- Department of Chemical Engineering, Virginia Polytechnic Institute and State University, Blacksburg, VA, United States
Biodegradable poly(α-hydroxy acids) can be synthesized by means of ring-opening polymerization (ROP) of O-carboxyanhydrides (OCAs). Numerous catalysts have been developed to control the living polymerization of OCAs. Here we review the rationale for the use of OCA, the desirable features for and important attributes of catalysts for the ROP of OCAs, and specific examples that have been developed.
Introduction
Polymers, commonly called plastics, can be categorized as degradable and non-degradable. Non-degradable plastics, mostly from petrochemical resources, tend to have difficulty in recycling and ultimately pollute the environment (Jambeck et al., 2015; Sardon and Dove, 2018). Substantial efforts have been devoted to develop degradable polymers (Zhu et al., 2016). Poly(α-hydroxy acids), including polylactide (PLA) and polyglycolide, and other polyesters are arguably the most successful examples (Middleton and Tipton, 2000; Dechy-Cabaret et al., 2004; Danhier et al., 2012). However, the mechanical and thermal properties of these materials still need to be improved to match non-degradable polymers (Jacobsen et al., 1999; Farah et al., 2016).
Besides efforts in processing with additives or developing new processing techniques (Di et al., 2005; Anderson et al., 2008; Lim et al., 2008; Rasal et al., 2010; Armentano et al., 2013; Nofar and Park, 2014; Kühnert et al., 2018), one major focus within polymer chemistry society is to generate new sets of monomers from natural resources to produce new degradable polymers that potentially replace many commodity polymers in the market (Yu et al., 2014; Gregory et al., 2017; Tong, 2017; Becker and Wurm, 2018). Among these new monomers, 1,3-dioxolane-2,4-diones, so-called O-carboxyanhydrides (OCAs), have emerged as active monomers for the synthesis of poly(α-hydroxy acids) (du Boullay et al., 2006; Martin Vaca and Bourissou, 2015; Yin et al., 2015). OCAs can be prepared from α-amino acid or α-hydroxy acids with a rich variety of side-chain functionalities (Figure 1; Martin Vaca and Bourissou, 2015; Yin et al., 2015). Note that the functionalization of corresponding lactide monomers often involves more synthetic steps with lower yields; and the polymerization of those functionalized lactide monomers can be difficult to achieve high molecular-weight (MW) polymers (Bourissou et al., 2007; Yu et al., 2014).
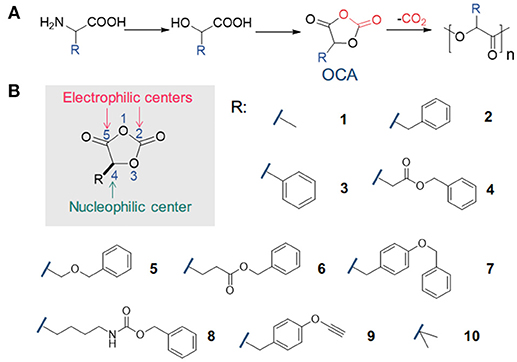
Figure 1. Synthesis and polymerization of O-carboxyanhydrides (OCAs). (A) Synthesis of OCAs from α-amino acids, and the polymerization of OCAs to prepare poly(α-hydroxy acids). (B) The scheme of reactivity sites in OCA, and representative OCA monomers bearing various functional groups.
OCAs are polymerized via ring-opening polymerization (ROP). This ROP process has been regarded as thermodynamically more favorable than that of lactide due to the liberation of a carbon dioxide molecule during the polymerization process (du Boullay et al., 2006). However, challenges remain in exploring desirable catalysts for controlled ROP of OCAs, especially for potential industrial application. A number of review articles have discussed the development of polymerization of OCAs (Martin Vaca and Bourissou, 2015; Yin et al., 2015; Feng et al., 2017). It is not the intention of this review to attempt another similar comprehensive review of OCA polymerization, but rather to discuss the problems in ROP of OCAs. We shall outline some general considerations about living ROP of OCA, followed by a discussion of the recent literature from a catalyst-development perspective. On occasion, we refer to some polymerization reactions for purely illustrative purposes. Their mention is not an endorsement, nor is omission to be considered as a negative judgment.
Considerations in OCA Polymerizations
Living and Controlled Polymerization of OCAs
Living polymerization—that is, all polymer chains grow at the same rate with no irreversible transfer or termination reactions—is central to current polymer chemistry (Szwarc, 1956; Grubbs and Grubbs, 2017). Generally, the rate of initiation (ki) should be greater than the rate of propagation (kp); the addition of a monomer to polymer chain ends occurs irreversibly, without chain termination and side reactions; and the breadth of the MW distribution (-D) becomes extremely narrow (-D approaches 1) (Grubbs and Grubbs, 2017). Living polymerizations can often be distinguished from kinetically-controlled (i.e., free radical) polymerizations by analyzing the evolution of the polymer's MW as a function of time and/or monomer conversion: MW is directly proportional to monomer conversion in living polymerizations since all chain ends are growing at essentially the same rate.
Based on the consensus of the “living and controlled” polymerization, the generic properties of an ideal OCA polymerization catalytic system are summarized as follows: (1) fast and complete initiation; (2) regioselective ring-opening of OCAs; (3) for practical reasons, converting monomers to growing polymer chains quantitatively and rapidly; (4) a linear relationship between the degree of polymerization (DP, typically measured as the number-average MW of the polymer, Mn) and monomer consumption; (5) - D < 1.2, which means the polymerization proceeding without an appreciable amount of (intramolecular or intermolecular) chain transfer or premature termination; (6) capable of controlling MW over a wide range (synthesis of high MW polymers). Last, but not least, for future industrial application, the catalyst should show high stability toward moisture and air, and maintain cost-effectiveness.
Thermodynamics for OCA Polymerization
At first glance, OCA bears multiple possible sites for nucleophilic attack (Figure 1B), similar to its analog NCA (N-carboxyanhydrides) molecule. Nevertheless, early studies by Smith and Tighe suggested that OCA is very stable and shows little tendency to polymerize compared with NCA (Smith and Tighe, 1976):the dimethyl-substituted OCA monomer (10) had a half-life over 1,000 h in a 90°C nitrobenzene solvent. However, these results remained relatively obscure over years; instead, the liberation of CO2 from OCA monomers has been regarded as a considerable driving force for polymerization (du Boullay et al., 2006), in addition to the ring strain as in many other cyclic monomers for ROPs (Saiyasombat et al., 1998; Odian, 2004; Houk et al., 2008).
One calculation showed that the ring-opening of L-1 is thermodynamically more favorable in terms of Gibbs free energy (ΔG° = −14.0 kcal/mol) than that of lactide (1.2 kcal/mol), catalyzed by 4-dimethylaminopyridine (DMAP) and methanol (du Boullay et al., 2006; Bonduelle et al., 2008). However, such a calculation only considers the initiation step, and the results can be complicated when factors such as chain propagation and different catalysts are involved in. For instance, the ROP of L-1 mediated by DMAP/neo-pentanol affords a controlled polymerization at room temperature (Mn = 62.3 kDa; -D = 1.18) (du Boullay et al., 2006), superior to that of lactide by the same catalyst requiring few days in refluxing solvent (Nederberg et al., 2001). However, recent experimental studies on the yttrium complex-mediated ROPs of 1 showed that the Gibbs free energy of activation of L-1 and L-lactide were essentially the same (16.5 vs. 16.7 kcal/mol, respectively) (Ouyang et al., 2017). The obtained kapp (kapp, the apparent rate constant) values for both polymerizations were also in the same order of magnitude with <20% difference; though prolonged induction time for the ROP of L-lactide was observed (Ouyang et al., 2017). In addition, in many cases (Breslow et al., 1957; Penczek et al., 1980; Duda et al., 2005), the fulfillment of thermodynamic requirements is a necessary—but not sufficient—prerequisite for a living polymerization to occur. The effects of catalysts can be seen as pivotal to the success of ROPs; performing polymerization kinetic studies is essential for mechanistic studies.
Molecular Weight of the Polyester
PLAs with Mn exceeding 100 kDa can be synthesized by Al(OiPr)3 or Sn(II) octanoate-based initiating systems (Dubois et al., 1991; Degée et al., 1999; Kowalski et al., 2000a). For example, the use of Sn(OBu)2 allows the polymer Mn over 900 kDa (Kowalski et al., 2000b). However, until recently, most polymers obtained from the ROP of OCAs have relatively low MWs (<50 kDa). Research in PLA shows that the mechanical properties and crystallization behaviors of PLA are dependent on the MW of the polymer (Garlotta, 2001). For instance, the tensile modulus of PLA increases by a factor of 2 when MW is raised from 50 to 100 kDa (Södergård and Stolt, 2002), whereas tensile strengths increase from 15.5 to 150 MPa when MW varies from 50 to 200 kDa (Van de Velde and Kiekens, 2002). Though PLAs used for biomedical applications often present a MW of about 5–30 kDa (Lasprilla et al., 2012), PLA materials for orthopedic and other temporary implants used in bone surgery usually have MWs from 150 to 300 kDa (Slomkowski et al., 2014). Those used to produce packaging materials necessitate high MW PLAs to exhibit decent mechanical properties (Garlotta, 2001; Auras et al., 2004). Therefore, it is critical for chemists to develop catalysts to enable the synthesis of high-MW polyesters.
OCA Monomer: Synthesis and Purification
In 1951 Davies first reported the synthesis of OCA by reacting α–hydroxy acid with phosgene, similar to NCA synthesis (Davies, 1951). To date, various OCAs have been synthesized (Figure 1B). In general, α-hydroxy acids are carbonylated using phosgene, diphosgene (Toyooka et al., 1989; Tang and Deng, 2002) or triphosgene (He et al., 2013; Chen et al., 2014). In case of the latter two carbonylation agents, activated charcoal is often used to promote the decomposition to phosgene and sometimes a tertiary amine (e.g., N-methylmorpholine) is added as an acid scavenger (Kricheldorf and Jont, 1983; Vandenbossche et al., 2010).
In many cases, repetitive crystallization is enough to obtain pure OCA monomers (du Boullay et al., 2006; Yin et al., 2013). However, methods are still needed for preparation of highly functional or low-melting-point OCAs that are difficult to recrystallize. A few reports suggested the use of flash chromatography for some OCA monomers purification (Vandenbossche et al., 2010; Lu Y. et al., 2012). Notably, NCAs can be purified by flash chromatography in anhydrous environments; (Kramer and Deming, 2010) however, the stability of OCAs in the column and the scope of such a method have not been well studied. The reported rapid and facile microflow synthesis of NCAs is also worth experimenting for OCAs (Otake et al., 2018).
Organocatalyst for OCA Polymerization
In early studies, the use of amines (e.g., pyridine and trimethylamine) for ROP of OCAs failed to initiate controlled polymerizations, with Mns < 3 kDa (Smith and Tighe, 1981; Kricheldorf and Jont, 1983). Besides, acidic catalysts (e.g., triflic acid) do not work for the ROP of OCAs (Martin Vaca and Bourissou, 2015). In 2006, the Bourissou group started to apply the organocatalysts that had achieved success in the ROP of lactones to OCA polymerization (du Boullay et al., 2006). Over the years, both DMAP and N-heterocyclic carbenes (NHCs) have been utilized for the ROP of OCAs (1, 2, 6, 8) and obtained reasonable results (du Boullay et al., 2006, 2008; Lu Y. et al., 2012; Zhang et al., 2012; Chen et al., 2014; Xia et al., 2014). However, most polymers catalyzed by organocatalysts exhibited MWs <30 kDa or low DPs (≤200) (Martin Vaca and Bourissou, 2015).
The Epimerization of α-Proton
Early studies by Kricheldorf and Jonté showed that the ROP of L-1 mediated by bases was accompanied by epimerization, as the optical rotations of the polymers decreased with the increase of the catalyst basicity (Kricheldorf and Jont, 1983). The racemization of α-proton in the 5-aryl-OCA monomers (e.g., 3) was also found in the alcoholysis mediated by a modified cinchona alkaloid, an aprotic nucleophile bearing tertiary amine and quinoline (Figure 2A) at −70°C (Tang and Deng, 2002). The kinetic studies showed that the interconversion between S- and R-3 was much faster than the enantioselective alcoholysis. When the aryl groups were replaced by alkyl groups, the reduced acidity of the α-proton rendered it unepimerizable by the cinchona alkaloid catalyst, which suggested the importance of the electronic property of the functional group on the 5-position of OCA monomers (Tang and Deng, 2002).
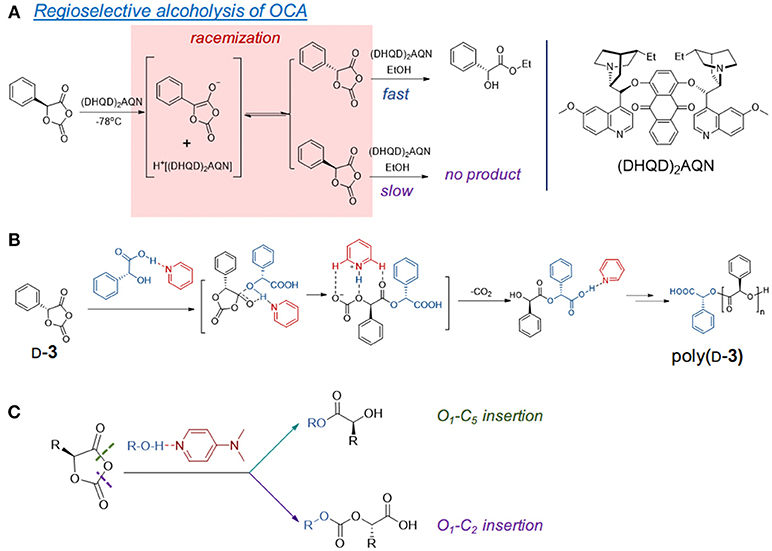
Figure 2. Epimerization of α-proton in the organocatalyst-mediated OCA polymerization. (A) The reported racemization of aryl-OCA (3) at low temperature in the presence of amine. (B) The use of D-mandelic acid/pyridine crystalline adduct can suppress the epimerization and provide isotactic-dominant poly(D-3). (C) Another proposed mechanism of DMAP/alcohol induced epimerization by non-regioselective ring-opening of OCA.
However, recent studies showed that epimerization of the α-proton still occurred to the DMAP-mediated ROP of OCAs bearing alkyl groups (e.g., 4 and 5) (Pounder et al., 2011; Wang et al., 2016). The decreased epimerization of the α-proton occurred in poly(L-4) when DMAP (pKa = 9.7) was replaced by less basic 4-methoxypyridine (pKa = 6.6) (Pounder et al., 2011). Similarly, the improved isotacticity of the poly(D-3) was found by using pyridine derivatives with decreased basicity (Buchard et al., 2014).
The use of acid/base crystalline adducts of mandelic acid and pyridine for the ROP of D-3 could suppress the racemization, and thereby (Figure 2B) could produce highly stereoregular isotactic polymers up to 48.0 kDa (over 80 h with Ð* of 1.17), which display enhanced thermal properties compared with the atactic poly(3) (Buchard et al., 2014). Similarly, the adducts of 4-methoxypyridine with L-lactic acid and β-benzyl α-L-malate could also initiate ROP of L-1 minimized epimerization of α-protons; however, those adducts failed to provide isotactic poly(L-4) (Bexis et al., 2017). Note that at low [OCA]/[initiator] ratios, epimerization still occurred in both polymers when using the acid-base adducts, suggesting that the pyridine adduct can still lead to epimerization even with decreased basicity (Bexis et al., 2017).
Lewis Pair Catalyst for OCA Polymerization
The use of a Lewis pair complex, that is a combination of Lewis acid with a base, has achieved recent success in controlled linear polymerization of acrylate, lactones and the synthesis of cyclic poly(lactide) (Hong et al., 2018). A very recent report showed that the use of the Lewis pair of Zn(C6F5)2 with primary or secondary amines could initiate the polymerization of L-2 and L-3 (Nie et al., 2018). The obtained polymers had Mns up to 26.8 kDa with -Ds < 1.1. However, severe epimerization (isotacticity <80%) occurred in both polymers (Nie et al., 2018). The use of bases in the Lewis pairs could therefore be detrimental to obtaining stereo-regular polymers from OCAs. Note that the same Lewis pair afforded cyclic PLAs, presumably via the zwitterionic intermediate with Zn(C6F5)2 and amine on each polymer chain terminus (Piedra-Arroni et al., 2013). The discrepancy between the two ROPs by the same Lewis pair indicated that chain propagation in the polymerization of OCAs was dominantly mediated by the Zn moiety without the influence of the amine. This was also attributed to the relatively low MWs, similar to those polymerizations promoted only by Zn-alkoxides (Wang et al., 2016; Feng and Tong, 2017a), which is discussed section Metal catalyst for OCA polymerization.
Other Concerns in Organocatalyst-Mediated OCA Polymerization
Computational studies by Bourissou et al. proposed that DMAP acts in a bifunctional nature by activating both the initiating alcohol and the carboxy oxygen O5 in OCA (Bonduelle et al., 2008). However, another computational study hinted that the pyridine-catalyzed ROP of OCAs could occur in both O1-C5 (ester formation) and O1-C2 (carbonate formation), which probably leads to epimerization (Figure 2C). Besides these computation studies, no detailed mechanistic studies revealed the initiation and chain propagation for DMAP or pyridine-analog mediated ROP. There also lacks the kinetic studies to reflect the reactivity order of DMAP and kapp during chain propagation.
In addition, studies using most organocatalysts to promote the ROP of OCAs usually started from 1, assuming that the success in 1 can be translated to other OCAs meaningfully. As observed in the ROP of lactones, when the methyl group of lactide is replaced with other groups, the polymerization conditions became harsh with incomplete monomer conversions and low DPs (Pounder and Dove, 2010; Chen et al., 2014). The ROP of OCAs using organocatalysts is similar. We found that at a high monomer-to-initiator ratio (500), the combination of DMAP/BnOH was not able to efficiently initiate the polymerization of L-2 (conversion of 2 = 57% in 24 h), in contrast to the results of the ROP of L-1 using the same catalysts (Feng and Tong, 2017a). Similarly, incomplete conversion of L-2 with a low MW (90% in 24 h, Mn = 3.2 kDa, -D = 2.19) was found in the reaction catalyzed by NHC/BnOH (NHC, 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene) (Feng and Tong, 2017a). As the motivation of studying OCA polymerization is to synthesize polyesters with pendant functional groups, we suggest that researchers should start from the OCA monomers bearing functional groups (e.g., 2) and validate the results in other monomers, instead of only reporting the results from 1.
Metal Catalyst for OCA Polymerization
Development of Metal Catalysts
Compared with the organocatalysts, the development of organometallic catalysts in OCA polymerization is surprisingly slow. Many organometallics that successfully mediated the ROP of lactide, lactones, and NCAs failed to translate to the polymerization of OCAs. Metal complexes that can promote ROPs of lactones or the copolymerization of epoxides and CO2, including Ti(IV), K, Sn(II), Al(III), Co(III), Nd(III), and Cr(III) complexes, did not mediate controlled ROP of L-1 (Figure 3; Kricheldorf and Jont, 1983; Zhuang et al., 2010; He et al., 2013; Jia et al., 2015).
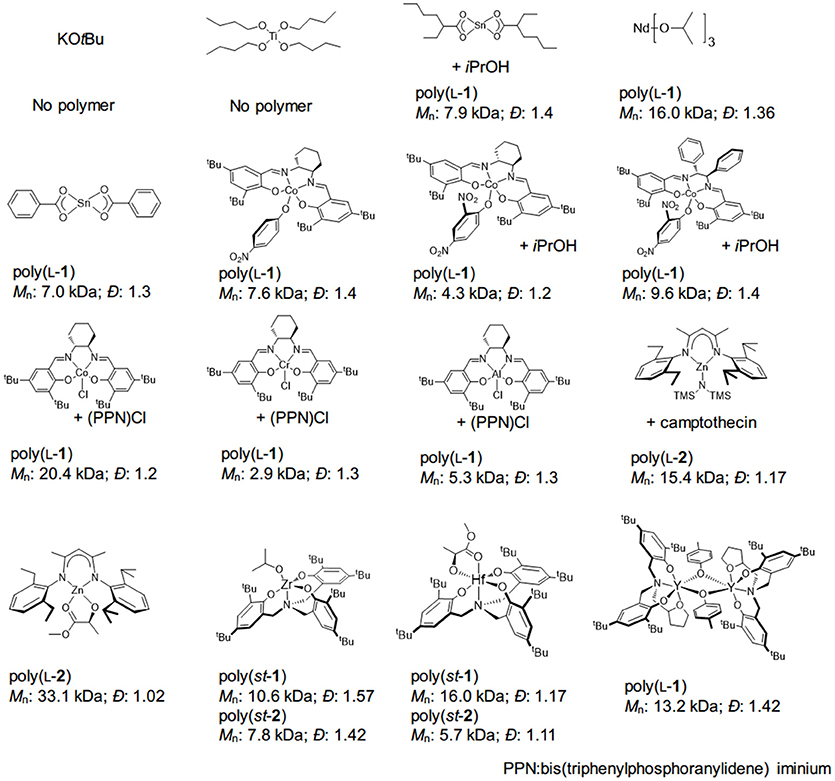
Figure 3. Representative results of various metal catalysts-mediated OCA polymerization. st, syndiotactic.
The Zn complexes with β-diiminate (BDI) ligands represented the first metal catalyst system that could mediate controlled ROP of OCAs (Figure 3; Yin et al., 2013; Wang et al., 2016). Similar to the well-known ROP of lactide (Chamberlain et al., 2001), the BDI-Zn complex requires an alcohol, even a very bulky one such as camptothecin or PEG (Yin et al., 2013), to promote polymerization, presumably through a coordination-insertion mechanism (Wang et al., 2016). It is worth noting that the monomeric BDI-Zn/alcohol complex performs more efficiently than a dimeric complex, which also agrees well with the reaction rate obtained in kinetic studies (Wang et al., 2016). In addition, no epimerization was found in the ROP of OCAs (for 2, 3, 5), indicating that no nucleophilic attack toward α-protons occurred for BDI-Zn complexes (Wang et al., 2016). As BDI-Zn-alkoxide can mediate the polymerization of either lactone or OCA, the sequential polymerization of lactone (including lactide) with OCAs can be smoothly achieved, regardless of monomer addition sequence (Wang et al., 2016).
Nevertheless, the BDI-Zn/alcohol complex cannot efficiently produce polymers with a high DP (≥300) (Feng and Tong, 2017a). This may be due to inefficient chain propagation; the insertion of Zn-alkoxide into L-2 was not followed by immediate decarboxylation, resulting in the inactive Zn-carbonate species at the chain end (Feng and Tong, 2017a). The mechanistic studies of using BDI-Zn to copolymerize epoxide and CO2 indicate the equilibrium between Zn-alkoxide and Zn-carbonate (Cheng et al., 2001; Moore et al., 2003; Jeske et al., 2007; Longo et al., 2016). Note that very recent studies involving the use of Zr, Hf (Sun et al., 2017), La and Y (Ouyang et al., 2017) complexes did not intend for high-MW polyester synthesis (Figure 3). For the reasons alluded to above (section Molecular weight of the polyester on the polymer MW), it is therefore crucial to develop a highly efficient decarboxylation process for rapid chain propagation in OCA polymerization.
We noticed that many metal catalysts for lactone polymerization, such as BDI-Zn, are disqualified for decarboxylation as they have been also used for polycarbonate synthesis (e.g., Al, Fe, Cr, Co) (Lu X. B. et al., 2012; Paul et al., 2015; Tong, 2017) (as have many organocatalysts Kiesewetter et al., 2010). Aware of substantial studies on metal catalyst-mediated NCA polymerization (Deming, 1997, 1998; Deming and Curtin, 2000) and the recent surge of interest in the photoredox catalysis (Prier et al., 2013; Zuo et al., 2014; Le and MacMillan, 2015), we developed a protocol for controlled photoredox ROP of enantiopure OCAs (1, 2, 5, 6) to afford isotactic polyesters with high MWs (>140 kDa) and narrow - Ds (<1.1) without epimerization at the α-methine hydrogen (Figure 4A) (Feng and Tong, 2017a). In such a system, the combination of the (bpy)Ni(0) complex (bpy, 2,2'-bipyridyl), a catalyst for NCA polymerization (Deming, 1997, 1998) but not reactive for controlled OCA polymerization per se (Feng and Tong, 2017a), and the photoredox catalyst Ir-1, is employed to efficiently promote the decarboxylation process under light irradiation based on the decarboxylation mechanism reported by the MacMillan lab (Zuo et al., 2014; Le and MacMillan, 2015). Zn(HMDS)2 was identified after screening a number of Zn complexes whereas the bulky BDI-Zn complexes do not provide high-MW polymers in the photoredox setting (Feng and Tong, 2017a). Kinetic studies indicated that the use of alcohol was only involved in the initiation to form Zn-alkoxide for ring-opening reactions and Ir-1 only influenced the Ni complex's oxidative state but did not affect chain-end reactivity. Mechanistic studies suggested that a Ni(0) complex regioselectively inserted at the O1-C5 bond in the OCA monomer, followed by Ir-mediated photoredox decarboxylation and transmetalation with a Zn complex, formed a reactive Zn-alkoxide terminus for chain propagation (Feng and Tong, 2017a). Notably, the polymerization has to be performed at low temperature (−15 to 20°C) to avoid the undesired Ni-mediated decarbonylation that occurs at room temperature (Yamamoto et al., 1980; Sano et al., 1984; Johnson et al., 2007).
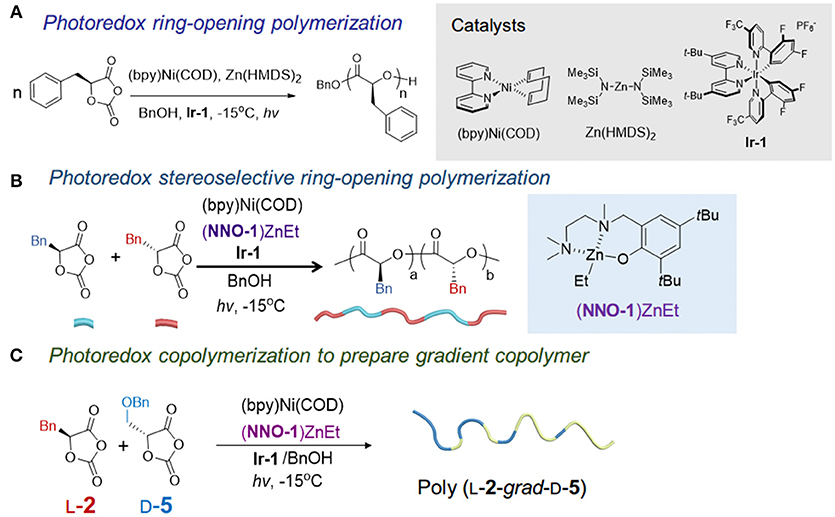
Figure 4. Photoredox polymerizations of OCAs. (A) The photoredox ROP of OCAs to prepare high MW isotactic polymers using (bpy)Ni/Zn(HMDS)2/Ir-1 catalysts. (B) Photoredox stereoselective copolymerization of racemic OCAs to prepare stereoblock polymers. (C) Photoredox copolymerization of two monomers with opposite chirality and significantly different reactivities to synthesize gradient copolymer. hv, blue LED with wavelengths of 400–500 nm.
Stereoselective ROP by Metal Catalysts
Organometallic catalysts are advantageous for preparing polyesters with various microstructures from lactides and β-lactones (Ajellal et al., 2010; Carpentier, 2010; Stanford and Dove, 2010; Thomas, 2010), although there have been reports on the utilization of organocatalysts (Dove et al., 2006; Zhang et al., 2007; Zhu and Chen, 2015). Nevertheless, the stereoselective synthesis of polyesters with pendant side-chain functional groups remains challenging. In 2017, Wu et al. identified a Hf-alkoxide complex with a C3 symmetric amino-tris(phenolate) ligand for syndioselective ROP of racemic OCAs (1, 2, and 7, Figure 3) (Sun et al., 2017). Most obtained polymers have MWs <20 kDa with a relatively broad - D (>1.1). The origin of such syndioselectivity (chirality influence of the metal), and the chain propagation mechanism, was not well-understood [chain-end or enantiomorphic mechanism; see the discussion on the Ge complex with the same C3 symmetric for lactide polymerization (Chmura et al., 2007, 2008)]. The Hf complex was utilized to mediate alternative copolymerization of the co-monomers with opposite chirality; however, all obtained copolymers had relatively low MWs (<10 kDa), and monomer conversions were even incomplete in many cases (Sun et al., 2017).
We have recently applied the photoredox Ni/Zn catalysts to stereoselective polymerization of OCAs (Feng et al., 2018). A (NNO-1)Zn complex with less bulky tridentate Schiff base ligands, compared with BDI, was identified to mediate the stereoselective and controlled polymerization of racemic OCAs (1, 2, 5, 6) that afforded stereoblock polymers (Figure 4B). The obtained stereoblock copolymers are highly isotactic with high MWs (>70 kDa) and narrow Ð*s (Mw/Mn < 1.1), with a probability of meso dyad formation (Pm, i.e., isotactic enchainment) of 0.97. To elucidate the microstructures and polymerization mechanism, deuterated [D2]-L-2 was synthesized from [D2]-L-phenylalanine and used for kinetic studies together with D-2 in the polymerization (Feng et al., 2018), as the methine deuterium in [D2]-L-2 does not show up in the 1H NMR spectrum. The NMR results suggested the polymer chain end did not have a kinetic preference for a specific enantiomer in the (NNO-1)ZnEt-mediated photoredox ROP of rac-2. Different from the enantiomorphic site-control by using racemic chiral aluminum catalysts for stereoblock PLA synthesis, the (bpy)Ni/(NNO-1)Zn/Ir-1 mediated stereoselective ROPs proceeds via the chain-end control; a stereoerror occurs during the chain propagation and the other enantiomer is incorporated and enchained (Feng et al., 2018). The computational study suggests the stereo-hindrance in the NNO-1 ligand affected the isoselectivity of the Zn complex. Notably, the obtained stereoblock polymers exhibited melting temperatures close to the stereocomplex of two isotactic polymers (Feng et al., 2018).
Subsequent copolymerization studies expand the use of (NNO-1) Zn photoredox system for the gradient copolymer synthesis. We found that using monomers with opposite chirality and significant difference in the polymerization rates (i.e., kapp) result in the formation of gradient copolymers with Mns close to the calculated MWs (over 40 kDa) and Ð* values of <1.1 (Figure 4C). The polymerization rates for OCA monomers in Ni/Zn systems have the following orders: k(2) > k(1) ≈ k(6) > k(5). No obvious difference exists between the rates of the two enantiomers of a given monomer. On the other hand, copolymerizations of monomers with the same chirality or similar polymerization rates can lead to the random copolymers.
Perspective
Despite the substantial number of catalysts that have been developed (Feng et al., 2017), few have really held promise for industrial production or commercialization. It remains difficult for organocatalysts to mediate stereoregular polymerization from OCAs as the racemization of α-proton persists when using most bases, even at low temperature (Tang and Deng, 2002) or with decreased basicity (Bexis et al., 2017). The detailed chain-propagation mechanism, including non-regioselective ring-opening of OCA (Pounder et al., 2011) and the existence of an active-monomer mechanism (Kricheldorf and Jont, 1983; Bonduelle et al., 2008), has not been well-studied compared with those results in the ROP of NCAs (Kricheldorf, 2006; Hadjichristidis et al., 2009; Cheng and Deming, 2012). On the other hand, substantial progress has been achieved in the use of metal complexes for controlled OCA polymerization, which allows for the synthesis of high MW polymers and stereoselective polymerizations (Feng and Tong, 2017a; Feng et al., 2018). However, the use of low temperature and relatively exotic experimental conditions could prevent the direct translation of the photoredox Ni/Zn catalysts to industrial production. Our review is not able to impart a perfect ability to predict what will work: the lessons learned in one context (e.g., polymerizations of lactide or NCAs) do not always translate into the ROP of OCAs, even when one might expect them to. Such instances reflect the fact that our understanding of the OCA polymerization mechanism remains incomplete.
Notably, as has been the case for decades, a comparison of results from different catalytic systems remains difficult, with many inconclusive or incomprehensive studies. The standardized and systemic studies can be helpful for future chemistry development, together with the use of visualized experimental procedures (Feng and Tong, 2017b).
Additionally, as many new polymers have been synthesized from OCAs, it is important to characterize their physiochemical properties, including their degradation profiles, to identify their potential applications. It is also important to start to design and perform studies on how to recycle the polymers for sustainable applications (Hillmyer and Tolman, 2014; Hong and Chen, 2017; Rahimi and García, 2017; Schneiderman and Hillmyer, 2017; Sardon and Dove, 2018; Zhu et al., 2018). Irrespective of the industrial prospects for the polyesters, the rapidly developing OCA chemistry can be suggestive for other polymerizations, in such a way as it benefits from the great expansion of the polymer field.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors acknowledge the financial support from ACS-PRF (57926-DNI-7) and National Science Foundation (CHE-1807911). The authors wish to thank the researchers who have made significant contributions to ROP of OCAs and related areas.
References
Ajellal, N., Carpentier, J.-F., Guillaume, C., Guillaume, S. M., Helou, M., Poirier, V., et al. (2010). Metal-catalyzed immortal ring-opening polymerization of lactones, lactides and cyclic carbonates. Dalton Trans. 39, 8363–8376. doi: 10.1039/c001226b
Anderson, K. S., Schreck, K. M., and Hillmyer, M. A. (2008). Toughening polylactide. Polym Rev. 48, 85–108. doi: 10.1080/15583720701834216
Armentano, I., Bitinis, N., Fortunati, E., Mattioli, S., Rescignano, N., Verdejo, R., et al. (2013). Multifunctional nanostructured PLA materials for packaging and tissue engineering. Prog. Polym. Sci. 38, 1720–1747. doi: 10.1016/j.progpolymsci.2013.05.010
Auras, R., Harte, B., and Selke, S. (2004). An overview of polylactides as packaging materials. Macromol. Biosci. 4, 835–864. doi: 10.1002/mabi.200400043
Becker, G., and Wurm, F. R. (2018). Functional biodegradable polymers via ring-opening polymerization of monomers without protective groups. Chem. Soc. Rev. 47, 7739–7782. doi: 10.1039/C8CS00531A
Bexis, P., De Winter, J., Coulembier, O., and Dove, A. P. (2017). Isotactic degradable polyesters derived from O-carboxyanhydrides of l-lactic and l-malic acid using a single organocatalyst/initiator system. Eur. Polym. J. 95, 660–670. doi: 10.1016/j.eurpolymj.2017.05.038
Bonduelle, C., Martín-Vaca, B., Cossío, F. P., and Bourissou, D. (2008). Monomer versus alcohol activation in the 4-Dimethylaminopyridine-Catalyzed Ring-opening polymerization of lactide and Lactic O-Carboxylic Anhydride. Chem. Eur. J. 14, 5304–5312. doi: 10.1002/chem.200800346
Bourissou, D., Moebs-Sanchez, S., and Martín-Vaca, B. (2007). Recent advances in the controlled preparation of poly(α-hydroxy acids): metal-free catalysts and new monomers. Comptes Rendus Chim. 10, 775–794. doi: 10.1016/j.crci.2007.05.004
Breslow, D. S., Hulse, G. E., and Matlack, A. S. (1957). Synthesis of Poly-β-alanine from Acrylamide. A novel synthesis of β-Alanine1. J. Am. Chem. Soc. 79, 3760–3763. doi: 10.1021/ja01571a039
Buchard, A., Carbery, D. R., Davidson, M. G., Ivanova, P. K., Jeffery, B. J., Kociok-Köhn, G. I., et al. (2014). Preparation of Stereoregular Isotactic Poly(mandelic acid) through Organocatalytic ring-opening polymerization of a cyclic O-carboxyanhydride. Angew. Chem. Int. Chem. 53, 13858–13861. doi: 10.1002/anie.201407525
Carpentier, J.-F. (2010). Discrete metal catalysts for stereoselective ring-opening polymerization of chiral Racemic β-Lactones. Macromol. Rapid Commun. 31, 1696–1705. doi: 10.1002/marc.201000114
Chamberlain, B. M., Cheng, M., Moore, D. R., Ovitt, T. M., Lobkovsky, E. B., and Coates, G. W. (2001). Polymerization of lactide with zinc and magnesium β-Diiminate complexes: stereocontrol and mechanism. J. Am. Chem. Soc. 123, 3229–3238. doi: 10.1021/ja003851f
Chen, X., Lai, H., Xiao, C., Tian, H., Chen, X., Tao, Y., et al. (2014). New bio-renewable polyester with rich side amino groups from l-lysine via controlled ring-opening polymerization. Polym. Chem. 5, 6495–6502. doi: 10.1039/C4PY00930D
Cheng, J., and Deming, T. J. (2012). “Synthesis of polypeptides by ring-opening polymerization of α-Amino Acid N-Carboxyanhydrides,” in Peptide-Based Materials, ed T. Deming (Berlin; Heidelberg: Springer), 1–26.
Cheng, M., Moore, D. R., Reczek, J. J., Chamberlain, B. M., Lobkovsky, E. B., and Coates, G. W. (2001). Single-site β-Diiminate zinc catalysts for the alternating copolymerization of CO2 and epoxides: catalyst synthesis and unprecedented polymerization activity. J. Am. Chem. Soc. 123, 8738–8749. doi: 10.1021/ja003850n
Chmura, A. J., Chuck, C. J., Davidson, M. G., Jones, M. D., Lunn, M. D., Bull, S. D., et al. (2007). A germanium alkoxide supported by a C3-symmetric ligand for the stereoselective synthesis of highly heterotactic polylactide under solvent-free conditions. Angew. Chem. Int. Ed. 46, 2280–2283. doi: 10.1002/anie.200603944
Chmura, A. J., Davidson, M. G., Frankis, C. J., Jones, M. D., and Lunn, M. D. (2008). Highly active and stereoselective zirconium and hafnium alkoxide initiators for solvent-free ring-opening polymerization of rac-lactide. Chem. Commun. 1293–1295. doi: 10.1039/b718678a
Danhier, F., Ansorena, E., Silva, J. M., Coco, R., Le Breton, A., and Préat, V. (2012). PLGA-based nanoparticles: an overview of biomedical applications. J. Control. Release 161, 505–522. doi: 10.1016/j.jconrel.2012.01.043
Davies, W. H. (1951). 302. Anhydrocarboxy-derivatives of hydroxy- and mercapto-acids. J. Chem. Soc 1357–1359.
Dechy-Cabaret, O., Martin-Vaca, B., and Bourissou, D. (2004). Controlled ring-opening polymerization of lactide and glycolide. Chem. Rev. 104, 6147–6176. doi: 10.1021/cr040002s
Degée, P., Dubois, P., Jérome, R., Jacobsen, S., and Fritz, H.-G. (1999). New catalysis for fast bulk ring-opening polymerization of lactide monomers. Macromol. Symp. 144, 289–302. doi: 10.1002/masy.19991440126
Deming, T. J. (1997). Facile synthesis of block copolypeptides of defined architecture. Nature 390, 386–389.
Deming, T. J. (1998). Amino acid derived nickelacycles: intermediates in nickel-mediated polypeptide synthesis. J. Am. Chem. Soc. 120, 4240–4241. doi: 10.1021/ja980313i
Deming, T. J., and Curtin, S. A. (2000). Chain initiation efficiency in cobalt- and nickel-mediated polypeptide synthesis. J. Am. Chem. Soc. 122, 5710–5717. doi: 10.1021/ja994281q
Di, Y. W., Iannace, S., Di Maio, E., and Nicolais, L. (2005). Poly(lactic acid)/organoclay nanocomposites: thermal, rheological properties and foam processing. J. Polym. Sci. B 43, 689–698. doi: 10.1002/polb.20366
Dove, A. P., Li, H. B., Pratt, R. C., Lohmeijer, B. G. G., Culkin, D. A., Waymouth, R. M., et al. (2006). Stereoselective polymerization of rac- and meso-lactide catalyzed by sterically encumbered N-heterocyclic carbenes. Chem. Commun. 2881–2883. doi: 10.1039/b601393g
du Boullay, O. T., Bonduelle, C., Martin-Vaca, B., and Bourissou, D. (2008). Functionalized polyesters from organocatalyzed ROP of gluOCA, the O-carboxyanhydride derived from glutamic acid. Chem. Commun. 1786–1788. doi: 10.1039/b800852c
du Boullay, O. T., Marchal, E., Martin-Vaca, B., Cossio, F. P., and Bourissou, D. (2006). An activated equivalent of lactide toward organocatalytic ring-opening polymerization. J. Am. Chem. Soc. 128, 16442–16443. doi: 10.1021/ja067046y
Dubois, P., Jacobs, C., Jerome, R., and Teyssie, P. (1991). Macromolecular engineering of polylactones and polylactides. 4. Mechanism and kinetics of lactide homopolymerization by aluminum isopropoxide. Macromolecules 24, 2266–2270. doi: 10.1021/ma00009a022
Duda, A., Kowalski, A., Libiszowski, J., and Penczek, S. (2005). Thermodynamic and kinetic polymerizability of cyclic esters. Macromol. Symp. 224, 71–84. doi: 10.1002/masy.200550607
Farah, S., Anderson, D. G., and Langer, R. (2016). Physical and mechanical properties of PLA, and their functions in widespread applications — A comprehensive review. Adv. Drug Deliv. Rev. 107, 367–392. doi: 10.1016/j.addr.2016.06.012
Feng, Q., and Tong, R. (2017a). Controlled photoredox ring-opening polymerization of O-Carboxyanhydrides. J. Am. Chem. Soc. 139, 6177–6182. doi: 10.1021/jacs.7b01462
Feng, Q., and Tong, R. (2017b). Controlled photoredox ring-opening polymerization of O-carboxyanhydrides mediated by Ni/Zn complexes. J. Vis. Exp. e56654. doi: 10.3791/56654
Feng, Q., Yang, L., Zhong, Y., Guo, D., Liu, G., Xie, L., et al. (2018). Stereoselective photoredox ring-opening polymerization of O-carboxyanhydrides. Nat. Commun. 9:1559. doi: 10.1038/s41467-018-03879-5
Feng, Q., Zhong, Y., Xie, L., and Tong, R. (2017). Recent advances in ring-opening polymerization of O-Carboxyanhydrides. Synlett 28, 1857–1866. doi: 10.1055/s-0036-1590841
Garlotta, D. A. (2001). Literature review of Poly(Lactic Acid). J. Polym. Environ. 9, 63–84. doi: 10.1023/A:1020200822435
Gregory, G. L., López-Vidal, E. M., and Buchard, A. (2017). Polymers from sugars: cyclic monomer synthesis, ring-opening polymerisation, material properties and applications. Chem. Commun. 53, 2198–2217. doi: 10.1039/C6CC09578J
Grubbs, R. B., and Grubbs, R. H. (2017). 50th anniversary perspective: living polymerization—emphasizing the molecule in macromolecules. Macromolecules 50, 6979–6997. doi: 10.1021/acs.macromol.7b01440
Hadjichristidis, N., Iatrou, H., Pitsikalis, M., and Sakellariou, G. (2009). Synthesis of well-defined polypeptide-based materials via the ring-opening polymerization of α-Amino Acid N-Carboxyanhydrides. Chem. Rev. 109, 5528–5578. doi: 10.1021/cr900049t
He, Z., Jiang, L., Chuan, Y., Li, H., and Yuan, M. (2013). Ring-opening polymerization of l-Lactic Acid O-Carboxyanhydrides initiated by alkoxy rare earth compounds. Molecules 18, 12768–12776. doi: 10.3390/molecules181012768
Hillmyer, M. A., and Tolman, W. B. (2014). Aliphatic polyester block polymers: renewable, degradable, and sustainable. Acc. Chem. Res. 47, 2390–2396. doi: 10.1021/ar500121d
Hong, M., and Chen, E. Y. X. (2017). Chemically recyclable polymers: a circular economy approach to sustainability. Green Chem. 19, 3692–3706. doi: 10.1039/C7GC01496A
Hong, M., Chen, J., and Chen, E. Y. X. (2018). Polymerization of polar monomers mediated by main-group lewis Acid–base pairs. Chem. Rev. 118, 10551–10616 doi: 10.1021/acs.chemrev.8b00352
Houk, K. N., Jabbari, A., Hall, H. K., and Alemán, C. (2008). Why δ-valerolactone polymerizes and γ-Butyrolactone does not. J. Org. Chem. 73, 2674–2678. doi: 10.1021/jo702567v
Jacobsen, S., Fritz, H. G., Degée, P., Dubois, P., and Jérôme, R. (1999). Polylactide (PLA)—a new way of production. Polym. Eng. Sci. 39, 1311–1319. doi: 10.1002/pen.11518
Jambeck, J. R., Geyer, R., Wilcox, C., Siegler, T. R., Perryman, M., Andrady, A., et al. (2015). Plastic waste inputs from land into the ocean. Science, 347, 768–771. doi: 10.1126/science.1260352
Jeske, R. C., DiCiccio, A. M., and Coates, G. W. (2007). Alternating copolymerization of epoxides and cyclic anhydrides: an improved route to aliphatic polyesters. J. Am. Chem. Soc. 129, 11330–11331. doi: 10.1021/ja0737568
Jia, F., Chen, X., Zheng, Y., Qin, Y., Tao, Y., and Wang, X. (2015). One-pot atom-efficient synthesis of bio-renewable polyesters and cyclic carbonates through tandem catalysis. Chem. Commun. 51, 8504–8507. doi: 10.1039/C5CC01329A
Johnson, J. B., Bercot, E. A., Rowley, J. M., Coates, G. W., and Rovis, T. (2007). Ligand-dependent catalytic cycle and role of styrene in nickel-catalyzed anhydride cross-coupling: evidence for turnover-limiting reductive elimination. J. Am. Chem. Soc. 129, 2718–2725. doi: 10.1021/ja067845g
Kiesewetter, M. K., Shin, E. J., Hedrick, J. L., and Waymouth, R. M. (2010). Organocatalysis: opportunities and challenges for polymer synthesis. Macromolecules 43, 2093–2107. doi: 10.1021/ma9025948
Kowalski, A., Duda, A., and Penczek, S. (2000a). Mechanism of cyclic ester polymerization initiated with Tin(II) Octoate. 2. Macromolecules fitted with Tin(II) Alkoxide species observed directly in MALDI–TOF spectra. Macromolecules 33, 689–695. doi: 10.1021/ma9906940
Kowalski, A., Libiszowski, J., Duda, A., and Penczek, S. (2000b). Polymerization of l,l-Dilactide Initiated by Tin(II) Butoxide. Macromolecules 33, 1964–1971. doi: 10.1021/ma991751s
Kramer, J. R., and Deming, T. J. (2010). General method for purification of α-Amino acid-N-carboxyanhydrides using flash chromatography. Biomacromolecules 11, 3668–3672. doi: 10.1021/bm101123k
Kricheldorf, H. R. (2006). Polypeptides and 100 years of chemistry of α-Amino Acid N-Carboxyanhydrides. Angew. Chem. Int. Chem. 45, 5752–5784. doi: 10.1002/anie.200600693
Kricheldorf, H. R., and Jonté, J. M. (1983). New polymer syntheses. 8. Synthesis and polymerization of l-Lactic Acid O-Carboxyanhydride (5-Methyl-Dioxolan-2,4-dione). Polym. Bull. 9, 276–283. doi: 10.1007/BF00262719
Kühnert, I., Spörer, Y., Brünig, H., Tran, N. H. A., and Rudolph, N. (2018). “Processing of Poly(lactic Acid),” in Industrial Applications of Poly(lactic acid), eds M. L. Di Lorenzo, R. Androsch (Cham: Springer International Publishing), 1–33.
Lasprilla, A. J. R., Martinez, G. A. R., Lunelli, B. H., Jardini, A. L., and Filho, R. M. (2012). Poly-lactic acid synthesis for application in biomedical devices — A review. Biotechnol. Adv. 30, 321–328. doi: 10.1016/j.biotechadv.2011.06.019
Le, C., and MacMillan, D. W. C. (2015). Fragment couplings via CO2 extrusion-recombination: expansion of a classic bond-forming strategy via metallaphotoredox. J. Am. Chem. Soc. 137, 11938–11941. doi: 10.1021/jacs.5b08304
Lim, L. T., Auras, R., and Rubino, M. (2008). Processing technologies for poly(lactic acid). Prog. Polym. Sci. 33, 820–852. doi: 10.1016/j.progpolymsci.2008.05.004
Longo, J. M., Sanford, M. J., and Coates, G. W. (2016). Ring-opening copolymerization of epoxides and cyclic anhydrides with discrete metal complexes: structure–property relationships. Chem. Rev. 116, 15167–15197. doi: 10.1021/acs.chemrev.6b00553
Lu, X. B., Ren, W. M., and Wu, G. P. (2012). CO2 copolymers from epoxides: catalyst activity, product selectivity, and stereochemistry control. Acc. Chem. Res. 45, 1721–1735. doi: 10.1021/ar300035z
Lu, Y., Yin, L., Zhang, Y., Zhang, Z., Xu, Y., Tong, R., et al. (2012). Synthesis of Water-Soluble Poly(α-hydroxy acids) from Living Ring-opening polymerization of O-Benzyl-l-serine carboxyanhydrides. ACS Macro Lett. 1, 441–444. doi: 10.1021/mz200165c
Martin Vaca, B., and Bourissou, D. (2015). O-Carboxyanhydrides: useful tools for the preparation of well-defined functionalized polyesters. ACS Macro Lett. 4, 792–798. doi: 10.1021/acsmacrolett.5b00376
Middleton, J. C., and Tipton, A. J. (2000). Synthetic biodegradable polymers as orthopedic devices. Biomaterials 21, 2335–2346. doi: 10.1016/S0142-9612(00)00101-0
Moore, D. R., Cheng, M., Lobkovsky, E. B., and Coates, G. W. (2003). Mechanism of the alternating copolymerization of epoxides and CO2 using β-Diiminate zinc catalysts: evidence for a bimetallic epoxide enchainment. J. Am. Chem. Soc. 125, 11911–11924. doi: 10.1021/ja030085e
Nederberg, F., Connor, E. F., Möller, M., Glauser, T., and Hedrick, J. L. (2001). New paradigms for organic catalysts: the first organocatalytic living polymerization. Angew. Chem. Int. Chem. 40, 2712–2715. doi: 10.1002/1521-3773(20010716)40:14<2712::AID-ANIE2712>3.0.CO;2-Z
Nie, Y., Wang, P., Du, H., Meng, W., and Yang, J. (2018). An efficient strategy for achieving controlled ring-opening polymerization of O-carboxyanhydrides via amine initiation in collaboration with metal-alkoxide catalysis. Polym. Chem. 9, 5014–5023. doi: 10.1039/C8PY01090K
Nofar, M., and Park, C. B. (2014). Poly (lactic acid) foaming. Prog. Polym. Sci. 39, 1721–1741. doi: 10.1016/j.progpolymsci.2014.04.001
Odian, G. (2004). “Ring-opening polymerization,” in Principle of Polymerization (Hoboken, NJ: John Wiley & Sons, Inc.), 544–618.
Otake, Y., Nakamura, H., and Fuse, S. (2018). Rapid and mild synthesis of amino acid n-carboxy anhydrides: basic-to-acidic flash switching in a microflow reactor. Angew. Chem. Int. Chem. 57, 11389–11393. doi: 10.1002/anie.201803549
Ouyang, H., Nie, K., Yuan, D., and Yao, Y. (2017). Synthesis of amine-bridged bis(phenolate) rare-earth metal aryloxides and their catalytic performances for the ring-opening polymerization of l-lactic acid O-carboxyanhydride and l-lactide. Dalton Trans. 46, 15928–15938. doi: 10.1039/C7DT03001K
Paul, S., Zhu, Y., Romain, C., Brooks, R., Saini, P. K., and Williams, C. K. (2015). Ring-opening copolymerization (ROCOP): synthesis and properties of polyesters and polycarbonates. Chem. Commun. 51, 6459–6479. doi: 10.1039/C4CC10113H
Penczek, S., Kubisa, P., and Matyjaszewski, K. (1980). “Monomer structures, ring strains and nucleophilicities (Basicities),” in Cationic Ring-Opening Polymerization of Heterocyclic Monomers, eds S. Penczek, P. Kubisa, K. Matyjaszewski (Berlin; Heidelberg: Springer), 3–7.
Piedra-Arroni, E., Ladavière, C., Amgoune, A., and Bourissou, D. (2013). Ring-opening polymerization with Zn(C6F5)2-based lewis pairs: original and efficient approach to cyclic polyesters. J. Am. Chem. Soc. 135, 13306–13309. doi: 10.1021/ja4069968
Pounder, R. J., and Dove, A. P. (2010). Synthesis and organocatalytic ring-opening polymerization of cyclic esters derived from l-Malic Acid. Biomacromolecules 11, 1930–1939. doi: 10.1021/bm1004355
Pounder, R. J., Fox, D. J., Barker, I. A., Bennison, M. J., and Dove, A. P. (2011). Ring-opening polymerization of an O-carboxyanhydride monomer derived from L-malic acid. Polym. Chem. 2, 2204–2212. doi: 10.1039/c1py00254f
Prier, C. K., Rankic, D. A., and MacMillan, D. W. C. (2013). Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 113, 5322–5363. doi: 10.1021/cr300503r
Rahimi, A., and García, J. M. (2017). Chemical recycling of waste plastics for new materials production. Nat. Rev. Chem. 1:0046. doi: 10.1038/s41570-017-0046
Rasal, R. M., Janorkar, A. V., and Hirt, D. E. (2010). Poly(lactic acid) modifications. Prog. Polym. Sci. 35, 338–356. doi: 10.1016/j.progpolymsci.2009.12.003
Saiyasombat, W., Molloy, R., Nicholson, T. M., Johnson, A. F., Ward, I. M., and Poshyachinda, S. (1998). Ring strain and polymerizability of cyclic esters. Polymer 39, 5581–5585. doi: 10.1016/S0032-3861(97)10370-6
Sano, K., Yamamoto, T., and Yamamoto, A. (1984). Preparation of Ni-containing or Pt-containing cyclic esters by oxidative addition of cyclic carboxylic anhydrides and their properties. Bull. Chem. Soc. Jpn. 57, 2741–2747. doi: 10.1246/bcsj.57.2741
Sardon, H., and Dove, A. P. (2018). Plastics recycling with a difference. Science 360, 380–381. doi: 10.1126/science.aat4997
Schneiderman, D. K., and Hillmyer, M. A. (2017). 50th anniversary perspective: there is a great future in sustainable polymers. Macromolecules 50, 3733–3750. doi: 10.1021/acs.macromol.7b00293
Slomkowski, S., Penczek, S., and Duda, A. (2014). Polylactides—an overview. Polym. Adv. Technol. 25, 436–447. doi: 10.1002/pat.3281
Smith, I. J., and Tighe, B. J. (1976). Studies in ring-opening polymerization. 4. Thermal polymerization of phenyl substituted 1,3-dioxolan-2,4-diones. J. Polym. Sci. Polym. Chem. 14, 949-960. doi: 10.1002/pol.1976.170140415
Smith, I. J., and Tighe, B. J. (1981). Studies in ring-opening polymerization. 6. Tertiary base initated polymerization of 5-phenyl-1,3-dioxolan-2,4-dione. Macromol. Chem. Phys. 182, 313-324. doi: 10.1002/macp.1981.021820204
Södergård, A., and Stolt, M. (2002). Properties of lactic acid based polymers and their correlation with composition. Prog. Polym. Sci. 27, 1123–1163. doi: 10.1016/S0079-6700(02)00012-6
Stanford, M. J., and Dove, A. P. (2010). Stereocontrolled ring-opening polymerisation of lactide. Chem. Soc. Rev. 39, 486–494. doi: 10.1039/B815104K
Sun, Y., Jia, Z., Chen, C., Cong, Y., Mao, X., and Wu, J. (2017). Alternating sequence controlled copolymer synthesis of α-Hydroxy acids via syndioselective ring-opening polymerization of O-Carboxyanhydrides using zirconium/hafnium alkoxide initiators. J. Am. Chem. Soc. 139, 10723–10732. doi: 10.1021/jacs.7b04712
Tang, L., and Deng, L. (2002). Dynamic kinetic resolution via dual-function catalysis of modified cinchona alkaloids: asymmetric synthesis of α-Hydroxy carboxylic acids. J. Am. Chem. Soc. 124, 2870–2871. doi: 10.1021/ja0255047
Thomas, C. M. (2010). Stereocontrolled ring-opening polymerization of cyclic esters: synthesis of new polyester microstructures. Chem. Soc. Rev. 39, 165–173. doi: 10.1039/B810065A
Tong, R. (2017). New chemistry in functional aliphatic polyesters. Ind. Eng. Chem. Res. 56, 4207–4219. doi: 10.1021/acs.iecr.7b00524
Toyooka, K., Takeuchi, Y., and Kubota, S. (1989). A noval and facile synthesis of 5-substituted 1,3-dioxolan-2,4-diones using trichloromethyl chloroformate. Heterocycles 29, 975–978. doi: 10.3987/COM-89-4939
Van de Velde, K., and Kiekens, P. (2002). Biopolymers: overview of several properties and consequences on their applications. Polym. Test. 21, 433–442. doi: 10.1016/S0142-9418(01)00107-6
Vandenbossche, C. P., de Croos, P., Singh, S. P., Bakale, R. P., and Wagler, T. R. (2010). Formation of (S)-5-Cyclohexyl-5-phenyl-1,3-dioxolane-2,4-dione: a key intermediate in the synthesis of (S)-Oxybutynin Hydrochloride. Org. Process Res. Dev. 14, 921–925. doi: 10.1021/op100021w
Wang, R., Zhang, J., Yin, Q., Xu, Y., Cheng, J., and Tong, R. (2016). Controlled ring-opening polymerization of O-Carboxyanhydrides using a β-Diiminate Zinc Catalyst. Angew. Chem. Int. Chem. 55, 13010–13014. doi: 10.1002/anie.201605508
Xia, H. D., Kan, S. L., Li, Z. J., Chen, J., Cui, S. D., Wu, W. Z., et al. (2014). N-Heterocyclic carbenes as organocatalysts in controlled/living ring-opening polymerization of O-Carboxyanhydrides derived from L-Lactic Acid and L-Mandelic Acid. J. Polym. Sci. Polym. Chem. 52, 2306–2315. doi: 10.1002/pola.27241
Yamamoto, T., Ishizu, J., Kohara, T., Komiya, S., and Yamamoto, A. (1980). Oxidative addition of aryl carboxylates to Ni(0) complexes involving cleavage of the aryl-O bond. J. Am. Chem. Soc. 102, 3758–3764. doi: 10.1021/ja00531a016
Yin, Q., Tong, R., Xu, Y., Baek, K., Dobrucki, L. W., Fan, T. M., et al. (2013). Drug-initiated ring-opening polymerization of O-Carboxyanhydrides for the preparation of anticancer drug-Poly(O-carboxyanhydride) nanoconjugates. Biomacromolecules 14, 920–929. doi: 10.1021/bm301999c
Yin, Q., Yin, L., Wang, H., and Cheng, J. (2015). Synthesis and biomedical applications of functional Poly(α-hydroxy acids) via ring-opening polymerization of O-Carboxyanhydrides. Acc. Chem. Res. 48, 1777–1787. doi: 10.1021/ar500455z
Yu, Y., Zou, J., and Cheng, C. (2014). Synthesis and biomedical applications of functional poly([small alpha]-hydroxyl acid)s. Polym. Chem. 5, 5854–5872. doi: 10.1039/C4PY00667D
Zhang, L., Nederberg, F., Messman, J. M., Pratt, R. C., Hedrick, J. L., and Wade, C. G. (2007). Organocatalytic stereoselective ring-opening polymerization of lactide with dimeric phosphazene bases. J. Am. Chem. Soc. 129, 12610–12611. doi: 10.1021/ja074131c
Zhang, Z., Yin, L., Xu, Y., Tong, R., Lu, Y., Ren, J., et al. (2012). Facile functionalization of polyesters through thiol-yne chemistry for the design of degradable, cell-penetrating and gene delivery dual-functional agents. Biomacromolecules 13, 3456–3462. doi: 10.1021/bm301333w
Zhu, J.-B., and Chen, E. Y. X. (2015). From meso-Lactide to isotactic polylactide: epimerization by B/N lewis pairs and kinetic resolution by organic catalysts. J. Am. Chem. Soc. 137, 12506–12509. doi: 10.1021/jacs.5b08658
Zhu, J.-B., Watson, E. M., Tang, J., and Chen, E. Y.-X. (2018). A synthetic polymer system with repeatable chemical recyclability. Science 360, 398–403. doi: 10.1126/science.aar5498
Zhu, Y., Romain, C., and Williams, C. K. (2016). Sustainable polymers from renewable resources. Nature 540, 354–362. doi: 10.1038/nature21001
Zhuang, X.-l., Yu, H.-y., Tang, Z.-h., Oyaizu, K., Nishide, H., and Chen, X.-s. (2010). Polymerization of lactic O-carboxylic anhydride using organometallic catalysts. Chin. J. Polym. Sci. 29, 197–202. doi: 10.1007/s10118-010-1013-7
Keywords: O-carboxyahydrides, polyester, ring-opening polymenzation, photoredox catalysis, living polvmerization, organocatalyst, stereoselective polymerization, poly(a-hydroxy acid)
Citation: Zhong Y and Tong R (2018) Living Ring-Opening Polymerization of O-Carboxyanhydrides: The Search for Catalysts. Front. Chem. 6:641. doi: 10.3389/fchem.2018.00641
Received: 28 October 2018; Accepted: 07 December 2018;
Published: 21 December 2018.
Edited by:
Hsuan-Ying Chen, Kaohsiung Medical University, TaiwanReviewed by:
Ming-Tsz Chen, Providence University, TaiwanKirill V. Zaitsev, Lomonosov Moscow State University, Russia
Wenjuan Zhang, Beijing Institute of Fashion Technology, China
Copyright © 2018 Zhong and Tong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rong Tong, cnRvbmdAdnQuZWR1
 Yongliang Zhong
Yongliang Zhong Rong Tong
Rong Tong