- 1Child Healthcare Department, The Fourth Affiliated Hospital of Jiangsu University, Zhenjiang, China
- 2Jiangsu Key Laboratory of Medical Science and Laboratory Medicine, School of Medicine, Jiangsu University, Zhenjiang, China
Cancer has become an important public health problem worldwide, and there is currently a lack of effective treatment and prevention strategies. Natural plant active ingredients have been proven to be a safe and highly promising method for preventing and treating cancer. It has been found that diallyl trisulfide have anticancer effects in multiple types of cancer via inhibiting cancer proliferation, enhancing chemotherapy sensitivity, inducing apoptosis/autophagy, suppressing invasion/migration, regulating microenvironment. With the deepening of research on new strategies for cancer prevention and treatment, the role of diallyl trisulfides in cancers occurrence, prognosis, and drug resistance is also receiving increasing attention. In order to better understand the relationship between diallyl trisulfides and various cancer, as well as the role and mechanism of diallyl trisulfides in cancer prevention and treatment, we briefly summarized the role and function of diallyl trisulfide in cancers.
1 Introduction
Because of its high incidence rate and mortality, cancer has gradually become a serious public health problem endangering life and health worldwide (Siegel et al., 2024). Despite the continuous advancement of cancer diagnosis and treatment technology, the effective rate of cancer treatment is still very low, resulting in a high recurrence rate and poor prognosis of cancer. Therefore, it is necessary to search for new interventions or treatment strategies for cancer. The development of new anti-cancer drugs is extremely challenging and time-consuming. On the other hand, due to the serious side effects of treatment methods such as chemotherapy and radiation therapy, it increases the burden and makes the treatment of cancer patients more challenging (Sohel et al., 2022). More and more evidence suggest that exercise and changing dietary habits may effectively prevent and play an important role in tumor treatment. Over the years, natural products have become increasingly popular in clinical settings. Natural products, such as plants, animals, and microorganisms, account for approximately 75% of clinical use of anticancer drugs, and have been used in medicine for decades, typically as supplements and substitutes (Sohel et al., 2022; Newman and Cragg, 2016). Among numerous natural products and dietary factors, phytochemistry is gradually showing their importance in anti-tumor research because they have many advantages such as wide sources, excellent anti-cancer effects, high safety, and ease of application.
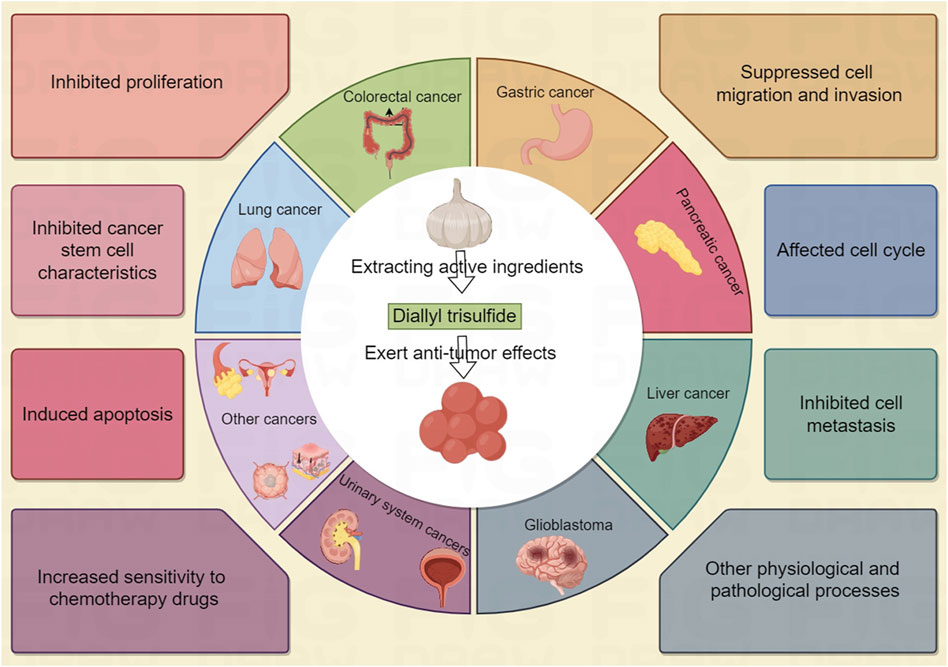
Currently, phytochemicals are the main candidate drugs most suitable for developing anti-cancer drugs (Malla et al., 2022). Studies have shown that natural products such as Diallyl trisulfide (DATS) selectively act on cancer cells by targeting multiple pathways related to cancers, with minimal impact on normal cells (Malla et al., 2022; Khan and Uddin, 2021). Allium plants, such as garlic and onions, have long been considered to have medicinal value (Puccinelli and Stan, 2017; Zhang et al., 2024). Many studies have shown that DATS is a biologically active organic sulfur compound found in garlic, which can regulate disease states such as cancer, infection, metabolic syndrome and various other diseases (Zhang et al., 2024; Rauf et al., 2022; Mondal et al., 2022). In this review, we summarize the role and molecular mechanisms of DATS in cancer treatment and prevention (Figure 1), and point out the shortcomings of existing research and challenges for future clinical applications.
2 The interventional effect and mechanism of DATS in cancer
2.1 DATS intervention in breast cancer
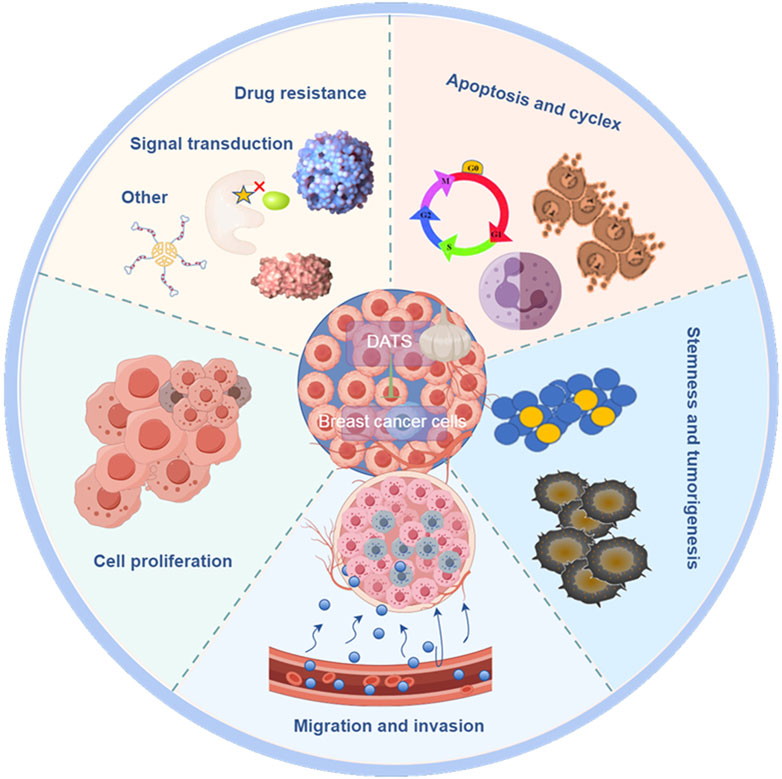
Breast cancer is one of the major causes of cancer related deaths among women worldwide (Malla et al., 2022). In this section, we discussed the potential anticancer activity of DATS in breast cancer, focusing on the intervention and treatment on proliferation, invasion, drug resistance cell cycle arrest, apoptosis, stem cell specificity and other aspects of breast cancer (Table 1; Figure 2). Understanding the anticancer activities of DATS provides insights into their potential in targeting the mechanism of occurrence and development of breast cancer.
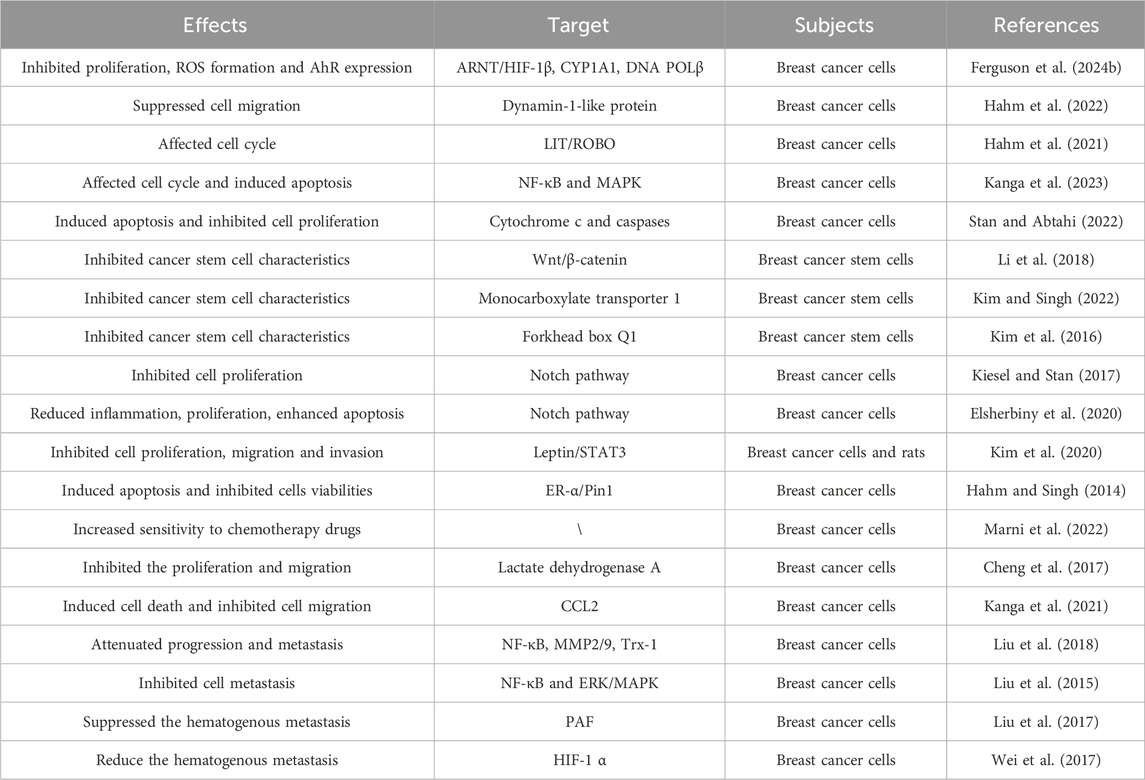
DATS inhibited proliferation, ROS formation, 8-OHdG levels, AhR expression and clonogenic formation in breast cancer cells compared to B [a] P group (Ferguson et al., 2024a). Diallyl trisulfide plays an anti-cancer role by reducing B [a] P induced oxidative stress, AhR expression and DNA damage in human breast cancer epithelial cells (Ferguson et al., 2024b). These results provided evidence for the intervention of DATS in breast cancer. Hahm et al. (2022) research found that actin cytoskeleton is a new target of DATS in SK-BR-3 cells, which may explain its inhibitory effect on migration of breast cancer cells. As is well known, cell cycle arrest plays a crucial role in the research of anti-tumor drugs. DATS regulated LIT/ROBO tumor suppressor signal related genes, thereby affecting cell cycle to play the role of anti-breast cancer (Hahm et al., 2021). DATS regulated cell cycle arrest, influenced caspase activity, induced apoptosis of breast cancer cells to play an intervention role (Kanga et al., 2023). Stan et al. showed that DATS inhibited proliferation and induced apoptosis of breast cancer cells, suggesting that DATS may be a potential cancer preventive agent (Stan and Abtahi, 2022). Breast cancer stem cells play an important role in the regulation of breast cancer. The research results of Li et al. (2018) indicate that DATS inhibits the Wnt/β-catenin pathway to inhibit the activities of breast cancer stem cells may provide a new strategy for the prevention of breast cancer. Kim and Singh (2022) also found that DATS suppress stem cell stemness of breast cancer and monocarboxylate transporter 1 can be a new target. These results of another study indicated that forkhead box Q1 is a new target for DATS to inhibit the stemness of breast cancer stem cells (Kim et al., 2016). DATS inhibits the expression of ADAM10 and ADAM17 by regulating Notch signaling pathway, and plays an intervention and therapeutic role in breast cancer (Kiesel and Stan, 2017). Elsherbiny et al. (2020) pointed out that the anti-cancer effect of DATS is mediated by inhibiting Notch signaling protein, reducing tumor inflammation, proliferation, and enhancing cell apoptosis. DATS suppressed leptin induced tumor signaling in breast cancer cells, thereby inhibiting proliferation, survival, migration and invasion of cells (Kim et al., 2020). DATS could induce apoptosis and inhibit cells viabilities by inhibiting estrogen receptor-α activity of breast cancer cells (Hahm and Singh, 2014). These data of this research indicated that DATS may be a safer alternative treatment scheme targeting estrogen receptor-α, and it has great application prospects in the prevention of breast cancer. DATS inhibited the cell proliferation of PTX resistant breast cancer cells by blocking cell cycle and inducing apoptosis, thereby affecting the drug sensing effect of breast cancer cells (Marni et al., 2022). DATS has an inhibitory effect on the Warburg effect in breast cancer cells, but has no significant effect on normal cells. DATS inhibits the cell proliferation and migration by downregulating the activity of lactate dehydrogenase A in breast cancer cells (Cheng et al., 2017). Warburg effect plays a crucial role in the occurrence of breast cancer and other cancers. Exploring specific drugs such as DATS targeting key proteins in the Warburg effect, may be a very promising strategy for treating cancer. Migration and invasion are two key steps of cancer metastasis, including breast cancer. Blocking the migration and invasion of cancer cells may be an effective strategy to reduce the risk of breast cancer, especially triple negative breast cancer. DATS reduced the expression of CCL2 in TNF-α-treated MDA-MB-231 cells, inducing cell death and inhibiting cell migration (Kanga et al., 2021). It is reported that DATS targeting Trx-1 system inhibits metastasis in nude mice inoculated with breast cancer cells, which may provide a promising strategy for the treatment of triple negative metastasis (Liu et al., 2018). Liu et al. showed that DATS inhibits the metastasis of triple negative breast cancer by suppressing NF-κB and ERK/MAPK pathways (Liu et al., 2015). It is found that DATS suppressed the hematogenous metastasis of MDA-MB-231 cells in the presence of platelets activated by PAF (Liu et al., 2017). DATS could also inhibit HIF-1 α synthesis to reduce the hematogenous metastasis of MDA-MB-231 cells (Wei et al., 2017). The hydrophobicity, short half-life, lack of target selectivity and limited bioavailability of DATS at tumor sites limit its efficacy in the treatment of breast cancer, especially triple negative breast cancer. In order to overcome these limitations, researchers have developed folate coupled DATS SLN, which improves the intervention effect of DATS on breast cancer (De et al., 2023; Hahm and Singh, 2022).
As a natural phytochemical, DATS is expected to become a new prevention and treatment strategy for breast cancer, especially triple negative breast cancer, which has great clinical application prospects. DATS play an intervention and therapeutic role in the proliferation, migration, invasion, drug resistance, cell cycle arrest, apoptosis, stem cell specificity and other aspects of breast cancer.
2.2 The intervention effect of DATS in gastric and colorectal cancer
Gastric cancer and colorectal cancer are the leading malignant tumors in terms of incidence rate and mortality worldwide. The stomach and colon are the main sites for the digestion and metabolism of phytochemicals such as DATS, so DATS may have good preventive and therapeutic effects on gastric and colorectal cancer (Wang et al., 2022). In this section, we discussed the potential anticancer activity of DATS in gastric cancer and colorectal cancer, focusing on the intervention and treatment on proliferation, recurrence, invasion cancer stem properties and drug resistance of breast cancer (Table 2). These are of great reference value for the search for new prevention and treatment strategies for digestive system cancers such as gastric cancer and colorectal cancer.
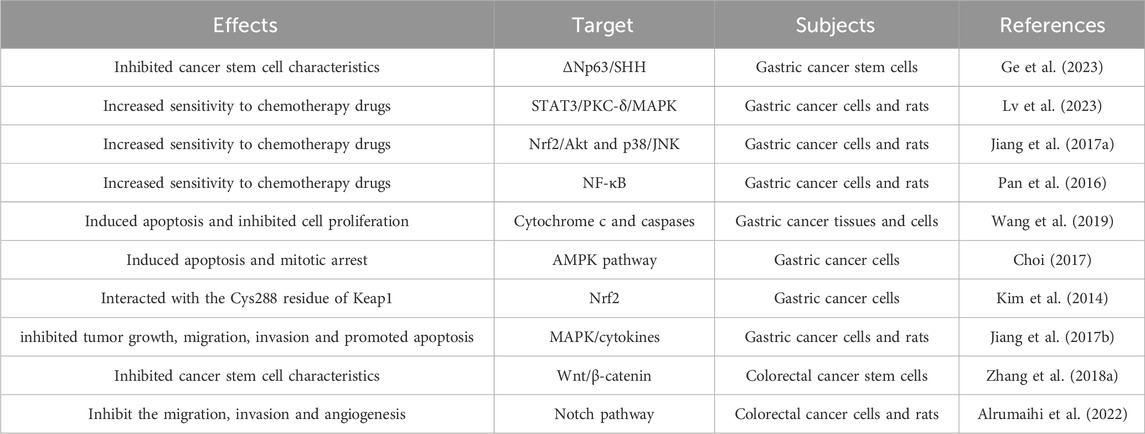
Gastric cancer is the common malignant cancer of the digestive system, and most patients are already in the advanced stage when diagnosed. Prevention and effective treatment strategies are particularly important. Gastric cancer stem cells play a crucial role in the progression, recurrence, and drug resistance of gastric cancer. Reported by Ge et al. (2023) that DATS inhibited the properties of gastric cancer stem cells via ΔNp63/SHH axis, exerting an intervention effect in gastric cancer and providing strategies for the prevention and treatment of gastric cancer. The research results indicate that the combination of DATS and cisplatin regulates endoplasmic reticulum stress and inhibits STAT3/PKC-δ collaborate with the MAPK pathway to enhance anti-tumor activity (Lv et al., 2023). DATS suppressed gastric cancer growth and enhanced the efficacy of cisplatin by inhibiting Nrf2/Akt and activating p38/JNK pathway (Jiang X. Y. et al., 2017). Epigenetic upregulation of metallothionein 2A by DATS through inhibition of NF-κB activation and enhancement the chemical sensitivity of gastric cancer cells to paclitaxel (Pan et al., 2016). Resistance to chemotherapy drugs is a very challenging task in cancer treatment. DATS can enhance the therapeutic effect of cisplatin, cisplatin, docetaxel and reduce cell toxicity, making it an ideal potential treatment option for gastric cancer patients. Sulfidoxine is a newly discovered antioxidant enzyme that is overexpressed in various cancers and may promote the occurrence and progress of gastric cancer. After treatment with DATS, the expression of sulfidoxine, MDA level, and ROS level in BGC823 cells were significantly inhibited, and DATS may exert anti-cancer effects by regulating sulfidoxine (Wang et al., 2019). It is reported that DATS induces gastric cancer cells apoptosis and mitotic arrest through activation of AMP activated protein kinase mediated by reactive oxygen species (Choi, 2017). These findings by Kim et al. (2014) suggested that DATS can interact with the Cys288 residue of Keap1, which to some extent explains its ability to induce Nrf2 activation and upregulate defense gene expression, playing an intervention role in gastric cancer. Jiang et al. (2017b) demonstrated that DATS exerts anti-gastric cancer effects in SGC-7901 tumor bearing mice by activating the MAPK pathway and regulating cytokines.
More and more evidence show that DATS has excellent intervention and therapeutic effects in multiple processes and pathological processes of gastric cancer occurrence and development, providing new strategies for early prevention and treatment of gastric cancer and enhancing chemotherapy efficacy.
Colorectal cancer is the third most common malignant cancer worldwide, with a high mortality rate due to treatment failure and cancer recurrence, with a 5 years relative survival rate of only 8%. Exploring the role of phytochemicals such as DATS in the intervention and treatment of colorectal cancer can provide new evidence for clinical prevention and treatment (Oravetz et al., 2023). Colorectal cancer stem cells play a crucial role in the initiation, development and metastasis of colorectal cancer (Liao et al., 2023). The results of Zhang et al. demonstrated that Wnt/β-catenin pathway mediates the inhibitory effect of DATS on colorectal cancer stem cells, playing an intervention and therapeutic role in colorectal cancer (Zhang Q. et al., 2018). Report has shown that DATS can inhibit the angiogenesis, migration, invasion of colon cancer cells, and can also inhibit the growth of transplanted tumors in nude mice. In order to enhance the anti-colorectal cancer effect of DATS, researchers constructed a lipid-based nanoparticle normula of DATS, which improved the anti-colorectal cancer effect of DATS (Alrumaihi et al., 2022). In summary, the above research results indicate that DATS is an effective anti-tumor phytochemistry and may become a clinically useful drug for the treatment of human colorectal cancer.
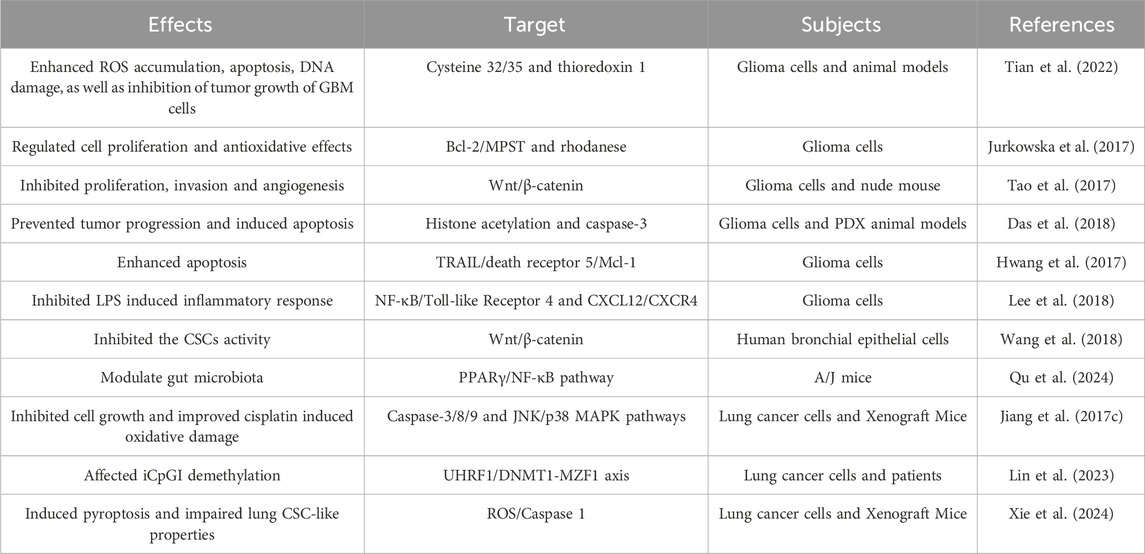
2.3 The preventive and therapeutic effects of DATS in gliomas
Gliomas is one of the most malignant brain tumor. Although multimodal treatment for glioblastoma includes surgery, radiotherapy, and chemotherapy, the extension of survival for gliomas patients is still limited. Therefore, there is an urgent need to find new strategies to improve the therapeutic efficacy of gliomas. DATS has shown excellent intervention effects in various cancers including gliomas (Choromanska et al., 2020). In this chapter, we summarize the preventive and therapeutic effects of DATS in gliomas (Table 3).
It is demonstrated that DATS sensitizes radiation therapy on glioblastoma through directly conjugates with cysteine 32/35 and targeting thioredoxin 1 (Tian et al., 2022). The unlimited proliferation of cancer cells is one of the main causes of cancer progression. The inhibition of glioma cells proliferation by DATS is associated with an increase in the inactivation form of Bcl-2 (Jurkowska et al., 2017). The research results of Tao demonstrated that DATS inactivate Wnt/β-catenin pathway inhibits the cell proliferation, angiogenesis and invasion of glioma cells (Tao et al., 2017). DATS regulates an increase in histone acetylation and activation of caspase-3, leading to a dose-dependent reduction in total tumor volume, proliferation, and angiogenesis in glioma (Das et al., 2018). It is reported that DATS upregulates death receptor 5 through ROS, making glioma cells sensitive to TRAIL mediated apoptosis (Hwang et al., 2017). These results of Lee indicated that DATS inhibits the activation of the CXCL12/CXCR4 axis associated with TLR4 antagonism and blocks NF-κB signal transduction, thereby demonstrating anti-inflammatory effects to intervene in glioma (Lee et al., 2018). DATS is an organic sulfur compound derived from garlic, which has been proven to have potential anti-inflammatory, anti-proliferative, angiogenic, enhanced radiation and other therapeutic effects.
2.4 The role of DATS in lung cancer
Lung cancer is the leading cause of cancer-related deaths worldwide, and there is currently a lack of effective and minimally toxic therapies in clinical practice. Researchers are increasingly focusing on the preventive and therapeutic effects of phytochemicals such as DATS on lung cancer (Table 3).
Wang et al. reported that DATS regulate the activity of the Wnt/β-catenin pathway modulates the acquisition of cancer stem cell characteristics induced by chronic tobacco smoke exposure (Wang et al., 2018). The results of Qu et al. indicated that DATS significantly decreased the number of lung tumors in A/J mice induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, which may be related to DATS regulating the gut microbiota, especially increasing the abundance of F. rodentium (Qu et al., 2024). It is reported that DATS regulates JNK and p38 pathway to inhibit the growth of NCI-H460 and improve the therapeutic effect of cisplatin induced oxidative damage on xenograft tumor mice and cells (Jiang et al., 2017c). The synergistic effect of DATS and 5-aza-2 ′- deoxycytidine allows for site-specific iCpGI demethylation of PRSS3-V3 upregulated by MZF1, exerting anti-cancer effects in lung adenocarcinoma cells (Lin et al., 2023). The research results of Xie et al. (2024) suggested that DATS promotes cell pyroptosis and attenuates lung cancer stem cells properties by activating the ROS/Aspase 1 signaling pathway, thereby delaying the progression of lung cancer.
At present, there is relatively little research on the prevention and treatment of DATS in lung cancer, and there are many types of lung cancer with different pathogenesis. In future research, it is necessary to observe the preventive and therapeutic effects of DATS on different types of lung cancer or the main risk factors for lung cancer, such as tobacco exposure.
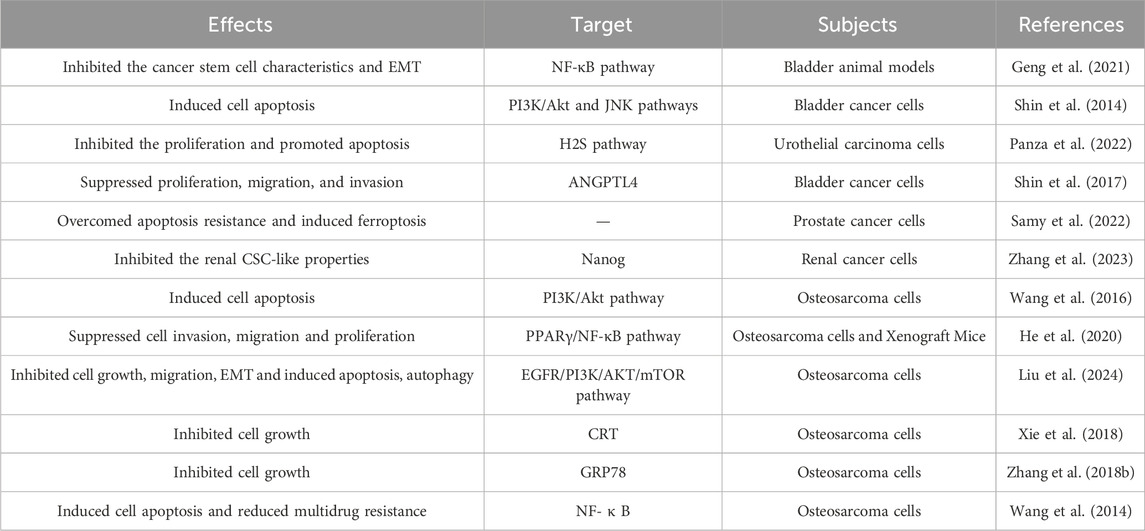
2.5 The preventive and therapeutic effects of DATS in the urinary system
DATS played an intervention role in smoke induced bladder cancer by regulating NF-κB pathway to inhibit the cancer stem cell characteristics and EMT (Table 4) (Geng et al., 2021). DATS induced apoptosis of bladder cancer cells to play an intervention effect, and PI3K/Akt and JNK pathways play a regulatory role in this process (Shin et al., 2014). DATS is a fully characterized H2S donor that inhibits the proliferation of urothelial carcinoma cells and promotes cell apoptosis in a time and concentration dependent manner (Panza et al., 2022). DATS mediated the inhibition of cancer cell proliferation, invasion and migration of bladder cancer. ANGPTL4 could regulate the intervention effect of DATS, which may become a prognostic marker of bladder cancer patients (Shin et al., 2017).
Apoptosis resistance is a potential mechanism for drug resistance in prostate cancer treatment. DATS effectively targeted prostate cancer cells by overcoming cell apoptosis resistance and inducing prostate cancer cell death mediated by ferroptosis (Samy et al., 2022). Cancer stem-like cells play an important role in cancer such as renal cancer initiation and progression. DATS inhibited the renal cancer stem-like cell properties by suppressing Nanog (Zhang et al., 2023).
2.6 Prevention and treatment of osteosarcoma
Osteosarcoma is the most common non hematopoietic primary bone cancer, mainly occurring in young people and adolescents (Table 4). At present, the preferred treatment for osteosarcoma includes neoadjuvant chemotherapy followed by surgery and chemotherapy, but most patients are prone to recurrence. We urgently need to discover new natural compounds such as DATS that have the potential to prevent osteosarcoma progression and improve patient survival.
Wang et al. (2016) reported that DATS induce apoptosis in osteosarcoma cells through downregulation of the PI3K/Akt pathway mediated by reactive oxygen species. The data of He et al. (2020) demonstrated that DATS exerts the anti-cancer effects by inhibiting cell invasion, migration and proliferation in vitro and in vivo. The study of Liu et al. (2024) also demonstrated that DATS inhibited the cell growth, migration, EMT and induced apoptosis, autophagy in osteosarcoma cells. Upregulation of CRT expression by DATS may be one of the mechanisms by which DATS inhibits the growth ability of human osteosarcoma Saos-2 cells (Xie et al., 2018). Zhang’s results indicated that DATS inhibits the growth of human osteosarcoma cells by regulating the expression of GRP78 (Zhang et al., 2018b). The relevant data indicated that DATS has significant anti-cancer effects on osteosarcoma cells, and its potential mechanisms include inducing cell apoptosis and reducing multidrug resistance (Wang et al., 2014).
These results provide evidence and strategies for using DATS alone or in combination with standard prevention and treatment of osteosarcoma.
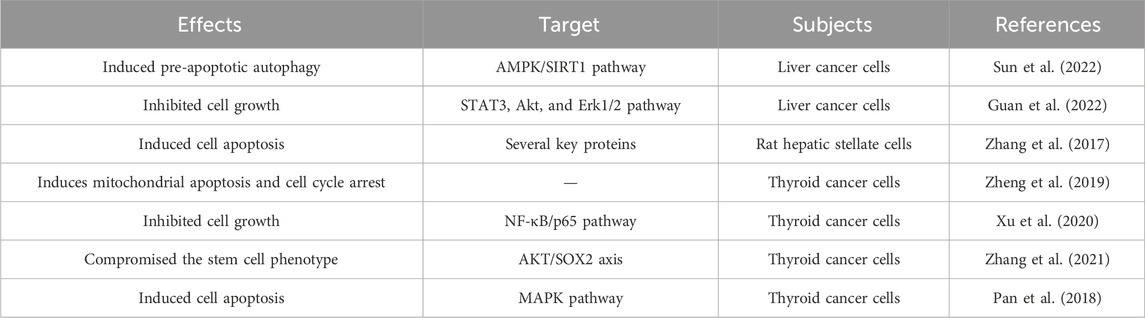
2.7 The anti-cancer effect of DATS in liver cancer
Liver cancer is a common malignant tumor in the world, with high incidence rate and mortality. There is no ideal treatment strategy at present. Many researchers have attempted to explore the anticancer effects of natural plant compounds such as DATS in liver cancer (Table 5).
DATS induces pre-apoptotic autophagy in human liver cancer HepG2 cells through the AMPK/SIRT1 signaling pathway (Sun et al., 2022). The results of Guan et al. (2022) indicated that APPL1 polyubiquitination may mediate the inhibitory effect of DAT on HepG2 cell survival by regulating the STAT3, Akt, and Erk1/2 pathways. It is reported that DATS is an inducer of hepatic stellate cells apoptosis, and multiple proteins may be involved in the molecular mechanism of DATS induced cell apoptosis (Zhang et al., 2017).
2.8 Thyroid cancer
Thyroid cancer is a common endocrine malignant tumor. The incidence rate of thyroid cancer has been rising. The existing treatment methods are not ideal. There is an urgent need to develop more promising new drugs or strategies to improve treatment efficiency. DATS, as one of the main active ingredients in garlic, is expected to become a new therapeutic strategy for thyroid cancer (Table 5).
The research results of Zheng et al. (2019) revealed that DATS induces mitochondrial apoptosis and cell cycle arrest by triggering DNA damage in anaplastic thyroid carcinoma cells, which may provide a new therapeutic approach for thyroid carcinoma treatment. As a H2 S donor, DATS suppressed the growth of human papillary thyroid cancer cells, inhibited cancer stem cell phenotype and restored thyroid-specific gene expression (Xu et al., 2020; Zhang et al., 2021). DATS induced apoptosis in human papillary thyroid cancer BCPAP cells by activating the MAPK pathway (Pan et al., 2018). The above study elucidates prospective therapeutic targets for thyroid cancer treatment.
2.9 Other cancer
In addition to the cancers that have been extensively studied, DATS also has intervention and therapeutic effects in many other cancers.
DATS could affect the apoptosis and immune status of leukemia cells, thereby exerting intervention effects (Suda et al., 2014; Hung et al., 2015). It was reported that DATS can interfere with pancreatic cancer by regulating cell cycle and apoptosis (Ma et al., 2014). Mitochondrial Ca2+ overload through voltage gated Ca2+ entry contributes to the anti-melanoma effect of DATS (Nakagawa et al., 2020). DATS inhibited the migration, invasion of melanoma cells by inhibiting the integrin/facial adhesion kinase pathway (Wang et al., 2017). DATS mitigated chemotherapy sensitivity of ovarian cancer by regulating the AMPK/SIRT1 pathway (Wang et al., 2023). DATS induced ROS mediated mitotic arrest and cell apoptosis, thereby inhibiting head and neck squamous cell carcinoma tumor growth and tumor stem cell characteristics (Mathan et al., 2024). DATS has inhibitory effects on the tumor biology of synovial sarcoma cells, including triggering cell apoptosis, inducing G2/M cell cycle arrest, increasing intracellular ROS, and damaging mitochondria (Xia et al., 2022). DATS promoted miR-127-3p and inactivates the PI3K/AKT signaling pathway, enhancing the anticancer activity of dexamethasone in multiple myeloma subpopulations of cells (He et al., 2021). DATS induced cell apoptosis through destabilization of TRAF6 by suppressing NF-κB signaling in primary effusion lymphoma (Shigemi et al., 2016). The finding of Wang et al. (2015) demonstrated that DATS inhibits the proliferation of ESCC cells by activating ERK1/2 in vitro and in vivo. Diallyl trisulfide, resveratrol, and epigallocatechin-3-gallate exerted synergistic inhibitory effects on skin cancer cell lines through the mitochondrial caspase dependent pathway (Arumugam and Alagar Yadav, 2024).
3 Discussion, challenges, and future research directions
The prevention and treatment of tumors, especially early prevention and treatment, is a global challenge. Currently commonly used tumor treatment drugs have strong toxicity and adverse reactions to normal cells, which can easily lead to drug resistance. Researchers need to develop and search for drugs with fewer side effects and less susceptibility to drug resistance. Natural plant chemicals are becoming alternative resources for combating cancer due to their unique advantages. Compared with traditional drugs, phytochemicals such as DATS have various advantages, including wide source, easy application, high security and excellent anti-cancer effect. DATS is a natural organosulfur compound derived from garlic that has been investigated as a very promising anticancer drug. DATS has multiple anti-cancer mechanisms, such as regulating immunity, inhibiting proliferation, suppressing migration, triggering apoptosis, and so on. DATS also enhanced the efficacy of traditional anti-cancer drugs, reduced the side effects of anti-cancer drugs and reduced incidence of drug resistance.
The application of DATS in the clinical treatment and prevention of cancers is still greatly limited. There are still many challenges that need to be addressed in order to better apply DATS to early clinical intervention and treatment of cancer. The clear role and precise molecular mechanism of DATS in early intervention and treatment of cancer need to be further explored and elucidated. There is an urgent need to establish methods that can accurately measure the concentration of DATS in blood or target tissues. More clinical and preclinical trials are needed to verify the anti-tumor effect of DATS, or its combined effect with commonly used anticancer drugs. We still need to find better forms of DATS delivery to improve the bioavailability of DATS. Attempts can be made to deliver DATS through exocrine pathways to enhance the bioavailability and targeting of DATS in cancer intervention and treatment. In addition, exosomes derived from plant chemistry from DATS can also be directly extracted, acting on malignant transformed cells, cancer stem cells and cancer cells to observe whether it can enhance the anti-cancer effect and bioavailability of DATS.
Author contributions
LL: Data curation, Funding acquisition, Investigation, Methodology, Writing–original draft. ZG: Methodology, Writing–review and editing. JS: Methodology, Writing–review and editing. LJ: Methodology, Supervision, Writing–review and editing. ZL: Funding acquisition, Investigation, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by National Natural Science Foundation of China (No. 81602883), “Jinshan Doctor” medical field talent training plan of Zhenjiang and Clinical Medical Science and Technology Development Foundation of Jiangsu University (No. JLY2021013), and Project of social development in Zhenjiang (SH2024070, SSH202402420145).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alrumaihi, F., Khan, M. A., Babiker, A. Y., Alsaweed, M., Azam, F., Allemailem, K. S., et al. (2022). Lipid-based nanoparticle formulation of diallyl trisulfide chemosensitizes the growth inhibitory activity of doxorubicin in colorectal cancer model: a novel in vitro, in vivo and in silico analysis. Molecules 27 (7), 2192. doi:10.3390/molecules27072192
Arumugam, G., and Alagar Yadav, S. (2024). Synergistic inhibitory actions of resveratrol, epigallocatechin-3-gallate, and diallyl trisulfide against skin cancer cell line A431 through mitochondrial caspase dependent pathway: a combinational drug approach. Med. Oncol. 41 (3), 64. doi:10.1007/s12032-023-02292-3
Cheng, S. Y., Yang, Y. C., Ting, K. L., Wen, S. Y., Viswanadha, V. P., Huang, C. Y., et al. (2017). Lactate dehydrogenase downregulation mediates the inhibitory effect of diallyl trisulfide on proliferation, metastasis, and invasion in triple-negative breast cancer. Environ. Toxicol. 32 (4), 1390–1398. doi:10.1002/tox.22333
Choi, Y. H. (2017). Diallyl trisulfide induces apoptosis and mitotic arrest in AGS human gastric carcinoma cells through reactive oxygen species-mediated activation of AMP-activated protein kinase. Biomed. Pharmacother. 94, 63–71. doi:10.1016/j.biopha.2017.07.055
Choromanska, A., Kulbacka, J., Saczko, J., and Surowiak, P. (2020). Effect of diallyl disulfide and garlic oil on different human astrocytoma cell lines. Biomed. Rep. 13 (4), 32. doi:10.3892/br.2020.1339
Das, A., Henderson, F., Lowe, S., Wallace, G. C. t., Vandergrift, W. A., Lindhorst, S. M., et al. (2018). Single agent efficacy of the HDAC inhibitor DATS in preclinical models of glioblastoma. Cancer Chemother. Pharmacol. 82 (6), 945–952. doi:10.1007/s00280-018-3684-7
De, A., Roychowdhury, P., Bhuyan, N. R., Ko, Y. T., Singh, S. K., Dua, K., et al. (2023). Folic acid functionalized diallyl trisulfide-solid lipid nanoparticles for targeting triple negative breast cancer. Molecules 28 (3), 1393. doi:10.3390/molecules28031393
Elsherbiny, N. M., El-Sherbiny, M., and Zaitone, S. A. (2020). Diallyl trisulfide potentiates chemotherapeutic efficacy of doxorubicin in experimentally induced mammary carcinoma: role of Notch signaling. Pathol. Res. Pract. 216 (10), 153139. doi:10.1016/j.prp.2020.153139
Ferguson, D. T., Taka, E., Messeha, S., Flores-Rozas, H., Reed, S. L., Redmond, B. V., et al. (2024a). The garlic compound, diallyl trisulfide, attenuates benzo[a]Pyrene-induced precancerous effect through its antioxidant effect, AhR inhibition, and increased DNA repair in human breast epithelial cells. Nutrients 16 (2), 300. doi:10.3390/nu16020300
Ferguson, D. T., Taka, E., Tilghman, S. L., Womble, T., Redmond, B. V., Gedeon, S., et al. (2024b). The anticancer effects of the garlic organosulfide diallyl trisulfide through the attenuation of B[a]P-induced oxidative stress, AhR expression, and DNA damage in human premalignant breast epithelial (MCF-10AT1) cells. Int. J. Mol. Sci. 25 (2), 923. doi:10.3390/ijms25020923
Ge, M., Zhu, J., Yi, K., Chen, Y., Cao, W., Wang, M., et al. (2023). Diallyl trisulfide inhibits gastric cancer stem cell properties through ΔNp63/sonic hedgehog pathway. Mol. Carcinog. 62 (11), 1673–1685. doi:10.1002/mc.23607
Geng, H., Guo, W., Feng, L., Xie, D., Bi, L., Wang, Y., et al. (2021). Diallyl trisulfide inhibited tobacco smoke-mediated bladder EMT and cancer stem cell marker expression via the NF-κB pathway in vivo. J. Int. Med. Res. 49 (3), 300060521992900. doi:10.1177/0300060521992900
Guan, F., Ding, Y., He, Y., Li, L., Yang, X., Wang, C., et al. (2022). Involvement of adaptor protein, phosphotyrosine interacting with PH domain and leucine zipper 1 in diallyl trisulfide-induced cytotoxicity in hepatocellular carcinoma cells. Korean J. Physiol. Pharmacol. 26 (6), 457–468. doi:10.4196/kjpp.2022.26.6.457
Hahm, E. R., Kim, S. H., Mathan, S. V., Singh, R. P., and Singh, S. V. (2021). Mechanistic targets of diallyl trisulfide in human breast cancer cells identified by RNA-seq analysis. J. Cancer Prev. 26 (2), 128–136. doi:10.15430/JCP.2021.26.2.128
Hahm, E. R., Mathan, S. V., Singh, R. P., and Singh, S. V. (2022). Breast cancer selective disruption of actin cytoskeleton by diallyl trisulfide. J. Cancer Prev. 27 (2), 101–111. doi:10.15430/JCP.2022.27.2.101
Hahm, E. R., and Singh, S. V. (2014). Diallyl trisulfide inhibits estrogen receptor-α activity in human breast cancer cells. Breast Cancer Res. Treat. 144 (1), 47–57. doi:10.1007/s10549-014-2841-x
Hahm, E. R., and Singh, S. V. (2022). Gene expression changes by diallyl trisulfide administration in chemically-induced mammary tumors in rats. J. Cancer Prev. 27 (1), 22–30. doi:10.15430/JCP.2022.27.1.22
He, P., Wang, Z., Sheng, B., Xu, Y., Feng, S., Huang, Y., et al. (2020). Diallyl trisulfide regulates cell apoptosis and invasion in human osteosarcoma U2OS cells through regulating PI3K/AKT/GSK3β signaling pathway. Histol. Histopathol. 35 (12), 1511–1520. doi:10.14670/HH-18-299
He, W., Fu, Y., Zheng, Y., Wang, X., Liu, B., and Zeng, J. (2021). Diallyl thiosulfinate enhanced the anti-cancer activity of dexamethasone in the side population cells of multiple myeloma by promoting miR-127-3p and deactivating the PI3K/AKT signaling pathway. BMC Cancer 21 (1), 125. doi:10.1186/s12885-021-07833-5
Hung, F. M., Shang, H. S., Tang, N. Y., Lin, J. J., Lu, K. W., Lin, J. P., et al. (2015). Effects of diallyl trisulfide on induction of apoptotic death in murine leukemia WEHI-3 cells in vitro and alterations of the immune responses in normal and leukemic mice in vivo. Environ. Toxicol. 30 (11), 1343–1353. doi:10.1002/tox.22005
Hwang, J. S., Lee, Y. Y., Lee, D. H., and Kwon, K. H. (2017). DATS sensitizes glioma cells to TRAIL-mediated apoptosis by up-regulation of death receptor 5 via ROS. Food Chem. Toxicol. 106 (Pt A), 514–521. doi:10.1016/j.fct.2017.05.056
Jiang, X., Zhu, X., Huang, W., Xu, H., Zhao, Z., Li, S., et al. (2017b). Garlic-derived organosulfur compound exerts antitumor efficacy via activation of MAPK pathway and modulation of cytokines in SGC-7901 tumor-bearing mice. Int. Immunopharmacol. 48, 135–145. doi:10.1016/j.intimp.2017.05.004
Jiang, X., Zhu, X., Liu, N., Xu, H., Zhao, Z., Li, S., et al. (2017c). Diallyl trisulfide inhibits growth of NCI-H460 in vitro and in vivo, and ameliorates cisplatin-induced oxidative injury in the treatment of lung carcinoma in xenograft mice. Int. J. Biol. Sci. 13 (2), 167–178. doi:10.7150/ijbs.16828
Jiang, X. Y., Zhu, X. S., Xu, H. Y., Zhao, Z. X., Li, S. Y., Li, S. Z., et al. (2017a). Diallyl trisulfide suppresses tumor growth through the attenuation of Nrf2/Akt and activation of p38/JNK and potentiates cisplatin efficacy in gastric cancer treatment. Acta Pharmacol. Sin. 38 (7), 1048–1058. doi:10.1038/aps.2016.176
Jurkowska, H., Wrobel, M., Kaczor-Kaminska, M., and Jasek-Gajda, E. (2017). A possible mechanism of inhibition of U87MG and SH-SY5Y cancer cell proliferation by diallyl trisulfide and other aspects of its activity. Amino Acids 49 (11), 1855–1866. doi:10.1007/s00726-017-2484-4
Kanga, K. J. W., Kanga, L. H. B., Mendonca, P., Soliman, K. F. A., Ferguson, D. T., Reed, S. L., et al. (2023). Attenuative effect of diallyl trisulfide on caspase activity in TNF-α-induced triple negative breast cancer cells. Anticancer Res. 43 (6), 2393–2405. doi:10.21873/anticanres.16407
Kanga, K. J. W., Mendonca, P., Soliman, K. F. A., Ferguson, D. T., and Darling-Reed, S. F. (2021). Effect of diallyl trisulfide on TNF-α-induced CCL2/MCP-1 release in genetically different triple-negative breast cancer cells. Anticancer Res. 41 (12), 5919–5933. doi:10.21873/anticanres.15411
Khan, A. Q., and Uddin, S. (2021). Anticancer activity of natural compounds. Asian Pac J. Cancer Prev. 22 (S1), 1–2. doi:10.31557/APJCP.2021.22.S1.1
Kiesel, V. A., and Stan, S. D. (2017). Diallyl trisulfide, a chemopreventive agent from Allium vegetables, inhibits alpha-secretases in breast cancer cells. Biochem. Biophys. Res. Commun. 484 (4), 833–838. doi:10.1016/j.bbrc.2017.01.184
Kim, S., Lee, H. G., Park, S. A., Kundu, J. K., Keum, Y. S., Cha, Y. N., et al. (2014). Keap1 cysteine 288 as a potential target for diallyl trisulfide-induced Nrf2 activation. PLoS One 9 (1), e85984. doi:10.1371/journal.pone.0085984
Kim, S. H., Hahm, E. R., Singh, K. B., and Singh, S. V. (2020). Diallyl trisulfide inhibits leptin-induced oncogenic signaling in human breast cancer cells but fails to prevent chemically-induced luminal-type cancer in rats. J. Cancer Prev. 25 (1), 1–12. doi:10.15430/JCP.2020.25.1.1
Kim, S. H., Kaschula, C. H., Priedigkeit, N., Lee, A. V., and Singh, S. V. (2016). Forkhead box Q1 is a novel target of breast cancer stem cell inhibition by diallyl trisulfide. J. Biol. Chem. 291 (26), 13495–13508. doi:10.1074/jbc.M116.715219
Kim, S. H., and Singh, S. V. (2022). Monocarboxylate transporter 1 is a novel target for breast cancer stem like-cell inhibition by diallyl trisulfide. Mol. Carcinog. 61 (8), 752–763. doi:10.1002/mc.23415
Lee, H. H., Jeong, J. W., Hong, S. H., Park, C., Kim, B. W., and Choi, Y. H. (2018). Diallyl trisulfide suppresses the production of lipopolysaccharide-induced inflammatory mediators in BV2 microglia by decreasing the NF-κB pathway activity associated with toll-like receptor 4 and CXCL12/CXCR4 pathway blockade. J. Cancer Prev. 23 (3), 134–140. doi:10.15430/JCP.2018.23.3.134
Li, X., Meng, Y., Xie, C., Zhu, J., Wang, X., Li, Y., et al. (2018). Diallyl Trisulfide inhibits breast cancer stem cells via suppression of Wnt/β-catenin pathway. J. Cell. Biochem. 119 (5), 4134–4141. doi:10.1002/jcb.26613
Liao, W., Zhang, L., Chen, X., Xiang, J., Zheng, Q., Chen, N., et al. (2023). Targeting cancer stem cells and signalling pathways through phytochemicals: a promising approach against colorectal cancer. Phytomedicine 108, 154524. doi:10.1016/j.phymed.2022.154524
Lin, S., Xu, H., Qin, L., Pang, M., Wang, Z., Gu, M., et al. (2023). UHRF1/DNMT1-MZF1 axis-modulated intragenic site-specific CpGI methylation confers divergent expression and opposing functions of PRSS3 isoforms in lung cancer. Acta Pharm. Sin. B 13 (5), 2086–2106. doi:10.1016/j.apsb.2023.02.015
Liu, X., Wang, N., He, Z., Chen, C., Ma, J., Liu, X., et al. (2024). Diallyl trisulfide inhibits osteosarcoma 143B cell migration, invasion and EMT by inducing autophagy. Heliyon 10 (5), e26681. doi:10.1016/j.heliyon.2024.e26681
Liu, Y., Zhao, Y., Wang, Y., Zhu, P., Wei, Z., Wang, S., et al. (2017). Suppressive role of diallyl trisulfide in the activated platelet-mediated hematogenous metastasis of MDA-MB-231 human breast cancer cells. Int. J. Mol. Med. 39 (6), 1516–1524. doi:10.3892/ijmm.2017.2953
Liu, Y., Zhao, Y., Wei, Z., Tao, L., Sheng, X., Wang, S., et al. (2018). Targeting thioredoxin system with an organosulfur compound, diallyl trisulfide (DATS), attenuates progression and metastasis of triple-negative breast cancer (TNBC). Cell. Physiol. Biochem. 50 (5), 1945–1963. doi:10.1159/000494874
Liu, Y., Zhu, P., Wang, Y., Wei, Z., Tao, L., Zhu, Z., et al. (2015). Antimetastatic therapies of the polysulfide diallyl trisulfide against triple-negative breast cancer (TNBC) via suppressing MMP2/9 by blocking NF-κB and ERK/MAPK signaling pathways. PLoS One 10 (4), e0123781. doi:10.1371/journal.pone.0123781
Lv, H., Jia, X., Yang, H., Zhu, X., Zhao, Z., and Jiang, X. (2023). Prediction of the mechanism for the combination of diallyl trisulfide and cisplatin against gastric cancer: a network pharmacology study and pharmacological evaluation. Front. Pharmacol. 14, 1269895. doi:10.3389/fphar.2023.1269895
Ma, H. B., Huang, S., Yin, X. R., Zhang, Y., and Di, Z. L. (2014). Apoptotic pathway induced by diallyl trisulfide in pancreatic cancer cells. World J. Gastroenterol. 20 (1), 193–203. doi:10.3748/wjg.v20.i1.193
Malla, R., Marni, R., Chakraborty, A., and Kamal, M. A. (2022). Diallyl disulfide and diallyl trisulfide in garlic as novel therapeutic agents to overcome drug resistance in breast cancer. J. Pharm. Anal. 12 (2), 221–231. doi:10.1016/j.jpha.2021.11.004
Marni, R., Kundrapu, D. B., Chakraborti, A., and Malla, R. (2022). Insight into drug sensitizing effect of diallyl disulfide and diallyl trisulfide from Allium sativum L. on paclitaxel-resistant triple-negative breast cancer cells. J. Ethnopharmacol. 296, 115452. doi:10.1016/j.jep.2022.115452
Mathan, S. V., Singh, R., Kim, S. H., Singh, S. V., and Singh, R. P. (2024). Diallyl trisulfide induces ROS-mediated mitotic arrest and apoptosis and inhibits HNSCC tumor growth and cancer stemness. Cancers (Basel) 16 (2), 378. doi:10.3390/cancers16020378
Mondal, A., Banerjee, S., Bose, S., Mazumder, S., Haber, R. A., Farzaei, M. H., et al. (2022). Garlic constituents for cancer prevention and therapy: from phytochemistry to novel formulations. Pharmacol. Res. 175, 105837. doi:10.1016/j.phrs.2021.105837
Nakagawa, C., Suzuki-Karasaki, M., Suzuki-Karasaki, M., Ochiai, T., and Suzuki-Karasaki, Y. (2020). The mitochondrial Ca(2+) overload via voltage-gated Ca(2+) entry contributes to an anti-melanoma effect of diallyl trisulfide. Int. J. Mol. Sci. 21 (2), 491. doi:10.3390/ijms21020491
Newman, D. J., and Cragg, G. M. (2016). Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 79 (3), 629–661. doi:10.1021/acs.jnatprod.5b01055
Oravetz, K., Todea, A. V., Balacescu, O., Cruceriu, D., and Rakosy-Tican, E. (2023). Potential antitumor activity of garlic against colorectal cancer: focus on the molecular mechanisms of action. Eur. J. Nutr. 62 (6), 2347–2363. doi:10.1007/s00394-023-03166-0
Pan, J., Zhang, L., Xu, S., Cheng, X., Yu, H., Bao, J., et al. (2018). Induction of apoptosis in human papillary-thyroid-carcinoma BCPAP cells by diallyl trisulfide through activation of the MAPK signaling pathway. J. Agric. Food Chem. 66 (23), 5871–5878. doi:10.1021/acs.jafc.8b02243
Pan, Y., Lin, S., Xing, R., Zhu, M., Lin, B., Cui, J., et al. (2016). Epigenetic upregulation of metallothionein 2A by diallyl trisulfide enhances chemosensitivity of human gastric cancer cells to docetaxel through attenuating NF-κB activation. Antioxid. Redox Signal 24 (15), 839–854. doi:10.1089/ars.2014.6128
Panza, E., Bello, I., Smimmo, M., Brancaleone, V., Mitidieri, E., Bucci, M., et al. (2022). Endogenous and exogenous hydrogen sulfide modulates urothelial bladder carcinoma development in human cell lines. Biomed. Pharmacother. 151, 113137. doi:10.1016/j.biopha.2022.113137
Puccinelli, M. T., and Stan, S. D. (2017). Dietary bioactive diallyl trisulfide in cancer prevention and treatment. Int. J. Mol. Sci. 18 (8), 1645. doi:10.3390/ijms18081645
Qu, Z., Tian, J., Sun, J., Shi, Y., Yu, J., Zhang, W., et al. (2024). Diallyl trisulfide inhibits 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung cancer via modulating gut microbiota and the PPARγ/NF-κB pathway. Food Funct. 15 (1), 158–171. doi:10.1039/d3fo03914e
Rauf, A., Abu-Izneid, T., Thiruvengadam, M., Imran, M., Olatunde, A., Shariati, M. A., et al. (2022). Garlic (allium sativum L.): its chemistry, nutritional composition, toxicity, and anticancer properties. Curr. Top. Med. Chem. 22 (11), 957–972. doi:10.2174/1568026621666211105094939
Samy, A., Shah, D., Shahagadkar, P., Shah, H., and Munirathinam, G. (2022). Can diallyl trisulfide, a dietary garlic-derived compound, activate ferroptosis to overcome therapy resistance in prostate cancer? Nutr. Health 28 (2), 207–212. doi:10.1177/02601060211018360
Shigemi, Z., Furukawa, Y., Hosokawa, K., Minami, S., Matsuhiro, J., Nakata, S., et al. (2016). Diallyl trisulfide induces apoptosis by suppressing NF-κB signaling through destabilization of TRAF6 in primary effusion lymphoma. Int. J. Oncol. 48 (1), 293–304. doi:10.3892/ijo.2015.3247
Shin, D. Y., Kim, G. Y., Hwang, H. J., Kim, W. J., and Choi, Y. H. (2014). Diallyl trisulfide-induced apoptosis of bladder cancer cells is caspase-dependent and regulated by PI3K/Akt and JNK pathways. Environ. Toxicol. Pharmacol. 37 (1), 74–83. doi:10.1016/j.etap.2013.11.002
Shin, S. S., Song, J. H., Hwang, B., Park, S. L., Kim, W. T., Park, S. S., et al. (2017). Angiopoietin-like protein 4 potentiates DATS-induced inhibition of proliferation, migration, and invasion of bladder cancer EJ cells; involvement of G(2)/M-phase cell cycle arrest, signaling pathways, and transcription factors-mediated MMP-9 expression. Food Nutr. Res. 61 (1), 1338918. doi:10.1080/16546628.2017.1338918
Siegel, R. L., Giaquinto, A. N., and Jemal, A. (2024). Cancer statistics, 2024. CA Cancer J. Clin. 74 (1), 12–49. doi:10.3322/caac.21820
Sohel, M., Sultana, H., Sultana, T., Al Amin, M., Aktar, S., Ali, M. C., et al. (2022). Chemotherapeutic potential of hesperetin for cancer treatment, with mechanistic insights: a comprehensive review. Heliyon 8 (1), e08815. doi:10.1016/j.heliyon.2022.e08815
Stan, S. D., and Abtahi, M. (2022). Diallyl trisulfide induces apoptosis in breast ductal carcinoma in situ derived and minimally invasive breast cancer cells. Nutrients 14 (7), 1455. doi:10.3390/nu14071455
Suda, S., Watanabe, K., Tanaka, Y., Watanabe, K., Tanaka, R., Ogihara, J., et al. (2014). Identification of molecular target of diallyl trisulfide in leukemic cells. Biosci. Biotechnol. Biochem. 78 (8), 1415–1417. doi:10.1080/09168451.2014.921563
Sun, S., Liu, X., Wei, X., Zhang, S., and Wang, W. (2022). Diallyl trisulfide induces pro-apoptotic autophagy via the AMPK/SIRT1 signalling pathway in human hepatocellular carcinoma HepG2 cell line. Food Nutr. Res. 66. doi:10.29219/fnr.v66.8981
Tao, Q., Wu, C., Xu, R., Niu, L., Qin, J., Liu, N., et al. (2017). Diallyl trisulfide inhibits proliferation, invasion and angiogenesis of glioma cells by inactivating Wnt/β-catenin signaling. Cell. Tissue Res. 370 (3), 379–390. doi:10.1007/s00441-017-2678-9
Tian, Y., Ge, Z., Xu, M., Ge, X., Zhao, M., Ding, F., et al. (2022). Diallyl trisulfide sensitizes radiation therapy on glioblastoma through directly targeting thioredoxin 1. Free Radic. Biol. Med. 189, 157–168. doi:10.1016/j.freeradbiomed.2022.07.019
Wang, F., Li, S., Wang, L., Zong, H., and Fan, Q. (2015). DATS suppresses growth of esophageal squamous cell carcinoma by regulation of ERK1/2. Clin. Lab. 61 (3-4), 315–322. doi:10.7754/clin.lab.2014.140806
Wang, H., Sun, N., Li, X., Li, K., Tian, J., and Li, J. (2016). Diallyl trisulfide induces osteosarcoma cell apoptosis through reactive oxygen species-mediated downregulation of the PI3K/Akt pathway. Oncol. Rep. 35 (6), 3648–3658. doi:10.3892/or.2016.4722
Wang, H. C., Chu, Y. L., Hsieh, S. C., and Sheen, L. Y. (2017). Diallyl trisulfide inhibits cell migration and invasion of human melanoma a375 cells via inhibiting integrin/facal adhesion kinase pathway. Environ. Toxicol. 32 (11), 2352–2359. doi:10.1002/tox.22445
Wang, J., Chen, J., Jiang, Y., Shi, Y., Zhu, J., Xie, C., et al. (2018). Wnt/β-catenin modulates chronic tobacco smoke exposure-induced acquisition of pulmonary cancer stem cell properties and diallyl trisulfide intervention. Toxicol. Lett. 291, 70–76. doi:10.1016/j.toxlet.2018.04.003
Wang, J., Si, L., Wang, G., Bai, Z., and Li, W. (2019). Increased sulfiredoxin expression in gastric cancer cells may Be a molecular target of the anticancer component diallyl trisulfide. Biomed. Res. Int. 2019, 4636804. doi:10.1155/2019/4636804
Wang, Y., Huang, P., Wu, Y., Liu, D., Ji, M., Li, H., et al. (2022). Association and mechanism of garlic consumption with gastrointestinal cancer risk: a systematic review and meta-analysis. Oncol. Lett. 23 (4), 125. doi:10.3892/ol.2022.13245
Wang, Z., Xia, Q., Cui, J., Diao, Y., and Li, J. (2014). Reversion of P-glycoprotein-mediated multidrug resistance by diallyl trisulfide in a human osteosarcoma cell line. Oncol. Rep. 31 (6), 2720–2726. doi:10.3892/or.2014.3154
Wang, Z., Yan, Y., Lou, Y., Huang, X., Liu, L., Weng, Z., et al. (2023). Diallyl trisulfide alleviates chemotherapy sensitivity of ovarian cancer via the AMPK/SIRT1/PGC1α pathway. Cancer Sci. 114 (2), 357–369. doi:10.1111/cas.15627
Wei, Z., Shan, Y., Tao, L., Liu, Y., Zhu, Z., Liu, Z., et al. (2017). Diallyl trisulfides, a natural histone deacetylase inhibitor, attenuate HIF-1α synthesis, and decreases breast cancer metastasis. Mol. Carcinog. 56 (10), 2317–2331. doi:10.1002/mc.22686
Xia, S. L., Ma, Z. Y., Wang, B., Gao, F., Yi, C. G., Zhou, X. X., et al. (2022). In vitro anti-synovial sarcoma effect of diallyl trisulfide and mRNA profiling. Gene 816, 146172. doi:10.1016/j.gene.2021.146172
Xie, C., Zhou, X., Chen, W., Ren, D., Li, X., Jiang, R., et al. (2024). Diallyl trisulfide induces pyroptosis and impairs lung CSC-like properties by activating the ROS/Caspase 1 signaling pathway. Chem. Biol. Interact. 397, 111083. doi:10.1016/j.cbi.2024.111083
Xie, W. P., Zhang, Y., Zhang, Y. K., Li, G., Xin, J., Bi, R. X., et al. (2018). Treatment of Saos-2 osteosarcoma cells with diallyl trisulfide is associated with an increase in calreticulin expression. Exp. Ther. Med. 15 (6), 4737–4742. doi:10.3892/etm.2018.6037
Xu, S., Pan, J., Cheng, X., Zheng, J., Wang, X., Guan, H., et al. (2020). Diallyl trisulfide, a H(2) S donor, inhibits cell growth of human papillary thyroid carcinoma KTC-1 cells through a positive feedback loop between H(2) S and cystathionine-gamma-lyase. Phytother. Res. 34 (5), 1154–1165. doi:10.1002/ptr.6586
Zhang, H., Wang, H., Qin, L., and Lin, S. (2024). Garlic-derived compounds: epigenetic modulators and their antitumor effects. Phytother. Res. 38 (3), 1329–1344. doi:10.1002/ptr.8108
Zhang, L., Xu, S., Cheng, X., Zheng, J., Wang, Y., Wu, J., et al. (2021). Diallyl trisulphide, a H(2) S donor, compromises the stem cell phenotype and restores thyroid-specific gene expression in anaplastic thyroid carcinoma cells by targeting AKT-SOX2 axis. Phytother. Res. 35 (6), 3428–3443. doi:10.1002/ptr.7065
Zhang, Q., Li, X. T., Chen, Y., Chen, J. Q., Zhu, J. Y., Meng, Y., et al. (2018a). Wnt/β-catenin signaling mediates the suppressive effects of diallyl trisulfide on colorectal cancer stem cells. Cancer Chemother. Pharmacol. 81 (6), 969–977. doi:10.1007/s00280-018-3565-0
Zhang, T., Cao, W., Sun, H., Yu, D., and Zhong, C. (2023). Diallyl trisulfide suppresses the renal cancer stem-like cell properties via Nanog. Nutr. Cancer 75 (3), 971–979. doi:10.1080/01635581.2022.2156553
Zhang, Y., Xie, W. P., Zhang, Y. K., Chen, Y. Q., Wang, D. L., Li, G., et al. (2018b). Experimental study of inhibitory effects of diallyl trisulfide on the growth of human osteosarcoma Saos-2 cells by downregulating expression of glucose-regulated protein 78. Onco Targets Ther. 11, 271–277. doi:10.2147/OTT.S150933
Zhang, Y., Zhou, X., Xu, L., Wang, L., Liu, J., Ye, J., et al. (2017). Apoptosis of rat hepatic stellate cells induced by diallyl trisulfide and proteomics profiling in vitro. Can. J. Physiol. Pharmacol. 95 (5), 463–473. doi:10.1139/cjpp-2015-0527
Keywords: diallyl trisulfide, cancer, prevention, treatment, mechanism
Citation: Lu L, Gao Z, Song J, Jin L and Liang Z (2024) The potential of diallyl trisulfide for cancer prevention and treatment, with mechanism insights. Front. Cell Dev. Biol. 12:1450836. doi: 10.3389/fcell.2024.1450836
Received: 18 June 2024; Accepted: 17 September 2024;
Published: 30 September 2024.
Edited by:
Subhadip Mukhopadhyay, New York University, United StatesReviewed by:
Zhaoji Pan, Xuzhou Central Hospital, ChinaSivapar V Mathan, All India Institute of Medical Sciences, India
Copyright © 2024 Lu, Gao, Song, Jin and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhaofeng Liang, bGlhbmd6aGFvZmVuZ0B1anMuZWR1LmNu
 Ling Lu1
Ling Lu1 Jiajia Song
Jiajia Song Zhaofeng Liang
Zhaofeng Liang