- 1Food and Pharmaceutical Engineering Institute, Guiyang University, Guiyang, China
- 2Center for Research and Development of Fine Chemicals, School of Pharmaceutical Sciences, Entomology of Institute, Guizhou University, Guiyang, China
- 3Material and Chemistry Engineering Institute, Tongren College, Tongren, China
- 4School of Chemical Engineering, Guizhou Institute of Technology, Guiyang, China
In this study, thirteen new pyridylpyrazolamide derivatives containing pyrimidine motifs were synthesized via six-step reactions. Bioassay results showed that some of the synthesized compounds revealed good antifungal properties against Sclerotinia sclerotiorum, Phytophthora infestans, Thanatephorus cucumeris, Gibberella zeae, Fusarium oxysporum, Cytospora mandshurica, Botryosphaeria dothidea, and Phompsis sp. at 50 μg/mL, which were similar to those of Kresoxim-methyl or Pyrimethanil. Meanwhile, bioassay results indicated that the synthesized compounds showed a certain insecticidal activity against Spodoptera litura, Mythimna separata, Pyrausta nubilalis, Tetranychus urticae, Rhopalosiphum maidis, and Nilaparvata lugens at 200 μg/mL, which was lower than that of Chlorantraniliprole. To the best of our knowledge, this study is the first report on the antifungal and insecticidal activities of pyridylpyrazol amide derivatives containing a pyrimidine moiety.
Introduction
Plant fungal and insect diseases have posed serious threats to crops in the world and caused a severe loss throughout the world (Strange and Scott, 2005; Yang et al., 2015). Nowadays, some of the available traditional fungicides and insecticides, such as Kresoxim-methyl, Pyrimethanil, Chlorantraniliprole, etc., are widely used to prevent plant harmful fungal and insect diseases. However, prolonged use of traditional pesticides can not only lead to drug resistance, but also have a harmful influence on the safety of the plants and the environment. Therefore, the development of novel and promising fungicides and insecticides is still an urgent task.
Pyrimidine derivatives, which play an important role in synthesis of various active molecules, have versatile properties in modern life, such as antifungal (Chen et al., 2008; Zhang et al., 2016), antibacterial (Triloknadh et al., 2018; Fang et al., 2019), insecticidal (Liu et al., 2017; Shen et al., 2018), herbicidal (Chen et al., 2015; Li et al., 2018), and antiviral (Xu et al., 2015; Wang Y. Y. et al., 2018) activities. In the previous work, some of the pyrimidine derivatives (for example, Mepanipyrim, Pyrimethanil, Diflumetorim, Azoxystrobin, and so on), which were known for their abilities to control severe fungal diseases, have been marketed as commercial pesticides worldwide. Meanwhile, in our preliminary work, several series of pyrimidine derivatives containing 1,3,4-oxadiazole (Figures 1A,E), 1,3,4-thiadiazole (Figures 1B,E), 1,2,4-triazole (Figure 1C) or amide (Figure 1D) moiety, as shown in Figure 1, were reported and revealed better antiviral, antifungal, and antibacterial activities (Wu et al., 2015, 2016a,b, 2019a,b).
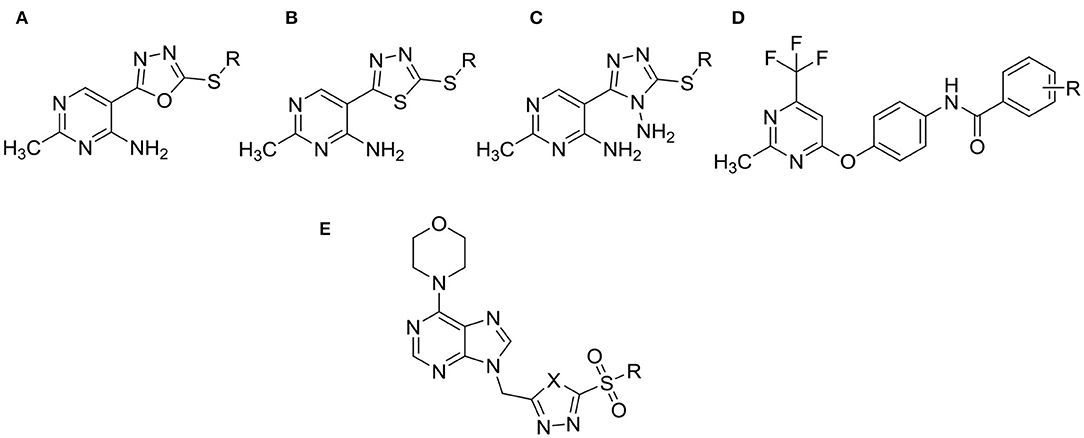
Figure 1. The structures of the pyrimidine derivatives containing 1,3,4-oxadiazole (A,E), 1,3,4-thiadiazole (B,E), 1,2,4-triazole (C) or amide (D) moiety reported by our research group.
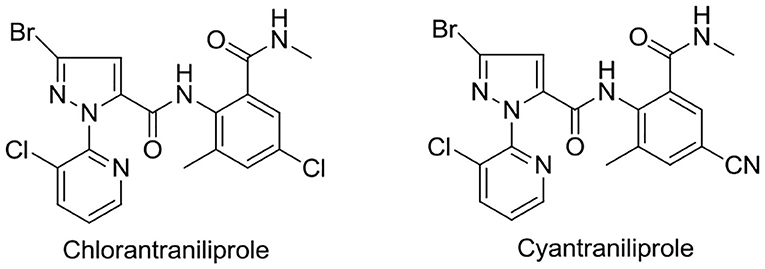
In recent years, pyridylpyrazole amide derivatives have attracted more and more considerable attention owing to their broad class of biological activities in pesticide chemistry, such as antifungal (Yan et al., 2012; Wang B. L. et al., 2018) and insecticidal (Wang et al., 2013, 2019; Shi et al., 2017; Wang B. L. et al., 2018) activity. In the past few years, some representative examples of pyridylpyrazole amide derivatives (for example, chlorantraniliprole and cyantraniliprole) were commercialized as pesticides, as shown in Figure 2.
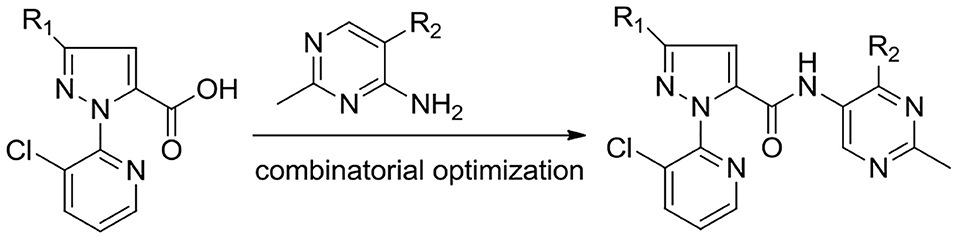
To develop effective pesticide agents, we aim to introduce the pyrimidine ring to the pyridiylpyrazol amide skeleton to design a series of novel pyridiylpyrazol amide derivatives containing a pyrimidine moiety (Figure 3). As far as we know, it is the first report on the antifungal and insecticidal activities of pyridylpyrazol amide derivatives containing a pyrimidine moiety.
Experimental and Methods
General Information
JEOL-ECX 500 NMR spectrometer (JEOL, Tokyo, Japan) was used to analyse the NMR spectral (1H NMR and 13C NMR) at room temperature using TMS as an internal standard and DMSO-d6 as the solvent. Elemental analysis was performed on the Elementar Vario-III CHN analyser (Elementar, Hanau, Germany). Mass spectral were conducted on the Agilent 5973 organic mass spectrometer (Agilent Technologies, Palo Alto, CA, USA). Melting points were determined on the XT-4 binocular microscope (Beijing Tech Instrument Co., China). All commercial reagents and solvents were used as they did not require any purification before use.
Synthesis
Preparation Procedure of the Key Intermediate 5
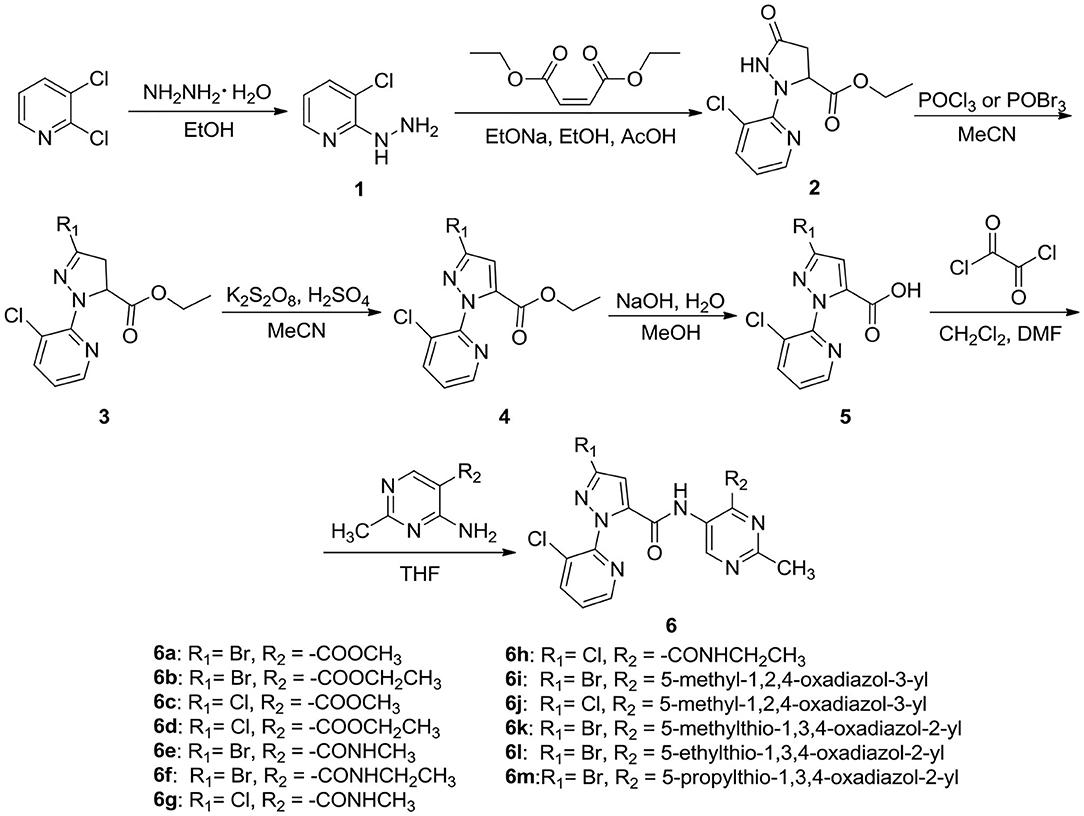
The synthetic procedure for the key intermediate 5 is shown in Scheme 1. To a mixture of 2,3-dichloropyridine (0.1 mol) dissolved in anhydrous ethanol (120 mL), 80% hydrazine hydrate (80 mL) was added dropwise and reacted under reflux. Upon completion of the reaction, the reaction solution was cooled to room temperature and the solvent was removed under reduced pressure. The residue was washed with water and recrystallized with ethanol to gain intermediate 1. Intermediate 1 (90 mmol) and diethyl maleate (90 mmol) were added to the mixture of sodium ethoxide (90 mmol) and ethanol (100 mL), then a moderate amount of glacial acetic acid was added when the temperature was below 60°C. Upon completion of the reaction, the reaction solution was poured into 100 mL distilled water to precipitate the solid, then the solid was recrystallized with ethanol to obtain intermediate 2. Then, a mixture of intermediate 2 (45 mmol), phosphorus oxychloride (POCl3, 50 mmol) or phosphorus oxybromide (POBr3, 50 mmol), and acetonitrile (CH3CN, 50 mL) was reacted under reflux. After ending the reaction, the reaction mixture was poured into 30 mL distilled water, extracted with dichloromethane (CH2Cl2), and dried with anhydrous sodium sulfate (Na2SO4) to give intermediate 3 (Wang B. L. et al., 2018). After that, a mixture of intermediate 3 (40 mmol), potassium persulfate (K2S2O8, 44 mmol), and CH3CN (50 mL) reacted under reflux. Upon completion of the reaction, the reaction mixture was poured into 100 mL of distilled water. The residue was washed with water and recrystallized with ethanol to obtain intermediate 4 (Wang B. L. et al., 2018). Finally, sodium hydroxide (NaOH, 30 mmol) dissolved in 10 mL of water was added to the mixture of intermediate 4 and methanol (20 mL) and reacted under reflux. When the reaction was completed, the reaction mixture was poured into 100 mL of distilled water and acidified the mixture to pH 5–6 using concentrated hydrochloric acid. The key intermediate 5 was attained after recrystallization with ethanol. 1H NMR spectral data for intermediates 1–5 are reported in the Supplementary Data.
General Procedure for the Preparation of the Target Compounds 6a−6m
As shown in Scheme 1, oxalyl chloride (2 mL) and 4 drops of N, N-dimethylformamide (DMF) were added to a mixture of intermediate 5 dissolved in 10 mL CH2Cl2, and reacted at room temperature. When the reaction was completed, the CH2Cl2 and excess oxaloyl chloride were removed under reduced pressure. Then, 5-substituted-4-amino-2-methylpyrimidine was added to the residue dissolved in 5 mL tetrahydrofuran (THF) and reacted under reflux. At the end of the reaction, the reaction mixture was poured into 20 mL of water, extracted with ethyl acetate, and recrystallized with ethanol to obtain the title compounds 6a–6m.
Methyl4-(3-bromo-1-(3-chloropyridin-2-yl)-1H-pyrazole-5-carboxamido)-2-methyl-pyrimidine-5-carboxylate (6a). White solid; yield 45.1%; m.p. 220–222°C; 1H NMR (DMSO-d6, 500 MHz) δ: 11.85 (s, 1H, CONH), 8.84 (s, 1H, Pyrimidine-H), 8.52 (d, 1H, J = 4.6 Hz, Pyridine-H), 8.23 (d, 1H, J = 6.9 Hz, pyridine-H), 7.67 (dd, 1H, J1 = 4.55 Hz, J2 = 8.0 Hz,pyridine-H), 7.58 (s, 1H, Pyrazole-H), 3.61 (s, 3H), 2.62 (s, 3H);13C NMR (DMSO-d6, 125 MHz) δ: 170.51, 165.33, 159.38, 156.62, 154.98, 148.73, 147.82, 140.40, 140.06, 138.27,1 128.50, 127.55, 115.07, 109.32, 52.83, 26.00; MS (ESI) m/z: 451.1 ([M+H]+); Anal. Calcd. for C16H12BrClN6O3: C 42.55, H 2.68, N 18.61; found: C 42.60, H 2.70, N 18.66.
Ethyl4-(3-bromo-1-(3-chloropyridin-2-yl)-1H-pyrazole-5-carboxamido)-2-methyl-pyrimidine-5-carboxylate (6b). White crystals; yield 50.4%; m.p. 176–177°C; 1H NMR (DMSO-d6, 500 MHz) δ: 11.88 (s, 1H, CONH), 8.84 (s, 1H, Pyrimidine-H), 8.51 (d, 1H, J = 4.0 Hz, Pyridine-H), 8.24 (d, 1H, J = 8.0 Hz, pyridine-H), 7.66 (dd, 1H, J1 = 4.55 Hz, J2 = 8.0 Hz, pyridine-H), 7.59 (s, 1H, Pyrazole-H), 4.09 (q, 2H, J = 6.9 Hz), 2.64 (s, 3H), 1.08 (t, 3H, J = 7.45 Hz); 13C NMR (DMSO-d6, 125 MHz) δ: 170.51, 164.80, 159.38, 156.64, 148.73, 147.80, 140.33, 140.07, 138.27, 128.46, 127.52, 115.38, 109.34, 61.68, 25.97, 14.32; MS (ESI) m/z: 465.1 ([M+H]+); Anal. Calcd. for C17H14BrClN6O3: C 43.85, H 3.03, N 18.05; found: C 43.90, H 3.00, N 18.06.
Methyl4-(3-chloro-1-(3-chloropyridin-2-yl)-1H-pyrazole-5-carboxamido)-2-methyl-pyrimidine-5-carboxylate (6c). White solid; yield 48.2%; m.p. 213–215°C; 1H NMR (DMSO-d6, 500 MHz) δ: 11.87 (s, 1H, CONH), 8.85 (s, 1H, Pyrimidine-H), 8.53 (d, 1H, J = 2.85 Hz, Pyridine-H), 8.25 (d, 1H, J = 7.65 Hz, pyridine-H), 7.68 (dd, 1H, J1 = 2.85 Hz, J2 = 8.0 Hz, pyridine-H), 7.55 (s, 1H, Pyrazole-H), 3.62 (s, 3H), 2.64 (s, 3H); 13C NMR (DMSO-d6, 125 MHz) δ: 170.54, 165.32, 159.41, 156.62, 155.01, 148.71, 147.85, 140.48, 140.01, 138.30, 128.51, 127.54, 115.05, 109.30, 52.82, 26.01; MS (ESI) m/z: 407.1 ([M+H]+); Anal. Calcd. for C16H12Cl2N6O3: C 47.19, H 2.97, N 20.64; found: C 47.20, H 3.00, N 20.62.
Ethyl4-(3-chloro-1-(3-chloropyridin-2-yl)-1H-pyrazole-5-carboxamido)-2-methyl-pyrimidine-5-carboxylate (6d). Yellow crystals; yield 38.5%; m.p. 191–192°C; 1H NMR (DMSO-d6, 500 MHz) δ: 11.88 (s, 1H, CONH), 8.84 (s, 1H, Pyrimidine-H), 8.51 (d, 1H, J = 2.85Hz, Pyridine-H), 8.23 (d, 1H, J = 7.65 Hz, pyridine-H), 7.66 (dd, 1H, J1 = 2.85 Hz, J2 = 8.00 Hz, pyridine-H), 7.53 (s, 1H, Pyrazole-H), 4.06 (q, 2H, J = 6.90 Hz), 2.64 (s, 3H), 1.08 (t, 3H, J = 6.85 Hz); 13C NMR (DMSO-d6, 125 MHz) δ: 170.52, 164.80, 159.38, 156.64, 148.73, 147.80, 140.33, 140.07, 138.27, 128.46, 127.52, 115.38, 109.34,61.68, 25.97,14.32; MS (ESI) m/z: 422.1 ([M+H]+); Anal. Calcd. for C17H14Cl2N6O3: C 48.47, H 3.35, N 19.95; found: C 48.50, H 3.40, N 20.02.
4-(3-Bromo-1-(3-chloropyridin-2-yl)-1H-pyrazole-5-carboxamido)-N-2-dimethylpyrimidine-5-carboxamide (6e). White solid; yield 52.6%; m.p. 228–233°C; 1H NMR (DMSO-d6, 500 MHz) δ: 12.33 (s, 1H, CONH), 8.78 (s, 1H, Pyrimidine-H), 8.74 (d, 1H, J = 4.55 Hz, CONH), 8.48 (d, 1H, J = 4.00 Hz, Pyridine-H), 8.19 (d, 1H, J = 8.05Hz, Pyridine-H), 7.62 (dd, 1H, J1 = 4.60 Hz, J2 = 8.00 Hz, Pyridine-H), 7.32 (s, 1H, Pyrazole-H), 2.67 (d, 3H, J = 4.6 Hz), 2.45 (s, 3H); 13C NMR (DMSO-d6, 125 MHz)δ: 169.38, 165.98, 157.28, 156.02, 154.80, 148.58, 147.82, 140.09, 139.38, 128.40, 127.68, 127.48, 114.27, 111.72, 26.67, 26.07; MS (ESI) m/z: 450.1 ([M+H]+); Anal. Calcd. for C16H13BrClN7O2: C 42.64, H 2.91, N 21.76; found: C 42.66, H 2.90, N 21.77.
4-(3-Bromo-1-(3-chloropyridin-2-yl)-1H-pyrazole-5-carboxamido)-N-ethyl-2-methylpyrimidine-5-carboxamide (6f). White solid; yield 60.1%; m.p. 236–237°C; 1H NMR (DMSO-d6, 500 MHz) δ: 12.33 (s, 1H, CONH), 8.78 (s, 1H, Pyrimidine-H), 8.72 (s, 1H, CONH), 8.48 (d, 1H, J = 4.00 Hz, Pyridine-H), 8.19 (d, 1H, J = 8.05 Hz, Pyridine-H), 7.62 (dd, 1H, J1 = 4.60 Hz, J2 = 8.00 Hz, Pyridine-H), 7.32 (s, 1H, Pyrazole-H), 2.84 (q, 2H, J = 6.85 Hz), 2.45 (s, 3H), 1.04 (t, 3H, J = 6.25 Hz); 13C NMR (DMSO-d6, 125 MHz)δ: 169.35, 166.00, 157.31, 156.04, 154.78, 148.60, 147.81, 140.11, 139.35, 128.42, 127.65, 127.49, 114.27, 111.76, 26.66, 26.03, 15.32; MS (ESI) m/z: 464.1 ([M+H]+); Anal. Calcd. for C17H15BrClN7O2: C 42.64, H 2.91, N 21.76; found: C 42.66, H 2.90, N 21.77.
4-(3-Chloro-1-(3-chloropyridin-2-yl)-1H-pyrazole-5-carboxamido)-N,2-dimethylpyrimidine-5-carboxamide (6g). White solid; yield 45.9%; m.p. 213–215°C; 1H NMR (DMSO-d6, 500 MHz) δ: 11.87 (s, 1H, CONH), 8.85 (s, 1H, Pyrimidine-H), 8.71 (s, 1H, CONH), 8.53 (d, 1H, J= 2.85 Hz, Pyridine-H), 8.25 (d, 1H, J = 7.65 Hz, Pyridine-H), 7.68 (dd, 1H, J1 = 2.85 Hz, J2 = 8.0 Hz, Pyridine-H), 7.55 (s, 1H, Pyrazole-H), 2.85 (s, 3H), 2.64 (s, 3H); 13C NMR (DMSO-d6, 125 MHz) δ: 170.51, 165.33, 159.38, 156.62, 154.98, 148.73, 147.82, 140.40, 140.06, 138.27, 128.50, 127.55, 115.07, 109.32, 52.83, 26.62, 26.04; MS (ESI) m/z: 406.1 ([M+H]+); Anal. Calcd. for C16H13Cl2N7O2: C 47.31, H 3.23, N 24.14; found: C 47.32, H 3.20, N 24.12.
4-(3-Chloro-1-(3-chloropyridin-2-yl)-1H-pyrazole-5-carboxamido)-N-ethyl-2-methylpyrimidine-5-carboxamide (6h). Yellow crystals; yield 47.8%; m.p. 191–192°C; 1H NMR (DMSO-d6, 500 MHz) δ: 11.88 (s, 1H, CONH), 8.84 (s, 1H, Pyrimidine-H), 8.71 (s, 1H, CONH), 8.51 (d, 1H, J = 2.85 Hz, Pyridine-H), 8.23 (d, 1H, J = 7.65 Hz, pyridine-H), 7.66 (dd, 1H, J1 = 2.85 Hz, J2 = 8.00 Hz, pyridine-H), 7.53 (s, 1H, Pyrazole-H), 4.06 (q, 2H, J = 6.90 Hz), 2.64 (s, 3H), 1.08 (t, 3H, J = 6.85 Hz); 13C NMR (DMSO-d6, 125 MHz) δ: 170.52, 164.89, 159.38, 156.64, 148.73, 147.80, 140.33, 140.07, 138.27, 128.46, 127.52, 115.38, 109.34, 61.68, 25.97,15.32; MS (ESI) m/z: 420.1 ([M+H]+); Anal. Calcd. for C17H15Cl2N7O2: C 48.59, H 3.60, N 23.33; found: C 48.61, H 3.62, N 23.30.
3-Bromo-1-(3-chloropyridin-2-yl)-N-(2-methyl-5-(5-methyl-1,2,4-oxadiazol-3-yl)pyrimidin-4-yl)-1H-pyrazole-5-carboxamide (6i). Yellow crystals; yield 36.5%; m.p. 180–181°C; 1H NMR (DMSO-d6, 500 MHz) δ: 11.64 (s, 1H, CONH), 8.99 (s, 1H, Pyrimidine-H), 8.49 (d, 1H, J = 4.55 Hz, Pyridine-H), 8.18 (d, 1H, J = 8.05 Hz, Pyridine-H), 7.63 (dd, 1H, J1 = 4.60 Hz, J2 = 8.0 Hz, Pyridine-H), 7.54 (s, 1H, Pyrazole-H), 2.67 (s, 3H), 2.61 (s, 3H); 13C NMR (DMSO-d6, 125 MHz)δ: 177.49, 169.89, 164.89, 158.92, 155.80, 155.08, 148.68, 147.69, 139.94, 138.68, 128.48, 127.52, 127.41, 112.34, 111.50, 25.96, 12.43; MS (ESI) m/z: 475.1 ([M+H]+); Anal. Calcd. for C17H12BrClN8O2: C 42.92, H 2.54, N 23.56; found: C 42.91, H 2.50, N 23.60.
3-Chloro-1-(3-chloropyridin-2-yl)-N-(2-methyl-5-(5-methyl-1,2,4-oxadiazol-3-yl)pyrimidin-4-yl)-1H-pyrazole-5-carboxamide (6j). Yellow crystals; yield 41.3%; m.p. 151–152°C; 1H NMR (DMSO-d6, 500 MHz) δ: 11.64 (s, 1H, CONH), 8.99 (s, 1H, Pyrimidine-H), 8.49 (d, 1H, J = 4.55 Hz, Pyridine-H), 8.18 (d, 1H, J = 8.05 Hz, Pyridine-H), 7.63 (dd, 1H, J1 = 4.60 Hz, J2 = 8.0 Hz, Pyridine-H), 7.54 (s, 1H, Pyrazole-H), 2.67 (s, 3H), 2.61 (s, 3H); 13C NMR (DMSO-d6, 125 MHz)δ: 177.52, 169.87, 164.92, 158.94, 155.85, 155.11, 148.65, 147.72, 139.99, 138.71, 128.50, 127.53, 127.45, 112.36, 111.32, 26.02, 12.45; MS (ESI) m/z: 431.1 ([M+H]+); Anal. Calcd. for C17H12BrClN8O2: C 47.35, H 2.80, N 25.98; found: 47.37, H 2.77, N 25.99.
3-Bromo-1-(3-chloropyridin-2-yl)-N-(2-methyl-5-(5-(methylthio)-1,3,4-oxadiazol-2-yl)pyrimidin-4-yl)-1H-pyrazole-5-carboxamide (6k). Yellow crystals; yield 46.8%; m.p. 166–168°C; 1H NMR (DMSO-d6, 500 MHz) δ: 11.84 (s, 1H, CONH), 9.04 (s, 1H, Pyrimidine-H), 8.46 (d, 1H, J = 5.15 Hz, Pyridine-H), 8.18 (d, 1H, J = 8.0 Hz, Pyridine-H), 7.62 (dd, 1H, J1 = 4.55 Hz, J2 = 7.7 Hz, Pyridine-H), 7.58 (s, 1H, Pyrazole-H), 2.72 (s, 3H), 2.66 (s, 3H); 13C NMR (DMSO-d6, 125 MHz) δ: 170.30, 164.72, 162.00, 158.79, 156.05, 148.51, 148.10, 147.81, 140.01, 138.54, 128.35, 127.60, 127.42, 112.61, 108.46, 26.10, 15.62; MS (ESI) m/z: 507.1 ([M+H]+); Anal. Calcd. for C17H12BrClN8O2S: C 40.21, H 2.38, N22.07; found: C 40.24, H 2.40, N22.05.
3-Bromo-1-(3-chloropyridin-2-yl)-N-(5-(5-(ethylthio)-1,3,4-oxadiazol-2-yl)-2-methylpyrimidin-4-yl)-1H-pyrazole-5-carboxamide (6l). Yellow crystals; yield 42.5%; m.p. 176–177°C; 1H NMR (DMSO-d6, 500 MHz) δ: 11.84 (s, 1H, CONH), 9.04 (s, 1H, Pyrimidine-H), 8.46 (d, 1H, J = 5.15 Hz, Pyridine-H), 8.18 (d, 1H, J = 8.0 Hz, Pyridine-H), 7.62 (dd, 1H, J1 = 4.55 Hz, J2 = 7.7 Hz, Pyridine-H), 7.58 (s, 1H, Pyrazole-H), 3.12 (q, 2H, J = 6.9 Hz), 2.66 (s, 3H), 1.35 (t, 3H, J = 7.45 Hz); 13C NMR (DMSO-d6, 125 MHz) δ: 170.25, 164.74, 161.95, 158.75, 156.08, 148.25, 148.12, 147.69, 140.07, 138.55, 128.27, 127.60, 127.44, 112.63, 108.56, 27.86, 26.10, 14.23; MS (ESI) m/z: 521.1 ([M+H]+); Anal. Calcd. for C18H14BrClN8O2S: C 41.43, H 2.70, N 21.48; found: C 41.45, H 2.74, N 21.49.
3-Bromo-1-(3-chloropyridin-2-yl)-N-(2-methyl-5-(5-(propylthio)-1,3,4-oxadiazol-2-yl)pyrimidin-4-yl)-1H-pyrazole-5-carboxamide (6m). Yellow crystals; yield 39.8%; m.p. 197–199°C; 1H NMR (DMSO-d6, 500 MHz) δ: 11.84 (s, 1H, CONH), 9.04 (s, 1H, Pyrimidine-H), 8.46 (d, 1H, J=5.15 Hz, Pyridine-H), 8.18 (d, 1H, J = 8.0 Hz, Pyridine-H), 7.62 (dd, 1H, J1 = 4.55 Hz, J2 = 7.7 Hz, Pyridine-H), 7.58 (s, 1H, Pyrazole-H), 3.15 (t, 2H, J = 6.9 Hz), 2.66 (s, 3H), 1.68 (m, 2H), 0.95 (t, 3H, J = 7.45 Hz); 13C NMR (DMSO-d6, 125 MHz)δ: 170.31, 164.70, 162.03, 158.75, 156.02, 148.48, 148.07, 147.77, 140.04, 138.50, 128.32, 127.60, 127.44, 112.67, 108.49, 34.43, 26.10, 22.92, 13.33; MS (ESI) m/z: 535.1 ([M+H]+); Anal. Calcd. for C19H16BrClN8O2S: C 42.59, H 3.01, N 20.91; found: C 42.53, H 3.00, N 20.92.
Antifungal Biological Assay
The antifungal activities of the title compounds 6a–6m against Sclerotinia sclerotiorum (S. sclerotiorum), Phytophthora infestans (P. infestans), Thanatephorus cucumeris (T. cucumeris), Gibberella zeae (G. zeae), Fusarium oxysporum (F. oxysporum), Cytospora mandshurica (C. mandshurica), Botryosphaeria dothidea (B. dothidea), and Phompsis sp. were evaluated at the concentration of 50 μg/mL (Min et al., 2016; Wu et al., 2019a,b). The target compounds 6a–6m (5 mg) were dissolved in dimethyl sulfoxide (1 mL) and sterile water (9 mL) before mixing with 90 mL potato dextrose agar (PDA) to generate a final concentration of 50 μg/mL. Then, 4 mm diameter of the mycelia dishes were cut from a culture medium of pathogenic fungi, then inoculated in the middle of PDA and cultivated at 27 ± 1°C for 4–5 days. DMSO in sterile distilled water served as a negative control, while Kresoxim-methyl and Pyrimethanil acted as positive controls. For each treatment, three replicates were conducted. The radial growth of the fungal colonies was measured and the data were statistically analyzed. The inhibition rate I (%) of the test compounds against eight pathogenic fungi were calculated by the following formula, where C represents the diameter of fungi growth on untreated PDA, and T represents the diameter of fungi on treated PDA.
Insecticidal Biological Assay
The insecticidal activities of all synthesized compounds 6a–6m against Spodoptera litura (S. litura), Mythimna separata (M. separata), Pyrausta nubilalis (P. nubilalis), Tetranychus urticae (T. urticae), Rhopalosiphum maidis (R. maidis), and Nilaparvata lugens (N. lugens) were performed according to the reported method (Wang B. L. et al., 2018; Wang et al., 2019). The target compounds 6a–6m were dissolved in NP-10 (0.1 mg/L) solution to generate a final concentration of 200 μg/mL. Then, 15 maize leaves (approximately 5 cm in length) and 5 sweet potato leaves (diameter 3 cm) were dipped in the test compounds solutions for 10 s, dried and placed into a tumbler. After that, 30 larvae of second-instar S. litura, M. separata, P. nubilalis, T. urticae, R. maidis, and N. lugens were transferred to the petri dish. Chlorantraniliprole was used as a control. All bioassays were performed in the laboratory at 27 ± 1°C for 48 h. Three replicates were performed for each treatment. The percentage of mortalities for the target compounds were determined using Abbott's formula.
Results and Discussion
In this study, using 2,3-dichloropyridine as the starting material, the title compounds 6a–6m were synthesized in six steps, including hydrazidation, cyclization, bromination or chlorination, oxidation, hydrolyzation, and condensation. The target compounds structures were confirmed by 1H NMR, 13C NMR, MS, and elemental analysis. In the 1H NMR spectra of compound 6a, a singlet at 2.45 ppm assigned to CH3 protons of pyrimidine-CH3, the doublet signal at 2.67 ppm indicated the presence of CH3 proton in CONH-CH3, meanwhile, a singlet at 8.78 and 7.32 ppm indicated the presence of pyrimidine and pyrazole ring. Two proton signals of two -CONH- in amide moiety was observed at 12.33 and 8.74 ppm. The structure of 6b was also confirmed by its mass spectral data. In its mass spectrum, the molecular ion peak was noticed m/z at 450.1 ([M+H]+) corresponding to its molecular weight.
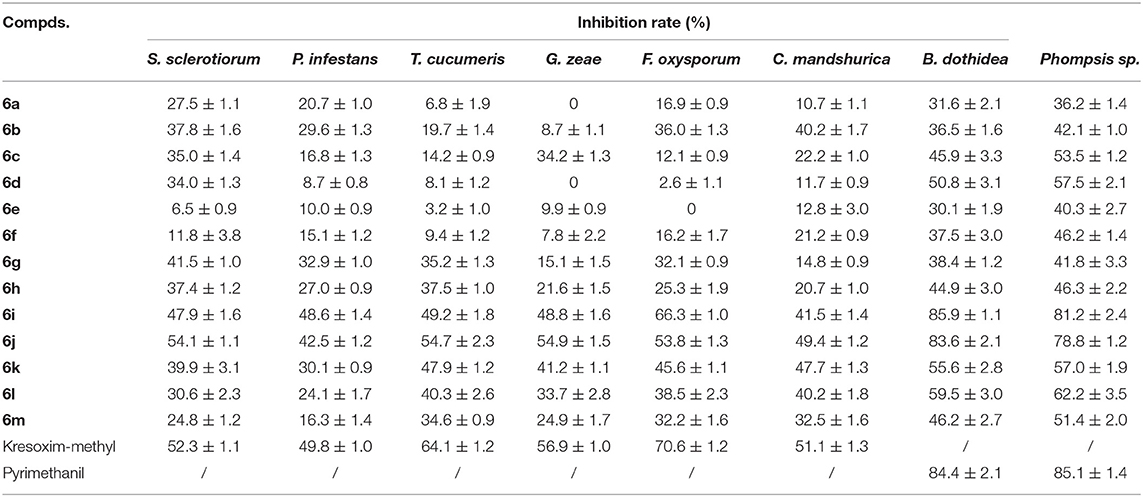
The in vitro antifungal activities at 50 μg/mL of the target compounds against eight plant fungi are listed in Table 1. Table 1 showed that, at 50 μg/mL, compounds 6a–6m indicated certain antifungal activities against S. sclerotiorum, P. infestans, T. cucumeris, G. zeae, F. oxysporum, C. mandshurica, B. dothidea, and Phompsis sp. with the inhibition rates of 6.5–54.1%, 8.7–48.6%, 3.2–54.7%, 0–54.9%, 0–66.3%, 10.7–49.4%, 30.1–85.9%, and 36.2–81.2%, respectively. Among the title compounds, compound 6i revealed good in vitro antifungal activities against P. infestans and B. dothidea, with the inhibition rates of 48.6% and 85.9%, respectively, which were equally to those of Kresoxim-methyl or Pyrimethanil. Meanwhile, compound 6j revealed good in vitro antifungal activities against S. sclerotiorum, G. zeae, C. mandshurica, and B. dothidea, with the inhibition rates of 54.1, 54.9, 49.4, and 85.9%, respectively, which were similar with those of Kresoxim-methyl or Pyrimethanil.
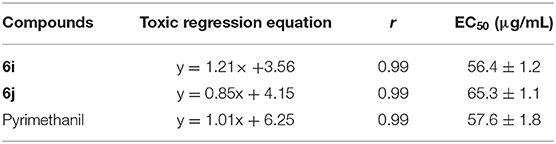
Based on the preliminary antifungal bioassays, the EC50 values of compounds 6i and 6j were also tested and presented in Table 2. Table 2 showed that compounds 6i and 6j showed good activities against B. dothidea, with EC50 values of 56.4 and 65.3 μg/mL, respectively, which were similar to that of Pyrimethanil (57.6 μg/mL).
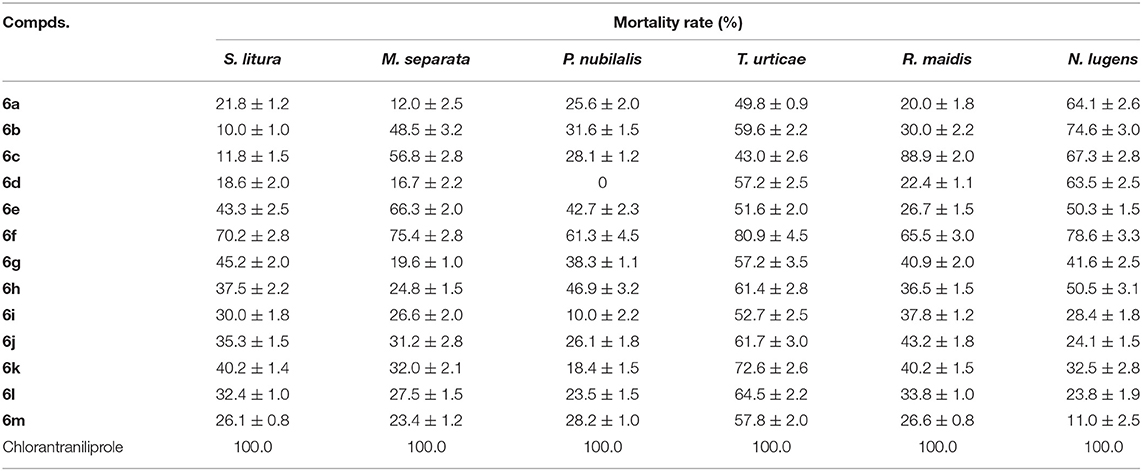
The in vitro insecticidal properties of title compounds against S. litura, M. separata, P. nubilalis, T. urticae, R. maidis, and N. lugens were evaluated and the insecticidal bioassay results are listed in Table 3. Table 3 showed that the target compounds 6a–6m indicated certain insecticidal activities against S. litura, M. separata, P. nubilalis, T. urticae, R. maidis, and N. lugens at 200 μg/mL, with the mortality rates of 10.0–70.2%, 12.0–75.4%, 0–61.3%, 43.0–80.9%, 20.0–88.9%, and 11.0–78.6%, respectively, which were lower than those of Chlorantraniliprole. Especially, compound 6f revealed good insecticidal activities against S. litura, M. separata, P. nubilalis, T. urticae, and N. lugens with mortality rates of 70.2, 75.4, 61.3, 80.9, and 78.6%, respectively. Meanwhile, compound 6c had a good mortality rate of 88.9% against R. maidis.
Conclusions
In summary, thirteen novel pyridylpyrazolamide derivatives containing pyrimidine motifs were synthesized, and their structures confirmed by 1H NMR, 13C NMR, MS and elemental analysis. Bioassay results showed that some of title compounds revealed good antifungal and insecticidal properties. The results provided strategy for leading the synthesis of novel pyridylpyrazolamide derivatives containing pyrimidine motifs.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
WW and QF contributed to the synthesis, purification, characterization of all compounds, and prepared the original manuscript. YZ and YG performed the activity research. MC and HC perfected the language and assisted with the structure elucidation and manuscript revision. GO and MY designed and supervised the research and revised the manuscript. All authors discussed, edited, and approved the final version.
Funding
This research was financially supported by China Postdoctoral Science Foundation (No. 2017M623070), the National Natural Science Foundation of China (No. 31701821 and 21762037), the Opening Foundation of the Key Laboratory of Green Pesticide and Agricultural Bioengineering, Ministry of Education, Guizhou University (No. 2018GDGP0102), the Guizhou Province Biological and Pharmaceutical engineering Research Centerand (No. QJHKY[2019]051), and the special Funding of Guiyang Science and Technology Bureau and Guiyang University [GYU-KYZ(2019~2020)PT10-06].
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a shared affiliation, though no other collaboration, with the authors YZ, MY, and GO.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2020.00522/full#supplementary-material
References
Chen, Q., Zhu, X. L., Jiang, L. L., Liu, Z. M., and Yang, G. F. (2008). Synthesis, antifungal activity and CoMFA analysis of novel1,2,4-triazolo[1,5-a]pyrimidine derivatives. Eur. J. Med. Chem. 43, 595–603. doi: 10.1016/j.ejmech.2007.04.021
Chen, X. M., Wang, S. H., and Cui, D. L. (2015). The synthesis and herbicidal activity of 5-(substituted-phenyl)-4,6-dioxo-4,5,6,7-tetrahydropyrazolo(3,4-d)pyrimidines. J. Heterocyclic Chem. 52, 607–610. doi: 10.1002/jhet.2047
Fang, Z., Zheng, S., Chan, K. F., Yuan, W., Guo, Q., Wu, W. Y., et al. (2019). Design, synthesis and antibacterial evaluation of 2, 4-disubstituted−6-thiophenyl-pyrimidines. Eur. J. Med. Chem. 161, 141–153. doi: 10.1016/j.ejmech.2018.10.039
Li, K. J., Qu, R. Y., Liu, Y. C., Yang, J. F., Devendar, P., Chen, Q., et al. (2018). Design, synthesis, and herbicidal activity of pyrimidine-biphenyl hybrids as novel acetohydroxyacid synthase inhibitors. J. Agric. Food Chem. 66, 3773–3782. doi: 10.1021/acs.jafc.8b00665
Liu, X. H., Wang, Q., Sun, Z. H., Wedge, D. P. E., Estep, J. J., Tan, C. X., et al. (2017). Synthesis and insecticidal activity of novel pyrimidine derivatives containing urea pharmacophore against aedes aegypti. Pest Manag. Sci. 73, 953–959. doi: 10.1002/ps.4370
Min, L. J., Shi, Y. X., Wu, H. K., Sun, Z. H., Liu, X. H., Li, B. J., et al. (2016). Microwave-assisted synthesis and antifungal activity of some novel thioethers containing 1,2,4-triazole moiety. Appl. Sci. 5, 1211–1220. doi: 10.3390/app5041211
Shen, Z. H., Sun, Z. H., Becnel, J. J., Estep, A., David, D. E., Tan, C. X., et al. (2018). Synthesis and mosquiticidal activity of novel hydrazone containing pyrimidine derivatives against aedes aegypt. Lett. Drug Des. Discov. 15, 951–956. doi: 10.2174/1570180815666180102141640
Shi, J. J., Ren, G. H., Wu, N. J., Weng, J. Q., Xu, T. M., Liu, X. H., et al. (2017). Design, synthesis and insecticidal activities of novel anthranilic diamides containing polyfluoroalkyl pyrazole moiety. Chinese Chem. Lett. 28, 1727–1730. doi: 10.1016/j.cclet.2017.05.015
Strange, R. N., and Scott, P. R. (2005). Plant disease: a threat to global food security. Annu. Rev. Phytopathol. 43, 83–116. doi: 10.1146/annurev.phyto.43.113004.133839
Triloknadh, S., Rao, C. V., Nagaraju, K., Krishna, N. H., Ramaiahb, C. V., Rajendra, W., et al. (2018). Design, synthesis, neuroprotective, antibacterial activities and docking studies of novel thieno[2,3-d]pyrimidine-alkyne Mannich base and oxadiazole hybrids. Bioorg. Med. Chem. Lett. 28, 1663–1669. doi: 10.1016/j.bmcl.2018.03.030
Wang, B., Zhu, H., Li, Z., Zhang, X., Yu, S., Ma, Y., et al. (2019). One-pot synthesis, structure and structure-activity relationship of novel bioactive diphenyl/diethyl (3-bromo-1-(3-chloropyridin-2-yl)-1 H- pyrazol-5-yl)(arylamino)methylphosphonates. Pest Manag. Sci. 75, 3273–3281. doi: 10.1002/ps.5449
Wang, B. L., Zhu, H. W., Li, Z. M., Wang, L. Z., Zhang, X., Xiong, L. X., et al. (2018). Synthesis, biological evaluation and SAR analysis of novel poly-heterocyclic compounds containing pyridylpyrazole group. Pest Manag. Sci. 74, 726–736. doi: 10.1002/ps.4770
Wang, B. L., Zhu, H. W., Ma, Y., Xiong, L. X., Li, Y. Q., Zhao, Y., et al. (2013). Synthesis, insecticidal activities, and SAR studies of novel pyridylpyrazole acid derivatives based on amide bridge modification of anthranilic diamide insecticides. J. Agric. Food Chem. 61, 5483–5493. doi: 10.1021/jf4012467
Wang, Y. Y., Xu, F. Z., Zhu, Y. Y., Song, B. A., Liu, D. X., Yu, G., et al. (2018). Pyrazolo[3,4-d]pyrimidine derivatives containing a schiff base moiety as potential antiviral agents. Bioorg. Med. Chem. Lett. 28, 2979–2984. doi: 10.1016/j.bmcl.2018.06.049
Wu, W. N., Chen, M. H., Wang, R., Tu, H. T., Yang, M. F., and Ouyang, G. P. (2019a). Novel pyrimidine derivatives containing an amide moiety: design, synthesis, and antifungal activity. Chem. Pap. 73, 719–729. doi: 10.1007/s11696-018-0583-7
Wu, W. N., Chen, Q., Tai, A. Q., Jiang, G. Q., and Ouyang, G. P. (2015). Synthesis and antiviral activity of 2-substituted methlthio-5-(4-amino-2-methylpyrimidin-5-yl)-1,3,4-oxadiazole derivatives. Bioorg. Med. Chem. Lett. 25, 2243–2246. doi: 10.1016/j.bmcl.2015.02.069
Wu, W. N., Gao, M. N., Tu, H., and Ouyang, G. P. (2016a). Synthesis and antibacterial of novel Substituted purine derivatives. J. Heterocyclic Chem. 53, 2042–2048. doi: 10.1002/jhet.2527
Wu, W. N., Jiang, Y. M., Fei, Q., and Du, H. T. (2019b). Synthesis and fungicidal activity of novel 1,2,4-triazole derivatives containing a pyrimidine moiety. Phosphorus Sulfur 194, 1171–1175. doi: 10.1080/10426507.2019.1633321
Wu, W. N., Tai, A. Q., Chen, Q., and Ouyang, G. P. (2016b). Synthesis and antiviral bioactivity of novel 2-substituted methlthio-5-(4-amino-2-methyl-pyrimidin-5yl)-1,3,4-thiadiazole derivatives. J. Heterocyclic Chem. 53, 626–632. doi: 10.1002/jhet.2435
Xu, X. J., Wang, J., and Yao, Q. Z. (2015). Synthesis and quantitative structure-activity relationship(QSAR) analysis of some novel oxadiazolo[3,4-d]pyrimidine nucleosides derivatives as antiviral agents. Bioorg. Med. Chem. Lett. 25, 241–244. doi: 10.1016/j.bmcl.2014.11.065
Yan, T., Yu, S., Liu, P., Liu, Z., Wang, B., Xiong, L., et al. (2012). Design, synthesis and biological activities of novel benzoyl hydrazines containing pyrazole. Chin. J. Chem. 30, 919–923. doi: 10.1002/cjoc.201100347
Yang, R., Gao, Z. F., Zhao, J. Y., Li, W. B., Zhou, L., and Miao, F. (2015). New class of 2-aryl-6-chloro-3,4-dihydro-isoquinolinium salts as potential antifungal agents for plant protection: synthesis, bioactivity and structure-activity relationships. J. Agric. Food Chem. 63, 1906–1914. doi: 10.1021/jf505609z
Keywords: pyridylpyrazol, amide, pyrimidine, antifungal activity, insecticidal activity
Citation: Wu W, Chen M, Fei Q, Ge Y, Zhu Y, Chen H, Yang M and Ouyang G (2020) Synthesis and Bioactivities Study of Novel Pyridylpyrazol Amide Derivatives Containing Pyrimidine Motifs. Front. Chem. 8:522. doi: 10.3389/fchem.2020.00522
Received: 01 May 2020; Accepted: 21 May 2020;
Published: 31 July 2020.
Edited by:
Hu Li, Guizhou University, ChinaReviewed by:
Pei Li, Kaili University, ChinaGuo Ping Zhang, Huaibei Normal University, China
Tianrui Ren, Shanghai Normal University, China
Copyright © 2020 Wu, Chen, Fei, Ge, Zhu, Chen, Yang and Ouyang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maofa Yang, Z2RnZGx5QDEyNi5jb20=; Guiping Ouyang, b3lncDcxMEAxNjMuY29t
 Wenneng Wu
Wenneng Wu Meihang Chen3
Meihang Chen3