
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Cell Dev. Biol., 13 April 2022
Sec. Signaling
Volume 10 - 2022 | https://doi.org/10.3389/fcell.2022.891763
This article is part of the Research TopicEmbryonic Developmental Signaling Pathways in Normal and Disease StatesView all 5 articles
The Ror-family proteins, Ror1 and Ror2, act as receptors or co-receptors for Wnt5a and its related Wnt proteins to activate non-canonical Wnt signaling. Ror1 and/or Ror2-mediated signaling plays essential roles in regulating cell polarity, migration, proliferation and differentiation during developmental morphogenesis, tissue-/organo-genesis and regeneration of adult tissues following injury. Ror1 and Ror2 are expressed abundantly in developing tissues in an overlapping, yet distinct manner, and their expression in adult tissues is restricted to specific cell types such as tissue stem/progenitor cells. Expression levels of Ror1 and/or Ror2 in the adult tissues are increased following injury, thereby promoting regeneration or repair of these injured tissues. On the other hand, disruption of Wnt5a-Ror2 signaling is implicated in senescence of tissue stem/progenitor cells that is related to the impaired regeneration capacity of aged tissues. In fact, Ror1 and Ror2 are implicated in age-related diseases, including tissue fibrosis, atherosclerosis (or arteriosclerosis), neurodegenerative diseases, and cancers. In these diseases, enhanced and/or sustained (chronic) expression of Ror1 and/or Ror2 is observed, and they might contribute to the progression of these diseases through Wnt5a-dependent and -independent manners. In this article, we overview recent advances in our understanding of the roles of Ror1 and Ror2-mediated signaling in the development, tissue regeneration and age-related diseases, and discuss their potential to be therapeutic targets for chronic inflammatory diseases and cancers.
In the developmental process, various cell behaviors, including cell polarization, migration, proliferation, and differentiation, are strictly regulated based on genetic programs, and thereby establishing proper morphogenesis and tissue-/organo-genesis. The regulation of these cell behaviors is also essential for proper tissue repair or regeneration following injury even in the adult tissues. For example, tissue stem/progenitor cells initiate their proliferation and differentiation in response to local environmental changes including inflammation caused by injury (Kizil et al., 2015; Xiao et al., 2020). The signaling mechanisms involved in the regulation of the developmental processes and responses following injury should be activated or inhibited at the appropriate strength and timing. Therefore, their aberrant activation under the pathological conditions such as chronic inflammation with aging might contribute to the progression of various diseases.
The Ror-family proteins, Ror1 and Ror2 act as receptors or co-receptors for non-canonical Wnt ligands such as Wnt5a, thereby regulating cell polarity, migration, proliferation, and differentiation that are required for proper developmental morphogenesis and tissue-/organo-genesis (Minami et al., 2010; Endo and Minami, 2018). Ror1 and Ror2 are expressed selectively in some undifferentiated cells including stem/progenitor cells, implying that in addition to the amount of their ligands, the gene regulation of the Ror-family receptors is important for their actions. In this review, we first overview the functions of the Ror-family receptors in regulating developmental processes, and then introduce how expression of Ror1 and/or Ror2 is regulated in certain types of cells, including tissue-resident stem/progenitor cells, in adult tissues during tissue repair or regeneration following injury. We also discuss the possible involvement of aberrant signaling mediated by the Ror-family receptors in the stem/progenitor cell aging and the age-related diseases including chronic inflammatory diseases and cancers.
In vertebrates, the Ror-family receptors consist of two structurally related proteins, Ror1 and Ror2. Both Ror1 and Ror2 (hereinafter, referred to as Ror1/Ror2) are type I transmembrane proteins, and their extracellular regions are composed of an immunoglobulin (Ig)-like domain, followed by a frizzled-like cysteine-rich domain (CRD), and then a kringle domain (KD) (Figure 1). The CRDs of Ror1/Ror2 can interact with Wnt5a (Oishi et al., 2003; Mikels and Nusse, 2006; Weissenböck et al., 2019), one of the most extensively studied non-canonical Wnt proteins. A growing body of evidence shows that Ror1/Ror2 act as receptors or co-receptors for various Wnt proteins, including Wnt5a, Wnt5b, Wnt9a, and Wnt11, to regulate various cellular responses (Oishi et al., 2003; Mikels and Nusse, 2006; Fukuda et al., 2008; Morioka et al., 2009; Paganoni et al., 2010; Sato et al., 2010; Endo et al., 2012; Ho et al., 2012; Bai et al., 2014; Weissenböck et al., 2019; Menck et al., 2021). The KD of Ror1 can interact with that of Ror2 to form Ror1/Ror2 heterooligomers that are required for Wnt5a-induced signaling at least in some cellular contexts (Yu et al., 2016). Although the function of the Ig-like domain in the Ror-family receptors remains elusive, Ror1 mutant mice lacking the Ig-like domain exhibit abnormal development of the kidneys when crossed with Ror2 deficient mice or Wnt5a heterozygous mutant mice (Qi et al., 2016), suggesting that Ror1/Ror2 have redundant functions in regulating Wnt5a-induced signaling.
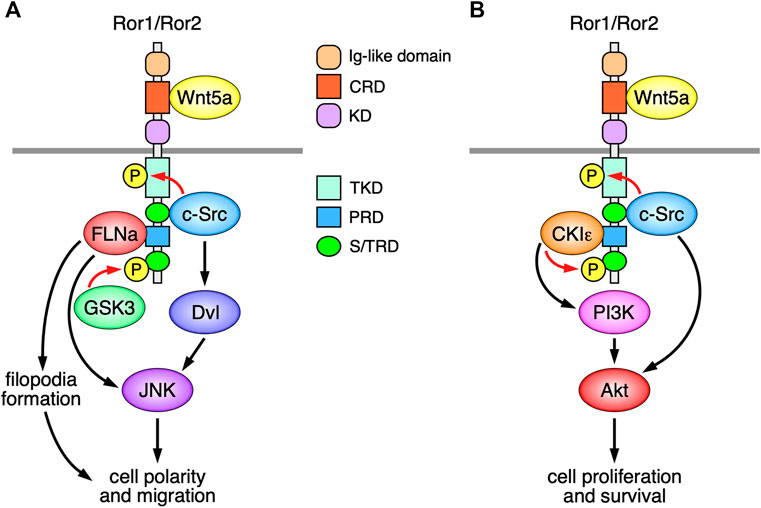
FIGURE 1. Schematic representation of the Ror-family receptors (Ror1/Ror2)-mediated signaling, implicated in the regulation of Wnt5a-induced cell polarity, migration, proliferation, and survival. (A) The Ror-family receptors induce filopodia formation and JNK activation via the interaction with FLNa, leading to the regulation of cell polarity and migration. Wnt5a-Ror signaling can also induce activation of c-Src and Dvl, leading to the activation of JNK. c-Src and GSK3, respectively, phosphorylate the Ror-family receptors on their tyrosine and serine/threonine residues, both of which are required for Wnt5a-induced cell polarity and migration. (B) Wnt5a-Ror signaling can activate PI3K-Akt pathway presumably via CKIε associated with Ror1/Ror2, which in turn promotes cell proliferation and survival. c-Src, activated by Wnt5a-Ror signaling, can also induce activation of Akt, thereby contributing to cell proliferation and survival.
The cytoplasmic regions of the Ror-family receptors contain a putative conserved tyrosine kinase domain (TKD), followed by a serine/threonine-rich domain (S/TRD1), a proline-rich domain (PRD), and another serine/threonine-rich domain (S/TRD2) (Figure 1). Recent studies demonstrate that the TKDs of Ror1/Ror2 act as pseudokinase domains that are incapable of binding to ATP by itself (Sheetz et al., 2020), although they can mediate Wnt5a-induced signaling and cellular responses (Enomoto et al., 2009; Sheetz et al., 2020). It has been shown that the non-receptor tyrosine kinase c-Src can phosphorylate the tyrosine residues within the activation loops in the TKDs of Ror1/Ror2 (Akbarzadeh et al., 2008; Gentile et al., 2014), and that the substitutions of these tyrosine residues with phenylalanine result in the impairment of their several functions (Mikels et al., 2009; Gentile et al., 2014; Sheetz et al., 2020). However, further studies will be required to determine the roles of the tyrosine phosphorylation within the TKDs of Ror1/Ror2 in regulating various cellular behaviors.
The S/TRD1 of Ror2 is required for recruitment and activation of c-Src following Wnt5a-stimulation (Akbarzadeh et al., 2008; Lai et al., 2012). Accumulating evidence demonstrates that c-Src plays essential roles in regulating Ror1/Ror2-mediated cell migration and proliferation (Enomoto et al., 2009; Gentile et al., 2014; Shojima et al., 2015). Ror2 can also bind to other signaling molecules, including filamin A (FLNa) and casein kinase I epsilon (CKIε), via the PRD (Kani et al., 2004; Nishita et al., 2006). Ror2 mediates filopodia formation and c-Jun N-terminal kinase (JNK) activation via the interaction with FLNa, thereby regulating cell polarity and migration (Nishita et al., 2006; Nomachi et al., 2008) (Figure 1A). Interestingly, c-Src has also been implicated in the activation of Dishevelled (Dvl) and c-Jun N-terminal kinase, downstream of Wnt5a-Ror2 signaling (Yamagata et al., 2012) (Figure 1A). Like Ror2, Ror1 might exhibit the same or similar function in regulating polarized cell migration presumably through filopodia formation (Kaucka et al., 2011). The serine/threonine residues within the C-terminal portion of Ror1/Ror2 can be phosphorylated via glycogen synthase kinase 3 (GSK3) and CKIε, which might be required for the activation of non-canonical Wnt signaling (Kani et al., 2004; Yamamoto et al., 2007; Grumolato et al., 2010; Konopelski Snavely et al., 2021). Indeed, it has been shown that GSK3-mediated phosphorylation of Serine 864 within the STD2 of Ror2 is required for Wnt5a-induced cell migration (Grumolato et al., 2010) (Figure 1A), and that Wnt5a-Ror signaling can also activate phosphoinositide three kinase (PI3K)-Akt pathway presumably via CKIε associated with Ror1/Ror2, which in turn promotes cell proliferation and survival (Zhang et al., 2012) (Figure 1B).
Wnt signaling can largely be classified into canonical (β-catenin-dependent) and non-canonical (β-catenin-independent) pathways. Wnt5a can activate the non-canonical Wnt/planar cell polarity (PCP) pathway, in which Dvl activates the Rho-family of small GTPases, including Rho, Rac, and Cdc42, and their downstream effectors, Rho-associated protein kinase and JNK, leading to reorganization of the actin cytoskeleton, and thereby regulating polarized cell morphology and migration. In vertebrates, Wnt/PCP pathway regulates polarized cell movements during convergent extension (CE), by which tissues undergo narrowing along one axis and concomitant extension along another axis. Indeed, Wnt5a-Ror2 signaling can regulate CE movements during gastrulation and neurulation in Xenopus embryos (Hikasa et al., 2002; Oishi et al., 2003; Schambony and Wedlich, 2007; Martinez et al., 2015). In zebrafish, Wnt11, but not Wnt5a, is expressed highly at gastrula-stage embryos and Wnt11-Ror2 signaling is implicated in regulating CE movements during gastrulation (Bai et al., 2014).
In mammals, Wnt/PCP pathway plays essential roles in regulating collective and directed cell movements involved in various developmental processes, including neural tube closure, neural crest migration, the anterior to posterior axis elongation, inner ear hair cell alignment, and heart morphogenesis (Kibar et al., 2001; Curtin et al., 2003; Montcouquiol et al., 2003; Wang J. et al., 2006; Wang Y. et al., 2006; Ybot-Gonzalez et al., 2007; Muñoz-Soriano et al., 2012). Indeed, Ror2−/− and Wnt5a−/- mouse embryos exhibit dwarfism, short limbs and tail, facial anomalies, and ventricular septal defect (Yamaguchi et al., 1999; DeChiara et al., 2000; Takeuchi et al., 2000; Oishi et al., 2003; Schwabe et al., 2004; He et al., 2008), reminiscent of features observed in patients with Robinow syndrome that is caused by loss-of-function mutations in Ror2 or Wnt5a genes (Atalay et al., 1993; Afzal et al., 2000; van Bokhoven et al., 2000; Person et al., 2010). These mutant mice also exhibit disrupted alignment and orientation of inner ear hair cells (Qian et al., 2007; Yamamoto et al., 2008), and disrupted morphogenesis of the kidney, lung, trachea, esophagus, and midgut (Yamada et al., 2010; Kishimoto et al., 2018). Although the phenotypes of Ror2−/− mouse embryos are somewhat milder than those of Wnt5a−/- mouse embryos, severer morphological phenotypes are observed in Ror1/Ror2 double mutant mice (Nomi et al., 2001; Ho et al., 2012), indicating that pleiotropic, yet redundant functions of Ror1/Ror2 during developmental morphogenesis.
During mouse development, Ror1/Ror2 are expressed in various tissues and organs, including the lung, kidney, tooth, and the skeletal and nervous systems (Oishi et al., 1999; Takeuchi et al., 2000; Al-Shawi et al., 2001; Matsuda et al., 2001), and play crucial roles in their establishment by regulating cell proliferation and differentiation as well as cell polarity and migration (Schwabe et al., 2004; Lin et al., 2011; Maeda et al., 2012; Nishita et al., 2014; Zhang K. et al., 2020; Ma et al., 2021).
In the nervous system of mouse embryos, Ror1/Ror2 are expressed in neural stem/progenitor cells (NPCs) within the neocortices (Oishi et al., 1999; Al-Shawi et al., 2001; Endo et al., 2012). At early embryonic stages, NPCs divide actively to maintain the NPC pool and thereby generating a large number of neurons via intermediate progenitor cells (IPCs), neuron-producing transient amplifying cells. Suppressed expression of Ror1 and/or Ror2 in NPCs results in reduced proportion of proliferating NPCs, IPCs, and their progeny neurons, probably due to increased cell cycle exit (Endo et al., 2012). In regulating the cell-cycle, Ror2-signaling can promote cell-cycle transition from the G1 to S phase by activating E2F1-mediated transcription (Endo et al., 2020). Expression levels of Ror1/Ror2 in NPCs are decreased gradually during neocortical development (Endo et al., 2017), suggesting that the relative activity of Wnt5a-Ror1/Ror2 signaling in NPCs might play a role in determining durations of the neocortical neurogenesis.
Ror2−/− mice exhibit short limbs with mesomelic dysplasia characterized by significant or complete loss of the distal long bones in the forelimbs (the radius and ulna) and hindlimbs (the tibia and fibula) (DeChiara et al., 2000; Takeuchi et al., 2000). Although Ror1 mutant mice do not show any apparent skeletal abnormalities, Ror1/Ror2 double mutant mice exhibit a drastic enhancement of the skeletal phenotypes observed in the Ror2−/− mice and additional phenotypes (Nomi et al., 2001), indicating that Ror1 and Ror2 interact genetically in regulating the developmental bone growth. Longitudinal growth of the bones is achieved by endochondral ossification, where a pre-formed cartilage template is replaced by newly formed bone. During this process, chondrocytes, which are derived from undifferentiated mesenchymal cells, proliferate and differentiate into hypertrophic chondrocytes within the cartilage template, subsequently instruct the differentiation of adjacent perichondrial cells into osteoblasts. Ror2 is expressed selectively in the resting and proliferating chondrocytes but not hypertrophic chondrocytes (DeChiara et al., 2000), and loss of Ror2 in limb bud mesenchyme leads to a decrease in chondrocyte differentiation and impaired ossification in the developing cartilages (Schwabe et al., 2004; Lei et al., 2020).
During embryonic stages of mouse tooth development, Ror2 is expressed in both the dental epithelium and mesenchyme, while Wnt5a is expressed only in the mesenchyme within the dental papilla adjacent to the dental epithelium (Lin et al., 2011). Teeth of Ror2−/− and Wnt5a−/- mice at P0 exhibit retarded growth with a delayed odontoblast differentiation (Lin et al., 2011). After the development of the dental crown, tooth root formation occurs extensively during the postnatal period in mice. Expression of Ror2 is widely distributed in the dental epithelium and mesenchyme at P0, but gradually become more prominent apically in the root-forming region during the root development of neonatal mice (Ma et al., 2021). Conditional deletion of Ror2 in the dental mesenchyme resulted in shortened roots without obvious abnormality in the crown patterning (Ma et al., 2021), suggesting that Ror2 expressed in the dental mesenchyme and epithelium contributes to the formation of the tooth root and dental crown, respectively. During root development, Ror2 signaling is required for cell proliferation of the dental mesenchyme and differentiation of odontoblasts (Ma et al., 2021).
Although overall expression levels of the Ror-family receptors in adult tissues are reduced compared to developing tissues, expression of Ror1 can be detected in satellite cells (SCs), one of the skeletal muscle-specific tissue stem cells, within the skeletal muscles of the adult mice (Kamizaki et al., 2017). It is well known that SCs play a crucial role in regulating the regeneration of skeletal muscles after injury (Mauro, 1961; Chargé and Rudnicki, 2004). Although SCs are in quiescent state under physiological conditions, they are activated and initiate their proliferation following skeletal muscle injury, then differentiate and fuse with each other to produce new myofibers, thereby promoting skeletal muscle regeneration (Seale et al., 2000). Importantly, expression level of Ror1 in SCs is further increased upon skeletal muscle injury (Kamizaki et al., 2017). Tumor necrosis factor-α and interleukin-1β (IL-1β), inflammatory cytokines produced from inflammatory cells, including neutrophils and macrophages infiltrated into the injured muscles, can induce the expression of Ror1 in SCs through the activation of NF-κB pathway. Further studies in SCs-specific Ror1 conditional knockout-mice have revealed that increased expression of Ror1 in SCs is required for their proliferation following injury and subsequent regeneration of skeletal muscles (Kamizaki et al., 2017).
The adult mammalian brains have a limited capacity to regenerate spontaneously following injury or diseases. Although NPCs exist in some parts even in the adult mammalian brains, there are no stem/progenitor cells involved in generating new neurons to compensate damaged neurons at least within the injured parenchyma. It has been shown that Ror2 expression is increased in the brains following traumatic injury (Endo et al., 2017). Interestingly, increased expression of Ror2 is observed in reactive astrocytes, surrounding the injured sites, that express Nestin, a marker of NPCs. Astrocytes are the most abundant glial cells in the brain and contribute to brain homeostasis. Under pathological conditions, astrocytes change to a state called reactive astrocytes that exhibit various specific properties, thereby affecting the brain functions. In the injured brains, reactive astrocytes acquire stem cell-like properties and start to proliferate (Götz et al., 2015). It has been reported that proliferative reactive astrocytes exhibit anti-inflammatory and tissue-repair promoting functions (Sofroniew, 2015; Frik et al., 2018; Williamson et al., 2021). In astrocyte-specific Ror2 conditional knockout-mice, number of proliferating reactive astrocytes is decreased (Endo et al., 2017), indicating that induced expression of Ror2 in reactive astrocytes plays an important role in promoting repair of the injured brains.
Cell-cycle entry of quiescent astrocytes is triggered by growth factors, including basic fibroblast growth factor (bFGF), where expression of Ror2 can be induced during the transition from G0/G1 to S-phase (Endo et al., 2017). It has been shown that Ror2 is a target gene of E2F1, and that Ror2 suppresses Foxo3a-mediated expression of the cyclin-dependent kinase inhibitors p21 and p27 via the PI3K-Akt pathway, leading to activation of E2F1-mediated transcription, thereby promoting the G1/S-phase transition (Endo et al., 2020). Although Wnt5a is not required for the cell-cycle progression stimulated by bFGF, the possible involvement of other Wnt ligands, including Wnt5b and Wnt11, has to be investigated.
It is known that the function of adult tissue stem/progenitor cells attenuates with aging that is causally linked to the age-associated impairment in tissue repair, regeneration, and homeostasis. Therefore, it is expected to develop clinical intervention methods for preventing tissue aging, by targeting tissue stem/progenitor cell aging. Dental pulp stem cells (DPSCs) play an important role in maintaining tooth homeostasis and repairing of postnatal tooth (Zheng et al., 2019). Furthermore, DPSCs exhibit multipotent differentiation capacity, and thus have the potential for use in clinical applications not only for dental diseases, but also for systemic diseases (Yamada et al., 2019). They can be isolated from human dental pulp tissues and expanded in culture, but gradually lose their proliferative ability and multipotent differentiation potential during the expansion because of entering cellular senescence. In cultured human DPSCs, Ror2 is expressed highly at earlier passages, but its expression is decreased in senescent DPSCs (Dong et al., 2021). Consistently, decreased expression of Ror2 is also detected in DPSCs isolated from elderly donors compared to those from young donors. Furthermore, Ror2 can inhibit induction of cellular senescence in cultured DPSCs by inhibiting STK4-Foxo1 pathway in a Wnt5a-independent manner (Dong et al., 2021). Therefore, supplementing expression of Ror2 or inhibiting decreased expression of Ror2 in DPSCs might lead to the maintenance of their stemness, which might be useful for the clinical use of DPSCs.
On the other hand, Wnt5a can induce cellular senescence in the tissue stem cells, including hematopoietic and hair follicle stem cells (Florian et al., 2013; Tiwari et al., 2021), although its receptors in regulating the cellular senescence remain elusive. In tendon stem/progenitor cells, however, Ror2 has been shown to mediate their Wnt5a-induced cellular senescence via JAK-STAT pathway (Chen et al., 2021). Therefore, it is important to clarify how Ror2 can regulate cellular senescence of these stem/progenitor cells in a cell-type specific manner.
Recently, it has become evident that chronic inflammation is a pervasive feature of aged tissues, and is also the common cause of various age-related diseases, including fibrosis, arteriosclerosis, and neurodegenerative diseases. Therefore, it’s a provocative issue to uncover the molecular mechanisms underlying the development or progression of the age-related diseases due to chronic inflammation, aiming to develop novel clinical intervention methods to prevent or ameliorate the pathologies of these age-related diseases. In this regard, enhanced and/or sustained activation of Ror1/Ror2-mediated signaling induced by prolonged inflammation might be associated with progression of these age-related diseases. In the kidney, expression levels of Ror1, Ror2, and Wnt5a are increased in the progressive fibrotic tissues with persistent inflammation after injury (Li et al., 2013). In the damaged kidneys from Ror2+/− mice, reduced disruption of the tubular basement membrane (TBM) along with reduced expression of MMP-2 in tubular epithelial cells were observed compared to Ror2+/+ mice (Li et al., 2013), suggesting that Wnt5a-Ror2 signaling might play an important role in disrupting TBM via MMP-2 during renal fibrosis. Furthermore, Wnt5a and Ror2 are expressed highly in foam cells within the atherosclerotic plaque (Ackers et al., 2018; Zhang C.-J. et al., 2020). Aberrant expression of Wnt5a in vascular smooth muscle cells (VSMCs) reduces expression of adenosine triphosphate-binding cassette A1, a key cholesterol transporter, via Ror2, resulting in the enhancement of cholesterol accumulation and inflammatory response in VSMCs (Zhang C.-J. et al., 2020), suggesting that Wnt5a-Ror2 signaling plays a critical role in the pathogenesis of atherosclerosis. A critical role of Wnt5a-Ror2 signaling has also been reported in the dextran sodium sulfate-induced colitis mouse model, where sustained up-regulation of Wnt5a can be observed in stromal fibroblasts in the ulcerative lesions of these mice, and Wnt5a-Ror2 signaling activated in dendritic cells can promote interferon-γ signaling, thereby promoting colitis (Sato et al., 2015). Ror2 expression is also increased in degenerating neurons by IL-1β secreted from activated microglia in the spinal cord of mice with experimental autoimmune encephalomyelitis, a mouse model of multiple sclerosis (Shimizu et al., 2016). Wnt5a- or Wnt11-Ror2 signaling can mediate IL-1β-induced neuronal cell death, suggesting that Ror2-mediated signaling might promote the pathology of the neurodegenerative diseases.
Since most of cancers are developed in elder people than young people, it is conceivable that aging and cancer development are tightly related. Accumulating evidence demonstrates that age-related chronic inflammation plays important roles in the progression of cancers (Coussens and Werb, 2002; Ostan et al., 2015). Interestingly, Ror1/Ror2 are expressed highly in various types of cancers (Zheng et al., 2016; Bayerlová et al., 2017; Hossein et al., 2017; Hasan et al., 2018; Astudillo, 2021). Enhanced expression of Ror1 and/or Ror2 in cancer cells can promote their proliferation, migration, invasion, survival or chemoresistance through activation of Rho-family GTPases, c-Src, MAP kinases or Akt in Wnt5a-dependent and/or -independent manners (Enomoto et al., 2009; Zhang et al., 2012; Ida et al., 2016; Nishita et al., 2017; Hasan et al., 2019). In addition, several studies have indicated that Ror1 might be ideal therapeutic target of cancers including leukemia and breast cancer (Karvonen et al., 2017; Choi et al., 2018; Wallstabe et al., 2019; Zhang et al., 2019; Stüber et al., 2020). These studies surmise that increased expression of Ror1 and/or Ror2 in cancer cells is important for their progression. Here, we describe the mechanism how expression of Ror1/Ror2 is upregulated in cancer cells by taking aging into account (see below).
Accumulating evidence has shown that expression of the Ror-family receptors in cancer cells can be regulated by multiple factors and drugs (Table 1). In breast cancers, increased expression of Ror1 can be attributable to reduced expression of miR30a, a suppressor of Ror1 (Wang et al., 2018), which is up-regulated by aging, and age-related up-regulation of miR30a induces cellular senescence (Muther et al., 2017). miR30a can inhibit expression of Ror1 to suppress the progression of breast cancer, however, its expression level is reduced in breast cancer cells, resulting in up-regulation of Ror1 and promotion of the progression of breast cancer.
Furthermore, up-regulated expression of Ror1 can be mediated by STAT3 in various types of cancer cells, thereby promoting proliferation of cancer cells in Wnt5a-dependent or -independent manners (Li et al., 2010; Ikeda et al., 2020; Wang et al., 2021). STAT3 has been shown to be activated in several aged tissues with chronic inflammation (Chazaud and Mouchiroud, 2014; O'Brown et al., 2015), and thus might contribute to the age-related progression of cancers through expression of Ror1. In the case of gastric cancers, constitutive Wnt5a-Ror2 signaling in bone marrow-derived mesenchymal stem cells (MSCs) induces expression of CXCL16, and that CXCL16 secreted from MSCs promotes proliferation and migration of undifferentiated gastric cancer cell line MKN45 cells through inducing expression of Ror1 via STAT3 activation (Takiguchi et al., 2016; Ikeda et al., 2020). Considering that senescent MSCs secrete cytokines and chemokines (Lunyak et al., 2017), it can be assumed that aging might be one of critical factors promoting progression of cancers.
The Ror-family receptors play important roles in establishing developmental morphogenesis and tissue-/organo-genesis in redundant, yet pleiotropic manners. The activation of the Ror1/Ror2-signaling might be regulated by the induction of their expression by themselves, in addition to the stimulation with their ligands, including Wnt5a. Although expression levels of Ror1/Ror2 are kept lower in most of adult tissues than developing ones, expression of Ror1 and/or Ror2 are increased transiently in specific cells, including stem/progenitor cells, following injury or inflammation, via environmental cues including inflammatory cytokines, contributing to the promotion of tissue repair or regeneration (Figure 2A). Furthermore, the Ror-family receptors seem to play a role in maintaining the stemness of tissue stem/progenitor cells, and thereby preventing the induction of their cellular senescence. Therefore, it can be assumed that decreased expression of the Ror-family receptors in the tissue stem/progenitor cells might lead to the dysfunction of these tissue stem/progenitor cells.
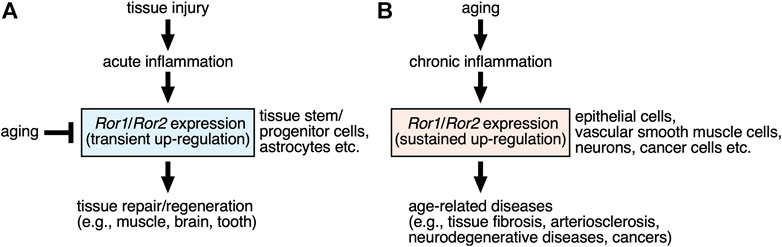
FIGURE 2. | Possible roles of the Ror-family receptors up-regulated by injury and aging in the tissue repair/regeneration and the age-related diseases. (A) Expression levels of Ror1 and/or Ror2 are increased transiently in somewhat restricted (specific) cells, including stem/progenitor cells and astrocytes, within the damaged tissues following injury, via environmental cues including inflammatory cytokines and growth factors. Up-regulated Ror1/Ror2 in turn contribute to the promotion of tissue repair or regeneration. Aging can mediate decreased expression of the Ror-family receptors in the tissue stem/progenitor cells, resulting in the dysfunction of these tissue stem/progenitor cells in regulating tissue repair or regeneration. (B) Sustained expression of Ror1 and/or Ror2 are induced in various types of cells under chronic inflammation caused by aging, thereby contributing to the development or progression of the age-related diseases, including fibrosis, arteriosclerosis, neurodegenerative diseases, and various cancers.
On the other hand, expression of Ror1 and/or Ror2 might be induced in various types of cells under chronic inflammation associated with aging, thereby contributing to the development or progression of the age-related diseases, including neurodegenerative diseases and cancers (Figure 2B). Therefore, it will be interesting to clarify the molecular mechanisms how expression of Ror1/Ror2 can be regulated in association with aging or cellular senescence.
ME, KK and YM wrote the manuscript, ME made the figures, KK made the table.
This work was supported by grants from Japan Agency for Medical Research, Development (AMED) [JP21gm1210005(YM) and JP21gm5010001(YM)], JST (Moonshot R and D) [JPMJMS 2022 (YM)] and KAKENHI from Japan Society for the Promotion Science (JSPS) [21K15504 (KK)]. This work also received funding from the collaborative research project program (PRISM) (ME) supported by Sumitomo Dainippon Pharma Co., Ltd. The funder was not involved in the writing of this article and the decision to submit it for publication. All authors declare no other competing interests.
This work received funding from the collaborative research project program (PRISM) (M.E.) supported by Sumitomo Dainippon Pharma Co., Ltd.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ackers, I., Szymanski, C., Duckett, K. J., Consitt, L. A., Silver, M. J., and Malgor, R. (2018). Blocking Wnt5a Signaling Decreases CD36 Expression and Foam Cell Formation in Atherosclerosis. Cardiovasc. Pathol. 34, 1–8. doi:10.1016/j.carpath.2018.01.008
Afzal, A. R., Rajab, A., Fenske, C. D., Oldridge, M., Elanko, N., Ternes-Pereira, E., et al. (2000). Recessive Robinow Syndrome, Allelic to Dominant Brachydactyly Type B, Is Caused by Mutation of ROR2. Nat. Genet. 25, 419–422. doi:10.1038/78107
Akbarzadeh, S., Wheldon, L. M., Sweet, S. M. M., Talma, S., Mardakheh, F. K., and Heath, J. K. (2008). The Deleted in Brachydactyly B Domain of ROR2 Is Required for Receptor Activation by Recruitment of Src. PLoS One 3, e1873. doi:10.1371/journal.pone.0001873
Al-Shawi, R., Ashton, S. V., Underwood, C., and Simons, J. P. (2001). Expression of the Ror1 and Ror2 Receptor Tyrosine Kinase Genes during Mouse Development. Dev. Genes Evol. 211, 161–171. doi:10.1007/s004270100140
Arabzadeh, S., Hossein, G., Salehi-Dulabi, Z., and Zarnani, A. H. (2016). WNT5A-ROR2 Is Induced by Inflammatory Mediators and Is Involved in the Migration of Human Ovarian Cancer Cell Line SKOV-3. Cell Mol Biol Lett 21, 9. doi:10.1186/s11658-016-0003-3
Astudillo, P. (2021). A Non-canonical Wnt Signature Correlates with Lower Survival in Gastric Cancer. Front. Cel Dev. Biol. 9, 633675. doi:10.3389/fcell.2021.633675
Atalay, S., Ege, B., Imamoglu, A., Suskan, E., ??Cal, B., and G??M????, H. (1993). Congenital Heart Disease and Robinow Syndrome. Clin. Dysmorphol. 2, 208–210. doi:10.1097/00019605-199307000-00003
Avincsal, M., Kamizaki, K., Jimbo, N., Shinomiya, H., Nibu, K.-I., Nishita, M., et al. (2021). Oncogenic E6 And/or E7 Proteins Drive Proliferation and Invasion of Human Papilloma Virus-positive H-ead and N-eck S-quamous C-ell C-ancer through U-pregulation of Ror2 E-xpression. Oncol. Rep. 46. doi:10.3892/or.2021.8099
Bai, Y., Tan, X., Zhang, H., Liu, C., Zhao, B., Li, Y., et al. (2014). Ror2 Receptor Mediates Wnt11 Ligand Signaling and Affects Convergence and Extension Movements in Zebrafish. J. Biol. Chem. 289, 20664–20676. doi:10.1074/jbc.M114.586099
Bayerlová, M., Menck, K., Klemm, F., Wolff, A., Pukrop, T., Binder, C., et al. (2017). Ror2 Signaling and its Relevance in Breast Cancer Progression. Front. Oncol. 7, 135. doi:10.3389/fonc.2017.00135
Cao, J., Wang, X., Dai, T., Wu, Y., Zhang, M., Cao, R., et al. (2018). Twist Promotes Tumor Metastasis in Basal-like Breast Cancer by Transcriptionally Upregulating ROR1. Theranostics 8, 2739–2751. doi:10.7150/thno.21477
Carbone, C., Piro, G., Gaianigo, N., Ligorio, F., Santoro, R., Merz, V., et al. (2018). Adipocytes Sustain Pancreatic Cancer Progression through a Non-canonical WNT Paracrine Network Inducing ROR2 Nuclear Shuttling. Int. J. Obes. 42, 334–343. doi:10.1038/ijo.2017.285
Chargé, S. B. P., and Rudnicki, M. A. (2004). Cellular and Molecular Regulation of Muscle Regeneration. Physiol. Rev. 84, 209–238. doi:10.1152/physrev.00019.2003
Chazaud, B., and Mouchiroud, G. (2014). Inflamm-aging: STAT3 Signaling Pushes Muscle Stem Cells off Balance. Cell Stem Cell 15, 401–402. doi:10.1016/j.stem.2014.09.010
Chen, M., Li, Y., Xiao, L., Dai, G., Lu, P., and Rui, Y. (2021). Noncanonical Wnt5a Signaling Regulates Tendon Stem/progenitor Cells Senescence. Stem Cel. Res. Ther. 12, 544. doi:10.1186/s13287-021-02605-1
Choi, M. Y., Widhopf, G. F., Ghia, E. M., Kidwell, R. L., Hasan, M. K., Yu, J., et al. (2018). Phase I Trial: Cirmtuzumab Inhibits ROR1 Signaling and Stemness Signatures in Patients with Chronic Lymphocytic Leukemia. Cell Stem Cell 22, 951–959.e953. doi:10.1016/j.stem.2018.05.018
Chow, M., Gao, L., Macmaniman, J. D., Bicocca, V. T., Chang, B. H., Alumkal, J. J., et al. (2018). Maintenance and Pharmacologic Targeting of ROR1 Protein Levels via UHRF1 in T(1;19) Pre-B-all. Oncogene 37, 5221–5232. doi:10.1038/s41388-018-0299-8
Coussens, L. M., and Werb, Z. (2002). Inflammation and Cancer. Nature 420, 860–867. doi:10.1038/nature01322
Curtin, J. A., Quint, E., Tsipouri, V., Arkell, R. M., Cattanach, B., Copp, A. J., et al. (2003). Mutation of Celsr1 Disrupts Planar Polarity of Inner Ear Hair Cells and Causes Severe Neural Tube Defects in the Mouse. Curr. Biol. 13, 1129–1133. doi:10.1016/s0960-9822(03)00374-9
Dechiara, T. M., Kimble, R. B., Poueymirou, W. T., Rojas, J., Masiakowski, P., Valenzuela, D. M., et al. (2000). Ror2, Encoding a Receptor-like Tyrosine Kinase, Is Required for Cartilage and Growth Plate Development. Nat. Genet. 24, 271–274. doi:10.1038/73488
Dong, X. Y., Huang, Y. X., Yang, Z., Chu, X. Y., Wu, J., Wang, S., et al. (2021). Downregulation of ROR2 Promotes Dental Pulp Stem Cell Senescence by Inhibiting STK4‐FOXO1/SMS1 axis in Sphingomyelin Biosynthesis. Aging Cell 20, e13430. doi:10.1111/acel.13430
Endo, M., Doi, R., Nishita, M., and Minami, Y. (2012). Ror-family Receptor Tyrosine Kinases Regulate Maintenance of Neural Progenitor Cells in the Developing Neocortex. J. Cel Sci. 125, 2017–2029. doi:10.1242/jcs.097782
Endo, M., and Minami, Y. (2018). Diverse Roles for the Ror-Family Receptor Tyrosine Kinases in Neurons and Glial Cells during Development and Repair of the Nervous System. Dev. Dyn. 247, 24–32. doi:10.1002/dvdy.24515
Endo, M., Tanaka, Y., Otsuka, M., and Minami, Y. (2020). E2F1‐Ror2 Signaling Mediates Coordinated Transcriptional Regulation to Promote G1/S Phase Transition in bFGF‐stimulated NIH/3T3 Fibroblasts. FASEB j. 34, 3413–3428. doi:10.1096/fj.201902849R
Endo, M., Ubulkasim, G., Kobayashi, C., Onishi, R., Aiba, A., and Minami, Y. (2017). Critical Role of Ror2 Receptor Tyrosine Kinase in Regulating Cell Cycle Progression of Reactive Astrocytes Following Brain Injury. Glia 65, 182–197. doi:10.1002/glia.23086
Enomoto, M., Hayakawa, S., Itsukushima, S., Ren, D. Y., Matsuo, M., Tamada, K., et al. (2009). Autonomous Regulation of Osteosarcoma Cell Invasiveness by Wnt5a/Ror2 Signaling. Oncogene 28, 3197–3208. doi:10.1038/onc.2009.175
Florian, M. C., Nattamai, K. J., Dörr, K., Marka, G., Überle, B., Vas, V., et al. (2013). A Canonical to Non-canonical Wnt Signalling Switch in Haematopoietic Stem-Cell Ageing. Nature 503, 392–396. doi:10.1038/nature12631
Frik, J., Merl‐Pham, J., Plesnila, N., Mattugini, N., Kjell, J., Kraska, J., et al. (2018). Cross‐talk between Monocyte Invasion and Astrocyte Proliferation Regulates Scarring in Brain Injury. EMBO Rep. 19. doi:10.15252/embr.201745294
Fukuda, T., Chen, L., Endo, T., Tang, L., Lu, D., Castro, J. E., et al. (2008). Antisera Induced by Infusions of Autologous Ad-Cd154-Leukemia B Cells Identify ROR1 as an Oncofetal Antigen and Receptor for Wnt5a. Proc. Natl. Acad. Sci. U.S.A. 105, 3047–3052. doi:10.1073/pnas.0712148105
Gentile, A., Lazzari, L., Benvenuti, S., Trusolino, L., and Comoglio, P. M. (2014). The ROR1 Pseudokinase Diversifies Signaling Outputs in MET-Addicted Cancer Cells. Int. J. Cancer 135, 2305–2316. doi:10.1002/ijc.28879
Götz, M., Sirko, S., Beckers, J., and Irmler, M. (2015). Reactive Astrocytes as Neural Stem or Progenitor Cells: In Vivo Lineage, In Vitro Potential, and Genome‐wide Expression Analysis. Glia 63, 1452–1468. doi:10.1002/glia.22850
Grumolato, L., Liu, G., Mong, P., Mudbhary, R., Biswas, R., Arroyave, R., et al. (2010). Canonical and Noncanonical Wnts Use a Common Mechanism to Activate Completely Unrelated Coreceptors. Genes Dev. 24, 2517–2530. doi:10.1101/gad.1957710
Hasan, M. K., Rassenti, L., Widhopf, G. F., YuYu, J., and Kipps, T. J. (2019). Wnt5a Causes ROR1 to Complex and Activate Cortactin to Enhance Migration of Chronic Lymphocytic Leukemia Cells. Leukemia 33, 653–661. doi:10.1038/s41375-018-0306-7
Hasan, M. K., Yu, J., Widhopf, G. F., Rassenti, L. Z., Chen, L., Shen, Z., et al. (2018). Wnt5a Induces ROR1 to Recruit DOCK2 to Activate Rac1/2 in Chronic Lymphocytic Leukemia. Blood 132, 170–178. doi:10.1182/blood-2017-12-819383
He, F., Xiong, W., Yu, X., Espinoza-Lewis, R., Liu, C., Gu, S., et al. (2008). Wnt5a Regulates Directional Cell Migration and Cell Proliferation via Ror2-Mediated Noncanonical Pathway in Mammalian Palate Development. Development 135, 3871–3879. doi:10.1242/dev.025767
Hikasa, H., Shibata, M., Hiratani, I., and Taira, M. (2002). TheXenopusreceptor Tyrosine Kinase Xror2 Modulates Morphogenetic Movements of the Axial Mesoderm and Neuroectoderm via Wnt Signaling. Development 129, 5227–5239. doi:10.1242/dev.129.22.5227
Ho, H.-Y. H., Susman, M. W., Bikoff, J. B., Ryu, Y. K., Jonas, A. M., Hu, L., et al. (2012). Wnt5a-Ror-Dishevelled Signaling Constitutes a Core Developmental Pathway that Controls Tissue Morphogenesis. Proc. Natl. Acad. Sci. U.S.A. 109, 4044–4051. doi:10.1073/pnas.1200421109
Hossein, G., Arabzadeh, S., Salehi-Dulabi, Z., Dehghani-Ghobadi, Z., Heidarian, Y., and Talebi-Juybari, M. (2017). Wnt5A Regulates the Expression of ROR2 Tyrosine Kinase Receptor in Ovarian Cancer Cells. Biochem. Cel Biol. 95, 609–615. doi:10.1139/bcb-2016-0216
Ida, L., Yamaguchi, T., Yanagisawa, K., Kajino, T., Shimada, Y., Suzuki, M., et al. (2016). Receptor Tyrosine Kinase‐like Orphan Receptor 1, a Target of NKX2‐1/TTF‐1 Lineage‐survival Oncogene, Inhibits Apoptosis Signal‐regulating Kinase 1‐mediated Pro‐apoptotic Signaling in Lung Adenocarcinoma. Cancer Sci. 107, 155–161. doi:10.1111/cas.12858
Ikeda, T., Nishita, M., Hoshi, K., Honda, T., Kakeji, Y., and Minami, Y. (2020). Mesenchymal Stem Cell‐derived CXCL16 Promotes Progression of Gastric Cancer Cells by STAT3‐mediated Expression of Ror1. Cancer Sci. 111, 1254–1265. doi:10.1111/cas.14339
Islam, S. S., Uddin, M., Noman, A. S. M., Akter, H., Dity, N. J., Basiruzzman, M., et al. (2019). Antibody-drug Conjugate T-DM1 Treatment for HER2+ Breast Cancer Induces ROR1 and Confers Resistance through Activation of Hippo Transcriptional Coactivator YAP1. EBioMedicine 43, 211–224. doi:10.1016/j.ebiom.2019.04.061
Kamizaki, K., Doi, R., Hayashi, M., Saji, T., Kanagawa, M., Toda, T., et al. (2017). The Ror1 Receptor Tyrosine Kinase Plays a Critical Role in Regulating Satellite Cell Proliferation during Regeneration of Injured Muscle. J. Biol. Chem. 292, 15939–15951. doi:10.1074/jbc.M117.785709
Kani, S., Oishi, I., Yamamoto, H., Yoda, A., Suzuki, H., Nomachi, A., et al. (2004). The Receptor Tyrosine Kinase Ror2 Associates with and Is Activated by Casein Kinase Iϵ. J. Biol. Chem. 279, 50102–50109. doi:10.1074/jbc.M409039200
Karvonen, H., Arjama, M., Kaleva, L., Niininen, W., Barker, H., Koivisto-Korander, R., et al. (2020). Glucocorticoids Induce Differentiation and Chemoresistance in Ovarian Cancer by Promoting ROR1-Mediated Stemness. Cell Death Dis 11, 790. doi:10.1038/s41419-020-03009-4
Karvonen, H., Niininen, W., Murumägi, A., and Ungureanu, D. (2017). Targeting ROR1 Identifies New Treatment Strategies in Hematological Cancers. Biochem. Soc. Trans. 45, 457–464. doi:10.1042/bst20160272
Kaucká, M., Krejčí, P., Plevová, K., Pavlová, Š., Procházková, J., Janovská, P., et al. (2011). Post-translational Modifications Regulate Signalling by Ror1. Acta Physiol. (Oxf.) 203, 351–362. doi:10.1111/j.1748-1716.2011.02306.x
Khaledian, B., Taguchi, A., Shin‐ya, K., Kondo‐Ida, L., Kagaya, N., Suzuki, M., et al. (2021). Inhibition of Heat Shock Protein 90 Destabilizes Receptor Tyrosine Kinase ROR1 in Lung Adenocarcinoma. Cancer Sci. 112, 1225–1234. doi:10.1111/cas.14786
Kibar, Z., Vogan, K. J., Groulx, N., Justice, M. J., Underhill, D. A., and Gros, P. (2001). Ltap, a Mammalian Homolog of Drosophila Strabismus/Van Gogh, Is Altered in the Mouse Neural Tube Mutant Loop-Tail. Nat. Genet. 28, 251–255. doi:10.1038/90081
Kishimoto, K., Tamura, M., Nishita, M., Minami, Y., Yamaoka, A., Abe, T., et al. (2018). Synchronized Mesenchymal Cell Polarization and Differentiation Shape the Formation of the Murine Trachea and Esophagus. Nat. Commun. 9, 2816. doi:10.1038/s41467-018-05189-2
Kizil, C., Kyritsis, N., and Brand, M. (2015). Effects of Inflammation on Stem Cells: Together They Strive? EMBO Rep. 16, 416–426. doi:10.15252/embr.201439702
Konopelski Snavely, S. E., Susman, M. W., Kunz, R. C., Tan, J., Srinivasan, S., Cohen, M. D., et al. (2021). Proteomic Analysis Identifies the E3 Ubiquitin Ligase Pdzrn3 as a Regulatory Target of Wnt5a-Ror Signaling. Proc. Natl. Acad. Sci. U.S.A. 118. doi:10.1073/pnas.2104944118
Lai, S.-s., Xue, B., Yang, Y., Zhao, L., Chu, C.-s., Hao, J.-y., et al. (2012). Ror2-Src Signaling in Metastasis of Mouse Melanoma Cells Is Inhibited by NRAGE. Cancer Genet. 205, 552–562. doi:10.1016/j.cancergen.2012.09.002
Lei, L., Huang, Z., Feng, J., Huang, Z., Tao, Y., Hu, X., et al. (2020). Loss of Receptor Tyrosine Kinase-like Orphan Receptor 2 Impairs the Osteogenesis of mBMSCs by Inhibiting Signal Transducer and Activator of Transcription 3. Stem Cel. Res. Ther. 11, 137. doi:10.1186/s13287-020-01646-2
Li, P., Harris, D., Liu, Z., Liu, J., Keating, M., and Estrov, Z. (2010). Stat3 Activates the Receptor Tyrosine Kinase like Orphan Receptor-1 Gene in Chronic Lymphocytic Leukemia Cells. PLoS One 5, e11859. doi:10.1371/journal.pone.0011859
Li, X., Yamagata, K., Nishita, M., Endo, M., Arfian, N., Rikitake, Y., et al. (2013). Activation of Wnt5a-Ror2 Signaling Associated with Epithelial-To-Mesenchymal Transition of Tubular Epithelial Cells during Renal Fibrosis. Genes Cells 18, 608–619. doi:10.1111/gtc.12064
Lin, M., Li, L., Liu, C., Liu, H., He, F., Yan, F., et al. (2011). Wnt5a Regulates Growth, Patterning, and Odontoblast Differentiation of Developing Mouse Tooth. Dev. Dyn. 240, 432–440. doi:10.1002/dvdy.22550
Liu, M., Shi, Y., Hu, Q., Qin, Y., Ji, S., Liu, W., et al. (2021). SETD8 Induces Stemness and Epithelial-Mesenchymal Transition of Pancreatic Cancer Cells by Regulating ROR1 Expression. Acta Biochim. Biophys. Sin (Shanghai) 53, 1614–1624. doi:10.1093/abbs/gmab140
Lunyak, V. V., Amaro-Ortiz, A., and Gaur, M. (2017). Mesenchymal Stem Cells Secretory Responses: Senescence Messaging Secretome and Immunomodulation Perspective. Front. Genet. 8, 220. doi:10.3389/fgene.2017.00220
Ma, Y., Jing, J., Feng, J., Yuan, Y., Wen, Q., Han, X., et al. (2021). Ror2-mediated Non-canonical Wnt Signaling Regulates Cdc42 and Cell Proliferation during Tooth Root Development. Development 148. doi:10.1242/dev.196360
Maeda, K., Kobayashi, Y., Udagawa, N., Uehara, S., Ishihara, A., Mizoguchi, T., et al. (2012). Wnt5a-Ror2 Signaling between Osteoblast-Lineage Cells and Osteoclast Precursors Enhances Osteoclastogenesis. Nat. Med. 18, 405–412. doi:10.1038/nm.2653
Martinez, S., Scerbo, P., Giordano, M., Daulat, A. M., Lhoumeau, A.-C., Thomé, V., et al. (2015). The PTK7 and ROR2 Protein Receptors Interact in the Vertebrate WNT/Planar Cell Polarity (PCP) Pathway. J. Biol. Chem. 290, 30562–30572. doi:10.1074/jbc.M115.697615
Matsuda, T., Nomi, M., Ikeya, M., Kani, S., Oishi, I., Terashima, T., et al. (2001). Expression of the Receptor Tyrosine Kinase Genes, Ror1 and Ror2, during Mouse Development. Mech. Dev. 105, 153–156. doi:10.1016/s0925-4773(01)00383-5
Mauro, A. (1961). Satellite Cell of Skeletal Muscle Fibers. J. Biophys. Biochem. Cytol. 9, 493–495. doi:10.1083/jcb.9.2.493
Menck, K., Heinrichs, S., Wlochowitz, D., Sitte, M., Noeding, H., Janshoff, A., et al. (2021). WNT11/ROR2 Signaling Is Associated with Tumor Invasion and Poor Survival in Breast Cancer. J. Exp. Clin. Cancer Res. 40, 395. doi:10.1186/s13046-021-02187-z
Mikels, A. J., and Nusse, R. (2006). Purified Wnt5a Protein Activates or Inhibits β-Catenin-TCF Signaling Depending on Receptor Context. Plos Biol. 4, e115. doi:10.1371/journal.pbio.0040115
Mikels, A., Minami, Y., and Nusse, R. (2009). Ror2 Receptor Requires Tyrosine Kinase Activity to Mediate Wnt5A Signaling. J. Biol. Chem. 284, 30167–30176. doi:10.1074/jbc.M109.041715
Minami, Y., Oishi, I., Endo, M., and Nishita, M. (2009). Ror-family Receptor Tyrosine Kinases in Noncanonical Wnt Signaling: Their Implications in Developmental Morphogenesis and Human Diseases. Dev. Dyn. 239, NA. doi:10.1002/dvdy.21991
Mohammadlou, M., Abdollahi, M., Hemati, M., Baharlou, R., Doulabi, E. M., Pashaei, M., et al. (2021). Apoptotic Effect of Berberine via Bcl‐2, ROR1, and Mir‐21 in Patients with B‐chronic Lymphocytic Leukemia. Phytotherapy Res. 35, 2025–2033. doi:10.1002/ptr.6945
Montcouquiol, M., Rachel, R. A., Lanford, P. J., Copeland, N. G., Jenkins, N. A., and Kelley, M. W. (2003). Identification of Vangl2 and Scrb1 as Planar Polarity Genes in Mammals. Nature 423, 173–177. doi:10.1038/nature01618
Morioka, K., Tanikawa, C., Ochi, K., Daigo, Y., Katagiri, T., Kawano, H., et al. (2009). Orphan Receptor Tyrosine Kinase ROR2 as a Potential Therapeutic Target for Osteosarcoma. Cancer Sci. 100, 1227–1233. doi:10.1111/j.1349-7006.2009.01165.x
Munoz-Soriano, V., Belacortu, Y., and Paricio, N. (2012). Planar Cell Polarity Signaling in Collective Cell Movements during Morphogenesis and Disease. Cg 13, 609–622. doi:10.2174/138920212803759721
Muther, C., Jobeili, L., Garion, M., Heraud, S., Thepot, A., Damour, O., et al. (2017). An Expression Screen for Aged-dependent microRNAs Identifies miR-30a as a Key Regulator of Aging Features in Human Epidermis. Aging 9, 2376–2396. doi:10.18632/aging.101326
Nishita, M., Park, S.-Y., Nishio, T., Kamizaki, K., Wang, Z., Tamada, K., et al. (2017). Ror2 Signaling Regulates Golgi Structure and Transport through IFT20 for Tumor Invasiveness. Sci. Rep. 7, 1. doi:10.1038/s41598-016-0028-x
Nishita, M., Qiao, S., Miyamoto, M., Okinaka, Y., Yamada, M., Hashimoto, R., et al. (2014). Role of Wnt5a-Ror2 Signaling in Morphogenesis of the Metanephric Mesenchyme during Ureteric Budding. Mol. Cel Biol 34, 3096–3105. doi:10.1128/mcb.00491-14
Nishita, M., Yoo, S. K., Nomachi, A., Kani, S., Sougawa, N., Ohta, Y., et al. (2006). Filopodia Formation Mediated by Receptor Tyrosine Kinase Ror2 Is Required for Wnt5a-Induced Cell Migration. J. Cel Biol. 175, 555–562. doi:10.1083/jcb.200607127
Nomachi, A., Nishita, M., Inaba, D., Enomoto, M., Hamasaki, M., and Minami, Y. (2008). Receptor Tyrosine Kinase Ror2 Mediates Wnt5a-Induced Polarized Cell Migration by Activating C-Jun N-Terminal Kinase via Actin-Binding Protein Filamin A. J. Biol. Chem. 283, 27973–27981. doi:10.1074/jbc.M802325200
Nomi, M., Oishi, I., Kani, S., Suzuki, H., Matsuda, T., Yoda, A., et al. (2001). Loss of mRor1 Enhances the Heart and Skeletal Abnormalities in mRor2 -Deficient Mice: Redundant and Pleiotropic Functions of mRor1 and mRor2 Receptor Tyrosine Kinases. Mol. Cel Biol 21, 8329–8335. doi:10.1128/MCB.21.24.8329-8335.2001
O'connell, M. P., Marchbank, K., Webster, M. R., Valiga, A. A., Kaur, A., Vultur, A., et al. (2013). Hypoxia Induces Phenotypic Plasticity and Therapy Resistance in Melanoma via the Tyrosine Kinase Receptors ROR1 and ROR2. Cancer Discov. 3, 1378–1393. doi:10.1158/2159-8290.Cd-13-0005
Obradović, M. M. S., Hamelin, B., Manevski, N., Couto, J. P., Sethi, A., Coissieux, M.-M., et al. (2019). Glucocorticoids Promote Breast Cancer Metastasis. Nature 567, 540–544. doi:10.1038/s41586-019-1019-4
O’Brown, Z. K., Van Nostrand, E. L., Higgins, J. P., and Kim, S. K. (2015). The Inflammatory Transcription Factors NFκB, STAT1 and STAT3 Drive Age-Associated Transcriptional Changes in the Human Kidney. Plos Genet. 11, e1005734. doi:10.1371/journal.pgen.1005734
Oishi, I., Suzuki, H., Onishi, N., Takada, R., Kani, S., Ohkawara, B., et al. (2003). The Receptor Tyrosine Kinase Ror2 Is Involved in Non-canonical Wnt5a/JNK Signalling Pathway. Genes Cells 8, 645–654. doi:10.1046/j.1365-2443.2003.00662.x
Oishi, I., Takeuchi, S., Hashimoto, R., Nagabukuro, A., Ueda, T., Liu, Z.-J., et al. (1999). Spatio-temporally Regulated Expression of Receptor Tyrosine Kinases, mRor1, mRor2, during Mouse Development: Implications in Development and Function of the Nervous System. Genes to Cells 4, 41–56. doi:10.1046/j.1365-2443.1999.00234.x
Ostan, R., Lanzarini, C., Pini, E., Scurti, M., Vianello, D., Bertarelli, C., et al. (2015). Inflammaging and Cancer: a challenge for the Mediterranean Diet. Nutrients 7, 2589–2621. doi:10.3390/nu7042589
Paganoni, S., Bernstein, J., and Ferreira, A. (2010). Ror1-Ror2 Complexes Modulate Synapse Formation in Hippocampal Neurons. Neuroscience 165, 1261–1274. doi:10.1016/j.neuroscience.2009.11.056
Person, A. D., Beiraghi, S., Sieben, C. M., Hermanson, S., Neumann, A. N., Robu, M. E., et al. (2009). WNT5Amutations in Patients with Autosomal Dominant Robinow Syndrome. Dev. Dyn. 239, NA. doi:10.1002/dvdy.22156
Qi, X., Okinaka, Y., Nishita, M., and Minami, Y. (2016). Essential Role of Wnt5a-Ror1/Ror2 Signaling in Metanephric Mesenchyme and Ureteric Bud Formation. Genes Cells 21, 325–334. doi:10.1111/gtc.12342
Qian, D., Jones, C., Rzadzinska, A., Mark, S., Zhang, X., Steel, K. P., et al. (2007). Wnt5a Functions in Planar Cell Polarity Regulation in Mice. Dev. Biol. 306, 121–133. doi:10.1016/j.ydbio.2007.03.011
Sato, A., Kayama, H., Shojima, K., Matsumoto, S., Koyama, H., Minami, Y., et al. (2015). The Wnt5a-Ror2 axis Promotes the Signaling Circuit between Interleukin-12 and Interferon-γ in Colitis. Sci. Rep. 5, 10536. doi:10.1038/srep10536
Sato, A., Yamamoto, H., Sakane, H., Koyama, H., and Kikuchi, A. (2010). Wnt5a Regulates Distinct Signalling Pathways by Binding to Frizzled2. EMBO J. 29, 41–54. doi:10.1038/emboj.2009.322
Schambony, A., and Wedlich, D. (2007). Wnt-5A/Ror2 Regulate Expression of XPAPC through an Alternative Noncanonical Signaling Pathway. Dev. Cel 12, 779–792. doi:10.1016/j.devcel.2007.02.016
Schwabe, G. C., Trepczik, B., Süring, K., Brieske, N., Tucker, A. S., Sharpe, P. T., et al. (2004). Ror2knockout Mouse as a Model for the Developmental Pathology of Autosomal Recessive Robinow Syndrome. Dev. Dyn. 229, 400–410. doi:10.1002/dvdy.10466
Seale, P., Sabourin, L. A., Girgis-Gabardo, A., Mansouri, A., Gruss, P., and Rudnicki, M. A. (2000). Pax7 Is Required for the Specification of Myogenic Satellite Cells. Cell 102, 777–786. doi:10.1016/s0092-8674(00)00066-0
Sheetz, J. B., Mathea, S., Karvonen, H., Malhotra, K., Chatterjee, D., Niininen, W., et al. (2020). Structural Insights into Pseudokinase Domains of Receptor Tyrosine Kinases. Mol. Cel 79, 390–405.e397. doi:10.1016/j.molcel.2020.06.018
Shimizu, T., Smits, R., and Ikenaka, K. (2016). Microglia-Induced Activation of Noncanonical Wnt Signaling Aggravates Neurodegeneration in Demyelinating Disorders. Mol. Cel Biol 36, 2728–2741. doi:10.1128/mcb.00139-16
Shojima, K., Sato, A., Hanaki, H., Tsujimoto, I., Nakamura, M., Hattori, K., et al. (2015). Wnt5a Promotes Cancer Cell Invasion and Proliferation by Receptor-Mediated Endocytosis-dependent and -independent Mechanisms, Respectively. Sci. Rep. 5, 8042. doi:10.1038/srep08042
Sofroniew, M. V. (2015). Astrocyte Barriers to Neurotoxic Inflammation. Nat. Rev. Neurosci. 16, 249–263. doi:10.1038/nrn3898
Stüber, T., Monjezi, R., Wallstabe, L., Kühnemundt, J., Nietzer, S. L., Dandekar, G., et al. (2020). Inhibition of TGF-β-Receptor Signaling Augments the Antitumor Function of ROR1-specific CAR T-Cells against Triple-Negative Breast Cancer. J. Immunother. Cancer 8, e000676. doi:10.1136/jitc-2020-000676
Takeuchi, S., Takeda, K., Oishi, I., Nomi, M., Ikeya, M., Itoh, K., et al. (2000). Mouse Ror2 Receptor Tyrosine Kinase Is Required for the Heart Development and Limb Formation. Genes to Cells 5, 71–78. doi:10.1046/j.1365-2443.2000.00300.x
Takiguchi, G., Nishita, M., Kurita, K., Kakeji, Y., and Minami, Y. (2016). Wnt5a‐Ror2 Signaling in Mesenchymal Stem Cells Promotes Proliferation of Gastric Cancer Cells by Activating CXCL16-CXCR6 axis. Cancer Sci. 107, 290–297. doi:10.1111/cas.12871
Tan, H., He, Q., Gong, G., Wang, Y., Li, J., Wang, J., et al. (2016). miR-382 Inhibits Migration and Invasion by Targeting ROR1 through Regulating EMT in Ovarian Cancer. Int. J. Oncol. 48, 181–190. doi:10.3892/ijo.2015.3241
Tao, J., Zhi, X., Zhang, X., Fu, M., Huang, H., Fan, Y., et al. (2015). miR-27b-3p Suppresses Cell Proliferation through Targeting Receptor Tyrosine Kinase like Orphan Receptor 1 in Gastric Cancer. J. Exp. Clin. Cancer Res. 34, 139. doi:10.1186/s13046-015-0253-3
Tiwari, R. L., Mishra, P., Martin, N., George, N. O., Sakk, V., Soller, K., et al. (2021). A Wnt5a-Cdc42 axis Controls Aging and Rejuvenation of Hair-Follicle Stem Cells. Aging 13, 4778–4793. doi:10.18632/aging.202694
Van Bokhoven, H., Celli, J., Kayserili, H., Van Beusekom, E., Balci, S., Brussel, W., et al. (2000). Mutation of the Gene Encoding the ROR2 Tyrosine Kinase Causes Autosomal Recessive Robinow Syndrome. Nat. Genet. 25, 423–426. doi:10.1038/78113
Veskimäe, K., Scaravilli, M., Niininen, W., Karvonen, H., Jaatinen, S., Nykter, M., et al. (2018). Expression Analysis of Platinum Sensitive and Resistant Epithelial Ovarian Cancer Patient Samples Reveals New Candidates for Targeted Therapies. Translational Oncol. 11, 1160–1170. doi:10.1016/j.tranon.2018.07.010
Wallstabe, L., Göttlich, C., Nelke, L. C., Kühnemundt, J., Schwarz, T., Nerreter, T., et al. (2019). ROR1-CAR T Cells Are Effective against Lung and Breast Cancer in Advanced Microphysiologic 3D Tumor Models. JCI Insight 4. doi:10.1172/jci.insight.126345
Wang, J., Hamblet, N. S., Mark, S., Dickinson, M. E., Brinkman, B. C., Segil, N., et al. (2006a). Dishevelled Genes Mediate a Conserved Mammalian PCP Pathway to Regulate Convergent Extension during Neurulation. Development 133, 1767–1778. doi:10.1242/dev.02347
Wang, Q., Lu, B., Zhang, Y., Yu, J., Guo, J., Zhou, Q., et al. (2021). STAT3 Inhibitor BBI608 Enhances the Antitumor Effect of Gefitinib on EGFR-Mutated Non-small Cell Lung Cancer Cells. Hum. Cel 34, 1855–1865. doi:10.1007/s13577-021-00582-4
Wang, X., Qiu, H., Tang, R., Song, H., Pan, H., Feng, Z., et al. (2018). miR-30a I-nhibits E-pithelial-mesenchymal T-ransition and M-etastasis in T-riple-negative B-reast C-ancer by T-argeting ROR1. Oncol. Rep. 39, 2635–2643. doi:10.3892/or.2018.6379
Wang, Y., Guo, N., and Nathans, J. (2006b). The Role of Frizzled3 and Frizzled6 in Neural Tube Closure and in the Planar Polarity of Inner-Ear Sensory Hair Cells. J. Neurosci. 26, 2147–2156. doi:10.1523/JNEUROSCI.4698-05.2005
Weissenböck, M., Latham, R., Nishita, M., Wolff, L. I., Ho, H. Y. H., Minami, Y., et al. (2019). Genetic Interactions between Ror2 and Wnt9a, Ror1 and Wnt9a and Ror2 and Ror1: Phenotypic Analysis of the Limb Skeleton and Palate in Compound Mutants. Genes Cells 24, 307–317. doi:10.1111/gtc.12676
Williamson, M. R., Fuertes, C. J. A., Dunn, A. K., Drew, M. R., and Jones, T. A. (2021). Reactive Astrocytes Facilitate Vascular Repair and Remodeling after Stroke. Cel Rep. 35, 109048. doi:10.1016/j.celrep.2021.109048
Wright, T. M., and Rathmell, W. K. (2010). Identification of Ror2 as a hypoxia-inducible factor target in von Hippel-Lindau-associated renal cell carcinoma. J. Biol. Chem. 285, 12916–12924. doi:10.1074/jbc.M109.073924
Xiao, T., Yan, Z., Xiao, S., and Xia, Y. (2020). Proinflammatory Cytokines Regulate Epidermal Stem Cells in Wound Epithelialization. Stem Cel. Res. Ther. 11, 232. doi:10.1186/s13287-020-01755-y
Yamada, M., Udagawa, J., Matsumoto, A., Hashimoto, R., Hatta, T., Nishita, M., et al. (2010). Ror2 Is Required for Midgut Elongation during Mouse Development. Dev. Dyn. 239, 941–953. doi:10.1002/dvdy.22212
Yamada, Y., Nakamura-Yamada, S., Kusano, K., and Baba, S. (2019). Clinical Potential and Current Progress of Dental Pulp Stem Cells for Various Systemic Diseases in Regenerative Medicine: A Concise Review. Ijms 20, 1132. doi:10.3390/ijms20051132
Yamagata, K., Li, X., Ikegaki, S., Oneyama, C., Okada, M., Nishita, M., et al. (2012). Dissection of Wnt5a-Ror2 Signaling Leading to Matrix Metalloproteinase (MMP-13) Expression. J. Biol. Chem. 287, 1588–1599. doi:10.1074/jbc.M111.315127
Yamaguchi, T. P., Bradley, A., Mcmahon, A. P., and Jones, S. (1999). A Wnt5a Pathway Underlies Outgrowth of Multiple Structures in the Vertebrate Embryo. Development 126, 1211–1223. doi:10.1242/dev.126.6.1211
Yamaguchi, T., Yanagisawa, K., Sugiyama, R., Hosono, Y., Shimada, Y., Arima, C., et al. (2012). NKX2-1/TITF1/TTF-1-Induced ROR1 Is Required to Sustain EGFR Survival Signaling in Lung Adenocarcinoma. Cancer Cell 21, 348–361. doi:10.1016/j.ccr.2012.02.008
Yamamoto, H., Yoo, S. K., Nishita, M., Kikuchi, A., and Minami, Y. (2007). Wnt5a Modulates Glycogen Synthase Kinase 3 to Induce Phosphorylation of Receptor Tyrosine Kinase Ror2. Genes Cells 12, 1215–1223. doi:10.1111/j.1365-2443.2007.01128.x
Yamamoto, S., Nishimura, O., Misaki, K., Nishita, M., Minami, Y., Yonemura, S., et al. (2008). Cthrc1 Selectively Activates the Planar Cell Polarity Pathway of Wnt Signaling by Stabilizing the Wnt-Receptor Complex. Dev. Cel 15, 23–36. doi:10.1016/j.devcel.2008.05.007
Yang, B., Teng, F., Chang, L., Wang, J., Liu, D.-L., Cui, Y.-S., et al. (2021). Tumor-derived Exosomal circRNA_102481 Contributes to EGFR-TKIs Resistance via the miR-30a-5p/ROR1 axis in Non-small Cell Lung Cancer. Aging 13, 13264–13286. doi:10.18632/aging.203011
Yang, H., Yu, G., Wang, Y., and Guo, X. (2022). Circ_0044520 Regulates the Progression of Laryngeal Squamous Cell Carcinoma via the miR-338-3p/ROR2 axis. Histol. Histopathol, 18420. doi:10.14670/hh-18-420
Ybot-Gonzalez, P., Savery, D., Gerrelli, D., Signore, M., Mitchell, C. E., Faux, C. H., et al. (2007). Convergent Extension, Planar-Cell-Polarity Signalling and Initiation of Mouse Neural Tube Closure. Development 134, 789–799. doi:10.1242/dev.000380
Yu, J., Chen, L., Cui, B., Widhopf, G. F., Shen, Z., Wu, R., et al. (2015). Wnt5a Induces ROR1/ROR2 Heterooligomerization to Enhance Leukemia Chemotaxis and Proliferation. J. Clin. Invest. 126, 585–598. doi:10.1172/jci83535
Zhang, C.-J., Zhu, N., Liu, Z., Shi, Z., Long, J., Zu, X.-Y., et al. (2020a). Wnt5a/Ror2 Pathway Contributes to the Regulation of Cholesterol Homeostasis and Inflammatory Response in Atherosclerosis. Biochim. Biophys. Acta (Bba) - Mol. Cel Biol. Lipids 1865, 158547. doi:10.1016/j.bbalip.2019.158547
Zhang, K., Yao, E., Lin, C., Chou, Y.-T., Wong, J., Li, J., et al. (2020b). A Mammalian Wnt5a-Ror2-Vangl2 axis Controls the Cytoskeleton and Confers Cellular Properties Required for Alveologenesis. Elife 9. doi:10.7554/eLife.53688
Zhang, S., Chen, L., Cui, B., Chuang, H.-Y., Yu, J., Wang-Rodriguez, J., et al. (2012). ROR1 Is Expressed in Human Breast Cancer and Associated with Enhanced Tumor-Cell Growth. PLoS One 7, e31127. doi:10.1371/journal.pone.0031127
Zhang, S., Zhang, H., Ghia, E. M., Huang, J., Wu, L., Zhang, J., et al. (2019). Inhibition of Chemotherapy Resistant Breast Cancer Stem Cells by a ROR1 Specific Antibody. Proc. Natl. Acad. Sci. U.S.A. 116, 1370–1377. doi:10.1073/pnas.1816262116
Zheng, C., Chen, J., Liu, S., and Jin, Y. (2019). Stem Cell-Based Bone and Dental Regeneration: a View of Microenvironmental Modulation. Int. J. Oral Sci. 11, 23. doi:10.1038/s41368-019-0060-3
Keywords: non-canonical wnt signaling, cell polarity, migration, proliferation, stem/progenitor cells, cellular senescence, inflammation, cancers
Citation: Endo M, Kamizaki K and Minami Y (2022) The Ror-Family Receptors in Development, Tissue Regeneration and Age-Related Disease. Front. Cell Dev. Biol. 10:891763. doi: 10.3389/fcell.2022.891763
Received: 08 March 2022; Accepted: 29 March 2022;
Published: 13 April 2022.
Edited by:
Henry Ho, UC Davis Health, United StatesReviewed by:
Bo Gao, The University of Hong Kong, Hong Kong SAR, ChinaCopyright © 2022 Endo, Kamizaki and Minami. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mitsuharu Endo, bWVuZG9AbWVkLmtvYmUtdS5hYy5qcA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.