- 1Faculty of Medicine and University Hospital of Cologne, Institute of Pathology, University of Cologne, Cologne, Germany
- 2Faculty of Medicine and University Hospital of Cologne, Department of Otorhinolaryngology, Head and Neck Surgery, University of Cologne, Cologne, Germany
- 3Center for Molecular Medicine Cologne, Faculty of Medicine and University Hospital of Cologne, University of Cologne, Cologne, Germany
- 4Faculty of Medicine and University Hospital of Cologne, Department of General, Visceral and Cancer Surgery, University of Cologne, Cologne, Germany
- 5Department I of Internal Medicine, Center for Integrated Oncology Aachen Bonn Cologne Duesseldorf, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany
Background: The incidence of oropharyngeal squamous cell carcinoma (OPSCC) is rapidly increasing in high income countries due to its association with persistent high-risk human papilloma virus (HPV) infection. Recent scientific advances have highlighted the importance of the tumor microenvironment in OPSCC. In this study, including 216 OPSCC patients, we analyze the composition of four established markers of cancer associated fibroblasts (CAFs) in the context of intratumoral CD8 T-cell infiltration.
Methods: Immunohistochemical staining for fibroblast activation protein (FAP), platelet-derived growth factor receptor beta (PDGFRb), periostin, alpha smooth muscle actin (α-SMA) and CD8 were analyzed digitally and their association with survival, tumor- and patient characteristics was assessed.
Results: Co-expression of CAF markers was frequent but not associated with HPV status. FAPhigh and PDGFRbhigh expression were associated with increased CD8 T-cell infiltration. Low expression of PDGFRb improved patient survival in female patients but not in male patients. We identified PDGFRblow periostinlow α-SMAlow status as an independent predictor of improved survival (hazard ratio 0.377, p = 0.006).
Conclusion: These findings elucidate the co-expression of four established CAF markers in OPSCC and underscore their association with T-cell infiltration and patient survival. Future analyses of CAF subgroups in OPSCC may enable the development of individualized therapies.
1 Introduction
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer entity worldwide. Oropharyngeal squamous cell carcinoma (OPSCC), comprising cancers of the base of tongue, tonsils, uvula, and soft palate, is a subtype of HNSCC distinguished by anatomical location and molecular characteristics. OPSCC, like other HNSCCs, has canonically been associated with alcohol and tobacco consumption as driving factors of carcinogenesis. However, persistent infection with carcinogenic high risk human papillomavirus (HPV) has emerged as an important risk factor of OPSCC in the past decades and the incidence of HPV-positive OPSCC is rising in high-income countries (Klussmann et al., 2003; Lechner et al., 2022). HPV driven OPSCC is characterized by a distinct pathogenesis and improved survival compared to HPV-negative cancers (Kian Ang et al., 2010; Mehanna et al., 2023).
In recent years, immune checkpoint blockade (ICB) has emerged as promising therapy for multiple cancer types including OPSCC (Ferris et al., 2016). As the efficacy of ICB remains limited in OPSCC and other types of solid cancer, they have gathered renewed interest in the tumor microenvironment (TME) (Elmusrati et al., 2021). The TME is comprised of extracellular matrix (ECM) and a variety of infiltrating cells including lymphocytes, tumor associated macrophages, myeloid derived suppressor cells, innate lymphoid cells, fibroblasts, and cancer associated fibroblasts (CAFs) (Pitt et al., 2016; Nakamura and Smyth, 2019; Elmusrati et al., 2021; Mao et al., 2021). CAFs arise from various cell types and are a primary source of ECM modulation, angiogenesis, cell migration and nutrition (Zhang et al., 2022). While some studies demonstrate a protumorigenic role of CAFs in HNSCC including cancer stem cell renewal, immune cell evasion and chemoresistance, evidence from other malignancies suggests a complimentary tumor-suppressive role of CAFs (Özdemir et al., 2014; Brechbuhl et al., 2017; Su et al., 2018; Galbo et al., 2021).
Currently, no definitive classification of CAFs has been established in HNSCC or other cancer types as this scientific field is still rapidly evolving and new insights often challenge the established paradigms of CAF-population defining signatures. (Brechbuhl et al., 2017; Chen et al., 2021; Galbo et al., 2021; Foster et al., 2022; Cords et al., 2023). Fibroblast activation protein (FAP), a serine protease and well-established CAF surface protein, is commonly overexpressed in activated fibroblasts and high expression is associated with worse survival in multiple cancer types (Liu et al., 2015). Targeted therapies directed against FAP including monoclonal antibodies, drug conjugates and FAP directed chimeric antigen receptor (CAR) T cells are currently under investigation (Busek et al., 1933; Ostermann et al., 2008). Furthermore, [68 Ga]Ga-labeled inhibitors of FAP ([68 Ga]Ga-FAPI-46) have been developed for positron emission tomography (PET/CT) imaging utilizing CAFs to improve detection rate of nodal metastasis and optimize radiotherapy planning (Kratochwil et al., 2019; Wegen et al., 2022). α smooth muscle actin (α-SMA) is involved in the contractile apparatus of smooth muscle cells and is commonly used to defined activated myofibroblasts (myCAFs) (Tomasek et al., 2002). myCAFs partake in the reorganization of the extracellular matrix and increase tissue stiffness contributing to impaired chemosensitivity (Shen et al., 2020). Periostin, canonically linked to pulmonary fibrosis and allergy (Sonnenberg-Riethmacher et al., 2021), is an extracellular matrix protein associated with epithelial-mesenchymal transition and tissue invasion and formation of metastases in HNSCC (Kudo et al., 2006). Expression of platelet-derived growth factor receptor beta (PDGFRb) regulates neo angiogenesis and is expressed in various malignancies including HNSCC (Bran et al., 2009).
As fibroblasts surrounding a cancerous lesion can promote tumor growth or act as tumor suppressors, evaluation of prognostic impact and specific identification of relevant targets for personalized therapy is warranted (Kanzaki and Pietras, 2020). Targeting tumor promoting CAFs may enable avenues for targeted therapy leading to the disinhibition of response to conventional therapies and minimize adverse events. In this study we aimed to describe the expression of FAP, PDGFRb, periostin, and α-SMA in a large cohort of HPV-positive and HPV-negative OPSCC patients and elucidate the association with CD8 T-cell infiltration and prognosis.
2 Materials and methods
2.1 Patient cohort and tumor samples
Patients included in this study were diagnosed with OPSCC [C09, C10, International Classification of Diseases for Oncology (ICD-O)] and treated at the University Hospital Cologne between 2005 and 2020. Patients with sufficient pretherapeutic tissue available were included in this study. Tissue microarrays (TMA) were prepared from formalin-fixed, paraffin-embedded (FFPE) cancer tissue resulting in 216 samples. Clinical and pathological features of the patient cohort were ascertained from medical records and are displayed in Table 1. The study was conducted in accordance with the declaration of Helsinki. The study protocol was approved by the Ethics committee of the University of Cologne (study number 19-1288).
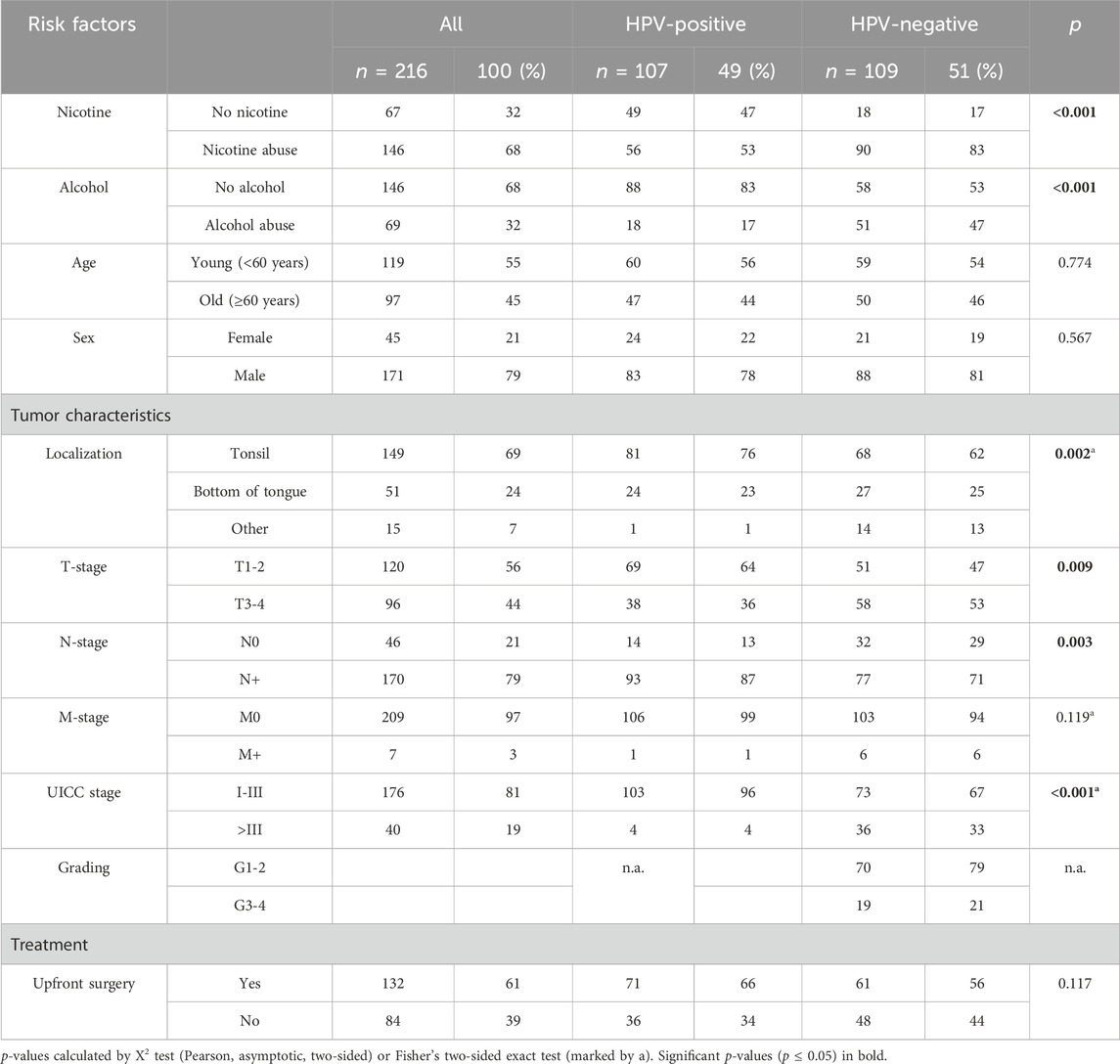
TABLE 1. Clinicopathological features of oropharyngeal squamous cell carcinoma patient cohort according to HPV status.
Disease extent was defined according to UICC TNM 7th or 8th edition according to the valid classification at the time of diagnosis. Based on established TNM status, tumor stage was defined according to the American Cancer Staging Classification 8 (AJCC8 I-IV) for all patients.
2.2 p16INK4a immunohistochemistry and HPV-DNA genotyping
To determine the HPV association of OPSCCs, p16INK4a (p16) immunohistochemistry (IHC) and HPV-DNA genotyping was performed for all patients. p16 expression was determined using the Zytomed histology kit (Zytomed Systems, Berlin, Germany) according to the supplier’s and standard protocols (Klussmann et al., 2003). Extracted DNA was analyzed for the presence of HPV-DNA and HPV genotypes (6, 11, 16, 18, 31, 33, 35, 39, 42, 44, 45, 51, 52, 53, 54, 56, 58, 59, 61, 62, 66, 67, 68, 70, 72, 73, 81, 82, 83, 84, 90, 91) by amplification of highly conserved regions of viral genome (L1 Region, primer pairs MY11/19, and 125′) followed by DNA/DNA hybridization to assess specificity. Cancers were considered HPV-positive when p16 IHC staining was positive on 70% of tumor cells and high-risk HPV DNA of the aforementioned types was detected. Other combinations (HPV DNA+/p16−, HPV DNA−/p16−, HPV DNA−/p16+) were considered HPV negative.
2.3 Preparation of tissue microarrays and immunohistochemistry
Sufficient FFPE cancer tissue with a thickness of at least 2 mm was mandatory to produce TMA cores. TMAs were assembled as previously described (Simon, 2008). Briefly, 1.2 mm cores were taken from a tumor area including invasive margins marked by an experienced pathologist. A 1.2 mm cylinder was removed using a semi-automated punch press and transferred to a fresh paraffin block. For staining, 4 µm thick slides were freshly cut from TMAs.
Antibodies targeting α-SMA, PDGFRb, periostin, FAP and CD8 were used for immunohistochemical staining according to the manufacturers recommended protocols. Antibody specifications are displayed in Supplementary Table S1. Automated staining was performed with the Leica BOND-MAX automated system (Leica Biosystems, Wetzlar, Germany) in accordance with the manufacturer’s protocol. Stained slides were scanned with the Aperio GT 450 DX (Leica Biosystems, Wetzlar, Germany). Digitalized slide scans were analyzed using an adapted QuPath v0.3.2 protocol as was described previously (Bankhead et al., 2017). After initial cell classification and identification of the cell stroma, the following parameters were defined to detect α-SMA, PDGFRb, periostin and FAP staining as published previously (Knipper et al., 2023): Threshold with the resolution of 2.11 µm/pixel, channel DAB, prefilter Gaussian, smoothing sigma 1, Threshold 0.05. For FAP, PDGFRb, periostin and α-SMA, the cutoff for high expression was defined as the median of the patient population. Values equal or lower to the median were defined as low. The following parameters were defined for CD8 T-cell detection: Detection image optical density sum, requested pixel size 0.5 µm; and cell parameters; cell expansion 5 μm, cell nucleus included; nucleus parameters; background radius 8 μm, median filter radius 0 μm, sigma 1.5 µm, minimum area 10 µm2, maximum area 400 μm2; intensity parameters; threshold 0.1, max background intensity 2. For CD8 T-cell infiltration, expression <50 lymphocytes/mm2 were defined as low and ≥50 lymphocytes/mm2 were defined as high. Human tonsil and appendix tissue on each of the TMA slides served as control for staining.
2.4 Statistical analysis
Statistical analyses were performed using SPSS statistical software (IBM SPSS 28.0, Armork, NY, United States) and Graphpad Prism (Graphpad Prism v8, San Diego, CA, United States). Differences in patient characteristics and correlation of fibroblast marker expression and expression of CD8 were calculated using Pearson’s Chi-squares test or Fisher’s exact test as appropriate. In Table 2, correction of false discovery rate (FDR) with a FDR of 5% using the original method of Benjamini-Hochberg was conducted (Benjamini and Hochberg, 1995). This resulted in a threshold for significant p-values (p ≤ 0.012). Overall survival (OS) was calculated from the initial date of histological diagnosis to date of death or loss to follow-up. Patients were followed for a maximum of 10 years. To plot survival curves, the Kaplan-Meier method was used and significance between groups was determined using the two-sided log-rank test. Cox proportional-hazards models were used to determine hazard ratios (HR) and a confidence interval (CI) of 95% in univariate and multivariate analyses. Upset plots were generated using the ComplexUpset packages in R v3.6.3 and Inkscape v1.0. All tests were two-sided and p-values ≤0.05 were considered significant if not specifically stated otherwise.

TABLE 2. Relation of fibroblast activation protein (FAP), platelet-derived growth factor receptor beta (PDGFRb), periostin and alpha-smooth muscle actin (α-SMA) expression to each other, wih CD8-positive TILs and clinical characteristics (n = 216).
3 Results
3.1 Patient and tumor characteristics
We included 107 (49%) HPV-positive and 109 (51%) HPV-negative OPSCC patients for a total of 216 analyzed tumors in this study. All patients were treated in curative intent. Patient characteristics are displayed in Table 1. The cohort was comprised of 171 (79%) male and 45 (21%) female patients and 119 patients (55%) were younger than 60 years old while 97 (45%) were under 60 years of age. In the entire cohort, 68% of patients reported nicotine abuse and 32% abused alcohol previously or at the time of diagnosis (self-reported “frequent” or “daily” consumption was considered abuse), while in HPV-negative patients the misuse of both substances was significantly more frequent (nicotine 83% vs. 53%, p < 0.001; alcohol 47% vs. 17%, p < 0.001). The most common tumor localization was the tonsil (n = 149, 69%), followed by the base of the tongue (n = 51, 24%). HPV-positive carcinomas were more frequently located in the tonsils (76% vs. 62%, p = 0.002).
Overall, 120 patients (56%) were classified as T1-2, whereas 170 (79%) showed lymphonodal infiltration at initial diagnosis. Seven patients suffered from metastatic disease (3%). In HPV-negative patients, stage four disease was significantly more common compared to HPV-positive patients (UICC stage > III in HPV neg. 33% vs. HPV pos. 4%, p < 0.001). HPV-negative tumors were classified as grade (G) 1-2 in 70 (79%) cases while 19 (21%) tumors were high grade (G 3-4). Grading was not applied for HPV-positive tumors (Pignon et al., 2009; Ferris and Westra, 2023). For curative treatment, 132 patients (61%) underwent upfront surgery while 84 (39%) of patients received primary (chemo-) radiation.
3.2 Distribution of cancer-associated fibroblast marker expression
As fibroblasts are an essential component of the TME, we performed immunohistochemical staining of FAP, α-SMA, PDGFRb and periostin. Following digital analysis, we divided the patient cohort according to median expression in tumor stroma (FAP: n = 106 low, n = 105 high; PDGFRb: n = 106 low, n = 106 high; periostin: n = 106 low, n = 105 high; α-SMA: n = 107 low, n = 106 high) (Table 2). Notably, expression of periostin was significantly associated with all other CAF markers (periostin-FAP p < 0.001; periostin-PDGFRb p = 0.003; periostin-α-SMA p = 0.009) while α-SMA was associated with periostin and PDGFRb (p = 0.01). Co-expression of fibroblast markers was common as 26 tumors had high expression of all four molecules while 24 were classified low for all markers (Figure 1A). The third most common expression pattern present in 16 patients was periostinhigh PDGFRbhigh FAPhigh α-SMAlow. Tumors with low expression of all four fibroblast activation markers were the largest population in HPV-positive tumors while combined high expression of all four markers was the most frequent pattern in HPV-negative OPSCC (Figures 1B–D). To elucidate the connection of fibroblast frequency with T cell infiltration in the TME, we measured CD8 T-cell infiltration. Considering a threshold of >50 CD8 T lymphocytes/mm2 as high, 117 (54%) tumors were highly infiltrated by CD8 T-cells while 98 (46%) were considered low. CD8 T-cell infiltration was positively associated PDGFRb (p = 0.003) high tumors. Expression of FAP, α-SMA, PDGFRb or periostin was independent from nicotine or alcohol abuse, UICC stage, sex or HPV status.
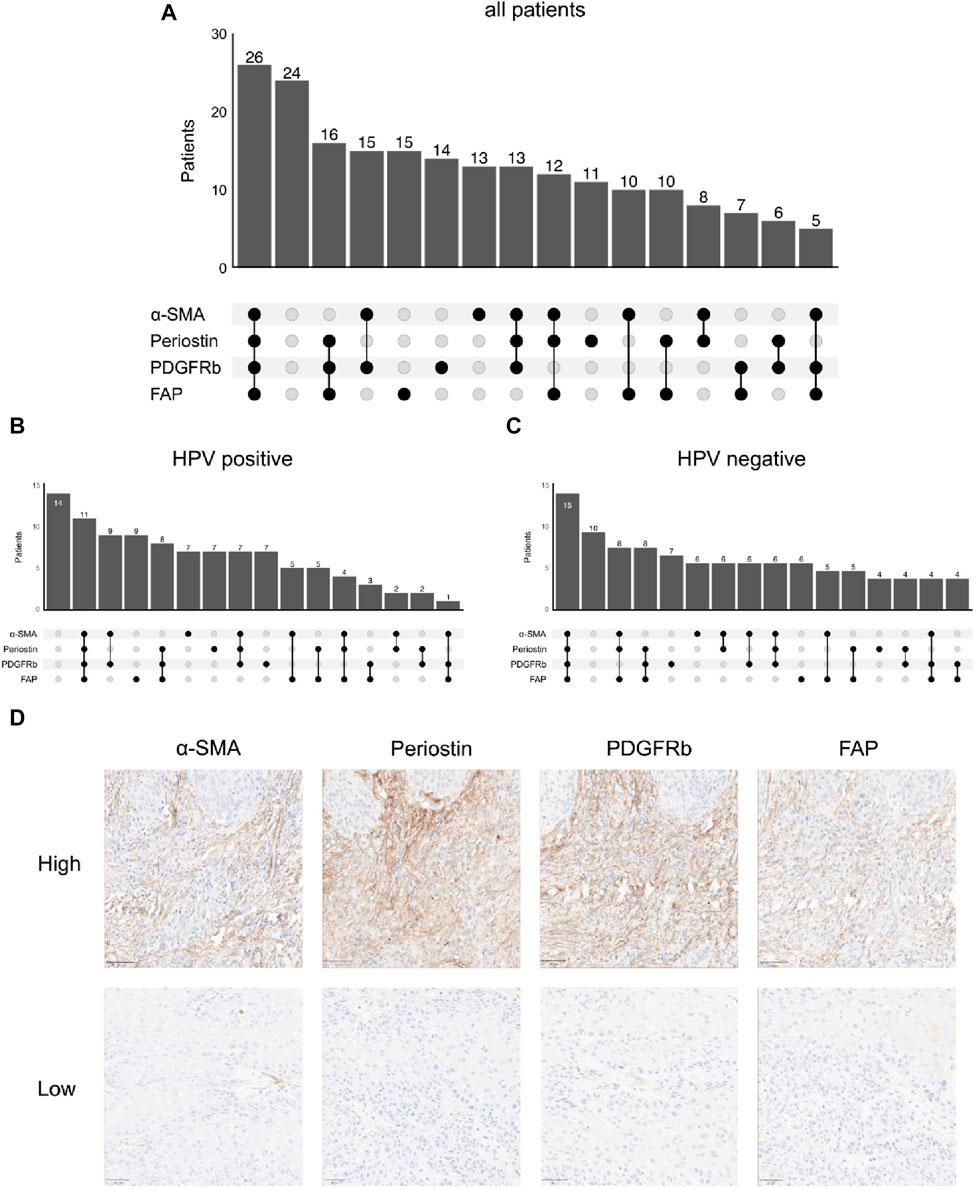
FIGURE 1. Co-expression patterns of fibroblast markers in immunohistochemistry; number of patients with high/low stromal expression of different combinations of α-smooth muscle actin (α-SMA), periostin, platelet derived growth factor receptor beta (PDGFRb) and fibroblast activation protein (FAP) in (A) all patients (n = 207), (B) human papilloma virus (HPV)-positive patients (n = 101) and (C) HPV-negative patients (n = 104), (D) Representative images of immunohistochemical stainings (top high, bottom low) for fibroblast activation protein (FAP), platelet derived growth factor receptor beta (PDGFRb), periostin and α-smooth muscle actin (α-SMA).
3.3 Survival analysis
Survival analysis was performed using the Log-Rank test as well as univariate and multivariate Cox regression to determine the association of CAFs and patient survival. Patient follow-up was conducted for a maximum of 10 years; mean follow up for survivors was 66 months. Periostin (p = 0.009) and α-SMA (p = 0.014) expression were associated with worse overall survival in univariate analyses (Figure 2). FAP (p = 0.557) and PDGFRb (p = 0.114) expression were not associated with OPSCC patient survival. High CD8 T-cell abundance was correlated to improved patient survival (p = 0.004) (Tables 3, 4). Stratifying patients according to CD8 T-cell infiltration revealed high PDGFRb expression as a predictor of impaired survival in the CD8high population (p = 0.006) (Figure 3A). PDGFRb status did not impact survival in the CD8low group (p = 0.970). In contrast, high periostin expression was associated with worse overall survival in the CD8low group (p = 0.009) while no impact on patient survival was observed in the CD8high group (p = 0.259) (Figure 3B). In the subgroup of HPV-positive tumors, individual expression of CAF markers did not reveal a significant association with overall survival (Supplementary Figure S2). Canonically, most OPSCC cases occur in men and the investigation of sex specific aspects and distinct molecular patterns in female patients have only recently been appreciated (Tosi et al., 2022). To elucidate the association of fibroblast activation markers with sex specific patient survival, we performed subgroup analysis by sex. Notably, PDGFRbhigh expression status was not associated with patient survival in men but in female patients, PDGFRbhigh status conferred poor overall survival (p = 0.007) (Figure 3C).
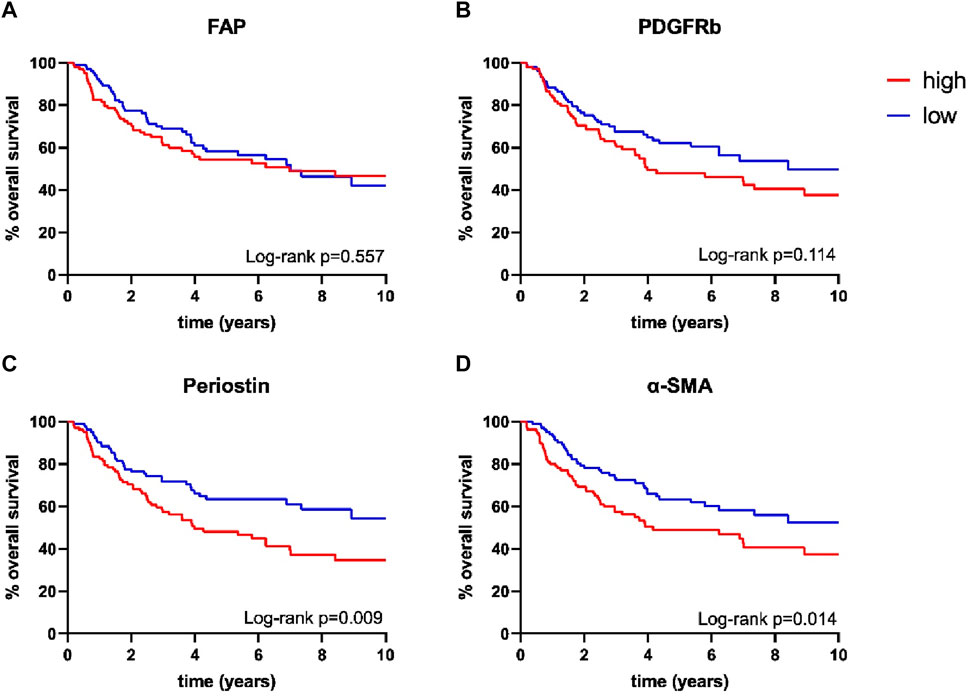
FIGURE 2. Kaplan-Meier curves for overall survival according to (A) fibroblast activation protein (FAP) (high n = 105, low n = 106), (B) platelet derived growth factor receptor beta (PDGFRb) (high n = 106, low n = 106), (C) periostin (high n = 105, low n = 106) and (D) α-smooth muscle actin (α-SMA) (high n = 106, low n = 107) expression; p-values calculated by log-rank test.
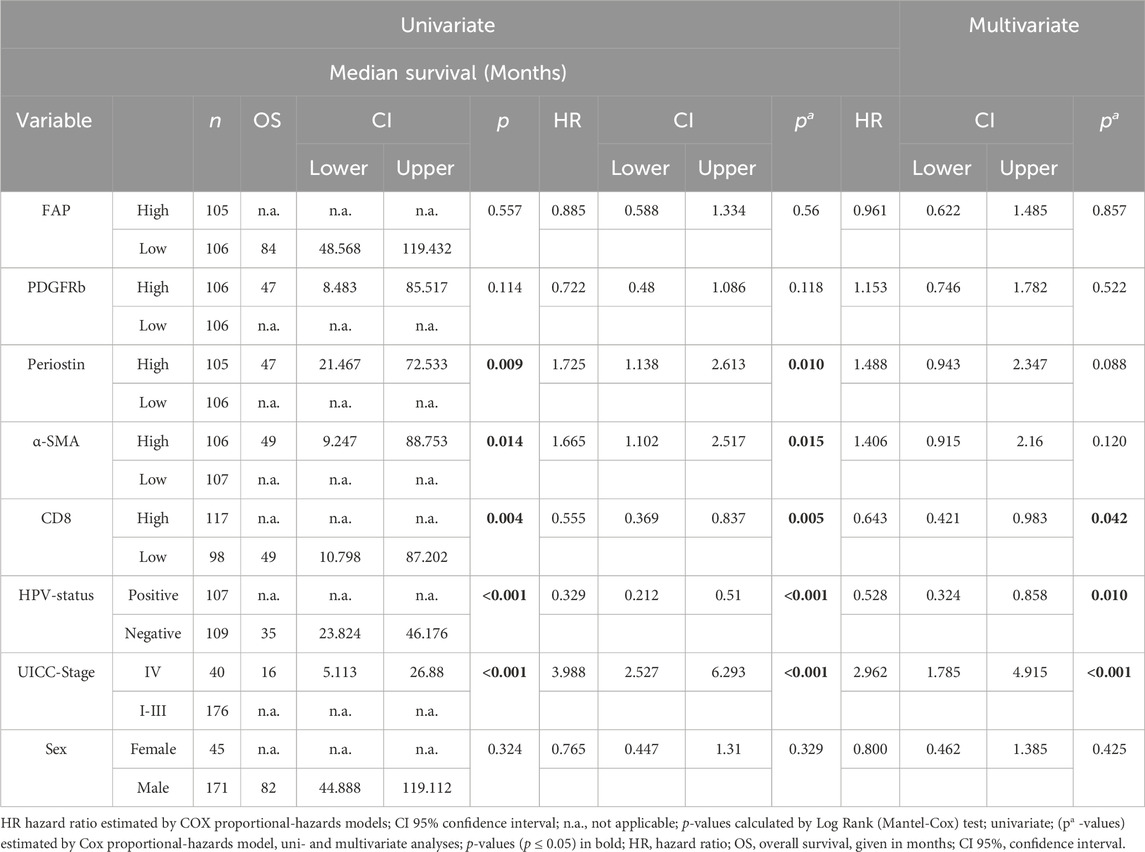
TABLE 3. Univariate and multivariate survival analysis according to tumor characteristics and risk factors (n = 216).
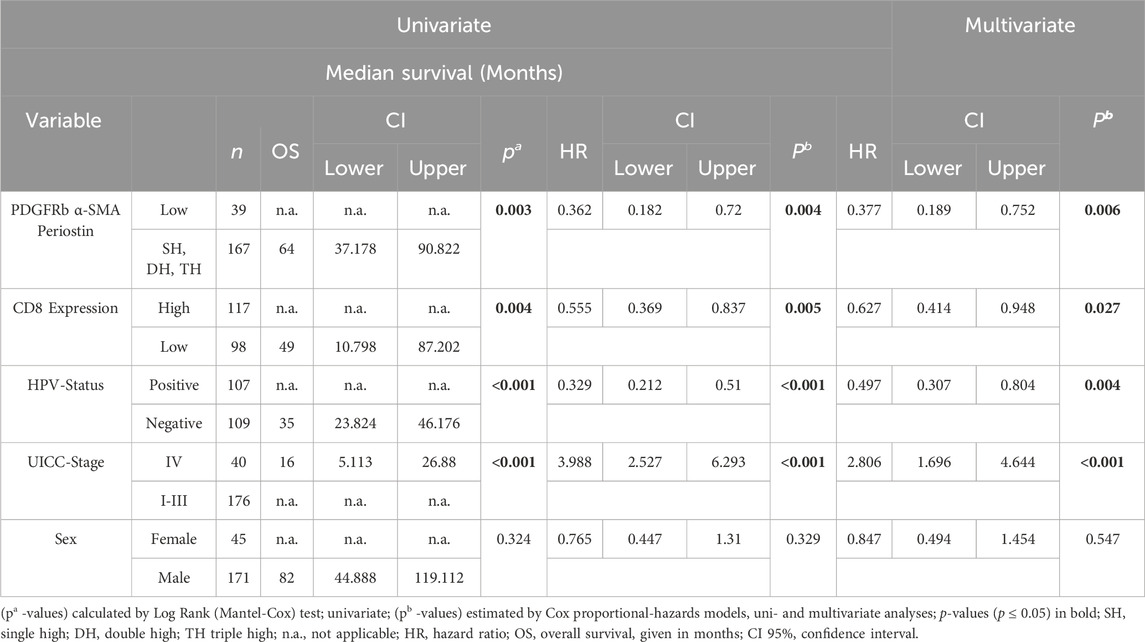
TABLE 4. Univariate and multivariate survival analysis according to tumor characteristics and combined expression of platelet-derived growth factor receptor beta (PDGFRb), periostin and α-smooth muscle actin (α-SMA).
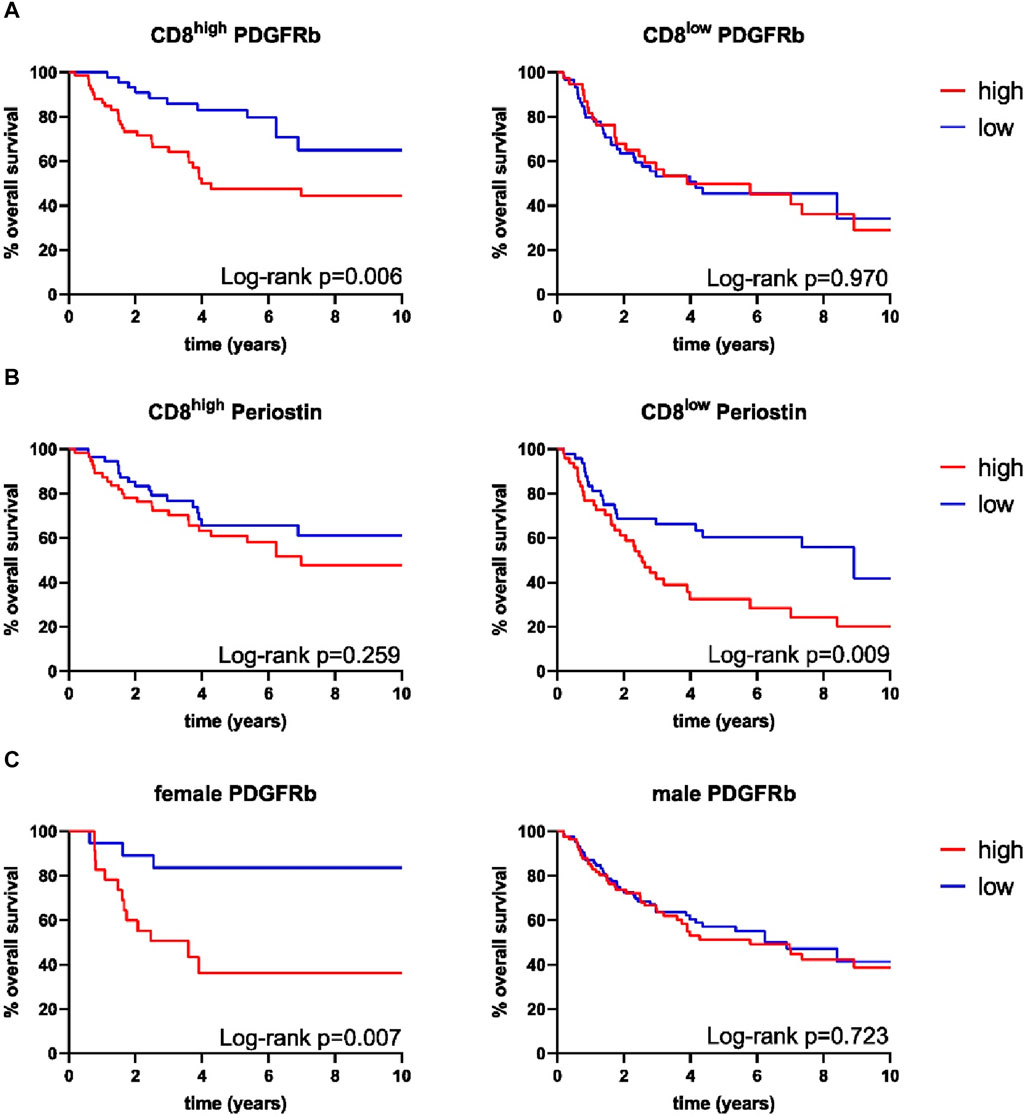
FIGURE 3. Kaplan-Meier curves for overall survival of combined expression of (A) platelet derived growth factor receptor beta (PDGFRb) in CD8high samples (high n = 67, low n = 46), PDGFRb in CD8low samples (high n = 38, low n = 60), (B) periostin in CD8high samples (high n = 38, low n = 60), periostin in CD8low samples (high n = 48, low n = 49), (C) PDGFRb in female samples (high n = 23, low n = 20), PDGFRb in male samples (high n = 83, low n = 86), p-values calculated by log-rank test.
In survival analysis by multivariate Cox regression, positive HPV-status was associated with improved survival in univariate and multivariate analyses (HR 0.528, CI 0.324–0.858, p = 0.01) (Table 1). Tumor infiltration of >50 CD8 T lymphocytes/mm2 improved patient survival significantly (HR 0.643, CI 0.421–0.983, p = 0.042) while a higher UICC stage (Stage I-III vs. Stage IV) was associated with worse survival (HR 2.962, CI 1.785–4.915, p < 0.001). FAP, PDGFRb, periostin and α-SMA as individual parameters did not predict patient survival (FAP p = 0.857, PDGFRb p = 0.522, periostin p = 0.088, α-SMA p = 0.120) in multivariate COX regression (Table 3).
To allow the further characterization of fibroblast activation marker expression patterns, we analyzed co-expression of the four examined fibroblast activation markers and assessed their correlation with patient survival (Figure 4). Expression patterns of FAPhigh/low periostinhigh/low (p = 0.084), FAPhigh/low α-SMAhigh/low (p = 0.139) and FAPhigh/low PDGFRbhigh/low (p = 0.578) were not associated with improved patient survival (Figures 4A–C). The combined expression status of PDGFRbhigh/low periostinhigh/low just reached a statistically significant impact on overall survival (p = 0.05) (Figure 4D). Combined status of periostinhigh/low α-SMAhigh/low (p = 0.009) and PDGFRbhigh/low α-SMAhigh/low (p = 0.017) facilitated the stratification of patient survival as for both combinations of CAF markers, double low expression status had the highest overall survival. As co-expression of other CAF markers with FAP did not reveal significant association with patient survival, we focused on the combined expression status of PDGFRb, α-SMA and periostin in relation to patient survival. PDGFRblow α-SMAlow periostinlow status was associated with improved overall survival in comparison to patients with high expression of one, two or three markers in univariate and multivariate survival analysis (HR 0.377, CI 0.189–0.752, p = 0.006) (Figure 4G) (Table 4).
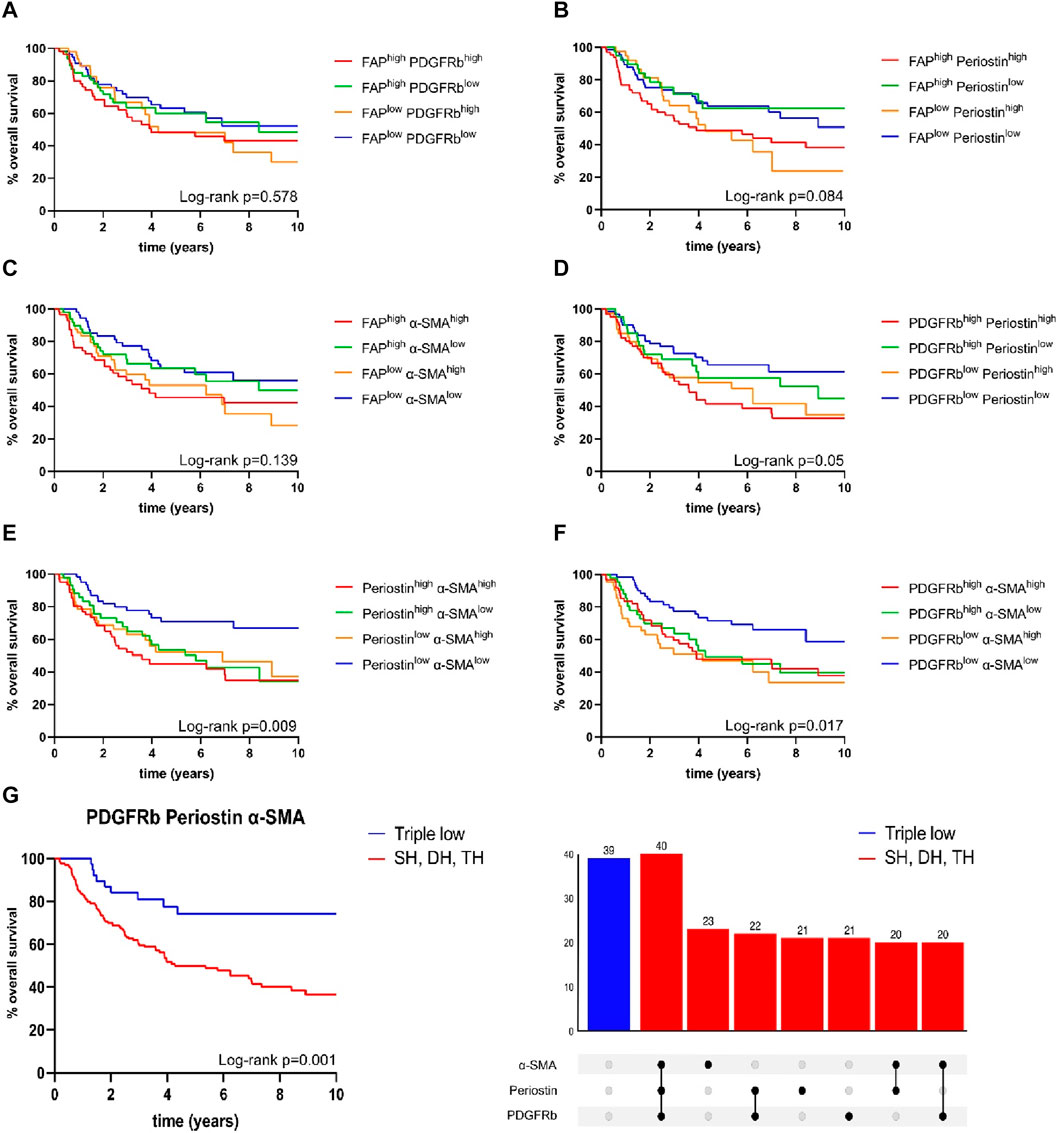
FIGURE 4. Kaplan-Meier curves for overall survival in correlation to expression status of (A) fibroblast activation protein (FAP)high/low platelet derived growth factor receptor beta (PDGFRb)high/low, (B) FAPhigh/low periostinhigh/low, (C) FAPhigh/low α-smooth muscle actin (α-SMA)high/low, (D) PDGFRbhigh/low periostinhigh/low, (E) periostinhigh/lowα-SMAhigh/low, (F) PDGFRbhigh/lowα-SMAhigh/low; (G) PDGFRblow, α-smooth muscle actin (α-SMA)low, periostinlow samples (n = 39) compared to samples high for one or more markers (n = 167); p-values calculated by log-rank test; SH single high, DH double high, TH triple high, p-values calculated by log-rank test.
4 Discussion
The heterogenous population of CAFs in OPSCC remains unsatisfactorily defined. However, our data clearly show an association of FAP and PDGFRb expression with intratumoral CD8 T-cell infiltration and we identify a PDGFRblow α-SMAlow periostinlow expression pattern as an independent predictor of improved survival in OPSCC. Therefore, the composition of intratumoral and peritumoral CAFs may be a biomarker to identify suitable patients for future treatment de-escalation trials. Targeting CAFs and specific CAF subpopulations, potentially in combination with immunotherapy, is an attractive avenue for the development of future targeted therapies in OPSCC.
Targeted immunotherapies have improved patient survival rates, but less than 20% of patients have achieved durable tumor regression (Ferris et al., 2016; Burtness et al., 2019). To further improve immunotherapy outcome, a comprehensive understanding of the TME, which includes diverse infiltrating cell types including CAFs, is required. Furthermore, PET/CT imaging using [68 Ga]-radiolabeled inhibitors of FAP utilizes CAFs to detect cancer tissue, thus enabling the classification of disease extent and spread based on CAF abundance. The heterogeneity of CAFs and their subtypes complicates the identification of one common marker for activated CAFs that can be established for routine clinical testing and patient stratification. The concept of α-SMA upregulation as a sign for activated CAFs is currently questioned due to existence of α-SMAlow CAFs (Patel et al., 2018). With the rise of high-resolution sequencing techniques, including single cell RNA sequencing, multiple gene signatures and biomarkers for CAFs have been identified primarily at the RNA level (Yamamoto et al., 2022; Zhou et al., 2022; Dong et al., 2023; Dwivedi et al., 2023). CAFs are presumed to be of various cell origins with diverse protumorigenic and arguably also tumor suppressive functions (Kalluri, 2016; Wang et al., 2022; Grout et al., 2022). This study investigated expression patterns of the previously established fibroblast markers FAP, α-SMA, PDGFRb and periostin in 216 OPSCC patients to not only be able to define co-expression patterns of CAF markers; but also create a tool that can find its way to routine clinical diagnostics in the future.
Oropharyngeal cancer caused by persistent high-risk HPV infection is a distinct cancer entity with different molecular and clinical features and improved patient survival when compared to HPV-negative OPSCC (Lechner et al., 2022; Mehanna et al., 2023). In line with extensive previously published evidence, HPV positivity conferred improved survival in our patient cohort (Ang et al., 2010; Lechner et al., 2022; Mehanna et al., 2023). HPV status was not correlated to the expression of examined fibroblast markers. These results contrast a previous report by Wang et al. (2022) who reported lower α-SMA expression in HPV-positive HNSCC compared to HPV-negative tumors. This discordance may be explained by the method used for HPV detection. While Wang et al. based HPV status solely on p16 expression, we used combined positivity of HPV DNA and p16 to define HPV-positive tumors as recent evidence suggests this classification to be a better predictor of the improved survival rates conferred by HPV-positive tumors (Mehanna et al., 2023). Stratification of HPV-positive tumors according to FAP, PDGFRb, periostin and α-SMA did not reveal significant association with overall survival in this subgroup (Supplementary Figure S2). Nonetheless, future studies should further examine HPV status in relation to specific CAF subgroups, as HPV serves as a crucial distinguishing characteristic in OPSCC.
Recent studies investigated possible subtypes of CAFs, categorizing them according to characteristic marker genes into inflammatory CAFs (iCAF), myo-cancer-associated fibroblasts (mCAF) and antigen presenting CAFs (apCAFs) (Elyada et al., 2019; Zhang et al., 2021; Wang et al., 2022). The role of intratumoral MHCII restricted antigen presentation by apCAFs remains elusive. While apCAFs isolated from orthotopic mouse tumor models were shown to induce lymphocyte activation, as measured by CD25 and CD69 upregulation, in an antigen specific manner, apCAFs express low levels of costimulatory molecules CD80, CD86 and CD40 when compared to professional antigen presenting cells (Elyada et al., 2019). A similar subset of apCAFs was detected in single cell RNA-seq of HNSCCs (Zhang et al., 2021). Expression of fibroblast markers FAP, PDGFRb and α-SMA is highest in mCAFs but PDGFRb and FAP gene expression was detectable in other CAF subsets as well (Elyada et al., 2019; Zhang et al., 2021). We included CD8 T-cell infiltration in our analysis to elucidate intratumoral cytotoxic lymphocyte abundance in the context of CAF background. In line with previous reports from HPV-positive and negative OPSCC, high CD8 T-cell infiltration facilitated improved patient survival (Jung et al., 2013; Haist et al., 2023). Furthermore, CD8 infiltration was positively associated with FAP and PDGFRb high tumors. CAFs can modulate intratumoral immune cell abundance via cytokines, growth factors and chemokines including CC-chemokine 2 (CCL2), CCL5, colony-stimulating factor 1 (CSF1), CXC-chemokine 5 (CXCL5), CXCL9 and CXCL10 (Chen et al., 2021). Grout et al. and others established a pattern of CAF mediated T-cell exclusion which is associated with resistance to immunotherapy (Ford et al., 2020; Grout et al., 2022). High levels of intratumoral periostin have been associated with increased infiltration of PD-L1 positive, immunosuppressive M2-like macrophages, thus the dismal overall survival of CD8low periostinhigh patients may be linked to high levels of M2-like macrophage mediated inhibition of effector T-cells (Wei et al., 2023). Within the CD8high population, PDGFRbhigh status delineates patients with impaired survival. Previous reports describe elevated evasion of immune surveillance mediated by intratumoral transforming growth factor-beta (TGF-β) signaling, which is positively associated to PDGFRb expression (Baba et al., 2022; Zhang Y. et al., 2022). In an analysis of metastatic urothelial cancer, a high TGF- β signature in fibroblasts attenuated response to PD-L1 blockade and contributed to CD8 T-cell exclusion (Mariathasan et al., 2018). Further analyses of the tumor stroma and invasive margin are warranted to specifically address differential heterogenous CAF populations in the context of tumor immune infiltration, patient survival and sex specific aspects in OPSCC.
These analyses may encompass a thorough exploration and enhanced comprehension of additional CAF markers, notably integrins. Integrins are recognized for their interactions with growth factors and exhibit co-expression patterns with various other CAF markers. Notably, the overexpression of Integrin α11 in head and neck squamous cell carcinoma demonstrated a positive correlation with α-SMA, underscoring the intricate interplay among these markers in the context of tumor biology (Parajuli et al., 2017).
An intriguing avenue for additional research could delve into the interaction of another PDGF receptor isoform, namely, PDGFRα. While both PDGFR isoforms exhibit some overlap in signaling pathways, there is compelling evidence pointing to a distinctly diverse impact on fibroblasts. This includes instances where PDGFR, depending on isoform, demonstrates the ability to either inhibit or stimulate fibroblast chemotaxis (Heldin et al., 1985; Heldin and Westermark, 1999). Exploring the co-expression patterns of PDGFRα with the markers discussed here could provide valuable insights, shedding light on the intricate interactions among CAF markers.
A limitation of the IHC analyses described here is the lack of an accepted cutoff for positivity of fibroblast markers. After careful evaluation and review of the literature we chose to use the median expression in our patient population as there is no established consensus on cutoffs and reported thresholds vary broadly (Tsujino et al., 2007; Liu et al., 2016; Fang et al., 2022). Previous publications from our group and others successfully used a similar classification to analyze CAFs in other tumor entities (Costa et al., 2018; Knipper et al., 2023). Investigating the association of treatment response and CAFs is a topic of great importance to allow patient selection based on CAF marker expression. Unfortunately, a rigorous investigation of response rates to different treatment modalities in relation to CAF marker expression was not feasible in the setting of this study, as no sufficient data regarding adjuvant are available to the authors and missing data points would have introduced a substantial bias.
Given the expanding complexity of differential fibroblast sets in cancers, the present study enhances the current understanding of CAFs in OPSCC. Importantly, we establish PDGFRblow α-SMAlow periostinlow status as an independent predictor of improved survival. Future studies exploring the reciprocal shaping of cancer cells, fibroblasts and tumor infiltrating immune cells are warranted and should consider multiplexed analyses to allow the specific classification of CAF subsets.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics committee of the University of Cologne. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because Tumor Tissue was obtained after patient treatment was concluded and analyses did not affect patients. Patients are not identifiable with the analyses performed.
Author contributions
SL: Data curation, Formal Analysis, Investigation, Methodology, Writing–original draft, Writing–review and editing. JJ: Resources, Writing–review and editing. AGS: Methodology, Writing–review and editing. KK: Formal Analysis, Writing–review and editing. NW: Investigation, Writing–review and editing. SS: Investigation, Writing–review and editing. MT: Validation, Visualization, Writing–review and editing. KH: Writing–review and editing. CF: Methodology, Writing–review and editing. CK: Investigation, Writing–review and editing. JE: Writing–original draft, Writing–review and editing. MS: Writing–review and editing. HA: Writing–review and editing. PZ: Writing–review and editing. AMS: Investigation, Methodology, Writing–review and editing. HS: Investigation, Writing–review and editing. JK: Conceptualization, Writing–review and editing. AQ: Investigation, Methodology, Resources, Supervision, Writing–review and editing. HE: Data curation, Formal Analysis, Investigation, Project administration, Resources, Visualization, Writing–original draft, Writing–review and editing.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article. HE was supported by the Koeln Fortune Program/Faculty of Medicine, University of Cologne. We acknowledge support for the Article Processing Charge from the DFG (German Research Foundation, 491454339).
Acknowledgments
We thank Wiebke Jeske for her continuous assistance.
Conflict of interest
HS: Funding for Research by Astra Zeneca and Tabby therapeutics; SAB for BMS. JK: Honoraria for advisory boards from BMS, MSD, for invited talks from Astra Zeneca, BMS, MSD, Merck and financial support for research projects from MSD. HE: Honoraria for advisory boards from MSD.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2024.1337361/full#supplementary-material
Abbreviations
OPSCC, oropharyngeal squamous cell carcinoma; CAF, carcinoma associated fibroblast; FAP, fibroblast activation protein; PDGFRb, platelet-derived growth factor receptor beta; α-SMA, alpha smooth muscle actin; HNSCC, head and neck squamous cell carcinoma; HPV, human papilloma virus; ICB, immune checkpoint blockade; TME, tumor micro environment; ECM, extracellular matrix; CAR T-cells, chimeric antigen receptor T-cells; FAPI, fibroblast activation protein inhibitor; PET, positron emission tomography; myCAF, myofibroblasts; ICD-O, International Classification of Diseases for Oncology; TMA, tissue microarrays; FFPE, Formalin fixed paraffin embedded; p16INK4a, p16; IHC, immunohistochemistry; OS, overall survival; HR, hazard ratio; CI, confidence interval; G, grade; iCAF, inflammatory carcinoma associated fibroblast; mCAF, myo carcinoma -associated fibroblast; apCAF, antigen presenting carcinoma associated fibroblast; TGF-β, transforming growth factor-beta; CCL2, CC-chemokine 2; CSF1, colony-stimulating factor 1; CXCL5, CXC-chemokine 5.
References
Ang, K. K., Harris, J., Wheeler, R., Weber, R., Rosenthal, D. I., Nguyen-Tân, P. F., et al. (2010). Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 363 (1), 24–35. doi:10.1056/nejmoa0912217
Baba, A. B., Rah, B., Bhat, G. R., Mushtaq, I., Parveen, S., Hassan, R., et al. (2022). Transforming growth factor-beta (TGF-β) signaling in cancer-A betrayal within. Front. Pharmacol. 13, 2022. doi:10.3389/fphar.2022.791272
Bankhead, P., Loughrey, M. B., Fernández, J. A., Dombrowski, Y., McArt, D. G., Dunne, P. D., et al. (2017). QuPath: open source software for digital pathology image analysis. Sci. Rep. 7 (1), 16878. doi:10.1038/s41598-017-17204-5
Benjamini, Y., and Hochberg, Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 57, 289–300. doi:10.1111/j.2517-6161.1995.tb02031.x
Bran, B., Bran, G., Hörmann, K., and Riedel, F. (2009). The platelet-derived growth factor receptor as a target for vascular endothelial growth factor-mediated anti-angiogenetic therapy in head and neck cancer. Int. J. Oncol. 34 (1), 255–261. doi:10.3892/ijo_00000147
Brechbuhl, H. M., Finlay-Schultz, J., Yamamoto, T. M., Gillen, A. E., Cittelly, D. M., Tan, A. C., et al. (2017). Fibroblast subtypes regulate responsiveness of luminal breast cancer to estrogen. Clin. Cancer Res. 23 (7), 1710–1721. doi:10.1158/1078-0432.CCR-15-2851
Burtness, B., Harrington, K. J., Greil, R., Soulières, D., Tahara, M., de Castro, G., et al. (2019). Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 394 (10212), 1915–1928. doi:10.1016/S0140-6736(19)32591-7
Busek, P., Mateu, R., Zubal, M., Kotackova, L., and Sedo, A. (1933). Targeting fibroblast activation protein in cancer-Prospects and caveats. Front. Biosci. 23, 1933–1968. doi:10.2741/4682
Chen, Y., McAndrews, K. M., and Kalluri, R. (2021). Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat. Rev. Clin. Oncol. 18 (12), 792–804. doi:10.1038/s41571-021-00546-5
Cords, L., Tietscher, S., Anzeneder, T., Langwieder, C., Rees, M., De Souza, N., et al. (2023). Cancer-associated fibroblast classification in single-cell and spatial proteomics data. Nat. Commun. 14 (1), 4294. doi:10.1038/s41467-023-39762-1
Costa, A., Kieffer, Y., Scholer-Dahirel, A., Pelon, F., Bourachot, B., Cardon, M., et al. (2018). Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell 33 (3), 463–479. doi:10.1016/j.ccell.2018.01.011
Dong, L., Sun, Q., Song, F., Song, X., Lu, C., Li, Y., et al. (2023). Identification and verification of eight cancer-associated fibroblasts related genes as a prognostic signature for head and neck squamous cell carcinoma. Heliyon 9 (3), e14003. doi:10.1016/j.heliyon.2023.e14003
Dwivedi, N., Shukla, N., Prathima, K. M., Das, M., and Dhar, S. K. (2023). Novel CAF-identifiers via transcriptomic and protein level analysis in HNSC patients. Sci. Rep. 13(1), 13899–13910. doi:10.1038/s41598-023-40908-w
Elmusrati, A., Wang, J., and Wang, C. Y. (2021). Tumor microenvironment and immune evasion in head and neck squamous cell carcinoma. Int. J. Oral Sci. 13, 24. doi:10.1038/s41368-021-00131-7
Elyada, E., Bolisetty, M., Laise, P., Flynn, W. F., Courtois, E. T., Burkhart, R. A., et al. (2019). Cross-Species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Discov. 9 (8), 1102–1123. doi:10.1158/2159-8290.CD-19-0094
Fang, Y., Chen, M., Li, G., Yang, Y., He, P., Chen, J., et al. (2022). Cancer-associated fibroblast-like fibroblasts in vocal fold leukoplakia suppress CD8+T cell functions by inducing IL-6 autocrine loop and interacting with Th17 cells. Cancer Lett. 546, 215839. doi:10.1016/j.canlet.2022.215839
Ferris, R. L., Blumenschein, G., Fayette, J., Guigay, J., Colevas, A. D., Licitra, L., et al. (2016). Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 375 (19), 1856–1867. doi:10.1056/NEJMoa1602252
Ferris, R. L., and Westra, W. (2023). Oropharyngeal carcinoma with a special focus on HPV-related squamous cell carcinoma. Annu. Rev. Pathol. 18, 515–535. doi:10.1146/annurev-pathmechdis-031521-041424
Ford, K., Hanley, C. J., Mellone, M., Szyndralewiez, C., Heitz, F., Wiesel, P., et al. (2020). NOX4 inhibition potentiates immunotherapy by overcoming cancer-associated fibroblast-mediated CD8 T-cell exclusion from tumors. Cancer Res. 80 (9), 1846–1860. doi:10.1158/0008-5472.CAN-19-3158
Foster, D. S., Januszyk, M., Delitto, D., Yost, K. E., Griffin, M., Guo, J., et al. (2022). Multiomic analysis reveals conservation of cancer-associated fibroblast phenotypes across species and tissue of origin. Cancer Cell 40 (11), 1392–1406.e7. doi:10.1016/j.ccell.2022.09.015
Galbo, P. M., Zang, X., and Zheng, D. (2021). Molecular features of cancer-associated fibroblast subtypes and their implication on cancer pathogenesis, prognosis, and immunotherapy resistance. Clin. Cancer Res. 27 (9), 2636–2647. doi:10.1158/1078-0432.CCR-20-4226
Grout, J. A., Sirven, P., Leader, A. M., Maskey, S., Hector, E., Puisieux, I., et al. (2022). Spatial positioning and matrix programs of cancer-associated fibroblasts promote T-cell exclusion in human lung tumors. Cancer Discov. 12 (11), 2606–2625. doi:10.1158/2159-8290.CD-21-1714
Haist, M., Kaufmann, J., Kur, I. M., Zimmer, S., Grabbe, S., Schmidberger, H., et al. (2023). Response to primary chemoradiotherapy of locally advanced oropharyngeal carcinoma is determined by the degree of cytotoxic T cell infiltration within tumor cell aggregates. Front. Immunol. 14, 1070203. doi:10.3389/fimmu.2023.1070203
Heldin, C. H., and Westermark, B. (1999). Mechanism of action and in vivo role of platelet-derived growth factor. Physiol. Rev. 79 (4), 1283–1316. doi:10.1152/physrev.1999.79.4.1283
Heldin, C. H., Å, W., and Westermark, B. (1985). Platelet-derived growth factor. Mol. Cell Endocrinol. 39 (3), 169–187. doi:10.1016/0303-7207(85)90061-9
Jung, A. C., Guihard, S., Krugell, S., Ledrappier, S., Brochot, A., Dalstein, V., et al. (2013). CD8-alpha T-cell infiltration in human papillomavirus-related oropharyngeal carcinoma correlates with improved patient prognosis. Int. J. Cancer 132 (2), E26–E36. doi:10.1002/ijc.27776
Kalluri, R. (2016). The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 16, 582–598. doi:10.1038/nrc.2016.73
Kanzaki, R., and Pietras, K. (2020). Heterogeneity of cancer-associated fibroblasts: opportunities for precision medicine. Cancer Sci. 111 (8), 2708–2717. doi:10.1111/cas.14537
Kian Ang, K., Harris, J., Wheeler, R., Weber, R., Rosenthal, D. I., Nguyen-Tân, F., et al. (2010). Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 363, 24–35. doi:10.1056/nejmoa0912217
Klussmann, J. P., Gültekin, E., Weissenborn, S. J., Wieland, U., Dries, V., Dienes, H. P., et al. (2003). Expression of p16 protein identifies a distinct entity of tonsillar carcinomas associated with human papillomavirus. Am. J. Pathol. 162 (3), 747–753. doi:10.1016/S0002-9440(10)63871-0
Knipper, K., Damanakis, A. I., Zhao, Y., Bruns, C. J., Schmidt, T., Popp, F. C., et al. (2023). Specific subtypes of carcinoma-associated fibroblasts are correlated with worse survival in resectable pancreatic ductal adenocarcinoma. Cancers (Basel) 15 (7), 2049. doi:10.3390/cancers15072049
Kratochwil, C., Flechsig, P., Lindner, T., Abderrahim, L., Altmann, A., Mier, W., et al. (2019). 68Ga-FAPI PET/CT: tracer uptake in 28 different kinds of cancer. J. Nucl. Med. 60 (6), 801–805. doi:10.2967/jnumed.119.227967
Kudo, Y., Ogawa, I., Kitajima, S., Kitagawa, M., Kawai, H., Gaffney, P. M., et al. (2006). Periostin promotes invasion and anchorage-independent growth in the metastatic process of head and neck cancer. Cancer Res. 66 (14), 6928–6935. doi:10.1158/0008-5472.CAN-05-4540
Lechner, M., Liu, J., Masterson, L., and Fenton, T. R. (2022). HPV-associated oropharyngeal cancer: epidemiology, molecular biology and clinical management. Nat. Rev. Clin. Oncol. 19, 306–327. doi:10.1038/s41571-022-00603-7
Liu, F., Qi, L., Liu, B., Liu, J., Zhang, H., Che, D. H., et al. (2015). Fibroblast activation protein overexpression and clinical implications in solid tumors: a meta-analysis. PLoS One 10 (3), e0116683. doi:10.1371/journal.pone.0116683
Liu, L., Liu, L., Yao, H. H., Zhu, Z. Q., Ning, Z. L., and Huang, Q. (2016). Stromal myofibroblasts are associated with poor prognosis in solid cancers: a meta-analysis of published studies. PLoS One 11 (7), e0159947. doi:10.1371/journal.pone.0159947
Mao, X., Xu, J., Wang, W., Liang, C., Hua, J., Liu, J., et al. (2021). Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol. Cancer 20 (1), 131–230. doi:10.1186/s12943-021-01428-1
Mariathasan, S., Turley, S. J., Nickles, D., Castiglioni, A., yuen, K., Wang, Y., et al. (2018). TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554 (7693), 544–548. doi:10.1038/nature25501
Mehanna, H., Taberna, M., von Buchwald, C., Tous, S., Brooks, J., Mena, M., et al. (2023). Prognostic implications of p16 and HPV discordance in oropharyngeal cancer (HNCIG-EPIC-OPC): a multicentre, multinational, individual patient data analysis. Lancet Oncol. 24, 239–251. doi:10.1016/S1470-2045(23)00013-X
Nakamura, K., and Smyth, M. J. (2019). Myeloid immunosuppression and immune checkpoints in the tumor microenvironment. Cell. Mol. Immunol. 17 (1), 1–12. doi:10.1038/s41423-019-0306-1
Ostermann, E., Garin-Chesa, P., Heider, K. H., Kalat, M., Lamche, H., Puri, C., et al. (2008). Effective immunoconjugate therapy in cancer models targeting a serine protease of tumor fibroblasts. Clin. Cancer Res. 14 (14), 4584–4592. doi:10.1158/1078-0432.CCR-07-5211
Özdemir, B. C., Pentcheva-Hoang, T., Carstens, J. L., Zheng, X., Wu, C. C., Simpson, T. R., et al. (2014). Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 25 (6), 719–734. doi:10.1016/j.ccr.2014.04.005
Parajuli, H., Teh, M. T., Abrahamsen, S., Christoffersen, I., Neppelberg, E., Lybak, S., et al. (2017). Integrin a11 is overexpressed by tumour stroma of head and neck squamous cell carcinoma and correlates positively with alpha smooth muscle actin expression. J. Oral Pathol. Med. 46, 267–275. doi:10.1111/jop.12493
Patel, A. K., Vipparthi, K., Thatikonda, V., Arun, I., Bhattacharjee, S., Sharan, R., et al. (2018). A subtype of cancer-associated fibroblasts with lower expression of alpha-smooth muscle actin suppresses stemness through BMP4 in oral carcinoma. Oncogenesis 7 (10), 78–15. doi:10.1038/s41389-018-0087-x
Pignon, J. P., Maître, A. le, Maillard, E., and Bourhis, J. (2009). Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiotherapy Oncol. 92 (1), 4–14. doi:10.1016/j.radonc.2009.04.014
Pitt, J. M., Marabelle, A., Eggermont, A., Soria, J. C., Kroemer, G., and Zitvogel, L. (2016). Targeting the tumor microenvironment: removing obstruction to anticancer immune responses and immunotherapy. Ann. Oncol. 27 (8), 1482–1492. doi:10.1093/annonc/mdw168
Shen, Y., Wang, X., Lu, J., Salfenmoser, M., Wirsik, N. M., Schleussner, N., et al. (2020). Reduction of liver metastasis stiffness improves response to bevacizumab in metastatic colorectal cancer. Cancer Cell 37 (6), 800–817. doi:10.1016/j.ccell.2020.05.005
Sonnenberg-Riethmacher, E., Miehe, M., and Riethmacher, D. (2021). Periostin in allergy and inflammation. Front. Immunol. 12, 722170. doi:10.3389/fimmu.2021.722170
Su, S., Chen, J., Yao, H., Liu, J., Yu, S., Lao, L., et al. (2018). CD10+GPR77+ cancer-associated fibroblasts promote cancer formation and chemoresistance by sustaining cancer stemness. Cell 172 (4), 841–856. doi:10.1016/j.cell.2018.01.009
Tomasek, J. J., Gabbiani, G., Hinz, B., Chaponnier, C., and Brown, R. A. (2002). Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 3 (5), 349–363. doi:10.1038/nrm809
Tosi, A., Parisatto, B., Menegaldo, A., Spinato, G., Guido, M., Del Mistro, A., et al. (2022). The immune microenvironment of HPV-positive and HPV-negative oropharyngeal squamous cell carcinoma: a multiparametric quantitative and spatial analysis unveils a rationale to target treatment-naïve tumors with immune checkpoint inhibitors. J. Exp. Clin. Cancer Res. 41 (1), 279. doi:10.1186/s13046-022-02481-4
Tsujino, T., Seshimo, I., Yamamoto, H., Yee Ngan, C., Ezumi, K., Takemasa, I., et al. (2007). Stromal myofibroblasts predict disease recurrence for colorectal cancer. Clin. Cancer Res. 13 (7), 2082–2090. doi:10.1158/1078-0432.CCR-06-2191
Wang, B., Zhang, S., Tong, F., Wang, Y., and Wei, L. (2022b). HPV+ HNSCC-derived exosomal miR-9-5p inhibits TGF-β signaling-mediated fibroblast phenotypic transformation through NOX4. Cancer Sci. 113 (4), 1475–1487. doi:10.1111/cas.15281
Wang, Z., Zhang, H., Zhai, Y., Li, F., Shi, X., and Ying, M. (2022a). Single-cell profiling reveals heterogeneity of primary and lymph node metastatic tumors and immune cell populations and discovers important prognostic significance of CCDC43 in oral squamous cell carcinoma. Front. Immunol. 13, 843322. doi:10.3389/fimmu.2022.843322
Wegen, S., van Heek, L., Linde, P., Claus, K., Akuamoa-Boateng, D., Baues, C., et al. (2022). Head-to-Head comparison of [68 Ga]Ga-FAPI-46-PET/CT and [18F]F-FDG-PET/CT for radiotherapy planning in head and neck cancer. Mol. Imaging Biol. 24 (6), 986–994. doi:10.1007/s11307-022-01749-7
Wei, T., Wang, K., Liu, S., Fang, Y., Hong, Z., Liu, Y., et al. (2023). Periostin deficiency reduces PD-1+ tumor-associated macrophage infiltration and enhances anti-PD-1 efficacy in colorectal cancer. Cell Rep. 42 (2), 112090. doi:10.1016/j.celrep.2023.112090
Yamamoto, Y., Kasashima, H., Fukui, Y., Tsujio, G., Yashiro, M., and Maeda, K. (2022). The heterogeneity of cancer-associated fibroblast subpopulations: their origins, biomarkers, and roles in the tumor microenvironment. Cancer Sci. 114, 16–24. doi:10.1111/cas.15609
Zhang, Q., Wang, Y., Xia, C., Ding, L., Pu, Y., Hu, X., et al. (2021). Integrated analysis of single-cell RNA-seq and bulk RNA-seq reveals distinct cancer-associated fibroblasts in head and neck squamous cell carcinoma. Ann. Transl. Med. 9 (12), 1017. doi:10.21037/atm-21-2767
Zhang, T., Ren, Y., Yang, P., Wang, J., and Zhou, H. (2022a). Cancer-associated fibroblasts in pancreatic ductal adenocarcinoma. Cell Death Dis. 13 (10), 897–911. doi:10.1038/s41419-022-05351-1
Zhang, Y., Manouchehri Doulabi, E., Herre, M., Cedervall, J., Qiao, Q., Miao, Z., et al. (2022b). Coxsackievirus group B3 has oncolytic activity against colon cancer through gasdermin E-mediated pyroptosis. Cancers (Basel). 14 (8), 6206. doi:10.3390/cancers14246206
Keywords: oropharyngeal cancer, human papillomavirus, head and neck squamous cell carcinoma, platelet-derived growth factor receptor beta, alpha smooth muscle actin, periostin, fibroblast activation protein, cancer associated fibroblasts
Citation: Lyu SI, Johannsen J, Simon AG, Knipper K, Wuerdemann N, Sharma SJ, Thelen M, Hansen KK, Fretter C, Klasen C, Esser J, Suchan MC, Abing H, Zimmermann PH, Schultheis AM, Schloesser HA, Klussmann JP, Quaas A and Eckel HNC (2024) Co-expression patterns of cancer associated fibroblast markers reveal distinct subgroups related to patient survival in oropharyngeal squamous cell carcinoma. Front. Cell Dev. Biol. 12:1337361. doi: 10.3389/fcell.2024.1337361
Received: 15 November 2023; Accepted: 05 January 2024;
Published: 24 January 2024.
Edited by:
Alessandro Arcucci, University of Naples Federico II, ItalyReviewed by:
Irina Primac, Université Libre de Bruxelles, BelgiumNingxin Ma, Fred Hutchinson Cancer Center, United States
Copyright © 2024 Lyu, Johannsen, Simon, Knipper, Wuerdemann, Sharma, Thelen, Hansen, Fretter, Klasen, Esser, Suchan, Abing, Zimmermann, Schultheis, Schloesser, Klussmann, Quaas and Eckel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hans Nikolaus Caspar Eckel, aGFucy5lY2tlbEB1ay1rb2Vsbi5kZQ==
 Su Ir Lyu1
Su Ir Lyu1 Adrian Georg Simon
Adrian Georg Simon Nora Wuerdemann
Nora Wuerdemann Shachi Jenny Sharma
Shachi Jenny Sharma Anne Maria Schultheis
Anne Maria Schultheis Hans Anton Schloesser
Hans Anton Schloesser Jens Peter Klussmann
Jens Peter Klussmann Alexander Quaas
Alexander Quaas Hans Nikolaus Caspar Eckel
Hans Nikolaus Caspar Eckel