
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell Dev. Biol. , 09 March 2022
Sec. Molecular and Cellular Pathology
Volume 10 - 2022 | https://doi.org/10.3389/fcell.2022.842320
This article is part of the Research Topic Extracellular Vesicles as Next Generation Therapeutics View all 11 articles
The vast majority of cells in the human body are capable of secreting exosomes. Exosomes have become an important vehicle for signaling between cells. Exosomes secreted by different cells have some of the structural and functional properties of that cell and thus have different regulatory functions. A large number of recent experimental studies have shown that exosomes from different sources have different regulatory effects on stroke, and the mechanisms still need to be elucidated. Microglia are core members of central intrinsic immune regulatory cells, which play an important regulatory role in the pathogenesis and progression of stroke. M1 microglia cause neuroinflammation and induce neurotoxic effects, while M2 microglia inhibit neuroinflammation and promote neurogenesis, thus exerting a series of neuroprotective effects. It was found that there is a close link between exosomes and microglia polarization, and that exosome inclusions such as microRNAs play a regulatory role in the M1/M2 polarization of microglia. This research reviews the role of exosomes in the regulation of microglia polarization and reveals their potential value in stroke treatment.
According to a report published by the World Health Organization in 2020, stroke is the second leading cause of death worldwide. The lethal and disabling nature of stroke greatly increases the burden on society and individual families. The main treatments are intravenous thrombolysis and mechanical thrombectomy. Both of therapeutic strategies are limited by the recommended treatment time window and the effect is still not ideal (Thiebaut et al., 2018; Ozaki et al., 2019). In order to achieve better treatment, research on new therapeutic targets is necessary.
Ischemic stroke is the main type of stroke pathogenesis, mainly due to impaired blood supply to the brain, which occurs followed by a series of ischemia-reperfusion injuries, such as inflammation and oxidative stress, while persistent neuroinflammation damages neurons and the blood-brain barrier, which in turn leads to disease progression (Zhuang et al., 2017; Xu et al., 2018; Azedi et al., 2019). In the pathological process of stroke, injury are mainly induced by ischemia and/or ischemia-reperfusion, and microglia play an important role in this process. When microglia are activated, they transform into two main M1 and M2 phenotypes. M1 is the pro-inflammatory type, which secretes substances such as interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor (TNF) that promote inflammation and neurotoxic substances that aggravate brain damage (Shu et al., 2016; Zhu et al., 2019). M2 phenotype is anti-inflammatory, which secretes anti-inflammatory cytokines such as interleukin-4 (IL-4), interleukin-10 (IL-10), transforming growth factor-β (TGF-β) and some neurotrophic factors, which facilitate brain function recovery and improve the prognosis of stroke (Hu et al., 2012; Liu et al., 2016; Shu et al., 2016).
Exosomes are involved in cell-to-cell regulation through their various inclusions (Braccioli et al., 2014). It not only regulates normal physiological processes, but also assumes an important role in the development of some diseases such as cancer (Pan and Johnstone, 1983). According to recent studies, it was found that exosome transport of miR-124-3p, a substance that promotes M2 microglial polarization, reduced brain damage and improved the outcome of stroke (Huang et al., 2018). This study will summarize the mechanisms of communications between microglia and stroke and elucidate roles of different sources of exosomes in regulating stroke by targeting microglia polarization, thus providing new therapeutic strategies for stroke.
Almost all prokaryotes and eukaryotes can release extracellular vesicles (EVs) for communication with other cells (Kalluri and LeBleu, 2020). The classification of EVs is constantly improving. Currently, the EVs are mainly divided into two categories: ectosome and exosome (Cocucci and Meldolesi, 2015; Théry et al., 2018). Exosome is a special type of secretory vesicle with small volume in EVs, which is wrapped with a phospholipid bilayer outside. And its diameter is about 50 nm to 1 um (Kalluri and LeBleu, 2020). Exosomes contain proteins, lipids, deoxyribonucleic acid, messenger RNA (mRNA), microRNA and so on (Braccioli et al., 2014). Exosomes emerge from the plasma membrane and endosomal membrane, diffuse into the intercellular fluid, fuse with the recipient cells, release their contents, and exert regulatory effects (Mittelbrunn and Sánchez-Madrid, 2012). they can be produced from a variety of cells such as reticulocytes, antigen-presenting cells, tumor cells, skeletal cells, etc., and the exosomes secreted by different cells have different biological effects and different intensity of effects (Harding et al., 1983; Anderson et al., 2005; Braccioli et al., 2014) (Fordjour et al., 2019). In terms of morphological structure, the exosomes have phosphatidylethanolamine (PE) and phosphatidylserine (PS) on the bilayer membrane, which is a distinctive feature of the exosome plasma membrane and also makes the exosomes have a high membrane curvature, which is conducive to maintaining the stability of the exosome membrane (Booth et al., 2006).
Exosome-mediated cell-to-cell interaction involves many physiological processes such as reproduction and development of mammals, immune response, metabolism, and many pathological changes such as neurodegenerative diseases, cardiovascular diseases and tumorigenesis (Kalluri and LeBleu, 2020). Exosomes play an important role in the physiological activities of cells, e.g., during fertilization, Juno receptors on the ovum are shed as exosomes, thus preventing other sperm from fertilizing (Bianchi et al., 2014). Exosomes, which originate from the embryonic trophectoderm, can transmit their characteristic high resistance to viral infection to other cells (Delorme-Axford et al., 2013). Exosomes, with their PS-rich outer membrane, play an important role in the osteogenesis process, and osteoblasts secrete exosomes to initiate the process of mineralization in vivo (Anderson et al., 2005). They also have an important regulatory role in the pathological processes of cells. In tumor cells, the exosome secretion pathway is held hostage by tumor cells to accomplish a variety of pathological activities, and exosomes contain substances that stimulate tumor proliferation (Skog et al., 2008). The exosomes contain substances that stimulate tumor proliferation. Metastatic melanoma cells suppress cellular immunity by releasing exosomes with Programmed Cell Death-Ligand 1 (PD-L1) on their surface and upregulating the intracellular PD-L1 concentration in target cells (Chen et al., 2018). HIV virus can also fuse with normal cells in the form of exosomes, thus infecting normal cells (Melikyan, 2014) (Pegtel et al., 2010). In summary, exosomes are used as a means to protect the normal cells. In conclusion, exosomes play an important role in the mutual regulation of cells as messengers of intercellular signaling.
Exosomes have an important role in the development of many diseases, especially neurological diseases. mRNA mutants and miRNAs characteristic of glioma can be detected in serum exosomes of glioblastoma patients, which may become a new target for the diagnosis and treatment of this disease (Skog et al., 2008). Prions are essentially a misfolded form of protein, and their spread in vivo is mainly in the form of exosomes (Fevrier et al., 2004). A large number of recent studies have shown the applicability of exosomes for the treatment of stroke. One reason is that exosomes can easily cross the blood-brain barrier and have the potential to be used as a vehicle for brain-targeted modulation or drug administration (Rufino-Ramos et al., 2017; Khan et al., 2021). Second, exosomes were found to transport miRNAs such as miR-124-3p, which can promote microglia polarization toward the anti-inflammatory M2 phenotype, thereby inhibiting neuroinflammation (Huang et al., 2018). These suggest that exosomes may become important therapeutic targets in the stroke.
In view of the heterogeneity of exosomes in source, content, function and size, exosomes have a good prospect in the diagnosis and treatment of different clinical diseases. The research shows that exosomes may become diagnostic markers of many diseases, including cardiovascular diseases (Zhang et al., 2017a; Jansen and Li, 2017), cancer (Fitts et al., 2019), liver-related diseases (Masyuk et al., 2013), central nervous system diseases and so on (Kanninen et al., 2016). In the mouse model, compared with the use of liposomes, exosomes are able to enter cells more effectively with less immune clearance in vivo (Ferguson and Nguyen, 2016; Barile and Vassalli, 2017; Liao et al., 2019). Exosomes play an important role in cardiovascular function regulation and cardiovascular and cancer therapy (Li et al., 2021a; Lim, 2021; Nasser et al., 2021; Robson, 2021). With the development of technology, artificial exosomes have been used to deliver various nanomedicines (Li et al., 2021b). Therefore, the clinical transformation of exosomes in the diagnosis and treatment of different diseases deserves further research.
Microglia are resident cells of the Central nervous system (CNS) and belong to the monocyte macrophage system, but microglia, unlike other individuals in the monocyte macrophage system, develop initially from c-KitloCD41lo progenitor cells produced in the yolk sac (YS) around embryonic day 7.25 (E7.25), well before the appearance of other glial cells (Ginhoux et al., 2010). Microglia exhibit different states and have different functions during developmental and adult stages. During development, microglia are “phagocytic” and mobile amoeba-like, reflecting the phagocytic role of microglia in removing dead cells and remodeling neural tissue (Frost and Schafer, 2016; Matcovitch-Natan et al., 2016; Hagemeyer et al., 2017). Microglia have multiple effects on synapses, removing non-functional synapses and remodeling synaptic circuits, as demonstrated by Paolicell et al., 2011, in which C-X3-C motif chemokine receptor 1 (CX3CR) 1-deficient animals exhibit a defective number of microglia and transient defects in synaptic connections during development (Paolicelli et al., 2011; Wake et al., 2013). Microglia at the adult stage appear to be quiescent, but in fact they are constantly scanning and monitoring their surroundings with their characteristic branches and interacting with neighboring cells. Their ability to clear cells is also very strong and does not even require activation (Hambardzumyan et al., 2016).
In the case of stroke, the activated microglia will promote neuroinflammation and thus further brain tissue damage (Jiang et al., 2020). Conversely, induction of M2 microglia polarization facilitates stroke recovery. Multiple pathways can induce different microglia polarization. Among transcription factors, Nuclear factor kappa B (NF-κB), signal transducer and activator of transcription (STAT) family members, and thrombospondin A2R receptor can promote microglial cell conversion to M1 type. Yang et al. used safranin which reduces the expression of M1 markers, inhibits microglial cell conversion to M1 type by reducing IκBα phosphorylation and NF-κB/p65 nuclear translocation (Yang et al., 2019a). Elena Butturini et al. showed that signal transducer and activator of transcription 1 (STAT1) gene silencing can counteract the hypoxic-M1 microglia phenotype, thus suggesting that STAT1 can promote microglia polarization to the M1 phenotype (Butturini et al., 2019).
In ischemia/reperfusion mice, increased Thromboxane A2 receptor (TXA2R) expression was detected in microglia/macrophages by double staining with immunofluorescence, and the TXA2R antagonist SQ29548 inhibited M1 microglia activation and subsequent inflammatory response (Yan et al., 2016). The transcription factors nuclear factor erythroid 2-related factor 2 (Nrf2) and translocation of proliferator-activated receptor gamma (PPARγ) promote microglial cell transition to the M2 phenotype (Jiang et al., 2020). It has been shown that l-F001, a novel multifunctional rho-related protein kinase inhibitor, can increase the expression level of the M2 microglia marker CD206 by activating the Nrf2 signaling pathway in vitro (Chen et al., 2017). A study by Liu et al. found that 10-o- (n,n-dimethylaminoethyl) -ginkgolide B methanesulfonate, a new derivative of ginkgolide B, could convert polarized BV2 microglia from M1 phenotype to M2 phenotype by promoting the translocation of PPARγ from the nucleus to the cytoplasm (Liu et al., 2018). Multiple ways have been found capable of inducing microglia polarization. At the same time, it has been found that some substances can convert polarized M1 microglia to the M2 phenotype. This suggests that modulating the conversion of microglia to M2 phenotype may be a practicable therapeutic strategy for stroke.
Recent studies have shown that different miRNAs transported by exosomes contribute to the polarization of microglia into different phenotypes. miRNAs are endogenous hairpin-loop structured non-coding RNAs, which mainly bind to mRNAs to block their translation and regulate gene expression. Exosomes containing miR-192-5p, miR-183, miR-378, miR-140-3p, miR-222 were found to inhibit the expression of TNF or/and IL-1β expression, thereby promoting microglia M2 polarization (Das et al., 2014; Ti et al., 2015; Eirin et al., 2017; Thomi et al., 2019; Bian et al., 2020). NF-κB is an important signaling hub that drives microglia M1 polarization (Taetzsch et al., 2015). It has been reported that a variety of miRNAs contained in exosomes can regulate the expression of microglia Toll-like receptors (TLRs), thus acting on NF-κB to regulate microglia polarization (Hajinejad and Sahab-Negah, 2021). miR-223, miR-101-3p, miR-26a -5p, miR-326, miR-182, miR-17-5p, miR-140-5p, miR-9, miR-let7, and miR-181c play a role in inhibiting M1 microglia polarization by downregulating TLR expression including TLR2 and TLR4 (Kumar et al., 2015; Wang et al., 2015; Li et al., 2016; Li et al., 2017; Qian et al., 2017; Li et al., 2019; Li et al., 2020a; Yang et al., 2020a; Chaurasiya et al., 2020; Huang et al., 2020). In addition, miR-26b-5p inhibited the TLR signaling pathway by regulating the reduction of CH25H protein expression, which in turn inhibited microglia M1 polarization (Li et al., 2020b).
In addition to regulating TLR expression, miRNAs can also directly regulate NF-κB expression levels. Studies have reported that miR-34a reduces the secretion of pro-inflammatory cytokines such as TNF and IL-1β by decreasing the expression of NF-κB and Notch-1 proteins (Domenis et al., 2018). miR-21 reduces the expression of TNF by inhibiting NF-κB pathway, increases the production of anti-inflammatory factor IL-10 and promotes microglia polarization to M2 phenotype (Das et al., 2014). miR-146a-5p downregulates the expression of M1-type microglia-related genes by inhibiting Interleukin-1 receptor-associated kinases (IRAK1) and Nuclear factor of activated T cells 5 (NFAT5) and thus downregulating the expression of genes characteristic of M1-polarized microglia (Duan et al., 2020). miRNAs have also been found to regulate both TLR and NF-κB expression. miR-216-3p shifts microglia from M1 to M2 phenotype by inhibiting the TLR/NF-κB signaling pathway (Liu et al., 2020). In addition, exosomes containing miR-21-5p and miR-29b-3p promote microglia polarization from M1 to M2 phenotype and inhibit M1 polarization by suppressing Inducible nitric oxide synthase (iNOS) mRNA expression and reducing the production of pro-inflammatory factors (Tsai et al., 2019; Jiang et al., 2020). It was found that miR-124 promoted microglia polarization to M2 type by inhibiting the transcription factor CCAAT/enhancer binding protein-α (C/EBP-α) and its downstream target protein Purine Rich Box-1 (PU.1) (Zha et al., 2021).
As mentioned above, exosomal miRNAs that act on the TLR/NF-κB pathway and its downstream signaling pathway can inhibit microglia M1 polarization. Uniquely, miR-155 was reported to promote M1 polarization in microglia. The mechanism may be related to the suppression of IL-13R, SMAD2 and CCAAT/enhancer-binding protein β (CEBPβ) expression (Allen et al., 2013; Dickey et al., 2017). However, miR-155 has been reported to promote M2 polarization by inhibiting TGF-Beta-Activated Kinase 1-Binding Protein 2 (TAB2) (Zhang et al., 2018). Most studies have reported the inhibitory roles of miRNAs in M1 polarization, while studies on miRNAs that inhibit M2 polarization-related pathways are still lacking and deserve further exploration (Figure 1).
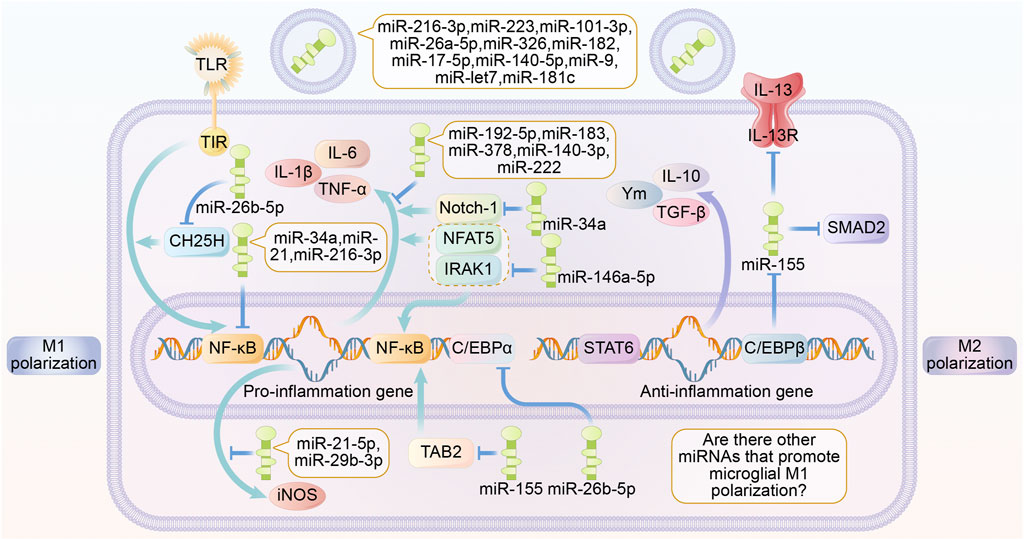
FIGURE 1. Roles of exosome miRNA in microglia polarization. Different miRNAs secreted by exosomes are able to play a role in regulating microglia polarization through multiple signaling pathways. miR-216-3p, miR-223, miR-101-3p, miR-26a-5p, miR-326, miR-182, miR-17-5p, miR-140-5p, miR-9, miR-let7, miR-181c are able to inhibit TLR expression. miR-192-5p, miR-183, miR-378, miR-140-3p, miR -222 are able to directly inhibit the expression of inflammatory factors. miR-26b-5p inhibits the TLR signaling pathway by suppressing the expression of CH25H. miR-34a, miR-21, miR-216-3p are able to directly inhibit the expression of NF-κB. miR—21-5p, and miR-29b-3p inhibits the expression of the pro-inflammatory factor iNOS. miR-155 inhibits NF-κB activation by suppressing TAB2. miR-26b-5p inhibits C/EBPα expression. miR-146a-5p inhibits the expression of IRAK1 and NFAT5, thereby suppressing the expression of inflammation-associated genes and products. miR-34a inhibits the expression of Notch-κB. MiR-34a is able to inhibit Notch-1. The above miRNAs promote microglia M2 polarization and anti-inflammatory factor production. miR-155 promotes microglia M1 polarization and inflammatory factor production by inhibiting the expression of IL-13 receptor, SMAD2 and C/EBPβ. Abbreviations: TLR, toll like receptor; iNOS, inducible nitric oxide synthase; TGF β, transforming growth factor β; Ym, chitinase-like proteins; C/EBPα, CCAAT/enhancer-binding protein α; C/EBPβ, CCAAT/enhancer-binding protein β; TNF-α, tumour necrosis factor α; STAT6, signal transducer and activator of transcription 6; NF-κB, Nuclear factor kappa B; NFAT5, nuclear factor of activated T cells 5; IRAK1, interleukin-1 receptor-associated kinase1; TAB2, TGF-Beta-Activated Kinase 1-Binding Protein 2; SMAD2, Sma- And Mad-Related Protein 2.
Acute cerebral ischemia is the initiating event of stroke. Inadequate blood supply leads to hypoxia and glucose shortage, disruption of homeostasis, which leads to inflammation, glutamate excitotoxicity, and mitochondrial dysfunction (Mo et al., 2020). Post-ischemia inflammation is mainly caused by necrotic tissue and activated inflammatory cells (Zhang et al., 2020). Microglia are the major intrinsic immune cells of the CNS and dominate the regulation of central inflammation. Damps (damage-associated molecular proteins) include high mobility group box 1tbox1 protein (HMGB-1), extracellular peroxiredoxin (Prx) family proteins and galectin-3 (Gal3), which are closely associated with microglia activation (Amruta et al., 2020). During stroke, hypoxic necrosis of brain cells leads to the production of various DAMPs (Xiong et al., 2016). HMGB-1 stabilizes nucleosome structure and participates in several physiological processes as a signaling factor. In stroke, HMGB-1 acts as a pro-inflammatory cytokine that activates microglia through the receptor of advanced glycation end-product (RAGE) and TLR-MyD88 pathways, producing immunosuppressive and lymphotoxic effects that further aggravate brain injury (Liesz et al., 2015; Jayaraj et al., 2019). Lin et al. found that in a mouse model of cerebral hemorrhage, heme activates TLR4 which in turn activates the MyD88/TRIF signaling pathway, ultimately allowing activation of the NF-κB pathway (Lin et al., 2012). Activated M1 microglia can release pro-inflammatory cytokines, reactive oxygen species (ROS) and Matrix metalloproteinase (MMP) to disrupt the blood-brain barrier and exacerbate neuroinflammation. After necrotic tissue is cleared, M2 microglia secrete the anti-inflammatory cytokine IL-10 and the neurotrophic factor IGF-1 to promote neural repair and reduce brain damage (Iadecola and Anrather, 2011). High expression of CD206, a marker of M2, and high expression of MHCII, a marker of M1, have been reported at about 3 and 7 days after stroke separately (Mirza et al., 2015). Microglia phenotypic shift and neuroinflammation are closely associated with the progression of stroke.
Many studies have now shown anti-neuroinflammatory effects in mouse models of stroke by modulating microglia polarization. These studies usually target TLR and Notch-related signaling pathways. In mouse/rat middle cerebral artery occlusion (MCAO) and oxygen-glucose deprivation (OGD) models, polyinosinic-polycytidylic acid activates TLR3/IRF3 signaling pathway and inhibits TLR4/NF-κB signaling pathway to reduce brain edema and improve prognosis (Wang et al., 2014). In hypoxia rat models, N-[N- (3,5-difluorophenacetyl) -1-alany1-S-phenyglycine t-butyl ester (DAPT) inhibited NF-κB/p65 expression by suppressing the Notch signaling pathway and TLR4/MyD88/TNF receptor associated factor 6 (TRAF6) pathway. Ultimately the expression levels of various inflammatory mediators such as TNF, IL-1β and iNOS proteins were reduced (Yao et al., 2013). In ischemia-reperfusion, NOSH-NBP, a novel mixture, can suppress the TLR4/MyD88/NF-κB pathway which leads to reduction of pro-inflammatory factors and microglia conversion to M1 phenotype. It also enhances the nuclear translocation of PPARγ and promotes the M2 polarization of microglia (Ji et al., 2017). cAMP-responsive element-binding protein (CREB) competes with NF-κB signaling pathway for the same coactivator molecule. By selectively activating CREB, the expression of M2-related genes may be increased and stroke related brain injury may be improved (Xia et al., 2015).
Oxidative stress is one of the important mechanisms that cause brain damage after stroke. Studies have shown that activated M1 glial cells such as microglia in the semidark zone in stroke will generate large amounts of oxidants including superoxide anion, hydrogen peroxide and peroxynitrite or nitrogen dioxide (Orellana-Urzúa et al., 2020). First, ROS lead to membrane damage and calcium overload, which in turn leads to mitochondrial dysfunction and ATP deficiency (Orellana-Urzúa et al., 2020). ATP deficiency will lead to sodium-potassium-ATPase and calcium pump dysfunction, ultimately exacerbating the disorder. In the case of stroke, oxidative stress is further exacerbated by the activation of enzyme xanthine oxidase enzyme (XO) enzymes, which further produce uric acid, superoxide radical anion and hydrogen peroxide by oxidizing hypoxanthine (Zhang et al., 2017b). In the mouse stroke model, the expression levels of antioxidants such as Superoxide dismutase (SOD) and Glutathione (GSH) are significantly reduced, followed by the exposure of proteins, lipids, and nucleic acids to ROS attack leading to neuronal cell death (Chamorro et al., 2016). Microglia located in the ischemic semidark zone of stroke are a major source of ROS (Chamorro et al., 2016). Ischemia and hypoxia lead to neuronal damage and release of DAMPs, which induce microglia to polarize to type M1. STAT family members play an important role in microglia polarization, with interferon γ (IFN-γ) activating STAT1 to promote M1 phenotype and IL-13 and IL-4 activating STAT6 to promote microglia polarization to type M2 (Allen et al., 2013). In stroke, activation of the TLR/NF-κB signaling pathway is associated with the induction of M1 microglia by IFNγ secreted by T helper 1 (Th1) cells (Zhao et al., 2017). NADPH oxidase 2 (NOX2) signaling activation is main pathway for ROS release from microglia. The regulatory part of NOX2 moves to the membrane and binds to the membrane-bound xanthochrome subunit upon induction by inflammatory stimuli such as IFN-γ, thus becoming a complex with nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity, which eventually promotes ROS production (Radak et al., 2017).
M2 phenotype of microglia are thought to be beneficial for neuronal regeneration. Microglia activated to M2 phenotype by IL-4, TGF-β will release brain-derived neurotrophic factor (BDNF), vascular endothelial growth factor (VEGF) and insulin-like growth factor-1 (IGF-1) (Hu et al., 2015; Spejo et al., 2018). BDNF is able to promote nerve survival, recovery, and regeneration (Allen et al., 2013). In a female mouse model, BDNF was found to interact with TrkB receptors expressed by oligodendrocytes to promote myelin formation, increase oligodendrocyte differentiation and myelin thickness after demyelination injury (Fletcher et al., 2018). Currently, various methods to polarize microglia M2 to promote neuronal regeneration are vigorous. Ultrasound intervention is a new approach that has recently emerged. This approach involves engineering platelet membrane (PM) decorated resting microglia, liposome-encapsulated IL-4 (here called CPIL4) and ultrasound to specifically polarize microglia to an anti-inflammatory phenotype. Intravenous injection of this specific microglia, followed by ultrasound irradiation to remotely control the local destruction of liposomes and release of IL-4, allows local conversion of microglia to the M2 phenotype, thereby inducing regeneration of vascular and neuronal structures in mice with ischemic stroke (Liu et al., 2021). The purinergic receptor (P2X4R) has a regulatory role in the phenotypic conversion of microglia. Ivermectin (IVM) promotes myelin regeneration by enhancing the P2X4R signaling pathway to promote the phenotypic conversion of microglia to the M2 phenotype (Zabala et al., 2018). In addition, miR-124-3P-containing exosomes secreted by M2 microglia were found to promote neurite outgrowth (Huang et al., 2018). Some drugs that inhibit M1 polarization of microglia have also been found to promote neurite regeneration under pathological conditions. In a mouse model of depression, Minocycline attenuated the inhibitory effect of neurotoxic M1 microglia on neurogenesis under chronic stress (Bassett et al., 2021). M1 microglia can exert synaptic phagocytic pruning by recognizing the complement component subunit 1q (C1q) complex at neuronal synapses (Allen et al., 2013). Short-Chain Fatty Acids promote post-stroke recovery by inhibiting microglia activation and thereby mitigating synaptic elimination (Kano et al., 2019). Collectively, promoting neuronal regeneration by facilitating microglia M2 polarization has great potential for application.
Brain tissue ischemia will result in insufficient supply of glucose and oxygen, which will disrupt the mitochondrial electron transport chain and lead to reduced ATP production (Ahsan et al., 2021). Dysregulation of ion concentrations caused by ATP deficiency will lead to a series of sequential events such as glutamate excitotoxicity. Excess glutamate will lead to calcium overload in peripheral cells, which induces neuronal cell death (Simpson and Oliver, 2020). Animal studies have shown that the expression of TNF, TNF-related inducing ligand (TRAIL), and Fas ligand (FasL) are all upregulated after ischemia, and these molecules worsen stroke prognosis to some extent (Radak et al., 2017). Microglia trigger neuronal cell death mainly through two pathways including death receptor pathway and mitochondrial pathway (Fricker et al., 2018). M1 microglia activate the death receptor pathway through the release of TNF. Through a cascade of reaction, TNF promotes activation of Bcl-2-associated X (Bid)and causes mitochondrial outer membrane permeabilization (MOMP). Ultimately, cytochrome c and caspase-9 activate downstream effectors to cleave cellular proteins and thus promote neuron apoptosis (Micheau and Tschopp, 2003; Haase et al., 2008). Many factors can activate the mitochondrial pathway, such as toxins, radiation and ROS released from M1 microglia. ROS can directly induce mitochondrial outer membrane permeabilization, which activates caspase-9 and acts downstream on caspase-3 and caspase-7, ultimately inducing apoptosis in neurons (Circu and Aw, 2010).
Autophagy affects stroke progression by regulating microglia polarization. Peroxisome proliferator-activated receptor γ (PPARγ) is a class of ligand-activated transcription factors that are important for inflammation regulation (Guo et al., 2017). It was shown that the PPARγ antagonist T0070907 could inhibit the expression of M1 markers such as iNOS and promote the expression of M2 markers such as CD206. This result was shown to be associated with enhanced cellular autophagy induced by LKB1-AMPK activation (Ji et al., 2018). In addition, it has been shown that Oxiracetam (ORC) promotes microglia autophagy through the AMPK/mTOR (AMPK inhibits mTOR) pathway, thereby promoting microglia M2 polarization (Wang et al., 2021a). It has also been demonstrated that peripheral macrophage-derived exosomes (PM-Exos) can locally induce M2 microglia production by promoting autophagy (Pandey et al., 2021). Furthermore, in the mouse oxygen glucose deprivation/reperfusion (OGD/R) model, microglia autophagic flux was inhibited by suppression of NF-κB pathway. Meanwhile, rise in M1 markers and decrease in M2 markers were detected when OGD/R reached 72 h (Xia et al., 2016). The above studies suggest that autophagy plays a role in the inhibition of microglia M2 polarization and neuroinflammation, and that inhibition of microglia autophagy would exacerbate neuroinflammation. However, miR-30d-5p-capsuling exosome secreted by adipose stem cells (ADSCs) was found to significantly reduce the area of infarct injury by inhibiting autophagy and promoting M2 microglia in AIS patients and rat models and in vitro models of hypoxia-glucose deprivation primary microglia (Jiang et al., 2018a). Heterogeneity of these results remains to be clarified.
In stroke, inflammasome is activated and then activates caspase-1 to shear gasdermin D, the nitrogen terminal of which form pores in the plasma membrane, finally leading to cell rupture and inflammatory responses (Tuo et al., 2021) (Fricker et al., 2018). Most recent studies have showed adverse effects of pyroptosis on the prognosis of ischemic stroke. Inhibition of platelet-activated factor receptors can inhibit the activation of down-regulated inflammasomeand thus reduce pyroptosis, while reducing ischemia-reperfusion injury (Zhao et al., 2021). In the Transient middle cerebral artery occlusion (tMCAO) mouse model, Peroxisome proliferator-activated receptor gamma coactivator 1α (PGC-1α) stimulated by medioresinol acts on the PPARα/Glutamate Oxalo (GOT1) axis to significantly reduce the expression of pyroptosis-related proteins and improve ischemic brain injury (Wang et al., 2021b). Microglia pyroptosis was found in ischemia-reperfusion injury (Wang et al., 2020). Bone marrow mesenchymal stem cell-derived exosomes (BMSCs-Exos) are neuroprotective by downregulating the expression of neuronal NLR family pyrin domain containing 3 (NLRP3) inflammasome and pyroptosis-related proteins (Liu et al., 2021). In pMCAO mice, dexmedetomidine inhibited microglia pyroptosis by acting on the P2X7R/NLRP3/Caspase-1 signaling pathway and play a protective role in ischemic brain injury (Sun et al., 2021). In retinal ischemic injury, a NOD-like receptor named NLR caspase recruitment domain (CARD) containing 5 (NLRC5) was identified, which binds to the inflammasome NLRP3 and NLR-family CARD-containing protein 4 (NLRC4) and mediates microglial cell pyroptosis (Deng et al., 2021). In addition, in spinal cord injury, CD73 may inhibit the activation of the NLRP3 inflammasome complex through the adenosine-A2B adenosine receptor- phosphoinositide 3-kinase (PI3K)-AKT-Foxo1 cascade, reducing the activation of gasdermin D and thus microglia pyroptosis (Xu et al., 2021). Microglia pyroptosis and stroke nerve injury are closely related. However, reports on microglia pyroptosis in stroke models are still lacking and need to be further added. Furthermore, despite the lack of studies linking microglia polarization and pyroptosis, it is known that NLRP3 activation is associated with both microglia M1 polarization and the occurrence of pyroptosis. Therefore, the relationship between microglia pyroptosis and their pro-inflammatory polarization deserves further exploration.
Glutamate excitotoxicity is an important mechanism associated with neuronal death in stroke. Ischemia and hypoxia lead to impaired ATP synthesis, which leads to sodium-potassium pump dysfunction and eventually causes depolarization of the cell membrane and activation of some calcium channels. This process will lead to increased release and decreased reuptake of glutamate (Fricker et al., 2018). Excessive concentrations of glutamate bound to N-methyl-D-aspartate (NMDA) receptors will lead to calcium overload and thus induce peripheral neuronal death (Yang et al., 2019b). After microglia activation, large amounts of glutamate are released through two glutamate transport systems, the glutamate transporter 1 (GLT-1) transport system and the xCT transport system (Belov Kirdajova et al., 2020). It has been shown that Post-synaptic density 93 (PSD-93) binds to CX3 chemokine ligand 1 (CX3CL1) and thereby activates microglia, mediating ischemia-induced glutamate excitotoxicity in acute ischemic stroke (Zhang et al., 2021a). Glutamate excitotoxicity can also be reduced by controlling the signaling pathway of glutamate release of microglia. Inhibition of endoplasmic reticulum inositol 1,4,5-trisphosphate (InsP3) receptor activation with vitamin C improves the permeability of microglia gap junctions and inhibits glutamate release (Socodato et al., 2018). Microglia reduce glutamate excitotoxicity by modulating neuronal calcium metabolism and reducing calcium overload (Szalay et al., 2016). In addition, G protein-coupled receptor 30 (GPR30) has been demonstrated to be neuroprotective and reduce glutamate-induced excitotoxity. G1, a stimulator of GPR30, can promote microglia polarization to M2 phenotype and thus promotes the neuroprotection (Yang et al., 2021).
Disruption of the blood-brain barrier is also one of the pathological features of stroke, mainly characterized by structural disruption of tight junction protein complexes and increased permeability, which usually implicates poor prognosis (Profaci et al., 2020). P2RY12 (purinergic receptor P2Y, G-protein coupled, 12) can mediate the movement of microglia to the site of blood-brain barrier damage. These aggregated microglia can not only patch up small breaches, but they can also temporarily assume the function of the blood-brain barrier (Lou et al., 2016). When microglia are polarized to the M2 phenotype, they can better protect the blood-brain barrier by producing IL-10 and TGF-β1, as well as VEGF, IL-8 and pro-MMP-9 to promote vascular remodeling (Jiang et al., 2018b). The use of tetramethylpyrazine (TMP) in experimental autoimmune encephalomyelitis animal models protects the blood-spinal cord barrier by activating the signal transducer and activator of transcription 3 (STAT3)/Suppressor of Cytokine Signaling 3 (SOCS3) signaling pathway and promoting microglia polarization from M1 to M2 phenotype (Zhang et al., 2021b). M1-type microglia not only secrete pro-inflammatory cytokines, but also secrete chemokines such as chemokine (C-C motif) ligand 2 (CCL2) and Chemokine C-X-C ligand 10 (CXCL10) to recruit more immune cells and compromise the integrity of the blood-brain barrier (Jiang et al., 2018b). Minocycline may be an ideal therapeutic agent because of its good blood-brain barrier permeability, high safety profile, and targeting of M1 microglia. In a transient MCAO model, the use of minocycline promoted microglia M2 polarization significantly reduced TNF and IL-1β levels and increased TGF-β, IL-10 and chitinase-like proteins (YM1) levels, thereby reducing cerebral infarct size, promoting vascular remodeling, and improving stroke prognosis (Yang et al., 2015).
IL-1β released from M1 microglia can promote astrocyte activation. In addition, IL-1α, TNF and C1q can also differentiate astrocytes into the A1 phenotype, which is detrimental to stroke recovery (Liddelow and Barres, 2017). Under physiological conditions, astrocytes can render microglia dormant, but in ischemic conditions, the ability of astrocytes to spread calcium ions over long distances may allow microglia to be activated even far from the infarct area (Nedergaard and Dirnagl, 2005). It has been shown that the use of antagonists of purinergic receptors can effectively block this transmission and slow down the expansion of the infarct volume (Xie et al., 2011). In vitro cultures of microglia and astrocytes were mixed. Astrocytes stimulated substantial proliferation and M2 polarization of microglia, while astrocytes appeared to differentiate to the beneficial A2 type (Kim and Son, 2021). In the stroke simulation experiment, M2 microglia and A2 astrocytes were highly susceptible to pro-inflammatory cytokines. Secretion of TNF and IFN-γ by microglia induced transition of A2 astrocytes to A1, and secretion of granulocyte-macrophage colony-stimulating factor (GM-CSF) by astrocytes caused M2 microglia polarization to M1 (Kim and Son, 2021). In a rat model of tMCAO, microglia-derived macrophage-like cells (MG-MΦ) upregulate astrocyte aquaporin-4 (AQP4) expression and exacerbate brain edema (Murata et al., 2020). In chronic recurrent experimental autoimmune encephalomyelitis, astrocyte-secreted TGF-β2 was found to exacerbate cerebral edema by possibly downregulating major histocompatibility complex class II expression and costimulatory/adhesion molecules, thereby affecting the antigen-presenting function of microglia. Besides, TGF-β2 secreted by prompted astrocytes appear to promote neuroinflammation (Siglienti et al., 2007). Complement C3 secreted by astrocytes can acts on neurons and microglia, resulting in impaired phagocytosis and subsequent abnormal synaptic pruning of microglia (Lian et al., 2016). In cerebral hemorrhage, IL-15 secretion by astrocytes is significantly upregulated and contributes to the microglia polarization to M1 phenotype, exacerbating brain injury (Shi et al., 2020).. (Figure 2)
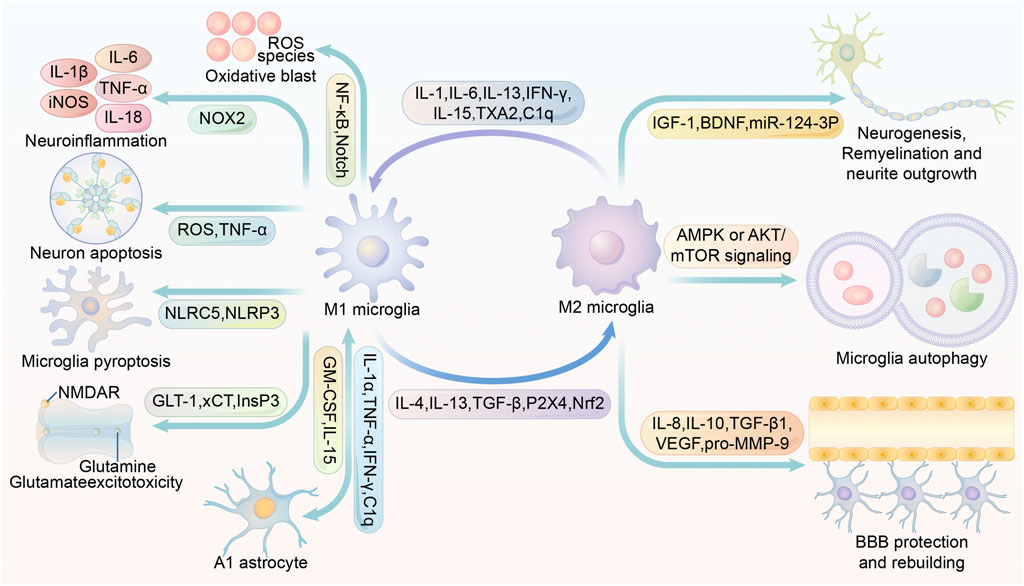
FIGURE 2. Multiple roles of M1/M2 microglia in stroke modulation. IL-1, IL-6, IL-13, IFN-γ, IL-15, TXA2 and C1q promote microglia M1 polarization or M2 microglia conversion to M1. IL-4, IL-13, TGF-β, P2X4 and Nrf2 promote microglia M2 conversion or M1 microglia conversion to M2. Activation of NF-κB and Notch in M1 microglia promotes inflammatory factor production. activation of NOX2 in M1 microglia promotes ROS production. M1 microglia are able to secrete ROS and TNF and thus induce neuronal apoptosis. activation of NLRP3 and NLRC5 in M1 microglia may contribute to microglia scorching. The inhibition of InsP3 receptors facilitates the inhibition of glutamate release. M1 microglia secrete IL-1α,TNF-α,IFN-γ, C1q to promote the A1 phenotype of astrocytes. A1 phenotype astrocytes secrete GM-CSF and IL-15 to promote microglia M1 polarization. glial cell M1 polarization. IL-4, IL-13, TGF-β, P2X4, Nrf2 induce microglia M2 polarization. M2 microglia promote neurogenesis, remyelination and neurite outgrowth by secreting IGF-1, BDNF and miR-124-3p-containing exosomes. Increased AMPK or AKT/mTOR signaling promotes microglia autophagy, and enhanced autophagy is closely associated with microglia M2 polarization. M2 microglia promote BBB protection and rebuilding by secreting IL-8, IL-10, TGF-β1, VEGF and pro-MMP-9. Abbreviations: iNOS, inducible nitric oxide synthase; TNF-α, tumour necrosis factor α; ROS, reactive oxygen species; NF-κB, Nuclear factor kappa B; NMDAR, N-methyl-D-aspartic acid or N-methyl-D-aspartate receptor; NOX2, NADPH oxidase 2; NLRC5, NOD-, LRR- and CARD-containing 5; NLRP3, NOD-like receptor protein 3; GLT-1, glutamate transporter-1; xCT, cystine/glutamate antiporter; InsP3, inositol 1,4,5-trisphosphate; GM-CSF, granulocyte-macrophage colony-stimulating factor; TXA2, thromboxane A2; TGF-β, tumor growth factor-β; Nrf2, Nuclear factor erythroid 2-related factor; IGF-1, insulin-like growth factor 1; BDNF, brain-derived neurotrophic factor; AMPK, AMP-activated protein kinase; mTOR, mammalian target of rapamycin; VEGF, vascular endothelial growth factor; BBB, blood-brain barrier.
Recently, a large number of studies have demonstrated that exosomes from different sources exert a significant regulatory effect on stroke-induced brain injury by targeting microglia polarization. Exosomes from endotoxemia mouse serum contain a large number of inflammation-associated miRNAs, which are phagocytosed by microglia and ultimately lead to increased neuroinflammation by promoting microglia activation and M1 polarization (Li et al., 2018). Young rat serum exosomes contain more CD46 and fewer complement components such as C1q. It was shown that serum exosomes from young rats could improve short- and long-term neurological outcomes in older rats with ischemic stroke by reducing M1 microglia and increasing M2 microglia (Zhang et al., 2021c). In acute ischemic stroke (AIS) patients and rat models, exosomes from different sources promote M2 polarization of microglia by releasing hsa-miR-124-3p and miR-30d-5p, respectively, thereby reducing brain damage caused by neuroinflammation (Jiang et al., 2018a; Qi et al., 2021). Exosomes derived from mesenchymal stem cells (MSCs) containing miR-223-3p were found to reduce infarct size and improve neurological deficits and learning memory in a mouse model of stroke by inhibiting the inflammatory response induced by M1 phenotype microglia (Zhao et al., 2020). Further studies found that exosomes from bone marrow mesenchymal stem cells (BMSCs) in Intracerebral hemorrhage (ICH) and MCAO mice reduced M1 microglia polarization and increased M2 polarization, thereby reducing neuroinflammation and neuronal apoptosis caused by ischemia or hemorrhage (Duan et al., 2020; Liu et al., 2021). In addition, human umbilical cord mesenchymal stem cells (hUMSCs) (Yang et al., 2020b; Zhang et al., 2021d), neural progenitor cells (Tian et al., 2021), lipopolysaccharide-stimulated macrophages (Zheng et al., 2019) and ADSCs (Geng et al., 2019) were found to alleviate ischemic injury caused by MCAO and promote neuroprotection and neurogenesis. These effects were shown to be closely related to exosome-induced microglia M2 polarization.
In addition to their own contained components that can exert stroke modulating effects, nanoscale exosomes can also act as carriers to transport relevant drugs or chemical molecules to modulate microglia polarization and thus influence stroke progression. Exosomes encapsulated with edaravone or melatonin can promote microglia M2 polarization and inhibit neuroinflammation (Li et al., 2020c; Liu et al., 2020). Exosomes derived from human embryonic kidney cells loaded with nerve growth factor (NGF) and its mRNA cause a decrease in the M1 phenotype and an increase in the M2 phenotype of microglia, thereby reducing ischemic damage caused by inflammation and cell death (Yang et al., 2020c). The above evidence provides a solid theoretical basis for the application of exosomes targeting microglia polarization in the treatment of stroke (Table 1).
Post-polarized microglia can secrete exosomes to influence stroke progression. Exosomes from microglia can promote neuronal necrosis and ICH injury by transmitting the pro-inflammatory miR-383-3p in ICH rats that sacrificed within 7 days (Wei et al., 2021). It was shown that exosomes derived from M2 microglia can reduce the volume of MCAO-induced cerebral infarcts, behavioral defects and promote neovascularization and neuronal survival by releasing miR-124, miR-137 and miR-26a within 3 days after ischemic attack (Song et al., 2019; Tian et al., 2019; Feng et al., 2021). OGD treated microglia can secrete exosomes containing miR-424-5p, which inhibit the fibroblast growth factor 2 (FGF2)/STAT3 signaling pathway and worsen endothelial cell damage (Xie et al., 2020). According to the in vivo study, activated microglia in the peri-infarct ischemic region were significantly increased in the first day and peaked at the 14th day after MCAO. The use of vinpocetine promotes microglia M2 polarization by enhancing microglia autophagy, ultimately attenuating neuronal damage caused by OGD treatment (Zang et al., 2020). The exosomes secreted by polarized microglia may also be an effective target for therapeutic modulation of stroke (Table 2).
Stroke remains to date a fatal and disabling serious neurological disease. Given the specificity of its lesion location and tissues and the complexity of its pathological mechanisms, the clinical outcome of stroke is still not particularly satisfactory. This is especially true for patients treated beyond the thrombolytic window. Therefore, research on new treatment modalities and therapeutic targets is particularly important.
Recent studies have shown that microglia polarization is strongly associated with stroke progression, and that M1 microglia will exacerbate stroke-induced brain injury by promoting neuroinflammation, oxidative stress, neuronal apoptosis, glutamate excitotoxicity, and astrocyte differentiation to the A1 phenotype through multiple signaling pathways. In addition, M1 microglia may further exacerbate inflammatory injury by promoting microglia pyroptosis. In contrast, M2 microglia exert a significant neuroprotective effect and promote neurological recovery. M2 microglia may promote neurogenesis, myelin regeneration, neurite growth and blood-brain barrier protection, thus exerting an important central protective function. Additionally, increased autophagic flux of microglia can promote their M2 polarization and thus reduce brain injury. Therefore, targeting microglia polarization and promoting the expression of M2 phenotype may become an important target for treatment and improving the prognosis of stroke.
Most cells are capable of secreting exosomes. Numerous studies have found that exosome pairs from different sources are able to regulate microglia polarization. This suggests that targeting the secretion of some specific exosomes may be able to regulate microglia polarization. In addition, the ability of exosomes to cross the blood-brain barrier makes them potentially an ideal vehicle for CNS drug delivery. The transport of specific drugs or molecules from exosomes to the CNS to regulate microglia polarization and improve the neurological environment has shown great potential for development. In animal models of stroke, the use of endogenous exosomes or exosomes as carriers to transport some drug molecules to promote the M2 polarization of microglia has achieved good therapeutic effects, and M2 polarized microglia can also play a corresponding neuroprotective role by secreting unique exosomes, and significantly inhibit the progression of stroke. Thus, targeting exosomes that regulate microglia and exosomes secreted by polarized microglia may be a new measure to treat stroke and improve its prognosis. However, the vast majority of exosomes have poor targeting properties. Currently, most studies have taken to implant molecules with targeting properties on the lipid membrane of exosomes, thus enhancing the targeting of exosomes. This will further increase the cost of exosome production and development, thus hindering their clinical application. Therefore, promoting the development of exosome acquisition and production technologies and increasing research on reagents that enhance exosome targeting will facilitate the clinical translation of relevant researches.
TW designed the article. TW, YH, and XG wrote the manuscript. WW and WG helped research and collect the materials. TW and XG prepared figures and tables. TW and WG critically revised the manuscript for important intellectual content. TW translated the article into English. All authors read and approved the final manuscript and agree to be accountable for all aspects of this work. Data authentication is not applicable.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank Haifeng Tan for his advice on some issues in the writing process of this article.
Ahsan, A., Liu, M., Zheng, Y., Yan, W., Pan, L., Li, Y., et al. (2021). Natural Compounds Modulate the Autophagy with Potential Implication of Stroke. Acta pharmaceutica Sinica B 11 (7), 1708–1720. doi:10.1016/j.apsb.2020.10.018
Allen, S. J., Watson, J. J., Shoemark, D. K., Barua, N. U., and Patel, N. K. (2013). GDNF, NGF and BDNF as Therapeutic Options for Neurodegeneration. Pharmacol. Ther. 138 (2), 155–175. doi:10.1016/j.pharmthera.2013.01.004
Amruta, N., Rahman, A. A., Pinteaux, E., and Bix, G. (2020). Neuroinflammation and Fibrosis in Stroke: The Good, the Bad and the Ugly. J. neuroimmunology 346, 577318. doi:10.1016/j.jneuroim.2020.577318
Anderson, H. C., Garimella, R., and Tague, S. E. (2005). The Role of Matrix Vesicles in Growth Plate Development and Biomineralization. Front. Biosci. 10, 822–837. doi:10.2741/1576
Azedi, F., Mehrpour, M., Talebi, S., Zendedel, A., Kazemnejad, S., Mousavizadeh, K., et al. (2019). Melatonin Regulates Neuroinflammation Ischemic Stroke Damage through Interactions with Microglia in Reperfusion Phase. Brain Res. 1723, 146401. doi:10.1016/j.brainres.2019.146401
Barile, L., and Vassalli, G. (2017). Exosomes: Therapy Delivery Tools and Biomarkers of Diseases. Pharmacol. Ther. 174, 63–78. doi:10.1016/j.pharmthera.2017.02.020
Bassett, B., Subramaniyam, S., Fan, Y., Varney, S., Pan, H., Carneiro, A. M. D., et al. (2021). Minocycline Alleviates Depression-like Symptoms by Rescuing Decrease in Neurogenesis in Dorsal hippocampus via Blocking Microglia Activation/phagocytosis. Brain Behav. Immun. 91, 519–530. doi:10.1016/j.bbi.2020.11.009
Belov Kirdajova, D., Kriska, J., Tureckova, J., and Anderova, M. (2020). Ischemia-Triggered Glutamate Excitotoxicity from the Perspective of Glial Cells. Front. Cel. Neurosci. 14, 51. doi:10.3389/fncel.2020.00051
Bian, H., Wang, G., Huang, J., Liang, L., Zheng, Y., Wei, Y., et al. (2020). Dihydrolipoic Acid Protects against Lipopolysaccharide-Induced Behavioral Deficits and Neuroinflammation via Regulation of Nrf2/HO-1/NLRP3 Signaling in Rat. J. Neuroinflammation 17 (1), 166. doi:10.1186/s12974-020-01836-y
Bianchi, E., Doe, B., Goulding, D., and Wright, G. J. (2014). Juno Is the Egg Izumo Receptor and Is Essential for Mammalian Fertilization. Nature 508 (7497), 483–487. doi:10.1038/nature13203
Booth, A. M., Fang, Y., Fallon, J. K., Yang, J.-M., Hildreth, J. E. K., GouldExosomes, S. J., et al. (2006). Exosomes and HIV Gag Bud from Endosome-like Domains of the T Cell Plasma Membrane. J. Cel. Biol. 172 (6), 923–935. doi:10.1083/jcb.200508014
Braccioli, L., van Velthoven, C., and Heijnen, C. J. (2014). Exosomes: a New Weapon to Treat the central Nervous System. Mol. Neurobiol. 49 (1), 113–119. doi:10.1007/s12035-013-8504-9
Butturini, E., Boriero, D., Carcereri de Prati, A., and Mariotto, S. (2019). STAT1 Drives M1 Microglia Activation and Neuroinflammation under Hypoxia. Arch. Biochem. Biophys. 669, 22–30. doi:10.1016/j.abb.2019.05.011
Chamorro, Á., Dirnagl, U., Urra, X., and Planas, A. M. (2016). Neuroprotection in Acute Stroke: Targeting Excitotoxicity, Oxidative and Nitrosative Stress, and Inflammation. Lancet Neurol. 15 (8), 869–881. doi:10.1016/s1474-4422(16)00114-9
Chaurasiya, V., Kumari, S., Onteru, S. K., and Singh, D. (2020). Up-regulation of miR-326 Regulates Pro-inflammatory Cytokines Targeting TLR-4 in buffalo Granulosa Cells. Mol. Immunol. 119, 154–158. doi:10.1016/j.molimm.2020.01.019
Chen, G., Huang, A. C., Zhang, W., Zhang, G., Wu, M., Xu, W., et al. (2018). Exosomal PD-L1 Contributes to Immunosuppression and Is Associated with Anti-PD-1 Response. Nature 560 (7718), 382–386. doi:10.1038/s41586-018-0392-8
Chen, J., Yin, W., Tu, Y., Wang, S., Yang, X., Chen, Q., et al. (2017). L-F001, a Novel Multifunctional ROCK Inhibitor, Suppresses Neuroinflammation In Vitro and In Vivo: Involvement of NF-Κb Inhibition and Nrf2 Pathway Activation. Eur. J. Pharmacol. 806, 1–9. doi:10.1016/j.ejphar.2017.03.025
Circu, M. L., and Aw, T. Y. (2010). Reactive Oxygen Species, Cellular Redox Systems, and Apoptosis. Free Radic. Biol. Med. 48 (6), 749–762. doi:10.1016/j.freeradbiomed.2009.12.022
Cocucci, E., and Meldolesi, J. (2015). Ectosomes and Exosomes: Shedding the Confusion between Extracellular Vesicles. Trends Cell Biology 25 (6), 364–372. doi:10.1016/j.tcb.2015.01.004
Das, A., Ganesh, K., Khanna, S., Sen, C. K., and Roy, S. (2014). Engulfment of Apoptotic Cells by Macrophages: A Role of MicroRNA-21 in the Resolution of Wound Inflammation. J.I. 192 (3), 1120–1129. doi:10.4049/jimmunol.1300613
Delorme-Axford, E., Donker, R. B., Mouillet, J.-F., Chu, T., Bayer, A., Ouyang, Y., et al. (2013). Human Placental Trophoblasts Confer Viral Resistance to Recipient Cells. Proc. Natl. Acad. Sci. 110 (29), 12048–12053. doi:10.1073/pnas.1304718110
Deng, Y., Fu, Y., Sheng, L., Hu, Y., Su, L., Luo, J., et al. (2021). The Regulatory NOD-like Receptor NLRC5 Promotes Ganglion Cell Death in Ischemic Retinopathy by Inducing Microglial Pyroptosis. Front. Cel Dev. Biol. 9, 669696. doi:10.3389/fcell.2021.669696
Dickey, L. L., Hanley, T. M., Huffaker, T. B., Ramstead, A. G., O'Connell, R. M., and Lane, T. E. (2017). MicroRNA 155 and Viral-Induced Neuroinflammation. J. neuroimmunology 308, 17–24. doi:10.1016/j.jneuroim.2017.01.016
Domenis, R., Cifù, A., Quaglia, S., Pistis, C., Moretti, M., Vicario, A., et al. (2018). Pro Inflammatory Stimuli Enhance the Immunosuppressive Functions of Adipose Mesenchymal Stem Cells-Derived Exosomes. Sci. Rep. 8 (1), 13325. doi:10.1038/s41598-018-31707-9
Duan, S., Wang, F., Cao, J., and Wang, C. (2020). Exosomes Derived from MicroRNA-146a-5p-Enriched Bone Marrow Mesenchymal Stem Cells Alleviate Intracerebral Hemorrhage by Inhibiting Neuronal Apoptosis and Microglial M1 Polarization. Dddt 14, 3143–3158. doi:10.2147/dddt.s255828
Eirin, A., Zhu, X. Y., Puranik, A. S., Woollard, J. R., Tang, H., Dasari, S., et al. (2017). Integrated Transcriptomic and Proteomic Analysis of the Molecular Cargo of Extracellular Vesicles Derived from Porcine Adipose Tissue-Derived Mesenchymal Stem Cells. PloS one 12, e0174303. doi:10.1371/journal.pone.0174303
Feng, Y.-S., Tan, Z.-X., Wu, L.-Y., Dong, F., and Zhang, F. (2021). The Involvement of NLRP3 Inflammasome in the Treatment of Neurodegenerative Diseases. Biomed. Pharmacother. 138, 111428. doi:10.1016/j.biopha.2021.111428
Ferguson, S. W., and Nguyen, J. (2016). Exosomes as Therapeutics: The Implications of Molecular Composition and Exosomal Heterogeneity. J. Controlled Release 228, 179–190. doi:10.1016/j.jconrel.2016.02.037
Fevrier, B., Vilette, D., Archer, F., Loew, D., Faigle, W., Vidal, M., et al. (2004). Cells Release Prions in Association with Exosomes. Proc. Natl. Acad. Sci. 101 (26), 9683–9688. doi:10.1073/pnas.0308413101
Fitts, C. A., Ji, N., Li, Y., and Tan, C. (2019). Exploiting Exosomes in Cancer Liquid Biopsies and Drug Delivery. Adv. Healthc. Mater. 8 (6), e1801268. doi:10.1002/adhm.201801268
Fletcher, J. L., Wood, R. J., Nguyen, J., Norman, E. M. L., Jun, C. M. K., Prawdiuk, A. R., et al. (2018). Targeting TrkB with a Brain-Derived Neurotrophic Factor Mimetic Promotes Myelin Repair in the Brain. J. Neurosci. 38 (32), 7088–7099. doi:10.1523/jneurosci.0487-18.2018
Fordjour, F. K., Daaboul, G. G., and Gould, S. J. (2019). A Shared Pathway of Exosome Biogenesis Operates at Plasma and Endosome Membranes. bioRxiv, 545228.
Fricker, M., Tolkovsky, A. M., Borutaite, V., Coleman, M., and Brown, G. C. (2018). Neuronal Cell Death. Physiol. Rev. 98 (2), 813–880. doi:10.1152/physrev.00011.2017
Frost, J. L., and Schafer, D. P. (2016). Microglia: Architects of the Developing Nervous System. Trends Cell Biology 26 (8), 587–597. doi:10.1016/j.tcb.2016.02.006
Geng, W., Tang, H., Luo, S., Lv, Y., Liang, D., Kang, X., et al. (2019). Exosomes from miRNA-126-Modified ADSCs Promotes Functional Recovery after Stroke in Rats by Improving Neurogenesis and Suppressing Microglia Activation. Am. J. Transl Res. 11 (2), 780–792.
Ginhoux, F., Greter, M., Leboeuf, M., Nandi, S., See, P., Gokhan, S., et al. (2010). Fate Mapping Analysis Reveals that Adult Microglia Derive from Primitive Macrophages. Science 330 (6005), 841–845. doi:10.1126/science.1194637
Guo, M., Li, C., Lei, Y., Xu, S., Zhao, D., and Lu, X.-Y. (2017). Role of the Adipose PPARγ-Adiponectin axis in Susceptibility to Stress and Depression/anxiety-Related Behaviors. Mol. Psychiatry 22 (7), 1056–1068. doi:10.1038/mp.2016.225
Haase, G., Pettmann, B., Raoul, C., and Henderson, C. E. (2008). Signaling by Death Receptors in the Nervous System. Curr. Opin. Neurobiol. 18 (3), 284–291. doi:10.1016/j.conb.2008.07.013
Hagemeyer, N., Hanft, K.-M., Akriditou, M.-A., Unger, N., Park, E. S., Stanley, E. R., et al. (2017). Microglia Contribute to normal Myelinogenesis and to Oligodendrocyte Progenitor Maintenance during Adulthood. Acta Neuropathol. 134 (3), 441–458. doi:10.1007/s00401-017-1747-1
Hajinejad, M., and Sahab-Negah, S. (2021). Neuroinflammation: The Next Target of Exosomal microRNAs Derived from Mesenchymal Stem Cells in the Context of Neurological Disorders. J. Cell. Physiol. doi:10.1002/jcp.30495
Hambardzumyan, D., Gutmann, D. H., and Kettenmann, H. (2016). The Role of Microglia and Macrophages in Glioma Maintenance and Progression. Nat. Neurosci. 19 (1), 20–27. doi:10.1038/nn.4185
Harding, C., Heuser, J., and Stahl, P. (1983). Receptor-mediated Endocytosis of Transferrin and Recycling of the Transferrin Receptor in Rat Reticulocytes. J. Cel. Biol. 97 (2), 329–339. doi:10.1083/jcb.97.2.329
Hu, X., Leak, R. K., Shi, Y., Suenaga, J., Gao, Y., Zheng, P., et al. (2015). Microglial and Macrophage Polarization-New Prospects for Brain Repair. Nat. Rev. Neurol. 11 (1), 56–64. doi:10.1038/nrneurol.2014.207
Hu, X., Li, P., Guo, Y., Wang, H., Leak, R. K., Chen, S., et al. (2012). Microglia/macrophage Polarization Dynamics Reveal Novel Mechanism of Injury Expansion after Focal Cerebral Ischemia. Stroke 43 (11), 3063–3070. doi:10.1161/strokeaha.112.659656
Huang, S., Ge, X., Yu, J., Han, Z., Yin, Z., Li, Y., et al. (2018). Increased miR‐124‐3p in Microglial Exosomes Following Traumatic Brain Injury Inhibits Neuronal Inflammation and Contributes to Neurite Outgrowthviatheir Transfer into Neurons. FASEB j. 32 (1), 512–528. doi:10.1096/fj.201700673r
Huang, T., Yang, J., Zhang, J., Ke, W., Zou, F., Wan, C., et al. (2020). MicroRNA-101-3p Downregulates TLR2 Expression, Leading to Reduction in Cytokine Production by Treponema Pallidum-Stimulated Macrophages. J. Invest. Dermatol. 140 (8), 1566–1575. doi:10.1016/j.jid.2019.12.012
Iadecola, C., and Anrather, J. (2011). The Immunology of Stroke: from Mechanisms to Translation. Nat. Med. 17 (7), 796–808. doi:10.1038/nm.2399
Jansen, F., and Li, Q. (2017). Exosomes as Diagnostic Biomarkers in Cardiovascular Diseases. Adv. Exp. Med. Biol. 998, 61–70. doi:10.1007/978-981-10-4397-0_4
Jayaraj, R. L., Azimullah, S., Beiram, R., Jalal, F. Y., and Rosenberg, G. A. (2019). Neuroinflammation: Friend and Foe for Ischemic Stroke. J. Neuroinflammation 16 (1), 142. doi:10.1186/s12974-019-1516-2
Ji, J., Xiang, P., Li, T., Lan, L., Xu, X., Lu, G., et al. (2017). NOSH-NBP, a Novel Nitric Oxide and Hydrogen Sulfide- Releasing Hybrid, Attenuates Ischemic Stroke-Induced Neuroinflammatory Injury by Modulating Microglia Polarization. Front. Cel. Neurosci. 11, 154. doi:10.3389/fncel.2017.00154
Ji, J., Xue, T.-F., Guo, X.-D., Yang, J., Guo, R.-B., Wang, J., et al. (2018). Antagonizing Peroxisome Proliferator-Activated Receptor γ Facilitates M1-To-M2 Shift of Microglia by Enhancing Autophagy via the LKB1-AMPK Signaling Pathway. Aging cell 17 (4), e12774. doi:10.1111/acel.12774
Jiang, C. T., Wu, W. F., Deng, Y. H., and Ge, J. W. (2020). Modulators of Microglia Activation and Polarization in Ischemic Stroke (Review). Mol. Med. Rep. 21 (5), 2006–2018. doi:10.3892/mmr.2020.11003
Jiang, M., Wang, H., Jin, M., Yang, X., Ji, H., Jiang, Y., et al. (2018). Exosomes from MiR-30d-5p-ADSCs Reverse Acute Ischemic Stroke-Induced, Autophagy-Mediated Brain Injury by Promoting M2 Microglial/Macrophage Polarization. Cell Physiol Biochem 47 (2), 864–878. doi:10.1159/000490078
Jiang, X., Andjelkovic, A. V., Zhu, L., Yang, T., Bennett, M. V. L., Chen, J., et al. (2018). Blood-brain Barrier Dysfunction and Recovery after Ischemic Stroke. Prog. Neurobiol. 163-164, 144–171. doi:10.1016/j.pneurobio.2017.10.001
Kalluri, R., and LeBleu, V. S. (2020). The Biology, Function, and Biomedical Applications of Exosomes. Science 367 (6478). doi:10.1126/science.aau6977
Kanninen, K. M., Bister, N., Koistinaho, J., and Malm, T. (2016). Exosomes as New Diagnostic Tools in CNS Diseases. Biochim. Biophys. Acta (Bba) - Mol. Basis Dis. 1862 (3), 403–410. doi:10.1016/j.bbadis.2015.09.020
Kano, S. I., Choi, E. Y., Dohi, E., Agarwal, S., Chang, D. J., Wilson, A. M., et al. (2019). Glutathione S-Transferases Promote Proinflammatory Astrocyte-Microglia Communication during Brain Inflammation. Sci. Signal. 12, 12. doi:10.1126/scisignal.aar2124
Khan, H., Pan, J.-J., Li, Y., Zhang, Z., YangNative, G.-Y., and Exosomes, Bioengineered, (2021). Native and Bioengineered Exosomes for Ischemic Stroke Therapy. Front. Cel Dev. Biol. 9, 619565. doi:10.3389/fcell.2021.619565
Kim, S., and Son, Y. (2021). Astrocytes Stimulate Microglial Proliferation and M2 Polarization In Vitro through Crosstalk between Astrocytes and Microglia. Int. J. Mol. Sci. 22.
Kumar, A., Bhatia, H. S., de Oliveira, A. C. P., and Fiebich, B. L. (2015). microRNA-26a Modulates Inflammatory Response Induced by Toll-like Receptor 4 Stimulation in Microglia. J. Neurochem. 135 (6), 1189–1202. doi:10.1111/jnc.13364
Li, B., Cao, Y., Sun, M., and Feng, H. (2021). Expression, Regulation, and Function of Exosome-Derived miRNAs in Cancer Progression and Therapy. FASEB Journal. official Publ. Fed. Am. Societies Exp. Biol. 35 (10), e21916. doi:10.1096/fj.202100294rr
Li, F., Zhao, L., Shi, Y., and Liang, J. (2020). Edaravone-Loaded Macrophage-Derived Exosomes Enhance Neuroprotection in the Rat Permanent Middle Cerebral Artery Occlusion Model of Stroke. Mol. Pharmaceutics 17 (9), 3192–3201. doi:10.1021/acs.molpharmaceut.0c00245
Li, G., Xiao, L., Qin, H., Zhuang, Q., Zhang, W., Liu, L., et al. (2020). Exosomes-carried microRNA-26b-5p Regulates Microglia M1 Polarization after Cerebral Ischemia/reperfusion. Cell Cycle 19 (9), 1022–1035. doi:10.1080/15384101.2020.1743912
Li, H., Guan, S.-B., Lu, Y., and Wang, F. (2017). MiR-140-5p Inhibits Synovial Fibroblasts Proliferation and Inflammatory Cytokines Secretion through Targeting TLR4. Biomed. Pharmacother. 96, 208–214. doi:10.1016/j.biopha.2017.09.079
Li, J. J., Wang, B., Kodali, M. C., Chen, C., Kim, E., Patters, B. J., et al. (2018). In Vivo evidence for the Contribution of Peripheral Circulating Inflammatory Exosomes to Neuroinflammation. J. Neuroinflammation 15 (1), 8. doi:10.1186/s12974-017-1038-8
Li, J., Xue, H., Li, T., Chu, X., Xin, D., Xiong, Y., et al. (2019). Exosomes Derived from Mesenchymal Stem Cells Attenuate the Progression of Atherosclerosis in ApoE−/- Mice via miR-Let7 Mediated Infiltration and Polarization of M2 Macrophage. Biochem. biophysical Res. Commun. 510 (4), 565–572. doi:10.1016/j.bbrc.2019.02.005
Li, X., Liu, L., Yang, J., Yu, Y., Chai, J., Wang, L., et al. (2016). Exosome Derived from Human Umbilical Cord Mesenchymal Stem Cell Mediates MiR-181c Attenuating Burn-Induced Excessive Inflammation. EBioMedicine 8, 72–82. doi:10.1016/j.ebiom.2016.04.030
Li, Y.-J., Wu, J.-Y., Liu, J., Xu, W., Qiu, X., Huang, S., et al. (2021). Artificial Exosomes for Translational Nanomedicine. J. Nanobiotechnol 19 (1), 242. doi:10.1186/s12951-021-00986-2
Li, Y., Guo, W., and Cai, Y. (2020). NEAT1 Promotes LPS-Induced Inflammatory Injury in Macrophages by Regulating MiR-17-5p/TLR4. Open Med. (Warsaw, Poland) 15, 38–49. doi:10.1515/med-2020-0007
Lian, H., Litvinchuk, A., Chiang, A. C.-A., Aithmitti, N., Jankowsky, J. L., and Zheng, H. (2016). Astrocyte-Microglia Cross Talk through Complement Activation Modulates Amyloid Pathology in Mouse Models of Alzheimer's Disease. J. Neurosci. 36 (2), 577–589. doi:10.1523/jneurosci.2117-15.2016
Liao, W., Du, Y., Zhang, C., Pan, F., Yao, Y., Zhang, T., et al. (2019). Exosomes: The Next Generation of Endogenous Nanomaterials for Advanced Drug Delivery and Therapy. Acta Biomater. 86, 1–14. doi:10.1016/j.actbio.2018.12.045
Liddelow, S. A., and Barres, B. A. (2017). Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 46 (6), 957–967. doi:10.1016/j.immuni.2017.06.006
Liesz, A., Dalpke, A., Mracsko, E., Roth, S., Zhou, W., Yang, H., et al. (2015). DAMP Signaling Is a Key Pathway Inducing Immune Modulation after Brain Injury. J. Neurosci. 35 (2), 583–598. doi:10.1523/jneurosci.2439-14.2015
Lim, G. B. (2021). Exosome-eluting Stents Improve Vascular Remodelling. Nat. Rev. Cardiol. 18 (6), 386. doi:10.1038/s41569-021-00557-w
Lin, S., Yin, Q., Zhong, Q., Lv, F. L., Zhou, Y., Li, J. Q., et al. (2012). Heme Activates TLR4-Mediated Inflammatory Injury via MyD88/TRIF Signaling Pathway in Intracerebral Hemorrhage. J. Neuroinflammation 9, 46. doi:10.1186/1742-2094-9-46
Liu, R., Diao, J., He, S., Li, B., Fei, Y., Li, Y., et al. (2018). XQ-1H Protects against Ischemic Stroke by Regulating Microglia Polarization through PPARγ Pathway in Mice. Int. immunopharmacology 57, 72–81. doi:10.1016/j.intimp.2018.02.014
Liu, W., Rong, Y., Wang, J., Zhou, Z., Ge, X., Ji, C., et al. (2020). Exosome-shuttled miR-216a-5p from Hypoxic Preconditioned Mesenchymal Stem Cells Repair Traumatic Spinal Cord Injury by Shifting Microglial M1/M2 Polarization. J. Neuroinflammation 17 (1), 47. doi:10.1186/s12974-020-1726-7
Liu, X., Liu, J., Zhao, S., Zhang, H., Cai, W., Cai, M., et al. (2016). Interleukin-4 Is Essential for Microglia/Macrophage M2 Polarization and Long-Term Recovery after Cerebral Ischemia. Stroke 47 (2), 498–504. doi:10.1161/strokeaha.115.012079
Liu, X., Zhang, M., Liu, H., Zhu, R., He, H., Zhou, Y., et al. (2021). Bone Marrow Mesenchymal Stem Cell-Derived Exosomes Attenuate Cerebral Ischemia-Reperfusion Injury-Induced Neuroinflammation and Pyroptosis by Modulating Microglia M1/M2 Phenotypes. Exp. Neurol. 341, 113700. doi:10.1016/j.expneurol.2021.113700
Lou, N., Takano, T., Pei, Y., Xavier, A. L., Goldman, S. A., and Nedergaard, M. (2016). Purinergic Receptor P2RY12-dependent Microglial Closure of the Injured Blood-Brain Barrier. Proc. Natl. Acad. Sci. USA 113 (4), 1074–1079. doi:10.1073/pnas.1520398113
Masyuk, A. I., Masyuk, T. V., and Larusso, N. F. (2013). Exosomes in the Pathogenesis, Diagnostics and Therapeutics of Liver Diseases. J. Hepatol. 59 (3), 621–625. doi:10.1016/j.jhep.2013.03.028
Matcovitch-Natan, O., Winter, D. R., Giladi, A., Vargas Aguilar, S., Spinrad, A., Sarrazin, S., et al. (2016). Microglia Development Follows a Stepwise Program to Regulate Brain Homeostasis. Science 353, aad8670.
Melikyan, G. B. (2014). HIV Entry: a Game of Hide-And-Fuse? Curr. Opin. Virol. 4, 1–7. doi:10.1016/j.coviro.2013.09.004
Micheau, O., and Tschopp, J. (2003). Induction of TNF Receptor I-Mediated Apoptosis via Two Sequential Signaling Complexes. Cell. 114 (2), 181–190. doi:10.1016/s0092-8674(03)00521-x
Mirza, M. A., Xu, Y., McCullough, L. D., and Liu, F. (2015). Abstract 20: Role of IRF5-IRF4 Regulatory Axis in Microglial Polarization after Neonatal Stroke. Stroke 46 (Suppl. l_1), A20. doi:10.1161/str.46.suppl_1.20()
Mittelbrunn, M., and Sánchez-Madrid, F. (2012). Intercellular Communication: Diverse Structures for Exchange of Genetic Information. Nat. Rev. Mol. Cel Biol 13 (5), 328–335. doi:10.1038/nrm3335
Mo, Y., Sun, Y. Y., and Liu, K. Y. (2020). Autophagy and Inflammation in Ischemic Stroke. Neural Regen. Res. 15 (8), 1388–1396. doi:10.4103/1673-5374.274331
Murata, Y., Sugimoto, K., Yang, C., Harada, K., Gono, R., Harada, T., et al. (2020). Activated Microglia-Derived Macrophage-like Cells Exacerbate Brain Edema after Ischemic Stroke Correlate with Astrocytic Expression of Aquaporin-4 and Interleukin-1 Alpha Release. Neurochem. Int. 140, 104848. doi:10.1016/j.neuint.2020.104848
Nasser, M., Masood, M., Adlat, S., Gang, D., Zhu, S., Li, G., et al. (2021). Mesenchymal Stem Cell-Derived Exosome microRNA as Therapy for Cardiac Ischemic Injury. Biomed. Pharmacother. 143, 112118. doi:10.1016/j.biopha.2021.112118
Nedergaard, M., and Dirnagl, U. (2005). Role of Glial Cells in Cerebral Ischemia. Glia 50 (4), 281–286. doi:10.1002/glia.20205
Orellana-Urzúa, S., Rojas, I., Líbano, L., and Rodrigo, R. (2020). Pathophysiology of Ischemic Stroke: Role of Oxidative Stress. Cpd 26 (34), 4246–4260. doi:10.2174/1381612826666200708133912
Ozaki, T., Nakamura, H., and Kishima, H. (2019). Therapeutic Strategy against Ischemic Stroke with the Concept of Neurovascular Unit. Neurochem. Int. 126, 246–251. doi:10.1016/j.neuint.2019.03.022
Pan, B.-T., and Johnstone, R. M. (1983). Fate of the Transferrin Receptor during Maturation of Sheep Reticulocytes In Vitro: Selective Externalization of the Receptor. Cell. 33 (3), 967–978. doi:10.1016/0092-8674(83)90040-5
Pandey, G. N., Zhang, H., Sharma, A., and Ren, X. (2021). Innate Immunity Receptors in Depression and Suicide: Upregulated NOD-like Receptors Containing Pyrin (NLRPs) and Hyperactive Inflammasomes in the Postmortem Brains of People Who Were Depressed and Died by Suicide. JPN 46 (5), E538–E547. doi:10.1503/jpn.210016
Paolicelli, R. C., Bolasco, G., Pagani, F., Maggi, L., Scianni, M., Panzanelli, P., et al. (2011). Synaptic Pruning by Microglia Is Necessary for normal Brain Development. Science 333 (6048), 1456–1458. doi:10.1126/science.1202529
Pegtel, D. M., Cosmopoulos, K., Thorley-Lawson, D. A., van Eijndhoven, M. A. J., Hopmans, E. S., Lindenberg, J. L., et al. (2010). Functional Delivery of Viral miRNAs via Exosomes. Proc. Natl. Acad. Sci. 107 (14), 6328–6333. doi:10.1073/pnas.0914843107
Profaci, C. P., Munji, R. N., Pulido, R. S., and Daneman, R. (2020). The Blood-Brain Barrier in Health and Disease: Important Unanswered Questions. J. Exp. Med. 217, 217. doi:10.1084/jem.20190062
Qi, Z., Zhao, Y., Su, Y., Cao, B., Yang, J.-J., and Xing, Q. (2021). Serum Extracellular Vesicle-Derived miR-124-3p as a Diagnostic and Predictive Marker for Early-Stage Acute Ischemic Stroke. Front. Mol. Biosci. 8, 685088. doi:10.3389/fmolb.2021.685088
Qian, D., Wei, G., Xu, C., He, Z., Hua, J., Li, J., et al. (2017). Bone Marrow-Derived Mesenchymal Stem Cells (BMSCs) Repair Acute Necrotized Pancreatitis by Secreting microRNA-9 to Target the NF-κB1/p50 Gene in Rats. Sci. Rep. 7 (1), 581. doi:10.1038/s41598-017-00629-3
Radak, D., Katsiki, N., Resanovic, I., Jovanovic, A., Sudar-Milovanovic, E., Zafirovic, S., et al. (2017). Apoptosis and Acute Brain Ischemia in Ischemic Stroke. Cvp 15 (2), 115–122. doi:10.2174/1570161115666161104095522
Robson, A. (2021). Exosome-derived microRNAs Improve Cardiac Function. Nat. Rev. Cardiol. 18 (3), 150–151. doi:10.1038/s41569-020-00498-w
Rufino-Ramos, D., Albuquerque, P. R., Carmona, V., Perfeito, R., Nobre, R. J., and Pereira de Almeida, L. (2017). Extracellular Vesicles: Novel Promising Delivery Systems for Therapy of Brain Diseases. J. Controlled Release 262, 247–258. doi:10.1016/j.jconrel.2017.07.001
Shi, S. X., Li, Y.-J., Shi, K., Wood, K., Ducruet, A. F., and Liu, Q. (2020). IL (Interleukin)-15 Bridges Astrocyte-Microglia Crosstalk and Exacerbates Brain Injury Following Intracerebral Hemorrhage. Stroke 51 (3), 967–974. doi:10.1161/strokeaha.119.028638
Shu, Z.-M., Shu, X.-D., Li, H.-Q., Sun, Y., Shan, H., Sun, X.-Y., et al. (2016). Ginkgolide B Protects against Ischemic Stroke via Modulating Microglia Polarization in Mice. CNS Neurosci. Ther. 22 (9), 729–739. doi:10.1111/cns.12577
Siglienti, I., Chan, A., Kleinschnitz, C., Jander, S., Toyka, K. V., Gold, R., et al. (2007). Downregulation of Transforming Growth Factor-Β2 Facilitates Inflammation in the Central Nervous System by Reciprocal Astrocyte/Microglia Interactions. J. Neuropathol. Exp. Neurol. 66 (1), 47–56. doi:10.1097/nen.0b013e31802d47b4
Simpson, D. S. A., and Oliver, P. L. (2020). ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants (Basel) 9, 9. doi:10.3390/antiox9080743
Skog, J., Würdinger, T., van Rijn, S., Meijer, D. H., Gainche, L., Curry, W. T., et al. (2008). Glioblastoma Microvesicles Transport RNA and Proteins that Promote Tumour Growth and Provide Diagnostic Biomarkers. Nat. Cel Biol 10 (12), 1470–1476. doi:10.1038/ncb1800
Socodato, R., Portugal, C. C., Rodrigues, A., Henriques, J., Rodrigues, C., Figueira, C., et al. (2018). Redox Tuning of Ca 2+ Signaling in Microglia Drives Glutamate Release during Hypoxia. Free Radic. Biol. Med. 118, 137–149. doi:10.1016/j.freeradbiomed.2018.02.036
Song, Y., Li, Z., He, T., Qu, M., Jiang, L., Li, W., et al. (2019). M2 Microglia-Derived Exosomes Protect the Mouse Brain from Ischemia-Reperfusion Injury via Exosomal miR-124. Theranostics 9 (10), 2910–2923. doi:10.7150/thno.30879
Spejo, A. B., Chiarotto, G. B., Ferreira, A. D. F., Gomes, D. A., Ferreira, R. S., Barraviera, B., et al. (2018). Neuroprotection and Immunomodulation Following Intraspinal Axotomy of Motoneurons by Treatment with Adult Mesenchymal Stem Cells. J. Neuroinflammation 15 (1), 230. doi:10.1186/s12974-018-1268-4
Sun, K., Zhang, J., Yang, Q., Zhu, J., Zhang, X., Wu, K., et al. (2021). Dexmedetomidine Exerts a Protective Effect on Ischemic Brain Injury by Inhibiting the P2X7R/NLRP3/Caspase-1 Signaling Pathway. Brain Res. Bull. 174, 11–21. doi:10.1016/j.brainresbull.2021.05.006
Szalay, G., Martinecz, B., Lénárt, N., Környei, Z., Orsolits, B., Judák, L., et al. (2016). Microglia Protect against Brain Injury and Their Selective Elimination Dysregulates Neuronal Network Activity after Stroke. Nat. Commun. 7, 11499. doi:10.1038/ncomms11499
Taetzsch, T., Levesque, S., McGraw, C., Brookins, S., Luqa, R., Bonini, M. G., et al. (2015). Redox Regulation of NF-Κb P50 and M1 Polarization in Microglia. Glia 63 (3), 423–440. doi:10.1002/glia.22762
Théry, C., Witwer, K. W., Aikawa, E., Alcaraz, M. J., Anderson, J. D., Andriantsitohaina, R., et al. (2018). Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): a Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell Vesicles 7 (1), 1535750. doi:10.1080/20013078.2018.1535750
Thiebaut, A. M., Gauberti, M., Ali, C., Martinez De Lizarrondo, S., Vivien, D., Yepes, M., et al. (2018). The Role of Plasminogen Activators in Stroke Treatment: Fibrinolysis and beyond. Lancet Neurol. 17 (12), 1121–1132. doi:10.1016/s1474-4422(18)30323-5
Thomi, G., Surbek, D., Haesler, V., Joerger-Messerli, M., and Schoeberlein, A. (2019). Exosomes Derived from Umbilical Cord Mesenchymal Stem Cells Reduce Microglia-Mediated Neuroinflammation in Perinatal Brain Injury. Stem Cel Res Ther 10 (1), 105. doi:10.1186/s13287-019-1207-z
Ti, D., Hao, H., Tong, C., Liu, J., Dong, L., Zheng, J., et al. (2015). LPS-preconditioned Mesenchymal Stromal Cells Modify Macrophage Polarization for Resolution of Chronic Inflammation via Exosome-Shuttled Let-7b. J. Transl Med. 13, 308. doi:10.1186/s12967-015-0642-6
Tian, T., Cao, L., He, C., Ye, Q., Liang, R., You, W., et al. (2021). Targeted Delivery of Neural Progenitor Cell-Derived Extracellular Vesicles for Anti-inflammation after Cerebral Ischemia. Theranostics 11 (13), 6507–6521. doi:10.7150/thno.56367
Tian, Y., Zhu, P., Liu, S., Jin, Z., Li, D., Zhao, H., et al. (2019). IL-4-polarized BV2 Microglia Cells Promote Angiogenesis by Secreting Exosomes. Adv. Clin. Exp. Med. 28 (4), 421–430. doi:10.17219/acem/91826
Tsai, T. Y., Liow, M. H. L., Li, G., Arauz, P., Peng, Y., Klemt, C., et al. (2019). Bi‐Cruciate Retaining Total Knee Arthroplasty Does Not Restore Native Tibiofemoral Articular Contact Kinematics during Gait. J. Orthop. Res. 37 (9), 1929–1937. doi:10.1002/jor.24333
Tuo, Q. Z., Zhang, S. T., and Lei, P. (2021). Mechanisms of Neuronal Cell Death in Ischemic Stroke and Their Therapeutic Implications. Med. Res. Rev. doi:10.1002/med.21817
Wake, H., Moorhouse, A. J., Miyamoto, A., and Nabekura, J. (2013). Microglia: Actively Surveying and Shaping Neuronal Circuit Structure and Function. Trends Neurosciences 36 (4), 209–217. doi:10.1016/j.tins.2012.11.007
Wang, D., Wei, Y., Tian, J., He, D., Zhang, R., Ji, X., et al. (2021). Oxiracetam Mediates Neuroprotection through the Regulation of Microglia under Hypoxia-Ischemia Neonatal Brain Injury in Mice. Mol. Neurobiol. 58 (8), 3918–3937. doi:10.1007/s12035-021-02376-z
Wang, K., Sun, Z., Ru, J., Wang, S., Huang, L., Ruan, L., et al. (2020). Ablation of GSDMD Improves Outcome of Ischemic Stroke through Blocking Canonical and Non-canonical Inflammasomes Dependent Pyroptosis in Microglia. Front. Neurol. 11, 577927. doi:10.3389/fneur.2020.577927
Wang, P.-F., Fang, H., Chen, J., Lin, S., Liu, Y., Xiong, X.-Y., et al. (2014). Polyinosinic-Polycytidylic Acid Has Therapeutic Effects against Cerebral Ischemia/Reperfusion Injury through the Downregulation of TLR4 Signaling via TLR3. J.I. 192 (10), 4783–4794. doi:10.4049/jimmunol.1303108
Wang, X., Gu, H., Qin, D., Yang, L., Huang, W., Essandoh, K., et al. (2015). Exosomal miR-223 Contributes to Mesenchymal Stem Cell-Elicited Cardioprotection in Polymicrobial Sepsis. Sci. Rep. 5, 13721. doi:10.1038/srep13721
Wang, Y., Guan, X., Gao, C.-L., Ruan, W., Zhao, S., Kai, G., et al. (2021). Medioresinol as a Novel PGC-1α Activator Prevents Pyroptosis of Endothelial Cells in Ischemic Stroke through PPARα-GOT1 axis. Pharmacol. Res. 169, 105640. doi:10.1016/j.phrs.2021.105640
Wei, M., Li, C., Yan, Z., Hu, Z., Dong, L., Zhang, J., et al. (2021). Activated Microglia Exosomes Mediated miR-383-3p Promotes Neuronal Necroptosis through Inhibiting ATF4 Expression in Intracerebral Hemorrhage. Neurochem. Res. 46 (6), 1337–1349. doi:10.1007/s11064-021-03268-3
Xia, C.-Y., Zhang, S., Chu, S.-F., Wang, Z.-Z., Song, X.-Y., Zuo, W., et al. (2016). Autophagic Flux Regulates Microglial Phenotype According to the Time of Oxygen-Glucose Deprivation/reperfusion. Int. Immunopharmacology 39, 140–148. doi:10.1016/j.intimp.2016.06.030
Xia, C.-Y., Zhang, S., Gao, Y., Wang, Z.-Z., and Chen, N.-H. (2015). Selective Modulation of Microglia Polarization to M2 Phenotype for Stroke Treatment. Int. immunopharmacology 25 (2), 377–382. doi:10.1016/j.intimp.2015.02.019
Xie, L., Zhao, H., Wang, Y., and Chen, Z. (2020). Exosomal Shuttled miR-424-5p from Ischemic Preconditioned Microglia Mediates Cerebral Endothelial Cell Injury through Negatively Regulation of FGF2/STAT3 Pathway. Exp. Neurol. 333, 113411. doi:10.1016/j.expneurol.2020.113411
Xie, M., Yi, C., Luo, X., Xu, S., Yu, Z., Tang, Y., et al. (2011). Glial gap Junctional Communication Involvement in Hippocampal Damage after Middle Cerebral Artery Occlusion. Ann. Neurol. 70 (1), 121–132. doi:10.1002/ana.22386
Xiong, X.-Y., Liu, L., and Yang, Q.-W. (2016). Functions and Mechanisms of Microglia/macrophages in Neuroinflammation and Neurogenesis after Stroke. Prog. Neurobiol. 142, 23–44. doi:10.1016/j.pneurobio.2016.05.001
Xu, S., Wang, J., Zhong, J., Shao, M., Jiang, J., Song, J., et al. (2021). CD73 Alleviates GSDMD-Mediated Microglia Pyroptosis in Spinal Cord Injury through PI3K/AKT/Foxo1 Signaling. Clin. Transl Med. 11 (1), e269. doi:10.1002/ctm2.269
Xu, X., Zhang, L., Ye, X., Hao, Q., Zhang, T., Cui, G., et al. (2018). Nrf2/ARE Pathway Inhibits ROS-Induced NLRP3 Inflammasome Activation in BV2 Cells after Cerebral Ischemia Reperfusion. Inflamm. Res. 67 (1), 57–65. doi:10.1007/s00011-017-1095-6
Yan, A., Zhang, T., Yang, X., Shao, J., Fu, N., Shen, F., et al. (2016). Thromboxane A2 Receptor Antagonist SQ29548 Reduces Ischemic Stroke-Induced Microglia/macrophages Activation and Enrichment, and Ameliorates Brain Injury. Sci. Rep. 6, 35885. doi:10.1038/srep35885
Yang, H.-C., Zhang, M., Wu, R., Zheng, H.-Q., Zhang, L.-Y., Luo, J., et al. (2020). C-C Chemokine Receptor Type 2-overexpressing Exosomes Alleviated Experimental post-stroke Cognitive Impairment by Enhancing Microglia/macrophage M2 Polarization. Wjsc 12 (2), 152–167. doi:10.4252/wjsc.v12.i2.152
Yang, J., Chen, Y., Jiang, K., Zhao, G., Guo, S., Liu, J., et al. (2020). MicroRNA‐182 Supplies Negative Feedback Regulation to Ameliorate Lipopolysaccharide‐induced ALI in Mice by Targeting TLR4. J. Cel Physiol 235 (9), 5925–5937. doi:10.1002/jcp.29504
Yang, J., Wu, S., Hou, L., Zhu, D., Yin, S., Yang, G., et al. (2020). Therapeutic Effects of Simultaneous Delivery of Nerve Growth Factor mRNA and Protein via Exosomes on Cerebral Ischemia. Mol. Ther. - Nucleic Acids 21, 512–522. doi:10.1016/j.omtn.2020.06.013
Yang, L.-k., Lu, L., Yue, J., Wang, X.-s., Qi, J.-y., Yang, F., et al. (2021). Activation of Microglial G-protein-C-oupled R-eceptor 30 P-rotects N-eurons against E-xcitotoxicity through NF-Κb-/MAPK P-athways. Brain Res. Bull. 172, 22–30. doi:10.1016/j.brainresbull.2021.04.005
Yang, Q., Huang, Q., Hu, Z., and Tang, X. (2019). Potential Neuroprotective Treatment of Stroke: Targeting Excitotoxicity, Oxidative Stress, and Inflammation. Front. Neurosci. 13, 1036. doi:10.3389/fnins.2019.01036
Yang, S., Wang, H., Yang, Y., Wang, R., Wang, Y., Wu, C., et al. (2019). Baicalein Administered in the Subacute Phase Ameliorates Ischemia-Reperfusion-Induced Brain Injury by Reducing Neuroinflammation and Neuronal Damage. Biomed. Pharmacother. 117, 109102. doi:10.1016/j.biopha.2019.109102
Yang, Y., Salayandia, V. M., Thompson, J. F., Yang, L. Y., Estrada, E. Y., and Yang, Y. (2015). Attenuation of Acute Stroke Injury in Rat Brain by Minocycline Promotes Blood-Brain Barrier Remodeling and Alternative Microglia/macrophage Activation during Recovery. J. neuroinflammation 12, 26. doi:10.1186/s12974-015-0245-4
Yao, L., Kan, E. M., Kaur, C., Dheen, S. T., Hao, A., Lu, J., et al. (2013). Notch-1 Signaling Regulates Microglia Activation via NF-Κb Pathway after Hypoxic Exposure In Vivo and In Vitro. PloS one 8 (11), e78439. doi:10.1371/journal.pone.0078439
Zabala, A., Vazquez-Villoldo, N., Rissiek, B., Gejo, J., Martin, A., Palomino, A., et al. (2018). P2X4 Receptor Controls Microglia Activation and Favors Remyelination in Autoimmune Encephalitis. EMBO Mol. Med. 10, 10. doi:10.15252/emmm.201708743
Zang, J., Wu, Y., Su, X., Zhang, T., Tang, X., Ma, D., et al. (2020). Inhibition of PDE1-B by Vinpocetine Regulates Microglial Exosomes and Polarization through Enhancing Autophagic Flux for Neuroprotection against Ischemic Stroke. Front Cel Dev Biol 8, 616590. doi:10.3389/fcell.2020.616590
Zha, Z., Gao, Y. F., Ji, J., Sun, Y. Q., Li, J. L., Qi, F., et al. (2021). Bu Shen Yi Sui Capsule Alleviates Neuroinflammation and Demyelination by Promoting Microglia toward M2 Polarization, Which Correlates with Changes in miR-124 and miR-155 in Experimental Autoimmune Encephalomyelitis. Oxid Med. Cel Longev 2021, 5521503. doi:10.1155/2021/5521503
Zhang, H., Lin, S., McElroy, C. L., Wang, B., Jin, D., Uteshev, V. V., et al. (2021). Circulating Pro-inflammatory Exosomes Worsen Stroke Outcomes in Aging. Circ. Res. 129 (7), e121–e40. doi:10.1161/CIRCRESAHA.121.318897
Zhang, L., Lu, X., Gong, L., Cui, L., Zhang, H., Zhao, W., et al. (2021). Tetramethylpyrazine Protects Blood-Spinal Cord Barrier Integrity by Modulating Microglia Polarization through Activation of STAT3/SOCS3 and Inhibition of NF-Кb Signaling Pathways in Experimental Autoimmune Encephalomyelitis Mice. Cell Mol Neurobiol 41 (4), 717–731. doi:10.1007/s10571-020-00878-3
Zhang, M., Gillaspy, A. F., Gipson, J. R., Cassidy, B. R., Nave, J. L., Brewer, M. F., et al. (2018). Neuroinvasive Listeria Monocytogenes Infection Triggers IFN-Activation of Microglia and Upregulates Microglial miR-155. Front. Immunol. 9, 2751. doi:10.3389/fimmu.2018.02751
Zhang, Q., Chen, Z., Zhang, Z., Jin, Z., Muratoglu, O. K., and Varadarajan, K. M. (2020). Leveraging Subject-specific Musculoskeletal Modeling to Assess Effect of Anterior Cruciate Ligament Retaining Total Knee Arthroplasty during Walking Gait. Proc. Inst. Mech. Eng. H 234 (12), 1445–1456. doi:10.1177/0954411920947204
Zhang, Q., He, L., Chen, M., Yang, H., Cao, X., Liu, X., et al. (2021). PSD‐93 Mediates the Crosstalk between Neuron and Microglia and Facilitates Acute Ischemic Stroke Injury by Binding to CX3CL1. J. Neurochem. 157 (6), 2145–2157. doi:10.1111/jnc.15324
Zhang, R., Xu, M., Wang, Y., Xie, F., Zhang, G., and Qin, X. (2017). Nrf2-a Promising Therapeutic Target for Defensing against Oxidative Stress in Stroke. Mol. Neurobiol. 54 (8), 6006–6017. doi:10.1007/s12035-016-0111-0
Zhang, Y., Hu, Y.-W., Zheng, L., and Wang, Q. (2017). Characteristics and Roles of Exosomes in Cardiovascular Disease. DNA Cel. Biol. 36 (3), 202–211. doi:10.1089/dna.2016.3496
Zhang, Z., Zou, X., Zhang, R., Xie, Y., Feng, Z., Li, F., et al. (2021). Human Umbilical Cord Mesenchymal Stem Cell-Derived Exosomal miR-146a-5p Reduces Microglial-Mediated Neuroinflammation via Suppression of the IRAK1/TRAF6 Signaling Pathway after Ischemic Stroke. Aging 13 (2), 3060–3079. doi:10.18632/aging.202466
Zhao, B., Fei, Y., Zhu, J., Yin, Q., Fang, W., and Li, Y. (2021). PAF Receptor Inhibition Attenuates Neuronal Pyroptosis in Cerebral Ischemia/Reperfusion Injury. Mol. Neurobiol. doi:10.1007/s12035-021-02537-0
Zhao, S.-c., Ma, L.-s., Chu, Z.-h., Xu, H., Wu, W.-q., and Liu, F. (2017). Regulation of Microglial Activation in Stroke. Acta Pharmacol. Sin 38 (4), 445–458. doi:10.1038/aps.2016.162
Zhao, Y., Gan, Y., Xu, G., Hua, K., and Liu, D. (2020). Exosomes from MSCs Overexpressing microRNA-223-3p Attenuate Cerebral Ischemia through Inhibiting Microglial M1 Polarization Mediated Inflammation. Life Sci. 260, 118403. doi:10.1016/j.lfs.2020.118403
Zheng, Y., He, R., Wang, P., Shi, Y., Zhao, L., and Liang, J. (2019). Exosomes from LPS-Stimulated Macrophages Induce Neuroprotection and Functional Improvement after Ischemic Stroke by Modulating Microglial Polarization. Biomater. Sci. 7 (5), 2037–2049. doi:10.1039/c8bm01449c
Zhu, J., Cao, D., Guo, C., Liu, M., Tao, Y., Zhou, J., et al. (2019). Berberine Facilitates Angiogenesis against Ischemic Stroke through Modulating Microglial Polarization via AMPK Signaling. Cel Mol Neurobiol 39 (6), 751–768. doi:10.1007/s10571-019-00675-7
Keywords: exosomes, microglia polarization, stroke treatment, microRNA, neuroinflammation, neuroprotection
Citation: Wan T, Huang Y, Gao X, Wu W and Guo W (2022) Microglia Polarization: A Novel Target of Exosome for Stroke Treatment. Front. Cell Dev. Biol. 10:842320. doi: 10.3389/fcell.2022.842320
Received: 23 December 2021; Accepted: 14 February 2022;
Published: 09 March 2022.
Edited by:
Sylwia Bobis-Wozowicz, Jagiellonian University, PolandReviewed by:
Zhijun Zhang, Shanghai Jiao Tong University, ChinaCopyright © 2022 Wan, Huang, Gao, Wu and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Teng Wan, d2FudGVuZ0B4dWVoYWl3dXlhLmNsdWI=; Weiming Guo, Z3Vvd2VpbWluZ0B4dWVoYWl3dXlhLmNsdWI=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.