
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Chem. , 03 July 2020
Sec. Theoretical and Computational Chemistry
Volume 8 - 2020 | https://doi.org/10.3389/fchem.2020.00566
This article is part of the Research Topic Changing the Perspectives of Noble Gas Reactivity View all 10 articles
Noble gas can be no noble in certain situations from the perspective of structure, bonding, and reactivity. These situations could be extreme experimental conditions or others. In this contribution, we systematically investigate the impact of fullerene encapsulation on molecular structure and chemical reactivity of noble gas dimers (Ng2) in a few fullerene molecules. To that end, we consider He2, Ne2, and Ar2 dimers encapsulated in C50, C60, and C70 fullerenes. We unveil that bond distances of Ng2 inside fullerene become substantially smaller and noble gas atoms become more electrophilic. In return, these noble gas dimers make fullerene molecules more nucleophilic. Using analytical tools from density functional theory, conceptual density functional theory, and information-theoretic approach, we appreciate the nature and origin of these structure and reactivity changes. The results and conclusions from this work should provide more new insights from the viewpoint of changing the perspectives of noble gas reactivity.
Regarded as the most unreactive elements in the periodic table, noble gas atoms could indeed be reactive and no noble at all under certain circumstances, [e.g., high temperature, high pressure, special conditions (e.g., confinement, etc.)]. The first successful experimental realization was the synthesis of xenon hexafluoro platinate XePtF6 in 1962 (Bartlett, 1962; Graham et al., 2000). Since then many more compounds with noble gas elements included have been reported (Turner and Pimentel, 1963; Nelson and Pimentel, 1967; Bondybey, 1971; Stein, 1973; Holloway and Hope, 1999) and a brand new discipline of noble gas chemistry has emerged thereafter.
In this contribution, we explore the possibility of fullerene encapsulation as another feasible way to make noble gas elements no longer noble. In specific, we consider the impact of the encapsulation by fullerene molecules on geometrical structure and chemical reactivity for noble gas species. Previously, by heating fullerenes at 650°C under 3,000 atmosphere, helium (Saunders et al., 1993), neon (Saunders et al., 1993), argon (DiCamillo et al., 1996), krypton (Yamamoto et al., 1999), and xenon (Syamala et al., 2002) noble gas atoms were experimentally introduced into the fullerene cage. This experimental realization of fullerene encapsulation demonstrates the feasibility, and there were reports of experimental realization of noble gas dimers encapsulation in fulerenes (Saunders et al., 1996; Laskin et al., 1998; Peres et al., 2001; Popov et al., 2013; Saha et al., 2019). Also, earlier, Krapp and Frenking performed a computational study on Ng2@C60 (Ng = He, Ne, Ar, Kr, and Xe) systems from the bonding perspective and concluded that He2@C60 and Ne2@C60 were weakly bonded van der Waals complexes (Krapp and Frenking, 2007). On the other hand, Solà et al. explored the same systems from the reactivity perspective and they attributed the changes in reactivity to the stabilized LUMO, increased fullerene strain energy, and compressed Ng2 unit (Osuna et al., 2009). There are other theoretical and computational studies on the encapsulation of either noble gas or other species in the literature (Osuna et al., 2011; Cheng and Sheng, 2013; Dolgonos and Peslherbe, 2014; Khatua et al., 2014; Bickelhaupt et al., 2015; Kryachko et al., 2015; Nikolaienko and Kryachko, 2015; Jalife et al., 2016; Srivastava et al., 2016; Nikolaienko et al., 2017; Foroutan-Nejad et al., 2018; Jaroš et al., 2018; Gómez and Restrepo, 2019; Das et al., 2020).
In this work, we reconsider the likelihood of these species from the computational perspective using a few newly developed theoretical tools. To that end, we examine noble gas dimers, He2, Ne2, and Ar2, inside a few fullerene systems, C50, C60, and C70, as illustrative examples in this work. In addition, to pinpoint the nature and origin of these structure and reactivity changes, we make use of a few analytical tools available in the literature, including density functional theory, conceptual density functional theory, information-theoretic approach, energy decomposition analysis, bonding energy analysis, natural population analysis, and noncovalent interaction analysis. In information theory, Shannon introduced a measure of information content or a lack of it as an entropy through an analogy with the statistical mechanical variant of thermodynamic entropy as proposed by Boltzmann. Hence the stability of a system and spontaneous evolution toward an equilibrium state can be understood by following the variation of the entropy during the thermodynamic process. These analyses should provide better understanding from the viewpoint of changing the perspectives of noble gas reactivity.
Nine model systems were built to examine the impact of fullerene encapsulation on structure and reactivity properties for noble gas dimers (Ng2) using three fullerenes C50, C60, and C70 and three Ng2 species, He2, Ne2, and Ar2. They are represented by following notations: He2@C50, He2@C60, He2@C70, Ne2@C50, Ne2@C60, Ne2@C70, Ar2@C50, Ar2@C60, and Ar2@C70. Also, as the reference, the structural and reactivity properties of these three fullerene molecules and three Ng2 dimers in vacuum were also simulated and compared.
Structure, bonding and reactivity properties for these species were analyzed through a number of well-established analytical tools available in the literature. They include analyses of the total energy decomposition (Parr and Yang, 1989; Liu, 2007), interaction energy decomposition (Bickelhaupt and Baerends, 2000), natural population (Glendening et al., 2012), non-covalent interactions (Johnson et al., 2010), conceptual density functional theory (Geerlings et al., 2003, 2020; Liu, 2009, 2013), and informational-theoretic approach (Liu, 2016; Rong et al., 2019). These analyses enable in-depth understanding about the origin and nature of the changes in structure, bonding, and reactivity. Formulations as well as the details of how to perform these analyses are available elsewhere.
Structure optimizations and natural population analyses were performed with the Gaussian 16 (Frisch et al., 2016) package (versionA03), with ultrafine integration grids and tight self-consistent field (SCF) convergence. To obtain the most stable orientation of Ng2 dimer in a fullerene cage, quantum molecular dynamics simulations were performed for each species at the DFT B3LYP/3-21G (Lee et al., 1988; Becke, 1993) level of theory at 3,000 K for 300 ps with a step size of 1 fs. A total of 20 structures with the lowest total energy were selected as the possible structure candidates from the trajectory and then optimized at the DFT M06-2X/6-311G(d) (Zhao and Truhlar, 2008) level of theory. The structure with the lowest total energy from these 20 candidates was selected as the global minimum for the species. The full structure optimization was followed by a single-point frequency calculation to ensure that the final optimized structure has no imaginary frequency. As a measure of quality control, we conducted a benchmark test on the impact of different choices of basis sets and approximate DFT functionals (Ma et al., 2014). The Multiwfn 3.6.1 program (Lu and Chen, 2012) was utilized to calculate the information-theoretic quantities by using the checkpoint file from the Gaussian calculations as the input file. The total energy components were obtained from Gaussian calculations with the keyword of iop(5/33 = 1). The ADF (Amsterdam Density Functional) (Lee et al., 1988) package was employed to perform the bond energy decomposition (BED) analysis. The BED analysis was conducted using the previously optimized structure and the DFT BP86 approximate functional with the double zeta basis set, zero order regular approximation for relativistic correction, and Grimme4 dispersion correction (Te Velde et al., 2001).
Table 1 is the benchmark results using Ar2@C60 as an example with 14 basis sets and 14 DFT functionals as well as HF and MP2 methods to examine their impact on the Ar-Ar bond distance. It is compared with Ar-Ar length in vacuum. As can be seen from the table, the bond length in Ar2@C60 is not significantly dependent on the choice of basis sets and functionals, which is unsurprising because the Ng2 molecule is confined. As a reference, the experimental Ar-Ar length in vacuum is 3.758 Å (Computational Chemistry Comparison Benchmark DataBase, 2019). There are a handful of computational results for the Ar-Ar length (Colburn and Douglas, 1976). In all analyses below, we chose M06-2X/6-311G(d).
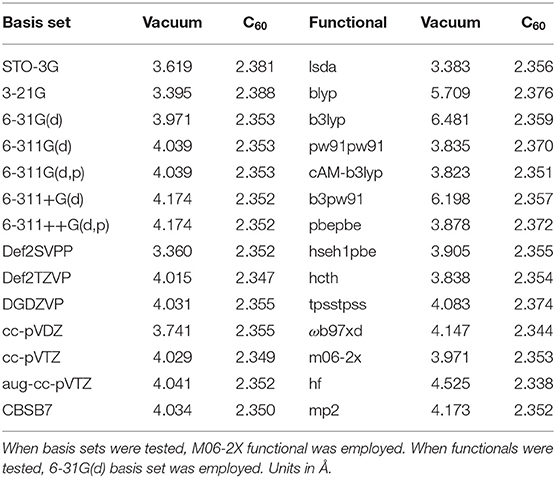
Table 1. Benchmark tests for Ar2@C60 with 14 basis sets and 14 functionals/methods to examine the impact of the methodology dependence on the Ar-Ar bond distance in both vacuum and C60 fullerene.
Figure 1 shows the final structure obtained for Ng2@C50, Ng2@C60, and Ng2@C70 with both top and side views. With a given fullerene, the binding mode is the same for different Ng2 dimers, but for different fullerenes, as shown in Figure 1, the same Ng2 dimer prefers to bind differently. This is not surprising either. This is because, for instance, C70 is elongated so the dimer aligns with the long axis, as one would expect from a simple steric argument. These similarity and difference illustrate the fact that the size and nature of fullerene molecules play more important role during the encapsulation process of Ng2 dimers.
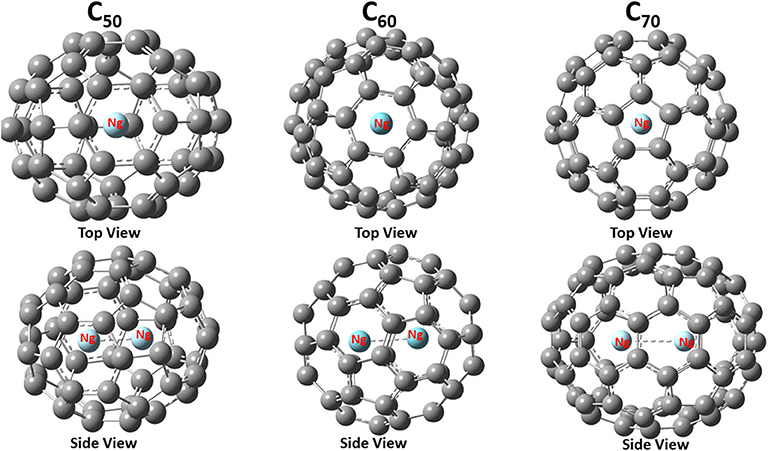
Figure 1. Optimized structures of noble gas dimers encapsulated in fullerene cages with Ng = Ar as an illustrative example. The point group for these three encapsulated systems are C2v, D3d, and D5h, respectively.
Table 2 exhibits a few selected structural and electronic properties of these species. As can be seen from the table, the bond length of Ng2 dimers becomes smaller when encapsulated in fullerenes, and the smaller the fullerene cage the shorter the Ng-Ng distance. This latter trend was resulted from the fact that a smaller fullerene has a smaller cage inside so Ng2 dimers are forced to have shorter bond distance. Energetically, both BSSE (basis set superposition error; Boys and Bernardi, 1970; Simon et al., 1996)-corrected and uncorrected interaction energy results show that for He2, all three fullerene molecules yield negative (favorable) interaction energies, whereas for larger Ng2 dimers, (e.g., Ne2 and Ar2, it appears that only larger-sized fullerenes such as C70 are able to yield attractive interactions between Ng2 and fullerene, albeit with the limitations of the method of computation used). This is because larger Ng2 dimers tend to occupy more space within the fullerene molecule, making it harder for smaller-sized fullerenes to compensate the energetic cost from electrostatic and other repulsions. We are, however, interested in analyzing the qualitative trends only.
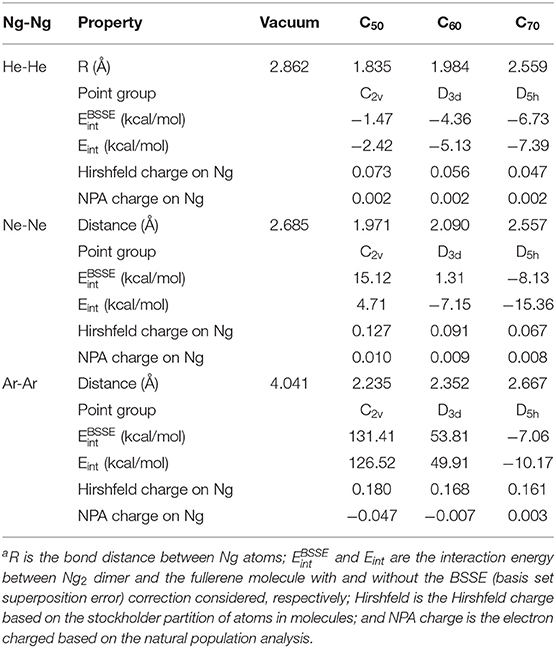
Table 2. A few selected structure and electronic properties at the DFT M06-2X/6-311G(d) level of theory for Ng2 (Ng = He, Ne, and Ar) dimers encapsulated in C50, C60, and C70 fullerenesa.
Shown in Table 2 are two charge results for Ng atoms encapsulated in fullerene molecules. The first one is the NPA (natural population analysis) charge from NBO (natural bond orbital) analysis (Glendening et al., 2012), and the other is the Hirshfeld charge based on the shareholder partition of atoms in molecules (Hirshfeld, 1977). NPA charges for Ng atoms could be either positive or negative, but the Hirshfeld charge value is always positive for Ng atoms. According to the reference literature on the Hirshfeld charge (Liu et al., 2014), which is a good descriptor of electrophilicity and nucleophilicity, a positive Hirshfeld charge is an indication that the atom is electrophilic. The larger value of a positive Hirshfeld charge indicates that it is more electrophilic, able to accept more electrons from a nucleophile. Hirshfeld charge results in Table 2 displays that Ng2 in smaller fullerenes tend to be more electrophilic.
Shown in Figure 2 are frontier molecular orbitals from both top and side views for Ar2@C70 as an illustrative example, from which one can see that they are both delocalized over the entire molecule. Notice that in both orbitals, we do not see discernible contributions from the Ng2 dimer overlapping with any of the atomic orbitals from the fullerene, suggesting that atomic orbitals from Ng atoms are not involved in the frontier molecular orbital delocalization. In Table 3, using the information from these frontier orbitals, we calculated a few global reactivity descriptors from conceptual DFT, including chemical potential μ (Parr et al., 1978), chemical hardness η (Parr and Pearson, 1983), and electrophilicity index ω (Parr et al., 1999) for the nine systems studied in this work. A larger value in hardness is usually an indication of being more stable, while a larger value in electrophilicity index suggests that the species is more reactive. From the results in Table 2, we can see that numerical values of these reactivity indices are slightly changed after Ng2 encapsulation. This should be mainly due to the charge transfer between Ng2 and fullerene. Ng2 in smaller fullerenes are more reactive with a larger ω value, whereas in larger fullerenes it becomes often more stable with a larger η value. These results agree well with the conventional chemical wisdom.
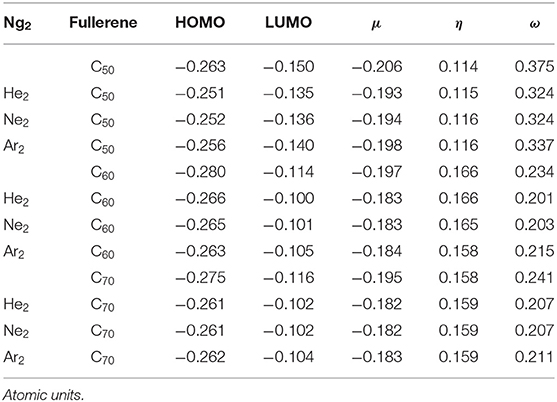
Table 3. Global descriptors from Conceptual DFT, including HOMO, LUMO, chemical potential μ, chemical hardness η, and electrophilicity index ω for the systems studied in this work.
Figure 3 shows the contour surface of the molecular electrostatic potential mapped onto the van der Waals surface for Ne2 and Ar2 dimers encapsulated in C60 fullerene. It shows that the carbon atoms perpendicular to the Ng-Ng bond is more electronegative. This result is consistent with our Hirshfeld charge result, showing that Ng atoms become more positive and the neighboring carbon atoms in fullerene become more negative. Since Hirshfeld charges are reliable descriptors of electrophilicity and nucleophilicity, Figure 3 also shows that carbon atoms of the fullerene molecule become more nucleophilic, capable of donating more electrons to an electrophile.
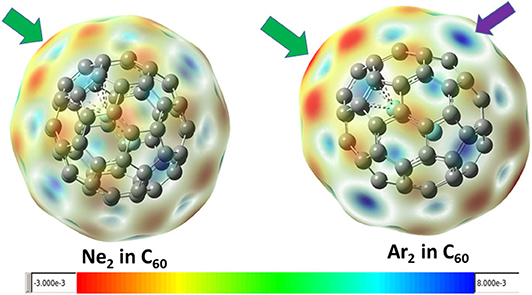
Figure 3. The mapped contour surface of the molecular electrostatic potential onto the van der Waals surface for Ne2 and Ar2 dimers encapsulated in C60 fullerene.
In conceptual DFT, Fukui functions (Yang et al., 1984) can also be employed as local descriptors to predict nucleophilic and electrophilic attacks. Shown in Figure 4 are these local functions, where both electrophilic (Figures 4A,B) and nucleophilic (Figures 4C,D) Fukui functions for Ar2 dimer encapsulated in C60 fullerene are exhibited. From the figure, we found that (i) carbon atoms in fullerene can be both electrophilic (dark blue areas in Figures 4A,B) and nucleophilic (dark blue regions in Figures 4C,D), and (ii) nucleophilic regions in Figures 4C,D are drastically different from those of Figure 3 (red areas), suggesting that using Hirshfeld charge and Fukui charge, we obtain different results. According our recent findings, this discrepancy is not unusual. As our recent study demonstrated (Wang et al., 2019), results from the Hirshfeld charge should be more robust and reliable. Notice that Krapp and Frenking (2007) also observed that Ng2 molecules were oxidized inside fullerenes, implying that Ng2 became more electrophilic in return as electrons were transferred to C60, and this also made fullerenes more nucleophilic. This result was confirmed by Solà et al. (Osuna et al., 2009, 2011), also who unveiled that the combination of the LUMO stabilization, increased fullerene strain energy, and greater compression of the encapsulated Ng2 contributed to the reactivity change.
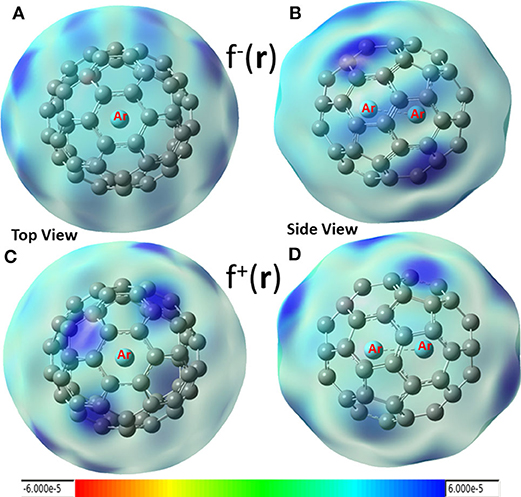
Figure 4. Electrophilic (A,B) and nucleophilic (C,D) Fukui functions for Ar2 dimer encapsulated in C60 fullerene.
To qualitatively identify and distinguish weak interactions between Ng2 and fullerene molecules, noncovalent interaction (NCI) analysis (Johnson et al., 2010) was employed, whose results are shown in Figure 5. The reduced density gradient is large and positive in the regions far from the molecule, but it becomes small in the vicinity of both covalent and non-covalent interactions. To identify different types of interactions, the sign of Laplacian of the density is utilized and compared. Plotting low-gradient isosurfaces subject to a further low-density constraint enables real-space visualization of non-covalent interactions. Results in Figure 5 show that when He2 encapsulated from C50 to C70, a “spike” area becomes more apparent from −0.02 to 0 a.u., indicating that there existed more attractive interactions within the complexes as the fullerene cage becomes larger. The same trend is also observed for Ne2 and Ar2. These results are also in qualitative agreement with the total energy difference result as shown in Table 2.
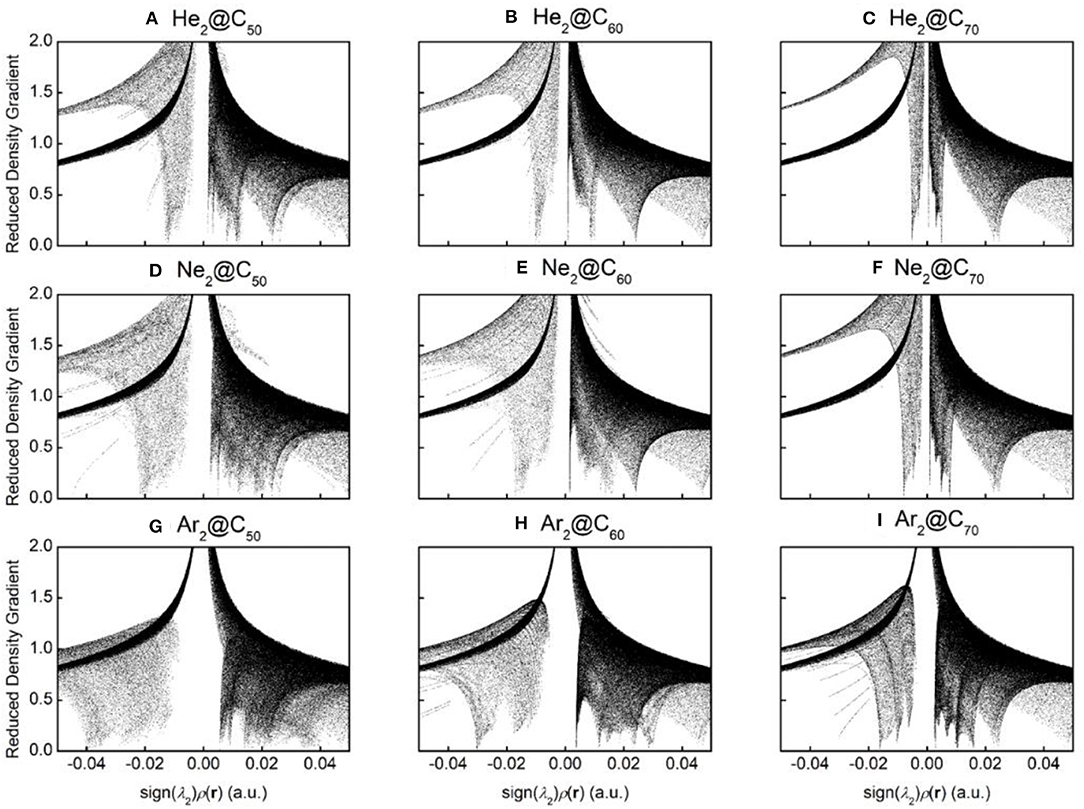
Figure 5. Diagrams of the reduced density gradient (RDG) vs. sign(λ2)ρ(r) for nine endohedral fullerenes: (A) He2@C50; (B) He2@C60; (C) He2@C70; (D) Ne2@C50; (E) Ne2@C60; (F) Ne2@C70; (G) Ar2@C50; (H) Ar2C60; and (I) Ar2@C70.
Table 4 is the results from the two schemes (Parr and Yang, 1989; Liu, 2007) of the total energy partition for the nine Ng2-fullerene systems studied in this work. Different from the traditional binding energy or interaction energy partition, the total energy partition decomposes the total energy E of a molecular system in terms of a few components that are physiochemically meaningful. In DFT (Parr and Yang, 1989), we have E = TS + Exc + Ee, where TS, Exc, and Ee are the non-interacting kinetic energy, exchange-correlation energy, and electrostatic interaction energy, respectively. An alternative scheme was proposed by one of us (Liu, 2007), where E = ES + Ee + Eq, where ES, Ee, and Eq stand for the energetic contribution from three physiochemical effects, steric, electrostatic, and quantum (due to the exchange-correlation effects). There is a common component in these two partition schemes, the electrostatic energy Ee. Considering the energy difference ΔE between two chemical processes sharing the same molecular fragments, we have ΔE = ΔTS + ΔExc + ΔEe, and ΔE = ΔES + ΔEe + ΔEq. Table 4 shows the values of these five components from the above two partition schemes using Ng2 and fullerene in vacuum as the reference. From the table, we found that in the first scheme (Parr and Yang, 1989; Liu, 2007), both ΔTs and ΔExc contribute positively to ΔE <0, and it is the electrostatic contribution ΔEe that contributes negatively to their stability, in order to make ΔE <0. Since Ee itself is negative in value (Liu, 2007), this result suggests that to put Ng2 dimer in the fullerene cage, one has to overcome the large electrostatic repulsion between Ng2 and fullerene. This result is further verified by the second total energy partition scheme (Liu, 2007), where we found that in this case both electrostatic and quantum (due to exchange-correlation effects) contribute negatively to ΔE <0 but the steric contribution ΔES is positive. This result shows that after Ng2 dimer is put into the cage, smaller space is occupied, but to do so, large electrostatic and Fermionic quantum repulsions have to be overcome. Notice that this total energy difference is different from the BSSE corrected interaction energy in Table 2 and our present results are consistent with what we observed elsewhere for other systems.
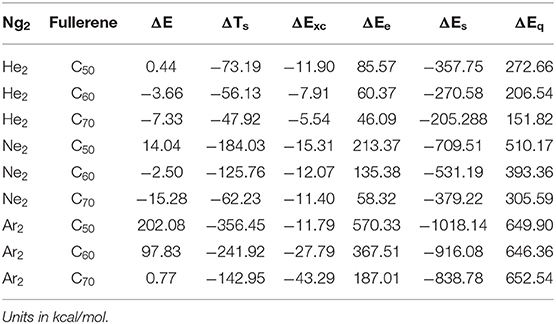
Table 4. Numerical results of the two total energy partition schemes using Ng2 and fullerene in vacuum as the references for the nine Ng2-fullerene systems studied in this work.
Figure 6 illustrates the total energy partition analysis to elucidate which energy component is dominant over others in contributing to the total energy difference. A strong linear correlation of the total energy difference ΔE with the electrostatic energy difference ΔEe is shown in Figure 6A, suggesting that it is the electrostatic repulsion that dominates the energetic contribution, which agrees well with the numerical results in Table 4. If the strategy of two-variable fitting is employed, shown in Figures 6B,C, we found that ΔEe combining with either ΔExc and ΔES yields even stronger correlations, indicating that contributions from the exchange-correlation and steric effects are minor yet indispensable.
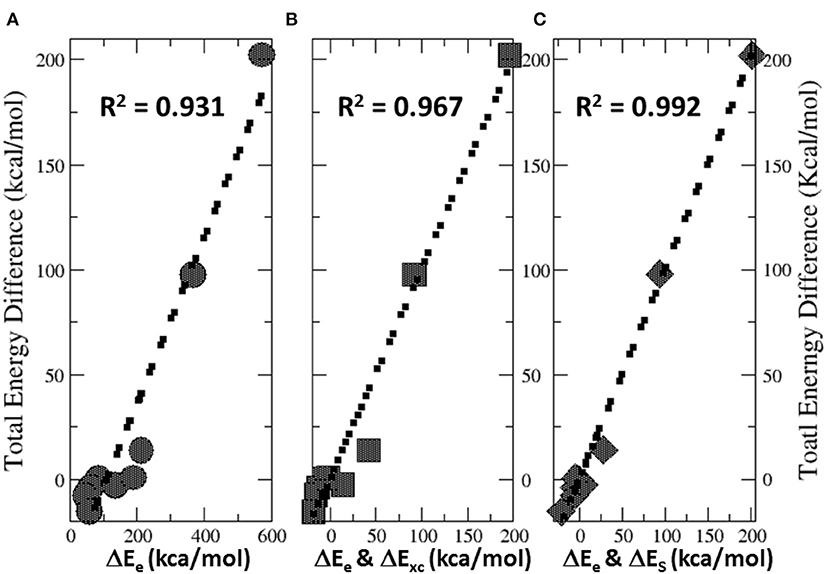
Figure 6. Strong linear correlations of the total energy difference ΔE with (A) the electrostatic energy difference ΔEe, (B) two-variable fitting with ΔEe and ΔExc, and (C) two-variable fitting with ΔEe and ΔES.
To confirm the results in Tables 4, 5 shows the total bonding energy ΔEbond analysis (Bickelhaupt and Baerends, 2000) results using the ADF package. In this analysis, ΔEbond consists of contributions from the Pauli repulsion ΔEPauli, steric interaction ΔEsteric, electrostatic attraction ΔEelstat, orbital interactions ΔEorb, and dispersion Pauli repulsion ΔEdisp, ΔEbond = ΔEPauli + ΔEsteric + ΔEorb + ΔEdisp + ΔEelstat. From the results in Table 5, it can be seen that the steric effect (even though its definition is substantially different) contributes favorably and the major opposite contribution is from the electrostatic interaction, agreeing well with our results in Table 4.
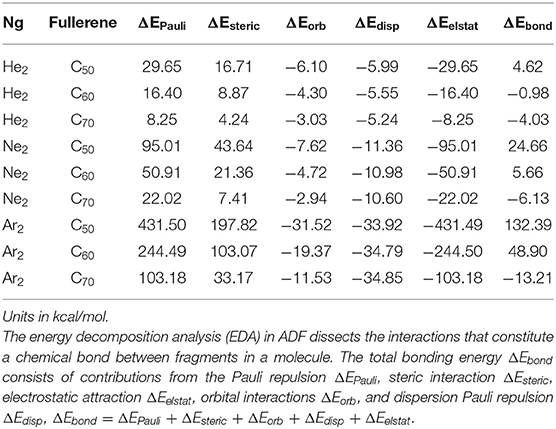
Table 5. The chemical bonding analysis results using the ADF package to analyze the bond energy between the Ng2dimer and fullerene fragments.
Lastly, we examine the results of eight quantities from the information-theoretic approach, which are shown in Table 6, including Shannon entropy (Shannon, 1948), Fisher information (Fisher, 1925), Ghosh-Berkowitz-Parr entropy (Ghosh et al., 1984), information gain (Kullback and Leibler, 1951), 2nd and 3rd orders of absolute Rényi and relative Rényi entropy (Rényi, 1970; Nagy and Romera, 2015). These quantities each have their own physio-chemical meaning and thus representing different yet intrinsic properties of molecular systems. For example, Shannon entropy is a measure of the electron density distribution uniformity, Fisher information gauges the heterogeneity of the same electron density distribution, and information gain is a robust measurement of regioselectivity, electrophilicity, and nucleophilicity, with its first-order approximation yielding the Hirshfeld charge (Liu et al., 2014). To make sense of these ITA results, Table 7 lists the correlation coefficient of these quantities with respect to either Ng2 or fullerene types, and Figure 7 displays illustrative example of strong linear relationships of the total energy difference ΔE with Shannon entropy, Fisher information, and information gain for He2, Ne2, and Ar2 encapsulated in three fullerenes. Strong correlations are seen in most cases, as shown in the figure, but as highlighted in Table 7, these strong correlations are true only for Ng2 dimers cross different fullerenes. If one fits a fullerene with three Ng2 dimers, no such strong correlation is often observed. Also, we tried to put all data points in one plot, and no significant correlation was observed either. Strong correlations in Figure 7 show that ITA quantities could be good descriptors of change trends for Ng2 dimers encapsulated in fullerene cages. For instance, for He2 encapsulated in from C50 to C70, binding energy increases, so does the Shannon entropy, due to the more delocalization of the electron density distribution. Another example is the information gain, which is a direct measure of the Hirshfeld charge. Again, for He2 dimer, from C50 to C70, the difference in information gain decreases, leading to the decrease in Hirshfeld charge, which is in good agreement with the result in Table 2. We noticed the abnormality of Shannon entropy for Ar2 dimer in Figure 7, whose trend is opposite to that of He2 and Ne2. The reason is unknown. More studies are in need.
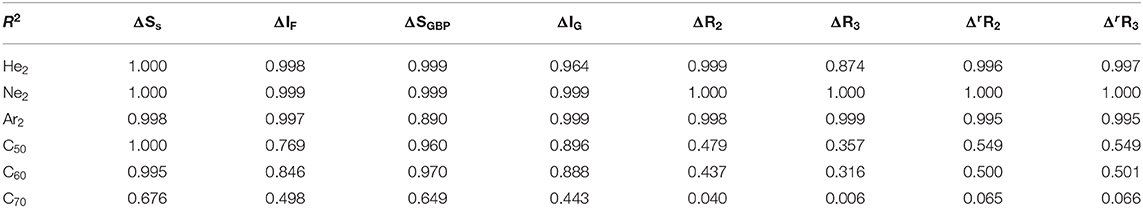
Table 7. Correlation coefficients R2 values between ΔE and eight ITA quantities for three Ng2 dimers with different fullerene systems (first three rows) and for three fullerenes with different Ng2 dimers (last three rows).
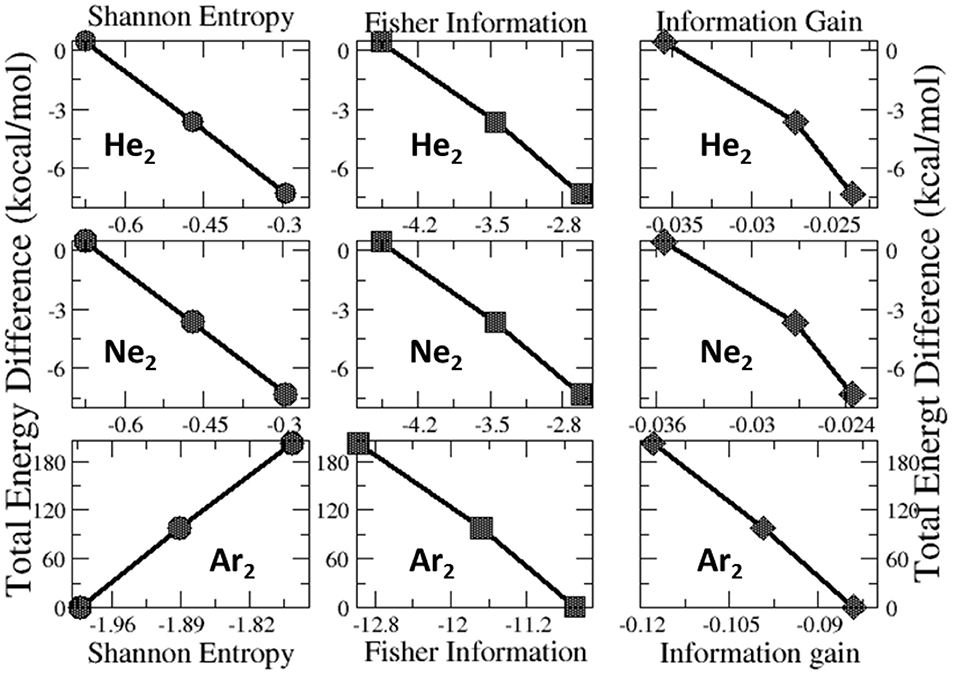
Figure 7. Strong linear relationships of the total energy difference ΔE with ITA quantities such as Shannon entropy, Fisher information, and information gain for He2, Ne2, and Ar2 encapsulated in C50, C60, and C70 fullerenes.
To summarize, in this work, we investigated the possibility of making noble gas reactive through the means of fullerene encapsulation. To that end, we built a total of nine model systems, with three Ng2 dimers (He2, Ne2, and Ar2) encapsulated in three fullerene (C50, C60, and C70) cages. Quantum molecular dynamics simulations were employed to explore the potential energy surface of Ng2 inside the fullerene molecule. To obtain the lowest energy structure it was further examined through a few well-established analytical tools such as conceptual density functional theory, information-theoretic approach, total energy decomposition, bonding energy decomposition, and others. Our results show that bond distances of Ng2 inside fullerene become substantially smaller and noble gas atoms become more electrophilic. In return, these noble gas dimers make fullerene molecules more nucleophilic. Using these analytical tools, we appreciate the nature and origin of these structure and reactivity changes. Our results and conclusions drawn from the present study should provide more understanding and new insights from the viewpoint of changing the perspectives of noble gas reactivity.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
ML performed the calculations and analyses and prepared the draft. XH and BW helped in calculations and analyses. DZ and CR managed progression of the project. PC initiated the project and revised the draft. SL worked on the plan, assisted the calculations and analyses, and revised the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
ML and CR acknowledge support from the Excellent Undergraduate Research Fund of Haoqing Experimental Class, Hunan Normal University and the National Natural Science Foundation of China (No. 21503076). PC would like to thank the DST, New Delhi for the J. C. Bose National Fellowship.
Bartlett, N. (1962). Xenon hexafluoroplatinate(V) Xe+[PtF 6]-. Proc. Chem. Soc. 115:218. doi: 10.1039/ps9620000197
Becke, A. D. (1993). Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652. doi: 10.1063/1.464913
Bickelhaupt, F. M., and Baerends, E. J. (2000). “Kohn-Sham density functional theory: predicting and understanding chemistry,” in Rev. Comput. Chem., eds K. B. Lipkowitz and D. B. Boyd (New York, Ny: Wiley-VCH), 1–86. doi: 10.1002/9780470125922.ch1
Bickelhaupt, F. M., Solà, M., and Fernández, I. (2015). Understanding the reactivity of endohedral metallofullerenes: C78 versus Sc3N@C78. Chem. Eur. J. 21, 5760–5768. doi: 10.1002/chem.201500067
Bondybey, V. E. (1971). Matrix Isolation Search for Transient Species. (Thesis). University of California, Berkeley, CA, United States.
Boys, S. F., and Bernardi, F. (1970). Calculation of small molecular interactions by differences of separate total energies—some procedures with reduced errors. Mol. Phys. 19, 553–566. doi: 10.1080/00268977000101561
Cheng, C., and Sheng, L. (2013). Ab-Initio study of Helium-small carbon cage systems. Int. J. Quantum Chem. 113, 35–38. doi: 10.1002/qua.24303
Colburn, E. Q., and Douglas, A. E. (1976). The spectrum and ground state potential curve of Ar2. J. Chem. Phys. 65:1741. doi: 10.1063/1.433319
Computational Chemistry Comparison Benchmark DataBase (2019). NIST Chemistry Webbook. Available online at: https://cccbdb.nist.gov/exp2x.asp?casno=12595594, https://cccbdb.nist.gov/geom2x.asp (accessed June 23, 2020).
Das, P., Saha, R., and Chattaraj, P. K. (2020). Encapsulation of Mg2 inside a C60 cage forms an electride. J. Comput. Chem. 41, 1645–1653. doi: 10.1002/jcc.26207
DiCamillo, B. A., Hettich, R. L., Guiochon, G., Compton, R. N., Saunders, M., Jimenez-Vazquez, H. A., et al. (1996). Enrichment and characterization of a noble gas fullerene: Ar@C60. J. Phys. Chem. 100, 9197–9201. doi: 10.1021/jp960049k
Dolgonos, G. A., and Peslherbe, G. H. (2014). Encapsulation of diatomic molecules in fullerene C60: implications for their main properties. Phys. Chem. Chem. Phys. 16, 26294–26305. doi: 10.1039/C4CP04069D
Fisher, R. A. (1925). Theory of statistical estimation. Proc. Cambridge Philos. Soc. 22, 700–725. doi: 10.1017/S0305004100009580
Foroutan-Nejad, C., Straka, M., Fernández, I., and Frenking, G. (2018). Buckyball difluoride @–a single-molecule crystal. Angew. Chem. Int. Ed. 57, 13931–13934. doi: 10.1002/anie.201809699
Frisch, M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., et al. (2016). Gaussian Inc., Wallingford, CT.
Geerlings, P., Chamorro, E., Chattaraj, P. K., De Proft, F., Gazquez, J. L., Liu, S. B., et al. (2020). Conceptual density functional theory: status, prospects, issues. Theor. Chem. Acc. 139:36. doi: 10.1007/s00214-020-2546-7
Geerlings, P., De Proft, F., and Langenaeker, W. (2003). Conceptual density functional theory. Chem. Rev. 103, 1793–1847. doi: 10.1021/cr990029p
Ghosh, S. K., Berkowitz, M., and Parr, R. G. (1984). Transcription of ground-state density-functional theory into a local thermodynamics. Proc. Natl. Acad. Sci. U. S. A. 81, 8028–8031. doi: 10.1073/pnas.81.24.8028
Glendening, E. D., Landis, C. R., and Weinhold, F. (2012). Natural bond orbital methods. WIREs Comput. Mol. Sci. 2, 1–42. doi: 10.1002/wcms.51
Gómez, S., and Restrepo, A. (2019). Noble gas dimers confined inside C70. Phys. Chem. Chem. Phys. 21, 15815–15822. doi: 10.1039/C9CP03015H
Graham, L., Graudejus, O., Jha, N. K., and Bartlett, N. (2000). Concerning the nature of XePtF6. Coord. Chem. Rev. 197, 321–334. doi: 10.1016/S0010-8545(99)00190-3
Hirshfeld, F. (1977). Bonded-atom fragments for describing molecular charge densities. Theor. Chim. Acc. 44, 129–138. doi: 10.1007/BF00549096
Holloway, J. H., and Hope, E. G. (1999). Recent advances in noble-gas chemistry. Adv. Inorg. Chem. 46, 51–100. doi: 10.1016/S0898-8838(08)60149-X
Jalife, S., Mondal, S., Cabellos, J. L., Pan, S., Méndez-Rojas, M. Á., Fernández, I., et al. (2016). Breaking the isolated pentagon rule by encapsulating Xe2 in C60: the guest defines the shape of the host. Chemistry Select 1, 2405–2408. doi: 10.1002/slct.201600525
Jaroš, A., Badri, Z., Bora, P. L., Bonab, E. F., Marek, R., Straka, M., et al. (2018). How does a container affect acidity of its content: charge-depletion bonding inside fullerenes. Chem. Eur. J. 24, 4245–4249. doi: 10.1002/chem.201706017
Johnson, E. R., Keinan, S., Mori-Sánchez, P., Contreras-García, J., Cohen, A. J., and Yang, W. (2010). Revealing noncovalent interactions. J. Am. Chem. Soc. 132, 6498–6506. doi: 10.1021/ja100936w
Khatua, M., Pan, S., and Chattaraj, P. K. (2014). Movement of Ng2 molecules confined in a C60 cage: an ab initio molecular dynamics study. Chem. Phys. Lett. 610–611, 351–356. doi: 10.1016/j.cplett.2014.06.052
Krapp, A., and Frenking, G. (2007). Is this a chemical bond? A theoretical study of Ng2@C60 (Ng=He, Ne, Ar, Ke, Xe). Chem. Eur. J. 13, 8256–8270. doi: 10.1002/chem.200700467
Kryachko, E. S., Nikolaienko, T., and Yu. (2015). He2@C60: thoughts of the concept of a molecule and of the concept of a bond in quantum chemistry. Int. J. Quantum Chem. 115, 859–867. doi: 10.1002/qua.24916
Kullback, S., and Leibler, R. A. (1951). On information and sufficiency. Ann. Math. Stat. 22, 79–86. doi: 10.1214/aoms/1177729694
Laskin, J., Peres, T., Lifshitz, C., Saunders, M., Cross, R. J., and Khong, A. (1998). An artificial molecule of Ne2 inside C70. Chem. Phys. Lett. 285, 7–9. doi: 10.1016/S0009-2614(97)01473-5
Lee, C., Yang, W., and Parr, R. G. (1988). Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. B 37, 785–789. doi: 10.1103/PhysRevB.37.785
Liu, S. B. (2007). Steric effect: a quantitative description from density functional theory. J. Chem. Phys. 126:244103. doi: 10.1063/1.2747247
Liu, S. B. (2009). Conceptual density functional theory and some recent developments. Acta Phys. Chim. Sin. 25, 590–600. doi: 10.3866/PKU.WHXB20090332
Liu, S. B. (2013). Origin and nature of bond rotation barriers: a unified view. J. Phys. Chem. A 17, 962–965. doi: 10.1021/jp312521z
Liu, S. B. (2016). Information-theoretic approach in density functional reactivity theory. Acta Phys. Chim. Sin. 32, 98–118. doi: 10.3866/PKU.WHXB201510302
Liu, S. B., Rong, C. Y., and Lu, T. (2014). Information conservation principle determines electrophilicity, nucleophilicity, and regioselectivity. J. Phys. Chem. A 118, 3698–3704. doi: 10.1021/jp5032702
Lu, T., and Chen, F. (2012). Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592. doi: 10.1002/jcc.22885
Ma, Y., Ai, Y., Song, X., Wang, C., and Luo, Y. (2014). The equilibrium geometry of A@C60: a test case for conventional density functional theory. Chem. Phys. Lett. 591, 312–316. doi: 10.1016/j.cplett.2013.11.050
Nagy, Á., and Romera, E. (2015). Relative Rényi entropy and fidelity susceptibility. Europhys. Lett. 109:60002. doi: 10.1209/0295-5075/109/60002
Nelson, L. Y., and Pimentel, G. C. (1967). Infrared detection of xenon dichloride. Inorg. Chem. 6, 1758–1759. doi: 10.1021/ic50055a038
Nikolaienko, T., Yu Kryachko, E. S., and Dolgonos, G. A. (2017). On the existence of He–He bond in the endohedral fullerene H?2@C60. J. Comput. Chem. 39, 1090–1102. doi: 10.1002/jcc.25061
Nikolaienko, T. Y., and Kryachko, E. S. (2015). Formation of dimers of light noble atoms under encapsulation within fullerene's voids. Nanoscale Res. Lett. 30:185. doi: 10.1186/s11671-015-0871-x
Osuna, S., Swart, M., and Solà, M. (2009). Reactivity and regioselectivity of noble gas endohedral fullerenes Ng@C60 and Ng2@C60 (Ng=He–Xe). Chem. Eur. J. 15, 13111–13123. doi: 10.1002/chem.200901224
Osuna, S., Swart, M., and Solà, M. (2011). The reactivity of endohedral fullerenes. What can be learnt from computational studies? Phys. Chem. Chem. Phys. 13, 3585–3603. doi: 10.1039/C0CP01594F
Parr, R. G., Donnelly, R. A., Levy, M., and Palke, W. E. (1978). Electronegativity: the density functional viewpoint. J. Chem. Phys. 68, 3801–3807. doi: 10.1063/1.436185
Parr, R. G., and Pearson, R. G. (1983). Absolute hardness: companion parameter to absolute electronegativity. J. Am. Chem. Soc. 105, 7512–7516. doi: 10.1021/ja00364a005
Parr, R. G., Szentpaly, L. V., and Liu, S. B. (1999). Electrophilicity index. J. Am. Chem. Soc. 121, 1922–1924. doi: 10.1021/ja983494x
Parr, R. G., and Yang, W. (1989). Density Functional Theory of Atoms and Molecules. Oxford: Oxford University Press.
Peres, T., Cao, B., Cui, W., Khong, A., Cross, R. J. Jr., Saunders, M., et al. (2001). Some new diatomic molecule containing endohedral fullerenes. Int. J. Mass Spec. 210–211, 241–247. doi: 10.1016/S1387-3806(01)00420-1
Popov, A. A., Yang, S., and Dunsch, L. (2013). Endohedral fullerenes. Chem. Rev. 113, 5989–6113. doi: 10.1021/cr300297r
Rong, C., Wang, B., Zhao, D., and Liu, S. (2019). Information-theoretic approach in density functional theory and its recent applications to chemical problems. WIREs Comput. Mol. Sci. 10:e1461. doi: 10.1002/wcms.1461
Saha, R., Jana, G., Pan, S., Merino, G., and Chattaraj, P. K. (2019). How far can one push the noble gases towards bonding?: a personal account. Molecules 24:2933. doi: 10.3390/molecules24162933
Saunders, M., Cross, R. J., Jiménez-Vázquez, H. A., Shimshi, R., and Khong, A. (1996). Noble gas atoms inside fullerenes. Science 271, 1693–1697. doi: 10.1126/science.271.5256.1693
Saunders, M., Jiménez-Vázquez, H. A., Cross, R. J., and Poreda, R. J. (1993). Stable compounds of helium and neon: He@C60 and Ne@C60. Science 259, 1428–1430. doi: 10.1126/science.259.5100.1428
Shannon, C. E. (1948). A mathematical theory of communication. Bell Syst. Tech. J. 27, 379–423. doi: 10.1002/j.1538-7305.1948.tb01338.x
Simon, S., Duran, M., and Dannenberg, J. J. (1996). How does basis set superposition error change the potential surfaces for hydrogen bonded dimers? J. Chem. Phys. 105, 11024–11031. doi: 10.1063/1.472902
Srivastava, A. K., Pandey, S. K., and Misra, N. (2016). (CH3Br?NH3)@C60: the effect of nanoconfinement on halogen bonding. Chem. Phys. Lett. 662, 240–243. doi: 10.1016/j.cplett.2016.09.036
Stein, L. (1973). Removal of xenon and radon from contaminated atmospheres with dioxygenyl hexafluoroantimonate, O2SbF6. Nature 243, 30–32. doi: 10.1038/243030a0
Syamala, M. S., Cross, R. J., and Saunders, M. (2002). 129Xe NMR spectrum of Xenon inside C60. J. Am. Chem. Soc. 124, 6216–6219. doi: 10.1021/ja012676f
Te Velde, G. T., Bickelhaupt, F. M., Baerends, E. J., Fonseca Guerra, C., van Gisbergen, S. J., Snijders, J. G., et al. (2001). Chemistry with ADF. J. Comput. Chem. 22, 931–967. doi: 10.1002/jcc.1056
Turner, J. J., and Pimentel, G. C. (1963). Krypton fluoride: preparation by the matrix isolation technique. Science 140, 974–975. doi: 10.1126/science.140.3570.974-a
Wang, B., Rong, C. Y., Chattaraj, P. K., and Liu, S. B. (2019). A comparative study to predict regioselectivity, electrophilicity and nucleophilicity with Fukui function and Hirshfeld charge. Theor. Chem. Acc. 138:124. doi: 10.1007/s00214-019-2515-1
Yamamoto, K., Saunders, M., Khong, A., Cross, R. J., Grayson, M., Gross, M. L., et al. (1999). Isolation and spectral properties of Kr@C60, a stable van der Waals molecule. J. Am. Chem. Soc. 121, 1591–1596. doi: 10.1021/ja9831498
Yang, W., Parr, R. G., and Pucci, R. (1984). Electron density, Kohn–Sham frontier orbitals, and Fukui functions. J. Chem. Phys. 81, 2862–2863. doi: 10.1063/1.447964
Zhao, Y., and Truhlar, D. G. (2008). The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 120, 215–241. doi: 10.1007/s00214-007-0310-x
Keywords: noble gas dimer, fullerene, density functional theory, information-theoretic approach, encapsulation
Citation: Li M, He X, Wang B, Zhao D, Rong C, Chattaraj PK and Liu S (2020) Changes in Structure and Reactivity of Ng2 Encapsulated in Fullerenes: A Density Functional Theory Study. Front. Chem. 8:566. doi: 10.3389/fchem.2020.00566
Received: 24 February 2020; Accepted: 02 June 2020;
Published: 03 July 2020.
Edited by:
Yong Wang, Ningbo University, ChinaReviewed by:
Cina Foroutan-Nejad, Masaryk University, CzechiaCopyright © 2020 Li, He, Wang, Zhao, Rong, Chattaraj and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunying Rong, cm9uZ2NodW55aW5nQGFsaXl1bi5jb20=; Shubin Liu, c2h1YmluQGVtYWlsLnVuYy5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.