
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Cell Dev. Biol. , 21 November 2023
Sec. Signaling
Volume 11 - 2023 | https://doi.org/10.3389/fcell.2023.1225055
This article is part of the Research Topic Myc as a Disease Target Beyond Cancer View all 16 articles
 Warren B. Nothnick1,2,3,4*
Warren B. Nothnick1,2,3,4* Sachith Polpitiya Arachchige5
Sachith Polpitiya Arachchige5 Paige Minchella1
Paige Minchella1 Edward B. Stephens5
Edward B. Stephens5 Amanda Graham1
Amanda Graham1Endometriosis is a benign gynecological disease in which eutopic endometrial tissue composed of glands and stroma grow within the pelvic cavity. The disease affects females of reproductive age and is characterized by pelvic pain, infertility and reduced quality of life. The majority of pharmacologic treatment modalities for endometriosis focus on suppression of estradiol production and/or action; an approach associated with adverse side effects. c-MYC is elevated in eutopic endometrium and endometriotic lesion tissue in patients with endometriosis and the disease shares many similar pathological characteristics with that of endometrial carcinoma. While targeting of c-MYC with Omomyc has recently gained substantial interest in the field of cancer research, there has been no recent attempt to evaluate the potential utility in targeting c-MYC for endometriosis treatment. The following perspective article compares the similarities between endometriosis and endometrial cancer and presents preliminary data suggesting that targeting c-MYC with Omomyc reduces endometriotic cell proliferation and viability in vitro. Future application of targeting c-MYC in endometriosis treatment and potential pros and cons are then discussed.
Endometriosis is a chronic inflammatory disease in which endometrial tissue grows outside the uterine cavity, predominantly within the pelvic cavity (Giudice and Kao, 2004). The disease affects approximately 10%–15% of women of reproductive age and is associated with pelvic pain, dysmenorrhea, infertility, and reduced quality of life (Giudice and Kao, 2004). The etiology of endometriosis is not clear although retrograde menstruation is widely accepted (Sampson, 1927). It is postulated that menstrual overflow leaves the uterine cavity through the fallopian tubes and implants into the peritoneal cavity and the ovaries. However, most women experience retrograde menstruation, yet not all women develop the disease (Jenkins et al., 1986). Therefore, multiple factors contribute to the development and progression of endometriosis such as aberrant immune response and altered hormonal balance (Sourial et al., 2014; MacLean and Hayashi, 2022). Given that the growth of endometrial lesions is estrogen-dependent, common pharmacologic treatments target estrogen production and subsequent estrogen action and include oral contraceptive pills (OCPs), gonadotropin-releasing hormone (GnRH) agonists, GnRH antagonists, levonorgestrel (progestin)-releasing intra-uterine devices and aromatase inhibitors (Nothnick et al., 2018). We discuss below the current pharmacologic approaches for endometriosis treatment and their limitations. We then discuss the similarities between endometriosis and endometrial cancer and review targeting of Myc as a therapeutic approach in cancer treatment. Lastly, we provide preliminary evidence supporting the potential of targeting c-MYC as a non-hormonal treatment option for endometriosis.
Oral contraceptive pills (OCPs), which contain low dose estrogens and high dose progesterone, are often the first line approach in treating endometriosis/endometriosis-associated pain (Menakaya et al., 2013; Dunselman et al., 2014). Low levels of estrogen are postulated to induced progesterone receptor expression and the progesterone/progestins contained within the OCP preparations inhibit estrogen production by the ovaries via suppression of gonadotropin release as well as via reduction of the inflammatory milieu associated with the disease. Progestins such as medroxyprogesterone acetate (MPA) are also effective in controlling pain, but a drawback associated with their use is side effects such as menstrual irregularities and weight gain (Brown et al., 2012). Levonorgestrel-releasing intrauterine system (LNG-IUS) is a popular treatment option effective in reducing dysmenorrhea, pelvic pain, deep dyspareunia as well as reducing lesion burden (Fedele et al., 2001; Bayoglu et al., 2011; Kim et al., 2022) and can be used as a long-term treatment option (Kim et al., 2022). While LNG-IUS offers many benefits, limitations include irregular uterine bleeding, vaginal bleeding and vaginal discharge. In summary, LNG-IUS treatment is an effective and feasible method to control pain as a long-term postoperative maintenance therapy for endometriosis patients. While progestin-based therapies are effective in many women, a substantial percentage of endometriosis patients exhibit progesterone resistance and therefore have insufficient therapeutic responses to these treatments.
The fact that endometriosis is an estrogen-dependent disease led to targeting production of this hormone as a means of treating the disease. Targeting gonadotropin release at the level of the hypothalamus to reduce circulating estrogen levels has proven effective and overcomes the issue of progesterone resistance. Gonadotropin hormone releasing analogs includes the use of both gonadotropin-releasing hormone (GnRH) agonists and, more recently antagonists. Both classes of drugs suppress ovarian estrogen production leading to a hypo-estrogenic state which is detrimental to endometriotic lesion survival. GnRH analogs are often prescribed when oral contraceptives and/or progestin analogues fail to produce successful outcomes. These compounds are effective in some, but not all women with endometriosis (Shaw, 1990). Further, GnRH agonist use is associated with significant side effects including altered lipid profile, hot flushes, loss of libido and reduction in bone mass/bone health (Prentice, 2001). The loss of bone mass can be overcome by estrogen add-back/hormone replacement therapy (Surrey, 2010) but this must be balanced to avoid estrogen action upon lesion survival and potential recurrence of disease and its symptomology.
One of the most common and successful therapies for endometriosis pain management is the GnRH agonist, leuprolide acetate (Geisler et al., 2004). However, while this is an advantage of GnRH agonist therapy, a disadvantage is the induction of a hypo-estrogenic state and negative impact on overall patient health. GnRH antagonists have also been used for treatment of endometriosis. Unlike GnRH agonists, they do not exhibit agonistic effects inducing estrogen “flare-up” prior to suppressing estrogen levels. Elagolix, relugolix, and linzagolix are three recent GnRH antagonist being used for endometriosis treatment (Rzewuska et al., 2023). Unlike earlier formulations, these three antagonists are taken orally, with both elagolix and relugolix already approved by the FDA and linzagolix currently under review. These GnRH antagonists may offer several benefits over older drug formulations which include lower levels of analgesics taken, significant reduction in pain scores and little to no irregular uterine bleeding. However, these GnRH antagonists are still associated with side effects including reduction in bone mineral density and risk of developing osteopenia and osteoporosis due to their induction of a hypoestrogenic state which limits their treatment regime duration (Rzewuska et al., 2023). It should be noted that combination therapy of relugolix with estradiol and norethisterone acetate for 24 weeks minimizes bone density loss and vasomotor symptoms while significantly reducing endometriosis associated pelvic pain (Giudice et al., 2022).
The use of aromatase inhibitors in the treatment of endometriosis have gained attention based upon the observation that endometriotic lesions express aromatase and are able to synthesize their own estrogen (Bulun et al., 2000). Current aromatase inhibitors prescribed include anastrozole and letrozole. Letrozole in combination with the synthetic progesterone analog, norethindrone acetate, was first reported to reduce disease burden at second look laparoscopy as well as significantly reduce pelvic pain scores in 2004 by Ailawadi and colleagues. Subsequent studies continue support the efficacy of letrozole in treatment of endometriosis-associated pelvic pain. Like letrozole, anastrozole, also inhibits estrogen production but the former is more potent in reducing levels of this steroid (Geisler et al., 2002). Anastrozole therapy combined with oral contraceptives was reported to significantly reduce pain in as little as 1 month after treatment initiation and this treatment regime was associated with minimal side effects (Amsterdam et al., 2005). However, the safety of aromatase inhibitors might be an issue especially since a recent systematic review and meta-analysis suggested an increased risk of cardiovascular events during endocrine therapy for early breast cancer (Yoo et al., 2023). None the less, aromatase inhibitors offer an additional treatment modality which is effective in treating endometriosis-associated pelvic pain.
In summary, the majority of currently prescribed endometriosis treatments rely upon reduction of estrogen production and/or estrogen action. As emphasized earlier in this article, while these treatments are effective in many women, they are not effective or well-tolerated in a large proportion of women suffering from endometriosis. These observations coupled with limitations due to side-effects and potential health complications emphasize the need for the development of novel, estrogen-sparing endometriosis treatments. In searching for such novel treatment targets, we next evaluate and compare the common mediators and mechanisms between endometriosis and cancer with the goal of identifying potential druggable targets which may be capable of reducing disease burden and symptomology associated with endometriosis.
Both endometriosis and endometrial cancer share numerous risk factors and common pathophysiological characteristics (Figure 1). Endometrial cancer is the most commonly diagnosed form of gynecological cancer in developed nations, accounting for approximately 5% of all cancers diagnosed in women (Contreras et al., 2022). Similar to the origins of endometriotic lesions, endometrial cancer originates in the endometrium and development and progression of both diseases is associated with estrogen exposure (Yu et al., 2015). With respect to endometrial cancer, one mechanism by which estrogen may promote progression of endometrial cancer is through the activation of the NLPR3 inflammasome (Liu et al., 2019), which has also recently been proposed to play a role in the pathophysiology of endometriosis (Irandoost, et al., 2023). Like endometriosis (Ailawadi et al., 2004; Patel et al., 2017), endometrial cancer also exhibits progesterone resistance (Gunderson et al., 2012) and displays altered expression of progesterone receptors (Saito et al., 2006; Jongen, et al., 2009).
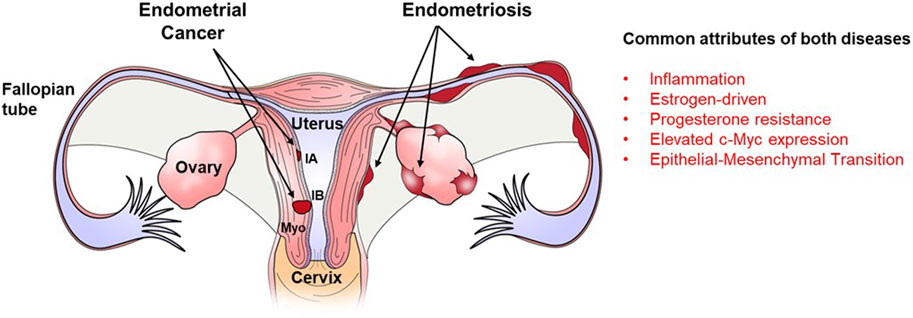
FIGURE 1. Localization of endometriotic implants and endometrial carcinoma within the female reproductive tract and major similarities between both diseases. Both endometrical cancer and endometriosis originate from the endometrial lining of the uterus. Endometrical cancer which remains confined to the endometrial lining is classified as stage IA while that which spreads into the myometrial smooth muscle (Myo) of the uterus is classified as stage IB. Endometriotic lesions develop outside of the uterus on the surface of the Fallopian tube, ovary and perimetrium of the uterus. Both diseases are characterized by inflammation, estrogen dominance, progesterone resistance, epithelial-mesenchymal transition and elevated expression of the oncogene. c-Myc.
It is further postulated that the hyper-estrogen and hypo-progesterone milieus associated with both disease contribute to the enhanced proliferation and invasiveness of the endometriotic lesions/endometrial cancer. Epithelial to mesenchymal transition (EMT) is a process by which epithelial cells lose polarity and cell-to-cell contacts, undergo remodeling of the cytoskeleton, and acquire migratory abilities and a mesenchymal-like gene expression program. The EMT process is proposed to play a role in the pathophysiology of both endometrial cancer (Colas et al., 2012; Mirantes et al., 2013) and endometriosis (Yang and Yang, 2017; Konrad et al., 2020) as both diseases are associated with the migration of endometrial cells into surrounding tissues as the diseases progress. One transcription factor whose overexpression induces EMT (Qiu et al., 2016) as well as immune evasion, angiogenesis, ECM remodeling, cell migration and invasion (Masso-Valles and Soucek, 2020) is c-MYC which is overexpressed in both endometriosis and endometrial cancer.
The Myc family of transcription factors is composed of c-MYC, N-MYC and L-MYC (Adhikary and Eilers, 2005) whose expression is dysregulated in over 70% of human cancers and associated with poor prognosis (Wang et al., 2021). Like other cancer types, c-MYC is highly expressed in endometrial tumors (Kim et al., 2013) and immunohistochemical localization studies revealed a 78.3% positive rate of c-MYC in endometrial cancer tissues with amplified c-MYC in 25% of the cases (Zhang et al., 2018; Buchynska et al., 2019). From a functional standpoint, upregulation of c-MYC in endometrial cancer cells in vitro was shown to induce EMT, drug resistance and invasion (Lv et al., 2012; Liu et al., 2015). Qiu and colleagues (2016) used a small molecule bromodomain 4 (BRD4) inhibitor, JQ1, to target c-MYC in endometrial cancer cells using both cell culture and tumor tissue xenograft models. In that study, JQ1 inhibited endometrial cancer growth in both models and this was associated with a reduction in c-MYC protein expression as well as reduced expression of c-MYC downstream targets. Additional studies demonstrated that inactivation of c-MYC resulted in tumor regression and was associated with cellular differentiation and apoptosis in transgenic mouse models (Jain et al., 2002; Tansey, 2014). Lastly, a more recent study using JQ1 demonstrated that JQ1-mediated reduction of c-MYC was associated with suppressed tumor growth in a xenograft mouse model as well as reduced proliferation and enhanced apoptosis of endometrial carcinoma cell lines in vitro (Pang et al., 2022). Thus, in addition to being a well-established target in multiple types of cancer, c-MYC also appears a plausible target for endometrial carcinoma.
Similar to expression levels in endometrial carcinoma, c-MYC expression is also elevated in endometriotic lesion tissue as well as matched eutopic endometrium from women with endometriosis (Schenken et al., 1991; Schneider et al., 1998; Johnson et al., 2005; Pellegrini et al., 2012; Proestling et al., 2015). Unfortunately, outside of these descriptive studies, little advancement has been made on our understanding as to why c-MYC is elevated in endometriotic lesion tissue and if this overexpression contributes to the pathophysiology of the disease. Based upon the similarities between endometrial cancer and endometriosis and the fact that c-MYC appears to a common transcription factor with augmented expression and signaling in both endometrial cancer and endometriosis, it may also be a viable, non-hormonal treatment for endometriosis. To date, the potential utility of targeting c-MYC as a therapeutic approach for endometriosis treatment has not been reported.
Given the aforementioned similarities between endometrial cancer and endometriosis and the necessity to identify novel, non-hormonal targets for endometriosis treatment, we conducted the following preliminary studies. To begin to evaluate potential targeting of c-MYC in endometriosis therapy, we utilized the well-characterized human endometriotic epithelial cell line, 12Z which expressed eGFP (Bulun et al., 2000) to evaluate the impact of blocking c-MYC signaling on cell proliferation and survival. To do so, 12Z-eGFP cells were transduced with lentiviral particles expressing a doxycycline (DOX)-inducible pTRIPZ-Omomyc-RFP (Omomyc) plasmid (Annibali et al., 2014) and were then subjected to puromycin-selected. Puromycin resistant Omomyc plasmid-infected 12Z cells (25,000 cells/mL) were cultured in Dulbecco’s Minimum Essential Medium (DMEM)/Ham’s F12 (Fisher Scientific, Pittsburgh, PA) containing 10% TET-FBS and Pen-Strep for 24 h in 10 cm tissue culture dishes after which the media was replaced with fresh media containing 2% TET-FBS with or without doxycycline (DOX, 2.0 μg/mL; Takara Bio catalog# 631311) to induce Omomyc expression. Cells were incubated for 48 h after which they were trypsinized and cell counts determined using a hemocytometer and all counts were conducted on duplicate dishes for 3 separate trials (N = 3). Compared to controls (-DOX), DOX induction (+DOX) of Omomyc resulted in a significant reduction in number of cells after 48 h of treatment. Figure 2 depicts representative immunofluorescence for Omomyc localization with white arrows highlighting nuclear expression of Dox-induced Omomyc expression. Based upon these preliminary studies, Omomyc reduces endometriotic epithelial cell survival in vitro. These preliminary observations are encouraging and may warrant further investigation into use of Omomyc to treat endometriosis and its associated symptomology using three-dimensional in vitro cell culture models and in vivo mouse models of experimental endometriosis routinely employed in our laboratory (Alali et al., 2020).
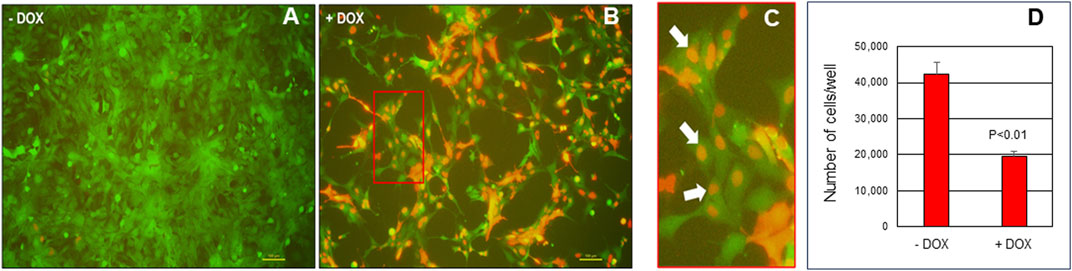
FIGURE 2. Induction of Omomyc in endometriotic epithelial 12Z cells reduces cell viability. 12Z cells were plated at 25,000 cells/mL of media for 24 h then cultured in the absence of doxycycline - DOX; (A) or presence of + DOX; (B) doxycycline to induce Omomyc expression (detected by the RFP tag). Red boxed area in panel B is enlarged and displayed in (C) with white arrows highlighting nuclear expression of Omomyc. Cell counts were then determined by manual counting and results are depicted in (D). Cell count data were analyzed by unpaired t-tests on three separate replicates independent experiments (N = 3) and are displayed as the mean +/- SEM with P < 0.01.
To interrogate potential down-stream pathways relevant to c-MYC signaling in the pathogenesis of endometriosis, we generated a list of c-MYC targets which have been reported in cancer (Zeller et al., 2003) and endometrial adenocarcinoma; Peterson et al., 2023) to those reported to be dysregulated in a similar manner in endometriosis. Down-stream targets of c-MYC relevant to endometriosis pathophysiology may include upregulated targets such as cyclin E1 (CCNE1; Park et al., 2019; Park et al., 2020), enolase 1 (ENO1; Nabeta et al., 2009), fatty acid synthase (FASN; Turathum et al., 2022), lactate dehydrogenase A (LDHA; Zheng et al., 2021), microsomal glutathione S-transferase 1 (MGST1; Ferrero et al., 2019), 60S acidic riboprotein 1 (RPLP1; Alali et al., 2020), and tumor protein p53 (TP53; Toki and Nakayama, 2000). c-MYC targets which are downregulated in cancer (Zeller et al., 2003) and endometriosis include cyclin dependent kinase 1A (CDKN1A; Kim et al., 2009) and fibronectin 1 (FN1; Holzer et al., 2020).
c-MYC has long been proposed to play a functional role in the pathophysiology of cancer. However, due to c-MYC’s unique properties with respect to a lack of a defined three-dimensional structure, nuclear localization and absence of a targetable enzymatic pocket, targeting c-MYC for cancer treatment has presented a challenge. Omomyc is a mutant basic helix–loop–helix leucine zipper (bHLHZip) domain which acts as a dominant negative and sequesters c-MYC in complexes preventing active transcription of c-MYC target genes while also allowing transcriptional repression (Masso-Valles and Soucek, 2020). Omomyc has been shown to be a potent inhibitor of tumor growth in multiple cancer types in both in vitro and in vivo studies (Soucek et al., 2004; Soucek et al., 2008; Sodir, et al., 2011; Soucek et al., 2013; Whitfield, et al., 2014; Fiorentino et al., 2016; Alimova et al., 2019; Sodir et al., 2020). Considering the similarities between cancer and endometriosis (Figure 1) and the limitations with current, anti-estrogen based treatment options for endometriosis, we evaluated the potential utility of Omomyc for endometriosis treatment. To do so, we transduced 12Z cells (a well-characterized endometriotic epithelial cell line) with lentiviral particles containing pTRIPZ-Omomyc-RFP (Omomyc) plasmid and treated them with DOX to induce Omomyc expression. Induction of Omomyc was associated with a reduction in cell viability (as reflected in total cell counts) compared to cells not treated with DOX. Although preliminary, these studies are encouraging and warrant further, more detailed studies. One limitation of current endometriosis treatments is the induction of a hypo-estrogenic state and unwanted side effects associated with it. For Omomyc to be an advancement over current therapies, it will be essential to assess if Omomyc could reduce disease burden and symptomology in vivo while concurrently avoiding induction of a hypo-estrogenic state. This would be one critical necessary first step in evaluating this c-MYC inhibitor for endometriosis treatment.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the University of Kansas Medical Center Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from gifted from another research group. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Conceptualization: WN; Methodology: WN, PM, SA, ES, and AG; Investigation: WN, PM, SA, ES, and AG; Resources: SA and ES; Writing Original Draft: WN; Writing–Reviewing and Editing: WN, PM, SA, ES, and AG; Supervision: WBN and EBS; All authors contributed to the article and approved the submitted version.
We thank Drs. Jonathan Whitfield and Laura Soucek at the Vall d’Hebron Institute of Oncology (VHIO), Barcelona, Spain for providing the pTRIPZ-Omomyc-RFP (Omomyc) plasmids and Dr. Joe Arosh, Texas A&M University for providing the eGFP-12Z cells.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adhikary, S., and Eilers, M. (2005). Transcriptional regulation and transformation by MYC proteins. Nat. Rev. Mol. Cell Biol. 6, 635–645. doi:10.1038/nrm1703
Ailawadi, R. K., Jobanputra, S., Kataria, M., Gurates, B., and Bulun, S. E. (2004). Treatment of endometriosis and chronic pelvic pain with letrozole and norethindrone acetate: a pilot study. Fertil. Steril. 81, 290–296. doi:10.1016/j.fertnstert.2003.09.029
Alali, Z., Graham, A., Swan, K., Flyckt, R., Falcone, T., Cui, W., et al. (2020). 60S acidic ribosomal protein P1 (RPLP1) is elevated in human endometriotic tissue and in a murine model of endometriosis and is essential for endometriotic epithelial cell survival in vitro. Mol. Hum. Reprod. 26, 53–64. doi:10.1093/molehr/gaz065
Alimova, I., Pierce, A., Danis, E., Donson, A., Birks, D. K., Griesinger, A., et al. (2019). Inhibition of MYC attenuates tumor cell self-renewal and promotes senescence in SMARCB1-deficient Group 2 atypical teratoid rhabdoid tumors to suppress tumor growth in vivo. Int. J. Cancer. 144, 1983–1995. doi:10.1002/ijc.31873
Amsterdam, L. L., Gentry, W., Jobanputra, S., Wolf, M., Rubin, S. D., and Bulun, S. E. (2005). Anastrazole and oral contraceptives: a novel treatment for endometriosis. Fertil. Steril. 84, 300–304. doi:10.1016/j.fertnstert.2005.02.018
Annibali, D., Whitfield, J. R., Favuzzi, E., Jauset, T., Serrano, E., Cuartas, I., et al. (2014). Myc inhibition is effective against glioma and reveals a role for Myc in proficient mitosis. Nat. Commun. 5, 4632. doi:10.1038/ncomms5632
Bayoglu, Y., Tekin, B., Dilbaz, S., Altinbas, K., and Dilbaz, S. (2011). Postoperative medical treatment of chronic pelvic pain related to severe endometriosis: levonorgestrel-releasing intrauterine system versus gonadotropin-releasing hormone analogue. Fertil. Steril. 95, 492–496. doi:10.1016/j.fertnstert.2010.08.042
Brown, J., Kives, S., and Akhtar, M. (2012). Progestagens and anti-progestagens for pain associated with endometriosis. Cochrane Database Syst. Rev. 3, CD002122. doi:10.1002/14651858.CD002122.pub2
Buchynska, L. G., Brieieva, O. V., and Iurchenko, N. P. (2019). Assessment of HER-2/neu, с-MYC and CCNE1 gene copy number variations and protein expression in endometrial carcinomas. Exp. Oncol. 41, 138–143. doi:10.32471/exp-oncology.2312-8852.vol-41-no-2.12973
Bulun, S., Zeitoun, K., Takayama, K., and Sasano, H. (2000). Molecular basis for treating endometriosis with aromatase inhibitors. Hum. Reprod. Update 6 (5), 413–418. doi:10.1093/humupd/6.5.413
Colas, E., Pedrola, N., Devis, L., Ertekin, T., Campoy, I., Martínez, E., et al. (2012). The EMT signaling pathways in endometrial carcinoma. Clin. Transl. Oncol. 14, 715–720. doi:10.1007/s12094-012-0866-3
Contreras, N.-A., Sabadell, J., Verdaguer, P., Julià, C., and Fernández-Montolí, M.-E. (2022). Fertility-sparing approaches in atypical endometrial hyperplasia and endometrial cancer patients: current evidence and future directions. Int. J. Mol. Sci. 235, 2531. doi:10.3390/ijms23052531
Dunselman, G. A., Vermeulen, N., Becker, C., Calhaz-Jorge, C., D’Hooghe, T., De Bie, B., et al. (2014). ESHRE guideline: management of women with endometriosis. Hum. Reprod. 29, 400–412. doi:10.1093/humrep/det457
Fedele, L., Bianchi, S., Zanconato, G., Portuese, A., and Raffaelli, R. (2001). Use of a levonorgestrel-releasing intrauterine device in the treatment of rectovaginal endometriosis. Fertil. Steril. 75, 485–488. doi:10.1016/s0015-0282(00)01759-3
Ferrero, H., Corachán, A., Aguilar, A., Quiñonero, A., Carbajo-García, M. C., Alamá, P., et al. (2019). Single-cell RNA sequencing of oocytes from ovarian endometriosis patients reveals a differential transcriptomic profile associated with lower quality. Hum. Reprod. 34, 1302–1312. doi:10.1093/humrep/dez053
Fiorentino, F. P., Tokgun, E., Sole-Sanchez, S., Giampaolo, S., Tokgun, O., Jauset, T., et al. (2016). Growth suppression by MYC inhibition in small cell lung cancer cells with TP53 and RB1 inactivation. Oncotarget 7, 31014–31028. doi:10.18632/oncotarget.8826
Geisler, J., Haynes, B., Anker, G., Dowsett, M., and Lonning, P. E. (2002). Influence of letrozole and anastrozole on total body aromatization and plasma estrogen levels in postmenopausal breast cancer patients evaluated in a randomized, cross-over study. J. Clin. Oncol. 20, 751–757. doi:10.1200/JCO.2002.20.3.751
Geisler, J. P., Geisler, H. E., Manahan, K. J., Miller, G. A., Wiemann, M. C., Zhou, Z., et al. (2004). Nuclear and cytoplasmic c-myc staining in endometrial carcinoma and their relationship to survival. Int. J. Gynecol. Cancer 14, 133–137. doi:10.1111/j.1048-891x.2004.14027.x
Giudice, L. C., As-Sanie, S., Arjona Ferreira, J. C., Becker, C. M., Abrao, M. S., Lessey, B. A., et al. (2022). Once daily oral relugolix combination therapy versus placebo in patients with endometriosis-associated pain: two replicate phase 3, randomised, double-blind, studies (SPIRIT 1 and 2). Lancet 399, 2267–2279. doi:10.1016/S0140-6736(22)00622-5
Giudice, L. C., and Kao, L. C. (2004). Endometriosis. Lancet 364, 1789–1799. doi:10.1016/S0140-6736(04)17403-5
Gunderson, C. C., Fader, A. N., Carson, K. A., and Bristow, R. E. (2012). Oncologic and reproductive outcomes with progestin therapy in women with endometrial hyperplasia and grade 1 adenocarcinoma: a systematic review. Gynecol. Oncol. 125, 477–482. doi:10.1016/j.ygyno.2012.01.003
Holzer, I., Machado Weber, A., Marshall, A., Freis, A., Jauckus, J., Strowitzki, T., et al. (2020). GRN, NOTCH3, FN1, and PINK1 expression in eutopic endometrium – potential biomarkers in the detection of endometriosis – a pilot study. J. Assist. Reprod. Genet. 37, 2723–2732. doi:10.1007/s10815-020-01905-4
Irandoost, E., Najibi, S., Talebbeigi, S., and Nassiri, S. (2023). Focus on the role of NLRP3 inflammasome in the pathology of endometriosis: a review on molecular mechanisms and possible medical applications. Naunyn Schmiedeb. Arch. Pharmacol. 396, 621–631. doi:10.1007/s00210-022-02365-6
Jain, M., Arvanitis, C., Chu, K., Dewey, W., Leonhardt, E., Trinh, M., et al. (2002). Sustained loss of a neoplastic phenotype by brief inactivation of MYC. Science 297, 102–104. doi:10.1126/science.1071489
Jenkins, S., Olive, D. L., and Haney, A. F. (1986). Endometriosis: pathogenetic implications of the anatomic distribution. Obstet. Gynecol. 67, 335–338.
Johnson, M. C., Torres, M., Alves, A., Bacallao, K., Fuentes, A., Vega, M., et al. (2005). Augmented cell survival in eutopic endometrium from women with endometriosis: expression of c-myc, TGF-beta1 and bax genes. Reprod. Biol. Endocrinol. 3, 45. doi:10.1186/1477-7827-3-45
Jongen, V., Briët, J., de Jong, R., Ten, H. K., Boezen, M., van der Zee, A., et al. (2009). Expression of estrogen receptor-alpha and -beta and progesterone receptor-A and -B in a large cohort of patients with endometrioid endometrial cancer. Gynecol. Oncol. 112, 537–542. doi:10.1016/j.ygyno.2008.10.032
Kim, H. Y., Song, S. Y., Jung, S. H., Song, H. J., Lee, M., Lee, K. H., et al. (2022). Long-term efficacy and safety of levonorgestrel-releasing intrauterine system as a maintenance treatment for endometriosis. Medicine 101, e29023. doi:10.1097/MD.0000000000029023
Kim, J. J., Kurita, T., and Bulun, S. E. (2013). Progesterone action in endometrial cancer, endometriosis, uterine fibroids and breast cancer. Endocr. Rev. 34, 130–162. doi:10.1210/er.2012-1043
Kim, S. H., Lee, H. W., Kim, Y. H., Koo, Y. H., Chae, H. D., Kim, C. H., et al. (2009). Down-regulation of p21-activated kinase 1 by progestin and its increased expression in the eutopic endometrium of women with endometriosis. Hum. Reprod. 24, 1133–1141. doi:10.1093/humrep/den484
Konrad, L., Dietze, R., Riaz, M. A., Scheiner-Bobis, G., Behnke, J., Horné, F., et al. (2020). Epithelial-mesenchymal transition in endometriosis-when does it happen? J. Clin. Med. 9, 1915. doi:10.3390/jcm9061915
Liu, L., Zhang, J., Yang, X., Fang, C., Xu, H., and Xi, X. (2015). SALL4 as an epithelial-mesenchymal transition and drug resistance inducer through the regulation of c-Myc in endometrial cancer. PLoS One 10, e0138515. doi:10.1371/journal.pone.0138515
Liu, S.-G., Wu, X.-X., Hua, T., Xin, X.-Y., Feng, D.-L., Chi, S.-Q., et al. (2019). NLRP3 inflammasome activation by estrogen promotes the progression of human endometrial cancer. Onco. Targets Ther. 12, 6927–6936. doi:10.2147/OTT.S218240
Lv, X. H., Chen, J. W., Zhao, G., Feng, Z. Z., Yang, D. H., Sun, W. W., et al. (2012). N-myc downstream-regulated gene 1/Cap43 may function as tumor suppressor in endometrial cancer. J. Cancer Res. Clin. Oncol. 138, 1703–1715. doi:10.1007/s00432-012-1249-4
MacLean, J. A., and Hayashi, K. (2022). Progesterone actions and resistance in gynecological disorders. Cells 11, 647. doi:10.3390/cells11040647
Masso-Valles, D., and Soucek, L. (2020). Blocking Myc to treat cancer: reflecting on two decades of Omomyc. Cells 9, 883. doi:10.3390/cells9040883
Menakaya, U., Infante, F., and Condous, G. (2013). Consensus on current management of endometriosis. Hum. Reprod. 28, 3162–3163. doi:10.1093/humrep/det346
Mirantes, C., Espinosa, I., Ferrer, I., Dolcet, X., Prat, J., and Matias-Guiu, X. (2013). Epithelial-to-mesenchymal transition and stem cells in endometrial cancer. Hum. Pathol. 44, 1973–1981. doi:10.1016/j.humpath.2013.04.009
Nabeta, M., Abe, Y., Kagawa, L., Haraguchi, R., Kito, K., Ueda, N., et al. (2009). Identification of anti-α-enolase autoantibody as a novel serum marker for endometriosis. Proteomics Clin. Appl. 10, 1201–1210. doi:10.1002/prca.200900055
Nothnick, W. B., Marsh, C., and Alali, Z. (2018). Future directions in endometriosis research and therapeutics. Curr. Womens Health Rev. 14, 189–194. doi:10.2174/1573404813666161221164810
Pang, Y., Bai, G., Zhao, J., Wei, X., Li, R., Li, J., et al. (2022). The BRD4 inhibitor JQ1 suppresses tumor growth by reducing c-Myc expression in endometrial cancer. J. Transl. Med. 20, 336. doi:10.1186/s12967-022-03545-x
Park, S., Lim, W., You, S., and Song, G. (2019). Ameliorative effects of luteolin against endometriosis progression in vitro and in vivo. J. Nutr. Biochem. 67, 161–172. doi:10.1016/j.jnutbio.2019.02.006
Park, S., Song, G., and Lim, W. (2020). Myricetin inhibits endometriosis growth through cyclin E1 down-regulation in vitro and in vivo. J. Nutr. Biochem. 78, 108328. doi:10.1016/j.jnutbio.2019.108328
Patel, B. G., Rudnicki, M., Yu, J., Shu, Y., and Taylor, R. N. (2017). Progesterone resistance in endometriosis: origins, consequences and interventions. Acta. Obstet. Gynecol. Scand. 96, 623–632. doi:10.1111/aogs.13156
Pellegrini, C., Gori, I., Achtari, C., Hornung, D., Chardonnens, E., Wunder, D., et al. (2012). The expression of estrogen receptors as well as GREB1, c-MYC, and cyclin D1, estrogen-regulated genes implicated in proliferation, is increased in peritoneal endometriosis. Fertil. Steril. 98, 1200–1208. doi:10.1016/j.fertnstert.2012.06.056
Peterson, R., Minchella, P., Cui, W., Graham, A., and Nothnick, W. B. (2023). RPLP1 is up-regulated in human adenomyosis and endometrial adenocarcinoma epithelial cells and is essential for cell survival and migration in vitro. Int. J. Mol. Sci. 24, 2690. doi:10.3390/ijms24032690
Prentice, A. (2001). Regular review: endometriosis. Br. Med. J. 323, 93–95. doi:10.1136/bmj.323.7304.93
Proestling, K., Birner, P., Gamperl, S., Nirtl, N., Marton, E., Yerlikaya, G., et al. (2015). Enhanced epithelial to mesenchymal transition (EMT) and upregulated MYC in ectopic lesions contribute independently to endometriosis. Reprod. Biol. Endocrinol. 13, 75. doi:10.1186/s12958-015-0063-7
Qiu, H., Li, J., Clark, L. H., Jackson, A. L., Zhang, L., Guo, H., et al. (2016). JQ1 suppresses tumor growth via PTEN/PI3K/AKT pathway in endometrial cancer. Oncotarget 7, 66809–66821. doi:10.18632/oncotarget.11631
Rzewuska, A. M., Żybowska, M., Sajkiewicz, I., Spiechowicz, I., Żak, K., Abramiuk, M., et al. (2023). Gonadotropin-releasing hormone antagonists – a new hope in endometriosis treatment? J. Clin. Med. 12, 1008. doi:10.3390/jcm12031008
Saito, S., Ito, K., Nagase, S., Suzuki, T., Akahira, J., Okamura, K., et al. (2006). Progesterone receptor isoforms as a prognostic marker in human endometrial carcinoma. Cancer Sci. 97, 1308–1314. doi:10.1111/j.1349-7006.2006.00332.x
Sampson, J. A. (1927). Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am. J. Obstet. Gynecol. 14, 422–469. doi:10.1016/s0002-9378(15)30003-x
Schenken, R. S., Johnson, J. V., and Riehl, R. M. (1991). c-myc protooncogene polypeptide expression in endometriosis. Am. J. Obstet. Gynecol. 164, 1031–1036. doi:10.1016/0002-9378(91)90580-k
Schneider, J., Jimenez, E., Rodriguez, F., and del Tanago, J. G. (1998). c-myc, c-erb-B2, nm23 and p53 expression in human endometriosis. Oncol. Rep. 5, 49–52. doi:10.3892/or.5.1.49
Shaw, R. W. (1990). “GnRH analogues in the treatment of endometriosis-rationale and efficacy,” in Modern approaches to endometriosis. Editors E. J. Thomas, and J. A. Rock 1st ed. (London: Kluwer Academic Publishers), 257–274.
Sodir, N. M., Kortlever, R. M., Barthet, V. J. A., Campos, T., Pellegrinet, L., Kupczak, S., et al. (2020). MYC instructs and maintains pancreatic adenocarcinoma phenotype. Cancer Discov. 19, 588–607. doi:10.1158/2159-8290.CD-19-0435
Sodir, N. M., Swigart, L. B., Karnezis, A. N., Hanahan, D., Evan, G. I., and Soucek, L. (2011). Endogenous Myc maintains the tumor microenvironment. Genes Dev. 25, 907–916. doi:10.1101/gad.2038411
Soucek, L., Nasi, S., and Evan, G. I. (2004). Omomyc expression in skin prevents Myc-induced papillomatosis. Cell Death Differ. 11, 1038–1045. doi:10.1038/sj.cdd.4401443
Soucek, L., Whitfield, J., Martins, C. P., Finch, A. J., Murphy, D. J., Sodir, N. M., et al. (2008). Modelling Myc inhibition as a cancer therapy. Nature 455, 679–683. doi:10.1038/nature07260
Soucek, L., Whitfield, J. R., Sodir, N. M., Masso-Valles, D., Serrano, E., Karnezis, A. N., et al. (2013). Inhibition of Myc family proteins eradicates Kras-driven lung cancer in mice. Genes Dev. 27, 504–513. doi:10.1101/gad.205542.112
Sourial, S., Tempest, N., and Hapangama, D. K. (2014). Theories on the pathogenesis of endometriosis. Int. J. Reprod. Med. 2014, 179515. doi:10.1155/2014/179515
Surrey, E. S. (2010). Gonadotropin-releasing hormone agonist and add-back therapy: what do the data show? Curr. Opin. Obstet. Gynecol. 22, 283–288. doi:10.1097/GCO.0b013e32833b35a7
Toki, T., and Nakayama, K. (2000). Proliferative activity and genetic alterations in TP53 in endometriosis. Gynecol. Obstet. Invest. 50 (1), 33–38. doi:10.1159/000052876
Turathum, B., Gao, E.-M., Grataitong, K., Liu, Y.-B., Wang, L., Dai, X., et al. (2022). Dysregulated sphingolipid metabolism and autophagy in granulosa cells of women with endometriosis. Front. Endocrinol. (Lausanne). 13, 906570. doi:10.3389/fendo.2022.906570
Wang, C., Zhang, J., Yin, J., Gan, Y., Xu, S., Gu, Y., et al. (2021). Alternative approaches to target Myc for cancer treatment. Signal Transduct. Target Ther. 6, 117. doi:10.1038/s41392-021-00500-y
Yang, Y. M., and Yang, W. X. (2017). Epithelial-to-mesenchymal transition in the development of endometriosis. Oncotarget 8, 41679–41689. doi:10.18632/oncotarget.16472
Yoo, J. J., Jung, E. A., Kim, Z., and Kim, B. Y. (2023). Risk of cardiovascular events and lipid profile change in patients with breast cancer taking aromatase inhibitor: a systematic review and meta-analysis. Curr. Oncol. 30, 1831–1843. doi:10.3390/curroncol30020142
Yu, H. C., Lin, C. Y., Chang, W. C., Shen, B. J., Chang, W. P., Chuang, C. M., et al. (2015). Increased association between endometriosis and endometrial cancer: a nationwide population-based retrospective cohort study. Int. J. Gynecol. Cancer. 25, 447–452. doi:10.1097/IGC.0000000000000384
Zeller, K. I., Jegga, A. G., Aronow, B. J., O’Donnel, K. A., and Dang, C. V. (2003). An integrated database of genes responsive to the Myc oncogenic transcription factor: identification of direct genomic targets. Genome Biol. 4, R69. doi:10.1186/gb-2003-4-10-r69
Zhang, Q., Xu, P., Lu, Y., and Dou, H. (2018). Correlation of MACC1/c-myc expression in endometrial carcinoma with clinical/pathological features or prognosis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 24, 4738–4744. doi:10.12659/MSM.908812
Keywords: c-Myc, endometrial cancer, endometriosis, Omomyc, treatment
Citation: Nothnick WB, Arachchige SP, Minchella P, Stephens EB and Graham A (2023) Targeting c-MYC: a potential non-hormonal therapeutic approach for endometriosis treatment. Front. Cell Dev. Biol. 11:1225055. doi: 10.3389/fcell.2023.1225055
Received: 18 May 2023; Accepted: 01 November 2023;
Published: 21 November 2023.
Edited by:
Jonathan R. Whitfield, Vall d’Hebron Institute of Oncology (VHIO), SpainReviewed by:
Joe Arosh, Texas A and M University, United StatesCopyright © 2023 Nothnick, Arachchige, Minchella, Stephens and Graham. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Warren B. Nothnick, d25vdGhuaWNAa3VtYy5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.