- 1Department of Thoracic Surgery, Shengjing Hospital of China Medical University, Shenyang, China
- 2Devission of Pulmonary, Allergy and Critical Care Medicine, School of Medicine, Stanford University, Stanford, CA, United States
- 3Clinical Skills Practice Teaching Center, Shengjing Hospital of China Medical University, Shenyang, China
Long non-coding RNAs (lncRNAs) are endogenously expressed RNAs longer than 200 nt that are not translated into proteins. In general, lncRNAs bind to mRNA, miRNA, DNA, and proteins and regulate gene expression at various cellular and molecular levels, including epigenetics, transcription, post-transcription, translation, and post-translation. LncRNAs play important roles in many biological processes, such as cell proliferation, apoptosis, cell metabolism, angiogenesis, migration, endothelial dysfunction, endothelial-mesenchymal transition, regulation of cell cycle, and cellular differentiation, and have become an important topic of study in genetic research in health and disease due to their close link with the development of various diseases. The exceptional stability, conservation, and abundance of lncRNAs in body fluids, have made them potential biomarkers for a wide range of diseases. LncRNA MALAT1 is one of the best-studied lncRNAs in the pathogenesis of various diseases, including cancers and cardiovascular diseases. A growing body of evidence suggests that aberrant expression of MALAT1 plays a key role in the pathogenesis of lung diseases, including asthma, chronic obstructive pulmonary diseases (COPD), Coronavirus Disease 2019 (COVID-19), acute respiratory distress syndrome (ARDS), lung cancers, and pulmonary hypertension through different mechanisms. Here we discuss the roles and molecular mechanisms of MALAT1 in the pathogenesis of these lung diseases.
Background
Lung diseases, such as asthma, chronic obstructive pulmonary diseases (COPD), lung cancer, Coronavirus Disease 2019 (COVID-19), acute respiratory distress syndrome (ARDS), idiopathic pulmonary fibrosis (IPF), and pulmonary hypertension (PH) are one of the major causes of death in the world. The effective therapies for lung diseases are limited. There is an urgent unmet need for developing effecting novel therapies for the diseases. To identify novel treatment or targeted interventions, understanding the molecular mechanisms of the signaling pathways involved in the pathogenesis of the disease is still required.
Long non-coding RNAs (lncRNAs) are RNAs longer than 200 nucleotides that lack the ability to code for proteins (they lack functional open reading frames) (Kung et al., 2013). According to GENCODE consortium (Version 42 and M31), 19,933 and 14,764 lncRNAs compared to 19,379 and 21,657 protein-coding genes have been annotated in human and mouse, respectively. Although lncRNAs do not make proteins, they regulate a range of biological processes, such as cell proliferation, apoptosis, migration, angiogenesis, and metabolism (Zhang et al., 2019). LncRNAs act as molecular decoys, scaffolding in signaling transduction, and guiding ribonucleoprotein complexes (Li et al., 2013). LncRNAs plays a significant role in various diseases, such as cancers, cardiovascular and lung diseases (Jiang et al., 2019; Fang et al., 2020). A growing body of evidence suggest that lncRNAs plays a significant role in the onset and progression of lung disease (Groot et al., 2018).
One of the most extensively studied lncRNA is metastasis-associated lung adenocarcinoma transcript 1 (MALAT1, ENSG00000251562), located in human chromosome 11q13.1 (mouse chromosome 19qA). MALAT1 is also known as nuclear-enriched abundant transcript 2 (NEAT2, originally described to be associated with non-small cell lung cancer (Ji et al., 2003). MALAT1 is a nuclear enriched and highly conserved lncRNA that is the most abundantly expressed lncRNA in cells and tissues (Hutchinson et al., 2007). The mouse MALAT1 is 6.7 kb, but human MALAT1 is approximately 8.7 kb in length (Djebali et al., 2012).
At the molecular level, MALAT1 has been reported to play a role in regulating pre-mRNA splicing (Hutchinson et al., 2007; Tripathi et al., 2010), transcription (Arun et al., 2020), and post-transcriptional modifications, such as 5-methylcytosine (Amort et al., 2017) and N6-methyladenosine (Liu et al., 2017). In addition, MALAT1 can act as a competing endogenous RNA or miRNA sponge to sequester miRNAs and regulate gene expression. MALAT1 has been shown to be aberrantly expressed and have prognostic and therapeutic significance in different cancers, including pancreatic cancer, lung cancer, breast cancer, colorectal cancer, gastric cancer, nasopharyngeal carcinoma, hepatocellular carcinoma, osteosarcoma (Sun and Ma, 2019). A growing body of evidence reveal that MALAT1 plays a significant role in lung diseases, such as asthma, COPD, COVID-19, IPF and PAH. Here, we summarize the roles and molecular mechanisms, therapeutic and biomarker potential of MALAT1 in lung diseases.
Role of MALAT1 in lung homeostasis and pathophysiology
Lung homeostasis refers to the balance of physiological processes and cellular activities necessary to maintain the normal structure and function of the lungs. This includes the proper functioning of the airways, alveoli, blood vessels, and other components of lung tissue. Key processes involved in lung homeostasis include gas exchange, immune defense, airway clearance, and proper cell growth and differentiation. The airway plays a vital role in regulating lung fluid balance, removing inhaled agents, attracting, and activating inflammatory cells, and maintaining airway smooth muscle function. The lung alveoli are the basic functional units of the gaseous exchange of oxygen and carbon dioxide between inhaled air and the bloodstream. Under normal circumstances, the airway epithelium forms a crucial tissue barrier and serves as the primary defense against both external and internal challenges (Knight and Holgate, 2003; Crystal et al., 2008). Exposure to toxic stimuli can cause damage to lung epithelial cells, resulting in the breakdown of the epithelial barrier and airway injury (Shaykhiev et al., 2011). This damage to the epithelium triggers the activation of various types of immune cells, including macrophages, dendritic cells (DCs), and others, which further contributes to the inflammatory immune response (Fujita et al., 2015). Proper lung homeostasis is crucial to maintain proper respiratory function and overall health. It ensures that the lungs can effectively exchange oxygen and carbon dioxide while also protecting against harmful pathogens, irritants, and toxins. Disruptions in lung homeostasis can lead to respiratory diseases, such as asthma, COPD, and lung cancer, which can significantly impact quality of life and mortality rates. Therefore, maintaining lung homeostasis is essential for optimal respiratory health.
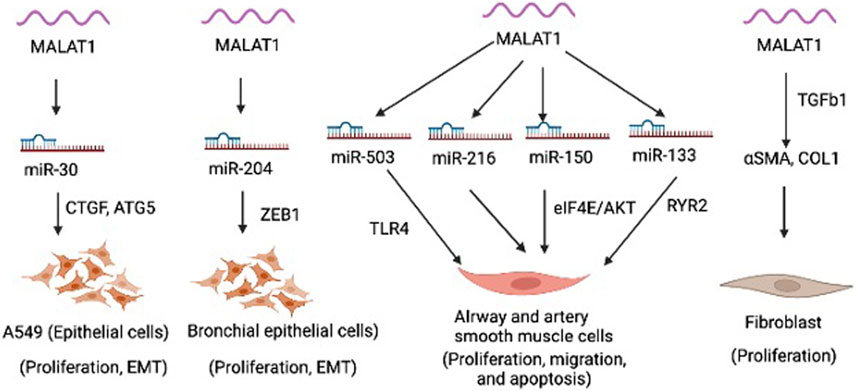
MALAT1 is expressed in various cell types within the lung, including epithelial cells, smooth muscle cells, fibroblasts, and immune cells. MALAT1 is involved in regulating various cellular processes, such as proliferation, migration, apoptosis, epithelial mesenchymal transition (EMT) (Figure 1). MALAT1 has been implicated in several aspects of lung homeostasis and dysregulation of MALAT1 is involved in the development and progression of different lung diseases, such as asthma, COPD, and lung cancer (Lai et al., 2022). Silencing of MALAT1 in airway smooth muscle cells using siRNA resulted in decreased cell viability, migration and invasion and induced apoptosis as evidenced by upregulated Caspase 3, Bax, E-cadherin and downregulated N-cadherin, Bcl-2 and β-catenin (Huang et al., 2021). MALAT1 was shown to regulate the function of airway smooth muscle cells through the modulation of microRNA-216a (Huang et al., 2021). Another study found that MALAT1 expression in ASMCs increased with PDGF-BB treatment, but knockdown of Malat1 reduced PDGF-BB-induced proliferation and migration (Lin et al., 2019). Malat1 directly targeted miR-150, inhibition of which promoted PDGF-BB-induced proliferation and migration, which was reversed by Malat1 overexpression. miR-150 targets eIF4E, and eIF4E knockdown and Akt inhibitor GSK690693 inhibited PDGF-BB-induced proliferation and migration (Lin et al., 2019). Malat1 was found to operate as a ceRNA for miR-150, derepressing eIF4E expression and activating the Akt pathway, facilitating PDGF-BB-induced ASMC proliferation and migration (Lin et al., 2019). Yang and Wang demonstrated that silencing MALAT1 provides protection against injury to bronchial/tracheal smooth muscle cells by regulating the microRNA-133a/ryanodine receptor 2 axis (Yang and Wang, 2021). Li et al., showed that MALAT1 expression increases in AECs co-cultured with dendritic cells (DCs), but down-regulating MALAT1 expression in this co-culture system leads to increased expression of maturity surface markers (CD86, CD80, and MH class II), production of inflammatory cytokines (TNF-α, IL-6, and IFN-γ), and secretion of chemokines (CXCR2 and CXCR4) in DCs (Li et al., 2018a). Furthermore, suppression of MALAT1 was shown to reduce apoptosis and promotes cell viability in DCs co-cultured with AECs (Li et al., 2018a). These findings suggest that MALAT1-mediated crosstalk between AECs and DCs affects DC maturation, and apoptosis, chemotaxis, and cytokine production. MALAT1 was also shown to promote the proliferation and migration of pulmonary artery smooth muscle cells via modulating mir-503/Toll-Like Receptor 4 (TLR4) Signal Axis (He et al., 2020). Furthermore, as mentioned above, epithelial cells in the respiratory system act as a crucial barrier to protect the internal tissues from the environment. They regulate gas exchange, maintain hydration, and defend against toxins, irritants, and pathogens. MALAT1 was shown to regulate the EMT process in alveolar epithelial cells (A549) by binding to miR-30c-5p and consequently influencing downstream targets such as CTGF, ATG5, and autophagy (Xia et al., 2023). In this study, the author elucidated fibrosis mechanism in silicosis and offered new therapeutic targets. a biological process by which epithelial cells undergo a series of molecular changes that enable them to acquire mesenchymal properties, such as increased motility and invasiveness. This process is essential for embryonic development, tissue repair, and wound healing, but it can also contribute to cancer progression and metastasis. During EMT, epithelial cells lose their characteristic morphology and polarity, and acquire a spindle-like shape and increased motility. Another study showed that MALAT1 mediates PM2.5-induced EMT through miR-204/ZEB1 pathway in bronchial epithelial cell lines (HBE and BEAS-2B) (Luo et al., 2019). These findings suggest that Malat1 may present a new target to modulate smooth muscle cells and epithelial cells in the lung to limit pulmonary vascular and airway remodeling in lung disease.
Role of MALAT1 in lung disease
Respiratory diseases, such as asthma, COPD, lung cancer, IPF, and CF are among the leading causes of mortality and morbidity, and they represent a major socio-economic burden worldwide (Xie et al., 2020). More specifically, asthma affects over 300 million people in the world and around 14% of all children suffer from the disease (Pearce et al., 2007; Fergeson et al., 2017). IPF, while less common, is a progressive disease that leads to shortness of breath, pulmonary embolisms, pneumonia, and other deadly conditions. Emerging studies suggest that lncRNA MALAT1 plays a significant role in the pathogenesis of lung diseases. In the following sections, we will discuss the involvement of MALAT1 in the pathogenesis of lung diseases, especially asthma, COPD, IPF, ARDS, and PAH.
MALAT1 and asthma
Asthma is characterised by features, such as cough, shortness of breath, wheezing and chest pain (Mattiuzzi and Lippi, 2020). The National Heart, Lung, and Blood Institute defines asthma as a chronic disease which affects lung airways, and most asthmatic patients may have narrowed and inflamed airways, and obstructed breathing. The researchers have added that pollen, virus infections and exercise can induce or worsen asthma symptoms (Ramsahai et al., 2019). Asthma is no respecter of age as according to the National Health Service; people of all ages can acquire this lung disease. The Centres for Disease Control and Prevention has made it clear that there is at least one asthmatic patient in a group of thirteen in the United States. Patient death is imminent because of failure in timely treatment of symptoms (Yan et al., 2019). Hence, insights into some of the underlying aberrant molecular mechanisms would be helpful in dealing with asthma more effectively.
It is evident that MALAT1 plays significant roles in the asthma pathogenesis and progression, and the work by Huang et al. is a typical example (Huang et al., 2021). Using preclinical asthmatic rat models and with the aim to probe the effects of MALAT1 on airway smooth muscle cells in asthma using various biochemical and molecular techniques, the researchers reported that asthma was associated with upregulated MALAT1 and downregulated microRNA-216a, a target of MALAT1 through inhibition. Essentially, downregulated MALAT1 and/or upregulated microRNA-216a significantly elevated apoptosis while cell proliferation, migration and invasion saw tremendous reductions (Huang et al., 2021). In another study, RT-qPCR analysis on blood samples revealed an upregulated MALAT1 in patients with asthma compared to healthy controls (Liang and Tang, 2020). In addition, dysregulated T-cell receptor signalling pathway was found to be a major hallmark in eosinophilic asthma patients (Zhu et al., 2018). However, the only concern, at least per what we could identify, which may present as a limitation to their study, was the small sample size used (n = 9 for eosinophilic asthma patients and n = 3 for healthy controls).
A recent study has reported aberrant proliferation and smooth muscle layer thickening at where airways are located, in lung tissues of newborn rat models of asthma (Yang and Wang, 2021). Using RT-qPCR, the authors discovered a significant upregulation of MALAT1 in the tracheal tissues of rats. The authors also observed synchronous changes in cell apoptosis and secretion of inflammatory factors, such as interleukin-6, tumour necrosis factor alpha, and interleukin-1β, or ryanodine receptor 2 (RyR2) level when MALAT1 was either silenced or overexpressed in the bronchial and tracheal smooth muscle cells. However, changes in miR-133a level with MALAT1 occurred at unrelated times. The authors added that since MALAT1 drives apoptosis in bronchial and tracheal smooth muscle cells, and either miR-133a upregulation or RyR2 silencing can partly reverse inflammation, the knockdown of MALAT1 can mitigate bronchial and tracheal smooth muscle cell injury through the regulation of miR-133a/RyR2 (Yang and Wang, 2021). This study proposes a mechanism by which MALAT1 is involved in asthma in newborn rats, which is an intriguing finding. When taken together with the three previous studies, it becomes evident that MALAT1 plays a crucial role in the pathogenesis of asthma, as demonstrated through preclinical and clinical investigations.
MALAT1 and IPF
Termed as the most occurring form of permanent interstitial pneumonia (Wang et al., 2020), IPF is a lung disease with no clear etiology. The major hallmark features of IPF are characterized by extensive lung architectural remodeling and subsequent chronic lung malfunction. IPF has an average mortality rate of 4 years, and there is currently no effective therapy available to treat the disease (Raghu et al., 2011; Guiot et al., 2017; Guiot et al., 2018). The prevalence of IPF is estimated to range from 14 to 28 cases per 100,000 in the United States, and 1 to 23 cases per 100,000 in Europe (Nalysnyk et al., 2012). The disease is age and sex-related, with men being more susceptible than women (Nalysnyk et al., 2012). Aside the lack of effective therapeutic intervention, there are other challenges which include unclear physiopathology, lack of a simple-but-effective diagnosis (currently only requires multidisciplinary methods) and a nonspecific radiological image (Poulet et al., 2020).
While the major regulatory genes associated with IPF onset and progression are not clearly known, recent arising evidence shows that MALAT1 is a major regulator of IPF pathogenesis and one of such evidence is the study conducted by Wang et al. (2020). In detail, the authors main aim was to investigate the major roles of lncRNAs, including MALAT1, and transcription factors, in the pathogenesis of IPF using blood samples from human subjects. To achieve this, the approach involved analysis of three Gene Expression Omnibus (GEO) dataset-derived gene expression profiles; GSE 2052, GSE44723, and GSE24206. While hypergeometric testing revealed that several genes were found to relate to ubiquitin-mediated proteolysis, spliceosome, and cell cycle in both IPF patients and healthy controls, MALAT1 was one of the only three expressed genes that differed in peripheral blood samples from the two groups, as measured by qRT-PCR. While this information provides great insight into the involvement of MALAT1 in IPF pathogenesis, the authors have also highlighted several limitations. For instance, although the genes associated with IPF pathogenesis were identified through dataset integration and bioinformatics, no casual molecular and histological investigations of the direct role of MALAT1 in IPF were conducted to support their findings. The authors also acknowledged small sample sizes and imbalances in the datasets as a limitation. Lastly, although the authors successfully confirmed gene expression levels in their clinical investigations, no in vivo or in vitro loss- and gain-of-function experiments were conducted to facilitate further functional verification of the genes (Wang et al., 2020).
MALAT1 and ARDS
ARDS is known to be a critical type of acute lung injury, which is distinguished by intense inflammatory reactions that may result in cellular and tissue damage and mortality (Wang et al., 2019a). While the role of MALAT1 in ARDS has still not been completely found, various studies are tirelessly trying to unlock this puzzle. For example, MALAT1, as detected by qRT-PCR, was found to be overexpressed in plasma and peripheral blood mononuclear cells (PBMCs) of ARDS patients relative to healthy controls. In addition, there was an interaction between MALAT1 and miR-425, which in turn resulted in the protection of the expression of phosphatase and tensin homolog (PTEN) in A549 and HFL-1 cells. Lastly, it was discovered that the delivery of miR-425 by exosomes into A549 and HFL-1 cells in ARDS patients was minimal compared to that delivered in healthy controls. The researchers concluded that MALAT1 is upregulated amidst downregulated miR-425 in ARDS, signifying the involvement of MALAT1 in disease pathogenesis (Wang et al., 2019a). A major limitation identified was the lack of the detection of levels of MALAT1 in pathological tissues. Though just a couple of studies detailing the evidence of MALAT1’s involvement in ARDS have been stated in this review, other researchers (Juan et al., 2018; Huang and Zhao, 2019; Yao et al., 2020) have undertaken similar studies and interested readers can also have a look at those.
MALAT1 and COPD
COPD is a chronic lung inflammatory disease characterized by airflow obstruction in the lung. COPD accounts for the fourth leading cause of death globally (Vogelmeier et al., 2017). The leading risk factor for COPD is smoking, while quitting smoking early on could significantly slow down or even reverse the progression of lung function reduction (Damkjaer et al., 2021). Currently, there is no cure for COPD, but treatment control the symptoms and slow the progression of the disease.
A growing body of evidence suggests that MALAT1 plays a significant role in COPD pathogenesis. A previous study by Hu et al., measured expression levels of six selected lncRNAs based on their involvement in different lung diseases (HOTAIR, HULC, MEG3, NEAT1, UCA1, and MALAT1) by RT-qPCR in the lung tissues collected from three pairs of age-matched COPD and non-COPD individuals (Hu et al., 2020). Among the six lncRNAs, lncRNAs MALAT1 and MEG3 showed a significant upregulation in COPD lung tissues. The authors then further confirm MALAT1 and MEG3 expression in a second cohort consisting of 10 pairs of age-matched COPD and non-COPD individuals and found that lncRNA MALAT1 is consistently upregulated in COPD lung tissues when compared with non-COPD lung tissues. Silencing of MALAT1 with siRNA in human fetal lung fibroblasts treated with TGFb showed a decrease in cell viability and reduction in protein expression of two mesenchymal markers, α-SMA and fibronectin. Mechanistically, the authors demonstrated that the α-SMA and fibronectin expression occurred independently of mTORC1, because p-S6K1 protein expression was not affected following MALAT1 silencing, which further warrants futures studies to investigate this mechanism in COPD.
By comparing MALAT1 expression in the blood of 120 healthy subjects, 120 stable COPD patients, and 120 acute exacerbation COPD patients, Liu et al., observed higher expression of MALAT1 in acute exacerbation COPD patients compared to stable COPD patients and healthy subjects (Liu et al., 2020). The ROC curve analysis revealed that MALAT1 expression discriminates from acute exacerbation COPD patients from the healthy subjects (sensitivity and specificity 99.2% and 83.3%, respectively) and the stable COPD patients (64.2% and 83.3%, respectively), indicating that MALAT1 could be used as a biomarker in COPD. The authors also found a significant positive correlation between expression of MALAT1 and GOLD stage and inflammatory cytokines, such as IL-1β, IL-6, IL-17, IL-8, IL-23 and TNF-α) in both patients with acute exacerbation and stable cohorts, indicating MALAT1 could induce inflammation and inappropriate immune response in COPD. The authors also showed a significant negative correlation of MALAT1 expression with its targets miR-146a, miR-125b, and miR-203 in acute exacerbation COPD patients and a reverse correlation with miR-146a and miR-125b in stable COPD patients. Importantly, acute exacerbation COPD patients had the lowest levels of miR-125b, miR-146a, miR-133, and miR-203, followed by in stable COPD patients and HCs. In both stable and acute exacerbation COPD patients, miR-125b, miR-146a, miR-133, and miR-203 were inversely linked with inflammation and GOLD stage. According to these findings, MALAT1 and its microRNA targets may be useful in helping predict the severity of acute exacerbations and the progression of COPD. There are, however, several limitations to these studies. Firstly, it is unclear how MALAT1 contributes to acute exacerbations of COPD. Secondly, acute exacerbation and stable COPD patients were not investigated for the effect of MALAT1 expression on short- and long-term prognosis. Finally, these findings may result in bias since blood samples were collected within 24 h of enrollment of acute exacerbation COPD patients, however, there was a wide range of time intervals between the onset of acute exacerbation COPD and hospital admissions.
MALAT1 and PAH
PAH is a serious, life-threatening progressive medical condition characterized by high blood pressure within the arteries of lungs. This leads to decreased cardiac function, results in right ventricular failure that eventually causes multiorgan dysfunction and ultimately death. It is a rare disease, affecting 15 peoples per million globally with 3–5 times more common in females than males. The key hallmark histopathological manifestation of PH is pulmonary vascular remodeling, such as, medial hypertrophy, neointima formation, plexiform lesion. Pulmonary vascular remodeling is majorly driven by the dysfunction of key cellular components of the pulmonary vasculature, namely, smooth muscle, endothelial, and inflammatory cells, pericytes, and adventitial fibroblasts. Existing PAH drugs majorly dilate pulmonary arteries, increasing survival and improving quality of life, but do not target pulmonary vascular remodeling. There is a gap between our treatment and the need to reverse the pulmonary vascular remodeling to improve PAH effectively. While the exact cause of PAH is not known, genetic defects (mutations or epigenetic changes), environmental factors (e.g., hypoxia, viral infections, anorectic agents, etc.), and immune or inflammatory triggers may contribute to the cause or progression of the disease. Emerging studies suggests that lncRNAs play a significant role in pulmonary vascular and RV remodeling and PAH (Han et al., 2021).
Through a selective 84 lncRNA profiling study in hypoxic HPASMCs by a PCR array, Brock et al., identified lncRNA MALAT1 as a promising candidate in PH (Brock et al., 2017). The authors demonstrated that MALAT1 was upregulated in hypoxic hPAECs and hPASMCs and in the lung of mice exposed to hypoxia. Furthermore, MALAT1 was shown to be majorly driven by HIF1a in hPAECs and hPASMCs. Silencing of MALAT1 with LNA Gapmers showed a significant inhibition of hPASMCs proliferation and migration, assessed by BrdU incorporation and wound-healing assays, respectively. Further in vitro studies showed that MALAT1 regulated PASMCs proliferation through targeting cyclin-dependent kinase (CDK) inhibitors. Importantly, intraperitoneal injections of LNA gapmers mediated inhibition of MALAT1 reduced heart weight in hypoxia-induced established PH in mice. Although RVSP was not changed by MALAT1 inhibition in this PH mouse model. While these findings are interesting, especially in the context of in vivo translation of MALAT1 findings in mice, but the authors did not able to show the direct causal effect of MALAT1 on PASMCs proliferation in mice.
Wang et al., found a significantly higher expression of MALAT1 in the pulmonary arteries and hPASMCs from 8 PAH patients compared to 8 healthy control samples (Wang et al., 2019b). ShRNA-mediated MALAT1 knockdown reduced hPASMCs proliferation and migration while overexpression of the lncRNA by overexpression plasmid showed the opposite cellular phenotypes. Subsequent in vitro mechanistic analysis revealed that MALAT1/hsa-miR-124-3p.1/KLF5 axis plays important role in hPASMCs proliferations.
Recently, He et al., also measured expression of MALAT1 and microRNA-503 by qRT-PCR in PBMCs of 45 PAH patients and 45 healthy donor controls and in HPASMCs exposed to hypoxia (He et al., 2020). Consistent with the above previous studies, the authors also found a significant upregulation of MALAT1 in PAH patients and hypoxic PASMCs. Importantly, through ROC analysis, the authors observed that MALAT1 and microRNA-503 could be used as a potential diagnostic biomarker in PAH. Additionally, the authors demonstrated that MALAT1 knockdown inhibited PASMCs proliferation and migration and induced apoptosis while overexpression studies had the opposite effects in PASMCs. MALAT1 was found to be sponged with miR-503 that controls TLR4 expression. Together, these results indicated that MALAT1 could regulate proliferation, apoptosis, and migration of PASMCs potentially through regulating miR-503/TLR4 axis. While the results of these studies are promising, there in vitro data need validation in vivo PH models as well as since all the PAH patients were recruited from only one center, their findings need to confirm in other larger cohorts of PAH patients.
MALAT1 as a therapeutic target in lung disease
As already indicated above, one of the main challenges with lung disease management lies in the difficulty to lay hands on an effective therapeutic intervention. MALAT1 is an important lncRNA that, when extensively explored, can end our eager search for the much-anticipated breakthroughs in efficient treatments for lung diseases, making MALAT1 a potential therapeutic target for lung disease treatment. For instance, in the asthma study by Yang and Wang detailed above, the knockdown of MALAT1 reversed inflammation and mitigated bronchial and tracheal smooth muscle cell injury through the regulation of miR-133a and RyR2 (Yang and Wang, 2021). This mechanism provides the suggestion that MALAT1/miR-133a/RyR2 axis signalling network is a potential therapeutic target for asthma treatment. Also, since Huang et al. showed that a downregulated MALAT1, via the regulation of microRNA-216a, can significantly induce apoptosis and reduce cell proliferation, migration, and invasion, it may inform that the MALAT1/microRNA-216a signalling network can be a therapeutic target in the treatment of asthma (Huang et al., 2021). Consistently, the fact that the study by Wang et al. showed that an upregulated MALAT1, through interactions with miR-425 and subsequently, PTEN, was found to associate with ARDS, suggests that the MALAT1/miR-425/PTEN axis signalling network is a potential therapeutic target for ARDS treatment (Wang et al., 2019a). Lastly, since MALAT1 also plays important roles in other lung diseases some of which have been abovementioned, the following signalling networks are thought to also be key targets of therapeutic treatment for the prevention or cure of other lung diseases: MALAT1/miR-503/TLR4 (He et al., 2020) and MALAT1/hsa-miR-124-3p.1/KLF5 (Wang et al., 2019b) for PAH treatment, MALAT1/miR-125b, MALAT1/miR-146a, MALAT1/miR-133 and MALAT1/miR-203 for COPD treatment (Liu et al., 2020) and MALAT1/antisense oligonucleotides (ASOs) for lung cancer treatment (Gutschner et al., 2013). Targeting MALAT1 with RNAi approach, microRNA response elements, and ASOs could be potentially useful for developing MALAT1 targeted therapies in lung diseases.
Several drugs have also shown to improve tissue injury through targeting MALAT1 in experimental models of lung diseases. For example, by impairing the MALAT1-miR-22-3p-NLRP3 axis, resveratrol effectively blocked cardiac injury caused by pulmonary embolism (Yang et al., 2019). Suppression of MALAT1 by minocycline contributed to the amelioration of septic lung injury in ALI mice by blocking oxidative stress and inflammatory responses (Cui et al., 2021). Propofol, an intravenous anaesthetic, was shown to impedes autophagy activation and secretion of inflammatory factors (e.g., TNF-α, IL-18, and IL-1β) through reducing the MALAT1-miR-144-GSK3β signaling axis. Propofol eventually alleviated lung ischemia-reperfusion in mice (Zhang et al., 2021). Although these findings imply that the use of these drugs targeting MALAT1 could be effective in treating various lung diseases, further research is necessary to fully understand their precise mechanisms of action.
Biomarker potential of MALAT1 in lung disease
Since MALAT1 plays important roles in the pathogenesis of lung diseases, identifying its biomarker potential would help in successful prognosis, diagnosis, and treatment of disease. Huang and colleagues asserted in their preclinical study that since MALAT1 and microRNA-216a can together regulate airway smooth muscle cell proliferation in rat models of asthma, it would be worthwhile starting to investigate both MALAT1 and microRNA-216a as potential biomarkers of asthma, and also investigate their relationship in the diagnosis and prognosis of asthma (Huang et al., 2021). MALAT1 expression is inversely associated with lung function and the Th1/Th2 ratio, and it affects GATA-3, a transcription factor that promotes the development of ILC2. ILC2, in turn, induces the biosynthesis of TH2 cytokines, such as IL-5, IL-6, and IL-13, which are implicated in the pathophysiology and clinical diagnosis of bronchial asthma (Wu et al., 2022). Poulet et al., (Poulet et al., 2020), has reported the involvement of MALAT1 in lung-related diseases. First, the authors briefly discussed about how MALAT1 dysregulation can influence the course of asthma and COPD. Now focusing on lung cancer, they mentioned in detail some of the regulators of MALAT1 (e.g., TDP-43, ESR2, Oct3/4) (Guo et al., 2015a; Jen et al., 2017; Yu et al., 2019), some genes regulated by MALAT1 (e.g., TP53, cleaved-PARP1, cleaved-CASP3, phospho-STAT3) (Tano et al., 2018; Yang et al., 2018), some miRNAs regulated by MALAT1 (e.g., hsa-miR-145, hsa-miR-204, hsa-miR-124-1) (Li et al., 2016; Li et al., 2018b; Yu et al., 2019) and MALAT1 behavior against treatment at clinical level. Given its various roles in lung cancer, the authors insisted on the need for additional studies on MALAT1 mechanisms, and their confirmation as potential biomarkers through further research. Liu and colleagues also found that upregulation of MALAT1, as well as regulation of its targets miR-125b, miR-146a, and miR-203, are important for detecting increased risk of COPD or for managing COPD (Liu et al., 2020). Patients with lung cancer have lower levels of MALAT1 in their blood compared to healthy individuals, with an area under the receiver operator curve of 0.718 (Guo et al., 2015b). The expression of MALAT1 was found to be stronger in the whole blood of lung cancer patients with metastasis compared to those without metastasis. As MALAT1 can be detected in whole blood, has the potential to be a useful biomarker for identifying and facilitating the treatment of lung cancer. MALAT1 is also involved in various other lung diseases as detailed in earlier sections of this review. In summary, with these clues, it is worthwhile to start thinking about MALAT1 is a potential biomarker for lung diseases.
Concluding remark and future direction
MALAT1 has recently become one of the most studied lncRNAs for genetic, epigenetic, and molecular research due to their various impacts on health and diseases. They are efficient in facilitating vital processes such as gene regulation, upholding of physiological conditions (Ma et al., 2015), and their help to maintain normal ageing process (Lei et al., 2017) and immunity (Sookoian et al., 2018). Through interaction with target proteins such as miRNAs, MALAT1 can alleviate lung disease progression, or at least mitigate it. However, they can become toxic and drive disease pathogenesis and progression when aberrant, because of dysregulation. This evidence suggests that MALAT1 is an important entity in lung disease study and management, and it has the potential of being both a reliable biomarker in lung diseases and therapeutic target for lung disease treatment. Current and future researchers and/or clinicians can capitalize on this for an improved research and treatment of lung diseases. However, to achieve this, additional work has to be done, which relates to addressing some of the limitations indicated in previous studies on MALAT1 and lung disease relationships.
Author contributions
JL: Conceptualization, Writing—Original Draft, Revision; MA: Manuscript discussion, review, editing and revision; YM: Conceptualization, Writing—Review and Editing, Funding acquisition. All authors have read and agreed to the published version of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work is funded by the People’s Livelihood Science and Technology Program Joint Plan of Liaoning Province, China (Grant No. 2021JH2/10300131).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Amort, T., Rieder, D., Wille, A., Khokhlova-Cubberley, D., Riml, C., Trixl, L., et al. (2017). Distinct 5-methylcytosine profiles in poly(A) RNA from mouse embryonic stem cells and brain. Genome Biol. 18 (1), 1. doi:10.1186/s13059-016-1139-1
Arun, G., Aggarwal, D., and Spector, D. L. (2020). MALAT1 long non-coding RNA: Functional implications. Noncoding RNA 6 (2), 22. doi:10.3390/ncrna6020022
Brock, M., Schuoler, C., Leuenberger, C., Buhlmann, C., Haider, T. J., Vogel, J., et al. (2017). Analysis of hypoxia-induced noncoding RNAs reveals metastasis-associated lung adenocarcinoma transcript 1 as an important regulator of vascular smooth muscle cell proliferation. Exp. Biol. Med. (Maywood) 242 (5), 487–496. doi:10.1177/1535370216685434
Crystal, R. G., Randell, S. H., Engelhardt, J. F., Voynow, J., and Sunday, M. E. (2008). Airway epithelial cells: Current concepts and challenges. Proc. Am. Thorac. Soc. 5 (7), 772–777. doi:10.1513/pats.200805-041HR
Cui, N., Liang, Y., Wang, J., Liu, B., Wei, B., and Zhao, Y. (2021). Minocycline attenuates oxidative and inflammatory injury in a intestinal perforation induced septic lung injury model via down-regulating lncRNA MALAT1 expression. Int. Immunopharmacol. 100, 108115. doi:10.1016/j.intimp.2021.108115
Damkjaer, M., Hakansson, K., Kallemose, T., Ulrik, C. S., and Godtfredsen, N. (2021). Statins in high-risk chronic obstructive pulmonary disease outpatients: No impact on time to first exacerbation and all-cause mortality - the STATUETTE cohort study. Int. J. Chron. Obstruct Pulmon Dis. 16, 579–589. doi:10.2147/COPD.S296472
Djebali, S., Davis, C. A., Merkel, A., Dobin, A., Lassmann, T., Mortazavi, A., et al. (2012). Landscape of transcription in human cells. Nature 489 (7414), 101–108. doi:10.1038/nature11233
Fang, Y., Xu, Y., Wang, R., Hu, L., Guo, D., Xue, F., et al. (2020). Recent advances on the roles of LncRNAs in cardiovascular disease. J. Cell Mol. Med. 24 (21), 12246–12257. doi:10.1111/jcmm.15880
Fergeson, J. E., Patel, S. S., and Lockey, R. F. (2017). Acute asthma, prognosis, and treatment. J. Allergy Clin. Immunol. 139 (2), 438–447. doi:10.1016/j.jaci.2016.06.054
Fujita, Y., Kosaka, N., Araya, J., Kuwano, K., and Ochiya, T. (2015). Extracellular vesicles in lung microenvironment and pathogenesis. Trends Mol. Med. 21 (9), 533–542. doi:10.1016/j.molmed.2015.07.004
Groot, M., Zhang, D., and Jin, Y. (2018). Long non-coding RNA review and implications in lung diseases. JSM Bioinform Genom Proteom 3 (2), 1033.
Guiot, J., Duysinx, B., Seidel, L., Henket, M., Gester, F., Bonhomme, O., et al. (2018). Clinical experience in idiopathic pulmonary fibrosis: A retrospective study. Acta Clin. Belg. 73 (2), 139–143. doi:10.1080/17843286.2017.1399228
Guiot, J., Henket, M., Corhay, J.-L., Moermans, C., and Louis, R. (2017). Sputum biomarkers in IPF: Evidence for raised gene expression and protein level of IGFBP-2, IL-8 and MMP-7. PLoS One 12 (2), e0171344. doi:10.1371/journal.pone.0171344
Guo, F., Jiao, F., Song, Z., Li, S., Liu, B., Yang, H., et al. (2015). Regulation of MALAT1 expression by TDP43 controls the migration and invasion of non-small cell lung cancer cells in vitro. Biochem. Biophys. Res. Commun. 465 (2), 293–298. doi:10.1016/j.bbrc.2015.08.027
Guo, F., Yu, F., Wang, J., Li, Y., Li, Y., Li, Z., et al. (2015). Expression of MALAT1 in the peripheral whole blood of patients with lung cancer. Biomed. Rep. 3 (3), 309–312. doi:10.3892/br.2015.422
Gutschner, T., Hammerle, M., Eissmann, M., Hsu, J., Kim, Y., Hung, G., et al. (2013). The noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Res. 73 (3), 1180–1189. doi:10.1158/0008-5472.CAN-12-2850
Han, Y., Ali, M. K., Dua, K., Spiekerkoetter, E., and Mao, Y. (2021). Role of long non-coding RNAs in pulmonary arterial hypertension. Cells 10 (8), 1892. doi:10.3390/cells10081892
He, M., Shen, J., Zhang, C., Chen, Y., Wang, W., and Tao, K. (2020). Long-chain non-coding RNA metastasis-related lung adenocarcinoma transcript 1 (MALAT1) promotes the proliferation and migration of human pulmonary artery smooth muscle cells (hPASMCs) by regulating the MicroRNA-503 (miR-503)/Toll-Like receptor 4 (TLR4) signal Axis. Med. Sci. Monit. 26, e923123. doi:10.12659/MSM.923123
Hu, T. J., Huang, H. B., Shen, H. B., Chen, W., and Yang, Z. H. (2020). Role of long non-coding RNA MALAT1 in chronic obstructive pulmonary disease. Exp. Ther. Med. 20 (3), 2691–2697. doi:10.3892/etm.2020.8996
Huang, J., Wang, F. H., Wang, L., Li, Y., Lu, J., and Chen, J. (2021). LncRNA MALAT1 promotes proliferation and migration of airway smooth muscle cells in asthma by downregulating microRNA-216a. Saudi J. Biol. Sci. 28 (8), 4124–4131. doi:10.1016/j.sjbs.2021.03.076
Huang, X., and Zhao, M. (2019). High expression of long non-coding RNA MALAT1 correlates with raised acute respiratory distress syndrome risk, disease severity, and increased mortality in sepstic patients. Int. J. Clin. Exp. Pathol. 12 (5), 1877–1887.
Hutchinson, J. N., Ensminger, A. W., Clemson, C. M., Lynch, C. R., Lawrence, J. B., and Chess, A. (2007). A screen for nuclear transcripts identifies two linked noncoding RNAs associated with SC35 splicing domains. BMC Genomics 8, 39. doi:10.1186/1471-2164-8-39
Jen, J., Tang, Y.-A., Lu, Y.-H., Lin, C.-C., Lai, W.-W., and Wang, Y.-C. (2017). Oct4 transcriptionally regulates the expression of long non-coding RNAs NEAT1 and MALAT1 to promote lung cancer progression. Mol. cancer 16 (1), 104–112. doi:10.1186/s12943-017-0674-z
Ji, P., Diederichs, S., Wang, W., Boing, S., Metzger, R., Schneider, P. M., et al. (2003). MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene 22 (39), 8031–8041. doi:10.1038/sj.onc.1206928
Jiang, M. C., Ni, J. J., Cui, W. Y., Wang, B. Y., and Zhuo, W. (2019). Emerging roles of lncRNA in cancer and therapeutic opportunities. Am. J. Cancer Res. 9 (7), 1354–1366.
Juan, C., Wang, Q., Mao, Y., Cao, Q., Li, S., Qiao, C., et al. (2018). Knockdown of LncRNA MALAT1 contributes to cell apoptosis via regulating NF-κB/CD80 axis in neonatal respiratory distress syndrome. Int. J. Biochem. Cell Biol. 104, 138–148. doi:10.1016/j.biocel.2018.09.009
Knight, D. A., and Holgate, S. T. (2003). The airway epithelium: Structural and functional properties in health and disease. Respirology 8 (4), 432–446. doi:10.1046/j.1440-1843.2003.00493.x
Kung, J. T., Colognori, D., and Lee, J. T. (2013). Long noncoding RNAs: Past, present, and future. Genetics 193 (3), 651–669. doi:10.1534/genetics.112.146704
Lai, X., Zhong, J., Zhang, A., Zhang, B., Zhu, T., and Liao, R. (2022). Focus on long non-coding RNA MALAT1: Insights into acute and chronic lung diseases. Front. Genet. 13, 1003964. doi:10.3389/fgene.2022.1003964
Lei, L., Zeng, Q., Lu, J., Ding, S., Xia, F., Kang, J., et al. (2017). MALAT1 participates in ultraviolet B-induced photo-aging via regulation of the ERK/MAPK signaling pathway. Mol. Med. Rep. 15 (6), 3977–3982. doi:10.3892/mmr.2017.6532
Li, J., Wang, J., Chen, Y., Li, S., Jin, M., Wang, H., et al. (2016). LncRNA MALAT1 exerts oncogenic functions in lung adenocarcinoma by targeting miR-204. Am. J. cancer Res. 6 (5), 1099–1107.
Li, S., Mei, Z., Hu, H. B., and Zhang, X. (2018). The lncRNA MALAT1 contributes to non-small cell lung cancer development via modulating miR-124/STAT3 axis. J. Cell. Physiology 233 (9), 6679–6688. doi:10.1002/jcp.26325
Li, X., Wu, Z., Fu, X., and Han, W. (2013). Long noncoding RNAs: Insights from biological features and functions to diseases. Med. Res. Rev. 33 (3), 517–553. doi:10.1002/med.21254
Li, Z., Zhang, Q., Wu, Y., Hu, F., Gu, L., Chen, T., et al. (2018). lncRNA Malat1 modulates the maturation process, cytokine secretion and apoptosis in airway epithelial cell-conditioned dendritic cells. Exp. Ther. Med. 16 (5), 3951–3958. doi:10.3892/etm.2018.6687
Liang, Z., and Tang, F. (2020). The potency of lncRNA MALAT1/miR-155/CTLA4 axis in altering Th1/Th2 balance of asthma. Biosci. Rep. 40 (2). doi:10.1042/BSR20190397
Lin, L., Li, Q., Hao, W., Zhang, Y., Zhao, L., and Han, W. (2019). Upregulation of LncRNA Malat1 induced proliferation and migration of airway smooth muscle cells via miR-150-eIF4E/Akt signaling. Front. Physiol. 10, 1337. doi:10.3389/fphys.2019.01337
Liu, N., Zhou, K. I., Parisien, M., Dai, Q., Diatchenko, L., and Pan, T. (2017). N6-methyladenosine alters RNA structure to regulate binding of a low-complexity protein. Nucleic Acids Res. 45 (10), 6051–6063. doi:10.1093/nar/gkx141
Liu, S., Liu, M., and Dong, L. (2020). The clinical value of lncRNA MALAT1 and its targets miR-125b, miR-133, miR-146a, and miR-203 for predicting disease progression in chronic obstructive pulmonary disease patients. J. Clin. Lab. Anal. 34 (9), e23410. doi:10.1002/jcla.23410
Luo, F., Wei, H., Guo, H., Li, Y., Feng, Y., Bian, Q., et al. (2019). LncRNA MALAT1, an lncRNA acting via the miR-204/ZEB1 pathway, mediates the EMT induced by organic extract of PM(2.5) in lung bronchial epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 317 (1), L87–L98. doi:10.1152/ajplung.00073.2019
Ma, X. Y., Wang, J. H., Wang, J. L., Ma, C. X., Wang, X. C., and Liu, F. S. (2015). Malat1 as an evolutionarily conserved lncRNA, plays a positive role in regulating proliferation and maintaining undifferentiated status of early-stage hematopoietic cells. BMC Genomics 16 (1), 676. doi:10.1186/s12864-015-1881-x
Mattiuzzi, C., and Lippi, G. (2020). Worldwide asthma epidemiology: Insights from the global health data exchange database. Int. Forum Allergy Rhinol. 10 (1), 75–80. doi:10.1002/alr.22464
Nalysnyk, L., Cid-Ruzafa, J., Rotella, P., and Esser, D. (2012). Incidence and prevalence of idiopathic pulmonary fibrosis: Review of the literature. Eur. Respir. Rev. 21 (126), 355–361. doi:10.1183/09059180.00002512
Pearce, N., Ait-Khaled, N., Beasley, R., Mallol, J., Keil, U., Mitchell, E., et al. (2007). Worldwide trends in the prevalence of asthma symptoms: Phase III of the international study of asthma and allergies in childhood (ISAAC). Thorax 62 (9), 758–766. doi:10.1136/thx.2006.070169
Poulet, C., Njock, M. S., Moermans, C., Louis, E., Louis, R., Malaise, M., et al. (2020). Exosomal long non-coding RNAs in lung diseases. Int. J. Mol. Sci. 21 (10), 3580. doi:10.3390/ijms21103580
Raghu, G., Collard, H. R., Egan, J. J., Martinez, F. J., Behr, J., Brown, K. K., et al. (2011). An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary fibrosis: Evidence-based guidelines for diagnosis and management. Am. J. Respir. Crit. Care Med. 183 (6), 788–824. doi:10.1164/rccm.2009-040GL
Ramsahai, J. M., Hansbro, P. M., and Wark, P. A. B. (2019). Mechanisms and management of asthma exacerbations. Am. J. Respir. Crit. Care Med. 199 (4), 423–432. doi:10.1164/rccm.201810-1931CI
Shaykhiev, R., Otaki, F., Bonsu, P., Dang, D. T., Teater, M., Strulovici-Barel, Y., et al. (2011). Cigarette smoking reprograms apical junctional complex molecular architecture in the human airway epithelium in vivo. Cell Mol. Life Sci. 68 (5), 877–892. doi:10.1007/s00018-010-0500-x
Sookoian, S., Flichman, D., Garaycoechea, M. E., San Martino, J., Castano, G. O., and Pirola, C. J. (2018). Metastasis-associated lung adenocarcinoma transcript 1 as a common molecular driver in the pathogenesis of nonalcoholic steatohepatitis and chronic immune-mediated liver damage. Hepatol. Commun. 2 (6), 654–665. doi:10.1002/hep4.1184
Sun, Y., and Ma, L. (2019). New insights into long non-coding RNA MALAT1 in cancer and metastasis. Cancers (Basel) 11 (2), 216. doi:10.3390/cancers11020216
Tano, K., Onoguchi-Mizutani, R., Yeasmin, F., Uchiumi, F., Suzuki, Y., Yada, T., et al. (2018). Identification of minimal p53 promoter region regulated by MALAT1 in human lung adenocarcinoma cells. Front. Genet. 8, 208. doi:10.3389/fgene.2017.00208
Tripathi, V., Ellis, J. D., Shen, Z., Song, D. Y., Pan, Q., Watt, A. T., et al. (2010). The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol. Cell 39 (6), 925–938. doi:10.1016/j.molcel.2010.08.011
Vogelmeier, C. F., Criner, G. J., Martinez, F. J., Anzueto, A., Barnes, P. J., Bourbeau, J., et al. (2017). Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am. J. Respir. Crit. Care Med. 195 (5), 557–582. doi:10.1164/rccm.201701-0218PP
Wang, D., Xu, H., Wu, B., Jiang, S., Pan, H., Wang, R., et al. (2019). Long noncoding RNA MALAT1 sponges miR1243p.1/KLF5 to promote pulmonary vascular remodeling and cell cycle progression of pulmonary artery hypertension. Int. J. Mol. Med. 44 (3), 871–884. doi:10.3892/ijmm.2019.4256
Wang, F., Li, P., and Li, F. S. (2020). Integrated analysis of a gene correlation network identifies critical regulation of fibrosis by lncRNAs and TFs in idiopathic pulmonary fibrosis. Biomed. Res. Int. 2020, 6537462. doi:10.1155/2020/6537462
Wang, L., Liu, J., Xie, W., Li, G., Yao, L., Zhang, R., et al. (2019). Overexpression of MALAT1 relates to lung injury through sponging miR-425 and promoting cell apoptosis during ARDS. Can. Respir. J. 2019, 1871394. doi:10.1155/2019/1871394
Wu, W., Wang, S., Zhang, L., Mao, B., Wang, B., Wang, X., et al. (2022). Mechanistic studies of MALAT1 in respiratory diseases. Front. Mol. Biosci. 9, 1031861. doi:10.3389/fmolb.2022.1031861
Xia, J., Tian, Y., Shao, Z., Li, C., Ding, M., Qi, Y., et al. (2023). MALAT1-miR-30c-5p-CTGF/ATG5 axis regulates silica-induced experimental silicosis by mediating EMT in alveolar epithelial cells. Ecotoxicol. Environ. Saf. 249, 114392. doi:10.1016/j.ecoenv.2022.114392
Xie, M., Liu, X., Cao, X., Guo, M., and Li, X. (2020). Trends in prevalence and incidence of chronic respiratory diseases from 1990 to 2017. Respir. Res. 21 (1), 49. doi:10.1186/s12931-020-1291-8
Yan, Y. R., Luo, Y., Zhong, M., and Shao, L. (2019). MiR-216a inhibits proliferation and promotes apoptosis of human airway smooth muscle cells by targeting JAK2. J. Asthma 56 (9), 938–946. doi:10.1080/02770903.2018.1509991
Yang, K., Li, W., Duan, W., Jiang, Y., Huang, N., Li, Y., et al. (2019). Resveratrol attenuates pulmonary embolism associated cardiac injury by suppressing activation of the inflammasome via the MALAT1-miR-22-3p signaling pathway. Int. J. Mol. Med. 44 (6), 2311–2320. doi:10.3892/ijmm.2019.4358
Yang, M., and Wang, L. I. (2021). MALAT1 knockdown protects from bronchial/tracheal smooth muscle cell injury via regulation of microRNA-133a/ryanodine receptor 2 axis. J. Biosci. 46, 28. doi:10.1007/s12038-021-00149-3
Yang, Q., Chen, W., Xu, Y., Lv, X., Zhang, M., and Jiang, H. (2018). Polyphyllin I modulates MALAT1/STAT3 signaling to induce apoptosis in gefitinib-resistant non-small cell lung cancer. Toxicol. Appl. Pharmacol. 356, 1–7. doi:10.1016/j.taap.2018.07.031
Yao, M. Y., Zhang, W. H., Ma, W. T., Liu, Q. H., Xing, L. H., and Zhao, G. F. (2020). Long non-coding RNA MALAT1 exacerbates acute respiratory distress syndrome by upregulating ICAM-1 expression via microRNA-150-5p downregulation. Aging (Albany NY) 12 (8), 6570–6585. doi:10.18632/aging.102953
Yu, W., Ding, J., He, M., Chen, Y., Wang, R., Han, Z., et al. (2019). Estrogen receptor β promotes the vasculogenic mimicry (VM) and cell invasion via altering the lncRNA-MALAT1/miR-145-5p/NEDD9 signals in lung cancer. Oncogene 38 (8), 1225–1238. doi:10.1038/s41388-018-0463-1
Zhang, J. P., Zhang, W. J., Yang, M., and Fang, H. (2021). Propofol attenuates lung ischemia/reperfusion injury though the involvement of the MALAT1/microRNA-144/GSK3β axis. Mol. Med. 27 (1), 77. doi:10.1186/s10020-021-00332-0
Zhang, X., Wang, W., Zhu, W., Dong, J., Cheng, Y., Yin, Z., et al. (2019). Mechanisms and functions of long non-coding RNAs at multiple regulatory levels. Int. J. Mol. Sci. 20 (22), 5573. doi:10.3390/ijms20225573
Keywords: MALAT1, asthma, COPD, COVID-19, acute respiratory distress syndrome, lung cancer
Citation: Liu J, Ali MK and Mao Y (2023) Emerging role of long non-coding RNA MALAT1 related signaling pathways in the pathogenesis of lung disease. Front. Cell Dev. Biol. 11:1149499. doi: 10.3389/fcell.2023.1149499
Received: 22 January 2023; Accepted: 02 May 2023;
Published: 11 May 2023.
Edited by:
Vladislav Victorovich Khrustalev, Belarusian State Medical University, BelarusReviewed by:
Melina M. Musri, Medical Research Institute Mercedes and Martín Ferreyra (INIMEC), ArgentinaAnna Popinako, Research Center of Biotechnology of the Russian Academy of Sciences, Russia
Copyright © 2023 Liu, Ali and Mao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuqiang Mao, bWFveXFAc2otaG9zcGl0YWwub3Jn
 Jun Liu1
Jun Liu1 Md Khadem Ali
Md Khadem Ali Yuqiang Mao
Yuqiang Mao