
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Cell Dev. Biol., 26 October 2023
Sec. Molecular and Cellular Pathology
Volume 11 - 2023 | https://doi.org/10.3389/fcell.2023.1269724
This article is part of the Research TopicBone and Joint Diseases: Mechanism, Diagnosis and TherapyView all 7 articles
Osteoarthritis (OA) is one of the leading causes of pain and disability in the elderly. Synovitis, cartilage destruction and osteophyte formation histologically manifest OA. Unfortunately, there is currently no effective therapy to delay its progression and the underlying mechanisms of OA require further exploration. Macrophage is a main cellular component of joint synovium. It is highly plastic and can be stimulated to polarize to different phenotypes, namely, the pro-inflammatory phenotype (M1) and the anti-inflammatory/tissue-repairing phenotype (M2). Ample evidence has demonstrated the vital roles of macrophages in the progression of OA. Imbalanced M1/M2 ratio is significantly related to OA severity indicating macrophage polarization might be a promising therapeutic target for OA. In this review, we summarized the involvements of polarized macrophages in synovitis, cartilage degradation, osteophyte formation and OA-related chronic pain. Promising therapies targeting macrophage polarization including the intra-articular cell/derivates-based therapy and the alternative non-invasive intervention such as photobiomodulation therapy were reviewed as well.
Osteoarthritis (OA) is a prevalent and heterogeneous degenerative disease, which is the leading cause of pain among elderly (Miller et al., 2014). It is characterized by synovitis, cartilage degeneration and osteophyte formation, and usually results in pain, joint swelling, joint space narrowing, physical impairment and even disability (Martel-Pelletier et al., 2016). Over 300 million people worldwide are affected by OA (Atukorala et al., 2016; Roos and Arden, 2016; Hunter and Bierma-Zeinstra, 2019). Despite the high prevalence, unfortunately, it is still a big challenge to treat OA patients in clinic because there’s no disease-modified drug to delay OA development or cure it. The most commonly used treatments for OA at present include non-steroid anti-inflammatory drugs (NSAIDs) and corticosteroids, which are accompanied with an extensive economic burden, unavoidable side effects and unsatisfactory outcomes (da Costa et al., 2021; Katz et al., 2021; Khumaidi et al., 2022). The potential mediators governing the initiation and progression of OA are not totally clear. New insights into the underlying mechanisms of OA and targeted therapeutics are urgently required.
Macrophages scatter in many joint tissue components including synovium, ligament, bone marrow, subchondral bone, adipose tissue and so on. In physiological conditions, macrophages engulf pathogens and debris of aged tissues to maintain the microenvironment homeostasis of joint, while in OA joint, abnormally activated macrophages might worsen joint destruction (Zhang et al., 2018). Intriguingly, due to the highly plasticity of macrophages, they are demonstrated to be induced polarization to different phenotypes by different stimuli. For instance, macrophages can be polarized from an unstimulated M0 status to the pro-inflammatory M1 phenotype by lipopolysaccharide (LPS) and interferon-γ (IFN-γ), while interleukin (IL)-4/IL-13 is efficient to enhance the anti-inflammatory/tissue-repairing M2 phenotype macrophage polarization (Figure 1A) (Wynn and Vannella, 2016; Thomson and Hilkens, 2021). That is, to say, macrophages play a dual role in driving inflammation as well as promoting tissue regeneration. So far, ample evidence has demonstrated the pivotal roles of macrophage polarization in a variety of diseases including cancer (Li et al., 2023a), atherosclerosis (Tabas and Bornfeldt, 2016; Jinnouchi et al., 2020), osteoporosis (Wang et al., 2022), wound healing (Louiselle et al., 2021) and so on. In 2016, Kraus and colleagues (Kraus et al., 2016) provided the first direct in vivo evidence for macrophage involvements in human OA by applying a new 99mTc-EC20 agent-based SPECT-CT imaging. Activated macrophages were found present in 76% of the knees. The quantity of knee-related activated macrophages was significantly associated with knee pain and radiographic knee OA severity including joint space narrowing and osteophyte formation, suggesting that macrophages-targeted interventions might work in attenuating OA progression. Subsequent experimental evidence from Zhang et al. (2018) showed that M1 but not M2-polarized macrophages accumulated in human and mouse OA synovium samples. Meanwhile, M1 macrophage polarization in synovium exacerbated collagenase-induced knee OA, shedding light on the potential of polarized macrophage as a promising therapeutic target for OA treatment.
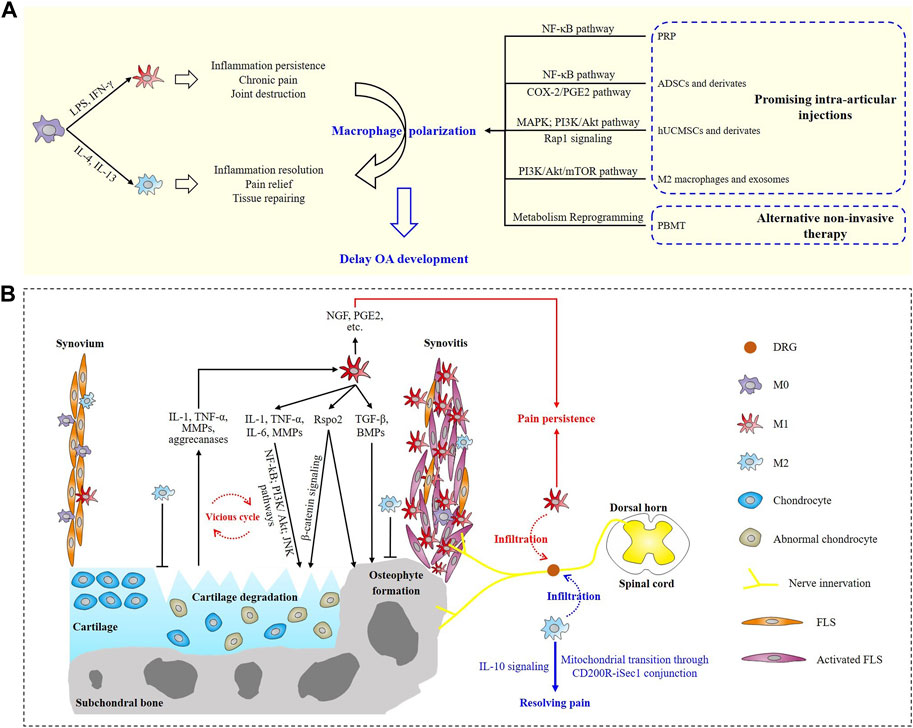
FIGURE 1. Schematic overview of macrophage polarization involved in osteoarthritis progression and potential treatments. (A) Roles of M1 and M2 macrophages in OA and promising macrophage polarization-targeted therapeutics. (B) Involvements of polarized macrophages in synovitis, cartilage degeneration, osteophyte formation and OA pain.
In this review, we focused on summarizing the involvements of polarized macrophages in synovitis, cartilage degeneration, osteophyte formation and pain in OA. Promising therapies targeting macrophage polarization including intra-articular injections and alternative non-invasive interventions to OA were briefly introduced as well.
OA has now been recognized as a whole-joint disease instead of a “wear and tear” disease induced by mechanical issues. Inflammation, synovitis in particular, significantly contributes to OA progression (Fernandez-Madrid et al., 1994; Benito et al., 2005). Cumulative evidence collectively proves the strong correlations between synovitis and OA symptoms, disease severity as well as outcomes (Ayral et al., 2005; Baker et al., 2010; Bacon et al., 2020), indicating synovitis may be a predictor or precursor of OA progression.
Macrophage and fibroblast-like synoviocyte (FLS) are the two main cellular components of synovium (Figure 1B). In OA joint, a large number of macrophage aggregation in the intimal layer of synovium manifests synovitis and it is suggested to drive the subsequent cartilage degeneration and osteophyte formation (Zhang et al., 2018). Kraus and colleagues preliminarily evaluated the involvements of the two macrophage phenotypes by performing immunostaining on synovial fluid samples of two patients, and the results suggested a potential block in the transition of macrophages from M1 to M2 phenotype (Kraus et al., 2016). This block might prolong the ongoing joint degeneration and prevents tissue repairing in OA, but it requires confirmation in a larger sample clinical trial. Liu et al. (2018) explored the status of M1 and M2 macrophages in knee OA patients for the first time. They collected the samples of both synovial fluids and peripheral whole blood from 80 knee OA patients and 80 healthy controls. M1 macrophages were shown to predominantly exist in OA knees and the ratio of M1/M2 macrophages was remarkably higher in OA knees and was significantly associated with Kellgren-Lawrence (KL) grading level, which indicated the imbalanced ratio of M1/M2 macrophages might be a sign of knee OA damage and severity (Liu et al., 2018). Actually, it’s further certified that M1 rather than M2 macrophages predominantly aggregated in the synovium of both OA patients and OA experimental animals, and M1 macrophages secreted R-spondin-2 (Rspo2) to exacerbate experimental OA progression (Zhang et al., 2018). Downregulating the polarization of M1 macrophages or abolishing the expression of Rspo2 may be a potential therapeutic target for OA.
To further evaluate the roles of polarized macrophages, macrophage depletion, which was considered a potential anti-OA intervention, was performed in experimental animals. By peritoneally injecting a small molecule (AP20187) in macrophage Fas-induced apoptosis (MaFIA)-transgenic mice, macrophages can be conditionally deleted (Burnett et al., 2004). Using this method, Wu et al. (2017) investigated the roles of macrophages in MaFIA knee OA mice in the setting of obesity. In this study, the injection of AP20187 was later demonstrated to successfully reduce the number of both M1 and M2 macrophages in the knees of OA mice compared to controls. However, systemic macrophage depletion did not attenuate the severity of OA in obese mice as expected; instead, it induced systemic inflammation, led to an extensive infiltration of CD3+ T cells and neutrophils, and remarkably increased the amount of pro-inflammatory factors; thereby exacerbated synovitis (Wu et al., 2017). This result was intriguing and it showed that the inflammatory state in macrophage-depleted OA mice transited from macrophage-associated to neutrophil-dependent. That is, to say, macrophage may be a double-edged sword in regulating OA synovitis. However, whether the failure of macrophage phenotype transition from M1 to M2 accounts for the post-depletion systemic inflammation is not concluded in this study. In another research, the effects of transient synovial depletion of macrophages on the inflammatory responses in post-trauma OA (PTOA) mice were evaluated (Bailey et al., 2020). Consistent with Wu’s research, intra-articular macrophage depletion 2 days prior to the joint injury induced intensive synovitis. In MaFIA mice, the depletion paradoxically altered macrophage polarization to a dominance of M1 phenotype within the joint synovium, while the expression of M2 macrophages in the synovial stroma was reduced (Bailey et al., 2020). The shift in M1/M2 macrophage ratio indicated macrophages might be critical immunomodulators in the acute inflammatory response to joint injury. These studies suggested that failure to correct polarity from the M1 to M2 subtype rather than the quantity of M1 or M2 macrophages may indeed mediate the progression and development of OA. But the precise mechanisms of macrophage phenotype transition in synovium warranted further investigation.
Irreversible cartilage loss is the main manifestation of OA. Activated macrophages mediate cartilage degradation and erosion mainly through autocrine and paracrine interactions. Both the inflammatory and destructive effects in OA joint are established to be largely dependent on synovial macrophages and these responses are driven by two main factors secreted by activated macrophages, namely, interleukin-1 (IL-1) and tumor necrosis factor-α (TNF-α) (Bondeson et al., 2006). Other potential mediators including matrix metalloproteinases (MMPs), cytokines, and growth factors were secreted into synovial fluid (SF) by activated synovial macrophages, FLS and damaged chondrocytes. The debris of destructed cartilage and mediators (MMP-9, MMP-13, aggrecanases, etc.) released by injured chondrocytes in turn facilitates M1 polarization by providing a proinflammatory microenvironment (Samavedi et al., 2017; Kulkarni et al., 2022). This interaction between synovial macrophages and chondrocytes provokes a vicious cycle that intensifies cartilage destruction and synovitis (Figure 1B) (Yin et al., 2022). CD14+ (a marker of M1 macrophage) synovial macrophages were shown to drive this effect. After specifically depleting CD14+ synovial macrophages in vitro, the synovial macrophages no longer produced significant amounts of macrophage-related cytokines (IL-1 and TNF-α), meanwhile, FLS-related mediators (IL-6, IL-8, MMP-1, and MMP-3) were also strongly downregulated (Bondeson et al., 2006). Activated macrophages can also lead to abnormal secretion of chondrocytes. The expression of MMP-1, MMP-3, MMP-9, MMP-13, IL-1β, TNF-α, IL-6, IL-8, and IFN-γ were observed to be significantly higher when chondrocytes were cocultured with activated macrophages, suggesting proinflammatory macrophages promoted the abnormal matrix degradation and chondrocyte-associated cytokine secretion (Samavedi et al., 2017). Mahon et al. (2020) treated human monocyte derived macrophages with basic calcium phosphate (BCP) crystals, known as OA-associated damage-associated molecular patterns (DAMPs), and assessed the expression of M1 and M2-associated markers. Treatment with BCP crystals promoted M1 macrophage polarization according to their results. Thereby, dampening the inflammatory responses provoked by M1 macrophages will definitely contribute to the amelioration of the vicious synovitis-cartilage degeneration cycle during OA development. Zhang et al. (2018) further explored the underlying mechanism for the macrophage-driving cartilage degeneration. In their study, tuberous sclerosis complex 1 (TSC1) deletion in mice was demonstrated to constitutively activate mechanistic target of rapamycin complex 1 (mTORC1), increase synovial M1 macrophage polarization and in this way exacerbate knee OA in experimental mice. And this effect was partially mediated through secretion of Rspo2 and activation of β-catenin signaling in chondrocytes (Figure 1B). Besides, inhibition of mTORC1 enhanced M2 polarization and in this way alleviated OA development in mice (Zhang et al., 2018). To date, several signaling pathways, including nuclear factor κB (NF-κB), PI3K/Akt, JNK and so on, are established participating in the cartilage degeneration of OA (Zhang et al., 2020; Zhao et al., 2023), however, associations between macrophage polarization and these pathways during OA pathogenesis are still under exploration.
Osteophyte formation is a hallmark for advanced or end-stage OA. Systemic depletion of macrophages was capable of significantly reducing osteophyte formation in knee OA mice (Wu et al., 2017). Dominant M1 macrophages in synovium are shown to be associated with decreased bone mineral density (BMD) in macrophage-depleted MaFIA PTOA mice, suggesting M1 macrophages might be detrimental for bone homeostasis (Bailey et al., 2020). The secretion of growth factors such as transforming growth factor-β (TGF-β) and bone morphogenetic protein (BMP) is established to be responsible for regulating osteophyte formation in OA, because they are efficient in promoting osteogenesis, chondrogenesis and osteogenic differentiation of mesenchymal stem cells (MSCs) (Blom et al., 2004; Zhang et al., 2020). By establishing a collagenase-induced OA (CIOA) model, Blom et al. (2004) investigated the involvements of synovial macrophages in osteophyte formation and demonstrated macrophages provoke osteophyte formation through regulating the expressions of TGF-β and BMPs (Figure 1B). In their study, prior to OA induction, macrophages were selectively removed using clodronate liposomes. Depletion of synovial macrophages resulted in spectacular reduction of osteophyte formation, fibrosis and synovial activation were also significantly ameliorated. In addition, production of TGF-β, BMP-2 and BMP-4 in the lining was largely prevented but production of them in deeper layers of the synovium and the periosteum did not differ from controls. Other cytokines like IL-1β and TNF-α are able to activate osteoclast formation and promote bone resorption, which exacerbates osteophyte formation in OA (Zhang et al., 2020). Intra-articular injections of recombinant human IL-1 receptor antagonist (IL-1Ra) were demonstrated protective in inhibiting the development of osteophytes and cartilage lesions in an experimental canine OA model, showing the positive effects of functional suppression of pro-inflammatory macrophages in OA osteophyte formation (Caron et al., 1996). In addition, M1 macrophages were also shown to secret Rspo2 to exacerbate osteophyte formation and worsen OA progression (Zhang et al., 2018). All these findings suggest the crucial roles of polarized macrophages in osteophyte formation.
Pain is the first and most common symptom in OA patients, and is the main reason that patients seek for medical assistance. 25% of adults over 55 years old experience knee pain at least once a year, which may be a marker of potential knee OA (Shamsi et al., 2021). The current interventional strategies for OA dominantly aim at relieving pain and improving functions. However, the existing pain management methods are inadequate and unsatisfactory. NSAIDs are the most frequently used and are recommended in most of the clinical guidelines for OA, but it’s accompanied with potential risks of side effects, for instance gastrointestinal toxicity and bleeding; while opiates bring risks of addiction (Conaghan et al., 2019; Hunter and Bierma-Zeinstra, 2019; Stausholm et al., 2019). Total joint replacement surgery is thought to be the most effective method for advanced and end-stage OA patients, however, about 7%–23% patients after hip replacement and 10%–34% after knee replacement reported an unfavorable long-term pain (Beswick et al., 2012). Therefore, it’s essential to figure out the mechanisms of OA pain in order to provide new insights for developing effective targeted pain management interventions. Macrophages might be an etiologic factor for OA pain because joint pain is shown to be significantly positively associated with the presence of activated macrophages in OA joint (Kraus et al., 2016).
It has been established that tissue injury, inflammatory response and central sensitization collectively contribute to OA-related pain (Raoof et al., 2018; Conaghan et al., 2019). M1 macrophages are illuminated to mediate OA pain through regulating local secretion of pain-related inflammatory factors including IL-1β, TNF-α, prostaglandin E2 (PGE2) etc., which not only promote synovitis and joint destruction, but also directly activate innervating nociceptors (Miller et al., 2014; Conaghan et al., 2019). Meanwhile, macrophage-derived IL-1β and TNF-α can also regulate the expression of nerve growth factor (NGF) in synovial macrophages, indicating the development and persistence of OA pain (Figure 1B) (Takano et al., 2017).
Central sensitization gives an explanation for why approximately 7%–34% of patients reported chronic post-surgical pain after the receiving total joint arthroplasty surgery and why OA pain is often found to be not always associated with radiographic manifestations. Apart from the secretion of pro-inflammatory factors, polarized macrophages also involve in central sensitization of OA pain. Raoof et al. (2021) identified that dorsal root ganglion (DRG) containing the somata of sensory neurons innervating the damaged knee were infiltrated with M1 macrophages. These M1 macrophages in DRG actively maintained OA joint pain remotely and independent of joint damage. Inhibiting M1 macrophages in DRG by intrathecal injection of IL4-10 fusion protein or M2 macrophages resolved persistent OA pain. Their work reveals a novel mechanism that help to maintain OA pain distant from the affected knee and suggests that DRG macrophages will be a new therapeutic target to treat OA chronic pain. In their later study, M2 macrophages were found to infiltrate in the DRG containing the somata of sensory neurons during the resolution of inflammatory pain. And M2 phenotype macrophages could actively control OA inflammatory pain resolution remotely from the site of inflammation by transferring mitochondria to sensory neurons, which was mediated by conjunction of CD200 receptor (CD200R) on macrophages and iSec1 (a non-canonical CD200R ligand) on sensory neurons. This research revealed a novel and direct mechanism for M2 macrophages in actively resolving inflammatory pain (van der Vlist et al., 2022). In addition to the direct participation of macrophages in OA pain resolution, monocytes/macrophages are uncovered to resolve the inflammatory hyperalgesia via IL-10 signaling-dependent mechanism in DRG (Figure 1B), suggesting the cardinal roles of M2 macrophages in pain resolution (Willemen et al., 2014).
Macrophage polarization sheds lights on developing new anti-OA interventions. To date, compelling experiments have been performed to test the effects of different anti-OA therapeutics, including medicine, cell therapy, nanomaterials, surgery, etc., on driving macrophage polarization (Ma et al., 2021; Robb et al., 2022; Li et al., 2023b; Liu et al., 2023; Zheng et al., 2023; Zhou et al., 2023). All of these preclinical researches are shown to be effective in inhibiting pro-inflammatory cytokines release, suppressing M1 polarization and enhancing M2 polarization. Herein, we simply focused on promising intra-articular cells/derivates-based therapies and alternative non-invasive interventions that target macrophage polarization for treating OA.
Intra-articular injection of medicine, including glucocorticoid, has long been applied for treating OA patients. It is effective by providing short-term analgesia and anti-inflammatory effects (Li et al., 2022a). However, long-term repeated injections will cause loss of cartilage or injury of other joint components. Therefore, to investigate bio-modulatory medium that are able to meanwhile promote cartilage proliferation and modulate joint homeostasis will definitely be necessary and useful for developing new intra-articular therapeutics.
Cells (usually stem cells) derived from adipose, umbilical cord and bone marrow, etc., or their derivates including extracellular vesicles (EVs) and exosomes are now under extensive investigation for treating OA. One example that has been increasingly applied in clinic is the platelet-rich plasma (PRP). PRP is derived from autologous blood and is suggested to maintain the homeostasis of joints by providing an analgesic effect, facilitating inflammation resolving and promoting anabolic metabolism (Uchiyama et al., 2021). Preclinical experiments have demonstrated that PRP suppressed M1 macrophage polarization and promoted M2 macrophage polarization in OA (Uchiyama et al., 2021). PRP exerts anti-inflammatory properties through its effects on the canonical NF-κB signaling pathway in multiple cell types including synoviocytes, macrophages and chondrocytes (Andia and Maffulli, 2013). A series of randomized clinical trials (RCTs) and meta-analysis have been performed to investigate the efficacy and safety of intra-articular PRP injections towards OA, however, the current findings do not really support the use of PRP injections for knee or ankle OA patients (Bennell et al., 2021; Paget et al., 2021).
Adipose-derived mesenchymal stem cells (ADMSCs) are indicated as one of the most promising cell sources, and they may exert their powerful immunomodulatory and anti-inflammatory activities through the cyclooxygenase-2 (COX-2)/prostaglandin E2 (PGE2) pathway (Manferdini et al., 2013). Besides, NF-κB1 and NF-κBIA (the NF-κB signaling inhibitor) were significantly decreased by ADMSC treatment, suggesting ADMSCs potentially switching off macrophages’ activity strictly dependent on NF-κB signaling (Paolella et al., 2019). The therapeutic potential of EVs derived from human adipose-derived stem cells (hADSCs) in alleviating OA was evaluated (Woo et al., 2020). Intra-articular injection of hADSC-EVs significantly attenuated OA progression and protected cartilage from degeneration in knee OA mouse models by inhibiting M1 macrophages infiltration in the synovium. KEGG pathway enrichment analysis indicated “ECM-receptor interaction”, and “glycosphingolipid biosynthesis–lacto and neolacto series” as dominant pathways controlled by hADSC-EVs (Woo et al., 2020). Both human umbilical cord mesenchymal stem cells (hUCMSCs) and EVs derived from hUCMSCs are efficient to promote M2 macrophage polarization, inhibit M1 macrophage expression (Tang et al., 2021). Notably, compared with hUCSMCs, small EVs derived from hUCMSCs exert stronger effects in maintaining cartilage homeostasis and alleviating cartilage damage by upregulating proteins involved in immune effector process, extracellular matrix organization, PI3K-Akt and Rap1 signaling pathway (Tang et al., 2021). In another study, hUCMSCs-EVs can alleviate cartilage degradation during the OA progression, mechanically may through modulating the PI3K-Akt signaling pathway mediated by miRNAs to promote polarization of M2 macrophage and exhibit the potent immunomodulatory potential (Li et al., 2022b). Besides, exosomes derived from hUCMSC resist IL-1β or M1 macrophages-induced chondrocyte inflammation dominantly through the MAPK and PI3K-Akt signaling pathways, which was demonstrated by performing a KEGG pathway enrichment analysis (Wang et al., 2023). All of these results suggest exosomes or EVs derived from ADMSC or hUCMSC might serve as a new reagent for treating OA.
Recently, Ma et al. (2021) proposed and fabricated an intriguing artificial M2 macrophage (AM2M) with yolk-shell structure to enhance the therapeutic efficacy of M2 macrophages in treating OA. AM2M was composed of macrophage membrane as “shell” and inflammation-responsive nanogel as “yolk”. AM2M exhibited the targeting and long-term residence in the inflamed area and blocked the immune stimulation of macrophages, indicating AM2M might be a promising medium applied for future intra-articular therapies for OA (Ma et al., 2021). But the potent mechanical signaling pathways accounting for the effects of AM2M in OA are uncovered in this research. Apart from M2 macrophages themselves, exosomes derived from M2 macrophages also exert positive effects towards protecting articular cartilage and attenuating inflammatory responses in knee OA rats mainly through the PI3K/AKT/mTOR signal pathway (Da-Wa et al., 2021).
Preclinical experiments indicate the potential of intra-articular injections of cells and derivates in driving synovial macrophage polarization for OA joints. However, few clinical evidence has elucidated the presence of macrophage phenotype transition in OA patients. Cell and derivates-based therapies are promising, but unnecessary injuries might happen when injecting the cells or derivates into joints. Besides, larger-sample and long-term studies are mandatory to confirm whether these therapies can improve symptoms and induce structural benefits in OA patients.
Photobiomodulation therapy (PBMT), also known as low-level light therapy (LLLT), is an intriguing intervention method using low-intensity laser or light emitting diodes (LEDs) with wavelengths in the range of visible (400–700 nm) or near-infrared (NIR, 700–1,000 nm) (Amaroli et al., 2019). PBM displays great advantages in treating OA because it is totally non-invasive with rarely reported side effects. In vivo studies have shown the efficacy of PBM in relieving pain, decreasing synovial inflammatory factors (IL-1β, TNF-α, IL-6 etc.) expression and attenuating joint swelling in OA animals (Alves et al., 2013; da Rosa et al., 2012; Trevisan et al., 2020; Wang et al., 2014; Yamada et al., 2020). In Zhang et al. (2022) latest study, they provided the first in vivo evidence for the efficacy of PBMT in modulating synovial macrophage polarization in collagenase-induced knee OA mice. A 630 nm Light Emitting Diode (LED) device was applied to illuminate the right knees of OA mice with different power densities. After 4-week consecutive treatments (1 hour irradiation per time per day), the pain and gait of OA mice were significantly attenuated in the group irradiated with a power density of 10 mW/cm2. Histological analysis and immunostaining results suggested a great amelioration of synovitis, cartilage degeneration and reduction of osteophyte formation in experimental mice, which might be partially mediated by the synovial macrophage polarity transition from M1 to M2 phenotype. Unfortunately, they didn’t further explore the potential mechanistic pathways of the phenotype transition caused by PBMT. Metabolically, inflammatory M1 macrophages display enhanced glycolytic metabolism, conversely, anti-inflammatory M2 macrophages show high mitochondrial oxidative phosphorylation (OXPHOS) (Van den Bossche et al., 2015). PBMT is believed to be sufficient in enhancing mitochondrial OXPHOS by increasing mitochondrial activity and ATP production (Amaroli et al., 2019; Zhang and Qu, 2023). Given this, metabolism reprogramming induced by light irradiation might be a potential cellular mechanism for PBMT in enhancing macrophage polarization and treating OA (Figure 1A). But it still requires further experimental verification in OA animals and patients.
It’s now widely acknowledged that OA is a multifactorial disease. Synovitis and macrophages play primordial roles in the onset and progression of OA. Macrophages function as a bridge between synovium, cartilage, adipose tissue, and subchondral bone. The cellular interactions among polarized macrophage, chondrocyte, FLS, etc., contribute to both homeostasis maintenance and pathological destruction of joint. Targeting macrophage polarization provides new insights for breaking the vicious and self-perpetuating cycle of OA. Future studies should focus on the following topics. First, detailed interactions between macrophages and other cellular components in OA joint should be uncovered. Second, the concise subsets of macrophages that participate in the whole process of OA progression need more clarification. Finally, understanding the underlying mechanisms of macrophage phenotypes transition and developing alternative targeted therapeutic methods. Larger sample clinical trials are required to evaluate the existence of macrophage polarization in human body.
YZ: Writing–review and editing, Investigation, Writing–original draft. QJ: Writing–review and editing, Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Validation.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was approved by National Natural Science Foundation of China (81902250, 82272558, and 82072464). Beijing Natural Science Foundation, 7212093 (grant number), QJ (Funder).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Alves, A. C., Vieira, R., Leal-Junior, E., dos Santos, S., Ligeiro, A. P., Albertini, R., et al. (2013). Effect of low-level laser therapy on the expression of inflammatory mediators and on neutrophils and macrophages in acute joint inflammation. Arthritis Res. Ther. 15, R116. doi:10.1186/ar4296
Amaroli, A., Ravera, S., Baldini, F., Benedicenti, S., Panfoli, I., and Vergani, L. (2019). Photobiomodulation with 808-nm diode laser light promotes wound healing of human endothelial cells through increased reactive oxygen species production stimulating mitochondrial oxidative phosphorylation. Lasers Med. Sci. 34, 495–504. doi:10.1007/s10103-018-2623-5
Andia, I., and Maffulli, N. (2013). Platelet-rich plasma for managing pain and inflammation in osteoarthritis. Nat. Rev. Rheumatol. 9, 721–730. doi:10.1038/nrrheum.2013.141
Atukorala, I., Kwoh, C., Guermazi, A., Roemer, F., Boudreau, R., Hannon, M., et al. (2016). Synovitis in knee osteoarthritis: a precursor of disease? Ann. Rheum. Dis. 75, 390–395. doi:10.1136/annrheumdis-2014-205894
Ayral, X., Pickering, E., Woodworth, T., Mackillop, N., and Dougados, M. (2005). Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis -- results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthr. Cartil. 13, 361–367. doi:10.1016/j.joca.2005.01.005
Bacon, K., LaValley, M., Jafarzadeh, S., and Felson, D. (2020). Does cartilage loss cause pain in osteoarthritis and if so, how much? Ann. Rheum. Dis. 79, 1105–1110. doi:10.1136/annrheumdis-2020-217363
Bailey, K. N., Furman, B. D., Zeitlin, J., Kimmerling, K. A., Wu, C. L., Guilak, F., et al. (2020). Intra-articular depletion of macrophages increases acute synovitis and alters macrophage polarity in the injured mouse knee. Osteoarthr. Cartil. 28, 626–638. doi:10.1016/j.joca.2020.01.015
Baker, K., Grainger, A., Niu, J., Clancy, M., Guermazi, A., Crema, M., et al. (2010). Relation of synovitis to knee pain using contrast-enhanced MRIs. Ann. Rheum. Dis. 69, 1779–1783. doi:10.1136/ard.2009.121426
Benito, M., Veale, D., FitzGerald, O., van den Berg, W., and Bresnihan, B. (2005). Synovial tissue inflammation in early and late osteoarthritis. Ann. Rheum. Dis. 64, 1263–1267. doi:10.1136/ard.2004.025270
Bennell, K. L., Paterson, K. L., Metcalf, B. R., Duong, V., Eyles, J., Kasza, J., et al. (2021). Effect of intra-articular platelet-rich plasma vs placebo injection on pain and medial tibial cartilage volume in patients with knee osteoarthritis: the RESTORE randomized clinical trial. Jama 326, 2021–2030. doi:10.1001/jama.2021.19415
Beswick, A. D., Wylde, V., Gooberman-Hill, R., Blom, A., and Dieppe, P. (2012). What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open 2, e000435. doi:10.1136/bmjopen-2011-000435
Blom, A., van Lent, P., Holthuysen, A., van der Kraan, P., Roth, J., van Rooijen, N., et al. (2004). Synovial lining macrophages mediate osteophyte formation during experimental osteoarthritis. Osteoarthr. Cartil. 12, 627–635. doi:10.1016/j.joca.2004.03.003
Bondeson, J., Wainwright, S. D., Lauder, S., Amos, N., and Hughes, C. E. (2006). The role of synovial macrophages and macrophage-produced cytokines in driving aggrecanases, matrix metalloproteinases, and other destructive and inflammatory responses in osteoarthritis. Arthritis Res. Ther. 8, R187. doi:10.1186/ar2099
Burnett, S. H., Kershen, E. J., Zhang, J., Zeng, L., Straley, S. C., Kaplan, A. M., et al. (2004). Conditional macrophage ablation in transgenic mice expressing a Fas-based suicide gene. J. Leukoc. Biol. 75, 612–623. doi:10.1189/jlb.0903442
Caron, J., Fernandes, J., Martel-Pelletier, J., Tardif, G., Mineau, F., Geng, C., et al. (1996). Chondroprotective effect of intraarticular injections of interleukin-1 receptor antagonist in experimental osteoarthritis. Suppression of collagenase-1 expression. Arthritis Rheum. 39, 1535–1544. doi:10.1002/art.1780390914
Conaghan, P. G., Cook, A. D., Hamilton, J. A., and Tak, P. P. (2019). Therapeutic options for targeting inflammatory osteoarthritis pain. Nat. Rev. Rheumatol. 15, 355–363. doi:10.1038/s41584-019-0221-y
da Costa, B. R., Pereira, T. V., Saadat, P., Rudnicki, M., Iskander, S. M., Bodmer, N. S., et al. (2021). Effectiveness and safety of non-steroidal anti-inflammatory drugs and opioid treatment for knee and hip osteoarthritis: network meta-analysis. Bmj 375, n2321. doi:10.1136/bmj.n2321
da Rosa, A. S., dos Santos, A. F., da Silva, M. M., Facco, G. G., Perreira, D. M., Alves, A. C., et al. (2012). Effects of low-level laser therapy at wavelengths of 660 and 808 nm in experimental model of osteoarthritis. Photochem Photobiol. 88, 161–166. doi:10.1111/j.1751-1097.2011.01032.x
Da-Wa, Z. X., Jun, M., Chao-Zheng, L., Sen-Lin, Y., Chuan, L., De-Chun, L., et al. (2021). Exosomes derived from M2 macrophages exert a therapeutic effect via inhibition of the PI3K/AKT/mTOR pathway in rats with knee osteoarthritic. Biomed. Res. Int. 2021, 7218067. doi:10.1155/2021/7218067
Fernandez-Madrid, F., Karvonen, R., Teitge, R., Miller, P., and Negendank, W. (1994). MR features of osteoarthritis of the knee. Magn. Reson Imaging 12, 703–709. doi:10.1016/0730-725x(94)92194-6
Hunter, D. J., and Bierma-Zeinstra, S. (2019). Osteoarthritis. Lancet 393, 1745–1759. doi:10.1016/s0140-6736(19)30417-9
Jinnouchi, H., Guo, L., Sakamoto, A., Torii, S., Sato, Y., Cornelissen, A., et al. (2020). Diversity of macrophage phenotypes and responses in atherosclerosis. Cell Mol. Life Sci. 77, 1919–1932. doi:10.1007/s00018-019-03371-3
Katz, J. N., Arant, K. R., and Loeser, R. F. (2021). Diagnosis and treatment of hip and knee osteoarthritis: a review. Jama 325, 568–578. doi:10.1001/jama.2020.22171
Khumaidi, M. A., Paturusi, I., Nusdwinuringtyas, N., Islam, A. A., Gunawan, W. B., Nurkolis, F., et al. (2022). Is low-level laser therapy effective for patients with knee joint osteoarthritis? implications and strategies to promote laser therapy usage. Front. Bioeng. Biotechnol. 10, 1089035. doi:10.3389/fbioe.2022.1089035
Kraus, V., McDaniel, G., Huebner, J., Stabler, T., Pieper, C., Shipes, S., et al. (2016). Direct in vivo evidence of activated macrophages in human osteoarthritis. Osteoarthr. Cartil. 24, 1613–1621. doi:10.1016/j.joca.2016.04.010
Kulkarni, P., Srivastava, V., Tootsi, K., Electricwala, A., Kharat, A., Bhonde, R., et al. (2022). Synovial fluid in knee osteoarthritis extends proinflammatory niche for macrophage polarization. Cells 11, 4115. doi:10.3390/cells11244115
Li, D., Ruan, G., Zhang, Y., Zhao, Y., Zhu, Z., Ou, Q., et al. (2023a). Metformin attenuates osteoarthritis by targeting chondrocytes, synovial macrophages and adipocytes. Rheumatol. Oxf. 62, 1652–1661. doi:10.1093/rheumatology/keac467
Li, D., Yang, Y., Xiong, L., Jiang, P., Wang, J., and Li, C. (2023b). Metabolism, metabolites, and macrophages in cancer. J. Hematol. Oncol. 16, 80. doi:10.1186/s13045-023-01478-6
Li, K., Tu, Q., Xie, D., Chen, S., Gao, K., Xu, X., et al. (2022b). Triamcinolone acetonide-loaded nanoparticles encapsulated by CD90+ MCSs-derived microvesicles drive anti-inflammatory properties and promote cartilage regeneration after osteoarthritis. J. nanobiotechnology 20, 150. doi:10.1186/s12951-022-01367-z
Li, K., Yan, G., Huang, H., Zheng, M., Ma, K., Cui, X., et al. (2022a). Anti-inflammatory and immunomodulatory effects of the extracellular vesicles derived from human umbilical cord mesenchymal stem cells on osteoarthritis via M2 macrophages. J. Nanobiotechnol 20, 38. doi:10.1186/s12951-021-01236-1
Liu, B., Zhang, M., Zhao, J., Zheng, M., and Yang, H. (2018). Imbalance of M1/M2 macrophages is linked to severity level of knee osteoarthritis. Exp. Ther. Med. 16, 5009–5014. doi:10.3892/etm.2018.6852
Liu, S., Zhang, C., Zhou, Y., Zhang, F., Duan, X., Liu, Y., et al. (2023). MRI-visible mesoporous polydopamine nanoparticles with enhanced antioxidant capacity for osteoarthritis therapy. Biomaterials 295, 122030. doi:10.1016/j.biomaterials.2023.122030
Louiselle, A., Niemiec, S., Zgheib, C., and Liechty, K. (2021). Macrophage polarization and diabetic wound healing. Transl. Res. 236, 109–116. doi:10.1016/j.trsl.2021.05.006
Ma, Y., Yang, H., Zong, X., Wu, J., Ji, X., Liu, W., et al. (2021). Artificial M2 macrophages for disease-modifying osteoarthritis therapeutics. Biomaterials 274, 120865. doi:10.1016/j.biomaterials.2021.120865
Mahon, O., Kelly, D., McCarthy, G., and Dunne, A. (2020). Osteoarthritis-associated basic calcium phosphate crystals alter immune cell metabolism and promote M1 macrophage polarization. Osteoarthr. Cartil. 28, 603–612. doi:10.1016/j.joca.2019.10.010
Manferdini, C., Maumus, M., Gabusi, E., Piacentini, A., Filardo, G., Peyrafitte, J. A., et al. (2013). Adipose-derived mesenchymal stem cells exert antiinflammatory effects on chondrocytes and synoviocytes from osteoarthritis patients through prostaglandin E2. Arthritis Rheum. 65, 1271–1281. doi:10.1002/art.37908
Martel-Pelletier, J., Barr, A. J., Cicuttini, F. M., Conaghan, P. G., Cooper, C., Goldring, M. B., et al. (2016). Osteoarthritis. Nat. Rev. Dis. Prim. 2, 16072. doi:10.1038/nrdp.2016.72
Miller, R. E., Miller, R. J., and Malfait, A. M. (2014). Osteoarthritis joint pain: the cytokine connection. Cytokine 70, 185–193. doi:10.1016/j.cyto.2014.06.019
Paget, L. D. A., Reurink, G., de Vos, R. J., Weir, A., Moen, M. H., Bierma-Zeinstra, S. M. A., et al. (2021). Effect of platelet-rich plasma injections vs placebo on ankle symptoms and function in patients with ankle osteoarthritis: a randomized clinical trial. Jama 326, 1595–1605. doi:10.1001/jama.2021.16602
Paolella, F., Manferdini, C., Gabusi, E., Gambari, L., Filardo, G., Kon, E., et al. (2019). Effect of microfragmented adipose tissue on osteoarthritic synovial macrophage factors. J. Cell Physiol. 234, 5044–5055. doi:10.1002/jcp.27307
Raoof, R., Martin Gil, C., Lafeber, F., de Visser, H., Prado, J., Versteeg, S., et al. (2021). Dorsal root ganglia macrophages maintain osteoarthritis pain. J. Neurosci. 41, 8249–8261. doi:10.1523/jneurosci.1787-20.2021
Raoof, R., Willemen, H., and Eijkelkamp, N. (2018). Divergent roles of immune cells and their mediators in pain. Rheumatol. Oxf. 57, 429–440. doi:10.1093/rheumatology/kex308
Robb, K., Audet, J., Gandhi, R., and Viswanathan, S. (2022). Putative critical quality attribute matrix identifies mesenchymal stromal cells with potent immunomodulatory and angiogenic "fitness" ranges in response to culture process parameters. Front. Immunol. 13, 972095. doi:10.3389/fimmu.2022.972095
Roos, E. M., and Arden, N. K. (2016). Strategies for the prevention of knee osteoarthritis. Nat. Rev. Rheumatol. 12, 92–101. doi:10.1038/nrrheum.2015.135
Samavedi, S., Diaz-Rodriguez, P., Erndt-Marino, J., and Hahn, M. (2017). A three-dimensional chondrocyte-macrophage coculture system to probe inflammation in experimental osteoarthritis. Tissue Eng. Pt A 23, 101–114. doi:10.1089/ten.TEA.2016.0007
Shamsi, M., Safari, A., Soroush, A., and Safari, Y. (2021). The survey of knee osteoarthritis in the population over age 50 visited in the health bus in kermanshah, Iran. J. Aging Res. 2021, 9809565. doi:10.1155/2021/9809565
Stausholm, M. B., Naterstad, I. F., Joensen, J., Brandao Lopes-Martins, R. A., Saebo, H., Lund, H., et al. (2019). Efficacy of low-level laser therapy on pain and disability in knee osteoarthritis: systematic review and meta-analysis of randomised placebo-controlled trials. BMJ Open 9, e031142. doi:10.1136/bmjopen-2019-031142
Tabas, I., and Bornfeldt, K. (2016). Macrophage phenotype and function in different stages of atherosclerosis. Circ. Res. 118, 653–667. doi:10.1161/circresaha.115.306256
Takano, S., Uchida, K., Inoue, G., Miyagi, M., Aikawa, J., Iwase, D., et al. (2017). Nerve growth factor regulation and production by macrophages in osteoarthritic synovium. Clin. Exp. Immunol. 190, 235–243. doi:10.1111/cei.13007
Tang, S., Chen, P., Zhang, H., Weng, H., Fang, Z., Chen, C., et al. (2021). Comparison of curative effect of human umbilical cord-derived mesenchymal stem cells and their small extracellular vesicles in treating osteoarthritis. Int. J. Nanomedicine 16, 8185–8202. doi:10.2147/ijn.S336062
Thomson, A., and Hilkens, C. M. U. (2021). Synovial macrophages in osteoarthritis: the key to understanding pathogenesis? Front. Immunol. 12, 678757. doi:10.3389/fimmu.2021.678757
Trevisan, E. S., Martignago, C. C. S., Assis, L., Tarocco, J. C., Salman, S., Dos Santos, L., et al. (2020). Effectiveness of led photobiomodulation therapy on treatment with knee osteoarthritis: a rat study. Am. J. Phys. Med. Rehabil. 99, 725–732. doi:10.1097/phm.0000000000001408
Uchiyama, R., Toyoda, E., Maehara, M., Wasai, S., Omura, H., Watanabe, M., et al. (2021). Effect of platelet-rich plasma on M1/M2 macrophage polarization. Int. J. Mol. Sci. 22, 2336. doi:10.3390/ijms22052336
van der Vlist, M., Raoof, R., Willemen, H., Prado, J., Versteeg, S., Martin Gil, C., et al. (2022). Macrophages transfer mitochondria to sensory neurons to resolve inflammatory pain. Neuron 110, 613–626.e9. doi:10.1016/j.neuron.2021.11.020
Van den Bossche, J., Baardman, J., and de Winther, M. P. (2015). Metabolic characterization of polarized M1 and M2 bone marrow-derived macrophages using real-time extracellular flux analysis. J. Vis. Exp. 2015, 53424. doi:10.3791/53424
Wang, P., Liu, C., Yang, X., Zhou, Y., Wei, X., Ji, Q., et al. (2014). Effects of low-level laser therapy on joint pain, synovitis, anabolic, and catabolic factors in a progressive osteoarthritis rabbit model. Lasers Med. Sci. 29, 1875–1885. doi:10.1007/s10103-014-1600-x
Wang, S., Jiang, W., Lv, S., Sun, Z., Si, L., Hu, J., et al. (2023). Human umbilical cord mesenchymal stem cells-derived exosomes exert anti-inflammatory effects on osteoarthritis chondrocytes. Aging (Albany NY) 15, 9544–9560. doi:10.18632/aging.205034
Wang, W., Liu, H., Liu, T., Yang, H., and He, F. (2022). Insights into the role of macrophage polarization in the pathogenesis of osteoporosis. Oxid. Med. Cell Longev. 2022, 2485959. doi:10.1155/2022/2485959
Willemen, H. L., Eijkelkamp, N., Garza Carbajal, A., Wang, H., Mack, M., Zijlstra, J., et al. (2014). Monocytes/Macrophages control resolution of transient inflammatory pain. J. Pain 15, 496–506. doi:10.1016/j.jpain.2014.01.491
Woo, C. H., Kim, H. K., Jung, G. Y., Jung, Y. J., Lee, K. S., Yun, Y. E., et al. (2020). Small extracellular vesicles from human adipose-derived stem cells attenuate cartilage degeneration. J. Extracell. Vesicles 9, 1735249. doi:10.1080/20013078.2020.1735249
Wu, C. L., McNeill, J., Goon, K., Little, D., Kimmerling, K., Huebner, J., et al. (2017). Conditional macrophage depletion increases inflammation and does not inhibit the development of osteoarthritis in obese macrophage fas-induced apoptosis-transgenic mice. Arthritis Rheumatol. 69, 1772–1783. doi:10.1002/art.40161
Wynn, T., and Vannella, K. (2016). Macrophages in tissue repair, regeneration, and fibrosis. Immunity 44, 450–462. doi:10.1016/j.immuni.2016.02.015
Yamada, E. F., Bobinski, F., Martins, D. F., Palandi, J., Folmer, V., and da Silva, M. D. (2020). Photobiomodulation therapy in knee osteoarthritis reduces oxidative stress and inflammatory cytokines in rats. J. Biophot. 13, e201900204. doi:10.1002/jbio.201900204
Yin, J., Zeng, H., Fan, K., Xie, H., Shao, Y., Lu, Y., et al. (2022). Pentraxin 3 regulated by miR-224-5p modulates macrophage reprogramming and exacerbates osteoarthritis associated synovitis by targeting CD32. Cell Death Dis. 13, 567. doi:10.1038/s41419-022-04962-y
Zhang, H., Cai, D., and Bai, X. (2020). Macrophages regulate the progression of osteoarthritis. Osteoarthr. Cartil. 28, 555–561. doi:10.1016/j.joca.2020.01.007
Zhang, H., Lin, C., Zeng, C., Wang, Z., Wang, H., Lu, J., et al. (2018). Synovial macrophage M1 polarisation exacerbates experimental osteoarthritis partially through R-spondin-2. Ann. Rheum. Dis. 77, 1524–1534. doi:10.1136/annrheumdis-2018-213450
Zhang, R., and Qu, J. (2023). The mechanisms and efficacy of photobiomodulation therapy for arthritis: a comprehensive review. Int. J. Mol. Sci. 24, 14293. doi:10.3390/ijms241814293
Zhang, Y., Ji, Q., Chen, D., Chang, B., Xu, Y., Qiu, H., et al. (2022). Efficacy of 630 nm LED-based non-damage phototherapy on early stage knee osteoarthritis in mice. Chin. J. Laser Med. Surg. 31, 61–118. doi:10.13480/j.issn1003-9430.2022.0061
Zhao, K., Ruan, J., Nie, L., Ye, X., and Li, J. (2023). Effects of synovial macrophages in osteoarthritis. Front. Immunol. 14, 1164137. doi:10.3389/fimmu.2023.1164137
Zheng, M., Zhu, Y., Wei, K., Pu, H., Peng, R., Xiao, J., et al. (2023). Metformin attenuates the inflammatory response via the regulation of synovial M1 macrophage in osteoarthritis. Int. J. Mol. Sci. 24, 5355. doi:10.3390/ijms24065355
Keywords: osteoarthritis, synovitis, macrophage polarization, cell therapy, tissue repairing
Citation: Zhang Y and Ji Q (2023) Macrophage polarization in osteoarthritis progression: a promising therapeutic target. Front. Cell Dev. Biol. 11:1269724. doi: 10.3389/fcell.2023.1269724
Received: 30 July 2023; Accepted: 16 October 2023;
Published: 26 October 2023.
Edited by:
Roland Wohlgemuth, Lodz University of Technology, PolandReviewed by:
Yao Lu, Southern Medical University, ChinaCopyright © 2023 Zhang and Ji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Quanbo Ji, cXVhbmJvMzAxQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.