
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Cell Dev. Biol. , 28 April 2023
Sec. Membrane Traffic and Organelle Dynamics
Volume 11 - 2023 | https://doi.org/10.3389/fcell.2023.1177303
This article is part of the Research Topic Endocytic and Extracellular Vesicles: Physiology, Mechanisms, and Modeling View all 4 articles
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by chronic synovitis and the destruction of bones and joints. Exosomes are nanoscale lipid membrane vesicles originating from multivesicular bodies and are used as a vital means of intercellular communication. Both exosomes and the microbial community are essential in RA pathogenesis. Multiple types of exosomes from different origins have been demonstrated to have effects on various immune cells through distinct mechanisms in RA, which depend on the specific cargo carried by the exosomes. Tens of thousands of microorganisms exist in the human intestinal system. Microorganisms exert various physiological and pathological effects on the host directly or through their metabolites. Gut microbe-derived exosomes are being studied in the field of liver disease; however, information on their role in the context of RA is still limited. Gut microbe-derived exosomes may enhance autoimmunity by altering intestinal permeability and transporting cargo to the extraintestinal system. Therefore, we performed a comprehensive literature review on the latest progress on exosomes in RA and provided an outlook on the potential role of microbe-derived exosomes as emerging players in clinical and translational research on RA. This review aimed to provide a theoretical basis for developing new clinical targets for RA therapy.
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by chronic persistent synovitis, systemic inflammation, and the presence of autoantibodies such as rheumatoid factor and anti-cyclic citrullinated peptide (anti-CCP) (Lee and Weinblatt, 2001; Scott et al., 2010). Untreated RA may lead to progressive disability and reduced quality of life, profoundly affecting the patient’s and family’s physical and mental health and increasing the burden on the whole family and society (Meier et al., 2013). For the clinical management of patients with RA, the first treatment principle is early detection, early intervention, and clinical remission with immunosuppression under the guidance of a physician (Meier et al., 2013). Currently, the main therapeutic drugs for RA include disease-modifying anti-rheumatic drugs, non-steroidal anti-inflammatory drugs, hormonal drugs, and new biologics to improve the treatment of the disease. Although certain clinical efficacy has been achieved, due to the complex factors of the disease itself, some patients still show no or poor/little response; therefore, the discovery of new disease targets and the development of clinical drugs with this is a top priority (Zhao et al., 2021).
RA risk factors include genetic, environmental, metabolic, immune, and microbial interactions, which lead to abnormal pathological features (Scott et al., 2010). Among these, multiple mechanisms of microbial community disorders are considered important pathological mechanisms (de Oliveira et al., 2017). Humans are multicellular organisms that co-exist as macroscopic beings with a diverse symbiotic microbial community, which is conservatively estimated to substantially exceed the number of constituent cells of humans (approximately 100 trillion). The microbial community also expresses more unique genes in its genome (Belkaid and Harrison, 2017). The existence of a large number of microbial communities contributes to the physiological functions of the body through a variety of mechanisms. The interaction between the microbial community and the host immune system is crucial to the local and systemic immune responses. Maintaining the balance of the microbiota is an essential factor for maintaining homeostasis (Lu et al., 2022). The primary association between gut microbes and RA is that preclinical and clinical patients with RA have abnormal changes in gut microbiota and gut microbial metabolites. These abnormal changes in microbial communities and metabolites may lead to abnormal immune recognition responses mediating the autoimmune response in RA.
The International Society for Extracellular Vesicles proposed Minimal Information for Studies of Extracellular Vesicles (“MISEV”) guidelines suggest the use of “EVs” to generally denote a heterogeneous extracellular vesicle population, and “exosomes” are defined as small extracellular vesicles (Cocucci and Meldolesi, 2015; Théry et al., 2018). Multiple sources of extracellular vesicles exist in RA, which exerts effects on various immune cells. As for the extracellular vesicles themselves, they are an emerging and widely researched topic; they are being used as a diagnostic and therapeutic tool in the treatment of various diseases as a critical mechanism of intercellular communication, organ homeostasis, and disease (Kalluri and LeBleu, 2020). Microbe-derived exosomes are emerging stars in the field of exosomes that may play a crucial role in liver diseases. Their effects may include anti-inflammatory, intestinal barrier restorative, and immune modulatory effects, among other mechanisms (Zhang et al., 2022). Given the link between microbes and exosomes and RA, we present a summary and review of both and further examine the role of microbe-derived exosomes in the clinical management of RA to provide new theoretical support and options for the clinical management of RA.
Exosomes are defined as a set of vesicle-like structures released by all cell types, whose contain and exposure at their membrane surface protein and lipid components as well as nucleic acids originating from their original cell (Turpin et al., 2016). Exosomes are unable to replicate independently and are known by various names, such as microvesicles and microparticles (Miao et al., 2022). The extracellular vesicle can primarily be classified into two major categories, namely, ectosomes and exosomes. Ectosomes represent vesicles that result from the direct outward protrusion of the plasma membrane, generating entities such as microvesicles, microparticles, and large vesicles with sizes ranging from approximately 50 nm to 1 μm in diameter. Conversely, exosomes originate from endosomes and exhibit sizes within the range of approximately 40 nm–160 nm in diameter, with an average size of around 100 nm (Kalluri and LeBleu, 2020). The production of exosomes involves a double invagination of the plasma membrane and the creation of intracellular multivesicular bodies (MVBs) containing intraluminal vesicles (ILVs). The ILVs are eventually released as exosomes, which have a diameter ranging from approximately 40 nm–160 nm, through the process of MVB fusion with the plasma membrane and subsequent exocytosis (Kalluri and LeBleu, 2020). In accordance with the guidelines of The International Society for Extracellular Vesicles 2018 and consistency throughout the text, we use exosomes consistently throughout the text (Cocucci and Meldolesi, 2015; Théry et al., 2018). Exosomes mainly mediate intercellular messaging and are involved in numerous physiological processes, such as inflammation, immune response, angiogenesis, cellular stress, cellular senescence, cell proliferation, and differentiation (Turpin et al., 2016). Transcriptomic analysis revealed that exosomes transport a variety of cargoes, including many functional small coding miRNAs, messenger RNA (mRNA), and non-coding RNA (ncRNAs) (Lee et al., 2007; Kaparakis-Liaskos and Ferrero, 2015). Proteomic analysis has demonstrated that exosomes also transport other cargo, including membrane-bound proteins, enzymes (e.g., autolysins), toxins, polysaccharides, and peptidoglycans (Lee et al., 2007; Kaparakis-Liaskos and Ferrero, 2015). There exist different patterns of extracellular vesicle in RA. For instance, Hirotaka et al. found significant differences in the serum exosomes protein profile between active RA patients, osteoarthritis (OA) patients, and healthy individuals. Specifically, RA patients exhibited higher levels of TLR3 and pro-neuregulin-3 proteins, which may reflect the hyperactive state of RA fibroblast-like synoviocytes (FLS) (Tsuno et al., 2018). Proteomic analysis of plasma exosomes from RA patients and healthy controls identified 32 upregulated proteins and 5 downregulated proteins. Bioinformatics analysis indicated that these differentially expressed proteins interact with each other and mainly participate in inflammation pathways affecting RA (Qin et al., 2021). Results from a systematic review and meta-analysis showed that the total number of exosomes in both plasma and synovial fluid was increased in RA patients compared to healthy individuals, which is mainly associated with the release of cytokines and chemokines(Schioppo et al., 2021).Compared with osteoarthritis, gout, and axial spondyloarthritis, it was found that 28 proteins were uniquely upregulated in exosomes derived from RA synovial fluid. Bioinformatics analysis indicated their significant involvement in “serine-type endopeptidase inhibitor activity” and “complement and coagulation cascades” (Huang et al., 2022a). Therefore, We demonstrate the therapeutic potential of exosomes from different sources by elucidating their effects on different immune cell populations in RA.
RA fibroblast-like synovial cells (FLS) are a crucial population responsible for abnormal synovial proliferation, inflammation, and bone destruction. Exosomes originating from RA FLS themselves are shown to promote their abnormal proliferation and synovial hyperplasia by carrying certain substances secreted by the cells. RA FLS is an essential group of cells in RA that causes abnormal synovial proliferation, inflammation, and bone destruction. Exosomes from RA FLS promote their abnormal proliferation and synovial proliferation by carrying some substances secreted by themselves. For example, Exosomes from RA FLS contain membrane-bound forms of tumor necrosis factor-α (TNF-α), which promotes the activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-KB) and induction of membrane-type matrix metalloproteinase (MMP)-1 in RA FLS (Zhang et al., 2006). RA FLS overexpress and releases cluster of differentiation (CD) 13, which acts mainly as an intracellular soluble molecule and is encapsulated in exosomes, enhancing RA FLS proliferation and migration by acting on itself. Inhibition of CD13 can inhibit this effect (Morgan et al., 2016). Transforming growth factor beta (TGF-β) stimulates FLS to produce an inhibitor of DNA binding 1, which is passed to endothelial progenitor cells and human dermal microvascular endothelial cells and activates the c-Jun N-terminal kinases (JNKs) signaling pathway to promote angiogenesis through exosomes from RA FLS (Edhayan et al., 2016). Some exosomes from other sources have different effects on promoting and inhibiting RA FLS proliferation and inflammation, which depend on the functional roles of the cargo types carried by the exosomes. For example, the peripheral blood mononuclear cell-derived exosome lncRNA nuclear-enriched abundant transcript 1 (NEAT1) targets and regulates the miR-23a/mouse double minute 2 homolog (MDM2)/sirtuin 6 (SIRT6) axis to promote proliferation and inflammation in RA FLS (Rao et al., 2020). However, T cells release large amounts of derived exosomes, miR-204-5p, to transfer to FLS and inhibit cell proliferation (Wu et al., 2022). It is noteworthy that exosomes derived from mesenchymal stem cells have been extensively studied and proven to be beneficial for RA, including: i) Reducing vascular endothelial growth factor (VEGF) expression and angiogenesis: For example, Synovial mesenchymal cell-derived exosomes inhibit miR-485-3p/signal transducer and activator of transcription 3 (STAT3) by transmitting circRNA-circEDIL3 to reduce VEGF expression and angiogenic activity in RA FLS induced in vitro and reduce the extent of arthritis in collagen-induced arthritis (CIA) models (Zhang et al., 2021). Similarly, mesenchymal stem cell-derived miR-150-5p targeting membrane-type matrix metalloproteinase 14(MMP14), and VEGF reduced angiogenic activity and clinical arthritis scores in arthritic mice (Chen et al., 2018). ii) Inhibiting synovial inflammation, synovial hyperplasia, and bone destruction. For example, human umbilical cord mesenchymal stem cells (MSCs)-derived exosome miR-140-3p inhibited inflammation, oxidative stress, and fibrotic response and suppressed synovial proliferation by silencing serum- and glucocorticoid-induced kinase 1 (SGK1) in CIA models (Huang et al., 2022b). MSCs-derived exosomes of circFBXW7 and miR-320a can inhibit cell proliferation, migration, inflammatory response, and bone damage in RA FLS by directly targeting miR-216a-3p to upregulate histone deacetylase 4 (HDAC4) and C-X-C motif chemokine 9 (CXCL9) (Meng and Qiu, 2020; Chang and Kan, 2021). Chondrogenic bone marrow mesenchymal stem cell-derived exosomes directly inhibit RA FLS pro-inflammatory factors, MMP production, and activation of mitogen-activated protein kinase (MAPK) and NF-KB pathways and ameliorate arthritis in CIA mice (Ma et al., 2022).
T helper 17 (Th17) cells, a subset of T cells, are a pro-inflammatory cell population in RA. Exosomes from different sources can affect disease progression by influencing the proliferation of CD4+ T cells and their differentiation towards Th17 cells. For example, autoimmune T cells engulfing RA FLS-derived exosomes cause increased resistance to apoptosis and prolong the survival time (Zhang et al., 2006). The lncRNA NEAT in RA blood exosomes promotes CD4+ T cell proliferation and Th17 cell differentiation by regulating the miR-144-3p/rho associated coiled-coil containing protein kinase 2(ROCK2)/WNT axis for disease progression (Liu et al., 2021). Interleukin (IL)-17, a pro-inflammatory cytokine secreted by Th17 cells, is an important pro-inflammatory factor in RA. Exosomes derived from human gingival mesenchymal stem cells inhibited IL-17A secretion and promoted the secretion of the anti-inflammatory factor IL-10, reduced bone erosion, and improved arthritis in CIA models and in vitro CD4+ T cell co-culture models (Tian et al., 2022). This further supports the link between the mouth and joints. A reduction in T cell differentiation towards the regulatory T (Treg) cells is a key factor in inflammation in RA, as Treg cells are an important cell population that suppresses Th17 differentiation. The effects of exosomes from different sources on Treg cell may be bidirectional. On the one hand, they may promote inflammation by inhibiting Treg cells. For example, exosomes derived from RA FLS lncRNA HOXA distal transcript antisense RNA (HOTTIP) negatively regulate miR-1908-5p and positively regulate STAT3, leading to upregulation of forkhead box P3 (FOXP3) and retinoid-related orphan receptor gamma (RORγt) expression and Th17/Treg cell ratio imbalance (Yao et al., 2021). Similarly, the RA joint cavity exhibited an overall hypoxic microenvironment owing to the over-proliferation of FLS. miR-424 in exosomes derived from RA FLS significantly induced Th17 differentiation and inhibited Treg cells differentiation under hypoxic conditions (Ding et al., 2020). On the other hand, they may promote the suppression of inflammation by enhancing Treg cells. For example, exosomes from MSCs can significantly suppress T- and B cell immune responses, increase Treg cells in vitro, and significantly suppress inflammatory responses in CIA models (Cosenza et al., 2018). miR-146a/miR-155 in MSCs exosomes suppress aberrant immune responses by increasing FOXP3, RORγt, TGF-β, IL-10, IL-17, and IL-6 expression and increasing the Treg cell population in CIA models (Tavasolian et al., 2020).
In addition, autoimmune B cells are the source of many autoantibodies, and regulatory B (Breg) cells suppress inflammation by secreting IL-10 and other anti-inflammatory factors in RA. Exosomal prostaglandin E2 secreted by granulocytic myeloid-derived suppressor cells can significantly upregulate glycogen synthase kinase-3 beta (GSK-3 beta), and adenosine triphosphate response element-binding protein phosphorylation can promote IL-10+ Breg production to suppress inflammation in CIA models (Wu et al., 2020).
The destruction of osteoblasts and the excessive production of osteoclasts are important factors in bone destruction in RA. On the one hand, some exosomes may promote the process of bone destruction. Reacher found that the levels of exosomal receptor activator of nuclear factor kappa-B ligand (RANKL) isolated from the synovial fluid of patients with RA were significantly higher than those of patients with several other types of arthritis and induced higher numbers of osteoclasts involved in bone destruction (Song et al., 2021). Smoking is considered a risk factor for RA (Zhao et al., 2021). Cigarette smoke promotes exosomal miR-132 secretion by Th17 cells, downregulating cyclooxygenase-2 (COX2) to promote osteoclastogenesis, and knockdown of miR132 in an arthritis model reduced the number of osteoclasts (Donate et al., 2021). Exosomes originating from RA FLS affect the RANKL/RANK/osteoprotegerin (OPG) pathway by transporting miR-106b to chondrocytes and inhibiting pyruvate dehydrogenase kinase isozyme 4 (PDK4), which inhibits chondrocyte proliferation and migration and accelerates apoptosis. In vivo inhibition of miR-106b improves arthritic symptoms in a CIA model (Liu et al., 2020). On the other hand, the researcher also found that in vitro osteoblasts can phagocytose miR-486-5p of exosomes originating from RA FLS and increase miRNA expression, which inhibits transducer of erb-b2 receptor tyrosine kinase 2, 1(TOB1) and activates the bone morphogenetic protein (BMP)/suppressor of mothers against decapentaplegic (SMAD) pathway to promote osteoblast differentiation, which is potentially beneficial in RA treatment (Chen et al., 2020).
Research on exosomes from different sources for their effects on macrophages in RA is still relatively limited. Secretion of miR-let-7b-containing exosomes by macrophages themselves promotes the differentiation of M1-type pro-inflammatory macrophages in RA joint inflammation by binding Toll-like receptor 7 through the GU structural domain (Kim et al., 2016). Additionally, serum-derived exosome miR-6089 and miR-548a-3p can regulate macrophage proliferation and differentiation, as well as inflammatory factor production, by targeting Toll-like receptor 4 (TLR4) (Wang et al., 2018; Xu et al., 2019). This is also in line with the pro-inflammatory role of macrophages themselves. miR-223 from exosomes sourced from bone marrow mesenchymal stem cells inhibits the release of IL-1β, TNF-α, IL-18 and the activation of the NOD-like receptor protein 3 (NLRP3) to attenuate macrophage inflammation (Huang et al., 2022c). Notably, a new type of nanovesicle was made by combining the membrane of an M1 macrophage with nanovesicles that mimic exosomes from M2 macrophages (Zhao et al., 2022). The nanovesicle has the ability to effectively reduce inflammation by binding to proinflammatory molecules and releasing anti-inflammatory mediators (Zhao et al., 2022). This comprehensive anti-inflammatory activity makes it a powerful tool for treating inflammatory diseases such as rheumatoid arthritis (Zhao et al., 2022) Figure 1.
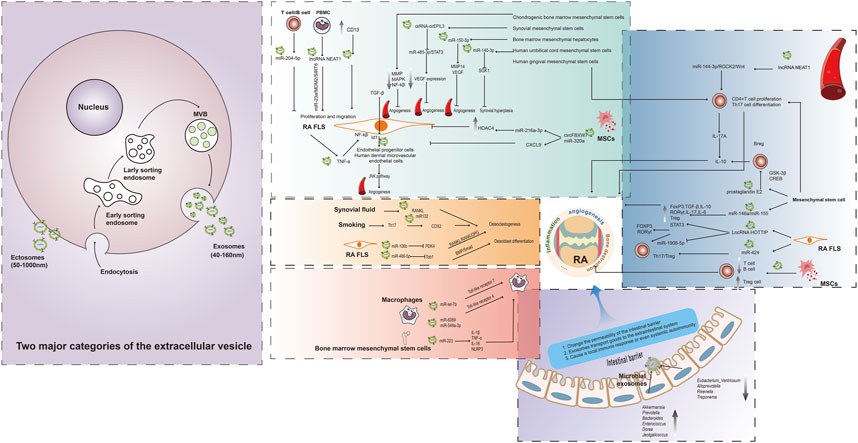
FIGURE 1. Association of multiple exosomes with RA. The extracellular vesicle can primarily be classified into two major categories, namely, ectosomes and exosomes. Ectosomes represent vesicles that result from the direct outward protrusion of the plasma membrane, generating entities such as microvesicles, microparticles, and large vesicles with sizes. The production of exosomes involves a double invagination of the plasma membrane and the creation of intracellular multivesicular bodies (MVBs) containing intraluminal vesicles (ILVs). The ILVs are eventually released as exosomes through the process of MVB fusion with the plasma membrane and subsequent exocytosis. Blood, FLS, mesenchymal stem cells, and other cell-derived exosomes are all associated with RA through different mechanisms. Gut microbe-derived exosomes are mainly involved in altering the permeability of the intestinal barrier and transporting cargo to the extraintestinal system affecting the immune system, and may be a novel target for future RA therapy.
The intestine is the organ with the largest surface area in the body. Various microorganisms are colonized in the intestine, and these microbial communities and their products play a vital role in the immune system (Round and Mazmanian, 2009). The pathophysiological relevance of gut microbes in RA may vary (Table 1). The possible mechanism by which gut microbes influence autoimmunity is as follows:
(i). Intestinal barrier dysfunction: Intestinal barrier dysfunction caused by intestinal microbial disorders is an important checkpoint for inflammation in CIA models and RA patients, and the use of the intestinal barrier modulator butyrate can effectively inhibit arthritis (Tajik et al., 2020). There are abnormal changes at the phylum or genus level in various microorganisms in RA. For example, the α- and β-diversity indices of the microbiome are significantly reduced in mice with experimental RA, and they have an elevated proportion of Firmicutes and Bacteroidetes. Four core genera, including Eubacterium_Ventriosum, Alloprevotella, Rikenella, and Treponema, were reduced in fecal and mucus content (Nguyen et al., 2022).
(ii). Molecular mimicry, in which gut microbes or metabolites trigger an autoimmune response of immune cells. The epitopes of Prevotella sp., Parabacteroides sp., and Butyricimonas sp. have obvious homology sequence with two autoantigens, N-acetylglucosamine-6-sulfatase, and filamin A, presented by major histocompatibility complex II cell surface receptor in RA (Pianta et al., 2017).Pattern recognition receptors on the surface of antigen-presenting cells constantly recognize and respond to potential antigens in the intestinal lumen, which often include some autoantigens. For example, antigen-presenting cells recognize gut microbes and metabolites (such as LPS and polysaccharide analogs) as potential antigens, leading to downstream T cell cascade differentiation and immune responses, which may initially result in local immunity. However, they can cause systemic autoimmune responses with lymph nodes or humoral circulation (Horta-Baas et al., 2017).
(iii). Regulation of multiple immune cells by multiple metabolites derived from intestinal microorganisms: mainly short-chain fatty acids (butyrate, acetate) and amino acid metabolites (tryptophan metabolism), which can also affect systemic immunity through abnormal intestinal barrier function into the circulation, and the detailed mechanisms can be found in the excellent review (Yang and Cong, 2021). In addition to changes in the microbial community, there were changes in its primary metabolites (short-chain fatty acids, bile acids, amino acids, and tryptophan metabolites) in RA. For example, the Firmicutes/Bacteroidetes ratio was low at the gate level in experimental arthritic mice with adjuvant induced arthritis (AIA). At the genus level, there was an abundance of Akkermansia, Prevotella, Bacteroides, Enterococcus, Dorea, and Jeotgalicoccus and a lower abundance of Oscillospira. Metabolites included acetic acid, propionic acid, butyric acid, valeric acid, capric acid, lactic acid, primary bile acids, secondary bile acids, conjugated bile acids, lipopolysaccharides (LPS), and conjugated bile acids (Cheng et al., 2022).
Abnormal responses to RA can be ameliorated by interventions to improve abnormal microbial changes including some RA clinical agents. For example, increased beneficial microbiota in patients with RA treated with the anti-tumor necrosis factor antibody etanercept (Picchianti-Diamanti et al., 2018). Methotrexate (MTX) may predominantly affect Bacteroidetes, and transplantation of microorganisms from patients with RA receiving MTX into germ-free mice under inflammatory stimuli results in a reduced inflammatory response, including reduced activation of multiple immune cells (Nayak et al., 2021). Therefore, correcting the abnormal changes in gut microbiota and metabolites in RA is one of the important research directions of the gut-joint axis. It should be noted that exosomes derived from gut microbiota may be an important component of the gut-joint axis, but as relevant research is still relatively scarce, we will speculate and prospect on the relevant theoretical basis in the following, in anticipation of providing a theoretical basis for future experimental research.
Gut microbe-derived exosomes have been suggested as vital mediators of immune transmission in the intestinal mucosa (Kaparakis-Liaskos and Ferrero, 2015; Olsen and Amano, 2015). There are four main types of exosomes derived from intestinal microorganisms: outer membrane vesicles, outer inner membrane vesicles, explosive outer membrane vesicles produced by gram-negative bacteria, and cytoplasmic membrane vesicles produced by gram-positive bacteria (Zhang et al., 2022). In regards to how exosomes from gut microbiota participate in the gut-joint axis, we have the following speculations:
(i). The intact intestinal barrier can prevent excessive activation of the mucosal immune system and inflammatory responses in the intestine (1). For example, exosomes secreted by the probiotic E. coli strain Nissle 1917 or commensal E. coli strain ECOR12 can promote the secretion of various inflammatory cytokines and chemokines, including IL-6, IL-8, TNF-α, and chemokine (C-C motif) ligand 3 (CCL3), from peripheral blood mononuclear cells in vitro and were unable to elevate these factors in an in vitro model of intestinal mucosa co-cultured with Caco-2/peripheral blood mononuclear cells, suggesting that this intact intestinal barrier prevents transitory activation of intestinal mucosal immunity and inflammatory responses (Fábrega et al., 2016).
The gut microbiota and its metabolic products can exert their effects beyond the intestinal area through various complex mechanisms. For example, studies have reported that probiotic intake can improve abnormal disease behaviors through communication from the peripheral immune system to the brain, such as immobility, which is commonly seen in patients with RA, by mechanisms that may involve reducing the number of TNF-α signals in the peripheral immune system that are recruited to monocytes in the brain in response to inflammation and the subsequent activation of brain microglia (D'Mello et al., 2015). Complicated lung disease is a leading cause of death in patients with RA. segmented filamentous bacteria (SFB) induce pulmonary autoimmunity by amplifying Th17 cells expressing dual T cell receptors, recognizing their antigenic epitopes, and inducing pulmonary injury in combination with pulmonary expression of C-C motif chemokine ligand 17 (CCL17) (Bradley et al., 2017).
Disturbances in the microbial communities or metabolic can lead to local inflammatory responses in the intestine and increase intestinal permeability (Alghamdi et al., 2021), which may contribute to the systemic diffusion of autoantibodies and the inflammatory response of the joints in RA. For example, the overall gut microbial diversity was reduced in patients with RA and correlated with disease duration and autoantibody levels, and Collinsella was significantly associated with the production of pro-inflammatory cytokines and altered intestinal permeability (Chen et al., 2016). In addition, the gut microbial metabolite short-chain fatty acid butyrate was reduced in patients with RA and animal models of arthritis, which may also lead to altered intestinal permeability (Rosser et al., 2020).
(ii). Autoantibody production is a distinctive characteristic of RA, and these autoantibodies may form immune complexes in the joints, leading to chemokine and cytokine secretion through complement activation or direct activation of immune cells, thereby attracting immune cells and enhancing the immune response, ultimately resulting in chronic inflammation and bone destruction (van Delft and Huizinga, 2020).The intestine may be an essential source of autoantibody production. Gut microbe-derived exosomes can be found in tissues and blood throughout the body and interact with multiple immune cells, suggesting that gut-derived exosomes can act as communicators between the intestinal and parenteral systems (Jones et al., 2020; Bittel et al., 2021). They may act as carriers to transport a variety of substances to extraintestinal systems, including joints. When multiple cells release intracellular mitochondrial DNA due to injury, dendritic cells can recognize this DNA to drive Th1 cell polarization in autoimmunity. Exosomes derived from intestinal microbes may initiate an immune response by transporting bacterial DNA, leading to the recognition of CpG motifs by dendritic cells (Lin and Zhang, 2017). In addition, there is a significant increase in gram-negative bacteria in RA, which induces the activation of Th17 cells and immune responses to autoantigens. This is also consistent with the hypothesis of an additional RA-gut link, in which toxic metabolites secreted by intestinal gram-negative bacteria enter the circulation, leading to systemic immunity and autoinflammation (Brusca et al., 2014; de Oliveira et al., 2017). Therefore, exosomes derived from intestinal microbes may also serve as important carriers for transporting self-antibodies produced in the gut to other parts of the body, leading to the inflammatory and bone-destructive processes. Research suggests that synovial-derived exosomes in RA can transport self-antibodies to enhance the autoreactive immune response through the process of antigen presentation (Alghamdi et al., 2021). The symptoms of arthritis in mice were greatly reduced when exposed to sterile conditions, in conjunction with a notable decrease in levels of serum autoantibody titers, splenic autoantibody-secreting cells, and Th17 cells. However, the loss of Th17 cells and autoantibody production in the lamina propria of the small intestine was restored when a single SFB was introduced (Wu et al., 2010).
In addition, miRNA-microbe interactions have been explored as a crucial mechanism in various diseases, and miRNA expression patterns in mouse and human fecal and intestinal lumen contents have also been explored (Liu et al., 2016a). Among them, gut microbe-derived exosomes can act as communication mediators to regulate host gene expression and even affect the microbial community (Liu et al., 2016a; Dong et al., 2019; Tavasolian and Inman, 2021). For example, transplantation of gut microbe-derived exosomal miRNAs from wild-type mice ameliorates intestinal barrier dysfunction and inflammation in systemic lupus erythematosus (Liu et al., 2016a). In the context of RA, miRNAs occupy an important position (Chang et al., 2022) and may also interact with the gut-joint axis through exosomes derived from gut microbes.
(iii). The link between gut microbes and joint inflammation has been established and in conjunction with the role of exosomes in RA, we speculate that gut microbe-derived exosomes are involved in the gut-joint axis:
As previously mentioned, there are pathological changes in gut microbiota in RA, including alterations in microbiota abundance and composition, which may result in the production of abnormal bioactive substances (such as metabolites), and impact the pathological features of RA in areas beyond the gut through various mechanisms (such as impaired intestinal barrier function). The abnormal changes in gut microbiota may transmit these aberrant bioactive substances through extracellular vesicles and lead to the subsequent progression of inflammation, angiogenesis, and bone destruction in RA, but further experiments are needed to investigate this phenomenon.
Different sources of exosomes may affect various immune cells in RA through multiple mechanisms, primarily including FLS, T cells, macrophages, osteoclasts, osteoblasts, and chondrocytes. The effects of exosomes from different sources on immune cells depend on the types and functions of the cargoes they carry. It is noteworthy that exosomes derived from mesenchymal stem cells appear to have an overall beneficial effect on RA, which is a promising direction for future development. Abnormal changes in the intestinal flora and multiple sources of exosomes have been observed to have multiple effects on the pathophysiological processes of RA. Although the quantitative proportions of gut microbiota, the most extensive symbiotic system in the human body, are abnormally altered in RA with specific alterations at the phylum and genus levels, metabolites have also been intensively studied and have given birth to many hypotheses. There are still many issues that need to be further elucidated, for example, to clarify the clear linkage of one or several gut-joint axes, widely demonstrated by experiments; nevertheless, there is no doubt that the primary principle is to maintain a reasonable balance between the microbial community and the organism and to prevent ecological imbalances, which may prevent harmful consequences in the gut as well as in the extraintestinal system. Based on the present hypothesis, using samples of preclinical models and clinical patients combined with proteomics, metabolomics, metagenomics technologies, and bioinformatics are essential tools for analysis. Finally, adopting multiple basic molecular biologies and microbiology approaches for continued validation is a necessary direction for the future. Due to the current technical limitations of exosomes, there are still various limitations for their isolation, extraction, and classification, which are proposed to be solved in the future. This is a blank field in the field of RA for microbe-derived exosomes. Gut-derived exosomes may serve as a crucial bridge between gut microbiota and RA. Nevertheless, we have provided reasonable hypotheses on the link between microbe-derived exosomes and the disease based on the association of microbial communities and exosomes with RA. Unfortunately, current relevant studies do not provide sufficient depth; therefore, we can only make appropriate hypotheses on the mechanism of action based on existing studies, mainly related to the alteration of intestinal permeability and possible transport of cargo to extraintestinal systems. Further experimental corroboration is required for in-depth studies. The future direction of our team is to build on this knowledge and gradually enhance future strategic research on the gut-joint axis, which is of great economic and clinical value.
JZ and BZ is responsible for the collection, collation, and writing of the original manuscript. MW is responsible for the collection. JH are responsible for the concept development, revision, and manuscript review. All authors reviewed and accepted the final version.
This work was funded by the Famous Traditional Chinese Medicine” Talent Training Plan of the Seventh People’s Hospital affiliated to Shanghai University of Traditional Chinese Medicine (MZY2021-01); “Medical Craftsman” Talent Training Plan of the Seventh People’s Hospital affiliated to Shanghai University of Traditional Chinese Medicine (GJ2021-06); Pudong New Area Traditional Chinese Medicine Brand Multiplication Plan—Chronic Nephropathy (PDZY-2021-0302), Construction of He Liqun’s famous TCM studio. Project supported by Shanghai Municipal Science and Technology Major Project (ZD2021CY001).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdollahi-Roodsaz, S., Joosten, L. A., Koenders, M. I., Devesa, I., Roelofs, M. F., Radstake, T. R., et al. (2008). Stimulation of TLR2 and TLR4 differentially skews the balance of T cells in a mouse model of arthritis. J. Clin. investigation 118 (1), 205–216. doi:10.1172/jci32639
Alghamdi, M., Alamry, S. A., Bahlas, S. M., Uversky, V. N., and Redwan, E. M. (2021). Circulating extracellular vesicles and rheumatoid arthritis: A proteomic analysis. Cell. Mol. Life Sci. 79 (1), 25. doi:10.1007/s00018-021-04020-4
Alpizar-Rodriguez, D., Lesker, T. R., Gronow, A., Gilbert, B., Raemy, E., Lamacchia, C., et al. (2019). Prevotella copri in individuals at risk for rheumatoid arthritis. Ann. rheumatic Dis. 78 (5), 590–593. doi:10.1136/annrheumdis-2018-214514
Belkaid, Y., and Harrison, O. J. (2017). Homeostatic immunity and the microbiota. Immunity 46 (4), 562–576. doi:10.1016/j.immuni.2017.04.008
Bittel, M., Reichert, P., Sarfati, I., Dressel, A., Leikam, S., Uderhardt, S., et al. (2021). Visualizing transfer of microbial biomolecules by outer membrane vesicles in microbe-host-communication in vivo. J. Extracell. Vesicles 10 (12), e12159. doi:10.1002/jev2.12159
Bradley, C. P., Teng, F., Felix, K. M., Sano, T., Naskar, D., Block, K. E., et al. (2017). Segmented filamentous bacteria provoke lung autoimmunity by inducing gut-lung Axis Th17 cells expressing dual TCRs. Cell Host Microbe 22 (5), 697–704. doi:10.1016/j.chom.2017.10.007
Brusca, S. B., Abramson, S. B., and Scher, J. U. (2014). Microbiome and mucosal inflammation as extra-articular triggers for rheumatoid arthritis and autoimmunity. Curr. Opin. Rheumatol. 26 (1), 101–107. doi:10.1097/bor.0000000000000008
Chang, C., Xu, L., Zhang, R., Jin, Y., Jiang, P., Wei, K., et al. (2022). MicroRNA-mediated epigenetic regulation of rheumatoid arthritis susceptibility and pathogenesis. Front. Immunol. 13, 838884. doi:10.3389/fimmu.2022.838884
Chang, L., and Kan, L. (2021). Mesenchymal stem cell-originated exosomal circular RNA circFBXW7 attenuates cell proliferation, migration and inflammation of fibroblast-like synoviocytes by targeting miR-216a-3p/HDAC4 in rheumatoid arthritis. J. Inflamm. Res. 14, 6157–6171. doi:10.2147/jir.S336099
Chen, J., Liu, M., Luo, X., Peng, L., Zhao, Z., He, C., et al. (2020). Exosomal miRNA-486-5p derived from rheumatoid arthritis fibroblast-like synoviocytes induces osteoblast differentiation through the Tob1/BMP/Smad pathway. Biomater. Sci. 8 (12), 3430–3442. doi:10.1039/c9bm01761e
Chen, J., Wright, K., Davis, J. M., Jeraldo, P., Marietta, E. V., Murray, J., et al. (2016). An expansion of rare lineage intestinal microbes characterizes rheumatoid arthritis. Genome Med. 8 (1), 43. doi:10.1186/s13073-016-0299-7
Chen, Z., Wang, H., Xia, Y., Yan, F., and Lu, Y. (2018). Therapeutic potential of mesenchymal cell-derived miRNA-150-5p-expressing exosomes in rheumatoid arthritis mediated by the modulation of MMP14 and VEGF. J. Immunol. 201 (8), 2472–2482. doi:10.4049/jimmunol.1800304
Cheng, X., Pi, Z., Zheng, Z., Liu, S., Song, F., and Liu, Z. (2022). Combined 16S rRNA gene sequencing and metabolomics to investigate the protective effects of Wu-tou decoction on rheumatoid arthritis in rats. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1199, 123249. doi:10.1016/j.jchromb.2022.123249
Chiang, H. I., Li, J. R., Liu, C. C., Liu, P. Y., Chen, H. H., Chen, Y. M., et al. (2019). An association of gut microbiota with different phenotypes in Chinese patients with rheumatoid arthritis. J. Clin. Med. 8 (11), 1770. doi:10.3390/jcm8111770
Cocucci, E., and Meldolesi, J. (2015). Ectosomes and exosomes: Shedding the confusion between extracellular vesicles. Trends Cell Biol. 25 (6), 364–372. doi:10.1016/j.tcb.2015.01.004
Cosenza, S., Toupet, K., Maumus, M., Luz-Crawford, P., Blanc-Brude, O., Jorgensen, C., et al. (2018). Mesenchymal stem cells-derived exosomes are more immunosuppressive than microparticles in inflammatory arthritis. Theranostics 8 (5), 1399–1410. doi:10.7150/thno.21072
D'Mello, C., Ronaghan, N., Zaheer, R., Dicay, M., Le, T., MacNaughton, W. K., et al. (2015). Probiotics improve inflammation-associated sickness behavior by altering communication between the peripheral immune system and the brain. J. Neurosci. 35 (30), 10821–10830. doi:10.1523/jneurosci.0575-15.2015
de Oliveira, G. L. V., Leite, A. Z., Higuchi, B. S., Gonzaga, M. I., and Mariano, V. S. (2017). Intestinal dysbiosis and probiotic applications in autoimmune diseases. Immunology 152 (1), 1–12. doi:10.1111/imm.12765
Ding, Y., Wang, L., Wu, H., Zhao, Q., and Wu, S. (2020). Exosomes derived from synovial fibroblasts under hypoxia aggravate rheumatoid arthritis by regulating Treg/Th17 balance. Exp. Biol. Med. (Maywood) 245 (14), 1177–1186. doi:10.1177/1535370220934736
Donate, P. B., Alves de Lima, K., Peres, R. S., Almeida, F., Fukada, S. Y., Silva, T. A., et al. (2021). Cigarette smoke induces miR-132 in Th17 cells that enhance osteoclastogenesis in inflammatory arthritis. Proc. Natl. Acad. Sci. U. S. A. 118 (1), e2017120118. doi:10.1073/pnas.2017120118
Dong, J., Tai, J. W., and Lu, L. F. (2019). miRNA-microbiota interaction in gut homeostasis and colorectal cancer. Trends Cancer 5 (11), 666–669. doi:10.1016/j.trecan.2019.08.003
Edhayan, G., Ohara, R. A., Stinson, W. A., Amin, M. A., Isozaki, T., Ha, C. M., et al. (2016). Inflammatory properties of inhibitor of DNA binding 1 secreted by synovial fibroblasts in rheumatoid arthritis. Arthritis Res. Ther. 18, 87. doi:10.1186/s13075-016-0984-3
Fábrega, M. J., Aguilera, L., Giménez, R., Varela, E., Alexandra Cañas, M., Antolín, M., et al. (2016). Activation of immune and defense responses in the intestinal mucosa by outer membrane vesicles of commensal and probiotic Escherichia coli strains. Front. Microbiol. 7, 705. doi:10.3389/fmicb.2016.00705
Horta-Baas, G., Romero-Figueroa, M. D. S., Montiel-Jarquín, A. J., Pizano-Zárate, M. L., García-Mena, J., and Ramírez-Durán, N. (2017). Intestinal dysbiosis and rheumatoid arthritis: A link between gut microbiota and the pathogenesis of rheumatoid arthritis. J. Immunol. Res. 2017, 4835189. doi:10.1155/2017/4835189
Huang, Y., Chen, L., Chen, D., Fan, P., and Yu, H. (2022). Exosomal microRNA-140-3p from human umbilical cord mesenchymal stem cells attenuates joint injury of rats with rheumatoid arthritis by silencing SGK1. Mol. Med. 28 (1), 36. doi:10.1186/s10020-022-00451-2
Huang, Y., Liu, Y., Huang, Q., Sun, S., Ji, Z., Huang, L., et al. (2022). TMT-based quantitative proteomics analysis of synovial fluid-derived exosomes in inflammatory arthritis. Front. Immunol. 13, 800902. doi:10.3389/fimmu.2022.800902
Huang, Y., Lu, D., Ma, W., Liu, J., Ning, Q., Tang, F., et al. (2022). miR-223 in exosomes from bone marrow mesenchymal stem cells ameliorates rheumatoid arthritis via downregulation of NLRP3 expression in macrophages. Mol. Immunol. 143, 68–76. doi:10.1016/j.molimm.2022.01.002
Jones, E. J., Booth, C., Fonseca, S., Parker, A., Cross, K., Miquel-Clopés, A., et al. (2020). The uptake, trafficking, and biodistribution of Bacteroides thetaiotaomicron generated outer membrane vesicles. Front. Microbiol. 11, 57. doi:10.3389/fmicb.2020.00057
Kalluri, R., and LeBleu, V. S. (2020). The biology, function, and biomedical applications of exosomes. Science 367 (6478), eaau6977. doi:10.1126/science.aau6977
Kaparakis-Liaskos, M., and Ferrero, R. L. (2015). Immune modulation by bacterial outer membrane vesicles. Nat. Rev. Immunol. 15 (6), 375–387. doi:10.1038/nri3837
Kim, S. J., Chen, Z., Essani, A. B., Elshabrawy, H. A., Volin, M. V., Volkov, S., et al. (2016). Identification of a novel toll-like receptor 7 endogenous ligand in rheumatoid arthritis synovial fluid that can provoke arthritic joint inflammation. Arthritis Rheumatol. 68 (5), 1099–1110. doi:10.1002/art.39544
Kishikawa, T., Maeda, Y., Nii, T., Motooka, D., Matsumoto, Y., Matsushita, M., et al. (2020). Metagenome-wide association study of gut microbiome revealed novel aetiology of rheumatoid arthritis in the Japanese population. Ann. rheumatic Dis. 79 (1), 103–111. doi:10.1136/annrheumdis-2019-215743
Lee, D. M., and Weinblatt, M. E. (2001). Rheumatoid arthritis. Lancet 358 (9285), 903–911. doi:10.1016/s0140-6736(01)06075-5
Lee, E. Y., Bang, J. Y., Park, G. W., Choi, D. S., Kang, J. S., Kim, H. J., et al. (2007). Global proteomic profiling of native outer membrane vesicles derived from Escherichia coli. Proteomics 7 (17), 3143–3153. doi:10.1002/pmic.200700196
Lee, J. Y., Mannaa, M., Kim, Y., Kim, J., Kim, G. T., and Seo, Y. S. (2019). Comparative analysis of fecal microbiota composition between rheumatoid arthritis and osteoarthritis patients. Genes 10 (10), 748. doi:10.3390/genes10100748
Lin, L., and Zhang, J. (2017). Role of intestinal microbiota and metabolites on gut homeostasis and human diseases. BMC Immunol. 18 (1), 2. doi:10.1186/s12865-016-0187-3
Liu, D., Fang, Y., Rao, Y., Tan, W., Zhou, W., Wu, X., et al. (2020). Synovial fibroblast-derived exosomal microRNA-106b suppresses chondrocyte proliferation and migration in rheumatoid arthritis via down-regulation of PDK4. J. Mol. Med. Berl. 98 (3), 409–423. doi:10.1007/s00109-020-01882-2
Liu, R., Jiang, C., Li, J., Li, X., Zhao, L., Yun, H., et al. (2021). Serum-derived exosomes containing NEAT1 promote the occurrence of rheumatoid arthritis through regulation of miR-144-3p/ROCK2 axis. Ther. Adv. Chronic Dis. 12, 2040622321991705. doi:10.1177/2040622321991705
Liu, S., da Cunha, A. P., Rezende, R. M., Cialic, R., Wei, Z., Bry, L., et al. (2016). The host shapes the gut microbiota via fecal MicroRNA. Cell Host Microbe 19 (1), 32–43. doi:10.1016/j.chom.2015.12.005
Liu, X., Zeng, B., Zhang, J., Li, W., Mou, F., Wang, H., et al. (2016). Role of the gut microbiome in modulating arthritis progression in mice. Sci. Rep. 6, 30594. doi:10.1038/srep30594
Liu, X., Zou, Q., Zeng, B., Fang, Y., and Wei, H. (2013). Analysis of fecal Lactobacillus community structure in patients with early rheumatoid arthritis. Curr. Microbiol. 67 (2), 170–176. doi:10.1007/s00284-013-0338-1
Lu, Y., Yuan, X., Wang, M., He, Z., Li, H., Wang, J., et al. (2022). Gut microbiota influence immunotherapy responses: Mechanisms and therapeutic strategies. J. Hematol. Oncol. 15 (1), 47. doi:10.1186/s13045-022-01273-9
Ma, W., Tang, F., Xiao, L., Han, S., Yao, X., Zhang, Q., et al. (2022). miR-205-5p in exosomes divided from chondrogenic mesenchymal stem cells alleviated rheumatoid arthritis via regulating MDM2 in fibroblast-like synoviocytes. J. Musculoskelet. Neuronal Interact. 22 (1), 132–141.
Meier, F. M., Frerix, M., Hermann, W., and Müller-Ladner, U. (2013). Current immunotherapy in rheumatoid arthritis. Immunotherapy 5 (9), 955–974. doi:10.2217/imt.13.94
Meng, Q., and Qiu, B. (2020). Exosomal MicroRNA-320a derived from mesenchymal stem cells regulates rheumatoid arthritis fibroblast-like synoviocyte activation by suppressing CXCL9 expression. Front. Physiol. 11, 441. doi:10.3389/fphys.2020.00441
Miao, H. B., Wang, F., Lin, S., and Chen, Z. (2022). Update on the role of extracellular vesicles in rheumatoid arthritis. Expert Rev. Mol. Med. 24, e12. doi:10.1017/erm.2021.33
Morgan, R. L., Behbahani-Nejad, N., Endres, J., Amin, M. A., Lepore, N. J., Du, Y., et al. (2016). Localization, shedding, regulation and function of aminopeptidase N/CD13 on fibroblast like synoviocytes. PLoS One 11 (9), e0162008. doi:10.1371/journal.pone.0162008
Muñiz Pedrogo, D. A., Chen, J., Hillmann, B., Jeraldo, P., Al-Ghalith, G., Taneja, V., et al. (2019). An increased abundance of clostridiaceae characterizes arthritis in inflammatory bowel disease and rheumatoid arthritis: A cross-sectional study. Inflamm. bowel Dis. 25 (5), 902–913. doi:10.1093/ibd/izy318
Nayak, R. R., Alexander, M., Deshpande, I., Stapleton-Gray, K., Rimal, B., Patterson, A. D., et al. (2021). Methotrexate impacts conserved pathways in diverse human gut bacteria leading to decreased host immune activation. Cell Host Microbe 29 (3), 362–377.e11. doi:10.1016/j.chom.2020.12.008
Nguyen, N. T., Sun, W. H., Chen, T. H., Tsai, P. C., Chen, C. C., and Huang, S. L. (2022). Gut mucosal microbiome is perturbed in rheumatoid arthritis mice and partly restored after TDAG8 deficiency or suppression by salicylanilide derivative. Int. J. Mol. Sci. 23 (7), 3527. doi:10.3390/ijms23073527
Olsen, I., and Amano, A. (2015). Outer membrane vesicles - offensive weapons or good Samaritans? J. Oral Microbiol. 7, 27468. doi:10.3402/jom.v7.27468
Pianta, A., Arvikar, S. L., Strle, K., Drouin, E. E., Wang, Q., Costello, C. E., et al. (2017). Two rheumatoid arthritis-specific autoantigens correlate microbial immunity with autoimmune responses in joints. J. Clin. Invest. 127 (8), 2946–2956. doi:10.1172/jci93450
Picchianti-Diamanti, A., Panebianco, C., Salemi, S., Sorgi, M. L., Di Rosa, R., Tropea, A., et al. (2018). Analysis of gut microbiota in rheumatoid arthritis patients: Disease-related dysbiosis and modifications induced by etanercept. Int. J. Mol. Sci. 19 (10), 2938. doi:10.3390/ijms19102938
Qin, Q., Song, R., Du, P., Gao, C., Yao, Q., and Zhang, J. A. (2021). Systemic proteomic analysis reveals distinct exosomal protein profiles in rheumatoid arthritis. J. Immunol. Res. 2021, 9421720. doi:10.1155/2021/9421720
Rao, Y., Fang, Y., Tan, W., Liu, D., Pang, Y., Wu, X., et al. (2020). Delivery of long non-coding RNA NEAT1 by peripheral blood monouclear cells-derived exosomes promotes the occurrence of rheumatoid arthritis via the MicroRNA-23a/MDM2/SIRT6 Axis. Front. Cell Dev. Biol. 8, 551681. doi:10.3389/fcell.2020.551681
Rodrigues, G. S. P., Cayres, L. C. F., Gonçalves, F. P., Takaoka, N. N. C., Lengert, A. H., Tansini, A., et al. (2019). Detection of increased relative expression units of Bacteroides and Prevotella, and decreased Clostridium leptum in stool samples from Brazilian rheumatoid arthritis patients: A pilot study. Microorganisms 7 (10), 413. doi:10.3390/microorganisms7100413
Rosser, E. C., Piper, C. J. M., Matei, D. E., Blair, P. A., Rendeiro, A. F., Orford, M., et al. (2020). Microbiota-derived metabolites suppress arthritis by amplifying aryl-hydrocarbon receptor activation in regulatory B cells. Cell Metab. 31 (4), 837–851. doi:10.1016/j.cmet.2020.03.003
Round, J. L., and Mazmanian, S. K. (2009). The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 9 (5), 313–323. doi:10.1038/nri2515
Scher, J. U., Sczesnak, A., Longman, R. S., Segata, N., Ubeda, C., Bielski, C., et al. (2013). Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife 2, e01202. doi:10.7554/eLife.01202
Schioppo, T., Ubiali, T., Ingegnoli, F., Bollati, V., and Caporali, R. (2021). The role of extracellular vesicles in rheumatoid arthritis: A systematic review. Clin. Rheumatol. 40 (9), 3481–3497. doi:10.1007/s10067-021-05614-w
Scott, D. L., Wolfe, F., and Huizinga, T. W. (2010). Rheumatoid arthritis. Lancet 376 (9746), 1094–1108. doi:10.1016/s0140-6736(10)60826-4
Song, J. E., Kim, J. S., Shin, J. H., Moon, K. W., Park, J. K., Park, K. S., et al. (2021). Role of synovial exosomes in osteoclast differentiation in inflammatory arthritis. Cells 10 (1), 120. doi:10.3390/cells10010120
Sun, Y., Chen, Q., Lin, P., Xu, R., He, D., Ji, W., et al. (2019). Characteristics of gut microbiota in patients with rheumatoid arthritis in Shanghai, China. Front. Cell. Infect. Microbiol. 9, 369. doi:10.3389/fcimb.2019.00369
Tajik, N., Frech, M., Schulz, O., Schälter, F., Lucas, S., Azizov, V., et al. (2020). Targeting zonulin and intestinal epithelial barrier function to prevent onset of arthritis. Nat. Commun. 11 (1), 1995. doi:10.1038/s41467-020-15831-7
Tavasolian, F., Hosseini, A. Z., Soudi, S., and Naderi, M. (2020). miRNA-146a improves immunomodulatory effects of MSC-derived exosomes in rheumatoid arthritis. Curr. Gene Ther. 20 (4), 297–312. doi:10.2174/1566523220666200916120708
Tavasolian, F., and Inman, R. D. (2021). Gut microbiota-microRNA interactions in ankylosing spondylitis. Autoimmun. Rev. 20 (6), 102827. doi:10.1016/j.autrev.2021.102827
Théry, C., Witwer, K. W., Aikawa, E., Alcaraz, M. J., Anderson, J. D., Andriantsitohaina, R., et al. (2018). Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 7 (1), 1535750. doi:10.1080/20013078.2018.1535750
Tian, X., Wei, W., Cao, Y., Ao, T., Huang, F., Javed, R., et al. (2022). Gingival mesenchymal stem cell-derived exosomes are immunosuppressive in preventing collagen-induced arthritis. J. Cell Mol. Med. 26 (3), 693–708. doi:10.1111/jcmm.17086
Tsuno, H., Arito, M., Suematsu, N., Sato, T., Hashimoto, A., Matsui, T., et al. (2018). A proteomic analysis of serum-derived exosomes in rheumatoid arthritis. BMC Rheumatol. 2 (1), 35. doi:10.1186/s41927-018-0041-8
Turpin, D., Truchetet, M. E., Faustin, B., Augusto, J. F., Contin-Bordes, C., Brisson, A., et al. (2016). Role of extracellular vesicles in autoimmune diseases. Autoimmun. Rev. 15 (2), 174–183. doi:10.1016/j.autrev.2015.11.004
van Delft, M. A. M., and Huizinga, T. W. J. (2020). An overview of autoantibodies in rheumatoid arthritis. J. Autoimmun. 110, 102392. doi:10.1016/j.jaut.2019.102392
Wang, Y., Zheng, F., Gao, G., Yan, S., Zhang, L., Wang, L., et al. (2018). MiR-548a-3p regulates inflammatory response via TLR4/NF-κB signaling pathway in rheumatoid arthritis. J. Cell Biochem. 120, 1133–1140. doi:10.1002/jcb.26659
Wu, H. J., Ivanov, , Darce, J., Hattori, K., Shima, T., Umesaki, Y., et al. (2010). Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity 32 (6), 815–827. doi:10.1016/j.immuni.2010.06.001
Wu, L. F., Zhang, Q., Mo, X. B., Lin, J., Wu, Y. L., Lu, X., et al. (2022). Identification of novel rheumatoid arthritis-associated MiRNA-204-5p from plasma exosomes. Exp. Mol. Med. 54 (3), 334–345. doi:10.1038/s12276-022-00751-x
Wu, X., Zhu, D., Tian, J., Tang, X., Guo, H., Ma, J., et al. (2020). Granulocytic myeloid-derived suppressor cell exosomal prostaglandin E2 ameliorates collagen-induced arthritis by enhancing IL-10(+) B cells. Front. Immunol. 11, 588500. doi:10.3389/fimmu.2020.588500
Xu, D., Song, M., Chai, C., Wang, J., Jin, C., Wang, X., et al. (2019). Exosome-encapsulated miR-6089 regulates inflammatory response via targeting TLR4. J. Cell Physiol. 234 (2), 1502–1511. doi:10.1002/jcp.27014
Yang, W., and Cong, Y. (2021). Gut microbiota-derived metabolites in the regulation of host immune responses and immune-related inflammatory diseases. Cell Mol. Immunol. 18 (4), 866–877. doi:10.1038/s41423-021-00661-4
Yao, X., Wang, Q., Zeng, P., Hou, L., Yang, Y., Lu, D., et al. (2021). LncRNA HOTTIP from synovial fibroblast-derived exosomes: A novel molecular target for rheumatoid arthritis through the miR-1908-5p/STAT3 axis. Exp. Cell Res. 409 (2), 112943. doi:10.1016/j.yexcr.2021.112943
Zhang, B., Zhao, J., Jiang, M., Peng, D., Dou, X., Song, Y., et al. (2022). The potential role of gut microbial-derived exosomes in metabolic-associated fatty liver disease: Implications for treatment. Front. Immunol. 13, 893617. doi:10.3389/fimmu.2022.893617
Zhang, H. G., Liu, C., Su, K., Yu, S., Zhang, L., Zhang, S., et al. (2006). A membrane form of TNF-alpha presented by exosomes delays T cell activation-induced cell death. J. Immunol. 176 (12), 7385–7393. doi:10.4049/jimmunol.176.12.7385
Zhang, J., Zhang, Y., Ma, Y., Luo, L., Chu, M., and Zhang, Z. (2021). Therapeutic potential of exosomal circRNA derived from synovial mesenchymal cells via targeting circEDIL3/miR-485-3p/PIAS3/STAT3/VEGF functional module in rheumatoid arthritis. Int. J. Nanomedicine 16, 7977–7994. doi:10.2147/ijn.S333465
Zhang, X., Zhang, D., Jia, H., Feng, Q., Wang, D., Liang, D., et al. (2015). The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat. Med. 21 (8), 895–905. doi:10.1038/nm.3914
Zhao, C., Song, W., Ma, J., and Wang, N. (2022). Macrophage-derived hybrid exosome-mimic nanovesicles loaded with black phosphorus for multimodal rheumatoid arthritis therapy. Biomater. Sci. 10 (23), 6731–6739. doi:10.1039/d2bm01274j
Zhao, J., Guo, S., Schrodi, S. J., and He, D. (2021). Molecular and cellular heterogeneity in rheumatoid arthritis: Mechanisms and clinical implications. Front. Immunol. 12, 790122. doi:10.3389/fimmu.2021.790122
Keywords: rheumatoid arthritis, exosomes, microbial communities, blood, mesenchymal stem cells, fibroblast-like synovial cells, gut microbe-derived exosomes
Citation: Zhao J, Zhang B, Meng W and Hu J (2023) Elucidating a fresh perspective on the interplay between exosomes and rheumatoid arthritis. Front. Cell Dev. Biol. 11:1177303. doi: 10.3389/fcell.2023.1177303
Received: 01 March 2023; Accepted: 11 April 2023;
Published: 28 April 2023.
Edited by:
Shiro Suetsugu, Nara Institute of Science and Technology (NAIST), JapanReviewed by:
Wenjing Yu, University of Pennsylvania, United StatesCopyright © 2023 Zhao, Zhang, Meng and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Hu, NjI2NDU3MEBxcS5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.