- 1Department of Internal Medicine, Section of Cardiovascular Medicine, Section of Molecular Medicine, Wake Forest University School of Medicine, Winston-Salem, NC, United States
- 2Department of Internal Medicine, Section of Gerontology and Geriatric Medicine, Section of Molecular Medicine, Wake Forest University School of Medicine, Winston-Salem, NC, United States
Mitochondria play a critical role in energy metabolism and signal transduction, which is tightly regulated by proteins, metabolites, and ion fluxes. Metabolites and ion homeostasis are mainly mediated by channels and transporters present on mitochondrial membranes. Mitochondria comprise two distinct compartments, the outer mitochondrial membrane (OMM) and the inner mitochondrial membrane (IMM), which have differing permeabilities to ions and metabolites. The OMM is semipermeable due to the presence of non-selective molecular pores, while the IMM is highly selective and impermeable due to the presence of specialized channels and transporters which regulate ion and metabolite fluxes. These channels and transporters are modulated by various post-translational modifications (PTMs), including phosphorylation, oxidative modifications, ions, and metabolites binding, glycosylation, acetylation, and others. Additionally, the mitochondrial protein quality control (MPQC) system plays a crucial role in ensuring efficient molecular flux through the mitochondrial membranes by selectively removing mistargeted or defective proteins. Inefficient functioning of the transporters and channels in mitochondria can disrupt cellular homeostasis, leading to the onset of various pathological conditions. In this review, we provide a comprehensive overview of the current understanding of mitochondrial channels and transporters in terms of their functions, PTMs, and quality control mechanisms.
1 Introduction
Mitochondria are the cellular power station and signaling hub, consisting of a double membrane system that forms the outer mitochondrial membrane (OMM) and inner mitochondrial membrane (IMM), creating the intermembrane space (IMS) and mitochondrial matrix (MM). The OMM is porous, allowing free passage of molecules less than 10,000 Da, with no membrane potential across it. On the other hand, the IMM acts as a highly discerning barricade against small molecules and ions, permitting regulated passage exclusively through specific membrane transport/channel proteins (Gray, 2001; Kuhlbrandt, 2015). The IMM is divided into two distinct regions: the inner boundary membrane (IBM), which connects with the outer mitochondrial membrane (OMM), and the cristae membrane (CM), which forms inward-folded structures within the MM (Hoshino et al.). IBM and CM are functionally distinguished and have different protein compositions. The CM is enriched with respiratory chain complexes, iron-sulfur cluster assembly proteins, and selective channels/transporters while the IBM contains the protein translocation and membrane fusion machinery (Vogel et al., 2006; Suppanz et al., 2009). Cristae junctions are tube-like membrane invaginations of ∼25 nm diameter that separate IBM and CM (Rabl et al., 2009; Davies et al., 2012; Busch, 2020). The cristae occupy most of the inner membrane surface, indicating their crucial role in cellular physiology. Mitochondria are essential for various cellular processes such as oxidative phosphorylation, calcium (Ca2+) and redox regulation, mitochondrial protein folding, mitophagy, metabolic pathways such as tricarboxylic acid-, β-oxidation- and urea cycle TCA cycle, and controlling signaling events from cell survival to cell death (Manoli et al., 2007; Murphy et al., 2016). To carry out these high-energy-requiring cellular processes, the crosstalk between mitochondria and the cytoplasm occurs uni- or bi-directionally through various channels and transporters present on the mitochondrial membranes, which function as gatekeepers, restricting access to the organelle from the cytoplasm. This selectivity of both the OMM and IMM helps to maintain MM’s volume by regulating influx and efflux mechanisms for ions and metabolites, primarily K+ and Ca2+. Additionally, these fluxes help to balance the levels of other important ions such as Na+, Cl−, and H+. Disruptions in ion homeostasis in the cytosol and MM can lead to detrimental consequences, ultimately causing cell death (Javadov et al., 2018).
Mitochondrial (∼99%) proteins are mostly encoded by the nucleus and equipped with specific targeting sequences that guide them from the cytosol to the mitochondrial surface receptors, ensuring correct protein localization and assisting in protein quality control. The translocases not only transport newly synthesized proteins to their correct mitochondrial destinations but also establish a complex dynamic network (Dolezal et al., 2006; Neupert and Herrmann, 2007). The efficiency of protein import into mitochondria is a significant indicator of the healthy energetic state of mitochondria. Dysfunction in mitochondrial metabolism decreases inner membrane potential (ΔΨm) and impairs protein import (Nunnari and Suomalainen, 2012; Frazier et al., 2019). The mitochondrial transport machinery plays a vital role in protein biogenesis, and its quality control is essential for understanding mitochondrial stress response-related diseases. The mitochondrial proteome quality is necessary for cellular survival and is maintained by various interlinked cellular processes (Calvo et al., 2016; Gómez-Serrano et al., 2018). The mitochondrial protein quality control system ensures proper protein production, maintenance, functional switching, and degradation (Anand et al., 2013). Various interlinked cellular processes, from the molecular to the organellar level, participate to maintain mitochondrial proteome/homeostasis based on cellular need and this is achieved by the mitochondrial protein quality control (MPQC) system (Jadiya and Tomar, 2020). The cytoplasmic protein quality control machinery helps eliminate misfolded proteins in the OMM, while each interior compartment of mitochondria (IMS, MM) possesses its own specialized machinery, including mitochondrial chaperones, proteases, and disaggregases (Baker and Haynes, 2011; Song et al., 2021). As mitochondria play a crucial role in cellular events, including essential pathways of amino acid biosynthesis, fatty acid oxidation, and signal transduction, stress response apoptotic pathways, maintaining a healthy mitochondrial environment is crucial for cell function (Schon and Manfredi, 2003). Therefore, uninterrupted checks and repair of mitochondria are necessary to keep a healthy mitochondrial pool in cells.
Post-translational modifications (PTMs) are crucial for maintaining the proteome and mitochondrial homeostasis. Throughout the protein molecular transition process, which includes transcription, post-transcription, translation, and post-translation, mitochondrial channels and transporters are tightly regulated by PTMs. These modifications, such as acetylation, phosphorylation, ubiquitination, nitrosylation, and oxidation, alter the chemical properties of amino acid residues, thereby influencing protein distribution, stability, and function (Doyle and Mamula, 2001; Beltrao et al., 2013). Although the influence of PTMs on misfolded proteins is extensively studied, our understanding of the underlying molecular pathways governing these PTMs and their significance in mitochondrial proteins, specifically channels and transporters, is still incomplete. Moreover, the molecular identity of numerous mitochondrial channels and transporters has been recently discovered or remains unknown, which has limited exploration of their PTMs. In this review, we aim to present an up-to-date overview of various mitochondrial channels and transporters, along with their associated PTMs and MPQC mechanisms (Figures 1, 2), shedding light on their roles in both normal physiological processes and pathological conditions.

FIGURE 1. Channels and transporters present at the OMM and IMM. The transporters and channel proteins present on OMM and IMM are depicted with the exchange ion/molecule machineries. VDAC-Voltage-dependent anion channel; SAM- Sorting and Assembly Machinery; MAC-Mitochondrial Apoptosis-induced Channel; TOM-Translocase of the OMM; MIM-Mitochondrial import; CLIC-Mitochondrial Chloride (Cl−) channel; UCP- Uncoupling proteins; mtCU- Mitochondrial calcium uniporter channel; MICOS- Mitochondrial contact site and cristae organizing system; ANT-ADP, ATP translocase; TIM- Translocase of the Inner Membrane; PiC- Mitochondrial phosphate carrier; MPTP- MPermeability Transition Pore; NCLX- Na+/Ca2+/Li+ exchanger; HCX- H+/Ca2+ exchanger; IMAC-Mitochondrial inner membrane anion channel.
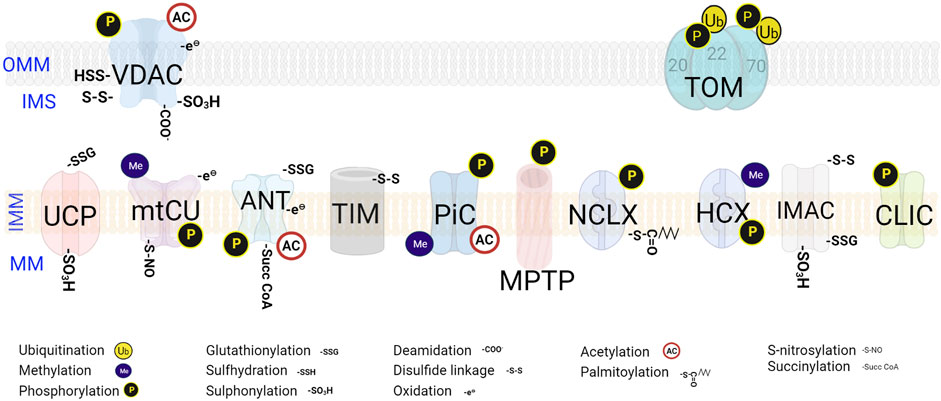
FIGURE 2. Post-translational modifications on mitochondrial channels and transporters. The PTMs occur at specific sites on the transporters. The various regulatory PTMs exhibited by both OMM and IMM transporters and channels are denoted. VDAC-Voltage-dependent anion channel; TOM-Translocase of the OMM; UCP- Uncoupling proteins; mtCU- Mitochondrial calcium uniporter channel; ANT-ADP, ATP translocase; TIM- Translocase of the Inner Membrane; PiC- Mitochondrial phosphate carrier; PTP- Permeability Transition Pore; NCLX- Na+/Ca2+/Li+ exchanger; HCX- H+/Ca2+ exchanger; IMAC-Mitochondrial inner membrane anion channel; CLIC-Mitochondrial Chloride (Cl−) channel.
2 Mitochondrial transporters/channels
Mitochondrial transporters and channels play a critical role in regulating the exchange of metabolites between the MM and the cytoplasm while maintaining the electrochemical proton gradient required for oxidative phosphorylation (OXPHOS). These channels are located in both the OMM and IMM and the exchange of metabolites/ion fluxes is maintained by various chemical signals and mechanical stimuli, allowing metabolites compartmentalization and regulation of cell volume and ΔΨm (O'Rourke, 2007; Szabo and Zoratti, 2014). Understanding the molecular processes of mitochondrial channels and transporters is critical to comprehend their biological significance.
2.1 OMM channels/transporters, their control mechanisms, and PTMs
The OMM contains around 200 proteins that act as the first line of defense against the influx of metabolites into the mitochondria (Pfanner et al., 2019). The main transporters and channels present in the OMM, along with their PTMs and quality control mechanisms, are discussed in detail here.
2.1.1 Translocation of proteins through OMM
2.1.1.1 Translocase of the OMM (TOM) complex
The TOM complex serves as the main access point for proteins into the mitochondria. High-resolution cryo-electron microscopy studies show that it consists of three receptors, Tom20, Tom22, and Tom70, the channel forming unit, Tom40, and three small proteins, namely, Tom5, Tom6, and Tom7. Tom20 and Tom70, the peripheral TOM receptors, bind to incoming proteins and recognize pre-sequence or mitochondrial localization sequences. The main subunit of the TOM complex is Tom40, the β-barrel protein which can form a protein-conducting channel. Tom22 is the docking site for Tom20 and Tom70, enabling protein precursor transfer from the receptor toward the translocation channel (Wang et al., 2020; Guan et al., 2021).
PTMs of TOM subunits play a crucial role in quality control. For instance, during mitochondrial stress, ubiquitylation of Tom22 can lead to mitochondrial membrane depolarization consequently (Wu et al., 2018). Additionally, the phosphorylation of Tom22 by casein kinase 2 (CSNK2) has been found to be essential for TOM complex biogenesis in yeast (Kravic et al., 2018). In contrast, in mammalian cells, phosphorylation of Tom22 is required for regulating Pink1/Parkin-related mitophagy, as it enhances Pink1 import. The loss of this phosphorylation impedes Pink1 import and promotes mitophagy (Horie et al., 2003). Furthermore, Tom70 undergoes ubiquitylation and phosphorylation as PTMs, but their precise functions remain to be fully explored (Zhang et al., 2017). To ensure the proper functioning of the TOM complex, the mitochondrial protein translocation-associated degradation (mitoTAD) pathway plays a critical role in quality control at the entry gate of the OMM. This pathway aims to prevent blocking of the TOM complex and retro-translocate IMS and IMM proteins through TOM complex into the cytoplasm for ubiquitin-mediated proteasomal degradation (Figure 3) (Ravanelli et al., 2020). To initiate the mitoTAD pathway, first Ubx2 binds to the TOM complex. Secondly, the cytosolic AAA ATPase Cdc48 then binds to the UBX domain of Ubx2 and pulls the substrate. Thirdly, it further causes the extraction and unfolding of the bound protein at the expense of ATP hydrolysis, leading to proteasomal degradation of the unbound protein (Bodnar and Rapoport, 2017). This process blocks the fusion of damaged mitochondria and accelerates their removal by mitophagy (Onishi et al., 2021). When mitochondrial proteins are stalled in the TOM complex due to defects in mitochondrial import, the Cis1-Msp1 recruitment mechanism is activated by the mitochondrial compromised protein import response (mitoCPR). Cis1, a cytosolic protein, is induced and localized to the OMM, where it connects AAA ATPase Msp1 and Tom70. Msp1 removes precursors from the import channel for proteasomal degradation (Figure 3).
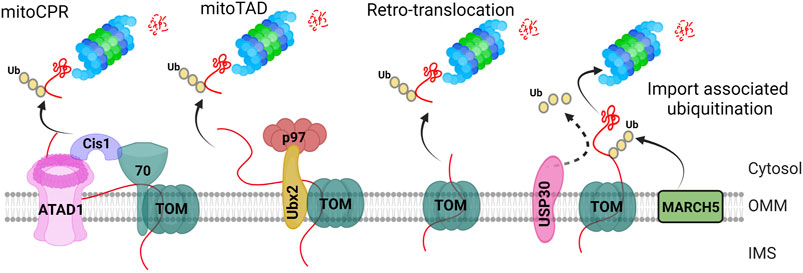
FIGURE 3. TOM complex associated ubiquitination of mitochondrial proteins. The “mitoCPR” pathway initiates through stress-stimulated dysfunctional mitochondrial protein import. It further causes localization of Cis1 at the TOM complex through its physical interaction with TOM70 subunit. Cis1 then recruits the AAA ATPase ATAD1/Msp1 at the TOM complex to remove the ubiquitinated protein and put them for proteasome mediated degradation. The “MitoTAD” pathway continuously checks the import channel, removing the blockade through TOM-Ubx2-p97/Cdc48 complex. The “retrotranslocation” pathway includes the export of unfolded intra-mitochondrial proteins to the cytoplasm for their ubiquitin-mediated proteasomal degradation. The “import associated ubiquitination” is mediated by OMM localized E3 ligase, March5, which constitutively ubiquitinates the incoming pre-proteins whereas USP30 deubiquitinase remove the ubiquitin, mediating mitochondrial protein import.
In addition, the accumulation of Pink1 due to mitochondrial dysfunctions leads to interactions with various components of the TOM complex, such as Tom22, and Tom70 has been found to be critical for maintaining mitochondrial quality control (Lazarou et al., 2012; Maruszczak et al., 2022). The Tom70 and Parkin interaction triggers mitophagy upon mitochondrial import blockage, and Parkin mutant cells exhibit reduced Tom70-Parkin. Furthermore, Tom70 is important for Pink1 import (Kato et al., 2013), although further studies are required to understand the mechanistic insights on Pink1/Parkin import with the TOM complex.
2.1.1.2 Mitochondrial import (MIM) complex
MIM complex is also one of the major translocases to insert the proteins having a single or multiple α-helical membrane anchors. It is made up of two membrane-spanning alpha-helical proteins, Mim1 and Mim2, with Mim1 as a major constituent (Dimmer et al., 2012). Mim1 can form pores in a lipid bilayer, while Mim2 may recognizes positively charged residues in precursor proteins (Krüger et al., 2017).
The MIM complex is a highly dynamic system that operates in three distinct states or pools to import various precursor proteins into the OMM. In the first pool, the MIM-TOM complex accepts precursor proteins from Tom70. Tom70 imports single-spanning proteins such as Pth2 and Mcy1, while Tom20 helps import Gem1. Once these proteins are brought to the MIM complex, they are incorporated into the OMM. The second pool, known as the MIM-SAM complex, plays a crucial role in enhancing the early assembly events of small TOM subunits. The third pool, free MIM, inserts single-spanning proteins like Msp1 and Tom20 that are imported in a Tom70-independent manner. This dynamic coupling to partner proteins allows the MIM complex to import various types of precursor proteins, making it an essential regulator in the biogenesis of OMM proteins (Doan et al., 2020; Vitali et al., 2020). The MIM complex comprises multiple copies of Mim1, which form a channel in a lipid membrane, and one or two copies of Mim2 (Krüger et al., 2017). Recent studies have shed light on the critical role of the MIM complex in facilitating the import, assembly, and localization of OMM proteins (Vitali et al., 2020). By working together with TOM receptors and the SAM complex in a sequential and co-operative manner, the MIM complex can integrate different types of proteins into the OMM, including signal-anchored, tail-anchored, and single-spanning proteins. Nevertheless, further research is required to fully understand the molecular mechanisms by which sorting, and insertion of alpha-helical outer membrane proteins occur.
MIM is also involved in the biogenesis of various OMM proteins, such as Tom20, Tom70, and tail-anchored receptors Tom5, Tom6, and Tom7 (Thornton et al., 2010). So far, PTMs of MIM are not well studied. However, import of Tom70 precursor depends on phosphorylation at Ser12/16 of MIM1 by casein kinase 2 in Yeast (Schmidt et al., 2011). The cytosolic domains of Atg32 and Gem1, which are targeted to and integrated into the OMM, depend on MIM involvement, indicating its critical role in OMM protein biogenesis (Vitali et al., 2020). Moreover, MIM has been found to regulate the assembly of Fzo1 and Ugo1, two proteins involved in mitochondrial fusion (Dimmer et al., 2012), as well as Ubx2, an ER-resident protein involved in the ER-associated degradation pathway (Mårtensson et al., 2019), ultimately contributing to MPQC. The beta-barrel precursor proteins initially cross the TOM channel and are then incorporated via the SAM complex from IMS. The TOM receptors also bind to alpha-helical precursor proteins and bring them to the MIM complex. The MIM and SAM complexes work together to assemble the alpha-helical embedded and beta-barrel protein subunits of the TOM complex, highlighting the crucial role of the MIM complex in OMM protein biogenesis.
2.1.1.3 Sorting and Assembly Machinery (SAM)
The sorting and assembly of complex β-barrel proteins in OMM requires additional machinery beyond the TOM complex, such as the SAM complex. The SAM complex mainly contains three subunits Sam50 (Tob55, Omp85), and the two peripheral components, Sam35 (Tom38, Tob38), and Sam37 (Mas37). Homology studies identified that human Sam50 is essential for the biogenesis of human Tom40 which is a β-barrel protein and Sam37 stabilizes mature Tom40 protein through electrostatic interactions, consequently facilitating successive TOM assembly (Humphries et al., 2005; Wang et al., 2021). Sam50 plays a key role in β-barrel precursor recognition and binding, as well as interacting with the MICOS complex for respiratory complex assembly (Ding et al., 2015). Additionally, Sam50 is involved in PINK1-Parkin-mediated mitophagy and mitochondrial dynamics, and recent research suggests its cooperation with p62/SQSTM1 mediates efficient mitophagy (Abudu et al., 2021). Sam37 is involved in the release of precursor proteins from SAM, indicating SAM’s substrate specificity is not limited to β-barrel proteins but also involves most α-helical Tom proteins. In C. elegans, the Sam50 homolog is essential for mitochondrial maintenance (Jian et al., 2018), suggesting its importance in mitochondrial function is evolutionarily conserved.
2.1.2 Transport of metabolite across OMM
Being the most abundant key protein of the OMM, voltage-dependent anion channels (VDAC) is a β-barrel membrane protein that spans the inner and outer environment of OMM where it participates in and regulates most of the metabolites such as inorganic phosphates, ADP, pyruvate and certain amino acids (approximately 4 kDa of molecular weight) transported across the OMM based on their conductance property (Colombini, 2012; De Pinto, 2021; Mannella, 2021). It has two different conductance states, permitting a selective route for metabolites and ions: an open (anion selective high conductance) and a closed (slightly cation-selective low conductance). Regardless of little variation in the conformation states of the N-terminus, all possible structures were demonstrated to correspond to the open high conductance state whereas no structural explanation of the closed state has yet been evidenced (Choudhary et al., 2014). This different conductance is important for maintaining the membrane permeability of ATP/ADP, Ca2+ homeostasis, and apoptotic signaling. The dissociation of hexokinase from VDAC triggers apoptosis due to influx of Ca2+ into mitochondria (Keinan et al., 2010). Three VDAC isoforms, namely, VDAC1, VDAC2 & VDAC3 in humans have been found. VDAC2 and VDAC3 structures are less studied, however, all VDACs are believed to contribute to the same general structural features (Böhm et al., 2020). Although isoforms of VDAC are characterized by slight differences in their amino acid sequences, studies suggest different function in physiological and disease conditions for each VDAC isoform (Anflous et al., 2001; Cheng et al., 2003; Menzel et al., 2009).
VDAC undertakes various PTMs, namely, oxidative PTMs (which modify the redox status of a cell), acetylation, phosphorylation, GlcNAcetylation and ubiquitination. Different numbers of cysteines found in VDAC isoforms led researchers to study their PTMs. Mutagenesis studies in Yeast with the individual or cluster cysteines of VDAC3 showed an inverse relationship between the number of cysteines and the pore-forming activity, likely due to the formation of intrachain disulfide bridges, signifying PTMs role in modulating VDAC3 (Reina et al., 2016; Queralt-Martín et al., 2020). The study by the Saletti group revealed that VDAC cysteine residues can undergo progressive oxidation of sulfur which may be reversible or irreversible under physiological conditions. This oxidation consists of a series of events from redox reactive thiol (–SH) to disulfide bonds (RS-SR), sulfinic acid (SO2H), or sulfonic acid (SO3H), sulfhydration (SSH), and sulfenylation (SOH) (Reina et al., 2020). Oxidative PTMs of cysteines suggested a preferential sensitivity to oxidation for each sulfur-containing amino acid of VDAC (De Pinto et al., 2016). A distinct modification of each cysteine can correlate to a specific functional/structural role of VDAC, understanding the oxidative potential for reactive oxygen species (ROS) generation, especially in IMS which has acidic pH and is favorable for oxidation. Other common PTMs such as phosphorylation at Ser104 by a protein kinase C (PKC), a cAMP-dependent protein kinase A (PKA), and a glycogen synthase kinase-3β (GSK3β) and acetylation at the N-terminal amino acid have also been found in VDACs. PKA and c-Jun N-terminal kinase-3 (JNK3) mediated phosphorylation of VDAC1 controls apoptotic activity, modulating its interaction with cytoskeletal components (Kerner et al., 2012; Reina et al., 2020). Elevated levels of VDAC O-GlcNAcylation are seen in rats having low running capacity, indicating this modification may serve increased mitochondrial stability (Johnsen et al., 2013). Moreover, the extent of ubiquitination of VDAC3 is observed directly proportional to the duration of hypothermia, may serving as a protective pathway in hypothermia against oxygen-glucose deprivation/recovery (OGD/R) (Zhao et al., 2020). Furthermore, a very unusual PTM, the deamidation at asparagine (Asn37, Asn106, Asn207, Asn214, and Asn239) and glutamine (Gln166 and Gln226) of VDAC1 was discovered in response to oxidative stress and known to interact with superoxide dismutase1 (SOD1) mutant (SOD1G93A) in NSC34 cells of amyotrophic lateral sclerosis (ALS) (Pittalà et al., 2020). The structural changes in VDAC1 due to these PTMs clarifies the interaction between VDAC1 and mutant form of SOD1 as well as ALS pathogenesis (Pittalà et al., 2020). Although the evolutionary roots highlight the importance of VDACs in mitochondria, gaining a deeper understanding of the connection between the structure and function of VDAC conductance, the structural variances influenced by the placement of PTMs in amino acid sequences, and their voltage-dependent mechanism would simplify the investigation of VDAC isoforms' functions and their involvement in neurodegenerative therapeutics.
Not all three isoforms of VDAC but VDAC1 is known to have a function in MPQC. An OMM localized translocator protein, TSPO was found to interact with VDAC1, stimulating a ROS-mediated inhibition of activation of PINK1/Parkin-dependent mitophagy (Gatliff et al., 2014). In Pink1/Parkin-dependent mitophagy, depolarization of the IMM results in the accumulation of Pink1 on the OMM, recruiting Parkin, an ubiquitin ligase. This in turn leads to the ubiquitination of the OMM proteins, including VDAC, and consequent degradation by the proteasome. Under TSPO-VDAC1 interaction, increased ROS levels cause a change in ΔΨm that enables Pink1 kinase import and blocks recruitment of Parkin and thereby mitophagy. TSPO overexpressing cancerous cells caused increased VDAC1 binding that led to dysfunctional mitochondria, signifying that coordinated TSPO-VDAC1 interaction is required to maintain mitochondrial homeostasis (Shoshan-Barmatz et al., 2019). Further information is needed on the structure of the closed state, how binding partners or ΔΨm could lead to the open/closed states, the function and mobility of the N-terminal α-helical domain of VDAC, and the physiological role of VDAC oligomers.
2.1.3 OMM channels involved in cell death
Mitochondrial Apoptosis-induced Channel (MAC) is a voltage-independent and gigantic channel having high conductance for both heterogeneous and small proteins like cytochrome c and is regulated by Bcl-2 family proteins. Nonetheless, MAC and Permeability Transition Pore (PTP) opening may behave alone or accompanied, based on cell type and death stimuli (Pavlov et al., 2001). Moreover, MAC activity is considerably unique from the VDAC and TOM complex which are integral channels of OMM. The very basic function of MAC is to provide the passage for the release of cytochrome c (12.5 kDa) in early apoptotic events. The onset of MAC activity corresponds to the release of cytochrome c in several cell systems (Martinez-Caballero et al., 2009). Bcl-2 family members are responsible for MAC formation and its activity. The loss of MAC activity and oligomeric Bax (pro-apoptotic studies) provide strong data that oligomeric activated Bax is a structural component of MAC (Dejean et al., 2005). On the contrary, anti-apoptotic molecules like Bcl-2 prevent Bax oligomerization, directly binding to pro-apoptotic effectors (Leber et al., 2007). Regulation of MAC is also mediated through BH-3-only proteins. These proteins are required to decipher the survival and death signals throughout the cell. They act either as activators or sensitizers. Bid, Bim, p53, and perhaps PUMA directly interact with Bax and Bak, causing a conformational change, which activates their oligomerization leading to MAC formation (Chipuk and Green, 2008).
MAC directly is not involved in the modulation of MPQC. However, Bcl-2 family members were found to take part in the maintenance of a healthy mitochondrial milieu, regulating Parkin/PINK1-dependent mitophagy. Bcl-xL and Mcl-1 bind to Parkin/PINK1 and prevent Parkin translocation to depolarized mitochondria and thus block Parkin-dependent ubiquitination of mitochondrial substrates and downstream signaling, implying Bcl-2 effectors can also be a target for therapeutics (Hollville et al., 2014; Zhang et al., 2020).
2.1.4 OMM as a site of inter-organelle contacts and lipid transfer
Mitochondria–Endoplasmic reticulum contact sites (MERCs) [Endoplasmic reticulum–mitochondria encounter structure (ERMES) in yeast] is a multimeric protein complex that joins the endoplasmic reticulum (ER) and OMM. The tethering proteins from both ER and mitochondria build a bridge to form MERCs. The tethering molecules include vesicle-associated membrane protein associated protein B (VAPB) (De Vos et al., 2012), protein tyrosine phosphatase-interacting protein-51 (PTPIP51) (De Vos et al., 2012), mitofusin1/2 (MFN1/2) (Sebastián et al., 2012), inositol 1,4,5-trisphosphate receptor (IP3R)-glucose-regulated protein 75 (GPR75)-voltage-dependent anion channel (VDAC1) complex (Szabadkai et al., 2006a), and B-cell receptor-associated protein 31 (BAP31) and fission 1 homolog (FIS1) (Iwasawa et al., 2011). MERCs are notable to shape the flux of Ca2+ and to maintain synthesis and composition of membrane lipids, shuttling import and export of lipids (Petrungaro and Kornmann, 2019). MERCs are enriched with two transmembrane proteins; Sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) and inositol 1,4,5-trisphosphate receptors (IP3R) provide Ca2+ shuttle service from the cytosol into the ER lumen (Camello et al., 2002) and Ca2+ from the ER lumen into the mitochondria (Szabadkai et al., 2006b). The ER-mitochondrial resident structural proteins such as GTPase MFN2 also participate in Ca2+ signaling (Naon et al., 2016). It directly binds and transfers phosphatidyl serine (PS) across the ER-mitochondria juncture (Hernández-Alvarez et al., 2019) to convert it into phosphatidylethanolamine (PE) (Ardail et al., 1991). PS to PE conversion warrants the suitable environment for PS transport and mitochondrial health (Horvath and Daum, 2013). Apart from Ca2+ signaling, homeostasis of membrane lipids is one of the signatory roles of MERCs. The precursor of cardiolipin (CL) synthesis, phosphatidic acid mostly comes from the ER through MERCs (Potting et al., 2013) and CL is very well known to be involved in various mitochondrial physiological processes like mitochondrial bioenergetics, structure, and mitophagy (Hsu et al., 2015). Besides CL, MERCs also serves as site for phosphatidyl serine and other lipid synthesis pathways. Lipid droplets bud from ER and provide fatty acids to mitochondria for regulating fatty acid oxidation and the tricarboxylic acid (TCA) cycle. The abundance of cholesteryl esters (CE) and triacylglycerols (TG) producing enzymes; acyl-CoA cholesterol acyltransferase-1 (ACAT1) and diacylglycerol acyltransferase 2 (DGAT2) respectively at MERCS implies their role in the biogenesis of lipid droplets (Stone and Vance, 2000; Stone et al., 2009). Another entity of ER-mitochondria tethering proteins, PDZ domain-containing protein 8 (PDZD8) ER transmembrane protein is recognized as a regulator of Ca2+ dynamics in neurons (Hirabayashi et al., 2017).
In yeast, ERMES is mainly formed by the mitochondrial distribution, and morphology (MDM) complex. The OMM protein, mitochondrial distribution, and morphology protein 10 (Mdm10) is a subunit of both MDM and SAM complexes, physically connecting the OMM and ER membrane to help in lipid transport between the two membranes (Esposito et al., 2019). The switching between MDM and SAM is controlled by Tom7. Thus, these interlinking TOM, SAM, and ERMES complexes behave as a functional network, maintaining mitochondrial structure and the transport of metabolites. Along with Mdm10, other OMM proteins like Mdm12, Mmm1, and Mmm2 are also components of ERMES. Moreover, other transporters, like VDAC, Tom70, etc., are also involved in the formation of ER-mitochondria contact sites (Meisinger et al., 2006; Kornmann et al., 2009; Murley et al., 2015). The SAM-Mdm10 complex offers an assembly platform for the TOM complex and the Tom22 precursor uses Mdm10 for its biogenesis, fostering an effective assembly of the TOM complex. Interestingly, ERMES is connected to SAM via the Mdm10, and ERMES subunits show strong genetic interactions with mitochondrial contact site and cristae organizing system (MICOS) subunits to maintain mitochondrial organization (Hoppins et al., 2011). Give and take relationship between ER and mitochondria provides a platform to understand the lipid metabolism and signaling in both ER and mitochondria compartments, extending the crucial role of MERCs in diseases linked with alterations in lipid pool.
2.2 IMM channels, their control mechanisms, and PTMs
IMM is the second level of entry barrier which is highly selective and more secure for entry into the MM. The transporters or ion channels, the part and parcel of IMM, are keenly engaged in maintaining mitochondrial processes.
2.2.1 ADP, ATP translocase (ANT)
ANT is an antiporter in nature and belongs to the mitochondrial carrier family (SLC25). It exchanges ATP from the MM and ADP from ATP consumption in the remainder of the cell. The ANT can be found in two states; the matrix and the cytoplasmic states, compatible with the establishment of a high-conductance carrier to dissipate the ΔΨm and lead to mitochondrial swelling (Vieira et al., 2000). Initially, it was supposed as a dimer, but structural, functional, and biophysical data now prove that this exists and works as a monomer (Kunji and Ruprecht, 2020). Other than ADP/ATP exchange, it also involves in stimulation of the Mitochondrial Permeability transition Pore Complex (MPTP) by Ca2+ and ROS, a mild uncoupling movement that is activated by anion superoxide, peroxidized lipids, and fatty acids. All these functions are controlled by ΔΨm (Brenner et al., 2011). ANT also modulates mitophagy, the coordinated degradation of mitochondria, irrespective of its nucleotide translocase catalytic activity. In response to the bioenergetic collapse due to mitochondrial stress causing agents (either CCCP or suppressors of OXPHOS), the ANT complex is necessary for inhibition of the presequence translocase TIM23, stabilizing PINK1. ANT controls TIM23 indirectly, interacting with TIM44. ANT1 knockout mice led to blunted mitophagy, accumulating aberrant mitochondria, and indicating a novel function of ANT as a fundamental facilitator of mitophagy (Hoshino et al., 2019).
ANT undergoes numerous PTMs such as phosphorylation, acetylation, succinylation at lysine, etc. ANT phosphorylation at T84 and Y191 in the hearts of mice and humans have been observed vital for cell fate and heart conductance (Lam et al., 2013). Acetylation of ANT is associated with compromised cardiac metabolism in mitochondria in the absence of Cyclophilin D (CypD) in mice (Nguyen et al., 2013). Moreover, ANT S-glutathionylation has been found on Cys57 in cardiac-specific inducible NO synthase transgenic mice (Finoshin et al.). The CypD binding site is situated alongside the glutathiolated Cys57, modulating its function (West et al., 2006). Oxidation of the thiol group of ANT is recently noticed to lead to mitochondrial dysfunction through MPTP opening upon silicon dioxide (SiO2) nanoparticles treatment (Lozano et al., 2020). The structural aspects of the conformational changes for the interconversion of matrix and cytoplasmic states and the free and substrate-bound intermediates still ought to be solved. Further functional investigations are necessary to find out PTMs, deepening insights into molecular mechanisms and physiological relevance in diseases.
2.2.2 Translocase of the inner membrane (TIM)
TIM is the major gateway of IMM. TIM23, translocase of IMM comprises Tim17, Tim21, Tim23, and Tim50 proteins which are incorporated into the IMM displaying functional domains to the IMS side, and Tim44, Tim14/Pam18, Tim16/Pam16, Pam17, and Tim44 facing matrix side. Tim17 shares sequence similarity with Tim23 presumably to form the translocation pore in the IMM. Tim50 guides preproteins released from TOM to enter the TIM23 complex and this transport is further controlled by Tim21. The small TIM chaperone molecules are imported to the OMM by an N-terminal end of Tom40. Tim54 interacts with small TIM chaperones through its IMS domain and sends the precursors to the carrier translocase of the inner membrane (TIM22) in a ΔΨm-dependent manner to release the proteins laterally into IM(Herrmann and Neupert, 2013). The translocation from TOM to TIM is regulated by the membrane potential, bolstering the passage of the positively charged pre-sequences to the negatively charged matrix side of IMM. The mitochondrial HSP70 chaperone protein (mtHSP70) then forms a complex with the pre-sequence associated motor (Hernández-Alvarez et al.), interacting with TIM23 core components to force the proteins inside the matrix at the expense of ATP. TIM23 also interacts with complexes III and IV to translocate pre-protein into the matrix in an ATP-driven manner. Mostly, the pre-sequence is cleaved by matrix mitochondrial processing peptidase (MPP). The TIM23 complex also confirms the lateral insertion of precursor proteins into the IMM(Schulz et al., 2011). The mitochondrial intermembrane space import and assembly (MIA) machinery brings in cysteine-rich proteins such as the small TIM proteins into the IMS for their oxidative folding. In addition to this, TOM complex precursors are also guided by TIM to SAM complex. The oxidase assembly (OXA) translocase mediates the sorting of IMM proteins imported by TOM and Tim23-PAM machinery and exports them to IMM. Interestingly, the N terminus of Pink1 is also imported through the Tim23 import pathway (Silvestri et al., 2005), proposing the possible role of TIM23 in mitophagy.
Although PTMs of TIMs are poorly understood, it has been studied that oxidation of Tim17 by sulfhydryl oxidase Erv1 is mediated by the mitochondrial disulfide relay. Tim17 contains a pair of highly conserved cysteine residues which form a disulfide bond when it is exposed to the IMS. This disulfide bond mediates efficient protein translocation through the TIM23 complex and dynamic gating of its pre-protein-conducting channel (Ramesh et al., 2016).
2.2.3 Ion exchangers
Mitochondrial calcium (mCa2+) plays an essential role in many cellular functions maintaining proper levels of both cytosolic and mitochondrial Ca2+ which is critical for cellular homeostasis. mCa2+ efflux is regulated by both Na+-dependent (mitochondrial Na+–Ca2+ exchanger, NCLX) pathway and an Na+-independent (H+–Ca2+ exchanger) pathway. NCLX is a distinct exchanger from the plasma membrane Na+–Ca2+ exchanger (NCX) and is found in most cell types and tissues, with activity mainly observed in excitable cells (Jiang et al., 2009; Palty et al., 2010). The activity of the H+–Ca2+ exchanger is mainly found in non-excitable cells. The ΔΨm, stoichiometry of NCLX, and electrochemical gradient for Na+ entry into the MM from the cytosol all influence Ca2+ extrusion from the matrix (Jiang et al., 2009). NCLX is endogenously expressed in the IMM (Palty et al., 2010), consisting of two transmembrane domains, α1 and α2, which bind ions and translocate them across the membrane (Khananshvili, 1991). The regulation of NCLX by ΔΨm is mediated through catalytic and regulatory domains, which can link mitochondrial metabolic state and Ca2+ signaling (Kostic et al., 2015). NCLX is also post-translationally regulated by phosphorylation at Ser258 by protein kinase A (Yang et al., 2003; Kostic et al., 2015) and palmitoylation at cysteine (Reilly et al., 2015), which ubiquitously regulates Ca2+ homeostasis and ΔΨm in cells.
HCX (H+/Ca2+): HCX activity is shown to be dominant in certain tissues such as lung, liver, smooth muscles, and kidney where NCLX activity is weak (Gunter and Pfeiffer, 1990). Though the molecular identity of HCX is yet to be revealed, high-throughput RNA interference screening in fruit fly, Drosophila identified a gene responsible for mitochondrial Ca2+ and H+ [LETM1 (leucine–zipper–EF hand-containing transmembrane region), the human homolog] (Jiang et al., 2009). Initial studies suggested that LETM1 functions as a Ca2+ influx candidate (Jiang et al., 2009) but later studies demonstrated Ca2+ efflux from cardiac mitochondria in a free matrix Ca2+ concentration dependent manner (Natarajan et al., 2020). The function of LETM1 is controversial and debated as to whether it acts a K+/H+ exchanger (KHE) as originally supposed or an Ca2+/H+ exchanger (CHE) as evidenced in studies (Austin and Nowikovsky, 2019; Lin and Stathopulos, 2019). LETM1-KD H9c2 cells altered Ca2+ efflux, indicating LETM1-HCX mediated Ca2+ efflux (Natarajan et al., 2020) whereas another finding showed that LETM1 mediated mitochondrial Ca2+ regulation is facilitated by a LETM1-KHE activity, modulating the activity of the mitochondrial Na+/H+ exchanger (mNHE) eventually affecting NCLX-mediated Ca2+ efflux (Austin et al., 2017). A recent study demonstrates that MICS1 is the long-sought mitochondrial CHE and interacts with LETM1, modulating its activity (Austin et al., 2021). Irrespective of its individuality, LETM1 has been implicated in interaction with signaling proteins such as MRPL36, BCS1L and CTMP to modulate protein import and assembly in IMM, mitochondrial morphology and metabolism (Park et al., 2014; Li et al., 2019).
LETM1 is known to be a direct target for phosphorylation by PINK1, which constitutively phosphorylates LETM1 and regulates Ca2+ signaling through a LETM1-CHE activity (Huang et al., 2017). LETM1 methylation has also been identified as an epigenetic marker in obese patients associated with fasting insulin levels (Liu et al., 2019). While LETM1’s primary role as HCX or CHE is still unclear, loss of LETM1 has been shown to adversely affect mitochondrial Ca2+ signaling.
In addition to LETM1, Transmembrane BAX Inhibitor-1 Motif 5 (TMBIM5) has been recently investigated as a Ca2+/H+ exchanger in the IMM that allows Ca2+ efflux from mitochondria, preventing mitochondrial membrane hyperpolarization (Austin et al., 2022; Patron et al., 2022). In vivo and in vitro gene alteration studies show that TMBIM5 is an essential unit of mitochondrial ion transport. Overexpression of TMBIM5 carries out mitochondrial Ca2+ uptake through aspartate residue of channel pore whereas TMBIM5 loss modulates matrix ion composition with no major effect on Ca2+ uptake (Zhang et al., 2022). Further studies are required to explore the mechanism of mitochondrial ion homeostasis by both TMBIM5 and LETM1.
2.2.4 Mitochondrial calcium uniporter channel (mtCU/MCU)
mCa2+ is a versatile molecule that regulates intracellular signaling events under both physiological and pathological conditions. The fundamental physiological role of the mitochondrial calcium uniporter (MCU) is to modulate the Ca2+ signaling, aerobic metabolism as well as apoptosis (De Stefani et al., 2015). However, excessive Ca2+ accumulation in the MM can trigger cell death through the opening of the MPTP. Therefore, maintaining the balance of Ca2+ levels is essential, which is achieved through the activity of IMM Ca2+ permeable transporters and channels (Zavodnik, 2016). MCU is a conserved protein found throughout eukaryotes, except in yeast. MCU is primary route for the Ca2+ influx in MM and only known channel to allow acute Ca2+ entry in the MM (Palty et al., 2010; Baughman et al., 2011). MCU’s function has been well documented in cellular proliferation and apoptosis (Bachmann et al., 2019). This multi-subunit complex consists of MCU (pore-forming protein), MCUB (an MCU paralog), EMRE (essential MCU regulator), the regulatory Ca2+ sensing MICU proteins (MICU1, MICU2 & MICU3), and MCUR1 (mitochondrial calcium uniport regulator 1) (Perocchi et al., 2010; Baughman et al., 2011; De Stefani et al., 2011; Mallilankaraman et al., 2012a; Mallilankaraman et al., 2012b; Plovanich et al., 2013; Raffaello et al., 2013; Sancak et al., 2013; Tomar et al., 2016; Patron et al., 2019). EMRE and MCUR1 act as scaffolding regulators of MCU channel and essential for mCa2+ flux. MCUR1 directly interacts with MCU and enhances Ca2+ uptake inside mitochondria (Vais et al., 2015). Downregulation of MCUR1 reduces mitochondrial Ca2+ uptake, OXPHOS activity, and hence ATP production (Mallilankaraman et al., 2012b; Tomar et al., 2016). The cytosolic Ca2+ threshold is set by MICU1, if the level of Ca2+ is exceeded above the threshold, i.e. 1-2 μM in most cell types, the EF-hands of MICU1 sense this elevation and switch the status of the channel from closed to open conformation (Mallilankaraman et al., 2012b).
The activity and expression of MCU and its regulators are regulated at several levels, transcriptional, post-transcriptional, and post-translational levels. Small non-coding regulatory microRNAs, such as miR-25 and miR-138, regulate the expression of MCU subunits (Hong et al., 2017). The majority of PTMs of MCU include methylation, oxidation, and phosphorylation. Oxidation of conserved cysteines, including C67, C97, and C191, in the N-terminal domain (NTD) by increased levels of ROS leads to S-glutathionylation of C97 (Dong et al., 2017). This modification remodels the conformational change in NTD of MCU, boosting MCU activity, and increasing the rate of Ca2+ entry into the MM (Lee et al., 2016; Dong et al., 2017). Another PTM is phosphorylation by Calmodulin kinase II (CaMKII) at S57 and S92 of the NTD of the MCU complex (Joiner et al., 2012) as well as phosphorylation by the proline-rich tyrosine kinase 2 (Pyk2) and AMP-activated protein kinase (AMPK). Both Pyk2 and AMPK-dependent MCU phosphorylation enhances Ca2+ entry to the matrix and intensifies mitochondrial respiration and energy production (Zhao et al., 2019).
Moreover, the MCU regulator, MICU1 is modulated through arginine methyltransferase 1 (PRMT1), reducing Ca2+ uptake into the matrix (Madreiter-Sokolowski et al., 2016). Recent studies have also shown that the S92 of MCU NTD is phosphorylated by protein kinase C isoforms, namely, PKCβII, PKCδ, and PKCε, which amazingly switch the exchange between cytosolic Ca2+ and mitochondrial Ca2+ and manipulate the expression level of MCU (Lee et al., 2020). Moreover, MCU, previously known solely as a Ca2+ transporter, has been found to play a role in mitochondrial quality control. Cho and colleagues demonstrated that during mitochondrial fission, the recruitment of Drp1 occurs at regions where the ΔΨm is reduced. At this point, Drp1 interacts with the mitochondrial zinc transporter Zip1-MCU complex, allowing for Zn2+ entry and subsequent fission. Healthy mitochondria are able to restore MMP levels through the fusion-fission cycle, while dysfunctional mitochondria undergo mitophagy (Cho et al., 2019). Additionally, MCU upregulation has been shown to activate calpain and reduce OPA1 levels during ischemia/reperfusion (IR) injury, resulting in an imbalance in mitochondrial dynamics (Guan et al., 2019). However, further research is needed to fully understand the molecular mechanisms by which MCU contributes to mitochondrial quality surveillance.
2.2.5 Mitochondrial chloride (Cl−) channel proteins
Chloride channels (Cl−) are a diverse group of proteins predominantly present in intracellular organelles, serving important roles in many physiological processes. The different types of Cl-channels include the voltage-sensitive ClC subfamily, glycine receptors, transmitter-gated GABAA, Ca2+-activated Cl-channel, the cystic fibrosis transmembrane conductance regulator (CFTR), volume-regulated channel, and high (maxi) conductance Cl-channel (Ashley, 2003). In addition, a few Chloride Intracellular Ion Channels (CLICs) have recently been shown to be localized to mitochondria. Two mitochondrial chlorides (mtCl) channels, namely, CLIC4 and CLIC5 have been identified in mitochondria (Ponnalagu and Singh, 2017). CLIC4 was first identified to be present on IMM of mouse keratinocyte mitochondria (Fernández-Salas et al., 1999) whereas in adult cardiomyocytes, it is found in OMM. CLIC5 is located in IMM of adult cardiomyocytes (Ponnalagu et al., 2016). These belong to the class of intracellular anion channels (CLICs) and have been shown to play a role in various physiological processes.
Recent studies have shown the presence of CLIC4 in mitochondrial-associated membranes, implicating its involvement in cardioprotection (Ponnalagu et al., 2022). There are six paralogs of CLICs (CLIC1-CLIC6) stated in mammals, four (AtDHAR1-AtDHAR4) in Arabidopsis thaliana, three in invertebrates in Drosophila Melanogaster (DmCLIC) and two in Caenorhabditis elegans (EXC4 and EXL1) (Gururaja Rao et al., 2017; Ponnalagu and Singh, 2017; Gururaja Rao et al., 2018). It is suggested to have a pro-apoptotic role in p53-mediated apoptosis (Fernández-Salas et al., 2002). The other chloride channel is from the ClC family, ClC-Nt, and has been intended as a component of IMAC (Lurin et al., 2000). Despite several articles on mtCl channels, understanding their molecular identity, structure, and role in physiological and pathological states is yet inadequate.
CLICs have also been found to be modulated by PTMs such as phosphorylation. Cyclin-dependent kinase 5 mediated serine phosphorylation of CLIC4 stimulates its stability and regulates cell death (Guo et al., 2018). In addition to CLICs, Maxi-Chloride channels are also present in mitochondria. While their molecular structure remains unidentified, it is believed that they have a strong relationship with the permeability transition pore complex (PTPC) as their activity is similar to that of monomeric PTPC (Sabirov et al., 2006). It is well known that CLICs modify mitochondrial activity through modulation of ROS levels. For example, the loss of CLIC5 in cardiac mitochondria has been shown to enhance mitochondrial ROS and modulate the Ca2+ retention capacity of mitochondria (Ponnalagu et al., 2016; Ponnalagu et al., 2019). However, the direct influence of mitochondrial CLICs on mitochondrial function remains unclear and requires further investigation. Overall, the role of mitochondrial chloride channels in regulating mitochondrial activities is complex and requires further research. A better understanding of the molecular identity, structure, and function of these channels in physiological and pathological states could potentially lead to the development of new therapeutic strategies for the treatment of various diseases.
2.2.6 Mitochondrial inner membrane anion channel (IMAC)
The inner mitochondrial membrane (IMM) harbors various chloride (Cl-) anion-selective channels, including the inner membrane anion channel (IMAC). IMAC has a single-channel conductance of approximately 100 pS, with slight anion selectivity. Small molecules such as bicarbonate, phosphate, chloride, citrate, ATP, and succinate can pass through this channel. The modulation of IMAC by palmitoyl-CoA Mg2+, amphiphilic amines, and pH has also been observed. Although the function of IMAC in pathophysiological conditions is well established, its physiological role is still uncertain, as it appears to facilitate ion movement only in the presence of an alkaline matrix environment. It has been proposed that anion efflux through IMAC can be a protective mechanism against excessive matrix swelling (Beavis, 1992). Recently, mitochondrial anion efflux has been linked to hypoxic preconditioning, further highlighting the potential importance of IMAC in cellular homeostasis (Vanden Hoek et al., 1998). Despite its significance, the molecular identity of IMAC remains elusive, which is the major limitation in researching this channel. The regulation of IMAC activity has been linked to glutathione levels, where inhibition of mitochondrial glutathione uptake, the NADPH-dependent glutathione reductase, or the NADH/NADPH transhydrogenase was found to stimulate IMAC opening (Aon et al., 2007). These findings suggest that redox-dependent activities might play a role in modulating IMAC activity. Further studies are required to understand the molecular identity of IMAC and the underlying redox-dependent PTMs.
2.2.7 Mitochondrial phosphate carrier (PiC)
The PiC is an important mitochondrial phosphate carrier protein, encoded by SLC25A3 in humans. It facilitates the transport of inorganic phosphate, a key substrate of OXPHOS, across the IMM. The mammalian mitochondrial PiC is ubiquitously expressed and exists in two distinct isoforms, PiC-A and PiC-B. Together with ANT and complex V, PiC forms the ATP synthase microdomain for ATP production. Furthermore, PiC transport is significant for efficient mitochondrial Ca2+ handling. mCa2+ uptake requires the presence of anions such as Pi, acetate, bicarbonate, and glutamate to provide protons for the respiratory chain. Reduced anions restrict Ca2+ uptake, leading to low O2 consumption. Pi may be required for both boosting the MCU-mediated Ca2+ influx and inhibiting the exchanger-mediated Ca2+ efflux (Chalmers and Nicholls, 2003; Wei et al., 2012). PiC transgenic mice with elevated and shRNA mediated diminished levels of cardiac PiC have been characterized by cardiac hypertrophy, unaltered respiration, ATP content, Ca2+ retention, and activation of the MPTP (Gutiérrez-Aguilar et al., 2014). Alongside, inducible and cardiac-specific deletion of PiC also showed the same phenotype as seen in shRNA mediated deletion of PiC. Notably, time-dependent mitochondrial phosphate uptake was eliminated, and the mitochondrial ATP content was reduced with no impaired Ca2+ retention and was in fact elevated. However, the absence of phenotypes of the PiC transgenic mice might be due to the abundance of PiC which does not regulate the transport in accord with PiC’s high turnover rate. The diminished ATP and cardiac hypertrophy in PiC depleted model are in line with the phenotype of the ANT knockout mouse, in which another component of the ATP synthasome is absent (Narula et al., 2011). Hence, insufficient Pi transport and oxidative phosphorylation might be the reasons for PiC phenotypes. Pathogenic mutations in PiC show different phenotypes based on tissue-specific expression and distinctive enzymatic activity of isoforms. Three PTMs for PiC, namely, methylation at lysine 112, phosphorylations at tyrosine 196 and 246, and acetylation at lysine 99, 209, and 234 are associated with cardiac diseases (Alves-Figueiredo et al., 2021). More studies are warranted to uncover PiC mutations in various models in stress response.
2.2.8 Potassium (K+) channels
Mitochondrial K+ channels play an important role in regulating the electrochemical gradient and mitochondrial function. Two types of K+ channels located at the IMM, mitoKATP and mitochondrial Ca2+ activated K+ channel (mitoKCa), are regulated by various factors including ROS, ATP, Ca2+ and free fatty acids (Wrzosek et al., 2020).
2.2.8.1 Mitochondrial KATP (mitoKATP)
MitoKATP was first observed in the liver and is similar to the KATP channel present in the sarcolemma of cardiac cells, albeit with a lower conductance. The elevated mitochondrial inner membrane permeability to K+ has been hypothesized to enhance tolerance to IR injury in a cell (O'Rourke, 2007). Recently, a novel protein complex consisting of a channel forming subunit (MITOK) and a regulatory subunit carrying the ATP-binding domain (MITOSUR) was identified for ATP-sensitive K+ transport across the IMM (Paggio et al., 2019). MitoKATP facilitates the uptake of potassium (K+) driven by the negative ΔΨm and is repressed by physiological ATP levels. Endogenous molecules such as adenosine and bradykinin can decrease infarct size by stimulating mitoKATP channels in a PKC-dependent approach.
2.2.8.2 Mitochondrial KCa
KCa channel family members are categorized through their differing single channel conductance into three types, namely, big conductance (BKCa, 200–300 pS), intermediate conductance (IKCa, 32–39 pS) and small conductance (SKCa, 4–14 pS) channels (Diness et al., 2015). In addition to plasma membrane, KCa channels have also been localized in mitochondria. The large-conductance Ca2+ -activated K+ channel (BK channel) is also present in the IMM of cardiomyocytes and brain, suggesting that the molecular identity of mitoKCa could be similar to the BK channel. These channels take part in oxidative regulation at the plasma membrane. However, no study is done in the context of their redox-sensitive PTMs (Nishida et al., 2009). Therefore, elucidating the molecular basis of redox-sensitive PTMs in these channels could provide new insights into oxidative regulation at the plasma membrane. Another selective K+ channel detected in IMM from human glioma cells by direct patch-clamp of mitoplasts is the Ca2+-stimulated K+ channel (mitoKCa) (Siemen et al., 1999). mCa2+ uptake can be raised through mitoKCa against extreme mitochondrial Ca2+ accumulation and that might control the tuning between mitochondrial volume and/or Ca2+ accumulation under increased cardiac workload conditions (O'Rourke, 2007). SKCa channels are shown to be localized in IMM of cardiomyocytes and neurons, regulating mitochondrial respiration and mCa2+ uptake (Stowe et al., 2013; Honrath et al., 2017). MitoSK channel activation generates a protective decrease in ischaemia-induced mitochondrial Ca2+ overload and ROS, balancing sarcoplasmic reticular (Ponnalagu et al.) Ca2+ release (Stowe et al., 2013). These findings together imply SK2 channels as potential targets for ameliorating ROS mediated mitochondrial stress.
2.2.8.3 Kv1.3
The Kv1.3 channel, which is identical to the plasma membrane Kv1.3 channel, has been detected in the IMM of Jurkat T lymphocytes (Szabò et al., 2005). This channel has increased expression in cancerous cells, making it a potential target for drug therapy (Szabò et al., 2005). However, the molecular identity and pharmacology of these channels are not well studied, and further research is necessary to develop treatments against diseases by targeting these channels. The experimental evidence on PTMs of mitoK channels is far limited. It has been found that the activity of mitoKATP and mitoBKCa is regulated by PKC, PKA and PKG through phosphorylation. The phosphorylation of mitochondrial potassium channels might induce cardioprotective cascade (Rotko et al., 2020b; Checchetto et al., 2021). mitoK channels are regulated by gaseous transmitters such as carbon monoxide (CO), hydrogen sulfide (H2S) or nitric oxide (NO) through PTMs such as S-nitrosylation or S-sulfhydration (Walewska et al., 2018; Rotko et al., 2020a). A very recent study showed that applications of the H2S donor NaHS causes mitoBKCa S-sulfhydration of cysteines without hampering the channel activity (Walewska et al., 2022).
2.2.9 Uncoupling proteins (UCPs)
Uncoupling proteins (UCPs) are a group of mitochondrial transporter proteins that play a crucial role in the regulation of energy metabolism and redox homeostasis (Ponnalagu and Singh, 2017). In response to stress conditions, ROS overproduction and dysfunction in ATP synthesis, mitoKATP channel and UCP get activated and produce mild uncoupling. This in turn attenuates mitochondrial ROS formation (Zhang et al., 2001). This feedback-induced decreased ROS protects mitochondria and allows for rapid oxidation of the reducing equivalents that overfeed the mitochondrial respiratory chain. However, under physiological conditions, the mitoKATP channel and UCPs are likely inhibited, preserving the efficiency of ATP synthesis (Slocińska et al., 2016). Among the UCP family, UCP1-UCP3 are the most well-studied. UCP1 is primarily expressed in brown adipose tissue and is responsible for the production of heat through thermogenesis. UCP2 and UCP3 are widely expressed and involved in regulating ROS levels, protecting against oxidative damage and balancing mCa2+ uptake (Trenker et al., 2007; Azzu and Brand, 2010). Electrophysiological analysis has proven that UCP2 and UCP3 modulate MCU-dependent Ca2+ current in mitoplasts (Bondarenko et al., 2015). On the other hand, UCP4, UCP5, and UCP6 are mainly expressed in the central nervous system and carry out the transport of thiosulfate, sulfate, and inorganic ions (Monteiro et al., 2021).
Apart from their traditional role in proton gradient and ROS homeostasis, UCPs are implicated in several mitochondrial quality control pathways, such as mitophagy and MAD. UCP2 and UCP3 are substrates for ubiquitination and proteasome degradation (Mookerjee and Brand, 2011). Compared to UCP1, UCP2 protein has a very short half-life which is rapidly degraded by the ubiquitin-proteasome system (Azzu and Brand, 2010). The implication of UCP2 degradation in diseases is not yet fully understood. UCPs are also known to be involved in redox signaling and homeostasis. For instance, UCP1 is regulated by ROS levels through sulfonylation of the Cys253 residue, increasing thermogenesis (Oelkrug et al., 2010; Chouchani et al., 2016). UCP2/3 can be modulated by glutathionylation, where GSH is conjugated to cysteine residues. When mitochondrial ROS levels are low, UCP2/3 remains inactive in the presence of glutathionylation. Elevated ROS levels turn on GSH, inducing de-glutathionylation, and subsequent stimulation of UCP2/3 (Mailloux et al., 2011), regulating ROS in a cell. Overall, UCPs play a critical role in regulating energy metabolism, redox homeostasis, mitochondrial quality control pathways, and redox signaling. However, further research is needed to fully understand the implications of UCP degradation in diseases and the broader implications of UCPs in redox signaling and homeostasis.
2.2.10 Other mitochondrial ion transporters
Mitochondria transport various metal ions, including magnesium (Mg2+), zinc (Zn2+), iron (Fe2+), and manganese (Mn2+). Mg2+ is important for respiratory function, and its concentration is required for Mg2+-sensitive matrix enzymes (e.g., pyruvate dehydrogenase) and transporters (e.g., Ca2+ uniporter) (Pradhan et al., 2011; Yamanaka et al., 2016). Mitochondrial Mg2+ levels are tenfold greater than the cytoplasm, mediating ADP/ATP exchange (Gout et al., 2014) and participating in the release of cytochrome c. The effects of Mg2+ on mitochondrial functions emphasize energy metabolism, mitochondrial Ca2+ processing, and apoptosis (Pilchova et al., 2017). The transporter of Mg2+ of mitochondrial RNA splicing protein 2 (MRS2) channel uptakes Mg2+ selectively (Schindl et al., 2007; Yamanaka et al., 2016) and expression levels of MRS2 are linked to the levels of Mg2+ (Piskacek et al., 2009). The impaired Mg2+ in cells and mitochondria has been studied to be implicated in the neurodegenerative process of Parkinson’s disease (Shindo et al., 2015).
Zn2+ is one of the most abundant ions in mitochondria as compared with other subcellular organelles (Sun et al., 2016) and is important for maintaining the antioxidant status of a cell (Adebayo et al., 2016). However, excessive Zn2+ within the mitochondria can lead to negative consequences such as loss of MMP, increased ROS, and reduced ATP levels (Pochwat et al., 2015). Studies on MCU knockout mice indicate that Zn2+ is transported into mitochondria via MCU (Ji et al., 2020), and it is suggested that the regulatory proteins MICU1 and MICU2 indirectly modulate the transport of both Zn2+ and Ca2+ levels. To better understand the compartment-specific levels of Zn2+ in mitochondria, further investigation is needed.
Mitochondria play a crucial role in iron metabolism, serving as the primary site for hemoglobin production, heme biosynthesis, and Fe-S cluster protein assembly. Mitochondrial solute carrier protein MFRN (SLC25A37) has been proposed to transport iron ions into the MM. Disruptions in MFRN function can lead to severe hypochromic anemia and stagnant red blood cell maturation (Finoshin et al., 2020; Seguin et al., 2020). DMT1, a divalent metal transporter protein located on the mitochondrial outer membrane, has also been shown to transport iron ions (Wolff et al., 2018). However, little is known about the mitochondrial iron ion efflux channels/transporters and further investigation is needed in this area.
Mitochondria generates ROS which can be detrimental to cells. Therefore, it is essential to remove ROS, and this process relies on SOD (Wang et al., 2018). In mammals, Mn2+ uptake is specifically mediated by cellular SLC39A8 (ZIP8) and SLC39A14 (ZIP14) (Park et al., 2015; Tuschl et al., 2016), while SLC30A10 (ZnT10) regulates Mn2+ efflux from cells (Mercadante et al., 2019). However, the mechanism of Mn2+ transport in mitochondria is still unclear, although it has been proposed that Mn2+ uptake from the cytosol to mitochondria may be mediated via MCU or MFRN1 (Liu et al., 2021). Though altered Mn2+ homeostasis and toxicity have been reported in neurodegenerative diseases, whether this is associated with mitochondrial Mn2+ transport remains to be explored.
2.2.11 Mitochondrial permeability transition pore (MPTP)
The MPTP is a large conductance channel protein located in the IMM. Under physiological conditions, it facilitates the exchange of small metabolites between the matrix and the cytoplasm through its “open and shut” conformation. However, under pathological or stress conditions, the MPTP can transition into a high-conductance state, allowing the uncontrolled access of solutes into the MM, leading to the permeability transition (PT). PT is a reversible process but is tightly controlled and activated by various factors such as Cyclophilin D (CyPD), ROS, matrix Ca2+ accumulation, and low ΔΨm (Hodge and Colombini, 1997). The MPTP is a supramolecular non-selective channel that forms at the intersections between the outer and inner mitochondrial membranes. It can transport metabolites up to ∼1,500 Da molecular weight, provided the diameter of the pore is ∼3 nm. The opening of the MPTP can be triggered by Ca2+ overload, depolarization of the transmembrane potential, ROS, and Pi (Brustovetsky and Klingenberg, 1996). Inhibitors of the MPTP include Mg2+, adenine nucleotides, and mild acidic matrix pH. There are multiple proteins proposed to be the MPTP. Earlier, it was believed that the ANT and FoF1-ATP synthase were responsible for pore formation, but recent evidence has shown that MPTP formation can occur in their absence (Gerle, 2016; Carraro et al., 2018; Karch et al., 2019; Gerle, 2020). SPG7 is identified as a potential MPTP core component in a targeted screening (Shanmughapriya et al., 2015). Therefore, the molecular identify of MPTP is still debated and there is a possibility of multiple proteins acting as MPTP based on upstream signal and cell types. CyPD, a protein found in the MM, can also modulate the opening of the MPTP. PTMs of CyPD have been linked to pore formation, and protein kinases like AKT, cdk5, ERK, PKA, PKC, and PKG can facilitate CyPD interaction with the MPTP through Ser/Thr phosphorylation (Gerle, 2016; Carraro et al., 2018; Karch et al., 2019; Gerle, 2020).
3 Pathophysiological implications
Biological cell systems rely on uninterrupted directional flow through metabolic pathways to convert organic molecules into structural entities and energy supply from the mitochondrion. Maintaining electroneutrality within the mitochondrion is essential for regulating the electric field and mitochondrial membrane potential, which is achieved through the prompt exchange of metabolites by carriers or transporters. Mitochondria perform several key cellular activities, including ATP generation (Wang and Youle, 2009), Ca2+ and ROS homeostasis (Bernardi, 1999; Murphy, 2009), cell death, and ion transport metabolism (Tzameli, 2012). Dysfunction in these activities has been associated with various pathophysiological conditions, such as cancer, neurodegenerative, cardiovascular, inflammatory, metabolic diseases and others. Numerous studies have demonstrated the implications of mitochondria in cardiovascular (Limongelli et al., 2012), neurodegenerative (Karbowski and Neutzner, 2012), inflammatory (Vringer and Tait, 2019), and metabolic diseases (Joseph et al., 2012). Given the crucial role of mitochondrial transporters and channels in modulation of mitochondrial activities, their dysregulation may contribute to pathophysiological implications observed. Here, we briefly discuss the involvement of mitochondrial channels and transporters in pathophysiology.
Mitochondria have been implicated in cancer progression, exhibiting several cancer hallmarks, including increased cellular proliferation, cell death resistance, induction of angiogenesis, and metabolic reprogramming. The outer mitochondrial anion channel, VDAC, is overexpressed in cancerous cells and is associated with hexokinase 1 (HK1) and hexokinase 2 (HK2) (Wolf et al., 2011; Rosa and César, 2016; Shoshan-Barmatz et al., 2019). VDAC also plays a role in apoptosis, as its interaction with the BAK/BAX complex results an increase in VDAC pore size, leading to the release of cytochrome c and triggering apoptosis (Banerjee and Ghosh, 2004). VDAC opening is also responsible for the release of mtDNA fragments and the onset of lupus-like disease (Kim et al., 2019). Additionally, UCPs, which are major antioxidants in mitochondria, are involved in cancer by favoring oxidative metabolism and AMPK activation in cancerous cells (Robbins and Zhao, 2011). Furthermore, members of the CLIC family, CLIC1 to CLIC6 are also involved in pathological conditions, like cancer initiation and progression (Hernandez-Fernaud et al., 2017), cardiac dysfunction (Takano et al., 2012), and Alzheimer’s Disease (AD) (Milton et al., 2008). For instance, CLIC5 is the first Cl−channel discovered in the IMM (Ponnalagu et al., 2016), is expressed in hepatocellular carcinoma and participates in the invasion and migration of tumors (Flores-Téllez et al., 2015). CLIC5 is also overexpressed in acute lymphoblastic leukemia (Neveu et al., 2016). The ubiquitous nature of the CLIC proteins and their involvement in various physiological processes make them a potential target for studying pathological conditions, including cancer. Another ion exchange molecule, LETM1 is also found to be highly expressed in various human malignancies with low survival rates such as lung, ovary, colon, breast, stomach and esophagus (Piao et al., 2019; Wang et al., 2020; Piao et al., 2020). Additionally, reduced NCLX levels are associated with human colorectal tumors, indicating that the regulation of mCa2+ can be a novel therapeutic approach in metastatic colorectal cancer (Pathak et al., 2020).
Mitochondrial dysfunction and disrupted Ca2+ homeostasis play significant roles in brain and neurodegenerative diseases. Our previous research has demonstrated that impaired mCa2+ efflux contributes to mCa2+ overload, accelerating the progression of Alzheimer’s disease (AD) and leading to cognitive decline in 3xTg-AD mouse models expressing amyloid beta (Aβ) and tau proteins. Notably, restoring neuronal expression of NCLX in 3xTg-AD mice effectively mitigates cognitive deficits and neuropathology (Jadiya et al., 2019). Moreover, our recent findings reveal that the loss of neuronal NCLX expression induces AD-like dysfunction in aged mice without any genetic predisposition to AD or neurodegenerative conditions (Jadiya et al., 2023). This dysfunction includes memory impairment, accumulation of Aβ plaques, tau hyperphosphorylation, oxidative stress, and synaptic integrity loss (Jadiya et al., 2023). These observations highlight the significant contribution of neuronal NCLX loss and resulting mCa2+ overload to neurodegenerative pathology and age-associated cognitive decline. Furthermore, our study demonstrates that specific deletion of MCU, a protein responsible for mCa2+ uptake, in 3xTg-AD mice reduces Aβ and tau pathology, synaptic dysfunction, and cognitive decline (Jadiya et al., 2021). Additionally, recent investigations reveal that a loss-of-function mutation in NCLX is associated with severe mental retardation and that the absence of NCLX leads to synaptic dysfunction and deficits in long-term plasticity (Stavsky et al., 2021). In addition, phosphorylation of NCLX by protein kinase A (PKA) has been found to enhance its ability to facilitate the efflux of mCa2+, effectively preventing the degeneration of neurons lacking PINK1, a cellular model of Parkinson’s disease (PD) (Kostic et al., 2015). Interestingly, a recent study has unveiled that increased mCa2+ uptake, mediated by ERK1/2-dependent upregulation of MCU, contributes to dendritic injury in a model of late-onset familial PD harboring a mutation in Leucine-Rich Repeat Kinase 2 (LRRK2). In various studies, the inhibition of MPTP has shown beneficial effects in mitigating neuronal cell death caused by glutamate excitotoxicity, premature aging, traumatic brain injury, ischemia-reperfusion injury, hepatic IR injury, Parkinson’s Disease, and Alzheimer’s Disease (Uchino et al., 1995; Schinder et al., 1996; Caspersen et al., 2005; Du et al., 2008; Hånell et al., 2015; Morciano et al., 2017; Ludtmann et al., 2018; Panel et al., 2019; Zhou et al., 2019). These findings highlight the potential therapeutic implications of targeting MPTP in neurodegenerative conditions. Furthermore, we have revealed reduced expression of proteins associated with mitochondrial calcium uptake, such as MICU1 and MCUB, in the frontal cortex of both sporadic AD patients and 3xTg-AD mice (Jadiya et al., 2019). Loss of MICU1 due to homozygous deletion of exon 1 has been implicated in sporadic neurological and muscular disorders characterized by mCa2+ overload, impaired metabolism, early muscle weakness, myofiber damage, cognitive impairment, and extrapyramidal movement disorders (Logan et al., 2014; Lewis-Smith et al., 2016; Debattisti et al., 2019). MICU1 variants have also been associated with congenital brain malformations featuring white matter abnormalities, cerebellar dysplasia, and acute encephalopathy (Wilton et al., 2020). Additionally, patients carrying nonsense mutations in the MICU1 gene exhibit myopathy accompanied by extrapyramidal symptoms (Kohlschmidt et al., 2021). Studies in mice with Micu1 deletion have further demonstrated significant ataxia and muscular defects (Antony et al., 2016; Liu et al., 2016). Additionally, reduced expression levels of UCP2, UCP4, and UCP5 have been observed in patients with AD, which upregulates nitric oxide synthases and compromises mitochondrial functions (de la Monte and Wands, 2006; Finoshin et al.). Moreover, LETM1, a contributing factor in PD and epilepsy, plays a role in calcium handling through constitutive phosphorylation by PINK1 under normal physiological conditions (Huang et al., 2017). In epileptic patients, reduced expression of LETM1 is observed, leading to mitochondrial swelling and increased seizure duration and frequency (Zhang et al., 2014). Collectively, these findings provide compelling evidence linking dysregulation of mCa2+ exchange to various neurodegenerative diseases.
Dysregulated Ca2+ handling in mitochondria is also implicated in various cardiovascular diseases such as cardiac hypertrophy, cardiomyopathies, arrythmia, myocardial infarction, and cardiac IR injury (Bonora et al., 2019). MCU/mtCU plays a crucial role in the heart’s ability to respond to acute increases in workload and regulates the opening of the mitochondrial permeability transition pore (Kwong et al., 2015; Luongo et al., 2015). Several studies report that all subunits/partners of mtCU complex assist in maintaining physiological Ca2+ levels in cells. MCUB, a negative regulator of mtCU complex has been found to protect against cardiac injury by reducing Ca2+ influx in mitochondria and inhibiting MPTP opening after IR injury to lessen pathological remodeling (Lambert et al., 2019; Huo et al., 2020). Another important component is MCUR1, which binds to MCU and EMRE to serve as a scaffold protein for the assembly of the MCU complex. We have reported that mouse cardiomyocytes and endothelial cells lacking MCUR1 exhibit severe impairment in mCa2+ uptake (Tomar et al., 2016). Collectively, these studies highlight the essential role of the MCU transporter in facilitating cardiac adaptation to acute physiological stress.
Diabetes mellitus is a growing healthcare disease worldwide that affects multiple organs and tissues, leading to multiple pathologies, such as cardiovascular disease, leading to structural and functional abnormalities in the absence of major coronary artery disease, hypertension, a valvular or neuromuscular disease called diabetic cardiomyopathy (Rubler et al., 1972). Studies have shown increased levels of MCUB protein and decreased levels of MCU protein in the hearts of mouse models of both type 1 (Suarez et al., 2018) and type 2 (Bækkerud et al., 2019) diabetes as well as in mouse neonatal cardiomyocytes exposed to hyperglycemia (Diaz-Juarez et al., 2016). Furthermore, we have shown that CRISPR/Cas9 mediated deletion of MCU in mouse liver and in Danio rerio (zebrafish) prevents mCa2+ uptake, delays cytosolic Ca2+ clearance, impairs OXPHOS, and leads to hepatic lipid accumulation through dephosphorylation of AMPK by protein phosphatase-4 (Tomar et al., 2016). These findings highlight the involvement of mCa2+ handling in the pathogenesis of diabetes-related complications.
Mitochondrial dysfunction is commonly associated with inflammatory responses and is implicated in the development of various diseases. Mitochondrial transporters play a crucial role in modulating inflammatory responses (Ball et al., 2011). For example, the activation of the NLRP3 inflammasome complex via the VDAC indicates its involvement in the inflammatory response (Zhou et al., 2011). UCP2, another mitochondrial transporter, is involved in regulating the inflammatory response by increasing ROS levels in macrophages (Ball et al., 2011). Downregulation of UCP2 is associated with a pro-inflammatory response in autoimmune encephalomyelitis, where T-cell proliferation and B-cell response is increased in Ucp2−/− mice (Vogler et al., 2006). CLICs, when translocated to the cellular membrane, enhance inflammasome assembly, interleukin-1β release, and caspase-1 activation, contributing to inflammation. (Tang et al., 2017). Additionally, elevated levels of myocardial UCP2 under hypoxic conditions can induce ischemic insult (Tian et al., 2018).
Mitochondrial channels and transporters play significant roles in both physiological and pathophysiological conditions. However, due to their dual conductance states (open and closed), establishing a clear structure-function relationship during disease conditions can be challenging. Further studies are necessary to understand the nature of their conductance states in healthy and diseased states. Such research is essential for designing potential therapeutics and advancing medical science.
4 Future outlook of mitochondrial transporters or channels: avenues for the development of potential therapeutic target
Mitochondrial transporters and channels play a critical role in maintaining cellular homeostasis, cell death, and signaling. As carriers of metabolites, they help maintain the balance between energy supply and demand, making them crucial determinants of a cell’s survival. Although the functional and biophysical characteristics of these transporters have been studied extensively, their molecular identity and regulatory mechanisms at the molecular level remains largely unknown. Understanding how these channels are regulated could lead to the identification of promising therapeutic targets for mitochondria-related diseases. One major challenge in targeting PTMs associated with channels is that they share biophysical properties with plasma membrane channels. However, a molecular understanding of channels during both physiological and disease states can provide insight into designing new therapeutic approaches for various diseases such as cancer, diabetes, cardiovascular, and neurodegenerative diseases. Research efforts should be directed towards exploring the structural conformations of these channels, their transport mechanisms, and the identification of functional interactors. Such studies could shed light on the molecular mechanisms underlying mitochondrial transporters and channels and pave the way for the development of new and effective treatments for a range of diseases.
Author contributions
Conceptualization, PJ and DT; writing–original draft preparation, AK; writing–review and editing, PJ and DT; funding acquisition, PJ and DT. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by National Institute of Diabetes and Digestive and Kidney Diseases grant number R00DK120876, Harold S. Geneen Charitable Trust Awards Program, and Wake Startup Fund to DT; National Institute on Aging grant numbers R00AG065445, Wake ADRC REC and development grant (P30 AG072947), and Wake Startup Fund to PJ; and American Heart Association (AHA) post-doctoral fellowship award 23POST1020344 to AK.
Acknowledgments
Figures are created with BioRender.com.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abudu, Y. P., Shrestha, B. K., Zhang, W., Palara, A., Brenne, H. B., Larsen, K. B., et al. (2021). SAMM50 acts with p62 in piecemeal basal- and OXPHOS-induced mitophagy of SAM and MICOS components. J. Cell. Biol. 220 (8), e202009092. doi:10.1083/jcb.202009092
Adebayo, O. L., Adenuga, G. A., and Sandhir, R. (2016). Selenium and zinc protect brain mitochondrial antioxidants and electron transport chain enzymes following postnatal protein malnutrition. Life Sci. 152, 145–155. doi:10.1016/j.lfs.2016.03.008
Alves-Figueiredo, H., Silva-Platas, C., Lozano, O., Vázquez-Garza, E., Guerrero-Beltrán, C. E., Zarain-Herzberg, A., et al. (2021). A systematic review of post-translational modifications in the mitochondrial permeability transition pore complex associated with cardiac diseases. Biochimica Biophysica Acta (BBA) - Mol. Basis Dis. 1867 (1), 165992. doi:10.1016/j.bbadis.2020.165992
Anand, R., Langer, T., and Baker, M. J. (2013). Proteolytic control of mitochondrial function and morphogenesis. Biochim. Biophys. Acta 1833 (1), 195–204. doi:10.1016/j.bbamcr.2012.06.025
Anflous, K., Armstrong, D. D., and Craigen, W. J. (2001). Altered mitochondrial sensitivity for ADP and maintenance of creatine-stimulated respiration in oxidative striated muscles from VDAC1-deficient mice. J. Biol. Chem. 276 (3), 1954–1960. doi:10.1074/jbc.M006587200
Antony, A. N., Paillard, M., Moffat, C., Juskeviciute, E., Correnti, J., Bolon, B., et al. (2016). MICU1 regulation of mitochondrial Ca(2+) uptake dictates survival and tissue regeneration. Nat. Commun. 7, 10955. doi:10.1038/ncomms10955
Aon, M. A., Cortassa, S., Maack, C., and O'Rourke, B. (2007). Sequential opening of mitochondrial ion channels as a function of glutathione redox thiol status. J. Biol. Chem. 282 (30), 21889–21900. doi:10.1074/jbc.M702841200
Ardail, D., Lerme, F., and Louisot, P. (1991). Involvement of contact sites in phosphatidylserine import into liver mitochondria. J. Biol. Chem. 266 (13), 7978–7981. doi:10.1016/S0021-9258(18)92926-1
Ashley, R. H. (2003). Challenging accepted ion channel biology: p64 and the CLIC family of putative intracellular anion channel proteins (review). Mol. Membr. Biol. 20 (1), 1–11. doi:10.1080/09687680210042746
Austin, S., Mekis, R., Mohammed, S. E. M., Scalise, M., Pfeiffer, C., Galluccio, M., et al. (2021). MICS1 is the Ca2+/H+ antiporter of mammalian mitochondria. bioRxiv. 468204. doi:10.1101/2021.11.11.468204
Austin, S., Mekis, R., Mohammed, S. E. M., Scalise, M., Wang, W. A., Galluccio, M., et al. (2022). TMBIM5 is the Ca(2+)/H(+) antiporter of mammalian mitochondria. EMBO Rep. 23 (12), e54978. doi:10.15252/embr.202254978
Austin, S., and Nowikovsky, K. (2019). LETM1: Essential for mitochondrial biology and cation homeostasis? Trends Biochem. Sci. 44 (8), 648–658. doi:10.1016/j.tibs.2019.04.002
Austin, S., Tavakoli, M., Pfeiffer, C., Seifert, J., Mattarei, A., De Stefani, D., et al. (2017). LETM1-Mediated K(+) and Na(+) homeostasis regulates mitochondrial Ca(2+) efflux. Front. Physiol. 8, 839. doi:10.3389/fphys.2017.00839
Azzu, V., and Brand, M. D. (2010). The on-off switches of the mitochondrial uncoupling proteins. Trends Biochem. Sci. 35 (5), 298–307. doi:10.1016/j.tibs.2009.11.001
Bachmann, M., Pontarin, G., and Szabo, I. (2019). The contribution of mitochondrial ion channels to cancer development and progression. Cell. Physiol. Biochem. 53 (S1), 63–78. doi:10.33594/000000198
Baker, B. M., and Haynes, C. M. (2011). Mitochondrial protein quality control during biogenesis and aging. Trends Biochem. Sci. 36 (5), 254–261. doi:10.1016/j.tibs.2011.01.004
Bækkerud, F. H., Salerno, S., Ceriotti, P., Morland, C., Storm-Mathisen, J., Bergersen, L. H., et al. (2019). High intensity interval training ameliorates mitochondrial dysfunction in the left ventricle of mice with type 2 diabetes. Cardiovasc Toxicol. 19 (5), 422–431. doi:10.1007/s12012-019-09514-z
Ball, W. B., Kar, S., Mukherjee, M., Chande, A. G., Mukhopadhyaya, R., and Das, P. K. (2011). Uncoupling protein 2 negatively regulates mitochondrial reactive oxygen species generation and induces phosphatase-mediated anti-inflammatory response in experimental visceral leishmaniasis. J. Immunol. 187 (3), 1322–1332. doi:10.4049/jimmunol.1004237
Banerjee, J., and Ghosh, S. (2004). Bax increases the pore size of rat brain mitochondrial voltage-dependent anion channel in the presence of tBid. Biochem. Biophys. Res. Commun. 323 (1), 310–314. doi:10.1016/j.bbrc.2004.08.094
Baughman, J. M., Perocchi, F., Girgis, H. S., Plovanich, M., Belcher-Timme, C. A., Sancak, Y., et al. (2011). Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476 (7360), 341–345. doi:10.1038/nature10234
Beavis, A. D. (1992). Properties of the inner membrane anion channel in intact mitochondria. J. Bioenerg. Biomembr. 24 (1), 77–90. doi:10.1007/bf00769534
Beltrao, P., Bork, P., Krogan, N. J., and van Noort, V. (2013). Evolution and functional cross-talk of protein post-translational modifications. Mol. Syst. Biol. 9, 714. doi:10.1002/msb.201304521
Bernardi, P. (1999). Mitochondrial transport of cations: Channels, exchangers, and permeability transition. Physiol. Rev. 79 (4), 1127–1155. doi:10.1152/physrev.1999.79.4.1127
Bodnar, N. O., and Rapoport, T. A. (2017). Molecular mechanism of substrate processing by the Cdc48 ATPase complex. Cell. 169 (4), 722–735. doi:10.1016/j.cell.2017.04.020
Böhm, R., Amodeo, G. F., Murlidaran, S., Chavali, S., Wagner, G., Winterhalter, M., et al. (2020). The structural basis for low conductance in the membrane protein VDAC upon β-NADH binding and voltage gating. Structure 28 (2), 206–214. doi:10.1016/j.str.2019.11.015
Bondarenko, A. I., Parichatikanond, W., Madreiter, C. T., Rost, R., Waldeck-Weiermair, M., Malli, R., et al. (2015). UCP2 modulates single-channel properties of a MCU-dependent Ca(2+) inward current in mitochondria. Pflugers Arch. 467 (12), 2509–2518. doi:10.1007/s00424-015-1727-z
Bonora, M., Wieckowski, M. R., Sinclair, D. A., Kroemer, G., Pinton, P., and Galluzzi, L. (2019). Targeting mitochondria for cardiovascular disorders: Therapeutic potential and obstacles. Nat. Rev. Cardiol. 16 (1), 33–55. doi:10.1038/s41569-018-0074-0
Brenner, C., Subramaniam, K., Pertuiset, C., and Pervaiz, S. (2011). Adenine nucleotide translocase family: Four isoforms for apoptosis modulation in cancer. Oncogene 30 (8), 883–895. doi:10.1038/onc.2010.501
Brustovetsky, N., and Klingenberg, M. (1996). Mitochondrial ADP/ATP carrier can be reversibly converted into a large channel by Ca2+. Biochemistry 35 (26), 8483–8488. doi:10.1021/bi960833v
Busch, K. B. (2020). Inner mitochondrial membrane compartmentalization: Dynamics across scales. Int. J. Biochem. Cell. Biol. 120, 105694. doi:10.1016/j.biocel.2020.105694
Calvo, S. E., Clauser, K. R., and Mootha, V. K. (2016). MitoCarta2.0: An updated inventory of mammalian mitochondrial proteins. Nucleic Acids Res. 44 (D1), D1251–D1257. doi:10.1093/nar/gkv1003
Camello, C., Lomax, R., Petersen, O. H., and Tepikin, A. V. (2002). Calcium leak from intracellular stores-the enigma of calcium signalling. Cell. Calcium 32 (5-6), 355–361. doi:10.1016/s0143416002001926
Carraro, M., Checchetto, V., Sartori, G., Kucharczyk, R., di Rago, J. P., Minervini, G., et al. (2018). High-Conductance Channel Formation in Yeast Mitochondria is Mediated by F-ATP Synthase e and g Subunits. Cell. Physiol. Biochem. 50 (5), 1840–1855. doi:10.1159/000494864
Caspersen, C., Wang, N., Yao, J., Sosunov, A., Chen, X., Lustbader, J. W., et al. (2005). Mitochondrial abeta: A potential focal point for neuronal metabolic dysfunction in Alzheimer's disease. FASEB J. 19 (14), 2040–2041. doi:10.1096/fj.05-3735fje
Chalmers, S., and Nicholls, D. G. (2003). The relationship between free and total calcium concentrations in the matrix of liver and brain mitochondria. J. Biol. Chem. 278 (21), 19062–19070. doi:10.1074/jbc.M212661200
Checchetto, V., Leanza, L., De Stefani, D., Rizzuto, R., Gulbins, E., and Szabo, I. (2021). Mitochondrial K(+) channels and their implications for disease mechanisms. Pharmacol. Ther. 227, 107874. doi:10.1016/j.pharmthera.2021.107874
Cheng, E. H., Sheiko, T. V., Fisher, J. K., Craigen, W. J., and Korsmeyer, S. J. (2003). VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science 301 (5632), 513–517. doi:10.1126/science.1083995
Chipuk, J. E., and Green, D. R. (2008). How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell. Biol. 18 (4), 157–164. doi:10.1016/j.tcb.2008.01.007
Cho, H. M., Ryu, J. R., Jo, Y., Seo, T. W., Choi, Y. N., Kim, J. H., et al. (2019). Drp1-Zip1 interaction regulates mitochondrial quality surveillance system. Mol. Cell. 73 (2), 364–376. doi:10.1016/j.molcel.2018.11.009
Chouchani, E. T., Kazak, L., Jedrychowski, M. P., Lu, G. Z., Erickson, B. K., Szpyt, J., et al. (2016). Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1. Nature 532 (7597), 112–116. doi:10.1038/nature17399
Choudhary, O. P., Paz, A., Adelman, J. L., Colletier, J. P., Abramson, J., Grabe, M., et al. (2014). Structure-guided simulations illuminate the mechanism of ATP transport through VDAC1. Nat. Struct. Mol. Biol. 21 (7), 626–632. doi:10.1038/nsmb.2841
Colombini, M. (2012). VDAC structure, selectivity, and dynamics. Biochim. Biophys. Acta 1818 (6), 1457–1465. doi:10.1016/j.bbamem.2011.12.026
Davies, K. M., Anselmi, C., Wittig, I., Faraldo-Gómez, J. D., and Kühlbrandt, W. (2012). Structure of the yeast F1Fo-ATP synthase dimer and its role in shaping the mitochondrial cristae. Proc. Natl. Acad. Sci. U. S. A. 109 (34), 13602–13607. doi:10.1073/pnas.1204593109
de la Monte, S. M., and Wands, J. R. (2006). Molecular indices of oxidative stress and mitochondrial dysfunction occur early and often progress with severity of Alzheimer's disease. J. Alzheimers Dis. 9 (2), 167–181. doi:10.3233/jad-2006-9209
De Pinto, V., Reina, S., Gupta, A., Messina, A., and Mahalakshmi, R. (2016). Role of cysteines in mammalian VDAC isoforms' function. Biochim. Biophys. Acta 1857 (8), 1219–1227. doi:10.1016/j.bbabio.2016.02.020
De Pinto, V. (2021). Renaissance of VDAC: New insights on a protein family at the interface between mitochondria and cytosol. Biomolecules 11 (1), 107. doi:10.3390/biom11010107
De Stefani, D., Patron, M., and Rizzuto, R. (2015). Structure and function of the mitochondrial calcium uniporter complex. Biochim. Biophys. Acta 1853 (9), 2006–2011. doi:10.1016/j.bbamcr.2015.04.008
De Stefani, D., Raffaello, A., Teardo, E., Szabo, I., and Rizzuto, R. (2011). A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476 (7360), 336–340. doi:10.1038/nature10230
De Vos, K. J., Mórotz, G. M., Stoica, R., Tudor, E. L., Lau, K. F., Ackerley, S., et al. (2012). VAPB interacts with the mitochondrial protein PTPIP51 to regulate calcium homeostasis. Hum. Mol. Genet. 21 (6), 1299–1311. doi:10.1093/hmg/ddr559
Debattisti, V., Horn, A., Singh, R., Seifert, E. L., Hogarth, M. W., Mazala, D. A., et al. (2019). Dysregulation of mitochondrial Ca(2+) uptake and sarcolemma repair underlie muscle weakness and wasting in patients and mice lacking MICU1. Cell. Rep. 29 (5), 1274–1286. doi:10.1016/j.celrep.2019.09.063
Dejean, L. M., Martinez-Caballero, S., Guo, L., Hughes, C., Teijido, O., Ducret, T., et al. (2005). Oligomeric Bax is a component of the putative cytochrome c release channel MAC, mitochondrial apoptosis-induced channel. Mol. Biol. Cell. 16 (5), 2424–2432. doi:10.1091/mbc.e04-12-1111
Diaz-Juarez, J., Suarez, J., Cividini, F., Scott, B. T., Diemer, T., Dai, A., et al. (2016). Expression of the mitochondrial calcium uniporter in cardiac myocytes improves impaired mitochondrial calcium handling and metabolism in simulated hyperglycemia. Am. J. Physiol. Cell. Physiol. 311 (6), C1005–C1013. doi:10.1152/ajpcell.00236.2016
Dimmer, K. S., Papić, D., Schumann, B., Sperl, D., Krumpe, K., Walther, D. M., et al. (2012). A crucial role for Mim2 in the biogenesis of mitochondrial outer membrane proteins. J. Cell. Sci. 125 (14), 3464–3473. doi:10.1242/jcs.103804
Diness, J. G., Kirchhoff, J. E., Sheykhzade, M., Jespersen, T., and Grunnet, M. (2015). Antiarrhythmic effect of either negative modulation or blockade of small conductance Ca2+-activated K+ channels on ventricular fibrillation in Guinea pig langendorff-perfused heart. J. Cardiovasc Pharmacol. 66 (3), 294–299. doi:10.1097/fjc.0000000000000278
Ding, C., Wu, Z., Huang, L., Wang, Y., Xue, J., Chen, S., et al. (2015). Mitofilin and CHCHD6 physically interact with Sam50 to sustain cristae structure. Sci. Rep. 5 (1), 16064. doi:10.1038/srep16064
Doan, K. N., Grevel, A., Mårtensson, C. U., Ellenrieder, L., Thornton, N., Wenz, L. S., et al. (2020). The mitochondrial import complex MIM functions as main translocase for α-helical outer membrane proteins. Cell. Rep. 31 (4), 107567. doi:10.1016/j.celrep.2020.107567
Dolezal, P., Likic, V., Tachezy, J., and Lithgow, T. (2006). Evolution of the molecular machines for protein import into mitochondria. Science 313 (5785), 314–318. doi:10.1126/science.1127895
Dong, Z., Shanmughapriya, S., Tomar, D., Siddiqui, N., Lynch, S., Nemani, N., et al. (2017). Mitochondrial Ca(2+) uniporter is a mitochondrial luminal redox sensor that augments MCU channel activity. Mol. Cell. 65 (6), 1014–1028. doi:10.1016/j.molcel.2017.01.032
Doyle, H. A., and Mamula, M. J. (2001). Post-translational protein modifications in antigen recognition and autoimmunity. Trends Immunol. 22 (8), 443–449. doi:10.1016/s1471-4906(01)01976-7
Du, H., Guo, L., Fang, F., Chen, D., Sosunov, A. A., McKhann, G. M., et al. (2008). Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer's disease. Nat. Med. 14 (10), 1097–1105. doi:10.1038/nm.1868
Esposito, M., Hermann-Le Denmat, S., and Delahodde, A. (2019). Contribution of ERMES subunits to mature peroxisome abundance. PLOS ONE 14 (3), e0214287. doi:10.1371/journal.pone.0214287
Fernández-Salas, E., Sagar, M., Cheng, C., Yuspa, S. H., and Weinberg, W. C. (1999). p53 and tumor necrosis factor alpha regulate the expression of a mitochondrial chloride channel protein. J. Biol. Chem. 274 (51), 36488–36497. doi:10.1074/jbc.274.51.36488
Fernández-Salas, E., Suh, K. S., Speransky, V. V., Bowers, W. L., Levy, J. M., Adams, T., et al. (2002). mtCLIC/CLIC4, an organellular chloride channel protein, is increased by DNA damage and participates in the apoptotic response to p53. Mol. Cell. Biol. 22 (11), 3610–3620. doi:10.1128/mcb.22.11.3610-3620.2002
Finoshin, A. D., Adameyko, K. I., Mikhailov, K. V., Kravchuk, O. I., Georgiev, A. A., Gornostaev, N. G., et al. (2020). Iron metabolic pathways in the processes of sponge plasticity. PLOS ONE 15 (2), e0228722. doi:10.1371/journal.pone.0228722
Flores-Téllez, T. N. J., Lopez, T. V., Vásquez Garzón, V. R., and Villa-Treviño, S. (2015). Co-expression of ezrin-CLIC5-podocalyxin is associated with migration and invasiveness in hepatocellular carcinoma. PLOS ONE 10 (7), e0131605. doi:10.1371/journal.pone.0131605
Frazier, A. E., Thorburn, D. R., and Compton, A. G. (2019). Mitochondrial energy generation disorders: Genes, mechanisms, and clues to pathology. J. Biol. Chem. 294 (14), 5386–5395. doi:10.1074/jbc.R117.809194
Gatliff, J., East, D., Crosby, J., Abeti, R., Harvey, R., Craigen, W., et al. (2014). TSPO interacts with VDAC1 and triggers a ROS-mediated inhibition of mitochondrial quality control. Autophagy 10 (12), 2279–2296. doi:10.4161/15548627.2014.991665
Gerle, C. (2020). Mitochondrial F-ATP synthase as the permeability transition pore. Pharmacol. Res. 160, 105081. doi:10.1016/j.phrs.2020.105081
Gerle, C. (2016). On the structural possibility of pore-forming mitochondrial FoF1 ATP synthase. Biochim. Biophys. Acta 1857 (8), 1191–1196. doi:10.1016/j.bbabio.2016.03.008
Gómez-Serrano, M., Camafeita, E., Loureiro, M., and Peral, B. (2018). Mitoproteomics: Tackling mitochondrial dysfunction in human disease. Oxid. Med. Cell. Longev. 2018, 1435934. doi:10.1155/2018/1435934
Gout, E., Rébeillé, F., Douce, R., and Bligny, R. (2014). Interplay of Mg2+, ADP, and ATP in the cytosol and mitochondria: Unravelling the role of Mg2+ in cell respiration. Proc. Natl. Acad. Sci. U. S. A. 111 (43), E4560–E4567. doi:10.1073/pnas.1406251111
Gray, M. W. (2001). “Mitochondrial genome,” in Encyclopedia of genetics. Editors S. Brenner, and J. H. Miller (New York: Academic Press), 1220–1222.
Guan, L., Che, Z., Meng, X., Yu, Y., Li, M., Yu, Z., et al. (2019). MCU Up-regulation contributes to myocardial ischemia-reperfusion Injury through calpain/OPA-1-mediated mitochondrial fusion/mitophagy Inhibition. J. Cell. Mol. Med. 23 (11), 7830–7843. doi:10.1111/jcmm.14662
Guan, Z., Yan, L., Wang, Q., Qi, L., Hong, S., Gong, Z., et al. (2021). Structural insights into assembly of human mitochondrial translocase TOM complex. Cell. Discov. 7 (1), 22. doi:10.1038/s41421-021-00252-7
Gunter, T. E., and Pfeiffer, D. R. (1990). Mechanisms by which mitochondria transport calcium. Am. J. Physiol. 258 (51), C755–C786. doi:10.1152/ajpcell.1990.258.5.C755
Guo, D., Xie, W., Xiong, P., Li, H., Wang, S., Chen, G., et al. (2018). Cyclin-dependent kinase 5-mediated phosphorylation of chloride intracellular channel 4 promotes oxidative stress-induced neuronal death. Cell. Death Dis. 9 (10), 951. doi:10.1038/s41419-018-0983-1
Gururaja Rao, S., Ponnalagu, D., Patel, N. J., and Singh, H. (2018). Three decades of chloride intracellular channel proteins: From organelle to organ physiology. Curr. Protoc. Pharmacol. 80 (1), 11. doi:10.1002/cpph.36
Gururaja Rao, S., Ponnalagu, D., Sukur, S., Singh, H., Sanghvi, S., Mei, Y., et al. (2017). Identification and characterization of a bacterial homolog of chloride intracellular channel (CLIC) protein. Sci. Rep. 7 (1), 8500. doi:10.1038/s41598-017-08742-z
Gutiérrez-Aguilar, M., Douglas, D. L., Gibson, A. K., Domeier, T. L., Molkentin, J. D., and Baines, C. P. (2014). Genetic manipulation of the cardiac mitochondrial phosphate carrier does not affect permeability transition. J. Mol. Cell. Cardiol. 72, 316–325. doi:10.1016/j.yjmcc.2014.04.008
Hånell, A., Greer, J. E., McGinn, M. J., and Povlishock, J. T. (2015). Traumatic brain injury-induced axonal phenotypes react differently to treatment. Acta Neuropathol. 129 (2), 317–332. doi:10.1007/s00401-014-1376-x
Hernández-Alvarez, M. I., Sebastián, D., Vives, S., Ivanova, S., Bartoccioni, P., Kakimoto, P., et al. (2019). Deficient endoplasmic reticulum-mitochondrial phosphatidylserine transfer causes liver disease. Cell. 177 (4), 881–895. doi:10.1016/j.cell.2019.04.010
Hernandez-Fernaud, J. R., Ruengeler, E., Casazza, A., Neilson, L. J., Pulleine, E., Santi, A., et al. (2017). Secreted CLIC3 drives cancer progression through its glutathione-dependent oxidoreductase activity. Nat. Commun. 8 (1), 14206. doi:10.1038/ncomms14206
Herrmann, J. M., and Neupert, W. (2013). “Protein import into mitochondria,” in Encyclopedia of biological chemistry. Editors W. J. Lennarz, and M. D. Lane (Waltham: Academic Press), 632–636.
Hirabayashi, Y., Kwon, S.-K., Paek, H., Pernice, W. M., Paul, M. A., Lee, J., et al. (2017). ER-mitochondria tethering by PDZD8 regulates Ca2+ dynamics in mammalian neurons. Science 358 (6363), 623–630. doi:10.1126/science.aan6009
Hodge, T., and Colombini, M. (1997). Regulation of metabolite flux through voltage-gating of VDAC channels. J. Membr. Biol. 157 (3), 271–279. doi:10.1007/s002329900235
Hollville, E., Carroll, R. G., Cullen, S. P., and Martin, S. J. (2014). Bcl-2 family proteins participate in mitochondrial quality control by regulating Parkin/PINK1-dependent mitophagy. Mol. Cell. 55 (3), 451–466. doi:10.1016/j.molcel.2014.06.001
Hong, Z., Chen, K. H., DasGupta, A., Potus, F., Dunham-Snary, K., Bonnet, S., et al. (2017). MicroRNA-138 and MicroRNA-25 down-regulate mitochondrial calcium uniporter, causing the pulmonary arterial hypertension cancer phenotype. Am. J. Respir. Crit. Care Med. 195 (4), 515–529. doi:10.1164/rccm.201604-0814OC
Honrath, B., Matschke, L., Meyer, T., Magerhans, L., Perocchi, F., Ganjam, G. K., et al. (2017). SK2 channels regulate mitochondrial respiration and mitochondrial Ca(2+) uptake. Cell. Death Differ. 24 (5), 761–773. doi:10.1038/cdd.2017.2
Hoppins, S., Collins, S. R., Cassidy-Stone, A., Hummel, E., Devay, R. M., Lackner, L. L., et al. (2011). A mitochondrial-focused genetic interaction map reveals a scaffold-like complex required for inner membrane organization in mitochondria. J. Cell. Biol. 195 (2), 323–340. doi:10.1083/jcb.201107053
Horie, C., Suzuki, H., Sakaguchi, M., and Mihara, K. (2003). Targeting and assembly of mitochondrial tail-anchored protein Tom5 to the TOM complex depend on a signal distinct from that of tail-anchored proteins dispersed in the membrane. J. Biol. Chem. 278 (42), 41462–41471. doi:10.1074/jbc.M307047200
Horvath, S. E., and Daum, G. (2013). Lipids of mitochondria. Prog. Lipid Res. 52 (4), 590–614. doi:10.1016/j.plipres.2013.07.002
Hoshino, A., Wang, W. J., Wada, S., McDermott-Roe, C., Evans, C. S., Gosis, B., et al. (2019). The ADP/ATP translocase drives mitophagy independent of nucleotide exchange. Nature 575 (7782), 375–379. doi:10.1038/s41586-019-1667-4
Hsu, P., Liu, X., Zhang, J., Wang, H.-G., Ye, J.-M., and Shi, Y. (2015). Cardiolipin remodeling by TAZ/tafazzin is selectively required for the initiation of mitophagy. Autophagy 11 (4), 643–652. doi:10.1080/15548627.2015.1023984
Huang, E., Qu, D., Huang, T., Rizzi, N., Boonying, W., Krolak, D., et al. (2017). PINK1-mediated phosphorylation of LETM1 regulates mitochondrial calcium transport and protects neurons against mitochondrial stress. Nat. Commun. 8 (1), 1399. doi:10.1038/s41467-017-01435-1
Humphries, A. D., Streimann, I. C., Stojanovski, D., Johnston, A. J., Yano, M., Hoogenraad, N. J., et al. (2005). Dissection of the mitochondrial import and assembly pathway for human Tom40. J. Biol. Chem. 280 (12), 11535–11543. doi:10.1074/jbc.M413816200
Huo, J., Lu, S., Kwong, J. Q., Bround, M. J., Grimes, K. M., Sargent, M. A., et al. (2020). MCUb induction protects the heart from postischemic remodeling. Circ. Res. 127 (3), 379–390. doi:10.1161/circresaha.119.316369
Iwasawa, R., Mahul-Mellier, A. L., Datler, C., Pazarentzos, E., and Grimm, S. (2011). Fis1 and Bap31 bridge the mitochondria-ER interface to establish a platform for apoptosis induction. Embo J. 30 (3), 556–568. doi:10.1038/emboj.2010.346
Jadiya, P., Cohen, H. M., Kolmetzky, D. W., Kadam, A. A., Tomar, D., and Elrod, J. W. (2023). Neuronal loss of NCLX-dependent mitochondrial calcium efflux mediates age-associated cognitive decline. iScience 26 (3), 106296. doi:10.1016/j.isci.2023.106296
Jadiya, P., Kolmetzky, D. W., Tomar, D., Di Meco, A., Lombardi, A. A., Lambert, J. P., et al. (2019). Impaired mitochondrial calcium efflux contributes to disease progression in models of Alzheimer's disease. Nat. Commun. 10 (1), 3885. doi:10.1038/s41467-019-11813-6
Jadiya, P., Kolmetzky, D. W., Tomar, D., Thomas, M., Khaledi, S., Garbincius, J. F., et al. (2021). Genetic ablation of neuronal mitochondrial calcium uptake halts Alzheimer's disease progression. SSRN Electron. J. doi:10.2139/ssrn.3872940
Jadiya, P., and Tomar, D. (2020). Mitochondrial protein quality control mechanisms. Genes. (Basel) 11 (5), 563. doi:10.3390/genes11050563
Javadov, S., Chapa-Dubocq, X., and Makarov, V. (2018). Different approaches to modeling analysis of mitochondrial swelling. Mitochondrion 38, 58–70. doi:10.1016/j.mito.2017.08.004
Ji, S. G., Medvedeva, Y. V., and Weiss, J. H. (2020). Zn(2+) entry through the mitochondrial calcium uniporter is a critical contributor to mitochondrial dysfunction and neurodegeneration. Exp. Neurol. 325, 113161. doi:10.1016/j.expneurol.2019.113161
Jian, F., Chen, D., Chen, L., Yan, C., Lu, B., Zhu, Y., et al. (2018). Sam50 regulates PINK1-parkin-mediated mitophagy by controlling PINK1 stability and mitochondrial morphology. Cell. Rep. 23 (10), 2989–3005. doi:10.1016/j.celrep.2018.05.015
Jiang, D., Zhao, L., and Clapham, D. E. (2009). Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca2+/H+ antiporter. Science 326 (5949), 144–147. doi:10.1126/science.1175145
Johnsen, V. L., Belke, D. D., Hughey, C. C., Hittel, D. S., Hepple, R. T., Koch, L. G., et al. (2013). Enhanced cardiac protein glycosylation (O-GlcNAc) of selected mitochondrial proteins in rats artificially selected for low running capacity. Physiol. Genomics 45 (1), 17–25. doi:10.1152/physiolgenomics.00111.2012
Joiner, M. L., Koval, O. M., Li, J., He, B. J., Allamargot, C., Gao, Z., et al. (2012). CaMKII determines mitochondrial stress responses in heart. Nature 491 (7423), 269–273. doi:10.1038/nature11444
Joseph, A. M., Joanisse, D. R., Baillot, R. G., and Hood, D. A. (2012). Mitochondrial dysregulation in the pathogenesis of diabetes: Potential for mitochondrial biogenesis-mediated interventions. Exp. Diabetes Res. 2012, 642038. doi:10.1155/2012/642038
Karbowski, M., and Neutzner, A. (2012). Neurodegeneration as a consequence of failed mitochondrial maintenance. Acta Neuropathol. 123 (2), 157–171. doi:10.1007/s00401-011-0921-0
Karch, J., Bround, M. J., Khalil, H., Sargent, M. A., Latchman, N., Terada, N., et al. (2019). Inhibition of mitochondrial permeability transition by deletion of the ANT family and CypD. Sci. Adv. 5 (8), eaaw4597. doi:10.1126/sciadv.aaw4597
Kato, H., Lu, Q., Rapaport, D., and Kozjak-Pavlovic, V. (2013). Tom70 is essential for PINK1 import into mitochondria. PLOS ONE 8 (3), e58435. doi:10.1371/journal.pone.0058435
Keinan, N., Tyomkin, D., and Shoshan-Barmatz, V. (2010). Oligomerization of the mitochondrial protein voltage-dependent anion channel is coupled to the induction of apoptosis. Mol. Cell. Biol. 30 (24), 5698–5709. doi:10.1128/mcb.00165-10
Kerner, J., Lee, K., Tandler, B., and Hoppel, C. L. (2012). VDAC proteomics: Post-translation modifications. Biochim. Biophys. Acta 1818 (6), 1520–1525. doi:10.1016/j.bbamem.2011.11.013
Khananshvili, D. (1991). Mechanism of partial reactions in the cardiac Na(+)-Ca2+ exchange system. Ann. N. Y. Acad. Sci. 639, 85–95. doi:10.1111/j.1749-6632.1991.tb17291.x
Kim, J., Gupta, R., Blanco, L. P., Yang, S., Shteinfer-Kuzmine, A., Wang, K., et al. (2019). VDAC oligomers form mitochondrial pores to release mtDNA fragments and promote lupus-like disease. Science 366 (6472), 1531–1536. doi:10.1126/science.aav4011
Kohlschmidt, N., Elbracht, M., Czech, A., Hausler, M., Phan, V., Topf, A., et al. (2021). Molecular pathophysiology of human MICU1 deficiency. Neuropathol. Appl. Neurobiol. 47, 840–855. doi:10.1111/nan.12694
Kornmann, B., Currie, E., Collins, S. R., Schuldiner, M., Nunnari, J., Weissman, J. S., et al. (2009). An ER-mitochondria tethering complex revealed by a synthetic biology screen. Science 325 (5939), 477–481. doi:10.1126/science.1175088
Kostic, M., Ludtmann, M. H., Bading, H., Hershfinkel, M., Steer, E., Chu, C. T., et al. (2015). PKA phosphorylation of NCLX reverses mitochondrial calcium overload and depolarization, promoting survival of PINK1-deficient dopaminergic neurons. Cell. Rep. 13 (2), 376–386. doi:10.1016/j.celrep.2015.08.079
Kravic, B., Harbauer, A. B., Romanello, V., Simeone, L., Vögtle, F. N., Kaiser, T., et al. (2018). In mammalian skeletal muscle, phosphorylation of TOMM22 by protein kinase CSNK2/CK2 controls mitophagy. Autophagy 14 (2), 311–335. doi:10.1080/15548627.2017.1403716
Krüger, V., Becker, T., Becker, L., Montilla-Martinez, M., Ellenrieder, L., Vögtle, F. N., et al. (2017). Identification of new channels by systematic analysis of the mitochondrial outer membrane. J. Cell. Biol. 216 (11), 3485–3495. doi:10.1083/jcb.201706043
Kuhlbrandt, W. (2015). Structure and function of mitochondrial membrane protein complexes. Bmc Biol. 13, 89. doi:10.1186/s12915-015-0201-x
Kunji, E. R. S., and Ruprecht, J. J. (2020). The mitochondrial ADP/ATP carrier exists and functions as a monomer. Biochem. Soc. Trans. 48 (4), 1419–1432. doi:10.1042/bst20190933
Kwong, J. Q., Lu, X., Correll, R. N., Schwanekamp, J. A., Vagnozzi, R. J., Sargent, M. A., et al. (2015). The mitochondrial calcium uniporter selectively matches metabolic output to acute contractile stress in the heart. Cell. Rep. 12 (1), 15–22. doi:10.1016/j.celrep.2015.06.002
Lam, M. P., Lau, E., Scruggs, S. B., Wang, D., Kim, T. Y., Liem, D. A., et al. (2013). Site-specific quantitative analysis of cardiac mitochondrial protein phosphorylation. J. Proteomics 81, 15–23. doi:10.1016/j.jprot.2012.09.015
Lambert, J. P., Luongo, T. S., Tomar, D., Jadiya, P., Gao, E., Zhang, X., et al. (2019). MCUB regulates the molecular composition of the mitochondrial calcium uniporter channel to limit mitochondrial calcium overload during stress. Circulation 140 (21), 1720–1733. doi:10.1161/CIRCULATIONAHA.118.037968
Lazarou, M., Jin, S. M., Kane, L. A., and Youle, R. J. (2012). Role of PINK1 binding to the TOM complex and alternate intracellular membranes in recruitment and activation of the E3 ligase Parkin. Dev. Cell. 22 (2), 320–333. doi:10.1016/j.devcel.2011.12.014
Leber, B., Lin, J., and Andrews, D. W. (2007). Embedded together: The life and death consequences of interaction of the bcl-2 family with membranes. Apoptosis 12 (5), 897–911. doi:10.1007/s10495-007-0746-4
Lee, S. K., Shanmughapriya, S., Mok, M. C. Y., Dong, Z., Tomar, D., Carvalho, E., et al. (2016). Structural insights into mitochondrial calcium uniporter regulation by divalent cations. Cell. Chem. Biol. 23 (9), 1157–1169. doi:10.1016/j.chembiol.2016.07.012
Lee, Y., Park, J., Lee, G., Yoon, S., Min, C. K., Kim, T. G., et al. (2020). S92 phosphorylation induces structural changes in the N-terminus domain of human mitochondrial calcium uniporter. Sci. Rep. 10 (1), 9131. doi:10.1038/s41598-020-65994-y
Lewis-Smith, D., Kamer, K. J., Griffin, H., Childs, A. M., Pysden, K., Titov, D., et al. (2016). Homozygous deletion in MICU1 presenting with fatigue and lethargy in childhood. Neurol. Genet. 2 (2), e59. doi:10.1212/NXG.0000000000000059
Li, Y., Tran, Q., Shrestha, R., Piao, L., Park, S., Park, J., et al. (2019). LETM1 is required for mitochondrial homeostasis and cellular viability (Review). Mol. Med. Rep. 19 (5), 3367–3375. doi:10.3892/mmr.2019.10041
Limongelli, G., Masarone, D., D’Alessandro, R., and Elliott, P. M. (2012). Mitochondrial diseases and the heart: An overview of molecular basis, diagnosis, treatment and clinical course. Future Cardiol. 8 (1), 71–88. doi:10.2217/fca.11.79
Lin, Q. T., and Stathopulos, P. B. (2019). Molecular mechanisms of leucine zipper EF-hand containing transmembrane protein-1 function in health and disease. Int. J. Mol. Sci. 20 (2), 286. doi:10.3390/ijms20020286
Liu, J., Carnero-Montoro, E., van Dongen, J., Lent, S., Nedeljkovic, I., Ligthart, S., et al. (2019). An integrative cross-omics analysis of DNA methylation sites of glucose and insulin homeostasis. Nat. Commun. 10 (1), 2581–2611. doi:10.1038/s41467-019-10487-4
Liu, J. C., Liu, J., Holmstrom, K. M., Menazza, S., Parks, R. J., Fergusson, M. M., et al. (2016). MICU1 serves as a molecular gatekeeper to prevent in vivo mitochondrial calcium overload. Cell. Rep. 16 (6), 1561–1573. doi:10.1016/j.celrep.2016.07.011
Liu, Q., Barker, S., and Knutson, M. D. (2021). Iron and manganese transport in mammalian systems. Biochim. Biophys. Acta Mol. Cell. Res. 1868 (1), 118890. doi:10.1016/j.bbamcr.2020.118890
Logan, C. V., Szabadkai, G., Sharpe, J. A., Parry, D. A., Torelli, S., Childs, A. M., et al. (2014). Loss-of-function mutations in MICU1 cause a brain and muscle disorder linked to primary alterations in mitochondrial calcium signaling. Nat. Genet. 46 (2), 188–193. doi:10.1038/ng.2851
Lozano, O., Silva-Platas, C., Chapoy-Villanueva, H., Pérez, B. E., Lees, J. G., Ramachandra, C. J. A., et al. (2020). Amorphous SiO2 nanoparticles promote cardiac dysfunction via the opening of the mitochondrial permeability transition pore in rat heart and human cardiomyocytes. Part. Fibre Toxicol. 17 (1), 15. doi:10.1186/s12989-020-00346-2
Ludtmann, M. H., Angelova, P. R., Horrocks, M. H., Choi, M. L., Rodrigues, M., Baev, A. Y., et al. (2018). α-synuclein oligomers interact with ATP synthase and open the permeability transition pore in Parkinson's disease. Nat. Commun. 9, 2293. doi:10.1038/s41467-018-04422-2
Luongo, T. S., Lambert, J. P., Yuan, A., Zhang, X., Gross, P., Song, J., et al. (2015). The mitochondrial calcium uniporter matches energetic supply with cardiac workload during stress and modulates permeability transition. Cell. Rep. 12 (1), 23–34. doi:10.1016/j.celrep.2015.06.017
Lurin, C., Güclü, J., Cheniclet, C., Carde, J. P., Barbier-Brygoo, H., and Maurel, C. (2000). CLC-Nt1, a putative chloride channel protein of tobacco, co-localizes with mitochondrial membrane markers. Biochem. J. 348 (2), 291–295. doi:10.1042/bj3480291
Madreiter-Sokolowski, C. T., Klec, C., Parichatikanond, W., Stryeck, S., Gottschalk, B., Pulido, S., et al. (2016). PRMT1-mediated methylation of MICU1 determines the UCP2/3 dependency of mitochondrial Ca(2+) uptake in immortalized cells. Nat. Commun. 7, 12897. doi:10.1038/ncomms12897
Mailloux, R. J., Seifert, E. L., Bouillaud, F., Aguer, C., Collins, S., and Harper, M. E. (2011). Glutathionylation acts as a control switch for uncoupling proteins UCP2 and UCP3. J. Biol. Chem. 286 (24), 21865–21875. doi:10.1074/jbc.M111.240242
Mallilankaraman, K., Cardenas, C., Doonan, P. J., Chandramoorthy, H. C., Irrinki, K. M., Golenar, T., et al. (2012a). MCUR1 is an essential component of mitochondrial Ca(2+) uptake that regulates cellular metabolism. Nat. Cell. Biol. 15 (1), 1336–1343. doi:10.1038/ncb2622
Mallilankaraman, K., Doonan, P., Cardenas, C., Chandramoorthy, H. C., Muller, M., Miller, R., et al. (2012b). MICU1 is an essential gatekeeper for MCU-mediated mitochondrial Ca(2+) uptake that regulates cell survival. Cell. 151 (3), 630–644. doi:10.1016/j.cell.2012.10.011
Mannella, C. A. (2021). VDAC—a primal perspective. Int. J. Mol. Sci. 22 (4), 1685. doi:10.3390/ijms22041685
Manoli, I., Alesci, S., and Chrousos, G. P. (2007). “Mitochondria,” in Encyclopedia of stress. Editor G. Fink (New York: Academic Press), 754–761.
Mårtensson, C. U., Priesnitz, C., Song, J., Ellenrieder, L., Doan, K. N., Boos, F., et al. (2019). Mitochondrial protein translocation-associated degradation. Nature 569 (7758), 679–683. doi:10.1038/s41586-019-1227-y
Martinez-Caballero, S., Dejean, L. M., Kinnally, M. S., Oh, K. J., Mannella, C. A., and Kinnally, K. W. (2009). Assembly of the mitochondrial apoptosis-induced channel, MAC. J. Biol. Chem. 284 (18), 12235–12245. doi:10.1074/jbc.M806610200
Maruszczak, K. K., Jung, M., Rasool, S., Trempe, J. F., and Rapaport, D. (2022). The role of the individual TOM subunits in the association of PINK1 with depolarized mitochondria. J. Mol. Med. Berl. 100 (5), 747–762. doi:10.1007/s00109-022-02191-6
Meisinger, C., Wiedemann, N., Rissler, M., Strub, A., Milenkovic, D., Schönfisch, B., et al. (2006). Mitochondrial protein sorting: Differentiation of beta-barrel assembly by tom7-mediated segregation of Mdm10. J. Biol. Chem. 281 (32), 22819–22826. doi:10.1074/jbc.M602679200
Menzel, V. A., Cassará, M. C., Benz, R., de Pinto, V., Messina, A., Cunsolo, V., et al. (2009). Molecular and functional characterization of VDAC2 purified from mammal spermatozoa. Biosci. Rep. 29 (6), 351–362. doi:10.1042/bsr20080123
Mercadante, C. J., Prajapati, M., Conboy, H. L., Dash, M. E., Herrera, C., Pettiglio, M. A., et al. (2019). Manganese transporter Slc30a10 controls physiological manganese excretion and toxicity. J. Clin. Investigation 129 (12), 5442–5461. doi:10.1172/JCI129710
Milton, R. H., Abeti, R., Averaimo, S., DeBiasi, S., Vitellaro, L., Jiang, L., et al. (2008). CLIC1 function is required for beta-amyloid-induced generation of reactive oxygen species by microglia. J. Neurosci. 28 (45), 11488–11499. doi:10.1523/jneurosci.2431-08.2008
Monteiro, B. S., Freire-Brito, L., Carrageta, D. F., Oliveira, P. F., and Alves, M. G. (2021). Mitochondrial uncoupling proteins (UCPs) as key modulators of ROS homeostasis: A crosstalk between diabesity and male infertility? Antioxidants (Basel) 10 (11), 1746. doi:10.3390/antiox10111746
Mookerjee, S. A., and Brand, M. D. (2011). Characteristics of the turnover of uncoupling protein 3 by the ubiquitin proteasome system in isolated mitochondria. Biochim. Biophys. Acta 1807 (11), 1474–1481. doi:10.1016/j.bbabio.2011.07.011
Morciano, G., Bonora, M., Campo, G., Aquila, G., Rizzo, P., Giorgi, C., et al. (2017). Mechanistic role of mPTP in ischemia-reperfusion injury. Mitochondrial Dyn. Cardiovasc. Med. 982, 169–189. doi:10.1007/978-3-319-55330-6_9
Murley, A., Sarsam, R. D., Toulmay, A., Yamada, J., Prinz, W. A., and Nunnari, J. (2015). Ltc1 is an ER-localized sterol transporter and a component of ER-mitochondria and ER-vacuole contacts. J. Cell. Biol. 209 (4), 539–548. doi:10.1083/jcb.201502033
Murphy, E., Ardehali, H., Balaban, R. S., DiLisa, F., Dorn, G. W., Kitsis, R. N., et al. (2016). Mitochondrial function, biology, and role in disease: A scientific statement from the American heart association. Circulation Res. 118 (12), 1960–1991. doi:10.1161/RES.0000000000000104
Murphy, M. P. (2009). How mitochondria produce reactive oxygen species. Biochem. J. 417 (1), 1–13. doi:10.1042/bj20081386
Naon, D., Zaninello, M., Giacomello, M., Varanita, T., Grespi, F., Lakshminaranayan, S., et al. (2016). Critical reappraisal confirms that Mitofusin 2 is an endoplasmic reticulum–mitochondria tether. Proc. Natl. Acad. Sci. 113 (40), 11249–11254. doi:10.1073/pnas.1606786113
Narula, N., Zaragoza, M. V., Sengupta, P. P., Li, P., Haider, N., Verjans, J., et al. (2011). Adenine nucleotide translocase 1 deficiency results in dilated cardiomyopathy with defects in myocardial mechanics, histopathological alterations, and activation of apoptosis. JACC Cardiovasc Imaging 4 (1), 1–10. doi:10.1016/j.jcmg.2010.06.018
Natarajan, G. K., Glait, L., Mishra, J., Stowe, D. F., Camara, A. K. S., and Kwok, W. M. (2020). Total matrix Ca(2+) modulates Ca(2+) efflux via the Ca(2+)/H(+) exchanger in cardiac mitochondria. Front. Physiol. 11, 510600. doi:10.3389/fphys.2020.510600
Neupert, W., and Herrmann, J. M. (2007). Translocation of proteins into mitochondria. Annu. Rev. Biochem. 76, 723–749. doi:10.1146/annurev.biochem.76.052705.163409
Neveu, B., Spinella, J. F., Richer, C., Lagacé, K., Cassart, P., Lajoie, M., et al. (2016). CLIC5: A novel ETV6 target gene in childhood acute lymphoblastic leukemia. Haematologica 101 (12), 1534–1543. doi:10.3324/haematol.2016.149740
Nguyen, T. T. M., Wong, R., Menazza, S., Sun, J., Chen, Y., Wang, G., et al. (2013). Cyclophilin D modulates mitochondrial acetylome. Circulation Res. 113 (12), 1308–1319. doi:10.1161/CIRCRESAHA.113.301867
Nishida, H., Sato, T., Ogura, T., and Nakaya, H. (2009). New aspects for the treatment of cardiac diseases based on the diversity of functional controls on cardiac muscles: Mitochondrial ion channels and cardioprotection. J. Pharmacol. Sci. 109 (3), 341–347. doi:10.1254/jphs.08R24FM
Nunnari, J., and Suomalainen, A. (2012). Mitochondria: In sickness and in health. Cell. 148 (6), 1145–1159. doi:10.1016/j.cell.2012.02.035
O'Rourke, B. (2007). Mitochondrial ion channels. Annu. Rev. Physiol. 69, 19–49. doi:10.1146/annurev.physiol.69.031905.163804
Oelkrug, R., Kutschke, M., Meyer, C. W., Heldmaier, G., and Jastroch, M. (2010). Uncoupling protein 1 decreases superoxide production in brown adipose tissue mitochondria. J. Biol. Chem. 285 (29), 21961–21968. doi:10.1074/jbc.M110.122861
Onishi, M., Yamano, K., Sato, M., Matsuda, N., and Okamoto, K. (2021). Molecular mechanisms and physiological functions of mitophagy. Embo J. 40 (3), e104705. doi:10.15252/embj.2020104705
Paggio, A., Checchetto, V., Campo, A., Menabò, R., Di Marco, G., Di Lisa, F., et al. (2019). Identification of an ATP-sensitive potassium channel in mitochondria. Nature 572 (7771), 609–613. doi:10.1038/s41586-019-1498-3
Palty, R., Silverman, W. F., Hershfinkel, M., Caporale, T., Sensi, S. L., Parnis, J., et al. (2010). NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc. Natl. Acad. Sci. U. S. A. 107 (1), 436–441. doi:10.1073/pnas.0908099107
Panel, M., Ruiz, I., Brillet, R., Lafdil, F., Teixeira-Clerc, F., Nguyen, C. T., et al. (2019). Small-molecule inhibitors of cyclophilins block opening of the mitochondrial permeability transition pore and protect mice from hepatic ischemia/reperfusion injury. Gastroenterology 157 (5), 1368–1382. doi:10.1053/j.gastro.2019.07.026
Park, J. H., Hogrebe, M., Grüneberg, M., DuChesne, I., von der Heiden, A. L., Reunert, J., et al. (2015). SLC39A8 deficiency: A disorder of manganese transport and glycosylation. Am. J. Hum. Genet. 97 (6), 894–903. doi:10.1016/j.ajhg.2015.11.003
Park, J., Li, Y., Kim, S. H., Yang, K. J., Kong, G., Shrestha, R., et al. (2014). New players in high fat diet-induced obesity: LETM1 and CTMP. Metabolism 63 (3), 318–327. doi:10.1016/j.metabol.2013.10.012
Pathak, T., Gueguinou, M., Walter, V., Delierneux, C., Johnson, M. T., Zhang, X., et al. (2020). Dichotomous role of the human mitochondrial Na(+)/Ca2(+)/Li(+) exchanger NCLX in colorectal cancer growth and metastasis. Elife 9, e59686. doi:10.7554/eLife.59686
Patron, M., Granatiero, V., Espino, J., Rizzuto, R., and De Stefani, D. (2019). MICU3 is a tissue-specific enhancer of mitochondrial calcium uptake. Cell. Death Differ. 26 (1), 179–195. doi:10.1038/s41418-018-0113-8
Patron, M., Tarasenko, D., Nolte, H., Kroczek, L., Ghosh, M., Ohba, Y., et al. (2022). Regulation of mitochondrial proteostasis by the proton gradient. Embo J. 41 (16), e110476. doi:10.15252/embj.2021110476
Pavlov, E. V., Priault, M., Pietkiewicz, D., Cheng, E. H., Antonsson, B., Manon, S., et al. (2001). A novel, high conductance channel of mitochondria linked to apoptosis in mammalian cells and Bax expression in yeast. J. Cell. Biol. 155 (5), 725–731. doi:10.1083/jcb.200107057
Perocchi, F., Gohil, V. M., Girgis, H. S., Bao, X. R., McCombs, J. E., Palmer, A. E., et al. (2010). MICU1 encodes a mitochondrial EF hand protein required for Ca(2+) uptake. Nature 467 (7313), 291–296. doi:10.1038/nature09358
Petrungaro, C., and Kornmann, B. (2019). Lipid exchange at ER-mitochondria contact sites: A puzzle falling into place with quite a few pieces missing. Curr. Opin. Cell. Biol. 57, 71–76. doi:10.1016/j.ceb.2018.11.005
Pfanner, N., Warscheid, B., and Wiedemann, N. (2019). Mitochondrial proteins: From biogenesis to functional networks. Nat. Rev. Mol. Cell. Biol. 20 (5), 267–284. doi:10.1038/s41580-018-0092-0
Piao, L., Feng, Y., Yang, Z., Qi, W., Li, H., Han, H., et al. (2019). LETM1 is a potential cancer stem-like cell marker and predicts poor prognosis in colorectal adenocarcinoma. Pathology-Research Pract. 215 (7), 152437. doi:10.1016/j.prp.2019.152437
Piao, L., Li, H., Feng, Y., Li, X., Cui, Y., and Xuan, Y. (2020). Leucine zipper-EF-hand containing transmembrane protein 1 is a potential prognostic biomarker and promotes cell progression in prostate cancer. Cancer Manag. Res. 12, 1649–1660. doi:10.2147/CMAR.S236482
Pilchova, I., Klacanova, K., Tatarkova, Z., Kaplan, P., and Racay, P. (2017). The involvement of Mg(2+) in regulation of cellular and mitochondrial functions. Oxid. Med. Cell. Longev. 2017, 6797460. doi:10.1155/2017/6797460
Piskacek, M., Zotova, L., Zsurka, G., and Schweyen, R. J. (2009). Conditional knockdown of hMRS2 results in loss of mitochondrial Mg(2+) uptake and cell death. J. Cell. Mol. Med. 13 (4), 693–700. doi:10.1111/j.1582-4934.2008.00328.x
Pittalà, M. G. G., Reina, S., Cubisino, S. A. M., Cucina, A., Formicola, B., Cunsolo, V., et al. (2020). Post-translational modification analysis of VDAC1 in ALS-SOD1 model cells reveals specific asparagine and glutamine deamidation. Antioxidants (Basel) 9 (12), 1218. doi:10.3390/antiox9121218
Plovanich, M., Bogorad, R. L., Sancak, Y., Kamer, K. J., Strittmatter, L., Li, A. A., et al. (2013). MICU2, a paralog of MICU1, resides within the mitochondrial uniporter complex to regulate calcium handling. PLoS One 8 (2), e55785. doi:10.1371/journal.pone.0055785
Pochwat, B., Nowak, G., and Szewczyk, B. (2015). Relationship between zinc (Zn (2+)) and glutamate receptors in the processes underlying neurodegeneration. Neural Plast. 2015, 591563. doi:10.1155/2015/591563
Ponnalagu, D., Gururaja Rao, S., Farber, J., Xin, W., Hussain, A. T., Shah, K., et al. (2016). Molecular identity of cardiac mitochondrial chloride intracellular channel proteins. Mitochondrion 27, 6–14. doi:10.1016/j.mito.2016.01.001
Ponnalagu, D., Hamilton, S., Sanghvi, S., Antelo, D., Schwieterman, N., Hansra, I., et al. (2022). CLIC4 localizes to mitochondrial-associated membranes and mediates cardioprotection. Sci. Adv. 8 (42), eabo1244. doi:10.1126/sciadv.abo1244
Ponnalagu, D., Hussain, A. T., Thanawala, R., Meka, J., Bednarczyk, P., Feng, Y., et al. (2019). Chloride channel blocker IAA-94 increases myocardial infarction by reducing calcium retention capacity of the cardiac mitochondria. Life Sci. 235, 116841. doi:10.1016/j.lfs.2019.116841
Ponnalagu, D., and Singh, H. (2017). Anion channels of mitochondria. Handb. Exp. Pharmacol. 240, 71–101. doi:10.1007/164_2016_39
Potting, C., Tatsuta, T., König, T., Haag, M., Wai, T., Aaltonen, M. J., et al. (2013). TRIAP1/PRELI complexes prevent apoptosis by mediating intramitochondrial transport of phosphatidic acid. Cell. Metab. 18 (2), 287–295. doi:10.1016/j.cmet.2013.07.008
Pradhan, R. K., Qi, F., Beard, D. A., and Dash, R. K. (2011). Characterization of Mg2+ inhibition of mitochondrial Ca2+ uptake by a mechanistic model of mitochondrial Ca2+ uniporter. Biophys. J. 101 (9), 2071–2081. doi:10.1016/j.bpj.2011.09.029
Queralt-Martín, M., Bergdoll, L., Teijido, O., Munshi, N., Jacobs, D., Kuszak, A. J., et al. (2020). A lower affinity to cytosolic proteins reveals VDAC3 isoform-specific role in mitochondrial biology. J. Gen. Physiol. 152 (2), e201912501. doi:10.1085/jgp.201912501
Rabl, R., Soubannier, V., Scholz, R., Vogel, F., Mendl, N., Vasiljev-Neumeyer, A., et al. (2009). Formation of cristae and crista junctions in mitochondria depends on antagonism between Fcj1 and Su e/g. J. Cell. Biol. 185 (6), 1047–1063. doi:10.1083/jcb.200811099
Raffaello, A., De Stefani, D., Sabbadin, D., Teardo, E., Merli, G., Picard, A., et al. (2013). The mitochondrial calcium uniporter is a multimer that can include a dominant-negative pore-forming subunit. EMBO J. 32 (17), 2362–2376. doi:10.1038/emboj.2013.157
Ramesh, A., Peleh, V., Martinez-Caballero, S., Wollweber, F., Sommer, F., van der Laan, M., et al. (2016). A disulfide bond in the TIM23 complex is crucial for voltage gating and mitochondrial protein import. J. Cell. Biol. 214 (4), 417–431. doi:10.1083/jcb.201602074
Ravanelli, S., den Brave, F., and Hoppe, T. (2020). Mitochondrial quality control governed by ubiquitin. Front. Cell. Dev. Biol. 8, 270. doi:10.3389/fcell.2020.00270
Reilly, L., Howie, J., Wypijewski, K., Ashford, M. L., Hilgemann, D. W., and Fuller, W. (2015). Palmitoylation of the Na/Ca exchanger cytoplasmic loop controls its inactivation and internalization during stress signaling. Faseb J. 29 (11), 4532–4543. doi:10.1096/fj.15-276493
Reina, S., Checchetto, V., Saletti, R., Gupta, A., Chaturvedi, D., Guardiani, C., et al. (2016). VDAC3 as a sensor of oxidative state of the intermembrane space of mitochondria: The putative role of cysteine residue modifications. Oncotarget 7 (3), 2249–2268. doi:10.18632/oncotarget.6850
Reina, S., Pittalà, M. G. G., Guarino, F., Messina, A., De Pinto, V., Foti, S., et al. (2020). Cysteine oxidations in mitochondrial membrane proteins: The case of VDAC isoforms in mammals. Front. Cell. Dev. Biol. 8, 397. doi:10.3389/fcell.2020.00397
Robbins, D., and Zhao, Y. (2011). New aspects of mitochondrial Uncoupling Proteins (UCPs) and their roles in tumorigenesis. Int. J. Mol. Sci. 12 (8), 5285–5293. doi:10.3390/ijms12085285
Rosa, J., and César, M. (2016). Role of hexokinase and VDAC in neurological disorders. Curr. Mol. Pharmacol. 09, 320–331. doi:10.2174/1874467209666160112123036
Rotko, D., Bednarczyk, P., Koprowski, P., Kunz, W. S., Szewczyk, A., and Kulawiak, B. (2020a). Heme is required for carbon monoxide activation of mitochondrial BK(Ca) channel. Eur. J. Pharmacol. 881, 173191. doi:10.1016/j.ejphar.2020.173191
Rotko, D., Kunz, W. S., Szewczyk, A., and Kulawiak, B. (2020b). Signaling pathways targeting mitochondrial potassium channels. Int. J. Biochem. Cell. Biol. 125, 105792. doi:10.1016/j.biocel.2020.105792
Rubler, S., Dlugash, J., Yuceoglu, Y. Z., Kumral, T., Branwood, A. W., and Grishman, A. (1972). New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am. J. Cardiol. 30 (6), 595–602. doi:10.1016/0002-9149(72)90595-4
Sabirov, R. Z., Sheiko, T., Liu, H., Deng, D., Okada, Y., and Craigen, W. J. (2006). Genetic demonstration that the plasma membrane maxianion channel and voltage-dependent anion channels are unrelated proteins. J. Biol. Chem. 281 (4), 1897–1904. doi:10.1074/jbc.M509482200
Sancak, Y., Markhard, A. L., Kitami, T., Kovacs-Bogdan, E., Kamer, K. J., Udeshi, N. D., et al. (2013). EMRE is an essential component of the mitochondrial calcium uniporter complex. Science 342 (6164), 1379–1382. doi:10.1126/science.1242993
Schinder, A. F., Olson, E. C., Spitzer, N. C., and Montal, M. (1996). Mitochondrial dysfunction is a primary event in glutamate neurotoxicity. J. Neurosci. 16 (19), 6125–6133. doi:10.1523/JNEUROSCI.16-19-06125.1996
Schindl, R., Weghuber, J., Romanin, C., and Schweyen, R. J. (2007). Mrs2p forms a high conductance Mg2+ selective channel in mitochondria. Biophys. J. 93 (11), 3872–3883. doi:10.1529/biophysj.107.112318
Schmidt, O., Harbauer, A. B., Rao, S., Eyrich, B., Zahedi, R. P., Stojanovski, D., et al. (2011). Regulation of mitochondrial protein import by cytosolic kinases. Cell. 144 (2), 227–239. doi:10.1016/j.cell.2010.12.015
Schon, E. A., and Manfredi, G. (2003). Neuronal degeneration and mitochondrial dysfunction. J. Clin. Invest. 111 (3), 303–312. doi:10.1172/jci17741
Schulz, C., Lytovchenko, O., Melin, J., Chacinska, A., Guiard, B., Neumann, P., et al. (2011). Tim50's presequence receptor domain is essential for signal driven transport across the TIM23 complex. J. Cell. Biol. 195 (4), 643–656. doi:10.1083/jcb.201105098
Sebastián, D., Hernández-Alvarez, M. I., Segalés, J., Sorianello, E., Muñoz, J. P., Sala, D., et al. (2012). Mitofusin 2 (Mfn2) links mitochondrial and endoplasmic reticulum function with insulin signaling and is essential for normal glucose homeostasis. Proc. Natl. Acad. Sci. U. S. A. 109 (14), 5523–5528. doi:10.1073/pnas.1108220109
Seguin, A., Jia, X., Earl, A. M., Li, L., Wallace, J., Qiu, A., et al. (2020). The mitochondrial metal transporters mitoferrin1 and mitoferrin2 are required for liver regeneration and cell proliferation in mice. J. Biol. Chem. 295 (32), 11002–11020. doi:10.1074/jbc.RA120.013229
Shanmughapriya, S., Rajan, S., Hoffman, N. E., Higgins, A. M., Tomar, D., Nemani, N., et al. (2015). SPG7 is an essential and conserved component of the mitochondrial permeability transition pore. Mol. Cell. 60 (1), 47–62. doi:10.1016/j.molcel.2015.08.009
Shindo, Y., Yamanaka, R., Suzuki, K., Hotta, K., and Oka, K. (2015). Intracellular magnesium level determines cell viability in the MPP(+) model of Parkinson's disease. Biochim. Biophys. Acta 1853 (12), 3182–3191. doi:10.1016/j.bbamcr.2015.08.013
Shoshan-Barmatz, V., Pittala, S., and Mizrachi, D. (2019). VDAC1 and the TSPO: Expression, interactions, and associated functions in health and disease states. Int. J. Mol. Sci. 20 (13), 3348. doi:10.3390/ijms20133348
Siemen, D., Loupatatzis, C., Borecky, J., Gulbins, E., and Lang, F. (1999). Ca2+-activated K channel of the BK-type in the inner mitochondrial membrane of a human glioma cell line. Biochem. Biophys. Res. Commun. 257 (2), 549–554. doi:10.1006/bbrc.1999.0496
Silvestri, L., Caputo, V., Bellacchio, E., Atorino, L., Dallapiccola, B., Valente, E. M., et al. (2005). Mitochondrial import and enzymatic activity of PINK1 mutants associated to recessive parkinsonism. Hum. Mol. Genet. 14 (22), 3477–3492. doi:10.1093/hmg/ddi377
Slocińska, M., Rosinski, G., and Jarmuszkiewicz, W. (2016). Activation of mitochondrial uncoupling protein 4 and ATP-sensitive potassium channel cumulatively decreases superoxide production in insect mitochondria. Protein Pept. Lett. 23 (1), 63–68. doi:10.2174/0929866523666151106121943
Song, J., Herrmann, J. M., and Becker, T. (2021). Quality control of the mitochondrial proteome. Nat. Rev. Mol. Cell. Biol. 22 (1), 54–70. doi:10.1038/s41580-020-00300-2
Stavsky, A., Stoler, O., Kostic, M., Katoshevsky, T., Assali, E. A., Savic, I., et al. (2021). Aberrant activity of mitochondrial NCLX is linked to impaired synaptic transmission and is associated with mental retardation. Commun. Biol. 4 (1), 666. doi:10.1038/s42003-021-02114-0
Stone, S. J., Levin, M. C., Zhou, P., Han, J., Walther, T. C., and Farese, R. V. (2009). The endoplasmic reticulum enzyme DGAT2 is found in mitochondria-associated membranes and has a mitochondrial targeting signal that promotes its association with mitochondria. J. Biol. Chem. 284 (8), 5352–5361. doi:10.1074/jbc.M805768200
Stone, S. J., and Vance, J. E. (2000). Phosphatidylserine synthase-1 and-2 are localized to mitochondria-associated membranes. J. Biol. Chem. 275 (44), 34534–34540. doi:10.1074/jbc.M002865200
Stowe, D. F., Gadicherla, A. K., Zhou, Y., Aldakkak, M., Cheng, Q., Kwok, W. M., et al. (2013). Protection against cardiac injury by small Ca(2+)-sensitive K(+) channels identified in Guinea pig cardiac inner mitochondrial membrane. Biochim. Biophys. Acta 1828 (2), 427–442. doi:10.1016/j.bbamem.2012.08.031
Suarez, J., Cividini, F., Scott, B. T., Lehmann, K., Diaz-Juarez, J., Diemer, T., et al. (2018). Restoring mitochondrial calcium uniporter expression in diabetic mouse heart improves mitochondrial calcium handling and cardiac function. J. Biol. Chem. 293 (21), 8182–8195. doi:10.1074/jbc.RA118.002066
Sun, Q., Zhong, W., Zhang, W., and Zhou, Z. (2016). Defect of mitochondrial respiratory chain is a mechanism of ROS overproduction in a rat model of alcoholic liver disease: Role of zinc deficiency. Am. J. Physiol. Gastrointest. Liver Physiol. 310 (3), G205–G214. doi:10.1152/ajpgi.00270.2015
Suppanz, I. E., Wurm, C. A., Wenzel, D., and Jakobs, S. (2009). The m-AAA protease processes cytochrome c peroxidase preferentially at the inner boundary membrane of mitochondria. Mol. Biol. Cell. 20 (2), 572–580. doi:10.1091/mbc.e07-11-1112
Szabadkai, G., Bianchi, K., Várnai, P. t., De Stefani, D., Wieckowski, M. R., Cavagna, D., et al. (2006b). Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J. Cell. Biol. 175 (6), 901–911. doi:10.1083/jcb.200608073
Szabadkai, G., Simoni, A. M., Bianchi, K., De Stefani, D., Leo, S., Wieckowski, M. R., et al. (2006a). Mitochondrial dynamics and Ca2+ signaling. Biochim. Biophys. Acta 1763 (5-6), 442–449. doi:10.1016/j.bbamcr.2006.04.002
Szabò, I., Bock, J., Jekle, A., Soddemann, M., Adams, C., Lang, F., et al. (2005). A novel potassium channel in lymphocyte mitochondria. J. Biol. Chem. 280 (13), 12790–12798. doi:10.1074/jbc.M413548200
Szabo, I., and Zoratti, M. (2014). Mitochondrial channels: Ion fluxes and more. Physiol. Rev. 94 (2), 519–608. doi:10.1152/physrev.00021.2013
Takano, K., Liu, D., Tarpey, P., Gallant, E., Lam, A., Witham, S., et al. (2012). An X-linked channelopathy with cardiomegaly due to a CLIC2 mutation enhancing ryanodine receptor channel activity. Hum. Mol. Genet. 21 (20), 4497–4507. doi:10.1093/hmg/dds292
Tang, T., Lang, X., Xu, C., Wang, X., Gong, T., Yang, Y., et al. (2017). CLICs-dependent chloride efflux is an essential and proximal upstream event for NLRP3 inflammasome activation. Nat. Commun. 8 (1), 202–212. doi:10.1038/s41467-017-00227-x
Thornton, N., Stroud, D. A., Milenkovic, D., Guiard, B., Pfanner, N., and Becker, T. (2010). Two modular forms of the mitochondrial sorting and assembly machinery are involved in biogenesis of alpha-helical outer membrane proteins. J. Mol. Biol. 396 (3), 540–549. doi:10.1016/j.jmb.2009.12.026
Tian, X. Y., Ma, S., Tse, G., Wong, W. T., and Huang, Y. (2018). Uncoupling protein 2 in cardiovascular health and disease. Front. Physiol. 9, 1060. doi:10.3389/fphys.2018.01060
Tomar, D., Dong, Z., Shanmughapriya, S., Koch, D. A., Thomas, T., Hoffman, N. E., et al. (2016). MCUR1 is a scaffold factor for the MCU complex function and promotes mitochondrial bioenergetics. Cell. Rep. 15 (8), 1673–1685. doi:10.1016/j.celrep.2016.04.050
Trenker, M., Malli, R., Fertschai, I., Levak-Frank, S., and Graier, W. F. (2007). Uncoupling proteins 2 and 3 are fundamental for mitochondrial Ca2+ uniport. Nat. Cell. Biol. 9 (4), 445–452. doi:10.1038/ncb1556
Tuschl, K., Meyer, E., Valdivia, L. E., Zhao, N., Dadswell, C., Abdul-Sada, A., et al. (2016). Mutations in SLC39A14 disrupt manganese homeostasis and cause childhood-onset parkinsonism–dystonia. Nat. Commun. 7 (1), 11601. doi:10.1038/ncomms11601
Tzameli, I. (2012). The evolving role of mitochondria in metabolism. Trends Endocrinol. Metab. 23 (9), 417–419. doi:10.1016/j.tem.2012.07.008
Uchino, H., Elmer, E., Uchino, K., Lindvall, O., and Siesjo, B. K. (1995). Cyclosporin A dramatically ameliorates CA1 hippocampal damage following transient forebrain ischaemia in the rat. Acta Physiol. Scand. 155 (4), 469–471. doi:10.1111/j.1748-1716.1995.tb09999.x
Vais, H., Tanis, J. E., Müller, M., Payne, R., Mallilankaraman, K., and Foskett, J. K. (2015). MCUR1, CCDC90A, is a regulator of the mitochondrial calcium uniporter. Cell. Metab. 22 (4), 533–535. doi:10.1016/j.cmet.2015.09.015
Vanden Hoek, T. L., Becker, L. B., Shao, Z., Li, C., and Schumacker, P. T. (1998). Reactive oxygen species released from mitochondria during brief hypoxia induce preconditioning in cardiomyocytes. J. Biol. Chem. 273 (29), 18092–18098. doi:10.1074/jbc.273.29.18092
Vieira, H. L., Haouzi, D., El Hamel, C., Jacotot, E., Belzacq, A. S., Brenner, C., et al. (2000). Permeabilization of the mitochondrial inner membrane during apoptosis: Impact of the adenine nucleotide translocator. Cell. Death Differ. 7 (12), 1146–1154. doi:10.1038/sj.cdd.4400778
Vitali, D. G., Drwesh, L., Cichocki, B. A., Kolb, A., and Rapaport, D. (2020). The biogenesis of mitochondrial outer membrane proteins show variable dependence on import factors. iScience 23 (1), 100779. doi:10.1016/j.isci.2019.100779
Vogel, F., Bornhövd, C., Neupert, W., and Reichert, A. S. (2006). Dynamic subcompartmentalization of the mitochondrial inner membrane. J. Cell. Biol. 175 (2), 237–247. doi:10.1083/jcb.200605138
Vogler, S., Pahnke, J., Rousset, S., Ricquier, D., Moch, H., Miroux, B., et al. (2006). Uncoupling protein 2 has protective function during experimental autoimmune encephalomyelitis. Am. J. pathology 168 (5), 1570–1575. doi:10.2353/ajpath.2006.051069
Vringer, E., and Tait, S. W. G. (2019). Mitochondria and inflammation: Cell death heats up. Front. Cell. Dev. Biol. 7, 100. doi:10.3389/fcell.2019.00100
Walewska, A., Szewczyk, A., and Koprowski, P. (2018). Gas signaling molecules and mitochondrial potassium channels. Int. J. Mol. Sci. 19 (10), 3227. doi:10.3390/ijms19103227
Walewska, A., Szewczyk, A., Krajewska, M., and Koprowski, P. (2022). Targeting mitochondrial large-conductance calcium-activated potassium channel by hydrogen sulfide via heme-binding site. J. Pharmacol. Exp. Ther. 381 (2), 137–150. doi:10.1124/jpet.121.001017
Wang, C., and Youle, R. J. (2009). The role of mitochondria in apoptosis. Annu. Rev. Genet. 43, 95–118. doi:10.1146/annurev-genet-102108-134850
Wang, J., Ding, W., Xu, Y., Tao, E., Mo, M., Xu, W., et al. (2020a). Long non-coding RNA RHPN1-AS1 promotes tumorigenesis and metastasis of ovarian cancer by acting as a ceRNA against miR-596 and upregulating LETM1. Aging (Albany NY) 12 (5), 4558–4572. doi:10.18632/aging.102911
Wang, Q., Guan, Z., Qi, L., Zhuang, J., Wang, C., Hong, S., et al. (2021). Structural insight into the SAM-mediated assembly of the mitochondrial TOM core complex. Science 373 (6561), 1377–1381. doi:10.1126/science.abh0704
Wang, W., Chen, X., Zhang, L., Yi, J., Ma, Q., Yin, J., et al. (2020b). Atomic structure of human TOM core complex. Cell. Discov. 6, 67. doi:10.1038/s41421-020-00198-2
Wang, Y., Branicky, R., Noë, A., and Hekimi, S. (2018). Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell. Biol. 217 (6), 1915–1928. doi:10.1083/jcb.201708007
Wei, A. C., Liu, T., Winslow, R. L., and O'Rourke, B. (2012). Dynamics of matrix-free Ca2+ in cardiac mitochondria: Two components of Ca2+ uptake and role of phosphate buffering. J. Gen. Physiol. 139 (6), 465–478. doi:10.1085/jgp.201210784
West, M. B., Hill, B. G., Xuan, Y. T., and Bhatnagar, A. (2006). Protein glutathiolation by nitric oxide: An intracellular mechanism regulating redox protein modification. Faseb J. 20 (10), 1715–1717. doi:10.1096/fj.06-5843fje
Wilton, K. M., Morales-Rosado, J. A., Selcen, D., Muthusamy, K., Ewing, S., Agre, K., et al. (2020). Developmental brain abnormalities and acute encephalopathy in a patient with myopathy with extrapyramidal signs secondary to pathogenic variants in MICU1. JIMD Rep. 53 (1), 22–28. doi:10.1002/jmd2.12114
Wolf, A., Agnihotri, S., Micallef, J., Mukherjee, J., Sabha, N., Cairns, R., et al. (2011). Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J. Exp. Med. 208 (2), 313–326. doi:10.1084/jem.20101470
Wolff, N. A., Garrick, M. D., Zhao, L., Garrick, L. M., Ghio, A. J., and Thévenod, F. (2018). A role for divalent metal transporter (DMT1) in mitochondrial uptake of iron and manganese. Sci. Rep. 8 (1), 211. doi:10.1038/s41598-017-18584-4
Wrzosek, A., Augustynek, B., Żochowska, M., and Szewczyk, A. (2020). Mitochondrial potassium channels as druggable targets. Biomolecules 10 (8), 1200. doi:10.3390/biom10081200
Wu, X., Li, L., and Jiang, H. (2018). Mitochondrial inner-membrane protease Yme1 degrades outer-membrane proteins Tom22 and Om45. J. Cell. Biol. 217 (1), 139–149. doi:10.1083/jcb.201702125
Yamanaka, R., Tabata, S., Shindo, Y., Hotta, K., Suzuki, K., Soga, T., et al. (2016). Mitochondrial Mg(2+) homeostasis decides cellular energy metabolism and vulnerability to stress. Sci. Rep. 6, 30027. doi:10.1038/srep30027
Yang, F., He, X. P., Russell, J., and Lu, B. (2003). Ca2+ influx-independent synaptic potentiation mediated by mitochondrial Na(+)-Ca2+ exchanger and protein kinase C. J. Cell. Biol. 163 (3), 511–523. doi:10.1083/jcb.200307027
Zavodnik, I. B. (2016). Mitochondria, calcium homeostasis and calcium signaling. Biomed. Khim 62 (3), 311–317. doi:10.18097/pbmc20166203311
Zhang, D. X., Chen, Y. F., Campbell, W. B., Zou, A. P., Gross, G. J., and Li, P. L. (2001). Characteristics and superoxide-induced activation of reconstituted myocardial mitochondrial ATP-sensitive potassium channels. Circ. Res. 89 (12), 1177–1183. doi:10.1161/hh2401.101752
Zhang, L., Dietsche, F., Seitaj, B., Rojas-Charry, L., Latchman, N., Tomar, D., et al. (2022). TMBIM5 loss of function alters mitochondrial matrix ion homeostasis and causes a skeletal myopathy. Life Sci. Alliance 5 (10), e202201478. doi:10.26508/lsa.202201478
Zhang, M., Yu, Q., Liu, Z., Liang, C., Zhang, B., and Li, M. (2017). UBX domain-containing proteins are involved in lipid homeostasis and stress responses in Pichia pastoris. Int. J. Biochem. Cell. Biol. 90, 136–144. doi:10.1016/j.biocel.2017.08.006
Zhang, X., Chen, G., Lu, Y., Liu, J., Fang, M., Luo, J., et al. (2014). Association of mitochondrial letm1 with epileptic seizures. Cereb. cortex 24 (10), 2533–2540. doi:10.1093/cercor/bht118
Zhang, Z., Chen, Z., Liu, R., Liang, Q., Peng, Z., Yin, S., et al. (2020). Bcl-2 proteins regulate mitophagy in lipopolysaccharide-induced acute lung injury via PINK1/parkin signaling pathway. Oxidative Med. Cell. Longev. 2020, 6579696. doi:10.1155/2020/6579696
Zhao, H., Li, T., Wang, K., Zhao, F., Chen, J., Xu, G., et al. (2019). AMPK-mediated activation of MCU stimulates mitochondrial Ca2+ entry to promote mitotic progression. Nat. Cell. Biol. 21 (4), 476–486. doi:10.1038/s41556-019-0296-3
Zhao, S., Xiao, P., Cui, H., Gong, P., Lin, C., Chen, F., et al. (2020). Hypothermia-induced ubiquitination of voltage-dependent anion channel 3 protects BV2 microglia cells from cytotoxicity following oxygen-glucose deprivation/recovery. Front. Mol. Neurosci. 13, 100. doi:10.3389/fnmol.2020.00100
Zhou, B., Kreuzer, J., Kumsta, C., Wu, L., Kamer, K. J., Cedillo, L., et al. (2019). Mitochondrial permeability uncouples elevated autophagy and lifespan extension. Cell. 177 (2), 299–314. doi:10.1016/j.cell.2019.02.013
Keywords: mitochondrial transporters, mitochondrial channels, posttranslational modifications, MPQC, MCU, VDAC, MPTP, SLCs
Citation: Kadam A, Jadiya P and Tomar D (2023) Post-translational modifications and protein quality control of mitochondrial channels and transporters. Front. Cell Dev. Biol. 11:1196466. doi: 10.3389/fcell.2023.1196466
Received: 29 March 2023; Accepted: 24 July 2023;
Published: 03 August 2023.
Edited by:
Karthik Babu Mallilankaraman, National University of Singapore, SingaporeReviewed by:
Diego De Stefani, University of Padua, ItalyGayathri K Natarajan, Medical College of Wisconsin, United States
Copyright © 2023 Kadam, Jadiya and Tomar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pooja Jadiya, cGphZGl5YUB3YWtlaGVhbHRoLmVkdQ==; Dhanendra Tomar, ZHRvbWFyQHdha2VoZWFsdGguZWR1
 Ashlesha Kadam
Ashlesha Kadam Pooja Jadiya
Pooja Jadiya Dhanendra Tomar
Dhanendra Tomar