
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Chem. , 11 January 2021
Sec. Green and Sustainable Chemistry
Volume 8 - 2020 | https://doi.org/10.3389/fchem.2020.574622
This article is part of the Research Topic Chemical Modification of Adsorbents for Enhanced Carbon Capture Performance View all 6 articles
Different types of amine-functionalized MOF structures were analyzed in this work using molecular simulations in order to determine their potential for post-combustion carbon dioxide capture and separation. Six amine models -of different chain lengths and degree of substitution- grafted to the unsaturated metal sites of the M2(dobdc) MOF [and its expanded version, M2(dobpdc)] were evaluated, in terms of adsorption isotherms, selectivity, cyclic working capacity and regenerability. Good agreement between simulation results and available experimental data was obtained. Moreover, results show two potential structures with high cyclic working capacities if used for Temperature Swing Adsorption processes: mmen/Mg/DOBPDC and mda-Zn/DOBPDC. Among them, the -mmen functionalized structure has higher CO2 uptake and better cyclability (regenerability) for the flue gas mixtures and conditions studied. Furthermore, it is shown that more amine functional groups grafted on the MOFs and/or full functionalization of the metal centers do not lead to better CO2 separation capabilities due to steric hindrances. In addition, multiple alkyl groups bonded to the amino group yield a shift in the step-like adsorption isotherms in the larger pore structures, at a given temperature. Our calculations shed light on how functionalization can enhance gas adsorption via the cooperative chemi-physisorption mechanism of these materials, and how the materials can be tuned for desired adsorption characteristics.
Rapid economic growth and continued industrial development have led to an increase of carbon dioxide in the atmosphere (Spigarelli and Kawatra, 2013). Hence, mitigation strategies such as Carbon Capture, Utilization, and Storage (CCUS) play an important role to limit the contribution of these emissions to the global climate change (IPCC, 2014). CO2 post-combustion capture from flue gas at power stations and chemical manufacturing plants is one of the key approaches for reducing these emissions (D'Alessandro et al., 2010). The concentration of CO2 in the flue gas produced by combustion is around 5–15%, under atmospheric pressure and at temperatures between 30 and 60°C (D'Alessandro et al., 2010; Sumida et al., 2011). The remaining components of the flue gas are mainly N2 and a small amount of secondary components including O2, H2O, CO, SOx, and NOx. In most cases the exhaust stream is treated before entering the CCUS system to reduce the concentration of these secondary species in the flue gas, as they might significantly affect the operation, even if present in trace concentrations (Spigarelli and Kawatra, 2013).
Absorption with aqueous amine solutions (e.g., monoethanolamine, MEA) is conventionally used to capture CO2 at large scale (Bourzac, 2017; Rochelle, 2009). The low solvent cost and good trapping effect make it the most popular and developed carbon capture technology. This strategy has a wide range of applications and can be applied to capture CO2 from streams with low concentrations. However, it presents several disadvantages such as high-energy consumption required for regeneration, degradation in the presence of oxygenated compounds, loss of amines by evaporation, and corrosion (Sumida et al., 2011; Huck et al., 2014; Alkhatib et al., 2020).
Alternatively, solid adsorbents are becoming popular aimed at improving the shortcomings of amine solutions. As such, the use of adsorbents has been proposed as an alternative for CO2 capture (Hedin et al., 2010; Mukherjee et al., 2019). Adsorbents can exhibit high affinity for selective CO2 adsorption under flue gas conditions. In this regard, zeolites, activated carbons, silicas, porous organic networks, and metal–organic frameworks (MOFs), among others, may offer enhanced stability, greater CO2 cycling capacities, and inherently lower regeneration energies (Choi et al., 2009; Bae and Snurr, 2011; Bollini et al., 2011; Sumida et al., 2011; Huck et al., 2014; Webley, 2014; Bui et al., 2018; Bahamon et al., 2019; Siegelman et al., 2019).
Among them, MOFs are a class of porous crystalline materials consisting of metal nodes connected by polytopic organic linkers, bearing high surface areas and highly tunable pore characteristics (Eddaoudi et al., 2002; Zhou et al., 2012; Furukawa et al., 2013). Research interest in MOFs has grown dramatically in the past decade, largely driven by their potential in gas storage, separation, catalysis, sensing, and drug delivery, to name a few (Eddaoudi et al., 2002; Lee et al., 2009; D'Alessandro et al., 2010; Keskin et al., 2010; McKinlay et al., 2010; Bae and Snurr, 2011; Mason et al., 2011; Shah et al., 2012). MOFs with high porosity, open metal uncoordinated sites and good thermal stability have demonstrated excellent qualities for CO2/N2 separations (Yazaydin et al., 2009; Zhang et al., 2010; Sumida et al., 2011; Yu et al., 2017) and are emerging as promising candidates for CO2 separation at industrial conditions. For instance, the M-MOF-74 family (with M being the divalent metal used), also known as M2(dobdc) or CPO27-M, presents one of the highest found CO2 adsorption capacities at low to moderate CO2 partial pressures, relevant at the required conditions for CO2 capture from flue gas (Britt et al., 2008; Caskey et al., 2008; Dietzel et al., 2009; Yazaydin et al., 2009; Valenzano et al., 2010; Alonso et al., 2018; Bahamon et al., 2020). However, in some cases, because CO2 typically adsorbs via weak physisorption interactions, most of these synthesized structures cannot satisfy industrial requirements. MOFs usually exhibit moderate CO2/N2 selectivity at low CO2 partial pressures, and are unable to remove most of the CO2 captured in the regeneration step (Vega and Bahamon, 2016). Furthermore, the use of the most promising MOFs require partial or complete drying of the gas stream because high affinity for CO2 entails hydrophilicity, and thus water can competitively adsorb with CO2 (Keskin et al., 2010; Sayari et al., 2011; Bahamon et al., 2018).
Therefore, amine-functionalized MOFs are attracting great attention, as they are more effective than the corresponding original solid adsorbents (McDonald et al., 2012; Planas et al., 2013; Qiao et al., 2016). In fact, functionalization is recognized today as an effective technique to improve the adsorption and separation of CO2 by MOFs and other adsorbent materials. Studies have shown that amine-functionalized MOFs have potential for greater adsorption capacity, higher selectivity, faster CO2 adsorption kinetics, and lower regeneration temperatures, making them good candidates for process adsorbents (Arstad et al., 2008; Vaidhyanathan et al., 2010; Choi et al., 2012; Das et al., 2012; Chen et al., 2013; Wang et al., 2013; Bernini et al., 2015; McDonald et al., 2015; Fracaroli et al., 2016; Huang et al., 2016; Liao et al., 2016; Lin et al., 2016; Qiao et al., 2016; Kang et al., 2019). For instance, by grafting ethylenediamine (-en) onto the open metal sites, Choi et al. (2012) modified Mg-MOF-74 and found that both CO2 adsorption capacity and the regenerability of the material were enhanced, especially in the lower pressure range. The secondary amine N,N'-dimethylethylenediamine (-mmen) was grafted onto the exposed Cu2+ sites of the Cu-BTTri framework, ensuing in a significantly enhanced CO2 capacity at low concentrations, related to the formation of zwitterionic carbamate or carbamic acid (Lee et al., 2014). Ultrahigh CO2/N2 selectivity was observed for polyethyleneimine (PEI)-impregnated MIL-101 (up to 1,200 at 50°C), alkylamine-tethered MIL-101 (up to 346), mmen-Cu-BTTri (327 under post-combustion conditions) and diamine-grafted Mg2(dobpdc) (dobpdc=4,4'-dihydroxy-(1,10-biphenyl)-3,3'-dicarboxylic acid) (up to 230) (McDonald et al., 2012; Lin et al., 2013; Lee et al., 2014).
Recently, functionalization with alkyldiamines on the unsaturated metal sites of M2(dobpdc) (herein after called M/DOBPDC), an expanded version of the well-studied MOF-74, have demonstrated to be a simple methodology for increasing low pressure CO2 adsorption selectivity and capacity (Demessence et al., 2009; Lee et al., 2014; McDonald et al., 2015; Jo et al., 2016; Milner et al., 2017, 2018; Kang et al., 2019). Formation of ammonium carbamate chains takes place for different diamine molecules on the adsorbent, leading to isotherms exhibiting step-like shapes at given temperatures (Choi et al., 2012; McDonald et al., 2015; Vlaisavljevich et al., 2015; Yeon et al., 2015; Jo et al., 2016; Milner et al., 2017, 2018; Siegelman et al., 2017; Forse et al., 2018; Lee et al., 2018). This type of material has been tested experimentally to be stable for at least 1,000 adsorption–desorption cycles (Forse et al., 2018), and this step-like adsorption can be tuned to meet different specifications. Moreover, functionalized M/DOBPDC materials showed greater stability than the non-functionalized counterparts when exposed to humidity and atmospheric conditions (McDonald et al., 2015; Siegelman et al., 2017).
Although recent achievements in post-functionalization have partially revealed the underlying CO2 adsorption mechanism for some materials, the optimal (i) MOF/amine combinations, and (ii) number of amine functional groups required for optimizing the performance of such materials for real-world CO2 capture applications, remain elusive (Kang et al., 2019). Hence, it is of great significance to understand how functionalized MOFs can be rationally designed from a computational approach to guide their efficient synthesis (Qiao et al., 2016). As molecular simulations allow the systematic and precise study of the various relevant variables of the system, they allow to isolate and quantify the effect of each of them on the performance of the system (Bahamon and Vega, 2019), being an excellent tool for the rational design of materials. Therefore, molecular simulations were used in this contribution to explore the relationship between the structure of MOFs and their CO2 adsorption performance when amino-functionalized. A series of amine-grafted MOF-74, and the expanded version M/DOBPDC, were screened, establishing the most promising materials for adsorbing low-concentration CO2, while considering their regeneration performance (cyclability). This computational study offers a molecular understanding on how the functionalization takes place on such materials and how it affects their final performance, providing guidance on the design of the best material/amine combination for optimal post-combustion CO2 capture.
M-MOF-74 possess a high density of exposed M2+ (M = Mg, Zn, Mn, Co, etc; dobdc4− = 4,6-dioxido-1,3-benzenedicarboxylate) sites (Rowsell and Yaghi, 2006). This family-type of MOFs has a honeycomb network topology, and the one-dimensional hexagonal channels are covered with bare metal ion adsorption sites (Pentyala et al., 2016). Such bare metal sites are the preferred location for adsorption of CO2 molecules at lower pressures. The coordinatively unsaturated M2+ sites in these materials lead to superior performance for the physisorptive separation of CO2 compared to other MOFs (Caskey et al., 2008; Alonso et al., 2018). Moreover, such unsaturated sites can be occupied by amines via coordination for improving the adsorption capabilities (Choi et al., 2012).
Expanded versions of materials belonging to the MOF-74 family have been synthesized using longer linker analogues, providing materials with extremely high porosity (Deng et al., 2012, Yeon et al., 2015; Forse et al., 2018). For instance, Mg/DOBPDC has a surface area (SBET) of 2,451 m2·g−1 greater than the 816 m2·g−1 for Mg-MOF-74 (McDonald et al., 2012). Therefore, the expanded form, with average pore diameter of 21Å (McDonald et al., 2012), was also chosen to be studied in this contribution. The enlarged pores provide enough space to accommodate longer alkylamines onto the open metal sites of the framework compared to M-MOF-74 materials. Hence, in this work, the open metal sites in the microporous channels were functionalized with different amines, including primary amines ethylenediamine (-en) and methanediamine (-mda); secondary amine N,N′-dimethylethylenediamine (-mmen); while 1,1-dimethylethylenediamine (-dmen) and dimethylamine (dma) were evaluated as ternary amines. Also, ammonia [(-a) as abbreviation] was included as a functionalization moiety. Crystallographic information for the solid-state structures (without and with amine-grafted molecules) can be found elsewhere (Choi et al., 2012; Milner et al., 2017; Siegelman et al., 2017; Lee et al., 2018). Structures of the different materials studied in this work are shown in Figure 1. The focus of this contribution is to gain understanding of the CO2 adsorption performance depending on the channel size and amine groups appended onto the metal sites. Moreover, different degrees of functionalization have been included by molecular simulations (e.g., 100% functionalization means one amine grafted molecule bounded to each metal center of the crystallographic structure) as part of the study, in order to quantify the effect of the functionalization degree on the adsorption behavior.
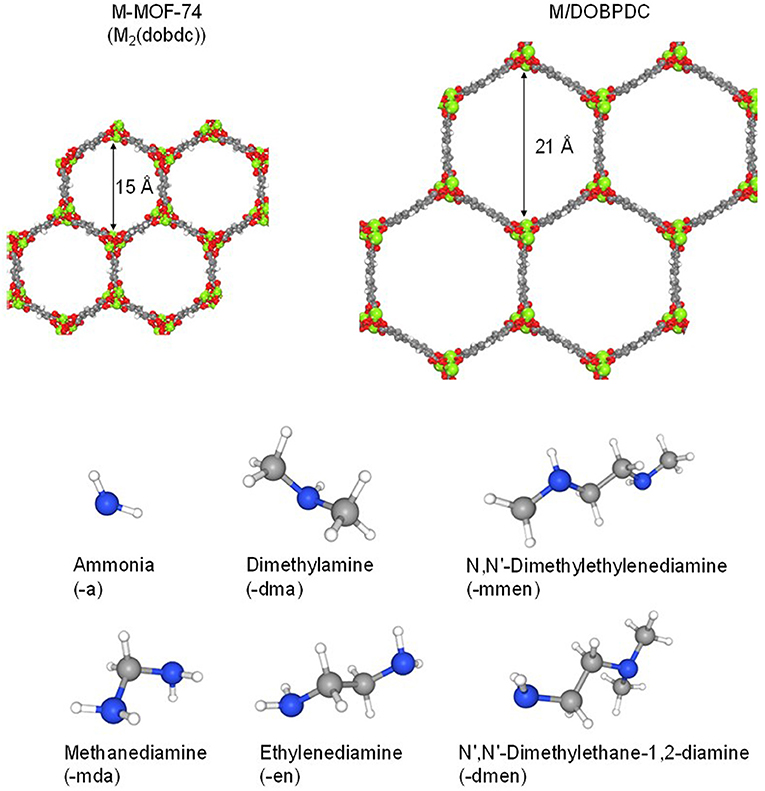
Figure 1. Molecular representation of the MOF structures studied in this work, and amine molecules used for functionalization [Color code: C, H, O, N, Mg (metal) in gray, white, red, blue, and green, respectively].
Grand Canonical Monte Carlo (GCMC, i.e., at constant chemical potential, volume and temperature) simulations were used to obtain adsorption properties for pressures ranging from 1 to 140 kPa. All simulations were performed using the LAMMPS code (Plimpton, 1995). Monte Carlo moves/steps were done with equal probability for translation, rotation, insertion, deletion, and random reinsertion of an existing molecule at a new position. The average number of adsorbed molecules of the system was calculated sequentially at each pressure point, to construct the adsorption isotherm. 1 × 106 MC equilibration steps and 1 × 106 MC production steps were used for each pressure point. Pure CO2 as well as a binary mixture containing 15% CO2 and 85% N2 (based on typical flue gas compositions) were simulated. Water and other impurities were omitted as multi-component mixtures are out of the scope of this study.
Molecular models of CO2 and N2 molecules were taken from TraPPE (Potoff and Siepmann, 2001) forcefield, which uses a rigid geometry considering only non-bonded interactions. Dzubak and Mercado force fields were used to account for the parameters of the MOFs (Dzubak et al., 2012; Mercado et al., 2016), while Lennard Jones (LJ) and Coulomb parameters of C, H, and N atoms belonging to the amine molecules were taken from the extension of TraPPE force field for nitrogen containing molecules (Wick et al., 2005). Moreover, the paired interactions between the framework atoms were excluded since the structures were considered frozen (including the amine-grafted molecules) to save computational time. The simulation cell was replicated to create 2x2x4 supercell (dimensions of ~ 40 × 40 × 28 Å). In each simulation step, the total energy calculation of the system was computed as the sum of the interaction energies of the adsorbate-adsorbent material and the adsorbate-adsorbate molecules. Lorentz–Berthelot standard mixing rules were applied for all cross terms, and Lennard-Jones interactions beyond 12.8 Å were neglected. The electrostatic interactions were computed with a relative accuracy of 10−5 by means of the Ewald summation method.
Since it is known that the amine groups chemically react with CO2 and enable adsorption enhancement at very low pressures, chemisorption was explicitly considered as proposed by Builes and Vega (2012, 2013), Builes et al. (2015). According to previous reports in the literature (Lee et al., 2014; Siegelman et al., 2017), CO2 molecules are inserted into the metal-amine bonds, promoting a reorganization of the amines into well-ordered chains. The interaction between the amines and CO2 in the pores leads to the formation of carbamic acid, as previously documented from DFT calculations (Lee et al., 2014). Therefore, in this work, instead of randomly binding selected metal centers first by the functional groups (either amine or carbamate), and then growing the rest of the amine molecule using a configurational bias algorithm (Builes and Vega, 2012), we started from a configuration extracted from reported crystallographic data files (Choi et al., 2012; Milner et al., 2017; Siegelman et al., 2017; Lee et al., 2018), where carbamic acid formation was already present. Additional CO2 physisorption was then evaluated starting from this configuration, for different degrees of functionalization. Furthermore, we considered that the metal centers of the MOFs only bind with carbon dioxide and then these with the amine groups, and no further amine-bridges or polymerization were formed between additional neighboring groups than 100% functionalization.
In mixtures, one additional indicator for determining the separation capacity of porous adsorbent is its adsorption selectivity. Selectivity was calculated as:
where x represents the molar fraction in the adsorbed phase and y the molar fraction in the bulk phase (i.e., feed conditions). The selectivity values presented in this work were directly calculated from the GCMC simulations of the mixtures, rather than from the pure isotherm data published in the theoretical and experimental studies.
For process evaluation, a particularly important attribute frequently used as an evaluation criterion in the adsorption process is the cyclic working capacity. The calculation method is:
where and stand for the uptake per mass of adsorbent under adsorption (feed) and desorption (regeneration) conditions, respectively, both involving pressure, temperature and mixture composition. For materials highly selective to CO2, a good assumption is that pure CO2 is recovered at the outlet of the adsorber (Vega and Bahamon, 2016; Prats et al., 2017). Usually this working capacity is more important than the total absorbed capacity because it defines the amount that can be recovered in each adsorption cycle. Moreover, regenerability is defined as the fraction of the bed used to collect the captured CO2, and was calculated as:
To confirm the reliability of the force fields adopted in this work, the simulated adsorption isotherms of pure CO2 were compared with experimental data at their corresponding temperatures (Caskey et al., 2008; Mason et al., 2011; Lee et al., 2014; McDonald et al., 2015). Compared materials include amine-functionalized en-Mg/DOBPDC, mmen-Mg/DOBPDC, and mmen-Zn/DOBPDC, as well as the bare Mg-MOF-74, Zn-MOF-74, and Mg/DOBPDC frameworks (see Figure 2). A good agreement with the corresponding experimental data was obtained for the amine-grafted materials after using the explicit chemisorption technique (Builes and Vega, 2012; Builes et al., 2015). However, it should be mentioned that a discrepancy of ca. 10% is presented for bare Mg-MOF-74 between the experimental and simulated isotherm, due to the use of a perfect crystal (i.e., no defects and no solvent impurities affecting accessibility for all the Mg sites), as previously explained by Dzubak et al. (2012). This difference is diminished when using a scaling factor for the simulated isotherm. Moreover, note that such treatment has more effect in this bare structure, since highly coordinated Mg-CO2 interactions are dominant at low pressures. Nevertheless, the effect is expected to show a minor contribution in the functionalized structures, since the metal sites are already occupied by a chemisorbed CO2 molecule and a bulky amine. For the other eleven studied materials, to the best of our knowledge, there are no experimental data available. Yet, we feel confident that the simulations enable good reproduction of the experimental process, as the validated structures/molecules are representative of the additional functional groups studied.
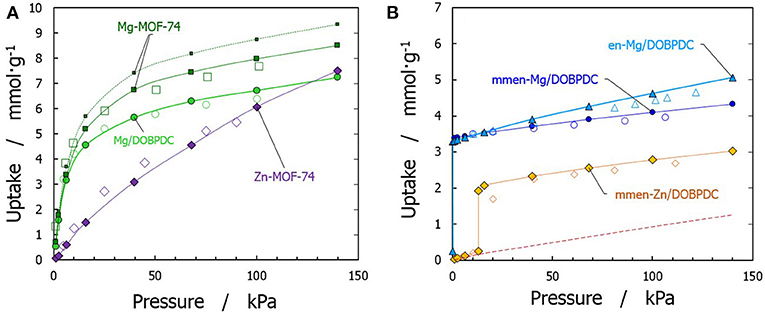
Figure 2. Validation of simulated (filled symbols) adsorption isotherms of carbon dioxide with available experimental data (open symbols) for (A) bare materials [squares from Mason et al. (2011), at 313 K; circles from McDonald et al. (2015), at 298 K; diamonds from Caskey et al. (2008), at 296 K], and (B) functionalized materials [triangles from Lee et al. (2014), at 298 K; circles and diamonds from McDonald et al. (2015), at 313 K]. Lines are to guide the eyes. For bare Mg-MOF-74, dotted line represents pristine material and straight line is the isotherm after using a scaling factor according to Dzubak et al. (2012).
At this point, is important to mention that most of the structures that have been experimentally explored use Magnesium as the open metal site (Lee et al., 2018; Kang et al., 2019). Therefore, stronger interactions are achieved but the regeneration will be more difficult because the adsorption uptake can still be significant at high temperatures. Therefore, simulated structures using a less attractive metal, such as Zinc, were also performed, as it is expected that the steepness of the isotherms gradually diminish as the temperature increases in these materials.
Figure 2 also shows the obtained CO2 adsorption isotherm for mmen-Zn/DOBPDC without considering the chemisorption process (red dashed line). As previously mentioned, the isotherms of amine grafted M/DOBPDC materials display step-like adsorption behavior (sigmoidal) at low pressures (McDonald et al., 2015), corresponding to strong interactions of CO2 with the frameworks and the amine moiety. The simulations show that this step originates from a mechanism wherein the CO2 gas molecule is cooperatively and reversibly inserted into metal–amine bond followed by formation of ammonium carbamate chain structure (Lee et al., 2018), as shown in Figure 3. When this critical step is reached and chemisorption occurs, then adsorption gradually increases with pressure due to the physisorption phenomenon. Such critical uptake was found to be 0.25 mmol·g−1 for the mmen-Zn/DOBPDC structure validated (i.e., one CO2 molecule in a crystal with 18 metal centers) and was assumed to be similar for the other hypothetical similar structures evaluated. Nevertheless, it should be mentioned that such critical point cannot be generalized to other metal centers and structures, because is highly dependent on the specific surface area (Builes et al., 2015) and the solid-fluid interactions. In fact, the assumption of both frozen frameworks and grafted-amine molecules appears as a good approximation for the pressure range studied, where adsorbents saturation has not been reached and steric effects can have a less important influence. This was validated by the agreement with experimental data at these conditions. However, the reader must be aware that enthalpic and entropic contributions when moving to higher pressures and temperatures must be considered, in this case, allowing the amines to move and reach their relaxed energetic position inside the MOF (Builes and Vega, 2012).
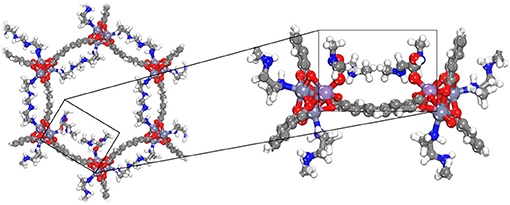
Figure 3. Three-dimensional structure representation of carbamic acid functionalization for mmen-Zn/DOBPDC (Color code: C, H, O, N, Zn in gray, white, red, blue, and purple, respectively].
A comparison of CO2 adsorption isotherms for all the studied structures at 313 K is displayed in Figure 4, with Figures 4A–C displaying the performance of bare and functionalized M-MOF-74 structures. Mg-MOF-74 represented with the scaled isotherm. Amine-grafted Mg/DOBPDC adsorbents (see Figure 4D) show significant capture capabilities at very low pressures, which are similar to the uptake for the narrower bare Mg-MOF-74 and greater than the observed in Zn-MOF-74. Note that not all the amine groups were explicitly linked to CO2 in the GCMC simulations to capture the experimentally observed isotherm step. It was found that the functionalization degree for Mg/DOBPDC and Zn/DOBPDC with the studied diamine-appended molecules is typically 72 and 61%, respectively.
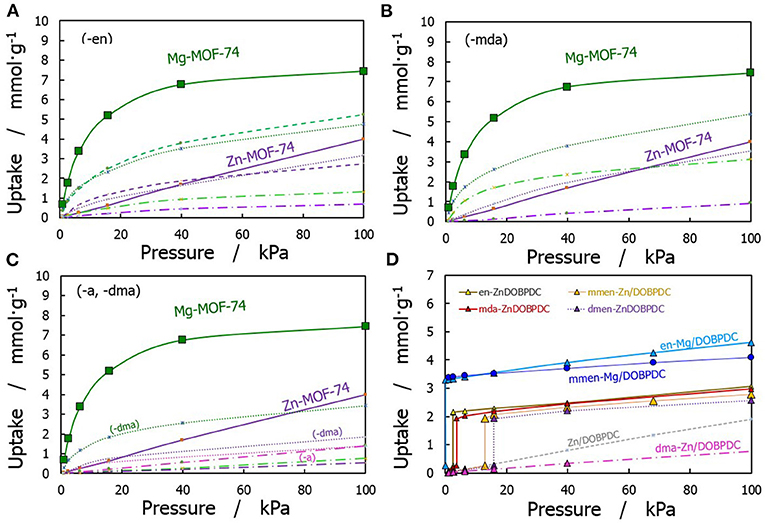
Figure 4. Comparison of simulated CO2 adsorption isotherms at 313 K. (A) MOF-74 functionalized with ethylenediamine, (B) MOF-74 functionalized with methanediamine, and (C) MOF-74 functionalized with dimethylamine and ammonia moieties (dashed lines for 16%, dotted lines for 50%, and long dash dotted lines for 100% functionalization); (D) M/DOBPDC structures functionalized with different amines.
At 15 kPa, the adsorption capacity of Mg-MOF-74 is 5.7 mmol·g−1, and 0.6 mmol·g−1 for Zn-MOF-74. The adsorption uptake for the functionalized Mg-MOF-74 structures decreases about 50–60% at this condition, mostly due to lower interactions between CO2 molecules and the highly attractive metallic centers. In contrast, some functionalized structures for Zn-MOF-74 show slightly higher values up to 0.9 mmol·g−1. However, saturation is reached at lower pressure due to the reduction of the available void fraction in the amine-containing frameworks. The degree of functionalization in MOF-74 structures show that amine substitution below 100% provide better performance than the fully substituted material, as already stated in the literature (Qiao et al., 2016). This is more noticeable as the size of the grafted amine increases. A compromise must be achieved between the increase in the CO2-adsorbent interactions and the loss of capacity due to steric effects.
As shown in Figure 4D, the capacities of the bare materials at typical flue gas conditions are reduced by using the expanded hexagonal version (M/DOBPDC), however a more interesting phenomena appears in the functionalized materials. For instance, -en and -mmen versions of Mg/DOBPDC present a capacity of ~3.6 mmol·g−1 at 15 kPa, 15% higher than the one achieved with the bare material (not shown). Furthermore, amine-functionalized versions of Zn/DOBPDC show a significant increase in the uptake at low pressures. The adsorption capacities at 15 kPa with Zn/ and Mg/DOBPDCs range from 2.1 to 3.5 mmol·g−1 respectively, higher than the representative amine-functionalized silica MCM-41-PEI-50 (Mason et al., 2015; Liao et al., 2016), with 1.5 mmol·g−1 under equivalent conditions. Hypothetically speaking, structures with an adsorption capacity of 5 mmol·g−1 can be achieved with the studied structures after 100% functionalization and carbamic acid formation in the expanded M/DOBPDC. However, no materials exhibiting more than 70–80% of functionalization have been experimentally synthesized yet for this MOF family (Sumida et al., 2011; Bernini et al., 2015).
According to the results presented in Figure 4D, the performance order for the adsorption uptake of the different studied amine functional groups was found to be –en, –mda > -mmen > -dmen. Two main reasons for this behavior are: -mda and –en have two active amine functional groups, which allow more specific interactions with CO2 molecules. The other reason is due to steric hindrance (Didas et al., 2012), since bigger and bulkier molecules reduce the CO2 accessibility.
Figure 5 shows the simulated selectivity values from 15% CO2/85% N2 mixtures at 313 K and a total pressure of 100 kPa. Compared with the bare MOF-74, the functionalized versions with magnesium as metal center do not present enhancement on the CO2-over-N2 affinity. Conversely, some zinc structures such as en-Zn-MOF-74 and mda-Zn-MOF-74 tripled their selectivity values. Mg-MOF-74 has the highest carbon dioxide selectivity (i.e., 97) among the bare studied structures. However, the selectivity becomes exceptionally high when it comes to amine-grafted Mg/DOBPDC MOFs. Obtained values for the structures with explicit chemisorption (highlighted in gray in Figure 5) range from 200 up to 350, in good agreement with the reported values of ~230 for functionalized Mg/DOBPDC (Lee et al., 2014). Moreover, it should be noted that even the functionalized expanded hexagonal frameworks with zinc as metal center could reach such high values, making them potential materials to be explored for CO2 capture from flue gas since high purity can be achieved.
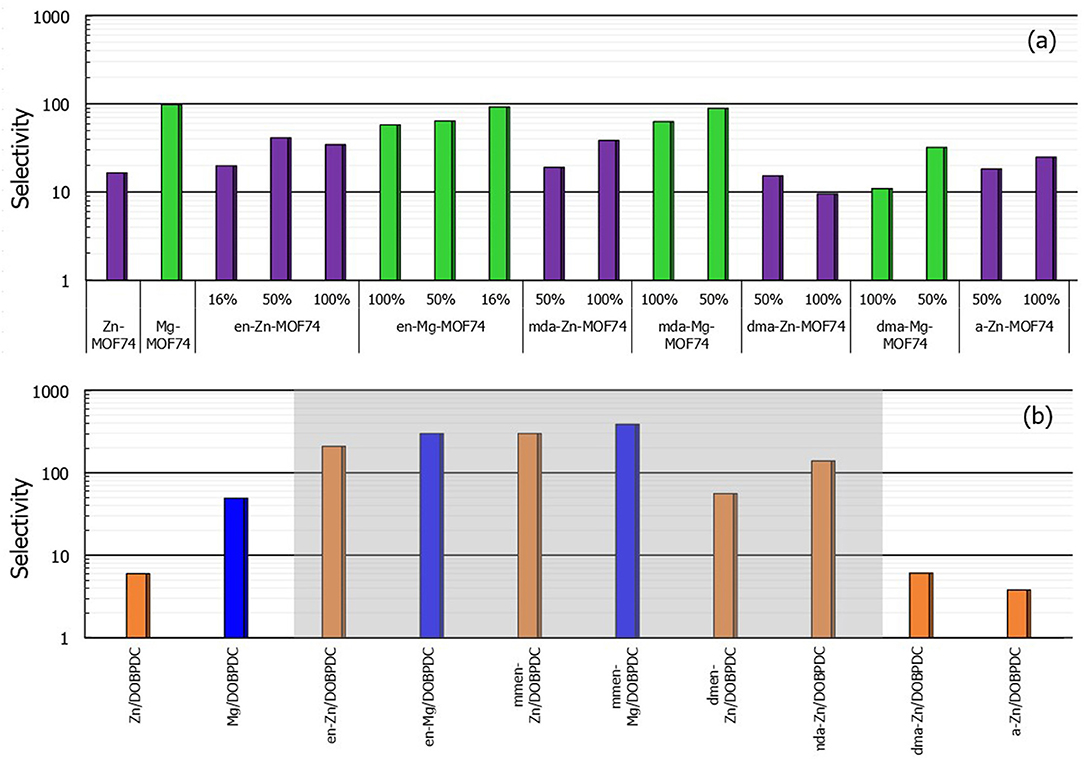
Figure 5. (a,b) Calculated selectivity values of binary 15% CO2 / 85% N2 mixtures (T = 313 K and Ptotal = 100 kPa) for M-MOF-74 and M/DOBPDC materials studied in this work (Color code: Mg-MOF-74 based structures in green, Zn-MOF-74 in violet, Mg/DOBPDC in blue and Zn/DOBPDC in orange). Percentages correspond to the degree of functionalization.
In a CO2 adsorption process, the adsorbent material should be regenerated after each CO2 uptake cycle. The most common method to regenerate the adsorbent is to recover CO2 by heating the adsorbent bed until full desorption is attained. Therefore, we calculated the working capacity related to temperature swing adsorption (TSA) cycles. The feed condition was taken as the binary 15% CO2/ 85% N2 mixture, and regeneration was set at 373 K. Since the amount adsorbed in bare materials gradually reduces as the temperature increases, these materials require a high desorption temperature to achieve large working capacities for separation. In the functionalized ones, with the explicit chemisorption analyzed in this study, it is observed that as the temperature increases the position of the isotherm step shifts to higher pressures (McDonald et al., 2015), allowing large working capacities and almost complete regenerability under specific conditions. However, using an adsorbent that binds CO2 too strongly would increase the temperature (energy) required to break the framework–CO2 interactions, hence making it less attractive from the regeneration point of view. Therefore, the adsorption step of these materials can be tuned by selecting the most promising amine balancing the conditions for the desired performance: the adsorption strength should be lower for the material to be readily regenerated, but not too low to strongly affect the selectivity and working capacity (Sumida et al., 2011, Bahamon et al., 2018).
Figure 6 shows a relationship between carbon dioxide regenerability (percentage) and the working capacity achieved, where the circle size represents the selectivity. The chart can be divided into four sections: MOFs grafted with primary diamines constitute the right bottom section (i.e., high adsorption at low CO2 pressures due to the strong interaction between the CO2 and the amine ends). Conversely, the left bottom section is mostly composed of the amine-functionalized M-MOF-74 structures, with a low cyclic working capacity due to reduced interactions between the heterodiamine and CO2. The right top section (highlighted in gray) contains mmen-Mg/DOBPDC, in which the methyl groups help in the facile desorption by weakening the Mg–carbamate interaction. Suitable adsorbents for a continuous CO2 capture TSA process should achieve the conditions of the highlighted section. In addition, mda-Zn/DOBPDC shows performance in the optimum range for post-combustion CO2 capture from flue gas, enabling a working capacity of 2.9 mmol·g−1 with a ΔT = 60°C for regeneration.
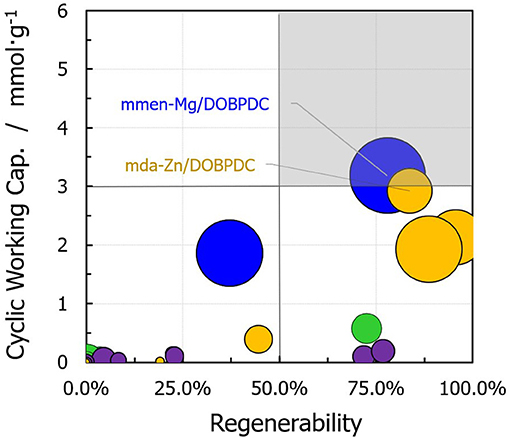
Figure 6. Carbon dioxide working capacity and regenerability values for functionalized structures under TSA conditions (Tregen = 373 K). The sizes of the circles represent the selectivity of the given material for CO2/N2 separation (Color code: same as in Figure 5).
In this study, GCMC molecular simulations were used to investigate the effect of different MOF amino-functionalized materials for carbon dioxide post-combustion capture by adsorption. A screening of synthetized and hypothetical materials, including different degrees of functionalization, was performed to identify key features of CO2 adsorbent materials for flue-gas applications.
The incorporation of amine groups grafted to the open metal sites of the MOFs can enhance the adsorption selectivity for CO2/N2 mixture separation. Results show that the functionalized expanded mmen-Mg/DOBPDC framework exhibits superior performance to their counterparts and the highest selectivities among the studied structures. Moreover, amine functionalization allows exceptionally high CO2 working capacity, and high regenerability, with potential promise for CO2 capture processes. mda-Zn/DOBPDC stands also as a promising material for such endeavor.
This study demonstrates that the CO2 adsorption properties of functionalized MOF materials can be tuned as a function of amine structures attached to the open metal sites, which can also be applied to other functionalized materials. Simulations have shown that the available void space left by the amine and the type of amine functional group plays a crucial role in achieving optimal CO2 capture properties, of great importance for the ad-hoc design of adsorbents using this feature. Further studies will include the performance evaluation of such adsorbent materials under more realistic industrial conditions, including trace impurities in the feed stream, and for Pressure/Vacuum Swing Adsorption processes with techno-economic assessment.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
DB is a research scientist who carried out some of the GCMC simulations work, data analyses, interpretation, and original writing of the manuscript. WA was an undergraduate research assistant who performed some of the GCMC calculations for functionalized MOFs. MK and SB are assistant professors who helped in the selection of the systems, the employed methodology, and the writing of the manuscript. LV is a professor and the academic supervisor of the work, together with DB, she defined the strategy of the work, followed up on the results, interpretation and revised the overall work, and final version of the manuscript. All authors contributed to the article and approved the submitted version.
Financial support for this work has been provided by Khalifa University (projects CIRA 2018-103, RC2-2019-007, and RC2-2018-024). Computational resources from KU-RICH are highly appreciated. DB and LV acknowledge support from Alya Technology & Innovations S.L. for the early development of this work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Alkhatib, I. I. I., Pereira, L. M. C., AlHajaj, A., and Vega, L. F. (2020). Performance of non-aqueous amine hybrid solvents mixtures for CO2 capture: a study using a molecular-based model. J. CO2 Util. 35, 126–144. doi: 10.1016/j.jcou.2019.09.010
Alonso, G., Bahamon, D., Keshavarz, F., Giménez, X., Gamallo, P., and Sayós, R. (2018). Density functional theory-based adsorption isotherms for pure and flue gas mixtures on Mg-MOF-74. Application in CO2 capture swing adsorption processes. J. Phys. Chem. C 122, 3945–3957. doi: 10.1021/acs.jpcc.8b00938
Arstad, B., Fjellvåg, H., Kongshaug, K. O., Swang, O., and Blom, R. (2008). Amine functionalised metal organic frameworks (MOFs) as adsorbents for carbon dioxide. Adsorption 14, 755–762. doi: 10.1007/s10450-008-9137-6
Bae, Y.-S., and Snurr, R. Q. (2011). Development and evaluation of porous materials for carbon dioxide separation and capture. Angew. Chem. Int. Ed. 50, 11586–11596. doi: 10.1002/anie.201101891
Bahamon, D., Abu-Zahra, M. R. M., and Vega, L. F. (2019). Molecular simulations of carbon-based materials for selected CO2 separation and water treatment processes. Fluid Phase Eq. 492, 10–25. doi: 10.1016/j.fluid.2019.03.014
Bahamon, D., Alkhatib, I. I. I., Alkhatib, N., Builes, S., Sinnokrot, M., and Vega, L. F. (2020). A comparative assessment of emerging solvents and adsorbents for mitigating CO2 emissions from the industrial sector by using molecular modeling tools. Front. Energy Res. 8:165. doi: 10.3389/fenrg.2020.00165
Bahamon, D., Díaz-Márquez, A., Gamallo, P., and Vega, L. F. (2018). Energetic evaluation of swing adsorption processes for CO2 capture in selected MOFs and zeolites: effect of impurities. Chem. Eng. J. 342, 458–473. doi: 10.1016/j.cej.2018.02.094
Bahamon, D., and Vega, L. F. (2019). Molecular simulations of phenol and ibuprofen removal from water using multilayered graphene oxide membranes. Mol. Phys. 117, 3703–3714. doi: 10.1080/00268976.2019.1662129
Bernini, M. C., Garcia-Blanco, A. A., Villarroel-Rocha, J., Fairen-Jimenez, D., Sapag, K., Ramirez-Pastor, A. J., et al. (2015). Tuning the target composition of amine-grafted CPO-27-Mg for capture of CO2 under post-combustion and air filtering conditions: a combined experimental and computational study. Dalton Trans. 44, 18970–18982. doi: 10.1039/C5DT03137K
Bollini, P., Didas, S. A., and Jones, C. W. (2011). Amine-oxide hybrid materials for acid gas separations. J Mater. Chem. 21:15100. doi: 10.1039/c1jm12522b
Britt, D., Tranchemontagne, D., and Yaghi, O. M. (2008). Metal-organic frameworks with high capacity and selectivity for harmful gases. Proc. Natl. Acad. Sci. U.S.A. 105, 11623–11627. doi: 10.1073/pnas.0804900105
Bui, M., Adjiman, C. S., Bardow, A., Anthony, E. J., Boston, A., Brown, S., et al. (2018). Carbon capture and storage (CCS): the way forward. Energy Environ. Sci. 11, 1062–1176. doi: 10.1039/C7EE02342A
Builes, S., Lopez-Aranguren, P., Fraile, J., and Vega LF Domingo, C. (2015). Analysis of CO2 adsorption in amine-functionalized porous silicas by molecular simulations. Energy Fuels 29, 3855–3862. doi: 10.1021/acs.energyfuels.5b00781
Builes, S., and Vega, L. F. (2012). Understanding CO2 capture in amine-functionalized MCM-41 by molecular simulation. J. Phys. Chem. C 116, 3017–3024. doi: 10.1021/jp210494f
Builes, S., and Vega, L. F. (2013). Effect of immobilized amines on the sorption properties of solid materials: impregnation versus grafting. Langmuir 29, 199–206. doi: 10.1021/la3038507
Caskey, S. R., Wong-Foy, A. G., and Matzger, A. J. (2008). Dramatic tuning of carbon dioxide uptake via metal substitution in a coordination polymer with cylindrical pores. J. Am. Chem. Soc. 130, 10870–10871. doi: 10.1021/ja8036096
Chen, Z., Deng, S., Wei, H., Wang, B., Huang, J., and Yu, G. (2013). Activated carbons and amine-modified materials for carbon dioxide capture—a review. Front. Environ. Sci. Eng. 7, 326–340. doi: 10.1007/s11783-013-0510-7
Choi, S., Drese, J. H., and Jones, C. W. (2009). Adsorbent materials for carbon dioxide capture from large anthropogenic point sources. ChemSusChem 2, 796–854. doi: 10.1002/cssc.200900036
Choi, S., Watanabe, T., Bae, T.-H., Sholl, D. S., and Jones, C. W. (2012). Modification of the Mg/DOBDC MOF with amines to enhance CO2 adsorption from ultradilute gases. J. Phys. Chem. Lett. 3, 1136–1141. doi: 10.1021/jz300328j
D'Alessandro, D. M., Smit, B., and Long, J. R. (2010). Carbon dioxide capture: prospects for new materials. Angew. Chem. Int. Ed. 49, 6058–6082. doi: 10.1002/anie.201000431
Das, A., Southon, P. D., Zhao, M., Kepert, C. J., Harris, A. T., and D'Alessandro, D. M. (2012). Carbon dioxide adsorption by physisorption and chemisorption interactions in piperazine-grafted Ni2(dobdc) (dobdc = 1,4-dioxido-2,5-benzenedicarboxylate). Dalton Trans. 41:11739. doi: 10.1039/c2dt31112g
Demessence, A., D'Alessandro, D. M., Foo, M. L., and Long, J. R. (2009). Strong CO2 binding in a water-stable, triazolate-bridged metal–organic framework functionalized with ethylenediamine. J. Am. Chem. Soc. 131, 8784–8786. doi: 10.1021/ja903411w
Deng, H., Grunder, S., Cordova, K. E., Valente, C., Furukawa, H., Hmadeh, M., et al. (2012). Large-pore apertures in a series of metal-organic frameworks. Science 336, 1018–1023. doi: 10.1126/science.1220131
Didas, S. A., Kulkarni, A. R., Sholl, D. S., and Jones, C. W. (2012). Role of amine structure on carbon dioxide adsorption from ultradilute gas streams such as ambient air. ChemSusChem 5, 2058–2064. doi: 10.1002/cssc.201200196
Dietzel, P. D. C., Besikiotis, V., and Blom, R. (2009). Application of metal–organic frameworks with coordinatively unsaturated metal sites in storage and separation of methane and carbon dioxide. J. Mater. Chem. 19:7362. doi: 10.1039/b911242a
Dzubak, A. L., Lin, L.-C., Kim, J., Swisher, J. A., Poloni, R., Maximoff, S. N., et al. (2012). Ab initio carbon capture in open-site metal–organic frameworks. Nat. Chem. 4, 810–816. doi: 10.1038/nchem.1432
Eddaoudi, M., Kim, J., Rosi, N., Vodak, D., Wachter, J., O'Keeffe, M., et al. (2002). Systematic design of pore size and functionality in isoreticular mofs and their application in methane storage. Science 295, 469–472. doi: 10.1126/science.1067208
Forse, A. C., Milner, P. J., Lee, J.-H., Redfearn, H. N., Oktawiec, J., Siegelman, R. L., et al. (2018). Elucidating CO2 chemisorption in diamine-appended metal–organic frameworks. J. Am. Chem. Soc. 140, 18016–18031. doi: 10.1021/jacs.8b10203
Fracaroli, A. M., Siman, P., Nagib, D. A., Suzuki, M., Furukawa, H., Toste, F. D., et al. (2016). Seven post-synthetic covalent reactions in tandem leading to enzyme-like complexity within metal–organic framework crystals. J. Am. Chem. Soc. 138, 8352–8355. doi: 10.1021/jacs.6b04204
Furukawa, H., Cordova, K. E., O'Keeffe, M., and Yaghi, O. M. (2013). The chemistry and applications of metal-organic frameworks. Science 341, 1230444–1230444. doi: 10.1126/science.1230444
Hedin, N., Chen, L., and Laaksonen, A. (2010). Sorbents for CO2 capture from flue gas—aspects from materials and theoretical chemistry. Nanoscale 2, 1819–1841. doi: 10.1039/c0nr00042f
Huang, X., Lu, J., Wang, W., Wei, X., and Ding, J. (2016). Experimental and computational investigation of CO2 capture on amine grafted metal-organic framework NH2-MIL-101. App. Surf. Sci. 371, 307–313. doi: 10.1016/j.apsusc.2016.02.154
Huck, J. M., Lin, L., Berger, A. H., Shahrak, M. N., Martin, R. L., Bhown, A. S., et al. (2014). Evaluating different classes of porous materials for carbon capture. Energy Environ. Sci. 7, 4132–4146. doi: 10.1039/C4EE02636E
IPCC (2014). Climate change 2014: synthesis report. contribution of working groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team, R.K. Pachauri, L.A. Meyer (eds.)]. Available online at: https://www.ipcc.ch/site/assets/uploads/2018/05/SYRAR5FINALfullwcover.pdf (accessed May 2020). doi: 10.1017/CBO9781107415416
Jo, H., Lee, W. R., Kim, N. W., Jung, H., Lim, K. S., Kim, J. E., et al. (2016). Fine-tuning of the carbon dioxide capture capability of diamine-grafted metal-organic framework adsorbents through amine functionalization. ChemSusChem 10, 541–550. doi: 10.1002/cssc.201601203
Kang, M., Kang, D. W., and Hong, C. S. (2019). Post-synthetic diamine-functionalization of MOF-74 type frameworks for effective carbon dioxide separation. Dalton Trans. 48, 2263–2270. doi: 10.1039/C8DT04339F
Keskin, S., van Heest, T. M., and Sholl, D. S. (2010). Can metal-organic framework materials play a useful role in large-scale carbon dioxide separations? ChemSusChem 3, 879–891. doi: 10.1002/cssc.201000114
Lee, J., Farha, O. K., Roberts, J., Scheidt, K. A., Nguyen, S. T., and Hupp, J. T. (2009). Metal–organic framework materials as catalysts. Chem. Soc. Rev. 38, 1450–1459. doi: 10.1039/b807080f
Lee, J.-H., Siegelman, R. L., Maserati, L., Rangel, T., Helms, B. A., Long, J. R., et al. (2018). Enhancement of CO2 binding and mechanical properties upon diamine functionalization of M2(dobpdc) metal–organic frameworks. Chem. Sci. 9, 5197–5206. doi: 10.1039/C7SC05217K
Lee, W. R., Hwang, S. Y., Ryu, D. W., Lim, K. S., Han, S. S., Moon, D., et al. (2014). Diamine-functionalized metal-organic framework: exceptionally high CO2 capacities from ambient air and flue gas, ultrafast CO2 uptake rate, and adsorption mechanism. Energy Environ. Sci. 7, 744–751. doi: 10.1039/C3EE42328J
Liao, P.-Q., Chen, X.-W., Liu, S.-Y., Li, X.-Y., Xu, Y.-T., Tang, M., et al. (2016). Putting an ultrahigh concentration of amine groups into a metal–organic framework for CO2 capture at low pressures. Chem. Sci. 7, 6528–6533. doi: 10.1039/C6SC00836D
Lin, Y., Kong, C., and Chen, L. (2016). Amine-functionalized metal–organic frameworks: structure, synthesis and applications. RSC Adv. 6, 32598–32614. doi: 10.1039/C6RA01536K
Lin, Y., Yan, Q., Kong, C., and Chen, L. (2013). Polyethyleneimine incorporated metal-organic frameworks adsorbent for highly selective CO2 capture. Sci. Rep. 3:1859. doi: 10.1038/srep01859
Mason, J. A., McDonald, T. M., Bae, T.-H., Bachman, J. E., Sumida, K., Dutton, J. J., et al. (2015). Application of a high-throughput analyzer in evaluating solid adsorbents for post-combustion carbon capture via multicomponent adsorption of CO2, N2, and H2O. J. Am. Chem. Soc. 137, 4787–4803. doi: 10.1021/jacs.5b00838
Mason, J. A., Sumida, K., Herm, Z. R., Krishna, R., and Long, J. R. (2011). Evaluating metal–organic frameworks for post-combustion carbon dioxide capture via temperature swing adsorption. Energy Environ. Sci. 4:3030. doi: 10.1039/c1ee01720a
McDonald, T. M., Lee, W. R., Mason, J. A., Wiers, B. M., Hong, C. S., and Long, J. R. (2012). Capture of carbon dioxide from air and flue gas in the alkylamine-appended metal–organic framework mmen-Mg2(dobpdc). J. Am. Chem. Soc. 134, 7056–7065. doi: 10.1021/ja300034j
McDonald, T. M., Mason, J. A., Kong, X., Bloch, E. D., Gygi, D., Dani, A., et al. (2015). Cooperative insertion of CO2 in diamine-appended metal-organic frameworks. Nature 519, 303–311. doi: 10.1038/nature14327
McKinlay, A. C., Morris, R. E., Horcajada, P., Férey, G., Gref, R., Couvreur, P., et al. (2010). BioMOFs: metal-organic frameworks for biological and medical applications. Angew. Chem. Int. Ed. 49, 6260–6266. doi: 10.1002/anie.201000048
Mercado, R., Vlaisavljevich, B., Lin, L.-C., Lee, K., Lee, Y., Mason, J. A., et al. (2016). Force field development from periodic density functional theory calculations for gas separation applications using metal–organic frameworks. J. Phys. Chem. C 120, 12590–12604. doi: 10.1021/acs.jpcc.6b03393
Milner, P. J., Martell, J. D., Siegelman, R. L., Gygi, D., Weston, S. C., and Long, J. R. (2018). Overcoming doule-step CO2 adsorption and minimizing water co-adsorption in bulky diamine-appended variants of Mg2(dobpdc). Chem. Sci. 9, 160–174. doi: 10.1039/C7SC04266C
Milner, P. J., Siegelman, R. L., Forse, A. C., Gonzalez, M. I., Runčevski, T., Martell, J. D., et al. (2017). A diaminopropane-appended metal–organic framework enabling efficient CO2 capture from coal flue gas via a mixed adsorption mechanism. J. Am. Chem. Soc. 139, 13541–13553. doi: 10.1021/jacs.7b07612
Mukherjee, A., Okolie, J. A., Abdelrasoul, A., Niu, C., and Dalai, A. K. (2019). Review of post-combustion carbon dioxide capture technologies using activated carbon. J. Environ. Sci. 83, 46–63. doi: 10.1016/j.jes.2019.03.014
Pentyala, V., Davydovskaya, P., Ade, M., Pohle, R., and Urban, G. (2016). Carbon dioxide gas detection by open metal site metal organic frameworks and surface functionalized metal organic frameworks. Sensors Actuat. B Chem. 225, 363–368. doi: 10.1016/j.snb.2015.11.071
Planas, N., Dzubak, A. L., Poloni, R., Lin, L.-C., McManus, A., McDonald, T. M., et al. (2013). The mechanism of carbon dioxide adsorption in an alkylamine-functionalized metal–organic framework. J. Am. Chem. Soc. 135, 7402–7405. doi: 10.1021/ja4004766
Plimpton, S. (1995). Fast parallel algorithms for short-range molecular dynamics. J. Comp. Phys. 117, 1–19. doi: 10.1006/jcph.1995.1039
Potoff, J. J., and Siepmann, J. I. (2001). Vapor–liquid equilibria of mixtures containing alkanes, carbon dioxide, and nitrogen. AICHE J. 47, 1676–1682. doi: 10.1002/aic.690470719
Prats, H., Bahamon, D., Giménez, X., Gamallo, P., and Sayós, R. (2017). Computational simulation study of the influence of faujasite Si/Al ratio on CO2 capture by temperature swing adsorption. J. CO2 til. 21, 261–269. doi: 10.1016/j.jcou.2017.07.013
Qiao, Z., Wang, N., Jiang, J., and Zhou, J. (2016). Design of amine-functionalized metal–organic frameworks for CO2 separation: the more amine, the better? Chem. Commun. 52, 974–977. doi: 10.1039/C5CC07171B
Rochelle, G. T. (2009). Amine scrubbing for CO2 capture. Science 325, 1652–1654. doi: 10.1126/science.1176731
Rowsell, J. L. C., and Yaghi, O. M. (2006). Effects of functionalization, catenation, and variation of the metal oxide and organic linking units on the low-pressure hydrogen adsorption properties of metal–organic frameworks. J. Am. Chem. Soc. 128, 1304–1315. doi: 10.1021/ja056639q
Sayari, A., Belmabkhout, Y., and Serna-Guerrero, R. (2011). Flue gas treatment via CO2 adsorption. Chem. Eng. J. 171, 760–774. doi: 10.1016/j.cej.2011.02.007
Shah, M., McCarthy, M. C., Sachdeva, S., Lee, A. K., and Jeong, H. K. (2012). Current status of metal–organic framework membranes for gas separations: promises and challenges. Ind. Eng. Chem. Res. 51, 2179–2199. doi: 10.1021/ie202038m
Siegelman, R. L., McDonald, T. M., Gonzalez, M. I., Martell, J. D., Milner, P. J., Mason, J. A., et al. (2017). Controlling cooperative CO2 adsorption in diamine-appended Mg2(dobpdc) metal–organic frameworks. J.Am. Chem. Soc. 139, 10526–10538. doi: 10.1021/jacs.7b05858
Siegelman, R. L., Milner, P. J., Forse, A. C., Lee, J.-H., Colwell, K. A., Neaton, J. B., et al. (2019). Water enables efficient CO2 capture from natural gas flue emissions in an oxidation-resistant diamine-appended metal– organic framework. J. Am. Chem. Soc. 141, 13171–13186. doi: 10.1021/jacs.9b05567
Spigarelli, B. P., and Kawatra, S. K. (2013). Opportunities and challenges in carbon dioxide capture. J. CO2 Util. 1, 69–87. doi: 10.1016/j.jcou.2013.03.002
Sumida, K., Rogow, D. L., Mason, J. A., McDonald, T. M., Bloch, E. D., Herm, Z. R., et al. (2011). Carbon dioxide capture in metal–organic frameworks. Chem. Rev. 112, 724–781. doi: 10.1021/cr2003272
Vaidhyanathan, R., Iremonger, S. S., Shimizu, G. K. H., Boyd, P. G., Alavi, S., and Woo, T. K. (2010). Direct observation and quantification of CO2 binding within an amine-functionalized nanoporous solid. Science 330, 650–653. doi: 10.1126/science.1194237
Valenzano, L., Civalleri, B., Chavan, S., Palomino, G. T., Areán, C. O., and Bordiga, S. (2010). Computational and experimental studies on the adsorption of CO, N2, and CO2 on Mg-MOF-74. J. Phys. Chem. C 114, 11185–11191. doi: 10.1021/jp102574f
Vega, L. F., and Bahamon, D. (2016). Comparative study of MOFs and zeolites for CO2 capture and separation at process conditions. Soc. Pet. Eng. J. doi: 10.2118/183480-MS
Vlaisavljevich, B., Odoh, S. O., Schnell, S. K., Dzubak, A. L., Lee, K., Planas, N., et al. (2015). CO2 induced phase transitions in diamine-appended metal–organic frameworks. Chem. Sci. 6, 5177–5185. doi: 10.1039/C5SC01828E
Wang, Z., Fang, M., Pan, Y., Yan, S., and Luo, Z. (2013). Amine-based absorbents selection for CO2 membrane vacuum regeneration technology by combined absorption–desorption analysis. Chem. Eng. Sci. 93, 238–249. doi: 10.1016/j.ces.2013.01.057
Webley, P. A. (2014). Adsorption technology for CO2 separation and capture: a perspective. Adsorption 20, 225–231. doi: 10.1007/s10450-014-9603-2
Wick, C. D., Stubbs, J. M., Rai, N., and Siepmann, J. I. (2005). Transferable potentials for phase equilibria. 7. Primary, secondary, and tertiary amines, nitroalkanes and nitrobenzene, nitriles, amides, pyridine, and pyrimidine. J. Phys. Chem. B 109, 18974–18982. doi: 10.1021/jp0504827
Yazaydin, A. O., Snurr, R. Q., Park, T. H., Koh, K., Liu, J., LeVan, M. D., et al. (2009). Screening of metal–organic frameworks for carbon dioxide capture from flue gas using a combined experimental and modeling approach. J. Am. Chem. Soc. 131, 18198–18199. doi: 10.1021/ja9057234
Yeon, J. S., Lee, W. R., Kim, N. W., Jo, H., Lee, H., Song, J. H., et al. (2015). Homodiamine-functionalized metal–organic frameworks with a MOF-74-type extended structure for superior selectivity of CO2 over N2. J. Mater. Chem. A 3, 19177–19185. doi: 10.1039/C5TA02357B
Yu, J., Xie, L.-H., Li, J.-R., Ma, Y., Seminario, J. M., and Balbuena, P. B. (2017). CO2 capture and separations using MOFs: computational and experimental studies. Chem. Rev. 117, 9674–9754. doi: 10.1021/acs.chemrev.6b00626
Zhang, J., Wu, H., Emge, T. J., and Li, J. (2010). A flexible MMOF exhibiting high selectivity for CO2 over N2, CH4 and other small gases. Chem. Commun. 46:9152. doi: 10.1039/c0cc02942d
Keywords: CO2 capture, metal-organic frameworks, MOF-74, amine, functionalization, Monte Carlo simulation, chemisorption
Citation: Bahamon D, Anlu W, Builes S, Khaleel M and Vega LF (2021) Effect of Amine Functionalization of MOF Adsorbents for Enhanced CO2 Capture and Separation: A Molecular Simulation Study. Front. Chem. 8:574622. doi: 10.3389/fchem.2020.574622
Received: 20 June 2020; Accepted: 04 December 2020;
Published: 11 January 2021.
Edited by:
Pu-Xian Gao, University of Connecticut, United StatesReviewed by:
Concepcion Domingo, Consejo Superior de Investigaciones Científicas (CSIC), SpainCopyright © 2021 Bahamon, Anlu, Builes, Khaleel and Vega. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lourdes F. Vega, bG91cmRlcy52ZWdhQGt1LmFjLmFl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.