
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Cell Dev. Biol. , 01 June 2022
Sec. Membrane Traffic and Organelle Dynamics
Volume 10 - 2022 | https://doi.org/10.3389/fcell.2022.883841
This article is part of the Research Topic Unconventional Protein Secretion: From Basic Mechanisms to Dysregulation in Disease View all 19 articles
Mechanisms devoted to the secretion of proteins via extracellular vesicles (EVs) have been found in mammals, yeasts, and plants. Since they transport a number of leader-less proteins to the plasma membrane or the extracellular space, EVs are considered part of Unconventional protein secretion (UPS) routes. UPS involving EVs are a relatively new field in plants. Aside from their role in plant physiology and immunity, plant extracts containing EVs have also been shown to be beneficial for human health. Therefore, exploring the use of plant EVs in biomedicine and their potential as drug delivery tools is an exciting avenue. Here we give a summary of the state of knowledge on plant EVs, their crosstalk with mammalian systems and potential research routes that could lead to practical applications in therapeutic drug delivery.
Extracellular vesicles (EVs) are a collection of vesicles with different origins, size ranges, and molecular composition. Originally considered as cellular waste, their discovery has revolutionised our understanding of cell-cell communications and transfer of biological information from 1 cell to another. Since leaderless proteins loaded in these vesicles bypass the Golgi and are recruited in EVs from the cytosol, most EVs are considered part of the unconventional secretion pathway (UPS). Exosomes, a particular type of EV, are particularly interesting in this context for the following reasons: the mechanism of cargo loading in vivo and in vitro are being better understood in human cells (Xu et al., 2020), exosomes have the ability to cross natural barriers (Blood brain barrier and placenta) and are described as safe and stable nanoparticles (Banks et al., 2020; Elliott and He, 2021). Consequently, mammalian exosomes are being investigated for their potential in drug delivery (Xu et al., 2020; Choi et al., 2021). Plants also secrete extracellular vesicles, and exosomes have been identified (He et al., 2021). While keeping the benefits of human exosomes, the use of plant exosomes as drug delivery tools in biomedicine might offer various additional advantages such as lower production costs involved in biopharming and reduced cross-human contaminations. In this mini-review, we are summarising the current knowledge on plant UPS specifically focusing on EVs and exosomes. We are then clarifying the extraction procedures of various plant EVs and finally we are proposing a view on the potential benefits of using plant EVs as drug delivery tools in human health.
Unconventional protein secretion (UPS) involves a range of mechanisms that allow proteins to reach the extracellular medium, bypassing at least part of the conventional ER-Golgi-PM secretory pathway. While this conventional pathway usually involves the presence of signal peptides at the N-terminus of proteins, UPS leads to the secretion of leaderless soluble proteins in the extracellular medium or trafficking of membrane proteins via an alternative route than through the Golgi (Rabouille et al., 2012; Rabouille, 2017). These mechanisms are being intensively studied in mammals and yeasts because they are often associated with stress and pathologies such as inflammatory diseases or cancer (Kim et al., 2018; Cohen et al., 2020). Therefore, understanding the mechanisms of UPS is a promising new route into identifying new therapeutic targets. Extracellular vesicles, in particular, represent a specific type of vesicular UPS that has been extensively studied since their discovery 40 years ago (Harding et al., 2013). Their ability to pack biological information which is then transmitted to adjacent or long-distance cells have triggered extensive research into their use as a drug delivery system. There are various types of extracellular vesicles that can be classified depending on their origin and content (Théry et al., 2018). This classification is constantly updated with new knowledge. Exosomes, a specific class of small EVs (sEVs) released by the fusion of MVBs with the membrane, are of particular interest for targeted drug delivery since they have been shown to cross natural barriers such as the Blood brain barrier and placenta (for review Elliott and He, 2021). The use of mammalian exosomes in drug delivery presents various advantages described above but also some challenges (Meng et al., 2020; Chen et al., 2021). Three of these challenges are the lack of homogeneity, the lack of large-scale cost-effective production, and ethical issues linked with transferring human material.
To address some of these challenges in terms of cost-effective production and lack of ethical issues, plants might offer an alternative source of exosomes and EVs. As a result, a growing number of studies are looking into their potential health benefits. For example, the effect of plant extracellular vesicles loaded with curcumin are currently being tested in clinical trials (NCT01294072) to evaluate their impact on surgery of newly diagnosed colon cancer patients (https://clinicaltrials.gov/ct2/show/NCT01294072).
Unfortunately, plant unconventional protein secretion pathways have attracted only late interests and our current knowledge of plant UPS and EVs is growing but still limited (Ding et al., 2014a; Robinson et al., 2016; Hansen and Nielsen 2017; Cui et al., 2019). The presence of leaderless proteins in apoplastic extracellular vesicles has confirmed that these EVs represent genuine plant UPS pathways involved in cell wall remodelling and resistance to infection (Delaunois et al., 2013, 2014). Investigations around these vesicular mechanisms have uncovered the existence of at least three pathways that result in the release of extracellular vesicles in plants: exocyst-positive organelle mediated secretion (EXPO), vesicle budding from the PM (including microvesicles), and multivesicular body (MVB)-PM fusion (Wang et al., 2010; Regente et al., 2012; Cui et al., 2019). A growing number of studies report the beneficial effect of crude and pure extracts of plant EVs on human health (Akuma et al., 2019; Alfieri et al., 2021; Urzì et al., 2021). To evaluate their potential as drug delivery tools, the current state of the field in terms of plant EVs classification, purification, and biomedical applications is presented below.
The term “plant extracellular vesicles” generally refers to apoplastic vesicles. Plant-derived nanovesicles (PDNVs) or exosomes-like nanoparticles (ELNs) are terms used to refer to vesicles that have been isolated from total plant extracts and usually contain a mix of EVs and other cellular microvesicles (Pinedo et al., 2021). Since the identification of specific markers for different EV subclasses is only recent, the classification of plant EVs is not well established, but three main classes have been described (Cai et al., 2021). One class involves EXPO vesicles secreted into the apoplast after the fusion of EXPO double membrane organelles with the plasma membrane. The second class includes microvesicles (or ectosomes), suggested to be smaller (150nm-1um) and originate by budding from the plasma membrane. Finally, exosomes (30–150 nm) are the third class of plant EVs and are released by fusion of MVBs (containing intraluminal vesicle) with the plasma membrane (Figure 1). The mechanisms by which all these fusions and releases in the extracellular space occur are not well understood in plants.
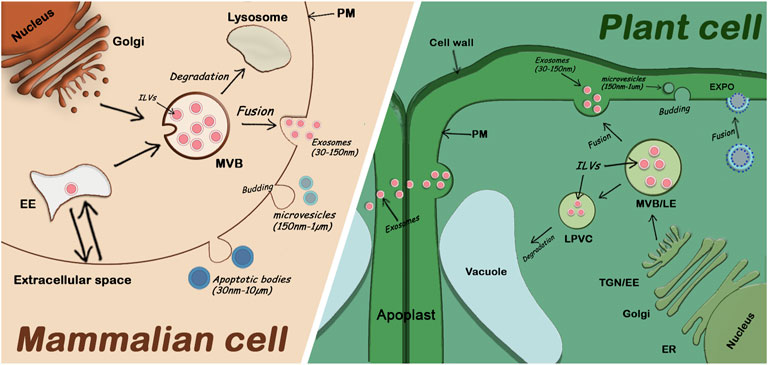
FIGURE 1. Comparison of extracellular vesicle secretion in mammalian cells and plant cells. Mammalian EVs including apoptotic bodies, microvesicles, and exosomes are secreted in the extracellular medium. Plant EVs are also secreted in the extracellular medium (the apoplast). Exosomes are secreted by fusion of MVBs with the PM, EXPO vesicles are also secreted by fusion with the PM while microvesicles and apoptotic bodies are released through budding of the PM. EE: Early Endosome; ER: Endoplasmic Reticulum; ILVs: Intraluminal Vesicles; LE: Late Endosome; LPVC: Late Pre-vacuolar Compartment; MVB: Multivesicular Body; PM: Plasma Membrane; TGN: Trans Golgi Network; (proportions of organelle sizes not conserved).
While Exo70E2 protein has been identified as a marker of EXPO vesicles, it has been reported that exosomes specifically contain TET8, a tetraspanin protein (Wang et al., 2010; Cai et al., 2018). This assumption is supported by the fact that TET8 is a plant orthologue for the human exosomal marker CD63 (Théry et al., 2018). In addition, the density of TET8 fraction (1.12–1.19 g/ml) isolated at 100,000 g correlates with the density of human exosomes, and TET8 is found to colocalize with MVB markers (He et al., 2021). Microvesicles, on the other hand, appear to be positive for the syntaxin SYP121, which has often been referred to as PEN1 (Ding et al., 2014; Rutter and Innes, 2017; He et al., 2021). The SYP121/PEN1-positive fraction appears to be slightly less dense (1.029–1.056 g/ml), and contains larger vesicles ranging from 50 to 300 nm that can be pelleted at 40 000 g (Rutter and Innes, 2017). SYP121/PEN1 has also been reported to be involved in Golgi-PM trafficking, reinforcing the fact that SYP121/PEN1 positive vesicles might not be of MVB origin (Nielsen et al., 2012; He et al., 2021).
The processes described to isolate plant EVs depend on the nature of the plant material. Apoplastic fluids are usually extracted from leaves, while blending/juicing is performed on fruits or roots. EVs can also be isolated from liquid plant exudates (Araya et al., 2015). Although the purities of different EV fractions will vary, they have all been found to have therapeutic potential in biomedicine.
The apoplast is the space outside the plasma membrane of plant cells where material can freely move (Sattelmacher, 2001). Although it is unknown how EVs cross the cell wall, their presence in the apoplast has been confirmed (Regente et al., 2012; Rutter et al., 2017; He et al., 2021). To recover these vesicles, a standard technique based on vacuum-infiltration and ultracentrifugation is performed (O’Leary et al., 2014). Applying sequential rounds of negative and atmospheric pressure onto leaves forces a buffer into the apoplastic space that can be recovered after centrifugation of the leaf. This method ensures that plant cells remain mostly undamaged and results in a relatively pure fraction containing EVs but depleted of intracellular components. It has been mostly used to purify EVs from leaf material (Arabidopsis thaliana, Nicotiana benthamiana) or seeds (sunflower) (Regente et al., 2009; Rutter and Innes, 2017; Zhang et al., 2020). Additional purification steps will allow further isolation of different types of EVs as described above (Regente et al., 2009; Rutter and Innes, 2017; He et al., 2021). Recently, a comparative analysis of two major methods for isolating EVs from apoplastic wash fluids has provided a guide into the selection of the right method adapted to the type of downstream applications desired (Huang et al., 2021).
Enriched EV fractions have been obtained through blending plant matter such as ginger roots, herbs, wheat, and dandelion (Mu et al., 2014; Xiao et al., 2018; Chen et al., 2019). Juicing of citrus fruits, pears, grapefruit, watermelons, and coconut water has also been used to prepare EV extracts (Liang et al., 2015; Raimondo et al., 2015; Xiao et al., 2018; Zhao et al., 2018). However, unless they are subjected to further purification steps, these methods often result in a mix of EVs and intracellular content (vesicles, organelles, membranes), meaning they are not solely products of UPS (Pinedo et al., 2021). They are, therefore, referred to as Plant-derived nanovesicles (PDNVs) or Exosome-like nanovesicles (ELNs) rather than EVs which refer to the purer fractions. There is increasing evidence that these PDNVs have significant biological effects on human cells and have brought new hope into novel forms of natural drug delivery systems (Di Gioia et al., 2020; Alfieri et al., 2021; Urzì et al., 2021).
Plant exudates are substances excreted from plants that include liquids flowing through and out of plants. This includes sap, gum, resins or root exudates. They have been used for many years in traditional medicine. Exudates contain many bioactive compounds, amongst them peptides, with beneficial effects on human health such as reduction of oedema and inflammation (Licá et al., 2018). Plant EVs derived from exudates are a relatively new research topic. EVs isolated from the sap of two plants (namely Dendropanax morbifera, and Pinus densiflora) have shown cytotoxic and anti-metastatic effects on human tumour cells (Kim et al., 2020a; Kim et al., 2020b). Furthermore, EVs from a hydroponic solution containing tomato (Solanum lycopersicum L.) root exudates were shown to inhibit the spore germination of three fungal phytopathogens (Fusarium oxysporum, Botrytis cinerea and Alternaria alternata) suggesting an antifungal activity in plants (De Palma et al., 2020). Whether this activity can be applied to mammalian fungal pathogens has not been tested. More research is needed to understand if exudates EVs could hold promising therapeutic applications.
Plants have been known for centuries to be beneficial for human health. Yet the identification of extracellular vesicles and their molecular content shed a new light on our understanding of cross-kingdom interaction and transfer of bioactive molecules.
In the past decade, numerous reports have described the beneficial effects of plant PDNVs/EVs in mammalian health. While PDNV proteomes from various plant origins have been characterised and some common proteins frequently identified in these vesicles, the variety of PDNVs and the lack of specific protein markers limits their classification which may prove problematic for large scale good manufacturing practices (GMPs). Nevertheless, PDNVs contain a range of bioactive molecules such as proteins, lipids, or metabolites with therapeutic effects summarised in (Woith et al., 2019; Di Gioia et al., 2020; Kocak et al., 2020; Alfieri et al., 2021; Urzì et al., 2021). Amongst the most studied plant PDNVs are those originating from ginger. These EV-containing PDNV isolates have many natural therapeutic potentials and can induce physiological changes in mammals. They were shown to influence the human gut microbiota (Teng et al., 2018), inhibit inflammasome activation (Chen, Zhou and Yu, 2019), and found to have a positive effect on inflammatory bowel disease and colitis-associated cancer (Zhang et al., 2016). They have also been shown to be taken up by, and inhibit the pathogenicity of, the periodontitis-causing Porphyromonas gingivalis (Sundaram et al., 2019). In parallel, wheat derived nanovesicles have been shown to aid in vitro wound healing by promoting proliferation and migration of dermal fibroblasts, endothelial, and epithelial cells (Şahin et al., 2018). Nanovesicles derived from various fruits and vegetables were also shown to inhibit cancer cell growth (Kameli et al., 2021). Despite their numerous health benefits, it is unclear however, if this positive impact is attributable to the combined action of various bioactive components in the crude fraction or to particular compounds that may be isolated from purer EVs preparations.
Research on EVs (obtained from the apoplast of plants) as potential drug delivery systems is far more restricted than those on PDNVs. So far, to our knowledge, only one study has shown that purified apoplastic small EVs (sEVs) are efficiently taken up by human ovarian cancer cells OVAR5 (Liu et al., 2020). This paper compared the uptake of apoplastic sEVs (purified from the apoplast of Arabidopsis leaves) and nanovesicles (obtained from disrupted leaf material). OVAR5 cells were found to be significantly more susceptible to apoplastic sEV uptake than leaf nanovesicle uptake, based on elevated numbers of fluorescent cells. These results suggest that pure EV samples have the same, if not greater, drug delivery potentials than PDNV isolates have, and that EVs may be the contributing factor to PDNV success. Unfortunately, to our knowledge, this is the only study that uses purified apoplastic EVs in human cells and more data is required to conclude. In addition, an assessment of immunogenicity and toxicity should be undertaken to validate pure plant EVs as a drug delivery system.
Based on the successes of PDNVs, efficient uptake of sEVs, and the potential of engineering exosomes in plants, biopharming is an attractive solution to produce cheap pharmaceuticals with a rapid turnover. Biopharming, or plant molecular farming, refers to the use of genetic tools to produce a wide range of pharmaceuticals. Plants have already been used to produce antibodies and vaccines for humans, animals, and aquaculture (Shoji et al., 2012; Takeyama et al., 2015; Yao et al., 2015; Lefebvre and Lécuyer, 2017; Zahara et al., 2017; Su et al., 2021). Recently, plants have been explored as a rapid alternative biofactory for the production of COVID vaccines through the expression of Virus-like particles exposing an immunogenic part of the Spike S protein (Dhama et al., 2020; Maharjan and Choe, 2021). Regarding clinical trials, intravenous administration of β-glucocerebrosidase protein expressed in carrots has been approved as being safe and efficient and successfully used for 2 decades (Shaaltiel et al., 2015). The advantages of using plants as Biofactories include their ability to produce functional proteins in large amounts, and at lower costs (Shaaltiel et al., 2007). One additional advantage is the possibility of relatively simple engineering associated with plants, potentially allowing in vivo packaging of exogenous cargo into EVs, ready for extraction. More data on the mechanisms of loading into plant EVs is still required, but with this possibility in mind, and given that delivery of therapeutic molecules by mammalian EVs has already been demonstrated by several studies (Alvarez-Erviti et al., 2011; Batrakova and Kim, 2016; Elsharkasy et al., 2020), biopharming plants to isolate therapeutic pure EVs is a very exciting avenue that needs to be explored.
If plant EVs are to be potential drug delivery systems, their administration and bioavailability must be considered. The first strong evidence of cross kingdom effects was provided when isolated PDNVs were fed to mice and found to reach intestinal macrophages. The vesicle uptake in these cells increased the expression of interleukins and alleviated colitis symptoms (Ju et al., 2013; Mu et al., 2014). This study has demonstrated that PDNVs are able to resist gastric and intestinal digestion, suggesting oral administration methods of plant nanoparticles are suitable for targeting these organs. In order to reach other organs, alternative administration methods have been investigated. In particular, intravenous injection is normally considered to have the advantage of avoiding the first-pass effect of hepatic metabolism, producing the highest bioavailability. When intravenous administration of edible tea flower nanoparticles was compared to oral administration, no difference was noted in terms of body weight and main pro-inflammatory cytokines levels. However, a sharp increase of complement C3 concentrations was detected, suggesting a slight immune reaction induced by these nanoparticles when they are administered intravenously (IV) (Chen et al., 2022). Other studies have suggested that IV administration of ginger derived exosome-like nanovesicles (GDELN) did not promote an immune reaction, though only body weight was examined (Li et al., 2018). The slight immune reaction induced by repetitive intravenous injection of EVs appears non-specific to plant EVs since a mild immune response has also been reported for human EVs (Saleh et al., 2019). The authors found that EVs purified from different sources could induce different responses. Therefore, this could also be the case for plant EVs, and more information needs to be collected before a conclusion could be drawn on intravenous injections of plant EVs. In parallel, one study has reported that intranasal administration of engineered grapefruit-derived nanovectors (GNVs) could slow down tumour brain progression in mice (Zhuang et al., 2016). This brings hope for the use of plant EVs as therapeutic tools in neurodegenerative diseases. It is noticeable that EV biodistribution changes with the administration method. While intravenous injection of mammalian and plant EVs results in the wide uptake by various organs (including spleen, liver, kidney, lung, heart, and brain) (Lai et al., 2014; Garaeva et al., 2021), the gut is more specifically targeted in oral administration of edible EVs (Ju et al., 2013; Mu et al., 2014; Zhang et al., 2016; Deng et al., 2017; Teng et al., 2018). In addition, plant EVs have been shown to penetrate a human skin model, which encourages their consideration for skin care treatments (Lee et al., 2020). Altogether, the data accumulated suggests that specific administration methods would have to be developed depending on the pathology targeted and that plant EVs present a lot of potential in therapeutic processes.
Extracellular vesicles (EVs) are associated with Unconventional protein secretion (UPS) routes. They are released in the extracellular space through mechanisms that are still poorly understood. The field of plant EVs is relatively new but is proving to have great prospects in biomedicine. The potential to produce pure plant EV subtypes such as exosomes through biopharming and be able to deliver therapeutic molecules is very appealing. Additional advantages include the engineering capability of in vivo cargo loading associated with low production costs and easy extraction procedures. Before validating plant EVs as putative drug delivery tools, further research investigating their toxicity and immunogenicity needs to be undertaken. In addition, a more robust composition and characterization of plant EVs is also essential in order to standardise production for good manufacturing practice (GMPs). Nevertheless, preliminary data seem very promising such as the efficient uptake of plant EVs by human cells, their expected low immunogenic character (associated with nutrition) and their positive effect on human health. As a consequence, using plant EVs as a drug delivery tool might represent a powerful future alternative to classical therapeutic systems.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
JF and ME are supported by a PhD scholarship from Leeds Beckett University.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
EVs, extracellular vesicles; ER, endoplasmic reticulum; PM, plasma membrane; MVB, multivesicular body; TGN, trans-Golgi network; EE, Early endosome; ILVs, Intraluminal vesicles
Akuma, P., Okagu, O. D., and Udenigwe, C. C. (2019). Naturally Occurring Exosome Vesicles as Potential Delivery Vehicle for Bioactive Compounds. Front. Sustain. Food Syst. 3, 23.doi:10.3389/fsufs.2019.00023
Alfieri, M., Leone, A., and Ambrosone, A. (2021). Plant-Derived Nano and Microvesicles for Human Health and Therapeutic Potential in Nanomedicine. Pharmaceutics 13 (4), 498. doi:10.3390/pharmaceutics13040498
Alvarez-Erviti, L., Seow, Y., Yin, H., Betts, C., Lakhal, S., and Wood, M. J. A. (2011). Delivery of siRNA to the Mouse Brain by Systemic Injection of Targeted Exosomes. Nat. Biotechnol. 29, 341–345. doi:10.1038/nbt.1807
Araya, T., Bohner, A., and von Wirén, N. (2015). Extraction of Apoplastic Wash Fluids and Leaf Petiole Exudates from Leaves of Arabidopsis thaliana. Bio-protocol 5 (24), e1691. doi:10.21769/bioprotoc.1691
BanksSharma, W. A. P., Sharma, P., Bullock, K. M., Hansen, K. M., Ludwig, N., and Whiteside, T. L. (2020). Transport of Extracellular Vesicles across the Blood-Brain Barrier: Brain Pharmacokinetics and Effects of Inflammation. Ijms 21 (12), 4407. doi:10.3390/ijms21124407
Batrakova, E. V., and Kim, M. S. (2016). Development and Regulation of Exosome‐based Therapy Products. WIREs Nanomed Nanobiotechnol 8 (5), 744–757. doi:10.1002/wnan.1395
Cai, Q., He, B., Wang, S., Fletcher, S., Niu, D., Mitter, N., et al. (2021). Message in a Bubble: Shuttling Small RNAs and Proteins between Cells and Interacting Organisms Using Extracellular Vesicles. Annu. Rev. Plant Biol. 72 (June), 497–524. doi:10.1146/annurev-arplant-081720-010616
Cai, Q., Qiao, L., Wang, M., He, B., Lin, F.-M., Palmquist, J., et al. (2018). Plants Send Small RNAs in Extracellular Vesicles to Fungal Pathogen to Silence Virulence Genes. Science 360, 1126–1129. doi:10.1126/science.aar4142
Chen, H., Wang, L., Zeng, X., Schwarz, H., Nanda, H. S., Peng, X., et al. (2021). Exosomes, a New Star for Targeted Delivery. Front. Cell. Dev. Biol. 9, 751079. doi:10.3389/fcell.2021.751079
Chen, Q., Li, Q., Liang, Y., Zu, M., Chen, N., Canup, B. S. B., et al. (2022). Natural Exosome-like Nanovesicles from Edible Tea Flowers Suppress Metastatic Breast Cancer via ROS Generation and Microbiota Modulation. Acta Pharm. Sin. B 12, 907–923. doi:10.1016/j.apsb.2021.08.016
Chen, X., Zhou, Y., and Yu, J. (2019). Exosome-like Nanoparticles from Ginger Rhizomes Inhibited NLRP3 Inflammasome Activation. Mol. Pharm. 16 (6), 2690–2699. doi:10.1021/acs.molpharmaceut.9b00246
Choi, H., Choi, Y., Yim, H. Y., Mirzaaghasi, A., Yoo, J.-K., and Choi, C. (2021). Biodistribution of Exosomes and Engineering Strategies for Targeted Delivery of Therapeutic Exosomes. Tissue Eng. Regen. Med. 18 (4), 499–511. doi:10.1007/s13770-021-00361-0
Cohen, M. J., Chirico, W. J., and Lipke, P. N. (2020). Through the Back Door: Unconventional Protein Secretion. Cell. Surf. 6, 100045. doi:10.1016/j.tcsw.2020.100045
Cui, Y., Gao, J., He, Y., and Jiang, L. (2019). Plant Extracellular Vesicles. Protoplasma 257, 3–12. doi:10.1007/s00709-019-01435-6
De Palma, M., Ambrosone, A., Leone, A., Del Gaudio, P., Ruocco, M., Turiák, L., et al. (2020). Plant Roots Release Small Extracellular Vesicles with Antifungal Activity. Plants 9 (12), 1777. doi:10.3390/plants9121777
Delaunois, B., Colby, T., Belloy, N., Conreux, A., Harzen, A., Baillieul, F., et al. (2013). Large-scale Proteomic Analysis of the Grapevine Leaf Apoplastic Fluid Reveals Mainly Stress-Related Proteins and Cell Wall Modifying Enzymes. BMC Plant Biol. 13, 24. doi:10.1186/1471-2229-13-24
Delaunois, B., Jeandet, P., Clément, C., Baillieul, F., Dorey, S. p., and Cordelier, S. (2014). Uncovering Plant-Pathogen Crosstalk through Apoplastic Proteomic Studies. Front. Plant Sci. 5, 249. doi:10.3389/fpls.2014.00249
Deng, Z., Rong, Y., Teng, Y., Mu, J., Zhuang, X., Tseng, M., et al. (2017). Broccoli-Derived Nanoparticle Inhibits Mouse Colitis by Activating Dendritic Cell AMP-Activated Protein Kinase. Mol. Ther. 25 (7), 1641–1654. doi:10.1016/j.ymthe.2017.01.025
Dhama, K., Natesan, S., Iqbal Yatoo, M., Patel, S. K., Tiwari, R., Saxena, S. K., et al. (2020). Plant-based Vaccines and Antibodies to Combat COVID-19: Current Status and Prospects. Hum. vaccines Immunother. 16 (12), 2913–2920. doi:10.1080/21645515.2020.1842034
Di Gioia, S., Hossain, M. N., and Conese, M. (2020). Biological Properties and Therapeutic Effects of Plant-Derived Nanovesicles. open-access J. 15 (1), 1096–1122. doi:10.1515/med-2020-0160
Ding, Y., Robinson, D. G., and Jiang, L. (2014a). Unconventional Protein Secretion (UPS) Pathways in Plants. Curr. Opin. Cell. Biol. 29 (August), 107–115. doi:10.1016/j.ceb.2014.05.008
Ding, Y., Wang, J., Chun Lai, J. H., Ling Chan, V. H., Wang, X., Cai, Y., et al. (2014b). Exo70E2 Is Essential for Exocyst Subunit Recruitment and EXPO Formation in Both Plants and Animals. MBoC 25 (3), 412–426. doi:10.1091/mbc.e13-10-0586
Elliott, R. O., and He, M. (2021). Unlocking the Power of Exosomes for Crossing Biological Barriers in Drug Delivery. Pharmaceutics 13 (1), 122. doi:10.3390/pharmaceutics13010122
Elsharkasy, O. M., Nordin, J. Z., Hagey, D. W., de Jong, O. G., Schiffelers, R. M., Andaloussi, S. E., et al. (2020). Extracellular Vesicles as Drug Delivery Systems: Why and How? Adv. drug Deliv. Rev. 159, 332–343. doi:10.1016/j.addr.2020.04.004
Garaeva, L., Kamyshinsky, R., Kil, Y., Varfolomeeva, E., Verlov, N., Komarova, E., et al. (2021). Delivery of Functional Exogenous Proteins by Plant-Derived Vesicles to Human Cells In Vitro. Sci. Rep. 11 (1), 1–12. doi:10.1038/s41598-021-85833-y
Hansen, L. L., and Nielsen, M. E. (2017). Plant Exosomes: Using an Unconventional Exit to Prevent Pathogen Entry? J. Exp. Bot. 69 (1), 59–68. doi:10.1093/jxb/erx319
Harding, C. V., Heuser, J. E., and Stahl, P. D. (2013). Exosomes: Looking Back Three Decades and into the Future. J. Cell Biol. 200, 367–371. doi:10.1083/jcb.201212113
He, B., Cai, Q., Qiao, L., Huang, C. Y., Wang, S., Miao, W., et al. (2021). RNA-binding Proteins Contribute to Small RNA Loading in Plant Extracellular Vesicles. Nat. Plants 7 (3), 342–352. doi:10.1038/s41477-021-00863-8
Huang, Y., Wang, S., Cai, Q., and Jin, H. (2021). Effective Methods for Isolation and Purification of Extracellular Vesicles from Plants. J. Integr. Plant Biol. 63 (12), 2020–2030. doi:10.1111/jipb.13181
Ju, S., Mu, J., Dokland, T., Zhuang, X., Wang, Q., Jiang, H., et al. (2013). Grape Exosome-like Nanoparticles Induce Intestinal Stem Cells and Protect Mice from DSS-Induced Colitis. Mol. Ther. 21 (7), 1345–1357. doi:10.1038/mt.2013.64
Kameli, N., Dragojlovic-Kerkache, A., Savelkoul, P., and Stassen, F. R. (2021). Plant-Derived Extracellular Vesicles: Current Findings, Challenges, and Future Applications. Membranes 11 (6), 411. doi:10.3390/membranes11060411
Kim, J., Gee, H. Y., and Lee, M. G. (2018). Unconventional Protein Secretion - New Insights into the Pathogenesis and Therapeutic Targets of Human Diseases. J. Cell Sci. 131 (12), jcs213686. doi:10.1242/jcs.213686
Kim, K., Jung, J.-H., Yoo, H. J., Hyun, J.-K., Park, J.-H., Na, D., et al. (2020). Anti-Metastatic Effects of Plant Sap-Derived Extracellular Vesicles in a 3D Microfluidic Cancer Metastasis Model. Jfb 11 (3), 49. doi:10.3390/jfb11030049
Kim, K., Yoo, H. J., Jung, J.-H., Lee, R., Hyun, J.-K., Park, J.-H., et al. (2020). Cytotoxic Effects of Plant Sap-Derived Extracellular Vesicles on Various Tumor Cell Types. Jfb 11 (2), 22. doi:10.3390/jfb11020022
Kocak, P., Kala, E. Y., Gunes, M., Unsal, N., Yilmaz, H., Metin, B., et al. (2020). Edible Plant-Derived Exosomes and Their Therapeutic Applicatons. J. Biomed. Imag. Bioeng. 4 (1), 130–135. Available at: https://www.alliedacademies.org/articles/edible-plantderived-exosomes-and-their-therapeutic-applicatons.pdf
Lai, C. P., Mardini, O., Ericsson, M., Prabhakar, S., Maguire, C. A., Chen, J. W., et al. (2014). Dynamic Biodistribution of Extracellular Vesicles In Vivo Using a Multimodal Imaging Reporter. ACS Nano 8 (1), 483–494. doi:10.1021/nn404945r
Lee, R., Ko, H. J., Kim, K., Sohn, Y., Min, S. Y., Kim, J. A., et al. (2020). Anti‐melanogenic Effects of Extracellular Vesicles Derived from Plant Leaves and Stems in Mouse Melanoma Cells and Human Healthy Skin. J. Extracell. Vesicles 9 (1), 1703480. doi:10.1080/20013078.2019.1703480
Lefebvre, F. A., and Lécuyer, E. (2017). Small Luggage for a Long Journey: Transfer of Vesicle-Enclosed Small RNA in Interspecies Communication. Front. Microbiol. 8, 377. doi:10.3389/fmicb.2017.00377
Li, Z., Wang, H., Yin, H., Bennett, C., Zhang, H. G., Guo, P., et al. (2018). Arrowtail RNA for Ligand Display on Ginger Exosome-like Nanovesicles to Systemic Deliver siRNA for Cancer Suppression. Sci. Rep. 8 (1), 14644. doi:10.1038/s41598-018-32953-7
Liang, H., Zhang, S., Fu, Z., Wang, Y., Wang, N., Liu, Y., et al. (2015). Effective Detection and Quantification of Dietetically Absorbed Plant microRNAs in Human Plasma. J. Nutr. Biochem. 26 (5), 505–512. doi:10.1016/j.jnutbio.2014.12.002
Licá, I. C. L., Soares, A. M. d. S., de Mesquita, L. S. S., and Malik, S. (2018). Biological Properties and Pharmacological Potential of Plant Exudates. Food Res. Int. 105, 1039–1053. doi:10.1016/j.foodres.2017.11.051
Liu, Y., Wu, S., Koo, Y., Yang, A., Dai, Y., Khant, H., et al. (2020). Characterization of and Isolation Methods for Plant Leaf Nanovesicles and Small Extracellular Vesicles. Nanomedicine 29, 102271. doi:10.1016/j.nano.2020.102271
Maharjan, P. M., and Choe, S. (2021). Plant-Based COVID-19 Vaccines: Current Status, Design, and Development Strategies of Candidate Vaccines. Vaccines 9 (9), 992. doi:10.3390/vaccines9090992
Meng, W., He, C., Hao, Y., Wang, L., Li, L., and Zhu, G. (2020). Prospects and Challenges of Extracellular Vesicle-Based Drug Delivery System: Considering Cell Source. Drug Deliv. 27 (1), 585–598. doi:10.1080/10717544.2020.1748758
Mu, J., Zhuang, X., Wang, Q., Jiang, H., Deng, Z. B., Wang, B., et al. (2014). Interspecies Communication between Plant and Mouse Gut Host Cells through Edible Plant Derived Exosome‐like Nanoparticles. Mol. Nutr. Food Res. 58 (7), 1561–1573. doi:10.1002/mnfr.201300729
Nielsen, M. E., Feechan, A., Böhlenius, H., Ueda, T., and Thordal-Christensen, H. (2012). Arabidopsis ARF-GTP Exchange Factor, GNOM, Mediates Transport Required for Innate Immunity and Focal Accumulation of Syntaxin PEN1. Proc. Natl. Acad. Sci. U.S.A. 109 (28), 11443–11448. doi:10.1073/pnas.1117596109
O'Leary, B. M., Rico, A., McCraw, S., Fones, H. N., and Preston, G. M. (2014). The Infiltration-Centrifugation Technique for Extraction of Apoplastic Fluid from Plant Leaves Using Phaseolus vulgaris as an Example. JoVE (94), e52113. doi:10.3791/52113
Pinedo, M., de la Canal, L., and de Marcos Lousa, C. (2021). A Call for Rigor and Standardization in Plant Extracellular Vesicle Research. J. Extracell. Vesicles 10 (6), e12048. doi:10.1002/jev2.12048
Rabouille, C., Malhotra, V., and Nickel, W. (2012). Diversity in Unconventional Protein Secretion. J. Cell. Sci. 125 (Pt 22), 5251–5255. doi:10.1242/jcs.103630
Rabouille, C. (2017). Pathways of Unconventional Protein Secretion. Trends Cell. Biol. 27 (3), 230–240. doi:10.1016/j.tcb.2016.11.007
Raimondo, S., Naselli, F., Fontana, S., Monteleone, F., Lo Dico, A., Saieva, L., et al. (2015). Citrus Limon-Derived Nanovesicles Inhibit Cancer Cell Proliferation and Suppress CML Xenograft Growth by Inducing TRAIL-Mediated Cell Death. Oncotarget 6 (23), 19514–19527. doi:10.18632/oncotarget.4004
Regente, M., Corti-Monzón, G., Maldonado, A. M., Pinedo, M., Jorrín, J., and De la Canal, L. (2009). Vesicular Fractions of Sunflower Apoplastic Fluids Are Associated with Potential Exosome Marker Proteins. FEBS Lett. 583 (20), 3363–3366. doi:10.1016/j.febslet.2009.09.041
Regente, M., Pinedo, M., Elizalde, M., and de la Canal, L. (2012). Apoplastic Exosome-like Vesicles: a New Way of Protein Secretion in Plants? Plant Signal. Behav. 7 (5), 544–546. doi:10.4161/psb.19675
Robinson, D. G., Ding, Y., and Jiang, L. (2016). Unconventional Protein Secretion in Plants: A Critical Assessment. Protoplasma 253 (1), 31–43. doi:10.1007/s00709-015-0887-1
Rutter, B. D., and Innes, R. W. (2017). Extracellular Vesicles Isolated from the Leaf Apoplast Carry Stress-Response Proteins. Plant Physiol. 173 (1), 728–741. doi:10.1104/pp.16.01253
Şahin, F., Koçak, P., Güneş, M. Y., Özkan, İ., Yıldırım, E., and Kala, E. Y. (2018). In Vitro Wound Healing Activity of Wheat-Derived Nanovesicles. Appl. Biochem. Biotechnol. 188 (2), 381–394. doi:10.1007/s12010-018-2913-1
Saleh, A. F., Lázaro-Ibáñez, E., Forsgard, M. A.-M., Shatnyeva, O., Osteikoetxea, X., Karlsson, F., et al. (2019). Extracellular Vesicles Induce Minimal Hepatotoxicity and Immunogenicity. Nanoscale 11 (14), 6990–7001. doi:10.1039/c8nr08720b
Sattelmacher, B. (2001). The Apoplast and its Significance for Plant Mineral Nutrition. New Phytol. 149 (2), 167–192. doi:10.1046/j.1469-8137.2001.00034.x
Shaaltiel, Y., Bartfeld, D., Hashmueli, S., Baum, G., Brill-Almon, E., Galili, G., et al. (2007). Production of Glucocerebrosidase with Terminal Mannose Glycans for Enzyme Replacement Therapy of Gaucher's Disease Using a Plant Cell System. Plant Biotechnol. J. 5 (5), 579–590. doi:10.1111/j.1467-7652.2007.00263.x
Shaaltiel, Y., Gingis-Velitski, S., Tzaban, S., Fiks, N., Tekoah, Y., and Aviezer, D. (2015). Plant-based Oral Delivery of β-glucocerebrosidase as an Enzyme Replacement Therapy for Gaucher's Disease. Plant Biotechnol. J. 13 (8), 1033–1040. doi:10.1111/pbi.12366
Shoji, Y., Farrance, C. E., Bautista, J., Bi, H., Musiychuk, K., Horsey, A., et al. (2012). A Plant-Based System for Rapid Production of Influenza Vaccine Antigens. Influenza other Respir. viruses 6 (3), 204–210. doi:10.1111/j.1750-2659.2011.00295.x
Su, H., Yakovlev, I. A., van Eerde, A., Su, J., and Clarke, J. L. (2021). Plant-Produced Vaccines: Future Applications in Aquaculture. Front. Plant Sci. 12, 718775. doi:10.3389/fpls.2021.718775
Sundaram, K., Miller, D. P., Kumar, A., Teng, Y., Sayed, M., Mu, J., et al. (2019). Plant-Derived Exosomal Nanoparticles Inhibit Pathogenicity of Porphyromonas Gingivalis. iScience 21, 308–327. doi:10.1016/j.isci.2019.10.032
Takeyama, N., Kiyono, H., and Yuki, Y. (2015). Plant-based Vaccines for Animals and Humans: Recent Advances in Technology and Clinical Trials. Ther. Adv. Vaccines 3 (5-6), 139–154. doi:10.1177/2051013615613272
Teng, Y., Ren, Y., Sayed, M., Hu, X., Lei, C., Kumar, A., et al. (2018). Plant-Derived Exosomal MicroRNAs Shape the Gut Microbiota. Cell. Host Microbe 24 (5), 637–e8. e8. doi:10.1016/j.chom.2018.10.001
Théry, C., Witwer, K. W., Aikawa, E., Alcaraz, M. J., Anderson, J. D., Andriantsitohaina, R., et al. (2018). Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): a Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 7 (1), 1535750. doi:10.1080/20013078.2018.1535750
Urzì, O., Raimondo, S., and Alessandro, R. (2021). Extracellular Vesicles from Plants: Current Knowledge and Open Questions. Ijms 22 (10), 5366. doi:10.3390/ijms22105366
Wang, J., Ding, Y., Wang, J., Hillmer, S., Miao, Y., Lo, S. W., et al. (2010). EXPO, an Exocyst-Positive Organelle Distinct from Multivesicular Endosomes and Autophagosomes, Mediates Cytosol to Cell Wall Exocytosis inArabidopsisand Tobacco Cells. Plant Cell 22 (12), 4009–4030. doi:10.1105/tpc.110.080697
Woith, E., Fuhrmann, G., and Melzig, M. F. (2019). Extracellular Vesicles-Connecting Kingdoms. Ijms 20 (22), 5695. doi:10.3390/ijms20225695
Xiao, J., Feng, S., Wang, X., Long, K., Luo, Y., Wang, Y., et al. (2018). Identification of Exosome-like Nanoparticle-Derived microRNAs from 11 Edible Fruits and Vegetables. PeerJ 6, e5186. doi:10.7717/peerj.5186
Xu, M., Yang, Q., Sun, X., and Wang, Y. (2020). Recent Advancements in the Loading and Modification of Therapeutic Exosomes. Front. Bioeng. Biotechnol. 8, 586130. doi:10.3389/fbioe.2020.586130
Yao, J., Weng, Y., Dickey, A., and Wang, K. (2015). Plants as Factories for Human Pharmaceuticals: Applications and Challenges. Ijms 16 (12), 28549–28565. doi:10.3390/ijms161226122
Zahara, K., Bibi, Y., Bibi, Y., Ajmal, M., Sadaf, H. M., Bibi, F., et al. (2017). Tobacco Plant: A Possible Key to Ebola Vaccine. Jclm 5 (5), 206–211. doi:10.12980/jclm.5.2017j7-9
Zhang, J., Qiu, Y., and Xu, K. (2020). Characterization of GFP-AtPEN1 as a Marker Protein for Extracellular Vesicles Isolated from Nicotiana Benthamiana Leaves. Plant Signal. Behav. 15 (9), 1791519. doi:10.1080/15592324.2020.1791519
Zhang, M., Viennois, E., Prasad, M., Zhang, Y., Wang, L., Zhang, Z., et al. (2016). Edible Ginger-Derived Nanoparticles: A Novel Therapeutic Approach for the Prevention and Treatment of Inflammatory Bowel Disease and Colitis-Associated Cancer. Biomaterials 101, 321–340. doi:10.1016/j.biomaterials.2016.06.018
Zhao, Z., Yu, S., Li, M., Gui, X., and Li, P. (2018). Isolation of Exosome-like Nanoparticles and Analysis of MicroRNAs Derived from Coconut Water Based on Small RNA High-Throughput Sequencing. J. Agric. Food Chem. 66 (11), 2749–2757. doi:10.1021/acs.jafc.7b05614
Keywords: extracellular vesicles, unconventional protein secretion (UPS), plant EVs, biomedicine, biopharming, exosomes
Citation: Farley JT, Eldahshoury MK and de Marcos Lousa C (2022) Unconventional Secretion of Plant Extracellular Vesicles and Their Benefits to Human Health: A Mini Review. Front. Cell Dev. Biol. 10:883841. doi: 10.3389/fcell.2022.883841
Received: 25 February 2022; Accepted: 09 May 2022;
Published: 01 June 2022.
Edited by:
Marioara Chiritoiu-Butnaru, Institute of Biochemistry of the Romanian Academy, RomaniaReviewed by:
Hailing Jin, University of California, Riverside, United StatesCopyright © 2022 Farley, Eldahshoury and de Marcos Lousa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carine de Marcos Lousa, Yy5kZS1tYXJjb3MtbG91c2FAbGVlZHNiZWNrZXR0LmFjLnVr;, ZmJzY2RAbGVlZHMuYWMudWs=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.