- 1Faculty of Geosciences and Environmental Engineering, Southwest Jiaotong University, Chengdu, China
- 2Department of Civil and Environmental Engineering, National University of Singapore, Singapore, Singapore
- 3College of Education for the Future/ Faculty of Arts and Sciences, Beijing Normal University, Zhuhai, China
- 4College of Chemistry and Environmental Engineering, Shenzhen University, Shenzhen, China
The studies on materials for decontamination in aqueous solutions have increasingly received greater attentions. Such contaminants as heavy metals, arsenic, fluoride and phosphate are harmful to humans and aqueous species due to higher toxicity. Zirconium based adsorbents have become more attractive due to outstanding performance in decontamination. This article provides a comprehensive review of the performance and mechanisms of five types adsorbents: zirconium (hydro)oxides, zirconium hydrogen sulfate, zirconium based multiple metal typed adsorbents and zirconium impregnated complexes. The pseudo-first order and pseudo-second order equations and the intraparticle diffusion model can be applied in describing the adsorption kinetics, while Langmuir and Freundlich equations are the most commonly used adsorption isotherms. The important mechanisms for uptake of contaminants are: ligand exchange between adsorbate and adsorbent, surface complexation formation, and Lewis acid–base and electrostatic interactions. A series of successful studies demonstrate that the adsorbents are promising for removing aqueous contaminants.
1 Introduction
Zirconium is a metallic element with the atomic number of 40. Its elementary substance is greyish-white and soft with ideal ductility. It is a malleable metal which is solid at room temperature, and would become hard and fragile under lower purities (Kloprogge et al., 2020). Zirconium can form various compounds and be used as promising engineering materials in many industrial processes, such as manufacturing of photoflash bulbs, surgical equipment, and tanning of leather (Lee et al., 1988; Akhtar et al., 2008). In recent years, zirconium-based materials have widely been studied for their applications in water treatment. Some of them have great potentials to be used as adsorbents in water purification due to the promising performances.
In recent studies, zirconium-based materials have attracted more attentions due to their higher binding capacities towards important anionic and cationic contaminants, such as copper, lead, arsenic, fluoride and phosphate (Wei et al., 2011; Chowdhury et al., 2019; Mukherjee and Singh, 2020; Khatun et al., 2022; Helleckes et al., 2023). Heavy metals are harmful to humans and aqueous species as they are highly toxic and non-degradable. Such pollution has become a great global environmental thrust, as they can enter the human body through several pathways and can cause irreversible damages to human organs. Discharge of too much heavy metals can destroy the ecological balance. Fluoride contamination in groundwater is a serious environmental problem as excessive intake would cause skeletal fluorosis to humans. Arsenic contamination can cause several diseases, such as liver cancer. As a result, the World Health Organization (WHO), U.S. Environmental Protection Agency (USEPA) and many governmental environmental protection agencies have set the maximum contaminant levels (MCLs) for the heavy metals, arsenic, and fluoride in drinking water and the effluent of wastewater. The MCL of main heavy metals and fluoride are shown in Table 1.
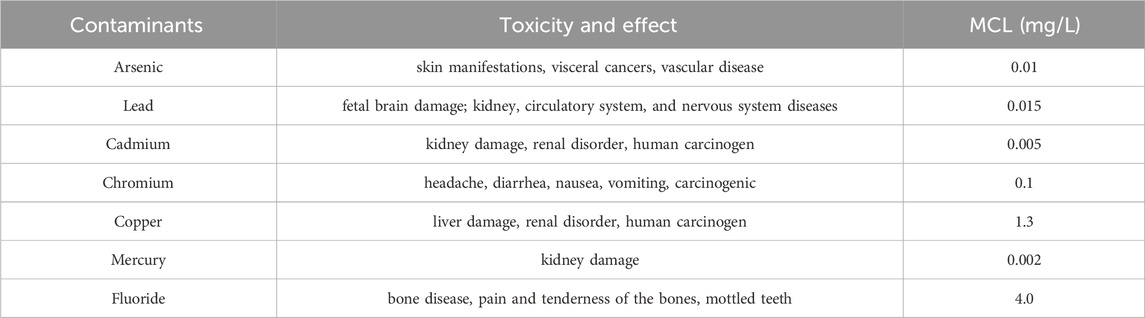
Table 1. Effects of toxic heavy metals and anions on health and MCL (US Environmental Protection Agency, 2012).
The main concern of excess phosphorous in environment is the risk of eutrophication, since too much phosphorous can accelerate biological growth in waters, leading to the destruction of aquatic ecological system. The regulated phosphorus concentration is set as 0.02 mg/L.
Adsorption is one of better technologies for water and wastewater treatment due to its low cost, high efficiency, and easy handling (Bavi et al., 2023; Filho et al. 2023; Scanlon et al., 2023; Waghmare et al., 2023; Yang et al., 2023). A number of studies were carried out on zirconium-based adsorbents. The typical ones are zirconium oxide/hydroxide based adsorbents, zirconium based hydrogen sulfates, zirconium based multiple metal oxide, zirconium ion impregnated materials and zirconium phosphate adsorbent. Compared with traditional adsorbents such as activated carbon and ferric oxides, zirconium-based adsorbents have advantages like higher adsorption capacity, and ease in operation and regeneration.
In this article, the adsorption performances and mechanisms of different types of zirconium-based adsorbents are reviewed and discussed in details. It is anticipated that it would provide a summary of current scientific and technological studies for scientists and engineers in fields of environmental engineering. The review work will be useful for future development of innovative and highly effective adsorbent(s) for water and wastewater treatment and resource recovery.
2 Zirconium based Adsorbents
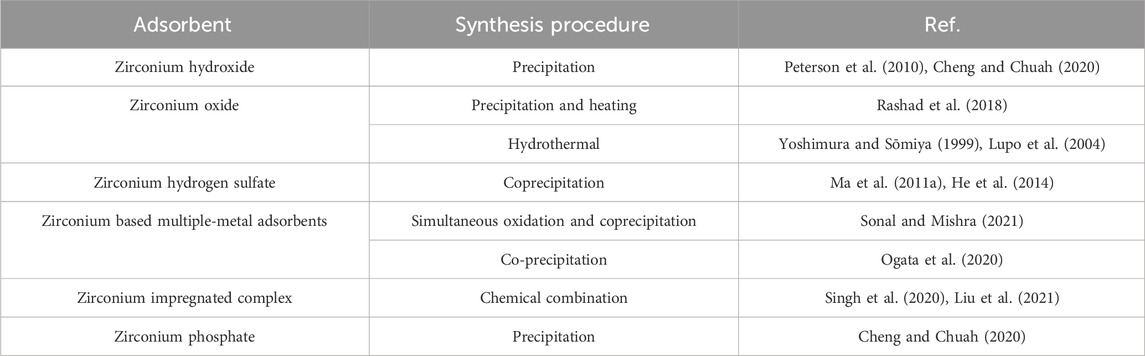
Generally, Zirconium based Adsorbents can be classified into five types, including Zirconium (hydro)oxide, Zirconium hydrogen sulfate, Zirconium based multiple-metal adsorbents, Zirconium impregnated complex and Zirconium phosphate. Their synthesis procedures are given in Table 2.
2.1 Zirconium oxide and hydroxide adsorbents
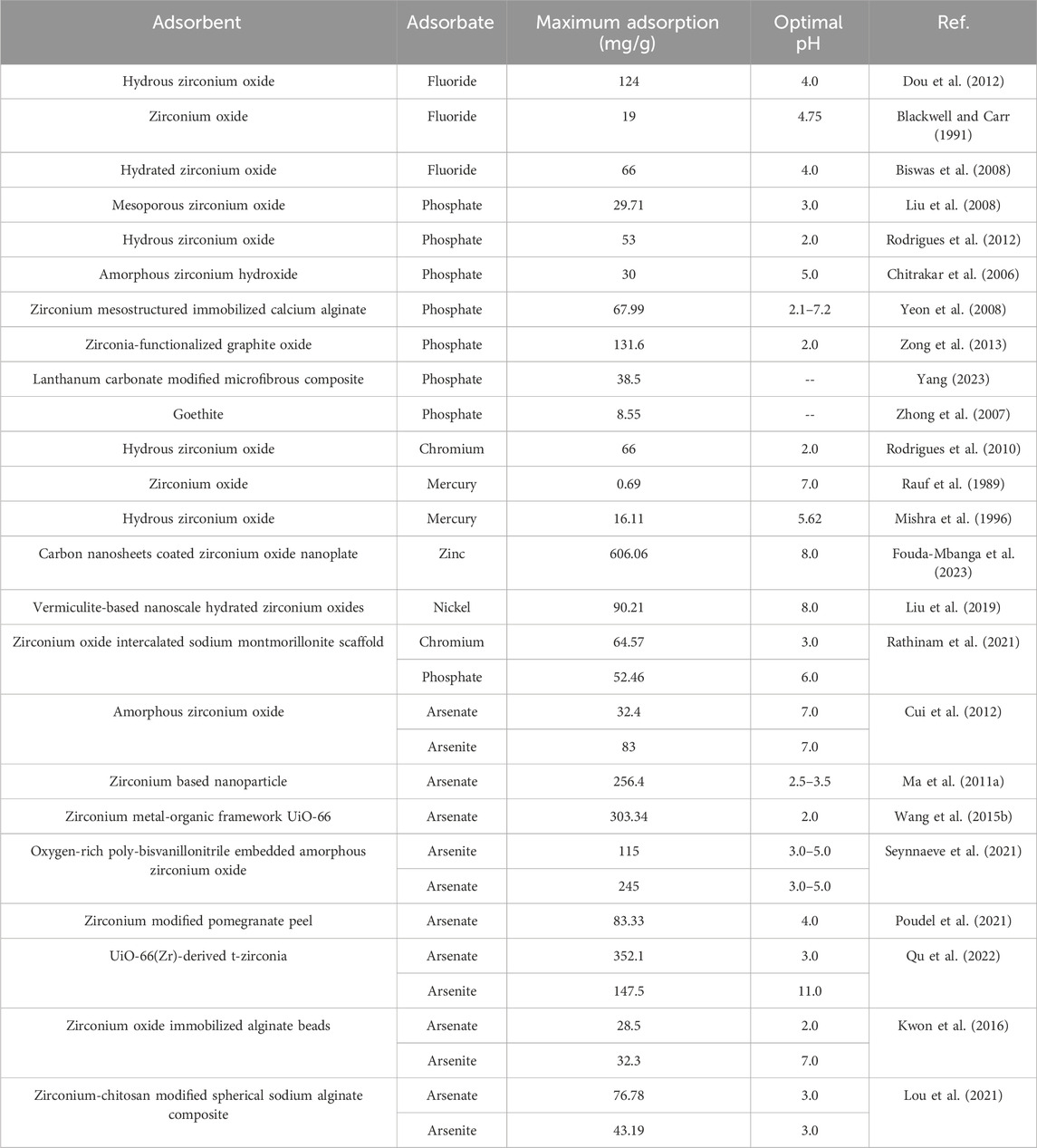
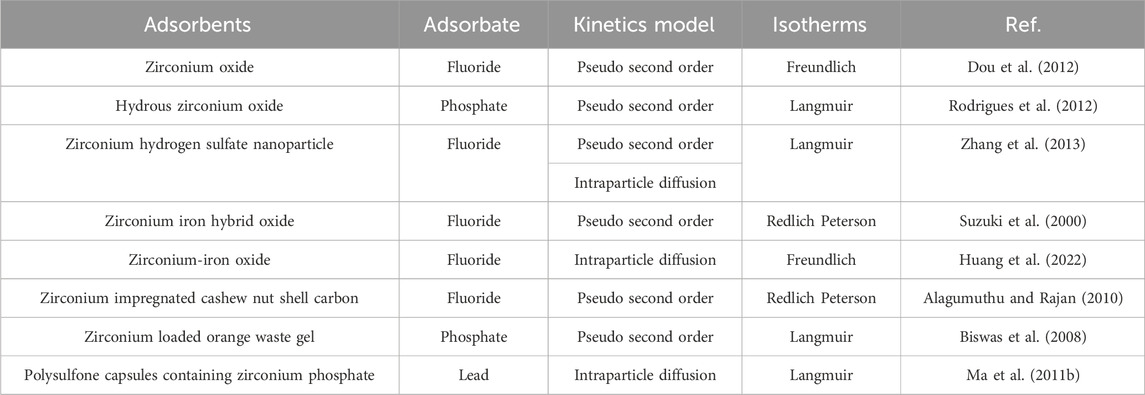
Zirconium oxide/hydroxide is the most traditional zirconium adsorbent owning many ideal properties like chemical stability and unique surface chemistry. Their performance in removal of mercury, chromium, phosphate and fluoride has been extensively studied, and some of the key results are summarized in Table 3.
Zirconium oxide (ZrO2) (called as zirconia) is a white crystalline oxide form of zirconium. It is the most natural form of zirconium which can be found in mineral baddeleyite with a monoclinic crystalline structure. Zirconium hydroxide, known as hydrous zirconia, has an uncertain chemical formula which is mostly described as ZrO2.nH2O. It is also written in the form of common hydroxide description as Zr(OH)4. The hydrous zirconia can be prepared facially by precipitation method. Zirconium oxychloride is first diluted in HCl solution, and then ammonia is added dropwise with constant stirring at room temperature until precipitate produces. The hydrous zirconia can be obtained after the precipitate is filtered, washed and dried (Rashad et al., 2018). On the other hand, the zirconium oxide can be obtained by heating the zirconium hydroxide for the removal of chemically bound water.
Mesoporous, micro or even nano sized zirconium oxides with large surface area and specific functionality are synthesized. The structure and morphology of zirconium oxides depends on different synthesis routes. Typically, they have high porous structure and high surface area (Ho, 1982; Saridag et al., 2013; Kwaśny and Balcerzak, 2017). The nano-sized zirconium oxide can be produced by the hydrothermal reaction; zirconium hydroxide can be heated at 200°C for 8 h for the production. This hydrothermal process at high temperatures/pressures can convert the hydroxide into the oxide (Zr(OH)4 → ZrO2 + 2H2O) (Yoshimura and Sōmiya, 1999; Lupo et al., 2004).
The adsorption capacities of several types of zirconium (hydro)oxides are summarized in Table 3. Generally speaking, zirconium oxide adsorbents have higher adsorption efficiency for anions (e.g., fluoride and phosphate). The zirconium (hydro)oxides with smaller sizes have higher adsorption capacity due to larger specific surface areas. The hydrous zirconium oxide has better adsorption capacity for fluoride than zirconium oxide (124 mg/g or 6.53 mmol/g); the adsorption of phosphate by zirconia-functionalized graphite oxide was as high as 131.6 mg/g.
2.2 Zirconium based hydrogen sulfate nanoparticles
Apart from the zirconium (hydro)oxide particles, newly zirconium based hydrogen sulfate nanoparticles were successfully synthesized; the thermal gravimetric and elemental analyses indicated that its molecular formula was Zr2(OH)6SO4
The adsorbent was incorporated in the adsorptive hollow fiber membrane for water treatment. It overcame the limitation of the nanoparticles and demonstrated effectiveness for fluoride and arsenic removal. The working mechanism was the ion exchange between arsenic ion and hydrogen sulfate ions (Wiechert et al., 2023). Through membrane filtration, the anionic contaminants can be treated by just one step operation and no sludge is generated.
Various zirconium modified materials were fabricated for arsenic removal from water, such as zirconium modified pomegranate peel, zirconium oxide immobilized alginate beads and zirconium-chitosan modified spherical sodium alginate composite. In such materials, zirconium ions are grafted onto support materials, which are easy to collect after adsorption process. In recent years, some metal-organic frameworks (MOFs) have been applied for arsenic removal from water. The UiO-66 MOF was first studied for arsenate removal from water. The adsorption capacity was as high as 303.34 mg/g, which was much higher than other zirconium-based adsorbents for arsenic removal (Wang Y. et al., 2015). The UiO-66(Zr)-derived t-zirconia was developed based on UiO-66, which shows very high adsorption capacity for both arsenate and arsenite (Qu et al., 2022).
2.3 Zirconium based multiple-metal typed adsorbents
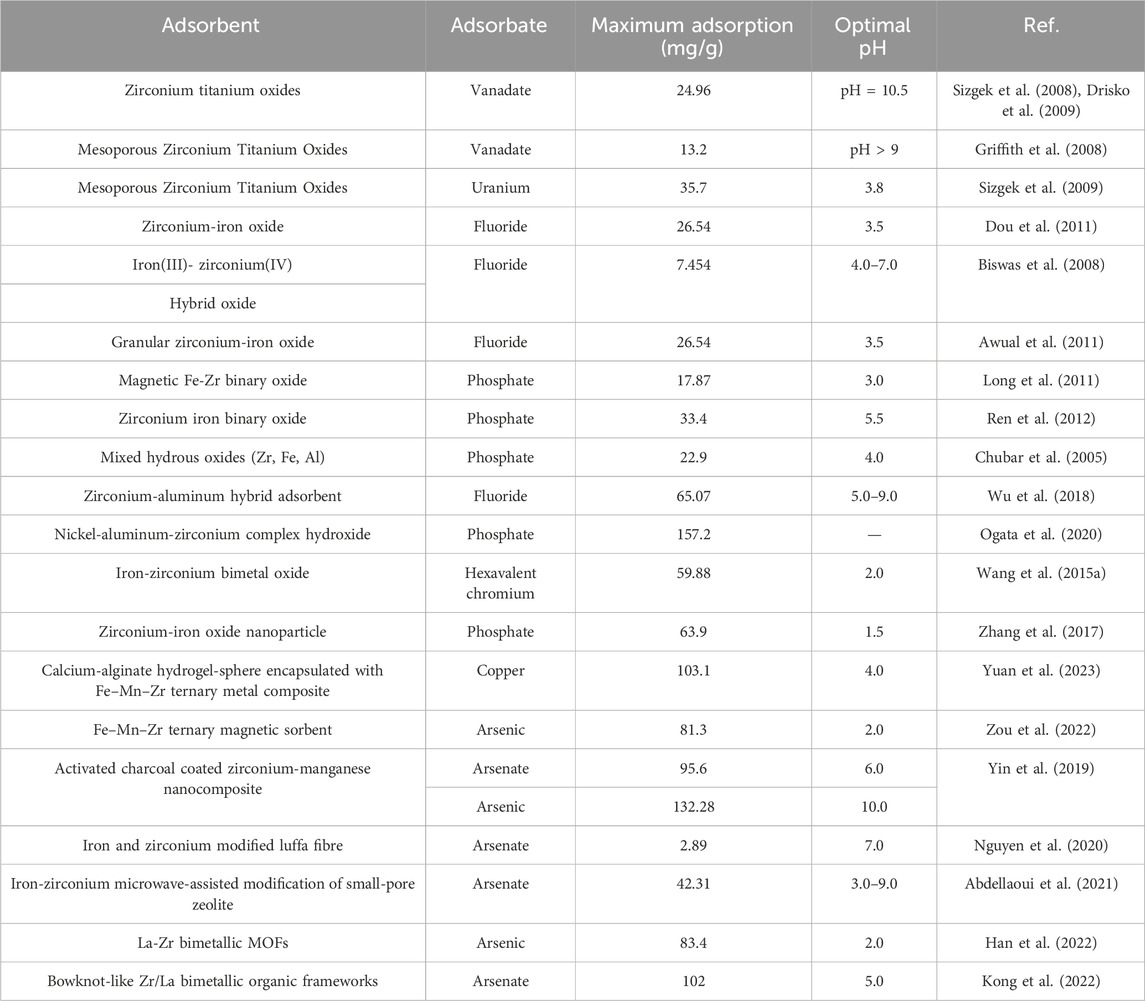
In recent studies, the work on synthesis and applications of multiple-metal typed adsorbents (namely, Zr with low-cost metals) increasingly has more attention. The zirconium based multiple-metal adsorbents can not only have advantages of each single metal oxide/hydroxides but also show significant synergistic effects through each component (Zhang et al., 2013). Iron (Zeng, 2003), titanium (Bekkouche et al., 2004), manganese (Gadde and Laitinen, 1974), and aluminum (Davis and Gloor, 1981) were reportedly used. These had much higher adsorption capacities than traditional mono-metallic adsorbents due to newly created active sites by the incorporation of new metals. These metals (Zr and associated metals used in fabrication of adsorbents) may work as Lewis acidic centers and form a list of complexes with target compounds (e.g., arsenic) (Fukuzaki et al., 1996). Because of the high electro-negativity properties, the anions (e.g., arsenate, arsenite, fluoride and phosphate) have strong affinities towards multivalent metal ions (e.g., Al (III), Fe (III) and Zr (IV)) (Luo and Inoue, 2004), leading to much better adsorption performances.
In the multiple-metal typed adsorbents, the surface areas vary substantially with zirconium content. Crystallization generally results in crystal growth, which in turn leads to larger interparticle pores. The zirconium titanium oxides were mainly X-ray amorphous (Sizgek et al., 2008). The pure metal oxides contained a larger mean mesopore diameter and a wider distribution than the mixed oxides. This may be due to the agglomeration of nanoparticles. In the iron-zirconium binary oxide, both morphologically heterogeneous, amorphous aggregations and crystalline domains with well-defined lattice fringes exist. Two sets of well defined peaks were identified. One was assigned to goethite and the other to lepidocrocite. They are slightly diverted from the standard references. The shifting of the diffraction positions was likely due to the doping state of the aggregated nanoparticles of the components (Dou et al., 2018).
A number of studies was conducted on these adsorbents, especially for removal of fluoride, arsenic, natural organic matters (NOMs) and phosphate. They can be prepared by simultaneous oxidation and coprecipitation techniques, like zirconium–manganese binary hydrous oxide (Zhang et al., 2013), zirconium-iron oxide (Dou et al., 2011) and magnetic Fe-Zr binary oxide (Long et al., 2011). In some cases, the adsorbent consisting of several metals can be synthesized through the co-precipitation approach (Ogata et al., 2020). Typically, zirconium (IV) solution is mixed with a multiple-metal containing solution, then sodium hydroxide solution or ammonia solution is added into the mixture to produce the fine-sized adsorbents. The performances of adsorbents summarized in Table 4 are much better than the commercial adsorbents.
Due to the characteristic of the metallic species as Lewis acid during adsorption, the optimal pH of most zirconium based multiple metal oxide adsorbents is still in acid range, as electrostatic attraction plays an important role during the pollutant removal process. It should be noted that after addition of different metals like titanium and manganese, the mixed metal oxide adsorbents can remove metal cations, like vanadate, uranium and strontium which could not be removed by single zirconium metal. Molar ratio between added metal(s) and Zr is important for adsorption performance. Hence, the optimization should be conducted to obtain better uptake.
2.4 Zirconium impregnated complex adsorbents
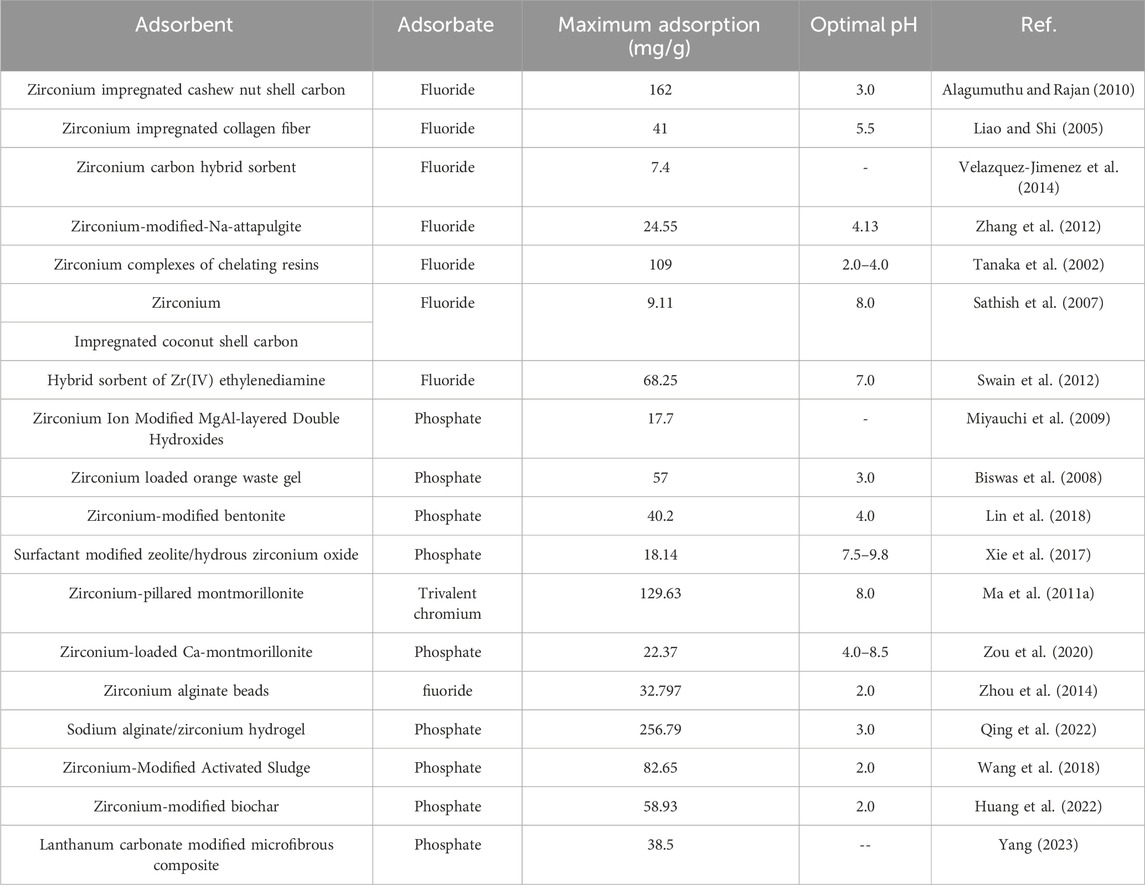
Hard Lewis metals such as zirconium, iron, aluminum, and lanthanum have higher affinities towards the anions through the ligand exchange mechanism (Awual et al., 2011). As a strong Lewis acid, zirconium ions can be combined with other support materials to form hybrid complexes to enhance the performance as shown in Table 5. The most common support material is carbon-typed materials, e.g., biochar (e.g., cashew nut shell carbon (Alagumuthu and Rajan, 2010), coconut shell carbon (Sathish et al., 2007), pea peel waste (Swain et al., 2012), peanut shell (Huang et al., 2022) sludge (Wang et al., 2018) and activated carbon (Schmidt et al., 2008).
The zirconium ions can combine with the carbon through chemical reactions with the unsaturated bonds on carbon surface. This combination remarkably generates larger surface area and improves the surface acidity and pore structure of carbon materials such as carbon nanotube (CNT) (Ntim and Mitra, 2012), which is beneficial for metal uptake as shown in Table 5. In activated carbon, the adsorption sites for metal can be divided into two major groups: hydrophobic surfaces containing the graphene layers and hydrophilic surface comprising of oxygen functional groups (Sathish et al., 2007). When combining with zirconium, there strong interaction and synergistic effect caused by the motion of the free electrons between the support and the metal further improve the adsorption capacity (Yang Y. et al., 2023; Yang et al., 2024). Different types of activated carbon contain different adsorption sites, making their affinity various towards different contaminants.
Zirconium can be combined with ion exchange resin(s) to form effective adsorbents like Zr-loaded lysine diabetics acid resin (Balaji et al., 2005), zirconium loaded EDTA polymer resin (Suzuki et al., 2000), and zirconium (IV) complexes of the chelating resin (Tanaka et al., 2002). These resins contain groups like EDTA, phosphoric acid, hydroxyl and epoxy groups, which show much higher affinities to metal ions. Reported support materials include orange waste gel (Biswas et al., 2008), collagen fiber (Liao and Shi, 2005), fibrous adsorbent (Awual et al., 2011), zeolite (Xie et al., 2017; Ma et al., 2019), montmorillonite (Ma Y. et al., 2011; Zou et al., 2020), and bead (Qiusheng et al., 2015; Qing et al., 2022). Due to their abundant functional groups, they can be great support for zirconium, leading to the better uptake as shown in Table 5.
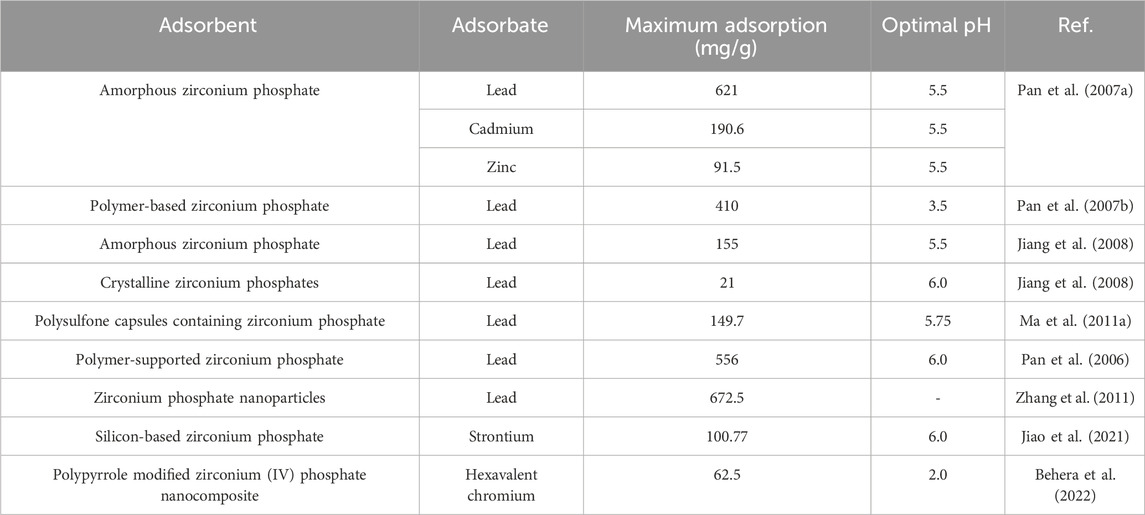
2.5 Zirconium phosphate adsorbents
Zirconium based adsorbents are widely used for phosphate removal, leading to formation of zirconium phosphate. It has low solubility in water and can further be used as an adsorbent/cation exchanger for heavy metals (e.g., lead, copper, cadmium and zinc). In practical applications, the zirconium phosphate adsorbent can be prepared by adding phosphate acid into zirconium solution (Azeroual et al., 2003; Gorbunoff, 1984; Nawrocki et al., 1993).
Similar to zirconium (hydro)oxide, zirconium phosphate can be developed into small sized particles which have larger surface area and specific functionality. The composite is usually grafted onto other engineering materials for column filtration study and for better industrial applications. Zirconium phosphate immobilized silica gel,
As shown in Table 6, the adsorption capacities of zirconium phosphate for lead are different. The adsorption was strongly relative to the adsorbent structure. Normal crystalline zirconium phosphate adsorbent has an adsorption capacity of 21 mg/g, while that by the nano-sized one can be as high as 672.5 mg/g. Other heavy metals, like zinc, copper, cadmium, chromium and strontium can well be removed by the adsorbents.
3 Adsorption kinetic models
Adsorption kinetic study is critical in evaluating performance of an adsorbent. It plays an important role in selection and design of adsorption reactors. The commonly used kinetics models for adsorption process of zirconium adsorbents are given in Table 7.
To better understand the modes of adsorption kinetics, several models are used, e.g., pseudo-first order, pseudo-second order, double constant, and Elovich models (equations); among them, pseudo-first order and pseudo-second order equations are commonly used due to their simplicity (Ali et al., 2023):
where qe and qt are the adsorption capacities (mg/g) of the adsorbent at equilibrium and at time t (h), respectively; k1 (h-1) and k2 (g/mg h) are the pseudo-first order and pseudo-second order adsorption rate constants, respectively. The suitability of equation is evaluated by the values of correlation coefficient (r2); the higher r2 value means the more suitable model.
As shown, most adsorption processes follow the pseudo second order kinetic model. This suggests the importance of chemical adsorption reaction(s) (Yang et al., 2020; Yang et al., 2021b).
Unlike the pseudo-first and second order equations, the intraparticle diffusion model is based on theoretical considerations and provides mechanisms in adsorption kinetics. It is more meaningful and theoretically sound to use it to simulate uptake history.
The model is based on an assumption of “two-step mass transport mechanism” where adsorbate first transfers through the external liquid film from the bulk solution to the adsorbent surface, and subsequently diffuses into the adsorbent before finally being adsorbed by functional groups. Depending upon the structures of adsorbents, the diffusion inside of adsorbent can be either surface diffusion or pore diffusion when specific surface area is either larger or smaller than 100 m3/g-adsorbent (Choy et al., 2004). The mathematical equations and corresponding initial and boundary conditions with the assumption of surface diffusion are expressed as follows (Chen, 2012):
where C and q are the concentrations of the adsorbate in bulk and in solid phases, respectively.
4 Adsorption isotherms
Several adsorption isotherms are extensively used to evaluate the maximum adsorption capacity, predict the concentrations of treated effluent and estimate the dosage of adsorbent to be used. The distribution of targets (e.g., arsenic, fluoride and phosphorous) in the bulk solution and on the adsorbents can be described by one or even a few isotherm equations: Gaseous slip, Langmuir, Freundlich, Tempkin, Dubinin-Radushkevich and Brunauer-Emmet-Teller (BET) equations. Among them Langmuir and Freundlich isotherms given below are the most commonly used due to simplicity and accuracy (Chen, 2012; Ezzati, 2020).
where, qe (mg/g) and Ce (mg/L) are amount of adsorbate adsorbed and residual adsorbate concentration;
Langmuir isotherm assumes that the adsorption takes place on a homogeneous surface and monolayer adsorption occurs on the surface. It can be used to evaluate the maximum adsorption capacity of an adsorbent (qmax). On the other hand, Freundlich isotherm is for multilayer adsorption process on heterogeneous surfaces.
Redlich-Peterson isotherm given below combines the characteristics of both Langmuir and Freundlich isotherms, and can describe adsorption with both uniform and non-uniform adsorption (Chen, 2012).
where A (L/g) and B ((L/mg)n) are Redlich-Peterson model constants and n is Redlich-Peterson model exponent.
Table 7 provides the models for adsorption isotherm and constants from several reported studies. In general, there is no common rule on selection of suitable model(s). Based on the observation of uptake kinetics, it is widely accepted that the adsorption of metals and anions is due to chemical interactions/reactions. In the processes, both physical process and chemical reactions are involved. For example, the electrostatic attraction first occurred between the adsorbents and the phosphate, by which the later was attracted onto the surfaces of adsorbents; the surface complexation and ligand exchange subsequently occurred, leading to the uptake (Yang et al., 2020; Yang et al., 2021b).
The selection of equilibrium mode(s) is mainly dependent on properties of adsorbents (e.g., type, size and specific surface area) and adsorbates (e.g., type, pH, ionic strength, and concentration). It should be noted that these models can only describe adsorption of single-component contaminant (e.g., arsenic or fluoride) and fail to do in cases of multiple-component ones (e.g., co-existence of arsenic and fluoride, or that of copper and zinc). Therefore, such theoretical models as surface complex formation (SCF) models and ion exchange-SCF model can be used (Lim et al., 2008; Chen, 2012).
5 Adsorption mechanisms
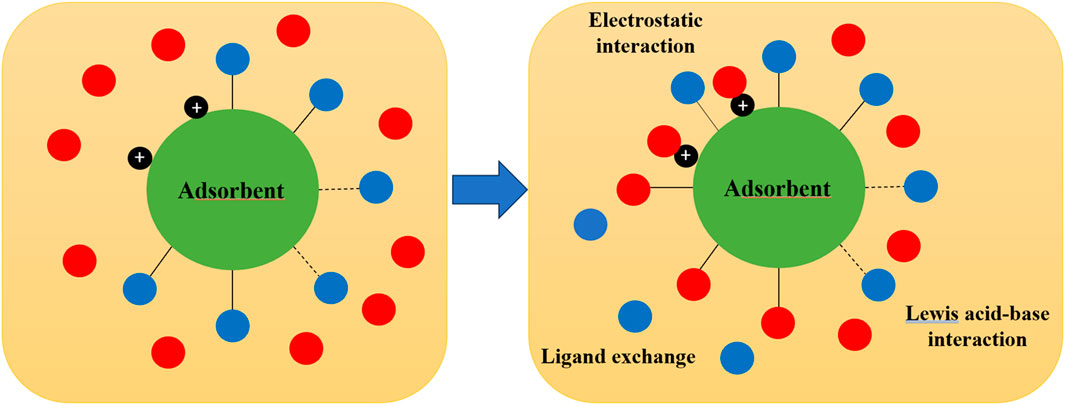
Zirconium is a typical Lewis acid and its electronic orbit is unsaturated. It has available coordination sites to accept electron pairs. In order to satisfy the coordination sites of the zirconium ions, electron pair donors could be coordinated to the available sites. In aqueous solutions, these donors would be water molecules, hydroxide ions and other Lewis bases (Blackwell and Carr, 1991; Blackwell and Carr, 1992). For example, fluoride and phosphate ions can provide electron pairs. Target contaminants are therefore strongly coordinated with zirconium ions and better uptake is achieved (Blackwell and Carr, 1992). The following chemical reactions can be used to describe the interaction between various species and different active sites on zirconium adsorbent surface (Blesa et al., 1988):
where L1 and L2 represent Lewis base in the solution and on the zirconium ions, respectively. The zirconium ion combines with H2O and OH− ligand in aqueous solution.
Equations (10)–(12) describe the structural change in the surface sites for the zirconium ion impregnated complex adsorbents or zirconium (hydro) oxide. The ligand exchange happens between H2O/OH− with L1. For the zirconium based hydrogen sulfate nanoparticles or other zirconium combined adsorbent, Eq. 12 can be used to describe the structural change of the surface sites, on which the ligand exchange happens between L1 and L2.
Single-component zirconium metal adsorbent is usually less efficient in uptake of contaminants due to the similarity in Lewis acid characteristics between adsorbent and adsorbate. When the zirconium is incorporated with other materials such as metals and anions (e.g., zirconium phosphate), the roles of ion exchange and electrostatic attraction become more significant, leading to better uptake (Zhao et al., 2016; McGowan et al., 2023).
The zirconium based adsorbents may have exchangeable anions (e.g., SO42-, and Cl−) or cations (e.g., H+). They can exchange of the targe compounds based on the characteristics of charges. Such ligand exchange commonly occurs in uptake process. Various evidences demonstrate that ion exchange plays a key role in uptake of contaminants.
The zirconium based nanoparticle can treat arsenic and fluoride with excellent perforamance due to the enriched content of sulfate ions in the adsorbent (Ma Y. et al., 2011; He et al., 2014). The arsenic adsorption was studied by using Zirconium/polyvinyl alcohol modified flat-sheet polyvinylidene fluoride membrane (Zhao et al., 2016). Through the batch experiment and the spectrum analysis, it was found that the ligand exchange between chloride and arsenate lead to the arsenate uptake as illustrated in Figure 1.
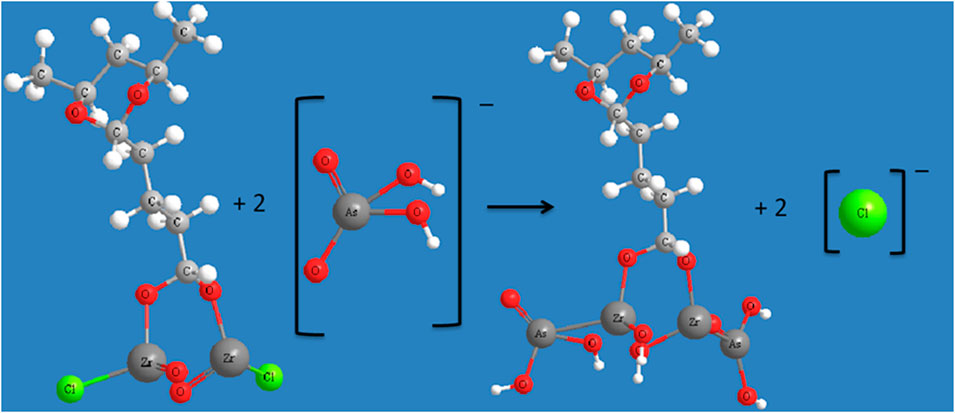
Figure 1. Illustration of ligand exchange between arsenate and zirconium based adsorbent (Zhao et al., 2023).
The point of zero charge (PZC) of adsorbent is an important factor in adsorption of either anions or cations. The PZC not only determines the surface charge property of adsorbent in different solutions, but also affects the chemical reactions and the electrostatic interaction between the ions and the adsorbent (Yang et al., 2021a; Yang et al., 2022).
Adsorbent surface becomes positively charged at solution pH < PZC, while it is negatively charged at pH > PZC. Electric attraction contributes to the pollutant as this interaction has some similarity with ionic bonds, and the ion will be attracted to the oppositely charged adsorbent surface. On the other hand, electrostatic repulsion occurs when the adsorbent surface is charged the same as the ions and causes an adverse effect on the performance.
The important mechanisms are illustrated in Figure 2 and summarized as follows: ligand exchange between adsorbate and adsorbent, surface complexation with electron donation from adsorbate, and Lewis acid–base and electrostatic interactions. These were confirmed through energy dispersive X-ray spectroscopy (EDX), X-ray photoelectron spectroscopy (XPS), Fourier transform infrared spectroscopy (FTIR), nuclear magnetic resonance (NMR) and zeta potential analyses in a larger number of published research articles (Wei et al., 2011; Sonal and Mishra, 2021; Yang L. et al., 2023; Zhang et al., 2023).
6 Conclusion
The findings from the studies on zirconium based adsorbents for removal of cationic heavy metals, and anionic contaminants are discussed and summarized. The adsorbents with better performances are zirconium oxide/hydroxide, zirconium hydrogen sulfate nanoparticles, zirconium based multiple metal oxide, zirconium ion impregnated complex and zirconium phosphate. The adsorption capacities and kinetics for aqueous contaminants are much better than many reported adsorbents; the adsorption capacities are as high as several micromoles per gram of adsorbent and uptake can be completed within several hours. The Lewis acid-base interaction and ligand exchange are main mechanisms for the better uptake. The adsorption isotherm can be well described by either Langmuir and Freundlich equation, while the intraparticle diffusion model as well as the empirical equations (pseudo-first and second order equations) can be used to describe adsorption history. This review work clearly indicates that zirconium-typed adsorbents are promising for treatment of heavy metal and anionic contaminants and the recovery.
Author contributions
DZ: Writing–original draft, Writing–review and editing. YY: Conceptualization, Writing–review and editing. JC: Conceptualization, Writing–review and editing, Funding acquisition, Supervision.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The PhD scholarship to DZ by the National University of Singapore is highly appreciated.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author JC declared that he was an editorial board member of Frontiers at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fceng.2024.1282076/full#supplementary-material
References
Abdellaoui, Y., El Ibrahimi, B., Abou Oualid, H., Kassab, Z., Quintal-Franco, C., Giácoman-Vallejos, G., et al. (2021). Iron-zirconium microwave-assisted modification of small-pore zeolite W and its alginate composites for enhanced aqueous removal of As(V) ions: experimental and theoretical studies. Chem. Eng. J. 421, 129909. doi:10.1016/j.cej.2021.129909
Akhtar, K., Akhtar, M. W., and Khalid, A. M. (2008). Removal and recovery of zirconium from its aqueous solution by Candida tropicalis. J. Hazard. Mater. 156, 108–117. doi:10.1016/j.jhazmat.2007.12.002
Alagumuthu, G., and Rajan, M. (2010). Equilibrium and kinetics of adsorption of fluoride onto zirconium impregnated cashew nut shell carbon. Chem. Eng. J. 158, 451–457. doi:10.1016/j.cej.2010.01.017
Ali, S. I., Lalji, S. M., Awan, Z., Hashmi, S., Khan, G., and Asad, M. (2023). Comprehensive performance analysis of kinetic models used to estimate asphaltene adsorption kinetics on nanoparticles. Chem. Pap. 77, 1017–1031. doi:10.1007/s11696-022-02539-9
Awual, M. R., Jyo, A., Ihara, T., Seko, N., Tamada, M., and Lim, K. T. (2011). Enhanced trace phosphate removal from water by zirconium (IV) loaded fibrous adsorbent. Water Res. 45, 4592–4600. doi:10.1016/j.watres.2011.06.009
Azeroual, S., Wattati, H., Belfkira, A., Taourirte, M., and Jalal, R. (2023). Preparation and application of phosphorylated starch as a flocculant for cationic dyes and heavy metal. Colloids and surfaces C: Environ. Aspects 1, 100019. doi:10.1016/j.colsuc.2023.100019
Balaji, T., Yokoyama, T., and Matsunaga, H. (2005). Adsorption and removal of as (V) and as (III) using Zr-loaded lysine diacetic acid chelating resin. Chemosphere 59, 1169–1174. doi:10.1016/j.chemosphere.2004.12.007
Bavi, A., Sadegh Jafari, M. S., Heydari, M., Ebrahimi, F., and Sadeghizadeh, A. (2023). Batch and continuous mode adsorption of methylene blue cationic dye onto synthesized titanium dioxide/polyurethane nanocomposite modified by sodium dodecyl sulfate. Colloids and surfaces C: Environ. Aspects 1, 100012. doi:10.1016/j.colsuc.2023.100012
Behera, A., Sahu, S., Pahi, S., kumar Singh, S., Mahapatra, B., and Patel, R. K. (2022). Polypyrrole modified zirconium (IV) phosphate nanocomposite: an effective adsorbent for Cr (VI) removal by adsorption-reduction mechanism. Mater. Chem. Phys. 290, 126540. doi:10.1016/j.matchemphys.2022.126540
Bekkouche, S., Bouhelassa, M., Salah, N. H., and Meghlaoui, F. (2004). Study of adsorption of phenol on titanium oxide (TiO2). Desalination 166, 355–362. doi:10.1016/j.desal.2004.06.090
Biswas, B. K., Inoue, K., Ghimire, K. N., Harada, H., Ohto, K., and Kawakita, H. (2008). Removal and recovery of phosphorus from water by means of adsorption onto orange waste gel loaded with zirconium. Bioresour. Technol. 99, 8685–8690. doi:10.1016/j.biortech.2008.04.015
Blackwell, J., and Carr, P. (1991). Study of the fluoride adsorption characteristics of porous microparticulate zirconium oxide. J. Chromatogr. A 549, 43–57. doi:10.1016/s0021-9673(00)91417-1
Blackwell, J. A., and Carr, P. W. (1992). Ion-and ligand-exchange chromatography of proteins using porous zirconium oxide supports in organic and inorganic Lewis base eluents. J. Chromatogr. A 596, 27–41. doi:10.1016/0021-9673(92)80199-5
Blesa, M. A., Regazzoni, A. E., and Maroto, A. J. (1988). “Reactions of metal oxides with aquesous solutions,” in Materials science forum (Stafa-Zurich, Switzerland: Trans Tech Publ), 31–98.
Chen, J. P. (2012). Decontamination of heavy metals: processes, mechanisms, and applications. Boca Raton, FL, USA: CRC Press.
Cheng, Y., and Chuah, G. K. (2020). The synthesis and applications of α-zirconium phosphate. Chin. Chem. Lett. 31, 307–310. doi:10.1016/j.cclet.2019.04.063
Chitrakar, R., Tezuka, S., Sonoda, A., Sakane, K., Ooi, K., and Hirotsu, T. (2006). Selective adsorption of phosphate from seawater and wastewater by amorphous zirconium hydroxide. J. colloid interface Sci. 297, 426–433. doi:10.1016/j.jcis.2005.11.011
Chowdhury, A., Adak, M. K., Mukherjee, A., Dhak, P., Khatun, J., and Dhak, D. (2019). A critical review on geochemical and geological aspects of fluoride belts, fluorosis and natural materials and other sources for alternatives to fluoride exposure. J. Hydrology 574, 333–359. doi:10.1016/j.jhydrol.2019.04.033
Choy, K. K., Porter, J. F., and McKay, G. (2004). Film–pore diffusion models—analytical and numerical solutions. Chem. Eng. Sci. 59, 501–512. doi:10.1016/j.ces.2003.10.012
Chubar, N., Kanibolotskyy, V., Strelko, V., Gallios, G., Samanidou, V., Shaposhnikova, T., et al. (2005). Adsorption of phosphate ions on novel inorganic ion exchangers. Colloids Surfaces A Physicochem. Eng. Aspects 255, 55–63. doi:10.1016/j.colsurfa.2004.12.015
Cui, H., Li, Q., Gao, S., and Shang, J. K. (2012). Strong adsorption of arsenic species by amorphous zirconium oxide nanoparticles. J. Industrial Eng. Chem. 18, 1418–1427. doi:10.1016/j.jiec.2012.01.045
Davis, J. A., and Gloor, R. (1981). Adsorption of dissolved organics in lake water by aluminum oxide. Effect of molecular weight. Environ. Sci. Technol. 15, 1223–1229. doi:10.1021/es00092a012
Dou, X., Mohan, D., Pittman, C. U., and Yang, S. (2012). Remediating fluoride from water using hydrous zirconium oxide. Chem. Eng. J. 198, 236–245. doi:10.1016/j.cej.2012.05.084
Dou, X., Wang, G.-C., Zhu, M., Liu, F., Li, W., Mohan, D., et al. (2018). Identification of Fe and Zr oxide phases in an iron-zirconium binary oxide and arsenate complexes adsorbed onto their surfaces. J. Hazard. Mater. 353, 340–347. doi:10.1016/j.jhazmat.2018.04.004
Dou, X., Zhang, Y., Wang, H., Wang, T., and Wang, Y. (2011). Performance of granular zirconium–iron oxide in the removal of fluoride from drinking water. Water Res. 45, 3571–3578. doi:10.1016/j.watres.2011.04.002
Drisko, G. L., Luca, V., Sizgek, E., Scales, N., and Caruso, R. A. (2009). Template synthesis and adsorption properties of hierarchically porous zirconium titanium oxides. Langmuir 25, 5286–5293. doi:10.1021/la804030h
Ezzati, R. (2020). Derivation of pseudo-first-order, pseudo-second-order and modified pseudo-first-order rate equations from Langmuir and Freundlich isotherms for adsorption. Chem. Eng. J. 392, 123705. doi:10.1016/j.cej.2019.123705
Filho, E. D. S., Cavalcante, C. D. M., Brizola, V. Y., Pereira, M. R., and Fonseca, J. L. C. (2023). A facile method for studying competitive sorption from binary mixtures of dyes. Colloids and surfaces C: Environ. Aspects 1, 100006. doi:10.1016/j.colsuc.2023.100006
Fouda-Mbanga, B. G., Prabakaran, E., and Pillay, K. (2023). Carbon nanosheets coated on zirconium oxide nanoplate nanocomposite for Zn2+ ion adsorption and reuse of spent adsorbent for fingerprint detection. Korean J. Chem. Eng. 40, 824–840. doi:10.1007/s11814-022-1187-z
Fukuzaki, S., Urano, H., and Nagata, K. (1996). Adsorption of bovine serum albumin onto metal oxide surfaces. J. Ferment. Bioeng. 81, 163–167. doi:10.1016/0922-338x(96)87596-9
Gadde, R. R., and Laitinen, H. A. (1974). Heavy metal adsorption by hydrous iron and manganese oxides. Anal. Chem. 46, 2022–2026. doi:10.1021/ac60349a004
Gorbunoff, M. J. (1984). The interaction of proteins with hydroxyapatite: II. Role of acidic and basic groups. Anal. Biochem. 136, 433–439. doi:10.1016/0003-2697(84)90240-9
Griffith, C. S., Sizgek, G. D., Sizgek, E., Scales, N., Yee, P. J., and Luca, V. (2008). Mesoporous zirconium titanium oxides. Part 1: porosity modulation and adsorption properties of xerogels. Langmuir 24, 12312–12322. doi:10.1021/la801464s
Han, C., Xie, J., and Min, X. (2022). Efficient adsorption H3AsO4 and Cr(VI) from strongly acidic solutions by La-Zr bimetallic MOFs: crystallinity role and mechanism. J. Environ. Chem. Eng. 10, 108982. doi:10.1016/j.jece.2022.108982
He, J., Siah, T.-S., and Chen, J. P. (2014). Performance of an optimized Zr-based nanoparticle-embedded PSF blend hollow fiber membrane in treatment of fluoride contaminated water. Water Res. 56, 88–97. doi:10.1016/j.watres.2014.02.030
Helleckes, L. M., Hemmerich, J., Wiechert, W., von Lieres, E., and Grünberger, A. (2023). Machine learning in bioprocess development: from promise to practice. Trends Biotechnol. 41, 817–835. doi:10.1016/j.tibtech.2022.10.010
Ho, S.-M. (1982). On the structural chemistry of zirconium oxide. Mater. Sci. Eng. 54, 23–29. doi:10.1016/0025-5416(82)90026-x
Huang, Q., Luo, K., Pi, Z., He, L., Yao, F., Chen, S., et al. (2022). Zirconium-modified biochar as the efficient adsorbent for low-concentration phosphate: performance and mechanism. Environ. Sci. Pollut. Res. 29, 62347–62360. doi:10.1007/s11356-022-20088-2
Jiang, P., Pan, B., Pan, B., Zhang, W., and Zhang, Q. (2008). A comparative study on lead sorption by amorphous and crystalline zirconium phosphates. Colloids Surfaces A Physicochem. Eng. Aspects 322, 108–112. doi:10.1016/j.colsurfa.2008.02.035
Jiao, Z., Meng, Y., He, C., Yin, X., Wang, X., and Wei, Y. (2021). One-pot synthesis of silicon-based zirconium phosphate for the enhanced adsorption of Sr (II) from the contaminated wastewater. Microporous Mesoporous Mater. 318, 111016. doi:10.1016/j.micromeso.2021.111016
Khatun, M. A., Yousuf, M. A., Ahmed, S., Uddin, M. Z., Alyami, S. A., Al-Ashhab, S., et al. (2022). Deep CNN-LSTM with self-attention model for human activity recognition using wearable sensor. IEEE J. Transl. Eng. Health Med. 10, 1–16. doi:10.1109/jtehm.2022.3177710
Kloprogge, J. T., Ponce, C. P., and Loomis, T. (2020). “The periodic table: nature’s building blocks: an introduction to the naturally occurring elements,” in Their origins and their uses (Amsterdam, Netherlands: Elsevier).
Kong, L., Zhang, J., Wang, Y., Yan, Q., Xu, J., Quan, X., et al. (2022). Bowknot-like Zr/La bimetallic organic frameworks for enhanced arsenate and phosphate removal: combined experimental and DFT studies. J. Colloid Interface Sci. 614, 47–57. doi:10.1016/j.jcis.2022.01.033
Kwaśny, J., and Balcerzak, W. (2017). Characteristics of selected methods for the synthesis of nanometric zirconium oxide–critical review. Tech. Trans. 114, 105–118. doi:10.4467/2353737xct.17.021.6214
Kwon, O.-H., Kim, J.-O., Cho, D.-W., Kumar, R., Baek, S. H., Kurade, M. B., et al. (2016). Adsorption of As(III), As(V) and Cu(II) on zirconium oxide immobilized alginate beads in aqueous phase. Chemosphere 160, 126–133. doi:10.1016/j.chemosphere.2016.06.074
Lee, H., Kepley, L. J., Hong, H. G., Akhter, S., and Mallouk, T. E. (1988). Adsorption of ordered zirconium phosphonate multilayer films on silicon and gold surfaces. J. Phys. Chem. 92, 2597–2601. doi:10.1021/j100320a040
Liao, X.-p., and Shi, B. (2005). Adsorption of fluoride on zirconium (IV)-impregnated collagen fiber. Environ. Sci. Technol. 39, 4628–4632. doi:10.1021/es0479944
Lim, S., Zheng, Y., Zou, S., and Chen, J. P. (2008). Characterization of copper adsorption onto an alginate encapsulated magnetic sorbent by a combined FT-IR, XPS, and mathematical modeling study. Environ. Sci. Technol. 42, 2551–2556. doi:10.1021/es7021889
Lin, J., Jiang, B., and Zhan, Y. (2018). Effect of pre-treatment of bentonite with sodium and calcium ions on phosphate adsorption onto zirconium-modified bentonite. J. Environ. Manag. 217, 183–195. doi:10.1016/j.jenvman.2018.03.079
Liu, D., Deng, S., Vakili, M., Du, R., Tao, L., Sun, J., et al. (2019). Fast and high adsorption of Ni (II) on vermiculite-based nanoscale hydrated zirconium oxides. Chem. Eng. J. 360, 1150–1157. doi:10.1016/j.cej.2018.10.178
Liu, H., Sun, X., Yin, C., and Hu, C. (2008). Removal of phosphate by mesoporous ZrO2. J. Hazard. Mater. 151, 616–622. doi:10.1016/j.jhazmat.2007.06.033
Liu, M., Zang, Z., Zhang, S., Ouyang, G., and Han, R. (2021). Enhanced fluoride adsorption from aqueous solution by zirconium (IV)-impregnated magnetic chitosan graphene oxide. Int. J. Biol. Macromol. 182, 1759–1768. doi:10.1016/j.ijbiomac.2021.05.116
Long, F., Gong, J.-L., Zeng, G.-M., Chen, L., Wang, X.-Y., Deng, J.-H., et al. (2011). Removal of phosphate from aqueous solution by magnetic Fe–Zr binary oxide. Chem. Eng. J. 171, 448–455. doi:10.1016/j.cej.2011.03.102
Lou, S., Liu, B., Qin, Y., Zeng, Y., Zhang, W., and Zhang, L. (2021). Enhanced removal of As(III) and As(V) from water by a novel zirconium-chitosan modified spherical sodium alginate composite. Int. J. Biol. Macromol. 176, 304–314. doi:10.1016/j.ijbiomac.2021.02.077
Luo, F., and Inoue, K. (2004). The removal of fluoride ion by using metal (III)-loaded amberlite resins. Solvent Extr. ion Exch. 22, 305–322. doi:10.1081/sei-120028007
Lupo, F., Kamalakaran, R., Scheu, C., Grobert, N., and Rühle, M. (2004). Microstructural investigations on zirconium oxide–carbon nanotube composites synthesized by hydrothermal crystallization. Carbon 42, 1995–1999. doi:10.1016/j.carbon.2004.03.037
Ma, H., Zhang, J., Wang, M., and Sun, S. (2019). Modification of Y-zeolite with zirconium for enhancing the active component loading: preparation and sulfate adsorption performance of ZrO (OH) 2/Y-zeolite. ChemistrySelect 4, 7981–7990. doi:10.1002/slct.201901519
Ma, X., Li, Y., Li, X., Yang, L., and Wang, X. (2011b). Preparation of novel polysulfone capsules containing zirconium phosphate and their properties for Pb2+ removal from aqueous solution. J. Hazard. Mater. 188, 296–303. doi:10.1016/j.jhazmat.2011.01.107
Ma, Y., Zheng, Y.-M., and Chen, J. P. (2011a). A zirconium based nanoparticle for significantly enhanced adsorption of arsenate: synthesis, characterization and performance. J. colloid interface Sci. 354, 785–792. doi:10.1016/j.jcis.2010.10.041
McGowan, S., Degueldre, C., and Aiouache, F. (2023). Modelling the reaction of uranium with carboxylic groups on surfaces through mono-and multi-dentate surface complexes on the basis of pH and redox potential. Colloids Surfaces C Environ. Aspects 1, 100002. doi:10.1016/j.colsuc.2023.100002
Mishra, S. P., Singh, V. K., and Tiwari, D. (1996). Radiotracer technique in adsorption study: part XIV. Efficient removal of mercury from aqueous solutions by hydrous zirconium oxide. Appl. Radiat. Isotopes 47, 15–21. doi:10.1016/0969-8043(95)00260-x
Miyauchi, H., Yamamoto, T., Chitrakar, R., Makita, Y., Wang, Z., Kawai, J., et al. (2009). Phosphate adsorption site on zirconium ion modified MgAl-layered double hydroxides. Top. Catal. 52, 714–723. doi:10.1007/s11244-009-9209-1
Mukherjee, I., and Singh, U. K. (2020). Fluoride abundance and their release mechanisms in groundwater along with associated human health risks in a geologically heterogeneous semi-arid region of east India. Microchem. J. 152, 104304. doi:10.1016/j.microc.2019.104304
Nawrocki, J., Rigney, M., McCormick, A., and Carr, P. (1993). Chemistry of zirconia and its use in chromatography. J. Chromatogr. A 657, 229–282. doi:10.1016/0021-9673(93)80284-f
Nguyen, T. T. Q., Loganathan, P., Nguyen, T. V., Vigneswaran, S., and Ngo, H. H. (2020). Iron and zirconium modified luffa fibre as an effective bioadsorbent to remove arsenic from drinking water. Chemosphere 258, 127370. doi:10.1016/j.chemosphere.2020.127370
Ntim, S. A., and Mitra, S. (2012). Adsorption of arsenic on multiwall carbon nanotube–zirconia nanohybrid for potential drinking water purification. J. colloid interface Sci. 375, 154–159. doi:10.1016/j.jcis.2012.01.063
Ogata, F., Iijima, S., Toda, M., Otani, M., Nakamura, T., and Kawasaki, N. (2020). Characterization and phosphate adsorption capability of novel Nickel–Aluminum–Zirconium complex hydroxide. Chem. Pharm. Bull. 68, 292–297. doi:10.1248/cpb.c19-01053
Pan, B., Pan, B., Chen, X., Zhang, W., Zhang, X., Zhang, Q., et al. (2006). Preparation and preliminary assessment of polymer-supported zirconium phosphate for selective lead removal from contaminated water. Water Res. 40, 2938–2946. doi:10.1016/j.watres.2006.05.028
Pan, B., Zhang, Q., Du, W., Zhang, W., Pan, B., Zhang, Q., et al. (2007a). Selective heavy metals removal from waters by amorphous zirconium phosphate: behavior and mechanism. Water Res. 41, 3103–3111. doi:10.1016/j.watres.2007.03.004
Pan, B., Zhang, Q., Zhang, W., Pan, B., Du, W., Lv, L., et al. (2007b). Highly effective removal of heavy metals by polymer-based zirconium phosphate: a case study of lead ion. J. colloid interface Sci. 310, 99–105. doi:10.1016/j.jcis.2007.01.064
Peterson, G. W., Wagner, G. W., Keller, J. H., and Rossin, J. A. (2010). Enhanced cyanogen chloride removal by the reactive zirconium hydroxide substrate. Industrial Eng. Chem. Res. 49, 11182–11187. doi:10.1021/ie101204e
Poudel, B. R., Aryal, R. L., Gautam, S. K., Ghimire, K. N., Paudyal, H., and Pokhrel, M. R. (2021). Effective remediation of arsenate from contaminated water by zirconium modified pomegranate peel as an anion exchanger. J. Environ. Chem. Eng. 9, 106552. doi:10.1016/j.jece.2021.106552
Qing, Z., Wang, L., Liu, X., Song, Z., Qian, F., and Song, Y. (2022). Simply synthesized sodium alginate/zirconium hydrogel as adsorbent for phosphate adsorption from aqueous solution: performance and mechanisms. Chemosphere 291, 133103. doi:10.1016/j.chemosphere.2021.133103
Qiusheng, Z., Xiaoyan, L., Jin, Q., Jing, W., and Xuegang, L. (2015). Porous zirconium alginate beads adsorbent for fluoride adsorption from aqueous solutions. RSC Adv. 5, 2100–2112. doi:10.1039/c4ra12036a
Qu, G., Jia, P., Zhang, T., Li, Z., Chen, C., and Zhao, Y. (2022). UiO-66(Zr)-derived t-zirconia with abundant lattice defect for remarkably enhanced arsenic removal. Chemosphere 288, 132594. doi:10.1016/j.chemosphere.2021.132594
Rashad, G. M., Soliman, M. A., and Mahmoud, M. R. (2018). Removal of radioselenium oxyanions from aqueous solutions by adsorption onto hydrous zirconium oxide. J. Radioanalytical Nucl. Chem. 317, 593–603. doi:10.1007/s10967-018-5916-z
Rathinam, K., Atchudan, R., and Edison, T. N. J. I. (2021). Zirconium oxide intercalated sodium montmorillonite scaffold as an effective adsorbent for the elimination of phosphate and hexavalent chromium ions. J. Environ. Chem. Eng. 9, 106053. doi:10.1016/j.jece.2021.106053
Rauf, M., Hasany, S., and Hussain, M. (1989). Adsorption studies of mercury on zirconium oxide from aqueous solution. J. radioanalytical Nucl. Chem. 132, 397–408. doi:10.1007/bf02136099
Ren, Z., Shao, L., and Zhang, G. (2012). Adsorption of phosphate from aqueous solution using an iron–zirconium binary oxide sorbent. Water, Air, & Soil Pollut. 223, 4221–4231. doi:10.1007/s11270-012-1186-5
Rodrigues, L. A., Maschio, L. J., Coppio, L. d.S. C., Thim, G. P., and Pinto da Silva, M. L. C. (2012). Adsorption of phosphate from aqueous solution by hydrous zirconium oxide. Environ. Technol. 33, 1345–1351. doi:10.1080/09593330.2011.632651
Rodrigues, L. A., Maschio, L. J., da Silva, R. E., and da Silva, M. L. C. P. (2010). Adsorption of Cr (VI) from aqueous solution by hydrous zirconium oxide. J. Hazard. Mater. 173, 630–636. doi:10.1016/j.jhazmat.2009.08.131
Saridag, S., Tak, O., and Alniacik, G. (2013). Basic properties and types of zirconia: an overview. World J. Stomatology 2, 40–47. doi:10.5321/wjs.v2.i3.40
Sathish, R. S., Raju, N., Raju, G., Nageswara Rao, G., Kumar, K. A., and Janardhana, C. (2007). Equilibrium and kinetic studies for fluoride adsorption from water on zirconium impregnated coconut shell carbon. Sep. Sci. Technol. 42, 769–788. doi:10.1080/01496390601070067
Scanlon, B. R., Fakhreddine, S., Rateb, A., de Graaf, I., Famiglietti, J., Gleeson, T., et al. (2023). Global water resources and the role of groundwater in a resilient water future. Nat. Rev. Earth Environ. 4, 87–101. doi:10.1038/s43017-022-00378-6
Schmidt, G. T., Vlasova, N., Zuzaan, D., Kersten, M., and Daus, B. (2008). Adsorption mechanism of arsenate by zirconyl-functionalized activated carbon. J. colloid interface Sci. 317, 228–234. doi:10.1016/j.jcis.2007.09.012
Seynnaeve, B., Folens, K., Krishnaraj, C., Ilic, I. K., Liedel, C., Schmidt, J., et al. (2021). Oxygen-rich poly-bisvanillonitrile embedded amorphous zirconium oxide nanoparticles as reusable and porous adsorbent for removal of arsenic species from water. J. Hazard. Mater. 413, 125356. doi:10.1016/j.jhazmat.2021.125356
Singh, S., German, M., Chaudhari, S., and Sengupta, A. K. (2020). Fluoride removal from groundwater using zirconium impregnated anion exchange resin. J. Environ. Manag. 263, 110415. doi:10.1016/j.jenvman.2020.110415
Sizgek, G. D., Griffith, C. S., Sizgek, E., and Luca, V. (2009). Mesoporous zirconium titanium oxides. Part 3. Synthesis and adsorption properties of unfunctionalized and phosphonate-functionalized hierarchical polyacrylonitrile-F-127-templated beads. Langmuir 25, 11874–11882. doi:10.1021/la9015299
Sizgek, G. D., Sizgek, E., Griffith, C. S., and Luca, V. (2008). Mesoporous zirconium titanium oxides. Part 2: synthesis, porosity, and adsorption properties of beads. Langmuir 24, 12323–12330. doi:10.1021/la801490k
Sonal, S., and Mishra, B. K. (2021). A comprehensive review on the synthesis and performance of different zirconium-based adsorbents for the removal of various water contaminants. Chem. Eng. J. 424, 130509. doi:10.1016/j.cej.2021.130509
Suzuki, T. M., Bomani, J. O., Matsunaga, H., and Yokoyama, T. (2000). Preparation of porous resin loaded with crystalline hydrous zirconium oxide and its application to the removal of arsenic. React. Funct. Polym. 43, 165–172. doi:10.1016/s1381-5148(99)00038-3
Swain, S., Mishra, S., Patnaik, T., Patel, R., Jha, U., and Dey, R. (2012). Fluoride removal performance of a new hybrid sorbent of Zr (IV)–ethylenediamine. Chem. Eng. J. 184, 72–81. doi:10.1016/j.cej.2011.12.091
Tanaka, D. A. P., Kerketta, S., Tanco, M. A. L., Yokoyama, T., and Suzuki, T. M. (2002). Adsorption of fluoride ion on the zirconium (IV) complexes of the chelating resins functionalized with amine-N-acetate ligands. Sep. Sci. Technol. 37, 877–894. doi:10.1081/ss-120002221
Us Environmental Protection Agency, (2012). Public drinking water systems: facts and figures Office of Water. Washington, DC, USA: USEPA.
Velazquez-Jimenez, L. H., Hurt, R. H., Matos, J., and Rangel-Mendez, J. R. (2014). Zirconium–carbon hybrid sorbent for removal of fluoride from water: oxalic acid mediated Zr (IV) assembly and adsorption mechanism. Environ. Sci. Technol. 48, 1166–1174. doi:10.1021/es403929b
Waghmare, A., Rathore, R., Pandey, A., and Chandra, V. (2023). Highly efficient adsorption of aqueous iodine on polythiophene/α-manganese dioxide nanocomposites. Colloids and surfaces C: Environ. Aspects 1, 100017. doi:10.1016/j.colsuc.2023.100017
Wang, C., Liu, X., Chen, J. P., and Li, K. (2015b). Superior removal of arsenic from water with zirconium metal-organic framework UiO-66. Sci. Rep. 5, 16613. doi:10.1038/srep16613
Wang, J., Tong, X., and Wang, S. (2018). Zirconium-modified activated sludge as a low-cost adsorbent for phosphate removal in aqueous solution, Water. Air, & Soil Pollut. 229, 1–10. doi:10.1007/s11270-018-3704-6
Wang, Y., Liu, D., Lu, J., and Huang, J. (2015a). Enhanced adsorption of hexavalent chromium from aqueous solutions on facilely synthesized mesoporous iron–zirconium bimetal oxide. Colloids Surfaces A Physicochem. Eng. Aspects 481, 133–142. doi:10.1016/j.colsurfa.2015.01.060
Wei, T., Zheng, Y.-M., and Chen, J. (2011). Functionalization of regenerated cellulose membrane via surface initiated atom transfer radical polymerization for boron removal from aqueous solution. Langmuir ACS J. surfaces colloids 27, 6018–6025. doi:10.1021/la200154y
Wiechert, A. I., Yiacoumi, S., McFarlane, J., Weber, C. F., and Tsouris, C. (2023). Effect of particle size on the capture of uranium oxide colloidal particles from aqueous suspensions via high-gradient magnetic filtration. Colloids Surfaces C Environ. Aspects 1, 100005. doi:10.1016/j.colsuc.2023.100005
Wu, K., Chen, Y., Ouyang, Y., Lei, H., and Liu, T. (2018). Adsorptive removal of fluoride from water by granular zirconium–aluminum hybrid adsorbent: performance and mechanisms. Environ. Sci. Pollut. Res. 25, 15390–15403. doi:10.1007/s11356-018-1711-1
Xie, Q., Lin, Y., Wu, D., and Kong, H. (2017). Performance of surfactant modified zeolite/hydrous zirconium oxide as a multi-functional adsorbent. Fuel 203, 411–418. doi:10.1016/j.fuel.2017.04.141
Yang, L., Liang, C., Shen, F., Hu, M., Zhu, W., and Dai, L. (2023a). A critical review on the development of lanthanum-engineered biochar for environmental applications. J. Environ. Manag. 332, 117318. doi:10.1016/j.jenvman.2023.117318
Yang, Y. (2023). Fixed bed adsorption of phosphate by lanthanum carbonate modified microfibrous composite. Colloids Surfaces C Environ. Aspects 1, 100007. doi:10.1016/j.colsuc.2023.100007
Yang, Y., Koh, K. Y., Huang, H., Zhang, H., Yan, Y., and Chen, J. P. (2021a). Great enhancement in phosphate uptake onto lanthanum carbonate grafted microfibrous composite under a low-voltage electrostatic field. Chemosphere 264, 128378. doi:10.1016/j.chemosphere.2020.128378
Yang, Y., Koh, K. Y., Li, R., Zhang, H., Yan, Y., and Chen, J. P. (2020). An innovative lanthanum carbonate grafted microfibrous composite for phosphate adsorption in wastewater. J. Hazard. Mater. 392, 121952. doi:10.1016/j.jhazmat.2019.121952
Yang, Y., Liu, M., You, X., Lin, H., and Chen, J. P. (2024). A novel bimetallic Fe-Cu-CNT catalyst for effective catalytic wet peroxide oxidation: reaction optimization and mechanism investigation. Chem. Eng. J. 479, 147320. doi:10.1016/j.cej.2023.147320
Yang, Y., Tang, S., Lin, H., Fu, H., Mei, Y., and Long, Y. (2023b). Catalytic reaction intensification by a novel cryogenic auxiliary synthesized Fe-PAN membrane. Industrial Eng. Chem. Res. 62, 20677–20688. doi:10.1021/acs.iecr.3c03497
Yang, Y., Wang, Y., Zheng, C., Lin, H., Xu, R., Zhu, H., et al. (2022). Lanthanum carbonate grafted ZSM-5 for superior phosphate uptake: investigation of the growth and adsorption mechanism. Chem. Eng. J. 430, 133166. doi:10.1016/j.cej.2021.133166
Yang, Y., Zhu, H., Xu, X., Bao, L., Wang, Y., Lin, H., et al. (2021b). Construction of a novel lanthanum carbonate-grafted ZSM-5 zeolite for effective highly selective phosphate removal from wastewater. Microporous Mesoporous Mater. 324, 111289. doi:10.1016/j.micromeso.2021.111289
Yeon, K.-H., Park, H., Lee, S.-H., Park, Y.-M., Lee, S.-H., and Iwamoto, M. (2008). Zirconium mesostructures immobilized in calcium alginate for phosphate removal. Korean J. Chem. Eng. 25, 1040–1046. doi:10.1007/s11814-008-0170-7
Yin, Y., Zhou, T., Luo, H., Geng, J., Yu, W., and Jiang, Z. (2019). Adsorption of arsenic by activated charcoal coated zirconium-manganese nanocomposite: performance and mechanism. Colloids Surfaces A Physicochem. Eng. Aspects 575, 318–328. doi:10.1016/j.colsurfa.2019.04.093
Yoshimura, M., and Sōmiya, S. (1999). Hydrothermal synthesis of crystallized nano-particles of rare earth-doped zirconia and hafnia. Mater. Chem. Phys. 61, 1–8. doi:10.1016/s0254-0584(99)00104-2
Yuan, B., Huang, X., Yang, S., Yang, Y., Lin, Z., Semiat, R., et al. (2023). Development of a magnetic calcium-alginate hydrogel-sphere encapsulated with Fe–Mn–Zr ternary metal composite for heavy metal adsorption. Sep. Purif. Technol. 306, 122531. doi:10.1016/j.seppur.2022.122531
Zeng, L. (2003). A method for preparing silica-containing iron (III) oxide adsorbents for arsenic removal. Water Res. 37, 4351–4358. doi:10.1016/s0043-1354(03)00402-0
Zhang, C., Li, Y., Wang, F., Yu, Z., Wei, J., Yang, Z., et al. (2017). Performance of magnetic zirconium-iron oxide nanoparticle in the removal of phosphate from aqueous solution. Appl. Surf. Sci. 396, 1783–1792. doi:10.1016/j.apsusc.2016.11.214
Zhang, G., Alam, AKMK., and Chen, J. P. (2023). Nanostructured Zr-Mn binary hydrous oxide as an effective adsorbent for arsenic removal from water and groundwater. Colloids Surfaces C Environ. Aspects 1, 100022. doi:10.1016/j.colsuc.2023.100022
Zhang, G., He, Z., and Xu, W. (2012). A low-cost and high efficient zirconium-modified-Na-attapulgite adsorbent for fluoride removal from aqueous solutions. Chem. Eng. J. 183, 315–324. doi:10.1016/j.cej.2011.12.085
Zhang, G., Khorshed, A., and Chen, J. P. (2013). Simultaneous removal of arsenate and arsenite by a nanostructured zirconium–manganese binary hydrous oxide: behavior and mechanism. J. colloid interface Sci. 397, 137–143. doi:10.1016/j.jcis.2012.11.056
Zhang, Q., Pan, B., Zhang, S., Wang, J., Zhang, W., and Lv, L. (2011). New insights into nanocomposite adsorbents for water treatment: a case study of polystyrene-supported zirconium phosphate nanoparticles for lead removal. J. Nanoparticle Res. 13, 5355–5364. doi:10.1007/s11051-011-0521-x
Zhao, D., Fu, C., Wu, Z., and Yu, Y. (2023). Understanding the process in the removal of dimethylarsenic by a zirconium-based nanoparticle. Colloids Surfaces C Environ. Aspects 1, 100016. doi:10.1016/j.colsuc.2023.100016
Zhao, D., Yu, Y., and Chen, J. P. (2016). Zirconium/polyvinyl alcohol modified flat-sheet polyvinyldene fluoride membrane for decontamination of arsenic: material design and optimization, study of mechanisms, and application prospects. Chemosphere 155, 630–639. doi:10.1016/j.chemosphere.2016.03.131
Zhong, B., Stanforth, R., Wu, S., and Chen, J. P. (2007). Proton interaction in phosphate adsorption onto goethite. J. Colloid Interface Sci. 308, 40–48. doi:10.1016/j.jcis.2006.12.055
Zhou, Q., Lin, X., Li, B., and Luo, X. (2014). Fluoride adsorption from aqueous solution by aluminum alginate particles prepared via electrostatic spinning device. Chem. Eng. J. 256, 306–315. doi:10.1016/j.cej.2014.06.101
Zong, E., Wei, D., Wan, H., Zheng, S., Xu, Z., and Zhu, D. (2013). Adsorptive removal of phosphate ions from aqueous solution using zirconia-functionalized graphite oxide. Chem. Eng. J. 221, 193–203. doi:10.1016/j.cej.2013.01.088
Zou, S.-W., Koh, K. Y., Chen, Z., Wang, Y.-Y., Chen, J. P., and Zheng, Y.-M. (2022). Adsorption of organic and inorganic arsenic from aqueous solution: optimization, characterization and performance of Fe–Mn–Zr ternary magnetic sorbent. Chemosphere 288, 132634. doi:10.1016/j.chemosphere.2021.132634
Keywords: zirconium, adsorption, heavy metal, anions, ligand exchange
Citation: Zhao D, Yang Y and Chen JP (2024) Performance of removing aqueous contaminant by zirconium based adsorbents: a critical review. Front. Chem. Eng. 6:1282076. doi: 10.3389/fceng.2024.1282076
Received: 23 August 2023; Accepted: 25 March 2024;
Published: 26 April 2024.
Edited by:
Costas Tsouris, Oak Ridge National Laboratory (DOE), United StatesReviewed by:
Raihan Choudhury, NETZSCH-Feinmahltechnik GmbH, GermanyShujuan Zhang, Nanjing University, China
Copyright © 2024 Zhao, Yang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: J. Paul Chen, anBhdWxjaGVuQGdhdGVjaC5lZHU=
 Dandan Zhao
Dandan Zhao Yi Yang
Yi Yang J. Paul Chen
J. Paul Chen