
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell Dev. Biol. , 09 June 2022
Sec. Stem Cell Research
Volume 10 - 2022 | https://doi.org/10.3389/fcell.2022.877254
This article is part of the Research Topic Ovarian Cancer Stem Cells and Drug Resistance View all 4 articles
 Zhijie Xu1,2,3†
Zhijie Xu1,2,3† Bi Peng2,4†
Bi Peng2,4† Fanhua Kang3
Fanhua Kang3 Wenqin Zhang3
Wenqin Zhang3 Muzhang Xiao5
Muzhang Xiao5 Jianbo Li3
Jianbo Li3 Qianhui Hong3
Qianhui Hong3 Yuan Cai2
Yuan Cai2 Wei Liu6
Wei Liu6 Yuanliang Yan7*
Yuanliang Yan7* Jinwu Peng2,3,4*
Jinwu Peng2,3,4*Background: The different pharmacological effects of drugs in different people can be explained by the polymorphisms of drug metabolism-related genes. Emerging studies have realized the importance of drug metabolism-related genes in the treatment and prognosis of cancers, including ovarian cancer (OV). In this study, using comprehensive bioinformatics and western blot, we identified that the drug metabolism-related gene, ADH1B, was significantly down-regulated in OV cells and tissues. The patients with a high level of ADH1B presented a good prognosis. We also found a negative correlation between ADH1B expression and the activity of chemotherapeutic agents, such as cyclophosphamide. In addition, positive correlations were observed between ADH1B expression and multiple immune checkpoints, including LAG3 and HAVCR2. The immune infiltration analysis further indicated that aberrantly expressed ADH1B might have important roles in regulating the infiltration of macrophages and neutrophils in OV tissues. Then, the co-expression analysis was conducted and the top three enriched KEGG pathways were spliceosome, RNA transport, and DNA replication. In conclusion, the drug metabolism-related gene ADH1B and its interactive network play an essential role in the immune regulation and therapeutic response and maybe identified as promising therapeutic targets for OV patients.
Ovarian cancer (OV) is one of the most frequently diagnosed and the most lethal type of cancer among all gynecological malignancies throughout the world (Siegel et al., 2021). Moreover, high-grade serous ovarian cancer is the most common and invasive histological type of epithelial ovarian cancer (Govindarajan et al., 2020). Despite advances in surgery, chemotherapy, and immunotherapy (Kuroki and Guntupalli, 2020), survival in patients with OV has stagnated for decades, mainly due to diagnosis at an advanced stage and rapidly increasing prevalence of resistance (Muñoz-Galván et al., 2020). Therefore, it is imperative to provide a promising new therapeutic strategy to improve the outcomes of OV patients.
Pharmacogenomics (PGx) is the study of the mechanism between genes and drug metabolism to predict efficacy and relieve side effects (Wake et al., 2019). With the progress of medicine, the idea that genetic variations could be used to personalize drug treatment become possible (Roden et al., 2019). Drug metabolism genes, as the name suggests, are genes that affect drug metabolism. Examples include that mutation in the DPYD gene cause more severe toxicity in patients treated with fluorouracil and loss-of-function alleles in the NUDT15 gene induce a reduction in the degradation of active nucleotide metabolites and result in myelosuppression (Henricks et al., 2018; Relling et al., 2019).
Alcohol dehydrogenase 1B (ADH1B) is located on human chromosome 4q21-q23 and its primordially well-known function is alcohol metabolism (Strommer, 2011; Wei et al., 2021). To date, emerging evidence has demonstrated that ADH1B-associated signaling pathways are key factors for tumorigenesis and progression, including esophageal squamous cell cancer (ESCC) (Suo et al., 2019) and colorectal cancer (CRC) (Choi et al., 2021). Nevertheless, the research on ADH1B and ovarian cancer are much less. It is a discussible question about the roles of ADH1B on prognosis and immune infiltration in ovarian cancer.
To reveal the important role of ADH1B in the treatment of ovarian cancer, our work mainly focuses on exploring the clinical and immunotherapeutic implications of it. First, several public databases were used to demonstrate that aberrantly expressed ADH1B influences the progression and prognosis of ovarian cancer. Furthermore, we identified the expression levels of ADH1B in ovarian cancer from several aspects. Then, the potential signaling pathways and biological functions of ADH1B were found through KEGG and GO annotation. Additionally, the correlations between ADH1B and tumor-infiltrating immune cells (TIICs) and immune checkpoints in ovarian cancer were deeply studied for an underlying immunotherapy target and ready-made targeted drugs. These results provide a novel and significant insight into the treatment of ovarian cancer.
Datasets of ovarian cancer were obtained from the gene expression omnibus (GEO) database which contained a lot of clinical information and all drug metabolism-related genes. We ultimately identified two ovarian cancer datasets (GSE26712 and GSE18520) (Mok et al., 2009; Vathipadiekal et al., 2015) according to the following conditions: 1) cancer ype: ovarian cancer, 2) study type: expression profiling by array, and 3) analysis type: umor tissue vs. normal tissue. Furthermore, differentially expressed genes (DEGs) were screened out (p-value <0.01 and | log FC | >3), which got ready for Venn diagram. Then, we obtained the intersection that is co-differentially expressed genes (co-DGEs) between DEGs and drug metabolism-related genes.
The Kaplan-Meier plotter is a web-based tool to evaluate clinical outcomes and explore survival biomarkers. It currently contains more than 25,000 samples from 21 tumor types (Lánczky and Győrffy, 2021). We utilized the Kaplan-Meier plotter to assess the correlation between the expression of co-DEGs and the patients’ prognosis, including overall survival (OS) and progressive survival (PPS).
Multiple databases were used for the evaluation of ADH1B expression levels in ovarian cancer. The Cancer Genome Atlas (TCGA) is a free data collection library which includes the clinicopathology and molecular expression information from 33 types of tumors (Liu et al., 2018). We extracted the expression data of 427 ovarian cancer tissues and 88 normal tissues from it for further validation. The ADH1B expressed levels between tumor group and normal control were analyzed to take advantage of GSE26712 and GSE18520 which were two datasets related to ovarian cancer from the GEO database. Furthermore, these results gave a further verification of ADH1B expression levels using the TCGA database, TNM plot, and GEPIA2 database. TNM plot, a convenient website, contains gene arrays from the GEO and RNA-seq data from TCGA and makes a comparison of the gene expressed levels in normal, tumor, and metastatic groups (Bartha and Győrffy, 2021). And GEPIA2, an updated and enhanced web, be appropriate for retrieving massive gene expression profiles and conducting interaction analysis (Tang et al., 2019).
We collected human ovarian epithelial cells IOSE80 and ovarian cancer cells A2780 and TOV112D from the Center for Molecular Medicine, Xiangya Hospital, Central South University and inoculated them onto Dulbecco’s Modified Eagle’s Medium (DMEM, Gibco, United States) with 10% fetal bovine serum (FBS, Gibco, United States) and 1% penicillin/streptomycin (Gibco, United States). These culture mediums were stored at a temperature of 37 with 5% CO2 in a sterile.
The experiment began with the addition of RIPA buffer supplemented with a protease inhibitor to cell lysates. The BCA protein assay kit was applied to test total protein concentration and ads-polyacrylamide gel electrophoresis was used to load equal amounts of protein in each lane. In addition, we passed on the bands to the PVDF membranes which were sealed for 1 h at room temperature with TBST and added 5% non-fat dry milk. Subsequently, these membranes were incubated at 4°C overnight with primary antibodies called ADH1B monoclonal antibody (Item no: 66939-1-Ig). At last, we tested protein bands using the Immobilon Western Chemiluminescent HRP reagents.
LinkedOmics is an efficient platform for the access, evaluation, and comparison of cancer multi-omics data (Vasaikar et al., 2018). Correlation analysis was conducted via the LinkFinder module of this database to screen positively and negatively co-expressed genes, the results were shown in the form of volcano plots and heat maps. Subsequently, gene enrichment analysis was performed through the LinkInterpreter module, gene ontology biological process (GO_BP), gene ontology cellular component (GO_CC), gene ontology molecular function (GO_MF), and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways were realized.
The evaluation of the ADH1B expression and 24 types of tumor-infiltrating immune cells (TIICs) in ovarian cancer was finished using the single-sample GSEA (ssGSEA) algorithm. Further validations were realized through Tumor IMmune Estimation Resource 2.0 (TIMER) database contributing to TIICs assessment (Li et al., 2020). More convincingly, we employed the TISIDB database (Ru et al., 2019) to verify these immunological results thirdly. Additionally, immunostimulators, immunoinhibitors, chemokines, and receptors targeting ADH1B expression were also explored with the TISIDB platform.
CellMiner Cross-Database (CellMinerCDB), a publicly available web, integrates and analyzes molecular and pharmacological data from the National Cancer Institute’s cancer cell line and cross cell line datasets (Luna et al., 2021). The correlation between chemotherapeutic drug activity and ADH1B expression was analyzed by CellMinerCDB. In addition, the relationship between immune checkpoints and ADH1B expression was evaluated with Spearman’s correlation test.
We analyzed gene expression profiles from GSE18520 and GSE26712 and identified the differentially expressed genes between the normal group and the ovarian cancer group under the filter criteria (p <0.01 and | log FC | >3). Then, we found 143 upregulated genes and 161 downregulated genes in GSE18520, and 17 and 35 in GSE26712. In recent years, many studies have suggested that certain genes play a significant role in drug metabolism and disposition and cancer chemical resistance (Mazor et al., 2019; Wang et al., 2020; Akkaif et al., 2021). A genetic approach to reversing chemotherapy resistance could be a new and promising breakthrough. Thus, co-DEGs were obtained from two GEO database and a drug metabolism-related gene dataset by Venn analysis. We initially identified three down-regulated genes whose names were ADH1B, AOX1, and DPYD (Figures 1A,B). Further investigations were focused on their prognostic significance.
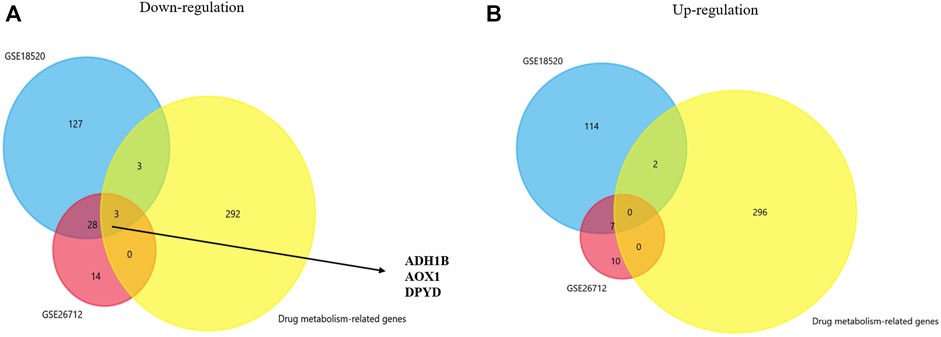
FIGURE 1. Venn plot for identifying co-DEGs. (A,B) Two GEO datasets and the drug metabolism-related gene dataset were used to identify the upregulated and downregulated co-DEGs, respectively.
The prognostic values of ADH1B, AOX1, and DPYD were investigated in the GSE30161 dataset (Ferriss et al., 2012) through Kaplan-Meier curves. As shown in Figure 2, ovarian cancer patients with higher ADH1B expression have favorable OS and PPS. On the contrary, the expression of AOX1 and DPYD did not consistently affect the patients’ outcomes in the GSE30161 dataset (Supplementary Figures S1,S2). Therefore, these results brought ADH1B into sharper focus of further study.
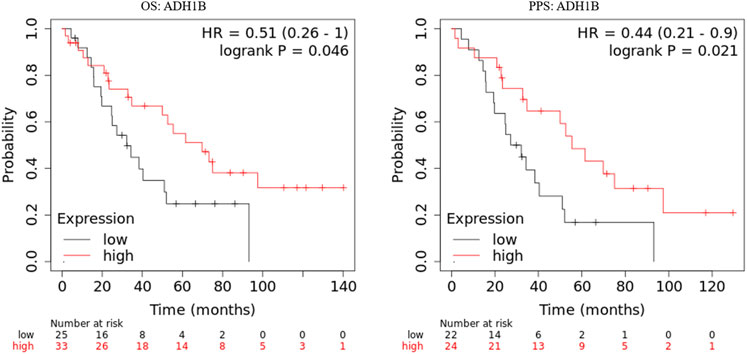
FIGURE 2. Kaplan-Meier curves of ADH1B in GSE30161. Kaplan-Meier plotter database was applied to obtain the OS and PPS curves of ADH1B in patients with ovarian cancer.
We compared the ADH1B expression between ovarian cancer tissue and normal tissues in GSE18520 and GSE26712, and found the reduced ADH1B expression in tumor tissues (Figures 3A,B). Then, data from TCGA-OV suggested that ADH1B expression is significantly down-regulated in ovarian cancer (Figure 3C). Furthermore, the lowly-expressed ADH1B in OV patients was also obtained from the GEPIA2 platform (p < 0.05) (Figure 3D). To enhance the credibility of these findings, the TNMplot was further applied to validate the decreased ADH1B in tumor groups from Gene-chip data (p = 2.9e-22) and RNA-seq data (p = 1.67e-59) (Figures 3E,F). Moreover, the downregulated ADH1B was further confirmed in two OV cells (A2780 and TOV112D) (Figures 3G,H) and OV tissues (Figure 3I).
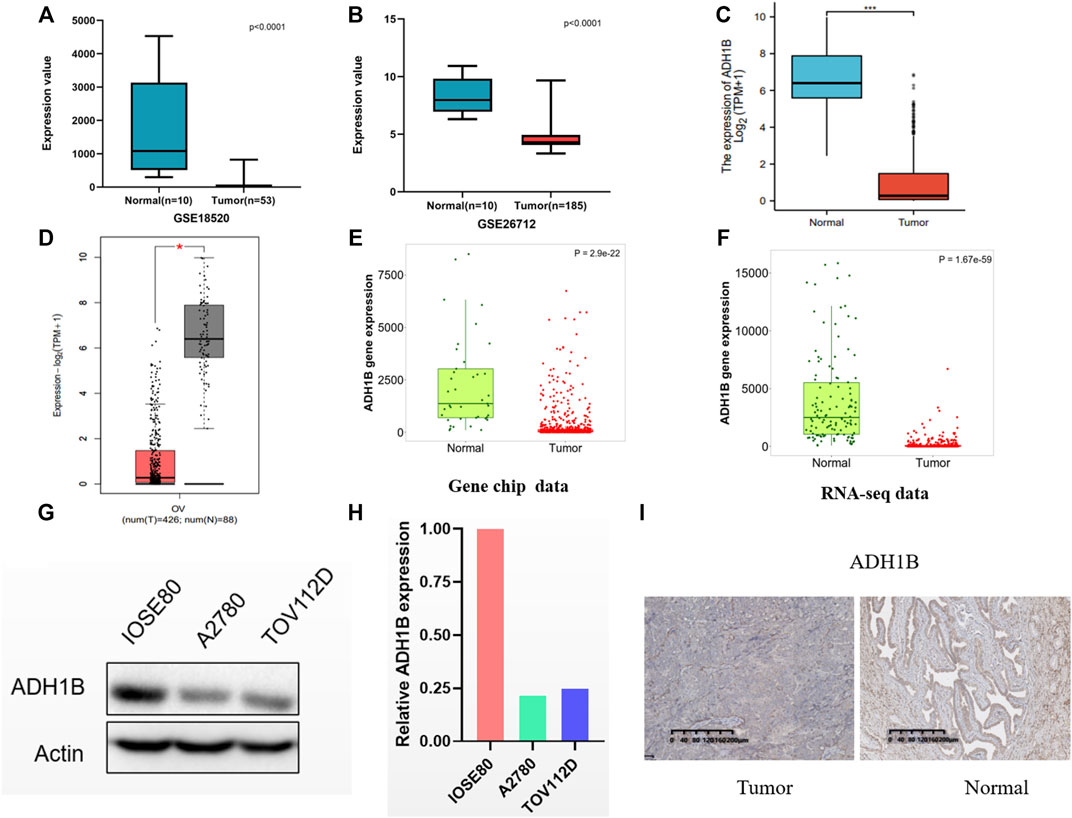
FIGURE 3. The expression of ADH1B in ovarian cancer tissues. (A–D) Compared with normal ovarian tissue, a significantly reduced ADH1B could be obtained in GSE18520 (A), GSE26712 (B), TCGA-OV (C), and GEPTA2 (D). (E,F) The verification of down-regulated expression level of ADH1B using TNMplot database from gene chip data (E) and RNA-seq data (F). (G,H) ADH1B was downregulated in two OV cells, A2780 and TOV112D. (I) Immunohistochemical analysis indicated the downregulated ADH1B in OV patients. *p < 0.05; ***p < 0.001.
To investigate the relationship between clinical characteristics and ADH1B expression, we divided ovarian cancer patients into two groups with high and low ADH1B expression and performed statistical analysis using the clinical data from the TCGA database. As shown in Table 1, in the “Fédération Internationale de Gynécologie et d’Obstétrique” (FIGO) stage, primary therapy outcome, race, histologic grade, and venous invasion, some differences were noticed but they did not achieve statistical significance. However, age, anatomic neoplasm subdivision, lymphatic invasion, tumor residual, and tumor status were all significantly different between the low ADH1B expression group and the high ADH1B expression group. These findings provided a novel and hopeful perspective on clinical biomarkers for ovarian cancer.
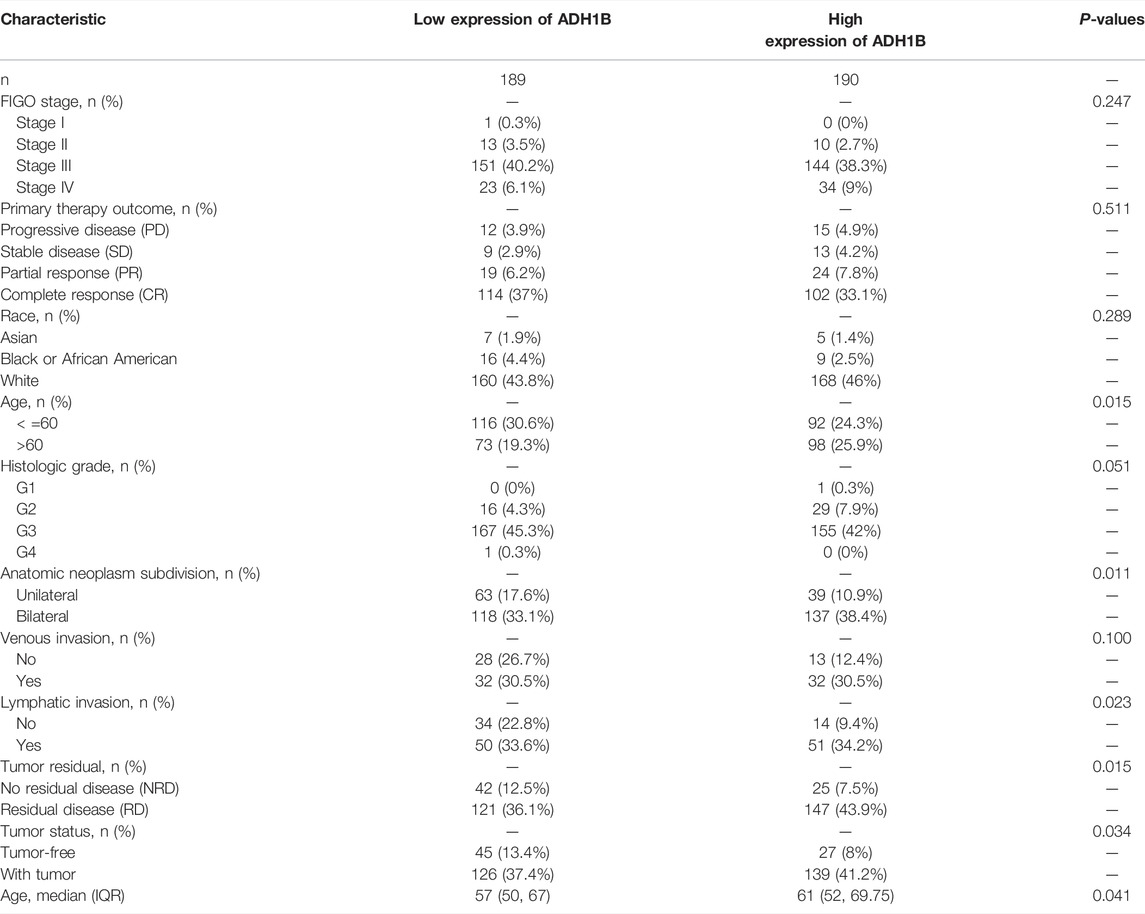
TABLE 1. The demographic parameters for ovarian cancer patients from the TCGA database based on the ADH1B levels.
In order to evaluate the biological functions of ADH1B in OV, the ADH1B co-expression pattern in the TCGA-OV cohort was considered an important event which was implemented by LinkedOmics. We observed that there were 3413 genes negatively associated with ADH1B and 3841 genes positively (Figure 4A). Then, the prognostic significance of partial genes having high relative coefficients was explored. The results were presented in the form of heatmaps (Figures 4B,C). It was worth noting that a protective hazard ratio was more likely to be owned by most negative co-expressed genes (34 out of 50) while an adverse hazard ratio by most positive co-expressed genes (32 out of 50) (Figures 4D,E). Meanwhile, the KEGG Pathway and GO enrichment analysis of ADH1B were conducted. As shown in Figure 4F, spliceosome, RNA transport, DNA replication, and aminoacyl-tRNA biosynthesis were the top four enriched KEGG pathways. Ribonucleoprotein complex localization, spliceosomal complex, and helicase activity were the top enriched terms in biological process, cellular component, and molecular function (Supplementary Figures S3A–C).
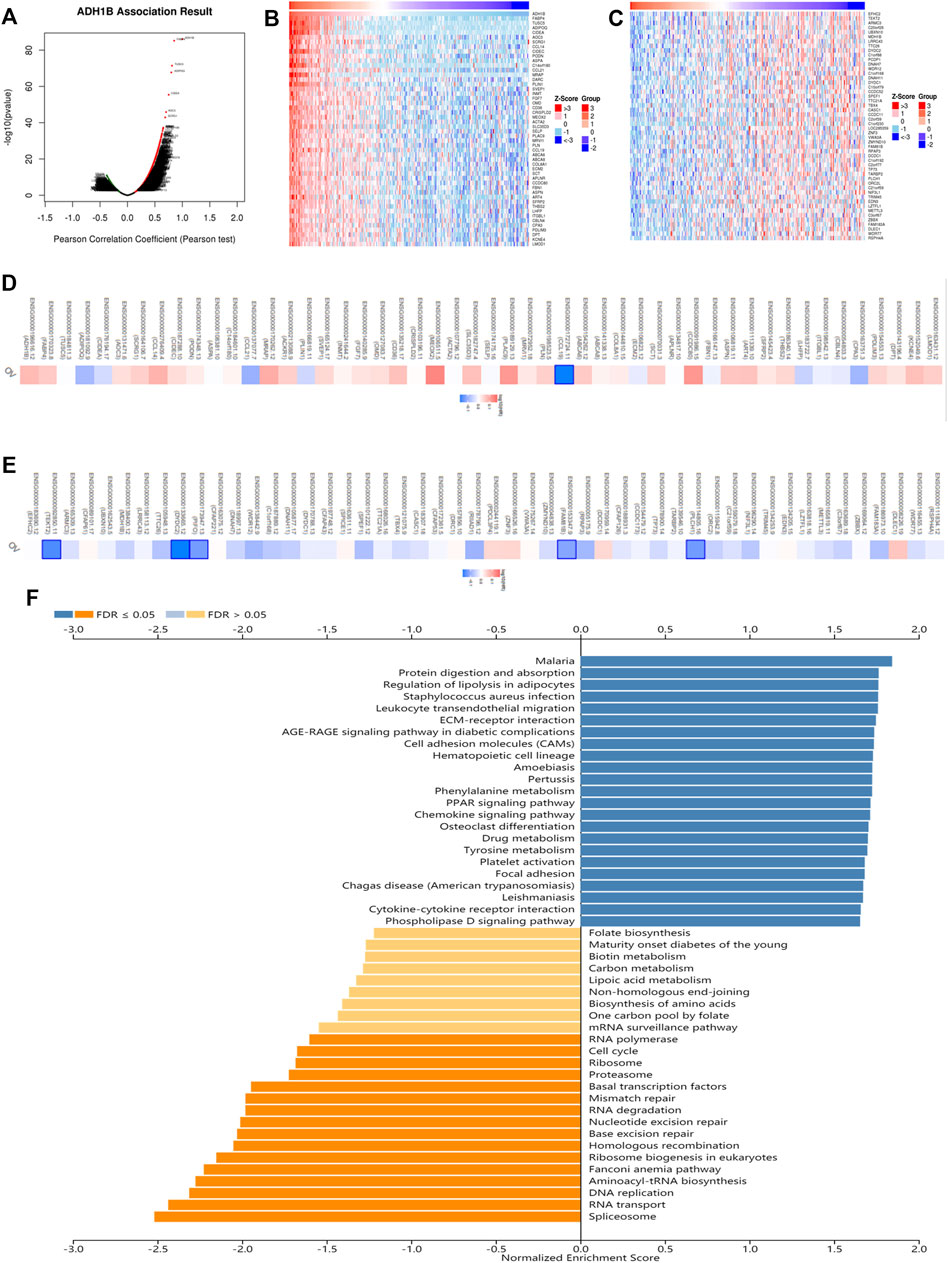
FIGURE 4. The co-expression analysis of ADH1B in ovarian cancer. (A) The identification of co-expressed genes remarkably related to ADH1B using the LinkedOmics database. (B,C) The top 50 genes positively and negatively in connection with ADH1B were shown on the heat map. Red indicated positive correlations while blue negative. (D,E) The top 50 genes having positive and negative associations with ADH1B were presented on the survival heat map. (F) Bar chart of the KEGG enrichment analysis of ADH1B.
A survey was carried out for the relationship between ADH1B and immune infiltration cells following these steps. We primarily evaluated the immune infiltration cells related to ADH1B in ovarian cancer through the ssGSEA algorithm with Pearson correlation. The results revealed that iDC, mast cells, B cells, macrophages, Th1, Tem, CD8 T-cells, T-cells, NK CD56dim cells, Tgd, T-helper cells, neutrophils, and cytotoxic cells were a positive correlation with ADH1B expression level (p < 0.001) (Figure 5A). Subsequently, TISIDB and TIMER databases were used for further validation. We finally concluded that macrophages and neutrophils were positively correlated with the expression of ADH1B (p <0.001) (Figures 5B–E).
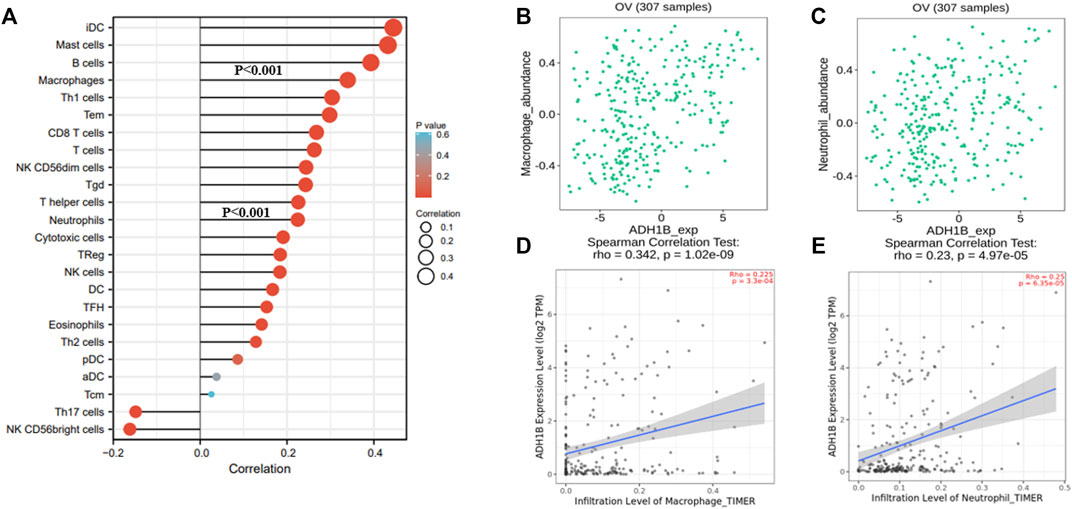
FIGURE 5. The relationship between ADH1B and immune infiltration. (A) 24 kinds of immune-infiltrating cells related to ADH1B expression. (B–E) Scatter plots were drawn through TIMER2.0 and TISIDB database to validate the correlations between ADH1B and immune-infiltrating cells, including macrophages and neutrophils.
In order to improve our understanding of the role of ADH1B in immune regulation, we shifted the focus of our research to the relationships between ADH1B between immunostimulators, immunoinhibitors, chemokines, and receptors. As shown in Supplementary Figures S4A–B, CD96 (Spearman r = 0.329, p = 4.26e-09), CD244 (Spearman r = 0.318, p = 1.5e-08), KDR (Spearman r = 0.352, p = 2.74e-10), and TGFB1 (Spearman r = 0.312, p = 2.66e-08) were the top four prominent markers of immune stimulation. Conversely, CXCL12 (Spearman r = 0.486, p = 2.22e-16), NT5E (Spearman r = 0.415, p = 2.19e-14), TNFRSF17 (Spearman r = 0.383, p = 5.4e-12), and TNFSF4 (Spearman r = 0.355, p = 1.96e-10) were the top four prominent markers of immune suppression. These findings indicated that ADH1B expression had a significantly positive relation with the molecules mentioned earlier. Supplementary Figure S5A suggested that the association between ADH1B expression and chemokines in patients with ovarian cancer, the result reported that the four most significant chemotactic agents were CCL21 (Spearman r = 0.629, p < 2.22e-16), CCL14 (Spearman r = 0.58, p < 2.22e-16), CCL19 (Spearman r = 0.518, p < 2.22e-16), and CCL21 (Spearman r = 0.486, p < 2.22e-16). Supplementary Figure S5B showed the most remarkable receptors closely related to ADH1B expression, CCR2 (Spearman r = 0.383, p = 5.4e-12), CCR4 (Spearman r = 0.355, p = 1.96e-10), CCR5 (Spearman r = 0.325, p = 6.77e-09), and CCR7 (Spearman r = 0.339, p = 1.4e-09) were included.
Overall, the implications of these results were that ADH1B was demonstrated to participate in a variety of immune regulation in ovarian cancer and able to be a new breakthrough in the treatment of ovarian cancer.
It is well known that chemotherapy is an important strategy for OV patients. Cyclophosphamide is one of the most used chemotherapeutic drugs (Ray-Coquard et al., 2020; Zsiros et al., 2021). Expectedly, we found that the expression of ADH1B was negatively associated with the activity of cyclophosphamide (Pearson r = -0.88, p = 0.047) (Supplementary Figure S6), indicating that high expression of ADH1B had an adverse effect on the chemotherapeutic effect. Additionally, positive correlations could be observed between ADH1B expression and multiple targets for immunotherapy, such as LAG3 and HAVCR2 (Figure 6A). Then, we employed the Human Protein Atlas portal (https://www.proteinatlas.org/) to validate the positive association between ADH1B and LAG3/HAVCR2. As shown in Figures 6B,C, among the 11 cases of OV tissues examined for ADH1B staining, six cases had negative expression and three cases had weak intensity. Meanwhile, all of the 11 cases displayed negative intensity for LAG3 and HAVCR2 staining. All these findings together suggested that ADH1B, LAG3, and HAVCR2 were significantly down-regulated in cancer tissues, which would offer a good prospect that targeted ADH1B could increase the therapeutic efficacy of OV patients.
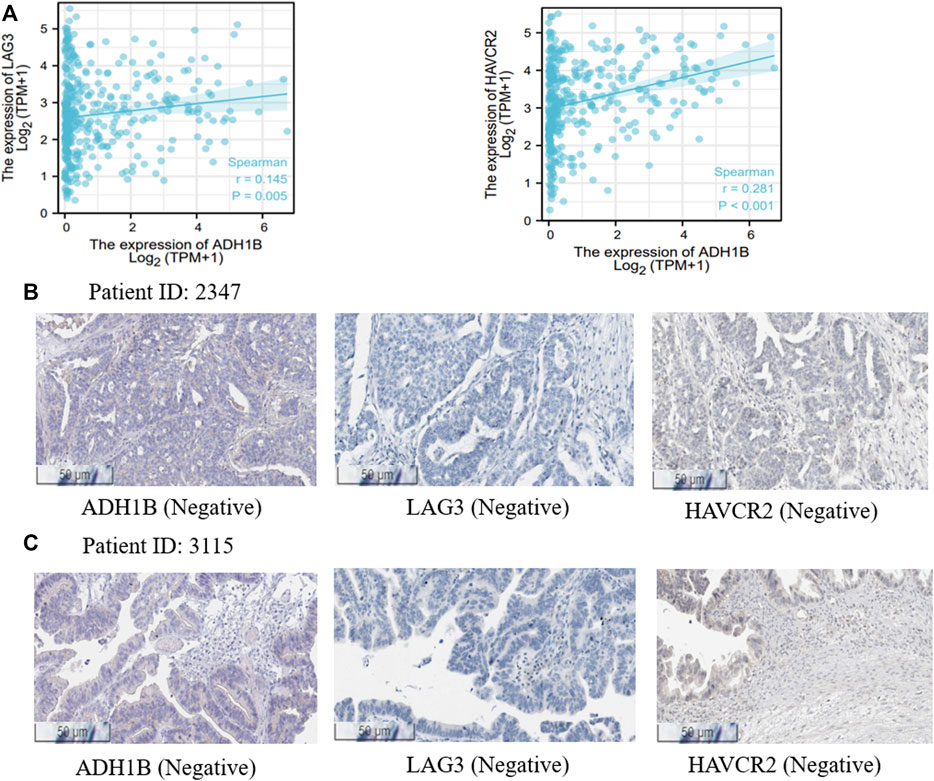
FIGURE 6. Treatment correlation analysis of ADH1B. (A) The associations between ADH1B with LAG3 and HAVCR2. (B,C) the Human Protein Atlas portal validated the downregulated ADH1B, LAG3, and HAVCR2 in OV tissues.
In this research, our aim is to find a candidate prognostic and targeted biomarker for the treatment of ovarian tumors. As a result, three down-regulated genes, ADH1B, AOX1, and DPYD, were identified using multiple bioinformation databases and drug metabolism-related genes datasets. Then, a series of survival analyses were conducted and ADH1B was the most significant biomarker. Moreover, we analyzed the co-expressed genes of ADH1B and found that these genes positively or negatively correlated to ADH1B might have a considerable influence on prognosis and treatment of ovarian cancer. In addition, ADH1B has shown great importance in the immune microenvironment in ovarian cancer. Hence, we conducted that ADH1B was allowed to function as a biomarker aiding in the treatment and prognosis of ovarian cancer.
Most human diseases, including cancer, have something to do with multiple genes and genetic variations. Similarly, the metabolic effects of most drugs are involved in polygenic products (Relling and Evans, 2015). There are several studies indicating the significance of drug metabolism-related genes and the outcome of different cancers. For instance, He et al. (2020) reported that there was a worse prognosis in both poor and ultrarapid CYP2D6 metabolizers of tamoxifen in comparison with normal metabolizers in breast cancer (He et al., 2020). Moore et al. (2018) proved that BRCA1/2 mutation carriers reap greater benefits from olaparib maintenance therapy in ovarian cancer (Moore et al., 2018). Additionally, UGT1A1 polymorphism influenced clinical outcome in patients undergoing cytarabine chemotherapy for moderate critical myeloid leukemia (Díaz-Santa et al., 2020). The evidence presented that drug metabolism-related genes are crucial for personalized cancer therapy to get a better outcome for patients. However, there have been few reports of the relationship between drug metabolism-related genes and prognosis in ovarian cancer patients. Based on acquired chemotherapy resistance in ovarian cancer (Christie and Bowtell, 2017), it is necessary to explore new therapeutic approaches from a pharmacogenomics perspective and find novel molecular biomarkers or drug targets.
Aberrantly expressed ADH1B could participate in the regulation of cellular metabolism and drug metabolism (Li et al., 2018). ADH1B can be considered as a molecular biomarker of residual disease and its overexpression promotes invasion and metastasis in ovarian cancer (Tucker et al., 2014). Moreover, the activity of the ADH1B is significantly increased in the OV group compared with normal ovarian tissues. As a result, increased acetaldehyde concentration induced by activated ADH1B produced carcinogenic effects in ovarian cells (Orywal et al., 2013). These findings may explain the roles of ADH1B in the development and progression of ovarian cancer. In addition, the higher ADH activity was observed in ESCC and bladder cancer. Zhang et al. (2010) found that the Han Chinese with ADH1B His47Arg locus have greater interaction with alcohol consumption and are prone to develop ESCC (Zhang et al., 2010). Masaoka et al. (2016) proved that the risk of bladder cancer was greatly increased in people with ADH1B Arg + due to gene-environment interaction (Masaoka et al., 2016). Therefore, ADH1B has a significant impact on tumorigenesis and progression. In our study, ADH1B, a drug metabolism-related gene, was down-regulated in ovarian cancer tissues, which indicated a better life span expectancy.
The results of KEGG annotation were shown in Figure 4F. Spliceosome, RNA transport, and DNA replication were the top three signal pathways regulated by ADH1B co-expressed genes in OV patients. The spliceosome is a powerful tool for removing introns from pre-mRNA molecules (Galej et al., 2014). Several splicing factors, including catenin β-like 1(CTNNBL1) and ubiquitin-specific peptidase 39 (USP39), could promote the growth and invasion of OV cells (Li et al., 2017; Wang et al., 2021). The intranuclear genetic information could be transferred into the cytoplasm through RNA transport, participating in cancer pathogenesis and therapeutic response (Gasparski et al., 2022). DNA replication is a key step in maintaining genome stability and is frequently disrupted in cancer cells. Inhibitors of poly (ADP-ribose) polymerase (PARP) targeting DNA repair were effective in treating patients with advanced OV (Yan et al., 2020).
Tumor microenvironment (TME) is a hot topic in recent years. Evidence has increasingly shown that tumor development, recurrence, and metastasis is in close relationship with the immune microenvironment as an acritical part of the tumor microenvironment (Fu et al., 2021). Meanwhile, a large number of studies on the immune microenvironment have contributed to the development of immunotherapy strategies and have increased the likelihood of favorable patient outcomes (Li et al., 2021). Ovarian cancer is usually found to be advanced and is prone to recurrence after surgery or chemotherapy, which leads to a high death rate (Lheureux et al., 2019) and survival for ovarian cancer has not improved significantly for decades (Torre et al., 2018). To improve the prognosis of ovarian cancer patients, special immunotherapy and developing new biomarkers become the development direction of therapeutic strategies now and even for a long time to come. At present, the major immunotherapies include oncolytic virus therapies, cancer vaccines, cytokine therapies, adoptive cell transfer, and immune checkpoint inhibitors which maybe be the new technologies and prospects for the treatment of ovarian cancer (Zhang and Zhang, 2020). Actually, the United States Food and Drug Administration recently has approved several immune checkpoint inhibitors for a variety of cancers, unfortunately not ovarian cancer (Odunsi, 2017). In our work, a comprehensive evaluation between ADH1B and the immune environment was conducted. The results suggested that the expression level of ADH1B had a positive relationship with macrophages and neutrophils. There has been a great deal of research indicating a connection between ovarian cancer and tumor-infiltrating immune cells including macrophages and neutrophils (Lee et al., 2019; Etzerodt et al., 2020). Macrophages can promote the invasion and chemoresistance of ovarian cancer (Raghavan et al., 2019). Neutrophils can release oxidized lipids to reactivate dormant tumor cells, resulting in early cancer recurrence (Perego et al., 2020). With a deeper understanding of the immune environment, the researchers have come up with various therapeutic ideas. Barkal et al. (2019) found that blocking the CD24 signaling pathway mediated by macrophage siglec-10 is promising as a new target of immunotherapy (Barkal et al., 2019). Raghavan et al. (2019) thought that the WNT pathway which promotes the interaction between ovarian cancer stem cells (CSC) and macrophages is a potential target for reducing the aggressiveness of ovarian cancer (Raghavan et al., 2019). Additionally, the upregulation of PD-L1 expressed in neutrophils leads to accelerate immune escape of ovarian cancer cells which can be solved by targeting HOXA transcript at the distal tip (HOTTIP) (Shang et al., 2019). Based on our research, there is reason to believe that ADH1B may be a potential treatment strategy with the regulation of macrophages and neutrophils for ovarian cancer.
Furthermore, the most valuable immunostimulators (CD96, CD244, KDR, and TGFB1), immunoinhibitors (CXCL12, NT5E, TNFSRF17, and TNFSF4), chemokines (CCL21, CCL14, CCL19, and CCL12), and chemokine receptors (CCR2, CCR4, CCR5, and CCR7) related to ADH1B were obtained. There has been a lot of research on these molecules and ovarian cancer. CD96 has been proved the key for natural killer (NK) cell reactivity to advanced ovarian cancer (Maas et al., 2020). TGFBI produced by macrophages can conduce to suppress the immune microenvironment of ovarian cancer (Lecker et al., 2021). The CXCL12 has been seen as a strong accelerant to promote metastasize and invasion in ovarian cancer (Luo et al., 2019). CCL21 has been shown an association with bad OS in TP53 wild-type serous ovarian cancer (Ignacio et al., 2018). CCR2 might make a difference in enhancing the ability of ovarian cancer cell peritoneal metastasis and portending a poor prognosis (Sun et al., 2020). Generally, these studies show that ADH1B is a key part of the immune microenvironment and regulates a variety of immune cells against ovarian cancer cells. On this basis, ADH1B may be a potential therapeutic target and contribute to the treatment of ovarian cancer.
In summary, the downregulation of ADH1B was illustrated in this study, which was shown with a good prognosis value for ovarian cancer. In addition, the expression of ADH1B has a significant correlation with the immune microenvironment. Immunostimulators, immunoinhibitors, chemokines, and chemokine receptors were included. Consequently, ADH1B could be significant for immune infiltration and be valuable in guidelines for treatment and evaluation of prognosis in patients with ovarian cancer. It was concluded that ADH1B might provide a new perspective for the treatment of ovarian cancer.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
The ethics of our study about the formalin-fixed, paraffin-embedded (FFPE) specimens of OV and adjacent tissues has been approved by the Ethical Committee of Xiangya Hospital of Central South University and the ethical approval number is 202204098.
XZ, BP, YY, and JP contributed to the conception and design of the study. BP, FK, WZ, JL, and QH contributed to the writing, review, and revision of the manuscript. WL, YC, and MX performed the administrative, technical, and material support. All authors approved the final version of the manuscript.
This study is supported by grants from the Horizontal project (1 43010100) and the Science and Technology Innovation Program of Hunan Province (2021RC3029).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2022.877254/full#supplementary-material
Akkaif, M. A., Daud, N. A. A., Sha’aban, A., Ng, M. L., Abdul Kader, M. A. S., Noor, D. A. M., et al. (2021). The Role of Genetic Polymorphism and Other Factors on Clopidogrel Resistance (CR) in an Asian Population with Coronary Heart Disease (CHD). Molecules 26 (7), 1987. doi:10.3390/molecules26071987
Barkal, A. A., Brewer, R. E., Markovic, M., Kowarsky, M., Barkal, S. A., Zaro, B. W., et al. (2019). CD24 Signalling through Macrophage Siglec-10 is a Target for Cancer Immunotherapy. Nature 572 (7769), 392–396. doi:10.1038/s41586-019-1456-0
Bartha, Á., and Győrffy, B. (2021). TNMplot.com: A Web Tool for the Comparison of Gene Expression in Normal, Tumor and Metastatic Tissues. Int. J. Mol. Sci. 22 (5), 2622. doi:10.3390/ijms22052622
Choi, C. K., Shin, M.-H., Cho, S.-H., Kim, H.-Y., Zheng, W., Long, J., et al. (2021). Association between ALDH2 and ADH1B Polymorphisms and the Risk for Colorectal Cancer in Koreans. Cancer Res. Treat. 53 (3), 754–762. doi:10.4143/crt.2020.478
Christie, E. L., and Bowtell, D. D. L. (2017). Acquired Chemotherapy Resistance in Ovarian Cancer. Ann. Oncol. 28 (Suppl. l_8), viii13–viii15. doi:10.1093/annonc/mdx446
Díaz-Santa, J., Rodriguez-Romanos, R., Rodríguez-Romanos, R., Osca, G., Pratcorona, M., Garrido, A., et al. (2020). UGT1A1 Genotype Influences Clinical Outcome in Patients with Intermediate-Risk Acute Myeloid Leukemia Treated with Cytarabine-Based Chemotherapy. Leukemia 34 (11), 2925–2933. doi:10.1038/s41375-020-0784-2
Etzerodt, A., Moulin, M., Doktor, T. K., Delfini, M., Mossadegh-Keller, N., Bajenoff, M., et al. (2020). Tissue-Resident Macrophages in Omentum Promote Metastatic Spread of Ovarian Cancer. J. Exp. Med. 217 (4), e20191869. doi:10.1084/jem.20191869
Ferriss, J. S., Kim, Y., Duska, L., Birrer, M., Levine, D. A., Moskaluk, C., et al. (2012). Multi-Gene Expression Predictors of Single Drug Responses to Adjuvant Chemotherapy in Ovarian Carcinoma: Predicting Platinum Resistance. PloS One 7 (2), e30550. doi:10.1371/journal.pone.0030550
Fu, T., Dai, L.-J., Wu, S.-Y., Xiao, Y., Ma, D., Jiang, Y.-Z., et al. (2021). Spatial Architecture of the Immune Microenvironment Orchestrates Tumor Immunity and Therapeutic Response. J. Hematol. Oncol. 14 (1), 98. doi:10.1186/s13045-021-01103-4
Galej, W. P., Nguyen, T. H. D., Newman, A. J., and Nagai, K. (2014). Structural Studies of the Spliceosome: Zooming into the Heart of the Machine. Curr. Opin. Struct. Biol. 25, 57–66. doi:10.1016/j.sbi.2013.12.002
Gasparski, A. N., Mason, D. E., Moissoglu, K., and Mili, S. (2022). Regulation and Outcomes of Localized RNA Translation. WIREs RNA, e1721. doi:10.1002/wrna.1721
Govindarajan, M., Wohlmuth, C., Waas, M., Bernardini, M. Q., and Kislinger, T. (2020). High-Throughput Approaches for Precision Medicine in High-Grade Serous Ovarian Cancer. J. Hematol. Oncol. 13 (1), 134. doi:10.1186/s13045-020-00971-6
He, W., Grassmann, F., Eriksson, M., Eliasson, E., Margolin, S., Thorén, L., et al. (2020). CYP2D6 Genotype Predicts Tamoxifen Discontinuation and Prognosis in Patients with Breast Cancer. J. Clin. Oncol. 38 (6), 548–557. doi:10.1200/JCO.19.01535
Henricks, L. M., Lunenburg, C. A. T. C., de Man, F. M., Meulendijks, D., Frederix, G. W. J., Kienhuis, E., et al. (2018). DPYD Genotype-Guided Dose Individualisation of Fluoropyrimidine Therapy in Patients with Cancer: A Prospective Safety Analysis. Lancet Oncol. 19 (11), 1459–1467. doi:10.1016/S1470-2045(18)30686-7
Ignacio, R. M. C., Lee, E.-S., Wilson, A. J., Beeghly-Fadiel, A., Whalen, M. M., and Son, D.-S. (2018). Chemokine Network and Overall Survival in TP53 Wild-Type and Mutant Ovarian Cancer. Immune Netw. 18 (4), e29. doi:10.4110/in.2018.18.e29
Kuroki, L., and Guntupalli, S. R. (2020). Treatment of Epithelial Ovarian Cancer. BMJ 371, m3773. doi:10.1136/bmj.m3773
Lánczky, A., and Győrffy, B. (2021). Web-Based Survival Analysis Tool Tailored for Medical Research (KMplot): Development and Implementation. J. Med. Internet Res. 23 (7), e27633. doi:10.2196/27633
Lecker, L. S. M., Berlato, C., Maniati, E., Delaine-Smith, R., Pearce, O. M. T., Heath, O., et al. (2021). TGFBI Production by Macrophages Contributes to an Immunosuppressive Microenvironment in Ovarian Cancer. Cancer Res. 81 (22), 5706–5719. doi:10.1158/0008-5472.CAN-21-0536
Lee, W., Ko, S. Y., Mohamed, M. S., Kenny, H. A., Lengyel, E., and Naora, H. (2019). Neutrophils Facilitate Ovarian Cancer Premetastatic Niche Formation in the Omentum. J. Exp. Med. 216 (1), 176–194. doi:10.1084/jem.20181170
Lheureux, S., Gourley, C., Vergote, I., and Oza, A. M. (2019). Epithelial Ovarian Cancer. Lancet 393 (10177), 1240–1253. doi:10.1016/S0140-6736(18)32552-2
Li, H., Toth, E., and Cherrington, N. J. (2018). Alcohol Metabolism in the Progression of Human Nonalcoholic Steatohepatitis. Toxicol. Sci. 164 (2), 428–438. doi:10.1093/toxsci/kfy106
Li, J.-Y., Chen, Y.-P., Li, Y.-Q., Liu, N., and Ma, J. (2021). Chemotherapeutic and Targeted Agents Can Modulate the Tumor Microenvironment and Increase the Efficacy of Immune Checkpoint Blockades. Mol. Cancer 20 (1), 27. doi:10.1186/s12943-021-01317-7
Li, T., Fu, J., Zeng, Z., Cohen, D., Li, J., Chen, Q., et al. (2020). TIMER2.0 for Analysis of Tumor-Infiltrating Immune Cells. Nucleic Acids Res. 48 (W1), W509–W514. doi:10.1093/nar/gkaa407
Li, Y., Guo, H., Jin, C., Qiu, C., Gao, M., Zhang, L., et al. (2017). Spliceosome-Associated Factor CTNNBL1 Promotes Proliferation and Invasion in Ovarian Cancer. Exp. Cell Res. 357 (1), 124–134. doi:10.1016/j.yexcr.2017.05.008
Liu, J., Lichtenberg, T., Hoadley, K. A., Poisson, L. M., Lazar, A. J., Cherniack, A. D., et al. (2018). An Integrated TCGA Pan-Cancer Clinical Data Resource to Drive High-Quality Survival Outcome Analytics. Cell 173 (2), 400–416. doi:10.1016/j.cell.2018.02.052
Luna, A., Elloumi, F., Varma, S., Wang, Y., Rajapakse, V. N., Aladjem, M. I., et al. (2021). CellMiner Cross-Database (CellMinerCDB) Version 1.2: Exploration of Patient-Derived Cancer Cell Line Pharmacogenomics. Nucleic Acids Res. 49 (D1), D1083–D1093. doi:10.1093/nar/gkaa968
Luo, N., Chen, D.-d., Liu, L., Li, L., and Cheng, Z.-p. (2019). CXCL12 Promotes Human Ovarian Cancer Cell Invasion through Suppressing ARHGAP10 Expression. Biochem. Biophysical Res. Commun. 518 (3), 416–422. doi:10.1016/j.bbrc.2019.07.098
Maas, R. J., Hoogstad-van Evert, J. S., Van der Meer, J. M., Mekers, V., Rezaeifard, S., Korman, A. J., et al. (2020). TIGIT Blockade Enhances Functionality of Peritoneal NK Cells with Altered Expression of DNAM-1/TIGIT/CD96 Checkpoint Molecules in Ovarian Cancer. Oncoimmunology 9 (1), 1843247. doi:10.1080/2162402X.2020.1843247
Masaoka, H., Ito, H., Soga, N., Hosono, S., Oze, I., Watanabe, M., et al. (2016). Aldehyde Dehydrogenase 2 (ALDH2) and Alcohol Dehydrogenase 1B (ADH1B) Polymorphisms Exacerbate Bladder Cancer Risk Associated with Alcohol Drinking: Gene-Environment Interaction. Carcinogenesis 37 (6), 583–588. doi:10.1093/carcin/bgw033
Mazor, G., Levin, L., Picard, D., Ahmadov, U., Carén, H., Borkhardt, A., et al. (2019). The lncRNA TP73-AS1 Is Linked to Aggressiveness in Glioblastoma and Promotes Temozolomide Resistance in Glioblastoma Cancer Stem Cells. Cell Death Dis. 10 (3), 246. doi:10.1038/s41419-019-1477-5
Mok, S. C., Bonome, T., Vathipadiekal, V., Bell, A., Johnson, M. E., Wong, k.-k., et al. (2009). A Gene Signature Predictive for Outcome in Advanced Ovarian Cancer Identifies a Survival Factor: Microfibril-Associated Glycoprotein 2. Cancer Cell 16 (6), 521–532. doi:10.1016/j.ccr.2009.10.018
Moore, K., Colombo, N., Scambia, G., Kim, B.-G., Oaknin, A., Friedlander, M., et al. (2018). Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 379 (26), 2495–2505. doi:10.1056/NEJMoa1810858
Muñoz-Galván, S., Felipe-Abrio, B., Verdugo-Sivianes, E. M., Perez, M., Jiménez-García, M. P., Suarez-Martinez, E., et al. (2020). Downregulation of MYPT1 Increases Tumor Resistance in Ovarian Cancer by Targeting the Hippo Pathway and Increasing the Stemness. Mol. Cancer 19 (1), 7. doi:10.1186/s12943-020-1130-z
Odunsi, K. (2017). Immunotherapy in Ovarian Cancer. Ann. Oncol. 28 (Suppl. l_8), viii1–viii7. doi:10.1093/annonc/mdx444
Orywal, K., Jelski, W., Zdrodowski, M., and Szmitkowski, M. (2013). The Activity of Class I, II, III and IV Alcohol Dehydrogenase Isoenzymes and Aldehyde Dehydrogenase in Ovarian Cancer and Ovarian Cysts. Adv. Med. Sci. 58 (2), 216–220. doi:10.2478/ams-2013-0012
Perego, M., Tyurin, V. A., Tyurina, Y. Y., Yellets, J., Nacarelli, T., Lin, C., et al. (2020). Reactivation of Dormant Tumor Cells by Modified Lipids Derived from Stress-Activated Neutrophils. Sci. Transl. Med. 12 (572), eabb5817. doi:10.1126/scitranslmed.abb5817
Raghavan, S., Mehta, P., Xie, Y., Lei, Y. L., and Mehta, G. (2019). Ovarian Cancer Stem Cells and Macrophages Reciprocally Interact through the WNT Pathway to Promote Pro-Tumoral and Malignant Phenotypes in 3D Engineered Microenvironments. J. Immunother. Cancer 7 (1), 190. doi:10.1186/s40425-019-0666-1
Ray-Coquard, I., Mirza, M. R., Pignata, S., Walther, A., Romero, I., and du Bois, A. (2020). Therapeutic Options Following Second-Line Platinum-Based Chemotherapy in Patients with Recurrent Ovarian Cancer: Comparison of Active Surveillance and Maintenance Treatment. Cancer Treat. Rev. 90, 102107. doi:10.1016/j.ctrv.2020.102107
Relling, M. V., and Evans, W. E. (2015). Pharmacogenomics in the Clinic. Nature 526 (7573), 343–350. doi:10.1038/nature15817
Relling, M. V., Schwab, M., Whirl‐Carrillo, M., Suarez‐Kurtz, G., Pui, C. H., Stein, C. M., et al. (2019). Clinical Pharmacogenetics Implementation Consortium Guideline for Thiopurine Dosing Based on TPMT and NUDT15 Genotypes: 2018 Update. Clin. Pharmacol. Ther. 105 (5), 1095–1105. doi:10.1002/cpt.1304
Roden, D. M., McLeod, H. L., Relling, M. V., Williams, M. S., Mensah, G. A., Peterson, J. F., et al. (2019). Pharmacogenomics. Lancet 394 (10197), 521–532. doi:10.1016/S0140-6736(19)31276-0
Ru, B., Wong, C. N., Tong, Y., Zhong, J. Y., Zhong, S. S. W., Wu, W. C., et al. (2019). TISIDB: An Integrated Repository Portal for Tumor-Immune System Interactions. Bioinformatics 35 (20), 4200–4202. doi:10.1093/bioinformatics/btz210
Shang, A., Wang, W., Gu, C., Chen, C., Zeng, B., Yang, Y., et al. (2019). Long Non-Coding RNA HOTTIP Enhances IL-6 Expression to Potentiate Immune Escape of Ovarian Cancer Cells by Upregulating the Expression of PD-L1 in Neutrophils. J. Exp. Clin. Cancer Res. 38 (1), 411. doi:10.1186/s13046-019-1394-6
Siegel, R. L., Miller, K. D., Fuchs, H. E., and Jemal, A. (2021). Cancer Statistics, 2021. CA A Cancer J. Clin. 71 (1), 7–33. doi:10.3322/caac.21654
Strommer, J. (2011). The Plant ADH Gene Family. Plant J. 66 (1), 128–142. doi:10.1111/j.1365-313X.2010.04458.x
Sun, C., Li, X., Guo, E., Li, N., Zhou, B., Lu, H., et al. (2020). MCP-1/CCR-2 Axis in Adipocytes and Cancer Cell Respectively Facilitates Ovarian Cancer Peritoneal Metastasis. Oncogene 39 (8), 1681–1695. doi:10.1038/s41388-019-1090-1
Suo, C., Yang, Y., Yuan, Z., Zhang, T., Yang, X., Qing, T., et al. (2019). Alcohol Intake Interacts with Functional Genetic Polymorphisms of Aldehyde Dehydrogenase (ALDH2) and Alcohol Dehydrogenase (ADH) to Increase Esophageal Squamous Cell Cancer Risk. J. Thorac. Oncol. 14 (4), 712–725. doi:10.1016/j.jtho.2018.12.023
Tang, Z., Kang, B., Li, C., Chen, T., and Zhang, Z. (2019). GEPIA2: An Enhanced Web Server for Large-Scale Expression Profiling and Interactive Analysis. Nucleic Acids Res. 47 (W1), W556–W560. doi:10.1093/nar/gkz430
Torre, L. A., Trabert, B., DeSantis, C. E., Miller, K. D., Samimi, G., Runowicz, C. D., et al. (2018). Ovarian Cancer Statistics, 2018. CA A Cancer J. Clin. 68 (4), 284–296. doi:10.3322/caac.21456
Tucker, S. L., Gharpure, K., Herbrich, S. M., Unruh, A. K., Nick, A. M., Crane, E. K., et al. (2014). Molecular Biomarkers of Residual Disease after Surgical Debulking of High-Grade Serous Ovarian Cancer. Clin. Cancer Res. 20 (12), 3280–3288. doi:10.1158/1078-0432.CCR-14-0445
Vasaikar, S. V., Straub, P., Wang, J., and Zhang, B. (2018). LinkedOmics: Analyzing Multi-Omics Data within and across 32 Cancer Types. Nucleic Acids Res. 46 (D1), D956–D963. doi:10.1093/nar/gkx1090
Vathipadiekal, V., Wang, V., Wei, W., Waldron, L., Drapkin, R., Gillette, M., et al. (2015). Creation of a Human Secretome: A Novel Composite Library of Human Secreted Proteins: Validation Using Ovarian Cancer Gene Expression Data and a Virtual Secretome Array. Clin. Cancer Res. 21 (21), 4960–4969. doi:10.1158/1078-0432.CCR-14-3173
Wake, D. T., Ilbawi, N., Dunnenberger, H. M., and Hulick, P. J. (2019). Pharmacogenomics: Prescribing Precisely. Med. Clin. N. Am. 103 (6), 977–990. doi:10.1016/j.mcna.2019.07.002
Wang, S., Wang, Z., Li, J., Qin, J., Song, J., Li, Y., et al. (2021). Splicing Factor USP39 Promotes Ovarian Cancer Malignancy through Maintaining Efficient Splicing of Oncogenic HMGA2. Cell Death Dis. 12 (4), 294. doi:10.1038/s41419-021-03581-3
Wang, Y., Fang, Z., Hong, M., Yang, D., and Xie, W. (2020). Long-Noncoding RNAs (lncRNAs) in Drug Metabolism and Disposition, Implications in Cancer Chemo-Resistance. Acta Pharm. Sin. B 10 (1), 105–112. doi:10.1016/j.apsb.2019.09.011
Wei, R., Li, P., He, F., Wei, G., Zhou, Z., Su, Z., et al. (2021). Comprehensive Analysis Reveals Distinct Mutational Signature and its Mechanistic Insights of Alcohol Consumption in Human Cancers. Brief. Bioinform 22 (3), bbaa066. doi:10.1093/bib/bbaa066
Yan, Y., Xu, Z., Huang, J., Guo, G., Gao, M., Kim, W., et al. (2020). The Deubiquitinase USP36 Regulates DNA Replication Stress and Confers Therapeutic Resistance through PrimPol Stabilization. Nucleic Acids Res. 48 (22), 12711–12726. doi:10.1093/nar/gkaa1090
Zhang, G. H., Mai, R. Q., and Huang, B. (2010). Meta-Analysis of ADH1B and ALDH2 Polymorphisms and Esophageal Cancer Risk in China. World J. Gastroenterol. 16 (47), 6020–6025. doi:10.3748/wjg.v16.i47.6020
Zhang, Y., and Zhang, Z. (2020). The History and Advances in Cancer Immunotherapy: Understanding the Characteristics of Tumor-Infiltrating Immune Cells and Their Therapeutic Implications. Cell Mol. Immunol. 17 (8), 807–821. doi:10.1038/s41423-020-0488-6
Zsiros, E., Lynam, S., Attwood, K. M., Wang, C., Chilakapati, S., Gomez, E. C., et al. (2021). Efficacy and Safety of Pembrolizumab in Combination with Bevacizumab and Oral Metronomic Cyclophosphamide in the Treatment of Recurrent Ovarian Cancer: A Phase 2 Nonrandomized Clinical Trial. JAMA Oncol. 7 (1), 78–85. doi:10.1001/jamaoncol.2020.5945
Keywords: ovarian cancer, ADH1B, drug metabolism-related genes, immune infiltration, therapeutic response
Citation: Xu Z, Peng B, Kang F, Zhang W, Xiao M, Li J, Hong Q, Cai Y, Liu W, Yan Y and Peng J (2022) The Roles of Drug Metabolism-Related ADH1B in Immune Regulation and Therapeutic Response of Ovarian Cancer. Front. Cell Dev. Biol. 10:877254. doi: 10.3389/fcell.2022.877254
Received: 16 February 2022; Accepted: 29 April 2022;
Published: 09 June 2022.
Edited by:
Dongling Zou, Chongqing University, ChinaCopyright © 2022 Xu, Peng, Kang, Zhang, Xiao, Li, Hong, Cai, Liu, Yan and Peng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuanliang Yan, eWFueXVhbmxpYW5nQGNzdS5lZHUuY24=; Jinwu Peng, amlud3VwZW5nQGNzdS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.