- 1Molecular Sciences Institute, School of Chemistry, University of Witwatersrand, Johannesburg, South Africa
- 2Center for Membrane Technology, Department of Chemistry and Bioscience, Aalborg University, Aalborg, Denmark
Membrane distillation crystallization (MDC) is an emerging technology envisaged to manage challenges affecting the desalination industry. This technology can sustainably treat concentrated solutions of produced water and industrially discharged saline wastewater. Simultaneous recovery of clean water and minerals is achieved through the integration of crystallization to membrane distillation (MD). MDC has received vast research interest because of its potential to treat hypersaline solutions. However, MDC still faces challenges in harnessing its industrial applications. Technically, MDC is affected by fouling/scaling and wetting thereby hindering practical application at the industrial level. This study reviews the occurrence of membrane fouling and wetting experienced with MDC. Additionally, existing developments carried out to address these challenges are critically reviewed. Finally, prospects suggesting the sustainability of this technology are highlighted.
Introduction
Presently, about four billion people are affected by water scarcity (Mekonnen and Hoekstra, 2016). Water scarcity is influenced by an increase in urbanization and industrialization, population growth, and climate change (Ahmed et al., 2020). Additionally, mineral resource depletion is emerging as an industrial problem. Thus, the decline in raw materials results in energy and financial challenges in several industries (Quist-Jensen et al., 2016). The shortage of raw materials consequently minimizes industrial production required to meet the market demand. Therefore, recycling mineral resources from waste streams while recovering freshwater is imperative. This avenue circumvents the search for freshwater sources due to their steady depletion. A progressively attractive technique addressing the issues of mineral and freshwater shortages is membrane distillation crystallization (MDC). Interestingly, MDC affords simultaneous recovery of both mineral crystals and freshwater from high saline wastewater (Quist-Jensen et al., 2016). Technically, MDC is a hybrid process consisting of membrane distillation (MD) and a crystallization reactor wherein the feed solution is concentrated in the MD system to reach supersaturation, followed by crystallization to recover the minerals (Quist-Jensen et al., 2017). Particularly, MDC can overcome challenges associated with common wastewater treatment options such as reverse osmosis (RO) and nanofiltration (NF) (Pramanik et al., 2017). Additionally, MDC operates at low temperatures and pressures, uses simple configuration, and consumes less energy compared to other thermal processes (Bouchrit et al., 2017; Pramanik et al., 2017). This review aims to unpack the principles and process characteristics of MDC for mineral and water recovery. Secondly, membrane fouling, and scale control measures are highlighted. Furthermore, process parameter optimization towards permeate flux, and crystal growth and selectivity are discussed. Additionally, membrane fabrication and modification strategies are reviewed to provide further insight into the development of more efficient and competitive membranes. Lastly, the latest developments towards MDC application are reported.
Principles of membrane distillation crystallization
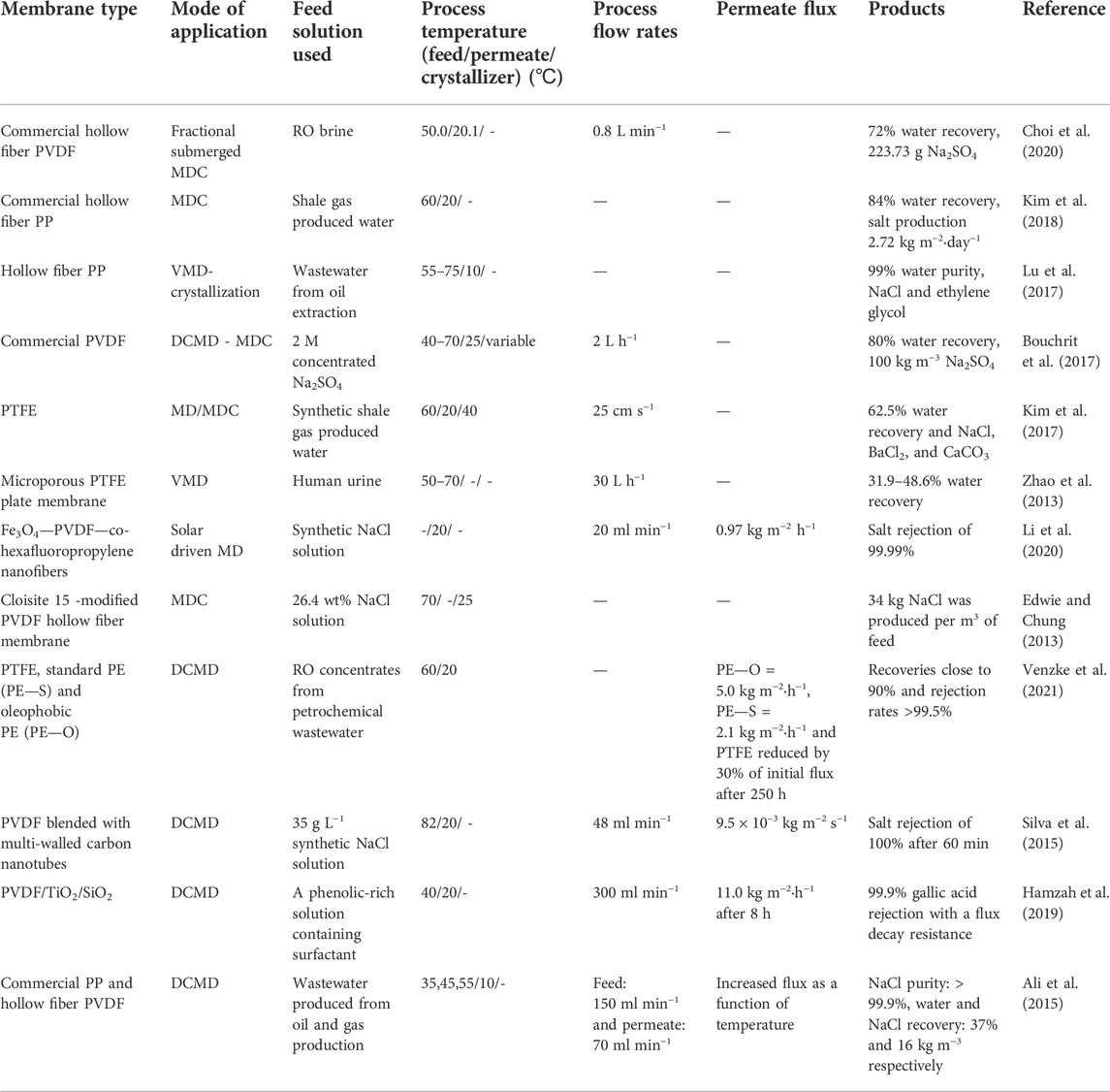
Membrane distillation (MD) has been extensively evaluated for the desalination of seawater and the treatment of high saline industrially discharged wastewater. During desalination processes, concentrated brines are generated and discharged to the environment. However, these brines could be treated further to recover mineral resources. Drioli et al. (2015) regard mineral resources to be more economically valuable compared to fresh water produced from MD processes. In their study, the researchers presented a proof-of-concept to extract mineral resources from MD desalination plants (Drioli et al., 2015). In this regard, MDC emerged as a new technology with similar mechanisms to MD. The MDC saturates the feed solution to recover mineral crystals. The feed solution is concentrated through the MD process while recovering fresh water (Pramanik et al., 2016). In this process, the feed solution becomes concentrated towards super-saturation, thus enabling nucleation and mineral crystallization while simultaneously recovering freshwater on the permeate side of the membrane (Figure 1) (Quist-Jensen et al., 2016). To facilitate selective passage of water in vapour state while exclusively retaining liquid, this technique requires the use of hydrophobic membrane (Das et al., 2021). This process operates in various MD modes namely, direct contact membrane distillation (DCMD), air gap membrane distillation (AGMD), sweep gas membrane distillation (SGMD) and vacuum membrane distillation (VMD) (Pramanik et al., 2016; Quist-Jensen et al., 2016). The detailed description of each mode is reported elsewhere (Nthunya et al., 2019a). Interestingly, the water recovery in MDC ranges from 50%–90%, thus emerging as an alternative water desalination technology (Quist-Jensen et al., 2019). According to Quist-Jensen et al. (2019), MDC can increase water production, mineral recovery and advance zero-liquid discharge (Quist-Jensen et al., 2019). The advantages and disadvantages of this technique are summarized in Table 1.
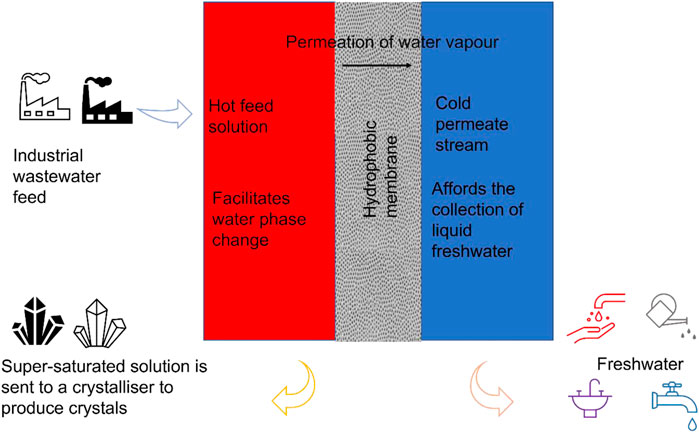
FIGURE 1. Schematic representation of MDC for recovery of freshwater and minerals from industrial wastewater.
Parameter optimization to enhance membrane distillation crystallization
The development of a viable MDC process requires optimization to prevent undesired crystallization inside the module and tubing. For this reason, the selection of appropriate MD and crystallization operating conditions are imperative. These parameters include process temperature, solution supersaturation, flow rates and duration of crystallization. Moreover, the temperatures and flow rates affect the crystal size distribution. Therefore, analysis of these parameters provides a better understanding of the MDC process and requirements to realize the maximum performance while ensuring zero liquid discharge to the environment.
Process temperature
The effect of process temperature on permeate flux is best described by Antoine equation, where α, β, and γ are constants relating to the specific substance and Pi is the vapour pressure (Pa) and T is the temperature (K).
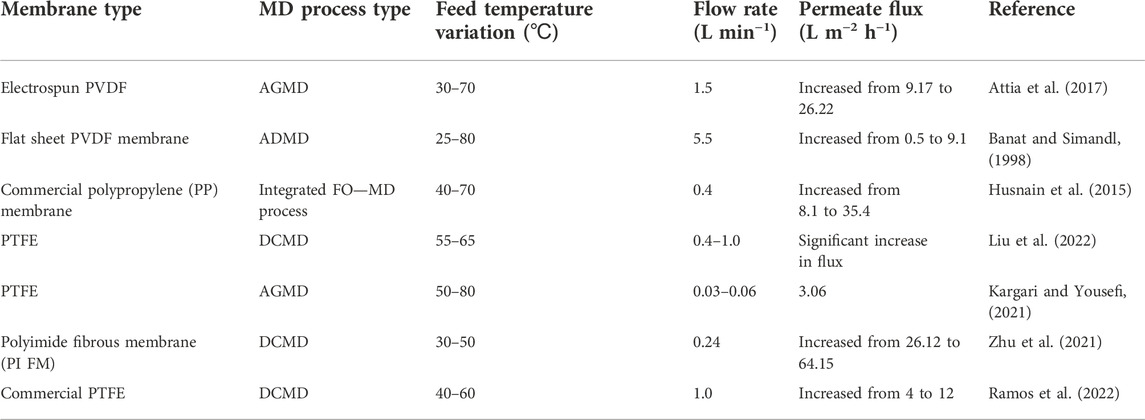
According to Antoine equation, vapour pressure exponentially increases with temperature (Choudhury et al., 2019). Furthermore, water flux is directly proportional to feed temperature (Banat and Simandl, 1998). However, flux increments are limited by the process temperature and declines once an optimum has been attained (Banat and Simandl, 1998). Moreover, Attia et al. (2017) evaluated the effect of temperature using synthetic electrospun PVDF, superhydrophobic alumina, and commercial PVDF membranes in a comparative AGMD process. A direct relationship between permeate flux and feed temperature was established (Attia et al., 2017). Liu et al. (2022) assessed the effect of temperature and flow velocity to obtain lithium chloride from air-conditioning systems via DCMD. Reportedly, an increase in feed temperature improved solute generation although the membrane’s hydrophobicity was altered. However, the increase in solute concentration reduced the water flux due to a decreased partial vapour pressure (Liu et al., 2022). Although high water fluxes are obtained at higher temperatures, the water recovery factor is reduced due to salt precipitation (Zhu et al., 2021). The effect of feed temperature on process operation is summarized in Table 2.
Solution supersaturation
The capability of the MD technique to progressively concentrate a feed solution to supersaturation gave rise to MDC (Yadav et al., 2022). The gradual passage of water vapor from the feed stream to the distillate results in the eventual concentration of the feed solution to its critical saturation. Further increase in feed supersaturation enables the recovery of crystal salts from the crystallization reactor (Das et al., 2021). Importantly, this process facilitates the recovery of higher quality mineral crystals in terms of size and purity. Other benefits include controlled rate of supersaturation and nucleation (Yadav et al., 2022). However, the increase in feed concentration towards solution supersaturation induces temperature and concentration polarization, thus reducing the permeate flux (Martínez, 2004). Moreover, pore blockage occurs due to the formation of crystals on the surface of membrane (Yadav et al., 2022). Martínez (2004) investigated the effect of feed concentration on the permeate flux using a flat sheet PTFE membrane and feed solutions of pure water, sodium chloride, and sucrose. Notably, the pure water flux remained stable towards supersaturation. However, the deposition of sodium chloride and sucrose crystal on the membrane surface resulted in a decrease in the permeate flux (Martínez, 2004). When supersaturation is attained in the bulk feed solution, nucleation is then induced which is succeeded by crystallization (Yadav et al., 2022). Moreover, a higher feed temperature increases rate of solvent evaporation, thus facilitating an increased rate of supersaturation compared to that experienced at low feed temperatures (Edwie and Chung, 2013).
Duration of crystallization
The formation and crystal growth are influenced by the solubility of the salt, rate of water recovery and process temperature. For instance, feed solutions with low concentration containing extremely soluble solutes requires a lengthy period to form crystals (Rudolph, 2010; Liu et al., 2021). Additionally, slow crystal growth rate facilitates formation of large crystals. Therefore, longer crystallization periods give rise to larger crystals (Alvarez et al., 2020). In their study, Wagstaff et al. (1964) evaluated the impact of crystallization duration to the size of cristobalite. Based on their findings, the size of the crystals increased quadratically upon increase in duration of process crystallization (Wagstaff et al., 1964). Essentially, the rate of crystal growth is governed by the various factors including flow of latent heat from the growing crystal, diffusion and reactions occurring at the crystal interface (Rudolph, 2010). In MDC processes, the inclusion of the membrane provides a site for heterogeneous nucleation. The Gibbs free energy is lower at the membrane-solution interface, thus favoring heterogeneous nucleation rather than homogenous nucleation (Ruiz Salmón and Luis, 2018). According to Edwie and Chung (2013), a high feed temperature encourages a higher rate of evaporation resulting in a lower average crystal size. Once nucleation has been established, the nuclei begin to grow until the critical cluster size has been achieved. Thereafter, crystals form and grow in saturation zones (i.e., metastable and unstable growth zones) (Yadav et al., 2022). Technically, rate of supersaturation and nucleation affect crystal network growth, consequently the duration of crystallization (Das et al., 2021).
Recirculation rate
High recovery rates of MDC processes are realized at higher recirculation rates (Swaminathan and Lienhard, 2018). For an efficient and high performing MDC process, the overall recovery factor should be greater than that of a single pass process (Lokare et al., 2018). To achieve high recovery factors, the retentate is mixed with the new feed solution prior to crystallization (Lokare et al., 2018). In addition to high recoveries, an increase in the recirculation rate enhances the heat transfer coefficient. Consequently, this minimizes the boundary layer thus improving the permeate flux (Srisurichan et al., 2006). Due to the improvement of water turbulence, a high recirculation rate reduces temperature polarization and membrane fouling, thus ensuring the stable water flux (Lokare et al., 2018).
Fouling of MDC membranes
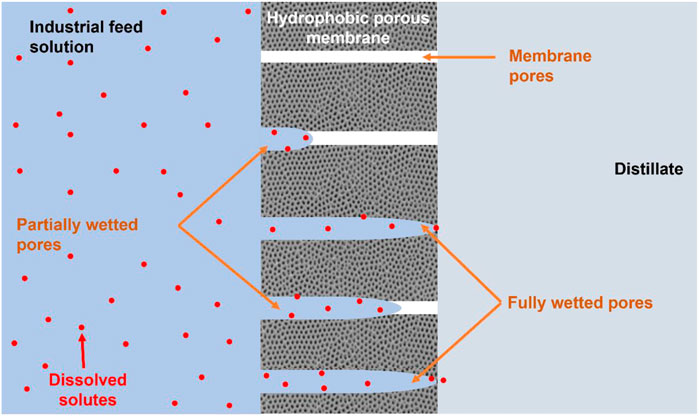
Occurrence of fouling in MDC is a common problem affecting process performance. To minimize fouling, its developments and successions should be established. Briefly, fouling occurs due to the deposition of microbial, colloidal, organic, or inorganic constituents on the surface or inner pores of the membrane, thus causing blockages (Choudhury et al., 2019; Mpala et al., 2022). Due to changes in the membrane physicochemical properties, fouling reduces permeate water flux, salt rejections and also increases the operating expenditure (OPEX) of the process (Nthunya et al., 2022). Additionally, fouling reduces membrane hydrophobicity leading to membrane wetting (Wang and Lin, 2017). Reduced membrane hydrophobicity encourages the passage of water in liquid state, thus reducing mineral salt rejection (Wang and Lin, 2017; Choudhury et al., 2019). Moreover, fouling is not limited to the membrane surface, but can also occur within the membrane pores. This was evident in a study conducted by Kim et al. (2018) reporting deposition of foulants within the membrane pores in conjunction with reduced water recoveries and permeate flux. Usually, permeate flux reduction is caused by partial and complete wetting while the latter is true for water quality deterioration (Figure 2) (Yao et al., 2020). Technically, the membrane is partially wetted by process conditions with limited passage of water in both liquid and vapour state. However, during full pore wetting, the water carrying salt ions passes through the membrane in liquid state, thus reducing the quality of the distillate.
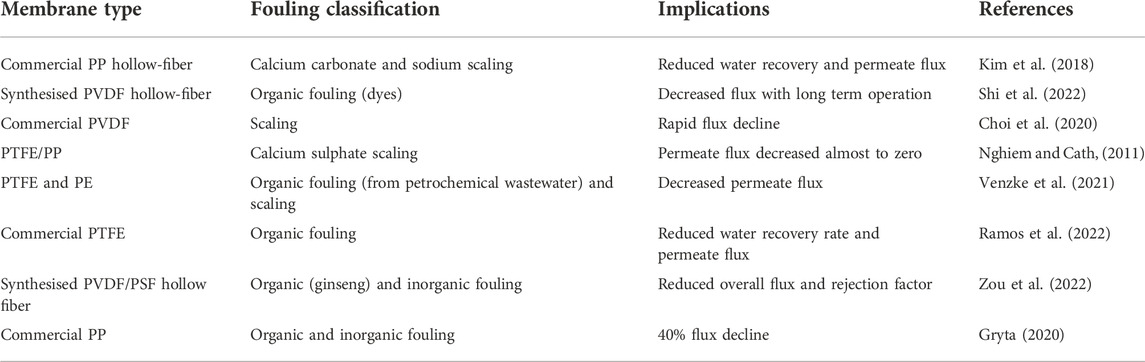
Common factors influencing fouling include feed solution properties, hydrodynamic conditions, and membrane characteristics (Yao et al., 2020). The most prevalent form of fouling in MDC is scaling caused by sparingly soluble salts (Pramanik et al., 2016; Char et al., 2021). Inorganic scaling occurs via two mechanisms, namely; 1) nucleation and precipitate growth on the surface or pores of the membrane and 2) the build-up of precipitates materializing in the bulk solution (Horseman et al., 2021). Common scalants causing membrane damage include calcium sulfate and calcium carbonate (Alkhatib et al., 2021). Fouling can be classified into porous and non-porous where the former causes thermal resistance and the latter results in both thermal and hydraulic resistance (Abdel-Karim et al., 2021; Alkhatib et al., 2021). Therefore, to maintain high MDC process performance, operational challenges associated with a high concentration of salts and a complex feed solution should be overcome. Fouling and its implications are presented in Table 3 below.
Fouling control
Membrane fouling is inevitable and therefore requires strategic measures to minimize its effects on process performance. Fouling control increases the membrane lifespan and maintains the performance of MDC processes (Laqbaqbi et al., 2017). Membrane fouling is controlled through several measures including pre-treatments, backwashing, and chemical cleaning. These processes lengthen membrane longevity. Chemical cleaning and backwashing are employed post membrane fouling to recover flux and salt rejection. To increase water recoveries, fouling control is optimized to minimize cost and damage of membranes.
Pre-treatment
Flux decline caused by membrane fouling requires frequent membrane cleaning and possibly replacement, consequently increasing operating and maintenance costs (OPEX). Therefore, wastewater pre-treatment integrated to MDC improves process performance. Primarily, pre-treatment strategies limit fouling by reducing foulants concentration in the feed water. The choice of pre-treatment depends on the feed water. Typically, a combination of pre-treatment strategies is required to improve efficiency of foulant removal from the feed solution. These combinations involve physical and chemical processes such as low-pressure membrane filtration, coagulation and flocculation, adsorption, pH adjustments and the addition of anti-scalants.
Mechanical pre-treatments consist of membrane processes such as microfiltration (MF), ultrafiltration (UF), and nanofiltration (NF). Particularly, NF is used for water softening and reduction of natural organic matter (NOM). The UF and MF reduces colloidal, suspended and biological matter (Alkhatib et al., 2021). These pre-treatment methods have been evaluated in water processing of various complexities (Nthunya et al., 2021). El-Abbassi et al. (2013) studied coagulation-flocculation and MF pre-treatment of olive mill wastewater in DCMD. Coagulation-flocculation pre-treatment reduced the concentration of TDS and phenolic compounds by 23% and 18%, respectively. The TDS removal was improved to 30% while that of phenolic compounds was reduced to 4.8% upon the MF treatment (El-Abbassi et al., 2013). In another study, Karakulski and Gryta (2005) investigated NF pre-treatment of tap water for use in MD. Reportedly, untreated feed water caused membrane scaling leading to rapid flux decay. However, NF pre-treatment removed scalants thus ensuring high process performance (Karakulski and Gryta, 2005). Additionally, adsorption has been proven to effectively remove organic matter prior to MD water purification. Nthunya et al. (2019c) reported removal of phenolic compounds from feed wastewater using a candle filter (pore size ∼100 µm) equipped with polyethyleneimine-functionalized polyacrylonitrile nanofibre membranes. The membranes presented 39.9 mg g−1 adsorption capacity (Nthunya et al., 2019b). Notably, MD process performance remained relatively stable upon feeding with pre-treated wastewater. Coagulation-flocculation is another process proven to effectively remove foulants prior to MD water processing. In this process, foulant particles are converted into larger flocs, thus reducing their adhesive interaction with the membranes. Moreover, coagulation-flocculation coupled with conventional treatment or membrane filtration processes remove the flocs from the feed water (Alkhatib et al., 2021). Li et al. (2016) investigated the purification of biologically treated coking wastewater using MD coupled with coagulation pre-treatment. A poly-aluminium chloride (PACl) flocculant reduced the foulants thus promoting the stable performance in MD (Li et al., 2016). Lastly, pH-adjustments have been extensively used to treat feed solutions in membrane processes. The increase in feed pH promotes formation of metal precipitates which are removed as insoluble metal hydroxides prior to MDC. Similarly, the feed solution is acidified to dissolve the foulant, thus impeding their interaction with the membranes (Karakulski and Gryta, 2005). A summary of MDC pre-treatment processes is presented in Figure 3.
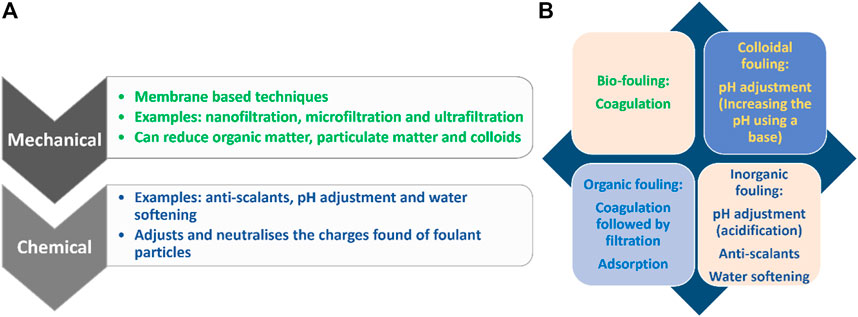
FIGURE 3. A summary of pretreatment processes in MDC (A) pre-treatment classifications and (B) process selection for specific foulant.
Use of anti-scalants
Anti-scalants are precipitation-inhibiting chemicals impeding nucleation or crystal growth of scalants on membrane surfaces. Anti-scalants adsorb on the nuclei surface to obstruct the rate of crystal growth and agglomeration (Lin and Singer, 2005; Gloede and Melin, 2008; Abdel-Karim et al., 2021). The anti-scaling mechanism of action takes place through ligand exchange or electrostatic interactions (Horseman et al., 2021). Commonly used anti-scalants include organophosphates, polyelectrolytes and polyphosphates (Ketrane et al., 2009). Yin et al. (2021) evaluated gypsum anti-scaling in reverse osmosis (RO) coupled with MD using Poly (acrylic) acid (PAA). A 1,300 min test recorded 95% water flux decay in the absence of an antiscalant. However, the decay was reduced by 30% upon addition of anti-scalant, thus corresponding to 40% water recovery (Yin et al., 2021). Lin and Singer (2005) utilized polyphosphates to minimize calcite crystal growth in MD. The process performance remained stable with minimal flux decay recorded. Though anti-scalants improve MDC processes, their addition beyond maximum threshold promote membrane biofouling (Tijing et al., 2015). Therefore, the anti-scalant dosage should be optimized to meet the process requirement upon treatment of a specific feed solution.
Membrane flushing and gas bubbling
Membrane flushing and gas bubbling are classified as physical fouling mitigation strategies. Flushing is often carried out to remove adsorbed solutes from the membrane surface using deionized water. Nonetheless, flushing fails to remove solutes within the membrane pores (Alkhatib et al., 2021). Flushing is often operated in two modes namely, forward and backwashing. Technically, deionized water is pumped in a forward direction during forward flushing while the reverse is true for backflushing (Alkhatib et al., 2021). Gas bubbling enhances shear rate and fluid dynamics thus reducing temperature and concentration polarization (Alkhatib et al., 2021). Reportedly, finely dispersed bubbles are more efficient compared to course bubbles (Lu et al., 2008). Choi et al. (2020) assessed the recovery of sodium sulfate from seawater brine using a hollow fiber PVDF membrane in fractionally submerged MD crystallization. Two cleaning procedures were used, namely air backwashing and deionized water flushing in the presence of ammonium sulfate. Air backwashing enabled 90% flux recovery. Similarly, flushing recovered 82% water flux from the original level. However, multiple air backwashing caused progressive permeate flux decline (Choi et al., 2020). To reduce scaling of a commercial PTFE membrane supported on polypropylene (PP), Nghiem and Cath (2011) used MilliQ water. Five cycles of membrane flushing recovered 30% of the original flux (Nghiem and Cath, 2011). Though flushing is more efficient for removal of inorganic foulants, it can also be used for removal of organic foulants upon treatment of an oil-contaminated feed (Gryta, 2020).
Temperature adjustments and backflow
Temperature and flow reversal (backflow) techniques are novel methods used to mitigate fouling in MD/MDC. This experimental procedure was evaluated by Hickenbottom and Cath (2014) to minimize scaling while ensuring stable process performance. The temperature swap between the feed and distillate effectively reversed the driving force across the membrane, thus reducing the surface interactions between the membrane and scalants. Water flux and rejection efficiencies were recovered to 95%. Remarkably, both methods minimized scaling, thus ensuring stable fluxes and maintaining high salt rejection (Hickenbottom and Cath, 2014). Notably, these mitigation strategies avoid the use of expensive and toxic chemicals. Therefore, temperature and flow reversal are attractive alternative measures to control membrane fouling. However, extensive research is required to ascertain their sustainability at an industrial scale.
Chemical cleaning
Chemical cleaning is the most evaluated reactive measure used to control membrane fouling. The mechanism of action involves breaking foulant-membrane interactions (Alkhatib et al., 2021). Chemical reagents include acids, bases, surfactants, chelating agents, enzymes, and oxidants (Al-Amoudi and Lovitt, 2007; Porcelli and Judd, 2010). Typically, bases and surfactants are used to address organic and biofouling (Alkhatib et al., 2021; Char et al., 2021) while acids and chelating agents are true for inorganic fouling (Alkhatib et al., 2021; Gryta, 2021). To ensure a synergistic cleaning process, a combination of chemicals is generally used during the treatment of complex feed solutions characterized by various foulants (Alkhatib et al., 2021). Charfi et al. (2021) optimized cleaning procedures of the MD process during treatment of anaerobic digestate. Reportedly, deionized water flushing was followed by 0.2% NaOCl and 3% citric acid for 60 min. NaOCl and citric acid were effective for organic and inorganic foulant removal, respectively thus ensuring 75.5% flux recovery. Furthermore, the cleaning process recovered 87% of membrane hydrophobicity with minimal membrane wetting (Char et al., 2021). In another study Guillen-Burrieza et al. (2014) evaluated a variety of cleaning agents in long-term scaling control in MD processes. As per reported findings, a combination of 0.1 wt% oxalic acid and 0.8 wt% citric acid recovered 97% of the membrane WCA. Furthermore, formic, and sulfuric acid recovered 96.7% and 94.6% of the membrane WCA respectively. Although these processes restored WCA, the integrity and mechanical strength of the membranes were affected (Guillen-Burrieza et al., 2014). The destruction of membrane integrity depends on cleaning conditions including the concentration of reagents, duration, and cleaning frequency. To understand the impact of chemical cleaning pertaining to physicochemical properties, various characterization techniques should be employed. These include chemical, morphological, topological, hydrophobic/hydrophilic, and mechanical analysis of the membrane. Puspitasari et al. (2010) investigated the cleaning and ageing of PVDF membranes using oxidative sodium hypochlorite (NaOCl). The effect of chemical concentration on the cleaning and ageing of the membrane is presented in Figure 4A. The cleaning efficiency improved with an increase in NaOCl. The same trend was observed for cyclical membrane cleaning. However, following the cleaning protocol, SEM micrographs showed presence of foulants on the membrane surface. Moreover, FTIR results presented changes in the chemical functional groups of membrane, thus alluding to an ageing effect. Furthermore, higher concentrations NaOCl damaged the integrity of the membrane (Figure 4B) (Puspitasari et al., 2010). To minimize the damage, a combination of cleaning reagents and anti-scalants is commonly used. This was evaluated by Peng et al. (2015) during the MD treatment of RO concentrated brine. A series of chemicals namely, NaCl, NaOH, KCOOH, citric acid, and EDTA-4Na were used. While operating at elevated temperatures, EDTA-4Na enabled highest flux recovery. Improved recovery was associated to chelation of calcium ions, thus reducing their interactions with the membranes (Peng et al., 2015). In another study, Zhang J et al. (2021) used a combination of organic phosphoric acid and hexamethylene diamine tetra (methylene phosphonic acid) (HDTMPA) during treatment of landfill leachate in FO/MD system. A combination of these chemicals reduced the foulant-membrane interactions, thus improving the process performance. Although 90% of flux was recovered in the first cycle, continuous cleaning did not show significant increase in performance recovery (Zhang P et al., 2021).
FIGURE 4. (A) Average cleaning efficiencies as a function of varying NaOCl concentrations, (B) overall cleaning efficiencies (OCE) observed for aged, fouled membranes (Puspitasari et al., 2010).
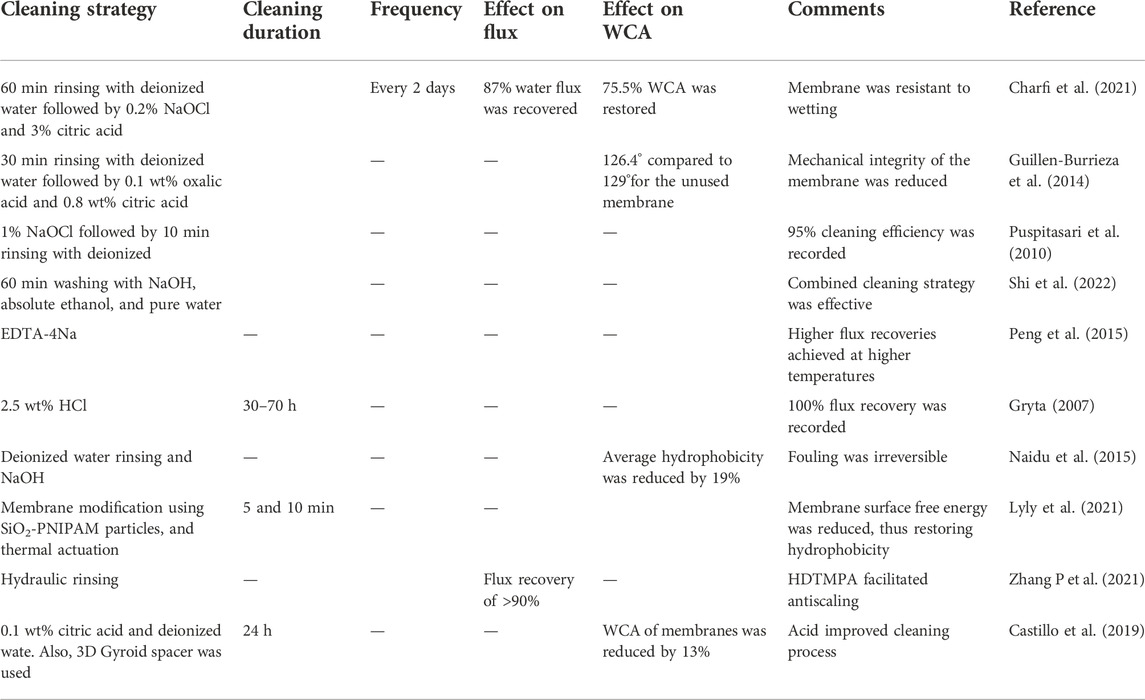
Some foulants bind strongly on membrane surfaces, thus causing irreversible fouling. This phenomenon was reported by Naidu et al. (2015) upon NaOH cleaning MD membranes fouled by humic substances. Partial regeneration of the membrane with 19% hydrophobicity recovery was reported (Naidu et al., 2015). Further improvements in chemical cleaning involves the use of 3D spacers. Spacers amplify flow turbulence, thus reducing foulant-membrane interaction. In their study, Castillo et al. (2019) investigated a step-wise cleaning of MD membrane using citric acid and water in the presence of spacers. Upon cleaning, 87% of membrane WCA was recovered (Castillo et al., 2019). The effect of various cleaning strategies is presented in Table 4.
Membrane modification
Membrane modification improves resistance to fouling and wetting. Typically, modification is achieved through systematic manipulation. Currently, superhydrophobic membranes characterized by self-cleaning properties are explored with low success rate. To improve membrane resistance to fouling while retaining high salt rejection, omniphobic and Janus membranes are also reported (Wang and Lin, 2017; Yao et al., 2020; Tjale et al., 2022). These membranes are characterized by asymmetric wettability to minimize fouling while retaining process stability (Afsari et al., 2021). Xiao et al. (2020) prepared omniphobic membranes through incorporation of silica nanoparticles (SiNPs)-coated micropillars (MP) to PVDF. Reportedly, SiNPs-MP-PVDF membrane reduced scaling and fouling, thus maintaining the process performance over a longer period. Figure 5 1) and 2) present the role of membrane modification towards preventing flux decay.
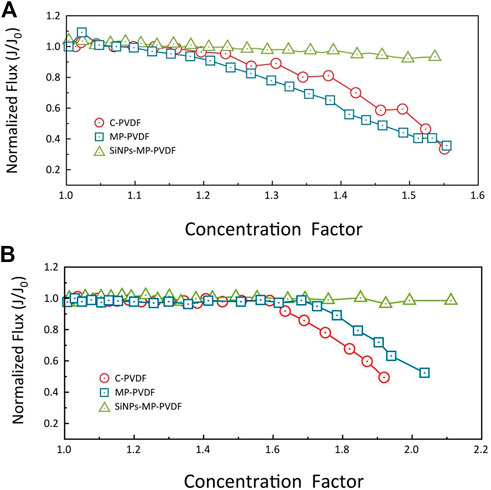
FIGURE 5. Normalized water flux (J/Jo) vs. concentration factor for the individual membranes upon evaluation of (A) CaSO4 scaling, (B) casein protein organic fouling (Xiao et al., 2020).
In another study, Toh et al. (2019) modified PVDF-co-hexafluropropylene membranes using silica nanoparticles to improve their resistance to wetting and fouling. The modified membranes were characterized by high WCA and low surface energy (Toh et al., 2019). Zhang J et al. (2021) reported hydrophilic surface modification of PVDF hollow fibre membrane through co-deposition of polydopamine (PDA) and poly (MPC-co-2-aminoethyl methacrylate hydrochloride) (MPC-co-AEMA). The smooth hydrophilic thin layer reduced the foulant-membrane interaction (Zhang P et al., 2021). In addition to hydrophilic coating, antimicrobial nanoparticles are embedded on hydrophobic membranes to combat organic, inorganic and biofouling. These additives include silver nanoparticles, cellulose nanocrystals and carbon nanotubes (Nthunya et al., 2019a; Nthunya et al., 2020). Membrane modifications processes addressing fouling are presented in Table 5.
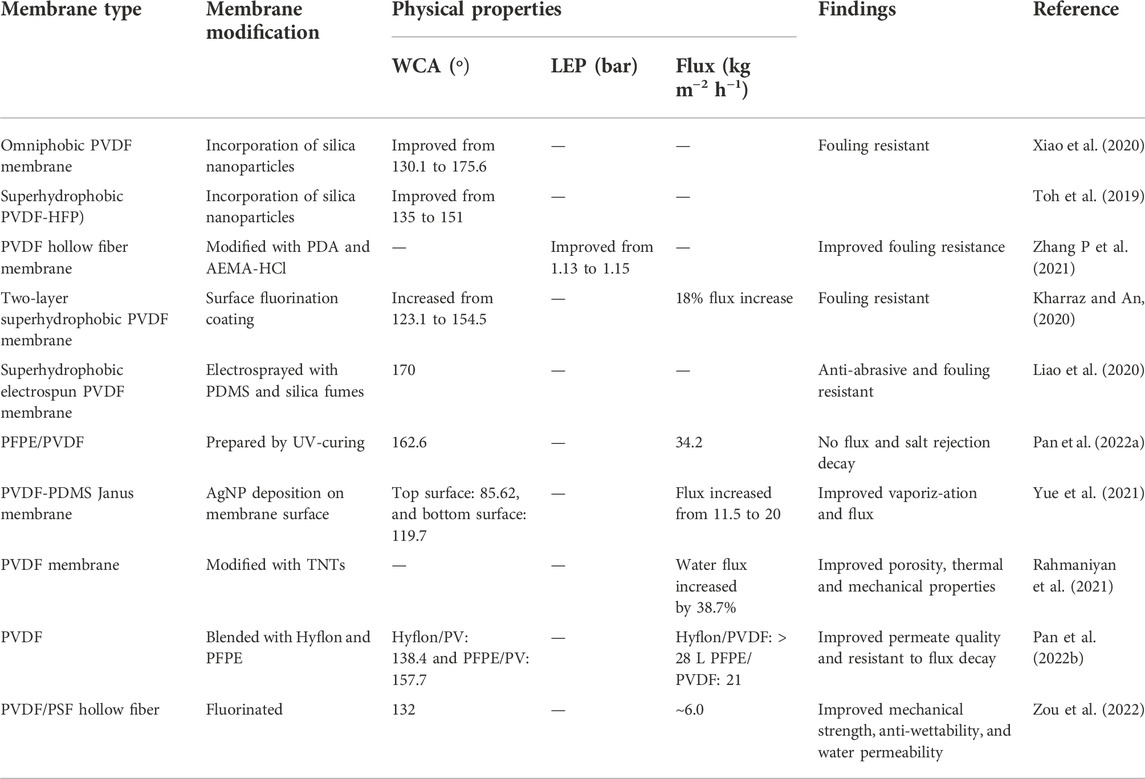
TABLE 5. Summary of various membrane modification strategies and their effects on membrane properties.
Application in wastewater treatment
Membrane distillation crystallization (MDC) emerged as a promising innovation in response to the global shortage of fresh water and mineral resources. Owing to the challenges associated with industrial application, MDC is extensively tested at laboratory-scale. Various applications of MDC are summarized in Table 6. Nonetheless, process optimization with sound findings has motivated its industrial use for treatment of wastewater. For instance, Hamzah et al. (2019) reported a flux of 11.0 kg m−2·hr−1 during the treatment of a phenolic-rich feed solution using a PVDF/TiO2/SiO2 composite membrane. Remarkably, TiO2-modification improved process resistance to organic fouling (Hamzah et al., 2019). Although fouling is minimized to some extent, it remains critically challenging (Kim et al., 2017). Notably, fouled membranes attract scaling and wetting (Kim et al., 2017). Despite all these challenges, MDC is relatively versatile towards treatment of complex feed solutions. In their study, Lu et al. (2017), reported 99% water purity recovered from oil-processing wastewater. Moreover, MDC is used as a finishing process to recover minerals and freshwater (90% water recovery and 99% salt rejection) from the RO concentrate (Venzke et al., 2021). Nonetheless, treatment of the RO concentrate causes concentration polarization and scaling (Venzke et al., 2021). Interestingly, MDC does not only treat industrial wastewater but also biological waste including human urine (Zhao et al., 2013). During this treatment, 31.9%–48.6% water recovery was reported along with ammonia-nitrogen recovery and COD reduction (Zhao et al., 2013). Among other factors related to the economics of the process, MDC is driven by renewable energy sources. The use of solar energy was evaluated by Li et al. (2020) in a pilot-scale where photothermal membrane was used. Although high flux (21.99 kg m−2·hr−1) was reported, water recovery factors were low and production of photothermal membrane was costly (Li et al., 2020). The successes achieved at lab scale supports the implementation of this technology toward pilot and industrial scale. Memstill ® reported first pilot testing of MD technology implemented at an incineration plant in Singapore in 2006 (Dotremont et al., 2010). Other pilot studies were established at BASF in Antwerp, Belgium in 2011 (Camacho et al., 2013).
Conclusion and future perspectives
MDC addresses financial challenges affecting developing countries. Various literature reports have documented the successes of this technique in effectively recovering freshwater and mineral salts from a myriad of wastewater feed sources (Quist-Jensen et al., 2016, 2017; Kim et al., 2017; Choi et al., 2020). In conjunction to emerging laboratory-scale studies, implementation of pilot studies at an industrial platform provides a promising trajectory for the future of this technology. Nevertheless, membrane fouling, and wetting requires special attention. Membrane fouling can be classified into organic, inorganic (i.e., scaling), biofouling, and/or colloidal fouling. In some circumstances, a combination of foulants may exist in the feed solutions thus resulting in more complex membrane fouling scenarios. To circumvent these issues, various fouling control measures have been registered including mechanical pre-treatment options such as microfiltration (MF) and nanofiltration (NF). Other pre-treatment strategies include anti-scalants, temperature adjustments, and membrane flushing. Moreover, chemical cleaning has been extensively evaluated to restore MDC performance. While commercial membranes have be used for MDC processes, further research has been directed towards synthesis and modification of various fouling resistant membranes. This includes incorporation of nanoparticles to induce self-cleaning through superhydrophicity enhancement (i.e., improving the lotus effect of the membrane). Similarly, Janus membranes characterized by asymmetric wettability have been evaluated to mitigate membrane fouling. The steady development in this technique and its accompanying components has probed further interest into its applicative potential. Promising feedback established with the use of this technique pave the way towards further implementation in an industrial setting for mineral and water recycling. Future perspectives include, though not limited to:
• Production of membranes using environmentally friendly reagents in addressing membrane fouling and wetting.
• Optimization of membrane cleaning strategies towards a feasible industrial application.
• Further research and implementation of pilot-scale studies to provide a realistic MDC suitability in industrial application.
• Further research establishing fouling mechanism is required to understand membrane longevity and process performance.
Author contributions
IC: Investigation; Methodology; Validation; Roles/Writing—original draft. LN: Conceptualization; Data curation; Formal analysis; Funding acquisition; Resources; Writing—review and editing. HR: Conceptualization; Funding acquisition; Project administration; Resources; Supervision; Writing—review and editing. CQ-J: Software; Visualization; Writing—review, editing and funding acqusition.
Funding
The authors would like to thank the University of the Witwatersrand, Aalborg University, Danish International Development Agency (Grant number: DANIDA1) and National Research Foundation (NRF-grant number: 132724) for funding this research work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdel-Karim, A., Leaper, S., Skuse, C., Zaragoza, G., Gryta, M., and Gorgojo, P. (2021). Membrane cleaning and pretreatments in membrane distillation – A review. Chem. Eng. J. 422, 129696. doi:10.1016/j.cej.2021.129696
Afsari, M., Shon, H. K., and Tijing, L. D. (2021). Janus membranes for membrane distillation: Recent advances and challenges. Adv. Colloid Interface Sci. 289, 102362. doi:10.1016/j.cis.2021.102362
Ahmed, F. E., Hashaikeh, R., and Hilal, N. (2020). Hybrid technologies: The future of energy efficient desalination – a review. Desalination 495, 114659. doi:10.1016/j.desal.2020.114659
Al-Amoudi, A., and Lovitt, R. W. (2007). Fouling strategies and the cleaning system of NF membranes and factors affecting cleaning efficiency. J. Membr. Sci. 303 (1–2), 4–28. doi:10.1016/j.memsci.2007.06.002
Ali, A., Quist-Jensen, C. A., Drioli, E., and Macedonio, F. (2018). Evaluation of integrated microfiltration and membrane distillation/crystallization processes for produced water treatment. Desalination 434, 161–168. doi:10.1016/j.desal.2017.11.035
Ali, A., Quist-Jensen, C., Macedonio, F., and Drioli, E. (2015). Application of membrane crystallization for minerals’ recovery from produced water. Membranes 5, 772–792. doi:10.3390/membranes5040772
Alkhatib, A., Ayari, M. A., and Hawari, A. H. (2021). Fouling mitigation strategies for different foulants in membrane distillation. Chem. Eng. Process. - Process Intensif. 167, 108517. doi:10.1016/j.cep.2021.108517
Alvarez, R., Nievergelt, P. P., Slyshkina, E., Muller, P., Alberto, R., and Spingler, B. (2020). Single crystal growth of water-soluble metal complexes with the help of the nano-crystallization method. Dalton Trans. 49 (28), 9632–9640. doi:10.1039/d0dt01236j
Attia, H., Osman, M. S., Johnson, D. J., Wright, C., and Hilal, N. (2017). Modelling of air gap membrane distillation and its application in heavy metals removal. Desalination 424, 27–36. doi:10.1016/j.desal.2017.09.027
Banat, F. A., and Simandl, J. (1998). Desalination by membrane distillation: A parametric study. Sep. Sci. Technol. 33 (2), 201–226. doi:10.1080/01496399808544764
Bouchrit, R., Boubakri, A., Mosbahi, T., Hafiane, A., and Bouguecha, S. A. T. (2017). Membrane crystallization for mineral recovery from saline solution: Study case Na2SO4 crystals. Desalination 412, 1–12. doi:10.1016/j.desal.2017.02.021
Camacho, L. M., Dumee, L., Zhang, J., Li, J. d., Duke, M., Gomez, J., et al. (2013). Advances in membrane distillation for water desalination and purification applications. Water 5 (1), 94–196. doi:10.3390/w5010094
Castillo, E. H. C., Thomas, N., Al-Ketan, O., Rowshan, R., Abu Al-Rub, R. K., Nghiem, L. D., et al. (2019). 3D printed spacers for organic fouling mitigation in membrane distillation. J. Membr. Sci. 581, 331–343. doi:10.1016/j.memsci.2019.03.040
Char, A., Kim, S., Yoon, Y., and Cho, J. (2021). Optimal cleaning strategy to alleviate fouling in membrane distillation process to treat anaerobic digestate. Chemosphere 279, 130524. doi:10.1016/j.chemosphere.2021.130524
Choi, Y., Naidu, G., Lee, S., and Vigneswaran, S. (2020). Recovery of sodium sulfate from seawater brine using fractional submerged membrane distillation crystallizer. Chemosphere 238, 124641. doi:10.1016/j.chemosphere.2019.124641
Choudhury, M. R., Anwar, N., Jassby, D., and Rahaman, M. S. (2019). Fouling and wetting in the membrane distillation driven wastewater reclamation process – a review. Adv. Colloid Interface Sci. 269, 370–399. doi:10.1016/j.cis.2019.04.008
Das, P., Dutta, S., and Kumar, K. (2021). Insights into membrane crystallization : A sustainable tool for value added product recovery from effluent streams. Sep. Purif. Technol. 257, 117666. doi:10.1016/j.seppur.2020.117666
Dotremont, C., Kregersman, B., Sih, R., Lai, K. C., Koh, K., and Seah, H. (2010). Seawater desalination with memstill technology - a sustainable solution for the industry. Water Pract. Technol. 5 (2), 1–7. doi:10.2166/wpt.2010.026
Drioli, E., Ali, A., and Macedonio, F. (2015). Membrane distillation: Recent developments and perspectives. Desalination 356, 56–84. doi:10.1016/j.desal.2014.10.028
Drioli, E., Profio, G. D., and Curcio, E. (2012). Progress in membrane crystallization. Curr. Opin. Chem. Eng. 1 (2), 178–182. doi:10.1016/j.coche.2012.03.005
Edwie, F., and Chung, T.-S. (2013). Development of simultaneous membrane distillation–crystallization (SMDC) technology for treatment of saturated brine. Chem. Eng. Sci. 98, 160–172. doi:10.1016/j.ces.2013.05.008
El-Abbassi, A., Hafidi, A., Khayet, M., and Garcia-Payo, M. (2013). Integrated direct contact membrane distillation for olive mill wastewater treatment. Desalination 323, 31–38. doi:10.1016/j.desal.2012.06.014
Gloede, M., and Melin, T. (2008). Physical aspects of membrane scaling. Desalination 224 (1–3), 71–75. doi:10.1016/j.desal.2007.02.081
Gryta, M. (2007). Influence of polypropylene membrane surface porosity on the performance of membrane distillation process. J. Membr. Sci. 287 (1), 67–78. doi:10.1016/j.memsci.2006.10.011
Gryta, M. (2020). Separation of saline oily wastewater by membrane distillation. Chem. Pap. 74 (7), 2277–2286. doi:10.1007/s11696-020-01071-y
Gryta, M. (2021). Surface modification of polypropylene membrane by helium plasma treatment for membrane distillation. J. Membr. Sci. 628, 119265. doi:10.1016/j.memsci.2021.119265
Guillen-Burrieza, E., Ruiz-Aguirre, A., Zaragoza, G., and Arafat, H. A. (2014). Membrane fouling and cleaning in long term plant-scale membrane distillation operations. J. Membr. Sci. 468, 360–372. doi:10.1016/j.memsci.2014.05.064
Hamzah, N., Leo, C. P., and Ooi, B. S. (2019). Superhydrophobic PVDF/TiO2-SiO2 membrane with hierarchical roughness in membrane distillation for water recovery from phenolic rich solution containing surfactant. Chin. J. Polym. Sci. 37, 609–616. doi:10.1007/s10118-019-2235-y
Hickenbottom, K. L., and Cath, T. Y. (2014). Sustainable operation of membrane distillation for enhancement of mineral recovery from hypersaline solutions. J. Membr. Sci. 454, 426–435. doi:10.1016/j.memsci.2013.12.043
Horseman, T., Yin, Y., Christie, K. S., Wang, Z., and Tong, T. (2021). Wetting, scaling, and fouling in membrane distillation: State-of-the-Art insights on fundamental mechanisms and mitigation strategies. ACS Est. Eng. 1 (1), 117–140. doi:10.1021/acsestengg.0c00025
Husnain, T., Liu, Y., Riffat, R., and Mi, B. (2015). Integration of forward osmosis and membrane distillation for sustainable wastewater reuse. Sep. Purif. Technol. 156, 424–431. doi:10.1016/j.seppur.2015.10.031
Karakulski, K., and Gryta, M. (2005). Water demineralisation by NF/MD integrated processes. Desalination 177, 109–119. doi:10.1016/j.desal.2004.11.018
Kargari, A., and Yousefi, A. (2021). Process intensification through magnetic treatment of seawater for production of drinking water by membrane distillation process: A novel approach for commercialization membrane distillation process. Chem. Eng. Process. - Process Intensif. 167, 108543. doi:10.1016/j.cep.2021.108543
Ketrane, R., Saidani, B., Gil, O., Leleyter, L., and Baraud, F. (2009). Efficiency of five scale inhibitors on calcium carbonate precipitation from hard water: Effect of temperature and concentration. Desalination 249 (3), 1397–1404. doi:10.1016/j.desal.2009.06.013
Kharraz, J. A., and An, A. K. (2020). Patterned superhydrophobic polyvinylidene fluoride (PVDF) membranes for membrane distillation: Enhanced flux with improved fouling and wetting resistance. J. Membr. Sci. 595, 117596. doi:10.1016/j.memsci.2019.117596
Kim, J., Kim, J., and Hong, S. (2018). Recovery of water and minerals from shale gas produced water by membrane distillation crystallization. Water Res. 129, 447–459. doi:10.1016/j.watres.2017.11.017
Kim, J., Kwon, H., Lee, S., Lee, S., and Hong, S. (2017). Membrane distillation (MD) integrated with crystallization (MDC) for shale gas produced water (SGPW) treatment. Desalination 403, 172–178. doi:10.1016/j.desal.2016.07.045
Laqbaqbi, M., Sanmartino, J., Khayet, M., Garcia-Payo, C., and Chaouch, M. (2017). Fouling in membrane distillation, osmotic distillation and osmotic membrane distillation. Appl. Sci. 7 (4), 334. doi:10.3390/app7040334
Li, J., Wu, J., Sun, H., Cheng, F., and Liu, Y. (2016). Advanced treatment of biologically treated coking wastewater by membrane distillation coupled with pre-coagulation. Desalination 380, 43–51. doi:10.1016/j.desal.2015.11.020
Li, W., Chen, Y., Yao, L., Ren, X., Li, Y., and Deng, L. (2020). Fe3O4/PVDF-HFP photothermal membrane with in-situ heating for sustainable, stable and efficient pilot-scale solar-driven membrane distillation. Desalination 478, 114288. doi:10.1016/j.desal.2019.114288
Liao, Y., Zheng, G., Huang, J. J., Tian, M., and Wang, R. (2020). Development of robust and superhydrophobic membranes to mitigate membrane scaling and fouling in membrane distillation. J. Membr. Sci. 601, 117962. doi:10.1016/j.memsci.2020.117962
Lin, Y. P., and Singer, P. C. (2005). Inhibition of calcite crystal growth by polyphosphates. Water Res. 39 (19), 4835–4843. doi:10.1016/j.watres.2005.10.003
Liu, G., Liu, J., Dunn, A. S., Nadazdy, P., Siffalovic, P., Resel, R., et al. (2021). Directional crystallization from the melt of an organic p-type and n-type semiconductor blend. Cryst. Growth & Des. 21 (9), 5231–5239. doi:10.1021/acs.cgd.1c00570
Liu, J., Albdoor, A. K., Lin, W., Hai, F. I., and Ma, Z. (2022). Membrane fouling in direct contact membrane distillation for liquid desiccant regeneration: Effects of feed temperature and flow velocity. J. Membr. Sci. 642, 119936. doi:10.1016/j.memsci.2021.119936
Lokare, O. R., Tavakkoli, S., Khanna, V., and Vidic, R. D. (2018). Importance of feed recirculation for the overall energy consumption in membrane distillation systems. Desalination 428, 250–254. doi:10.1016/j.desal.2017.11.037
Lu, D., Li, P., Xiao, W., He, G., and Jiang, X. (2017). Simultaneous recovery and crystallization control of saline organic wastewater by membrane distillation crystallization. AIChE J. 63, 2187–2197. doi:10.1002/aic.15581
Lu, Y., Ding, Z., Liu, L., and Wang, Z. (2008). The influence of bubble characteristics on the performance of submerged hollow fiber membrane module used in microfiltration. Sep. Purif. Technol. 61, 89–95. doi:10.1016/j.seppur.2007.09.019
Lyly, L. H. T., Chang, Y., Ng, W., Lim, J., Derek, C., and Ooi, B. (2021). Development of membrane distillation by dosing SiO2-PNIPAM with thermal cleaning properties via surface energy actuation. J. Membr. Sci. 636, 119193. doi:10.1016/j.memsci.2021.119193
Martínez, L. (2004). Comparison of membrane distillation performance using different feeds. Desalination 168 (1–3), 359–365. doi:10.1016/j.desal.2004.07.022
Mekonnen, M. M., and Hoekstra, A. Y. (2016). Four billion people facing severe water scarcity. Sci. Adv. 2 (2), e1500323–e1500327. doi:10.1126/sciadv.1500323
Mpala, T. J., Etale, A., Richards, H., and Nthunya, L. N. (2022). Biofouling phenomena in membrane distillation : Mechanisms and mitigation strategies. Environ. Sci. Adv. doi:10.1039/d2va00161f
Naidu, G., Jeong, S., and Vigneswaran, S. (2015). Interaction of humic substances on fouling in membrane distillation for seawater desalination. Chem. Eng. J. 262, 946–957. doi:10.1016/j.cej.2014.10.060
Nghiem, L. D., and Cath, T. (2011). A scaling mitigation approach during direct contact membrane distillation. Sep. Purif. Technol. 80 (2), 315–322. doi:10.1016/j.seppur.2011.05.013
Nthunya, L. N., Bopape, M. F., Mahlangu, O. T., Mamba, B. B., Van der Bruggen, B., Quist-Jensen, C. A., et al. (2022). Fouling, performance and cost analysis of membrane-based water desalination technologies: A critical review. J. Environ. Manag. 301, 113922. doi:10.1016/j.jenvman.2021.113922
Nthunya, L. N., Gutierrez, L., Derese, S., Edward, N., Verliefde, A. R., Mamba, B. B., et al. (2019a). A review of nanoparticle-enhanced membrane distillation membranes : Membrane synthesis and applications in water treatment. J. Chem. Technol. Biotechnol. 94 (9), 2757–2771. doi:10.1002/jctb.5977
Nthunya, L. N., Gutierrez, L., Derese, S., Mamba, B. B., Verliefde, A. R., and Mhlanga, S. D. (2019b). Adsorption of phenolic compounds by polyacrylonitrile nano fibre membranes : A pretreatment for the removal of hydrophobic bearing compounds from water. J. Environ. Chem. Eng. 7, 103254. doi:10.1016/j.jece.2019.103254
Nthunya, L. N., Gutierrez, L., Lapeire, L., Verbeken, K., Zaouri, N., Nxumalo, E. N., et al. (2019c). Fouling resistant PVDF nanofibre membranes for the desalination of brackish water in membrane distillation. Sep. Purif. Technol. 228, 115793. doi:10.1016/j.seppur.2019.115793
Nthunya, L. N., Gutierrez, L., Nxumalo, E. N., Verliefde, A. R., Mhlanga, S. D., and Onyango, M. S. (2020). f-MWCNTs/AgNPs-coated superhydrophobic PVDF nanofibre membrane for organic, colloidal, and biofouling mitigation in direct contact membrane distillation. J. Environ. Chem. Eng. 8 (2), 103654. doi:10.1016/j.jece.2020.103654
Nthunya, L. N., Mbakop, S., and Mhlanga, S. D. (2021). “Emerging nanoenhanced membrane-based hybrid processes for complex industrial wastewater treatment,” in Membrane-based hybrid processes for wastewater treatment (Netherlands: Elsevier B.V), 633–656. doi:10.1016/B978-0-12-823804-2.00024-0
Pan, J., Chen, M., Xu, X., Sun, S. P., Wang, Z., Cui, Z., et al. (2022a). Enhanced anti-wetted PVDF membrane for pulping RO brine treatment by vacuum membrane distillation. Desalination 526, 115533. doi:10.1016/j.desal.2021.115533
Pan, J., Zhang, F., Wang, Z., Sun, S. P., Cui, Z., Jin, W., et al. (2022b). Enhanced anti-wetting and anti-fouling properties of composite PFPE/PVDF membrane in vacuum membrane distillation. Sep. Purif. Technol. 282, 120084. doi:10.1016/j.seppur.2021.120084
Peng, Y., Ge, J., Li, Z., and Wang, S. (2015). Effects of anti-scaling and cleaning chemicals on membrane scale in direct contact membrane distillation process for RO brine concentrate. Sep. Purif. Technol. 154, 22–26. doi:10.1016/j.seppur.2015.09.007
Porcelli, N., and Judd, S. (2010). Chemical cleaning of potable water membranes: A review. Sep. Purif. Technol. 71 (2), 137–143. doi:10.1016/j.seppur.2009.12.007
Pramanik, B. K., Shu, L., and Jegatheesan, V. (2017). A review of the management and treatment of brine solutions. Environ. Sci. Water Res. Technol. 3, 625–658. doi:10.1039/C6EW00339G
Pramanik, B. K., Thangavadivel, K., Shu, L., and Jegatheesan, V. (2016). A critical review of membrane crystallization for the purification of water and recovery of minerals. Rev. Environ. Sci. Biotechnol. 15, 411–439. doi:10.1007/s11157-016-9403-0
Puspitasari, V., Granville, A., Le-Clech, P., and Chen, V. (2010). Cleaning and ageing effect of sodium hypochlorite on polyvinylidene fluoride (PVDF) membrane. Sep. Purif. Technol. 72 (3), 301–308. doi:10.1016/j.seppur.2010.03.001
Quist-Jensen, C. A., Ali, A., Drioli, E., and Macedonio, F. (2019). Perspectives on mining from sea and other alternative strategies for minerals and water recovery – the development of novel membrane operations. J. Taiwan Inst. Chem. Eng. 94, 129–134. doi:10.1016/j.jtice.2018.02.002
Quist-Jensen, C. A., Ali, A., Mondal, S., Macedonio, F., and Drioli, E. (2016). A study of membrane distillation and crystallization for lithium recovery from high-concentrated aqueous solutions. J. Membr. Sci. 505, 167–173. doi:10.1016/j.memsci.2016.01.033
Quist-Jensen, C. A., Macedonio, F., Horbez, D., and Drioli, E. (2017). Reclamation of sodium sulfate from industrial wastewater by using membrane distillation and membrane crystallization. Desalination 401, 112–119. doi:10.1016/j.desal.2016.05.007
Rahmaniyan, B., Mohammadi, T., and Tofighy, M. A. (2021). Development of high flux PVDF/modified TNTs membrane with improved properties for desalination by vacuum membrane distillation. J. Environ. Chem. Eng. 9 (6), 106730. doi:10.1016/j.jece.2021.106730
Ramos, R. L., Lebron, Y. A., Moreira, V. R., Martins, M. F., Santos, L. V., and Amaral, M. C. (2022). Direct contact membrane distillation as an approach for water treatment with phenolic compounds. J. Environ. Manag. 303, 114117. doi:10.1016/j.jenvman.2021.114117
Rudolph, P. (2010). “Defect Formation during crystal growth from the melt,” in Springer handbook of crystal growth. 1st edn. (Berlin: Springer), 159–201. doi:10.1007/978-3-540-74761-1_6
Ruiz Salmón, I., and Luis, P. (2018). Membrane crystallization via membrane distillation. Chem. Eng. Process. - Process Intensif. 123, 258–271. doi:10.1016/j.cep.2017.11.017
Shi, W., Tian, Y., Li, H., Fan, M., Zhang, H., et al. (2022). An innovative hollow fiber vacuum membrane distillation-crystallization (VMDC) coupling process for dye house effluent separation to reclaim fresh water and salts. J. Clean. Prod. 337, 130586. doi:10.1016/j.jclepro.2022.130586
Silva, T. L. S., Morales-Torres, S., Figueiredo, J. L., and Silva, A. M. (2015). Multi-walled carbon nanotube/PVDF blended membranes with sponge- and finger-like pores for direct contact membrane distillation. Desalination 357, 233–245. doi:10.1016/j.desal.2014.11.025
Srisurichan, S., Jiraratananon, R., and Fane, A. G. (2006). Mass transfer mechanisms and transport resistances in direct contact membrane distillation process. J. Membr. Sci. 277 (1–2), 186–194. doi:10.1016/j.memsci.2005.10.028
Swaminathan, J., and Lienhard, J. H. (2018). Design and operation of membrane distillation with feed recirculation for high recovery brine concentration. Desalination 445, 51–62. doi:10.1016/j.desal.2018.07.018
Tijing, L. D., Woo, Y. C., Choi, J. S., Lee, S., Kim, S. H., and Shon, H. K. (2015). Fouling and its control in membrane distillation-A review. J. Membr. Sci. 475, 215–244. doi:10.1016/j.memsci.2014.09.042
Tjale, L., Richards, H., Mahlangu, O., and Nthunya, L. N. (2022). Silica nanoparticle modified polysulfone/polypropylene membrane for separation of oil-water emulsions. Results Eng. 16, 100623. doi:10.1016/j.rineng.2022.100623
Toh, M. J., Oh, P. C., Chew, T. L., and Ahmad, A. L. (2019). Antiwettability enhancement of PVDF-HFP membrane via superhydrophobic modification by SiO2 nanoparticles. Comptes Rendus Chim. 22 (5), 369–372. doi:10.1016/j.crci.2019.05.004
Venzke, C. D., Rizzana, D., Giacobbo, A., Rodrigues, M., and Bernardes, A. (2021). Membrane distillation treating a real petrochemical reverse osmosis concentrate: Influence of membrane characteristics on the process performance. J. Water Process Eng. 39, 101722. doi:10.1016/j.jwpe.2020.101722
Wagstaff, F. E., Brown, S. D., and Cutler, I. (1964). The influence of H2O and O2 atmospheres on the crystallisation of vitreous silica. Phys. Chem. Glasses 5 (3), 76–81.
Wang, Z., and Lin, S. (2017). Membrane fouling and wetting in membrane distillation and their mitigation by novel membranes with special wettability. Water Res. 112, 38–47. doi:10.1016/j.watres.2017.01.022
Xiao, Z., Guo, H., He, H., Liu, Y., Li, X., Zhang, Y., et al. (2020). Unprecedented scaling/fouling resistance of omniphobic polyvinylidene fluoride membrane with silica nanoparticle coated micropillars in direct contact membrane distillation. J. Membr. Sci. 599, 117819. doi:10.1016/j.memsci.2020.117819
Yadav, A., Labhasetwar, P. K., and Shahi, V. K. (2022). Membrane distillation crystallization technology for zero liquid discharge and resource recovery: Opportunities, challenges and futuristic perspectives. Sci. Total Environ. 806, 150692. doi:10.1016/j.scitotenv.2021.150692
Yao, M., Tijing, L. D., Naidu, G., Kim, S. H., Matsuyama, H., Fane, A. G., et al. (2020). A review of membrane wettability for the treatment of saline water deploying membrane distillation. Desalination 479, 114312. doi:10.1016/j.desal.2020.114312
Yin, Y., Kalam, S., Livingston, J. L., Minjarez, R., Lee, J., et al. (2021). The use of anti-scalants in gypsum scaling mitigation: Comparison with membrane surface modification and efficiency in combined reverse osmosis and membrane distillation. J. Membr. Sci. 643, 120077. doi:10.1016/j.memsci.2021.120077
Yue, D., Wang, Y., Zhang, H., Sun, D., Ye, X., et al. (2021). A novel silver/activated - polyvinylidene fluoride - polydimethyl siloxane hydrophilic-hydrophobic Janus membrane for vacuum membrane distillation and its anti-oil-fouling ability. J. Membr. Sci. 638, 119718. doi:10.1016/j.memsci.2021.119718
Zhang, J., Wang, D., Chen, Y., Gao, B., and Wang, Z. (2021). Scaling control of forward osmosis-membrane distillation (FO-MD) integrated process for pre-treated landfill leachate treatment. Desalination 520, 115342. doi:10.1016/j.desal.2021.115342
Zhang, P., Liu, W., Rajabzadeh, S., Jia, Y., Shen, Q., Fang, C., et al. (2021). Modification of PVDF hollow fiber membrane by co-deposition of PDA/MPC-co-AEMA for membrane distillation application with anti-fouling and anti-scaling properties. J. Membr. Sci. 636, 119596. doi:10.1016/j.memsci.2021.119596
Zhao, Z. P., Xu, L., Shang, X., and Chen, K. (2013). Water regeneration from human urine by vacuum membrane distillation and analysis of membrane fouling characteristics. Sep. Purif. Technol. 118, 369–376. doi:10.1016/j.seppur.2013.07.021
Zhu, Z., Tan, G., Lei, D., Yang, Q., Tan, X., Liang, N., et al. (2021). Omniphobic membrane with process optimization for advancing flux and durability toward concentrating reverse-osmosis concentrated seawater with membrane distillation. J. Membr. Sci. 639, 119763. doi:10.1016/j.memsci.2021.119763
Keywords: water recovery, mineral mining, fouling, wetting, membrane distillation crystallization
Citation: Chimanlal I, Nthunya LN, Quist-Jensen C and Richards H (2022) Membrane distillation crystallization for water and mineral recovery: The occurrence of fouling and its control during wastewater treatment. Front. Chem. Eng. 4:1066027. doi: 10.3389/fceng.2022.1066027
Received: 10 October 2022; Accepted: 18 November 2022;
Published: 29 November 2022.
Edited by:
Le Han, Chongqing University, ChinaReviewed by:
Enrico Drioli, Department of Chemical Sciences and Materials Technologies, Institute for Membrane Technology (CNR), ItalyQingyao He, Huazhong Agricultural University, China
Copyright © 2022 Chimanlal, Nthunya, Quist-Jensen and Richards. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Heidi Richards, SGVpZGkucmljaGFyZHNAd2l0cy5hYy56YQ==
 Indira Chimanlal1,2
Indira Chimanlal1,2 Lebea N. Nthunya
Lebea N. Nthunya Cejna Quist-Jensen
Cejna Quist-Jensen Heidi Richards
Heidi Richards