- 1Department of Chemical and Biomolecular Engineering, Sogang University, Seoul, South Korea
- 2School of Biomedical Convergence Engineering, Pusan National University, Yangsan, South Korea
- 3Department of Chemical Engineering, Kwangwoon University, Seoul, South Korea
- 4Uniance Gene Inc., Seoul, South Korea
Numerous efforts have been made to develop efficient biosensors for detecting analytes in the human body. However, biosensors are often developed on rigid materials, which limits their application on skin, organs, and other tissues in the human body where good flexibility is required. Developing flexible materials for biosensors that can be used on soft and irregularly shaped surfaces would significantly expand the clinical application of biosensors. In this review, we will provide a selective overview of recently developed flexible electronic devices and their applications for monitoring in vivo metabolite and electrophysiology signals. The article provides guidelines for the development of an in vivo signal monitoring system and emphasizes research from various disciplines for the further development of flexible electronics that can be used in more biomedical applications in the future.
Introduction
A biosensor is a platform for detecting biological components. Applications may range from the medical field to environmental science, and both continuous and intermittent monitoring may be required (Brindha et al., 2018; Khansili et al., 2018; Yang and Gao, 2019). For a decade, researchers have focused on increasing the sensitivity and selectivity of biosensors to improve sensing performance (Lee et al., 2005; Jeong et al., 2018; Park et al., 2018). However, due to the soft and irregularly shaped structure of biological conditions, conventional biosensors developed on rigid materials are not suitable for monitoring signals from surfaces such as skin, organs, and other tissues in the human body (Kim et al., 2019; Salim and Lim, 2019). Therefore, a novel design for biosensors is required to monitor biological components, such as metabolites and electrophysiology signals, more accurately and precisely (Huang and Zhu, 2019; Xu et al., 2019; Yao et al., 2020). A suitable substrate material could conform to the shape of the biological structures without causing adverse effects such as tissue damage and inflammation (Park et al., 2014; Song et al., 2019).
To meet this demand, more research has focused on flexible electronics to expand the application of biosensors in clinical fields, particularly in the healthcare industry (Liu et al., 2018; Chung et al., 2019). Biocompatible and mechanically flexible substrates could be used on delicate, curvilinear interfaces such as the human body (e.g., wearable biosensors) or in vivo for monitoring biological components in humans (Boutry et al., 2019; Huang et al., 2019). For example, flexible, synthetic polymers, such as polyethylene terephthalate (PET), polyimide (PI), poly (dimethylsiloxane) (PDMS), and polyethersulfone, and natural polymers including cellulose and silk fibroin have been evaluated as base components for flexible electronic devices for biomedical applications, specifically for implantation (Yao et al., 2019; Park et al., 2020).
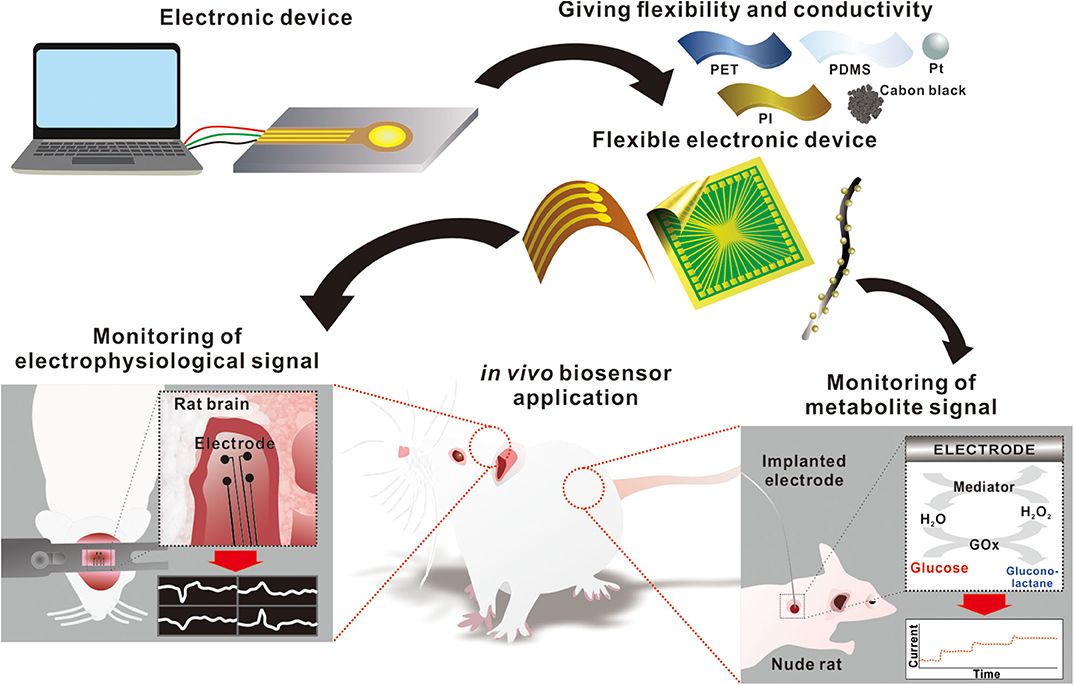
Although many reviews discuss flexible electronics for use in wearable biosensors, the recent research on the development of an in vivo signal monitoring device warrants a thorough review at this time. This mini-review will focus on some of the current state-of-the-art developments on flexible electronic devices that can be used to monitor in vivo electrophysiology and metabolite signals. This article provides a broad overview of the potential for in vivo signal monitoring devices for human healthcare, which may be useful for researchers across various disciplines (Figure 1).
In vivo Monitoring of Electrophysiological Signals
Many neuroscientists have studied the structural and functional relationships between the brain and brain potential mapping to understand and treat neurological diseases (Woo et al., 2017; Lake et al., 2018; Murphy et al., 2019). Although, there are many available imaging techniques for brain mapping, such as magnetic resonance imaging (MRI), positron emission tomography (PET), computed tomography (CT), and electroencephalography (EEG), electrical activity in the brain is difficult to monitor using current techniques due to slow response or low resolution (Damborská et al., 2019; Glaab et al., 2019; Hou et al., 2019; Huhn et al., 2019; Klauser et al., 2019). Numerous electronics, such as cortical probes and electrodes, which use penetrating probes or surface electrodes, have been developed with various materials to reliably acquire an accurate electrical signal. However, integrating conventional electrodes into the neural system of living animals is complicated by the differences in the mechanical properties of biological surfaces compared to glass or metal (Ganesana et al., 2019; Ji et al., 2019; Liu et al., 2019; Peng et al., 2019). As a result, the development of electrophysiological electrodes with appropriate mechanical (flexible and soft) properties, biocompatibility, and high electrical/electrochemical performance has become a promising field of research for monitoring and modulating neural interfaces with soft tissues, especially for implantation (Hong and Lieber, 2019; Hossain et al., 2019; Jayant et al., 2019; Das et al., 2020).
In an effort to develop biosensors capable of both in vitro and potentially in vivo sensing, Pothof et al. fabricated biocompatible stereo-electroencephalography (SEEG) probes (minimal feature size of 5 μm) on a flexible polyimide (PI) foil with platinum electrodes and leads (Pothof et al., 2016). The stability and functionality of the device were tested with saline solution. The PI probe was rolled into a cylindrical shape with a diameter of 0.8 mm, and probes with 32 and 64 electrode sites were implanted 22 mm deep into the posterior parietal cortex of the brain of one monkey (Macaca mulatta), which was trained to perform different motor tasks. Local field potentials (LFP) and multi-unit activity (MUA) were measured 1 h after implantation. Moreover, stable single-unit activity (SUA) was achieved up to 26 d after implantation. Similarly, Kim et al. fabricated a PI-based flexible neural probe for precise site stimulation and recording in the deep brain (Kim et al., 2018). The probe was composed of five electrodes, including ground, stimulation, and three recording electrodes at the inserted tip. Because of its soft and flexible mechanical properties, this probe was expected to be foldable, enabling easy insertion into the deep brain tissue via temporarily used tungsten guide sticks. Due to the flexibility and biocompatibility of the PI and the small size (cross-sectional area) of the electrode, the probe did not cause significant damage to the neural tissue or show evidence of serious immune reactions (high density of macrophage or microglia) for 30 d. The additional ground electrode around the stimulation electrode reduced the leakage power by up to 80% (in vitro) and 40% (in vivo) and allowed selective site stimulation in the brain. The performance of the probe was tested in animal experiments using rats. The probe showed stable impedance and cyclic voltammogram (CV) response for 30 d under continuous electrical stimulation, and neural spike signals from the subthalamic nucleus (STh) in the 7 mm deep brain were successfully recorded after implantation.
On the other hand, Ji et al. developed a fabrication method of enhanced micro-scale wrinkles on a hyperelastic substrate (Polydimethylsiloxane, PDMS, and Ecoflex) based on oil-pretreating, not only to provide comparable flexibility on the curved cortical surface, but also to improve sensing capability due to the increased surface area (Ji et al., 2019). The wrinkled gold microelectrodes showed promising electrochemical properties with a relatively larger effective surface area (33.5% and 41.6% larger) compared to the flat microelectrodes, and no crack or delamination was observed even after electroplating poly(3,4-ethylene dioxythiophene) polystyrene sulfonate (PEDOT:PSS) and platinum black on the wrinkled microelectrodes. The adhesion and stability of the modified electrodes were tested with 2,500 repetitions of cyclic voltammetry scanning. Neural recording ability was further verified by in vivo electrocorticogram (ECoG) signal measurements combined with optogenetics in mice.
Expanding from the brain to other organs, Xue et al. fabricated a PI-based 2D cuff electrode to wrap around the vagus nerve (Xue et al., 2018). Because this nerve provides parasympathetic innervation to human organs, stimulation of the vagus nerve has emerged as a new strategy to treat and modulate cardiac function. Utilizing the bendable property of the contact tips of the device, the electrode sites, which are located on the contact tips, can touch the nerve and selectively record and stimulate the vagus nerve. Among the different kinds of materials (Au, Pt, and Pt-black) tested for electrode and electrochemical measurement (electrochemical impedance spectroscopy (EIS) and CV), the Pt-black exhibited ~30 times larger charge delivery capacity (CDC). The in vitro measurements of EIS and CV for Au, Pt, and Pt-black, were 405 kΩ, 41 kΩ, and 10.5 kΩ @1 kHz and 0.81 mC/cm2, 4.26 mC/cm2, and 25.5 mC/cm2, respectively (n = 3). During the cell viability test over 24 h, no obvious cell damage was observed. For the in vivo experiment, the device was implanted into the right-sided vagus nerve of rats. A biphasic current was used to stimulate the vagus nerve with a frequency of 10 Hz, pulse duration of 300 μs, and varying current stimulus. The result showed that the successful stimulation of the vagus nerve reduced the heartbeat rate by up to 36%, and the heartbeat resumed a regular frequency when stimulation was removed. As these studies show, recent advances in flexible electronics have expanded the in vivo application for monitoring electrophysiological signals using devices with suitable mechanical and biocompatible properties that do not cause significant damage to the tissues of interest.
In vivo Monitoring of Metabolite Signals
Glucose is one of the essential metabolites in the human body which functions as fuel for cells (Pellerin and Magistretti, 1994; Simpson et al., 2007; Lee et al., 2012; López-Gambero et al., 2019), and abnormal glucose levels can lead to a range of adverse conditions and diseases including diabetes (Santiago et al., 2007; Chen et al., 2018). Therefore, continuous blood glucose monitoring devices are widely utilized in the diagnosis and treatment of diabetes mellitus to provide information about blood glucose levels (Klonoff, 2005; Bruen et al., 2017; Tripathy and Kim, 2018). However, biofouling, inflammation, fibrosis, and extracellular release of lysosomal contents are crucial barriers for the design of reliable, implantable glucose biosensors. For example, electrochemical glucose biosensors are known to lose their sensitivity as soon as they are implanted. The immobilized enzyme (glucose oxidase, GOD) activity on the surface of the electrode is a fundamental limiting factor for in vivo conditions due to the accumulation of H2O2 in the blood (Pickup et al., 1989). In addition, biofouling such as the eventual adhesion and spreading of macrophages on the sensor surface significantly affects the sensitivity of implanted sensors (Yu et al., 2008). To improve the stability and biocompatibility of implanted glucose sensors, Burugapalli et al. fabricated a biomimetic membrane of PU and gelatin (GE) on the electrode using the electrospinning method to act as a mass transport limiting membrane (Burugapalli et al., 2018). Fibro-porous structured membranes with optimized fiber diameters, pore sizes, and permeability were generated using three different polymer solution concentrations (wt %) (8PU, 12PU, and 6PU10GE). The developed electrode with fibro-porous structured membranes was implanted in rats for an in vivo functional efficacy test. While the fibro-porous PU-GE structure with an average pore size of approximately 1.5 mm allowed host cell infiltration, PU structure with a similar pore size (~1 mm) was not permeable to host cells at all. Since the fibro-porous PU membrane successfully acted as a mass transport-limiting membrane, 8PU membranes showed the highest sensitivity for both in vitro and in vivo conditions. However, the biomimetic PU-GE structure most effectively prevented fibrous capsule formation on the sensor surface, producing the slowest decrement of sensing ability in in vivo conditions. Though the advantage of the PU-GE coating was diminished after 9 weeks due to the resorption of GE and replacement with collagen, the results demonstrated that the formation of the mass-transport limiting membrane such as a fibro-porous structure could improve the lifespan of implanted in vivo glucose biosensors.
Another approach for increasing the lifespan of in vivo glucose biosensors includes employing nanomaterials to maintain enzyme activity. Fang et al. utilized Cu nanoflowers to construct a minimally invasive glucose microelectrode (Fang et al., 2018). Electrodeposited flower-shaped Cu nanostructures on the Pt microelectrode provided a large surface area and a high number of active sites to promote electrocatalysis of glucose on the surface of the electrode. The subsequent Nafion layer improved the anti-interference performance of the sensor devices. Glucose oxidase was immobilized using covalent bonding with glutaraldehyde and bovine serum albumin (BSA). In addition, the biocompatible polyurethane (PU) layer was used as an outer membrane to improve the stability and biocompatibility of the implanted glucose sensors. The outer membrane worked as a limiting diffusion layer to reduce enzyme leaching and also limit the diffusion of glucose relative to oxygen. As a result, in vitro electrochemical performance with good sensitivity and a large linear response range was obtained by CV and chronoamperometry (CA). Furthermore, a real-time response to the variation of blood glucose concentration was successfully observed in the in vivo implantable experiments using anesthetized rats.
Similarly, Pu et al. used a 3D nanostructure consisting of graphene and platinum nanoparticles on a cylindrical, flexible, substrate-based electrode (polyetheretherketone, PEEK, diameter: 1 mm, length of the fabricated sensor: 5 mm, stiffness: 62.5 kN m−1) to enhance the sensitivity of an in vivo glucose sensor (Pu et al., 2018). The cylindrical PEEK substrate was pre-modified by (3-aminopropyl) trimethoxysilane (ATPMS) and (3-mercaptopropyl) trimethoxysilane (MPTMS) to achieve a hydrophilic and metal adhesive surface to construct an electrode. After rotated inkjet printing and a synchronous heating technique, microstructures were directly developed on a curved surface, providing a larger active surface compared to the traditional pin-type implantable glucose monitoring system. The in vitro experimental results demonstrated a sensing ability ranging from 0 to 570 mg/L of glucose, which aligns with the range of physiological glucose levels. After in vitro characterization, an in vivo experiment was conducted in which the sensor was implanted into the subcutaneous tissue of a rat. The results demonstrated the ability of the device to monitor glucose continuously in subcutaneous tissue.
In a different study, Zhang et al. developed a self-powered, implantable, skin-like glucose sensor for real-time detection of in vivo blood glucose levels (Zhang et al., 2018). Based on the piezo-enzymatic-reaction coupling effect of a GOx@ZnO nanowire array, the device converted the mechanical deformation into a piezoelectric impulse, which acted as both the biosensing signal and electrical power. The skin-like device was implanted in a mouse, and the blood glucose concentration was successfully measured in real time. Chen et al. also presented a skin-like electronic device for non-invasive, in situ, and highly accurate intravascular blood glucose monitoring (Chen et al., 2017). The ultrathin (~3 mm), nanostructured glucose sensor with high sensitivity (130.4 mA/mM) was developed by integrating multiple layers, including poly(methyl methacrylate) (PMMA), PI, a nanostructured gold thin film, a transducer layer (PB), and a transfer/glucose oxidase (GOx). Additionally, by incorporating electrochemical twin channels (ETC) and reverse iontophoresis, high-density hyaluronic acid (HA) was inserted into the interstitial fluid (ISF) and raised the ISF osmotic pressure to promote intravascular blood glucose transportation from the vessel. The change in glucose concentration in the ISF resulted in reverse iontophoresis at a low-current level to drive intravascular blood glucose to the skin surface. Then, in vivo human clinical trials were conducted with hourly measurements over a 1-day period, and the results showed a good correlation (>0.9) with both ISF and blood glucose levels.
Similarly, Gao et al. used a flexible printed circuit board on a mechanically flexible polyethylene terephthalate (PET) substrate to monitor metabolites and achieved in situ monitoring of multiple analytes from sweat, including glucose, lactate, K+, and Na+ (Gao et al., 2016). Although this experiment was performed on the skin instead of in in vivo conditions, the results indicate the successful monitoring of sweat analytes in real time, providing an increased ability to understand and diagnose health conditions. For example, ex-situ measurements of Na+ levels in sweat substantially increased when the subjects had lost a large amount of water (~2.5% of body weight), which indicates that Na+ levels in sweat are a potential biomarker for monitoring dehydration. Although body fluids including sweat, saliva, and tears are considered potential candidates for glucose monitoring, the density of glucose in those fluids is quite low compared to blood (only ~1-10%). In addition, the accuracy of fluid glucose measurements is significantly affected by several factors, such as water evaporation and other internal components. Considering the complex physiology of the human body, the developed device will have to overcome several limitations before it can be applied to an in vivo system. Furthermore, multiplexed sensing of other metabolite biomarkers would be a key advancement in healthcare monitoring in in vivo conditions as well (Chung et al., 2019; Zhang et al., 2020). Glutamate, which is one of the major excitatory neurotransmitters in the central nervous system, has been investigated through flexible electronics in an in vivo condition as well (Cao et al., 2012; Nguyen et al., 2019). Additionally, lactate, which is the one of energy metabolites, can also be a suitable candidate for metabolite biomarkers in in vivo monitoring (Weltin et al., 2014a,b).
Concluding Remarks and Future Prospects
Due to their unique properties, flexible electronic devices have gained interest as a promising platform for non-invasive, real-time, and continuous monitoring systems in the healthcare field. In this review, we have summarized some of the most advanced developments in flexible electronic devices to monitor in vivo electrophysiology signals and metabolite (e.g., glucose) signals (Table 1). The major advantage of in vivo metabolite signal monitoring, compared to in vitro monitoring methods, is the continuous and real-time monitoring of the abnormal level of a metabolite which could be an indicator of impending disease. For example, as the abnormal level of glucose can lead to diseases including diabetes, the rapid, sensitive, and continuous monitoring of glucose is critical. Although current in vitro glucose monitoring systems are highly sensitive and selective, continuous and real-time monitoring is not currently possible due to fundamental obstacles such as the requirement of sufficient amounts of continuous biological fluids (i.e., blood, or sweat) for reliable monitoring. Furthermore, continuous and real-time monitoring could lead to a personalized care system based on individualized reports such as pharmacokinetic drug effect.
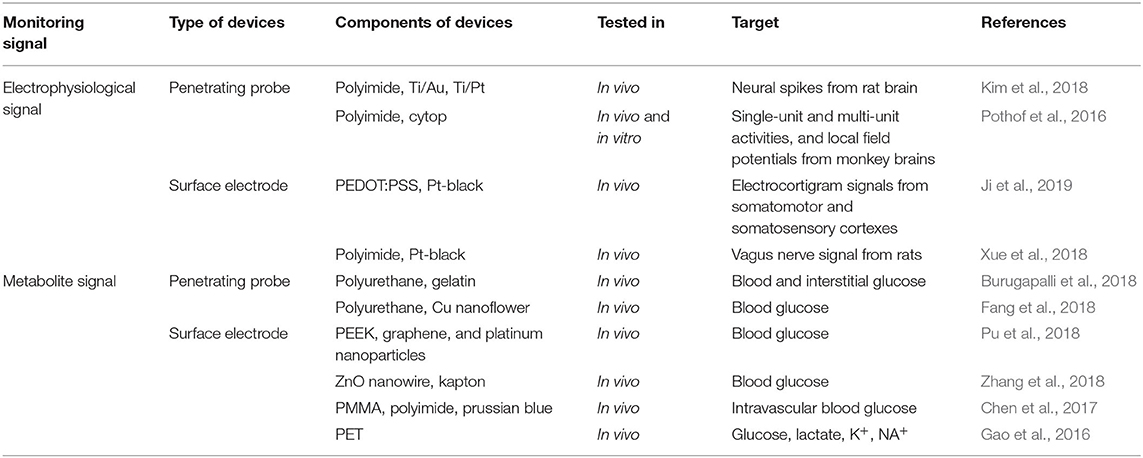
Table 1. Flexible electronic devices for the monitoring of electrophysiological and metabolite signals.
Although various synthetic and natural materials are being utilized to design better flexible biosensors, some major limitations remain for in vivo application. For example, to be effective in clinical applications, overall devices should be designed for minimally invasive implantation and the base material must be flexible and soft to avoid tissue-substrate mechanical mismatch. The mechanical properties of conventional electrodes are not suitable for biological tissues due to their high modulus and stiffness, which can cause damage to the tissues. In addition, the electronic system is supposed to be bendable and flexible to be utilized in an in vivo condition. Materials based on flexible and bending properties with stiffness could sufficiently allow us to monitor the micro-motion related to physiological processes including heartbeat and respiration, which is not critical or required for skin applications.
The flexible in vivo signal monitoring device should be completely non-toxic, while maintaining its sensitivity, selectivity, and reproducibility compared to conventional biosensors developed on rigid materials. To monitor the metabolites in an in vivo condition, the flexible electronics have to be implanted in the body. Therefore, if the flexible electronic system is composed of toxic or non-biocompatible components, it could lead to harmful side effects such as tissue necrosis. For example, due to the complex matrix and harsh conditions inside the body, the degradation of system components can occur. The degradation of the system could release toxic chemicals and cause unexpected side effects. Thus, for the development of flexible electronics for in vivo applications, material selection should be made cautiously to avoid any side effects. However, as the degradation property is not crucial for flexible electronics on the skin, there are more possibilities in the choice of the material component. In addition, challenges in the fabrication of flexible in vivo signal monitoring devices for continuously monitoring body fluids do not only include the sensing components, but also integrate different technical components, such as fluid delivery, power source, etc. For example, energy consumption will be a limitation in continuous, long-term monitoring. Although many aspects of flexible electronic devices for in vivo metabolites monitoring require improvement, these advancements could have a significant impact on biological, biochemical, and medical applications, especially for continuous monitoring in healthcare.
Author Contributions
The manuscript was written through the contributions of all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the project by Uniance Gene Inc. (2019).
Conflict of Interest
S-NL was employed by the company; Uniance Gene Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Boutry, C. M., Beker, L., Kaizawa, Y., Vassos, C., Tran, H., Hinckley, A. C., et al. (2019). Biodegradable and flexible arterial-pulse sensor for the wireless monitoring of blood flow. Nat. Biomed. Engin. 3, 47–57. doi: 10.1038/s41551-018-0336-5
Brindha, J., Chanda, K., and Balamurali, M. (2018). Biosensors for pathogen surveillance. Environ. Chem. Lett. 16, 1325–1337. doi: 10.1007/s10311-018-0759-y
Bruen, D., Delaney, C., Florea, L., and Diamond, D. (2017). Glucose sensing for diabetes monitoring: recent developments. Sensors 17:1866. doi: 10.3390/s17081866
Burugapalli, K., Wijesuriya, S., Wang, N., and Song, W. (2018). Biomimetic electrospun coatings increase the in vivo sensitivity of implantable glucose biosensors. J. Biomed. Mater. Res. 106, 1072–1081. doi: 10.1002/jbm.a.36308
Cao, H., Li, A., Nguyen, C. M., Peng, Y., and Chiao, J. (2012). An integrated flexible implantable micro-probe for sensing neurotransmitters. IEEE Sensors J. 12, 1618–1624. doi: 10.1109/JSEN.2011.2173674
Chen, X., Shen, W.-B., Yang, P., Dong, D., Sun, W., and Yang, P. (2018). High glucose inhibits neural stem cell differentiation through oxidative stress and endoplasmic reticulum stress. Stem Cells Dev. 27, 745–755. doi: 10.1089/scd.2017.0203
Chen, Y., Lu, S., Zhang, S., Li, Y., Qu, Z., Chen, Y., et al. (2017). Skin-like biosensor system via electrochemical channels for noninvasive blood glucose monitoring. Sci. Adv. 3:e1701629. doi: 10.1126/sciadv.1701629
Chung, M., Fortunato, G., and Radacsi, N. (2019). Wearable flexible sweat sensors for healthcare monitoring: a review. J. R. Soc. Interf. 16:20190217. doi: 10.1098/rsif.2019.0217
Damborská, A., Tomescu, M. I., Honzírková, E., Barteček, R., Horínková, J., Fedorová, S., et al. (2019). EEG resting-state large-scale brain network dynamics are related to depressive symptoms. Front. Psychiatry 10:548. doi: 10.3389/fpsyt.2019.00548
Das, P. S., Park, S. H., Baik, K. Y., Lee, J. W., and Park, J. Y. (2020). Thermally reduced graphene oxide-nylon membrane based epidermal sensor using vacuum filtration for wearable electrophysiological signals and human motion monitoring. Carbon 158, 386–393. doi: 10.1016/j.carbon.2019.11.001
Fang, Y., Wang, S., Liu, Y., Xu, Z., Zhang, K., and Guo, Y. (2018). Development of Cu nanoflowers modified the flexible needle-type microelectrode and its application in continuous monitoring glucose in vivo. Biosensors and Bioelectronics 110, 44–51. doi: 10.1016/j.bios.2018.03.024
Ganesana, M., Trikantzopoulos, E., Maniar, Y., Lee, S. T., and Venton, B. J. (2019). Development of a novel micro biosensor for in vivo monitoring of glutamate release in the brain. Biosens. Bioelectr. 130, 103–109. doi: 10.1016/j.bios.2019.01.049
Gao, W., Emaminejad, S., Nyein, H. Y. Y., Challa, S., Chen, K., Peck, A., et al. (2016). Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 529, 509–514. doi: 10.1038/nature16521
Glaab, E., Trezzi, J.-P., Greuel, A., Jäger, C., Hodak, Z., Drzezga, A., et al. (2019). Integrative analysis of blood metabolomics and PET brain neuroimaging data for Parkinson's disease. Neurobiol. Dis. 124, 555–562. doi: 10.1016/j.nbd.2019.01.003
Hong, G., and Lieber, C. M. (2019). Novel electrode technologies for neural recordings. Nat. Rev. Neurosci. 20, 330–345. doi: 10.1038/s41583-019-0140-6
Hossain, F. M., Heo, S. J., Nelson, J., and Kim, I. (2019). Paper-based flexible electrode using chemically-modified graphene and functionalized multiwalled carbon nanotube composites for electrophysiological signal sensing. Information 10:325. doi: 10.3390/info10100325
Hou, R., Zhou, D., Nie, R., Liu, D., and Ruan, X. (2019). Brain CT and MRI medical image fusion using convolutional neural networks and a dual-channel spiking cortical model. Med. Biol. Engin. Comput. 57, 887–900. doi: 10.1007/s11517-018-1935-8
Huang, Q., and Zhu, Y. (2019). Printing conductive nanomaterials for flexible and stretchable electronics: a review of materials, processes, and applications. Adv. Mater. Technol. 4:1800546. doi: 10.1002/admt.201800546
Huang, S., Liu, Y., Zhao, Y., Ren, Z., and Guo, C. F. (2019). Flexible electronics: stretchable electrodes and their future. Adv. Funct. Mater. 29:1805924. doi: 10.1002/adfm.201805924
Huhn, K., Engelhorn, T., Linker, R. A., and Nagel, A. M. (2019). Potential of sodium MRI as a biomarker for neurodegeneration and neuroinflammation in multiple sclerosis. Front. Neurol. 10:84. doi: 10.3389/fneur.2019.00084
Jayant, K., Wenzel, M., Bando, Y., Hamm, J. P., Mandriota, N., Rabinowitz, J. H., et al. (2019). Flexible nanopipettes for minimally invasive intracellular electrophysiology in vivo. Cell Rep. 26:266-278.e265. doi: 10.1016/j.celrep.2018.12.019
Jeong, Y., Kook, Y.-M., Lee, K., and Koh, W.-G. (2018). Metal enhanced fluorescence (MEF) for biosensors: General approaches and a review of recent developments. Biosens. Bioelectr. 111, 102–116. doi: 10.1016/j.bios.2018.04.007
Ji, B., Wang, M., Ge, C., Xie, Z., Guo, Z., Hong, W., et al. (2019). Flexible bioelectrodes with enhanced wrinkle microstructures for reliable electrochemical modification and neuromodulation in vivo. Biosensors and Bioelectronics 135, 181–191. doi: 10.1016/j.bios.2019.04.025
Khansili, N., Rattu, G., and Krishna, P. M. (2018). Label-free optical biosensors for food and biological sensor applications. Sens. Actuat. Chem. 265, 35–49. doi: 10.1016/j.snb.2018.03.004
Kim, J., Campbell, A. S., De vila, B. E.-F., and Wang, J. (2019). Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 37, 389–406. doi: 10.1038/s41587-019-0045-y
Kim, J. H., Lee, G. H., Kim, S., Chung, H. W., Lee, J. H., Lee, S. M., et al. (2018). Flexible deep brain neural probe for localized stimulation and detection with metal guide. Biosens. Bioelectr. 117, 436–443. doi: 10.1016/j.bios.2018.06.035
Klauser, A., Courvoisier, S., Kasten, J., Kocher, M., Guerquin-Kern, M., Van De Ville, D., et al. (2019). Fast high-resolution brain metabolite mapping on a clinical 3T MRI by accelerated H-FID-MRSI and low-rank constrained reconstruction. Magn. Reson. Med. 81, 2841–2857. doi: 10.1002/mrm.27623
Klonoff, D. C. (2005). Continuous glucose monitoring. Diabetes Care 28:1231. doi: 10.2337/diacare.28.5.1231
Lake, B. B., Chen, S., Sos, B. C., Fan, J., Kaeser, G. E., Yung, Y. C., et al. (2018). Integrative single-cell analysis of transcriptional and epigenetic states in the human adult brain. Nat. Biotechnol. 36, 70–80. doi: 10.1038/nbt.4038
Lee, W., Park, K.-S., Kim, Y.-W., Lee, W. H., and Choi, J.-W. (2005). Protein array consisting of sol-gel bioactive platform for detection of E. coli O157: H7. Biosens. Bioelectr. 20, 2292–2299. doi: 10.1016/j.bios.2004.11.010
Lee, Y., Morrison, B. M., Li, Y., Lengacher, S., Farah, M. H., Hoffman, P. N., et al. (2012). Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 487, 443–448. doi: 10.1038/nature11314
Liu, F., Dong, H., and Tian, Y. (2019). Real-time monitoring of peroxynitrite (ONOO–) in the rat brain by developing a ratiometric electrochemical biosensor. Analyst 144, 2150–2157. doi: 10.1039/C9AN00079H
Liu, Y., Wang, H., Zhao, W., Zhang, M., Qin, H., and Xie, Y. (2018). Flexible, stretchable sensors for wearable health monitoring: sensing mechanisms, materials, fabrication strategies and features. Sensors 18:645. doi: 10.3390/s18020645
López-Gambero, A. J., Martínez, F., Salazar, K., Cifuentes, M., and Nualart, F. (2019). Brain glucose-sensing mechanism and energy homeostasis. Mol. Neurobiol. 56, 769–796. doi: 10.1007/s12035-018-1099-4
Murphy, M. C., Huston, J., and Ehman, R. L. (2019). MR elastography of the brain and its application in neurological diseases. NeuroImage 187, 176–183. doi: 10.1016/j.neuroimage.2017.10.008
Nguyen, T. N. H., Nolan, J. K., Park, H., Lam, S., Fattah, M., Page, J. C., et al. (2019). Facile fabrication of flexible glutamate biosensor using direct writing of platinum nanoparticle-based nanocomposite ink. Biosens. Bioelectr. 131, 257–266. doi: 10.1016/j.bios.2019.01.051
Park, G., Chung, H. J., Kim, K., Lim, S. A., Kim, J., Kim, Y. S., et al. (2014). Immunologic and tissue biocompatibility of flexible/stretchable electronics and optoelectronics. Advanced healthcare materials 3, 515–525. doi: 10.1002/adhm.201300220
Park, J., Sempionatto, J. R., Kim, J., Jeong, Y., Gu, J., Wang, J., et al. (2020). Microscale biosensor array based on flexible polymeric platform toward lab-on-a-needle: real-time multiparameter biomedical assays on curved needle surfaces. ACS Sensors 5, 1363–1373. doi: 10.1021/acssensors.0c00078
Park, Y. M., Lim, S. Y., Jeong, S. W., Song, Y., Bae, N. H., Hong, S. B., et al. (2018). Flexible nanopillar-based electrochemical sensors for genetic detection of foodborne pathogens. Nano convergence 5:15. doi: 10.1186/s40580-018-0147-0
Pellerin, L., and Magistretti, P. J. (1994). Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci. U.S.A. 91:10625. doi: 10.1073/pnas.91.22.10625
Peng, Q., Yan, X., Shi, X., Ou, S., Gu, H., Yin, X., et al. (2019). In vivo monitoring of superoxide anion from Alzheimer's rat brains with functionalized ionic liquid polymer decorated microsensor. Biosens. Bioelectr. 144, 111665. doi: 10.1016/j.bios.2019.111665
Pickup, J. C., Shaw, G. W., and Claremont, D. J. (1989). Potentially-implantable, amperometric glucose sensors with mediated electron transfer: improving the operating stability. Biosensors 4, 109–119. doi: 10.1016/0265-928X(89)80026-4
Pothof, F., Bonini, L., Lanzilotto, M., Livi, A., Fogassi, L., Orban, G., et al. (2016). Chronic neural probe for simultaneous recording of single-unit, multi-unit, and local field potential activity from multiple brain sites. J. Neural Engin. 13:046006. doi: 10.1088/1741-2560/13/4/046006
Pu, Z., Tu, J., Han, R., Zhang, X., Wu, J., Fang, C., et al. (2018). A flexible enzyme-electrode sensor with cylindrical working electrode modified with a 3D nanostructure for implantable continuous glucose monitoring. Lab on a Chip 18, 3570–3577. doi: 10.1039/C8LC00908B
Salim, A., and Lim, S. (2019). Recent advances in noninvasive flexible and wearable wireless biosensors. Biosens. Bioelectr. 11:1422. doi: 10.1016/j.bios.2019.111422
Santiago, A. R., Cristóvão, A. J., Santos, P. F., Carvalho, C. M., and Ambrósio, A. F. (2007). High glucose induces caspase-independent cell death in retinal neural cells. Neurobiol. Dis. 25, 464–472. doi: 10.1016/j.nbd.2006.10.023
Simpson, I. A., Carruthers, A., and Vannucci, S. J. (2007). Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J. Cerebr. Blood Flow Metab. 27, 1766–1791. doi: 10.1038/sj.jcbfm.9600521
Song, E., Chiang, C.-H., Li, R., Jin, X., Zhao, J., Hill, M., et al. (2019). Flexible electronic/optoelectronic microsystems with scalable designs for chronic biointegration. Proc. Natl. Acad. Sci. U.S.A. 116, 15398–15406. doi: 10.1073/pnas.1907697116
Tripathy, N., and Kim, D.-H. (2018). Metal oxide modified ZnO nanomaterials for biosensor applications. Nano Converg. 5:27. doi: 10.1186/s40580-018-0159-9
Weltin, A., Enderle, B., Kieninger, J., and Urban, G. A. (2014a). Multiparametric, flexible microsensor platform for metabolic monitoring in vivo. IEEE Sens. J. 14, 3345–3351. doi: 10.1109/JSEN.2014.2323220
Weltin, A., Kieninger, J., Enderle, B., Gellner, A.-K., Fritsch, B., and Urban, G. A. (2014b). Polymer-based, flexible glutamate and lactate microsensors for in vivo applications. Biosens. Bioelectr. 61, 192–199. doi: 10.1016/j.bios.2014.05.014
Woo, C.-W., Chang, L. J., Lindquist, M. A., and Wager, T. D. (2017). Building better biomarkers: brain models in translational neuroimaging. Nature Neurosci. 20, 365–377. doi: 10.1038/nn.4478
Xu, M., Jiang, Y., Pradhan, S., and Yadavalli, V. K. (2019). Use of silk proteins to form organic, flexible, degradable biosensors for metabolite monitoring. Front. Mater. 6:331. doi: 10.3389/fmats.2019.00331
Xue, N., Martinez, I. D., Sun, J., Cheng, Y., and Liu, C. (2018). Flexible multichannel vagus nerve electrode for stimulation and recording for heart failure treatment. Biosens. Bioelectr. 112, 114–119. doi: 10.1016/j.bios.2018.04.043
Yang, Y., and Gao, W. (2019). Wearable and flexible electronics for continuous molecular monitoring. Chem. Soc. Rev. 48, 1465–1491. doi: 10.1039/C7CS00730B
Yao, G., Yin, C., Wang, Q., Zhang, T., Chen, S., Lu, C., et al. (2019). Flexible bioelectronics for physiological signals sensing and disease treatment. J. Materiomics, 6, 397–413. doi: 10.1016/j.jmat.2019.12.005
Yao, S., Ren, P., Song, R., Liu, Y., Huang, Q., Dong, J., et al. (2020). Nanomaterial-enabled flexible and stretchable sensing systems: processing, integration, and applications. Adv. Mater. 32:1902343. doi: 10.1002/adma.201902343
Yu, B., Wang, C., Ju, Y. M., West, L., Harmon, J., Moussy, Y., et al. (2008). Use of hydrogel coating to improve the performance of implanted glucose sensors. Biosens. Bioelectr. 23, 1278–1284. doi: 10.1016/j.bios.2007.11.010
Zhang, Q., Jiang, D., Xu, C., Ge, Y., Liu, X., Wei, Q., et al. (2020). Wearable electrochemical biosensor based on molecularly imprinted Ag nanowires for noninvasive monitoring lactate in human sweat. Sens Actuat. 320:128325. doi: 10.1016/j.snb.2020.128325
Keywords: flexible electronics, biosensor, in vivo monitoring, electrophysiological signal, metabolite signal
Citation: Choi HK, Lee J-H, Lee T, Lee S-N and Choi J-W (2020) Flexible Electronics for Monitoring in vivo Electrophysiology and Metabolite Signals. Front. Chem. 8:547591. doi: 10.3389/fchem.2020.547591
Received: 31 March 2020; Accepted: 07 October 2020;
Published: 19 November 2020.
Edited by:
Shangsheng Feng, Xi'an Jiaotong University, ChinaReviewed by:
Diego Centonze, University of Foggia, ItalyZedong Li, Xi'an Jiaotong University, China
Copyright © 2020 Choi, Lee, Lee, Lee and Choi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jeong-Woo Choi, andjaG9pQHNvZ2FuZy5hYy5rcg==; Sang-Nam Lee, c25sZWU5MTkxQGhhbm1haWwubmV0
†These authors have contributed equally to this work
 Hye Kyu Choi
Hye Kyu Choi Jin-Ho Lee
Jin-Ho Lee Taek Lee3
Taek Lee3 Jeong-Woo Choi
Jeong-Woo Choi