- 1Wolfson Centre for Mathematical Biology, Mathematical Institute, University of Oxford, Oxford, United Kingdom
- 2Computational Developmental Biology Group, Institute of Biodynamics and Biocomplexity, Utrecht University, Utrecht, Netherlands
- 3Northern Institute for Cancer Research, Newcastle University, Newcastle upon Tyne, United Kingdom
- 4LaserLab, Department of Physics and Astronomy, Vrije Universiteit Amsterdam, Amsterdam, Netherlands
- 5Optics11 life, Amsterdam, Netherlands
- 6Watson School of Mathematics, University of Birmingham, Birmingham, United Kingdom
- 7Department of Applied Physics and Science Education, Eindhoven University of Technology, Eindhoven, Netherlands
- 8Department of Intelligent Systems Engineering, Indiana University, Bloomington, IN, United States
- 9Department of Informatics, Indiana University, Bloomington, IN, United States
- 10Institut Curie, Université PSL, Paris, France
- 11Altos Labs, Redwood City, CA, United States
The extracellular matrix (ECM) is a highly complex structure through which biochemical and mechanical signals are transmitted. In processes of cell migration, the ECM also acts as a scaffold, providing structural support to cells as well as points of potential attachment. Although the ECM is a well-studied structure, its role in many biological processes remains difficult to investigate comprehensively due to its complexity and structural variation within an organism. In tandem with experiments, mathematical models are helpful in refining and testing hypotheses, generating predictions, and exploring conditions outside the scope of experiments. Such models can be combined and calibrated with in vivo and in vitro data to identify critical cell-ECM interactions that drive developmental and homeostatic processes, or the progression of diseases. In this review, we focus on mathematical and computational models of the ECM in processes such as cell migration including cancer metastasis, and in tissue structure and morphogenesis. By highlighting the predictive power of these models, we aim to help bridge the gap between experimental and computational approaches to studying the ECM and to provide guidance on selecting an appropriate model framework to complement corresponding experimental studies.
1 Introduction
The extracellular matrix (ECM) encompasses all biological components outside of cells. It provides biochemical cues and acts as a physical scaffold for cells and tissues, facilitating cell-cell and cell-microenvironment communication (Bowers et al., 2010). The ECM is present within all tissues and organs and its composition is highly diverse, varying with the type and location of tissue in an organism (Karamanos et al., 2021). This structural variation is further amplified by cell-mediated ECM remodeling. For example, cells can realign or crosslink ECM components, thereby changing matrix stiffness (Karsdal et al., 2013), or directly degrade the ECM to expedite invasion (Artym et al., 2009). Additionally, the ECM provides essential biochemical and biomechanical cues for cell differentiation, tissue morphogenesis, and homeostasis (Yamada et al., 2019; He et al., 2022a). The mechanical effects of the ECM are particularly important in facilitating cell movement during embryonic development, wound healing, and in the progression of diseases such as fibrosis and cancer (Cruz Walma and Yamada, 2020; Diller and Tabor, 2022; Dzobo and Dandara, 2023). For example, an increase in ECM stiffness is associated with the progression of tumor malignancy (Guo et al., 2022a).
The highly complex nature of the ECM means that it remains difficult to fully quantify and understand the role of all components through experiments alone. Mathematical and computational models, therefore, are well-poised to provide further insights via rapid simulation of complex biological processes involving the ECM at a lower cost than corresponding experiments.
The aim of this review is to highlight the various approaches used to model biological systems in which the ECM plays a major role. We begin by briefly reviewing ECM structure and function, as well as experimental and modeling approaches. We provide both a glossary (Table 1) and a list of abbreviations (Table 2) of important biological and mathematical terms found throughout the review. We then give an in-depth overview of theoretical work that includes model representations of the ECM, focusing on models of 1) cell migration (including wound healing and cancer invasion) and 2) tissue structure and morphology (including morphogenesis). We selectively highlight models that explicitly represent the ECM and cover a breadth of techniques. Each section begins with a short overview of the underlying biological processes and reviews the different types of models that have been developed in each context, highlighting key results. Finally, we provide guidance on choosing the most appropriate modeling framework for a given scientific inquiry and comment on the current open challenges surrounding modeling of the ECM. We also list potential limitations of these models and highlight the importance of collaborations to overcome these difficulties in further uncovering the role of the ECM in biological systems.
1.1 ECM composition and properties
The average mammalian tissue consists of over 150 ECM and ECM-associated proteins, and several hundred proteins are catalogued in the matrisome database (Shao et al., 2020), testifying to the enormous quantity of molecular components and the diversity of ECM composition of different tissues within an organism. This diversity is amplified by continuous ECM remodeling through both enzymatic and non-enzymatic interactions. Remodeling not only alters ECM composition, but also the three-dimensional (3D) structural organization of its molecular components, which determines the ECM’s physical properties (Chaudhuri et al., 2020). The composition and structure of the ECM changes most greatly during times of stress, for example, during aging, tissue wounding, and tumor development.
There are two major forms of ECM in vivo: interstitial ECM, and basement membrane (BM) ECM (Cruz Walma and Yamada, 2020). Interstitial ECM fills spaces between organs. It is rich in fibrous proteins and proteoglycans that form 3D structures, whilst BMs form two-dimensional (2D) sheet-like ECM, that line organ boundaries. A major structural component of both interstitial ECM and BMs is fibroblast-secreted collagen. There are currently 28 known types of collagens with collagen I being the most common in interstitial ECM, and collagen IV being the most abundant within BMs (Ricard-Blum, 2011). In interstitial ECM, collagen I fibers undergo crosslinking to form networks, either with themselves or with other fibrous proteins, such as fibronectin and elastin, in processes facilitated by the enzyme lysyl oxidase (LOX) (Sun, 2021). Of the other fibrous proteins, fibronectin facilitates the adherence of cells to the ECM, aiding their migration by providing new sites of potential adhesion (Parisi et al., 2020). Elastin, on the other hand, confers resilience to plastic deformation and is found most frequently in blood vessels, skin, and lung tissue (Kristensen and Karsdal, 2016).
In the BM, the main structural scaffold consists of two interconnected polymeric networks of laminin and collagen IV. Including splice variants, there are 16 known laminin complexes in humans (McKee et al., 2021). The laminin and collagen IV networks do not interact directly but are crosslinked by other macromolecules such as nidogen and perlecan (Töpfer, 2023).
The ECM also contains a number of macromolecules, such as proteoglycans (PGs) and glycosaminoglycans (GAGs) (Frantz et al., 2010). Additionally, the ECM stores proteases, such as matrix-degrading enzymes (MDEs) that target specific components of the ECM (Parsons et al., 1997; Brinckerhoff and Matrisian, 2002). These proteases play a key role in many biological processes, for example, during wound healing, where matrix metalloproteinases (MMPs) degrade damaged collagen fibers, creating space for cells to migrate towards the site of wounding for tissue repair (Kandhwal et al., 2022). Similarly, MMPs also play a significant role during tumor progression by degrading and remodeling the tumor microenvironment to facilitate cancer cell migration through the ECM (Visse and Nagase, 2003). A schematic highlighting some of the main components of ECM is shown in Figure 1A, whilst Table 3 lists the major ECM components and their functions.
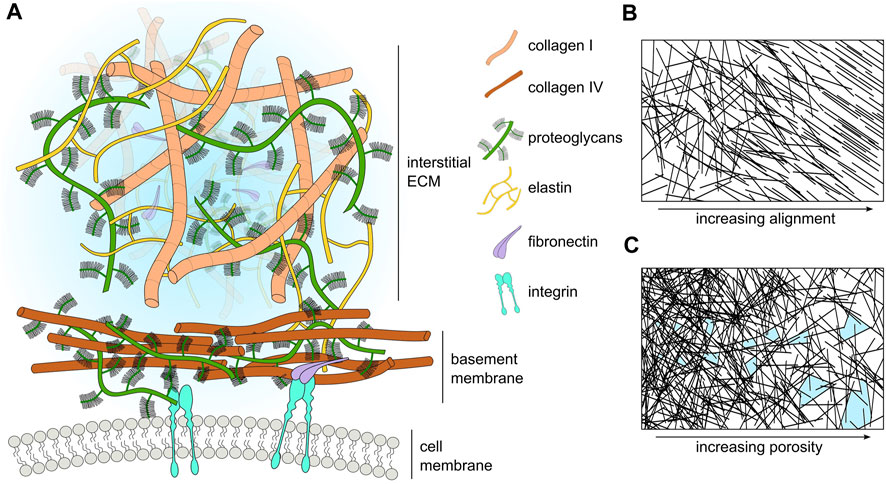
FIGURE 1. (A) Schematic representation of the major ECM compartments and their components. Macromolecular components of the ECM (size in the order of hundreds of nanometers) are smaller than cells (size in the order of tens of micrometers). However, many components such as laminin and collagen polymerize to form extended sheets or fibers (size order ranging from tens of micrometers to a few millimeters). For visual clarity, we do not represent small molecules such as cytokines, enzymes, or soluble factors. (B) Schematic representation of ECM fiber crosslinking and alignment. (C) Schematic representation of the change in ECM pore size with respect to ECM fiber density; pores highlighted in light blue.
With such a rich and diverse collection of components, and different ways of arranging those components into 3D architectures, it is natural that the biochemical and biomechanical properties of the ECM can vary greatly between tissues. Cells can interact with ECM via various receptors, such as integrins, discoidin domain receptors, and syndecans (Muncie and Weaver, 2018; Karamanos et al., 2021). Cell-ECM interactions, in turn, modulate a number of intracellular signaling pathways, which results in changes to migratory behavior, proliferation, and adhesion in cell populations (Muncie and Weaver, 2018). PGs facilitate signaling between cells and their environment. For example, PGs can bind growth factors, cytokines, and morphogens, thus modulating their availability (Muncie and Weaver, 2018; Barkovskaya et al., 2020). Additionally, PGs and in particular GAGs such as hyaluronic acid, can bind large amounts of water, and thereby modulate tissue hydration (Karamanos et al., 2021). Hydration impacts biochemical properties such as osmotic balance and the speed of molecular diffusion, and biomechanical properties such as porosity or the stiffness of other ECM components. For example, collagen becomes softer with increasing hydration (Andriotis et al., 2018).
The mechanical properties of the ECM are determined by its composition and structural organization at different length scales, ranging from the stiffness of molecular bonds at the nanoscale to protein fiber entanglement at the microscale (Chaudhuri et al., 2020). ECM biomechanical properties are often complex and time-dependent; important ECM material characteristics include stiffness, elasticity, plasticity, and viscosity. All of these properties can affect cell behavior, including alterations in cell spreading, proliferation, matrix deposition, and cell migration.
Perhaps the best-studied ECM mechanical property is stiffness. Stiffness describes a material’s ability to resist deformation to an applied force. A simple example is a linear spring, whose spring constant determines its stiffness. More complex materials can have non-linear stiffness, time-dependent stiffness, or stiffness dependent on the rate and direction of force application. All of these responses can occur in different ECMs (Chaudhuri et al., 2020). ECM stiffness depends primarily on the organization of collagen fibrils, with stiff matrices having a high density of collagen and fiber crosslinking (Cox and Erler, 2011). Conversely, softer ECM has a less dense collagen network with a lower occurrence of fiber crosslinking (Figure 1B). Matrix stiffness has consequences for many cellular processes. Membrane-bound mechanosensitive ion channels such as Piezo1 (Chen et al., 2018) are over-expressed in stiff ECM and can cause uncontrolled cell proliferation in cancerous tissue. Additionally, stiff ECM tends to have reduced pore sizes (Figure 1C), limiting cell migration in the absence of any proteolytic processes (He et al., 2022b). Each tissue has an optimal matrix stiffness, for example, load-bearing tissue such as bone and cartilage are very stiff in comparison to internal organs such as the liver and kidney (Handorf et al., 2015; Chaudhuri et al., 2020). However, during aging and disease, mechanical homeostasis of the ECM is disrupted and matrix stiffness is altered, either increasing in stiffness through the increased deposition of collagen and increases in fiber crosslinking (Rahman et al., 2020), or decreasing in stiffness due to an increase in MMP secretion that results in high levels of ECM degradation (Wang et al., 2023).
Elasticity refers to a material’s ability to return to its original shape after an applied force has been removed. Crimped collagen fibers, elastic proteins such as elastin, and reversible swelling due to water-binding pores confer elasticity to the ECM (Kristensen and Karsdal, 2016; Chaudhuri et al., 2020; Karamanos et al., 2021). Conversely, plasticity is the ability to retain a deformed shape after an applied force is removed. Intermolecular crosslinking can tune the balance between elasticity and plasticity in the ECM. Weaker non-covalent crosslinks enable flexible macromolecules to permanently rearrange, a feature of plasticity, whereas stronger or denser fiber crosslinking prevent deformation and confer elastic properties (Chaudhuri et al., 2020). Viscosity refers to time-dependent features of the mechanical response. Everyday examples are water (a low-viscosity fluid) and honey (a high-viscosity fluid). A high density of water-binding molecules such as PGs and GAGs can increase ECM viscosity (Chaudhuri et al., 2020; Karamanos et al., 2021). Additionally, weak non-bonded interactions between macromolecules and crosslinks that dynamically unbind upon force loading can also lead to ECM viscosity (Chaudhuri et al., 2020).
For a more in-depth review of the biochemical and biomechanical properties of the ECM, we refer the reader to excellent reviews elsewhere (Frantz et al., 2010; Muncie and Weaver, 2018; Chaudhuri et al., 2020; Karamanos et al., 2021).
1.2 Experimental approaches and data collection
Experiments can be designed to characterize several features of the ECM and its components, namely, its chemical composition, structural arrangement, and mechanical response to environmental stresses and strains. In such studies, an emphasis is often placed on characterizing the topography, viscoelastic properties such as poroelasticity (Ehret et al., 2017), and ligand presentation of the ECM (Young et al., 2016).
To explore the chemical composition of the ECM, commonly utilized methods include mass spectrometry, alongside immunostaining and fluorescence microscopy to characterize the ECM proteome (Naba et al., 2017; Malakpour-Permlid et al., 2021). Spectroscopic methods such as Fourier transform infrared spectroscopy (Chrabaszcz et al., 2020), and Raman spectroscopy (Ettema et al., 2022), allow for the collection of spatially resolved spectra containing the vibrational fingerprint of the ECM. These, in turn, permit the location and quantification of any molecular species present in a sample, as well as any variations in ECM structure (Tiwari et al., 2020). As signal detection in these methods relies on changes in vibrational states of molecules, neither require stains or dyes (in contrast with fluorescence microscopy). This is particularly useful when analyzing evolving structures non-invasively, as in the case the zona pellucida of oocytes during maturation (Jimenez et al., 2019).
The structural characterization of tissues is typically performed via a combination of microscopy methods. Common approaches include widefield and point scanning fluorescence methods such as confocal microscopy, a method that has recently been used to measure long-range cell-cell mechanical interactions via the ECM (Nahum et al., 2023). Other fluorescence-based super-resolution techniques exploit sophisticated illumination systems, for example, stimulated emission depletion microscopy (STED), or the stochastic activation of fluorophores to improve on resolution limits (Poole and Mostaço-Guidolin, 2021). Further methods worth noting are based on higher harmonic generation. In this approach to ECM characterization, contrast in images arises from the intrinsic optical properties of the sample which avoids the need for staining and other methods of sample preparation that are otherwise necessary (van Huizen et al., 2020). Due to their performance in imaging collagen fibers, second harmonic generation microscopes are especially well-suited to the quantification of fibrous structures (Woessner and Quinn, 2022). If observations on smaller length scales are required, scanning or transmission electron microscopy offer viable solutions (Leonard et al., 2018).
The mechanical properties of tissues have been investigated across several different scales using a variety of experimental techniques. At the nanoscale and microscale, the most common characterization method is indentation, conducted using an atomic force microscope (Plodinec et al., 2012), or an instrumented nanoindenter (Martinez-Vidal et al., 2023). The selection of indenters of different dimensions affords great flexibility in quantifying both elastic and viscous properties, as well as providing the capacity to collect correlative measurements by pairing the system with brightfield or fluorescence microscopy. As a measurement typically consists of programmatically deflecting a cantilever over a set region, starting from an out-of-contact condition, it is also possible to extrapolate the sample topography. Other notable approaches include optical and magnetic tweezers (Lehmann et al., 2020), as well as fluorescence resonance energy transfer (FRET) biosensors (Arnoldini et al., 2017).
Common methods to investigate the mechanical properties of cells within the ECM are tensile testing (Yang et al., 2015), and rheometry (Vos et al., 2020). The former is more common when investigating tissues like skin and bone, and the latter is more convenient when studying reconstituted systems or samples that are particularly soft. A less common approach is that of optical coherence elastography, a method that pairs an optical coherence tomography system with mechanical loading of the tissue (Kennedy et al., 2014). Typically, this is realized by means of compression (Hepburn et al., 2020), suction (Berardi et al., 2023), or acoustic radiation force (Li et al., 2020). By virtue of light-tissue interactions, optical coherence elastography allows the rapid collection of 3D maps of scattering and mechanical contrast, spanning hundreds of microns in each direction. A relatively recent innovation is Brillouin microscopy (Prevedel et al., 2019), a non-contact mechanical imaging method based on acoustically induced inelastic light scattering. Example applications include measuring mechanical properties of diseased cornea (Shao et al., 2019) and developing zebrafish tissue (Bevilacqua et al., 2019).
1.3 Modeling approaches
Mathematical models can be used to describe a wide range of biological processes and explore hypotheses, providing a complementary means of investigation to laboratory experiments. In general, experimental design should consider the equipment required to answer the research question at hand, and the same is true for choosing an appropriate mathematical modeling framework. However, it is important to stress that there is no ‘best’ modeling approach for a given problem, and what is most suitable for a given study depends on the scientific question at hand and any prior knowledge of a system. In this section, we introduce some common approaches for mathematical modeling of the ECM, before detailing specific biological applications of these models in Sections 2, 3.
1.3.1 Continuum models
Continuum models describe the density of cells and ECM constituents as smoothly-varying continuous functions (Figure 2), with models of cell movement and interactions with the ECM taking the form of either ordinary differential equations (ODEs) or partial differential equations (PDEs). ODE models are used to represent the evolution of a single quantity in space or time (cell density, for example), but are often limited in their utility as they cannot describe evolution in both space and time, as is often desirable for biological processes. However, in many contexts, a series of ODEs can be coupled to describe complex biological systems such as cell signaling pathways in cancer (Itani et al., 2010). Simple PDE models, on the other hand, represent cell density, n, as a scalar field, n(x, t), where x denotes position in one-dimensional space and t represents time. The evolution of cell density has been well-studied via a class of PDE models called reaction-diffusion equations. Perhaps the most famous model for this is the Fisher-Kolmogorov-Pietrovskii-Piskunov (Fisher-KPP) model (Fisher, 1937; Kolmogorov et al., 1937), whose numerical and analytical solutions have been studied extensively in the context of cell biology (Maini et al., 2004; Gerlee and Nelander, 2016). However, these models only explicitly consider changes in cell density with respect to time. More sophisticated models for cell movement can be constructed by coupling cell and ECM densities to capture their chemo-mechanical interactions. Some continuum models are exactly solvable (Petrovskii and Li, 2005), however, in more complex cases where exact solutions cannot be obtained, there exists a suite of numerical techniques to approximate solutions at a relatively low computational cost. In general, ODE and PDE models are amenable to mathematical analysis, for example, using boundary layer techniques (Farrell et al., 2000), or asymptotic methods (Keller and Lewis, 1995), wherein the behavior of a system under certain limits (for example, long-time behavior) may be studied.
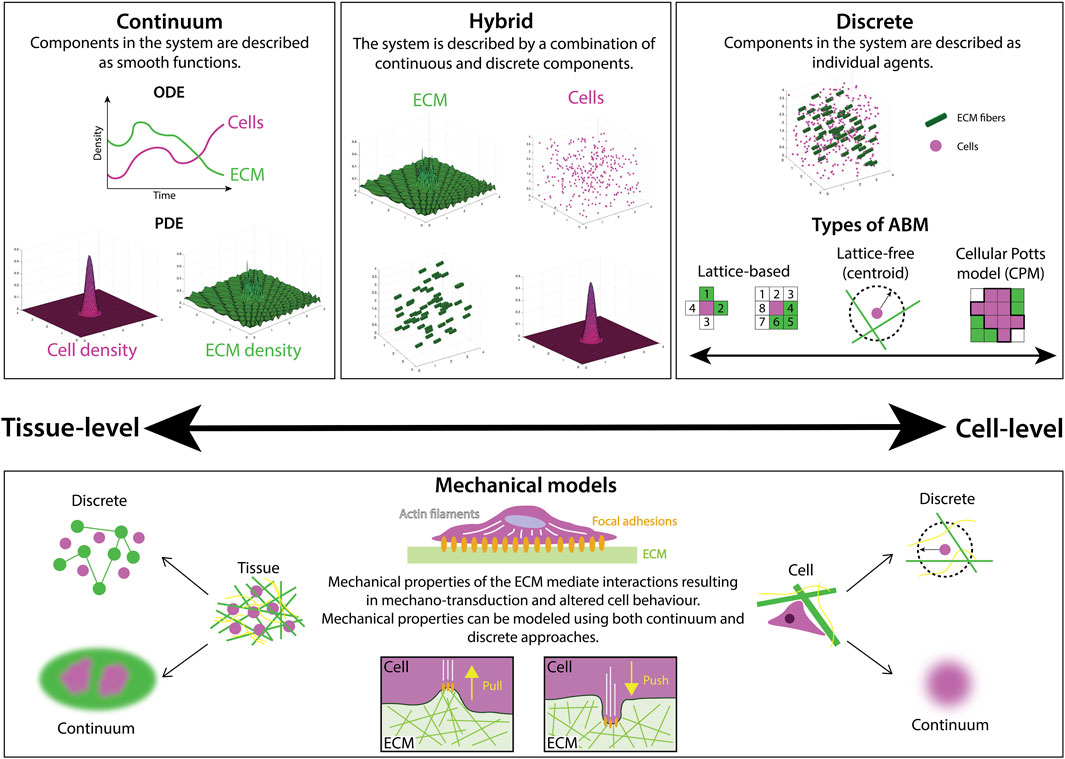
FIGURE 2. There are many different mathematical modeling approaches to studying ECM and cell-ECM interactions, ranging from continuum to discrete frameworks. Continuum models describe the densities of cells and ECM components with either ordinary differential equations (ODEs), typically used to describe total population size via a spatially-averaged density, or partial differential equations (PDEs) that often describe both the spatial distribution and temporal evolution of a population density. On the other hand, discrete frameworks, such as agent-based models (ABMs) represent biological components as separate, interacting agents. ABMs can be further categorized as either lattice-based, where positions are restricted to a finite set of points, or lattice-free, where positions lie in a continuous range. In some cases, multiple modeling approaches are combined in a hybrid fashion to balance the computational complexity of frameworks with many interacting agents with the accuracy of their outputs. Mechanical models are a subset of models focused on representing material properties such as stiffness and elasticity. Both continuum and discrete mechanical models are utilized to study the mechanical interactions between cells and the ECM.
When conducting studies at the tissue scale, both the cells and individual ECM components are of a small size relative to the characteristic length scale of tissue, and are often densely packed. Thus, in many cases, a continuum framework for modeling spatially averaged behavior at the cell scale and its effects at the macroscopic scale of the tissue is often appropriate. An important consideration, however, is that continuum-based models often overlook smaller scale interactions. If small-scale behavior is also to be studied, it can be advantageous to consider model frameworks that capture behavior at higher spatial resolutions, such as discrete models.
1.3.2 Discrete agent-based models
Agent-based models (ABMs), also referred to as individual-based models, establish independent agents that each interact and evolve according to a specific set of rules and predetermined behaviors (Metzcar et al., 2019). At the cell scale, ABMs typically represent cells as agents, and in this context are sometimes referred to as cell-based models. However, agents can also represent components of the ECM, and can be assigned characteristics such as size, shape, and polarity. ABMs can be broadly split into two categories: lattice-free and lattice-based models (Figure 2). Lattice-free models are often center-based, approximating the cell surface as a sphere (or a circular disc in 2D models). This approach does not confine the cells to a specific set of points in the domain, such as a lattice, and allows free movement of the cells in any direction. Other popular lattice-free approaches include vertex models, where cells are modeled as polygons, and subcellular element models, where each cell is described as a collection of spheres or circles (Metzcar et al., 2019). Conversely, lattice-based models restrict cell position and movement to a lattice, where space is represented as a discrete set of points. In restricting position, lattice-based models are often more computationally efficient than their off-lattice counterparts. However, this simplicity can come at the expense of accuracy, particularly in processes where the position of agents is a major determinant of system behavior. Commonly used approaches include cellular automata, where each cell is represented as the occupation of a lattice site, and Cellular Potts models (CPMs), where each cell is a collection of multiple lattice sites.
ABMs excel both at modeling stochastic environments and giving a fine-grained investigation of cell-cell mechanical interactions and dynamics of cell phenotypes (e.g., motile versus immotile cells). Due to their focus on individual cell behaviors, ABMs are generally better equipped to capture cell-level processes when compared with continuum models. Moreover, ABMs can incorporate subcellular processes which inform and drive cell behavior (Letort et al., 2019; Ponce-de Leon et al., 2023; Verstraete et al., 2023). By representing cell-cell communication in models, agents can also interact with one another to reproduce the emergence of population dynamics. Disadvantages of using ABMs include that they are often computationally expensive and analytically intractable, particularly for large systems with many agents.
1.3.3 Mechanical models
Mechanical models are a subset of models that place a focus on describing the material properties of biological structures. They can be applied both at the tissue or cell level, using either continuum or discrete approaches (Figure 2). We highlight this category due to its relevance for modeling ECM mechanobiology.
At the heart of a mechanical model lie the constitutive equations, mathematical relations that describe how a material responds to deformation (strain) caused by an applied force (stress) (Chaudhuri et al., 2020). The types of constitutive equations range in complexity from simple isotropic linear models (e.g., a linear spring) to complex non-linear models that consider how anisotropy in the microstructure, for example, ECM fiber alignment, influences the material response to forces applied from different directions. Continuum mechanical models typically represent biological structures as geometrical objects onto which continuous fields of stresses and strains are mapped (Guo et al., 2022c), with the Finite Element Method (FEM) a popular choice for solving such stress-strain problems.
Generally, continuum mechanical models average tissue microstructure, making it challenging to represent highly diverse environments such as ECMs with spatially varying architecture, or tissue regions with different cell types. Inhomogeneous and anisotropic constitutive equations can to some extent address this limitation by incorporating spatially-varying and direction-dependent terms. Further, continuum models generally assume that displacement within the modeled domain is negligibly small, an assumption that is violated when cellular forces substantially rearrange the ECM. In these cases, a more suitable approach is to break up the domain into smaller discrete regions. Discrete mechanical models typically represent cells or ECM as discrete objects, such as points distributed in space that can be mechanically linked to each other. As in continuum mechanical models, a constitutive equation is chosen to describe how each discrete object physically behaves upon force loading. ABM frameworks that include mechanical interactions between agents are thus also a type of discrete mechanical model. Discrete mechanical ECM models are particularly suited to investigate emergent biophysical properties of matrix polymers (Broedersz and MacKintosh, 2014), and long-range mechanical cues through the microenvironment (Alisafaei et al., 2021). However, they come at the price of lower analytical tractability and increased computational cost.
1.3.4 Hybrid models
Many of the models above are tailored to a biological problem with a specific characteristic length or time scale. However, there is often a disparity in such scales, for example, the size of cells is typically within micrometer range, whereas the thickness of collagen fibrils are usually of a nanometer-scale size (Siadat et al., 2022). Biological questions with such differences in scales motivates the use of hybrid models (Figure 2). Hybrid models employ a combination of different modeling techniques, coupling continuum and discrete approaches to better capture the dynamics and evolution of a system (Stéphanou and Volpert, 2016). A key advantage of hybrid models is that the rules governing the evolution of an individual cell can be easily decoupled from the underlying dynamics of continuous ECM constituent densities, resulting in a more comprehensive model of cell-cell and cell-ECM dynamics than purely discrete or continuum equivalents. However, this extra layer of complexity can also introduce a number of disadvantages, the most notable being computational expense.
2 Cell migration
The ECM influences the migratory properties of cells in many ways, including cell morphology, polarization, matrix deposition, and matrix degradation (Saraswathibhatla et al., 2023). Experimentally, cell morphology changes depending on the local geometry of the ECM and on the distribution of adhesion sites in the region through which a cell migrates (Wu et al., 2021). Cells interact with the ECM through several types of actin-rich protrusions, such as filopodia and lamellipodia that form focal adhesions with the ECM to facilitate directed cell migration along environmental gradients (Figure 3) (Caswell and Zech, 2018). Here, we review mathematical and computational models considering the role of the ECM in cell migration during development, cancer cell migration, and wound healing.
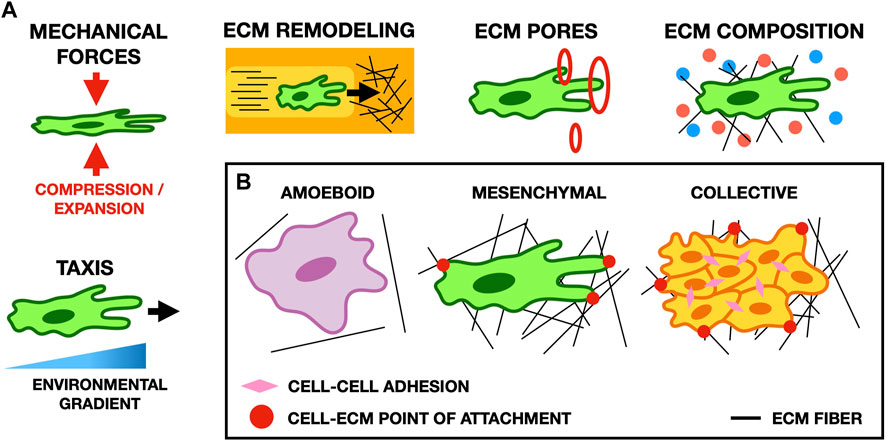
FIGURE 3. (A) Schematic illustrating typical cell-ECM interactions in migration. Cell morphology and migratory behavior are affected by many properties of the ECM, such as its composition, the forces it exerts on cells, the size of pores in the ECM, and gradients in the microenvironment of the cell (for example, the stiffness of the ECM or the concentration of potential points of focal adhesion). (B) Schematic showing common migratory cell phenotypes. Amoeboid cells form weak focal adhesions to the ECM, and move primarily by cortical actin flow and cell deformation. Conversely, mesenchymal cells form strong focal adhesions and pull on the ECM to move. In collective migration, cells mechanically adhere to one another and their microenvironment to facilitate cohesive movement as a group.
2.1 Mechanisms of cell migration
The ECM impacts cell migration in a multitude of ways through both biochemical signaling and biophysical interactions (Pally and Naba, 2024). Mathematical and computational models can unravel this complexity by selectively including only a subset of ECM components and their properties, and thereby explore the role of specific mechanisms in cell migration (Figure 3).
In vivo tissues present a notable degree of spatial heterogeneity, primarily in the arrangement of ECM collagen fibers (Yuan, 2016; Filipe et al., 2018). The role of fiber orientation has been the focus of several models. An early continuum model developed in the late 1990s describing cell migration through collagen-rich networks studies the relationship between fibroblast polarization and fiber orientation (Dallon and Sherratt, 1998). This work formed the basis of many subsequent models of cancer cell migration and wound healing that are discussed later in Sections 2.3, 2.4. Using a different approach to account for fiber orientation, Painter (2009) represents the ECM as a probability density function that includes a description of fiber alignment. Here, cell motion is modeled with run-and-tumble motility, in which the change in migration velocity and direction is given by a discrete random process, known as a ‘velocity-jump process’ (Othmer et al., 1988). In this model, the distribution of ECM fibers influences the rate and direction of each ‘jump’. In amoeboid cell populations, their model shows that the structure of the ECM influences cell organization in the absence of any additional cues such as adhesion or chemotactic gradients. Furthermore, this model demonstrates that the realignment of the ECM fibers causes the formation of cell chains, a phenomenon also observed in mesenchymal-type cells such as neural crest cells (Alhashem et al., 2022). Although this model permits ECM fiber reorientation, it does not model mechanical forces. To focus on the mechanics of fiber reorientation by cells, Schlüter et al. (2012) develop an ABM. Their model predicts that stiffer ECM prevents the reorientation of fibers and that faster, persistent migration strategies emerge in environments with highly aligned ECM fibers. Furthermore, the model also suggests that fiber realignment by migrating cells promotes other nearby cells to trail behind, inducing leader-follower migration (Qin et al., 2021).
The ability of cells to change their shape to adapt to their surroundings is essential for migration and invasive capacity (Van Helvert et al., 2018). Among ABMs, the CPM explicitly incorporates cell morphology, which Scianna et al. (2013) leverage to study the interplay between cell and nucleus deformability and ECM properties such as density, pore size, and fiber elasticity. They find that intermediate pore size permits cell passage while allowing optimal adhesion to ECM. ECM stiffness modulates this relationship: highly deformable soft ECM facilitates migration through small pores, while stiff ECM facilitates migration through large pores. Similarly, overly dense ECM prevents motility, while loose ECM reduces contact guidance. Follow-up work shows that a highly deformable nucleus promotes cell migration through constrictions in 2D and 3D (Scianna and Preziosi, 2021). To investigate the role of focal adhesions in cell migration, Rens and Merks (2020) develop a hybrid CPM–FEM framework. Here, cells can distinguish between soft and stiff ECM via the dynamic and force-dependent nature of focal adhesion growth, which is modeled by catch-slip bonds—a type of interaction where the bond strengthens as it is pulled, and which is known to occur for integrin-ECM binding (Guo et al., 2022c). As a result of self-reinforcing focal adhesion stabilization on stiffer substrates, cells spontaneously migrate toward stiffer matrix. Thus, this model proposes a mechanism for the emergence of durotaxis.
Several works focused on detailed mechanical modeling of cell and ECM interactions in migration, for example, using frameworks such as the subcellular element model (Newman, 2007). A highly detailed 3D ABM represents the cell surface as a collection of membrane elements that mechanically interface with the nucleus via elastic cytoskeletal strings (He and Jiang, 2017). This model predicts more persistent cell migration on concave surfaces than convex surfaces due to the different contact angle of adhesions. Another model by Kim et al. (2018) imposes durotaxis as an assumption, and studies the mechanism of stiffness sensing. In their 3D discrete mechanical model, a dynamically-shaped cell extends thin filopodia inside an ECM fiber network. Migration direction is biased towards the stiffest ECM sensed at the filopodial tips. This model reproduces experimental observations that cells are more likely to remain stationary when the distance between soft and stiff ECM increases, but steer towards stiffer ECM if they are within the reach of filopodia. This model was later extended to integrate intracellular mechanosensing (Kim et al., 2022).
In mechanosensing, intracellular cascades are triggered when cells adhere to the ECM through focal adhesions (Hanna and El-Sibai, 2013). The ECM is considered a key regulator of many intracellular signaling pathways, with it being noted that varying ECM density can alter the activation potential of proteins in the Rho-family of small GTPases (Bhadriraju et al., 2007). Mathematical models have been developed to explore these complex signaling networks and uncover intracellular dynamics; we refer the reader to (Hastings et al., 2019) for a concise review of models of ECM-mediated signaling pathways.
One of the strengths of models is their ability to predict the emergence of complex behaviors from simple underlying mechanisms. Different models can lead to the same conclusion, such as the more abstract continuum model by Painter (2009) and the discrete model by Schlüter et al. (2012) that accounts for mechanics. Both these models predict that mutual feedback between cell migration direction and ECM fiber orientation leads to the spontaneous emergence of cell chains and leader-follower behavior. This highlights how fiber reorientation is a critical mechanism, regardless of how it is achieved. In other cases, more detailed models are necessary to recover certain features. Accounting explicitly for cell deformation allows models such as the CPM to study the influence of matrix microstructure such as pores. As a general trend recovered by such models, many ECM properties influence cell migration in a non-trivial manner, with an intermediate optimum (Scianna et al., 2013; Scianna and Preziosi, 2021). However, to predict durotaxis, a more accurate biomechanical model of focal adhesions is required (Rens and Merks, 2020). Highly detailed mechanical models aim to comprehensively describe the physics of the cytoskeleton, membrane deformation, and adhesion to the ECM (He and Jiang, 2017; Kim et al., 2018; 2022). These efforts, however, come at a price of a large amount of unknown parameters which limits model interpretability, and considerable computational cost of simulation. Finally, from a molecular standpoint, models including mechanosensing can be developed through the integration of intracellular signaling pathways, such as those involving Rho GTPases (Hastings et al., 2019).
2.2 Neural crest cell (NCC) migration
The collective migration of neural crest cells (NCCs) underpins a range of developmental processes during embryogenesis (Mayor and Theveneau, 2013). Defects in neural crest biology can result in a range of congenital diseases and birth defects termed neurocristopathies (Vega-Lopez et al., 2018). Mathematical models are of particular interest in the context of NCC migration, often directing experiments towards important parameter ranges and identifying particular points of interest in the development of therapies that aim to prevent or counteract the effects of disrupted or misdirected migration. For a comprehensive review of mathematical models of NCC migration, we refer the reader to the review by Giniunaité et al. (2020). In this section, we briefly outline two recent hybrid models that explicitly consider the ECM in this context.
One approach to modeling the migration of NCCs considers the influence of fibronectin network remodeling in NCC migration (Martinson et al., 2023). Using PhysiCell, an open source physics-based cell simulator (Ghaffarizadeh et al., 2018), cells are modeled as freely moving 2D agents, whose velocities are functions of friction, cell-ECM interactions, and cell-cell repulsion. Simulations of this model predict that fibronectin deposition and remodeling by cells at the leading edge of collectives is an important mechanism in facilitating long-distance migration by preventing the collective arrest of cell motion, known as cell jamming (Lawson-Keister and Manning, 2021).
A later developed hybrid model for collective migration in the chick cranial neural crest represents the ECM via a tunneling mechanism, in which follower cells move along tunnels of aligned ECM formed by leader cells (McLennan et al., 2023). This model was used highlight the importance of spatial confinement in chick cranial NCC migration, suggesting that factors such as Colec12 and Trail, which are expressed primarily in NCC-free zones adjacent to NCC collectives, play a key role in confining NCCs to stereotypical migratory pathways during migration and maintaining coherence within collectives.
In the context of NCC migration, these recent hybrid modeling developments reveal the importance of fibronectin and alignment of fibers in the ECM in guiding long-range migration.
2.3 Cancer cell migration and tumor development
During cancer cell migration, changes in the composition, stiffness, and topography of the ECM can significantly influence cell behavior and migratory capacity (Park et al., 2018; Eble and Niland, 2019; Najafi et al., 2019). Cancer cells remodel the ECM to facilitate migration (Mohan et al., 2020), for example, by secreting enzymes such as MMPs (Castro-Castro et al., 2016). Additionally, changes in ECM structure can induce a more invasive phenotype (Leight et al., 2017), further promoting migration. In this section, we review models of cancer cell migration that explicitly consider the ECM during tumor progression.
In mathematical studies of cancer cell migration, traveling waves are often used to describe the speed and shape of spatial processes in cancer progression (Gerlee and Nelander, 2016). A simple continuum model that has been utilized extensively in the context of cell migration is the Fisher-KPP model (Fisher, 1937; Kolmogorov et al., 1937; Maini et al., 2004). This seminal continuum model only explicitly considers changes in cell density with respect to space and time, and does not explicitly consider cellular interactions with the ECM. To more closely align theoretical predictions with in vivo and in vitro experiments, more sophisticated traveling wave models explicitly represent both cells and the ECM as distinct, smoothly-varying quantities that interact with one another. Newer models of this nature suggest that the speed of tumor invasion depends on the ECM density ahead of the invading front, and on the rate of ECM degradation by motile cancer cells (Colson et al., 2021; El-Hachem et al., 2021). Similar results have also been found using a PDE model derived from the underlying individual cell-cell and cell-ECM interactions (Crossley et al., 2023), which, unlike the majority of continuum models, considers the volume-filling effects of both the cells and ECM during invasion. The resulting model describes non-linear cross-diffusion (Simpson et al., 2009b), and proliferation terms akin to those considered in a different model for melanoma invasion into human skin (Browning et al., 2019). By considering asymptotic parameter regimes, this work justifies the use of simpler models such as the Fisher-KPP model to understand qualitative behaviors of model solutions, and employs boundary layer analysis to profile the traveling wave solutions for small ECM degradation rates.
In addition to the ECM, there are several other extrinsic factors that also influence cell behavior during migration. A recent model describing acid-mediated tumor invasion (Strobl et al., 2020), considers the role of both the ECM and stroma in impeding tumor cell movement and inhibiting growth. Tumors often consist of a heterogeneous population of cells (Sinha et al., 2020), so this model considers two tumor cell types that are able to antagonize either the ECM or stroma, and subsequently constructs a model of five coupled differential equations as an extension of Gatenby-Gawlinksi models of acid-mediated invasion (Gatenby et al., 2006; Martin et al., 2010). The model predicts that heterogeneous tumors are more invasive than spatially separated tumor populations. Additionally, it concludes that the biological barrier of the stroma provides a stronger prevention of tumor growth than the physical ECM barrier.
The ECM not only influences cell migration, but also tumor morphology and its expansion. In this context, multi-scale moving boundary approaches are employed to understand behaviors across scales, ranging from the cell scale to the tissue scale. An initial framework introduced by Trucu et al. (2013) is later extended to examine tumor morphology by considering the direct interaction of cancer cells with a two-phase ECM comprised of both a fibrous and non-fibrous component (Shuttleworth and Trucu, 2019). The inclusion of bi-directional cell-ECM interactions, including cell adhesion and dynamic fiber reorientation, enables an exploration of pattern formation, highlighting the characteristic fingering pattern observed in tumor modeling (Drasdo and Hoehme, 2012). By considering different compositions of ECM, such as varied initial fiber distributions and orientations, the authors show that tumors display more aggressive growth patterns when seeded in a heterogeneous ECM.
While continuum models are excellent at capturing population trends such as collective migration, ABMs are more suitable for lower cell densities, such as in single cell migration or for modeling the transition between collective and individual cell migration. Harjanto and Zaman (2013) develop a 3D cellular automaton model with explicitly modeled ECM fibers to investigate four types of cell-matrix interactions: collagen-density dependent cell-mediated deposition, degradation, realignment, and displacement of collagen fibers. After parameter fitting to two prostate cancer cell lines, the model reveals an elevated probability of collagen degradation and motility even in denser ECM, which correlates to a more invasive tumor and matches experimental observations.
In an effort to make ABMs more accessible, computational packages for hybrid CPM such as CompuCell3D (Izaguirre et al., 2004; Swat et al., 2012) and ABM software such as PhysiCell (Ghaffarizadeh et al., 2018) facilitate model development with minimal programming experience. Recent work in PhysiCell represents the ECM as a drag force to study how cancer cell migration and tumor cluster formation are affected by a 3D ECM architecture (Gonçalves and Garcia-Aznar, 2021). This work also introduces a representation of the distribution of cell locomotive forces and intercellular adhesion and repulsion to capture the spread in cell velocities found in vitro. This model predicts a dichotomy between cell migration and tumor size: if the former is hindered by increased ECM density, multicellular clusters increase in area, and vice versa, such that even tumors with low levels of proliferation can be highly invasive. A PhysiCell extension, PhysiBoSS, allows for the tracking of intracellular dynamics such as gene regulatory networks (Letort et al., 2019; Ponce-de Leon et al., 2023). Ruscone et al. (2023) use PhysiBoSS to model cancer invasion through the ECM, which is represented as a scalar field in the base structure of the microenvironment. The ECM acts as a barrier for cells and cell-ECM interactions, such as repulsion and adhesion, along with intracellular regulation, are modeled using Boolean logic. This model successfully reproduces experimental results and demonstrates non-reversible epithelial-mesenchymal transitions (EMTs), a phenotypic switch from epithelial cells to mesenchymal cells, wherein cells lose cell-cell adhesion capabilities and gain both migratory and invasive properties (Kalluri and Weinberg, 2009).
Cancer cell migration has long been framed by the concept of EMT. Recent studies taking inspiration from the physics of active matter reframed tumor cell migration as state transitions, where a non-migratory tumor is likened to a solid or glass-like material, and a tumor exhibiting more migratory cells to a liquid or gas. In analogy to granular materials, this transition from immotile to motile is called the jamming-unjamming transition (Oswald et al., 2017). Interestingly, at least in some experimental systems, distinct biological mechanisms underlie EMT and jamming-unjamming transitions (Mitchel et al., 2020). A key regulator of EMT is the cell-cell adhesion protein E-cadherin, whose loss of expression is associated with increased tumor invasiveness. To study how breast cancer cells switch from collective to single-cell migration, Ilina et al. (2020) complement their experimental study with a discrete lattice-based cellular automata model based on a previously-developed ABM framework (Deutsch et al., 2021). Key parameters in this model are cell-cell adhesion mediated by E-cadherin, and ECM density which confines cell movement. Increasing intercellular adhesion favors collective motion but also reduces cell migration speed, while increasing confinement by dense ECM reduces cell migration speed and forces cells into a solid-like jammed state. When both ECM density and intercellular adhesion are low, cells tend to move as individuals in a gas-like state. When ECM density is high and intercellular adhesion low, cells move collectively in loose flocks in a fluid-like state.
As in non-malignant cell populations, cancer cells utilize the ECM to facilitate migration. Models agree with experimental data that cancer cells adhere to dense ECM structures at a stronger rate than to soft ECM, where they secrete high levels of MMPs that degrade the surrounding matrix, which in turn frees up space for cell migration (Harjanto and Zaman, 2013). On the other hand, in a soft ECM, cancer cells are less likely to adhere to the matrix and therefore have a slower migration speed. Mathematical modeling has also been used to shown that heterogeneity in either the ECM or tumor cell population can increase tumor invasiveness (Shuttleworth and Trucu, 2019; Strobl et al., 2020). The vast array of mathematical models discussed here highlight the power of models to predict cellular responses to a range of mechanical ECM properties, and by understanding how tumors spread in surrounding tissue, we can develop therapies aiming to reduce the speed at which tumors grow and invade healthy tissues.
2.3.1 The role of ECM remodeling enzymes
Many mathematical models of cell-ECM interactions, including those discussed previously, assume that cancer cells themselves are responsible for degrading and remodeling the ECM. MDEs are generally localized: either on the cell membrane, at the tips of invadopodia (Weaver, 2006; Lu et al., 2011), or with very small diffusivity (Werb, 1997). Thus, the highly localized degradation of ECM by cells is a suitable approximation to employ, in particular because the degradation of MMPs occur on a much shorter time scale than degradation of ECM (Perumpanani and Byrne, 1999; Webb et al., 1999). However, some MMPs are freely diffusible, allowing degradation of the ECM without direct cell contact (Cabral-Pacheco et al., 2020). This behavior can be modeled by introducing a diffusible population of MDEs which are secreted by cells and degrade the ECM (Anderson et al., 2000), and analyzed using partial integro-differential equations to describe the spatial-temporal dynamics of cancer invasion (Chaplain et al., 2011).
Many CPMs are hybridized with PDEs to model diffusible MDEs. An early example by Szabó et al. (2012) combines both diffusible and immobile ECM components to investigate how cell-ECM adhesion, cell motility, and ECM degradation impact invasive dynamics into an aligned fiber array. Whilst cell-ECM adhesion can lead to invasion as cells progressively spread to maximize their adhesive contact with the ECM, the addition of cell motility accelerates the invasive front. The effect of ECM degradation then depends on the relative strength of cell-ECM adhesion; weakly adherent cells preferentially migrate along tracts where matrix has been degraded, while strongly adherent cells prefer to migrate along ECM-rich paths. Similarly, in their model of cell migration, Scianna et al. (2013) find that matrix degradation can enhance migration when the ECM is too dense or pore size too small. In contrast, ECM degradation is deleterious when the ECM is too sparse, as some ECM contact is necessary for cells to move. Using a similar model, Pal et al. (2021) study how different ECM patterns affect migration. Varying adhesion parameters and the elasticity of fibers in the network is found to change the invasive potential of a cluster of cells. Moreover, randomly curved fibers decrease invasion while wave-like and parallel fibers increase cancer invasion speed and distance. Further work by Kumar et al. (2016) shows that increased MMP secretion and fiber alignment enhances cell migration, while increased ECM density inhibits the migration of cells. This implementation has since been extended to study how differences in cell size and deformability promote invasion during cancer cell migration (Asadullah et al., 2021). In a similar hybrid model, Pally et al. (2019) adapt the rules specifying the agent morphology such that the BM forms clusters, while collagen forms elongated structures. Pramanik et al. (2021) later extended this work to systematically investigate the impact of cell proliferation, and MMP diffusivity and inhibitor cooperativity, revealing five migratory phenotypes: non-invasive, dispersed individual invasion, multimodal invasion with both non-invasive and dispersed individual invasive cells, invasion as a non-adherent flock, and invasion as an adherent cluster.
In addition to MMPs, LOX is another important diffusible ECM-remodeling enzyme. A continuum framework by Nguyen Edalgo and Ford Versypt (2018) couples a PDE for cancer cell movement to two PDEs governing the evolution of randomly oriented and crosslinked ECM fibers, both represented as distinct scalar fields. They include reaction-diffusion PDEs to capture ECM degradation, remodeling, and fiber crosslinking by MMPs and LOX. By distinguishing crosslinked and randomly oriented ECM fibers, the model reproduces the enhanced migration of cancer cells in the presence of LOX-induced crosslinking. This work highlights the importance of LOX in facilitating cancer cell migration and metastasis (Cox et al., 2013), with implications for the developments of novel therapeutics combating the progression of cancer.
The importance of LOX in remodeling the ECM to facilitate cancer cell metastasis has also been studied using CPMs and other seminal hybrid ABM-continuum frameworks (Anderson et al., 2000) that describe tumor growth within the microenvironment (Nguyen Edalgo et al., 2019). All ECM components, including collagen fibers and enzymes such as MMPs and LOX, are modeled using PDEs, with cancer cells modeled as discrete agents. Simulations show that the rate of invasion by cancer cells is higher in a uniformly distributed fiber concentration, rather than a random distribution, and that as fiber concentration increases, pore size increases and migration is more efficient, agreeing with experimental studies such as Paul et al. (2017). As in prior work by Nguyen Edalgo and Ford Versypt (2018), the study by Nguyen Edalgo et al. (2019) highlights the importance of LOX in migration and highlights LOX inhibition as a potential therapy to reduce cancer metastasis.
Remodeling ECM enzymes, such as those that can degrade the matrix (MMPs) or those that help remodel the matrix (LOX), are crucial to tissue homeostasis. However, they are also exploited by cancer cells to help them invade the surrounding tissue. Through the upregulated secretion of MMPs, cancer cells are able to degrade large amounts of the ECM which frees up space for proliferation and migration. Another important ECM enzyme is LOX, which plays a key role in cross-linking collagen with elastin fibers. Through mathematical modeling, it has been shown that migration of cancer cells is enhanced in the presence of LOX-mediated fiber cross-links, highlighting the importance of LOX during tumor invasion.
2.3.2 Tumor spheroids
Continuum mechanics-based models are often used to study 3D tumor spheroids embedded in fibrous collagen gel. Chemo-mechanical free energy of the ECM, cells and adhesion determines cell evolution in an ODE model by Ahmadzadeh et al. (2017), where the ECM is modeled with radially aligned fibers using stress–strain relations for transversely isotropic materials. The work shows that below a predicted critical elastic modulus for the ECM, cells favor adhesion and remain within the tumor, whereas above the critical value, it is energetically favorable for cells to detach from the spheroid. Similar energy-based approaches to modeling mechanical deformations, motor binding energy and mechano-chemical feedback are used to predict age-related differences in ECM structure that impact melanoma progression (Kaur et al., 2019).
Experimental results have shown that various breast cancer cell lines demonstrate different modes of cell invasion. Confirming earlier findings from Ilina et al. (2020) and using new experimental results in spheroids, Kang et al. (2021) present a hybrid model combining vertex and particle-based approaches for spheroid growth within an ECM. They propose a jamming phase diagram to describe how two key parameters, ECM confinement and cell motility, control the transition from a solid-like jammed state, to a liquid-like collective migration in loose strands, up to a more gas-like migration as single detached cells.
A model by Caiazzo and Ramis-Conde (2015) is used to simulate ECM fibers, and extended to model blood vessels and cells using a 3D ABM coupled to an FEM solver for oxygen evolution in time and space (Macnamara et al., 2020). The interactions between agents in this model are mainly mechanical, showing that tumor shape is driven by local structures. Cells grow in alignment with the fibers and around existing vasculature, and migration is led by interactions with the microenvironment, including ECM fibers, and as cells seek out regions of higher oxygen concentration. An alternative approach applied to glioblastoma spheroid growth uses a mechanochemical model, where the ECM is modeled using a PDE that describes its diffusion towards the tumor spheroid core and its uptake by cells (Carrasco-Mantis et al., 2023b). An ODE describes nutrient-dependent cell proliferation and death, and further PDEs model the mechanical properties of cells within the spheroid. Through parameter analysis and validation against experimental data, the authors conclude that this mechanical model effectively approximates glioblastoma spheroid growth and shrinkage.
These results, similarly to other cell migration contexts, demonstrate that ECM elasticity and fiber alignment strongly influence tumor spheroid growth - both in speed and shape. Additionally, age-related structural and density changes in the ECM will impact modes of tumor progression across various cancers, where this observation can be successfully reproduced using experimental data.
2.4 Wound healing
Wound healing is essential for restoring tissue integrity and function after an injury and requires a cascade of coordinated events at both the cell and molecular levels. Human skin, for example, is composed of two distinct layers - an outer layer, the epidermis, and an inner layer, the dermis (McGrath et al., 2004). The epidermis is mainly composed of keratinocytes, while the dermis is predominantly composed of ECM, but also contains blood and lymphatic vessels, immune cells, and fibroblasts that locally deposit ECM constituents (Pfisterer et al., 2021). Skin wound healing proceeds in four major phases: haemostasis, inflammation, proliferation, and tissue remodeling (Wilkinson and Hardman, 2020). Anomalies during any stage can cause improper tissue repair and complications, including significant tissue scarring (Guo and DiPietro, 2010). Haemostasis, the first stage of wound healing, involves blood coagulation at the wound site promoted by platelets and fibrin fibers (Arnout et al., 2006). After coagulation, inflammatory cells clean the wound and generate chemokine gradients that attract keratinocytes, fibroblasts, and other cells towards the wound center (Koh and DiPietro, 2011). In the proliferation phase, growth factors stimulate keratinocytes to proliferate and restore the epidermis (Landén et al., 2016), while blood vessels are re-established by angiogenesis. The final phase consists of ECM remodeling (Guerra et al., 2018). The ECM plays critical roles in coagulation, during migration of cells towards the wound center, and in the final remodeling stage. In this section, we review modeling approaches that explicitly describe the interactions between cells and the ECM during wound healing. For in-depth reviews of broader mathematical models of wound healing and closure and wound healing angiogenesis, that do not necessarily consider ECM, we refer the reader to excellent reviews elsewhere (Flegg et al., 2015; Jorgensen and Sanders, 2016).
Simple continuum mathematical frameworks represent cell density in wound healing as a scalar field. A seminal model considers the effect of insoluble, fibrillar ECM during wound-healing angiogenesis (Olsen et al., 1997), where the ECM mediates the movement of endothelial cells towards the center of a wound (Lamalice et al., 2007). Cells and the ECM interact with one another through haptotaxis (Ricoult et al., 2015), haptokinesis (Friedl and Bröcker, 2000), ECM-mediated cell proliferation (Rabie et al., 2021), and ECM production and degradation (Winkler et al., 2020). Steady-state analysis and traveling wave solutions predicts that the ECM and cell densities evolve with approximately constant speed prior to reaching the center of the wound, with cell density increasing beyond baseline levels at the wound edge before settling to a pre-wounding equilibrium (Dale et al., 1994).
ECM orientation and alignment are also of importance during the healing process and can influence the invasive capacity of cells during migration towards the centre of a wound (Lin et al., 2020; Ray and Provenzano, 2021). Furthermore, ECM modulates the direction of cell migration through a process known as contact guidance (Dunn and Heath, 1976). Thus, an important extension to traditional continuum models is a representation of ECM fiber orientations, pioneered by Dallon and Sherratt (1998). Models of this sort have been used to study fiber alignment as a dynamic and reversible process - first in two fixed orthogonal directions (Olsen et al., 1998b), and then for a continuous range of directions (Olsen et al., 1999), in the context of angiogenesis.
To model the final remodeling stage of skin wound healing, Dallon et al. (1999) develop a hybrid framework with cells as discrete agents and collagen fibers as a continuous bi-directional vector field describing fiber density and directionality. In the model, ECM orientation biases the direction of cell migration and migrating cells also reorient ECM. This reciprocal interaction is sufficient to generate fiber alignment in model simulations, with the precise nature of patterns depending on cell speed, the degree of cell polarization, and the initial fiber structure. Cumming et al. (2010) extend this work by including both collagen fibers and fibrins, both modeled as continuous tensorial fields in 2D, and model fibroblasts as discrete circular discs, whose behaviors are determined by chemotaxis, volume exclusion, and contact guidance. This work successfully reproduces ECM remodeling dynamics observed both with and without scarring. Recent experimental work highlights the importance of viscoelastic behaviors in cell and tissue function (Alegre-Cebollada et al., 2023; Eroles et al., 2023), which is subsequently incorporated into corresponding hybrid models (Pensalfini and Tepole, 2023). To investigate dermal wound healing, Guo et al. (2022b) couple a microscale stochastic cell adhesion model with a macroscale continuum mechanics ECM modeled as a fiber-reinforced material with hyper-viscoelastic constitutive behavior to represent fibroblast contraction. This model qualitatively captures the cutaneous wound healing process, with initial active contractions, increased fibroblast population at the wounded site, and consequential stress distribution changes.
To model epidermal-dermal interactions during wound healing, Wang et al. (2019) model the epidermis and dermis as separate compartments. Keratinocytes in the epidermis compartment are modeled as individuals, whereas fibroblast and immune cells in the dermis compartment are modeled as continuous fields using Keller-Segel reaction-diffusion-advection PDEs (Arumugam and Tyagi, 2021). Diffusible signals across the BM, such as signaling molecules for ECM deposition and degradation are also modeled explicitly as continuous quantities. The model predicts that dermal wounds heal by forming scar tissue with different ECM and fibroblast compositions than healthy tissue, in agreement with experimental studies (Pastar et al., 2014). Furthermore, the model predicts wound depth to be a critical determinant in scarring. In particular, shallow but wide wounds are predicted to repair with smaller scars than narrow but deep wounds (Hinshaw and Miller, 1965; Marshall et al., 2018). The benefit of this compartmentalized framework is that it is easily extensible without affecting existing model components, enabling a model representation of sharp boundaries within the skin such as the follicle/dermal boundary.
The ECM also impacts regeneration in tissues other than skin. In a model of tendon healing, Dudziuk et al. (2019) employ a continuous integro-PDE model to study the orientation of collagen fibers during scar formation. This work highlights the role of the initial fiber orientations in determining the final structure of the collagen network. Later work using a similar mathematical framework (Carrillo et al., 2021), adds local alignment interactions between collagen fibers and simplifies this model under appropriate limits, allowing for a parameterization based on experimental data.
A large fraction of mathematical models currently investigating wound healing focus on the role of ECM fibers, in particular, fiber alignment and orientation. Through different mathematical approaches, these models agree that the ECM structure and configuration of the fibers facilitate cell migration and guide the cells towards the wounded site, contributing to the success of wound healing.
3 Tissue structure and morphology
One of the major functions of the ECM is to mechanically support and maintain tissue shape both during tissue formation and in homeostasis (Cruz Walma and Yamada, 2020). The ECM provides not only mechanical support, but also regulatory input that can determine cell fates. For example, stem cells in mammalian skin need contact with the BM to retain their proliferative potential (Rousselle et al., 2022; Shen et al., 2023). Here, we review modeling approaches that focus on the ECM’s impact on tissue structure (Sections 3.1, 3.2) and its effects in patterning and morphogenesis (Section 3.3).
3.1 Cell-ECM mechanics and ECM structure
A fascinating observation is the transmission of long-ranged cellular forces via the ECM. Experimental work in the 1980s showed that fibroblasts exert sufficient force to densify and align collagen fibers over distances much larger than the cells themselves, and that these aligned ECM tracts encourage cell migration (Stopak and Harris, 1982). Cellular forces lead to the formation of so-called intercellular ECM bridges, which are visible bands of dense and aligned ECM between distant cells (Panchenko et al., 2022). These experimental observations inspired the development of Turing-type mechanochemical models. Turing models, first proposed by Turing (1952), are a class of pattern formation models that rely on the reaction and diffusion of chemicals. A well-known example consists of two chemical species, a short-ranged activator and a long-ranged inhibitor (Kondo and Miura, 2010). Mechanochemical Turing models, pioneered by Oster et al. (1983), substitute one or more chemical species by mechanical constitutive equations coupling cell and ECM fields. These models explicitly describe the ECM as a continuum field with variable concentration and displacement via diffusive, advective, and convective flows. Like its chemical cousin, the mechanochemical Turing model predicts the formation of spatial patterns of spots or stripes denoting regions of high cell and ECM density.
In the seminal model by Oster et al. (1983), fibroblast contraction takes the role of both activator and inhibitor of the typical biochemical Turing model. On the one hand, contraction pulls in both nearby collagen and nearby cells; the local increase in cells and collagen leads to greater local contraction in a positive feedback loop, thus acting as a short-ranged activator. On the other hand, this leads to a decrease of both cell and collagen density at a longer range, thus acting as a long-ranged inhibitor. An extensive body of work has built on this, for instance, to include more complex cellular behavior such as cell division and haptotaxis (Murray et al., 1988; Holmes and Sleeman, 2000; Tranqui and Tracqui, 2000). Models of this type are widely applied to in vitro endothelial cell sprouting on ECM substrates (Manoussaki, 2003; Murray, 2003; Namy et al., 2004; Tosin et al., 2006), patterning of epidermal placodes and dermal papillae (Murray et al., 1988), patterning during limb morphogenesis (Oster et al., 1985; Murray et al., 1988), wound healing (Tranquillo and Murray, 1992; Olsen et al., 1995; 1998a), and tumor metastasis (Tracqui, 1995). Variations of the model have been developed to include other types of ECM mechanics such as osmotic swelling and de-swelling due to the secretion or degradation of hyaluronic acid (Oster et al., 1985). Several studies also analyze how the choice of constitutive equation for the cell forces and matrix displacement impacts the patterns predicted by the model (Byrne and Chaplain, 1996; Villa et al., 2021). Patterns consistent with mechanochemical Turing models are observed experimentally and are suggested to lay down the pre-pattern for skin follicle morphogenesis (Palmquist et al., 2022).
Another widely-used approach to understand how cell forces spread through the ECM and ultimately form intercellular ECM bridges is based on mechanical models (Wang and Xu, 2020). Many models of this type consider a large (sometimes infinite) ECM gel seeded with a relatively small number of contractile cells, often modeled as shrinking circles or ellipses. Multiscale models combining a microscopic discrete mechanical fiber model with a macroscopic continuum ECM biogel model have also been used (Aghvami et al., 2013). This body of work predicts various contributing mechanisms to intercellular ECM bridge formation, including cell elongation (Abhilash et al., 2014; Han et al., 2018), ECM fiber buckling (Notbohm et al., 2015; Han et al., 2018), focal adhesion mechanics (Cao et al., 2017), non-linear ECM fiber mechanics (Goren et al., 2020; Grekas et al., 2021; Sopher et al., 2023), fiber re-orientation (Goren et al., 2020; Panchenko et al., 2022), force-dependent crosslink breakage and plastic ECM deformation (Kim et al., 2017; Ban et al., 2018; 2019; Malandrino et al., 2019). Clearly, many different mechanisms can qualitatively explain long-ranged force transmission. However, the precise ECM network architecture can quantitatively affect the range and heterogeneity of propagation of cellular forces through ECM (Humphries et al., 2017). Thus, a close matching of simulated ECM to quantitative experimental data is paramount.
Although the aforementioned models successfully capture realistic ECM mechanics, they tend to oversimplify cell shape. To address this shortcoming, some approaches incorporate deformable cells coupled to discrete mechanical models of ECM networks. Perhaps the earliest such model was developed by Reinhardt and Gooch (2014), in which cells are made up of multiple membrane elements that interface with fibers from a 2D ECM network model. In follow-up work, Reinhardt and Gooch (2018) calibrate their model against experimental measurements of collagen biophysical properties. Both models successfully replicate the formation of intercellular ECM bridges. A similar approach is used by Slater et al. (2021) and combined with a more sophisticated ECM network model that includes force-dependent crosslinker unbinding. Eichinger et al. (2021) hybridized an ABM with an ECM fiber network that is fitted to confocal microscopy data of collagen gels. In follow up work, Paukner et al. (2023) use this model to investigate the minimal criteria needed to obtain durotaxis. They identify two sufficient conditions for durotaxis. Firstly, the cell must adhere to ECM via catch-slip bonds. Secondly, the cell must continuously pull on their adhesions to the ECM via actomyosin contraction of its internal cytoskeletal network. Interestingly, both of these factors are included in a different model of a CPM cell on a continuum ECM substrate (discussed in Section 2 in the context of cell migration), which also predicts emerging durotaxis (Rens and Merks, 2020). The fact that two models with such different implementations come to the same conclusion supports the notion that indeed, the biomechanics of integrin bonds and cytoskeletal activity are key to durotaxis. To allow even more cell shape flexibility, Tsingos et al. (2023) hybridized a CPM with a discrete mechanical ECM model that captures long-ranged strain and force transmission through the ECM network. They highlight a transition from viscoplastic to viscoelastic ECM mechanical properties as the density of fiber crosslinking is increased.
Intercellular ECM bridges have been proposed to be involved in long-ranged sensing and potentially communication between distant cells (Panchenko et al., 2022; Nahum et al., 2023). By considering a range of mathematical approaches, the models highlight the key role of cell-derived forces and fiber realignment in the formation of intercellular ECM bridges. Furthermore, many different ECM mechanical properties are permissive to long-ranged force transmission, suggesting this mode of mechanosensing is robust to variations in ECM composition, and could be co-opted to regulate cell function in different tissue contexts. Indeed, long-ranged mechanical interactions mediated by ECM have been proposed to guide collective cell migration, orchestrate wound healing, and allow cells to coordinate during morphogenesis (Sapir and Tzlil, 2017). The pattern-formation potential of relatively simple force-based interactions between cells and ECM are beautifully demonstrated by the Turing-type models. As continuum models, Turing-type models provide an excellent tool at tissue length-scales, while mechanical ECM models tend to focus on smaller cellular scales, but with few exceptions have not incorporated detailed cellular behavior models typically seen in ABM. Recent advances in coupling mechanical network models of the ECM with ABM frameworks such as CPM will enable to investigate how long-ranged sensing and communication impact on migration and pattern formation at the cellular level.
3.2 Tissue integrity
The role of ECM in maintaining tissue integrity has long been appreciated in biomedical engineering, where continuum mechanical modeling of biological tissues including ECM is applied to predict the effects of injury in joint cartilages, the cardiovascular system, and bones, as well as to design synthetic biomaterials for prosthetics, such as arterial stents and heart valve replacements (Khaniki et al., 2023). The strong focus on biomedical engineering has led to the development of many sophisticated material models, for which the reader is referred to excellent reviews elsewhere (Holzapfel et al., 2019; Guo et al., 2022c).
In many ABMs, ECM surrounding the tissue of interest is included by creating a new type of agent. Frequently, these ECM agents are made immobile and act as a physical boundary as well as attachment points for the freely-moving cells. Although clearly a simplification, this approach permits an investigation of how ECM structure affects cell behavior. For example, Buske et al. (2011) models the BM lining the mammalian intestinal epithelium by discrete points with pre-specified positions in the shape of a typical intestinal crypt. A key assumption of this model is that local tissue curvature affects cell proliferation via Wnt-signaling (Huelsken and Behrens, 2002), enabling self-regulation of the proliferative compartment size. Similarly, in a model of mammalian skin epidermis by Sütterlin et al. (2017), cells are simulated using ellipsoids and the BM either as a flat or curved static surface. In this model, feedback regulation between surface skin layers and deep skin layers in contact with the BM ensures that the epidermis achieves a minimum homeostatic depth regardless of BM curvature, and showed that cell proliferation adjusts to fill deeper ECM clefts. A static reticular network—a structure made of close association of fibroblasts with ECM—is used in a CPM of T cell motility in the lymph nodes (Beltman et al., 2007). This model suggests that many features of T cell motility such as stop-and-go motion can be explained by cells colliding with the maze-like reticular network.
Discrete mechanical models of laminin structure have been used to explore how BM integrity affects adjacent tissues, using, for example, a 2D honeycomb lattice (Reuten et al., 2021). By removing nodes in this lattice, ECM softening by Netrin-4, a BM component associated with reduced metastases and better prognoses in cancer patients, is modeled. A similar laminin polygonal model is constructed to explain how laminin ECM aids in epithelial sheet migration during optic cup morphogenesis in zebrafish embryos (Soans et al., 2022). The model proposes that weakening of the laminin matrix creates holes in the BM that prevent collective migration.
The ECM plays a key role in maintaining the integrity of surrounding tissues. The models reviewed in this section simplify the ECM as a static barrier. Despite this strong simplification, such models permit the study of how the mere presence of a steric obstacle can regulate cell motility and proliferation dynamics.
3.3 Morphogenesis
Morphogenesis refers to the acquisition of tissue shape during development, which may involve formation of patterns of cell clumps, but also cell migration, cell adhesion, cell shape change, and differentiation—all processes that can be regulated by the ECM (Cruz Walma and Yamada, 2020). In this section, we first review models of cell and ECM pattern formation before focusing on models considering the influence of ECM on the generation of tissue shape.
3.3.1 Cell and ECM patterning
Besides the aforementioned mechanochemical Turing models, other ECM-mediated pattern-forming mechanisms have been studied with computational models. A general study of pattern-forming potential uses a cellular automaton model in which each lattice site contains either a cell, ECM particle, or fluid medium (Grant et al., 2006). Phenomenological rules are used to explore how cells self-organize into different structures, including cysts surrounding a fluid-filled lumen, cysts surrounding an ECM lumen, or boundaries separating a fluid phase from an ECM interior like an epithelium. In a more involved approach, Checa et al. (2015) combine a cellular automaton model with a continuum mechanics-based description of the ECM to study how cell behavior such as stiffness-mediated realignment coupled to ECM mechanics leads to the formation of patterns of aligned cells and fibers.
To study how patterned ECM emerges from fibroblast migration, Wershof et al. (2019) use a Vicsek model (a type of ABM) coupled to an ECM model on a lattice. Three mechanisms contribute to fibroblast motility: migration noise, cell-cell guidance, and cell-matrix guidance. Cells affect the underlying ECM via fiber deposition, realignment, and degradation. In the absence of cell-matrix guidance, the ECM is either completely disorganized (high migration noise, low cell-cell guidance), or organizes into long coherent strands (low migration noise, high cell-cell guidance). Cell-matrix guidance leads to the generation of wavy fibers and swirls reminiscent of patterns in various tissue types observed by the authors.
To explain the formation of cell clumps in their experimental in vitro work on mesenchymal cell condensation, Zeng et al. (2004) develop a CPM where cells secrete fibronectin on the lattice sites they occupy, forming insoluble non-diffusing deposits. Cells perform haptotaxis toward fibronectin, and cell-fibronectin adhesion leads to the upregulation of cell-cell adhesion proteins. Rapidly and randomly moving cells progressively clump and slow down due to the self-reinforcing loop of fibronectin secretion and increased cell-cell adhesion. This model correctly recapitulates that cell seeding density is correlated with the transition from spots to stripes, and the fact that (unlike Turing patterns) the clump patterns are highly irregular.
White adipose tissue is organized into lobules separated by collagen-rich septa, which Peurichard et al. (2017) sought to model with an ABM representing adipocytes as spheres and ECM fibers as lines. They recapitulate the formation of lobules and septa by simple mechanical interactions; as islands of adipocytes expand by cell proliferation and growth, they compress collagen fibers into septa until the tissue is densely packed to an extent that no new cell growth can occur.
During development, the mammalian skin BM changes from an initially flat surface to an undulated one (Shen et al., 2023). In this context, the interaction between cell proliferation and the ECM is also the focus of several models where the BM is represented as a collection of small spheres (Kobayashi et al., 2018; Ohno et al., 2021). The authors argue that mechanical buckling alone cannot explain undulation, as buckling neither gives a direction of undulation nor does it lead to irregular undulations, as observed in the skin. In contrast, their model shows that cell division dynamics could bias the ECM curvature due to cells pinching off the ECM when detaching. In addition, the positioning of stem cells along the tips of the undulated BM emerges spontaneously due to the combined action of cell-ECM adhesion and cell division.
Realignment of the ECM by migrating cells leads to the formation of patterns, an observation that has also been made in other models of cell migration (Painter, 2009). In particular, cell-ECM guidance causes the organization of migrating cells into long coherent, directed strands. Cell clumping, on the other hand, is a result of a feedback loop between cell secretion of fibronectin and increased cell-cell adhesion. Different patterns can emerge by steric interactions due to cell proliferation. As shown by Peurichard et al. (2017), cell growth can compress strands of initially loose ECM to form thick septa. In the skin, cell proliferation may underlie the formation of undulations in the BM (Kobayashi et al., 2018; Ohno et al., 2021).
3.3.2 Blood vessel morphogenesis
3.3.2.1 Models of angiogenesis
Angiogenesis refers to the formation of new blood vessels from pre-existing ones (Folkman, 2006) (Figure 4A). During angiogenesis, hypoxic tissues, such as tumors, secrete vascular endothelial growth factor (VEGF), which diffuses around cells and acts as a chemotactic cue for endothelial cells. Endothelial cells sprout from pre-existing blood vessels and migrate towards the source of VEGF. The ECM plays central role in angiogenesis and vascular remodeling, which has led to extensive theoretical work explicitly including the ECM. Here, we highlight some of these modeling works, and refer the reader to more in-depth reviews elsewhere (Heck et al., 2015; Abdalrahman and Checa, 2022; Crawshaw et al., 2023).
FIGURE 4. Schematic depiction of angiogenesis and vasculogenesis. (A) Angiogenesis is a process in which new blood vessels form from a pre-existing network, whilst (B) vasculogenesis is the process by which randomly scattered cells spontaneously form vessel networks. During both of these processes, the underlying ECM plays an important role.
The role of different cues in angiogenesis is a long-standing question. In this context, Anderson and Chaplain (1998) study the balance between chemotaxis and haptotaxis. Their continuum model predicts that, when chemotaxis prevails, cells quickly reach their target tissue. However when haptotaxis dominates, the sprout moves slower and can fail to reach its target tissue. As the continuum approach cannot track individual cells, and thus precludes analysis of sprout morphology, the authors also derive a discrete cellular automaton model. In this model, migration via chemotaxis is predicted to favor straight sprouts, whilst haptotaxis favors the formation of sprouts looping onto themselves to form anastomoses. In contrast to Anderson and Chaplain (1998), Levine et al. (2001) argue, based on experimental evidence, for ‘negative’ haptotaxis, that is, cell movement from high to low substrate concentrations. In their continuum model, endothelial cells secrete MMPs in response to VEGF, locally degrading fibronectin. Thus, the cells at the migrating front carve a path for following cells, promoting further migration despite the absence of chemotaxis.
The seminal ABM of Anderson and Chaplain (1998) inspired many other ABMs of angiogenesis, including CPMs. Bauer et al. (2007) introduce a model of interstitial ECM as line segments in the CPM lattice, and model how cells crawl on top of, adhere to, and degrade ECM. The ECM is a static obstacle around which cells must move, a task well-suited to be modeled with the CPM. Here and in a follow-up model (Bauer et al., 2009), an optimum ECM fiber density is required for successful angiogenesis. In sparse ECM, degradation reduces sprout extension speed, while in dense ECM, degradation enhances sprout extension speed. However, beyond a threshold ECM density, sprout migration slows down considerably. A similar ECM density optimum is also found in a hybrid model by Milde et al. (2008). They represent the ECM as a tensor with density and directionality in 3D, while the cells at the sprout tip are modeled as agents. The model predicts that low ECM densities lead to slowly advancing but unbranched vessels, intermediate ECM densities cause the vessels to advance quickly but form many branches, while at very high ECM densities, vessels advance slowly and branch frequently. In line with the model by Anderson and Chaplain (1998), increased fibronectin-directed haptotaxis leads to increased vessel tortuosity, as haptotaxis acts as a local autocrine directional cue.
Daub and Merks (2013) investigate the roles of MMPs in the balance of chemotaxis, haptotaxis and haptokinesis (faster cell speed toward denser ECM). The model combines CPM with a mechanical continuum ECM model, and predicts that strong chemotaxis favors long unbranched sprouts, while stronger haptotaxis or -kinesis favors short branched sprouts. These predictions are similar to those by Anderson and Chaplain (1998). Boas et al. (2012) also study sprout morphology, but include both MMPs and soluble MMP inhibitors. At low secretion of both factors or high levels of MMP secretion, sprouts showed tubular morphology; at high levels of MMP activator secretion, sprouts developed into cyst-like structures; and high secretion of both MMP and their activators led to collective movement of the entire endothelial cell population without sprouting. In follow-up work the authors explored and experimentally validated the positive feedback loop between ECM degradation, and release of bound growth factors that further stimulate MMP production (Boas et al., 2018).
Several models focus on mechanical cues in angiogenesis. Edgar et al. (2013) represent local ECM orientation as a vector field that biases the growth direction of blood vessels. They later expanded this model to simulate how macroscopic gel deformations affected local ECM fiber orientation and vessel sprouting (Edgar et al., 2015). Stéphanou et al. (2015) model individual endothelial cells superimposed on a continuum chemo-mechanical model of the ECM. They include a strain-biased diffusion tensor, allowing matrix deformation to feed back directly on cell migration direction. They identify an intermediate parameter range for cell traction forces and ECM stiffness that enable angiogenesis.
Recent models have extended previous work by introducing Delta-Notch signaling pathways to model phenotype acquisition of sprout tip and stalk cells. Extending a model by van Oers et al. (2014) where a CPM is hybridized with a continuum mechanics model of the ECM, Vega et al. (2020) introduce Delta-Notch-Jagged signaling. The model predicts that higher Delta reduces the number of sprouts and thickens them, while higher Jagged leads to more branching as it promotes lateral sprout induction. ECM mechanics play into this dynamic by promoting cell extension and lateral branch anastomosis. In follow-up work, the authors combine their angiogenesis model with a model of retinal cells and BM to simulate age-related macular degeneration (Vega et al., 2021). They predict that impaired intercellular adhesion, excess VEGF and Jagged expression contribute to the disease. More recently, Stepanova et al. (2021) develop a 2D hybrid, multiscale model for angiogenesis with phenotype-dependent cell-ECM interactions. At the subcellular level, a stochastic model of VEGF-Delta-Notch signaling determines two phenotypes: Delta-high tip cells and Notch-high stalk cells. The phenotypes differ at the cellular scale, as tip cells are more motile, degrade interstitial ECM, and deposit BM. Interstitial ECM prevents cell migration, while the deposited BM prevents sprout branching. Finally, the tissue scale employs ODEs to track changes in ECM component concentrations, and ECM fiber orientation. As in many other models, cell migration direction and ECM fiber alignment mutually influence each other. In contrast to other models, here both branching and chemotactic sensitivity emerge dynamically. Further, this model identifies interstitial ECM degradation and BM deposition as crucial processes in determining number and length of vessel branches.
A few common characteristics emerge in these studies. First, chemotaxis and haptotaxis are often predicted to have opposing effects on sprout migration velocity and morphology. Second, mechanical features—ECM density, stiffness, cell forces—display a non-monotonic relationship with cell migration, being optimal at an intermediate value. Finally, newer models have begun extending the scales to include biochemical signalling. Nevertheless, due to the different mechanisms investigated in each study, comparing the validity of these models remains challenging. Quantitative comparisons of models to experimental data will be invaluable to identify the leading mechanisms of angiogenesis.
3.3.2.2 Models of vasculogenesis
An alternative way of blood vessel formation is vasculogenesis, wherein blood vessels are created de novo from scattered endothelial cells (Figure 4B). Experimental vasculogenesis in the tube formation assay begins with isolated cells plated on ECM. The cells then coalesce into strands, forming a loose cell network (Arnaoutova et al., 2009). As mentioned earlier, mechanochemical Turing models have been applied to vasculogenesis (Manoussaki, 2003; Murray, 2003; Namy et al., 2004; Tosin et al., 2006). In general, these models predict the formation of stable patterns of cell strands, which correspond to positions where cell forces densified ECM.
To model the formation of vascular networks in embryos, Köhn-Luque et al. (2011) propose and experimentally validate a model where VEGF, secreted by adjacent tissue, can be immobilized through binding to the ECM (Köhn-Luque et al., 2013). Thus, in contrast to angiogenesis, secreted VEGF does not form a gradient, but is uniformly present. In their model, randomly distributed CPM cells deposit ECM on the lattice sites they occupy. Because deposited ECM binds VEGF, sequestered VEGF locally accumulates. These local bound VEGF gradients induce cell chemotaxis, effectively encouraging cells to stretch and move towards locations they previously visited.
Using a CPM hybridized with a continuum mechanics model of ECM, van Oers et al. (2014) model cell-generated ECM strains, while the reciprocal coupling of the ECM to cells occurs indirectly by biasing cell motility towards regions of higher ECM strain. Follow-up work by Ramos et al. (2018) shows that cells elongate and align along tracts of high strain, demonstrating that patterning requires an intermediate level of ECM stiffness. More recently, Carrasco-Mantis et al. (2023a) study the effects of the ECM on vasculogenesis using a 3D ABM. They predict a strong relationship between ECM stiffness and vascular network proliferation, and in particular, that as the viscoelasticity of the ECM is increased, the vascular network proliferates less, such that network growth occurs primarily in regions of stiffer ECM. These predictions are partially validated through comparisons with existing experimental studies of vasculogenesis. Noerr et al. (2023) use an ABM in which cells align along directions of strongest ECM strain through the exertion of dipole forces. Three parameters are found to affect the ability to form a vascular network in the model: cell density, cell contractility, and substrate stiffness. Substrates that enable long-ranged force transmission are found to be most suited to lead to vascular network formation even at low cell densities.
The reviewed studies predict that the success of vasculogenesis strongly depends on the ECM. Although playing a different role during angiogenesis, VEGF facilitates vasculogenesis through local accumulation by binding to ECM, creating pockets of high VEGF concentration to which cells preferentially migrate towards. Another common theme among models is the role of ECM mechanics, as vasculature development requires some level of ECM stiffness to enable cells to sense each other over longer distances and thus coordinate their alignment.
3.3.3 Tissue morphogenesis
3.3.3.1 Cell-driven morphogenesis
Morphogenetic events can often be traced back to cellular changes, such as cell shape changes and cell rearrangements. A textbook example is invagination, which is often ascribed to apical cell constriction (Figure 5). Davidson et al. (1995) develops a highly detailed continuum mechanical model of invagination in the early sea urchin gastrula that includes separate layers for apical, lateral, and basal cell sides, as well as various layers of the ECM. They then systematically test five competing hypotheses: apical cell constriction, lateral cell tractoring, apical ring contraction, apicobasal contraction, and ECM swelling. Surprisingly, all of these mechanisms are found to reproduce invagination, but require different mechanical parameters. For example, the model predicts that apical cell constriction requires similar cell and ECM stiffnesses, while an ECM swelling hypotheses requires the ECM to be much stiffer than the cells.
FIGURE 5. Schematic depiction of processes of invagination. (A) Apical constriction: a cell shape change whereby the apical side of the cell actively contracts and shrinks, forming a concave indentation (Martin and Goldstein, 2014). (B) Buckling: external mechanical forces cause a tissue sheet to bend, cell shapes change passively as a consequence of the change in tissue curvature (Nelson, 2016).
Cell rearrangement is another paradigmatic morphogenetic event, and an excellent use-case for ABMs. Longo et al. (2004) develop a cellular automaton model of radial intercalation in tissue extension of the blastocoel roof of Xenopus laevis embryos. The model consists of several stacked cell layers, where at the very bottom they are lined with a layer of fibronectin ECM. Using phenomenological rules for cell motility and fibronectin deposition, they recapitulate the pattern of tissue elongation and basal fibronectin enrichment. The model predicts that groups of initially adjacent cells disperse laterally during intercalation, which they subsequently verify with cell transplantation experiments. The model also shows that the fibronectin layer reduces cell dispersion by promoting cell adhesion, and thus prevents overextension of the tissue.
3.3.3.2 Somitogenesis
During vertebrate development, somites form by segmental epithelialization of mesenchymal cells derived from the presomitic mesoderm at the posterior end of the embryo. The cells in each segment become patterned by spatio-temporal gene activity oscillations in a mechanism known as the clock-and-wavefront model (Baker et al., 2006). Truskinovsky et al. (2014) argues that chemical models of somitogenesis are insufficient to explain physical separation of the tissue into segments, and propose a mechanical model where inter-tissue coupling via fibronectin ECM plays a crucial role. This model predicts that segments form because the presomitic mesoderm is a relatively soft tissue that extends while mechanically connected to and confined by relatively rigid layers. A similar physical mechanism is used by Nelemans et al. (2020) where the ECM is represented as a mesenchymal cell type in the context of a CPM. This work predicts that imposed exterior mechanical strain leads to the separation of somites, which is also experimentally verified by mechanical manipulation of tissue explants. Supportive of the predictions of these modeling approaches, new intriguing experimental evidence indicates that the fibronectin ECM plays a role in regulating left-right somite symmetry (Naganathan et al., 2022) and inter-tissue growth coordination (Guillon et al., 2020).
3.3.3.3 Folding morphogenesis
A recurring theme in morphogenesis is the reshaping of flat or tubular tissue into more complex topologies with folds, bends, and loops. Tissue folding can occur via multiple mechanisms, including cell growth, migration either of single cells or epithelial sheets, confinement-imposed forces by adjacent tissues, and also from a mechanical mismatch between adjacent tissue layers such as epithelia and ECM-rich mesenchyme (Hughes et al., 2018; Agarwal et al., 2022). For example, Gardiner et al. (2015) exert compressive forces on a subcellular ABM where cells and the BM are represented by clusters of particles. When lateral cell-cell adhesion is high, the tissue responds by mechanical buckling. In contrast, when vertical cell-ECM adhesion prevails, the tissue resists buckling and instead adapts to compression by cell shape changes to columnar morphology. Although tissue folding is a highly reproducible process in embryonic morphogenesis, mimicking it in vitro remains a challenge. To address this, Hughes et al. (2018) developed an experimental bioengineered tissue scaffold whose folding pattern is predicted using a continuum mechanical model. By combining experimental work with modeling, cell contraction is found to condense ECM into specific locations, which allows the tissue to locally support greater tensile strength. These bands of condensed ECM are thus akin to predetermined faultlines that facilitate tissue folding in a robust, predictable pattern.
An example of the flat to folded transition is seen in the Drosophila wing imaginal disc - a bilayered epithelium surrounded by BM that develops stereotypical folds and eventually gives rise to the adult wing of the fly (Martín et al., 2009). Using a continuum mechanical model, Keller et al. (2018) estimate mechanical properties of the tissue through comparison with stretching experiments. Interestingly, a stiffer layer of ECM at one side of the tissue is necessary to replicate experimental measurements. Tozluoǧlu et al. (2019) develop a continuum mechanics model of the wing disc and the underlying BM. In their model, folding at the stereotypic positions requires both differential planar tissue growth and mechanical constraining by uniform elastic compression from the BM. Though this model correctly predicts folding, it cannot resolve individual cells and the contribution of cell shape changes. To address this, Nematbakhsh et al. (2020) develop a 2D subcellular element model that includes detailed cell shape, nuclear shape and position, and homotypic and heterotypic cell-cell and cell-ECM contacts. Using a combination of modeling and experiments, they find that actomyosin contractility is required to initiate tissue folding, but is dispensable afterwards. In contrast, ECM tension cannot create the initial fold, but is necessary to maintain it after actomyosin activity ceases.
Several embryonic tissues start as a straight tube-like shape, then fold and twist to produce loops. A classic example is the vertebrate heart which undergoes cardiac looping, (Taber, 2003). The embryonic heart consists of two epithelial cell layers enclosing a thick layer of ECM, the cardiac jelly. Ramasubramanian et al. (2006) develop a continuum mechanics model of both the outer myoepithelium cell layer and the cardiac jelly. Each layer’s material properties are adapted to match experimental data, including deformation terms to account for cell migration, cell growth, active contraction, and ECM swelling. This model predicts that swelling of the cardiac jelly, though not responsible for organ shape change, enhances structural integrity of the epithelial layer by balancing cell forces.
The vertebrate intestine is another example of a tubular organ that bends and loops. The first step of gut looping is a leftward tilt in the tissue, which Kurpios et al. (2008) model by representing cells as a collection of six membrane nodes and one internal node, all mechanically connected via spring-dashpot mechanics (Figure 6B). The ECM is represented indirectly by a spring-like forces between adjacent cells. The model shows that, while cell shape change leads to a slight leftward tilt, it fails to explain the full range of motion of the tissue. Instead, the model predicts a synergistic effect of asymmetric swelling of the ECM combined with asymmetric cell shape changes. Looking at the scale of the entire organ, Savin et al. (2011) elegantly combine ex vivo tissue explants with physical rubber models and computational discrete mechanical models to explain gut looping. Using a similar mechanical model as Kurpios et al. (2008), Savin et al. (2011) show that looping emerges from a mismatch of the mechanical parameters of two adjacent and connected tissue layers, the intestinal epithelium and the ECM-rich mesentery. Much like in a bimetallic strip, differential expansion or shrinkage of one tissue leads to wrinkling, which manifests as looping in the tube-shaped tissue.
FIGURE 6. Schematic of material models. (A) A spring-like model where the strain is linearly proportional to stress, and (B) a spring-dashpot model that depicts the stress-strain relationship in viscoelastic materials. The spring permits fast, unrestricted motion, whilst the dashpot is effectively a damper that slows motion in the opposite direction.
On a smaller spatial scale, the intestinal tissue starts as a flat epithelium on top of a BM that folds to form multiple undulations—the bottom of these undulations will form the intestinal villi and crypts that house stem cells. Hannezo et al. (2011) develop a mechanical continuum model of the intestinal tissue, where epithelium, BM, and stroma are each modeled as layers with separate mechanical parametrization. They predict that different patterns can emerge by mechanical buckling—spots, checkerboards, herringbone-stripes, and labyrinthine stripes—and depend on parameter values for coupling of cell division and ECM curvature, and the parameters for pressure exerted by the intestinal epithelium. The elasticity of the BM is predicted to play a crucial role in stabilizing the amplitude of the villus folds. Dunn et al. (2012) develop an ABM of the BM to study how intestinal crypts attain their morphology. They model the BM as discrete regions between epithelium and stroma, each with a preferred curvature maintained via spring-like forces (Figure 6A). Starting from a flat sheet, a gradual change in BM local preferred curvature is imposed, which results in gradual invagination of the intestinal crypt. The depth of invagination also depends on the density of epithelial cells, which results from a combination of proliferation, death, and intercellular forces. For example, when cells adhere more strongly they become denser, and their collective force overcomes the ECM force and thereby reduces crypt depth. The model thus predicts that deeper crypts emerge when intercellular connections are weak. Shyer et al. (2013) develop a more detailed continuum mechanics model of a section of the gut tube to model villus morphogenesis. They take into account not only the epidermal and mesenchymal layers (which includes the BM), but also surrounding muscular layers, each modeled as a separate material. This study elegantly demonstrates how mechanical coupling of layers growing or shrinking at different rates can lead to development of tissue folds. Interestingly, their model also recovers the same sort of patterns found by Hannezo et al. (2011) in their more abstract model.
Another tube-like structure that becomes looped is the C. elegans gonad, which consists of an epithelium ensheathed by a BM. Agarwal et al. (2022) model the gonad as a rectangular structure with elastic walls representing the BM, and include cell-ECM adhesions using elastic springs. To test their hypothesis that asymmetric BM degradation induces looping, they impose different spring constants on one side of the tissue. This mechanical imbalance directs the tissue to always bend in the direction opposite to weaker adhesions, which the authors verify experimentally.
Many epithelial tissues such as lungs and secretory glands develop by a branching of initially symmetric epithelial structures. As in gut morphogenesis, these epithelia are usually ensheathed by a BM, and further surrounded by an ECM-rich mesenchymal tissue with contractile fibroblasts (Varner et al., 2015). The interplay between epithelium and mesenchyme plays a central role in determining the position of branch points, but the nature of this interplay—chemical or mechanical—is under debate (Varner et al., 2015). In an exploratory model, Wan et al. (2008) investigate how mesenchymal fibroblast contraction may deform an epithelial bud at the onset of branching. Their mechanical continuum model explicitly includes both fibroblasts and ECM in the mesenchyme using a fluid mixture model. Variations in density and spatial distribution of contractile fibroblasts give rise to different morphologies in the epithelial bud such as dimples and clefts. Varner et al. (2015) combine experimental work in lung bud explants with a continuum mechanical model of a growing flat epithelial sheet surrounded by viscoelastic medium. Their model predicts that explants branch due to growth in a confined environment, which they verify experimentally by embedding explants in increasing concentrations of Matrigel, a commercially available matrix. Notably, the mechanism of shape generation is highly reminiscent of the models of gut and gonad morphogenesis discussed earlier, and suggest an elegant underlying principle of tissue morphogenesis: adjacent tissues each with different mechanical properties and different growth rates. Crucially, both of these properties are tunable by cells—tissue mechanics by changing ECM composition, and growth by cell proliferation or ECM swelling. In essence, this suggests that ECM composition is part of evolution’s toolbox in shaping the diversity of forms in the animal kingdom.
4 Discussion
The aim of this review is to highlight the variety of mathematical models and computational tools available to study the composition and function of the ECM across a range of biological systems and scales, and to direct the interested reader to relevant papers. From the vast number of examples presented, it is clear that mathematical and computational investigations can help illuminate key behaviors and mechanisms of interaction between cells and the ECM.
Throughout this review, we highlight models of cell migration, including cancer cell progression and wound healing, alongside models of tissue structure and morphology, that concern the integrity and composition of a tissue. Notably, ECM structure, particularly the mechanical properties, play a key role in both cell migration and tissue morphology. A prime example of how matrix stiffness affects cell migration is durotaxis, which different modeling approaches demonstrate requires force-dependent focal adhesion dynamics and contractile cytoskeletal activity (Rens and Merks, 2020; Paukner et al., 2023). ECM stiffness also drives vasculature formation, with simulations from Carrasco-Mantis et al. (2023a) and Noerr et al. (2023) highlighting its importance in coordinating cell behaviors via mechanical feedback. Fiber alignment and orientation play a central role in several models, including in migration and pattern formation. One such model of wound healing (Cumming et al., 2010) produces simulations concurrent with experimental data, emphasizing the importance and role of ECM fiber properties during scar formation.
A major benefit of the use of mathematical models in biology is their predictive power in guiding further experiments. Often, hypotheses generated in silico are used to predict key parameters or mechanisms within a system, which in turn expedite corresponding experimental investigations by reducing their required scope. For example, the ABM for NCC migration considered by McLennan et al. (2023) was used to predict that Trail and Colec12, factors expressed adjacent to the pathways along which cranial NCCs migrate in chick, must only be highly expressed for a fraction of the regions adjacent to NCC collectives for the restriction of NCC movement to stereotypical migratory pathways. This hypothesis was then tested in chick, where Colec12 and Trail were also found to be highly expressed for around one-third of the regions adjacent to the pathways along which NCC migration occurs. In the context of neural crest biology, a similar ABM was also used to predict distinct phenotypes in cranial NCCs at the leading and trailing edge of collectives. This prediction motivated a gene expression profile analysis of chick cranial NCCs, which also found differential gene expression across NCC collectives McLennan et al. (2012). Such findings highlight the predictive power of mathematical and computational modeling in helping to guide experiments towards mechanisms and parameters of interest in understanding observations in vivo.
Another key purpose of mathematical models in biology is in elucidating unexplained processes, for example, the role of the ECM remodeling enzyme LOX during cancer cell migration (Nguyen Edalgo and Ford Versypt, 2018). This particular model provides predictions that could guide therapeutic treatments, such as inhibiting LOX production to slow down ECM remodeling, thus preventing the spread of cancer. Another example is the investigation of how the ECM regulates the underpinning molecular mechanisms of cell migration (Park et al., 2017). In understanding how ECM structure impacts the molecular mechanisms of cells, greater insight is developed into the design of targeted pharmaceutical treatments that can inhibit or express more migratory cell phenotypes.
Nevertheless, in order to refine and validate these models, it is crucial to couple modeling with experimental investigations. To facilitate such collaborative efforts, in the following discussion, we aim to provide brief guidance for determining the most appropriate modeling framework for a given study. We discuss the challenges faced when pairing mathematical and computational models with sparse experimental data and also the benefits of fostering interdisciplinary collaborations between theoreticians and experimentalists.
4.1 Selecting an appropriate modeling framework
Many mathematical models of biological processes neglect the structure of the ECM and its interactions with cells, and this review serves to highlight modeling efforts that explicitly consider the ECM and its role in processes such as tumor progression (Section 2.3), wound healing (Section 2.4), and tissue morphogenesis (Section 3.3). The most appropriate modeling framework to adopt depends on various factors including the complexity of the system at hand, the characteristic time and length scales of relevant processes, and the overall purpose of a study. In this section, we highlight key considerations for selecting a model framework and list the relative advantages and disadvantages of each framework considered.
When considering the composition of the ECM itself, the most suitable modeling approach depends primarily on the spatial scale at which the ECM is to be studied. For example, at the tissue level, simulating each constituent cell and ECM particle will incur significant computational costs (Saxena et al., 2021; Stack et al., 2022) that make repeated simulations or parameter sweeping infeasible. Therefore, over large length scales, continuum representations of the ECM are often favored over ABMs due to their lower computational demands. The coarse-graining of ABMs offers a potential compromise between microscopic detail and macroscopic simplicity (Simpson et al., 2009a; Penington et al., 2011). For example, work by Voulgarelis et al. (2017) derives a continuum representation of an ABM for tumor cell reprogramming, which is found to produce similar results to simulations of the underlying ABM for certain parameter regimes. However, an advantage of using purely agent-based representations of the ECM is that they permit greater interpretability than continuum models through their detailed descriptions of cell behaviors and cell-cell communication. Furthermore, ABMs allow an even greater emphasis to be placed on behaviors observed at the cell-scale and can often capture interactions between cells and the ECM that continuum frameworks cannot. For example, Bull et al. (2018) develop a hybrid ABM model that is required to capture microbead infiltration patterns in tumor spheroids. For subcellular level studies, discrete mechanical models may be most suitable, as they allow a more detailed representation of cell morphology and cell-cell interactions based on the underlying physical properties of cells. However, the use of such models is also primarily limited by the computational resources required to simulate many cells at a subcellular resolution. Alternatively, when a system of interest comprises elements at vastly different characteristic length-scales, combining agent-based and continuum approaches, for example, into a hybrid model may be the most desirable solution in that it offers accuracy of each relative counterpart in the system, without the computational cost of a fully agent-based system. It is, therefore, clear that the scale of the tissue and the scale at which the interactions of interest are occurring is vital in establishing the most appropriate modeling framework.
As well as defining the spatial scale of the system, it is also important to consider the availability of experimental data for a computational investigation. Mechanical models require precise material properties and geometrical data to be accurately calibrated, whereas continuum models require only macroscopic data, such as spatially averaged mechanical properties and concentrations of ECM constituents. In many cases, obtaining biomechanical measurements requires specialized expertise that may not be available to most experimental researchers. In contrast, ABMs necessitate granular data at the cellular or molecular level, such as individual cell behaviors and interaction rules, that are ideal when high-resolution experimental data is abundant. Hybrid models, such as coupled continuum and mechanical models, are versatile and can be adapted to the available data, making them suitable for scenarios with variable data resolutions. The choice among these frameworks should, therefore, be closely aligned with the quality, quantity, and scale of the available data, ensuring that the chosen model can be adequately parameterized and validated to produce reliable insights into ECM composition and its effects on biological processes.
Using data generated in experiments, a common paradigm in biological modeling is to iteratively refine models upon comparing model predictions with corresponding in vitro and in vivo studies. As such, another important consideration in selecting a model is the extent to which it may be adapted and refined to account for new experimental findings. Continuum models are inherently adaptable at the macroscopic level, easily integrating changes in material properties and boundary conditions, which makes them suitable for a broad range of tissue-scale scenarios of varying degrees of complexity. Conversely, ABMs are easily mechanistically interpretable and as a result, are particularly well suited to refinement at an individual cell/ECM constituent level by permitting biological mechanisms to be built into models with relative ease. Furthermore, ABMs often contain more parameters that can be independently varied for comparison to wide-ranging biological phenomena. In general, mechanical models are less flexible in terms of biological adaptability but can be highly amenable to incorporating new mechanical data or in simulating different physical scenarios. The flexibility of mechanical models lies in their capacity to adjust to new geometries, loading conditions, and material properties, all of which are crucial when exploring the bio-mechanical aspects of the ECM.
Finally, the complexity of the biological system often dictates the choice of mathematical model and resulting number of parameters in the system, which represent quantities such as exchange rates, substrate concentrations, and forces. In general, it is best to consider the number of parameters attainable via experimental exploration when choosing the most suitable modeling framework. It is also possible to determine unknown parameter values through model fitting. With this in mind, the larger the number of parameters, the harder it can be to fit the results of the model to in vivo and in silico data. Moreover, some of these parameters may be difficult to interpret biologically, and may in fact be an artifact of the model development, for example, ABMs often include a parameter that sets a threshold distance to limit cell-cell interactions.
In cases where fitting the model to experimental data is not possible, other approaches can be used, such as machine learning algorithms (Preen et al., 2019), that explore the parameter space to find the optimum values and reach an objective function (Ozik et al., 2018). These, however, have the drawback of requiring a large amount of data, upwards of ten times the number of samples versus number of features or parameters. Additionally, interpretation of these models can be very difficult, with the ‘black box’ nature of many of these models providing metrics for the predictive accuracy of a model, but inhibiting interpretation of the underlying biological meaning (Xu and Jackson, 2019).
In summary, carefully assessing and balancing elements such as the scale of the biological system, the specific objectives of the study, and the accessibility and type of relevant data, is crucial when considering the most suitable modeling framework for the biological system of interest.
4.2 Open challenges
Due to the inherent complexity of the ECM, containing components that vary vastly in composition and size, it can be difficult to quantify its properties experimentally. Methods mentioned in Section 1.2 can identify ECM components and characterize ECM structure, chemical composition, and mechanics. However, the specialized expertise essential to each method underscores the importance of interdisciplinary collaborations. Advances in obtaining quantitive and qualitative data of the ECM will greatly enhance in silico models by allowing for more accurate predictions to be made through enhanced model parameterization.
One of the most pertinent aspects of mathematical modeling in biology is the integration of models over multiple spatial and temporal scales. For example, molecular interactions occur at a vastly different rate from cellular or tissue level dynamics and therefore must be modeled at a different temporal scale. Linking such scales can be difficult depending on the numerical approaches taken, where the simulations and results from one scale define the parameters and variables at another. Multi-scale models are an excellent tool for simulating processes that occur over vastly different time or length-scales, but can become prohibitively complex as more interactions and biological phenomena are considered. The development of a multi-scale model most certainly means an increase in the size of the model and computational power needed for simulations. As a model increases in size, typically the number of variables and parameters will also increase. Some of these issues can be tackled, for example, if there are sufficient data available, such that unknown parameters can be constrained to a realistic regime. However, without such constraints, these models can become too complex to systematically analyze and biological interpretation can be lost.
Computational implementation can also be a barrier to developing such models. Although there are many available software packages for building computational models (see Table 4 for a list of resources), they are built with a specific modeling approach in mind. Thus, often one needs to develop new codes tailored to a specific biological question, reducing the possibility of fully leveraging hybrid or multi-scale systems, and requiring extensive programming ability.
TABLE 4. A list of computational resources for modeling cell-ECM interactions in cell migration and tissue morphogenesis.
All of these challenges apply to models that either do or do not include the ECM. Models that include the ECM are intrinsically more complex due to at least one additional variable in the model (or more if including specific components of the ECM) and their associated properties. The implementation of large, all-encompassing models is something many aspire to, and work is ongoing in the field of mathematical biology to improve and perfect these models to elucidate the most relevant and important information (Eftimie, 2022).
4.3 Mathematical modeling as a tool in collaborative biological research
This review highlights that the development of models and the interpretation of wet lab results are intricately linked, drawing insights and questions from the existing literature and data. In general, mathematical models are developed to investigate previously unexplored experimental conditions or to predict behavior in conditions that are experimentally intractable. Hence, mathematical models provide an alternative way to explore new theories and hypotheses that can then be tested and validated experimentally.
A major benefit of using mathematical models to study biological systems is their ability to provide an efficient approach to rapidly executing numerous simulations with wide-ranging parameter sets, such that relevant biological parameter ranges may be quickly identified for further testing in experiments. This route also proves to be faster and vastly less expensive than the cost and time-intensive nature associated with setting up and performing experiments both in vitro and in vivo. Instead, models excel in exploring conditions that, in the lab, are challenging to replicate or analyze with available equipment. In the development of novel therapies, models may be used to study the effects of biological perturbations in a system. This, in turn, can be used to predict or highlight parameters of particular importance in the prevention of diseases, eliminating the need for costly interventions after the onset of pathogenesis. Furthermore, it is often difficult, or impossible, to experimentally stain numerous cell phenotypes for identification in microscopy. In such cases, mathematical models can be used to predict the presence of certain phenotypes that experiments cannot replicate, in order to enhance the understanding of the underlying cell-ECM interactions, and help develop targeted treatments specific to a given phenotype. Additionally, mathematical simulations provide a systematic approach to exploring mechanical perturbations within systems, for example, stiffness of the ECM (Aparicio-Yuste et al., 2022), and structure of fiber networks and their alignment (Lee et al., 2017).
In highly complex systems where designing experiments and gathering data can be difficult, model predictions aim to help in determining significant molecules or pathways in a system. This approach simplifies the system and narrows down the scope of focus towards interesting processes and behaviors, helping to guide experimental design.
In this way, mathematical models work most effectively when there is direct synergy between both the experimental and computational counterparts. As such, collaborative efforts often unveil new research questions during the construction and validation of biological hypotheses, establishing a continuous cycle of model development, refinement, and experimental data generation that yields new insights and perspectives.
Author contributions
RMC: Conceptualization, Investigation, Writing–original draft, Writing–review and editing. SJ: Conceptualization, Investigation, Visualization, Writing–original draft, Writing–review and editing. ET: Conceptualization, Investigation, Writing–original draft, Writing–review and editing. ZB: Investigation, Writing–original draft. MBe: Investigation, Visualization, Writing–original draft. MBo: Investigation, Writing–original draft. QJSB: Writing–original draft, Writing–review and editing. JM: Conceptualization, Writing–original draft, Writing–review and editing. MR: Writing–original draft, Writing–review and editing, Investigation. YY: Investigation, Writing–original draft, Writing–review and editing. RS: Conceptualization, Investigation, Supervision, Visualization, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. RMC is supported by funding from the Engineering and Physical Sciences Research Council (EP/T517811/1) and the Oxford-Wolfson-Marriott scholarship at Wolfson College, University of Oxford. SJ receives funding from the Biotechnology and Biological Sciences Research Council (BBSRC) (BB/T008784/1). ET is funded by the Dutch Research Council (NWO) grant number VI. Veni.222.323. ZB is supported by funding from the Engineering and Physical Sciences Research Council (EP/T517914/1). QJSB is funded by Dutch Research Council (NWO) by the ENW-XL program ‘Active Matter Physics of Collective Metastasis’ (OCENW.GROOT.2019.022). JM was supported in part by NSF Grant 1735095 - NRT: Interdisciplinary Training in Complex Networks and Systems, the Jayne Koskinas Ted Giovanis Foundation for Health and Policy, and the Breast Cancer Research Foundation. MR is funded by PerMedCoE (H2020-ICT-951773). YY is funded by the Engineering and Physical Sciences Research Council (EP/W524311/1) and supported by the Travel for Research and Study Grant at St Hilda’s College, University of Oxford.
Acknowledgments
MBo acknowledges the University of Birmingham. Authors would like to acknowledge Cicely Macnamara and RS, alongside Centre Européen de Calcul Atomique et Moléculaire (CECAM) and the Lorentz Centre for hosting the workshop “The Extracellular Matrix: How to model structure complexity” in which this collaborative group was formed.
Conflict of interest
Author MBe was employed by Optics11 Life Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdalrahman, T., and Checa, S. (2022). On the role of mechanical signals on sprouting angiogenesis through computer modeling approaches. Biomech. Model. Mechanobiol. 21, 1623–1640. doi:10.1007/s10237-022-01648-4
Abhilash, A. S., Baker, B. M., Trappmann, B., Chen, C. S., and Shenoy, V. B. (2014). Remodeling of fibrous extracellular matrices by contractile cells: predictions from discrete fiber network simulations. Biophys. J. 107, 1829–1840. doi:10.1016/j.bpj.2014.08.029
Agarwal, P., Shemesh, T., and Zaidel-Bar, R. (2022). Directed cell invasion and asymmetric adhesion drive tissue elongation and turning in C. elegans gonad morphogenesis. Dev. Cell 57, 2111–2126.e6. doi:10.1016/j.devcel.2022.08.003
Aghvami, M., Barocas, V. H., and Sander, E. A. (2013). Multiscale mechanical simulations of cell compacted collagen gels. J. Biomech. Eng. 135, 071004–0710049. doi:10.1115/1.4024460
Ahmadzadeh, H., Webster, M. R., Behera, R., Jimenez Valencia, A. M., Wirtz, D., Weeraratna, A. T., et al. (2017). Modeling the two-way feedback between contractility and matrix realignment reveals a nonlinear mode of cancer cell invasion. PNAS 114, E1617–E1626. doi:10.1073/pnas.1617037114
Alegre-Cebollada, J., Huerta-Lopez, C., Clemente-Manteca, A., Velazquez-Carreras, D., Espinosa, F. M., Saez, P., et al. (2023). Cell response to substrate energy dissipation outweighs rigidity sensing. Biophys. J. 122, 292a. doi:10.1016/j.bpj.2022.11.1652
Alhashem, Z., Camargo-Sosa, K., Kelsh, R. N., and Linker, C. (2022). Trunk neural crest migratory position and asymmetric division predict terminal differentiation. Front. Cell Dev. Biol. 10, 887393. doi:10.3389/fcell.2022.887393
Alisafaei, F., Chen, X., Leahy, T., Janmey, P. A., and Shenoy, V. B. (2021). Long-range mechanical signaling in biological systems. Soft Matter 17, 241–253. doi:10.1039/d0sm01442g
Alnaes, M. S., Blechta, J., Hake, J., Johansson, A., Kehlet, B., Logg, A., et al. (2015). The FEniCS project version 1.5. Archive Numer. Softw. 3. doi:10.11588/ans.2015.100.20553
Anderson, A. R. A., and Chaplain, M. A. J. (1998). Continuous and discrete mathematical models of tumor-induced angiogenesis. Bull. Math. Biol. 60, 857–899. doi:10.1006/bulm.1998.0042
Anderson, A. R. A., Chaplain, M. A. J., Newman, E. L., Steele, R. J. C., and Thompson, A. M. (2000). Mathematical modelling of tumour invasion and metastasis. Comput. Math. Methods Med. 2, 129–154. doi:10.1080/10273660008833042
Andriotis, O. G., Desissaire, S., and Thurner, P. J. (2018). Collagen fibrils: nature’s highly tunable nonlinear springs. ACS Nano 12, 3671–3680. doi:10.1021/acsnano.8b00837
Aparicio-Yuste, R., Muenkel, M., Clark, A., Gómez-Benito, M., and Bastounis, E. (2022). A stiff extracellular matrix favors the mechanical cell competition that leads to extrusion of bacterially-infected epithelial cells. Front. Cell Dev. Biol. 10, 912318. doi:10.3389/fcell.2022.912318
Arnaoutova, I., George, J., Kleinman, H. K., and Benton, G. (2009). The endothelial cell tube formation assay on basement membrane turns 20: state of the science and the art. Angiogenesis 12, 267–274. doi:10.1007/s10456-009-9146-4
Arnoldini, S., Moscaroli, A., Chabria, M., Hilbert, M., Hertig, S., Schibli, R., et al. (2017). Novel peptide probes to assess the tensional state of fibronectin fibers in cancer. Nat. Commun. 8, 1793. doi:10.1038/s41467-017-01846-0
Arnout, J., Hoylaerts, M. F., and Lijnen, H. R. (2006). Haemostasis. Handb. Exp. Pharmacol. 176 (2), 1–41. doi:10.1007/3-540-36028-X_1
Artym, V. V., Yamada, K. M., and Mueller, S. C. (2009). ECM degradation assays for analyzing local cell invasion. Extracell. Matrix Protoc. 522, 211–219. doi:10.1007/978-1-59745-413-1_15
Arumugam, G., and Tyagi, J. (2021). Keller-segel chemotaxis models: a review. Acta Appl. Math. 171, 6–82. doi:10.1007/s10440-020-00374-2
Asadullah, , Kumar, S., Saxena, N., Sarkar, M., Barai, A., and Sen, S. (2021). Combined heterogeneity in cell size and deformability promotes cancer invasiveness. J. Cell Sci. 134, jcs250225. doi:10.1242/jcs.250225
Baker, R. E., Schnell, S., and Maini, P. K. (2006). A clock and wavefront mechanism for somite formation. Dev. Biol. 293, 116–126. doi:10.1016/j.ydbio.2006.01.018
Ban, E., Franklin, J. M., Nam, S., Smith, L. R., Wang, H., Wells, R. G., et al. (2018). Mechanisms of plastic deformation in collagen networks induced by cellular forces. Biophys. J. 114, 450–461. doi:10.1016/j.bpj.2017.11.3739
Ban, E., Wang, H., Franklin, J. M., Liphardt, J. T., Janmey, P. A., and Shenoy, V. B. (2019). Strong triaxial coupling and anomalous Poisson effect in collagen networks. PNAS 116, 6790–6799. doi:10.1073/pnas.1815659116
Barkovskaya, A., Buffone, A., Žídek, M., and Weaver, V. M. (2020). Proteoglycans as mediators of cancer tissue mechanics. Front. Cell Dev. Biol. 8, 569377. doi:10.3389/fcell.2020.569377
Bauer, A. L., Jackson, T. L., and Jiang, Y. (2007). A cell-based model exhibiting branching and anastomosis during tumor-induced angiogenesis. Biophys. J. 92, 3105–3121. doi:10.1529/biophysj.106.101501
Bauer, A. L., Jackson, T. L., and Jiang, Y. (2009). Topography of extracellular matrix mediates vascular morphogenesis and migration speeds in angiogenesis. PLoS Comput. Biol. 5, e1000445. doi:10.1371/journal.pcbi.1000445
Beltman, J. B., Marée, A. F. M., Lynch, J. N., Miller, M. J., and De Boer, R. J. (2007). Lymph node topology dictates T cell migration behavior. J. Exp. Med. 204, 771–780. doi:10.1084/jem.20061278
Berardi, M., Gnanachandran, K., Jiang, J., Bielawski, K., Visser, C. W., Lekka, M., et al. (2023). Dynamic mechanical analysis of suspended soft bodies via hydraulic force spectroscopy. Soft Matter 19, 615–624. doi:10.1039/D2SM01173E
Bevilacqua, C., Sánchez-Iranzo, H., Richter, D., Diz-Muñoz, A., and Prevedel, R. (2019). Imaging mechanical properties of sub-micron ECM in live zebrafish using brillouin microscopy. Biomed. Opt. Express 10, 1420–1431. doi:10.1364/boe.10.001420
Bhadriraju, K., Yang, M., Ruiz, S., Pirone, D., Tan, J., and Chen, C. (2007). Activation of ROCK by RhoA is regulated by cell adhesion, shape, and cytoskeletal tension. Exp. Cell Res. 313, 3616–3623. doi:10.1016/j.yexcr.2007.07.002
Boas, S. E., Carvalho, J., van den Broek, M., Weijers, E. M., Goumans, M.-J., Koolwijk, P., et al. (2018). A local uPAR-plasmin-TGFβ1 positive feedback loop in a qualitative computational model of angiogenic sprouting explains the in vitro effect of fibrinogen variants. PLoS Comput. Biol. 14, e1006239. doi:10.1371/journal.pcbi.1006239
Boas, S. E. M., Palm, M. M., Koolwijk, P., and Merks, R. M. H. (2012). “Computational modeling of angiogenesis: towards a multi-scale understanding of cell–cell and cell–matrix interactions,” in Mechanical and chemical signaling in angiogenesis (Berlin Heidelberg: Springer), 161–183. doi:10.1007/978-3-642-30856-7_8
Bowers, S. L., Banerjee, I., and Baudino, T. A. (2010). The extracellular matrix: at the center of it all. J. Mol. Cell. Cardiol. 48, 474–482. doi:10.1016/j.yjmcc.2009.08.024
Brinckerhoff, C. E., and Matrisian, L. M. (2002). Matrix metalloproteinases: a tail of a frog that became a prince. Nat. Rev. Mol. Cel. Biol. 3, 207–214. doi:10.1038/nrm763
Broedersz, C. P., and MacKintosh, F. C. (2014). Modeling semiflexible polymer networks. Rev. Mod. Phys. 86, 995–1036. doi:10.1103/revmodphys.86.995
Browning, A. P., Haridas, P., and Simpson, M. J. (2019). A Bayesian sequential learning framework to parameterise continuum models of melanoma invasion into human skin. Bull. Math. Biol. 81, 676–698. doi:10.1007/s11538-018-0532-1
Bull, J., Mech, F., Quaiser, T., Waters, S., and Byrne, H. (2018). Mathematical modelling reveals cellular dynamics within tumour spheroids. PLoS Comput. Biol. 16, e1007961. doi:10.1371/journal.pcbi.1007961
Buske, P., Galle, J., Barker, N., Aust, G., Clevers, H., and Loeffler, M. (2011). A comprehensive model of the spatio-temporal stem cell and tissue organisation in the intestinal crypt. PLoS Comput. Biol. 7, e1001045. doi:10.1371/journal.pcbi.1001045
Byrne, H. M., and Chaplain, M. A. J. (1996). The importance of constitutive equations in mechanochemical models of pattern formation. Appl. Math. Lett. 9, 85–90. doi:10.1016/0893-9659(96)00099-7
Cabral-Pacheco, G. A., Garza-Veloz, I., Castruita-De la Rosa, C., Ramirez-Acuña, J. M., Perez-Romero, B. A., Guerrero-Rodriguez, J. F., et al. (2020). The roles of matrix metalloproteinases and their inhibitors in human diseases. Int. J. Mol. Sci. 21, 9739. doi:10.3390/ijms21249739
Caiazzo, A., and Ramis-Conde, I. (2015). Multiscale modelling of palisade formation in gliobastoma multiforme. J. Theor. Biol. 383, 145–156. doi:10.1016/j.jtbi.2015.07.021
Cao, X., Ban, E., Baker, B. M., Lin, Y., Burdick, J. A., Chen, C. S., et al. (2017). Multiscale model predicts increasing focal adhesion size with decreasing stiffness in fibrous matrices. PNAS 114, E4549–E4555. doi:10.1073/pnas.1620486114
Carrasco-Mantis, A., Alarcón, T., and Sanz-Herrera, J. A. (2023a). An in silico study on the influence of extracellular matrix mechanics on vasculogenesis. Comput. Methods Programs Biomed. 231, 107369. doi:10.1016/j.cmpb.2023.107369
Carrasco-Mantis, A., Randelovic, T., Castro-Abril, H., Ochoa, I., Doblaré, M., and Sanz-Herrera, J. A. (2023b). A mechanobiological model for tumor spheroid evolution with application to glioblastoma: a continuum multiphysics approach. Comput. Biol. Med. 159, 106897. doi:10.1016/j.compbiomed.2023.106897
Carrillo, J. A., Parisot, M., and Szymańska, Z. (2021). Mathematical modelling of collagen fibres rearrangement during the tendon healing process. Kinet. Relat. Models 14, 283–301. doi:10.3934/krm.2021005
Castro-Castro, A., Marchesin, V., Monteiro, P., Lodillinsky, C., Rossé, C., and Chavrier, P. (2016). Cellular and molecular mechanisms of MT1-MMP-dependent cancer cell invasion. Annu. Rev. Cell Dev. Biol. 32, 555–576. doi:10.1146/annurev-cellbio-111315-125227
Caswell, P. T., and Zech, T. (2018). Actin-based cell protrusion in a 3D matrix. Trends Cell Biol. 28, 823–834. doi:10.1016/j.tcb.2018.06.003
Chaplain, M. A. J., Lachowicz, M., Szymanska, Z., and Wrzosek, D. (2011). Mathematical modelling of cancer invasion: the importance of cell-cell adhesion and cell-matrix adhesion. Math. Model. Meth. Appl. Sci. 21, 719–743. doi:10.1142/s0218202511005192
Chaudhuri, O., Cooper-White, J., Janmey, P. A., Mooney, D. J., and Shenoy, V. B. (2020). Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 584, 535–546. doi:10.1038/s41586-020-2612-2
Checa, S., Rausch, M. K., Petersen, A., Kuhl, E., and Duda, G. N. (2015). The emergence of extracellular matrix mechanics and cell traction forces as important regulators of cellular self-organization. Biomech. Model. Mechanobiol. 14, 1–13. doi:10.1007/s10237-014-0581-9
Chen, X., Wanggou, S., Bodalia, A., Zhu, M., Dong, W., Fan, J. J., et al. (2018). A feedforward mechanism mediated by mechanosensitive ion channel piezo1 and tissue mechanics promotes glioma aggression. Neuron 100, 799–815. doi:10.1016/j.neuron.2018.09.046
Chrabaszcz, K., Kaminska, K., Augustyniak, K., Kujdowicz, M., Smeda, M., Jasztal, A., et al. (2020). Tracking extracellular matrix remodeling in lungs induced by breast cancer metastasis. Fourier transform infrared spectroscopic studies. Molecules 25, 236. doi:10.3390/molecules25010236
Colson, C., Sánchez-Garduño, F., Byrne, H. M., Maini, P. K., and Lorenzi, T. (2021). Travelling-wave analysis of a model of tumour invasion with degenerate, cross-dependent diffusion. Proc. R. Soc. A 477, 20210593. doi:10.1098/rspa.2021.0593
Cooper, F., Baker, R., Bernabeu, M., Bordas, R., Bowler, L., Bueno-Orovio, A., et al. (2020). Chaste: cancer, heart and soft tissue environment. J. Open Source Softw. 5, 1848. doi:10.21105/joss.01848
Cowan, A., Moraru, I., Schaff, J., Slepchenko, B., and Loew, L. (2012). Spatial modeling of cell signaling networks. Methods Cell Biol. 110, 195–221. doi:10.1016/B978-0-12-388403-9.00008-4
Cox, T. R., Bird, D., Baker, A.-M., Barker, H. E., Ho, M. W., Lang, G., et al. (2013). LOX-mediated collagen crosslinking is responsible for fibrosis-enhanced metastasis. Cancer Res. 73, 1721–1732. doi:10.1158/0008-5472.can-12-2233
Cox, T. R., and Erler, J. T. (2011). Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis. Model. Mech. 4, 165–178. doi:10.1242/dmm.004077
Crawshaw, J. R., Flegg, J. A., Bernabeu, M. O., and Osborne, J. M. (2023). Mathematical models of developmental vascular remodelling: a review. PLoS Comput. Biol. 19, e1011130. doi:10.1371/journal.pcbi.1011130
Crossley, R. M., Maini, P. K., Lorenzi, T., and Baker, R. E. (2023). Traveling waves in a coarse-grained model of volume-filling cell invasion: simulations and comparisons. Stud. Appl. Math. 151, 1471–1497. doi:10.1111/sapm.12635
Cruz Walma, D. A., and Yamada, K. M. (2020). The extracellular matrix in development. Development 147, dev175596. doi:10.1242/dev.175596
Cumming, B. D., McElwain, D. L. S., and Upton, Z. (2010). A mathematical model of wound healing and subsequent scarring. J. R. Soc. Interface 7, 19–34. doi:10.1098/rsif.2008.0536
Dale, P. D., Sherratt, J. A., and Maini, P. K. (1994). The speed of corneal epithelial wound healing. Appl. Math. Lett. 7, 11–14. doi:10.1016/0893-9659(94)90022-1
Dallon, J. C., and Sherratt, J. A. (1998). A mathematical model for fibroblast and collagen orientation. Bull. Math. Biol. 60, 101–129. doi:10.1006/bulm.1997.0027
Dallon, J. C., Sherratt, J. A., and Maini, P. K. (1999). Mathematical modelling of extracellular matrix dynamics using discrete cells: fiber orientation and tissue regeneration. J. Theor. Biol. 199, 449–471. doi:10.1006/jtbi.1999.0971
Daub, J. T., and Merks, R. M. H. (2013). A cell-based model of extracellular-matrix-guided endothelial cell migration during angiogenesis. Bull. Math. Biol. 75, 1377–1399. doi:10.1007/s11538-013-9826-5
Davidson, L. A., Koehl, M., Keller, R., and Oster, G. F. (1995). How do sea urchins invaginate? Using biomechanics to distinguish between mechanisms of primary invagination. Development 121, 2005–2018. doi:10.1242/dev.121.7.2005
Deutsch, A., Nava-Sedeño, J. M., Syga, S., and Hatzikirou, H. (2021). BIO-LGCA: a cellular automaton modelling class for analysing collective cell migration. PLoS Comput. Biol. 17, e1009066. doi:10.1371/journal.pcbi.1009066
Diller, R. B., and Tabor, A. J. (2022). The role of the extracellular matrix (ECM) in wound healing: a review. Biomimetics (Basel) 7, 87. doi:10.3390/biomimetics7030087
Drasdo, D., and Hoehme, S. (2012). Modeling the impact of granular embedding media, and pulling versus pushing cells on growing cell clones. New J. Phys. 14, 055025. doi:10.1088/1367-2630/14/5/055025
Dudziuk, G., Lachowicz, M., Leszczyński, H., and Szymańska, Z. (2019). A simple model of collagen remodeling. Discrete Contin. Dyn. Syst. - B 24, 2205–2217. doi:10.3934/dcdsb.2019091
Dunn, G. A., and Heath, J. P. (1976). A new hypothesis of contact guidance in tissue cells. Exp. Cell Res. 101, 1–14. doi:10.1016/0014-4827(76)90405-5
Dunn, S.-J., Appleton, P. L., Nelson, S. A., Näthke, I. S., Gavaghan, D. J., and Osborne, J. M. (2012). A two-dimensional model of the colonic crypt accounting for the role of the basement membrane and pericryptal fibroblast sheath. PLoS Comput. Biol. 8, e1002515. doi:10.1371/journal.pcbi.1002515
Dzobo, K., and Dandara, C. (2023). The extracellular matrix: its composition, function, remodeling, and role in tumorigenesis. Biomimetics 8, 146. doi:10.3390/biomimetics8020146
Eble, J. A., and Niland, S. (2019). The extracellular matrix in tumor progression and metastasis. Clin. Exp. Metastasis 36, 171–198. doi:10.1007/s10585-019-09966-1
Edgar, L. T., Maas, S. A., Guilkey, J. E., and Weiss, J. A. (2015). A coupled model of neovessel growth and matrix mechanics describes and predicts angiogenesis in vitro. Biomech. Model. Mechanobiol. 14, 767–782. doi:10.1007/s10237-014-0635-z
Edgar, L. T., Sibole, S. C., Underwood, C. J., Guilkey, J. E., and Weiss, J. A. (2013). A computational model of in vitro angiogenesis based on extracellular matrix fibre orientation. Comput. Methods Biomech. Biomed. Eng. 16, 790–801. doi:10.1080/10255842.2012.662678
Eftimie, R. (2022). Grand challenges in mathematical biology: integrating multi-scale modeling and data. Front. Appl. Math. Stat. 8. doi:10.3389/fams.2022.1010622
Ehret, A. E., Bircher, K., Stracuzzi, A., Marina, V., Zündel, M., and Mazza, E. (2017). Inverse poroelasticity as a fundamental mechanism in biomechanics and mechanobiology. Nat. Commun. 8, 1002. doi:10.1038/s41467-017-00801-3
Eichinger, J. F., Grill, M. J., Kermani, I. D., Aydin, R. C., Wall, W. A., Humphrey, J. D., et al. (2021). A computational framework for modeling cell–matrix interactions in soft biological tissues. Biomech. Model. Mechanobiol. 20, 1851–1870. doi:10.1007/s10237-021-01480-2
El-Hachem, M., McCue, S. W., and Simpson, M. J. (2021). Travelling wave analysis of cellular invasion into surrounding tissues. Phys. D. 428, 133026. doi:10.1016/j.physd.2021.133026
Eroles, M., Lopez-Alonso, J., Ortega, A., Boudier, T., Gharzeddine, K., Lafont, F., et al. (2023). Coupled mechanical mapping and interference contrast microscopy reveal viscoelastic and adhesion hallmarks of monocyte differentiation into macrophages. Nanoscale 15, 12255–12269. doi:10.1039/d3nr00757j
Ettema, L., Lochocki, B., Hoozemans, J. J. M., De Boer, J. F., and Ariese, F. (2022). Label-free Raman and fluorescence imaging of amyloid plaques in human alzheimer’s disease brain tissue reveal carotenoid accumulations. J. Opt. 24, 054005. doi:10.1088/2040-8986/ac5b51
Farrell, P., Hegarty, A., Miller, J. M., O’Riordan, E., and Shishkin, G. I. (2000). Robust computational techniques for boundary layers (Chapman and Hall/CRC). Available at: https://doi.org/10.1201/9781482285727.
Filipe, E. C., Chitty, J. L., and Cox, T. R. (2018). Charting the unexplored extracellular matrix in cancer. Int. J. Exp. Pathol. 99, 58–76. doi:10.1111/iep.12269
Fisher, R. A. (1937). The wave of advance of advantageous genes. Ann. Eugen. 7, 355–369. doi:10.1111/j.1469-1809.1937.tb02153.x
Flegg, J., Menon, S., Maini, P., and McElwain, D. (2015). On the mathematical modeling of wound healing angiogenesis in skin as a reaction-transport process. Front. Physiol. 6, 262. doi:10.3389/fphys.2015.00262
Folkman, J. (2006). Angiogenesis. Annu. Rev. Med. 57, 1–18. doi:10.1146/annurev.med.57.121304.131306
Frantz, C., Stewart, K. M., and Weaver, V. M. (2010). The extracellular matrix at a glance. J. Cell Sci. 123, 4195–4200. doi:10.1242/jcs.023820
Friedl, P., and Bröcker, E.-B. (2000). The biology of cell locomotion within three-dimensional extracellular matrix. CMLS, Cell. Mol. Life Sci. 57, 41–64. doi:10.1007/s000180050498
Gardiner, B. S., Wong, K. K. L., Joldes, G. R., Rich, A. J., Wee Tan, C., Burgess, A. W., et al. (2015). Discrete element framework for modelling extracellular matrix, deformable cells and subcellular components. PLoS Comput. Biol. 11, e1004544. doi:10.1371/journal.pcbi.1004544
Gatenby, R. A., Gawlinski, E. T., Gmitro, A. F., Kaylor, B., and Gillies, R. J. (2006). Acid-mediated tumor invasion: a multidisciplinary study. Cancer Res. 66, 5216–5223. doi:10.1158/0008-5472.can-05-4193
Gerlee, P., and Nelander, S. (2016). Travelling wave analysis of a mathematical model of glioblastoma growth. Math. Biosci. 276, 75–81. doi:10.1016/j.mbs.2016.03.004
Ghaffarizadeh, A., Heiland, R., Friedman, S. H., Mumenthaler, S. M., and Macklin, P. (2018). PhysiCell: an open source physics-based cell simulator for 3-D multicellular systems. PLoS Comput. Biol. 14, e1005991. doi:10.1371/journal.pcbi.1005991
Giniunaité, R., Baker, R., Kulesa, P., and Maini, P. (2020). Modelling collective cell migration: neural crest as a model paradigm. J. Math. Biol. 80, 481–504. doi:10.1007/s00285-019-01436-2
Gonçalves, I. G., and Garcia-Aznar, J. M. (2021). Extracellular matrix density regulates the formation of tumour spheroids through cell migration. PLoS Comput. Biol. 17, e1008764. doi:10.1371/journal.pcbi.1008764
Goren, S., Koren, Y., Xu, X., and Lesman, A. (2020). Elastic anisotropy governs the range of cell-induced displacements. Biophys. J. 118, 1152–1164. doi:10.1016/j.bpj.2019.12.033
Grant, M. R., Mostov, K. E., Tlsty, T. D., and Hunt, C. A. (2006). Simulating properties of in vitro epithelial cell morphogenesis. PLoS Comput. Biol. 2, e129. doi:10.1371/journal.pcbi.0020129
Grekas, G., Proestaki, M., Rosakis, P., Notbohm, J., Makridakis, C., and Ravichandran, G. (2021). Cells exploit a phase transition to mechanically remodel the fibrous extracellular matrix. J. R. Soc. Interface 18, 20200823. doi:10.1098/rsif.2020.0823
Guerra, A., Belinha, J., and Jorge, R. N. (2018). Modelling skin wound healing angiogenesis: a review. J. Theor. Biol. 459, 1–17. doi:10.1016/j.jtbi.2018.09.020
Guillon, E., Das, D., Jülich, D., Hassan, A.-R., Geller, H., and Holley, S. (2020). Fibronectin is a smart adhesive that both influences and responds to the mechanics of early spinal column development. Elife 9, e48964. doi:10.7554/eLife.48964
Guo, S., and DiPietro, L. A. (2010). Factors affecting wound healing. J. Dent. Res. 89, 219–229. doi:10.1177/0022034509359125
Guo, T., He, C., Venado, A., and Zhou, Y. (2022a). Extracellular matrix stiffness in lung health and disease. Compr. Physiol. 12, 3523–3558. doi:10.1002/cphy.c210032
Guo, Y., Calve, S., and Buganza Tepole, A. (2022b). Multiscale mechanobiology: coupling models of adhesion kinetics and nonlinear tissue mechanics. Biophys. J. 121, 525–539. doi:10.1016/j.bpj.2022.01.012
Guo, Y., Mofrad, M. R. K., and Tepole, A. B. (2022c). On modeling the multiscale mechanobiology of soft tissues: challenges and progress. Biophys. Rev. 3. doi:10.1063/5.0085025
Han, Y. L., Ronceray, P., Xu, G., Malandrino, A., Kamm, R. D., Lenz, M., et al. (2018). Cell contraction induces long-ranged stress stiffening in the extracellular matrix. PNAS 115, 4075–4080. doi:10.1073/pnas.1722619115
Handorf, A., Zhou, Y., Halanski, M., and Li, W. (2015). Tissue stiffness dictates development, homeostasis, and disease progression. Organogenesis 11, 1–15. doi:10.1080/15476278.2015.1019687
Hanna, S., and El-Sibai, M. (2013). Signaling networks of rho gtpases in cell motility. Cell. Signal. 25, 1955–1961. doi:10.1016/j.cellsig.2013.04.009
Hannezo, E., Prost, J., and Joanny, J.-F. (2011). Instabilities of monolayered epithelia: shape and structure of villi and crypts. Phys. Rev. Lett. 107, 078104. doi:10.1103/PhysRevLett.107.078104
Harjanto, D., and Zaman, M. H. (2013). Modeling extracellular matrix reorganization in 3D environments. PLoS One 8, e52509. doi:10.1371/journal.pone.0052509
Hastings, J., Skhinas, J., Fey, D., Croucher, D., and Cox, T. (2019). The extracellular matrix as a key regulator of intracellular signalling networks. Br. J. Pharmacol. 176, 82–92. doi:10.1111/bph.14195
He, X., and Jiang, Y. (2017). Substrate curvature regulates cell migration. Phys. Biol. 14, P035006. doi:10.1088/1478-3975/aa6f8e
He, X., Lee, B., and Jiang, Y. (2022a). Extracellular matrix in cancer progression and therapy. Med. Rev. Berl. 2, 125–139. doi:10.1515/mr-2021-0028
He, X., Yang, Y., Han, Y., Cao, C., Zhang, Z., Xi, L., et al. (2022b). Extracellular matrix physical properties govern the diffusion of nanoparticles in tumor microenvironment. PNAS 120, e2209260120. doi:10.1073/pnas.2209260120
Heck, T., Vaeyens, M.-M., and Van Oosterwyck, H. (2015). Computational models of sprouting angiogenesis and cell migration: towards multiscale mechanochemical models of angiogenesis. Math. Model. Nat. Phenom. 10, 108–141. doi:10.1051/mmnp/201510106
Hepburn, M. S., Wijesinghe, P., Major, L. G., Li, J., Mowla, A., Astell, C., et al. (2020). Three-dimensional imaging of cell and extracellular matrix elasticity using quantitative micro-elastography. Biomed. Opt. Express 11, 867–884. doi:10.1364/boe.383419
Hinshaw, J., and Miller, E. (1965). Histology of healing split-thickness, full-thickness autogenous skin grafts and donor sites. Archives Surg. 91, 658–670. doi:10.1001/archsurg.1965.01320160112027
Hoehme, S., and Drasdo, D. (2010). A cell-based simulation software for multi-cellular systems. Bioinformatics 26, 2641–2642. doi:10.1093/bioinformatics/btq437
Holmes, M. J., and Sleeman, B. D. (2000). A mathematical model of tumour angiogenesis incorporating cellular traction and viscoelastic effects. J. Theor. Biol. 202, 95–112. doi:10.1006/jtbi.1999.1038
Holzapfel, G. A., Ogden, R. W., and Sherifova, S. (2019). On fibre dispersion modelling of soft biological tissues: a review. Proc. R. Soc. A 475, 20180736. doi:10.1098/rspa.2018.0736
Huelsken, J., and Behrens, J. (2002). The Wnt signalling pathway. J. Cell Sci. 115, 3977–3978. doi:10.1242/jcs.00089
Hughes, A. J., Miyazaki, H., Coyle, M. C., Zhang, J., Laurie, M. T., Chu, D., et al. (2018). Engineered tissue folding by mechanical compaction of the mesenchyme. Dev. Cell 44, 165–178. doi:10.1016/j.devcel.2017.12.004
Humphries, D. L., Grogan, J. A., and Gaffney, E. A. (2017). Mechanical cell–cell communication in fibrous networks: the importance of network geometry. Bull. Math. Biol. 79, 498–524. doi:10.1007/s11538-016-0242-5
Ilina, O., Gritsenko, P., Syga, S., Lippoldt, J., Porta, C. L., Chepizhko, O., et al. (2020). Cell-cell adhesion and 3d matrix confinement determine jamming transitions in breast cancer invasion. Nat. Cell Biol. 22, 1103–1115. doi:10.1038/s41556-020-0552-6
Itani, S., Gray, J., and Tomlin, C. J. (2010). “An ode model for the her2/3-akt signaling pathway in cancers that overexpress her2,” in Proceedings of the 2010 American Control Conference (IEEE), 1235–1241.
Izaguirre, J. A., Chaturvedi, R., Huang, C., Cickovski, T., Coffland, J., Thomas, G., et al. (2004). Compucell, a multi-model framework for simulation of morphogenesis. Bioinformatics 20, 1129–1137. doi:10.1093/bioinformatics/bth050
Jimenez, L. E., Roldán-Olarte, M., and Álvarez, R. M. S. (2019). Raman microscopy analysis of the biochemical changes in the cytoplasm of bovine oocytes induced by an in vitro maturation process: interference of the zona pellucida. ChemistrySelect 4, 3706–3716. doi:10.1002/slct.201803454
Jorgensen, S., and Sanders, J. (2016). Mathematical models of wound healing and closure: a comprehensive review. Med. Biol. Eng. Comput. 54, 1297–1316. doi:10.1007/s11517-015-1435-z
Kalluri, R., and Weinberg, R. (2009). The basics of epithelial-mesenchymal transition. J. Clin. Invest. 119, 1420–1428. doi:10.1172/JCI39104
Kandhwal, M., Behl, T., Singh, S., Sharma, N., Arora, S., Bhatia, S., et al. (2022). Role of matrix metalloproteinase in wound healing. Am. J. Transl. Res. 14, 4391–4405.
Kang, W., Ferruzzi, J., Spatarelu, C.-P., Han, Y. L., Sharma, Y., Koehler, S. A., et al. (2021). A novel jamming phase diagram links tumor invasion to non-equilibrium phase separation. iScience 24, 103252. doi:10.1016/j.isci.2021.103252
Karamanos, N. K., Theocharis, A. D., Piperigkou, Z., Manou, D., Passi, A., Skandalis, S. S., et al. (2021). A guide to the composition and functions of the extracellular matrix. FEBS J. 288, 6850–6912. doi:10.1111/febs.15776
Karsdal, M. A., Nielsen, M. J., Sand, J. M., Henriksen, K., Genovese, F., Bay-Jensen, A.-C., et al. (2013). Extracellular matrix remodeling: the common denominator in connective tissue diseases. Possibilities for evaluation and current understanding of the matrix as more than a passive architecture, but a key player in tissue failure. Assay. Drug Dev. Technol. 11, 70–92. doi:10.1089/adt.2012.474
Kaur, A., Ecker, B. L., Douglass, S. M., Kugel, C. H., Webster, M. R., Almeida, F. V., et al. (2019). Remodeling of the collagen matrix in aging skin promotes melanoma metastasis and affects immune cell motility. Cancer Discov. 9, 64–81. doi:10.1158/2159-8290.CD-18-0193
Keller, A., Lanfranconi, F., and Aegerter, C. M. (2018). The influence of geometry on the elastic properties of the drosophila wing disc. Phys. A 510, 208–218. doi:10.1016/j.physa.2018.06.106
Keller, J. B., and Lewis, R. M. (1995). “Asymptotic methods for partial differential equations: the reduced wave equation and Maxwell’s equations,” in Surveys in applied mathematics (Boston, MA.: Springer), 1–82. doi:10.1007/978-1-4899-0436-2_1
Kennedy, B. F., Gomes Malheiro, F., Chin, L., and Sampson, D. D. (2014). Three-dimensional optical coherence elastography by phase-sensitive comparison of C-scans. J. Biomed. Opt. 19, 076006. doi:10.1117/1.jbo.19.7.076006
Khaniki, H. B., Ghayesh, M. H., Chin, R., and Amabili, M. (2023). Hyperelastic structures: a review on the mechanics and biomechanics. Int. J. Non Linear Mech. 1480, 104275. doi:10.1016/j.ijnonlinmec.2022.104275
Kim, J., Feng, J., Jones, C. A. R., Mao, X., Sander, L. M., Levine, H., et al. (2017). Stress-induced plasticity of dynamic collagen networks. Nat. Commun. 8, 842. doi:10.1038/s41467-017-01011-7
Kim, M.-C., Li, R., Abeyaratne, R., Kamm, R. D., and Asada, H. H. (2022). A computational modeling of invadopodia protrusion into an extracellular matrix fiber network. Sci. Rep. 12, 1231. doi:10.1038/s41598-022-05224-9
Kim, M.-C., Silberberg, Y. R., Abeyaratne, R., Kamm, R. D., and Asada, H. H. (2018). Computational modeling of three-dimensional ECM-rigidity sensing to guide directed cell migration. PNAS 115, E390–E399. doi:10.1073/pnas.1717230115
Kobayashi, Y., Yasugahira, Y., Kitahata, H., Watanabe, M., Natsuga, K., and Nagayama, M. (2018). Interplay between epidermal stem cell dynamics and dermal deformation. npj Comput. Mat. 4, 45. doi:10.1038/s41524-018-0101-z
Koh, T. J., and DiPietro, L. A. (2011). Inflammation and wound healing: the role of the macrophage. Expert Rev. Mol. Med. 13, e23. doi:10.1017/s1462399411001943
Köhn-Luque, A., De Back, W., Starruß, J., Mattiotti, A., Deutsch, A., Pérez-Pomares, J. M., et al. (2011). Early embryonic vascular patterning by matrix-mediated paracrine signalling: a mathematical model study. PLoS One 6, e24175. doi:10.1371/journal.pone.0024175
Köhn-Luque, A., De Back, W., Yamaguchi, Y., Yoshimura, K., Herrero, M., and Miura, T. (2013). Dynamics of VEGF matrix-retention in vascular network patterning. Phys. Biol. 10, 066007. doi:10.1088/1478-3975/10/6/066007
Kolmogorov, A. N., Petrovskii, I. G., and Piskunov, N. S. (1937). A study of the equation of diffusion with increase in the quantity of matter, and its application to a biological problem. Byul. Mosk. Gos. Univ. 1, 1–25.
Kondo, S., and Miura, T. (2010). Reaction-diffusion model as a framework for understanding biological pattern formation. Science 329, 1616–1620. doi:10.1126/science.1179047
Kristensen, J. H., and Karsdal, M. A. (2016). Biochemistry of collagens, laminins and elastin. Academic Press, 197–201. chap. 30 - Elastin. doi:10.1016/B978-0-12-809847-9.00030-1
Kumar, S., Kapoor, A., Desai, S., Inamdar, M. M., and Sen, S. (2016). Proteolytic and non-proteolytic regulation of collective cell invasion: tuning by ECM density and organization. Sci. Rep. 6, 19905. doi:10.1038/srep19905
Kurpios, N. A., Ibañes, M., Davis, N. M., Lui, W., Katz, T., Martin, J. F., et al. (2008). The direction of gut looping is established by changes in the extracellular matrix and in cell:cell adhesion. PNAS 105, 8499–8506. doi:10.1073/pnas.0803578105
Lamalice, L., Le Boeuf, F., and Huot, J. (2007). Endothelial cell migration during angiogenesis. Circ. Res. 100, 782–794. doi:10.1161/01.res.0000259593.07661.1e
Landén, N. X., Li, D., and Ståhle, M. (2016). Transition from inflammation to proliferation: a critical step during wound healing. Cell. Mol. Life Sci. 73, 3861–3885. doi:10.1007/s00018-016-2268-0
Lawson-Keister, E., and Manning, M. (2021). Jamming and arrest of cell motion in biological tissues. Curr. Opin. Cell Biol. 72, 146–155. doi:10.1016/j.ceb.2021.07.011
Lee, B., Konen, J., Wilkinson, S., Marcus, A. I., and Jiang, Y. (2017). Local alignment vectors reveal cancer cell-induced ECM fiber remodeling dynamics. Sci. Rep. 7, 39498. doi:10.1038/srep39498
Lehmann, K., Shayegan, M., Blab, G. A., and Forde, N. R. (2020). Optical tweezers approaches for probing multiscale protein mechanics and assembly. Front. Mol. Biosci. 7, 577314. doi:10.3389/fmolb.2020.577314
Leight, J. L., Drain, A. P., and Weaver, V. M. (2017). Extracellular matrix remodeling and stiffening modulate tumor phenotype and treatment response. Annu. Rev. Cancer Biol. 1, 313–334. doi:10.1146/annurev-cancerbio-050216-034431
Leonard, A. K., Loughran, E. A., Klymenko, Y., Liu, Y., Kim, O., Asem, M., et al. (2018). Methods for the visualization and analysis of extracellular matrix protein structure and degradation. Methods Cell Biol. 143, 79–95. doi:10.1016/bs.mcb.2017.08.005
Letort, G., Montagud, A., Stoll, G., Heiland, R., Barillot, E., Macklin, P., et al. (2019). PhysiBoSS: a multi-scale agent-based modelling framework integrating physical dimension and cell signalling. Bioinforma. Oxf. Engl. 35, 1188–1196. doi:10.1093/bioinformatics/bty766
Levine, H. A., Sleeman, B. D., and Nilsen-Hamilton, M. (2001). Mathematical modeling of the onset of capillary formation initiating angiogenesis. J. Theor. Biol. 42, 195–238. doi:10.1007/s002850000037
Li, Y., Moon, S., Chen, J. J., Zhu, Z., and Chen, Z. (2020). Ultrahigh-sensitive optical coherence elastography. Light Sci. Appl. 9, 58. doi:10.1038/s41377-020-0297-9
Lin, J., Shi, Y., Men, Y., Wang, X., Ye, J., and Zhang, C. (2020). Mechanical roles in formation of oriented collagen fibers. Tissue Eng. Part B Rev. 26, 116–128. doi:10.1089/ten.teb.2019.0243
Longo, D., Peirce, S. M., Skalak, T. C., Davidson, L., Marsden, M., Dzamba, B., et al. (2004). Multicellular computer simulation of morphogenesis: blastocoel roof thinning and matrix assembly in Xenopus laevis. Dev. Biol. 271, 210–222. doi:10.1016/j.ydbio.2004.03.021
Lu, P., Takai, K., Weaver, V. M., and Werb, Z. (2011). Extracellular matrix degradation and remodeling in development and disease. Cold Spring. Harb. Perspect. Biol. 3, a005058. doi:10.1101/cshperspect.a005058
Macnamara, C. K., Caiazzo, A., Ramis-Conde, I., and Chaplain, M. A. J. (2020). Computational modelling and simulation of cancer growth and migration within a 3D heterogeneous tissue: the effects of fibre and vascular structure. J. Comput. Sci. 40, 101067. doi:10.1016/j.jocs.2019.101067
Maini, P. K., McElwain, D. L. S., and Leavesley, D. I. (2004). Traveling wave model to interpret a wound-healing cell migration assay for human peritoneal mesothelial cells. Tissue Eng. 10, 475–482. doi:10.1089/107632704323061834
Malakpour-Permlid, A., Buzzi, I., Hegardt, C., Johansson, F., and Oredsson, S. (2021). Identification of extracellular matrix proteins secreted by human dermal fibroblasts cultured in 3D electrospun scaffolds. Sci. Rep. 1, 6655. doi:10.1038/s41598-021-85742-0
Malandrino, A., Trepat, X., Kamm, R. D., and Mak, M. (2019). Dynamic filopodial forces induce accumulation, damage, and plastic remodeling of 3D extracellular matrices. PLoS Comput. Biol. 15, e1006684. doi:10.1371/journal.pcbi.1006684
Manoussaki, D. (2003). A mechanochemical model of angiogenesis and vasculogenesis. ESAIM Math. Model. Numer. Anal. 37, 581–599. doi:10.1051/m2an:2003046
Marshall, C. D., Hu, M. S., Leavitt, T., Barnes, L. A., Lorenz, H. P., and Longaker, M. T. (2018). Cutaneous scarring: basic science, current treatments, and future directions. Adv. wound care 7, 29–45. doi:10.1089/wound.2016.0696
Martin, A., and Goldstein, B. (2014). Apical constriction: themes and variations on a cellular mechanism driving morphogenesis. Development 141, 1987–1998. doi:10.1242/dev.102228
Martín, F. A., Herrera, S. C., and Morata, G. (2009). Cell competition, growth and size control in the drosophila wing imaginal disc. Development 136, 3747–3756. doi:10.1242/dev.038406
Martin, N. K., Gaffney, E. A., Gatenby, R. A., and Maini, P. K. (2010). Tumour–stromal interactions in acid-mediated invasion: a mathematical model. J. Theor. Biol. 267, 461–470. doi:10.1016/j.jtbi.2010.08.028
Martinez-Vidal, L., Chighizola, M., Berardi, M., Alchera, E., Locatelli, I., Pederzoli, F., et al. (2023). Micro-mechanical fingerprints of the rat bladder change in actinic cystitis and tumor presence. Commun. Biol. 6, 217. doi:10.1038/s42003-023-04572-0
Martinson, W. D., McLennan, R., Teddy, J. M., McKinney, M. C., Davidson, L. A., Baker, R. E., et al. (2023). Dynamic fibronectin assembly and remodeling by leader neural crest cells prevents jamming in collective cell migration. eLife 12, 83792. doi:10.7554/eLife.83792
Mayor, R., and Theveneau, E. (2013). The neural crest. Development 140, 2247–2251. doi:10.1242/dev.091751
McGrath, J. A., Eady, R. A. J., and Pope, F. M. (2004). Anatomy and organization of human skin. John Wiley and Sons, Ltd, 45–128. chap. 3. doi:10.1002/9780470750520.ch3
McKee, K. K., Hohenester, E., Aleksandrova, M., and Yurchenco, P. D. (2021). Organization of the laminin polymer node. Matrix Biol. 98, 49–63. doi:10.1016/j.matbio.2021.05.004
McLennan, R., Dyson, L., Prather, K. W., Morrison, J. A., Baker, R. E., Maini, P. K., et al. (2012). Multiscale mechanisms of cell migration during development: theory and experiment. Development 139, 2935–2944. doi:10.1242/dev.081471
McLennan, R., Giniunaité, R., Hildebrand, K., Teddy, J. M., Kasemeier-Kulesa, J. C., Bolanos, L., et al. (2023). Colec12 and trail signaling confine cranial neural crest cell trajectories and promote collective cell migration. Dev. Dyn. 252, 629–646. doi:10.1002/dvdy.569
Merks, R., and Guravage, M. (2012). Building simulation models of developing plant organs using VirtualLeaf. Plant Organog. 959, 333–352. doi:10.1007/978-1-62703-221-6_23
Merks, R., Guravage, M., Inzé, D., and Beemster, G. (2011). VirtualLeaf: an open-source framework for cell-based modeling of plant tissue growth and development. Plant Phys. 155, 656–666. doi:10.1104/pp.110.167619
Metzcar, J., Wang, Y., Heiland, R., and Macklin, P. (2019). A review of cell-based computational modeling in cancer biology. JCO Clin. cancer Inf. 2, 1–13. doi:10.1200/CCI.18.00069
Milde, F., Bergdorf, M., and Koumoutsakos, P. (2008). A hybrid model for three-dimensional simulations of sprouting angiogenesis. Biophys. J. 95, 3146–3160. doi:10.1529/biophysj.107.124511
Mirams, G. R., Arthurs, C. J., Bernabeu, M. O., Bordas, R., Cooper, J., Corrias, A., et al. (2013). Chaste: an open source C++ library for computational physiology and biology. PLoS Comput. Biol. 9, e1002970. doi:10.1371/journal.pcbi.1002970
Mitchel, J. A., Das, A., O’Sullivan, M. J., Stancil, I. T., DeCamp, S. J., Koehler, S., et al. (2020). In primary airway epithelial cells, the unjamming transition is distinct from the epithelial-to-mesenchymal transition. Nat. Commun. 11, 5053. doi:10.1038/s41467-020-18841-7
Mohan, V., Das, A., and Sagi, I. (2020). Emerging roles of ECM remodeling processes in cancer. Semin. Cancer Biol. 62, 192–200. doi:10.1016/j.semcancer.2019.09.004
Muncie, J. M., and Weaver, V. M. (2018). The physical and biochemical properties of the extracellular matrix regulate cell fate. Curr. Top. Dev. Biol. 130, 1–37. doi:10.1016/bs.ctdb.2018.02.002
Murray, J. D. (2003). On the mechanochemical theory of biological pattern formation with application to vasculogenesis. C. R. Biol. 326, 239–252. doi:10.1016/s1631-0691(03)00065-9
Murray, J. D., Maini, P. K., and Tranquillo, R. T. (1988). Mechanochemical models for generating biological pattern and form in development. Phys. Rep. 171, 59–84. doi:10.1016/0370-1573(88)90003-8
Naba, A., Pearce, O. M. T., Del Rosario, A., Ma, D., Ding, H., Rajeeve, V., et al. (2017). Characterization of the extracellular matrix of normal and diseased tissues using proteomics. J. Proteome Res. 16, 3083–3091. doi:10.1021/acs.jproteome.7b00191
Naganathan, S. R., Popović, M., and Oates, A. C. (2022). Left–right symmetry of zebrafish embryos requires somite surface tension. Nature 605, 516–521. doi:10.1038/s41586-022-04646-9
Nahum, A., Koren, Y., Ergaz, B., Natan, S., Miller, G., Tamir, Y., et al. (2023). Inference of long-range cell-cell force transmission from ECM remodeling fluctuations. Commun. Biol. 6, 811. doi:10.1038/s42003-023-05179-1
Najafi, M., Farhood, B., and Mortezaee, K. (2019). Extracellular matrix (ECM) stiffness and degradation as cancer drivers. J. Cell Biochem. 120, 2782–2790. doi:10.1002/jcb.27681
Namy, P., Ohayon, J., and Tracqui, P. (2004). Critical conditions for pattern formation and in vitro tubulogenesis driven by cellular traction fields. J. Theor. Biol. 227, 103–120. doi:10.1016/j.jtbi.2003.10.015
Nelemans, B. K. A., Schmitz, M., Tahir, H., Merks, R. M. H., and Smit, T. H. (2020). Somite division and new boundary formation by mechanical strain. iScience 23, 100976. doi:10.1016/j.isci.2020.100976
Nematbakhsh, A., Levis, M., Kumar, N., Chen, W., Zartman, J. J., and Alber, M. (2020). Epithelial organ shape is generated by patterned actomyosin contractility and maintained by the extracellular matrix. PLoS Comput. Biol. 16, e1008105. doi:10.1371/journal.pcbi.1008105
Newman, T. (2007). Modeling multicellular structures using the subcellular element model. Basel: Birkhäuser Basel, 221–239. chap. III. doi:10.1007/978-3-7643-8123-3_10
Nguyen Edalgo, Y. T., and Ford Versypt, A. N. (2018). Mathematical modeling of metastatic cancer migration through a remodeling extracellular matrix. Processes 6, 58. doi:10.3390/pr6050058
Nguyen Edalgo, Y. T., Zornes, A. L., and Ford Versypt, A. N. (2019). A hybrid discrete–continuous model of metastatic cancer cell migration through a remodeling extracellular matrix. AIChE J. 65, e16671. doi:10.1002/aic.16671
Noerr, P. S., Zamora Alvarado, J. E., Golnaraghi, F., McCloskey, K. E., Gopinathan, A., and Dasbiswas, K. (2023). Optimal mechanical interactions direct multicellular network formation on elastic substrates. PNAS 120, e2301555120. doi:10.1073/pnas.2301555120
Notbohm, J., Lesman, A., Rosakis, P., Tirrell, D. A., and Ravichandran, G. (2015). Microbuckling of fibrin provides a mechanism for cell mechanosensing. J. R. Soc. Interface. 12, 20150320. doi:10.1098/rsif.2015.0320
Ohno, K., Kobayashi, Y., Uesaka, M., Gotoda, T., Denda, M., Kosumi, H., et al. (2021). A computational model of the epidermis with the deformable dermis and its application to skin diseases. Sci. Rep. 11, 13234. doi:10.1038/s41598-021-92540-1
Olsen, L., Maini, P. K., and Sherratt, J. A. (1998a). Spatially varying equilibria of mechanical models: application to dermal wound contraction. Math. Biosci. 147, 113–129. doi:10.1016/s0025-5564(97)00075-8
Olsen, L., Maini, P. K., Sherratt, J. A., and Dallon, J. (1999). Mathematical modelling of anisotropy in fibrous connective tissue. Math. Biosci. 158, 145–170. doi:10.1016/s0025-5564(99)00005-x
Olsen, L., Maini, P. K., Sherratt, J. A., and Marchant, B. (1998b). Simple modelling of extracellular matrix alignment in dermal wound healing I. Cell flux induced alignment. Comput. Math. Methods Med. 1, 175–192. doi:10.1080/10273669808833018
Olsen, L., Sherratt, J. A., and Maini, P. K. (1995). A mechanochemical model for adult dermal wound contraction and the permanence of the contracted tissue displacement profile. J. Theor. Biol. 177, 113–128. doi:10.1006/jtbi.1995.0230
Olsen, L., Sherratt, J. A., Maini, P. K., and Arnold, F. (1997). A mathematical model for the capillary endothelial cell-extracellular matrix interactions in wound-healing angiogenesis. IMA J. Math. Appl. Med. Biol. 14, 261–281. doi:10.1093/imammb/14.4.261
Oster, G., Murray, J., and Harris, A. (1983). Mechanical aspects of mesenchymal morphogenesis. Development 78, 83–125. doi:10.1242/dev.78.1.83
Oster, G. F., Murray, J. D., and Maini, P. K. (1985). A model for chondrogenic condensations in the developing limb: the role of extracellular matrix and cell tractions. Development 89, 93–112. doi:10.1242/dev.89.1.93
Oswald, L., Grosser, S., Smith, D. M., and Käs, J. A. (2017). Jamming transitions in cancer. J. Phys. D Appl. Phys. 50, 483001. doi:10.1088/1361-6463/aa8e83
Othmer, H., Dunbar, S., and Alt, W. (1988). Models of dispersal in biological systems. J. Math. Biol. 26, 263–298. doi:10.1007/BF00277392
Ozik, J., Collier, N., Wozniak, J. M., Macal, C., Cockrell, C., Friedman, S. H., et al. (2018). High-throughput cancer hypothesis testing with an integrated PhysiCell-EMEWS workflow. BMC Bioinforma. 19, 483. doi:10.1186/s12859-018-2510-x
Painter, K. J. (2009). Modelling cell migration strategies in the extracellular matrix. J. Math. Biol. 58, 511–543. doi:10.1007/s00285-008-0217-8
Pal, A., Haliti, P., Dharmadhikari, B., Qi, W., and Patra, P. (2021). Manipulating extracellular matrix organizations and parameters to control local cancer invasion. IEEE/ACM Trans. Comput. Biol. Bioinform. 18, 2566–2576. doi:10.1109/TCBB.2020.2989223
Pally, D., and Naba, A. (2024). Extracellular matrix dynamics: a key regulator of cell migration across length-scales and systems. Curr. Opin. Cell Biol. 86, 102309. doi:10.1016/j.ceb.2023.102309
Pally, D., Pramanik, D., and Bhat, R. (2019). An interplay between reaction-diffusion and cell-matrix adhesion regulates multiscale invasion in early breast carcinomatosis. Front. physiology 10, 790. doi:10.3389/fphys.2019.00790
Palmquist, K. H., Tiemann, S. F., Ezzeddine, F. L., Yang, S., Pfeifer, C. R., Erzberger, A., et al. (2022). Reciprocal cell-ECM dynamics generate supracellular fluidity underlying spontaneous follicle patterning. Cell 185, 1960–1973. doi:10.1016/j.cell.2022.04.023
Panchenko, A. Y., Tchaicheeyan, O., Berinskii, I. E., and Lesman, A. (2022). Does the extracellular matrix support cell–cell communication by elastic wave packets? ACS Biomaterials Sci. Eng. 8, 5155–5170. doi:10.1021/acsbiomaterials.2c01049
Parisi, L., Toffoli, A., Ghezzi, B., Mozzoni, B., Lumetti, S., and Macaluso, G. M. (2020). A glance on the role of fibronectin in controlling cell response at biomaterial interface. Jpn. Dent. Sci. Rev. 56, 50–55. doi:10.1016/j.jdsr.2019.11.002
Park, J., Holmes, W., Lee, S., Kim, H., Kim, D., Kwak, M., et al. (2017). Mechanochemical feedback underlies coexistence of qualitatively distinct cell polarity patterns within diverse cell populations. PNAS 114, E5750–E5759. doi:10.1073/pnas.1700054114
Park, J., Kim, D.-H., and Levchenko, A. (2018). Topotaxis: a new mechanism of directed cell migration in topographic ECM gradients. Biophys. J. 114, 1257–1263. doi:10.1016/j.bpj.2017.11.3813
Parsons, S. L., Watson, S. A., Brown, P. D., Collins, H. M., and Steele, R. J. (1997). Matrix metalloproteinases. Brit. J. Surg. 84, 160–166. doi:10.1046/j.1365-2168.1997.02719.x
Pastar, I., Stojadinovic, O., Yin, N. C., Ramirez, H., Nusbaum, A. G., Sawaya, A., et al. (2014). Epithelialization in wound healing: a comprehensive review. Adv. Wound Care 3, 445–464. doi:10.1089/wound.2013.0473
Paukner, D., Eichinger, J. F., and Cyron, C. J. (2023). What are the key mechanical mechanisms governing integrin-mediated cell migration in three-dimensional fiber networks? Biomech. Model. Mechanobiol. 22, 1177–1192. doi:10.1007/s10237-023-01709-2
Paul, C. D., Mistriotis, P., and Konstantopoulos, K. (2017). Cancer cell motility: lessons from migration in confined spaces. Nat. Rev. Cancer 17, 131–140. doi:10.1038/nrc.2016.123
Penington, C. J., Hughes, B. D., and Landman, K. A. (2011). Building macroscale models from microscale probabilistic models: a general probabilistic approach for nonlinear diffusion and multispecies phenomena. Phys. Rev. E 84, 041120. doi:10.1103/physreve.84.041120
Pensalfini, M., and Tepole, A. B. (2023). Mechano-biological and bio-mechanical pathways in cutaneous wound healing. PLoS Comput. Biol. 19, e1010902. doi:10.1371/journal.pcbi.1010902
Perumpanani, A. J., and Byrne, H. M. (1999). Extracellular matrix concentration exerts selection pressure on invasive cells. Eur. J. Cancer 35, 1274–1280. doi:10.1016/s0959-8049(99)00125-2
Petrovskii, S. V., and Li, B.-L. (2005). Exactly solvable models of biological invasion. United States: CRC Press. doi:10.1201/9781420034967
Peurichard, D., Delebecque, F., Lorsignol, A., Barreau, C., Rouquette, J., Descombes, X., et al. (2017). Simple mechanical cues could explain adipose tissue morphology. J. Theor. Biol. 429, 61–81. doi:10.1016/j.jtbi.2017.06.030
Pfisterer, K., Shaw, L. E., Symmank, D., and Weninger, W. (2021). The extracellular matrix in skin inflammation and infection. Front. Cell. Dev. Biol. 9, 682414. doi:10.3389/fcell.2021.682414
Pitt-Francis, J., Pathmanathan, P., Bernabeu, M. O., Bordas, R., Cooper, J., Fletcher, A. G., et al. (2009). Chaste: a test-driven approach to software development for biological modelling. Comput. Phys. Commun. 180, 2452–2471. doi:10.1016/j.cpc.2009.07.019
Plodinec, M., Loparic, M., Monnier, C. A., Obermann, E. C., Zanetti-Dallenbach, R., Oertle, P., et al. (2012). The nanomechanical signature of breast cancer. Nat. Nanotech 7, 757–765. doi:10.1038/nnano.2012.167
Ponce-de Leon, M., Montagud, A., Noël, V., Meert, A., Pradas, G., Barillot, E., et al. (2023). PhysiBoSS 2.0: a sustainable integration of stochastic Boolean and agent-based modelling frameworks. npj Syst. Biol. Appl. 9, 1–12. doi:10.1038/s41540-023-00314-4
Poole, J. J. A., and Mostaço-Guidolin, L. B. (2021). Optical microscopy and the extracellular matrix structure: a review. Cells 10, 1760. doi:10.3390/cells10071760
Pramanik, D., Jolly, M. K., and Bhat, R. (2021). Matrix adhesion and remodeling diversifies modes of cancer invasion across spatial scales. J. Theor. Biol. 524, 110733. doi:10.1016/j.jtbi.2021.110733
Preen, R. J., Bull, L., and Adamatzky, A. (2019). Towards an evolvable cancer treatment simulator. BioSystems 182, 1–7. doi:10.1016/j.biosystems.2019.05.005
Prevedel, R., Diz-Muñoz, A., Ruocco, G., and Antonacci, G. (2019). Brillouin microscopy: an emerging tool for mechanobiology. Nat. Methods 16, 969–977. doi:10.1038/s41592-019-0543-3
Qin, L., Yang, D., Yi, W., Cao, H., and Xiao, G. (2021). Roles of leader and follower cells in collective cell migration. Mol. Biol. Cell 32, 1267–1272. doi:10.1091/mbc.E20-10-0681
Rabie, E. M., Zhang, S. X., Kourouklis, A. P., Kilinc, A. N., Simi, A. K., Radisky, D. C., et al. (2021). Matrix degradation and cell proliferation are coupled to promote invasion and escape from an engineered human breast microtumor. Integr. Biol. 13, 17–29. doi:10.1093/intbio/zyaa026
Rahman, N., O’Neill, E., Irnaten, M., Wallace, D., and O’Brien, C. (2020). Corneal stiffness and collagen cross-linking proteins in glaucoma: potential for novel therapeutic strategy. J. Ocul. Pharma. Ther. 36, 582–594. doi:10.1089/jop.2019.0118
Ramasubramanian, A., Latacha, K. S., Benjamin, J. M., Voronov, D. A., Ravi, A., and Taber, L. A. (2006). Computational model for early cardiac looping. Ann. Biomed. Eng. 34, 1655–1669. doi:10.1007/s10439-005-9021-4
Ramos, J. R. D., Travasso, R., and Carvalho, J. (2018). Capillary network formation from dispersed endothelial cells: influence of cell traction, cell adhesion, and extracellular matrix rigidity. Phys. Rev. E 97, 012408. doi:10.1103/physreve.97.012408
Ray, A., and Provenzano, P. P. (2021). Aligned forces: origins and mechanisms of cancer dissemination guided by extracellular matrix architecture. Curr. Opin. Cell Biol. 72, 63–71. doi:10.1016/j.ceb.2021.05.004
Reinhardt, J. W., and Gooch, K. J. (2014). Agent-based modeling traction force mediated compaction of cell-populated collagen gels using physically realistic fibril mechanics. J. Biomech. Eng. 136, 021024. doi:10.1115/1.4026179
Reinhardt, J. W., and Gooch, K. J. (2018). An agent-based discrete collagen fiber network model of dynamic traction force-induced remodeling. J. Biomech. Eng. 140, 051003. doi:10.1115/1.4037947
Rens, E. G., and Merks, R. M. (2020). Cell shape and durotaxis explained from cell-extracellular matrix forces and focal adhesion dynamics. iScience 23, 101488. doi:10.1016/j.isci.2020.101488
Reuten, R., Zendehroud, S., Nicolau, M., Fleischhauer, L., Laitala, A., Kiderlen, S., et al. (2021). Basement membrane stiffness determines metastases formation. Nat. Mat. 20, 892–903. doi:10.1038/s41563-020-00894-0
Ricard-Blum, S. (2011). The collagen family. Cold Spring Harb. Perspect. Biol. 3, a004978. doi:10.1101/cshperspect.a004978
Ricoult, S. G., Kennedy, T. E., and Juncker, D. (2015). Substrate-bound protein gradients to study haptotaxis. Front. Bioeng. Biotechnol. 3, 40. doi:10.3389/fbioe.2015.00040
Rousselle, P., Laigle, C., and Rousselet, G. (2022). The basement membrane in epidermal polarity, stemness, and regeneration. Am. J. Physiology-Cell Physiology 323, C1807–C1822. doi:10.1152/ajpcell.00069.2022
Ruscone, M., Montagud, A., Chavrier, P., Destaing, O., Bonnet, I., Zinovyev, A., et al. (2023). Multiscale model of the different modes of cancer cell invasion. Bioinformatics 39, btad374. doi:10.1093/bioinformatics/btad374
Sapir, L., and Tzlil, S. (2017). Talking over the extracellular matrix: how do cells communicate mechanically? Seminars Cell and Dev. Biol. 71, 99–105. doi:10.1016/j.semcdb.2017.06.010
Saraswathibhatla, A., Indana, D., and Chaudhuri, O. (2023). Cell–extracellular matrix mechanotransduction in 3D. Nat. Rev. Mol. Cell Biol. 24, 495–516. doi:10.1038/s41580-023-00583-1
Savin, T., Kurpios, N. A., Shyer, A. E., Florescu, P., Liang, H., Mahadevan, L., et al. (2011). On the growth and form of the gut. Nature 476, 57–62. doi:10.1038/nature10277
Saxena, G., Ponce-de Leon, M., Montagud, A., Vicente Dorca, D., and Valencia, A. (2021). “BioFVM-X: an MPI+OpenMP 3-D simulator for biological systems,” in Computational methods in systems biology. Editors E. Cinquemani,, and L. Paulevé (Cham: Springer International Publishing), 266–279. doi:10.1007/978-3-030-85633-5
Schaff, J., Fink, C., Slepchenko, B., Carson, J., and Loew, L. (1997). A general computational framework for modeling cellular structure and function. Biophys. J. 73, 1135–1146. doi:10.1016/S0006-3495(97)78146-3
Schlüter, D. K., Ramis-Conde, I., and Chaplain, M. A. J. (2012). Computational modeling of single-cell migration: the leading role of extracellular matrix fibers. Biophys. J. 103, 1141–1151. doi:10.1016/j.bpj.2012.07.048
Scianna, M., and Preziosi, L. (2021). A cellular potts model for analyzing cell migration across constraining pillar arrays. Axioms 10, 32. doi:10.3390/axioms10010032
Scianna, M., Preziosi, L., and Wolf, K. (2013). A cellular potts model simulating cell migration on and in matrix environments. Math. Biosci. Eng. 10, 235–261. doi:10.3934/mbe.2013.10.235
Sego, T., Sluka, J., Sauro, H., and Glazier, J. (2023). Tissue Forge: interactive biological and biophysics simulation environment. PLoS Comput. Biol. 19, e1010768. doi:10.1371/journal.pcbi.1010768
Shao, P., Eltony, A. M., Seiler, T. G., Tavakol, B., Pineda, R., Koller, T., et al. (2019). Spatially-resolved brillouin spectroscopy reveals biomechanical abnormalities in mild to advanced keratoconus in vivo. Sci. Rep. 9, 7467. doi:10.1038/s41598-019-43811-5
Shao, X., Taha, I. N., Clauser, K. R., Gao, Y., and Naba, A. (2020). Matrisomedb: the ecm-protein knowledge database. Nucleic acids Res. 48, D1136–D1144. doi:10.1093/nar/gkz849
Shen, Z., Sun, L., Liu, Z., Li, M., Cao, Y., Han, L., et al. (2023). Rete ridges: morphogenesis, function, regulation, and reconstruction. Acta Biomater. 155, 19–34. doi:10.1016/j.actbio.2022.11.031
Shuttleworth, R., and Trucu, D. (2019). Multiscale modelling of fibres dynamics and cell adhesion within moving boundary cancer invasion. Bull. Math. Biol. 81, 2176–2219. doi:10.1007/s11538-019-00598-w
Shyer, A. E., Tallinen, T., Nerurkar, N. L., Wei, Z., Gil, E. S., Kaplan, D. L., et al. (2013). Villification: how the gut gets its villi. Science 342, 212–218. doi:10.1126/science.1238842
Siadat, S., Silverman, A., DiMarzio, C., and Ruberti, J. (2022). Measuring collagen fibril diameter with differential interference contrast microscopy. J. Struct. Biol. 213, 107697. doi:10.1016/j.jsb.2021.107697
Simpson, M. J., Hughes, B. D., and Landman, K. A. (2009a). Diffusing populations: ghosts or folks? Australas. J. Eng. Educ. 15, 59–68. doi:10.1080/22054952.2009.11464027
Simpson, M. J., Landman, K. A., and Hughes, B. D. (2009b). Multi-species simple exclusion processes. Phys. A Stat. 388, 399–406. doi:10.1016/j.physa.2008.10.038
Sinha, S., Malmi-Kakkada, A. N., Li, X., Samanta, H. S., and Thirumalai, D. (2020). Spatially heterogeneous dynamics of cells in a growing tumor spheroid: comparison between theory and experiments. Soft matter 16, 5294–5304. doi:10.1039/c9sm02277e
Slater, B., Li, J., Indana, D., Xie, Y., Chaudhuri, O., and Kim, T. (2021). Transient mechanical interactions between cells and viscoelastic extracellular matrix. Soft Matter 17, 10274–10285. doi:10.1039/d0sm01911a
Soans, K. G., Ramos, A. P., Sidhaye, J., Krishna, A., Solomatina, A., Hoffmann, K. B., et al. (2022). Collective cell migration during optic cup formation features changing cell-matrix interactions linked to matrix topology. Curr. Biol. 32, 4817–4831.e9. doi:10.1016/j.cub.2022.09.034
Sopher, R. S., Goren, S., Koren, Y., Tchaicheeyan, O., and Lesman, A. (2023). Intercellular mechanical signalling in a 3D nonlinear fibrous network model. Mech. Mat. 184, 104739. doi:10.1016/j.mechmat.2023.104739
Stack, M., Macklin, P., Searles, R., and Chandrasekaran, S. (2022). OpenACC acceleration of an agent-based biological simulation framework. Comput. Sci. Eng. 24, 53–63. doi:10.1109/MCSE.2022.3226602
Starruß, J., de Back, W., Brusch, L., and Deutsch, A. (2014). Morpheus: a user-friendly modeling environment for multiscale and multicellular systems biology. Bioinformatics 30, 1331–1332. doi:10.1093/bioinformatics/btt772
Stepanova, D., Byrne, H. M., Maini, P. K., and Alarcón, T. (2021). A multiscale model of complex endothelial cell dynamics in early angiogenesis. PLoS Comput. Biol. 17, e1008055. doi:10.1371/journal.pcbi.1008055
Stéphanou, A., Le Floc’h, S., and Chauvière, A. (2015). A hybrid model to test the importance of mechanical cues driving cell migration in angiogenesis. Math. Model. Nat. Phenom. 10, 142–166. doi:10.1051/mmnp/201510107
Stéphanou, A., and Volpert, V. (2016). Hybrid modelling in biology: a classification review. Math. Model. Nat. Phenom. 11, 37–48. doi:10.1051/mmnp/201611103
Stopak, D., and Harris, A. K. (1982). Connective tissue morphogenesis by fibroblast traction: I. Tissue culture observations. Dev. Biol. 90, 383–398. doi:10.1016/0012-1606(82)90388-8
Strobl, M. A. R., Krause, A. L., Damaghi, M., Gillies, R., Anderson, A. R. A., and Maini, P. K. (2020). Mix and match: phenotypic coexistence as a key facilitator of cancer invasion. Bull. Math. Biol. 82, 15. doi:10.1007/s11538-019-00675-0
Sun, B. (2021). The mechanics of fibrillar collagen extracellular matrix. Cell Rep. Phys. Sci. 2, 100515. doi:10.1016/j.xcrp.2021.100515
Sütterlin, T., Tsingos, E., Bensaci, J., Stamatas, G. N., and Grabe, N. (2017). A 3D self-organizing multicellular epidermis model of barrier formation and hydration with realistic cell morphology based on episim. Sci. Rep. 7, 43472. doi:10.1038/srep43472
Swat, M. H., Thomas, G. L., Belmonte, J. M., Shirinifard, A., Hmeljak, D., and Glazier, J. A. (2012). Multi-scale modeling of tissues using CompuCell3D. Methods Cell Biol. 110, 325–366. doi:10.1016/B978-0-12-388403-9.00013-8
Szabó, A., Varga, K., Garay, T., Hegedűs, B., and Czirók, A. (2012). Invasion from a cell aggregate—the roles of active cell motion and mechanical equilibrium. Phys. Biol. 9, 016010. doi:10.1088/1478-3975/9/1/016010
Taber, L. A. (2003). Biophysical mechanisms of cardiac looping. Int. J. Dev. Biol. 50, 323–332. doi:10.1387/ijdb.052045lt
Tiwari, S., Triulzi, T., Holton, S., Regondi, V., Paolini, B., Tagliabue, E., et al. (2020). Infrared spectroscopic imaging visualizes a prognostic extracellular matrix-related signature in breast cancer. Sci. Rep. 10, 5442. doi:10.1038/s41598-020-62403-2
Töpfer, U. (2023). Basement membrane dynamics and mechanics in tissue morphogenesis. Biol. Open 12, bio059980. doi:10.1242/bio.059980
Tosin, A., Ambrosi, D., and Preziosi, L. (2006). Mechanics and chemotaxis in the morphogenesis of vascular networks. Bull. Math. Biol. 68, 1819–1836. doi:10.1007/s11538-006-9071-2
Tozluoǧlu, M., Duda, M., Kirkland, N. J., Barrientos, R., Burden, J. J., Munoz, J. J., et al. (2019). Planar differential growth rates initiate precise fold positions in complex epithelia. Dev. Cell 51, 299–312. doi:10.1016/j.devcel.2019.09.009
Tracqui, P. (1995). From passive diffusion to active cellular migration in mathematical models of tumour invasion. Acta Biotheor. 43, 443–464. doi:10.1007/bf00713564
Tranqui, L., and Tracqui, P. (2000). Mechanical signalling and angiogenesis. The integration of cell–extracellular matrix couplings. C. R. Acad. Sci. III 323, 31–47. doi:10.1016/s0764-4469(00)00110-4
Tranquillo, R. T., and Murray, J. D. (1992). Continuum model of fibroblast-driven wound contraction: inflammation-mediation. J. Theor. Biol. 158, 135–172. doi:10.1016/s0022-5193(05)80715-5
Trucu, D., Lin, P., Chaplain, M. A. J., and Wang, Y. (2013). A multiscale moving boundary model arising in cancer invasion. Multisc. Model Simul. 11, 309–335. doi:10.1137/110839011
Truskinovsky, L., Vitale, G., and Smit, T. H. (2014). A mechanical perspective on vertebral segmentation. Int. J. Eng. Sci. 83, 124–137. doi:10.1016/j.ijengsci.2014.05.003
Tsingos, E., Bakker, B. H., Keijzer, K. A. E., Hupkes, H. J., and Merks, R. M. H. (2023). Hybrid cellular Potts and bead-spring modeling of cells in fibrous extracellular matrix. Biophys. J. 112, 2609–2622. doi:10.1016/j.bpj.2023.05.013
Turing, A. M. (1952). The chemical basis of morphogenesis. Philosophical Trans. R. Soc. Lond. Ser. B, Biol. Sci. 237, 37–72.
Van Helvert, S., Storm, C., and Friedl, P. (2018). Mechanoreciprocity in cell migration. Nat. Cell Biol. 20, 8–20. doi:10.1038/s41556-017-0012-0
van Huizen, L. M. G., Radonic, T., van Mourik, F., Seinstra, D., Dickhoff, C., Daniels, J. M. A., et al. (2020). Compact portable multiphoton microscopy reveals histopathological hallmarks of unprocessed lung tumor tissue in real time. Transl. Biophot. 2, e202000009. doi:10.1002/tbio.202000009
van Oers, R. F. M., Rens, E. G., LaValley, D. J., Reinhart-King, C. A., and Merks, R. M. H. (2014). Mechanical cell-matrix feedback explains pairwise and collective endothelial cell behavior in vitro. PLoS Comput. Biol. 10, e1003774. doi:10.1371/journal.pcbi.1003774
Varner, V. D., Gleghorn, J. P., Miller, E., Radisky, D. C., and Nelson, C. M. (2015). Mechanically patterning the embryonic airway epithelium. PNAS 112, 9230–9235. doi:10.1073/pnas.1504102112
Vega, R., Carretero, M., and Bonilla, L. L. (2021). Anomalous angiogenesis in retina. Biomedicines 9, 224. doi:10.3390/biomedicines9020224
Vega, R., Carretero, M., Travasso, R. D., and Bonilla, L. L. (2020). Notch signaling and taxis mechanisms regulate early stage angiogenesis: a mathematical and computational model. PLoS Comput. Biol. 16, e1006919. doi:10.1371/journal.pcbi.1006919
Vega-Lopez, G. A., Cerrizuela, S., Tribulo, C., and Aybar, M. J. (2018). Neurocristopathies: new insights 150 years after the neural crest discovery. Dev. Biol. 444, S110–S143. doi:10.1016/j.ydbio.2018.05.013
Verstraete, N., Marku, M., Domagala, M., Arduin, H., Bordenave, J., Fournié, J.-J., et al. (2023). An agent-based model of monocyte differentiation into tumour-associated macrophages in chronic lymphocytic leukemia. iScience 26, 106897. doi:10.1016/j.isci.2023.106897
Villa, C., Chaplain, M. A. J., Gerisch, A., and Lorenzi, T. (2021). Mechanical models of pattern and form in biological tissues: the role of stress–strain constitutive equations. Bull. Math. Biol. 83, 80. doi:10.1007/s11538-021-00912-5
Visse, R., and Nagase, H. (2003). Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure function and biochemistry. Circ. Res. 92, 827–839. doi:10.1161/01.RES.0000070112.80711.3D
Vos, B. E., Martinez-Torres, C., Burla, F., Weisel, J. W., and Koenderink, G. H. (2020). Revealing the molecular origins of fibrin’s elastomeric properties by in situ x-ray scattering. Acta Biomater. 104, 39–52. doi:10.1016/j.actbio.2020.01.002
Voulgarelis, D., Velayudhan, A., and Smith, F. (2017). Derivation of continuum models from an agent-based cancer model: optimization and sensitivity analysis. Curr. Pharm. Biotechnol. 18, 1249–1263. doi:10.2174/1389201019666180329111909
Wan, X., Li, Z., and Lubkin, S. R. (2008). Mechanics of mesenchymal contribution to clefting force in branching morphogenesis. Biomech. Model. Mechanobiol. 7, 417–426. doi:10.1007/s10237-007-0105-y
Wang, H., and Xu, X. (2020). Continuum elastic models for force transmission in biopolymer gels. Soft Matter 16, 10781–10808. doi:10.1039/d0sm01451f
Wang, K., Wen, D., Xu, X., Zhou, R., Jiang, F., Yuan, S., et al. (2023). Extracellular matrix stiffness - the central cue for skin fibrosis. Front. Mol. Biosci. 10, 1132353. doi:10.3389/fmolb.2023.1132353
Wang, Y., Guerrero-Juarez, C. F., Qiu, Y., Du, H., Chen, W., Figueroa, S., et al. (2019). A multiscale hybrid mathematical model of epidermal-dermal interactions during skin wound healing. Exp. Dermatol. 28, 493–502. doi:10.1111/exd.13909
Weaver, A. M. (2006). Invadopodia: specialized cell structures for cancer invasion. Clin. Exp. Metastasis 23, 97–105. doi:10.1007/s10585-006-9014-1
Webb, S. D., Sherratt, J. A., and Fish, R. G. (1999). Alterations in proteolytic activity at low pH and its association with invasion: a theoretical model. Clin. Exp. Metastasis. 17, 397–407. doi:10.1023/a:1006667303583
Werb, Z. (1997). ECM and cell surface proteolysis: regulating cellular ecology. Cell 91, 439–442. doi:10.1016/s0092-8674(00)80429-8
Wershof, E., Park, D., Jenkins, R. P., Barry, D. J., Sahai, E., and Bates, P. A. (2019). Matrix feedback enables diverse higher-order patterning of the extracellular matrix. PLoS Comput. Biol. 15, e1007251. doi:10.1371/journal.pcbi.1007251
Wilkinson, H. N., and Hardman, M. J. (2020). Wound healing: cellular mechanisms and pathological outcomes. Open Biol. 10, 200223. doi:10.1098/rsob.200223
Winkler, J., Abisoye-Ogunniyan, A., Metcalf, K. J., and Werb, Z. (2020). Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 11, 5120. doi:10.1038/s41467-020-18794-x
Woessner, A. E., and Quinn, K. P. (2022). Improved segmentation of collagen second harmonic generation images with a deep learning convolutional neural network. J. Biophot. 15, e202200191. doi:10.1002/jbio.202200191
Wu, J.-s., Jiang, J., Chen, B.-j., Wang, K., Tang, Y.-l., and Liang, X.-h. (2021). Plasticity of cancer cell invasion: patterns and mechanisms. Transl. Oncol. 14, 100899. doi:10.1016/j.tranon.2020.100899
Xu, C., and Jackson, S. (2019). Machine learning and complex biological data. Genome Biol. 20, 76. doi:10.1186/s13059-019-1689-0
Yamada, K. M., Collins, J. W., Cruz Walma, D. A., Doyle, A. D., Morales, S. G., Lu, J., et al. (2019). Extracellular matrix dynamics in cell migration, invasion and tissue morphogenesis. Int. J. Exp. Path. 100, 144–152. doi:10.1111/iep.12329
Yang, W., Sherman, V. R., Gludovatz, B., Schaible, E., Stewart, P., Ritchie, R. O., et al. (2015). On the tear resistance of skin. Nat. Commun. 6, 6649–6710. doi:10.1038/ncomms7649
Young, J. L., Holle, A. W., and Spatz, J. P. (2016). Nanoscale and mechanical properties of the physiological cell-ECM microenvironment. Exp. Cell Res. 343, 3–6. doi:10.1016/j.yexcr.2015.10.037
Yuan, Y. (2016). Spatial heterogeneity in the tumor microenvironment. Cold Spring Harb. Perspect. Med. 6, a026583. doi:10.1101/cshperspect.a026583
Keywords: extracellular matrix, mathematical modeling, ECM structure, cancer, wound healing, tissue morphogenesis
Citation: Crossley RM, Johnson S, Tsingos E, Bell Z, Berardi M, Botticelli M, Braat QJS, Metzcar J, Ruscone M, Yin Y and Shuttleworth R (2024) Modeling the extracellular matrix in cell migration and morphogenesis: a guide for the curious biologist. Front. Cell Dev. Biol. 12:1354132. doi: 10.3389/fcell.2024.1354132
Received: 11 December 2023; Accepted: 12 February 2024;
Published: 01 March 2024.
Edited by:
Karin Pfisterer, Medical University of Vienna, AustriaReviewed by:
Simon Syga, Technical University Dresden, GermanyJinseok Park, Children’s Hospital of Los Angeles, United States
Copyright © 2024 Crossley, Johnson, Tsingos, Bell, Berardi, Botticelli, Braat, Metzcar, Ruscone, Yin and Shuttleworth. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Robyn Shuttleworth, rshuttleworth@altoslabs.com
†These authors have contributed equally to this work and share first authorship
 Rebecca M. Crossley
Rebecca M. Crossley