
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell Dev. Biol. , 13 February 2024
Sec. Stem Cell Research
Volume 12 - 2024 | https://doi.org/10.3389/fcell.2024.1344705
This article is part of the Research Topic Stem Cells and Extracellular Vesicles in Aging-Related Diseases View all 8 articles
Exosomes, extracellular vesicles secreted by cells, have garnered significant attention in recent years for their remarkable therapeutic potential. These nanoscale carriers can be harnessed for the targeted delivery of therapeutic agents, such as pharmaceuticals, proteins, and nucleic acids, across biological barriers. This versatile attribute of exosomes is a promising modality for precision medicine applications, notably in the realm of cancer therapy. However, despite their substantial therapeutic potential, exosomes still confront challenges tied to standardization and scalability that impede their practice in clinical applications. Moreover, heterogeneity in isolation methodologies and limited cargo loading mechanisms pose obstacles to ensuring consistent outcomes, thereby constraining their therapeutic utility. In contrast, exosomes exhibit a distinct advantage in cancer diagnosis, as they harbor specific signatures reflective of the tumor’s genetic and proteomic profile. This characteristic endows them with the potential to serve as valuable liquid biopsies for non-invasive and real-time monitoring, making possible early cancer detection for the development of personalized treatment strategies. In this review, we provide an extensive evaluation of the advancements in exosome research, critically examining their advantages and limitations in the context of cancer therapy and early diagnosis. Furthermore, we present a curated overview of the most recent technological innovations utilizing exosomes, with a focus on enhancing the efficacy of early cancer detection.
Cancer, an intricate and pervasive disease, presents a formidable global threat to human life. With a reach that spans across age, gender, and diverse backgrounds, cancer strikes fear into millions of the population (Thill et al., 2023; Wolf et al., 2023). Moreover, cancer places a substantial economic burden on healthcare systems and societies worldwide due to escalating costs associated with treatment and supportive care (Amini et al., 2022; Munshi et al., 2022). What makes cancer particularly challenging is its capacity to infiltrate any organ or tissue, from the lungs to the skin, and from the brain to the bones (Hayashi et al., 2023; Li and Zheng, 2023). Often, its stealthy progression remains concealed until it develops an advanced stage (i.e., usually hard to treat), rendering early detection and treatment a critical challenge (Aleksandrowicz et al., 2023; Wang W T et al., 2023).
Curing cancer is an exceptionally tough endeavor, largely due to the obstacle known as metastasis (Hu Z et al., 2023; Zhou et al., 2023). Metastasis entails cancer cells breaking away from the primary tumor, traversing the bloodstream or lymphatic system, and establishing secondary tumors in distant body parts. This ability to spread and infiltrate new organs or tissues renders cancer highly elusive and resistant to conventional treatment approaches (Hayashi et al., 2023; Li and Zheng, 2023). By the time metastasis is detected, cancer is often at an advanced stage, making complete eradication considerably more challenging. Each secondary tumor presents unique challenges, demanding specific approaches, and the intricate complexity of metastatic disease necessitates a multifaceted treatment strategy (Feng et al., 2023; Gao et al., 2023). Furthermore, cancer cells can evolve, developing resistance to previously effective therapies (Gera et al., 2023; Mahmoud et al., 2023). Therefore, early detection of cancer before its metastasis is critical for cancer treatment and enhances the survival of cancer patients. However, tackling metastasis remains one of the most formidable hurdles on the path to finding a cure for cancer (Wang et al., 2023a; Holladay et al., 2023).
Traditional cancer diagnostic techniques, while historically invaluable, grapple with significant limitations (Xiao, 2015). Many of these methods require invasive procedures like surgeries to obtain tissue samples for analysis, which can be painful and carry risks, making them unsuitable for the patients (Zhang et al., 2013). Additionally, these conventional diagnostic approaches can incur prohibitively high costs, burdening both healthcare systems and patients, particularly when multiple tests are necessary to confirm a diagnosis or monitor treatment progress. Furthermore, their sensitivity, especially in detecting early-stage cancers, can be limited, resulting in smaller tumors or cancerous cells missing until they reach more advanced and less treatable stages (Bassani et al., 2023; Kawada et al., 2023; Tan and Hosein, 2023; Zhang Y et al., 2023). To overcome these limitations, there has been a growing endeavor on developing non-invasive, cost-effective, and highly sensitive diagnostic methods, such as liquid biopsies and advanced imaging technologies (Young et al., 2023). These innovations hold the potential to revolutionize cancer diagnosis by offering earlier detection, reduced invasiveness, and more precise monitoring, ultimately enhancing our ability to combat this devastating disease.
Exosomes have emerged as a burgeoning field of research with vast potential in the realm of cancer detection (Hyun et al., 2018; Xia et al., 2020). These nanoscale particles, once dismissed as cellular debris, are now recognized for their pivotal role in intercellular communication and their capacity to transport molecular cargo, including nucleic acids and proteins (Zhang et al., 2016; Maia et al., 2018). In the context of cancer development, exosomes assume a critical role by ferrying tumor-specific molecules that can serve as biomarkers for early detection (Regev-Rudzki et al., 2013). Their presence in bodily fluids like blood and urine renders them a highly attractive source for non-invasive and easily accessible diagnostic tests (Agnoletto et al., 2023; Wang, et al., 2023b). Researchers are increasingly harnessing the exosomes to detect cancer at its earliest stages, especially before the metastasis when treatment interventions are most effective (Lin et al., 2021; Yu et al., 2021). Furthermore, the diverse array of information conveyed within exosomes offers a plethora of potential markers, enabling not only the detection of cancer but also the identification of specific cancer types and their distinctive molecular characteristics (Sharma and Salomon, 2020). This approach holds significant promise for developing specific treatment strategies for individual patients, thereby improving treatment outcomes and reducing the burden of unnecessary treatments (Kumata et al., 2018; Luo et al., 2018; Smith and Lam, 2018).
In conclusion, the investigation of exosomes as a method for cancer detection marks a promising and innovative Frontier in the field of oncology research (Li G et al., 2020; Zhang W et al., 2021). It holds the tremendous potential to reform the field of early diagnosis, monitoring, and treatment decisions on cancers. As innovative methods continue to emerge in this burgeoning field, exosomes have the potential to become a pivotal element in combatting cancer. They offer patients more efficient and minimally invasive diagnostic alternatives, potentially enhancing treatment efficacy, prolonging survival, and significantly improving the quality of life for those affected by cancer.
Exosomes play a crucial role in cellular quality control, ensuring the maintenance of cellular health. This assures their potential therapeutic applications, especially in conditions marked by impaired waste removal processes (Kakiuchi et al., 2023; Zhao J et al., 2023). The primary functions of exosomes are sketched in Figure 1.

FIGURE 1. Sketch of the activity and functions of the micro vehicles/exosome in vivo. (1) Cell-Cell Communication: Mediate intercellular communication by transporting essential molecules, fostering coordination in physiological processes. (2) Efficient Waste Removal: Serve as carriers for cellular waste removal, contributing to cellular cleanliness (e.g., apoptotic bodies) and overall tissue health. (3) Developmental Regulation and Tissue Repair: Regulate developmental processes and tissue repair by delivering signaling molecules (e.g., DNA, micro RNAs, and proteins) that influence cellular activities. (4) Immune Response Modulation: Actively modulate immune responses by conveying signals via targeting ligand and covalent linkage between immune cells and somatic cells, contributing to inflammation regulation and immune system balance.
Exosomes play a fundamental role in the intricate network of cell-to-cell communication within biological systems. These nanoscale vesicles serve as essential messengers between cells, functioning as conduits for the intercellular exchange of diverse molecular cargo, including proteins, nucleic acids (both DNA and RNA), and lipids (Luo et al., 2023; Shang et al., 2023). When a cell secretes exosomes, they can be internalized by neighboring or distant recipient cells, thereby facilitating the transfer of vital information (Altintas and Saylan, 2023). This molecular exchange enables cells to coordinate and regulate a wide array of cellular activities and responses. For instance, immune cells utilize exosomes to convey signals that orchestrate immune responses, while cancer cells exploit exosome communication to promote tumor growth and metastasis (De La Pena et al., 2009; Kang et al., 2023).
In the network of cellular metabolite maintenance, exosomes serve as indispensable tools for waste removal and cellular quality control mechanisms (Lu et al., 2023). Cells harness the remarkable capacity of exosomes to encapsulate and transport a variety of unwanted or degraded cellular components, such as misfolded proteins, broken organelles, and waste of metabolites, to facilitate their efficient elimination from the cell (Gudbergsson and Johnsen, 2019; Schubert, 2020). This waste disposal process not only ensures the preservation of cellular integrity and functionality but also contributes to the overall health and homeostasis of multicellular organisms. Moreover, the role of exosomes in waste removal extends beyond the individual cell, as they can be released into extracellular environments, such as bodily fluids, where they can aid in systemic waste clearance and potentially play a role in intercellular signaling (Nik Mohamed Kamal and Shahidan, 2021; Lai et al., 2023).
Exosomes also serve as pivotal mediators in critical biological processes, including tissue regeneration and development (Li et al., 2015; Pan et al., 2023). They orchestrate the process of growth and repair in tissues by facilitating the transfer of growth factors and an array of signaling molecules to target cells (Wang and Yang, 2023; Zhao X et al., 2023). In the context of tissue regeneration, exosomes act as specialized messengers, guiding and stimulating cellular responses necessary for the healing and rebuilding of damaged or injured tissues. Notably, in the nervous system, exosomes play an indispensable role in neuronal communication (i.e., synapse) and maintenance (Liu et al., 2022). They serve as nanocarriers, shuttling neurotransmitters, with a myriad of signaling molecules between neurons and other supporting cells within the brain. This intercellular exchange not only underpins fundamental processes in neural development but also contributes to the fine-tuning of neural circuits, highlighting the profound impact of exosomes on neurological function and giving potential therapeutic applications in neurodegenerative diseases and neurological disorders (Hu T et al., 2023; Liu et al., 2023; Zhong et al., 2023).
Exosomes assume a crucial role in orchestrating immune responses, wielding their influence in the intricate domain of immune system function (Schwarzenbach and Gahan, 2021). Immune cells harness the power of exosomes to disseminate signaling molecules that serve as directives for the regulation of immune responses, effectively coordinating the complex interplay of immune mechanisms (Greening et al., 2015; Chen et al., 2017; Li et al., 2019). Moreover, exosomes act as couriers of information, transferring crucial insights about pathogens or abnormalities to other immune cells, thereby enabling swift and coordinated countermeasures against infections or aberrant cell behavior (Kaminski et al., 2019; Lema and Burlingham, 2019). In the realm of disease, exosomes take on added significance as potential diagnostic tools. For instance, cancer cells exploit exosomes as conveyors of disease-specific cargo, including tumor-specific proteins and genetic material which further modulate the immune response (Anel et al., 2019; Shen et al., 2019).
Of note, exosomes have garnered significant attention in the field of cancer research, as they are believed to play a critical role in cancer progression, metastasis, and drug resistance (Lema and Burlingham, 2019; Shen et al., 2019). Researchers are exploring the potential of exosomes as diagnostic markers and therapeutic targets in various diseases, including cancer, neurodegenerative disorders, and autoimmune conditions (Figure 2). Their ability to carry specific molecular cargo makes them a promising avenue for understanding disease mechanisms and developing novel medical treatments (Table 1).
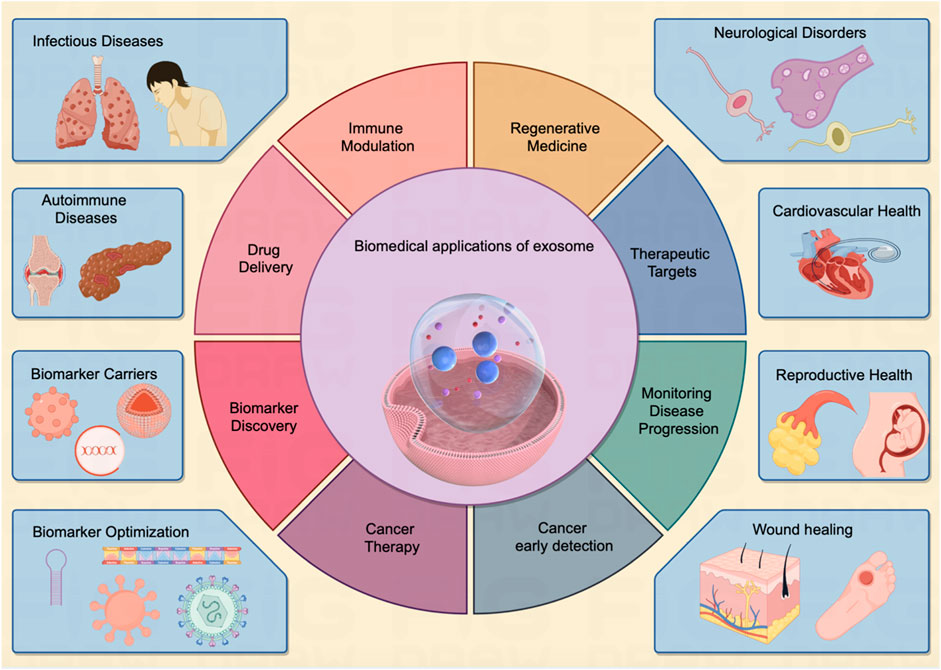
FIGURE 2. The exosome exerts multi-faceted biomedical potentials for human disease. Exosomes demonstrate a vast array of biomedical applications pertinent to human diseases affecting various organs throughout the body. These entities serve as pivotal biomarkers in diagnostic processes and hold considerable potential across multiple domains, including targeted drug delivery, immunomodulation, and tissue regeneration. As multifunctional agents, they are increasingly acknowledged as instrumental in driving forward the Frontier of innovative biomedical research, heralding a notable shift in the paradigms of disease detection and therapeutic strategies, as illustrated in the center panel. The inherent attributes of exosomes establish them as an emergent and significant field within biomedical research, poised to radically reshape methodologies in disease diagnosis, therapeutic interventions, and the broader spectrum of healthcare practices.
Exosomes have gained immense significance in the field of biomedicine due to their diverse and versatile applications (Greening et al., 2015). These exosomes carry a variant of molecular cargo, making them valuable tools for numerous biomedical applications (Whiteside, 2017). The key biomedical applications of exosomes are indicated in Figure 2.
Exosomes derived from stem cells contain growth factors and signaling molecules that can stimulate cell proliferation and tissue healing. Exosome-based therapies are being explored for conditions like heart disease and tissue injuries (Zhang and Cheng, 2023). It can be used in more multiple-system disease treatments such as follows:
Neurological disorders: Exosomes are involved in intercellular communication in the nervous system. They may play a role in the pathogenesis of neurodegenerative diseases such as Alzheimer’s and Parkinson’s. Studying exosomes in these contexts can provide insights into disease mechanisms and potential therapeutic interventions (Xiao et al., 2021). Recent studies show that exosomes from neural progenitor cells promote neuronal differentiation and neurogenesis via miR-21a (Ma et al., 2019). Similarly, exosomes from human induced pluripotent stem cell (hiPSC)-derived neurons enhance proliferation in human primary neural cultures in vitro. Injection of exosomes from rodent primary neural cultures into P4 mouse brains also increases neurogenesis in the dentate gyrus of the hippocampus (Sharma et al., 2019).
Cardiovascular health: Exosomes are being explored as diagnostic and therapeutic tools in cardiovascular diseases. They can carry molecules associated with heart conditions and may be used to monitor and treat heart diseases (Li N et al., 2023). Wang et al. report that exosomes from induced pluripotent stem cells (iPSCs) transfer cardioprotective miRNAs, including miR-21 and miR-210, to cardiomyocytes. Elevated miRNA levels in iPSC-exosomes contribute to protection against oxidative stress in H9C2 cells by inhibiting caspase 3/7 activation. These anti-apoptotic effects were validated in a mouse model of acute myocardial ischemia/reperfusion (Zhang et al., 2015).
Reproductive health: Exosomes play roles in reproductive biology, including sperm function and embryo development. They may have applications in fertility treatments and the study of reproductive disorders (Ranjbaran et al., 2019; Pu et al., 2022). Zhao et al. explored the impact of exosomal miR-323-3p from adipose mesenchymal stem cells (AMSCs) on cumulus cells (CCs) of polycystic ovary syndrome (PCOS) patients, finding that it inhibited apoptosis by targeting programmed cell death protein 4 (PDCD4) and alleviated PCOS (Zhao et al., 2019). Another study revealed that exosomes from the serum of PCOS patients significantly stimulated the migration and invasion of endometrial cancer cell lines. The study identified miR-27a-5p as highly induced in serum exosomes from PCOS patients and demonstrated its direct targeting of the tumor suppressor gene SMAD4 in the TGF-β signaling pathway (Che et al., 2020).
Wound healing: Exosomes have shown great promise in wound healing due to their ability to carry and deliver bioactive molecules, such as growth factors and microRNAs, to the damaged tissue. These exosomes can promote tissue regeneration, reduce inflammation, and accelerate the overall wound-healing process (Kudinov et al., 2021; Geng et al., 2022). A study engineered MSCs to produce exosomes enriched with long non-coding RNA H19, regulating the PI3K/AKT pathway and suppressing inflammation and apoptosis in a diabetic foot ulcer mouse model (Li B et al., 2020). Additionally, nanoparticles loaded into exosomes, specifically BMSCs-exosomes with magnetic Fe3O4 nanoparticles and increased exosome miR-1260a, were found to boost angiogenesis (Wu et al., 2021).
Exosomes derived from immune cells play a pivotal role in orchestrating immune responses within the body (Gangadaran et al., 2022b). These tiny vesicles serve as potent messengers of intercellular communication, carrying a cargo of bioactive molecules such as cytokines, chemokines, and antigens (Trams et al., 1981). When immune cells encounter pathogens or foreign invaders, they release exosomes that can activate or suppress immune reactions, depending on the context (Table 1). For instance, dendritic cell-derived exosomes can present antigens to T cells, initiating adaptive immune responses against infections or cancer (Shenoda and Ajit, 2016). Conversely, regulatory T cell-derived exosomes can exert immunosuppressive effects, modulating excessive inflammation and autoimmunity (Arenaccio et al., 2014). Numerous studies highlight the role of CD8+ T cell-derived exosomes in facilitating communication between immune cells and tumor cells, regulating tumor development. Fully activated CD8+ CTLs release exosomes that boost the activation of low-affinity CD8+ T cells, contributing to the tumor-killing process (Li et al., 2017; Wu S W et al., 2019). Understanding the intricate role of immune cell-derived exosomes is crucial for advancing our knowledge of immune regulation and developing novel therapeutic strategies for immune-related disorders.
Exosomes also have emerged as promising natural nanocarriers for drug delivery in recent years (Gangadaran et al., 2022a). First, their natural origin minimizes immunogenicity and toxicity concerns, making them well-tolerated in vivo. Second, exosomes are equipped with cell-targeting proteins and bioactive molecules on their surface, facilitating specific delivery to target cells or tissues (Gangadaran et al., 2022c). Furthermore, their stability in circulation and ability to traverse biological barriers, such as the blood-brain barrier, offer a versatile platform for encapsulating various therapeutic cargoes, including small molecules, nucleic acids, and proteins (Krishnan et al., 2022). Harnessing exosomes as nanocarriers holds great potential to enhance the precision and efficacy of drug delivery in diverse therapeutic applications.
Exosome cargo analysis can facilitate the discovery of disease-specific biomarkers. These biomarkers can be used for early disease detection, monitoring disease progression, and assessing treatment responses. Of note, the exosome is promising for the cancer diagnostics (Si et al., 2023). Exosomes derived from cancer cells contain specific biomarkers, such as mutant DNA, proteins, and microRNAs, that can serve as indicators of cancer presence and progression (Ji et al., 2013; Miaomiao et al., 2023). Liquid biopsies based on exosome cargo analysis offer non-invasive methods for early cancer detection and monitoring treatment responses (Feng et al., 2019). Moreover, exosome-based diagnostics and therapeutics represent a promising avenue in cancer therapy (Kalluri and LeBleu, 2020). Researchers are exploring the use of exosomes for targeted drug delivery. By engineering exosomes to carry therapeutic molecules, drugs can be directed specifically to cancer cells, reducing off-target effects and improving treatment efficacy (Barile and Vassalli, 2017; Chen H et al., 2021; Jin et al., 2021). Briefly, enhancing exosome targeting for drug delivery platforms involves selecting specific exosome donors or employing bioengineering techniques. This can be achieved by modifying the exosome surface with homing molecules through ligands, magnetic materials, charge affinity, and pH-responsive motifs (Butreddy et al., 2021). Ultimately, by loading the drug into these modified exosomes, targeted carriers can be created for specific cells or organs, leading to improved clinical treatment outcomes (He et al., 2022). The accumulation of drugs in target sites enhances the efficacy of exosomes while simultaneously reducing off-target effects.
Overall, the biomedical applications of exosomes are vast and continually expanding. Their unique properties as carriers of molecular information, combined with their potential for non-invasive sampling and targeted delivery, make exosomes a promising area of research and development in the quest to better understand, diagnose, and treat a wide range of diseases and medical conditions.
Exosomes play a crucial role in cancer progression and metastasis by facilitating the transfer of bioactive molecules between cancer cells and diverse cells in both local and distant microenvironments. This intercellular communication leads to alterations in various cellular and biological functions within the recipient cells (Jiang et al., 2021). Additionally, exosomes serve as indicators of the heterogeneity present in cancer tumors (Al-Nedawi et al., 2008). Distinct subpopulations of cancer cells within a single tumor release exosomes with unique molecular profiles (Maia et al., 2018). Recognizing and understanding this heterogeneity is essential as it can guide treatment decisions and potentially pave the way for personalized therapy strategies.
Exosomes have emerged as a promising Frontier in cancer research and diagnostics, driven by their distinctive cargo (Tai et al., 2018). These microscopic vesicles encapsulate a wealth of information derived from cancer cells, serving as invaluable reservoirs of diverse cancer biomarkers, including genetic mutations, oncogenic proteins, and a spectrum of cancer-specific molecules (Jiang et al., 2021; Liu et al., 2021). The encapsulation of such biomarkers within exosomes is of paramount significance, as they can serve as pivotal indicators of cancer initiation, progression, and response to therapeutic interventions (Table 2).
A particularly noteworthy attribute of exosome-based diagnostics lies in their non-invasive nature. The isolation and analysis of exosomes from easily accessible bodily fluids, such as blood or urine, present a transformative approach for clinicians and researchers (Wang et al., 2014; Sohn et al., 2015). This minimally invasive method not only facilitates early cancer detection but also enables the continuous monitoring of cancer dynamics over time (Thind and Wilson, 2016). The escalating comprehension of exosomes and their cargo augurs well for revolutionizing cancer diagnostics and tailoring personalized treatment strategies. Researchers and clinicians are actively harnessing the potential of exosomes to usher in novel horizons in the detection and management of cancer (Patel et al., 2019).
The retrieval and scrutiny of exosomes from common bodily fluids represent a paradigm shift in cancer diagnostics, underscoring its pivotal role in advancing healthcare. This innovative methodology assumes particular significance due to its capacity for early cancer detection. The identification of cancers in their early stages holds immense clinical importance, as prompt recognition often correlates with higher treatment success rates and substantially improved patient outcomes (Hoshino et al., 2015; Hinestrosa et al., 2022). The accessibility of bodily fluids such as blood, urine, and saliva for exosome retrieval streamlines the diagnostic process, augmenting its potential impact on healthcare. The utilization of exosomes for early cancer detection heralds a promising era in medicine—one with the potential to transform the oncological landscape by saving lives through early intervention and enhancing treatment outcomes (Kalluri and LeBleu, 2020; Yu et al., 2021). As ongoing research continues to unravel the intricacies of exosomes, their role in advancing cancer diagnostics and personalized medicine is poised to play an increasingly pivotal role in shaping the future of oncology.
The isolation and analysis of exosomes offer a dynamic window into the complex landscape of cancer, as they can be collected at various stages of disease progression (Khan et al., 2012). This versatility provides invaluable insights into the entire continuum of cancer development, from its initial onset to metastasis and response to treatment. By examining the molecular cargo of exosomes over time, clinicians gain a nuanced understanding of how cancers evolve, adapt, and potentially resist therapy (Tang et al., 2020). This real-time monitoring becomes a critical tool in tailoring treatment strategies for individual patients. For example, in the early stages, exosomes may contain biomarkers indicative of tumor initiation, offering an opportunity for timely intervention (Jalalian et al., 2019). As cancer progresses, exosomes can carry information about metastatic potential, aiding in the prediction of disease spread (Dai et al., 2020). Moreover, tracking changes in exosome content during treatment can guide clinicians in optimizing therapeutic regimens, making them more effective and personalized (Corradetti et al., 2021). Ultimately, the ability to harness exosomes as dynamic indicators of cancer progression has the potential to revolutionize cancer care, leading to more precise and successful treatment approaches.
Beyond their diagnostic potential, exosomes emerge as direct therapeutic targets in the fight against cancer (Zhou et al., 2021). Exosomes offer a highly efficient method for delivering small molecules, proteins, and RNAs to target cancer cells. Additionally, manipulating exosomes derived from cancer cells provides a novel approach to disrupting key processes in tumorigenesis (Scavo et al., 2020). By inhibiting the release or interfering with the function of these exosomes, it becomes possible to impede critical facets of cancer progression (Moitra et al., 2011). For example, interfering with exosome release from cancer cells may curtail their ability to communicate with neighboring cells and create a conducive environment for tumor growth (Wu M et al., 2019). Additionally, targeting the specific functions of cancer-derived exosomes can hinder metastasis, potentially preventing the spread of cancer to distant organs (Tai et al., 2018). Furthermore, disrupting the exosome-mediated transfer of molecules that confer resistance to treatment can enhance the effectiveness of therapies (Steinbichler et al., 2019; Zeng and Fu, 2020). Generally, the therapeutic targeting of exosomes holds great promise for developing innovative strategies to combat cancer, offering hope for more effective treatments and improved patient outcomes.
In conclusion, exosomes represent a promising avenue for cancer research and diagnosis. Their unique ability to carry and transfer cancer-specific information opens new possibilities for early detection, monitoring, and personalized treatment strategies. As researchers continue to unravel the complexities of exosome biology and their role in cancer, we can anticipate groundbreaking advancements in cancer diagnostics and therapies in the years to come.
While exosomes offer significant potential for drug application and delivery, they are not without their limitations, which need to be carefully considered in research and development. The key limitations of exosomes for drug application include:
The intricate nature of exosomes, characterized by their diverse molecular cargo, poses significant challenges in harnessing them for precise drug delivery applications (McAndrews and Kalluri, 2019). Achieving consistent and effective drug loading into exosomes is a formidable task due to its inherent complexity (Mathieu et al., 2019; Kalluri and LeBleu, 2020). Ensuring that the therapeutic cargo is properly encapsulated within exosomes can be challenging, as it demands meticulous control over the encapsulation process to maintain therapeutic efficacy.
Furthermore, while exosomes possess a natural propensity to target specific cells or tissues, engineering them for precise targeting of disease sites adds a layer of complexity (He et al., 2022). The intricacies of achieving this level of precision involve modifying exosomes to recognize and bind exclusively to the intended target cells while minimizing off-target effects (Sun et al., 2020). Striking this delicate balance between specificity and avoiding unintended consequences remains a significant challenge in the development of exosome-based drug delivery systems (Chen H et al., 2021). Despite these challenges, ongoing research and innovation in exosome engineering hold great promise for advancing the field of targeted therapeutics.
Exosomes, while offering immense potential as drug delivery vehicles, do have limitations that must be considered in their application (Lee et al., 2023; Sadeghi et al., 2023). Their finite cargo capacity is one such constraint, which may pose challenges for drugs requiring high payloads (Jayaseelan and Arumugam, 2022; Malekian et al., 2023). Larger therapeutic molecules or those with low solubility might not be efficiently encapsulated within the limited confines of exosomes. This limitation necessitates careful selection of drugs to ensure compatibility with exosome-based delivery systems (Zhang L et al., 2021; Wu et al., 2022). Another concern is the fragility of exosomes. They can be susceptible to degradation, particularly when exposed to extreme temperatures or mechanical stress (Chen H et al., 2023; Zhang M et al., 2023). This fragility underscores the importance of maintaining proper storage and transportation conditions to preserve their integrity and functionality (Sanz-Ros et al., 2022). Ensuring stability throughout these processes can be a significant logistical challenge, but it is crucial for the successful deployment of exosome-based therapeutics.
In addressing these limitations, ongoing research focuses on optimizing exosome-based drug delivery by exploring innovative loading techniques, enhancing cargo capacity, and developing improved storage and transportation protocols. Despite these challenges, the versatility and potential of exosomes as drug carriers make them an exciting avenue for the future of targeted and personalized medicine.
When administered in vivo, exosomes encounter a series of biological barriers (e.g., including the blood-brain barrier, blood–cerebrospinal fluid barrier, blood–retinal barrier, and gastrointestinal tract) that must be overcome to effectively reach their intended target sites (Elliott and He, 2021). These barriers encompass the complex milieu of bodily fluids and tissues, which can hinder the distribution and efficacy of exosomes. Their journey through the circulatory system, extracellular matrix, and cellular barriers necessitates a thorough understanding of their interactions within these environments (Lakhal and Wood, 2011; Das et al., 2019).
Moreover, the immunogenicity of exosomes remains a topic of ongoing research and scrutiny. While they are generally considered to have low immunogenic potential, there are still gaps in our understanding (Zhao et al., 2022). Concerns about potential immune reactions to exosomes used for drug delivery persist. Undesirable immune responses could not only reduce the therapeutic efficacy of the delivered cargo but also trigger adverse effects in the recipient (Gyorgy et al., 2015). Therefore, meticulous investigation into the immune aspects of exosome-based therapies is essential for their safe and effective implementation in clinical settings.
The large-scale production of exosomes with consistent quality presents significant challenges in terms of both technical feasibility and cost-effectiveness (Maumus et al., 2020). Establishing scalable manufacturing processes for exosome production continues to be a paramount concern within the field of exosome-based therapies. The complexity of isolating and purifying exosomes, coupled with the need for customization for specific therapeutic applications, can drive up production costs considerably (Sadeghi et al., 2023). The cost-intensive nature of exosome production has the potential to impact the affordability and accessibility of exosome-based treatments. As healthcare systems and patients alike seek cost-effective solutions, it becomes essential to develop more efficient and cost-efficient production methods (Ahn et al., 2022). This will not only make exosome therapies more financially accessible but also enable wider adoption within the medical community.
Recently, research endeavors have been dedicated to streamlining and optimizing exosome production processes, seeking ways to reduce costs without compromising quality, and ensuring that exosome-based treatments can reach a broader spectrum of patients in need. Achieving these goals will be pivotal in realizing the full therapeutic potential of exosomes.
Studying exosomes can lead to the identification of novel biomarkers and therapeutic targets, advancing our understanding of cancer biology (Eassa, 2023). Importantly, Exosomes offer several advantages for cancer early detection, making them a promising avenue for improving the accuracy and effectiveness of diagnostic methods. For example, Exosomes can be collected at multiple time points, enabling dynamic monitoring of disease progression and treatment response (Chanteloup et al., 2020). Changes in the molecular cargo of exosomes over time can guide treatment adjustments, providing a real-time view of the disease (Sharma et al., 2017; Armstrong et al., 2018). The key advantages of using exosomes for cancer early detection include:
Exosome-based assays represent a significant advancement in cancer diagnostics due to their exceptional specificity and sensitivity (Salciccia et al., 2023). These assays are adept at detecting cancer-specific molecules, which greatly reduces the likelihood of false positives. This precision not only minimizes unnecessary diagnostic procedures but also alleviates the anxiety often associated with false alarms, ensuring a more patient-centric approach to the healthcare (Logozzi et al., 2023). Logzzi et al. reported that specific prostate-specific antigen (PSA) exosomes efficiently differentiated between prostate cancer (PC) and non-PC patients (benign prostatic hyperplasia (BPH) and healthy controls), outperforming the traditional serum PSA test. IC-ELISA achieved 98.57% sensitivity and 80.28% specificity in PC vs. BPH discrimination. Combining IC-ELISA with NFSC increased sensitivity to 96% and specificity to 100% (Logozzi et al., 2019).
What sets exosome-based assays apart is their capacity to detect even trace amounts of cancer biomarkers, even when they are present in low concentrations (Saad et al., 2021). This heightened sensitivity becomes a potent tool in the early detection of cancer, potentially identifying the disease before clinical symptoms manifest (Hu et al., 2022). This early detection, because of exosome-based assays, substantially enhances the chances of successful treatment outcomes, ultimately improving patient prognosis.
Moreover, exosomes offer a more comprehensive representation of the entire tumor’s molecular profile compared to traditional biopsies, where only a small portion of the tumor is sampled (Yu et al., 2021). This advantage significantly reduces the potential for sampling bias and ensures that clinicians have a more accurate and holistic understanding of the disease (Li L et al., 2023). In essence, exosome-based assays usher in a new era of cancer diagnostics, characterized by enhanced accuracy, sensitivity, and patient-centered care.
Exosomes represent a groundbreaking avenue for non-invasive diagnostics as they can be conveniently isolated from readily accessible bodily fluids, including blood, urine, saliva, and cerebrospinal fluid (Lin et al., 2015; Yi et al., 2022). This non-invasive sampling method eliminates the need for painful and invasive procedures such as biopsies, which can be uncomfortable and entail inherent risks (Chen J et al., 2021). Consequently, exosome-based tests offer a patient-friendly approach, requiring nothing more than simple blood or urine collection (Liu et al., 2018). This simplicity in sampling not only enhances patient comfort but also encourages a broader spectrum of individuals to undergo regular screenings, contributing significantly to early cancer detection efforts.
Furthermore, the molecular cargo of exosomes can be meticulously analyzed to identify specific cancer types and discern their unique characteristics (Mitchell et al., 2022). This valuable information provides a foundation for the development of personalized treatment plans tailored to the patient’s specific cancer subtype (Srivastava et al., 2018). Recent studies demonstrated that exosomes from lung tumor cells reflected the genetic mutations of the parent cell lines, notably EGFR status (Thakur et al., 2014). In a separate in vitro study, exosomes from various cancer cell lines facilitated the transfer of activated EGFR to endothelial cells, triggering MAPK and AKT pathways (Al-Nedawi et al., 2009). Therefore, specific target methods can be developed in precise treatment strategies not only to improve therapeutic efficacy but also to minimize potential side effects, enhancing the overall quality of care and patient outcomes (Sancho-Albero et al., 2019; He et al., 2022). In summary, exosomes hold significant promise for cancer early detection due to their non-invasive nature, ability to detect cancer-specific biomarkers, high sensitivity, and potential for dynamic monitoring. Moreover, exosome-based diagnostics represent a transformative shift towards patient-friendly and personalized approaches to cancer detection and treatment.
In the pursuit of harnessing the potential of exosomes to improve efficiency and precise cancer detection, several up-to-date innovative strategies and cutting-edge technologies have been developed (Chen J et al., 2021; Cheng et al., 2022). These approaches have the power to transform cancer diagnostics, providing more precise, non-invasive, and early detection methods. The key strategies and technologies that are paving the way for the utilization of exosomes in cancer detection.
Liquid biopsies are a groundbreaking strategy in cancer detection, utilizing the non-invasive collection and analysis of exosomes from body fluids like blood, urine, and saliva (Shegekar et al., 2023). This technique is a significant evolution from traditional tissue biopsies, which are often invasive and not always suitable for all patients. Exosomes, when released by cancer cells into the bloodstream, contain critical biomarkers (mutated DNA, proteins, microRNAs) that offer real-time insights into tumor status, are more convenient and authentic than the circulating tumor DNA (ctDNA) and circulating tumor cells (CTCs) (Heitzer et al., 2019; Yu et al., 2022). For example, the abundance of exosomes, with concentrations around 10^9 particles per milliliter in biological fluids, facilitates the collection of these vesicles. In contrast, only a few circulating tumor cells (CTCs) are present in 1 mL of blood samples (Cai et al., 2015). On the other hand, due to their lipid bilayer composition, exosomes inherently possess stability, enabling them to circulate consistently under physiological conditions, even within the rigorous conditions of the tumor microenvironment. This intrinsic biological stability facilitates the prolonged preservation of samples for the isolation and detection of exosomes (Yu et al., 2021). Therefore, liquid biopsies on exosomes may facilitate early cancer detection, potentially before symptoms appear, and allow continuous monitoring of disease progression and treatment response. This method enhances early detection capabilities and provides a less invasive alternative to conventional biopsies.
Nanotechnology has been instrumental in developing exosome-based biosensors and platforms for the sensitive detection of cancer-associated biomarkers (Mukhtar et al., 2021). Nanoparticles and nanomaterials are used to isolate exosomes from biological samples accurately, ensuring precise detection of cancer-related exosomal cargo. These nano-based methods increase the sensitivity of assays, enabling the detection of minimal biomarker levels, thus improving the accuracy of cancer diagnoses and aiding in identifying specific cancer types and molecular signatures. For example, Patolsky et al. developed a nanotechnology-based method for DNA detection, which has the potential for cancer diagnosis. They achieved this by integrating biotin labels into DNA replicas. These replicas were attached to magnetic particles, forming a nanodevice. This innovative approach enhances the accuracy of DNA-based detection in cancer diagnostics (Patolsky et al., 2003). Similarly, the research team led by Xu made significant advancements in the field of nanoparticle technology for cancer diagnosis. They created shell-engineered nanoparticles (NPs) that were coated with silver (Ag) and gold (Au). This coating substantially improved the sensitivity of PCR-based DNA detection methods which can be used in identifying cancer (Zhao et al., 2014).
The vast and complex data from exosome profiling necessitate advanced analysis tools, where machine learning and bioinformatics play a crucial role (Chen J et al., 2023). Machine learning algorithms are adept at identifying patterns in large datasets, making them ideal for interpreting exosome profiles (Li J et al., 2023). Li et al. present a machine learning approach for non-invasive cancer diagnosis using exosome protein markers, achieving high accuracy in identifying cancer types with an advanced biomarker signature and sophisticated data models, marking a significant leap in early cancer detection methodologies (Li B et al., 2023). Wang et al. employed the Least Absolute Shrinkage and Selection Operator (LASSO) regression algorithm to construct a prognostic model based on differentially expressed genes (DEGs). The model’s predictive accuracy and sensitivity were validated through prognostic analysis and receiver operating characteristic curve analysis (Wang Z et al., 2023). These algorithms can differentiate between cancerous and non-cancerous samples, classify cancer subtypes, and predict treatment outcomes. Additionally, bioinformatics tools are essential for managing and interpreting the extensive data from exosome analysis, helping to derive meaningful insights from the complex molecular profiles of exosomes.
The integration of liquid biopsies, nanotechnology, machine learning, and bioinformatics represents a paradigm shift in cancer diagnostics. This convergence facilitates non-invasive, precise early cancer detection, and dynamic disease monitoring through exosome analysis. The precision of nanotechnology and the analytical power of machine learning and bioinformatics in interpreting complex data highlight the transformative potential of exosomes in cancer detection. As these technologies continue to evolve, exosome-based cancer detection is poised to become a fundamental aspect of early diagnosis and personalized cancer treatment, significantly impacting oncology by improving patient outcomes and expanding our understanding of cancer biology.
Exosomes hold immense potential for cancer detection and are more practicable for achieving compared to cancer treatment per the accessibility in the clinical applications (Figure 3). However, several critical challenges need to be confronted to fully harness their potential: A pressing concern is the absence of standardized protocols for exosome isolation, characterization, and analysis. The establishment of rigorous standards is imperative to ensure the reproducibility and reliability of exosome-based assays across various laboratories and therapeutic companies. Standardization efforts will bolster the credibility of exosome-based diagnostics. Moreover, the success of exosome-based assays hinges on achieving heightened specificity and sensitivity. The aim is to minimize false positives and false negatives, which can have significant clinical implications. This necessitates the development of more selective and reliable biomarkers, as well as advancements in detection technologies.
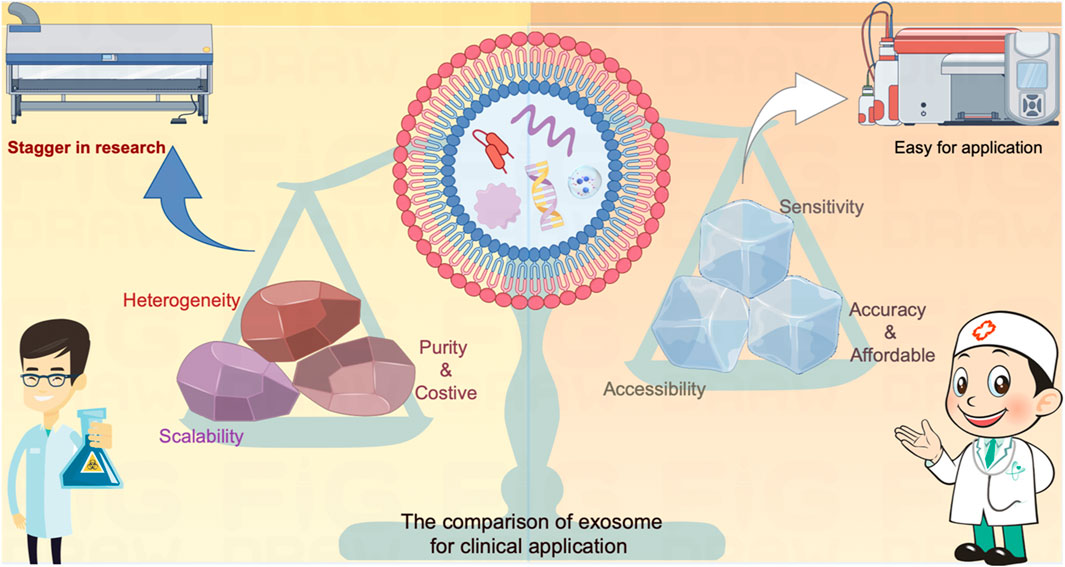
FIGURE 3. The comparison of exosome on diagnosis versus treatment for clinical application. The exosome serves as a carrier for various contents that actively influence cellular function or provide real-time insights into the developmental processes of target cells. These attributes have demonstrated significant value in the realm of cancer diagnosis and treatment. Nevertheless, the direct utilization of exosomes as mediators for cancer treatment faces inherent limitations due to contemporary technical challenges, encompassing issues related to heterogeneity, scalability, and purity. These constraints impede the seamless translation of exosome-based therapies from laboratory research to clinical applications. In contrast, leveraging exosomes as sensors offers a promising alternative. By repurposing them into highly sensitive, easily accessible, accurate, and cost-effective tools, these challenges can be transformed into opportunities for detecting cancers. The incorporation of state-of-the-art diagnostic equipment facilitates a more expeditious and practical application of exosomes in cancer diagnosis compared to treatment methodologies.
On the other hand, exosomes exhibit great promise in the research field which has a well-controlled environment, but their real-world utility as cancer biomarkers needs to be rigorously validated through large-scale clinical trials. These trials are essential to establish the relevance and efficacy of exosome-based diagnostics. Demonstrating their performance in diverse patient populations is a crucial step toward clinical application. In addition, the ethical dimensions of employing exosomes for cancer detection require rigid contemplation. Therefore, obtaining informed consent from patients, ensuring data privacy, and adhering to ethical guidelines are very important. Safeguarding patient rights and privacy is an ethical requirement in the development and application of exosome-based diagnostics for cancer.
In conclusion, exosomes represent a promising avenue for cancer detection because they are enriched with cancer-specific biomolecules, and offer non-invasive, sensitive, and specific avenues to identify cancer-related markers. As research continues to advance, exosome-based assays can hold the potential to revolutionize cancer diagnosis, facilitating earlier detection and improving patient survival and life quality. Nevertheless, addressing the challenges associated with standardization, specificity, sensitivity, clinical validation, and ethical considerations is significant to comprehensively realize the translational potential of exosomes for cancer detection in clinical practice.
ZW: Writing–original draft. QW: Visualization, Writing–review and editing. FQ: Writing–review and editing. JC: Conceptualization, Project administration, Resources, Supervision, Writing–review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work is supported by the National Natural Science Foundation of China (No. 8197211).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abd Elmageed, Z. Y., Yang, Y., Thomas, R., Ranjan, M., Mondal, D., Moroz, K., et al. (2014). Neoplastic reprogramming of patient-derived adipose stem cells by prostate cancer cell-associated exosomes. Stem Cells 32 (4), 983–997. doi:10.1002/stem.1619
Agnoletto, C., Pignochino, Y., Caruso, C., and Garofalo, C. (2023). Exosome-based liquid biopsy approaches in bone and soft tissue sarcomas: review of the literature, prospectives, and hopes for clinical application. Int. J. Mol. Sci. 24 (6), 5159. doi:10.3390/ijms24065159
Ahn, S. H., Ryu, S. W., Choi, H., You, S., Park, J., and Choi, C. (2022). Manufacturing therapeutic exosomes: from bench to industry. Mol. Cells 45 (5), 284–290. doi:10.14348/molcells.2022.2033
Aleksandrowicz, K., Hempel, D., Politynska, B., Wojtukiewicz, A. M., Honn, K. V., Tang, D. G., et al. (2023). The complex role of thrombin in cancer and metastasis: focus on interactions with the immune system. Semin. Thromb. Hemost. 2023, 1776875. doi:10.1055/s-0043-1776875
Ali, S. A., Huang, M. B., Campbell, P. E., Roth, W. W., Campbell, T., Khan, M., et al. (2010). Genetic characterization of HIV type 1 Nef-induced vesicle secretion. AIDS Res. Hum. Retroviruses 26 (2), 173–192. doi:10.1089/aid.2009.0068
Al-Nedawi, K., Meehan, B., Kerbel, R. S., Allison, A. C., and Rak, J. (2009). Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc. Natl. Acad. Sci. U. S. A. 106 (10), 3794–3799. doi:10.1073/pnas.0804543106
Al-Nedawi, K., Meehan, B., Micallef, J., Lhotak, V., May, L., Guha, A., et al. (2008). Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 10 (5), 619–624. doi:10.1038/ncb1725
Alonso-Curbelo, D., Riveiro-Falkenbach, E., Perez-Guijarro, E., Cifdaloz, M., Karras, P., Osterloh, L., et al. (2014). RAB7 controls melanoma progression by exploiting a lineage-specific wiring of the endolysosomal pathway. Cancer Cell 26 (1), 61–76. doi:10.1016/j.ccr.2014.04.030
Altintas, O., and Saylan, Y. (2023). Exploring the versatility of exosomes: a review on isolation, characterization, detection methods, and diverse applications. Anal. Chem. 95 (44), 16029–16048. doi:10.1021/acs.analchem.3c02224
Amini, A., Verma, V., Simone, C. B., Chetty, I. J., Chun, S. G., Donington, J., et al. (2022). American radium society appropriate use criteria for radiation therapy in oligometastatic or oligoprogressive non-small cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 112 (2), 361–375. doi:10.1016/j.ijrobp.2021.09.022
Anel, A., Gallego-Lleyda, A., de Miguel, D., Naval, J., and Martinez-Lostao, L. (2019). Role of exosomes in the regulation of T-cell mediated immune responses and in autoimmune disease. Cells 8 (2), 154. doi:10.3390/cells8020154
Anfossi, S., Giordano, A., Gao, H., Cohen, E. N., Tin, S., Wu, Q., et al. (2014). High serum miR-19a levels are associated with inflammatory breast cancer and are predictive of favorable clinical outcome in patients with metastatic HER2+ inflammatory breast cancer. PLoS One 9 (1), e83113. doi:10.1371/journal.pone.0083113
Arenaccio, C., Chiozzini, C., Columba-Cabezas, S., Manfredi, F., Affabris, E., Baur, A., et al. (2014). Exosomes from human immunodeficiency virus type 1 (HIV-1)-infected cells license quiescent CD4+ T lymphocytes to replicate HIV-1 through a Nef- and ADAM17-dependent mechanism. J. Virol. 88 (19), 11529–11539. doi:10.1128/JVI.01712-14
Armstrong, E. A., Beal, E. W., Chakedis, J., Paredes, A. Z., Moris, D., Pawlik, T. M., et al. (2018). Exosomes in pancreatic cancer: from early detection to treatment. J. Gastrointest. Surg. 22 (4), 737–750. doi:10.1007/s11605-018-3693-1
Barile, L., and Vassalli, G. (2017). Exosomes: therapy delivery tools and biomarkers of diseases. Pharmacol. Ther. 174, 63–78. doi:10.1016/j.pharmthera.2017.02.020
Bassani, S., Lee, Y. K., Campagnari, V., Eccher, A., Monzani, D., Nocini, R., et al. (2023). From hype to reality: a narrative review on the promising role of artificial intelligence in larynx cancer detection and transoral microsurgery. Crit. Rev. Oncog. 28 (3), 21–24. doi:10.1615/CritRevOncog.2023049134
Bernard, M. A., Zhao, H., Yue, S. C., Anandaiah, A., Koziel, H., and Tachado, S. D. (2014). Novel HIV-1 miRNAs stimulate TNFα release in human macrophages via TLR8 signaling pathway. PLoS One 9 (9), e106006. doi:10.1371/journal.pone.0106006
Bozdag, G., Mumusoglu, S., Zengin, D., Karabulut, E., and Yildiz, B. O. (2016). The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum. Reprod. 31 (12), 2841–2855. doi:10.1093/humrep/dew218
Butreddy, A., Kommineni, N., and Dudhipala, N. (2021). Exosomes as naturally occurring vehicles for delivery of biopharmaceuticals: insights from drug delivery to clinical perspectives. Nanomater. (Basel) 11 (6), 1481. doi:10.3390/nano11061481
Cai, X., Janku, F., Zhan, Q., and Fan, J. B. (2015). Accessing genetic information with liquid biopsies. Trends Genet. 31 (10), 564–575. doi:10.1016/j.tig.2015.06.001
Cambier, L. D., Stachelek, K., Triska, M., Jubran, R., Huang, M. Y., Li, W. Y., et al. (2021). Extracellular vesicle-associated repetitive element DNAs as candidate osteosarcoma biomarkers. Sci. Rep. 11 (1), 94. doi:10.1038/s41598-020-77398-z
Campanella, C., Rappa, F., Sciumè, C., Marino Gammazza, A., Barone, R., Bucchieri, F., et al. (2015). Heat shock protein 60 levels in tissue and circulating exosomes in human large bowel cancer before and after ablative surgery. Cancer 121 (18), 3230–3239. doi:10.1002/cncr.29499
Chanteloup, G., Cordonnier, M., Isambert, N., Bertaut, A., Hervieu, A., Hennequin, A., et al. (2020). Monitoring HSP70 exosomes in cancer patients' follow up: a clinical prospective pilot study. J. Extracell. Vesicles 9 (1), 1766192. doi:10.1080/20013078.2020.1766192
Che, X., Jian, F., Chen, C., Liu, C., Liu, G., and Feng, W. (2020). PCOS serum-derived exosomal miR-27a-5p stimulates endometrial cancer cells migration and invasion. J. Mol. Endocrinol. 64 (1), 1–12. doi:10.1530/JME-19-0159
Chen, B., Li, M. Y., Guo, Y., Zhao, X., and Lim, H. C. (2017). Mast cell-derived exosomes at the stimulated acupoints activating the neuro-immune regulation. Chin. J. Integr. Med. 23 (11), 878–880. doi:10.1007/s11655-016-2269-8
Chen, H., Wang, L., Zeng, X., Schwarz, H., Nanda, H. S., Peng, X., et al. (2021). Exosomes, a new star for targeted delivery. Front. Cell Dev. Biol. 9, 751079. doi:10.3389/fcell.2021.751079
Chen, H., Yao, H., Chi, J., Li, C., Liu, Y., Yang, J., et al. (2023). Engineered exosomes as drug and RNA co-delivery system: new hope for enhanced therapeutics? Front. Bioeng. Biotechnol. 11, 1254356. doi:10.3389/fbioe.2023.1254356
Chen, J., Dong, H., Fu, R., Liu, X., Xue, F., Liu, W., et al. (2023). Machine learning analyses constructed a novel model to predict recurrent thrombosis in adults with essential thrombocythemia. J. Thromb. Thrombolysis 56 (2), 291–300. doi:10.1007/s11239-023-02833-7
Chen, J., Li, P., Zhang, T., Xu, Z., Huang, X., Wang, R., et al. (2021). Review on strategies and technologies for exosome isolation and purification. Front. Bioeng. Biotechnol. 9, 811971. doi:10.3389/fbioe.2021.811971
Cheng, H., Yang, Q., Wang, R., Luo, R., Zhu, S., Li, M., et al. (2022). Emerging advances of detection strategies for tumor-derived exosomes. Int. J. Mol. Sci. 23 (2), 868. doi:10.3390/ijms23020868
Chung, Y. C., Wei, W. C., Huang, S. H., Shih, C. M., Hsu, C. P., Chang, K. J., et al. (2014). Rab11 regulates E-cadherin expression and induces cell transformation in colorectal carcinoma. BMC Cancer 14, 587. doi:10.1186/1471-2407-14-587
Corradetti, B., Gonzalez, D., Mendes Pinto, I., and Conlan, R. S. (2021). Editorial: exosomes as therapeutic systems. Front. Cell Dev. Biol. 9, 714743. doi:10.3389/fcell.2021.714743
Dai, J., Su, Y., Zhong, S., Cong, L., Liu, B., Yang, J., et al. (2020). Exosomes: key players in cancer and potential therapeutic strategy. Signal Transduct. Target Ther. 5 (1), 145. doi:10.1038/s41392-020-00261-0
Das, C. K., Jena, B. C., Banerjee, I., Das, S., Parekh, A., Bhutia, S. K., et al. (2019). Exosome as a novel shuttle for delivery of therapeutics across biological barriers. Mol. Pharm. 16 (1), 24–40. doi:10.1021/acs.molpharmaceut.8b00901
de Carvalho, J. V., de Castro, R. O., da Silva, E. Z. M., Silveira, P. P., da Silva-Januario, M. E., Arruda, E., et al. (2014). Nef neutralizes the ability of exosomes from CD4+ T cells to act as decoys during HIV-1 infectionT Cells to Act as Decoys during HIV-1 Infection. PLoS One 9 (11), e113691. doi:10.1371/journal.pone.0113691
De La Pena, H., Madrigal, J. A., Rusakiewicz, S., Bencsik, M., Cave, G. W., Selman, A., et al. (2009). Artificial exosomes as tools for basic and clinical immunology. J. Immunol. Methods 344 (2), 121–132. doi:10.1016/j.jim.2009.03.011
Eassa, H. A. (2023). Exosomes: double-edged weapon in cancer therapy. Curr. Pharm. Des. 29 (30), 2366–2368. doi:10.2174/0113816128272352231013074525
Elliott, R. O., and He, M. (2021). Unlocking the power of exosomes for crossing biological barriers in drug delivery. Pharmaceutics 13 (1), 122. doi:10.3390/pharmaceutics13010122
Fabbri, M., Paone, A., Calore, F., Galli, R., Gaudio, E., Santhanam, R., et al. (2012). MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc. Natl. Acad. Sci. U. S. A. 109 (31), E2110–E2116. doi:10.1073/pnas.1209414109
Feng, C., She, J., Chen, X., Zhang, Q., Zhang, X., Wang, Y., et al. (2019). Exosomal miR-196a-1 promotes gastric cancer cell invasion and metastasis by targeting SFRP1. Nanomedicine (Lond) 14 (19), 2579–2593. doi:10.2217/nnm-2019-0053
Feng, K., Xing, Z., Dai, Q., Cheng, H., and Wang, X. (2023). Role of aggressive locoregional surgery in treatment strategies for ipsilateral supraclavicular lymph node metastasis of breast cancer: a real-world cohort study. Front. Mol. Biosci. 10, 1248410. doi:10.3389/fmolb.2023.1248410
Gangadaran, P., Gunassekaran, G. R., Rajendran, R. L., Oh, J. M., Vadevoo, S. M. P., Lee, H. W., et al. (2022a). Interleukin-4 receptor targeting peptide decorated extracellular vesicles as a platform for in vivo drug delivery to thyroid cancer. Biomedicines 10 (8), 1978. doi:10.3390/biomedicines10081978
Gangadaran, P., Madhyastha, H., Madhyastha, R., Rajendran, R. L., Nakajima, Y., Watanabe, N., et al. (2022b). The emerging role of exosomes in innate immunity, diagnosis and therapy. Front. Immunol. 13, 1085057. doi:10.3389/fimmu.2022.1085057
Gangadaran, P., Rajendran, R. L., Kwack, M. H., Jeyaraman, M., Hong, C. M., Sung, Y. K., et al. (2022c). Application of cell-derived extracellular vesicles and engineered nanovesicles for hair growth: from mechanisms to therapeutics. Front. Cell Dev. Biol. 10, 963278. doi:10.3389/fcell.2022.963278
Gao, X., Zhang, F., Zhou, Q., Xu, H., and Bian, J. (2023). Metastasis, characteristic, and treatment of breast cancer in young women and older women: a study from the Surveillance, Epidemiology, and End Results registration database. PLoS One 18 (11), e0293830. doi:10.1371/journal.pone.0293830
García-Romero, N., Carrión-Navarro, J., Esteban-Rubio, S., Lázaro-Ibáñez, E., Peris-Celda, M., Alonso, M. M., et al. (2017). DNA sequences within glioma-derived extracellular vesicles can cross the intact blood-brain barrier and be detected in peripheral blood of patients. Oncotarget 8 (1), 1416–1428. doi:10.18632/oncotarget.13635
Geng, X., Qi, Y., Liu, X., Shi, Y., Li, H., and Zhao, L. (2022). A multifunctional antibacterial and self-healing hydrogel laden with bone marrow mesenchymal stem cell-derived exosomes for accelerating diabetic wound healing. Biomater. Adv. 133, 112613. doi:10.1016/j.msec.2021.112613
Gera, K., Kahramangil, D., Fenton, G. A., Martir, D., Rodriguez, D. N., Ijaz, Z., et al. (2023). Prognosis and treatment outcomes of bone metastasis in gallbladder adenocarcinoma: a SEER-based study. Cancers (Basel) 15 (20), 5055. doi:10.3390/cancers15205055
Gobbo, J., Marcion, G., Cordonnier, M., Dias, A. M. M., Pernet, N., Hammann, A., et al. (2016). Restoring anticancer immune response by targeting tumor-derived exosomes with a HSP70 peptide aptamer. J. Natl. Cancer Inst. 108 (3), djv330. doi:10.1093/jnci/djv330
Greening, D. W., Gopal, S. K., Xu, R., Simpson, R. J., and Chen, W. (2015). Exosomes and their roles in immune regulation and cancer. Semin. Cell Dev. Biol. 40, 72–81. doi:10.1016/j.semcdb.2015.02.009
Gudbergsson, J. M., and Johnsen, K. B. (2019). Exosomes and autophagy: rekindling the vesicular waste hypothesis. J. Cell Commun. Signal 13 (4), 443–450. doi:10.1007/s12079-019-00524-8
Gyorgy, B., Hung, M. E., Breakefield, X. O., and Leonard, J. N. (2015). Therapeutic applications of extracellular vesicles: clinical promise and open questions. Annu. Rev. Pharmacol. Toxicol. 55, 439–464. doi:10.1146/annurev-pharmtox-010814-124630
Hasegawa, H., and Matsumoto, T. (2018). Mechanisms of tolerance induction by dendritic cells in vivo. Front. Immunol. 9, 350. doi:10.3389/fimmu.2018.00350
Hayashi, K., Kitago, M., Abe, Y., Yagi, H., Hasegawa, Y., Hori, S., et al. (2023). Long-term survival after surgical resection for bone metastasis from pancreatic cancer: a case report. Med. Baltim. 102 (46), e35856. doi:10.1097/MD.0000000000035856
He, J., Ren, W., Wang, W., Han, W., Jiang, L., Zhang, D., et al. (2022). Exosomal targeting and its potential clinical application. Drug Deliv. Transl. Res. 12 (10), 2385–2402. doi:10.1007/s13346-021-01087-1
He, N. H., Liu, M., Hsu, J., Xue, Y. H., Chou, S., Burlingame, A., et al. (2010). HIV-1 tat and host AFF4 recruit two transcription elongation factors into a bifunctional complex for coordinated activation of HIV-1 transcription. Mol. Cell 38 (3), 428–438. doi:10.1016/j.molcel.2010.04.013
Heitzer, E., Haque, I. S., Roberts, C. E. S., and Speicher, M. R. (2019). Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat. Rev. Genet. 20 (2), 71–88. doi:10.1038/s41576-018-0071-5
Hinestrosa, J. P., Kurzrock, R., Lewis, J. M., Schork, N. J., Schroeder, G., Kamat, A. M., et al. (2022). Early-stage multi-cancer detection using an extracellular vesicle protein-based blood test. Commun. Med. (Lond) 2, 29. doi:10.1038/s43856-022-00088-6
Holladay, L., Luu, J., Balendra, V., and Kmetz, K. (2023). Current and potential treatment of colorectal cancer metastasis to bone. Cancer Treat. Res. Commun. 37, 100763. doi:10.1016/j.ctarc.2023.100763
Hoshino, A., Costa-Silva, B., Shen, T. L., Rodrigues, G., Hashimoto, A., Tesic Mark, M., et al. (2015). Tumour exosome integrins determine organotropic metastasis. Nature 527 (7578), 329–335. doi:10.1038/nature15756
Hsu, D. H., Paz, P., Villaflor, G., Rivas, A., Mehta-Damani, A., Angevin, E., et al. (2003). Exosomes as a tumor vaccine: enhancing potency through direct loading of antigenic peptides. J. Immunother. 26 (5), 440–450. doi:10.1097/00002371-200309000-00007
Hu, C., Jiang, W., Lv, M., Fan, S., Lu, Y., Wu, Q., et al. (2022). Potentiality of exosomal proteins as novel cancer biomarkers for liquid biopsy. Front. Immunol. 13, 792046. doi:10.3389/fimmu.2022.792046
Hu, T., Chang, S., Qi, F., Zhang, Z., Chen, J., Jiang, L., et al. (2023). Neural grafts containing exosomes derived from Schwann cell-like cells promote peripheral nerve regeneration in rats. Burns Trauma 11, tkad013. doi:10.1093/burnst/tkad013
Hu, Z., Yang, S., Xu, Z., Zhang, X., Wang, H., Fan, G., et al. (2023). Prevalence and risk factors of bone metastasis and the development of bone metastatic prognostic classification system: a pan-cancer population study. Aging (Albany NY) 15, 13134–13149. doi:10.18632/aging.205224
Hyun, K. A., Gwak, H., Lee, J., Kwak, B., and Jung, H. I. (2018). Salivary exosome and cell-free DNA for cancer detection. Micromachines (Basel) 9 (7), 340. doi:10.3390/mi9070340
Jalalian, S. H., Ramezani, M., Jalalian, S. A., Abnous, K., and Taghdisi, S. M. (2019). Exosomes, new biomarkers in early cancer detection. Anal. Biochem. 571, 1–13. doi:10.1016/j.ab.2019.02.013
Jayaseelan, V. P., and Arumugam, P. (2022). Exosome engineering: a promising step toward cancer therapeutics. Epigenomics 14 (9), 503–506. doi:10.2217/epi-2021-0474
Ji, H., Greening, D. W., Barnes, T. W., Lim, J. W., Tauro, B. J., Rai, A., et al. (2013). Proteome profiling of exosomes derived from human primary and metastatic colorectal cancer cells reveal differential expression of key metastatic factors and signal transduction components. Proteomics 13 (10-11), 1672–1686. doi:10.1002/pmic.201200562
Jiang, C., Zhang, N., Hu, X., and Wang, H. (2021). Tumor-associated exosomes promote lung cancer metastasis through multiple mechanisms. Mol. Cancer 20 (1), 117. doi:10.1186/s12943-021-01411-w
Jin, C., Wu, P., Li, L., Xu, W., and Qian, H. (2021). Exosomes: emerging therapy delivery tools and biomarkers for kidney diseases. Stem Cells Int. 2021, 7844455. doi:10.1155/2021/7844455
Kakiuchi, Y., Kuroda, S., Kanaya, N., Kagawa, S., Tazawa, H., and Fujiwara, T. (2023). Exosomes as a drug delivery tool for cancer therapy: a new era for existing drugs and oncolytic viruses. Expert Opin. Ther. Targets 27 (9), 807–816. doi:10.1080/14728222.2023.2259102
Kalluri, R., and LeBleu, V. S. (2020). The biology, function, and biomedical applications of exosomes. Science 367 (6478), eaau6977. doi:10.1126/science.aau6977
Kaminski, V. L., Ellwanger, J. H., and Chies, J. A. B. (2019). Extracellular vesicles in host-pathogen interactions and immune regulation - exosomes as emerging actors in the immunological theater of pregnancy. Heliyon 5 (8), e02355. doi:10.1016/j.heliyon.2019.e02355
Kang, C., He, H., Liu, P., Liu, Y., Li, X., Zhang, J., et al. (2023). Role of dendritic cell-derived exosomes in allergic rhinitis (Review). Int. J. Mol. Med. 52 (6), 117. doi:10.3892/ijmm.2023.5320
Kawada, T., Shim, S. R., Quhal, F., Rajwa, P., Pradere, B., Yanagisawa, T., et al. (2023). Diagnostic accuracy of liquid biomarkers for clinically significant prostate cancer detection: a systematic review and diagnostic meta-analysis of multiple thresholds. Eur. Urol. Oncol. 2023, 29. doi:10.1016/j.euo.2023.10.029
Keseru, J. S., Soltesz, B., Lukacs, J., Marton, E., Szilagyi-Bonizs, M., Penyige, A., et al. (2019). Detection of cell-free, exosomal and whole blood mitochondrial DNA copy number in plasma or whole blood of patients with serous epithelial ovarian cancer. J. Biotechnol. 298, 76–81. doi:10.1016/j.jbiotec.2019.04.015
Khan, S., Jutzy, J. M., Valenzuela, M. M., Turay, D., Aspe, J. R., Ashok, A., et al. (2012). Plasma-derived exosomal survivin, a plausible biomarker for early detection of prostate cancer. PLoS One 7 (10), e46737. doi:10.1371/journal.pone.0046737
Krishnan, A., Gangadaran, P., Chavda, V. P., Jogalekar, M. P., Muthusamy, R., Valu, D., et al. (2022). Convalescent serum-derived exosomes: attractive niche as COVID-19 diagnostic tool and vehicle for mRNA delivery. Exp. Biol. Med. (Maywood) 247 (14), 1244–1252. doi:10.1177/15353702221092984
Kudinov, V. A., Artyushev, R. I., Zurina, I. M., Lapshin, R. D., Snopova, L. B., Mukhina, I. V., et al. (2021). Antimicrobial and regenerative effects of placental multipotent mesenchymal stromal cell secretome-based chitosan gel on infected burns in rats. Pharm. (Basel) 14 (12), 1263. doi:10.3390/ph14121263
Kumata, Y., Iinuma, H., Suzuki, Y., Tsukahara, D., Midorikawa, H., Igarashi, Y., et al. (2018). Exosome-encapsulated microRNA-23b as a minimally invasive liquid biomarker for the prediction of recurrence and prognosis of gastric cancer patients in each tumor stage. Oncol. Rep. 40 (1), 319–330. doi:10.3892/or.2018.6418
Kurakevich, E., Hennet, T., Hausmann, M., Rogler, G., and Borsig, L. (2013). Milk oligosaccharide sialyl(α2,3)lactose activates intestinal CD11c+ cells through TLR4. Proc. Natl. Acad. Sci. U. S. A. 110 (43), 17444–17449. doi:10.1073/pnas.1306322110
Lai, Z., Liang, J., Zhang, J., Mao, Y., Zheng, X., Shen, X., et al. (2023). Exosomes as a delivery tool of exercise-induced beneficial factors for the prevention and treatment of cardiovascular disease: a systematic review and meta-analysis. Front. Physiol. 14, 1190095. doi:10.3389/fphys.2023.1190095
Lakhal, S., and Wood, M. J. (2011). Exosome nanotechnology: an emerging paradigm shift in drug delivery: exploitation of exosome nanovesicles for systemic in vivo delivery of RNAi heralds new horizons for drug delivery across biological barriers. Bioessays 33 (10), 737–741. doi:10.1002/bies.201100076
Lee, C. S., Fan, J., Hwang, H. S., Kim, S., Chen, C., Kang, M., et al. (2023). Bone-targeting exosome mimetics engineered by bioorthogonal surface functionalization for bone tissue engineering. Nano Lett. 23 (4), 1202–1210. doi:10.1021/acs.nanolett.2c04159
Lema, D. A., and Burlingham, W. J. (2019). Role of exosomes in tumour and transplant immune regulation. Scand. J. Immunol. 90 (5), e12807. doi:10.1111/sji.12807
Lenassi, M., Cagney, G., Liao, M. F., Vaupotic, T., Bartholomeeusen, K., Cheng, Y. F., et al. (2010). HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cellsT Cells. Traffic 11 (1), 110–122. doi:10.1111/j.1600-0854.2009.01006.x
Li, B., Kugeratski, F. G., and Kalluri, R. (2023). A novel machine learning algorithm selects proteome signature to specifically identify cancer exosomes. bioRxiv. Available at: https://doi.org/10.1101/2023.07.18.549557.
Li, B., Luan, S., Chen, J., Zhou, Y., Wang, T., Li, Z., et al. (2020). The MSC-derived exosomal lncRNA H19 promotes wound healing in diabetic foot ulcers by upregulating PTEN via MicroRNA-152-3p. Mol. Ther. Nucleic Acids 19, 814–826. doi:10.1016/j.omtn.2019.11.034
Li, G., Tang, W., and Yang, F. (2020). Cancer liquid biopsy using integrated microfluidic exosome analysis platforms. Biotechnol. J. 15 (5), e1900225. doi:10.1002/biot.201900225
Li, J., Liang, Y., Zhao, X., and Wu, C. (2023). Integrating machine learning algorithms to systematically assess reactive oxygen species levels to aid prognosis and novel treatments for triple -negative breast cancer patients. Front. Immunol. 14, 1196054. doi:10.3389/fimmu.2023.1196054
Li, L., Jay, S. M., Wang, Y., Wu, S. W., and Xiao, Z. (2017). IL-12 stimulates CTLs to secrete exosomes capable of activating bystander CD8(+) T cells. Sci. Rep. 7 (1), 13365. doi:10.1038/s41598-017-14000-z
Li, L., Zhang, L., Montgomery, K. C., Jiang, L., Lyon, C. J., and Hu, T. Y. (2023). Advanced technologies for molecular diagnosis of cancer: state of pre-clinical tumor-derived exosome liquid biopsies. Mater Today Bio 18, 100538. doi:10.1016/j.mtbio.2022.100538
Li, M., and Zheng, W. (2023). Metastasis of endometrial adenocarcinoma masquerading as a primary rectal cancer: a rare case report with literature review. Med. Baltim. 102 (46), e36170. doi:10.1097/MD.0000000000036170
Li, Q., Wang, H., Peng, H., Huyan, T., and Cacalano, N. A. (2019). Exosomes: versatile nano mediators of immune regulation. Cancers (Basel) 11 (10), 1557. doi:10.3390/cancers11101557
Li, W., Hu, Y., Jiang, T., Han, Y., Han, G., Chen, J., et al. (2014). Rab27A regulates exosome secretion from lung adenocarcinoma cells A549: involvement of EPI64. APMIS 122 (11), 1080–1087. doi:10.1111/apm.12261
Li, X., Liu, L., and Chai, J. (2015). Progress of mesenchymal stem cell-derived exosomes in tissue repair. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 29 (2), 234–238.
Lin, B., Lei, Y., Wang, J., Zhu, L., Wu, Y., Zhang, H., et al. (2021). Microfluidic-based exosome analysis for liquid biopsy. Small Methods 5 (3), e2001131. doi:10.1002/smtd.202001131
Lin, J., Li, J., Huang, B., Liu, J., Chen, X., Chen, X. M., et al. (2015). Exosomes: novel biomarkers for clinical diagnosis. ScientificWorldJournal 2015, 657086. doi:10.1155/2015/657086
Li N, N., Zhang, T., Zhu, L., Sun, L., Shao, G., and Gao, J. (2023). Recent advances of using exosomes as diagnostic markers and targeting carriers for cardiovascular disease. Mol. Pharm. 20 (9), 4354–4372. doi:10.1021/acs.molpharmaceut.3c00268
Liu, C., Yang, Y., and Wu, Y. (2018). Recent advances in exosomal protein detection via liquid biopsy biosensors for cancer screening, diagnosis, and prognosis. AAPS J. 20 (2), 41. doi:10.1208/s12248-018-0201-1
Liu, X., Wang, J., Wang, P., Zhong, L., Wang, S., Feng, Q., et al. (2022). Hypoxia-pretreated mesenchymal stem cell-derived exosomes-loaded low-temperature extrusion 3D-printed implants for neural regeneration after traumatic brain injury in canines. Front. Bioeng. Biotechnol. 10, 1025138. doi:10.3389/fbioe.2022.1025138
Liu, X. Y., Feng, Y. H., Feng, Q. B., Zhang, J. Y., Zhong, L., Liu, P., et al. (2023). Low-temperature 3D-printed collagen/chitosan scaffolds loaded with exosomes derived from neural stem cells pretreated with insulin growth factor-1 enhance neural regeneration after traumatic brain injury. Neural Regen. Res. 18 (9), 1990–1998. doi:10.4103/1673-5374.366497
Liu, Y., Wen, J., and Huang, W. (2021). Exosomes in nasopharyngeal carcinoma. Clin. Chim. Acta 523, 355–364. doi:10.1016/j.cca.2021.10.013
Logozzi, M., Angelini, D. F., Giuliani, A., Mizzoni, D., Di Raimo, R., Maggi, M., et al. (2019). Increased plasmatic levels of PSA-expressing exosomes distinguish prostate cancer patients from benign prostatic hyperplasia: a prospective study. Cancers (Basel) 11 (10), 1449. doi:10.3390/cancers11101449
Logozzi, M., Orefice, N. S., Di Raimo, R., Mizzoni, D., and Fais, S. (2023). The importance of detecting, quantifying, and characterizing exosomes as a new diagnostic/prognostic approach for tumor patients. Cancers (Basel) 15 (11), 2878. doi:10.3390/cancers15112878
Loscocco, F., Visani, G., Galimberti, S., Curti, A., and Isidori, A. (2019). BCR-ABL independent mechanisms of resistance in chronic myeloid leukemia. Front. Oncol. 9, 939. doi:10.3389/fonc.2019.00939
Lu, J., Zhang, Y., Yang, X., and Zhao, H. (2023). Harnessing exosomes as cutting-edge drug delivery systems for revolutionary osteoarthritis therapy. Biomed. Pharmacother. 165, 115135. doi:10.1016/j.biopha.2023.115135
Luga, V., Zhang, L., Viloria-Petit, A. M., Ogunjimi, A. A., Inanlou, M. R., Chiu, E., et al. (2012). Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell 151 (7), 1542–1556. doi:10.1016/j.cell.2012.11.024
Luo, M., Luan, X., Jiang, G., Yang, L., Yan, K., Li, S., et al. (2023). The dual effects of exosomes on glioma: a comprehensive review. J. Cancer 14 (14), 2707–2719. doi:10.7150/jca.86996
Luo, X., An, M., Cuneo, K. C., Lubman, D. M., and Li, L. (2018). High-performance chemical isotope labeling liquid chromatography mass spectrometry for exosome metabolomics. Anal. Chem. 90 (14), 8314–8319. doi:10.1021/acs.analchem.8b01726
Ma, Y., Li, C., Huang, Y., Wang, Y., Xia, X., and Zheng, J. C. (2019). Exosomes released from neural progenitor cells and induced neural progenitor cells regulate neurogenesis through miR-21a. Cell Commun. Signal 17 (1), 96. doi:10.1186/s12964-019-0418-3
Mahmoud, A. M., Childs, D. S., Ahmed, M. E., Tuba Kendi, A., Johnson, G. B., Orme, J. J., et al. (2023). Treatment modalities and survival outcomes in prostate cancer parenchymal brain metastasis. Prostate 84, 237–244. doi:10.1002/pros.24643
Maia, J., Caja, S., Strano Moraes, M. C., Couto, N., and Costa-Silva, B. (2018). Exosome-based cell-cell communication in the tumor microenvironment. Front. Cell Dev. Biol. 6, 18. doi:10.3389/fcell.2018.00018
Maji, S., Chaudhary, P., Akopova, I., Nguyen, P. M., Hare, R. J., Gryczynski, I., et al. (2017). Exosomal annexin II promotes angiogenesis and breast cancer metastasis. Mol. Cancer Res. 15 (1), 93–105. doi:10.1158/1541-7786.MCR-16-0163
Malekian, F., Shamsian, A., Kodam, S. P., and Ullah, M. (2023). Exosome engineering for efficient and targeted drug delivery: current status and future perspective. J. Physiol. 601 (22), 4853–4872. doi:10.1113/JP282799
Mallawaaratchy, D. M., Hallal, S., Russell, B., Ly, L., Ebrahimkhani, S., Wei, H., et al. (2017). Comprehensive proteome profiling of glioblastoma-derived extracellular vesicles identifies markers for more aggressive disease. J. Neurooncol 131 (2), 233–244. doi:10.1007/s11060-016-2298-3
Mason, R. J. (2020). Pathogenesis of COVID-19 from a cell biology perspective. Eur. Respir. J. 55 (4), 2000607. doi:10.1183/13993003.00607-2020
Mathieu, M., Martin-Jaular, L., Lavieu, G., and Thery, C. (2019). Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 21 (1), 9–17. doi:10.1038/s41556-018-0250-9
Maumus, M., Rozier, P., Boulestreau, J., Jorgensen, C., and Noel, D. (2020). Mesenchymal stem cell-derived extracellular vesicles: opportunities and challenges for clinical translation. Front. Bioeng. Biotechnol. 8, 997. doi:10.3389/fbioe.2020.00997
McAndrews, K. M., and Kalluri, R. (2019). Mechanisms associated with biogenesis of exosomes in cancer. Mol. Cancer 18 (1), 52. doi:10.1186/s12943-019-0963-9
Miaomiao, S., Xiaoqian, W., Yuwei, S., Chao, C., Chenbo, Y., Yinghao, L., et al. (2023). Cancer-associated fibroblast-derived exosome microRNA-21 promotes angiogenesis in multiple myeloma. Sci. Rep. 13 (1), 9671. doi:10.1038/s41598-023-36092-6
Mitchell, M. I., Ma, J., Carter, C. L., and Loudig, O. (2022). Circulating exosome cargoes contain functionally diverse cancer biomarkers: from biogenesis and function to purification and potential translational utility. Cancers (Basel) 14 (14), 3350. doi:10.3390/cancers14143350
Moitra, K., Lou, H., and Dean, M. (2011). Multidrug efflux pumps and cancer stem cells: insights into multidrug resistance and therapeutic development. Clin. Pharmacol. Ther. 89 (4), 491–502. doi:10.1038/clpt.2011.14
Mukhtar, M., Sargazi, S., Barani, M., Madry, H., Rahdar, A., and Cucchiarini, M. (2021). Application of nanotechnology for sensitive detection of low-abundance single-nucleotide variations in genomic DNA: a review. Nanomater. (Basel) 11 (6), 1384. doi:10.3390/nano11061384
Munshi, V. N., Perkins, R. B., Sy, S., and Kim, J. J. (2022). Cost-effectiveness analysis of the 2019 American Society for Colposcopy and Cervical Pathology Risk-Based Management Consensus Guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Am. J. Obstet. Gynecol. 226 (2), 228.e1–228.e9. doi:10.1016/j.ajog.2021.09.012
Narayanan, A., Iordanskiy, S., Das, R., Van Duyne, R., Santos, S., Jaworski, E., et al. (2013). Exosomes derived from HIV-1-infected cells contain trans-activation response element RNA. J. Biol. Chem. 288 (27), 20014–20033. doi:10.1074/jbc.M112.438895
Nik Mohamed Kamal, N. N. S., and Shahidan, W. N. S. (2021). Salivary exosomes: from waste to promising periodontitis treatment. Front. Physiol. 12, 798682. doi:10.3389/fphys.2021.798682
Niu, L. M., Song, X. G., Wang, N., Xue, L. L., Song, X. E., and Xie, L. (2019). Tumor-derived exosomal proteins as diagnostic biomarkers in non-small cell lung cancer. Cancer Sci. 110 (1), 433–442. doi:10.1111/cas.13862
Okoye, I. S., Coomes, S. M., Pelly, V. S., Czieso, S., Papayannopoulos, V., Tolmachova, T., et al. (2014). MicroRNA-Containing T-regulatory-cell-derived exosomes suppress pathogenic T helper 1 cells. Immunity 41 (3), 503. doi:10.1016/j.immuni.2014.08.008
Ostenfeld, M. S., Jeppesen, D. K., Laurberg, J. R., Boysen, A. T., Bramsen, J. B., Primdal-Bengtson, B., et al. (2014). Cellular disposal of miR23b by RAB27-dependent exosome release is linked to acquisition of metastatic properties. Cancer Res. 74 (20), 5758–5771. doi:10.1158/0008-5472.Can-13-3512
Palmisano, G., Jensen, S. S., Le Bihan, M. C., Laine, J., McGuire, J. N., Pociot, F., et al. (2012). Characterization of membrane-shed microvesicles from cytokine-stimulated β-cells using proteomics strategies. Mol. Cell Proteomics 11 (8), 230–243. doi:10.1074/mcp.M111.012732
Pan, Y., Li, Y., Dong, W., Jiang, B., Yu, Y., and Chen, Y. (2023). Role of nano-hydrogels coated exosomes in bone tissue repair. Front. Bioeng. Biotechnol. 11, 1167012. doi:10.3389/fbioe.2023.1167012
Patel, G. K., Khan, M. A., Zubair, H., Srivastava, S. K., Khushman, M., Singh, S., et al. (2019). Comparative analysis of exosome isolation methods using culture supernatant for optimum yield, purity and downstream applications. Sci. Rep. 9 (1), 5335. doi:10.1038/s41598-019-41800-2
Patolsky, F., Weizmann, Y., Katz, E., and Willner, I. (2003). Magnetically amplified DNA assays (MADA): sensing of viral DNA and single-base mismatches by using nucleic acid modified magnetic particles. Angew. Chem. Int. Ed. Engl. 42 (21), 2372–2376. doi:10.1002/anie.200250379
Pols, M. S., and Klumperman, J. (2009). Trafficking and function of the tetraspanin CD63. Exp. Cell Res. 315 (9), 1584–1592. doi:10.1016/j.yexcr.2008.09.020
Pu, Q., Chai, J., Chen, L., Liu, C., Yang, C., Huang, Y., et al. (2022). Exosome miRNA expression in umbilical cord blood of high-parity sows regulates their reproductive potential. Anim. (Basel) 12 (18), 2456. doi:10.3390/ani12182456
Qian, Z., Travanty, E. A., Oko, L., Edeen, K., Berglund, A., Wang, J., et al. (2013). Innate immune response of human alveolar type II cells infected with severe acute respiratory syndrome-coronavirus. Am. J. Respir. Cell Mol. Biol. 48 (6), 742–748. doi:10.1165/rcmb.2012-0339OC
Ranjbaran, A., Latifi, Z., Nejabati, H. R., Abroon, S., Mihanfar, A., Sadigh, A. R., et al. (2019). Exosome-based intercellular communication in female reproductive microenvironments. J. Cell Physiol. 234 (11), 19212–19222. doi:10.1002/jcp.28668
Regev-Rudzki, N., Wilson, D. W., Carvalho, T. G., Sisquella, X., Coleman, B. M., Rug, M., et al. (2013). Cell-cell communication between malaria-infected red blood cells via exosome-like vesicles. Cell 153 (5), 1120–1133. doi:10.1016/j.cell.2013.04.029
Rozmyslowicz, T., Majka, M., Kijowski, J., Murphy, S. L., Conover, D. O., Poncz, M., et al. (2003). Platelet- and megakaryocyte-derived microparticles transfer CXCR4 receptor to CXCR4-null cells and make them susceptible to infection by X4-HIV. Aids 17 (1), 33–42. doi:10.1097/00002030-200301030-00006
Saad, M. G., Beyenal, H., and Dong, W. J. (2021). Exosomes as powerful engines in cancer: isolation, characterization and detection techniques. Biosens. (Basel) 11 (12), 518. doi:10.3390/bios11120518
Sadeghi, S., Tehrani, F. R., Tahmasebi, S., Shafiee, A., and Hashemi, S. M. (2023). Exosome engineering in cell therapy and drug delivery. Inflammopharmacology 31 (1), 145–169. doi:10.1007/s10787-022-01115-7
Sakwe, A. M., Koumangoye, R., Guillory, B., and Ochieng, J. (2011). Annexin A6 contributes to the invasiveness of breast carcinoma cells by influencing the organization and localization of functional focal adhesions. Exp. Cell Res. 317 (6), 823–837. doi:10.1016/j.yexcr.2010.12.008
Salciccia, S., Frisenda, M., Bevilacqua, G., Gobbi, L., Bucca, B., Moriconi, M., et al. (2023). Exosome analysis in prostate cancer: how they can improve biomarkers' performance. Curr. Issues Mol. Biol. 45 (7), 6085–6096. doi:10.3390/cimb45070384
Sancho-Albero, M., Navascues, N., Mendoza, G., Sebastian, V., Arruebo, M., Martin-Duque, P., et al. (2019). Exosome origin determines cell targeting and the transfer of therapeutic nanoparticles towards target cells. J. Nanobiotechnology 17 (1), 16. doi:10.1186/s12951-018-0437-z
Sanz-Ros, J., Romero-Garcia, N., Mas-Bargues, C., Monleon, D., Gordevicius, J., Brooke, R. T., et al. (2022). Small extracellular vesicles from young adipose-derived stem cells prevent frailty, improve health span, and decrease epigenetic age in old mice. Sci. Adv. 8 (42), eabq2226. doi:10.1126/sciadv.abq2226
Scavo, M. P., Depalo, N., Tutino, V., De Nunzio, V., Ingrosso, C., Rizzi, F., et al. (2020). Exosomes for diagnosis and therapy in gastrointestinal cancers. Int. J. Mol. Sci. 21 (1), 367. doi:10.3390/ijms21010367
Schubert, D. (2020). A brief history of adherons: the discovery of brain exosomes. Int. J. Mol. Sci. 21 (20), 7673. doi:10.3390/ijms21207673
Schwarzenbach, H., and Gahan, P. B. (2021). Exosomes in immune regulation. Noncoding RNA 7 (1), 4. doi:10.3390/ncrna7010004
Shang, Z., Wanyan, P., Wang, M., Zhang, B., Cui, X., and Wang, X. (2023). Stem cell-derived exosomes for traumatic spinal cord injury: a systematic review and network meta-analysis based on a rat model. Cytotherapy 26, 1–10. doi:10.1016/j.jcyt.2023.09.002
Sharma, P., Mesci, P., Carromeu, C., McClatchy, D. R., Schiapparelli, L., Yates, J. R., et al. (2019). Exosomes regulate neurogenesis and circuit assembly. Proc. Natl. Acad. Sci. U. S. A. 116 (32), 16086–16094. doi:10.1073/pnas.1902513116
Sharma, S., and Salomon, C. (2020). Techniques associated with exosome isolation for biomarker development: liquid biopsies for ovarian cancer detection. Methods Mol. Biol. 2055, 181–199. doi:10.1007/978-1-4939-9773-2_8
Sharma, S., Zuniga, F., Rice, G. E., Perrin, L. C., Hooper, J. D., and Salomon, C. (2017). Tumor-derived exosomes in ovarian cancer - liquid biopsies for early detection and real-time monitoring of cancer progression. Oncotarget 8 (61), 104687–104703. doi:10.18632/oncotarget.22191
Shegekar, T., Vodithala, S., and Juganavar, A. (2023). The emerging role of liquid biopsies in revolutionising cancer diagnosis and therapy. Cureus 15 (8), e43650. doi:10.7759/cureus.43650
Shen, Y., Xue, C., Li, X., Ba, L., Gu, J., Sun, Z., et al. (2019). Effects of gastric cancer cell-derived exosomes on the immune regulation of mesenchymal stem cells by the NF-kB signaling pathway. Stem Cells Dev. 28 (7), 464–476. doi:10.1089/scd.2018.0125
Shenoda, B. B., and Ajit, S. K. (2016). Modulation of immune responses by exosomes derived from antigen-presenting cells. Clin. Med. Insights Pathol. 9 (1), 1–8. doi:10.4137/CPath.S39925
Si, Q., Wu, L., Pang, D., and Jiang, P. (2023). Exosomes in brain diseases: pathogenesis and therapeutic targets. MedComm 4 (3), e287. doi:10.1002/mco2.287
Silva, P., Mendoza, P., Rivas, S., Diaz, J., Moraga, C., Quest, A. F., et al. (2016). Hypoxia promotes Rab5 activation, leading to tumor cell migration, invasion and metastasis. Oncotarget 7 (20), 29548–29562. doi:10.18632/oncotarget.8794
Smith, R. A., and Lam, A. K. (2018). Liquid biopsy for investigation of cancer DNA in esophageal adenocarcinoma: cell-free plasma DNA and exosome-associated DNA. Methods Mol. Biol. 1756, 187–194. doi:10.1007/978-1-4939-7734-5_17
Sohn, W., Kim, J., Kang, S. H., Yang, S. R., Cho, J. Y., Cho, H. C., et al. (2015). Serum exosomal microRNAs as novel biomarkers for hepatocellular carcinoma. Exp. Mol. Med. 47 (9), e184. doi:10.1038/emm.2015.68
Srivastava, A., Amreddy, N., Razaq, M., Towner, R., Zhao, Y. D., Ahmed, R. A., et al. (2018). Exosomes as theranostics for lung cancer. Adv. Cancer Res. 139, 1–33. doi:10.1016/bs.acr.2018.04.001
Steinbichler, T. B., Dudas, J., Skvortsov, S., Ganswindt, U., Riechelmann, H., and Skvortsova, I. I. (2019). Therapy resistance mediated by exosomes. Mol. Cancer 18 (1), 58. doi:10.1186/s12943-019-0970-x
Sugimachi, K., Matsumura, T., Hirata, H., Uchi, R., Ueda, M., Ueo, H., et al. (2015). Identification of a bona fide microRNA biomarker in serum exosomes that predicts hepatocellular carcinoma recurrence after liver transplantation. Br. J. Cancer 112 (3), 532–538. doi:10.1038/bjc.2014.621
Sun, W., Xing, C., Zhao, L., Zhao, P., Yang, G., and Yuan, L. (2020). Ultrasound assisted exosomal delivery of tissue responsive mRNA for enhanced efficacy and minimized off-target effects. Mol. Ther. Nucleic Acids 20, 558–567. doi:10.1016/j.omtn.2020.03.016
Tadokoro, H., Umezu, T., Ohyashiki, K., Hirano, T., and Ohyashiki, J. H. (2013). Exosomes derived from hypoxic leukemia cells enhance tube formation in endothelial cells. J. Biol. Chem. 288 (48), 34343–34351. doi:10.1074/jbc.M113.480822
Tai, Y. L., Chen, K. C., Hsieh, J. T., and Shen, T. L. (2018). Exosomes in cancer development and clinical applications. Cancer Sci. 109 (8), 2364–2374. doi:10.1111/cas.13697
Takenaka, M. C., and Quintana, F. J. (2017). Tolerogenic dendritic cells. Semin. Immunopathol. 39 (2), 113–120. doi:10.1007/s00281-016-0587-8
Tan, H., and Hosein, P. J. (2023). Detection and therapeutic implications of homologous recombination repair deficiency in pancreatic cancer: a narrative review. J. Gastrointest. Oncol. 14 (5), 2249–2259. doi:10.21037/jgo-23-85
Tang, S., Cheng, J., Yao, Y., Lou, C., Wang, L., Huang, X., et al. (2020). Combination of four serum exosomal MiRNAs as novel diagnostic biomarkers for early-stage gastric cancer. Front. Genet. 11, 237. doi:10.3389/fgene.2020.00237
Taylor, D. D., and Gercel-Taylor, C. (2008). MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol. Oncol. 110 (1), 13–21. doi:10.1016/j.ygyno.2008.04.033
Thakur, B. K., Zhang, H., Becker, A., Matei, I., Huang, Y., Costa-Silva, B., et al. (2014). Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res. 24 (6), 766–769. doi:10.1038/cr.2014.44
Thill, M., Kolberg-Liedtke, C., Albert, U. S., Banys-Paluchowski, M., Bauerfeind, I., Blohmer, J. U., et al. (2023). AGO recommendations for the diagnosis and treatment of patients with locally advanced and metastatic breast cancer: update 2023. Breast Care (Basel) 18 (4), 306–315. doi:10.1159/000531579
Thind, A., and Wilson, C. (2016). Exosomal miRNAs as cancer biomarkers and therapeutic targets. J. Extracell. Vesicles 5, 31292. doi:10.3402/jev.v5.31292
Trams, E. G., Lauter, C. J., Salem, N., and Heine, U. (1981). Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim. Biophys. Acta 645 (1), 63–70. doi:10.1016/0005-2736(81)90512-5
Umezu, T., Tadokoro, H., Azuma, K., Yoshizawa, S., Ohyashiki, K., and Ohyashiki, J. H. (2014). Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1. Blood 124 (25), 3748–3757. doi:10.1182/blood-2014-05-576116
Valcz, G., Galamb, O., Krenacs, T., Spisak, S., Kalmar, A., Patai, A. V., et al. (2016). Exosomes in colorectal carcinoma formation: ALIX under the magnifying glass. Mod. Pathol. 29 (8), 928–938. doi:10.1038/modpathol.2016.72
Wang, H., Hou, L., Li, A., Duan, Y., Gao, H., and Song, X. (2014). Expression of serum exosomal microRNA-21 in human hepatocellular carcinoma. Biomed. Res. Int. 2014, 864894. doi:10.1155/2014/864894
Wang, J., and Yang, L. (2023). The role of exosomes in central nervous system tissue regeneration and repair. Biomed. Mater 18 (5), 052003. doi:10.1088/1748-605X/ace39c
Wang, J. C., Bégin, L. R., Bérube, N. G., Chevalier, S., Aprikian, A. G., Gourdeau, H., et al. (2007). Down-regulation of CD9 expression during prostate carcinoma progression is associated with CD9 mRNA modifications. Clin. Cancer Res. 13 (8), 2354–2361. doi:10.1158/1078-0432.Ccr-06-1692
Wang, L., Skotland, T., Berge, V., Sandvig, K., and Llorente, A. (2017). Exosomal proteins as prostate cancer biomarkers in urine: from mass spectrometry discovery to immunoassay-based validation. Eur. J. Pharm. Sci. 98, 80–85. doi:10.1016/j.ejps.2016.09.023
Wang, M., Hu, D., Yang, Y., Shi, K., Li, J., Liu, Q., et al. (2023a). Enhanced chemo-immunotherapy strategy utilizing injectable thermosensitive hydrogel for the treatment of diffuse peritoneal metastasis in advanced colorectal cancer. Adv. Sci. (Weinh) 10, e2303819. doi:10.1002/advs.202303819
Wang, M., Wang, Y., Tian, X., Wang, Q., Huang, H., Lu, X., et al. (2023b). Diagnostic and predictive value of liquid biopsy-derived exosome miR-21 for breast cancer: a systematic review and meta-analysis. Expert Rev. Mol. Diagn 23 (4), 315–324. doi:10.1080/14737159.2023.2195552
Wang, T. W., Chao, H. S., Chiu, H. Y., Lu, C. F., Liao, C. Y., Lee, Y., et al. (2023). Radiomics of metastatic brain tumor as a predictive image biomarker of progression-free survival in patients with non-small-cell lung cancer with brain metastasis receiving tyrosine kinase inhibitors. Transl. Oncol. 39, 101826. doi:10.1016/j.tranon.2023.101826
Wang, Z., Du, X., Lian, W., Chen, J., Hong, C., Li, L., et al. (2023). A novel disulfidptosis-associated expression pattern in breast cancer based on machine learning. Front. Genet. 14, 1193944. doi:10.3389/fgene.2023.1193944
Whiteside, T. L. (2017). The effect of tumor-derived exosomes on immune regulation and cancer immunotherapy. Future Oncol. 13 (28), 2583–2592. doi:10.2217/fon-2017-0343
Wolf, A. M. D., Oeffinger, K. C., Shih, T. Y., Walter, L. C., Church, T. R., Fontham, E. T. H., et al. (2023). Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J. Clin. 68, 250–281. doi:10.3322/caac.21457
Wu, D., Chang, X., Tian, J., Kang, L., Wu, Y., Liu, J., et al. (2021). Bone mesenchymal stem cells stimulation by magnetic nanoparticles and a static magnetic field: release of exosomal miR-1260a improves osteogenesis and angiogenesis. J. Nanobiotechnology 19 (1), 209. doi:10.1186/s12951-021-00958-6
Wu, M., Wang, G., Hu, W., Yao, Y., and Yu, X. F. (2019). Emerging roles and therapeutic value of exosomes in cancer metastasis. Mol. Cancer 18 (1), 53. doi:10.1186/s12943-019-0964-8
Wu, S. W., Li, L., Wang, Y., and Xiao, Z. (2019). CTL-derived exosomes enhance the activation of CTLs stimulated by low-affinity peptides. Front. Immunol. 10, 1274. doi:10.3389/fimmu.2019.01274
Wu, T., Liu, Y., Cao, Y., and Liu, Z. (2022). Engineering macrophage exosome disguised biodegradable nanoplatform for enhanced sonodynamic therapy of glioblastoma. Adv. Mater 34 (15), e2110364. doi:10.1002/adma.202110364
Xia, Y., Hu, X., Di, K., Liu, C., Tan, T., Lin, Y., et al. (2020). Combined detection of exosome concentration and tumor markers in gastric cancer. J. Biomed. Nanotechnol. 16 (2), 252–258. doi:10.1166/jbn.2020.2887
Xiao, L., Hareendran, S., and Loh, Y. P. (2021). Function of exosomes in neurological disorders and brain tumors. Extracell. Vesicles Circ. Nucl. Acids 2, 55–79. doi:10.20517/evcna.2021.04
Xiao, X. (2015). Screening, dignosis and follow-up in lung cancer in early stage. Zhonghua Yi Xue Za Zhi 95 (43), 3481–3483.
Yang, M., Chen, J. Q., Su, F., Yu, B., Su, F. X., Lin, L., et al. (2011). Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol. Cancer 10, 117. doi:10.1186/1476-4598-10-117
Yang, S. J., Wang, D. D., Li, J., Xu, H. Z., Shen, H. Y., Chen, X., et al. (2017). Predictive role of GSTP1-containing exosomes in chemotherapy-resistant breast cancer. Gene 623, 5–14. doi:10.1016/j.gene.2017.04.031
Yi, X., Chen, J., Huang, D., Feng, S., Yang, T., Li, Z., et al. (2022). Current perspectives on clinical use of exosomes as novel biomarkers for cancer diagnosis. Front. Oncol. 12, 966981. doi:10.3389/fonc.2022.966981
Young, E., Edwards, L., and Singh, R. (2023). The role of artificial intelligence in colorectal cancer screening: lesion detection and lesion characterization. Cancers (Basel) 15 (21), 5126. doi:10.3390/cancers15215126
Yu, D., Li, Y., Wang, M., Gu, J., Xu, W., Cai, H., et al. (2022). Exosomes as a new frontier of cancer liquid biopsy. Mol. Cancer 21 (1), 56. doi:10.1186/s12943-022-01509-9
Yu, W., Hurley, J., Roberts, D., Chakrabortty, S. K., Enderle, D., Noerholm, M., et al. (2021). Exosome-based liquid biopsies in cancer: opportunities and challenges. Ann. Oncol. 32 (4), 466–477. doi:10.1016/j.annonc.2021.01.074
Zeng, Y., and Fu, B. M. (2020). Resistance mechanisms of anti-angiogenic therapy and exosomes-mediated revascularization in cancer. Front. Cell Dev. Biol. 8, 610661. doi:10.3389/fcell.2020.610661
Zhang, J., Guan, J., Niu, X., Hu, G., Guo, S., Li, Q., et al. (2015). Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J. Transl. Med. 13, 49. doi:10.1186/s12967-015-0417-0
Zhang, K., and Cheng, K. (2023). Stem cell-derived exosome versus stem cell therapy. Nat. Rev. Bioeng. 1, 608–609. doi:10.1038/s44222-023-00064-2
Zhang, L., He, F., Gao, L., Cong, M., Sun, J., Xu, J., et al. (2021). Engineering exosome-like nanovesicles derived from Asparagus cochinchinensis can inhibit the proliferation of hepatocellular carcinoma cells with better safety profile. Int. J. Nanomedicine 16, 1575–1586. doi:10.2147/IJN.S293067
Zhang, M., Wan, L., Li, R., Li, X., Zhu, T., and Lu, H. (2023). Engineered exosomes for tissue regeneration: from biouptake, functionalization and biosafety to applications. Biomater. Sci. 11 (22), 7247–7267. doi:10.1039/d3bm01169k
Zhang, W., Peng, P., and Shen, K. (2016). Role of exosome shuttle RNA in cell-to-cell communication. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 38 (4), 480–483. doi:10.3881/j.issn.1000-503X.2016.04.020
Zhang, W., Pham, T. H., and Wang, D. H. (2021). A "clue" to improving liquid biopsies for cancer: microfluidic multiparametric exosome analysis. Clin. Chem. 67 (2), 335–337. doi:10.1093/clinchem/hvaa209
Zhang, X. C., Lu, S., Zhang, L., Wang, C. L., Cheng, Y., Li, G. D., et al. (2013). Consensus on dignosis for ALK positive non-small cell lung cancer in China, the 2013 version. Zhonghua Bing Li Xue Za Zhi 42 (6), 402–406. doi:10.3760/cma.j.issn.0529-5807.2013.06.012
Zhang, Y., Ali, A., and Xie, J. (2023). Detection of clinically important BRCA gene mutations in ovarian cancer patients using next generation sequencing analysis. Am. J. Cancer Res. 13 (10), 5005–5020.
Zhao, J., Yang, J., Jiao, J., Wang, X., Zhao, Y., and Zhang, L. (2023). Biomedical applications of artificial exosomes for intranasal drug delivery. Front. Bioeng. Biotechnol. 11, 1271489. doi:10.3389/fbioe.2023.1271489
Zhao, X., Fu, L., Zou, H., He, Y., Pan, Y., Ye, L., et al. (2023). Optogenetic engineered umbilical cord MSC-derived exosomes for remodeling of the immune microenvironment in diabetic wounds and the promotion of tissue repair. J. Nanobiotechnology 21 (1), 176. doi:10.1186/s12951-023-01886-3
Zhao, Y., Liu, L., Sun, R., Cui, G., Guo, S., Han, S., et al. (2022). Exosomes in cancer immunoediting and immunotherapy. Asian J. Pharm. Sci. 17 (2), 193–205. doi:10.1016/j.ajps.2021.12.001
Zhao, Y., Tao, M., Wei, M., Du, S., Wang, H., and Wang, X. (2019). Mesenchymal stem cells derived exosomal miR-323-3p promotes proliferation and inhibits apoptosis of cumulus cells in polycystic ovary syndrome (PCOS). Artif. Cells Nanomed Biotechnol. 47 (1), 3804–3813. doi:10.1080/21691401.2019.1669619
Zhao, Y., Xu, L., Ma, W., Wang, L., Kuang, H., Xu, C., et al. (2014). Shell-engineered chiroplasmonic assemblies of nanoparticles for zeptomolar DNA detection. Nano Lett. 14 (7), 3908–3913. doi:10.1021/nl501166m
Zhong, L., Wang, J., Wang, P., Liu, X., Liu, P., Cheng, X., et al. (2023). Neural stem cell-derived exosomes and regeneration: cell-free therapeutic strategies for traumatic brain injury. Stem Cell Res. Ther. 14 (1), 198. doi:10.1186/s13287-023-03409-1
Zhou, K., Li, Z. Z., Cai, Z. M., Zhong, N. N., Cao, L. M., Huo, F. Y., et al. (2023). Nanotheranostics in cancer lymph node metastasis: the long road ahead. Pharmacol. Res. 198, 106989. doi:10.1016/j.phrs.2023.106989
Zhou, W., Fong, M. Y., Min, Y., Somlo, G., Liu, L., Palomares, M. R., et al. (2014). Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell 25 (4), 501–515. doi:10.1016/j.ccr.2014.03.007
Keywords: exosome, cancer, diagnosis, therapy, translational research
Citation: Wang Z, Wang Q, Qin F and Chen J (2024) Exosomes: a promising avenue for cancer diagnosis beyond treatment. Front. Cell Dev. Biol. 12:1344705. doi: 10.3389/fcell.2024.1344705
Received: 26 November 2023; Accepted: 31 January 2024;
Published: 13 February 2024.
Edited by:
Li Yan, University of Maryland, College Park, United StatesReviewed by:
Dwijendra K. Gupta, Allahabad University, IndiaCopyright © 2024 Wang, Wang, Qin and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Chen, Y2hlbmppZXdlc3RjaGluYUAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.