
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell Dev. Biol. , 09 January 2025
Sec. Embryonic Development
Volume 12 - 2024 | https://doi.org/10.3389/fcell.2024.1520429
 Na Wang1
Na Wang1 Han Yang1
Han Yang1 Yelei Chen1
Yelei Chen1 Hekun Wang2
Hekun Wang2 Chaorui Wang1
Chaorui Wang1 Jianglin Fan1
Jianglin Fan1 Yajie Chen1*
Yajie Chen1* Yinghua Li1*
Yinghua Li1* Maobi Zhu1*
Maobi Zhu1*Increasing evidence has demonstrated that oxidative stress impairs oocyte maturation and embryonic development. Conventionally, antioxidants have been applied in vitro systems to improve oocyte maturation and blastocyst rates. Formononetin (FMN) is a flavonoid that has been shown to have various pharmacological effects, including antioxidants. In this study, we delved into the impact of FMN, acting as an antioxidant, on the in vitro development of oocytes and blastocysts within the culture system. FMN supplementation at 0.5 μM enhanced the rate of first polar body extrusion and blastocyst formation post parthenogenetic activation. It also increased mitochondrial function and ATP levels, reduced intracellular reactive oxygen species, and elevated intracellular GSH levels in both oocytes and embryos. Moreover, FMN significantly decreased autophagy and apoptosis levels in blastocyst cells, potentially via regulation of the Nrf2/Keap1 pathway. This is the first study to report that FMN supplementation benefits the in vitro culture of oocytes and early embryo development, potentially by regulating oxidative stress, mitochondrial function, and autophagy.
In vitro oocyte maturation (IVM), in vitro fertilization (IVF), and in vitro embryo culture (IVC) techniques have been widely used in agricultural and biomedical research (Gil et al., 2010). Especially, these techniques serve as the basis of modern biomedical assisted reproductive technologies, such as assisted reproductive technology (ART), somatic cell nuclear transfer (SCNT), and embryo cryopreservation (Grupen, 2014). However, external factors associated with a high-oxygen environment and in vitro procedures can adversely affect oocyte quality and suppress embryonic development.
During the process of oocyte and embryo in vitro, unstable extracellular environments, such as excessive oxygen stress, light, water, oocyte handling, culture medium composition (Ciray et al., 2009; Bennemann et al., 2018; Lee et al., 2017; Li et al., 2015), and other uncertain factors, could impair oocyte maturation and embryo development. Excessive ROS causes a series of injuries to oocytes and embryos, such as induction of mitochondrial dysfunction, DNA fragmentation, apoptosis, and cytoplasmic abnormalities (Amin et al., 2014; Agarwal et al., 2005; Agarwal et al., 2006). The use of substances with antioxidant properties reducing excessive ROS could ameliorate oocyte quality and enhance embryo developmental competence in vitro. Therefore, exploring the role of antioxidants in oxidative stress during oocyte and embryo in vitro is crucial. Meanwhile, it is also beneficial to the treatment of woman infertility (Li et al., 2019).
Formononetin (7-hydroxy, 4′-methoxy isoflavone, FMN) is a representative flavonoid found in Chinese medicinal plants, such as Red Clover, Astragalus, Licorice, and Pueraria (Machado Dutra et al., 2021). FMN has been reported to have a variety of biological activities. Such as antioxidant, anti-inflammatory, anti-allergic, neuroprotective, anti-hypertensive, and anti-cancer (Li et al., 2018; Sun et al., 2011; Mu et al., 2009; Tian et al., 2013; Chen et al., 2014). Moreover, previous studies have shown that FMN is a kind of phytoestrogens of relevant interest in the areas of nutrition, medicine, and cosmetics can be used in hormone replacement therapy (Kulling et al., 2002; Dornstauder et al., 2001), which indicates it may have potential benefits for female and reproductive health. FMN exhibits strong free radical scavenging activity and is attributed to their influence on the antioxidant enzymes and non-enzymatic system by scavenging free radical (Mu et al., 2009).
Although FMN is a flavonoid in herbal medicine and has well-known protective properties, the role of FMN in mammalian oocytes and embryos has not been studied. Our study aimed to explore the benefits of FMN on oocyte maturation and early embryonic development. Oocytes and embryos were exposed to FMN and evaluated oocyte first polar body extrusion rate, blastocyst rate, ROS, apoptosis, mitochondrial function, and autophagy.
Unless otherwise stated, all reagents and chemicals were purchased from Sigma-Aldrich (St. Louis, MO, United States). Porcine ovaries used in this study were obtained from a slaughterhouse (Jiangmen, China). Formononetin (Selleck) was dissolved in DMSO to make a 50 mM stock solution. Different concentrations of FMN were tested in vitro maturation (IVM) or in vitro culture (IVC) media to assess their dose-dependent effects.
Cumulus–oocyte complexes were collected from 3- to 8-mm follicles. Oocytes with a minimum of three layers of cumulus cells were selected with a stereomicroscope and cultured in vitro in maturation medium (IVM; M199 medium with 100 μL penicillin-streptomycin, 1 mL porcine follicular fluid, 0.2 mg epidermal growth factor, 0.22 mg sodium pyruvate, 100 IU luteinizing hormone, 100 IU follicle stimulating hormone, and 0.9 mg L-cysteine) for 44 h at 38.5°C in an incubator of 5% CO2 and 100% humidity.
At the end of maturation, COCs were removed from IVM and were repeatedly pipetted with 0.25% hyaluronidase. Oocytes with polar bodies were activated by 120 V direct current stimulation twice, each time for 60 μs followed by 0.1 s of pause using an electro cell fusion generator and transferred to 7.5 mg/mL cytochalasin B to balance for 3 h. Finally, they were cultured in bicarbonate-buffered porcine zygote medium 5 (PZM- 5) with 4 mg/mL BSA for 7 days at 38.5°C, 5% CO2, and 100% humidity.
MII-stage oocytes and 4-cell stage embryos were cultured with 10 µM 2′,7′-dichlorodihydrofluorescein diacetate (DCFH, #S0033, Beyotime, Shanghai, China) or 10 µM 4-chloromethyl-6,8-difluoro-7-hydroxycoumarin (CMF2HC, #C12881, Invitrogen,) at 37°C for 45 min and 30 min in darkness, respectively. Fluorescence intensity was analyzed using a fluorescence microscope and ImageJ software.
Oocytes were cultured with 100 ng/mL Mito-Tracker Red CMXRos (#C1049B Beyotime, Shanghai, China) for 30 min in darkness. Oocytes were rinsed thrice and photographed by fluorescence microscope. Fluorescence intensity was analyzed using a fluorescence microscope and ImageJ software.
Embryos were cultured with 10 µM 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanineiodide (JC-1; #C2006, Invitrogen) for 1 h in darkness, with red fluorescence indicating the aggregated form (J-aggregate) and green fluorescence indicating the monomer form (J-monomer). The red/green fluorescence intensity was used to calculate the levels of MMP.
In brief, blastocysts were cultured in 5 μM EdU (#C10310-1, Ribobio, China) for 8 h at 38.5°C. Subsequently, the blastocysts were fixed in 4% paraformaldehyde and permeabilized using 0.3% Triton X-100% and 5% fetal bovine serum for 30 min. The detection of EdU was performed according to the manufacturer’s protocol and the number of fluorescent cells was analyzed using a fluorescence microscope and ImageJ software. The ratio of the number of EdU-positive nuclei to the total number of nuclei was used to compute the cell proliferation rate.
After being fixed with 4% paraformaldehyde, blastocysts were permeabilized and blocked with 0.3% Triton X-100% and 5% fetal bovine serum for 30 min. Next, blastocysts were treated with primary antibodies to LC3B antibody (1:200; #ab48394, Abcam) and anti-NRF2 antibody (1:200; #ab31163, Abcam) overnight at 4°C. After washing, blastocysts were incubated with corresponding secondary antibodies for 1 h. TUNEL staining was carried out using the Situ Cell Death Detection kit (#11684795910, Roche) according to the manufacturer’s instructions. Nuclei were counterstained with DAPI (#62248, Thermo Fisher Scientific). Finally, fluorescent microscope and ImageJ software were used to analyze the fluorescence intensity.
ATP in 4-cell stage embryos was measured using an ATP detection kit (#S0026, Beyotime, China). 4-cell stage embryos were lysed and the supernatant was collected. Then, supernatant and ATP working solution were mixed and analyzed using a multifunctional enzyme marker (Synergy Neo2, BioTek) via chemiluminescence.
60 blastocysts were lysed with RIPA buffer (#89900, Thermo Fisher Scientific) containing a protease inhibitor cocktail. The protein samples were separated by 8% SDS-polyacrylamide gel and wet-transferred to a PVDF membrane at 200 mA and 4°C for 2 h. The membrane was blocked using 5% bovine serum albumin with Tween 20 (TBS-T), followed by the incubation with primary antibodies overnight at 4°C. And incubation with horseradish peroxidase-conjugated secondary antibodies at RT for 1 h. Primary antibodies were rabbit monoclonal antibodies against NRF2 (1:2000, #16396-1-AP, Proteintech), KEAP1 (1:2000, #10503-2-AP, Proteintech), and β-Actin (1:5000, #20536-1-AP, Proteintech). Secondary antibodies were goat anti-rabbit IgG (#31460, Thermo Fisher Scientific). Signals were developed using an enhanced chemiluminescence substrate (#34580, Thermo Fisher Scientific), and images were acquired using an Azure Sapphire RGBNIR detection system.
Total RNA was extracted using the Dynabeads mRNA DIRECT Purification Kit (#61012, Invitrogen) and reverse transcribe the RNA into cDNA using the iScript cDNA Synthesis Kit (#1708891, iScript cDNA Synthesis, BIO-RAD). qRT-PCR was performed using KAPA SYBR FAST Universal qPCR Kit (#KK4601, Kapa Biosystems, United States) and LightCycle96 devices (Roche) (Liu et al., 2023). The 2−ΔΔCt method were used to quantify the mRNA expression of genes, and GAPDH was used as the standard. The primer sequences are listed in Table 1.
Statistical analysis was performed with the SPSS and Graphpad Prism. One-way ANOVA and Duncan’s tests were used to process data. Data were presented by average ±SEM. Different letters (p < 0.05) denoted significant differences. There were at least three biological replicates in each group.
FMN has been shown to have antioxidant and anti-inflammatory effects at concentration of 10 μM, particularly inhibiting Aβ-induced vascular inflammation (Fan et al., 2022). In our initial experiments, we designed a concentration range of 0.5–50 μM (Supplementary Data). However, we found that concentrations exceeding 10 μM inhibited oocyte polar body extrusion and blastocyst formation. On the other hand, the addition of 0.5 μM FMN to the IVM and IVC media significantly increased the rate of polar body extrusion at the MII stage (a: with polar body, b: without polar body) and the blastocyst formation rate after parthenogenetic activation.
During in vitro oocyte maturation, the rates of polar body extrusion in the FMN treatment groups (0, 0.5, 5, 10, 20, and 50 μM,) were (50.05% ± 5.73%, 64.22% ± 8.37%, 44.16% ± 4.31%, 35.21% ± 4.89%, 33.22% ± 4.22%, and 28.81% ± 3.40%, respectively; Figures 1A, B). Furthermore, we examined genes associated with cumulus expansion, including pentraxin-3 (PTX3), hyaluronan synthase 2 (HAS2), prostaglandin endoperoxide synthase 2 (PTGS2), prostaglandin endoperoxide synthase 1 (PTGS1) and hyaluronan receptor (CD44), among which PTGS2, PTGS1, and CD44 were significantly increased compared to the control (1.59 ± 0.16, 1.36 ± 0.07, 1.12 ± 0.05, respectively; Figure 1C).
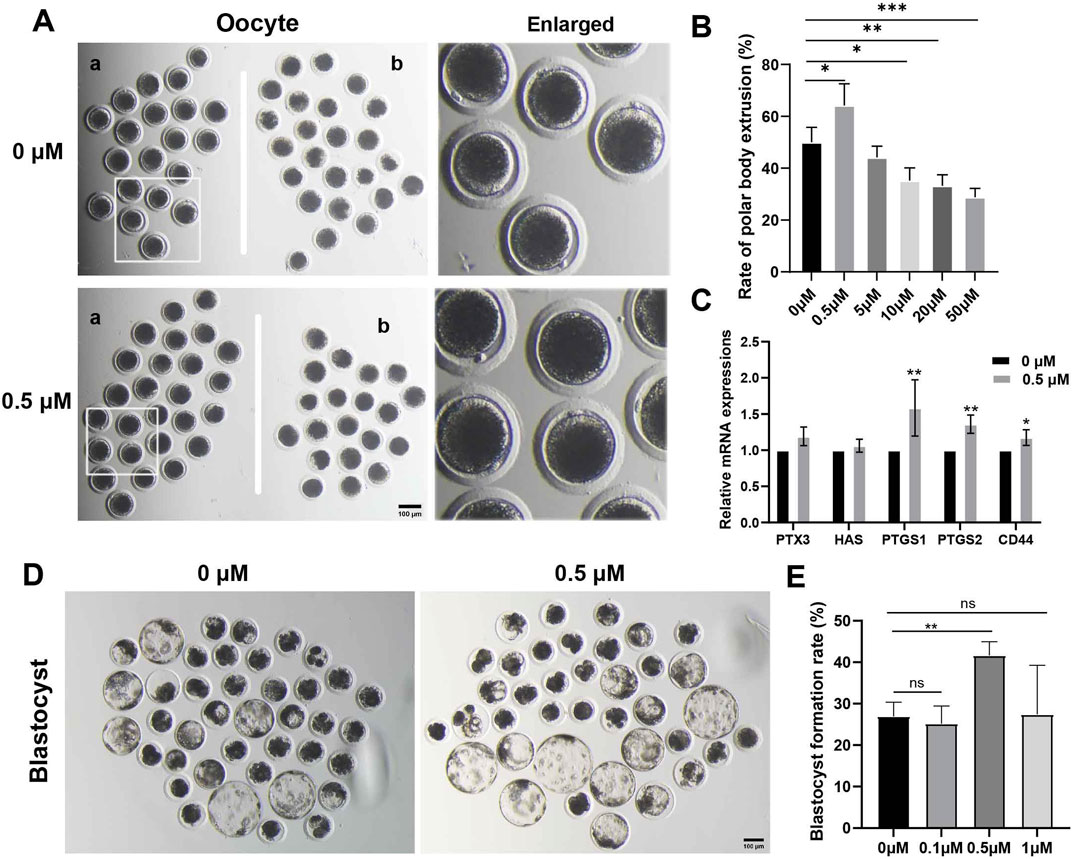
Figure 1. Effects of FMN on the oocyte polar body extrusion and blastocysts development in vitro. (A) Oocyte polar body extrusion in the control and FMNtreated groups. (a) Oocytes with polar body, (b) oocytes without polar body, scale bar, 100 μm. (B) The rate of different concentrations of FMN treatment on the polar body extrusion in porcine oocytes (C) Relative mRNA levels of cumulus expansion related genes PTX3, HAS2, PTGS2, PTGS1, and CD44 in oocytes. (D) Embryonic development rate on day 7 in the control and FMN-treated groups, scale bar, 100 μm. (E) The rate of blastocyst formation under treatment of various concentrations of FMN. Data are presented as the mean ± standard deviation (SD). *p < 0.05; **p < 0.01, ***p < 0.001 vs. 0 μM FMN group.
During in vitro embryo cultivation, the blastocyst rates in the FMN treatment groups (0, 0.1, 0.5, and 1 μM) were (26.95% ± 3.40%, 25.18% ± 4.27%, 41.63% ± 3.31% and 27.45% ± 11.83%, respectively Figures 1D, E). In conclusion, the group treated with 0.5 μM FMN showed a significant increase in the rate of blastocyst formation.
To explore whether FMN promotes oocyte maturation and embryonic development in association with the antioxidant properties, we measured the levels of ROS and GSH, an endogenous antioxidant, in porcine MII stage oocytes and 4-cell stage embryos [a critical period for porcine early embryo zygotic genome activation (ZGA)] (Liu et al., 2023), respectively.
In MII stage oocytes, comparison to the control group, the FMN-treated group’s fluorescence intensity of ROS was significantly lower (0.88 ± 0.06), whereas the fluorescence intensity of GSH was significantly higher (1.24 ± 0.06; Figures 2A, B). Antioxidant-related genes peroxisome proliferator-activated receptor γ coactivator 1-alpha (PGC1α), peroxiredoxin 2 (PRDX2), glutathione peroxidase (GPX), and catalase (CAT), were also higher than those in the control group (1.36 ± 0.33, 1.32 ± 0.05, 1.64 ± 0.46, and 1.20 ± 0.21, respectively Figure 2C).
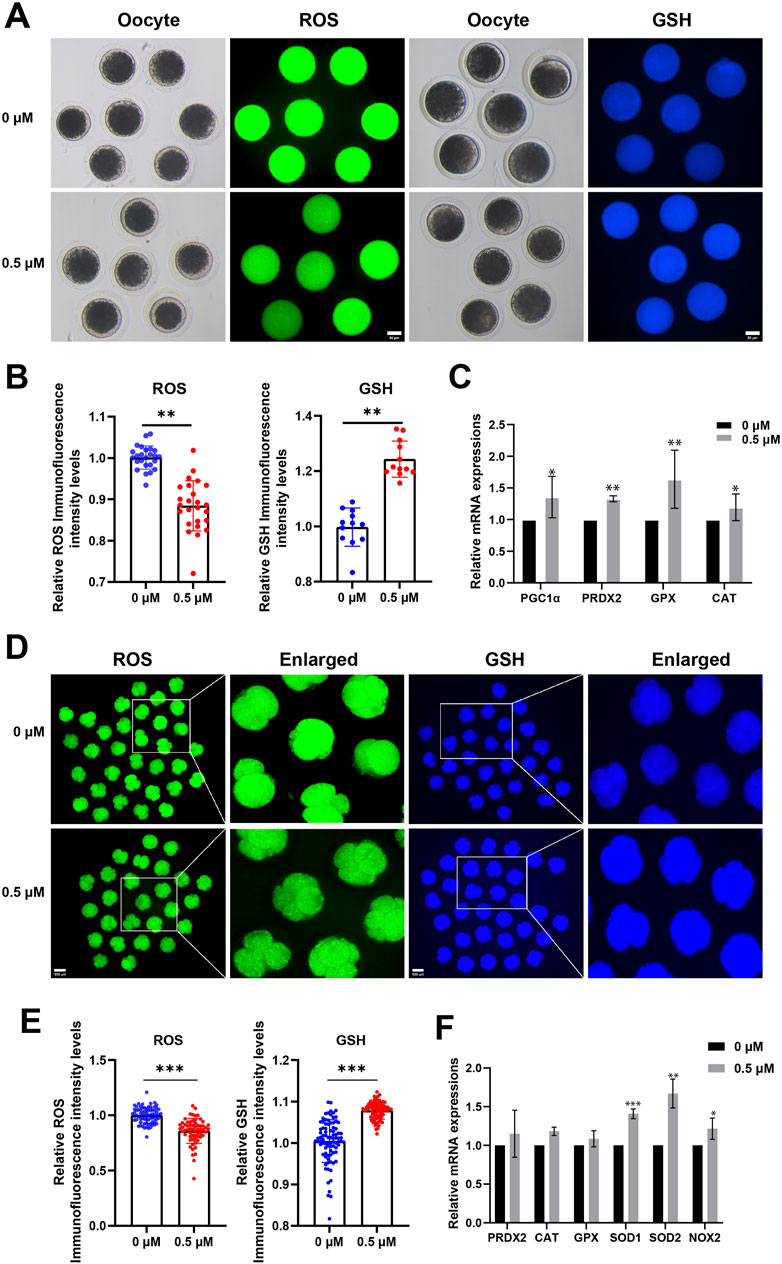
Figure 2. Effect of FMN on the antioxidant capacity of oocytes and early embryos. (A) Oocyte ROS (green) and GSH (blue) expression at MII stage in control and FMN-treated groups, scale bar, 50 μm. (B) Relative fluorescence intensity levels of ROS were analyzed for control and FMN-treated group. (C) Relative mRNA levels of antioxidant-related genes PGC1α, PRDX2, GPX and CAT in oocytes treated with or without FMN. (D) Embryonic ROS (green) and GSH (blue) expression in 4-cell stage in control and FMN-treated groups, scale bar, 100 μm. (E) Relative fluorescence intensity levels of embryonic ROS and GSH were analyzed for control and FMN-treated group. (F): Relative mRNA levels of antioxidant-related genes PRDX2, CAT, GPX, SOD1, SOD2 and NOX2 in embryos treated with or without FMN. Data were presented as the mean ± standard deviation (SD). *p < 0.05,**p < 0.01, ***p < 0.001 vs. 0 μM FMN group.
In 4-cell stage embryos comparison to the control group, the FMN-treated group’s fluorescence intensity of ROS was significantly lower (0.86 ± 0.11), whereas the fluorescence intensity of GSH was significantly higher (1.37 ± 0.05; Figures 2D, E). Antioxidant-related genes peroxisome superoxide dismutase 1 (SOD1), superoxide dismutase 2 (SOD2), and oxidative stress-related gene NADPH oxidase 2 (NOX2)—were more significantly elevated in the FMN-treated group (1.40 ± 0.06, 1.67 ± 0.19, and 1.22 ± 0.14, respectively Figure 2F). This suggests that FMN can boost the antioxidant activity of porcine oocytes and early embryos, mitigating ROS, and increasing GSH levels.
As mitochondrial dysfunction can significantly contribute to increased ROS levels and hinder oocyte and embryo development, we used Mito-Tracker for MII-stage oocytes and JC-1 for 4-cell stage porcine embryos to assess their mitochondrial function.
The results show a significant increase in the functional mitochondrial fluorescence levels of MII-stage oocytes in the FMN treatment group compared to the control group (1.53 ± 0.46-fold higher; Figures 3A, B). In addition, the expression levels of mitochondrial function-related genes, ATP synthase F1 subunit beta (ATP5β) and transcription factor nuclear factor erythroid 2-related factor 2 (NRF2), were elevated in FMN treatment group (1.40 ± 0.06; 1.37 ± 0.19, respectively; Figure 3C).
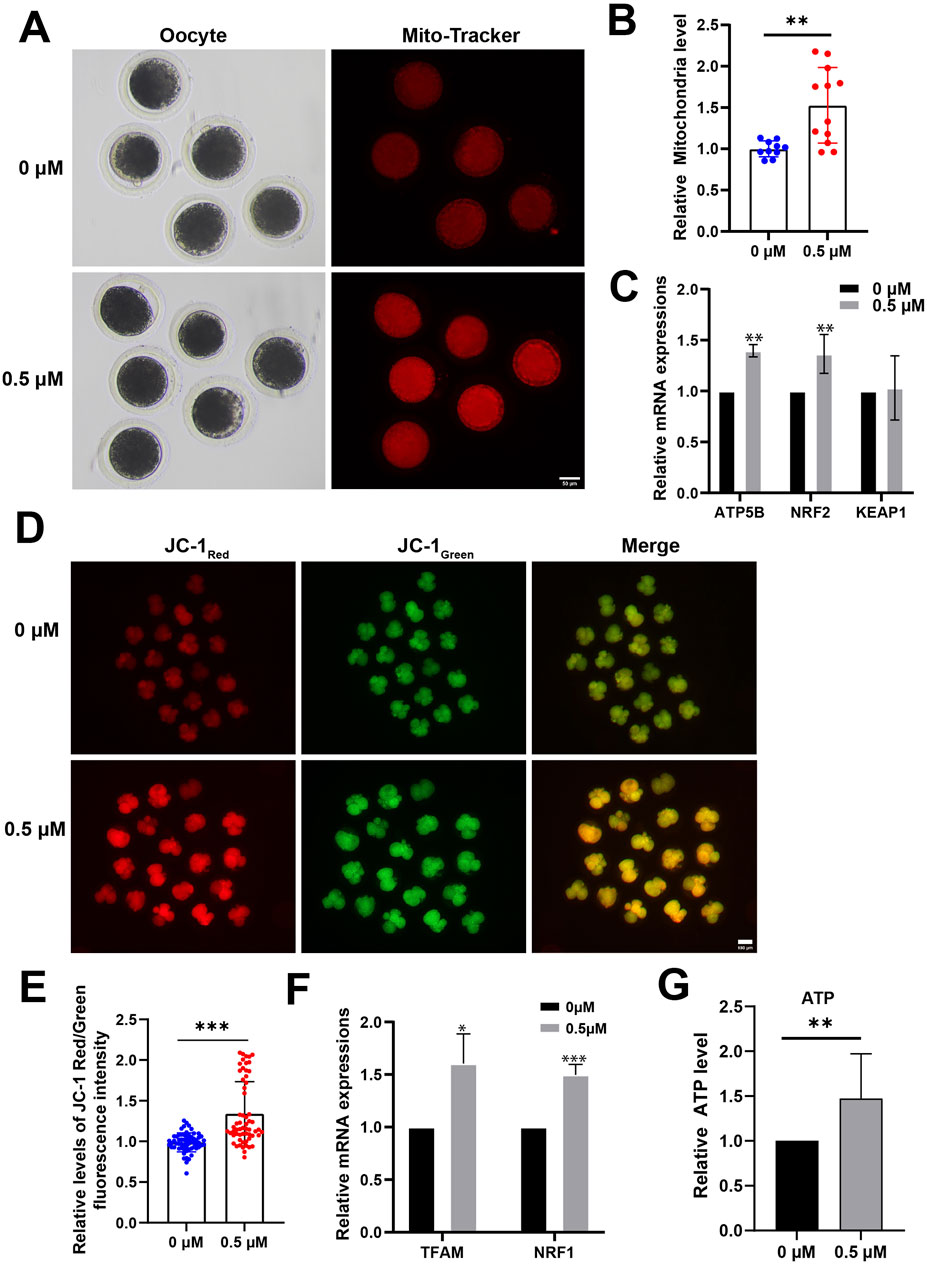
Figure 3. Effect of FMN on mitochondrial function in oocytes and early embryos. (A) The mitochondria distribution at MII stage were stained with Mito-Tracker Red; scale bar, 50 μm. (B) The relative abundance of mitochondria in oocyte were analyzed for control and FMN-treated group. (C) Relative mRNA levels of ATP5B, NRF2 and KEAP1 in oocytes. (D) 4-cell stage embryos stained with JC-1. Scale bar, 100 μm. (E) Relative levels of JC-1 Red/Green fluorescence intensity in embryos were analyzed for control and FMN-treated group. (F) Relative mRNA levels of TFAM and NRF1 in embryos. (G) ATP levels of 4-cell stage embryos in both control and FMN-treated groups. Data are presented as the mean ± standard deviation (SD). *p < 0.05,**p < 0.01, ***p < 0.001 vs. 0 μM FMN group.
In 4-cell stage embryos, comparison to the control group JC-1 Red/Green fluorescence intensity in FMN-treated embryos was 1.32 ± 5.20-fold higher (Figures 3D, E) and FMN significantly increased the MMP. In addition, the mRNA levels of mitochondrial biogenesis-related genes mitochondrial transcription factor A (TFAM), and nuclear respiratory factor 1 (NRF1) were elevated in the embryos treated with FMN (1.64 ± 0.28 and 1.50 ± 0.10, respectively; Figure 3F). In addition, the ATP levels of 4-cell stage embryos were measured. The results showed a 1.47 ± 0.20-fold increase in FMN-treated embryos compared to the control group (Figure 3G).
Next, we investigated how FMN regulates antioxidant activity and improves mitochondrial function in embryos. The transcription factor NF-E2P45-related factor 2 (Nrf2) has been reported to regulate oxidative damage in cells by expressing genes related to the oxidative stress response. It also protects mitochondrial function, thereby regulating cell adaptation and survival under stress conditions (Bellezza, 2018; He et al., 2020; Fang et al., 2020).
On the day 7, we collected blastocysts for immunofluorescence staining. The results revealed a significant 1.63 ± 15.10-fold enhancement in the immunofluorescence staining of Nrf2 in blastocysts from the FMN-treated group compared to the control group (Figures 4A, B). A recent study suggests that FMN disrupts the dissociation of Nrf2/Keap1 by selectively binding to Keap1. This disruption increases the nuclear expression of Nrf2, potentially addressing oxidative stress-induced peripheral neuropathy and mitochondrial dysfunction (Fang et al., 2020). In addition, we also evaluated the levels of the mRNA and protein associated with the Nrf2/Keap1 pathway. As expected, FMN significantly increased Nrf2 levels while decreasing Keap1 levels (Figures 4C, D).
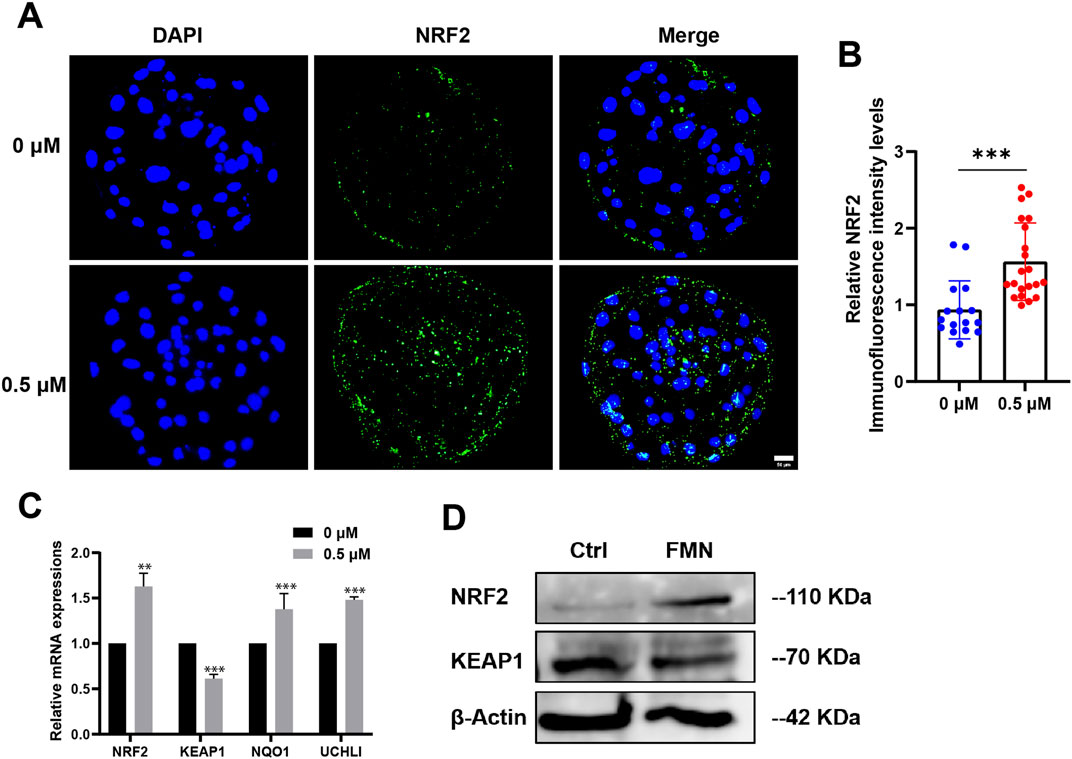
Figure 4. Effect of FMN on the expression levels of the Nrf2/Keap1 signaling pathway-related protein and genes in porcine blastocysts. (A) Porcine blastocysts incubated with or without FMN were stained with NRF2 (green) and DAPI (blue), scale bar, 50 μm. (B) Relative levels of NRF2 fluorescence intensity in blastocysts were analyzed for the control and FMN-treated group. (C) Relative mRNA expression levels of genes related to Nrf2/Keap1 pathway, NRF2, KEAP1, NQO1, UCHL1 in blastocysts. (D) Western blot analysis of NRF2 and KEAP1 protein expressions in blastocysts in the control and FMN-treated groups. Data were presented as the mean ± standard deviation (SD). **p < 0.01,***p < 0.001 vs. 0 μM FMN group.
An increasing number of studies have demonstrated crosstalk between the Nrf2 pathway and autophagy, and ROS stress generated by mitochondria can induce autophagy (Scherz-Shouval and Elazar, 2011; Bartolini et al., 2018). We collected blastocysts, examined the levels of the autophagy marker LC3B and assessed the expression of autophagy-related genes. After FMN treatment, the relative fluorescence intensity of LC-3B decreased significantly to 0.89 ± 0.11 (Figures 5A, B). The expression levels of autophagy-related genes ATG5, P62, BECLIN1 and LC3 were also reduced in the FMN-treated group (0.31 ± 0.18, 0.46 ± 0.03, 0.46 ± 0.07, and 0.38 ± 0.17, respectively; Figure 5C).
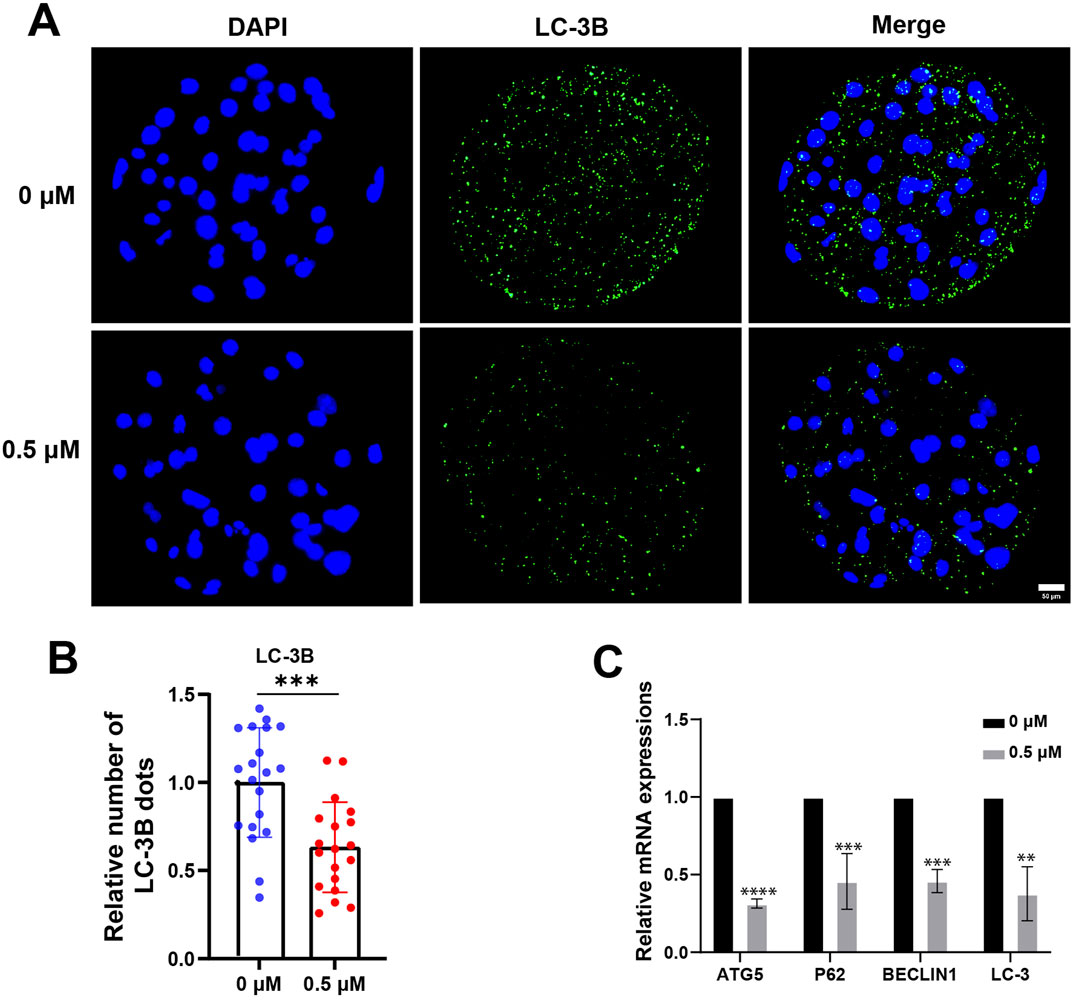
Figure 5. Effect of FMN on autophagy in porcine blastocysts. (A) Porcine blastocysts were stained with LC-3B (green) and DAPI (blue), scale bar, 50 μm. (B) Relative levels of LC-3B fluorescence intensity in blastocysts were analyzed for the control and FMN-treated group. (C) Relative mRNA expression levels of genes related to autophagy ATG5, P62, BECLIN1and LC-3 in blastocysts. Data were presented as the mean ± standard deviation (SD). **p < 0.01, ***p < 0.001, ****p < 0.0001 vs. 0 μM FMN group.
In summary, we assessed blastocyst quality by examining the proliferation and apoptosis of embryonic cells. On day 7, blastocysts were collected, proliferation and apoptosis of blastocysts were detected by EdU and TUNEL assay respectively. The proportions of EdU-positive nuclei in the control and FMN-treated groups were 31.68% ± 11.33% and 42.29% ± 12.85%, respectively (Figures 6A, B). The proportions of TUNEL-positive nuclei in the control and FMN-treated groups were 12.26% ± 4.03% and 3.99% ± 3.28%, respectively (Figures 6C, D). Additionally, FMN treatment also upregulated the expression of the anti-apoptotic gene BCL2 (1.61% ± 9.34%) but downregulated the expression of the apoptosis-promoting genes Caspase 3 (CASP3) and Caspase 8 (CASP8) (0.60 ± 0.11, 0.54 ± 0.43, respectively; (Figure 6E).
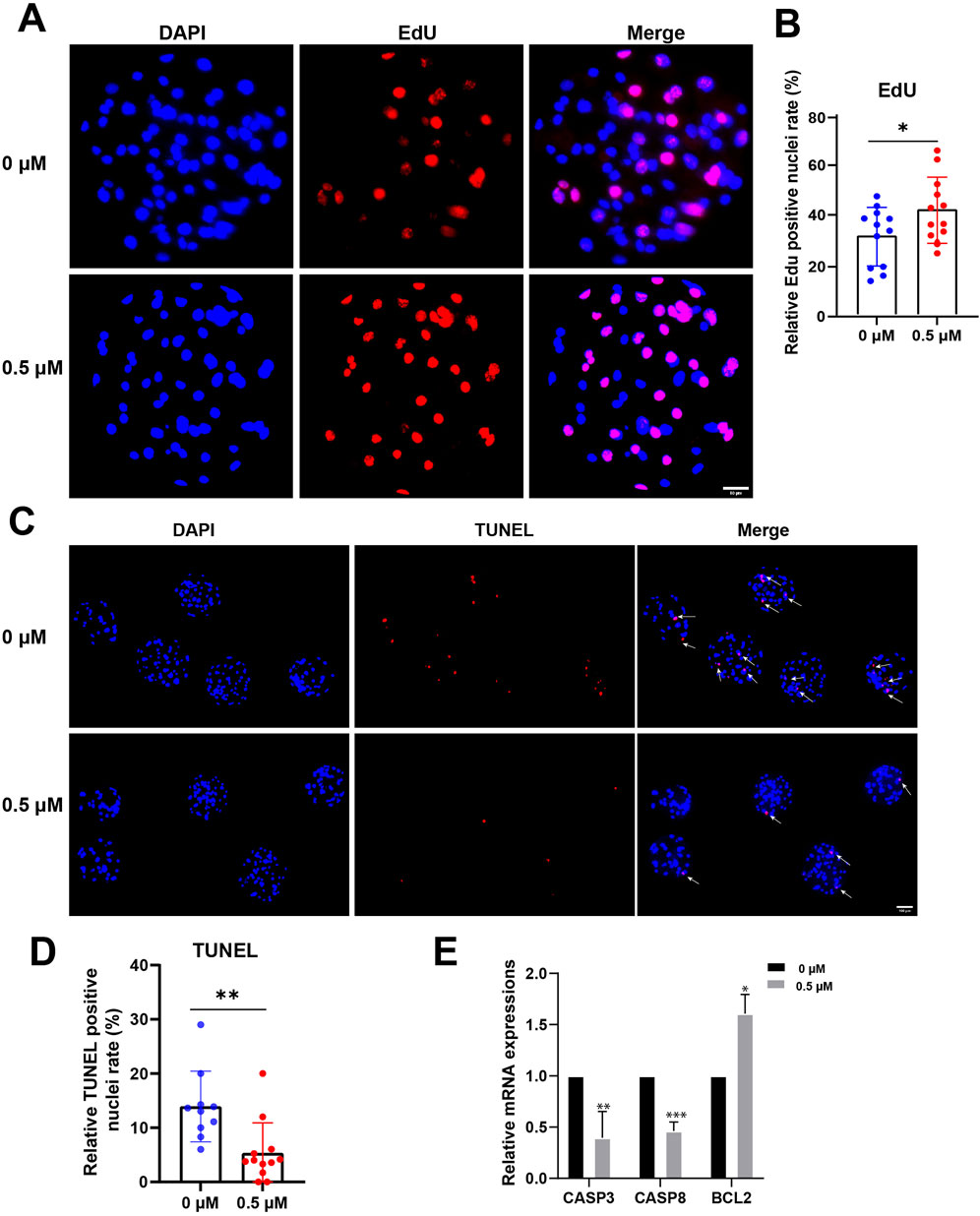
Figure 6. Effect of FMN on proliferation and apoptosis in early embryos. (A) Porcine blastocysts were stained with EdU (red) and DAPI (blue), scale bar, 50 μm. (B) The rate of EdU-positive cells in blastocysts with control and FMN-treated group. (C) Porcine blastocysts were stained with TUNEL (red) and DAPI (blue), scale bar, 100 μm. (D) The rate of TUNEL-positive nuclei in blastocysts with control and FMN-treated group. (E) Relative mRNA expression levels of CASP3, CASPS8, and BCL2 genes related to anti-apoptosis in blastocysts. Data were presented as the mean ± standard deviation (SD). *p < 0.05, **p < 0.01, ***p < 0.001 vs. 0 μM FMN group.
In this study, we confirmed that FMN significantly increased the polar body extrusion of oocytes and early embryo development in vitro. FMN is a flavonoid that can be found in herbal dietary supplements like Astragalus and safflower (Machado Dutra et al., 2021). Natural flavonoid compounds, due to their antioxidant activity, have drawn extensive attention for their role in promoting oocyte development. Similar studies have shown that quercetin (Kang et al., 2016; Kang et al., 2013), kaempferol (Zhao et al., 2020), and isorhamnetin (Li et al., 2024) promoted porcine oocyte maturation in vitro by reducing oxidative stress and improving mitochondrial functions or increased the cleavage and blastocyst rate of porcine embryos through antioxidant activity. Indicating that the promotion of oocyte maturation and embryo development by FMN may be related to its antioxidant capacity.
The cumulus cells (CCs) support oocyte maturation, and the expansion of CCs contributes to the growth and maturation of the oocyte within the follicle (Turathum et al., 2021). Our study found that FMN effectively promoted the expansion of CCs by increasing the expression of oocyte expansion-related genes, including PTGS2, PTGS1, and CD44. In addition, we also observed an increase in the first polar body extrusion rate (Figure 1). Furthermore, the addition of FMN during the IVC stage significantly enhances the rate of blastocyst formation. Previous studies have indicated that FMN has antioxidant properties, which can effectively eliminate free radicals (Mu et al., 2009). Our study found that during the oocyte period, the expression levels of oocyte redox-related genes, including PGC1α, PRDX2, GPX, and CAT, were significantly higher than those of the control group. Different from the IVC stage, during the embryonic period, the expression levels of SOD1, SOD2, and the oxidative stress-related gene NOX2 were higher. In both periods, FMN significantly reduced ROS and increased GSH levels. Taken together, FMN is capable of exerting antioxidant effects to promote the development of porcine oocytes and embryos in vitro.
Mitochondria are the primary site of ROS production in cells and play a crucial role in the oxidative-reductive balance of oocytes and embryos. The physiological processes of oocyte development and early embryo development are notably complex and energy-intensive events (Zhao et al., 2022). Our research showed that, during the oocyte stage, mitochondrial abundance was higher after FMN treatment compared to controls. In the embryonic stage, FMN treatment additionally enhanced mitochondrial membrane potential (MMP) and increased ATP levels (Figure 3). FMN may increase mitochondrial abundance through its antioxidant effect and alleviate the damage of oxidative stress to the mitochondrial membrane, thus stabilizing the membrane potential. Meanwhile, the increase in ATP level directly provides the necessary energy guarantee for the rapid division and differentiation of embryos.
Previous studies have shown that FMN can bind to Keap1 and then change the activity of Nrf2, prompting the dissociation of the Nrf2/Keap1 complex. This enables Nrf2 to translocate to the nucleus, initiate antioxidant components and enhance mitochondrial function (Chen et al., 2023; Yang et al., 2023). In mammals, the Nrf2-Keap1 system serves as an important anti-stress mechanism. Under oxidative stress, Nrf2 is released from Keap1, preventing its ubiquitination and degradation. This leads to the upregulation of antioxidant-related components, enhancement of mitochondrial function, and maintenance of physiological homeostasis (Zhang et al., 2020; Zhang et al., 2013; Fang et al., 2024). Our study found FMN can similarly regulate the Nrf2/Keap1 pathway during the embryonic period. Our research reveals that the expression of Nrf2 is upregulated while that of Keap1 is downregulated under the treatment of FMN. FMN facilitates the nuclear expression of Nrf2, which is confirmed by immunofluorescence and Western blot (Figure 4). Furthermore, at the gene level, FMN also downregulated KEAP1 gene expression and increase NRF2 gene expression. The downstream genes, including NQO-1, SOD1, and SOD2 significant upregulate in response. So we predicted that FMN exerts antioxidant effects to promote embryonic development through the Nrf2/Keap1 pathway.
The activation and modulation of Nrf2 constitute a complex and multifaceted process. Experimental investigations have evinced the crosstalk existing between autophagy and the Nrf2/Keap1 signaling pathway. The adaptor protein P62 can compete with Nrf2 for binding to Keap1, thereby facilitating Nrf2 activation. This activation occurs in response to the reduction of peroxidase activity and the suppression of excessive autophagy as well as ROS generation (Jain et al., 2010; Ichimura et al., 2013; Kong et al., 2021). We examined the impact of FMN on autophagy-related genes in both oocytes and embryos. At the embryonic stage, FMN markedly reduced the protein level of the autophagy marker LC3 (Figure 5). Additionally, it led to a significant decrease in the expression of autophagy-associated genes such as P62, LC3, BECLIN1, and ATG5 (Figure 5). The regulation of oxidative stress levels and autophagy is a dynamically changing process. In this dynamic process, the regulation of the Keap1-Nrf2 pathway by FMN downregulates the levels of oxidative stress and autophagy, thereby promoting the development of oocytes and embryos (Figure 7).

Figure 7. Summary of the effect of Formononetin (FMN) on porcine oocyte in vitro maturation and early embryo in vitro. This schematic view illustrates that during the IVM stage, FMN enhances oocyte maturation in vitro by up-regulating genes related to CCs expansion and antioxidants, reducing oxidative stress, and improving mitochondrial function. During the IVC stage, FMN promotes the nuclear expression of Nrf2 through the P62-Keap1-Nrf2 pathway, which exerts antioxidant function and enhances mitochondrial function. Then, FMN promotes cell proliferation, reduces apoptosis, and lowers autophagy levels.
In conclusion, adding the same concentration of FMN to the oocyte and embryo culture media respectively enhanced development and exerted antioxidant effects. Moreover, FMN’s antioxidant mechanism varies between the oocyte and embryonic stages. The potential relationship between such differences in these two phases, like the level of autophagy, requires further exploration.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because Porcine ovaries used in this study were obtained from a slaughterhouse (Jiangmen, China). They were not required to be screened by the Institutional Animal Care and Use Committee (IACUC) because our university does not regard the use of these ovaries as an animal experiment.
NW: Data curation, Formal Analysis, Investigation, Validation, Writing–original draft, Writing–review and editing. HY: Investigation, Software, Writing–original draft. YeC: Investigation, Software, Writing–original draft. HW: Supervision, Validation, Writing–original draft. CW: Investigation, Methodology, Software, Writing–original draft. JF: Funding acquisition, Project administration, Supervision, Writing–review and editing. YaC: Funding acquisition, Project administration, Supervision, Writing–review and editing. YL: Methodology, Project administration, Resources, Supervision, Writing–review and editing. MZ: Conceptualization, Formal Analysis, Funding acquisition, Resources, Supervision, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by the Science and Technology Planning Project of Guangdong Province (2021A1515110916), Foundation and Application Foundation project support by Jiangmen City (2220002000296, 2021030103170007301), Innovation team program supported by Guangdong Province (2020KCXTD038), Guangdong Province Key Program of Discipline (2021ZDJS091), National Key Research and Development Program of China Stem Cell and Translational Research (2022YFA1105403).
We would like to acknowledge the lab workers who collected the porcine ovary. We appreciate the opinions and critical reading of the manuscript provided by each group member.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2024.1520429/full#supplementary-material
Agarwal, A., Gupta, S., and Sharma, R. K. (2005). Role of oxidative stress in female reproduction. Reproductive Biol. Endocrinol. RB&E 3, 28. doi:10.1186/1477-7827-3-28
Agarwal, A., Said, T. M., Bedaiwy, M. A., Banerjee, J., and Alvarez, J. G. (2006). Oxidative stress in an assisted reproductive techniques setting. Fertil. Steril. 86 (3), 503–512. doi:10.1016/j.fertnstert.2006.02.088
Amin, A., Gad, A., Salilew-Wondim, D., Prastowo, S., Held, E., Hoelker, M., et al. (2014). Bovine embryo survival under oxidative-stress conditions is associated with activity of the NRF2-mediated oxidative-stress-response pathway. Mol. reproduction Dev. 81 (6), 497–513. doi:10.1002/mrd.22316
Bartolini, D., Dallaglio, K., Torquato, P., Piroddi, M., and Galli, F. (2018). Nrf2-p62 autophagy pathway and its response to oxidative stress in hepatocellular carcinoma. Transl. Res. J. laboratory Clin. Med. 193, 54–71. doi:10.1016/j.trsl.2017.11.007
Bellezza, I. (2018). Oxidative stress in age-related macular degeneration: Nrf2 as therapeutic target. Front. Pharmacol. 9, 1280. doi:10.3389/fphar.2018.01280
Bennemann, J., Grothmann, H., and Wrenzycki, C. (2018). Reduced oxygen concentration during in vitro oocyte maturation alters global DNA methylation in the maternal pronucleus of subsequent zygotes in cattle. Mol. reproduction Dev. 85 (11), 849–857. doi:10.1002/mrd.23073
Chen, H., Lou, Y., Lin, S., Tan, X., Zheng, Y., Yu, H., et al. (2023). Formononetin, a bioactive isoflavonoid constituent from Astragalus membranaceus (Fisch.) Bunge, ameliorates type 1 diabetes mellitus via activation of Keap1/Nrf2 signaling pathway: an integrated study supported by network pharmacology and experimental validation. J. Ethnopharmacol. 322, 117576. doi:10.1016/j.jep.2023.117576
Chen, S. M., Tsai, Y. S., Lee, S. W., Liu, Y. H., Liao, S. K., Chang, W. W., et al. (2014). Astragalus membranaceus modulates Th1/2 immune balance and activates PPARγ in a murine asthma model. Biochem. cell Biol. = Biochimie Biol. Cell. 92 (5), 397–405. doi:10.1139/bcb-2014-0008
Ciray, H. N., Aksoy, T., Yaramanci, K., Karayaka, I., and Bahceci, M. (2009). In vitro culture under physiologic oxygen concentration improves blastocyst yield and quality: a prospective randomized survey on sibling oocytes. Fertil. Steril. 91 (4), 1459–1461. doi:10.1016/j.fertnstert.2008.07.1707
Dornstauder, E., Jisa, E., Unterrieder, I., Krenn, L., Kubelka, W., and Jungbauer, A. (2001). Estrogenic activity of two standardized red clover extracts (Menoflavon) intended for large scale use in hormone replacement therapy. J. steroid Biochem. Mol. Biol. 78 (1), 67–75. doi:10.1016/s0960-0760(01)00075-9
Fan, M., Li, Z., Hu, M., Zhao, H., Wang, T., Jia, Y., et al. (2022). Formononetin attenuates Aβ25-35-induced adhesion molecules in HBMECs via Nrf2 activation. Brain Res. Bull. 183, 162–171. doi:10.1016/j.brainresbull.2022.03.009
Fang, Y., Ye, J., Zhao, B., Sun, J., Gu, N., Chen, X., et al. (2020). Formononetin ameliorates oxaliplatin-induced peripheral neuropathy via the KEAP1-NRF2-GSTP1 axis. Redox Biol. 36, 101677. doi:10.1016/j.redox.2020.101677
Fang, Y., Zheng, Y., Gao, Q., Pang, M., Wu, Y., Feng, X., et al. (2024). Activation of the Nrf2/Keap1 signaling pathway mediates the neuroprotective effect of Perillyl alcohol against cerebral hypoxic-ischemic damage in neonatal rats. Redox Rep. Commun. free Radic. Res. 29 (1), 2394714. doi:10.1080/13510002.2024.2394714
Gil, M. A., Cuello, C., Parrilla, I., Vazquez, J. M., Roca, J., and Martinez, E. A. (2010). Advances in swine in vitro embryo production technologies. Reproduction Domest. Animals 45, 40–48. doi:10.1111/j.1439-0531.2010.01623.x
Grupen, C. G. (2014). The evolution of porcine embryo in vitro production. Theriogenology 81 (1), 24–37. doi:10.1016/j.theriogenology.2013.09.022
He, F., Ru, X., and Wen, T. (2020). NRF2, a transcription factor for stress response and beyond. Int. J. Mol. Sci. 21 (13), 4777. doi:10.3390/ijms21134777
Ichimura, Y., Waguri, S., Sou, Y. S., Kageyama, S., Hasegawa, J., Ishimura, R., et al. (2013). Phosphorylation of p62 activates the Keap1-Nrf2 pathway during selective autophagy. Mol. cell 51 (5), 618–631. doi:10.1016/j.molcel.2013.08.003
Jain, A., Lamark, T., Sjøttem, E., Larsen, K. B., Awuh, J. A., Øvervatn, A., et al. (2010). p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J. Biol. Chem. 285 (29), 22576–22591. doi:10.1074/jbc.M110.118976
Kang, J. T., Kwon, D. K., Park, S. J., Kim, S. J., Moon, J. H., Koo, O. J., et al. (2013). Quercetin improves the in vitro development of porcine oocytes by decreasing reactive oxygen species levels. J. veterinary Sci. 14 (1), 15–20. doi:10.4142/jvs.2013.14.1.15
Kang, J. T., Moon, J. H., Choi, J. Y., Park, S. J., Kim, S. J., Saadeldin, I. M., et al. (2016). Effect of antioxidant flavonoids (quercetin and taxifolin) on in vitro maturation of porcine oocytes. Asian-Australasian J. animal Sci. 29 (3), 352–358. doi:10.5713/ajas.15.0341
Kong, L., Deng, J., Zhou, X., Cai, B., Zhang, B., Chen, X., et al. (2021). Sitagliptin activates the p62-Keap1-Nrf2 signalling pathway to alleviate oxidative stress and excessive autophagy in severe acute pancreatitis-related acute lung injury. Cell death and Dis. 12 (10), 928. doi:10.1038/s41419-021-04227-0
Kulling, S. E., Lehmann, L., and Metzler, M. (2002). Oxidative metabolism and genotoxic potential of major isoflavone phytoestrogens. J. Chromatogr. B, Anal. Technol. Biomed. life Sci. 777 (1-2), 211–218. doi:10.1016/s1570-0232(02)00215-5
Lee, J., Lee, H., Lee, Y., Park, B., Elahi, F., Lee, S. T., et al. (2017). In vitro oocyte maturation in a medium containing reduced sodium chloride improves the developmental competence of pig oocytes after parthenogenesis and somatic cell nuclear transfer. Reproduction, Fertil. Dev. 29 (8), 1625–1634. doi:10.1071/RD15488
Li, L., Wang, Y., Wang, X., Tao, Y., Bao, K., Hua, Y., et al. (2018). Formononetin attenuated allergic diseases through inhibition of epithelial-derived cytokines by regulating E-cadherin. Clin. Immunol. Orl. Fla 195, 67–76. doi:10.1016/j.clim.2018.07.018
Li, R., Liu, Y., Pedersen, H. S., and Callesen, H. (2015). Effect of ambient light exposure of media and embryos on development and quality of porcine parthenogenetically activated embryos. Zygote 23 (3), 378–383. doi:10.1017/S096719941300066X
Li, X., Cheng, J., Yao, Q., Duan, J., Chen, H., Zhang, Z., et al. (2024). Isorhamnetin improves oocyte maturation by activating the pi3k/akt signaling pathway. Mol. Nutr. and food Res. 68, e2300904. doi:10.1002/mnfr.202300904
Li, Y., Liu, H., Wu, K., Liu, H., Huang, T., Chen, Z. J., et al. (2019). Melatonin promotes human oocyte maturation and early embryo development by enhancing clathrin-mediated endocytosis. J. pineal Res. 67 (3), e12601. doi:10.1111/jpi.12601
Liu, R. P., Wang, J., Wang, X. Q., Wang, C. R., He, S. Y., Xu, Y. N., et al. (2023). Xanthoangelol promotes early embryonic development of porcine embryos by relieving endoplasmic reticulum stress and enhancing mitochondrial function. Reprod. Biomed. online 47 (2), 103211. doi:10.1016/j.rbmo.2023.04.002
Machado Dutra, J., Espitia, P. J. P., and Andrade Batista, R. (2021). Formononetin: biological effects and uses - a review. Food Chem. 359, 129975. doi:10.1016/j.foodchem.2021.129975
Mu, H., Bai, Y. H., Wang, S. T., Zhu, Z. M., and Zhang, Y. W. (2009). Research on antioxidant effects and estrogenic effect of formononetin from Trifolium pratense (red clover). Phytomedicine Int. J. phytotherapy Phytopharm. 16 (4), 314–319. doi:10.1016/j.phymed.2008.07.005
Scherz-Shouval, R., and Elazar, Z. (2011). Regulation of autophagy by ROS: physiology and pathology. Trends Biochem. Sci. 36 (1), 30–38. doi:10.1016/j.tibs.2010.07.007
Sun, T., Liu, R., and Cao, Y. X. (2011). Vasorelaxant and antihypertensive effects of formononetin through endothelium-dependent and -independent mechanisms. Acta Pharmacol. Sin. 32 (8), 1009–1018. doi:10.1038/aps.2011.51
Tian, Z., Liu, S. B., Wang, Y. C., Li, X. Q., Zheng, L. H., and Zhao, M. G. (2013). Neuroprotective effects of formononetin against NMDA-induced apoptosis in cortical neurons. Phytotherapy Res. PTR 27 (12), 1770–1775. doi:10.1002/ptr.4928
Turathum, B., Gao, E. M., and Chian, R. C. (2021). The function of cumulus cells in oocyte growth and maturation and in subsequent ovulation and fertilization. Cells 10 (9), 2292. doi:10.3390/cells10092292
Yang, J., Sha, X., Wu, D., Wu, B., Pan, X., Pan, L. L., et al. (2023). Formononetin alleviates acute pancreatitis by reducing oxidative stress and modulating intestinal barrier. Chin. Med. 18 (1), 78. doi:10.1186/s13020-023-00773-1
Zhang, M., An, C., Gao, Y., Leak, R. K., Chen, J., and Zhang, F. (2013). Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog. Neurobiol. 100, 30–47. doi:10.1016/j.pneurobio.2012.09.003
Zhang, Y., Ma, Y., Zhao, C., Zhang, H., Pu, Y., and Yin, L. (2020). Synergistic carcinogenesis of HPV18 and MNNG in het-1A cells through p62-KEAP1-NRF2 and PI3K/AKT/mTOR pathway. Oxidative Med. Cell. Longev. 2020, 6352876. doi:10.1155/2020/6352876
Zhao, S., Heng, N., Wang, H., Wang, H., Zhang, H., Gong, J., et al. (2022). Mitofusins: from mitochondria to fertility. Cell. Mol. life Sci. CMLS 79 (7), 370. doi:10.1007/s00018-022-04386-z
Keywords: formononetin, mitochondrial function, oxidative stress, Nrf2/Keap1, autophagy
Citation: Wang N, Yang H, Chen Y, Wang H, Wang C, Fan J, Chen Y, Li Y and Zhu M (2025) Formononetin promotes porcine oocytes maturation and improves embryonic development by reducing oxidative stress. Front. Cell Dev. Biol. 12:1520429. doi: 10.3389/fcell.2024.1520429
Received: 31 October 2024; Accepted: 26 December 2024;
Published: 09 January 2025.
Edited by:
Xiangpeng Dai, Jilin University, ChinaReviewed by:
Yingzheng Wang, Cornell University, United StatesCopyright © 2025 Wang, Yang, Chen, Wang, Wang, Fan, Chen, Li and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yajie Chen, ajAwMjY3NkB3eXUuZWR1LmNu; Yinghua Li, eWhsaUB3eXUuZWR1LmNu; Maobi Zhu, ajAwMjY3NUB3eXUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.