- Department of Immunology, Center of Immunomolecular Engineering, Innovation and Practice Base for Graduate Students Education, Zunyi Medical University, Zunyi, China
Insulin-like growth factor binding protein 7 (IGFBP7) serves as a crucial extracellular matrix protein, exerting pivotal roles in both physiological and pathological processes. This comprehensive review meticulously delineates the structural attributes of IGFBP7, juxtaposing them with other members within the IGFBP families, and delves into the expression patterns across various tissues. Furthermore, the review thoroughly examines the multifaceted functions of IGFBP7, encompassing its regulatory effects on cell proliferation, apoptosis, and migration, elucidating the underlying mechanistic pathways. Moreover, it underscores the compelling roles in tumor progression, acute kidney injury, and reproductive processes. By rigorously elucidating the diverse functionalities and regulatory networks of IGFBP7 across various physiological and pathological contexts, this review aims to furnish a robust theoretical framework and delineate future research trajectories for leveraging IGFBP7 in disease diagnosis, therapeutic interventions, and pharmaceutical innovations.
1 Introduction
Insulin-like growth factor binding protein 7 (IGFBP7), as a crucial member of the IGFBP family, has been extensively investigated and recognized for its significant roles in cellular biology and pathophysiology. Serving as an extracellular matrix protein, IGFBP7 not only participates in regulating fundamental biological processes such as cell proliferation (Xia et al., 2020), apoptosis (Tang et al., 2021), and migration (Hong et al., 2023) but also exerts important regulatory effects in tumor development (Yi et al., 2022; Artico et al., 2023), angiogenesis (Bracun et al., 2022; Lam et al., 2022; Liu et al., 2023; Tan et al., 2023), renal diseases (Waskowski et al., 2021; Chapman et al., 2023; Hu et al., 2023; Stanski et al., 2023), and reproduction (Wandji et al., 2000; Huang et al., 2021; Wu et al., 2022). In recent years, continuous research focus on the relationship between the structure and function of IGFBP7, as well as its mechanistic involvement in various diseases have gradually been acknowledged. However, numerous mysteries persist regarding the functional mechanisms of IGFBP7, its potential applications in disease diagnosis, treatment, and drug development. Therefore, this review aims to systematically summarize the recent advances in the study of IGFBP7, encompassing its structural characteristics, expression pattern, as well as its functional and mechanistic roles in different physiological and pathological processes. The comprehensive understanding of IGFBP7’s biological functions provided herein is intended to lay a theoretical foundation and guide future research directions for its further development in clinical applications.
2 Structure and function of the IGFBP family
IGFBPs, a family of proteins that bind to insulin-like growth factors (IGFs) and regulate their biological activity, play a crucial role in the IGF signaling pathway (Ma et al., 2023). By binding to IGFs, IGFBPs modulate their biological activity and availability, prolonging their half-life in vivo and regulating their access to IGF receptors, thus impacting the activity of the IGF signaling pathway and regulating biological processes such as cell growth, proliferation, and apoptosis (Baxter, 2023; Galal et al., 2023; Werner, 2023). Based on their different affinities for IGF, IGFBPs are divided into two classes: high-affinity binding proteins (IGFBP1-6) and low-affinity binding proteins (IGFBP-rP1-10).
IGFBPs are a family of proteins characterized by multiple conserved domains. They typically consist of three distinct domains: the N-terminal domain, the C-terminal domain, and the central domain. The N-terminal domain contains approximately 16–18 conserved cysteine residues, including a common IGFBP motif (GCGCCXXC), which is a key region for binding to IGFs (Vorwerk et al., 2002). In contrast, the C-terminal domain usually contains about six conserved cysteine residues, with potential variations among different members of the IGFBP family (Zhou et al., 2023). The central domain, also known as the binding domain, exhibits structural differences from other domains and typically contains glycosylation and phosphorylation sites, which can influence the activity and stability of IGFBPs. The central domain mediates the binding of IGFBPs to IGFs, thereby regulating the biological activity of IGFs and the activation of cellular signaling pathways (Fowlkes et al., 1997).
The IGFBP-rPs, including IGFBP-rP1 to IGFBP-rP10, share structural and functional similarities with IGFBPs. IGFBP-rP1, initially named IGFBP7, was the first discovered IGFBP-related protein component due to its ability to connect with IGF via the N-terminal domain (Song et al., 2021). IGFBP7 has been cloned from various cellular systems and is known by multiple names such as mac25 (Kato, 2000), tumor adhesion factor (Albelda, 1993), prostate stromal factor (Yarosh et al., 2015), and angiostatin (Jin et al., 2020). Structurally, IGFBP7 differs significantly from other IGFBPs, particularly in its C-terminal domain, which lacks conserved cysteine residues, possessing only one cysteine residue (Oh et al., 1996). Moreover, IGFBP7 exhibits 100-fold lower affinity for binding to IGF-1 and is the only member of the family that binds insulin with strong affinity, limiting its binding to insulin receptors (Yamanaka et al., 1997). Unlike IGFBP3 and IGFBP5, IGFBP7 is not subject to glycosylation or phosphorylation effects and is distinguished from other IGFBPs by its regulation mechanisms at the RNA and DNA levels (Kutsukake et al., 2008). These structural and post-translational modification differences suggest that IGFBP7 may possess unique functions independent of IGF.
3 Expression of IGFBP7
IGFBP7 expression was detected in various normal tissues (Figure 1) including brain, liver, heart, small intestine, spleen, kidney, placenta, lung, skeletal muscle, thymus, prostate, testis, ovary, pancreas, and colon (Hwa et al., 1998). Immunohistochemical analysis revealed strong positive staining of IGFBP7 in peripheral nerves, respiratory cilia, epididymis, and fallopian tubes; smooth muscle cells in intestines, bladder, prostate, and endothelial cell walls also exhibited strong positive staining (Degeorges et al., 2000). Conversely, lymphocytes, plasma cells, and adipocytes displayed negative staining (Artico et al., 2021). Within the kidneys, stronger staining was observed in the epithelium of distal tubules compared to proximal tubules (Sekiuchi et al., 2012). Moreover, cells from the reticular zone and glomerular zone showed stronger staining than those from the cortical zone, with some studies indicating stronger expression of IGFBP7 in proximal tubules and localization along the brush border of certain proximal convoluted tubules (Emlet et al., 2017). In the liver, analysis via serial analysis of gene expression revealed that activated stellate cells were the major contributors to IGFBP7 expression (Degeorges et al., 2000). Notably, compared to isolated activated stellate cells, IGFBP7 exhibited lower expression throughout the entire liver. Immunohistochemical studies conducted on human prostate tissue (normal) demonstrated universally intense staining (Degeorges et al., 1999). IGFBP7 is also detectable in various body fluids such as serum, urine, cerebrospinal fluid, and amniotic fluid of pregnant women (Anderlová et al., 2022). The cell-specific differential expression pattern of IGFBP7 within tissues may suggest its potential specific functions in these organs.
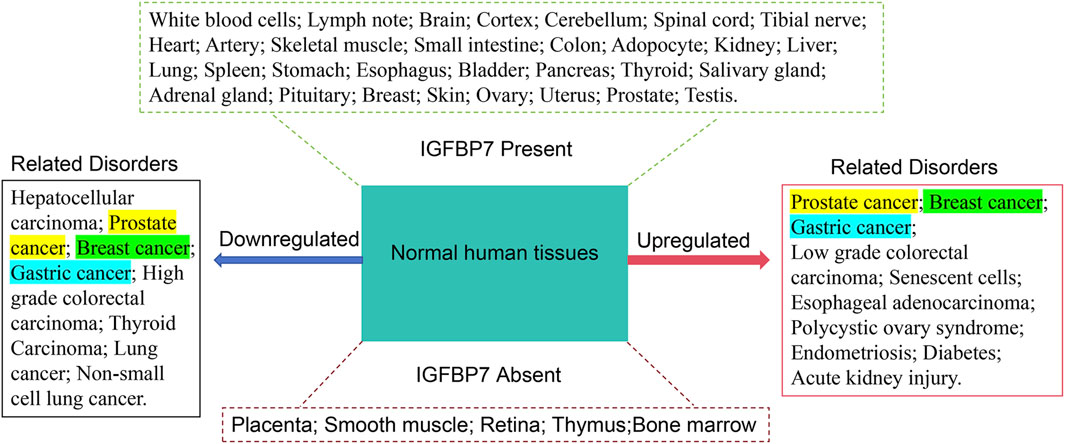
Figure 1. Expression of IGFBP7 in various tissues and its association with diseases related to its downregulation and upregulation. The left side lists diseases associated with downregulation of IGFBP7 expression, while the right side lists diseases associated with upregulation of IGFBP7 expression. The upper part shows tissues and organs where IGFBP7 expression is detected, and the lower part shows tissues and organs where IGFBP7 expression is not detected. Diseases marked with the same color indicate the same disease. Data are sourced from transcriptome sequencing data in the GEO and GeneCards databases.
4 Functional mechanisms of IGFBP7
IGFBP7, a novel member of the IGFBP superfamily, possesses a unique molecular structure characterized by a conserved N-terminal domain similar to other IGFBPs, as well as distinctive Kazal-type serine protease inhibitor domains and immunoglobulin-like C2 domains (Yamanaka et al., 1997). Apart from its canonical role in modulating the effects of IGFs, IGFBP7 independently regulates cellular processes such as apoptosis, proliferation, and migration (Kim et al., 1997). In particular, IGFBP7 is implicated in cell adhesion and tumor cell proliferation processes, with its N-terminal fragments post-degradation retaining cell membrane adhesion properties (Oh et al., 1996; Vorwerk et al., 2002). Studies have demonstrated an upregulation of IGFBP7 expression in cells treated with TGF-β1 and retinoic acid (Oh, 1998). Additionally, IGFBP7 has been shown to bind to cell surface heparan sulfate, although this interaction may be influenced by the cleavage of IGFBP7 by pancreatic trypsin-like integral membrane serine protease, matriptase (Godfried Sie et al., 2012). Cleavage by matriptase at the P1 site, involving Arg or Lys residues, has been associated with breast cancer invasion and metastasis. Proteolytic cleavage, particularly at the N-terminus, including the heparin-binding domain, reduces heparin binding and IGF-1R occupancy (Werner, 2023).
Furthermore, researchers have observed co-localization of IGFBP7 with the basement membrane in the vasculature, and subsequent direct measurement of IGFBP7 binding to extracellular matrix proteins, revealing its ability to bind to Type IV collagen (Pen et al., 2007). Moreover, IGFBP7 was found to stimulate adhesion of human umbilical vein endothelial cells to Type IV collagen matrices, inducing morphological changes. St Croix et al. (2000) also identified a role for IGFBP7 in binding to Type IV collagen protein. They demonstrated elevated expression of IGFBP7 compared to healthy endothelial cells, suggesting IGFBP7 as a potential tumor endothelial cell marker, as determined by serial analysis of gene expression.
5 The role of IGFBP7 in tumor development
The role of IGFBP7 in cancer has been a highly researched area of interest. Numerous studies have confirmed the association between IGFBP7 and various cancers (Jin et al., 2020; Li et al., 2023), including hepatocellular carcinoma, breast cancer (Godina et al., 2021; Wilcox et al., 2021), esophageal cancer (Li et al., 2022), colorectal cancer, and prostate cancer (Singh et al., 2020). However, the role of IGFBP7 appears to exhibit a complex pattern across different types of cancer. Utilizing detection techniques such as qRT-PCR, immunohistochemistry, Northern blot, and Western blot, studies have revealed that IGFBP7 expression is generally downregulated in hepatocellular carcinoma, melanoma, and lung cancer, while showing an upregulation trend in esophageal cancer. In breast, gastric, prostate, colorectal, and glioma cancers, some studies have reported upregulation of IGFBP7 expression, while others have reported downregulation, indicating a dual role of IGFBP7 in cancer cell proliferation, progression, and prognosis (Lin et al., 2019). Furthermore, research on IGFBP7 has shown its ability to alter cancer cell sensitivity to chemotherapy drugs, suggesting its potential beneficial value in anticancer therapy (Roška et al., 2020; Tang et al., 2021). However, despite a wealth of studies elucidating the significant role of IGFBP7 in tumor development, its specific mechanisms and roles in different types of cancer still require further investigation.
IGFBP7 primarily exerts its anti-tumor effects by inhibiting tumor cell growth and accelerating tumor cell apoptosis (Figure 2). This is achieved through inhibition of the expression of cell cycle proteins D1 and p21, and promotion of the expression of cell cycle proteins A, E, p16, and p27, or by suppressing Akt kinase activity, leading to upregulation of cyclin-dependent kinase (CDK) inhibitory factors p27Kip1 and p21Cip1, thereby inducing cell cycle arrest at the G0/G1 phase. Overexpression of IGFBP7 or addition of exogenous IGFBP7 in cell culture can induce cell cycle arrest at the G2 phase through non-IGF-1 receptor, AKT, and ERK pathways, subsequently leading to cell apoptosis (Sato et al., 2007; Wang et al., 2017; Zhang et al., 2019). Despite some conflicting conclusions, the majority of evidence currently suggests that IGFBP7 inhibits tumor cell growth and promotes tumor cell apoptosis, rendering it a potential candidate for tumor suppression. Overall, IGFBP7 exhibits a dual role in tumor development, inhibiting tumor cell growth and accelerating tumor cell apoptosis, thus emerging as a potential candidate for tumor suppression. However, further research is needed to elucidate its specific mechanisms and roles in different types of cancer.
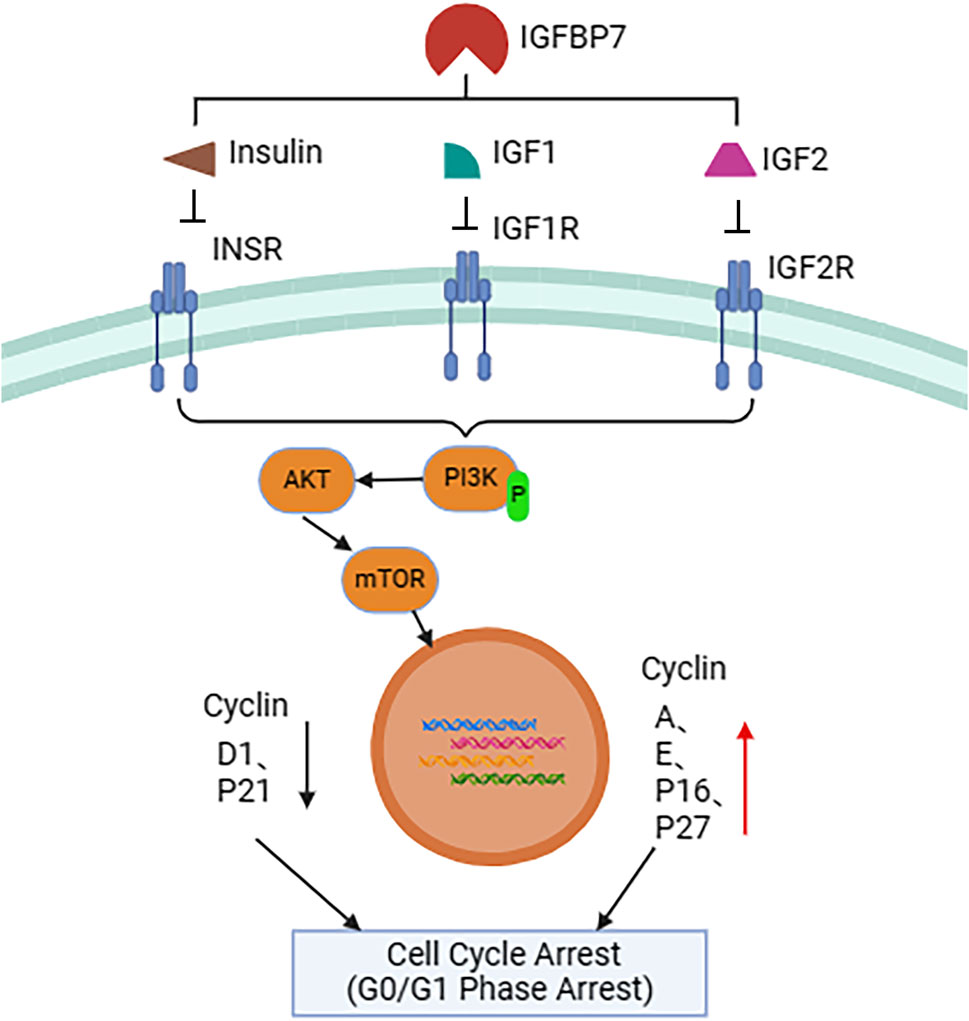
Figure 2. Schematic representation of IGFBP7-mediated regulation of the cell cycle. IGFBP7 interacts with insulin (INS), insulin-like growth factor 1 (IGF1), and insulin-like growth factor 2 (IGF2), inhibiting their binding to corresponding cell membrane receptors such as insulin receptor (INSR), IGF1 receptor (IGF1R), and IGF2 receptor (IGF2R). This process affects the activation of the intracellular PI3K-AKT-mTOR signaling pathway, ultimately leading to upregulation of nuclear cell cycle-related proteins A (Cyclin A), E (Cyclin E), P16 (CDKN2A), and P27 (CDKN1B), while downregulating D1 (Cyclin D1) and P21 (CDKN1A), resulting in cell cycle arrest at the G0/G1 phase. This IGFBP7-mediated cell cycle regulation mechanism may have significant implications for tumor growth and treatment.
6 The role of IGFBP7 in acute kidney injury
IGFBP7 has been proposed as a biomarker for acute kidney injury (AKI), aiming to enhance early detection, discrimination, and prognosis assessment, complementing serum creatinine and urine output (Meena et al., 2023; Murugan et al., 2023; Stanski et al., 2023). Insights derived from studies on TIMP2 and IGFBP7, which modulate cell cycle, exhibit differential expression and distribution, and undergo alterations in severity of AKI, along with changes in protein distribution, are crucial for guiding the diagnosis of renal injury across various etiologies, extents, and locations (proximal tubule, distal tubule, collecting duct, or interstitium). In 2013, was identified by Kashani et al. (2013) as a biomarker for AKI(56). From a screening of 340 candidate biomarkers, IGFBP7 was found to predict AKI based on creatinine standards. Released from proximal tubules, IGFBP7 facilitates pinpointing specific segments of damaged renal tubules. In the early phases of cellular stress, IGFBP-7 and TIMP2 induce G1 cell cycle arrest by inhibiting cyclin-dependent protein kinases. A TIMP2×IGFBP7 > 0.3 has demonstrated a sensitivity of 92% for moderate to severe AKI (Luthra and Tyagi, 2019; Zaouter and Ouattara, 2019). Moreover, elevated IGFBP7 mRNA levels have been observed in uranium nitrate-induced acute renal failure in mice (Taulan et al., 2006).
The mechanism of IGFBP7 in AKI involves its ability to regulate cell cycle progression (Zang et al., 2019), inflammation, fibrosis, apoptosis, and oxidative stress (Yu et al., 2022). IGFBP7 induces G1 phase cell cycle arrest in renal tubular epithelial cells, thereby inhibiting their proliferation. This effect is mediated through the upregulation of CDK inhibitors such as p21 and p27, which suppress CDK activity, thus halting cell cycle progression (Wang et al., 2019). Additionally, IGFBP7 is implicated in the modulation of renal inflammation and fibrosis, hallmark features of AKI progression. It can regulate the expression of pro-inflammatory cytokines and chemokines, including IL-6 and TNF-α, thereby mitigating inflammatory responses in the kidney (Zwaag et al., 2019). Moreover, IGFBP7 has been shown to inhibit the activation of the TGF-β signaling pathway (van Duijl et al., 2022), a key mediator of renal fibrosis, thereby ameliorating fibrotic changes in the kidney. In summary, IGFBP7 serves as a promising biomarker for acute kidney injury, aiding in early detection and prognosis assessment. Its involvement in regulating cell cycle progression, inflammation, fibrosis, apoptosis, and oxidative stress underscores its significance in AKI pathogenesis and highlights its potential as a therapeutic target.
7 The role of IGFBP7 in reproduction
IGFBP7 plays a regulatory role in folliculogenesis (Wijesena et al., 2024). IGFBP7 exhibits significant homology with follicular inhibin (Kato, 2000). Follicular inhibin is considered an inhibitor of FSH secretion, playing a pivotal role in follicular development and ovarian function (Appiah Adu-Gyamfi et al., 2020). Similarly to follicular inhibin, IGFBP7 can bind with activin A, thereby influencing the growth inhibitory effects of the TGF-β superfamily on granulosa cells (Figure 3) (Tamura et al., 2007). Recent studies have shown that IGFBP7 is expressed in granulosa cells of pig antral follicles and bovine corpora lutea, capable of suppressing estrogen production in granulosa cells (Ożegowska et al., 2018). RNA-seq results have revealed high expression of IGFBP7 in granulosa cells of buffalo antral follicles, and expression of IGFBP7 has been identified in granulosa cells of bovine large antral follicles and bovine corpora lutea (Li et al., 2018). Knockdown of IGFBP7 has been observed to affect the number of apoptotic cells, cell cycle, cell proliferation, as well as estrogen and progesterone production (Kim et al., 2018). Treatment of granulosa cells with FSH and activin has significantly increased the expression of Cyp19a1 mRNA and secretion of 17β-estradiol (E2), whereas the addition of exogenous recombinant mouse IGFBP7 in the culture medium inhibits this promotion (Tamura et al., 2007). Treatment of granulosa cells with IGFBP7-specific small interfering RNA (siRNA) reduces IGFBP7 expression, enhancing FSH-stimulated E2 secretion into the culture medium. These results suggest that IGFBP7 inhibits estrogen production in granulosa cells, indicating that this protein secreted into the follicular fluid may serve as an ovarian intrinsic factor, negatively regulating granulosa cell differentiation (Yoshie et al., 2021). Furthermore, invertebrate insulin-like growth factor-binding proteins (ILPBPs) share structural homology with vertebrate IGFBP7, and ILPBPs have been shown to potentially function in ovarian development in the invertebrate red deep-sea crab (Huang et al., 2021).
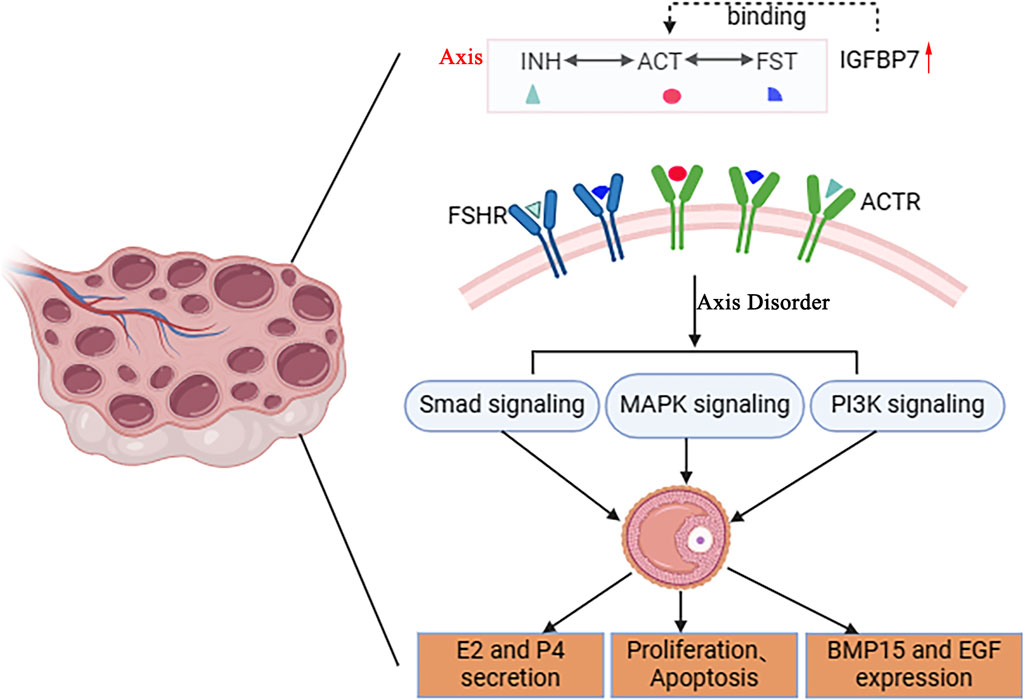
Figure 3. Potential mechanism of IGFBP7 regulation in follicular development. IGFBP7 is structurally homologous to follistatin (FST) and has been shown to interact with activins (ACT) on protein level. The INH-ACT-FST axis plays a crucial role in regulating follicular development, modulating follicular cell secretion of E2 and P4, proliferation, apoptosis, and expression of BMP15, EGF, through Smad, MAPK, and PI3K signaling pathways. This schematic diagram illustrates the potential molecular mechanism by which IGFBP7 regulates follicular development, highlighting its interactions with key players in the INH-ACT-FST axis and downstream signaling pathways.
IGFBP7 is also significantly associated with embryo implantation and the success rate of pregnancy. IGFBP7 is present in the uterine glandular epithelial cells and uterine stromal cells, with elevated expression during the mid-to-late secretory phase of the menstrual cycle in women (Domínguez et al., 2003). In vitro studies have demonstrated that IGFBP7 acts as a decidualization regulator in uterine stromal cells, potentially exerting its effects during the decidualization process of uterine stromal cells (Kutsukake et al., 2007; Yoshie et al., 2021). IGFBP7 participates in embryo implantation and uterine decidualization. Inhibition of IGFBP7 significantly increases the TH1-type cytokine IFNγ and decreases the Th2-type cytokines IL-4 and IL-10, thereby inhibiting uterine decidualization and reducing uterine receptivity. This can significantly lower embryo implantation and pregnancy rates, leading to pregnancy failure in a mouse model (Liu et al., 2012). In human umbilical vein endothelial cells, IGFBP7 treatment inhibits exogenous VEGF-induced angiogenesis, proliferation, and phosphorylation of MEK and ERK (Tamura et al., 2009). Using the human endometrial epithelial cell line (EM1) to study the significance of IGFBP7 in endometrial glandular function, the results indicate that IGFBP7 regulates glandular cell morphological changes by interfering with normal PKA and MAPK signaling pathways associated with the transformation and/or differentiation of endometrial glands, which is crucial for the initiation of embryo implantation (Kutsukake et al., 2010).
IGFBP7 plays a crucial role in pathological pregnancies, including complete hydatidiform mole, pregnancy-related nausea and vomiting (hyperemesis gravidarum), and endometriosis. IHC analysis revealed that downregulation of IGFBP7 may play a significant role in the progression of complete hydatidiform mole (Xiao et al., 2014). Common variants of IGFBP7 are susceptibility loci for the diagnosis of pregnancy-related nausea and vomiting (Fejzo et al., 2019b), with serum levels of IGFBP7 significantly increased in hyperemesis gravidarum women at 12 weeks of pregnancy (Fejzo et al., 2019a). Moreover, the homologue of fruit fly IGFBP7 has been shown to play a role in coordinating neurons between metabolic states and feeding behavior, potentially conveying food preferences and pregnancy intentions (Bader et al., 2013). IGFBP7 is associated with the pathophysiology of endometriosis, as serum IGFBP7 concentrations in patients with endometriosis are significantly higher than those in the control group (Kutsukake et al., 2008), and metformin can upregulate the expression of IGFBP7 in both human and mouse models of endometriosis (Huang et al., 2022). IGFBP7 is also involved in male reproductive processes. A study conducted at the Federal University of São Paulo from May 2014 to April 2016 detected increased expression levels of IGFBP7 protein in the semen of patients with varicocele using Western blot analysis (Belardin et al., 2016).
In summary, IGFBP7 plays a multifaceted role in folliculogenesis, embryo implantation, pregnancy success, and pathological pregnancies, including conditions like complete hydatidiform mole, hyperemesis gravidarum, and endometriosis. Its involvement in regulating decidualization, angiogenesis, glandular function, and neuronal coordination underscores its significance in reproductive processes and highlights its potential as a diagnostic and therapeutic target in reproductive disorders.
In this review, we focus on the roles of IGFBP7 in tumor development, acute kidney injury, and reproduction due to their significant impact on clinical outcomes and the extensive research supporting IGFBP7’s involvement in these areas. These functions are critical in understanding IGFBP7’s diverse biological activities and its potential as a therapeutic target. Furthermore, IGFBP7’s interaction with key signaling pathways such as the AKT/ERK pathway, which are common to these conditions, underscores its multifaceted role in cellular processes. In the realm of future research, IGFBP7 holds promise across multiple fronts. In cancer, its tumor-suppressive properties in melanoma, breast, and colorectal cancers beckon exploration into underlying mechanisms and its potential as a biomarker for early detection and therapeutic target. Additionally, its role in fibrosis regulation in organs like the liver, lungs, and kidneys warrants investigation into fibrotic disease pathogenesis and therapeutic potential. In metabolic disorders such as diabetes and obesity, IGFBP7’s influence on metabolic processes hints at diagnostic and therapeutic applications. By delving into these avenues, IGFBP7 could emerge as a pivotal player in disease diagnosis, prognosis, and treatment strategies, offering hope for improved healthcare outcomes.
Author contributions
LC: Data curation, Formal Analysis, Methodology, Resources, Software, Writing–original draft. LH: Data curation, Writing–original draft, Conceptualization, Investigation, Visualization. JL: Visualization, Funding acquisition, Supervision, Validation, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work financially supported by the National Natural Science Foundation of China (82060272), the Guizhou Province General Higher Education Youth Science and Technology Talent Growth Project ([2022] No. 278), and the Zunyi Science and Technology Plan Project ([2020] No. 73).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Albelda, S. M. (1993). Role of integrins and other cell adhesion molecules in tumor progression and metastasis. Lab. Invest 68, 4–17.
Anderlová, K., Cinkajzlová, A., Šimják, P., Kloučková, J., Kratochvílová, H., Lacinová, Z., et al. (2022). Association between gestational diabetes mellitus and bioavailability of insulin-like growth factors and role of their binding proteins. Growth Horm. IGF Res. 67, 101511. doi:10.1016/j.ghir.2022.101511
Appiah Adu-Gyamfi, E., Tanam Djankpa, F., Nelson, W., Czika, A., Kumar Sah, S., Lamptey, J., et al. (2020). Activin and inhibin signaling: from regulation of physiology to involvement in the pathology of the female reproductive system. Cytokine 133, 155105. doi:10.1016/j.cyto.2020.155105
Artico, L. L., Laranjeira, A., Campos, L. W., Corrêa, J. R., Zenatti, P. P., Carvalheira, J., et al. (2021). Physiologic IGFBP7 levels prolong IGF1R activation in acute lymphoblastic leukemia. Blood Adv. 5, 3633–3646. doi:10.1182/bloodadvances.2020003627
Artico, L. L., Ruas, J. S., Teixeira Júnior, J. R., Migita, N. A., Seguchi, G., Shi, X., et al. (2023). IGFBP7 fuels the glycolytic metabolism in B-cell precursor acute lymphoblastic leukemia by sustaining activation of the IGF1R-Akt-GLUT1 Axis. Int. J. Mol. Sci. 24, 9679. doi:10.3390/ijms24119679
Bader, R., Sarraf-Zadeh, L., Peters, M., Moderau, N., Stocker, H., Köhler, K., et al. (2013). The IGFBP7 homolog Imp-L2 promotes insulin signaling in distinct neurons of the Drosophila brain. J. Cell Sci. 126, 2571–2576. doi:10.1242/jcs.120261
Baxter, R. C. (2023). Signaling pathways of the insulin-like growth factor binding proteins. Endocr. Rev. 44, 753–778. doi:10.1210/endrev/bnad008
Belardin, L. B., Del Giudice, P. T., Camargo, M., Intasqui, P., Antoniassi, M. P., Bertolla, R. P., et al. (2016). Alterations in the proliferative/apoptotic equilibrium in semen of adolescents with varicocele. J. Assist. Reprod. Genet. 33, 1657–1664. doi:10.1007/s10815-016-0808-z
Bracun, V., van Essen, B., Voors, A. A., van Veldhuisen, D. J., Dickstein, K., Zannad, F., et al. (2022). Insulin-like growth factor binding protein 7 (IGFBP7), a link between heart failure and senescence. Esc. Heart Fail 9, 4167–4176. doi:10.1002/ehf2.14120
Chapman, C. L., Holt, S. M., O'Connell, C. T., Brazelton, S. C., Howells, W., Medved, H. N., et al. (2023). Acute kidney injury biomarkers and hydration assessments following prolonged mild hypohydration in healthy young adults. Am. J. Physiol. Ren. Physiol. 325, F199–F213. doi:10.1152/ajprenal.00086.2023
Degeorges, A., Wang, F., Frierson, H. F., Seth, A., Chung, L. W., and Sikes, R. A. (1999). Human prostate cancer expresses the low affinity insulin-like growth factor binding protein IGFBP-rP1. Cancer Res. 59, 2787–2790.
Degeorges, A., Wang, F., Frierson, H. F., Seth, A., and Sikes, R. A. (2000). Distribution of IGFBP-rP1 in normal human tissues. J. Histochem Cytochem 48, 747–754. doi:10.1177/002215540004800603
Domínguez, F., Avila, S., Cervero, A., Martín, J., Pellicer, A., Castrillo, J. L., et al. (2003). A combined approach for gene discovery identifies insulin-like growth factor-binding protein-related protein 1 as a new gene implicated in human endometrial receptivity. J. Clin. Endocrinol. Metab. 88, 1849–1857. doi:10.1210/jc.2002-020724
Emlet, D. R., Pastor-Soler, N., Marciszyn, A., Wen, X., Gomez, H., Humphries, W. H., et al. (2017). Insulin-like growth factor binding protein 7 and tissue inhibitor of metalloproteinases-2: differential expression and secretion in human kidney tubule cells. Am. J. Physiol. Ren. Physiol. 312, F284–F296. doi:10.1152/ajprenal.00271.2016
Fejzo, M. S., Fasching, P. A., Schneider, M. O., Schwitulla, J., Beckmann, M. W., Schwenke, E., et al. (2019a). Analysis of GDF15 and IGFBP7 in hyperemesis gravidarum support causality. Geburtshilfe Frauenheilkd 79, 382–388. doi:10.1055/a-0830-1346
Fejzo, M. S., Trovik, J., Grooten, I. J., Sridharan, K., Roseboom, T. J., Vikanes, A., et al. (2019b). Nausea and vomiting of pregnancy and hyperemesis gravidarum. Nat. Rev. Dis. Prim. 5, 62. doi:10.1038/s41572-019-0110-3
Fowlkes, J. L., Thrailkill, K. M., George-Nascimento, C., Rosenberg, C. K., and Serra, D. M. (1997). Heparin-binding, highly basic regions within the thyroglobulin type-1 repeat of insulin-like growth factor (IGF)-binding proteins (IGFBPs) -3, -5, and -6 inhibit IGFBP-4 degradation. Endocrinology 138, 2280–2285. doi:10.1210/endo.138.6.5182
Galal, M. A., Alouch, S. S., Alsultan, B. S., Dahman, H., Alyabis, N. A., Alammar, S. A., et al. (2023). Insulin receptor isoforms and insulin growth factor-like receptors: implications in cell signaling, carcinogenesis, and chemoresistance. Int. J. Mol. Sci. 24, 15006. doi:10.3390/ijms241915006
Godfried Sie, C., Hesler, S., Maas, S., and Kuchka, M. (2012). IGFBP7’s susceptibility to proteolysis is altered by A-to-I RNA editing of its transcript. FEBS Lett. 586, 2313–2317. doi:10.1016/j.febslet.2012.06.037
Godina, C., Khazaei, S., Tryggvadottir, H., Visse, E., Nodin, B., Jirström, K., et al. (2021). Prognostic impact of tumor-specific insulin-like growth factor binding protein 7 (IGFBP7) levels in breast cancer: a prospective cohort study. Carcinogenesis 42, 1314–1325. doi:10.1093/carcin/bgab090
Hong, Z., Xie, W., Zhuo, H., Wei, X., Wang, K., Cheng, J., et al. (2023). Crosstalk between cancer cells and cancer-associated fibroblasts mediated by TGF-β1-IGFBP7 signaling promotes the progression of infiltrative gastric cancer. Cancers (Basel) 15, 3965. doi:10.3390/cancers15153965
Hu, B. C., Zhu, J. W., Wu, G. H., Cai, J. J., Yang, X., Shao, Z. Q., et al. (2023). Auto- and paracrine rewiring of NIX-mediated mitophagy by insulin-like growth factor-binding protein 7 in septic AKI escalates inflammation-coupling tubular damage. Life Sci. 322, 121653. doi:10.1016/j.lfs.2023.121653
Huang, X., Green, S., and Sook Chung, J. (2021). The presence of an insulin-like peptide-binding protein (ILPBP) in the ovary and its involvement in the ovarian development of the red deep-sea crab, Chaceon quinquedens. Gen. Comp. Endocrinol. 301, 113653. doi:10.1016/j.ygcen.2020.113653
Huang, X., Xiao, L., Long, Y., Pei, T., Luo, B., Liao, T., et al. (2022). Comparative proteomic analysis reveals metformin improves the expression of biomarkers of endometrial receptivity in infertile women with minimal/mild endometriosis. Reprod. Sci. 29, 2593–2606. doi:10.1007/s43032-022-00869-3
Hwa, V., Tomasini-Sprenger, C., Bermejo, A. L., Rosenfeld, R. G., and Plymate, S. R. (1998). Characterization of insulin-like growth factor-binding protein-related protein-1 in prostate cells. J. Clin. Endocrinol. Metab. 83, 4355–4362. doi:10.1210/jcem.83.12.5341
Jin, L., Shen, F., Weinfeld, M., and Sergi, C. (2020). Insulin growth factor binding protein 7 (IGFBP7)-Related cancer and IGFBP3 and IGFBP7 crosstalk. Front. Oncol. 10, 727. doi:10.3389/fonc.2020.00727
Kashani, K., Al-Khafaji, A., Ardiles, T., Artigas, A., Bagshaw, S. M., Bell, M., et al. (2013). Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit. Care 17, R25. doi:10.1186/cc12503
Kato, M. V. (2000). A secreted tumor-suppressor, mac25, with activin-binding activity. Mol. Med. 6, 126–135. doi:10.1007/s0089400060126
Kim, H. S., Nagalla, S. R., Oh, Y., Wilson, E., Roberts, C. T., and Rosenfeld, R. G. (1997). Identification of a family of low-affinity insulin-like growth factor binding proteins (IGFBPs): characterization of connective tissue growth factor as a member of the IGFBP superfamily. Proc. Natl. Acad. Sci. U. S. A. 94, 12981–12986. doi:10.1073/pnas.94.24.12981
Kim, J., Kim, W. H., Byeon, S. J., Lee, B. L., and Kim, M. A. (2018). Epigenetic downregulation and growth inhibition of IGFBP7 in gastric cancer. Asian Pac J. Cancer Prev. 19, 667–675. doi:10.22034/APJCP.2018.19.3.667
Kutsukake, M., Ishihara, R., Momose, K., Isaka, K., Itokazu, O., Higuma, C., et al. (2008). Circulating IGF-binding protein 7 (IGFBP7) levels are elevated in patients with endometriosis or undergoing diabetic hemodialysis. Reprod. Biol. Endocrinol. 6, 54. doi:10.1186/1477-7827-6-54
Kutsukake, M., Ishihara, R., Yoshie, M., Kogo, H., and Tamura, K. (2007). Involvement of insulin-like growth factor-binding protein-related protein 1 in decidualization of human endometrial stromal cells. Mol. Hum. Reprod. 13, 737–743. doi:10.1093/molehr/gam058
Kutsukake, M., Tamura, K., Yoshie, M., and Tachikawa, E. (2010). Knockdown of IGF-binding protein 7 inhibits transformation of the endometrial gland in an in vitro model. Mol. Reprod. Dev. 77, 265–272. doi:10.1002/mrd.21143
Lam, J., Lee, B., Yu, J., Kwee, B. J., Kim, Y., Kim, J., et al. (2022). A microphysiological system-based potency bioassay for the functional quality assessment of mesenchymal stromal cells targeting vasculogenesis. Biomaterials 290, 121826. doi:10.1016/j.biomaterials.2022.121826
Li, D., Xia, L., Huang, P., Wang, Z., Guo, Q., Huang, C., et al. (2023). Cancer-associated fibroblast-secreted IGFBP7 promotes gastric cancer by enhancing tumor associated macrophage infiltration via FGF2/FGFR1/PI3K/AKT axis. Cell Death Discov. 9, 17. doi:10.1038/s41420-023-01336-x
Li, J., Liu, J., Campanile, G., Plastow, G., Zhang, C., Wang, Z., et al. (2018). Novel insights into the genetic basis of buffalo reproductive performance. BMC Genomics 19, 814. doi:10.1186/s12864-018-5208-6
Li, X., Zhang, J., Wu, Y., Ma, C., Wei, D., Pan, L., et al. (2022). IGFBP7 remodels the tumor microenvironment of esophageal squamous cell carcinoma by activating the TGFβ1/SMAD signaling pathway. Oncol. Lett. 24, 251. doi:10.3892/ol.2022.13371
Lin, H., Ruan, G. Y., Sun, X. Q., Chen, X. Y., Zheng, X., and Sun, P. M. (2019). Effects of RNAi-induced Skp2 inhibition on cell cycle, apoptosis and proliferation of endometrial carcinoma cells. Exp. Ther. Med. 17, 3441–3450. doi:10.3892/etm.2019.7392
Liu, F., Yu, T., Liu, J., Yang, Q., Wu, J., Ren, J., et al. (2023). IGFBP-7 secreted by adipose-derived stem cells inhibits keloid formation via the BRAF/MEK/ERK signaling pathway. J. Dermatol Sci. 111, 10–19. doi:10.1016/j.jdermsci.2023.05.004
Liu, Z. K., Wang, R. C., Han, B. C., Yang, Y., and Peng, J. P. (2012). A novel role of IGFBP7 in mouse uterus: regulating uterine receptivity through Th1/Th2 lymphocyte balance and decidualization. PLoS One 7, e45224. doi:10.1371/journal.pone.0045224
Luthra, A., and Tyagi, A. (2019). [TIMP-2]*[IGFBP7] for predicting early AKI. Anaesth. Crit. Care Pain Med. 38, 677. doi:10.1016/j.accpm.2018.11.011
Ma, L., Zhao, W., Huang, S., Xu, F., Wang, Y., Deng, D., et al. (2023). IGF/IGF-1R signal pathway in pain: a promising therapeutic target. Int. J. Biol. Sci. 19, 3472–3482. doi:10.7150/ijbs.84353
Meena, J., Thomas, C. C., Kumar, J., Mathew, G., and Bagga, A. (2023). Biomarkers for prediction of acute kidney injury in pediatric patients: a systematic review and meta-analysis of diagnostic test accuracy studies. Pediatr. Nephrol. 38, 3241–3251. doi:10.1007/s00467-023-05891-4
Murugan, R., Boudreaux-Kelly, M. Y., Kellum, J. A., Palevsky, P. M., and Weisbord, S. (2023). Contrast-associated acute kidney injury and cardiovascular events: a secondary analysis of the PRESERVE cohort. Clin. Kidney J. 16, 2626–2638. doi:10.1093/ckj/sfad214
Oh, Y. (1998). IGF-independent regulation of breast cancer growth by IGF binding proteins. Breast Cancer Res. Treat. 47, 283–293. doi:10.1023/a:1005911319432
Oh, Y., Nagalla, S. R., Yamanaka, Y., Kim, H. S., Wilson, E., and Rosenfeld, R. G. (1996). Synthesis and characterization of insulin-like growth factor-binding protein (IGFBP)-7. Recombinant human mac25 protein specifically binds IGF-I and -II. J. Biol. Chem. 271, 30322–30325. doi:10.1074/jbc.271.48.30322
Ożegowska, K., Dyszkiewicz-Konwińska, M., Celichowski, P., Nawrocki, M. J., Bryja, A., Jankowski, M., et al. (2018). Expression pattern of new genes regulating female sex differentiation and in vitro maturational status of oocytes in pigs. Theriogenology 121, 122–133. doi:10.1016/j.theriogenology.2018.08.019
Pen, A., Moreno, M. J., Martin, J., and Stanimirovic, D. B. (2007). Molecular markers of extracellular matrix remodeling in glioblastoma vessels: microarray study of laser-captured glioblastoma vessels. Glia 55, 559–572. doi:10.1002/glia.20481
Roška, J., Wachsmannová, L., Hurbanová, L., Šestáková, Z., Mueller, T., Jurkovičová, D., et al. (2020). Differential gene expression in cisplatin-resistant and -sensitive testicular germ cell tumor cell lines. Oncotarget 11, 4735–4753. doi:10.18632/oncotarget.27844
Sato, Y., Chen, Z., and Miyazaki, K. (2007). Strong suppression of tumor growth by insulin-like growth factor-binding protein-related protein 1/tumor-derived cell adhesion factor/mac25. Cancer Sci. 98, 1055–1063. doi:10.1111/j.1349-7006.2007.00502.x
Sekiuchi, M., Kudo, A., Nakabayashi, K., Kanai-Azuma, M., Akimoto, Y., Kawakami, H., et al. (2012). Expression of matrix metalloproteinases 2 and 9 and tissue inhibitors of matrix metalloproteinases 2 and 1 in the glomeruli of human glomerular diseases: the results of studies using immunofluorescence, in situ hybridization, and immunoelectron microscopy. Clin. Exp. Nephrol. 16, 863–874. doi:10.1007/s10157-012-0633-3
Singh, C. K., Chhabra, G., Ndiaye, M. A., Siddiqui, I. A., Panackal, J. E., Mintie, C. A., et al. (2020). Quercetin-resveratrol combination for prostate cancer management in TRAMP mice. Cancers (Basel) 12, 2141. doi:10.3390/cancers12082141
Song, F., Zhou, X. X., Hu, Y., Li, G., and Wang, Y. (2021). The roles of insulin-like growth factor binding protein family in development and diseases. Adv. Ther. 38, 885–903. doi:10.1007/s12325-020-01581-x
Stanski, N. L., Rodrigues, C. E., Strader, M., Murray, P. T., Endre, Z. H., and Bagshaw, S. M. (2023). Precision management of acute kidney injury in the intensive care unit: current state of the art. Intensive Care Med. 49, 1049–1061. doi:10.1007/s00134-023-07171-z
St Croix, B., Rago, C., Velculescu, V., Traverso, G., Romans, K. E., Montgomery, E., et al. (2000). Genes expressed in human tumor endothelium. Science. 289, 1197–1202. doi:10.1126/science.289.5482.1197
Tamura, K., Hashimoto, K., Suzuki, K., Yoshie, M., Kutsukake, M., and Sakurai, T. (2009). Insulin-like growth factor binding protein-7 (IGFBP7) blocks vascular endothelial cell growth factor (VEGF)-induced angiogenesis in human vascular endothelial cells. Eur. J. Pharmacol. 610, 61–67. doi:10.1016/j.ejphar.2009.01.045
Tamura, K., Matsushita, M., Endo, A., Kutsukake, M., and Kogo, H. (2007). Effect of insulin-like growth factor-binding protein 7 on steroidogenesis in granulosa cells derived from equine chorionic gonadotropin-primed immature rat ovaries. Biol. Reprod. 77, 485–491. doi:10.1095/biolreprod.106.058867
Tan, E., Chan, S. P., Choi, Y. C., Pemberton, C. J., Troughton, R., Poppe, K., et al. (2023). Regional handling and prognostic performance of circulating insulin-like growth factor binding protein-7 in heart failure. JACC Heart Fail 11, 662–674. doi:10.1016/j.jchf.2023.01.016
Tang, X., Mu, J., Ma, L., Tan, Q., Wang, J., Tan, J., et al. (2021). IGFBP7 overexpression promotes acquired resistance to AZD9291 in non-small cell lung cancer. Biochem. Biophys. Res. Commun. 571, 38–45. doi:10.1016/j.bbrc.2021.07.055
Taulan, M., Paquet, F., Argiles, A., Demaille, J., and Romey, M. C. (2006). Comprehensive analysis of the renal transcriptional response to acute uranyl nitrate exposure. BMC Genomics 7, 2. doi:10.1186/1471-2164-7-2
van Duijl, T. T., Ruhaak, L. R., Hoogeveen, E. K., de Mutsert, R., Rosendaal, F. R., le Cessie, S., et al. (2022). Reference intervals of urinary kidney injury biomarkers for middle-aged men and women determined by quantitative protein mass spectrometry. Ann. Clin. Biochem. 59, 420–432. doi:10.1177/00045632221121780
Vorwerk, P., Hohmann, B., Oh, Y., Rosenfeld, R. G., and Shymko, R. M. (2002). Binding properties of insulin-like growth factor binding protein-3 (IGFBP-3), IGFBP-3 N- and C-terminal fragments, and structurally related proteins mac25 and connective tissue growth factor measured using a biosensor. Endocrinology 143, 1677–1685. doi:10.1210/endo.143.5.8760
Wandji, S. A., Gadsby, J. E., Barber, J. A., and Hammond, J. M. (2000). Messenger ribonucleic acids for MAC25 and connective tissue growth factor (CTGF) are inversely regulated during folliculogenesis and early luteogenesis. Endocrinology 141, 2648–2657. doi:10.1210/endo.141.7.7576
Wang, X., Li, Y., Zhao, Z., Meng, Y., Bian, J., Bao, R., et al. (2019). IGFBP7 regulates sepsis-induced epithelial-mesenchymal transition through ERK1/2 signaling. Acta Biochim. Biophys. Sin. (Shanghai) 51, 799–806. doi:10.1093/abbs/gmz072
Wang, Y., Zou, Z., Jin, J., Teng, J., Xu, J., Shen, B., et al. (2017). Urinary TIMP-2 and IGFBP7 for the prediction of acute kidney injury following cardiac surgery. BMC Nephrol. 18, 177. doi:10.1186/s12882-017-0592-8
Waskowski, J., Pfortmueller, C. A., Schenk, N., Buehlmann, R., Schmidli, J., Erdoes, G., et al. (2021). (TIMP2) x (IGFBP7) as early renal biomarker for the prediction of acute kidney injury in aortic surgery (TIGER). A single center observational study. PLoS One 16, e0244658. doi:10.1371/journal.pone.0244658
Werner, H. (2023). The IGF1 signaling pathway: from basic concepts to therapeutic opportunities. Int. J. Mol. Sci. 24, 14882. doi:10.3390/ijms241914882
Wijesena, H. R., Keel, B. N., Nonneman, D. J., Cushman, R. A., and Lents, C. A. (2024). Clustering of multi-tissue transcriptomes in gilts with normal cyclicity or delayed puberty reveals genes related to pubertal development. Biol. Reprod. 110, 261–274. doi:10.1093/biolre/ioad145
Wilcox, N. S., Demessei, B. G., Smith, A. M., Carver, J. R., Zhang, L., Liu, P., et al. (2021). Sustained increases in IGFBP-7 may Be related to doxorubicin in breast cancer patients. JACC CardioOncol 3 (3), 444–446. doi:10.1016/j.jaccao.2021.06.005
Wu, P. L., Zhu, J. W., Zeng, C., Li, X., Xue, Q., and Yang, H. X. (2022). IGFBP7 enhances trophoblast invasion via IGF-1R/c-Jun signaling in unexplained recurrent spontaneous abortion. Reproduction 164, 231–241. doi:10.1530/REP-21-0501
Xia, C., Yang, F., He, Z., and Cai, Y. (2020). iTRAQ-based quantitative proteomic analysis of the inhibition of cervical cancer cell invasion and migration by metformin. Biomed. Pharmacother. 123, 109762. doi:10.1016/j.biopha.2019.109762
Xiao, P., Yin, R. T., Xie, D., Tong, L. X., and Li, K. M. (2014). Expression of CLIC1 and IGFBP7 in complete hydatidiform mole. Sichuan Da Xue Xue Bao Yi Xue Ban. 45, 612–617.
Yamanaka, Y., Wilson, E. M., Rosenfeld, R. G., and Oh, Y. (1997). Inhibition of insulin receptor activation by insulin-like growth factor binding proteins. J. Biol. Chem. 272, 30729–30734. doi:10.1074/jbc.272.49.30729
Yarosh, C. A., Iacona, J. R., Lutz, C. S., and Lynch, K. W. (2015). PSF: nuclear busy-body or nuclear facilitator. Wiley Interdiscip. Rev. RNA 6, 351–367. doi:10.1002/wrna.1280
Yi, X., Zheng, X., Xu, H., Li, J., Zhang, T., Ge, P., et al. (2022). IGFBP7 and the tumor immune landscape: a novel target for immunotherapy in bladder cancer. Front. Immunol. 13, 898493. doi:10.3389/fimmu.2022.898493
Yoshie, M., Kusama, K., Tanaka, R., Okubo, T., Kojima, J., Takaesu, Y., et al. (2021). Possible roles of calreticulin in uterine decidualization and receptivity in rats and humans. Int. J. Mol. Sci. 22, 10505. doi:10.3390/ijms221910505
Yu, J. T., Hu, X. W., Yang, Q., Shan, R. R., Zhang, Y., Dong, Z. H., et al. (2022). Insulin-like growth factor binding protein 7 promotes acute kidney injury by alleviating poly ADP ribose polymerase 1 degradation. Kidney Int. 102, 828–844. doi:10.1016/j.kint.2022.05.026
Zang, H., Yang, Q., and Li, J. (2019). Eleutheroside B protects against acute kidney injury by activating IGF pathway. Molecules 24, 3876. doi:10.3390/molecules24213876
Zaouter, C., and Ouattara, A. (2019). [TIMP-2]*[IGFBP7] for predicting early AKI, response to prof. Tyagi. Anaesth. Crit. Care Pain Med. 38, 679. doi:10.1016/j.accpm.2019.10.007
Zhang, L., Lian, R., Zhao, J., Feng, X., Ye, R., Pan, L., et al. (2019). IGFBP7 inhibits cell proliferation by suppressing AKT activity and cell cycle progression in thyroid carcinoma. Cell Biosci. 9, 44. doi:10.1186/s13578-019-0310-2
Zhou, H., Sun, Q., Feng, M., Gao, Z., Jia, S., Cao, L., et al. (2023). Regulatory mechanisms and therapeutic implications of insulin-like growth factor 2 mRNA-binding proteins, the emerging crucial m(6)A regulators of tumors. Theranostics 13, 4247–4265. doi:10.7150/thno.86528
Zwaag, J., Beunders, R., Warlé, M. C., Kellum, J. A., Riksen, N. P., Pickkers, P., et al. (2019). Remote ischaemic preconditioning does not modulate the systemic inflammatory response or renal tubular stress biomarkers after endotoxaemia in healthy human volunteers: a single-centre, mechanistic, randomised controlled trial. Br. J. Anaesth. 123, 177–185. doi:10.1016/j.bja.2019.03.037
Keywords: acute kidney injury, IGFBP7, insulin-like growth factor, reproduction, tumor
Citation: Chen L, Hui L and Li J (2024) The multifaceted role of insulin-like growth factor binding protein 7. Front. Cell Dev. Biol. 12:1420862. doi: 10.3389/fcell.2024.1420862
Received: 24 April 2024; Accepted: 01 July 2024;
Published: 16 July 2024.
Edited by:
Essa M. Saied, Humboldt University of Berlin, GermanyReviewed by:
Swetha Ramadesikan, Nationwide Children’s Hospital Columbus OH, United StatesZhejun Chen, Zhejiang Chinese Medical University, China
Maria C Arroyo Lopez, Darmstadt University of Technology, Germany
Victoria Rozés-Salvador, CONICET Centre for Research in Clinical Biochemistry and Immunology (CIBICI), Argentina
Copyright © 2024 Chen, Hui and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Li, aHVvc3UyMDE0QDE2My5jb20=
†These authors have contributed equally to this work
 Li Chen†
Li Chen† Jun Li
Jun Li