- 1The ŁUKASIEWICZ Research Network - The Institute of Heavy Organic Synthesis “Blachownia”, Kedzierzyn-Kozle, Poland
- 2Institute of Chemical Technology and Engineering, Faculty of Chemical Technology, Poznan University of Technology, Poznań, Poland
The processes of direct ethoxylation of methyl and ethyl esters of unsaturated fatty acids have been described. Two different types of catalysts were employed in those tests: calcium-based catalyst and aluminum-magnesium-based catalyst. Compositions of the synthesized oxyethylates were analyzed with the use of the GC/FID, GC/MS, GPC, and HPLC, HPLC/MS, MALDI methods. Comparative evaluation of the obtained products for both types of catalysts was run in terms of reaction by-products. Biodegradation tests of the oxyethylated products were carried out according to OECD guidelines and physical and chemical properties of oxyethylates were specified which affects the directions of their applications.
Introduction
Surfactants are a group of substances that is used in many industrial processes and everyday goods. The unusual universality of these compounds results from their characteristic chemical structure and physicochemical properties. The size of the global surfactant market was valued at $ 41.3 billion in 2019 and it is expected to reach $ 58.5 billion by 2027, with an annual growth rate (CAGR) of 5.3% (Allied Market Research., 2020). The expansion of the surfactants market is being driven by the growing population and urbanization in the Asia-Pacific region. In addition, greater awareness of these products, and the need for COVID-19 sanitizers and disinfectants, are other factors driving the market demand. In Europe, the leading manufacturers of surfactants are BASF, Nourynon, Clariant, Evonik, Sasol Olefins & Surfactants, Solvay, Dow Chemical, Stepan Corporation, Shell, Cepsa, Kolb, Enaspol, ICO, Wilmar (Barbacki, 2020). The largest market share in the world is held by anionic (≈39%) and non-ionic (≈37%) surfactants. It is forecasted that for non-ionic surfactants, the increase in CAGR by 2025 will reach 5.8%. About two-thirds of the surfactant market value is accounted for the production of detergents and personal care products. It is currently the fastest growing industry in terms of revenues. The CAGR index is expected to remain at 6% by 2025 (Chemia i Biznes, 2018). The dynamic increase in the production of non-ionic surfactants is related to the increased demand for raw materials for the production of liquid detergents, where ethoxylates are proved to be the most suitable. Furthermore, non-ionic surfactants benefit from their low toxicity. The conducted research on the cytotoxicity of surfactants allows to arrange them in the following order: cationic>anionic>amphoteric>non-ionic (Grant et al., 1992). Moreover, the more easily biodegradable non-ionic surfactants authorized for sale, thanks to the Regulation (EC) No. 648/2004 on detergents, began to replace anionic surfactants in large-volume household chemicals (Kuberska-Maciejewska, 2020). Non-ionic surfactants are most often obtained by ethoxylation of alcohols (mainly C12–C14 fractions) of synthetic or natural origin. For economic and ecological reasons, less processed fatty materials such as fats (mainly vegetable oils), fatty acids, and fatty acid methyl esters (FAME) play an increasingly important role in these processes. Vegetable oil ethoxylates (VOE) are produced by direct ethoxylation of triglycerides or transesterification of triglycerides with ethoxylated glycerin. They exhibit good surface activity and are extremely gentle on the skin and eyes, and are therefore mainly used in body care as emollients and foam boosters in rinse-off cosmetic products (Smith, 2019). Fatty acid ethoxylates are also obtained by direct ethoxylation. In their case, apart from the main product, a mixture of by-products (Di Serio et al., 1994; Bheler et al., 2001; Lukosek and Alejski, 2016) that affect the physicochemical and functional properties namely diesters and polyethylene glycols is obtained. These products are attractive due to their good emulsifying properties and low foaming properties. They are widely used in the textile industry, agrotechnics, or pulp industry, where their good dispersing, greasing, and anti-electrostatic properties are applied. FAME is an ecologically and economically attractive alternative to fatty alcohols. Due to the presence of an ester group and linear alkyl chains, they are environmentally friendly compounds (Kolano et al., 2012). The main driver of the global market of methyl ester ethoxylates (FAMEE) is low cost of production, mainly due to subsidies to their production introduced by governments in Europe and Southeast Asia. It is estimated that the global FAMEE market by 2021 will reach the value of USD 139.8 million with the assumed annual growth of 3.4% (Markets Markets., 2017).
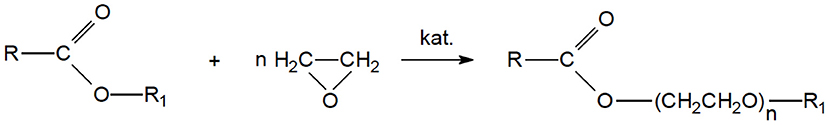
The direct reaction of ethoxylation of esters (Figure 1) requires the use of a catalyst that will enable the introduction of ethylene oxide between oxygen and carbon in the alkoxy group (Hama et al., 1995, 1997b; Cox and Weerasooriya, 1997).
The most commonly used catalysts are based on group IIA metal alkoxides modified with mineral acids and composite oxide catalysts containing two or three metal components (Cox and Weerasooriya, 2000; Kolano et al., 2013; Di Serio et al., 2015). Composite catalysts of magnesium and aluminum oxides, additionally containing small amounts of metals from groups VIA, VIIA, and VIII, exhibit particularly interesting properties (Alejski et al., 2004, 2012; Białowas and Szymanowski, 2004). The mechanism of the direct ester ethoxylation reaction depends on the catalyst used. In the case of an acidic catalyst (e.g., calcium salts modified with sulfuric acid), the C-OR bond is weakened, and the oxyethylene group is introduced between the carbonyl carbon atom of the ester bond and the oxygen atom of the alkoxy group, where the oxyethylene chain may also propagate (Alejski et al., 2004; Di Serio et al., 2015). The use of a catalyst with alkali or transition metals allows for the effective introduction of ethylene oxide to the ester bond through two stages: ethoxylation and transesterification (Hama et al., 1997a; Di Serio et al., 2015). With the use of a composite aluminum-magnesium oxide catalyst, the ethoxylation reaction of methyl esters is probably preceded by chemisorption, and a methoxy group undergoes the ethoxylation reaction on the catalyst surface. This catalyst performs a double role, namely it has acid reaction centers and works on the principles of chemisorption (Hama et al., 1997a).
FAMEE show surface activity and physicochemical properties very similar to the corresponding ethoxylated fatty alcohols. They are distinguished by the possibility of hydrolysis in an aqueous solution of high or low pH to non-toxic biodegradable products (Johansson and Svensson, 2001; Renkin et al., 2005; Smidrkal et al., 2008; Liu et al., 2017; Wasilewski et al., 2019). Moreover, compared to ethoxylated alcohols, FAMEE show low ecotoxicity and a favorable carbon footprint. The conducted studies indicate that ethoxylated methyl esters from rapeseed oil are characterized by a 5–8% higher biodegradability and lower toxicity to aquatic organisms compared to alcohol ethoxylates (Szwach and Lukosek, 2017). In addition to the typical uses for the production of household chemicals and industrial cleaners, FAMEE can be used as pharmaceutical auxiliaries. Surfactants commonly used in pharmaceuticals, such as Tweeny (e.g., polyoxyethylene sorbitan monostearate), are, like FAMEE, a complex mixture of chemical compounds (Alejski et al., 2003; Abrar et al., 2018). The results of the comparative studies of the effect of FAMEE and surfactants commonly used in pharmacy (Tween 80, Pluronic F68) on the solubility profile of lipophilic drugs belonging to the BCS 2 class suggest that FAMEE can successfully replace the surfactants used in drugs so far (Zgoda et al., 2005; Lachowicz et al., 2016). Another advantage of FAMEE is the fact that they are produced from cheap, large volume renewable resources. Methyl esters are currently extensively produced by the biodiesel industry and the technology for producing ethylene oxide from bioethanol is nowadays widely available. There are currently several plants that carry out the ethoxylation of methyl esters on an industrial scale. However, the process of ester ethoxylation is not yet fully explored and there are still both scientific and technological challenges in this area.
Materials and Methods
Raw Materials
For the synthesis of ethoxylateds used ethylene oxide production ChemoGas NV Belgium with a purity of min. 99%, the catalyst K-4/XII-O (Ca) containing calcium salts obtained by the ŁUKASIEWICZ Research Network ICSO “Blachownia” in Kedzierzyn-Kozle (Polish Patent No. 360, 448), the catalyst of Al–Mg—oxide catalyst magnesium aluminum composite character (Polish Patent No. 391,571). As hydrophobic raw materials were used fatty acid ethyl esters of linseed oil production Ollen-Pol Sp. Z o.o. (Zary) content of ethyl esters 95.16%, LJ = 167 gJ/100 g, FAME from rapeseed oil—Biodiesel RME/FAME made by ORLEN Południe SA, content of methyl esters 96.5%, LJ = 120 gJ/100 g. The percentage composition of rapeseed oil acid methyl ester and linseed oil ethyl ester was determined using GC/FID in ŁUKASIEWICZ Research Network ICSO “Blachownia” in Kedzierzyn-Kozle (Table 1).
Synthesis Methodology
Oxyethylation reactions were carried out in a semi-periodic pressure reactor made of stainless steel (Autoclave Engineers, Erie, PA, USA) of 2 L capacity, equipped with a mechanical stirrer, electric heating jacket and cooling coil, pressure gauge, and safety valve. The synthesis was started by loading the reactor with a defined mass of hydrophobic substrate and catalyst (0.3%, 0.5% wt. of the product, for fatty acid ethyl esters and FAME, respectively). The hydrophobic substrates used were fatty acid ethyl esters of linseed oil and FAME from rapeseed oil. After loading the reactor and checking its tightness, the feedstock was purged with nitrogen at 130°C for 30 min to remove trace amounts of water. After raising the reactor temperature to a set value of 185°C, automatic dosing of ethylene oxide was started. Having completed dosing of ethylene oxide, the reaction mixture was kept for 60 min at the reaction temperature to ensure the maximum conversion of substrates. After cooling to 60–80°C, the reactor was purged with nitrogen and the resulting product was discharged, weighed, and analyzed.
Analytical Methods
Determination of the Composition of Hydrophobic Raw Materials Using Gas Chromatography (GC/FID)
The GC/FID chromatographic separation of the components of the reaction residues was done on gas chromatograph type Agilent 6890N Network System (Agilent Technologies). It is equipped with a flame ionization detector (FID) and capillary column made of 15 m length and 0.25 mm inner diameter fused silica, filled with methyl phenyl silicone stationary phase ZB-5HT with film thickness 0.10 μm. The chromatography column was heated from 100 to 400°C at a rate of 15°C/min, applying at the beginning 1 min temperature isotherm and at the end 9 min isotherm. The temperature of the split/splitless dispenser was 380°C, the temperature of the FID was 400°C. The flow rate of carrier gas (helium) through the column was equal to 2 ml/min. The split ratio of the carrier gas in the dispenser was 100: 1. Before the analysis the test samples underwent silanization, were diluted with benzene and dosed in 0.2 μl quantities to the chromatographic column by a microsyringe (SGE Analytical Science).
Analysis GC (Ethylene Oxide and 1,4-Dioxane Contents)
Content of ethylene oxide and 1,4-dioxane was performed using a Perkin Elmer Clarus 500 gas chromatograph with FID detection, Perkin Elmer TurboMatrix 40 Trap headspace adapter, chromatographic column: HP-INNOWAX (30 m × 0.53 mm × 1 μm). Headspace temperatures: 160–170°C, GC parameters: initial temp: 55°C, ramp rate: 8°C/min to 110°C, next ramp rate: 45°C/min to 240°C, detector temp: 280°C.
Method of Analysis by High Performance Liquid Chromatography (HPLC) and Gel Permeation Chromatography (GPC)
Molecular weight distribution was analyzed by gel permeation chromatography (GPC) on a reverse phase column at a critical point of adsorption and/or normal phase. Polystyrene columns were used and tetrahydrofuran were applied as solvent and mobile phase. The DIONEX UltiMate 3000 chromatograph and refraction detector (Varian) was used.
High performance chromatography (HPLC) was used to separate polyglycol homologs and determine the total concentration of analytes in samples. A reversed phase analytical column (ODS 250 × 2.1 mm) was used. The mobile phase was water-acetonitrile (35:65, v:v). Analytes were detected with an evaporative light scattering detector (ELSD 2000 Alltech), which is an ideal instrument for detecting compounds with no UV chromophores. The eluent stream passes through the column, where the solvent is evaporated and a mist of tiny sample particles is left. These scatter a light beam, and the extent of the light scattering is proportional to the amount of sample present.
Mass Spectroscopy MALDI-TOF
The registration of the mass spectra was performed using a Bruker autoflex speed Matrix Assisted Laser Desorption Ionization-Time of flight (MALDI-TOF) mass spectrometer. A Sartorius MC5 microbalance was used to weigh the sample, templates, and salts applied in these studies. The sample was spotted on a MALDI plate by a “dried droplet method.” The analyzed sample is dissolved in a volatile solvent. The sample solution is then mixed with excess solution template and if necessary, small quantities of substances facilitating ion formation (e.g., Trifluoroacetic acid salts). The samples was dissolved in 1 ml of tetrahydrofuran (THF-T. Baker). The used parameters:mode of operation of the spectrometer: reflectron, ionization: positive, spectra were registered in the mass range to 5,500 Da. The best spectra obtained for the solution of 3-indoleacrylic acid (IAA): solution of sample = 10:10 μL. The analysis was performed in accordance with the PN-EN ISO 10927:2011.
Spectroscopy ESI-MS
ESI-MS detection was performed in a 4000 QTRAP Mass spectrometer (Sciex, USA) equipped with an electrospray ionization source (ESI) and a triple quadrupole-ion trap mass analyzer that was controlled by the Analyst 1.5 software. ESI worked in the positive-ion mode at the following conditions: curtain gas at 20 psi, nebulizer gas at 10 psi, and negative ionization mode source voltage 4,800 V. Nitrogen was used as curtain and collision gas. Sample (10 mg) was diluted in 10 ml of methanol. Prior to analysis the sample was diluted 100X with methanol and infused directly with rate of 10 μl/min via syringe pomp to mass spectrometer and scanned from m/z 20 to 1,500 Da under positive-ion mode.
Iodine Number LJ
In order to confirm that the ethoxylation process has no effect on unsaturated bonds in the molecules of ethyl esters of fatty acids of linseed oil, the iodine number of the obtained products was determined. The tests was performed in accordance with the EN ISO 3961:2011 “Animal and vegetable fats and oils—Determination of iodine value.”
Cloud Point Temperature (CP)
The temperature of the turbidity was determined according to international standard No. PN-EN 1890:2006. Five grams of surfactant dissolved in 45 g of a 25% aqueous solution of butyldiglicol ether. A solution of the test samples were heated until complete turbidity and then cooled. As the cloud point temperature adopted in which the solution became clear during cooling.
Average Consumption Rate of Ethylene Oxide by the Reaction System (VAV)
Due to the complexity of the ethoxylation reaction esters and complicated composition of the products to estimate the dynamics of the process that parameter average consumption rate of ethylene oxide by the reaction system—VAV. Thus, understood, the process rate (actually the average rate of reaction) reflecting the consumption rate of ethylene oxide by the reaction, which is the average rate of consumption of ethylene oxide in the process introduced in moles of ethylene oxide per mole of feedstock per hour (moles EO/mole of h). This parameter is calculated by the following formula:
where: mTE, weight of introduced ethylene oxide (g); msub, weight of feedstock (g); Msub, molecular weight of feedstock (g/mol); MTE, molecular weight of ethylene oxide (g/mol); and t, dosing time ethylene oxide (min).
Biodegradability Test
Biodegradability tests was performed in accordance with the OECD guideline 301 A “Ready Biodegradability. DOC Die-Away” and the method C.4-A “Commission Regulation (EC) No. 440/2008 of 30 May 2008 laying down test methods pursuant to Regulation (EC) No. 1907/2006 of the European Parliament and of the Council on the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) (J. of Laws L 142, 31.05.2008).” The methods selected for testing are based on the determination of biodegradability of organic substances which are easily dissolve in water. The percentage biodegradation is calculated on the basis of the removal of DOC (Dissolved Organic Carbon) of the tested compound. Incubation was proceeded under diffuse light at 22 ± 2°C. The study was conducted in an incubator. Aeration of the solutions was obtained by a shaker. Biodegradation process was monitored by determining the value of DOC after 3 h ± 30 min, and then on 4th, 7th, 14th, 21th, and 28th day of the study. Samples for analysis were filtered on the suitable membranes (pore size 0.45 mm). Based on the obtained results, the DOC content in the mixture after a period of incubation was calculated, following by an assessment of biodegradability of the tested sample in the aquatic environment. The result higher that 70% percent biodegradation (determined by the OECD 301A method) means that tested substance is ready biodegradability.
Toxicity
According to guidelines of OECD in a toxicity test, containing both the test substance and a reference compound, <35% degradation (based on total DOC) occurred within 14 days, the test substance can be assumed to be inhibitors.
Results
As it was specified in the introduction, the research conducted on FAMEE clearly indicates that in most cases, they can successfully replace FAE, especially due to their high biodegradability and lower toxicity to aquatic organisms. When designing non-ionic ethoxylated surfactants, attention should be paid primarily to the high availability of relatively cheap renewable raw materials constituting a hydrophobic substrate. These are the ethyl and methyl esters of rapeseed and linseed oil being the subject of this paper.
In the literature, studies on the physicochemical properties and the use of ethoxylated methyl esters from rapeseed oil (RAMEE) may be found.
Renkin et al. (2005) investigated the possibility of using ethoxylated methyl esters from rapeseed oil with an average degree of ethoxylation (NAV) in the range of 5–15 (RAME-E5—RAME-E15) as an ingredient of liquid laundry detergents. They stated that the formulation containing RAME-E7 gives the best global performance, which is equal to that of fatty alcohol ethoxylate. Use of RAME-E in washing products can prevent the use of expensive low foaming, end-capped surfactants, as well as any other silicone antifoamers or paraffin oils. Comparative evaluation of environmental impact of RAME (NAV 5–15) and 1-dodecanol ethoxylates as non-ionic surfactants was conducted by Szwach and Lukosek (2017). Based on the results from measurements of biodegradability and aquatic toxicity it was found that ethoxylated rapeseed acid methyl esters represent environmentally superior surfactants. They exhibit relatively higher biodegradation and lower toxicity against water organisms compared to dodecanolethoxylates. Liu et al. (2017) analyzed the issue of Interfacial Tensions of Ethoxylated Fatty Acid Methyl Ester Solutions Against Crude Oil. However, only selected methyl esters of myristic acid, palmitic acid, and oleic acid, respectively, differing in structure and properties from their equivalents derived from shorter hydrocarbon chains compared to rapeseed and linseed oil, were taken into account.
Lachowicz et al. (2016) tested ethoxylated methyl esters of rapeseed oil with very long hydrophilic chains (NAV = 70) in terms of improved solubility of megestrol acetate. Rapeseed methyl ester ethoxylate was found to improve the solubility of the BCS Class II drug and increase its bioavailability. It increased drug solubility more effectively than Pluronic F68. Actually, there are no studies on the properties and applications of linseed oil ester oxyethylates.
The discussed research results encouraged us to evaluate the physicochemical properties of methyl and ethyl ester oxyethylates from rapeseed and linseed oils with longer hydrophilic chains in terms of their applicability in cosmetic or pharmaceutical preparations.
Synthesis
Comparative syntheses of fatty acid methyl and ethyl esters oxyethylates were performed with the use of two different types of catalysts: calcium (Ca) and aluminum–magnesium (Al–Mg). The syntheses were carried out periodically in a pressure autoclave at a temperature of 185°C. The ester oxyethylates were obtained with the degree of ethoxylation (NAV) from 10 to 70 moles of ethylene oxide per mole of ester.
Assessing the kinetics of the process, the average rate of ethylene oxide consumption during syntheses (VAV) was calculated. This parameter is commonly used in the evaluation of industrial processes. The obtained products were initially characterized by determining the cloud point (CP) for 1% oxyethylate solutions. It is characteristic for non-ionic surfactants of the oxyethylate type that the cloud point increases with the increase of the ethoxylation degree, which proves the higher proportion of oxyethylene groups in the molecule. The test results are presented in Table 2.
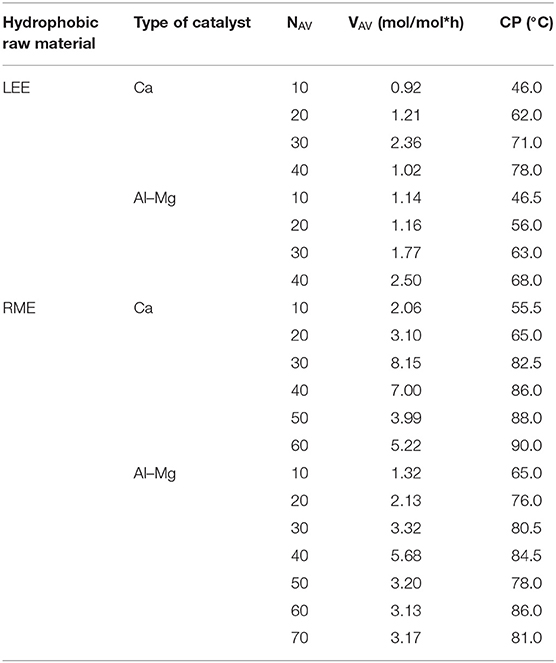
Table 2. Oxyethylates of rapeseed oil fatty acid methyl esters (RME) and linseed oil fatty acid ethyl esters (LEE).
Qualitative Analysis
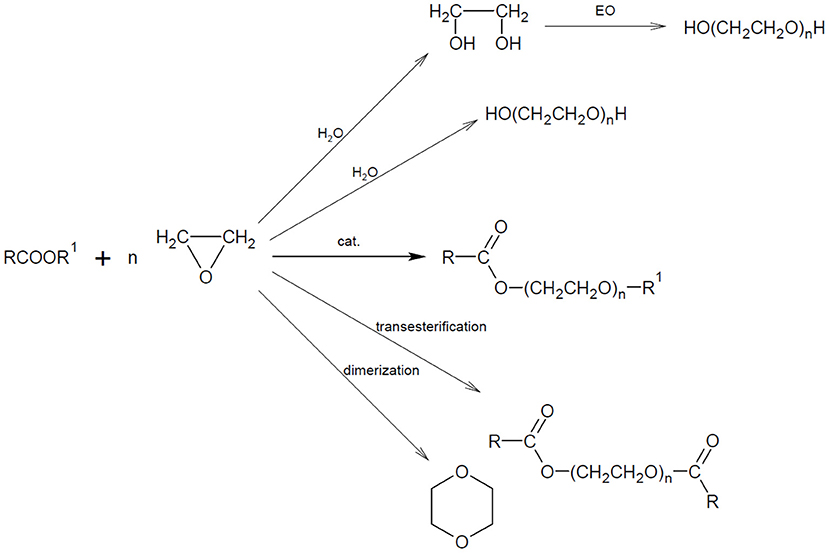
Regardless of the synthesis conditions, a mixture of the expected oxyethylene homologs with different molecular weights and various types of by-products are virtually always produced in the ethoxylation processes. Possible products formed during the direct ethoxylation of carboxylic acid esters are presented in Figure 2 below.
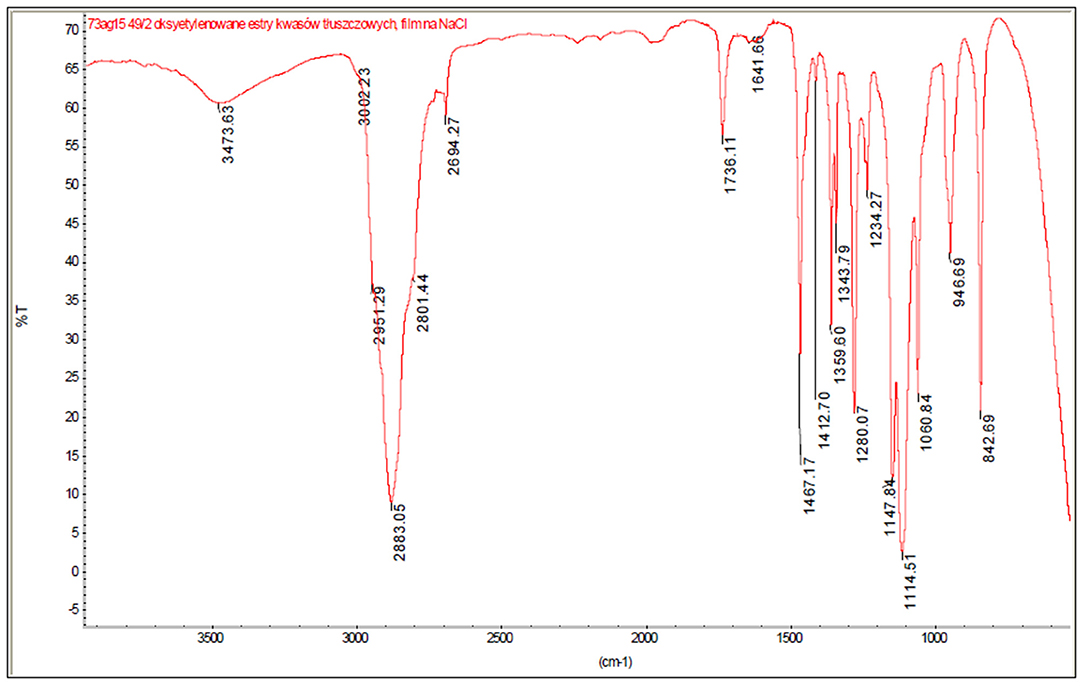
In order to identify the qualitative chemical composition of ester oxyethylation products, three spectroscopic methods were proposed, namely FT-IR, MALDI-TOF, and ESI MS. Methyl and ethyl ester oxyethylates with higher degrees of oxyethylation (for RME NAV = 70, for LEE NAV = 40) were selected for the research due to potentially higher concentrations of by-products. The products of FAME ethoxylation (RME NAV = 70) were analyzed by the MALDI-TOF method, while the ethyl ester oxyethylates (LEE NAV = 40) were analyzed by the ESI MS method. Both methods gave comparable results identifying analogous groups of compounds. The FT-IR method was used to perform transmission spectra of the samples applied as a film onto a NaCl plate in the range of 4,000–450 cm−1. An exemplary spectrum is presented in Figure 3.
In the IR spectra, characteristic bands were identified for bonds occurring in the following chemical compounds:
• 3,500 cm−1–γO−H in alcohols
• 3,002 cm−1–γ and δC−H in alkenes
• 2,950, 2,883 cm−1–γC−H groups -CH3 and -CH2-, -CH in alkanach and n alkanes
• 1,736 cm−1–γC=O in ester groups
• 1,640 cm−1–γC=C in alkenes
• 1,470–1,340 cm−1–δC−H groups CH3 and CH2, CH in alkanach
• 1,412–1,150 cm−1–γC−O and δO−H in alcohols III rz.
• 1,280 cm−1–γC−O in unsaturated fatty acid esters
• 1,150–1,060 cm−1–γC−O in ether groups
• 947 and 843 cm−1–γC−O in ether groups eterach, on plane C-O-C.
Based on the results of the IR analysis, it can be concluded that the following substances are present in the tested samples:
• fatty acid esters,
• ethers: polioxyethylated chaines and methyl ethers.
The MALDI-TOF-MS assay (for RME NAV = 70) was performed in order to identify the molecular weight and the molecular weight distribution of individual groups of polyoxyethylene compounds and homologs. An example of the spectrum is presented in Figure 4.
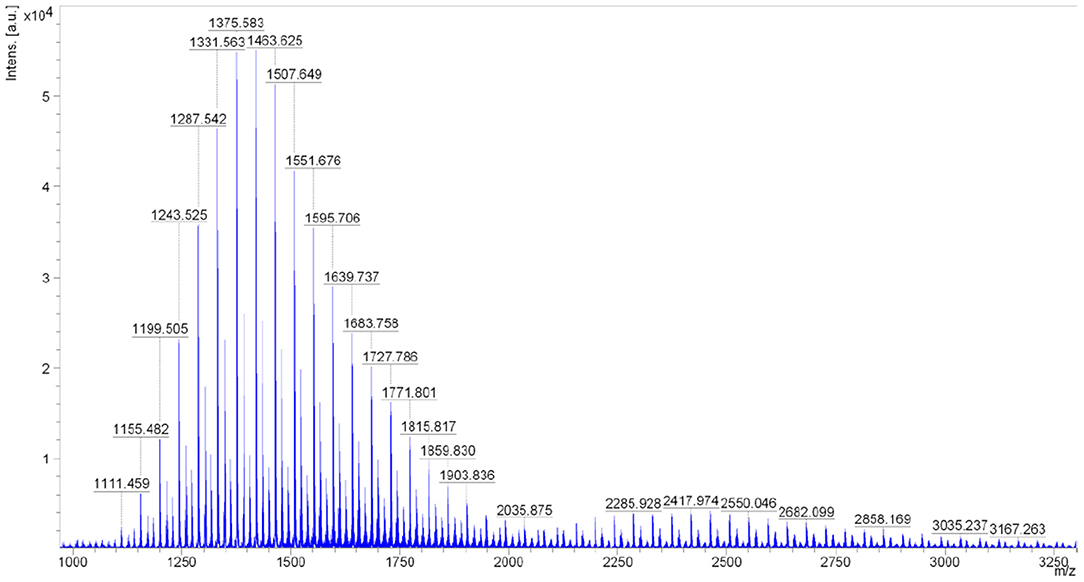
Figure 4. MALDI-TOF spectrum of the RME sample (NAV = 70) obtained with the IAA matrix (range 1,000–3,200 Da).
Two main series of peaks grouping into characteristic maxima were noted in the spectra. The first series is observed in the weight range from 1,100 to 2,100 Da and the second series from 2,000 to 4,700 Da. The first major series of peaks in the mass range from 1,100 to 2,100 Da corresponds to the structure of fatty acid methyl ethoxylates. The second major series of peaks in the mass range from 2,000 to 4,700 Da corresponds to the structure of fatty acid diesters and polyethylene glycols. On the basis of the detailed interpretation of the spectra, it was found that they contain a series of pseudomolecular ion bands that indicate the presence of two main groups of chemical compounds in the analyzed oxyethylates:
1. R-COO(CH2CH2O)nCH3–oxyethylated FAME, maximum on n = 22–29,
2. R-COO(CH2CH2O)zOC-R—diesters of oxyethylated glycols and fatty acid, maximum on n = 35–50.
The remaining peaks visible in the spectra as less intense series may indicate the presence of by-products that can be identified with high probability as:
1. H(OCH2CH2)mOCH3–oxyethylated methanol,
2. H(OCH2CH2)yOH—polyglycols.
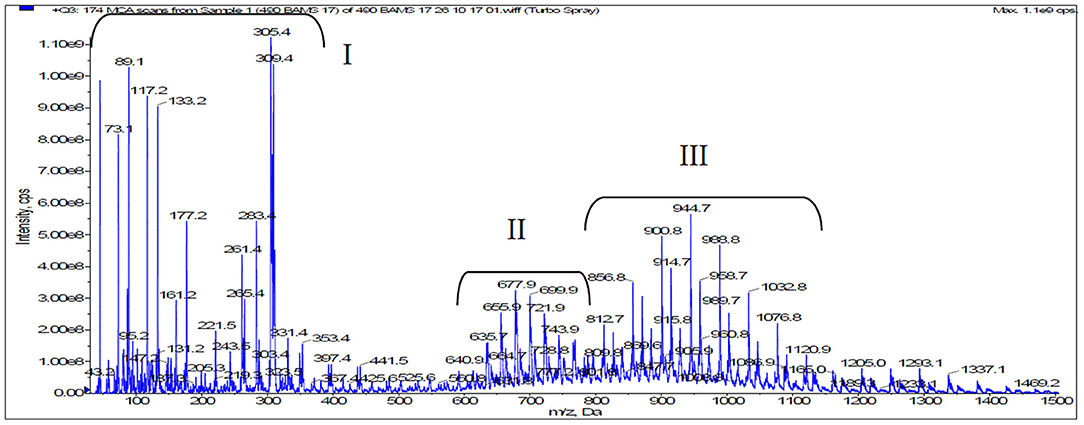
Qualitative analysis of the another selected oxyethylate was performed using the ESI MS mass spectroscopy method. In the ESI MS spectrum developed for ethyl ester oxyethylates (LEE NAV = 40), signals grouping into three main series, originating from different types of compounds with cyclically repeating 44 Da mers were identified. Mass signals corresponding to the likely reaction products of ethylene oxide and fatty acid ethyl esters were identified. An example of the spectrum is presented in Figure 5.
Based on the interpretation of the spectra, it was found that they contain a series of ion bands grouping into characteristic maxima that correspond to the structure of the following compounds:
The series of peaks I
• Polyethylene glycols, Mw = n × 44 where n = 1–10,
• Oxyethylated ethanol, Mw = 29 + n × 44, where n = 1–4.
The series of peaks II
• Diesters of oxyethylated glycols and fatty acid C16-C18, with varying degrees of saturation, ethoxylation degree n = 2–7,
e.g., series of peaks for diesters C18 (Mw = 502 + n × 44): n = 3 (634 Da), n = 4 (678 Da), n = 5 (722 Da), n = 6 (766 Da),
e.g., series of peaks for diesters C16 (Mw = 480+ n × 44): n = 4 (656 Da), n = 5 (700 Da), n = 6 (744 Da), n = 7 (788 Da).
The series of peaks III
• Oxyethylated fatty acid with varying degrees of saturation, ethoxylation degree n = 12–19
e.g., series of peaks for fatty acid C18 (Mw = 282 + n × 44): n = 12 (812 Da), n = 13 (857 Da), n = 14 (900 Da), n = 15 (944 Da), n = 16 (988 Da), n = 17 (1,032 Da), n = 18 (1,076 Da), n = 19 (1,120 Da).
• Oxyethlated fatty acid ethyl esters, mainly C16-C18, with varying degrees of saturation, ethoxylation degree n = 11–18
e.g., series of peaks for ethyl esters C18 (Mw = 312 + n × 44): n = 11 (796 Da), n = 12 (840 Da), n = 13 (884 Da), n = 14 (928 Da), n = 15 (972 Da), n = 16 (1,016), n = 17 (1,060), n = 18 (1,104 Da),
e.g., series of peaks for ethyl esters C16 (Mw =298 + n × 44): n = 13 (870 Da), n = 14 (914 Da), n = 15 (958 Da), n = 16 (1,002), n = 17 (1,046 Da), n = 18 (1,090).
On the basis of the obtained results of the qualitative analysis using the ESI MS method, it can be concluded that ethyl ethyl esters of fatty acids contain a mixture of the following chemical compounds:
1. Oxyethylated fatty acid mainly C16-C18 with nEO = 11–20,
2. Oxyethlated fatty acid ethyl esters and diesters, mainly C16-C18, ethoxylation degree n = 11–20,
3. Oxyethylated ethanol, ethoxylation degree n = 1–12,
4. Polyethylene glycols n = 1–12.
Summarizing the results of qualitative analyzes performed by MALDI TOF and ESI MS spectroscopy methods, it was found that the products of ethoxylation of methyl/ethyl fatty acid esters contain a mixture consisting mainly of:
• Oxyethylated fatty acid methyl/ethyl esters
• Diesters of oxyethylated glycols and fatty acid,
• Oxyethylated fatty acid,
• Oxyethylated methanol/ethanol and polyglycols with varying ethoxylation degree.
Quantitative Analysis
Based on the interpretation of the results of qualitative analyzes by means of spectroscopic methods, quantitative analyzes of the investigated ester oxyethylates were carried out using liquid chromatography (GPC, HPLC) methods, determining the shares of individual fractions that differ significantly in molecular weights. GPC gel chromatography is a method that can determine both average molecular weights and molecular weight distribution. The GPC determination consists in separating a sample according to the hydrodynamic volume of the particles, assuming that the separation depends solely on the particle size. The obtained GPC chromatograms show clearly separated three fractions corresponding to different types of compounds with significantly different molecular weights. An exemplary chromatogram is presented in Figure 6. On the basis of the results of the qualitative analysis, the individual fractions were interpreted as follows:
• Fraction 1—diesters of polyglycols and fatty acid,
• Fraction 2—a mixture of oxyethylated fatty acid and oxyethylated fatty acid ethyl/methyl esters,
• Fraction 3—polyglycols and ethoxylated ethanol/methanol.
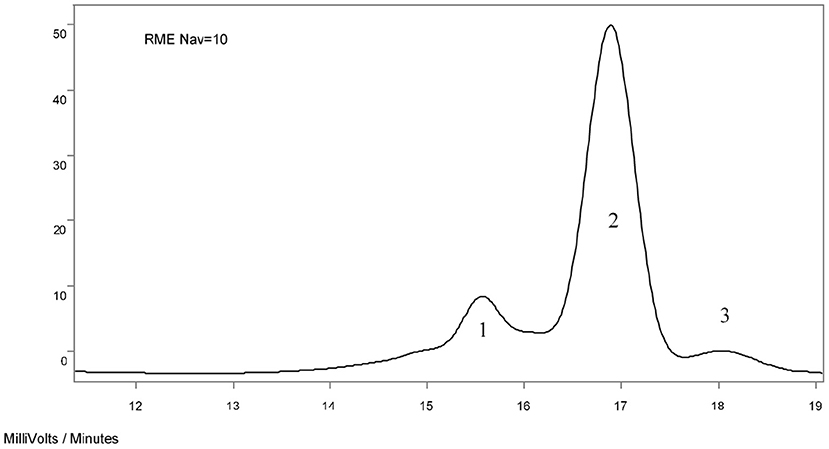
Figure 6. Exemplary GPC chromatogram of the ethoxylation products of rapeseed methyl esters with NAV = 10.
The content of polyethylene glycols (PEG) in the tested oxyethylates was determined by high performance liquid chromatography with a scattered light detector (HPLC/ELSD). The results of the quantitative analysis are presented in Table 3.
Table 3. The results of the chromatographic analysis of the obtained ethyl and methyl esters oxyethylates (GPC/HPLC).
The conducted analytical assessment of the composition of the products of ethoxylation of fatty acid methyl and ethyl esters shows that the tested oxyethylates differ in the content of the main product (fraction 2) depending on the catalyst used. The use of a homogeneous calcium catalyst in the case of the methyl esters results in a higher main product content in comparison with the Al–Mg catalyst. In the case of ethyl esters, the reverse tendency is observed (Figure 7). The content of by-products (fraction 1) depends on the type of the catalyst used and the average degree of ethoxylation, however, no unequivocal relationships were found here. Taking into account all the products obtained, the content of the main product decreases with an increase in the degree of ethoxylation, while the content of fraction 3 increases.
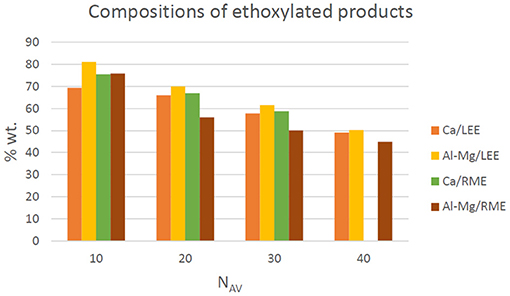
Figure 7. Content of ethoxylated methyl esters (main product—fraction 2) depending on the catalyst used and the hydrophobic raw material.
As has been shown, regardless of the catalyst, the direct ethoxylation of esters produces a relatively complicated mixture of products. It is a typical phenomenon confirmed in numerous literature reports (Hama et al., 1995; Cox and Weerasooriya, 1997; Alejski et al., 2003). However, fractions 1 and 2 present in the products are surface active substances, giving the mixture interesting functional properties. Therefore, diester ethoxylates should not be considered as undesirable by-products. With the increase of Nav, the amount of polyglycols (fraction 3), which are a ballast in ethoxylates, not only in the case of esters but also in other products of this type, also increases (Abrar et al., 2018). Consequently, the key aspect of the development of the industrial sector seems to be the improvement of the catalysts used to enhance the reaction selectivity. Activities in this direction are being performed all over the world, as evidenced by the revival in the professional literature.
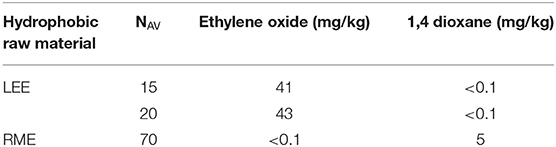
In ethoxylation products, substances such as 1,4-dioxane and unreacted ethylene oxide, i.e., compounds posing a health hazard, may also be present. For the selected products of ester ethoxylation, the content of the above mentioned substances was analyzed. The results are presented in Table 4. In the tested cases, the content of 1,4-dioxane and ethylene oxide was at a relatively low level.
Taking into account the complex composition of the tested products of direct ester ethoxylation, the quality and the directions of their application will be determined by the physicochemical and functional properties of the obtained oxyethylates. This approach is commonly used in practice for surfactants based on natural raw materials, which are usually already a mixture of chemical compounds.
Physicochemical Properties
Iodine Number LJ
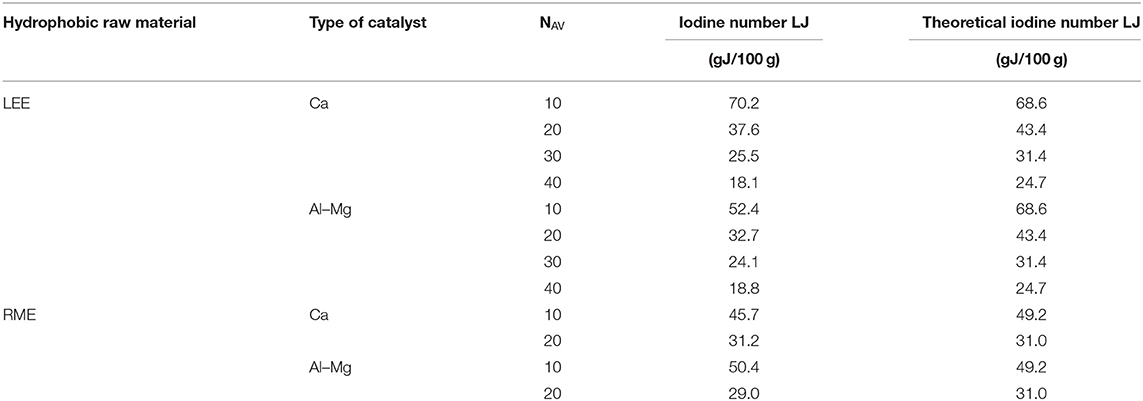
In order to investigate whether the ethoxylation process changes the degree of unsaturation in fatty acid ester molecules, the iodine number of the obtained products was determined. The results for the selected products are presented in Table 5.
The obtained results were related to the theoretically calculated LJ values (based on the LJ values for the starting material). It was observed that the obtained LJ values slightly differ from the theoretical ones, but it is difficult to find a clear tendency. In some cases, there was an increase in LJ and in others a decrease in LJ. The maximum difference in relation to the theoretical value (over 24%) was observed for oxyethylates with NAV = 40. There can be several reasons for the partial saturation of double bonds at the temperature of ethoxylation (185°C) (e.g., polymerization, reactions with acid catalyst components initiated, etc.). However, in order to explicitly state whether the ethoxylation process affects the content of polyunsaturated groups, additional studies should be carried out.
Biodegradability
In the case of surfactants, legal regulations require from producers the eco-assessment of the product in terms of its biodegradability. Ecological safety aspects of marketed products should be taken into account already at the stage of designing the structures of active substances. Therefore, it was purposeful to investigate the biodegradability of the obtained ethoxylated esters and their potential toxic properties.
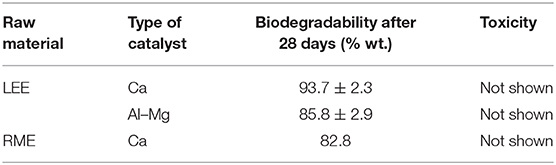
The test results for the oxyethylene products of methyl and ethyl esters with an average degree of ethoxylation of NAV = 10 are presented in Table 6.
The obtained results confirm that the ethoxylated methyl and ethyl esters of fatty acids show very good biodegradability and do not exhibit toxic properties for the microorganisms contained in the activated sludge. The biodegradation values for the tested products are above the required minimum of 80%. Interpretation of the results of the biodegradation tests carried out allows to draw a conclusion that ethoxylated fatty acid esters are characterized by better biodegradation in relation to commonly used ethoxylated alcohols.
Conclusions
To conclude, the products of ethoxylation of FAME from rapeseed oil and ethyl esters of fatty acids from linseed oil were analyzed. The presented research material gives the evidence of the complexity of the chemical composition of ester ethoxylation products. The results of the analyzes confirmed that the products of oxyethylation of fatty acid methyl or ethyl esters contain a mixture consisting mainly of:
• oxyethylene derivatives of methyl/ethylene esters,
• diesters of polyethylene glycols and fatty acid,
• oxyethylated fatty acids,
• oxyethylated methanol/ethanol, and polyglycols with varying ethoxylation degree.
Based on the results of biodegradation research, it was proved that the tested ester oxyethylates are well-biodegradable in the aquatic environment and do not show any toxic properties. Due to their natural origin and good biodegradability, these substances can be considered as ecological and gentle surfactants for use in cosmetic or pharmaceutical preparations.
Data Availability Statement
The original contributions generated for the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the Ministry of Education and Science.
References
Abrar, S., Trathnigg, B., Javed, S., Kiran, S., and Gulzar, T. (2018). Characterization of tween surfactants by MALDI TOF-MS and high performance liquid chromatography in a ternary mobile phase. Tenside Surf. Det. 55, 447–454. doi: 10.3139/113.110590
Alejski, K., Bartkowiak, P., and Szymanowski, J. (2004). Modeling of fatty acid methyl ester oxyethylation in a semi-batch reactor with successive dosing of ethylene oxide. Tenside Surf. Det. 41, 130–134. doi: 10.3139/113.100217
Alejski, K., Bialowas, E., Hreczuch, W., Trathnigg, B., and J., Szymanowski, J. (2003). Oxyethylation of fatty acid methyl esters. Molar ratio and temperature effects. Pressure drop modeling. Ind. Eng. Chem. Res. 42, 2924–2933. doi: 10.1021/ie020471p
Alejski, K., Emmons, M., Lukosek, M., Miesiac, I., and Wesołowski, P. (2012). Method for Obtaining Oxyalkylation Catalysts. Polish Patent No. 391,571. Warsaw: Patent Office of the Republic of Poland.
Allied Market Research. (2020). Surfactants Market Outlook – 2027; Global Opportunity Analysis and Industry Forecast, 2020–2027. Available online at: https://www.alliedmarketresearch.com/surfactant-market (accessed August 05, 2020).
Barbacki, Ł. (2020). Exports in uncertain times. Chem. Biz. Rynek Kosmetyc. Chem. Gospodar. 2020, 10–11.
Bheler, A., Biermann, M., Hill, K., Raths, H. C., Saint Victor, M. E., and Uphues, G. (2001). “Industrial surfactant syntheses,” in Reactions and Synthesis in Surfactant Systems, ed. J. Texter (Boca Raton, FL: CRC Press), 1–44.
Białowas, E., and Szymanowski, J. (2004). Catalysts for oxyethylation of alcohols and fatty acid methyl esters. Ind. Eng. Chem. Res. 43, 6267–6280. doi: 10.1021/ie049898h
Chemia i Biznes (2018). The Detergent Industry Determines the Dynamics of the Surfactant Market. Available online at: https://www.chemiaibiznes.com.pl/aktualnosc/branza-detergentowa-determinuje-dynamike-rynku-surfaktantow (accessed April 10, 2020).
Cox, M. F., and Weerasooriya, U. (1997). Methyl ester ethoxylates. J. Am. Oil Chem. Soc. 74, 847–859. doi: 10.1007/s11746-997-0228-4
Cox, M. F., and Weerasooriya, U. (2000). Partially saponified triglyceride ethoxylates. J. Surf. Deterg. 3, 213–220. doi: 10.1007/s11743-000-0128-x
Di Serio, M., Di Martino, S., and Santacesaria, E. (1994). Kinetics of fatty acids polyethoxylation. Ind. Eng. Chem. Res. 33, 509–514. doi: 10.1021/ie00027a006
Di Serio, M., Tesser, R., Russo, V., Turco, R., Vitiello, R., Sun, Y., et al. (2015). Catalysts for the ethoxylation of esters. J. Surfact. Deterg. 18, 913–918. doi: 10.1007/s11743-015-1719-1
Grant, R. L., Yao, C., Gabaldon, D., and Acosta, D. (1992). Evaluation of surfactant cytotoxicity potential by primary cultures of ocular tissues: I. Characterization of rabbit corneal epithelial cells and initial injury and delayed toxicity studies. Toxicology 76, 153–176. doi: 10.1016/0300-483X(92)90162-8
Hama, I., Okamoto, T., Hidai, E., and Yamada, K. (1997a). Direct ethoxylation of fatty methyl ester over Al-Mg composite oxide catalyst. J. Am. Oil Chem. Soc. 74, 19–24. doi: 10.1007/s11746-997-0113-1
Hama, I., Okamoto, T., and Nakamura, H. J. (1995). Preparation and properties of ethoxylated fatty methyl ester nonionics. Am. Oil Chem. Soc. 72, 781–784. doi: 10.1007/BF02541025
Hama, I., Samasoto, H., and Okamoto, T. (1997b). Influence of catalyst structure on direct ethoxylation of fatty methyl esters over Al-Mg composite oxide catalyst. J. Am. Oil Chem. Soc. 74, 817–822. doi: 10.1007/s11746-997-0224-8
Johansson, I., and Svensson, M. (2001). Surfactants based on fatty acids and other natural hydrophobes. Curr. Opin. Colloid Interface Sci. 6, 178–188. doi: 10.1016/S1359-0294(01)00076-0
Kolano, C., Mohle, H. L., and Richner, R. (2013). Alkoxylation Method Of Fatty Acid Alkyl Esters. European Patent No. 2,611,768. Munich, European Patent Office.
Kolano, C., and Richner, R., Sahebi, M. (2012). Methyl ester ethoxylates – an approach to use renewable raw materials. HandPCToday 7, 52–54.
Kuberska-Maciejewska, M. (2020). Ecological, natural and organic products of household chemicals and cosmetics. Chem. Biz. Rynek Kosmetyc. Chem. Gospodar. 2020, 46–49.
Lachowicz, M., Kołodziejczyk, M., Lukosek, M., Kosno, J., Olszewska, P., and Szymański, P. (2016). New biopolymer nanoparticles improve the solubility of lipophilic megestrol acetate. Molecules 21:197. doi: 10.3390/molecules21020197
Liu, M., Fang, H., Jin, Z., Xu, Z., Zhang, L., Zhang, L., et al. (2017). Interfacial tensions of ethoxylated fatty acid methyl ester solutions against crude oil. J. Surfact. Deterg. 20, 961–967. doi: 10.1007/s11743-017-1973-5
Lukosek, M., and Alejski, K. (2016). Comparative study on two types of ethoxylation catalysts applicable for natural raw materials which contain hydroxyl. Przem. Chem. 95, 2352–2358. doi: 10.15199/62.2016.11.43
Markets and Markets. (2017). Markets and Markets, 9 Feb 2017. Available online at: http://www.marketsandmarkets.com (accessed April 10, 2020).
Renkin, M., Fleurackers, S., Szwach, I., and Hreczuch, W. (2005). Rapeseed methyl ester ethoxylates: a new class of surfactants of environmental and commercial interest. Tenside Surf. Det. 42, 280–287. doi: 10.3139/113.100269
Smidrkal, J., Vokacova, M., Polakova, L., Filip, V., and Machkova, K. (2008). Properties of oxyethylenated fatty acid methyl esters and ethyl esters. Hydrolysis, critical micelle concentrations and foaming. Tenside Surf. Det. 45, 249–253. doi: 10.3139/113.100381
Smith, G. A. (2019). “Fatty acid, methyl ester, and vegetable oil ethoxylates,” in Biobased Surfactants Synthesis, Properties, and Applications, eds D. G. Hayes, D. K. Y. Solaiman, and R. P. Ashby (Academic Press, AOCS Press), 287–301. doi: 10.1016/B978-0-12-812705-6.00008-3
Szwach, I., and Lukosek, M. (2017). Comparative evaluation of environmental impact of selected ethoxylate fatty alcohols and ethoxylate fatty acid methyl esters as nonionic surfactants. Pol. J. Environ. Stud. 26, 1245–1250. doi: 10.15244/pjoes/67748
Wasilewski, T., Sun, Y.-Q., Hreczuch, W., Seweryn, A., and Bujak, T. (2019). Evaluation of ethoxylated rapeseed oil fatty acids methyl esters as nonionic co-surfactants in hand dishwashing liquids. Tenside Surf. Det. 56, 279–286. doi: 10.3139/113.110630
Keywords: ethoxylation, catalyst of ethoxylation, oxyethylated esters, catalyst, natural raw materials
Citation: Lukosek M, Emmons-Burzyńska M, Alejski K and Szwach I (2021) Physicochemical Characterization of Ethoxylation Products of Fatty Acid Esters. Front. Chem. Eng. 3:617701. doi: 10.3389/fceng.2021.617701
Received: 15 October 2020; Accepted: 11 March 2021;
Published: 01 April 2021.
Edited by:
Riccardo Tesser, University of Naples Federico II, ItalyReviewed by:
Vincenzo Vaiano, University of Salerno, ItalyZenon Lukaszewski, Poznań University of Technology, Poland
Copyright © 2021 Lukosek, Emmons-Burzyńska, Alejski and Szwach. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marek Lukosek, bWFyZWsubHVrb3Nla0BpY3NvLmx1a2FzaWV3aWN6Lmdvdi5wbA==
 Marek Lukosek
Marek Lukosek Magdalena Emmons-Burzyńska2
Magdalena Emmons-Burzyńska2