- Department of Orthopedics, Peking University Third Hospital, Beijing, China
Traditional intravenous chemotherapy is relative to many systemic side effects, including myelosuppression, liver or kidney dysfunction, and neurotoxicity. As an alternative method, the injectable hydrogel can efficiently avoid these problems by releasing drugs topically at the tumor site. With advantages of localized drug toxicity in the tumor site, proper injectable hydrogel as the drug delivery system has become a research hotspot. Based on different types and stages of cancer, a variety of hydrogel drug delivery systems were developed, including thermosensitive, pH-sensitive, photosensitive, and dual-sensitive hydrogel. In this review, the latest developments of these hydrogels and related drug delivery systems were summarized. In summary, our increasing knowledge of injectable hydrogel for localized cancer therapy ensures us that it is a more durable and effective approach than traditional chemotherapy. Smart release system reacting to different stimuli at different time according to the micro-environment changes in the tumor site is a promising tendency for further studies.
Introduction
With the deterioration of the environment, the incidence of cancer is increasing year by year. In 2018, there were 18.1 million new cancer cases worldwide (9.5 million males and 8.6 million females), and the death toll was 9.6 million (5.4 million males and 4.2 million females) (Bray et al., 2018). The global cancer burden is further aggravated. One in five men and one in six women worldwide will develop cancer, and 1 in 8 men and 1 in 11 women will die for cancer (Bray et al., 2018; Sivaram et al., 2018).
Different strategies and methods are applied for cancer patients according to their different diagnose and stage. Possible therapies include chemotherapy, radiation, surgery, immunotherapy, targeted therapy, and gene therapy (Wang et al., 2013; Roy and Trinchieri, 2017; Bykov et al., 2018; Senft et al., 2018). Among those options, chemotherapy plays a pivotal role in tumor control and prevention of recurrence. The anti-tumor activity of chemotherapeutic drugs kill tumor cells with its drug toxicity (Zhou et al., 2017). Without selective or targeted killing, normal tissues could be harmed by chemical drugs along with tumor cells (Andreyev et al., 2014).
With the development of hydrogel, traditional chemotherapy drugs are booming a new life. Drugs are injected within the hydrogel directly into the tumor or adjacent to it (Elias et al., 2015; Ma et al., 2015; Pan et al., 2018). Drugs could be localized in a crosslinked 3D network of hydrophilic polymer chains (Wang et al., 2013, 2017; Bu et al., 2017). In this way, drug toxicity is limited within a localized area where tumor cells lie. Meanwhile, localized hydrogel reveals the ability for continuous efficient drug delivery in the tumor site (Yang Y. Y. et al., 2016; Yang et al., 2018). To enhance this character, a variety of systems have been developed with different composition, including polyphosphazene (PPZ), polyethylene glycol (PEG), and Polylactate glycolic acid PLGA (Bu et al., 2017; Ahmad et al., 2018; Cheng et al., 2018; Gajendiran et al., 2019).
Most of the hydrogel is insoluble in water but excellent in water absorption capacity (from 10% to thousands of times their dry weight) (Bin Imran et al., 2014; Murgia et al., 2018) (Figure 1). The soft, moist surface, and affinity with the tissue greatly reduce the stimulation of the human body. Most materials that make hydrogels are non-toxic (Zhang et al., 2015; Batista et al., 2019; Gajendiran et al., 2019). According to the response to external stimuli, hydrogels can be divided into ordinary hydrogels and smart hydrogels (Gu et al., 2017). Original hydrogels are not sensitive to environmental changes (Castelletto et al., 2019; Kerdsirichairat et al., 2019; Luo et al., 2019). While smart hydrogels could be affected by pH, temperature, and photoelectricity. It results in changeable gel volume and traits for smart hydrogels (Deepa et al., 2012; Chen and Liu, 2016; Chen X. et al., 2016; Milcovich et al., 2017; Chen et al., 2019). These hydrogels are currently widely used in tissue engineering, drug delivery, and other fields (Bae et al., 2013; Del Bufalo et al., 2016; Casey et al., 2017; Castelletto et al., 2019).
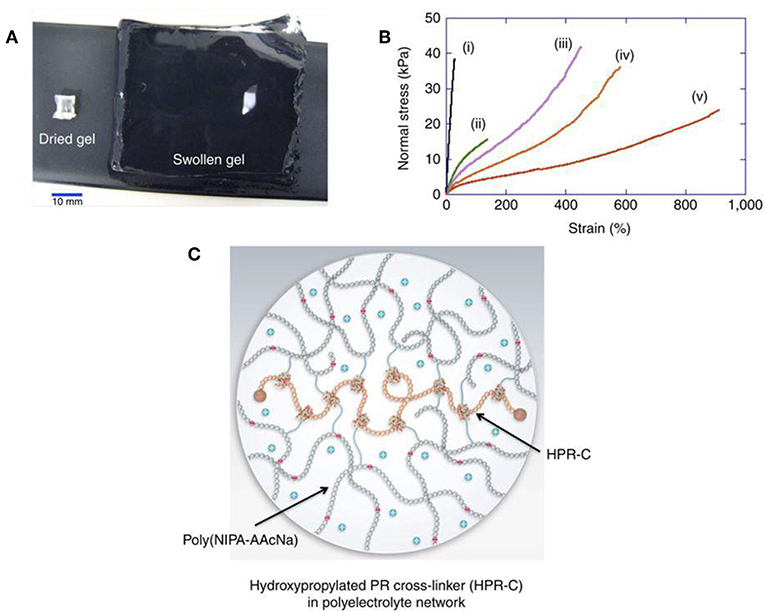
Figure 1. Hydrogel forms crosslinked 3D network and shows excellent water absorption capacity. (A) Hydrogel swelling from 129 mg (dry gel) to 80,000 mg (water-swollen gel). (B) Stress-strain curves of different hydrogels (i–v). (C) Schematic of swollen hydrogel (Bin Imran et al., 2014). Reproduced, with permission, from Bin Imran et al. (2014).
Thermosensitive Hydrogels
Temperature-sensitive hydrogels are hydrogels that change in volume as the ambient temperature changes (Klouda, 2015; Chen et al., 2018). The gel always has a certain proportion of hydrophobic and hydrophilic groups. Temperature changes can affect the hydrophobic interaction of these groups. The hydrogen bonding between the macromolecular chains causes the gel structure to change, and volume changes occur (Fan et al., 2015). The temperature at which the volume changes is referred as lower critical solution temperature (LCST) (Sapino et al., 2019). Under this temperature, the gel swells in aqueous solution. Once the temperature rises to LCST, the gel shrinks (Lei et al., 2012; Wang et al., 2015). Its unique properties can be used as a drug carrier, which is injected into the body after being combined with a drug at a low temperature (Le et al., 2018). Forming a colloidal state with the help of body temperature makes it a drug sustained-release system, which simplifies not only medical treatment but also patients' suffering (Wang et al., 2015, 2016; Yang Y. et al., 2016). Main thermosensitive injectable hydrogels include chitosan/glycerophosphate (C/GP), hyaluronic acid (HA), PLGA based hydrogel, PEG-based hydrogel, PECE, and PECT (Guo et al., 2012; Klouda, 2015; Huang et al., 2016; Le et al., 2018; Sapino et al., 2019). Their characters and drug delivery systems were summarized in Table 1.
In one study (Huang et al., 2016), injectable thermosensitive doxorubicin (DOX) delivery system was developed with PECT hydrogel. Instead of hydrogel based on free DOX diffusion, which suffered from rapid drug clearance and poor drug penetration in tumor tissue, self-polymerized drug-loaded nanoparticles were encapsulated into PECT hydrogel (Huang et al., 2016). After in vivo injection, PECT gel exhibited a transition phase between sol and gel. The viscosity increased abruptly once the temperature is higher than 28°C. With the highest viscosity at 37°C, the hydrogel turned to gel from sol (Figure 2). Loaded nanoparticles were dissociated from hydrogel and diffused within tumor tissue by EPR effect. Intracellular chemical drug release limited its toxic effects and enhanced its anti-tumor effectiveness. Contrasted with intravenous drug injections (I.V.), a thermosensitive hydrogel with nanomedicine loaded is an efficient drug delivery system, which enabled continuous drug release around tumor tissues (Huang et al., 2016).
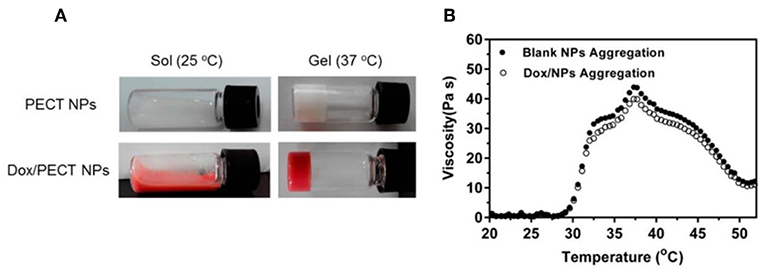
Figure 2. The thermosensitive property of hydrogel. (A) State of the thermosensitive hydrogel at different temperatures (25°C, 37°C). (B) Hydrogel viscosity increase of aqueous solution as a function of temperature. Source: Reproduced, with permission, from Huang et al. (2016).
Two or more elements loaded in one thermosensitive hydrogel has emerged as a promising drug delivery system for its superior anti-tumor efficacy. Polo-like kinase 1 (PLK1) gene is recognized as a key regulator of tumor cell meiosis and mitosis (Ma et al., 2014). RNA interference-based on PLK1shRNA can specifically reduce the function of the target gene in the tumor. A strategy of DOX and PLK1shRNA/PEI-Lys co-delivery hydrogel was developed for the treatment of osteosarcoma (Ma et al., 2014). In this method, PLK1shRNA/PEI-Lys in the hydrogel can greatly enhance the anti-tumor effect of DOX. With the synergistic effect from PLK1shRNA/PEI-Lys and DOX, significant osteosarcoma apoptosis was caused by the co-loaded hydrogel (Figure 3) (Ma et al., 2014).
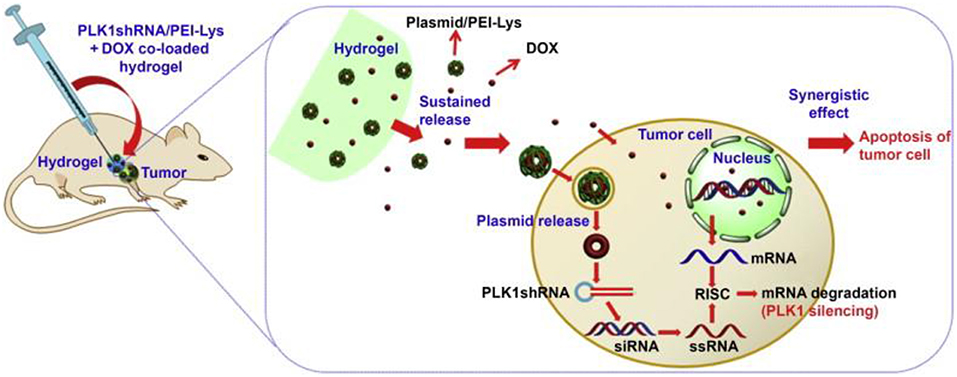
Figure 3. Schematic for synergism of PLK1shRNA/PEI-Lys and DOX from the hydrogel promotes tumor apoptosis on osteosarcoma in nude mice. Reproduced, with permission, from Ma et al. (2014).
pH-Sensitive Hydrogels
Glycolysis of tumor cells can cause acidification in the environment next to tumor tissues, resulting in lower pH value in the extracellular matrix than normal tissues (Kenney et al., 2018; Hu et al., 2019). A pH-sensitive hydrogel is a polymer gel in which the volume of the hydrogel changes depending on the pH of the external environment and the ionic strength (Liao et al., 2017; Liu et al., 2019). Such gels contain a large number of readily hydrolyzable or protonated acids, base groups such as carboxyl groups and amino groups (Lym et al., 2016). The dissociation of these groups is affected by the external pH. When the external pH changes, the degree of dissociation of these groups changes correspondingly, causing the internal and external ion concentration to change (Norouzi et al., 2016). In addition, the dissociation of these groups will destroy the corresponding hydrogen in the gel. The bond reduces the cross-linking point of the gel network, causing a change in the structure of the gel network and the degree of swelling of the hydrogel (Qu et al., 2017; Oroojalian et al., 2018). With this property, the rate of diffusion and release of the drug in the gel can be conveniently adjusted and controlled (Samanta et al., 2015).
A variety of elements for pH-sensitive hydrogel were explored in the past decades. Their characters and drug delivery systems were summarized in Table 2. One of the choices is based on chitosan-grafted-dihydrocaffeic acid (CS-DA) and oxidized pullulan (OP) (Liang et al., 2019). With classical drug for anti-tumor therapy, DOX-loaded hydrogel was tested to explore its reactions for the pH changes in the tumor environment. With glycolysis in the tumor site, a decrease of pH triggered the drug release (Liang et al., 2019). Compared with the morphology of hydrogel at pH 7.4, significant disintegration of hydrogel resulted in larger pore size at pH 5.5 (Figure 4) (Liang et al., 2019). After 60 h at pH 5.5, more than 80% of DOX was released from the hydrogel. The hydrogel was co-cultured with Hct116 cells (colon tumor cells) to test its anti-tumor effect (Liang et al., 2019). DOX is continuously stable released from hydrogel at pH 5.5 and 7.4. In both conditions, DOX can be effectively released for more than 3 days (Liang et al., 2019).
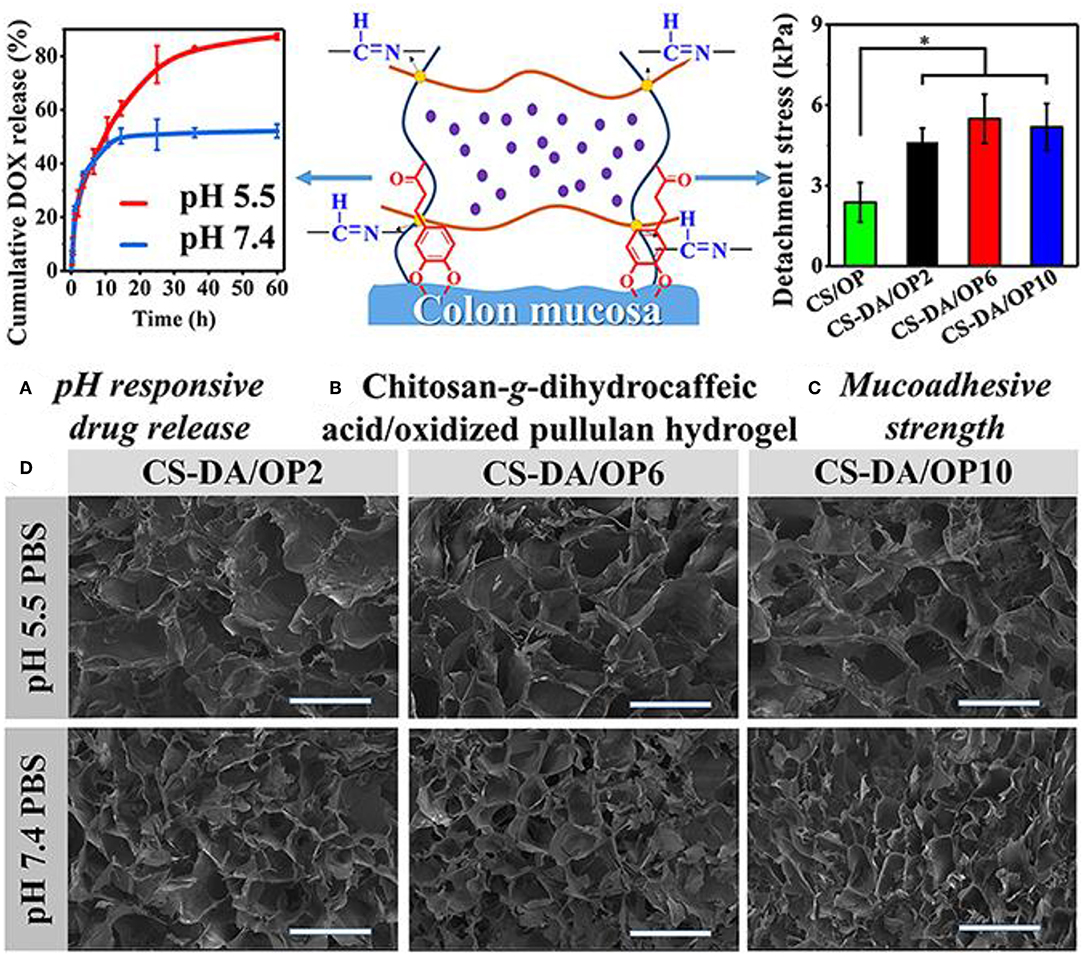
Figure 4. Characters of the pH-sensitive hydrogel. (A) pH-responsive drug release. (B) Schematic for pH-sensitive hydrogel. (C) Mucoadhesive strength of different elements. (D) SEM images of the pH-sensitive hydrogel after swelling in PBS with different pH values. *P < 0.05. Reproduced, with permission, from Liang et al. (2019).
In recent years, aspirin has been found to inhibit carbon monoxide synthase, inhibit nitrite-mediated DNA damage, reduce surviving, inhibit nuclear transcription factors, proteasomes, and calcium-activated neutral proteasome genes by inhibiting cyclooxygenase (Lu et al., 2008; Choi et al., 2013). The expression and other mechanisms play an anti-tumor effect. After loading aspirin into the hemicellulose hydrogel, it was found that 85% of the drug could be released continuously for 5–6 h under pH 7.4 (Choi et al., 2013). Sun et al. successfully prepared a series of acrylic acid and acrylamide copolymer grafted hemicellulose hydrogels by free radical polymerization (Sun et al., 2013). The combination of aspirin and the drug showed that the release rate of the drug in the simulated gastric juice was slower, and the release rate in the simulated intestinal fluid was significantly faster than that of the simulated gastric juice. When the release time was 12 h, the cumulative release rate reached 90%, which shows excellent sustained release properties (Sun et al., 2013).
In addition, Wang et al. first inserted dexamethasone phosphate into molecularly imprinted polymer nanospheres and loaded the polymer onto a pH-sensitive hydrogel, making it a biosensor that inhibits inflammation (Wang et al., 2010). Since the inflammatory reaction can lead to an acidic environment, this pH-sensitive hydrogel can rapidly release the drug at pH 6.0~7.4 to inhibit the inflammation (Wang et al., 2010). This different method is resistant to pH-sensitive hydrogels. The application of oncology drugs has brought new ideas.
Photosensitive Hydrogels
The mechanism of a light-sensitive hydrogel is divided into two types according to the properties of the photosensitive material (Chang et al., 2019). One is to directly add the photosensitive molecular material to the temperature-sensitive gel, and convert the light energy into heat energy to make the temperature inside the gel reach the phase transition temperature. In this way, hydrogel produces photosensitivity. Another kind of chromophore is introduced into the gel structure (Norouzi et al., 2016). The physicochemical properties of the chromophore are changed when subjected to light stimulation. By changing the network structure, hydrogel macroscopically exhibits photosensitivity. Usually, a structure such as azobenzene, spiropyran, o-naphthoquinone, anthracene, nitrophenyl, and coumarin is introduced into the gel (Tam et al., 2017). Among them, ruthenium, nitrophenyl, and coumarin compounds mainly take advantage of photo-cleavage photosensitive groups, which are bonded to the hydrophobic end through an aryl methyl bond (Norouzi et al., 2016; Tam et al., 2017). Under ultraviolet light or near-infrared light, the ester group is broken. The photosensitive reaction is caused. The hydrophobic end is converted to a hydrophilic end, causing the gel to dissociate. The azobenzene compound is controlled by the conversion of the cis-trans structure. Their characters and drug delivery systems were summarized in Table 3.
One of the applications for photosensitive hydrogels is the platform for cell culture and 3D tumor micro-environment studies. With advantages of its photosensitive character, cell detachment on the surface of hydrogel was done layer-by-layer to form a 3D cell culture medium (Figure 5) (Wang et al., 2014). Photoinitiated copolymerization of P (OEGMA-co-VDT-co-SPAA) (POVSP) hydrogels happened with UV irradiation. The compressive strengths of hydrogels were up to 5.1 MPa, which is strong enough for cell culture (Wang et al., 2014). It is revealed that photosensitive hydrogel is suitable for 3D cell culture model, which is vital for the study of the mechanism for tumor development.
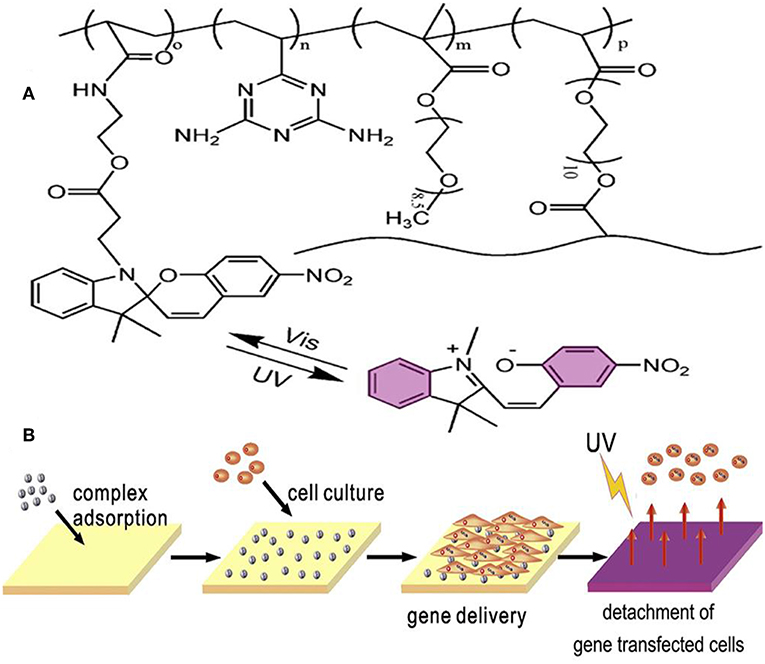
Figure 5. Schematic structure and mechanism of the photosensitive hydrogel. (A) Schematic molecular structure of hydrogel. (B) UV irradiation triggers the detachment of cells from the surface of the hydrogel. Reproduced, with permission, from Wang et al. (2014).
To achieve the same purpose, a photocleavable terpolymer hydrogel was developed as the basic technique for 3D bio-printing. This hydrogel is capable of self-shaping directly to the UV irradiation. It is designable by using selective illumination to UV light with the specific area covered with darkness (Figure 6) (Liao et al., 2015). The printable hydrogel is an inspiring design for controlled drug delivery with district distribution. It is a key technique for the realization of 4D drug delivery with both dimensions of time and space. With drugs loaded in the 3D space of hydrogels, dynamic drug release can be realized. In this process, different drug could be controlled to be released in different time with purpose (Xu et al., 2015; Chen Y. et al., 2016; Kim et al., 2017; Guo et al., 2018). This is the typical way of 4D drug delivery with additional dimensions of time.
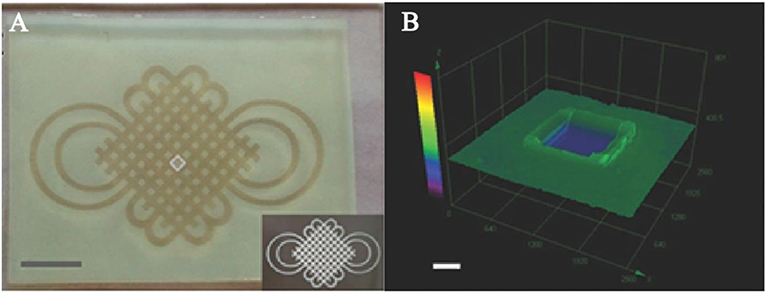
Figure 6. Photoactive self-shaping hydrogel spontaneous swelling caused by UV irradiation. (A) Hydrogel shaped by selected UV irradiation to form a designed pattern. (B) 3D image of a square unit for the design of UV irradiation. Reproduced, with permission, from Liao et al. (2015).
Dual-Sensitive Hydrogels
With the increasing requirements for the precision of controlled release of drugs, multi-sensitive hydrogels have received more and more attention (Bardajee et al., 2017). In particular, co-sensitive hydrogels for temperature and pH is widely researched. Temperature and pH are two important factors in physiological, biological, and chemical systems (Bardajee et al., 2017; Fathi et al., 2019). The temperature-pH double-sensitive hydrogel consists of a temperature-sensitive and pH-sensitive two-part hydrophilic polymer network (Lym et al., 2016). Usually formed with two or more monomers or polymers, which respond to temperature and pH, respectively.
The combination of temperature and pH sensitivity is crucial for the management of locoregional tumor recurrence (Mackiewicz et al., 2019). A novel pH-sensitive thermosensitive hydrogel loaded with modified doxorubicin-based prodrug nanoparticles (PDNPs), which is more efficient for tumor management than free DOX (Liu et al., 2019). Good biocompatibility and anti-tumor activity were verified by in vitro uptake and cell toxicity. For in vivo experiment, 4T1 cells with luciferase-tagged expression were implanted into mice. Management by temperature and pH co-sensitive hydrogel was remarkable (Figure 7) (Liu et al., 2019). It is a promising strategy for preventing the locoregional recurrence of the tumor.
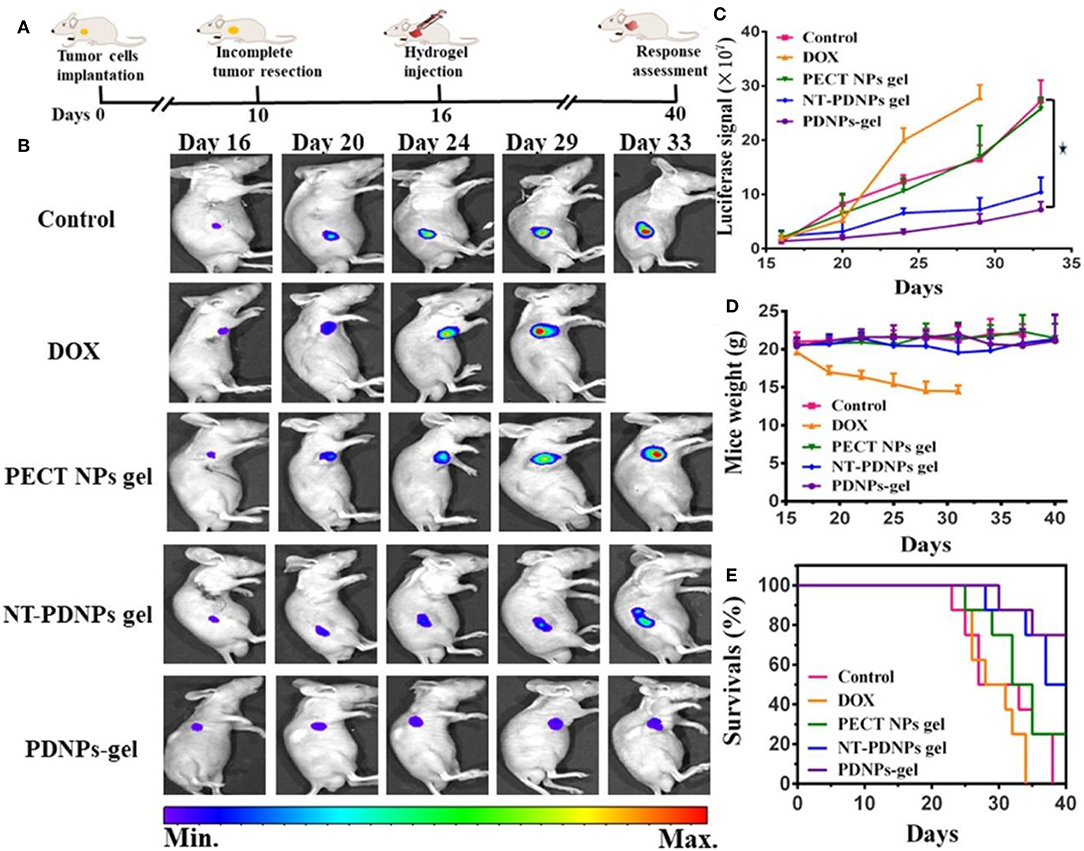
Figure 7. In vivo luciferase-tagged tumor model with the management of hydrogel. (A) Experiment process. (B) Bioluminescence images of mice treated with different formulations, (C) Quantified bioluminescence for tumors in mice. (D) Mice weight changes. (E) Survival rates of mice treated with different methods. Reproduced, with permission, from Liu et al. (2019).
Co-sensitive hydrogel with dual photoluminescence was developed with PL and PNIPAM (Zhao et al., 2016). This hydrogel contains a core which was made up of a red-emission complex and a blue-emission d-TPE. This nanoparticle is sensitive to the change of temperature and pH (Zhao et al., 2016). This hydrogel is stimulated by both temperature and pH and is more adaptable to the complex environment of human body fluids. In addition, the application of two or more materials, through their interaction, not only can improve the mechanical strength of the hydrogel, but also improve the precision of controlled release. With this character, the stimuli-responsive hydrogel has a wide application in medical imaging, cancer diagnosis, and advanced antitumor drug delivery (Zhao et al., 2016).
Conclusions
The unique character of hydrogel makes it an efficient functional medium for drug delivery (Zhang et al., 2018; Fathi et al., 2019; Mackiewicz et al., 2019). Given the limits from chemical drug toxicity for normal tissue and organs, localized drug delivery system by hydrogel has been a crucial method for cancer management. Related studies mainly focused on the delivery function and the methods of stimuli-response (Lym et al., 2016; Bardajee et al., 2017; Wei et al., 2017; Fathi et al., 2019). The smart hydrogel was developed with accurate responses to tiny changes in temperature, pH, and light. For now, drugs can be easily delivered to cancer tissue at the right time point. In the future, co-loaded drugs, including DNA, RNA, protein, and related products, would be a key point. The constantly accurate drug delivery system can realize anti-tumor drugs release followed by tissue repair factors. In this way, demission of time and space for drug delivery would be mixed in one hydrogel, making it a 4D functional hydrogel. It can make hydrogel a perfect choice for local chemotherapy and cancer management.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The financial support from Ministry of Science and Technology of China (2016YFB1101501) is gratefully acknowledged.
References
Ahmad, R., Kaus, N. H. M., and Hamid, S. (2018). Synthesis and characterization of PLGA-PEG thymoquinone nanoparticles and its cytotoxicity effects in tamoxifen-resistant breast cancer cells. Adv. Exp. Med. Biol. 1, 1–18. doi: 10.1007/5584_2018_302
Andreyev, J., Ross, P., Donnellan, C., Lennan, E., Leonard, P., Waters, C., et al. (2014). Guidance on the management of diarrhoea during cancer chemotherapy. Lancet Oncol. 15, e447–e460. doi: 10.1016/S1470-2045(14)70006-3
Bae, W. K., Park, M. S., Lee, J. H., Hwang, J. E., Shim, H. J., Cho, S. H., et al. (2013). Docetaxel-loaded thermoresponsive conjugated linoleic acid-incorporated poloxamer hydrogel for the suppression of peritoneal metastasis of gastric cancer. Biomaterials 34, 1433–1441. doi: 10.1016/j.biomaterials.2012.10.077
Bardajee, G. R., Hooshyar, Z., Farsi, M., Mobini, A., and Sang, G. (2017). Synthesis of a novel thermo/pH sensitive nanogel based on salep modified graphene oxide for drug release. Mater. Sci. Eng. C Mater. Biol. Appl. 72, 558–565. doi: 10.1016/j.msec.2016.11.109
Batista, R. A., Espitia, P. J. P., Quintans, J. S. S., Freitas, M. M., Cerqueira, M. A., Teixeira, J. A., et al. (2019). Hydrogel as an alternative structure for food packaging systems. Carbohydr. Polym. 205, 106–116. doi: 10.1016/j.carbpol.2018.10.006
Bin Imran, A., Esaki, K., Gotoh, H., Seki, T., Ito, K., Sakai, Y., et al. (2014). Extremely stretchable thermosensitive hydrogels by introducing slide-ring polyrotaxane cross-linkers and ionic groups into the polymer network. Nat. Commun. 5:5124. doi: 10.1038/ncomms6124
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., and Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424. doi: 10.3322/caac.21492
Bu, Y., Shen, H., Yang, F., Yang, Y., Wang, X., and Wu, D. (2017). Construction of tough, in situ forming double-network hydrogels with good biocompatibility. ACS Appl. Mater. Interfaces 9, 2205–2212. doi: 10.1021/acsami.6b15364
Bykov, V. J. N., Eriksson, S. E., Bianchi, J., and Wiman, K. G. (2018). Targeting mutant p53 for efficient cancer therapy. Nat. Rev. Cancer 18, 89–102. doi: 10.1038/nrc.2017.109
Casey, J., Yue, X., Nguyen, T. D., Acun, A., Zellmer, V. R., Zhang, S., et al. (2017). 3D hydrogel-based microwell arrays as a tumor microenvironment model to study breast cancer growth. Biomed. Mater. 12:025009. doi: 10.1088/1748-605X/aa5d5c
Castelletto, V., Edwards-Gayle, C. J. C., Greco, F., Hamley, I. W., Seitsonen, J., and Ruokolainen, J. (2019). Self-assembly, tunable hydrogel properties and selective anti- cancer activity of a carnosine-derived lipidated peptide. ACS Appl. Mater. Interfaces 11, 33573–33580. doi: 10.1021/acsami.9b09065
Chang, G., Zhang, H., Li, S., Huang, F., Shen, Y., and Xie, A. (2019). Effective photodynamic therapy of polymer hydrogel on tumor cells prepared using methylene blue sensitized mesoporous titania nanocrystal. Mater. Sci. Eng. C Mater. Biol. Appl. 99, 1392–1398. doi: 10.1016/j.msec.2019.02.056
Chen, C. H., Kuo, C. Y., Chen, S. H., Mao, S. H., Chang, C. Y., Shalumon, K. T., et al. (2018). Thermosensitive injectable hydrogel for simultaneous intraperitoneal delivery of doxorubicin and prevention of peritoneal adhesion. Int. J. Mol. Sci. 19:E1373. doi: 10.3390/ijms19051373
Chen, X., and Liu, Z. (2016). A pH-responsive hydrogel based on a tumor-targeting mesoporous silica nanocomposite for sustained cancer labeling and therapy. Macromol. Rapid Commun. 37, 1533–1539. doi: 10.1002/marc.201600261
Chen, X., Liu, Z., Parker, S. G., Zhang, X., Gooding, J. J., Ru, Y., et al. (2016). Light-induced hydrogel based on tumor-targeting mesoporous silica nanoparticles as a theranostic platform for sustained cancer treatment. ACS Appl. Mater. Interfaces 8, 15857–15863. doi: 10.1021/acsami.6b02562
Chen, Y., Hao, Y., Huang, Y., Wu, W., Liu, X., Li, Y., et al. (2019). An injectable, near-infrared light-responsive click cross-linked azobenzene hydrogel for breast cancer chemotherapy. J. Biomed. Nanotechnol. 15, 1923–1936. doi: 10.1166/jbn.2019.2821
Chen, Y., Zhang, F., Fu, Q., Liu, Y., Wang, Z., and Qi, N. (2016). In vitro proliferation and osteogenic differentiation of human dental pulp stem cells in injectable thermo-sensitive chitosan/beta-glycerophosphate/hydroxyapatite hydrogel. J. Biomater. Appl. 31, 317–327. doi: 10.1177/0885328216661566
Cheng, C., Meng, Y., Zhang, Z., Li, Y., and Zhang, Q. (2018). Tumoral acidic pH-responsive cis-diaminodichloroplatinum-incorporated Cy5.5-PEG- g-A-HA nanoparticles for targeting delivery of CDDP against cervical cancer. ACS Appl. Mater. Interfaces 10, 26882–26892. doi: 10.1021/acsami.8b07425
Choi, B. H., Chakraborty, G., Baek, K., and Yoon, H. S. (2013). Aspirin-induced Bcl-2 translocation and its phosphorylation in the nucleus trigger apoptosis in breast cancer cells. Exp. Mol. Med. 45:e47. doi: 10.1038/emm.2013.91
Deepa, G., Thulasidasan, A. K., Anto, R. J., Pillai, J. J., and Kumar, G. S. (2012). Cross-linked acrylic hydrogel for the controlled delivery of hydrophobic drugs in cancer therapy. Int. J. Nanomed. 7, 4077–4088. doi: 10.2147/IJN.S30149
Del Bufalo, F., Manzo, T., Hoyos, V., Yagyu, S., Caruana, I., Jacot, J., et al. (2016). 3D modeling of human cancer: a PEG-fibrin hydrogel system to study the role of tumor microenvironment and recapitulate the in vivo effect of oncolytic adenovirus. Biomaterials 84, 76–85. doi: 10.1016/j.biomaterials.2016.01.030
Demirdirek, B., and Uhrich, K. E. (2017). Novel salicylic acid-based chemically crosslinked pH-sensitive hydrogels as potential drug delivery systems. Int. J. Pharm. 528, 406–415. doi: 10.1016/j.ijpharm.2017.05.047
Dong, X., Wei, C., Lu, L., Liu, T., and Lv, F. (2016). Fluorescent nanogel based on four-arm PEG-PCL copolymer with porphyrin core for bioimaging. Mater. Sci. Eng. C Mater. Biol. Appl. 61, 214–219. doi: 10.1016/j.msec.2015.12.037
Elias, P. Z., Liu, G. W., Wei, H., Jensen, M. C., Horner, P. J., and Pun, S. H. (2015). A functionalized, injectable hydrogel for localized drug delivery with tunable thermosensitivity: synthesis and characterization of physical and toxicological properties. J. Control. Release 208, 76–84. doi: 10.1016/j.jconrel.2015.03.003
Fan, R., Tong, A., Li, X., Gao, X., Mei, L., Zhou, L., et al. (2015). Enhanced antitumor effects by docetaxel/LL37-loaded thermosensitive hydrogel nanoparticles in peritoneal carcinomatosis of colorectal cancer. Int. J. Nanomed. 10, 7291–7305. doi: 10.2147/IJN.S89066
Fathi, M., Alami-Milani, M., Geranmayeh, M. H., Barar, J., Erfan-Niya, H., and Omidi, Y. (2019). Dual thermo-and pH-sensitive injectable hydrogels of chitosan/(poly(N-isopropylacrylamide-co-itaconic acid)) for doxorubicin delivery in breast cancer. Int. J. Biol. Macromol. 128, 957–964. doi: 10.1016/j.ijbiomac.2019.01.122
Gajendiran, M., Jo, H., Kim, K., and Balasubramanian, S. (2019). Green synthesis of multifunctional PEG-carboxylate pi back-bonded gold nanoconjugates for breast cancer treatment. Int. J. Nanomed. 14, 819–834. doi: 10.2147/IJN.S190946
Gu, D., O'Connor, A. J., G, G. H. Q., and Ladewig, K. (2017). Hydrogels with smart systems for delivery of hydrophobic drugs. Expert Opin. Drug Deliv. 14, 879–895. doi: 10.1080/17425247.2017.1245290
Guo, D., Xu, S., Huang, Y., Jiang, H., Yasen, W., Wang, N., et al. (2018). Platinum(IV) complex-based two-in-one polyprodrug for a combinatorial chemo-photodynamic therapy. Biomaterials 177, 67–77. doi: 10.1016/j.biomaterials.2018.05.052
Guo, D. D., Hong, S. H., Jiang, H. L., Kim, J. H., Minai-Tehrani, A., Kim, J. E., et al. (2012). Synergistic effects of Akt1 shRNA and paclitaxel-incorporated conjugated linoleic acid-coupled poloxamer thermosensitive hydrogel on breast cancer. Biomaterials 33, 2272–2281. doi: 10.1016/j.biomaterials.2011.12.011
Hu, S. W., Wang, J., Zhang, T. T., Li, X. L., Chen, H. Y., and Xu, J. J. (2019). Targeted transmembrane delivery of Ca(2+) via FA-nanogel for synergistically enhanced chemotherapy. ACS Appl. Mater. Interfaces 11, 16412–16420. doi: 10.1021/acsami.9b04967
Huang, P., Song, H., Zhang, Y., Liu, J., Zhang, J., Wang, W., et al. (2016). Bridging the gap between macroscale drug delivery systems and nanomedicines: a nanoparticle-assembled thermosensitive hydrogel for peritumoral chemotherapy. ACS Appl. Mater. Interfaces 8, 29323–29333. doi: 10.1021/acsami.6b10416
Huang, Z., Xiao, H., Lu, X., Yan, W., and Ji, Z. (2018). Enhanced photo/chemo combination efficiency against bladder tumor by encapsulation of DOX and ZnPC into in situ-formed thermosensitive polymer hydrogel. Int. J. Nanomed. 13, 7623–7631. doi: 10.2147/IJN.S179226
Kang, H., Liu, H., Zhang, X., Yan, J., Zhu, Z., Peng, L., et al. (2011). Photoresponsive DNA-cross-linked hydrogels for controllable release and cancer therapy. Langmuir 27, 399–408. doi: 10.1021/la1037553
Kenney, R. M., Boyce, M. W., Whitman, N. A., Kromhout, B. P., and Lockett, M. R. (2018). A pH-sensing optode for mapping spatiotemporal gradients in 3D paper-based cell cultures. Anal. Chem. 90, 2376–2383. doi: 10.1021/acs.analchem.7b05015
Kerdsirichairat, T., Narang, A. K., Thompson, E., Kim, S. H., Rao, A., Ding, K., et al. (2019). Hydrogel spacer for borderline resectable and locally advanced pancreatic cancer: a feasibility human study. Gastroenterology 157, 933–935. doi: 10.1053/j.gastro.2019.07.012
Kim, Y. M., Potta, T., Park, K. H., and Song, S. C. (2017). Temperature responsive chemical crosslinkable UV pretreated hydrogel for application to injectable tissue regeneration system via differentiations of encapsulated hMSCs. Biomaterials 112, 248–256. doi: 10.1016/j.biomaterials.2016.10.025
Klouda, L. (2015). Thermoresponsive hydrogels in biomedical applications: a seven-year update. Eur. J. Pharm. Biopharm. 97(Pt B), 338–349. doi: 10.1016/j.ejpb.2015.05.017
Le, P. N., Huynh, C. K., and Tran, N. Q. (2018). Advances in thermosensitive polymer-grafted platforms for biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 92, 1016–1030. doi: 10.1016/j.msec.2018.02.006
Lei, N., Gong, C., Qian, Z., Luo, F., Wang, C., Wang, H., et al. (2012). Therapeutic application of injectable thermosensitive hydrogel in preventing local breast cancer recurrence and improving incision wound healing in a mouse model. Nanoscale 4, 5686–5693. doi: 10.1039/c2nr30731f
Liang, Y., Zhao, X., Ma, P. X., Guo, B., Du, Y., and Han, X. (2019). pH-responsive injectable hydrogels with mucosal adhesiveness based on chitosan-grafted-dihydrocaffeic acid and oxidized pullulan for localized drug delivery. J. Colloid Interface Sci. 536, 224–234. doi: 10.1016/j.jcis.2018.10.056
Liao, W. C., Lilienthal, S., Kahn, J. S., Riutin, M., Sohn, Y. S., Nechushtai, R., et al. (2017). pH- and ligand-induced release of loads from DNA-acrylamide hydrogel microcapsules. Chem. Sci. 8, 3362–3373. doi: 10.1039/C6SC04770J
Liao, Y., An, N., Wang, N., Zhang, Y., Song, J., Zhou, J., et al. (2015). Photoactive self-shaping hydrogels as noncontact 3D macro/microscopic photoprinting platforms. Macromol. Rapid Commun. 36, 2129–2136. doi: 10.1002/marc.201500390
Lin, Z., Xu, S., Gao, W., Hu, H., Chen, M., Wang, Y., et al. (2016). A comparative investigation between paclitaxel nanoparticle- and nanocrystal-loaded thermosensitive PECT hydrogels for peri-tumoural administration. Nanoscale 8, 18782–18791. doi: 10.1039/C6NR05498F
Liu, H., Shi, X., Wu, D., Kahsay Khshen, F., Deng, L., Dong, A., et al. (2019). Injectable, biodegradable, thermosensitive nanoparticles-aggregated hydrogel with tumor-specific targeting, penetration, and release for efficient postsurgical prevention of tumor recurrence. ACS Appl. Mater. Interfaces 11, 19700–19711. doi: 10.1021/acsami.9b01987
Liu, Z., Xu, G., Wang, C., Li, C., and Yao, P. (2017). Shear-responsive injectable supramolecular hydrogel releasing doxorubicin loaded micelles with pH-sensitivity for local tumor chemotherapy. Int. J. Pharm. 530, 53–62. doi: 10.1016/j.ijpharm.2017.07.063
Lu, M., Strohecker, A., Chen, F., Kwan, T., Bosman, J., Jordan, V. C., et al. (2008). Aspirin sensitizes cancer cells to TRAIL-induced apoptosis by reducing survivin levels. Clin. Cancer Res. 14, 3168–3176. doi: 10.1158/1078-0432.CCR-07-4362
Luo, Y., Wei, X., Wan, Y., Lin, X., Wang, Z., and Huang, P. (2019). 3D printing of hydrogel scaffolds for future application in photothermal therapy of breast cancer and tissue repair. Acta Biomater. 92, 37–47. doi: 10.1016/j.actbio.2019.05.039
Lym, J. S., Nguyen, Q. V., Ahn da, W., Huynh, C. T., Jae, H. J., Kim, Y. I., et al. (2016). Sulfamethazine-based pH-sensitive hydrogels with potential application for transcatheter arterial chemoembolization therapy. Acta Biomater. 41, 253–263. doi: 10.1016/j.actbio.2016.05.018
Ma, H., He, C., Cheng, Y., Li, D., Gong, Y., Liu, J., et al. (2014). PLK1shRNA and doxorubicin co-loaded thermosensitive PLGA-PEG-PLGA hydrogels for osteosarcoma treatment. Biomaterials 35, 8723–8734. doi: 10.1016/j.biomaterials.2014.06.045
Ma, H., He, C., Cheng, Y., Yang, Z., Zang, J., Liu, J., et al. (2015). Localized co-delivery of doxorubicin, cisplatin, and methotrexate by thermosensitive hydrogels for enhanced osteosarcoma treatment. ACS Appl. Mater. Interfaces 7, 27040–27048. doi: 10.1021/acsami.5b09112
Mackiewicz, M., Romanski, J., Drabczyk, K., Waleka, E., Stojek, Z., and Karbarz, M. (2019). Degradable, thermo-, pH- and redox-sensitive hydrogel microcapsules for burst and sustained release of drugs. Int. J. Pharm. 569:118589. doi: 10.1016/j.ijpharm.2019.118589
Milcovich, G., Lettieri, S., Antunes, F. E., Medronho, B., Fonseca, A. C., Coelho, J. F. J., et al. (2017). Recent advances in smart biotechnology: hydrogels and nanocarriers for tailored bioactive molecules depot. Adv. Colloid Interface Sci. 249, 163–180. doi: 10.1016/j.cis.2017.05.009
Murgia, X., Loretz, B., Hartwig, O., Hittinger, M., and Lehr, C. M. (2018). The role of mucus on drug transport and its potential to affect therapeutic outcomes. Adv. Drug Deliv. Rev. 124, 82–97. doi: 10.1016/j.addr.2017.10.009
Norouzi, M., Nazari, B., and Miller, D. W. (2016). Injectable hydrogel-based drug delivery systems for local cancer therapy. Drug Discov. Today 21, 1835–1849. doi: 10.1016/j.drudis.2016.07.006
Oroojalian, F., Babaei, M., Taghdisi, S. M., Abnous, K., Ramezani, M., and Alibolandi, M. (2018). Encapsulation of thermo-responsive gel in pH-sensitive polymersomes as dual-responsive smart carriers for controlled release of doxorubicin. J. Control. Release 288, 45–61. doi: 10.1016/j.jconrel.2018.08.039
Pan, A., Wang, Z., Chen, B., Dai, W., Zhang, H., He, B., et al. (2018). Localized co-delivery of collagenase and trastuzumab by thermosensitive hydrogels for enhanced antitumor efficacy in human breast xenograft. Drug Deliv. 25, 1495–1503. doi: 10.1080/10717544.2018.1474971
Pesoa, J. I., Rico, M. J., Rozados, V. R., Scharovsky, O. G., Luna, J. A., and Mengatto, L. N. (2018). Paclitaxel delivery system based on poly(lactide-co-glycolide) microparticles and chitosan thermo-sensitive gel for mammary adenocarcinoma treatment. J. Pharm. Pharmacol. 70, 1494–1502. doi: 10.1111/jphp.13006
Qu, J., Zhao, X., Ma, P. X., and Guo, B. (2017). pH-responsive self-healing injectable hydrogel based on N-carboxyethyl chitosan for hepatocellular carcinoma therapy. Acta Biomater. 58, 168–180. doi: 10.1016/j.actbio.2017.06.001
Ren, S., Dai, Y., Li, C., Qiu, Z., Wang, X., Tian, F., et al. (2016). Pharmacokinetics and pharmacodynamics evaluation of a thermosensitive chitosan based hydrogel containing liposomal doxorubicin. Eur. J. Pharm. Sci. 92, 137–145. doi: 10.1016/j.ejps.2016.07.002
Roy, S., and Trinchieri, G. (2017). Microbiota: a key orchestrator of cancer therapy. Nat. Rev. Cancer 17, 271–285. doi: 10.1038/nrc.2017.13
Salis, A., Rassu, G., Budai-Szucs, M., Benzoni, I., Csányi, E., Berkó, S., et al. (2015). Development of thermosensitive chitosan/glicerophospate injectable in situ gelling solutions for potential application in intraoperative fluorescence imaging and local therapy of hepatocellular carcinoma: a preliminary study. Expert Opin. Drug Deliv. 12, 1583–1596. doi: 10.1517/17425247.2015.1042452
Samanta, D., Meiser, J. L., and Zare, R. N. (2015). Polypyrrole nanoparticles for tunable, pH-sensitive and sustained drug release. Nanoscale 7, 9497–9504. doi: 10.1039/C5NR02196K
Sapino, S., Chirio, D., Peira, E., Abellán Rubio, E., Brunella, V., Jadhav, S. A., et al. (2019). Ocular drug delivery: a special focus on the thermosensitive approach. Nanomaterials 9:884. doi: 10.3390/nano9060884
Senft, D., Qi, J., and Ronai, Z. A. (2018). Ubiquitin ligases in oncogenic transformation and cancer therapy. Nat. Rev. Cancer 18, 69–88. doi: 10.1038/nrc.2017.105
Sheu, M. T., Jhan, H. J., Su, C. Y., Chen, L. C., Chang, C. E., Liu, D. Z., et al. (2016). Codelivery of doxorubicin-containing thermosensitive hydrogels incorporated with docetaxel-loaded mixed micelles enhances local cancer therapy. Colloids Surf. B Biointerfaces 143, 260–270. doi: 10.1016/j.colsurfb.2016.03.054
Sivaram, S., Majumdar, G., Perin, D., Nessa, A., Broeders, M., Lynge, E., et al. (2018). Population-based cancer screening programmes in low-income and middle-income countries: regional consultation of the International Cancer Screening Network in India. Lancet Oncol. 19, e113–e122. doi: 10.1016/S1470-2045(18)30003-2
Sun, X. F., Wang, H. H., Jing, Z. X., and Mohanathas, R. (2013). Hemicellulose-based pH-sensitive and biodegradable hydrogel for controlled drug delivery. Carbohydr. Polym. 92, 1357–1366. doi: 10.1016/j.carbpol.2012.10.032
Tam, R. Y., Smith, L. J., and Shoichet, M. S. (2017). Engineering cellular microenvironments with photo- and enzymatically responsive hydrogels: toward biomimetic 3D cell culture models. Acc. Chem. Res. 50, 703–713. doi: 10.1021/acs.accounts.6b00543
Wang, C., Javadi, A., Ghaffari, M., and Gong, S. (2010). A pH-sensitive molecularly imprinted nanospheres/hydrogel composite as a coating for implantable biosensors. Biomaterials 31, 4944–4951. doi: 10.1016/j.biomaterials.2010.02.073
Wang, N., Zhang, J., Sun, L., Wang, P., and Liu, W. (2014). Gene-modified cell detachment on photoresponsive hydrogels strengthened through hydrogen bonding. Acta Biomater. 10, 2529–2538. doi: 10.1016/j.actbio.2014.02.017
Wang, W., Song, H., Zhang, J., Li, P., Li, C., Wang, C., et al. (2015). An injectable, thermosensitive and multicompartment hydrogel for simultaneous encapsulation and independent release of a drug cocktail as an effective combination therapy platform. J. Control. Release 203, 57–66. doi: 10.1016/j.jconrel.2015.02.015
Wang, X., Li, D., Yang, F., Shen, H., Li, Z., and Wu, D. (2013). Controlled cross-linking strategy: from hybrid hydrogels to nanoparticle macroscopic aggregates. Polym. Chem. 4, 4596–4600. doi: 10.1039/c3py00811h
Wang, X., Wang, J., Wu, W., and Li, H. (2016). Vaginal delivery of carboplatin-loaded thermosensitive hydrogel to prevent local cervical cancer recurrence in mice. Drug Deliv. 23, 3544–3551. doi: 10.1080/10717544.2016.1205158
Wang, X., Wang, J., Yang, Y., Yang, F., and Wu, D. (2017). Fabrication of multi-stimuli responsive supramolecular hydrogels based on host-guest inclusion complexation of a tadpole-shaped cyclodextrin derivative with the azobenzene dimer. Polym. Chem. 8, 3901–3909. doi: 10.1039/C7PY00698E
Wei, L., Chen, J., Zhao, S., Ding, J., and Chen, X. (2017). Thermo-sensitive polypeptide hydrogel for locally sequential delivery of two-pronged antitumor drugs. Acta Biomater. 58, 44–53. doi: 10.1016/j.actbio.2017.05.053
Xu, Y., Li, Z., Li, X., Fan, Z., Liu, Z., Xie, X., et al. (2015). Regulating myogenic differentiation of mesenchymal stem cells using thermosensitive hydrogels. Acta Biomater. 26, 23–33. doi: 10.1016/j.actbio.2015.08.010
Yang, Y., Wang, X., Yang, F., Wang, L., and Wu, D. (2018). Highly elastic and ultratough hybrid ionic-covalent hydrogels with tunable structures and mechanics. Adv. Mater. Weinheim. 30:e1707071. doi: 10.1002/adma.201707071
Yang, Y., Zhao, H., Jia, Y., Guo, Q., Qu, Y., Su, J., et al. (2016). A novel gene delivery composite system based on biodegradable folate-poly (ester amine) polymer and thermosensitive hydrogel for sustained gene release. Sci. Rep. 6:21402. doi: 10.1038/srep21402
Yang, Y. Y., Wang, X., Yang, F., Shen, H., and Wu, D. (2016). A universal soaking strategy to convert composite hydrogels into extremely tough and rapidly recoverable double-network hydrogels. Adv. Mater. 28, 7178–7184. doi: 10.1002/adma.201601742
Yue, Z., Che, Y., Jin, Z., Wang, S., Ma, Q., Zhang, Q., et al. (2019). A facile method to fabricate thermo- and pH-sensitive hydrogels with good mechanical performance based on poly(ethylene glycol) methyl ether methacrylate and acrylic acid as a potential drug carriers. J. Biomater. Sci. Polym. Ed. 30, 1375–1398. doi: 10.1080/09205063.2019.1634859
Zhang, J., Wang, X., Wang, L., Yang, F., and Wu, D. (2015). Controlled cross-linking strategy for formation of hydrogels, microgels and nanogels. J. Control. Release 213:e25. doi: 10.1016/j.jconrel.2015.05.038
Zhang, W., Jin, X., Li, H., Zhang, R. R., and Wu, C. W. (2018). Injectable and body temperature sensitive hydrogels based on chitosan and hyaluronic acid for pH sensitive drug release. Carbohydr. Polym. 186, 82–90. doi: 10.1016/j.carbpol.2018.01.008
Zhao, Y., Shi, C., Yang, X., Shen, B., Sun, Y., Chen, Y., et al. (2016). pH- and temperature-sensitive hydrogel nanoparticles with dual photoluminescence for bioprobes. ACS Nano 10, 5856–5863. doi: 10.1021/acsnano.6b00770
Keywords: smart hydrogels, injectable, localized chemotherapy, stimuli responsive, drug delivery
Citation: Fan D, Tian Y and Liu Z (2019) Injectable Hydrogels for Localized Cancer Therapy. Front. Chem. 7:675. doi: 10.3389/fchem.2019.00675
Received: 19 August 2019; Accepted: 25 September 2019;
Published: 11 October 2019.
Edited by:
Xing Wang, Institute of Chemistry (CAS), ChinaReviewed by:
Daifeng Li, Stanford Bio-X, Stanford University, United StatesXiangjie Su, Utrecht University, Netherlands
Sophie Sun, Chinese Academy of Sciences, China
Copyright © 2019 Fan, Tian and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun Tian, dGlhbnlAYmptdS5lZHUuY24=; Zhong-jun Liu, empsaXVAYmptdS5lZHUuY24=
 Dao-yang Fan
Dao-yang Fan Yun Tian
Yun Tian Zhong-jun Liu
Zhong-jun Liu