- Department of Reproductive Medicine, Department of Prenatal Genetics, First Hospital of Jilin University, Changchun, China
Long noncoding RNAs (lncRNAs) have been characterized to play an essential role in ovarian tumorigenesis via controlling a variety of cellular processes, such as cell proliferation, invasion, apoptotic death, metastasis, cell cycle, migration, metabolism, immune evasion, and chemoresistance. The one obstacle for the therapeutic efficacy is due to the development of drug resistance in ovarian cancer patients. Therefore, in this review article, we describe the role of lncRNAs in chemoresistance in ovarian cancer. Moreover, we discuss the molecular mechanism of lncRNAs-involved drug resistance in ovarian cancer. We conclude that lncRNAs could be useful targets to overcome chemoresistance and improve therapeutic outcome in ovarian cancer patients.
Introduction
Ovarian cancer is one of common malignancies in females (Giaquinto et al., 2022; Siegel et al., 2022). The key obstacle for the therapy outcome is the development of drug resistance in ovarian cancer patients (Ortiz et al., 2022). Genetic and epigenetic changes have been known to participate in chemotherapy resistance in a variety of cancers, including ovarian cancer (Meng et al., 2021; Zhang et al., 2022; Zhao et al., 2022). LncRNAs are remarkably involved in carcinogenesis via regulating a variety of cellular processes, such as cell proliferation, invasion, apoptotic death, metastasis, cell cycle, migration, metabolism, immune evasion (Jiang et al., 2021; Chen et al., 2022a; Chen et al., 2022b; Liu and Shang, 2022). LncRNAs can epigenetically regulate gene expression and take part in drug resistance (Jiang et al., 2020a; Xie et al., 2022). LncRNAs bind to proteins and nucleic acids and regulate the expression of tumor suppressive genes and oncogenes through working as decoys, scaffolds, or enhancers (Liu et al., 2021; Statello et al., 2021).
Noncoding RNAs have been involved in drug resistance and can become the therapeutic targets for cancer therapy (Lan et al., 2021; Xie et al., 2021; Liu et al., 2022). Drug resistance is developed due to decreased drug uptake, enhanced drug efflex, dysregulated drug metabolism, and drug compartmentalization. In addition, dysregulation of DNA damage repair and decreased sensitivity to apoptosis also lead to drug resistance. A ferroptosis and iron-metabolism-related lncRNA profile has been reported for prediction of therapeutic responses by analysis of integrated clinical data and omics data in ovarian cancer (Feng et al., 2022). LncRNAs have been emerged in regulating drug sensitivity in ovarian cancer (Tripathi et al., 2018; Abildgaard et al., 2019; Wambecke et al., 2020; Wambecke et al., 2021). In the following sections, we will describe the role of various lncRNAs in regulation of cisplatin, paclitaxel, 5-FU, rapamycin and carboplatin resistance.
LncRNAs regulate cisplatin resistance
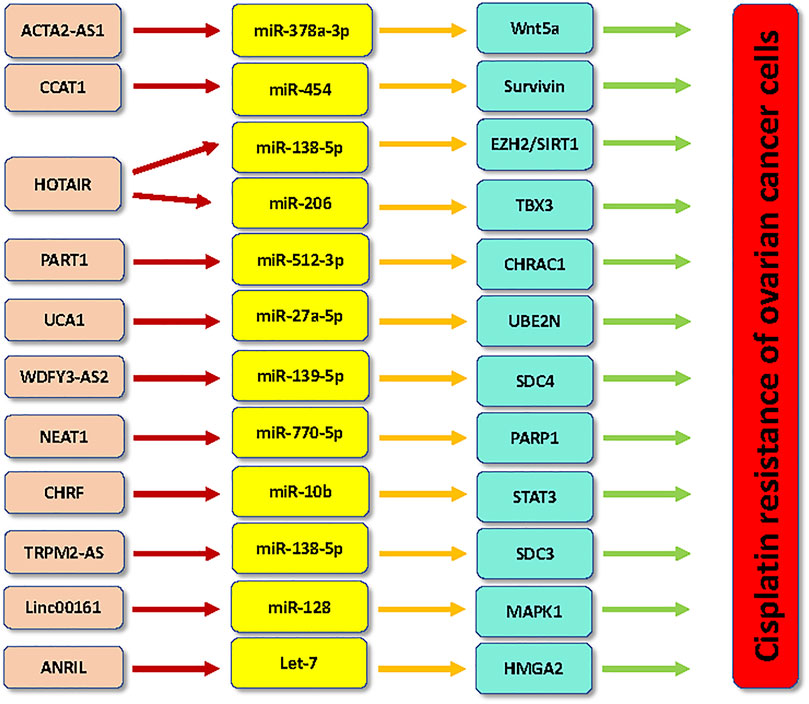
Cisplatin is platinum-based drug, which has been widely used for the therapy of ovarian cancer patients (Song et al., 2022). One study showed that lncRNA PVT1 was elevated in cisplatin resistant ovarian cancer tissues, while lncRNAs TUG1 and MEG3 were decreased in ovarian cancer patients with cisplatin resistance (El-Khazragy et al., 2020). Several lncRNAs have been reported to promote the cisplatin resistance in ovarian cancer, including ACTA2-AS1, CCAT1, CRNDE, HOTAIR, HOXA11-AS, TCF7, PANDAR, PART1, MALAT1, UCA1, WDFY3-AS2. However, numerous lncRNAs have been found to inhibit the cisplatin resistance in ovarian cancer, such as LINC01508 and GAS5.
LncRNAs promote cisplatin resistance
LncRNA ACTA2-AS1
The high expression of lncRNA ACTA2-AS1 was found in cisplatin-resistant A2780 and SKOV3 cells. Silencing of lncRNA ACTA2-AS1 in cisplatin-resistant ovarian cancer cells reduced cell proliferation (Lin et al., 2022). Moreover, lncRNA ACTA2-AS1 can sponge the expression of miR-378a-3p, which increased the expression of Wnt5a. Knockdown of miR-378a-3p reversed lncRNA ACTA2-AS1 knockdown-mediated inhibition of cisplatin-resistant cell viability (Lin et al., 2022). Consistently, miR-378a-3p alleviated cell resistance to cisplatin via suppression of Wnt5a. Hence, lncRNA ACTA2-AS1 boosted cisplatin resistance via sponging miR-378a-3p and targeting Wnt5a in ovarian cancer cells (Lin et al., 2022).
LncRNA CCAT1
LncRNA CCAT1 has been known to involve in cisplatin resistance in ovarian cancer cells (Wang et al., 2020a). Cisplatin-resistant ovarian cancer cells had the higher expression of CCAT1. Silencing of CCAT1 triggered cisplatin-mediated apoptosis via regulation of Bax, survivin and Bcl-1. Notably, CCAT1 can bind to miR-454 and increase the expression of survivin (Wang et al., 2020a). Hence, CCAT1/miR-454/survivin pathway contributed to cisplatin resistance in ovarian cancer (Wang et al., 2020a).
LncRNA CRNDE
Wu et al. reported that lncRNA colorectal neoplasia differentially expressed gene (CRNDE) promoted cisplatin resistance via regulation of SRSF1/TIA1 signaling pathway in ovarian cancer (Wu et al., 2022a). Cisplatin resistant ovarian cancer cells have a higher expression of lncRNA CRNDE compared with control cells. Overexpression of lncRNA CRNDE caused cisplatin resistance, whereas depletion of lncRNA CRNDE sensitized cancer cells to cisplatin in vitro and in vivo (Wu et al., 2022a). Moreover, lncRNA CRNDE increased SRSF1 expression and subsequently elevated TIA1 expression, which caused cisplatin resistance in ovarian cancer (Wu et al., 2022a).
LncRNA HOTAIR
LncRNA HOTAIR had a lower expression in benign ovarian tissues compared with ovarian tumor samples (Wang et al., 2015). A higher expression of lncRNA HOTAIR was observed in late stage malignant tumors compared with the early stage samples in ovarian cancer (Wang et al., 2015). The higher expression of HOTAIR was also observed in cisplatin-resistant SKOV3 cells than cisplatin-sensitivity control. Downregulation of HOTAIR retarded cell proliferation and migratory ability and reversed cisplatin resistance in cisplatin-resistant SKOV3 cells (Wang et al., 2015). Similarly, silencing of lncRNA HOTAIR abolished cisplatin resistance via suppression of miR-138-5p-involved EZH2 and SIRT1 in ovarian cancer cells (Zhang et al., 2020a). Zhang et al. found that HOTAIR kept the stemness features by sponging miR-206 and increasing the expression of T-box transcription factor 3 (TBX3) in ovarian cancer stem cells, contributing to cisplatin resistance (Zhang et al., 2020b). Moreover, HOTAIR promoted cisplatin resistance via regulation of DNA damage response and senescence by activation of NF-κB signaling pathway via suppression of IκBα in ovarian cancer cells (Ozes et al., 2016). Consistently, depletion of HOTAIR reduced cisplatin resistance via attenuating cisplatin-mediated autophagy in ovarian cancer (Yu et al., 2018).
LncRNA HOXA11-AS
Knockdown of lncRNA HOXA11-AS suppressed cell proliferation and overcame cisplatin resistance in ovarian cancer (Chen et al., 2022c). Cisplatin-resistance ovarian cancer cells had a higher expression of lncRNA HOXA11-AS compared with normal cells. Silencing of lncRNA HOXA11-AS in ovarian cancer cells suppressed cell viability, invasion and migration, but promoted apoptosis (Chen et al., 2022c). Knockout of lncRNA HOXA11-AS increased cisplatin sensitivity in cisplatin-resistant ovarian cancer cells. LncRNA HOXA11-AS depletion elevated cellular autophagy in ovarian cancer cells (Chen et al., 2022c).
LncRNA TCF7
LncRNA TCF7 has been found to enhance cell viability, migration, invasion via modulation of ITGB8 in epithelial ovarian cancer (Su and Huang, 2021). Moreover, lncRNA TCF7 promoted the stemness of ovarian cancer cells via acceleration of spheres formation by promotion of CD44 and CD133 expression. Furthermore, lncRNA TCF7 accelerated cisplatin resistance in epithelial ovarian cancer cells (Su and Huang, 2021). Molecular experimental data showed that lncRNA TCF7 promoted the expression of ITGB8 and exerted its oncogenic function in ovarian cancer (Su and Huang, 2021).
LncRNA PANDAR
LncRNA PANDAR participated in the drug resistance in ovarian cancer cells (Wang et al., 2018a). Cisplatin treatment induced higher expression levels of lncRNA PANDAR than paclitaxel and doxorubicin exposure in ovarian cancer. In cisplatin-sensitive ovarian cancer cells displayed lower expression of lncRNA PANDAR compared with cisplatin-resistant groups (Wang et al., 2018a). Moreover, overexpression of lncRNA PANDAR elevated the tumor growth and cell survival after cisplatin treatment, whereas downregulation of lncRNA PANDAR retarded tumor growth (Wang et al., 2018a). PANDAR interacted with SFRS2, a key factor to negatively govern p53 phosphorylation, leading to inhibition of PUMA in ovarian cancer (Wang et al., 2018a).
LncRNA PART1
LncRNA PART1 participated in promotion of resistance of cancer cells to cisplatin exposure (Yang et al., 2021). LncRNA PART1 was highly expressed in cisplatin-resistant ovarian cancer cells. Silencing of lncRNA PART1 led to cisplatin sensitivity in cisplatin-resistant ovarian cancer cells, which is shown by suppression of proliferation, invasion and migration (Yang et al., 2021). YY1 transcription factor can induce the expression of lncRNA PART1. PART1 can target miR-512-3p and regulate the expression of CHRAC1 in ovarian cancer cells. Taken together, lncRNA PART1 targeted miR-512-3p/CHRAC1 axis to increase cisplatin resistance in ovarian cancer (Yang et al., 2021).
LncRNA UCA1
LncRNA UCA1 reduced cisplatin response of OAW42 ovarian cancer cells via direct sponging to miR-27a-5p and decreasing the expression of UBE2N levels, resulting in the inhibition of BIM expression, a member of the Bcl-2 family to induce apoptotic death (Wambecke et al., 2021). UCA1 accelerated cisplatin resistance via miR-27a-5p/UBE2N/BIM axis in ovarian cancer cells (Wambecke et al., 2021).
LncRNA WDFY3-AS2
Evidence revealed that lncRNA WDFY3-AS2 participates in regulation of cisplatin sensitivity in ovarian cancer cells (Wu et al., 2021). In cisplatin-resistant A2780 ovarian cancer cells, WDFY3-AS2 had an increased expression. Depletion of WDFY3-AS2 blocked migration and invasion of cisplatin-resistant A2780 cells, but induced apoptosis and proliferation inhibition (Wu et al., 2021). Moreover, WDFY3-AS2 stimulated tumor-spheres in cisplatin-resistant A2780 cells. WDFY3-AS2 interacted with miR-139-5p and elevated the expression of SDC4 (Wu et al., 2021). Silencing of WDFY3-AS2 caused tumor growth reduction in xenografts. Hence, WDFY3-AS2 could regulate cisplatin resistance via targeting miR-139-5p/SDC4 axis in ovarian cancer (Wu et al., 2021). Deletion of LINC00152 elevated cisplatin sensitivity via upregulation of Bax and cleaved caspase-3, and downregulation of Bcl-2 in ovarian cancer (Zou and Li, 2019). Moreover, deletion of LINC00152 reduced the expression of several drug resistant genes, such as MDR1, MRP1 and GSTπ in ovarian cancer (Zou and Li, 2019).
Other lncRNAs promote cisplatin resistance
LncRNA NEAT1 depletion abrogated the cisplatin resistance via modulation of miR-770-5p and PARP1 in ovarian cancer (Zhu et al., 2020). LncRNA NEAT1 overexpression sponged miR-770-5p and inhibited the expression of PARP1, leading to cisplatin resistance in ovarian cancer (Zhu et al., 2020). Tan et al. found that lncRNA CHRF downregulation inhibited EMT and inactivated STAT3 pathway and abolish cisplatin resistance via regulation of miR-10b in ovarian cancer cells (Tan et al., 2020). LncRNA TRPM2-AS stimulated cisplatin resistance via binding to miR-138-5p and releasing SDC3 mRNA, leading to ovarian cancer malignant progression (Ding et al., 2021). Knockdown of lncRNA PVT1 repressed tumor progression via targeting JAK2/STAT3/PD-L1 in cisplatin-resistant ovarian cancer cells (Chen et al., 2021). Silencing of MALAT1 accelerated cisplatin sensitivity in ovarian cancer by suppression of the Notch1 pathway and ABCC1 expression (Bai et al., 2018). Knockdown of MALAT1 upregulated Bax protein and downregulated Bcl-2 protein in ovarian cancer cells (Bai et al., 2018). Upregulation of lncRNA PVT1 increased cisplatin resistance via targeting apoptotic pathway in ovarian cancer (Liu et al., 2015). LncRNA EBIC also facilitated cisplatin resistance via modulating Wnt/β-catenin in ovarian cancer cells (Xu et al., 2018). Linc00161 upregulation led to cisplatin resistance via regulating miR-128 and MAPK1 in ovarian cancer cells (Xu et al., 2019). LncRNA ANRIL depletion enhanced cisplatin resistance via sponging let-7 and increasing HMGA2 in ovarian cancer cells (Miao et al., 2019).
LncRNAs inhibit cisplatin resistance
LINC01508
LINC01508 expression was decreased in ovarian cancer cells with cisplatin resistance and ovarian cancer patients with platinum resistance (Xiao et al., 2021). In addition, tumor size and platinum resistance were linked to lower expression of LINC01508. Upregulation of LINC01508 increased cisplatin sensitivity of ovarian cancer cells via the suppression of the Hippo signaling pathway (Xiao et al., 2021). This study indicated that LINC01508 could be a biomarker for prediction of platinum resistance in ovarian cancer patients.
LINC01125 and LINC00312
LINC01125 promoted cisplatin sensitivity through targeting miR-1972 in ovarian cancer (Guo and Pan, 2019). Cisplatin-resistant ovarian cancer specimens had a lower expression of LINC01125. Increased LINC01125 retarded proliferation of ovarian cancer cells and induced the cisplatin cytotoxicity (Guo and Pan, 2019). LINC01125 interacted with miR-1972 and induced cell apoptosis pathways in ovarian cancer. Hence, LINC01125 could work as an antitumor lncRNA to increase cisplatin sensitivity of ovarian cancer (Guo and Pan, 2019). Linc00312 overexpression increased the cisplatin sensitivity via regulation of the Bcl-2 and caspase-3 pathways in cisplatin-resistant ovarian cancer cells (Zhang et al., 2018).
LncRNA GAS5
Using microarray and RT-PCR analysis, one group found that lncRNA GAS5 was dramatically downregulated in ovarian cancer specimens (Long et al., 2019). Low expression of lncRNA GAS5 was associated with poor prognosis in patients with ovarian cancer. In line with the role of GAS5, cisplatin-resistant ovarian cancer cells displayed lower expression of lncRNA GAS5 (Long et al., 2019). Increased expression of lncRNA GAS5 led to G0/G1 phase arrest and triggered apoptosis in ovarian cancer cells. Notably, overexpression of lncRNA GAS5 elevated the cisplatin sensitivity in ovarian cancer cells and in mice. LncRNA GAS5 recruited E2F4 to PARP1 and modulate the activation of MAPK pathway, leading to inhibition of cisplatin resistance (Long et al., 2019).
LncRNA ENST00000457645
One study showed that lncRNA ENST00000457645 reduced cisplatin resistance in ovarian cancer cells (Yan et al., 2017). LncRNA ENST00000457645 overexpression suppressed viability and migratory capacity of ovarian cancer. This lncRNA upregulation increased the expression of Bax protein and cleaved caspase-3 in CP70 ovarian cancer cells (Yan et al., 2017). Further investigation is pivotal for determining the mechanism of lncRNA ENST00000457645-mediated cisplatin resistance.
LncRNAs regulate carboplatin resistance
LncRNA SNHG12
RNA sequencing and global DNA methylation assays were performed in four carboplatin-sensitive ovarian cancer cell lines and their resistant cells, and two ovarian cancer cells (OVCAR8 and Ovc316) with inherent carboplatin-resistant cell lines (Abildgaard et al., 2022). TCGA dataset and internal database were used to validate lncRNA candidates. This study found that 4,255 DEGs and 14,529 DMPs (differentially methylated CpG positions) in carboplatin-resistant cells (Abildgaard et al., 2022). They found that 50 lncRNAs were linked to carboplatin resistance. Moreover, 11 lncRNAs exhibited DMPs, including lncRNA SNHG12 (Abildgaard et al., 2022). Furthermore, depletion of lncRNA SNHG12 promoted carboplatin resistance in OVCAR8 and Ovc316 cells. Therefore, lncRNA SNHG12 accelerated carboplatin resistance in ovarian cancer (Abildgaard et al., 2022).
LncRNA TLR8-AS1
TLR8-AS1 knockdown suppressed cell migration and invasion, and reduced resistance to carboplatin in OV90 and SKOV3 (Xu et al., 2020). TLR8-AS1 can stabilize TLR8 mRNA and increase the expression of TLR8, leading to activation of NF-κB signaling pathway (Xu et al., 2020). TLR8-AS1 expression level was upregulated in ovarian cancer tissues, especial in metastatic ovarian cancer, which was linked to poor prognosis. Altogether, TLR8-AS1 targeted NF-κB pathway to govern tumor metastasis and carboplatin resistance (Xu et al., 2020).
LncRNA HOTAIR
LncRNA HOTAIR expression was linked to poor survival in ovarian cancer patients with carboplatin treatment (Teschendorff et al., 2015). HOTAIR and its DNA methylation file suggest carboplatin resistance in ovarian cancer patients (Teschendorff et al., 2015). However, it is necessary to determine and find direct evidence to demonstrate the function of HOTAIR in carboplatin resistance in ovarian cancer.
LncRNAs regulate paclitaxel resistance
LncRNA HOTAIR
LncRNA HOTAIR was identified to play an important role in ovarian carcinogenesis.
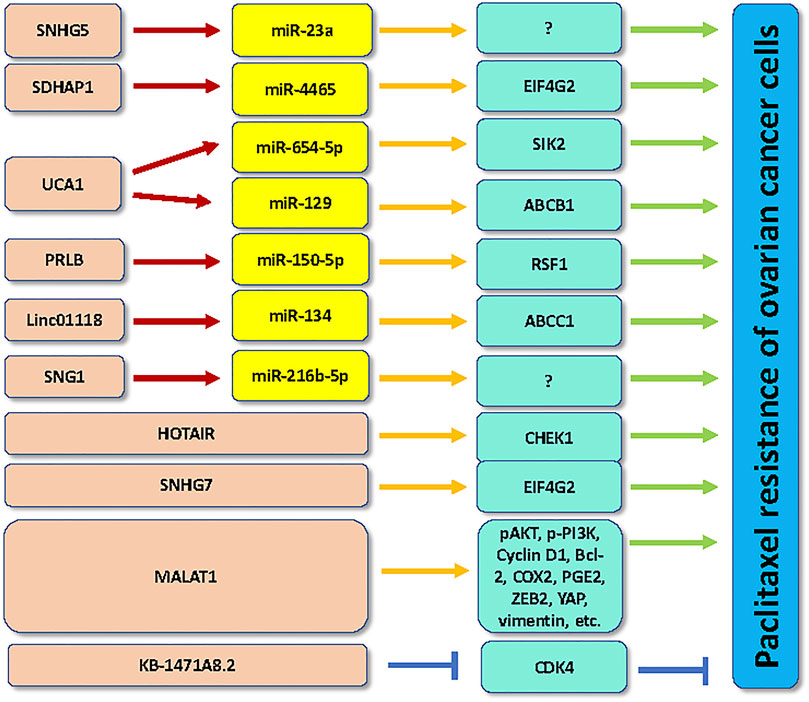
HOTAIR facilitated paclitaxel resistance by regulation of checkpoint kinase 1 (CHEK1) in ovarian cancer (Jiang et al., 2020b). Paclitaxel treatment increased the expression of HOTAIR in ovarian cancer cells. Moreover, downregulation of HOTAIR suppressed cell proliferation and enhanced paclitaxel sensitivity as well as caused G2/M phase arrest in ovarian cancer cells (Jiang et al., 2020b). Overexpression of CHEK1 weakened HOTAIR knockdown-induced paclitaxel sensitivity in ovarian cancer (Jiang et al., 2020b).
LncRNA SNHG5
Lin et al. reported that lncRNA SNHG5 promoted paclitaxel sensitivity via repressing the expression of miR-23a in ovarian cancer cells (Lin et al., 2020). SNHG5 was remarked downregulated in patients with ovarian cancer and associated with poor prognosis. SNHG5 can act as a decoy to repress miR-23a in ovarian cancer cells (Lin et al., 2020). Knockdown of miR-23a or overexpression of SNHG5 overcame the paclitaxel resistance in ovarian cancer cells (Lin et al., 2020).
LncRNA SNHG7
LncRNA SNHG7 has been revealed to govern ovarian tumorigenesis. For instance, lncRNA SNHG7 can be activated by SP1 and interact with EZH2 and confer oncogenic functions in ovarian cancer (Bai et al., 2020). LncRNA SNHG7 knockdown by siRNA transfection retarded migration and invasion of paclitaxel-resistant cells in ovarian cancer (Zhang et al., 2021). LncRNA SNHG7 interacted with EIF4G2 and affected the degradation of EIF4G2, leading to upregulation of EIF4G2. LncRNA SNHG7 promoted cell viability and motility as well as paclitaxel resistance via promotion of EIF4G2 (Zhang et al., 2021).
LncRNA SDHAP1
LncRNA SDHAP1 had a higher expression in paclitaxel-resistant Hey-8 and SKOV3 cells (Zhao et al., 2020). Depletion of lncRNA SDHAP1 stimulated paclitaxel sensitivity in ovarian cancer cells. Mechanically, lncRNA SDHAP1 sponged miR-4465 and promoted the expression of EIF4G2, which conferred paclitaxel-induced cell apoptotic death in ovarian cancer cells (Zhao et al., 2020).
LncRNA MIR17HG
LncRNA MIR17HG upregulation inhibited paclitaxel resistance and glycolysis via regulation of Claudin in epithelial ovarian cancer (EOC) cells (Wu et al., 2022b). Moreover, KHDRBS3 interacted with MIR17HG and blocked the function of MIR17HG in EOC cells (Wu et al., 2022b). KHDRBS3 expression was increased in paclitaxel-resistant EOC cells, while KHDRBS3 downregulation reduced the IC50 of paclitaxel-resistant paclitaxel in EOC cells. MIR17HG overexpression abrogated the KHDRBS3-mediated paclitaxel-resistance in EOC cells (Wu et al., 2022b).
LncRNA MALAT1
Overexpression of lncRNA MALAT1 led to promotion of cyclin D1, pAkt and p-PI3K, contributing to acceleration of ovarian cancer cell proliferation (Mao et al., 2021). MALAT1 elevated the expression of IL-1β, p-P38, p-NF-κB, COX2 and PGE2 signaling, and reduced caspase-3 level and promoted Bcl-2 expression in ovarian cancer cells. MALAT1 upregulation also elevated the expression of ZEB2, YAP, vimentin and decreased E-cadherin, suggesting that MALAT1 triggered EMT and tumor metastasis in ovarian cancer cells (Mao et al., 2021). Moreover, MALAT1 upregulation increased paclitaxel resistance of ovarian cancer cells in the tumor microenvironment (Mao et al., 2021).
LncRNA UCA1
LncRNA UCA1 has been reported to enhance paclitaxel resistance via targeting the miR-654-5p and SIK2 in ovarian cancer (Li et al., 2020a). LncRNA UCA1 upregulation was found in ovarian cancer tissues and paclitaxel-resistant ovarian cancer cells (Wang et al., 2018b; Li et al., 2020a). Paclitaxel resistance was restrained by inhibition of lncRNA UCA1 in paclitaxel-resistant ovarian cancer cells (Li et al., 2020a). Moreover, UCA1 sponged the miR-654-5p and upregulated the expression of its target SIK2 in ovarian cancer cells (Li et al., 2020a). In line with this finding, downregulation of SIK2 blocked paclitaxel resistance and attenuated the phenomenon of paclitaxel-resistant ovarian cancer cells, but inhibition of miR-654-5p by inhibitors rescued this inhibitory phenotype (Li et al., 2020a). Another study revealed that knockdown of UCA1 sensitized the paclitaxel-resistant ovarian cancer cells to paclitaxel treatment via induction of apoptosis (Wang et al., 2018b). Mechanistically, UCA1 can sponge miR-129 and increase the expression of ABCB1, contributing to paclitaxel resistance in ovarian cancer (Wang et al., 2018b).
LncRNA KB-1471A8.2
LncRNA KB-1471A8.2 upregulation reduced proliferation and migratory ability of ovarian cancer cells (Zhang et al., 2019). Increased expression of lncRNA KB-1471A8.2 also antagonized the paclitaxel resistance in ovarian cancer cells (Zhang et al., 2019). KB-1471A8.2 was decreased in ovarian cancer specimens and chemo-resistant ovarian cancer cell lines. KB-1471A8.2 upregulation can inhibit the expression of CDK4 in ovarian cancer cells, leading to G0/G1 phase arrest (Zhang et al., 2019).
LncRNA PRLB
LncRNA PRLB was found to regulate the paclitaxel resistance in ovarian cancer cells (Zhao and Hong, 2021). Paclitaxel-resistant ovarian cancer samples and cell lines exhibited the increased expression of lncRNA PRLB. Silencing of lncRNA PRLB reduced the IC50 value of the paclitaxel-resistant SKOV3 and CAOV3 ovarian cancer cells to paclitaxel treatment due to upregulation of apoptosis. LncRNA PRLB can bind to miR-150-5p and suppress the expression of miR-150-5p in ovarian cancer cells (Zhao and Hong, 2021). RSF1 was further identified as a target of miR-150-5p in ovarian cancer cells. RSF1 can activate the NF-κB signaling pathway in ovarian cancer cells (Zhao and Hong, 2021). Hence, lncRNA PRLB enhanced paclitaxel resistance via targeting miR-150-5p/RSF1/NF-κB in ovarian cancer cells.
Other lncRNAs regulate paclitaxel resistance
LncRNA SNHG1 knockdown enhanced paclitaxel sensitivity in A2780 cells via suppression of cell growth and migration and induction of apoptosis by regulating miR-216b-5p (Pei et al., 2020). LINC01118 plays an oncogenic role in promotion of paclitaxel resistance via modulation of miR-134 and upregulation of ABCC1 in ovarian cancer cells (Shi and Wang, 2018).
LncRNAs regulate 5-FU and rapamycin resistance
LncRNA TMPO-AS1 was reported to take part in 5-fluorouracil resistance in ovarian cancer cells (Li et al., 2020b). LncRNA TMPO-AS1 governed the expression of the TMEFF2 via sponging miR-200c, and activated the PI3K/Akt pathway. Depletion of TMPO-AS1 suppressed the EMT and motility and 5-FU resistance in ovarian cancer cells (Li et al., 2020b). LncRNA EPIC1 enhanced rapamycin resistance via activation of AKT-mTORC1 pathway in ovarian cancer (Wang et al., 2020b). Myc was involved in EPIC1-mediated oncogenesis in ovarian cancer cells. Overexpression of lncRNA EPIC1 activated the AKT-mTORC1 pathway via Myc and caused rapamycin resistance in ovarian cancer (Wang et al., 2020b).
Conclusion and perspective
In conclusion, lncRNAs regulate chemoresistance via sponging miRNAs in ovarian cancer cells (Figures 1, 2). It has several issues that need to be mentioned. Firstly, evidence suggests that cicrRNAs also regulate chemoresistance in ovarian cancer. For instance, silencing of circNRIP1 enhanced the sensitivity of paclitaxel in ovarian cancer cells through regulation of miR-211-5p and homeobox C8 (HOXC8) pathways (Li et al., 2020c). Paclitaxel-resistant ovarian cancer tissues and cells exhibited the higher expression of circNRIP1 (Li et al., 2020c). Knockdown of circNRIP1 abolished the paclitaxel resistance in ovarian cancer cells and mice. Furthermore, circNRIP1 interacted with miR-211-5p and subsequently increased the expression of HOXC8 in ovarian cancer cells (Li et al., 2020c). One study demonstrated that increased expression of circ_CELSR1 enhanced paclitaxel resistance via targeting miR-149-5p/SIK2 axis in ovarian cancer (Wei et al., 2021). Consistently, silencing of circ_CELSR1 reduced resistance of ovarian cancer cells to paclitaxel treatment (Wei et al., 2021).
Secondly, several compounds have been reported to target the expression of lncRNAs to control drug resistance in ovarian cancer. For example, Metformin inhibited the expression of lncRNA SNHG7 and increased the miR-3127-5p expression levels as well as regulated autophagy, which increased paclitaxel sensitivity (Yu et al., 2020). Curcumin elevated the lncRNA MEG3 levels via demethylation of MEG3, leading to inhibition of extracellular vesicle-involved transfer of miR-214, thereby attenuation of cisplatin resistance in ovarian cancer (Zhang et al., 2017). Thirdly, one lncRNA can regulate resistance of several chemotherapeutic drugs in ovarian cancer. For example, lncRNA ZEB1-AS1 attenuated cisplatin and paclitaxel sensitivity in epithelial ovarian cancer cells via suppression of MMP19 (Dai et al., 2021). LncRNA HOTAIR regulated cisplatin resistance and paclitaxel resistance in ovarian cancer cells (Zhang et al., 2020a; Jiang et al., 2020b). LncRNA MALAT1 facilitated both cisplatin and paclitaxel resistance in ovarian cancer cells via inhibition of cell apoptosis and promotion of invasion and proliferation (Mao et al., 2021). Taken together, multiple lncRNAs regulate the drug resistance in ovarian cancer. Due to that lncRNAs are involved in drug resistance in ovarian cancer, targeting these lncRNAs could be useful strategy for overcoming chemoresistance in ovarian cancer patients.
Author contributions
LC and JW drafted the manuscript and made the figure. QL revised this manuscript and supervised this work. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CCAT1, colon cancer-associated transcript 1; CircNRIP1, circRNA nuclear receptor-interacting protein 1; EIF4G2, eukaryotic translation initiation factor 4 gamma 2; HOTAIR, Hox transcript antisense intergenic RNA; KHDRBS3, KH domain-containing, RNA-binding signal transduction-associated protein 3; NEAT1, nuclear paraspeckle assembly transcript 1; PANDAR, the promoter of CDKN1A antisense DNA damage activated RNA; SDHAP1, succinate dehydrogenase complex flavoprotein subunit A pseudogene 1; SFRS2, arginine/serine-rich 2; SIK2, salt inducible kinase 2; TMEFF2, transmembrane protein with epidermal growth factor and two follistatin motifs 2; UCA1, urothelial carcinoma associated 1.
References
Abildgaard, C., do Canto, L. M., Rainho, C. A., Marchi, F. A., Calanca, N., Waldstrom, M., et al. (2022). The long non-coding RNA SNHG12 as a mediator of carboplatin resistance in ovarian cancer via epigenetic mechanisms. Cancers (Basel) 14 (7), 1664. Epub 20220325. doi:10.3390/cancers14071664
Abildgaard, C., Do Canto, L. M., Steffensen, K. D., and Rogatto, S. R. (2019). Long non-coding RNAs involved in resistance to chemotherapy in ovarian cancer. Front. Oncol. 9, 1549. Cited in: Pubmed; PMID 32039022. doi:10.3389/fonc.2019.01549
Bai, L., Wang, A., Zhang, Y., Xu, X., and Zhang, X. (2018). Knockdown of MALAT1 enhances chemosensitivity of ovarian cancer cells to cisplatin through inhibiting the Notch1 signaling pathway. Exp. Cell. Res. 366 (2), 161–171. Cited in: Pubmed; PMID 29548748. doi:10.1016/j.yexcr.2018.03.014
Bai, Z., Wu, Y., Bai, S., Yan, Y., Kang, H., Ma, W., et al. (2020). Long non-coding RNA SNGH7 Is activated by SP1 and exerts oncogenic properties by interacting with EZH2 in ovarian cancer. J. Cell. Mol. Med. 24 (13), 7479–7489. Epub 20200518. doi:10.1111/jcmm.15373
Chen, T., Liu, J., Zhang, H., Li, J., and Shang, G. (2022). Long intergenic noncoding RNA00265 enhances cell viability and metastasis via targeting miR-485-5p/USP22 Axis in osteosarcoma. Front. Oncol. 12, 907472. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Epub 20220526. doi:10.3389/fonc.2022.907472
Chen, X., Liu, Y., Liu, H., Wang, Z. W., and Zhu, X. (2022). Unraveling diverse roles of noncoding RNAs in various human papillomavirus negative cancers. Pharmacol. Ther. 238, 108188. Cited in: Pubmed; PMID 35421419. doi:10.1016/j.pharmthera.2022.108188
Chen, Y., Cui, Z., Wu, Q., Wang, H., Xia, H., and Sun, Y. (2022). Long non-coding RNA HOXA11-AS knockout inhibits proliferation and overcomes drug resistance in ovarian cancer. Bioengineered 13 (5), 13893–13905. in: Pubmed; PMID 35706412. doi:10.1080/21655979.2022.2086377
Chen, Y., Li, F., Li, D., Liu, W., and Zhang, L. (2021). Atezolizumab and blockade of LncRNA PVT1 attenuate cisplatin resistant ovarian cancer cells progression synergistically via JAK2/STAT3/PD-L1 pathway. Clin. Immunol. 227, 108728. Cited in: Pubmed; PMID 33878452. doi:10.1016/j.clim.2021.108728
Dai, C., Xu, P., Liu, S., Xu, S., Xu, J., Fu, Z., et al. (2021). Long noncoding RNA ZEB1-AS1 affects paclitaxel and cisplatin resistance by regulating MMP19 in epithelial ovarian cancer cells. Arch. Gynecol. Obstet. 303 (5), 1271–1281. Cited in: Pubmed; PMID 33151424. doi:10.1007/s00404-020-05858-y
Ding, Y., Tan, X., Abasi, A., Dai, Y., Wu, R., Zhang, T., et al. (2021). LncRNA TRPM2-AS promotes ovarian cancer progression and cisplatin resistance by sponging miR-138-5p to release SDC3 mRNA. Aging (Albany NY) 13 (5), 6832–6848. Epub 20210217. doi:10.18632/aging.202541
El-Khazragy, N., Mohammed, H. F., Yassin, M., Elghoneimy, K. K., Bayoumy, W., Hewety, A., et al. (2020). Tissue-based long non-coding RNAs "PVT1, TUG1 and MEG3" signature predicts Cisplatin resistance in ovarian Cancer. Genomics 112 (6), 4640–4646. Epub 20200808. doi:10.1016/j.ygeno.2020.08.005
Feng, S., Yin, H., Zhang, K., Shan, M., Ji, X., Luo, S., et al. (2022). Integrated clinical characteristics and omics analysis identifies a ferroptosis and iron-metabolism-related lncRNA signature for predicting prognosis and therapeutic responses in ovarian cancer. J. Ovarian Res. 15 (1), 10. Cited in: Pubmed; PMID 35057848. doi:10.1186/s13048-022-00944-y
Giaquinto, A. N., Broaddus, R. R., Jemal, A., and Siegel, R. L. (2022). The changing landscape of gynecologic cancer mortality in the United States. Obstet. Gynecol. 139 (3), 440–442. Financial Disclosure The authors did not report any potential conflicts of interest. doi:10.1097/AOG.0000000000004676
Guo, J., and Pan, H. (2019). Long noncoding RNA LINC01125 enhances cisplatin sensitivity of ovarian cancer via miR-1972. Med. Sci. Monit. 25, 9844–9854. Epub 20191222. doi:10.12659/MSM.916820
Jiang, J., Wang, S., Wang, Z., Cai, J., Han, L., Xie, L., et al. (2020). HOTAIR promotes paclitaxel resistance by regulating CHEK1 in ovarian cancer. Cancer Chemother. Pharmacol. 86 (2), 295–305. Cited in: Pubmed; PMID 32743678. doi:10.1007/s00280-020-04120-1
Jiang, W., Pan, S., Chen, X., Wang, Z. W., and Zhu, X. (2021). The role of lncRNAs and circRNAs in the PD-1/PD-L1 pathway in cancer immunotherapy. Mol. Cancer 20 (1), 116. Cited in: Pubmed; PMID 34496886. doi:10.1186/s12943-021-01406-7
Jiang, W., Xia, J., Xie, S., Zou, R., Pan, S., Wang, Z. W., et al. (2020). Long non-coding RNAs as a determinant of cancer drug resistance: Towards the overcoming of chemoresistance via modulation of lncRNAs. Drug Resist Updat 50, 100683. Cited in: Pubmed; PMID 32146422. doi:10.1016/j.drup.2020.100683
Lan, H., Yuan, J., Zeng, D., Liu, C., Guo, X., Yong, J., et al. (2021). The emerging role of non-coding RNAs in drug resistance of ovarian cancer. Front. Genet. 12, 693259. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. doi:10.3389/fgene.2021.693259
Li, H., Zhou, Y., Cheng, H., Tian, J., and Yang, S. (2020). Roles of a TMPO-AS1/microRNA-200c/TMEFF2 ceRNA network in the malignant behaviors and 5-FU resistance of ovarian cancer cells. Exp. Mol. Pathol. 115, 104481. Declaration of Competing Interest All authors declare that there is no conflict of interests in this study. doi:10.1016/j.yexmp.2020.104481
Li, M., Cai, J., Han, X., and Ren, Y. (2020). Downregulation of circNRIP1 suppresses the paclitaxel resistance of ovarian cancer via regulating the miR-211-5p/HOXC8 Axis. Cancer Manag. Res. 12, 9159–9171. Epub 20200928. doi:10.2147/CMAR.S268872
Li, Z. Y., Wang, X. L., Dang, Y., Zhu, X. Z., Zhang, Y. H., Cai, B. X., et al. (2020). Long non-coding RNA UCA1 promotes the progression of paclitaxel resistance in ovarian cancer by regulating the miR-654-5p/SIK2 axis. Eur. Rev. Med. Pharmacol. Sci. 24 (2), 591–603. in: Pubmed; PMID 32016960. doi:10.26355/eurrev_202001_20035
Lin, C., Zheng, M., Yang, Y., Chen, Y., Zhang, X., Zhu, L., et al. (2022). Knockdown of lncRNA ACTA2-AS1 reverses cisplatin resistance of ovarian cancer cells via inhibition of miR-378a-3p-regulated Wnt5a. Bioengineered 13 (4), 9829–9838. in: Pubmed; PMID 35412951. doi:10.1080/21655979.2022.2061181
Lin, H., Shen, L., Lin, Q., Dong, C., Maswela, B., Illahi, G. S., et al. (2020). SNHG5 enhances Paclitaxel sensitivity of ovarian cancer cells through sponging miR-23a. Biomed. Pharmacother. 123, 109711. Cited in: Pubmed; PMID 31884343. doi:10.1016/j.biopha.2019.109711
Liu, E., Liu, Z., Zhou, Y., Mi, R., and Wang, D. (2015). Overexpression of long non-coding RNA PVT1 in ovarian cancer cells promotes cisplatin resistance by regulating apoptotic pathways. Int. J. Clin. Exp. Med. 8 (11), 20565–20572. Epub 20151115. Cited in: Pubmed; PMID 26884974.
Liu, J., and Shang, G. (2022). The roles of noncoding RNAs in the development of osteosarcoma stem cells and potential therapeutic targets. Front. Cell. Dev. Biol. 10, 773038. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. doi:10.3389/fcell.2022.773038
Liu, M., Zhang, H., Li, Y., and Wang, S. (2022). Noncoding RNAs interplay in ovarian cancer therapy and drug resistance. Cancer biother. Radiopharm. 37 (3), 186–198. Cited in: Pubmed; PMID 35133881. doi:10.1089/cbr.2021.0339
Liu, S. J., Dang, H. X., Lim, D. A., Feng, F. Y., and Maher, C. A. (2021). Long noncoding RNAs in cancer metastasis. Nat. Rev. Cancer 21 (7), 446–460. Cited in: Pubmed; PMID 33953369. doi:10.1038/s41568-021-00353-1
Long, X., Song, K., Hu, H., Tian, Q., Wang, W., Dong, Q., et al. (2019). Long non-coding RNA GAS5 inhibits DDP-resistance and tumor progression of epithelial ovarian cancer via GAS5-E2F4-PARP1-MAPK axis. J. Exp. Clin. Cancer Res. 38 (1), 345. Cited in: Pubmed; PMID 31391118. doi:10.1186/s13046-019-1329-2
Mao, T. L., Fan, M. H., Dlamini, N., and Liu, C. L. (2021). LncRNA MALAT1 facilitates ovarian cancer progression through promoting chemoresistance and invasiveness in the tumor microenvironment. Int. J. Mol. Sci. 22 (19), 10201. Epub 20210922. doi:10.3390/ijms221910201
Meng, Y., Qiu, L., Zhang, S., and Han, J. (2021). The emerging roles of E3 ubiquitin ligases in ovarian cancer chemoresistance. Cancer Drug resist. 4 (2), 365–381. All authors declared that there are no conflicts of interest. Epub 20210619. doi:10.20517/cdr.2020.115
Miao, J. T., Gao, J. H., Chen, Y. Q., Chen, H., Meng, H. Y., and Lou, G. (2019). LncRNA ANRIL affects the sensitivity of ovarian cancer to cisplatin via regulation of let-7a/HMGA2 axis. Biosci. Rep. 39 (7), BSR20182101. Epub 20190705. doi:10.1042/BSR20182101
Ortiz, M., Wabel, E., Mitchell, K., and Horibata, S. (2022). Mechanisms of chemotherapy resistance in ovarian cancer. Cancer Drug resist. 5 (2), 304–316. All authors declared that there are no conflicts of interest. Epub 20220403. doi:10.20517/cdr.2021.147
Ozes, A. R., Miller, D. F., Ozes, O. N., Fang, F., Liu, Y., Matei, D., et al. (2016). NF-κB-HOTAIR axis links DNA damage response, chemoresistance and cellular senescence in ovarian cancer. Oncogene 35 (41), 5350–5361. The authors have declared that no conflict of interest exists. Epub 20160404. doi:10.1038/onc.2016.75
Pei, M. L., Zhao, Z. X., and Shuang, T. (2020). Dysregulation of lnc-SNHG1 and miR-216b-5p correlate with chemoresistance and indicate poor prognosis of serous epithelial ovarian cancer. J. Ovarian Res. 13 (1), 144. Epub 20201210. doi:10.1186/s13048-020-00750-4
Shi, C., and Wang, M. (2018). LINC01118 modulates paclitaxel resistance of epithelial ovarian cancer by regulating miR-134/ABCC1. Med. Sci. Monit. 24, 8831–8839. Epub 20181206. doi:10.12659/MSM.910932
Siegel, R. L., Miller, K. D., Fuchs, H. E., and Jemal, A. (2022). Cancer statistics, 2022. Ca. Cancer J. Clin. 72 (1), 7–33. Epub 20220112. doi:10.3322/caac.21708
Song, M., Cui, M., and Liu, K. (2022). Therapeutic strategies to overcome cisplatin resistance in ovarian cancer. Eur. J. Med. Chem. 232, 114205. Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. doi:10.1016/j.ejmech.2022.114205
Statello, L., Guo, C. J., Chen, L. L., and Huarte, M. (2021). Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell. Biol. 22 (2), 96–118. Cited in: Pubmed; PMID 33353982. doi:10.1038/s41580-020-00315-9
Su, C., and Huang, K. (2021). LncRNA TCF7 promotes epithelial ovarian cancer viability, mobility and stemness via regulating ITGB8. Front. Oncol. 11, 649655. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. doi:10.3389/fonc.2021.649655
Tan, W. X., Sun, G., Shangguan, M. Y., Gui, Z., Bao, Y., Li, Y. F., et al. (2020). Novel role of lncRNA CHRF in cisplatin resistance of ovarian cancer is mediated by miR-10b induced EMT and STAT3 signaling. Sci. Rep. 10 (1), 14768. Cited in: Pubmed; PMID 32901049. doi:10.1038/s41598-020-71153-0
Teschendorff, A. E., Lee, S. H., Jones, A., Fiegl, H., Kalwa, M., Wagner, W., et al. (2015). HOTAIR and its surrogate DNA methylation signature indicate carboplatin resistance in ovarian cancer. Genome Med. 7, 108. Cited in: Pubmed; PMID 26497652. doi:10.1186/s13073-015-0233-4
Tripathi, M. K., Doxtater, K., Keramatnia, F., Zacheaus, C., Yallapu, M. M., Jaggi, M., et al. (2018). Role of lncRNAs in ovarian cancer: Defining new biomarkers for therapeutic purposes. Drug Discov. Today 23 (9), 1635–1643. Cited in: Pubmed; PMID 29698834. doi:10.1016/j.drudis.2018.04.010
Wambecke, A., Ahmad, M., Lambert, B., Joly, F., Poulain, L., Denoyelle, C., et al. (2020). The influence of long non-coding RNAs on the response to chemotherapy in ovarian cancer. Gynecol. Oncol. 156 (3), 726–733. Declaration of competing interest The authors declare no conflict of interest. Epub 20191226. doi:10.1016/j.ygyno.2019.12.020
Wambecke, A., Ahmad, M., Morice, P. M., Lambert, B., Weiswald, L. B., Vernon, M., et al. (2021). The lncRNA 'UCA1' modulates the response to chemotherapy of ovarian cancer through direct binding to miR-27a-5p and control of UBE2N levels. Mol. Oncol. 15 (12), 3659–3678. Epub 20210713. doi:10.1002/1878-0261.13045
Wang, D. Y., Li, N., and Cui, Y. L. (2020). Long non-coding RNA CCAT1 sponges miR-454 to promote chemoresistance of ovarian cancer cells to cisplatin by regulation of surviving. Cancer Res. Treat. 52 (3), 798–814. Epub 20200303. doi:10.4143/crt.2019.498
Wang, H., Fang, L., Jiang, J., Kuang, Y., Wang, B., Shang, X., et al. (2018). The cisplatin-induced lncRNA PANDAR dictates the chemoresistance of ovarian cancer via regulating SFRS2-mediated p53 phosphorylation. Cell. Death Dis. 9 (11), 1103. Cited in: Pubmed; PMID 30375398. doi:10.1038/s41419-018-1148-y
Wang, J., Ye, C., Liu, J., and Hu, Y. (2018). UCA1 confers paclitaxel resistance to ovarian cancer through miR-129/ABCB1 axis. Biochem. Biophys. Res. Commun. 501 (4), 1034–1040. Epub 20180519. doi:10.1016/j.bbrc.2018.05.104
Wang, Y., Wang, H., Song, T., Zou, Y., Jiang, J., Fang, L., et al. (2015). HOTAIR is a potential target for the treatment of cisplatinresistant ovarian cancer. Mol. Med. Rep. 12 (2), 2211–2216. Epub 20150327. doi:10.3892/mmr.2015.3562
Wang, Y., Zhang, M., Wang, Z., Guo, W., and Yang, D. (2020). MYC-binding lncRNA EPIC1 promotes AKT-mTORC1 signaling and rapamycin resistance in breast and ovarian cancer. Mol. Carcinog. 59 (10), 1188–1198. Epub 20200818. doi:10.1002/mc.23248
Wei, S., Qi, L., and Wang, L. (2021). Overexpression of circ_CELSR1 facilitates paclitaxel resistance of ovarian cancer by regulating miR-149-5p/SIK2 axis. Anticancer. Drugs 32 (5), 496–507. Cited in: Pubmed; PMID 33735118. doi:10.1097/CAD.0000000000001058
Wu, J., Ni, X., Yu, Z., Wu, S., and Liu, Z. (2022). CRNDE inducing cisplatin resistance through SRSF1/TIA1 signaling pathway in ovarian cancer. Pathol. Res. Pract. 235, 153957. Cited in: Pubmed; PMID 35653925. doi:10.1016/j.prp.2022.153957
Wu, X., Qiu, L., Feng, H., Zhang, H., Yu, H., Du, Y., et al. (2022). KHDRBS3 promotes paclitaxel resistance and induces glycolysis through modulated MIR17HG/CLDN6 signaling in epithelial ovarian cancer. Life Sci. 293, 120328. Epub 20220117. doi:10.1016/j.lfs.2022.120328
Wu, Y., Wang, T., Xia, L., and Zhang, M. (2021). LncRNA WDFY3-AS2 promotes cisplatin resistance and the cancer stem cell in ovarian cancer by regulating hsa-miR-139-5p/SDC4 axis. Cancer Cell. Int. 21 (1), 284. Cited in: Pubmed; PMID 34051810. doi:10.1186/s12935-021-01993-x
Xiao, L., Shi, X. Y., Li, Z. L., Li, M., Zhang, M. M., Yan, S. J., et al. (2021). Downregulation of LINC01508 contributes to cisplatin resistance in ovarian cancer via the regulation of the Hippo-YAP pathway. J. Gynecol. Oncol. 32 (5), e77. No potential conflict of interest relevant to this article was reported. doi:10.3802/jgo.2021.32.e77
Xie, W., Chu, M., Song, G., Zuo, Z., Han, Z., Chen, C., et al. (2022). Emerging roles of long noncoding RNAs in chemoresistance of pancreatic cancer. Semin. Cancer Biol. 83, 303–318. Cited in: Pubmed; PMID 33207266. doi:10.1016/j.semcancer.2020.11.004
Xie, W., Sun, H., Li, X., Lin, F., Wang, Z., and Wang, X. (2021). Ovarian cancer: Epigenetics, drug resistance, and progression. Cancer Cell. Int. 21 (1), 434. Cited in: Pubmed; PMID 34404407. doi:10.1186/s12935-021-02136-y
Xu, M., Zhou, K., Wu, Y., Wang, L., and Lu, S. (2019). Linc00161 regulated the drug resistance of ovarian cancer by sponging microRNA-128 and modulating MAPK1. Mol. Carcinog. 58 (4), 577–587. Epub 20190122. doi:10.1002/mc.22952
Xu, Q., Lin, Y. B., Li, L., and Liu, J. (2020). LncRNA TLR8-AS1 promotes metastasis and chemoresistance of ovarian cancer through enhancing TLR8 mRNA stability. Biochem. Biophys. Res. Commun. 526 (4), 857–864. Cited in: Pubmed; PMID 32278547. doi:10.1016/j.bbrc.2020.03.087
Xu, Q. F., Tang, Y. X., and Wang, X. (2018). LncRNA EBIC promoted proliferation, metastasis and cisplatin resistance of ovarian cancer cells and predicted poor survival in ovarian cancer patients. Eur. Rev. Med. Pharmacol. Sci. 22 (14), 4440–4447. in: Pubmed; PMID 30058681. doi:10.26355/eurrev_201807_15495
Yan, H., Xia, J. Y., and Feng, F. Z. (2017). Long non-coding RNA ENST00000457645 reverses cisplatin resistance in CP70 ovarian cancer cells. Genet. Mol. Res. 16 (1). Epub 20170123. doi:10.4238/gmr16019411
Yang, H., Zhang, X., Zhu, L., Yang, Y., and Yin, X. (2021). YY1-Induced lncRNA PART1 enhanced resistance of ovarian cancer cells to cisplatin by regulating miR-512-3p/CHRAC1 Axis. DNA Cell. Biol. 40 (6), 821–832. Epub 20210524. doi:10.1089/dna.2021.0059
Yu, Y., Zhang, X., Tian, H., Zhang, Z., and Tian, Y. (2018). Knockdown of long non-coding RNA HOTAIR increases cisplatin sensitivity in ovarian cancer by inhibiting cisplatin-induced autophagy. J. BUON 23 (5), 1396–1401. Cited in: Pubmed; PMID 30570864.
Yu, Z., Wang, Y., Wang, B., and Zhai, J. (2020). Metformin affects paclitaxel sensitivity of ovarian cancer cells through autophagy mediated by long noncoding RNASNHG7/miR-3127-5p Axis. Cancer Biother. Radiopharm.. Online ahead of print New Rochelle: Mary Ann Liebert, Inc.. doi:10.1089/cbr.2019.3390
Zhang, C., Wang, M., Shi, C., Shi, F., and Pei, C. (2018). Long non-coding RNA Linc00312 modulates the sensitivity of ovarian cancer to cisplatin via the Bcl-2/Caspase-3 signaling pathway. Biosci. Trends 12 (3), 309–316. Epub 20180628. doi:10.5582/bst.2018.01052
Zhang, J., Liu, J., Xu, X., and Li, L. (2017). Curcumin suppresses cisplatin resistance development partly via modulating extracellular vesicle-mediated transfer of MEG3 and miR-214 in ovarian cancer. Cancer Chemother. Pharmacol. 79 (3), 479–487. Cited in: Pubmed; PMID 28175963. doi:10.1007/s00280-017-3238-4
Zhang, J., Zhang, R., and Ye, Y. (2021). Long non-coding RNA (LncRNA) SNHG7/ Eukaryotic translation initiation factor 4 gamma 2 (EIF4G2) involves in the malignant events of ovarian cancer cells with paclitaxel resistant. Bioengineered 12 (2), 10541–10552. in: Pubmed; PMID 34709112. doi:10.1080/21655979.2021.1999555
Zhang, M., Liu, S., Fu, C., Wang, X., Zhang, M., Liu, G., et al. (2019). LncRNA KB-1471A8.2 overexpression suppresses cell proliferation and migration and antagonizes the paclitaxel resistance of ovarian cancer cells. Cancer biother. Radiopharm. 34 (5), 316–324. Epub 20190320. doi:10.1089/cbr.2018.2698
Zhang, Q., Ding, J., Wang, Y., He, L., and Xue, F. (2022). Tumor microenvironment manipulates chemoresistance in ovarian cancer (Review). Oncol. Rep. 47 (5), 102. Epub 20220401. doi:10.3892/or.2022.8313
Zhang, Y., Ai, H., Fan, X., Chen, S., Wang, Y., and Liu, L. (2020). Knockdown of long non-coding RNA HOTAIR reverses cisplatin resistance of ovarian cancer cells through inhibiting miR-138-5p-regulated EZH2 and SIRT1. Biol. Res. 53 (1), 18. Cited in: Pubmed; PMID 32349783. doi:10.1186/s40659-020-00286-3
Zhang, Y., Guo, J., Cai, E., Cai, J., Wen, Y., Lu, S., et al. (2020). HOTAIR maintains the stemness of ovarian cancer stem cells via the miR-206/TBX3 axis. Exp. Cell. Res. 395 (2), 112218. Epub 20200806. doi:10.1016/j.yexcr.2020.112218
Zhao, H., Wang, A., and Zhang, Z. (2020). LncRNA SDHAP1 confers paclitaxel resistance of ovarian cancer by regulating EIF4G2 expression via miR-4465. J. Biochem. 168 (2), 171–181. in: Pubmed; PMID 32211849. doi:10.1093/jb/mvaa036
Zhao, L., Guo, H., Chen, X., Zhang, W., He, Q., Ding, L., et al. (2022). Tackling drug resistance in ovarian cancer with epigenetic targeted drugs. Eur. J. Pharmacol. 927, 175071. Epub 20220527. doi:10.1016/j.ejphar.2022.175071
Zhao, Y., and Hong, L. (2021). lncRNA-PRLB confers paclitaxel resistance of ovarian cancer cells by regulating RSF1/NF-κB signaling pathway. Cancer biother. Radiopharm. 36 (2), 202–210. Epub 20201106. doi:10.1089/cbr.2019.3363
Zhu, M., Yang, L., and Wang, X. (2020). NEAT1 knockdown suppresses the cisplatin resistance in ovarian cancer by regulating miR-770-5p/PARP1 Axis. Cancer Manag. Res. 12, 7277–7289. The authors report no fundin and no conflicts of interest for this work. Epub 20200814. doi:10.2147/CMAR.S257311
Keywords: lncRNA, epigenetic, chemoresistance, ovarian cancer, cisplatin, paclitaxel, target
Citation: Chen L, Wang J and Liu Q (2022) Long noncoding RNAs as therapeutic targets to overcome chemoresistance in ovarian cancer. Front. Cell Dev. Biol. 10:999174. doi: 10.3389/fcell.2022.999174
Received: 20 July 2022; Accepted: 08 August 2022;
Published: 29 August 2022.
Edited by:
Zichuan Liu, Tianjin University, ChinaReviewed by:
Zhiwei Wang, Wenzhou Medical University, ChinaJianfeng Guo, Huazhong University of Science and Technology, China
Copyright © 2022 Chen, Wang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qian Liu, bGl1cWlhbjAyNjJAamx1LmVkdS5jbg==
 Linjiao Chen
Linjiao Chen Jie Wang
Jie Wang Qian Liu
Qian Liu