- 1Department of Microbiology and Immunology, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
- 2Department of Biostatistics, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
- 3Center for AIDS Research, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
- 4UNC HIV Cure Center, Institute of Global Health and Infectious Diseases, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
- 5Department of Dermatology, Indiana University, Indianapolis, IN, United States
HIV-associated Kaposi’s sarcoma (KS), which is caused by Kaposi’s sarcoma-associated herpesvirus, usually arises in the context of uncontrolled HIV replication and immunosuppression. However, disease occasionally occurs in individuals with durable HIV viral suppression and CD4 T cell recovery under antiretroviral therapy (ART). The underlying mechanisms associated with this phenomenon are unclear. Suppression of viral infections can be mediated by CD8 T cells, which detect infected cells via their T cell receptor and the CD8 coreceptor. However, CD8 T cells exhibit signs of functional exhaustion in untreated HIV infection that may not be fully reversed under ART. To investigate whether KS under ART was associated with phenotypic and functional perturbations of CD8 T cells, we performed a cross-sectional study comparing HIV-infected individuals with persistent KS under effective ART (HIV+ KS+) to HIV-infected individuals receiving effective ART with no documented history of KS (HIV+ KSneg). A subset of T cells with low cell surface expression of CD8 (“CD8dim T cells”) was expanded in HIV+ KS+ compared with HIV+ KSneg participants. Relative to CD8bright T cells, CD8dim T cells exhibited signs of senescence (CD57) and mitochondrial alterations (PGC-1α, MitoTracker) ex vivo. Mitochondrial activity (MitoTracker) was also reduced in proliferating CD8dim T cells. These findings indicate that an expanded CD8dim T cell population displaying features of senescence and mitochondrial dysfunction is associated with KS disease under ART. CD8 coreceptor down-modulation may be symptomatic of ongoing disease.
Introduction
Kaposi’s sarcoma (KS), a cancer of epithelial and endothelial cells characterized by dark plaques and nodules, is a common AIDS-related morbidity in individuals with untreated HIV infection (Shiels et al., 2011). However, while the etiologic agent of KS, Kaposi’s sarcoma-associated herpesvirus (KSHV), generates a lifelong infection, it rarely causes disease in immunocompetent individuals. The introduction of HIV antiretroviral therapy (ART), and resulting immune recovery in treated individuals, has been accompanied by a steep decline in HIV-associated KS cases (Eltom et al., 2002; Franceschi et al., 2008). However, a minority of HIV-infected KSHV-seropositive individuals develop KS despite durable HIV suppression and CD4 T cell recovery under ART (Maurer et al., 2007; Mani et al., 2002; von Braun et al., 2014). The underlying causes of this phenomenon are not currently understood. The recent discovery that latent KSHV can be reactivated by proteins from SARS-CoV-2 and some anti-COVID-19 drugs further underscores the need to better understand the control and pathogenesis of KS (Chen et al., 2021).
CD8 T cells are major mediators of anti-viral immunity, detecting virus-infected cells via the T cell receptor (TCR) and CD8 coreceptor. KSHV-infected individuals harbor CD8 T cells capable of secreting antiviral cytokines and killing cells expressing KSHV antigens in vitro (Osman et al., 1999; Bihl et al., 2007; Bihl et al., 2009; Robey et al., 2009; Lepone et al., 2010; Lepone et al., 2017; Lambert et al., 2006). These KSHV-specific CD8 T cells are detected at higher frequencies in KSHV-seropositive individuals who do not have KS compared with individuals with active disease (Lambert et al., 2006). These observations suggest that in immunocompetent individuals, CD8 T cells may play a lifelong role in preventing KSHV from causing disease. However, during untreated progressive HIV infection, T cells exhibit signs of functional exhaustion that may not be fully reversed by ART (Trautmann et al., 2006; Day et al., 2006; Day et al., 2007; Migueles et al., 2009; Gaiha et al., 2014). Notably, these defects include metabolic alterations such as impaired mitochondrial oxidative phosphorylation and increased reliance on glycolysis (Korencak et al., 2019; Angin et al., 2019). Mitochondrial metabolism is crucial for lasting CD8 T cell control of viral infections: while effector T cells upregulate glycolysis to rapidly generate ATP, memory T cells use mitochondrial oxidative phosphorylation to support their long-term persistence (Cham and Gajewski, 2005; Jacobs et al., 2008; van der Windt et al., 2012; van der Windt et al., 2013; Chang et al., 2013). Indeed, loss of mitochondrial function is associated with senescence, a state where T cells lose proliferative capacity (Callender et al., 2021). Defects in CD8 T cell metabolic fitness in HIV-infected individuals on ART could be particularly detrimental in the tumor microenvironment, where rapidly proliferating malignant cells create an environment of hypoxia and mitochondrial stress (Kim et al., 2007; Delgado et al., 2010; Siska et al., 2017).
We investigated the possibility that altered CD8 T cell phenotype and metabolism could be associated with persistent KS in HIV-infected individuals on ART by comparing HIV-infected individuals with and without KS. We observed an expanded population of CD8dim T cells in individuals with HIV-associated KS. These cells expressed elevated levels of the senescence marker CD57, lower levels of the mitochondrial master-regulator PGC-1α, and contained mitochondria with a dysfunctional phenotype. Persistent KS is therefore associated with the expansion of a subset of CD8 T cells with metabolic hallmarks of senescence.
Materials and methods
Study participants
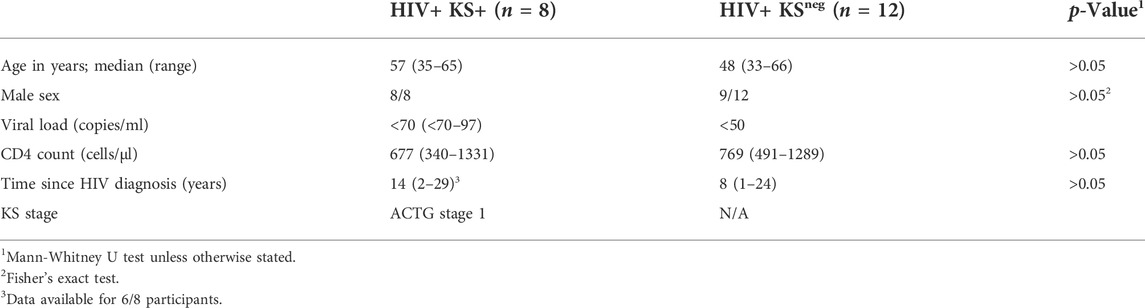
HIV-1-infected participants with biopsy-confirmed KS (“HIV+ KS+”) were recruited from primary care practices in San Francisco and the adjacent counties, UCLA-related primary care clinics, and the Study of the Consequences of the Protease Inhibitor Era (SCOPE). All participants had received ART (including protease inhibitors, NNRTIs, and early integrase inhibitors) and maintained plasma viral loads <75 copies/ml for ≥2 years. 7/8 HIV+ KS+ participants had been receiving ART at the time of KS presentation. CD4 T cell counts were ≥340/µl. All participants had stage 1 tumors according to ACTG criteria (tumor confined to skin and/or lymph nodes and/or minimal oral disease) (Krown et al., 1989). The study was approved by the Institutional Review Board of the University of California, San Francisco (approval no. 10-02850). HIV-1-infected participants with no documented history of KS (“HIV+ KSneg”) were recruited from the UNC HIV Clinical Trials Unit. Participants had received ART for ≥2 years and maintained plasma viral loads <50 copies/ml and CD4 T cell counts >300/µl for ≥6 months. Participant characteristics are detailed in Table 1. Initial collection of UNC samples was approved by the UNC Institutional Review Board (ethics numbers 11-0228; 14-0741; and 15-1626). Retrospective use of all samples was approved by the UNC Institutional Review Board (ethics number 17-2415).
Ethical approval for retrospective sample use
This study exclusively used retrospective, de-identified samples. Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the participants was not required to participate in this study in accordance with the national legislation and the institutional requirements.
CD8 T cell phenotyping
Cryopreserved peripheral blood mononuclear cells (PBMC) were rested in R10 medium (RPMI 1640 supplemented with 10% fetal bovine serum; penicillin/streptomycin; 2 mM l-glutamine; 10 mM sodium pyruvate; and 10 mM HEPES) at 37°C overnight. PBMC were stained with Zombie NIR viability dye, then cell surface antibodies CD3-PerCP-Cy5.5 (clone UCHT1); CD4-PE-Cy5 (OKT4); CD8-Brilliant Violet 510 (SK1); CD14 (M5E2), CD16 (3G8), CD19 (HIB19), and CD56 (HCD56)-Brilliant Violet 650; CD45RO-PE (UCHL1); and CD57-PE-Dazzle 594 (HNK-1) (all Biolegend). Cells were fixed and permeabilized using eBioscience’s FoxP3 Transcription Factor Staining Buffer Set and stained intracellularly for 1 hour with T-bet-Brilliant Violet 421 (4B10; Biolegend); Eomes-eFluor 660 (WD 1928; eBioscience); and rabbit polyclonal anti-PGC-1α antibody (PA5-38022; Invitrogen). They were treated with normal serum block (Biolegend) to prevent non-specific binding of secondary antibody, then stained with goat anti-rabbit PE-Cy7 secondary antibody (Santa Cruz Biotechnology). A control sample stained with secondary antibody but not PGC-1α primary antibody was included for each donor to confirm the specificity of secondary antibody binding (Supplementary Figure S1). Samples were acquired on an LSRII flow cytometer and analyzed using FlowJo 10 (BD Biosciences). Live lymphocytes were defined by dim staining with Zombie viability dye, forward scatter height vs. area (to identify single events), and forward scatter versus side scatter. CD8 T lymphocytes were defined as CD3+ CD4− CD14/16/19/56- and CD8 bright or dim. For phenotypic markers, positive events were gated using fluorescence minus one controls (Supplementary Figure S1).
CD8 T cell proliferation
Cryopreserved PBMC were rested overnight, then pulsed under rotation with 5 µM carboxyfluorescein succinimidyl ester (CFSE, Biolegend). Staining was quenched with ice-cold R10. Cells were stimulated with vehicle (0.5% DMSO) or 3 μg/ml phytohaemagglutinin (PHA, Sigma) for 5 days at 37°C. PBMC were stained with Zombie NIR; CD3-PE-Cy7; CD4-BV421; CD8-BV510; CD14, CD16, CD19, and CD56-BV650 (clones as previously; Biolegend). To assess mitochondrial activity, cells were stained with 25 nM MitoTracker® Deep Red (MTDR, Thermo Fisher) at 37°C. Cells were acquired on an LSR Fortessa and analyzed using FlowJo 10 and Modfit LT 4 (Verity Software House). Proliferation was assessed using proliferation index, defined as the mean number of proliferative cycles undergone by each proliferating cell (Roederer, 2011). MTDRhigh cells were gated using a previously described method (Clutton et al., 2019). Briefly, after excluding outliers (the brightest and dimmest 0.1% of events), the fluorescence intensities of the brightest and dimmest cells were used to calculate the fluorescence range (brightest—dimmest). Cells that fell within the top 90% of this range were considered MTDRhigh (Supplementary Figure S2).
Mitochondrial phenotyping
Cryopreserved PBMC were rested overnight, then stained with viability dye and surface antibodies CD3-PE-Cy7; CD4-BV421; CD8-BV510; and CD14, CD16, CD19, CD56-BV650 as above (clones as previously; Biolegend). Cells were stained at 37°C with 100 nM MitoTracker® Green (MTG) and 5 µM MitoSOX® to measure mitochondrial mass and mitochondrial superoxide, respectively (Thermo Fisher). Cells were immediately acquired, unfixed on an LSR Fortessa and analyzed using FlowJo 10. MTGhigh cells were gated as above (Clutton et al., 2019).
Statistical analysis
Data were analyzed using GraphPad Prism version 8. Between-group differences were analyzed using an exact, two-sided Mann-Whitney U test. Within-individual differences between CD8bright and CD8dim T cells were analyzed using an exact, two-sided Wilcoxon signed-rank test. The monotonic (strictly increasing or decreasing) relationship between variables such as CD8dim percentage and proliferation index was assessed using Spearman’s rank correlation coefficient.
Results
CD8dim T cells are expanded in HIV+ individuals with persistent KS, and exhibit features of senescence
We compared the phenotype of CD8 T cells between HIV-infected individuals with persistent KS under ART (HIV+ KS+) and HIV-infected individuals receiving ART with no documented history of KS (HIV+ KSneg). CD8 T cells were defined as CD3+ CD8+ CD4− CD14/CD16/CD56 to exclude NKT cells (Supplementary Figure S1). We observed two populations of CD8 T cells: A CD8bright subset with high cell surface expression of CD8 and a CD8dim subset with lower CD8 surface expression (Figure 1A). CD3ε, which forms part of the TCR complex, was also expressed at a lower level on the surface of CD8dim cells compared with CD8bright cells (median of differences = −4358 MFI; Wilcoxon signed-rank test p = 0.007; Supplementary Figure S3). The CD8dim subset was significantly expanded, as a percentage of total CD8 T cells, in HIV+ KS+ compared with HIV+ KSneg participants (difference of medians = 12.28%; Mann-Whitney test p = 0.0006; Figure 1B). Since highly differentiated T cells accumulate during chronic untreated infections (Papagno et al., 2004; Unemori et al., 2013), we next compared the differentiation state of CD8bright and CD8dim cells within participants. CD57 expression was higher on CD8dim than CD8bright T cells (median of differences = 8%; Wilcoxon signed-rank test p = 0.008), indicating that late-differentiated or senescent cells were overrepresented in the CD8dim population (Figure 1C and Supplementary Figure S3). Supporting this observation, Eomesodermin (Eomes), a transcription factor expressed in terminal memory cells, was expressed in a higher percentage of CD8dim than CD8bright cells (median of differences = 13.5%; Wilcoxon signed-rank test p = 0.008, Figure 1D and Supplementary Figure S3) (Paley et al., 2012; Hasley, et al., 2013). T-bet, a transcription factor expressed by effector cells, was expressed at similar levels in CD8bright and CD8dim cells (Supplementary Figure S3).
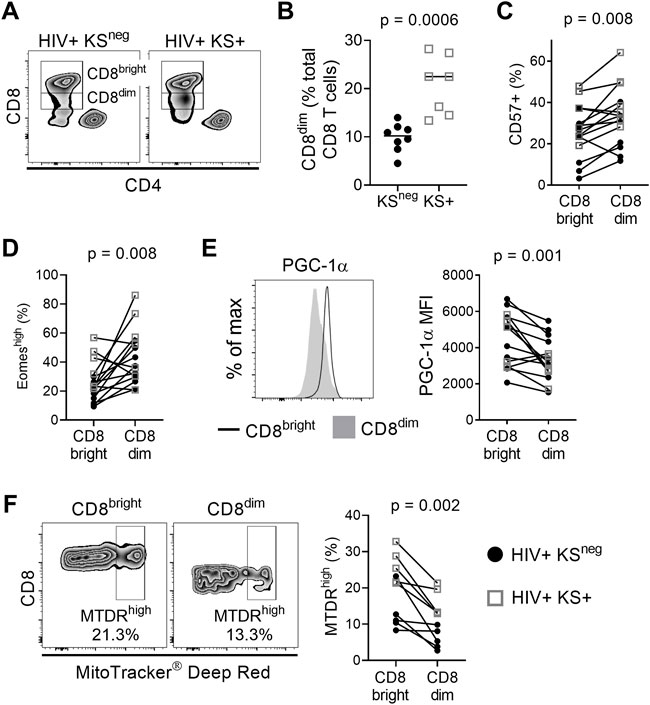
FIGURE 1. CD8dim T cells with low mitochondrial activity are expanded in individuals with persistent KS. (A) Representative plots showing CD8bright and CD8dim T cells in a HIV+ KSneg and a HIV+ KS+ participant. (B) The frequency of CD8dim T cells (as a percentage of total CD8 T cells) is significantly elevated in HIV+ individuals with persistent KS under ART (KS+; n = 7) compared with the KSneg group (n = 8) (difference of medians = 12.28%; Mann-Whitney test). (C) A significantly higher percentage of CD8dim T cells express CD57 compared with CD8bright T cells (median of differences = 8%; Wilcoxon signed-rank test). (D) A significantly higher percentage of CD8dim T cells express Eomes compared with CD8bright T cells (median of differences = 13.5%; Wilcoxon signed-rank test). (E) Expression of the mitochondrial master regulator PGC-1α is significantly reduced in CD8dim T cells (median of differences = −789 MFI; Wilcoxon signed-rank test). (F) The frequency of MitoTracker Deep Red high cells is significantly lower for CD8dim T cells compared with CD8bright T cells (median of differences = −8.99%; Wilcoxon signed-rank test). Gray open squares, HIV+ KS+ participants; black circles, HIV+ KSneg participants.
CD8dim T cells have an altered mitochondrial phenotype
The development, persistence, and recall function of memory CD8 T cells is highly dependent on mitochondrial metabolism (van der Windt et al., 2012; van der Windt et al., 2013). Compared with CD8bright T cells, expression of the nuclear-encoded mitochondrial master regulator PGC-1α was significantly reduced in CD8dim T cells (median of differences = −789 MFI; Wilcoxon signed-rank test p = 0.001; Figure 1E). To further investigate mitochondrial phenotype in CD8bright vs. CD8dim T cells, we used MitoTracker® Deep Red (MTDR), which selectively binds actively respiring mitochondria (Bengsch et al., 2016). MTDRhigh cells were defined using a previously described objective gating strategy (Clutton et al., 2019); Supplementary Figure S2). The frequency of MTDRhigh cells was lower in the CD8dim T cell population than in CD8bright T cells (median of differences = −8.99%; Wilcoxon signed-rank test p = 0.002; Figure 1F). This was not due to a loss of mitochondrial mass, as the frequency of MitoTracker® Greenhigh cells was higher in CD8dim than CD8bright cells (Supplementary Figure S4). However, mitochondrial superoxide, which can contribute to senescence, was elevated in CD8dim compared to CD8bright T cells (MitoSOX®; Supplementary Figure S4) (Miwa et al., 2022). These observations indicate that individuals with HIV-associated KS have an expanded population of CD8dim T cells with a highly differentiated/senescent phenotype and signs of mitochondrial dysfunction.
Mitochondrial activity is reduced in CD8dim proliferating cells
We next examined whether cell surface expression of the CD8 coreceptor was related to replicative capacity and mitochondrial activity in proliferating cells. PBMC were stimulated with the polyclonal stimulus PHA for five days and proliferation was measured by CFSE dilution (Figure 2A). Proliferative capacity was reported as proliferation index (the mean number of proliferative cycles undergone by each responding cell).
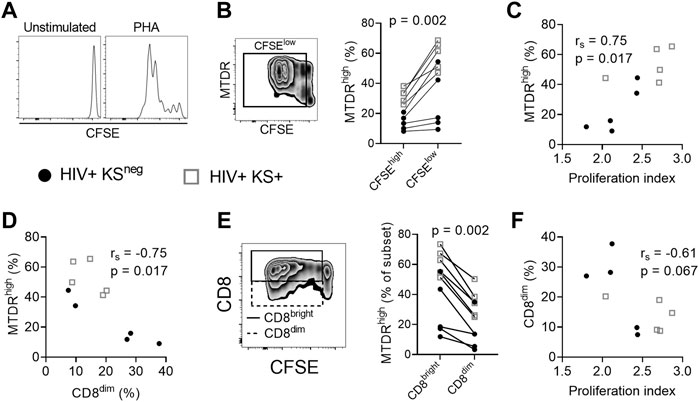
FIGURE 2. Mitochondrial activity is reduced in CD8dim proliferating cells. (A) Histograms showing CFSE dilution in unstimulated and PHA-stimulated CD8 T cells. (B) MitoTracker Deep Red (MTDR) fluorescence in PHA-stimulated proliferating (CFSElow) cells. A significantly higher percentage of proliferating (CFSElow) CD8 T cells are MTDRhigh compared with non-proliferating (CFSEhigh) CD8 T cells, indicating that proliferating cells have higher mitochondrial activity (n = 10; median of differences = 23.25%; Wilcoxon signed-rank test). (C) Positive association between mitochondrial activity (% MTDRhigh) of all CD8 T cells in the culture and the proliferation index of proliferating cells in response to PHA stimulation (Spearman correlation). (D) Negative association between mitochondrial activity and the frequency of CD8dim T cells following stimulation with PHA (Spearman correlation). (E) The frequency of MTDRhigh cells, comparing CD8bright and CD8dim proliferating (CFSElow) cells. CD8dim proliferating cells are significantly less likely to be MTDRhigh, indicating lower mitochondrial activity (median of differences = −23.35%; Wilcoxon signed-rank test). (F) Proliferation index of PHA-stimulated CD8 T cells versus the frequency of CD8dim T cells in the culture (Spearman correlation). Gray open squares, HIV+ KS+ participants; black circles, HIV+ KSneg participants.
CD8 T cells that had proliferated (CFSElow) exhibited greater mitochondrial activity (% of cells MTDRhigh) than non-proliferating cells (CFSEhigh) (median of differences = 23.25%; Wilcoxon signed-rank test p = 0.002; Figure 2B). There was a strong positive correlation between mitochondrial activity of all CD8 T cells and proliferation index at day five, demonstrating the importance of mitochondrial respiration to CD8 T cell proliferation (rs = 0.75, p = 0.017; Figure 2C) (Chang et al., 2013). Conversely, there was a strong negative correlation between mitochondrial activity of all CD8 T cells and the percentage of CD8dim T cells in the culture at five days (rs = −0.75, p = 0.017; Figure 2D), suggesting that low CD8 expression is associated with reduced mitochondrial activity. Further supporting this hypothesis, CD8dim cells that had proliferated exhibited lower mitochondrial activity (% MTDRhigh) than CD8bright cells that had proliferated (median of differences = −23.35%; Wilcoxon signed-rank test p = 0.002; Figure 2E). There was also evidence of a moderate negative correlation between the percentage of CD8dim T cells in the culture and proliferation index (rs = −0.61, p = 0.067; Figure 2F). Collectively these results indicate that low surface expression of CD8 is associated with reduced mitochondrial respiration and replicative capacity of proliferating CD8 T cells.
Discussion
The underlying causes of KS in a minority of HIV-infected individuals with durable viral suppression and CD4 T cell recovery under ART are unknown. Here, we have identified an expanded population of CD8dim T cells with phenotypic characteristics of senescence and mitochondrial dysfunction in these individuals.
The CD8 coreceptor amplifies signals through the TCR (van den Berg et al., 2007; Wooldridge et al., 2010). Following antigenic stimulation (e.g., by a virus-infected cell), CD8, along with the TCR, is downregulated from the cell surface, possibly to limit the strength or duration of signaling (Park et al., 2007; Lachmann et al., 2012; Balyan et al., 2018). This suggests that the expansion of CD8dim T cells may be a response to high and/or persistent antigen stimulation. Supporting this hypothesis, elevated frequencies of CD8dim T cells have been reported during acute HIV infection, and in children exposed to a high cumulative pathogen burden during the first years of life (Eller et al., 2016; Falanga et al., 2017). Our observation that CD8dim T cells are also expanded in individuals with persistent KS under ART supports the notion that CD8 downregulation is a general phenomenon in settings of unresolved infection.
Chronic viral infections are also associated with CD8 T cell terminal differentiation and/or senescence (Koch et al., 2007). Senescent CD8 T cells exhibit reduced expression of PGC-1α, the master-regulator of mitochondrial biogenesis, and lower mitochondrial activity (Henson et al., 2014; Callender et al., 2020). Conversely, forced expression of PGC-1α promotes robust CD8 T cell memory responses (Dumauthioz et al., 2020). These observations underscore the importance of mitochondrial respiration for long-term CD8 T cell anti-viral function. We observed that the CD8dim T cell population, which is expanded in persistent KS, expressed elevated levels of Eomesodermin and CD57, proteins respectively associated with terminal differentiation and senescence, and had reduced expression of PGC-1α. Mitochondrial membrane potential (MTDR) was reduced in CD8dim cells, both ex vivo and during proliferation, while mitochondrial mass (MTG) and superoxide production (MitoSOX) were elevated. Importantly, this phenotype (increased mitochondrial mass and ROS production coupled with reduced mitochondrial membrane potential) has previously been identified as a driver of senescence (Passos et al., 2007). Collectively, our observations suggest that mitochondrial dysfunction may underlie the accumulation of senescent CD8 T cells that has previously been reported in KS (Unemori et al., 2013). However, this hypothesis must be tested in future studies, and additional data regarding mitochondrial morphology and the expression of other mitochondria-related genes would further add to our understanding of this phenomenon. It is unclear from our current data whether CD8 expression directly regulates mitochondrial activity in CD8 T cells; however a recent study reported that CD8 agonism boosted CD8 T cell metabolism and anti-tumor responses (Madi et al., 2022). This suggests there may be a direct link between CD8 levels on T cells and their metabolic activity, and that CD8 may be a viable therapeutic target to boost anti-tumor T cell immunity.
A key question is whether the CD8dim T cells we observed are specific for KSHV, for HIV, or for other persistent viral infections such as CMV, which is highly seroprevalent in HIV-infected individuals (Compston et al., 2009). It is possible that CD8 down-modulation could be driven by multiple concurrent infections, by ongoing immune activation, or by a combination of factors. In this initial study, we were unable to determine whether a subpopulation of the CD8dim T cells we observed were KSHV-specific, as KSHV is a large virus and immunoprevalent epitopes eliciting responses in a high percentage of seropositive individuals have not yet been identified. The question of the antigen specificity of CD8dim T cells will be the subject of subsequent investigations.
Our work has some limitations. As this was an observational study, we were unable to determine whether the expansion of CD8dim T cells plays a causative role in the failure to control KSHV under ART or is a consequence of this lack of suppression. Due to lack of available tissue, we were unable to assess whether CD8 T cells infiltrating the tumor microenvironment also exhibit a CD8dim phenotype. This question, together with the antigen specificity of CD8dim T cells and a direct examination of their functional profile, is the subject of ongoing investigations. If KSHV-specific CD8 T cells infiltrating the tumor microenvironment express low levels of CD8 and exhibit reduced mitochondrial activity, this will have important implications for immunotherapeutic approaches to KS treatment.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study exclusively used retrospective, de-identified samples. Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the participants was not required to participate in this study in accordance with the national legislation and the institutional requirements. Initial collection of samples from HIV+ KS+ participants was approved by the Institutional Review Board of the University of California, San Francisco (approval no. 10-02850). Initial collection of UNC samples was approved by the UNC Institutional Review Board (ethics numbers 11-0228; 14-0741; and 15-1626). Retrospective use of all samples was approved by the UNC Institutional Review Board (ethics number 17-2415).
Author contributions
The manuscript was written by GC, AW, NG, and TM. GC and NG contributed to study design. GC performed experimentation and collection of data. GC and AW performed the statistical analyses. GC acquired funding for the study. TM facilitated participant recruitment and sample collection. All authors provided review of the final manuscript.
Funding
This study was supported by a Young Investigator Pilot Award awarded to GC by the AIDS and Cancer Specimen Resource, funded by the National Cancer Institute (UM1 CA181255). Initial sample collection was supported by NIH grants U01 AI117844, U01 AI095052, and R01 HL132791. The UNC Flow Cytometry Core Facility is supported in part by P30 CA016086 Cancer Center Core Support Grant to the UNC Lineberger Comprehensive Cancer Center and by the Center for AIDS Research award number 5P30AI050410. Statistical expertise was provided by the University of North Carolina at Chapel Hill Center for AIDS Research, an NIH funded program P30 AI050410. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Acknowledgments
We thank Joann Kuruc and Cynthia Gay for their work recruiting participants to UNC cohorts that were retrospectively utilized for this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2022.961021/full#supplementary-material
References
Angin, M., Volant, S., Passaes, C., Lecuroux, C., Monceaux, V., Dillies, M.-A., et al. (2019). Metabolic plasticity of HIV-specific CD8+ T cells is associated with enhanced antiviral potential and natural control of HIV-1 infection. Nat. Metab. 1, 704–716. doi:10.1038/s42255-019-0081-4
Balyan, R., Gund, R., Chawla, A. S., Khare, S. P., Pradhan, S. J., Rane, S., et al. (2018). Correlation of cell-surface CD8 levels with function, phenotype and transcriptome of naive CD8 T cells. Immunology 156, 384–401. doi:10.1111/imm.13036
Bengsch, B., Johnson, A. L., Kurachi, M., Odorizzi, P. M., Pauken, K. E., Attanasio, J., et al. (2016). Bioenergetic insufficiencies due to metabolic alterations regulated by the inhibitory receptor PD-1 are an early driver of CD8+ T cell exhaustion. Immunity 45, 358–373. doi:10.1016/j.immuni.2016.07.008
Bihl, F., Narayan, M., Chisholm, J. V., Henry, L. M., Suscovich, T. J., Brown, E. E., et al. (2007). Lytic and latent antigens of the human gammaherpesviruses Kaposi's sarcoma-associated herpesvirus and Epstein-Barr virus induce T-cell responses with similar functional properties and memory phenotypes. J. Virol. 81, 4904–4908. doi:10.1128/jvi.02509-06
Bihl, F., Berger, C., Chisholm, J. V., Henry, L. M., Bertisch, B., Trojan, A., et al. (2009). Cellular immune responses and disease control in acute AIDS-associated Kaposi's sarcoma. AIDS 23, 1918–1922. doi:10.1097/qad.0b013e3283300a91
Callender, L. A., Carroll, E. C., Bober, E. A., Akbar, A. N., Solito, E., and Henson, S. M. (2020). Mitochondrial mass governs the extent of human T cell senescence. Aging Cell 19, e13067. doi:10.1111/acel.13067
Callender, L. A., Carroll, E. C., Garrod-Ketchley, C., Schroth, J., Bystrom, J., Berryman, V., et al. (2021). Altered nutrient uptake causes mitochondrial dysfunction in senescent CD8+ EMRA T cells during type 2 diabetes. Front. Aging 2, 681428. doi:10.3389/fragi.2021.681428
Chang, C.-H., Curtis, J. D., Maggi, L. B., Faubert, B., Villarino, A. V., O’Sullivan, D., et al. (2013). Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 153, 1239–1251. doi:10.1016/j.cell.2013.05.016
Cham, C. M., and Gajewski, T. F. (2005). Glucose availability regulates IFN-γ production and p70S6 kinase activation in CD8+Effector T cells. J. Immunol. 174, 4670–4677. doi:10.4049/jimmunol.174.8.4670
Chen, J., Dai, L., Barrett, L., James, J., Plaisance-Bonstaff, K., Post, S. R., et al. (2021). SARS-CoV-2 proteins and anti-COVID-19 drugs induce lytic reactivation of an oncogenic virus. Commun. Biol. 4, 682. doi:10.1038/s42003-021-02220-z
Clutton, G., Mollan, K., Hudgens, M., Goonetilleke, N., and Reproducible, A. (2019). A reproducible, objective method using MitoTracker fluorescent dyes to assess mitochondrial mass in T cells by flow Cytometry. Cytometry 95, 450–456. doi:10.1002/cyto.a.23705
Compston, L. I. C., Li, C., Sarkodie, F., Owusu-Ofori, S., Opare-Sem, O., and Allain, J.-P. (2009). Prevalence of persistent and latent viruses in untreated patients infected with HIV-1 from Ghana, West Africa. J. Med. Virol. 81, 1860–1868. doi:10.1002/jmv.21614
Day, C. L., Kaufmann, D. E., Kiepiela, P., Brown, J. A., Moodley, E. S., Reddy, S., et al. (2006). PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443, 350–354. doi:10.1038/nature05115
Day, C. L., Kiepiela, P., Leslie, A. J., van der Stok, M., Nair, K., Ismail, N., et al. (2007). Proliferative capacity of epitope-specific CD8 T-cell responses is inversely related to viral load in chronic human immunodeficiency virus type 1 infection. J. Virol. 81, 434–438. doi:10.1128/jvi.01754-06
Delgado, T., Carroll, P. A., Punjabi, A. S., Margineantu, D., Hockenbery, D. M., and Lagunoff, M. (2010). Induction of the Warburg effect by Kaposi's sarcoma herpesvirus is required for the maintenance of latently infected endothelial cells. Proc. Natl. Acad. Sci. U.S.A. 107, 10696–10701. doi:10.1073/pnas.1004882107
Dumauthioz, N., Tschum, B., Wenes, M., Marti, B., Wang, H., Franco, F., et al. (2020). Enforced PGC-1alpha expression promotes CD8 T cell fitness, memory formation and antitumor immunity. Cell. Mol. Immunol. 18, 1761–1771. doi:10.1038/s41423-020-0365-3
Eller, M. A., Goonetilleke, N., Tassaneetrithep, B., Eller, L. A., Costanzo, M. C., Johnson, S., et al. (2016). Expansion of inefficient HIV-specific CD8 T cells during acute infection. J. Virol. 90, 4005–4016. doi:10.1128/jvi.02785-15
Eltom, M. A., Jemal, A., Mbulaiteye, S. M., Devesa, S. S., and Biggar, R. J. (2002). Trends in Kaposi's sarcoma and non-Hodgkin's lymphoma incidence in the United States from 1973 through 1998. J. Natl. Cancer Inst. 94, 1204–1210. doi:10.1093/jnci/94.16.1204
Falanga, Y. T., Frascoli, M., Kaymaz, Y., Forconi, C., Ong'echa, J. M., Bailey, J. A., et al. (2017). High pathogen burden in childhood promotes the development of unconventional innate-like CD8+ T cells. JCI Insight 2. doi:10.1172/jci.insight.93814
Franceschi, S., Maso, L. D., Rickenbach, M., Polesel, J., Hirschel, B., Cavassini, M., et al. (2008). Kaposi sarcoma incidence in the Swiss HIV Cohort Study before and after highly active antiretroviral therapy. Br. J. Cancer 99, 800–804. doi:10.1038/sj.bjc.6604520
Gaiha, G. D., McKim, K. J., Woods, M., Pertel, T., Rohrbach, J., Barteneva, N., et al. (2014). Dysfunctional HIV-specific CD8+ T cell proliferation is associated with increased caspase-8 activity and mediated by necroptosis. Immunity 41, 1001–1012. doi:10.1016/j.immuni.2014.12.011
Hasley, R. B., Hong, C., Li, W., Friesen, T., Nakamura, Y., Kim, G. Y., et al. (2013). HIV immune activation drives increased Eomes expression in memory CD8 T cells in association with transcriptional downregulation of CD127. Aids 27, 1867–1877. doi:10.1097/qad.0b013e3283618487
Henson, S. M., Lanna, A., Riddell, N. E., Franzese, O., Macaulay, R., Griffiths, S. J., et al. (2014). p38 signaling inhibits mTORC1-independent autophagy in senescent human CD8+ T cells. J. Clin. Invest. 124, 4004–4016. doi:10.1172/jci75051
Jacobs, S. R., Herman, C. E., MacIver, N. J., Wofford, J. A., Wieman, H. L., Hammen, J. J., et al. (2008). Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J. Immunol. 180, 4476–4486. doi:10.4049/jimmunol.180.7.4476
Kim, J. W., Gao, P., Liu, Y. C., Semenza, G. L., and Dang, C. V. (2007). Hypoxia-inducible factor 1 and dysregulated c-Myc cooperatively induce vascular endothelial growth factor and metabolic switches hexokinase 2 and pyruvate dehydrogenase kinase 1. Mol. Cell Biol. 27, 7381–7393. doi:10.1128/mcb.00440-07
Koch, S., Larbi, A., Ozcelik, D., Solana, R., Gouttefangeas, C., Attig, S., et al. (2007). Cytomegalovirus infection: A driving force in human T cell immunosenescence. Ann. N. Y. Acad. Sci. 1114, 23–35. doi:10.1196/annals.1396.043
Korencak, M., Byrne, M., Richter, E., Schultz, B. T., Juszczak, P., Ake, J. A., et al. (2019). Effect of HIV infection and antiretroviral therapy on immune cellular functions. JCI Insight 4. doi:10.1172/jci.insight.126675
Krown, S. E., Metroka, C., and Wernz, J. C. (1989). Kaposi's sarcoma in the acquired immune deficiency syndrome: A proposal for uniform evaluation, response, and staging criteria. AIDS clinical Trials group oncology committee. Jco 7, 1201–1207. doi:10.1200/jco.1989.7.9.1201
Lachmann, R., Bajwa, R., Vita, S., Smith, H., Cheek, E., Akbar, A., et al. (2012). Polyfunctional T cells accumulate in large human cytomegalovirus-specific T cell responses. J. Virol. 86, 1001–1009. doi:10.1128/jvi.00873-11
Lambert, M., Gannagé, M., Karras, A., Abel, M., Legendre, C., Kerob, D., et al. (2006). Differences in the frequency and function of HHV8-specific CD8 T cells between asymptomatic HHV8 infection and Kaposi sarcoma. Blood 108, 3871–3880. doi:10.1182/blood-2006-03-014225
Lepone, L., Rappocciolo, G., Knowlton, E., Jais, M., Piazza, P., Jenkins, F. J., et al. (2010). Monofunctional and polyfunctional CD8 + T cell responses to human herpesvirus 8 lytic and latency proteins. Clin. Vaccine Immunol. 17, 1507–1516. doi:10.1128/cvi.00189-10
Lepone, L. M., Rappocciolo, G., Piazza, P. A., Campbell, D. M., Jenkins, F. J., and Rinaldo, C. R. (2017). Regulatory T cell effect on CD8+T cell responses to human herpesvirus 8 infection and development of kaposi's sarcoma. AIDS Res. Hum. Retroviruses 33, 668–674. doi:10.1089/aid.2016.0155
Madi, A., Weisshaar, N., Buettner, M., Poschet, G., Ma, S., Wu, J., et al. (2022). CD8 agonism functionally activates memory T cells and enhances antitumor immunity. Int. J. Cancer 151, 797–808. doi:10.1002/ijc.34059
Mani, D., Neil, N., Israel, R., and Aboulafia, D. M. (2002). A retrospective analysis of AIDS-associated Kaposi's sarcoma in patients with undetectable HIV viral loads and CD4 counts greater than 300 cells/mm(3). J. Int. Assoc. Physicians AIDS Care (Chic) 8, 279–285doi:10.1177/1545109709341852
Maurer, T., Ponte, M., and Leslie, K. (2007). HIV-associated Kaposi's sarcoma with a high CD4 count and a low viral load. N. Engl. J. Med. 357, 1352–1353. doi:10.1056/nejmc070508
Migueles, S. A., Weeks, K. A., Nou, E., Berkley, A. M., Rood, J. E., Osborne, C. M., et al. (2009). Defective human immunodeficiency virus-specific CD8 + T-cell polyfunctionality, proliferation, and cytotoxicity are not restored by antiretroviral therapy. J. Virol. 83, 11876–11889. doi:10.1128/jvi.01153-09
Miwa, S., Kashyap, S., Chini, E., and von Zglinicki, T. (2022). Mitochondrial dysfunction in cell senescence and aging. J. Clin. Invest. 132. doi:10.1172/JCI158447
Osman, M., Kubo, T., Gill, J., Neipel, F., Becker, M., Smith, G., et al. (1999). Identification of human herpesvirus 8-specific cytotoxic T-cell responses. J. Virol. 73, 6136–6140. doi:10.1128/jvi.73.7.6136-6140.1999
Paley, M. A., Kroy, D. C., Odorizzi, P. M., Johnnidis, J. B., Dolfi, D. V., Barnett, B. E., et al. (2012). Progenitor and terminal subsets of CD8 + T cells cooperate to contain chronic viral infection. Science 338, 1220–1225. doi:10.1126/science.1229620
Papagno, L., Spina, C. A., Marchant, A., Salio, M., Rufer, N., Little, S., et al. (2004). Immune activation and CD8+ T-cell differentiation towards senescence in HIV-1 infection. PLoS Biol. 2, E20. doi:10.1371/journal.pbio.0020020
Park, J.-H., Adoro, S., Lucas, P. J., Sarafova, S. D., Alag, A. S., Doan, L. L., et al. (2007). 'Coreceptor tuning': Cytokine signals transcriptionally tailor CD8 coreceptor expression to the self-specificity of the TCR. Nat. Immunol. 8, 1049–1059. doi:10.1038/ni1512
Passos, J. F., Saretzki, G., Ahmed, S., Nelson, G., Richter, T., Peters, H., et al. (2007). Mitochondrial dysfunction accounts for the stochastic heterogeneity in telomere-dependent senescence. PLoS Biol. 5, e110. doi:10.1371/journal.pbio.0050110
Robey, R. C., Lagos, D., Gratrix, F., Henderson, S., Matthews, N. C., Vart, R. J., et al. (2009). The CD8 and CD4 T-cell response against Kaposi's sarcoma-associated herpesvirus is skewed towards early and late lytic antigens. PLoS One 4, e5890. doi:10.1371/journal.pone.0005890
Roederer, M. (2011). Interpretation of cellular proliferation data: Avoid the panglossian. Cytometry 79A, 95–101. doi:10.1002/cyto.a.21010
Siska, P. J., Beckermann, E. K., Mason, F. M., Andrejeva, G., Greenplate, A. R., Sendor, A. B., Chiang, Y. J., et al. (2017). Mitochondrial dysregulation and glycolytic insufficiency functionally impair CD8 T cells infiltrating human renal cell carcinoma. JCI Insight 2. doi:10.1172/jci.insight.93411
Shiels, M. S., Pfeiffer, R. M., Gail, M. H., Hall, H. I., Li, J., Chaturvedi, A. K., et al. (2011). Cancer burden in the HIV-infected population in the United States. J. Natl. Cancer Inst. 103, 753–762. doi:10.1093/jnci/djr076
Trautmann, L., Janbazian, L., Chomont, N., Said, E. A., Gimmig, S., Bessette, B., et al. (2006). Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 12, 1198–1202. doi:10.1038/nm1482
Unemori, P. A., Leslie, K. S., Hunt, P. W., Sinclair, E., Epling, L., Mitsuyasu, R., Effros, R. B., et al. (2013). Immunosenescence is associated with presence of Kaposi's sarcoma in antiretroviral treated HIV infection. AIDS 27, 1735–1742. doi:10.1097/qad.0b013e3283601144
van den Berg, H. A., Wooldridge, L., Laugel, B., and Sewell, A. K. (2007). Coreceptor CD8-driven modulation of T cell antigen receptor specificity. J. Theor. Biol. 249, 395–408. doi:10.1016/j.jtbi.2007.08.002
van der Windt, G. J., Everts, B., Chang, C. H., Curtis, J. D., Freitas, T. C., Amiel, E., et al. (2012). Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity 36, 68–78. doi:10.1016/j.immuni.2011.12.007
van der Windt, G. J., O’Sullivan, D., Everts, B., Huang, S. C.-C., Buck, M. D., Curtis, J. D., et al. (2013). CD8 memory T cells have a bioenergetic advantage that underlies their rapid recall ability. Proc. Natl. Acad. Sci. U.S.A. 110, 14336–14341. doi:10.1073/pnas.1221740110
von Braun, A., Braun, D. L., Kamarachev, J., and Günthard, H. F. (2014). New onset of kaposi sarcoma in a human immunodeficiency virus-1-infected homosexual man, despite early antiretroviral treatment, sustained viral suppression, and immune restoration. Open Forum Infect. Dis. 1, ofu005. doi:10.1093/ofid/ofu005
Keywords: Kaposi’s sarcoma, KSHV, HIV, T cells, CD8 coreceptor, metabolism, mitochondria, senescence
Citation: Clutton GT, Weideman AMK, Goonetilleke NP and Maurer T (2022) An expanded population of CD8dim T cells with features of mitochondrial dysfunction and senescence is associated with persistent HIV-associated Kaposi’s sarcoma under ART. Front. Cell Dev. Biol. 10:961021. doi: 10.3389/fcell.2022.961021
Received: 03 June 2022; Accepted: 12 September 2022;
Published: 29 September 2022.
Edited by:
Ioannis S. Pateras, National and Kapodistrian University of Athens, GreeceReviewed by:
Lianjun Zhang, Suzhou Institute of Systems Medicine (ISM), ChinaLeila B. Giron, Wistar Institute, United States
Copyright © 2022 Clutton, Weideman, Goonetilleke and Maurer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Genevieve T. Clutton, Z2VuX2NsdXR0b25AbWVkLnVuYy5lZHU=
 Genevieve T. Clutton
Genevieve T. Clutton Ann Marie K. Weideman
Ann Marie K. Weideman Nilu P. Goonetilleke
Nilu P. Goonetilleke Toby Maurer5
Toby Maurer5