- 1Department of Molecular Biology, Pusan National University, Busan, Republic of Korea
- 2Institute of Systems Biology, Pusan National University, Busan, Republic of Korea
Ca2+ is a key secondary messenger that modulates sperm motility by tuning flagellar movement in various species. The sperm-specific Ca2+ channel, CatSper, is a primary Ca2+ gate that is essential for male fertility in mammals. CatSper-mediated Ca2+ signaling enables sperm to develop hyperactivated motility and fertilize the eggs in the female tract. Therefore, altered CatSper function compromises the entry of Ca2+ into the sperm, followed by impairing hyperactivation and male fertility. However, methods to evaluate the function of the CatSper channel are limited to patch clamping and functional imaging using Ca2+ dye. Previous studies have revealed that various parameters for sperm motility are highly correlated with intracellular Ca2+ levels in mouse. Here, I cover a step-by-step protocol to analyze the change in Ca2+-mediated sperm motility by using computer-assisted semen analysis (CASA) to evaluate the functional normality of the CatSper channel in sperm. This approach analyzes sperm motility parameters during intracellular Ca2+ chelation followed by in vitro capacitation to recover intracellular Ca2+ via the activated CatSper channel. Thus, this Ca2+-handling method is handy and could be broadly applied in reproductive biology labs and clinics that have CASA equipment to examine the functional normality of the CatSper channel.
Introduction
Sperm from various species apply Ca2+-mediated signaling pathways to modulate their flagellar movement and motility pattern. In marine invertebrates, such as sea urchins, the sperm acquire chemotactic movement by coordinating Ca2+ entry and downstream signaling pathways (Wood et al., 2005; Seifert et al., 2015). Mammalian sperm develop a unique motility pattern called hyperactivated motility via extracellular Ca2+ influx in the female reproductive tract (Suarez et al., 1993). The changed motility patterns enable the sperm to successfully migrate to eggs followed by fertilization, highlighting that Ca2+-mediated motility change is crucial for sperm fertility.
In the female reproductive tract, mammalian sperm acquire fertilizing ability in a process called capacitation, which triggers the sperm to develop hyperactivated motility by introducing extracellular Ca2+ (Yanagimachi, 1970; Suarez et al., 1993). The major pathway for Ca2+ entry in mammalian sperm is the sperm-specific ion channel, known as CatSper, on their flagella (Ren et al., 2001). The CatSper channel is a multi-protein complex comprising at least 14 proteins (Hwang and Chung, 2023). Previous studies using genetically engineered mouse models revealed that CatSper deficiency (Ren et al., 2001; Quill et al., 2003; Qi et al., 2007; Chung et al., 2011) or its altered function (Chung et al., 2017; Hwang et al., 2019) causes male infertility or subfertility due to the impaired sperm hyperactivation. In addition, mutations in the genes encoding CatSper subunits, such as CatSper1 (Avenarius et al., 2009), CatSper2 (Avidan et al., 2003; Smith et al., 2013; Luo et al., 2019; Schiffer et al., 2020), and CatSperε (Brown et al., 2018), have been identified in infertile males whose sperm failed to develop hyperactivated motility (Schiffer et al., 2020; Young et al., 2023). Thus, examining CatSper function is important to explain the physiological defects in sperm hyperactivation and male infertility. Yet, despite its significance, evaluating CatSper function is limited to patch-clamping techniques (Kirichok et al., 2006; Lishko et al., 2011; Strunker et al., 2011) and functional imaging using Ca2+ dye.
A previous study revealed that the intracellular Ca2+ level ([Ca2+]i) in sperm is correlated with male fertility (Kelly et al., 2018). Sperm from subfertile patients showed reduced sensitivity against progesterone (Kelly et al., 2018), which activates the CatSper channel in human sperm (Lishko et al., 2011; Strunker et al., 2011); this resulted in a significantly reduced [Ca2+]i and Ca2+ oscillation. In addition, another study revealed that flagellar movement can be switched by the threshold [Ca2+]i in the sperm (Sanchez-Cardenas et al., 2018). These studies suggest that [Ca2+]i determined by CatSper function could be highly correlated with sperm motility patterns.
Here, I provide another detailed method and protocol—a sperm Ca2+-handling assay—which evaluates CatSper function by analyzing the [Ca2+]i-dependent motility change of mouse sperm, as described previously (Hwang et al., 2019; Hwang et al., 2022). This technique uses a computer-assisted semen analysis (CASA) system, instead of equipment for patch clamping and/or functional imaging. Thus, this protocol could be broadly used in andrology and reproductive biology labs that have a CASA system to evaluate CatSper function in mammalian sperm.
Materials and equipment
Materials and reagents
• Mice—all the mouse lines used in this study are on a C57BL/6 background.
- Wildtype (WT) mice (Charles River Laboratory)
- Mice of your interest
- CatSper-deficient mice (if available)
Note: CatSper-deficient mice [CatSper1- or CatSperd-null mice (Ren et al., 2001; Chung et al., 2011)] are used as a negative control to support the proof-of-concept in the study. If the lines are not applicable, the negative control can be replaced by pharmacological treatment using a CatSper inhibitor, such as NNC 05-0396.
• Nunc™ 4-Well Dishes for IVF (Thermo Fisher Scientific™, 144444)
• Hemocytometer
• A 1.5-mL microcentrifuge tube
• A 15-mL centrifuge tube
• CellVision 4 Chamber 20 micron (CellVision, CV 1020-4CH)
• Phosphate-buffered saline (Sigma-Aldrich, P4417)
• A measure of 0.4% HCl in distilled water (J.T. Baker, 9535-01)
• EmbryoMax® M2 Medium (1X), Liquid, with Phenol Red (EMD Millipore, MR-015-D)
• EmbryoMax® Human Tubal Fluid (HTF) (1X), Liquid, for Mouse IVF (EMD Millipore, MR-070-D)
• Modified HEPES-buffered HTF, H-HTF, pH 7.4 (Chung et al., 2017; Hwang et al., 2019)
− 92 mM NaCl (AmericanBio, AB01915)
− 4.7 mM KCl (J.T.Baker, 3040-01)
− 0.2 mM MgCl2 (J.T.Baker, 2444-01)
− 0.37 mM KH2PO4 (J.T.Baker, 3246-01)
− 2.78 mM dextrose (J.T.Baker, 1920-01)
− 0.33 mM sodium pyruvate (Sigma-Aldrich, P5280)
− 18.3 mM sodium lactate (J.T.Baker, V034-08)
− 10 mM HEPES (AmericanBio, AB00892)
• Dimethyl sulfoxide, DMSO (AmericanBio, AB03091)
• Pluronic™ F127, 20% solution in DMSO (Invitrogen, P3000MP)
• 20 mM stock of BAPTA-AM (Calbiochem, 196419) in DMSO
• Distilled water (Ultrapure grade)
• 5 mM stock of NNC 55-0396 hydrate (Sigma-Aldrich, N0287) in distilled water
Equipment and instruments
• Microcentrifuge
• CO2 incubator
• Computer-assisted semen analysis system
Note: The protocol described here is based on the SCA CASA® system.
- Inverted microscope (Nikon E200)
- CMOS camera (Basler AG, acA1300-200 μm)
- Warm stage
- SCA® CASA system analyzer (MICROPTIC)
• Slide warmer (C&A Scientific Co., Inc.)
Methods
Basic principles and the overall procedure
The current protocol has been established to examine normal CatSper activity by analyzing motility changes depending on [Ca2]i (Hwang et al., 2019). Sperm lose their motility by intracellular Ca2+ chelation (Marquez et al., 2007); CatSper is the main Ca2+ channel that introduces extracellular Ca2+ into the mammalian sperm. Thus, the previous study (Hwang et al., 2019) hypothesized the following:
Impaired sperm motility by intracellular Ca2+ chelation could be recovered if [Ca2+]i is restored by the normal function of the CatSper channel.
This protocol compares the motility parameters of sperm depending on BAPTA-AM treatment for intracellular Ca2+ chelation. Thus, the sperm motility parameters are analyzed in three statuses, as described below (Figure 1A):
Step 1. Basal status: Non-capacitated, before inducing [Ca2+]i chelation
Step 2. Intracellular Ca2+ chelation status: Non-capacitated, with BAPTA-AM
Step 3. Intracellular Ca2+ recovery status: Capacitation induced, without BAPTA-AM
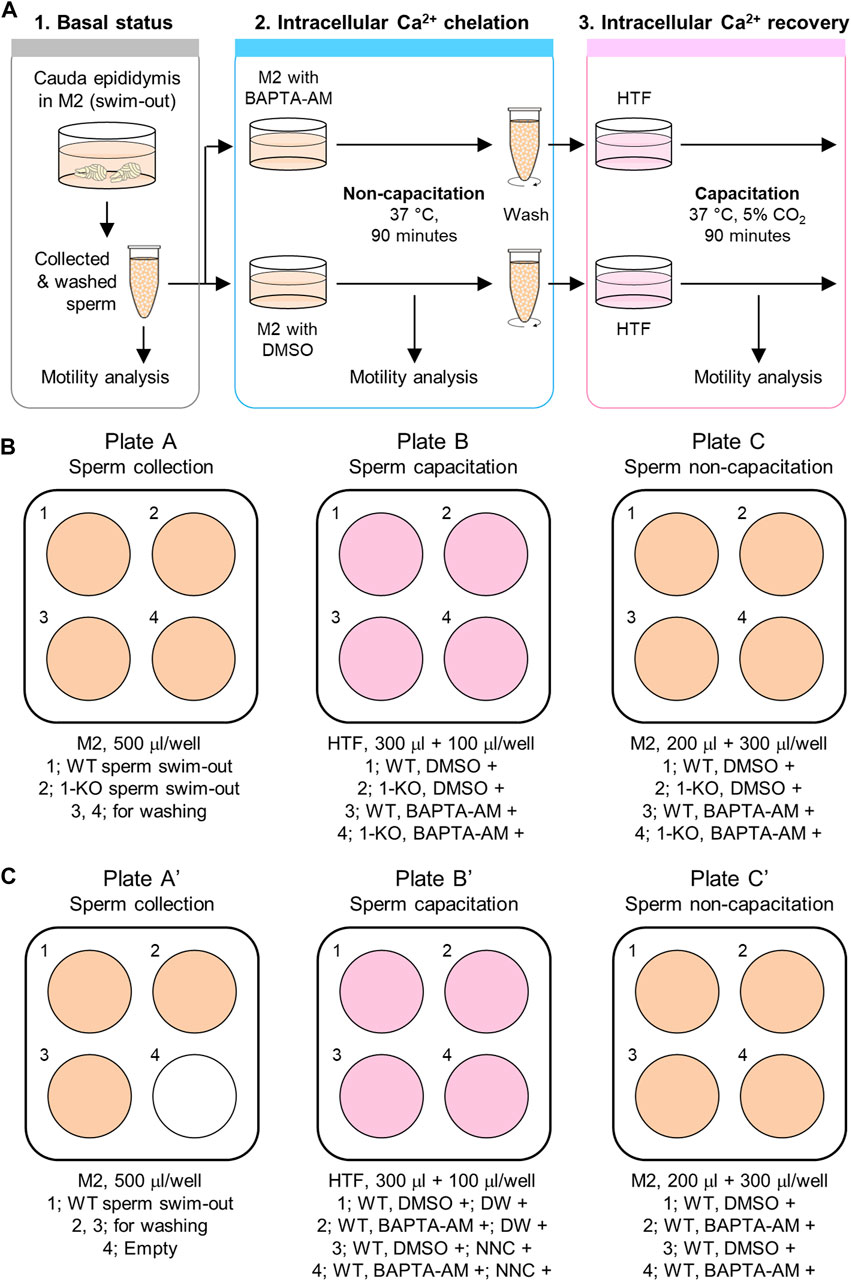
FIGURE 1. Procedure of the sperm Ca2+-handling assay. (A) Ca2+-handling assay to analyze [Ca2+]i-dependent sperm motility changes. Sperm motility is analyzed under three different conditions. First, the motility of fresh epididymal sperm is examined after swim-out (1. Basal status). Next, the sperm are incubated in a non-capacitating medium, M2, supplemented with an intracellular Ca2+ chelator, BAPTA-AM (2. Intracellular Ca2+ chelation status). Last, after incubation with BAPTA-AM for intracellular Ca2+ chelation, the sperm are washed and incubated in a capacitating medium, HTF, to activate the CatSper channel for extracellular Ca2+ influx into the sperm (3. Intracellular Ca2+ recovery status). Sperm motility is analyzed for 90 min of incubation in both the second and third steps. DMSO is used as a vehicle in this assay. (B,C) Media and plate preparation for the assay with the CatSper-deficient sperm (B) or pharmacological inhibition of the CatSper channel using NNC 05-0396 (C) for negative control. Shown are the 4-well plates and media information described in the Methods. WT, wild type; 1-KO, CatSper1-null; DW, distilled water; and NNC, NNC 05-0396.
First, the motility parameters of non-capacitated sperms are analyzed before BAPTA-AM treatment to chelate the intracellular Ca2+ (step 1). Next, sperm motility is examined during intracellular Ca2+ chelation under the non-capacitating condition supplemented with BAPTA-AM (step 2). Since sperm motility is altered following the removal of free intracellular Ca2+, the sperm will gradually lose motility during intracellular Ca2+ chelation by BAPTA-AM. Once the [Ca2+]i is buffered at a marginal level, a severe reduction in the total sperm motility is seen. Following this, the sperm is washed, capacitation is induced without BAPTA-AM, and motility changes are analyzed (step 3). If CatSper is functioning normally, the channel is activated during capacitation and extracellular Ca2+ is introduced into the sperm. This will elevate [Ca2+]i in the sperm and enable it to develop hyperactivated motility as well as recover motility. If CatSper is functioning abnormally, such as CatSper-deficient sperm, the sperm will not be able to recover motility and will fail to develop hyperactivated motility. Thus, the functional normality of the CatSper channel can be evaluated by analyzing motility changes during intracellular Ca2+ chelation and restoration.
Before the start
The procedure to prepare the required reagents described in this study is basically for experiments that compare the motility changes of sperm from two animals (WT and CatSper1-null males). An experimental procedure using 1 animal (WT) with the pharmacological treatment of a CatSper inhibitor, NNC 05-0396, is also described— !) for negative control with pharmacological treatment—as an alternative negative control instead of the CatSper1-null sperm. Thus, the number of tubes, wells in plates, and/or the amount of the medium will need to be adjusted according to the number of animals being used in the experiment.
1. All wells of a 4-well plate (plate A; Figure 1B) are filled with 500 μL of M2 medium and kept warm at 37 °C.
!) For negative control with pharmacological treatment, three wells of a 4-well plate (plate A’; Figure 1C) are filled with 500 μL of M2 medium.
2. All wells of another 4-well plate are filled with 300 μL of HTF medium (plate B; Figure 1B), and two microcentrifuge tubes are prepared with 1.5 mL of HTF medium. The HTF medium in the 4-well plate and the tube (cap opened) need to be pre-incubated at 5% CO2, 37 °C humidifying environment for at least 1 h.
!) For negative control with pharmacological treatment, 0.4 μL of distilled water or the same volume of 5 mM NNC 05-0396 stock are added to two wells per each (plate B’; Figure 1C).
3. 5 mL of modified H-HTF medium is prepared in a 15-mL centrifugal tube and kept warm at 37 °C.
4. 2 mL of M2 medium is prepared in a centrifugal tube and kept warm at 37 °C.
5. 190 μL of 0.4% HCl solution is added in a microcentrifuge tube.
6. Two microcentrifuge tubes are prepared with 500 μL of PBS.
7. 2 μL of 20 mM of BAPTA-AM is mixed in DMSO and 2 μL of Pluronic F-127 (F-127). Also, DMSO and F-127 are mixed to the same ratio for control (vehicle).
Note: F-127 is required to disperse the esterification of the acetoxymethyl (AM) group in BAPTA-AM, which will cage the Ca2+ chelator in the treated cell. Thus, BAPTA-AM treatment without F-127 is functional.
Note: BAPTA-AM stock should be preserved at −20 °C until use; the stock should be mixed with F-127 just before euthanizing the mice.
Note: F-127 in DMSO is sticky at room temperature (RT). Thus, it should be collected slowly from the tube.
Collection of epididymal sperm
1. Mice are euthanized in accordance with the guidelines approved by the Institutional Animal Care and Use Committee (IACUC).
2. Two cauda epididymides are taken out from a euthanized mouse, and the tissues are gently squeezed to remove blood. The collected tissues are placed in PBS in the microcentrifuge tubes at RT.
3. The tissues are moved to the M2 medium in plate A. Both epididymides from the same animal are placed into one well and cut 2–3 times using scissors followed by incubation on a 37°C warm plate for 10 min to let the sperm swim out of the epididymis.
Note: The M2 medium is buffered with HEPES. Thus, the medium should not be incubated in a CO2 incubator as this will alter its pH.
4. A new 4-well plate is prepared with 200 μL of pre-warmed M2 medium, and 0.25 μL of BAPTA-AM/F-127 or DMSO/F-127 mixture (plate C and C’; Figures 1B, C, respectively) is added and mixed well by gently pipetting.
Note: The sperm are placed in each well of these plates (plate C and C’; Figures 1B, C, respectively) in a 3.5 × 106 cells/mL concentration. As the final volume in the wells will be 500 μL, the concentrations of BAPTA-AM and DMSO will be 5 μM and 0.05% (v/v), respectively. The F-127 concentration will be 0.01% (w/v).
5. The M2 medium with sperm is transferred to new microcentrifuge tubes using a micropipette. The tubes are placed upright for 5 min so that any non-sperm cells and debris can sediment.
Note: All pipetting for the sperm should be performed gently to minimize any physical damage to the cells.
6. 400 μL of the sperm in the M2 medium are gently taken from the top and transferred to a new microcentrifuge tube followed by centrifugation at 500 g for 3 min at RT.
7. The supernatant is discarded, and the cells are resuspended with 300 μL of pre-warmed M2 medium at 37 °C.
Note: The sperm cells are sedimented rather than pelleted tightly.
8. 10 μL of the sperm in the M2 medium are taken and mixed with 190 μL of 0.4% HCl in a microcentrifuge tube by gentle tapping; 6–8 μL of the mixture is placed in the hemocytometer to count the sperm number and calculate the concentration.
9. A small volume of the sperm in the M2 medium is taken and mixed with the pre-warmed M2 medium to prepare 50 μL of sperm in the M2 medium with a concentration of 3.5 × 106 cells/mL. The sperm in this small volume of M2 are used to record their motility before intracellular Ca2+ chelation with BAPTA-AM.
10. 1.75 × 106 cells in the M2 medium are added to the wells containing BAPTA-AM or DMSO in plate C (3.5 × 106 cells/mL) and filled up to 500 μL with the pre-warmed M2 medium. The 4-well plate (plate C) is placed on the slide warmer, and the sperm cells are incubated at 37 °C for 90 min to analyze their motility changes using CASA.
!) For negative control with pharmacological treatment, 1.75 × 106 cells in the M2 medium are added to all wells containing either BAPTA-AM or DMSO (plate C’; Figure 1C). Later, sperm in two wells under the same condition are capacitated with or without NNC 05-0396.
Motility analyses using the CASA system
1. The SCA CASA system, microscope, and slide warmer equipped with a microscope are turned on. The temperature of the slide warmer should be maintained at 37 °C.
Note: As the operation of CASA systems may be different depending on the brands and companies, a detailed configuration and protocol on how to use the software are not described here.
Note: Sperm motility should be measured on the slide warmer equipped with a microscope, which is set to 37 °C, throughout the experiment.
2. 20 μL of the sperm-containing M2 medium (non-capacitated) is taken, which does not carry either vehicle (DMSO) or BAPTA-AM, and placed in the chamber.
Note: The sperm-containing medium is loaded from one side. Once the solution fills up the chamber, extra solution is placed on each side of the path to prevent evaporation during the experiment, as depicted in Figure 2.
3. The chamber is placed on the warm stage equipped with the microscope, and sperm motility parameters are analyzed using the CASA system. Usually, over 200 total sperms need to be measured per experiment.
4. The recorded information is saved, and a new experiment tab is opened in the software.
5. Steps 2–4 are repeated to measure sperm motility after 15, 30, and 90 min of incubation in an M2 medium supplemented with DMSO or BAPTA-AM.
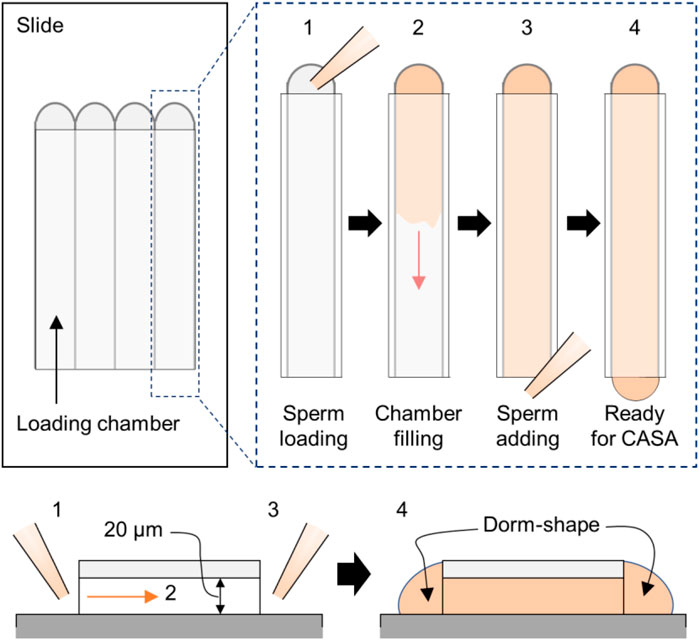
FIGURE 2. CASA application in the Ca2+-handling assay. The cartoon shows sample loading in the CASA imaging chamber. Shown are the top–down (top) and side (bottom) views. In brief, the sperm-containing medium is loaded first into one side (step 1), and we wait until the solution spreads thoroughly to the end of the other side (step 2). Next, the remaining sperm solution is added to the open-end side to create a dome shape on each end to prevent evaporation (step 3). Then, sperm in the chambers are ready to be analyzed (step 4).
Notes: The time points to measure the motility parameters could be changed according to the experiment.
Note: Each experiment carried out to analyze sperm motility will take some time. Therefore, the incubation of sperm from different animals might be initiated with 5–10-min intervals.
Note: In general, the curvilinear velocity (VCL) of WT sperm will drop significantly and be lowered to the basal level 15–30 min following incubation with 5–10 µM of BAPTA-AM. If the sperm’s swimming speed is not altered rapidly, it could be due to the activity loss of BAPTA-AM.
6. After 90 min of incubation, the remaining sperm (approximately 400 μL) are transferred into microcentrifuge tubes and centrifuged at 700 g for 2 min.
!) For negative control with pharmacological treatment, the sperm incubated under the same condition (approximately 900 μL) are transferred to one tube and centrifuged under the same condition as above.
7. The supernatant is removed, and the sperm are pelleted with 800 μL of H-HTF medium followed by another centrifugation at 700 g for 2 min to wash out the vehicle and BAPTA-AM in the media. This step is repeated one more time.
Note: A modified H-HTF medium lacking HCO3- and Ca2+ is used in this study, as per the original publication (Hwang et al., 2019). However, a normal H-HTF medium could be also applicable which does not affect the results dramatically (Hwang et al., 2022).
8. The supernatant is removed, and the sperm pellet is resuspended with 300 μL of pre-incubated HTF medium and centrifuged at 700 g for 2 min.
9. The supernatant is removed, and sperm pellets are resuspended with 100 μL of HTF pre-incubated in a microcentrifuge tube. The sperm elute is transferred to each well of plate B (Figure 1B). Incubation is started at 5% CO2, 37 °C condition to induce capacitation.
!) For negative control with pharmacological treatment, the sperm pellet is eluted in each tube with 200 μL of the HTF media in a microcentrifuge tube and 100 μL of the elute is transferred to the HTF medium supplemented with distilled water or NNC 05-0396 in a 4-well plate. The final volume in each well is 400 μL, and the concentration of NNC 05-0396 is 5 μM.
10. The sperm motility is measured at 15, 60, and 90 min after inducing capacitation using the CASA system.
Note: These time points can be changed according to the experiment.
11. The results are exported, and the motility changes are compared statistically.
Anticipated results and discussion
The expected results are described based on the previous study (Hwang et al., 2019).
Lowering [Ca2+]i impairs sperm motility
As depicted in a previous study (Marquez et al., 2007), treatment with the intracellular Ca2+ chelator, BAPTA-AM, dramatically reduces total sperm motility and severely alters the motility parameters (Figure 3). The total motility of both WT and CatSper1-null sperm was severely reduced following a 90-min incubation with 5-µM BAPTA-AM under the non-capacitating condition (Figure 3A, left; WT, 79.83% ± 3.29% to 28.36% ± 0.93%; CatSper1-null, 78.2% ± 2.09% to 29.3% ± 2.09%). In addition, the motility parameters, VCL, and amplitude of the lateral head (ALH), were also severely reduced after 90 min of incubation with BAPTA-AM in the sperm from both WT (VCL, 224.57 ± 12.22 μm/s to 123.03 ± 1.43 μm/s; ALH, 7.82 ± 0.42 µm to 4.87 ± 0.05 µm) and CatSper1-null (VCL, 236.46 ± 10.83 μm/s to 126.71 ± 4.05 μm/s; ALH, 7.45 ± 0.31 µm to 4.38 ± 0.21 µm) mice (Figures 3B, C, both left). Notably, despite the gradual decrease in the total motility for 90 min, the VCL and ALH values dropped more rapidly, and 15–30 min of incubation with BAPTA-AM is enough to lower these motility parameters to the basal level (Figures 3A–C). These results clearly demonstrate that lowering [Ca2+]i by BAPTA-AM rapidly alters the overall sperm swimming ability. Interestingly, despite the absence of BAPTA-AM, CatSper1-null sperm motility was also reduced after 90 min of incubation under the non-capacitating condition (Figure 3A, right; 78.25% ± 2.09% to 58.49% ± 4.58%). In addition, the VCL and ALH values were also severely decreased after 90 min of incubation without BAPTA-AM (Figures 3B, C, right each; VCL, 236.46 ± 10.83 μm/s to 157.96 ± 13.49 μm/s; ALH, 7.45 ± 0.31 µm to 5.13 ± 0.41 µm). These results are consistent with previous studies that analyzed the motility changes of CatSper1-, 2-, 3-, and 4-null sperm (Qi et al., 2007) or CatSperd-null sperm (Hwang et al., 2022) under non-capacitating conditions. One possibility could be that the intracellular Ca2+ is rapidly depleted and failed to maintain proper [Ca2+]i in the CatSper-deficient sperm. CatSper could marginally introduce extracellular Ca2+ into the sperm under non-capacitating and capacitating conditions (Kirichok et al., 2006; Hwang et al., 2019). Thus, the CatSper-deficient sperm may keep consuming and/or extruding free intracellular Ca2+ but fail to recharge it, which will eventually impair sperm motility even under the non-capacitating condition without BAPTA-AM.
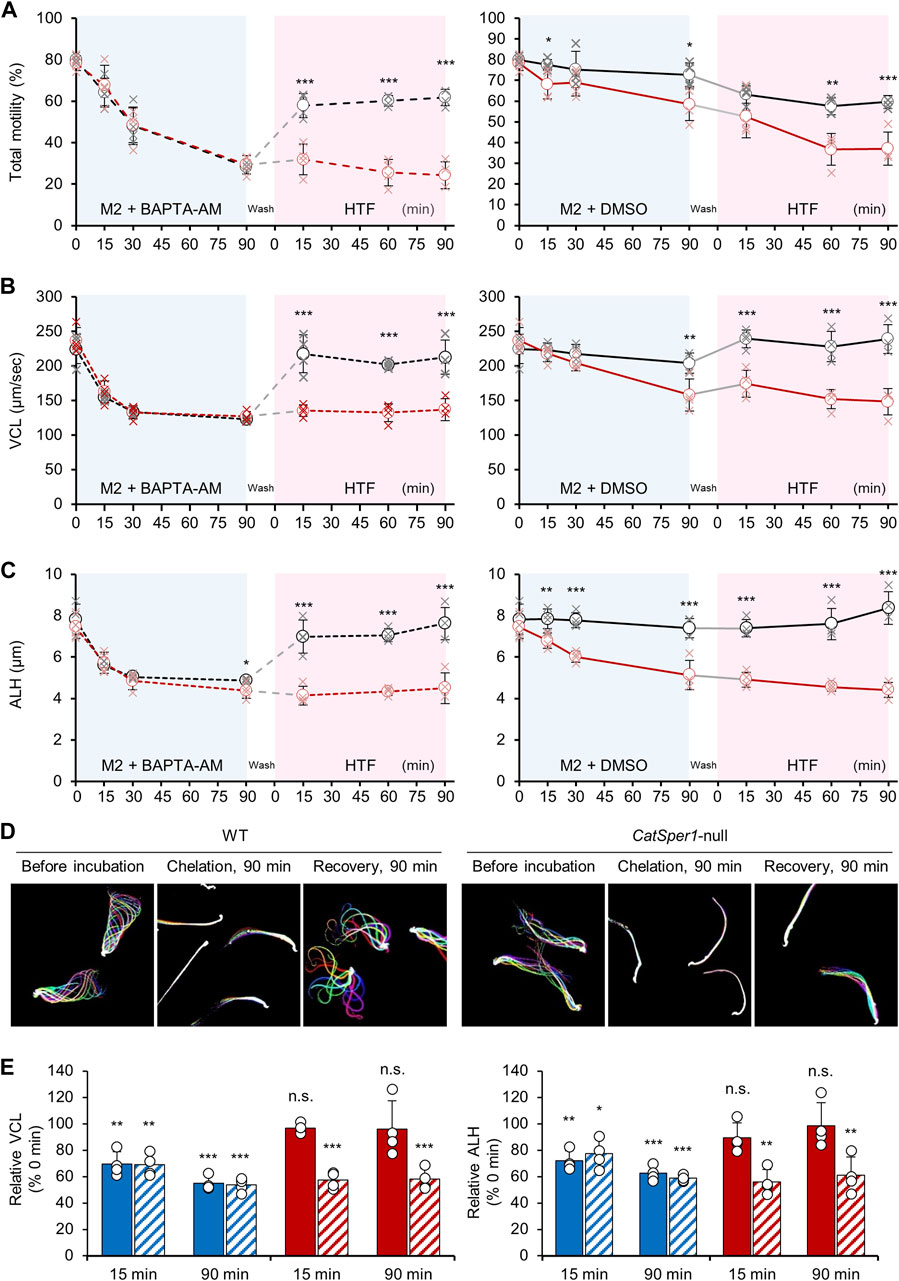
FIGURE 3. CatSper-dependent sperm motility changes by handling [Ca2+]i. (A–C) Changes in the total sperm motility (A), curvilinear motility [VCL, (B)], and amplitude of head displacement [ALH, (C)] during the Ca2+-handling assay. Shown are the changes in the motility parameters of WT (black, N = 4) and CatSper1-null (red, N = 4) sperm by chelating intracellular Ca2+ followed by inducing capacitation (left). DMSO is used as the vehicle (right). The data are represented by the mean ± SD (N = 4 for each strain). Cross marks at each time point represent values for sperm motility parameters from individual males. Statistical comparisons are performed between the WT and CatSper1-null sperm at each time point using Student’s t-test. (D) Images for the flagellar waveform changes of the sperm from WT (left) and CatSper1-null (right) males during the Ca2+-handling assay. Flagellar waveforms of head-tethered WT and CatSper1-null sperm are imaged before (each left) and after 90 min (each middle) incubation with BAPTA-AM and 90 min recovery in the HTF medium after washing (each right). Shown are merged images taken for two beating cycles. (E) Relative changes in the VCL (left) and ALH (right) of the WT (filled) and CatSper1-null (sashed) sperm in the Ca2+-handling assay. Shown are the relative changes after intracellular Ca2+ chelation (blue bars) and inducing capacitation (red bars). The values at each time point are normalized to those at 0 min and statically compared using Student’s t-test. The data are represented as the mean ± SD (N = 4 for each strain). Circles indicate the relative changes in the motility parameters in the sperm from each male. *p < 0.05; **p < 0.01; and ***p < 0.001. n.s., non-significant. The charts and flagellar waveform images are reproduced from a previous study (Hwang et al., 2019) with the permission of Cell.
[Ca2+]i-lowered sperm recover motility in a CatSper-dependent manner during capacitation
Both the WT and CatSper1-null sperm were treated with BAPTA-AM for 90 min followed by washing with a modified H-HTF medium to terminate any additional BAPTA caging in the sperm. The washed sperm were further incubated under the capacitating condition for 90 min. Although the WT sperm could successfully rescue their motility, the CatSper1-null sperm failed to do this for 90 min of capacitation. The total motility of the WT sperm increased rapidly (Figure 3A, left) after inducing capacitation for 15 min (28.36% ± 0.93% to 57.80% ± 3.35%), and the value was maintained for 90 min of capacitation (61.78% ± 2.31%). In contrast, the total motility of the CatSper1-null sperm did not significantly increase after washing and inducing capacitation for 90 min (29.34% ± 2.55% to 31.83% ± 4.32%). In addition, the VCL (Figure 3B, left) and ALH (Figure 3C, left) values in the WT sperm increased dramatically after only 15 min of capacitation (VCL, 123.03 ± 1.43 μm/s to 217.37 ± 15.81 μm/s; ALH, 4.87 ± 0.05 µm to 6.98 ± 0.46 µm) and were maintained or further increased after 90 min of capacitation (VCL, 212.59 ± 14.23 μm/s; ALH, 7.62 ± 0.44 µm). Furthermore, the WT sperm recovered from lowered [Ca2+]i can successfully develop hyperactivated motility (Figure 3D; Hwang et al., 2019). However, the motility parameters were not rescued in the CatSper1-null sperm following 90 min of capacitation (VCL, 126.71 ± 4.05 μm/s to 136.84 ± 9.13 μm/s; ALH, 4.38 ± 0.21 µm to 4.51 ± 0.43 µm). The WT sperm capacitated with NNC 055-0396 failed to recover the motility parameters after intracellular Ca2+ chelation (Supplementary Figure S1) like the CatSper-deficient sperm. All the results clearly demonstrate that sperm motility parameters are recovered in a CatSper-dependent manner after intracellular Ca2+ chelation. The CatSper-mediated recovery of sperm motility is also supported by a relative comparison of the motility parameters before and after inducing capacitation (Figure 3D). Notably, chelating intracellular Ca2+ for 90 min seems to barely affect the recovery process due to the potential introduction of excessive BAPTA-AM. Recovery statuses by inducing capacitation are not significantly different within the groups incubated with BAPTA-AM for 30 or 90 min, which lowers the motility parameters to the basal level (Supplementary Figure S2).
Previous studies demonstrated that VCL and ALH values were recovered in the Efcab9-null sperm but not in the CatSpert-null sperm, in which the CatSper current is at ∼50% and 10% level compared to the current in the WT sperm, respectively (Hwang et al., 2019; Hwang et al., 2022). These results indicate that the Ca2+-handling assay is applicable to detect the altered CatSper function in which the current is lower than 50% of the normal ranges despite its lesser sensitivity than patch clamping.
The total motility and motility parameters in the CatSper-deficient sperm could transiently increase after washing (Hwang et al., 2019; Hwang et al., 2022). Originally, the CatSper1-or CatSperd-null sperm were expected to fail to recover their motility parameters after washing and inducing capacitation due to the absence of CatSper. However, the motility parameters were marginally recovered in the CatSper-deficient sperm after washing (refer to the 15-min point after inducing capacitation in Figures 3A, B). Presumably, this might be due to the transient increase of free Ca2+ released from the storage organelle (e.g., mitochondria) during the washing step, which needs to be further examined.
Although the marginal motility rescue was observed shortly, total motility and the parameters in CatSper-deficient sperm are eventually compromised during capacitation. The above results clearly demonstrate that sperm motility is recovered by restoring [Ca2+]i via CatSper-mediated Ca2+ entry during capacitation.
This new method can examine the functional normality of the CatSper channel by analyzing sperm motility changes. Although this method is currently validated in mouse sperm, optimizing this method in sperm from other species would contribute to expanding the methodology to analyze CatSper function. There is a possibility that altered upstream pathways also affect abnormal CatSper function in sperm. Thus, direct methods to open CatSper specifically, such as intracellular alkalinization using NH4Cl, could be applicable in this protocol to distinguish altered CatSper function by upstream signaling pathways. Those modifications remain to be further tested, and updated protocols will also contribute to examining the functional normality of the CatSper channel in mammalian sperm.
Data availability statement
The data analyzed in this study are subject to the following licenses/restrictions: raw CASA data are from a previous study (Hwang et al., 2019; Cell) and this study. Requests to access these datasets should be directed to JH, jyhwang@pusan.ac.kr.
Ethics statement
The animal study was approved by the Institutional Animal Care and Use Committee (IACUC) for Pusan National University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
JH: conceptualization, data curation, funding acquisition, methodology, project administration, visualization, writing–original draft, and writing–review and editing.
Funding
The authors declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (grant nos. RS-2023-00210046 and RS-2023-00262293).
Acknowledgments
The author appreciates Professor Jean-Ju Chung from Yale School of Medicine and Professor Bum-Joon Park and Eui-Man Jung from Pusan National University for sharing the research materials.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2024.1284988/full#supplementary-material
SUPPLEMENTARY FIGURE S1 | Motility changes of WT sperm capacitated with or without NNC 05-0396 (NNC) after intracellular Ca2+ chelation. Shown are the changes in total motility (A), curvilinear velocity [VCL, (B)], and amplitude of head displacement [ALH, (C)] of WT sperm capacitated with 5 μM of NNC 05-0396, a CatSper inhibitor (NNC +, dashed lines). Distilled water was used for control (control, solid lines). Impaired motility changes by NNC 05-0396 were examined in the capacitating sperm, which were incubated in the M2 medium supplemented with BAPTA-AM (left, red lines) or DMSO (right, black lines) The data are represented by the mean ± SD (N=3). Triangles and cross marks represent the values for the motility parameters from individual males, whose sperm were capacitated with or without NNC 05-0396, respectively. Statistical comparisons are performed within control and NNC-treated groups at each time point using Student’s t-test. *p<0.05; **<0.01; and ***<0.001.
SUPPLEMENTARY FIGURE S2 | Effects of incubation time for intracellular Ca2+ chelation to rescue motility parameters. (A) Changes of curvilinear motility (VCL, top) and amplitude of head displacement (ALH, bottom) values after incubation with BAPTA-AM for 30 (left) or 90 (right) minutes. Shown are the changes in VCL and ALH values incubated with an M2 medium supplemented with BAPTA-AM (red) or DMSO (black). Statistical comparisons are conducted within groups incubated with DMSO or BAPTA-AM using Student’s t-test (N=3). *p<0.05; **p<0.01; and ***P<0.001. (B) Relative comparison of VCL (top) and ALH (bottom) values in the capacitating (Cap) sperm after chelating intracellular Ca2+ for 30 or 90 minutes. Shown are the relative VCL and ALH values of sperm capacitated for 15, 60, and 90 minutes after intracellular Ca2+ chelation. The values are normalized by those from sperm incubated with DMSO at the same time points. None of the relative values between 30 and 90 minutes of intracellular Ca2+ chelation are significantly different at each time point (N=3, each). Statistical comparisons are performed with Student’s t-test. The data are represented by the mean ± SD. Values from individual animals are marked in crosses (A) and circles (B) at each time point. Data for the 90-minute intracellular Ca2+ chelation are from those shown in Supplementary Figure 1.
References
Avenarius, M. R., Hildebrand, M. S., Zhang, Y., Meyer, N. C., Smith, L. L., Kahrizi, K., et al. (2009). Human male infertility caused by mutations in the CATSPER1 channel protein. Am. J. Hum. Genet. 84 (4), 505–510. doi:10.1016/j.ajhg.2009.03.004
Avidan, N., Tamary, H., Dgany, O., Cattan, D., Pariente, A., Thulliez, M., et al. (2003). CATSPER2, a human autosomal nonsyndromic male infertility gene. Eur. J. Hum. Genet. 11 (7), 497–502. doi:10.1038/sj.ejhg.5200991
Brown, S. G., Miller, M. R., Lishko, P. V., Lester, D. H., Publicover, S. J., Barratt, C. L. R., et al. (2018). Homozygous in-frame deletion in CATSPERE in a man producing spermatozoa with loss of CatSper function and compromised fertilizing capacity. Hum. Reprod. 33 (10), 1812–1816. doi:10.1093/humrep/dey278
Chung, J. J., Miki, K., Kim, D., Shim, S. H., Shi, H. F., Hwang, J. Y., et al. (2017). CatSperζ regulates the structural continuity of sperm Ca2+ signaling domains and is required for normal fertility. Elife 6, e23082. doi:10.7554/eLife.23082
Chung, J. J., Navarro, B., Krapivinsky, G., Krapivinsky, L., and Clapham, D. E. (2011). A novel gene required for male fertility and functional CATSPER channel formation in spermatozoa. Nat. Commun. 2, 153. doi:10.1038/ncomms1153
Hwang, J. Y., and Chung, J. J. (2023). CatSper calcium channels: 20 Years on. Physiol. (Bethesda) 38 (3), 0. doi:10.1152/physiol.00028.2022
Hwang, J. Y., Mannowetz, N., Zhang, Y., Everley, R. A., Gygi, S. P., Bewersdorf, J., et al. (2019). Dual sensing of physiologic pH and calcium by EFCAB9 regulates sperm motility. Cell 177 (6), 1480–1494. doi:10.1016/j.cell.2019.03.047
Hwang, J. Y., Wang, H., Lu, Y., Ikawa, M., and Chung, J. J. (2022). C2cd6-encoded CatSperτ targets sperm calcium channel to Ca2+ signaling domains in the flagellar membrane. Cell Rep. 38 (3), 110226. doi:10.1016/j.celrep.2021.110226
Kelly, M. C., Brown, S. G., Costello, S. M., Ramalingam, M., Drew, E., Publicover, S. J., et al. (2018). Single-cell analysis of [Ca2+] i signalling in sub-fertile men: characteristics and relation to fertilization outcome. Hum. Reprod. 33 (6), 1023–1033. doi:10.1093/humrep/dey096
Kirichok, Y., Navarro, B., and Clapham, D. E. (2006). Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature 439 (7077), 737–740. doi:10.1038/nature04417
Lishko, P. V., Botchkina, I. L., and Kirichok, Y. (2011). Progesterone activates the principal Ca2+ channel of human sperm. Nature 471 (7338), 387–391. doi:10.1038/nature09767
Luo, T., Chen, H. Y., Zou, Q. X., Wang, T., Cheng, Y. M., Wang, H. F., et al. (2019). A novel copy number variation in CATSPER2 causes idiopathic male infertility with normal semen parameters. Hum. Reprod. 34 (3), 414–423. doi:10.1093/humrep/dey377
Marquez, B., Ignotz, G., and Suarez, S. S. (2007). Contributions of extracellular and intracellular Ca2+ to regulation of sperm motility: release of intracellular stores can hyperactivate CatSper1 and CatSper2 null sperm. Dev. Biol. 303 (1), 214–221. doi:10.1016/j.ydbio.2006.11.007
Qi, H., Moran, M. M., Navarro, B., Chong, J. A., Krapivinsky, G., Krapivinsky, L., et al. (2007). All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility. Proc. Natl. Acad. Sci. U. S. A. 104 (4), 1219–1223. doi:10.1073/pnas.0610286104
Quill, T. A., Sugden, S. A., Rossi, K. L., Doolittle, L. K., Hammer, R. E., and Garbers, D. L. (2003). Hyperactivated sperm motility driven by CatSper2 is required for fertilization. Proc. Natl. Acad. Sci. U. S. A. 100 (25), 14869–14874. doi:10.1073/pnas.2136654100
Ren, D., Navarro, B., Perez, G., Jackson, A. C., Hsu, S., Shi, Q., et al. (2001). A sperm ion channel required for sperm motility and male fertility. Nature 413 (6856), 603–609. doi:10.1038/35098027
Sanchez-Cardenas, C., Montoya, F., Navarrete, F., Hernandez-Cruz, A., Corkidi, G., Visconti, P., et al. (2018). Intracellular Ca2+ threshold reversibly switches flagellar beat off and on. Biol. reproduction 99 (5), 1010–1021. doi:10.1093/biolre/ioy132
Schiffer, C., Rieger, S., Brenker, C., Young, S., Hamzeh, H., Wachten, D., et al. (2020). Rotational motion and rheotaxis of human sperm do not require functional CatSper channels and transmembrane Ca(2+) signaling. EMBO J. 39 (4), e102363. doi:10.15252/embj.2019102363
Seifert, R., Flick, M., Bonigk, W., Alvarez, L., Trotschel, C., Poetsch, A., et al. (2015). The CatSper channel controls chemosensation in sea urchin sperm. EMBO J. 34 (3), 379–392. doi:10.15252/embj.201489376
Smith, J. F., Syritsyna, O., Fellous, M., Serres, C., Mannowetz, N., Kirichok, Y., et al. (2013). Disruption of the principal, progesterone-activated sperm Ca2+ channel in a CatSper2-deficient infertile patient. Proc. Natl. Acad. Sci. U. S. A. 110 (17), 6823–6828. doi:10.1073/pnas.1216588110
Strunker, T., Goodwin, N., Brenker, C., Kashikar, N. D., Weyand, I., Seifert, R., et al. (2011). The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature 471 (7338), 382–386. doi:10.1038/nature09769
Suarez, S. S., Varosi, S. M., and Dai, X. (1993). Intracellular calcium increases with hyperactivation in intact, moving hamster sperm and oscillates with the flagellar beat cycle. Proc. Natl. Acad. Sci. U. S. A. 90 (10), 4660–4664. doi:10.1073/pnas.90.10.4660
Wood, C. D., Nishigaki, T., Furuta, T., Baba, S. A., and Darszon, A. (2005). Real-time analysis of the role of Ca(2+) in flagellar movement and motility in single sea urchin sperm. J. Cell Biol. 169 (5), 725–731. doi:10.1083/jcb.200411001
Yanagimachi, R. (1970). The movement of golden hamster spermatozoa before and after capacitation. J. Reprod. Fertil. 23 (1), 193–196. doi:10.1530/jrf.0.0230193
Keywords: sperm motility, hyperactivation, CatSper, Ca2+, computer-assisted semen analysis
Citation: Hwang JY (2024) Analysis of Ca2+-mediated sperm motility to evaluate the functional normality of the sperm-specific Ca2+ channel, CatSper. Front. Cell Dev. Biol. 12:1284988. doi: 10.3389/fcell.2024.1284988
Received: 29 August 2023; Accepted: 16 January 2024;
Published: 07 February 2024.
Edited by:
Pascale Lybaert, Université libre de Bruxelles, BelgiumReviewed by:
Christoph Brenker, University Hospital Münster, GermanyJuan J. Ferreira, Washington University in St. Louis, United States
Copyright © 2024 Hwang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jae Yeon Hwang, jyhwang@pusan.ac.kr
 Jae Yeon Hwang
Jae Yeon Hwang