- 1Department of Anatomy and Cellular Biology, Basic Medical Science, Tokai University School of Medicine, Isehara, Japan
- 2Department of Orthopaedic Surgery, Surgical Science, Tokai University School of Medicine, Isehara, Japan
It has been reported that degenerated and herniated lumbar intervertebral discs show high expression of IL-17, suggesting that local immune reactions occur in patients with low back pain. While clinical sample analyses from different laboratories confirm this, it is not deeply not known on how IL-17 is induced in the pathology and their interactions with other inflammatory responses. This conscience review organizes current laboratory findings on this topic and present trajectory for full understanding on the role of IL-17 in pathology of intervertebral disc disease.
Introduction
The intervertebral disc (IVD) consists of an outer fibrocartilaginous annulus fibrosus (AF) that surrounds a gel-like nucleus pulposus (NP). Its main functions are to act as a shock absorber and maintain the backbone mobility, including the cartilaginous endplates that cover this assembly on both the top and bottom sides. The AF is characterised by 15–25 concentric lamellae consisting of fibres of collagen types I and II, with smaller amounts of collagenⅢ, proteoglycans and elastin (Urban and Roberts, 2003; Önnerfjord et al., 2012). A small number of capillaries that penetrate only a few millimetres into the outermost AF (Sakai and Grad, 2015). NP is an avascular tissue. NP is comprised mainly of water along with collagen fibrils (including types VI, IX, and XI), various proteoglycans for shock absorption, and cells of the NP, which are adapted to survive in this hypoxic environment (Urban and Roberts, 2003; Hiyama et al., 2015; Sakai and Grad, 2015). Due to its avascular nature, the nutrients and metabolites are exchanged by diffusion to and from microvessels in the cartilaginous endplates and outer AF (Vo et al., 2016).
With aging, trauma, genetic susceptibility, and other factors, the IVDs gradually degenerate due to many factors, such as microenvironment changes and cell death (Zhang F et al., 2016). During IVD degeneration, the structure of the disc changes and homeostasis in disc become disturbed (Sakai and Grad, 2015). IVD degeneration is linked to low back pain and sciatica, which lead the physical disability of the patients (Urban and Roberts, 2003). Current studies demonstrate that IVD degeneration progresses in consequence of many factors, such as biomolecular damage, inflammatory response, IVD cell loss, extracellular matrix (ECM) degradation increase, and synthesis reduction (Vo et al., 2016; Zhang F et al., 2016).
The frequently reported inflammatory cytokines that are secreted and promote IVD degeneration include tumor necrosis factor-α (TNFα), IL-1 α/β, IL-6, IL-17, IL-8, IL-2, IL-4, IL-10, COX-2, IFN-γ, chemokines, and prostaglandin (PGE)2. Moreover, the factors promoting the extracellular matrix (ECM) degradation often reported are matrix metalloprotease (MMP)-1, -3, -7, -9, and -13, and A disintegrin-like and metalloprotease with thrombospondin type-1 motif (ADAMTS)-1, -4, -5, -9, and -15 (Risbud and Shapiro, 2014; Vo et al., 2016; Willems et al., 2016; Zhang F et al., 2016; Sutovsky et al., 2017). Conversely, TGF-β is essential in maintaining IVD homeostasis (Risbud and Shapiro, 2014; Chen et al., 2019).
Recently, high levels of IL-17A were associated with IVD degeneration (IDD) and IVD herniation (LDH) and IL-17A is considered as the crucial factor in IVD pathology (Shamji et al., 2010; Gabr et al., 2011; Cheng et al., 2013; Suyama et al., 2018). Furthermore, the rat tail suspended or punctured model showed the expression of IL-17A in NP cells and AF cells with tissue degeneration or injury (Han et al., 2015; Wang et al., 2018; Ding and Li, 2020). In this review, based on the current literature, we will focus on the IL-17 signaling pathway, its interaction with other factors, and multiple functions in IVD pathology.
Production of IL-17
IL-17 (IL-17A, CTLA8) cDNA was isolated and cloned from murine hybridomas in 1993 (Rouvier et al., 1993). IL-17 (also called IL-17A) is produced by the T helper 17 (Th17), a subset of CD4+ T cells that are distinct from classic Th1 and Th2 lineages (Gaffen, 2011; Iwakura et al., 2011). These Th17 cells are characterized by the expression of the “master” transcription factor RAR-related orphan receptor gamma (RORγt) and are activated by the IL-12 family cytokine IL-23 (McGeachy et al., 2019). The IL-17A producing CD4+ Th17 cells arise from multiple differentiation triggers, including TGFβ, IL-6, IL-1β, and IL-21 (Gaffen, 2011). Besides IL-17A, IL-17 consists of six other family molecules (IL-17B, IL-17C, IL-17D, IL-17E (IL-25), and IL-17F) with structural identity. IL-17A and IL-17F are closely related linked genes that are usually coproduced by Type 17 cells (Aggarwal and Gurney, 2002).
It was reported that Th17 cells are the major source of IL-17A, and other innate immune cells produce IL-17A in response to pathogens or tissue injury (Cua and Tato, 2010). Overall, IL-17A is produced from Th17 cells and other innate immune cells, inducing various products, including cytokines (IL-6, granulocyte-colony-stimulating factor [G-CSF], TNFα), chemokines (CXCL1, CXCL2, CCL20, among many others), inflammatory effectors (acute-phase proteins, complement), and antimicrobial proteins (defensins, mucins) (Onishi and Gaffen, 2010).
Recently, numerous studies in humans and mice have suggested that IL-17A plays a leading role in the pathogenesis of different immune-mediated diseases, including rheumatoid arthritis, psoriasis, asthma, and inflammatory bowel disease (Cua and Tato, 2010; Onishi and Gaffen, 2010; Gaffen, 2011; Amatya et al., 2017).
Th17 and IL-17A in IVD
In patients with lumbar IDD, the percentage of Th17 that are the main source of IL-17A and IL-17A expression in peripheral blood, demonstrated significant increase (Zhang et al., 2014). Furthermore, in LDH, which NP herniated with AF rupture, compared with the healthy controls, the elevated levels of Th17 lymphocytes and IL-17A correlated with the patients’ pain intensity of sciatica, suggesting that the rupture of the AF and herniation of the NP are initiators of an autoimmune response to a ruptured lumbar disc (Cheng et al., 2013). Interestingly, the Th17 cell frequency and IL-17A concentration positively correlated with the visual analog scale score of low back pain and PGE2 expression levels (Zhang et al., 2014).
Many reports have shown that the cytokines trigger Th17 to produce IL-17A in IVD. The Th17 cells producing IL-17A arise from CD4+ Th17 cells by stimulating cytokines, such as IL-6, and they secrete IL-17A in IDD and LDH pathologies. When Shamji et al. analyzed IDD and LDH patients’ samples, the percentage of CD4+ lymphocytes and CD68+ macrophages were significantly higher in NP of both IDD and LDH compared with healthy IVD, and the expression of IL-4, IL-6, IL-12, IL-17, and IFNγ were significantly higher in NP of LDH compared with IDD; notably, IL-17A was particularly elevated. These findings suggest that Th17 differentiation and activation inducing IL-17A production mediate the inflammatory processes underlying IVD pathology (Shamji et al., 2010). Similarly, high IL-6, IL-17A, and TNFα levels were observed in the serum of lumbar radiculopathy patient group compared with the neuropathic pain group, and Th17 was higher in the venous blood of lumbar radiculopathy patients group compared with the neuropathic pain group (Shamji et al., 2017).
Further, IL-23 (Jiang et al., 2016; Schinocca et al., 2021) and IL-21 (Cua and Tato, 2010) were reported as the cytokines stimulating Th17 to produce IL-17A in IDD or LDH. IL-23 expression was significantly increased in human LDH tissues, and it showed significant positive correlations between IL-23 and IL-17A expression. Therefore, the canonical inflammatory-related signaling IL-23/IL-17A axis may play a critical role in degenerated IVD (Jiang et al., 2016). Moreover, as an illustration of the IL-17A and IL-21 correlation, LDH patients exhibited significantly higher serum IL-21 and IL-17 than healthy controls (Xue et al., 2015).
Th17 and CC Chemokine
There are many studies on the relation between Th17 and chemokines. Th17-related cytokines such as IL-17A, IL-22, and TNFα increased the expression of CC chemokine ligand (CCL) 20 and its only receptor, the CC chemokine ligand-receptor (CCR)6, in human keratinocytes (Harper et al., 2009). Th17 cells predominantly express CCR6 and produce CCL20 as its ligand in rheumatoid arthritis models (Hirota et al., 2007). CCR6 is specifically expressed on the surfaces of Th17 cells, and it is associated with Th17 infiltration (Liu and Rohowsky-Kochan, 2008; Pene et al., 2008). A study of human T cells revealed that CD4+CD45RO+CCR6+ cells contain and secrete much more IL-17A mRNA and more IL-17 protein than CD4+CD45RO+CCR6− cells (Singh et al., 2008).
The studies analyzing CCL20-CC6 in the disc tissues of IVD disease patients reported that IL-17A-producing cells (CD4+IL-17A+ and CD4+CCR6+) appeared in the NP tissues if the AF was ruptured. Furthermore, these studies revealed that NP cells could produce abundant CCL20 and that Th17 associated cytokines (IL-17A and TNFα) can upregulate CCL20 production (Zhang et al., 2013). It was also reported that TNFα stimulation contributes to the gene expression level of CCL20 in IDD cells (Liu et al., 2015; Wang et al., 2020).
In an in vivo study, the expression levels of CCL20, IL-17A, and CCR6 of IVD tissues were dramatically elevated compared with the control groups and needle punctured disc groups in the IVD degenerated model that the needle punctured NP tissue grafted on the nerve root. Furthermore, using ELISA, it was shown that the circulating IL-17A in the serum was elevated in the same models (Zhang Y et al., 2016).
The analysis of the correlation between chemokines and inflammatory cytokine gene expression in humans reported a significant correlation between CCR6 and IL-17A expression both in IVD tissues and blood samples (Singh et al., 2008; Hiyama et al., 2021). These studies indicate that IL-17A-producing cells might participate in the degeneration of disc tissues via an interaction with the CCL20/CCR6 system in vivo.
IL-17A Receptor
The IL-17 receptor family includes five members (IL-17RA to IL-17RE) that have such conserved structural characteristics as extracellular fibronectin III-like domains and cytoplasmic expression similar to that of fibroblast growth factor genes and IL-17Rs, and Toll-IL-1R family (SEFIR) domains (Iwakura et al., 2011; Gu et al., 2013).
IL-17A binds the heterodimeric receptor complex composed of IL-17RA and IL-17RC (Toy et al., 2006; Gu et al., 2013) (Figure 1) The initial event in IL-17A receptor signaling is recruitment of NF-κB activator 1 (Act1), a multifunctional signaling protein that also contains a SEFIR domain necessary for IL-17A receptor-Act1 association (Gu et al., 2013; McGeachy, et al., 2019). Then, IL-17A signaling activates several intracellular pathways or factors. For example, the IL-17A signaling activates Activator protein-1 (AP-1), TNF receptor-associated factor (TRAF)6, NF-κB, Jun N-terminal kinase (JNK), Erk1/2 and p38, CCAAT/enhancer-binding proteins (C/EBPs), Janus kinase (JAK) and phosphoinositol-3 kinase (PI3K). It also induces several proinflammatory cytokines (including IL-1β, IL-6, TNFα, and CCL2), antimicrobial peptides (β-defensin), and matrix metalloproteinases (Huang et al., 2007; Gaffen, 2009; Gaffen, 2011; Amatya et al., 2017; Chen et al., 2019; Schinocca et al., 2021).
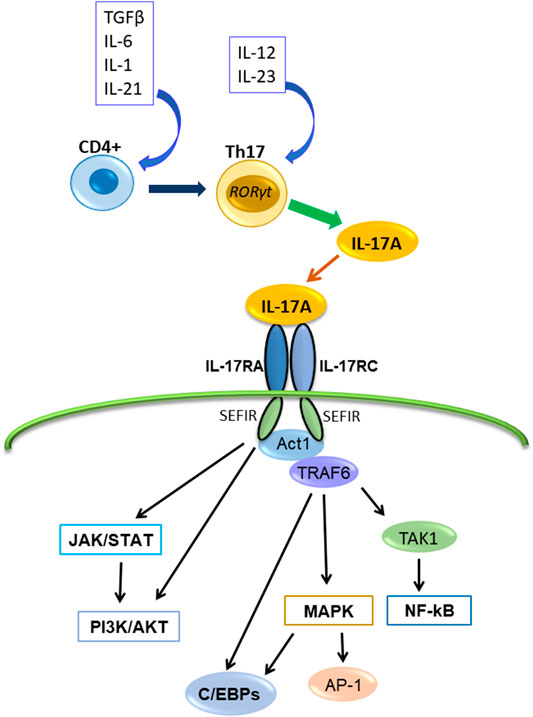
FIGURE 1. The production of IL-17A, its receptor and transduction signaling pathways. IL-17A is produced by Th17cell and binds the receptor composed of IL-17RA and IL-17RC, and interacts with Act1. Subsequently, Act1 activates multiple independent signaling pathways.
IL-17A Receptor of IVD Cell
There are many reports based on the addition of IL-17A to culture NP cells showing that IL-17A affects NP cells directly in vitro (Gabr et al., 2011; Lin et al., 2015; Wang et al., 2015; Hu et al., 2016; Li et al., 2016; Yao et al., 2016; Suyama et al., 2018; Liu et al., 2019; He et al., 2020). In an in vitro study, IL-17A treatment of NP cells isolated from IVD revealed that IL-17A inhibits the proliferation of cells and extracellular matrix synthesis (Lin et al., 2015). Furthermore, treatment with IL-17A and anti-IL-17A neutralizing antibodies caused a significant decrease in the response of IL-6, COX-2, MMP-3, and MMP-13. The small-molecule compounds identified as inhibitors by binding to the IL-17A-binding region of IL-17R by in silico analysis revealed effects similar to the evaluation of the IL-17A-neutralizing antibody (Suyama et al., 2018). According to these studies, NP cells have an IL-17A receptor (IL-17R) on their cell surface, IL-17A could affect intracellular reactions by forming an IL-17A/IL-17R complex, and IL-17A signaling participates in IVD pathology.
IL-17A Signaling
The major downstream pathway of IL-17A signaling is NF-κB pathway (Xie et al., 2010; Gu et al., 2013). NF-κB activator 1 (Act1) contains a SEFIR domain and TRAF6 binding motif (Chang et al., 2006; Li et al., 2007). After IL-17A binding to IL-17R (IL-17RA/IL-17RC), Act1 interacts with IL-17A receptor through the SEFIR domain, and TRAF6 interacts with the TRAF6 binding motif of Act1; subsequently, TRAF6 activates the transforming growth factor β-activated kinase (TAK)1 and the inhibitor of NF-κB kinase (IKK) complex composed of IKKα, IKKβ, and IKKγ, and then, NF-κB activation occurs (Chang et al., 2006; Li et al., 2007; Amatya et al., 2017) (Figure 1). Because it was reported that IL-17A alone is insufficient for strong activation of NF-κB, IL-17A synergizes with other cytokines like TNFα to stimulate NF-κB and enhances the stabilization of proinflammatory cytokine and chemokine mRNA expression (Hartupee et al., 2007; Onishi and Gaffen, 2010; Gu et al., 2013).
Moreover, IL-17A–IL-17R–TRAF6 can promote the activation of the mitogen-activated protein kinase (MAPK) pathway and AP-1 (Walsh et al., 2015). And IL-17A stimulation recruits TRAF4, which binds the same binding site of TRAF6 on Act-1 competitively (Zepp et al., 2012; Gu et al., 2013). Act1-TRAF4 interaction specifically directs the activation of the MEKK3–MEK5–ERK5 cascade and results in MAPK pathway activation (Wu et al., 2015). Additionally, TRAF3 binds directly to the IL-17RA and interferes with IL-17RA-Act1-TRAF6 interaction (Zhu et al., 2010). These reports show that IL-17A activates the canonical NF-κB pathway but not the noncanonical pathway.
NF-κB Pathways
NF-κB signaling pathways play a crucial role in the pathophysiology of IVD degeneration (IDD) (Sakai and Grad, 2015; Zhang G. Z et al., 2021). The activation of the NF-κB pathway releases of inflammatory cytokines, such as TNFα, IL-2, IL-6, INF-γ, and catabolic enzymes, including matrix metalloproteinase (MMP)-3, MMP-9, MMP-13, ADAMTS-4, and ADAMS-5 (Zhang G. Z et al., 2021). These IL-17A-induced factors contribute to the progression of IDD and LDH pathology.
A study analyzing the samples of NP tissue obtained from the IDD patients by the surgical intervention reported that the expression levels of IL-17A and TNFα in the discs of the AF-disrupted group were significantly higher than those in the AF intact group, and the IL-17A and TNFα expression levels were correlated (Wang et al., 2015; Liu et al., 2016). Furthermore, ADMTS-7 expression levels were significantly elevated with a degenerative grade of discs collected from degenerative patients with IVD. Etanercept, an antagonist of TNFα, decreased the expression of ADMTS-7 induced by IL-17A in NP cell culture evaluations (Wang et al., 2015). Yao et al. reported that IL-17A increased the production of MMP-13 and decreased expression of collagen type II alpha 1 (COL2A1) and Aggrecan (ACAN) via the NF-κB pathway in NP cells (Yao et al., 2016). Similarly, peroxisome proliferator-activated receptor γ (PPAR-γ), which inhibits the NF-κB signal pathway, decreased in degenerative IVD tissues compared with nondegenerated tissues, and a PPAR-γ agonist downregulated the production of IL-1β, CCL20, COX-2, PGE-2, MMP-13, and ADAMTS-7 induced by IL-17A along with TNFα in NP cells (Liu et al., 2019). Overall, these studies indicate that IL-17A synergize with TNFα to stimulate the NF-κB signaling pathway and contributes to the production of cytokines, chemokines, and extracellular matrix-degrading factors in IVD (Figure 2).
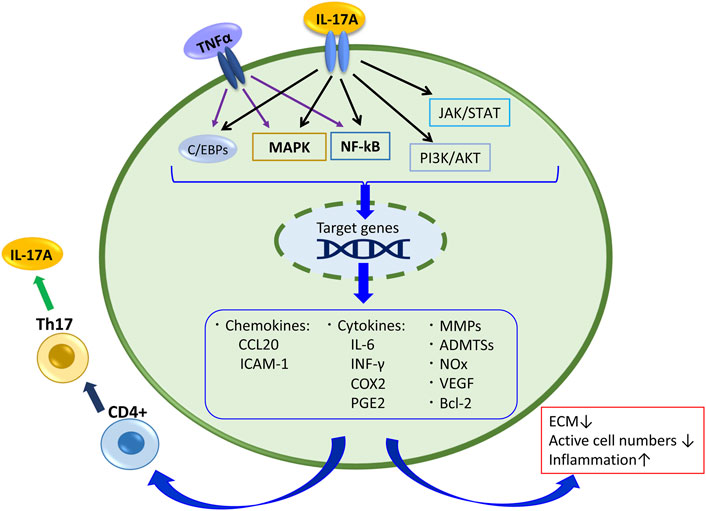
FIGURE 2. IL-17A signaling and its related products in disc cell and tissue. Also,TNFα stimulates the NF-kB and MAPK pathways and C/EBPs, and contributes to the production of cytokines, chemokines, and extracellular matrix-degrading factors in IVD.
MAPK Pathways
The effects of the MAPK pathways, including three major MAPK, p38 kinase, JNK, and extracellular signal-regulated kinase (ERK), play a vital role in IDD (Zhang F et al., 2016; Zhang H. J et al., 2021). IL-17A signaling mediates the MAPK pathway in NP cells, and IL-17A increased COX-2 and PGE2 production by activating the p38/c-Fos and JNK/c-Jun signaling pathways in an AP-1-dependent manner (Li et al., 2016). Similarly, IL-17A increased IL-6 expression via p38 activation in NP cells (Suyama et al., 2018). AP-1 is a downstream transcription factor of the MAPK pathway, and it regulates the expression of several genes, including COX-2 and IL-6, cell proliferation, apoptosis, and inflammation (Shaulian and Karin, 2002; Ye et al., 2014; Yoon and Park, 2021). AP-1 protein is primarily regulated at the level of both Jun and Fos gene transcription involving MAPK pathways and by post-translational modifications via phosphorylation and dephosphorylation, and the complex of c-Jun with c-Fos are the members of the AP-1 family of transcription factors (Shaulian and Karin, 2002; Ye et al., 2014). These reports indicate that MAPKs are the IL-17A-induced signals that cause disc cell degeneration. Additionally, a chemokine N-acetylated proline-glycine-proline (N-Ac-PGP) was reported to induce proinflammatory cytokines, including IL-17A and matrix catabolic enzymes in NP cells via both NF-κB and MAPK signaling pathways (Feng et al., 2017). These observations suggest that both NF-κB and MAPK pathways involve IL-17A transcription and production in NP cells.
Meanwhile, there are different reports about the role of c-Jun and AP-1 for IDD. Lei et al. reported that c-Jun overexpression upregulated the expression levels of TGF-β, TIMP-3, and COL2A1 in the mRNA and proteins, but simultaneously downregulated the expression levels of inflammatory factors IL-1β, IL-17, IL-6, and TNFα both in vivo and in vitro studies (Lei et al., 2020; Lei et al., 2021). The downregulation of IL-17A expression and NP cells decreases IL-17A secretion from NP cells and its effects on IL-17A signaling. Furthermore, a more detailed investigation about the relation of IL-17A-MAPK-c-Jun is required.
CCAAT/Enhancer-Binding Proteins
In addition to the NF-κB and MAPK pathways, IL-17A signaling stimulates the transcription factor CCAAT/enhancer-binding proteins (C/EBPs). IL-17A-IL-17R-Act1-TRAF6 are required for the activation of C/EBPβ and C/EBPδ (Gaffen, 2009; Chang and Ding, 2011; Iwakura et al., 2011). Both NF-κB and C/EBPs transcriptional factor binding sites are over-represented in promoters of IL-17A target genes (Shen et al., 2006). C/EBPs binds to the IL-1β response element in the IL-6 promoter region (Akira et al., 1990), and IL-17A collaborates with TNFα to regulate C/EBPδ (or the related transcription factor C/EBPβ) involved in IL-6 gene expression (Ruddy et al., 2004). On the other hand, a negative regulation of C/EBPs mediated through IL-17RA has been reported. IL-17RA can be mediated through ERK and glycogen synthase kinase 3β (GSK-3β)-dependent mechanisms to phosphorylate C/EBPβ, leading to suppression of the cytokines induced by IL-17RA (Shen, et al., 2009; Chang and Ding, 2011).
With regards to the IVD, both human and rat NP cells expressed C/EBPβ, and C/EBPβ acts as a potent proinflammatory mediator by inducing the TNFα expression levels via the ERK1/2 and p38 pathways in rat NP cells in IDD (Hiyama et al., 2016). These reports show that IL-17A can participate in the various responses with TNFα, IL-6, and IL-1β in IVD pathology.
JAK/STAT and PI3K/AKT Pathways
JAK/STAT and PI3K/AKT pathways are reported as IL-17A mediates and affects several factors in disc cells (Gu et al., 2011; Lee et al., 2013; Hu et al., 2016). IL-17A activates the JAK/STAT pathway by mediating a rapid tyrosine phosphorylation of the JAK family (Tyk 2, JAK 1,2, and 3) and STAT 1, 2, 3, and 4 (Subramaniam et al., 1999). In addition, the activation of JAK by IL-17A induces PI3K/AKT activation (Huang et al., 2007).
A study on IVD revealed that vascular endothelial growth factor (VEGF) and IL-17A are highly expressed in human degenerated NP tissue, and IL-17A upregulated VEGF expression through the JAK/STAT pathway in NP cells (Hu et al., 2016). VEGF contributes to NP cells survival under hypoxic conditions because of the avascular environment of the disc (Fujita et al., 2008). Although under IVD degeneration conditions, some cytokines, including IL-17A, elevated VEGF, which then promoted angiogenesis and vasculogenesis in disc lesions to exacerbate IDD (Lee et al., 2011; Studer et al., 2011; Binch et al., 2014).
With regards to the relation between IL-17A and PI3K/AKT, He et al. reported that IL-17A increases the activation levels of the PI3K/AKT pathway, which then induces the Bcl-2 expression in NP cells, and that led to the suppression of NP cells autophagy (He et al., 2020). In NP tissues, it was reported that autophagy regulates the catabolic effects and apoptosis in inflammatory conditions (Shen et al., 2011; Xu et al., 2015). Therefore, these findings that IL-17A-PI3K/AKT activation inhibits autophagy might lead the progression of IVD degeneration (Shen et al., 2011; Xu et al., 2015).
The Interaction of IL-17A and Other Cytokines in IVD Cells
In the NP cells from IDD patient’s tissue, the production of NOx, PEG2, IL-6, and ICAM-1 was upregulated by the synergy of IL-17A and TNFα or IFNγ (Gabr et al., 2011). Similarly, it was reported that IL-17A interacts with many cytokines, such as TNFα or chemokines in IVD cells (Shamji et al., 2010; Wang et al., 2015; Zhang Y et al., 2016; Liu et al., 2019). As mentioned above, IL-6 is necessary for Th17 differentiation to produce IL-17A, and it was also expressed by IL-17A signaling in IVD cells via MAPK pathways (Suyama et al., 2018). Furthermore, IL-1β was reported to induce IL-17A expression in IDD (Johnson et al., 2015; Wang et al., 2020).
Regarding the cooperation of IL-17A and TNFα in IVD pathology, besides the IL-17A receptor- NF-κB pathway, the TNF receptor TNFR1 and TNFR2 are involved in the IL-17A response. TNFR1 can be activated either by tmTNFα (transmembrane TNFα) or sTNFα (a soluble form), whereas TNFR2 is activated mainly by sTNFα. Under tmTNFα and sTNFα stimulation, the TNFR1/SODD complex releases the inhibitory SODD protein, and TNFR1 becomes activated. Then TNFR1 binds TNF receptor-associated death domain, recruiting other adaptor proteins, including TNF receptor-associated factor 2 (TRAF2), receptor-interacting protein-1 (RIP-1), and cellular inhibitor of apoptosis protein (cIAP) 1, resulting in the formation of complex I that signal through either the NF-κB or MAPK pathways to activate p65 or AP-1 (Johnson et al., 2015; Wang et al., 2020). TNFR2 recruits TRAF3, TRAF2, cIAP1/2, and TRAF1 to establish a complex that also activates NF-κB, AP-1, and ERK, and it activates PI3K/AKT consequently (Wang et al., 2020). This regulation concerns many responses of IL-17A in IVD cells. Further, these pathways regulate the production of pro-inflammatory mediators such as TNFα, IL-1β or IL-6, and these mediators can recruit Th17 cells, which produce IL-17A, again (Figure 2). Indeed, when the factor that inhibits TNFα-TNFR1 signaling was suppressed in murine models of IDD, IL-17A expression was significantly increased by TNFα stimulating the NF-κB and MAPK pathways via TNFα receptors (Wang et al., 2018). TNFα can stimulate IVD cells directly, and it affects IL-17A signaling. Therefore, it results in IDD progression.
Discussion
IL-17A plays an important role in IDD and LDH. IL-17A regulates many factors, including inflammatory cytokines, chemokines, PGE2, and the factors promoting ECM degradation, consequently promoting IVD pathology. However, there are many unknowns in IL-17A about their function, interaction, and gene regulation without immune cells or antagonists. It has yet been unclear how IL-17A is produced from IVD cells, though, it was reported that exposing degenerated disc cells to IL-1β or TNFα stimulates IL-17A production (Yang et al., 2015; Wang et al., 2020). These reports indicate the possibility that several cells can produce IL-17A in disc tissue. Similarly, Risbud and Shapiro suggested that resident disc cells may contribute to the production of lymphokine, including IL-17A (Risbud and Shapiro, 2014) because Shamji et al. showed the prominent IL-17 staining in non-degenerate control tissues (Shamji et al., 2010).
NF-κB and MAPK pathways which IL-17A effected on, regulate pro-inflammatory mediators such as TNF-α, IL-1β or IL-6, as both pathways have been identified as master regulators of inflammation and catabolism in IDD and LDH (Sakai and Grad, 2015). Furthermore, other cytokines or chemokines may be involved in IL-17A functions. Therefore, IL-17A could be used for therapy targets of IVD diseases.
According to current reports, IL-17A receptors are at the surface of NP cells, it indicates that IL-17A inhibitor, such as biologicals or small molecule compounds likely to be efficacious against IDD. These findings show the practical utility of IL-17A inhibitor. The therapy of IL-17A regulation may effect on pro-inflammatory cytokines including TNFα, IL-6, and IL-1β, and may suppress the degradation of ECM. Further, it was suggested the possibility that IL-17A correlates with IVD disease patients’ pain intensity, IL-17A is expected to be a biological marker of curative effects. It suggests the possibility that IL-17A inhibitors can be used as the medication for IDD to suppress the inflammation and the degradation of ECM by improving the IVD specific microenvironment via the related pathways in NP cells. It may lead to the improvement of physical functioning and quality of life of IDD patients. Indeed, as the reports of IL-17A medication in other diseases, it was reported that the treatment with IL-17A blockers improved joint symptoms of psoriatic arthritis compared with placebo (Mease et al., 2017; Nash et al., 2017; Blauvelt and Chiricozzi. 2018).
Additionally, although only IL-17A has been associated with IVD, the IL-17 family contains six members (IL-17A–IL-17F) (Iwakura et al., 2011; McGeachy et al., 2019). Therefore, the involvement of additional members of the IL-17 family in IVD could be reported in the future.
Author Contributions
DS and KS conception and writing, MW suggestion, editing and supervision.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aggarwal, S., and Gurney, A. L. (2002). IL-17: Prototype Member of an Emerging Cytokine Family. J. Leukoc. Biol. 71, 1–8.
Akira, S., Isshiki, H., Sugita, T., Tanabe, O., Kinoshita, S., Nishio, Y., et al. (1990). A Nuclear Factor for IL-6 Expression (NF-IL6) Is a Member of a C/EBP Family. EMBO J. 9, 1897–1906. doi:10.1002/j.1460-2075.1990.tb08316.x
Amatya, N., Garg, A. V., and Gaffen, S. L. (2017). IL-17 Signaling: the Yin and the Yang. Trends Immunol. 38, 310–322. doi:10.1016/j.it.2017.01.006
Blauvelt, A., and Chiricozzi, A. (2018). The Immunologic Role of IL-17 in Psoriasis and Psoriatic Arthritis Pathogenesis. Clinic Rev. Allerg Immunol. 55, 379–390. doi:10.1007/s12016-018-8702-3
Chang, S. H., and Dong, C. (2011). Signaling of Interleukin-17 Family Cytokines in Immunity and Inflammation. Cell Signal. 23, 1069–1075. doi:10.1016/j.cellsig.2010.11.022
Chang, S. H., Park, H., and Dong, C. (2006). Act1 Adaptor Protein Is an Immediate and Essential Signaling Component of Interleukin-17 Receptor. J. Biol. Chem. 281, 35603–35607. doi:10.1074/jbc.c600256200
Chen, S., Liu, S., Ma, K., Zhao, L., Lin, H., and Shao, Z. (2019). TGF-β Signaling in Intervertebral Disc Health and Disease. Osteoarthritis and Cartilage 27, 1109–1117. doi:10.1016/j.joca.2019.05.005
Cheng, L., Fan, W., Liu, B., Wang, X., and Nie, L. (2013). Th17 Lymphocyte Levels Are Higher in Patients with Ruptured Than Non-ruptured Lumbar Discs, and Are Correlated with Pain Intensity. Injury 44, 1805–1810. doi:10.1016/j.injury.2013.04.010
Cua, D. J., and Tato, C. M. (2010). Innate IL-17-producing Cells: the Sentinels of the Immune System. Nat. Rev. Immunol. 10, 479–489. doi:10.1038/nri2800
Ding, F., and Li, X. (2020). Apigenin Mitigates Intervertebral Disc Degeneration through the Amelioration of Tumor Necrosis Factor α (TNF-α) Signaling Pathway. Med. Sci. Monit. 26, e924587. doi:10.12659/MSM.924587
Feng, C., He, J., Zhang, Y., Lan, M., Yang, M., Liu, H., et al. (2017). Collagen-derived N-Acetylated Proline-Glycine-Proline Upregulates the Expression of Pro-inflammatory Cytokines and Extracellular Matrix Proteases in Nucleus Pulposus Cells via the NF-Κb and MAPK Signaling Pathways. Int. J. Mol. Med. 40, 164–174. doi:10.3892/ijmm.2017.3005
Fujita, N., Imai, J., Suzuki, T., Yamada, M., Ninomiya, K., Miyamoto, K., et al. (2008). Vascular Endothelial Growth Factor-A Is a Survival Factor for Nucleus Pulposus Cells in the Intervertebral Disc. Biochem. Biophysical Res. Commun. 372, 367–372. doi:10.1016/j.bbrc.2008.05.044
Gabr, M. A., Jing, L., Helbling, A. R., Sinclair, S. M., Allen, K. D., Shamji, M. F., et al. (2011). Interleukin-17 Synergizes with IFNγ or TNFα to Promote Inflammatory Mediator Release and Intercellular Adhesion Molecule-1 (ICAM-1) Expression in Human Intervertebral Disc Cells. J. Orthop. Res. 29, 1–7. doi:10.1002/jor.21206
Gaffen, S. L. (2011). Recent Advances in the IL-17 Cytokine Family. Curr. Opin. Immunol. 23, 613–619. doi:10.1016/j.coi.2011.07.006
Gaffen, S. L. (2009). Structure and Signalling in the IL-17 Receptor Family. Nat. Rev. Immunol. 9, 556–567. doi:10.1038/nri2586
Gu, C., Wu, L., and Li, X. (2013). IL-17 Family: Cytokines, Receptors and Signaling. Cytokine 64, 477–485. doi:10.1016/j.cyto.2013.07.022
Gu, F.-M., Li, Q.-L., Gao, Q., Jiang, J.-H., Zhu, K., Huang, X.-Y., et al. (2011). IL-17 Induces AKT-dependent IL-6/JAK2/STAT3 Activation and Tumor Progression in Hepatocellular Carcinoma. Mol. Cancer 10, 150. doi:10.1186/1476-4598-10-150
Han, C., Ma, X.-l., Wang, T., Ma, J.-x., Tian, P., Zang, J.-c., et al. (2015). Low Magnitude of Tensile Stress Represses the Inflammatory Response at Intervertebral Disc in Rats. J. Orthop. Surg. Res. 10, 26. doi:10.1186/s13018-015-0159-y
Harper, E. G., Guo, C., Rizzo, H., Lillis, J. V., Kurtz, S. E., Skorcheva, I., et al. (2009). Th17 Cytokines Stimulate CCL20 Expression in Keratinocytes In Vitro and In Vivo: Implications for Psoriasis Pathogenesis. J. Invest. Dermatol. 129, 2175–2183. doi:10.1038/jid.2009.65
Hartupee, J., Liu, C., Novotny, M., Li, X., and Hamilton, T. (2007). IL-17 Enhances Chemokine Gene Expression through mRNA Stabilization. J. Immunol. 179, 4135–4141. doi:10.4049/jimmunol.179.6.4135
He, W.-S., Zou, M.-X., Yan, Y.-G., Yao, N.-Z., Chen, W.-K., Li, Z., et al. (2020). Interleukin-17A Promotes Human Disc Degeneration by Inhibiting Autophagy through the Activation of the Phosphatidylinositol 3-kinase/Akt/Bcl2 Signaling Pathway. World Neurosurg. 143, e215–e223. doi:10.1016/j.wneu.2020.07.117
Hirota, K., Yoshitomi, H., Hashimoto, M., Maeda, S., Teradaira, S., Sugimoto, N., et al. (2007). Preferential Recruitment of CCR6-Expressing Th17 Cells to Inflamed Joints via CCL20 in Rheumatoid Arthritis and its Animal Model. J. Exp. Med. 204, 2803–2812. doi:10.1084/jem.20071397
Hiyama, A., Hiraishi, S., Sakai, D., and Mochida, J. (2016). CCAAT/enhancer Binding Protein β Regulates the Expression of Tumor Necrosis Factor-α in the Nucleus Pulposus Cells. J. Orthop. Res. 34, 865–875. doi:10.1002/jor.23085
Hiyama, A., Suyama, K., Sakai, D., Tanaka, M., and Watanabe, M. (2021). Correlational Analysis of Chemokine and Inflammatory Cytokine Expression in the Intervertebral Disc and Blood in Patients with Lumbar Disc Disease. J. Orthop. Res.
Hiyama, A., Yokoyama, K., Nukaga, T., Sakai, D., and Mochida, J. (2015). Response to Tumor Necrosis Factor-α Mediated Inflammation Involving Activation of Prostaglandin E2 and Wnt Signaling in Nucleus Pulposus Cells. J. Orthop. Res. 33, 1756–1768. doi:10.1002/jor.22959
Hu, B., Wang, J., Wu, X., Chen, Y., Yuan, W., and Chen, H. (2016). Interleukin-17 Upregulates Vascular Endothelial Growth Factor by Activating the JAK/STAT Pathway in Nucleus Pulposus Cells. Jt. Bone Spine 84, 327–334. doi:10.1016/j.jbspin.2016.05.014
Huang, F., Kao, C.-Y., Wachi, S., Thai, P., Ryu, J., and Wu, R. (2007). Requirement for Both JAK-Mediated PI3K Signaling and ACT1/TRAF6/TAK1-dependent NF-Κb Activation by IL-17A in Enhancing Cytokine Expression in Human Airway Epithelial Cells. J. Immunol. 179, 6504–6513. doi:10.4049/jimmunol.179.10.6504
Iwakura, Y., Ishigame, H., Saijo, S., and Nakae, S. (2011). Functional Specialization of Interleukin-17 Family Members. Immunity 34, 149–162. doi:10.1016/j.immuni.2011.02.012
Jiang, H., Deng, Y., Wang, T., Ma, J., Li, P., Tian, P., et al. (2016). Interleukin-23 May Contribute to the Pathogenesis of Lumbar Disc Herniation through the IL-23/IL-17 Pathway. J. Orthop. Surg. Res. 11, 12. doi:10.1186/s13018-016-0343-8
Johnson, Z., Schoepflin, Z. R., Schoepflin, Z., Choi, H., Shapiro, I., and Risbud, M. (2015). Disc in Flames: Roles of TNF-α and IL-1β in Intervertebral Disc Degeneration. eCM 30, 104–117. doi:10.22203/ecm.v030a08
LA Binch, A., Cole, A. A., Breakwell, L. M., Michael, A. L., Chiverton, N., Cross, A. K., et al. (2014). Expression and Regulation of Neurotrophic and Angiogenic Factors during Human Intervertebral Disc Degeneration. Arthritis Res. Ther. 16, 416. doi:10.1186/s13075-014-0416-1
Lee, J. M., Song, J. Y., Baek, M., Jung, H.-Y., Kang, H., Han, I. B., et al. (2011). Interleukin-1β Induces Angiogenesis and Innervation in Human Intervertebral Disc Degeneration. J. Orthop. Res. 29, 265–269. doi:10.1002/jor.21210
Lee, S. Y., Kwok, S. K., Son, H. J., Ryu, J. G., Kim, E. K., Oh, H. J., et al. (2013). IL-17-mediated Bcl-2 Expression Regulates Survival of Fibroblast-like Synoviocytes in Rheumatoid Arthritis through STAT3 Activation. Arthritis Res. Ther. 15, R31. doi:10.1186/ar4179
Lei, M., Wang, K., Li, S., Zhao, K., Hua, W., Wu, X., et al. (2020). The C-Jun Signaling Pathway Has a Protective Effect on Nucleus Pulposus Cells in Patients with Intervertebral Disc Degeneration. Exp. Ther. Med. 20, 123. doi:10.3892/etm.2020.9251
Lei, M., Zhao, K., Hua, W., Wang, K., Li, S., Wu, X., et al. (2021). An In Vivo Study of the Effect of C-Jun on Intervertebral Disc Degeneration in Rats. Bioengineered 12, 4320–4330. doi:10.1080/21655979.2021.1946459
Li, J. K., Nie, L., Zhao, Y. P., Zhang, Y. Q., Wang, X., Wang, S. S., et al. (2016). IL-17 Mediates Inflammatory Reactions via P38/c-Fos and JNK/c-Jun Activation in an AP-1-dependent Manner in Human Nucleus Pulposus Cells. J. Transl. Med. 14, 77. doi:10.1186/s12967-016-0833-9
Lin, X., Lin, Q., and Ye, J.-J. (2015). Role of IL-17 in Nucleus Pulposus Cell Proliferation and Metabolism Cultured In Vitro. Asian Pac. J. Trop. Med. 8, 41–47. doi:10.1016/s1995-7645(14)60185-1
Liu, C., Fei, H. D., Sun, Z. Y., and Tian, J. W. (2015). Bioinformatic Analysis of the Microarray Gene Expression Profile in Degenerative Intervertebral Disc Cells Exposed to TNF-α. Eur. Rev. Med. Pharmacol. Sci. 19, 3332–3339.
Liu, H., and Rohowsky-Kochan, C. (2008). Regulation of IL-17 in Human CCR6+ Effector Memory T Cells. J. Immunol. 180, 7948–7957. doi:10.4049/jimmunol.180.12.7948
Liu, X.-G., Hou, H.-W., and Liu, Y.-L. (2016). Expression Levels of IL-17 and TNF-α in Degenerated Lumbar Intervertebral Discs and Their Correlation. Exp. Ther. Med. 11, 2333–2340. doi:10.3892/etm.2016.3250
Liu, Y., Qu, Y., Liu, L., Zhao, H., Ma, H., Si, M., et al. (2019). PPAR-γ Agonist Pioglitazone Protects against IL-17 Induced Intervertebral Disc Inflammation and Degeneration via Suppression of NF-Κb Signaling Pathway. Int. Immunopharmacology 72, 138–147. doi:10.1016/j.intimp.2019.04.012
McGeachy, M. J., Cua, D. J., and Gaffen, S. L. (2019). The IL-17 Family of Cytokines in Health and Disease. Immunity 50, 892–906. doi:10.1016/j.immuni.2019.03.021
Mease, P. J., van der Heijde, D., Ritchlin, C. T., Okada, M., Cuchacovich, R. S., Shuler, C. L., et al. (2017). Ixekizumab, an interleukin-17A Specific Monoclonal Antibody, for the Treatment of Biologic-Naive Patientswith Active Psoriatic Arthritis: Results from the 24-week Randomised, Double-Blind, Placebo-Controlled and Active (Adalimumab)-controlled Period of the Phase III Trial SPIRIT-P1. Ann. Rheum. Dis. 76, 79–87. doi:10.1136/annrheumdis-2016-209709
Nash, P., Kirkham, B., Okada, M., Rahman, P., Combe, B., Burmester, G. R., et al. (2017). SPIRITP2Study GroupIxekizumab for the Treatment of Patientswith Active Psoriatic Arthritis and an Inadequate Response to Tumournecrosis Factor Inhibitors: Results from the 24-week Randomised,double-Blind, Placebo-Controlled Period of the SPIRIT-P2 Phase 3 Trial. Lancet 389, 2317–2327.
Onishi, R. M., and Gaffen, S. L. (2010). Interleukin-17 and its Target Genes: Mechanisms of Interleukin-17 Function in Disease. Immunology 129, 311–321. doi:10.1111/j.1365-2567.2009.03240.x
Önnerfjord, P., Khabut, A., Reinholt, F. P., Svensson, O., and Heinegård, D. (2012). Quantitative Proteomic Analysis of Eight Cartilaginous Tissues Reveals Characteristic Differences as Well as Similarities between Subgroups. J. Biol. Chem. 287, 18913–18924. doi:10.1074/jbc.m111.298968
Pène, J., Chevalier, S., Preisser, L., Vénéreau, E., Guilleux, M.-H., Ghannam, S., et al. (2008). Chronically Inflamed Human Tissues Are Infiltrated by Highly Differentiated Th17 Lymphocytes. J. Immunol. 180, 7423–7430. doi:10.4049/jimmunol.180.11.7423
Qian, Y., Liu, C., Hartupee, J., Altuntas, C. Z., Gulen, M. F., Jane-Wit, D., et al. (2007). The Adaptor Act1 Is Required for Interleukin 17-dependent Signaling Associated with Autoimmune and Inflammatory Disease. Nat. Immunol. 8, 247–256. doi:10.1038/ni1439
Risbud, M. V., and Shapiro, I. M. (2014). Role of Cytokines in Intervertebral Disc Degeneration: Pain and Disc Content. Nat. Rev. Rheumatol. 10, 44–56. doi:10.1038/nrrheum.2013.160
Rouvier, E., Luciani, M. F., Mattéi, M. G., Denizot, F., and Golstein, P. (1993). CTLA-8, Cloned from an Activated T Cell, Bearing AU-Rich Messenger RNA Instability Sequences, and Homologous to a Herpesvirus Saimiri Gene. J. Immunol. 150, 5445–5456.
Ruddy, M. J., Wong, G. C., Liu, X. K., Yamamoto, H., Kasayama, S., Kirkwood, K. L., et al. (2004). Functional Cooperation between Interleukin-17 and Tumor Necrosis Factor-α Is Mediated by CCAAT/Enhancer-binding Protein Family Members. J. Biol. Chem. 279, 2559–2567. doi:10.1074/jbc.m308809200
Sakai, D., and Grad, S. (2015). Advancing the Cellular and Molecular Therapy for Intervertebral Disc Disease. Adv. Drug Deliv. Rev. 84, 159–171. doi:10.1016/j.addr.2014.06.009
Schinocca, C., Rizzo, C., Fasano, S., Grasso, G., La Barbera, L., Ciccia, F., et al. (2021). Role of the IL-23/IL-17 Pathway in Rheumatic Diseases: an Overview. Front. Immunol. 12, 637829. doi:10.3389/fimmu.2021.637829
Shamji, M. F., Setton, L. A., Jarvis, W., So, S., Chen, J., Jing, L., et al. (2010). Proinflammatory Cytokine Expression Profile in Degenerated and Herniated Human Intervertebral Disc Tissues. Arthritis Rheum. 62, 1974–1982. doi:10.1002/art.27444
Shamji, M. F., Guha, D., Paul, D., and Shcharinsky, A. (2017). Systemic Inflammatory and Th17 Immune Activation Among Patients Treated for Lumbar Radiculopathy Exceeds that of Patients Treated for Persistent Postoperative Neuropathic Pain. Neurosurgery 81, 537–544. doi:10.1093/neuros/nyx052
Shaulian, E., and Karin, M. (2002). AP-1 as a Regulator of Cell Life and Death. Nat. Cell Biol 4, E131–E136. doi:10.1038/ncb0502-e131
Shen, C., Yan, J., Jiang, L. S., and Dai, L. Y. (2011). Autophagy in Rat Annulus Fibrosus Cells: Evidence and Possible Implications. Arthritis Res. Ther. 13, R132. doi:10.1186/ar3443
Shen, F., Hu, Z., Goswami, J., and Gaffen, S. L. (2006). Identification of Common Transcriptional Regulatory Elements in Interleukin-17 Target Genes. J. Biol. Chem. 281, 24138–24148. doi:10.1074/jbc.m604597200
Shen, F., Li, N., Gade, P., Kalvakolanu, D. V., Weibley, T., Doble, B., et al. (2009). IL-17 Receptor Signaling Inhibits C/EBPbeta by Sequential Phosphorylation of the Regulatory 2 Domain. Sci. Signal. 2, ra8. doi:10.1126/scisignal.2000066
Singh, S. P., Zhang, H. H., Foley, J. F., Hedrick, M. N., and Farber, J. M. (2008). Human T Cells that Are Able to Produce IL-17 Express the Chemokine Receptor CCR6. J. Immunol. 180, 214–221. doi:10.4049/jimmunol.180.1.214
Studer, R. K., Vo, N., Sowa, G., Ondeck, C., and Kang, J. (2011). Human Nucleus Pulposus Cells React to IL-6. Spine 36, 593–599. doi:10.1097/BRS.0b013e3181da38d5
Subramaniam, S. V., Cooper, R. S., and Adunyah, S. E. (1999). Evidence for the Involvement of JAK/STAT Pathway in the Signaling Mechanism of Interleukin-17. Biochem. Biophysical Res. Commun. 262, 14–19. doi:10.1006/bbrc.1999.1156
Sutovsky, J., Benco, M., Sutovska, M., Kocmalova, M., Pappova, L., Miklusica, J., et al. (2017). Cytokine and Chemokine Profile Changes in Patients with Lower Segment Lumbar Degenerative Spondylolisthesis. Int. J. Surg. 43, 163–170. doi:10.1016/j.ijsu.2017.06.024
Suyama, K., Sakai, D., Hirayama, N., Nakamura, Y., Matsushita, E., Terayama, H., et al. (2018). Effects of interleukin-17A in Nucleus Pulposus Cells and its Small-Molecule Inhibitors for Intervertebral Disc Disease. J. Cell. Mol. Med 22, 5539–5551. doi:10.1111/jcmm.13828
Toy, D., Kugler, D., Wolfson, M., Bos, T. V., Derry, J., Tocker, J., et al. (2006). Cutting Edge: Interleukin 17 Signals through a Heteromeric Receptor Complex. J. Immunol. 177, 36–39. doi:10.4049/jimmunol.177.1.36
Urban, J. P., and Roberts, S. (2003). Degeneration of the Intervertebral Disc. Arthritis Res. Ther. 5, 120–130. doi:10.1186/ar629
Vo, N. V., Hartman, R. A., Patil, P. R., Risbud, M. V., Kletsas, D., Iatridis, J. C., et al. (2016). Molecular Mechanisms of Biological Aging in Intervertebral Discs. J. Orthop. Res. 34, 1289–1306. doi:10.1002/jor.23195
Walsh, M. C., Lee, J., and Choi, Y. (2015). Tumor Necrosis Factor Receptor- Associated Factor 6 (TRAF6) Regulation of Development, Function, and Homeostasis of the Immune System. Immunol. Rev. 266, 72–92. doi:10.1111/imr.12302
Wang, S.-S., Zhang, W., Zhang, Y.-Q., Zhao, Y., Liu, Y., Li, J.-k., et al. (2015). IL-17A Enhances ADAMTS-7 Expression through Regulation of TNF-α in Human Nucleus Pulposus Cells. J. Mol. Hist. 46, 475–483. doi:10.1007/s10735-015-9640-5
Wang, S., Wei, J., Fan, Y., Ding, H., Tian, H., Zhou, X., et al. (2018). Progranulin Is Positively Associated with Intervertebral Disc Degeneration by Interaction with IL-10 and IL-17 through TNF Pathways. Inflammation 41, 1852–1863. doi:10.1007/s10753-018-0828-1
Wang, Y., Che, M., Xin, J., Zheng, Z., Li, J., and Zhang, S. (2020). The Role of IL-1β and TNF-α in Intervertebral Disc Degeneration. Biomed. Pharmacother. 131, 110660. doi:10.1016/j.biopha.2020.110660
Willems, N., Tellegen, A. R., Bergknut, N., Creemers, L. B., Wolfswinkel, J., Freudigmann, C., et al. (2016). Inflammatory Profiles in Canine Intervertebral Disc Degeneration. BMC Vet. Res. 12, 10. doi:10.1186/s12917-016-0635-6
Wu, L., Chen, X., Zhao, J., Martin, B., Zepp, J. A., Ko, J. S., et al. (2015). A Novel IL-17 Signaling Pathway Controlling Keratinocyte Proliferation and Tumorigenesis via the TRAF4-ERK5 axis. J. Exp. Med. 212, 1571–1587. doi:10.1084/jem.20150204
Xie, S., Li, J., Wang, J. H., Wu, Q., Yang, P., Hsu, H.-C., et al. (2010). IL-17 Activates the Canonical NF-Κb Signaling Pathway in Autoimmune B Cells of BXD2 Mice to Upregulate the Expression of Regulators of G-Protein Signaling 16. J.I. 184, 2289–2296. doi:10.4049/jimmunol.0903133
Xu, K., Chen, W., Wang, X., Peng, Y., Liang, A., Huang, D., et al. (2015). Autophagy Attenuates the Catabolic Effect during Inflammatory Conditions in Nucleus Pulposus Cells, as Sustained by NF-Κb and JNK Inhibition. Int. J. Mol. Med. 36, 661–668. doi:10.3892/ijmm.2015.2280
Xue, H., Yao, Y., Wang, X., Zhang, F., Jiang, X., Liu, J., et al. (2015). Interleukin-21 Is Associated with the Pathogenesis of Lumbar Disc Herniation. Iran J. Allergy Asthma Immunol. 14, 509–518.
Yang, W., Yu, X.-H., Wang, C., He, W.-S., Zhang, S.-J., Yan, Y.-G., et al. (2015). Interleukin-1β in Intervertebral Disk Degeneration. Clinica Chim. Acta 450, 262–272. doi:10.1016/j.cca.2015.08.029
Yao, Z., Nie, L., Zhao, Y., Zhang, Y., Liu, Y., Li, J., et al. (2016). Salubrinal Suppresses IL-17-induced Upregulation of MMP-13 and Extracellular Matrix Degradation through the NF-kB Pathway in Human Nucleus Pulposus Cells. Inflammation 39, 1997–2007. doi:10.1007/s10753-016-0435-y
Ye, N., Ding, Y., Wild, C., Shen, Q., and Zhou, J. (2014). Small Molecule Inhibitors Targeting Activator Protein 1 (AP-1). J. Med. Chem. 57, 6930–6948. doi:10.1021/jm5004733
Yoon, H.-S., and Park, C. (2021). Chrysoeriol Ameliorates COX-2 Expression through NF-Κb, AP-1 and MAPK Regulation via the TLR4/MyD88 Signaling Pathway in LPS-Stimulated Murine Macrophages. Exp. Ther. Med. 22, 718. doi:10.3892/etm.2021.10150
Zepp, J. A., Liu, C., Qian, W., Wu, L., Gulen, M. F., Kang, Z., et al. (2012). Cutting Edge: TNF Receptor-Associated Factor 4 Restricts IL-17-mediated Pathology and Signaling Processes. J.I. 189, 33–37. doi:10.4049/jimmunol.1200470
Zhang, F., Zhao, X., Shen, H., and Zhang, C. (2016). Molecular Mechanisms of Cell Death in Intervertebral Disc Degeneration (Review). Int. J. Mol. Med. 37, 1439–1448. doi:10.3892/ijmm.2016.2573
Zhang, G. Z., Liu, M. Q., Chen, H. W., Wu, Z. L., Gao, Y. C., Ma, Z. J., et al. (2021). NF-κB Signalling Pathways in Nucleus Pulposus Cell Function and Intervertebral Disc Degeneration. Cell Prolif 54, e13057. doi:10.1111/cpr.13057
Zhang H. J, H.-J., Liao, H.-Y., Bai, D.-Y., Wang, Z.-Q., and Xie, X.-W. (2021). MAPK /ERK Signaling Pathway: A Potential Target for the Treatment of Intervertebral Disc Degeneration. Biomed. Pharmacother. 143, 112170. doi:10.1016/j.biopha.2021.112170
Zhang, W., Nie, L., Guo, Y. J., Han, L. X., Wang, X., Zhao, H., et al. (2014). Th17 Cell Frequency and IL-17 Concentration Correlate with Pre- and Postoperative Pain Sensation in Patients with Intervertebral Disk Degeneration. Orthopedics 37, e685–91. doi:10.3928/01477447-20140626-62
Zhang, W., Nie, L., Wang, Y., Wang, X.-p., Zhao, H., Dongol, S., et al. (2013). CCL20 Secretion from the Nucleus Pulposus Improves the Recruitment of CCR6-Expressing Th17 Cells to Degenerated IVD Tissues. PLOS ONE 8, e66286. doi:10.1371/journal.pone.0066286
Zhang, Y., Liu, L., Wang, S., Zhao, Y., Liu, Y., Li, J., et al. (2016). Production of CCL20 on Nucleus Pulposus Cells Recruits IL-17-producing Cells to Degenerated IVD Tissues in Rat Models. J. Mol. Hist. 47, 81–89. doi:10.1007/s10735-015-9651-2
Keywords: interleukin-17, intervertebral disc, nucleus pulposus, disc herniation, cytokine
Citation: Suyama K, Sakai D and Watanabe M (2022) The Role of IL-17-Mediated Inflammatory Processes in the Pathogenesis of Intervertebral Disc Degeneration and Herniation: A Comprehensive Review. Front. Cell Dev. Biol. 10:857164. doi: 10.3389/fcell.2022.857164
Received: 18 January 2022; Accepted: 17 February 2022;
Published: 03 March 2022.
Edited by:
Zhen Sun, Fourth Military Medical University, ChinaReviewed by:
Ziya Levent Gokaslan, Brown University, United StatesNorimasa Iwasaki, Hokkaido University, Japan
Copyright © 2022 Suyama, Sakai and Watanabe. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daisuke Sakai, ZGFpc2FrYWlAaXMuaWNjLnUtdG9rYWkuYWMuanA=
 Kaori Suyama
Kaori Suyama Daisuke Sakai
Daisuke Sakai Masahiko Watanabe2
Masahiko Watanabe2