- Department of Reproductive Medicine, The Second Hospital of Hebei Medical University, Shijiazhuang, China
Objective: To investigate the effect of progesterone elevation during late follicular phase on early pregnancy outcomes and live births after fresh embryo transfers.
Methods: Patients who underwent IVF/ICSI treatment cycles were retrospectively enrolled. The effect of progesterone elevation was analyzed on early pregnancy outcome and live births after fresh embryo transfers.
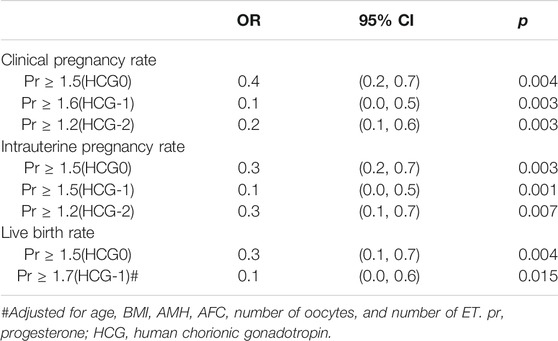
Results: A total of 2,404 patients were enrolled on the day of HCG triggering (HCG0), 1,584 patients on the day before HCG triggering (HCG-1), and 800 patients 2 days before HCG triggering (HCG-2). With a 1 ng/ml increase in the progesterone level on HCG0 day when the progesterone level was ≥1.5 ng/ml, the clinical pregnancy rate decreased by 60% (95% CI: 0.2–0.7, p = 0.004), the intrauterine pregnancy rate decreased by 70% (95% CI: 0.2–0.7, p = 0.003), and the live birth rate decreased by 70% (95% CI: 0.1–0.7, p = 0.004). With a 1 ng/ml increase in the progesterone level on HCG-1 day, the clinical pregnancy rate decreased by 90% (95% CI: 0.0–0.5, p = 0.003) when the progesterone level was ≥1.6 ng/ml, the intrauterine pregnancy rate decreased by 90% (95% CI: 0.0–0.5, p = 0.001) when the progesterone was ≥1.5 ng/ml, and the live birth rate decreased by 90% (95% CI: 0.0–0.6, p = 0.015) when the progesterone was ≥1.7 ng/ml. On HCG-2 day when the progesterone was ≥1.2 ng/ml, the clinical pregnancy rate decreased by 80% (95% CI: 0.1–0.6, p = 0.003), and the intrauterine pregnancy rate decreased by 70% (95% CI: 0.1–0.7, p = 0.007) with a 1 ng/ml increase in the progesterone level.
Conclusion: Elevated progesterone level during the late follicular phase is an independent risk factor affecting the clinical pregnancy rate, intrauterine pregnancy rate, and live birth rate among infertile patients undergoing IVF/ICSI after fresh embryo transfers. When the progesterone level exceeds a certain level, the early pregnancy and live birth rates after fresh embryo transfers show a rapid downward trend.
Introduction
For more than 2 decades, the effect of elevated progesterone on the outcome of in vitro fertilization and embryo transfer (IVF-ET) has been the focus of intensive scientific research. Although the application of gonadotropin-releasing hormone (GnRH) analogues could significantly reduce the incidence of premature luteinization in controlled ovarian stimulation (COS), the incidence of progesterone elevation in late follicular phase still fluctuated at 5%–30%. Since Edelstein et al. published their first report in 1990 (Edelstein et al., 1990), there have been many studies investigating the effect of elevated progesterone during the late follicular phase on IVF-ET outcomes with conflicting conclusions (Arvis et al., 2019).
To our knowledge, the most commonly used cutoff value for elevated progesterone on the day of human chorionic gonadotrophin (HCG) administration is 1.5 ng/ml (Bosch et al., 2010; Venetis et al., 2013). However, this cutoff value of progesterone elevation should be treated with caution because of the high variability of assays used for progesterone measurement, making it difficult to compare the results of different studies (Bosch et al., 2003; Kiliçdag et al., 2010; Santos-Ribeiro et al., 2014; Connell et al., 2016; Cui et al., 2017). In most of these studies, the serum progesterone concentration had been divided into different levels, and in the current study, the appropriate serum progesterone threshold that negatively affected the IVF outcome was investigated without dividing the serum concentration of progesterone into different levels, given that the progesterone level is a continuous variable. The second objective of this study was to evaluate the effect of elevated progesterone levels on the pregnancy outcomes of IVF-ET in the late follicular phase and the live birth rate.
Materials and Methods
Subjects
This was a retrospective, one-center case-cohort study on patients undergoing routine IVF-ET. The study protocol was approved by the Medical Ethics Committee of the Second Hospital of Hebei Medical University (No. 2017-P033). The criteria for inclusion were as follows: (1) patients undergoing IVF/intracytoplasmic sperm injection (ICSI) for the first time, (2) age ≤38 years, and (3) fresh embryo transfers. The criteria for exclusion were as follows: (1) abnormal uterine development, (2) preimplantation genetic testing (PGT), (3) endocrine diseases, and (4) missing progesterone values and unknown live birth at follow-up. A total of 2,404 patients undergoing IVF/ICSI treatment at our hospital from January 2018 to December 2019 were enrolled.
Controlled Ovarian Stimulation Protocol
The patients underwent COS with the use of luteal phase short-acting long protocol, GnRH antagonist protocol, and long-acting long protocol at the early follicular stage. According to the patient’s ovarian reserve and body mass index (BMI), ovarian stimulation was started with administration of 150–300 IU/day recombinant FSH (Gonal-F; Serono, Pharma S. p.A., Bari, Italy). The development of follicles was monitored by transvaginal ultrasonography. HCG 6500–10000U was given to trigger ovulation when two leading follicles reached a mean diameter of 18 mm. Oocytes were retrieved transvaginally 36–38 h after HCG administration. IVF or ICSI was performed according to the cause of infertility in the patients. Fewer than two embryos were transferred on day 3 after oocyte retrieval if the serum progesterone level was ≤2.0 ng/ml on the day of HCG administration, and excessive good-quality embryos were cryopreserved. Patients received conventional corpus luteum support, and embryo transfer was performed 3 days after oocyte retrieval. Preliminary diagnosis of biochemical pregnancy was made based on blood β-HCG 14 days after transplantation. Clinical pregnancy indicated the gestational sac, fetal bud, and fetal heart beat on ultrasound imaging approximately 30 days after transplantation.
Hormone Measurements
Whole blood was collected between 7:30 and 9 a.m. Serum follicle stimulating hormone (FSH), luteinizing hormone (LH), estradiol (E2), and progesterone were measured using the Immulite 2000 Immunoassay System (Siemens Healthcare Global, Munich, Germany) throughout the research process to minimize the bias in results related to time and reagent batch updates.
Indicators
The laboratory indicators included number of oocytes, number of two pronuclear (2 PN) embryos, number of normal cleavage embryos (2 PN cleavage), number of embryos transferred, and number of available embryos (the sum of number of transferred embryos and frozen embryos).
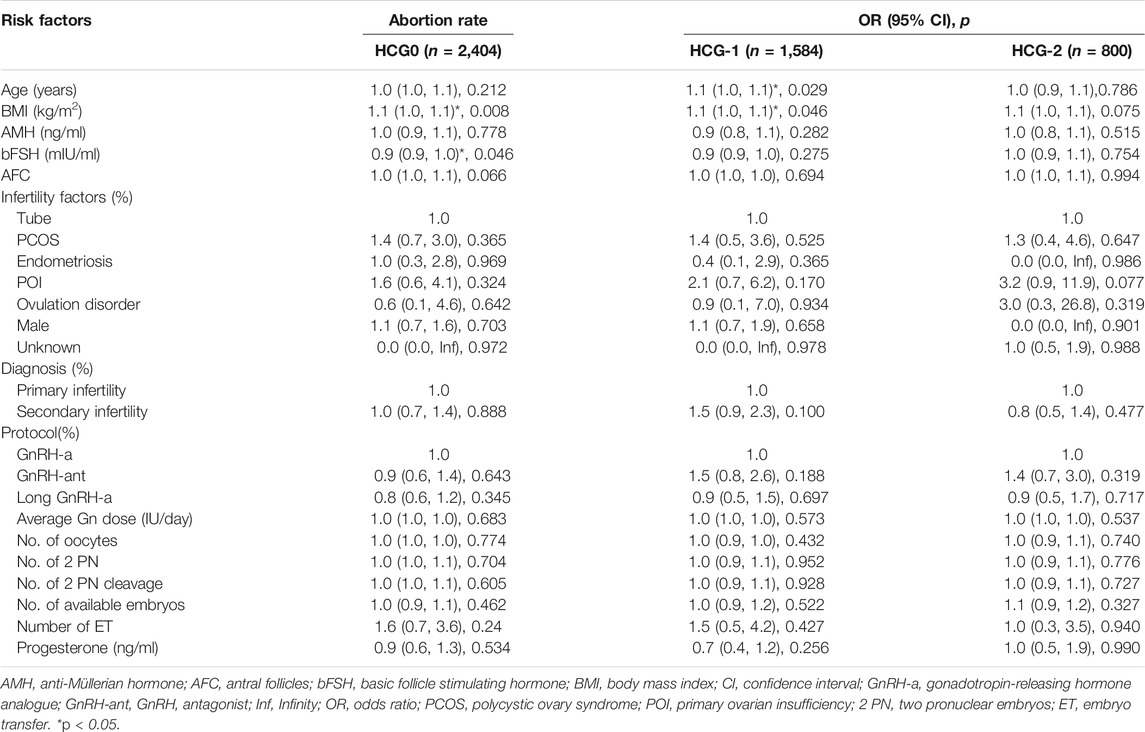
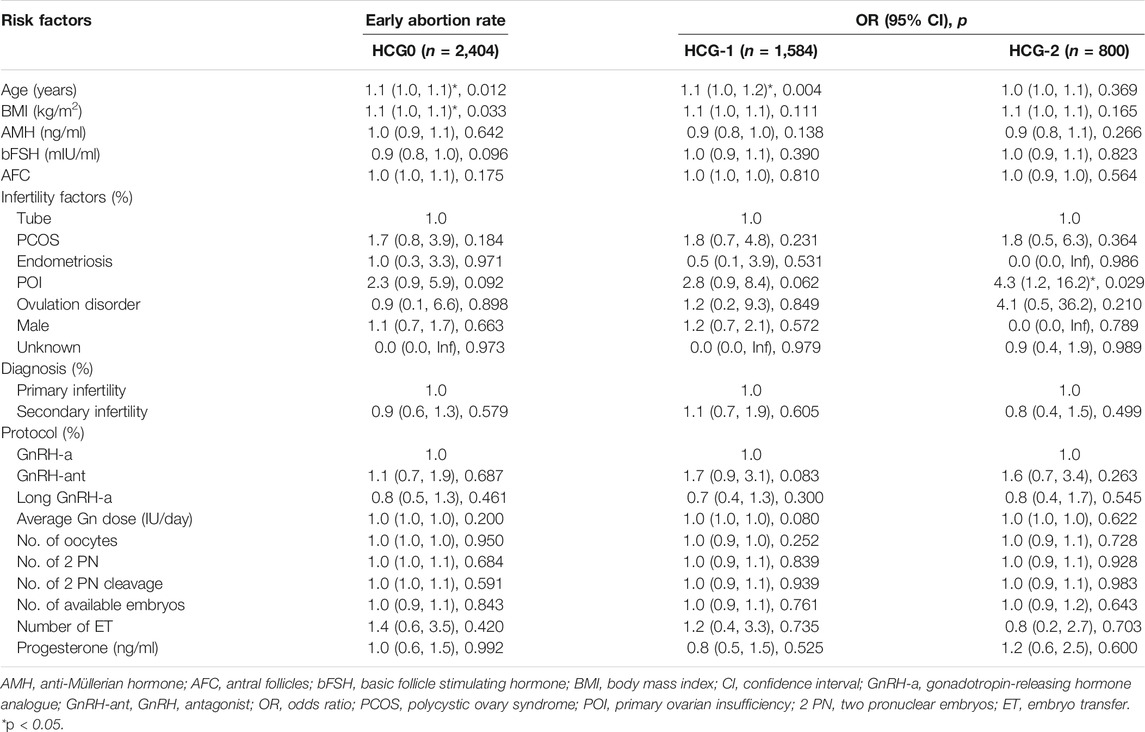
The clinical indicators included age, BMI, anti-Müllerian hormone (AMH), basic FSH level (bFSH), number of antral follicles (AFC), infertility factors (tubal factor infertility, polycystic ovary syndrome, endometriosis, primary ovarian insufficiency, ovulation disorder, male factor, and unknown factors), diagnosis (primary infertility and secondary infertility), COS protocols (luteal phase short-acting long protocol, GnRH antagonist protocol, and long-acting long protocol at early follicular stage), average Gn dosage, clinical pregnancy rate (clinical pregnancy cycles/transplantation cycles × 100%), intrauterine pregnancy rate (intrauterine pregnancy cycles/transplantation cycles × 100%), abortion (termination of pregnancy for less than 28 weeks of pregnancy and less than 1,000 g of fetus weight) rate (abortion cycles/transplantation cycles × 100%), early abortion (abortion occurring before 12 weeks of pregnancy) rate (early abortion cycles/transplantation cycles × 100%), and live birth rate (live birth cycles/transplantation cycles × 100%).
Statistical Analysis
All statistical analyses were performed with the Empower (R) (X&Y solutions, Boston, MA, United States) and the SPSS 23.0 statistical software (IBM, Chicago, IL, United States ) (Yu et al., 2014; Park et al., 2017; Sun et al., 2020). Continuous variables of normal distribution were expressed as the mean ± standard deviation (mean ± SD), and variables of non-normal distribution were presented as median ± quartile range (median ± QR). Classified variables were expressed as frequency and percentage (%). The independent sample t-test was used to analyze the continuous variables, whereas the odds ratio (OR) and chi-square test were used to analyze the categorical variables. The covariate screening, regression analysis, and curve fitting were performed using the Empower (R) statistical software. Univariate regression analysis was performed to select confounding factors, and multivariate logistic regression analysis was performed to adjust confounding factors for the effect of elevated progesterone on pregnancy outcomes. A generalized additive model was then applied to estimate the independent relationship between the progesterone level and pregnancy outcomes, with adjustment for potential confounders. A two-piece-wise linear regression model was further applied to examine the threshold effect of progesterone level on pregnancy outcomes according to the smoothing plot. The turning point of progesterone level where the relationship between the pregnancy outcomes and progesterone level started to change significantly was determined using the trial method, which was to move the trial turning point along the pre-defined interval and pick up the one that gave the maximal model likelihood. The cutoff value was determined in combination with the threshold effect analysis. p < 0.05 was considered statistically significant.
Results
Univariate Analysis of Pregnancy Outcomes
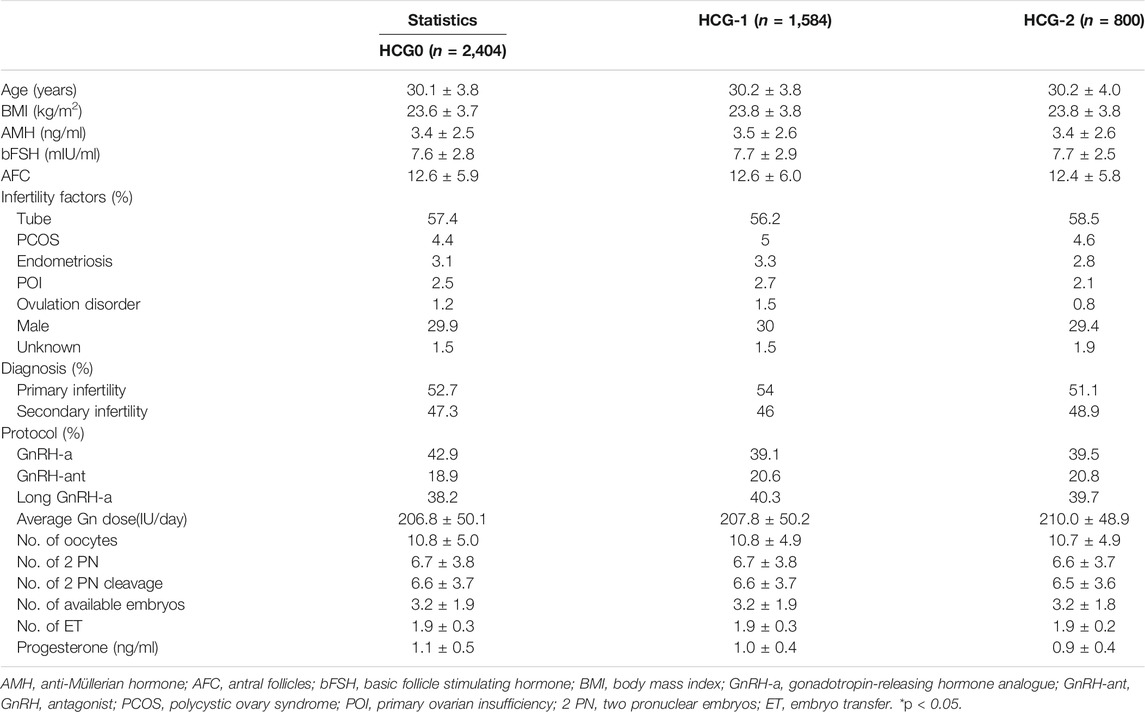
Based on the inclusion criteria, 2,404 patients were enrolled on the day of HCG triggering (HCG0), 1,584 patients on the day before HCG triggering (HCG-1), and 800 patients 2 days before HCG triggering (HCG-2). No significant (p > 0.05) differences were detected in the baseline data among three groups (Table 1).
To determine the risk factors for pregnancy outcomes, a logistic regression model was established and analyzed. Clinical pregnancy rate, intrauterine pregnancy rate, early abortion rate, and live birth rate were used as the dependent variable, respectively. Different risk factors identified in the univariable analysis were used as independent variables (Tables 2–6).
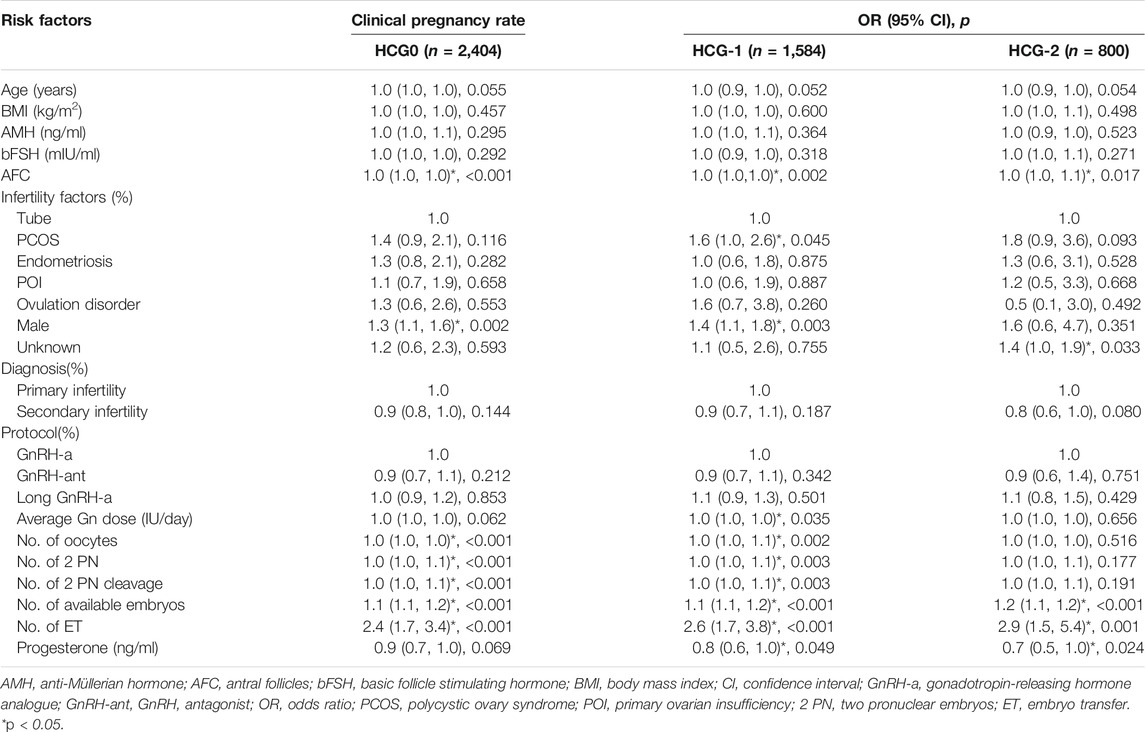
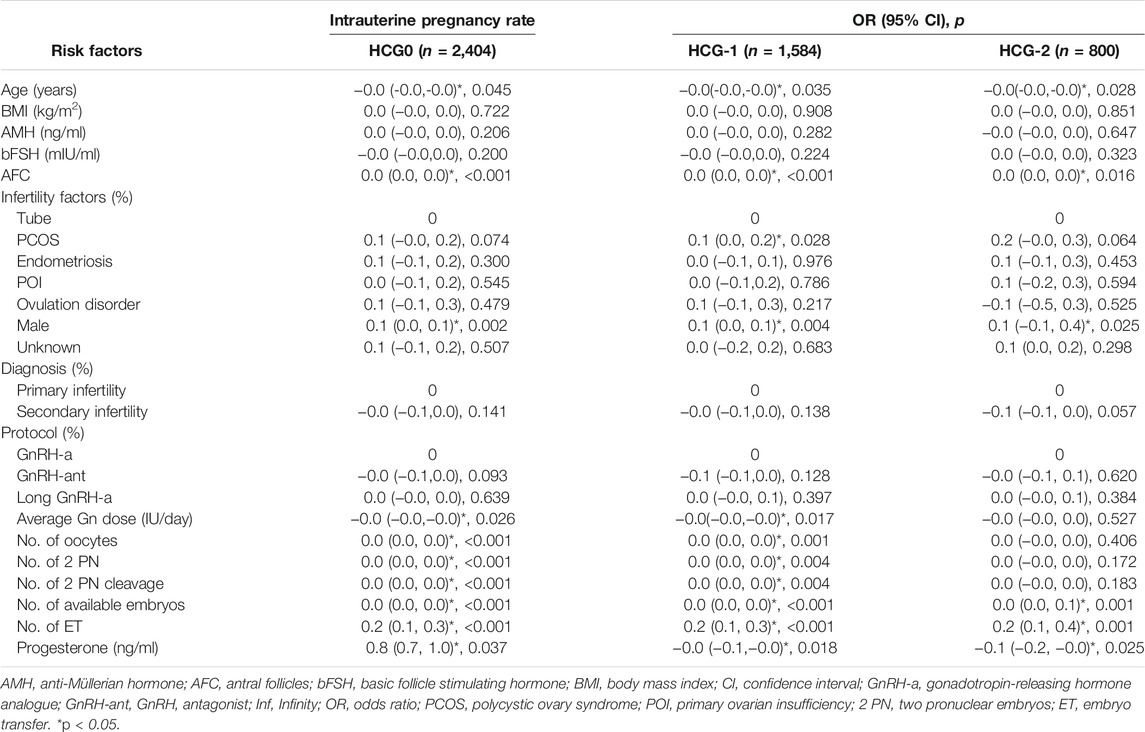
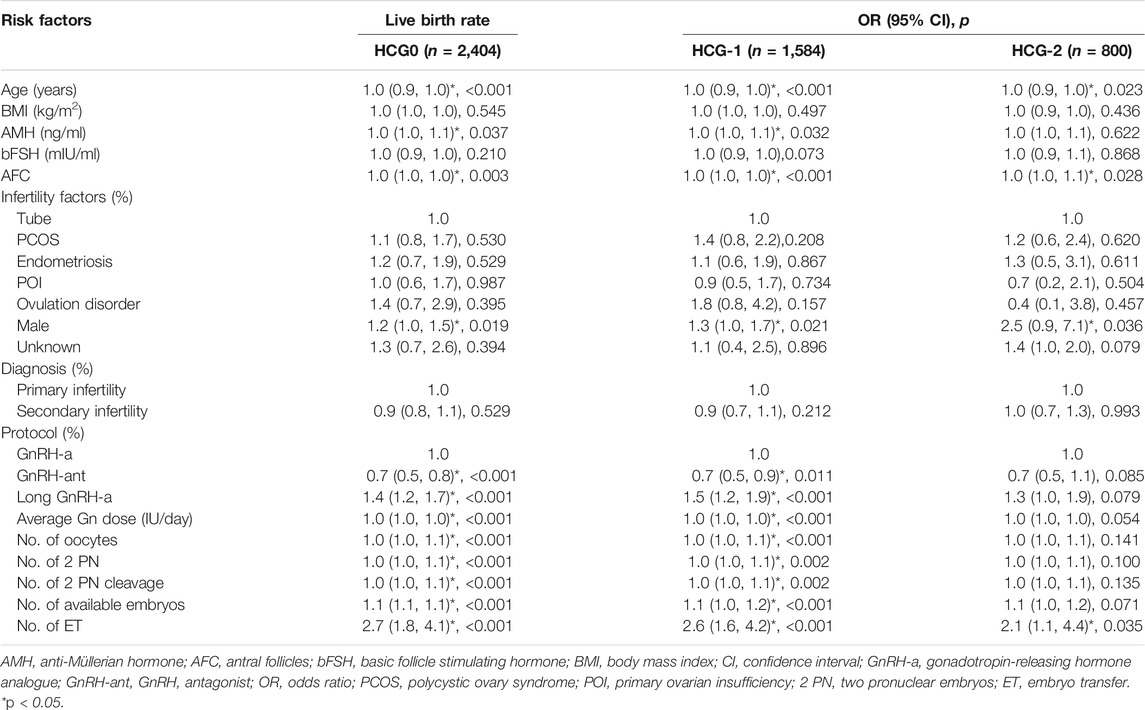
The mean level of serum progesterone on HCG0 day was 1.1 ± 0.5 ng/ml. There were significant associations of the live birth rate with age, AMH, AFC, male factor, COS protocol, average Gn dosage, number of oocytes, number of 2 PN embryos, number of 2 PN cleavage embryos, number of available embryos, or number of transferred embryos (p < 0.05) (Table 4), whereas AFC, male factor, number of oocytes, number of 2 PN embryos, number of 2 PN cleavage embryos, number of available embryos, and number of transferred embryos were significantly associated with the clinical pregnancy rate (p < 0.05) (Table 2). Age, AFC, male factor, average Gn dosage, number of oocytes, number of 2 PN embryos, number of 2 PN cleavage embryos, number of available embryos, number of transferred embryos, and progesterone level were significantly (p < 0.05) correlated with the intrauterine pregnancy rate (Table 3). Only BMI and bFSH were significantly (p < 0.05) associated with the abortion rate, and age and BMI were significantly (p < 0.05) associated with the early abortion rate (Tables 5, 6).
The mean level of serum progesterone on HCG-1 day was 1.0 ± 0.4 ng/ml. Age, AMH, AFC, male factor, COS protocol, average Gn dose, number of oocytes, number of 2 PN embryos, number of 2 PN cleavage embryos, number of available embryos, and number of transferred embryos were significantly (p < 0.05) correlated with the live birth rate (Table 4), whereas AFC, PCOS, male factor, average Gn dose, number of oocytes, number of 2 PN embryos, number of 2 PN cleavage embryos, number of available embryos, number of transferred embryos, and progesterone level were significantly (p < 0.05) associated with the clinical pregnancy rate (Table 2). Age, AFC, male factor, average Gn dose, number of oocytes, number of 2 PN embryos, number of 2 PN cleavage embryos, number of available embryos, number of transferred embryos, and progesterone level were significantly (p < 0.05) correlated with the intrauterine pregnancy rate (Table 3). Age and BMI were significantly (p < 0.05) related to the abortion rate, and age was significantly (p < 0.05) related to the early abortion rate (Tables 5, 6).
The mean level of serum progesterone on HCG-2 day was 0.9 ± 0.4 ng/ml. Age, AFC, unknown infertility, and number of transferred embryos were significantly (p < 0.05) associated with the live birth rate (Table 4). AFC, unknown infertility, number of available embryos, number of transferred embryos, and progesterone level were significantly (p < 0.05) related to the clinical pregnancy rate (Table 2). Age, unknown infertility, number of available embryos, number of transferred embryos, and progesterone level were significantly (p < 0.05) related to the intrauterine pregnancy rate (Table 3). Premature ovarian insufficiency (POI) was significantly (p < 0.05) associated with the early abortion rate (Table 6). No risk factors were found to be significantly associated with abortion.
Multivariate Logistic Regression Analysis
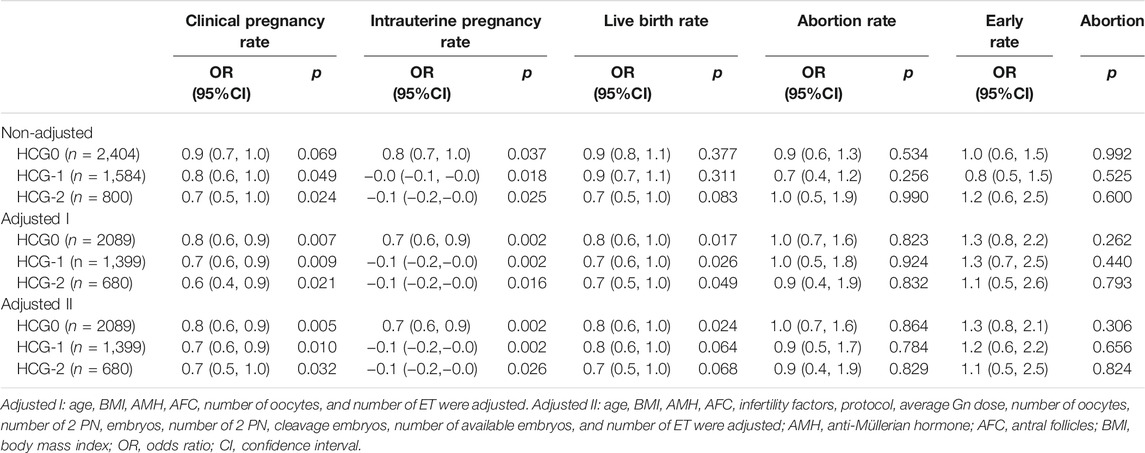
After adjustment of variables affecting the association between the progesterone level and pregnancy outcomes, there were 2,089 patients on HCG0, 1,399 on HCG-1, and 680 on HCG-2. In model I, the potential confounders were chosen based on the clinical experience: age, BMI, AMH, AFC, number of oocytes, and number of embryos transferred. In model II, the following variables were adjusted based on the clinical experience as well as results of the univariate analysis: age, BMI, AMH, AFC, factors of infertility, COS protocol, average Gn dosage, number of oocytes, number of 2 PN embryos, number of 2 PN cleavage embryos, number of available embryos, and number of transferred embryos. The progesterone level on HCG0 was an independent risk factor for clinical pregnancy rate (OR = 0.8, 95% CI: 0.6–0.9, p = 0.005), intrauterine pregnancy rate (OR = 0.7, 95% CI: 0.6–0.9, p = 0.002), and live birth rate (OR = 0.8, 95% CI: 0.6–1.0, p = 0.024). The progesterone level on HCG-1 was an independent risk factor for clinical pregnancy rate (OR = 0.7, 95% CI: 0.6–0.9, p = 0.010), intrauterine pregnancy rate (OR = −0.1, 95% CI: 0.2–0.0, p = 0.002), and live birth rate (OR = 0.7, 95% CI: 0.6–1.0, p = 0.026, model I). The progesterone level on HCG-2 was an independent risk factor for clinical pregnancy rate (OR = 0.7, 95% CI: 0.5–1.0, p = 0.032) and intrauterine pregnancy rate (OR = −0.1, 95% CI: 0.2–0.0, p = 0.026). The progesterone level in the late follicular phase had no significant (p > 0.05) predictive effect on the abortion rate and early abortion rate (Table 7).
Smooth Curve Fitting
After adjusting for confounding factors, there was a non-linear relationship of the HCG0 or HCG-1 progesterone levels with the clinical pregnancy rate, intrauterine pregnancy rate, and live birth rate (Figures 1, 2). The HCG-2 progesterone level had a non-linear relationship with the clinical pregnancy rate and intrauterine pregnancy rate (Figure 3). When the progesterone level exceeded a certain level, the clinical pregnancy rate, intrauterine pregnancy rate, and live birth rate all showed a rapid downward trend.
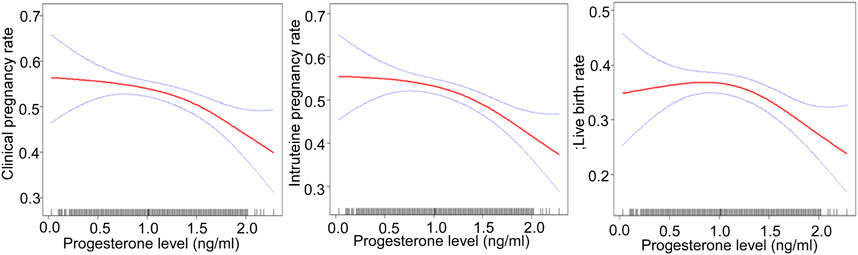
FIGURE 1. Association of progesterone levels on the day of HCG triggering with the IVF outcomes including the clinical pregnancy rate, intrauterine pregnancy rate, and live birth rate. Adjusted for age, BMI, AMH, AFC, infertility factors, protocol, average Gn dose, number of oocytes, number of 2 PN embryos, number of 2 PN cleavage embryos, number of available embryos, and number of ET. HCG, human chorionic gonadotropin; IVF, in vitro fertilization; BMI, body mass index, AFC, antral follicles; AMH, anti-Müllerian hormone; 2 PN, pronucleus; Gn, gonadotropin.
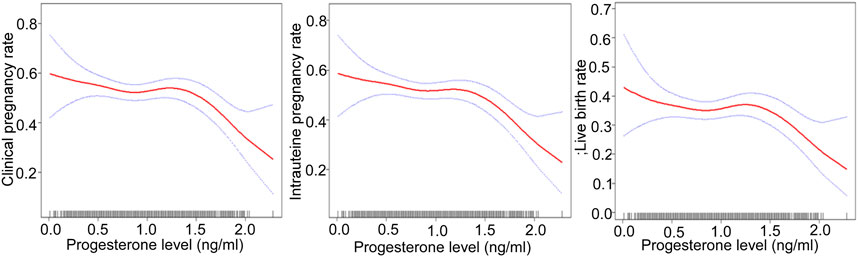
FIGURE 2. Association of progesterone levels on the day before HCG triggering with the IVF outcomes including the clinical pregnancy rate, the intrauterine pregnancy rate, and the live birth rate. The association of progesterone levels with the clinical and intrauterine pregnancy rate was calculated after adjustment for age, BMI, AMH, AFC, infertility factors, protocol, average Gn dose, number of oocytes, number of 2 PN embryos, number of 2 PN cleavage embryos, number of available embryos, and number of ET. The live birth rate was calculated after adjustment for age, BMI, AMH, AFC, number of oocytes, and number of ET. HCG, human chorionic gonadotropin; IVF, in vitro fertilization; BMI, body mass index, AFC, antral follicles; AMH, anti-Müllerian hormone; 2 PN, pronucleus; Gn, gonadotropin; ET, embryo transfer.
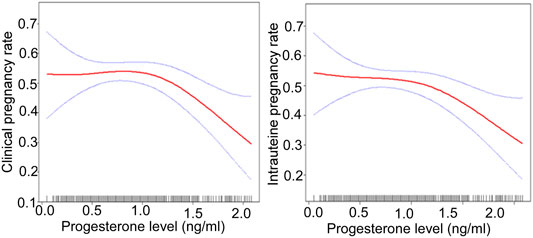
FIGURE 3. The association of the progesterone level on 2 days before HCG triggering with the IVF outcomes including the clinical pregnancy rate and the intrauterine pregnancy rate. Adjusted for age, BMI, AMH, AFC, infertility factors, protocol, average Gn dose, number of oocytes, number of 2 PN embryos, number of 2 PN cleavage embryos, number of available embryos, and number of ET. HCG, human chorionic gonadotropin; IVF, in vitro fertilization; BMI, body mass index, AFC, antral follicles; AMH, anti-Müllerian hormone; 2 PN, pronucleus; Gn, gonadotropin; ET, embryo transfer.
Threshold Effect Analysis
The threshold effect was analyzed after adjusting for confounding factors. When the progesterone level was ≥1.5 ng/ml on HCG0 day, with a 1 ng/ml increase in the progesterone level, the clinical pregnancy rate decreased by 60% (95% CI: 0.2–0.7, p = 0.004), the intrauterine pregnancy rate decreased by 70% (95% CI: 0.2–0.7, p = 0.003), and the live birth rate decreased by 70% (95% CI: 0.1–0.7, p = 0.004).
When the progesterone was ≥1.6 ng/ml on HCG-1 day, with a 1 ng/ml increase in the progesterone level, the clinical pregnancy rate decreased by 90% (95% CI: 0.0–0.5, p = 0.003), and the intrauterine pregnancy rate decreased by 90% (95% CI: 0.0–0.5, p = 0.001) when the progesterone level was ≥1.5 ng/ml. Similarly, the live birth rate decreased by 90% (95% CI: 0.0–0.6, p = 0.015) when the progesterone level was ≥1.7 ng/ml.
On HCG-2 day when the progesterone was ≥1.2 ng/ml, the clinical pregnancy rate decreased by 80% (95% CI: 0.1–0.6, p = 0.003), and the intrauterine pregnancy rate decreased by 70% (95% CI: 0.1–0.7, p = 0.007) with a 1 ng/ml increase in the progesterone level (Table 8).
Discussion
This study discovered that the increase of progesterone level during the late follicular phase in the COS process had a negative effect on the early pregnancy outcome and live birth rate in the IVF fresh cycle. However, currently, no consensus has been reached on the statistical methods for determining the critical value of serum progesterone elevation, and most researchers used the receiver operating characteristic (ROC) curves for analysis. Since the relationship between the serum progesterone level and the pregnancy outcome is not linear (Saleh et al., 2009; Bosch et al., 2010), the area under the ROC curve may not be the most suitable analysis method. Therefore, our study applied the smooth curve fitting and threshold effect analysis for the first time to determine the quantitative relationship between the increase in the progesterone level during the late follicular period and the pregnancy outcome of IVF fresh cycle.
To date, the largest meta-analysis of more than 60,000 IVF-ET cycles stratified according to different progesterone thresholds found that once the progesterone level exceeded 0.8 ng/ml, the increase in progesterone was significantly negatively correlated with pregnancy outcome (Venetis et al., 2013). When all the data were integrated, the threshold for increased progesterone that had the greatest impact on the pregnancy rate was between 1.5 ng/ml and 1.75 ng/ml (OR: 0.64, 95% CI: 0.54–0.76; p < 0.001) (Venetis et al., 2013). In addition, there did not seem to be a negative correlation between the progesterone elevation on the day of HCG triggering in fresh cycles and the pregnancy rate of frozen–thawed embryos transferred from this cycle, suggesting that elevated progesterone did not affect the quality of oocytes.
The study by Xiong et al. showed that the progesterone levels higher than 1.7 ng/ml on the day of HCG triggering affected the epigenetic modification of endometrium, including glandular epithelium, luminal epithelium, and stroma in the peri-implantation period, which may disrupt the endometrial receptivity and lead to a decrease in pregnancy rate (Xiong et al., 2017). However, some recent research has found a significant difference in the top embryo quality (TEQ) rate between serum progesterone levels <2.0 ng/ml and >2.0 ng/ml. Obviously, progesterone levels higher than 2.0 ng/ml before oocyte maturation were harmful to the quality of oocytes (Huang et al., 2016). Studies emphasized that elevated progesterone levels resulted in a potential decline in high-quality embryos and even a decrease in the cumulative pregnancy rates (Vanni et al., 2017; Racca et al., 2018). The latest research showed a significant negative correlation in the progesterone concentration exceeding 1 ng/ml with the proportion of high-quality embryos and the implantation rate (Simon et al., 2019).
In a study with 1,784 women, the clinical pregnancy rate decreased with the increase of high progesterone exposure time after dividing progesterone elevation (>1 ng/ml) time into three groups: 0, one to two, and ≥3 days (OR: 0.77, 95% CI: 0.66–0.89, p = 0.001). Therefore, not only the presence of high progesterone, but also the duration of high progesterone exposure seems to have a negative effect on the outcome of IVF (Huang et al., 2012). Our study found that the increase of serum progesterone concentration in different periods during the late follicular phase had adverse effects on clinical pregnancy outcome. The earlier the progesterone elevation, the lower the threshold and the greater the impact on pregnancy outcome. When the progesterone level was ≥1.5 ng/ml on HCG0 day, with a 1 ng/ml increase of the progesterone level, the clinical pregnancy rate decreased by 60% (95% CI: 0.2–0.7, p = 0.004), and the intrauterine pregnancy rate decreased by 70% (95% CI: 0.2–0.7, p = 0.003). When the progesterone level was ≥1.6 ng/ml on HCG-1 day, with a 1 ng/ml increase of progesterone, the clinical pregnancy rate decreased by 90% (95% CI: 0.0–0.5, p = 0.003). When the progesterone level was ≥1.5 ng/ml, the intrauterine pregnancy rate decreased by 90% (95% CI: 0.0–0.5, p = 0.001) with a 1 ng/ml increase in the progesterone level. When the progesterone level was ≥1.2 ng/ml on HCG-2 day, with a 1 ng/ml increase of progesterone, the clinical pregnancy rate decreased by 80% (95% CI: 0.1–0.6, p = 0.003), and the intrauterine pregnancy rate decreased by 70% (95% CI: 0.1–0.7, p = 0.007). However, our study did not find significant effects of elevated progesterone on abortion, including early abortion.
In the study by Santos-Ribeiro et al. focusing on exploring the relationship between the progesterone level on the day of HCG administration with GnRH antagonist protocol and the live birth rate, it was found that the lower (≤0.50 ng/ml) and upper (>1.5 ng/ml) limits of the progesterone level could significantly reduce the live birth rate (Santos-Ribeiro et al., 2014). One study that included 817 IVF fresh cycles with single birth showed that with the increase in the progesterone level, especially when the progesterone level was >2.0 ng/ml, the average birth weight decreased significantly (Ibrahim et al., 2017). A recent study suggested that in a cohort of women undergoing IVF, the serum concentration of progesterone in the range of 0.64–1.04 ng/ml (RR: 1.6, 95% CI: 1.1–2.5) on the day of HCG administration was significantly associated with an increased risk of ischemic placental disease (IPD), which might lead to fetal growth restriction; however, this was not seen in women with the highest progesterone levels (Modest et al., 2021). Our study found that regardless of the routine COS protocols, with a 1 ng/ml increase in the progesterone level on HCG0 day, the live birth rate decreased by 70% (95% CI: 0.1–0.7, p = 0.004) when the progesterone level was ≥1.5 ng/ml. When the progesterone level was ≥1.7 ng/ml on HCG-1 day, with a 1 ng/ml increase in the progesterone level, the live birth rate decreased by 90% (95% CI: 0.0–0.6, p = 0.015). However, the decrease in progesterone levels caused no significant adverse effects on the live birth rate. At present, the mechanism by which a high progesterone level leads to a decrease in the live birth rate and low birth weight is not clear, and the decrease of the progesterone level may have an impact on fetal development itself, or may be related to the internal factors of infertility and super-physiological hormone levels.
It has been shown that reducing the intensity of stimulation in the late follicular phase of COS could decrease the incidence of progesterone elevation (Lawrenz et al., 2016). The duration of COS should not be prolonged or exceeded beyond the optimal standard of final oocyte maturation. In this regard, the key to ovarian stimulation in IVF is an individualized therapy, which means to select the appropriate Gn dose according to the patient’s ovarian reserve and BMI, and to adjust the Gn dose according to hormone levels and follicular development during COS. In addition, compared with fresh embryo transfers, the application of “freeze-all” strategy could improve the perinatal outcome, including a lower incidence of preterm delivery and improved build for gestational age infants (Maheshwari et al., 2012; Shapiro et al., 2014), besides eliminating the adverse effect of elevated progesterone levels on endometrial receptivity (Fatemi and Garcia-Velasco, 2015).
Our study is the first known study to quantify the relationship between progesterone elevation in the late follicular phase and pregnancy outcomes in the IVF fresh cycle by smoothing curve fitting and threshold effect analysis. An observational retrospective study demonstrated that different progesterone assays had a limited reproducibility and results depending on the specific assay used and the range of progesterone level (Lawrenz et al., 2018). Consequently, the reliability of progesterone thresholds, suggested by a previously published meta-analysis on the topic (Venetis et al., 2013), has to be questioned because studies using different assays were included in that meta-analysis. Due to the differences in the performance and efficacy of progesterone detection methods (Patton et al., 2014) and inherent changes in secretion, it may be wise for each center to define its own progesterone threshold according to its own test results and clinical data.
Although strict inclusion and exclusion criteria had been adopted and many potential confounders had been adjusted as far as possible in this study, there were still some limitations, including the retrospective study design, single center study, and Chinese patients enrolled only, which may all affect the generalization of the outcomes of our study. Future studies will have to resolve these issues for better outcomes.
In summary, an increase of the serum progesterone level in the late follicular phase reduces the clinical pregnancy rate, intrauterine pregnancy rate, and live birth rate of IVF fresh cycles. The earlier the progesterone level rises, the lower the threshold and the greater impact on pregnancy outcomes. Therefore, we should not only pay attention to the progesterone level on the HCG triggering day, but also focus on whether the progesterone level increases in the late follicular phase to prevent possible adverse effect on pregnancy. The pregnancy outcomes of IVF can be improved by reducing the intensity of ovarian stimulation and freezing all embryos when the progesterone level is ≥1.5 ng/ml. Nonetheless, further studies are necessary to confirm this outcome.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the Second Hospital of Hebei Medical University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Study design: YX and GH. Data collection: YX, JZ, AL, NY, and NC. Study analysis: YX and B-LG. Study supervision: NC. Writing of the original version: YX. Revision of the version: B-LG. Approval of the article: all authors.
Funding
This work was supported by the Beijing, Tianjin, and Hebei Cooperation Project (H2019206707), the National Key R and D Plan (2018YFC1002014), Hebei People’s Livelihood Science and Technology Project (20377714D), and the General Project of Hebei Natural Science Foundation (H2019206674).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Arvis, P., Lehert, P., and Guivarc'h-Levêque, A. (2019). Both high and Low Hcg Day Progesterone Concentrations Negatively Affect Live Birth Rates in Ivf/icsi Cycles. Reprod. BioMedicine Online 39, 852–859. doi:10.1016/j.rbmo.2019.07.001
Bosch, E., Labarta, E., Crespo, J., Simón, C., Remohí, J., Jenkins, J., et al. (2010). Circulating Progesterone Levels and Ongoing Pregnancy Rates in Controlled Ovarian Stimulation Cycles for In Vitro Fertilization: Analysis of over 4000 Cycles. Hum. Reprod. 25, 2092–2100. doi:10.1093/humrep/deq125
Bosch, E., Valencia, I., Escudero, E., Crespo, J., Simón, C., Remohí, J., et al. (2003). Premature Luteinization during Gonadotropin-Releasing Hormone Antagonist Cycles and its Relationship with In Vitro Fertilization Outcome. Fertil. Sterility 80, 1444–1449. doi:10.1016/j.fertnstert.2003.07.002
Connell, M. T., Patounakis, G., Healy, M. W., DeCherney, A. H., Devine, K., Widra, E., et al. (2016). Is the Effect of Premature Elevated Progesterone Augmented by Human Chorionic Gonadotropin versus Gonadotropin-Releasing Hormone Agonist Trigger? Fertil. Sterility 106, 584–589. doi:10.1016/j.fertnstert.2016.04.024
Cui, N., Zhang, J., Xu, Y., Jiang, L., Yang, A., and Hao, G. (2017). Premature Progesterone Rise Positively Correlates with Clinical Pregnancy Rate in In Vitro Fertilization (Ivf) and Intracytoplasmic Sperm Injection (Icsi) Patients with Good Ovarian Response. Horm. Metab. Res. 49, 372–379. doi:10.1055/s-0043-104384
Edelstein, M. C., Seltman, H. J., Cox, B. J., Robinson, S. M., Shaw, R. A., and Muasher, S. J. (1990). Progesterone Levels on the Day of Human Chorionic Gonadotropin Administration in Cycles with Gonadotropin-Releasing Hormone Agonist Suppression Are Not Predictive of Pregnancy Outcome. Fertil. Sterility 54, 853–857. doi:10.1016/s0015-0282(16)53945-4
Fatemi, H. M., and Garcia-Velasco, J. (2015). Avoiding Ovarian Hyperstimulation Syndrome with the Use of Gonadotropin-Releasing Hormone Agonist Trigger. Fertil. Sterility 103, 870–873. doi:10.1016/j.fertnstert.2015.02.004
Huang, B., Ren, X., Wu, L., Zhu, L., Xu, B., Li, Y., et al. (2016). Elevated Progesterone Levels on the Day of Oocyte Maturation May Affect Top Quality Embryo Ivf Cycles. PLoS One 11, e0145895. doi:10.1371/journal.pone.0145895
Huang, C.-C., Lien, Y.-R., Chen, H.-F., Chen, M.-J., Shieh, C.-J., Yao, Y.-L., et al. (2012). The Duration of Pre-ovulatory Serum Progesterone Elevation before Hcg Administration Affects the Outcome of Ivf/icsi Cycles. Hum. Reprod. 27, 2036–2045. doi:10.1093/humrep/des141
Ibrahim, Y., Haviland, M. J., Hacker, M. R., Penzias, A. S., Thornton, K. L., and Sakkas, D. (2017). Elevated Progesterone and its Impact on Birth Weight after Fresh Embryo Transfers. J. Assist. Reprod. Genet. 34, 759–764. doi:10.1007/s10815-017-0920-8
Kiliçdag, E. B., Haydardedeoglu, B., Cok, T., Hacivelioglu, S. O., and Bagis, T. (2010). Premature Progesterone Elevation Impairs Implantation and Live Birth Rates in Gnrh-Agonist Ivf/icsi Cycles. Arch. Gynecol. Obstet. 281, 747–752. doi:10.1007/s00404-009-1248-0
Lawrenz, B., Sibal, J., Garrido, N., Abu, E., Jean, A., Melado, L., et al. (2018). Inter-assay Variation and Reproducibility of Progesterone Measurements during Ovarian Stimulation for Ivf. Plos One 13, e0206098. doi:10.1371/journal.pone.0206098
Lawrenz, B., Beligotti, F., Engelmann, N., Gates, D., and Fatemi, H. M. (2016). Impact of Gonadotropin Type on Progesterone Elevation during Ovarian Stimulation in Gnrh Antagonist Cycles. Hum. Reprod. 31, 2554–2560. doi:10.1093/humrep/dew213
Maheshwari, A., Pandey, S., Shetty, A., Hamilton, M., and Bhattacharya, S. (2012). Obstetric and Perinatal Outcomes in Singleton Pregnancies Resulting from the Transfer of Frozen Thawed versus Fresh Embryos Generated through In Vitro Fertilization Treatment: A Systematic Review and Meta-Analysis. Fertil. Sterility 98, 368–377. doi:10.1016/j.fertnstert.2012.05.019
Modest, A. M., Johnson, K. M., Aluko, A., Joshi, A., Wise, L. A., Fox, M. P., et al. (2021). Elevated Serum Progesterone during In Vitro Fertilization Treatment and the Risk of Ischemic Placental Disease. Pregnancy Hypertens. 24, 7–12. doi:10.1016/j.preghy.2021.02.004
Park, S.-Y., Freedman, N. D., Haiman, C. A., Le Marchand, L., Wilkens, L. R., and Setiawan, V. W. (2017). Association of Coffee Consumption with Total and Cause-specific Mortality Among Nonwhite Populations. Ann. Intern. Med. 167, 228–235. doi:10.7326/m16-2472
Patton, P. E., Lim, J. Y., Hickok, L. R., Kettel, L. M., Larson, J. M., and Pau, K. Y. F. (2014). Precision of Progesterone Measurements with the Use of Automated Immunoassay Analyzers and the Impact on Clinical Decisions for In Vitro Fertilization. Fertil. Sterility 101, 1629–1636. doi:10.1016/j.fertnstert.2014.02.037
Racca, A., Santos-Ribeiro, S., De Munck, N., Mackens, S., Drakopoulos, P., Camus, M., et al. (2018). Impact of Late-Follicular Phase Elevated Serum Progesterone on Cumulative Live Birth Rates: Is There a Deleterious Effect on Embryo Quality? Hum. Reprod. 33, 860–868. doi:10.1093/humrep/dey031
Saleh, H. A., Omran, M. S. E.-A., and Draz, M. (2009). Does Subtle Progesterone Rise on the Day of Hcg Affect Pregnancy Rate in Long Agonist Icsi Cycles? J. Assist. Reprod. Genet. 26, 239–242. doi:10.1007/s10815-009-9309-7
Santos-Ribeiro, S., Polyzos, N. P., Haentjens, P., Smitz, J., Camus, M., Tournaye, H., et al. (2014). Live Birth Rates after Ivf Are Reduced by Both Low and High Progesterone Levels on the Day of Human Chorionic Gonadotrophin Administration. Hum. Reprod. 29, 1698–1705. doi:10.1093/humrep/deu151
Shapiro, B. S., Daneshmand, S. T., Garner, F. C., Aguirre, M., and Hudson, C. (2014). Clinical Rationale for Cryopreservation of Entire Embryo Cohorts In Lieu of Fresh Transfer. Fertil. Sterility 102, 3–9. doi:10.1016/j.fertnstert.2014.04.018
Simon, C., Moreau, J., Gatimel, N., Cohade, C., Parinaud, J., and Leandri, R. (2019). Impact of Estradiol and Progesterone Levels during the Late Follicular Stage on the Outcome of Gnrh Antagonist Protocols. Gynecol. Endocrinol. 35, 481–484. doi:10.1080/09513590.2018.1538346
Sun, Y.-F., Zhang, J., Xu, Y.-M., Luo, Z.-Y., Sun, Y., Hao, G.-M., et al. (2020). Effects of Age on Pregnancy Outcomes in Patients with Simple Tubal Factor Infertility Receiving Frozen-Thawed Embryo Transfer. Sci. Rep. 10, 18121. doi:10.1038/s41598-020-75124-3
Vanni, V. S., Somigliana, E., Reschini, M., Pagliardini, L., Marotta, E., Faulisi, S., et al. (2017). Top Quality Blastocyst Formation Rates in Relation to Progesterone Levels on the Day of Oocyte Maturation in Gnrh Antagonist Ivf/icsi Cycles. PLoS One 12, e0176482. doi:10.1371/journal.pone.0176482
Venetis, C. A., Kolibianakis, E. M., Bosdou, J. K., and Tarlatzis, B. C. (2013). Progesterone Elevation and Probability of Pregnancy after Ivf: A Systematic Review and Meta-Analysis of over 60 000 Cycles. Hum. Reprod. Update 19, 433–457. doi:10.1093/humupd/dmt014
Xiong, Y., Wang, J., Liu, L., Chen, X., Xu, H., Li, T. C., et al. (2017). Effects of High Progesterone Level on the Day of Human Chorionic Gonadotrophin Administration in In Vitro Fertilization Cycles on Epigenetic Modification of Endometrium in the Peri-Implantation Period. Fertil. Sterility 108, 269–276. doi:10.1016/j.fertnstert.2017.06.004
Keywords: elevated progesterone, in vitro fertilization, fresh embryo transfer, early pregnancy outcome, live birth rate
Citation: Xu Y, Zhang J, Li A, Yang N, Cui N, Hao G and Gao B-L (2022) Impact of Elevated Progesterone in Late Follicular Phase on Early Pregnancy Outcomes and Live Birth Rate After Fresh Embryo Transfers. Front. Cell Dev. Biol. 10:855455. doi: 10.3389/fcell.2022.855455
Received: 15 January 2022; Accepted: 10 February 2022;
Published: 11 March 2022.
Edited by:
Albert Salas-Huetos, Harvard University, United StatesReviewed by:
Yentel Mateo Otero, University of Girona, SpainJianyuan Song, Zhejiang University, China
Copyright © 2022 Xu, Zhang, Li, Yang, Cui, Hao and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Na Cui, Y25uYzk5OUAxNjMuY29t; Guimin Hao, aGFvZ3VpbWluQDE2My5jb20=
 Yueming Xu
Yueming Xu Jie Zhang
Jie Zhang Aimin Li
Aimin Li Guimin Hao
Guimin Hao Bu-Lang Gao
Bu-Lang Gao