- 1Department of Neurosurgery, Xiangya Hospital, Central South University, Changsha, China
- 2Department of Pathophysiology, Xiangya School of Medicine, Central South University, Changsha, China
- 3Sepsis Translational Medicine Key Lab of Hunan Province, Changsha, China
- 4China-Africa Research Center of Infectious Diseases, Central South University, Changsha, China
Ferroptosis is a new form of regulatory cell death named by Dixon in 2012, which is characterized by the accumulation of lipid peroxides and iron ions. Molecular chaperones are a class of evolutionarily conserved proteins in the cytoplasm. They recognize and bind incompletely folded or assembled proteins to help them fold, transport or prevent their aggregation, but they themselves do not participate in the formation of final products. As the largest number of molecular chaperones, heat shock proteins can be divided into five families: HSP110 (HSPH), HSP90 (HSPC), HSP70 (HSPA), HSP40 (DNAJ) and small heat shock proteins (HSPB). Different heat shock proteins play different roles in promoting or inhibiting ferroptosis in different diseases. It is known that ferroptosis is participated in tumors, nervous system diseases, renal injury and ischemia-reperfusion injury. However, there are few reviews about the relationship of heat shock proteins and ferroptosis. In this study, we systematically summarize the roles of heat shock proteins in the occurrence of ferroptosis, and predict the possible mechanisms of different families of heat shock proteins in the development of ferroptosis.
Introduction
Ferroptosis is an iron-dependent regulatory cell death mode, which is different from apoptosis, necrosis and pyrodeath in morphology, biochemistry and other aspects. Studies have found that ferroptosis is not only related to tumorigenesis, but also involved in the occurrence of a variety of nervous system diseases. What’s more, it also plays an important role in many diseases such as ischemia-reperfusion injury.
The ability of cells to respond to external stress is important for homeostasis, and stress of all kinds is particularly harmful for proteins that need to fold to function (Morimoto and Cuervo, 2014). Folded proteins have very little thermodynamic stability and are susceptible to environmental changes even when cell conditions change slightly (Kim Y. E. et al., 2013). To do this, a complex network of networks formed inside the cell to cope with these stressful conditions, that is, a quality control system for proteins (Thirumalai et al., 2020). The system regulates the entire process from initial synthesis of proteins on ribosomes to final degradation of proteins. As main members of this protein quality control system, molecular chaperones play an indispensable role in maintaining protein stability. Among them, heat shock proteins (HSP) are the most abundant molecular chaperone proteins. In addition to normal expression under physiological conditions, HSPs expression increases when cells are exposed to stress conditions, such as high temperature or hypoxia (Rylander et al., 2005). According to the order of molecular weight, HSPs were classified by predecessors, including HSP110, HSP90, HSP70, HSP40 and small heat shock protein (sHSP) (Kampinga et al., 2009). What is more, there are also HSP related proteins, such as co-chaperone, TCP-1 ring complex (TriC), protein disulfide isomerases (PDIs) and cis-trans proline isomerase, cadherin/calreticulin, etc., (Hageman and Kampinga, 2009; Kampinga et al., 2009).
In recent years, more and more studies have shown that HSPs participate in the pathophysiological process of ferroptosis. They play different roles in the process of ferroptosis in occurrence, development and regulation. Therefore, this paper makes a systematic review on the regulation of HSPs on ferroptosis, in which we focus on the important role of different HSPs in the process of ferroptosis, which may provide innovative ideas for further research on ferroptosis.
Ferroptosis
Regulated cell death (RCD) is an important process that maintains the metabolism and homeostasis of multicellular organisms, including apoptosis, necrosis, pyroptosis, ferroptosis and other forms of RCD (Galluzzi et al., 2018; Tang et al., 2019). Among them, ferroptosis has been revealed to be involved in more and more diseases in recent years, such as neurodegenerative diseases (Ayton et al., 2015), cancer (Chen X. et al., 2021), ischemia-reperfusion injury (IRI) (Swaminathan, 2018), intestinal diseases (Xu S. et al., 2021) and so on. Unlike apoptosis (cell atrophy, chromatin concentration, cytoskeleton disintegration and the formation of apoptotic bodies), necrosis (cell membrane rupture, organelle swelling) and pyroptosis (cell swelling, nuclear shrinkage, cell membrane rupture and content leakage), the biochemical aspects of ferroptosis are characterized by the accumulation of intracellular iron and lipid reactive oxygen. As major sites of iron utilization and master regulators of oxidative metabolism, mitochondria are the main source of reactive oxygen species (ROS) and, thus, the severity of damage to mitochondrial morphology, bioenergetics and metabolism is closely related to ferroptosis (Battaglia et al., 2020). Morphologically, when ferroptosis occurs in cells, the volume of mitochondria becomes smaller, accompanied by the increase of mitochondrial membrane density, the decrease or disappearance of cristae and the rupture of mitochondrial outer membrane, which may be caused by the dysfunction of voltage-dependent anion channels (VDCAs) and the change of mitochondrial membrane fluidity caused by lipid peroxidation products (DeHart et al., 2018). Although ferroptosis is widely involved in the pathological process of many different diseases, there is still no specific molecular detection index for it. The existence of ferroptosis can only detected by the level of lipid peroxidation and the inhibition of ferroptosis inhibitors or iron chelators on cell death.
In recent years, the complex and unknown regulatory mechanism network of ferroptosis is slowly being revealed, including cysteine/glutamate antiporter system (system Xc−), glutathione peroxidase (GPX), mitochondrial membrane voltage dependent anion channel, iron metabolism, ferroptosis suppressor protein 1(FSP1), GTP cyclohydrolase 1 (GCH1), p53, HO-1, p62-Keap1-Nrf2, ATG5-ATG7-NCOA4 and other related signal pathways. This paper will focus on the mechanism of ferroptosis from five aspects: iron metabolism, GSH/GPX4 antioxidant pathway, FSP1/CoQ10 antioxidant pathway, GCH1/BH4 antioxidant pathway, lipid peroxidation and the production of reactive oxygen species.
Iron Metabolism
Iron is one of the most basic life-sustaining elements and is involved in many physiological processes, such as oxygen transport, cell respiration, DNA synthesis, myelination and neurotransmitter biosynthesis. Hemoglobin in the blood contains iron, which carried by red blood cells and carries oxygen through the blood circulation to different tissues to maintain the body’s blood oxygen supply (Martínková et al., 2013). Most of the iron in the human body is mainly stored in hemoglobin and myosin, the rest combined with iron storage proteins, such as ferritin, transferrin, cytochrome, etc. There is no doubt that this is necessary for normal cell and tissue physiology.
Under normal conditions, iron exists in the form of Fe2+ and Fe3+. However, during the intracellular iron cycle, iron binds to transferrin (TF) in the form of Fe3+, is absorbed into cells through transferrin receptor 1 (TFR1) and stored in endosomes. In the endosomes, Fe3+ is transformed into Fe2+ by six-transmembrane epithelial antigen of prostate 3(STEAP3) (Yan and Zhang, 2019). Then Fe2+ is transported from endosomes to cytosol via divalent metal transporter 1 (DMT1) (Xie et al., 2016). After that, some Fe2+ is stored in ferritin heavy chain 1(FTH1) and ferritin light chain (FLC) of iron storage protein complex, while others are transported out of cells through ferroportin1(FPN1). FPN1 is the only known protein that controls iron output in mammalian cells, and plays a critical role in the transport of Fe2+ and maintenance of intracellular iron balance (Trujillo-Alonso et al., 2019). However, based on this regulated mechanism of precise intracellular input-transport-storage or exocytosis, any process of iron uptake, transportation, storage or utilization will cause abnormal metabolism of intracellular iron ions, resulting in abnormalities in the Fe2+ metabolic chain, and then may lead to Fenton reaction to generate ROS (Xu et al., 2020). Then, ROS subsequently modifies and interferes with proteins, lipids and DNA, and finally induces the termination of cell life (Singh et al., 2014; Bogdan et al., 2016). It is worth noting that the release of mitochondrial ROS caused by mitochondrial damage also aggravates this process and promotes ferroptosis (Wu et al., 2021).
Glutathione/Glutathione Peroxidase 4 Antioxidant Pathway
Cells transport substances to the cells for digestion, absorption and uptake of nutrients to maintain biochemical reactions. Nutrients such as sugar, fat and amino acids cannot directly enter cells, but they must transported through specific transporters. System Xc−, a cysteine/glutamate reverse transporter widely distributed in phospholipid bilayer, is one of these transporters. System Xc− is a disulfide heterodimer composed of light chain subunit (SLC7A11) and heavy chain subunit (SLC3A2). It plays an important role in the upstream events of ferroptosis and is responsible for 1:1 input of cystine and excretion of glutamate (Dixon et al., 2012). Cystine transported into the cell and reduced to cysteine. In addition to relying on System Xc−, cysteine can also enter cells through sulfur-transfer pathway. Cysteine entering cells through these two pathways and becomes the raw material for the synthesis of glutathione (GSH) and participates in the synthesis of GSH (Xu W. et al., 2021). GSH is the core substance of amino acid metabolism in the process of ferroptosis (Friedmann Angeli et al., 2014; Yang et al., 2014), which is an important antioxidant to protect cells from oxidative damage and is also the substrate of GPX4 lipid repair function. In the process of ferroptosis, GPX4 as the central regulator is an important enzyme that scavenge anaerobic free radicals from lipids (Yang et al., 2014). Once activated, GPX4 catalyzes toxic lipid hydroperoxides (L-OOH) into non-toxic lipid alcohols (L-OH) (Ursini et al., 1982). Under this catalysis, GPX4 uses two molecules of GSH as substrate to produce one molecule of oxidized glutathione (GSSG). GSSG reduced to GSH by GSH reductase in a NADPH dependent manner. Overexpression of GPX4 can inhibit ROS production and lipid peroxidation (Koppula et al., 2018), while the decrease of GPX4 activity or expression will lead to the accumulation of intracellular lipid peroxide, resulting in ferroptosis (Kim et al., 2021). Thus, the expression of GPX4 may represent the process of ferroptosis to some extent. It is worth mentioning that some studies have shown that knockout of GPX4 can also cause cell death and tissue damage by means of necrosis (Canli et al., 2016), pyroptosis (Kang et al., 2018) or apoptosis (Ran et al., 2007). Therefore, it has important pathological significance to continue to explore the role and mechanism of GPX4.
Ferroptosis Suppressor Protein 1/CoQ10 Antioxidant Pathway
Initially, the GSH/GPX4 pathway was thought to be the only ferroptosis inhibiting pathway, but later it was found that there is another parallel inhibitory pathway: in the absence of GPX4, ferroptosis suppressor protein 1 (FSP1) adequately counteracts lethal lipid peroxidation and ferroptosis (Bersuker et al., 2019; Doll et al., 2019). FSP1 is a member of the type II NADH: quinone oxidoreductase (NDH-2) family. Thus, its intrinsic role is to reduce CoQ10 using NADH (Elguindy and Nakamaru-Ogiso, 2015). During this resistance to ferroptosis, FSP1 terminates lipid autoxidation by reducing ubiquinone to ubiquinol, which in turn can directly reduce lipid free radicals. In addition, it prevents lipid peroxidation and ferroptosis by regenerating oxidized α-tocopherol radicals, the most powerful natural chain-breaking antioxidants in lipids, into non-radical forms.
GCH1/BH4 Antioxidant Pathway
As research on ferroptosis progresses more rapidly, another highly effective endogenous ferroptosis inhibitor, GTP Cyclohydrolase 1 (GCH1), was reported by Kraft in 2020 (Kraft et al., 2020). GCH1 is the rate-limiting enzyme for the synthesis of the antioxidant tetrahydrobiopterin (BH4). Like FSP1/CoQ10 axis, GCH1/BH4 pathway also blocks ferroptosis in a GPX4-independent form. In this process, BH4 needs to regenerated by reducing BH2 by dihydrofolate reductase (DHFR). What’s more, BH4 converts phenylalanine to tyrosine, which in turn is converted to 4-OH-benzoate (a precursor of CoQ10) to promote the synthesis of CoQ10, thereby inhibiting ferroptosis.
Production of Reactive Oxygen Species and Lipid Peroxidation
ROS is a by-product of cellular oxygen metabolism, which is responsible for maintaining the stability of the body and participating in the signal transduction of cell normal metabolism. ROS include peroxide (H2O2、ROOH) superoxide (O2−) singlet oxygen (1O2) and free radicals (HO−, HO2−, R−, NO−and NO2−). Under pathological conditions, excessive ROS can destroy all types of cellular components, such as nucleic acids, proteins and lipids, resulting in cell death (Imlay, 2013). Most ferroptosis related ROS come from Fenton reaction and Haber Weiss reaction (Shen et al., 2018), then ROS interacts with polyunsaturated fatty acids (PUFAs) on lipid membrane to form lipid ROS. When a large amount of lipid ROS accumulates in cells, it will cause ferroptosis (Florean et al., 2019).
Lipid peroxidation refers to the lipid oxidative degradation reaction in which lipids lose hydrogen atoms under the action of free radicals or lipid peroxidase, resulting in the oxidation, fracture and shortening of lipid carbon chain, and produce cytotoxic substances resulting in cell damage (Ayala et al., 2014). More and more studies have shown that lipid peroxide is a key mediator of many pathological states, including inflammation (Kang and Yang, 2020), cancer (Rodríguez-García et al., 2020) and neurodegenerative diseases (Zhao et al., 2021). Among them, lipid peroxides have toxic effects on cells mainly through two mechanisms: at the molecular level, lipid peroxides are further decomposed into active substances, which consume nucleic acids and proteins, leading to ferroptosis; at the structural level, lipid peroxidation leads to thinning and increased bending of the biofilm, which ultimately leads to membrane instability and micellar formation (Gaschler and Stockwell, 2017).
Before lipid peroxidation, activated long-chain acyl CoA synthase 4 (ACSl4) induced esterification of free PUFAs and incorporated into membrane phospholipids with the assistance of lysophosphatidylcholine acyltransferase 3 (LPCAT3). Therefore, the upregulation of ACSL4 generally regarded as a sign of ferroptosis. In this process, the level and location of intracellular PUFAs determine the degree of lipid peroxidation (Liu et al., 2021). It is worth noting that free PUFAs, such as arachidonic acid (AA) and adrenaline (AdA), must be esterified into phospholipids (PLs) for peroxidation (Sato et al., 1999). Subsequently, lipoxygenases (LOXs), such as cyclooxygenases (COXs) and cytochrome P450 enzymes (CYPs), catalyze PL-PUFAs to form lipid hydroperoxides (Doll and Conrad, 2017; Lei et al., 2019). As mentioned above, GPX4 reduces PL-PUFAs lipid peroxides (L-OOH) to lipid alcohols (L-OH). Excess iron ions in the cytoplasm trigger free radical induced lipid hydroperoxides that damage cells. In addition, these free radicals can transfer protons, leading to a new wave of lipid peroxidation with positive feedback.
Heat Shock Proteins and Ferroptosis
Molecular chaperones are a class of evolutionarily conserved proteins in the cytoplasm. They recognize and bind incompletely folded or assembled proteins to help them fold, transport or prevent their aggregation. However, molecular chaperones themselves do not participate in the formation of final products (Hoter et al., 2018). In cell homeostasis, molecular chaperones regulate the activity and interaction of mature proteins by assisting in the proper folding and assembly of newborn polypeptide chains, guide damaged or short-lived proteins into the degradation pathway. All these chaperones work together to maintain the mass and weight balance of protein and avoid misfolding and/or aggregation of client proteins.
Based on the naming method specified by HUGO Gene Nomenclature Committee, the HSP family divided into five subfamilies according to molecular weight: HSP110 (HSPH), HSP90 (HSPC), HSP70 (HSPA), HSP40 (DNAJ) and small HSP (HSPB). Although, the latest nomenclature contains heat shock 70 kDa proteins, DNAJ (HSP40) heat shock proteins, small heat shock proteins, heat shock 90 kDa proteins, and chaperonins.
Based on different molecular weights, different types of HSPs also have great differences in configuration. For example, the macromolecule HSP104 forms a hexameric ring and promotes unfolding through a ratchet mechanism. HSP90 forms a multi domain V-shaped structure, and its scissors like movement helps to refine the receptor protein. HSP70 and small HSP use modular “clamps” to protect the extended hydrophobic structure in the target protein. HSP60 adopts barrel “Anfinsen” cage structure for isolation and folding of target protein (Bascos and Landry, 2019).
Ferroptosis is an oxidative stress-dependent regulation of cell death related to iron accumulation and lipid peroxidation. Since it named in 2012, there are still many unexplored mechanisms (Dixon et al., 2012). Members of the HSP family play an important role in the occurrence and development of ferroptosis. However, there are few relevant reviews on the interaction between molecular chaperones and ferroptosis. In this paper, the existing research results on molecular chaperones in ferroptosis reviewed to provide clues for further revealing the unknown mechanism of ferroptosis and to clarify the potential role of molecular chaperones in ferroptosis.
HSP90 (HSPC) and Ferroptosis
HSP90 family is one of the most widely studied molecular chaperones of HSP family. It is also one of the most abundant proteins in cells, accounting for 1–2% of cellular proteins and 4–6% in stressed cells (Picard, 2002; Taipale et al., 2010).HSP90 family include cytoplasmic HSP90α and HSP90β, endoplasmic reticulum glucose regulatory protein 94 (GRP94) and mitochondrial TNF receptor associated protein 1 (TRAP1) (DeZwaan and Freeman, 2010). HSP90 have conserved domains in the process of evolution, including an amino terminal domain (NTD) and a carboxyl terminal domain (CTD) (Csermely et al., 1998). The evolutionary conservation and expression universality reflect the basic and necessary role of HSP90 in cell physiology.
As ATPase, ATP binding and hydrolysis are the key characteristics of HSP90’s activity on client proteins. In addition to the hydrolysis of ATP, the function of HSP90 also regulated by its interacting co-chaperones (Sreedhar et al., 2004; Zhao et al., 2005; Prodromou, 2016). In addition, the role of HSP90 is also essential in genomic DNA. In mammalian cells, HSP90 enhances the enzymatic activity of histone methyltransferase SMYD3, which is involved in RNA polymerase II-mediated transcriptional regulation (Hamamoto et al., 2004). Moreover, Drosophila HSP90 also participates in the interaction with chromatin modifying enzyme trithorax protein, so as to participate in gene regulation (Tariq et al., 2009).
Compared with other HSPs, HSP90 has received more attention as a promising anticancer drug target because of its clear importance in regulating the stability and activity of many proteins involved in human cancer. It is now clear that siRNA interference with HSP90 can protect mouse nerve cells from the toxic effect of ferroptosis (Wu et al., 2019). Besides, necrotic apoptosis and ferroptosis are two different ways of necrotic cell death, there is no common regulatory mechanism. Studies have shown that HSP90 plays a complex role in necrotic apoptosis by binding and regulating the activity of RIPK1, RIPK3 or MLKL in a strictly cell environmental-dependent manner (Li et al., 2015; Jacobsen et al., 2016). However, recent studies have found that chaperone mediated autophagy (CMA) can be used as a common regulatory point to regulate necrotic apoptosis and ferroptosis (Wu et al., 2019). CMA degrades its substrate GPX4 by interacting with Lamp-2a and GPX4-HSC70-HSP90 trimers located in lysosomes. Inhibition of CMA can stabilize GPX4 and reduce ferroptosis. Furthermore, in IRI or folate-induced acute kidney injury (AKI) models, HSC70-HSP90-GPX4 interacts with legumain in the kidney to promote chaperone mediated degradation of GPX4, thereby promoting renal tubular cell ferroptosis in AKI (Chen C. A. et al., 2021).
Therefore, based on the existing scientific research results, HSP90 family may act on GPX4, inhibit the antioxidant capacity of GPX4 by inhibiting its activity (Zhou et al., 2021), then participate in the regulation of ferroptosis through GSH/GPX4 pathway and inhibite lipid peroxidation, therefore influencing ferroptosis.
HSP70 (HSPA) and Ferroptosis
The molecular chaperones of HSP70 family exist in different forms, including cytoplasmic HSP73 (also named HSC70), cytoplasmic inducible HSP72, mitochondrial HSP75/lethal protein mortalin and endoplasmic reticulum HSP78/BIP. Other variants include HSP72 and HSP70i (Kim et al., 2020).
The neuroprotective properties of HSP70 have been widely studied. In the ischemic stroke model, HSP70 plays an endogenous protective mechanism in the penumbra of the hippocampus (States et al., 1996; Weinstein et al., 2004). And HSP70 can protect cells from apoptosis, necrosis and other types of cell death (Frebel and Wiese, 2006). It is reported that HSP70 seems to improve nerve injury by blocking cell death and immune response. Besides, HSP70 is also involved in the regulation of inflammatory pathways (Kim J. Y. et al., 2013). Therefore, more and more drugs take HSP70 as a potential therapeutic target for nervous system diseases.
HSPA5, also known as GRP78 or Bip, is an important member of HSP70 family. As a receptor of endoplasmic reticulum stress, HSPA5 is responsible for regulating the folding and transport of unfolded proteins in endoplasmic reticulum, so as to maintain the homeostasis. In human pancreatic ductal adenocarcinoma cells (PDAC), HSPA5 negatively regulates ferroptosis of PDAC cells through HSPA5-GPX4 signaling pathway, and mediates resistance to ferroptosis (Zhu et al., 2017). Similarly, dihydroartemisinin (DHA) can induce ferroptosis in glioma cells. This is because DHA causes endoplasmic reticulum stress in glioma cells, which up regulates the expression of activated transcription factor 4 (ATF4) and induces the expression of HSPA5 by activating protein kinase R-like endoplasmic reticulum kinase (PERK). The upregulation of HSPA5 increases the expression and activity of GPX4, GPX4 protects glioma cells from ferroptosis by neutralizing DHA induced lipid peroxidation (Chen et al., 2019).
Thus, based on the existing research status, HSP70 family members may enhance cell resistance to ferroptosis by promoting GPX4 expression and its antioxidant activity, inhibiting the production of lipid ROS.
HSP40 (DNAJ) and Ferroptosis
In all known protein homeostasis processes, HSP70 exerts chaperone activity assisted by two types of well-defined and essential chaperones: HSP40 family and nucleotide exchange factors (NEFs) (Hartl and Hayer-Hartl, 2009). Therefore, to some extent, HSP40 and HSP70 functions are inseparable. In fact, HSP40 protein determines the activity of HSP70 by stabilizing the interaction with substrate proteins. Functionally conserved HSP40 has a J-domain that interacts with HSP70 and then referred to as a J-domain-containing protein. This domain is characterized by its conserved histidine, proline and aspartate residues (Qiu et al., 2006). The binding of J domain to HSP70 enhances the ATPase activity of HSP70, thereby regulating protein folding, translation and translocation.
Based on the unique structure and precise function of HSP40, it has been shown to be highly relevant to the development of cancer. Interestingly, HSP40 family proteins have dual properties and play different roles in anticancer and cancer promotion. For example, studies on lung cancer specimens have shown that HSP40 is highly expressed in cancerous lung tissues, detection of HSP40 level in serum of tumor patients with anti-HSP antibodies can be used for tumor diagnosis (Oka et al., 2001). On the contrary, DNAJB4 (also known as HLJ1) is a tumor suppressor that can inhibit the proliferation and invasion of lung cancer cells. High DNAJB4 levels can slow down lung cancer cell cycle progression through the STAT1/P21 pathway (Wang et al., 2005; Zhang et al., 2011).
At present, there are few studies on the relationship between HSP40 family and ferroptosis. It is of pioneering significance to explore the regulation and occurrence of HSP40 family members and ferroptosis. Studies have confirmed that in esophageal squamous adenocarcinoma, overexpression of DNAJB6 enhances the degradation of GSH, down regulates GPX4, enhances lipid peroxidation and promotes ferroptosis. This confirmed the adverse role of HSP40 family members in regulating ferroptosis (Jiang et al., 2020).
Similarly, based on previous studies, we predict that, like DNAJB6, other HSP40 family members may promote the occurrence of ferroptosis by inhibiting GSH/GPX4 and enhancing lipid peroxidation. So, it may be of great significance to continue to explore the relationship between HSP40 family members and ferroptosis.
Small Heat Shock Protein and Ferroptosis
sHSP is usually defined as an ATP independent HSP with a subunit molecular weight of 12–43 kDa. Members of the sHSP family possess a homologous core domain, a highly conserved α-crystalline domain (ACD) consisting of 80–100 amino acids (Kriehuber et al., 2010). In the sHSP family, HO-1(HSP32), HSPB1 (HSP27), HSPB4 (αA crystal protein) and HSPB5 (αB crystal protein) have been studied and reported the most. sHSP is usually present in oligomeric complexes involving one or more family members and thus may provide cells with molecular chaperone-specific diversity. This is because the most striking feature of sHSP is its oligomerization, in which sHSP adjusts its quaternary structure through the assembly of monomers or dimers (Benesch et al., 2008). They can interact with themselves or other sHSPs to form homo or hetero oligomers containing up to 50 subunits (Garrido et al., 2012). This dynamic oligomerization process can cause rapid subunit exchange. The assembly of different substructures affects the exposure of hydrophobic surfaces, thus providing a molecular mechanism to regulate their binding activity.
Ferroptosis is a process dependent on both iron and ROS. After oxidative stress, cells respond through the inherent adaptive defense mechanism to restore healthy cell redox homeostasis. One mechanism involves the haem oxygenase enzyme system, especially its inducible isomer HO-1, a member of sHSP family. This is a kind of cell protective, anti-inflammatory and antioxidant enzyme. HO-1 expression was up-regulated in cancer cells when ferroptosis occurs (Kwon et al., 2015). For example, in human renal tubular epithelial cells, HO-1 has an important anti-ferroptosis effect. Its mechanism may be that HO-1 protects AKI from ferroptosis by promoting GSH depletion (Zhang et al., 2009; Adedoyin et al., 2018). In addition, in the pathogenesis of adriamycin induced myocardial toxicity and ischemia-reperfusion mediated ferroptosis in cardiomyopathy, Nrf2/HO-1 axis promotes the release of free iron, and excessive free iron accumulates in mitochondria, resulting in lipid peroxidation on mitochondrial membrane, mitochondrial dysfunction, and then aggravate ferroptosis (Fang et al., 2019).
HSPB1, a member of sHSP, also plays an integral role in ferroptosis. Previously, HSPB1 was considered to be a negative regulator of iron accumulation and uptake in fibroblasts or cardiac cells (Chen et al., 2006; Zhang et al., 2010). Sun et al. found that HSPB1 overexpression also inhibited erastin-induced iron uptake, while down regulating HSPB1 expression increased erastin-induced iron uptake in cancer cells (Sun et al., 2015). Therefore, when cancer cells contain abnormally elevated iron and HSPB1, if HSPB1 pathway is down regulated, cells may be prone to ferroptosis (Oesterreich et al., 1993; Torti and Torti, 2013). Inhibition of HSF1-HSPB1-PRKC pathway promotes ferroptosis in cancer cells induced by erastin (Sun et al., 2015). Besides, proteomic analysis of animal models of depression found that, the levels of alpha crystallin B (Cryab) and brain-derived neurotrophic factor (BDNF) of sHSP family members decreased, can explain the loss of some neurons during ferroptosis (Cao et al., 2021). However, the mechanism involved is unclear.
Based on the relationship and discovery of existing sHSP family members in ferroptosis, it can regulate ferroptosis through iron metabolism and GSH/GPX4 pathway. This regulation may be beneficial, such as HO-1 and HSPB1; it may also be harmful, such as Nrf2/HO-1 pathway. Therefore, in-depth study of their relationship may have some enlightenment for the research progress of ferroptosis.
Ubiquitin and Ferroptosis
Ubiquitin is a small protein with a molecular weight of 8.5 kDa and 76 amino acids. It is highly conformed in eukaryotes and named by its widespread expression in cells. There are eight amino acid sites of ubiquitin itself (Met1, Lys6, Lys11, Lys27, Lys29, Lys33, Lys48, Lys63), which can be modified by other ubiquitin molecules to form eight poly polymerized ubiquitin chains. Ubiquitin modification system is one of the most common post-translational modification (PTM) systems. It serves as a label or signal to determine the fate and/or function of its labeled substrate protein, and has complex and important physiological functions.
The process of ubiquitin modification of target protein depends on the cascade reaction catalyzed by three kinds of ubiquitin enzymes, namely, ubiquitin activase (E1) binding enzyme (E2) and ligase (E3). Firstly, E1 mediates ATP-dependent activation of ubiquitin to initiate a reaction in which E1 and the 76th amino acid glycine carboxyl group of ubiquitin converted to thiocyanates. Secondly, E2 enzyme acts on the chemical bond for thioesterification, coupling ubiquitin to cysteine residues in E2. Finally, ubiquitin attached to the lysine residue of the target protein by E3. Among them, the specificity of E3 enzyme determines the specificity of ubiquitinated substrate (Hershko and Ciechanover, 1998; Pickart and Eddins, 2004). Single ubiquitin protein can continue to link ubiquitin protein to form polymerized ubiquitin chain. The specificity of ubiquitin chain type determined by E3 enzymes and some specific E2 enzymes (Stewart et al., 2016; Swatek and Komander, 2016).
Unlike other HSPs, ubiquitin exhibits certain uniqueness in functional roles, such as protein degradation pathways (Dikic et al., 2006). As a unique member of the HSP family, ubiquitin closely related to ferroptosis. For example, ubiquitin specific protease 35(USP35) belongs to a family of de-ubiquitinase related to cell proliferation and mitosis. In lung cancer cells, USP35 interacts directly with FPN1 to maintain its protein stability and prevent iron overload and ferroptosis (Tang Z. et al., 2021). Down regulation of USP35 promotes ferroptosis and inhibits cell growth, colony formation and tumor progression. Therefore, it is a promising therapeutic target for lung cancer. 6-Gingerol improves autophagy-dependent-ferroptosis by inhibiting the expression of USP14 and increasing the contents of ROS and Fe2+, which verified the protective effect of USP14 on ferroptosis (Tsai et al., 2020). In addition, overexpression of USP22 can reduce ferroptosis, accompanied by an increase in GSH and a decrease in ROS production, lipid peroxidation and iron accumulation (Ma et al., 2020). In I/R treated rat hearts, USP7, p53 and TfR1 formed a unique pathway of USP7/p53/TfR1. Inhibition of USP7 can inhibit the ubiquitination process and then activate p53, resulting in the down-regulation of TfR1, accompanied by the reduction of ferroptosis and myocardial injury (Tang L.-J. et al., 2021).
In addition to the above, different members of the E3 ligase family also play important roles in regulating ferroptosis. TRIM26 is an E3 ubiquitin ligase, which plays a role as a tumor suppressor in hepatocellular carcinoma. TRIM26 can inhibit liver fibrosis by mediating the ubiquitination of SLC7A11 and promoting the ferroptosis of hepatic stellate cells (HSCs), which can be used as a new therapeutic strategy for liver fibrosis (Zhu et al., 2021). Similarly, E3 ubiquitin ligase S-phase kinase-associated protein 2(SKP2) also plays a role in ferroptosis: YAP (Yes-associated protein 1) is the only analogue of TAZ (a regulator of the Hippo pathway, which regulates ferroptosis in renal and ovarian cancer cells), which promotes the production of ROS by regulating the SKP2 thus aggravates ferroptosis. It provides a new treatment for YAP driven tumors (Yang et al., 2021).Besides, the ubiquitin-E3 ligase TRIM21 interacts with and ubiquitinizes p62, negatively regulating the antioxidant pathway of p62-keap1-Nrf2, thereby exacerbating ferroptosis (Hou et al., 2021). Knockout of TRIM21 protects cardiomyocytes from doxorubicin-induced ferroptosis. However, from the perspective of mechanism, it should be noted that, as a ubiquitin-E3 ligase, TRIM21 can promote proteasome degradation of various proteins, and may play an anti-ferroptosis role through regulating other pathways, one of which may be regulated by the immune system (Hou et al., 2021). Similarly, as a member of the E3 ligase family, neuronal precursor cell expressed developmentally downregulated 4(Nedd4) is an important member of this family. Down-regulation Nedd4 saves erastin-induced elimination of the voltage-dependent anionic channel VDAC2/3 protein, increases the resistance of melanoma cells to erastin (Yang et al., 2020).
To sum up, ubiquitin family proteins closely related to the mechanism of ferroptosis, which may involve many cytokines or mechanisms, including GSH/GPX4 antioxidant pathway and iron metabolism, etc. The continued study of ubiquitin system has prospective significance and broad prospects for mastering the regulatory mechanism of ferroptosis.
Perspectives
In conclusion, after the above review, we summarized and predicted the mechanism of different HSP family members in ferroptosis (Figure 1). It seems that each HSP family has members either promote ferroptosis (such as HSP90 family, HSP40 family, partial members of sHSP family and partial ubiquitin ligases) or inhibit ferroptosis (such as HSP70 family, partial members of sHSP family and some ubiquitin ligases) by targeting the GSH/GPX4 antioxidant pathway. In addition to GSH/GPX4 axis, other antioxidant pathways, such as FSP1/CoQ10 axis and GCH1/BH4 axis, also have great exploration potential. Therefore, future studies can target these antioxidant pathways to explore the association between HSP and ferroptosis.
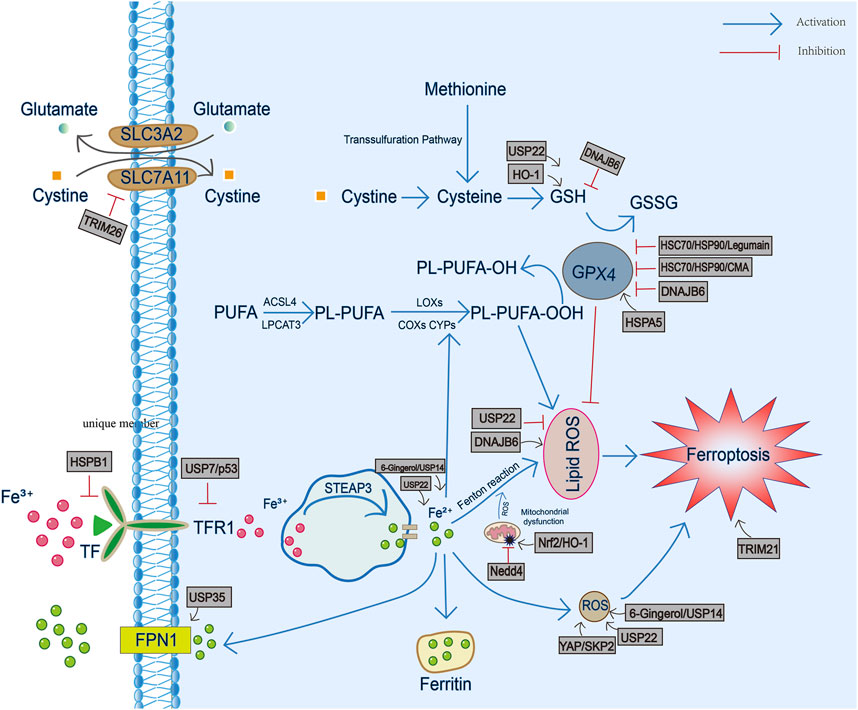
FIGURE 1. The involvement of heat shock proteins in iron metabolism, GSH/GPX4 antioxidant pathway and production of reactive oxygen species and lipid peroxidation during ferroptosis. TF, transferrin; TFR1, transferrin receptor 1; FPN1, ferroportin1; PL-PUFA, phospholipid-bound polyunsaturated fatty acids; STEAP3, six-transmembrane epithelial antigen of prostate 3; ROS, reactive oxygen species; GSH, glutathione; GSSG, oxidized glutathione; GPX4, Glutathione peroxidase 4.
Summary and Prospect
The research on fine regulation mechanism related to ferroptosis is increasing rapidly. However, different HSP family members seem to regulate ferroptosis differently in different diseases/organ microenvironments. In this review, we summarized the effects of HSP on ferroptosis in existing studies. They may participate in the regulation of ferroptosis through iron metabolism, GSH/GPX4 axis, production of ROS and lipid peroxidation. Future research needs to determine the pathophysiological effects of HSP family in ferroptosis, especially in tumorigenesis and neurodegenerative diseases, so as to provide new ideas and strategies for defining the new mechanism of ferroptosis.
Author Contributions
YL and JZ designed and directed the project. YL, LZ, YX, KL, YZ, HQ, QX, and JZ analyzed the data. All authors discussed the results and contributed to the final manuscript. YL and LZ wrote the manuscript.
Funding
This work was supported by National Natural Science Foundation of China (Nos. 82172147, 81571880, 81373147, 30901555, 30972870, 81360080) and Natural Science Foundation of Hunan Province (2021JJ30900, 2016JJ2157), Fundamental Research Funds for the Central Universities of Central South University (2021zzts0938) to LZ. All the funding bodies funded in the study design, collection, analysis, interpretation of data and writing the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adedoyin, O., Boddu, R., Traylor, A., Lever, J. M., Bolisetty, S., George, J. F., et al. (2018). Heme Oxygenase-1 Mitigates Ferroptosis in Renal Proximal Tubule Cells. Am. J. Physiology-Renal Physiol. 314 (5), F702–F714. doi:10.1152/ajprenal.00044.2017
Ayala, A., Muñoz, M. F., and Argüelles, S. (2014). Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid Med. Cel Longev 2014, 360438. doi:10.1155/2014/360438
Ayton, S., Faux, N. G., and Bush, A. I. (2015). Ferritin Levels in the Cerebrospinal Fluid Predict Alzheimer's Disease Outcomes and Are Regulated by APOE. Nat. Commun. 6, 6760. doi:10.1038/ncomms7760
Bascos, N. A. D., and Landry, S. J. (2019). A History of Molecular Chaperone Structures in the Protein Data Bank. Int. J. Mol. Sci. 20 (24), 6195. doi:10.3390/ijms20246195
Battaglia, A. M., Chirillo, R., Aversa, I., Sacco, A., Costanzo, F., and Biamonte, F. (2020). Ferroptosis and Cancer: Mitochondria Meet the "Iron Maiden" Cell Death. Cells 9 (6), 1505. doi:10.3390/cells9061505
Benesch, J. L. P., Ayoub, M., Robinson, C. V., and Aquilina, J. A. (2008). Small Heat Shock Protein Activity Is Regulated by Variable Oligomeric Substructure. J. Biol. Chem. 283 (42), 28513–28517. doi:10.1074/jbc.m804729200
Bersuker, K., Hendricks, J. M., Li, Z., Magtanong, L., Ford, B., Tang, P. H., et al. (2019). The CoQ Oxidoreductase FSP1 Acts Parallel to GPX4 to Inhibit Ferroptosis. Nature 575 (7784), 688–692. doi:10.1038/s41586-019-1705-2
Bogdan, A. R., Miyazawa, M., Hashimoto, K., and Tsuji, Y. (2016). Regulators of Iron Homeostasis: New Players in Metabolism, Cell Death, and Disease. Trends Biochem. Sci. 41 (3), 274–286. doi:10.1016/j.tibs.2015.11.012
Canli, Ö., Alankuş, Y. B., Grootjans, S., Vegi, N., Hültner, L., Hoppe, P. S., et al. (2016). Glutathione Peroxidase 4 Prevents Necroptosis in Mouse Erythroid Precursors. Blood 127 (1), 139–148. doi:10.1182/blood-2015-06-654194
Cao, H., Zuo, C., Huang, Y., Zhu, L., Zhao, J., Yang, Y., et al. (2021). Hippocampal Proteomic Analysis Reveals Activation of Necroptosis and Ferroptosis in a Mouse Model of Chronic Unpredictable Mild Stress-Induced Depression. Behav. Brain Res. 407, 113261. doi:10.1016/j.bbr.2021.113261
Chen, H., Zheng, C., Zhang, Y., Chang, Y.-Z., Qian, Z.-M., and Shen, X. (2006). Heat Shock Protein 27 Downregulates the Transferrin Receptor 1-Mediated Iron Uptake. Int. J. Biochem. Cel Biol 38 (8), 1402–1416. doi:10.1016/j.biocel.2006.02.006
Chen, Y., Mi, Y., Zhang, X., Ma, Q., Song, Y., Zhang, L., et al. (2019). Dihydroartemisinin-Induced Unfolded Protein Response Feedback Attenuates Ferroptosis via PERK/ATF4/HSPA5 Pathway in Glioma Cells. J. Exp. Clin. Cancer Res. 38 (1), 402. doi:10.1186/s13046-019-1413-7
Chen, X., Kang, R., Kroemer, G., and Tang, D. (2021). Broadening Horizons: The Role of Ferroptosis in Cancer. Nat. Rev. Clin. Oncol. 18 (5), 280–296. doi:10.1038/s41571-020-00462-0
Chen, C. A., Wang, D., Yu, Y., Zhao, T., Min, N., Wu, Y., et al. (2021). Legumain Promotes Tubular Ferroptosis by Facilitating Chaperone-Mediated Autophagy of GPX4 in AKI. Cell Death Dis 12 (1), 65. doi:10.1038/s41419-020-03362-4
Csermely, P., Schnaider, T., Soti, C., Prohászka, Z., and Nardai, G. (1998). The 90-kDa Molecular Chaperone Family: Structure, Function, and Clinical Applications. A Comprehensive Review. Pharmacol. Ther. 79 (2), 129–168. doi:10.1016/s0163-7258(98)00013-8
DeHart, D. N., Fang, D., Heslop, K., Li, L., Lemasters, J. J., and Maldonado, E. N. (2018). Opening of Voltage Dependent Anion Channels Promotes Reactive Oxygen Species Generation, Mitochondrial Dysfunction and Cell Death in Cancer Cells. Biochem. Pharmacol. 148, 155–162. doi:10.1016/j.bcp.2017.12.022
DeZwaan, D. C., and Freeman, B. C. (2010). HSP90 Manages the Ends. Trends Biochem. Sci. 35 (7), 384–391. doi:10.1016/j.tibs.2010.02.005
Dikic, I., Crosetto, N., Calatroni, S., and Bernasconi, P. (2006). Targeting Ubiquitin in Cancers. Eur. J. Cancer 42 (18), 3095–3102. doi:10.1016/j.ejca.2006.05.041
Dixon, S. J., Lemberg, K. M., Lamprecht, M. R., Skouta, R., Zaitsev, E. M., Gleason, C. E., et al. (2012). Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 149 (5), 1060–1072. doi:10.1016/j.cell.2012.03.042
Doll, S., and Conrad, M. (2017). Iron and Ferroptosis: A Still Ill‐Defined Liaison. IUBMB Life 69 (6), 423–434. doi:10.1002/iub.1616
Doll, S., Freitas, F. P., Shah, R., Aldrovandi, M., da Silva, M. C., Ingold, I., et al. (2019). FSP1 Is a Glutathione-Independent Ferroptosis Suppressor. Nature 575 (7784), 693–698. doi:10.1038/s41586-019-1707-0
Elguindy, M. M., and Nakamaru-Ogiso, E. (2015). Apoptosis-Inducing Factor (AIF) and its Family Member Protein, AMID, Are Rotenone-Sensitive NADH:Ubiquinone Oxidoreductases (NDH-2). J. Biol. Chem. 290 (34), 20815–20826. doi:10.1074/jbc.m115.641498
Fang, X., Wang, H., Han, D., Xie, E., Yang, X., Wei, J., et al. (2019). Ferroptosis as a Target for protection against Cardiomyopathy. Proc. Natl. Acad. Sci. U.S.A. 116 (7), 2672–2680. doi:10.1073/pnas.1821022116
Florean, C., Song, S., Dicato, M., and Diederich, M. (2019). Redox Biology of Regulated Cell Death in Cancer: A Focus on Necroptosis and Ferroptosis. Free Radic. Biol. Med. 134, 177–189. doi:10.1016/j.freeradbiomed.2019.01.008
Frebel, K., and Wiese, S. (2006). Signalling Molecules Essential for Neuronal Survival and Differentiation. Biochem. Soc. Trans. 34 (Pt 6), 1287–1290. doi:10.1042/BST0341287
Friedmann Angeli, J. P., Schneider, M., Proneth, B., Tyurina, Y. Y., Tyurin, V. A., Hammond, V. J., et al. (2014). Inactivation of the Ferroptosis Regulator Gpx4 Triggers Acute Renal Failure in Mice. Nat. Cel Biol 16 (12), 1180–1191. doi:10.1038/ncb3064
Galluzzi, L., Vitale, I., Aaronson, S. A., Abrams, J. M., Adam, D., Agostinis, P., et al. (2018). Molecular Mechanisms of Cell Death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ 25 (3), 486–541. doi:10.1038/s41418-017-0012-4
Garrido, C., Paul, C., Seigneuric, R., and Kampinga, H. H. (2012). The Small Heat Shock Proteins Family: The Long Forgotten Chaperones. Int. J. Biochem. Cel Biol 44 (10), 1588–1592. doi:10.1016/j.biocel.2012.02.022
Gaschler, M. M., and Stockwell, B. R. (2017). Lipid Peroxidation in Cell Death. Biochem. Biophysical Res. Commun. 482 (3), 419–425. doi:10.1016/j.bbrc.2016.10.086
Hageman, J., and Kampinga, H. H. (2009). Computational Analysis of the Human HSPH/HSPA/DNAJ Family and Cloning of a Human HSPH/HSPA/DNAJ Expression Library. Cell Stress and Chaperones 14 (1), 1–21. doi:10.1007/s12192-008-0060-2
Hamamoto, R., Furukawa, Y., Morita, M., Iimura, Y., Silva, F. P., Li, M., et al. (2004). SMYD3 Encodes a Histone Methyltransferase Involved in the Proliferation of Cancer Cells. Nat. Cel Biol 6 (8), 731–740. doi:10.1038/ncb1151
Hartl, F. U., and Hayer-Hartl, M. (2009). Converging Concepts of Protein Folding In Vitro and In Vivo. Nat. Struct. Mol. Biol. 16 (6), 574–581. doi:10.1038/nsmb.1591
Hershko, A., and Ciechanover, A. (1998). The Ubiquitin System. Annu. Rev. Biochem. 67, 425–479. doi:10.1146/annurev.biochem.67.1.425
Hoter, A., El-Sabban, M. E., and Naim, H. Y. (2018). The HSP90 Family: Structure, Regulation, Function, and Implications in Health and Disease. Int. J. Mol. Sci. 19 (9), 2560. doi:10.3390/ijms19092560
Hou, K., Shen, J., Yan, J., Zhai, C., Zhang, J., Pan, J.-A., et al. (2021). Loss of TRIM21 Alleviates Cardiotoxicity by Suppressing Ferroptosis Induced by the Chemotherapeutic Agent Doxorubicin. EBioMedicine 69, 103456. doi:10.1016/j.ebiom.2021.103456
Imlay, J. A. (2013). The Molecular Mechanisms and Physiological Consequences of Oxidative Stress: Lessons from a Model Bacterium. Nat. Rev. Microbiol. 11 (7), 443–454. doi:10.1038/nrmicro3032
Jacobsen, A. V., Lowes, K. N., Tanzer, M. C., Lucet, I. S., Hildebrand, J. M., Petrie, E. J., et al. (2016). HSP90 Activity Is Required for MLKL Oligomerisation and Membrane Translocation and the Induction of Necroptotic Cell Death. Cel Death Dis 7, e2051. doi:10.1038/cddis.2015.386
Jiang, B., Zhao, Y., Shi, M., Song, L., Wang, Q., Qin, Q., et al. (2020). DNAJB6 Promotes Ferroptosis in Esophageal Squamous Cell Carcinoma. Dig. Dis. Sci. 65 (7), 1999–2008. doi:10.1007/s10620-019-05929-4
Kampinga, H. H., Hageman, J., Vos, M. J., Kubota, H., Tanguay, R. M., Bruford, E. A., et al. (2009). Guidelines for the Nomenclature of the Human Heat Shock Proteins. Cell Stress and Chaperones 14 (1), 105–111. doi:10.1007/s12192-008-0068-7
Kang, Q., and Yang, C. (2020). Oxidative Stress and Diabetic Retinopathy: Molecular Mechanisms, Pathogenetic Role and Therapeutic Implications. Redox Biol. 37, 101799. doi:10.1016/j.redox.2020.101799
Kang, R., Zeng, L., Zhu, S., Xie, Y., Liu, J., Wen, Q., et al. (2018). Lipid Peroxidation Drives Gasdermin D-Mediated Pyroptosis in Lethal Polymicrobial Sepsis. Cell Host Microbe 24 (1), 97–108. doi:10.1016/j.chom.2018.05.009
Kim, Y. E., Hipp, M. S., Bracher, A., Hayer-Hartl, M., and Ulrich Hartl, F. (2013). Molecular Chaperone Functions in Protein Folding and Proteostasis. Annu. Rev. Biochem. 82, 323–355. doi:10.1146/annurev-biochem-060208-092442
Kim, J. Y., Kim, N., Zheng, Z., Lee, J. E., and Yenari, M. A. (2013). The 70 kDa Heat Shock Protein Protects against Experimental Traumatic Brain Injury. Neurobiol. Dis. 58, 289–295. doi:10.1016/j.nbd.2013.06.012
Kim, J. Y., Barua, S., Huang, M. Y., Park, J., Yenari, M. A., and Lee, J. E. (2020). Heat Shock Protein 70 (HSP70) Induction: Chaperonotherapy for Neuroprotection after Brain Injury. Cells 9 (9), 2020. doi:10.3390/cells9092020
Kim, S., Kang, S.-W., Joo, J., Han, S. H., Shin, H., Nam, B. Y., et al. (2021). Characterization of Ferroptosis in Kidney Tubular Cell Death under Diabetic Conditions. Cel Death Dis 12 (2), 160. doi:10.1038/s41419-021-03452-x
Koppula, P., Zhang, Y., Zhuang, L., and Gan, B. (2018). Amino Acid Transporter SLC7A11/xCT at the Crossroads of Regulating Redox Homeostasis and Nutrient Dependency of Cancer. Cancer Commun. 38 (1), 12. doi:10.1186/s40880-018-0288-x
Kraft, V. A. N., Bezjian, C. T., Pfeiffer, S., Ringelstetter, L., Müller, C., Zandkarimi, F., et al. (2020). GTP Cyclohydrolase 1/Tetrahydrobiopterin Counteract Ferroptosis through Lipid Remodeling. ACS Cent. Sci. 6 (1), 41–53. doi:10.1021/acscentsci.9b01063
Kriehuber, T., Rattei, T., Weinmaier, T., Bepperling, A., Haslbeck, M., and Buchner, J. (2010). Independent Evolution of the Core Domain and its Flanking Sequences in Small Heat Shock Proteins. FASEB J. 24 (10), 3633–3642. doi:10.1096/fj.10-156992
Kwon, M.-Y., Park, E., Lee, S.-J., and Chung, S. W. (2015). Heme Oxygenase-1 Accelerates Erastin-Induced Ferroptotic Cell Death. Oncotarget 6 (27), 24393–24403. doi:10.18632/oncotarget.5162
Lei, P., Bai, T., and Sun, Y. (2019). Mechanisms of Ferroptosis and Relations with Regulated Cell Death: A Review. Front. Physiol. 10, 139. doi:10.3389/fphys.2019.00139
Li, D., Xu, T., Cao, Y., Wang, H., Li, L., Chen, S., et al. (2015). A Cytosolic Heat Shock Protein 90 and Cochaperone CDC37 Complex Is Required for RIP3 Activation during Necroptosis. Proc. Natl. Acad. Sci. U.S.A. 112 (16), 5017–5022. doi:10.1073/pnas.1505244112
Liu, Y., Fang, Y., Zhang, Z., Luo, Y., Zhang, A., Lenahan, C., et al. (2021). Ferroptosis: An Emerging Therapeutic Target in Stroke. J. Neurochem. 160 (1), 64–73. doi:10.1111/jnc.15351
Ma, S., Sun, L., Wu, W., Wu, J., Sun, Z., and Ren, J. (2020). USP22 Protects against Myocardial Ischemia-Reperfusion Injury via the SIRT1-p53/SLC7A11-Dependent Inhibition of Ferroptosis-Induced Cardiomyocyte Death. Front. Physiol. 11, 551318. doi:10.3389/fphys.2020.551318
Martínková, M., Kitanishi, K., and Shimizu, T. (2013). Heme-based Globin-Coupled Oxygen Sensors: Linking Oxygen Binding to Functional Regulation of Diguanylate Cyclase, Histidine Kinase, and Methyl-Accepting Chemotaxis. J. Biol. Chem. 288 (39), 27702–27711. doi:10.1074/jbc.r113.473249
Morimoto, R. I., and Cuervo, A. M. (2014). Proteostasis and the Aging Proteome in Health and Disease. J. Gerontol. A. Biol. Sci. Med. Sci. 69 (Suppl. 1), S33–S38. doi:10.1093/gerona/glu049
Oesterreich, S., Weng, C. N., Qiu, M., Hilsenbeck, S. G., Osborne, C. K., and Fuqua, S. A. (1993). The Small Heat Shock Protein Hsp27 Is Correlated with Growth and Drug Resistance in Human Breast Cancer Cell Lines. Cancer Res. 53 (19), 4443–4448.
Oka, M., Sato, S.-i., Soda, H., Fukuda, M., Kawabata, S., Nakatomi, K., et al. (2001). Autoantibody to Heat Shock Protein Hsp40 in Sera of Lung Cancer Patients. Jpn. J. Cancer Res. 92 (3), 316–320. doi:10.1111/j.1349-7006.2001.tb01097.x
Picard, D. (2002). Heat-shock Protein 90, a Chaperone for Folding and Regulation. Ell Mol. Life Sci. 59 (10), 1640–1648. doi:10.1007/pl00012491
Pickart, C. M., and Eddins, M. J. (2004). Ubiquitin: Structures, Functions, Mechanisms. Biochim. Biophys. Acta 1695 (1-3), 55–72. doi:10.1016/j.bbamcr.2004.09.019
Prodromou, C. (2016). Mechanisms of Hsp90 Regulation. Biochem. J. 473 (16), 2439–2452. doi:10.1042/bcj20160005
Qiu, X.-B., Shao, Y.-M., Miao, S., and Wang, L. (2006). The Diversity of the DnaJ/Hsp40 Family, the Crucial Partners for Hsp70 Chaperones. Cell. Mol. Life Sci. 63 (22), 2560–2570. doi:10.1007/s00018-006-6192-6
Ran, Q., Liang, H., Ikeno, Y., Qi, W., Prolla, T. A., Roberts, L. J., et al. (2007). Reduction in Glutathione Peroxidase 4 Increases Life Span through Increased Sensitivity to Apoptosis. J. Gerontol. A. Biol. Sci. Med. Sci. 62 (9), 932–942. doi:10.1093/gerona/62.9.932
Rodríguez-García, A., García-Vicente, R., Morales, M. L., Ortiz-Ruiz, A., Martínez-López, J., and Linares, M. (2020). Protein Carbonylation and Lipid Peroxidation in Hematological Malignancies. Antioxidants (Basel) 9 (12), 1212. doi:10.3390/antiox9121212
Rylander, M. N., Feng, Y., Bass, J., and Diller, K. R. (2005). Thermally Induced Injury and Heat-Shock Protein Expression in Cells and Tissues. Ann. N.Y Acad. Sci. 1066, 222–242. doi:10.1196/annals.1363.009
Sato, H., Tamba, M., Ishii, T., and Bannai, S. (1999). Cloning and Expression of a Plasma Membrane Cystine/Glutamate Exchange Transporter Composed of Two Distinct Proteins. J. Biol. Chem. 274 (17), 11455–11458. doi:10.1074/jbc.274.17.11455
Shen, Z., Liu, T., Li, Y., Lau, J., Yang, Z., Fan, W., et al. (2018). Fenton-Reaction-Acceleratable Magnetic Nanoparticles for Ferroptosis Therapy of Orthotopic Brain Tumors. ACS Nano 12 (11), 11355–11365. doi:10.1021/acsnano.8b06201
Singh, N., Haldar, S., Tripathi, A. K., Horback, K., Wong, J., Sharma, D., et al. (2014). Brain Iron Homeostasis: From Molecular Mechanisms to Clinical Significance and Therapeutic Opportunities. Antioxid. Redox Signal. 20 (8), 1324–1363. doi:10.1089/ars.2012.4931
Sreedhar, A. S., Kalmár, E., Csermely, P., and Shen, Y. F. (2004). Hsp90 Isoforms: Functions, Expression and Clinical Importance. FEBS Lett. 562 (1-3), 11–15. doi:10.1016/s0014-5793(04)00229-7
States, B. A., Honkaniemi, J., Weinstein, P. R., and Sharp, F. R. (1996). DNA Fragmentation and HSP70 Protein Induction in hippocampus and Cortex Occurs in Separate Neurons Following Permanent Middle Cerebral Artery Occlusions. J. Cereb. Blood Flow Metab. 16 (6), 1165–1175. doi:10.1097/00004647-199611000-00011
Stewart, M. D., Ritterhoff, T., Klevit, R. E., and Brzovic, P. S. (2016). E2 Enzymes: More Than Just Middle Men. Cell Res 26 (4), 423–440. doi:10.1038/cr.2016.35
Sun, X., Ou, Z., Xie, M., Kang, R., Fan, Y., Niu, X., et al. (2015). HSPB1 as a Novel Regulator of Ferroptotic Cancer Cell Death. Oncogene 34 (45), 5617–5625. doi:10.1038/onc.2015.32
Swaminathan, S. (2018). Iron Homeostasis Pathways as Therapeutic Targets in Acute Kidney Injury. Nephron 140 (2), 156–159. doi:10.1159/000490808
Swatek, K. N., and Komander, D. (2016). Ubiquitin Modifications. Cel Res 26 (4), 399–422. doi:10.1038/cr.2016.39
Taipale, M., Jarosz, D. F., and Lindquist, S. (2010). HSP90 at the Hub of Protein Homeostasis: Emerging Mechanistic Insights. Nat. Rev. Mol. Cel Biol 11 (7), 515–528. doi:10.1038/nrm2918
Tang, D., Kang, R., Berghe, T. V., Vandenabeele, P., and Kroemer, G. (2019). The Molecular Machinery of Regulated Cell Death. Cel Res 29 (5), 347–364. doi:10.1038/s41422-019-0164-5
Tang, Z., Jiang, W., Mao, M., Zhao, J., Chen, J., and Cheng, N. (2021). Deubiquitinase USP35 Modulates Ferroptosis in Lung Cancer via Targeting Ferroportin. Clin. Transl Med. 11 (4), e390. doi:10.1002/ctm2.390
Tang, L.-J., Zhou, Y.-J., Xiong, X.-M., Li, N.-S., Zhang, J.-J., Luo, X.-J., et al. (2021). Ubiquitin-Specific Protease 7 Promotes Ferroptosis via Activation of the p53/TfR1 Pathway in the Rat Hearts after Ischemia/reperfusion. Free Radic. Biol. Med. 162, 339–352. doi:10.1016/j.freeradbiomed.2020.10.307
Tariq, M., Nussbaumer, U., Chen, Y., Beisel, C., and Paro, R. (2009). Trithorax Requires Hsp90 for Maintenance of Active Chromatin at Sites of Gene Expression. Proc. Natl. Acad. Sci. U.S.A. 106 (4), 1157–1162. doi:10.1073/pnas.0809669106
Thirumalai, D., Lorimer, G. H., and Hyeon, C. (2020). Iterative Annealing Mechanism Explains the Functions of the GroEL and RNA Chaperones. Protein Sci. 29 (2), 360–377. doi:10.1002/pro.3795
Torti, S. V., and Torti, F. M. (2013). Iron and Cancer: More Ore to Be Mined. Nat. Rev. Cancer 13 (5), 342–355. doi:10.1038/nrc3495
Trujillo-Alonso, V., Pratt, E. C., Zong, H., Lara-Martinez, A., Kaittanis, C., Rabie, M. O., et al. (2019). FDA-Approved Ferumoxytol Displays Anti-Leukaemia Efficacy against Cells with Low Ferroportin Levels. Nat. Nanotechnol. 14 (6), 616–622. doi:10.1038/s41565-019-0406-1
Tsai, Y., Xia, C., and Sun, Z. (2020). The Inhibitory Effect of 6-Gingerol on Ubiquitin-Specific Peptidase 14 Enhances Autophagy-Dependent Ferroptosis and Anti-Tumor In Vivo and In Vitro. Front. Pharmacol. 11, 598555. doi:10.3389/fphar.2020.598555
Ursini, F., Maiorino, M., Valente, M., Ferri, L., and Gregolin, C. (1982). Purification from Pig Liver of a Protein Which Protects Liposomes and Biomembranes from Peroxidative Degradation and Exhibits Glutathione Peroxidase Activity on Phosphatidylcholine Hydroperoxides. Biochim. Biophys. Acta 710 (2), 197–211. doi:10.1016/0005-2760(82)90150-3
Wang, C.-C., Tsai, M.-F., Hong, T.-M., Chang, G.-C., Chen, C.-Y., Yang, W.-M., et al. (2005). The Transcriptional Factor YY1 Upregulates the Novel Invasion Suppressor HLJ1 Expression and Inhibits Cancer Cell Invasion. Oncogene 24 (25), 4081–4093. doi:10.1038/sj.onc.1208573
Weinstein, P. R., Hong, S., and Sharp, F. R. (2004). Molecular Identification of the Ischemic Penumbra. Stroke 35 (11 Suppl. 1), 2666–2670. doi:10.1161/01.STR.0000144052.10644.ed
Wu, Z., Geng, Y., Lu, X., Shi, Y., Wu, G., Zhang, M., et al. (2019). Chaperone-Mediated Autophagy Is Involved in the Execution of Ferroptosis. Proc. Natl. Acad. Sci. U.S.A. 116 (8), 2996–3005. doi:10.1073/pnas.1819728116
Wu, H., Wang, F., Ta, N., Zhang, T., and Gao, W. (2021). The Multifaceted Regulation of Mitochondria in Ferroptosis. Life (Basel) 11 (3), 222. doi:10.3390/life11030222
Xie, Y., Hou, W., Song, X., Yu, Y., Huang, J., Sun, X., et al. (2016). Ferroptosis: Process and Function. Cel Death Differ 23 (3), 369–379. doi:10.1038/cdd.2015.158
Xu, Y.-Y., Wan, W.-P., Zhao, S., and Ma, Z.-G. (2020). L-Type Calcium Channels Are Involved in Iron-Induced Neurotoxicity in Primary Cultured Ventral Mesencephalon Neurons of Rats. Neurosci. Bull. 36 (2), 165–173. doi:10.1007/s12264-019-00424-2
Xu, S., He, Y., Lin, L., Chen, P., Chen, M., and Zhang, S. (2021). The Emerging Role of Ferroptosis in Intestinal Disease. Cel Death Dis 12 (4), 289. doi:10.1038/s41419-021-03559-1
Xu, W., Deng, H., Hu, S., Zhang, Y., Zheng, L., Liu, M., et al. (2021). Role of Ferroptosis in Lung Diseases. J. Inflamm. Res. 14, 2079–2090. doi:10.2147/jir.s307081
Yan, N., and Zhang, J.-J. (2019). The Emerging Roles of Ferroptosis in Vascular Cognitive Impairment. Front. Neurosci. 13, 811. doi:10.3389/fnins.2019.00811
Yang, W. S., SriRamaratnam, R., Welsch, M. E., Shimada, K., Skouta, R., Viswanathan, V. S., et al. (2014). Regulation of Ferroptotic Cancer Cell Death by GPX4. Cell 156 (1-2), 317–331. doi:10.1016/j.cell.2013.12.010
Yang, Y., Luo, M., Zhang, K., Zhang, J., Gao, T., Connell, D. O., et al. (2020). Nedd4 Ubiquitylates VDAC2/3 to Suppress Erastin-Induced Ferroptosis in Melanoma. Nat. Commun. 11 (1), 433. doi:10.1038/s41467-020-14324-x
Yang, W.-H., Lin, C.-C., Wu, J., Chao, P.-Y., Chen, K., Chen, P.-H., et al. (2021). The Hippo Pathway Effector YAP Promotes Ferroptosis via the E3 Ligase SKP2. Mol. Cancer Res. 19 (6), 1005–1014. doi:10.1158/1541-7786.mcr-20-0534
Zhang, D., Shen, J., Wang, C., Zhang, X., and Chen, J. (2009). GSH-Dependent iNOS and HO-1 Mediated Apoptosis of Human Jurkat Cells Induced by Nickel(II). Environ. Toxicol. 24 (4), 404–414. doi:10.1002/tox.20440
Zhang, X., Min, X., Li, C., Benjamin, I. J., Qian, B., Zhang, X., et al. (2010). Involvement of Reductive Stress in the Cardiomyopathy in Transgenic Mice with Cardiac-Specific Overexpression of Heat Shock Protein 27. Hypertension 55 (6), 1412–1417. doi:10.1161/hypertensionaha.109.147066
Zhang, L., Cai, X., Chen, K., Wang, Z., Wang, L., Ren, M., et al. (2011). Hepatitis B Virus Protein Up-Regulated HLJ1 Expression via the Transcription Factor YY1 in Human Hepatocarcinoma Cells. Virus. Res. 157 (1), 76–81. doi:10.1016/j.virusres.2011.02.009
Zhao, R., Davey, M., Hsu, Y.-C., Kaplanek, P., Tong, A., Parsons, A. B., et al. (2005). Navigating the Chaperone Network: An Integrative Map of Physical and Genetic Interactions Mediated by the Hsp90 Chaperone. Cell 120 (5), 715–727. doi:10.1016/j.cell.2004.12.024
Zhao, T., Guo, X., and Sun, Y. (2021). Iron Accumulation and Lipid Peroxidation in the Aging Retina: Implication of Ferroptosis in Age-Related Macular Degeneration. Aging Dis. 12 (2), 529–551. doi:10.14336/ad.2020.0912
Zhou, Y., Liao, J., Mei, Z., Liu, X., and Ge, J. (2021). Insight into Crosstalk between Ferroptosis and Necroptosis: Novel Therapeutics in Ischemic Stroke. Oxid Med. Cel Longev 2021, 9991001. doi:10.1155/2021/9991001
Zhu, S., Zhang, Q., Sun, X., Zeh, H. J., Lotze, M. T., Kang, R., et al. (2017). HSPA5 Regulates Ferroptotic Cell Death in Cancer Cells. Cancer Res. 77 (8), 2064–2077. doi:10.1158/0008-5472.can-16-1979
Keywords: ferroptosis, heat shock proteins, ubiquitin, molecular chaperone, GPx4
Citation: Liu Y, Zhou L, Xu Y, Li K, Zhao Y, Qiao H, Xu Q and Zhao J (2022) Heat Shock Proteins and Ferroptosis. Front. Cell Dev. Biol. 10:864635. doi: 10.3389/fcell.2022.864635
Received: 31 January 2022; Accepted: 23 March 2022;
Published: 11 April 2022.
Edited by:
Patrice X. Petit, Centre National de la Recherche Scientifique (CNRS), FranceReviewed by:
Gabriele Multhoff, Technical University of Munich, GermanyPatrick Raymond D'Silva, Indian Institute of Science (IISc), India
Copyright © 2022 Liu, Zhou, Xu, Li, Zhao, Qiao, Xu and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Liu, bGl1MTk3N3lpbmdAMTI2LmNvbQ==; Jie Zhao, c3RlZWx6akBjc3UuZWR1LmNu
†These authors have contributed equally to this work
 Ying Liu
Ying Liu Lin Zhou
Lin Zhou Yunfei Xu
Yunfei Xu Kexin Li
Kexin Li Yao Zhao
Yao Zhao Haoduo Qiao
Haoduo Qiao Qing Xu
Qing Xu Jie Zhao
Jie Zhao