
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Chem. Eng. , 23 January 2024
Sec. Environmental Chemical Engineering
Volume 5 - 2023 | https://doi.org/10.3389/fceng.2023.1303357
This article is part of the Research Topic Innovative Uses of Biochar in Environmental Applications View all 5 articles
The excessive application of dairy manure to soils to supply plant nutrients can result in increased offsite nutrient transport and degraded water quality. However, by concentrating nutrients from dairy-waste onto biochar or as biosolids, a viable alternative fertilizer can be produced that will benefit soil health, promote carbon sequestration, and decrease nutrient leaching into surface waters. In this study, a greenhouse experiment was conducted to assess soil phosphorus (P) speciation and barley plant growth in soils amended with dairy-waste treated biochar and fermented manure. Phosphorus characterization in the post-harvest soils was determined using selective extractions, 31P-NMR and XANES spectroscopy, and isotopic tracing (P-δ18O). Selective extractions and NMR spectroscopy revealed that most of the P in the amended soils occurred as inorganic species (>85%). XANES spectroscopy further showed that the soil P occurred as either calcium-P minerals (54%–87%) or adsorbed P (0%–46%) species. Analysis by P-δ18O in water and sodium bicarbonate extractions of the treated soils showed that the water-extracted P pool is cycled much faster than the sodium bicarbonate extracted P. Although less than 10% of the total P in the dairy-derived bioproducts was extracted using water, plant productivity in the soils treated with these amendments was the same as that in soils treated with equivalent amounts of conventional nitrogen and P fertilizer. This suggests that dairy-derived bioproducts are good soil amendments to supply nutrients and limit leaching.
Utilization of dairy manure as a nutrient source for agricultural production is a potential way to sustainably use dairy waste products (De Rosa et al., 2022). However, the application of raw dairy manure to agricultural fields often leads to nitrogen (N) and phosphorus (P) leaching that threatens water quality (Cao and Harris, 2010; Ghezzehei et al., 2014; Pagliari et al., 2020). Manures are typically applied to soils at rates needed to meet plant N requirements, thus exceeding P requirements by up to six times (Carey et al., 2011), which may lead to legacy P accumulation in the soil (McDowell and Sharpley, 2001). Due to the widespread threat from dairies to surface water quality (US EPA, 2013; Femeena et al., 2023), there is a major imperative to develop nutrient recycling technology from dairy waste that will reduce nutrient leaching and improve the dairy bioeconomy, soil health, and nutrient resource sustainability (Adhikari et al., 2005; Van Zanten et al., 2019; Bach et al., 2021; Muscat et al., 2021; De Rosa et al., 2022). Meeting this imperative requires research to develop technology to recover P and N from dairy waste and test its efficacy as a beneficial soil amendment (Kushwaha et al., 2011; Kolev, 2017; Ahmad et al., 2019).
Availability of P in soils for plant uptake or leaching is dependent on the P species (Supplementary Table S1), which can be precipitates such as iron (Fe) or calcium (Ca) minerals, adsorbed on soil minerals or soil organic matter surfaces, or biologically-derived organic P compounds. When P fertilizer is applied to calcareous soils, such as those in the main dairy-producing region of Idaho, United States, it remains soluble for a short time following application before it becomes fixed on soil clay and CaCO3 surfaces and/or precipitates as calcium phosphate (Ca-P) minerals (Leytem and Mikkelsen, 2005; Carreira et al., 2006). These reactions decrease the solubility and plant availability of P compounds, requiring, in some cases, an increase in the amount of fertilizer applied; estimates of 0.1 Mg ha−1 increased fertilizer for every 1% free lime (CaCO3) measured in the soil have been suggested (Leytem and Mikkelsen, 2005). Application of P sources with organic matter, such as in dairy manure, may hinder the reaction of P compounds with Ca, making P more available for plant uptake, but may also increase the risks for leaching losses (Leytem and Mikkelsen, 2005; Weyers et al., 2017).
The reuse of waste streams such as liquid manure effluent from dairy lagoons and residual solids from anaerobic digestion offers a source of sustainable P and N fertilizers, decreasing the need for chemical fertilizers derived from non-renewable mined phosphate rocks (Metson et al., 2016; Krounbi et al., 2021; Ndoung et al., 2021). Liquid manure has high concentrations of total P, with P in variable molecular forms that have different availabilities for plant uptake (Pagliari and Laboski, 2013), including 10%–70% organic P compounds (Po) that vary depending on the lagoon properties and the season (Hansen et al., 2004; Leytem and Westermann, 2005; McDowell et al., 2008; Li et al., 2014).
Anaerobic digestion (AD) systems are used on dairies to produce renewable energy from dairy wastes, reducing manure storage and leaving behind residual forms of digested products that are high in nutrients (Bach et al., 2021). Mazzini et al. (2020) found that AD solids of cattle manure contain 65%–81% inorganic P compounds (Pi), depending on the operation methods of the AD plant. Amongst the Pi species, anaerobic digestion waste contains more Ca-P species than undigested manure sources (Güngör et al., 2007). Since many Ca-P minerals have low solubility, they can have reduced leaching potential as compared to other soil P species such as phosphate adsorbed on soil particles (Doydora et al., 2020), which can decrease P availability for plant uptake.
A recent technology to improve dairy waste nutrient reuse efficiency is to use biochar as a substrate to mix with the dairy waste to recover nutrients. The dairy waste-amended biochar can be used as an enriched soil amendment to promote nutrient retention and reduce leaching losses (Sarkhot et al., 2012; Streubel et al., 2012; Sarkhot et al., 2013; Ghezzehei et al., 2014; Liang et al., 2014). It can also add stable organic carbon (C) to soils that can persist for centuries, thus adding a C sequestration potential (Osman et al., 2022). In alkaline soils, biochar’s ability to add P to soil is dependent on total P content and its bioavailability.
Biochar has been used to remove up to 65% of P from liquid manure sources (Sarkhot et al., 2012; Streubel et al., 2012; Wang et al., 2020). Some biochars may release P into solution instead of sorbing it, and thus require modifications to increase sorption capacity (Hale et al., 2013). Modifying biochar with cations, such as iron (Fe), allows more P to be sorbed onto biochar from solution than in unmodified biochars (Liu et al., 2015; Ren et al., 2015; Wu et al., 2020; Strawn et al., 2023). Iron-modified biochars have been used to remove over 90% of P from wastewaters (He et al., 2017; Yang et al., 2018; Dalahmeh et al., 2020). The availability of P from the modified biochar fortified with dairy waste-derived nutrients will depend on soil and plant properties, the biochar modification, and the dairy waste source (e.g., anaerobic digest, stall washout, etc.), and thus needs to be evaluated for different products and soils.
Phosphorus recovered on biochars has been shown to be plant-available (Streubel et al., 2012; Wang et al., 2020; Wu et al., 2020). Streubel et al. (2012) found a 29-fold increase in water extractable P from biochar with sorbed P as compared to untreated biochar, indicating that recovered P biochars may increase bioavailable P in soils. Although soil total P concentration increases with added P from dairy waste streams, low plant availability of P on biochar may reduce yield compared to chemical fertilizers (Collins et al., 2020). Forms of relatively low-solubility Ca-P are the dominant inorganic P species in most biochars, suggesting that biochar may be a good slow-release fertilizer (Streubel et al., 2012; Robinson et al., 2017; Rose et al., 2019; Strawn et al., 2023). Over multiple cropping seasons, biochars continue to release P, therefore one application may provide all the P required for multiple years (Lustosa Filho et al., 2020).
If biochar is modified to maximize removal of phosphate from wastewater using Fe-modification, the final product could be a beneficial slow-release P source that could supply P to plants and limit its leaching into surface and ground waters (Yao et al., 2013; An et al., 2021; Wang et al., 2022). Several recent reviews have indicated the need for availability studies and speciation studies to better understand the mechanisms controlling P uptake and release from biochar so that it can be effectively used as a sustainable P fertilizer source (Kopecky et al., 2020; Zhang et al., 2020; Gelardi and Parikh, 2021). Thus, the goal of this project was to investigate P species and availability in calcareous soils amended with various forms of dairy-derived nutrients and biochar in a barley (Hordeum vulgare L.) greenhouse growth trial. We used multiple experimental methods to elucidate major species controlling P availability, including Hedley sequential extraction, 31P nuclear magnetic resonance (31P -NMR) and P K-edge X-ray absorption near edge structure (XANES) spectroscopies, and P-δ18O isotopic signatures of dairy-derived phosphate. Using P species characterization and availability extractions together with greenhouse growth studies allowed for direct assessment of how soil processes affect plant availability. Information from this study will support future long-term field-scale studies using biochar enriched from dairy waste streams as alternative fertilizers.
Barley was grown in a greenhouse experiment in soils collected from the University of Idaho Research and Extension Center in Parma, Idaho, United States. Three different dairy-derived P fertilizer treatments were used and compared to two non-dairy biochar treatments, a conventional multi-nutrient fertilizer, and an unfertilized control.
Amendments were generated from two dairy-waste streams sourced from the University of Idaho dairy in Moscow, Idaho, United States. Dairy-derived amendments used in this study were fermented dairy manure biosolids (FS), dairy lagoon P sorbed to 1% Fe-modified biochar (dairy-BC), and fermented dairy solids mixed with unmodified biochar (FS/BC). The two non-dairy biochar treatments used were the unmodified biochar (UBC) and a 1% Fe-modified biochar (Fe-BC). This study used a commercially available biochar (Biochar Now, Berthoud, Colorado, United States) that is produced from beetle-killed and fire-damaged mixed conifers trees. Details of the biochar preparation and Fe modification are described in supplemental information S1.1 Text.
The Fe-modified biochar was loaded with nutrients from the dairy lagoon water from the University of Idaho dairy, Moscow, Idaho, United States: 1500 L lagoon water was pumped into a polycarbonate tank and 1 kg of air-dried 1% Fe-modified biochar was added and mixed for 2 hours. After mixing, the biochar was allowed to settle for 10 minutes, and then filtered through an 80-μm mesh nylon bag filter. The recovered biochar was air dried over 3 days until it was a loose powder. Water samples were taken from the top foot of the tank before biochar was added and after the 2-h biochar incubation (allowing biochar to settle for 10 minutes after mixing prior to sampling) to estimate biochar effects on water quality (Supplementary Table S2).
Fermented manure biosolids were collected from fermentation experiments operated at a total volume of 16 L with a solids retention time of 4 days. Fresh, wet manure was collected bi-weekly from the University of Idaho dairy and refrigerated until use. Manure was added to maintain 8.75 g volatile solids L−1 day−1 and mixed to 4 L with warm tap water before being added back to the fermenter. Each day, 4 L of liquid were decanted from the fermenter. Coarse solids were strained out of the mixture and the remaining liquid was centrifuged at 14,334 relative centrifugal force for 30 min before being filtered to collect the fine solids. Coarse and fine fermented manure biosolids were collected over 1 week for use in this study. The solids were dried in a 50°C oven for about 4 days until mass was stable before being combined at a 1:1 w/w ratio of fine and coarse solids.
The UBC, Fe-BC, FS and dairy-BC were all autoclaved at 123°C and 124 kPa for 30 min before use in the greenhouse. Biochars and fermented manure were analyzed for chemical properties to determine their viability for use in the greenhouse. Details on the characterization methods for the amendments are provided in the S1.2 Text.
Surface soils (0–30 cm) were collected for use in the greenhouse from the University of Idaho Parma Research Extension Center (43°48″03.2″ N; 116°56′18.0″ W). The soil is a Greenleaf-Owyhee silt loam, a fine-silty, mixed, superactive mesic Calciargid (Soil Survey Staff, 1999). The site receives annual rainfall of 229 mm. Amended and control soil samples were sent out for characterization of soil physicochemical properties (Kuo Testing Labs, Pasco, WA, United States). All analyses were performed according to Gavlak et al. (2005) for the Western region; details are described in Supplementary Table S3.
In the greenhouse UBC, Fe-BC, dairy-BC, FS, FS/BC, and conventional fertilizer (CF) treatments were applied at two rates (high (H) and low (L)), with the high treatment rate adding five times more total P than the low treatment. Each treatment had five replicate pots. The conventional fertilizer (Expert Gardener, All-Purpose Plant Food (Gro Tec. Inc., Madison, Georgia, United States)) was chosen based on having a N/P ratio of 6 that is between the N/P ratio of the dairy-derived biochar (4) and fermented solids (9). The fertilizer had an N–P–potassium (K) value of 12–5–7 [12% N, 5% P2O5 (2.2% P), 7% K2O (5.8% K)], and was composed of urea, ammonium phosphate, potash, iron sucrate, manganese sucrate, and zinc sucrate. Biochars were applied at 20 Mg ha−1 and 100 Mg ha-1 based on values suggested in a meta-analysis by Glaser and Lehr (2019), which, for the dairy-BC treatment resulted in 12 and 59 mg P per pot, respectively. The amounts of FS, FS/BC and CF treatments were applied to match the equimolar total P applied in the dairy-BC treatment (Supplementary Table S4).
All amendments were homogenously mixed with the soil and filled into rectangular plastic pots (10 × 10 cm, 35 cm tall). Approximately 2.85 kg of soil were added to each pot. Biochars were added at 28.5 and 142.5 g per pot to meet low and high application rates, totaling 12 and 59 mg total P per pot, respectively. Pots were watered to 25.6% (mass basis) soil moisture, which corresponded to 70% water holding capacity in these soils using greenhouse tap water with a pH of 7.35 and electrical conductivity of 254 μS. In the greenhouse, pots were randomized in a complete block design and allowed to equilibrate for 2 days after watering. Two-row spring barley (GemCraft cultivar) seeds were initially sprouted in plastic bags, and then five sprouted barley plants were planted at a depth of one inch in moist soil that had equilibrated for 5 days prior to planting. Eleven days after planting, plants were clipped to three per pot. Moisture conditions in the pots were measured every 1–2 days with a soil moisture probe (HydroSense II, Campbell Scientific, Pullman, WA, United States). Pots were watered when necessary to maintain moisture conditions at 70% water holding capacity. To meet N and S requirements, pots were fertilized four times with (NH4)2SO4 and NH4NO3 (Thermo Scientific, Waltham, MA, United States) at rates of 5.6 kg S ha−1 and 14.0 kg N ha−1 per application starting 2 weeks after planting and continuing every 2 weeks until the fourth application, totaling 22 kg S ha−1 and 56 kg N ha−1. Throughout the study, light was provided at a photoperiod of 16/8 h day/night and temperature varied between 16°C–27°C. Starting at week four, blocks were rotated weekly to account for differences in light.
After 109 days, barley was cut above the soil surface. Dry biomass was recorded after drying at 50°C for 48 h. Roots were removed from the soil and washed to remove residual soil and weighed to obtain root mass. Barley yield was estimated by weighing the mass of heads, and the heads per plant were also counted. After plant harvest, soil in each pot was carefully separated from the plant roots, air dried in the greenhouse, and crushed to disaggregate and homogenize.
Soil samples were analyzed after use in greenhouse experiments for total P (TP), total Pi and total Po concentrations by H2SO4 extraction with and without ignition (Saunders and Williams, 1955; Cade-Menun and Lavkulich, 1997). Details on the TP method can be found in the S1.3 Text. The untreated soil and a Standard Reference Soil (SRM 2711 NIST) were also digested using EPA Method 3050 (aqua regia and H2O2 at 90°C) and the digest analyzed for total P via inductively coupled plasma optical emission (ICP-OES) spectrometry. The P recovery of the SRM by EPA 3050 was 84% of certified value. The untreated soil was also submitted to Bureau Veritas (Vancouver, British Columbia, Canada) for TP analysis via four-acid digestion.
A modified Hedley sequential extraction was used to quantify soil P pools using operationally defined soil extractants: H2O-P, NaHCO3-P, NaOH-P, and HNO3-P (Hedley et al., 1982; Joshi et al., 2018). The H2O-P fraction was analyzed colorimetrically and by ICP-OES. The difference between the total H2O-P measured by ICP-OES and H2O-PMR is molybdate unreactive phosphorus (H2O-PMU), which primarily consists of P associated with organic, colloidal, and non-hydrolysable P compounds (Haygarth and Sharpley, 2000). Aliquots from the H2O, NaOH, and HNO3 extractants were analyzed for P by ICP-OES. The NaHCO3 extracts were not analyzed on the ICP-OES due to high Na concentrations that needed high dilution, causing P to be below detection level (0.01 mg/L). However, as a test, eight NaHCO3 extract samples that were only diluted by 1:10 were analyzed by ICP-OES. The ICP measured P concentrations of the NaHCO3 extract samples were within 7% of the colorimetrically determined concentrations, indicating that the colorimetric data from the NaHCO3 extracts is a good estimate for total NaHCO3-extracted P.
Isotope tracing using the stable oxygen isotope of phosphate (P-δ18O) allows for the tracing of P sourced from different soil pools. This can be complicated by temperature-dependent intracellular microbial cycling of P, which causes an equilibrium fractionation value between the P-δ18O and the ambient cellular water (δ18Ow), often overwriting other system fractionation effects. Recent work by Joshi et al. (2016) suggests that by comparing P-δ18O signatures of P inputs, the relative cycling between soil pools can be estimated.
For P-δ18O isotopic analysis, soils were sequentially extracted using DI H2O, and 0.5 M NaHCO3, as described above (Hedley et al., 1982; Joshi et al., 2018). Replicate treatment samples were extracted to estimate greenhouse sample variation. The NaOH and HNO3 soil extracts were tested for P-δ18O isotopic analysis, but due to inefficient recovery of Ag3PO4 (NaOH extract) or high replicate variability, the P-δ18O data for these extracts were not included in this manuscript.
The extract preparation for isotope analysis used a modified protocol developed by Nisbeth et al. (2019), which produces silver phosphate (Ag3PO4) crystals (details are in the S1.4 Text). To adapt the protocol for soil matrix extracts, an organic matter removal step (DAX-8) and a cation removal step were added to the protocol, as described in Tamburini et al. (2010). The P-δ18O isotope ratios were measured using a high temperature conversion elemental analyzer coupled to a Delta V Advantage continuous flow isotope ratio monitoring mass spectrometer (TCEA-IRMS, Thermo Fisher Scientific, Waltham, MA, United States). All in-house isotopic standards were normalized to the Vienna Standard Mean Oceanic Water (VSMOW) -SLAP scale through silver phosphate provided by the USGS Renton Stable Isotope Lab (USGS 80 and USGS 81). The standard deviation of multiple runs of the standards was 0.4‰, thus differences of less than 0.4‰ were within instrument resolution. The measured P-δ18O values are reported relative to VSMOW in per mil (‰) notation. The temperature-dependent equilibrium was predicted by the following equation (41):
Temperature (T) is in Kelvin and Ow is the δ18O value of the greenhouse irrigation water.
Soil samples at high amendment rates were selected for 31P-NMR analysis to identify concentrations and speciation of organic P in soils. The dairy-BC and FS/BC samples were extracted in duplicate. The raw amendments were also analyzed. Standard procedures for 31P-NMR analysis were used for extraction (Cade-Menun and Preston, 1996; Cade-Menun and Liu, 2014) (details are in the S1.5 Text). Ferric chloride was spiked in the extracts to achieve the correct delay times (McDowell et al., 2006). Spectra were plotted with 10 Hz line-broadening for the main spectra and 3 Hz line-broadening to assess finer details. Peak areas were computed by integration and visual inspection using SpinWorks Software (SpinWorks 4.2.11, Marat (2017)), with correction for the degradation of orthophosphate diesters (Cade-Menun and Liu, 2014). Peak assignments were made from the literature and confirmed using a phytate spike (Supplementary Table S5) (Cade-Menun, 2015; Schneider et al., 2016).
The P K-edge XANES spectra were collected from the soils with amendments applied at the highest rates. The soil samples were analyzed for P K-edge XANES at Beamline 14-3a at the Stanford Synchrotron Radiation Lightsource (SSRL) (details of analysis and processing are in the S1.6 Text). The XANES spectra of the standards were collected at the Soft X-ray Micro-Characterization Beamline (SXRMB) at the Canadian Light Source (CLS) in Saskatoon, Canada. Reference standards were collected at the two beamlines to allow for alignment.
The collected scans were analyzed using the Athena software program (Ravel and Newville, 2005) (details are in the S1.6 Text). Linear combination fitting was performed to determine speciation of each spectrum. Initially, nine standards (Supplementary Figure S1) were used to fit the spectra, followed by reduction to five standards that represented the major species possible in the sample spectra (adsorbed P, apatite, dicalcium phosphate dihydrate (DCPD), DCPD 50:50 Ca:Mg, and phytic acid). Standards were iteratively removed if they comprised less than zero percent of the sample fit. Previous tests of P soil XANES LCF indicated that the accuracy is approximately 5–15 percent absolute (Ingall et al., 2011; Gustafsson et al., 2020). In fitting of the spectra, it was observed that the different Ca-P mineral standards could be substituted for each other, with only a slight decrease in the fit quality (as judged by reduced chi square fit statistic); thus, the fit of all Ca-P minerals is grouped into a single category.
Data analysis was performed using R version 4.1.2 (R Studio Team, 2020). Hedley sequential extraction data, plant biomass data, and total soil P were analyzed in a generalized linear mixed model with treatment and rate of application as fixed effects and block as a random effect. Model fit was assessed by examining log-likelihoods and inspecting residual plots. Package “lme4” (Bates et al., 2015) was used for model building and ANOVA. Mean comparisons were performed using R package “emmeans” (Lenth et al., 2022). Significance was determined at a p ≤ 0.05 using a Tukey HSD test.
Following Fe modification and incubation in lagoon water, the pH of biochar amendments decreased (Table 1). The EC of the fermented manure was over seven times greater than for the biochars, likely due to increased Ca, K, Mg, and Na ions after fermentation. Biochar amendments had nearly twice as much total C as the fermented manure, but the fermented manure had up to 10 times as much total N.
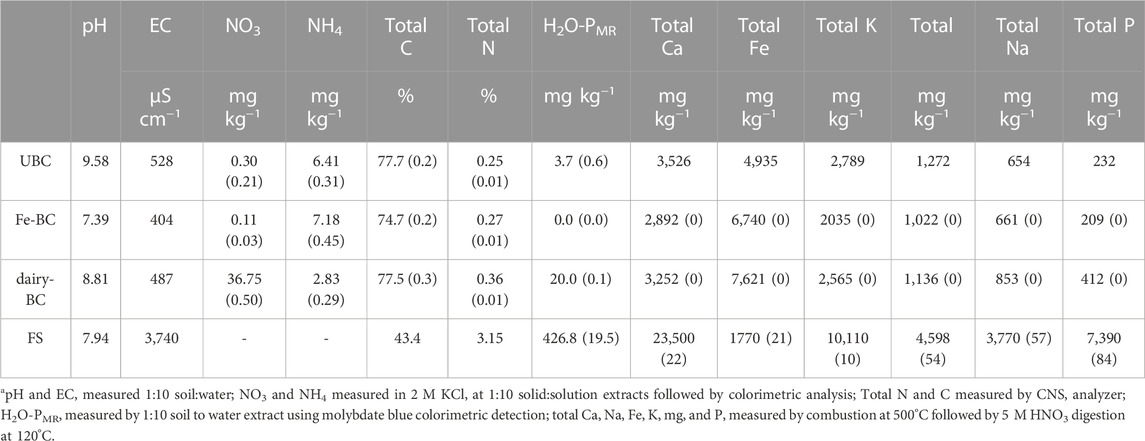
TABLE 1. Biochar and fermented biosolids propertiesa. Values are averages of analytical replicates, where applicable (standard error in parenthesis; n = 2). dairy-BC, dairy manure-amended Fe-modified biochar; Fe-BC, 1% Fe modified biochar; FS, fermented biosolids; UBC, unmodified biochar.
Compared to the biochars without dairy treatments, dairy-treated biochar had almost twice as much total P, and over five times more H2O-PMR (Table 1). Fermented manure had over 20-times more H2O-PMR than the dairy-amended biochar. Using 31P-NMR spectroscopy, orthophosphate was identified as the main species in all treatments, ranging from 66%–93% of the NaOH-EDTA extracted P (Figure 1, Supplementary Table S6 Supplementary Figure S2. Other identified Pi compounds included pyrophosphate, and polyphosphates. The Po compounds identified by 31P-NMR were grouped into the compound classes phosphonates, orthophosphate monoesters, and orthophosphate diesters after correction for degradation of diesters to monoesters during analysis (Figure 1, Supplementary Table S7). Extracts of biochar amendments were 5.2%–5.9% Po as determined by 31P-NMR, while the extract from the fermented manure amendment was 28.4% Po.

FIGURE 1. The percentage of P within the main organic P compound classes (orthophosphate diesters, orthophosphate monoesters, phosphonates) in NaOH-EDTA extracts analyzed by 31P-NMR spectroscopy. Inorganic P is the sum of the inorganic orthophosphate and polyphosphate compounds. cDiester, cMonoester, orthophosphate diesters and monoesters after correction for degradation; CF, conventional fertilizer; dairy-BC, dairy manure-amended Fe-modified biochar; Fe-BC, 1% Fe-modified biochar; FS, fermented biosolids; FS/BC, fermented biosolids plus unmodified biochar; -H, high application rates; UBC, unmodified biochar; untreated, soil sampled prior to greenhouse experiment; (2), sample analyzed in duplicate.
Prior to use in the greenhouse, Olsen P was slightly greater in the amended soils than the control soil (Supplementary Table S3). Percent organic matter, micronutrients (Zn, Mn, Fe), and soluble salts were higher in the amended soils than the control soils. Base saturation and Mg and Ca concentrations slightly decreased in the amended soils. Soil pH of the treated soils ranged from 8.25 to 8.40 prior to the greenhouse growth trial (Supplementary Table S8), which is close to the pH of the untreated soil (8.32). After the greenhouse trial, the soil pH ranged from 8.33 to 8.55, which is only about 0.1 pH unit higher than soil prior to planting. The EC of the untreated soil was initially 85 μS cm−1, but after treatment, ranged from 149 to 285 μS cm−1 (Supplementary Table S8).
Estimated TP in soils post-harvest measured by ignition and H2SO4 extraction ranged from 1,072 mg kg−1–1,142 mg kg−1 (Figure 2). The untreated soil was also digested using both a complete four-acid digestion and a partial three-acid digestion (EPA 3050); TP concentrations were 1,020 mg kg−1 and 993 mg kg−1, respectively. These are close to the value from ignition and H2SO4 extraction (1,102 mg kg−1), suggesting that estimated TP from ignition and H2SO4 extraction is an accurate measure of TP for this soil. Total organic P (Porg) concentrations in the amended soils ranged from 104 mg kg−1–170 mg kg−1 (10%–16% of TP). There were no significant differences in post-harvest TP or Porg concentration between treatments or application rates.
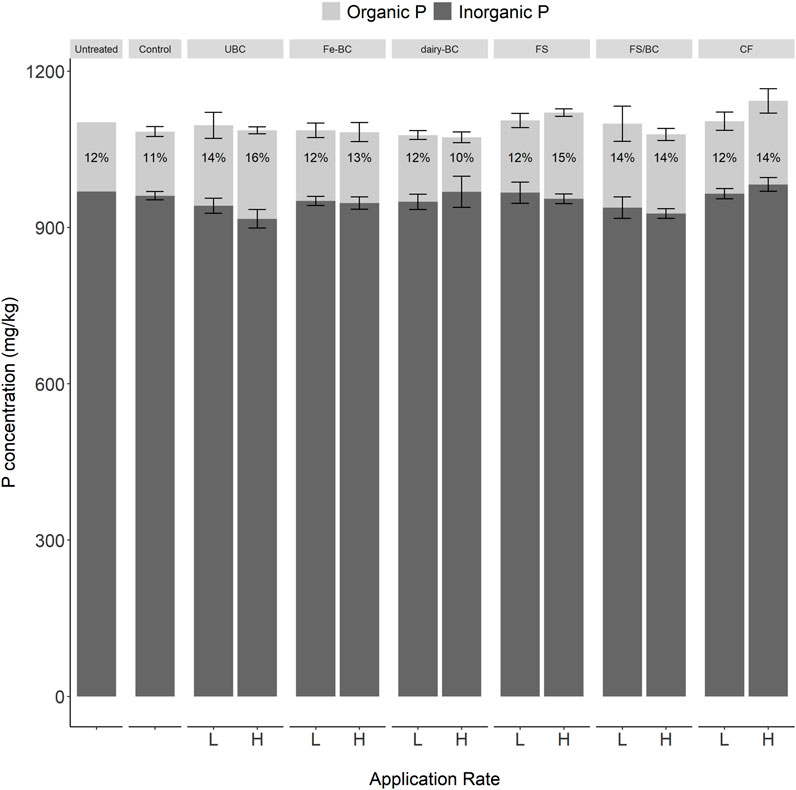
FIGURE 2. Post-harvest organic and inorganic P determined by the ignition and H2SO4 extraction method for all treatments and application rates. Error bars represent standard errors (n = 5). CF, conventional fertilizer; control, untreated soil used in greenhouse experiment; dairy-BC, dairy manure-amended Fe-modified biochar; Fe-BC, 1% Fe-modified biochar; FS, fermented biosolids; FS-BC, FS/BC, fermented biosolids plus unmodified biochar; -L, low application rates; -H, high application rates; UBC, unmodified biochar; untreated, soil sampled prior to greenhouse experiment.
Extraction of soils with NaOH-EDTA removed 194–212 mg P kg−1 (Supplementary Table S9), giving an extraction efficiency of 18.2%–21.2% of total P. Two samples were extracted and analyzed by NMR in duplicate. Differences between the Pi and Po percentages measured by 31P-NMR for both sample replicates were less than 1%. The 31P-NMR analysis showed that most of the NaOH-EDTA extracted P was inorganic P in the form of orthophosphate (ranging from 86%–89%) (Figure 1, Supplementary Table S10, S11). Other Pi forms, including pyrophosphate and polyphosphate, were less than 1% of the samples. Organic P ranged from 10%–14% of the soil extracts. Most of the Po in the soil extracts was monoester P species of inositol hexakisphosphate. The concentrations of orthophosphate diester P compounds (including P in DNA) were approximately half those of the orthophosphate monoester compounds. There were observed differences or trends in Po compound groups or concentrations across the different soil sample treatments.
Normalized XANES spectra for all the amended soils were similar (Supplementary Figure S3), and Ca-P species ranged from 54% to 87% of the overall fit in the soil samples (Figure 3, Supplementary Table S12). Adsorbed P was also fit (13.2%–46.4%) in all samples except CF-H. Organic P (phytic acid) was only able to be fit in the CF-H sample, comprising 16.3% of the fit, which is a minor component of the fit, and is close to the limits of fit accuracy. Compared to other soil XANES spectra fits, UBC-H had a poorer fit quality (Supplementary Figure S3, Supplementary Table S13), suggesting that there may be a species in the sample that is not represented in the standard set. Evaluation of the fit of the spectrum from UBC-H showed that the shoulder at 2,155 eV is poorly fit. This peak is indicative of Ca-P species, thus it is likely that a Ca-P species may be an important phase that is underrepresented in the LCF results of the UBC-H sample.
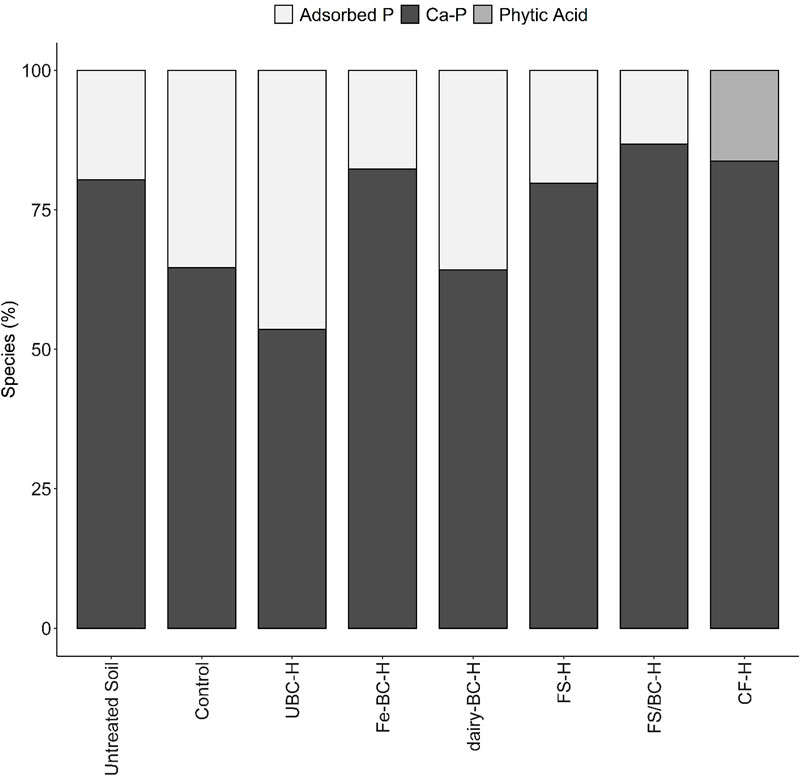
FIGURE 3. Phosphorus species composition of soil samples used in greenhouse pot trial determined by linear combination fitting of K-edge XANES spectra. Soil phosphorus was fit with phosphorus adsorbed on goethite, apatite, dicalcium phosphate dihydrate (DCPD), DCPD 50:50 Ca:Mg, and phytic acid. All Ca-P minerals were combined into one category. CF, conventional fertilizer; control, untreated soil used in greenhouse experiment; dairy-BC, dairy manure-amended Fe-modified biochar; Fe-BC, 1% Fe-modified biochar; FS, fermented biosolids; FS/BC, fermented biosolids plus unmodified biochar; -H, high application rate; UBC, unmodified biochar; untreated soil, sampled prior to the greenhouse experiment.
Total extracted P concentration was greatest in the HNO3 extraction and lowest in the H2O extraction (Table 2). The concentrations of H2O-PMR and H2O-P (determined by ICP-OES) were within 3% of each other (Supplementary Table S14), suggesting that most of the P in this extracted pool is inorganic. The sum of all the sequentially extracted pools in the soils accounted for 76%–81% of TP measured by ignition and H2SO4 digestion. The unextracted P (19%–24% of remaining TP) comprises soil P that is not extractable with these reagents, and is expected to have limited availability for plant uptake or leaching (Hedley et al., 1982). The Hedley extraction is operationally defined, and several papers have shown that the extracts can be inaccurate for the targeted phases on some soils (Hunger et al., 2005; Gul and Whalen, 2016; Gu and Margenot, 2021). However, the procedure sequentially extracts the soils using increasingly more aggressive extract solutions, and thus results provide insight into the relative solubility of P in the soils, which can be related to soil P pools and species given the soil properties and other P speciation information.
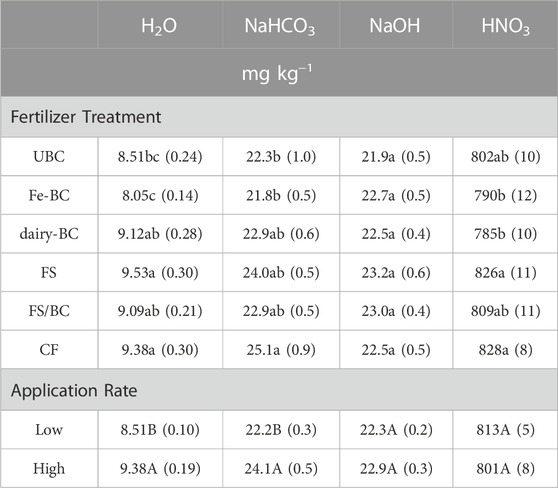
TABLE 2. Estimated marginal means for main effects of treatment and level on Hedley H2O, NaHCO3, NaOH, and HNO3 extractable P in soils after the greenhouse experiments. Values in parentheses are standard errors (n = 5). Means in each column followed by the same letter for fertilizer treatment or application rate are not statistically different (α = 0.05). CF, conventional fertilizer; dairy-BC, dairy manure-amended Fe-modified biochar; Fe-BC, 1% Fe-modified biochar; FS, fermented biosolids; FS/BC, fermented biosolids plus unmodified biochar; UBC, unmodified biochar.
Phosphorus concentrations in the H2O-P or NaHCO3-P extracts from the soils amended with dairy treatments (dairy-BC, FS, and FS/BC) were not significantly different from those of the CF treatments (Table 2). Additionally, the soils amended with biochar that did not have P treatments (UBC and Fe-BC) had significantly less H2O-P and NaHCO3-P than the CF treatment, suggesting that biochar alone does not increase the readily-extractable P pools. Amendments applied at the high level produced significantly more H2O-P and NaHCO3-P than the low amendment treatments, except for the Fe-BC-L treatment. There were no significant differences in NaOH-P concentrations across the treatments. The only difference in the HNO3-P concentrations between the different dairy treatments was in the dairy-BC treatment, which had less HNO3-P than the CF treatment (Table 2).
Pairwise comparisons of treatments within each amendment rate as well as the comparison of treatments to each other at the high and low amendment rates provides additional insight into P dynamics in the extracted pools (Table 3). At the highest amendment rate, the dairy-derived amendments (FS/BC-H, dairy-BC-H, and FS-H) had greater H2O-P concentrations than the control soil and the Fe-BC-H treatments. Soils amended with CF-H, dairy-BC-H, and FS-H had significantly greater NaHCO3-P concentrations than the control soils. The high rate of treatments did not significantly affect the HNO3-P fraction compared to the control. At the low application rates, there were no significant differences in P fractions in the post-greenhouse soil among any of the treatments.
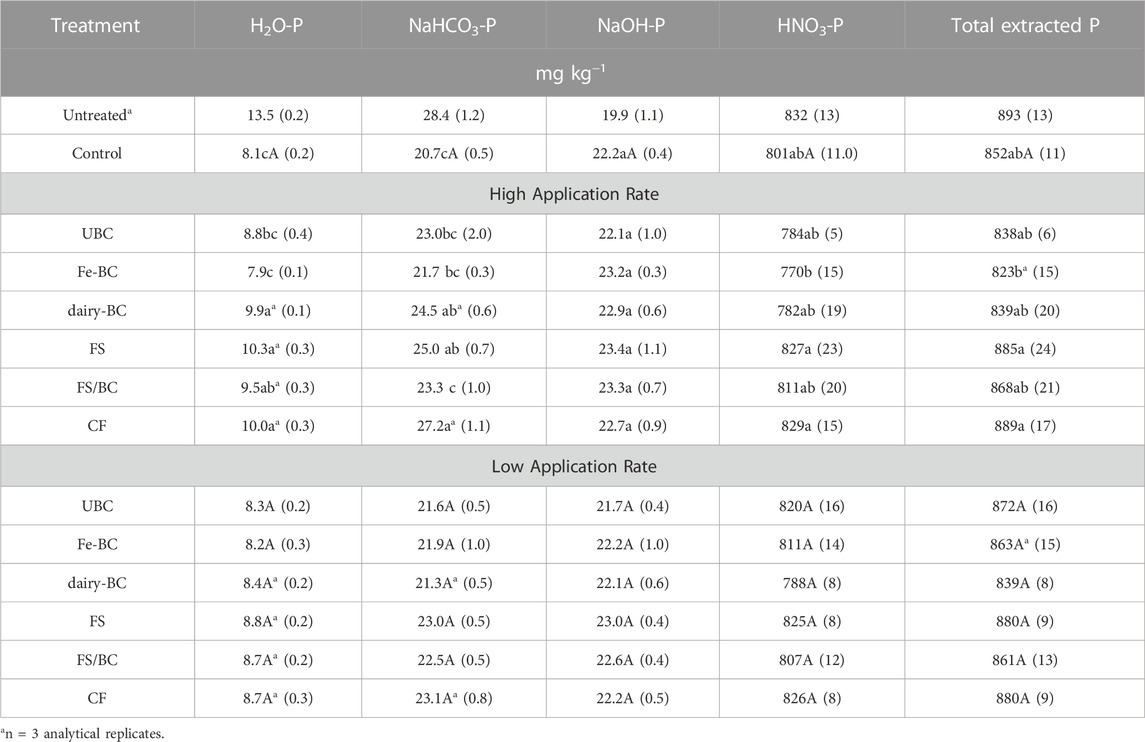
TABLE 3. Estimated marginal means for Hedley H2O-, NaHCO3-, NaOH-, and HNO3-extractable P in soils after the greenhouse experiments. Values in parentheses are standard errors (n = 5). Means in each column within each application rate that have the same letters are not statistically different (α = 0.05). Treatment means at the two application rates that are followed by an asterisk are significantly different (α = 0.05). CF, conventional fertilizer; control, untreated soil used in greenhouse experiment; dairy-BC, dairy manure-amended Fe-modified biochar; Fe-BC, 1% Fe-modified biochar; FS, fermented biosolids; FS/BC, fermented biosolids plus unmodified biochar; UBC, unmodified biochar; Untreated, sampled prior to the greenhouse experiment (included for comparison but not included in statistical analysis.
Before use in the greenhouse, P-δ18O values of the H2O- and NaHCO3-extractable P fractions from the Parma soil were determined (Supplementary Table S15). The isotopic composition of the different extraction pools for the initial untreated Parma soil ranged from 11‰–12.8‰ (Figure 4). The dairy biochar H2O-extractable P-δ18O was 13.1‰, which is too close to the Parma soil P-δ18O for comparison; thus, isotopic analysis was not done on the soils with dairy biochar treatments. The conventional fertilizer had a H2O-extractable P-δ18O value of 24.2‰, which was 11.4‰ greater than the most enriched extraction from the Parma soil. The fermented manure had a H2O-extractable P-δ18O value of 6.9‰, which was 2.5‰ lower than the most depleted extraction from the Parma soil. Because the H2O-extractable P-δ18O values of the conventional fertilizer and fermented manure treatments were significantly different from the soil, the soils treated with these amendments were chosen for P-δ18O extractions to gain insights into P cycling in the amended soils.
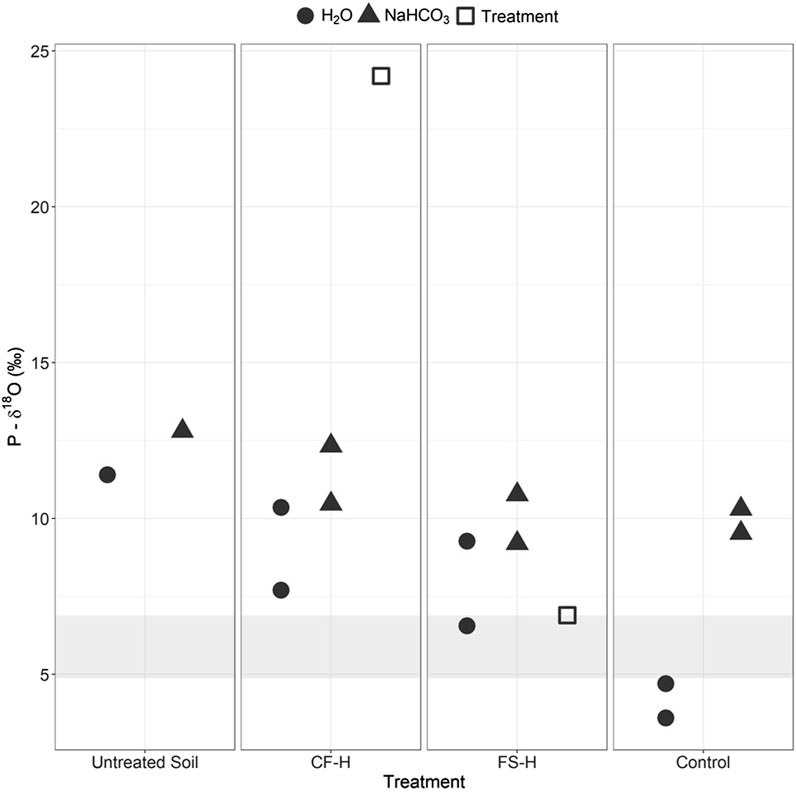
FIGURE 4. Average isotopic values (± standard deviation) for the H2O- and NaHCO3-extracted P fractions from the CF-H, (conventional fertilizer, high application rate) FS-H (fermented biosolids, high application rate), and pre-greenhouse treatment (untreated) and control soils (no amendment). Equilibrium range is denoted by gray bar across graph. Treatment symbols indicate the isotopic characterization of the raw CF and FS amendments.
The microbially-mediated equilibrium range was calculated over the last 2 months of the growth trial using the δ18Ow values from the greenhouse water and average greenhouse temperatures (Supplementary Table S16). Small variations in δ18Ow and average temperatures led to an equilibrium range of 4.9‰–6.9‰. The equilibrium range upper-bound had the same P-δ18O value as the fermented manure (Figure 4).
Comparison of the H2O-extractable P-δ18O of the untreated Parma soil (pre-greenhouse planting) to the control soil (no treatment, post-greenhouse planting) shows a depletion in P-δ18O occurred during the greenhouse growth trial, indicating that the amount of P-δ18O decreased through soil cycling to near the δ18Ow equilibrium values (Figure 4); the H2O-extractable P-δ18O values are actually slightly lower than equilibrium values, which has been observed in other studies (Blake et al., 2005; Zohar et al., 2010; Angert et al., 2012; Gross and Angert, 2015; Joshi et al., 2018; Liang et al., 2018). The H2O-extractable P-δ18O values in the CF-H and FS-H soils were 2‰–6‰ greater than the control soil (Figure 4), suggesting that amendment addition changed the H2O-extractable fraction P-δ18O values in the treated soils.
The P-δ18O values in the NaHCO3 fraction of the CF-H and FS-H treated soils were similar to the pre-greenhouse Parma soil and control soil, although there was a slight decrease towards equilibrium in the treated samples. The variation in NaHCO3-extracted P-δ18O was lower than in the H2O-extracted pool. Low variations in NaHCO3-extracted P-δ18O signatures have been found in other studies as well (Zohar et al., 2010).
Root mass, plant biomass, and plant yield varied across treatment type and level (Table 4). At the low application rate, plant biomass, yield, and number of heads were significantly greater than at the high application rate. Plant biomass was not significantly different for the CF, FS, and dairy-BC treatments. However, the yields of FS and dairy-BC treatments were significantly greater than the yields of the CF treatment.
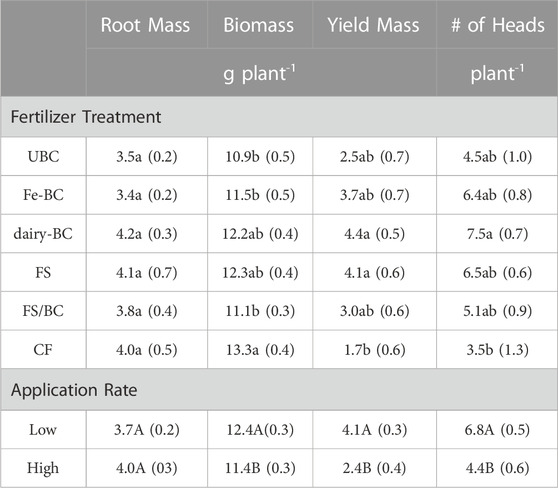
TABLE 4. Estimated marginal means for main effects of treatment and level on root mass, dried plant biomass, yield, and number of barley heads per plant. Values in parentheses are standard errors (n = 5). Means in the same column followed by the same letters are not statistically different (α = 0.05). CF, conventional fertilizer; dairy-BC, dairy manure-amended Fe-modified biochar; Fe-BC, 1% Fe-modified biochar; FS, fermented biosolids; FS/BC, fermented biosolids plus unmodified biochar; UBC, unmodified biochar.
Pairwise comparisons of treatments within the high and low application rates and comparisons of each treatment are shown in Table 5. Plant biomass of dairy-BC and Fe-BC treatments were significantly greater at the low application rate. At the high rate, Fe-BC-H biomass was significantly less than CF-H. Compared to the control, biomasses for the CF-H, CF-L, Fe-BC-L, dairy-BC-L, FS-L, and FS-H treatments were significantly greater.
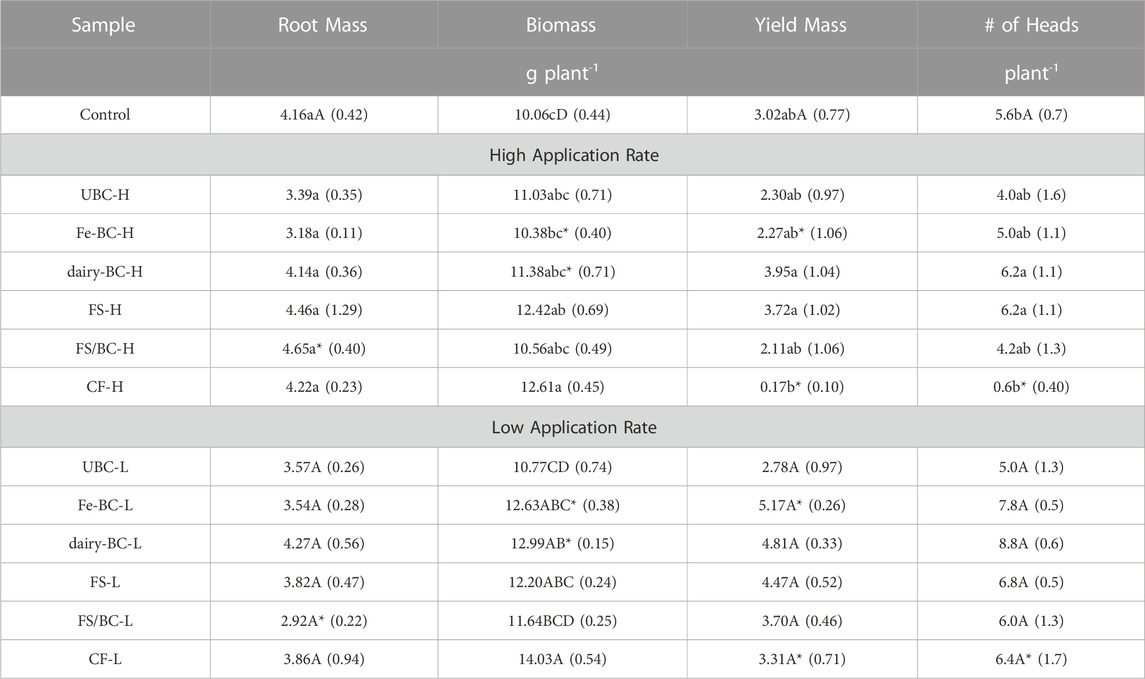
TABLE 5. Estimated marginal means for biomass, root biomass, yield mass, and number of grain heads for barley harvested from greenhouse pot trial. Values in parentheses are standard errors (n = 5). Pairwise comparisons of treatments within each application rate and comparisons to control with the same letters are not statistically different (α = 0.05) within each application rate. Means within application rate followed by the same letter are not significant differences at a 95% confidence level. Comparison of each treatment type at both application rates that are followed by an asterisk are significantly different (α = 0.05). CF, conventional fertilizer; control, untreated soil used in greenhouse experiment; dairy-BC, dairy manure-amended Fe-modified biochar; Fe-BC, 1% Fe-modified biochar; FS, fermented biosolids; FS/BC, fermented biosolids plus unmodified biochar; H, high application rate; UBC, unmodified biochar; untreated soil, sampled prior to the greenhouse experiment.
Grain yield mass for the Fe-BC and CF treatments were significantly greater at the low application rate than high application rate. In the CF-H treatment, the barley did not form any heads, thus yield for this sample was not compared to other treatments; instead, only biomass was compared to assess CF-H treatment effects on plant growth. The reason for the failed head production in the CF-H treatment is unclear but could be due to overapplication of nutrients in a readily available form (conventional fertilizer) that stimulated the plants to continue to grow with little energy being directed toward reproduction. Yields for other treatments were not significantly different from the control.
Phosphorus availability to crops from dairy-manure derived soil amendments can be highly variable, depending on the source and handling of the dairy manure. In contrast, nutrients in conventional fertilizers are designed to be readily available for crop uptake upon application to the soil. The dairy-derived biochar and fermented manure used in this study had more total P than the unmodified biochar (Table 1), and greater water-extractable P (H2O-P). However, the water-extractable P in the treated dairy materials was less than 10% of the total P, suggesting the majority of P in the fermented manure and dairy-derived biochar has limited availability for plant uptake. Streubel et al. (2012) observed that P recovered on biochar from anaerobic digestate had nearly 30- and five-times more H2O- and NaHCO3-extractable P concentrations, respectively. The low extractable P in the dairy-BC from this study is likely a result of the Fe-modification that facilitated strong adsorption of phosphate, preventing it from being released (Strawn et al., 2023). Compared to other studies (e.g., Jorgensen et al., 2010), the fermented manure used in this study had lower concentrations of water-extractable P, possibly because the coarse and fine fermented manure fractions were mixed in this study, and the coarse fraction may contain a significant amount of P fixed in incompletely digested plant fibers.
The low H2O-P concentrations extracted from the dairy-derived amendments suggests low plant availability. Water-extractable P is also a good predictor of the amount of soil P that is soluble and available for leaching (McDowell and Sharpley, 2001); thus the dairy-derived amendments will likely have reduced P leaching as compared to conventional fertilizers and raw or composted manures. Since P availability in plant rhizospheres is impacted by microbial processes and plant exudates that enhance P solubility, the availability of P from the dairy-derived amendments used in this study may be more plant available than indicated by the H2O-P and NaHCO3-P concentrations (Hinsinger, 2001; Richardson et al., 2009). Thus, while the decreased H2O-P from the dairy-derived amendments may be plant available in the rhizosphere, the reduced P availability in the bulk soils may facilitate less P leaching into surface waters (Shen et al., 2011).
Total P in the soil after greenhouse plant growth did not vary with treatment (Figure 2). However, based on sequential extraction results, partitioning of P into soil pools did vary (Table 2). When applied at a high rate, plant-available P (H2O-P and NaHCO3- P) increased in soils amended with dairy-derived fertilizers (dairy-BC, FS, FS/BC) compared to the control soil, but were not significantly different from the chemical fertilizer. Collins et al. (2020) observed that anaerobically digested dairy solids provided similar amounts of plant available P as synthetic fertilizers, and there were no differences in crop yield.
The P-δ18O values from H2O and NaHCO3 extraction of soils provide insight into transformations between extractable P pools in the soil. The P-δ18O values in the H2O extracts were closer to equilibrium values than the NaHCO3 extracts (Figure 4), indicating that P compounds in this fraction are actively cycled by microbes (Blake et al., 2005; Zohar et al., 2010; Angert et al., 2012; Gross et al., 2015; Joshi et al., 2018; Liang et al., 2018).
The most enriched P-δ18O values occurred in the CF-H treatment (Figure 4). Average H2O-extractable P-δ18O values in the amended soils (CF-H and FS-H) were higher than the control treatment. Gross et al. (2015) also observed that in P-amended soils, P-δ18O values were farther from equilibrium than in control soils, suggesting that the rate of microbial P turnover is slower when microbes are not P-limited. This agrees with the CF-H and FS-H treated soils, which had greater concentrations of available P compared to the control soils (Table 3), thus promoting slower microbial cycling of the H2O-extractable soil P.
At the end of the growth trials, the NaHCO3-extracted P-δ18O values in the CF-H and FS-H treated soils were enriched by 3.5‰ from the top of the equilibrium range. Gross et al. (2015) and Blake et al. (2005) also reported a 3‰–3.5‰ enrichment in labile-P pools in lab and field studies. The apparent steady-state enrichment suggests uptake of lighter isotopologues by plants and microorganisms, leaving the remaining soil pool enriched (Gross et al., 2015). Additionally, the enrichment in NaHCO3-extracted P-δ18O values as compared to the H2O-P pool indicates that the rate of microbial cycling is slower in the NaHCO3-extracted P pool, likely because it is more tightly bound and thus is consumed by microorganisms after the more available H2O-P (Joshi et al., 2016).
To predict P-δ18O progression towards steady-state equilibrium, an exponential decay model was used to calculate turnover times of the H2O- and NaHCO3-extracted P-δ18O from the untreated Parma soils. The model uses initial P-δ18O values in the soil extracts (t = 0), the measured isotopic values at t = 150 days incubation in the greenhouse, and the steady-state equilibrium values predicted based on Eq. (1). The model assumes that cycling to equilibrium follows an exponential decay function:
where τ is the effective time constant of the system in days and t is the sampling time in days from the beginning of the greenhouse study. Subscripts i, 1, and e represent P-δ18O values at initial, day 150, and equilibrium. Values of τ were optimized for the untreated Parma soil after greenhouse incubation. The derivation of Eq. (2) is based on the model proposed by Olson (1963) for the net rate of change in material in ecological systems, where τ represents the approximate time it takes for decomposition to 37% of the initial level (multiply by 0.693 to convert to the degradation half-life).
A main finding from the P-δ18O tracing is that the average half-life for the H2O-extractable P-δ18O from the Parma soil was 47 days (Supplementary Table S17, Supplementary Figure S4), which is much faster than the 233 days P-δ18O half-life for the NaHCO3-extracted P. This supports the main idea that the H2O and NaHCO3 extractions selectively remove different P pools, and that H2O-extractable P is more available to the soil solution than NaHCO3-extractable P. Further research to measure P-δ18O in plant tissues would allow for additional confirmation of the plant uptake of P from the applied fertilizer and fermented manure treatments.
Results from the ignition and H2SO4 extraction, 31P-NMR, and P K-edge XANES analyses revealed that the majority of P in the treated and control soil samples existed as inorganic P species. Low Po concentrations were similar to concentrations found by other researchers investigating dairy-manure amended soils from the same region (Hansen et al., 2004; Weyers et al., 2016). Although the fermented manure treatment had more Po than the dairy-BC, the soil amendment rate was not enough to significantly change the Po concentrations in the soil as measured by ignition followed by H2SO4 extraction.
The NaOH-EDTA soil extracts used for NMR analysis only extracted a small amount of the total soil P (Supplementary Table S9), which is common in calcareous soils (Turner et al., 2003; Hansen et al., 2004; Weyers et al., 2016). Phosphorus not extracted by NaOH-EDTA is considered mineral-bound inorganic P that is not readily available for biological cycling (Cade-Menun, 2015). Results from 31P-NMR analysis showed that the majority of P extracted from the soil samples was orthophosphate (86%–90%; Figure 3). Leytem and Westermann (2005) found that dairy lagoon water was comprised of less than 10% Po, which is similar to the low levels of Po in the dairy lagoon water-amended biochars in this study. Mazzini et al. (2020) also observed that in anaerobic-digested cattle manure, Po was much lower than Pi.
Based on results from the NMR analysis of the soil extracts, the FS- and FS/BC-treated soil samples were the most enriched in Po compounds (Figure 1, Supplementary Table S1), which is a result of the greater amount of Po compounds in the fermented-manure amendment (32% in NaOH-EDTA extracts detected by NMR, Figure 1). The ratio of monoesters to diesters after correction for degradation was greater than one for all soils, indicating that orthophosphate monoesters are the dominant compound class of organic P in these samples. Phytate (myo-IHP) was the dominant orthophosphate monoester compound detected in the soil samples. This is a storage compound in higher plants and seeds, and is often detected in manures and agricultural soils (Hill and Cade-Menun, 2009; Cade-Menun et al., 2010). The FS also had more orthophosphate diester P compounds than the dairy-BC amendment, likely reflecting high microbial activity during fermentation. However, in the soils amended with FS, the concentrations and proportions of orthophosphate diester P compounds were not different from the other soils, which suggests that they must have been degraded after they were applied to the soil. The FS-treated soil had the highest concentration of total IHP compounds, which include myo-IHP and its stereoisomers scyllo-, neo- and chiro-IHP. These compounds can be strongly sorbed to calcite in calcareous soils, reducing their availability (Celi et al., 2000), but the IHP associated with Ca minerals can be readily mineralized, releasing the P to soil solution (Doolette et al., 2010).
Analysis of the soils using XANES spectroscopy indicated that two main groups of P species were present: adsorbed P (0%–46%) and Ca-P (54%–87%; Figure 3). Both groups can include several P species, including both Pi and Po compounds associated with Ca minerals. Phytic acid was used as a model Po compound and was only fit in the CF sample at 16%, which is near the sensitivity level for P XANES fitting. Adsorbed P species are considered to have a greater potential to be released to soil solution than stable Ca-P mineral species like hydroxyapatite (Hansen et al., 2004; Weyers, 2016); less stable Ca-P minerals such as brushite (DCPD) and octacalcium phosphate (OCP) are more soluble than hydroxyapatite (Lindsay, 1979). The CF-H treated soil was the only sample with no adsorbed P, suggesting that much of the P in this soil was quickly fixed as low solubility Ca-P compounds (e.g., hydroxyapatite) that may limit plant uptake and reduce leaching losses. This is in contrast to Kar et al. (2011), who showed that application of synthetic P fertilizer resulted in both apatite and adsorbed P species in the soil. The predominance of Ca-P compounds in these soils agrees with the high amounts of HNO3-extractable P in the Hedley fractionation, which is thought to contain low-solubility Ca-P minerals such as hydroxyapatite (Hunger et al., 2005; Gu and Margenot, 2021). Past studies of soils amended with biochar show an increase in insoluble Ca-P species (Siebers et al., 2013; Morshedizad et al., 2018; Peng et al., 2021). Beauchemin et al. (2003) also found agreement between Ca-P species determined by sequential extraction and P K-edge XANES fitting in calcareous soils and suggested that P was removed from the plant available pool through precipitation as calcium phosphate minerals that have lower solubility.
The effects of experimental soil treatments on plant growth were assessed using biomass and yield data. At the low amendment application rates, biomass and yield main effects were greater than at the high rates (Table 4), suggesting that there is a maximum amendment rate benefit, and beyond this level there may be an inhibitory effect. Other studies have also reported decreased plant production at high biochar application rates (>60 Mg ha−1) (Rondon et al., 2007; Mia et al., 2014; Aller et al., 2018). Decreased yield with biochar application may be due to high C:N ratios of biochar stimulating microbial N immobilization and removing N from the plant available pool (Rondon et al., 2007; Deenik et al., 2010; Aller et al., 2018). The biochar could also affect micronutrient plant availability or cause salinity issues. There were slight increases in Fe, Mn and Zn in the amended soils compared to the control (Supplementary Table S3), but since the Parma soil was sufficient in all micronutrient requirements (Havlin, 2013), and the increases also occurred in the non-dairy derived amendments in the control treatments, these micronutrients are ruled out as the reason for the increased barley growth.
Compared to the control, the CF treatment at both high and low application rates had a significant increase in biomass, but not yield (Table 5). Since grain production is a reproductive stage of the barley life cycle, it can be triggered by many different factors, including soil and environmental conditions (temperature, light, water); even plant stress can trigger seed heading. Furthermore, during kernel development, plant energy sent to the grain may be diverted from stems and leaves, causing biomass to decrease. Thus, while interpretation of the barley yield and plant biomass response to treatments is tenuous, some general observations can be made. The main effects of the dairy amendments show no significant difference in biomass as compared to the CF amendment. Amongst the dairy amendments, the FS treatment had a significant increase in biomass compared to the control at both high and low rates. The dairy-BC treatment had greater biomass than the control only at the low rate. Based on biomass production, the FS and dairy-BC supplied adequate nutrients to the plants to support growth.
Many studies have noted a positive effect of biochar on plant growth. Melo et al. (2022) completed a meta-analysis of biochar effects on plant growth and observed that biochar amendment increased plant productivity by an average of 10% compared to a traditional fertilizer. In the barley growth greenhouse trial, there were no significant differences in biomass or yield in the untreated biochar amendments (UBC) as compared to the control at either amendment rate. Thus, it is concluded that the biochar alone had no effects on barley growth. It has been noted that benefits from biochar amendment may take several years (Jones et al., 2012), thus the one season barley growth in a greenhouse trial may not be enough to capture the complete physical or chemical benefits from the non-enhanced biochar (UBC) amendment.
Applying unprocessed manure to soil has implications for environmental health. As an alternative, dairy-derived fertilizers utilizing P from liquid and solid dairy waste streams are potential value-added products that can help avoid high application rates of manure-sourced nutrients to soils located near dairies. Two bioproducts evaluated in this paper are biochar enhanced with dairy wastewater and fermented manure biosolids. Less than 10% of the total P from the dairy-manure derived bioproducts was water-extractable, yet when applied at a P loading rate equivalent to conventional fertilizer, barley plant productivity was the same. This suggests that the manure-loaded biochar and fermented manure can supply sufficient P for plant growth. Given the reduced extractability of P from the dairy-derived amendments, P losses from the soil will be reduced, leading to improved water quality.
Improving the use of dairy-derived bioproducts as soil amendments requires studies that will elucidate the mechanisms controlling P chemistry and availability in soils (Ajiboye et al., 2007; Peak et al., 2012; Gelardi and Parikh, 2021). In this paper, research showed that the majority of P in the amended soils was either absorbed P or Ca-P species such as hydroxyapatite. Organic P compounds in the amended soils were observed to be only minor species, even in the fermented manure treatment that had the highest total organic P (28% of the NaOH-EDTA extractable P). Assessment of P-δ18O cycling showed that microbial cycling of P from available pools was slower when soils were not P limited, and further that the P pool extracted with bicarbonate was cycled more slowly than the P pool extracted with water.
Results from this study provide mechanistic details of P cycling in soils when amended with P from dairy waste streams and biochar. This information is crucial for advancing the use of these potential fertilizers and producing bioproducts that can help close the gap in the circular bioeconomy of the dairy industry while simultaneously maintaining crop yields and preventing P losses to surface water. Additionally, recycling dairy-derived nutrients on biochar can be an important source of organic carbon addition to soils, supporting carbon sequestration efforts (Osman et al., 2022).
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
DS: Conceptualization, Funding acquisition, Investigation, Methodology, Resources, Supervision, Writing–original draft, Writing–review and editing. ML: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing–original draft, Writing–review and editing. ZK: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing–review and editing. BC-M: Investigation, Methodology, Validation, Writing–review and editing. GM: Conceptualization, Funding acquisition, Investigation, Methodology, Resources, Writing–review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This publication was developed under Agreement No. 2020-69012-31871, funded by the U.S. Department of Agriculture (USDA), National Institute of Food and Agriculture (to DS, ZK, and GM). This work was also supported by the Idaho Agricultural Experiment Station, USDA, NIFA Project Number IDA01711 (to DS, ZK and GM).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fceng.2023.1303357/full#supplementary-material
Adhikari, M., Paudel, K. P., Martin, N. R., and Gauthier, W. M. (2005). Economics of dairy waste use as fertilizer in central Texas. Waste Manag. 25, 1067–1074. doi:10.1016/j.wasman.2005.06.012
Ahmad, T., Aadil, R. M., Ahmed, H., Rahman, U. u., Soares, B. C. V., Souza, S. L. Q., et al. (2019). Treatment and utilization of dairy industrial waste: a review. Trends Food Sci. Technol. 88, 361–372. doi:10.1016/j.tifs.2019.04.003
Ajiboye, B., Akinremi, O. O., and Jürgensen, A. (2007). Experimental validation of quantitative XANES analysis for phosphorus speciation. Soil Sci. Soc. Am. J. 71, 1288–1291. doi:10.2136/sssaj2007.0007
Aller, D. M., Archontoulis, S. V., Zhang, W., Sawadgo, W., Laird, D. A., and Moore, K. (2018). Long term biochar effects on corn yield, soil quality and profitability in the US Midwest. Field Crops Res. 227, 30–40. doi:10.1016/j.fcr.2018.07.012
An, X., Wu, Z., Liu, X., Shi, W., Tian, F., and Yu, B. (2021). A new class of biochar-based slow-release phosphorus fertilizers with high water retention based on integrated co-pyrolysis and co-polymerization. Chemosphere 285, 131481. doi:10.1016/j.chemosphere.2021.131481
Angert, A., Weiner, T., Mazeh, S., and Sternberg, M. (2012). Soil phosphate stable oxygen isotopes across rainfall and bedrock gradients. Environ. Sci. Technol. 46, 2156–2162. doi:10.1021/es203551s
Bach, I.-M., Essich, L., and Müller, T. (2021). Efficiency of recycled biogas digestates as phosphorus fertilizers for maize. Agriculture 11, 553. doi:10.3390/agriculture11060553
Bates, D., Machler, M., Bolker, B., and Walker, (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. doi:10.18637/jss.v067.i01
Beauchemin, S., Hesterberg, D., Chou, J., Beauchemin, M., al, e., and Sayers, D. E. (2003). Speciation of phosphorus in phosphorus-enriched agricultural soils using x-ray absorption near-edge structure spectroscopy and chemical fractionation. J. Environ. Qual. 32, 1809–1819. doi:10.2134/jeq2003.1809
Blake, R. E., O’Neil, J. R., and Surkov, A. V. (2005). Biogeochemical cycling of phosphorus: insights from oxygen isotope effects of phosphoenzymes. Am. J. Sci. 305, 596–620. doi:10.2475/ajs.305.6-8.596
Cade-Menun, B., and Liu, C. W. (2014). Solution phosphorus-31 nuclear magnetic resonance spectroscopy of soils from 2005 to 2013: a review of sample preparation and experimental parameters. Soil Sci. Soc. Am. J. 78, 19–37. doi:10.2136/sssaj2013.05.0187dgs
Cade-Menun, B. J. (2015). Improved peak identification in 31P-NMR spectra of environmental samples with a standardized method and peak library. Geoderma 257-258, 102–114. doi:10.1016/j.geoderma.2014.12.016
Cade-Menun, B. J., Carter, M. R., James, D. C., and Liu, C. W. (2010). Phosphorus forms and chemistry in the soil profile under long-term conservation tillage: a phosphorus-31 nuclear magnetic resonance study. J. Environ. Qual. 39, 1647–1656. doi:10.2134/jeq2009.0491
Cade-Menun, B. J., and Lavkulich, L. M. (1997). A comparison of methods to determine total, organic, and available phosphorus in forest soils. Commun. Soil Sci. Plant Analysis 28, 651–663. doi:10.1080/00103629709369818
Cade-Menun, B. J., and Preston, C. M. (1996). A comparison of soil extraction procedures for 31P NMR Spectroscopy. Soil Sci. 161, 770–785. doi:10.1097/00010694-199611000-00006
Cao, X., and Harris, W. (2010). Properties of dairy-manure-derived biochar pertinent to its potential use in remediation. Bioresour. Technol. 101, 5222–5228. doi:10.1016/j.biortech.2010.02.052
Carey, A., Moore, A., and Leytem, A. (2011). “Phosphorus in the calcareous soils of Southern Idaho: a literature review with implications for crop production, manure management and water quality,” in University of Idaho extension (Moscow, Idaho: University of Idaho).
Carreira, J. A., Viñegla, B., and Lajtha, K. (2006). Secondary CaCO3 and precipitation of P–Ca compounds control the retention of soil P in arid ecosystems. J. Arid Environ. 64, 460–473. doi:10.1016/j.jaridenv.2005.06.003
Celi, L., Lamacchia, S., and Barberis, E. (2000). Interaction of inositol phosphate with calcite. Nutrient Cycl. Agroecosyst. 57, 271–277. doi:10.1023/a:1009805501082
Collins, H. P., Kimura, E., and Smith, D. R. (2020). Sweet corn phosphorus uptake from sandy soil amended with anaerobically-digested manure. Commun. Soil Sci. Plant Analysis 51, 2398–2413. doi:10.1080/00103624.2020.1836208
Dalahmeh, S. S., Stenström, Y., Jebrane, M., Hylander, L. D., Daniel, G., and Heinmaa, I. (2020). Efficiency of iron- and calcium-impregnated biochar in adsorbing phosphate from wastewater in onsite wastewater treatment systems. Front. Environ. Sci. 8. doi:10.3389/fenvs.2020.538539
Deenik, J. L., McClellan, T., Uehara, G., Antal, M. J., and Campbell, S. (2010). Charcoal volatile matter content influences plant growth and soil nitrogen transformations. Soil Sci. Soc. Am. J. 74, 1259–1270. doi:10.2136/sssaj2009.0115
De Rosa, D., Biala, J., Nguyen, T. H., Mitchell, E., Friedl, J., Scheer, C., et al. (2022). Environmental and economic trade-offs of using composted or stockpiled manure as partial substitute for synthetic fertilizer. J. Environ. Qual. 51, 589–601. doi:10.1002/jeq2.20255
Doolette, A. L., Smernik, R. J., and Dougherty, W. J. (2010). Rapid decomposition of phytate applied to a calcareous soil demonstrated by a solution 31P NMR study. Eur. J. Soil Sci. 61, 563–575. doi:10.1111/j.1365-2389.2010.01259.x
Doydora, S., Gatiboni, L., Grieger, K., Hesterberg, D., Jones, J. L., McLamore, E. S., et al. (2020). Accessing legacy phosphorus in soils. Soil Syst. 4, 74. doi:10.3390/soilsystems4040074
Femeena, P. V., Costello, C., and Brennan, R. A. (2023). Spatial optimization of nutrient recovery from dairy farms to support economically viable load reductions in the Chesapeake Bay Watershed. Agric. Syst. 207, 103640. doi:10.1016/j.agsy.2023.103640
Gavlak, R., Horneck, D., and Miller, R. O. (2005). Soil, plant and water reference methods for the western region, WREP 125. Wisconsin: North American Proficiency Testing.
Gelardi, D. L., and Parikh, S. J. (2021). Soils and beyond: optimizing sustainability opportunities for biochar. Sustainability 13, 10079. doi:10.3390/su131810079
Ghezzehei, T. A., Sarkhot, D. V., and Berhe, A. A. (2014). Biochar can be used to capture essential nutrients from dairy wastewater and improve soil physico-chemical properties. Solid earth. 5, 953–962. doi:10.5194/se-5-953-2014
Glaser, B., and Lehr, V.-I. (2019). Biochar effects on phosphorus availability in agricultural soils: a meta-analysis. Sci. Rep. 9, 9338. doi:10.1038/s41598-019-45693-z
Gross, A., and Angert, A. (2015). What processes control the oxygen isotopes of soil bio-available phosphate? Geochimica Cosmochimica Acta 159, 100–111. doi:10.1016/j.gca.2015.03.023
Gross, A., Turner, B. L., Wright, S. J., Tanner, E. V. J., Reichstein, M., Weiner, T., et al. (2015). Oxygen isotope ratios of plant available phosphate in lowland tropical forest soils. Soil Biol. Biochem. 88, 354–361. doi:10.1016/j.soilbio.2015.06.015
Gu, C., and Margenot, A. J. (2021). Navigating limitations and opportunities of soil phosphorus fractionation. Plant Soil 459, 13–17. doi:10.1007/s11104-020-04552-x
Gul, S., and Whalen, J. K. (2016). Biochemical cycling of nitrogen and phosphorus in biochar-amended soils. Soil Biol. Biochem. 103, 1–15. doi:10.1016/j.soilbio.2016.08.001
Güngör, K., Jürgensen, A., and Karthikeyan, K. G. (2007). Determination of phosphorus speciation in dairy manure using XRD and XANES spectroscopy. J. Environ. Qual. 36, 1856–1863. doi:10.2134/jeq2006.0563
Gustafsson, J. P., Braun, S., Tuyishime, J. R. M., Adediran, G. A., Warrinnier, R., and Hesterberg, D. (2020). A probabilistic approach to Phosphorus speciation of soils using P K-edge XANES spectroscopy with linear combination fitting. Soil Syst. 4, 26. doi:10.3390/soilsystems4020026
Hale, S. E., Alling, V., Martinsen, V., Mulder, J., Breedveld, G. D., and Cornelissen, G. (2013). The sorption and desorption of phosphate-P, ammonium-N and nitrate-N in cacao shell and corn cob biochars. Chemosphere 91, 1612–1619. doi:10.1016/j.chemosphere.2012.12.057
Hansen, J. C., Cade-Menun, B. J., and Strawn, D. G. (2004). Phosphorus speciation in manure-amended alkaline soils. J. Environ. Qual. 33, 1521–1527. doi:10.2134/jeq2004.1521
Havlin, J. (2013). Soil fertility and fertilizers: an introduction to nutrient management. Upper Saddle River, N.J.: Pearson.
Haygarth, P. M., and Sharpley, A. N. (2000). Terminology for phosphorus transfer. J. Environ. Qual. 29, 10–15. doi:10.2134/jeq2000.00472425002900010002x
He, S., Zhong, L., Duan, J., Feng, Y., Yang, B., and Yang, L. (2017). Bioremediation of wastewater by iron oxide-biochar nanocomposites loaded with photosynthetic bacteria. Front. Microbiol. 8, 823. doi:10.3389/fmicb.2017.00823
Hedley, M. J., Stewart, J. W. B., and Chauhan, B. S. (1982). Changes in inorganic and organic soil phosphorus fractions induced by cultivation practices and by laboratory incubations. Soil Sci. Soc. Am. J. 46, 970–976. doi:10.2136/sssaj1982.03615995004600050017x
Hill, J. E., and Cade-Menun, B. J. (2009). Phosphorus-31 nuclear magnetic resonance spectroscopy transect study of poultry operations on the Delmarva Peninsula. J. Environ. Qual. 38, 130–138. doi:10.2134/jeq2007.0587
Hinsinger, P. (2001). Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant Soil 237, 173–195. doi:10.1023/a:1013351617532
Hunger, S., Sims, J. T., and Sparks, D. L. (2005). How accurate is the assessment of phosphorus pools in poultry litter by sequential extraction? J. Environ. Qual. 34, 382–389. doi:10.2134/jeq2005.0382
Ingall, E. D., Brandes, J. A., Diaz, J. M., de Jonge, M. D., Paterson, D., McNulty, I., et al. (2011). Phosphorus K-edge XANES spectroscopy of mineral standards. J. Synchrotron Radiat. 18, 189–197. doi:10.1107/s0909049510045322
Jones, D. L., Rousk, J., Edwards-Jones, G., DeLuca, T. H., and Murphy, D. V. (2012). Biochar-mediated changes in soil quality and plant growth in a three year field trial. Soil Biol. Biochem. 45, 113–124. doi:10.1016/j.soilbio.2011.10.012
Jorgensen, K., Magid, J., Luxhoi, J., and Jensen, L. S. (2010). Phosphorus distribution in untreated and composted solid fractions from slurry separation. J. Environ. Qual. 39, 393–401. doi:10.2134/jeq2009.0168
Joshi, S. R., Li, W., Bowden, M., and Jaisi, D. P. (2018). Sources and pathways of formation of recalcitrant and residual phosphorus in an agricultural soil. Soil Syst. 2, 45. doi:10.3390/soilsystems2030045
Joshi, S. R., Li, X., and Jaisi, D. P. (2016). Transformation of phosphorus pools in an agricultural soil: an application of oxygen-18 labeling in phosphate. Soil Sci. Soc. Am. J. 80, 69–78. doi:10.2136/sssaj2015.06.0219
Kar, G., Hundal, L. S., Schoenau, J. J., and Peak, D. (2011). Direct chemical speciation of P in sequential chemical extraction residues using P K-edge X-ray absorption near-edge structure spectroscopy. Soil Sci. 176, 589–595. doi:10.1097/ss.0b013e31823939a3
Kolev, S. A. (2017). General characteristics and treatment possibilities of dairy wastewater – a review. Food Technol. Biotechnol. 55, 14–28. doi:10.17113/ftb.55.01.17.4520
Kopecky, M., Kolar, L., Konvalina, P., Strunecky, O., Teodorescu, F., Mraz, P., et al. (2020). Modified biochar-A tool for wastewater treatment. Energies 13, 5270. doi:10.3390/en13205270
Krounbi, L., Enders, A., Gaunt, J., Ball, M., and Lehmann, J. (2021). Plant uptake of nitrogen adsorbed to biochars made from dairy manure. Sci. Rep. 11, 15001. doi:10.1038/s41598-021-94337-8
Kushwaha, J. P., Srivastava, V. C., and Mall, I. D. (2011). An overview of various technologies for the treatment of dairy wastewaters. Crit. Rev. Food Sci. Nutr. 51, 442–452. doi:10.1080/10408391003663879
Lenth, R. V., Buerkner, P., Herve, M., Love, J., Miguez, F., Riebl, H., et al. (2022). Emmeans: estimated marginal means, aka least-squares means. 1.7.3 ed.
Leytem, A. B., and Mikkelsen, R. L. (2005). The nature of phosphorus in calcareous soils. Better Crops 89 (2), 11–13.
Leytem, A. B., and Westermann, D. T. (2005). Phosphorus availability to barley from manures and fertilizers on a calcareous soil. Soil Sci. 170, 401–412. doi:10.1097/01.ss.0000169914.17732.69
Li, G., Li, H., Leffelaar, P. A., Shen, J., and Zhang, F. (2014). Characterization of phosphorus in animal manures collected from three (dairy, swine, and broiler) farms in China. PLoS One 9, e102698. doi:10.1371/journal.pone.0102698
Liang, X., Jin, Y., He, M., Niyungeko, C., Zhang, J., Liu, C., et al. (2018). Phosphorus speciation and release kinetics of swine manure biochar under various pyrolysis temperatures. Environ. Sci. Pollut. Res. Int. 25, 25780–25788. doi:10.1007/s11356-017-0640-8
Liang, Y., Cao, X., Zhao, L., Xu, X., and Harris, W. (2014). Phosphorus release from dairy manure, the manure-derived biochar, and their amended soil: effects of phosphorus nature and soil property. J. Environ. Qual. 43, 1504–1509. doi:10.2134/jeq2014.01.0021
Liu, F., Zuo, J., Chi, T., Wang, P., and Yang, B. (2015). Removing phosphorus from aqueous solutions by using iron-modified corn straw biochar. Front. Environ. Sci. Eng. 9, 1066–1075. doi:10.1007/s11783-015-0769-y
Lustosa Filho, J. F., Carneiro, J. S. d. S., Barbosa, C. F., de Lima, K. P., Leite, A. d. A., and Melo, L. C. A. (2020). Aging of biochar-based fertilizers in soil: effects on phosphorus pools and availability to Urochloa brizantha grass. Sci. Total Environ. 709, 136028. doi:10.1016/j.scitotenv.2019.136028
Marat, K. (2017). SpinWorks 4.2.1. Available at: https://science.umanitoba.ca/chemistry/davinci/SpinWorks/SpinWorks_4.pdf.
Mazzini, S., Borgonovo, G., Scaglioni, L., Bedussi, F., D’Imporzano, G., Tambone, F., et al. (2020). Phosphorus speciation during anaerobic digestion and subsequent solid/liquid separation. Sci. Total Environ. 734, 139284. doi:10.1016/j.scitotenv.2020.139284
McDowell, R. W., Dou, Z., Toth, J. D., Cade-Menun, B. J., Kleinman, P. J. A., Soder, K., et al. (2008). A comparison of phosphorus speciation and potential bioavailability in feed and feces of different dairy herds using 31P nuclear magnetic resonance spectroscopy. J. Environ. Qual. 37, 741–752. doi:10.2134/jeq2007.0086
McDowell, R. W., and Sharpley, A. N. (2001). Approximating phosphorus release from soils to surface runoff and subsurface drainage. J. Environ. Qual. 30, 508–520. doi:10.2134/jeq2001.302508x
McDowell, R. W., Stewart, I., and Cade-Menun, B. J. (2006). An examination of spin-lattice relaxation times for analysis of soil and manure extracts by liquid state phosphorus-31 nuclear magnetic resonance spectroscopy. J. Environ. Qual. 35, 293–302. doi:10.2134/jeq2005.0285
Melo, L. C. A., Lehmann, J., Carneiro, J. S. d. S., and Camps-Arbestain, M. (2022). Biochar-based fertilizer effects on crop productivity: a meta-analysis. Plant Soil 472, 45–58. doi:10.1007/s11104-021-05276-2
Metson, G. S., MacDonald, G. K., Haberman, D., Nesme, T., and Bennett, E. M. (2016). Feeding the corn belt: opportunities for phosphorus recycling in U.S. agriculture. Sci. Total Environ. 542, 1117–1126. doi:10.1016/j.scitotenv.2015.08.047
Mia, S., van Groenigen, J. W., van de Voorde, T. F. J., Oram, N. J., Bezemer, T. M., Mommer, L., et al. (2014). Biochar application rate affects biological nitrogen fixation in red clover conditional on potassium availability. Agric. Ecosyst. Environ. 191, 83–91. doi:10.1016/j.agee.2014.03.011
Morshedizad, M., Panten, K., Klysubun, W., and Leinweber, P. (2018). Bone char effects on soil: sequential fractionations and XANES spectroscopy. Soil 4, 23–35. doi:10.5194/soil-4-23-2018
Muscat, A., de Olde, E. M., Ripoll-Bosch, R., Van Zanten, H. H. E., Metze, T. A. P., Termeer, C. J. A. M., et al. (2021). Principles, drivers and opportunities of a circular bioeconomy. Nat. Food 2, 561–566. doi:10.1038/s43016-021-00340-7
Ndoung, O. C. N., de Figueiredo, C. C., and Ramos, M. L. G. (2021). A scoping review on biochar-based fertilizers: enrichment techniques and agro-environmental application. Heliyon 7, e08473. doi:10.1016/j.heliyon.2021.e08473
Nisbeth, C. S., Tamburini, F., Kidmose, J., Jessen, S., and O’Connell, D. W. (2019). Analysis of oxygen isotopes of inorganic phosphate δ18Op in freshwater: a detailed method description. Hydrology Earth Syst. Sci. Discuss., 1–18. doi:10.1016/j.mex.2022.101706
Olson, J. S. (1963). Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44, 322–331. doi:10.2307/1932179
Osman, A. I., Fawzy, S., Farghali, M., El-Azazy, M., Elgarahy, A. M., Fahim, R. A., et al. (2022). Biochar for agronomy, animal farming, anaerobic digestion, composting, water treatment, soil remediation, construction, energy storage, and carbon sequestration: a review. Environ. Chem. Lett. 20, 2385–2485. doi:10.1007/s10311-022-01424-x
Pagliari, P. H., and Laboski, C. A. (2013). Dairy manure treatment effects on manure phosphorus fractionation and changes in soil test phosphorus. Biol. Fertil. Soils 49, 987–999. doi:10.1007/s00374-013-0798-2
Pagliari, P. H., Wilson, M., Waldrip, H. M., and He, Z. (2020). Nitrogen and phosphorus characteristics of beef and dairy manure. Animal Manure Prod. Charact. Environ. Concerns, Manag., 45–62. doi:10.2134/asaspecpub67.c4
Peak, D., Kar, G., Hundal, L., and Schoenau, J. (2012). Kinetics and mechanisms of phosphorus release in a soil amended with biosolids or inorganic fertilizer. Soil Sci. 177, 183–187. doi:10.1097/ss.0b013e31823fd478
Peng, Y., Sun, Y., Fan, B., Zhang, S., Bolan, N. S., Chen, Q., et al. (2021). Fe/Al (hydr)oxides engineered biochar for reducing phosphorus leaching from a fertile calcareous soil. J. Clean. Prod. 279, 123877. doi:10.1016/j.jclepro.2020.123877
Ravel, B., and Newville, M. (2005). ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541. doi:10.1107/s0909049505012719
Ren, J., Li, N., Li, L., An, J.-K., Zhao, L., and Ren, N.-Q. (2015). Granulation and ferric oxides loading enable biochar derived from cotton stalk to remove phosphate from water. Bioresour. Technol. 178, 119–125. doi:10.1016/j.biortech.2014.09.071
Richardson, A. E., Barea, J.-M., McNeill, A. M., and Prigent-Combaret, C. (2009). Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 321, 305–339. doi:10.1007/s11104-009-9895-2
Robinson, J. S., Baumann, K., Hu, Y., Hagemann, P., Kebelmann, L., and Leinweber, P. (2017). Phosphorus transformations in plant-based and bio-waste materials induced by pyrolysis. Ambio 47, S73–S82. doi:10.1007/s13280-017-0990-y
Rondon, M. A., Lehmann, J., Ramírez, J., and Hurtado, M. (2007). Biological nitrogen fixation by common beans (Phaseolus vulgaris L.) increases with bio-char additions. Biol. Fertil. Soils 43, 699–708. doi:10.1007/s00374-006-0152-z
Rose, T. J., Schefe, C., Weng, Z., Rose, M. T., Zwieten, L. v., Liu, L., et al. (2019). Phosphorus speciation and bioavailability in diverse biochars. Plant Soil 443, 233–244. doi:10.1007/s11104-019-04219-2
Sarkhot, D. V., Berhe, A. A., and Ghezzehei, T. A. (2012). Impact of biochar enriched with dairy manure effluent on carbon and nitrogen dynamics. J. Environ. Qual. 41, 1107–1114. doi:10.2134/jeq2011.0123
Sarkhot, D. V., Ghezzehei, T. A., and Berhe, A. A. (2013). Effectiveness of biochar for sorption of ammonium and phosphate from dairy effluent. J. Environ. Qual. 42, 1545–1554. doi:10.2134/jeq2012.0482
Saunders, W. M. H., and Williams, E. G. (1955). Observations on the determination of total organic phosphorus in soils. J. Soil Sci. 6, 254–267. doi:10.1111/j.1365-2389.1955.tb00849.x
Schneider, K. D., Cade-Menun, B. J., Lynch, D. H., and Voroney, R. P. (2016). Soil phosphorus forms from organic and conventional forage fields. Soil Sci. Soc. Am. J. 80, 328–340. doi:10.2136/sssaj2015.09.0340
Shen, J., Yuan, L., Zhang, J., Li, H., Bai, Z., Chen, X., et al. (2011). Phosphorus dynamics: from soil to plant. Plant Physiol. 156, 997–1005. doi:10.1104/pp.111.175232
Siebers, N., Kruse, J., and Leinweber, P. (2013). Speciation of phosphorus and cadmium in a contaminated soil amended with bone char: sequential fractionations and XANES spectroscopy. Water, Air, Soil Pollut. 224. doi:10.1007/s11270-013-1564-7
Soil Survey Staff (1999). Official series description - Greenleaf series. Available at: https://soilseries.sc.egov.usda.gov/OSD_Docs/G/GREENLEAF.html.
Strawn, D. G., Crump, A. R., Peak, D., Garcia-Perez, M., and Moller, G. (2023). Reactivity of Fe-amended biochar for phosphorus removal and recycling from wastewater. Plos Water 2, e0000092. doi:10.1371/journal.pwat.0000092
Streubel, J. D., Collins, H. P., Tarara, J. M., and Cochran, R. L. (2012). Biochar produced from anaerobically digested fiber reduces phosphorus in dairy lagoons. J. Environ. Qual. 41, 1166–1174. doi:10.2134/jeq2011.0131
Tamburini, F., Bernasconi, S. M., Angert, A., Weiner, T., and Frossard, E. (2010). A method for the analysis of the δ18O of inorganic phosphate extracted from soils with HCl. Eur. J. Soil Sci. 61, 1025–1032. doi:10.1111/j.1365-2389.2010.01290.x
Turner, B. L., Cade-Menun, B. J., and Westermann, D. T. (2003). Organic phosphorus composition and potential bioavailability in semi-arid arable soils of the Western United States. Soil Sci. Soc. Am. J. 67, 1168–1179. doi:10.2136/sssaj2003.1168
US EPA (2013). Literature review of contaminants in livestock and poultry manure and implications for water quality. United States: EPA.
Van Zanten, H. H. E., Van Ittersum, M. K., and De Boer, I. J. M. (2019). The role of farm animals in a circular food system. Glob. Food Secur. 21, 18–22. doi:10.1016/j.gfs.2019.06.003
Wang, C., Luo, D., Zhang, X., Huang, R., Cao, Y., Liu, G., et al. (2022). Biochar-based slow-release of fertilizers for sustainable agriculture: a mini review. Environ. Sci. Ecotechnology 10, 100167. doi:10.1016/j.ese.2022.100167
Wang, H., Xiao, K., Yang, J., Yu, Z., Yu, W., Xu, Q., et al. (2020). Phosphorus recovery from the liquid phase of anaerobic digestate using biochar derived from iron−rich sludge: a potential phosphorus fertilizer. Water Res. 174, 115629. doi:10.1016/j.watres.2020.115629
Weyers, E. (2016). Phosphorus speciation in dairy manure-amended calcareous soils. Idaho: M.S., University of Idaho.
Weyers, E., Strawn, D. G., Peak, D., and Baker, L. L. (2017). Inhibition of phosphorus sorption on calcite by dairy manure-sourced DOC. Chemosphere 184, 99–105. doi:10.1016/j.chemosphere.2017.05.141
Weyers, E., Strawn, D. G., Peak, D., Moore, A. D., Baker, L. L., and Cade-Menun, B. (2016). Phosphorus speciation in calcareous soils following annual dairy manure amendments. Soil Sci. Soc. Am. J. 80, 1531–1542. doi:10.2136/sssaj2016.09.0280
Wu, L., Zhang, S., Wang, J., and Ding, X. (2020). Phosphorus retention using iron (II/III) modified biochar in saline-alkaline soils: adsorption, column and field tests. Environ. Pollut. 261, 114223. doi:10.1016/j.envpol.2020.114223
Yang, Q., Wang, X., Luo, W., Sun, J., Xu, Q., Chen, F., et al. (2018). Effectiveness and mechanisms of phosphate adsorption on iron-modified biochars derived from waste activated sludge. Bioresour. Technol. 247, 537–544. doi:10.1016/j.biortech.2017.09.136
Yao, Y., Gao, B., Chen, J., and Yang, L. (2013). Engineered biochar reclaiming phosphate from aqueous solutions: mechanisms and potential application as a slow-release fertilizer. Environ. Sci. Technol. 47, 8700–8708. doi:10.1021/es4012977
Zhang, M., Song, G., Gelardi, D. L., Huang, L., Khan, E., Mašek, O., et al. (2020). Evaluating biochar and its modifications for the removal of ammonium, nitrate, and phosphate in water. Water Res. 186, 116303. doi:10.1016/j.watres.2020.116303
Keywords: biochar, dairy nutrients, phosphorus, anaerobic digest, soil speciation
Citation: Laan M, Strawn DG, Kayler ZE, Cade-Menun BJ and Möller G (2024) Phosphorus availability and speciation in soils amended with upcycled dairy-waste nutrients. Front. Chem. Eng. 5:1303357. doi: 10.3389/fceng.2023.1303357
Received: 27 September 2023; Accepted: 14 December 2023;
Published: 23 January 2024.
Edited by:
Antoni Sánchez, Autonomous University of Barcelona, SpainReviewed by:
Parameswari E., Tamil Nadu Agricultural University, IndiaCopyright © 2024 Maggi Laan, Daniel G. Strawn, Zachary E. Kayler, Gregory Möller, and His Majesty the King in Right of Canada, as represented by the Minister of Agriculture and Agri-Food Canada for the contribution of Barbara J. Cade-Menun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel G. Strawn, ZGdzdHJhd25AdWlkYWhvLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.