
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
EDITORIAL article
Front. Cell Dev. Biol. , 17 June 2022
Sec. Molecular and Cellular Pathology
Volume 10 - 2022 | https://doi.org/10.3389/fcell.2022.918511
This article is part of the Research Topic Molecular Mechanisms of Autophagy in Cancer View all 8 articles
Editorial on the Research Topic
Molecular Mechanisms of Autophagy in Cancer
Autophagy (self-eating) is an evolutionary conserved process. It plays an important role in keeping cells clear of damaging insults by degrading damaged proteins and organelles as well as fighting against infections. Autophagy also provides energy during fasting or exercise through the lysosomal degradation of cellular components. Therefore, it is not surprising that autophagy is involved in protection against many diseases, including cancer (Mizushima and Levine, 2020). Multiple studies have investigated the role of autophagy in cancer development and progression showing that this cellular process could play onco-suppressive or oncogenic functions (Vega-Rubin-de-Celis, 2021). Thus, autophagy dysregulation has been proposed to contribute to cancer generation and progression by facilitating some of the events that lead to cell transformation. Likewise, autophagy stimulation could be one of the mechanisms by which primary or metastatic tumors adapt to the hypoxic and nutrient-deprived environment characteristic of the cancer niche. However, the precise mechanisms underlying these effects as well as the events that regulate the expression of key autophagy regulatory genes in cancer cells are far from being fully understood. Furthermore, several clinical trials in different tumor types have been developed using autophagy modulators (especially the non-specific autophagy inhibitor chloroquine) (Mohsen et al., 2022). Unfortunately, results from these studies have been disappointing, probably reflecting the need for a better understanding of the role of autophagy in tumorigenesis. This special issue includes a total of seven papers, including six original articles and one review that might help to improve our understanding of these processes (Figure 1).
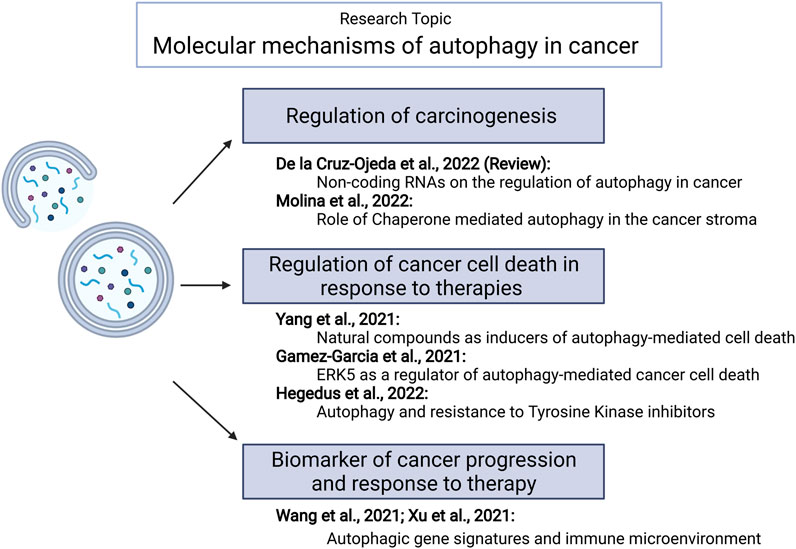
FIGURE 1. Summary of papers published within this Research Topic. Generated using BioRender.com.
The importance of autophagy-related non-coding RNAs in carcinogenesis is summarized in a review by de la Cruz-Ojeda et al. This manuscript discusses some of the mechanisms that could be involved in the regulation of the expression of autophagic genes during cancer generation and progression underlining the key role played by ncRNAs in the control of macroautophagy (de la Cruz-Ojeda et al.).
As discussed above, depending on the cellular context and the stage of tumor progression, activation or inhibition of autophagic flux will impact oncogenesis differently. Following this interesting question, one article included in this special issue explores the role of chaperone-mediated autophagy (CMA) in pericytes - one of the cell types that plays a crucial role in the glioblastoma microenvironment (Molina et al.). The possibility of selectively targeting macroautophagy or CMA in certain cell types is an issue of great interest that may have potential therapeutic implications.
Autophagy plays a dual role in the regulation of cell survival. Accordingly, promoting the activation of cytotoxic autophagy (autophagy-mediated cell death) has been a significant part of the work in the autophagy and cancer field. Likewise, avoiding the activation of protective autophagy as a mechanism of resistance to cancer therapies is an issue of utmost importance that has been the subject of intensive research. Three articles included in this issue of Frontiers in Cell and Developmental Biology explore these questions in further detail. Thus, in the article by Yang and colleagues (Yang et al.), the authors investigate the autophagic mechanism by which a natural compound activates cancer cell death. In their study, they demonstrated that TEOA induced autophagic cell death in pancreatic ductal adenocarcinoma cells by promoting mitochondria dysfunction and increasing ROS production. Another article (Gamez-Garcia et al.) identifies a new signaling mechanism involved in the regulation of autophagy-mediated cancer cell death that may have very interesting therapeutic implications. Briefly, ERK5 inhibition induces ER stress which leads in turn to autophagy-mediated cancer cell death. Finally, Hegedüs et al. identified a potential resistance mechanism to treatment with tyrosine kinase inhibitors in malignant pleural mesothelioma that is associated with autophagy stimulation. Interestingly, such a mechanism does not seem to rely on the regulation of the AKT/mTORC1 and ERK pathways (Hegedus et al.).
A third important aspect of autophagy research in the cancer field is focused on the investigation of the potential use of readouts of the autophagic process (including the expression of autophagy-associated genes) as potential biomarkers of cancer progression or response to therapy. Two articles within this Research Topic explore this question and, specifically, the potential connection between autophagy and the immune microenvironment (Wang et al.; Xu et al.).
Research in the autophagic field has yielded spectacular advances in the last couple of decades but there are still many burning questions that remain to be investigated and, specifically, in the context of the role of this cellular process in cancer. Altogether, studies published in the present topic highlight the importance of more precisely dissecting the mechanisms that regulate the activation of autophagy.
All authors contributed equally to this work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Mizushima, N., and Levine, B. (2020). Autophagy in Human Diseases. N. Engl. J. Med. 383 (16), 1564–1576. doi:10.1056/NEJMra2022774
Mohsen, S., Sobash, P. T., Algwaiz, G. F., Nasef, N., Al-Zeidaneen, S. A., and Karim, N. A. (2022). Autophagy Agents in Clinical Trials for Cancer Therapy: A Brief Review. Curr. Oncol. 29 (3), 1695–1708. doi:10.3390/curroncol29030141
Keywords: autophagy, cancer, therapy, CMA, biomarkers
Citation: Humbert M, Vega-Rubin-de-Celis S, Velasco G and Wei Y (2022) Editorial: Molecular Mechanisms of Autophagy in Cancer. Front. Cell Dev. Biol. 10:918511. doi: 10.3389/fcell.2022.918511
Received: 12 April 2022; Accepted: 25 April 2022;
Published: 17 June 2022.
Edited and reviewed by:
Ramani Ramchandran, Medical College of Wisconsin, United StatesCopyright © 2022 Humbert, Vega-Rubin-de-Celis, Velasco and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Magali Humbert, bWFnYWxpLmh1bWJlcnRAaGVtb3N0b2QuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.