- 1Department of Mechanical and Mechatronics Engineering, Tshwane University Technology, Pretoria, South Africa
- 2Department of Mechanical Engineering, The Federal Polytechnic, Bida, Nigeria
In recent years, there has been an increasing focus on renewable and biodegradable energy sources among lubricant manufacturers due to the environmental impacts and limited availability of fossil-based engine oils. Biomass sources present a cost-effective and eco-friendly alternative to traditional mineral oil sources. This study aims to produce and characterize biodiesel and biolubricant from desert date seed oil through transesterification. The result of the study was compared with the properties of conventional and commercial lubricants. The study employed transesterification to convert desert date seed oil into biodiesel and biolubricant. The produced biolubricant and biodiesel were characterized to determine their kinematic viscosity at 40°C, specific gravity, flash point, and pour point. These properties were then compared with those of other bio-lubricants and commercial base lubricants. For biodiesel, the yield was 56%, with a favorable acid value (0.98 mg KOH/g), iodine value (43.41 mg/g), and saponification value (197.4 mg KOH/g). Although, the specific gravity (1.876) was higher than ASTM standard. However, the flash point (112°C) and cloud point (11°C) were within acceptable ranges. The biolubricant produced from desert date oil showed promising results with a high kinematic viscosity of 67.54 mm2/s, a specific gravity of 1.876, a flash point of 120°C and a pour point (−5°C). These results obviously suggest the produced lubricant a suitable for automotive applications possessing good low-temperature performance. The flash point result and the physicochemical properties of the oil aligned well with industrial standards. The comparisons revealed that the produced biolubricant closely matched the properties of SAE VG 220 and SAE VG 40. The findings suggest that the biolubricant and biodiesel derived from desert date seed oil can serve as a viable substitute for petroleum-based lubricants in light gear applications and can be effectively used in two-stroke engines, providing a sustainable alternative to conventional lubricants.
1 Introduction
The global demand for energy sources is increasing. Each year, approximately 30–40 million tons of lubricants are produced. These lubricants are mainly used in industrial applications to reduce friction and heat, protect against corrosion and wear, transmit energy, and eliminate contaminants or seal processes (Usman et al., 2023). Conventional lubricants, mainly derived from fossil fuels, have sparked controversy due to their environmental impact and the urgent need for cleaner, and renewable energy sources (Zhukovskiy et al., 2021). This has spurred the search for renewable, cleaner, and cheaper lubricant sources, leading to the development of biolubricants. Biolubricants are non-toxic, biodegradable lubricants produced from plant or animal oils and (Heikal et al., 2017), primarily derived from non-edible seeds.
Lubricants are crucial for engine and other metal surfaces as they sustain machine performance by reducing friction and wear (Masripan et al., 2020). In contrast, bio-lubricants are environmentally friendly and safe for health. They offer several benefits over conventional lubricants, including adherence to green chemistry principles, biodegradability, sustainability, and alignment with current legislation and consumer demands for eco-friendly products (Salimon et al., 2012). In the last century, societies have extensively depended on fossil fuels, leading to a slow depletion of these resources. It is predicted that these non-renewable energy sources will be exhausted in the medium term. This prediction prompts a need for greater efforts to find and develop alternative chemicals and energy sources to replace traditional fossil fuels (Cecilia et al., 2020). The search for alternative feedstocks and sustainable technologies has intensified due to the limitations of non-renewable energy sources. Vegetable oils, a renewable energy source, are used to produce bio-lubricants. These bio-lubricants are preferred over mineral oils because they offer higher lubricity, a higher flash point, lower volatility, a higher viscosity index, biodegradability, non-toxicity, cost-effectiveness, and sustainable origins compared to traditional mineral-based oils (Singh et al., 2017). Plant-based bio-lubricants have several advantages, including improved lubrication leading to reduced friction losses, lower volatility resulting in decreased exhaust emissions, and a higher viscosity index. In many African countries, oils are typically produced from groundnut, coconut, Moringa, castor, and jatropha seeds. However, there are other possible sources of vegetable oil that have not been fully utilized yet.
Previous research has shown that biolubricants can be produced from plant oils such as jatropha, rapeseed, sunflower, soybean, castor, and linseed (Usman et al., 2023). These biolubricants have many desirable qualities and minimal toxic effects on the environment. For example, a study by Bilal et al. (2013) found that Jatropha curcas biolubricant has viscosities of 66.74 and 14.28 cSt (cSt) at 40°C and 100°C, respectively, with minimal toxicity. (Bilal et al., 2013). Similarly, research on castor bean oil has shown that biolubricants possess qualities comparable to those of fossil-based lubricants. Recently, Aseibichin et al. (2024) optimized the esterification of Jathropha oil into fatty acid and methyl ester by using response surface methodology and the Taguchi orthogonal method (Aseibichin et al., 2024). It was observed from the study that optimizing the esterification of Jatropha oil is an innovative approach that enhances the yield and efficiency of the transesterification process. The method also boosts the overall production of fatty acid methyl ester from Jatropha oil. In addition, the method can efficiently generate biodiesel from renewable resources in an environmentally friendly manner while maximizing the effectiveness of the process parameters (Aseibichin et al., 2024).
Despite significant progress in alternative fuels, fossil fuels continue to negatively impact the environment, water bodies, atmosphere, plants, animals, and humans. For instance, fossil fuels release large amounts of greenhouse gases into the atmosphere. Lubricants can contaminate soils and groundwater, and accumulate in the tissues of plants, terrestrial, and aquatic animals. They have also been reported to be carcinogenic and harmful when they come into contact with human tissue (Pareek et al., 2020; Usman et al., 2023).
While fossil fuels remain the primary sustainable energy sources in the short and medium term, their availability is also limited (Folorunso et al., 2021; Folorunso et al., 2022; Folorunso et al., 2023). Therefore, this study carried out the production of biolubricants from Desert date seed oil extracted through transesterification methods. The significance of this is that desert date seed, from the Balanites Aegyptiaca plant, is a fruit that is rich in oil. Balanites aegyptiaca commonly called Desert date is an underutilized fruit-growing tree that is mostly found in African countries. It is distributed in tropical and subtropical regions of Africa, from Senegal in the west (16°W) to Somali in the East (49°E) and Jordan in the north (35°N) to Zimbabwe in the south (19°S) (Murthy et al., 2020). It is a semi-evergreen, usually spiny, extremely variable shrub or small tree that grows up to 12 m high, with many branches, and small flowers (Kabo et al., 2020). The plant thrive in various habitats, tropical and desert areas. Besides, the plant can grow in various soil types, from sandy to heavy clay, as well as different levels of climatic moisture (Elfeel, 2010). Balanites Aegyptiaca is a perennial plant commonly utilised in food preparations, paticularlly in developing and most Africa countries. Balanites aegyptiaca, commonly known as the desert date, is an arid land tree valued for its diverse products and uses, including food, fodder, shade, oil, traditional medicine, and potential for use in shelterbelts and agroforestry systems (Al-Thobaiti and Abu Zeid, 2018). The fruit of the Balanites tree is particularly important, as it is edible and its seeds contain approximately 40%–87% oil (Kabo et al., 2020). This seed oil is comparable in quality to sesame and groundnut oils, boasting a high percentage of fatty acids (Khadra et al., 2022). It is suitable for human consumption, can be converted into biodiesel, and has potential applications as a biofuel or lubricant (Gutti et al., 2012; Usman et al., 2022). Additionally, Balanites oil has medicinal uses, and the residual cake post-oil extraction serves as a valuable animal feed supplement (Morkaz et al., 2011). Various extraction methods yield different quantities of oil from Balanites seeds. For example, Aboje et al. (Aboje et al., 2023) reported an 82% yield using the Soxhlet extraction technique with n-hexane as the solvent. More recently, Wakawa and Akinyele (Wakawa and Akinyele, 2024) found that oil yields varied significantly (21.09% ± 1.04% to 43.95% ± 1.85%) across different locations. Physicochemical analysis by Muhammad et al. (Muhammad et al., 2023) indicated that the seed oil had an acid value of 0.75 ± 0.84 mg KOH/g, an iodine value of 96.44 ± 2.53 mg/g, a saponification value of 147.26 ± 3.13 KOH mg/g, and a peroxide value of 7.50 ± 0.09 meq/kg. Given its renewable nature and environmental benefits, Balanites seed oil is a promising resource for sustainable applications, this study focuses on producing Bio-diesel and Bio-lubricant through the transesterification process. The results is compared alongside with the standard value and the most commonly produced seed oils (Palm kernel seed oil and Jathropha seed oil) whose results are closely alligned with the ASTM standard.
2 Materials and methods
The materials used in this study comprise desert date seeds, potassium hydroxide catalyst, ethanol, iodine solution, ethylene glycol, hexane, and N-hexane.
2.1 Methods
2.1.1 Seed preparation and oil extraction
The Desert date seeds utilised in this study were obtained from a local market in Bida, Niger State, Nigeria. The fruits were initially screened to remove any defective ones and then soaked in a large bowl of water for 3 days to remove the glycoside pulp from the seed coat through washing and filtration. After collection, the seeds were dried in the Sun for 7 days. The shells were cracked using a mechanical method (a metal hammer) and then sun-dried for an additional 2 days to facilitate easier oil extraction. The dried seeds were ground to the desired size using a Thomas Wiley Mill grinding machine and further dried in an oven to reduce moisture content. Oil extraction from the milled seeds was carried out with a Soxhlet extractor connected with a round-bottomed flask and a reflux condenser. The extraction process lasted for 4 hours with 150 mL of refluxing n-hexane at 65°C. After extraction, the solvent was evaporated, and the extracted oil was filtered and degummed (Kabo et al., 2020). The yield of the extracted oil was calculated according to the available method in (Ulakpa et al., 2022), and Eq. 1. Figure 1 shows the raw Date seed, washed, and cracked.
2.1.2 Biodiesel production
The extracted crude oil from Desert date seed as shown in Figure 2, went through transesterification to minimize its free fatty acid (FFA) content, using sulfuric acid as a catalyst. 100 g of the oil was measured and transferred into a 2-L, three-necked round-bottom flask. A separate mixture of 20% w/w methanol and 5% w/w sulfuric acid was also prepared. Both the methanol-acid mixture and the oil were then heated in a water bath to 60°C before being combined in the flask. The mixture was mechanically stirred at 800 rpm for 1 h and 20 min at 60°C (Bilal et al., 2013).
2.1.3 Biolubricant production
The bio-lubricant was synthesized using another transesterification (second transesterification similar to the first one) process. Methyl ester (produced biodiesel) was transesterified with 15 mL of ethylene glycol in 50 mL batches, using sodium methoxide as a catalyst (prepared by dissolving fresh clean sodium in 30% methanol). The oil to methanol weight ratio was found to be 3.5:1, with the catalyst amounting to 0.8% w/w of the total reactants. The reaction was conducted at 100°C for one and a half hours, as explained in previous studies (Bilal et al., 2013; Usman et al., 2023).
2.2 Characterization
The physicochemical properties of the extracted desert date seed oil, biodiesel, and bio-lubricant conducted, include: density, viscosity at 40°C, acid value, specific gravity, flash point, pour point, refractive index, saponification value, iodine value, and moisture content. These were carried out using the procedure outlined by ASTM D287, ASTM D445, ASTM D93, and ASTM D97 respectively.
2.2.1 Density
A 50 cm3 of the oil sample was then poured into empty beaker which was weighed before and after pouring the sample. From the sample weight obtained, the density was determined by taking the ratio of the weight of the oil to the known volume (50 cm3) according to Eq. (2).
2.2.2 Moisture content
The moisture content of the extracted oil was determined by heating 5 g of the sample placed in a clean dish, which had been previously oven dried, at 105°C for about 24 h in a thermosetting oven. The weights of the dish with its content were measured before and after heating and recorded. The percentage moisture content of the oil was calculated using Eq. (3) (MercyAkaagerger et al., 2016).
2.2.3 Viscosity
The viscosity of the samples were measured at temperatures of 40°C, using viscometer. For consistency and comparability, 40°C was used to measure the temperatures. It is also a standardized practice that ensures practical relevance of the study. The kinematic viscosity measurement of the samples were carried out in accordance with ASTM D445 using Redwood viscometer (Oluwaniyi et al., 2023). The viscometer was washed and calibrated with distilled water and the calibration constant of the viscometer was recorded. About 50 cm³ of the oil sample was placed into a clean, dry viscosity tube and adjusted (sucked) to a point in the capillary arm about 5 mm ahead of the first timing mark. The oil was allowed to flow freely. The time taken for the meniscus to move from the first to the next was recorded (Indhumathi et al., 2014). Kinematic viscosity (ν) was then calculated using the appropriate Eq. (4) stated below.
where V represents kinematic viscosity (mm2/s), t is the time in seconds for the 50 cm³ sample to flow, and C is the viscosity tube constant (0.09757), as adapted from Dallatu et al. (2017) (Dallatu et al., 2017).
2.2.4 Specific gravity
Density bottle was used to determine the specific gravity of the oil samples by hydrometer method (Dallatu et al., 2017). A clean 50 mL specific gravity bottle was weighed while empty (W0), when filled completely with water (W1), and then when filled with oils (W2). S.G. of the oils were calculated using Eq. (5) as shown below.
where W0 is the Weight of empty specific gravity bottle W1 is Weight of the water + the specific gravity bottle and W2 is the Weight of the test sample + specific gravity bottle.
2.2.5 Saponification value
The saponification values were determined by dissolving 1 g of the oil samples into 12.50 mL of 0.5 M ethanolic potassium hydroxide. The mixture was refluxed until oil droplets disappear and was left to cool to room temperature. The solution was then titrated with 0.5 M HCl using phenolphthalein indicator until the pink colour disappears. A blank titration was similarly carried out under the same conditions (Ouattara et al., 2015). The Saponification value was then calculated using Eq. (6).
where a = sample titre value, b = blank titre value, M = molarity of the HCl and 56.1 = molecular weight of ethanolic potassium hydroxide.
2.2.6 Iodine value
To determine the iodine value, 2.0 g of the crude oil was measured into a 100 mL conical flask and 5 mL Dam’s iodine was added. The flask was corked and placed in a dark cupboard for 5 min 5 mL of 10% KI was added followed by 20 mL of distilled water. The solution was titrated with 6.6% sodium thiosulphate in the presence of 1 mL of 1% starch indicator until the blue colour turned colourless (Manji et al., 2013). The iodine value was calculated using Eq. (7).
2.2.7 Free fatty acid (FFA)
The free fatty acid (FFA) content of the oil was determined by using 1 g of the oil sample placed in a 250 mL conical flask and warmed. 2.5 mL of methanol was then added with thorough stirring, followed by two (2) drops of phenolphthalein indicator and a drop of 0.14Npotassium hydroxide solution. The content containing the oil sample was then titrated against 0.14N potassium hydroxide solution while shaking vigorously until a permanent light pink colour, which persisted for 1 min, was observed (MercyAkaagerger et al., 2016). The end point was recorded, and the FFA value was calculated using Eq. (8).
2.2.8 Acid value
The acid value of the samples were determined by mixing the samples with equal volume (25 mL) of ethanol and four drops of phenolphthalein indicator in a flask that contained 2 g of the oils. The mixture were titrated against 0.1M KOH solution with proper shaking till a pink colour which persisted for 15 s was observed as shown in Eq. (9) (Ali and Tay, 2013).
where S = volume of standard base in mL, B = normality of the standard base.
2.2.9 Refractive index
Abbe’s refractometer was used to determine the refractive index of the oil samples by transferring a few drops of the sample into the glass slide of the refractometer. Water at 30°C was circulated round the glass slide to keep its temperature uniform. Through the space of the refractometer, the dark portion viewed was adjusted to be in line with the intersection of the cross. At no parallax error, the pointer on the scale pointed to the refractive index. The refractometer will be calibrated using distilled water here the refractive index of water at that temperature was then obtained. The procedure was repeated for triplicate samples and their refractive indices were recorded at 30°C and the mean valve for each sample were calculated as the refractive index (Dallatu et al., 2017).
2.2.10 Cloud point
Cloud point of the oil sample will be determined according to ASTM D 2500 as reported by Indhumathi et al., 2014. A test jar containing a small sample of the prepared sample was placed in a cooling bath while monitoring the temperature at the bottom of the test jar. The temperature at which the sample begins to form cloud was noted and recorded.
2.2.11 Pour point
The pour point of the oil samples were determined by filling a cylindrical test tube with the oil sample to a 5 mL level and held with a wooden clamp bearing a thermometer. The sample was then allowed to cool below 0°C in ice bath after which it was removed, tilted on the clamp and observed at some intervals. The lowest temperature at which the oil was observed to flow was recorded as its pour point (MercyAkaagerger et al., 2016).
2.2.12 Flash point
The flash point of the sample oils were determined by 150 mL conical flask filled to a 6 mL level with the sample oils and heated at slow constant rate on a hot plate. The lowest temperature when the application of a test flame caused the vapour above the sample to be ignited was then taken as the flash point. This is an improved method reported by (MercyAkaagerger et al., 2016).
2.3 Fourier transform infrared spectrometry (FTIR)
The functional groups present in the bio-lubricant was analyzed using Fourier Transform Infrared Spectrometry (FTIR). Analysis of FTIR spectra at wave numbers (ranging between 350 and 4000 cm−1) of the bio-oil provides a quick and simple qualitative technique that uses the standard IRspectra to identify the functional group(s) of the components of the bio-lubricant.
3 Results and discussion
3.1 Characterization of the desert date seed oil
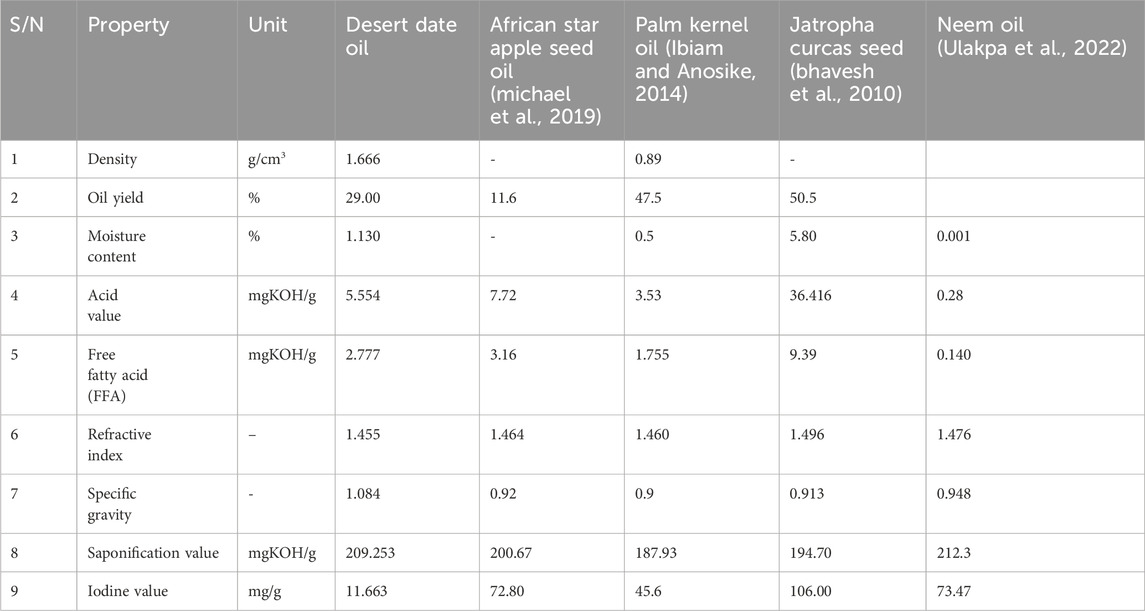
The results from the analysis of the characteristic parameters of the extracted oil are presented in Table 1, along with a comparison to the reported values.
3.1.1 Oil yield
Figure 2 Shows the extracted seed oil, produced biodiesel and produced biolubricant. The result for the percentage of oil content of Desert date seed (29%) falls below the value found in Palm kernel oil, and Jatropha curcas seed (50.5%) (Nayak and Patel, 2010), but higher than the valve found in African star apple seed oil (11.6%) (Anang et al., 2019). The extraction method and fruit species are crucial factors influencing the percentage yield and quality of the oil. The oil content and its composition are key determinants of an oil source’s viability. In this study, the oil content of all oil-bearing seeds was appreciable and showed significant variations.
3.1.2 Moisture content
The result from the percentage moisture content for desert date oil extracted is (1.130%) which is slightly higher than the value found in Palm Kernel Oil (0.5%) (Ibiam and Anosike, 2014), and lesser than the reported value for Jatropha curcas oil (5.80%) (Nayak and Patel, 2010). The low moisture content could be attributed to the proper treatment and processing of the oil samples.
3.1.3 Acid value
The acid value obtained for the desert date seed oil was 5.554 which fall below the value of African star apple seed oil (7.72 mgKOH/g) (Anang et al., 2019) and Jatropha curcas Seed oil (36.416 mg/KOH/g) (Nayak and Patel, 2010) and higher than the Palm Kernel Oil (3.53 mgKOH/g). This result indicates that Jatropha curcas seed oil is more acidic compared to desert date seed oil and African star apple seed oil. The higher acidity suggests a greater free fatty acid content in the oil.
3.1.4 Free fatty acid
The free fatty acid content is an important indicator of oil quality. Lower free acid content makes the oil more desirable for consumption. Edible oils are recommended to have free fatty acid values below 5% (Maliki et al., 2020). Free fatty acid values for the desert date seed oil recorded about 2.777 mgKOH/g and was found to be below the value of Jatropha curcas Seed oil (9.39 mg/koH/g) (Nayak and Patel, 2010) and African star apple seed oil (3.16 mgKOH/g) (Anang et al., 2019) and slightly higher than the value of Palm Kernel Oil (1.755 mgKOH/g). However, the free fatty acids values for Palm Kernel Oil and African star apple seed oil being lower than 5% makes them desirable as edible oils.
3.1.5 The refractive index
The refractive index (1.455) was found to be in same range as compared to African star apple seed oil (1.460) (Anang et al., 2019), Palm kernel oil (1.464), and also Jatropha curcas seed oil (1.496) (Nayak and Patel, 2010). The specific gravity (1.084) was found to be higher compared to that of African star apple oil (0.92) (Anang et al., 2019), Palm kernel oil (0.9) and Jatropha curcas Seed (0.913) (Nayak and Patel, 2010).
3.1.6 Saponification value
This represents the ratio of alkalis required to saponify a specific mass of oil. The saponification value obtained from the study was (209.253 mgKOH/g) and this is higher when compare with saponification value African star apple seed oil (200.67 mgKOH/g) (Anang et al., 2019), Palm kernel oil (187.93 mgKOH/g), and Jatropha curcas seed (194.70 mgKOH/g) (Nayak and Patel, 2010) found in literature and the high saponification values obtained suggest that the oils could be good for soap making.
3.1.7 Iodine value
The experimental analysis for the desert date seed shows that the iodine value was 11.663 mg/g and it falls below the values reported for African star apple seed oil (72.80 mg/g) (Anang et al., 2019), Palm Kernel Oil (45.6 mg/g) and Jatropha curcas Seed (106.00 mg/g) (Nayak and Patel, 2010). This low value indicates the level of unsaturation present in the oil. A lower iodine value contributes to the oil’s stability against oxidation. Understanding the iodine value helps assess the oil’s combustion temperature (Roger et al., 2010).
3.2 Physicochemical characterization of biodiesel
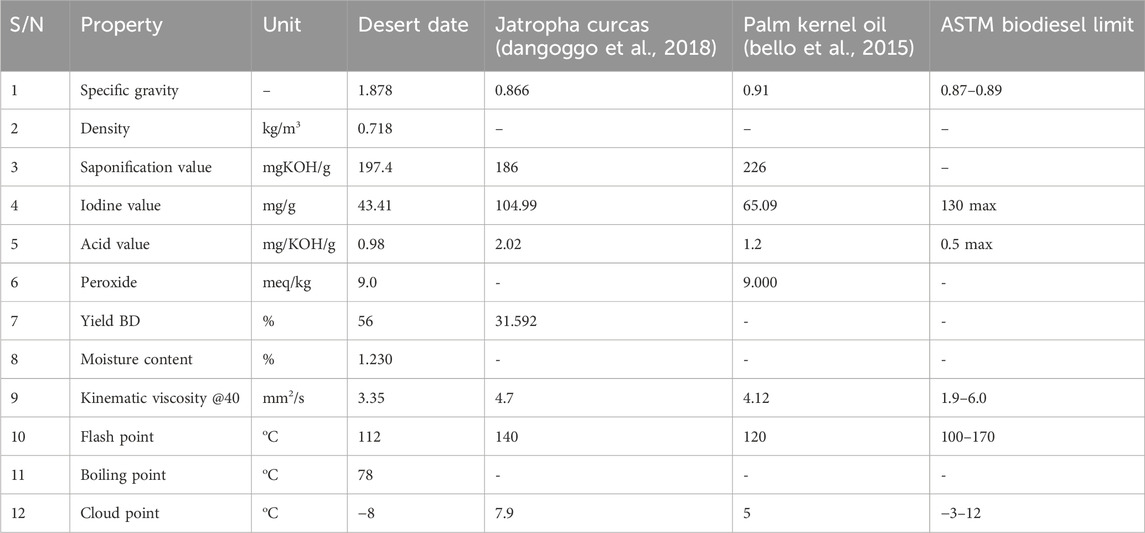
The result of the physicochemical characteristics of the biodiesel produced from desert date oil is presented in Table 2. Comparisons were made with ASTM and values of vegetable oils biodiesel reported literature.
3.2.1 Percentage biodiesel yield
The biodiesel yield obtained under the optimal transesterification conditions was determined to be 56%, slightly surpassing the 31.592% reported for Jatropha biodiesel by Dangoggo et al., in 2018. The differences in the optimal biodiesel yields could be attributed to variations in catalyst preparation methods, catalyst amounts, reaction temperatures, and oil to methanol ratios.
3.2.2 Acid value
The acid value of biodiesel indicates the presence of free fatty acids in the fuel. The biodiesel produced exhibited an acid value of 0.980 mgKOH/g, as depicted in Table 4.2, which is lower than the values reported for Jatropha biodiesel (2.02 mg/KOH/g) by Dangoggo et al., in 2018, and for palm kernel oil (1.2 mg/KOH/g) by Bello et al., in 2015. However, both the obtained value and those reported in the literature exceed the maximum ASTM standards of 0.5. To mitigate this, neutralizing the oil before use is recommended to align with engine specifications, thereby minimizing the risk of corrosion and wear in fuel systems and storage tanks.
3.2.3 Iodine value
The iodine value of biodiesel produced was 43.410 mg/g as can be seen in Table 3.2, it was found to be lower than the value of 104.99 mg/g for Jatropha biodiesel obtained by Dangoggo, Dhikrah, Sani, Baki, Bagudo and Jibrin (Dangoggo et al., 2018) and (65.09 mg/g) for palm kernel oil obtained by (Bello et al., 2015). Which falls within the range specified by the maximum ASTM standards of 130.
3.2.4 Saponification value
The saponification value obtained for desert date seed oil is 197.4 mg/KOH/g which was higher than the value (186 mgKOH/g) for Jatropha biodiesel obtained by Dangoggo, Dhikrah, Sani, Baki, Bagudo and Jibrin (Dangoggo et al., 2018) and lower than the value (226 mgKOH/g) for palm kernel oil obtained by Bello, Oguntuase, Osasona and Mohammed (Bello et al., 2015). The saponification value implies that the biodiesel possesses a higher molecular weight, which could account for its elevated specific gravity of 1.876. This elevated saponification value suggests a greater concentration of methyl esters, as the ester value is derived from the difference between the saponification value and the acid value.
3.2.5 Flash point
The biodiesel obtained exhibited a flash point of 112°C, as indicated in Table 3.2. This value is lower compared to the 140°C reported for Jatropha biodiesel by Dangoggo et al., in 2018, and the 120°C reported for palm kernel oil by Bello et al., in 2015. However, the obtained result falls within the range specified by the 170 ASTM standards. Despite not directly influencing engine performance, the flash point is inversely related to fuel volatility (Ramírez-Verduzco et al., 2012). The biodiesel’s flash point suggests lower volatility, implying safer handling procedures.
3.2.6 Cloud point
Cloud point of the produced biodiesel is 11°C, a lower result (7.9°C) for Jatropha biodiesel obtained by Dangoggo et al., 2018 and (5°C) for palm kernel oil obtained by Bello et al., 2015. Both values fall within the (−3–12) ASTM standards range for biodiesel. The variation may be attributed to differences in weather and climatic conditions.
3.2.7 Specific gravity
The specific gravity of the biodiesel under optimal yield conditions was recorded as 1.876. A lower result (0.866) was obtained for Jatropha biodiesel and (0.9) for palm kernel oil (Dangoggo et al., 2018; Bello et al., 2015). The result obtained for desert date biodiesel exceeds the ASTM standards range of 0.95 maximum. A higher specific gravity in fuel leads to increased mass injected into the engine, resulting in greater power (Rao et al., 2010).
3.2.8 Kinematic viscosity
The kinematic viscosity of the biodiesel obtained from desert date seed oil was recorded at 3.35 mm2/s. This is lower than results obtained by Dangoggo et al., 2018; Bello et al., 2015 (4.7 mm2/s; 4.12 mm2/s). The value obtained for the desert date biodiesel was found to be within the ASTM standard range.
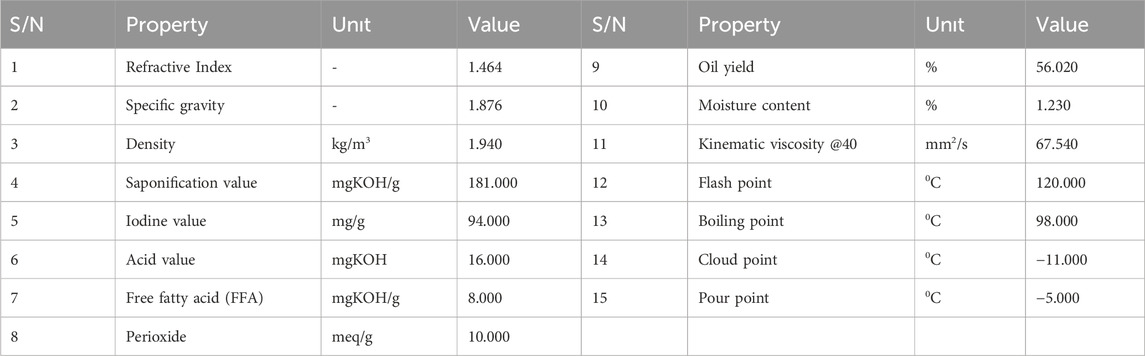
3.3 Physicochemical characterization of bio-lubricant
The physiochemical analysis of the bio lubricant produce carried is presented in Table 3. The highest value saponification of 181.000 mgKOH/g was recorded, while the lowest value of pour point obtained was–5°C. The following parameter were also obtained refractive index 1.464, specific gravity 1.876, density 1.940, iodine value 94.00, acid value 16 mg/g, free fatty acid 8.0mgKOH/g, perioxide value 10Meq/g, % oil yield 56.02, moisture content 1.23%, kinematic viscosity @40°C 67.54 CSt, flash point 120°C, boiling point 98°C, cloud point −11°C.
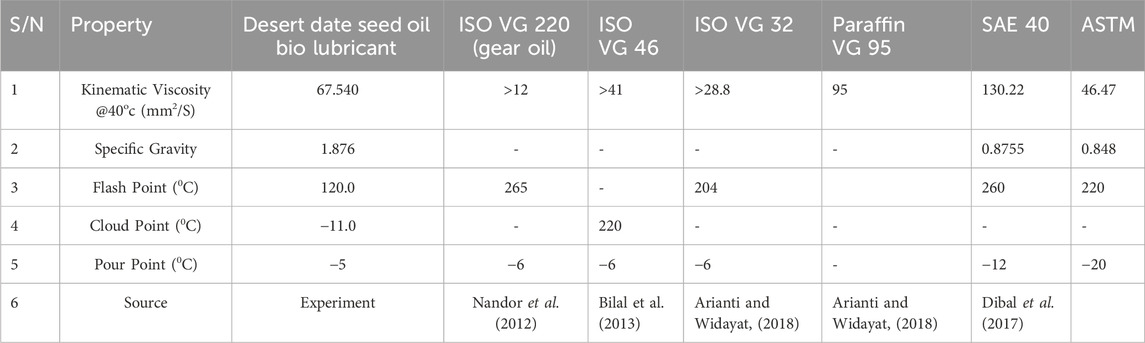
Kinematic viscosity: The produced bio-lubricant recorded higher viscosity value of 67.540 mm2/s as compared to value for the jatropha bio lubricant (35.4 mm2/s) and lower viscosity when compared with the SAE 40. The obtained viscosity value for the biolubricant meets the requirements for ISO viscosity grades and falls within the specified range (Nemestóthy et al., 2012; Bilal et al., 2013). Variations observed may stem from varied fatty acid composition of the base oil or variations in process conditions/catalytic modification techniques utilized. These findings suggest that desert date polyol ester (biolubricant) holds potential for application as a gear oil in automobiles, as its properties align with the requirements of ISO VG 46 and ISO VG 220 for this type of lubricant. Among the three standard specifications for lubricants, ISO Viscosity Grade (VG) 46 is predominant, representing over 80% of all lubricants consumed (Bilal et al., 2013).
The pour point and the flash point for the bio lubricants and mineral base lubricant also varies as presented in Table 4. The pour point of a biolubricant is a crucial characteristic that indicates its suitability for use at low temperatures. It represents the minimum temperature at which the oil flows when its container is tilted for a specified duration. This property is among the most significant factors influencing the performance of lubricants. The desert date derived bio lubricant has a pour point of −5°C which is appreciable and is higher than the value (−18°C) for the castor bio lubricant and also that of the value (−12°C) for SAE40, but found to be in close proximity to the ISO viscosity requirement (−6°C) for (Nemestóthy et al., 2012; Bilal et al., 2013). The reported pour point value for the synthesized biolubricant demonstrates its exceptional performance at low temperatures.
The flash point is the minimum temperature at which a lubricant emits sufficient vapor to create a flammable mixture with air. It serves as an indicator of the hazardous nature of a lubricant. The analysis results in Table 4 indicate that the flash point of the biolubricant produced is 120°C, which is lower in comparison to other values reported (Nemestóthy et al., 2012; Bilal et al., 2013).
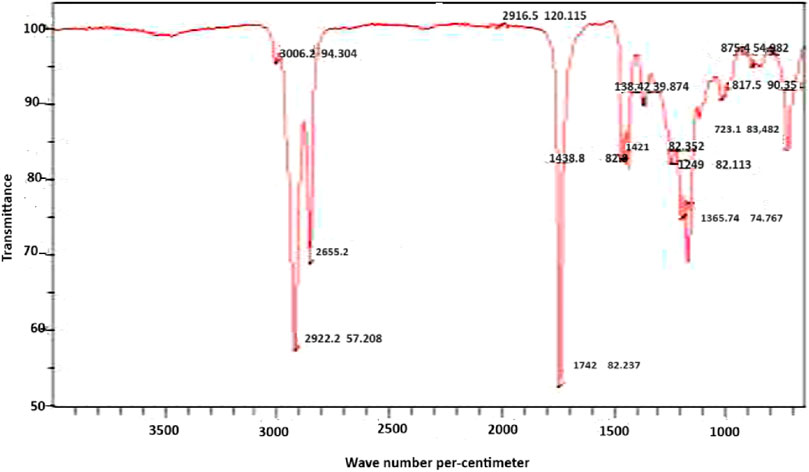
3.4 Fourier transform infrared spectrometry (FTIR)
Figure 3 displays the Fourier Transform Infrared Spectrum of the Lubricant, revealing specific wavelengths associated with various molecular vibrations. These include 3,006.0 cm−3for C-H stretching, 2,810.5 cm−1 for -OH (acid), 1,635.4 cm−1 for C=O, 1,370.8 cm−1 for C=C stretching, 1,124.9 cm−1 for C-O, 1,127.4 cm−1 for C-C, and 632.5 cm−1 or C-H bending. Notably, these wavelengths align closely with those observed in biolubricants produced from palm kernel oil and castor oil seed, as documented by previous studies (Musa et al., 2015; Anang et al., 2019; Usman et al., 2023).
4 Conclusion
This study successfully extracted and produced biodiesel and bio-lubricant from desert date seed oil through transesterification. The produced bio-lubricant and biodiesel were characterized by determining their kinematic viscosity at 40°C, specific gravity, flash point, and pour point. These properties were then compared with those of other bio-lubricants and commercial base lubricants. For biodiesel, the yield was 56%, with favorable acid value (0.98 mg KOH/g), iodine value (43.41 mg/g), and saponification value (197.4 mg KOH/g). Although the specific gravity (1.876) was higher than the ASTM standard, the flash point (112°C) and cloud point (11°C) were within acceptable ranges. The bio-lubricant produced from desert date oil showed promising results with a high kinematic viscosity of 67.54 mm2/s, a specific gravity of 1.876, a flash point of 120°C, and a pour point of −5°C. These results suggest the produced lubricant is suitable for automotive applications, possessing good low-temperature performance. The flash point result and the physicochemical properties of the oil aligned well with industrial standards. Comparisons revealed that the produced bio-lubricant closely matched the properties of SAE VG 220 and SAE VG 40.
The findings indicate that the bio-lubricant and biodiesel derived from desert date seed oil can serve as viable substitutes for petroleum-based lubricants in light gear applications and can be effectively used in two-stroke engines, providing a sustainable alternative to conventional lubricants. The derived products also exhibit significant potential for various industrial applications, particularly due to their favorable physicochemical properties and stability. Future research can explore the optimization of extraction and transesterification processes to enhance oil and biodiesel yields. Additionally, the potential of desert date seed oil in other applications, such as bio-polymers, could be investigated given its favorable physicochemical properties. Comparative analysis of the long-term stability and performance of desert date seed oil biodiesel in various engine types is also recommended.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
MA: Conceptualization, Data curation, Methodology, Project administration, Supervision, Validation, Writing–original draft, Writing–review and editing. JT: Formal Analysis, Funding acquisition, Project administration, Resources, Supervision, Writing–original draft, Writing–review and editing. AA: Data curation, Formal Analysis, Funding acquisition, Software, Supervision, Visualization, Writing–original draft, Writing–review and editing. AM: Conceptualization, Investigation, Methodology, Resources, Visualization, Writing–original draft, Writing–review and editing. IS: Data curation, Formal Analysis, Software, Validation, Visualization, Writing–original draft, Writing–review and editing. BA: Data curation, Formal Analysis, Validation, Visualization, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The authors acknowledge the financial support from Tshwane University of Technology (TUT), Pretoria, South Africa, without which this work would not have been published.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aboje, A., Ohile, S., Uthman, H., Olutoye, M., and Nwachukwu, C. (2023). Optimization and characterization of biodiesel production from Desert Date seed oil (Balanites aegyptiaca) via transesterification reaction. J. Energy Technol. Environ. 5 (2). doi:10.5281/zenodo.8025638
Ali, E. N., and Tay, C. I. (2013). Characterization of biodiesel produced from palm oil via base catalyzed transesterification. Procedia Eng. 53, 7–12. doi:10.1016/j.proeng.2013.02.002
Al-Thobaiti, S. A., and Abu Zeid, I. (2018). Medicinal properties of desert date plants (Balanites aegyptiaca)-an overview. Glob. J. Pharmacol. 12 (1), 01–12. doi:10.5829/idosi.gjp.2018.01.12
Anang, M. A., Oteng-Peprah, M., and Opoku-Boadu, K. (2019). Extraction and characterisation of african star apple (Chrysophyllum albidum) seed oil and the adsorptive properties of the fruit shell in Ghana. Int. J. food Sci. 2019, 1–8. doi:10.1155/2019/4959586
Aseibichin, C., Ulakpa, W. C., Omenogor, I., Doyah, E., Olaseinde, A. A., Anakpoha, O. C., et al. (2024). Modeling and optimization of transesterification of Jatropha oil to fatty acid methyl ester: application of response surface methodology (CCD) and Taguchi orthogonal method. RSC Adv. 14 (17), 11784–11796. doi:10.1039/d4ra01149j
Bello, E., Oguntuase, B., Osasona, A., and Mohammed, T. (2015). Characterization and engine testing of palm kernel oil biodiesel. Eur. J. Eng. Technol. 3 (3).
Bilal, S., Mohammed-Dabo, I., Nuhu, M., Kasim, S., Almustapha, I., and Yamusa, Y. (2013). Production of biolubricant from Jatropha curcas seed oil. J. Chem. Eng. Mater. Sci. 4 (6), 72–79. doi:10.5897/jcems2013.0164
Cecilia, J. A., Ballesteros Plata, D., Alves Saboya, R. M., Tavares de Luna, F. M., Cavalcante, Jr C. L., and Rodríguez-Castellón, E. (2020). An overview of the biolubricant production process: challenges and future perspectives. Processes 8 (3), 257. doi:10.3390/pr8030257
Dallatu, Y., Agbaji, E., and Ajibola, V. (2017). The influence of physicochemical characteristics of a non-edible oil of yellow oleander seed on its fuel properties. Bayero J. Pure Appl. Sci. 10 (2), 283–291. doi:10.4314/bajopas.v10i2.46
Dangoggo, S., Dhikrah, I., Sani, N., Baki, A., Bagudo, B., and Jibrin, M. (2018). Preparation and characterization of biodiesel produced from Jatropha seed oil using sulphated zirconia as catalyst. Ind. Chem. 4 (1), 2–5. doi:10.4172/2469-9764.1000126
Elfeel, A. A. (2010). Variability in Balanites aegyptiaca var. aegyptiaca seed kernel oil, protein and minerals contents between and within locations. Agric. Biol. J. N. Am. 1 (2), 170–174.
Folorunso, O., Kumar, N., Hamam, Y., Sadiku, R., and Ray, S. S. (2021). Recent progress on 2D metal carbide/nitride (MXene) nanocomposites for lithium-based batteries. FlatChem 29, 100281. doi:10.1016/j.flatc.2021.100281
Folorunso, O., Olukanmi, P. O., and Shongwe, T. (2023). Progress towards sustainable energy storage: a concise review. Eng. Rep. 5 (11), e12731. doi:10.1002/eng2.12731
Folorunso, O., Sadiku, R., Hamam, Y., and Ray, S. S. (2022). An investigation of copper oxide-loaded reduced graphene oxide nanocomposite for energy storage applications. Appl. Phys. A 128 (1), 54. doi:10.1007/s00339-021-05205-1
Gutti, B., Kiman, S., and Murtala, A. M. (2012). Solar dryer-an effective tool for agricultural products preservation. J. Appl. Technol. Environ. Sanitation 2 (1).
Heikal, E. K., Elmelawy, M., Khalil, S. A., and Elbasuny, N. (2017). Manufacturing of environment friendly biolubricants from vegetable oils. Egypt. J. Petroleum 26 (1), 53–59. doi:10.1016/j.ejpe.2016.03.003
Ibiam, J. A., and Anosike, P. O. (2014). Extraction and characterization of palm kernel oil from the kernel of palm tree (Elaeis guineensis). Int. J. Curr. Res. 6 (05), 6696–6698.
Indhumathi, P., Syed Shabudeen, P. S., and Shoba, U. S. (2014). A method for production and characterization of biodiesel from green micro algae. Int. J. Bio Sci. Bio Technol. 6 (5), 111–122. doi:10.14257/ijbsbt.2014.6.5.11
Kabo, K. S., Ali, T., and Ogbesejana, A. B. (2020). Extraction and physico-chemical parameter analysis of desert date (Balanite aegyptiaca) oil from Dutsin-Ma. FUDMA J. Sci. 4 (2), 409–413. doi:10.33003/fjs-2020-0402-225
Khadra, B., Ahmed, M., Somia, B., Ahmed, B., and Nassima, F. (2022). Physico-chemical properties of Balanites aegyptiaca’s seeds and seed oil from southern Algeria. Egypt. J. Chem. 65 (10), 39–45. doi:10.21608/ejchem.2022.98766.4596
Maliki, M., Ikhuoria, E., and Ifijen, I. (2020). Extraction and physiochemical characterization of oils obtained from selected under-utilized oil bearing seeds in Nigeria. ChemSearch J. 11 (1), 110–117.
Manji, A., Sarah, E., and Modibbo, U. (2013). Studies on the potentials of Balanites aegyptiaca seed oil as raw material for the production of liquid cleansing agents. Int. J. Phys. Sci. 8 (33), 1655–1660. doi:10.5897/IJPS07.049
Masripan, N. A., Salim, M. A., Omar, G., Mansor, M. R., Saad, A. M., Hamid, N. A., et al. (2020). Vegetable oil as bio-lubricant and natural additive in lubrication: a review. Int. J. Nanoelectron. Mater. 13.
MercyAkaagerger, S., Giwa, S. O., Ibrahim, M., and Giwa, A. (2016). Production of biodiesel from desert date seed oil. Int. J. ChemTech Res. 9, 453–463.
Morkaz, M., Elamin, K., Ahmed, S., and Omer, S. (2011). Effects of feeding different levels of Balanites aegyptiaca (HEGLIG) kernel cake on cattle rumen environment.
Muhammad, H. S., Agada, R., Ogaji, I. J., and Ngwuluka, N. C. (2023). Physicochemical characterization and fatty acids composition of four indigenous plant oils. Sci. Afr. 20, e01669. doi:10.1016/j.sciaf.2023.e01669
Murthy, H. N., Yadav, G. G., Dewir, Y. H., and Ibrahim, A. (2020). Phytochemicals and biological activity of desert date (Balanites aegyptiaca (L.) Delile). Plants 10 (1), 32. doi:10.3390/plants10010032
Musa, U., Isah, A. G., Mohammed, I., Garba, M., Usman, Z., and Alhassan, B. (2015). Extraction of Chrysophyllum albidum seed oil: optimization and characterization.
Nayak, B., and Patel, K. (2010). Physicochemical characterization of seed and seed oil of Jatropha curcas L. collected from Bardoli (South Gujarat). Sains Malays. 39 (6), 951–955.
Nemestóthy, N., Bányai, T., Bélafi-Bakó, K., Bartha, L., and Gubicza, L. (2012). Biotechnological utilisation of fusel oil for biolubricant production. Food Industrial Processes-Methods Equip. IntechOpen.
Oluwaniyi, O. O., Oloruntele, I. O., Olaniyi, O. B., Sekoni, H. A., and Hamza, M. I., "Production and characterization of biodiesel from prunus amygdalus “dulcis” seed oil," Int. J. Sci. Res. Chem. Sci. Vol, vol. 10, no. 4, 2023.
Ouattara, C. A. T., Somda, M. K., Moyen, R., and Traore, A. S. (2015). Comparative physico-chemical and proximate analysis of oils of Shea nut, Sesamum indicum, Cucurbita pepo, Cucumis melo seeds commonly cultivated in West Africa. Afr. J. Biotechnol. 14 (31), 2449–2454. doi:10.5897/ajb2015.14642
Pareek, A., Dom, R., Gupta, J., Chandran, J., Adepu, V., and Borse, P. H. (2020). Insights into renewable hydrogen energy: recent advances and prospects. Mater. Sci. Energy Technol. 3, 319–327. doi:10.1016/j.mset.2019.12.002
Ramírez-Verduzco, L. F., Rodríguez-Rodríguez, J. E., and del Rayo Jaramillo-Jacob, A. (2012). Predicting cetane number, kinematic viscosity, density and higher heating value of biodiesel from its fatty acid methyl ester composition. Fuel 91 (1), 102–111. doi:10.1016/j.fuel.2011.06.070
Rao, G. L. N., Ramadhas, A., Nallusamy, N., and Sakthivel, P. (2010). Relationships among the physical properties of biodiesel and engine fuel system design requirement. Int. J. energy Environ. 1 (5), 919–926.
Roger, A., Rebecca, R., Georges, A., and Mathias, I. (2010). Chemical characterization of oil form Germinated nuts of several coconut cultivars (Cocos nuciferh L.). Eur. J. Sci. Res. 391 (4), 514–522.
Salimon, J., Salih, N., and Yousif, E. (2012). Industrial development and applications of plant oils and their biobased oleochemicals. Arabian J. Chem. 5 (2), 135–145. doi:10.1016/j.arabjc.2010.08.007
Singh, Y., Farooq, A., Raza, A., Mahmood, M. A., and Jain, S. (2017). Sustainability of a non-edible vegetable oil based bio-lubricant for automotive applications: a review. Process Saf. Environ. Prot. 111, 701–713. doi:10.1016/j.psep.2017.08.041
Ulakpa, W. C., Ulakpa, R. O., Egwunyenga, M. C., and Egbosiuba, T. C. (2022). Transesterification of non-edible oil and effects of process parameters on biodiesel yield. Clean. Waste Syst. 3, 100047. doi:10.1016/j.clwas.2022.100047
Usman, M., Ibrahim, H., and Agbaji, E. (2022). Extraction, characterization and determination of physicochemical properties of biodiesel obtained from Desert Date (Balanites aegyptiaca) seed oil. J. Appl. Sci. Environ. Manag. 26 (12), 2045–2051. doi:10.4314/jasem.v26i12.19
Usman, M., Ibrahim, H., and Agbaji, E. (2023). Production of biolubricants from Balanites aegyptiaca seed oil via epoxidation and double transesterification techniques. J. Appl. Sci. Environ. Manag. 27 (1), 79–86. doi:10.4314/jasem.v27i1.12
Wakawa, L. D., and Akinyele, A. O. (2024). Natural variability in yield and properties of Balanites aegyptiaca (L.) Delile kernel oil from different locations in Nigeria. J. Bioresour. Environ. Sci. 3 (2), 61–69. doi:10.61435/jbes.2024.19921
Keywords: biodiesel, biolubricant, desert date seed, transesterification, characterization
Citation: Adeoti MO, Jamiru T, Adegbola TA, Abdullahi M, Sulaiman I and Aramide BP (2024) Comparative study on lubrication properties of biodiesel and bio-lubricant trans-esterified from desert seed oil with conventional lubricants. Front. Chem. Eng. 6:1451187. doi: 10.3389/fceng.2024.1451187
Received: 18 June 2024; Accepted: 25 July 2024;
Published: 14 August 2024.
Edited by:
Congrui Grace Jin, University of Nebraska-Lincoln, United StatesReviewed by:
Titus Egbosiuba, Texas A and M University System, United StatesGokul Raghavendra Srinivasan, Steamax Envirocare Private Limited, India
Copyright © 2024 Adeoti, Jamiru, Adegbola, Abdullahi, Sulaiman and Aramide. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: M. O. Adeoti, bHltYXRoMjAxNUBnbWFpbC5jb20=, QWRlb3RpTU9AdHV0LmFjLnph
 M. O. Adeoti
M. O. Adeoti T. Jamiru
T. Jamiru T. A. Adegbola1
T. A. Adegbola1