
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Chem. , 21 January 2020
Sec. Supramolecular Chemistry
Volume 7 - 2019 | https://doi.org/10.3389/fchem.2019.00933
This article is part of the Research Topic Host-Guest Chemistry of Macrocycles View all 17 articles
The formation and decomposition of inclusion compounds with a solid-solid phase transition may be very selective to the guest molecular structure. This selectivity may function in essentially different ways than defined by the classical concept of molecular recognition, which implies the preferential binding of complementary molecules. Solid inclusion compounds may take part as an initial or/and final state in several processes of different types summarized in this review, which selectivity is boosted by cooperativity of participating molecular crystals. Some of these processes resemble switching electronic devices and can be called smart giving practically absolute molecular recognition.
Molecular recognition of neutral molecules is one of the key problems in chemical technologies and in analytical and biotechnological applications (Reinhoudt, 2013; Persch et al., 2015; Shu et al., 2018). To reach a sufficient selectivity, host compounds with very complex structure are synthesized (Ariga et al., 2012; Zhang et al., 2019) to fit the well-known key-to-lock concept of molecular recognition formulated by Fischer (1894). This concept later developed in supramolecular chemistry is based on complementarity of two interacting molecules, where the host interacts with guest cooperatively through several more or less strong coordinate, donor-acceptor, and hydrogen bonds having a specific spatial arrangement (Joyce et al., 2010; Sonnenberg et al., 2012). The most studies of molecular recognition are conducted in liquid solutions (Ariga et al., 2012; Persch et al., 2015; Shu et al., 2018; Zhang et al., 2019) and perform a sufficient selectivity only if guest forms at least two such bonds with host (Yao et al., 2018).
This review describes the possible alternatives to the classical key-to-lock principle with a higher selectivity of molecular recognition. These alternatives are based on cooperativity of phase transitions, which adds up the small differences in molecular structure of different included guests. Some of the described recognition principles can be called smart because they resemble the function of electronic devices.
Quantitatively, the cooperativity of phase transition at guest inclusion by solid host can be seen in a stepwise sigmoidal shape of guest sorption isotherm (Gorbatchuk et al., 1997a; Dewa et al., 1998). According to the Gibbs phase rule, a sorption isotherm in system with two independent components (guest and host) should have a threshold concentration, vapor pressure or thermodynamic activity of guest corresponding to formation of three phases of guest, host,and clathrate (inclusion compound) at constant temperature, Figure 1A (Gorbatchuk et al., 2002). Below this threshold activity, the guest is not included, and below and above this threshold the composition of the solid phase does not change.
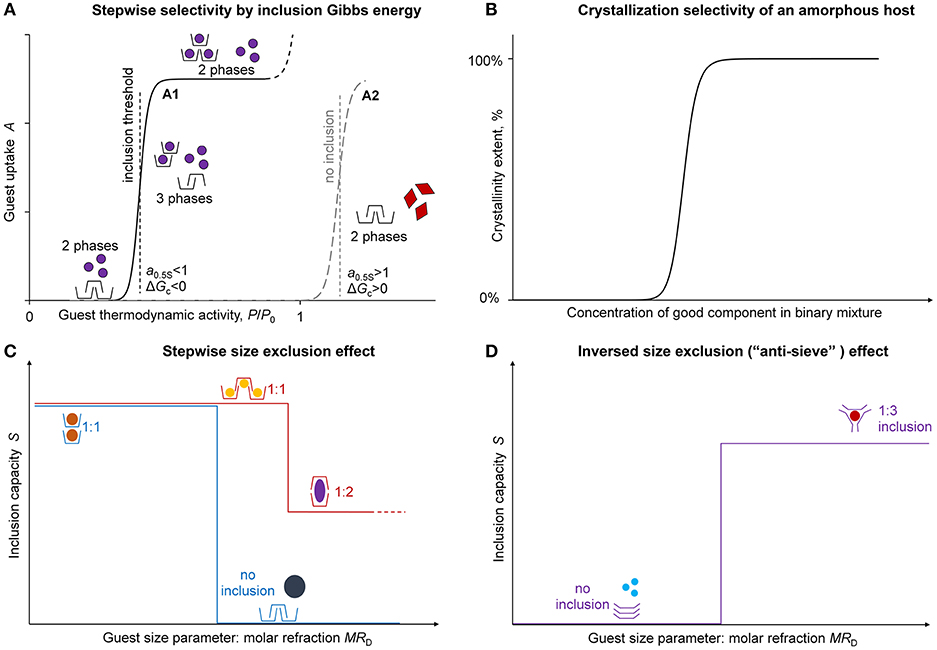
Figure 1. Stepwise inclusion selectivity of solid hosts. (A) Stepwise selectivity by inclusion Gibbs energy; (B) crystallization selectivity of an amorphous host; (C) stepwise size exclusion effect; (D) inversed size exclusion effect (“anti-sieve”).
In solid state, this phase transition is observed if the initial host is non-porous (Gorbatchuk et al., 2002). If the host has a permanent porosity combined with flexible structure, like that of some metal organic frameworks (MOFs) (Hiraide et al., 2016; Engel et al., 2017) or silicalites (DeJaco et al., 2019), the initial part of sorption isotherm may have the shape of Langmuir isotherm followed by a sigmoidal step. This step is called the gate-opening or breathing (Afonso et al., 2012; Lee et al., 2019). A similar cooperative phenomena were observed for biological objects, e.g., for oxygen binding by aqueous solution of hemoglobin (Yuan et al., 2015).
The sigmoidal isotherms of guest inclusion by solid host and related cooperativity of guest release from the inclusion compound may boost the selectivity of these processes. Depending on the initial and final states of host, several specific types of selectivity may be observed, which are described in this review.
Selectivity of guest inclusion may be visualized if the initial state of host is amorphous. The amorphous state is a high-energy state, so its transition to the crystalline state may be spontaneous (Faizullin et al., 2019). The activation of this process with guest vapors may be selective. Such selectivity was observed visually for a compact glass of calixarene (Gataullina et al., 2015, 2017) and using an atomic force microscopy for thin amorphous films of dipeptides (Ziganshin et al., 2015). Amorphous dipeptides may have three options in contact with guest vapors depending on the guest molecular structure: (1) crystallization, (2) gel formation, (3) intact host morphology (Ziganshin et al., 2017).
The amorphous calixarenes in the form of a compact transparent glass can be used to detect visually the composition of a binary guest mixture, where only one (good) component has an ability to induce the host crystallization. The mixture should have the concentration of this guest above a certain threshold value for this crystallization to be apparent, Figure 1B. For example, glassy tert-butylthiacalix[4]arene derivative crystallizes in contact with vapors of the aqueous solution of ethanol if its concentration is above 24 vol.% (Gataullina et al., 2015). The glass of the same calixarene in another conformation allows detecting 1% vol. of benzene in hexane (Gataullina et al., 2017). A similar crystallization behavior was observed for glassy polymers (Gao et al., 2012), which have a less pronounced concentration threshold for the good component in binary solvent due to the incomplete crystallization.
The guest inclusion by the host with the phase transition complicates much the structure-property relationships for this process. The related selectivity can be described using approximation parameters of sigmoidal isotherms of guest inclusion, Figure 1A. These isotherms may be fitted with Hill equation adjusted to “guest uptake A vs. relative vapor pressure P/P0” coordinates (Gorbatchuk et al., 1997a):
where S is guest contents in a saturated inclusion compound (clathrate) in mol of guest per 1 mol of host, C is a sorption constant, N is a cooperativity parameter, which in ideal case of phase transition should have an infinitely high value, N → ∞. The integration of sigmoidal sorption isotherms fitted by this equation gives the inclusion Gibbs energy ΔGc of guest transfer from its pure liquid or solid state to the saturated inclusion compound (Gorbatchuk et al., 1999a):
Here Y = A/S is the extent of host saturation with guest, a0.5S is the guest activity P/P0 at Y = 0.50.
The thermodynamics defined by Equations (1–3) means the stepwise selectivity of guest inclusion. If two guests have very small difference in molecular structure, but the first guest has sorption constant C slightly below unity and for the second one this parameter should be slightly above this level, only the first guest will be included, Figure 1A. As a result, a high selectivity of guest inclusion may be observed discriminating the close homologs. For example, tert-butylthiacalix[4]arene includes methanol from the vapor phase, but not ethanol (Galyaltdinov et al., 2012).
The same inclusion thermodynamics may produce a stepwise change in the guest inclusion capacity S at the variation of the guest molecular structure, Figure 1C. A good example is tert-butylcalix[4]arene including a lot of guests inside its molecular cavity (Ripmeester et al., 2006) with a regular stepwise size exclusion effect between the inclusion capacity S and guest molar refraction MRD, which is a good molecular size parameter (Gorbatchuk et al., 1999b). The exclusions are the guests, which can break the host intramolecular cyclic H-bond, like 1-butylamine (Udachin et al., 2002).
In those cases, where also interstitial guest inclusion is possible, the structure-property relationship for the host inclusion capacity S may be more complex. tert-Butylcalix[5]arene with such structure of inclusion compounds has a very irregular relationship between S and MRD values (Ziganshin et al., 2007). The same was observed for diol host (Gorbatchuk et al., 2000), adamantylcalix[4]arene (Yakimova et al., 2008), and tert-butylcalix[6]arene (Safina et al., 2013).
Rather regular size exclusion effect may be expected for hosts with strong intermolecular H-bonding in their crystals. This was observed for dry hydrophilic receptors α-cyclodextrin (Gatiatulin et al., 2018) and β-cyclodextrin (Gatiatulin et al., 2016). In both cases, hydrophilic guests are included better than hydrophobic ones. In this relation, the inclusion selectivity of dry cyclodextrins is similar to those of dry glassy hydrophilic receptors like human serum albumin (Gorbatchuk et al., 1997b, 1999c), β-lactoglobulin (Mironov et al., 2003), and cross-linked polyacrylamide derivative (Gorbatchuk et al., 2004).
The second type of host selectivity to the guest size is an inverted size exclusion or “anti-sieve” effect, where the host prefers larger molecules, while the smaller are not included, Figure 1D. Such selectivity was observed for thiacalix[4]arene, which may include guests into the interstitial space formed by too many calixarene macrocycles where a sufficient driving force apparently needed to push them aside (Galyaltdinov et al., 2014).
The solid-phase transition at guest inclusion by solid host implies also the host selectivity by inclusion threshold of guest thermodynamic activity, and accordingly, by inclusion Gibbs energy ΔGc, Figure 1A. The range of the observed ΔGc values depends much on the size of host cavity that does not require work to be created (Gorbatchuk et al., 2002; Gatiatulin et al., 2018). For example, for the tert-butylcalix[4]arene, which includes the most guests studied inside its molecular cavity (Ripmeester et al., 2006; Ramon et al., 2011), there is a significant variation in ΔGc from −1.2 to −8.9 kJ/mol for different guests (Gorbatchuk et al., 2002). tert-Butylthiacalix[4]arene with the same type of guest inclusion but with a smaller effective cavity has the ΔGc values from −0.4 to −2.0 kJ/mol (Gorbatchuk et al., 2002). tert-Butylcalix[5]arene (Ziganshin et al., 2007), adamantylcalix[4]arene (Yakimova et al., 2008), and β-cyclodextrin (Gorbatchuk et al., 2013), which may include guests into interstitial space of their crystal packing, have an intermediate position by this parameter: with ΔGc less negative than −4.6, −3.6 and −3.8 kJ/mol, respectively. If the interstitial inclusion is possible, the higher values of inclusion capacity S corresponds mostly to the less negative ΔGc values (Ziganshin et al., 2007; Yakimova et al., 2008).
This type of selectivity explains the described above stepwise size exclusion effect in the guest inclusion by solid hosts. When the guest molecule is too big for the host molecular cavity, the structure-property relationship may have two options. Either there is a stepwise change to no inclusion, e.g., for tert-butylthiacalix[4]arene (Gorbatchuk et al., 2002), or a stepwise change to a different packing pattern with a lower guest content observed for tert-butylcalix[4]arene (Gorbatchuk et al., 1999b). One should not compare the selectivity by inclusion Gibbs energy ΔGc and the selectivity by host-guest association constants Ka in liquid solutions from NMR titration experiments, which may give a huge overestimation of Ka values (Gorbatchuk et al., 2017).
Cooperativity of the guest inclusion process creates the additional selectivity options that can be used to enhance the efficient molecular recognition. Molecular structure of host and guest may have a strong impact also on the process of guest release, Figure 2A, e.g., in host regeneration of the sensor experiment. Being kinetically controlled through a strong sorption/desorption hysteresis (Dewa et al., 1998), guest release from the host-guest clathrate may have a different structure-property relationship than guest inclusion, which is under a thermodynamic control described above. This irreversibility may be detrimental in sensor experiments (Yakimova et al., 2008; Gorbatchuk et al., 2017), and the undesired history effect may be removed by high-temperature treatment of the host layer giving a normal sigmoidal shape of sorption isotherm by sensor unit (Matsuura et al., 2000).
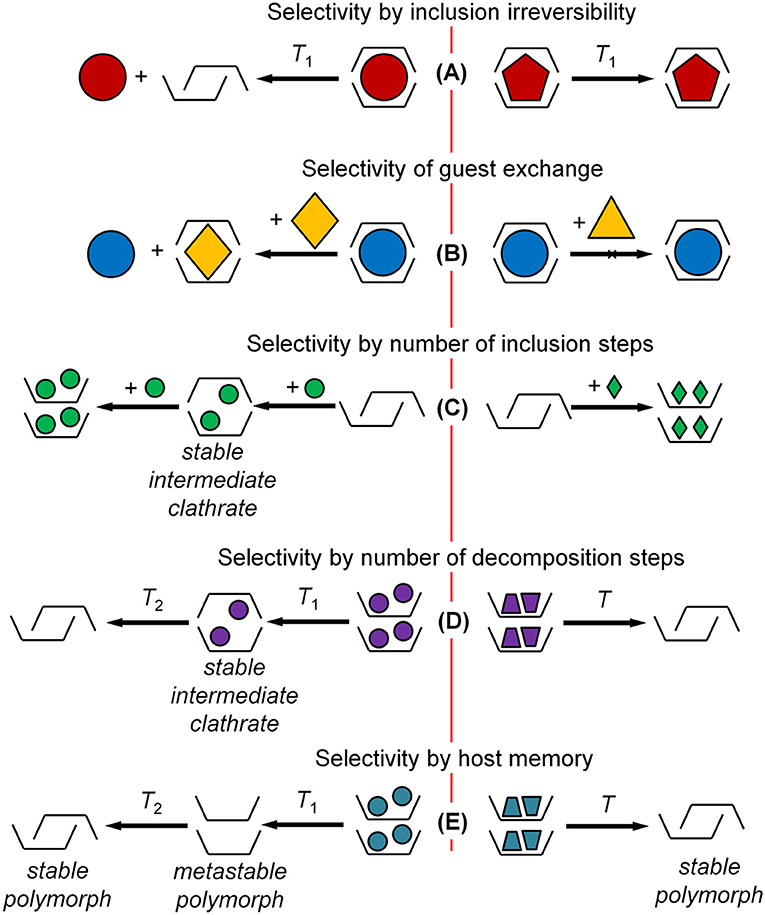
Figure 2. Specific types of molecular recognition using solid-solid phase transitions. (A) Selectivity by inclusion irreversibility; (B) selectivity of guest exchange; (C) selectivity by number of inclusion steps; (D) selectivity by number of decomposition steps; (E) selectivity by host memory.
The dependence of inclusion irreversibility on the guest molecular structure may be used to increase the selectivity of sensor experiment. A good example is the vapor sensor with a thin layer of adamantylcalix[4]arene on the quartz microbalance (Yakimova et al., 2008). The first run of this sensor experiment at 25°C and the second run after the host intermediate regeneration at 45°C by air purge give the sensor responses R1 and R2, respectively. The ratio of these responses R2/R1 is mostly different for different guests being a parameter of guest inclusion reversibility with R2/R1 ≤ 1. Using this parameter helps to increase the selectivity of single sensor analysis and to ensure recognition of more individual guests.
Along with the inclusion selectivity in binary host-guest systems, the selectivity of guest exchange in the solid phase of inclusion compound may be used for molecular recognition, Figure 2B. An efficiency of this exchange may depend on guest molecular structure in a different way than that of guest inclusion in binary system (Galyaltdinov et al., 2012; Amombo Noa et al., 2016). This gives an additional dimension to molecular recognition of guest compounds using the same host. For example, for thiacalix[4]arene (Galyaltdinov et al., 2014) and tert-butylthiacalix[4]arene (Galyaltdinov et al., 2012; Morohashi et al., 2019), the guest exchange increases the range of included compounds thus decreasing the inclusion selectivity. Still, this selectivity remains essentially stepwise. In some cases, the guest capable of inclusion in binary system cannot replace another guest in inclusion compound.
The guest inclusion by the host with a partial exchange of the already included water is a standard experimental procedure for solid hydrophilic hosts, such as native cyclodextrins (Ho et al., 2011, 2016; Gatiatulin et al., 2019) that do not include large hydrophobic guests in binary host-guest systems in the absence of water (Gorbatchuk et al., 2013; Gatiatulin et al., 2018). To activate this inclusion without water, the guest exchange in anhydrous inclusion compounds of cyclodextrins may be used (Gorbatchuk et al., 2013; Gatiatulin et al., 2014), which selectivity and efficiency depends much on molecular structure of the leaving guest. For example, 1-propanol and propionitrile cannot replace water in the saturated β-cyclodextrin hydrate but can exchange benzene, ethanol and acetonitrile in anhydrous clathrates with this host (Gorbatchuk et al., 2013; Gatiatulin et al., 2016).
The geometric constraints for guest inclusion changing with the variation of guest content in inclusion compound (clathrate) may give another type of selectivity. This is the selective formation of stable intermediate clathrates, Figures 2C,D, which can be seen in two-step sorption isotherms (Ziganshin et al., 2007; Safina et al., 2010) and thermogravimetric (TG) curves (Yakimov et al., 2008). Sorption isotherms and TG curves of this type are relatively rare. So for tert-butylcalix[4]arene (Ziganshin et al., 2007), tert-butylcalix[5]arene (Ziganshin et al., 2007), and adamantylcalix[4]arene (Yakimova et al., 2008), two-step sorption isotherms or TG curves are observed for 2 out of 15, 3 out of 8, 2 out of 7 studied guests, respectively.
An example of absolute molecular recognition of benzene by a number of guest inclusion steps was observed for tetra(ethoxycarbonyl)methoxy thiacalix[4]arene (Safina et al., 2010). This calixarene performs a two-step inclusion only for benzene in experiments with quartz-crystal microbalance sensors, while all other studied guests are included in one step. This type of selectivity was observed also for benzene in mixtures with its close homologs. It fundamentally differs from the classical key-to-lock model.
The irreversibility of guest inclusion and release with solid-solid phase transition can be a source of one more type of selectivity. This is selectivity of guest-induced polymorphism, which is a well-studied phenomenon used for screening of polymorphs (Braga et al., 2010; Petkune et al., 2012; Newman, 2013; Lee, 2014). A corresponding screening technique involves preparing the inclusion compound and removing the included guest (Lee et al., 2013; Gataullina et al., 2017). This is a smart process, where the host may remember molecular structure of a released guest by formation of a specific metastable polymorph (Gataullina et al., 2015).
An ideal case for molecular recognition is the host ability to form two polymorphs: stable and metastable ones, Figure 2E, where the metastable polymorph is formed after inclusion and release of only one guest and not of any other. Such an absolute selectivity for chloroform and methanol was found for N-(2-hydroxyethyl)carbamoylmethoxy) tert-butylthiacalix[4]arene (Safina et al., 2011) and for tert-butylthiacalix[4]arene (Galyaltdinov et al., 2012), respectively. For tert-butylthiacalix[4]arene, metastable polymorph is formed from its clathrate prepared only by solid-phase exchange of included 1,2-dichloroethane with methanol. In both cases, the formation of metastable polymorph can be detected by exothermic solid-solid phase transition of guest-free host in simultaneous experiment of TG and differential scanning calorimetry (DSC).
For comparison, tert-butylcalix[6]arene is less selective breaking the studied guest compounds into two groups: (1) remembered guests inducing formation of metastable polymorphs, and (2) non-remembered guests without such ability (Yakimov et al., 2008). This selectivity of tert-butylcalix[6]arene may be used in the analysis of binary mixtures if at least one of their components is from the first group. The efficiency of this analysis was demonstrated using DSC for the binary mixtures with one (Safina et al., 2013) and two (Gabdulkhaev et al., 2016) remembered components.
Guest-induced metastable polymorphs of calixarenes capable of an exothermic solid-phase transition have also a potential in 100% separation of binary mixtures of close homologs (Morohashi et al., 2017; Morohashi and Hattori, 2018) or compounds with close boiling points (Gabdulkhaev et al., 2016).
The phenomenon of polymorphism is more variable than the examples given in this review. In many cases, metastability of a polymorph is in its lower melting point than that of the stable form. Such polymorphs may have more than one melting point with an intermediate exothermic cold crystallization to the more stable forms (Gataullina et al., 2017, 2019). The formation of such polymorphs by guest inclusion and release may be also a kind of molecular recognition when it is selective enough, but in this case the problem is to find sufficient experimental proofs that the host treatment with different guests gives different polymorphs.
Cooperativity of guest inclusion by solid host with phase transition provides specific types of selectivity for neutral guest compounds that cannot be observed in liquid solutions. In some cases, this selectivity gives practically absolute molecular recognition and may be called smart because it uses the host polymorphism with a very selective and easily detectable memory of the guest included and released. Besides, of the same molecular recognition level is the very selective formation of stable intermediate inclusion compounds, which may be detected by mass-sensitive sensor and in thermogravimetric curves. This process resembles a smart switch of the initial host crystals recognizing only one guest or few guest compounds.
The specific types of structure-property relationships and molecular recognition caused by phase transition at guest inclusion and release may be expected for any solid host capable of clathrate formation. Still, discovery of the host-guest systems with a genuine selectivity for neutral molecules requires an extensive screening, which success cannot be predicted.
VG supervised the project and mainly wrote the paper. AG and MZ co-wrote the paper. All authors discussed the reviewed results and commented on the manuscript.
This work was financially supported by RFBR, grant No. 17-03-01311.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Afonso, R., Mendes, A., and Gales, L. (2012). Peptide-based solids: porosity and zeolitic behavior. J. Mater. Chem. 22, 1709–1723. doi: 10.1039/C1JM13568F
Amombo Noa, F. M., Bourne, S. A., Su, H., and Nassimbeni, L. R. (2016). Guest exchange in halogenated host-guest compounds: structures and kinetics. Cryst. Growth Des. 16, 1636–1642. doi: 10.1021/acs.cgd.5b01728
Ariga, K., Ito, H., Hill, J. P., and Tsukube, H. (2012). Molecular recognition: from solution science to nano/materials technology. Chem. Soc. Rev. 41, 5800–5835. doi: 10.1039/c2cs35162e
Braga, D., Grepioni, F., and Maini, L. (2010). The growing world of crystal forms. Chem. Commun. 46, 6232–6242. doi: 10.1039/c0cc01195a
DeJaco, R. F., de Mello, M. D., Nguyen, H. G. T., Jeon, M. Y., Zee, R. D., Tsapatsis, M., et al. (2019). Vapor and liquid phase adsorption of alcohol and water in silicalite-1 synthesized in fluoride media. AIChE J. e16868. doi: 10.1002/aic.16868. [Epub ahead of print].
Dewa, T., Endo, K., and Aoyama, Y. (1998). Dynamic aspects of lattice inclusion complexation involving a phase change. Equilibrium, kinetics, and energetics of guest-binding to a hydrogen-bonded flexible organic network. J. Am. Chem. Soc. 120, 8933–8940. doi: 10.1021/ja9812453
Engel, E. R., Jouaiti, A., Bezuidenhout, C. X., Hosseini, M. W., and Barbour, L. J. (2017). Activation-dependent breathing in a flexible metal-organic framework and the effects of repeated sorption/desorption cycling. Angew. Chemie Int. Ed. 56, 8874–8878. doi: 10.1002/anie.201704044
Faizullin, M. Z., Vinogradov, A. V., Tomin, A. S., and Koverda, V. P. (2019). Kinetics of decay of highly non-equilibrium metastable states of gas-saturated amorphous ice in the presence of artificially introduced crystal centers. Int. J. Heat Mass Transf. 143:118592. doi: 10.1016/j.ijheatmasstransfer.2019.118592
Fischer, E. (1894). Einfluss der configuration auf die wirkung der enzyme. II. Berichte der dtsch. Chem. Gesellschaft 27, 3479–3483. doi: 10.1002/cber.189402703169
Gabdulkhaev, M. N., Gatiatulin, A. K., Ziganshin, M. A., and Gorbatchuk, V. V. (2016). Nonlinear effect of two remembered guests in their mixtures on the host memory for guest inclusion and release. J. Therm. Anal. Calorim. 126, 627–632. doi: 10.1007/s10973-016-5558-8
Galyaltdinov, S. F., Ziganshin, M. A., Drapailo, A. B., and Gorbatchuk, V. V. (2012). Unusually high selectivity of guest exchange in tert-butylthiacalix[4] arene clathrate producing more thermostable inclusion and memory of guest. J. Phys. Chem. B 116, 11379–11385. doi: 10.1021/jp3065739
Galyaltdinov, S. F., Ziganshin, M. A., Gubaidullin, A. T., Vyshnevsky, S. G., Kalchenko, O. I., and Gorbatchuk, V. V. (2014). Anti-sieve effect in guest inclusion by thiacalix[4]arene giving a surge in thermal stability of its clathrates prepared by solid-phase guest exchange. CrystEngComm 16, 3781–3787. doi: 10.1039/C3CE42304B
Gao, J., Duan, L., Yang, G., Zhang, Q., Yang, M., and Fu, Q. (2012). Manipulating poly(lactic acid) surface morphology by solvent-induced crystallization. Appl. Surf. Sci. 261, 528–535. doi: 10.1016/j.apsusc.2012.08.050
Gataullina, K. V., Buzyurov, A. V., Ziganshin, M. A., Padnya, P. L., Stoikov, I. I., Schick, C., et al. (2019). Using fast scanning calorimetry to detect guest-induced polymorphism by irreversible phase transitions in the nanogram scale. CrystEngComm 21, 1034–1041. doi: 10.1039/C8CE01865K
Gataullina, K. V., Ziganshin, M. A., Stoikov, I. I., Gubaidullin, A. T., and Gorbatchuk, V. V. (2015). Twice as smart behavior of tert-butylthiacalix[4]arene derivative in glassy and crystalline form. Phys. Chem. Chem. Phys. 17, 15887–15895. doi: 10.1039/C5CP02042E
Gataullina, K. V., Ziganshin, M. A., Stoikov, I. I., Klimovitskii, A. E., Gubaidullin, A. T., Suwinska, K., et al. (2017). Smart polymorphism of thiacalix[4]arene with long-chain amide containing substituents. Cryst. Growth Des. 17, 3512–3527. doi: 10.1021/acs.cgd.7b00463
Gatiatulin, A. K., Osel'Skaya, V. Y., Ziganshin, M. A., and Gorbatchuk, V. V. (2018). Size exclusion effect in binary inclusion compounds of α-cyclodextrin. Phys. Chem. Chem. Phys. 20, 26105–26116. doi: 10.1039/C8CP03104E
Gatiatulin, A. K., Osel'skaya, V. Y., Ziganshin, M. A., and Gorbatchuk, V. V. (2019). Smart control of guest inclusion by α-cyclodextrin using its hydration history. RSC Adv. 9, 37778–37787. doi: 10.1039/C9RA08710A
Gatiatulin, A. K., Ziganshin, M. A., and Gorbatchuk, V. V. (2014). Selective preparation of beta-cyclodextrin clathrates by solid-phase exchange of included tetrahydrofurane for volatile guests in absence of water. J. Therm. Anal. Calorim. 118, 987–992. doi: 10.1007/s10973-014-3800-9
Gatiatulin, A. K., Ziganshin, M. A., Yumaeva, G. F., Gubaidullin, A. T., Suwinska, K., and Gorbatchuk, V. V. (2016). Using water-mimic organic compounds to activate guest inclusion by initially dry beta-cyclodextrin. RSC Adv. 6, 61984–61995. doi: 10.1039/C6RA11378H
Gorbatchuk, V. V., Antipin, I. S., Tsifarkin, A. G., Solomonov, B. N., and Konovalov, A. I. (1997a). The cooperative effect of the third component on the isotherms of guest vapour inclusion in solid tert-butylcalix[4]arene. Mendeleev Commun. 7, 215–217. doi: 10.1070/MC1997v007n06ABEH000807
Gorbatchuk, V. V., Gatiatulin, A. K., and Ziganshin, M. A. (2017). “Gas/solid complexation and inclusion” in Comprehensive Supramolecular Chemistry II, Vol II, ed J. L. Atwood, G. W. Gokel, and L. J. Barbour (Oxford: Elsevier), 139–150. doi: 10.1016/B978-0-12-409547-2.12499-0
Gorbatchuk, V. V., Gatiatulin, A. K., Ziganshin, M. A., Gubaidullin, A. T., and Yakimova, L. S. (2013). Unusually high efficiency of β-cyclodextrin clathrate preparation by water-free solid-phase guest exchange. J. Phys. Chem. B 117, 14544–14556. doi: 10.1021/jp408059b
Gorbatchuk, V. V., Mironov, N. A., Solomonov, B. N., and Habicher, W. D. (2004). Biomimetic cooperative interactions of dried cross-linked poly(N-6-aminohexylacrylamide) with binary mixtures of solvent vapors. Biomacromolecules 5, 1615–1623. doi: 10.1021/bm049743t
Gorbatchuk, V. V., Tsifarkin, A. G., Antipin, I. S., Solomonov, B. N., and Konovalov, A. I. (1999b). Influence of the guest molecular size on the thermodynamic parameters of host–guest complexes between solid tert-butylcalix[4]arene and vapours of organic compounds. Mendeleev Commun. 9, 11–13. doi: 10.1070/MC1999v009n01ABEH000989
Gorbatchuk, V. V., Tsifarkin, A. G., Antipin, I. S., Solomonov, B. N., and Konovalov, A. I. (1999a). Estimation of the free energy of the supramolecular effect on host–guest complex formation between solid tert-butylcalix[4]arene and vapors of organic compounds. J. Incl. Phenom. Macrocycl. Chem. 35, 389–396. doi: 10.1023/A:1008136124183
Gorbatchuk, V. V., Tsifarkin, A. G., Antipin, I. S., Solomonov, B. N., Konovalov, A. I., Lhotak, P., et al. (2002). Nonlinear structure - affinity relationships for vapor guest inclusion by solid calixarenes. J. Phys. Chem. B 106, 5845–5851. doi: 10.1021/jp014352j
Gorbatchuk, V. V., Tsifarkin, A. G., Antipin, I. S., Solomonov, B. N., Konovalov, A. I., Seidel, J., et al. (2000). Thermodynamic comparison of molecular recognition of vaporous guests by solid calixarene and diol hosts. J. Chem. Soc. Perkin Trans. 2, 2287–2294. doi: 10.1039/b003477k
Gorbatchuk, V. V., Ziganshin, M. A., and Solomonov, B. N. (1999c). Supramolecular interactions of solid human serum albumin with binary mixtures of solvent vapors. Biophys. Chem. 81, 107–123. doi: 10.1016/S0301-4622(99)00087-3
Gorbatchuk, V. V., Ziganshin, M. A., Solomonov, B. N., and Borisover, M. D. (1997b). Vapor sorption of organic compounds on human serum albumin. J. Phys. Org. Chem. 10, 901–907. doi: 10.1002/(SICI)1099-1395(199712)10:12<901::AID-POC956>3.0.CO;2-J
Hiraide, S., Tanaka, H., and Miyahara, M. T. (2016). Understanding gate adsorption behaviour of CO2 on elastic layer-structured metal-organic framework-11. Dalton Trans. 45, 4193–4202. doi: 10.1039/C5DT03476K
Ho, B. T., Joyce, D. C., and Bhandari, B. R. (2011). Encapsulation of ethylene gas into α-cyclodextrin and characterisation of the inclusion complexes. Food Chem. 127, 572–580. doi: 10.1016/j.foodchem.2011.01.043
Ho, T. M., Howes, T., and Bhandari, B. R. (2016). Encapsulation of CO2 into amorphous alpha-cyclodextrin powder at different moisture contents – part 1: encapsulation capacity and stability of inclusion complexes. Food Chem. 203, 348–355. doi: 10.1016/j.foodchem.2016.02.076
Joyce, L. A., Shabbir, S. H., and Anslyn, E. V. (2010). The uses of supramolecular chemistry in synthetic methodology development: examples of anion and neutral molecular recognition. Chem. Soc. Rev. 39, 3621–3632. doi: 10.1039/b926224p
Lee, E. H. (2014). A practical guide to pharmaceutical polymorph screening & selection. Asian J. Pharm. Sci. 9, 163–175. doi: 10.1016/j.ajps.2014.05.002
Lee, J., Boerrigter, S. X. M., Jung, Y. W., Byun, Y., Yuk, S. H., Byrn, S. R., et al. (2013). Organic vapor sorption method of isostructural solvates and polymorph of tenofovir disoproxil fumarate. Eur. J. Pharm. Sci. 50, 253–262. doi: 10.1016/j.ejps.2013.07.004
Lee, J. H., Jeoung, S., Chung, Y. G., and Moon, H. R. (2019). Elucidation of flexible metal-organic frameworks: research progresses and recent developments. Coord. Chem. Rev. 389, 161–188. doi: 10.1016/j.ccr.2019.03.008
Matsuura, K., Ariga, K., Endo, K., Aoyama, Y., and Okahata, Y. (2000). Dynamic analyses on induced-fit gaseous guest binding to organic crystals with a quartz-crystal microbalance. Chem. Eur. J. 6, 1750–1756. doi: 10.1002/(sici)1521-3765(20000515)6:10<1750::aid-chem1750>3.0.co;2-a
Mironov, N. A., Breus, V. V., Gorbatchuk, V. V., Solomonov, B. N., and Haertlé, T. (2003). Effects of hydration, lipids, and temperature on the binding of the volatile aroma terpenes by β-lactoglobulin powders. J. Agric. Food Chem. 51, 2665–2673. doi: 10.1021/jf020896m
Morohashi, N., and Hattori, T. (2018). Selective guest inclusion by crystals of calixarenes: potential for application as separation materials. J. Incl. Phenom. Macrocycl. Chem. 90, 261–277. doi: 10.1007/s10847-018-0783-3
Morohashi, N., Miyoshi, I., Sasaki, T., Nakaji, Y., Nakayama, H., and Hattori, T. (2019). Inclusion of alkanes with a crystal consisting of exocavity complexes of p-tert-butylthiacalix[4]arene with diethylamine: extension of guest scope by changing the structure of inclusion crystals. Cryst. Growth Des. 19, 7022–7029. doi: 10.1021/acs.cgd.9b00837
Morohashi, N., Tonosaki, A., Kitagawa, T., Sasaki, T., Ebata, K., and Hattori, T. (2017). Competitive inclusion of disubstituted benzene regioisomers with crystals of p-tert-butylcalix[4]arene. Cryst. Growth Des. 17, 5038–5043. doi: 10.1021/acs.cgd.7b01007
Newman, A. (2013). Specialized solid form screening techniques. Org. Process Res. Dev. 17, 457–471. doi: 10.1021/op300241f
Persch, E., Dumele, O., and Diederich, F. (2015). Molecular recognition in chemical and biological systems. Angew. Chemie Int. Ed. 54, 3290–3327. doi: 10.1002/anie.201408487
Petkune, S., Bobrovs, R., and Actinš, A. (2012). Organic solvents vapor pressure and relative humidity effects on the phase transition rate of α and β forms of tegafur. Pharm. Dev. Technol. 17, 625–631. doi: 10.3109/10837450.2011.565346
Ramon, G., Jacobs, A., Nassimbeni, L. R., and Yav-Kabwit, R. (2011). Inclusion compounds of p-tert-butylcalixarenes: structures, kinetics, and selectivity. Cryst. Growth Des. 11, 3172–3182. doi: 10.1021/cg2004084
Reinhoudt, D. N. (2013). “Supramolecular chemistry and heterocycles,” in Reference Module in Chemistry, Molecular Sciences, and Chemical Engineering (Elsevier), 1–2. doi: 10.1016/B978-0-12-409547-2.05396-8
Ripmeester, J. A., Enright, G. D., Ratcliffe, C. I., Udachin, K. A., and Moudrakovski, I. L. (2006). What we have learned from the study of solid p-tert-butylcalix[4]arene compounds. Chem. Commun. 48, 4986–4996. doi: 10.1039/b605275d
Safina, G. D., Gavrilova, O. M., Ziganshin, M. A., Stoikov, I. I., Antipin, I. S., and Gorbatchuk, V. V. (2011). Molecular recognition of chloroform by divergent polymorphic transitions in tert-butylthiacalix[4]arene tetrasubstituted with N-(2-hydroxyethyl)carbamoylmethoxy groups in a lower rim. Mendeleev Commun. 21, 291–292. doi: 10.1016/j.mencom.2011.09.022
Safina, G. D., Validova, L. R., Ziganshin, M. A., Stoikov, I. I., Antipin, I. S., and Gorbatchuk, V. V. (2010). Using clathrate pseudopolymorphism for a single sensor detection of target component in the headspace of liquid mixture. Sens. Actu. B Chem. 148, 264–268. doi: 10.1016/j.snb.2010.04.032
Safina, G. D., Ziganshin, M. A., Gubaidullin, A. T., and Gorbatchuk, V. V. (2013). Analysis of guest binary mixtures by tert-butylcalix[6]arene using host memory of previously bound guests. Org. Biomol. Chem. 11, 1318–1325. doi: 10.1039/c2ob27164h
Shu, X., Xu, K., Hou, D., and Li, C. (2018). Molecular recognition of water-soluble pillar[n]arenes towards biomolecules and drugs. Isr. J. Chem. 58, 1230–1240. doi: 10.1002/ijch.201800115
Sonnenberg, C., Hartmann, A., and Mazik, M. (2012). Molecular recognition of carbohydrates: evaluation of the binding properties of pyrazole-based receptors and their comparison with imidazole- and indole-based systems. Nat. Prod. Commun. 7, 321–326. doi: 10.1177/1934578X1200700311
Udachin, K. A., Enright, G. D., Brown, P. O., and Ripmeester, J. A. (2002). Pseudopolymorphism in the p-tert-butylcalix[4]arene-n-butylamine system: directing the structural motifs. Chem. Commun. 2162–2163. doi: 10.1039/B204313K
Yakimov, A. V., Ziganshin, M. A., Gubaidullin, A. T., and Gorbatchuk, V. V. (2008). Metastable tert-butylcalix[6]arene with unusually large tunable free volume for non-threshold enclathration of volatiles. Org. Biomol. Chem. 6, 982–985. doi: 10.1039/b800187a
Yakimova, L. S., Ziganshin, M. A., Sidorov, V. A., Kovalev, V. V., Shokova, E. A., Tafeenko, V. A., et al. (2008). Molecular recognition of organic vapors by adamantylcalix[4]arene in QCM sensor using partial binding reversibility. J. Phys. Chem. B 112, 15569–15575. doi: 10.1021/jp804277u
Yao, H., Ke, H., Zhang, X., Pan, S.-J., Li, M.-S., Yang, L.-P., et al. (2018). Molecular recognition of hydrophilic molecules in water by combining the hydrophobic effect with hydrogen bonding. J. Am. Chem. Soc. 140, 13466–13477. doi: 10.1021/jacs.8b09157
Yuan, Y., Tam, M. F., Simplaceanu, V., and Ho, C. (2015). New look at hemoglobin allostery. Chem. Rev. 115, 1702–1724. doi: 10.1021/cr500495x
Zhang, Y., Xu, Q., and Liu, Y. (2019). Molecular recognition and biological application of modified β-cyclodextrins. Sci. China Chem. 62, 549–560. doi: 10.1007/s11426-018-9405-3
Ziganshin, M. A., Gubina, N. S., Gerasimov, A. V., Gorbatchuk, V. V., Ziganshina, S. A., Chuklanov, A. P., et al. (2015). Interaction of L-alanyl-L-valine and L-valyl-L-alanine with organic vapors: thermal stability of clathrates, sorption capacity and the change in the morphology of dipeptide films. Phys. Chem. Chem. Phys. 17, 20168–20177. doi: 10.1039/C5CP03309H
Ziganshin, M. A., Safiullina, A. S., Ziganshina, S. A., Gerasimov, A. V., and Gorbatchuk, V. V. (2017). Non-zeolitic properties of the dipeptide L-leucyl-L-leucine as a result of the specific nanostructure formation. Phys. Chem. Chem. Phys. 19, 13788–13797. doi: 10.1039/C7CP01393K
Keywords: molecular recognition, selectivity, inclusion compound, clathrate, phase transition
Citation: Gatiatulin AK, Ziganshin MA and Gorbatchuk VV (2020) Smart Molecular Recognition: From Key-to-Lock Principle to Memory-Based Selectivity. Front. Chem. 7:933. doi: 10.3389/fchem.2019.00933
Received: 27 November 2019; Accepted: 23 December 2019;
Published: 21 January 2020.
Edited by:
Yong Yao, Nantong University, ChinaReviewed by:
Pi Wang, Taiyuan University of Technology, ChinaCopyright © 2020 Gatiatulin, Ziganshin and Gorbatchuk. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Valery V. Gorbatchuk, dmFsZXJ5LmdvcmJhdGNodWtAa3BmdS5ydQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.