
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell Dev. Biol., 24 April 2023
Sec. Cancer Cell Biology
Volume 11 - 2023 | https://doi.org/10.3389/fcell.2023.1170444
This article is part of the Research TopicKinases for Cancer Prevention and TherapyView all 4 articles
There are major hurdles to the use of tyrosine kinase inhibitors (TKIs) and any other agents with significant toxicities (which means practically the preponderance of potential effective agents) in the context of prevention/anti-progression (interception) studies. We will discuss epidermal growth factor receptor (EGFR) inhibitors as examples, both in a primary prevention setting, where agent(s) are administered to individuals with no cancer but who might be considered at higher risk due to a variety of factors, and in anti-progression/interception studies, where agent(s) are administered to persons with known preinvasive lesions (e.g., colon adenomas, lung nodules, ductal carcinoma in situ (DCIS), or pancreatic intraepithelial neoplasia (PanIN) lesions in the pancreas) in an attempt to reverse or inhibit progression of these lesions. Multiple potential hurdles will be examined, including: a) toxicity of agents, b) the likely range of subtypes of cancers affected by a given treatment (e.g., EGFR inhibitors against EGFR mutant lung adenocarcinomas), c) the availability of practical endpoints besides the blocking of cancer formation or pharmacokinetics related to the agents administered in a primary prevention study, and d) the interpretation of the regression or blockage of new preinvasive lesions in the anti-progression study. Such an anti-progression approach may help address some of the factors commented on regarding primary prevention (toxicity, potential target organ cancer subtypes) but still leaves major questions regarding interpretation of modulation of preinvasive endpoints when it may not be clear how frequently they progress to clinical cancer. Additionally, we address whether certain recent preclinical findings might be able to reduce the toxicities associated with these agents and perhaps even increase their potential efficacy. Antibodies and TKIs other than the EGFR inhibitors are not discussed because few if any had been tested as monotherapies in humans, making their efficacy harder to predict, and because a number have relatively rare but quite striking toxicities. Furthermore, most of the practical hurdles raised regarding the EGFR inhibitors are relevant to the other TKIs. Finally, we briefly discuss whether early detection employing blood or serum samples may allow identification of high-risk groups more amenable to agents with greater toxicity.
The primary focus of this review is on some of the first generation EGFR inhibitors, e.g., gefitinib, erlotinib, lapatinib. This has been in contrast to certain of the third generation inhibitors, e.g., osmertinib, which preferentially interact with mutated forms of EGFR. This is predicated on the consideration that, as proposed for use in bladder, colon and pancreas, it appears that one is dealing with inhibition of wild type EGFR. Therefore, osmertinib which minimally effects wild type EGFR is unlikely to be highly effective. However, one could probably run a test for CT-scan identified lesions in an Asian population which has a very high incidence of EGFR mutant tumors with osmertinib since a high percentage of those lesions even without sequencing would be expected to have EGFR mutations. A systematic discussion of the widest range of small molecule EGFR inhibitors examining their specificity, target kinases, etc., has recently been published (Abourehab et al., 2021), with informative diagrams where EGFR inhibitors block various proteins. The additional anti-HER1/EGFR and anti-HER2 inhibitors that might be used are the antibodies, e.g., cetuximab, panitumumab and trastuzumab and their biosimilars. The difficulties with those agents are that they require intravenous administration, are likely to be quite expensive, and their combination with additional agents such as NSAIDs is not known. Examples of potential prevention and interception trials which include EGFR inhibitors that are discussed below, are on Figure 1.
Primary prevention involves administering an agent(s) to a group of individuals with no cancer but who might be considered at higher risk for that type of cancer based on other known factors (e.g., age, hormonal status, known genetic factors) (Table 1).
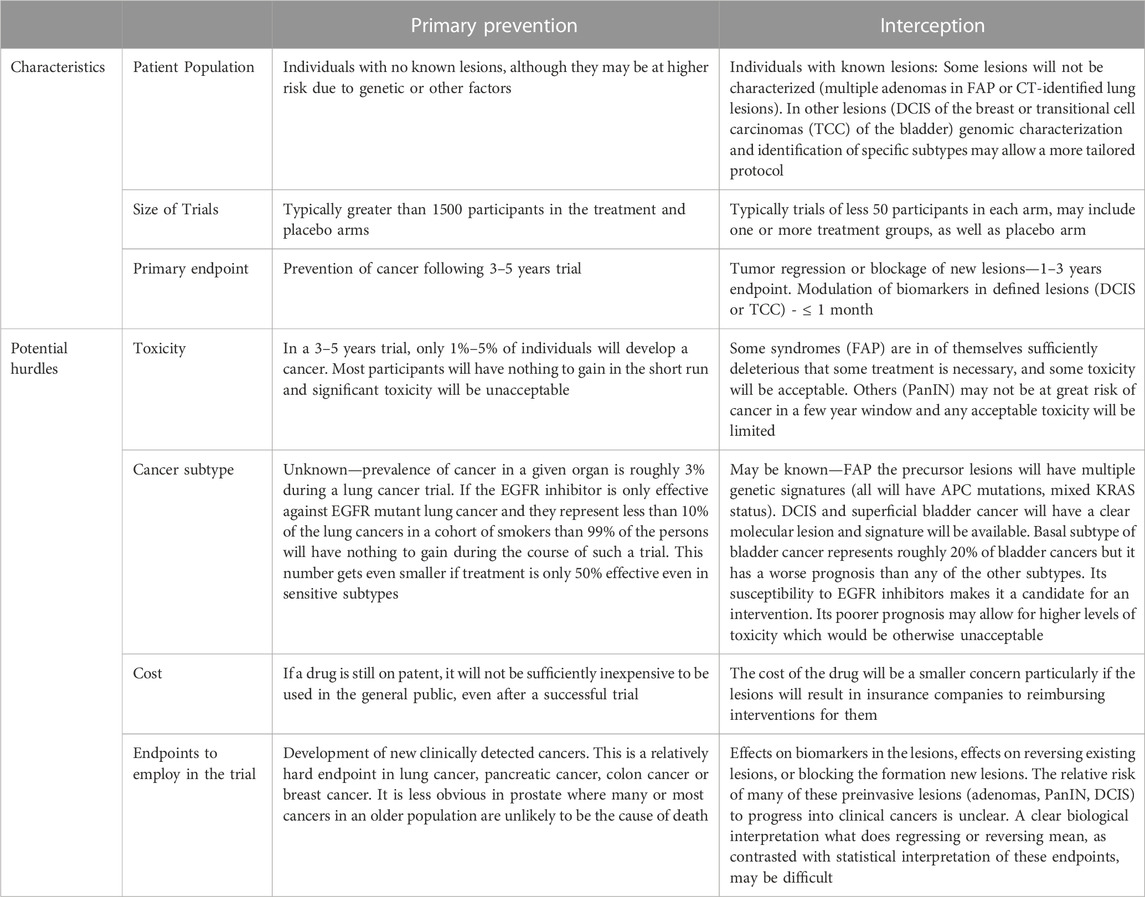
TABLE 1. Trial characteristics and potential hurdles in primary prevention and interception studies using EGFR inhibitors.
A primary obstacle to long-term clinical primary chemoprevention studies and acceptance is toxicity. For example, tamoxifen and the aromatase inhibitors have proven highly effective in the prevention of estrogen receptor-positive (ER+) breast cancer in large-scale clinical trials (Fisher et al., 1998; Goss et al., 2011). In the prevention trials, these agents were tested at the same doses employed in the therapy of estrogen/progesterone receptor-positive (ER + PR+) tumors. Non-etheless, it has been difficult for at-risk women to take these agents. In the case of tamoxifen, a major hurdle is the increase in endometrial cancer associated with treatment. In the case of the aromatase inhibitors, drawbacks include postmenopausal symptoms, muscle and joint pain, and osteoporosis. Another major class of agents that has shown therapeutic efficacy but has minimally advanced to a prevention setting are the tyrosine kinase inhibitors (TKIs). Specifically, epidermal growth factor receptor (EGFR) inhibitors are clearly effective clinically in the treatment of EGFR mutant lung cancer (Ramalingam et al., 2020), and HER2 inhibitors are active in HER2-positive breast cancer (Cortes et al., 2022) (Park et al., 2009). Furthermore, the EGFR inhibitor erlotinib proved active when combined with the NSAID sulindac in a polyp trial with familial adenomatous polyposis (FAP) patients (Samadder et al., 2016; Samadder et al., 2018). However, these agents at their recommended daily dosing cause significant and common toxicities, such as acneiform rash and diarrhea (Guttman-Yassky et al., 2010; Shepherd et al., 2005; Burstein et al., 2008; Yang et al., 2012), which have been a barrier to their routine use in a prevention setting. Preclinical prevention studies (see below) have raised the possibility of reducing such toxicities by employing intermittent dosing, weekly dosing, or a combination of agents. If validated in clinical trials, these methods could be key to improving the relative efficacy to toxicity index and increase the potential use of these inhibitors in prevention/anti-progressions studies.
In the case of tamoxifen and the aromatase inhibitors, one had clear data from large adjuvant trials that these agents used as monotherapies would prevent the development of the preponderance of ER + PR + HER2-negative breast cancers (Luminal A) (Perou et al., 2000). This was based on their ability to affect breast cancer recurrence and/or metastasis in the adjuvant setting (Howell et al., 2005). However, there was a second parameter observed in the large adjuvant trials with tamoxifen and the aromatase inhibitors that closely paralleled prevention trials, specifically the ability of these agents to prevent the development of breast cancer in the contralateral breast (Goss et al., 2005; Ruhstaller et al., 2019). These contralateral tumors are thought to represent newly arising tumors in contrast with recurrences or metastatic cancers. These studies showed that tamoxifen was relatively effective in the prevention of contralateral breast cancers (60%), while the aromatase inhibitors were very effective in preventing >80% of contralateral cancers. The prevention trials reproduced these results with tamoxifen, reducing the incidence of ER + breast cancer 35%–55% in various trials, while the aromatase inhibitors decreased ER + tumors by almost two-thirds. ER + breast cancers represent 70%–80% of all breast cancers in most studies. Thus, if an agent inhibited ER + tumors by 75% in a large prevention trial of breast cancer, it would be expected to inhibit 50%–60% of all breast cancers. This is a reasonable approximation of what was seen in the two large prevention trials (Fisher et al., 1998; Goss et al., 2011). Furthermore, neither tamoxifen nor the aromatase inhibitors appeared to affect the expected numbers of ER-negative breast cancers relative to placebo, implying you are not shunting or specifically augmenting the development of ER-negative tumors. In contrast, adjuvant results with agents in most other forms of cancer (lung, colon, pancreas, bladder) do not yield any similar contralateral type “prevention” results, so one must try to extrapolate from results in an adjuvant setting that uses endpoints such as recurrence free survival (RFS) or overall survival (OS), which may seem less relevant for prevention. However, a second problem is that with the exception of treating EGFR mutant lung cancers, the majority of examples of the use of EGFR inhibitors, and most other TKIs have been in combination with a second agent, most typically a standard cytotoxic agent (Geyer et al., 2006). However, there appears to be sufficient data in HER2 tumors to propose that HER2 small molecule inhibitors by themselves will be effective. Therefore, it is more difficult to determine whether a given agent will be functional on its own. This leads to the question whether positive results in a preclinical setting or effective preclinical results with EGFR inhibitors alone or in combination with other known preventive agents would be sufficient to initiate at least a small phase II prevention/anti-progression trial. One could certainly hope that preclinical data in a relevant animal model with or without clinical data on a compound or a preclinical combination with a second standard agent would be sufficient to perform a small presurgical neoadjuvant trial which might support a prevention trial.
These specific examples of EGFR inhibitors or HER2 inhibitors exemplify the problem for TKIs, or probably most targeted therapies in a primary prevention setting. EGFR mutant lung adenocarcinomas in a Caucasian population represent <15% of all adenocarcinomas. Since adenocarcinomas represent at most 50% of all lung cancers worldwide, EGFR mutant lung adenocarcinomas will represent <7% of all lung cancers. Similarly, an HER2 inhibitor by itself is likely to affect only the roughly 20% of breast cancers that overexpress HER2 and are either ER positive or ER negative. Thus, it would not be the basis for a primary prevention trial, although it might significantly improve a trial in conjunction with an anti-hormonal agent (Fisher et al., 1998; Goss et al., 2011).
In the metastatic setting, one routinely expects to continue treatment until the tumor progresses or until you have a striking pathologic response. In the adjuvant setting, as in the case of tamoxifen and the aromatase inhibitors, it was demonstrated that 5 years of treatment is roughly as good as 10 years. However, the prevention trial with the aromatase inhibitor anastrozole raises the question of how long to treat (Cuzick, 2003; Cuzick et al., 2014; Forbes et al., 2016; Cuzick et al., 2020). This recent trial showed that while total breast cancers were reduced by roughly 50% during the first 5 years when the agent was administered, this dropped to roughly 30% efficacy during the next 5 years during which anastrozole was withdrawn. In a phase I or phase II prevention trial, the endpoints are likely to be biomarkers of efficacy and/or blocking some preinvasive lesion. In a large prevention trial, the question of duration of treatment is a major question. You do not have a progressing lesion. You can look for the development of new cancers and that would presumably be the endpoint for any large-scale trial (Fisher et al., 1998; Goss et al., 2011). Thus, optimistically, the incidence of the cancer of interest is reduced by 50%. That is likely to be sufficient to yield a statistically positive trial. However, do you have to keep administering the agent chronically? Only 2%–3% of persons will develop a tumor in a large primary prevention trial (Fisher et al., 1998; Goss et al., 2011). Do you keep the 97%–98% of the group who have not developed a cancer on the agent? This is a significant question. Furthermore, particularly for the TKIs, the possibility of developing resistance is always an open question. Quite obviously for the TKIs, this is perhaps the major question in therapy trials where one has a tumor which is likely to keep turning out new mutated variants. However, from limited highly effective prevention trials with tamoxifen or aromatase inhibitors in breast or NSAIDs in colon, it does not appear that you are getting resistant tumors readily. The rationale for this lack of resistance may be that you do not have a significant number of cells from more advanced lesions which are more likely to undergo mutations. However, there is certainly a question of whether these results with hormonal agents and NSAIDs are relevant for the TKIs.
The cost of a large-scale clinical prevention trial is quite substantial. However, if the result obtained is relatively striking then it may be more than worth the cost. However, if the agent employed is still on patent, then the cost is likely to prove prohibitive for general use in a population. Quite obviously, if an agent has significant toxicity and may require observation by medical practitioners due to toxicity, this likely makes it untenable for primary prevention.
Although primary prevention is often what people think of when they discuss prevention as listed above, the use of such an approach is complex in general and potentially fraught for a class of agents such as the TKI inhibitors (using as an example the EGFR inhibitors) (Table 1). The existence of populations with precursor lesions partially overcomes certain of the problems associated with pure primary prevention trials: 1) The mere presence of a lesion: If a lesion is available which helps to define the subtype of cancer, then one may be able to use a specific class of agents more rationally. 2) Precursor lesions and potential endpoints: If there are specific precursor lesions, either biomarker alterations in the lesion or lesions that one can measure, or regression of the lesion or blockage of development of new lesions, one may be able to define endpoints for a phase I/II trial. 3) Persons with precursor lesions must be considered at higher risk: Presumably, the existence of the lesion puts the individual at greater risk of developing the specific caner and therefore makes somewhat higher levels of toxicity more acceptable. 4) Precursor lesions may make some treatment necessary: Certain precursor lesions (e.g., multiple polyps associated with FAP) virtually require some treatment. Furthermore, precursor lesions such as ductal carcinoma in situ (DCIS) in the breast or transitional cell carcinomas (TCCs) in the bladder are routinely treated with surgery and some adjuvant treatment.
(Table 1) CT scanning of lungs, although far from foolproof, may help identify persons with early lesions (nodules) in the lung (National Lung Screening Trial Research et al., 2011). These and other techniques are felt to determine lesions which may be the precursors to primarily lung adenocarcinomas (Hammer and Byrne, 2022). Regarding the use of EGFR inhibitors, such an approach would be of limited use regarding potential prevention trials, particularly in Caucasian smokers, since few of these lung adenocarcinomas have EGFR mutations. However, if such a study were performed in an East Asian population of smokers or non-smokers, then the prevalence of EGFR mutations (Shi et al., 2014) is likely to be sufficient to justify such a trial, particularly if one might enhance the efficacy of these inhibitors and potentially partially reduce their toxicity (see below). There is a rapidly evolving field of risk assessment tools in addition to CT screening for inclusion in lung cancer prevention/interception studies (Tammemagi et al., 2013; Mathios et al., 2021).
Due to germline mutations in APC (adenomatous polyposis coli), individuals with FAP have literally scores to hundreds of adenomatous polyps. Because of the presence of a great number of lesions and the expectation that one or more of these lesions will progress to invasive colon cancer, some clinical intervention appears necessary. There was a positive FAP trial combining the EGFR inhibitor erlotinib together with a relatively low dose of the NSAID sulindac which resulted in a fairly striking clinical effect (Samadder et al., 2016; Samadder et al., 2018). This small trial was based on strong preclinical data showing that the combination of an EGFR inhibitor and an NSAID was profoundly effective in animal models of FAP (Buchanan et al., 2007). This is in contrast to adjuvant clinical data which show that anti-EGFR antibodies, but not EGFR small molecule inhibitors, are effective in colon cancer (Taieb et al., 2014). This raises the question whether these data reflect a greater sensitivity of the preinvasive lesions or whether a small molecule EGFR inhibitor specifically combined with an NSAID might be effective even in an adjuvant setting.
DCIS are precursor pre-invasive lesions that are frequently identified at screening mammography or during breast cancer surgery. These are commonly treated with surgery, often followed by adjuvant radiation and hormonal therapy. While this is considered therapy by most oncologists, the objective is to prevent the development of invasive cancer and could serve as an interception (anti-progression) model. For DCIS lesions overexpressing HER2, trastuzumab has demonstrated activity in clinical trials (Cobleigh et al., 2021; von Minckwitz et al., 2012). A trial of the small molecule HER2 inhibitor lapatinib in DCIS was terminated early for low accrual, despite strong preclinical evidence for activity (Farnie et al., 2014).
PanIN is a histologically well-defined precursor to invasive ductal adenocarcinoma of the pancreas. PanINs are relatively common lesions, particularly in an elderly population. These lesions typically have a KRAS mutation and often alterations in P16 as well. These are the two most common genomic alterations observed in invasive ductal adenocarcinomas of the pancreas. However, it is still not clear what percentage of PanIN lesions progress to pancreatic adenocarcinoma (Delpu et al., 2011).
TCC is the most frequently observed tumor in the bladder. TCC arises from the cells lining the bladder and is routinely diagnosed during the pre-invasive stage. At the molecular level, there are 5 major subtypes of bladder cancer, including luminal (HER2/3 high, papillary) (Cancer Genome Atlas Research, 2014), HER2-expressing, and basal (squamous cell, mesenchymal cell) (Hedegaard et al., 2016; Choi et al., 2014; Kamoun et al., 2020). The basal cell cancers, which represent roughly 20%–25% of bladder cancers, appear to be responsive to EGFR inhibitors. Similarly, the HER2-overexpressing category, which has a far better prognosis than the basal, might be a candidate for treatment with HER2 inhibitors. Lapatinib, a combined EGFR inhibitor and HER2 inhibitor, when combined with the NSAID piroxicam, profoundly increased progression free survival and overall survival in dogs with invasive bladder cancers (Maeda et al., 2022).
Carcinogenesis of the head and neck area provides a classical example of the multistep process leading to invasive malignancy. With easily observable oral premalignant lesions that could undergo serial biopsies, several trials assessed the activity of EGFR TKIs alone or in combinations with other agents for prevention of invasive cancer, or evaluating surrogate biomarkers in patients with oral premalignant lesions. The combination of erlotinib and celecoxib led to 63% histologic response rate that correlated with EGFR pathway inhibition (Saba et al., 2014). Green tea polyphenon E and erlotinib resulted in 47% complete pathologic response with an excellent 66.3% 5-year cancer-free survival. Phosphorylated ERK was correlated with response to treatment, among the biomarkers that were studied. (Shin et al., 2020). However, a randomized trial with erlotinib vs. placebo did not show improvement in the primary endpoint of cancer-free survival (William et al., 2016). Preclinical data suggest that MET activation may be present as an early driver in premalignant lesions and could become a target for chemoprevention of oral cancer (Saintigny et al., 2018). A proof of concept phase II clinical trial of metformin to target PI3K/mTOR signaling for patients with oral premalignant lesions showed modest clinical responses and decreased mTOR activity correlating with the histological and clinical responses. The observed significant modulation of the PI3K/mTOR pathway indicated that trials with other PI3K inhibitors are warranted (Gutkind et al., 2021).
Preclinical prevention studies have raised the possibility of reducing toxicity by employing intermittent dosing, weekly dosing, lower dosing, or a combination of agents (Padda et al., 2013) (Table2). Once validated in clinical trials, these methods could be a key to improving the therapeutic index of preventive/anti-progression (interception) agents and thus their public acceptance.
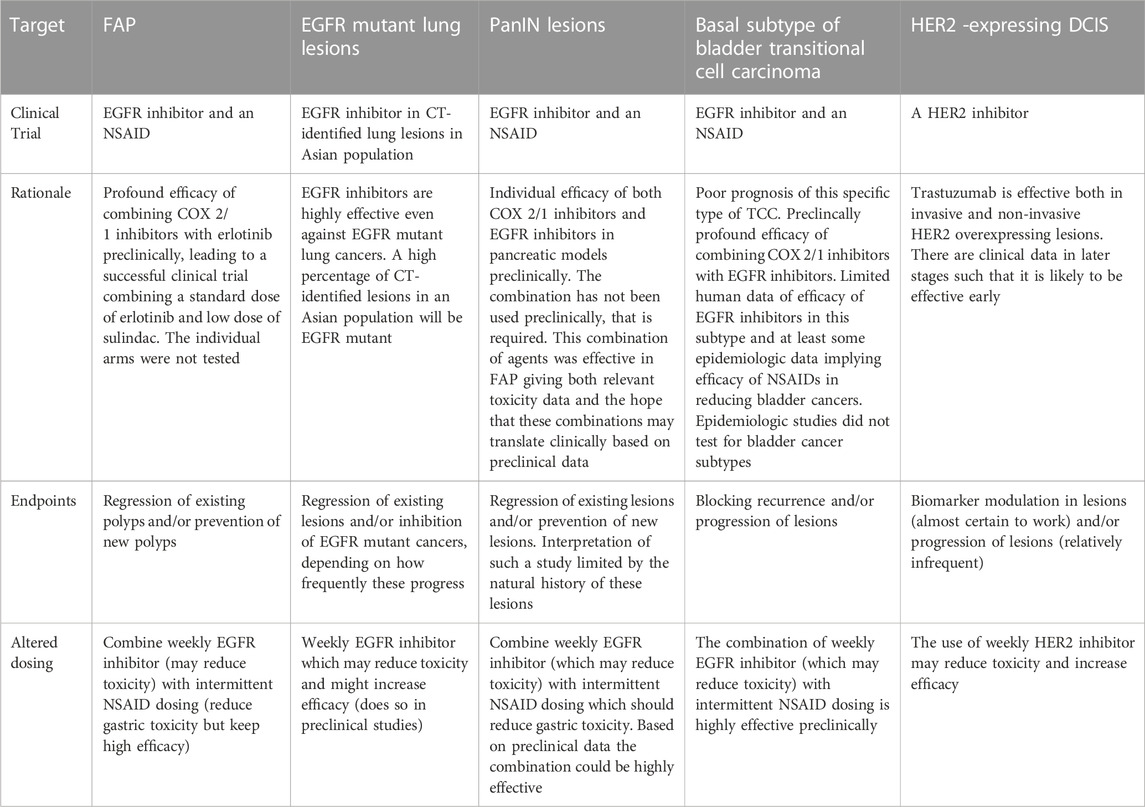
TABLE 2. Clinical interception trials which might be performed: the rationale for such trials, potential trial endpoints and altered dosing which might reduce toxicity and/or increase efficacy (See also Figure 1).
A problem with targeted therapies developed for cancer treatment, such as the EGFR inhibitors erlotinib, gefitinib, and lapatinib, is that they all can cause acneiform rash and diarrhea when given daily. Studies in humans treating brain metastases from either EGFR mutant lung cancers or HER2-overexpressing breast cancers demonstrated that pulsatile weekly dosing with erlotinib or lapatinib, at up to 7-fold the standard daily dose, was effective and paradoxically caused less toxicity than daily dosing. This high dose pulsatile dosing was initiated to deal with brain metastases from EGFR mutant lung cancers and HER2-overexpressing breast cancers. Daily dosing of these agents did not reach effective levels in the brain following standard daily dosing, presumably due to the blood brain barrier. Therefore, weekly pulsatile dosing was employed on the rationale that one would achieve higher levels in the brain. It appears that the approach has been relatively effective in numerous human trials (Milton et al., 2006; Grommes et al., 2011; Morikawa et al., 2019). In preclinical studies, weekly dosing with a number of EGFR inhibitors, including erlotinib and gefitinib, EGFR inhibitors were equally effective as daily dosing in breast, bladder and colon cancer models, and potentially more effective in two lung cancer models (Lubet et al., 2013; Zhang et al., 2017; Mohammed et al., 2020; Ulusan et al., 2021). A particularly striking example of the efficacy of EGFR inhibitors combined with an NSAID has been recently reported in dogs (Maeda et al., 2022). The combination of the HER inhibitor lapatinib (which inhibits both HER1/EGFR and HER2) increased progression free survival in dogs with invasive bladder tumors almost 2-fold, and increased overall survival roughly 2-fold as well. The comparator arm for these trials was piroxicam alone, which is a fairly standard way to treat these tumors. These results similarly appear more effective than standard chemotherapies (e.g., mitoxane). However, there was not a direct comparator arm in this study. These results raise the question whether this type of approach might be relevant to human bladder cancers as well. These studies did not test altered dosing, which we have recently examined. However, our observation of efficacy with multiple models in rats and mice and the implication that it will at a minimum reduce toxicity and might increase efficacy warrants consideration. As a pure aside, most of our early studies in bladder used erlotinib and gefitinib because our model paralleled human basal bladder cancer and overexpressed EGFR but not HER2. However, HER2 is overexpressed in many other human and canine subtypes of bladder cancer and may be relevant to the use of this approach for a wide variety of bladder tumors (preinvasive and invasive) in humans. Parenthetically, as is to be expected, lapatinib, an EGFR and HER2 inhibitor, was similarly effective when given alone or in combination with an NSAID in our basal bladder cancer model (Lubet et al., 2021). Examples of the efficacy of this approach are shown in Table 2. A number of papers have shown reduced toxicity with weekly dosing in animal models, although the skin lesions examined are not exactly the same as acneiform rash in humans (Zhang et al., 2017; Ulusan et al., 2021). Also, the combination of weekly dosing of an EGFR inhibitor together with an NSAID has looked striking in preclinical models of colon and bladder cancer.
Potential trials employing weekly EGFR inhibitor administration (Table 2):
1. Weekly dosing with an EGFR inhibitor in an Asian population (that has a high incidence of EGFR mutant lung cancers) with pulmonary nodules determined by CAT scans (Shi et al., 2014)
2. Weekly dosing of an EGFR inhibitor together with an NSAID (see below) in individuals with a) FAP, b) pancreatic precursor lesions (PanINs), and c) basal TCC of the bladder (Mohammed et al., 2020)
3. Potential trial of lapatinib in DCIS. HER2 overexpression is often observed in DCIS in the breast and appears to be associated with a higher incidence of recurrence and progression to invasive breast cancer. This is routinely treated with surgery plus radiation. Trastuzumab can be employed in this setting with at least hints of efficacy, but it requires intravenous administration.
There are both preclinical and some clinical data that combinations of certain agents may yield strong efficacy. However, any potential toxicity problems that may arise may have to be determined clinically. Common toxicities, such as acneiform rash or diarrhea for EGFR inhibitors or gastric bleeding for NSAIDs, could be determined in a limited Phase I trial.
Two of the more successful Phase II clinical prevention trials that involved combinations of agents were performed in colon and were based on preclinical data. In the first, the combination of an NSAID (piroxicam) and DFMO was shown to be highly effective preclinically in a colon model almost 30 years ago (Reddy et al., 1990). This led to a colon adenoma prevention trial combining the NSAID sulindac and DFMO, which prevented adenomas by roughly 60% (Meyskens et al., 2008). In the second, which is immediately relevant to the present paper, the EGFR inhibitor erlotinib administered with sulindac was highly effective in persons with FAP (Samadder et al., 2016; Samadder et al., 2018). The efficacy of this combination was first observed in Min mice by DuBois and coworkers (Buchanan et al., 2007) and most importantly, was confirmed in a human trial of FAP which looked at the regression of preexisting polyps. As mentioned above, the toxicities observed were a combination of those seen with the individual agents. Regrettably, for each of these trials, which were relatively small phase II trials, neither of the individual agents were administered concurrently as comparator arms. This makes it more difficult to determine whether the combination of agents was more or less toxic than the sum of the individual agents. In the case of the NSAIDs, this is particularly relevant since they were administered at less than their standard doses.
Potential trials employing EGFR inhibitor plus an NSAID:
1. Repeat of FAP Study: The prior FAP study examining erlotinib plus an NSAID can be performed using a weekly dose of erlotinib. This is likely to be both highly effective and plausibly less toxic than the standard dosing. The combination of pulsatile EGFR inhibitor and intermittent NSAID was found to be profoundly effective in a rat model of FAP (Ulusan et al., 2021).
2. Interception (anti-progression study) on pre-invasive pancreatic lesions (e.g., PanIN lesions): The combination of an EGFR inhibitor and a Cox 1/2 inhibitor is highly effective preclinically (Rao et al., 2015).
3. Interception (anti-progression) study in basal subtype of bladder cancer: Erlotinib plus an NSAID can be administered using a weekly dose of erlotinib. This is likely to be both more effective and plausibly less toxic. The combination of pulsatile EGFR inhibitor and intermittent NSAID was found to be profoundly effective in a rat model of basal bladder cancer (Mohammed et al., 2020). Perhaps even more surprisingly the combination of lapatinib plus piroxicam was highly effective against all bladder cancers in dogs (Maeda et al., 2022).
In science in general and oncology in particular, significant advances, at least conceptually, often come out of some technical advance. The potential technical advance that is starting to come into clearer focus is the possibility of early detection of incipient tumors in blood or some other body fluid (Liu et al., 2020; Mathios et al., 2021). By definition, the signal will reflect some existing lesion that has to be of a sufficient size to give a significant signal in any relevant assay. The signal would identify both a type of cancer and hopefully some subtype of cancer (e.g., EGFR mutant lung cancer or basal bladder cancer) but would not offer a complete genome to sequence. At the time of diagnosis, it might not offer a clear lesion to examine. Would administering agents to such a lesion constitute prevention or is it a therapy? That may be somewhat of a semantic argument. This yields a number of advantages in terms of a patient population. First, it offers a population that is likely to advance to frank clinical cancer. Second, the biomarkers that allowed for the identification of patients with incipient cancers are likely to be useful in monitoring the efficacy of any treatments. Third, the incipient lesions are more advanced and therefore require more effective agents (e.g., TKIs). This greater likelihood of progressing to clinical cancer would appear to make this group of patients more receptive to treatments with some but easily manageable toxicities. Progressing into this population will hopefully allow the use of TKIs in certain of these populations, particularly if some of the preclinical methods mentioned above do reduce the toxicity of some of the TKIs.
A. As outlined above, there are multiple hurdles to any potential use of EGFR inhibitors in particular or any other TKIs generally in a primary prevention trial. These include the fact that a potential subtype of sensitive tumors (EGFR mutant lung cancer or HER2-amplified breast cancer) to target in such a blind primary prevention trial is unclear. Furthermore, there are too many toxicities associated with daily dosing to make it viable unless some of the newer TKIs have more limited toxicity.
B. In an interception trial, it would seem that one can determine subtypes of cancers of particular organs (EGFR mutant lung adenocarcinomas) or precursor lesions (HER2-overexpressing DCIS) that might be susceptible to an EGFR receptor inhibitor. There are still questions about potential endpoints to employ. However, the presence of lesions raises the possibility of examining alterations in lesions by various -omic methodologies. Furthermore, if someone has existing lesions, some increase in toxicity will be more acceptable.
C. There are alterations in dosing and potential combinations of agents which might make the use of TKIs more acceptable. These were discussed above. It appears that pulsatile dosing of TKI inhibitors may prove useful in certain subtypes of cancer. Furthermore, the combination of an EGFR inhibitor plus an NSAID (a known preventive agent) appears particularly promising in anti-progression studies such as FAP.
KHD and RAL contributed to conception and design of the study. CPCD organized the database. RAL wrote the first draft of the manuscript. KHD and CPCD wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
The authors wish to thank Dr. Jennifer Fox for her critical review of the manuscript. The opinions expressed by the authors are their own and this material should not be interpreted as representing the official viewpoint of the US Department of Health and Human Services, the National Institutes of Health, or the National Cancer Institute.
KHD has received research funding to the institution from Roche/Genentech, Eli Lilly, Novartis, Amgen, Merck, Molecular Templates, Io Therapeutics.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abourehab, M. A. S., Alqahtani, A. M., Youssif, B. G. M., and Gouda, A. M. (2021). Globally approved EGFR inhibitors: Insights into their syntheses, target kinases, biological activities, receptor interactions, and metabolism. Molecules 26, 6677. doi:10.3390/molecules26216677
Buchanan, F. G., Holla, V., Katkuri, S., Matta, P., and Dubois, R. N. (2007). Targeting cyclooxygenase-2 and the epidermal growth factor receptor for the prevention and treatment of intestinal cancer. Cancer Res. 67, 9380–9388. doi:10.1158/0008-5472.CAN-07-0710
Burstein, H. J., Storniolo, A. M., Franco, S., Forster, J., Stein, S., Rubin, S., et al. (2008). A phase II study of lapatinib monotherapy in chemotherapy-refractory HER2-positive and HER2-negative advanced or metastatic breast cancer. Ann. Oncol. 19, 1068–1074. doi:10.1093/annonc/mdm601
Cancer Genome Atlas Research, N. (2014). Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 507, 315–322. doi:10.1038/nature12965
Choi, W., Czerniak, B., Ochoa, A., Su, X., Siefker-Radtke, A., Dinney, C., et al. (2014). Intrinsic basal and luminal subtypes of muscle-invasive bladder cancer. Nat. Rev. Urol. 11, 400–410. doi:10.1038/nrurol.2014.129
Cobleigh, M. A., Anderson, S. J., Siziopikou, K. P., Arthur, D. W., Rabinovitch, R., Julian, T. B., et al. (2021). Comparison of radiation with or without concurrent trastuzumab for HER2-positive ductal carcinoma in situ resected by lumpectomy: A phase III clinical trial. J. Clin. Oncol. 39, 2367–2374. doi:10.1200/JCO.20.02824
Cortes, J., Kim, S. B., Chung, W. P., Im, S. A., Park, Y. H., Hegg, R., et al. (2022). Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N. Engl. J. Med. 386, 1143–1154. doi:10.1056/NEJMoa2115022
Cuzick, J. (2003). Aromatase inhibitors in prevention--data from the ATAC (arimidex, tamoxifen alone or in combination) trial and the design of IBIS-II (the second International Breast Cancer Intervention Study). Recent Results Cancer Res. 163, 96–103. discussion 264-6. doi:10.1007/978-3-642-55647-0_9
Cuzick, J., Sestak, I., Forbes, J. F., Dowsett, M., Cawthorn, S., Mansel, R. E., et al. (2020). Use of anastrozole for breast cancer prevention (IBIS-II): Long-term results of a randomised controlled trial. Lancet 395, 117–122. doi:10.1016/S0140-6736(19)32955-1
Cuzick, J., Sestak, I., Forbes, J. F., Dowsett, M., Knox, J., Cawthorn, S., et al. (2014). Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): An international, double-blind, randomised placebo-controlled trial. Lancet 383, 1041–1048. doi:10.1016/S0140-6736(13)62292-8
Delpu, Y., Hanoun, N., Lulka, H., Sicard, F., Selves, J., Buscail, L., et al. (2011). Genetic and epigenetic alterations in pancreatic carcinogenesis. Curr. Genomics 12, 15–24. doi:10.2174/138920211794520132
Farnie, G., Johnson, R. L., Williams, K. E., Clarke, R. B., and Bundred, N. J. (2014). Lapatinib inhibits stem/progenitor proliferation in preclinical in vitro models of ductal carcinoma in situ (DCIS). Cell Cycle 13, 418–425. doi:10.4161/cc.27201
Fisher, B., Costantino, J. P., Wickerham, D. L., Redmond, C. K., Kavanah, M., Cronin, W. M., et al. (1998). Tamoxifen for prevention of breast cancer: Report of the national surgical adjuvant breast and bowel project P-1 study. J. Natl. Cancer Inst. 90, 1371–1388. doi:10.1093/jnci/90.18.1371
Forbes, J. F., Sestak, I., Howell, A., Bonanni, B., Bundred, N., Levy, C., et al. (2016). Anastrozole versus tamoxifen for the prevention of locoregional and contralateral breast cancer in postmenopausal women with locally excised ductal carcinoma in situ (IBIS-II DCIS): A double-blind, randomised controlled trial. Lancet 387, 866–873. doi:10.1016/S0140-6736(15)01129-0
Geyer, C. E., Forster, J., Lindquist, D., Chan, S., Romieu, C. G., Pienkowski, T., et al. (2006). Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N. Engl. J. Med. 355, 2733–2743. doi:10.1056/NEJMoa064320
Goss, P. E., Ingle, J. N., Ales-Martinez, J. E., Cheung, A. M., Chlebowski, R. T., Wactawski-Wende, J., et al. (2011). Exemestane for breast-cancer prevention in postmenopausal women. N. Engl. J. Med. 364, 2381–2391. doi:10.1056/NEJMoa1103507
Goss, P. E., Ingle, J. N., Martino, S., Robert, N. J., Muss, H. B., Piccart, M. J., et al. (2005). Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: Updated findings from NCIC CTG MA.17. J. Natl. Cancer Inst. 97, 1262–1271. doi:10.1093/jnci/dji250
Grommes, C., Oxnard, G. R., Kris, M. G., Miller, V. A., Pao, W., Holodny, A. I., et al. (2011). Pulsatile" high-dose weekly erlotinib for CNS metastases from EGFR mutant non-small cell lung cancer. Neuro Oncol. 13, 1364–1369. doi:10.1093/neuonc/nor121
Gutkind, J. S., Molinolo, A. A., Wu, X., Wang, Z., Nachmanson, D., Harismendy, O., et al. (2021). Inhibition of mTOR signaling and clinical activity of metformin in oral premalignant lesions. JCI Insight 6, e147096. doi:10.1172/jci.insight.147096
Guttman-Yassky, E., Mita, A., de Jonge, M., Matthews, L., Mccarthy, S., Iwata, K. K., et al. (2010). Characterisation of the cutaneous pathology in non-small cell lung cancer (NSCLC) patients treated with the EGFR tyrosine kinase inhibitor erlotinib. Eur. J. Cancer 46, 2010–2019. doi:10.1016/j.ejca.2010.04.028
Hammer, M. M., and Byrne, S. C. (2022). Cancer risk in nodules detected at follow-up lung cancer screening CT. AJR Am. J. Roentgenol. 218, 634–641. doi:10.2214/AJR.21.26927
Hedegaard, J., Lamy, P., Nordentoft, I., Algaba, F., Hoyer, S., Ulhoi, B. P., et al. (2016). Comprehensive transcriptional analysis of early-stage urothelial carcinoma. Cancer Cell 30, 27–42. doi:10.1016/j.ccell.2016.05.004
Howell, A., Cuzick, J., Baum, M., Buzdar, A., Dowsett, M., Forbes, J. F., et al. (2005). Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet 365, 60–62. doi:10.1016/S0140-6736(04)17666-6
Kamoun, A., de Reynies, A., Allory, Y., Sjodahl, G., Robertson, A. G., Seiler, R., et al. (2020). A consensus molecular classification of muscle-invasive bladder cancer. Eur. Urol. 77, 420–433. doi:10.1016/j.eururo.2019.09.006
Liu, M. C., Oxnard, G. R., Klein, E. A., Swanton, C., Seiden, M. V., and Consortium, C. (2020). Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann. Oncol. 31, 745–759. doi:10.1016/j.annonc.2020.02.011
Lubet, R. A., Kumar, A., Fox, J. T., You, M., Mohammed, A., Juliana, M. M., et al. (2021). Efficacy of EGFR inhibitors and NSAIDs against basal bladder cancers in a rat model: Daily vs. Weekly dosing, combining EGFR inhibitors with naproxen, and effects on RNA expression. Bladder Cancer 7, 335–345. doi:10.3233/blc-200423
Lubet, R. A., Szabo, E., Iwata, K. K., Gill, S. C., Tucker, C., Bode, A., et al. (2013). Effect of intermittent dosing regimens of erlotinib on methylnitrosourea-induced mammary carcinogenesis. Cancer Prev. Res. (Phila) 6, 448–454. doi:10.1158/1940-6207.CAPR-12-0322
Maeda, S., Sakai, K., Kaji, K., Iio, A., Nakazawa, M., Motegi, T., et al. (2022). Lapatinib as first-line treatment for muscle-invasive urothelial carcinoma in dogs. Sci. Rep. 12, 4. doi:10.1038/s41598-021-04229-0
Mathios, D., Johansen, J. S., Cristiano, S., Medina, J. E., Phallen, J., Larsen, K. R., et al. (2021). Detection and characterization of lung cancer using cell-free DNA fragmentomes. Nat. Commun. 12, 5060. doi:10.1038/s41467-021-24994-w
Meyskens, F. L., Mclaren, C. E., Pelot, D., Fujikawa-Brooks, S., Carpenter, P. M., Hawk, E., et al. (2008). Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: A randomized placebo-controlled, double-blind trial. Cancer Prev. Res. (Phila) 1, 32–38. doi:10.1158/1940-6207.CAPR-08-0042
Milton, D. T., Azzoli, C. G., Heelan, R. T., Venkatraman, E., Gomez, J. E., Kris, M. G., et al. (2006). A phase I/II study of weekly high-dose erlotinib in previously treated patients with nonsmall cell lung cancer. Cancer 107, 1034–1041. doi:10.1002/cncr.22088
Mohammed, A., Miller, M. S., Lubet, R. A., Suen, C. S., Sei, S., Shoemaker, R. H., et al. (2020). Combination of erlotinib and naproxen employing pulsatile or intermittent dosing profoundly inhibits urinary bladder cancers. Cancer Prev. Res. (Phila) 13, 273–282. doi:10.1158/1940-6207.CAPR-19-0339
Morikawa, A., de Stanchina, E., Pentsova, E., Kemeny, M. M., Li, B. T., Tang, K., et al. (2019). Phase I study of intermittent high-dose lapatinib alternating with capecitabine for HER2-positive breast cancer patients with central nervous system metastases. Clin. Cancer Res. 25, 3784–3792. doi:10.1158/1078-0432.CCR-18-3502
Padda, S. K., Chhatwani, L., Zhou, L., Jacobs, C. D., Lopez-Anaya, A., and Wakelee, H. A. (2013). Phase I and pharmacokinetic study of bexarotene in combination with gefitinib in the third-line treatment of non-small-cell lung cancer: Brief report. Anticancer Drugs 24, 731–735. doi:10.1097/CAD.0b013e32836100d7
Park, Y. H., Park, M. J., Ji, S. H., Yi, S. Y., Lim, D. H., Nam, D. H., et al. (2009). Trastuzumab treatment improves brain metastasis outcomes through control and durable prolongation of systemic extracranial disease in HER2-overexpressing breast cancer patients. Br. J. Cancer 100, 894–900. doi:10.1038/sj.bjc.6604941
Perou, C. M., Sorlie, T., Eisen, M. B., van de Rijn, M., Jeffrey, S. S., Rees, C. A., et al. (2000). Molecular portraits of human breast tumours. Nature 406, 747–752. doi:10.1038/35021093
Ramalingam, S. S., Vansteenkiste, J., Planchard, D., Cho, B. C., Gray, J. E., Ohe, Y., et al. (2020). Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N. Engl. J. Med. 382, 41–50. doi:10.1056/NEJMoa1913662
Rao, C. V., Janakiram, N. B., Madka, V., Devarkonda, V., Brewer, M., Biddick, L., et al. (2015). Simultaneous targeting of 5-LOX-COX and EGFR blocks progression of pancreatic ductal adenocarcinoma. Oncotarget 6, 33290–33305. doi:10.18632/oncotarget.5396
Reddy, B. S., Nayini, J., Tokumo, K., Rigotty, J., Zang, E., and Kelloff, G. (1990). Chemoprevention of colon carcinogenesis by concurrent administration of piroxicam, a nonsteroidal antiinflammatory drug with D,L-alpha-difluoromethylornithine, an ornithine decarboxylase inhibitor, in diet. Cancer Res. 50, 2562–2568.
Ruhstaller, T., Giobbie-Hurder, A., Colleoni, M., Jensen, M. B., Ejlertsen, B., de Azambuja, E., et al. (2019). Adjuvant letrozole and tamoxifen alone or sequentially for postmenopausal women with hormone receptor-positive breast cancer: Long-term follow-up of the BIG 1-98 trial. J. Clin. Oncol. 37, 105–114. doi:10.1200/JCO.18.00440
Saba, N. F., Hurwitz, S. J., Kono, S. A., Yang, C. S., Zhao, Y., Chen, Z., et al. (2014). Chemoprevention of head and neck cancer with celecoxib and erlotinib: Results of a phase ib and pharmacokinetic study. Cancer Prev. Res. (Phila) 7, 283–291. doi:10.1158/1940-6207.CAPR-13-0215
Saintigny, P., William, W. N., Foy, J. P., Papadimitrakopoulou, V., Lang, W., Zhang, L., et al. (2018). Met receptor tyrosine kinase and chemoprevention of oral cancer. J. Natl. Cancer Inst. 110, 250–257. doi:10.1093/jnci/djx186
Samadder, N. J., Kuwada, S. K., Boucher, K. M., Byrne, K., Kanth, P., Samowitz, W., et al. (2018). Association of sulindac and erlotinib vs placebo with colorectal neoplasia in familial adenomatous polyposis: Secondary analysis of a randomized clinical trial. JAMA Oncol. 4, 671–677. doi:10.1001/jamaoncol.2017.5431
Samadder, N. J., Neklason, D. W., Boucher, K. M., Byrne, K. R., Kanth, P., Samowitz, W., et al. (2016). Effect of sulindac and erlotinib vs placebo on duodenal neoplasia in familial adenomatous polyposis: A randomized clinical trial. JAMA 315, 1266–1275. doi:10.1001/jama.2016.2522
Shepherd, F. A., Rodrigues Pereira, J., Ciuleanu, T., Tan, E. H., Hirsh, V., Thongprasert, S., et al. (2005). Erlotinib in previously treated non-small-cell lung cancer. N. Engl. J. Med. 353, 123–132. doi:10.1056/NEJMoa050753
Shi, Y., Au, J. S., Thongprasert, S., Srinivasan, S., Tsai, C. M., Khoa, M. T., et al. (2014). A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J. Thorac. Oncol. 9, 154–162. doi:10.1097/JTO.0000000000000033
Shin, D. M., Nannapaneni, S., Patel, M. R., Shi, Q., Liu, Y., Chen, Z., et al. (2020). Phase Ib study of chemoprevention with green tea polyphenon E and erlotinib in patients with advanced premalignant lesions (APL) of the head and neck. Clin. Cancer Res. 26, 5860–5868. doi:10.1158/1078-0432.CCR-20-2276
The National Lung Screening Trial Research Team Aberle, D. R., Adams, A. M., Berg, C. D., Black, W. C., Clapp, J. D., Fagerstrom, R. M., et al. (2011). Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 365, 395–409. doi:10.1056/NEJMoa1102873
Taieb, J., Tabernero, J., Mini, E., Subtil, F., Folprecht, G., van Laethem, J. L., et al. (2014). Oxaliplatin, fluorouracil, and leucovorin with or without cetuximab in patients with resected stage III colon cancer (PETACC-8): An open-label, randomised phase 3 trial. Lancet Oncol. 15, 862–873. doi:10.1016/S1470-2045(14)70227-X
Tammemagi, M. C., Katki, H. A., Hocking, W. G., Church, T. R., Caporaso, N., Kvale, P. A., et al. (2013). Selection criteria for lung-cancer screening. N. Engl. J. Med. 368, 728–736. doi:10.1056/NEJMoa1211776
Ulusan, A. M., Rajendran, P., Dashwood, W. M., Yavuz, O. F., Kapoor, S., Gustafson, T. A., et al. (2021). Optimization of erlotinib plus sulindac dosing regimens for intestinal cancer prevention in an APC-mutant model of familial adenomatous polyposis (FAP). Cancer Prev. Res. (Phila) 14, 325–336. doi:10.1158/1940-6207.CAPR-20-0262
von Minckwitz, G., Darb-Esfahani, S., Loibl, S., Huober, J., Tesch, H., Solbach, C., et al. (2012). Responsiveness of adjacent ductal carcinoma in situ and changes in HER2 status after neoadjuvant chemotherapy/trastuzumab treatment in early breast cancer--results from the GeparQuattro study (GBG 40). Breast Cancer Res. Treat. 132, 863–870. doi:10.1007/s10549-011-1621-0
William, W. N., Papadimitrakopoulou, V., Lee, J. J., Mao, L., Cohen, E. E., Lin, H. Y., et al. (2016). Erlotinib and the risk of oral cancer: The erlotinib prevention of oral cancer (EPOC) randomized clinical trial. JAMA Oncol. 2, 209–216. doi:10.1001/jamaoncol.2015.4364
Yang, J. C., Shih, J. Y., Su, W. C., Hsia, T. C., Tsai, C. M., Ou, S. H., et al. (2012). Afatinib for patients with lung adenocarcinoma and epidermal growth factor receptor mutations (LUX-Lung 2): A phase 2 trial. Lancet Oncol. 13, 539–548. doi:10.1016/S1470-2045(12)70086-4
Keywords: tyrosine kinase inhibitor, cancer interception, EGFR inhibitiors, HER-2 inhibitors, clinical cancer prevention, cancer prevention trials
Citation: Dragnev KH, Dragnev CPC and Lubet RA (2023) Major hurdles to the use of tyrosine kinase inhibitors in clinical prevention/interception studies: Do preclinical studies with EGFR inhibitors suggest approaches to overcome some of the limitations. Front. Cell Dev. Biol. 11:1170444. doi: 10.3389/fcell.2023.1170444
Received: 20 February 2023; Accepted: 11 April 2023;
Published: 24 April 2023.
Edited by:
Feng Zhu, Affiliated Hospital of Guilin Medical University, ChinaReviewed by:
Qiuhong Duan, Huazhong University of Science and Technology, ChinaCopyright © 2023 Dragnev, Dragnev and Lubet. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Konstantin H. Dragnev, a29uc3RhbnRpbi5oLmRyYWduZXZAaGl0Y2hjb2NrLm9yZw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.