- 1School of Nursing and Midwifery, Bonab University of Medical Sciences, Bonab, Iran
- 2Department of Medical Genetics and Molecular Biology, School of Medicine, Iran University of Medical Sciences, Tehran, Iran
- 3School of Nursing and Midwifery, Tehran Medical Branch, Islamic Azad University, Tehran, Iran
- 4Tabriz Islamic Azad University of Medical Sciences, Tabriz, Iran
- 5Department of obstetrics and gynecology, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
- 6Department of Pathology, Faculty of Medicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
- 7Nervous System Stem Cells Research Center, Semnan University of Medical Sciences, Semnan, Iran
- 8Department of Medical Microbiology, Faculty of Medicine, Shahed University, Tehran, Iran
- 9Department of Immunology, School of Medicine, Semnan University of Medical Sciences, Semnan, Iran
- 10Iran University of Medical Sciences, Deputy of Health, Tehran, Iran
- 11Department of Anatomy, Faculty of Medicine, Qom Medical Sciences, Islamic Azad University, Qom, Iran
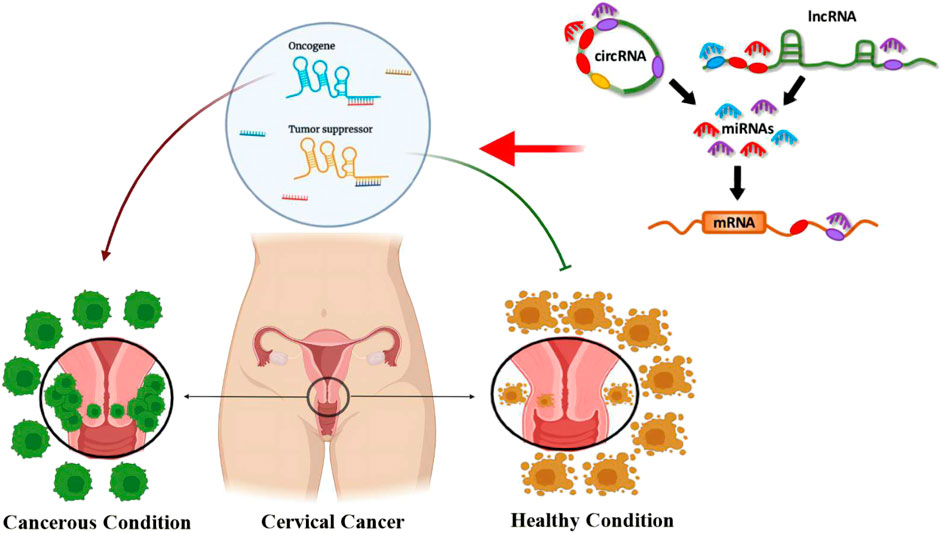
Cervical cancer (CC) is a primary global health concern, ranking as the fourth leading cause of cancer-related death in women. Despite advancements in prognosis, long-term outcomes remained poor. Beyond HPV, cofactors like dietary deficiencies, immunosuppression, hormonal contraceptives, co-infections, and genetic variations are involved in CC progression. The pathogenesis of various diseases, including cancer, has brought to light the critical regulatory roles of microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs). The aberrant expression of these miRNAs, lncRNAs, and circRNAs plays a pivotal role in the initiation and progression of CC. This review provides a comprehensive summary of the recent literature regarding the involvement of lncRNAs and circRNAs in modulating miRNA functions in cervical neoplasia and metastasis. Studies have shown that lncRNAs and circRNAs hold great potential as therapeutic agents and innovative biomarkers in CC. However, more clinical research is needed to advance our understanding of the therapeutic benefits of circRNAs and lncRNAs in CC.
Introduction
Uterine cervix carcinoma ranks among the most frequently diagnosed gynecological malignancies and is recognized as the fourth most fatal cancer in women on a global scale (He et al., 2020). In 2020, the approximate incidence, prevalence, and fatality rate accounted for 604,127, 1.495.211, and 341,831, respectively (Bray et al., 2018; Singh et al., 2023). Cervical cancer (CC) predominantly manifests in two primary histological forms: squamous cell carcinomas (SCC) and adenocarcinoma (ADC). Adenosquamous carcinoma (ADSC) is the less common type of CC (Nicolás-Párraga et al., 2017; Small et al., 2017). Notably, there has been an increased upsurge in ADC incidence, which is more prevalent among young adult women (Fujiwara et al., 2014). The primary risk factor contributing to CC is the presence of oncogenic human papillomavirus (HPV) (Savira et al., 2022). Previous studies have revealed that HPV 16 and 18 types account for approximately 70% of HPV infections (Aalijahan and Ghorbian, 2019). HPV18 is particularly common in individuals with ADC, while HPV16 is predominant in SCC cases (He et al., 2020). Despite advancements in CC prognosis, long-term outcomes remain poor due to resistance and recurrence (He et al., 2020). Addressing this issue necessitates further research to identify new biomarkers for precise CC progression monitoring and therapeutic targets to enhance survival rates.
The progression from HPV infection to cancer necessitates the involvement of other cofactors, including specific dietary deficiencies, immunosuppression, prolonged use of hormonal contraceptives, co-infection with Chlamydia trachomatis (CT), co-infection with herpes simplex virus type 2 (HSV-2) and HIV, high parity, and tobacco smoking, among other potential cofactors (Aalijahan and Ghorbian, 2019). Furthermore, individual genetic variations and distinct epigenetic modifications are pivotal in carcinogenesis. DNA methylation, histone modifications, and non-coding RNAs (ncRNAs) influence gene expression without altering the DNA sequence. The ncRNAs are prevalent throughout the entire genome and can be classified into two subclasses: small ncRNAs with 20–200 nucleotides and long ncRNAs (lncRNAs) with over 200 nucleotides (Hosseini et al., 2017).
Biogenesis of lncRNAs, circRNAs, and microRNAs
The biogenesis of lncRNAs can be divided into various categories, including exonic, intronic, intergenic, and overlapping (Hosseini et al., 2017). The lncRNAs, through both cis- and trans-regulation, can alter the phenotypes of cancer cells by targeting their final genes (Guttman and Rinn, 2012). Given their capacity to induce dysregulation in various human diseases and foster cancer development, lncRNAs hold significant potential as active biomarkers and, specifically, as therapeutic targets in cancer (He et al., 2020).
Small non-coding RNAs, particularly microRNAs (miRNAs), have been identified as key players in various cellular processes such as cell cycle regulation, inflammation, apoptosis, migration, and differentiation. Additionally, they influence messenger RNA (mRNA) translation and stability (Di Leva et al., 2014). Studies have substantiated that miRNAs can function as oncogenes or tumor suppressors in various cancer types (Romano et al., 2017; Rupaimoole and Slack, 2017). Understanding the regulatory mechanisms governing miRNA expression is closely intertwined with cancer diagnosis, prognosis, treatment, and underlying pathogenesis (Shen et al., 2020).
Circular RNAs (circRNAs) are a large family of non-coding RNAs in the transcript of eukaryotes. They appear as covalently closed loops formed by successive exons in the nucleus. CircRNAs have polar 5′ and 3′ ends and polyadenylated tails. There are many types of circRNAs in different cell lines and species, involving diverse ranges of biological processes between cells (Chen and Yang, 2015). After transferring to the cytoplasm, circRNAs perform various biological actions by binding to microRNAs and proteins (Ren et al., 2019). They play a vital role in transcriptional regulation and are potential biomarkers (Liang et al., 2021). Additionally, circRNAs are secreted into body fluids both in free form and encapsulation in multi-vesicular endosomes and exosomes along with lipids, proteins, and nucleic acids, controlling various biological functions in different areas of the body (Wang et al., 2019a), including fetal growth and brain synaptic plasticity (Liang et al., 2021). Moreover, abnormal expression of circRNAs is detected in the occurrence of diseases such as human malignancies, Alzheimer’s, and Parkinson’s disease (Nisar et al., 2021).
Biological functions of long non-coding RNAs
Long non-coding RNAs exhibit a broad spectrum of biological and regulatory functions, including proliferation, differentiation, development, and DNA damage repair (Wang and Chang, 2011). They interfere with transcription, translation, and post-transcriptional and post-translation processes, enabling a direct interaction with DNA, RNA, and proteins (He et al., 2020). Cis-acting lncRNAs are closely associated with specific transcription sites, affecting neighboring genes’ chromatin structure or expression state on the same allele. In contrast, trans-acting lncRNAs act from a distance, triggering the expression of genes, proteins, or RNA molecules within the cell (Guttman and Rinn, 2012; Kopp and Mendell, 2018).
LncRNAs are involved in epigenetic gene regulation through three main mechanisms. First, they influence the transcription of target genes by regulating histone modifications and the chromatin status. Second, lncRNAs actively recruit repressors or cell transcription factors to the promoters of their target genes. Third, they tightly bind to proteins involved in transcription, preventing them from attaching to their specific DNA targets (Long et al., 2017).
In addition, lncRNAs act as competing endogenous RNAs (ceRNAs) by binding to miRNAs, thereby preventing miRNA-mediated gene suppression (Gutschner and Diederichs, 2012). Some lncRNAs serve as templates or precursors for various small RNAs (Cheetham et al., 2013) and influence alternative mRNA splicing (Romero-Barrios et al., 2018). At the post-translational level, lncRNAs can bind to target proteins, affecting their localization and transportation. Previous studies have reported that lncRNAs function as scaffolds, facilitating protein–protein interactions, and the formation of protein complexes (Yoon et al., 2013) (Figure 1).
Relevant lncRNAs in cervical cancer
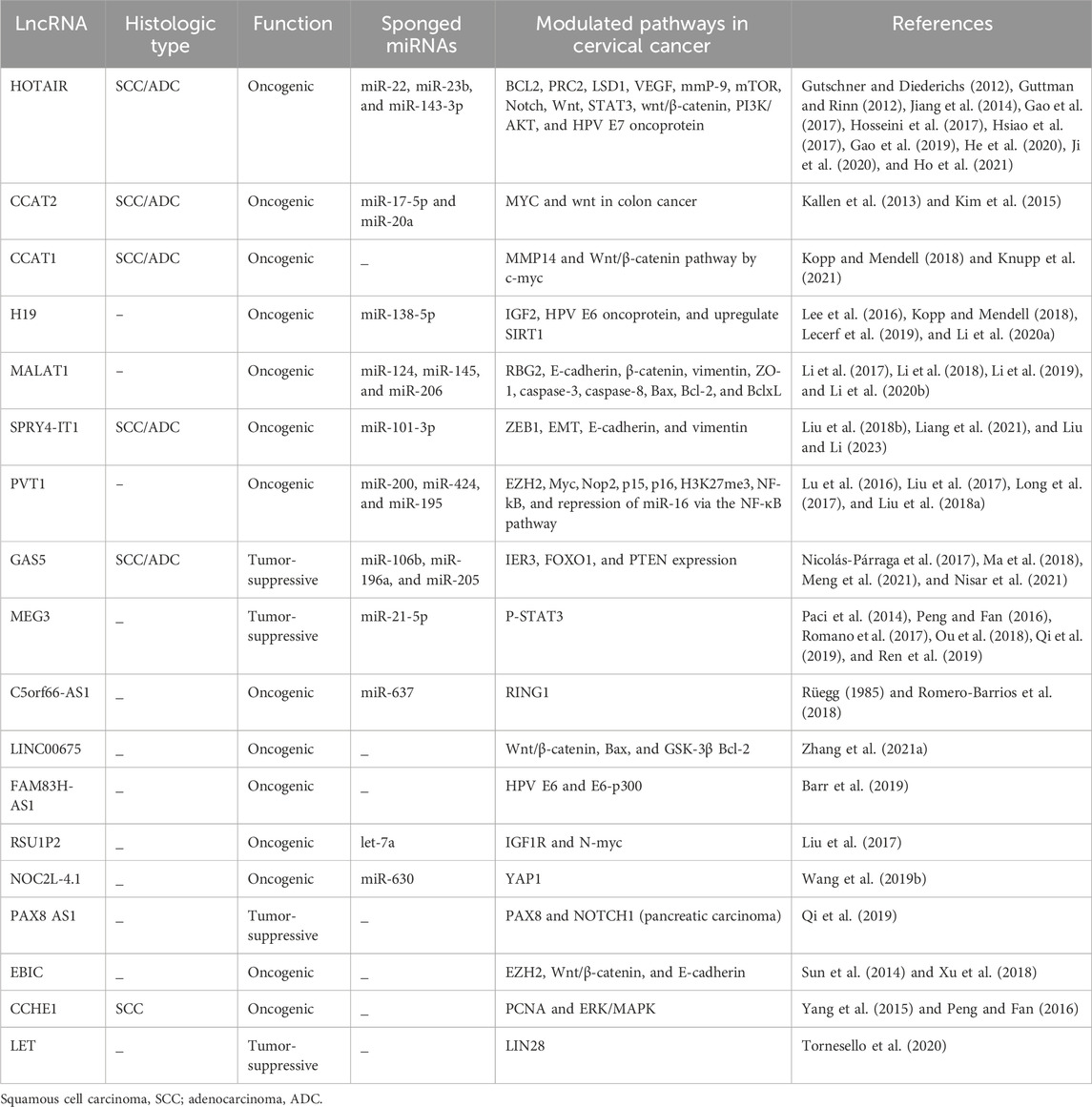
Long non-coding RNAs interact with proteins, mRNAs, and miRNAs and play a critical role in cancer progression. Several lncRNAs, including HOTAIR, CCAT2, H19, MALT1, SPRY4-IT1, PVT1, GAS5, MEG3, and C5orf66-AS1, have been identified as critical players in the development, invasion, metastasis, and radio resistance of CC (see Table 1) (Larrieta-Carrasco et al., 2018; Tornesello et al., 2020).
HOTAIR lncRNA
HOTAIR, “2.2 kb HOX transcript antisense intergenic RNA,” is one of the extensively studied oncogenic lncRNAs. It is encoded by the antisense strand of the HOXC gene and is located on chromosome 12 at position q13.13 (Rüegg, 1985). Like other lncRNAs, HOTAIR recruits chromatin-modifying proteins and influences the epigenome of cancer (Ren et al., 2019). In CC, the levels of HOTAIR are tightly controlled by the HPV E7 protein (Sharma et al., 2015). Furthermore, HOTAIR functions as a sponge for specific miRNAs, resulting in the dysregulation of their respective target genes. For instance, HOTAIR has been reported to alter the miR-143-3p/BCL2 axis, promoting CC cell growth (Liu et al., 2018a). It also modulates the miR-23b/MAPK1 axis, which is involved in cell proliferation and metastasis (Li et al., 2018). Additionally, HOTAIR overexpression has regulatory effects on various metabolic functions, such as the activation of the Notch-Wnt signaling pathway in SiHa cells (Lee et al., 2016) and the mTOR pathway in different CC cell lines, including C33A, HeLa, and CaSki (Zhang et al., 2015). The expression of HOTAIR leads to the upregulation of matrix metalloproteinase-9 (MMP-9), vascular endothelial growth factor (VEGF), and genes associated with the epithelial–mesenchymal transition (EMT), thereby promoting tumor aggressiveness in CC (Kim et al., 2015). It is worth noting that the HOTAIR enhancer contains a single nucleotide polymorphism, rs920778T, which is associated with HOTAIR overexpression and increased susceptibility to cancer (Xiao et al., 2011). In cases of HPV-positive CC, the rs920778 C HOTAIR allele is more prevalent, but its expression level may be reduced due to the binding of miR22 to the rs920778C sequence, leading to the suppression of the HOTAIR expression (Sh et al., 2016).
CCAT2 lncRNA
Substantial upregulation of the long non-coding RNA, known as colon cancer-related transcript 2 (CCAT2), has been observed in CC cells, including HeLa, SiHa, and CaSki, as well as in CC tissues (Lee et al., 2016).
CCAT1 lncRNA
The lncRNA CCAT1 is located within the c-Myc gene region, belonging to the Myc proto-oncogene family, which is known for its critical role in tumorigenesis. CCAT1, acting as a sponge for miR-181a-5p, promotes the proliferation and invasion of CC cells. This function is accomplished through the upregulation of heparin-binding EGF-like growth factor (HB-EGF) and matrix metalloproteinase-14 (MMP14) (Shen et al., 2019). Furthermore, upon binding to c-Myc, CCAT1 activates the Wnt/β-catenin signaling pathway, leading to increased cell progression (Zhang and Gao, 2017).
H19 lncRNA
The lncRNA H19 is encoded by the H19 gene and is situated in chromosome 11 at 11p15.5. It is expressed exclusively from the maternally inherited chromosome and is recognized as the first riboregulator lncRNA. H19 is expressed in various tissues, including fetal tissues, adult muscles, and cancers (Kallen et al., 2013). The HPV16 E6 oncoprotein modulates lncRNA H19, which acts as a molecular sponge for miR-138-5p in epithelial cells (Ou et al., 2018; Barr et al., 2019). However, H19 promotes cell proliferation and inhibits apoptosis by silencing miR-143-3p and upregulating sirtuin-1 (SIRT1) (Ou et al., 2018).
Metastasis-associated lung adenocarcinoma transcript 1 lncRNA
The metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) consists of 8,000 nucleotides and is located in chromosome 11q13.1. It was first recognized in non-small cell lung cancer (Sun and Ma, 2019). Overexpression of MALAT1 has been observed in CC cell lines and cancer tissues infected with high-risk HPV (Jiang et al., 2014). MALAT1 is a sponge for several miRNAs, including miR-124, miR-145, and miR-20 (Lu et al., 2016). Consequently, downregulation of MALAT1 in both CC tissues and CC cell lines leads to a significant reduction in invasion and metastasis, achieved through the modulation of the MALAT1-miR-124-RBG2 axis and the inhibition of the epithelial–mesenchymal transition (Lee et al., 2016). Additionally, MALAT1 plays a role in the mechanisms of radio resistance by acting as a sponge for miR-145 during CC radiotherapy (Lu et al., 2016).
SPRY4-IT1 lncRNA
Intron two of the SPRY4 gene generates SPRY4 intronic transcript 1 (SPRY4-IT1), a molecule that assumes the role of either a tumor suppressor or an oncogenic factor in various cancer types (Xie et al., 2015). However, in CC, SPRY4-IT1 expression levels surpass those of normal tissues and are closely associated with advanced clinical stages, ultimately diminishing the survival rates of CC patients (Cao et al., 2016). Recent research has unveiled the pivotal role of SPRY4-IT1 silencing in CC cell lines, particularly in inhibiting migration and invasion via the SPYR4-IT1/miR-101-3p/ZEB1 axis. This function is directly linked to the active suppression of epithelial–mesenchymal transition (EMT) alterations, leading to increased E-cadherin levels and reduced expression levels of both N-cadherin and vimentin (Fan et al., 2019).
PVT1 lncRNA
Plasmacytoma variant translocation 1 (PVT1) is an exceptionally conserved long non-coding RNA (lncRNA) located downstream of the MYC gene. It is frequently co-amplified with MYC in various types of cancer (Li et al., 2019). PVT1 downregulates the expression levels of miR-195 and miR-200b in CC through direct binding or the enhancement of histone H3K27me3 in the promoter regions of miR-195 and miR-200b (Zhang et al., 2016; Shen et al., 2017). MiR-195 is associated with chemoresistance and the epithelial–mesenchymal transition, while miR-200b plays a role in cellular proliferation, invasion, and migration of CC cells. A positive correlation between serum PVT1 levels and the expression of PVT1 in cancer tissues has been reported, suggesting PVT1 as a potential diagnostic biomarker for CC (Yang et al., 2016).
GAS5 lncRNA
Growth arrest-specific transcript 5 (GAS5) exhibits tumor suppressor activity in various cancer types (Schneider et al., 1988; Pickard and Williams, 2016). Therefore, in CC, lower GAS5 expression levels are linked to tumor progression and poorer clinical outcomes for individuals with CC (Cao et al., 2014). The inhibition of GAS5 in CC cells has been observed to enhance proliferation, migration, and invasion, further underscoring its potential as a tumor suppressor in the development of CC (Cao et al., 2014). Notably, GAS5 functions as a sponge for miR-106b, resulting in the increased expression of IER3 (immediate early response 3) and enhanced radio sensitivity of CC cells (Gao et al., 2019).
Maternally expressed gene 3 lncRNA
Maternally expressed gene 3 (MEG3), a long non-coding RNA derived from the DLK1-MEG3 locus at the chromosomal location 14q32.3, is well-established as a cancer-suppressive factor (Zink et al., 2018), which was initially recognized as the ortholog of gene trap locus 2 (Gtl2) in mice. MEG3 overexpression demonstrates preventive effects on cell proliferation and enhances apoptosis in cancer cells, both in a p53-dependent and independent manner (Paci et al., 2014). A notable reduction in the MEG3 expression level was observed in CC tissues and cell lines. Enhancing MEG3 in CC cell lines prevented proliferation and induced cell cycle arrest and apoptosis. It was achieved by direct binding to P-STAT3, leading to subsequent ubiquitination and degradation of P-STAT3 (Zhang and Gao, 2019). Additionally, MEG3 exerts its cancer-suppressive effect by downregulating the levels of miR-21-5p in CC cell lines (Avila et al., 2023). Knockdown of MEG3 in HeLa and CaSki cells resulted in a significant upregulation of miR21-5p expression (Yadav et al., 2023).
C5orf66-AS1 lncRNA
C5orf66-AS1 lncRNA primarily resides in the cytoplasm, suggesting its modulation of CC cell functions through the competing endogenous RNA (ceRNA) mechanism (Begliarzade et al., 2023). Previous research has identified interactions between C5orf66-AS1 and various miRNAs, including miR-621, miR-637, miR-663b, miR-3184, miR-3187, miR-4449, miR-4706, and miR-5001, depicting its role as a sponge for these miRNAs. Alterations in the expression levels of C5orf66-AS1 in CC cells (C-4 I and SiHa) were observed to correlate with changes in the miR-637 expression. Specifically, the upregulation of C5orf66-AS1 led to a significant decrease in miR-637 expression levels and vice versa (Rui et al., 2018).
Novel lncRNAs in cervical tumorigenesis
In addition to the mentioned lncRNAs, several novel sequences of lncRNAs, such as LINC00675, FAM83H-AS1, RSU1P2, NOC2L-4.1, EBIC, PAX8 AS1, CCHE1, and LET, have been identified in cervical tumorigenesis (refer to Table 1) (Figure 2).
Biological functions of circular RNAs
Circular RNAs (circRNAs) represent a recently discovered class of widespread endogenous non-coding RNA molecules characterized by their covalent circular structures, which serve as transcription factors, miRNA sponges, and RNA-binding proteins (RBPs) (Hsiao et al., 2017). CircRNAs have been observed to exhibit abnormal expression patterns in cancer cells and play diverse roles in the development and progression of cancer. Due to their unique stable loop-like structure, circRNAs have shown promise as potential biomarkers and therapeutic targets for various types of cancer (Zhu et al., 2021). CircRNAs have various biological functions such as regulation of gene expression (Hansen et al., 2013). They act as microRNA sponges, sequestering and inhibiting the activity of microRNAs, thus modulating the expression of target genes (Hansen et al., 2013). CircRNAs can interact with proteins (Du et al., 2016) and regulate alternative splicing of mRNA by interacting with splicing factors, thereby regulating the diversity of the transcriptome (Ashwal-Fluss et al., 2014). They also play roles in cellular proliferation, apoptosis, and cellular senescence (Abdelmohs et al., 2015; Li et al., 2015). CircRNAs are also associated with various aspects of cancer, including tumorigenesis, metastasis, and resistance to therapy (Guarnerio et al., 2016). Additionally, they have been implicated in neurological disorders, synaptic function, neuronal development, and neurodegenerative diseases (Rybak-Wolf et al., 2015) (Figure 3).
Regulation of circRNA biogenesis
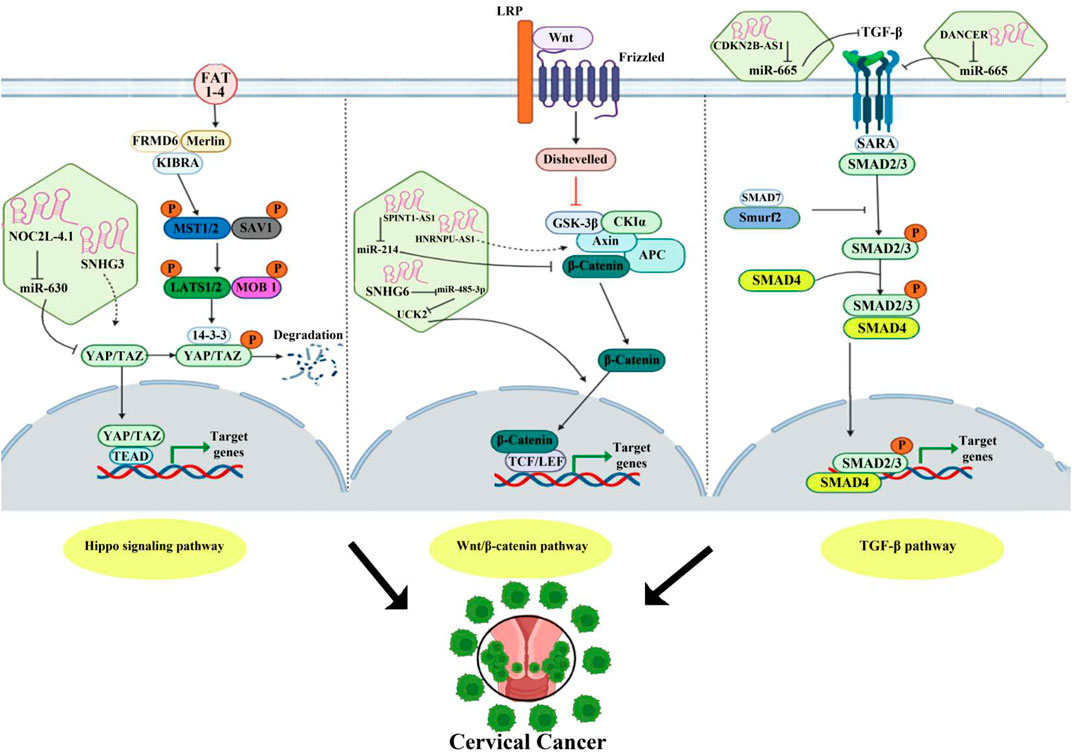
CircRNA biogenesis is intricately controlled by repetitive intronic elements, particularly sequences derived from transposons (Wilusz, 2015). The production of circRNA involves alternative splicing of one or several exons of a gene in the presence of an RNA-mediated silencing complex (Liang et al., 2021). Splice sites bring exons closer together and facilitate the formation of closed loops (Wilusz, 2015). Backsplicing, mediated by inversion repeats flanking cyclic exons, further aids circRNA production. When producing circRNAs, different versions of the closed loop are created depending on repeated base pairs. Therefore, the specific arrangement of intronic repeats regulates the potential function of protein-coding genes (Wilusz, 2015).
Protein factors in circRNA biogenesis
Numerous protein factors play pivotal roles in circRNA biogenesis. NF90/NF110 is a crucial regulator that assembles exons to form circRNAs in the nucleus and interacts with mature circRNAs in the cytoplasm (Li et al., 2017). NOVA2, an enriched RNA-binding protein, plays a crucial role in the circRNA expression by binding to specific sites inside the introns flanking circRNA exons (Knupp et al., 2021). Heterogeneous nuclear ribonucleoprotein M (HNRNPM) is another factor involved in controlling circRNA biogenesis by preventing misplaced exon splicing and backsplicing after binding to homeostatic gene transcripts (Ho et al., 2021). The RNA-binding protein SFPQ regulates circRNA biogenesis by overseeing long-intron splicing, ensuring precise splicing, and preserving the intron integrity (Stagsted et al., 2021).
Circular RNA and modulation microRNAs in cervical cancer
circRNAs have emerged as a novel therapeutic target in CC (Chaichian et al., 2020). A recent study highlighted the role of circRNA-VPRBP in blocking galectin-1-mediated CC metastasis by binding to RACK1 O-GlcNAcylation (Zhang et al., 2023). Aberrant expression of circRNAs has been linked to the initiation of pathological processes in cervical tissue, influencing cell proliferation, migration, and apoptosis. CircRNAs are considered a potential clinical biomarker for early detection of CC, offering a prognosis for early treatment (Chen et al., 2021). Understanding the circRNA–miRNA–mRNA network’s role in CC pathogenesis opens avenues for designing chemotherapy drugs targeting this network, providing new possibilities for CC treatment (Yi et al., 2019).
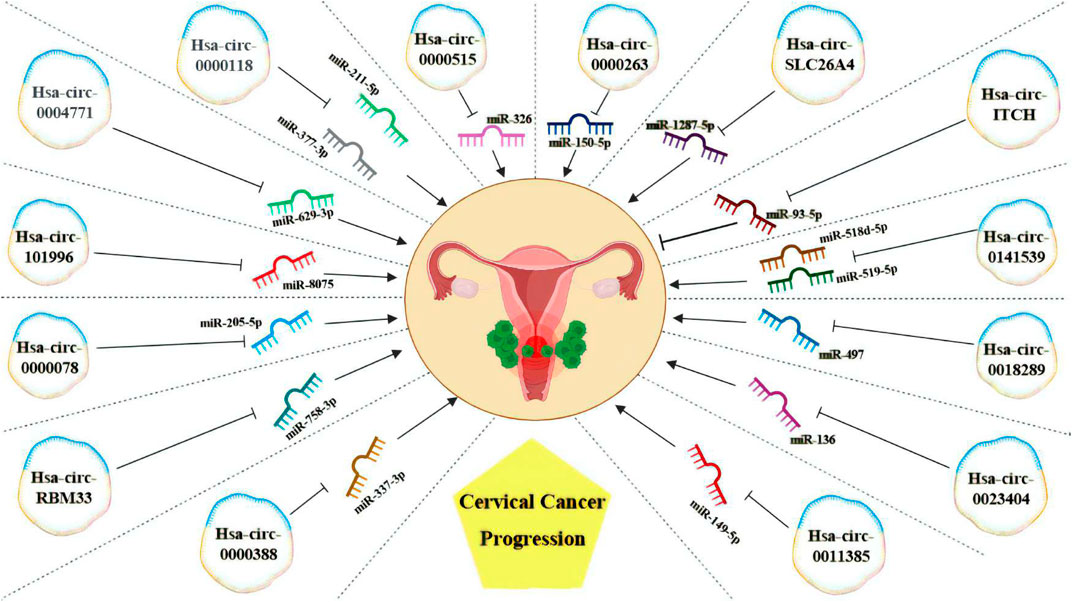
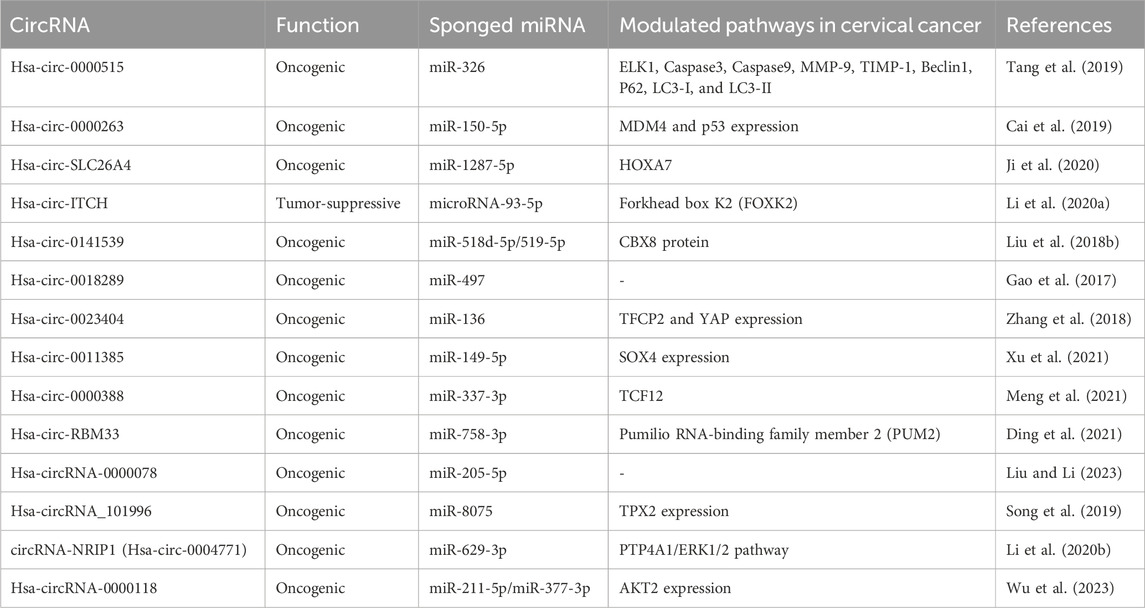
Several circRNAs act as competing endogenous RNAs or microRNA sponges, regulating mRNA expression (Arnaiz et al., 2019). For instance, circRNA hsa_circ_0000515 acts as a sponge for miR-326, mediating CC progression. Silencing hsa_circ_0000515 reduces proliferation and invasion while promoting apoptosis and autophagy in CC cells. Another study revealed that hsa_circ_0000263 knockdown suppresses cell migration and proliferation by controlling the target gene of miR-150-5p (Cai et al., 2019). Hsa-circSLC26A4 accelerates CC progression via the QKI/circSLC26A4/miR-1287-5p/HOXA7 axis (Ji et al., 2020). Overexpression of circRNA hsa-Circ-ITCH inhibits malignant behaviors in CC by regulating microRNA-93-5p and forkhead box K2 (FOXK2) expression (Li et al., 2020a).
Hsa_circ_0141539, acting as a sponge for miR-518d-5p/519-5p, leads to increased CBX8 expression, promoting malignant transformation and proliferation of cervical cells (Liu et al., 2018b). Additionally, this circRNA has been shown to sponge miR-506, resulting in the upregulation of Snail-2 expression, a direct target of miR-506 (Ma et al., 2018). Gao et al. demonstrated that Hsa_circ_0018289 binds to miR-497, enhancing invasion, cell proliferation, and migration of CC cells (Gao et al., 2017). Hsa_circ_0023404 acts as a miR-136 sequester, promoting the upregulation of TFCP2 expression, activating the YAP signaling pathway, and contributing to CC progression (Zhang et al., 2018). Hsa_circ_0011385 increased expression in CC influences SOX4 expression by targeting miR-149-5p, affecting the malignant biological behaviors of CC cells (Xu et al., 2021). Hsa_Circ_0000388 accelerates CC progression by modulating miR-337-3p and TCF12 expression (Meng et al., 2021). Circ_0043280 regulates CC through the miR-203a-3p/PAQR3 axis, competitively binding miR-203a-3p and restoring PAQR3 expression, thereby preventing tumor growth and metastasis (Zhang et al., 2021b).
Circular RNA (hsa_circ_0001772) is implicated in cancer tumorigenesis, and its knockdown reduces tumor growth, migration, invasion, and glycolysis and promotes CC cell apoptosis (Ding et al., 2021). Hsa_circ_0001772 functions as a sponge for miR-758-3p, modulating PUM2 expression, which reverses the suppressive influence of upregulated miR-758-3p on CC cell malignant behaviors. Hsa_circ_0001772 fosters CC advancement by absorbing miR-758-3p and enhancing PUM2 expression.
In another study, circRNA-0000078 was found to suppress CC cell survival and proliferation by regulating the miRNA-205-5p/EREG pathway, preventing tumorigenesis development in this cancer type (Liu and Li, 2023). Hsa_circRNA_101996 promotes the proliferation and invasion of cancer by increasing TPX2 factor expression, inhibiting miR-8075, and is directly correlated with the tumor size and lymph node metastasis (Stagsted et al., 2021). CircRNA-SLC26A4 plays a crucial role in promoting CC proliferation and invasion by targeting the QKI/circSLC26A4/miR-1287-5p/HOXA7 axis. CircRNA_0018289 contributes to cervix tumorigenesis by targeting miRNA-497 (Gao et al., 2017). CircRNA-NRIP1 (Hsa-circ-0004771) enhances CC oncogenic effects and lymph node invasion by impacting miRNA-629-3p and subsequently on the PTP4A1/ERK1/2 signaling pathway (Li et al., 2020b). CircRNA-00284 arrests the cell cycle at the G0/G1 phase, increasing CC invasion through miRNA-506 sponging and Snail-2 upregulation (Ma et al., 2018). Hsa-circRNA-0000118 is implicated in cervical cancer malignancy by targeting miR-211-5p and miR-377-3p, preserving AKT2 levels (Wu et al., 2023) (Table 2).
Conclusion and future directions
Due to various limitations and shortcomings, such as challenges in early detection, a lack of targeted therapies, limited HPV vaccination coverage, treatment side effects, and resistance to therapy, CC stands as one of the leading causes of death among women worldwide. LncRNAs and circRNAs serve pivotal roles in modulating miRNA activity, thereby influencing the initiation and progression of cervical carcinogenesis. These non-coding RNAs engage in intricate regulatory networks, directly interacting with miRNAs to impact downstream target mRNA expression. Examples of such interactions include lncRNA HOTAIR, which acts as a sponge for miR-23b-3p, relieving the suppression of its target gene ZEB1. This leads to enhanced EMT and increased metastatic potential in CC cells. Similarly, circRNA CDR1as sequesters miR-7, leading to increased expression of its target gene EGFR, promoting cell proliferation and migration in CC. The dysregulation of these non-coding RNA-mediated interactions contributes significantly to key aspects of cervical carcinogenesis, including uncontrolled proliferation, evasion of apoptosis, and increased metastatic potential. Furthermore, the intricate interplay between lncRNAs, circRNAs, and miRNAs contributes to the establishment of a conducive tumorigenic microenvironment. Numerous studies have explored the diagnostic and therapeutic potential of miRNAs in various cancers. This review focuses on summarizing the role of lncRNAs and circRNAs in the context of CC. Understanding these regulatory mechanisms at the molecular level provides valuable insights into the complexity of CC pathogenesis. Targeting the dysregulated interactions between lncRNAs, circRNAs, and miRNAs holds promise for developing innovative diagnostic and therapeutic strategies against cervical carcinogenesis. Thus, elucidating these molecular networks enhances our comprehension of CC biology and informs potential avenues for precision medicine in its management. Additionally, the review emphasizes the potential of lncRNAs as biomarkers and therapeutic targets in CC. It provides a detailed examination of specific lncRNAs implicated in CC development, such as HOTAIR, CCAT2, H19, MALAT1, SPRY4-IT1, PVT1, GAS5, MEG3, and C5orf66-AS1. We present a comprehensive list of circRNAs and their functions in CC, detailing how they act as miRNA sponges, influencing mRNA expression, and contributing to the pathogenesis of CC.
This article is a valuable resource for researchers, highlighting the potential of lncRNAs and circRNAs as promising targets for diagnostic and therapeutic interventions in CC. Efforts to identify early detection biomarkers in CC focus on specific lncRNA–miRNA–target mRNA networks associated with initial stages of the disease. The dysregulation of a lncRNA, which modulates miRNA activity and influences the expression of oncogenic mRNAs, holds potential as an early diagnostic biomarker, enabling timely intervention and improving prognosis. Investigating diverse interactions within lncRNA–miRNA–target mRNA networks in CC subtypes can lead to the development of molecular signatures for precise subtyping. These specific networks may indicate distinct subtypes, aiding in the formulation of tailored treatment strategies and enhancing therapeutic outcomes. Additionally, exploring liquid biopsy approaches to detect lncRNAs, miRNAs, and target mRNAs in bodily fluids offers non-invasive diagnostic possibilities. Circulating nucleic acids associated with regulatory networks can serve as liquid biopsy markers, providing a convenient method for monitoring disease progression and treatment response. Integrating diverse lncRNA–miRNA–target mRNA networks with established molecular markers contributes to comprehensive molecular profiling, enhancing CC diagnosis accuracy by considering multiple layers of molecular information. This understanding guides clinicians in developing personalized treatment plans. Furthermore, identifying specific lncRNA–miRNA–target mRNA networks linked to treatment response or resistance serves as therapeutic monitoring markers. Monitoring dynamic changes in these networks provides real-time insights, allowing for timely adjustments in treatment strategies. In conclusion, recognizing the potential of lncRNAs and circRNAs as diagnostic markers in CC prompts specific exploration of molecular networks for practical clinical applications. Spanning from early detection to subtype-specific diagnostics and non-invasive monitoring, these applications offer actionable directions for translating molecular discoveries into tangible benefits for CC patients. However, the utilization of these emerging lncRNAs and circRNAs as biomarkers for both the prognosis and diagnosis of CC requires further exploration through extensive clinical research in this field.
Author contributions
SH: data curation, investigation, and writing–original draft. PM: data curation, investigation, and writing–original draft. BA: data curation, investigation, and writing–original draft. FM: writing–review and editing. SA: writing–review and editing. MZ: writing–review and editing. HA: project administration, supervision, and writing–review and editing. AS: project administration, supervision, and writing–review and editing. NS: project administration, supervision, and writing–review and editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aalijahan, H., and Ghorbian, S. (2019). Long non-coding RNAs and cervical cancer. Exp. Mol. Pathology 106, 7–16. doi:10.1016/j.yexmp.2018.11.010
Abdelmohsen, K., Panda, A. C., De, S., Grammatikakis, I., Kim, J., Ding, J., et al. (2015). Circular RNAs in monkey muscle: age-dependent changes. Aging (Albany NY) 7 (11), 903–910. doi:10.18632/aging.100834
Arnaiz, E., Sole, C., Manterola, L., Iparraguirre, L., Otaegui, D., and Lawrie, C. H. (2019). CircRNAs and cancer: biomarkers and master regulators. Semin. Cancer Biol. 58, 90–99. doi:10.1016/j.semcancer.2018.12.002
Ashwal-Fluss, R., Meyer, M., Pamudurti, N. R., Ivanov, A., Bartok, O., Hanan, M., et al. (2014). circRNA biogenesis competes with pre-mRNA splicing. Mol. Cell 56 (1), 55–66. doi:10.1016/j.molcel.2014.08.019
Avila, J. P., Carvalho, B. M., and Coimbra, E. C. (2023). A comprehensive view of the cancer-immunity cycle (CIC) in HPV-mediated cervical cancer and prospects for emerging therapeutic opportunities. Cancers (Basel). 15 (4), 1333. doi:10.3390/cancers15041333
Barr, J. A., Hayes, K. E., Brownmiller, T., Harold, A. D., Jagannathan, R., Lockman, P. R., et al. (2019). Long non-coding RNA FAM83H-AS1 is regulated by human papillomavirus 16 E6 independently of p53 in cervical cancer cells. Sci. Rep. 9 (1), 3662. doi:10.1038/s41598-019-40094-8
Begliarzade, S., Beilerli, A., Sufianov, A., Tamrazov, R., Kudriashov, V., Ilyasova, T., et al. (2023). Long non-coding RNAs as promising biomarkers and therapeutic targets in cervical cancer. Noncoding RNA Res. 8 (2), 233–239. doi:10.1016/j.ncrna.2023.02.006
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., and Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68 (6), 394–424. doi:10.3322/caac.21492
Cai, H., Zhang, P., Xu, M., Yan, L., Liu, N., and Wu, X. (2019). Circular RNA hsa_circ_0000263 participates in cervical cancer development by regulating target gene of miR-150-5p. J. Cell Physiol. 234 (7), 11391–11400. doi:10.1002/jcp.27796
Cao, S., Liu, W., Li, F., Zhao, W., and Qin, C. (2014). Decreased expression of lncRNA GAS5 predicts a poor prognosis in cervical cancer. Int. J. Clin. Exp. Pathol. 7 (10), 6776–6783.
Cao, Y., Liu, Y., Lu, X., Wang, Y., Qiao, H., and Liu, M. (2016). Upregulation of long noncoding RNA SPRY4-IT1 correlates with tumor progression and poor prognosis in cervical cancer. FEBS Open Bio 6 (9), 954–960. doi:10.1002/2211-5463.12102
Chaichian, S., Shafabakhsh, R., Mirhashemi, S. M., Moazzami, B., and Asemi, Z. (2020). Circular RNAs: a novel biomarker for cervical cancer. J. Cell Physiol. 235 (2), 718–724. doi:10.1002/jcp.29009
Cheetham, S. W., Gruhl, F., Mattick, J. S., and Dinger, M. E. (2013). Long noncoding RNAs and the genetics of cancer. Br. J. Cancer 108 (12), 2419–2425. doi:10.1038/bjc.2013.233
Chen, L. L., and Yang, L. (2015). Regulation of circRNA biogenesis. RNA Biol. 12 (4), 381–388. doi:10.1080/15476286.2015.1020271
Chen, S., Yang, X., Yu, C., Zhou, W., Xia, Q., Liu, Y., et al. (2021). The potential of circRNA as a novel diagnostic biomarker in cervical cancer. J. Oncol. 2021, 5529486. doi:10.1155/2021/5529486
Di Leva, G., Garofalo, M., and Croce, C. M. (2014). MicroRNAs in cancer. Annu. Rev. Pathol. 9, 287–314. doi:10.1146/annurev-pathol-012513-104715
Ding, Y., Yuan, X., and Gu, W. (2021). Circular RNA RBM33 contributes to cervical cancer progression via modulation of the miR-758-3p/PUM2 axis. J. Mol. Histol. 52 (2), 173–185. doi:10.1007/s10735-020-09933-1
Du, W. W., Yang, W., Liu, E., Yang, Z., Dhaliwal, P., and Yang, B. B. (2016). Foxo3 circular RNA retards cell cycle progression via forming ternary complexes with p21 and CDK2. Nucleic Acids Res. 44 (6), 2846–2858. doi:10.1093/nar/gkw027
Fan, M. J., Zou, Y. H., He, P. J., Zhang, S., Sun, X. M., and Li, C. Z. (2019). Long non-coding RNA SPRY4-IT1 promotes epithelial-mesenchymal transition of cervical cancer by regulating the miR-101-3p/ZEB1 axis. Biosci. Rep. 39 (6). doi:10.1042/BSR20181339
Fujiwara, K., Monk, B., and Devouassoux-Shisheboran, M. (2014). Adenocarcinoma of the uterine cervix: why is it different? Curr. Oncol. Rep. 16 (12), 416. doi:10.1007/s11912-014-0416-y
Gao, J., Liu, L., Li, G., Cai, M., Tan, C., Han, X., et al. (2019). LncRNA GAS5 confers the radio sensitivity of cervical cancer cells via regulating miR-106b/IER3 axis. Int. J. Biol. Macromol. 126, 994–1001. doi:10.1016/j.ijbiomac.2018.12.176
Gao, Y. L., Zhang, M. Y., Xu, B., Han, L. J., Lan, S. F., Chen, J., et al. (2017). Circular RNA expression profiles reveal that hsa_circ_0018289 is up-regulated in cervical cancer and promotes the tumorigenesis. Oncotarget 8 (49), 86625–86633. doi:10.18632/oncotarget.21257
Guarnerio, J., Bezzi, M., Jeong, J. C., Paffenholz, S. V., Berry, K., Naldini, M. M., et al. (2016). Oncogenic role of fusion-circRNAs derived from cancer-associated chromosomal translocations. Cell 165 (2), 289–302. doi:10.1016/j.cell.2016.03.020
Gutschner, T., and Diederichs, S. (2012). The hallmarks of cancer: a long non-coding RNA point of view. RNA Biol. 9 (6), 703–719. doi:10.4161/rna.20481
Guttman, M., and Rinn, J. L. (2012). Modular regulatory principles of large non-coding RNAs. Nature 482 (7385), 339–346. doi:10.1038/nature10887
Hansen, T. B., Jensen, T. I., Clausen, B. H., Bramsen, J. B., Finsen, B., Damgaard, C. K., et al. (2013). Natural RNA circles function as efficient microRNA sponges. Nature 495 (7441), 384–388. doi:10.1038/nature11993
He, J., Huang, B., Zhang, K., Liu, M., and Xu, T. (2020). Long non-coding RNA in cervical cancer: from biology to therapeutic opportunity. Biomed. Pharmacother. 127, 110209. doi:10.1016/j.biopha.2020.110209
Ho, J. S., Di Tullio, F., Schwarz, M., Low, D., Incarnato, D., Gay, F., et al. (2021). HNRNPM controls circRNA biogenesis and splicing fidelity to sustain cancer cell fitness. Elife 10, e59654. doi:10.7554/eLife.59654
Hosseini, E. S., Meryet-Figuiere, M., Sabzalipoor, H., Kashani, H. H., Nikzad, H., and Asemi, Z. (2017). Dysregulated expression of long noncoding RNAs in gynecologic cancers. Mol. Cancer 16 (1), 107. doi:10.1186/s12943-017-0671-2
Hsiao, K. Y., Sun, H. S., and Tsai, S. J. (2017). Circular RNA - new member of noncoding RNA with novel functions. Exp. Biol. Med. (Maywood). 242 (11), 1136–1141. doi:10.1177/1535370217708978
Ji, F., Du, R., Chen, T., Zhang, M., Zhu, Y., Luo, X., et al. (2020). Circular RNA circSLC26A4 accelerates cervical cancer progression via miR-1287-5p/HOXA7 Axis. Mol. Ther. Nucleic Acids 19, 413–420. doi:10.1016/j.omtn.2019.11.032
Jiang, Y., Li, Y., Fang, S., Jiang, B., Qin, C., Xie, P., et al. (2014). The role of MALAT1 correlates with HPV in cervical cancer. Oncol. Lett. 7 (6), 2135–2141. doi:10.3892/ol.2014.1996
Kallen, A. N., Zhou, X. B., Xu, J., Qiao, C., Ma, J., Yan, L., et al. (2013). The imprinted H19 lncRNA antagonizes let-7 microRNAs. Mol. Cell 52 (1), 101–112. doi:10.1016/j.molcel.2013.08.027
Kim, H. J., Lee, D. W., Yim, G. W., Nam, E. J., Kim, S., Kim, S. W., et al. (2015). Long non-coding RNA HOTAIR is associated with human cervical cancer progression. Int. J. Oncol. 46 (2), 521–530. doi:10.3892/ijo.2014.2758
Knupp, D., Cooper, D. A., Saito, Y., Darnell, R. B., and Miura, P. (2021). NOVA2 regulates neural circRNA biogenesis. Nucleic Acids Res. 49 (12), 6849–6862. doi:10.1093/nar/gkab523
Kopp, F., and Mendell, J. T. (2018). Functional classification and experimental dissection of long noncoding RNAs. Cell 172 (3), 393–407. doi:10.1016/j.cell.2018.01.011
Larrieta-Carrasco, E., Flores, Y. N., Macías-Kauffer, L. R., Ramírez-Palacios, P., Quiterio, M., Ramírez-Salazar, E. G., et al. (2018). Genetic variants in COL13A1, ADIPOQ and SAMM50, in addition to the PNPLA3 gene, confer susceptibility to elevated transaminase levels in an admixed Mexican population. Exp. Mol. Pathology 104 (1), 50–58. doi:10.1016/j.yexmp.2018.01.001
Lecerf, C., Le Bourhis, X., and Adriaenssens, E. (2019). The long non-coding RNA H19: an active player with multiple facets to sustain the hallmarks of cancer. Cell Mol. Life Sci. 76 (23), 4673–4687. doi:10.1007/s00018-019-03240-z
Lee, M., Kim, H. J., Kim, S. W., Park, S. A., Chun, K. H., Cho, N. H., et al. (2016). The long non-coding RNA HOTAIR increases tumour growth and invasion in cervical cancer by targeting the Notch pathway. Oncotarget 7 (28), 44558–44571. doi:10.18632/oncotarget.10065
Li, J., Guo, R., Liu, Q., Sun, J., and Wang, H. (2020a). Circular RNA circ-ITCH inhibits the malignant behaviors of cervical cancer by microRNA-93-5p/FOXK2 Axis. Reprod. Sci. 27 (3), 860–868. doi:10.1007/s43032-020-00140-7
Li, M. Y., Tang, X. H., Fu, Y., Wang, T. J., and Zhu, J. M. (2019). Regulatory mechanisms and clinical applications of the long non-coding RNA PVT1 in cancer treatment. Front. Oncol. 9, 787. doi:10.3389/fonc.2019.00787
Li, Q., Feng, Y., Chao, X., Shi, S., Liang, M., Qiao, Y., et al. (2018). HOTAIR contributes to cell proliferation and metastasis of cervical cancer via targetting miR-23b/MAPK1 axis. Biosci. Rep. 38 (1). doi:10.1042/BSR20171563
Li, X., Liu, C. X., Xue, W., Zhang, Y., Jiang, S., Yin, Q. F., et al. (2017). Coordinated circRNA biogenesis and function with NF90/NF110 in viral infection. Mol. Cell 67 (2), 214–227. doi:10.1016/j.molcel.2017.05.023
Li, X., Ma, N., Zhang, Y., Wei, H., Zhang, H., Pang, X., et al. (2020b). Circular RNA circNRIP1 promotes migration and invasion in cervical cancer by sponging miR-629-3p and regulating the PTP4A1/ERK1/2 pathway. Cell Death Dis. 11 (5), 399. doi:10.1038/s41419-020-2607-9
Li, Y., Zheng, Q., Bao, C., Li, S., Guo, W., Zhao, J., et al. (2015). Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res. 25 (8), 981–984. doi:10.1038/cr.2015.82
Liang, Y., Liu, N., Yang, L., Tang, J., Wang, Y., and Mei, M. (2021). A brief review of circRNA biogenesis, detection, and function. Curr. Genomics 22 (7), 485–495. doi:10.2174/1389202922666210331130722
Liu, C., and Li, Y. (2023). Hsa_circ_0000078 regulates miR-205-5p/EREG pathway to inhibit cervical cancer progression. Mol. Biotechnol. 65 (9), 1453–1464. doi:10.1007/s12033-023-00658-6
Liu, J., Wang, D., Long, Z., Liu, J., and Li, W. (2018b). CircRNA8924 promotes cervical cancer cell proliferation, migration and invasion by competitively binding to MiR-518d-5p/519-5p family and modulating the expression of CBX8. Cell Physiol. Biochem. 48 (1), 173–184. doi:10.1159/000491716
Liu, M., Jia, J., Wang, X., Liu, Y., Wang, C., and Fan, R. (2018a). Long non-coding RNA HOTAIR promotes cervical cancer progression through regulating BCL2 via targeting miR-143-3p. Cancer Biol. Ther. 19 (5), 391–399. doi:10.1080/15384047.2018.1423921
Liu, Q., Guo, X., Que, S., Yang, X., Fan, H., Liu, M., et al. (2017). LncRNA RSU1P2 contributes to tumorigenesis by acting as a ceRNA against let-7a in cervical cancer cells. Oncotarget 8 (27), 43768–43781. doi:10.18632/oncotarget.10844
Long, Y., Wang, X., Youmans, D. T., and Cech, T. R. (2017). How do lncRNAs regulate transcription? Sci. Adv. 3 (9), eaao2110. doi:10.1126/sciadv.aao2110
Lu, H., He, Y., Lin, L., Qi, Z., Ma, L., Li, L., et al. (2016). Long non-coding RNA MALAT1 modulates radiosensitivity of HR-HPV+ cervical cancer via sponging miR-145. Tumour Biol. 37 (2), 1683–1691. doi:10.1007/s13277-015-3946-5
Ma, H. B., Yao, Y. N., Yu, J. J., Chen, X. X., and Li, H. F. (2018). Extensive profiling of circular RNAs and the potential regulatory role of circRNA-000284 in cell proliferation and invasion of cervical cancer via sponging miR-506. Am. J. Transl. Res. 10 (2), 592–604.
Meng, Q. H., Li, Y., Kong, C., Gao, X. M., and Jiang, X. J. (2021). Circ_0000388 exerts oncogenic function in cervical cancer cells by regulating miR-337-3p/TCF12 Axis. Cancer Biother Radiopharm. 36 (1), 58–69. doi:10.1089/cbr.2019.3159
Nicolás-Párraga, S., Alemany, L., de Sanjosé, S., Bosch, F. X., and Bravo, I. G.RIS HPV TT and HPV VVAP study groups (2017). Differential HPV16 variant distribution in squamous cell carcinoma, adenocarcinoma and adenosquamous cell carcinoma. Int. J. Cancer 140 (9), 2092–2100. doi:10.1002/ijc.30636
Nisar, S., Bhat, A. A., Singh, M., Karedath, T., Rizwan, A., Hashem, S., et al. (2021). Insights into the role of CircRNAs: biogenesis, characterization, functional, and clinical impact in human malignancies. Front. Cell Dev. Biol. 9, 617281. doi:10.3389/fcell.2021.617281
Ou, L., Wang, D., Zhang, H., Yu, Q., and Hua, F. (2018). Decreased expression of miR-138-5p by lncRNA H19 in cervical cancer promotes tumor proliferation. Oncol. Res. 26 (3), 401–410. doi:10.3727/096504017X15017209042610
Paci, P., Colombo, T., and Farina, L. (2014). Computational analysis identifies a sponge interaction network between long non-coding RNAs and messenger RNAs in human breast cancer. BMC Syst. Biol. 8, 83. doi:10.1186/1752-0509-8-83
Peng, W., and Fan, H. (2016). Long noncoding RNA CCHE1 indicates a poor prognosis of hepatocellular carcinoma and promotes carcinogenesis via activation of the ERK/MAPK pathway. Biomed. Pharmacother. 83, 450–455. doi:10.1016/j.biopha.2016.06.056
Pickard, M. R., and Williams, G. T. (2016). The hormone response element mimic sequence of GAS5 lncRNA is sufficient to induce apoptosis in breast cancer cells. Oncotarget 7 (9), 10104–10116. doi:10.18632/oncotarget.7173
Qi, C., Xiaofeng, C., Dongen, L., Liang, Y., Liping, X., Yue, H., et al. (2019). Long non-coding RNA MACC1-AS1 promoted pancreatic carcinoma progression through activation of PAX8/NOTCH1 signaling pathway. J. Exp. Clin. Cancer Res. 38 (1), 344. doi:10.1186/s13046-019-1332-7
Ren, Y., Wang, Y. F., Zhang, J., Wang, Q. X., Han, L., Mei, M., et al. (2019). Targeted design and identification of AC1NOD4Q to block activity of HOTAIR by abrogating the scaffold interaction with EZH2. Clin. Epigenetics 11 (1), 29. doi:10.1186/s13148-019-0624-2
Romano, G., Veneziano, D., Acunzo, M., and Croce, C. M. (2017). Small non-coding RNA and cancer. Carcinogenesis 38 (5), 485–491. doi:10.1093/carcin/bgx026
Romero-Barrios, N., Legascue, M. F., Benhamed, M., Ariel, F., and Crespi, M. (2018). Splicing regulation by long noncoding RNAs. Nucleic Acids Res. 46 (5), 2169–2184. doi:10.1093/nar/gky095
Rüegg, J. C. (1985). Modulation of calcium sensitivity in cardiac muscle cells. Basic Res. Cardiol. 80 (2), 79–82.
Rui, X., Xu, Y., Jiang, X., Ye, W., Huang, Y., and Jiang, J. (2018). Long non-coding RNA C5orf66-AS1 promotes cell proliferation in cervical cancer by targeting miR-637/RING1 axis. Cell Death Dis. 9 (12), 1175. doi:10.1038/s41419-018-1228-z
Rupaimoole, R., and Slack, F. J. (2017). MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 16 (3), 203–222. doi:10.1038/nrd.2016.246
Rybak-Wolf, A., Stottmeister, C., Glažar, P., Jens, M., Pino, N., Giusti, S., et al. (2015). Circular RNAs in the mammalian brain are highly abundant, conserved, and dynamically expressed. Mol. Cell 58 (5), 870–885. doi:10.1016/j.molcel.2015.03.027
Savira, M., Suhaimi, D., Putra, A. E., Yusrawati, Y., and Lipoeto, N. I. (2022). Prevalence oncogenic human papillomavirus in cervical cancer patients in riau province Indonesia. Rep. Biochem. Mol. Biol. 10 (4), 573–579. doi:10.52547/rbmb.10.4.573
Schneider, C., King, R. M., and Philipson, L. (1988). Genes specifically expressed at growth arrest of mammalian cells. Cell 54 (6), 787–793. doi:10.1016/s0092-8674(88)91065-3
Sharma, S., Mandal, P., Sadhukhan, T., Roy Chowdhury, R., Ranjan Mondal, N., Chakravarty, B., et al. (2015). Bridging links between long noncoding RNA HOTAIR and HPV oncoprotein E7 in cervical cancer pathogenesis. Sci. Rep. 5, 11724. doi:10.1038/srep11724
Sharma Saha, S., Roy Chowdhury, R., Mondal, N. R., Chakravarty, B., Chatterjee, T., Roy, S., et al. (2016). Identification of genetic variation in the lncRNA HOTAIR associated with HPV16-related cervical cancer pathogenesis. Cell Oncol. (Dordr) 39 (6), 559–572. doi:10.1007/s13402-016-0298-0
Shen, C. J., Cheng, Y. M., and Wang, C. L. (2017). LncRNA PVT1 epigenetically silences miR-195 and modulates EMT and chemoresistance in cervical cancer cells. J. Drug Target 25 (7), 637–644. doi:10.1080/1061186X.2017.1307379
Shen, H., Wang, L., Xiong, J., Ren, C., Gao, C., Ding, W., et al. (2019). Long non-coding RNA CCAT1 promotes cervical cancer cell proliferation and invasion by regulating the miR-181a-5p/MMP14 axis. Cell Cycle 18 (10), 1110–1121. doi:10.1080/15384101.2019.1609829
Shen, S., Zhang, S., Liu, P., Wang, J., and Du, H. (2020). Potential role of microRNAs in the treatment and diagnosis of cervical cancer. Cancer Genet. 248-249, 25–30. doi:10.1016/j.cancergen.2020.09.003
Singh, D., Vignat, J., Lorenzoni, V., Eslahi, M., Ginsburg, O., Lauby-Secretan, B., et al. (2023). Global estimates of incidence and mortality of cervical cancer in 2020: a baseline analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Glob. Health 11 (2), e197–e206. doi:10.1016/S2214-109X(22)00501-0
Small, W., Bacon, M. A., Bajaj, A., Chuang, L. T., Fisher, B. J., Harkenrider, M. M., et al. (2017). Cervical cancer: a global health crisis. Cancer 123 (13), 2404–2412. doi:10.1002/cncr.30667
Song, T., Xu, A., Zhang, Z., Gao, F., Zhao, L., Chen, X., et al. (2019). CircRNA hsa_circRNA_101996 increases cervical cancer proliferation and invasion through activating TPX2 expression by restraining miR-8075. J. Cell Physiol. 234 (8), 14296–14305. doi:10.1002/jcp.28128
Stagsted, L. V. W., O'Leary, E. T., Ebbesen, K. K., and Hansen, T. B. (2021). The RNA-binding protein SFPQ preserves long-intron splicing and regulates circRNA biogenesis in mammals. Elife 10, e63088. doi:10.7554/eLife.63088
Sun, N. X., Ye, C., Zhao, Q., Zhang, Q., Xu, C., Wang, S. B., et al. (2014). Long noncoding RNA-EBIC promotes tumor cell invasion by binding to EZH2 and repressing E-cadherin in cervical cancer. PLoS One 9 (7), e100340. doi:10.1371/journal.pone.0100340
Sun, Y., and Ma, L. (2019). New insights into long non-coding RNA MALAT1 in cancer and metastasis. Cancers (Basel) 11 (2), 216. doi:10.3390/cancers11020216
Tang, Q., Chen, Z., Zhao, L., and Xu, H. (2019). Circular RNA hsa_circ_0000515 acts as a miR-326 sponge to promote cervical cancer progression through up-regulation of ELK1. Aging (Albany NY) 11 (22), 9982–9999. doi:10.18632/aging.102356
Tornesello, M. L., Faraonio, R., Buonaguro, L., Annunziata, C., Starita, N., Cerasuolo, A., et al. (2020). The role of microRNAs, long non-coding RNAs, and circular RNAs in cervical cancer. Front. Oncol. 10, 150. doi:10.3389/fonc.2020.00150
Wang, K. C., and Chang, H. Y. (2011). Molecular mechanisms of long noncoding RNAs. Mol. Cell 43 (6), 904–914. doi:10.1016/j.molcel.2011.08.018
Wang, Q., Ding, J., Nan, G., Lyu, Y., and Ni, G. (2019b). LncRNA NOC2L-4.1 functions as a tumor oncogene in cervical cancer progression by regulating the miR-630/YAP1 pathway. J. Cell Biochem. 120 (10), 16913–16920. doi:10.1002/jcb.28949
Wang, Y., Liu, J., Ma, J., Sun, T., Zhou, Q., Wang, W., et al. (2019a). Exosomal circRNAs: biogenesis, effect and application in human diseases. Mol. Cancer 18 (1), 116. doi:10.1186/s12943-019-1041-z
Wilusz, J. E. (2015). Repetitive elements regulate circular RNA biogenesis. Mob. Genet. Elem. 5 (3), 1–7. doi:10.1080/2159256X.2015.1045682
Wu, L., Xiao, H., Hong, Y., Xie, M., Yu, Y., and Jiang, L. (2023). CircRNA Circ_0000118 regulates malignancy of cervical cancer cells by regulating miR-211-5p/miR-377-3p/AKT2 Axis. Biochem. Genet. 61 (4), 1625–1644. doi:10.1007/s10528-023-10332-w
Xiao, X., Wu, Z. C., and Chou, K. C. (2011). A multi-label classifier for predicting the subcellular localization of gram-negative bacterial proteins with both single and multiple sites. PLoS One 6 (6), e20592. doi:10.1371/journal.pone.0020592
Xie, M., Nie, F. Q., Sun, M., Xia, R., Liu, Y. W., Zhou, P., et al. (2015). Decreased long noncoding RNA SPRY4-IT1 contributing to gastric cancer cell metastasis partly via affecting epithelial-mesenchymal transition. J. Transl. Med. 13, 250. doi:10.1186/s12967-015-0595-9
Xu, A. L., Wang, W. S., Zhao, M. Y., Sun, J. N., Chen, X. R., and Hou, J. Q. (2021). Circular RNA circ_0011385 promotes cervical cancer progression through competitively binding to miR-149-5p and up-regulating SOX4 expression. Kaohsiung J. Med. Sci. 37 (12), 1058–1068. doi:10.1002/kjm2.12432
Xu, Q. F., Tang, Y. X., and Wang, X. (2018). LncRNA EBIC promoted proliferation, metastasis and cisplatin resistance of ovarian cancer cells and predicted poor survival in ovarian cancer patients. Eur. Rev. Med. Pharmacol. Sci. 22 (14), 4440–4447. doi:10.26355/eurrev_201807_15495
Yadav, C., Yadav, R., Chabbra, R., Nanda, S., Ranga, S., Kadian, L., et al. (2023). Overview of genetic and epigenetic regulation of human papillomavirus and apoptosis in cervical cancer. Apoptosis 28 (5-6), 683–701. doi:10.1007/s10495-023-01812-w
Yang, J. P., Yang, X. J., Xiao, L., and Wang, Y. (2016). Long noncoding RNA PVT1 as a novel serum biomarker for detection of cervical cancer. Eur. Rev. Med. Pharmacol. Sci. 20 (19), 3980–3986.
Yang, M., Zhai, X., Xia, B., Wang, Y., and Lou, G. (2015). Long noncoding RNA CCHE1 promotes cervical cancer cell proliferation via upregulating PCNA. Tumour Biol. 36 (10), 7615–7622. doi:10.1007/s13277-015-3465-4
Yi, Y., Liu, Y., Wu, W., Wu, K., and Zhang, W. (2019). Reconstruction and analysis of circRNA-miRNA-mRNA network in the pathology of cervical cancer. Oncol. Rep. 41 (4), 2209–2225. doi:10.3892/or.2019.7028
Yoon, J. H., Abdelmohsen, K., Kim, J., Yang, X., Martindale, J. L., Tominaga-Yamanaka, K., et al. (2013). Scaffold function of long non-coding RNA HOTAIR in protein ubiquitination. Nat. Commun. 4, 2939. doi:10.1038/ncomms3939
Zhang, B., Liu, Z., Liu, J., Cao, K., Shan, W., Wen, Q., et al. (2021a). Long non-coding RNA ST8SIA6-AS1 promotes the migration and invasion of hypoxia-treated hepatocellular carcinoma cells through the miR-338/MEPCE axis. Oncol. Rep. 45 (1), 73–82. doi:10.3892/or.2020.7864
Zhang, C., Jiang, H., Yuan, L., Liao, Y., Liu, P., Du, Q., et al. (2023). CircVPRBP inhibits nodal metastasis of cervical cancer by impeding RACK1 O-GlcNAcylation and stability. Oncogene 42 (11), 793–807. doi:10.1038/s41388-023-02595-9
Zhang, C., Liu, P., Huang, J., Liao, Y., Pan, C., Liu, J., et al. (2021b). Circular RNA hsa_circ_0043280 inhibits cervical cancer tumor growth and metastasis via miR-203a-3p/PAQR3 axis. Cell Death Dis. 12 (10), 888. doi:10.1038/s41419-021-04193-7
Zhang, D., Zhou, X. H., Zhang, J., Zhou, Y. X., Ying, J., Wu, G. Q., et al. (2015). Propofol promotes cell apoptosis via inhibiting HOTAIR mediated mTOR pathway in cervical cancer. Biochem. Biophys. Res. Commun. 468 (4), 561–567. doi:10.1016/j.bbrc.2015.10.129
Zhang, J., and Gao, Y. (2017). CCAT-1 promotes proliferation and inhibits apoptosis of cervical cancer cells via the Wnt signaling pathway. Oncotarget 8 (40), 68059–68070. doi:10.18632/oncotarget.19155
Zhang, J., and Gao, Y. (2019). Long non-coding RNA MEG3 inhibits cervical cancer cell growth by promoting degradation of P-STAT3 protein via ubiquitination. Cancer Cell Int. 19 (1), 175. doi:10.1186/s12935-019-0893-z
Zhang, J., Zhao, X., Zhang, J., Zheng, X., and Li, F. (2018). Circular RNA hsa_circ_0023404 exerts an oncogenic role in cervical cancer through regulating miR-136/TFCP2/YAP pathway. Biochem. Biophys. Res. Commun. 501 (2), 428–433. doi:10.1016/j.bbrc.2018.05.006
Zhang, S., Zhang, G., and Liu, J. (2016). Long noncoding RNA PVT1 promotes cervical cancer progression through epigenetically silencing miR-200b. Apmis 124 (8), 649–658. doi:10.1111/apm.12555
Zhu, S., Chen, X., Wang, J. N., Xu, J. J., Wang, A., Li, J. J., et al. (2021). Circular RNA circUbe2k promotes hepatic fibrosis via sponging miR-149-5p/TGF-β2 axis. Faseb J. 35 (6), e21622. doi:10.1096/fj.202002738R
Keywords: cervical cancer, long non-coding RNAs, circular RNAs, miRNA, microRNAs
Citation: Heidari-Ezzati S, Moeinian P, Ahmadian-Nejad B, Maghbbouli F, Abbasi S, Zahedi M, Afkhami H, Shadab A and Sajedi N (2024) The role of long non-coding RNAs and circular RNAs in cervical cancer: modulating miRNA function. Front. Cell Dev. Biol. 12:1308730. doi: 10.3389/fcell.2024.1308730
Received: 09 October 2023; Accepted: 24 January 2024;
Published: 16 February 2024.
Edited by:
Zhizhou Shi, Kunming University of Science and Technology, ChinaReviewed by:
Abraham Pedroza-Torres, Instituto Nacional de Cancerología, MexicoNaoshad Muhammad, Washington University in St. Louis, United States
Copyright © 2024 Heidari-Ezzati, Moeinian, Ahmadian-Nejad, Maghbbouli, Abbasi, Zahedi, Afkhami, Shadab and Sajedi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hamed Afkhami, aGFtZWRhZmtoYW1pNzBAZ21haWwuY29t; Alireza Shadab, c2hhZGFiLmFsaXJlemFAZ21haWwuY29t; Nayereh Sajedi, bmF5ZXJlaHNhamVkaUB5YWhvby5jb20=
†These authors have contributed equally to this work and share first authorship
 Sama Heidari-Ezzati1†
Sama Heidari-Ezzati1† Hamed Afkhami
Hamed Afkhami