- 1Department of Digestive endoscopy, Jiangsu Province Hospital of Traditional Chinese Medicine, Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing, Jiangsu, China
- 2Xin-Huangpu Joint Innovation Institute of Chinese Medicine, Guangzhou, Guangdong, China
- 3China Science and Technology Development Center of Chinese Medicine, Beijing, China
- 4The Comprehensive Cancer Centre of Nanjing Drum Tower Hospital, The Affiliated Hospital of Nanjing University Medical School, Nanjing, Jiangsu, China
- 5Acupuncture and Tuina college, Nanjing University of Chinese Medicine, Nanjing, Jiangsu, China
Gastric cancer (GC) is a potential dominant disease in tumor immunotherapy checkpoint inhibitors, and adoptive cell therapy have brought great hope to GC patients. However, only some patients with GC can benefit from immunotherapy, and some patients develop drug resistance. More and more studies have shown that long non-coding RNAs (lncRNAs) may be important in GC immunotherapy’s prognosis and drug resistance. Here, we summarize the differential expression of lncRNAs in GC and their impact on the curative effect of GC immunotherapy, discuss potential mechanisms of activity in GC immunotherapy resistance regulated by lncRNAs. This paper reviews the differential expression of lncRNA in GC and its effect on immunotherapy efficacy in GC. In terms of genomic stability, inhibitory immune checkpoint molecular expression, the cross-talk between lncRNA and immune-related characteristics of GC was summarized, including tumor mutation burden (TMB), microsatellite instability (MSI), and Programmed death 1 (PD-1). At the same time, this paper reviewed the mechanism of tumor-induced antigen presentation and upregulation of immunosuppressive factors, as well as the association between Fas system and lncRNA, immune microenvironment (TIME) and lncRNA, and summarized the functional role of lncRNA in tumor immune evasion and immunotherapy resistance.
1 Introduction
Gastric cancer (GC) has become the fifth commonest cancer in 2020, with a 5.6% incidence rate and a 7.7% death rate, according to the updated research (Sung et al., 2021). As a kind of cancer with poor prognosis, there are several risk factors, such as helicobacter pylori infection, age and high salt intake (Smyth et al., 2020). Apart from common surgeries, including surgical or endoscopic resection, adjuvant and perioperative chemotherapy, immunotherapy, especially immunotherapy checkpoint inhibitors (ICI) therapy, is now established as an essential strategy for chemo refractory GC therapy (Kang et al., 2017; Wang et al., 2021a). Besides, the use of cytotoxic immunocytes and gene transferred vaccines also grow rapidly (Joshi and Badgwell, 2021). Unfortunately, GC has variable responsiveness to immunotherapy due to the heterogeneity of the disease, which is a great challenge to this cancer treatment.
Many factors influence the efficacy of immunotherapy for that gastric tumorigenesis involves a series of genetic, epigenetic, and epitranscriptomic alterations (Xie et al., 2021). Currently, long non-coding RNAs (lncRNAs) has more than 200 nucleotides at length and takes emerging roles in the immunosuppressive tumor microenvironment (Xiao et al., 2022a). As a cluster of RNAs regulating multiple protein-coding genes, the alterations in lncRNAs expression and their mutations promote tumorigenesis and metastasis (Bhan et al., 2017; Luo et al., 2020). When it comes to GC, the abnormal expressions of lncRNAs are strongly related to its chemoresistance, drug resistance, and immunotherapy (Wei et al., 2020a; Yuan et al., 2020). However, definite mechanisms under the interplay between lncRNA and GC are still unclear.
In the following paragraphs, we summarize the interferences between immunological characteristics of GC and lncRNAs. Meanwhile, we conclude the factors associated with the response to immunotherapy of GC, focusing on the functional roles of lncRNAs in tumor immune evasion and immunotherapy resistance emphatically.
2 LncRNAs have superior values in optimizing patients’ selection for ICIs therapy and predicting patients’ outcomes of ICI therapy
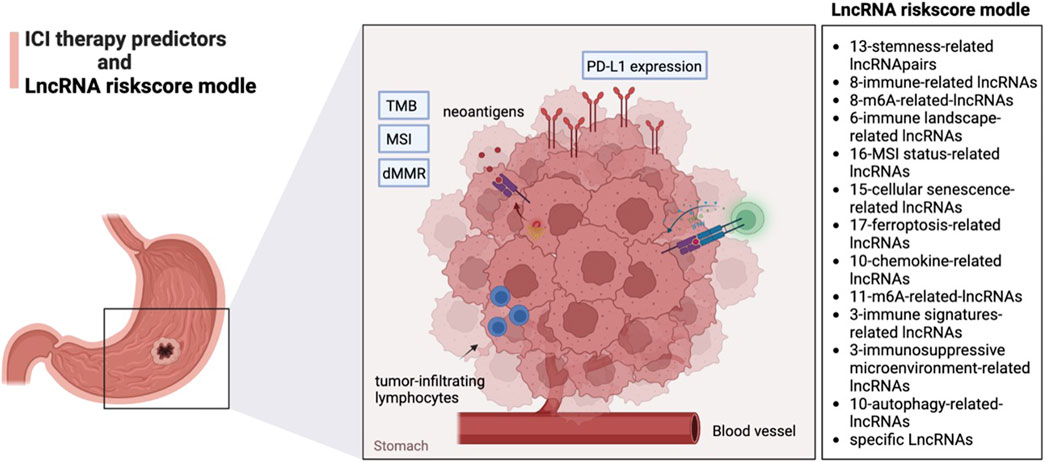
Programmed death 1 (PD-1) ligand (PD-L1) (CD274) expression, tumor mutation burden (TMB), and microsatellite instability (MSI) status of tumor tissue are potential predictors of anti-PD-1 treatment response (Schreiber et al., 2011; Galon and Bruni, 2019). The expression of PD-L1 was correlated with the tumor infiltration level, TMB, MSI, and dMMR of different types of cancers (Dai et al., 2021). Many researchers have investigated the relationship between various biomarkers, including TMB, MSI, PD-L1 expression, etc., and the lncRNAs risk models in guiding the treatment of ICI (Figure 1).
2.1 Genome stability and lncRNAs risk models
As a widely used indicator of tumor immunogenicity, TMB is a quantifiable biomarker affecting immune checkpoint inhibitors. It reflects the number of mutations in a tumor cell, usually expressed as mutations per megabase. In other words, TMB is a statistic and calculation of the number of tumor mutations (Alexandrov et al., 2013). The higher the value of TMB, the more mutations that can produce neoantigens, the higher the immune response rate, and the better the effect of tumor immunotherapy. At present, TMB detection methods are mainly based on high-throughput sequencing platforms of whole exon sequencing and targeted Panel sequencing (Pardoll, 2012). It is worth noting that the definitions of TMB-H and TMB-L are not set in stone (Carbone et al., 2017; Goodman et al., 2017; Marabelle et al., 2020). In the current studies, TMB-H (≥10 mutations/MB) and TMB-L (<10 mutations/MB) are mainly used to distinguish them (Marabelle et al., 2020). For the distribution of tumor patients with low, medium, and high load TMB expression, some studies believe that the proportion of low TMB tumor patients is about 50%, the proportion of medium TMB tumor patients is about 40%, and the proportion of high TMB tumor patients is only about 10% (Goodman et al., 2017).
In a targeted sequencing study of 529 Chinese patients with gastric adenocarcinoma, the genetic mutations of TMB-H GC patients were mainly in ARID1A, KMT2D, RNF43, TGFBR2 and CIC. The gene mutations in TMB-L GC patients were mainly in ERBB2, CCNE1, CDK12 and CCND1 (Yu et al., 2021). There have also been other reports that mutations in the LRP1B gene are so prominent, in Chinese GC patients with high TMB (Zhang et al., 2021).
Many researchers have built the lncRNAs risk score models in TMB in guiding the treatment of ICI. A negative correlation is prevalent between cancer stemness and anticancer immunity (Miranda et al., 2019). Jiang Q et al. established a 13-DEsrlncRNA pair-based signature. This study could provide a stemness-related lncRNA signature for survival prediction in GC patients and establish a model with predictive potentials for GC patients’ sensitivity to chemotherapy and immunotherapy. The risk score presented negative correlations with TMB values based on the Spearman correlation analysis. Compared with CTLA4, the results may indicate a better efficacy of this risk score model for PD-1 therapy response prediction (Jiang et al., 2021). Yujiao Wang et al. established a risk model involving 8 immune-related lncRNA (irlncRNA) pairs and found that patients in the high-risk group had lower TMB scores and poorer prognoses (Wang et al., 2021b). Based on lncRNA, Yi Wang et al. selected 8 lncRNAs to build a feature classifier for predicting TMB level, which is associated with the expression of immune checkpoint tumor-infiltrating lymphocytes and microsatellite instability (Wang et al., 2022a).
Microsatellites are simple repeats of 2-6 nucleotides in the DNA genome, also known as Short Tandem Repeat (STR). When the function of MMR is abnormal, the alignment errors in microsatellite replication cannot be corrected. With the accumulation of replication errors, the base composition or sequence length of microsatellite changes, and the genome shows a hypermutant phenotype. It is called MSI. According to the microsatellite state, it can be divided into MSI-H, low microsatellite instability (MSI-L) and microsatellite stability (MSS). The detection method of MSI mainly uses PCR technology to directly amplify the bases of MSI sites, and then uses capillary electrophoresis to analyze the amplified products. This method is currently considered as the gold standard for MSI detection. Five loci “2B3D” (BAT25, BAT26, D2S123, D5S346 and D17S250) were universally detected. The diagnostic criteria were as follows: instability of 2 or more loci was MSI-H; The instability of one locus was microsatellite MSI-L. If all loci are stable, MSS. The updated NCCN guidelines in 2021 will recommend cancer indications for MSI testing, including GC, which explicitly states that MSI sites select 5-site panels (2B3D National Cancer Institute (NCI) Panel and 5-single nucleotide Panel). In addition, the 2021 CSCO guidelines explicitly state the selection of 2B3D as recommended by the NCI for MSI sites.
In GC, lncRNAs associated with MSI mainly include LINC02678, HOXA10-AS, RHOXF1-AS1, AC010789.1, LINC01150, and TGFB2-AS1 (Sun et al., 2021). A model composed of 16 lncRNA features was established to classify MSI status in patients with GC (Chen et al., 2019). Zeng et al. established a risk score model of 15 lncRNAs. The study found a higher proportion of MSI-H in the low-risk group of GC patients (Zeng et al., 2022). Xiao S et al. constructed a 17-ferroptosis-related-lncRNA signature via multivariate Cox analysis to divide patients into low- and high-risk groups. The risk score was significantly higher in the MSI-H or MSI subtype, respectively. Meanwhile, TMB was pronounced in the low-risk group and negatively correlated with the risk score (Xiao et al., 2021a). Liang X et al. first constructed a multi-lncRNA risk model composed of 10 chemokine-related lncRNAs based on The Cancer Genome Atlas (TCGA) expression data. The results demonstrated that the lncRNA risk model better predicts patient survival, immune cell infiltration, and immunotherapy effectiveness. The risk score obtained from the risk model is negatively correlated with TMB. Low-risk patients with single positivity for CTLA4 or PD-1 and double positivity for CTLA4+PD-1 had higher immunotherapy scores. The chemokine-related lncRNA risk model could be used to predict the immunotherapy sensitivity of GC (Liang et al., 2021).
Genomic instability-associated lncRNA signature can show a distinct immune landscape and predict prognosis in GC. To further reveal the potential role of lncRNAs in guiding the treatment of ICI. Genomic instability-associated lncRNA risk models were not completely independent. At the molecular level, TMB is associated with deficient mismatch repair (dMMR), high microsatellite instability (MSI-H), and mutations in DNA polymerase correction domains encoding POLE and POLD1 genes (Jardim et al., 2021).
2.2 Expression of inhibitory immune checkpoint molecules and lncRNAs risk models
PD-1/PD-L1 is a key member of the immunoglobulin superfamily B7-CD28 co-stimulatory molecules. The PD-1 receptor on the surface of T cells binds to the PD-L1 ligand expressed on the surface of tumor cells, inhibiting the activation and proliferation of T cells, leading to tumor cells produce immune escape (Okazaki and Honjo, 2007). Therefore, PD-1/PD-L1, as a negative immune regulator, is involved in the regulation of various tumor immunity. At the same time, TAMs, tumor-infiltrating lymphocytes, and circulating tumor cells (CTCs) may be related to regulating the expression of PD-L1 and affecting tumor prognosis. TAMs secrete a variety of cytokines, including VEGF, IL-1β, TNF, IL-10, etc., which further attract Tregs, and promote tumor cells to express PD-L1, inhibiting the immune function of T cells. In addition, its associated exosomes interact with tumor cells to further promote tumor cell proliferation, invasion, migration, and angiogenesis (Gordon et al., 2017). Tumor-infiltrating CD4+ T cells express high levels of Helios and upregulate PD-1 expression (Toor et al., 2019). In addition, CTCs mediate the expression level of PD-L1 and promote the distant metastasis of tumor cells. It is worth noting that after CTCs constitute circulating colonies, the probability of tumor progression and metastasis increases (Winograd et al., 2020).
ICI therapy has become one of the most popular immunotherapies, which has significantly changed the current pattern of cancer treatment, and PD-1 immunoblockade therapy is one of the most typical representatives. Studies have confirmed that the expression of PD-1 on the surface of tumor cells is significantly increased in tumor tissues, including non-small cell lung cancer, melanoma, kidney cancer, ovarian cancer, colorectal cancer, pancreatic cancer, GC, breast cancer, etc (Brahmer et al., 2012). Therefore, researchers focused on blocking the PD-1/PD-L1 pathway through immunotherapy to inhibit tumor development. Major breakthroughs have occurred in non-small cell lung cancer (Reck et al., 2022) and Hodgkin lymphoma (Reinke et al., 2020). A variety of immune checkpoint inhibitors related to this have been approved by the FDA for immunotherapy of cancer patients.
The expression of PD-L1 has been shown to correlate with response to ICIs in GC (Rizzo et al., 2020). Liangliang Lei et al. identified 11 m6A-related lncRNA pairs associated with GC prognosis. Patients in the low-risk group had more prolonged overall survival versus the high-risk group. Infiltration of cancer-associated fibroblasts, endothelial cells, macrophages, particularly M2 macrophages, and monocytes was more severe in high-risk patients than low-risk individuals, who exhibited high CD4+ Th1 cell infiltration in GC. Altered expressions of immune-related genes were observed in both groups. PD-1 and LAG3 expressions were higher in low-risk patients than in high-risk patients. Immunotherapy, either single or combined use of PD-1 or CTLA4 inhibitors, had better efficacy in low-risk patients than high-risk patients (Lei et al., 2022). Three lncRNAs (AC022706.1, LINC01871, and AC006033.2) have been identified as associated with GC immunotherapy responses through multi-omics data analysis. At the same time, seven gene mutations (ARID1A, BCOR, MTOR, CREBBP, SPEN, NOTCH4, and TET1) were identified that were associated with the prognosis of GC patients receiving anti-PD-1/PD-L1 immunotherapy (He and Wang, 2020) which suggests that lncRNA may be used for risk stratification in GC patients by anti-PD-1/PD-L1 immunotherapy. In subsequent studies, GC patients were divided into high-risk and low-risk groups based on immune-related lncRNA characteristics. PD-1 and PD-L1 were highly expressed in the high-risk group (Ma et al., 2021a). Other studies have shown similar results, with CD274 (PD-L1), PDCD1 (PD-1), and PDCD1LG2 (PD-L2) significantly upregulated in high-risk groups (Wang et al., 2022b) which helps identify GC patients who might benefit from immune checkpoint therapy.
There were also some studies that screen out specific lncRNAs. ZFPM2-AS1, located in 8q23.1, plays an oncogenic role in several tumors. ZFPM2-AS1 was correlated with several known immune checkpoints, including CTLA4, PD-1, PD-L1, TIGIT, LAG-3, HAVCR2 (TIM-3), and IDO1, in most tumors. ZFPM2-AS1 expression was correlated with TMB and MSI in GAC. NUP107 and C8orf76 were identified as potential target mRNAs of ZFPM2-AS1, ZFPM2-AS, NUP107, and C8orf76 were highly expressed in GC cells (Chen et al., 2022).
3 LncRNAs and the mechanism of immunotherapy resistance in GC
3.1 Tumor induced antigen presentation
LncRNA is related to the tumor induced antigen presentation. T cells are lymphoid stem cells derived from bone marrow. T cell receptor (TCR) is a specific receptor for T cells to recognize and bind foreign antigens. TCR cannot directly recognize and bind free soluble antigens, but only recognize antigen molecules processed by antigen presenting cells and connected with major histocompatibility complexes (MHC-I and -II) (Gaud et al., 2018). The initiation of tumor immune response begins with the recognition of tumor specific antigens by MHC on the surface of antigen cells (Boyne et al., 2021). Downregulation of antigen presentation mechanism (MHC-I) will inhibit immunogenicity and accelerate immune escape (Di Tomaso et al., 2010). In patients with GC, the expression of MHC-I is generally reduced, and the frequency of downregulation of MHC-I in metastatic cells is higher than that in primary tumor cells (Erdogdu, 2019). Histocompatibility leukocyte antigen complex P5 (HCP5), an important lncRNA located between the MICA and MICB genes in MHC-I region (Zou and Chen, 2021). Targeting lncRNA HCP5 may be a novel approach to enhancing the efficacy of chemotherapy in GC through miR-3619-5p/AMPK/PGC1α/CEBPB axis (Kulski, 2019).
3.2 LncRNA is a regulator of immunosuppressive factors in GC
More and more evidence showed that lncRNAs are associated with the upregulation of inhibitory immune checkpoints and thus involved in the development and progression of GC.
LncRNA hypoxia-inducible factor 1 alpha-antisense RNA 2 (HIF1A-AS2) expression is elevated in GC tissues and is associated with poor prognosis of GC (Wen-Ming et al., 2015). It is found that HIF1A-AS2 directly binds to microRNA 429 (miR-429) and negatively regulates miR-429 expression, while MiR-429 directly targets PD-L1and inhibits PD-L1 expression. In summary, HIF1A-AS2 can promote PD-L1 expression by targeting and inhibiting miR-429 (Mu et al., 2021). Urothelial carcinoma-associated 1 (UCA1), also significantly highly expressed in GC tissues, could act as competing endogenous RNA (ceRNA) for miR-193a and miR-214, reducing its transcriptional inhibition of PD-L1, thus promoting PD-L1 expression (Wang et al., 2019). Small nucleolar RNA host gene 15 (SNHG15), a ceRNA of miR-141, increased the expression level of PD-L1 on GC cells, thereby improving the resistance of GC cells to tumor immune response (Dang et al., 2020). Ji Wang et al. demonstrated that targeting the NUT family member 2A antisense RNA 1/miR-376a/Tet-eleven translocation 1/PD-L1 (NUTM2A-AS1/miR-376a/TET1/PD-L1) axis may provide a new strategy for GC diagnosis and treatment (Wang et al., 2020a). Specifically, NUTM2A-AS1 is important in GC drug resistance. MiR-376a targets suppressing the expression levels of downstream TET1 and HIF-1A, while the TET1/HIF-1A complex positively regulated PD-L1. Other lncRNAs, such as prospero homeobox 1-antisense RNA 1 (PROX1-AS1), is also extremely highly expressed in GC and can promote GC cell proliferation and invasion via miR-877-5p/PD-L1 axis (Guo et al., 2021).
3.3 LncRNAs and fas ligand
Recently, lots of researches proved that lncRNAs and Fas ligand (FasL) are participated in a majority of tumor immune progression. FasL induces programmed cell death through receptors. FasL expression may kill infiltrating lymphocytes and inflammatory cells. On the other hand, some relevant studies have shown that when FasL is expressed in tumors or transplants, the proinflammatory function of FasL may cause rejection (Simon et al., 2002; Newsom-Davis et al., 2009). Some important ceRNAs, such as FAS and hsa-miR-125b-5p, and tumor-infiltrating immune cells might relate to distance metastasis and prognosis of Colon Adenocarcinoma Metastasis (Ai et al., 2020; Chang et al., 2020). In addition, lncRNA cancer susceptibility candidate 7 (CASC7) can downgrade the malignant behaviors of breast cancer with miR-21-5p/FasL axis (Wang et al., 2021c).
3.4 LncRNA and immune microenvironment (TIME)
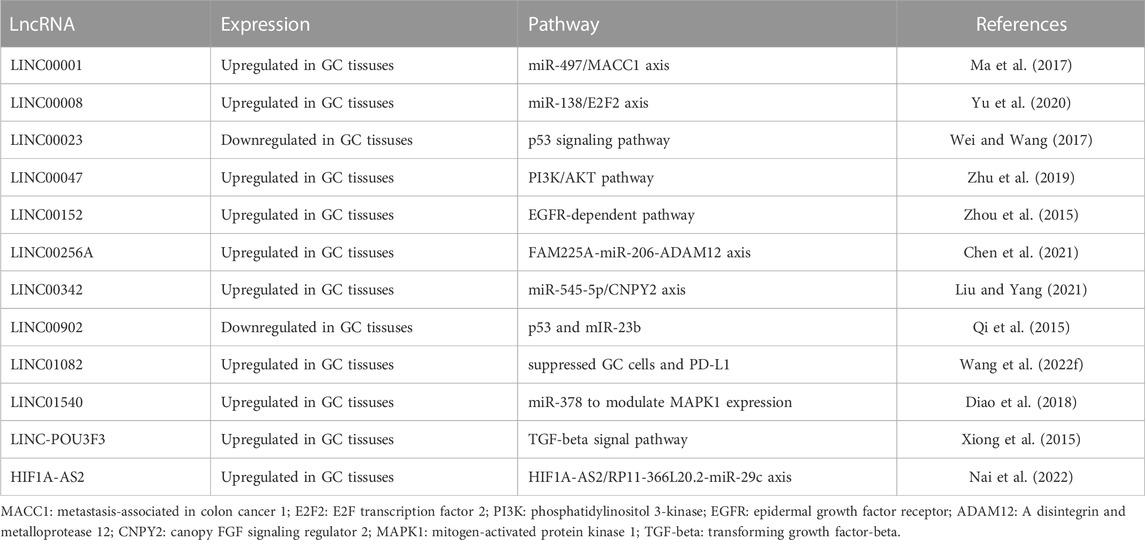
Tumor microenvironment plays an important role in the development of GC. LncRNA is also involved in the regulation of tumor microenvironment (Wang et al., 2022c). Many abnormally expressed lncRNAs have been recognized in GC tissues, which affect the occurrence and prognosis of tumors (Table 1). Moreover, lncRNA regulate immunity in several ways in GC through influencing the polarization of GC-associated macrophages (Xie et al., 2020), the differentiation of natural killer cells (Ou et al., 2021), the regulation of dendritic cells (Demaria et al., 2019) and so on (Xiao et al., 2022b).
Moreover, lncRNA in tumor microenvironment is often used as a prognostic marker of tumors. LncRNA and focal cell apoptosis can be used as a prognostic tool for gastric adenocarcinoma (Wang et al., 2022d). Ferroptosis-related lncRNAs in tumor microenvironment also related to the prognosis of GC (Ma et al., 2021b; Xiao et al., 2021b). LncRNA and immune microenvironment can help us better identify the stage and prognosis of GC (Wang et al., 2020b). LncRNA HOX transcript antisense RNA (lncRNA HOTAIR) promotes the metastasis of GC according to miR-1277-5p and increasing Collagen type V alpha 1 chain (COL5A1) (Wei et al., 2020b). The tumor immune microenvironment and prognosis of N6-methyladenosine (m6A) related lncRNA in GC (Wang et al., 2022e). Meanwhile, ferroptosis-related lncRNA can predict the treatment and prognosis of GC (Li et al., 2022).
Conclusion
To date, surgeries, cytotoxic immunocytes, gene transferred vaccines and immunotherapy, remain the mainstay of clinical therapies for GC. Especially ICI, has been employed as an essential strategy for refractory GC. LncRNA had superior values in optimizing patients’ selection for ICIs therapy and predicting patients’ outcomes of ICI therapy, as revealed in the following. Firstly, lncRNA risk score models have been built in TMB in guiding the treatment of ICI. Secondly, inhibitory immune checkpoint molecules related lncRNA have been shown to correlate with response to ICIs in GC. The mechanisms of lncRNA in immunotherapy resistance are revealed in the following. On the one hand, lncRNA is related to the tumor induced antigen presentation and a regulator upregulation of immunosuppressive factors in GC. On the other hand, lncRNA can regulate the malignant behaviors via FasL axis. In particular, lncRNA regulate immunity in several ways to GC tumor growth and progression. LncRNA in tumor microenvironment is often used as a prognostic marker of tumors. Therefore, lncRNA targeting GC immunotherapy has a wide range of potential applications, the use of lncRNA as a therapeutic target will contribute to the development of novel GC treatment strategies.
Author contributions
ZL, RX, and YY designed the manuscript. QZ and CW wrote the manuscript. ZL, RX, and QZ drew the figure and table. ZL revised the manuscript.
Funding
This study was supported by the Fundamental Research Funds for the Central public welfare research institutes, No. ZZ15-YQ-069.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2023.1052942/full#supplementary-material
References
Ai, Y., Chen, M., Liu, J., Ren, L., Yan, X., and Feng, Y. (2020). lncRNA TUG1 promotes endometrial fibrosis and inflammation by sponging miR-590-5p to regulate Fasl in intrauterine adhesions. Int. Immunopharmacol. 86, 106703. doi:10.1016/j.intimp.2020.106703
Alexandrov, L. B., Nik-Zainal, S., Wedge, D. C., Aparicio, S. A. J. R., Behjati, S., Biankin, A. V., et al. (2013). Signatures of mutational processes in human cancer. Nature 500, 415–421. doi:10.1038/nature12477
Bhan, A., Soleimani, M., and Mandal, S. S. (2017). Long noncoding RNA and cancer: A new paradigm. Cancer Res. 77, 3965–3981. doi:10.1158/0008-5472.CAN-16-2634
Boyne, C., Lennox, D., Beech, O., Powis, S. J., and Kumar, P. (2021). What is the role of HLA-I on cancer derived extracellular vesicles? Defining the challenges in characterisation and potential uses of this ligandome. Int. J. Mol. Sci. 22 (24), 13554. doi:10.3390/ijms222413554
Brahmer, J. R., Tykodi, S. S., Chow, L. Q., Hwu, W. J., Topalian, S. L., Hwu, P., et al. (2012). Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 366, 2455–2465. doi:10.1056/NEJMoa1200694
Carbone, D. P., Reck, M., Paz-Ares, L., Creelan, B., Horn, L., Steins, M., et al. (2017). First-Line nivolumab in stage IV or recurrent non-small-cell lung cancer. N. Engl. J. Med. 376, 2415–2426. doi:10.1056/NEJMoa1613493
Chang, Z., Huang, R., Fu, W., Li, J., Ji, G., Huang, J., et al. (2020). The construction and analysis of ceRNA network and patterns of immune infiltration in colon adenocarcinoma metastasis. Front. Cell Dev. Biol. 8, 688. doi:10.3389/fcell.2020.00688
Chen, D., Wang, M., Jiang, X., and Xiong, Z. (2022). Comprehensive analysis of ZFPM2-AS1 prognostic value, immune microenvironment, drug sensitivity, and co-expression network: From gastric adenocarcinoma to pan-cancers. Discov. Oncol. 13, 24. doi:10.1007/s12672-022-00487-0
Chen, N., Zhu, X., Zhu, Y., Shi, J., Zhang, J., Tang, C., et al. (2021). The regulatory relationship and function of LncRNA fam225a-miR-206-ADAM12 in gastric cancer. Am. J. Transl. Res. 13 (8), 8632–8652.
Chen, T., Zhang, C., Liu, Y., Zhao, Y., Lin, D., Hu, Y., et al. (2019). A gastric cancer LncRNAs model for MSI and survival prediction based on support vector machine. BMC Genomics 20, 846. doi:10.1186/s12864-019-6135-x
Dai, L., Huang, Z., and Li, W. (2021). Analysis of the PD-1 ligands among gastrointestinal cancer patients: Focus on cancer immunity. Front. Oncol. 11, 637015. doi:10.3389/fonc.2021.637015
Dang, S., Malik, A., Chen, J., Qu, J., Yin, K., Cui, L., et al. (2020). LncRNA SNHG15 contributes to immuno-escape of gastric cancer through targeting miR141/PD-L1. Onco Targets Ther. 13, 8547–8556. doi:10.2147/OTT.S251625
Demaria, O., Cornen, S., Daëron, M., Morel, Y., Medzhitov, R., and Vivier, E. (2019). Harnessing innate immunity in cancer therapy. Nature 574 (7776), 45–56. doi:10.1038/s41586-019-1593-5
Di Tomaso, T., Mazzoleni, S., Wang, E., Sovena, G., Clavenna, D., Franzin, A., et al. (2010). Immunobiological characterization of cancer stem cells isolated from glioblastoma patients. Clin. Cancer Res. 16 (3), 800–813. doi:10.1158/1078-0432.CCR-09-2730
Diao, L., Wang, S., and Sun, Z. (2018). Long noncoding RNA GAPLINC promotes gastric cancer cell proliferation by acting as a molecular sponge of miR-378 to modulate MAPK1 expression. Onco Targets Ther. 11, 2797–2804. doi:10.2147/OTT.S165147
Erdogdu, I. H. (2019). MHC class 1 and PDL-1 status of primary tumor and lymph node metastatic tumor tissue in gastric cancers. Gastroenterol. Res. Pract. 2019, 4785098. doi:10.1155/2019/4785098
Galon, J., and Bruni, D. (2019). Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 18, 197–218. doi:10.1038/s41573-018-0007-y
Gaud, G., Lesourne, R., and Love, P. E. (2018). Regulatory mechanisms in T cell receptor signalling. Nat. Rev. Immunol. 18 (8), 485–497. doi:10.1038/s41577-018-0020-8
Goodman, A. M., Kato, S., Bazhenova, L., Patel, S. P., Frampton, G. M., Miller, V., et al. (2017). Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol. Cancer Ther. 16, 2598–2608. doi:10.1158/1535-7163.MCT-17-0386
Gordon, S. R., Maute, R. L., Dulken, B. W., Hutter, G., George, B. M., McCracken, M. N., et al. (2017). PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 545, 495–499. doi:10.1038/nature22396
Guo, T., Wang, W., Ji, Y., Zhang, M., Xu, G., and Lin, S. (2021). LncRNA PROX1-AS1 facilitates gastric cancer progression via miR-877-5p/PD-L1 Axis. Cancer Manag. Res. 13, 2669–2680. doi:10.2147/CMAR.S275352
He, Y., and Wang, X. (2020). Identification of molecular features correlating with tumor immunity in gastric cancer by multi-omics data analysis. Ann. Transl. Med. 8, 1050. doi:10.21037/atm-20-922
Jardim, D. L., Goodman, A., de Melo Gagliato, D., and Kurzrock, R. (2021). The challenges of tumor mutational burden as an immunotherapy biomarker. Cancer Cell 39, 154–173. doi:10.1016/j.ccell.2020.10.001
Jiang, Q., Chen, H., Tang, Z., Sun, J., Ruan, Y., Liu, F., et al. (2021). Stemness-related LncRNA pair signature for predicting therapy response in gastric cancer. BMC Cancer 21, 1067. doi:10.1186/s12885-021-08798-1
Joshi, S. S., and Badgwell, B. D. (2021). Current treatment and recent progress in gastric cancer. CA Cancer J. Clin. 71, 264–279. doi:10.3322/caac.21657
Kang, Y. K., Boku, N., Satoh, T., Ryu, M. H., Chao, Y., Kato, K., et al. (2017). Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 390, 2461–2471. doi:10.1016/S0140-6736(17)31827-5
Kulski, J. K. (2019). Long noncoding RNA HCP5, a hybrid HLA class I endogenous retroviral gene: Structure, expression, and disease associations. Cells 8 (5), 480. doi:10.3390/cells8050480
Lei, L., Li, N., Yuan, P., and Liu, D. (2022). A new risk model based on a 11-m (6)A-related lncRNA signature for predicting prognosis and monitoring immunotherapy for gastric cancer. BMC Cancer 22, 365. doi:10.1186/s12885-021-09062-2
Li, J., Xiang, R., Song, W., Wu, J., Kong, C., and Fu, T. (2022). A novel ferroptosis-related LncRNA pair prognostic signature predicts immune landscapes and treatment responses for gastric cancer patients. Front. Genet. 13, 899419. doi:10.3389/fgene.2022.899419
Liang, X., Yu, G., Zha, L., Guo, X., Cheng, A., Qin, C., et al. (2021). Identification and comprehensive prognostic analysis of a novel chemokine-related lncRNA signature and immune landscape in gastric cancer. Front. Cell Dev. Biol. 9, 797341. doi:10.3389/fcell.2021.797341
Liu, R., and Yang, X. (2021). LncRNA LINC00342 promotes gastric cancer progression by targeting the miR-545-5p/CNPY2 Axis. BMC Cancer 21 (1), 1163. doi:10.1186/s12885-021-08829-x
Luo, Y., Yang, J., Yu, J., Liu, X., Yu, C., Hu, J., et al. (2020). Long non-coding RNAs: Emerging roles in the immunosuppressive tumor microenvironment. Front. Oncol. 10, 48. doi:10.3389/fonc.2020.00048
Ma, E., Hou, S., Wang, Y., Xu, X., Wang, Z., and Zhao, J. (2021). Identification and validation of an immune-related lncRNA signature to facilitate survival prediction in gastric cancer. Front. Oncol. 11, 666064. doi:10.3389/fonc.2021.666064
Ma, L., Zhou, Y., Luo, X., Gao, H., Deng, X., and Jiang, Y. (2017). Long non-coding RNA XIST promotes cell growth and invasion through regulating miR-497/MACC1 Axis in gastric cancer. Oncotarget 8 (3), 4125–4135. doi:10.18632/oncotarget.13670
Marabelle, A., Fakih, M., Lopez, J., Shah, M., Shapira-Frommer, R., Nakagawa, K., et al. (2020). Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 21, 1353–1365. doi:10.1016/S1470-2045(20)30445-9
Miranda, A., Hamilton, P. T., Zhang, A. W., Pattnaik, S., Becht, E., Mezheyeuski, A., et al. (2019). Cancer stemness, intratumoral heterogeneity, and immune response across cancers. Proc. Natl. Acad. Sci. U. S. A. 116, 9020–9029. doi:10.1073/pnas.1818210116
Mu, L., Wang, Y., Su, H., Lin, Y., Sui, W., Yu, X., et al. (2021). HIF1A-AS2 promotes the proliferation and metastasis of gastric cancer cells through miR-429/PD-L1 Axis. Dig. Dis. Sci. 66, 4314–4325. doi:10.1007/s10620-020-06819-w
Nai, A., Zeng, H., Wu, Q., He, Z., Zeng, S., Bashir, S., et al. (2022). lncRNA/miR-29c-Mediated high expression of LOX can influence the immune status and chemosensitivity and can forecast the poor prognosis of gastric cancer. Front. Cell Dev. Biol. 9, 760470. doi:10.3389/fcell.2021.760470
Newsom-Davis, T. E., Wang, D., Steinman, L., Chen, P. F. T., Wang, L. X., Simon, A. K., et al. (2009). Enhanced immune recognition of cryptic glycan markers in human tumors. Cancer Res. 69 (5), 2018–2025. doi:10.1158/0008-5472.CAN-08-3589
Okazaki, T., and Honjo, T. (2007). PD-1 and PD-1 ligands: From discovery to clinical application. Int. Immunol. 19, 813–824. doi:10.1093/intimm/dxm057
Ou, J., Lei, P., Yang, Z., Yang, M., Luo, L., Mo, H., et al. (2021). LINC00152 mediates CD8+ T-cell infiltration in gastric cancer through binding to EZH2 and regulating the CXCL9, 10/CXCR3 axis. J. Mol. Histol. 52 (3), 611–620. doi:10.1007/s10735-021-09967-z
Pardoll, D. M. (2012). The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264. doi:10.1038/nrc3239
Qi, P., Xu, M. D., Shen, X. H., Ni, S. J., Huang, D., Tan, C., et al. (2015). Reciprocal repression between TUSC7 and miR-23b in gastric cancer. Int. J. Cancer 137 (6), 1269–1278. doi:10.1002/ijc.29516
Reck, M., Remon, J., and Hellmann, M. D. (2022). First-Line immunotherapy for non-small-cell lung cancer. J. Clin. Oncol. 40, 586–597. doi:10.1200/JCO.21.01497
Reinke, S., Bröckelmann, P. J., Iaccarino, I., Garcia-Marquez, M. A., Borchmann, S., Jochims, F., et al. (2020). Tumor and microenvironment response but no cytotoxic T-cell activation in classic Hodgkin lymphoma treated with anti-PD1. Blood 136, 2851–2863. doi:10.1182/blood.2020008553
Rizzo, A., Mollica, V., Ricci, A. D., Maggio, I., Massucci, M., Rojas Limpe, F. L., et al. (2020). Third- and later-line treatment in advanced or metastatic gastric cancer: A systematic review and meta-analysis. Future Oncol. 16, 4409–4418. doi:10.2217/fon-2019-0429
Schreiber, R. D., Old, L. J., and Smyth, M. J. (2011). Cancer immunoediting: Integrating immunity's roles in cancer suppression and promotion. Science 331, 1565–1570. doi:10.1126/science.1203486
Simon, A. K., Gallimore, A., Jones, E., Sawitzki, B., Cerundolo, V., and Screaton, G. R. (2002). Fas ligand breaks tolerance to self-antigens and induces tumor immunity mediated by antibodies. Cancer Cell 2 (4), 315–322. doi:10.1016/s1535-6108(02)00151-4
Smyth, E. C., Nilsson, M., Grabsch, H. I., van Grieken, N. C., and Lordick, F. (2020). Gastric cancer. Lancet 396, 635–648. doi:10.1016/S0140-6736(20)31288-5
Sun, J., Jiang, Q., Chen, H., Zhang, Q., Zhao, J., Li, H., et al. (2021). Genomic instability-associated lncRNA signature predicts prognosis and distinct immune landscape in gastric cancer. Ann. Transl. Med. 9, 1326. doi:10.21037/atm-21-3569
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249. doi:10.3322/caac.21660
Toor, S. M., Murshed, K., Al-Dhaheri, M., Khawar, M., Abu Nada, M., and Elkord, E. (2019). Immune checkpoints in circulating and tumor-infiltrating CD4(+) T cell subsets in colorectal cancer patients. Front. Immunol. 10, 2936. doi:10.3389/fimmu.2019.02936
Wang, C-J., Chun-Chao, Z., Jia, X., Wang, M., Zhao, W. Y., Liu, Q., et al. (2019). The lncRNA UCA1 promotes proliferation, migration, immune escape and inhibits apoptosis in gastric cancer by sponging anti-tumor miRNAs. Mol. Cancer 18, 115. doi:10.1186/s12943-019-1032-0
Wang, F. H., Zhang, X. T., Li, Y. F., Tang, L., Qu, X. J., Ying, J. E., et al. (2021). The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun. (Lond) 41, 747–795. doi:10.1002/cac2.12193
Wang, G., Duan, P., Liu, F., and Wei, Z. (2021). Long non-coding RNA CASC7 suppresses malignant behaviors of breast cancer by regulating miR-21-5p/FASLG axis. Bioengineered 12 (2), 11555–11566. doi:10.1080/21655979.2021.2010372
Wang, J., Wang, B., Zhou, B., Chen, J., Qi, J., Shi, L., et al. (2022). A novel immune-related lncRNA pair signature for prognostic prediction and immune response evaluation in gastric cancer: A bioinformatics and biological validation study. Cancer Cell Int. 22 (1), 69. doi:10.1186/s12935-022-02493-2
Wang, J. B., Li, P., Liu, X. L., Zheng, Q. L., Ma, Y. B., Zhao, Y. J., et al. (2020). An immune checkpoint score system for prognostic evaluation and adjuvant chemotherapy selection in gastric cancer. Nat. Commun. 11 (1), 6352. doi:10.1038/s41467-020-20260-7
Wang, J., Yu, Z., Wang, J., Shen, Y., Qiu, J., and Zhuang, Z. (2020). LncRNA NUTM2A-AS1 positively modulates TET1 and HIF-1A to enhance gastric cancer tumorigenesis and drug resistance by sponging miR-376a. Cancer Med. 9, 9499–9510. doi:10.1002/cam4.3544
Wang, W., Pei, Q., Wang, L., Mu, T., and Feng, H. (2022). Construction of a prognostic signature of 10 autophagy-related lncRNAs in gastric cancer. Int. J. Gen. Med. 15, 3699–3710. doi:10.2147/IJGM.S348943
Wang, Y., Zhang, X., Dai, X., and He, D. (2021). Applying immune-related lncRNA pairs to construct a prognostic signature and predict the immune landscape of stomach adenocarcinoma. Expert Rev. Anticancer Ther. 21, 1161–1170. doi:10.1080/14737140.2021.1962297
Wang, Y., Zhu, G. Q., Tian, D., Zhou, C. W., Li, N., Feng, Y., et al. (2022). Comprehensive analysis of tumor immune microenvironment and prognosis of m6A-related lncRNAs in gastric cancer. BMC Cancer 22, 316. doi:10.1186/s12885-022-09377-8
Wang, Y., Zhu, G. Q., Tian, D., Zhou, C. W., Li, N., Feng, Y., et al. (2022). Comprehensive analysis of tumor immune microenvironment and prognosis of m6A-related lncRNAs in gastric cancer. BMC Cancer 22 (1), 316. doi:10.1186/s12885-022-09377-8
Wang, Z., Cao, L., Zhou, S., Lyu, J., Gao, Y., and Yang, R. (2022). Construction and validation of a novel pyroptosis-related four-lncRNA prognostic signature related to gastric cancer and immune infiltration. Front. Immunol. 13, 854785. doi:10.3389/fimmu.2022.854785
Wei, G. H., and Wang, X. (2017). lncRNA MEG3 inhibit proliferation and metastasis of gastric cancer via P53 signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 21 (17), 3850–3856.
Wei, L., Sun, J., Zhang, N., Zheng, Y., Wang, X., Lv, L., et al. (2020). Noncoding RNAs in gastric cancer: Implications for drug resistance. Mol. Cancer 19, 62. doi:10.1186/s12943-020-01185-7
Wei, Z., Chen, L., Meng, L., Han, W., Huang, L., and Xu, A. (2020). LncRNA HOTAIR promotes the growth and metastasis of gastric cancer by sponging miR-1277-5p and upregulating COL5A1. Gastric Cancer 23 (6), 1018–1032. doi:10.1007/s10120-020-01091-3
Wen-Ming, Chen, Huang, Ming-de, Kong, Rong, Xu, T. P., Zhang, E. B., Xia, R., et al. (2015). Antisense long noncoding RNA HIF1A-AS2 is upregulated in gastric cancer and associated with poor prognosis. J. .Dig Dis. Sci. 60, 1655–1662. doi:10.1007/s10620-015-3524-0
Winograd, P., Hou, S., Court, C. M., Lee, Y. T., Chen, P. J., Zhu, Y., et al. (2020). Hepatocellular carcinoma-circulating tumor cells expressing PD-L1 are prognostic and potentially associated WithResponse to checkpoint inhibitors. Hepatol. Commun. 4, 1527–1540. doi:10.1002/hep4.1577
Xiao, S., Liu, X., Yuan, L., and Wang, F. (2021). A ferroptosis-related lncRNAs signature predicts prognosis and therapeutic response of gastric cancer. Front. Cell Dev. Biol. 9, 736682. doi:10.3389/fcell.2021.736682
Xiao, X., Cheng, W., Zhang, G., Wang, C., Sun, B., Zha, C., et al. (2022). Long noncoding RNA: Shining stars in the immune microenvironment of gastric cancer. Front. Oncol. 12, 862337. doi:10.3389/fonc.2022.862337
Xie, C., Guo, Y., and Lou, S. (2020). LncRNA ANCR promotes invasion and migration of gastric cancer by regulating FoxO1 expression to inhibit macrophage M1 polarization. Dig. Dis. Sci. 65 (10), 2863–2872. doi:10.1007/s10620-019-06019-1
Xie, J., Fu, L., and Jin, L. (2021). Immunotherapy of gastric cancer: Past, future perspective and challenges. Pathol. Res. Pract. 218, 153322. doi:10.1016/j.prp.2020.153322
Xiong, G., Yang, L., Chen, Y., and Fan, Z. (2015). Linc-POU3F3 promotes cell proliferation in gastric cancer via increasing T-reg distribution. Am. J. Transl. Res. 7 (11), 2262–2269.
Yu, J., Fang, C., Zhang, Z., Zhang, G., Shi, L., Qian, J., et al. (2020). H19 rises in gastric cancer and exerts a tumor-promoting function via miR-138/E2F2 Axis. Cancer Manag. Res. 12, 13033–13042. doi:10.2147/CMAR.S267357
Yu, P., Wang, Y., Yu, Y., Wang, A., Huang, L., Zhang, Y., et al. (2021). Deep targeted sequencing and its potential implication for cancer therapy in Chinese patients with gastric adenocarcinoma. Oncologist 26, e756–e768. doi:10.1002/onco.13695
Yuan, L., Xu, Z. Y., Ruan, S. M., Mo, S., Qin, J. J., and Cheng, X. D. (2020). Long non-coding RNAs towards precision medicine in gastric cancer: Early diagnosis, treatment, and drug resistance. Mol. Cancer 19, 96. doi:10.1186/s12943-020-01219-0
Zeng, C., Liu, Y., He, R., Lu, X., Dai, Y., Qi, G., et al. (2022). Identification and validation of a novel cellular senescence-related lncRNA prognostic signature for predicting immunotherapy response in stomach adenocarcinoma. Front. Genet. 13, 935056. doi:10.3389/fgene.2022.935056
Zhang, L., Wang, Y., Li, Z., Lin, D., Liu, Y., Zhou, L., et al. (2021). Clinicopathological features of tumor mutation burden, Epstein-Barr virus infection, microsatellite instability and PD-L1 status in Chinese patients with gastric cancer. Diagn Pathol. 16, 38. doi:10.1186/s13000-021-01099-y
Zhou, J., Zhi, X., Wang, L., Wang, W., Li, Z., Tang, J., et al. (2015). Linc00152 promotes proliferation in gastric cancer through the EGFR-dependent pathway. J. Exp. Clin. Cancer Res. 34, 135. doi:10.1186/s13046-015-0250-6
Zhu, K., Ren, Q., and Zhao, Y. (2019). lncRNA MALAT1 overexpression promotes proliferation, migration and invasion of gastric cancer by activating the PI3K/AKT pathway. Oncol. Lett. 17 (6), 5335–5342. doi:10.3892/ol.2019.10253
Keywords: gastric cancer, lncRNA, immunity, therapy, mechanism
Citation: Zhang Q, Wang C, Yang Y, Xu R and Li Z (2023) LncRNA and its role in gastric cancer immunotherapy. Front. Cell Dev. Biol. 11:1052942. doi: 10.3389/fcell.2023.1052942
Received: 24 September 2022; Accepted: 30 January 2023;
Published: 16 February 2023.
Edited by:
Xiqing Li, Henan Provincial People’s Hospital, ChinaReviewed by:
Qun Zhao, Fourth Hospital of Hebei Medical University, ChinaCopyright © 2023 Zhang, Wang, Yang, Xu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Yang, 632387011@qq.com; Ruihan Xu, ruihanxu@126.com; Ziyun Li, liziyun0412@126.com
†These authors have contributed equally to this work
 Qiang Zhang
Qiang Zhang