- 1Department of Cell Biology and Anatomy, Arnie Charbonneau Cancer and Alberta Children’s Hospital Research Institutes, University of Calgary, Calgary, AB, Canada
- 2Unit of Immunology and General Pathology, Department of Molecular Medicine, University of Pavia, Pavia, Italy
Editorial on the Research Topic
Biomarkers and therapeutic strategies in acute lymphoblastic leukemia
Acute lymphoblastic leukemia (aLL) is a malignancy characterized by an expeditious increase in immature B- (in ∼85% of cases) and T-(in ∼15% of cases) lymphocytes in the blood and bone marrow. It is the most prevalent cancer in children and the primary cause of death from pediatric cancer (Hunger and Mullighan, 2015). Chemotherapy continues to be the main treatment for aLL (Lee et al., 2019). There is considerable evidence for superior outcome from multiple rounds of highly intensive chemotherapy (Pui and Evans, 2006). However, the risk of acquiring resistance and toxicity from chemotherapy could be fatal. Although novel therapies such as monoclonal antibodies have been developed, their effectiveness is greatly enhanced when used in combination with chemotherapy. Thus, there is continued interest in chemotherapy. However, some of these combinations are effective in certain patients but have no clinical benefit to others, and their non-specific effects make them intolerable to many. Thus, unnecessary toxicity- and resistance-induced patient suffering or mortality occurs frequently, and relapsed and refractory aLL continue to be a major concern. A growing number of resistance biomarkers for aLL drugs have been identified (Kang et al., 2017; Lee et al., 2019), increasing the current understanding of the molecular mechanisms by which chemotherapy resistance develops. On the other hand, the extensive genetic heterogeneity in B- and T-acute lymphoblastic leukemia precursor cells indicates a range of biomarkers that promote disease development and recurrence.
The objective of this Research Topic is to bring forth recent advances in biomarkers and potential therapeutic strategies in aLL as well as their implications in managing the disease. The ultimate goal is to foster exploitation of these discoveries and provide insight into the development of more innovative and effective therapeutic approaches for aLL.
1 Novel biomarkers and therapeutics
Utilizing novel innovative tools such as high throughput small molecular drug screening and gene expression analyses/datasets, a number of new biomarkers and potential therapeutics have been identified. For example, a large-scale screening of small molecule drugs for aLL performed by (Nevado et al.) led to the identification of synthetic oleanane triterpenoids (OTs) as sensitizers of T-aLL cells to doxorubicin, a common component of aLL chemotherapy regimens. OTs attenuate the MYB transcription factor and its downstream targets, and downregulate the expression of DNA repair genes. The authors proposed that OTs may be utilized in the treatment of aggressive T-aLL with poor prognosis.
Through gene expression analyses of 207 children with high-risk pre-B-aLL, (Ogana et al.) found that upregulation of Artemis, an endonuclease in V(D)J recombination and non-homologous end joining (NHEJ) of DNA double-strand break (DSB) repair, is correlated with poor prognosis. Artemis inhibition sensitizes RAG-expressing T- and B-aLL cells by causing DSBs. Through a large Artemis targeted drug screen, the authors identified four compounds (8,27,171, 8,27,032, 8,26,941, and 8,25,226) that markedly reduce proliferation of B-aLL cells, while sparing normal B Cells. This provides a basis for the authors to suggest that Artemis inhibition as a therapeutic strategy for B-aLL.
Alsuwaidi et al. analyzed datasets to identify genetic biomarkers for early detection, risk stratification, and prognosis in childhood B-aLL. The authors found that ADAM6 is a novel biomarker for B-aLL development and progression.
Torres-López et al. demonstrate that T-aLL cells express G-protein–coupled estrogen receptor (GPER), which is stimulated by the non-steroidal agonist, G-1. The authors show that G-1 kills T-aLL cells by causing a premature rise in intracellular Ca2+ and arresting cells in G2/M, resulting in apoptosis. They suggest that G-1 is a promising T-aLL therapeutic drug.
Mukherjee et al. tackled early T precursor-aLL (ETP-aLL), which still exhibits poor clinical outcome. Gene expression of ETP-aLL blasts was compared to non-ETP-aLL blasts and nine genes (KIT, HGF, NT5E, PROM1, CD33, ANPEP, CDH2, IL1B, and CXCL2) were validated as possible diagnostic ETP-aLL markers using another gene expression dataset (GSE78132) with 17 ETP-aLL and 27 non-ETP-aLL samples. B-lineage-skewed markers were also identified, with distinct functionality and possible druggability in ETP-aLL. These data could help identifying the dominant lineage specification programmes in the ETP-aLL blasts on a personalized level, thus providing new drug targets.
Zhang et al. studied NRAS mutations, which affect relapse susceptibility in the ∼15% of aLL cases with negative outcomes. About one-third of the NRAS mutations significantly transformed Ba/F3 cells as measured by IL3-independent growth and, through a high-throughput drug screening method, the authors uncovered that leukemogenic-NRAS mutations might respond to MEK, autophagy, Akt, EGFR signaling, Polo−like kinase, Src signaling, and TGF-β receptor inhibition depending on the mutation profile.
2 Novel tools for assessing prognosis
Lu et al. studied the role of immune genes in the prognosis and microenvironment of acute myeloid leukaemia (AML) by single-cell analysis. Comparison of the gene expression between high and low immune cell infiltration clusters identified nine prognostic immune genes. The authors validated a constructed model that can serve as a new method to assess the prognosis of AML patients.
Pan et al. built a novel prognostic model to improve risk stratification in chronic lymphoid leukaemia patients based on ferroptosis, a lipid peroxidation–induced cell death. Using the ferroptosis-related prognostic score (FPS) model, which was based on nine ferroptosis-related genes (FRGs: AKR1C3, BECN1, CAV1, CDKN2A, CXCL2, JDP2, SIRT1, SLC1A5, and SP1), they showed that patients with high FPS correlated with worse overall and treatment free survival.
3 Allogeneic hematopoietic stem cell transplantation (allo-HSCT) and anti-CD19 chimeric antigen receptor (CAR) T Cell therapy
Cao et al. focus their work on an uncommon complication from allogeneic hematopoietic stem cell transplantation (allo-HSCT) and anti-CD19 chimeric antigen receptor (CAR) T Cell therapy for B Cell lymphoblastic lymphoma/aLL. The authors discussed the development of a cardiac mass and myocardial infiltration, which are associated with increased cytokine levels following allo-HSCT and CAR T Cell therapy. They indicated that monitoring of cardiac functions in B Cell lymphoblastic lymphoma/aLL patients undergoing allo-HSCT and CAR T Cell therapy is of paramount importance.
The current Research Topic highlights new biomarkers and prognostic models for aLL and other types of leukemias (Figure 1). They have the advantage of expanding the potential targets for personalized medicine, which, as it is emerging from clinical practice, paves the way for improved therapy outcome, reducing off-target side effects, and preventing relapse (Rosen et al., 2022).
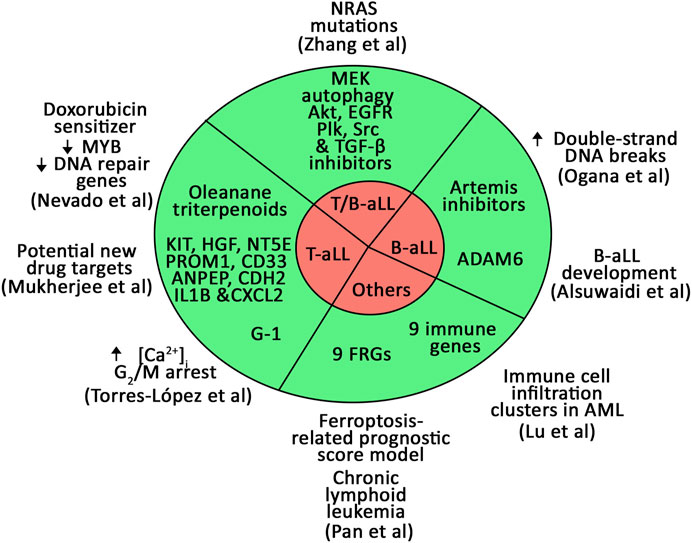
FIGURE 1. Novel biomarkers and prognostic models for B- and T-aLL and other types of leukemias identified in the current Research Topic.
Author contributions
K-YL wrote the initial draft of the editorial. MM and CS provided constructive comments and revision to the editorial. K-YL edited the final version of the editorial. All authors contributed to the editorial and approved it for publication.
Funding
This work was supported by a grant from CIHR (PJT-174983) to K-YL. This research was funded by: “Associazione Un sorriso alla vita—ONLUS” and a grant from the Italian Ministry of Education, University and Research (MIUR) to the Department of Molecular Medicine, University of Pavia, under the initiative “Dipartimento di Eccellenza (2023-2027)”.
Conflict of interest
CS and MM are coinventors of a patent on L-asparaginase.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Hunger, S. .P, and Mullighan, C. .G (2015). Acute lymphoblastic leukemia in children. N. Engl. J. Med. 373 (16), 1541–1552. doi:10.1056/NEJMra1400972
Kang, S. .M, Rosales, J. .L, Meier-Stephenson, V, Kim, S, Lee, K. .Y, and Narendran, A (2017). Genome-wide loss-of-function genetic screening identifies opioid receptor μ1 as a key regulator of L-asparaginase resistance in pediatric acute lymphoblastic leukemia. Oncogene 36 (42), 5910–5913. doi:10.1038/onc.2017.211
Lee, J. .K, Kang, S, Wang, X, Rosales, J. L., Gao, X., Byun, H. G., et al. (2019). HAP1 loss confers l-asparaginase resistance in ALL by downregulating the calpain-1-Bid-caspase-3/12 pathway. Blood 133 (20), 2222–2232. doi:10.1182/blood-2018-12-890236
Pui, C. .H, and Evans, W. .E (2006). Treatment of acute lymphoblastic leukemia. N. Engl. J. Med. 354 (2), 166–178. doi:10.1056/NEJMra052603
Keywords: acute lymphoblastic leukemia, biomarkers, chemotherapy, blood-related disorders, leukemia
Citation: Lee K-Y, Maggi M and Scotti C (2023) Editorial: Biomarkers and therapeutic strategies in acute lymphoblastic leukemia. Front. Cell Dev. Biol. 11:1211569. doi: 10.3389/fcell.2023.1211569
Received: 24 April 2023; Accepted: 09 May 2023;
Published: 17 May 2023.
Edited and reviewed by:
Ramani Ramchandran, Medical College of Wisconsin, United StatesCopyright © 2023 Lee, Maggi and Scotti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ki-Young Lee, a3lsZWVAdWNhbGdhcnkuY2E=
 Ki-Young Lee
Ki-Young Lee Maristella Maggi
Maristella Maggi Claudia Scotti
Claudia Scotti