
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Chem. , 31 March 2020
Sec. Inorganic Chemistry
Volume 8 - 2020 | https://doi.org/10.3389/fchem.2020.00179
This article is part of the Research Topic Inorganic Chemistry Editor’s Pick 2021 View all 20 articles
This review summarizes the syntheses and applications of metal oxysulfides. Bulk compounds of rare earth and transition metals are discussed in the section Introduction. After a presentation of their main properties and applications, their structures are presented and their syntheses are discussed. The section Bulk Materials and Their Main Applications is dedicated to the growing field of nanoscaled metal oxysulfides. Synthesis and applications of lanthanide-based nanoparticles are more mature and are discussed first. Then, works on transition-metal based nanoparticles are presented and discussed. Altogether, this review highlights the opportunities offered by metal oxysulfides for application in a range of technological fields, in relation with the most advanced synthetic routes and characterization techniques.
A “metal oxysulfide” is a compound composed of at least a metal, oxygen and sulfur, with negative oxidation states (e.g., –II) for both oxygen and sulfur. The generic formula for ternary oxysulfide is MxOySz. Due to its negative oxidation state, sulfur forms no bounds with oxygen in oxysulfides, in contrast with more common metal sulfates Mx(SVIO4)y where the sulfur is +IV.
In 1951, Eastman et al. recommended the following distinction (Eastman et al., 1951): MxOySz compounds should be designed by the general term “oxide-sulfide” and named after the similarities of their crystalline structure with the corresponding oxide or sulfide. If the oxide-sulfide has the same crystalline structure than the oxide, it should be named “thio-oxide”; if its crystalline structure is the same than the sulfide, it should be called “oxy-sulfide” and if its structure is none of the two, it should be called “sulfoxide.”
However, in an article of 1958 published in French, Flahaut et al. questioned this nomenclature (Flahaut et al., 1958). They argued that all the Ln2O2S (Ln, lanthanide) compounds crystallize in the same structure and showed similar chemical properties. With Eastman's nomenclature, because Ln2O3 oxides crystallize in the two different structures Ce2O3 and Tl2O3, the Ln2O2S compounds would have been named “thioxyde” (French word for thio-oxide) from lanthanum to praseodymium and “sulfoxyde” (sulfoxide) for the others.
Although the terms “thio-oxide” and “oxide-sulfide” are still present in the literature, “oxysulfide” is now employed in a large majority of the works to name any combination on one or several metals to oxygen and sulfur anions.
Altogether, metal oxysulfides represent a class of compounds that is independent from both metal oxides and metal sulfides, though common properties may be punctually identified depending on the metal, the crystal structure, and the anion substitution scheme.
Oxysulfides are scarce in nature and are the most often synthetic. One reason for this is the competitive formation of sulfates, which are found in numerous minerals and are more stable toward oxidation. This competition between sulfate and sulfide is also at stake when designing a synthetic route.
To the best of our knowledge, the first occurrence of an oxysulfide compound was reported in 1827 by Mosander, who was working on the sulfidation of Ce2O3 into Ce2S3 using H2S (Figure 1). He noticed the presence of oxygen and sulfur combined with the metal in a single product, along with the formation of cerium sulfate. Later, Sterba (1904) and Biltz (1908) also reported this observation. Biltz even proposed the formula Ce2S2.5O·S as he identified remaining sulfur as polysulfide on its final product. In 1907, Hauser prepared using H2S on oxides two oxysulfides of tetravalent metal, namely ZrOS and ThOS based on their composition (Hauser, 1907). Hauser indicated that the zirconium and thorium oxysulfides were pyrophoric. Without knowing it, Klemm et al. were probably the first to obtain a pure phase of Ln2O2S by heating Er(SO4)3 in H2S and consequently getting Er2O2S, that they only described as pale pink and resistant to other heating treatments in H2S (Klemm et al., 1930).
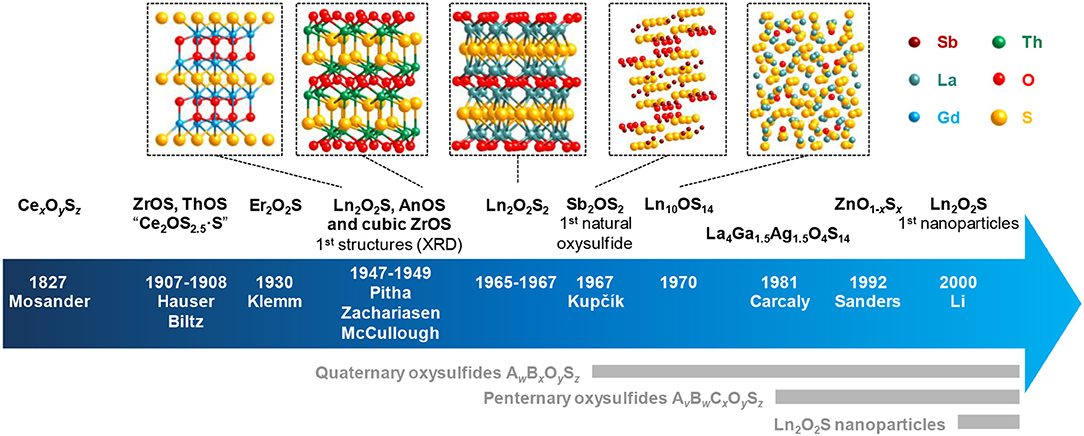
Figure 1. Key dates and authors of the oxysulfide research with some related structures. Ln stands for lanthanide and An for actinide. An2O2S compounds are also known since 1949 (Pu2O2S) and possess the same structure as Ln2O2S.
The first crystalline oxysulfide structures were elucidated by Pitha et al. (1947) (La2O2S) and Zachariasen (1949a) (La2O2S, Ce2O2S, and Pu2O2S). The samples often contained impurities and were prepared either by reducing the corresponding sulfate Ln2(SO4)3 using H2 or by gently heating in air sesquisulfide compounds (Ln2S3). The two authors noticed that the metal was coordinated to seven atoms: four atoms of oxygen and three atoms of sulfur. The Ln2O2S structure derives from the hexagonal oxide Ln2O3 and crystallizes in the P-3m1 space group. This lamellar structure can be described as alternating sheets of [Ln2O2]2+ and S2− (Figure 2). Since this discovery, the entire series of lanthanide oxysulfide Ln2O2S (except promethium) was prepared (Flahaut et al., 1958).
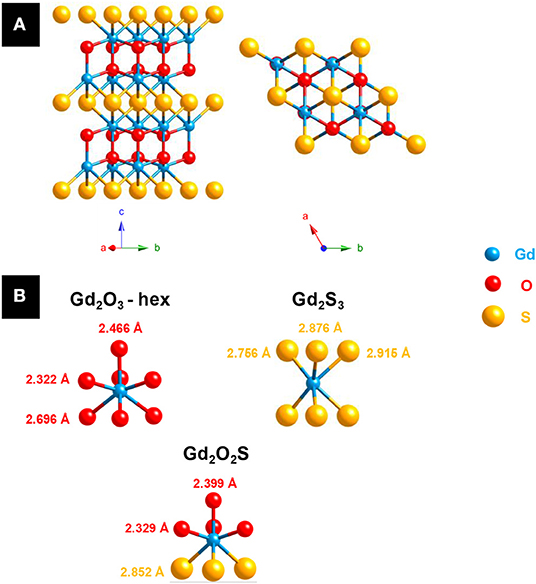
Figure 2. (A) Ln2O2S structure: a hexagonal layered structure (Ln = Gd). (B) Lanthanide environment in hexagonal lanthanide oxide Ln2O3 (JCPDS 02-1878), lanthanide sesquisulfide Ln2S3 (JCPDS 03-2364) and lanthanide oxysulfide Ln2O2S (JCPDS 06-8819) with the example of gadolinium.
In 1948, McCullough et al. established the structure of cubic ZrOS (McCullough et al., 1948) prepared similarly to Hauser and in 1962, Jellinek described a new tetragonal form with the same composition (Jellinek, 1962). In the 1960's and the 1970's, more M2O2S compounds were also reported. The work on radioactive elements gave the actinide oxysulfides Np2O2S (Marcon, 1967a), Am2O2S (Haire and Fahey, 1977), Cm2O2S (Haire and Fahey, 1977), Bk2O2S (Haire and Fahey, 1977), and Cf2O2S (Baybarz et al., 1974). Similarly to Ln2O2S compounds, An2O2S (An, actinide) materials crystallize in the P-3m1 space group. On the contrary, Sc2O2S crystallizes in the hexagonal P63/mmc space group. Its structure remains very close to Ln2O2S with a coordinence of seven for scandium atoms and a structure based on alternative layers of [Sc2O2]2+ and S2− (Julien-Pouzol et al., 1978).
In 1949, Zachariasen described the structure of tetravalent actinide oxysulfides ThOS, UOS, and NpOS as presenting a PbFCl structure type with tetragonal symmetry in P4/nmm space group. The tetragonal form of ZrOS described by Jellinek is isostructural of these compounds (Zachariasen, 1949b). On the contrary, cubic HfOS (isostructural to cubic ZrOS) was first identified by Stocks et al. (1980) and prepared as a pure phase by Eisman and Steinfink (1982). The latter were also able to prepare solid solutions of zirconium and hafnium oxysulfides Zr1−xHfxOS (0 ≤ x ≤ 1) such as Zr0.25Hf0.75OS and Zr0.75Hf0.25OS.
A few years later, Khodadad et al. and Ballestracci demonstrated the existence of several Ln2O2S2 compounds (Ln = La, Pr, and Nd), where the disulfide [S2]2− anion is present (Khodadad et al., 1965; Ballestracci, 1967; Wichelhaus, 1978a). They have to be distinguished from AnOS (An, actinide) compounds in which the actinide is at the +IV oxidation state while the lanthanide in Ln2O2S2 remains at the +III oxidation state.
In 1967, Kupčík reported the kermesite's structure (Kupčík, 1967). The antimony-based compound Sb2OS2 is a rare crystalline natural oxysulfide mineral, which can form thanks to a partial oxidation of stibnite Sb2S3. This so-called oxydisulfide M2OS2 composition was also synthetically obtained for lanthanide compounds Ln2OS2 [Ln = Sm (Lissner and Schleid, 1992), Gd (Wontcheu and Schleid, 2003), Tb (Schleid, 1991a), Dy (Schleid, 1991b), Er (Range et al., 1990), Tm (Range et al., 1990), Yb (Range et al., 1990), Y (Schleid, 1992)]. The erbium, thulium, and ytterbium compounds were obtained at 10 kbar and 1,600°C.
A sulfur-rich phase was also discovered by trying to solve the crystalline structure of what was thought to be β-Ln2S3. It happened to be Ln10OS14 (Ln = La, Ce, Pr, Nd, Sm) that formed because of traces of water or oxygen during the reaction (Carré et al., 1970; Besançon, 1973). Besançon et al. showed that the oxygen content of Ln10S14OxS1−x can be lowered down to a value close to 0.1 mol% for La, Ce, and Pr (Besançon et al., 1970, 1973). Later, Schleid et al. also reported the gadolinium compound Gd10OS14 (Schleid and Weber, 1998).
The work of Marcon with actinides led to the first description of more complex compounds, namely MIII2MIV2O4S3 (Pu4O4S3, U2Pu2O4S3, U2Gd2O4S3, and Ce4O4S3), based on composition analysis (Marcon, 1967b). He also completed the work of Zachariasen by obtaining PuOS (Marcon, 1967b). In the same time, based on the work of Marcon, the compositions of cerium oxysulfides Ce4O4S3 (Dugué et al., 1978; Wichelhaus, 1978b) and Ce6O6S4 (Dugué et al., 1979) were confirmed and their structures were elucidated by X-Ray diffraction on monocrystals by Dugué et al. and Wichelhaus. Ce4O4S3 and Ce6O6S4 monocrystals were obtained by heating Ce2O2S and sulfur or CeO2, Ce2S3, and sulfur together. In the lanthanide series, only cerium allows both oxidation states +III and +IV. In CeIII2O2S, partial oxidation of cerium led to CeIII2CeIV2O4S3 and CeIII4CeIV2O6S4.
A decade after the discovery of Bi2O2Se (Boller, 1973), Koyama et al. published in 1984 a study about the combination of bismuth with chalcogens. They obtained the ternary oxysulfide Bi2O2S from Bi2O3 and Bi2S3 via a hydrothermal synthesis (Koyama et al., 1984). The Bi2O2S structure differs from Ln2O2S (Ln, lanthanide), as it crystallizes in the Pnnm space group.
The coordination number of the bismuth is eight: bismuth is bound to four atoms of oxygen and four atoms of sulfur (Figure 3). In comparison with Ln2O2S in which Ln forms four Ln-O and three Ln-S bonds, bismuth-oxygen bonds are in the same length range (between 2.2 and 2.5 Å) but bismuth-sulfur bonds are significantly longer (3.4 Å for Bi2O2S, <3 Å for Ln2O2S). Further works showed that bismuth can form several oxysulfides, leading to superconductive Bi4O4S3 (Zhang et al., 2015) (containing both sulfide and sulfate ions) and to Bi9O7.5S6 (Meng et al., 2015).
We already cited the work of Marcon who isolated actinide oxysulfides U2Pu2O4S3 and U2Gd2O4S3 (Marcon, 1967b). These structures contain UIV and LnIII. This mixed valence allowed the formation of the AnIV2LnIII2O4S3 and AnIV2LnIII4O6S4 (of general formula AnIV2LnIII2nO2+2nS2+n) structures by a shearing mechanism of the Ln2O2S structure when similar mixed-valent uranium-lanthanide oxysulfides were obtained (Tien et al., 1988). With Okabe et al. (1988), they also exhibited a series of U2La2n−2O2nSn+1 compounds.
Besides, in the 1980's, a considerable amount of quaternary oxysulfides containing other metals than lanthanides or actinides were synthesized. Firstly, the idea was to insert another metal in the lamellar structure of a lanthanide oxysulfide Ln2O2S. The easiest way to get a quaternary oxysulfide was to put the other metal in the layer of sulfur anions, and consequently obtain a structure composed by sheets of lanthanide oxide and metal sulfide. This compound, in which oxygen is bound only to the lanthanide and sulfur only to the additional metal, exhibits a particular order that one can call selective bonding. As the quaternary oxysulfides can be formed with a large variety of precursors (mainly oxides and sulfides, but also elemental sulfur, H2S, metals, …) and not only using lamellar preformed structures such as Ln2O2S, this selective bonding can be extended to any resulting oxysulfide in which one of the anions is preferentially bound to one of the metals and conversely. It generally led to layered compounds. On the contrary, when no such order is present in the structure (at least one metal site in the structure is bound to the two anions), the compound exhibits unselective bonding.
To illustrate this difference, we chose to study a family of quaternary oxysulfides Ln2Ti2S2O5 (with TiIV) reported in the late 1990's (Figures 4A–D). These structures turned out to be defective Ruddlesden-Popper phases which alternate [Ln2S2]2+ and [Ti2O5]2− layers (Figure 4A). However, it is also possible to get compounds where both metals are equally bound to both oxygen and sulfur without particular arrangement (unselective bonding). It can be illustrated by the previously reported quaternary titanium oxysulfides La4Ti3O8S4 and La6Ti2S8O5 that do not show any selective bonding (Figures 4B,D; Cody and Ibers, 1995). In the 1980's, the study of the LawGaxOySz compounds already started the reflexion on the selectivity of the bonds in quaternary oxysulfides (selective bonding for LaGaOS2-α, La4Ga1.33O4S4, and La3GaOS5; unselective bonding for LaGaOS2-β and La3.33Ga6O2S12, Table 1; Guittard et al., 1985).
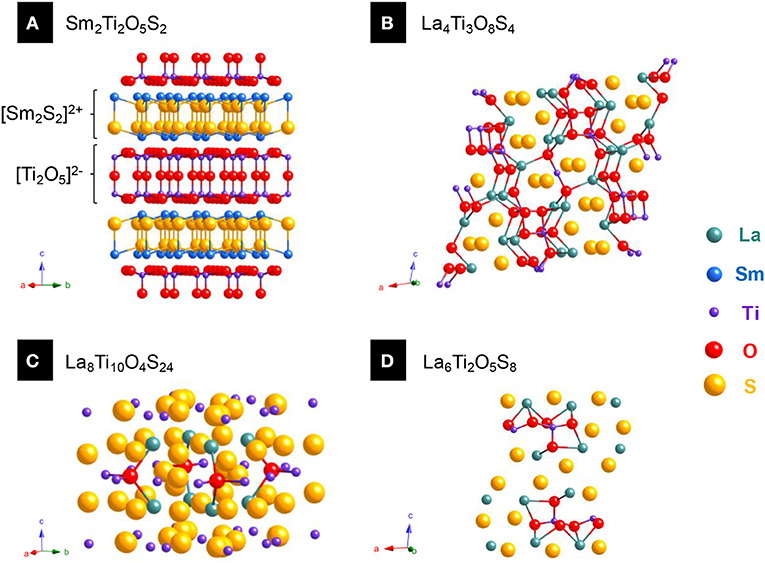
Figure 4. Various quarternary oxysulfide structures containing titanium. (A) Sm2Ti2O5S2 (JCPDS 13-1325) exhibits selective bonding as sulfur is preferentially bound to titanium and oxygen to samarium. (B) La4Ti3O8S4 (JCPDS 09-7018), (C) La8Ti10O4S24 (JCPDS 09-8085), and (D) La6Ti2O5S8 (JCPDS 09-7017) show different structures with unselective bonding.
Using high temperatures and long reaction times, monovalent (CuI, AgI), trivalent (CrIII, GaIII, AsIII, SbIII, BiIII), tetravalent (SnIV) and pentavalent elements (NbV) were shown to be able to crystallize along with a lanthanide in various types of oxysulfide compounds (Table 1). In some cases, the second metal can also present mixed oxidation states (TiIII, IV, VIII, IV).
More recently, lanthanide-free quaternary oxysulfide compounds CaMOS [M = Fe (Selivanov et al., 2004; Delacotte et al., 2015), Co (Pitha et al., 1947), Zn (McCullough et al., 1948)] and BaM'OS [with M' = Co (Pitha et al., 1947; Valldor et al., 2015), Zn (Broadley et al., 2005)] were synthesized and characterized. This shows the growing interest in obtaining metal oxysulfides without rare earth (which are strategic resources) in order to explore their magnetic and catalytic properties.
In this table are not referenced the quaternary phases reported by Umarji et al. in 1980: M2Mo6S6O2 (M = Co, Ni, Cu) and PbMo6S6O2 (Umarji et al., 1980). A few years after this publication, Selwyn et al. tried to obtain the copper-based phase and demonstrated that Umarji et al. reached only a mixture of the Chevrel phase Cu2.7Mo6S8, Mo, and MoO2 (Selwyn et al., 1987). Then Selwyn et al. also concluded that obtaining the ternary Mo6S6O2 oxysulfide from the claimed M2Mo6S6O2 was impossible.
Quinary oxysulfides also exist, but are not exhaustively listed in this review. Most of them are layered compounds with selective interactions and contain earth-alkaline atoms, as evidenced by Teske in 1985 with CaLaGa3OS6, SrLaGa3OS6, La2ZnGa2OS6, and Sr2ZnGe2OS6 (Teske, 1985). A similar Sr2MnGe2OS6 phase was synthesized and studied recently (Endo et al., 2017). Doped phosphors CaLaGa3OS6 (Yu et al., 2008, 2012; Zhang et al., 2010, 2011, 2012) and SrLaGa3OS6 (Zhang et al., 2005a, 2016; Yu et al., 2011, 2012) were extensively studied by Zhang, Yu, and Zhang since 2005. Zhu, Hor, and Otzschi also detailed different quinary oxysulfide families: (i) the Sr2Cu2MO2S2 [M = Mn (Zhu and Hor, 1997a), Co (Zhu et al., 1997; Smura et al., 2011), Zn (Zhu and Hor, 1997a), Ni (Otzschi et al., 1999)] and Ba2Cu2CoO2S2 (Zhu et al., 1997; Smura et al., 2011) family that displays an unusual square planar MO2 layer and the two perovskite-based families (ii) Sr3Cu2M2O5S2 [M = Sc (Otzschi et al., 1999), Fe (Zhu and Hor, 1997b)] and (iii) Sr2CuMO3S [M = Sc (Ogino et al., 2012), Cr (Zhu and Hor, 1997b), Fe (Zhu and Hor, 1997b), Ga (Zhu and Hor, 1997c), In (Zhu and Hor, 1997b)] with the work of Ogino on scandium. Later, Blandy transformed Sr2Cu2MnO2S2 in Sr2Cu1.5MnO2S2 by oxidative deintercalation of copper to obtain a mixed-valent perovskite (Blandy et al., 2015).
The study of the quasi-binary system La2O2S-AgGaS2 (La2O2S – 0.75 Ga2S3 – 0.75 Ag2S) by Carcaly et al. (1981) led to the formation of La4Ag1.5Ga1.5O4S5 in which silver and gallium are randomly distributed in the same sites. Along with La3MO5S2 (M = Nb, Ta; Table 1), Cario et al. reported bilanthanide lamellar La2YMO5S2 phases very close to the Ln2Ti2O5S2 structure (Eisman and Steinfink, 1982). The works of Tranchitella on La/Ti quaternary oxysulfide (Table 1) led him to the quinary compound Sr5.8La4.4Ti7.8S24O4 with the same [(Ti4S2O4)(TiS6)4/2]12− layer than La14Ti8S33O4 (Tranchitella et al., 1996). La5Ti2MS5O7 (M = Cu, Ag), an alkaline-free structure with perovskite layers was also evidenced by Meignen et al. (2004b) and studied for its photocatalytic properties for water reduction and oxidation (Suzuki et al., 2012). Meignen et al. (2005) also prepared La5Ti~3.25Zr~0.25S5O9.25 with mixed Ti/Zr sites. In 2003, Rutt et al. obtained KY2Ti2O5S2 by topotactic potassium intercalation of potassium in Y2Ti2O5S2 (Rutt et al., 2003). As a perspective, in 2015, Yee et al. designed by DFT modeling a new high-temperature superconductor Ca2HgCuO2S2 whose superconducting transition temperature should be close to mercury cuprates' ones (Yee et al., 2015).
For a long time, ternary oxysulfides MxOySz were limited to lanthanides, actinides, and bismuth. Despite the presence of numerous metals in quaternary oxysulfides, the transition metals did not give any crystalline ternary oxysulfide (except ZrOS and HfOS) until the synthesis of ZnO1−xSx in the 1990's. This phase is the most often found as crystalline thin films. It is also the case for titanium, tungsten and molybdenum oxysulfides except that they are amorphous.
The first-raw transition metals ternary oxysulfides represent a challenge, because the coordination of the metal commonly does not exceed six, and consequently cannot bear the M2O2S structure of Ln2O2S (Ln, lanthanide) where the lanthanide coordination is seven or the Bi2O2S structure where the coordination of bismuth is eight. Alternative crystal structures may be obtained in the case of first-raw transition metals.
In 2013, Meyer et al. reported the synthesis of ternary compounds Cu2O1−xSx with various compositions (Meyer et al., 2013). These were obtained using radio-frequency magnetron sputtering (RFS) with a copper target and a flow of O2 and H2S with various gas ratios. The authors showed that for x > 0.39, the compounds did not crystallize in the cubic structure of Cu2O and became amorphous. The lattice constant of cubic Cu2O1−xSx evolved with the composition toward bigger values because of sulfur insertion. The variation was linear only up to x = 0.13 and did not follow the Vegard's law. Unfortunately, direct information about the sulfur oxidation state is missing: the oxysulfide nature of the compound remains unsubstantiated.
Despite their electronegativity and size differences, sulfur atoms are able to replace the oxygen atoms of the wurtzite structure which progressively turns into the ZnS blende structure. It evidences another challenge of metal oxysulfide identification: they could be isostructural of metal sulfides or metal oxides.
Zinc oxysulfide was first reported as thin films grown by atomic layer deposition (ALD) in 1992 by Sanders et al. The oxygen and water traces in the gases were responsible for the oxygen in the resulting film. Since 2010, extensive characterization of ZnO1−xSx thin films were reported, not only involving ALD (Bakke et al., 2012) but also pulsed-laser deposition (Deulkar et al., 2010), chemical spray pyrolysis (Polat et al., 2011a,b, 2012; Thankalekshmi and Rastogi, 2012) or thioacetate-capped ZnO nanocrystals (Lee and Jeong, 2014). Because of the active research on bandgap engineering, zinc oxysulfide was envisaged as buffer layer in solar cells (Platzer-Björkman et al., 2006; Sinsermsuksakul et al., 2013). X-Ray photoemission spectroscopy (XPS) showed that the sulfur in these films is reduced and thus in agreement with the announced oxysulfide nature (Thankalekshmi and Rastogi, 2012; Lee and Jeong, 2014).
In 1986, Inoue et al. crystallized two MoxOySz compounds while studying the MoS2:MoS3 system (Inoue et al., 1986). The deep-bluish crystal of MoO2.74S0.12 (otherwise written as Mo4O10.96S0.48) was isostructural to γ-Mo4O11 and exhibited charge density wave instabilities similar to these of quasi-2D materials. The similar properties of MoO2.74S0.12 and γ-Mo4O11 supported the hypothesis of a true oxysulfide compound. Also, reddish crystals of MoO1.88S0.15 were obtained and presented structural and electronic similarities with monoclinic MoO2.
The decomposition of molybdenum oxodithiocarbamate as a single source precursor also enabled the formation of crystalline thin films (Olofinjana et al., 2010). Rutherford backscattering spectroscopy (RBS) indicated a pure phase. Unfortunately, the final product shared the XRD patterns of Mo8O23, Mo9O26, and Mo2S3 but the structure was not fully solved.
In this section are referenced the oxysulfides of three elements: titanium, tungsten, and molybdenum. In the 1990's, thin films of these oxysulfides were obtained and studied for their electrochemical properties.
In 1993, Tchangbedji et al. announced the formation of a hydrated amorphous phase of vanadium oxysulfide by reacting Na2S·9H2O and VOCl2 (Tchangbedji et al., 1993). The first described formula for this compound was V2O4S·2H2O, but was adjusted to V2O3S·3H2O in latter studies (electron paramagnetic resonance and XANES at V K-edge demonstrated the presence of VIV species; Tchangbédji et al., 1994; Ouvrard et al., 1995). Water was believed to stabilize the compounds, as its evaporation was accompanied by the loss of the sulfur in the structure. Unfortunately, the authors did not provide enough convincing arguments to justify the oxysulfide nature and the purity of their phase without ambiguity. In particular, the absence of the IR and XANES at S K-edge spectra, which are discussed in the articles, is detrimental. Because of this lack of information, we did not focus on this phase.
Titanium oxysulfides were obtained under the form of thin films to serve as positive electrode material for solid state batteries. Reported for the first time in 1989 by Meunier et al. (1989, 1991) they were extensively characterized in the same group by X-ray photoemission spectroscopy (XPS) that was shown well-adapted for thin films characterization (Levasseur et al., 1999).
Titanium oxysulfides (TiOySz) of various compositions were obtained using RFS of hydrolyzed TiS2 targets. The composition can be adjusted via the partial pressure of oxygen during the sputtering process. XPS showed that titanium oxysulfides thin films contain three titanium species (TiIV as in TiO2, TiIV as in TiS2 and Ti in mixed environment) and three sulfur species (S−II of S2− anions, S−I in disulfide S22− ions and undefined Sn2− ions). For high oxygen contents (TiOS for instance), SVI species of sulfate ions attributed to surface species were also observed, although in a lesser extent due to mechanical erosion (Gonbeau et al., 1991; Dupin et al., 2001; Martinez et al., 2004; Lindic et al., 2005a) Besides, the presence of ordered domains, observed by TEM and XRD, revealed the existence of TiS2 nanocrystals in the amorphous materials (Lindic et al., 2005b). Lithiated titanium oxysulfides thin films were recently obtained with RFS using LiTiS2 targets (Dubois et al., 2017). Their characterization show similar properties than TiOySz. Their capacities of around 85 μAh.cm−2.μm−1 made them usable in a Li-ion cell.
Aside these thin films, “sulfur-doped TiO2” can be obtained by reacting TiO2 with thiourea or hexamethyldisilathiane, for instance. However, in this case, the products should not be named “oxysulfides,” because they only contain oxidized sulfur under the form of SIV and SVI species (Yang et al., 2012; Ramacharyulu et al., 2014; Smith et al., 2016).
Similarly to titanium oxysulfides, amorphous tungsten oxysulfides thin films with adjustable composition were obtained by RFS on WS2 targets and mainly characterized by XPS (Martin et al., 1999). Along with the three species of sulfur described in the titanium section, three different species of tungsten (WVI as in WO3, WIV as in WS2, and WV in a mixed environment of O2−, S2−, and S22−) were observed (Dupin et al., 2001; Martinez et al., 2004). TEM and XRD showed the presence of nano-crystallites of WS2, but the polymorphs 3R-WS2 and 2H-WS2 could not be distinguished (Martin-Litas et al., 2002). The incorporation of lithium in these thin films and their electrochemical properties were studied (Martin et al., 1999; Martin-Litas et al., 2001). It revealed that 1.1 lithium atoms per formula can be incorporated, providing a capacity of 75 μA.cm−2. XPS also demonstrated that the tungsten ions are reduced to W(0) and that sulfide ions participated to the redox process with irreversible behaviors (Martin-Litas et al., 2003).
Abraham, Pasquariello et al. synthesized various MoOySz amorphous compounds from the thermal decomposition of ammonium dithiomolybdate (NH4)2MoO2S2 (Abraham et al., 1989; Pasquariello et al., 1990). This precursor was obtained by bubbling H2S on ammonium paramolybdate [(NH4)6Mo7O24·4H2O] in an ammonia solution. Depending on the thermal treatment (temperature, number of steps), significant amounts of hydrogen and/or nitrogen could be found in the solids. Reacting a mixture of [(NH4)6Mo7O24·4H2O] and (NH4)2MoS4 also led to a solid precursor whose thermal decomposition yielded MoOySz. Based on the electrochemical properties of these amorphous compounds, the authors suggested different structures for them, with different O/S ratios and involving both S22− and S2− anions (Abraham and Pasquariello, 1993). Infrared spectroscopy and XPS supported the presence of Mo–O and Mo–S bonds in the solid, but Mo–Mo bonding could not be evidenced.
The solution obtained by reflux of (NH4)2Mo2S12 in acetone dispersed in different aqueous electrolyte solutions led to original morphologies of amorphous molybdenum oxysulfides (water/acetone = 1/10 v/v; Afanasiev and Bezverkhy, 2003). For instance, tubular morphologies (with the following electrolyte: 10% KCl, 10% NH4SCN), hollow spheres [with 10% (NH2OH)H2SO4] and fractal sponge-like solids (with 20% NH4SCN) were obtained. A solid was collected by evaporation of the solvent. EXAFS at Mo K-edge spectra showed one oxygen atom and four sulfur atoms in the first coordination shell of molybdenum atoms. XPS supported the hypothesis of mainly reduced sulfur, even if a broad peak in the region 167–171 eV indicated oxidized species. In the same group, Genuit et al. (2005) performed the condensation in acidic medium of MoO2S22− to amorphous MoOS2. The addition of HCl in a (NH4)2MoO2S2 aqueous solution led to MoOS2.
Similarly to titanium and tungsten, RFS gave amorphous thin films of molybdenum oxysulfides (Schmidt et al., 1994, 1995a). The target was a pellet of MoS2. Pure oxygen was flowed into the chamber to get oxygen-rich oxysulfides (MoO~1.3S~1.9), but the traces of oxygen in the glovebox were originally sufficient to get MoOySz thin films. For oxysulfides with a low content of oxygen (MoO~0.5S~2.0), TEM showed ordered domains that are isostrucural of MoS2, based on electronic diffraction. XRD evidenced both MoS2 and MoO2 phases when the films were annealed under inert atmosphere. As shown by Buck for contaminated MoS2 films, substitution of sulfur by oxygen atoms is likely to explain the changes in lattice parameters observed in the MoS2-like phase (Buck, 1991). Later, XPS analysis provided clues about the oxidation states of molybdenum and sulfur in MoOySz films which strongly varied with the film composition (Levasseur et al., 1995; Schmidt et al., 1995b; Dupin et al., 2001). For y <0.6 and z > 2 (oxygen-poor oxysulfides), MoIV cations and S−II (as in MoS2) were dominant. For y > 3 and z <1 (oxygen-rich oxysulfides), only MoVI in octahedral sites (as in MoO3) was observed. For 0.6 < y <3 and 1 < z <2, MoV was observed in addition to MoIV and MoVI and was likely surrounded by O−II (O2−) and S−I (S22−) species. Moreover, in MoO0.6S1.9 thin films, extended X-ray absorption fine structure (EXAFS) at molybdenum K-edge also showed the presence of oxygen atoms in the coordination sphere of molybdenum atoms (Schmidt et al., 1995b).
During the same decade, useful XPS and IR references for molybdenum oxysulfides were established by Muijsers et al. (1995) and Weber et al. (1996) in the study of MoO3 films sulfidation. The formation of oxysulfide intermediate species with their corresponding probable structures was detailed.
In the 1980's, potential applications for doped Ln2O2S materials were identified and led to their use as lamps, lasers, scintillators, screens, etc. For example, they can be found in X-ray detectors used for tomography or medical imaging. They have also been used for oxygen storage. Often, Y2O2S, La2O2S, or Gd2O2S are used as the lattice and doped with one or several lanthanide ions to obtain the desirable luminescence features. The oxysulfide was compared with the corresponding oxide Sm2Ti2O7 that has a higher bandgap. Also, electrochemical properties of transition metal oxysulfides were investigated for their use in lithium-ion batteries. A brief summary is given below on these applications.
Doped oxysulfides were primary employed in cathode ray tubes (CRTs) of television screens and later in computer monitors. In 1968, Royce patented a “family of new cathodoluminescent phosphors” by describing the potential use of doped Y2O2S and Gd2O2S (Royce, 1968). Lutetium and lanthanum were also envisaged as efficient matrixes for the doping ions (mainly SmIII or EuIII).
Three classes of phosphors, respectively, associated to red, blue, and green, are necessary for a proper screen to emit the colors of the visible spectrum. Thanks to their good luminescence properties, lanthanide doping ions equip the main phosphors used for industrial applications (Jüstel et al., 1998). Red color is provided by EuIII: Y2O3:Eu, Y2O2S:Eu, YVO4:Eu, Y2(WO4)3:Eu; blue emission is enabled by EuII in compounds such as: Sr5(PO4)3Cl:Eu, BaMgAl11O7:Eu, Sr2Al6O11:Eu; and green is emitted thanks to TbIII: CeMgAl11O19:Tb, (Ce,Gd)MgB5O10:Tb, (La,Ce)PO4:Tb, Y2SiO5:Tb, Y3Al5O12:Tb (Ronda et al., 1998). However, for CRTs, blue and green are preferentially obtained with ZnS:Ag and ZnS:(Cu,Au), respectively.
In current computer monitors, the amount of europium-doped yttrium oxysulfide Y2O2S:Eu (0.73% w/w for Eu, 13.4% w/w for Y) used for red emission has become large enough to implement and develop the rare-earths recovery (Resende and Morais, 2015).
The first study on metal oxysulfides as laser-emitting material was reported in the earliest years of the design of laser devices. “Laser” stands for light amplification by stimulated emission of radiation and is a general term for a device that emits light through a process of optical amplification based on the stimulated emission of electromagnetic radiation. It is characterized and differs from other light sources by the spatial and temporal coherences of the resulting light. Thus, there are countless applications of the laser devices. Two kinds of applications can be distinguished: information transfer (fiber-optic communication, length measurements, fingerprint detection, barcode scanner, thermometers, laser pointers, weapon guidance…) and power transfer (cutting, welding, superficial fusion, marking materials…).
In 1971, while the most famous laser crystal, namely YAG:Nd (neodymium-doped yttrium aluminum garnet), had already been extensively studied, Alves et al. (1971) carried out the first experiments dealing with an oxysulfide-based laser. They grew and studied millimetric La2O2S:Nd crystals with many defects, but also estimated the properties of crystals with less imperfections. Similarly to YAG:Nd, the stimulated emission takes place between the 4F3/2 and 4I11/2 energy levels of the NdIII ion in La2O2S:Nd with an emission wavelength of 1,075 nm (9,300 cm−1), while YAG:Nd emits at 1,064 nm (9,400 cm−1).
In 1990, Markushev et al. (1990) presented preliminary results on the stimulation emission kinetics at the temperature of liquid nitrogen for 1 mol% of neodymium. In 2012, the stimulated emission properties of La2O2S:Nd were studied with oxysulfide powders (Iparraguirre et al., 2012). In particular, Iparraguirre et al. (2012) estimated and experimentally investigated the influence of the doping ion concentration and pumping wavelengths on the different laser properties.
The counterpart of laser emission is laser absorption. Because of the coherence of the emitted light, laser devices can be harmful for human skin or eyes. Protecting glasses or clothes are then required for safety issues. Absorption materials must display a low reflectivity and a good thermal stability because of local heating induced by the laser beam.
The research on absorption devices deals with materials that can absorb the 1,064 nm radiation of the widespread YAG:Nd3+ laser. In particular, samarium-based compounds were found to be efficient absorption materials because of electronic transitions between the ground state 6H5/2 to the 6F9/2 excited state.
Undoped Sm2O2S was found to absorb a large proportion of the 1,064 nm laser radiation with a reflectivity of around 0.74% and it is stable up to 2,000°C (Zhu et al., 2016). In comparison, SmBO3 presents a reflectivity of 0.6% but endures a phase transition at 1,200°C (He et al., 2009). Doping with erbium or thulium may also be an efficient way to slightly enhance the absorption properties of Sm2O2S (Sun et al., 2017).
A scintillator is a material that emits light when it is excited by an ionizing radiation (X-rays or gamma rays for example). Scintillators are mainly used in the field of medical imaging. Their role is to lower the dose of X-rays endured by a patient during an analysis. To enable a good absorption of the X-ray beam, the requirements for a good scintillator phosphor is the presence of heavy atoms (cadmium, bismuth, lanthanides, tungsten for instance), a high material density (≥ 4 g.cm−3) and a high stability regarding the radiations. The photon must be converted into photons in the visible range (500-800 nm) with a good efficiency, a fast decay and a short afterglow. Moreover, mechanical strength, absence of toxicity, and chemical stability are desired features (Rossner and Grabmaier, 1991).
Thus, a scintillator is generally composed by a dense ceramic and converts X-rays in visible light. It is connected to photodiodes that convert the visible photons in electrons that form an image on a layer of amorphous silica. Considering their efficient absorption of X-rays, Y2O2S:Tb, La2O2S:Tb, Gd2O2S:Tb were considered to replace CaWO4, which was commonly used as scintillator (Brixner, 1987). Gd2O2S:Tb was finally chosen for its higher density and better absorption properties in comparison to the other lanthanides (Brixner, 1987). Later, Gd2O2S:Pr was shown to be an efficient scintillator by Rossner et al. They demonstrated that the main differences between the PrIII and the TbIII doping lie in the incident beam conversion efficiency (for a 40-80 keV X-ray beam, 8.5% for Gd2O2S:Pr, Ce, F, and 15% for Gd2O2S:Tb) and the luminescence lifetime of the doping ion (~3 μs for Gd2O2S:Pr, Ce, F; 600 μs for Gd2O2S:Tb; Rossner and Grabmaier, 1991). PrIII shows a very rapid decay, cerium decreases the trap states and fluorine causes an important decrease of the afterglow. Gd2O2S:Eu was also studied. Its absorption and luminescence properties were competitive enough and it enables the emission of red photons (instead of green photons for Pr and Tb) which can be useful for compatibility issues with digital imaging systems (Michail et al., 2010).
Nowadays, gadolinium oxysulfides are used as scintillators for Single-Photon Emission Computed Tomography (SPECT), X-ray Computed Tomography (CT), and Positron Emitting Tomography (PET).
Lithium intercalation and electrochemical properties of bulk metal oxysulfides were discussed because of possible oxido-reduction reactions with transition metals such as titanium or molybdenum. We already mentioned that titanium (Meunier et al., 1989; Lindic et al., 2005a; Dubois et al., 2017) or tungsten (Martin et al., 1999; Martin-Litas et al., 2001, 2003) thin films were studied as cathodes in solid-state lithium-ion batteries. As cathodes, molybdenum oxysulfide thin films were also developed (Abraham et al., 1989; Pasquariello et al., 1990; Gonbeau et al., 1991; Abraham and Pasquariello, 1993; Levasseur et al., 1995; Schmidt et al., 1995a; Yufit et al., 2003; Golodnitsky et al., 2006a,b) More recently, a TiO2@MoOySz composite was investigated as anode material (Qiao et al., 2013). The external layer of molybdenum oxysulfide was supposed to enhance the conductivity of the hybrid material.
At the end of this section, we wanted to underline several crucial points:
(i) Oxysulfide materials are mainly reached by chemical synthesis and much fewer compositions were obtained compared to monochalcogenide compounds.
(ii) The lack of oxysulfide compositions is mainly based on the strong differences between oxygen and sulfur. Metals tend to preferentially bind to one compared to the other.
(iii) Transition metals oxysulfides are particularly rare and their crystalline phases even more.
In this context, a periodic table showing the reported oxysulfide compounds is presented in Figure 5.
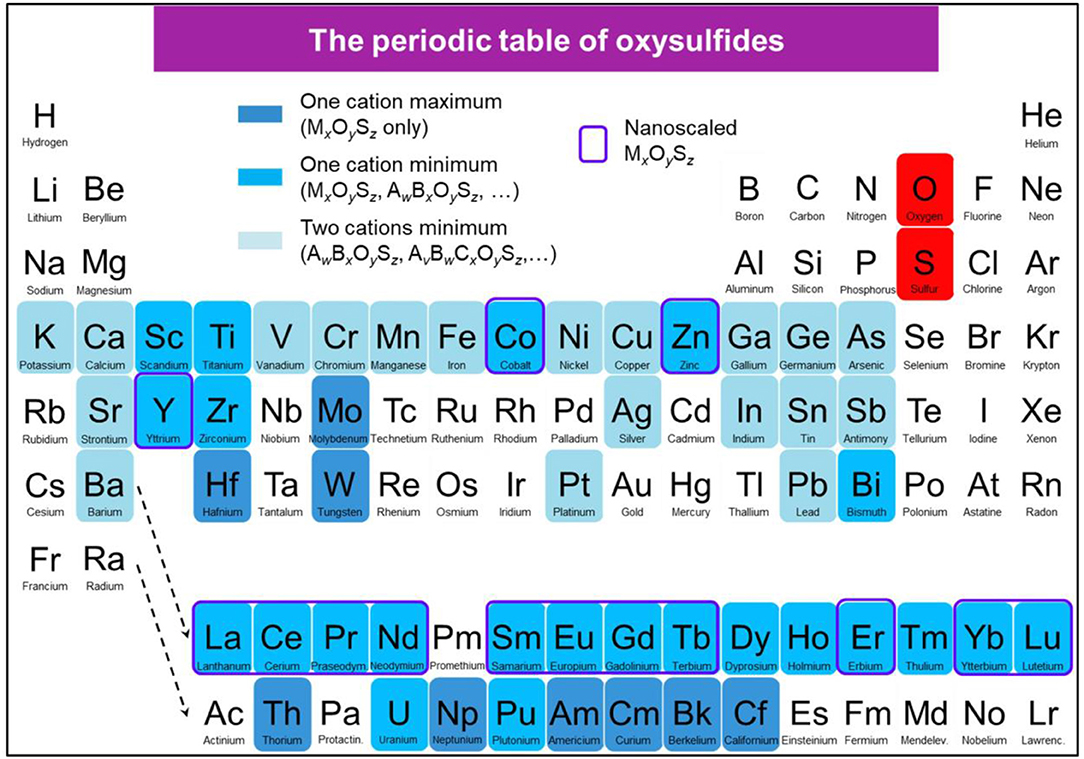
Figure 5. Periodic table showing the reported oxysulfide compounds. In blue are indicated the elements that can be found in synthetic or natural oxysulfides. Blue shades indicate the compositions (ternary, quaternary, and more) that can be achieved for each element. Surrounded in violet are the elements for which MxOySz nanoparticles were reported.
Following the hype for nanotechnology in the twenty-first century, researchers have recently worked on producing metal oxysulfide materials as nanoparticles (Figure 6). This trend is justified by the applications that could emerge from nanomaterials, especially in the domain of biology and medicine. In particular, nano-scale objects can cross biologic barriers and be metabolized by living beings. Also, because of their wide range of morphologies, compositions, and grafting, the nanoparticles can reach targeted zones using specific interactions to provide local information, deliver drugs at precise places or stimulate organs and tissues with an internal or external stimulus.
Lanthanide oxysulfide nanomaterials present many advantages for imaging in biological medium. They have a good chemical and thermal stability. Their size and shape is highly tunable from very small crystals around 5 nm to micrometer spheres, rods, belts, tubes and so on. Moreover, the Ln2O2S crystalline phase bears many lanthanide/transition metal or lanthanide/lanthanide substitutions, which guarantees a generous variety of luminescent properties.
In the fields of therapy and in vivo imaging, using direct light composed of high energy photons, typically X-rays or gamma rays, leads to potential harmful effects for the patient. Organic dyes, radioisotopes and quantum dots are currently used in order to perform bioimaging. However, toxicity of radioactive isotopes, and quantum dots is problematic. Also, organic fluorophores and quantum dots (QD) are sometimes excited through ultraviolet (UV) irradiation that can lead to autofluorescence (excitation of natural targets, such as elastin, collagen…), photobleaching (destruction of the dye), and luminescence blinking.
Another indirect but efficient way to excite phosphors at low energy for bioimaging is infrared (IR) irradiation, taking advantage of the biological transparency windows: 750–950 nm (BW−1), 1,000–1,450 nm (BW–II), and 1,500–1,700 nm (BW–III). The main advantage is the high signal-to-noise ratio, because biological tissues (containing melanin, hemoglobin and water) absorb less light in these spectral ranges (Shi et al., 2016). Consequently, IR bioimaging does not result in parasitic fluorescence. Moreover, it causes low tissue damage and enables local irradiation along with high penetration depth.
Lanthanide-based upconverting phosphors are based (in the simplest case) on the combination of two absorbed low-energy photons in one of a higher energy, resulting for instance in the absorption of IR wavelengths and emission of visible light (Auzel, 2004). This way, many advantages are conferred to the imaging system (Ajithkumar et al., 2013): the chemical stability and low toxicity of rare-earth compounds, the absence of photobleaching, the low and easy available required energy.
Oxysulfide nanomaterials based on the upconverting properties of lanthanide dopants have been studied as potential upconverting phosphors for biomedical imaging. Ytterbium and erbium co-doped materials are being investigated in detail, but other dopants, such as holmium and thulium have also been reported for upconverting materials.
The phenomenon of persistent luminescence is the emission of light by a material after excitation has stopped. It must be distinguished from fluorescence and phosphorescence. Its mechanism is complex and still debated (Jain et al., 2016). In persistent luminescence, the origin of the extended emission in an insulator or semi-conductor is the entrapment of electrons or holes that are progressively released (Leverenz, 1949). Either an electron is trapped in an energy level near the conduction band or a hole is trapped in an energy level near the valence band.
The traps can be point defects with intrinsic defects of the lattice such as vacancies, interstitial defects, antisite defects, or extrinsic defects when doping ions substitute lattice atoms or occupy interstitial sites. Extended defects (dislocations, surface, or grain boundaries) of the lattice can also play the role of traps.
Oxysulfide materials containing titanium and europium have been developed for persistent luminescence. Here, the doping ions substitute the rare-earth of the matrix and correspond to extrinsic defects. Y2O2S:Ti in 2005 was the first example (Zhang et al., 2005b), but numerous articles focused on the promising properties of Ln2O2S:Eu3+, Mg2+, Ti4+ (Ln = Gd, Y) which will be named Ln2O2S:Eu, Mg, Ti for simplification (Mao et al., 2008; Li et al., 2010; Cui et al., 2013a, 2014a; Liu et al., 2014a).
Because of their remaining 4f electrons, most of the lanthanide ions present magnetic properties. Lanthanide oxysulfides were found to be paramagnetic in a large range of temperatures, and their magnetic properties at low temperatures were extensively studied (Ballestracci et al., 1968; Quezel et al., 1970; Biondo et al., 2014).
Lanthanides can exhibit high magnetic susceptibility, which is major interest for chemicals that can be injected in a living organism. For instance, GdIII complexes are used as positive contrast agents in magnetic resonance imaging (MRI) due to the 4f7 electronic configuration of the ion (μ = 7.94 μB). The role of a contrast agent is to enhance the MRI signal by locally perturbing the magnetic field. The spin relaxation time of GdIII is long enough to optimize the dipole-dipole interactions of electron and protons (biological tissues, water) in the neighborhood of the contrast agent. The MRI signal is then enhanced by the acceleration of the spin relaxation of the protons caused by these interactions. Gadolinium ions in molecular complexes are toxic because of polarizing effects and competition with calcium. Special hydrosoluble complexes were then developed to prevent the toxicity of GdIII (Tóth et al., 2002).
An alternative to lanthanide complexes is lanthanide nanoparticles. A better detection occurs as the consequence of the concentration of several thousand atoms in a little volume. Iron oxide nanoparticles have been widely studied and used as negative contrast agents, but many artifacts were observed on the resulting images (Bulte and Kraitchman, 2004). Gd2O3 nanoparticles were found to have a similar or better relaxivity than gadolinium complexes, without the drawbacks of iron oxides. They were then chosen for the precise visualization of locally injected cells (Engström et al., 2006; Petoral et al., 2009).
With doping ions, gadolinium oxide nanoparticles were then applied for bimodal imaging (MRI and luminescence) (Kryza et al., 2011). Because of their very good luminescence properties, similar results are expected for oxysulfide Gd2O2S nanoparticles. Bimodal agents are especially useful to get various information of the environment of the nanoparticles from the luminescence properties (wavelength, lifetime, and so on) in short times coupled with long term data and precise localization with magnetic resonance imaging (Cherry, 2006). Ajithkumar et al. (2013) demonstrated the possibility of performing multimodal bioimaging using oxysulfide material choosing the Gd2O2S:Yb, Er phosphor. Besides, Gd2O2S:Eu micronic particles were used as a colloidal solution for X-ray Luminescence Computed Tomograghy (XLCT), a technique that could be applied in vivo (Pratx et al., 2010a,b). Drug delivery can also be tracked in vivo. Gd2O2S:Tb nanoparticles coated with SiO2 were employed as radioluminescent markers to evaluate the release of doxorubicin as a function of pH, using X-ray Excited Optical Luminescence (XEOL) (Chen et al., 2013).
Recently, sub-micronic powder of Sm2Ti2O5S2 was used as a stable photocatalyst for water oxidation and reduction under visible-light irradiation, and this was later further extended to other Ln2Ti2O5S2 (Ln = Pr, Nd, Gd, Tb, Dy, Ho, and Er) phases (Ishikawa et al., 2002, 2004). Moreover, because the majority of lanthanides are often restricted to the +III oxidation state, catalysis based on oxido-reduction reactions is not the preferential application of oxysulfide materials. Nevertheless, cerium (CeIII and CeIV) and europium (EuII and EuIII) are notable exceptions. In particular, Ce2O2S nanoparticles on carbon was tested for oxygen reduction reaction (ORR) (Yang et al., 2017). Also, Eu2O2S nanoparticles showed catalytic activity for the water-gas shift reaction (reaction of CO with water that yields CO2 and H2) (Tan et al., 2016). They can also act as a peroxidase mimic for the catalytic oxidation of 3,3′,5,5′-tetramethylbenzidine (TMB) (Ghosh et al., 2016).
Several strategies can be employed to yield oxysulfides nanoparticles. Historically, bulk oxysulfides were formed by partial sulfidation of oxides, oxidation of sulfides or reduction of sulfates (Figure 7). However, solid-gas or solid-solid reactions at high temperatures inevitably lead to sintering and large particles. This should be avoided to control the growth of nanoparticles. Moreover, avoiding sulfates is challenging: their formation is thermodynamically favored.
Four major strategies are employed to yield Ln2O2S (bulk and nanoparticles). The two first methods are the sulfidation of an oxygenated phase such as an oxide or a hydroxide (Figure 7, pathway A) and the oxidation of sulfides (Figure 7, pathway B). In the latter case, the term “oxidation” names a substitution between sulfur and oxygen and does not imply oxido-reduction processes. This process is challenging: the partial oxygenation of sulfides is hard to control because sulfates are easily formed. To the best of our knowledge, only bulk materials were synthesized this way.
The reduction of sulfates and oxysulfates is also possible (Figure 7, pathway C). It is generally excluded for the formation of nanoparticles as it demands high temperatures (≥800°C). Finally, another way to achieve the synthesis of metal oxysulfides is the co-insertion of oxygen and sulfur. Decompositions of organic precursors containing oxygen or sulfur are especially helpful for this method (Figure 7, pathway D). For syntheses in which oxygen rate has to be finely controlled, inert atmosphere assured by N2 or argon is mandatory.
Since 20 years, a broad spectrum of techniques has been developed to yield Ln2O2S nanoparticles, which remains by far the center of the oxysulfide research. Here, we chose to classify them in three groups mainly depending on the reaction medium: water, organic solvent, and others. As we focused our study on the synthesis of nanomaterials, we excluded the works dealing with particles which were systematically sub-micronic or micronic (>700-800 nm).
Oxygen source The oxygen source for the formation of Ln2O2S nanoparticles highly depends on the synthetic route (Figure 7).
Commonly, in the water-based syntheses, oxygen is brought by hydroxide ions with the precipitation of an intermediate oxygenated phase in basic medium. Oxygen insertion in sulfides Ln2S3 has never been performed for nanoparticles, to the best of our knowledge. Molecular precursors such as lanthanide formate or lanthanide acetylacetonate contain enough oxygen for the targeted composition. In organic medium, the use of ketones as ligands enables the formation of in situ water when an amine is present. The thermal decomposition of single-source precursors with sulfide ligands can be performed in air or pure dioxygen to give Ln2O2S nanoparticles. In the case of reduction of sulfates and oxysulfates, no additional source of oxygen is required.
Sulfur sources (Scheme 1). In water, sulfidation is mainly carried out by solid-gas reaction with H2S or in situ formed CS2 using elemental sulfur heated in graphite or in presence of carbon. Nevertheless, a significant amount of syntheses also use sulfur sources soluble in water, such as thiourea or thioacetamide that initiate the sulfidation process. Elemental sulfur can also be used in organic medium especially dissolved in amines. Recently, substitution of oxygen by sulfur was carried out by ammonium sulfide and hexamethyldisilathiane (HMDTS).
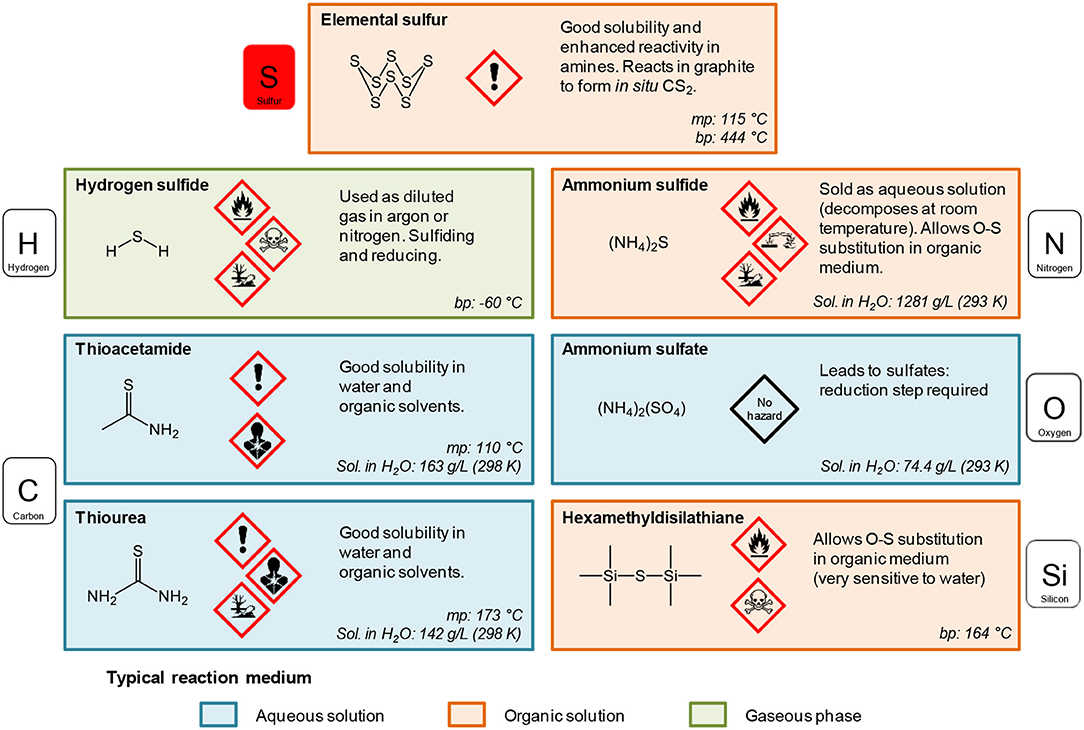
Scheme 1. Sulfur sources typically used for the sulfidation processes leading to oxysulfides (mp, melting point; bp, boiling point; Sol., solubility). The first neighbor of sulfur in the molecule is indicated.
Classical nanoparticles syntheses consist in heating hydrophobic or water-soluble inorganic precursors in aqueous or organic media, possibly sealed and/or pressurized and often followed by a thermal treatment which helps sulfidation and/or crystallization. In marge of these techniques, unconventional synthetic methods can be found. They involve unusual solvents, like molten salts, or are performed in uncommon conditions (electrospinning, combustion, and so on). This section describes such syntheses.
In 2008, Huang et al. adapted the boron-sulfur method, originally destined to the synthesis of sulfides, to the synthesis of La2O2S and Nd2O2S (Huang et al., 2008). In this synthesis, nanowires of the lanthanide hydroxide Ln(OH)3 (formed by reaction between Ln(NO3)3 and KOH) are directly heated in presence of boron and elemental sulfur S8 placed in a neighboring crucible. The driving force of the reaction is the strong affinity of boron with oxygen, which leads to the formation of B2O3 as a by-product.
When the reaction is maintained for 24 h at 400°C, LnS2 nanowires are obtained. Using shorter reactions times (500°C, 10 min), sulfidation of the wire is partial and Ln2O2S can be obtained (Figure 8A).

Figure 8. Ln2O2S nanoparticles obtained from unconventional synthetic methods: (A) Boron-sulfur method (La2O2S:Eu nanowires); Adapted with permission from (Huang et al., 2008), copyright (2008) American Chemical Society. (B) Combustion (La2O2S:Yb, Er nanoparticles); Adapted from Hakmeh et al. (2015) with permission of Elsevier. (C) Thermal decomposition of a gel of Pomelo skins (Ce2O2S nanoparticles supported on carbon); Adapted with permission from Yang et al. (2017), copyright (2017) American Chemical Society. (D) Emulsion liquid membrane system (Y2O2S:Yb, Er nanoparticles); Adapted with permission from Hirai et al. (2002), copyright (2002) American Chemical Society. (E) Electrospinning (Y2O2S: Yb, Er hollow nanofiber). Adapted from Han et al. (2015a) with permission of The Royal Society of Chemistry.
This solid-state reaction preserves the shape of the precursor. Also, it is one of the rare techniques that enable the formation of Ln2O2S2 nanomaterials using in some conditions an excess amount of sulfur compared with the targeted stoichiometry to ensure complete reactions. Nevertheless, only a small quantity of reactants were loaded in the crucible, leading to <15 mg of product per reaction. Also, the remaining species (B2O3, sulfur in excess) were washed with toxic CS2.
In order to get a swift synthesis, Hakmeh et al. (2015) developed a combustion synthesis by mixing lanthanide nitrates [La(NO3)3, Er(NO3)3 and Yb(NO3)3] with thioacetamide in ethanol. The precursors were rapidly inserted in a furnace at 500°C. Two successive flames evidenced first the ignition of ethanol, then the exothermic decomposition of the organic compounds, leading to an increase of the temperature and eventually to the formation of particles. A post-treatment at high temperature was also necessary (H2S in N2, 2 h, 1,000°C) and resulted in large particles with a typical size around 300-500 nm (Figure 8B).
Recently, an original catalyst for oxygen reduction reaction (ORR) was obtained by using the thermal decomposition of a vegetal, which provides the carbon support for the inorganic catalyst (Yang et al., 2017). Cerium nitrate [Ce(NO3)3] was dissolved in water along with thiourea and then pomelo skins were added to the solution in order to form a gel. After drying, the gel was annealed at 900-950°C for 2 h to get Ce2O2S supported on carbon doped by nitrogen and sulfur. When the reaction temperature was set to 850 or 1,000°C, the reaction led to the formation of CeO2. The TEM observation of the catalyst shows 50-100 nm crystals of Ce2O2S disseminated on the surface of the samples (Figure 8C). The porous structure, inherited from the pomelo precursor and the oxygen vacancies evidenced by the authors make this material suitable for the ORR.
Emulsion Liquid Membrane System (ELM) employs a water-in-oil-in-water (W/O/W) double emulsion. Originally, ELM was applied to separate metals. Here, the double emulsion is used for the formation of doped yttrium and gadolinium oxalates. These intermediates are converted to oxysulfides, Y2O2S:Yb, Er, and Gd2O2S:Eu, by a solid-state reaction with sulfur vapor (Hirai et al., 2002; Hirai and Orikoshi, 2004). Typically, a first emulsion is obtained by mechanical agitation of an organic phase containing kerosene with bis(1,1,3,3-tetramethylbutyl)phosphinic acid (DTMBPA) (or 2-methyl-2-ethylheptanoïc acid, VA-10) as extractant and sorbitan sesquioleate as surfactant and an aqueous phase containing oxalic acid. This emulsion is then added to the external water phase which contains the metal ions (chloride or nitrates) and the double emulsion is produced by mechanical stirring. The oxalate compounds are thus produced at ambient temperature, and the system is demulsified using ethylene glycol. Oxysulfides nanoparticles of 50-100 nm are then obtained by annealing the powders at 600-1,000°C in sulfur vapor generated at 200°C by elemental sulfur and carried by a N2 flow (Figure 8D).
The synthesis in molten salts is an emerging technique which consists in the use of one or several salts as solvents for an inorganic reaction. An eutectic mixture can even be used to benefit from a lower melting point. Molten salts are typically suitable for reaction temperatures between 300 and 1,000°C, which enable the formation of nanoparticles while avoiding their sintering (Portehault et al., 2011; Gouget et al., 2017). After cooling, the particles are obtained in a matrix composed by the salts that are washed with water or alcohols.
Molten sodium chloride (melting point: 801°C) was chosen for the one-pot synthesis of Y2O2S:Eu. Y(NO3)3, Eu(NO3)3 and NaOH were mixed and stirred before the addition of NaCl, S8 and a surfactant (Wang et al., 2014). After grinding, the mixture was heated to 850°C in a CO atmosphere for 4 h, and then cooled and washed.
Depending on the surfactant, the particles were either sub-micrometric or nanoscaled, but the morphology was quite irregular and the size polydisperse in all cases. For instance, polyoxyethylene (9) nonylphenyl ether (Scheme 2) gave 150-250 nm particles while sodium dodecylbenzenesulfonate (Scheme 2) gave 0.5-1.5 μm particles.
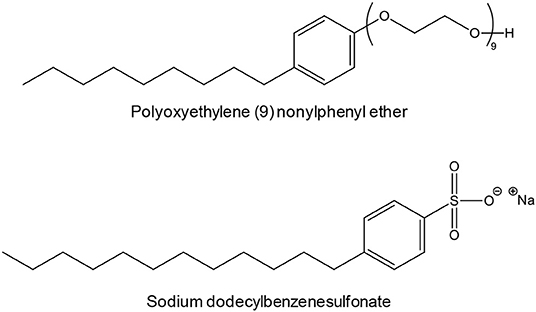
Scheme 2. Examples of surfactants employed by Wang et al. to synthesize Y2O2S:Eu in molten NaCl (Wang et al., 2014).
The composite-hydroxide-mediated method is also a synthesis in molten salts, but with hydroxides. Thirumalai et al. (2011a) adapted this method to the synthesis of Eu-doped yttrium oxysulfide by heating concentrated yttrium acetate Y(CH3COO)3 in an eutectic mixture of NaOH and KOH in an autoclave. As the eutectic of the mixture is 165°C, the autoclave was heated at 200°C to yield Y(OH)3 nanobelts (48 h) and nanorods (24 h) (Figure 9).
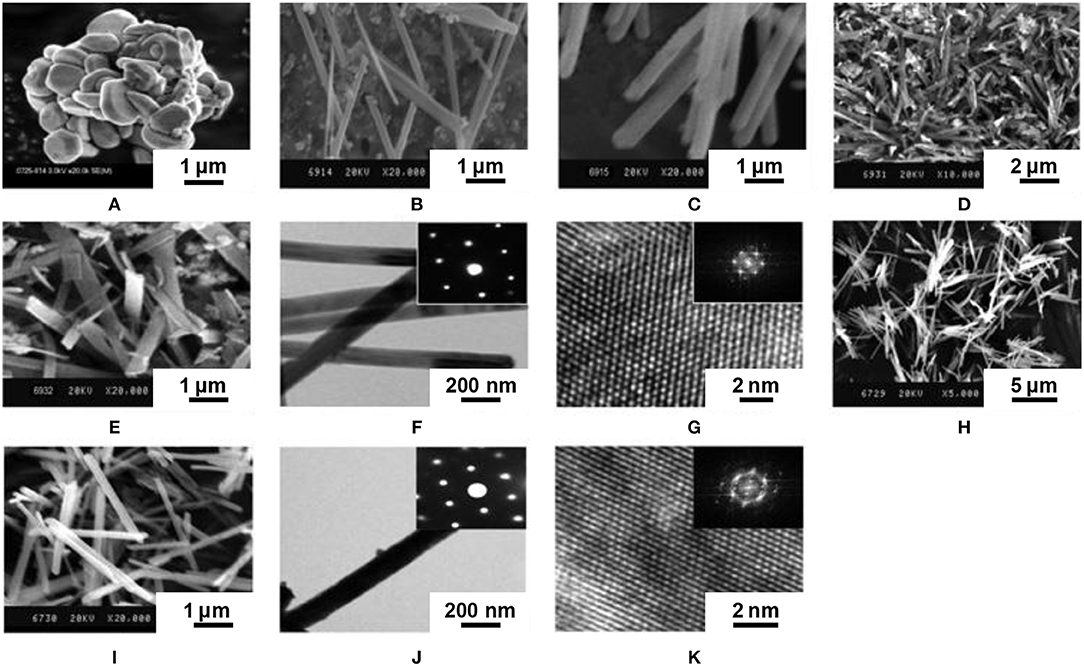
Figure 9. Europium-doped yttrium oxysulfide nanoparticles with different morphologies obtained via the composite-hydroxide-mediated method and their precursors. (A) SEM image of bulk Y2O2S:Eu. (B) SEM image of Y(OH)3 nanobelts. (C) SEM image of Y(OH)3 nanorods. (D) SEM image of Y2O2S:Eu nanobelts. (E) SEM image of Y2O2S:Eu nanorods. (F) TEM and (G) HRTEM images of Y2O2S:Eu nanobelts. (H) High-magnification SEM image of Y2O2S:Eu nanobelts. (I) High-magnification SEM image of Y2O2S:Eu nanorods. (J) TEM and (K) HRTEM images of Y2O2S:Eu nanorods. Insets are corresponding SAED patterns. Adapted from Thirumalai et al. (2011a), copyright (2011) The Institution of Engineering and Technology.
Europium and S8 were then mixed with the Y(OH)3 nanomaterial at 70-80°C and underwent an undescribed sulfidation process. In any case, the product was then annealed at 600°C for 2 h in an (Ar or N2)/sulfur atmosphere to form Y2O2S:Eu. Interestingly, the final product retained the morphology of the Y(OH)3 precursor. On the other hand, the step where the product was sulfidated was particularly unclear here, as three sulfidation processes are mentioned.
Electrospinning is based on the application of a high potential difference between a polymer solution or a polymer melt and a collector. The electrical field creates charged threads that can be assembled depending on the experimental parameters such as tension, temperature, relative humidity (RH), concentration of the precursors, viscosity, distance between capillary screen and collection screen, etc.
Lanthanide nitrates Y(NO3)3, Yb(NO3)3, and Er(NO3)3 and polyvinyl pyrrolidone (PVP) were dissolved in DMF and stirred 8 h (Han et al., 2015a). Fibers were produced by electrospinning. They were annealed twice: (i) at 700°C for 8 h under air to get Y2O3:Tb, Er fibers and (ii) at 800°C for 4 h in a CS2 atmosphere (obtained by heating S8 in presence of carbon) to yield Y2O2S:Yb, Er hollow nanofibers (Figure 8E). The same strategy was used to yield Y2O2S:Er hollow nanofibers (Han et al., 2015b). With slightly different electrospinning parameters, full nanofibers of Y2O2S:Yb, Er with a diameter comprised between 80 and 140 nm were obtained and studied by Lu et al. (2015).
In 2013, Cui et al. (2013a,b) elaborated a synthesis for doped oxysulfide nanoarrays using an anodic aluminum oxide template (AAO). A nitrate solution obtained by dissolution of Y2O3, Eu2O3, and Mg(OH)2·4MgCO3·2H2O in hot HNO3 (65%) was diluted by ethanol. Titanium doping was then obtained by adding the reaction product of Ti(OBu)4 with acetylacetone. The pH was adjusted to 1 with HNO3. The sol was eventually obtained by evaporation at 80-90°C. The AAO template was dipped in the sol, dried, calcined at 600°C for 2 h and etched by NaOH (2.0 M) to give Y2O3:Eu, Mg, Ti nanoarrays. The whole process involved numerous steps and the resulting nanoarrays had to be sulfurated to Y2O2S:Eu, Mg, Ti using S8 in graphite at 850°C sharp (Cui et al., 2013a). Lower and higher temperatures were indeed not adequate: they resulted, respectively, in uncomplete sulfidation or oxide formation. Besides, an optimal concentration of europium dopant for the luminescence properties was determined (6.5 mol% Eu vs. Y) (Cui et al., 2013b).
In the following syntheses, the reaction medium is water. It is an available, green, and ideal solvent for the dissolution of numerous metallic precursors, especially nitrates and chlorides.
Water also brings two main advantages: first, the availability of lanthanide precursors, and especially nitrates (that can be prepared from oxides in HNO3) and water-soluble sulfur sources (thioacetamide, thiourea, ammonium sulfide, sodium sulfide, and so on; see Scheme 1); second, the substantial knowledge on inorganic polymerization in water. So far, in more than 90% of the articles dealing with Ln2O2S nanoparticles, the desired feature of the material was luminescence. Luminescence is due to a controlled doping of the oxysulfide phase (Ln12O2S:Ln2, M3, M4) that is achieved by co-precipitation of the main cation (Ln1) with the cations that trigger the luminescence and influence its properties (Ln2, and possibly M3, M4,…).
Water is however limiting metal oxysulfide synthesis by its relatively low boiling point. Even hydrothermal syntheses with autoclaves do not provide enough energy to obtain crystalline oxysulfide nanoparticles. In general, syntheses lead to an intermediate nanoscaled phase (which sometimes already contains sulfur) that is subsequently fully converted in oxysulfide nanoparticles with a solid-gas sulfidation (Figure 7). This last step remains an important drawback. It requires relatively high temperatures for nanoparticles synthesis (typically between 600 and 1,100°C) and a large excess of inert gas and sulfur which is often present under the active but toxic gaseous forms of H2S or CS2. Also, it can affect the morphology of the solid by sintering or degradation of the desired phase.
The high-temperature sulfidation step remains the most challenging process here, but can be useful for other features. For luminescence purposes, the energy provided during the thermal treatment gives better-crystallized nanoparticles that present better photoluminescence properties. Moreover, doping ions can be inserted during this step.
Reported in 2008 by Liu et al., this synthesis stands out through the original use of gelatin and the way the oxysulfide phase is obtained (Liu et al., 2008).
First, the appropriate amounts of lanthanum, terbium, and europium nitrates obtained from dissolution of La2O3, Tb4O7, and Eu2O3 in nitric acid are mixed and heated with gelatin at 80°C in H2O. The obtained translucent gelatin sol turns into a gel at 0°C. Small pieces of the gel are soaked into NH3·H2O and La(OH)3:Eu, Tb precipitates inside the gel. Violent stirring can then turn the gel into sol again, and (NH4)2(SO4) is added in stoichiometric amount. After drying and annealing at 500°C for 2 h in air, a powder of oxysulfate La2O2SO4:Eu, Tb nanoparticles is formed. The oxysulfate nanoparticles are then converted to oxysulfide nanoparticles by solid-gas reaction using H2 as reducing gas (700-800°C, 2 h).
The pathway of oxysulfate reduction is quite rare in the oxysulfide nanoparticles literature, as it often requires high temperatures and long reaction times. Here, the nanoparticles however keep a reasonable 50 nm diameter. On the other hand, this synthesis comprises a myriad of steps, generates two intermediary phases and requires two heat treatments above 500°C.
This strategy is based on the elaboration of an organic network in which the inorganic nanoparticles nucleate and grow in a controlled way. The network is then burnt to free the nanoparticles. It is analogous to the Pechini method used for oxide synthesis for which a tridimensional polyester network is elaborated by reaction of trisodium citrate and ethylene glycol for instance (Pechini, 1967).
Dhanaraj et al. published in 2003 a first version of a sol-gel polymer thermolysis strategy to yield Y2O2S:Eu nanoparticles (Dhanaraj et al., 2003). Y(NO3)3 and Eu(NO3)3 were obtained from the corresponding oxides. Urea, formaldehyde and elemental sulfur were then added and the network was formed at 60°C. By condensation of urea and formaldehyde along with water evaporation, a gel was obtained. After thermolysis at 500°C in sulfidating atmosphere, Y2O2S:Eu nanoparticles were formed. Based on the XRD pattern, the product was not pure (small peaks of impurities). Despite the treatment at 500°C, the nanoparticles were quite small (around 30–50 nm) but presented an unclear morphology and aggregation. The work of Dai et al. in 2008 on La2O2S:Eu which deals with the effects of Eu3+ concentration on the photoluminescence is based on the same synthetic route (Dai et al., 2008).
One year later, Dhanaraj et al. published a second version of the protocol that led to hexagonal nanoplates with a size between 7 and 15 nm, tunable via the reactants concentrations (Dhanaraj et al., 2004). The thermolysis process was divided in two steps: first, the sol/network solid was heated at 500°C for 2 h to get Y2O3:Eu nanoparticles, and was subsequently digested by a thiosulfate solution. After water evaporation, a second thermal treatment at 500°C (1 h) burnt the mixture to yield Y2O2S:Eu nanoparticles. The authors did not obtain a pure product yet, based on XRD analysis, but this time they identified sodium polysulfides as side-products. Later, Thirumalai and Nakkiran reused this strategy, succeeded in washing the by-products (Thirumalai et al., 2007) and deeply investigated the nanoparticles: optical (Thirumalai et al., 2007, 2008a) and electronic properties (Thirumalai et al., 2008a) were discussed as well as the photo-assisted relaxation of surface states (Nakkiran et al., 2007).
Because of the attractiveness of luminescent water-dispersible nanoparticles, the pursuit of doped oxysulfide nanoparticles led to the publication and the refinement of synthetic strategies in water. However, the reported syntheses illustrate the complexity of obtaining oxysulfides at low temperatures in water: most often, the authors choose to precipitate an unsulfurated intermediary doped phase [Ln(OH)3, Ln(OH)(CO3) for instance] that can be amorphous or not. Thus, the syntheses presented in this section are worthwhile for oxide-, hydroxide-, or hydroxycarbonate-based nanomaterials. The intermediate nanoparticles are then sulfidated, most often with a solid-gas or alternatively with a solid-solid reaction.
Interestingly, the conditions for lanthanide oxysulfide nanoparticles syntheses in water are majorly optimized on Gd2O2S and Y2O2S because of their well-known luminescent properties and also maybe for the relatively low price of the related precursors (Table 2).
Decomposition of urea in water. Generally, the precipitation of the lanthanide salts is performed via the basification of the reaction medium. Thus, a significant amount of research has focused on the cheap, safe, highly available, and water-soluble urea. Urea is indeed known to decompose in ammonia [pKa(,NH3) = 9.25] and aqueous carbon dioxide which can carbonate aqueous lanthanide species (Scheme 3).
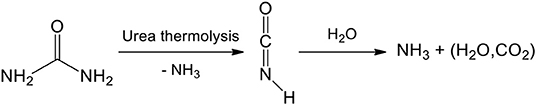
Scheme 3. Urea decomposition in water. Isocyanic acid is slowly obtained by urea thermolysis and ammoniac release. A second ammoniac molecule is released by hydrolysis which gives aqueous carbon dioxide “H2CO3.”
The concomitant release of ammonia and aqueous carbon dioxide is used in particular for the precipitation of lanthanide hydroxycarbonates Ln(OH)CO3 that turned out to be a suitable precursor of lanthanide oxysulfide nanoparticles. In the absence of sulfur source, further decomposition of this intermediate leads instead to lanthanide oxide. This was demonstrated in the pioneering work of Matijević and Hsu (1987) in the context of the fabrication of well-calibrated lanthanide colloids.
Syntheses with urea in water. The first aqueous synthesis of oxysulfide nanoparticles was reported by Kawahara et al. (2006; Table 3). Using yttrium and europium nitrates Y(NO3)3 and Eu(NO3)3 along with urea, an europium-doped hydroxide precursor Y(OH)3:Eu was obtained by heating the mixture possibly in the presence of a glycol (ethylene glycol, propylene glycol, or hexamethylene glycol). The isolated powder of Y(OH)3:Eu was then heated between 800 and 1,200°C with Na2CO3 and sulfur to create a sulfidating vapor and yield Y2O2S:Eu nanoparticles. XRD showed that the crystalline phase was pure Y2O2S. The obtained nanoparticles were facetted crystals of 100-300 nm length. Above 1,100°C, sintering made the particles sub-micrometric (≥600 nm).
Xing et al. (2009) then developed an inspiring but complex protocol to synthesize Y2O2S:Yb, Ho upconversion nanoparticles (Xing et al., 2009). A solution of lanthanide nitrates Y(NO3)3, Yb(NO3)3, and Ho(NO3)3 and a solution of urea were separately prepared. The latter solution was added to the first that had been pre-heated at 60°C and the mixture was then heated at 82°C. After cooling and aging during 48 h, a white amorphous precipitate [likely Y(OH)CO3] (Tian et al., 2017) was dried and converted to Y2O3:Yb, Ho via calcination (600°C, 1 h, air). Then, the oxide was sulfidated at 800°C for 1 h with a sulfur vapor created by S8 at 400°C and conveyed by an argon flow. It enabled the formation of size-monodisperse and non-aggregated nanoparticles with an average diameter of ca 80 nm. The diameter could also be tuned by adjusting the reaction time (aging step). Several works are based on Xing's synthesis with slight modifications. Luo et al. added a small amount of oleic acid in the urea mixture and performed the sulfidation at only 600°C to form the same Y2O2S:Yb, Ho nanoparticles (Luo et al., 2009). In the same group, Pang et al. (2010) reported additional reactions that coated the nanoparticles with functionalized silica using a derived Stöber process with polyvinylpyrrolidone (PVP), aqueous ammonia, teraethylorthosilicate (TEOS), and aminopropyltriethoxysilane (APTES) in a second step (Figure 10). Sulfidation of hydrated Ln(OH)CO3:Eu3+ nanoparticles (Ln = Gd, Dy, Ho) was alternatively performed under a flow of H2S at 750°C for 90 min followed by an annealing under Ar at 850°C for 4 h (Verelst et al., 2010; Osseni, 2012) This constitutes the sole reported route to Dy2O2S and Ho2O2S nanoparticles, to the best of our knowledge.
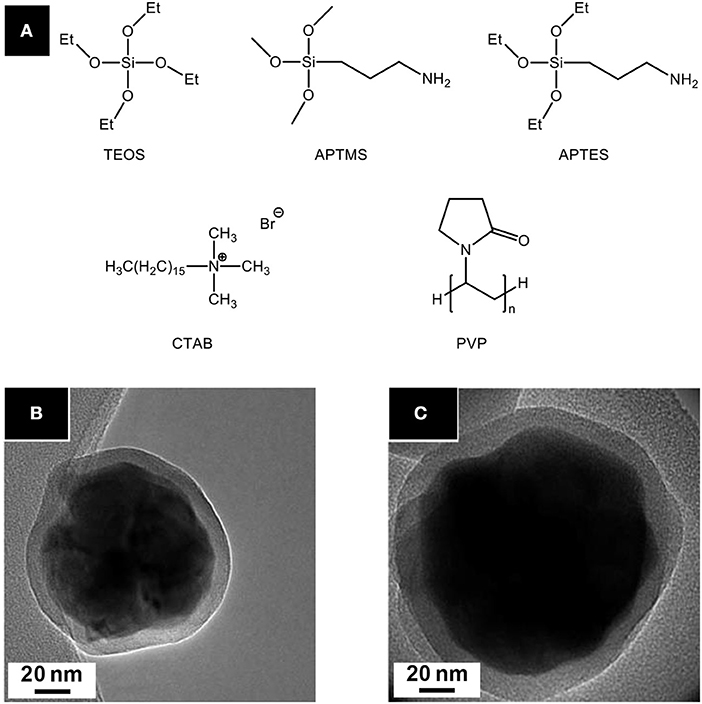
Figure 10. (A) Precursors and additives commonly used for nanoparticles silica coating. Tetraethylorthosilicate (TEOS) is used as silica precursor; 3-aminopropyltrimethoxysilane (APTMS), and 3-aminopropyltriethoxysilane (APTES) are rather employed for silica functionalization. TEM micrographs of Gd2O2S:Eu@SiO2-APTMS (B) and Gd2O2S:Eu@mSiO2 (C) nanoparticles from Osseni et al. (mSiO2 stands for mesoporous silica). Adapted from Osseni et al. (2011) with permission of The Royal Society of Chemistry.
Also based on Xing's work, Bakhtiari et al. (2015) later studied the effect of europium concentration on Y2O2S:Eu nanoparticles size and luminescence. Very recently, Tian et al. succeeded in forming upconverting core-shell nanoparticles Y2O2S:Er@Y2O2S:Yb,Tm by applying Xing's method twice to form the oxide-oxide compound Y2O3:Er@Y2O3:Yb,Tm as an intermediate (Tian et al., 2017). Solid-gas reaction with sulfur vapor at 800°C finally provided the oxysulfide nanoparticles. After the shell formation, Y2O3:Er@Y2O3:Yb,Tm nanoparticles were well-separated (Figure 11A). After sulfidation, the nanoparticles were aggregated because of sintering (Figure 11B). Nevertheless, the shell prevented the quenching of the ErIII luminescence and multicolor fluorescence was achieved thanks to ErIII/TmIII co-doping (Figure 11C).
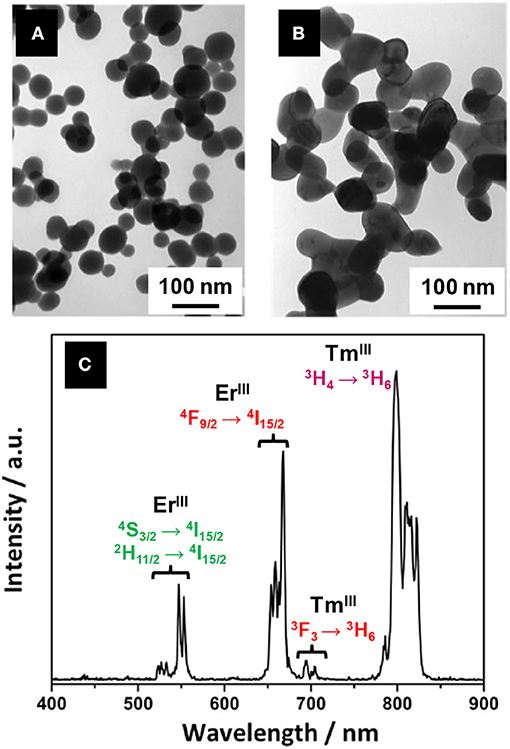
Figure 11. TEM micrographs of Y2O3:Er (A) and Y2O2S:Er@Y2O2S:Yb,Tm nanoparticles (B) synthesized by Tian et al. (C) Luminescence spectrum of Y2O2S:Er@Y2O2S:Yb, Tm nanoparticles under 1,550 nm excitation that exhibits the multicolor fluorescence of the core-shell nanoparticles (in the near-infrared region, between 780 and 830 nm, the 4I9/2→4I15/2 transition of ErIII was proven to play a minor role). Adapted from Tian et al. (2017) with permission of Elsevier.
Y2O2S:Eu, Mg, Ti nanoparticles were also synthesized for persistent luminescence applications by Ai et al. (2010a). Y(OH)CO3:Eu was obtained by heating a mixture of Y(NO3)3, Eu(NO3)3, and urea at 90°C for 2 h. The final product is obtained by a two-step thermal treatment developed by Li et al. (2009), It involves first S8 in graphite at 800°C for 4 h, which creates in situ reactive CS2, and then solid-solid reaction with doping solids (here Mg(OH)2·4MgCO3·6H2O and TiO2). The same year, Ai et al. (2010b) presented an original morphology for the same phase. Hollow submicrospheres were obtained using templating 350-400 nm carbon submicrospheres obtained by hydrothermal glucose decomposition (autoclave, 160°C, 9 h). Before sulfidation and Mg/Ti doping, Y2O3:Eu was obtained when removing carbon by thermal treatment at 700°C (2 h, air).
In 2011, Osseni et al. reported the first synthesis of Gd2O2S:Eu nanoparticles starting from nitrates and urea in a water/ethanol mixture (H2O/EtOH = 80/20 v/v) (Osseni et al., 2011). After dissolution, the reactants were heated to 85°C to form a doped hydroxycarbonate precursor Gd(OH)CO3·H2O:Eu. After isolation and drying, a heat treatment in two steps was performed. First, sulfidation was performed by Ar/H2S at 750°C for 90 min and then the nanoparticles were maintained at 850°C for 4 h under argon atmosphere only. The final nanoparticles were crystalline and spherical. Diameter was tunable by varying the H2O/EtOH ratio and reaction time. Interestingly, two techniques of deposition of silica on the nanoparticles were presented. The shell was either formed of mesoporous silica using TEOS and cetyltrimethylammonium bromide (CTAB) or functionalized by a silica/APTMS shell using TEOS and 3-aminopropyltrimethoxysilane (APTMS). In particular, mesoporous silica was found to enhance the luminescence properties of the nanoparticles. Multimodal imaging was recently applied using these Gd2O2S:Eu nanoparticles (Santelli et al., 2018).
A slightly different strategy, close to the work of Xing et al. on yttrium, was adopted in 2013 by Yan et al. (2013a) for the formation of terbium-doped oxysulfide nanoparticles of gadolinium and yttrium. Tb(NO3)3 and Gd(NO3)3 were dissolved in water around 100°C, and then urea was added. After filtration and drying, Gd(OH)CO3·H2O:Tb was obtained. The sulfidation process was quite complex: the precursor is mixed with Na2CO3 and sulfur but is also covered by a second mixture composed of Gd2O3, Na2CO3, and S8. The bottom layer was washed in hot water and filtrated after being fired at 900°C for 1 h. The crystalline phases Gd2O2S or alternatively Y2O2S were pure (based on XRD) and the polydispersity of the diameter was significant (average diameter around 100–120 nm). Yan et al. also studied the role of the doping ions in the luminescence mechanism of Y2O2S:Tb, Er nanoparticles (Yan et al., 2013b). In 2016, Bagheri et al. fabricated a scintillator screen composed of Gd2O2S:Pr nanoparticles synthesized via a similar nitrate/urea reaction (Bagheri et al., 2016). However, the sulfidation process is a solid-solid reaction with S8 at 900°C for 1 h.
In 2014, Hernández-Adame et al. extensively studied the influence of the reaction conditions on the morphology of Gd(OH)CO3:Tb and Gd2O2S:Tb, by mixing an urea aqueous solution with an aqueous solution of Tb(NO3)3 and Gd(NO3)3, and performing two thermal treatments (at 800°C under air and at 900°C under a N2/S atmosphere; Hernández-Adame et al., 2014). The precursor concentrations, the temperature of the stock solutions of nitrates and urea and the time and temperature of reactions were varied (Figure 12). Eventually, only one set of conditions gave regular spherical nanoparticles (Ø ≈ 100 nm): a nitrate solution at 6.0 10−3 M, pre-heated at 65°C, and a urea solution at 0.5 M, at room temperature, reacting for 90 min at 85°C (Figure 12B). Hernández-Adame et al. recently completed their work with a comprehensive study of the effects of the terbium concentration on the luminescence properties of their nanoparticles (Hernandez-Adame et al., 2018).
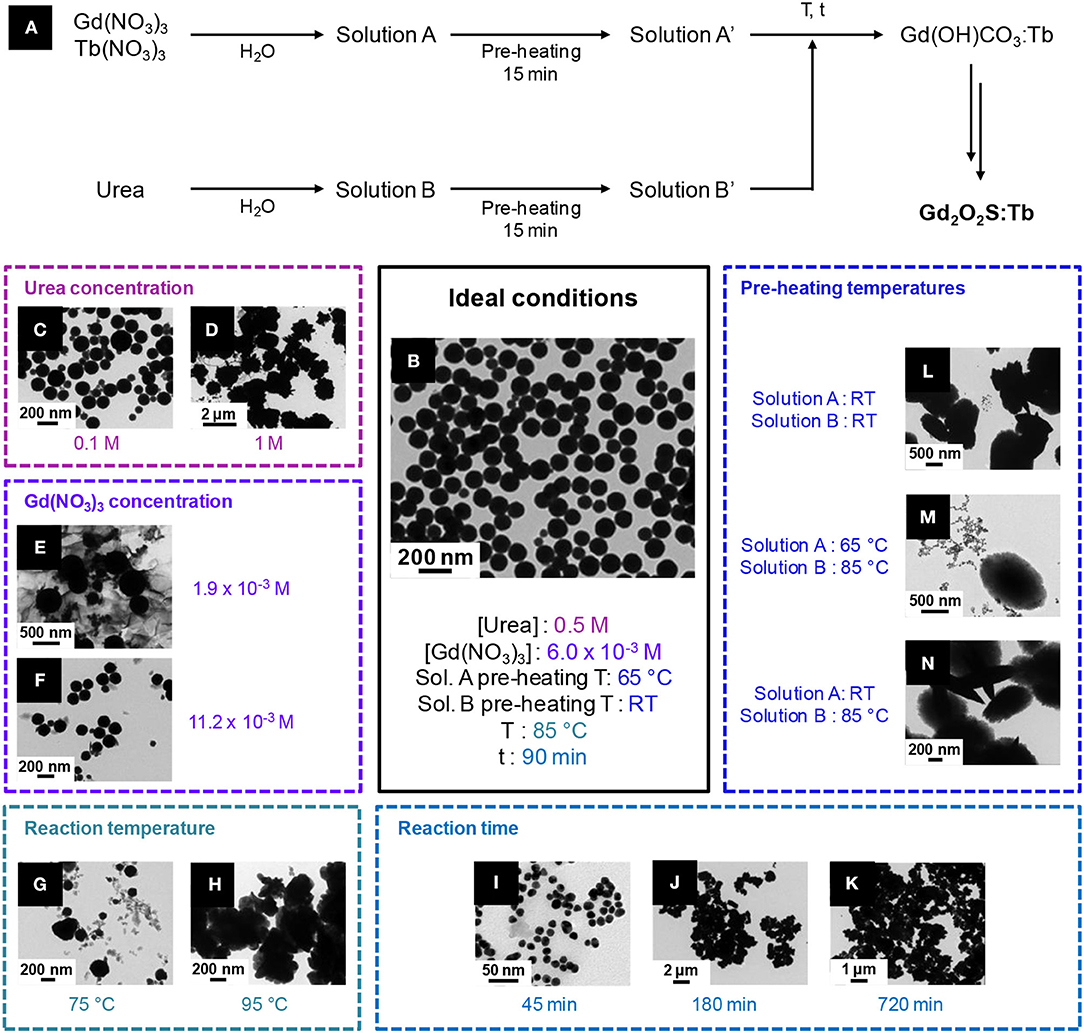
Figure 12. Optimized synthesis of Gd2O2S:Tb nanoparticles by Hernández-Adame et al. (2014) (A) Synthetic strategy: the authors first synthesized from nitrates and urea a doped hydroxycarbonate precursor that was later converted to the oxysulfide. TEM micrographs of the final Gd2O2S:Tb nanoparticles in the optimized conditions (B) and of the different Gd(OH)(CO3) particles obtained with through the optimization (C–N). Only one parameter is changed at once, the others being identical to the optimal conditions. Adapted from Hernández-Adame et al. (2014) with permission of Elsevier.
Recently, Cichos et al. studied three different syntheses of europium-doped Gd2O2S nanoparticles starting from nitrates and urea: (i) heating water at around 100°C for 2 h using an oil bath, (ii) heating a Teflon bottle at 100°C for 24 h, and (iii) heating an autoclave at 120°C for 12 h (see the autoclave section; Cichos et al., 2016). After reaction, the isolated solids were heated with an excess of sulfur under argon at 950°C for 1 h to yield Gd2O2S:Eu particles. In case (i), the intermediary solid was amorphous but the particles were spherical and quite monodisperse in diameter (Figure 13A). After sulfidation, crystalline Gd2O2S:Eu nanoparticles with a diameter close to 135 nm were obtained. The surface was rougher than the amorphous precursor's one. The Teflon bottle method [case (ii)] gave micrometric hydroxycarbonate Gd(OH)CO3 particles (Figure 13B) that were converted to Gd2O2S:Eu micrometric crystals and was thus not suitable for nanoparticles synthesis.
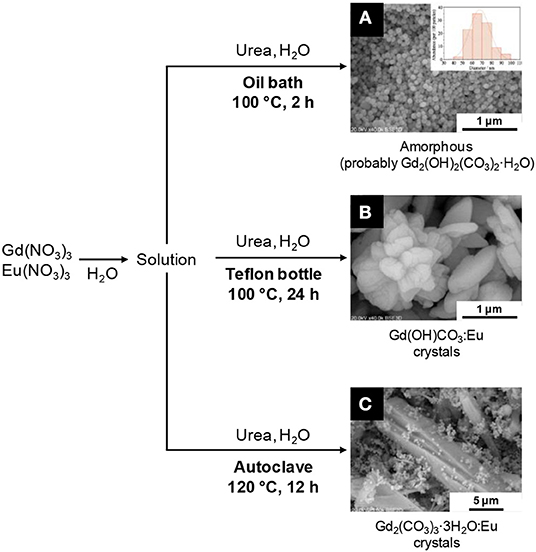
Figure 13. Structural and morphological variations of Gd2O2S precursors obtained by Cichos et al. (2016). Depending on the heating process, strong variations are observed: amorphous spherical nanoparticles (A with the size distribution in inset), hydroxycarbonate microcrystals (B) or carbonate microcrystals (C) can be obtained. Adapted from Cichos et al. (2016) with permission of Elsevier.
Closely related to urea's precipitating method, an aqueous ammonia/ammonium hydrogenocarbonate precipitation of nitrates was reported by Tian et al. (2015). A NH4HCO3/NH3·H2O solution was added dropwise to a nitrate solution including Y(NO3)3, Yb(NO3)3, and Er(NO3)3. A white precipitate of Y(OH)x(CO3)y:Yb, Er was obtained and dried. The Y2O2S:Yb, Er nanoparticles were obtained using sulfur vapor (S8 heated at 400°C) carried by N2 at 900°C for 1 h. The small but aggregated crystalline nanoparticles (Ø ≈ 30 nm) were phase-pure, based on XRD. Here, the use of ammonium hydrogenocarbonate and aqueous ammonia enabled the authors to carry out the reaction without heating whereas urea needed thermolysis.
Regarding upconverting oxysulfide nanoparticles, Fu et al. (2010) chose Na2CO3 to form intermediate solids which were then sulfidated. After dissolution of Y(NO3)3, Yb(NO3)3, and Ho(NO3)3, the nitrate solution was added in a 0.1 M solution of Na2CO3 containing PEG 4000 as surfactant. A solid precipitated, was isolated and dried. It was heated at 600°C to yield Y2O3:Yb, Ho. Then, the oxide was converted to oxysulfide using Xing's thermal treatment described in the previous section. Interestingly, Na2CO3 enables the authors to work at ambient temperature in the first step whereas urea required thermolysis. However, two thermal treatments were necessary to reach the oxysulfide product. Moreover, an irregular faceted morphology and a significant polydispersity in size were found in the final sample.
This section is dedicated synthesis in aqueous solution under pressure, in autoclave. We already mentioned the low boiling point of water as a strong limitation if we consider the temperatures commonly required for crystalline nanoparticles synthesis. Synthesis under pressure might be a way to overcome this limitation. Unfortunately, like the precipitation reactions at atmospheric pressure, the reported syntheses in hydrothermal conditions mainly focus on producing an intermediate solid that requires sulfidation in a second step (Table 4). Nevertheless, these syntheses expanded the range of available morphologies for the final oxysulfide nanoparticles.
In the late 2000's, Thirumalai et al. (2008b, 2009a) reported the hydrothermal synthesis of Gd2O2S:Eu (Table 4, entries 1, 2). Starting with an amorphous precipitate (obtained by adjusting the pH of an aqueous solution of Gd(NO3)3 with NaOH), they obtained Gd(OH)3 nanoscaled materials (hexagonal nanocrystals, nanotubes, nanobelts, …) after the hydrothermal treatment. The influence of the pH of precipitation and the temperature and duration of the hydrothermal define the morphology of the material. After impregnation of the solid with Eu3+ ions in a aqueous solution, sulfidation was performed using a CS2 atmosphere generated by reaction of sulfur and carbon. The morphology of Gd(OH)3 was retained in the final Gd2O2S:Eu nanopowder, with only slight size decreases. The nanomaterials are well-crystallized and the morphology is finely adjustable varying the reaction conditions. Unfortunately, an undescribed sulfidation process is performed before the annealing step. It is probably similar to the one mentioned before for the composite hydroxide method conducted by the same group (Thirumalai et al., 2011a). Moroever, an original study on the photo-induced impedance is presented. Interestingly, the morphology is retained also with other lanthanides, as similar results were obtained by Thirumalai et al. (2009b) on Y2O2S:Eu (Table 4, entry 3).
The oxysulfides nanoparticles obtained by hydrothermal syntheses were also extensively studied by Li, Ai, Liu et al. who obtained Y2O2S:Eu,Mg,Ti nanoparticles (Table 4, entries 4, 5, and 6; Li et al., 2009, 2010; Ai et al., 2010c). This combination of doping ions is typical for persistent luminescence. Aqueous ammonia NH3·H2O was used as a base for precipitation of hydroxides. The authors then inserted the dopants by solid-solid reaction in the annealing step with Eu2O3, Mg(OH)2·4Mg(CO3)·6H2O, and TiO2. Moreover, they noticed that using CS2 formed in situ, rather than solid S8, is crucial to keep the morphology. With S8, the Y(OH)3 nanotubes turned into hexagonal nanoparticles after the annealing step.
The group of Cui and Liu also put great efforts on the characterization of such nanoparticles (Table 4, entries 7 and 8; Cui et al., 2014b; Huang et al., 2014; Liu et al., 2014a,c). Soluble sources [Eu(NO3)3, Mg(NO3)2 and Ti(OBu)4] were employed as reactants rather than solids for doping. Thus, a moderated sulfidation annealing temperature ( ≤ 800°C) was employed to yield Y2O2S:Eu, Mg, Ti nanotubes. The synthesis (Cui et al., 2014b), the influence of earth-alkaline or metal MII ion (Huang et al., 2014; Liu et al., 2014b), the effect of the relative concentration of MgII and TiIV (Liu et al., 2014a), and the EuIII concentration were separately studied (Cui et al., 2014a). Later, Yuan et al. (2016) also reported mild conditions to synthesize composite Y2O3:Eu/Y2O2S:Eu nanoparticles starting from soluble nitrate precursors (Table 4, entry 12; Yuan et al., 2016). The particles were crystalline but presented an irregular morphology. They were incorporated in dye-sensitized solar cells that were fabricated by the group. An enhancement of the cell efficiency was measured thanks to the light scattering properties of the nanocomposite.
A rare example of Lu2O2S:Eu nanocrystals was reported in 2015 by Wang et al. (Table 4, entry 9). PVP K30 followed by a solution of thiourea in ethanol were added to lutetium nitrate dissolved in a mixture of water and ethylene glycol. Perfectly regular nanorods were obtained after a thermal treatment with a sulfidizing atmosphere. Here again, the sulfidizing step mechanism was not studied in detail.
In 2016, Cichos et al. tested an hydrothermal synthesis (Table 4, entry 10) to yield doped Gd2(CO3)3:Eu particles in comparison with reactions at atmospheric pressure (Cichos et al., 2016). The authors noticed that this method is rather not adapted to the synthesis of nanoparticles: several populations are obtained, including micrometric irregular crystals (Figure 13C).
The synthesis reported by Rosticher et al. (2016) is a promising exception (Table 4, entry 11). The crucial difference lies in the sulfidation method. An excess of water-soluble thioacetamide was incorporated before the hydrothermal heating after precipitation of amorphous Gd(OH)3:Eu, Mg, Ti with NaOH. This allowed incorporation of sulfur before the annealing step, which could conveniently be performed under inert atmosphere. Its role was only to improve the cristallinity and the luminescence performances of the powder.
The formation of oxysulfide nanoparticles in water encounters several limits. Because of the aqueous solvent, excess oxygen favors the formation of intermediary phases such as hydroxides, hydroxycarbonates, or oxides. Only an adequate sulfidation annealing step at high temperatures enables the formation of the oxysulfide nanoparticles. Nevertheless, it can affect the morphology of the nanoparticles with aggregation and sintering.
Moreover, the synthesis of the intermediary phases is also challenging. Precise reaction parameters have to be employed, with long optimization processes. In Figures 12, 13, we reminded for instance the works of Hernández-Adame et al. and Cichos et al. on the synthesis of doped gadolinium oxysulfide nanoparticles with urea. Not only the reaction temperature and time had a great effect on the final morphology of the intermediates: concentrations of the reactants, pre-heating temperatures, heating techniques are also crucial to obtain the desired product.
Working in organic medium then seems to be a suitable solution to overcome the excess available oxygen.
The following section is dedicated to the reactions mainly performed in organic medium. Thanks to the availability of high boiling-point solvents, the temperature of the reaction medium can reach 200-300°C much easier than in water. Moreover, the control of the nanoparticles size and morphology in organic solvents is easily attainable using surfactants.
In the case of lanthanide oxysulfide nanoparticles, we also note several benefits of organic medium for the stoichiometry:
(i) the control of the oxygen concentration that is assured by the absence of excess reactive oxygen brought by water and reactions under inert atmosphere,
(ii) the use of molecular sulfur sources with for instance the possibility of decomposing hydrophobic single-source precursors (typically, lanthanide complexes with sulfur-containing ligands) or the dissolution and activation of elemental sulfur in primary amines, as they react to form reactive alkylammonium polysulfides which release in situ H2S (Scheme 4; Thomson et al., 2011).
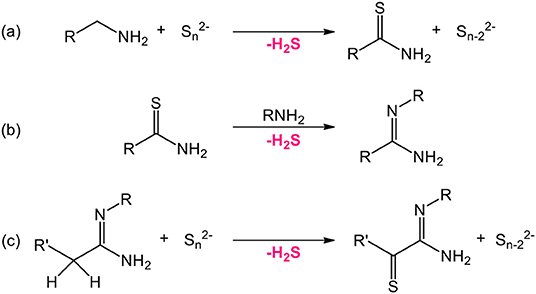
Scheme 4. Reaction pathways releasing H2S in a primary amine/elemental sulfur medium at 130°C as proposed by Thomson et al. (2011) Water traces can also react with the thioamide to form the corresponding amide and H2S similarly to reaction (b).
In both cases, the amount of reactive anions can be set to the desired value by playing on the concentration and the nature of the reactants. In water-based reactions, an excess of water in the precipitation step was followed by an excess of sulfur during the annealing step. Thus, organic medium brings the possibility to finely control the stoichiometry of the anions and one could expect that it leads to different oxysulfide compositions apart from thermodynamics considerations.
The decomposition of lanthanide complexes bearing ligands with sulfur in the presence of dioxygen can lead to oxysulfide nanoparticles. It was shown for the first time in 2006 in a communication by Zhao et al. who developed the synthesis of thin monodisperse hexagonal nanoplates of Eu2O2S, Sm2O2S and Gd2O2S (Zhao et al., 2006a). In a mixture of organic solvents and surfactants typical for colloidal synthesis (1-octadecene, oleic acid and oleylamine), [Eu(phen)(ddtc)3] (phen = 1,10-phenanthroline, ddtc = diethyldithiocarbamate; Scheme 5) was decomposed under air at 290°C in 45 min, forming anistropic nanocrystals (15 × 1.7 nm (Flahaut et al., 1958; Figure 14). For the first time, the observation of self-assembled oxysulfide nanoplates to nanowires is made (Figures 14A–C). The nanoplates are piled one above each other, because of the hydrophobic interaction between the surface surfactant chains of oleic acid (oleylamine-metal bonds are weaker than oleic acid-metal bonds; Cheon et al., 2004).
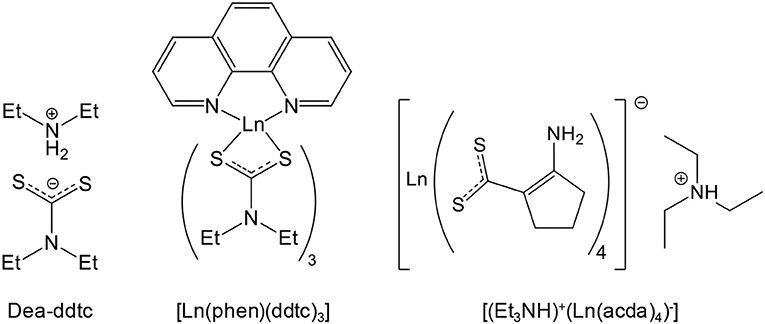
Scheme 5. Chemical formulas of diethylammonium diethyldithiocarbamate (dea-ddtc), [Ln(1,10-phenanthroline)(diethyldithiocarbamate)3] complex ([Ln(phen)(ddtc)3]), and Triethylammonium of tetra(2-aminocyclopentenedithiocarbamate) lanthanide ([(Et3NH)+(Ln(acda)4)−]).
Figure 14. Characterization of Eu2O2S nanoparticles obtained by decomposition of [Eu(phen)(ddtc)3] by Zhao et al. (2006a) TEM micrographs of self-assemblies formed by the hexagonal nanoplates (A), of a single nanowire (B) with a zoom in inset and HRTEM micrograph of two nanowires (C). (D) TEM micrographs of hexagonal nanoplates lying on their flat surface. Inset: representation of a single nanoplate. HRTEM images of Eu2O2S nanoplates lying on their flat surface (E) and of short nanorods (F). Insets are FFT of the indicated regions. (G) Powder XRD pattern of the Eu2O2S nanoparticles. Adapted with permission from Zhao et al. (2006a), copyright (2006) American Chemical Society.
Interestingly, EuS (EuII) nanocrystals were obtained with the same synthesis but under inert atmosphere with oleylamine alone (which played the role of reducing agent; Zhao et al., 2006b). A more detailed study on the pyrolysis of the [Ln(phen)(ddtc)3] precursor and the nanoparticles properties was also reported. A noticeable work using the same strategy was conducted by Tan et al. (2016). In comparison with europium, the decomposition of [La(phen)(ddtc)3] and [Pr(phen)(ddtc)3] only yielded LaS and PrS. From oxidation of the sulfides, oxysulfates nanoparticles of La2O2SO4 and Pr2O2SO4 were obtained. The nanoparticles of Eu2O2S, La2O2SO4, and Pr2O2SO4 were then tested for the water-gas-shift reaction.
Lin et al. (2016) obtained europium- and terbium-doped Gd2O2S and europium-doped Tb2O2S by decomposition of the same precursor. Although the morphology of the nanoparticles was not perfectly regular, and the crystallinity not optimal, an extensive luminescence study was performed and biologic tests (in vivo imaging, cell viability) were conducted. The latter required a coating with 3-aminopropyltriethoxysilane (Figure 10) and grafting of methoxy-polyethyleneglycol and Alexa Fluor 660 (photostable red dye which emits photons in the wavelength range of 630-650 nm).
In 2012, He et al. (2009) described a similar decomposition of a precursor formed in situ. The reaction yielded europium oxysulfide nanorods. In this synthesis, europium oleate, oleylamine, 1,10-phenanthroline, and dodecanethiol were heated at 320°C under inert atmosphere before hot injection of diethylammonium diethyldithiocarbamate (dea-ddtc, Scheme 5) dissolved in oleylamine. Nanorods were isolated after 1 h of reaction. The oxygen source was not explicitly discussed, but it was likely the oleate ions in the europium-oleate complex. Even if dea-ddtc is the most probable sulfur source, the introduction of dodecanethiol was not discussed. Nevertheless, this report showed that forming the single-source precursor in situ was a viable strategy. The non-stoichiometric character of the Eu2+xO2S nanoparticles was evidenced by the Eu/S ratio measured by EDS. Non-stoichiometry is attributed to EuII in the solid and was already observed for the bulk phase in the 1960's by Ballestracci and Quezel who estimated that 1% of the europium atoms were divalent thanks to neutron diffraction and magnetic measurements (Ballestracci et al., 1968; Quezel et al., 1970). He et al. (2009) also described magnetic properties of europium oxysulfide nanoparticles and confirm the EuII/EuIII ratio, even if the average composition Eu2.11O2S corresponds to about 15% of EuII. Moreover, an electrophoretic deposition of the nanorods was proposed.
Ghosh et al. reported another precursor to obtain Eu2O2S nanoparticles (Ghosh et al., 2016). According to the authors, La2O2S and Nd2O2S can also be prepared with a similar procedure. Synthesized from europium nitrate, triethylamine (Et3N) and 2-aminocyclopentene-1-dithiocarboxylic acid (Hacda), [(Et3NH)+(Eu(acda)4)−] was decomposed via three different methods (Scheme 6).
Scheme 6. Triethylammonium of tetra(2-aminocyclopentenedithiocarbamate) europium ([(Et3NH)+(Eu(acda)4)−]) decomposition in organic solvents by (Ghosh et al., 2016)
By heating the precursor in an OM/OA/ODE mixture, ultrathin nanoplates of Eu2O2S were obtained. However, the 0.3 nm reported thickness is quite surprising, as it would represent a single monolayer of the solid, and there is no correlated peak extinction in the corresponding XRD pattern. Using similar conditions with OM only led to rod-like nanoparticles (7 × 3 nm2). Finally, hot injection of [(Et3NH)+(Eu(acda)4)−] and trioctylphosphine (TOP) led to polydisperse nanospheres with an average diameter of 13 nm. The catalytic activity of Eu2O2S, and especially its activity as a peroxidase mimic, was deeply investigated. Because Eu2O2S catalyzed the oxidation of 3,3′,5,5′-tetramethylbenzidine (TMB) in presence of H2O2 and neither La2O2S nor Nd2O2S succeeded in it, the authors concluded to a mechanism involving the EuIII/EuII redox couple.
Also colloidal synthesis in organic solvents have been used for years in the synthesis of metal and metal oxide nanoparticles, the first report for metal oxysulfides was published by Ding et al. (2011). Lanthanide acetylacetonate Ln(acac)3 (1 equiv.), elemental sulfur (1 equiv.), and sodium acetylacetonate (1 equiv.) were added in an OM/OA/ODE mixture and heated for 45 min. at 310°C under inert atmosphere after degassing under vacuum at 120°C (Figure 15). Size-monodisperse hexagonal nanoplates of Ln2O2S were obtained. They were thin (a few monolayers) and 5–40 nm wide depending on the lanthanide. The composition of the powder showed a lack of sulfur (Na0.4La1.6O2S0.6), which was attributed to terminal [Ln2O2]2+ layers. The crucial advantage of this method is its high versatility: La2O2S, Pr2O2S, Nd2O2S, Sm2O2S, Eu2O2S, Gd2O2S, Tb2O2S were prepared. The sodium ions, added in stoichiometric amounts, were proposed to help the crystallization and favor the oxysulfide formation. The hypothesis of the authors is that the close ionic radii of sodium [r(NaI(VII)) = 1.26 Å] and larger lanthanide ions [r(LaIII(VII)) = 1.24 Å to r(TbIII(VII)) = 1.12 Å] enables cation exchanges in the solid and favors the oxysulfide crystallization. Lithium ions were tested and were efficient for Y2O2S synthesis. In 2013, a more complete study (experimental study and calculations based on density functional theory) on the alkaline additives on the formation and morphology of the obtained nanocrystals also showed the possible use of potassium to synthesize oxysulfide nanoparticles (La2O2S, Eu2O2S, Gd2O2S, and Yb2O2S; Zhang et al., 2013). The hypothesis of alkali insertion in the crystal structure was recently disputed: the alkali would serve as a stabilizing species for the formation of a lamellar alkali-oleate phase (as observed with sodium) rather than as a doping ion in the Ln2O2S structure (Larquet et al., 2020). In 2017, Lei et al. investigated the roles of yttrium and sodium in the formation and growth of Gd2O2S, by using them separately or combined. They also demonstrated that a large excess of sulfur allows forming gadolinium oxysulfide nanoplates without adding sodium ions (Lei et al., 2017).
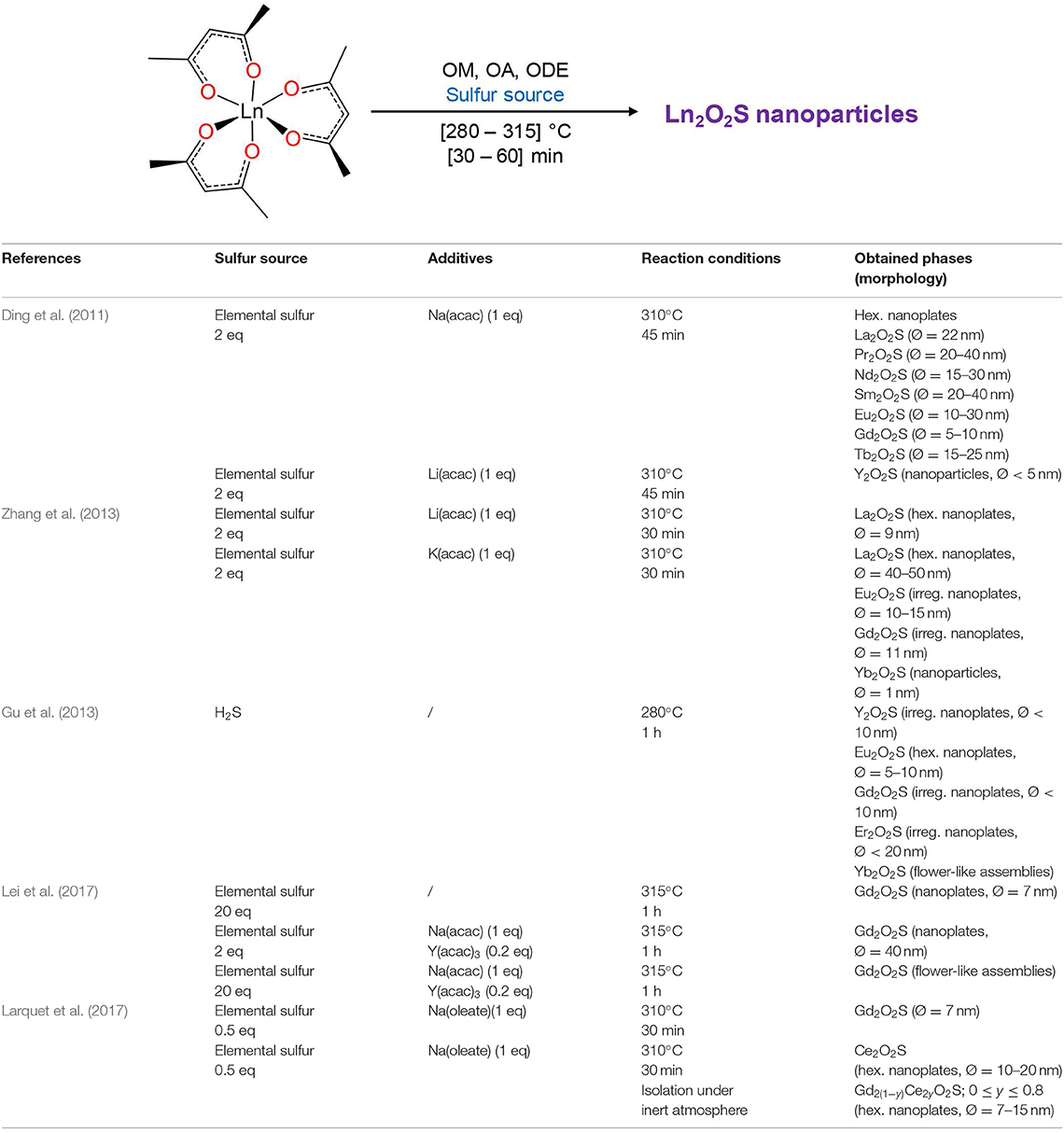
Figure 15. Ding's alkali-based synthesis of lanthanide oxysulfide and its derivatives. The number of equivalent (“eq” in the table) is the molar ratio between the reactant and metal. The term “hex.” stands for “hexagonal” and “irreg.” for “irregular.”
In 2017, Tan and Li announced the formation iron/sodium co-doped lanthanum oxysulfide nanoparticles (Na, La)2O2S:Fe (Tan and Li, 2017). Such doping with light transition metal is very rare due to ionic radii mismatch. Thus, according to the authors, only a limited amount of iron would have been able to substitute lanthanum, and surprisingly, no contraction of the lattice was observed despite the ionic radii difference [r(LaIII(VII)) = 1.24 Å; r(FeIII(VII)) ≈ 0.85 Å]. Even though such iron doping would be very interesting for catalytic features, it is quite unclear that iron was well-inserted in the La2O2S phase. In 2015, Jiang et al. employed Ding's synthesis and demonstrated the possible use of La2O2S:Eu nanoparticles as optical temperature sensors (“nanothermometer”) (Jiang et al., 2015). Our group recently investigated the reactivity of Ln2O2S hexagonal nanoplates formed with a stoichiometric amount of sulfur and demonstrated different oxidation processes in bimetallic Gd2(1−y)Ce2yO2S nanoparticles. It enabled us to prove that highly unstable Ce2O2S nanoparticles can also be formed with the help of sodium ions, as long as it is isolated and stored under strict inert conditions (Larquet et al., 2017). The thermal stability of the nanoparticles was investigated under inert and oxidizing atmosphere, highlighting the possibility to remove the oleate surface ligands by a mild thermal treatment (Larquet et al., 2019a). Moreover, both magnetic properties (Larquet et al., 2019b) and optical ones (bandgap) (Larquet et al., 2019c) could be tuned as a function of the Gd:Ce ratio.
Gu et al. managed to obtain yttrium, gadolinium, erbium, and ytterbium oxysulfide nanoplates using oleylamine as only solvent and H2S as sulfurating agent. Ln(acac)3 and oleylamine were degassed at 120°C and then heated at 280°C for 1 h under a H2S/N2 flow (20/80 v/v, 60 mL/min) to yield Ln2O2S nanoplates (Gu et al., 2013). Again, sodium ions were shown to help the crystallization of the nanoplates but were not necessary in this case. Y2O2S, Eu2O2S, Gd2O2S, Er2O2S, and Yb2O2S were prepared by this route.
Another method was reported in 2013 by Ma et al. (2013) to synthesize europium-doped lanthanum oxysulfide La2O2S:Eu. Lanthanide formates La(HCOO)3 and Eu(HCOO)3 were heated at 260°C in the presence of elemental sulfur (2 equiv. of S) in triethylenetetramine (TETA) and dodecanethiol (DT) (Figure 16). After 12 h, La2O2S:Eu nanocrystals were obtained with triethylenetetramine/dodecanethiol ratio being 1:2 and the nanospheres diameter was around 100 nm. Without dodecanethiol, 100 nm in width and 10 nm in thickness La2O2S:Eu nanoplates were obtained.
Figure 16. Reaction of Ma et al. (2013) to yield La2O2S nanoparticles.
The amine/thiol ratio influenced the morphology. When the TETA/DT ratio was 3:1 or 1:1, micronic structures were obtained. Interestingly, other precursors [La(NO3)3, LaCl3, La2O3, and La(OH)3] were not selective enough or did not completely react [impurities of La(OH)3 or La2O3 based on XRD]. Also, shorter reaction times with TETA/DT = 3:1 for the exhibited the rare La10OS14 intermediary phase with LaOOH and La2(SO4)3. Although the crystals are large and the selectivity can be improved, it is to the best of our knowledge the only occurrence of a promising protocol for nanoscaled Ln10OS14.
In 2000, Li et al. tested a direct and simple solvothermal sulfidation process for numerous lanthanide oxides. LnxOy powders (Ln = Y, Sc, La, Pr, Nd, Eu, Sm, Gd, Ho, Er, Yb, or Lu) and S8 were suspended in ethylenediamine and heated in autoclave at 150°C for 8 h (Li et al., 2000). Aggregated and irregularly-shaped crystalline spherical nanoparticles of Pr2O2S, Eu2O2S, and Gd2O2S were obtained with this method (<50 nm). The authors suggested an anion-exchange mechanism between the O−II in the LnxOy crystal and the S−II available in polyanions when S8 reacts with TETA. They supposed next that oxysulfide nuclei could leave the surface of the oxide to grow apart.
For Ln = La, Nd, Sm, Ho, and Er, the conversion was incomplete (Ln2O2S and LnxOy on the XRD pattern) and no change was observed with longer reaction times. Only the starting oxide was observed for Ln = Y, Sc, Yb, and Lu. In 2012, Gd2O2S:Eu and Gd2O2S:Er, Yb nanoplates were also obtained by Liu et al. in ethylenediamine using gadolinium nitrate and elemental sulfur (Liu et al., 2012). PVP (K29-32) or OM was added to a solution of the lanthanide nitrate in ethanol. The resulting solution was added dropwise into ethylenediamine and sulfur. The autoclave was heated at 220°C for at least 4 h to form crystalline hexagonal nanoplates. With OM, subsequent aggregation in flower-like structures was observed. Separated nanocrystals of irregular shape were obtained with PVP. Y2O2S:Eu and Y2O2S:Er, Yb were obtained from yttrium acetate by the same group with PVP and a thermal treatment at 250°C for 24 h (Liu et al., 2014a). Various self-assemblies of the nanoparticles were formed, depending on the presence of PVP, sulfur concentration, and so on.
Song et al. (2010) obtained Gd2O2S:Eu and Gd2O2S:Tb nanospheres from the solvothermal treatment of lanthanide nitrates in a mixture of ethanol and ethylene glycol, containing polyvinylpyrrolidone (PVP K30, M = 40,000 g/mol) and thiourea. The autoclave was heated at 200°C for 24 h and the isolated solid was then sulfidized in a N2/S atmosphere at 600-800°C to form doped gadolinium oxysulfide nanoparticles. PVP is believed to be responsible for the spherical morphology, and polymer residues were evidenced on the rough surface of the nanoparticles. They presented a good crystallinity and a good monodispersity in diameter. Their size was tunable between 150 nm and 1.25 μm by varying the PVP content and the ethanol/ethylene glycol ratio. A similar strategy was used by Deng et al. to yield Y2O2S:Sm hollow nanospheres (Ø = 140-200 nm; Deng et al., 2012a). The thermal treatment was based on Li's work (Y2O2S nanoparticles hydrothermal synthesis, Table 4, entry 4; Li et al., 2009). The authors first proposed a mechanism involving H2S/CO2 bubbles to explain the holes, but finally declared in a second paper on Y2O2S:Eu, Mg, Ti nanoparticles that NH3/CO2 bubbles were more likely the templating agents (Deng et al., 2012b). Similarly to Song's spheres, the surface was rough and the nanoparticles seemed to be constituted with smaller units.
Thirumalai et al. (2011b) have mainly focused their work on water-based syntheses, but also prepared various morphologies of Gd2O2S:Eu nanoparticles in oleylamine. GdCl3·6H2O and EuCl3·6H2O were introduced in hot oleylamine and various amounts of thioacetamide were added. The resulting solution was heated in an autoclave at 120-240°C for 12-24 h. Flower-like nanocrystals (≈10 nm), nanospheres (Ø = 5-10 nm) and nanorods of various lengths (Ø = 6 nm) were obtained depending on the reaction conditions and the thioacetamide amount. An excess of sulfur was proposed to be mandatory to ensure a high chemical potential, which promoted the formation of nanorods. Despite the good morphology control, the XRD patterns of the nanoparticles showed a poor crystallinity of the materials: only broad peaks were observed. It is intriguing because nanorods presented big crystal domains, and HRTEM images showed large and regular lattices.
Transition metal bulk oxysulfides are quite rare. Zinc, titanium, molybdenum, and tungsten oxysulfide were nevertheless obtained. Most of the time, they were obtained under the form of amorphous thin films or particles.
Because of the electronegativity and atomic number differences between the two anions, transition metals will preferentially bind to one of them (in the hard and soft acids and bases theory, oxygen is a hard base and sulfur a soft base). Also, keeping reduced sulfur id est avoiding sulfates or other oxidized sulfur species is highly difficult because of their good thermodynamic stability.
In the previous section, we detailed numerous syntheses of Ln2O2S nanoparticles. It is an exception in the oxysulfide family, as it remains to the best of our knowledge the only structure for which monophasic crystalline nanoparticles could be formed. Today, a vast and promising land of metal oxysulfide nanoparticles, especially involving transition metals, must be explored. With such nanoparticles with transitions metals and chalcogens, new applications could emerge, such as heterogeneous catalysis, photocatalysis, battery materials, superconduction, and so on.
Several advantages are intrinsically brought by soft reaction conditions (compared with typical synthesis of bulk crystals) and the nanoscale. Mild temperatures and small grain size can unlock metastable structures. Also, diffusion processes are much faster over nanometric distances and lead to efficient substitution reactions with nanoscaled materials. It opens new synthetic strategies to transform preformed oxide, sulfide, or metal nanoparticles in oxysulfide nanoparticles.
However, synthesizing transition metal ternary oxysulfides is particularly challenging. The ionic radius difference between O−II (1.26 Å) and S−II (1.70 Å) associated with the variable affinities with the metal make the substitution reactions highly difficult. Energy input by heating is especially not recommended for nanoparticles synthesis because of excessive growth and sintering.
Despite these difficulties, transition metal oxysulfide nanoparticles were already prepared. A few examples will be detailed in the next section. The main issue consists of identifying and evidencing the oxysulfide nature of the compound. Because excessive heating tends to stabilize sulfate or separate oxides and sulfides rather than crystallize an oxysulfide structure, the reported structures are mainly amorphous.
Identification and characterization of such phases is much harder than crystalline nanoparticles. In particular, inductively coupled plasma atomic emission spectroscopy (ICP-AES), X-ray fluorescence (XRF), and energy dispersive X-ray spectroscopy (EDS) are suitable techniques to evidence the presence of sulfur, but the determination of the nanoparticles' precise oxygen content remains a challenge. High resolution transmission electron microscopy and energy filtered transmission electron microscopy (EFTEM) constitute an elegant solution, but requires well-dispersed nanoparticles and will not provide accurate quantitative data. Moreover, it is hard to conclude about the precise localization of the atoms: are they in the whole particle or only at the surface (because of ligands for instance)?
The identification of the nature of the chemical bonds and oxidation states inside the material is a supplementary issue, yet this is required to differentiate oxysulfides from sulfates. It becomes highly problematic when the composition of a solid is unclear. Infrared and Raman spectroscopies are particularly appropriate for amorphous oxysulfide identification because M-O and M-S bonds generally present very distinguishable signatures. However, only qualitative analysis is possible. X-Ray photoemission spectroscopy (XPS) brings some clues but investigates only the very surface. X-ray absorption spectroscopy, such as XANES or EXAFS (at O K-edge, S K-edge, M K, L, or M-edge) is able to characterize the whole sample and gives precious information on the oxidation states and chemical bonds. However, surface and core cannot be distinguished and only average information is obtained, so that one should be very careful about hypothesis and interpretations. Furthermore, it must be noticed that the required energies for the different edges involves the use of different X-ray ranges (soft for oxygen, tender for sulfur, hard for the metal K-edge) and consequently the use of distinct beamlines. The analysis of the pair distribution function of the diffuse background of X-ray diffraction patterns (PDF) is expected to bring solutions as it can be applied to the analysis of amorphous compounds. Still, it remains a poorly studied technique in the field of nanoparticles analysis.
Finally, we emphasized the fact that oxysulfide nanoparticles can be metastable or unstable phases. It reinforces the difficulty to store, transfer, manipulate, and characterize them (for instance in air-filled room atmosphere and devices or when heating upon irradiation by electron or X-ray beams).
In 2016, Nelson et al. reported the formation of cobalt oxysulfide CoOxSy hollow nanoparticles (Nelson et al., 2016). The strategy consisted in the substitution of oxygen anions by sulfur anions in cobalt oxide hollow nanoparticles, using ammonium sulfide dissolved in oleylamine at 100°C (Figure 17A). The sulfur content was adjustable via the nominal (NH4)2S amount, with a saturation of the sulfur content at y ≈ 1.3. With low sulfur contents (y < 0.2), the particles keep the crystalline structure of CoO but with higher sulfur contents, the nanoparticles became amorphous (Figure 17F). The hollow nanosphere morphology was preserved during the whole experiment (Figures 17B–E).
Figure 17. Adapted from Nelson et al. (2016) (A) Synthetic strategy of CoOxSy hollow nanoparticles. TEM micrographs of CoOxSy nanoparticles with y = 0 (B); 0.18 (C); 1.03 (D); 1.27 (E). (F) Rotationally averaged SAED patterns of the various CoOxSy nanoparticles. Reference lines in green indicate CoO Bragg peaks (JCPDS 00-048-1719). Adapted from Nelson et al. (2016) with permission of the Royal Chemical Society.
No direct proof of the oxidation state of sulfur is brought by the authors. Nevertheless, annealing the sulfur-rich nanoparticles led to the formation of cobalt sulfides (possibly in a mixture with CoO). It supported the presence of reduced sulfur in the nanoparticles.
Crystalline zinc oxysulfide was obtained at the nanoscale. In 2009, Park et al. carried out the substitution of oxygen atoms in ZnO by sulfur using hexamethyldisilathiane (Scheme 1) and obtained ZnS crystalline hollow nanoparticles (Figure 18; Park et al., 2009). The driving force of the reaction with ZnO is the formation of very stable Si–O bonds.
Figure 18. TEM (A) and EFTEM (B) images of ZnO@ZnS nanoparticles. (C) Oxygen and sulfur composition along the cross-section in (B). (D) XRD patterns of the nanoparticles from ZnO to ZnS through ZnO@ZnS core-shell nanoparticles. Inset: Normalized pattern in the [38.5°; 44.5°] 2θ region. The small shifts of the diffraction peaks toward low 2θ values indicate a lattice dilatation caused by sulfur insertion. Adapted with permission from Park et al. (2009), copyright (2006) American Chemical Society.
During the substitution process, the authors were able to isolate ZnO@ZnS core-shell crystalline nanoparticles, which are composed by a core of ZnO and a shell of isostructural ZnS wurtzite structure.
The process was accompanied by the so-called “nanoscale Kirkendall effect,” which refers to the hollowing of the nanoparticles as a consequence of unbalanced diffusion rates (Wang et al., 2013). Because ZnII diffuse outwards faster than S−II inwards, the reaction finally led to a hollow ZnS structure. HRTEM and EFTEM also showed that the final ZnS nanoparticles were obtained through the formation of heteroepitaxial ZnO@ZnS intermediates that release the high interface energy by the diffusion of the core into the shell (Figure 19). The composition analyses of core-shell intermediates indeed showed that oxygen is not only localized in the core of the nanoparticle, but also in the shell. It suggested that the substitution process with hexamethyldisilathiane took place in the shell region where oxygen had migrated.
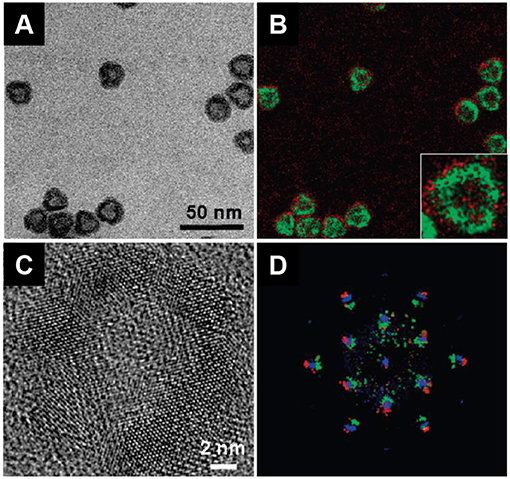
Figure 19. TEM (A), EFTEM (B), and HRTEM (C) of annealed core-shell nanoparticles. EFTEM strongly suggest the formation of a ZnO1−xSx nanoalloy. (D) FFT image of (C) overlaid on FFT image of the precursor ZnO@ZnS core-shell nanoparticles (red: ZnO; green: ZnS; blue: annealed nanoparticles). These images support the heteroepitaxial formation of the ZnS shell and ZnO1−xSx when thermally annealed. Adapted with permission from Park et al. (2009), copyright (2006) American Chemical Society.
The reaction resulted in the formation of crystalline ZnO1−xSx located in the shell. Furthermore, the authors were able to obtain pure hollow ZnO1−xSx nanoparticles by thermally annealed the core-shell intermediates. Interestingly, the diffusion processes spontaneously occurred without sulfurating reagents and led to hollow ZnO1−xSx alloys.
The research on zinc oxysulfide nanoparticles has grown in the last years. Pandey et al. (2013, 2014) managed to obtain the whole composition range (0 ≤ x ≤ 1) of nanoparticles. They obtained ZnO1−xSx crystalline nanoparticles by a solution combustion method. Zn(acetate)2 and thiourea were incorporated in a mixture of ethanol and ethylene glycol (4:1) and then placed in a hot furnace (350°C) for 2 h. They showed that the bandgap varied with the sulfur content and that ZnO1−xSx nanoparticles can photocatalyze the degradation of methyl orange. In 2017, Zhang et al. underlined the importance of doped zinc oxide to understand the intriguing ferromagnetic properties of certain d0 components and used the same combustion method than Pandey (Zhang et al., 2017). As a consequence of oxidation by air, they measured a significant amount of sulfate groups at the surface of their nanoparticles using XPS. Abdullah et al. (2017) synthesized ZnO1−xSx nanoparticles using zinc(II) acetate and thioacetamide for the photocatalysis of the hydrogen evolution reaction. Gultom et al. (2017) in the same group also showed that nickel-doped ZnO1−xSx nanoparticles were suitable for hydrogen production.
Crystalline nano-aggregates of cobalt nickel oxysulfides (CoNi)OySz were claimed by Liu (2013). However, the author noticed that sulfur only existed in oxidized species (SIV, SVI) in the material using XPS. By definition, it cannot be called “oxysulfide” but should rather be named “oxysulfate.”
In 2017, Liu et al. reported “fullerene-like oxysulfide hollow nanospheres” (Liu et al., 2017). The name is also abusively employed in this case, as the authors demonstrated that their nanoparticles are composed of crystalline nickel sulfide mixed with amorphous nickel oxide.
The oxysulfide family is full of surprises. Most of its members are strictly synthetic because of size and electronegativity differences of oxygen and sulfur. It explains why these compounds were obtained as pure materials and identified relatively late in the history of Chemistry. However, since the 1950's, many compositions have been synthesized, from the simplest ternary compounds to oxysulfide materials containing five or more different atoms.
Among this family, Ln2O2S must be pointed out. It was the first discovered phase, and represents one of the simplest oxysulfide compositions. It is nearly the only one for which the scientific community proposed various applications especially in the domain of imaging. Consequently, it led to the development of numerous synthetic approaches for Ln2O2S nanoparticles. Size, morphology, composition, and reactivity of Ln2O2S nanoparticles are more and more controlled and understood. However, there is still an open door for the study of novel Ln2O2S nanoparticles. Surprisingly, nanoscaled Ln2O2S with heavy lanthanides remain hard to obtain. To the best of our knowledge, Tm2O2S nanoparticles were never prepared. Also, most of the articles focus on the luminescence applications. Yet, in the domain of catalysis for instance, europium, cerium, or ytterbium redox properties have only been poorly explored in such compounds. Lastly, at this point there is no general method to control the size and shape of these nanoparticles on a broad range: only slight adjustments are proposed so far. Such control would enable understanding the influence of the nanoscale on the electric, magnetic, and optical properties. In particular, controlling the nanoparticles thickness could allow to identify the transition between a regime of indirect bandgap (as in the bulk) to the direct one.
Regarding transition metals, their integration in quaternary oxysulfides at bulk scale is successful. Recently, lanthanide-free compositions were obtained. Still, crystalline ternary oxysulfides are very rare, and the characterization of the amorphous products is still incomplete, even if molybdenum or titanium oxysulfide thin films for instance were explored in many studies. One of the challenges is to identify preparation route that lead to crystalline compounds that are easier analyzed by structural techniques. The synthesis of nanoscaled materials with accelerated diffusion processes and possible metastable phases, the developments of characterization techniques and the promising field of applications of transition metals oxysulfides should start an unprecedented area of novel oxysulfide syntheses, identifications and applications.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
This work was supported by French state funds managed by the ANR within the Investissements d'Avenir programme under reference ANR-11-IDEX-0004-02, and more specifically within the framework of the Cluster of Excellence MATISSE led by Sorbonne Universités.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abdullah, H., Kuo, D. H., and Chen, X. (2017). High efficient noble metal free Zn(O,S) nanoparticles for hydrogen evolution. Int. J. Hydrogen Energy 42, 5638–5648. doi: 10.1016/j.ijhydene.2016.11.137
Abraham, K. M., and Pasquariello, D. M. (1993). Synthesis, characterization, and lithium battery applications of molybdenum oxysulfides. Chem. Mater. 5, 1233–1241. doi: 10.1021/cm00033a009
Abraham, K. M., Pasquariello, D. M., and Willstaedt, E. B. (1989). Lithium/molybdenum oxysulfide secondary batteries. J. Electrochem. Soc. 136:576. doi: 10.1149/1.2096686
Afanasiev, P., and Bezverkhy, I. (2003). Genesis of vesicle-like and tubular morphologies in inorganic precipitates: amorphous Mo oxysulfides. J. Phys. Chem. B 107, 2678–2683. doi: 10.1021/jp021655k
Ai, P. F., Li, W. Y., Xiao, L. Y., Li, Y. D., Wang, H. J., and Liu, Y. L. (2010a). Monodisperse nanospheres of yttrium oxysulfide: synthesis, characterization, and luminescent properties. Ceram. Int. 36, 2169–2174. doi: 10.1016/j.ceramint.2010.05.025
Ai, P. F., Liu, Y. L., Li, W. Y., and Xiao, L. Y. (2010c). Synthesis and luminescent characterization of Y2O2S: Eu3+, Mg2+, Ti4+ nanotubes. Phys. B Condens. Matter 405, 3360–3364. doi: 10.1016/j.physb.2010.05.005
Ai, P. F., Liu, Y. L., Xiao, L. Y., Wang, H. J., and Meng, J. X. (2010b). Synthesis of Y2O2S:Eu3+, Mg2+, Ti4+ hollow microspheres via homogeneous precipitation route. Sci. Technol. Adv. Mater. 11:035002. doi: 10.1088/1468-6996/11/3/035002
Ajithkumar, G., Yoo, B., Goral, D. E., Hornsby, P. J., Lin, A.-L., Ladiwala, U., et al. (2013). Multimodal bioimaging using a rare earth doped Gd2O2S:Yb/Er phosphor with upconversion luminescence and magnetic resonance properties. J. Mater. Chem. B 1, 1561–1572. doi: 10.1039/c3tb00551h
Aliev, O. M., and Tanryverdiev, V. S. (1997). The synthesis and some physical properties of rare-earth oxysulfostibnites. Zhurnal Neorg. Khimii 42, 1918–1921.
Altmannshofer, S., and Johrendt, D. (2008). Synthesis, crystal structure and magnetism of the new oxysulfide Ce3NbO4S3. Zeitschrift für Anorg. Allg. Chem. 634, 1361–1364. doi: 10.1002/zaac.200800078
Alves, R. V., Buchanan, R. A., Wickersheim, K. A., and Yates, E. A. C. (1971). Neodymium-activated lanthanum oxysulfide: a new high-gain laser material. J. Appl. Phys. 42, 3043–3048. doi: 10.1063/1.1660681
Auzel, F. (2004). Upconversion and anti-stokes processes with f and d ions in solids. Chem. Rev. 104, 139–174. doi: 10.1021/cr020357g
Bagheri, A., Rezaee Ebrahim Saraee, K., Shakur, H. R., and Zamani Zeinali, H. (2016). Synthesis and characterization of physical properties of Gd2O2S:Pr3+ semi-nanoflower phosphor. Appl. Phys. A Mater. Sci. Process. 122:553. doi: 10.1007/s00339-016-0058-z
Bakhtiari, H., Ghasemi, M. R., Hashemizadeh Aghda, A., Noorkojouri, H., Sarabadani, P., and Zeeb, M. (2015). Effect of europium dopant concentration on particle size and luminescence of yttrium oxysulfide nanoparticles prepared by urea homogenous precipitation. J. Clust. Sci. 26, 1671–1681. doi: 10.1007/s10876-015-0866-x
Bakke, J. R., Tanskanen, J. T., Hägglund, C., Pakkanen, T. A., and Bent, S. F. (2012). Growth characteristics, material properties, and optical properties of zinc oxysulfide films deposited by atomic layer deposition. J. Vac. Sci. Technol. 30:01A135. doi: 10.1116/1.3664758
Ballestracci, R. (1967). Structure cristalline des oxydisulfures de terres rares Ln2O2S2. Mater. Res. Bull. 2, 473–479. doi: 10.1016/0025-5408(67)90068-2
Ballestracci, R., Bertaut, E. F., and Quezel, G. (1968). Etude par diffraction neutronique et mesures magnetiques des oxysulfures de terres rares T2O2S. J. Phys. Chem. Solids 29, 1001–1014. doi: 10.1016/0022-3697(68)90236-9
BaQais, A., Curutchet, A., Ziani, A., Ait Ahsaine, H., Sautet, P., Takanabe, K., et al. (2017). Bismuth silver oxysulfide for photoconversion applications: structural and optoelectronic properties. Chem. Mater. 29, 8679–8689. doi: 10.1021/acs.chemmater.7b02664
Baybarz, R. D., Fahey, J. A., and Haire, R. G. (1974). The preparation, crystal structures and some properties of californium oxysulfate and oxysulfide. J. Inorg. Nucl. Chem. 36, 2023–2027. doi: 10.1016/0022-1902(74)80716-5
Besançon, P. (1973). teneur en oxygene et formule exacte d'une famille de composes habituellement appeles “Variété β” Ou “Phase Complexe” des sulfures de terres rares. J. Solid State Chem. 7, 232–240. doi: 10.1016/0022-4596(73)90159-X
Besançon, P., Carré, D., Guittard, M., and Flahaut, J. (1970). Sur une famille de composes usuellement appeles “Variété Beta Des Sulfures de Terres Rares.” Comptes Rendus Hebd. l'Acad. Sci. 271, 679–682.
Besançon, P., Carré, D., and Laruelle, P. (1973). Mécanisme de la solution solide des oxysulfures de terres rares l10s15–xox. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 29, 1064–1066. doi: 10.1107/S0567740873003869
Biltz, W. (1908). Über die sulfide der seltenen erden. 1. mitteilung: über cersulfide und ihre existenzgebiete. Berichte der Dtsch. Chem. Gesellschaft 41, 3341–3350. doi: 10.1002/cber.19080410310
Biondo, V., Sarvezuk, P. W. C., Ivashita, F. F., Silva, K. L., Paesano, A., and Isnard, O. (2014). Geometric magnetic frustration in RE2O2S oxysulfides (RE=Sm, Eu and Gd). Mater. Res. Bull. 54, 41–47. doi: 10.1016/j.materresbull.2014.03.008
Blandy, J. N., Abakumov, A. M., Christensen, K. E., Hadermann, J., Adamson, P., Cassidy, S. J., et al. (2015). Soft chemical control of the crystal and magnetic structure of a layered mixed valent manganite oxide sulfide. APL Mater. 3:041520. doi: 10.1063/1.4918973
Boller, H. (1973). Die Kristallstruktur von Bi2O2Se. Monatshefte Chem. 104, 916–919. doi: 10.1007/BF00903904
Boyer, C., Deudon, C., and Meerschaut, A. (1999). Synthesis and structure determination of the new Sm2Ti2O5S0 compound. Comptes Rendus l'Académie des Sci. IIC 2, 93–99. doi: 10.1016/S1387-1609(99)80007-3
Boyer-Candalen, C., Deudon, C., and Meerschaut, A. (2000a). Synthesis and structure determination of Nd16Ti5S17O17. J. Solid State Chem. 152, 554–559. doi: 10.1006/jssc.2000.8730
Boyer-Candalen, C., and Meerschaut, A. (2000). Synthesis and structure determination of the new compound La~10.8Nb5O20S10. J. Solid State Chem. 152, 348–352. doi: 10.1006/jssc.2000.8662
Boyer-Candalen, C., Meerschaut, A., and Palvadeau, P. (2000b). Crystal structure determination of the new compound Sm3NbO4S3. Mater. Res. Bull. 35, 1593–1601. doi: 10.1016/S0025-5408(00)00371-8
Brennan, T. D., and Ibers, J. A. (1992). Metal-metal bonding and mixed-valent tantalum in La2Ta3S2O8. J. Solid State Chem. 98, 82–89. doi: 10.1016/0022-4596(92)90072-4
Brixner, L. H. (1987). New X-ray phosphors. Mater. Chem. Phys. 16, 253–281. doi: 10.1016/0254-0584(87)90102-7
Broadley, S., Gál, Z. A., Corà, F., Smura, C. F., and Clarke, S. J. (2005). Vertex-linked ZnO2S2 tetrahedra in the oxysulfide BaZnOS: a new coordination environment for zinc in a condensed solid. Inorg. Chem. 44, 9092–9096. doi: 10.1021/ic051240o
Buck, V. (1991). Lattice parameters of sputtered MoS2 films. Thin Solid Films 198, 157–167. doi: 10.1016/0040-6090(91)90334-T
Bulte, J. W. M., and Kraitchman, D. L. (2004). Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed. 17, 484–499. doi: 10.1002/nbm.924
Carcaly, C., Flahaut, J., Guittard, M., and Palazzi, M. (1981). Un Composé à Structure Feuilletée (LaO)4Ag1,5Ga1,5S5. Mater. Res. Bull. 16, 1367–1374. doi: 10.1016/0025-5408(81)90055-6
Cario, L., Deudon, C., Meerschaut, A., and Rouxel, J. (1998). Synthesis and structure determination of La8Ti10S24O4. J. Solid State Chem. 136, 46–50. doi: 10.1006/jssc.1997.7649
Cario, L., Kabbour, H., Guillot-Deudon, C., and Meerschaut, A. (2003). A mixed-valent niobium oxysulfide, La2Nb3S2O8. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 59, i55–i56. doi: 10.1107/S0108270103009570
Cario, L., Popa, A. F., Lafond, A., Guillot-Deudon, C., Kabbour, H., Meerschaut, A., et al. (2007). Cation deficient layered ruddlesden–popper-related oxysulfides La2LnMS2O5 (Ln = La, Y; M = Nb, Ta). Inorg. Chem. 46, 9584–9590. doi: 10.1021/ic700422r
Carré, D., Laruelle, P., and Besançon, P. (1970). Structure cristalline de la pretendue variete β des sulfures de terres rares de composition Pr10S14O. Comptes Rendus Hebd. l'Académie des Sci. 270:537.
Céolin, R., and Rodier, N. (1976). Structure cristalline de l'oxysulfure de cerium et de bismuth CeBiOS2. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 32, 1476–1479. doi: 10.1107/S0567740876005591
Chan, G. H., Deng, B., Bertoni, M., Ireland, J. R., Hersam, M. C., Mason, T. O., et al. (2006). Syntheses, structures, physical properties, and theoretical studies of CeMxOS (M = Cu, Ag; x ≈ 0.8) and CeAgOS. Inorg. Chem. 45, 8264–8272. doi: 10.1021/ic061041k
Charkin, D. O., Plotnikov, V. A., Sadakov, A. V., Omel'yanovskii, O. E., and Kazakov, S. M. (2011). Synthesis of novel rare earth—iron oxide chalcogenides with the La2Fe2O3Se2 structure. J. Alloys Compd. 509, 7344–7348. doi: 10.1016/j.jallcom.2011.04.127
Chen, H., Moore, T., Qi, B., Colvin, D. C., Jelen, E. K., Hitchcock, D. A., et al. (2013). Monitoring PH-triggered drug release from radioluminescent nanocapsules with X-Ray excited optical luminescence. ACS Nano 7, 1178–1187. doi: 10.1021/nn304369m
Cheon, J., Kang, N. J., Lee, S. M., Lee, J. H., Yoon, J. H., and Oh, S. J. (2004). Shape evolution of single-crystalline iron oxide nanocrystals. J. Am. Chem. Soc. 126, 1950–1951. doi: 10.1021/ja038722o
Cherry, S. R. (2006). Multimodality in vivo imaging systems: twice the power or double the trouble? Annu. Rev. Biomed. Eng. 8, 35–62. doi: 10.1146/annurev.bioeng.8.061505.095728
Cichos, J., Karbowiak, M., Hreniak, D., and Strek, W. (2016). Synthesis and characterization of monodisperse Eu3+ doped gadolinium oxysulfide nanocrystals. J. Rare Earths 34, 850–856. doi: 10.1016/S1002-0721(16)60105-9
Cody, J. A., Deudon, C., Cario, L., and Meerschaut, A. (1997). Synthesis and structure determination of a new cerium titanium oxysulfide [Ce20Ti11S44O6]. Mater. Res. Bull. 32, 1181–1192. doi: 10.1016/S0025-5408(97)00094-9
Cody, J. A., and Ibers, J. A. (1995). Synthesis and characterization of the new rare-earth/transition-metal oxysulfides La6Ti2S8O5 and La4Ti3S4O8. J. Solid State Chem. 114, 406–412. doi: 10.1006/jssc.1995.1062
Cui, C., Jiang, G., Huang, P., Wang, L., and Liu, D. (2014a). Effect of Eu3+ concentration on the luminescence properties of Y2O2S:Eu3+, Mg2+, Ti4+ nanotubes. Ceram. Int. 40, 4725–4730. doi: 10.1016/j.ceramint.2013.09.015
Cui, C., Jiang, G., Huang, P., Wang, L., and Liu, D. (2014b). Synthesis and characterization of Y2O2S:Eu3+, Mg2+, Ti4+ nanotubes via hydrothermal method. J. Lumin. 145, 665–668. doi: 10.1016/j.jlumin.2013.08.055
Cui, C. E., Lei, X., Huang, P., Wang, L., and Yang, F. (2013a). Influence of sulfuretted temperature on the luminescent properties of Y2O2S:Eu3+, Mg2+, Ti4+ nanoarrays. J. Lumin. 138, 138–142. doi: 10.1016/j.jlumin.2013.02.013
Cui, C. E., Liu, H., Huang, P., and Wang, L. (2013b). Influence of Eu3+ doping concentration on the luminescence properties of Y2O2S: Eu3+, Mg2+, Ti4+ nanoarrays via sol–gel template method. Opt. Mater. 36, 495–499. doi: 10.1016/j.optmat.2013.10.016
Dai, Q., Song, H., Wang, M., Bai, X., Dong, B., Qin, R., et al. (2008). Size and concentration effects on the photoluminescence of La2O2S:Eu3+ nanocrystals. J. Phys. Chem. C 112, 19399–19404. doi: 10.1021/jp808343f
Delacotte, C., Pérez, O., Pautrat, A., Berthebaud, D., Hébert, S., Suard, E., et al. (2015). Magnetodielectric effect in crystals of the noncentrosymmetric CaOFeS at low temperature. Inorg. Chem. 54, 6560–6565. doi: 10.1021/acs.inorgchem.5b00879
Deng, S., Xue, Z., Liu, Y., Lei, B., Xiao, Y., and Zheng, M. (2012b). Synthesis and characterization of Y2O2S:Eu3+, Mg2+, Ti4+ hollow nanospheres via a template-free route. J. Alloys Compd. 542, 207–212. doi: 10.1016/j.jallcom.2012.07.060
Deng, S. Q., Xue, Z. P., Yang, Y. H., Yang, Q., and Liu, Y. L. (2012a). Template-free fabrication and luminescent characterization of highly uniform and monodisperse Y2O2S:Sm3+ hollow submicrospheres. J. Mater. Sci. Technol. 28, 666–672. doi: 10.1016/S1005-0302(12)60114-5
Deudon, C., Meerschaut, A., Cario, L., and Rouxel, J. (1995). Preparation and crystal structure determination of La20Ti11S44O6. J. Solid State Chem. 120, 164–169. doi: 10.1006/jssc.1995.1392
Deulkar, S. H., Huang, J. L., and Neumann-Spallart, M. (2010). Zinc oxysulfide thin films grown by pulsed laser deposition. J. Electron. Mater. 39, 589–594. doi: 10.1007/s11664-009-1069-8
Dhanaraj, J., Geethalakshmi, M., Jagannathan, R., and Kutty, T. R. (2004). Eu3+ doped yttrium oxysulfide nanocrystals – crystallite size and luminescence transition(S). Chem. Phys. Lett. 387, 23–28. doi: 10.1016/j.cplett.2004.01.079
Dhanaraj, J., Jagannathan, R., and Trivedi, D. C. (2003). Y2O2S: Eu3+ nanocrystals—synthesis and luminescent properties. J. Mater. Chem. 13, 1778–1782. doi: 10.1039/B302073H
Ding, Y., Gu, J., Ke, J., Zhang, Y. W. W., and Yan, C. H. H. (2011). Sodium doping controlled synthesis of monodisperse lanthanide oxysulfide ultrathin nanoplates guided by density functional calculations. Angew. Chem. Int. Ed. Engl. 50, 12330–12334. doi: 10.1002/anie.201105025
Doussier-Brochard, C., Chavillon, B., Cario, L., and Jobic, S. (2010). Synthesis of P-Type transparent LaOCuS nanoparticles via soft chemistry. Inorg. Chem. 49, 3074–3076. doi: 10.1021/ic902521r
Dubois, V., Pecquenard, B., Soulé, S., Martinez, H., and Le Cras, F. (2017). Dual cation- and anion-based redox process in lithium titanium oxysulfide thin film cathodes for all-solid-state lithium-ion batteries. ACS Appl. Mater. Interfaces 9, 2275–2284. doi: 10.1021/acsami.6b11987
Dugué, J., Carré, D., and Guittard, M. (1978). Etude structurale des oxysulfures de cerium(III) et cerium(IV). I. structure cristalline de l'oxysulfure de cerium Ce4O4S3. Acta Crystallogr. B 34, 3564–3568. doi: 10.1107/S0567740878011607
Dugué, J., Carré, D., and Guittard, M. (1979). Etude structurale des oxysulfures de cerium(III) et cerium(IV). II. structure cristalline de l'oxysulfure de cerium Ce6O6S4. Acta Crystallogr. B 35, 1550–1554. doi: 10.1107/S056774087900710X
Dugué, J., Tien, V., and Laruelle, P. (1985). Structure de l'oxysulfure de Lanthane et de Vanadium, La5V3O7S6. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 41, 1146–1148. doi: 10.1107/S0108270185006928
Dugué, J., Vovan, T., and Villers, J. (1980a). Etude structurale des oxysulfures de chrome(Ill) et de terres rares. I. Structure de I'oxysulfure LaCrOS2. Acta Crystallogr. B 36, 1291–1294. doi: 10.1107/S0567740880005948
Dugué, J., Vovan, T., and Villers, J. (1980b). Etude structurale des oxysulfures de chrome(III) et de terres rares. II. Structure de I'oxysulfure CeCrOS2. Acta Crystallogr. B 36, 1294–1297. doi: 10.1107/S056774088000595X
Dupin, J., Gonbeau, D., Martin-Litas, I., Vinatier, P., and Levasseur, A. (2001). Amorphous oxysulfide thin films MOySz (M=W, Mo, Ti) XPS characterization: structural and electronic pecularities. Appl. Surf. Sci. 173, 140–150. doi: 10.1016/S0169-4332(00)00893-X
Eastman, E. D., Brewer, L., Bromley, L. A., Gilles, P. W., and Lofgren, N. L. (1951). Preparation and properties of the oxide-sulfides of cerium, zirconium, thorium and uranium 2. J. Am. Chem. Soc. 73, 3896–3898. doi: 10.1021/ja01152a100
Eisman, G. A., and Steinfink, H. (1982). The synthesis of HfOS. J. Solid State Chem. 43, 225–226. doi: 10.1016/0022-4596(82)90233-X
Endo, T., Doi, Y., Wakeshima, M., Suzuki, K., Matsuo, Y., Tezuka, K., et al. (2017). Magnetic properties of the melilite-type oxysulfide Sr2MnGe2S6O: magnetic interactions enhanced by anion substitution. Inorg. Chem. 56, 2459–2466. doi: 10.1021/acs.inorgchem.6b02505
Engström, M., Klasson, A., Pedersen, H., Vahlberg, C., Käll, P.-O., and Uvdal, K. (2006). High proton relaxivity for gadolinium oxide nanoparticles. Magn. Reson. Mater. Physics, Biol. Med. 19, 180–186. doi: 10.1007/s10334-006-0039-x
Flahaut, J., Guittard, M., and Patrie, M. (1958). Les oxysulfures Me2O2S des élements du groupe des terres rares. Bull. Soc. Chim. Fr. 7, 990–994.
Fu, Y., Cao, W., Peng, Y., Luo, X., and Xing, M. (2010). The upconversion luminescence properties of the Yb3+-Ho3+ system in nanocrystalline Y2O2S. J. Mater. Sci. 45, 6556–6561. doi: 10.1007/s10853-010-4744-5
Gastaldi, L., Carré, D., and Pardo, M. P. (1982). Structure de l'oxysulfure d'indium et de lanthane In6La10O6S17. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 38, 2365–2367. doi: 10.1107/S0567740882008802
Genuit, D., Bezverkhyy, I., and Afanasiev, P. (2005). Solution preparation of the amorphous molybdenum oxysulfide MoOS2 and its use for catalysis. J. Solid State Chem. 178, 2759–2765. doi: 10.1016/j.jssc.2005.06.016
Ghosh, A. B., Saha, N., Sarkar, A., Dutta, A. K., Biswas, P., Nag, K., et al. (2016). Morphological tuning of Eu2O2S nanoparticles, manifestation of peroxidase-like activity and glucose assay use. New J. Chem. 40, 1595–1604. doi: 10.1039/C5NJ02705E
Goga, M., Seshadri, R., Ksenofontov, V., Gütlich, P., and Tremel, W. (1999). Ln2Ti2S2O5 (Ln = Nd, Pr, Sm): a novel series of defective ruddlesden–popper phases. Chem. Commun. 7, 979–980. doi: 10.1039/a809737b
Golodnitsky, D., Nathan, M., Yufit, V., Strauss, E., Freedman, K., Burstein, L., et al. (2006b). Progress in three-dimensional (3D) Li-Ion microbatteries. Solid State Ionics 177, 2811–2819. doi: 10.1016/j.ssi.2006.02.048
Golodnitsky, D., Yufit, V., Nathan, M., Shechtman, I., Ripenbein, T., Strauss, E., et al. (2006a). Advanced materials for the 3D microbattery. J. Power Sources 153, 281–287. doi: 10.1016/j.jpowsour.2005.05.029
Gonbeau, D., Guimon, C., Pfister-Guillouzo, G., Levasseur, A., Meunier, G., and Dormoy, R. (1991). XPS study of thin films of titanium oxysulfides. Surf. Sci. 254, 81–89. doi: 10.1016/0039-6028(91)90640-E
Gouget, G., Debecker, D. P., Kim, A., Olivieri, G., Gallet, J.-J., Bournel, F., et al. (2017). In situ solid–gas reactivity of nanoscaled metal borides from molten salt synthesis. Inorg. Chem. 56, 9225–9234. doi: 10.1021/acs.inorgchem.7b01279
Gu, J., Ding, Y., Ke, J., Zhang, Y., and Yan, C. (2013). Controllable synthesis of monodispersed middle and heavy rare earth oxysulfide nanoplates based on the principles of HSAB theory. Acta Chim. Sin. 71:360. doi: 10.6023/A12121014
Guittard, M., Benazeth, S., Dugue', J., Jaulmes, S., Palazzi, M., Laruelle, P., et al. (1984). Oxysulfides and oxyselenides in sheets, formed by a rare earth element and a second metal. J. Solid State Chem. 51, 227–238. doi: 10.1016/0022-4596(84)90338-4
Guittard, M., Jaulmes, S., Loireau-Lozac'h, A. M., Mazurier, A., Berguer, F., and Flahaut, J. (1985). Étude du systeme La2S3-La2O3-Ga2O3-Ga2S3: description structurale des phases quaternaires et approche du diagramme de phase. J. Solid State Chem. 58, 276–289. doi: 10.1016/0022-4596(85)90210-5
Guittard, M., Vovan, T., Julien-Pouzol, M., Jaulmes, S., Laruelle, P., and Flahaut, J. (1986). Mise en evidence et etude structurale d'une famille de composes en feuillet de formule generale (UO)2RS3 (R = Gd à Lu et Y). Zeitschrift Anorg. Allg. Chem. 540, 59–66. doi: 10.1002/zaac.19865400909
Gultom, N. S., Abdullah, H., and Kuo, D.-H. (2017). Enhanced photocatalytic hydrogen production of noble-metal free Ni-Doped Zn(O,S) in ethanol solution. Int. J. Hydrogen Energy 42, 25891–25902. doi: 10.1016/j.ijhydene.2017.08.198
Guo, G., Wang, Y., Chen, J., Zhuang, H., Huang, J., and Zhang, Q. (1995). Samarium tantalum oxysulfide, Sm2Ta3S2O8. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 51, 1964–1966. doi: 10.1107/S0108270195005026
Haire, R. G., and Fahey, J. A. (1977). The oxysulfates and oxysulfides of americium, curium and berkelium. J. Inorg. Nucl. Chem. 39, 837–841. doi: 10.1016/0022-1902(77)80165-6
Hakmeh, N., Chlique, C., Merdrignac-Conanec, O., Fan, B., Cheviré, F., Zhang, X., et al. (2015). Combustion synthesis and up-conversion luminescence of La2O2S:Er3+,Yb3+ nanophosphors. J. Solid State Chem. 226, 255–261. doi: 10.1016/j.jssc.2015.02.015
Han, L., Hu, Y., Pan, M., Xie, Y., Liu, Y., Li, D., et al. (2015a). A new tactic to achieve Y2O2S:Yb3+/Er3+ up-conversion luminescent hollow nanofibers. CrystEngComm. 17, 2529–2535. doi: 10.1039/C4CE02527J
Han, L., Pan, M., Hu, Y., Xie, Y., Liu, Y., Li, D., et al. (2015b). A novel scheme to obtain Y2O2S:Er3+ upconversion luminescent hollow nanofibers via precursor templating. J. Am. Ceram. Soc. 98, 2817–2822. doi: 10.1111/jace.13696
Hauser, O. (1907). Notiz über die darstellung der oxysulfide des zirkoniums und thoriums. Zeitschrift für Anorg. Chemie 53, 74–77. doi: 10.1002/zaac.19070530107
He, W., Zhang, J., Wang, L., and Zhang, Q. (2009). Preparation and properties of nanocrystal SmBO3 by nitrate-citrate sol-gel combustion method. J. Rare Earths 27, 231–233. doi: 10.1016/S1002-0721(08)60225-2
Hernández-Adame, L., Méndez-Blas, A., Ruiz-García, J., Vega-Acosta, J. R., Medellín-Rodríguez, F. J., and Palestino, G. (2014). Synthesis, characterization, and photoluminescence properties of Gd:Tb oxysulfide colloidal particles. Chem. Eng. J. 258, 136–145. doi: 10.1016/j.cej.2014.07.067
Hernandez-Adame, L., Palestino, G., Meza, O., Hernandez-Adame, P. L., Vega-Carrillo, H. R., and Sarhid, I. (2018). Effect of Tb3+ concentration in the visible emission of terbium-doped gadolinium oxysulfide microspheres. Solid State Sci. 84, 8–14. doi: 10.1016/j.solidstatesciences.2018.07.021
Hirai, T., and Orikoshi, T. (2004). Preparation of yttrium oxysulfide phosphor nanoparticles with infrared-to-green and -blue upconversion emission using an emulsion liquid membrane system. J. Colloid Interface Sci. 273, 470–477. doi: 10.1016/j.jcis.2003.12.013
Hirai, T., Orikoshi, T., and Komasawa, I. (2002). Preparation of Y2O3 :Yb,Er infrared-to-visible conversion phosphor fine particles using an emulsion liquid membrane system. Chem. Mater. 14, 3576–3583. doi: 10.1021/cm0202207
Huang, F. Q., Brazis, P., Kannewurf, C. R., and Ibers, J. A. (2000). Synthesis, structure, electrical conductivity, and band structure of the rare-earth copper oxychalcogenide La5Cu6O4S7. J. Solid State Chem. 155, 366–371. doi: 10.1006/jssc.2000.8926
Huang, P., Liu, D., Cui, C. E., Wang, L., and Jiang, G. (2014). Synthesis and luminescence properties of red long-lasting phosphor Y2O2S:Eu3+, Zn2+, Ti4+ nanotubes via hydrothermal method. Appl. Phys. A 116, 759–765. doi: 10.1007/s00339-013-8145-x
Huang, Y. Z., Chen, L., and Wu, L. M. (2008). Crystalline nanowires of Ln2O2S, Ln2O2S2, LnS2 (Ln) La, (Nd), and La2O2S:Eu3+. conversions via the boron-sulfur method that preserve shape. Cryst. Growth Des. 8, 739–743. doi: 10.1021/cg700751j
Ijjaali, I., Deng, B., and Ibers, J. A. (2005). Seven new rare-earth transition-metal oxychalcogenides: syntheses and characterization of Ln4MnOSe6 (Ln=La, Ce, Nd), Ln4FeOSe6 (Ln=La, Ce, Sm), and La4MnOS6. J. Solid State Chem. 178, 1503–1507. doi: 10.1016/j.jssc.2005.02.022
Inoue, M., Ueda, Y., Negishi, H., Sasaki, M., Ohba, T., Kitano, Y., et al. (1986). Effect of sulphur doping on the electrical properties of γ-Mo4O11 crystals. J. Less Common Met. 115, 261–268. doi: 10.1016/0022-5088(86)90148-7
Iparraguirre, I., Azkargorta, J., Merdrignac-Conanec, O., Al-Saleh, M., Chlique, C., Zhang, X., et al. (2012). Laser action in Nd3+-doped lanthanum oxysulfide powders. Opt. Express 20:23690. doi: 10.1364/OE.20.023690
Ishikawa, A., Takata, T., Kondo, J. N., Hara, M., Kobayashi, H., and Domen, K. (2002). Oxysulfide Sm2Ti2S2O5 as a stable photocatalyst for water oxidation and reduction under visible light irradiation (λ ≤ 650 Nm). J. Am. Chem. Soc. 124, 13547–13553. doi: 10.1021/ja0269643
Ishikawa, A., Takata, T., Matsumura, T., Kondo, J. N., Hara, M., Kobayashi, H., et al. (2004). Oxysulfides Ln2Ti2S2O5 as stable photocatalysts for water oxidation and reduction under visible-light irradiation. J. Phys. Chem. B 108, 2637–2642. doi: 10.1021/jp036890x
Jain, A., Kumar, A., Dhoble, S. J., and Peshwe, D. R. (2016). Persistent luminescence: an insight. Renew. Sustain. Energy Rev. 65, 135–153. doi: 10.1016/j.rser.2016.06.081
Jaulmes, S. (1978). Oxysulfure de gallium et de lanthane LaGaOS2. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 34, 2610–2612. doi: 10.1107/S0567740878008705
Jaulmes, S., Godlewski, E., Palazzi, M., and Etienne, J. (1982). Deux structures isotypes a sites anioniques et cationiques lacunaires: (CeO)4Ga2S5 et (LaO)4As2S5. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 38, 1707–1710. doi: 10.1107/S0567740882007006
Jaulmes, S., Julien-Pouzol, M., Dugué, J., Laruelle, P., Vovan, T., and Guittard, M. (1990). Structure de l'oxysulfure d'uranium et de lutécium, (UOS)4LuS. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 46, 1205–1207. doi: 10.1107/S0108270189011418
Jaulmes, S., Mazurier, A., and Guittard, M. (1983). Structure de l'oxypentasulfure de gallium et de trilanthane, GaLa3OS5. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 39, 1594–1597. doi: 10.1107/S0108270183009397
Jellinek, F. (1962). A tetragonal form of zirconium oxide sulfide, ZrOS. Acta Chem. Scand. 16, 791–792. doi: 10.3891/acta.chem.scand.16-0791
Jiang, G., Wei, X., Chen, Y., Duan, C., Yin, M., Yang, B., et al. (2015). Luminescent La2O2S:Eu3+ nanoparticles as non-contact optical temperature sensor in physiological temperature range. Mater. Lett. 143, 98–100. doi: 10.1016/j.matlet.2014.12.057
Julien-Pouzol, M., Jaulmes, S., Guittard, M., and Laruelle, P. (1978). Oxysulfure de scandium Sc2O2S. J. Solid State Chem. 26, 185–188. doi: 10.1016/0022-4596(78)90150-0
Jüstel, T., Nikol, H., and Ronda, C. (1998). New developments in the field of luminescent materials for lighting and displays. Angew. Chemie Int. Ed. 37, 3084–3103. doi: 10.1002/(SICI)1521-3773(19981204)37:22<3084::AID-ANIE3084>3.0.CO;2-W
Kabbour, H., Cario, L., Deudon, C., and Meerschaut, A. (2003). A gadolinium and niobium oxide sulfide, Gd3NbS3O4. Acta Crystallogr. Sect. E Struct. 59, i101–i102. doi: 10.1107/S1600536803013035
Kabbour, H., Cario, L., Moëlo, Y., and Meerschaut, A. (2004). Synthesis, X-Ray and optical characterizations of two new oxysulfides: LaInS2O and La5In3S9O3. J. Solid State Chem. 177, 1053–1059. doi: 10.1016/j.jssc.2003.10.012
Kawahara, Y., Petrykin, V., Ichihara, T., Kijima, N., and Kakihana, M. (2006). Synthesis of high-brightness sub-micrometer Y2O2S red phosphor powders by complex homogeneous precipitation method. Chem. Mater. 18, 6303–6307. doi: 10.1021/cm060609k
Khodadad, P., Tek, T., Flahaut, J., and Domange, L. (1965). Sur une nouvelle famille de combinaisons chimiques des terres rares les oxydisulfures de formule generale L2O2S2. Comptes Rendus Hebd. l'Acad. Sci. 260, 2235–2238.
Klemm, W., Meisel, K., and Vogel, H. U. (1930). Über die sulfide der seltenen erden. Zeitschrift Anorg. Allg. Chem. 190, 123–144. doi: 10.1002/zaac.19301900113
Koyama, E., Nakai, I., and Nagashima, K. (1984). Crystal chemistry of oxide–chalcogenides. II. Synthesis and crystal structure of the first bismuth oxide–sulfide, Bi2O2S. Acta Crystallogr. Sect. B Struct. Sci. 40, 105–109. doi: 10.1107/S010876818400183X
Kryza, D., Taleb, J., Janier, M., Marmuse, L., Miladi, I., Bonazza, P., et al. (2011). Biodistribution study of nanometric hybrid gadolinium oxide particles as a multimodal SPECT/MR/optical imaging and theragnostic agent. Bioconjug. Chem. 22, 1145–1152. doi: 10.1021/bc1005976
Kupčík, V. (1967). Die kristallstruktur des kermesits, Sb2S2O. Naturwissenschaften 54, 114. doi: 10.1007/BF00640574
Kusainova, A. M., Berdonosov, P. S., Akselrud, L. G., Kholodkovskaya, L. N., Dolgikh, V. A., and Popovkin, B. A. (1994). New layered compounds with the general composition (MO) (CuSe), where M = Bi, Nd, Gd, Dy, and BiOCuS: syntheses and crystal structure. J. Solid State Chem. 112, 189–191. doi: 10.1006/jssc.1994.1285
Larquet, C., Carriere, D., Nguyen, A. M., Le, T. K. C., Frogneux-Plé, X., Génois, I., et al. (2020). Unraveling the role of alkali cations in the growth mechanism of Gd2O2S nanoparticles. Chem. Mater. 32, 1131–1139. doi: 10.1021/acs.chemmater.9b04059
Larquet, C., Hourlier, D., Nguyen, A.-M., Torres-Pardo, A., Gauzzi, A., Sanchez, C., et al. (2019a). Thermal stability of oleate-stabilized Gd2O2S nanoplates in inert and oxidizing atmospheres. ChemNanoMat. 5, 539–546. doi: 10.1002/cnma.201800578
Larquet, C., Klein, Y., Hrabovsky, D., Gauzzi, A., Sanchez, C., and Carenco, S. (2019b). Tunable magnetic properties of (Gd,Ce)2O2S oxysulfide nanoparticles. Eur. J. Inorg. Chem. 2019, 762–765. doi: 10.1002/ejic.201801466
Larquet, C., Nguyen, A.-M., Glais, E., Paulatto, L., Sassoye, C., Selmane, M., et al. (2019c). Band gap engineering from cation balance: the case of lanthanide oxysulfide nanoparticles. Chem. Mater. 31, 5014–5023. doi: 10.1021/acs.chemmater.9b00450
Larquet, C., Nguyen, A. M., Ávila-Gutiérrez, M., Tinat, L., Lassalle-Kaiser, B., Gallet, J. J., et al. (2017). Synthesis of Ce2O2S and Gd2(1−−y)Ce2yO2S nanoparticles and reactivity from in situ X-Ray absorption spectroscopy and X-Ray photoelectron spectroscopy. Inorg. Chem. 56, 14227–14236. doi: 10.1021/acs.inorgchem.7b02336
Lauxmann, P., and Schleid, T. (2000). CuPrOS: kein einprägsames akronym, vielmehr ein echtes quaternäres chalkogenid mit aufgefüllter pbfcl-struktur. Zeitschrift Anorg. Allg. Chem. 626, 2253–2255. doi: 10.1002/1521-3749(200011)626:11<2253::AID-ZAAC2253>3.0.CO;2-N
Lee, D. S., and Jeong, H. D. (2014). Distinct band gap tunability of zinc oxysulfide (ZnOS) thin films synthesized from thioacetate-capped ZnO nanocrystals. Appl. Sci. Converg. Technol. 23, 376–386. doi: 10.5757/ASCT.2014.23.6.376
Lei, L., Zhang, S., Xia, H., Tian, Y., Zhang, J., and Xu, S. (2017). Controlled synthesis of lanthanide-doped Gd2O2S nanocrystals with novel excitation-dependent multicolor emissions. Nanoscale 9, 5718–5724. doi: 10.1039/C7NR00454K
Levasseur, A., Schmidt, E., Meunier, G., Gonbeau, D., Benoist, L., and Pfister-Guillouzo, G. (1995). New amorphous molybdenum oxysulfide thin films their characterization and their electrochemical properties. J. Power Sources 54, 352–355. doi: 10.1016/0378-7753(94)02100-H
Levasseur, A., Vinatier, P., and Gonbeau, D. (1999). X-Ray photoelectron spectroscopy: a powerful tool for a better characterization of thin film materials. Bull. Mater. Sci. 22, 607–614. doi: 10.1007/BF02749975
Leverenz, H. W. (1949). Luminescent solids (phosphors). Science 109, 183–195. doi: 10.1126/science.109.2826.183
Li, W., Liu, Y., and Ai, P. (2010). Synthesis and luminescence properties of red long-lasting phosphor Y2O2S:Eu3+, Mg2+, Ti4+ nanoparticles. Mater. Chem. Phys. 119, 52–56. doi: 10.1016/j.matchemphys.2009.07.037
Li, W., Liu, Y., Ai, P., and Chen, X. (2009). Synthesis and characterization of Y2O2S:Eu3+, Mg2+, Ti4+ nanorods via a solvothermal routine. J. Rare Earths 27, 895–899. doi: 10.1016/S1002-0721(08)60358-0
Li, Y., Huang, Y., Bai, T., and Li, L. (2000). Straightforward conversion route to nanocrystalline monothiooxides of rare earths through a high-temperature colloid technique. Inorg. Chem. 39, 3418–3420. doi: 10.1021/ic9912169
Lin, S. L., Liu, T. Y., Lo, C. L., Wang, B. S., Lee, Y. J., Lin, K. Y., et al. (2016). Synthesis, surface modification, and photophysical studies of Ln2O2S:Ln′3+ (Ln = Gd, Tb, Eu; Ln′= Tb and/or Eu) nanoparticles for luminescence bioimaging. J. Lumin. 175, 165–175. doi: 10.1016/j.jlumin.2016.01.037
Lindic, M. H., Martinez, H., Benayad, A., Pecquenard, B., Vinatier, P., Levasseur, A., et al. (2005a). XPS investigations of TiOySz amorphous thin films used as positive electrode in lithium microbatteries. Solid State Ionics 176, 1529–1537. doi: 10.1016/j.ssi.2005.04.007
Lindic, M. H., Pecquenard, B., Vinatier, P., Levasseur, A., Martinez, H., Gonbeau, D., et al. (2005b). Characterization of Rf sputtered TiOySz thin films. Thin Solid Films 484, 113–123. doi: 10.1016/j.tsf.2005.02.014
Lissner, F., and Schleid, T. (1992). Über sulfide und oxidsulfide des samariums/on sulfides and oxysulfides of samarium. Zeitschrift für Naturforsch. B 47, 1614–1620. doi: 10.1515/znb-1992-1116
Liu, D., Cui, C., Huang, P., Wang, L., and Jiang, G. (2014b). Luminescent properties of red long-lasting phosphor Y2O2S:Eu3+, M2+ (M=Mg, Ca, Sr, Ba), Ti4+ nanotubes via hydrothermal method. J. Alloys Compd. 583, 530–534. doi: 10.1016/j.jallcom.2013.08.196
Liu, D., Huang, P., Cui, C., Wang, L., and Jiang, G. (2014a). Effects of simultaneous change of Mg2+ and Ti4+ contents on the luminescence properties of Y2O2S:Eu3+, Mg2+, Ti4+ nanotubes. Ceram. Int. 40, 117–122. doi: 10.1016/j.ceramint.2013.05.111
Liu, H., Liu, P., Su, X., Liu, J., Li, X., Luo, H., et al. (2014c). One-pot solvothermal synthesis of singly doped Eu3+ and Codoped Er3+, Yb3+ heavy rare earth oxysulfide Y2O2S nano-aggregates and their luminescence study. RSC Adv. 4, 57048–57053. doi: 10.1039/C4RA10276B
Liu, J., Luo, H., Liu, P., Han, L., Zheng, X., Xu, B., et al. (2012). One-pot solvothermal synthesis of uniform layer-by-layer self-assembled ultrathin hexagonal Gd2O2S nanoplates and luminescent properties from single doped Eu3+ and Codoped Er3+, Yb3+. Dalt. Trans. 41, 13984–13988. doi: 10.1039/c2dt31610b
Liu, J., Yang, Y., Ni, B., Li, H., and Wang, X. (2017). Fullerene-like nickel oxysulfide hollow nanospheres as bifunctional electrocatalysts for water splitting. Small 13:1602637. doi: 10.1002/smll.201602637
Liu, L. (2013). Nano-aggregates of cobalt nickel oxysulfide as a high-performance electrode material for supercapacitors. Nanoscale 5, 11615–11619. doi: 10.1039/c3nr03533f
Liu, Z., Sun, X., Xu, S., Lian, J., Li, X., Xiu, Z., et al. (2008). Tb3+- and Eu3+-doped lanthanum oxysulfide nanocrystals. gelatin-templated synthesis and luminescence properties? J. Phys. Chem. C 112, 2353–2358. doi: 10.1021/jp0764687
Lu, X., Yang, M., Yang, L., Ma, Q., Dong, X., and Tian, J. (2015). Y2O2S:Yb3+, Er3+ nanofibers: novel fabrication technique, structure and up-conversion luminescent characteristics. J. Mater. Sci. Mater. Electron. 26, 4078–4084. doi: 10.1007/s10854-015-2947-x
Luo, X., Cao, W., and Xing, M. (2009). Upconversion luminescence properties of monodisperse spherical Y2O2S:Yb,Ho nanocrystals. J. Mater. Res. 24, 1756–1760. doi: 10.1557/jmr.2009.0208
Ma, D., Liu, S., Zhang, Y., Zhang, C., and Huang, S. (2013). Controlled synthesis of Eu3+-doped La2O2S nanophosphors by refluxing method. J. Exp. Nanosci. 8, 434–441. doi: 10.1080/17458080.2011.591002
Mao, S., Liu, Q., Gu, M., Mao, D., and Chang, C. (2008). Long lasting phosphorescence of Gd2O2S:Eu,Ti,Mg nanorods via a hydrothermal routine. J. Alloys Compd. 465, 367–374. doi: 10.1016/j.jallcom.2007.10.119
Markushev, V. M., Ter-Gabriélyan, N. É., Briskina, C. M., Belan, V. R., and Zolin, V. F. (1990). Stimulated emission kinetics of neodymium powder lasers. Sov. J. Quantum Electron. 20, 773–777. doi: 10.1070/QE1990v020n07ABEH006817
Martin, I., Vinatier, P., Levasseur, A., Dupin, J., and Gonbeau, D. (1999). XPS analysis of the lithium intercalation in amorphous tungsten oxysulfide thin films. J. Power Sources 81–82, 306–311. doi: 10.1016/S0378-7753(99)00129-9
Martinez, H., Benayad, A., Gonbeau, D., Vinatier, P., Pecquenard, B., and Levasseur, A. (2004). Influence of the cation nature of high sulfur content oxysulfide thin films MOySz (M=W, Ti) studied by XPS. Appl. Surf. Sci. 236, 377–386. doi: 10.1016/j.apsusc.2004.05.010
Martin-Litas, I., Vinatier, P., Levasseur, A., Dupin, J., Gonbeau, D., and Weill, F. (2002). Characterisation of r.f. sputtered tungsten disulfide and oxysulfide thin films. Thin Solid Films 416, 1–9. doi: 10.1016/S0040-6090(02)00717-4
Martin-Litas, I., Vinatier, P., Levasseur, A., Dupin, J. C., and Gonbeau, D. (2001). Promising thin films (WO1.05S2 and WO1.35S2.2) as positive electrode materials in microbatteries. J. Power Sources 97–98, 545–547. doi: 10.1016/S0378-7753(01)00730-3
Martin-Litas, I., Vinatier, P., Levasseur, A., Dupin, J. C., and Gonbeau, D. (2003). Electrochemical properties of tungsten oxysulphide thin films as positive electrodes for lithium microbatteries. Bull. Mater. Sci. 26, 673–681. doi: 10.1007/BF02706762
Matijević, E., and Hsu, W. P. (1987). Preparation and properties of monodispersed colloidal particles of lanthanide compounds. J. Colloid Interface Sci. 118, 506–523. doi: 10.1016/0021-9797(87)90486-3
Mayer, J. M., Schneemeyer, L. F., Siegrist, T., Waszczak, J. V., and Van Dover, B. (1992). New layered iron-lanthanum-oxide-sulfide and -selenide phases: Fe2La2O3E2(E=S,Se). Angew. Chemie Int. Ed. English 31, 1645–1647. doi: 10.1002/anie.199216451
Mazurier, A., Guittard, M., and Jaulmes, S. (1982). Structure cristalline d'un oxysulfure isotype de La Mélilite, La3, 33Ga6O2S12. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 38, 379–382. doi: 10.1107/S0567740882003021
McCullough, J. D., Brewer, L., and Bromley, L. A. (1948). The crystal structure of zirconium oxysulfide, ZrOS. Acta Crystallogr. 1, 287–289. doi: 10.1107/S0365110X4800079X
Meignen, V., Cario, L., Lafond, A., Moëlo, Y., Guillot-Deudon, C., and Meerschaut, A. (2004b). Crystal structures of two new oxysulfides La5Ti2MS5O7 (M=Cu, Ag): evidence of anionic segregation. J. Solid State Chem. 177, 2810–2817. doi: 10.1016/j.jssc.2004.04.023
Meignen, V., Lafond, A., Cario, L., Deudon, C., and Meerschaut, A. (2003). A new lanthanum titanium oxysulfide, La16Ti5S17+xO17, with x = 0.75 (9). Acta Crystallogr. Sect. C Cryst. Struct. Commun. 59, i63–i64. doi: 10.1002/chin.200341009
Meignen, V., Meerschaut, A., Cario, L., and Lafond, A. (2004a). Synthesis and crystal structure of a new oxysulfide Gd6+xTi4-XS10-YO6+y (Where x ~ 0.04 and y ~ 0.27). Zeitschrift fur Naturforsch. 59, 4–9. doi: 10.1515/znb-2004-0903
Meignen, V., Meerschaut, A., Cario, L., and Lafond, A. (2005). Synthesis and crystal structure of a new oxychalcogenide La5Ti~3.25Zr~0.25S5O9.25. J. Solid State Chem. 178, 1637–1643. doi: 10.1016/j.jssc.2005.03.003
Meng, S., Zhang, X., Zhang, G., Wang, Y., Zhang, H., and Huang, F. (2015). Synthesis, crystal structure, and photoelectric properties of a new layered bismuth oxysulfide. Inorg. Chem. 54, 5768–5773. doi: 10.1021/acs.inorgchem.5b00436
Meunier, G., Dormoy, R., and Levasseur, A. (1989). New positive-electrode materials for lithium thin film secondary batteries. Mater. Sci. Eng. B 3, 19–23. doi: 10.1016/0921-5107(89)90173-6
Meunier, G., Dormoy, R., and Levasseur, A. (1991). New amorphous titanium oxysulfides obtained in the form of thin films. Thin Solid Films 205, 213–217. doi: 10.1016/0040-6090(91)90302-E
Meyer, B. K., Merita, S., and Polity, A. (2013). On the synthesis and properties of ternary copper oxide sulfides (Cu2O1−XSx). Phys. status solidi 7, 360–363. doi: 10.1002/pssr.201206538
Michail, C. M., Fountos, G. P., Liaparinos, P. F., Kalyvas, N. E., Valais, I., Kandarakis, I. S., et al. (2010). Light emission efficiency and imaging performance of Gd2O2S:Eu powder scintillator under x-Ray radiography conditions. Med. Phys. 37, 3694–3703. doi: 10.1118/1.3451113
Muijsers, J. C., Weber, T., Vanhardeveld, R. M., Zandbergen, H. W., and Niemantsverdriet, J. W. (1995). Sulfidation study of molybdenum oxide using MoO3/SiO2/Si(100) model catalysts and Mo-IV3-sulfur cluster compounds. J. Catal. 157, 698–705. doi: 10.1006/jcat.1995.1335
Nakai, I., Nagashima, K., Koto, K., and Morimoto, N. (1978). Crystal chemistry of oxide–chalcogenide. I. The crystal structure of sarabauite CaSb10O10S6. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 34, 3569–3572. doi: 10.1107/S0567740878011619
Nakkiran, A., Thirumalai, J., and Jagannathan, R. (2007). Luminescence blinking in Eu3+ doped yttrium oxysulfide (Y2O2S:Eu3+) quantum-dot ensembles: photo-assisted relaxation of surface state(S). Chem. Phys. Lett. 436, 155–161. doi: 10.1016/j.cplett.2007.01.009
Nelson, A., Fritz, K. E., Honrao, S., Hennig, R. G., Robinson, R. D., and Suntivich, J. (2016). Increased activity in hydrogen evolution electrocatalysis for partial anionic substitution in cobalt oxysulfide nanoparticles. J. Mater. Chem. A 4, 2842–2848. doi: 10.1039/C5TA08706F
Ogino, H., Shimoyama, J., Kishio, K., Katsura, Y., Tsuboi, M., Yamanoi, K., et al. (2012). Excitonic luminescence in two-dimensionally confined layered sulfide oxides. Appl. Phys. Lett. 101:191901. doi: 10.1063/1.4764941
Okabe, T., Van Tendeloo, G., Van Landuyt, J., Amelinckx, S., and Guittard, M. (1988). Long-period stacking variants in the homologous series U2La2n−2O2nSn+1. J. Solid State Chem. 72, 376–389. doi: 10.1016/0022-4596(88)90041-2
Olofinjana, B., Egharevba, G. O., Eleruja, M. A., Jeynes, C., Adedeji, A. V., Akinwunmi, O. O., et al. (2010). Synthesis and some properties of metal organic chemical vapour deposited molybdenum oxysulphide thin films. J. Mater. Sci. Technol. 26, 552–557. doi: 10.1016/S1005-0302(10)60084-9
Orlandi, P., Moëlo, Y., Meerschaut, A., and Palvadeau, P. (1999). Lead-antimony sulfosalts from Tuscany (Italy). I. Scainiite, Pb14Sb30S54O5, the First Pb-Sb oxy-sulfosalt, from buca della vena mine. Eur. J. Mineral. 11, 949–954. doi: 10.1127/ejm/11/6/0949
Osseni, S. A. (2012). Nanoplateformes Hybrides Multimodales Pour l'imagerie Médicale. Toulouse: Université de Toulouse.
Osseni, S. A., Lechevallier, S., Verelst, M., Dujardin, C., Dexpert-Ghys, J., Neumeyer, D., et al. (2011). New nanoplatform based on Gd2O2S:Eu3+ core: synthesis, characterization and use for in vitro bio-labelling. J. Mater. Chem. 21, 18365–18372. doi: 10.1039/c1jm13542b
Otzschi, K., Ogino, H., Shimoyama, J., and Kishio, K. (1999). New candidates for superconductors; a series of layered oxysulfides (Cu2S2)(Srn+1MnO3n−1). J. Low Temp. Phys. 117, 729–733. doi: 10.1023/A:1022545228168
Ouvrard, G., Tchangbédji, G., Deniard, P., and Prouzet, E. (1995). Structural, physical and electrochemical characteristics of a vanadium oxysulfide, a cathode material for lithium batteries. J. Power Sources 54, 246–249. doi: 10.1016/0378-7753(94)02077-G
Palazzi, M. (1981). Préparation et affinement de la structure de (LaO) CuS. Comptes Rendus Hebd. l'Acad. Sci. 292, 789–791.
Palazzi, M., Carcaly, C., and Flahaut, J. (1980). Un nouveau conducteur ionique (LaO)AgS. J. Solid State Chem. 35, 150–155. doi: 10.1016/0022-4596(80)90487-9
Palazzi, M., and Jaulmes, S. (1981). Structure du conducteur ionique (LaO)AgS. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 37, 1337–1339. doi: 10.1107/S0567740881005876
Pandey, S. K., Pandey, S., Pandey, A. C., and Mehrotra, G. K. (2013). Zinc oxysulfide ternary alloy nanocrystals: a bandgap modulated photocatalyst. Appl. Phys. Lett. 102:233110. doi: 10.1063/1.4810910
Pandey, S. K., Pandey, S., Parashar, V., Yadav, R. S., Mehrotra, G. K., and Pandey, A. C. (2014). Bandgap engineering of colloidal zinc oxysulfide via lattice substitution with sulfur. Nanoscale 6, 1602–1606. doi: 10.1039/C3NR04457B
Pang, T., Cao, W., Xing, M., Feng, W., Xu, S., and Luo, X. (2010). Preparation and upconversion luminescence of monodisperse Y2O2S:Yb/Ho-Silica/Aminosilane core-shell nanoparticles. J. Rare Earths 28, 509–512. doi: 10.1016/S1002-0721(09)60142-3
Pardo, M.-P., Céolin, R., and Guittard, M. (1976). Sur les oxysulfures a deux élements metalliques, terre rare et bismuth, ou terre rare et antimoine. Comptes rendus Hebd. l'Acad. Sci. 283, 735–738.
Park, J., Zheng, H., Jun, Y., and Alivisatos, A. P. (2009). Hetero-epitaxial anion exchange yields single-crystalline hollow nanoparticles. J. Am. Chem. Soc. 131, 13943–13945. doi: 10.1021/ja905732q
Pasquariello, D. M., Dunn, W. J., and Abraham, K. M. (1990). “Rechargeable Lithium-Molybdenum Oxysulfide Batteries,” in Proceedings of the 34th International Power Sources Symposium (Cherry Hill, NJ: IEEE), 94–97. doi: 10.1109/IPSS.1990.145800
Pechini, M. P. (1967). Method of Preparing Lead and Alkaline Earth Titanates and Niobates and Coating Method Using the Same to Form a Capacitor. US Patent No. 3330697.
Petoral, R. M., Söderlind, F., Klasson, A., Suska, A., Fortin, M. A., Abrikossova, N., et al. (2009). Synthesis and characterization of Tb3+-doped Gd2O3 nanocrystals: a bifunctional material with combined fluorescent labeling and MRI contrast agent properties. J. Phys. Chem. C 113, 6913–6920. doi: 10.1021/jp808708m
Petrova, S. A., Mar'evich, V. P., Zakharov, R. G., Selivanov, E. N., Chumarev, V. M., and Udoeva, L. Y. (2003). Crystal structure of zinc calcium oxysulfide. Dokl. Chem. 393, 255–258. doi: 10.1023/B:DOCH.0000003458.35866.40
Pitha, J. J., Smith, A. L., and Ward, R. (1947). The preparation of lanthanum oxysulfide and its properties as a base material for phosphors stimulated by infrared 1. J. Am. Chem. Soc. 69, 1870–1871. doi: 10.1021/ja01200a009
Platzer-Björkman, C., Törndahl, T., Abou-Ras, D., Malmström, J., Kessler, J., and Stolt, L. (2006). Zn(O,S) buffer layers by atomic layer deposition in Cu(In,Ga)Se2 based thin film solar cells: band alignment and sulfur gradient. J. Appl. Phys. 100:044506. doi: 10.1063/1.2222067
Polat, I., Aksu, S., Altunbaş, M., and Bacaksiz, E. (2011b). Microstructural, optical and magnetic properties of cobalt-doped zinc oxysulfide thin films. Mater. Chem. Phys. 130, 800–805. doi: 10.1016/j.matchemphys.2011.07.069
Polat, I., Aksu, S., Altunbaş, M., and Bacaksiz, E. (2012). The influence of diffusion temperature on the structural, optical, and magnetic properties of nickel-doped zinc oxysulfide thin films. Phys. Status Solidi 209, 160–166. doi: 10.1002/pssa.201127248
Polat, I., Aksu, S., Altunbaş, M., Yilmaz, S., and Bacaksiz, E. (2011a). The influence of diffusion temperature on the structural, optical and magnetic properties of manganese-doped zinc oxysulfide thin films. J. Solid State Chem. 184, 2683–2689. doi: 10.1016/j.jssc.2011.07.017
Popovkin, B. A., Kusainova, A. M., Dolgikh, V. A., and Akselrud, L. G. (1998). New layered phases of the MOCuX (M = Ln, Bi; X = S, Se, TE) family: a geometric approach to the explanation of phase stability. Russ. J. Inorg. Chem. 43, 1471–1475.
Portehault, D., Devi, S., Beaunier, P., Gervais, C., Giordano, C., Sanchez, C., et al. (2011). A general solution route toward metal boride nanocrystals. Angew. Chemie Int. Ed. 50, 3262–3265. doi: 10.1002/anie.201006810
Pratx, G., Carpenter, C. M., Sun, C., Rao, R. P., and Xing, L. (2010b). Tomographic molecular imaging of X-Ray-excitable nanoparticles. Opt. Lett. 35, 3345–3347. doi: 10.1364/OL.35.003345
Pratx, G., Carpenter, C. M., Sun, C., and Xing, L. (2010a). X-Ray luminescence computed tomography via selective excitation: a feasibility study. IEEE Trans. Med. Imaging 29, 1992–1999. doi: 10.1109/TMI.2010.2055883
Qiao, Y., Hu, X., Liu, Y., Liang, G., Croft, M. C., and Huang, Y. (2013). Surface modification of MoOxSy on Porous TiO2 nanospheres as an anode material with highly reversible and ultra-fast lithium storage properties. J. Mater. Chem. A 1:15128. doi: 10.1039/c3ta13582a
Quezel, G., Ballestracci, R., and Rossat-Mignod, J. (1970). Proprietes magnetiques des oxysulfures de terres rares. J. Phys. Chem. Solids 31, 669–684. doi: 10.1016/0022-3697(70)90201-5
Ramacharyulu, P. V. R. K., Praveen Kumar, J., Prasad, G. K., and Sreedhar, B. (2014). Sulphur doped nano TiO2: synthesis, characterization and photocatalytic degradation of a toxic chemical in presence of sunlight. Mater. Chem. Phys. 148, 692–698. doi: 10.1016/j.matchemphys.2014.08.036
Range, K.-J., Lange, K. G., and Gietl, A. (1990). Rare earth sulphide oxides Ln2S2O (Ln = Er, Tm, Yb): high pressure synthesis and crystal structure. J. Less Common Met. 158, 137–145. doi: 10.1016/0022-5088(90)90440-U
Resende, L. V., and Morais, C. A. (2015). Process development for the recovery of europium and yttrium from computer monitor screens. Miner. Eng. 70, 217–221. doi: 10.1016/j.mineng.2014.09.016
Ronda, C., Jüstel, T., and Nikol, H. (1998). Rare earth phosphors: fundamentals and applications. J. Alloys Compd. 275–277, 669–676. doi: 10.1016/S0925-8388(98)00416-2
Rossner, W., and Grabmaier, B. C. (1991). Phosphors for X-Ray detectors in computed tomography. J. Lumin. 48–49, 29–36. doi: 10.1016/0022-2313(91)90072-4
Rosticher, C., Viana, B., Fortin, M. A., Lagueux, J., Faucher, L., and Chanéac, C. (2016). Gadolinium oxysulfide nanoprobes with both persistent luminescent and magnetic properties for multimodal imaging. RSC Adv. 6, 55472–55478. doi: 10.1039/C6RA05030A
Royce, M. R. (1968). Rare Earth Activated Yttrium and Gadolinium Oxy-Chalcogenide Phosphors. U.S. Patent N°3418246.
Rutt, O. J., Hill, T. L., Gál, Z. A., Hayward, M. A., and Clarke, S. J. (2003). The cation-deficient ruddlesden–popper oxysulfide Y2Ti2O5S2 as a layered sulfide: topotactic potassium intercalation to form KY2Ti2O5S2. Inorg. Chem. 42, 7906–7911. doi: 10.1021/ic0301730
Salter, E. J. T., Blandy, J. N., and Clarke, S. J. (2016). Crystal and magnetic structures of the oxide sulfides CaCoSO and BaCoSO. Inorg. Chem. 55, 1697–1701. doi: 10.1021/acs.inorgchem.5b02615
Sambrook, T., Smura, C. F., Clarke, S. J., Ok, K. M., and Halasyamani, P. S. (2007). Structure and physical properties of the polar oxysulfide CaZnOS. Inorg. Chem. 46, 2571–2574. doi: 10.1021/ic062120z
Santelli, J., Lechevallier, S., Baaziz, H., Vincent, M., Martinez, C., Mauricot, R., et al. (2018). Multimodal gadolinium oxysulfide nanoparticles: a versatile contrast agent for mesenchymal stem cell labeling. Nanoscale 10, 16775–16786. doi: 10.1039/C8NR03263G
Schleid, T. (1991a). A new oxysulfide of terbium - Tb2OS2. Eur. J. Solid State Inorg. Chem. 28, 557–562.
Schleid, T. (1991b). Zwei formen von Dy2OS2. Zeitschrift für Anorg. und Allg. Chemie 602, 39–47. doi: 10.1002/zaac.19916020105
Schleid, T. (1992). Crystal structures of D-Y2S3 and Y2OS2. Eur. J. Solid State Inorg. Chem. 29, 1015–1028.
Schleid, T., and Weber, F. A. (1998). Crystal structure of dekagadolìiiium(III) oxide tetradekasulfide, Gd10OS14. Zeitschrift für Krist 213:32. doi: 10.1524/ncrs.1998.213.14.32
Schmidt, E., Meunier, G., and Levasseur, A. (1995a). Electrochemical properties of new amorphous molybdenum oxysulfide thin films. Solid State Ionics 76, 243–247. doi: 10.1016/0167-2738(94)00284-Y
Schmidt, E., Sourisseau, C., Meunier, G., and Levasseur, A. (1995b). Amorphous molybdenum oxysulfide thin films and their physical characterization. Thin Solid Films 260, 21–25. doi: 10.1016/0040-6090(94)06463-6
Schmidt, E., Weill, F., Meunier, G., and Levasseur, A. (1994). New amorphous molybdenum oxysulfides obtained in the form of thin films and their characterization by TEM. Thin Solid Films 245, 34–39. doi: 10.1016/0040-6090(94)90873-7
Selivanov, E. N., Chumarev, V. M., Gulyaeva, R. I., Mar'evich, V. P., Vershinin, A. D., Pankratov, A. A., et al. (2004). Composition, structure, and thermal expansion of Ca3Fe4S3O6 and CaFeSO. Inorg. Mater. 40, 845–850. doi: 10.1023/B:INMA.0000037931.30753.56
Selwyn, L., McKinnon, W., and Dahn, J. (1987). Lack of oxygen substitution in the chevrel compound Mo6S8. Solid State Commun. 64, 1025–1028. doi: 10.1016/0038-1098(87)91023-4
Sheets, W. C., Stampler, E. S., Kabbour, H., Bertoni, M. I., Cario, L., Mason, T. O., et al. (2007). Facile synthesis of BiCuOS by hydrothermal methods. Inorg. Chem. 46, 10741–10748. doi: 10.1021/ic7014622
Shi, L., Sordillo, L. A., Rodríguez-Contreras, A., and Alfano, R. (2016). Transmission in near-infrared optical windows for deep brain imaging. J. Biophotonics 9, 38–43. doi: 10.1002/jbio.201500192
Sinsermsuksakul, P., Hartman, K., Bok Kim, S., Heo, J., Sun, L., Hejin Park, H., et al. (2013). Enhancing the efficiency of SnS solar cells via band-offset engineering with a zinc oxysulfide buffer layer. Appl. Phys. Lett. 102:053901. doi: 10.1063/1.4789855
Smith, L. A. C., Trudeau, M. L., Provencher, M., Smith, M. E., and Antonelli, D. M. (2016). Low-temperature synthesis and electrochemical properties of mesoporous titanium oxysulfides. ChemElectroChem 3, 256–265. doi: 10.1002/celc.201500463
Smura, C. F., Parker, D. R., Zbiri, M., Johnson, M. R., Gál, Z. A., and Clarke, S. J. (2011). High-spin cobalt(II) ions in square planar coordination: structures and magnetism of the oxysulfides Sr2CoO2Cu2S2 and Ba2CoO2Cu2S2 and their solid solution. J. Am. Chem. Soc. 133, 2691–2705. doi: 10.1021/ja109553u
So, W. W., LaCour, A., Aliev, V. O., and Dorhout, P. K. (2004). Synthesis and characterization of a new quaternary lanthanum oxythioantimonite: La6Sb4O12S3. J. Alloys Compd. 374, 234–239. doi: 10.1016/j.jallcom.2003.11.108
Song, Y., You, H., Huang, Y., Yang, M., Zheng, Y., Zhang, L., et al. (2010). Highly uniform and monodisperse Gd2O2S:Ln3+ (Ln = Eu, Tb) submicrospheres: solvothermal synthesis and luminescence properties. Inorg. Chem. 49, 11499–11504. doi: 10.1021/ic101608b
Sterba, J. (1904). Contribution to the study of several combinations of cerium. Ann. Chim. Phys. 2, 193–232.
Stocks, K., Eulenberger, G., and Hahn, H. (1980). Darstellung und kristallstruktur von HfOS. Zeitschrift Anorg. Allg. Chem. 463, 105–109. doi: 10.1002/zaac.19804630114
Sun, W., Zhu, K., Xu, H., Yang, X., Yu, M., Li, X., et al. (2017). Enhanced absorbing property of Sm2O2S laser absorbent by doping Er3+/Tm3+. J. Mater. Sci. Mater. Electron. 28, 697–701. doi: 10.1007/s10854-016-5578-y
Sutorik, A. C., and Kanatzidis, M. G. (1994). Ba6Ti5S15O: a new metal/oxysulfide resulting from the inclusion of BaO into the BaTiS3 structure type. Chem. Mater. 6, 1700–1704. doi: 10.1021/cm00046a023
Suzuki, T., Hisatomi, T., Teramura, K., Shimodaira, Y., Kobayashi, H., and Domen, K. (2012). A titanium-based oxysulfide photocatalyst: La5Ti2MS5O7 (M = Ag, Cu) for water reduction and oxidation. Phys. Chem. Chem. Phys. 14, 15475–15481. doi: 10.1039/c2cp43132g
Tan, S., and Li, D. (2017). Enhancing oxygen storage capability and catalytic activity of lanthanum oxysulfide (La2O2S) nanocatalysts by sodium and iron/sodium doping. ChemCatChem 10, 550–558. doi: 10.1002/cctc.201701117
Tan, S., Paglieri, S. N., and Li, D. (2016). Nano-scale sulfur-tolerant lanthanide oxysulfide/oxysulfate catalysts for water–gas-shift reaction in a novel reactor configuration. Catal. Commun. 73, 16–21. doi: 10.1016/j.catcom.2015.10.007
Tanryverdiev, V. S., Aliev, O. M., and Aliev, I. I. (1995). Synthesis and physicochemical properties of LnBiOS2. Inorg. Mater. 31, 1361–1363.
Tchangbedji, G., Odink, D. A., and Ouvrard, G. (1993). V2O4S — a new transition metal oxysulfide as positive for lithium batteries. J. Power Sources 44, 577–581. doi: 10.1016/0378-7753(93)80205-4
Tchangbédji, G., Prouzet, E. P., and Ouvrard, G. (1994). A new soft chemistry synthesized vanadium oxysulfide. Mater. Sci. Forum 152–153, 319–322. doi: 10.4028/www.scientific.net/MSF.152-153.319
Teske, C. L. (1985). Über oxidsulfide mit akermanitstruktur CaLaGa3S6O, SrLaGa3S6O, La2ZnGa2S6O und Sr2ZnGe2S6O. Zeitschrift Anorg. Allg. Chem. 531, 52–60. doi: 10.1002/zaac.19855311208
Thankalekshmi, R. R., and Rastogi, A. C. (2012). Structure and optical band gap of ZnO1−xSx thin films synthesized by chemical spray pyrolysis for application in solar cells. J. Appl. Phys. 112:063708. doi: 10.1063/1.4754014
Thirumalai, J., Chandramohan, R., Auluck, S., Mahalingam, T., and Srikumar, S. R. (2009b). Controlled synthesis, optical and electronic properties of Eu3+ doped yttrium oxysulfide (Y2O2S) nanostructures. J. Colloid Interface Sci. 336, 889–897. doi: 10.1016/j.jcis.2009.04.042
Thirumalai, J., Chandramohan, R., Divakar, R., Mohandas, E., Sekar, M., and Parameswaran, P. (2008b). Eu3+ doped gadolinium oxysulfide (Gd2O2S) nanostructures—synthesis and optical and electronic properties. Nanotechnology 19:395703. doi: 10.1088/0957-4484/19/39/395703
Thirumalai, J., Chandramohan, R., Sekar, M., and Rajachandrasekar, R. (2008a). Eu3+ doped yttrium oxysulfide quantum structures—structural, optical and electronic properties. J. Nanoparticle Res. 10, 455–463. doi: 10.1007/s11051-007-9276-9
Thirumalai, J., Chandramohan, R., Valanarasu, S., Vijayan, T.a, Somasundaram, R. M., et al. (2009a). Shape-selective synthesis and opto-electronic properties of Eu3+-doped gadolinium oxysulfide nanostructures. J. Mater. Sci. 44, 3889–3899. doi: 10.1007/s10853-009-3531-7
Thirumalai, J., Chandramohan, R., Valanarasu, S., Vijayan, T. A., and Ezhilvizhian, S. (2011a). Synthesis and chemical properties of Y2O2S:Eu3+ nanostructures using composite-hydroxide-mediated method. Micro Nano Lett. 6, 614–618. doi: 10.1049/mnl.2011.0252
Thirumalai, J., Chandramohan, R., and Vijayan, T. A. (2011b). Synthesis, characterization and formation mechanism of monodispersed Gd2O2S:Eu3+ nanocrystals. J. Mater. Sci. Mater. Electron. 22, 936–943. doi: 10.1007/s10854-010-0240-6
Thirumalai, J., Jagannathan, R., and Trivedi, D. C. (2007). Y2O2S:Eu3+ nanocrystals, a strong quantum-confined luminescent system. J. Lumin. 126, 353–358. doi: 10.1016/j.jlumin.2006.08.064
Thomson, J. W., Nagashima, K., Macdonald, P. M., and Ozin, G. A. (2011). From sulfur–amine solutions to metal sulfide nanocrystals: peering into the oleylamine–sulfur black box. J. Am. Chem. Soc. 133, 5036–5041. doi: 10.1021/ja1109997
Tian, Y., Fu, Y., Xing, M., and Luo, X. (2015). Upconversion luminescence properties of Y2O3:Yb, Er and Y2O2S:Yb, Er nanoparticles prepared by complex precipitation. J. Nanomater. 2015:573253. doi: 10.1155/2015/573253
Tian, Y., Lu, F., Xing, M., Ran, J., Fu, Y., Peng, Y., et al. (2017). Upconversion luminescence properties of Y2O2S:Er3+@Y2O2S:Yb3+,Tm3+ core-shell nanoparticles prepared via homogeneous co-precipitation. Opt. Mater. 64, 58–63. doi: 10.1016/j.optmat.2016.11.031
Tien, V., Guittard, M., Dugué, J., and Flahaut, J. (1988). Les combinaisons U2R2n−2O2nSn+1 Formées Par Les Lanthanides Légers (R = Ce à Tb) avec n = 2 et 3 et dans le cas du lanthane avec N= 2 à 6. J. Solid State Chem. 73, 11–18. doi: 10.1016/0022-4596(88)90047-3
Tillinski, R., Näther, C., Winkler, B., and Bensch, W. (2001). Synthesis and crystal structure of K6Ti6S18O: a new coordination compound containing discrete Ti6O units in a chalcogenide environment. Zeitschrift Anorg. Allg. Chem. 627, 2576–2580. doi: 10.1002/1521-3749(200112)627:12<2576::AID-ZAAC2576>3.0.CO;2-A
Tóth, É., Helm, L., and Merbach, A. E. (2002). “Relaxivity of MRI contrast agents,” in Topics in Current Chemistry, ed W. Krause (Berlin; Heidelberg: Springer), 221. doi: 10.1007/3-540-45733-X_3
Tranchitella, L. J., Fettinger, J. C., and Eichhorn, B. W. (1996). Synthesis and structural analysis of Sr5.8La4.4Ti7.8S24O4 and La14Ti8S33O4 : two new oxysulfides containing a common [(Ti4S2O4)(TiS6)4/2]12- layer. Chem. Mater. 8, 2265–2271. doi: 10.1021/cm960001j
Tranchitella, L. J., Fettinger, J. C., Heller-Zeisler, S. F., and Eichhorn, B. W. (1998). La8+x Ti8+y S24O4 compounds where x + y ≤ 2: a series of phases with mixed-valent titanium. Chem. Mater. 10, 2078–2085. doi: 10.1021/cm970663o
Tsujimoto, Y., Juillerat, C. A., Zhang, W., Fujii, K., Yashima, M., Halasyamani, P. S., et al. (2018). Function of tetrahedral ZnS3O building blocks in the formation of SrZn2S2O: a phase matchable polar oxysulfide with a large second harmonic generation response. Chem. Mater. 30, 6486–6493. doi: 10.1021/acs.chemmater.8b02967
Ueda, K., Takafuji, K., and Hosono, H. (2003). Preparation and crystal structure analysis of CeCuOS. J. Solid State Chem. 170, 182–187. doi: 10.1016/S0022-4596(02)00061-0
Umarji, A. M., Rao, G. V. S., Sankaranarayana, V., Rangarajan, G., and Srinivasan, R. (1980). Synthesis and properties of O-containing chevrel phases, AxMo6S6O2 (A = Co, Ni, Cu and Pb). Mater. Res. Bull. 15, 1025–1031. doi: 10.1016/0025-5408(80)90229-9
Valldor, M., Rößler, U. K., Prots, Y., Kuo, C.-Y., Chiang, J. C., Hu, Z., et al. (2015). Synthesis and characterization of Ba[CoSO]: magnetic complexity in the presence of chalcogen ordering. Chem. A Eur. J. 21, 10821–10828. doi: 10.1002/chem.201501024
Verelst, M. Dexpert-Ghys, J., Marchin, L., Mauricot, R., Osseni, S. A., and Lechevallier, S. (2010). Nanoparticules Luminescentes Utilisables en Tant que Marqueurs et Procede Pour Leur Preparation. French patent: FR1057296/14-09-2010.
Vovan, T., Dugué, J., and Guittard, M. (1978). Oxysulfures mixtes de chrome III et de terres rares. Mater. Res. Bull. 13, 1163–1166. doi: 10.1016/0025-5408(78)90204-0
Vovan, T., Dugué, J., and Guittard, M. (1981). Oxysulfures mixtes de vanadium et de terre rare de formule generale R5V3S6O7 (R = lanthane a neodyme). Comptes Rendus l'Acad. Sci. 292, 957–959.
Wang, G., Zou, H., Zhang, B., Sun, Y., Huo, Q., Xu, X., et al. (2015). Preparation and luminescent properties of 1D Lu2O2S:Eu3+ Nanorods. Opt. Mater. (Amst). 45, 131–135. doi: 10.1016/j.optmat.2015.03.020
Wang, W., Dahl, M., and Yin, Y. (2013). Hollow nanocrystals through the nanoscale kirkendall effect. Chem. Mater. 25, 1179–1189. doi: 10.1021/cm3030928
Wang, Z., Cheng, P., He, P., Hu, F., Luo, L., and Zhou, Q. (2014). Controlled Y2O2S: Eu3+ nanocrystals prepared by the molten salt synthesis for solid state lighting. Nanosci. Nanotechnol. Lett. 6, 1053–1057. doi: 10.1166/nnl.2014.1878
Weber, T., Muijsers, J. C., van Wolput, J. H. M. C., Verhagen, C. P. J., and Niemantsverdriet, J. W. (1996). Basic reaction steps in the sulfidation of crystalline MoO3 to MoS2 as studied by X-Ray photoelectron and infrared emission spectroscopy. J. Phys. Chem. 100, 14144–14150. doi: 10.1021/jp961204y
Wichelhaus, W. (1978a). The rare-earth oxide disulfides La2O2S2, Pr2O2S2, and Nd2O2S2. Naturwissenschaften 65, 593–594. doi: 10.1007/BF00364913
Wichelhaus, W. (1978b). Ce4O4S3: a mixed-valence cerium oxide sulfide. Angew. Chemie Int. Ed. 17, 451–452. doi: 10.1002/anie.197804511
Wontcheu, J., and Schleid, T. (2003). Crystal structure of digadolinium(III) oxide disulfide, Gd2OS2. Zeitschrift für Krist. 218, 285–286. doi: 10.1524/ncrs.2003.218.jg.307
Xing, M., Cao, W., Pang, T., and Ling, X. (2009). Synthesis of monodisperse spherical Y2O2S:Yb,Ho upconversion nanoparticles. Solid State Commun. 149, 911–914. doi: 10.1016/j.ssc.2009.03.031
Yan, X., Fern, G. R., Withnall, R., and Silver, J. (2013a). Effects of the host lattice and doping concentration on the colour of Tb3+ cation emission in Y2O2S:Tb3+ and Gd2O2S:Tb3+ nanometer sized phosphor particles. Nanoscale 5, 8640–8646. doi: 10.1039/c3nr01034a
Yan, X., Fern, G. R., Withnall, R., and Silver, J. (2013b). Contrasting behaviour of the co-activators in the luminescence spectra of Y2O2S:Tb3+, Er3+ nanometre sized particles under UV and red light excitation. Nanoscale 5, 1091–1096. doi: 10.1039/C2NR33391K
Yang, G., Yan, Z., and Xiao, T. (2012). Low-temperature solvothermal synthesis of visible-light-responsive S-doped TiO2 nanocrystal. Appl. Surf. Sci. 258, 4016–4022. doi: 10.1016/j.apsusc.2011.12.092
Yang, L., Cai, Z., Hao, L., Xing, Z., Dai, Y., Xu, X., et al. (2017). Nano Ce2O2S with highly enriched oxygen-deficient Ce3+ sites supported by N and S dual-doped carbon as an active oxygen-supply catalyst for the oxygen reduction reaction. ACS Appl. Mater. Interfaces 9, 22518–22529. doi: 10.1021/acsami.7b04997
Yee, C.-H., Birol, T., and Kotliar, G. (2015). Guided design of copper oxysulfide superconductors. Europhys. Lett. 111:17002. doi: 10.1209/0295-5075/111/17002
Yu, R., An, Y., Wang, C., Wang, H., Wu, Y., Chen, J., et al. (2012). Tunable yellowish-green to green (Ca1−XSrx)LaGa3S6O:Eu2+ phosphors for potential LED application. Electrochem. Solid-State Lett. 15:J1. doi: 10.1149/2.017201esl
Yu, R., Deng, B., Zhang, G., An, Y., Zhang, J., and Wang, J. (2011). Luminescence properties of Ce3+-activated SrLaGa3S6O and application in white LEDs. J. Electrochem. Soc. 158, J255–J259. doi: 10.1149/1.3601850
Yu, R., Wang, J., Zhang, M., Zhang, J., Yuan, H., and Su, Q. (2008). A new blue-emitting phosphor of Ce3+-activated CaLaGa3S6O for white-light-emitting diodes. Chem. Phys. Lett. 453, 197–201. doi: 10.1016/j.cplett.2008.01.039
Yuan, G., Li, M., Yu, M., Tian, C., Wang, G., and Fu, H. (2016). In Situ synthesis, enhanced luminescence and application in dye sensitized solar cells of Y2O3/Y2O2S:Eu3+ nanocomposites by reduction of Y2O3:Eu3+. Sci. Rep. 6:37133. doi: 10.1038/srep37133
Yufit, V., Nathan, M., Golodnitsky, D., and Peled, E. (2003). Thin-film lithium and lithium-ion batteries with electrochemically deposited molybdenum oxysulfide cathodes. J. Power Sources 122, 169–173. doi: 10.1016/S0378-7753(03)00401-4
Zachariasen, W. H. (1949a). Crystal chemical studies of the 5f-series of elements. VII. The crystal structure of Ce2O2S, La2O2S and Pu2O2S. Acta Crystallogr. 2, 60–62. doi: 10.1107/S0365110X49000138
Zachariasen, W. H. (1949b). Crystal chemical studies of the 5 f -series of elements. X. sulfides and oxysulfides. Acta Crystallogr. 2, 291–296. doi: 10.1107/S0365110X49000758
Zhang, G., Cui, Q., and Liu, G. (2016). Efficient near-infrared quantum cutting and downshift in Ce3+-Pr3+ Codoped SrLaGa3S6O suitable for solar spectral converter. Opt. Mater. 53, 214–217. doi: 10.1016/j.optmat.2016.01.042
Zhang, G., Liu, C., Wang, J., Kuang, X., and Su, Q. (2011). An intense charge transfer broadband sensitized near-infrared emitting CaLaGa3S6O:Yb3+ phosphor suitable for solar spectral convertor. Opt. Express 19, 24314–24319. doi: 10.1364/OE.19.024314
Zhang, G., Liu, C., Wang, J., Kuang, X., and Su, Q. (2012). A dual-mode solar spectral converter CaLaGa3S6O:Ce3+,Pr3+: UV-Vis-NIR luminescence properties and solar spectral converting mechanism. J. Mater. Chem. 22, 2226–2232. doi: 10.1039/C1JM14942C
Zhang, G., Wang, J., Chen, Y., and Su, Q. (2010). Two-Color Emitting of Ce3+ and Tb3+ co-doped CaLaGa3S6O for UV LEDs. Opt. Lett. 35, 2382–2384. doi: 10.1364/OL.35.002382
Zhang, H., Liu, G., Cao, Y., Chen, J., Shen, K., Kumar, A., et al. (2017). The magnetic and adsorption properties of ZnO1−x Sx nanoparticles. Phys. Chem. Chem. Phys. 19, 26918–26925. doi: 10.1039/C7CP03470A
Zhang, P., Hong, Z., Wang, M., Fang, X., Qian, G., and Wang, Z. (2005b). Luminescence characterization of a new long afterglow phosphor of single Ti-Doped Y2O2S. J. Lumin. 113, 89–93. doi: 10.1016/j.jlumin.2004.08.056
Zhang, T., Gu, J., Ding, Y., Zhang, Y. W., and Yan, C. H. (2013). Experimental and theoretical studies on the controlled synthesis of alkali-metal-doped rare-earth oxysulfide nanocrystals. Chempluschem 78, 515–521. doi: 10.1002/cplu.201300092
Zhang, X., Liu, Y., Zhang, G., Wang, Y., Zhang, H., and Huang, F. (2015). Thermal decomposition of bismuth oxysulfide from photoelectric Bi2O2S to superconducting Bi4O4S3. ACS Appl. Mater. Interfaces 7, 4442–4448. doi: 10.1021/am5092159
Zhang, X., Zhang, J., Xu, J., and Su, Q. (2005a). Luminescent properties of Eu2+-activated SrLaGa3S6O phosphor. J. Alloys Compd. 389, 247–251. doi: 10.1016/j.jallcom.2004.06.092
Zhao, F., Sun, H. L., Su, G., and Gao, S. (2006b). Synthesis and size-dependent magnetic properties of monodisperse EuS nanocrystals. Small 2, 244–248. doi: 10.1002/smll.200500294
Zhao, F., Yuan, M., Zhang, W., and Gao, S. (2006a). Monodisperse lanthanide oxysulfide nanocrystals. J. Am. Chem. Soc. 128, 11758–11759. doi: 10.1021/ja0638410
Zhu, K., Ding, W., Sun, W., Han, P., Wang, L., and Zhang, Q. (2016). 1.06 Mm laser absorption properties of Sm2O2S prepared by flux method. J. Mater. Sci. Mater. Electron. 27, 2379–2384. doi: 10.1007/s10854-015-4035-7
Zhu, W. J., and Hor, P. H. (1997a). Unusual layered transition-metal oxysulfides: Sr2Cu2MO2S2(M=Mn, Zn). J. Solid State Chem. 130, 319–321. doi: 10.1006/jssc.1997.7299
Zhu, W. J., and Hor, P. H. (1997b). Crystal structure of new layered oxysulfides: Sr3Cu2Fe2O5S2and Sr2CuMO3S (M=Cr, Fe, In). J. Solid State Chem. 134, 128–131. doi: 10.1006/jssc.1997.7556
Zhu, W. J., and Hor, P. H. (1997c). Sr2CuGaO3S, a rare example of square pyramidal gallium. Inorg. Chem. 36, 3576–3577. doi: 10.1021/ic970322c
Keywords: oxysulfide, lanthanides, sulfidation, transition metal, nanoparticles, synthesis, applications
Citation: Larquet C and Carenco S (2020) Metal Oxysulfides: From Bulk Compounds to Nanomaterials. Front. Chem. 8:179. doi: 10.3389/fchem.2020.00179
Received: 03 October 2019; Accepted: 26 February 2020;
Published: 31 March 2020.
Edited by:
Sidney J. L. Ribeiro, São Paulo State University, BrazilReviewed by:
Hongjie Zhang, Changchun University of Science and Technology, ChinaCopyright © 2020 Larquet and Carenco. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sophie Carenco, c29waGllLmNhcmVuY29Ac29yYm9ubmUtdW5pdmVyc2l0ZS5mcg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.