
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Cell Dev. Biol., 15 June 2022
Sec. Molecular and Cellular Pathology
Volume 10 - 2022 | https://doi.org/10.3389/fcell.2022.914788
This article is part of the Research TopicRegional and Molecular Fingerprint of AdipogenesisView all 9 articles
Substantial levels of lysozyme in adipose tissue in association to obesity have been recently demonstrated in mice and humans. In addition, experiments in mice suggest that lysozyme might impact on adipose tissue adipogenesis. To further investigate the relationship between lysozyme and adipogenesis, in the present study, we aimed to study lysozyme (Lyz2) during 3T3-L1 adipocyte differentiation and its possible role in adipogenesis. Time course experiment during 3T3-L1 adipocyte differentiation indicated that Lyz2 gene expression decreased at day 4, which was caused by isobutylmethylxanthine administration, and recovered at the end of the process (day 8). Importantly, the impact of isobutylmethylxanthine-induced downregulation of Lyz2 gene expression on adipogenesis was not comparable to that observed in the full cocktail, questioning whether the reduction in lysozyme at early stage of adipocyte differentiation is relevant to this process. In fact, the depletion in Lyz2 expression had a negative impact on adipogenesis, and rosiglitazone administration failed to compensate for the anti-adipogenic effect observed in Lyz2 gene knockdown cells. Otherwise, when Lyz2 gene knockdown cells were co-cultured with control cells, these cells had higher expression of adipogenic genes than those co-cultured with themselves at the end of adipocyte differentiation. In conclusion, this study suggests that lysozyme expression in 3T3-L1 cells sustains expression of adipogenic genes and adipocyte differentiation.
The generation of new adipocytes in adipose tissue is mediated by adipogenesis, which consists in the differentiation of adipose-derived mesenchymal stem cells into adipocytes. This process is crucial to maintain adipose tissue physiology and prevent obesity-associated metabolic disturbances (Vishvanath and Gupta, 2019).
In the searching of new factors that might modulate adipose tissue adipogenesis, in a recent study, we found that lysozyme was highly expressed in adipose tissue in association with obesity and inflammation, but negatively correlated with the expression of adipogenesis-related genes in both subcutaneous and visceral adipose tissue (Latorre et al., 2021). More importantly, adipose tissue lysozyme gene knockdown in mice fed with high-fat and high-sucrose diet improved adipogenesis in parallel to reduced inflammatory markers (Latorre et al., 2021).
Although the highest expression of lysozyme in adipose tissue occurs in macrophages, it is also expressed to a lesser extent in preadipocytes and adipocytes (Latorre et al., 2021). At cellular level, lysozyme expression during adipogenesis, and whether it has a direct role on this process remains to be explored. In the present study, we aimed to investigate lysozyme (Lyz2) during 3T3-L1 adipocyte differentiation and its possible role in this process.
3T3-L1 cells, an embryonic fibroblast mouse cell line (ATCC, LGC Standards GmbH, Wesel, Germany), were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 4.5 g/L glucose supplemented with 10% Fetal Bovine Serum (FBS), 1% penicillin and streptomycin, 1% Glutamine and 2% Hepes at 37°C and 5% CO2. At 2 days after confluence, to induce differentiation, a cocktail mixture containing insulin (INS, 5 μg/ml), dexamethasone (DEX, 0.25 μM), and isobutylmethylxanthine (IBMX, 0.25 mM) was added for 4 days (Day 4), followed by 4 days with INS (5 μg/ml) alone (Day 8). Cocktail components were tested separately at Day 4, comprising INS (5 μg/ml) or DEX (0.25 μM) or IBMX (0.25 mM) or all of them.
Permanent silencing was achieved in murine 3T3-L1 fibroblasts using Lyz2-targeted and control sh-RNA lentiviral particles, obtained as previously described (Latorre et al., 2021). Puromycin (2 ug/ml) was used to select positive adipocytes harboring sh-RNA cassette of Lyz2 or control. After selection, cells were induced to differentiate, comprising Day 0, Day 4 and Day 8. Afterwards, Rosiglitazone treatments at 0.5 μmol/L were applied in shRNA_SCR and shRNA_Lyz2 cells in parallel to vehicle-treated shRNA_SCR and shRNA_Lyz2 cells, all induced to differentiate into mature adipocytes.
Coculture was assessed using cell culture inserts 0.4 um pore size (#353180, Corning Inc., Corning, NY) in a TC-Platte 12 Well (#83.3921, Sarstedt, Nümbrecht, Germany) making the combinations of shRNA_Lyz2 (upper) and shRNA_Lyz2 or shRNA_SCR (down) and induced to differentiate into mature adipocytes.
Total RNA was extracted from cells using RNeasy Mini Kit (QIAgen, Izasa SA, Barcelona, Spain) and quantified using a Nanodrop ND-1000 Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, United States). The same amount of total RNA was reverse transcribed to cDNA using High Capacity cDNA Archive Kit (Applied Biosystems, Darmstadt, Germany). Commercial available TaqMan primer/probes sets were used to analyze gene expression with a Light Cycler 480 II (Roche Diagnostics SL, Barcelona, Spain). Primer/probe sets used were as follows: Peptidylprolyl isomerase A (Ppia, Mm02342430_g1 as endogenous control), lysozyme (Lyz2, Mm01612741_m1), CCAAT/enhancer binding protein (C/EBP), alpha (Cebpa, Mm00514283_s1), CCAAT/enhancer binding protein (C/EBP), beta (Cebpb, Mm00843434_s1), fatty acid binding protein 4, adipocyte (Fabp4, Mm00445880_m1), adiponectin (Adipoq, Mm04933656_m1), perilipin 1 (Plin1, Mm00558672_m1), solute carrier family 2 member 4 (Slc2a4, Mm00436615_m1) and peroxisome proliferator activated receptor gamma (Pparg, Mm00440940_m1).
Oil-Red O Staining was used to assess intracellular lipid content. Briefly, cells were washed with cold PBS, fixed with paraformaldehyde 4% (Sigma-Aldrich, Saint Louis, MO, United States) for 1 h, dipped in isopropanol 60% before completely dried, stained with filtered 5% Oil-Red O solution in isopropanol for 10 min at room temperature and washed several times with distilled water. Images were taken using an inverted microscope Anxiovert 40 CFL (Carl Zeiss, Jena, Germany). The stained area was quantified with ImageJ software (Rasband, W. S., ImageJ, United States National Institutes of Health, Bethesda, MD) by means of optimal applicable threshold tools. The image was first transformed to 8-bit format and inverted, followed by a constant threshold adjustment for all conditions, allowing to distinct the red-oil stained areas from the rest with red signal, which was analyzed as the % stained area in respect to image total area.
Statistical analyses were performed using SPSS statistical software (SPSS v21.0; IBM, Chicago, IL, United States). Data are presented as mean ± SEM. Unpaired t-test and nonparametric test (Mann Whitney test) was used to analyse in vitro experimental data. Levels of statistical significance were set at p < 0.05.
In a time course experiment, we found that Lyz2 gene expression decreased at day 4, but was recovered at the end of the process (day 8) (Figure 1A). Interestingly, the decrease of Lyz2 gene expression in this early stage of adipocyte differentiation was associated with the increase in gene expression of very early (Cebpb), early (Cebpa, Pparg) and middle-terminal stage (Adipoq, Plin1, Slc2a4 and Fabp4) markers of adipogenesis (Farmer, 2006; Kleiman et al., 2009; Burrell and Stephens, 2021; Yadav and Jang, 2021) (Figure 1A), suggesting that the decrease in Lyz2 gene expression at this stage might be required for adipocyte differentiation.
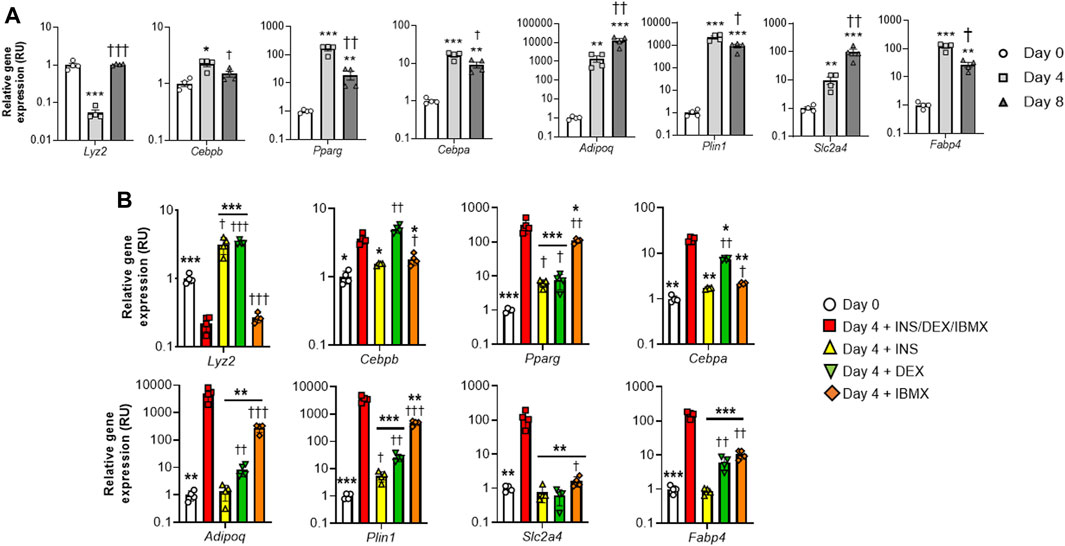
FIGURE 1. Lyz2 gene expression during 3T3-L1 adipocyte differentiation. (A) Lyz2, Cebpb, Pparg, Cebpa, Adipoq, Plin1, Slc2a4 and Fabp4 gene expression during 3T3-L1 adipocyte differentiation. **p < 0.01 and ***p < 0.001 compared to Day 0; †p < 0.05, ††p < 0.01 and †††p < 0.001 compared to Day 4. (B) The effect of individual compound administration (INS, DEX or IBMX) of differentiation cocktail on Lyz2, Cebpb, Pparg, Cebpa, Adipoq, Plin1, Slc2a4 and Fabp4 gene expression during 4 days. **p < 0.01 and ***p < 0.001 compared to complete differentiation cocktail (INS/DEX/IBMX); †p < 0.05, ††p < 0.01 and †††p < 0.001 compared to Day 0.
To study which compound of the differentiation cocktail could be responsible for the downregulation of Lyz2 gene expression at day 4, a second experiment in which individual administrations of insulin, dexamethasone or isobutylmethylxanthine (IBMX) was performed, only IBMX replicated the observed effects of the complete differentiation cocktail (Figure 1B). In fact, insulin and dexamethasone administrations displayed opposite effects (Figure 1B). In this experiment, even though insulin- and dexamethasone-induced increase of Lyz2 gene expression was associated to decreased expression in adipogenic genes compared to the full cocktail administration, the impact of IBMX-induced downregulation of Lyz2 gene expression on adipogenesis was not comparable to that observed in the full cocktail (Figure 1B), questioning whether the reduction in lysozyme is relevant to adipocyte differentiation.
To address this point, permanent Lyz2 gene knockdown (KD) was performed and assessed during adipocyte differentiation.
At day 0, Lyz2 gene KD slightly decreased Plin1 gene expression, without significant changes in the other adipogenic gene expression (Figures 2A−H). At day 4, Lyz2 gene KD reduced expression of early (Cebpa, Pparg) and middle-terminal stage (Adipoq, Plin1, Slc2a4 and Fabp4) of adipogenesis-related genes, and finally this attenuation was enhanced on Cebpa, Adipoq, Plin1, Slc2a4 and Fabp4 gene expression at day 8 (Figures 2A−H), indicating that the depletion in Lyz2 expression had a negative impact on adipogenesis. In line with this, intracellular lipid accumulation measured by Oil Red O staining at day 8 was significantly reduced in Lyz2 gene KD cells (Figure 3A).
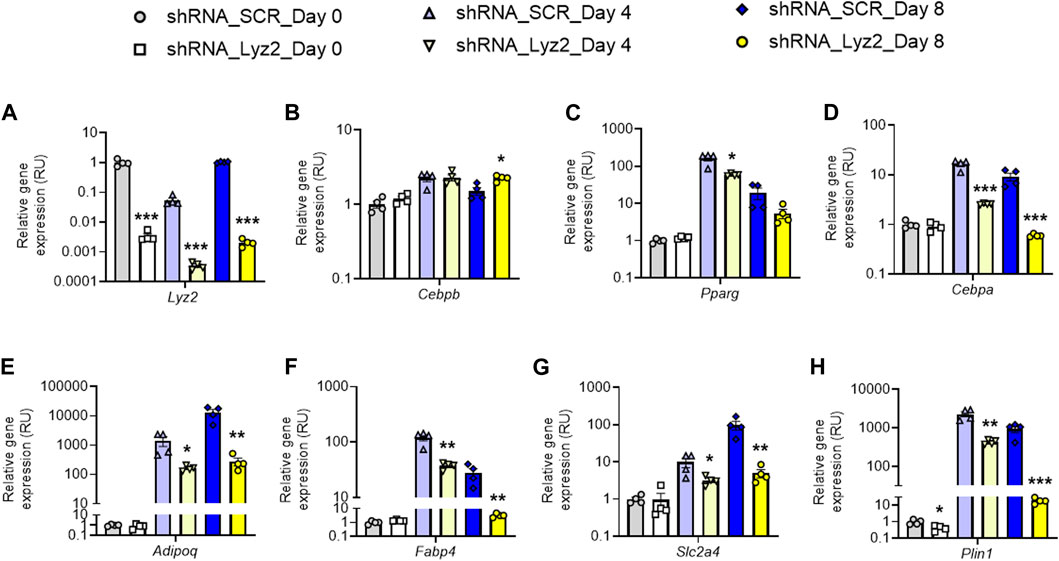
FIGURE 2. The impact of Lyz2 gene knockdown on expression of adipogenic genes during 3T3-L1 adipocyte differentiation. (A–H) Lyz2, Cebpb, Pparg, Cebpa, Adipoq, Plin1, Slc2a4 and Fabp4 gene expression during 3T3-L1 adipocyte differentiation at day 0, 4 and 8. *p < 0.05, **p < 0.01 and ***p < 0.001 compared to shRNA_SCR.
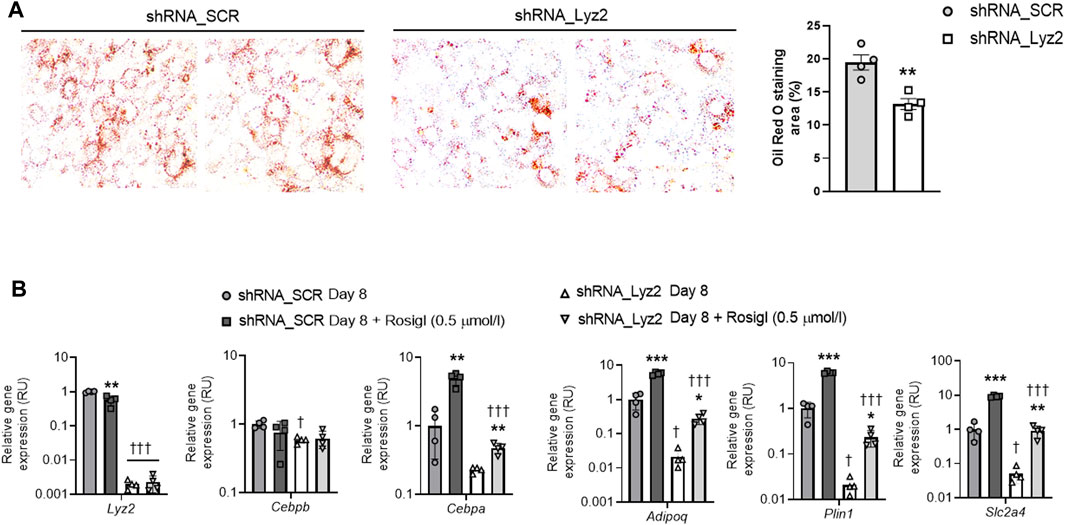
FIGURE 3. (A) The impact of Lyz2 gene knockdown on intracellular lipid accumulation, which was measured as Oil Red O staining area (%), at day 8 of adipocyte differentiation. The lens magnification used was ×10. **p < 0.01 compared to shRNA_SCR. (B) The impact of rosiglitazone (0.5 μmol/L) administration during adipocyte differentiation on Lyz2, Cebpb, Cebpa, Adipoq, Plin1 and Slc2a4 gene expression at day 8 in shRNA_SCR and shRNA_Lyz2 cells. *p < 0.05, **p < 0.01 and ***p < 0.001 compared to vehicle; †p < 0.05 and †††p < 0.001 compared to shRNA_SCR.
In control cells, rosiglitazone administration (0.5 μmol/L) led to decreased Lyz2 gene expression, and as expected, in both control and Lyz2 gene KD cells enhanced expression of adipogenic genes (Figure 3B). However, rosiglitazone administration (0.5 μmol/L) failed to compensate for the anti-adipogenic effect observed in Lyz2 gene KD compared rosiglitazone-treated control cells (Figure 3B).
Then, the effects of lysozyme biosynthesis from control cells on Lyz2 gene KD cells were assessed in a co-culture experiment using transwell insert microporous membranes, as previously reported (Moreno-Navarrete et al., 2015). Of note, Lyz2 gene KD cells co-cultured with control cells had higher expression of adipogenic genes than those co-cultured with themselves (Figure 4).
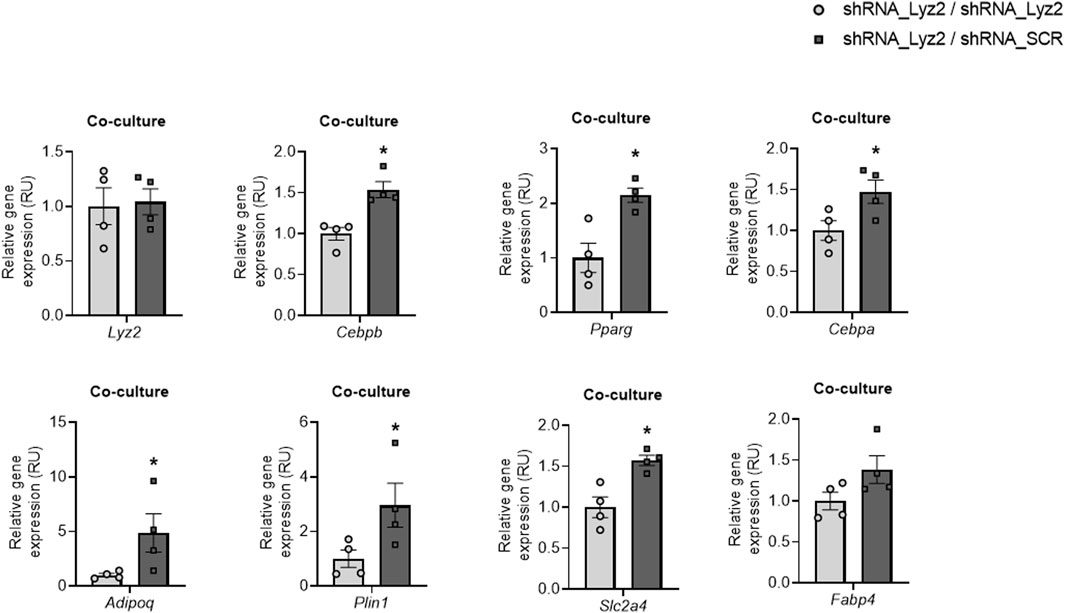
FIGURE 4. The effect of coculture Lyz2 gene knockdown cells (shRNA_Lyz2) with lysozyme-producing cells (shRNA_SCR) on Lyz2, Cebpb, Pparg, Cebpa, Adipoq, Plin1, Slc2a4 and Fabp4 gene expression at day 8 of adipocyte differentiation. *p < 0.05 compared to shRNA_Lyz2 cells co-cultured with themselves.
To the best of our knowledge this is the first study evaluating the direct role of preadipocyte- and adipocyte-produced lysozyme on adipogenesis. Altogether, these data indicate that Lyz2 gene knockdown (greater than 95%) resulted in a significant inhibition in 3T3-L1 adipocyte differentiation, characterized by reduced expression of adipogenic genes and decreased intracellular lipid accumulation. Compared to day 4, Plin1 and Fabp4 mRNA decreased at day 8. This might indicate that adipocyte maturation was completely achieved at day8. Even though previous studies demonstrated that gene expression of these adipogenic genes ran in parallel to their protein levels (Kleiman et al., 2009; Burrell and Stephens, 2021; Yadav and Jang, 2021), the impact of Lyz2 gene KD on these adipogenic genes at protein level should be confirmed in future studies.
Supporting the impact of lysozyme on fat mass expansion, studies in breastfeeding infants reported that calculated daily intake of lysozyme was positively associated to infant fat mass (Gridneva et al., 2021; Gridneva et al., 2022), and studies in humans, mice and rats demonstrated increased adipose tissue lysozyme in association to adiposity (Latorre et al., 2021) or increased plasma lysozyme in association to fat mass (Moreno-Navarrete et al., 2021). However, in contrast to the importance of lysozyme to maintain adipogenesis in 3T3-L1 cells, lysozyme expression in human and mice adipose tissue was negatively associated to gene expression markers of adipogenesis, but positively with adipose tissue inflammatory markers (Latorre et al., 2021). Taking into account that macrophages were the main source of lysozyme in adipose tissue (Latorre et al., 2021), these discrepancies might indicate that adipose tissue lysozyme reflects the proinflammatory effect of macrophage lysozyme (Shimada et al., 2010; Davis et al., 2011), and the consequent negative effect of inflammation on adipogenesis (Vila et al., 2014; Kursawe et al., 2016). In fact, experimentally, the downregulation of adipose tissue lysozyme impacted on adipose tissue, attenuating local adipose tissue inflammatory activity and improving adipogenesis (Latorre et al., 2021).
The beneficial effects of lysozyme on adipocyte biology could be explained in part by its protective role against the deleterious effects of advanced glycation end-products (AGEs) (Mitsuhashi et al., 1997; Cocchietto et al., 2008; Gallo et al., 2014). However, the absence of AGEs in current experiments suggest other mechanisms. Previous studies demonstrated that lysozyme can exert antioxidant activities through the suppression of endogenous reactive oxygen species (ROS) (Liu et al., 2006a; Liu et al., 2006b) and attenuating the ROS harmful effects in hepatocytes (Liu et al., 2006a). Taking into account that intracellular ROS levels increased during 3T3-L1 adipocyte differentiation, and that excess intracellular ROS impaired expression of adipogenic genes and resulted in a pathological adipocyte differentiation (Furukawa et al., 2004), a possible role of lysozyme buffering the negative impact of endogenous ROS on adipocyte differentiation might be postulated. However, further experiments should be designed to investigate the molecular mechanism that underlie in the requirement of lysozyme expression for an optimal adipocyte differentiation.
This study also reported different effects in the regulation of Lyz2 gene expression of the components that constitute the adipocyte differentiation cocktail. Of note, insulin administration led to increased Lyz2 gene expression in 3T3-L1 cells. These data suggest that obesity-associated hyperinsulinemia could contribute to increased circulating and adipose tissue lysozyme levels observed in subjects with obesity (Latorre et al., 2021; Moreno-Navarrete et al., 2021). Strikingly, dexamethasone administration also increased Lyz2 gene expression in 3T3-L1 cells. In contrast, dexamethasone administration impacted negatively on lysozyme expression in human monocytic cell lines, such as THP-1 and U937) (Hoff et al., 1992; Kulkarni et al., 2016). Otherwise, and in agreement with current findings, the phosphodiesterase inhibitor (isobutylmethylxanthine) also decreased lysozyme production in neutrophils and monocytes through the increase in cAMP levels (Herlin and Kragballe, 1982; Nagata et al., 1992).
An important limitation of co-culture experiment is that the chambers allow the flow of the entire secretome of control cells, not directly showing if lysozyme alone can rescue the defect. Further experiments treating Lyz2 gene KD cells with lysozyme should be performed to confirm if this is sufficient to improve adipogenesis in the absence of endogenous lysozyme production. To gain insight in the mechanism behind the observed Lyz2-associated changes in adipogenesis, additional experiments are required. For instance, the bioenergetic profiles of these cells might be evaluated.
In conclusion, these data indicate that lysozyme is required to achieve an optimal adipocyte differentiation in 3T3-L1 cells, suggesting a possible role of lysozyme in the maintenance of adequate adipogenic gene expression.
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
AL and JL participated in this study conducting experiments, acquiring and analyzing data and reviewed the manuscript. JF-R contributed to the discussion and reviewed the manuscript. JM-N contributed to research study design, conducting experiments, acquiring and analyzing data, and writing the manuscript.
This study was partially supported by research grants PI19/01712 from the Instituto de Salud Carlos III from Spain, Fundació Marató de TV3 (201612-31) and FEDER funds. The CIBEROBN is an initiative from the Instituto de Salud Carlos III (ISCIII). We did not received funds for open access publication fees.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Burrell, J. A., and Stephens, J. M. (2021). KAT8, Lysine Acetyltransferase 8, Is Required for Adipocyte Differentiation In Vitro. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 1867 (6), 166103. doi:10.1016/j.bbadis.2021.166103
Cocchietto, M., Zorzin, L., Toffoli, B., Candido, R., Fabris, B., Stebel, M., et al. (2008). Orally Administered Microencapsulated Lysozyme Downregulates Serum AGE and Reduces the Severity of Early-Stage Diabetic Nephropathy. Diabetes Metab. 34 (6 Pt 1), 587–594. doi:10.1016/j.diabet.2008.05.009
Davis, K. M., Nakamura, S., and Weiser, J. N. (2011). Nod2 Sensing of Lysozyme-Digested Peptidoglycan Promotes Macrophage Recruitment and Clearance of S. Pneumoniae Colonization in Mice. J. Clin. Invest. 121 (9), 3666–3676. doi:10.1172/JCI57761
Farmer, S. R. (2006). Transcriptional Control of Adipocyte Formation. Cell Metab. 4 (4), 263–273. doi:10.1016/j.cmet.2006.07.001
Furukawa, S., Fujita, T., Shimabukuro, M., Iwaki, M., Yamada, Y., Nakajima, Y., et al. (2004). Increased Oxidative Stress in Obesity and its Impact on Metabolic Syndrome. J. Clin. Invest. 114 (12), 1752–1761. doi:10.1172/JCI21625
Gallo, D., Cocchietto, M., Masat, E., Agostinis, C., Harei, E., Veronesi, P., et al. (2014). Human Recombinant Lysozyme Downregulates Advanced Glycation Endproduct-Induced Interleukin-6 Production and Release in an In-Vitro Model of Human Proximal Tubular Epithelial Cells. Exp. Biol. Med. (Maywood) 239 (3), 337–346. doi:10.1177/1535370213518281
Gridneva, Z., Lai, C. T., Rea, A., Tie, W. J., Ward, L. C., Murray, K., et al. (2021). Human Milk Immunomodulatory Proteins are Related to Development of Infant Body Composition during the First Year of Lactation. Pediatr. Res. 89 (4), 911–921. doi:10.1038/s41390-020-0961-z
Gridneva, Z., Rea, A., Lai, C. T., Tie, W. J., Kugananthan, S., Warden, A. H., et al. (2022). Human Milk Macronutrients and Bioactive Molecules and Development of Regional Fat Depots in Western Australian Infants during the First 12 Months of Lactation. Life 12 (4), 493. doi:10.3390/life12040493
Herlin, T., and Kragballe, K. (1982). Divergent Effects of Methylxanthines and Adenylate Cyclase Agonists on Monocyte Cytotoxicity and Cyclic AMP Levels. Eur. J. Clin. Invest. 12 (4), 293–299. doi:10.1111/j.1365-2362.1982.tb02235.x
Hoff, T., Spencker, T., Emmendoerffer, A., and Goppelt-Struebe, M. (1992). Effects of Glucocorticoids on the TPA-Induced Monocytic Differentiation. J. Leukoc. Biol. 52 (2), 173–182. doi:10.1002/jlb.52.2.173
Kleiman, E., Carter, G., Ghansah, T., Patel, N. A., and Cooper, D. R. (2009). Developmentally Spliced PKCβII Provides a Possible Link between mTORC2 and Akt Kinase to Regulate 3T3-L1 Adipocyte Insulin-Stimulated Glucose Transport. Biochem. Biophys. Res. Commun. 388 (3), 554–559. doi:10.1016/j.bbrc.2009.08.063
Kulkarni, N. N., Gunnarsson, H. I., Yi, Z., Gudmundsdottir, S., Sigurjonsson, O. E., Agerberth, B., et al. (2016). Glucocorticoid Dexamethasone Down-Regulates Basal and Vitamin D3 Induced Cathelicidin Expression in Human Monocytes and Bronchial Epithelial Cell Line. Immunobiology 221 (2), 245–252. doi:10.1016/j.imbio.2015.09.001
Kursawe, R., Dixit, V. D., Scherer, P. E., Santoro, N., Narayan, D., Gordillo, R., et al. (2016). A Role of the Inflammasome in the Low Storage Capacity of the Abdominal Subcutaneous Adipose Tissue in Obese Adolescents. Diabetes 65 (3), 610–618. doi:10.2337/db15-1478
Latorre, J., Lluch, A., Ortega, F. J., Gavaldà-Navarro, A., Comas, F., Morón-Ros, S., et al. (2021). Adipose Tissue Knockdown of Lysozyme Reduces Local Inflammation and Improves Adipogenesis in High-Fat Diet-Fed Mice. Pharmacol. Res. 166, 105486. doi:10.1016/j.phrs.2021.105486
Liu, H., Zheng, F., Cao, Q., Ren, B., Zhu, L., Striker, G., et al. (2006a). Amelioration of Oxidant Stress by the Defensin Lysozyme. Am. J. Physiol.-Endocrinol. Metab. 290 (5), E824–E832. doi:10.1152/ajpendo.00349.2005
Liu, H., Zheng, F., Li, Z., Uribarri, J., Ren, B., Hutter, R., et al. (2006b). Reduced Acute Vascular Injury and Atherosclerosis in Hyperlipidemic Mice Transgenic for Lysozyme. Am. J. Pathol. 169 (1), 303–313. doi:10.2353/ajpath.2006.050885
Mitsuhashi, T., Li, Y. M., Fishbane, S., and Vlassara, H. (1997). Depletion of Reactive Advanced Glycation Endproducts from Diabetic Uremic Sera Using a Lysozyme-Linked Matrix. J. Clin. Invest. 100 (4), 847–854. doi:10.1172/JCI119600
Moreno-Navarrete, J. M., Escoté, X., Ortega, F., Camps, M., Ricart, W., Zorzano, A., et al. (2015). Lipopolysaccharide Binding Protein Is an Adipokine Involved in the Resilience of the Mouse Adipocyte to Inflammation. Diabetologia 58 (10), 2424–2434. doi:10.1007/s00125-015-3692-7
Moreno-Navarrete, J. M., Latorre, J., Lluch, A., Ortega, F. J., Comas, F., Arnoriaga-Rodríguez, M., et al. (2021). Lysozyme is a Component of the Innate Immune System Linked to Obesity Associated-Chronic Low-Grade Inflammation and Altered Glucose Tolerance. Clin. Nutr. 40 (3), 1420–1429. doi:10.1016/j.clnu.2020.08.036
Nagata, S., Kebo, D. K., Kunkel, S., and Glovsky, M. (1992). Effect of Adenylate Cyclase Activators on C5a-Lnduced Human Neutrophil Aggregation, Enzyme Release and Superoxide Production. Int. Arch. Allergy Immunol. 97 (3), 194–199. doi:10.1159/000236118
Shimada, T., Park, B. G., Wolf, A. J., Brikos, C., Goodridge, H. S., Becker, C. A., et al. (2010). Staphylococcus A Evades Lysozyme-Based Peptidoglycan Digestion that Links Phagocytosis, Inflammasome Activation, and IL-1β Secretion. Cell Host Microbe 7 (1), 38–49. doi:10.1016/j.chom.2009.12.008
Vila, I. K., Badin, P.-M., Marques, M.-A., Monbrun, L., Lefort, C., Mir, L., et al. (2014). Immune Cell Toll-Like Receptor 4 Mediates the Development of Obesity- and Endotoxemia-Associated Adipose Tissue Fibrosis. Cell Rep. 7 (4), 1116–1129. doi:10.1016/j.celrep.2014.03.062
Vishvanath, L., and Gupta, R. K. (2019). Contribution of Adipogenesis to Healthy Adipose Tissue Expansion in Obesity. J. Clin. Invest. 129 (10), 4022–4031. doi:10.1172/JCI129191
Keywords: lysozyme, 3T3-L1, adipogenesis, gene knockdown, lentiviral particles
Citation: Lluch A, Latorre J, Fernández-Real JM and Moreno-Navarrete JM (2022) Lysozyme Gene Expression in 3T3-L1 Cells Sustains Expression of Adipogenic Genes and Adipocyte Differentiation. Front. Cell Dev. Biol. 10:914788. doi: 10.3389/fcell.2022.914788
Received: 07 April 2022; Accepted: 31 May 2022;
Published: 15 June 2022.
Edited by:
Rossella Labella, Columbia University, United StatesReviewed by:
Kripa Shankar, University of Texas Southwestern Medical Center, United StatesCopyright © 2022 Lluch, Latorre, Fernández-Real and Moreno-Navarrete. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: José María Moreno-Navarrete, jmoreno@idibgi.org
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.