
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Chem., 24 August 2017
Sec. Chemical Biology
Volume 5 - 2017 | https://doi.org/10.3389/fchem.2017.00063
This article is part of the Research TopicAntimicrobial and Anticancer PeptidesView all 15 articles
Over the past decades the use of medical devices, such as catheters, artificial heart valves, prosthetic joints, and other implants, has grown significantly. Despite continuous improvements in device design, surgical procedures, and wound care, biomaterial-associated infections (BAI) are still a major problem in modern medicine. Conventional antibiotic treatment often fails due to the low levels of antibiotic at the site of infection. The presence of biofilms on the biomaterial and/or the multidrug-resistant phenotype of the bacteria further impair the efficacy of antibiotic treatment. Removal of the biomaterial is then the last option to control the infection. Clearly, there is a pressing need for alternative strategies to prevent and treat BAI. Synthetic antimicrobial peptides (AMPs) are considered promising candidates as they are active against a broad spectrum of (antibiotic-resistant) planktonic bacteria and biofilms. Moreover, bacteria are less likely to develop resistance to these rapidly-acting peptides. In this review we highlight the four main strategies, three of which applying AMPs, in biomedical device manufacturing to prevent BAI. The first involves modification of the physicochemical characteristics of the surface of implants. Immobilization of AMPs on surfaces of medical devices with a variety of chemical techniques is essential in the second strategy. The main disadvantage of these two strategies relates to the limited antibacterial effect in the tissue surrounding the implant. This limitation is addressed by the third strategy that releases AMPs from a coating in a controlled fashion. Lastly, AMPs can be integrated in the design and manufacturing of additively manufactured/3D-printed implants, owing to the physicochemical characteristics of the implant material and the versatile manufacturing technologies compatible with antimicrobials incorporation. These novel technologies utilizing AMPs will contribute to development of novel and safe antimicrobial medical devices, reducing complications and associated costs of device infection.
The use of medical devices, including catheters, artificial heart valves, prosthetic joints, and other implants, increased dramatically over the past century (Darouiche, 2004; Anderson and Patel, 2013; Kwakman and Zaat, 2013), and has become a major part of modern medicine and our daily life. With the aging society, the demand for medical devices to restore body functions and quality of life increases, and so do the numbers of cases of biomaterial-associated infection (BAI). The risk for BAI may in part be explained by the reduced efficacy of the local immune defense induced by the foreign body. In agreement, the number of bacteria required to cause an infection is significantly lower in the presence of a foreign body, such as a stitch or an implant, than when such devices are not present (Elek and Conen, 1957; James and Macleod, 1961; Noble, 1965; Taubler and Kapral, 1966; Zimmerli et al., 1982; Southwood et al., 1987). Another contributing factor is that the bacteria—often derived from the commensal skin flora or the hospital environment—can adhere to the foreign body, replicate, and form a biofilm from which they can invade the peri-implant tissues and cause an infection. The most common causative microorganisms in BAI are Staphylococcus aureus and Staphylococcus epidermidis (Anderson and Marchant, 2000; O'Gara and Humphreys, 2001; Zimmerli et al., 2004). Depending on the type of device and location of application, other coagulase-negative staphylococci, enterococci, streptococci, Propionibacterium acnes, and yeasts such as Candida spp., can also cause BAI (Waldvogel and Bisno, 2000; Holmberg et al., 2009). Infections following primary implant surgery occur in 0.5–1% of the patients receiving an artificial hip or knee and in over 5% of those receiving a prosthetic elbow or ankle implant (Zimmerli et al., 2004; Krenek et al., 2011). As treatment of BAI is complex, combinations of antibiotics, such as vancomycin or ciprofloxacin with rifampicin, are recommended. Such combinations show some efficacy against biofilms, although much higher concentrations of antibiotics are required than effective against planktonic cells (Saginur et al., 2006). Nevertheless, treatments with antibiotic combinations often fail with the only option being removal of the medical device (Burns, 2006). Catheters suspected for infection are removed and replaced by a new device at a different location, as re-implantation at the original site is strongly discouraged because of the high re-infection risk (Safdar et al., 2002). Revision surgery of infected orthopedic devices in most cases involves removal of the implant, thorough debridement of the infected site and prolonged (4–8 weeks) antibiotic treatment before a new implant is placed (Zimmerli, 2006). Still, revision surgery is associated with high frequencies of infection due to extensive surgical procedures and more severe tissue damage.
Bacterial biofilm formation is considered to play a major role in the pathogenesis of BAI (Costerton et al., 1999; Holmberg et al., 2009; Anderson and Patel, 2013). Biofilm formation is initiated by bacterial cells attaching to the surfaces of medical devices. Subsequently, bacteria replicate and produce extracellular matrix forming complex communities consisting of bacteria, bacterial exopolysaccharides, proteins, extracellular DNA, and host proteins (Costerton et al., 1999). Bacteria in biofilms are considerably more tolerant to antibiotics and less accessible to cells and molecules of the human immune defense system than their planktonic counterparts (Otto, 2009; Chen et al., 2013). This might be due to the extracellular polymeric matrix of the biofilm, making the bacteria less accessible for phagocytes and effector molecules, and to the persister state of the bacteria. Persisters are metabolically-inactive, antibiotic tolerant bacteria that maintain the ability to multiply after antibiotic treatment (Harms et al., 2016), thus explaining the recurrence of BAI (Gerdes and Semsey, 2016; Fisher et al., 2017).
Another important element in the pathogenesis of BAI is bacterial colonization of the tissue surrounding the implant (Boelens et al., 2000a; Ciampolini and Harding, 2000). In vivo studies showed that S. epidermidis applied on the surface of titanium implants, both as adherent cells and as a pregrown biofilm, rapidly relocated from the implants to the surrounding tissue (Riool et al., 2014). Similarly, large numbers of S. aureus were cultured from mouse tissues around infected titanium (Riool et al., 2017a,b) and silicon elastomer implants (de Breij et al., 2016). In a murine model of chronic osteomyelitis, S. aureus was found in osteoblasts and osteocytes, as well as in canaliculi of live cortical bone (de Mesy Bentley et al., 2017).
Bacterial invasion of the peri-implant tissue and subsequent development of infection is facilitated by dysregulation of the local immune response resulting from the presence of a foreign body. The phagocytic and intracellular killing activities of neutrophils and macrophages are reduced due to altered cytokine tissue levels in the presence of a biomaterial (Boelens et al., 2000a,b,c; Broekhuizen et al., 2010; Zimmerli and Sendi, 2011). In agreement, microscopical examination has revealed that many of the bacteria reside within these inflammatory phagocytes (Broekhuizen et al., 2010). Interestingly, studies in mice infected with S. epidermidis as well as in infected peri-catheter tissue biopsies obtained from deceased intensive care unit patients showed that bacteria present in tissue surrounding the implants had incorporated bromodesoxyuridine, demonstrating that the bacteria can replicate in the peri-implant tissue (Broekhuizen et al., 2010). Furthermore, bacteria may adapt to the tissue and intracellular micro-environment by the formation of so-called small colony variants. The presence of such intracellular small colony variants further complicates treatment as they are more resistant to antimicrobial compounds (Tuchscherr et al., 2010; Zaat, 2013).
In addition to the limited activity of antibiotics against biofilm-encased bacteria, persisters, and intracellular bacteria, the emergence of resistance among staphylococci as well as other bacterial species causing BAI constitutes a major challenge to the efficacy of (combinations of) conventional antibiotics. The emergence of multidrug-resistant (resistant to at least one agent in three or more antimicrobial classes), extensively drug-resistant (resistant to at least one agent in all but one or two antimicrobial classes), and pan-drug-resistant (resistant to all agents in all antimicrobial classes) pathogens, is accelerated by the selective pressure exerted by extensive use and abuse of antimicrobials (Magiorakos et al., 2012). Bacteria belonging to the so-called ESKAPE panel (Enterococcus faecium, S. aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) are increasingly prevalent and resistant and thereby a particularly dangerous group of bacteria (Rice, 2008). Currently, the majority of hospital infections in the United States is caused by multidrug-resistant ESKAPE bacterial strains (Boucher et al., 2009). The World Health Organization recently endorsed a global action plan to tackle antibiotic resistance to avoid the dark scenario of a “post-antibiotic era” (Chan, 2015). One of the key objectives of this plan is to develop novel antimicrobial drugs with a mode of action different from those of current antibiotics.
Antimicrobial peptides (AMPs)—effector molecules of the innate defense of animals, plants, and microorganisms (Zasloff, 2002; Hancock and Sahl, 2006)—have recently attracted considerable interest as agents that may subvert many of the problems related to BAI, i.e., they display antimicrobial activity against bacteria resistant to antibiotics and residing within biofilms. A specialized biofilm-active AMPs database lists most of the published AMPs with anti-biofilm activity (Di Luca et al., 2015). AMPs are mostly amphipathic, cationic peptides that display antimicrobial activity against bacteria, fungi and (enveloped) viruses. They interact with specific constituents of the bacterial cell envelope resulting in depolarization, destabilization, and/or disruption of the bacterial plasma membrane leading to bacterial cell death within minutes (Pasupuleti et al., 2012). Due to the rapid and non-specific mechanisms of action, the risk of resistance development is generally thought to be low (Zasloff, 2002). Nonetheless, resistance to AMPs in bacteria does occur and several mechanisms of resistance have been described, including membrane and cell envelope structure alterations increasing positive charge, upregulation of efflux pumps, and proteolytic degradation of the peptides (Goytia et al., 2013; Ernst et al., 2015). For instance, resistance against the human cathelicidin LL-37 has been reported to involve degradation of the peptide by bacterial proteolytic enzymes, up-regulation of efflux pumps as well as bacterial-induced down-regulation of LL-37 expression in host cells (Bandurska et al., 2015). Under low calcium or magnesium ion concentrations, as in blood plasma, P. aeruginosa activates the pmr (polymyxin resistance) operon, which medicates the addition of N-arabinose to its lipopolysaccharide. This renders the outer surface of the bacterial cell more positively charged, repelling the cationic AMPs (Goytia et al., 2013). So, resistance of bacteria against AMPs is possible for several bacterial species, however development of such resistance against novel synthetic AMPs has not often been studied.
In addition to direct antimicrobial activity, AMPs display immunomodulatory activities. For example, they can prevent excessive activation of pro-inflammatory responses due to bacterial endotoxins such as lipopolysaccharide of Gram-negative bacteria, and peptidoglycan and lipoteichoic acid of Gram-positive bacteria. AMPs may improve clearance of bacterial biofilms by host defense systems (Mansour et al., 2014, 2015) as they may prevent derangement of immune responses after implantation of foreign bodies (Zaat et al., 2010; Heim et al., 2014, 2015). Other favorable characteristics of AMPs relate to wound healing (Nakatsuji and Gallo, 2012), angiogenesis (Salvado et al., 2013), and osteogenic activity (Kittaka et al., 2013; Zhang and Shively, 2013). Regarding the latter activity, it has been reported that in a trabecular bone growth in vivo study, cylindrical titanium implants coated with the antimicrobial peptide HHC36 had osteoconductive properties (Kazemzadeh-Narbat et al., 2012). Similarly, fusion peptide P15-CSP showed anti-biofilm activity and pro-osteogenic activity (Li et al., 2015) and LL-37 promoted bone regeneration in a rat calvarial bone defect model (Kittaka et al., 2013) and accelerated bone repair in NOD/SCID mice (Zhang and Shively, 2013).
Naturally occurring AMPs have been used as design templates for a large variety of synthetic AMPs, some of which have reached the stage of phase 2 and 3 clinical trials (Fox, 2013; Greber and Dawgul, 2016), such as OP-145 (Peek et al., 2009), LL-37 (Grönberg et al., 2014), Iseganan (IB-367; Mosca et al., 2000), Omiganan (MBI-226; Sader et al., 2004), and Pexiganan (MSI-78; Fuchs et al., 1998). With respect to the development of synthetic peptides for the treatment of BAI we will focus on a few of the most promising peptides. The synthetic peptide IDR-1018 prevented biofilm formation by S. aureus and various other species by blocking (p)ppGpp, which is a signal molecule in persister development (Harms et al., 2016) and biofilm formation (Mansour et al., 2015). In a murine model of S. aureus implant infection, IDR-1018 showed to be potentially useful in reducing orthopedic infections by recruiting macrophages to the infection site, blunting excess cytokine production and reducing osseointegration failures (Choe et al., 2015).
In an attempt to meet the requirements for the treatment of BAI as much as possible, a series of novel synthetic AMPs was recently developed based on two human AMPs, i.e., thrombocidin-1, the major antimicrobial protein of human blood platelets (Krijgsveld et al., 2000; Kwakman et al., 2011), and LL-37, a principal human AMP produced by mucosal epithelial cells and multiple immune cells. The LL-37-inspired peptide OP-145 (formerly designated as P60.4Ac; Nell et al., 2006) proved to be safe and efficacious for treatment of therapy-resistant otitis media patients (Peek et al., 2009). In vitro, OP-145 (de Breij et al., 2016), and the newer generation LL-37-inspired peptides SAAP-145 and SAAP-276 (Riool et al., 2017a) and the trombocidin-1-derived peptide TC19 (Zaat et al., 2014) inhibited biofilm formation by a clinical S. aureus BAI isolate in a dose-dependent fashion. The mode of action of these synthetic peptides may involve inhibition of adherence of bacteria to surfaces and/or reduction of expression of genes involved in biofilm formation, as has been reported for LL-37 (Overhage et al., 2008). These novel synthetic peptides all rapidly permeabilize the membrane of S. aureus bacteria (Riool et al., 2017a), explaining why they are highly effective against dividing as well as non-dividing, biofilm-encased bacteria whether or not resistant to antibiotics. Interestingly, these newer generation peptides display good bactericidal activity in the presence of human plasma, despite possible binding of the peptides to plasma components (de Breij et al., 2016). In contrast, the first generation AMP OP-145 showed strong reduction of antimicrobial activity in plasma in vitro. Despite this OP-145 proved to be effective in preventing S. aureus colonization of subcutaneous implants in mice and protected rabbits from experimental intramedullary nail-associated osteomyelitis (de Breij et al., 2016). Apparently, in vitro activities in the presence of human plasma do not necessarily predict the in vivo potency of AMPs.
The physical properties of synthetic AMPs, i.e., cationic charge and peptidic nature, present challenges to their biological stability and balance between antimicrobial efficacy and host cell toxicity. Fortunately, several solutions can be considered to address these issues. For example, PEGylation is a well-accepted method for minimizing cytotoxicity while maintaining antimicrobial activity of AMPs and reducing elimination of the peptides by the liver and kidneys (Morris et al., 2012). D-enantiomers—peptides that are comprised of unnatural amino acids—and (retro-)inverso peptides are insensitive to most peptidase activity (Guichard et al., 1994; Feng and Xu, 2016). In this connection, a series of modified HHC10 peptides were synthesized, including inverso-CysHHC10 (i.e., different stereo isomer). Inverso-CysHHC10 was stable in human serum, showed microbicidal activities at low micromolar concentrations against Escherichia coli, S. aureus, and S. epidermidis and was active in a polyethylene glycol (PEG)-based hydrogel in serum (Cleophas et al., 2014). Of note, serum may be a worst-case scenario for peptides, since Fibrinopeptide A peptides were degraded in serum, but not in fresh blood (Böttger et al., 2017). Serum may have a level of proteolytic activity not encountered in blood or plasma, since preparation of serum involves blood coagulation, which leads to activation of coagulation pathway proteases (Chambers and Laurent, 2002). Therefore, inhibition/degradation is best studied in plasma or fresh blood.
Another area of potential improvement of synthetic AMPs is that of intracellular antimicrobial activity, required to treat intracellular infections. In general, AMPs do not effectively penetrate host cells due to their high positive charge. Several cell-penetrating peptides have been developed for intracellular “delivery” of peptides. Such peptides may be utilized to deliver AMPs and PEGylated peptides into host cells to facilitate elimination of intracellular pathogens, e.g., staphylococci, residing within inflammatory and other cells, such as osteoblasts (Iwase et al., 2016).
For prevention of BAI, various types of antimicrobial biomaterials have been developed, including (i) antifouling surfaces, (ii) contact-killing surfaces, and (iii) surfaces which incorporate and release antimicrobials (Busscher et al., 2012). These approaches all have their benefits and limitations, which need to be taken into account when designing an antimicrobial strategy for a particular device (Brooks et al., 2013). Importantly, both biofilm formation on the implant and colonization of the peri-implant tissue need to be taken into consideration when designing preventive strategies against BAI. Here, we will discuss various combinations of these strategies and AMPs to prevent BAI (summarized in Figure 1).
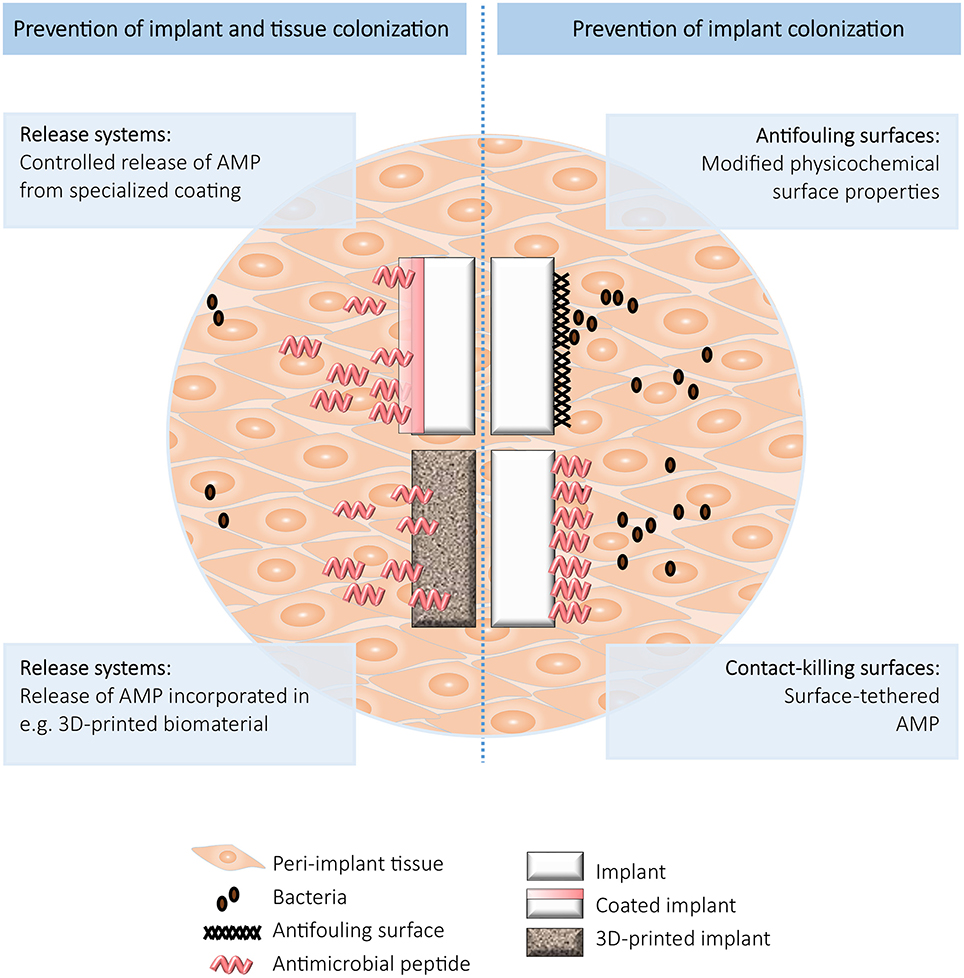
Figure 1. Schematic overview of the strategies to prevent implant (Right) and implant and tissue (Left) colonization.
Already in 1987, Gristina suggested that adhering tissue cells and bacteria compete for a spot on the implant's surface, the so-called “race for the surface” concept (Gristina, 1987). In case this race is won by the bacteria, this will result in infection instead of tissue integration. Gristina also realized that colonization of the tissue around implants was another possible mechanism of infection (Gristina, 1987). Bacterial adhesion and subsequent biofilm formation may be prevented by modifying the physicochemical surface properties of biomaterials such as the surface charge, hydrophobicity/hydrophilicity, and surface chemistry. One strategy is to use hydrophilic polymer coatings, e.g., immobilized PEG, as applied on contact lenses, shunts, endotracheal tubes, and urinary catheters (Banerjee et al., 2011; Busscher et al., 2012). Another approach is functionalization of the surface with a dense layer of polymer chains commonly known as polymer brush coatings (Nejadnik et al., 2008; Neoh et al., 2013; Keum et al., 2017). Large exclusion volumes of tethered polymer chains result in surfaces difficult to approach by proteins or bacteria.
Another approach to prevent implant colonization is the immobilization of AMPs on surfaces of medical devices, which can be performed with a variety of chemical techniques. An excellent overview of immobilization strategies has recently been published by Silva et al. (2016). There are several common “rules” for success. The structural characteristics important for the antimicrobial activity of the peptides should not be altered by the immobilization process. Length, flexibility, and kind of spacer connecting the peptide to the surface, orientation of the immobilized peptides, and the AMP surface density are additional important parameters (Costa et al., 2011). Interestingly, even short surface-attached peptides not likely to have a free interaction with the bacterial cytoplasmic membrane, have antimicrobial activity (Hilpert et al., 2009). This is thought to be due to destabilization of the bacterial membrane by displacement of positively charged counter-ions, disrupting the ionic balance, changing bacterial surface electrostatics, and activating autolytic enzymes (Hilpert et al., 2009). An example of a contact-killing surface is the hydrogel network with the covalently attached stabilized inverso-CysHHC10 peptide (Cleophas et al., 2014). This coating demonstrated high in vitro antimicrobial activity against S. aureus, S. epidermidis, and E. coli. Furthermore, brush coating molecules may also possess active functional groups with antimicrobial activity, e.g., by conjugation with the AMPs Tet20 (Gao et al., 2011a) and Tet213 (Gao et al., 2011b). Another example is polyurethane with a brush coating tethered with the AMP E6 for the prevention of catheter-associated infections (Yu et al., 2017). This surface coating reduced bacterial adhesion on the catheter surface in a mouse urinary catheter infection model. A variety of AMPs, like GZ3.27 (De Zoysa and Sarojini, 2017), GL13K (Chen et al., 2014; Zhou et al., 2015), SESB2V (Tan et al., 2014), bacitracin (Nie et al., 2016, 2017), hLF1-11 (Costa et al., 2014; Godoy-Gallardo et al., 2015), LL-37, Melimine, lactoferricin, and Mel-4 (Chen et al., 2016; Dutta et al., 2016) have been covalently coupled onto various surfaces, such as glass, silicon, and titanium, with different degrees of success (summarized in Table 1). Chimeric peptides comprised of both a titanium binding domain and an antimicrobial motif are also used to create contact-killing surfaces (Yucesoy et al., 2015; Liu et al., 2016; Yazici et al., 2016). Due to their titanium-binding domain, the peptides preferentially bind the implant, while the freely exposed antimicrobial domain is available for combatting invading bacteria. Titanium surfaces modified with these chimeric peptides were found to significantly reduce adhesion of different Streptococcus species, S. aureus, S. epidermidis, P. aeruginosa, and E. coli, compared to bare titanium. Immobilization of GL13K onto titanium dental implants even enabled osseointegration when tested in rabbit femurs (Chen et al., 2017). Another promising strategy is the development of multifunctional coatings by combining the well-known RGD cell adhesive sequence with the lactoferrin-derived AMP LF1-11, resulting in in vitro cell integration as well as inhibition of bacterial colonization by S. aureus and Streptococcus sanguinis (Hoyos-Nogués et al., 2017). Recently, others described a self-assembling coating of recombinant spider silk protein fused to the AMP Magainin I for different biomaterials, which reduced numbers of live bacteria on the coated surfaces (Nilebäck et al., 2017). It should be noted that not in all studies described above the absence of unbound peptide within the coating is verified. Thus, in those cases it cannot be excluded that the antimicrobial activity of the coating is caused by a combination of bound and released AMP.
It should be noted that surface attachment of peptides does suffer from some disadvantages. The antimicrobial activity of the surface with immobilized AMPs is critically dependent on the chemical tethering procedure and the orientation of the covalently attached AMPs. The antimicrobial activity of the resulting coating may be strongly reduced compared to the activity of the peptide in free form (Bagheri et al., 2009; Onaizi and Leong, 2011; Dutta et al., 2016). Apart from this reduction of activity due to the tethering process, proteins, blood platelets, and dead bacteria may block the antimicrobial groups on the surface. Moreover, since the antimicrobial activity is restricted to the surface of the implant, there is a lack of antimicrobial impact on bacteria in the tissue surrounding the implant. Contact-killing surfaces will only eradicate bacteria that are in direct contact with the active surface, meaning that clearance of any bacteria further away from the surface will depend on efficient phagocytosis and systemic or local antibiotics. However, as mentioned before, due to the presence of a biomaterial the local host immune response is dysregulated, and therefore phagocytosed bacteria may not be killed and may even persist intracellularly (Boelens et al., 2000a,b).
As described above, the peri-implant tissue is an important niche for bacterial survival. Therefore, antimicrobial-releasing surfaces or coatings from which the antimicrobial agent also reaches this niche are preferred to prevent BAI. Antibiotic-releasing coatings are widely used for medical devices such as sutures and central venous catheters and urinary tract catheters. However, these coatings have two major disadvantages: (i) a patient may be infected with a bacterium resistant to the released antibiotic, and (ii) due to the local release a gradient of the antibiotic will be present near the implant thereby increasing the risk to select for resistant bacteria. Coatings releasing antibiotics for orthopedic devices remain mainly experimental (Lucke et al., 2003; Kälicke et al., 2006; Darouiche, 2007; Moojen et al., 2009; Alt et al., 2011). The first commercially available gentamicin-releasing intramedullary tibia nail has recently shown promising results in a first prospective study (Fuchs et al., 2011; Metsemakers et al., 2015; Alt, 2017). In view of the increasing development of antibiotic resistance among bacteria, the use of antibiotics in medical devices is discouraged by government regulatory agencies like the American Food and Drug Administration (FDA, 2007; Brooks et al., 2013). Obviously, coatings releasing antimicrobial agents that are less likely to induce resistance, such as AMPs, are preferred in view of both managing resistance development and compatibility with use of antibiotics for prophylaxis or treatment. To prevent the spread of bacteria from the implant surface to the surrounding tissue, and to eradicate bacteria contaminating tissue during surgery, a rapid initial release of antimicrobials is required. If this release is delayed, bacteria may “escape” into host cells before effective levels of the antimicrobial agent have been established. Subsequently, prolonged local release of the antimicrobial agent at sufficiently high concentrations will be required to eradicate any residual bacteria (Zilberman and Elsner, 2008; Emanuel et al., 2012).
Application of AMPs in antimicrobial surface coatings is a subject of increasing interest and different types of release-coatings have been described, including hydrogels, nanotubes, microporous calcium phosphate coatings, and polymer coatings (summarized in Table 2). Hydrogels with the AMP Cateslytin strongly adhere to dental implant surfaces. The hydrogels showed potent antimicrobial activities against Porphyromonas gingivalis, an important causative agent of peri-implantitis, without signs of toxicity (Mateescu et al., 2015). Another example is a gelatin-based hydrogel on titanium surfaces allowing for the controlled release of the short cationic AMP HHC36 preventing S. aureus, S. epidermidis, E. coli, and P. aeruginosa biofilm formation (Cheng et al., 2017).
Self-organized and vertically oriented titanium oxide nanotubes loaded with the broad spectrum AMP HHC36 showed in vitro bactericidal activity against S. aureus in liquid surrounding the nanotubular surface and reduced bacterial colonization on the surface ~200-fold (Ma et al., 2012). GL13K-eluting coatings on these titanium oxide nanotubes prevented growth of Fusobacterium nucleatum and P. gingivalis in an in vitro disk-diffusion assay (Li et al., 2017). In vitro release of Tet213 from microporous calcium phosphate coatings applied on titanium showed bactericidal activity against S. aureus and P. aeruginosa (Kazemzadeh-Narbat et al., 2010). In a similar approach, release of PSI 10 from microporous calcium phosphate coated magnesium alloy inhibited S. aureus growth in vitro and promoted in vivo bone repair (Tian et al., 2015). Furthermore, controlled release of Tet213 linked to collagen IV inhibited S. aureus biofilm formation in vitro (Shi et al., 2015). However, these types of coatings have not yet been tested in vivo.
Injection of the LL-37-inspired AMPs OP-145 (de Breij et al., 2016), SAAP-145, and SAAP-276 (Riool et al., 2017a) along subcutaneous implants in mice did not reduce the numbers of S. aureus in the surrounding tissue. This might be because the AMPs did not effectively penetrate the tissue or were not taken up by the host cells and thereby not capable of killing internalized bacteria. However, when these AMPs were released from Polymer-Lipid Encapsulation Matrix (PLEX) coatings, the numbers of viable S. aureus bacteria were reduced in the peri-implant soft tissue in mice (Riool et al., 2017a) and even in bone in a rabbit humerus intramedullary nail infection model (de Breij et al., 2016). This clearly illustrates the benefit of the PLEX coating technology allowing controlled and prolonged release of the AMPs at the implant-tissue-interface. The SAAP-276-PLEX-coated implants were able to significantly, but not completely, reduce the number of doxycycline-resistant S. aureus in the peri-implant tissue, in contrast to the doxycycline-PLEX coated implants which failed to reduce their numbers in the tissue (Riool et al., 2017a). This underlines the potency of SAAP-276-PLEX coatings in the fight against BAI caused by multidrug-resistant staphylococci.
Although the AMPs mentioned above reduced the colonization of the peri-implant tissue in vivo when released from a coating, they might still not be able to act against intracellular bacteria. Apparently, the rapid initial release of the AMPs killed the vast majority of the infecting bacteria, preventing biofilm formation on the implant surface as well as colonization of the tissue, thereby protecting both these sites against colonization. Treatment of infections featuring intracellular bacteria remains difficult, as observed with the conventional antibiotic vancomycin (Broekhuizen et al., 2008), and likely with the novel AMPs as well. A possible way to improve the intracellular entry of AMPs is by adding a specific domain (“tag”) to the peptides as a signal for uptake by the host cells (Splith and Neundorf, 2011; Ye et al., 2016). However, intracellular localization of bacteria does not seem to occur to a large extent when AMPs are used in BAI prevention, as shown for instance with the AMP-PLEX coatings described above. By directly killing the bacteria on the implant-tissue interface the AMPs prevented bacterial invasion into the tissue and internalization by and survival in host cells.
Several novel technologies are arising for manufacturing implants with particular focus on the possibility of personalization. We will briefly address additive manufacturing and electrospinning with regards to the strategies of incorporation of antimicrobial agents and potential for AMPs.
Additive manufacturing (3D-printing) of medical devices is a major breakthrough that enables the production of implants customized in size and shape, and potentially with high porosity, thereby increasing the surface area. These aspects make this technique attractive for personalized implants. However, as with conventional implants, the 3D-printed implants are susceptible to infection. Therefore, different approaches are currently explored to develop 3D-printed medical devices with antimicrobial functionalities. For example, antimicrobials may be added by surface modification of the 3D-printed implants, using plasma electrolytic oxidation, also known as micro-arc oxidation (Fidan et al., 2017). In this process, a titanium oxide layer is generated and compounds or nanoparticles present in the electrolyte are incorporated in the growing surface oxide layer (Necula et al., 2009; Lara Rodriguez et al., 2014; Fidan et al., 2017). One antimicrobial agent often used for the implants is silver. Silver is used in numerous medical applications (Bach et al., 1999; Rupp et al., 2005; Osma et al., 2006; Kuehl et al., 2016) and has broad-spectrum antimicrobial activity (Bürgers et al., 2009; Sussman et al., 2015). In a recent study silver nanoparticles were embedded in the titanium oxide layer of 3D-printed titanium implants (van Hengel et al., 2017) using a plasma electrolytic oxidation protocol developed for conventional medical grade titanium implants (Necula et al., 2009, 2012). These 3D-printed implants released silver ions over time, and showed in vitro bactericidal activity against MRSA including prevention of biofilm formation, and eradicated MRSA in an ex vivo mouse femur implant infection model (van Hengel et al., 2017).
The antibiotics rifampin and vancomycin have been incorporated in 3D-printed calcium-phosphate scaffolds during manufacturing. Due to their local delivery these incorporated antibiotics rendered the scaffolds capable of controlling murine implant-associated bone infection (Inzana et al., 2015). A similar approach might very well be suitable to incorporate AMPs for local delivery, as an alternative for the use of conventional antibiotics. AMPs might also be incorporated in hydrogels to coat the 3D-printed implants, similar to approaches utilizing polymers to create antibiotic release systems (ter Boo et al., 2015, 2016).
Another novel approach currently explored for prevention or treatment of BAI is the use of gold nanoparticles with tethered AMPs to increase the in vivo stability of AMPs and decrease possible toxicity. This technology would be readily applicable to 3D-printed implants. Gold nanoparticles conjugated with the hydrophilic cationic peptide cecropin melittin (CM) demonstrated higher antimicrobial activity and stability in serum than the CM peptide in solution, The CM-gold nanoparticles had favorably low cytotoxicity for human cells and demonstrated high antimicrobial activity in mouse chronic wound infection and system infection models (Rai et al., 2016a,b).
Electrospinning is an entirely different technique which offers many possibilities for manufacturing medical devices. An example is the electrospun prosthetic heart valve, which has reached the phase of advanced preclinical testing (Kluin et al., 2017). By electrospinning, biocompatible nanofibers can be produced that have a large surface area mimicking the extracellular matrix of the body. The porosity of the matrices may however allow colonization by bacteria. To reduce the risk of infection of electrospun materials, antimicrobial agents have been incorporated in the polymers used for the electrospinning process. Examples include antibiotics such as vancomycin and/or rifampicin (Waeiss et al., 2014; Song et al., 2016), moxifloxacin (Song et al., 2016), silver nanoparticles (Tian et al., 2013; Almajhdi et al., 2014), or combinations of silver nitrate and chlorhexidine (Song et al., 2016). Recently, studies have also reported on the use of AMPs to render electrospun materials antimicrobial. Poly(E-caprolactone) nanofibers have been loaded with the synthetic AMP inverso-crabrolin (Eriksen et al., 2013), and poly(vinyl alcohol) nanofibers with pleurocidin (Wang et al., 2015) or the antifungal peptide Cm-p1 (Viana et al., 2015). In view of the increasing importance of electrospinning applications in the medical field, this is an area where additional studies on the use of AMPs will be highly relevant and novel forms of AMP structures may be highly desired. In this respect a novel class of antimicrobial agents is emerging. This is the class of structurally nanoengineered antimicrobial peptide polymers (SNAPPs). In the form of 16- or 32-arm star-shaped peptide polymer nanoparticles, these SNAPPs showed in vitro activity at sub-micromolar concentrations against a wide panel of Gram-negative bacteria, including multidrug-resistant pathogens. They were effective in vivo against an multidrug-resistant strain of A. baumannii and did not induce resistance (Lam et al., 2016).
Prevention and treatment of BAI is a major medical challenge, in particular due to the involvement of biofilm-encased and intracellular multidrug-resistant bacteria. Synthetic AMPs, displaying broad spectrum activity including activity against multidrug-resistant pathogens, anti-biofilm activities, little/no development of resistance, and in vivo activity in preventing BAI, are important candidates. Tethering of these AMPs to the biomaterial surfaces, and particularly combining AMPs with formulations to release the peptides in a controlled fashion is expected to protect both the implant and the surrounding tissue, both for conventional implants and biomedical devices manufactured by 3D-printing and electrospinning.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was supported by FP7-HEALTH-2011 grant 278890, BALI–Biofilm Alliance. MR and SZ would like to acknowledge networking support by the COST Action iPROMEDAI (Project No. TD1305), supported by COST (European Cooperation in Science and Technology).
AMPs, antimicrobial peptides; BAI, biomaterial-associated infections; CM, cecropin melittin; PEG, polyethylene glycol; PET, polyethylene terephthalate; pHEMA, poly-hydroxyethylmethacrylate; PLEX, polymer-lipid encapsulation; matrixPS, polystyrene; PU, polyurethane; SAAP, synthetic antimicrobial and anti-biofilm peptide; TiO2, titanium oxide.
Almajhdi, F. N., Fouad, H., Khalil, K. A., Awad, H. M., Mohamed, S. H. S., Elsarnagawy, T., et al. (2014). In-vitro anticancer and antimicrobial activities of PLGA/silver nanofiber composites prepared by electrospinning. J. Mater. Sci. Mater. Med. 25, 1045–1053. doi: 10.1007/s10856-013-5131-y
Alt, V. (2017). Antimicrobial coated implants in trauma and orthopaedics–A clinical review and risk-benefit analysis. Injury 48, 599–607. doi: 10.1016/j.injury.2016.12.011
Alt, V., Bitschnau, A., Böhner, F., Heerich, K. E., Magesin, E., Sewing, A., et al. (2011). Effects of gentamicin and gentamicin–RGD coatings on bone ingrowth and biocompatibility of cementless joint prostheses: an experimental study in rabbits. Acta Biomater. 7, 1274–1280. doi: 10.1016/j.actbio.2010.11.012
Anderson, J. M., and Marchant, R. E. (2000). “Biomaterials: factors favoring colonization and infection,” in Infections Associated with Indwelling Medical Devices, 3rd Edn, eds F. A. Waldvogel and A. L. Bisno (Washington, DC: American Society of Microbiology), 89–109.
Anderson, J. M., and Patel, J. D. (2013). “Biomaterial-dependent characteristics of the foreign body response and S. epidermidis biofilm interactions,” in Biomaterials Associated Infection, eds T. F. Moriarty, S. A. J. Zaat, and H. J. Busscher (New York, NY: Springer New York), 119–149.
Bach, A., Eberhardt, H., Frick, A., Schmidt, H., Böttiger, B. W., and Martin, E. (1999). Efficacy of silver-coating central venous catheters in reducing bacterial colonization. Crit. Care Med. 27, 515–521. doi: 10.1097/00003246-199903000-00028
Bagheri, M., Beyermann, M., and Dathe, M. (2009). Immobilization reduces the activity of surface-bound cationic antimicrobial peptides with no influence upon the activity spectrum. Antimicrob. Agents Chemother. 53, 1132–1141. doi: 10.1128/AAC.01254-08
Bandurska, K., Berdowska, A., Barczynska-Felusiak, R., and Krupa, P. (2015). Unique features of human cathelicidin LL-37. Biofactors 41, 289–300. doi: 10.1002/biof.1225
Banerjee, I., Pangule, R. C., and Kane, R. S. (2011). Antifouling coatings: recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Adv. Mater. Weinheim 23, 690–718. doi: 10.1002/adma.201001215
Boelens, J. J., Dankert, J., Murk, J. L., Weening, J. J., van der Poll, T., Dingemans, K. P., et al. (2000a). Biomaterial-associated persistence of Staphylococcus epidermidis in pericatheter macrophages. J. Infect. Dis. 181, 1337–1349. doi: 10.1086/315369
Boelens, J. J., van der Poll, T., Dankert, J., and Zaat, S. A. J. (2000b). Interferon-γ protects against biomaterial-associated Staphylococcus epidermidis infection in mice. J. Infect. Dis. 181, 1167–1171. doi: 10.1086/315344
Boelens, J. J., van Der Poll, T., Zaat, S. A. J., Murk, J. L., Weening, J. J., and Dankert, J. (2000c). Interleukin-1 receptor type I gene-deficient mice are less susceptible to Staphylococcus epidermidis biomaterial-associated infection than are wild-type mice. Infect. Immun. 68, 6924–6931. doi: 10.1128/IAI.68.12.6924-6931.2000
Böttger, R., Hoffmann, R., and Knappe, D. (2017). Differential stability of therapeutic peptides with different proteolytic cleavage sites in blood, plasma and serum. PLoS ONE 12:e0178943. doi: 10.1371/journal.pone.0178943
Boucher, H. W., Talbot, G. H., Bradley, J. S., Edwards, J. E., Gilbert, D., Rice, L. B., et al. (2009). Bad bugs, no drugs: no ESKAPE! an update from the infectious diseases society of America. Clin. Infect. Dis. 48, 1–12. doi: 10.1086/595011
Broekhuizen, C. A. N., de Boer, L., Schipper, K., Jones, C. D., Quadir, S., Vandenbroucke-Grauls, C. M. J. E., et al. (2008). Staphylococcus epidermidis is cleared from biomaterial implants but persists in peri-implant tissue in mice despite rifampicin/vancomycin treatment. J. Biomed. Mater. Res. A 85, 498–505. doi: 10.1002/jbm.a.31528
Broekhuizen, C. A. N., Sta, M., Vandenbroucke-Grauls, C. M. J. E., and Zaat, S. A. J. (2010). Microscopic detection of viable Staphylococcus epidermidis in peri-implant tissue in experimental biomaterial-associated infection, identified by bromodeoxyuridine incorporation. Infect. Immun. 78, 954–962. doi: 10.1128/IAI.00849-09
Brooks, B. D., Brooks, A. E., and Grainger, D. W. (2013). “Antimicrobial medical devices in preclinical development and clinical use,” in Biomaterials Associated Infection, eds T. F. Moriarty, S. A. J. Zaat, and H. J. Busscher (New York, NY: Springer), 307–354.
Bürgers, R., Eidt, A., Frankenberger, R., Rosentritt, M., Schweikl, H., Handel, G., et al. (2009). The anti-adherence activity and bactericidal effect of microparticulate silver additives in composite resin materials. Arch. Oral Biol. 54, 595–601. doi: 10.1016/j.archoralbio.2009.03.004
Burns, C. A. (2006). Daptomycin-rifampin for a recurrent MRSA joint infection unresponsive to vancomycin-based therapy. Scand. J. Infect. Dis. 38, 133–136. doi: 10.1080/00365540500277292
Busscher, H. J., van der Mei, H. C., Subbiahdoss, G., Jutte, P. C., van den Dungen, J. J., Zaat, S. A., et al. (2012). Biomaterial-associated infection: locating the finish line in the race for the surface. Sci. Transl. Med. 4:153rv10. doi: 10.1126/scitranslmed.3004528
Chambers, R. C., and Laurent, G. J. (2002). Coagulation cascade proteases and tissue fibrosis. Biochem. Soc. Trans. 30, 194–200. doi: 10.1042/bst0300194
Chen, M., Yu, Q., and Sun, H. (2013). Novel strategies for the prevention and treatment of biofilm related infections. Int. J. Mol. Sci. 14, 18488–18501. doi: 10.3390/ijms140918488
Chen, R., Willcox, M. D. P., Ho, K. K. K., Smyth, D., and Kumar, N. (2016). Antimicrobial peptide melimine coating for titanium and its in vivo antibacterial activity in rodent subcutaneous infection models. Biomaterials 85, 142–151. doi: 10.1016/j.biomaterials.2016.01.063
Chen, X., Hirt, H., Li, Y., Gorr, S. U., and Aparicio, C. (2014). Antimicrobial GL13K peptide coatings killed and ruptured the wall of Streptococcus gordonii and prevented formation and growth of biofilms. PLoS ONE 9:e111579. doi: 10.1371/journal.pone.0111579
Chen, X., Zhou, X. C., Liu, S., Wu, R. F., Aparicio, C., and Wu, J. Y. (2017). In vivo osseointegration of dental implants with an antimicrobial peptide coating. J. Mater. Sci. Mater. Med. 28:76. doi: 10.1007/s10856-017-5885-8
Cheng, H., Yue, K., Kazemzadeh-Narbat, M., Liu, Y., Khalilpour, A., Li, B., et al. (2017). Mussel-inspired multifunctional hydrogel coating for prevention of infections and enhanced osteogenesis. ACS Appl. Mater. Interfaces 9, 11428–11439. doi: 10.1021/acsami.6b16779
Choe, H., Narayanan, A. S., Gandhi, D. A., Weinberg, A., Marcus, R. E., Lee, Z., et al. (2015). Immunomodulatory peptide IDR-1018 decreases implant infection and preserves osseointegration. Clin. Orthop. Relat. Res. 473, 2898–2907. doi: 10.1007/s11999-015-4301-2
Ciampolini, J., and Harding, K. G. (2000). Pathophysiology of chronic bacterial osteomyelitis. why do antibiotics fail so often? Postgrad. Med. J. 76, 479–483. doi: 10.1136/pmj.76.898.479
Cleophas, R. T. C., Riool, M., Quarles van Ufford, H., Linda, C., Zaat, S. A. J., Kruijtzer, J. A. W., et al. (2014). Convenient preparation of bactericidal hydrogels by covalent attachment of stabilized antimicrobial peptides using thiol–ene click chemistry. ACS Macro Lett. 3, 477–480. doi: 10.1021/mz5001465
Costa, F., Carvalho, I. F., Montelaro, R. C., Gomes, P., and Martins, M. C. L. (2011). Covalent immobilization of antimicrobial peptides (AMPs) onto biomaterial surfaces. Acta Biomater. 7, 1431–1440. doi: 10.1016/j.actbio.2010.11.005
Costa, F., Maia, S., Gomes, J., Gomes, P., and Martins, M. C. L. (2014). Characterization of hLF1–11 immobilization onto chitosan ultrathin films, and its effects on antimicrobial activity. Acta Biomater. 10, 3513–3521. doi: 10.1016/j.actbio.2014.02.028
Costerton, J. W., Stewart, P. S., and Greenberg, E. P. (1999). Bacterial biofilms: a common cause of persistent infections. Science 284, 1318–1322. doi: 10.1126/science.284.5418.1318
Darouiche, R. O. (2004). Treatment of infections associated with surgical implants. N. Engl. J. Med. 350, 1422–1429. doi: 10.1056/NEJMra035415
Darouiche, R. O. (2007). In vivo efficacy of antimicrobial-coated devices. J. Bone Joint Surg. 89:792. doi: 10.2106/00004623-200704000-00014
de Breij, A., Riool, M., Kwakman, P. H. S., de Boer, L., Cordfunke, R. A., Drijfhout, J. W., et al. (2016). Prevention of Staphylococcus aureus biomaterial-associated infections using a polymer-lipid coating containing the antimicrobial peptide OP-145. J. Control. Release 222, 1–8. doi: 10.1016/j.jconrel.2015.12.003
de Mesy Bentley, K. L., Trombetta, R., Nishitani, K., Bello-Irizarry, S. N., Ninomiya, M., Zhang, L., et al. (2017). Evidence of Staphylococcus Aureus deformation, proliferation, and migration in canaliculi of live cortical bone in murine models of osteomyelitis. J. Bone Miner. Res. 32, 985–990. doi: 10.1002/jbmr.3055
De Zoysa, G. H., and Sarojini, V. (2017). Feasibility study exploring the potential of novel battacin lipopeptides as antimicrobial coatings. ACS Appl. Mater. Interfaces 9, 1373–1383. doi: 10.1021/acsami.6b15859
Di Luca, M., Maccari, G., Maisetta, G., and Batoni, G. (2015). BaAMPs: the database of biofilm-active antimicrobial peptides. Biofouling 31, 193–199. doi: 10.1080/08927014.2015.1021340
Dutta, D., Kumar, N., and Willcox, D. P. M. (2016). Antimicrobial activity of four cationic peptides immobilised to poly-hydroxyethylmethacrylate. Biofouling 32, 429–438. doi: 10.1080/08927014.2015.1129533
Elek, S., and Conen, P. (1957). The virulence of Staphylococcus pyogenes for man. a study of the problems of wound infection. Br. J. Exp. Pathol. 38, 573–586.
Emanuel, N., Rosenfeld, Y., Cohen, O., Applbaum, Y. H., Segal, D., and Barenholz, Y. (2012). A lipid-and-polymer-based novel local drug delivery system—BonyPidTM: from physicochemical aspects to therapy of bacterially infected bones. J. Control. Release 160, 353–361. doi: 10.1016/j.jconrel.2012.03.027
Eriksen, T. H. B., Skovsen, E., and Fojan, P. (2013). Release of antimicrobial peptides from electrospun nanofibres as a drug delivery system. J. Biomed. Nanotechnol. 9, 492–498. doi: 10.1166/jbn.2013.1553
Ernst, C. M., Kuhn, S., Slavetinsky, C. J., Krismer, B., Heilbronner, S., Gekeler, C., et al. (2015). The lipid-modifying multiple peptide resistance factor is an oligomer consisting of distinct interacting synthase and flippase subunits. MBio 6, e02340–e02314. doi: 10.1128/mBio.02340-14
FDA (2007). Draft Guidance for Industry and FDA Staff - Premarket Notification [510(k)] Submissions for Medical Devices that Include Antimicrobial Agents., 1–18.
Feng, Z., and Xu, B. (2016). Inspiration from the mirror: D-amino acid containing peptides in biomedical approaches. Biomol. Concepts 7, 179–187. doi: 10.1515/bmc-2015-0035
Fidan, S., Muhaffel, F., Riool, M., Cempura, G., de Boer, L., Zaat, S. A. J., et al. (2017). Fabrication of oxide layer on zirconium by micro-arc oxidation: structural and antimicrobial characteristics. Mater. Sci. Eng. C 71, 565–569. doi: 10.1016/j.msec.2016.11.035
Fisher, R. A., Gollan, B., and Helaine, S. (2017). Persistent bacterial infections and persister cells. Nat. Rev. Microbiol. 15, 453–464. doi: 10.1038/nrmicro.2017.42
Fox, J. L. (2013). Antimicrobial peptides stage a comeback. Nat. Biotechnol. 31, 379–382. doi: 10.1038/nbt.2572
Fuchs, P. C., Barry, A. L., and Brown, S. D. (1998). In vitro antimicrobial activity of MSI-78, a magainin analog. Antimicrob. Agents Chemother. 42, 1213–1216.
Fuchs, T., Stange, R., Schmidmaier, G., and Raschke, M. J. (2011). The use of gentamicin-coated nails in the tibia: preliminary results of a prospective study. Arch. Orthop. Trauma Surg. 131, 1419–1425. doi: 10.1007/s00402-011-1321-6
Gao, G., Lange, D., Hilpert, K., Kindrachuk, J., Zou, Y., Cheng, J. T. J., et al. (2011a). The biocompatibility and biofilm resistance of implant coatings based on hydrophilic polymer brushes conjugated with antimicrobial peptides. Biomaterials 32, 3899–3909. doi: 10.1016/j.biomaterials.2011.02.013
Gao, G., Yu, K., Kindrachuk, J., Brooks, D. E., Hancock, R. E. W., and Kizhakkedathu, J. N. (2011b). Antibacterial surfaces based on polymer brushes: investigation on the influence of brush properties on antimicrobial peptide immobilization and antimicrobial activity. Biomacromolecules 12, 3715–3727. doi: 10.1021/bm2009697
Gerdes, K., and Semsey, S. (2016). Microbiology: pumping persisters. Nature 534, 41–42. doi: 10.1038/nature18442
Godoy-Gallardo, M., Mas-Moruno, C., Yu, K., Manero, J. M., Gil, F. J., Kizhakkedathu, J. N., et al. (2015). Antibacterial properties of hLf1–11 peptide onto titanium surfaces: a comparison study between silanization and surface initiated polymerization. Biomacromolecules 16, 483–496. doi: 10.1021/bm501528x
Goytia, M., Kandler, J. L., and Shafer, W. M. (2013). “Mechanisms and significance of bacterial resistance to human cationic antimicrobial peptides,” in Antimicrobial Peptides and Innate Immunity, eds P. Hiemstra and S. Zaat (Basel: Springer), 219–254.
Greber, E. K., and Dawgul, M. (2016). Antimicrobial peptides under clinical trials. Curr. Top. Med. Chem. 17, 620–628. doi: 10.2174/1568026616666160713143331
Gristina, A. (1987). Biomaterial-centered infection: microbial adhesion versus tissue integration. Science 237, 1588–1595. doi: 10.1126/science.3629258
Grönberg, A., Mahlapuu, M., Ståhle, M., Whately-Smith, C., and Rollman, O. (2014). Treatment with LL-37 is safe and effective in enhancing healing of hard-to-heal venous leg ulcers: a randomized, placebo-controlled clinical trial. Wound Repair Regen. 22, 613–621. doi: 10.1111/wrr.12211
Guichard, G., Benkirane, N., Zeder-Lutz, G., van Regenmortel, M. H., Briand, J. P., and Muller, S. (1994). Antigenic mimicry of natural L-peptides with retro-inverso-peptidomimetics. Proc. Natl. Acad. Sci. U.S.A. 91, 9765–9769. doi: 10.1073/pnas.91.21.9765
Hancock, R. E. W., and Sahl, H.-G. (2006). Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 24, 1551–1557. doi: 10.1038/nbt1267
Harms, A., Maisonneuve, E., and Gerdes, K. (2016). Mechanisms of bacterial persistence during stress and antibiotic exposure. Science 354:aaf4268. doi: 10.1126/science.aaf4268
Heim, C. E., Vidlak, D., Scherr, T. D., Hartman, C. W., Garvin, K. L., and Kielian, T. (2015). IL-12 Promotes myeloid-derived suppressor cell recruitment and bacterial persistence during Staphylococcus aureus orthopedic implant infection. J. Immunol. 194, 3861–3872. doi: 10.4049/jimmunol.1402689
Heim, C. E., Vidlak, D., Scherr, T. D., Kozel, J. A., Holzapfel, M., Muirhead, D. E., et al. (2014). Myeloid-derived suppressor cells contribute to Staphylococcus aureus orthopedic biofilm infection. J. Immunol. 192, 3778–3792. doi: 10.4049/jimmunol.1303408
Hilpert, K., Elliott, M., Jenssen, H., Kindrachuk, J., Fjell, C. D., Körner, J., et al. (2009). Screening and characterization of surface-tethered cationic peptides for antimicrobial activity. Chem. Biol. 16, 58–69. doi: 10.1016/j.chembiol.2008.11.006
Holmberg, A., Lood, R., Mörgelin, M., Söderquist, B., Holst, E., Collin, M., et al. (2009). Biofilm formation by Propionibacterium acnes is a characteristic of invasive isolates. Clin. Microbiol. Infect. 15, 787–795. doi: 10.1111/j.1469-0691.2009.02747.x
Hoyos-Nogués, M., Velasco, F., Ginebra, M.-P., Manero, J. M., Gil, F. J., and Mas-Moruno, C. (2017). Regenerating Bone via multifunctional coatings: the blending of cell integration and bacterial inhibition properties on the surface of biomaterials. ACS Appl. Mater. Interfaces 9, 21618–21630. doi: 10.1021/acsami.7b03127
Inzana, J., Trombetta, R., Schwarz, E., Kates, S., and Awad, H. (2015). 3D printed bioceramics for dual antibiotic delivery to treat implant-associated bone infection. Eur. Cells Mater. 30, 232–247. doi: 10.22203/eCM.v030a16
Iwase, Y., Kamei, N., Khafagy, E.-S., Miyamoto, M., and Takeda-Morishita, M. (2016). Use of a non-covalent cell-penetrating peptide strategy to enhance the nasal delivery of interferon beta and its PEGylated form. Int. J. Pharm. 510, 304–310. doi: 10.1016/j.ijpharm.2016.06.054
James, R. C., and Macleod, C. J. (1961). Induction of staphylococcal infections in mice with small inocula introduced on sutures. Br. J. Exp. Pathol. 42, 266–277.
Kälicke, T., Schierholz, J., Schlegel, U., Frangen, T. M., Köller, M., Printzen, G., et al. (2006). Effect on infection resistance of a local antiseptic and antibiotic coating on osteosynthesis implants: an in vitro and in vivo study. J. Orthop. Res. 24, 1622–1640. doi: 10.1002/jor.20193
Kazemzadeh-Narbat, M., Kindrachuk, J., Duan, K., Jenssen, H., Hancock, R. E. W., and Wang, R. (2010). Antimicrobial peptides on calcium phosphate-coated titanium for the prevention of implant-associated infections. Biomaterials 31, 9519–9526. doi: 10.1016/j.biomaterials.2010.08.035
Kazemzadeh-Narbat, M., Noordin, S., Masri, B. A., Garbuz, D. S., Duncan, C. P., Hancock, R. E. W., et al. (2012). Drug release and bone growth studies of antimicrobial peptide-loaded calcium phosphate coating on titanium. J. Biomed. Mater. Res. B Appl. Biomater. 100B, 1344–1352. doi: 10.1002/jbm.b.32701
Keum, H., Kim, J. Y., Yu, B., Yu, S. J., Kim, J., Jeon, H., et al. (2017). Prevention of bacterial colonization on catheters by a one-step coating process involving an antibiofouling polymer in water. ACS Appl. Mater. Interfaces 9, 19736–19745. doi: 10.1021/acsami.7b06899
Kittaka, M., Shiba, H., Kajiya, M., Fujita, T., Iwata, T., Rathvisal, K., et al. (2013). The antimicrobial peptide LL37 promotes bone regeneration in a rat calvarial bone defect. Peptides 46, 136–142. doi: 10.1016/j.peptides.2013.06.001
Kluin, J., Talacua, H., Smits, A. I. P. M., Emmert, M. Y., Brugmans, M. C. P., Fioretta, E. S., et al. (2017). In situ heart valve tissue engineering using a bioresorbable elastomeric implant – From material design to 12 months follow-up in sheep. Biomaterials 125, 101–117. doi: 10.1016/j.biomaterials.2017.02.007
Krenek, L., Farng, E., Zingmond, D., and SooHoo, N. F. (2011). Complication and revision rates following total elbow arthroplasty. J. Hand Surg. Am. 36, 68–73. doi: 10.1016/j.jhsa.2010.09.036
Krijgsveld, J., Zaat, S. A. J., Meeldijk, J., van Veelen, P. A., Fang, G., Poolman, B., et al. (2000). Thrombocidins, microbicidal proteins from human blood platelets, are C-terminal deletion products of CXC chemokines. J. Biol. Chem. 275, 20374–20381. doi: 10.1074/jbc.275.27.20374
Kuehl, R., Brunetto, P. S., Woischnig, A.-K., Varisco, M., Rajacic, Z., Vosbeck, J., et al. (2016). Preventing implant-associated infections by silver coating. Antimicrob. Agents Chemother. 60, 2467–2475. doi: 10.1128/AAC.02934-15
Kwakman, P. H. S., Krijgsveld, J., de Boer, L., Nguyen, L. T., Boszhard, L., Vreede, J., et al. (2011). Native thrombocidin-1 and unfolded thrombocidin-1 exert antimicrobial activity via distinct structural elements. J. Biol. Chem. 286, 43506–43514. doi: 10.1074/jbc.M111.248641
Kwakman, P. H. S., and Zaat, S. A. J. (2013). “Preventive measures against transcutaneous device infections,” in Biomaterials Associated Infection, eds T. F. Moriarty, S. A. J. Zaat, and H. J. Busscher (New York, NY: Springer), 229–248.
Lam, S. J., O'Brien-Simpson, N. M., Pantarat, N., Sulistio, A., Wong, E. H. H., Chen, Y.-Y., et al. (2016). Combating multidrug-resistant gram-negative bacteria with structurally nanoengineered antimicrobial peptide polymers. Nat. Microbiol. 1:16162. doi: 10.1038/nmicrobiol.2016.162
Lara Rodriguez, L., Sundaram, P. A., Rosim-Fachini, E., Padovani, A. M., and Diffoot-Carlo, N. (2014). Plasma electrolytic oxidation coatings on ??tiAl alloy for potential biomedical applications. J. Biomed. Mater. Res. B Appl. Biomater. 102, 988–1001. doi: 10.1002/jbm.b.33079
Li, T., Wang, N., Chen, S., Lu, R., Li, H., and Zhang, Z. (2017). Antibacterial activity and cytocompatibility of an implant coating consisting of TiO2 nanotubes combined with a GL13K antimicrobial peptide. Int. J. Nanomedicine 12, 2995–3007. doi: 10.2147/IJN.S128775
Li, X., Contreras-Garcia, A., LoVetri, K., Yakandawala, N., Wertheimer, M. R., De Crescenzo, G., et al. (2015). Fusion peptide P15-CSP shows antibiofilm activity and pro-osteogenic activity when deposited as a coating on hydrophilic but not hydrophobic surfaces. J. Biomed. Mater. Res. A 103, 3736–3746. doi: 10.1002/jbm.a.35511
Liu, Z., Ma, S., Duan, S., Xuliang, D., Sun, Y., Zhang, X., et al. (2016). Modification of titanium substrates with chimeric peptides comprising antimicrobial and titanium-binding motifs connected by linkers to inhibit biofilm formation. ACS Appl. Mater. Interfaces 8, 5124–5136. doi: 10.1021/acsami.5b11949
Lucke, M., Schmidmaier, G., Sadoni, S., Wildemann, B., Schiller, R., Haas, N., et al. (2003). Gentamicin coating of metallic implants reduces implant-related osteomyelitis in rats. Bone 32, 521–531. doi: 10.1016/S8756-3282(03)00050-4
Ma, M., Kazemzadeh-Narbat, M., Hui, Y., Lu, S., Ding, C., Chen, D. D. Y., et al. (2012). Local delivery of antimicrobial peptides using self-organized TiO2 nanotube arrays for peri-implant infections. J. Biomed. Mater. Res. A 100, 278–285. doi: 10.1002/jbm.a.33251
Magiorakos, A.-P., Srinivasan, A., Carey, R. B., Carmeli, Y., Falagas, M. E., Giske, C. G., et al. (2012). Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 18, 268–281. doi: 10.1111/j.1469-0691.2011.03570.x
Mansour, S. C., de la Fuente-Núñez, C., and Hancock, R. E. W. (2015). Peptide IDR-1018: modulating the immune system and targeting bacterial biofilms to treat antibiotic-resistant bacterial infections. J. Pept. Sci. 21, 323–329. doi: 10.1002/psc.2708
Mansour, S. C., Pena, O. M., and Hancock, R. E. W. (2014). Host defense peptides: front-line immunomodulators. Trends Immunol. 35, 443–450. doi: 10.1016/j.it.2014.07.004
Mateescu, M., Baixe, S., Garnier, T., Jierry, L., Ball, V., Haikel, Y., et al. (2015). Antibacterial peptide-based gel for prevention of medical implanted-device infection. PLoS ONE 10:e0145143. doi: 10.1371/journal.pone.0145143
Metsemakers, W. J., Reul, M., and Nijs, S. (2015). The use of gentamicin-coated nails in complex open tibia fracture and revision cases: a retrospective analysis of a single centre case series and review of the literature. Injury 46, 2433–2437. doi: 10.1016/j.injury.2015.09.028
Moojen, D. J. F., Vogely, H. C., Fleer, A., Nikkels, P. G. J., Higham, P. A., Verbout, A. J., et al. (2009). Prophylaxis of infection and effects on osseointegration using a tobramycin-periapatite coating on titanium implants-an experimental study in the rabbit. J. Orthop. Res. 27, 710–716. doi: 10.1002/jor.20808
Morris, C. J., Beck, K., Fox, M. A., Ulaeto, D., Clark, G. C., and Gumbleton, M. (2012). Pegylation of antimicrobial peptides maintains the active peptide conformation, model membrane interactions, and antimicrobial activity while improving lung tissue biocompatibility following airway delivery. Antimicrob. Agents Chemother. 56, 3298–3308. doi: 10.1128/AAC.06335-11
Mosca, D. A., Hurst, M. A., So, W., Viajar, B. S. C., Fujii, C. A., and Falla, T. J. (2000). IB-367, a protegrin peptide with in vitro and in vivo activities against the microflora associated with oral mucositis. Antimicrob. Agents Chemother. 44, 1803–1808. doi: 10.1128/AAC.44.7.1803-1808.2000
Nakatsuji, T., and Gallo, R. L. (2012). Antimicrobial peptides: old molecules with new ideas. J. Invest. Dermatol. 132, 887–895. doi: 10.1038/jid.2011.387
Necula, B. S., Fratila-Apachitei, L. E., Zaat, S. A. J., Apachitei, I., and Duszczyk, J. (2009). In vitro antibacterial activity of porous TiO2–Ag composite layers against methicillin-resistant Staphylococcus aureus. Acta Biomater. 5, 3573–3580. doi: 10.1016/j.actbio.2009.05.010
Necula, B. S., van Leeuwen, J. P. T. M., Fratila-Apachitei, L. E., Zaat, S. A. J., Apachitei, I., and Duszczyk, J. (2012). In vitro cytotoxicity evaluation of porous TiO2–Ag antibacterial coatings for human fetal osteoblasts. Acta Biomater. 8, 4191–4197. doi: 10.1016/j.actbio.2012.07.005
Nejadnik, M. R., Engelsman, A. F., Saldarriaga Fernandez, I. C., Busscher, H. J., Norde, W., and van der Mei, H. C. (2008). Bacterial colonization of polymer brush-coated and pristine silicone rubber implanted in infected pockets in mice. J. Antimicrob. Chemother. 62, 1323–1325. doi: 10.1093/jac/dkn395
Nell, M. J., Tjabringa, G. S., Wafelman, A. R., Verrijk, R., Hiemstra, P. S., Drijfhout, J. W., et al. (2006). Development of novel LL-37 derived antimicrobial peptides with LPS and LTA neutralizing and antimicrobial activities for therapeutic application. Peptides 27, 649–660. doi: 10.1016/j.peptides.2005.09.016
Neoh, K. G., Shi, Z. L., and Kang, E. T. (2013). “Anti-adhesive and antibacterial polymer brushes,” in Biomaterials Associated Infection, eds T. F. Moriarty, S. A. J. Zaat, and H. J. Busscher (New York, NY: Springer), 405–432.
Nie, B., Ao, H., Long, T., Zhou, J., Tang, T., and Yue, B. (2017). Immobilizing bacitracin on titanium for prophylaxis of infections and for improving osteoinductivity: an in vivo study. Colloids Surf. B Biointerfaces 150, 183–191. doi: 10.1016/j.colsurfb.2016.11.034
Nie, B., Ao, H., Zhou, J., Tang, T., and Yue, B. (2016). Biofunctionalization of titanium with bacitracin immobilization shows potential for anti-bacteria, osteogenesis and reduction of macrophage inflammation. Colloids Surf. B Biointerfaces 145, 728–739. doi: 10.1016/j.colsurfb.2016.05.089
Nilebäck, L., Hedin, J., Widhe, M., Floderus, L. S., Krona, A., Bysell, H., et al. (2017). Self-Assembly of recombinant silk as a strategy for chemical-free formation of bioactive coatings: a real-time study. Biomacromolecules 18, 846–854. doi: 10.1021/acs.biomac.6b01721
Noble, W. C. (1965). The production of subcutaneous staphylococcal skin lesions in mice. Br. J. Exp. Pathol. 46, 254–262.
O'Gara, J. P., and Humphreys, H. (2001). Staphylococcus epidermidis biofilms: importance and implications. J. Med. Microbiol. 50, 582–587. doi: 10.1099/0022-1317-50-7-582
Onaizi, S. A., and Leong, S. S. J. (2011). Tethering antimicrobial peptides: current status and potential challenges. Biotechnol. Adv. 29, 67–74. doi: 10.1016/j.biotechadv.2010.08.012
Osma, S., Kahveci, S. F., Kaya, F. N., Akalin, H., Özakin, C., Yilmaz, E., et al. (2006). Efficacy of antiseptic-impregnated catheters on catheter colonization and catheter-related bloodstream infections in patients in an intensive care unit. J. Hosp. Infect. 62, 156–162. doi: 10.1016/j.jhin.2005.06.030
Otto, M. (2009). Staphylococcus epidermidis — the “accidental” pathogen. Nat. Rev. Microbiol. 7, 555–567. doi: 10.1038/nrmicro2182
Overhage, J., Campisano, A., Bains, M., Torfs, E. C. W., Rehm, B. H. A., and Hancock, R. E. W. (2008). Human host defense peptide LL-37 prevents bacterial biofilm formation. Infect. Immun. 76, 4176–4182. doi: 10.1128/IAI.00318-08
Pasupuleti, M., Schmidtchen, A., and Malmsten, M. (2012). Antimicrobial peptides: key components of the innate immune system. Crit. Rev. Biotechnol. 32, 143–171. doi: 10.3109/07388551.2011.594423
Peek, F., Nell, M. J., Brand, R., Jansen-Werkhoven, T., Van Hoogdalem, E., and Frijns, J. (2009). “Double-blind placebo-controlled study of the novel peptide drug P60.4Ac in cronic middle ear infection,” in ICAAC (San Francisco, CA), L1–L337.
Rai, A., Pinto, S., Evangelista, M. B., Gil, H., Kallip, S., Ferreira, M. G. S. S., et al. (2016a). High-density antimicrobial peptide coating with broad activity and low cytotoxicity against human cells. Acta Biomater. 33, 64–74. doi: 10.1016/j.actbio.2016.01.035
Rai, A., Pinto, S., Velho, T. R., Ferreira, A. F., Moita, C., Trivedi, U., et al. (2016b). One-step synthesis of high-density peptide-conjugated gold nanoparticles with antimicrobial efficacy in a systemic infection model. Biomaterials 85, 99–110. doi: 10.1016/j.biomaterials.2016.01.051
Rice, L. B. (2008). federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J. Infect. Dis. 197, 1079–1081. doi: 10.1086/533452
Riool, M., de Boer, L., Jaspers, V., van der Loos, C. M., van Wamel, W. J. B., Wu, G., et al. (2014). Staphylococcus epidermidis originating from titanium implants infects surrounding tissue and immune cells. Acta Biomater. 10, 5202–5212. doi: 10.1016/j.actbio.2014.08.012
Riool, M., de Breij, A., de Boer, L., Kwakman, P. H. S., Cordfunke, R. A., Cohen, O., et al. (2017a). Controlled release of LL-37-derived Synthetic Antimicrobial and Anti-Biofilm Peptides SAAP-145 and SAAP-276 prevents experimental biomaterial-associated Staphylococcus aureus infection. Adv. Funct. Mater. 27:1606623. doi: 10.1002/adfm.201606623
Riool, M., Dirks, A., Jaspers, V., de Boer, L., Loontjens, T., van der Loos, C., et al. (2017b). A chlorhexidine-releasing epoxy-based coating on titanium implants prevents Staphylococcus aureus experimental biomaterial-associated infection. Eur. Cells Mater. 33, 143–157. doi: 10.22203/eCM.v033a11
Rupp, M. E., Lisco, S. J., Lipsett, P. A., Perl, T. M., Keating, K., Civetta, J. M., et al. (2005). Effect of a second-generation venous catheter impregnated with chlorhexidine and silver sulfadiazine on central catheter-related infections: a randomized, controlled trial. Ann. Intern. Med. 143, 570–580. doi: 10.7326/0003-4819-143-8-200510180-00007
Sader, H. S., Fedler, K. A., Rennie, R. P., Stevens, S., and Jones, R. N. (2004). Omiganan pentahydrochloride (MBI 226), a topical 12-amino-acid cationic peptide: spectrum of antimicrobial activity and measurements of bactericidal activity. Antimicrob. Agents Chemother. 48, 3112–3118. doi: 10.1128/AAC.48.8.3112-3118.2004
Safdar, N., Kluger, D. M., and Maki, D. G. (2002). A review of risk factors for catheter-related bloodstream infection caused by percutaneously inserted, noncuffed central venous catheters: implications for preventive strategies. Medicine 81, 466–479. doi: 10.1097/00005792-200211000-00007
Saginur, R., St Denis, M., Ferris, W., Aaron, S. D., Chan, F., Lee, C., et al. (2006). Multiple combination bactericidal testing of Staphylococcal biofilms from implant-associated infections. Antimicrob. Agents Chemother. 50, 55–61. doi: 10.1128/AAC.50.1.55-61.2006
Salvado, M. D., Di Gennaro, A., Lindbom, L., Agerberth, B., and Haeggstrom, J. Z. (2013). Cathelicidin LL-37 induces angiogenesis via PGE2-EP3 signaling in endothelial cells, in vivo inhibition by aspirin. Arterioscler. Thromb. Vasc. Biol. 33, 1965–1972. doi: 10.1161/ATVBAHA.113.301851
Shi, J., Liu, Y., Wang, Y., Zhang, J., Zhao, S., and Yang, G. (2015). Biological and immunotoxicity evaluation of antimicrobial peptide-loaded coatings using a layer-by-layer process on titanium. Sci. Rep. 5:16336. doi: 10.1038/srep16336
Silva, R. R., Avelino, K. Y. P. S., Ribeiro, K. L., Franco, O. L., Oliveira, M. D. L., and Andrade, C. A. S. (2016). Chemical immobilization of antimicrobial peptides on biomaterial surfaces. Front. Biosci. 8, 129–142. doi: 10.2741/s453
Song, J., Chen, Q., Zhang, Y., Diba, M., Kolwijck, E., Shao, J., et al. (2016). Electrophoretic Deposition of chitosan coatings modified with gelatin nanospheres to tune the release of antibiotics. ACS Appl. Mater. Interfaces 8, 13785–13792. doi: 10.1021/acsami.6b03454
Southwood, R. T., Rice, J. L., McDonald, P. J., Hakendorf, P. H., and Rozenbilds, M. A. (1987). Infection in experimental arthroplasties. Clin. Orthop. Relat. Res. 33–36. doi: 10.1097/00003086-198711000-00005
Splith, K., and Neundorf, I. (2011). Antimicrobial peptides with cell-penetrating peptide properties and vice versa. Eur. Biophys. J. 40, 387–397. doi: 10.1007/s00249-011-0682-7
Sussman, E. M., Jayanti, P., Dair, B. J., and Casey, B. J. (2015). Assessment of total silver and silver nanoparticle extraction from medical devices. Food Chem. Toxicol. 85, 10–19. doi: 10.1016/j.fct.2015.08.013
Tan, X. W., Goh, T. W., Saraswathi, P., Nyein, C. L., Setiawan, M., Riau, A., et al. (2014). Effectiveness of antimicrobial peptide immobilization for preventing perioperative cornea implant-associated bacterial infection. Antimicrob. Agents Chemother. 58, 5229–5238. doi: 10.1128/AAC.02859-14
Taubler, J. H., and Kapral, F. A. (1966). Staphylococcal population changes in experimentally infected mice: infection with suture-adsorbed and unadsorbed organisms grown in vitro and in vivo. J. Infect. Dis. 116, 257–262. doi: 10.1093/infdis/116.3.257
ter Boo, G.-J. A., Arens, D., Metsemakers, W.-J., Zeiter, S., Richards, R. G., Grijpma, D. W., et al. (2016). Injectable gentamicin-loaded thermo-responsive hyaluronic acid derivative prevents infection in a rabbit model. Acta Biomater. 43, 185–194. doi: 10.1016/j.actbio.2016.07.029
ter Boo, G.-J. A., Grijpma, D. W., Moriarty, T. F., Richards, R. G., and Eglin, D. (2015). Antimicrobial delivery systems for local infection prophylaxis in orthopedic- and trauma surgery. Biomaterials 52, 113–125. doi: 10.1016/j.biomaterials.2015.02.020
Tian, J., Shen, S., Zhou, C., Dang, X., Jiao, Y., Li, L., et al. (2015). Investigation of the antimicrobial activity and biocompatibility of magnesium alloy coated with HA and antimicrobial peptide. J. Mater. Sci. Mater. Med. 26:66. doi: 10.1007/s10856-015-5389-3
Tian, L., Wang, P., Zhao, Z., and Ji, J. (2013). Antimicrobial activity of electrospun poly(butylenes succinate) fiber mats containing PVP-capped silver nanoparticles. Appl. Biochem. Biotechnol. 171, 1890–1899. doi: 10.1007/s12010-013-0461-2
Tuchscherr, L., Heitmann, V., Hussain, M., Viemann, D., Roth, J., von Eiff, C., et al. (2010). Staphylococcus aureus small-colony variants are adapted phenotypes for intracellular persistence. J. Infect. Dis. 202, 1031–1040. doi: 10.1086/656047
van Hengel, I. A. J., Riool, M., Fratila-Apachitei, L. E., Witte-Bouma, J., Farrell, E., Zadpoor, A. A., et al. (2017). Selective laser melting porous metallic implants with immobilized silver nanoparticles kill and prevent biofilm formation by methicillin-resistant Staphylococcus aureus. Biomaterials 140, 1–15. doi: 10.1016/j.biomaterials.2017.02.030
Viana, J. F. C., Carrijo, J., Freitas, C. G., Paul, A., Alcaraz, J., Lacorte, C. C., et al. (2015). Antifungal nanofibers made by controlled release of sea animal derived peptide. Nanoscale 7, 6238–6246. doi: 10.1039/C5NR00767D
Waeiss, R. A., Negrini, T. C., Arthur, R. A., and Bottino, M. C. (2014). Antimicrobial effects of drug-containing electrospun matrices on osteomyelitis-associated pathogens. J. Oral Maxillofac. Surg. 72, 1310–1319. doi: 10.1016/j.joms.2014.01.007
Waldvogel, F. A., and Bisno, A. L. (eds.) (2000). Infections Associated with Indwelling Medical Devices, 3rd Edn. Washington, DC: American Society of Microbiology.
Wang, X., Yue, T., and Lee, T. C. (2015). Development of pleurocidin-poly(vinyl alcohol) electrospun antimicrobial nanofibers to retain antimicrobial activity in food system application. Food Control 54, 150–157. doi: 10.1016/j.foodcont.2015.02.001
Yazici, H., O'Neill, M. B., Kacar, T., Wilson, B. R., Oren, E. E., Sarikaya, M., et al. (2016). Engineered chimeric peptides as antimicrobial surface coating agents toward infection-free implants. ACS Appl. Mater. Interfaces 8, 5070–5081. doi: 10.1021/acsami.5b03697
Ye, J., Liu, E., Yu, Z., Pei, X., Chen, S., Zhang, P., et al. (2016). CPP-assisted intracellular drug delivery, what is next? Int. J. Mol. Sci. 17:e1892. doi: 10.3390/ijms17111892
Yu, K., Lo, J. C. Y., Yan, M., Yang, X., Brooks, D. E., Hancock, R. E. W., et al. (2017). Anti-adhesive antimicrobial peptide coating prevents catheter associated infection in a mouse urinary infection model. Biomaterials 116, 69–81. doi: 10.1016/j.biomaterials.2016.11.047
Yucesoy, D. T., Hnilova, M., Boone, K., Arnold, P. M., Snead, M. L., and Tamerler, C. (2015). Chimeric Peptides as implant functionalization agents for titanium alloy implants with antimicrobial properties. JOM 67, 754–766. doi: 10.1007/s11837-015-1350-7
Zaat, S. A. J. (2013). “Tissue colonization in biomaterial-associated infection,” in Biomaterials Associated Infection, eds T. F. Moriarty, S. A. J. Zaat, and H. J. Busscher (New York, NY: Springer), 175–207.
Zaat, S. A. J., Kwakman, P. H. S., and Drijfhout, J. W. (2014). International Patent Application: “Thrombocidin-derived antimicrobial peptides.” No. PCT/NL2014/050909. Amsterdam.
Zaat, S., Broekhuizen, C., and Riool, M. (2010). Host tissue as a niche for biomaterial-associated infection. Future Microbiol. 5, 1149–1151. doi: 10.2217/fmb.10.89
Zasloff, M. (2002). Antimicrobial peptides of multicellular organisms. Nature 415, 389–395. doi: 10.1038/415389a
Zhang, Z., and Shively, J. E. (2013). Acceleration of Bone Repair in NOD/SCID mice by human monoosteophils, novel LL-37-activated monocytes. PLoS ONE 8:e67649. doi: 10.1371/journal.pone.0067649
Zhou, L., Lai, Y., Huang, W., Huang, S., Xu, Z., Chen, J., et al. (2015). Biofunctionalization of microgroove titanium surfaces with an antimicrobial peptide to enhance their bactericidal activity and cytocompatibility. Colloids Surf. B Biointerfaces 128, 552–560. doi: 10.1016/j.colsurfb.2015.03.008
Zilberman, M., and Elsner, J. J. (2008). Antibiotic-eluting medical devices for various applications. J. Control. Release 130, 202–215. doi: 10.1016/j.jconrel.2008.05.020
Zimmerli, W. (2006). Prosthetic-joint-associated infections. Best Pract. Res. Clin. Rheumatol. 20, 1045–1063. doi: 10.1016/j.berh.2006.08.003
Zimmerli, W., and Sendi, P. (2011). Pathogenesis of implant-associated infection: the role of the host. Semin. Immunopathol. 33, 295–306. doi: 10.1007/s00281-011-0275-7
Zimmerli, W., Trampuz, A., and Ochsner, P. E. (2004). Prosthetic-Joint Infections. N. Engl. J. Med. 351, 1645–1654. doi: 10.1056/NEJMra040181
Keywords: antimicrobial peptide, biomaterial-associated infection, biofilm, antimicrobial resistance, implant, device manufacturing
Citation: Riool M, de Breij A, Drijfhout JW, Nibbering PH and Zaat SAJ (2017) Antimicrobial Peptides in Biomedical Device Manufacturing. Front. Chem. 5:63. doi: 10.3389/fchem.2017.00063
Received: 15 July 2017; Accepted: 11 August 2017;
Published: 24 August 2017.
Edited by:
Ralf Hoffmann, Leipzig University, GermanyReviewed by:
Mare Cudic, Florida Atlantic University, United StatesCopyright © 2017 Riool, de Breij, Drijfhout, Nibbering and Zaat. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sebastian A. J. Zaat, cy5hLnphYXRAYW1jLnV2YS5ubA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.