
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell Dev. Biol. , 05 October 2022
Sec. Cell Adhesion and Migration
Volume 10 - 2022 | https://doi.org/10.3389/fcell.2022.998373
This article is part of the Research Topic Cell-Cell Adhesion Complexes as Key Conductors of Cell Behavior View all 8 articles
The function and structure of the mammalian epithelial cell layer is maintained by distinct intercellular adhesion complexes including adherens junctions (AJs), tight junctions, and desmosomes. The AJ is most integral for stabilizing cell-cell adhesion and conserving the structural integrity of epithelial tissues. AJs are comprised of the transmembrane protein E-cadherin and cytoplasmic catenin cofactors (α, β, γ, and p120-catenin). One organ where malfunction of AJ is a major contributor to disease states is the mammalian intestine. In the intestine, cell-cell adhesion complexes work synergistically to maintain structural integrity and homeostasis of the epithelium and prevent its malfunction. Consequently, when AJ integrity is compromised in the intestinal epithelium, the ensuing homeostatic disruption leads to diseases such as inflammatory bowel disease and colorectal carcinoma. In addition to their function at the plasma membrane, protein components of AJs also have nuclear functions and are thus implicated in regulating gene expression and intracellular signaling. Within the nucleus, AJ proteins have been shown to interact with transcription factors such as TCF/LEF and Kaiso (ZBTB33), which converge on the canonical Wnt signaling pathway. The multifaceted nature of AJ proteins highlights their complexity in modulating homeostasis and emphasizes the importance of their subcellular localization and expression in the mammalian intestine. In this review, we summarize the nuclear roles of AJ proteins in intestinal tissues; their interactions with transcription factors and how this leads to crosstalk with canonical Wnt signaling; and how nuclear AJ proteins are implicated in intestinal homeostasis and disease.
The gastrointestinal (GI) tract is a hollow continuous tube that begins at the mouth and ends at the anus. Digestion, waste elimination and nutrient absorption occurs over the folded epithelia of the small and large intestines (Michielan and D'Incà, 2015; Peterson and Artis, 2014). The epithelial layer is comprised of intestinal epithelial cells (IEC) that undergo rapid turnover and are replenished, on average, every 3–5 days (Rees et al., 2020).
Similar to other epithelial cell types, the IEC basolateral membrane consists of an intricate network of multi-protein complexes that regulate paracellular permeability via tight junctions (Landy et al., 2016), and cell-cell adhesion via adherens junctions (AJs) and desmosomes (Bondow et al., 2012; Mehta et al., 2015; Spindler et al., 2015; Ungewiß et al., 2017). As the most apical cell-cell adhesion complex, tight junctions contribute to maintaining cell polarity. Their primary role, however, is to establish the epithelial cell barrier and regulate the paracellular passage of ions, water, and macromolecules (Hartsock and Nelson, 2008; Ramena and Ramena, 2018). The AJ is located basal to tight junctions and plays a crucial role in mediating cell-cell adhesion (Hartsock and Nelson, 2008; Ramena and Ramena, 2018). Desmosomes, the basal-most junctional complex, provide structural support to the epithelial layer (Ramena and Ramena, 2018), and further strengthens cell–cell adhesion (Raya-Sandino et al., 2021) (Figure 1).
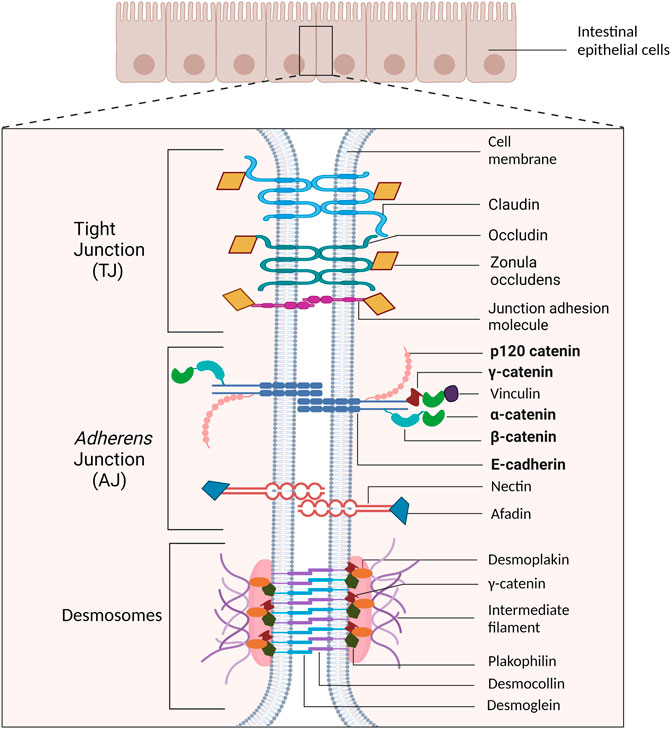
FIGURE 1. Schematic representation of cell-cell adhesion complexes in intestinal epithelial cells. The tight junction (TJ) is the most apical cell adhesion complex and functions to establish polarity and regulate paracellular permeability. Immediately basal to the TJ is the adherens junction (AJ), which plays a key role in mediating cell-cell adhesion. Desmosomal complexes are basal to AJ and are key for strengthening cell-cell adhesion and withstanding mechanical stress. The proteins emphasized in this review are bolded.
Collectively, cell-cell adhesion complexes regulate paracellular permeability in part by limiting passage of ions and molecules. They also restrict translocation of microbes from the gut lumen into the underlying lamina propria, thus serving as a physical barrier that participates in the innate immunity in the intestine (Ali et al., 2020). Indeed, prolonged intestinal barrier disruption leads to increased epithelial permeability and increases the risk of intestinal diseases such as inflammatory bowel disease (IBD) (Lechuga and Ivanov, 2017). Notably, chronic long term intestinal inflammation, concomitant with disruption of cell-cell adhesion, is a major risk factor for the development of colorectal cancer (Rubin et al., 2012; Bujko et al., 2015).
Apart from their roles mediating cell-cell adhesion at the plasma membrane, AJ proteins also have distinct nuclear functions where they participate in cell signaling and transcriptional regulation (van Hengel et al., 1999; Giannini et al., 2000; Kolligs et al., 2000; Cong et al., 2003; Suh and Gumbiner, 2003; Kelly et al., 2004; Reynolds and Roczniak-Ferguson, 2004; Daniel, 2007; Nagel et al., 2010; Valenta et al., 2012; Daugherty et al., 2014; Sun et al., 2014; Su et al., 2015; Aktary et al., 2017; Nagel et al., 2017; Serebryannyy et al., 2017; Zhao et al., 2019). Specifically, nuclear AJ proteins have been shown to interact with transcription factors such as TCF/LEF1 (Maeda et al., 2004; Shang et al., 2017) and Kaiso (Daniel et al., 2002; Jones et al., 2012; Liu et al., 2014) both of which are implicated in regulating canonical Wnt signaling and several human cancers including colorectal cancer (Valenta et al., 2012; Pierre et al., 2019). Interestingly, Kaiso was initially discovered as a binding partner of the catenin p120ctn before it’s role in AJ was fully elucidated (Daniel and Reynolds, 1999; Pierre et al., 2019). In fact, studies to characterize Kaiso as a p120ctn binding partner were pursued because of the first report that β-catenin interacted directly with TCF/LEF1 and localized to the nucleus (Behrens et al., 1996). That seminal publication linking β-catenin and TCF/LEF1 was the first hint that membrane-associated catenin cofactors shuttled in and out of the nucleus to possibly regulate transcription and gene expression. Together the findings of the Birchmeier and Reynolds labs (β-catenin and p120ctn interacting with nuclear transcription factors) laid the foundation for the past two decades of studies seeking to understand how misexpression and/or nucleocytoplasmic localization of AJ proteins contribute to human developmental disorders and cancers.
In this review we discuss cell-cell adhesion complexes in the intestinal epithelial layer with a focus on the nuclear functions of adherens junction proteins, their interaction with the transcription factor Kaiso, their impact on cell signaling pathway regulation, and their potential roles in intestinal homeostasis and disease.
AJs are comprised of cadherin-catenin complexes that mediate cell-cell adhesion and are anchored to the Actin cytoskeleton (Hartsock and Nelson, 2008). The core component of AJs is the transmembrane protein, epithelial cadherin (E-cadherin) (Hartsock and Nelson, 2008). E-cadherin initiates intercellular contact by forming calcium-dependent homophilic interactions with E-cadherin proteins on adjacent cells (Hartsock and Nelson, 2008; Solanas and Batlle, 2011; Maître and Heisenberg, 2013), and are anchored within the cytoplasm via interactions with cofactors called catenins (Yamada et al., 2005; Hartsock and Nelson, 2008). Most of the catenins in the AJ are members of the Armadillo protein family (β-, γ- and p120-catenin) (van Hengel et al., 1999; McCrea and Gottardi, 2016). Armadillo proteins possess several copies of a repeating 42-amino acid motif that facilitates protein-protein interaction and their participation in many essential cellular functions such as nucleocytoplasmic trafficking, transcriptional regulation, and ubiquitination (Tewari et al., 2010).
β-catenin binds the E-cadherin catenin-binding domain via its central Armadillo repeat region (Hartsock and Nelson, 2008; Tewari et al., 2010) and facilitates the connection between the AJ complexes and the Actin cytoskeleton through another molecule, α-catenin (Orsulic et al., 1999). Notably, loss of β-catenin in the intestine does not lead to cell-cell adhesion defects (Fevr et al., 2007). Instead, intestines lacking β-catenin display crypt loss and differentiation defects due to the loss of β-catenin’s signaling role as the downstream effector of canonical Wnt signaling (Fevr et al., 2007). This is in contrast to E-cadherin-null intestines that exhibit defects in cell polarity, cell-cell and cell-matrix adhesions, and an increase in paracellular permeability (Schneider et al., 2010), thus further supporting the critical role of E-cadherin in cell-cell adhesion (Tunggal et al., 2005).
Unlike β-catenin that functions to anchor E-cadherin to the Actin cytoskeleton, p120ctn binds E-cadherin at the juxtamembrane domain (Yap et al., 1998; Hartsock and Nelson, 2008; Hu, 2012) and regulates E-cadherin stability and turnover (Ireton et al., 2002; Davis and Reynolds, 2006; Xiao et al., 2007). Similar to E-cadherin-null intestines (Schneider et al., 2010), intestinal-specific p120ctn depletion in mice leads to cell-cell adhesion defects that result in mucosal erosion and terminal intestinal bleeding into the lumen (Smalley-Freed et al., 2010). Moreover, p120ctn ablation in murine intestines reduces E-cadherin and β-catenin levels resulting in an epithelial barrier defect (Smalley-Freed et al., 2010).
Another Armadillo catenin involved in AJ function is γ-catenin. Like β-catenin, γ-catenin also functions to establish a bridge between E-cadherin and α-catenin (Aktary et al., 2017). While not considered an essential component of AJ per se (Aktary et al., 2017), Lewis et al. showed that γ-catenin plays a vital role in the stability of these adhesive complexes as epithelial cells lacking both γ-catenin and E-cadherin were unable to form AJs. Reintroduction of E-cadherin only, which facilitated the formation of AJs, was insufficient to stabilize the AJs. However, reintroduction of both γ-catenin and E-cadherin resulted in AJ stabilization and facilitated the organization of desmosomal junctions (Aktary et al., 2017; Lewis et al., 1997). Interestingly γ-catenin is also found in desmosomal junctions where it binds to desmosomal cadherins Desmocollin and Desmoglein. However, γ-catenin’s interaction with α-catenin and the desmosomal cadherins appear to be mutually exclusive as there is partial overlap of the binding sites (Chitaev et al., 1998; Zhurinsky et al., 2000).
Unlike β, γ and p120ctn, α-catenin does not have Armadillo repeats (Hulpiau et al., 2013; McCrea and Gottardi, 2016). Instead, α-catenin contains vinculin homology domains and thus does not bind directly to E-cadherin (Herrenknecht et al., 1991; Maiden and Hardin, 2011). Nevertheless, α-catenin functions to connect and anchor the cadherin-catenin complex to the Actin cytoskeleton. α-catenin binds to β-catenin and F-Actin via its N- and C-terminus, respectively, thereby stabilizing the AJ and ensuring tissue integrity (Maiden and Hardin, 2011). Loss of α-catenin thus leads to defects in cell adhesion and has been shown to promote cancer development (Benjamin and Nelson, 2008). Collectively, these studies highlight that α-catenin is necessary for the adhesive role of cadherins (Benjamin and Nelson, 2008; Maiden and Hardin, 2011).
Vinculin, another Actin-binding protein found in cell–matrix adhesions and adherens junctions, binds to both α- and β-catenin, links cell adhesion complexes to the Actin cytoskeleton and functions in the maintenance of the AJ’s integrity (Peng et al., 2010; Peng et al., 2011). However, Peng et al. (2010) showed that only the binding between β-catenin and Vinculin, and not α-catenin, is crucial for Vinculin’s effect on the organization of AJ. They also showed that Vinculin regulates surface E-cadherin expression through its interaction with β-catenin. While loss of Vinculin did not affect the total levels of E-cadherin, there was reduced E-cadherin expression at the cell membrane which led to decreased cell-cell adhesion. The rescue of cell adhesion in this model by the addition of Vinculin underscores the importance of Vinculin in AJs (Peng et al., 2010).
Nectin-afadin complexes can also be found in AJ where they are known to participate in AJ organization and formation (Takai and Nakanishi, 2003; Takai et al., 2006; Meng and Takeichi, 2009). Afadin is an Actin filament-binding protein that binds to Nectin’s cytoplasmic tail and connects it to the Actin cytoskeleton (Takai et al., 2006; Takai et al., 2008a). Afadin also promotes the association of Nectins with other cell-cell adhesion molecules like E-cadherin via its interaction with α-catenin (Tachibana et al., 2000; Takai et al., 2006; Takai et al., 2008a; Takai et al., 2008b). Like E-cadherin, Nectin is a Ca2+-independent transmembrane cell-cell adhesion molecule that forms homophilic and heterophilic interactions between neighbouring cells (Takai and Nakanishi, 2003; Takai et al., 2006; Takai et al., 2008a; Okumura et al., 2018). During AJ formation Nectin regulates the rate of assembly of individual AJ components. At the initial stages of cell-cell contact, Nectin from neighbouring cells trans-dimerizes to generate clusters at these contact sites (Takai et al., 2006). These Nectin clusters then recruit E-cadherin, which also trans-dimerizes and results in the formation of E-cadherin clusters. The Nectin- and E-cadherin clusters eventually fuse and develop into mature AJ (Takai et al., 2006; Takai et al., 2008b). The importance of the Nectin-Afadin network in AJ formation was highlighted when Afadin knockout resulted in the loss of organization of cell-cell junctions and failure of cadherin-based AJ formation (Ikeda et al., 1999; Takai et al., 2008b). Importantly, when the homo- and hetero-trans-dimer of Nectin was inhibited, cadherin-based AJ formation did not occur (Takai et al., 2008a).
Once these AJ complexes bind to Actin, Actin binds to α-actinin, which crosslinks the Actin filaments to create a scaffold that stabilizes the attached junctions. α-actinin thus establishes a connection between the cytoskeleton and transmembrane proteins found in the AJ (Otey and Carpen, 2004; Sjöblom et al., 2008).
Loss of apical junctional complexes perturbs cell-cell adhesion and compromises intestinal barrier integrity. This was first demonstrated by Hermiston and Gordon using their chimeric transgenic mice that expressed dominant negative N-cadherin (NCADΔ) (Hermiston and Gordon, 1995). N-cadherin is a Ca2+-dependent adhesion molecule that functions like E-cadherin to mediate cell–cell adhesion in AJ (Van Patten et al., 2010). NCADΔ mice exhibit reduced endogenous E-cadherin, a defective intestinal epithelial barrier and increased infiltration of bacteria (Hermiston and Gordon, 1995). Similarly, intestinal-specific E-cadherin knockout (VilCre:Cdh1loxP/loxP) causes perinatal death that is likely due to an impaired epithelial barrier. Indeed, by embryonic day 18.5, VilCre:Cdh1loxP/loxP mice already exhibit gaps in the intestinal barrier and deterioration of the epithelium (Bondow et al., 2012). Consistent with these findings, inducible E-cadherin inactivation in adult mice also caused disintegration of the intestinal epithelium (Schneider et al., 2010).
It is well established that IBD patients exhibit enhanced intestinal permeability (Hollander, 1999; Secondulfo et al., 2001; Vivinus-Nébot et al., 2014) which is attributed in part to defective intercellular adhesion (Lechuga and Ivanov, 2017). Indeed, inflamed intestinal tissues in both humans and rodent models of IBD exhibit misexpression of several adhesion-associated proteins, a finding supported by genome-wide association studies of both Crohn’s disease (CD) and ulcerative colitis (UC), reviewed in (Mehta et al., 2015). Interestingly, in inflamed intestinal mucosa of both active CD and UC, a phenomenon known as cadherin switching, where the expression of E-cadherin decreases and the expression of P-cadherin increases, is observed (Sanders et al., 2000; Naydenov et al., 2022). While E-cadherin and P-cadherin are both classical cadherins and expressed in epithelial tissues, E-cadherin is the most highly expressed in healthy epithelial tissues in the gastrointestinal tract (Sanders et al., 2000), while P-cadherin is poorly expressed (Naydenov et al., 2022). Moreover, E-cadherin and P-cadherin have different adhesive properties and participate in different signaling activities, and thus the functional relevance of this switching in the inflamed intestinal mucosa remains to be determined (Naydenov et al., 2022). Regardless, the CDH1 and CDH3 loci, which encode E-cadherin and P-cadherin respectively, are among the various susceptibility loci implicated in IBD (Sanders et al., 2000; Barrett et al., 2009; Mehta et al., 2015).
Given p120ctn’s role in stabilizing E-cadherin at the AJ, it is not surprising that loss of p120ctn has also been associated with intestinal inflammation (Perez-Moreno et al., 2006; Perez-Moreno et al., 2008; Smalley-Freed et al., 2010). Intestinal-specific loss of p120ctn resulted in a concomitant reduction in E-cadherin and impaired cell-cell adhesion (Smalley-Freed et al., 2010). Moreover, p120ctn knockout mice also exhibited histological features of IBD, including crypt hyperplasia, cell-cell adhesion defects, mucosal thickening and increased neutrophil infiltration (Smalley-Freed et al., 2010). Similar findings were also observed in tamoxifen-induced p120ctn mosaic knockout mice, which developed intestinal adenomas as they aged (Smalley-Freed et al., 2011).
Interestingly, colon cancer usually presents with altered gene expression of many cell-cell adhesion proteins, which perturbs cell-cell adhesion and intestinal permeability, and enhances the invasive ability of colon cancer (Tsanou et al., 2008; Bujko et al., 2015). Indeed, reduced localization of β-catenin at the AJ, concomitant with its translocation to the nucleus activates transcription of tumour-promoting genes such as cyclin D1, matrilysin, and c-myc (Wong and Pignatelli, 2002). Collectively, these findings highlight the importance of functional apical junctional complexes in maintaining the normal physiology and homeostasis of the mammalian intestine and how their malfunction contributes to disease.
The fundamental role that membrane-associated cadherin and catenin proteins play in establishing and maintaining intestinal tissue integrity is well documented (Benjamin and Nelson, 2008; Schneider et al., 2010; Smalley-Freed et al., 2010; Schneider and Kolligs, 2015; Aktary et al., 2017). However, their nuclear roles and impacts on transcriptional regulation and epithelial barrier disruption are still being elucidated and are an emerging research area. Below, we summarize findings on the AJ proteins with characterized nuclear functions and their implications for intestinal diseases.
Recently, many published studies have highlighted a nuclear role for several AJ proteins, namely E-cadherin (Su et al., 2015; Zhao et al., 2019), β-catenin (Cong et al., 2003; Suh and Gumbiner, 2003; Valenta et al., 2012), γ-catenin (Kolligs et al., 2000; Nagel et al., 2010; Aktary et al., 2017; Nagel et al., 2017), α-catenin (Giannini et al., 2000; Daugherty et al., 2014; Sun et al., 2014; Serebryannyy et al., 2017), and p120ctn (van Hengel et al., 1999; Kelly et al., 2004; Reynolds and Roczniak-Ferguson, 2004; Daniel, 2007). These nuclear functions are linked to transcriptional regulation that ultimately contributes to intestinal diseases such as IBD and colon cancer (van Hengel et al., 1999; Giannini et al., 2000; Kolligs et al., 2000; Cong et al., 2003; Suh and Gumbiner, 2003; Kelly et al., 2004; Reynolds and Roczniak-Ferguson, 2004; Daniel, 2007; Nagel et al., 2010; Valenta et al., 2012; Daugherty et al., 2014; Sun et al., 2014; Su et al., 2015; Aktary et al., 2017; Nagel et al., 2017; Serebryannyy et al., 2017; Zhao et al., 2019). While many studies have reported that the nuclear functions of AJ proteins are distinct from their roles at the plasma membrane, nuclear localization of AJ proteins often coincides with a concomitant loss of expression at the epithelial cell membrane (Hao et al., 2001; El-Bahrawy et al., 2002; Daniel, 2007; Su et al., 2015). This suggests that nuclear AJ proteins may be a marker of intestinal disease.
In addition to catenin cofactors, the transmembrane protein E-cadherin interacts with several additional proteins of varying functions—these include GTPase regulators, kinases, phosphatases, and Actin dynamics regulators to name a few (Guo et al., 2014; Van Itallie et al., 2014). While some of these E-cadherin binding partners are localized at cell-cell junctions (e.g., Tropomodulin-3) some were found to localize in the cytoplasm (e.g., Filamin-A), within or around the nucleus (e.g., Ran GTPase-activating protein 1) and in vesicles (e.g., Retinoic acid-induced protein 3 (reviewed in Guo et al., 2014). Interestingly, most of the proteins involved in regulating Actin dynamics or function as adaptors localize at cell junctions, in contrast to those involved in transcription, translation and metabolism (Guo et al., 2014). These findings suggest that E-cadherin itself localizes in multiple subcellular compartments (cell membrane, cytoplasm, nucleus), to facilitate its binding to diverse proteins located in distinct subcellular compartments.
Indeed, nuclear localization of E-cadherin has been reported in several cell types, including lung and colon cancer cells (Su et al., 2015; Zhao et al., 2019). Zhao et al. (2019) found an enrichment of E-cadherin in the nuclei of HCT116 and SW480 colon cancer cells and in human colon cancer tissues concomitant with a loss of membrane localization. They also showed that E-cadherin nuclear translocation occurs in a p120ctn-dependent manner as E-cadherin does not possess a nuclear localization signal (NLS) (Zhao et al., 2019). They also showed that p120ctn overexpression enhanced E-cadherin’s nuclear localization in HCT116 colon cancer cells (Zhao et al., 2019). Interestingly, Ferber et al. also found that p120ctn enhanced the nuclear translocation of E-cadherin’s cleaved cytoplasmic domain (Ferber et al., 2008). E-cadherin’s intracellular domain is cleaved by γ-secretase, releasing the E-cadherin C-terminal fragment 2 (E-cad/CTF2) that then translocates into the nucleus where it regulates transcription. While E-cad/CTF2 can bind DNA, this binding is conditional upon it first binding to p120ctn. When bound to p120ctn, E-cad/CTF2 enhances p120ctn's inhibitory transcriptional effects. E-cad/CTF2 is also implicated in apoptosis, however the exact mechanism has yet to be determined (Ferber et al., 2008).
E-cadherin nuclear localization and function can also be regulated by post-translational modifications (Su et al., 2015; Zhao et al., 2019). For example, Su et al. (2015) showed that Src kinase-mediated tyrosine phosphorylation of E-cadherin in cancer stem cells resulted in E-cadherin endocytosis and nuclear translocation. In the nucleus, E-cadherin competed with TCF4 for binding to β-catenin’s Armadillo repeat domain, thus inhibiting β-catenin/TCF4 heterodimerization (Su et al., 2015) (Figure 2 and Table 1 described in more detail in the following section). Subsequently, there was a decrease in canonical Wnt-mediated signaling and metastasis by cancer stem cells (Su et al., 2015). In addition to tyrosine phosphorylation, nuclear E-cadherin can also be acetylated on lysine residues via the acetyltransferase CREB-binding protein (CBP) (Zhao et al., 2019). This prevents protein interactions between E-cadherin and β-catenin, therefore potentiating β-catenin’s transcriptional and oncogenic activities in HCT116 cells. Importantly, increased acetylated E-cadherin correlated with poorer clinical outcomes in colorectal cancers (Zhao et al., 2019). Collectively, these findings highlight a role for nuclear E-cadherin in colorectal tumorigenesis and metastasis (Zhao et al., 2019).
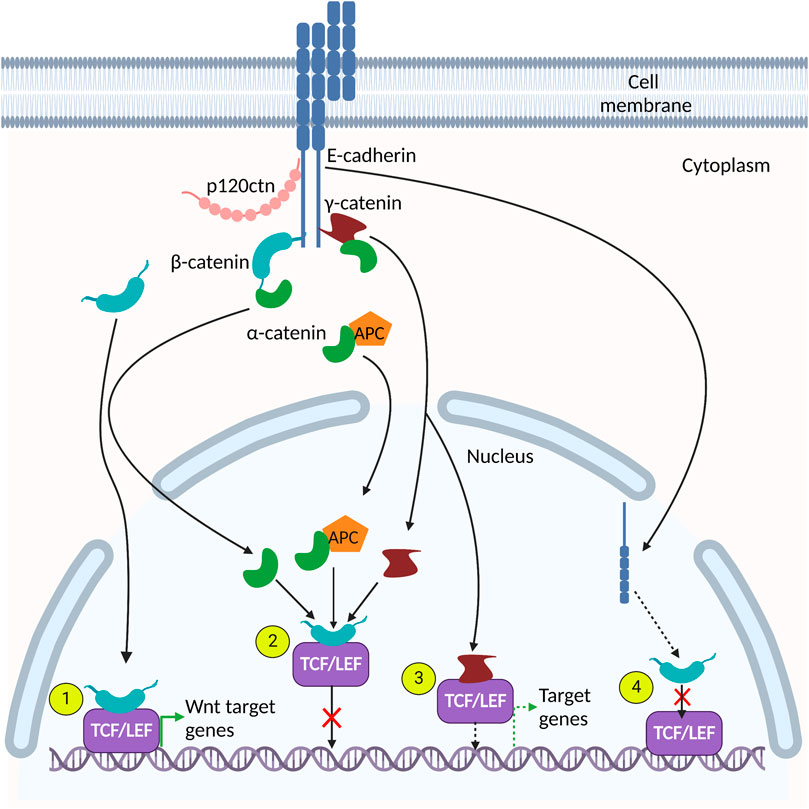
FIGURE 2. Adherens junction (AJ) protein interactions in the nucleus. Many AJ proteins interact with components of the canonical Wnt signaling pathway. (1). β-catenin interacts directly with TCF/LEF to activate Wnt target genes. (2). α-catenin, γ-catenin and α-catenin/APC complexes interact with the β-catenin-TCF/LEF heterodimer and prevents its association with DNA, thereby inhibiting expression of Wnt target genes. (3). γ-catenin binds directly to TCF/LEF and activates TCF/LEF-dependent gene expression. (4). E-cadherin interacts with β-catenin and disrupts its interaction with TCF/LEF cofactors.
The studies above highlight the versatility of E-cadherin and the possibility for both membrane-bound E-cadherin and E-cad/CTF2 to contribute to intestinal diseases and cancer. Additional studies are needed to determine the mechanism by which E-cadherin and E-cad/CTF2 translocate into the nucleus and to ascertain whether the p120ctn NLS plays a role in this process. Moreover, given that nuclear E-cadherin can modulate β-catenin’s transcriptional and oncogenic activities, studies to further understand this interaction and its implications for intestinal diseases are needed.
Of all the catenins involved in the AJ, β-catenin is the most studied and characterized with respect to its nuclear roles. Nuclear accumulation of β-catenin is a key event in canonical Wnt signaling (Shang et al., 2017; Tanaka et al., 2019; Cheng et al., 2019), which is fundamental to maintain the intestinal stem cell niche and integral to IEC differentiation. Importantly, ∼ 90% of colorectal cancers exhibit dysregulated Wnt signaling (Fevr et al., 2007).
β-catenin can be found in four distinct subcellular locations––at the plasma membrane in cell-cell contacts, in the cytoplasm, in the nucleus and at centrosomes (Mbom et al., 2013; Kam and Quaranta, 2009; Bienz, 2004). The membrane-bound pool is stabilized by binding to E-cadherin where it functions to anchor E-cadherin to the Actin cytoskeleton (Bienz, 2004). However, newly synthesized β-catenin and β-catenin released from the AJ—which makes up the cytoplasmic pool—are normally unstable and short-lived (Valenta et al., 2012). In the absence of Wnt signaling, the free cytoplasmic pool of β-catenin is phosphorylated by a destruction complex, comprised of Adenomatous polyposis coli (APC), Axin, Casein kinase 1 (CK1) and Glycogen synthase kinase 3 (GSK3), that targets cytoplasmic β-catenin for proteasomal degradation (Cong et al., 2003; Valenta et al., 2012; Shang et al., 2017). However, upon activation of the canonical Wnt pathway, β-catenin escapes degradation, accumulates in the cytoplasm and then translocates into the nucleus (Cong et al., 2003; Suh and Gumbiner, 2003; Valenta et al., 2012; Arnold et al., 2020). Nuclear translocation of β-catenin occurs independently of importin-β and Ran GTPase, proteins essential for nuclear transport. While β-catenin does not possess an NLS, it was shown to interact directly with nuclear pore proteins via its Armadillo domain and it was postulated that this interaction facilitates its nuclear translocation (Fagotto et al., 1998; Yokoya et al., 1999; Morgan et al., 2014; Anthony et al., 2020). Sharma et al., (2016) also highlighted a role for the hydrophobic amino acids in the N- and C- terminal regions of β-catenin in mediating its nuclear translocation. However, β-catenin was also suggested to utilize NLS-containing chaperone proteins such as BCL9, APC and LEF to facilitate its nuclear translocation (Morgan et al., 2014; Anthony et al., 2020). Recently however, in colon cancers carrying mutations in APC, a member of the importin-β family, IPO11, was found to interact with β-catenin in a Ran-dependent manner, mediating its import into the nucleus (Mis et al., 2020). Interestingly, IPO11-dependent nuclear translocation of β-catenin was not observed in colon cancer cells with wildtype APC (Mis et al., 2020). These findings suggest that the mechanisms whereby β-catenin translocates to the nucleus is complex and context-dependent (Mis et al., 2020).
The tumor suppressor APC interacts with β-catenin in two distinct sub-cellular locations—in the cytoplasm and the nucleus (Hankey et al., 2018). In the cytoplasm APC is important for the degradation of β-catenin (Munemitsu et al., 1995; Yang et al., 2006) while in the nucleus, it negatively regulates the transcriptional activity of β-catenin (Rosin-Arbesfeld et al., 2003; Xiong and Kotake, 2006), and facilitates its nuclear export and subsequent cytoplasmic degradation (Henderson, 2000; Xiong and Kotake, 2006; Hankey et al., 2018). When APC is mutated, it is still capable of binding to β-catenin (Henderson, 2000), and Yang et al. (2006) showed that β-catenin phosphorylation also still occurs. However, Ikeda et al. (1998) showed that phosphorylation of β-catenin also occurs in APC-deficient cells, suggesting that APC does not regulate β-catenin phosphorylation. Studies aimed at further elucidating APC’s function show that APC is necessary for the ubiquitination of β-catenin (Yang et al., 2006; Su et al., 2008) since mutation of APC prevented β-catenin ubiquitination and its subsequent degradation, and led to its nuclear accumulation (Yang et al., 2006).
Once in the nucleus, β-catenin binds TCF/LEF1 transcription factors, which on their own, lack transcriptional activity (Shang et al., 2017). However, the β-catenin/TCF/LEF1 complex forms a functional heterodimer that modulates transcription and upregulates the expression of Wnt target genes (Cong et al., 2003; Suh and Gumbiner, 2003; Valenta et al., 2012) (Figure 2 and Table 1). Interestingly, mutated APC can still enter the nucleus where it binds to and exports β-catenin from the nucleus, thus maintaining the nuclear export function of APC (Henderson, 2000). Collectively, these studies suggest that mutated APC can affect the transcriptional activity of β-catenin but not its expression levels (Henderson, 2000). In this review, we focus on the nuclear roles of β-catenin in mammalian intestines. For additional details on β-catenin nuclear interactions see reviews (Anthony et al., 2020; McCrea and Gottardi, 2016) and (Valenta et al., 2012).
In the intestine, canonical Wnt signaling plays a critical role in the maintenance and renewal of the intestinal epithelium by regulating stem cell proliferation and differentiation (Mah et al., 2016; Perochon et al., 2018). Since nuclear β-catenin is the key effector of canonical Wnt signaling, it plays a key role in intestinal homeostasis. Wnt/β-catenin-mediated transcription maintains many aspects of tissue homeostasis, including cell renewal (maintenance of stemness and the undifferentiated state of intestinal stem cells), and intestinal regeneration after injury (Fevr et al., 2007; Shang et al., 2017), both of which are characterized by increased proliferation within the crypt compartment (Perochon et al., 2018). This is accomplished specifically via the activation of Wnt target genes involved in cell proliferation (e.g., c-myc, cyclin D1). Wnt/β-catenin signaling also contributes to tissue homeostasis via regulation of the intestinal lineage differentiation of transit amplifying cells. (Fevr et al., 2007; Shang et al., 2017).
Elevated canonical Wnt signaling, and therefore increased nuclear β-catenin, perturbs intestinal homeostasis and increases the risk of colon cancer (Shang et al., 2017). In vitro and in vivo models of colorectal neoplasia displayed nuclear localization of β-catenin (Sheng et al., 1998). Nuclear β-catenin was also observed in aberrant crypt foci, which are early neoplastic lesions that precede malignant transformation in the colon (Hao et al., 2001). This finding suggests that aberrant localization of β-catenin is an early event in colorectal tumorigenesis (Sheng et al., 1998; Hao et al., 2001), a concept further strengthened by Sheng et al. (1998) who noted that nuclear β-catenin is a common occurrence in intestinal adenomas. Studies have also found that β-catenin localizes primarily to the plasma membrane in normal colon tissues, but exhibits decreased membrane association and enhanced nuclear localization in intestinal adenomas and carcinomas (Hao et al., 2001; Chen et al., 2008; Stanczak et al., 2011). Indeed, elevated levels of nuclear β-catenin has been observed in ∼ 80% of colorectal cancers (White et al., 2012; Shang et al., 2017), and this high expression of nuclear β-catenin correlated with poor overall survival (Mårtensson et al., 2007; Chen et al., 2008; Chen et al., 2013; Kim et al., 2019) and poor prognosis (Mårtensson et al., 2007; Chen et al., 2013).
While increased nuclear β-catenin localization due to aberrant canonical Wnt signaling is an early and important alteration in CRC progression (Zhang et al., 2016), genomic mutations in β-catenin also occur, albeit more rarely (Kitaeva et al., 1997; Kim et al., 2019; Arnold et al., 2020). Most mutations occur in exon 3 (Kitaeva et al., 1997; Sparks et al., 1998; Samowitz et al., 1999; Johnson et al., 2005; Kim et al., 2019; Arnold et al., 2020) and involve deletions, transitions or transversions, causing a loss of the serine/threonine phosphorylation sites (Kitaeva et al., 1997; Sparks et al., 1998; Samowitz et al., 1999; Arnold et al., 2020). These mutations allow β-catenin to escape proteasomal degradation (Sparks et al., 1998; Kim et al., 2019), leading to increased cytoplasmic accumulation, subsequent nuclear translocation, and ultimately increased Wnt signaling activity (Kim et al., 2019). β-catenin mutations have also been observed in adenomas suggesting that genomic mutations in β-catenin may also be an early occurrence in colorectal tumorigenesis (Sparks et al., 1998; Samowitz et al., 1999).
γ-catenin, also known as Plakoglobin, is another member of the catenin protein family with known nuclear localization and functions (Lifschitz-Mercer et al., 2001; Nagel et al., 2010; Lombardi et al., 2011; Aktary et al., 2013). Nuclear γ-catenin plays a role in biological processes such as adipogenesis and fibrogenesis (Garcia-Gras et al., 2006; Lombardi et al., 2011). Nuclear γ-catenin also interacts with and enhances the transcriptional activity of both the wild type and mutant form of p53, highlighting a role in regulating gene expression (Aktary et al., 2013). Protein interactions between γ-catenin and p53 have been observed in both the cytoplasm and nucleus. In the nucleus, γ-catenin/p53 complexes induced expression of 14-3-3σ, which negatively regulates cell cycle and positively regulates the transcriptional activity of p53 (Aktary et al., 2013).
Whether nuclear γ-catenin promotes or prevents CRC progression remains unresolved. For example, Lifschitz-Mercer et al. (2001) found more nuclear γ-catenin in normal compared to neoplastic human intestinal tissue samples. However, in a study by Nagel et al. (2010) strong nuclear γ-catenin localization was observed in invasive CRC samples relative to normal colon tissues where no nuclear localization was observed. These conflicting findings warrant further characterization of the role that nuclear γ-catenin plays in the malignant transformation and progression of CRC.
The Armadillo domains of γ- and β-catenin share ∼80% amino acid sequence identity and these catenins are sometimes deemed to be paralogs since they are reported to interact with many of the same proteins (Kolligs et al., 2000; Aktary et al., 2017). Indeed, immunoprecipitation of the cytoplasmic and nuclear fractions of γ-catenin-overexpressing HCT116 colorectal carcinoma cells revealed enriched γ-catenin/TCF4 interactions in the nuclear fraction (Pan et al., 2007). Maeda et al. (2004) also detected binding of γ-catenin to both TCF and LEF in immunoprecipitation assays and demonstrated that γ-catenin can alter TCF/LEF-mediated transcriptional regulation (Figure 2 and Table 1). Furthermore, γ-catenin disrupts β-catenin-TCF/LEF complexes, preventing their interaction with DNA and thus attenuating canonical Wnt signaling (Miravet et al., 2002; Aktary et al., 2017) (Figure 2 and Table 1). In agreement with this finding, γ-catenin was found to activate Wnt signaling in DLD-1 cells, and γ-catenin depletion led to decreased tumor cell invasion and increased apoptosis in anchorage-dependent cells (Nagel et al., 2017). Moreover, re-expression of γ-catenin in β-catenin-deficient RKO colon cancer cells resulted in activation of Wnt signaling, but decreased cell proliferation via modulation of the cell cycle (Nagel et al., 2017).
Collectively, these findings suggest that γ-catenin’s role in colon tumorigenesis is context-dependent, and that γ-catenin can influence CRC tumorigenesis via TCF/LEF-dependent and -independent mechanisms (Aktary and Pasdar, 2012; Aktary et al., 2017). Currently, much of what is known about nuclear γ-catenin’s role in CRC was determined in studies using cultured cell lines. Therefore, additional research using complementary approaches such as intestinal organoids and murine models of colorectal cancer and IBD are needed to fully elucidate the role of nuclear γ-catenin in intestinal disease. Moreover, given that p53 mutations are common in colon cancer (Kameyama et al., 2018) and γ-catenin interacts directly with both wild type and mutant p53, a thorough investigation into the role of these interactions in the context of intestinal homeostasis and disease is warranted. Additional research is also needed to elucidate the mechanism of nuclear translocation of γ-catenin.
Numerous studies have reported that α-catenin is capable of trafficking between the cytoplasm and the nucleus (Giannini et al., 2000; El-Bahrawy et al., 2002; Daugherty et al., 2014; Serebryannyy et al., 2017). α-catenin exhibits strong nuclear localization in colon cancer cell lines (Giannini et al., 2000; El-Bahrawy et al., 2002), which was more evident in dispersed rather than confluent cells (El-Bahrawy et al., 2002). This finding suggests that α-catenin nuclear localization is concomitant with a loss of cell-cell adhesive properties. Interestingly, α-catenin showed near mutually exclusive localization between the nucleus and plasma membrane — colorectal adenocarcinoma tissues with significant plasma membrane localization of α-catenin showed minimal nuclear localization and vice versa (El-Bahrawy et al., 2002).
Functionally, loss of α-catenin is implicated in colorectal cancer and cellular dysfunction (Raftopoulos et al., 1998; Cheng et al., 2016). Reduced expression of α-catenin led to increased cell proliferation, possibly due to its indirect interaction with pathways that modulate cell proliferation such as the Wnt signaling pathway (Benjamin and Nelson, 2008; Sun et al., 2014). Notably, α-catenin has been implicated in regulating the canonical Wnt pathway by binding to the β-catenin-TCF complex, disrupting its interaction with DNA (Giannini et al., 2000; Daugherty et al., 2014; Sun et al., 2014; McCrea and Gottardi, 2016; Serebryannyy et al., 2017), and inhibiting transcription of Wnt/β-catenin target genes such as c-myc (Daugherty et al., 2014) (Figure 2 and Table 1). α-catenin’s inhibition of Wnt/β-catenin signaling was also demonstrated by Giannini et al. (2000) who reported that α-catenin depletion increased β-catenin-TCF-dependent transcription. Thus, α-catenin may act to fine-tune the canonical Wnt pathway, since its nuclear localization is dependent on nuclear β-catenin, which itself depends on active Wnt signaling (Daugherty et al., 2014). Additionally, studies show that α-catenin also interacts with cytoplasmic APC protein, recruiting it to the β-catenin-TCF complex in the nucleus and suppressing the expression of Wnt target genes (Choi et al., 2013; Sun et al., 2014) (Figure 2).
Interestingly in colon cancer cells, nuclear α-catenin also interacts with nuclear F-Actin (Daugherty et al., 2014; Serebryannyy et al., 2017). Serebryannyy et al. (2017) showed that upon binding to nuclear F-Actin, α-catenin recruitment to sites of DNA damage was enhanced. This interaction between nuclear α-catenin and F-Actin also highlights a possible role for α-catenin in nuclear Actin dynamics. This possibility is further supported by the finding that nuclear α-catenin induces nuclear F-Actin formation which is associated with changes in chromatin organization (Daugherty et al., 2014). This implicates α-catenin as a regulator of gene expression via its effects on nuclear Actin organization. Indeed α-catenin’s inhibitory effect on the transcription of Wnt target genes also requires its Actin-binding domains (Daugherty et al., 2014).
Collectively, these findings hint that nuclear α-catenin inhibits tumorigenesis, although the molecular mechanisms involved in the nucleo-cytoplasmic shuttling of α-catenin needs to be further explored. Moreover, additional studies are warranted to fully elucidate whether nuclear α-catenin plays a functional role in CRC progression and whether nuclear translocation of α-catenin is a cause or consequence of intestinal dysfunction.
Aside from its role in providing stability to E-cadherin at the plasma membrane, the Src substrate p120ctn also shuttles in and out of the nucleus (Roczniak-Ferguson and Reynolds, 2003), hinting a nuclear role. This catenin has two classical NLSs—one localized in the phosphorylation domain and the other in Armadillo (Arm) repeat 6 (Roczniak-Ferguson and Reynolds, 2003). However, studies have shown that p120ctn nuclear translocation is mediated via alternate sequences in its Arm domain rather than via its conventional NLSs (Roczniak-Ferguson and Reynolds, 2003; Daniel, 2007; van Hengel and van Roy, 2007). Roczniak-Ferguson and Reynolds found that p120ctn Arm repeats 3 and 5 are essential for its entry into the nucleus (Roczniak-Ferguson and Reynolds, 2003). However, Kelly et al. (2004) showed that nuclear localization of p120ctn is mediated via another highly basic NLS located between Arm repeats 6 and 7. These findings suggest that p120ctn utilizes different mechanisms to shuttle into the nucleus, possibly due to it having many functional isoforms (Kelly et al., 2004). While the functional significance of the p120ctn isoforms generated as a result of alternative splicing are yet to be fully elucidated (Roczniak-Ferguson and Reynolds, 2003), it has been postulated that each p120ctn isoform participates in distinct cellular processes and utilizes different mechanisms to shuttle into the nucleus (Kelly et al., 2004). Intriguingly, using a DNA-cellulose binding assay, Ferber et al. showed that nuclear p120ctn can bind directly to DNA (Ferber et al., 2008). If it is experimentally validated that endogenous p120ctn binds directly to DNA, this will add another level of complexity to the role of AJ proteins in regulating transcription, gene expression and tumorigenesis.
Although p120ctn can localize to the nucleus, its nuclear function in intestinal diseases is not as well characterized as its membrane function, or the nuclear functions of β-catenin. However, a few studies have attempted to elucidate the nuclear functions of p120ctn in other pathologies. For example, p120ctn has been implicated in pancreatic cancer where its nuclear localization is associated with tumour malignancy by promoting cancer cell proliferation (Mayerle et al., 2003). Nuclear p120ctn was also found to bind to and relieve repression of the transcriptional regulators RE1-silencing transcription factor (REST) and CoREST, thus activating their target genes (Lee et al., 2014; McCrea and Gottardi, 2016). As REST and CoREST regulate the expression of genes involved in neuronal differentiation, nuclear p120ctn is implicated in neuronal differentiation (Lee et al., 2014; McCrea and Gottardi, 2016). Additionally, p120ctn nuclear localization during junction disintegration in endothelial cells (Beckers et al., 2008) suggests that its nuclear localization may occur as a result of loss of AJ integrity. Thus, the relationship between nuclear p120ctn and intestinal diseases needs further investigation to gain a better understanding of how p120ctn's nuclear roles affect intestinal function and homeostasis, and to discern cause verses consequence.
Chaudhary et al. (2013) has previously shown p120ctn is localized to the nucleus in a Kaiso over-expressing transgenic mouse model that exhibited intestinal inflammation. Whether nuclear p120ctn translocation is a cause or consequence of Kaiso-induced inflammation remains to be resolved. The implications of p120ctn's interaction with the transcription factor Kaiso (van Hengel et al., 1999; Kelly et al., 2004; Reynolds and Roczniak-Ferguson, 2004; Daniel, 2007; McCrea and Gottardi, 2016) (Figure 3), will be further discussed below.
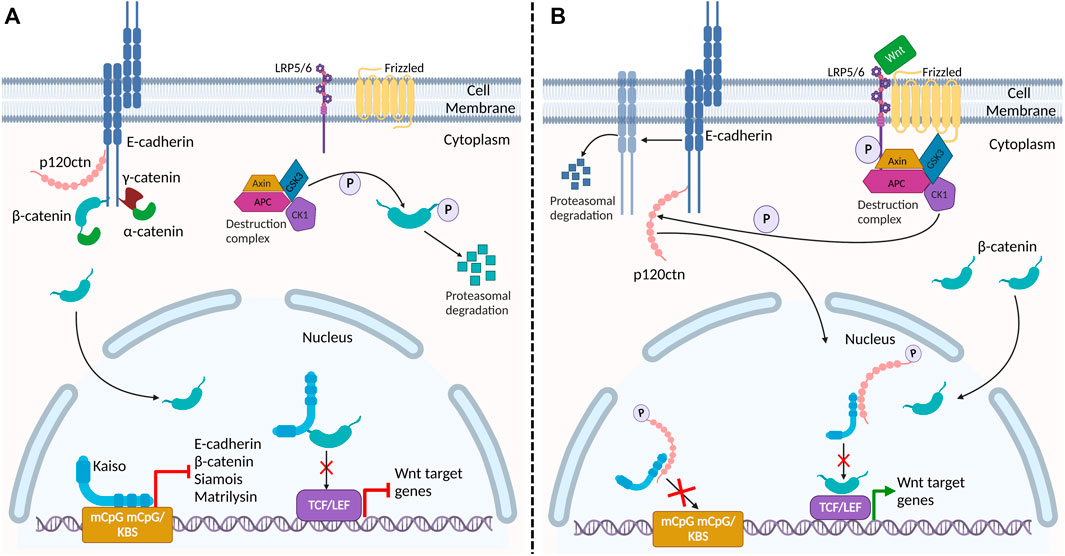
FIGURE 3. Proposed interaction of adherens junction proteins and Kaiso in the Wnt signaling pathway and epithelial barrier stability. (A) In the absence of Wnt signaling, β-catenin and p120ctn bind E-cadherin at the membrane, providing stability to E-cadherin at the plasma membrane. Cytosolic β-catenin is phosphorylated and targeted for degradation by the destruction complex. In the nucleus, Kaiso binds to the promoter region of target genes β-catenin (CTNNB1), E-cadherin (CDH1), Siamois and matrilysin (mmp7) repressing their transcription. Kaiso also binds to β-catenin, preventing its interaction with TCF/LEF. (B) Activation of the canonical Wnt signaling pathway inactivates the destruction complex, resulting in cytosolic accumulation and nuclear translocation of β-catenin. Wnt signaling also triggers CK1, a component of the destruction complex, to phosphorylate p120ctn. Phosphorylated p120ctn then dissociates from E-cadherin and translocates to the nucleus where it relieves Kaiso-mediated regulation of genes. Reduced E-cadherin-associated p120ctn at the plasma membrane results in increased E-cadherin degradation, leading to defective cell adhesion and increased permeability in the epithelial barrier. Given that nuclear p120ctn blocks Kaiso’s DNA binding domain, we propose that this Wnt signal may be a potential mechanism by which p120ctn attenuates Kaiso-mediated regulation of its target genes promoter. Additionally, this may be a potential mechanism by which Wnt signaling promotes the association between β-catenin and TCF/LEF, alleviating Kaiso’s inhibition of this heterodimer formation, and thus activating Wnt target genes.
Over two decades ago, Kaiso was discovered as a binding partner for p120ctn (Daniel and Reynolds, 1999; Kelly and Daniel, 2006). To gain insight into the role of p120ctn in AJs, a yeast-two hybrid screen was conducted to identify p120ctn binding partners. The most frequent gene identified encoded the novel uncharacterized protein that was named Kaiso (Daniel and Reynolds, 1999). Kaiso is a member of the Broad complex, Tramtrack and Bric à brac/Poxvirus and Zinc finger (BTB/POZ) transcription factor family, characterized by an amino-terminal BTB/POZ domain that facilitates protein-protein interactions and three zinc fingers at its carboxy-terminal that mediate DNA binding (Kelly and Daniel, 2006; Daniel, 2007; Pierre et al., 2019). Initial characterization showed that Kaiso binds to DNA via a specific sequence called the Kaiso binding site (KBS) or to methylated CpG dinucleotides (Daniel et al., 2002; Kelly and Daniel, 2006). It was later shown that Kaiso can also bind to a methylated TCTCGCGAGA motif (Raghav et al., 2012; Pierre et al., 2019). As a dual-specificity DNA-binding transcription factor, Kaiso functions as either a transcriptional activator or repressor (Daniel, 2007; Iioka et al., 2009; Pierre et al., 2019), which further adds to the complexity of its role in normal vertebrate development and disease states such as IBD and colon cancer. Notably, several Kaiso target genes are also canonical Wnt target genes (e.g., Cyclin D1, c-Myc and matrilysin) (Park et al., 2005; Spring et al., 2005; Pierre et al., 2019). Since canonical Wnt signaling is essential for normal intestinal homeostasis, studies from our lab and others have explored Kaiso’s role in the mammalian intestine.
Insights into Kaiso’s roles in the intestine were garnered primarily from transgenic and knock-out mouse models. In the context of the ApcMin/+ mouse model of colon cancer, Prokhortchouk et al., found that Kaisonull; ApcMin/+ mice displayed delayed polyp growth and an increased lifespan (Prokhortchouk et al., 2006). This was an unexpected finding given that Kaiso repressed several Wnt target genes and Kaiso overexpression in the Xenopus model rescued the Wnt/β-catenin duplicate axis phenotype (Park et al., 2005; Donaldson et al., 2012; Jiang et al., 2012). To better understand these paradoxical findings, Chaudhary et al. generated an intestinal-specific Kaiso overexpressing mouse model (KaisoTg) (Chaudhary et al., 2013). Surprisingly the KaisoTg mice exhibited morphological changes (e.g., villi blunting, villi fusion, crypt hyperplasia and expansion), intestinal inflammation characterized by neutrophilia, and increased intestinal permeability (Chaudhary et al., 2013; Robinson et al., 2019). When KaisoTg were mated with the ApcMin/+ mouse model of colon cancer, the resulting KaisoTg;ApcMin/+ progeny exhibited ∼3-fold more (but smaller) polyps and reduced life span (Pierre et al., 2015). Together, these findings coupled with the findings of Prokhortchouk et al. support a role for Kaiso in promoting intestinal tumorigenesis (Prokhortchouk et al., 2006; Pierre et al., 2015). This role is further underscored by the fact that ApcMin/+ polyps, human colon cancer and Crohn’s disease tissues display high Kaiso levels relative to normal tissues (Pierre et al., 2015; Robinson et al., 2019).
The contradictory findings observed from Kaiso overexpression and knock-out in different model systems may be due to the presence of the Kaiso-like transcription factor ZBTB4. Like Kaiso, ZBTB4 is a BTB/POZ protein that functions as a transcriptional repressor and like Kaiso, ZBTB4 also binds to DNA via both the KBS and methylated CpG sites (Filion et al., 2006). Thus, in the absence of Kaiso, ZBTB4 may compensate for some Kaiso functions and regulate the expression of essential Kaiso targets genes (Pierre et al., 2019). However, ZBTB4 does not heterodimerize with Kaiso and it does not interact with p120ctn (Filion et al., 2006). Interestingly, in contrast to Kaiso that is highly expressed in many aggressive cancers (Pierre et al., 2019), ZBTB4 is expressed at low levels in several cancers including colorectal cancer (Weber et al., 2008; Xiang et al., 2020) and is postulated to be a tumor suppressor since it inhibits cell proliferation and induces cell cycle arrest and apoptosis in Ewing sarcoma (Yu et al., 2018). As there have been few studies examining the function of ZBTB4 in the mammalian intestine or canonical Wnt signaling, further studies are needed to determine if ZBTB4 does in fact function somewhat redundantly in the absence of Kaiso and its potential roles in intestinal diseases.
Several studies have shown that Kaiso directly or indirectly regulates the expression of various proteins in apical junctional complexes (e.g., ZO-1, E-cadherin) (Jones et al., 2012; Bassey-Archibong et al., 2016; Pierre et al., 2019; Robinson et al., 2019), which has important implications for diseases such as IBD and colon cancer (Pierre et al., 2015; Robinson et al., 2019). Since Kaiso primarily localized to the nucleus (Daniel and Reynolds, 1999), Kaiso’s identification as a p120ctn binding partner was the first hint that p120ctn, like β-catenin, could translocate to the nucleus (Kelly et al., 2004; Daniel, 2007). Studies have since revealed that Kaiso binds preferentially to p120ctn isoform-3A compared to p120ctn isoform-1A in colorectal cancer and lung cancer cells (Daniel and Reynolds, 1999; Liu et al., 2014). p120ctn uses Arm repeats 1-7 to interact with a carboxy-terminal region flanking Kaiso’s DNA-binding zinc finger domain (Daniel and Reynolds, 1999). Consequently, nuclear p120ctn prevents Kaiso’s DNA-binding and inhibits Kaiso’s transcriptional activity and regulation of Wnt target genes such as Siamois and matrilysin (Daniel et al., 2002; Park et al., 2005; Spring et al., 2005; Daniel, 2007) (Figure 3 and Table 1).
Loss of p120ctn at the plasma membrane results in defective AJ structure and integrity, and contributes to intestinal inflammation in mice (Smalley-Freed et al., 2010). In the inflamed intestines of KaisoTg mice, p120ctn exhibits reduced membrane localization and increased nuclear localization, where it presumably interacts with Kaiso (Chaudhary et al., 2013). Importantly, p120ctn also utilizes Arm repeats 1-7 to bind to E-cadherin, suggesting that p120ctn’s association with Kaiso and E-cadherin is mutually exclusive (Daniel and Reynolds, 1995; Daniel and Reynolds, 1999; Daniel et al., 2002). This would affect E-cadherin stability and turnover at the AJ, rendering the integrity of the AJ non-functional. Robinson et al. found that E-cadherin protein expression and membrane localization were decreased in the KaisoTg mice (Robinson et al., 2019). As such, the change in p120ctn’s subcellular localization and concomitant decrease in E-cadherin expression hints at one possible mechanism by which Kaiso disrupts intestinal barrier integrity in KaisoTg mice (Chaudhary et al., 2013; Robinson et al., 2019). Importantly, however, Kaiso also binds to and represses the CDH1 gene (which encodes E-cadherin) in a methylation-dependent manner (Jones et al., 2012; Jones et al., 2014). Kaiso-depletion and demethylation treatment with 5-aza-2′-deoxycytidine in prostate and breast tumour cell lines resulted in a substantial increase in E-cadherin mRNA expression (Jones et al., 2012; Jones et al., 2014). The repressive impact of Kaiso on CDH1 gene expression offers another mechanism by which Kaiso overexpression may potentiate intestinal inflammation and permeability in KaisoTg mice. Whether the intestinal inflammation observed in KaisoTg mice is the result of nuclear p120ctn localization, CDH1 transcriptional repression, or both, warrants further investigation and is one current focus of our laboratory.
In addition to p120ctn, one study revealed that Kaiso also associates with β-catenin (del Valle-Pérez et al., 2016). β-catenin interacts with Kaiso using Arm repeats 1-6, which partially overlaps with the region required for binding TCF (Arm repeats 3–10). In fact, ectopic Kaiso expression has been found to abrogate binding between β-catenin and TCF (Park et al., 2005; del Valle-Pérez et al., 2016), while Kaiso depletion enhanced this interaction (Park et al., 2005). Furthermore, Liu et al. (2014) demonstrated that Kaiso binds to CpG dinucleotides in the CTNNB1 gene promoter (which encodes β-catenin) in a methylation-dependent manner and that high Kaiso expression repressed transcription of β-catenin mRNA. As such, Kaiso directly impacts canonical Wnt signaling at the DNA level (by repressing gene expression of canonical Wnt target genes and CTNNB1), and protein level (by binding to nuclear β-catenin) (Park et al., 2005; Liu et al., 2014). Importantly, p120ctn has been shown to interfere with Kaiso’s repression of the β-catenin/TCF4 transcription complex in SW480 colon cancer cells (del Valle-Pérez et al., 2016). del Valle-Pérez et al. (2016) showed that Wnt stimulation resulted in p120ctn nuclear translocation that disrupted Kaiso-mediated transcriptional repression and potentiated Wnt/β-catenin signaling. The antagonistic relationship between Kaiso and p120ctn suggests that these two proteins may have opposing roles in regulating the Wnt/β-catenin pathway in intestinal tissues (Figure 3). These observations may explain the increased tumour burden, decreased lifespan, and increased Wnt target gene expression in KaisoTg;ApcMin/+ mice (Pierre et al., 2015), although this is yet to be tested empirically.
Collectively, these findings demonstrate that several key AJ proteins interact with Kaiso in the nucleus of intestinal epithelial cells. However, more studies are necessary to fully elucidate how (or whether) Kaiso’s interaction with p120ctn, β-catenin and E-cadherin contribute to the neutrophil-driven inflammation in KaisoTg murine intestines, and the implications this has for diseases like IBD and CRC. Future studies also need to consider the role of ZBTB4 in Kaiso-mediated regulation of gene expression and biological processes such as inflammation, cell proliferation, apoptosis and cell motility.
AJ proteins have emerged as key players in the regulation of cellular signaling in addition to their classical structural functions at the epithelial cell membrane. Imbalances in the structural and signaling properties of AJ proteins can result in the development of intestinal diseases. Given the fundamental role that nuclear β-catenin plays in canonical Wnt signaling and the essential role Wnt plays in intestinal homeostasis, the biological functions of nuclear β-catenin in the intestine are well established. However, as highlighted above, additional investigation into the nuclear roles of other AJ proteins such as p120ctn is deserving of further attention to address some outstanding questions. For example, what are the cue(s) that signal the nuclear translocation of AJ proteins? Is nuclear translocation of AJ proteins a cause or consequence of loss of epithelial barrier integrity? Are all AJ proteins capable of binding to DNA and regulating transcription?
Additionally, several studies have shown that the transcription factor Kaiso plays a role in intestinal inflammation and tumorigenesis in mice, and that these pathologies may be influenced by Kaiso’s regulation of E-cadherin and nuclear interactions with p120ctn and β-catenin. However, the upstream signals that dictate Kaiso’s interaction with AJ proteins, and whether Kaiso interacts with other members of the AJ (e.g., α-and γ-catenin) or other proteins in the apical junctional complex (e.g., ZO-1, claudins) are currently unknown. Furthermore, the possibility of a redundant function between Kaiso and ZBTB4 warrants further investigation to fully understand the role of Kaiso in faulty cell adhesion, Wnt signaling, and intestinal disease.
Studies linking mislocalization of AJ proteins, ectopic Kaiso overexpression, and nuclear interactions between Kaiso and AJ proteins, hint at a possible mechanism by which Kaiso (and possibly other transcription factors) potentiates intestinal inflammation in mammalian tissues. These interactions have vast implications for disease processes where intestinal epithelial integrity is an important and essential factor to maintain intestinal homeostasis. Additional studies utilizing in vivo mouse models will be vital for elucidating and shedding much needed light on the cellular contexts involved in these interactions.
Conceptualization: LRL, SCR, RC, JMD. Co-first authors: LRL and SCR. Writing first draft: LRL, SCR, and RC. Figure creation: LRL. Figure editing: SCR, RC, and JMD. Manuscript review and iterative editing: LRL, SCR, RC, JMD.
These studies were funded by Natural Sciences and Engineering Research Council (NSERC; grant number RGPIN-2020-06822) and Cancer Research Society (CRS; grant number 840883).
The authors thank Drs. Blessing I Bassey-Archibong and Robert W. Cowan for their feedback on the manuscript. All images were created with BioRender.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aktary, Z., Alaee, M., and Pasdar, M. (2017). Beyond cell-cell adhesion: Plakoglobin and the regulation of tumorigenesis and metastasis. Oncotarget 8 (19), 32270–32291. doi:10.18632/oncotarget.15650
Aktary, Z., Kulak, S., Mackey, J., Jahroudi, N., and Pasdar, M. (2013). Plakoglobin interacts with the transcription factor p53 and regulates the expression of 14-3-3σ. J. Cell Sci. 126 (14), 3031–3042. doi:10.1242/jcs.120642
Aktary, Z., and Pasdar, M. (2012). Plakoglobin: Role in tumorigenesis and metastasis. Int. J. Cell Biol. 2012, 189521. doi:10.1155/2012/189521
Ali, A., Tan, H. Y., and Kaiko, G. E. (2020). Role of the intestinal epithelium and its interaction with the microbiota in food allergy. Front. Immunol. 1–12. doi:10.3389/fimmu.2020.604054
Anthony, C. C., Robbins, D. J., Ahmed, Y., and Lee, E. (2020). Nuclear regulation of wnt/β-catenin signaling: It's a complex situation. Genes (Basel) 11 (8), 1–11. doi:10.3390/genes11080886
Arnold, A., Tronser, M., Sers, C., Ahadova, A., Endris, V., Mamlouk, S., et al. (2020). The majority of β-catenin mutations in colorectal cancer is homozygous. BMC Cancer 20 (1), 1038–1110. doi:10.1186/s12885-020-07537-2
Barrett, J. C., Barrett, J. C., Lee, C. W., Lees, N. J., Prescott, C. A., Anderson, A., et al. (2009). Genome-wide association study of ulcerative colitis identifies three new susceptibility loci, including the HNF4A region. Nat. Genet. 41 (12), 1330–1334. doi:10.1038/ng.483
Bassey-Archibong, B. I., Kwiecien, J. M., Milosavljevic, S. B., Hallett, R. M., Rayner, L. G. A., Erb, M. J., et al. (2016). Kaiso depletion attenuates transforming growth factor-β signaling and metastatic activity of triple-negative breast cancer cells. Oncogenesis 5 (3), e208. doi:10.1038/oncsis.2016.17
Beckers, C. M. L., García-Vallejo, J. J., van Hinsbergh, V. W. M., and van Nieuw Amerongen, G. P. (2008). Nuclear targeting of β-catenin and p120ctn during thrombin-induced endothelial barrier dysfunction. Cardiovasc Res. 79 (4), 679–688. doi:10.1093/cvr/cvn127
Behrens, J., von Kries, J. P., Kühl, M., Bruhn, L., Wedlich, D., Grosschedl, R., et al. (1996). Functional interaction of β-catenin with the transcription factor LEF-1. Nature 382 (6592), 638–642. doi:10.1038/382638a0
Benjamin, J. M., and Nelson, W. J. (2008). Bench to bedside and back again: Molecular mechanisms of α-catenin function and roles in tumorigenesis. Seminars Cancer Biol. 18 (1), 53–64. doi:10.1016/j.semcancer.2007.08.003
Bienz, M. (2004). β-Catenin: A pivot between cell adhesion and Wnt signaling. Curr. Biol. 15, 64–67. doi:10.1016/j.cub.2004.12.058
Bondow, B. J., Faber, M. L., Wojta, K. J., Walker, E. M., and Battle, M. A. (2012). E-cadherin is required for intestinal morphogenesis in the mouse. Dev. Biol. 371 (1), 1–12. doi:10.1016/j.ydbio.2012.06.005
Bujko, M., Kober, P., Mikula, M., Ligaj, M., Ostrowski, O., and Siedlecki, J. A. (2015). Expression changes of cell-cell adhesion-related genes in colorectal tumors. Oncol. Lett. 9, 2463–2470. doi:10.3892/ol.2015.3107
Chaudhary, R., Pierre, C. C., Nanan, K., Wojtal, D., Morone, S., Pinelli, C., et al. (2013). The POZ-ZF transcription factor kaiso (ZBTB33) induces inflammation and progenitor cell differentiation in the murine intestine. PLoS One 8 (9), e74160. doi:10.1371/journal.pone.0074160
Chen, S., Liu, J., Li, G., Mo, F., Xu, X., Zhang, T., et al. (2008). Altered distribution of β-catenin and prognostic roles in colorectal carcinogenesis. Scand. J. Gastroenterology 43 (4), 456–464. doi:10.1080/00365520701785194
Chen, Z., He, X., Jia, M., Liu, Y., Qu, D., Wu, D., et al. (2013). β-Catenin overexpression in the nucleus predicts progress disease and unfavourable survival in colorectal cancer: A meta-analysis. PLoS One 8 (5), 1–9. doi:10.1371/journal.pone.0063854
Cheng, X., Xu, X., Chen, D., Zhao, F., and Wang, W. (2019). Therapeutic potential of targeting the Wnt/β-catenin signaling pathway in colorectal cancer. Biomed. Pharmacother. 110, 473–481.
Cheng, G., Yang, S., Zhang, G., Xu, Y., Liu, X., Sun, W., et al. (2016). Lipopolysaccharide-induced α-catenin downregulation enhances the motility of human colorectal cancer cells in an NF-κB signaling-dependent manner. Ott 9, 7563–7571. doi:10.2147/ott.s123986
Chitaev, N. A., Averbakh, A. Z., Troyanovsky, R. B., and Troyanovsky, S. M. (1998). Molecular organization of the desmoglein-plakoglobin complex. J. Cell Sci. 111 (14), 1941–1949. doi:10.1242/jcs.111.14.1941
Choi, S. H., Estarás, C., Moresco, J. J., Yates, J. R., and Jones, K. A. (2013). α-Catenin interacts with APC to regulate β-catenin proteolysis and transcriptional repression of Wnt target genes. Genes Dev. 27 (22), 2473–2488. doi:10.1101/gad.229062.113
Cong, F., Schweizer, L., Chamorro, M., and Varmus, H. (2003). Requirement for a nuclear function of β-catenin in Wnt signaling. Mol. Cell Biol. 23 (23), 8462–8470. doi:10.1128/mcb.23.23.8462-8470.2003
Daniels, D. L., and Weis, W. I. (2005). β-catenin directly displaces Groucho/TLE repressors from Tcf/Lef in Wnt-mediated transcription activation. Nat. Struct. Mol. Biol. 12 (4), 364–371.
Daniel, J. M. (2007). Dancing in and out of the nucleus: p120ctn and the transcription factor kaiso. Biochimica Biophysica Acta (BBA) - Mol. Cell Res. 1773 (1), 59–68. doi:10.1016/j.bbamcr.2006.08.052
Daniel, J. M., and Reynolds, A. B. (1999). The catenin p120 ctn interacts with kaiso, a novel BTB/POZ domain zinc finger transcription factor. Mol. Cell Biol. 19 (5), 3614–3623. doi:10.1128/mcb.19.5.3614
Daniel, J. M., and Reynolds, A. B. (1995). The tyrosine kinase substrate p120cas binds directly to E-cadherin but not to the adenomatous polyposis coli protein or alpha-catenin. Mol. Cell Biol. 15 (9), 4819–4824. doi:10.1128/mcb.15.9.4819
Daniel, J. M., Spring, C. M., Crawford, H. C., Reynolds, A. B., and Baig, A. (2002). The p120ctn-binding partner Kaiso is a bi-modal DNA-binding protein that recognizes both a sequence-specific consensus and methylated CpG dinucleotides. Nucleic Acids Res. 30 (13), 2911–2919. doi:10.1093/nar/gkf398
Daugherty, R. L., Serebryannyy, L., Yemelyanov, A., Flozak, A. S., Yu, H. J., Kosak, S. T., et al. (2014). α-Catenin is an inhibitor of transcription. Proc. Natl. Acad. Sci. U.S.A. 111 (14), 5260–5265. doi:10.1073/pnas.1308663111
Davis, M. A., and Reynolds, A. B. (2006). Blocked acinar development, E-cadherin reduction, and intraepithelial neoplasia upon ablation of p120-catenin in the mouse salivary gland. Dev. Cell 10 (1), 21–31. doi:10.1016/j.devcel.2005.12.004
del Valle-Pérez, B., Casagolda, D., Lugilde, E., Valls, G., Codina, M., Dave, N., et al. (2016). Wnt controls the transcriptional activity of Kaiso through CK1ε-dependent phosphorylation of p120-catenin. J. Cell Sci. 129 (4), 873. doi:10.1242/jcs.186288
Donaldson, N. S., Pierre, C. C., Anstey, M. I., Robinson, S. C., Weerawardane, S. M., and Daniel, J. M. (2012). Kaiso represses the cell cycle gene cyclin D1 via sequence-specific and methyl-CpG-dependent mechanisms. PLoS One 7 (11), e50398. doi:10.1371/journal.pone.0050398
El-Bahrawy, M., Talbot, I., Poulsom, R., and Alison, M. (2002). Variable nuclear localization of α-catenin in colorectal carcinoma. Lab. Invest. 82 (9), 1167–1174. doi:10.1097/01.lab.0000028821.41246.6a
Fagotto, F., Glück, U., and Gumbiner, B. M. (1998). Nuclear localization signal-independent and importin/karyopherin-independent nuclear import of β-catenin. Curr. Biol. 8 (4), 181–190. doi:10.1016/s0960-9822(98)70082-x
Ferber, E. C., Kajita, M., Wadlow, A., Tobiansky, L., Niessen, C., Ariga, H., et al. (2008). A role for the cleaved cytoplasmic domain of E-cadherin in the nucleus. J. Biol. Chem. 283 (19), 12691–12700. doi:10.1074/jbc.M708887200
Fevr, T., Robine, S., Louvard, D., and Huelsken, J. (2007). Wnt/β-Catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol. Cell Biol. 27 (21), 7551–7559. doi:10.1128/mcb.01034-07
Filion, G. J. P., Zhenilo, S., Salozhin, S., Yamada, D., Prokhortchouk, E., and Defossez, P-A. (2006). A family of human zinc finger proteins that bind methylated DNA and repress transcription. Mol. Cell Biol. 26 (1), 169–181. doi:10.1128/mcb.26.1.169-181.2006
Garcia-Gras, E., Lombardi, R., Giocondo, M. J., Willerson, J. T., Schneider, M. D., Khoury, D. S., et al. (2006). Suppression of canonical Wnt/ -catenin signaling by nuclear plakoglobin recapitulates phenotype of arrhythmogenic right ventricular cardiomyopathy. J. Clin. Investigation 116 (7), 2012–2021. doi:10.1172/jci27751
Giannini, A. L., Vivanco, M. D. M., and Kypta, R. M. (2000). α-Catenin inhibits β-catenin signaling by preventing formation of a β-Catenin·T-cell factor·DNA complex. J. Biol. Chem. 275 (29), 21883–21888. doi:10.1074/jbc.m001929200
Guo, Z., Neilson, L. J., Zhong, H., Murray, P. S., Zanivan, S., and Zaidel-Bar, R. (2014). E-cadherin interactome complexity and robustness resolved by quantitative proteomics. Sci. Signal 7 (354), rs7–13. doi:10.1126/scisignal.2005473
Hankey, W., Frankel, W. L., and Groden, J. (2018). Functions of the APC tumor suppressor protein dependent and independent of canonical WNT signaling: Implications for therapeutic targeting. Cancer Metastasis Rev. 37 (1), 159–172. doi:10.1007/s10555-017-9725-6
Hao, X. P., Pretlow, T. G., Rao, J. S., and Pretlow, T. P. (2001). β-Catenin expression is altered in human colonic aberrant crypt foci. Cancer Res. 216, 8085–8088.
Hartsock, A., and Nelson, W. J. (2008). Adherens and tight junctions: Structure, function and connections to the Actin cytoskeleton. Biochimica Biophysica Acta (BBA) - Biomembr. 1778 (3), 660–669. doi:10.1016/j.bbamem.2007.07.012
Henderson, B. R. (2000). Nuclear-cytoplasmic shuttling of APC regulates β-catenin subcellular localization and turnover. Nat. Cell Biol. 2 (9), 653–660. doi:10.1038/35023605
Hermiston, M. L., and Gordon, J. I. (1995). In vivo analysis of cadherin function in the mouse intestinal epithelium: Essential roles in adhesion, maintenance of differentiation, and regulation of programmed cell death. J. Cell Biol. 129 (2), 489–506. doi:10.1083/jcb.129.2.489
Herrenknecht, K., Ozawa, M., Eckerskorn, C., Lottspeich, F., Lenter, M., and Kemler, R. (1991). The uvomorulin-anchorage protein α catenin is a vinculin homologue. Proc. Natl. Acad. Sci. U. S. A. 88 (20), 9156–9160.
Hollander, D. (1999). Intestinal permeability, leaky gut, and intestinal disorders. Curr. Gastroenterol. Rep. 1 (5), 410–416. doi:10.1007/s11894-999-0023-5
Hu, G. (2012). p120-catenin: A novel regulator of innate immunity and inflammation. Crit. Rev. Immunol. 32 (2), 127–138. doi:10.1615/critrevimmunol.v32.i2.20
Hulpiau, P., Gul, I. S., and van Roy, F. (2013). New insights into the evolution of metazoan cadherins and catenins. Prog. Mol. Biol. Transl. Sci. 116, 71–94. doi:10.1016/b978-0-12-394311-8.00004-2
Iioka, H., Doerner, S. K., and Tamai, K. (2009). Kaiso is a bimodal modulator for Wnt/β-catenin signaling. FEBS Lett. [Internet] 583 (4), 627–632. doi:10.1016/j.febslet.2009.01.012
Ikeda, S., Kishida, S., Yamamoto, H., Murai, H., Koyama, S., and Kikuchi, A. (1998). Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta -catenin and promotes GSK-3beta -dependent phosphorylation of beta -catenin. EMBO J. 17 (5), 1371–1384. doi:10.1093/emboj/17.5.1371
Ikeda, W., Nakanishi, H., Miyoshi, J., Mandai, K., Ishizaki, H., Tanaka, M., et al. (1999). Afadin. J. Cell Biol. 146 (5), 1117–1132. doi:10.1083/jcb.146.5.1117
Ireton, R. C., Davis, M. A., van Hengel, J., Mariner, D. J., Barnes, K., Thoreson, M. A., et al. (2002). A novel role for p120 catenin in E-cadherin function. J. Cell Biol. 159 (3), 465–476. doi:10.1083/jcb.200205115
Jiang, G., Wang, Y., Dai, S., Liu, Y., Stoecker, M., Wang, E., et al. (2012). P120-catenin isoforms 1 and 3 regulate proliferation and cell cycle of lung cancer cells via β-catenin and Kaiso respectively. PLoS One 7 (1), e30303. doi:10.1371/journal.pone.0030303
Johnson, V., Volikos, E., Halford, S. E., Sadat, E. T. E., Popat, S., Talbot, I., et al. (2005). Exon 3 -catenin mutations are specifically associated with colorectal carcinomas in hereditary non-polyposis colorectal cancer syndrome. Gut 54 (2), 264–267. doi:10.1136/gut.2004.048132
Jones, J., Wang, H., Karanam, B., Theodore, S., Dean-Colomb, W., Welch, D. R., et al. (2014). Nuclear localization of Kaiso promotes the poorly differentiated phenotype and EMT in infiltrating ductal carcinomas. Clin. Exp. Metastasis 31 (5), 497–510. doi:10.1007/s10585-014-9644-7
Jones, J., Wang, H., Zhou, J., Hardy, S., Turner, T., Austin, D., et al. (2012). Nuclear kaiso indicates aggressive prostate cancers and promotes migration and invasiveness of prostate cancer cells. Am. J. Pathology 181 (5), 1836–1846. doi:10.1016/j.ajpath.2012.08.008
Kam, Y., and Quaranta, V. (2009). Cadherin-bound β-catenin feeds into the Wnt pathway upon adherens junctions dissociation: Evidence for an intersection between β-catenin pools. PLoS One. 4 (2).
Kameyama, H., Nagahashi, T., Shimada, T., Tajima, Y., Ichikawa, K., Nakano, J., et al. (2018). Genomic characterization of colitis-associated colorectal cancer. World J. Surg. Oncol. 16, 121–129. doi:10.1186/s12957-018-1428-0
Kelly, K. F., and Daniel, J. M. (2006). POZ for effect - POZ-ZF transcription factors in cancer and development. Trends Cell Biol. 16 (11), 578–587. doi:10.1016/j.tcb.2006.09.003
Kelly, K. F., Spring, C. M., Otchere, A. A., and Daniel, J. M. (2004). NLS-dependent nuclear localization of p120ctnis necessary to relieve Kaiso-mediated transcriptional repression. J. Cell Sci. 117 (13), 2675–2686. doi:10.1242/jcs.01101
Kim, W. K., Kwon, Y., Jang, M., Park, M., Kim, J., Cho, S., et al. (2019). β-catenin activation down-regulates cell-cell junction-related genes and induces epithelial-to-mesenchymal transition in colorectal cancers. Sci. Rep. 9 (1), 1–15. doi:10.1038/s41598-019-54890-9
Kitaeva, M. N., Grogan, L., Williams, J. P., Dimond, E., Nakahara, K., Hausner, P., et al. (1997). Mutations in beta-catenin are uncommon in colorectal cancer occurring in occasional replication error-positive tumors. Cancer Res. 57 (20), 4478–4481.
Kolligs, F. T., Hu, G., Dang, C. V., and Fearon, E. R. (1999). Neoplastic Transformation of RK3E by Mutant β-Catenin Requires Deregulation of Tcf/Lef Transcription but Not Activation of c- myc Expression. Mol. Cell. Biol. 19 (8), 5696–5706.
Kolligs, F. T., Kolligs, B., Hajra, K. M., Hu, H., Tani, M., Cho, R. C., et al. (2000). γ-Catenin is regulated by the APC tumor suppressor and its oncogenic activity is distinct from that of β-catenin. Genes Dev. 14 (11), 1319–1331. doi:10.1101/gad.14.11.1319
Landy, J., Ronde, E., English, N., Clark, S. K., Hart, A. L., Knight, S. C., et al. (2016). Tight junctions in inflammatory bowel diseases and inflammatory bowel disease associated colorectal cancer. Wjg 22 (11), 3117–3126. doi:10.3748/wjg.v22.i11.3117
Lechuga, S., and Ivanov, A. I. (2017). Disruption of the epithelial barrier during intestinal inflammation: Quest for new molecules and mechanisms. Biochimica Biophysica Acta (BBA) - Mol. Cell Res. 1864 (7), 1183–1194. doi:10.1016/j.bbamcr.2017.03.007
Lee, M., Ji, H., Furuta, Y., Park, J. I., and McCrea, P. D. (2014). p120-catenin regulates REST and CoREST, and modulates mouse embryonic stem cell differentiation. J. Cell Sci. 127 (18), 4037–4051. doi:10.1242/jcs.151944
Lewis, J. S., Wahl, J. K., Sass, K. M., Jensen, P. J., Johnson, K. R., and Wheelock, M. J. (1997). Cross-talk between adherens junctions and desmosomes depends on plakoglobin. J. Cell Biol. 136 (4), 919–934.
Lifschitz-Mercer, B., Amitai, R., Maymon, B. B. S., Shechtman, L., Czernobilsky, B., Leider-Trejo, L., et al. (2001). Nuclear localization of β-catenin and plakoglobin in primary and metastatic human colonic carcinomas, colonic adenomas, and normal colon. Int. J. Surg. Pathol. 9 (4), 273–279. doi:10.1177/106689690100900403
Liu, Y., Dong, Q. Z., Wang, S., Xu, H. T., Miao, Y., Wang, L., et al. (2014). Kaiso interacts with p120-catenin to regulate β-catenin expression at the transcriptional level. PLoS One 9 (2), e87537–9. doi:10.1371/journal.pone.0087537
Lombardi, R., da Graca Cabreira-Hansen, M., Bell, A., Fromm, R. R., Willerson, J. T., and Marian, A. J. (2011). Nuclear plakoglobin is essential for differentiation of cardiac progenitor cells to adipocytes in arrhythmogenic right ventricular cardiomyopathy. Circ. Res. 109 (12), 1342–1353. doi:10.1161/circresaha.111.255075
Maeda, O., Usami, N., Kondo, M., Takahashi, M., Goto, H., Shimokata, K., et al. (2004). Plakoglobin (γ-catenin) has TCF/LEF family-dependent transcriptional activity in β-catenin-deficient cell line. Oncogene 23 (4), 964–972. doi:10.1038/sj.onc.1207254
Mah, A. T., Yan, K. S., and Kuo, C. J. (2016). Wnt pathway regulation of intestinal stem cells. J. Physiol. 594 (17), 4837–4847. doi:10.1113/jp271754
Maiden, S. L., and Hardin, J. (2011). The secret life of α-catenin: Moonlighting in morphogenesis. J. Cell Biol. 195 (4), 543–552. doi:10.1083/jcb.201103106
Maître, J. L., and Heisenberg, C. P. (2013). Three functions of cadherins in cell adhesion. Curr. Biol. 23 (14), 626–633.
Mårtensson, A., Öberg, A., Jung, A., Cederquist, K., Stenling, R., and Palmqvist, R. (2007). Β-Catenin expression in relation to genetic instability and prognosis in colorectal cancer. Oncol. Rep. 17 (2), 447–452.
Mayerle, J., Friess, H., Büchler, M. W., Schnekenburger, J., Weiss, F. U., Zimmer, K. P., et al. (2003). Up-regulation, nuclear import, and tumor growth stimulation of the adhesion protein p120ctn in pancreatic cancer. Gastroenterology 124 (4), 949–960. doi:10.1053/gast.2003.50142
Mbom, B. C., Nelson, W. J., and Barth, A. (2013). β-catenin at the centrosome. BioEssays 35 (9), 804–809. doi:10.1002/bies.201300045
McCrea, P. D., and Gottardi, C. J. (2016). Beyond β-catenin: Prospects for a larger catenin network in the nucleus. Nat. Rev. Mol. Cell Biol. 17 (1), 55–64. doi:10.1038/nrm.2015.3
Mehta, S., Nijhuis, A., Kumagai, T., Lindsay, J., and Silver, A. (2015). Defects in the adherens junction complex (E-cadherin/ β-catenin) in inflammatory bowel disease. Cell Tissue Res. 360 (3), 749–760. doi:10.1007/s00441-014-1994-6
Meng, W., and Takeichi, M. (2009). Adherens junction: Molecular architecture and regulation. Cold Spring Harb. Perspect. Biol. 1, a002899. doi:10.1101/cshperspect.a002899
Michielan, A., and D'Incà, R. (2015). Intestinal permeability in inflammatory bowel disease: Pathogenesis, clinical evaluation, and therapy of leaky gut. Mediat. Inflamm. 2015, 628157. doi:10.1155/2015/628157
Miravet, S., Piedra, J., Miró, F., Itarte, E., Garcıa de Herreros, A. G., and Duñach, M. (2002). The transcriptional factor tcf-4 contains different binding sites for β-catenin and plakoglobin. J. Biol. Chem. 277 (3), 1884–1891. doi:10.1074/jbc.m110248200
Mis, M., O'Brien, S., Steinhart, Z., Lin, S., Hart, T., Moffat, J., et al. (2020). IPO11 mediates βcatenin nuclear import in a subset of colorectal cancers. J. Cell Biol. 219 (2), 1–13. doi:10.1083/jcb.201903017
Morgan, R. G., Ridsdale, J., Tonks, A., and Darley, R. L. (2014). Factors affecting the nuclear localization of β-catenin in normal and malignant tissue. J. Cell. Biochem. 115 (8), 1351–1361. doi:10.1002/jcb.24803
Munemitsu, S., Albert, I., Souza, B., Rubinfeld, D., and Polakis, P. (1995). Regulation of intracellular beta-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc. Natl. Acad. Sci. U.S.A. 92 (7), 3046–3050. doi:10.1073/pnas.92.7.3046
Nagel, J. M., Kriegl, L., Horst, D., Engel, J., Gautam, S., Mantzoros, C. S., et al. (2010). γ-Catenin is an independent prognostic marker in early stage colorectal cancer. Int. J. Colorectal Dis. 25 (11), 1301–1309. doi:10.1007/s00384-010-1046-y
Nagel, J. M., Lahm, H., Ofner, A., Göke, B., and Kolligs, F. T. (2017). γ-Catenin acts as a tumor suppressor through context-dependent mechanisms in colorectal cancer. Int. J. Colorectal Dis. 32 (9), 1243–1251. doi:10.1007/s00384-017-2846-0
Naydenov, N. G., Lechuga, S., Zalavadia, A., Mukherjee, P. K., Gordon, I. O., Skvasik, D., et al. (2022). P-cadherin regulates intestinal epithelial cell migration and mucosal repair, but is dispensable for colitis associated colon cancer. Cells 11 (9). doi:10.3390/cells11091467
Okumura, N., Kagami, T., Fujii, K., Nakahara, M., and Koizumi, N. (2018). Involvement of nectin-afadin in the adherens junctions of the corneal endothelium. Cornea 37 (5), 633–640. doi:10.1097/ico.0000000000001526
Orsulic, S., Huber, O., Aberle, H., Arnold, S., and Kemler, R. (1999). E-cadherin binding prevents beta-catenin nuclear localization and beta-catenin/LEF-1-mediated transactivation. J. Cell Sci. 112 (8), 1237–1245. doi:10.1242/jcs.112.8.1237
Otey, C. A., and Carpen, O. (2004). ?-actinin revisited: A fresh look at an old player. Cell Motil. Cytoskelet. 58, 104–111. doi:10.1002/cm.20007
Pan, H., Gao, F., Papageorgis, P., Abdolmaleky, H. M., Faller, D. V., and Thiagalingam, S. (2007). Aberrant activation of γ-catenin promotes genomic instability and oncogenic effects during tumor progression. Cancer Biol. Ther. 6 (10), 1638–1643. doi:10.4161/cbt.6.10.4904
Park, J. I., Kim, S. W., Lyons, J. P., Ji, H., Nguyen, T. T., Cho, K., et al. (2005). Kaiso/p120-Catenin and TCF/β-Catenin complexes coordinately regulate canonical Wnt gene targets. Dev. Cell 8 (6), 843–854. doi:10.1016/j.devcel.2005.04.010
Peng, X., Cuff, L. E., Lawton, C. D., and DeMali, K. A. (2010). Vinculin regulates cell-surface E-cadherin expression by binding to β-catenin. J. Cell Sci. 123 (4), 567–577. doi:10.1242/jcs.056432
Peng, X., Nelson, E. S., Maiers, J. L., and DeMali, K. A. (2011). New insights into vinculin function and regulation. Chem. Res. Toxicol. 28, 191–231. doi:10.1016/b978-0-12-386043-9.00005-0
Perez-Moreno, M., Davis, M. A., Wong, E., Pasolli, H. A., Reynolds, A. B., and Fuchs, E. (2006). P120-Catenin mediates inflammatory responses in the skin. Cell 124 (3), 631–644. doi:10.1016/j.cell.2005.11.043
Perez-Moreno, M., Song, W., Pasolli, H. A., Williams, S. E., and Fuchs, E. (2008). Loss of p120 catenin and links to mitotic alterations, inflammation, and skin cancer. Proc. Natl. Acad. Sci. U.S.A. 105 (40), 15399–15404. doi:10.1073/pnas.0807301105
Perochon, J., Carroll, L. R., and Cordero, J. B. (2018). Wnt signaling in intestinal stem cells: Lessons from mice and flies. Genes (Basel) 9 (3), 1–19. doi:10.3390/genes9030138
Peterson, L. W., and Artis, D. (2014). Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 14 (3), 141–153. doi:10.1038/nri3608
Pierre, C. C., Hercules, S. M., Yates, C., and Daniel, J. M. (2019). Dancing from bottoms up - roles of the POZ-ZF transcription factor kaiso in cancer. Biochimica Biophysica Acta (BBA) - Rev. Cancer 1871 (1), 64–74. [Internet]. doi:10.1016/j.bbcan.2018.10.005
Pierre, C. C., Longo, J., Mavor, M., Milosavljevic, S. B., Chaudhary, R., Gilbreath, E., et al. (2015). Kaiso overexpression promotes intestinal inflammation and potentiates intestinal tumorigenesis in ApcMin/+ mice. Biochimica Biophysica Acta (BBA) - Mol. Basis Dis. 1852 (9), 1846–1855. doi:10.1016/j.bbadis.2015.06.011
Prokhortchouk, A., Sansom, O., Selfridge, J., Caballero, I. M., Salozhin, S., Aithozhina, D., et al. (2006). Kaiso-deficient mice show resistance to intestinal cancer. Mol. Cell Biol. 26 (1), 199–208. doi:10.1128/mcb.26.1.199-208.2006
Raftopoulos, I., Davaris, P., Karatzas, G., Karayannacos, P., and Kouraklis, G. (1998). Level of α-catenin expression in colorectal cancer correlates with invasiveness, metastatic potential, and survival. J. Surg. Oncol. 68 (2), 92–99. doi:10.1002/(sici)1096-9098(199806)68:2<92:aid-jso4>3.0.co;2-f
Raghav, S. K., Waszak, S. M., Krier, I., Gubelmann, C., Isakova, A., Mikkelsen, T. S., et al. (2012). Integrative genomics identifies the corepressor SMRT as a gatekeeper of adipogenesis through the transcription factors C/EBPβ and KAISO. Mol. Cell 46 (3), 335–350. doi:10.1016/j.molcel.2012.03.017
Ramena, Y., and Ramena, G. (2018). Cell-cell junctions and epithelial differentiation. J. Morphol. Anat. 2 (1), 111.
Raya-Sandino, A., Luissint, A. C., Kusters, D. H. M., Narayanan, V., Flemming, S., Garcia-Hernandez, V., et al. (2021). Regulation of intestinal epithelial intercellular adhesion and barrier function by desmosomal cadherin desmocollin-2. MBoC 32 (8), 753–768. doi:10.1091/mbc.e20-12-0775
Rees, W. D., Tandun, R., Yau, E., Zachos, N. C., and Steiner, T. S. (2020). Regenerative intestinal stem cells induced by acute and chronic injury: The saving grace of the epithelium? Front. Cell Dev. Biol. 8, 583919–584015. doi:10.3389/fcell.2020.583919
Reynolds, A. B., and Roczniak-Ferguson, A. (2004). Emerging roles for p120-catenin in cell adhesion and cancer. Oncogene 23 (487), 7947–7956. doi:10.1038/sj.onc.1208161
Robinson, S. C., Chaudhary, R., Jiménez-Saiz, R., Rayner, L. G. A., Bayer, L., Jordana, M., et al. (2019). Kaiso-induced intestinal inflammation is preceded by diminished E-cadherin expression and intestinal integrity. PLoS One 14, e0217220–17. doi:10.1371/journal.pone.0217220
Roczniak-Ferguson, A., and Reynolds, A. B. (2003). Regulation of p120-catenin nucleocytoplasmic shuttling activity. J. Cell Sci. 116 (20), 4201–4212. doi:10.1242/jcs.00724
Rosin-Arbesfeld, R., Cliffe, A., Brabletz, T., and Bienz, M. (2003). Nuclear export of the APC tumour suppressor controls β-catenin function in transcription. EMBO J. 22 (5), 1101–1113. doi:10.1093/emboj/cdg105
Rubin, D. C., Shaker, A., and Levin, M. S. (2012). Chronic intestinal inflammation: Inflammatory bowel disease and colitis-associated colon cancer. Front. Immunol. 3, 107–110. doi:10.3389/fimmu.2012.00107
Samowitz, W. S., Powers, M. D., Spirio, L. N., Nollet, F., van Roy, F., and Slattery, M. L. (1999). Beta-catenin mutations are more frequent in small colorectal adenomas than in larger adenomas and invasive carcinomas. Cancer Res. 59 (7), 1442–1444.
Sanders, D. S. A., Perry, I., Hardy, R., and Jankowski, J. (2000). Aberrant P-cadherin expression is a feature of clonal expansion in the gastrointestinal tract associated with repair and neoplasia. J. Pathol. 190 (5), 526–530. doi:10.1002/(sici)1096-9896(200004)190:5<526:aid-path564>3.0.co;2-9
Schneider, M. R., Dahlhoff, M., Horst, D., Hirschi, B., Trülzsch, K., Müller-Höcker, J., et al. (2010). A key role for E-cadherin in intestinal homeostasis and paneth cell maturation. PLoS One 5 (12), e14325. doi:10.1371/journal.pone.0014325
Schneider, M. R., and Kolligs, F. T. (2015). E-cadherin's role in development, tissue homeostasis and disease: Insights from mouse models. BioEssays 37 (3), 294–304. doi:10.1002/bies.201400141
Secondulfo, M., de Magistris, L., Fiandra, R., Caserta, L., Belletta, M., Tartaglione, M. T., et al. (2001). Intestinal permeability in Crohn's disease patients and their first degree relatives. Dig. Liver Dis. 33 (8), 680–685. doi:10.1016/s1590-8658(01)80045-1
Serebryannyy, L. A., Yemelyanov, A., Gottardi, C. J., and de Lanerolle, P. (2017). Nuclear α-catenin mediates the DNA damage response via β-catenin and nuclear Actin. J. Cell Sci. 130 (10), 1717–1729. doi:10.1242/jcs.199893
Shang, S., Hua, F., and Hu, Z. W. (2017). The regulation of β-catenin activity and function in cancer: Therapeutic opportunities. Oncotarget 8 (20), 33972–33989. doi:10.18632/oncotarget.15687
Sharma, M., Jamieson, C., Lui, C., and Henderson, B. R. (2016). Distinct hydrophobic "patches" in the N- and C-tails of beta-catenin contribute to nuclear transport. Exp. Cell Res. 348 (2), 132–145. doi:10.1016/j.yexcr.2016.09.009
Sheng, H., Shao, J., Williams, C. S., Pereira, M. A., Taketo, M. M., Oshima, M., et al. (1998). Nuclear translocation of beta-catenin in hereditary and carcinogen- induced intestinal adenomas. Carcinogenesis 19 (4), 543–549. doi:10.1093/carcin/19.4.543
Sjöblom, B., Salmazo, A., and Djinović-Carugo, K. (2008). α-Actinin structure and regulation. Cell Mol. Life Sci. 65 (17), 2688–2701.
Smalley-Freed, W. G., Efimov, A., Burnett, P. E., Short, S. P., Davis, M. A., Gumucio, D. L., et al. (2010). P120-Catenin is essential for maintenance of barrier function and intestinal homeostasis in mice. J. Clin. Invest. 120 (6), 1824–1835. doi:10.1172/jci41414
Smalley-Freed, W. G., Efimov, A., Short, S. P., Jia, P., Zhao, Z., Washington, M. K., et al. (2011). Adenoma formation following limited ablation of p120-catenin in the mouse intestine. PLoS One 6 (5), e19880. doi:10.1371/journal.pone.0019880
Solanas, G., and Batlle, E. (2011). Control of cell adhesion and compartmentalization in the intestinal epithelium. Exp. Cell Res. 317 (19), 2695–2701. doi:10.1016/j.yexcr.2011.07.019
Sparks, A. B., Morin, P. J., Vogelstein, B., and Kinzler, K. W. (1998). Mutational analysis of the APC/beta-catenin/Tcf pathway in colorectal cancer. Cancer Res. 58 (6), 1130–1134.
Spindler, V., Meir, M., Vigh, B., Flemming, S., Hütz, K., Germer, C. T., et al. (2015). Loss of desmoglein 2 contributes to the pathogenesis of crohn's disease. Inflamm. Bowel Dis. 21 (10), 2349–2359. doi:10.1097/MIB.0000000000000486
Spring, C. M., Kelly, K. F., O'Kelly, I., Graham, M., Crawford, H. C., and Daniel, J. M. (2005). The catenin p120ctn inhibits Kaiso-mediated transcriptional repression of the β-catenin/TCF target gene matrilysin. Exp. Cell Res. 305 (2), 253–265. doi:10.1016/j.yexcr.2005.01.007
Stanczak, A., Stec, R., Bodnar, L., Olszewski, W., Cichowicz, M., Kozlowski, W., et al. (2011). Prognostic significance of wnt-1, β-catenin and E-cadherin expression in advanced colorectal carcinoma. Pathol. Oncol. Res. 17 (4), 955–963. doi:10.1007/s12253-011-9409-4
Su, Y. J., Chang, Y. W., Lin, W. H., Liang, C. L., and Lee, J. L. (2015). An aberrant nuclear localization of E-cadherin is a potent inhibitor of Wnt/β-catenin-elicited promotion of the cancer stem cell phenotype. Oncogenesis 4 (6), e157. doi:10.1038/oncsis.2015.17
Su, Y. Y., Fu, C., Ishikawa, S., Stella, A., Kojima, M., Shitoh, K., et al. (2008). APC is essential for targeting phosphorylated β-catenin to the scfβ-TrCP ubiquitin ligase. Mol. Cell 32 (5), 652–661. doi:10.1016/j.molcel.2008.10.023
Suh, E. K., and Gumbiner, B. M. (2003). Translocation of β-catenin into the nucleus independent of interactions with FG-rich nucleoporins. Exp. Cell Res. 290 (2), 447–456. doi:10.1016/s0014-4827(03)00370-7
Sun, Y., Zhang, J., and Ma, L. (2014). α-catenin. Cell Cycle 13 (15), 2334–2339. doi:10.4161/cc.29765
Tachibana, K., Nakanishi, H., Mandai, K., Ozaki, K., Ikeda, W., Yamamoto, Y., et al. (2000). Two cell adhesion molecules, nectin and cadherin, interact through their cytoplasmic domain-associated proteins. J. Cell Biol. 150 (5), 1161–1176. doi:10.1083/jcb.150.5.1161
Takai, Y., Ikeda, W., Ogita, H., and Rikitake, Y. (2008). The immunoglobulin-like cell adhesion molecule nectin and its associated protein afadin. Annu. Rev. Cell Dev. Biol. 24, 309–342. doi:10.1146/annurev.cellbio.24.110707.175339
Takai, Y., Irie, K., Shimizu, K., Sakisaka, T., and Ikeda, W. (2006). Nectins and nectin-like molecules: Roles in cell adhesion, migration, and polarization. Cancer Sci. 94 (8), 655–667. doi:10.1111/j.1349-7006.2003.tb01499.x
Takai, Y., Miyoshi, J., Ikeda, W., and Ogita, H. (2008). Nectins and nectin-like molecules: Roles in contact inhibition of cell movement and proliferation. Nat. Rev. Mol. Cell Biol. 9 (8), 603–615. doi:10.1038/nrm2457
Takai, Y., and Nakanishi, H. (2003). Nectin and afadin: Novel organizers of intercellular junctions. J. Cell Sci. 116 (1), 17–27. doi:10.1242/jcs.00167
Tanaka, H., Kawaguchi, M., Shoda, S., Miyoshi, T., Iwasaki, R., and Hyodo, F. (2019). Nuclear accumulation of β-catenin in cancer stem cell radioresistance and stemness in human colon cancer. Anticancer Res. 39 (12), 6575–6583.
Tetsu, O., and McCormick, F. (1999). Beta-Catenin regulates expression of cyclinD1 in colon carcinoma cells. Nature. 398, 422.
Tewari, R., Bailes, E., Bunting, K. A., and Coates, J. C. (2010). Armadillo-repeat protein functions: Questions for little creatures. Trends Cell Biol. 20 (8), 470–481. doi:10.1016/j.tcb.2010.05.003
Tsanou, E., Peschos, D., Batistatou, A., Charalabopoulos, A., and Charalabopoulos, K. (2008). The E-cadherin adhesion molecule and colorectal cancer. A global literature approach. Anticancer Res. 28, 3815–3826.
Tunggal, J. A., Helfrich, I., Schmitz, A., Schwarz, H., Günzel, D., Fromm, M., et al. (2005). E-cadherin is essential for in vivo epidermal barrier function by regulating tight junctions. EMBO J. 24 (6), 1146–1156. doi:10.1038/sj.emboj.7600605
Ungewiß, H., Vielmuth, F., Suzuki, S. T., Maiser, A., Harz, H., Leonhardt, H., et al. (2017). Desmoglein 2 regulates the intestinal epithelial barrier via p38 mitogen-activated protein kinase. Sci. Rep. 7 (1), 6329–6410. doi:10.1038/s41598-017-06713-y
Valenta, T., Hausmann, G., and Basler, K. (2012). The many faces and functions of β-catenin. Eur. Mol. Biol. Organ 31 (12), 2714–2736. doi:10.1038/emboj.2012.150
van Hengel, J., and van Roy, F. (2007). Diverse functions of p120ctn in tumors. Biochimica Biophysica Acta (BBA) - Mol. Cell Res. 1773 (1), 78–88. doi:10.1016/j.bbamcr.2006.08.033
van Hengel, J., Vanhoenacker, P., Staes, K., and van Roy, F. (1999). Nuclear localization of the p120 ctn Armadillo-like catenin is counteracted by a nuclear export signal and by E-cadherin expression. Proc. Natl. Acad. Sci. U.S.A. 96 (14), 7980–7985. doi:10.1073/pnas.96.14.7980
Van Itallie, C. M., Tietgens, A. J., Aponte, A., Fredriksson, K., Fanning, A. S., Gucek, M., et al. (2014). Biotin ligase tagging identifies proteins proximal to E-cadherin, including lipoma preferred partner, a regulator of epithelial cell-cell and cell-substrate adhesion. J. Cell Sci. 127 (4), 885–895. doi:10.1242/jcs.140475
Van Patten, K., Parkash, V., and Jain, D. (2010). Cadherin expression in gastrointestinal tract endometriosis: Possible role in deep tissue invasion and development of malignancy. Mod. Pathol. 23 (1), 38–44. doi:10.1038/modpathol.2009.127
Vivinus-Nébot, M., Frin-Mathy, G., Bzioueche, H., Dainese, R., Bernard, G., Anty, R., et al. (2014). Functional bowel symptoms in quiescent inflammatory bowel diseases: Role of epithelial barrier disruption and low-grade inflammation. Gut 63 (5), 744–752. doi:10.1136/gutjnl-2012-304066
Weber, A., Marquardt, J., Elzi, D., Forster, N., Starke, S., Glaum, A., et al. (2008). Zbtb4 represses transcription of P21CIP1 and controls the cellular response to p53 activation. EMBO J. 27 (11), 1563–1574. doi:10.1038/emboj.2008.85
White, B. D., Chien, A. J., and Dawson, D. W. (2012). Dysregulation of wnt/β-catenin signaling in gastrointestinal cancers. Gastroenterology 142 (2), 219–232. doi:10.1053/j.gastro.2011.12.001
Wong, N. A. C. S., and Pignatelli, M. (2002). β-Catenin-A linchpin in colorectal carcinogenesis? Am. J. Pathology 160 (2), 389–401. doi:10.1016/s0002-9440(10)64856-0
Xiang, T., He, K., Wang, S., Chen, W., and Li, H. (2020). Expression of zinc finger and BTB domain-containing 4 in colorectal cancer and its clinical significance. Cmar 12, 9621–9626. doi:10.2147/cmar.s266529
Xiao, K., Oas, R. G., Chiasson, C. M., and Kowalczyk, A. P. (2007). Role of p120-catenin in cadherin trafficking. Biochimica Biophysica Acta (BBA) - Mol. Cell Res. 1773 (1), 8–16. doi:10.1016/j.bbamcr.2006.07.005
Xiong, Y., and Kotake, Y. (2006). No exit strategy? No problem: APC inhibits β-catenin inside the nucleus: Figure 1. Genes Dev. 20 (6), 637–642. doi:10.1101/gad.1413206
Yamada, S., Pokutta, S., Drees, F., Weis, W. I., and Nelson, W. J. (2005). Deconstructing the cadherin-catenin-Actin complex. Cell 123 (5), 889–901. doi:10.1016/j.cell.2005.09.020
Yang, J., Zhang, W., Evans, P. M., Chen, X., He, X., and Liu, C. (2006). Adenomatous polyposis coli (APC) differentially regulates β-catenin phosphorylation and ubiquitination in colon cancer cells. J. Biol. Chem. 281 (26), 17751–17757. doi:10.1074/jbc.m600831200
Yap, A. S., Niessen, C. M., and Gumbiner, B. M. (1998). The juxtamembrane region of the cadherin cytoplasmic tail supports lateral clustering, adhesive strengthening, and interaction with p120(ctn). J. Cell Biol. 141 (3), 779–789.
Yokoya, F., Imamoto, N., Tachibana, T., and Yoneda, Y. (1999). β-Catenin can Be transported into the nucleus in a ran-unassisted manner. MBoC 10 (4), 1119–1131. doi:10.1091/mbc.10.4.1119
Yu, Y., Shang, R., Chen, Y., Li, J., Liang, Z., Hu, J., et al. (2018). Tumor suppressive ZBTB4 inhibits cell growth by regulating cell cycle progression and apoptosis in Ewing sarcoma. Biomed. Pharmacother. 100, 108–115. doi:10.1016/j.biopha.2018.01.132
Zhang, S., Wang, Z., Shan, J., Yu, X., Li, L., Lei, R., et al. (2016). Nuclear expression and/or reduced membranous expression of β-catenin correlate with poor prognosis in colorectal carcinoma. Med. (United States). 95 (49), e5546. doi:10.1097/md.0000000000005546
Zhao, Y., Yu, T., Zhang, N., Chen, J., Zhang, P., Li, S., et al. (2019). Nuclear E-cadherin acetylation promotes colorectal tumorigenesis via enhancing β-catenin activity. Mol. Cancer Res. 17 (2), 655–665. doi:10.1158/1541-7786.mcr-18-0637
Keywords: Adherens Junction, E-cadherin, catenins, Kaiso, Wnt signaling, inflammatory bowel disease, colon cancer
Citation: Lessey LR, Robinson SC, Chaudhary R and Daniel JM (2022) Adherens junction proteins on the move—From the membrane to the nucleus in intestinal diseases. Front. Cell Dev. Biol. 10:998373. doi: 10.3389/fcell.2022.998373
Received: 19 July 2022; Accepted: 29 August 2022;
Published: 05 October 2022.
Edited by:
Antonis Kourtidis, Medical University of South Carolina, United StatesReviewed by:
Andrei Ivanov, Cleveland Clinic, United StatesCopyright © 2022 Lessey, Robinson, Chaudhary and Daniel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juliet M. Daniel, ZGFuaWVsakBtY21hc3Rlci5jYQ==
†Present Address: Shaiya C. Robinson, School of Interdisciplinary Science, McMaster University, Hamilton, ON, Canada
‡These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.