1039/C8PY00226F
Zhang, M., Yin, S., Zhang, J., Zhou, Z., Saha, M. L., Lu, C., et al. (2017). Metallacycle-cored supramolecular assemblies with tunable fluorescence including white-light emission. Proc. Natl. Acad. Sci. U.S.A. 114, 3044–3049. doi: 10.1073/pnas.1702510114
Zhang, Q., Li, T., Duan, A., Dong, S., Zhao, W., and Stang, P. J. (2019). Formation of a supramolecular polymeric adhesive via water-participant hydrogen bond formation. J. Am. Chem. Soc. 141, 8058–8063. doi: 10.1021/jacs.9b02677
Keywords: supramolecular polymer, AIE, host-guest, fluorescent materials, B21C7
Citation: Wu H and Xiao T (2020) Supramolecular Polymers With AIE Property Fabricated From a Cyanostilbene Motif-Derived Ditopic Benzo-21-Crown-7 and a Ditopic Dialkylammonium Salt. Front. Chem. 8:610093. doi: 10.3389/fchem.2020.610093
Received: 25 September 2020; Accepted: 16 October 2020;
Published: 19 November 2020.
Edited by:
Suying Xu, Beijing University of Chemical Technology, ChinaReviewed by:
Xin Wu, The University of Sydney, AustraliaCopyright © 2020 Wu and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tangxin Xiao, xiaotangxin@cczu.edu.cn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.