- 1Sports Medicine Division, Institute of Orthopedics and Traumatology, Hospital das Clínicas, Faculdade de Medicina, Universidade de São Paulo, São Paulo, Brazil
- 2Hospital Sírio-Libanês, São Paulo, Brazil
- 3Department of Orthopaedic Surgery, Osaka University Graduate School of Medicine, Suita, Japan
- 4Institute of Sports Medicine, Peking University Third Hospital, Beijing, China
- 5Department of Orthopaedic Surgery, Hospital for Special Surgery, New York, NY, United States
Editorial on the Research Topic
Tissue Engineering and Cell Therapy for Cartilage Restoration
Articular cartilage is a connective tissue consisting of two phases: a solid composed of collagen, proteoglycans, proteins, and chondrocytes, and a liquid made up of water and electrolytes. It can be divided into four zones (superficial, middle, deep and calcified), each presenting a different cellular organization, collagen fiber architecture and depth (Hernandez et al., 2000; Meyers and Chawla, 2008; Sophia Fox et al., 2009). These structures and components establish the properties of cartilage, in terms of stiffness, elasticity and other important aspects for its characterization.
It is known that articular cartilage is an avascular tissue with little cellularity, making it difficult to heal. As a consequence, cartilage injuries can generate several complications for the individual, such as loss of mobility, degeneration and osteoarthritis (OA), directly affecting the quality of life. Not to mention that it represents an economic burden (Fernandes et al., 2018; Fernandes et al., 2020).
Current procedures that seek to repair chondral tissue are microfracture stimulation, autograft and osteochondral allograft, and cellular implant therapies based on tissue engineering principles. Cell therapies and regenerative techniques are important and there are two main examples: autologous chondrocyte implantation (ACI) and mesenchymal stromal cells (MSCs). MSCs have received considerable research attention, due to ease of collection, ability to proliferate and differentiate cells, non-rejection by the patient, and paracrine effect on local cellular machinery (Ando et al., 2007; Shimomura et al., 2015).
This Research Topic aimed to widening the knowledge on the strategies being used for articular cartilage regeneration, describing the state-of-the-art and exploring the innovative approaches in cartilage restoration field. This issue currently includes 14 articles related to different innovative propositions associated with cartilage injuries.
In Table 1, a summary and the innovative aspects of each article are presented.
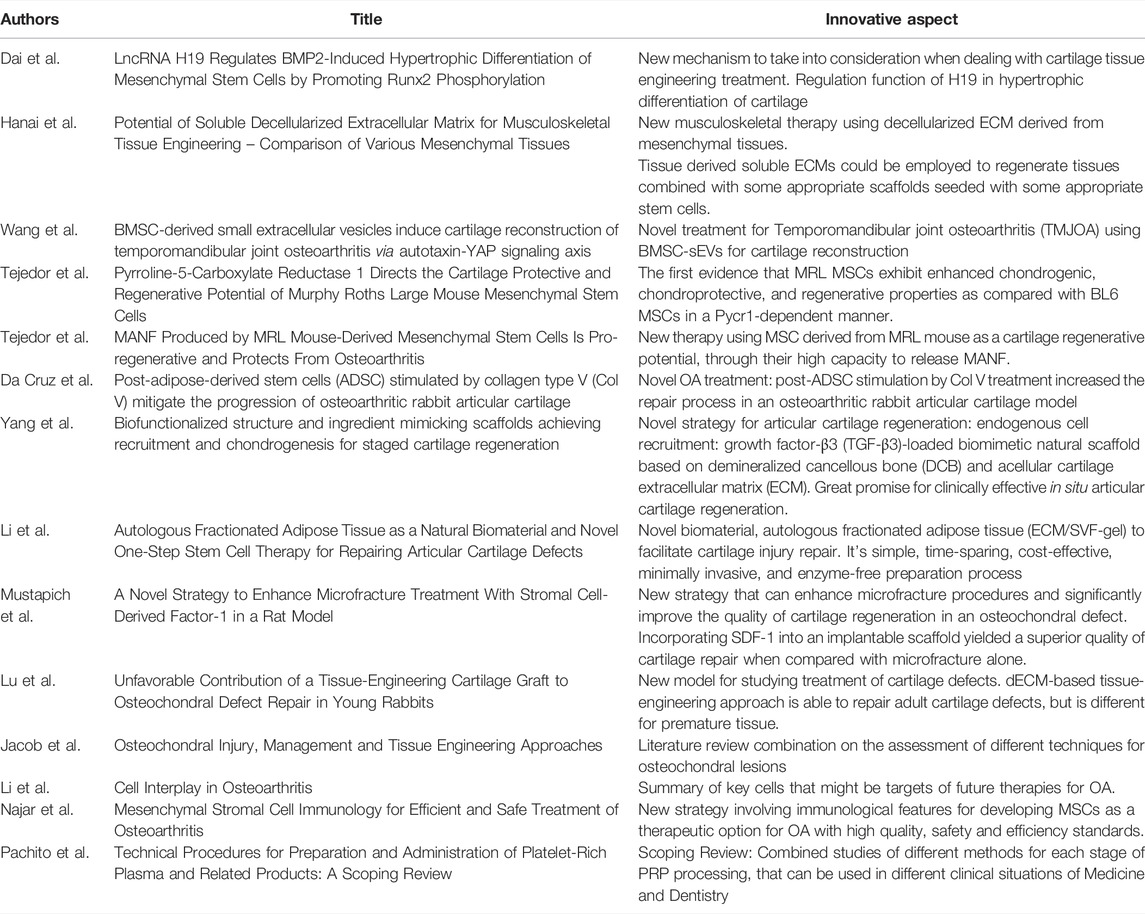
TABLE 1. Innovative contribution of the articles published in Research Topic Tissue Engineering and Cell Therapy for Cartilage Restoration.
Stem-cell-based and gene-enhanced tissue engineered cartilage is promising in the treatment of cartilaginous pathologies, especially traumatic cartilage defects (Bishop et al., 2017; Pirraco et al., 2018; Wang et al., 2019). Bone morphogenetic protein 2 (BMP2) triggers hypertrophic differentiation after chondrogenic differentiation of MSCs, which blocked the further application of BMP2-mediated cartilage tissue engineering. Dai et al. investigated the function of LncRNA H19 (H19) in BMP2, finding out that H19 regulates BMP2-mediated hypertrophic differentiation of MSCs by promoting the phosphorylation of Runx2, that maturates chondrocytes. H19 may play a role in cartilage differentiation, cartilage phenotype maintaining, and cartilage hypertrophic differentiation. They evaluated nine types of porcine tissue through a simple standard decellularization protocol.
At present, tissue engineering and regenerative medicine have focused on extracellular matrices (ECMs) to function as a natural scaffold (Harrison et al., 2014). Hanai et al. found that a soluble decellularized ECM (dECM) for each of the nine tissues harvested exhibited variations in their biochemical characteristics and growth factor distribution. On cell culture, it appeared to promote cell differentiation toward the specified used ECM tissue phenotype and decellularization was successful with reducing cellular components in every tissue. The present results are important in the field of musculoskeletal regeneration therapy, since tissue derived soluble ECMs could be employed to regenerate tissues combined with some appropriate scaffolds seeded with stem cells.
Researching less invasive treatments for Temporomandibular joint osteoarthritis (TMJOA), Wang et al. attempted to analyze the cartilage reconstruction effect of bone marrow MSC-derived small extracellular vesicles (BMSC-sEVs) and showed an increase of the tissue in the cartilage lacuna and hypertrophic cartilage cells in the deep area of the bone under the cartilage, besides higher rates of cell proliferation and migratory activity and alleviated G1 stagnation of the cell cycle of OAsEV which may provide guidance regarding their therapeutic applications as early and minimally invasive therapies for TMJOA.
Comparing Murphy Roths Large (MRL), who possess remarkable capacity to regenerate several musculoskeletal tissues, and BL6 mices regeneration to a ear punch, Tejedor et al. demonstrated that the enhanced regenerative potential of MRL mice is attributed, in part, to their MSCs that exhibit PYCR1-dependent higher glycolytic potential, differentiation capacities, chondroprotective abilities, and regenerative properties than BL6 MSCs, what could be a promising tool in the treatment of OA. Comparing the MSCs of these two mouse species, Tejedor et al. showed that the slow-proliferating MRL MSCs display a higher migration potential than the fast-proliferating BL6 MSC and found that mesencephalic astrocyte derived neurotrophic factor (MANF) was specifically highly produced by MRL MSC as compared to MSC derived from other mouse strains, and that MANF highly produced by MRL MSC contributes to their capacity to tend to reduce the OA score.
To delay the development of OA and promote joint cartilage regeneration, Da Cruz et al. demonstrated that post-adipose-derived stem cells (ADSC) stimulation by type V collagen (Col V) treatment induced a significant regeneration of cartilage in an osteoarthritic rabbit articular cartilage model suggesting that surgical-induced OA treated with ADSCs stimulated by Col V may prevent the progression of cartilage injury and indicating that ADSCs/Col V may be a therapeutic target for the treatment of osteoarthritis. Also using a defect produced in the articular cartilage of rabbits, Yang et al. developed a staged regeneration strategy that combines endogenous cell recruitment and pro-chondrogenesis approaches for in situ articular cartilage regeneration with growth factor (GF)-loaded scaffold who facilitated cell homing, migration, and chondrogenic differentiation and promoted the reconstructive effects of in vivo cartilage formation.
Studying the regeneration of defects in articular cartilage, Li et al. used extracellular matrix/stromal vascular fraction gel (ECM/SVF-gel) to evaluate the therapeutic effect of this natural biomaterial on this repair, comparing the control group treated with microfractures to the group with microfractures associated with ECM/SVF-gel, finding that autologous ECM/SVF-gel displays a curative effect on articular cartilage regeneration in a rabbit model, besides being a simple, time-sparing, cost-effective, enzyme-free and minimally invasive preparation process.
Microfracture is one of the most widely used techniques for the repair of articular cartilage. However, microfracture often results in filling of the chondral defect with fibrocartilage, which exhibits poor durability and sub-optimal mechanical properties. Mustapich et al. studied a strategy for enhanced microfracture treatment with stromal cell-derived factor-1 (SDF-1), demonstrating a simple cost-effective one-step process for improving the quality of cartilage defect repair in a rat model of microfracture. In this perspective, Lu et al. found that this approach played a unique role in cartilage resurfacing of adult rabbits despite the fact that self-healing dominates cartilage repair in young rabbits less than 9 months old.
After categorizing treatment options for osteochondral lesions into repair and regenerative techniques, Jacob et al. showed emerging techniques, exploring tissue engineering with new methods of cultures and their results, and stated that for improved chondrogenesis the cells require both growth factors and mechanical forces to bring about more physiological cellular responses. The author demonstrated satisfactory results in the medium-term follow-up with the MaioRegen presenting faster return to sports and a low rate of complication and failure, while the Agili-C showed encouraging results through significant improvement in the assessments.
OA is a multifactorial disease that presupposes local and systemic factors and has multiple pathogenetic mechanisms, which must be considered when exploring new treatment options. Li et al. presented the cells in the joint tissues and their role in OA pathogenesis, the numerous ways these cells communicate, and concluded that application of stem cell-derived EVs in OA treatment is an emerging field in regenerative medicine.
Cartilage tissue engineering has potential for the treatment of cartilage pathologies, providing biomaterials that can be adjuvants to already established treatments to improve the clinical outcome, providing a higher quality regeneration and in the shortest possible time. There are clearly several cellular regulatory pathways involved in the therapeutic effect of MSCs, and the broad cellular and molecular changes that accompany MSC apoptosis, autophagy, and senescence may be essential for their therapeutic effects according to Najar et al. This is an essential understanding of the immunological profile and functions of MSCs as a graft and understanding of the mechanisms involved in the effects of MSCs for better therapeutic targeting.
A treatment widely used in different clinical situations is Platelet-rich plasma (PRP), but universal standardization of procedures for its preparation is still lacking. Pachito et al. reviewed thirty-nine studies focusing on the comparison of PRP to a related product, types of anticoagulants, centrifugation protocols, commercial kits, processing time, methods for activation, and application concomitantly to other substances, finding a great variability embed in each step necessary for the preparation of PRP which may justify the variability of clinical effects of PRP across different clinical trials.
Final Considerations
In this editorial, it was possible to discover a large number of innovative aspects, with extremely promising research fields that could change the course of the treatment of OA and other pathologies that affect the articular cartilage. Despite that, the articles have some methodological limitations and further research should be carried out focusing on the difficulties raised, in order to increase the level of evidence, safety and efficacy of the new therapies.
Innovative technologies and discoveries take time to be translated from an idea to clinical practice (Fernandes et al., 2022). Moreover the continuing development of the mentioned studies is of key importance to create collaborative learning and reach research goals for cartilage treatment and, at the end, improve healthcare and patient’s quality of life.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication. TF wrote the manuscript. DB, KS, ZS, NN, and AG carried out the final review.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We’d like to acknowledge Rafaella Rogatto de Faria and Teófilo Josué Alecrim da Costa for contributing to this manuscript.
References
Ando, W., Tateishi, K., Hart, D. A., Katakai, D., Tanaka, Y., Nakata, K., et al. (2007). Cartilage Repair Using an In Vitro Generated Scaffold-free Tissue-Engineered Construct Derived from Porcine Synovial Mesenchymal Stem Cells. Biomaterials 28 (36), 5462–5470. doi:10.1016/j.biomaterials.2007.08.030
Bishop, E. S., Mostafa, S., Pakvasa, M., Luu, H. H., Lee, M. J., Wolf, J. M., et al. (2017). 3-D Bioprinting Technologies in Tissue Engineering and Regenerative Medicine: Current and Future Trends. Genes & Dis. 4, 185–195. doi:10.1016/j.gendis.2017.10.002
Fernandes, T. L., Cortez De Santanna, J. P., Frisene, I., Gazarini, J. P., Gomes Pinheiro, C. C., Gomoll, A. H., et al. (2020). Systematic Review of Human Dental Pulp Stem Cells for Cartilage Regeneration. Tissue Eng. Part B Rev. 26 (1), 1–12. doi:10.1089/ten.teb.2019.0140
Fernandes, T. L., de Faria, R. R., Gonzales, M. A., Sherman, S. L., Goldchmit, S., and Fleury, A. (2022). Innovation in Orthopaedics: Part 2—How to Translate Ideas and Research into Clinical Practice. Curr. Rev. Musculoskelet. Med. 15, 150–155. doi:10.1007/s12178-022-09749-4
Fernandes, T. L., Kimura, H. A., Pinheiro, C. C. G., Shimomura, K., Nakamura, N., Ferreira, J. R., et al. (2018). Human Synovial Mesenchymal Stem Cells Good Manufacturing Practices for Articular Cartilage Regeneration. Tissue Eng. Part C. Methods 24 (12), 709–716. doi:10.1089/ten.tec.2018.0219
Harrison, R. H., St-Pierre, J.-P., and Stevens, M. M. (2014). Tissue Engineering and Regenerative Medicine: a Year in Review. Tissue Eng. Part B Rev. 20, 1–16. doi:10.1089/ten.TEB.2013.0668
Hernandez, A. J., Camanho, G. L., and Amatuzzi, M. M. (2000). Cartilagem Articular e Osteoartrose. Acta ortop. bras. 8 (2), 100–104. doi:10.1590/s1413-78522000000200005
Meyers, M. A., and Chawla, K. K. (2008). Mechanical Behavior of Materials. 2nd Edn. Cambridge: Cambridge University Press, 137–138. doi:10.1017/CBO9780511810947
Pirraco, R. F., Pirraco, R. P., Oliveira, J. M., Reis, R. L., and Marques, A. P. (2018). Stem Cells for Osteochondral Regeneration. Adv. Exp. Med. Biol. 1059, 219–240. doi:10.1007/978-3-319-76735-2_10
Shimomura, K., Ando, W., Moriguchi, Y., Sugita, N., Yasui, Y., Koizumi, K., et al. (2015). Next Generation Mesenchymal Stem Cell (MSC)-based Cartilage Repair Using Scaffold-free Tissue Engineered Constructs Generated with Synovial Mesenchymal Stem Cells. Cartilage 6, 13S–29S. doi:10.1177/1947603515571002
Sophia Fox, A. J., Bedi, A., and Rodeo, S. A. (2009). The Basic Science of Articular Cartilage: Structure, Composition, and Function. Sports Health 1 (6), 461–468. doi:10.1177/1941738109350438
Keywords: tissue engineering, cell therapy, cartilage restoration, innovation, stem cells, cartilage, osteoarthritis
Citation: Fernandes TL, Bueno DF, Shimomura K, Shao Z and Gomoll AH (2022) Editorial: Tissue Engineering and Cell Therapy for Cartilage Restoration. Front. Cell Dev. Biol. 10:947588. doi: 10.3389/fcell.2022.947588
Received: 18 May 2022; Accepted: 06 June 2022;
Published: 01 July 2022.
Edited and Reviewed by:
Atsushi Asakura, University of Minnesota Twin Cities, United StatesCopyright © 2022 Fernandes, Bueno, Shimomura, Shao and Gomoll. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tiago Lazzaretti Fernandes, dGlhZ290ODZAaG90bWFpbC5jb20=
 Tiago Lazzaretti Fernandes
Tiago Lazzaretti Fernandes Daniela Franco Bueno2
Daniela Franco Bueno2 Kazunori Shimomura
Kazunori Shimomura Zhenxing Shao
Zhenxing Shao