- 1The Vice Chancellery, The University of Notre Dame Australia, Fremantle, WA, Australia
- 2Biomedical Biotechnology Research Unit, Department of Biochemistry and Microbiology, Rhodes University, Grahamstown, South Africa
- 3Biomedical Research and Drug Discovery Research Group, Faculty of Health Sciences, Higher Colleges of Technology, Sharjah, United Arab Emirates
Plasmodium falciparum is a unicellular protozoan parasite and causative agent of the most severe form of malaria in humans. The malaria parasite has had to develop sophisticated mechanisms to preserve its proteome under the changing stressful conditions it confronts, particularly when it invades host erythrocytes. Heat shock proteins, especially those that function as molecular chaperones, play a key role in protein homeostasis (proteostasis) of P. falciparum. Soon after invading erythrocytes, the malaria parasite exports a large number of proteins including chaperones, which are responsible for remodeling the infected erythrocyte to enable its survival and pathogenesis. The infected host cell has parasite-resident and erythrocyte-resident chaperones, which appear to play a vital role in the folding and functioning of P. falciparum proteins and potentially host proteins. This review critiques the current understanding of how the major chaperones, particularly the Hsp70 and Hsp40 (or J domain proteins, JDPs) families, contribute to proteostasis of the malaria parasite-infected erythrocytes.
Introduction
The deadliest human malaria parasite, Plasmodium falciparum, has a reduced genome, and yet appears to have dedicated a significant proportion of its genes (∼2%) to molecular chaperones (Sargeant et al., 2006), the guardians of protein folding. This suggests that the structural integrity of the proteome is an important aspect of the survival of the malaria parasite. Interestingly, an unusually high proportion of P. falciparum proteins (24–30%) are rich in asparagine (N) and glutamine (Q), particularly poly-N repeats (Singh et al., 2004; Pallarès et al., 2018), which have been found to have a tendancy to aggregate (Halfmann et al., 2011). Furthermore, a key phase in the pathology of malaria, is the invasion of host erythrocytes by the parasite, which it completely remodels by exporting over 400 parasite proteins, including a substantial proportion (∼5%) of molecular chaperones (Cortés et al., 2020). This massive renovation of the host cell potentially requires unique protein folding pathways involving both parasite and host molecular chaperones (Pesce and Blatch, 2014; Gabriela et al., 2022). This review will critique the evidence indicating that heat shock proteins serving as molecular chaperones, especially Hsp70 and Hsp40 (also called J domain proteins, JDPs) families, are highly adapted to maintaining the structural and functional integrity of the proteomes of the parasite and potentially the host erythrocyte.
PfHSP70s are the Guardians of the Parasite-Resident and Exported Proteome
P. falciparum has only six Hsp70s (PfHsp70s; Shonhai et al., 2007; Shonhai, 2021) and four Hsp90s (PfHsp90s; Shahinas and Pillai, 2021). However, there is a highly expanded complement of JDPs, with 49 members (PfJDPs; Botha et al., 2007; Njunge et al., 2013; Pesce and Blatch, 2014; Dutta et al., 2021a).
All six PfHsp70s appear to be finely tuned to the malaria parasite lifecycle, playing an important role in parasite survival and virulence (Przyborski et al., 2015), with most being essential (Zhang et al., 2018), and a number of them shown to be inhibited by small molecules with anti-malarial activity (Chiang et al., 2009; Cockburn et al., 2011; Cockburn et al., 2014; Zininga et al. 2017a; Zininga et al. 2017b; Zininga et al. 2017c). The canonical and highly abundant cytoplasmic and nuclear P. falciparum Hsp70-1 (PfHsp70-1; Kumar et al., 1991; Pesce et al., 2008) has been shown to be essential (Zhang et al., 2018). PfHsp70-1 is regulated by a number of co-chaperones, including JDPs which deliver specialized substrates (Pesce et al., 2008; Botha et al., 2011; Njunge et al., 2015), P. falciparum Hsp70/Hsp90 organizing protein which enables transfer of substrates to PfHsp90 (PfHop; Gitau et al., 2012; Zininga et al., 2015), and the cytosolic Hsp70-like protein, PfHsp70-z (an Hsp110), serving as a nucleotide exchange factor (Zininga et al., 2016). The cytoplasmic PfHsp70-z is also essential (Muralidharan et al., 2012; Zhang et al., 2018), which may well be due to its highly effective protein aggregation suppression activity (Zininga et al., 2016). PfHsp70-1 has also been shown to have high ATPase activity (Matambo et al., 2004; Misra and Ramachandran, 2009; Makumire et al., 2021) and strong aggregation suppression activity (Botha et al., 2011), suggesting that it is a superior chaperone compared to human Hsp70s (Anas et al., 2020). Overall, the evidence suggests that PfHsp70-1 and PfHsp70-z are major players in proteostasis of the parasite cytoplasm.
In the endoplasmic reticulum (ER), there are two Hsp70s, PfHsp70-2 (a BiP/Grp78 homologue) and PfHsp70-y (a Hsp110/Grp170 homologue). PfHsp70-2 is essential (Zhang et al., 2018), and has been proposed to be involved in protein translocation into the ER, working with a PfJDP (PfSec63) and the PfSec translocon (Tuteja, 2007; Blatch and Zimmermann, 2009; Cortés et al., 2020; Shonhai, 2021). PfHsp70-2 was found to functionally associate with another PfJDP (Pfj2) and a protein disulfide isomerase (PDI-8) in the oxidative folding of ER proteins (Cobb et al., 2017). PfHsp70-y has also been shown to be essential, and to potentially interact with, and serve as a nucleotide exchange factor for PfHsp70-2, analogous to the cytoplasmic PfHsp70-z-PfHsp70-1 interaction (Zhang et al., 2018; Kudyba et al., 2019). Overall, the data suggest that these chaperones play an important role in protein quality control and proteostasis within the ER.
Very little is known about the proposed mitochondrial PfHsp70-3, except for the observation that PfHsp70-3 interacted with at least two N-rich malarial antigens (LaCount et al., 2005). Like PfHsp70-3, other PfHsp70s (PfHsp70-1, PfHsp70-z, and PfHsp70-x) have been reported to exhibit a preference for N-rich substrates (Muralidharan et al., 2012; Mabate et al., 2018; Lebepe et al., 2020; Rajapandi, 2020). Therefore, there is growing evidence that PfHsp70s may be finely tuned for the protection of unstable N-rich P. falciparum proteins from misfolding and aggregation.
PfHsp70-x is the only exported member of the PfHsp70 family, and has been shown to be localized to the PV and erythrocyte cytosol where it is found free or associated with PfJDPs (PFE0055c and PFA0660w) in mobile lipid containing complexes called J-Dots (Külzer et al., 2012; Grover et al., 2013; Behl and Mishra. 2019). Interestingly, PfHsp70-x is not essential; however, knockout compromised virulence (Charnaud et al., 2017), while knockdown compromised growth under stressful conditions similar to febrile episodes (Day et al., 2019). Recently, the crystal structures of the ATPase (Day et al., 2019) and substrate binding domains (Schmidt and Vakonakis, 2020) of PfHsp70-x were elucidated. Interestingly, PfHsp70-x contains an N-terminal signal sequence for secretion through the ER, but not the Plasmodium export element (PEXEL; Marti et al., 2004; Hiller et al., 2004), which has been shown to be required for the export of many P. falciparum proteins through the Plasmodium translocon of exported proteins (PTEX; de Koning-Ward et al., 2009; Beck et al., 2014; Elsworth et al., 2014; Elsworth et al., 2016). PfHsp70-x, like certain other PEXEL-negative P. falciparum proteins (PNEPs), is also successfully exported through the PTEX translocon (Rhiel et al., 2016). We are yet to elucidate exactly how proteins synthesized off the ribosome in the parasite cytosol, are threaded through the ER, across the plasma membrane, through the PV and the PV membrane, and into the erythrocyte cytosol or via the Maurer’s Cleft to the membrane, where they are folded and begin functioning. However, there is some evidence emerging that suggests that PfHsp70-2 and PfHsp70-x (and potentially other chaperones/co-chaperones such as PfJDPs) may collaborate with the core threading machinery of PTEX, a class I AAA + ATPase (PfHsp101; Russo et al., 2010; Matthews et al., 2019) in the chaperoning of exported P. falciparum proteins. For example, it has been shown that PfHsp101 is localized to the ER and the PV (Russo et al., 2010), and is able to preferentially associate with certain PEXEL-containing proteins within these compartments (Gabriela et al., 2022). PfHsp70-2 has been shown to not only interact with proteins secreted into the PV, but also with exported proteins, including the main virulence factor P. falciparum erythrocyte membrane protein 1 (PfEMP1; Saridaki et al., 2008; Batinovic et al., 2017; Cortés et al., 2020). In addition, it has been reported that PfHsp70-x associates with PfHsp101 (Charnaud et al., 2017; Cobb et al., 2017; Zhang et al., 2018) and PfEMP1 (Külzer et al., 2012). Therefore, in addition to its role in protein quality control and proteostasis in the ER, PfHsp70-2 may be playing a major role in the protein trafficking of secreted proteins, and in collaboration with PfHsp70-x also involved in the trafficking of exported proteins. It is tempting to speculate that two protein trafficking and folding pathways exist for exported proteins: 1) a pathway for PEXEL-containing proteins with PfHsp101 being the main chaperone and PfHsp70-2 and PfHsp70-x being auxiliary chaperones; and 2) a pathway for PNEPs with PfHsp70-2 and PfHsp70-x being the main chaperones which hand over to PfHsp101 in the PV or as a component of PTEX.
PfJDPs Harness the Chaperone Power of Parasite and Host HSP70s
PfJDPs are ubiquitous, expressed in all compartments of the parasite, in the PV and in the host cell (Dutta et al., 2021a). There is emerging evidence that they play an important role in protein trafficking, folding, assembly and protection from misfolding and aggregation under stressful conditions (Daniyan et al., 2016; Dutta et al., 2021a). The majority of the PfJDPs are essential (Maier et al., 2008; Zhang et al., 2018), and nearly half them are exported into the erythrocyte and play a crucial role in promoting parasite virulence (Dutta et al., 2021a).
A number of the parasite-resident PfJDPs, have been identified as potential co-chaperone of PfHsp70-1 (PfHsp40/PF14_0359, Botha et al., 2011, Anas et al., 2020; PFB0595w, Njunge et al., 2015; and Pfj4/PFL0565w, Pesce et al., 2008). Like PfHsp70-1, PfHsp40 is essential (Zhang et al., 2018), cytosolic, constitutively expressed, and upregulated under stressful conditions (Botha et al., 2011). While they are the homologous chaperone pair to the canonical cytosolic human Hsp70-JDP pair (HSPA1A-DNAJA1), there are subtle but critical structural, biochemical and functional differences, with the P. falciparum pair shown to be a more effective chaperone machine (Anas et al., 2020). Furthermore, PfHsp40 has been found to be farnesylated and palmitoylated, leading to membrane localization (Mathews et al., 2021). Notably, farnesyl-PfHsp40 may well be the essential isoform of this PfJDP, as inhibition of farnesylation significantly compromised survival of the parasite under stress conditions.
PfHsp70-PfJDP pairs have also been identified within the ER, and appear to play an important role in protein translocation (PfHsp70-2 and PfSec63/PF13_0102; Marapana et al., 2018) and protein folding and quality control within that compartment (PfHsp70-2 and Pfj2/PF11_0099; Cobb et al., 2017). At least one PfJDP has been shown to be secreted through the ER, and partially localized to the PV (PFF1415c; Khosh-Naucke et al., 2018). PFF1415c was found to be essential for growth of erythrocyte-stage parasites; however, its precise function is yet to be determined. Pfj1 (PFD0462w) is the only PfJDP reported to be localized to the apicoplast (Kumar et al., 2010), which was contrary to a previous report which proposed it was targeted to the mitochondrion (Watanabe, 1987). Pfj1 has an unusually long and unique C-terminal region, and has been proposed to be capable of binding to the apicoplast genome and play a role in DNA replication (Kumar et al., 2010). Interesting, Pfj1 has been shown to have a functional J domain (Nicoll et al., 2007), and therefore is likely to associate with a partner Hsp70; however, none of the PfHsp70s have been shown to localize to the apicoplast.
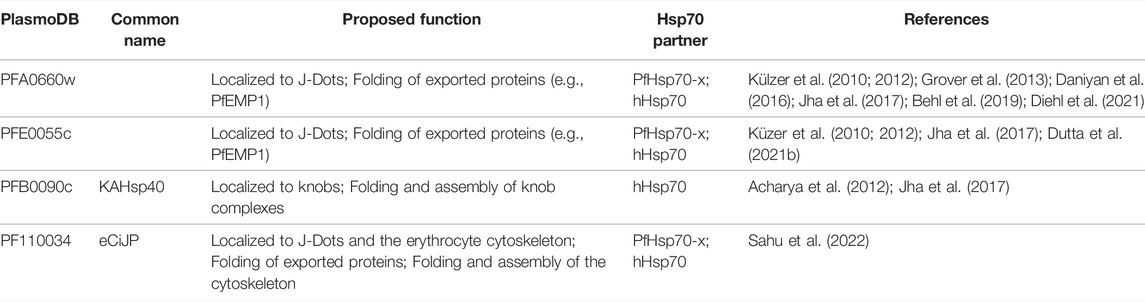
A number of recent reports suggest that the exported PfJDPs may serve as co-chaperones of not only the exported PfHsp70-x, but also the host Hsp70. As mentioned in the previous section, two of the exported PfJDPs (PFA0660w and PFE0055c) associate with PfHsp70-x in J-dots within the erythrocyte cytosol (Külzer et al., 2010; Külzer et al., 2012; Grover et al., 2013; Petersen et al., 2016), and have been shown to be co-chaperones of PfHsp70-x (Daniyan et al., 2016; Dutta et al., 2021b). It has been proposed that these J-dots play a role in the trafficking and folding of exported proteins (Külzer et al., 2012; Behl et al., 2019; Gabriela et al., 2022). Interestingly, one of the J-Dot PfJDPs, PFE0055c, was found to be essential (Zhang et al., 2018), while the other (PFA0660w) was not. However, functional disruption of PFA0660w was found to causes defects in knob formation and cytoadherence, with further genetic and biochemical studies suggesting that the role of PFA0660w in host cell modification involved host Hsp70 (Diehl et al., 2021; Table 1). This finding is consistent with a previous study using a yeast two-hybrid system, which reported an interaction between three exported PfJDPs (PFA0660w, PFE0055c, and PFB0090c) and human Hsp70 (Jha et al., 2017; Table 1). PFB0090c, also called knob-associated Hsp40 (KAHsp40; structurally similar to PFE0055c and PFA0660w), has been shown to interact with components of PTEX and knobs, and may be involved in the genesis of knob complexes (Acharya et al., 2012). It is well established that the knob protein complex does not contain PfHsp70-x, but rather, host chaperones (Hsp70, Hsp90, and Hop), and there is significant evidence that human Hsp70 is involved in the assembly of knob protein complexes (Banumathy et al., 2002; Alampalli et al., 2018). Hence, it is plausible that PFB0090c occurs in a common complex with human Hsp70, and potentially serves as its co-chaperone (Table 1).
The largest group of PfJDPs are those members containing a J domain with a corrupted HPD motif (so-called type IVs), most of which appear to be exported (Botha et al., 2007; Njunge et al., 2013; Daniyan and Blatch, 2017). In fact, there is evidence that a number of the exported type IV PfJDPs are essential for parasite survival (e.g., PFB0085c and PF14_0013; Zhang et al., 2018), required for growth or survival under febrile conditions [e.g., PFA0110w, the ring-infected erythrocyte surface antigen protein (RESA); Silva et al., 2005; Diez-Silva et al., 2012], or involved in pathogenesis (e.g., PF10_0381; knockout causes loss of knobs; Maier et al., 2008). Recently, an exported type IV PfJDP, called eCiJP/PF11_0034 (and a paralogue of PF10_0381), was found to localize to J-Dots, associate with the erythrocyte cytoskeleton, and to potentially interact with host Hsp70 (HSPA1A) (Sahu et al., 2022; Table 1).
Conclusion
The malaria parasite has adapted to its pathological co-existence with the human host, enabling it to overcome the extreme physiological and cellular challenges that it faces, particularly during the erythrocytic stages of the life cycle. P. falciparum appears to have finely tuned its molecular chaperone machinery to be highly efficient, particularly its PfHsp70-PfJDP pairs, which are found in most compartments of the parasite-infected erythrocyte. Most of these P. falciparum PfHsp70-PfJDP partnerships appears to have evolved to efficiently protect the N-repeat-rich parasite proteome from the toxic effects of aggregation and misfolding. Furthermore, and perhaps as importantly, the parasite appears to have harnessed the host Hsp70 chaperone machinery to enable it to renovate the infected erythrocyte for its survival and pathology. The molecular details of this host-parasite interface represent an important frontier of future research endeavours.
Author Contributions
GB developed the conceptual framework for this review and wrote the article.
Funding
GB acknowledges the financial support of Higher Colleges of Technology, UAE (Interdisciplinary Research Grant, IRG, grant number 213471), and Rhodes University, South Africa (Rated Researcher Grant).
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Acharya, P., Chaubey, S., Grover, M., and Tatu, U. (2012). An Exported Heat Shock Protein 40 Associates with Pathogenesis-Related Knobs in Plasmodium falciparum Infected Erythrocytes. PloS One 7, e44605. doi:10.1371/journal.pone.0044605
Alampalli, S. V., Grover, M., Chandran, S., Tatu, U., and Acharya, P. (2018). Proteome and Structural Organization of the Knob Complex on the Surface of the Plasmodium Infected Red Blood Cell. Proteomics Clin. Appl. 12, e1600177. doi:10.1002/prca.201600177
Anas, M., Shukla, A., Tripathi, A., Kumari, V., Prakash, C., Nag, P., et al. (2020). Structural-functional Diversity of Malaria Parasite's PfHSP70-1 and PfHSP40 Chaperone Pair Gives an Edge over Human Orthologs in Chaperone-Assisted Protein Folding. Biochem. J. 477, 3625–3643. doi:10.1042/bcj20200434
Banumathy, G., Singh, V., and Tatu, U. (2002). Host Chaperones Are Recruited in Membrane-Bound Complexes by Plasmodium falciparum. J. Biol. Chem. 277, 3902–3912. doi:10.1074/jbc.m110513200
Batinovic, S., McHugh, E., Chisholm, S. A., Matthews, K., Liu, B., Dumont, L., et al. (2017). An Exported Protein-Interacting Complex Involved in the Trafficking of Virulence Determinants in Plasmodium-Infected Erythrocytes. Nat. Commun. 8, 16044. doi:10.1038/ncomms16044
Beck, J. R., Muralidharan, V., Oksman, A., and Goldberg, D. E. (2014). PTEX Component HSP101 Mediates Export of Diverse Malaria Effectors into Host Erythrocytes. Nature 511, 592–595. doi:10.1038/nature13574
Behl, A., and Mishra, P. C. (2019). Structural Insights into the Binding Mechanism of Plasmodium falciparum Exported Hsp40-Hsp70 Chaperone Pair. Comput. Biol. Chem. 83, 107099. doi:10.1016/j.compbiolchem.2019.107099
Behl, A., Kumar, V., and Bisht, A. (2019). Cholesterol Bound Plasmodium falciparum Co-chaperone ‘PFA0660w’complexes with Major Virulence Factor ‘PfEMP1’via Chaperone ‘PfHsp70-X. Sci. Rep. 9, 1–7. doi:10.1038/s41598-019-39217-y
Botha, M., Pesce, E.-R., and Blatch, G. L. (2007). The Hsp40 Proteins of Plasmodium falciparum and Other Apicomplexa: Regulating Chaperone Power in the Parasite and the Host. Int. J. Biochem. Cell Biol. 39, 1781–1803. doi:10.1016/j.biocel.2007.02.011
Botha, M., Chiang, A. N., Needham, P. G., Stephens, L. L., Hoppe, H. C., Külzer, S., et al. (2011). Plasmodium falciparum Encodes a Single Cytosolic Type I Hsp40 that Functionally Interacts with Hsp70 and Is Upregulated by Heat Shock. Cell Stress Chaperones 16, 389–401. doi:10.1007/s12192-010-0250-6
Charnaud, S. C., Dixon, M. W. A., Nie, C. Q., Chappell, L., Sanders, P. R., Nebl, T., et al. (2017). The Exported Chaperone Hsp70-X Supports Virulence Functions for Plasmodium falciparum Blood Stage Parasites. PLoS One 12, e0181656. doi:10.1371/journal.pone.0181656
Chiang, A. N., Valderramos, J.-C., Balachandran, R., Chovatiya, R. J., Mead, B. P., Schneider, C., et al. (2009). Select Pyrimidinones Inhibit the Propagation of the Malarial Parasite, Plasmodium falciparum. Bioorg. Med. Chem. 17, 1527–1533. doi:10.1016/j.bmc.2009.01.024
Cobb, D. W., Florentin, A., Fierro, M. A., Krakowiak, M., Moore, J. M., and Muralidharan, V. (2017). The Exported Chaperone PfHsp70x Is Dispensable for the Plasmodium falciparum Intraerythrocytic Life Cycle. MSphere 2, e00363–17. doi:10.1128/mSphere.00363-17
Cockburn, I. L., Pesce, E. R., Pryzborski, J. M., Davies-Coleman, M. T., Clark, P. G., Keyzers, R. A., et al. (2011). Screening for Small Molecule Modulators of Hsp70 Chaperone Activity Using Protein Aggregation Suppression Assays: Inhibition of the Plasmodial Chaperone PfHsp70-1. Biol. Chem. 392, 431–438. doi:10.1515/BC.2011.040
Cockburn, I. L., Boshoff, A., Pesce, E.-R., and Blatch, G. L. (2014). Selective Modulation of Plasmodial Hsp70s by Small Molecules with Antimalarial Activity. Biol. Chem. 395, 1353–1362. doi:10.1515/hsz-2014-0138
Cortés, G. T., Wiser, M. F., and Gómez-Alegría, C. J. (2020). Identification of Plasmodium falciparum HSP70-2 as a Resident of the Plasmodium Export Compartment. Heliyon 6, e04037.
Daniyan, M. O., and Blatch, G. L. (2017). Plasmodial Hsp40s: New Avenues for Antimalarial Drug Discovery. Curr. Pharm. Des. 23, 4555–4570. doi:10.2174/1381612823666170124142439
Daniyan, M. O., Boshoff, A., Prinsloo, E., Pesce, E.-R., and Blatch, G. L. (2016). The Malarial Exported PFA0660w Is an Hsp40 Co-chaperone of PfHsp70-X. PLoS ONE 11, e0148517. doi:10.1371/journal.pone.0148517
Day, J., Passecker, A., Beck, H.-P., and Vakonakis, I. (2019). The Plasmodium falciparum Hsp70-X Chaperone Assists the Heat Stress Response of the Malaria Parasite. FASEB J. 33, 14611–14624. doi:10.1096/fj.201901741r
de Koning-Ward, T. F., Gilson, P. R., Boddey, J. A., Rug, M., Smith, B. J., Papenfuss, A. T., et al. (2009). A Newly Discovered Protein Export Machine in Malaria Parasites. Nature 459, 945–949. doi:10.1038/nature08104
Diehl, M., Roling, L., Rohland, L., Weber, S., Cyrklaff, M., Sanchez, C. P., et al. (2021). Co-chaperone Involvement in Knob Biogenesis Implicates Host-Derived Chaperones in Malaria Virulence. PLoS Pathog. 17, e1009969. doi:10.1371/journal.ppat.1009969
Diez-Silva, M., Park, Y., Huang, S., Bow, H., Mercereau-Puijalon, O., Deplaine, G., et al. (2012). Pf155/RESA Protein Influences the Dynamic Micro-Circulatory Behavior of Ring-Stage Plasmodium falciparum Infected Red Blood Cells. Sci. Rep. 2, 614. doi:10.1038/srep00614
Dutta, T., Pesce, E.-R., Maier, A. G., and Blatch, G. L. (2021a). Role of the J Domain Protein Family in the Survival and Pathogenesis of Plasmodium falciparum. Adv. Exp. Med. Biol. 1340, 97–123. doi:10.1007/978-3-030-78397-6_4
Dutta, T., Singh, H., Gestwicki, J. E., and Blatch, G. L. (2021b). Exported Plasmodial J Domain Protein, PFE0055c, and PfHsp70-X Form a Specific Co-chaperone-chaperone Partnership. Cell Stress Chaperones 26, 355–366. doi:10.1007/s12192-020-01181-2
Elsworth, B., Matthews, K., Nie, C. Q., Kalanon, M., Charnaud, S. C., Sanders, P. R., et al. (2014). PTEX Is an Essential Nexus for Protein Export in Malaria Parasites. Nature 511, 587–591. doi:10.1038/nature13555
Elsworth, B., Sanders, P. R., Nebl, T., Batinovic, S., Kalanon, M., Nie, C. Q., et al. (2016). Proteomic Analysis Reveals Novel Proteins Associated with the Plasmodium Protein Exporter PTEX and a Loss of Complex Stability upon Truncation of the Core PTEX Component, PTEX150. Cell. Microbiol. 18, 1551–1569. doi:10.1111/cmi.12596
Gabriela, M., Matthews, K. M., Boshoven, C., Kouskousis, B., Jonsdottir, T. K., Bullen, H. E., et al. (2022). A Revised Mechanism for How Plasmodium falciparum Recruits and Exports Proteins into its Erythrocytic Host Cell. PLoS Pathog. 18, e1009977. doi:10.1371/journal.ppat.1009977
Gitau, G. W., Mandal, P., Blatch, G. L., Przyborski, J., and Shonhai, A. (2012). Characterisation of the Plasmodium falciparum Hsp70-Hsp90 Organising Protein (PfHop). Cell Stress Chaperones 17, 191–202. doi:10.1007/s12192-011-0299-x
Grover, M., Chaubey, S., Ranade, S., and Tatu, U. (2013). Identification of an Exported Heat Shock Protein 70 in Plasmodium falciparum. Parasite 20, 2. doi:10.1051/parasite/2012002
Halfmann, R., Alberti, S., Krishnan, R., Lyle, N., O'Donnell, C. W., King, O. D., et al. (2011). Opposing Effects of Glutamine and Asparagine Govern Prion Formation by Intrinsically Disordered Proteins. Mol. Cell 43, 72–84. doi:10.1016/j.molcel.2011.05.013
Hiller, N. L., Bhattacharjee, S., van Ooij, C., Liolios, K., Harrison, T., LopezEstraño, C., et al. (2004). A Host-Targeting Signal in Virulence Proteins Reveals a Secretome in Malarial Infection. Science 306, 1934–1937. doi:10.1126/science.1102737
Jha, P., Laskar, S., Dubey, S., Bhattacharyya, M. K., and Bhattacharyya, S. (2017). Plasmodium Hsp40 and Human Hsp70: A Potential Cochaperone-Chaperone Complex. Mol. Biochem. Parasitol. 214, 10–13. doi:10.1016/j.molbiopara.2017.03.003
Khosh-Naucke, M., Becker, J., Mesén-Ramírez, P., Kiani, P., Birnbaum, J., Fröhlke, U., et al. (2018). Identification of Novel Parasitophorous Vacuole Proteins in P. falciparum Parasites Using BioID. Int. J. Med. Microbiol. 308, 13–24. doi:10.1016/j.ijmm.2017.07.007
Kudyba, H. M., Cobb, D. W., Fierro, M. A., Florentin, A., Ljolje, D., Singh, B., et al. (2019). The Endoplasmic Reticulum Chaperone PfGRP170 Is Essential for Asexual Development and Is Linked to Stress Response in Malaria Parasites. Cell Microbiol. 21, e13042. doi:10.1111/cmi.13042
Külzer, S., Rug, M., Brinkmann, K., Cannon, P., Cowman, A., Lingelbach, K., et al. (2010). Parasite-encoded Hsp40 Proteins Define Novel Mobile Structures in the Cytosol of the P. falciparum-Infected Erythrocyte. Cell Microbiol. 12, 1398–1420. doi:10.1111/j.1462-5822.2010.01477.x
Külzer, S., Charnaud, S., Dagan, T., Riedel, J., Mandal, P., Pesce, E. R., et al. (2012). Plasmodium falciparum-encoded Exported Hsp70/hsp40 Chaperone/co-Chaperone Complexes within the Host Erythrocyte. Cell Microbiol. 14, 1784–1795. doi:10.1111/j.1462-5822.2012.01840.x
Kumar, N., Koski, G., Harada, M., Aikawa, M., and Zheng, H. (1991). Induction and Localization of Plasmodium falciparum Stress Proteins Related to the Heat Shock Protein 70 Family. Mol. Biochem. Parasitol. 48, 47–58. doi:10.1016/0166-6851(91)90163-z
Kumar, A., Tanveer, A., Biswas, S., Ram, E. V. S. R., Gupta, A., Kumar, B., et al. (2010). Nuclear-encoded DnaJ Homologue of Plasmodium falciparum interacts with Replicationori of the Apicoplast Genome. Mol. Microbiol. 75, 942–956. doi:10.1111/j.1365-2958.2009.07033.x
LaCount, D. J., Vignali, M., Chettier, R., Phansalkar, A., Bell, R., Hesselberth, J. R., et al. (2005). A Protein Interaction Network of the Malaria Parasite Plasmodium falciparum. Nature 438, 103–107. doi:10.1038/nature04104
Lebepe, C. M., Matambanadzo, P. R., Makhoba, X. H., Achilonu, I., Zininga, T., and Shonhai, A. (2020). Comparative Characterization of Plasmodium falciparum Hsp70-1 Relative to E. coli DnaK Reveals the Functional Specificity of the Parasite Chaperone. Biomolecules 10, 856. doi:10.3390/biom10060856
Mabate, B., Zininga, T., Ramatsui, L., Makumire, S., Achilonu, I., Dirr, H. W., et al. (2018). Structural and Biochemical Characterization of Plasmodium falciparum Hsp70-X Reveals Functional Versatility of its C-Terminal EEVN Motif. Proteins 86, 1189–1201. doi:10.1002/prot.25600
Maier, A. G., Rug, M., O'Neill, M. T., Brown, M., Chakravorty, S., Szestak, T., et al. (2008). Exported Proteins Required for Virulence and Rigidity of Plasmodium falciparum-Infected Human Erythrocytes. Cell 134, 48–61. doi:10.1016/j.cell.2008.04.051
Makumire, S., Dongola, T. H., Chakafana, G., Tshikonwane, L., Chauke, C. T., Maharaj, T., et al. (2021). Mutation of GGMP Repeat Segments of Plasmodium falciparum Hsp70-1 Compromises Chaperone Function and Hop Co-chaperone Binding. Ijms 22, 2226. doi:10.3390/ijms22042226
Marapana, D. S., Dagley, L. F., Sandow, J. J., Nebl, T., Triglia, T., Pasternak, M., et al. (2018). Plasmepsin V Cleaves Malaria Effector Proteins in a Distinct Endoplasmic Reticulum Translocation Interactome for Export to the Erythrocyte. Nat. Microbiol. 3, 1010–1022. doi:10.1038/s41564-018-0219-2
Marti, M., Good, R. T., Rug, M., Knuepfer, E., and Cowman, A. F. (2004). Targeting Malaria Virulence and Remodeling Proteins to the Host Erythrocyte. Science 306, 1930–1933. doi:10.1126/science.1102452
Matambo, T. S., Odunuga, O. O., Boshoff, A., and Blatch, G. L. (2004). Overproduction, Purification, and Characterization of the Plasmodium falciparum Heat Shock Protein 70. Protein Expr. Purif. 33, 214–222. doi:10.1016/j.pep.2003.09.010
Mathews, E. S., Jezewski, A. J., and Odom John, A. R. (2021). Protein Prenylation and Hsp40 in Thermotolerance of Plasmodium falciparum Malaria Parasites. mBio 12, e0076021. doi:10.1128/mBio.00760-21
Matthews, K. M., Kalanon, M., and de Koning-Ward, T. F. (2019). Uncoupling the Threading and Unfoldase Actions of Plasmodium HSP101 Reveals Differences in Export between Soluble and Insoluble Proteins. mBio 10, e01106–19. doi:10.1128/mBio.01106-19
Misra, G., and Ramachandran, R. (2009). Hsp70-1 from Plasmodium falciparum: Protein Stability, Domain Analysis and Chaperone Activity. Biophys. Chem. 142, 55–64. doi:10.1016/j.bpc.2009.03.006
Muralidharan, V., Oksman, A., Pal, P., Lindquist, S., and Goldberg, D. E. (2012). Plasmodium falciparum Heat Shock Protein 110 Stabilizes the Asparagine Repeat-Rich Parasite Proteome during Malarial Fevers. Nat. Commun. 3, 1310. doi:10.1038/ncomms2306
Nicoll, W. S., Botha, M., McNamara, C., Schlange, M., Pesce, E.-R., Boshoff, A., et al. (2007). Cytosolic and ER J-Domains of Mammalian and Parasitic Origin Can Functionally Interact with DnaK. Int. J. Biochem. Cell Biol. 39, 736–751. doi:10.1016/j.biocel.2006.11.006
Njunge M., J., Ludewig H., M., Boshoff, A., Pesce, E.-R., and Blatch L., G. (2013). Hsp70s and J Proteins of Plasmodium Parasites Infecting Rodents and Primates: Structure, Function, Clinical Relevance, and Drug Targets. Curr. Pharm. Des. 19, 387–403. doi:10.2174/138161213804143734
Njunge, J. M., Mandal, P., Przyborski, J. M., Boshoff, A., Pesce, E.-R., and Blatch, G. L. (2015). PFB0595w Is a Plasmodium falciparum J Protein that Co-localizes with PfHsp70-1 and Can Stimulate its In Vitro ATP Hydrolysis Activity. Int. J. Biochem. Cell Biol. 62, 47–53. doi:10.1016/j.biocel.2015.02.008
Pallare`s, I., de Groot, N. S., Iglesias, V., Sant’Anna, R., Biosca, A., Ferna`ndez-Busquets, X., et al. (2018). Discovering Putative Prion-like Proteins in Plasmodium falciparum: a Computational and Experimental Analysis. Front. Microbiol. 9, 1737.
Pesce, E.-R., and Blatch, G. L. (2014). Plasmodial Hsp40 and Hsp70 Chaperones: Current and Future Perspectives. Parasitology 141, 1167–1176. doi:10.1017/s003118201300228x
Pesce, E.-R., Acharya, P., Tatu, U., Nicoll, W. S., Shonhai, A., Hoppe, H. C., et al. (2008). The Plasmodium falciparum Heat Shock Protein 40, Pfj4, Associates with Heat Shock Protein 70 and Shows Similar Heat Induction and Localisation Patterns. Int. J. Biochem. Cell Biol. 40, 2914–2926. doi:10.1016/j.biocel.2008.06.011
Petersen, W., Külzer, S., Engels, S., Zhang, Q., Ingmundson, A., Rug, M., et al. (2016). J-dot Targeting of an Exported HSP40 in Plasmodium falciparum-infected Erythrocytes. Int. J. Parasitol. 46, 519–525. doi:10.1016/j.ijpara.2016.03.005
Przyborski, J. M., Diehl, M., and Blatch, G. L. (2015). Plasmodial Hsp70s Are Functionally Adapted to the Malaria Parasite Life Cycle. Front. Mol. Biosci. 2, 34. doi:10.3389/fmolb.2015.00034
Rajapandi, T. (2020). Chaperoning of Asparagine Repeat-Containing Proteins in Plasmodium falciparum. J Parasit. Dis. 44, 687–693. doi:10.1007/s12639-020-01251-3
Rhiel, M., Bittl, V., Tribensky, A., Charnaud, S. C., Strecker, M., Müller, S., et al. (2016). Trafficking of the Exported P. falciparum Chaperone PfHsp70x. Sci. Rep. 6, 36174. doi:10.1038/srep36174
Russo, I., Babbitt, S., Muralidharan, V., Butler, T., Oksman, A., and Goldberg, D. E. (2010). Plasmepsin V Licenses Plasmodium Proteins for Export into the Host Erythrocyte. Nature 463, 632–636. doi:10.1038/nature08726
Sahu, W., Bai, T., Panda, P. K., Mazumder, A., Das, A., Ojha, D. K., et al. (2022). Plasmodium falciparum HSP40 Protein eCiJp Traffics to the Erythrocyte Cytoskeleton and Interacts with the Human HSP70 Chaperone HSPA1. FEBS Lett. 596, 95–111. doi:10.1002/1873-3468.14255
Sargeant, T. J., Marti, M., Caler, E., Carlton, J. M., Simpson, K., Speed, T. P., et al. (2006). Lineage-specific Expansion of Proteins Exported to Erythrocytes in Malaria Parasites. Genome Biol. 7, R12. doi:10.1186/gb-2006-7-2-r12
Saridaki, T., Sanchez, C. P., Pfahler, J., and Lanzer, M. (2008). A Conditional Export System Provides New Insights Into Protein Export in Plasmodium falciparum-Infected Erythrocytes. Cell Microbiol. 1, 2483–2495. doi:10.1111/j.1462-5822.2008.01223.x
Schmidt, J., and Vakonakis, I. (2020). Structure of the Substrate-Binding Domain of Plasmodium falciparum Heat-Shock Protein 70-x. Acta Cryst. Sect. F. 76, 495–500. doi:10.1107/s2053230x2001208x
Shahinas, D., and Pillai, D. R. (2021). Role of Hsp90 in Plasmodium falciparum Malaria. Adv. Exp. Med. Biol. 1340, 125–139. doi:10.1007/978-3-030-78397-6_5
Shonhai, A., Boshoff, A., and Blatch, G. L. (2007). The Structural and Functional Diversity of Hsp70 Proteins from Plasmodium falciparum. Protein Sci. 16, 1803–1818. doi:10.1110/ps.072918107
Shonhai, A. (2021). The Role of Hsp70s in the Development and Pathogenicity of Plasmodium falciparum. Adv. Exp. Med. Biol. 340, 75–95. doi:10.1007/978-3-030-78397-6_3
Silva, M. D., Cooke, B. M., Guillotte, M., Buckingham, D. W., Sauzet, J.-P., Scanf, C. L., et al. (2005). A Role for the Plasmodium falciparum RESA Protein in Resistance against Heat Shock Demonstrated Using Gene Disruption. Mol. Microbiol. 56, 990–1003. doi:10.1111/j.1365-2958.2005.04603.x
Singh, G. P., Chandra, B. R., Bhattacharya, A., Akhouri, R. R., Singh, S. K., and Sharma, A. (2004). Hyper-expansion of Asparagines Correlates with an Abundance of Proteins with Prion-like Domains in Plasmodium falciparum. Mol. Biochem. Parasitol. 137, 307–319. doi:10.1016/j.molbiopara.2004.05.016
Tuteja, R. (2007). Unraveling the Components of Protein Translocation Pathway in Human Malaria Parasite Plasmodium falciparum. Archives Biochem. Biophysics 467, 249–260. doi:10.1016/j.abb.2007.08.031
Watanabe, J. (1987). Cloning and Characterization of Heat Shock Protein DnaJ Homologues from Plasmodium falciparum and Comparison with Ring Infected Erythrocyte Surface Antigen. Mol. Biochem. Parasitol. 88, 253–258. doi:10.1016/s0166-6851(97)00073-x
Zhang, M., Wang, C., Otto, T. D., Oberstaller, J., Liao, X., Adapa, S. R., et al. (2018). Uncovering the Essential Genes of the Human Malaria Parasite Plasmodium falciparum by Saturation Mutagenesis. Science 360, eaap7847. doi:10.1126/science.aap7847
Zimmermann, R., and Blatch, G. L. (2009). A Novel Twist to Protein Secretion in Eukaryotes. Trends Parasitol. 25, 147–150. doi:10.1016/j.pt.2009.01.002
Zininga, T., Makumire, S., Gitau, G. W., Njunge, J. M., Pooe, O. J., Klimek, H., et al. (2015). Plasmodium falciparum Hop (PfHop) Interacts with the Hsp70 Chaperone in a Nucleotide-dependent Fashion and Exhibits Ligand Selectivity. PLoS One 10, e0135326. doi:10.1371/journal.pone.0135326
Zininga, T., Achilonu, I., Hoppe, H., Prinsloo, E., Dirr, H. W., and Shonhai, A. (2016). Plasmodium falciparum Hsp70-Z, an Hsp110 Homologue, Exhibits Independent Chaperone Activity and Interacts with Hsp70-1 in a Nucleotide-dependent Fashion. Cell Stress Chaperones 21, 499–513. doi:10.1007/s12192-016-0678-4
Zininga, T., Pooe, O. J., Makhado, P. B., Ramatsui, L., Prinsloo, E., Achilonu, I., et al. (2017a). Polymyxin B Inhibits the Chaperone Activity of Plasmodium falciparum Hsp70. Cell Stress Chaperones 22, 707–715. doi:10.1007/s12192-017-0797-6
Zininga, T., Anokwuru, C., Sigidi, M., Tshisikhawe, M., Ramaite, I., Traoré, A., et al. (2017b). Extracts Obtained from Pterocarpus Angolensis DC and Ziziphus Mucronata Exhibit Antiplasmodial Activity and Inhibit Heat Shock Protein 70 (Hsp70) Function. Molecules 22, 1224. doi:10.3390/molecules22071224
Zininga, T., Ramatsui, L., Makhado, P., Makumire, S., Achilinou, I., Hoppe, H., et al. (2017c). (−)-Epigallocatechin-3-Gallate Inhibits the Chaperone Activity of Plasmodium falciparum Hsp70 Chaperones and Abrogates Their Association with Functional Partners. Molecules 22, 2139. doi:10.3390/molecules22122139
Keywords: heat shock proteins, molecular chaperones and co-chaperones, malaria parasite, parasitophorous vacuole, proteostasis
Citation: Blatch GL (2022) Plasmodium falciparum Molecular Chaperones: Guardians of the Malaria Parasite Proteome and Renovators of the Host Proteome. Front. Cell Dev. Biol. 10:921739. doi: 10.3389/fcell.2022.921739
Received: 16 April 2022; Accepted: 28 April 2022;
Published: 16 May 2022.
Edited by:
Francesco Fazi, Sapienza University of Rome, ItalyReviewed by:
Paul R. Gilson, Burnet Institute, AustraliaCopyright © 2022 Blatch. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gregory L. Blatch, Zy5ibGF0Y2hAcnUuYWMuemE=
 Gregory L. Blatch
Gregory L. Blatch