- 1Key Laboratory of Biomedical Polymers of Ministry of Education, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, China
- 2State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Institute of Chemical Biology and Nanomedicine (ICBN), Hunan University, Changsha, China
Persistent luminescence phosphors (PLPs) are largely used in biomedical areas owing to their unique advantages in reducing the autofluorescence and light-scattering interference from tissues. Moreover, PLPs with long-lived luminescence in the near-infrared (NIR) region are able to be applied in deep-tissue bioimaging or therapy due to the reduced light absorption of tissues in NIR region. Because of their abundant election levels and energy transfer channels, lanthanides are widely doped in PLPs for the generation of NIR persistent emissions. In addition, the crystal defects introduced by lanthanides-doping can serves as charge traps in PLPs, which contributes to the enhancement of persistent luminescence intensity and the increase of persistent time. In this paper, the research progress in the synthesis and biomedical applications of lanthanides-doped PLPs with NIR emissions are systematically summarized, which can provide instructions for the design and applications of PLPs in the future.
Introduction
Persistent luminescence phosphors (PLPs) are photoluminescent materials that will remain luminescence after the excitation light is extinguished (Yang et al., 2016; Feng et al., 2018). It is generally accepted that the crystal defects in PLPs can store photogenerated electrons and holes during excitation. After the excitation light is closed, the electrons and holes in the defects can escape from the defects under stimulation, and their recombination generates the persistent luminescence phenomenon (Wang et al., 2018). The delayed luminescence in PLPs enables researchers to completely avoid the interference of autofluorescence in biological samples, which greatly improves the signal-to-noise ratio (SNR) of bioimaging (Rosticher et al., 2016; Gong et al., 2017). In addition, PLPs with near-infrared (NIR) emissions provides the possibility for bioimaging in deep tumor tissues. In 2007, Chermont et al. first applied PLPs to bioimaging, and they realized long-term in-vivo imaging for more than 1 h by using the delayed luminescence of PLPs (Chermont et al., 2007). Nowadays, persistent luminescence materials are widely studied in biomedical fields, including biosensing, bioimaging, and tumor therapy.
Generally, PLPs consist of host materials and the doped ions (Hu et al., 2018). The host materials usually display broad emission peaks at 350–600 nm, so their applications in bioimaging and so on are limited. Doping is an effective way to generate narrow band emission at different wavelengths in PLPs (Hai et al., 2020). Lanthanides have a lot of electron energy levels and long-lived excitation states, which can generate a variety of radiation absorption and emission (Zhao L. et al., 2019). Lanthanides-doping is often used to generate the desired visible or NIR emissions in PLPs. Moreover, the doped lanthanides can participate in the charge trapping and detrapping processes. These doped lanthanides may enhance the persistent luminescence intensity and prolong the persistent time (Zhang et al., 2019). In 1996, Matsuzawa et al. reported the milestone SrAl2O4:Eu2+, Dy3+ PLPs with bright and durable persistent luminescence (Matsuzawa et al., 1996). In SrAl2O4:Eu2+, Dy3+, the Eu2+ is the emission center, and Dy3+ participates in the charge trapping/detrapping processes. Inspired by Matsuzawa's work, many different lanthanides-doped PLPs have been synthesized, whose applications in biomedical areas have also been investigated (Zhang et al., 2015).
Recently, there are several good reviews on PLPs (Singh, 2014; Liang et al., 2019; Lin et al., 2020). In these reviews, the luminescence mechanisms, the different kinds of PLPs, the controlled synthesis of persistent luminescence materials, and the design of persistent luminescence nanoprobes for biomedical applications have been systematically reviewed (Lin et al., 2019; Ma et al., 2019). Whereas, the design of lanthanides-doped PLPs with NIR emissions and their biomedical applications have not been overviewed. In this review, the development of lanthanides-doped PLPs in recent years is systematically introduced from two aspects: synthesis and biomedical application. This paper mainly reviews the controlled synthesis of lanthanides-doped PLPs with emissions in the NIR region, and the applications of PLPs in biosensing, bioimaging, drug delivery, and phototherapy, which may provide instructions for the future studies on lanthanides-doped PLPs.
Synthesis of Lanthanides-Doped PLPs With NIR Emissions
Fluorescence imaging in the NIR window (650–1,700 nm) has been widely used in the field of biotechnology, such as bioimaging and targeted disease therapy (Gong et al., 2019). Generally, NIR biological window can be divided into NIR-I (650–1,000 nm) region and NIR-II (1,000–1,700 nm) region according to the wavelength (Liu Y. et al., 2019). Some PLPs whose emission located in the NIR biological window will have deep tissue penetration depth in bioimaging (Zhao H. et al., 2019). Due to having various electron energy levels, lanthanides are highly efficient in producing emissions in the NIR region. This section will introduce the methods for the design and synthesis of lanthanides-doped PLPs with NIR emissions.
Lanthanides-Doped PLPs With Emissions in NIR-I Region
In the process of NIR luminescence, the doped ions can be used as the luminescence centers to generate corresponding emissions (Zhang et al., 2018). Lanthanides atoms have abundant electron energy levels, long-lived excited states, and more than 200,000 transition channels, which can produce various radiation absorption and emission. Therefore, lanthanides-doping is widely used in the design of NIR luminescence materials, including PLPs. Li et al. prepared lanthanides-doped SrZrO3:Yb3+ PLPs by high-temperature solid-state reaction (HTSSR) (Li Z. et al., 2018). This phosphor could produce stable luminescence at around 986 nm under UV excitation. Calculations showed that the luminescence at 986 nm was originated from the doped Yb3+:2F2/5-2F2/7. The NIR persistent luminescence at 986 nm was also detected in SrZrO3:Yb3+, and the electrons stored in oxygen vacancies was proved to generate the persistent luminescence. Additionally, they showed the persistent luminescence intensity in SrZrO3:Yb3+ was related to the concentration of the doped Yb3+. The NIR persistent luminescence intensity increased with raising the concentration of Yb3+, whereas a high concentration of Yb3+ resulted in quench of the NIR luminescence. The optimal concentration of the doped Yb3+ in SrZrO3:Yb3+ was determined to be 2.5%. This work showed the good promise of lanthanides in the generation of NIR persistent luminescence by serving as the luminescence centers.
In addition to being luminescence centers, lanthanides can also serve as defects to regulate the energy storage and transfer process. Li et al. directly synthesized ZnSn2O4:Cr, Eu PLPs by a hydrothermal reaction (Figure 1A) (Li et al., 2017). In ZnSn2O4:Cr,Eu, Cr3+ acts as the NIR luminescence center with emission at 800 nm (Figure 1B), and Eu3+ acts as the trap center to capture photo-generated electrons and generate persistent luminescence after excitation ceases (Figure 1C). The ZnSn2O4:Cr,Eu PLPs can effectively avoid the light scattering interference and show deep-tissue penetration in bioimaging. Moreover, the authors realized the covalent modification of folic acids on the ZnSn2O4:Cr,Eu surface for target tumor imaging.
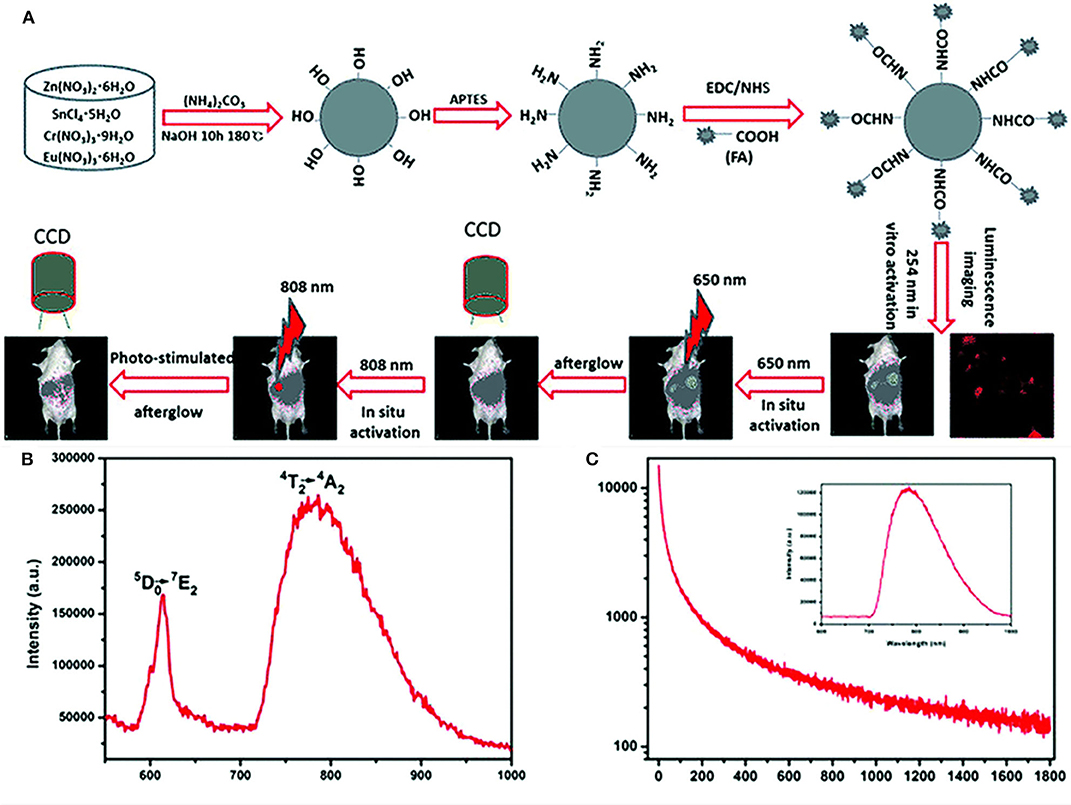
Figure 1. (A) Schematic diagram of the synthesis and bioimaging applications of the ZnSn2O4:Cr,Eu PLPs, (B) emission spectrum of the PLPs of excitation at 254 nm, (C) decay curve of the PLPs of excitation at 254 nm and the inset is PersL spectrum. Reproduced from a literature Li et al. (2017) with permission from Royal Society of Chemistry.
Generally, PLPs need to be charged by UV light, and this largely limits their biomedical applications. Considering the deep penetration of NIR light, the PLPs which are excited by NIR light have a better promise in biomedical areas. Qin et al. successfully synthesized a upconversion PLPs Zn1.3Ga1.4Sn0.3O4:Yb3+,Er3+,Cr3+ by HTSSR (Qin et al., 2019). Due to the tailored energy transfer (ET) between doping ions, this phosphor shows the NIR emission of Cr3+. In these PLPs, Yb3+ first absorbs NIR (980 nm) photons and transfers the energy to Er3+ by upconversion (UC) emission, and then Er3+ transfers the energy to Cr3+ by ET to realize NIR persistent luminescence emission of Cr3+ at 694 nm. Due to the special luminescence property, these PLPs will reduce photodamage to biological tissues as well as improving the penetrability of the excitation light, which provides the possibility for deep tumors imaging and therapy.
Lanthanides-Doped PLPs With Emissions in NIR-II Region
As previously introduced, a longer emission wavelength of PLPs can effectively improve the penetration depth of bioimaging (Zhang et al., 2018). Therefore, the PLPs in NIR-II have a better application prospect in biomedical fields. Xu et al. synthesized the Y3Al2Ga3O12:Er3+,Cr3+ PLPs with emissions in NIR-II region by HTSSR (Xu et al., 2018b). Due to the ET from Cr3+ to Er3+, these PLPs have the persistent luminescence of both Cr3+ (690 nm) and Er3+ (1,532 nm). The authors also proved that PLPs did have deep penetration depth in tissues, which improved SNR of bioimaging. In addition, by doping Ho3+ in LaAlO3 and LaGaO3 perovskite, Xu et al. also successfully developed two kinds of persistent luminescence perovskite particles with multi-wavelength emissions (NIR-I and NIR-II) (Xu et al., 2018a). This work indicated that the method of lanthanides doping can be used to adjust the emission wavelength of various types of PLPs. Therefore, this lanthanides co-doping method provides a reference for the synthesis of other PLPs with emissions in NIR-II.
Recently, Xu et al. synthesized a Y3Al2Ga3O12:Nd3+, Ce3+,Cr3+ PLPs with multi-wavelength emissions at about 880, 1,064, and 1,335 nm by HTSSR (Xu et al., 2015). Calculations showed that the multi-wavelength luminescence was originated from the doped Nd3+:4F3/2 to 4I9/2, 4I11/2, and 4I13/2, respectively. Since its emissions match with the NIR-I and NIR-II region, PLPs with long persistent luminescence have promising applications in bioimaging and tumor therapy.
In addition to inorganic materials, organic lanthanides co-doped system also shows the persistent luminescence phenomenon. Li et al. synthesized an organic Er3+ complex Er(F-TPIP)3 [tetrakis(pentafluorophenyl)imidodiphosphinate] successfully (Li H.-F. et al., 2020). This complex had the NIR emission at around 1,500 nm due to the 4I13/2-4I15/2 transition of Er3+. The lifetime of the Er(F-TPIP)3 is 2.65 ms, which was the longest Er3+ lifetime in the hydrogenous organic environment. The authors realized the enhancement of Er3+ emission through the co-doping of a photosensitizer (phosphororganic molecule), whose enhancement effect was up to 1,600 times. This photosensitizer and lanthanides co-doped method is expected to realize the design and synthesis of organic lanthanides system with strong NIR persistent emissions.
Biosensing Based on Lanthanides-Doped PLPs
PLPs are ideal materials for building fluorescent probes for biosensing due to their delayed luminescence properties (Kumar et al., 2017; Zhang X. et al., 2018). Li et al. synthesized the PLPs nanoprobe Ir(III)@SiNPs-Eu3+ with 653 nm emission for tetracycline (TC) detection (Li X. et al., 2020). Since TC will enhance the emission intensity of doped Er3+ and quench the original Ir(III)@SiNPs luminescence, this nanoprobe could realize the ratiometric analysis of the TC in complicated tissues. The authors demonstrated that the nanoprobe was sensitive to TC in the serum background, and the TC-nanoprobe complex could also detect Hg2+ sensitively through the ratiometric luminescence mode. In addition, the nanoprobe had low cytotoxicity. The developed nanoprobe has a great application potential in biological detection, including the detection of TC and Hg2+ in biological samples.
Recently, Dou et al. synthesized a La2O2CO3:Eu3+,Ho3+ PLPs with emission at around 704 nm, whose size and persistent luminescence could be adjusted by changing the reaction conditions such as time and reaction power (Dou et al., 2019). They found that the PLPs had good stability in water, which can achieve long-term preservation in liquid environment for more than 1 week. In addition, they demonstrated the La2O2CO3:Eu3+,Ho3+ nanoprobe could achieve highly sensitive detection of H2O2 in the serum environment because the H2O2 can quench the luminescence of this probe. Glucose, on the other hand, can produce H2O2 through enzymatic (glucose oxidase) reaction in vivo, so this probe can determine the serum glucose concentration. Different from traditional detecting methods based on enzymes, this PLP-based method isn't sensitive to the change of temperature and pH. Therefore, this probe will have a broader prospect in the clinical monitoring of patients with hyperglycemia or diabetes.
The application of PLPs has also been extended to the field of food safety. Liu et al. successfully synthesized a kind of persistent luminescence nanophosphors (PLNPs, ZnGa2O4:Ga,Er,Yb)@MIP (molecularly imprinted) with 700 nm emission, which can selectively adsorb the biological toxins such as ochratoxin and aflatoxin in vivo and in vitro (Liu J. et al., 2019). In this material, MIP has specific recognition ability for three biotoxins (sterigmatocystin, ochratoxin, and aflatoxin), and PLNPs act as fluorescent probes to eliminate background interference. In mice, the PLNPs@MIP in the tissues that contained biotoxins will have a brighter luminescence and a slower rate of being cleared out. In addition, the characteristic of persistent luminescence enabled PLNPs@MIP to realize the tracking of biological toxins, so as to explain the damage mechanism of biological toxins to human body. This work showed had a good application prospect in the detection of biological toxins in food.
Bioimaging Based on Lanthanides-Doped PLPs
As mentioned in the first part, PLPs are ideal materials for bioimaging since they can effectively avoid spontaneous fluorescence of biological tissues (Abdukayum et al., 2013; Li et al., 2020). However, the PLPs synthesized by traditional high-temperature reactions lack appropriate modifiable group on the surface (Du et al., 2017). Moreover, the persistent luminescence of PLPs will diminish over time in vivo, and they cannot be effectively reactivated by UV light due to the limited penetration of UV. Shi et al. proposed a synthesis method for the preparation of amino functionalized ZnGa2O4:Cr,Eu PLPs with emission at 700 nm (Figures 2A–C) (Shi et al., 2016). The particle size and persistent luminescence of the PLPs can be controlled by changing the reaction conditions including time, pH, and so on. Due to the surface amino groups, the PLPs can be easily modified with biological molecules, such as folic acid for bioimaging. Lanthanides-doping in the PLPs significantly enhanced the persistent emission of Cr3+ at 700 nm. As a result, a high SNR (>4.0) was achieved in tumor imaging (Figure 2D). The authors also demonstrated that the PLPs can be re-excited in vivo to restore the signal intensity by NIR stimulation at 808 nm. This work paves the way for the development of PLPs with easy surface modification and in-vivo reactivation, showing a great application prospect in sustainable biological imaging.
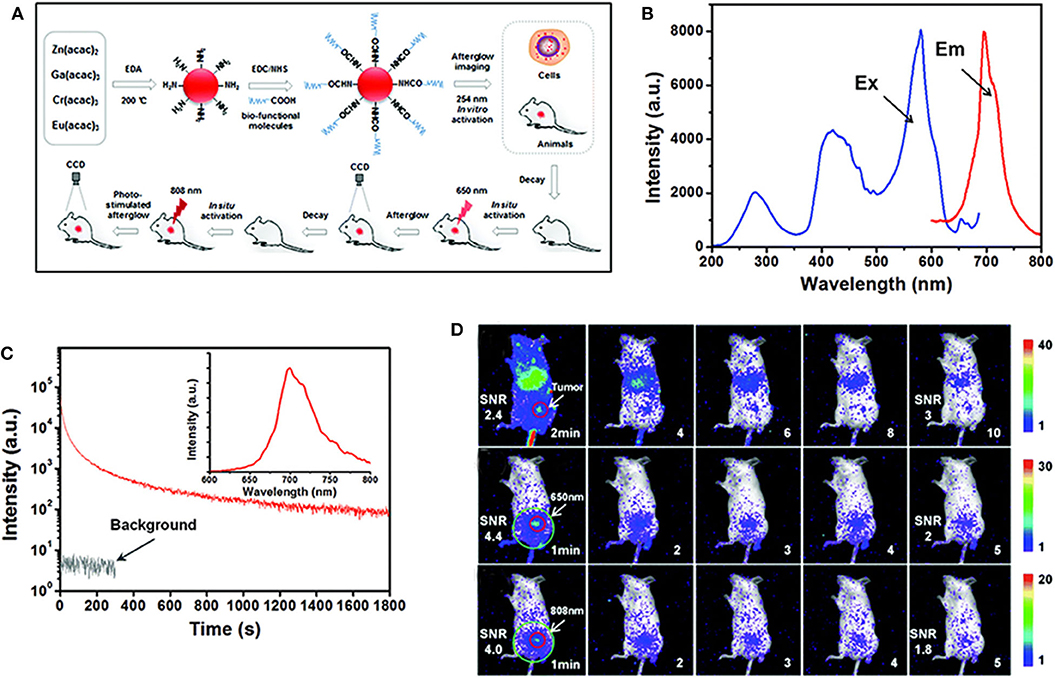
Figure 2. (A) Schematic diagram of synthesis and bioimaging application of the PLPs, (B) emission and excitation spectra of the PLPs, (C) decay curve of the PLPs of excitation at 254 nm and the inset is PersL spectrum, (D) bioimaging in mice after injection of PLPs. Reproduced from a literature Shi et al. (2016) with permission from Royal Society of Chemistry.
Recently, Li et al. proposed a hydrothermal method for the synthesis of monodisperse Zn1.25Ga1.5Ge0.25O4:Cr3+,Yb3+,Er3+ PLPs with a broad emission from 650 to 850 nm (Li and Yan, 2016). These triple-doped PLPs had high quantum yields (9.86%) and long persistent luminescence time (>20 days). They found that after modifying folic acid on the surface, the particle that was low in biotoxicity can achieve targeted imaging of the tumor. Moreover, the therapy effect of oral administration was better than intravenous administration. The oral administration imaging based on this lanthanides-doped PLPs can improve the imaging time window while avoiding the injection step. These PLPs were expected to achieve effective optical imaging of oral drugs.
Terminal cancer is often difficult to treat because of the metastasis of cancer cells, so it is necessary to develop an imaging method to track the cancer metastasis in order to achieve more accurate treatment (Sengar et al., 2019). Lanthanides-doped PLPs have good promise in tumor tracking and metastasis imaging due to their bright and rechargeable persistent luminescence. Zhao et al. synthesized alginate containing PLPs Zn1.1Ga1.8Ge0.1O4:Eu0.009,Cr0.09 with the emission at around 698 nm, and they covalently modified the PLPs with 4-carboxyphenylboric acid to target breast cancer cells (Zhao et al., 2020). The PLP probe was not interfered by spontaneous fluorescence and could achieve long-term tumor imaging. They found that the composite PLPs could target breast cancer cells precisely in mice by endocytosis. In addition, with the increase of labeled cells, the SNR increased as well. When the number of labeled cells was 10, the SNR is up to 3.0 ± 0.1. They also showed that these PLPs can be covalently modified with other targeted agents to achieve long-term tracking of other cancer metastasis. This tailored composite PLPs can solve the problems of cancer metastasis monitoring and provide a common platform for the accurate detection of cancer.
In bioimaging, the penetration depth of excitation light directly affects the imaging sensitivity in deep tissues (Rosticher et al., 2015; Zhong et al., 2019). Although X-rays have high energy and deep penetration, they are harmful to the human body (Song et al., 2017). So researchers hope to develop PLP probes that can be directly excited by NIR light. Xue et al. synthesized a kind of UC PLP (Zn3Ga2GeO8:Yb/Er/Cr). The PLPs have a NIR persistent luminescence emission at around 700 nm under NIR excitation and a duration of up to 15 h (Xue et al., 2017). Different from previous X-rays and UV excitation, NIR excitation shows less toxic effect on biological samples. The authors found can be recharged by stimulating light to recover the persistent luminescence in vivo for long-term bioimaging. This dual-mode NIR charging/emission imaging PLPs greatly improves the sensitivity and penetration depth of bioimaging, and the PLPs are expected to achieve long-term imaging of deep tissues.
Drug Delivery Based on Lanthanides-Doped PLPs
In addition to bioimaging, PLPs can also be used as drug carriers to construct a bioimaging-guided drug delivery system (Zhang D. et al., 2018). Previous studies have shown that folate-modified PLPs can precisely target tumor cells, so the researchers attempted to load the PLPs with anti-tumor drugs to treat tumors in situ (Jabalera et al., 2020). Shi et al. synthesized the PLP probe Zn1.1Ga1.8Ge0.1O4:Cr3+,Eu3+@SiO2 with a 696 nm emission (Shi et al., 2015). The PLPs were covalently modified with folic acid and further loaded with anticancer drug doxorubicin to achieve targeted drug delivery. The PLP probe had strong NIR luminescence and long persistent time (>10 days). The authors showed that the probe was sensitive enough to tumor cells and could deliver drugs in situ. In addition, the PLPs can be readily recharged by excitation light, which can be applied in monitoring tumor cells for a long time for exploring the therapeutic mechanisms of antitumor drugs. This targeted drug delivery system has a good promise in tumor detection and cancer therapy.
Most PLP-based drug carriers have poor biocompatibility, so they are easy to be swallowed by macrophages in delivering drugs. In order to solve this problem, Liu et al. combined Zn1.25Ga1.5Ge0.25O4:Cr3+,Er3+,Yb3+ PLPs with membrane structure to prepare a new type of nanocarrier with biological characteristics (Liu et al., 2018). This nanocarrier has the NIR emission at around 700 nm, and displayed long persistent luminescence for over 20 days. In a proof of concept study, the erythrocyte membrane-coated nanocarriers effectively evaded the body's immune system and delivered drugs efficiently. The erythrocyte membrane-coated nanocarriers also retained the excellent luminescence performance of PLPs. Additionally, the authors found that the nanocarriers not only have good biocompatibility and bright persistent luminescence but also can achieve the release of drugs guided by bioimaging. This new drug carrier has a great prospect in targeted tumor therapy and other biological fields.
Addition to the poor biocompatibility, the drug-loading amount of PLPs is usually limited, which will affect the efficiency of drugs delivery and cancer therapy. Recently, Li et al. synthesized porous PLPs GdAlO3:Cr3+,Sm3+ for drug delivery (Li J. et al., 2018). These PLPs showed strong persistent luminescence at around 732 nm under UV excitation. They modified the surface of the PLPs with carboxymethyl chitosan to reduce their biotoxicity. The drug carriers based on this porous PLPs had high drug-loading efficiency. They also demonstrated in this composite that PLPs can achieve slow drug release when loaded with ibuprofen, an anti-inflammatory drug. These porous PLPs have a broad application prospect in drug delivery guided by bioimaging and can serve as a potential platform to explore the kinetics of drug release in vivo.
Phototherapy Based on Lanthanides-Doped PLPs
Phototherapy has great advantages in tumor therapy because it has little toxicity to normal cells and can effectively kill cancer cells (Yang et al., 2018; Hu et al., 2019). The traditional photodynamic therapy (PDT) platform is usually composed of a fluorescent probe and photosensitizers. Due to its delayed luminescence, PLPs do not require long-term excitation light irradiation. Therefore, PLP-based fluorescent probes can effectively reduce the light damage to biological tissues and have a good application prospect in PDT. Most porphyrin-based photosensitizers are excited by UV light, but the long irradiation of UV is damage to biological tissues. To reduce the time of UV irradiation, Wang et al. synthesized Zn1.25Ga1.5Ge0.25O4:Cr3+,Yb3+,Er3+ PLPs for PDT (Wang et al., 2017). These PLPs had strong persistent luminescence at about 690 nm. They found that the persistent luminescence of these PLNPs can effectively activated the photosensitizers aluminum phthalocyanine to generate 1O2. They coated PLNPs with mesoporous silica to reduce its biotoxicity and conjugated photosensitizer to this the PLNPs to build the platform for PDT (The PLNPs were coated with mesoporous silica to reduce their biotoxicity and were further modified with the photosensitizer to construct the PDT platform). The authors demonstrated that the PDT platform can effectively eliminate cancer cells under short periods of UV irradiation. Therefore, the PLPs show good promise in UV-based PDT as the second excitation source to a photosensitizer and can provide possibilities for low-dose UV-excited PDT.
PLPs are often applied in phototherapy as fluorescent probes, but some PLPs do not have good water solubility, which affects their application in phototherapy. Homayoni et al. prepared Sr2MgSi2O7:Eu2+,Dy3+ PLPs with good water solubility by the sol-gel method, and used APTES to modify their surface (Homayoni et al., 2016). These PLPs had emission at around 660 nm under the X-ray excitation due to the 4F9/2-6H13/2 transitions of Dy3+. The PLPs were covalently modified with folic acid and were further modified with protoporphyrin, a photosensitizer, to achieve targeted PDT. The protoporphyrin was excited by the emission of PLPs in this PDT system, which enhanced the luminescence of protoporphyrin by 10 times. By integrating in-situ biological imaging and photodynamic therapy, this PDT system was able to produce 1O2 continuously upon the primary X-ray excitation to achieve efficient tumor therapy. This work showed the good promise of these PLPs in tumor phototherapy and radiotherapy.
Besides PDT, photothermal therapy (PTT) is also an effective phototherapy method, and the traditional PTT platform is usually composed of fluorescent probe and photothermal agent (Zheng et al., 2016; Zhao et al., 2017; Zhen et al., 2018). Different from the previous method of carrying photothermal agent, Wu et al. synthesized a Zn3Ga2SnO8:Cr3+,Nd3+,Gd3+ PLPs with photothermal effect (Wu et al., 2016). These PLPs had strong persistent luminescence at about 700 nm because of the 2E–4A2 transition of Cr3+. This use of PLPs that don't need the prolonged exposure of high-energy excitation light could effectively reduce light damage in PTT. The doped Nd3+ can absorb the energy of excitation light at 808 nm and convert it into heat energy for ablating the tumor cells. Due to the combination of persistent luminescence and photothermal effect, this PLP-based integrated platform is expected to realize efficient PTT guided by bioimaging, which can effectively reduce the side effects of PTT. This work provides a reference for the designing of non-composite PTT platform.
Conclusion
Lanthanides ions are often doped as luminescence centers or defects to regulate the persistent luminescence of PLPs. With proper lanthanides doping, the researchers have synthesized many kinds of PLPs with NIR persistent luminescence. These PLPs can effectively avoid spontaneous luminescence in tissues, and NIR luminescence allows PLPs to have deeper penetration. Therefore, the lanthanides-doped PLPs are ideal materials for biosensing, bioimaging, and cancer therapy. Various synthetic methods and modification strategies were proposed to improve the water solubility and biocompatibility of the PLPs. The PLP-based composite platforms have a broader prospect in biomedicine applications. In this paper, the synthesis and biomedical applications, including biosensing, bioimaging, drug delivery, and phototherapy of lanthanides-doped PLPs with NIR emission, are reviewed, aiming to provide instructions for the further studies on lanthanides-doped PLPs.
Although PLPs with NIR emission have great promise in biomedicine, great challenges are still confronted by PLPs before their practical applications. Currently, PLPs with NIR emission are generally synthesized with “top-down” methods, such as wet grinding, particularly for PLPs with emission in the NI-II region. Such PLPs usually show irregular size/shape, poor dispersibility, and surface modification. The “bottom-up” methods need to be developed for the controlled synthesis of PLPs with NIR emission. On the other hand, the PLPs with NIR emission are usually activated by UV light. The UV light has shallow tissue penetration and usually causes serious photo damage to tissues. Developing PLPs that can be directly charged by NIR light is highly desired for deep-tissue imaging and therapy. Last but not the least, research about the biosafety of the PLPs with NIR emission is rather limited. Many efforts have to be made to systematically investigate the biosafety of PLPs. With further research, these challenges are expected to be addressed, and the lanthanides-doped PLPs with NIR emission will be readily implemented into the clinical workflow for disease diagnosis and therapy.
Author Contributions
QY supervised the project and mainly wrote the paper. XQ and JW co-wrote the paper. All authors discussed the reviewed results and commented on the manuscript.
Funding
This research was supported by National Natural Science Foundation of China (21925401, 21675120, and 21904100) and the National Key R&D Program of China (2017YFA0208000).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abdukayum, A., Chen, J.-T., Zhao, Q., and Yan, X.-P. (2013). Functional near infrared-emitting Cr3+/Pr3+ co-doped zinc gallogermanate persistent luminescent nanoparticles with superlong afterglow for in vivo targeted bioimaging. J. Am. Chem. Soc. 135, 14125–14133. doi: 10.1021/ja404243v
Chermont, l. M. d. Q., Chaneac, C., Seguin, J., Pelle, F., Maitrejean, S., Jolivet, J. P., et al. (2007). Nanoprobes with near-infrared persistent luminescence for in vivo imaging. Proc. Natl. Acad. Sci. U.S.A. 104, 9266–9271. doi: 10.1073/pnas.0702427104
Dou, X., Li, Y., Vaneckova, T., Kang, R., Hu, Y., Wen, H., et al. (2019). Versatile persistent luminescent oxycarbonates: morphology evolution from nanorods through bamboo-like nanorods to nanoparticles. J. Lumin. 215:116635. doi: 10.1016/j.jlumin.2019.116635
Du, J., Clercq, O. Q. D., Korthout, K., and Poelman, D. (2017). LaAlO3:Mn4+ as near-infrared emitting persistent luminescence phosphor for medical imaging: a charge compensation study. Materials 10:1422. doi: 10.3390/ma10121422
Feng, F., Chen, X., Li, G., Liang, S., Hong, Z., and Wang, H. F. (2018). Afterglow resonance energy transfer inhibition for fibroblast activation protein-alpha assay. ACS Sensors 3, 1846–1854. doi: 10.1021/acssensors.8b00680
Gong, Z., Liu, Y., Yang, J., Yan, D., Zhu, H., Liu, C., et al. (2017). A Pr3+ doping strategy for simultaneously optimizing the size and near infrared persistent luminescence of ZGGO:Cr3+ nanoparticles for potential bio-imaging. Phys. Chem. Chem. Phys. 19, 24513–24521. doi: 10.1039/C7CP02909H
Gong, Z., Yang, J., Zhu, H., Yan, D., Liu, C., Xu, C., et al. (2019). The synergistically improved afterglow and magnetic resonance imaging induced by Gd3+ doping in ZGGO:Cr3+ nanoparticles. Mater. Res. Bull. 113, 122–132. doi: 10.1016/j.materresbull.2019.01.031
Hai, O., Yang, E., Wei, B., Ren, Q., Wu, X., and Zhu, J. (2020). The trap control in the long afterglow luminescent material (Ca,Sr)2MgSi2O7:Eu2+,Dy3+. J. Solid State Chem. 283:121174. doi: 10.1016/j.jssc.2020.121174
Homayoni, H., Ma, L., Zhang, J., Sahi, S. K., Rashidi, L. H., Bui, B., et al. (2016). Synthesis and conjugation of Sr2MgSi2O7:Eu2+, Dy3+ water soluble afterglow nanoparticles for photodynamic activation. Photodiagn. Photodyn. 16, 90–99. doi: 10.1016/j.pdpdt.2016.08.012
Hu, X., Yang, H., Guo, T., Shu, D., Shan, W., Li, G., et al. (2018). Preparation and properties of Eu and Dy co-doped strontium aluminate long afterglow nanomaterials. Ceram. Int. 44, 7535–7544. doi: 10.1016/j.ceramint.2018.01.157
Hu, Y., Yang, Y., Li, X., Wang, X., Li, Y., Li, T., et al. (2019). Super long green persistent luminescence from X-ray excited β-NaYF4: Tb3+. Preprints 02:0241. doi: 10.20944/preprints201902.0241.v1
Jabalera, Y., Oltolina, F., Prat, M., Jimenez-Lopez, C., Fernández-Sánchez, J. F., Choquesillo-Lazarte, D., et al. (2020). Eu-doped citrate-coated carbonated apatite luminescent nanoprobes for drug delivery. Nanomaterials 10:199. doi: 10.3390/nano10020199
Kumar, D., Umrao, S., Mishra, H., Srivastava, R. R., Srivastava, M., Srivastava, A., et al. (2017). Eu:Y2O3 highly dispersed fluorescent PVA film as turn off luminescent probe for enzyme free detection of H2O2. Sens. Actuators B Chem. 247, 170–178. doi: 10.1016/j.snb.2017.02.128
Li, H.-F., Liu, X.-Q., Lyu, C., Gorbaciova, J., Wen, L.-L., Shan, G.-G., et al. (2020). Enhanced 1.54-μm photo- and electroluminescence based on a perfluorinated Er(III) complex utilizing an iridium(III) complex as a sensitizer. Light Sci. Appl. 9:32. doi: 10.1038/s41377-020-0266-3
Li, J., Shi, J., Wang, C., Li, P., Yu, Z., and Zhang, H. (2017). Five-nanometer ZnSn2O4: Cr, Eu ultra-small nanoparticles as new near infrared-emitting persistent luminescent nanoprobes for cellular and deep tissue imaging at 800 nm. Nanoscale 9, 8631–8638. doi: 10.1039/C7NR02468A
Li, J., Wang, C., Shi, J., Li, P., Yu, Z., and Zhang, H. (2018). Porous GdAlO3: Cr3+, Sm3+ drug carrier for real-time long afterglow and magnetic resonance dual-mode imaging. J. Lumin. 199, 363–371. doi: 10.1016/j.jlumin.2018.03.071
Li, X., Fan, K., Yang, R., Du, X., Qu, B., Miao, X., et al. (2020). A long lifetime ratiometrically luminescent tetracycline nanoprobe based on Ir(III) complex-doped and Eu3+-functionalized silicon nanoparticles. J. Hazard. Mater. 386:121929. doi: 10.1016/j.jhazmat.2019.121929
Li, Y.-J., and Yan, X.-P. (2016). Synthesis of functionalized triple-doped zinc gallogermanate nanoparticles with superlong near-infrared persistent luminescence for long-term orally administrated bioimaging. Nanoscale 8, 14965–14970. doi: 10.1039/C6NR04950H
Li, Z., Wu, J., Wang, Q., Liang, T., Ge, J., Wang, P., et al. (2020). A universal strategy to construct lanthanide-doped nanoparticles-based activable NIR-II luminescence probe for bioimaging. iScience 23:100962. doi: 10.1016/j.isci.2020.100962
Li, Z., Zhang, S., Xu, Q., Duan, H., Lv, Y., Lin, X., et al. (2018). Long persistent phosphor SrZrO3:Yb3+ with dual emission in NUV and NIR region: a combined experimental and first-principles methods. J. Alloys Compd. 766, 663–671. doi: 10.1016/j.jallcom.2018.06.376
Liang, L., Chen, N., Jia, Y., Ma, Q., Wang, J., Yuan, Q., et al. (2019). Recent progress in engineering near-infrared persistent luminescence nanoprobes for time-resolved biosensing/bioimaging. Nano Res. 12, 1279–1292. doi: 10.1007/s12274-019-2343-6
Lin, Q., Li, Z., Ji, C., and Yuan, Q. (2020). Electronic structure engineering and biomedical applications of low energy-excited persistent luminescence nanoparticles. Nanoscale Adv. 2, 1380–1394. doi: 10.1039/C9NA00817A
Lin, Q., Li, Z., and Yuan, Q. (2019). Recent advances in autofluorescence-free biosensing and bioimaging based on persistent luminescence nanoparticles. Chin. Chem. Lett. 30, 1547–1556. doi: 10.1016/j.cclet.2019.06.016
Liu, J., Wang, Z., Li, C., Lv, S., Zhao, N., and Wang, S. (2019). Construction of molecularly imprinted nanoplatforms with persistent luminescence for in vitro specific adsorption and in vivo targeted regulation of food-borne biotoxins. New J. Chem. 43, 15097–15104. doi: 10.1039/C9NJ03231B
Liu, J.-M., Zhang, D.-D., Fang, G.-Z., and Wang, S. (2018). Erythrocyte membrane bioinspired near-infrared persistent luminescence nanocarriers for in vivo long-circulating bioimaging and drug delivery. Biomaterials 165, 39–47. doi: 10.1016/j.biomaterials.2018.02.042
Liu, Y., Liu, J., Chen, D., Wang, X., Liu, Z., Liu, H., et al. (2019). Quinoxaline-based semiconducting polymer dots for in vivo NIR-II fluorescence imaging. Macromolecules 52, 5735–5740. doi: 10.1021/acs.macromol.9b01142
Ma, Q., Wang, J., Li, Z., Lv, X., Liang, L., and Yuan, Q. (2019). Recent progress in time-resolved biosensing and bioimaging based on lanthanide-doped nanoparticles. Small 15:1804969. doi: 10.1002/smll.201804969
Matsuzawa, T., Aoki, Y., Takeuchi, N., and Murayama, Y. (1996). A new long phosphorescent phosphor with high brightness, SrAI2O4:Eu2+,Dy3+. J. Electrochem. Soc. 143:2670. doi: 10.1149/1.1837067
Qin, J., Xiang, J., Suo, H., Chen, Y., Zhang, Z., Zhao, X., et al. (2019). NIR persistent luminescence phosphor Zn1.3Ga1.4Sn0.3O4:Yb3+,Er3+,Cr3+ with 980 nm laser excitation. J. Mater. Chem. C 7, 11903–11910. doi: 10.1039/C9TC03882E
Rosticher, C., Viana, B., Fortin, M. A., Lagueux, J., Faucher, L., and Chanéac, C. (2016). Gadolinium oxysulfide nanoprobes with both persistent luminescent and magnetic properties for multimodal imaging. RSC Adv. 6, 55472–55478. doi: 10.1039/C6RA05030A
Rosticher, C., Viana, B., Laurent, G., Le Griel, P., and Chanéac, C. (2015). Insight into CaMgSi2O6:Eu2+,Mn2+,Dy3+ nanoprobes: influence of chemical composition and crystallinity on persistent red luminescence. Eur. J. Inorg. Chem. 2015, 3681–3687. doi: 10.1002/ejic.201500257
Sengar, P., García-Tapia, K., Can-Uc, B., Juárez-Moreno, K., Contreras-López, O. E., and Hirata, G. A. (2019). Simultaneous paramagnetic and persistence-luminescence in GAGG:Ce,Pr nanoparticles synthesized by sol-gel for biomedical applications. J. Appl. Phys. 126:083107. doi: 10.1063/1.5098788
Shi, J., Sun, X., Li, J., Man, H., Shen, J., Yu, Y., et al. (2015). Multifunctional near infrared-emitting long-persistence luminescent nanoprobes for drug delivery and targeted tumor imaging. Biomaterials 37, 260–270. doi: 10.1016/j.biomaterials.2014.10.033
Shi, J., Sun, X., Zhu, J., Li, J., and Zhang, H. (2016). One-step synthesis of amino-functionalized ultrasmall near infrared-emitting persistent luminescence nanoparticles for in vitro and in vivo bioimaging. Nanoscale 8, 9798–9804. doi: 10.1039/C6NR00590J
Singh, S. K. (2014). Red and near infrared persistent luminescence nano-probes for bioimaging and targeting applications. RSC Adv. 4, 58674–58698. doi: 10.1039/C4RA08847F
Song, L., Lin, X., Song, X., Chen, S., Chen, X., Li, J., et al. (2017). Repeatable deep-tissue activation of persistent luminescence nanoparticles by soft X-ray for high sensitivity long-term in vivo bioimaging. Nanoscale 9, 2718–2722 doi: 10.1039/C6NR09553D
Wang, J., Li, J., Yu, J., Zhang, H., and Zhang, B. (2018). Large hollow cavity luminous nanoparticles with near-infrared persistent luminescence and tunable sizes for tumor afterglow imaging and chemo-/photodynamic therapies. ACS Nano 12, 4246–4258. doi: 10.1021/acsnano.7b07606
Wang, J., Li, Y., Mao, R., Wang, Y., Yan, X., and Liu, J. (2017). Persistent luminescent nanoparticles as energy mediators for enhanced photodynamic therapy with fractionated irradiation. J. Mater. Chem. B 5, 5793–5805. doi: 10.1039/C7TB00950J
Wu, Y., Li, Y., Qin, X., and Qiu, J. (2016). A multifunctional biomaterial with NIR long persistent phosphorescence, photothermal response and magnetism. Chem. Asian J. 11, 2537–2541. doi: 10.1002/asia.201600569
Xu, J., Murata, D., So, B., Asami, K., Ueda, J., Heo, J., et al. (2018a). 1.2 lm persistent luminescence of Ho3+ in LaAlO3 and LaGaO3 perovskites. J. Mater. Chem. C 6:11374. doi: 10.1039/C8TC04393K
Xu, J., Murata, D., Ueda, J., Viana, B., and Tanabe, S. (2018b). Toward rechargeable persistent luminescence for the first and third biological windows via persistent energy transfer and electron trap redistribution. Inorg. Chem. 57, 5194–5203. doi: 10.1021/acs.inorgchem.8b00218
Xu, J., Tanabe, S., Sontakke, A. D., and Ueda, J. (2015). Near-infrared multi-wavelengths long persistent luminescence of Nd3+ ion through persistent energy transfer in Ce3+, Cr3+ co-doped Y3Al2Ga3O12 for the first and second bio-imaging windows. Appl. Phys. Lett. 107:081903. doi: 10.1063/1.4929495
Xue, Z., Li, X., Li, Y., Jiang, M., Ren, G., Liu, H., et al. (2017). A 980 nm laser-activated upconverted persistent probe for NIR-to-NIR rechargeable in vivo bioimaging. Nanoscale 9, 7276–7283. doi: 10.1039/C6NR09716B
Yang, Y., Wang, K. Z., and Yan, D. (2016). Ultralong persistent room temperature phosphorescence of metal coordination polymers exhibiting reversible pH-responsive emission. ACS Appl. Mater. Interfaces 8, 15489–15496. doi: 10.1021/acsami.6b03956
Yang, Y.-M., Li, Z.-Y., Zhang, J.-Y., Lu, Y., Guo, S.-Q., Zhao, Q., et al. (2018). X-ray-activated long persistent phosphors featuring strong UVC afterglow emissions. Light Sci. Appl. 7:88. doi: 10.1038/s41377-018-0089-7
Zhang, D., Liu, J., Song, N., Liu, Y., Dang, M., Fang, G., et al. (2018). Fabrication of mesoporous La3Ga5GeO14: Cr3+, Zn2+ persistent luminescence nanocarriers with super-long afterglow for bioimaging-guided in vivo drug delivery to gut. J. Mater. Chem. B 6, 1479–1488. doi: 10.1039/C7TB02759A
Zhang, J., Chen, W., and Chen, G. (2019). Luminescence of long-persistent Ca2MgSi2O7–1.5xNx:Eu2+,Dy3+ phosphors for LEDs applications. J. Mater. Sci. Mater. Electron. 30, 4056–4063. doi: 10.1007/s10854-019-00692-8
Zhang, L., Lei, J., Liu, J., Ma, F., and Ju, H. (2015). Persistent luminescence nanoprobe for biosensing and lifetime imaging of cell apoptosis via time-resolved fluorescence resonance energy transfer. Biomaterials 67, 323–334. doi: 10.1016/j.biomaterials.2015.07.037
Zhang, X., Xu, N.-Y., Ruan, Q., Lu, D.-Q., Yang, Y.-H., and Hu, R. (2018). A label-free and sensitive photoluminescence sensing platform based on long persistent luminescence nanoparticles for the determination of antibiotics and 2,4,6-trinitrophenol. RSC Adv. 8, 5714–5720. doi: 10.1039/C7RA12222E
Zhang, Y., Huang, R., Lin, Z., Li, H., Hou, D., Song, J., et al. (2018). Positive effect of codoping Yb3+ on the super-long persistent luminescence of Cr3+-doped zinc aluminum germanate. Ceram. Int. 44, 17377–17382. doi: 10.1016/j.ceramint.2018.06.202
Zhao, H., Liu, C., Gu, Z., Dong, L., Li, F., Yao, C., et al. (2020). Persistent luminescent nanoparticles containing hydrogel for targeted, sustained and autofluorescence-free tumor metastasis imaging. Nano Lett. 20, 252–260. doi: 10.1021/acs.nanolett.9b03755
Zhao, H., Shu, G., Zhu, J., Fu, Y., Gu, Z., and Yang, D. (2019). Persistent luminescent metal-organic frameworks with long-lasting near infrared emission for tumor site activated imaging and drug delivery. Biomaterials 217:119332. doi: 10.1016/j.biomaterials.2019.119332
Zhao, L., Mao, J., Jiang, B., Wei, X., Yin, M., and Chen, Y. (2019). A new yellow long persistent luminescence phosphor Ca2Al2SiO7:Eu2+,Tm3+ found by co-doping Ln3+ (Ln = Ce, Pr, Nd, Sm, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu) with Eu2+ in Ca2Al2SiO7 host. J. Lumin. 206, 6–10. doi: 10.1016/j.jlumin.2018.10.038
Zhao, P., Ji, W., Zhou, S., Qiu, L., Li, L., Qian, Z., et al. (2017). Upconverting and persistent luminescent nanocarriers for accurately imaging-guided photothermal therapy. Mater. Sci. Eng. C 79, 191–198. doi: 10.1016/j.msec.2017.05.046
Zhen, X., Xie, C., and Pu, K. (2018). Temperature-correlated afterglow of a semiconducting polymer nanococktail for imaging-guided photothermal therapy. Angew. Chem. Int. Ed. Engl. 57, 3938–3942. doi: 10.1002/anie.201712550
Zheng, B., Chen, H.-B., Zhao, P.-Q., Pan, H.-Z., Wu, X.-L., Gong, X.-Q., et al. (2016). Persistent luminescent nanocarrier as an accurate tracker in vivo for near infrared-remote selectively triggered photothermal therapy. ACS Appl. Mater. Interfaces 8, 21603–21611. doi: 10.1021/acsami.6b07642
Keywords: lanthanides, persistent luminescence, near-infrared, bioimaging, therapy
Citation: Qin X, Wang J and Yuan Q (2020) Synthesis and Biomedical Applications of Lanthanides-Doped Persistent Luminescence Phosphors With NIR Emissions. Front. Chem. 8:608578. doi: 10.3389/fchem.2020.608578
Received: 21 September 2020; Accepted: 06 November 2020;
Published: 14 December 2020.
Edited by:
Lining Sun, Shanghai University, ChinaCopyright © 2020 Qin, Wang and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Quan Yuan, eXVhbnF1YW5Ad2h1LmVkdS5jbg==
 Xinyuan Qin1
Xinyuan Qin1 Quan Yuan
Quan Yuan