
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell Dev. Biol. , 05 March 2025
Sec. Cell Death and Survival
Volume 13 - 2025 | https://doi.org/10.3389/fcell.2025.1460416
This article is part of the Research Topic Decoding Cell Fate: The Critical Roles of Extracellular Vesicles View all 4 articles
 Han Longfei1
Han Longfei1 Hou Wenyuan1
Hou Wenyuan1 Fang Weihua1
Fang Weihua1 Peng Peng1
Peng Peng1 Lu Sun1
Lu Sun1 Lin Kun1
Lin Kun1 He Mincong2,3
He Mincong2,3 Yang Fan2,3
Yang Fan2,3 He Wei2,3
He Wei2,3 Wei Qiushi2,3,4*
Wei Qiushi2,3,4*Osteoarthritis (OA) is a debilitating disease that predominantly impacts the hip, hand, and knee joints. Its pathology is defined by the progressive degradation of articular cartilage, formation of bone spurs, and synovial inflammation, resulting in pain, joint function limitations, and substantial societal and familial burdens. Current treatment strategies primarily target pain alleviation, yet improved interventions addressing the underlying disease pathology are scarce. Recently, exosomes have emerged as a subject of growing interest in OA therapy. Numerous studies have investigated exosomes to offer promising therapeutic approaches for OA through diverse in vivo and in vitro models, elucidating the mechanisms by which exosomes from various cell sources modulate the cartilage microenvironment and promote cartilage repair. Preclinical investigations have demonstrated the regulatory effects of exosomes originating from human cells, including mesenchymal stem cells (MSC), synovial fibroblasts, chondrocytes, macrophages, and exosomes derived from Chinese herbal medicines, on the modulation of the cartilage microenvironment and cartilage repair through diverse signaling pathways. Additionally, therapeutic mechanisms encompass cartilage inflammation, degradation of the cartilage matrix, proliferation and migration of chondrocytes, autophagy, apoptosis, and mitigation of oxidative stress. An increasing number of exosome carrier scaffolds are under development. Our review adopts a multidimensional approach to enhance comprehension of the pivotal therapeutic functions exerted by exosomes sourced from diverse cell types in OA. Ultimately, our aim is to pinpoint therapeutic targets capable of regulating the cartilage microenvironment and facilitating cartilage repair in OA.
Osteoarthritis (OA), recognized as the prevailing joint pathology globally, is distinguished by the degradation of articular cartilage, formation of bone redundancy, subchondral bone sclerosis, and inflammation and fibrosis in the synovium, predominantly affecting joints such as the hip, hand, and knee. This degenerative process leads to functional constraints and impairment in joint functionality. Current pharmacological interventions, serving as palliative measures, fail to halt or reverse the relentless advancement of the ailment, culminating in the necessity for joint replacement (Fan et al., 2022). Hence, there exists a pressing imperative to innovate novel therapeutic modalities that not only arrest but also potentially reverse the trajectory of OA. In OA, intricate biomechanical transformations transpire within the cartilage and chondrocytes, involving cartilage deterioration, mechanical stress, and modifications in the composition of the cartilage matrix, mediated by vital agents comprising matrix-degrading enzymes and inflammatory cytokines (Chen L. et al., 2024). While chondrocytes reside in a quiescent state in the pristine joint environment marked by diminished metabolic activity and regulated exchange of matrix constituents, upon injury to articular cartilage, chondrocytes mount a reparative response, paradoxically exacerbating arthritic progression due to constrained vascular supply to the cartilage matrix. Resultantly, an accelerated process of cartilage matrix degradation outpaces the chondrocytic synthesis of new matrix elements (Adam et al., 2024; Lee et al., 2024). Consequently, emphasis on the pivotal role of chondrocytes in the therapeutic paradigm of OA is warranted. Damage to articular cartilage or an inflammatory response accelerates joint degeneration, representing a significant pathological manifestation of OA. The challenges associated with repairing cartilage damage are attributed to the absence of blood supply, innervation, and lymphatic tissue within the joint cavity, compounded by the negative pressure environment resulting from ischemia and hypoxia (Chen J. et al., 2024). Formerly extolled as a promising intervention approach, cell-based therapy has manifested some efficacy through intra-articular instillations of pertinent cells, such as mesenchymal stem cells, showing promise in mitigating inflammatory cascades and alleviating pain (Zelinka et al., 2022; Copp et al., 2023). Conversely, empirical evidence underscores the potential unpredictability of cellular therapeutics, evoking adverse sequelae encompassing joint discomfort, edema, swelling, and even grave ramifications like carcinogenesis (Shang et al., 2023). These limitations are also evident in the loss of grafted cells, cellular senescence, apoptosis, hypertrophy, inflammation, and necrosis (Steinert et al., 2007; Alahdal et al., 2021). Exosomes (Exo) wield paramount significance in intercellular signaling mechanisms, serving as pivotal conduits for biological communication among cellular entities to regulate a myriad of physiological processes. Operating as vital signaling moieties, exosomes orchestrate the transfer of proteins, lipids, and nucleic acids, constituting a significant role in diverse physiopathological processes including tissue restitution and immune surveillance (Xu X. et al., 2024). Capitalizing on the multifaceted functions of exosomes, the manipulation of the cartilage microenvironment and facilitation of cartilage repair hold profound therapeutic promise in tackling OA (Yue et al., 2024). Despite elucidative advancements in delineating the molecular underpinnings by which exosomes foster cartilage regeneration, a comprehensive exploration of the diverse cellular origins of exosomes influencing cartilage repair in OA remains a nascent domain. Therefore, a meticulous review delineating the regulatory efficacy of exosomes derived from distinct cellular reservoirs in orchestrating cartilage healing in OA is warranted to furnish a seminal reference for subsequent investigational pursuits.
Extracellular vesicles (EVs) are lipid-membrane vesicles secreted by cells into the extracellular space. They exhibit diameters ranging from 30 nm to 2,000 nm and encompass three subtypes: microvesicles, exosomes, and apoptotic vesicles. EVs comprise approximately 0.1% of the RNA content found in parental cells and exhibit diverse mechanisms through which they influence biological processes. This involves proteins and biologically active lipid ligands present on the vesicle surface mediating membrane fusion by engaging cell surface receptors. Consequently, vesicles release their contents—comprising transcription factors, oncogenes, non-coding RNAs, mRNAs, and infectious particles—to recipient cells, thus modulating their functions (Andaloussi et al., 2013; Lin et al., 2016). Among extracellular vesicles, exosomes are extensively researched and can be emitted by all cell types. These membranous structures have been detected in various bodily fluids, including plasma, urine, saliva, lymph, and synovial fluid (Doyle and Wang, 2019). A schematic diagram of exosomes is shown in Figure 1.
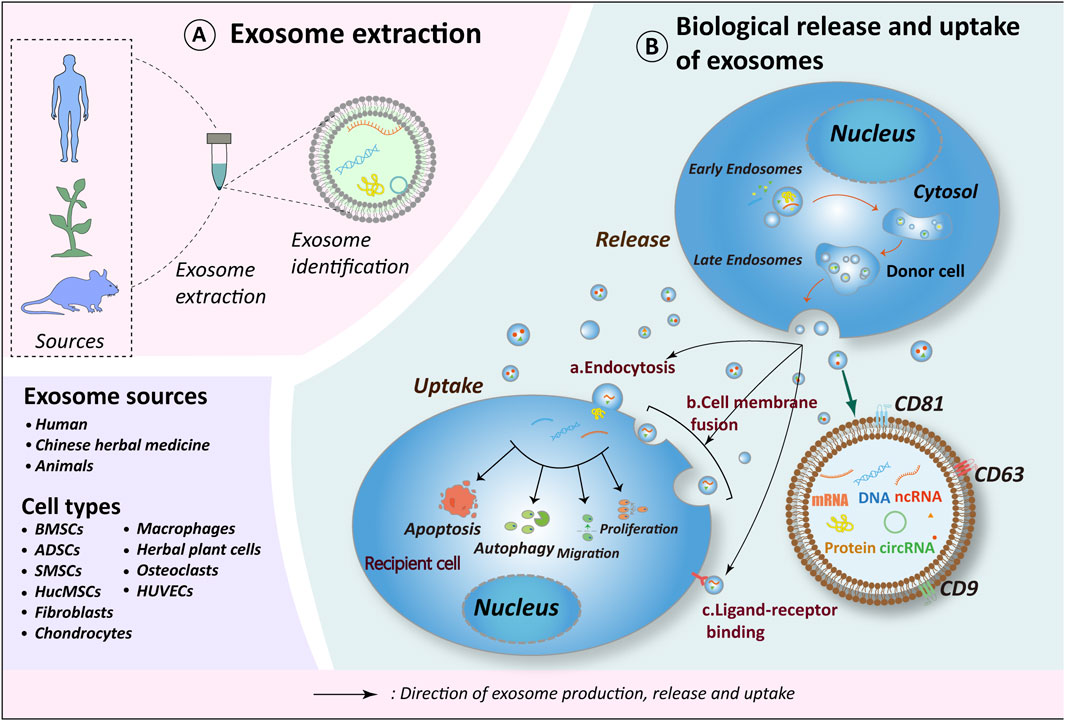
Figure 1. Exosome biorelease and uptake process. (A) Exosomes originate from diverse sources, with current research emphasizing those derived from human tissues, plants (including herbs), and animal tissues. The cell types of exosomes exhibiting therapeutic potential for OA include BMSCs, ADSCs, SMSCs, HucMSCs, Fibroblasts, Chondrocytes, Macrophages, Osteoclasts, HUVECs and herbs. (B) The biogenesis of exosomes occurs in four distinct stages: 1. Endocytosis, during which the cell membrane invaginates to form early endosomes; 2. Packaging of contents, wherein early endosomes further mature into late endosomes; 3. Fusion of late endosomes to form multivesicular bodies (MVBs); 4. MVBs subsequently fuse with the cell membrane to release their contents into the extracellular compartment, thereby forming exosomes. Recipient cells internalize exosomes via endocytosis, direct fusion, or ligand-receptor interactions. Through the release of materials encapsulated within exosomes (e.g., proteins, nucleic acids, lipids, metabolites), exosomes exert influence on the metabolic processes of recipient cells, including cell proliferation, migration, apoptosis, and autophagy, thereby further impacting the OA process. Schematic drawing reference from Chen J. et al. (2022). BMSCs, bone marrow mesenchymal stem cells; ADSCs, adipose mesenchymal stem cells; SMSCs, synovial mesenchymal stem cells; HucMSCs, human umbilical cord mesenchymal stem cells; HUVECs, human umbilical vein endothelial cells.
Several techniques exist for the extraction, isolation, and purification of exosomes, albeit lacking uniform standards. Common methodologies encompass differential ultracentrifugation, density gradient centrifugation, kit-based procedures, magnetic bead-based immunoaffinity capture, and size-exclusion chromatography (SEC). The rationale for these solutions is grounded in the unique physical and molecular biological properties of exosomes. While numerous methods for exosome isolation exist, each presents specific limitations (Ma X. et al., 2024). Ultracentrifugation leverages the size differential between exosomes and other components in the cell culture supernatant for effective separation. Although ultracentrifugation is regarded as the gold standard for exosome isolation, it is time-consuming, requires expensive centrifuge equipment, and is prone to contamination by various impurities (e.g., other particles, viruses, lipoprotein particles, and protein complexes). Additionally, it may cause structural disruption of the exosomes, potentially affecting downstream analyses (Yang et al., 2020). These disadvantages further restrict the applicability of ultracentrifugation. In contrast to ultracentrifugation, density gradient centrifugation operates on the principle that different vesicle types exhibit varying densities and sedimentation rates. The two primary methods include optiprep density medium separation and sucrose density medium separation. This method necessitates the use of a density gradient medium. Under high centrifugal force, the particles within the sample migrate at varying rates and remain suspended in the solution without the need for layering. This separation technique is particularly suitable for the purification of large-volume samples. However, this method is time-consuming, cumbersome, and susceptible to sample damage (Zhu et al., 2019). To enhance the specificity and targeting of exosome isolation, the magnetic bead-based immunoaffinity capture technique can be employed (Jiawei et al., 2022). This method employs immunological techniques to specifically recognize and isolate exosomal membrane proteins, such as CD9, CD63, and CD81, resulting in significantly higher purity compared to exosomes isolated solely based on physical properties (Tauro et al., 2012). However, the differential expression of exosomal membrane proteins frequently results in insufficient exosome yield. Size exclusion chromatography separates exosomes based on molecular weight and size and is currently more widely utilized for the removal of mixed proteins and lipids from exosome samples (Gámez-Valero et al., 2016). This method is advantageous for preserving the biological activity of exosomes; however, it necessitates expensive equipment, is time-consuming, and is best suited for processing small sample volumes (Gheinani et al., 2018). Nevertheless, the application of size exclusion chromatography for exosome extraction has garnered increasing attention in recent years (Yang et al., 2020). Notably, differential ultracentrifugation, the pioneering technique for exosome isolation, persists as the preferred method in current exosome research (Doyle and Wang, 2019; Fan et al., 2024; Yue et al., 2024).
Exosomes exert their distinct functions via intercellular signaling, influencing key physiological processes like development, cell proliferation, differentiation, and metabolism. Increasing research indicates the vast potential of exosomes in tissue regeneration, disease therapy across various conditions, and in conferring protective effects to the body (Tang et al., 2014; Chen C. Y. et al., 2022; Wei et al., 2023). Exosomes hold significance in orthopedic degenerative ailments such as OA, osteoporosis, and disc degeneration. Studies have highlighted 46 distinctively expressed miRNAs linked to processes like inflammation, autophagy, chondrocyte viability, differentiation, homeostasis, metabolism, and extracellular matrix degradation in individuals with OA (Cong et al., 2017). Due to their enhanced targeting capacity and simple storage and management feasibility (Li S. et al., 2024), exploring miRNAs further holds promise in elucidating OA pathogenesis and potentially altering the disease trajectory.
OA is therapeutically characterized by a variety of disease processes and pathologies, which complicates the development of universally effective treatments (Hart, 2022). Treatment strategies do not focus solely on a single joint’s pathology; instead, they integrate behavioral, psychosocial, educational, and physical interventions (Kolasinski et al., 2020). Based on the treatment modality, approaches may be categorized into two primary types: non-pharmacological and pharmacological (Kolasinski et al., 2020; Katz et al., 2021). Non-pharmacological interventions, including walking, swimming, tai chi, yoga, cognitive behavioral therapy (CBT), and acupuncture, have been shown to enhance joint function in patients with OA (Bannuru et al., 2019). Conversely, pharmacological approaches primarily consist of non-steroidal anti-inflammatory drugs (NSAIDs) (Conaghan et al., 2008), the development of disease-modifying OA drugs, and regenerative medicine (Cho et al., 2021). Stem cells have been rigorously investigated in tissue regeneration research due to their capacity for redifferentiation and the advantageous components found in their secreted factors. Given the instability of stem cells in the human body, their secreted exosomes are regarded as a safer alternative for the next-generation of cell-based therapies (Alcaraz et al., 2019).
Mesenchymal stem cells (MSCs), possessing self-renewal and differentiation capabilities, demonstrate significant potential in OA therapy. Serving as pivotal cells in regenerative approaches, reports exist on the transplantation of MSCs sourced from diverse origins such as bone marrow, adipose tissue, synovium, blood, and umbilical cord (Wen et al., 2012; Chen et al., 2016; Chen et al., 2014; Fu et al., 2016; Otsuki et al., 2018). In contrast to conventional cell transplantation techniques, extracellular vesicles (EVs) present a safer and innovative avenue for the prevention and treatment of OA. Progressive research findings increasingly indicate that the therapeutic benefits of stem cell transplantation may be facilitated through the actions of their extracellular vesicles (Nguyen et al., 2021; Rizzo et al., 2023). Exosomes from stem cells, such as MSCs, carry essential growth factors and other bioactive molecules that promote tissue repair and regeneration, facilitating healing processes and enhancing cell communication (José, 2024). Hence, there is a critical need to investigate the mechanism by which exosomes sourced from MSCs modulate the cartilage microenvironment and facilitate cartilage restoration.
The Wnt/β-catenin pathway is a well-established signaling cascade linked to bone formation, tissue regeneration, and joint equilibrium, playing a crucial role in the development of OA (Huang et al., 2020; Feng et al., 2024). Activation of the Wnt signaling pathway induces the stabilization and nuclear translocation of β-catenin, thereby further activating target gene expression related to cartilage degradation, inflammation, and bone regrowth (Usami et al., 2016; Aziz et al., 2024). The Wnt signaling pathway is categorized into two modes based on β-catenin dependence: the β-catenin-dependent mode, referred to as the classical Wnt/β-catenin signaling pathway, and its counterpart, known as the non-classical pathway. The classical Wnt pathway is initiated by the binding of Wnt to the Wnt receptor (i.e., Frizzled) and the Wnt co-receptor (i.e., lipoprotein receptor-related protein-LRP-5/6), which subsequently induces conformational changes in the downstream molecular complex. The downstream molecular complex comprises dishevelled, glycogen synthase kinase 3β (GSK3β), axin, adenomatosis polyposis coli (APC), β-catenin, and other associated proteins. Alterations in the interactions and phosphorylation order of constituent proteins disrupt the phosphorylation of the amino-terminal structural domain of β-catenin, leading to its stabilization and nuclear translocation. This process, in turn, stimulates the transcription of target genes in conjunction with T-cell factors and lymphoid enhancer-binding factors (TCF/LEF), among others. Wnt-associated proteins exhibit differential expression in various regions of cartilage (Witte et al., 2009). Notably, Wnt4, Wnt5a, Wnt5b, Wnt11, and Wnt14 are significantly differentially expressed in cartilage, perichondrium, and surrounding tissues (Hartmann and Tabin, 2000). Wnt4 and Wnt14 are predominantly localized in articular cartilage (Hartmann and Tabin, 2001); Wnt5a is highly expressed in cartilage spanning the epiphysis to the metaphysis (Church et al., 2002); Wnt5b is primarily expressed in the prehypertrophic region; and Wnt11 is predominantly found in chondrocytes during embryonic limb development. Notably, the activation of the Wnt/β-catenin pathway must be tightly regulated to facilitate normal cartilage development, as either excessive activation or insufficient activation of this signaling pathway can lead to skeletal diseases (Yuasa et al., 2009; Dao et al., 2012).
Exosomes originating from BMSCs have been shown to enhance cartilage regeneration through the stimulation of chondrocyte proliferation and migration while preventing apoptosis, mainly mediated by the Wnt/β-catenin pathway. Subsequent investigations have identified that the distinctive molecular underpinnings of BMSC-derived exosomes involve the enrichment of miR-127-3p, miR-92a-3p, miR-140-3p, along with proteins associated with cellular adhesion and tissue restoration capabilities (Mao et al., 2018; Dong et al., 2021; Hu Y. et al., 2023; Wang L. et al., 2024).
The PI3K/AKT/mTOR pathway stands as a critical cellular cascade governing cell proliferation, motility, growth, and metabolism, essential for maintaining the homeostasis of joint tissues and implicated in the pathogenesis of OA (Sun et al., 2020). Glucose, insulin, growth factors, and cytokines all activate the PI3K/AKT/mTOR signaling pathway (Engelman et al., 2006). These molecules function by activating receptor tyrosine kinases (RTKs) and G protein-coupled receptors (GPCRs), thereby stimulating PI3K to produce phospholipids and activate downstream proteins, including AKT and the mammalian target of rapamycin complex 1 (mTORC1) (De Santis et al., 2019). Protein kinase B (AKT) serves as a crucial signaling molecule within the PI3K pathway. Once activated, AKT translocates to various cellular compartments to activate several downstream substrates, including protein kinases, E3 ubiquitin ligases, small G-protein modulators, metabolic enzymes, transcription factors, and cell cycle regulators (Manning and Toker, 2017). A key downstream branch of AKT is mTORC1. Phosphorylation of AKT promotes the phosphorylation of mechanistic target of rapamycin kinase (mTOR) at Ser2448 and directly activates mTORC1. Altered mTORC1 activity subsequently affects its effectors, including S6 kinase 1 (S6K1), eukaryotic translation initiation factor 4E binding protein 1 (4EBP1), and unc-51-like kinase 1 (ULK1). Among these, S6K1 and 4EBP1 promote the translation of mRNAs for hypoxia inducible factor 1 subunit alpha (HIF-1α), cyclin D1, and myelocytomatosis oncogene (c-Myc), thereby participating in processes such as cell cycle regulation and angiogenesis (Laplante and Sabatini, 2012). Notably, mTORC1 serves as a major regulator of unc-51 like autophagy activating kinase 1 (ULK1) and is associated with the initiation of autophagy. Treatment with rapamycin, an inhibitor of mTORC1, enhances ULK1 kinase activity; conversely, the promotion of mTORC1 activity effectively inhibits ULK1 activity (Park et al., 2016; Nnah et al., 2019). Furthermore, ULK1 can form a complex by binding to autophagy related 13 (ATG13) and fak family kinase-interacting protein (FIP200), acting as a node that translates autophagic signaling into the initiation of autophagic biological processes (Ganley et al., 2009). These observations suggest that the PI3K/AKT/mTOR signaling pathway is critical for maintaining cellular homeostasis and is closely associated with various biological processes, including the cell cycle, cell survival, inflammation, metabolism, and apoptosis (Cravero et al., 2009; Tang et al., 2018).
Research has revealed that exosomes derived from BMSCs mediate KLF 3-AS 1, activating the PI3K/Akt/mTOR pathway. They also competitively adsorb miR-206 to enhance G protein-coupled receptor kinase interacting ArfGAP 1 (GIT 1) expression, thereby fostering chondrocyte proliferation and inhibiting apoptosis. Consequently, KLF 3-AS 1 emerges as a promising candidate for therapeutic interventions aimed at enhancing articular cartilage repair (Liu et al., 2018a; Wen et al., 2022). Another investigation demonstrated that exosomes derived from BMSCs treated with decellularized extracellular matrix (dECM) enhanced the expression of miR-3473b, mediating the phosphatase and tensin homolog (PTEN)/protein kinase b. (AKT) pathway to ameliorate cartilage damage in a similar manner (Liu et al., 2018b). Additionally, BMSC-derived exosomes exhibit the ability to suppress chondrocyte apoptosis by mediating miR-326 and targeting the histone deacetylase 3 (HDAC3), signal transducer and activator of transcription 1 (STAT1), and nuclear factor kappa b (NF-κB)/p65 signaling pathways (Xu and Xu, 2021).
Mechanical stress plays a crucial role in stimulating cell proliferation and differentiation. Studies have demonstrated that low-intensity pulsed ultrasound (LIPUS) promotes the inhibition of inflammation, enhances chondrocyte proliferation, and stimulates cartilage matrix synthesis through mechanisms closely associated with the NF-κB pathway (Liao et al., 2021). Additionally, pulsed electromagnetic fields (PEMF) at 75 Hz have shown comparable efficacy in these processes (Xu et al., 2023). Furthermore, exosomes derived from BMSCs overexpressing miRNA-210 have shown potential in safeguarding chondrocytes from damage responses in inflammatory conditions via this pathway (He et al., 2020). Besides mechanical factors, proteins influenced by mechanical stress have been linked to cartilage restoration. Research has indicated that the transfection of exosomes from bone marrow endothelial cells (BMECs) with silenced Piezo1 lentivirus not only hampers the inflammatory response but also enhances cartilage repair processes (Li et al., 2021). These findings imply that Piezo1 may hinder OA therapy and holds potential as a target for therapeutic interventions.
Macrophages are the predominant immune cells in synovial tissue involved in inflammation and tissue damage repair processes. Studies have shown that both BMSCs-Exos and HucMSCs-Exos possess the ability to modulate macrophage polarization from the M1 to M2 type (Li X. et al., 2024; Tian et al., 2024). Furthermore, BMSCs-Exos have been found to suppress inflammation and enhance cartilage repair by orchestrating the glutamic-oxaloacetic transaminase 1 (GOT1)/c-c motif chemokine receptor 2 (CCR2) pathway, resulting in increased expression of NF-E2-related factor 2 (Nrf2)/heme oxygenase 1 (HO-1), thereby inhibiting macrophage iron death (Peng et al., 2023). Additionally, MSCs exposed to aging and inflammatory conditions demonstrate reduced effectiveness in alleviating the pathological progression of OA (Jérémy et al., 2024). This indicates that while bone marrow MSCs have the potential to differentiate into cartilage, the cellular microenvironment and physiological conditions are crucial factors influencing the functionality of the exosomes they secrete. Moreover, BMSCs-Exos promote chondrocyte mitochondrial autophagy by regulating dynamin-related protein 1 (Drp1) expression, leading to the inhibition of chondrocyte apoptosis and cartilage matrix degradation (Tang et al., 2021).
Using pharmacological agents known for promoting cartilage regeneration and chondroprotection to treat BMSCs, followed by the extraction of exosomes for studying their effects on chondrocytes, represents valuable research strategies for elucidating drug mechanisms and exploring the therapeutic potential of the exosomal pathway (Wang and Xu, 2021). In vitro studies have demonstrated the substantial anti-inflammatory, antioxidant, antidiabetic, and immunomodulatory properties of fucoidan. Particularly noteworthy is the enrichment of miR-146b-5p in fucoidan-treated BMSCs-Exos, which effectively inhibited tumor necrosis factor receptor-associated factor 6 (TRAF 6) activation, leading to the attenuation of inflammatory reactions and preservation of extracellular matrix integrity (Lou et al., 2023). Transforming growth factor-β1 (TGF-β1) is synthesized and secreted by chondrocytes, where it remains latent in the extracellular matrix (ECM) of various tissues before activating and stimulating chondrocytes to produce essential ECM elements. When BMSCs-Exos were exposed to TGF-β1, enhanced cartilage repair efficacy was observed compared to BMSCs-Exos treatment alone. Discrepancies in the levels of miR-373 and miR-483 within their exosomal content were evident, alongside increased expression of miR-135b following TGF-β1 treatment, which in turn downregulated sp1 transcription factor (Sp1), ultimately facilitating cartilage repair (Wang et al., 2018).
In conclusion, the remarkable potential for OA treatment is evident, owing to the exceptional delivery capacity and diverse biological functionalities exhibited by exosomes released by BMSCs.
Adipose-derived stem cells (ADSCs), owing to their ease of procurement and notable chondrogenic differentiation potential in treating articular cartilage injuries, are categorized based on their origin as either infrapatellar fat pad (IPFP) or subcutaneous fat-derived MSCs. Notably, ADSCs exhibit superior chondrogenic capabilities compared to other MSCs and display resilience to inflammatory influences, thereby attracting significant research focus and scrutiny (Liao et al., 2022).
Kartogenin (KGN) is recognized as a potent stimulator of mesenchymal stem cells for chondrocyte differentiation. Studies have demonstrated that KGN effectively promotes chondrogenic differentiation in synovial MSCs (Xu et al., 2021). Furthermore, in comparative analyses with exosomes derived from BMSCs, exosomes obtained from MSCs pre-treated with KGN exhibited enhanced chondrogenic matrix formation and reduced levels of matrix degradation in both in vitro and in vivo investigations (Liu C. et al., 2020). These findings suggest that utilizing a chondrocyte inducer like KGN may enhance the bioefficacy of exosome preparation.
Cellular autophagy, the process wherein aging organelles fuse with and are degraded by lysosomes through autophagosomes, represents the cellular mechanism through which cells utilize their components to uphold biological equilibrium. Notably, mTOR functions as a negative regulator of autophagy, and inhibiting mTOR enhances cellular autophagy, thereby contributing to the protection of articular cartilage (Caramés et al., 2012). Several studies have validated that exosomes derived from infrapatellar fat pad stem cells can confer chondroprotective and reparative effects by modulating the mTOR autophagy pathway via miR-100-5p and inducing autophagy through miR-429 targeting of fasciculation and elongation protein zeta-2 (FEZ 2) (Wu et al., 2019; Meng C. et al., 2023). To simulate the impact of exosomes released in a hypoxic microenvironment on chondrocytes, research has compared the effects of exosomes generated by ADSCs on chondrocytes under normoxic and hypoxic conditions. Results revealed that hypoxic conditions can upregulate the expression of chondrocyte proliferation genes (NDRG family member 3 [NDRG3], collagen type II alpha 1 chain [Col2a1]), while downregulating the expression of chondrocyte matrix-degrading genes, such as matrix metallopeptidase 13 (MMP-13), ultimately facilitating cartilage repair (Zhao J. et al., 2023). Additionally, ADSCs-Exos suppresses the Wnt/β-catenin pathway by targeting Wnt3 and Wnt9a with miR-376c-3p (Li F. et al., 2023), leading to the attenuation of cartilage matrix degradation and synovial membrane fibrosis. Conversely, exosomes released from ADSCs treated with protoelastin (TE) enhance chondrocyte extracellular matrix synthesis by transporting miR-451-5p, which contributes to OA progression (Meng S. et al., 2023). ADAMTS has been identified as a potential arthritis biomarker. Intriguingly, ADSCs-Exos can deliver miR-93-5p to target ADAM metallopeptidase with thrombospondin type 1 motif 9 (ADAMTS9), inhibiting the inflammatory response and decelerating cartilage damage progression (Li Y. et al., 2023).
Oxidative stress triggers inflammatory and matrix catabolic pathways in articular cartilage, contributing to cartilage damage (Chen G. et al., 2024). Additionally, members of the peroxiredoxin family exhibit protective effects against oxidative stress-induced cartilage injury. Studies have demonstrated that exosomes from adipose mesenchymal stem cells mitigate chondrocyte oxidative stress, alleviate endoplasmic reticulum stress, and boost the expression of the autophagy marker microtubule-associated protein 1 light chain 3 beta (MAP1LC3B), with peroxiredoxin 6 (Prdx6) and miR-486-5p playing pivotal roles (Guillén et al., 2021; Wang et al., 2022).
Synovial mesenchymal stem cells (SMSCs) exhibit multidirectional differentiation potential and have been utilized in experimental interventions in animal models of OA (Fan et al., 2009; To et al., 2019; Wang et al., 2020; Wang et al., 2021). However, the precise mechanism of action remains to be elucidated. extracellular vesicles derived from synovial mesenchymal stem cells (SMSCs-Exos) exhibiting elevated expression of miR-302c have the capability to target ADAM metallopeptidase domain 19 (ADAM19), facilitating chondrocyte proliferation, suppressing cartilage inflammation and ECM degeneration, thereby fostering the repair of osteoarthritic cartilage (Kong et al., 2023b). Bone morphogenetic protein (BMP), a crucial growth factor within the transforming growth factor β (TGF-β) superfamily, is intricately linked to the maintenance and repair of cartilage homeostasis. Notably, SMSCs-Exos exhibiting elevated levels of bone morphogenetic protein 7 (BMP-7) facilitated the polarization of macrophages towards the M2 phenotype, leading to inflammation reduction and improvement in pathological alterations within the cartilage (Sun et al., 2023). Neurofibrillar protein-1 (NRP-1) is a transmembrane protein expressed across various tissues and has been recently implicated in the regulation of bone metabolism. Additionally, matrilin-3 (MATN-3) serves as a pivotal matrix protein secreted by chondrocytes, constituting the third member of the ECM protein family. Studies demonstrated that SMSCs-Exos upregulated miR-485-3p to suppress NRP-1 expression. Furthermore, these vesicles delivered MATN-3 to concomitantly modulate the interleukin 17a (IL-17)-mediated activation of the PI3K/AKT/mTOR pathway, resulting in the inhibition of cartilage matrix degradation (Long et al., 2023; Qiu et al., 2024). Furthermore, studies have revealed that SMSCs-Exos are enriched in miR-140-5p and miR-212-5p, which target and suppress e74 like ets transcription factor 3 (ELF3) expression, resulting in the downregulation of inflammatory mediators (interleukin 6 [IL-6], c-c motif chemokine ligand 2 [MCP-1], tumor necrosis factor α [TNF-α], cyclooxygenase-2 [COX-2], and inducible nitric oxide synthase [iNOS]). Additionally, miR-140-5p enhances chondrocyte proliferation and migration capacity without compromising the integrity of the cartilage matrix, representing a promising avenue for potential cartilage repair applications (Tao et al., 2017; Zheng et al., 2022).
Human umbilical cord mesenchymal stem cells (HucMSCs) are recognized for their angiogenic and osteogenic properties (Wang et al., 2023; Yang et al., 2024). Within chondrocytes, the involvement of NADPH oxidase 4 (NOX4) in oxidative stress processes is pivotal. Studies have revealed a downregulation of miR-21-5p expression in cartilage tissues from patients with osteonecrosis of the femoral head. It was elucidated that SRY-box transcription factor 5 (SOX5) could enhance enhancer of zeste homolog 2 (EZH2) transcription, collectively counteracting the effects of miR-21-5p. Simultaneously, miR-21-5p demonstrated the ability to suppress both SOX5 and enhancer of zeste 2 polycomb repressive complex 2 subunit (EZH2) expression (Fang et al., 2022), thus fostering angiogenesis and bone repair. Moreover, the delivery of miR-100-5p and miR-23a-3p to chondrocytes targeted NOX4 and phosphatase and tensin homolog (PTEN), consequently promoting AKT expression to mitigate oxidative stress and apoptosis, thereby facilitating cartilage regeneration (Hu et al., 2020; Li X. et al., 2021). In the realm of cartilage restoration, umbilical cord MSCs and pluripotent stem cell-differentiated MSCs showcased superiority over adipose MSCs. Notably, pluripotent stem cell-differentiated MSCs exhibited greater efficacy than synovial MSCs, followed by bone marrow MSCs, in OA treatment (Zhu et al., 2017; Li Q. et al., 2021; Xu T. et al., 2024). Long non-coding RNAs (lncRNAs), acting as “molecular sponges,” can absorb specific miRNAs, thereby suppressing their target gene expression. For instance, lncRNA H19 within HucMSCs-Exos can absorb miR-29b-3p, elevate TGF-β1 and SMAD family member 3 (Smad3) levels, and inhibit forkhead box o3 (FoxO3) expression, thereby propelling cartilage repair and regeneration (Yan et al., 2021). As the exploration of various MSC types continues to expand, the potential of additional MSCs remains to be unraveled in future investigations.
Synovial fibroblasts have garnered increased attention owing to their close proximity to articular cartilage within synovial tissue, comprising macrophage-like synoviocytes and fibroblast-like synoviocytes (Damerau et al., 2024; Hu Z. et al., 2024). Exosomes derived from both cell types have been identified as contributors to the exacerbation of cartilage inflammatory responses through modulation of the TLRs/NF-κB pathway (Ma T. et al., 2024). Notably, the pronounced impact of macrophage-like synoviocytes and the ability of fibroblast-like synoviocyte exosomes to induce macrophage glycolysis activation, consequently fostering M1 cell polarization, but HIF 1α inhibited this process, highlight novel insights into the role of fibroblast-like synoviocyte-derived exosomes in inflammatory-induced cartilage damage (Liu B. et al., 2024; Wang H. et al., 2024). Distinct variations were observed in the impact of small molecule RNAs from synovial fibroblast exosomes on chondrocytes; for instance, miR-19b-3p and miR-106b were identified to exacerbate articular cartilage damage (Liu D. et al., 2020; Kong et al., 2023a), whereas miR-182-5p, miR-214-3p, miR-126-3P, and miR-142-5p exhibited potential in promoting articular cartilage repair (Zhou et al., 2021; Zeng et al., 2022; Ji et al., 2023; Lai et al., 2023). Furthermore, the enrichment of miR-106b in synovial fibroblast exosomes, targeting pyruvate dehydrogenase kinase 4 (PDK4) and modulating the RANKL/RANK/OPG pathway, underscored its role in advancing cartilage damage progression, thus identifying miR-106b as an actionable target (Liu D. et al., 2020). Moreover, the interaction between miR-106b and long non-coding RNA H19 in competition for miR-106b adsorption offers a promising avenue to reverse cartilage damage and facilitate cartilage repair (Tan et al., 2020). Iron death, characterized by redox homeostasis disruption, mitochondrial dysfunction, and iron-dependent metabolic processes, emerges as a distinctive mode of cell demise impacting cartilage inflammatory responses. Noteworthy findings indicate that miR-19b-3p, transported by fibroblast-like synoviocyte exosomes, inhibits solute carrier family seven member 11 (SLC7A11), thereby exacerbating chondrocyte iron death and cartilage damage (Kong et al., 2023a). Collectively, the adverse impact of synovial fibroblasts on cartilage repair pathways underscores the destructive consequences of inflammation on cartilage integrity, emphasizing the imperative focus on inhibiting inflammation for successful cartilage repair strategies.
Chondrocyte-derived exosomes exhibit similarity to chondrocytes, ensuring a more reliable safety profile (Chen J. et al., 2024). Regenerated cartilage from BMSCs-Exos displays a fibrous nature, in contrast to the hyaline cartilage formation facilitated by chondrocyte-derived exosomes, maintaining chondrocyte phenotype integrity over an extended period (Chen J. et al., 2024). However, exosomes derived from degenerative chondrocytes have the potential to hasten the process of cartilage calcification (Liu Q. et al., 2022). Notably, a study investigating exosomes from osteoarthritic chondrocytes revealed their ability to inhibit autophagy related 4B cysteine peptidase (ATG4B) expression via miR-449a-5p, impeding macrophage autophagy and leading to reactive oxygen species (ROS) accumulation. This suggests a possible exacerbation of OA progression by miR-449a-5p, underscoring the significance of chondrocyte physiological conditions in determining the efficacy of miR-449a-5p for cartilage repair (Ni et al., 2019).
Research has elucidated the mechanosensitivity of condylar cartilage, demonstrating its ability to interpret mechanical stimuli into biochemical signals (Zhao et al., 2017). Furthermore, examination of individual chondrocyte cells from distinct weight-bearing zones within the tibial plateau has unveiled differential gene expression patterns in response to varying mechanical loads, emphasizing the importance of studies on chondrocytes under differing stress conditions (Wang J. et al., 2024). Circular RNAs (circRNAs) have emerged as key players in OA development, with a specific focus on circ-PRKCH, which exhibits significant involvement in disease progression. Elevated levels of Circ-PRKCH and ADAM metallopeptidase with thrombospondin type 1 motif 5 (ADAMTS 5) were detected in cartilage from arthritis patients, concomitant with decreased miR-502-5p expression. Subsequent investigations unveiled that Circ-PRKCH sequesters miR-502-5p, modulating ADAMTS 5 expression to drive inflammatory processes in articular cartilage. Additionally, interleukin 1 beta (IL-1β)-treated chondrocytes were found capable of transporting circ-PRKCH via exosomes, hinting at its potential as a biomarker (Liu Z. et al., 2022). Conversely, another study reported upregulation of Circ_0001846 and WNT 5B in OA patients and IL-1β-treated chondrocytes, accompanied by decreased miR-149-5p levels. Subsequent analyses demonstrated that silencing of Circ_0001846 reversed IL-1β-induced proliferation, apoptosis, migration, inflammation, and extracellular matrix degradation in chondrocytes. Furthermore, miR-149-5p hindered these processes by targeting WNT 5B. These results suggest that Circ_0001846, originating from chondrocytes in OA patients, sequesters miR-149-5p, subsequently disrupting its involvement in the WNT 5B axis and compromising its chondroprotective effects, thereby contributing to cartilage damage (Zhu et al., 2021), offering a promising avenue for potential OA therapies.
Macrophages, pivotal cells in bone immunity, undergo differentiation into pro-inflammatory M1 macrophages and anti-inflammatory M2 macrophages (Hu K. et al., 2023), crucial for regulating bone metabolism and maintaining bone homeostasis. Research demonstrated that exosomes derived from M1 macrophages upregulated the expression of interleukin 6 (IL-6), interleukin-8 (IL-8), matrix metallopeptidase 1 (MMP1), matrix metallopeptidase 3 (MMP3), matrix metallopeptidase 9 (MMP9), and matrix metallopeptidase 13 (MMP13) in chondrocytes. Subsequent investigations indicated that this impact was intricately linked to the suppression of glycogen synthase kinase 3 beta (GSK3β) and axis inhibition protein 2 (Axin2) expression by miR-1246, ultimately activating the Wnt/β-catenin pathway. Thus, this elucidated the mechanism by which M1 macrophage exocytosis exacerbates cartilage damage (Zhang et al., 2024). Furthermore, M2 macrophages perform a critical anti-inflammatory function during the advancement of OA. Studies indicated that exocytosis from M2 macrophages downregulates the PI3K-Akt-mTOR pathway, leading to the suppression of IL-1β, IL-6, and TNF-α expression, ultimately promoting cartilage restoration (Da-Wa et al., 2021). Moreover, by sequencing M1 and M2 macrophage exosomes, miR-26b-5p and miR-127-3p were significantly upregulated in M2-Exos, while miR-134-5p was significantly downregulated. Validation of miR-26b-5p in vitro showed that it inhibited toll like receptor 3 (TLR3) expression, thereby preventing chondrocyte hypertrophy, promoting the differentiation of M1-type macrophages to M2-type macrophages, and suppressing inflammatory responses (Qian et al., 2024). Stimulating macrophage polarization from pro-inflammatory M1 towards anti-inflammatory M2 using exosomes could represent a promising therapeutic strategy for OA management. A flowchart depicting exosome origins and altered cartilage biology is shown in Figure 2.
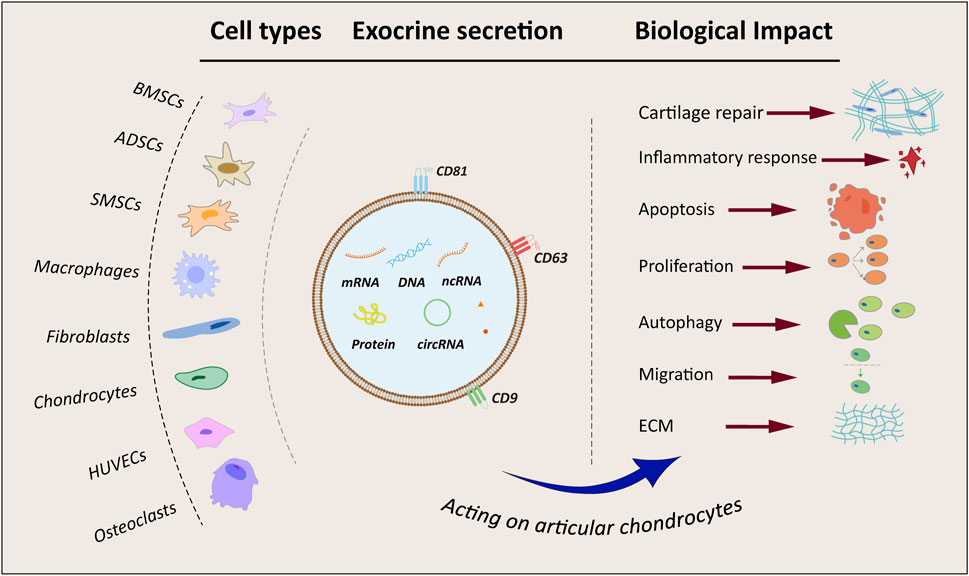
Figure 2. Flowchart and summary of exosomes from different sources. BMSCs, bone marrow mesenchymal stem cells; ADSCs, adipose-derived mesenchymal stem cells; SMSCs, synovial mesenchymal stem cells; HUVECs, human umbilical vein endothelial cells; ECM, extracellular matrix; mRNA, coding RNA; DNA, deoxyribonucleic acid; ncRNA, non-coding RNA; circRNA, circular RNA.
The secretion of extracellular vesicles (EVs) by plant cells has long been a subject of scrutiny owing to the presence of their robust cell walls. Nevertheless, advancements in research methodologies have increasingly unveiled compelling evidence supporting the capacity of plant cells to discharge plant-derived vesicles (PDVs) (Regente et al., 2009; Liu N. J. et al., 2020; Niu et al., 2023). These PDVs, characterized as intricate exosome-like structures with diverse and multifunctional traits, exhibit exceptional absorption capabilities in organisms (Garaeva et al., 2021). This property not only mitigates the longstanding challenge of limited bioavailability of active plant constituents but also opens avenues for the utilization and development of extracellular lipid nanoparticles (ELNs) as biotherapeutic agents and carriers for drug delivery (Cong et al., 2022; Zhao Y. et al., 2023). In recent times, there has been a surge in reports focusing on the use of herbal remedies for the management of bone and joint disorders. Contemporary studies have demonstrated that bioactive compounds extracted from medicinal herbs contain active ingredients that confer anti-inflammatory, anti-apoptotic, and anti-oxidative stress effects (Li and Zhang, 2020). The methodology for isolating vesicles rich in biologically potent substances from herbal remedies has reached a state of maturity, offering a novel perspective for the prospective application of herbal medications in addressing bone and joint ailments (Liu Y. et al., 2024). Notably, Morinda Officinalis encompasses a diverse range of anti-osteoporosis active constituents, and extracellular vesicles obtained from Morinda Officinalis exhibit the ability to stimulate osteoblast proliferation and differentiation via the mitogen-activated protein kinase (MAPK) pathway (Cao et al., 2024). Furthermore, extracellular vesicles sourced from Rhizoma Drynariae can modulate the ER-alpha (ERα) pathway to promote the differentiation of BMSCs into osteoblasts for the management of orthopaedic conditions (Zhao et al., 2024). Collectively, these investigations suggest that extracellular vesicles derived from herbs present a promising avenue for the effective treatment of OA.
Exosomes originate from a diverse array of sources, among which are exosomes derived from osteoclasts that likely play a pivotal role in modulating bone metabolism (Stegen and Carmeliet, 2024). Research has indicated that exosomes originating from osteoclasts have the capability to modulate the transforming growth factor beta 1 (TGF-β1)/SMAD family member 2 (Smad2) signaling pathway through miR-212-3p, thereby expediting the degradation of cartilage matrix. This highlights the potential benefit of suppressing miR-212-3p expression to facilitate cartilage repair, while also identifying the TGF-β1/Smad 2 axis as a plausible target for enhancing cartilage restoration (Dai et al., 2024). Furthermore, exosomes released by osteoclasts have been implicated in hastening the progression of OA. Conversely, the downregulation of RAB27A, member RAS oncogene family (Rab 27a), achieved through the inhibition of key miRNA processing enzymes or small interfering RNAs, results in diminished exosome secretion from osteoclasts, subsequently mitigating the advancement of OA (Liu et al., 2021).
Excessive free radicals in the joints trigger oxidative stress, posing a risk for the degradation of the cartilage matrix and the progression of OA (Da et al., 2024). Exosomes extracted from human umbilical vein endothelial cells (HUVEC-Exos) intriguingly stimulated the production of reactive oxygen species (ROS) in chondrocytes. Additionally, they suppressed autophagy and P21 expression, diminishing chondrocyte resilience to oxidative stress, consequently fostering chondrocyte apoptosis, which hampers the repair of OA cartilage (Yang et al., 2021). This observation suggests that the vasculature has an adverse impact on cartilage repair. Inhibiting exosome secretion from intra-articular vascular endothelial cells could potentially serve as a therapeutic approach. Due to its close proximity to the cartilage, the articular subchondral bone plays a pivotal role in the investigation of cartilage repair (Hu Y. J. et al., 2024). Studies have demonstrated a close association between exosomes originating from osteoblasts in the articular subchondral bone and cartilage degeneration. Subsequent investigations unveiled that this mechanism is linked to miR-210-5p, and inhibiting miR-210-5p substantially resulted in chondroprotective effects (Wu X. et al., 2021). Table 1 and Figure 3 depict a summary of the mechanisms by which exosomes from various cell types impact cartilage repair.
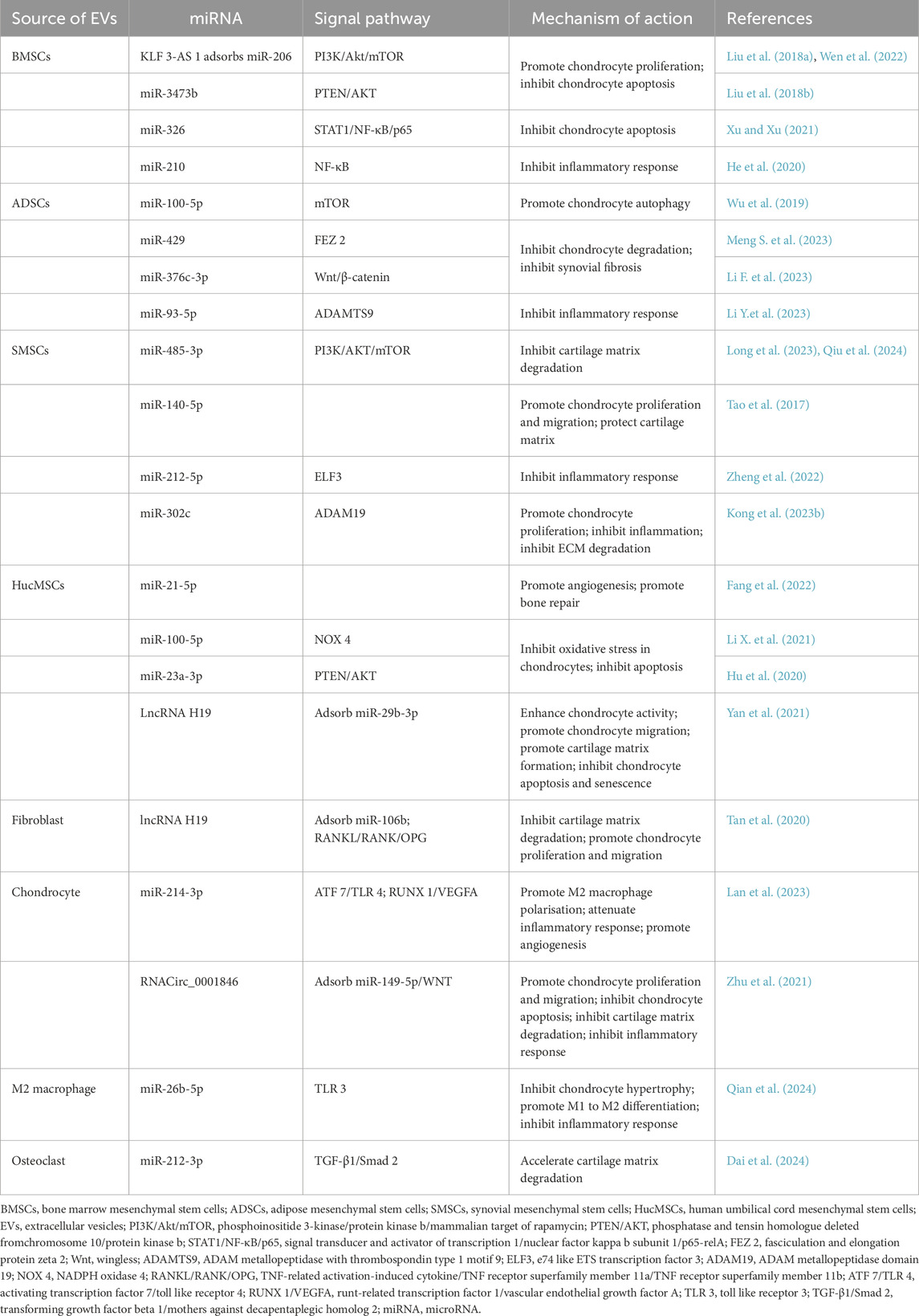
Table 1. Mechanism of different cell-derived exosomes in the cartilage repair process of osteoarthritis.
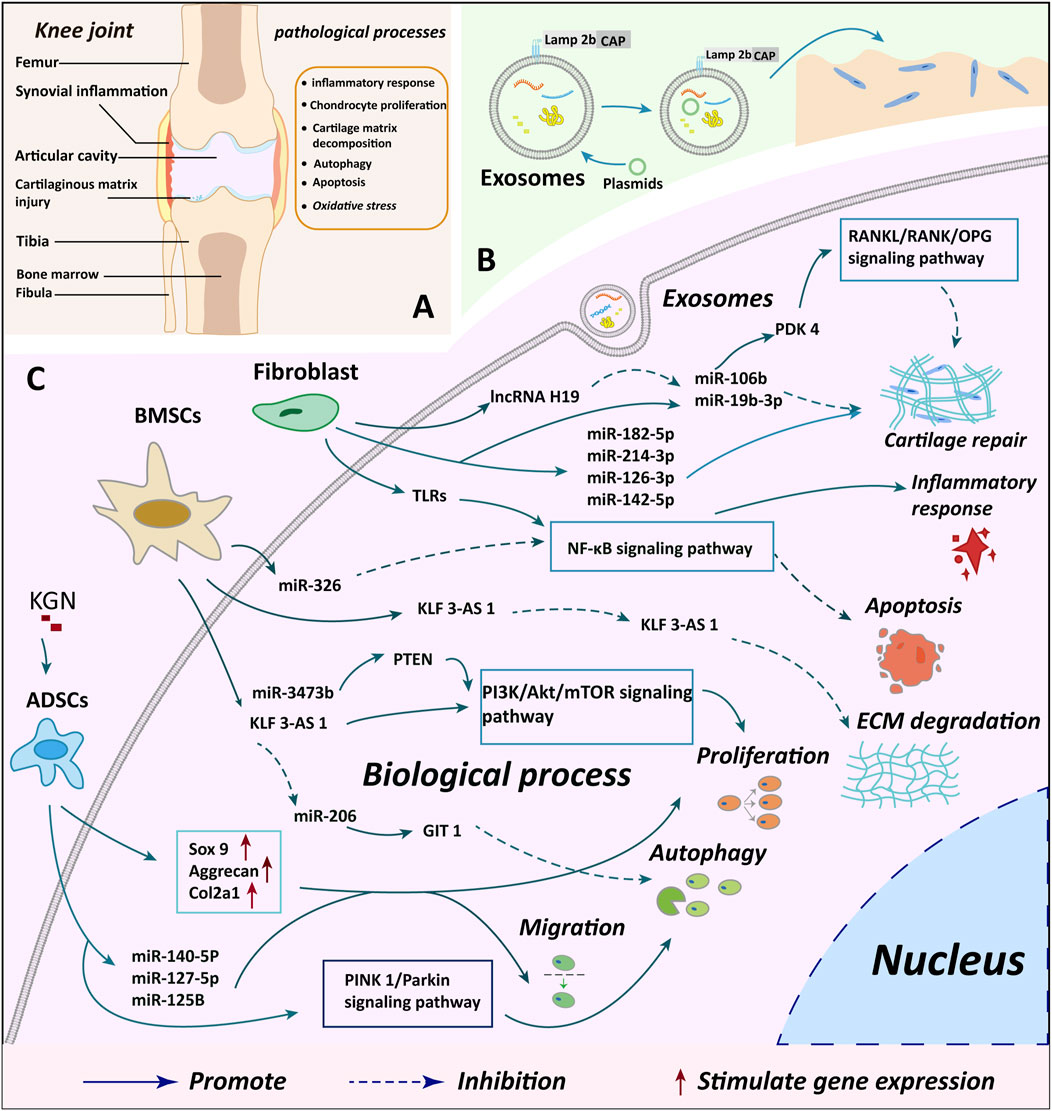
Figure 3. Summary of cartilage microenvironment regulation and cartilage repair mechanisms by different cellular exosomes. (A) A depicts the observed changes in injury and knee joint structure in knee OA cases, offering insights into the structural impacts of the condition (B) B illustrates the process of plasmid transfection of exosomes and genetic modification to enhance the targeting of exosomes, indicating potential avenues for novel treatment approaches (C) C delineates the specific molecular mechanisms through which exosomes from various cellular origins exert their biological effects on chondrocytes, providing a detailed understanding of the biological interactions involved in the disease. BMSCs, bone marrow mesenchymal stem cells; ADSCs, adipose mesenchymal stem cells; KGN, kartogenin; miR, microRNA.
Exosomes present a promising avenue for OA treatment (Xavier et al., 2023); however, they rely on specific matrix components to ensure enduring repair. Scaffold materials utilized in musculoskeletal disorders encompass organic elements (e.g., fibrin, hyaluronic acid, chitosan, collagen, alginate, and silk fibroin), inorganic compounds (e.g., hydroxyapatite, tricalcium phosphate, glass ceramics, and titanium), and biodegradable substances (e.g., polycaprolactone, polylactic acid [PLA], polyglycolic acid, and polylactic-co-glycolic acid [PLGA]) (Wu N. et al., 2021; Rahman et al., 2022; Zhang et al., 2023; Yuan et al., 2024). Furthermore, 3D cell culture systems have undergone comprehensive investigation in current studies (Lee and Lee, 2022). The 3D system’s capability to culture a higher number of cells concurrently for increased exosome production, in conjunction with the hypoxia-induced cell aggregation that stimulates cells to produce growth factors, has proven advantageous in fostering cartilage repair (Duan et al., 2022). In vitro studies demonstrated that 3D-Exos exhibited enhanced capacity in regulating the microenvironment of the joint cavity, with sequencing revealing its association with elevated miRNA expression; however, further investigation is required to elucidate the precise molecular mechanism (Yan et al., 2023).
To enhance the sustained release effect of exosomes, a study combined gelatin hydrogel with BMSCs-Exos, demonstrating its ability to maintain release for 14 days with an exosome release rate of 80%. This combination was shown to modulate immunity to support the repair of damaged cartilage, indicating a significant potential for the application of biomaterials and extracellular vesicles (Guan et al., 2022a). The advent of CRISPR technology in 2012 has offered promise for disease treatment. By incorporating chondrocyte affinity peptide (CAP) at the N-terminus of the exosome surface protein Lamp 2b to create exosomes targeted at chondrocytes, the efficiency of biosignalling within the exosome can be significantly enhanced, improving the exosome’s targeting capabilities (Liang et al., 2022). Lithium-containing scaffolds have been shown to effectively enhance cartilage regeneration. Treatment of BMSC-derived exosomes with lithium-containing blocking glass ceramics (Li-BGC) can employ miR-455-3p to inhibit histone deacetylase 2 (HDAC2) and enhance histone H3 acetylation, thereby promoting cartilage regrowth. This validates the critical role of lithium ions in promoting cartilage formation (Liu et al., 2023). To mimic the normal anatomical structure of cartilage, a double-layer gel was utilized to simulate cartilage and subchondral bone matrix components, combined with cartilage-derived exosomes. The results indicate an enhancement in chondrocyte migration ability, contributing to articular cartilage repair (Nikhil and Kumar, 2022). Chondroitin sulfate, a glycosaminoglycan secreted by chondrocytes, promotes cartilage regeneration and pain relief. By combining exosome carriers with pro-cartilage repair cytokines like chondroitin sulfate, not only is the release of exosomes promoted, but it also aids in cartilage matrix formation in a drug-delivery manner, providing valuable insights for the design and development of exosome carriers (Guan et al., 2022; Zhang et al., 2021). These findings suggest that incorporating cartilage repair-related cytokines into exosome-carrying hydrogels shows promising applications for cartilage repair.
Given that chondrocytes are the sole cell type found within cartilage, this review centers its attention on the impact of various cellular exosomes on chondrocytes. The influence of exosomes derived from different cellular origins on regulating the OA cartilage microenvironment and aiding in cartilage repair involves the modulation of multiple essential pathways and downstream genes. These effects significantly alter the biological functions of chondrocytes, particularly in terms of suppressing inflammation, remodeling the cartilage matrix, enhancing chondrocyte proliferation, regulating autophagy and apoptosis, promoting directed migration to injury sites, influencing mitochondrial metabolism, and reducing oxidative stress. Engineered exosomes have demonstrated enhanced precision in targeting and more pronounced interventional effects, representing a pivotal technology for advancing clinical investigations related to exosomes. However, there remains a scarcity of studies on the differences in the efficacy of exosomes in facilitating cartilage repair across various cell types. It is crucial to identify the optimal donor cells for exosomes and explore potential synergistic or antagonistic effects among exosomes released by diverse cell sources.
While the role of pertinent signaling molecules in exosomes for promoting cartilage repair is extensively demonstrated, existing studies have primarily focused on both cellular and animal models. Notably, there is a lack of clinical trials assessing the use of exosomes for OA treatment. Treatment with MSC-derived exosomes (MSC-Exos) has demonstrated significant preclinical efficacy in animal models of OA (Zhou et al., 2022). Although clinical reports on the use of MSC-Exos in osteoarthritis patients are limited, cases involving graft-versus-host disease (Kordelas et al., 2014) and chronic kidney disease (Nassar et al., 2016) indicate good tolerability following systemic administration of exosomes. This suggests that clinical studies on exosomes are still in the exploratory phase but hold positive implications for future research. Developing highly standardized manufacturing protocols for the production of clinical-grade exosome products with essential bioactivities presents a significant challenge for clinical research groups. Figueroa-Valdés et al. (2025) pioneered the standardized development and intra-articular validation of clinical-grade extracellular vesicles derived from human umbilical cord mesenchymal stem cells (HucMSCs). Consistency in exosome production was achieved through the establishment of a standardized production protocol. The potential for standardized production of exosomes for clinical translation can be further validated by identifying intra-exosomal miRNA and protein profiles. The efficacy and safety of HucMSCs-Exos were further validated through in vitro cell and animal studies. Finally, twelve patients with moderate knee OA received a single dose of HucMSCs-Exos via intra-articular injection and were followed for 12 months. The results indicated no adverse effects and demonstrated significant, long-lasting improvements in pain and dysfunction throughout the 12-month follow-up period. Imaging studies revealed that the administration of HucMSCs-Exos via intra-articular injection in OA patients did not result in structural joint destruction nor promote the progression of cartilage damage. This clinical study validates, for the first time, the initial safety of exosome therapy in knee osteoarthritis (KOA) and has significant implications for future clinical studies involving exosomes.
Consequently, exploring the efficacy and safety of exosomes holds critical clinical importance. However, before their practical application, it is imperative to address potential safety risks, ensure exosome purity, and optimize their yield. Therefore, further investigation into suitable scaffolds, safer and more effective bioactive factors, utilization of plant and animal sources, and exploration of genetic modification techniques is essential to establish more favorable conditions for exosomes, validating their safety and efficacy in human contexts. Despite the challenges, the ongoing research progress signifies the increasingly vital role of exosomes in OA prevention and treatment, with exosome therapy poised to emerge as a key option for early OA treatment and prevention.
HL: Writing–original draft. HW: Methodology, Supervision, Writing–review and editing. FW: Methodology, Supervision, Writing–review and editing. PP: Methodology, Supervision, Writing–review and editing. LS: Methodology, Supervision, Writing–review and editing. LK: Methodology, Supervision, Writing–review and editing. HM: Methodology, Project administration, Writing–review and editing. YF: Methodology, Project administration, Writing–review and editing. HW: Methodology, Project administration, Resources, Writing–review and editing. WQ: Funding acquisition, Methodology, Project administration, Resources, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by “2023 Cultivation Project of “Double First-class” and High-level University Discipline Reserve Talents of Guangzhou University of Chinese Medicine, Project Leader: WQ; 2022 Guangdong Institute of Traditional Chinese Medicine and Orthopaedic Traumatology Open Fund Project Youth Project (GYH202201-03), Constructing an efficacy prediction model for the treatment of postmenopausal OA of the knee with the method of tonifying the kidney and activating the blood based on machine learning, Project Leader: He Xiaoming; 2022 Guangzhou Science and Technology Plan Project Key R&D Programme (202206010048), Research on key technologies for the prevention, diagnosis and treatment of bone and joint degenerative diseases (OA of the knee), Co-responsor: WQ; 2022 The Third Affiliated Hospital of Guangzhou University of Traditional Chinese Medicine “Gazelle Plan” key projects, Chinese medicine diagnosis and treatment of bone and joint disease high-level speciality capacity building project.”
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adam, M. S., Zhuang, H., Ren, X., Zhang, Y., and Zhou, P. (2024). The metabolic characteristics and changes of chondrocytes in vivo and in vitro in osteoarthritis. Front. Endocrinol. (Lausanne) 15, 1393550. doi:10.3389/fendo.2024.1393550
Alahdal, M., Huang, R., Duan, L., Zhiqin, D., Hongwei, O., Li, W., et al. (2021). Indoleamine 2, 3 dioxygenase 1 impairs chondrogenic differentiation of mesenchymal stem cells in the joint of osteoarthritis mice model. Front. Immunol. 12, 781185. doi:10.3389/fimmu.2021.781185
Alcaraz, M. J., Compañ, A., and Guillén, M. I. (2019). Extracellular vesicles from mesenchymal stem cells as novel treatments for musculoskeletal diseases. Cells 9 (1), 98. doi:10.3390/cells9010098
Andaloussi, E. L. S., Mager, I., Breakefield, X. O., and Wood, M. J. (2013). Extracellular vesicles: biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 12 (5), 347–357. doi:10.1038/nrd3978
Aziz, A., Ganesan, N. K., Kamarul, T., Mobasheri, A., and Sharifi, A. (2024). The interplay between dysregulated metabolites and signaling pathway alterations involved in osteoarthritis: a systematic review. Ther. Adv. Musculoskelet. Dis. 16, 1759720X241299535. doi:10.1177/1759720X241299535
Bannuru, R. R., Osani, M. C., Vaysbrot, E. E., Arden, N. K., Bennell, K., Bierma-Zeinstra, S., et al. (2019). Oarsi guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 27 (11), 1578–1589. doi:10.1016/j.joca.2019.06.011
Cao, Y., Tan, X., Shen, J., Liu, F., Xu, Y., Chen, Y., et al. (2024). Morinda officinalis-derived extracellular vesicle-like particles: anti-osteoporosis effect by regulating mapk signaling pathway. Phytomedicine 129, 155628. doi:10.1016/j.phymed.2024.155628
Caramés, B., Hasegawa, A., Taniguchi, N., Miyaki, S., Blanco, F. J., and Lotz, M. (2012). Autophagy activation by rapamycin reduces severity of experimental osteoarthritis. Ann. Rheum. Dis. 71 (4), 575–581. doi:10.1136/annrheumdis-2011-200557
Chen, C., Qu, Z., Yin, X., Shang, C., Ao, Q., Gu, Y., et al. (2016). Efficacy of umbilical cord-derived mesenchymal stem cell-based therapy for osteonecrosis of the femoral head: a three-year follow-up study. Mol. Med. Rep. 14 (5), 4209–4215. doi:10.3892/mmr.2016.5745
Chen, C. Y., Rao, S. S., Yue, T., Tan, Y. J., Yin, H., Chen, L. J., et al. (2022). Glucocorticoid-induced loss of beneficial gut bacterial extracellular vesicles is associated with the pathogenesis of osteonecrosis. Sci. Adv. 8 (15), eabg8335. doi:10.1126/sciadv.abg8335
Chen, G., Cui, L., Luo, P., Fang, J., Xie, X., and Jiang, C. (2024). A reactive oxygen species “sweeper” based on hollow mesopore cerium oxide nanospheres for targeted and anti-inflammatory management of osteoarthritis. ACS Appl. Mater Interfaces 16, 34705–34719. doi:10.1021/acsami.4c06231
Chen, J., Ni, X., Yang, J., Yang, H., Liu, X., Chen, M., et al. (2024). Cartilage stem/progenitor cells-derived exosomes facilitate knee cartilage repair in a subacute osteoarthritis rat model. J. Cell. Mol. Med. 28 (8), e18327. doi:10.1111/jcmm.18327
Chen, J., Yu, X., and Zhang, X. (2022). Advances on biological functions of exosomal non-coding rnas in osteoarthritis. Cell biochem. Funct. 40 (1), 49–59. doi:10.1002/cbf.3679
Chen, L., Zhang, Z., and Liu, X. (2024). Role and mechanism of mechanical load in the homeostasis of the subchondral bone in knee osteoarthritis: a comprehensive review. J. Inflamm. Res. 17, 9359–9378. doi:10.2147/JIR.S492415
Chen, Y. P., Chen, W. C., Wang, K. C., and Chen, C. H. (2014). Effectiveness of synovial fluid mesenchymal stem cells embedded in alginate beads for treatment of steroid-induced avascular necrosis of the femoral head. J. Orthop. Sci. 19 (4), 657–666. doi:10.1007/s00776-014-0568-5
Cho, Y., Jeong, S., Kim, H., Kang, D., Lee, J., Kang, S. B., et al. (2021). Disease-modifying therapeutic strategies in osteoarthritis: current status and future directions. Exp. Mol. Med. 53 (11), 1689–1696. doi:10.1038/s12276-021-00710-y
Church, V., Nohno, T., Linker, C., Marcelle, C., and Francis-West, P. (2002). Wnt regulation of chondrocyte differentiation. J. Cell Sci. 115 (Pt 24), 4809–4818. doi:10.1242/jcs.00152
Conaghan, P. G., Dickson, J., and Grant, R. L.Guideline Development Group (2008). Care and management of osteoarthritis in adults: summary of nice guidance. BMJ 336 (7642), 502–503. doi:10.1136/bmj.39490.608009.AD
Cong, L., Zhu, Y., and Tu, G. (2017). A bioinformatic analysis of micrornas role in osteoarthritis. Osteoarthr. Cartil. 25 (8), 1362–1371. doi:10.1016/j.joca.2017.03.012
Cong, M., Tan, S., Li, S., Gao, L., Huang, L., Zhang, H. G., et al. (2022). Technology insight: plant-derived vesicles-how far from the clinical biotherapeutics and therapeutic drug carriers? Adv. Drug Deliv. Rev. 182, 114108. doi:10.1016/j.addr.2021.114108
Copp, G., Robb, K. P., and Viswanathan, S. (2023). Culture-expanded mesenchymal stromal cell therapy: does it work in knee osteoarthritis? A pathway to clinical success. Cell. Mol. Immunol. 20 (6), 626–650. doi:10.1038/s41423-023-01020-1
Cravero, J. D., Carlson, C. S., Im, H. J., Yammani, R. R., Long, D., and Loeser, R. F. (2009). Increased expression of the akt/pkb inhibitor trb3 in osteoarthritic chondrocytes inhibits insulin-like growth factor 1-mediated cell survival and proteoglycan synthesis. Arthritis Rheum. 60 (2), 492–500. doi:10.1002/art.24225
Da, W., Chen, Q., and Shen, B. (2024). The current insights of mitochondrial hormesis in the occurrence and treatment of bone and cartilage degeneration. Biol. Res. 57 (1), 37. doi:10.1186/s40659-024-00494-1
Dai, J., Hu, Z., Zeng, F., Gong, X., Tang, H., Deng, J., et al. (2024). Osteoclast-derived exosomal miR-212-3p suppressed the anabolism and accelerated the catabolism of chondrocytes in osteoarthritis by targeting TGF-β1/Smad2 signaling. Arch. Biochem. Biophys. 751, 109827. doi:10.1016/j.abb.2023.109827
Damerau, A., Rosenow, E., Alkhoury, D., Buttgereit, F., and Gaber, T. (2024). Fibrotic pathways and fibroblast-like synoviocyte phenotypes in osteoarthritis. Front. Immunol. 15, 1385006. doi:10.3389/fimmu.2024.1385006
Dao, D. Y., Jonason, J. H., Zhang, Y., Hsu, W., Chen, D., Hilton, M. J., et al. (2012). Cartilage-specific β-catenin signaling regulates chondrocyte maturation, generation of ossification centers, and perichondrial bone formation during skeletal development. J. Bone Min. Res. 27 (8), 1680–1694. doi:10.1002/jbmr.1639
Da-Wa, Z. X., Jun, M., Chao-Zheng, L., Sen-Lin, Y., Chuan, L., De-Chun, L., et al. (2021). Exosomes derived from m2 macrophages exert a therapeutic effect via inhibition of the pi3k/akt/mtor pathway in rats with knee osteoarthritic. Biomed. Res. Int. 2021, 7218067. doi:10.1155/2021/7218067
De Santis, M. C., Gulluni, F., Campa, C. C., Martini, M., and Hirsch, E. (2019). Targeting pi3k signaling in cancer: challenges and advances. Biochim. Biophys. Acta Rev. Cancer 1871 (2), 361–366. doi:10.1016/j.bbcan.2019.03.003
Dong, J., Li, L., Fang, X., and Zang, M. (2021). Exosome-encapsulated microRNA-127-3p released from bone marrow-derived mesenchymal stem cells alleviates osteoarthritis through regulating CDH11-mediated wnt/β-catenin pathway. J. Pain Res. 14, 297–310. doi:10.2147/JPR.S291472
Doyle, L., and Wang, M. (2019). Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells 8 (7), 727. doi:10.3390/cells8070727
Duan, L., Li, X., Xu, X., Xu, L., Wang, D., Ouyang, K., et al. (2022). Large-scale preparation of synovial fluid mesenchymal stem cell-derived exosomes by 3d bioreactor culture. J. Vis. Exp. 185. doi:10.3791/62221
Engelman, J. A., Luo, J., and Cantley, L. C. (2006). The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 7 (8), 606–619. doi:10.1038/nrg1879
Fan, J., Varshney, R. R., Ren, L., Cai, D., and Wang, D. A. (2009). Synovium-derived mesenchymal stem cells: a new cell source for musculoskeletal regeneration. Tissue Eng. Part B Rev. 15 (1), 75–86. doi:10.1089/ten.teb.2008.0586
Fan, X., Zhang, Y., Liu, W., Shao, M., Gong, Y., Wang, T., et al. (2024). A comprehensive review of engineered exosomes from the preparation strategy to therapeutic applications. Biomater. Sci. 12, 3500–3521. doi:10.1039/d4bm00558a
Fan, Y., Li, Z., and He, Y. (2022). Exosomes in the pathogenesis, progression, and treatment of osteoarthritis. Bioengineering 9 (3), 99. doi:10.3390/bioengineering9030099
Fang, S., Liu, Z., Wu, S., Chen, X., You, M., Li, Y., et al. (2022). Pro-angiognetic and pro-osteogenic effects of human umbilical cord mesenchymal stem cell-derived exosomal mir-21-5p in osteonecrosis of the femoral head. Cell death Discov. 8 (1), 226. doi:10.1038/s41420-022-00971-0
Feng, J., Zhang, Q., Pu, F., Zhu, Z., Lu, K., Lu, W. W., et al. (2024). Signalling interaction between β-catenin and other signalling molecules during osteoarthritis development. Cell Prolif. 57 (6), e13600. doi:10.1111/cpr.13600
Figueroa-Valdés, A. I., Luz-Crawford, P., Herrera-Luna, Y., Georges-Calderón, N., García, C., Tobar, H. E., et al. (2025). Clinical-grade extracellular vesicles derived from umbilical cord mesenchymal stromal cells: preclinical development and first-in-human intra-articular validation as therapeutics for knee osteoarthritis. J. Nanobiotechnol. 23 (1), 13. doi:10.1186/s12951-024-03088-x
Fu, Q., Tang, N. N., Zhang, Q., Liu, Y., Peng, J. C., Fang, N., et al. (2016). Preclinical study of cell therapy for osteonecrosis of the femoral head with allogenic peripheral blood-derived mesenchymal stem cells. Yonsei Med. J. 57 (4), 1006–1015. doi:10.3349/ymj.2016.57.4.1006
Gámez-Valero, A., Monguió-Tortajada, M., Carreras-Planella, L., Franquesa, M., Beyer, K., and Borràs, F. E. (2016). Size-exclusion chromatography-based isolation minimally alters extracellular vesicles’ characteristics compared to precipitating agents. Sci. Rep. 6, 33641. doi:10.1038/srep33641
Ganley, I. G., Lam, D. H., Wang, J., Ding, X., Chen, S., and Jiang, X. (2009). Ulk1.atg13.fip200 complex mediates mtor signaling and is essential for autophagy. J. Biol. Chem. 284 (18), 12297–12305. doi:10.1074/jbc.M900573200
Garaeva, L., Kamyshinsky, R., Kil, Y., Varfolomeeva, E., Verlov, N., Komarova, E., et al. (2021). Delivery of functional exogenous proteins by plant-derived vesicles to human cells in vitro. Sci. Rep. 11 (1), 6489. doi:10.1038/s41598-021-85833-y
Gheinani, A. H., Vögeli, M., Baumgartner, U., Vassella, E., Draeger, A., Burkhard, F. C., et al. (2018). Improved isolation strategies to increase the yield and purity of human urinary exosomes for biomarker discovery. Sci. Rep. 8 (1), 3945. doi:10.1038/s41598-018-22142-x
Guan, P., Cui, R., Wang, Q., and Sun, Y. (2022a). A 3d hydrogel loaded with exosomes derived from bone marrow stem cells promotes cartilage repair in rats by modulating immunological microenvironment. Nan Fang. Yi Ke Da Xue Xue Bao 42 (4), 528–537. doi:10.12122/j.issn.1673-4254.2022.04.08
Guan, P., Liu, C., Xie, D., Mao, S., Ji, Y., Lin, Y., et al. (2022b). Exosome-loaded extracellular matrix-mimic hydrogel with anti-inflammatory property facilitates/promotes growth plate injury repair. Bioact. Mater 10, 145–158. doi:10.1016/j.bioactmat.2021.09.010
Guillén, M. I., Tofiño-Vian, M., Silvestre, A., Castejón, M. A., and Alcaraz, M. J. (2021). Role of peroxiredoxin 6 in the chondroprotective effects of microvesicles from human adipose tissue-derived mesenchymal stem cells. J. Orthop. Transl. 30, 61–69. doi:10.1016/j.jot.2021.08.003
Hart, D. A. (2022). Osteoarthritis as an umbrella term for different subsets of humans undergoing joint degeneration: the need to address the differences to develop effective conservative treatments and prevention strategies. Int. J. Mol. Sci. 23 (23), 15365. doi:10.3390/ijms232315365
Hartmann, C., and Tabin, C. J. (2000). Dual roles of wnt signaling during chondrogenesis in the chicken limb. Development 127 (14), 3141–3159. doi:10.1242/dev.127.14.3141
Hartmann, C., and Tabin, C. J. (2001). Wnt-14 plays a pivotal role in inducing synovial joint formation in the developing appendicular skeleton. Cell 104 (3), 341–351. doi:10.1016/s0092-8674(01)00222-7
He, L., Chen, Y., Ke, Z., Pang, M., Yang, B., Feng, F., et al. (2020). Exosomes derived from mirna-210 overexpressing bone marrow mesenchymal stem cells protect lipopolysaccharide induced chondrocytes injury via the nf-κb pathway. Gene 751, 144764. doi:10.1016/j.gene.2020.144764
Hu, H., Dong, L., Bu, Z., Shen, Y., Luo, J., Zhang, H., et al. (2020). Mir-23a-3p-abundant small extracellular vesicles released from gelma/nanoclay hydrogel for cartilage regeneration. J. Extracell. Vesicles 9 (1), 1778883. doi:10.1080/20013078.2020.1778883
Hu, K., Shang, Z., Yang, X., Zhang, Y., and Cao, L. (2023). Macrophage polarization and the regulation of bone immunity in bone homeostasis. J. Inflamm. Res. 16, 3563–3580. doi:10.2147/JIR.S423819
Hu, Y., Liu, H. X., Xu, D., Xue, X., and Xu, X. (2023). The anti-inflammatory effect of mir-140-3p in bmscs-exosomes on osteoarthritis. Acta Chir. Orthop. Traumatol. Cech 90 (4), 267–276. doi:10.55095/achot2023/032
Hu, Y. J., Yu, Y. E., Cooper, H. J., Shah, R. P., Geller, J. A., Lu, X. L., et al. (2024). Mechanical and structural properties of articular cartilage and subchondral bone in human osteoarthritic knees. J. Bone Min. Res. 39, 1120–1131. doi:10.1093/jbmr/zjae094
Hu, Z., Chen, L., Zhao, J., Zhang, W., Jin, Z., Sun, Y., et al. (2024). Lipoxin A4 ameliorates knee osteoarthritis progression in rats by antagonizing ferroptosis through activation of the esr2/lpar3/nrf2 axis in synovial fibroblast-like synoviocytes. Redox Biol. 73, 103143. doi:10.1016/j.redox.2024.103143
Huang, J., Chen, C., Liang, C., Luo, P., Xia, G., Zhang, L., et al. (2020). Dysregulation of the wnt signaling pathway and synovial stem cell dysfunction in osteoarthritis development. Stem Cells Dev. 29 (7), 401–413. doi:10.1089/scd.2019.0260
Jérémy, B., Marie, M., Giuliana, B. M., Karine, T., Christian, J., and Danièle, N. (2024). Extracellular vesicles from senescent mesenchymal stromal cells are defective and cannot prevent osteoarthritis. J. Nanobiotechnology 22 (1), 255. doi:10.1186/s12951-024-02509-1
Ji, Y., Xiong, L., Zhang, G., Xu, M., Qiu, W., Xiu, C., et al. (2023). Synovial fluid exosome-derived mir-182-5p alleviates osteoarthritis by downregulating tnfaip8 and promoting autophagy through lc3 signaling. Int. Immunopharmacol. 125 (Pt A), 111177. doi:10.1016/j.intimp.2023.111177
Jiawei, S., Zhi, C., Kewei, T., and Xiaoping, L. (2022). Magnetic bead-based adsorption strategy for exosome isolation. Front. Bioeng. Biotechnol. 10, 942077. doi:10.3389/fbioe.2022.942077
José, A. M. (2024). Control of articular degeneration by extracellular vesicles from stem/stromal cells as a potential strategy for the treatment of osteoarthritis. Biochem. Pharmacol. 228, 116226. doi:10.1016/j.bcp.2024.116226
Katz, J. N., Arant, K. R., and Loeser, R. F. (2021). Diagnosis and treatment of hip and knee osteoarthritis: a review. JAMA 325 (6), 568–578. doi:10.1001/jama.2020.22171
Kolasinski, S. L., Neogi, T., Hochberg, M. C., Oatis, C., Guyatt, G., Block, J., et al. (2020). 2019 american college of rheumatology/arthritis foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol. 72 (2), 220–233. doi:10.1002/art.41142
Kong, R., Ji, L., Pang, Y., Zhao, D., and Gao, J. (2023a). Exosomes from osteoarthritic fibroblast-like synoviocytes promote cartilage ferroptosis and damage via delivering microrna-19b-3p to target slc7a11 in osteoarthritis. Front. Immunol. 14, 1181156. doi:10.3389/fimmu.2023.1181156
Kong, R., Zhang, J., Ji, L., Yu, Y., Gao, J., and Zhao, D. (2023b). Synovial mesenchymal stem cell-derived exosomal microrna-320c facilitates cartilage damage repair by targeting adam19-dependent wnt signalling in osteoarthritis rats. Inflammopharmacology 31 (2), 915–926. doi:10.1007/s10787-023-01142-y
Kordelas, L., Rebmann, V., Ludwig, A. K., Radtke, S., Ruesing, J., Doeppner, T. R., et al. (2014). Msc-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia 28 (4), 970–973. doi:10.1038/leu.2014.41
Lai, C., Liao, B., Peng, S., Fang, P., Bao, N., and Zhang, L. (2023). Synovial fibroblast-mir-214-3p-derived exosomes inhibit inflammation and degeneration of cartilage tissues of osteoarthritis rats. Mol. Cell. Biochem. 478 (3), 637–649. doi:10.1007/s11010-022-04535-9
Lan, X., Yu, R., Xu, J., and Jiang, X. (2023). Exosomes from chondrocytes overexpressing mir-214-3p facilitate m2 macrophage polarization and angiogenesis to relieve legg calvé-perthes disease. Cytokine 168, 156233. doi:10.1016/j.cyto.2023.156233
Laplante, M., and Sabatini, D. M. (2012). Mtor signaling in growth control and disease. Cell 149 (2), 274–293. doi:10.1016/j.cell.2012.03.017
Lee, S. Y., and Lee, J. W. (2022). 3d spheroid cultures of stem cells and exosome applications for cartilage repair. Life (Basel) 12 (7), 939. doi:10.3390/life12070939
Lee, Y. T., Mohd, Y. M., Yazid, M. D., and Ugusman, A. (2024). Unraveling the path to osteoarthritis management: targeting chondrocyte apoptosis for therapeutic intervention. Front. Cell Dev. Biol. 12, 1347126. doi:10.3389/fcell.2024.1347126
Li, F., Xu, Z., Xie, Z., Sun, X., Li, C., Chen, Y., et al. (2023). Adipose mesenchymal stem cells-derived exosomes alleviate osteoarthritis by transporting microrna -376c-3p and targeting the wnt-beta-catenin signaling axis. Apoptosis 28 (3-4), 362–378. doi:10.1007/s10495-022-01787-0
Li, L. L., Li, X. F., Zhang, J. Y., Zhou, Y. W., and Yang, Q. N. (2021). Repair of osteoarthritis in animal model with exosomes derived from bmscs transfected by the sirna -piezo1 through ct navigation. Zhongguo Gu Shang 34 (12), 1171–1178. doi:10.12200/j.issn.1003-0034.2021.12.015
Li, Q., Yu, H., Sun, M., Yang, P., Hu, X., Ao, Y., et al. (2021). The tissue origin effect of extracellular vesicles on cartilage and bone regeneration. Acta Biomater. 125, 253–266. doi:10.1016/j.actbio.2021.02.039
Li, S., Zheng, W., Deng, W., Li, Z., Yang, J., Zhang, H., et al. (2024). Logic-based strategy for spatiotemporal release of dual extracellular vesicles in osteoarthritis treatment. Adv. Sci. (Weinh) 11, e2403227. doi:10.1002/advs.202403227
Li, X., Si, Y., Liang, J., Li, M., Wang, Z., Qin, Y., et al. (2024). Enhancing bone regeneration and immunomodulation via gelatin methacryloyl hydrogel-encapsulated exosomes from osteogenic pre-differentiated mesenchymal stem cells. J. Colloid Interface Sci. 672, 179–199. doi:10.1016/j.jcis.2024.05.209
Li, X., Wang, Y., Cai, Z., Zhou, Q., Li, L., and Fu, P. (2021). Exosomes from human umbilical cord mesenchymal stem cells inhibit ros production and cell apoptosis in human articular chondrocytes via the mir-100-5p/nox4 axis. Cell Biol. Int. 45 (10), 2096–2106. doi:10.1002/cbin.11657
Li, X. Z., and Zhang, S. N. (2020). Recent advance in treatment of osteoarthritis by bioactive components from herbal medicine. Chin. Med. 15, 80. doi:10.1186/s13020-020-00363-5
Li, Y., Duan, J., Lin, W., and Liu, J. (2023). Exosomal mir-93-5p regulated the progression of osteoarthritis by targeting adamts9. Open Med. (Wars) 18 (1), 20230668. doi:10.1515/med-2023-0668
Liang, Y., Xu, X., Xu, L., Iqbal, Z., Ouyang, K., Zhang, H., et al. (2022). Chondrocyte-specific genomic editing enabled by hybrid exosomes for osteoarthritis treatment. Theranostics 12 (11), 4866–4878. doi:10.7150/thno.69368
Liao, H. J., Chang, C. H., Huang, C. F., and Chen, H. T. (2022). Potential of using infrapatellar-fat-pad-derived mesenchymal stem cells for therapy in degenerative arthritis: chondrogenesis, exosomes, and transcription regulation. Biomolecules 12 (3), 386. doi:10.3390/biom12030386
Liao, Q., Li, B. J., Li, Y., Xiao, Y., Zeng, H., Liu, J. M., et al. (2021). Low-intensity pulsed ultrasound promotes osteoarthritic cartilage regeneration by bmsc-derived exosomes via modulating the nf-κb signaling pathway. Int. Immunopharmacol. 97, 107824. doi:10.1016/j.intimp.2021.107824
Lin, Z., Rodriguez, N. E., Zhao, J., Ramey, A. N., Hyzy, S. L., Boyan, B. D., et al. (2016). Selective enrichment of micrornas in extracellular matrix vesicles produced by growth plate chondrocytes. Bone 88, 47–55. doi:10.1016/j.bone.2016.03.018
Liu, B., Xian, Y., Chen, X., Shi, Y., Dong, J., Yang, L., et al. (2024). Inflammatory fibroblast-like synoviocyte-derived exosomes aggravate osteoarthritis via enhancing macrophage glycolysis. Adv. Sci. (Weinh) 11, e2307338. doi:10.1002/advs.202307338
Liu, C., Li, Y., Yang, Z., Zhou, Z., Lou, Z., and Zhang, Q. (2020). Kartogenin enhances the therapeutic effect of bone marrow mesenchymal stem cells derived exosomes in cartilage repair. Nanomedicine (Lond). 15 (3), 273–288. doi:10.2217/nnm-2019-0208
Liu, D., Fang, Y., Rao, Y., Tan, W., Zhou, W., Wu, X., et al. (2020). Synovial fibroblast-derived exosomal microrna-106b suppresses chondrocyte proliferation and migration in rheumatoid arthritis via down-regulation of pdk4. J. Mol. Med. Berl. 98 (3), 409–423. doi:10.1007/s00109-020-01882-2
Liu, J., Wu, X., Lu, J., Huang, G., Dang, L., Zhang, H., et al. (2021). Exosomal transfer of osteoclast-derived mirnas to chondrocytes contributes to osteoarthritis progression. Nat. Aging 1 (4), 368–384. doi:10.1038/s43587-021-00050-6
Liu, L., Yu, F., Chen, L., Xia, L., Wu, C., and Fang, B. (2023). Lithium-containing biomaterials stimulate cartilage repair through bone marrow stromal cells-derived exosomal mir-455-3p and histone h3 acetylation. Adv. Healthc. Mater. 12 (11), e2202390. doi:10.1002/adhm.202202390
Liu, N. J., Wang, N., Bao, J. J., Zhu, H. X., Wang, L. J., and Chen, X. Y. (2020). Lipidomic analysis reveals the importance of gipcs in arabidopsis leaf extracellular vesicles. Mol. Plant. 13 (10), 1523–1532. doi:10.1016/j.molp.2020.07.016
Liu, Q., Wang, R., Hou, S., He, F., Ma, Y., Ye, T., et al. (2022). Chondrocyte-derived exosomes promote cartilage calcification in temporomandibular joint osteoarthritis. Arthritis Res. Ther. 24 (1), 44. doi:10.1186/s13075-022-02738-5
Liu, Y., Lin, L., Zou, R., Wen, C., Wang, Z., and Lin, F. (2018a). Msc-derived exosomes promote proliferation and inhibit apoptosis of chondrocytes via lncrna-klf3-as1/mir-206/git1 axis in osteoarthritis. Cell Cycle 17 (21-22), 2411–2422. doi:10.1080/15384101.2018.1526603
Liu, Y., Xiao, S., Wang, D., Qin, C., Wei, H., and Li, D. (2024). A review on separation and application of plant-derived exosome-like nanoparticles. J. Sep. Sci. 47 (8), e2300669. doi:10.1002/jssc.202300669
Liu, Y., Zou, R., Wang, Z., Wen, C., Zhang, F., and Lin, F. (2018b). Exosomal klf3-as1 from hmscs promoted cartilage repair and chondrocyte proliferation in osteoarthritis. Biochem. J. 475 (22), 3629–3638. doi:10.1042/BCJ20180675
Liu, Z., Cao, J., Zhang, L., Li, J., Yan, T., Zhou, P., et al. (2022). Knockdown of circ-PRKCH alleviates IL-1β-treated chondrocyte cell phenotypic changes through modulating miR-502-5p/ADAMTS5 axis. Autoimmunity 55 (3), 179–191. doi:10.1080/08916934.2022.2027918
Long, L., Zou, G., Cheng, Y., Li, F., Wu, H., and Shen, Y. (2023). Matn3 delivered by exosome from synovial mesenchymal stem cells relieves knee osteoarthritis: evidence from in vitro and in vivo studies. J. Orthop. Transl. 41, 20–32. doi:10.1016/j.jot.2023.06.003
Lou, C., Jiang, H., Lin, Z., Xia, T., Wang, W., Lin, C., et al. (2023). Mir-146b-5p enriched bioinspired exosomes derived from fucoidan-directed induction mesenchymal stem cells protect chondrocytes in osteoarthritis by targeting traf6. J. Nanobiotechnol. 21 (1), 486. doi:10.1186/s12951-023-02264-9
Ma, T., Wu, C. B., Shen, Q. X., Wang, Q., and Zhou, Q. (2024). TRIM52 knockdown inhibits proliferation, inflammatory responses and oxidative stress in IL-1β-induced synovial fibroblasts to alleviate temporomandibular joint osteoarthritis. J. Cell. Mol. Med. 28 (8), e18244. doi:10.1111/jcmm.18244
Ma, X., Peng, L., Zhu, X., Chu, T., Yang, C., Zhou, B., et al. (2024). Isolation, identification, and challenges of extracellular vesicles: emerging players in clinical applications. Apoptosis 30, 422–445. doi:10.1007/s10495-024-02036-2
Manning, B. D., and Toker, A. (2017). Akt/pkb signaling: navigating the network. Cell 169 (3), 381–405. doi:10.1016/j.cell.2017.04.001
Mao, G., Zhang, Z., Hu, S., Zhang, Z., Chang, Z., Huang, Z., et al. (2018). Exosomes derived from mir-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting wnt5a. Stem Cell Res. Ther. 9 (1), 247. doi:10.1186/s13287-018-1004-0
Meng, C., Na, Y., Han, C., Ren, Y., Liu, M., Ma, P., et al. (2023). Exosomal mir-429 derived from adipose-derived stem cells ameliorated chondral injury in osteoarthritis via autophagy by targeting fez2. Int. Immunopharmacol. 120, 110315. doi:10.1016/j.intimp.2023.110315
Meng, S., Tang, C., Deng, M., Yuan, J., Fan, Y., Gao, S., et al. (2023). Tropoelastin-pretreated exosomes from adipose-derived stem cells improve the synthesis of cartilage matrix and alleviate osteoarthritis. J. Funct. Biomater. 14 (4), 203. doi:10.3390/jfb14040203
Nassar, W., El-Ansary, M., Sabry, D., Mostafa, M. A., Fayad, T., Kotb, E., et al. (2016). Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomater. Res. 20, 21. doi:10.1186/s40824-016-0068-0
Nguyen, T. H., Duong, C. M., Nguyen, X. H., and Than, U. (2021). Mesenchymal stem cell-derived extracellular vesicles for osteoarthritis treatment: extracellular matrix protection, chondrocyte and osteocyte physiology, pain and inflammation management. Cells 10 (11), 2887. doi:10.3390/cells10112887
Ni, Z., Kuang, L., Chen, H., Xie, Y., Zhang, B., Ouyang, J., et al. (2019). The exosome-like vesicles from osteoarthritic chondrocyte enhanced mature IL-1β production of macrophages and aggravated synovitis in osteoarthritis. Cell Death Dis. 10 (7), 522. doi:10.1038/s41419-019-1739-2
Nikhil, A., and Kumar, A. (2022). Evaluating potential of tissue-engineered cryogels and chondrocyte derived exosomes in articular cartilage repair. Biotechnol. Bioeng. 119 (2), 605–625. doi:10.1002/bit.27982
Niu, G., Jian, T., Gai, Y., and Chen, J. (2023). Microbiota and plant-derived vesicles that serve as therapeutic agents and delivery carriers to regulate metabolic syndrome. Adv. Drug Deliv. Rev. 196, 114774. doi:10.1016/j.addr.2023.114774
Nnah, I. C., Wang, B., Saqcena, C., Weber, G. F., Bonder, E. M., Bagley, D., et al. (2019). Tfeb-driven endocytosis coordinates mtorc1 signaling and autophagy. Autophagy 15 (1), 151–164. doi:10.1080/15548627.2018.1511504
Otsuki, Y., Ii, M., Moriwaki, K., Okada, M., Ueda, K., and Asahi, M. (2018). W9 peptide enhanced osteogenic differentiation of human adipose-derived stem cells. Biochem. Biophys. Res. Commun. 495 (1), 904–910. doi:10.1016/j.bbrc.2017.11.056
Park, J. M., Jung, C. H., Seo, M., Otto, N. M., Grunwald, D., Kim, K. H., et al. (2016). The ulk1 complex mediates mtorc1 signaling to the autophagy initiation machinery via binding and phosphorylating atg14. Autophagy 12 (3), 547–564. doi:10.1080/15548627.2016.1140293
Peng, S., Sun, C., Lai, C., and Zhang, L. (2023). Exosomes derived from mesenchymal stem cells rescue cartilage injury in osteoarthritis through ferroptosis by got1/ccr2 expression. Int. Immunopharmacol. 122, 110566. doi:10.1016/j.intimp.2023.110566
Qian, Y., Chu, G., Zhang, L., Wu, Z., Wang, Q., Guo, J. J., et al. (2024). M2 macrophage-derived exosomal mir-26b-5p regulates macrophage polarization and chondrocyte hypertrophy by targeting tlr3 and col10a1 to alleviate osteoarthritis. J. Nanobiotechnol. 22 (1), 72. doi:10.1186/s12951-024-02336-4
Qiu, M., Xie, Y., Tan, G., Wang, X., Huang, P., and Hong, L. (2024). Synovial mesenchymal stem cell-derived exosomal mir-485-3p relieves cartilage damage in osteoarthritis by targeting the nrp1-mediated pi3k/akt pathway: exosomal mir-485-3p relieves cartilage damage. Heliyon 10 (2), e24042. doi:10.1016/j.heliyon.2024.e24042
Rahman, G., Frazier, T. P., Gimble, J. M., and Mohiuddin, O. A. (2022). The emerging use of asc/scaffold composites for the regeneration of osteochondral defects. Front. Bioeng. Biotechnol. 10, 893992. doi:10.3389/fbioe.2022.893992
Regente, M., Corti-Monzón, G., Maldonado, A. M., Pinedo, M., Jorrín, J., and de la Canal, L. (2009). Vesicular fractions of sunflower apoplastic fluids are associated with potential exosome marker proteins. FEBS Lett. 583 (20), 3363–3366. doi:10.1016/j.febslet.2009.09.041
Rizzo, M. G., Best, T. M., Huard, J., Philippon, M., Hornicek, F., Duan, Z., et al. (2023). Therapeutic perspectives for inflammation and senescence in osteoarthritis using mesenchymal stem cells, mesenchymal stem cell-derived extracellular vesicles and senolytic agents. Cells 12 (10), 1421. doi:10.3390/cells12101421
Shang, Z., Wanyan, P., Zhang, B., Wang, M., and Wang, X. (2023). A systematic review, umbrella review, and quality assessment on clinical translation of stem cell therapy for knee osteoarthritis: are we there yet? Stem Cell Res. Ther. 14 (1), 91. doi:10.1186/s13287-023-03332-5
Stegen, S., and Carmeliet, G. (2024). Metabolic regulation of skeletal cell fate and function. Nat. Rev. Endocrinol. 20 (7), 399–413. doi:10.1038/s41574-024-00969-x
Steinert, A. F., Ghivizzani, S. C., Rethwilm, A., Tuan, R. S., Evans, C. H., and Nöth, U. (2007). Major biological obstacles for persistent cell-based regeneration of articular cartilage. Arthritis Res. Ther. 9 (3), 213. doi:10.1186/ar2195
Sun, K., Luo, J., Guo, J., Yao, X., Jing, X., and Guo, F. (2020). The pi3k/akt/mtor signaling pathway in osteoarthritis: a narrative review. Osteoarthr. Cartil. 28 (4), 400–409. doi:10.1016/j.joca.2020.02.027
Sun, W., Qu, S., Ji, M., Sun, Y., and Hu, B. (2023). Bmp-7 modified exosomes derived from synovial mesenchymal stem cells attenuate osteoarthritis by m2 polarization of macrophages. Heliyon 9 (9), e19934. doi:10.1016/j.heliyon.2023.e19934
Tan, F., Wang, D., and Yuan, Z. (2020). The fibroblast-like synoviocyte derived exosomal long non-coding rna h19 alleviates osteoarthritis progression through the mir-106b-5p/timp2 axis. Inflammation 43 (4), 1498–1509. doi:10.1007/s10753-020-01227-8
Tang, F., Wang, Y., Hemmings, B. A., Rüegg, C., and Xue, G. (2018). Pkb/akt-dependent regulation of inflammation in cancer. Semin. Cancer Biol. 48, 62–69. doi:10.1016/j.semcancer.2017.04.018
Tang, P., Xiong, Q., Ge, W., and Zhang, L. (2014). The role of micrornas in osteoclasts and osteoporosis. RNA Biol. 11 (11), 1355–1363. doi:10.1080/15476286.2014.996462
Tang, S., Tang, T., Gao, G., Wei, Q., Sun, K., and Huang, W. (2021). Bone marrow mesenchymal stem cell-derived exosomes inhibit chondrocyte apoptosis and the expression of mmps by regulating drp1-mediated mitophagy. Acta Histochem. 123 (8), 151796. doi:10.1016/j.acthis.2021.151796
Tao, S. C., Yuan, T., Zhang, Y. L., Yin, W. J., Guo, S. C., and Zhang, C. Q. (2017). Exosomes derived from mir-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics 7 (1), 180–195. doi:10.7150/thno.17133
Tauro, B. J., Greening, D. W., Mathias, R. A., Ji, H., Mathivanan, S., Scott, A. M., et al. (2012). Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line lim1863-derived exosomes. Methods 56 (2), 293–304. doi:10.1016/j.ymeth.2012.01.002
Tian, H., Chen, A., Gao, P., Wang, F., Zhao, Y., Wang, F., et al. (2024). Human umbilical cord mesenchymal stem cell-derived exosomes induce macrophage m2 polarization by antagonizing lps-mediated stimulation of the nf-κb and stat3 pathways. Comb. Chem. High. Throughput Screen 27. doi:10.2174/0113862073314685240514050119
To, K., Zhang, B., Romain, K., Mak, C., and Khan, W. (2019). Synovium-derived mesenchymal stem cell transplantation in cartilage regeneration: a prisma review of in vivo studies. Front. Bioeng. Biotechnol. 7, 314. doi:10.3389/fbioe.2019.00314
Usami, Y., Gunawardena, A. T., Iwamoto, M., and Enomoto-Iwamoto, M. (2016). Wnt signaling in cartilage development and diseases: lessons from animal studies. Lab. Invest. 96 (2), 186–196. doi:10.1038/labinvest.2015.142
Wang, H., Zhang, Y., Zhang, C., Zhao, Y., Shu, J., and Tang, X. (2024). Exosomes derived from mir-146a-overexpressing fibroblast-like synoviocytes in cartilage degradation and macrophage m1 polarization: a novel protective agent for osteoarthritis? Front. Immunol. 15, 1361606. doi:10.3389/fimmu.2024.1361606
Wang, J., Sun, Z., Yu, C., Zhao, H., Yan, M., Sun, S., et al. (2024). Single-cell rna sequencing reveals the impact of mechanical loading on knee tibial cartilage in osteoarthritis. Int. Immunopharmacol. 128, 111496. doi:10.1016/j.intimp.2024.111496
Wang, K., Li, F., Yuan, Y., Shan, L., Cui, Y., Qu, J., et al. (2020). Synovial mesenchymal stem cell-derived ev-packaged mir-31 downregulates histone demethylase kdm2a to prevent knee osteoarthritis. Mol. Ther. Nucleic Acids 22, 1078–1091. doi:10.1016/j.omtn.2020.09.014
Wang, L., Li, F., Wang, L., Wu, B., Du, M., Xing, H., et al. (2024). Exosomes derived from bone marrow mesenchymal stem cells alleviate rheumatoid arthritis symptoms via shuttling proteins. J. Proteome Res. 23, 1298–1312. doi:10.1021/acs.jproteome.3c00697
Wang, R., and Xu, B. (2021). TGF-β1-modified MSC-derived exosomal miR-135b attenuates cartilage injury via promoting M2 synovial macrophage polarization by targeting MAPK6. Cell Tissue Res. 384 (1), 113–127. doi:10.1007/s00441-020-03319-1
Wang, R., Xu, B., and Xu, H. (2018). TGF-β1 promoted chondrocyte proliferation by regulating Sp1 through MSC-exosomes derived miR-135b. Cell Cycle 17 (24), 2756–2765. doi:10.1080/15384101.2018.1556063
Wang, S., Jiang, W., Lv, S., Sun, Z., Si, L., Hu, J., et al. (2023). Human umbilical cord mesenchymal stem cells-derived exosomes exert anti-inflammatory effects on osteoarthritis chondrocytes. Aging (Albany NY) 15 (18), 9544–9560. doi:10.18632/aging.205034
Wang, Y., Fan, A., Lu, L., Pan, Z., Ma, M., Luo, S., et al. (2022). Exosome modification to better alleviates endoplasmic reticulum stress induced chondrocyte apoptosis and osteoarthritis. Biochem. Pharmacol. 206, 115343. doi:10.1016/j.bcp.2022.115343
Wang, Z., Yan, K., Ge, G., Zhang, D., Bai, J., Guo, X., et al. (2021). Exosomes derived from mir-155-5p-overexpressing synovial mesenchymal stem cells prevent osteoarthritis via enhancing proliferation and migration, attenuating apoptosis, and modulating extracellular matrix secretion in chondrocytes. Cell Biol. Toxicol. 37 (1), 85–96. doi:10.1007/s10565-020-09559-9
Wei, J., Ou, Z., Tong, B., Liao, Z., and Yang, C. (2023). Engineered extracellular vesicles as therapeutics of degenerative orthopedic diseases. Front. Bioeng. Biotechnol. 11, 1162263. doi:10.3389/fbioe.2023.1162263
Wen, C., Lin, L., Zou, R., Lin, F., and Liu, Y. (2022). Mesenchymal stem cell-derived exosome mediated long non-coding rna klf3-as1 represses autophagy and apoptosis of chondrocytes in osteoarthritis. Cell Cycle 21 (3), 289–303. doi:10.1080/15384101.2021.2019411
Wen, Q., Zhou, L., Zhou, C., Zhou, M., Luo, W., and Ma, L. (2012). Change in hepatocyte growth factor concentration promote mesenchymal stem cell-mediated osteogenic regeneration. J. Cell. Mol. Med. 16 (6), 1260–1273. doi:10.1111/j.1582-4934.2011.01407.x
Witte, F., Dokas, J., Neuendorf, F., Mundlos, S., and Stricker, S. (2009). Comprehensive expression analysis of all wnt genes and their major secreted antagonists during mouse limb development and cartilage differentiation. Gene Expr. Patterns 9 (4), 215–223. doi:10.1016/j.gep.2008.12.009
Wu, J., Kuang, L., Chen, C., Yang, J., Zeng, W. N., Li, T., et al. (2019). Mir-100-5p-abundant exosomes derived from infrapatellar fat pad mscs protect articular cartilage and ameliorate gait abnormalities via inhibition of mtor in osteoarthritis. Biomaterials 206, 87–100. doi:10.1016/j.biomaterials.2019.03.022
Wu, N., Liu, J., Ma, W., Dong, X., Wang, F., Yang, D., et al. (2021). Degradable calcium deficient hydroxyapatite/poly(lactic-glycolic acid copolymer) bilayer scaffold through integral molding 3d printing for bone defect repair. Biofabrication 13 (2), 025005. doi:10.1088/1758-5090/abcb48
Wu, X., Crawford, R., Xiao, Y., Mao, X., and Prasadam, I. (2021). Osteoarthritic subchondral bone release exosomes that promote cartilage degeneration. Cells 10 (2), 251. doi:10.3390/cells10020251
Xavier, J., Jerome, W., Zaslav, K., and Grande, D. (2023). Exosome-laden scaffolds for treatment of post-traumatic cartilage injury and osteoarthritis of the knee: a systematic review. Int. J. Mol. Sci. 24 (20), 15178. doi:10.3390/ijms242015178
Xu, H., and Xu, B. (2021). Bmsc-derived exosomes ameliorate osteoarthritis by inhibiting pyroptosis of cartilage via delivering mir-326 targeting hdac3 and stat1//nf-κb p65 to chondrocytes. Mediat. Inflamm. 2021, 9972805. doi:10.1155/2021/9972805
Xu, T., Yu, X., Xu, K., Lin, Y., Wang, J., Pan, Z., et al. (2024). Comparison of the ability of exosomes and ectosomes derived from adipose-derived stromal cells to promote cartilage regeneration in a rat osteochondral defect model. Stem Cell Res. Ther. 15 (1), 18. doi:10.1186/s13287-024-03632-4
Xu, X., Liang, Y., Li, X., Ouyang, K., Wang, M., Cao, T., et al. (2021). Exosome-mediated delivery of kartogenin for chondrogenesis of synovial fluid-derived mesenchymal stem cells and cartilage regeneration. Biomaterials 269, 120539. doi:10.1016/j.biomaterials.2020.120539
Xu, X., Xu, L., Wang, J., Wen, C., Xia, J., Zhang, Y., et al. (2024). Bioinspired cellular membrane-derived vesicles for mrna delivery. Theranostics 14 (8), 3246–3266. doi:10.7150/thno.93755
Xu, Y., Wang, Q., Wang, X., Xiang, X., Peng, J., Zhang, J., et al. (2023). Effect of pulsed electromagnetic fields on mesenchymal stem cell-derived exosomes in inhibiting chondrocyte apoptosis. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 40 (1), 95–102. doi:10.7507/1001-5515.202209053
Yan, L., Liu, G., and Wu, X. (2021). The umbilical cord mesenchymal stem cell-derived exosomal lncrna h19 improves osteochondral activity through mir-29b-3p/foxo3 axis. Clin. Transl. Med. 11 (1), e255. doi:10.1002/ctm2.255
Yan, Z., Yin, H., Wu, J., Tian, G., Li, M., Liao, Z., et al. (2023). Engineering exosomes by three-dimensional porous scaffold culture of human umbilical cord mesenchymal stem cells promote osteochondral repair. Mater Today Bio 19, 100549. doi:10.1016/j.mtbio.2023.100549
Yang, D., Zhang, W., Zhang, H., Zhang, F., Chen, L., Ma, L., et al. (2020). Progress, opportunity, and perspective on exosome isolation - efforts for efficient exosome-based theranostics. Theranostics 10 (8), 3684–3707. doi:10.7150/thno.41580
Yang, H., Zhou, Y., Ying, B., Dong, X., Qian, Q., and Gao, S. (2024). Effects of human umbilical cord mesenchymal stem cell-derived exosomes in the rat osteoarthritis models. Stem Cells Transl. Med. 13, 803–811. doi:10.1093/stcltm/szae031
Yang, R. Z., Zheng, H. L., Xu, W. N., Zheng, X. F., Li, B., Jiang, L. S., et al. (2021). Vascular endothelial cell-secreted exosomes facilitate osteoarthritis pathogenesis by promoting chondrocyte apoptosis. Aging (Albany NY) 13 (3), 4647–4662. doi:10.18632/aging.202506
Yuan, C., Song, W., Jiang, X., Wang, Y., Li, C., Yu, W., et al. (2024). Adipose-derived stem cell-based optimization strategies for musculoskeletal regeneration: recent advances and perspectives. Stem Cell Res. Ther. 15 (1), 91. doi:10.1186/s13287-024-03703-6
Yuasa, T., Kondo, N., Yasuhara, R., Shimono, K., Mackem, S., Pacifici, M., et al. (2009). Transient activation of wnt/{beta}-catenin signaling induces abnormal growth plate closure and articular cartilage thickening in postnatal mice. Am. J. Pathol. 175 (5), 1993–2003. doi:10.2353/ajpath.2009.081173
Yue, Y., Dai, W., Wei, Y., Cao, S., Liao, S., Li, A., et al. (2024). Unlocking the potential of exosomes: a breakthrough in the theranosis of degenerative orthopaedic diseases. Front. Bioeng. Biotechnol. 12, 1377142. doi:10.3389/fbioe.2024.1377142
Zelinka, A., Roelofs, A. J., Kandel, R. A., and De Bari, C. (2022). Cellular therapy and tissue engineering for cartilage repair. Osteoarthr. Cartil. 30 (12), 1547–1560. doi:10.1016/j.joca.2022.07.012
Zeng, G., Deng, G., Xiao, S., and Li, F. (2022). Fibroblast-like synoviocytes-derived exosomal PCGEM1 accelerates IL-1β-induced apoptosis and cartilage matrix degradation by miR-142-5p/RUNX2 in chondrocytes. Immunol. Invest. 51 (5), 1284–1301. doi:10.1080/08820139.2021.1936010
Zhang, F. X., Liu, P., Ding, W., Meng, Q. B., Su, D. H., Zhang, Q. C., et al. (2021). Injectable mussel-inspired highly adhesive hydrogel with exosomes for endogenous cell recruitment and cartilage defect regeneration. Biomaterials 278, 121169. doi:10.1016/j.biomaterials.2021.121169
Zhang, H., Huang, J., and Alahdal, M. (2023). Exosomes loaded with chondrogenic stimuli agents combined with 3d bioprinting hydrogel in the treatment of osteoarthritis and cartilage degeneration. Biomed. Pharmacother. 168, 115715. doi:10.1016/j.biopha.2023.115715
Zhang, R., Li, M., Li, H., Ran, X., Jin, F., Tan, Q., et al. (2024). Immune cell-derived exosomes in inflammatory disease and inflammatory tumor microenvironment: a review. J. Inflamm. Res. 17, 301–312. doi:10.2147/JIR.S421649
Zhao, C., Jiang, W., Zhou, N., Liao, J., Yang, M., Hu, N., et al. (2017). Sox9 augments bmp2-induced chondrogenic differentiation by downregulating smad7 in mesenchymal stem cells (mscs). Genes Dis. 4 (4), 229–239. doi:10.1016/j.gendis.2017.10.004
Zhao, J., Sun, Y., Sheng, X., Xu, J., Dai, G., He, R., et al. (2023). Hypoxia-treated adipose mesenchymal stem cell-derived exosomes attenuate lumbar facet joint osteoarthritis. Mol. Med. 29 (1), 120. doi:10.1186/s10020-023-00709-3
Zhao, Q., Feng, J., Liu, F., Liang, Q., Xie, M., Dong, J., et al. (2024). Rhizoma drynariae-derived nanovesicles reverse osteoporosis by potentiating osteogenic differentiation of human bone marrow mesenchymal stem cells via targeting erα signaling. Acta Pharm. Sin. B 14 (5), 2210–2227. doi:10.1016/j.apsb.2024.02.005
Zhao, Y., Tan, H., Zhang, J., Pan, B., Wang, N., Chen, T., et al. (2023). Plant-derived vesicles: a new era for anti-cancer drug delivery and cancer treatment. Int. J. Nanomedicine 18, 6847–6868. doi:10.2147/IJN.S432279
Zheng, T., Li, Y., Zhang, X., Xu, J., and Luo, M. (2022). Exosomes derived from mir-212-5p overexpressed human synovial mesenchymal stem cells suppress chondrocyte degeneration and inflammation by targeting elf3. Front. Bioeng. Biotechnol. 10, 816209. doi:10.3389/fbioe.2022.816209
Zhou, H., Shen, X., Yan, C., Xiong, W., Ma, Z., Tan, Z., et al. (2022). Extracellular vesicles derived from human umbilical cord mesenchymal stem cells alleviate osteoarthritis of the knee in mice model by interacting with mettl3 to reduce m6a of nlrp3 in macrophage. Stem Cell Res. Ther. 13 (1), 322. doi:10.1186/s13287-022-03005-9
Zhou, Y., Ming, J., Li, Y., Li, B., Deng, M., Ma, Y., et al. (2021). Exosomes derived from mir-126-3p-overexpressing synovial fibroblasts suppress chondrocyte inflammation and cartilage degradation in a rat model of osteoarthritis. Cell Death Discov. 7 (1), 37. doi:10.1038/s41420-021-00418-y
Zhu, C., Shen, K., Zhou, W., Wu, H., and Lu, Y. (2021). Exosome-mediated circ_0001846 participates in IL-1β-induced chondrocyte cell damage by miR-149-5p-dependent regulation of WNT5B. Clin. Immunol. 232, 108856. doi:10.1016/j.clim.2021.108856
Zhu, J., Liu, B., Wang, Z., Wang, D., Ni, H., Zhang, L., et al. (2019). Exosomes from nicotine-stimulated macrophages accelerate atherosclerosis through mir-21-3p/pten-mediated vsmc migration and proliferation. Theranostics 9 (23), 6901–6919. doi:10.7150/thno.37357
Zhu, Y., Wang, Y., Zhao, B., Niu, X., Hu, B., Li, Q., et al. (2017). Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res. Ther. 8 (1), 64. doi:10.1186/s13287-017-0510-9
Keywords: exosomes, osteoarthritis, cartilage repair, cartilage microenvironment, mechanism of action
Citation: Longfei H, Wenyuan H, Weihua F, Peng P, Sun L, Kun L, Mincong H, Fan Y, Wei H and Qiushi W (2025) Exosomes in cartilage microenvironment regulation and cartilage repair. Front. Cell Dev. Biol. 13:1460416. doi: 10.3389/fcell.2025.1460416
Received: 06 July 2024; Accepted: 17 February 2025;
Published: 05 March 2025.
Edited by:
Yongqiang Chen, University of Manitoba, CanadaReviewed by:
Naresh Kumar, The Ohio State University, United StatesCopyright © 2025 Longfei, Wenyuan, Weihua, Peng, Sun, Kun, Mincong, Fan, Wei and Qiushi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Qiushi, d2VpcXNoaUAxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.